Note: Descriptions are shown in the official language in which they were submitted.
81625301
PIPETTE TIPS
Related Patent Applications
This patent application claims priority to U.S. provisional patent application
no. 61/297,658 and U.S.
design patent application no. 29/354,398, each filed January 22, 2010, and
entitled PIPETTE TIPS,
naming Arta Motadel, Peter Paul Blaszcak, Phillip Chad Hairfield, and Sean
Michael Callahan as
inventors, and designated by Attorney Docket Nos. PEL-1011-PV and PEL-1011-
DUS,
respectively. This patent application also claims priority to U.S. provisional
patent application
no. 61/411, 859, filed November 9, 2010, and entitled PIPETTE TIPS, naming
Arta Motadel, Peter
Paul Blaszcak, Phillip Chad Hairfield, and Sean Michael Callahan as inventors,
and designated by
Attorney Docket No. PEL-1011-PV2.
Field
The technology relates in part to pipette tips and methods for using them.
Background
Pipette tips are utilized in a variety of industries that have a requirement
for handling fluids, and are
used in facilities including medical laboratories and research laboratories,
for example. In many
instances pipette tips are used in large numbers, and often are utilized for
processing many
samples and/or adding many reagents to samples, for example.
Pipette tips often are substantially cone-shaped with an aperture at one end
that can engage a
dispensing device, and another relatively smaller aperture at the other end
that can receive and
emit fluid. Pipette tips generally are manufactured from a moldable plastic,
such as polypropylene,
for example. Pipette tips are made in a number of sizes to allow for accurate
and reproducible
liquid handling for volumes ranging from nanoliters to milliliters.
Pipette tips can be utilized in conjunction with a variety of dispensing
devices, including manual
dispensers (e.g., pipettors) and automated dispensers. A dispenser is a device
that, when
1
CA 2787274 2017-07-26
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
attached to the upper end of a pipette tip (the larger opening end), applies
negative pressure to
acquire fluids, and applies positive pressure to dispense fluids. The lower or
distal portion of a
dispenser (typically referred to as the barrel or nozzle) is placed in contact
with the upper end of
the pipette tip and held in place by pressing the barrel or nozzle of the
dispenser into the upper end
of the pipette tip. The combination then can be used to manipulate liquid
samples.
Summary
In some embodiments, provided are pipette tips comprising a proximal region
and a distal region,
where the proximal region comprises an exterior surface and an annular flange
at the proximal
terminus of the proximal region, the proximal region comprises a first set of
axially oriented ribs and
a second set of axially oriented ribs, the ribs of the first set and the
second set are circumferentially
spaced and alternately spaced around the exterior surface of the proximal
region, and ribs of the
first set have a maximum thickness greater than the maximum thickness of ribs
of the second set.
In certain embodiments, the distal region wall thickness tapers from (a) a
point at or between (i)
about the junction of the proximal region and distal region to (ii) about one-
quarter of the axial
distance from the terminus of the distal region to the junction, to (b) the
distal region terminus, and
the wall thickness at the distal region terminus is about 0.0040 inches to
about 0.0055 inches.
Provided also, in some embodiments, are pipette tips comprising a proximal
region and a distal
region, where the proximal region comprises an exterior surface and an annular
flange at the
proximal terminus of the proximal region, the distal region wall thickness
tapers from (a) a point at
or between (i) about the junction of the proximal region and distal region to
(ii) about one-quarter of
the axial distance from the terminus of the distal region to the junction, to
(b) the distal region
terminus, and the wall thickness at the distal region terminus is about 0.0040
inches to about
0.0055 inches. In certain embodiments, the proximal region comprises a first
set of axially oriented
ribs and a second set of axially oriented ribs. In some embodiments, the ribs
of the first set and
the second set are circumferentially spaced and alternately spaced around the
exterior surface of
the proximal region. In certain embodiments, ribs of the first set have a
maximum thickness
greater than the maximum thickness of ribs of the second set.
Some pipette tip embodiments can comprise rib sets of differing thickness
disposed on, or co-
extensive with, the flexible proximal region. In some embodiments, ribs can
have a profile shape
selected from an arc, pyramid, flat, rectangle, semi-circular, stepped,
triangle, rhombus,
2
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
parallelogram, trapezoid, and the like, and combinations thereof. In some
embodiments, ribs can
be disposed at a particular distance below the flange terminal opening of the
pipette tip (e.g., the
top boundary of each section of increased thickness can be offset from the
edge of the pipette tip).
A pipette tip sometimes includes a region of increased thickness (e.g., ribs)
at an outer or exterior
surface of the proximal region of the pipette tip, while retaining a
substantially smooth inner surface
in the proximal region, in specific embodiments. On a pipette tip, (i) one or
more ribs may be
coextensive with a portion of the flange, (ii) one or more ribs may be
coextensive with the
flange/proximal region junction, (iii) one or more ribs may terminate at a
point on the proximal
region before the flange/proximal region junction, (iv) one or more ribs may
be coextensive with the
junction between the proximal region and the distal region of the pipette tip,
(v) one or more ribs
may terminate at a point on the proximal region before the junction between
the proximal region
and the distal region of the pipette tip, or combinations of the foregoing, in
some embodiments.
In certain embodiments, the proximal region may comprise a frustum-shaped
cavity within the
interior of the proximal region. In some embodiments, the frustum-shaped
cavity can be
substantially smooth. In certain embodiments, the frustum-shaped cavity may
comprise an
optional annular groove.
In some embodiments, the wall thickness at the distal region terminus is about
0.0043 inches to
about 0.0050 inches. In certain embodiments, the wall thickness at the distal
region terminus is
about 0.0044 inches to about 0.0049 inches. In some embodiments, the interior
surface of the
distal region is substantially smooth, and in certain embodiments, the
exterior surface of the distal
region comprises a step.
In some embodiments, each rib of the first set alternates with each rib of the
second set. In certain
embodiments, one end of ribs in the first set, one end of ribs in the second
set, or one end of ribs in
the first and the second set is co-extensive with, or terminates at, the
flange. In some
embodiments, one end of ribs in the first set, one end of ribs in the second
set, or one end of ribs in
the first and the second set is co-extensive with, or terminates at the
junction between the flange
and proximal region. In certain embodiments, one end of ribs in the first set,
one end of ribs in the
second set, or one end of ribs in the first and the second set is co-extensive
with, or terminates at
the junction between the proximal region and the distal region.
3
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
Provided in some embodiments, are pipette tips comprising a proximal region
and a distal region,
where the proximal region has an average softness rating of less than about
1.75 pounds of force.
As used herein, the term "softness rating" is the amount of force required to
deflect a surface of the
pipette tip (e.g., deflection force) a given distance from a starting or
resting position. In certain
embodiments, the force for a softness rating is measured by pressing on the
side of a pipette tip,
often in the proximal region of the pipette tip, towards the axis extending
longitudinally from the
distal region terminus to the proximal region terminus (e.g., Example 1). In
some embodiments,
the softness rating is a mean, nominal, average, maximum or minimum value. In
certain
embodiments, pipette tips described herein have a mean, nominal or average
deflection force to
deflect a pipette tip a given amount from the resting position of below about
1.75 pounds of force,
below about 1.70 pounds of force, below about 1.65 pounds of force, below
about 1.60 pounds of
force, below about 1.55 pounds of force, below about 1.50 pounds of force,
below about 1.45
pounds of force, below about 1.40 pounds of force, below about 1.35 pounds of
force, below about
1.30 pounds of force, below about 1.25 pounds of force, below about 1.20
pounds of force, below
about 1.15 pounds of force, and below about 1.10 pounds of force required for
deflection of the
pipette tip proximal region. In some embodiments, a pipette tip proximal
region has a minimal
deflection force of about 1.07 pounds. In certain embodiments, a pipette tip
proximal region has a
maximal deflection force of about 1.75 pounds. In some embodiments, a pipette
tip has a
deflection force in the range of between about 1.07 pounds and about 1.26
pounds (e.g., about
1.07 pounds, about 1.08 pounds, about 1.09 pounds, about 1.10 pounds, about
1.11 pounds,
about 1.12 pounds, about 1.13 pounds, about 1.14 pounds, about 1.15 pounds,
about 1.16
pounds, about 1.17 pounds, about 1.18 pounds, about 1.19 pounds, about 1.20
pounds, about
1.21 pounds, about 1.22 pounds, about 1.23 pounds, about 1.24 pounds, about
1.25 pounds, and
about 1.26 pounds of force).
In some embodiments, provided are pipette tips comprising a proximal region
and a distal region,
where the proximal region comprises an exterior surface and an annular flange
at the proximal
terminus of the proximal region, the proximal region comprises a first set of
axially oriented ribs and
a second set of axially oriented ribs, the ribs of the first set and the
second set are circumferentially
__ spaced and alternately spaced around the exterior surface of the proximal
region, and ribs of the
first set have a maximum thickness greater than the maximum thickness of ribs
of the second set.
In certain embodiments, the distal region wall thickness tapers from (a) a
point at or between (i)
about the junction of the proximal region and distal region to (ii) about one-
quarter of the axial
distance from the terminus of the distal region to the junction, to (b) the
distal region terminus, the
4
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
wall thickness at the distal region terminus is about 0.0040 inches to about
0.0055 inches, and the
proximal region is deflected by a known amount from its starting or resting
position by a deflection
force of less than 1.75 pounds. In certain embodiments, the proximal region is
deflected by a
known amount from the starting position by a deflection force between about
1.07 pounds and
about 1.26 pounds.
Provided also, in some embodiments, are pipette tips comprising a proximal
region and a distal
region, where the proximal region comprises an exterior surface and an annular
flange at the
proximal terminus of the proximal region, the distal region wall thickness
tapers from (a) a point at
or between (i) about the junction of the proximal region and distal region to
(ii) about one-quarter of
the axial distance from the terminus of the distal region to the junction, to
(b) the distal region
terminus, the wall thickness at the distal region terminus is about 0.0040
inches to about 0.0055
inches, and the proximal region is deflected a by a known amount from its
starting or resting
position by a deflection force of less than 1.75 pounds. In certain
embodiments, the proximal
region is deflected by a known amount from the starting position by a
deflection force between
about 1.07 pounds and about 1.26 pounds. In certain embodiments, the proximal
region
comprises a first set of axially oriented ribs and a second set of axially
oriented ribs. In some
embodiments, the ribs of the first set and the second set are
circumferentially spaced and
alternately spaced around the exterior surface of the proximal region. In
certain embodiments, ribs
of the first set have a maximum thickness greater than the maximum thickness
of ribs of the
second set.
In some embodiments, provided also are pipette tips comprising a proximal
region and a distal
region, where the proximal region comprises an exterior surface and an annular
flange at the
proximal terminus of the proximal region, the proximal region comprises a
plurality of axially
oriented ribs, a thickness of the proximal region is about 0.005 inches to
about 0.015 inches, the
thickness is (i) at or near a sealing zone for a dispensing device, and (ii)
at a portion between the
ribs, the ribs or portion thereof extend over the sealing zone, and the
proximal region is deflected
by a known amount from its starting or resting position by a deflection force
of less than 1.75
pounds. In certain embodiments, the proximal region is deflected by a known
amount from the
starting position by a deflection force between about 1.07 pounds and about
1.26 pounds.
Also provided, in some embodiments, is a method of using a pipette tip,
comprising: (a) inserting a
pipettor into a pipette tip, and (b) contacting the pipette tip with a fluid,
where the pipette tip
5
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
comprises a proximal region and a distal region, and further where the
proximal region comprises
an exterior surface and an annular flange at the proximal terminus of the
proximal region, the
proximal region comprises a first set of axially oriented ribs and a second
set of axially oriented
ribs, the ribs of the first set and the second set are circumferentially
spaced and alternately spaced
around the exterior surface of the proximal region, and ribs of the first set
have a maximum
thickness greater than the maximum thickness of ribs of the second set.
Provided also, in some embodiments, is method of using a pipette tip,
comprising: (a) inserting a
pipettor into a pipette tip, and (b) contacting the pipette tip with a fluid,
where the pipette tip
comprises a proximal region and a distal region, the proximal region comprises
an exterior surface
and an annular flange at the proximal terminus of the proximal region, and
further where the distal
region wall thickness tapers from (a) a point at or between (i) about the
junction of the proximal
region and distal region to (ii) about one-quarter of the axial distance from
the terminus of the distal
region to the junction, to (b) the distal region terminus, and the wall
thickness at the distal region
terminus is about 0.0040 inches to about 0.0055 inches.
Also provided in some embodiments, is a method for manipulating a solution
using a pipette tip
described herein, comprising: (a) applying a pipette tip to a pipettor, (b)
aspirating a solution, (c)
dispensing the solution into a receptacle, and (d) ejecting the pipette tip
from the pipettor, where
.. the average time to complete 3 cycles of steps (a) to (d) is about 20.88
seconds or less. Provided
also in certain embodiments, is a method for measuring improved pipetting
efficiency, comprising:
(a) applying a pipette tip to a pipettor, (b) aspirating a solution, (c)
dispensing the solution into a
receptacle, and (d) ejecting the pipette tip from the pipettor, where the
average time to complete 3
cycles of steps (a) to (d) is about 20.88 seconds or less. In certain
embodiments, the thickness of
the tip wall at the distal region terminus is 0.0055 or less. In some
embodiments the average time
to complete a single cycle of steps (a) to (d) is about 6.7 seconds or less.
In certain embodiments,
dispensing includes touching the distal terminus of the pipette tip to a wall
of the receptacle after
the fluid is dispensed from the interior of the tip.
.. In some embodiments, a pipette tip having a wall thickness at the distal
region terminus of about
0.0040 inches to about 0.0055 inches is configured to retain less than 0.065%
of the fluid drawn
into the pipette tip, after the fluid is dispensed (e.g., less than about
0.065%, 0.060%, 0.055%,
0.050%, 0.045%, 0.040%, 0.035%, 0.030%, 0.025%, 0.020%, 0.015%, 0.010%,
0.0095%,
0.0090%, 0.0085%, 0.0080%, 0.0075%, 0.0070%, 0.0065%, 0.0060%, 0.0055%,
0.0050%,
6
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
0.0045%, 0.0040%, 0.0035%, 0.0030%, 0.0025%, 0.0020%, 0.0015%, 0.0010%,
0.00095%,
0.00090%, 0.00085%, 0.00080%, 0.00075%, 0.00070%, 0.00065%, 0.00060%,
0.00055%,
0.00050%, 0.00045%, 0.00040%, 0.00035%, 0.00030%, 0.00025%, 0.00020%,
0.00015%,
0.00014%, 0.00013%, 0.00012%, 0.00011%, or about 0.00010%). In certain
embodiments, the
pipette tip retains between about 0.00010% and about 0.00015% (e.g., about
0.00011%,
0.00012%, 0.00013%, or 0.00014%) of the fluid drawn into the tip, after the
fluid is dispensed. In
some embodiments, the pipette tip is configured to retain no more than
0.00012% of the fluid
drawn into the tip, after the fluid is dispensed. In certain embodiments,
provided is a method for
dispensing fluid from a pipette tip, comprising, (a) drawing a volume of fluid
into a pipette tip having
a wall thickness at the distal region terminus of about 0.0040 inches to about
0.0055 inches, and
(b) dispensing the fluid from the pipette tip, where the pipette tip retains
less than 0.065% of the
volume of the fluid that was drawn into the pipette tip, and in some
embodiments, the pipette tip is
configured to retain no more than 0.00012% of the volume of the fluid that was
drawn into the
pipette tip, after the fluid is dispensed. In some embodiments, the percentage
of the fluid drawn
into the pipette tip that is retained after dispensing is determined by
weight, and in certain
embodiments, the percentage of the fluid drawn into the pipette tip that is
retained after dispensing
is determined using a plurality of pipette tips. In some embodiments, the
method optionally
comprises one or more of (i) applying a pipette tip to a pipettor prior to
step (a), (ii) visually
inspecting the pipette tip after step (b), (iii) ejecting the pipette tip from
the pipettor after step (b),
and (iv) combinations thereof.
In certain embodiments, less than 3.72% of a plurality of pipette tips having
a wall thickness at the
distal region terminus of about 0.0040 inches to about 0.0055 inches retain a
portion of the liquid
drawn into the pipette tips after the liquid is dispensed (e.g., less than
3.72%, 3.70%, 3.65%,
3.60%, 3.55%, 3.50%, 3.45%, 3.40%, 3.35%, 3.30%, 3.25%, 3.20%, 3.15%, 3.10%,
3.05%, 3.00%,
2.95%, 2.90%, 2.80%, 2.70%, 2.60%, 2.50%, 2.40%, 2.30%, 2.20%, 2.10%, 2.00%,
1.90%, 1.80%,
1.70%, 1.60%, 1.50%, 1.40%, 1.35%, 1.30%, 1.25%, 1.20%, 1.15%, 1.10%, 1.05%,
1.00%, 0.95%,
0.90%, 0.85%, 0.80%, 0.75%, 0.70%, 0.65%, 0.60%, 0.55%, 0.50%, 0.45%, 0.40%,
0.35%, 0.34%,
0.33%, 0.32%, 0.31%, 0.30%, 0.29%, 0.28%, 0.26%, 0.25%, 0.24%, 0.23%, 0.22%,
0.21%, 0.20%,
0.19%, 0.18%, 0.17%, 0.16%, 0.15%, 0.14%, 0.13%, 0.12%, 0.11%, 0.10%, 0.09%,
0.08%, 0.07%,
0.06%, or less than about 0.05%). In some embodiments, between about 0.05% and
about 1.0%
of the plurality of pipette tips having a wall thickness at the distal region
terminus of about 0.0040
inches to about 0.0055 inches retain a portion of the liquid drawn into
pipette tips after the liquid is
dispensed. In certain embodiments, between about 0.15% and about 0.30% of the
plurality of
7
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
pipette tips having a wall thickness at the distal region terminus of about
0.0040 inches to about
0.0055 inches retain a portion of the liquid drawn into pipette tip after the
liquid is dispensed. In
some embodiments, between about 0.20% and about 0.26% of the plurality of
pipette tips having a
wall thickness at the distal region terminus of about 0.0040 inches to about
0.0055 inches retain a
portion of the liquid drawn into pipette tips after the liquid is dispensed.
In certain embodiments,
provided is a method for dispensing fluid from a pipette tip, comprising, (a)
drawing fluid into a
plurality of pipette tips having a wall thickness at the distal region
terminus of about 0.0040 inches
to about 0.0055 inches, and (b) dispensing the fluid from the pipette tips,
where less than 3.72% of
the pipette tips retain a portion of the liquid drawn into pipette tips after
the liquid is dispensed. In
.. some embodiments, provided is a method for dispensing fluid from a pipette
tip, comprising (a)
drawing fluid into a plurality of pipette tips having a wall thickness at the
distal region terminus of
about 0.0040 inches to about 0.0055 inches, and (b) dispensing the fluid from
the pipette tips,
where between about 0.15% and about 0.30% of the pipette tips retain a portion
of the liquid drawn
into pipette tips after the liquid is dispensed, and in certain embodiments,
between about 0.20%
and about 0.26% of the pipette tips retain a portion of the liquid drawn into
pipette tips after the
liquid is dispensed. In some embodiments, the number of pipette tips that
retain liquid after
dispensing is determined by visual inspection. In certain embodiments, the
method optionally
comprises one or more of (i) applying a pipette tip to a pipettor prior to
step (a), (ii) visually
inspecting the pipette tip after step (b), (iii) ejecting the pipette tip from
the pipettor after step (b),
and (iv) combinations thereof.
In some embodiments, a pipette tip having a wall thickness at the distal
region terminus of about
0.0040 inches to about 0.0055 inches contributes to a reduction of between
about 20% and about
90% in the average time to complete a cycle of steps in a fluid manipulation
procedure (e.g., about
20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, or up to
about
90%). In some embodiments, provided is a method for dispensing fluid from a
pipette tip,
comprising (a) drawing a volume of fluid into a pipette tip having a wall
thickness at the distal
region terminus of about 0.0040 inches to about 0.0055 inches, and (b)
dispensing the fluid from
the pipette tip, where the pipette tip contributes to a reduction of between
about 20% and about
90% in the average time to complete a cycle of steps in a method for
dispensing fluid from a
pipette tip. In certain embodiments, the method optionally comprises one or
more of (i) applying a
pipette tip to a pipettor prior to step (a), (ii) visually inspecting the
pipette tip after step (b), (iii)
ejecting the pipette tip from the pipettor after step (b), and (iv)
combinations thereof.
8
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
Also provided, in certain embodiments, is a method of manufacturing a pipette
tip, comprising: (a)
contacting a pipette tip mold with a molten polymer, and releasing the formed
pipette tip from the
mold after cooling, where the pipette tip comprises a proximal region and a
distal region, and
further where the proximal region comprises an exterior surface and an annular
flange at the
proximal terminus of the proximal region, the proximal region comprises a
first set of axially
oriented ribs and a second set of axially oriented ribs, the ribs of the first
set and the second set
are circumferentially spaced and alternately spaced around the exterior
surface of the proximal
region, and ribs of the first set have a maximum thickness greater than the
maximum thickness of
ribs of the second set.
Provided also, in some embodiments, is method of manufacturing a pipette tip
comprising: (a)
contacting a pipette tip mold with a molten polymer, and releasing the formed
pipette tip from the
mold after cooling, where the pipette tip comprises a proximal region and a
distal region, and
further where the proximal region comprises an exterior surface and an annular
flange at the
proximal terminus of the proximal region, the distal region wall thickness
tapers from (a) a point at
or between (i) about the junction of the proximal region and distal region to
(ii) about one-quarter of
the axial distance from the terminus of the distal region to the junction, to
(b) the distal region
terminus, and the wall thickness at the distal region terminus is about 0.0040
inches to about
0.0055 inches.
Also provided, in some embodiments, are pipette tips comprising a proximal
region and a distal
region, where the proximal region comprises an exterior surface and an annular
flange at the
proximal terminus of the proximal region, the proximal region comprises a
plurality of axially
oriented ribs; a thickness of the proximal region is about 0.005 inches to
about 0.015 inches; the
thickness is (i) at or near a sealing zone for a dispensing device, and (ii)
at a portion between the
ribs; and the ribs or portion thereof extend over the sealing zone. One end of
ribs is co-extensive
with, or terminates at, the flange, in certain embodiments. At times, one end
of ribs is co-extensive
with, or terminates at, the junction between the flange and the proximal
region. Sometimes one
end of ribs is co-extensive with, or terminates at, the junction between the
proximal region and the
distal region. In certain embodiments, the ribs extend from the junction of
the flange and proximal
region to the junction of the proximal and distal regions. In some
embodiments, the distal region
wall thickness tapers from (a) a point at or between (i) about the junction of
the proximal region and
distal region to (ii) about one-quarter of the axial distance from the
terminus of the distal region to
the junction, to (b) the distal region terminus, and the wall thickness at the
distal region terminus is
9
.81625301
about 0.0040 inches to about 0.0055 inches. The wall thickness at the distal
region
terminus sometimes is about 0.0043 inches to about 0.0050 inches, and at times
is
about 0.0044 inches to about 0.0049 inches. In certain embodiments, the
interior
surface of the distal region is substantially smooth, and sometimes the
exterior
surface of the distal region comprises a step. The proximal region sometimes
comprises a frustum-shaped cavity within the interior of the proximal region,
and at
the frustum-shaped cavity is substantially smooth and, in some embodiments,
comprises an optional annular groove. In certain embodiments, the thickness of
the
proximal region is about 0.007 inches to about 0.0013 inches, is aboutØ008
inches
to about 0.0012 inches, is about 0.009 inches to about 0.011 inches or is
about 0.010
inches. In some embodiments, the maximum thickness of the ribs is about 0.037
inches to about 0.060, is about 0.016 inches to about 0.027 inches, is about
0.015
inches to about 0.025 inches, is about 0.011 to about 0.021 inches or is about
0.003
inches to about 0.009 inches. Also included are methods of manufacturing and
using
such pipette tips, described in greater detail hereafter.
In some embodiments, the pipette tip is a unitary construction. In certain
embodiments, the pipette tip is made of not made of an elastonner. In some
embodiments, the interior surface of the proximal region does not include an
internal
shelf. In certain embodiments, the internal surface of the proximal region has
a
continuous circumferential thickness. In some embodiments, the internal
surface of
the proximal region does not have a continuous axial thickness. In certain
embodiments, the internal surface of the proximal region provides a continuous
contact zone. In some embodiments, the internal surface of the proximal region
does
not include internal spaced contact points.
According to an embodiment, there is provided a pipette tip comprising a
proximal
region and a distal region, wherein: the proximal region comprises an exterior
surface
and an annular flange at the proximal terminus of the proximal region; the
proximal
region comprises a first set of axially oriented ribs and a second set of
axially oriented
ribs; the ribs of the first set and the second set are circumferentially
spaced and
CA 2787274 2017-07-26
,81625301
alternately spaced around the exterior surface of the proximal region; and
ribs of the
first set have a maximum thickness greater than the maximum thickness of ribs
of the
second set.
According to another embodiment, there is provided a method of using a pipette
tip
comprising; (a) inserting a pipettor into the pipette tip as described herein;
and (b)
contacting the pipette tip with a fluid.
According to another embodiment, there is provided a method of manufacturing a
pipette tip comprising; (a) contacting a pipette tip mold with molten polymer;
and (b)
releasing the formed pipette tip from the mold after cooling; wherein the
pipette tip
has features imparted by the mold comprising the pipette tip as described
herein.
According to another embodiment, there is provided a method for manipulating a
solution using a pipette tip, comprising (a) applying the pipette tip as
described herein
to a pipettor; (b) aspirating a solution; (c) dispensing the solution into a
receptacle;
and (d) ejecting the pipette tip from the pipettor, wherein the average time
to
complete 3 cycles of steps (a) to (d) is 20.88 seconds or less.
Certain embodiments are described further in the following description,
examples,
claims and drawings.
Brief Description of the Drawings
The drawings illustrate embodiments of the invention and are not limiting. For
clarity
and ease of illustration, the drawings are not necessarily made to scale and,
in some
instances, various aspects may be shown exaggerated or enlarged to facilitate
an
understanding of particular embodiments.
10a
CA 2787274 2017-07-26
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
FIGS. 1A-1D illustrate perspective and cross-sectional views of a pipette tip
embodiment as
described herein, configured to manipulate volumes up to 200 microliters. FIG.
1A is a side
perspective view. FIG. 1B shows a side view with cross-section markings
indicating the view
shown in FIG. 1C. FIG. 1C is a midline cross-sectional view of the drawing
illustrated in FIG. 1B.
FIG. 1C contains detail (indicated by the circle B) illustrated in FIG. 1D.
FIG. 1D is an enlarged
view of the distal aperture, illustrating the decrease in taper ending in the
"blade" or "knife-edge"
tip.
FIG. 2 is an enlarged perspective view of the proximal portion of the pipette
tip embodiment
described in FIG. 1. FIG. 3 represents a side view of the pipette tip
embodiment described in
FIGS. 1 and 2, labeled to illustrate various cross-sections presented in FIGS.
4A-4D. FIGS. 4A-40
illustrate views looking down at the cross-sections taken along the lines
illustrated in FIG. 3.
FIG. 5 illustrates a perspective view of a pipette tip embodiment as described
herein, configured to
manipulate volumes in the range of about 1 to about 20 microliters (e.g.,
about 1, 2, 3, 4, 5, 6, 7, 8,
9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 18, or about 20 microliters), with a
mean or average volume of
about 10 microliters. FIG. 6 illustrates a perspective view of an extra long
pipette tip embodiment
as described herein, configured to manipulate volumes in the range of about 1
to about 20
microliters, with a mean or average volume of about 10 microliters. FIG. 7
illustrates a perspective
view of a pipette tip embodiment as described herein, configured to manipulate
volumes up to
about 300 microliters. FIG. 8 illustrates a perspective view of a pipette tip
embodiment as
described herein, configured to manipulate volumes up to about 1250
microliters.
FIG. 9 illustrates the experimental protocol used for the pipette tip
flexibility deformation test. In the
experiment, a pipette tip embodiment described herein is compared to pipette
tips currently
commercially available. The results are presented in graphical form in FIG.
10. FIG. 10 graphically
illustrates the data from the pipette tip deformation experiment. "TDH" in the
legend of FIG. 10,
and subsequent figures, refers to "Tip Described Herein". The data is also
presented in table form
in Example 1.
FIG. 11 is a photograph of a test participant wired for electomyographic
monitoring while
performing pipetting tasks. FIG. 12 graphically illustrates the distribution
of aches, pains or
discomfort during participants normal work activities. Experimental details
are given in Example 2,
and results are given in Example 3. FIG. 13 shows representative tracings of
electromyography
11
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
analysis of muscle effort associated with pipette tip usage. Experimental
details are given in
Example 2, and results are given in Example 3.
FIG. 14 graphically illustrates the total muscle work done as a measure of tip
performance.
Experimental details are given in Example 2, and results are given in Example
3. FIG. 15
graphically illustrates the total muscle work during a pipetting cycle as a
measure of tip
performance. Experimental details are given in Example 2, and results are
given in Example 3.
FIG. 16 graphically illustrates the average time to task completion for
pipette cycling time.
Experimental details are given in Example 2, and results are given in Example
5. FIG. 17
graphically illustrates the average time to perform a tip/de-tip cycle.
Experimental details are given
in Example 2, and results are given in Example 5.
FIG. 18 graphically illustrates the average overall ratings of perceived
exertion for all pipette tips
tested using all 5 pipettors. FIG. 19 graphically illustrates the perceived
exertion ratings for all
pipette tips tested using pipettor 2. FIG. 20 graphically illustrates the
perceived exertion ratings for
all pipette tips tested using pipettor 4. FIG. 21 graphically illustrated the
perceived exertion ratings
for all pipette tips tested using pipettor 5. FIG. 22 graphically illustrates
the perceived exertion
ratings for all pipette tips tested using pipettor 1. Experimental details for
FIGS. 18-22 are given in
Example 2, and results are given in Example 6.
FIG. 23 graphically illustrates the average overall performance rating with
respect to 'effort to apply
tip" to the various pipettors for each pipette tip. FIG. 24 graphically
illustrates the average overall
performance rating with respect to "ease of aligning pipette barrel and tip'',
for each pipette tip.
FIG. 25 graphically illustrates the average overall performance rating with
respect to "confidence
tip is sealed on pipettor", for each pipette tip. FIG. 26 graphically
illustrates the average overall
performance rating with respect to "effort to eject tip", from the various
pipettors for each pipette tip.
FIG. 27 graphically illustrates the average overall performance rating with
respect to "performance
during touching off", for each tip. FIG. 28 graphically illustrates the
average overall performance
__ rating with respect to "overall comfort of use" for each pipette tip.
Experimental details for FIGS.
23-28 are given in Example 2, and results are given in Example 6.
FIG. 29 graphically illustrates the overall tip rankings for; effort to apply
pipette tip to pipettor (e.g.,
"tip application effort" panel), effort to eject pipette tip from pipettor
(e.g., "tip ejection effort" panel),
12
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
and ease of aligning pipette tip with pipettor barrel (e.g., "ease of
alignment" panel) for each pipette
tip tested. FIG. 30 graphically illustrates the overall tip rankings for;
overall comfort of a particular
tip (e.g., "overall comfort" panel), overall speed and efficiency of task
completion with a particular
pipette tip (e.g., "speed/efficiency" panel), and overall preference of use
(e.g., "overall preference
panel") of a particular tip. Experimental details for FIGS. 29 and 30 are
given in Example 2, and
results are given in Example 7.
FIGS. 31-39 graphically illustrate pipette tip application and ejection forces
or each of the type of
pipette tips tested with each pipettor. Pipette tips of the 200 microliter and
1000 microliter
capacities were tested for each brand. FIGS. 31 and 32 present the results of
force measurements
performed using pipettor 1, where FIG. 31 presents the results of the 200
microliter tips and FIG.
32 presents the results of the 1000 microliter tips. FIGS. 33 and 34 present
the results of force
measurements performed using pipettor 2, where FIG. 33 presents the results of
the 200 microliter
tips and FIG. 34 presents the results of the 1000 microliter tips. FIG. 35
presents the results of the
force measurements performed using pipettor 3 using only brand specific custom
pipette tips in the
200 microliter and 1000 microliter capacities. FIGS 36 and 37 present the
results of force
measurements performed using pipettor 4, where FIG. 36 presents the results of
the 200 microliter
tips and FIG. 37 presents the results of the 1000 microliter tips. FIGS. 38
and 39 present the
results of force measurements performed using pipettor 5, where FIG. 38
presents the results of
the 200 microliter tips and FIG. 39 presents the results of the 1000
microliter tips. Experimental
details for FIGS. 31-39 are given in Example 2 and experimental results are
presented in Example
8.
FIG. 40 graphically illustrates differences in amount of liquid collected from
the tips (i.e., termini) of
each of the pipette tips used in a comparison. FIG. 41 graphically illustrates
the total number of
pipette tips of each type that retained fluid. FIG. 42 graphically illustrates
the time to complete a
defined pipette cycle for 430 pipette tips of each type. Experimental protocol
and results are
described in Example 10.
Detailed Description
Certain structural features of pipette tip embodiments described herein may
afford particular
advantages to some users. In some embodiments, one or more of the structural
features
13
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
described may be incorporated into a pipette tip embodiment in one or more
combinations.
Incorporation of a structural feature can result in an advantage described
hereafter, in certain
instances.
Pipette Tip General Features
Pipette tip embodiments described herein can be of any overall geometry useful
for dispensing
fluids in combination with a dispensing device. The pipette tips described
herein also can be of
any volume useful for dispensing fluids in combination with a dispensing
device. Non-limiting
examples of volumes useful for dispensing fluids in combination with a
dispensing device, and
described as non-limiting embodiments herein, include pipette tips configured
in sizes that hold
from 0 to 10 microliters, 0 to 20 microliters, 1 to 100 microliters, 1 to 200
microliters, 1 to 300
microliters, and from 1 to 1250 microliters, for example. In some embodiments,
the volumes
pipette tips described herein can manipulate are larger than the volume
designation given that
particular pipette tip. For example, a pipette tip designated as suitable to
manipulate volumes up
to 300 microliters, can sometimes be used to manipulate volumes up to about
1%, 2%, 3%, 5%,
10%, 15% or sometimes as much as up to about 20% larger than the designated
pipette tip
volume.
The external appearance of pipette tips may differ, and certain pipette tips
can comprise a
continuous tapered wall forming a central channel or tube that is roughly
circular in horizontal cross
section, in some embodiments. A pipette tip can have any cross-sectional
geometry that results in
a tip that (i) provides suitable flow characteristics, and (ii) can be fitted
to a dispenser (e.g., pipette),
for example.
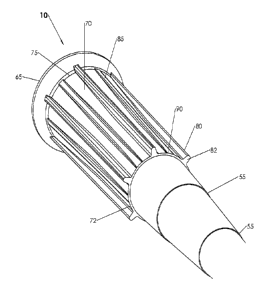
In certain embodiments, pipette tips comprise a proximal region 15 and a
distal region 20 (e.g.,
FIGS. 1A-1D). Proximal region 15 comprises an outer or exterior surface upon
which regions of
increased thickness (e.g., ribs) are disposed, in some embodiments. In certain
embodiments,
proximal region 15 comprises an annular flange at the proximal terminus of the
proximal region.
The bore of the top-most portion of the central channel or tube generally is
wide enough to accept
a particular dispenser apparatus (e.g., nozzle, barrel). Pipette tips
described herein often taper
from the widest point at the top-most portion of the pipette tip (pipette
proximal end or end that
engages a dispenser), to a narrow opening at the bottom most portion of the
pipette tip (pipette
distal end used to acquire or dispel fluid). In certain embodiments, a pipette
tip wall includes two or
14
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
more taper angles. In some embodiments, pipette tips described herein are of
unitary
construction.
Proximal region 15 also comprises an interior or inner surface. The inner
surface of the pipette tip
sometimes forms a tapered continuous wall, in some embodiments, and in certain
embodiments,
the external wall may assume an appearance ranging from a continuous taper to
a stepped taper
or a combination of smooth taper with external protrusions. In some
embodiments, the interior
surface of proximal region 15 is smooth and does not include an internal
shelf. That is, the inner
surface of proximal region 15 does not have internal walls or protrusions that
stop the axial
insertion of a pipette tip barrel or nozzle. In certain embodiments, the inner
surface of proximal
region 15 provides a continuous contact zone (e.g., sealing zone), for
engagement of a pipettor
nozzle or barrel. In some embodiments, the inner surface of proximal region 15
does not include
internal spaced contact points.
In some embodiments, a pipette tip can have (i) an overall length of about
1.10 inches to about
3.50 inches (e.g., about 1.25, 1.50, 1.75, 2.00, 2.25, 2.50, 2.75, 3.00, 3.25
inches); (ii) a fluid-
emitting distal section terminus having an inner diameter of about 0.01 inches
to about 0.03 inches
(e.g., about 0.015, 0.020, 0.025 inches) and an outer diameter of about 0.02
to about 0.7 inches
(e.g., about 0.025, 0.03, 0.04, 0.05, 0.06 inches); and (iii) a dispenser-
engaging proximal section
terminus having an inner diameter of about 0.10 inches to about 0.40 inches
(e.g., about 0.15,
0.20, 0.25, 0.30, 0.35 inches) and an outer diameter of about 0.15 to about
0.45 inches (e.g., about
0.20, 0.25, 0.30, 0.35, 0.45 inches). In the latter embodiments, the inner
diameter is less than the
outer diameter.
The wall of the proximal section of a pipette tip described herein sometimes
is continuously
tapered from the top portion, to a narrower terminus. The top portion
generally is open and often is
shaped to receive a pipette tip engagement portion of a dispensing device. The
wall of a proximal
section, in some embodiments, forms a stepped tapered surface. The angle of
each taper in the
proximal section is between about zero degrees to about thirty degrees from
the central
longitudinal vertical axis of the pipette tip (e.g., about 0, 1, 2, 3, 4, 5,
6, 7, 8, 9, 10 ,11 , 12, 13, 14,
15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29 or 30 degrees), in
certain embodiments.
The wall thickness of a proximal section may be constant over the length of
the section, or may
vary with the length of the proximal section (e.g., the wall of the proximal
section closer to the distal
section of the pipette tip may be thicker or thinner than the wall closer to
the top of the proximal
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
section; the thickness may continuously thicken or thin over the length of the
wall). In certain
embodiments, the walls of proximal region 15 do not have a continuous axial
thickness. That is,
the thickness of the walls in proximal region 15 sometimes decreases axially
towards the midpoint
of proximal region 15, then increases axially from the midpoint towards the
junction of proximal
region 15 and distal region 20. In some embodiments, the walls of proximal
thickness 15 have a
continuous circumferential thickness. That is, the thickness of the walls in
proximal region 15, as
viewed in a particular cross section, do not vary in thickness. A proximal
section of a pipette tip
may contain a filter, insert or other material.
The wall of the distal section of a pipette tip sometimes is continuously
tapered from the wider
portion, which is in effective connection with the proximal section, to a
narrower terminus. The wall
of the distal section, in some embodiments, forms a stepped tapered surface.
The angle of each
taper in a distal section is between about zero degrees to about thirty
degrees from the central
longitudinal vertical axis of the pipette tip (e.g., about 0, 1, 2, 3, 4, 5,
6, 7, 8, 9, 10, 11, 12, 13, 14,
15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29 or 30 degrees), in
certain embodiments. In
some embodiments, the wall of the distal section forms stepped vertical
sections. The wall
thickness of a distal section may be constant along the length of the section,
or may vary with the
length of the section (e.g., the wall of the distal section closer to the
proximal section of the pipette
tip may be thicker or thinner than the wall closer to the distal section
terminus; the thickness may
continuously thicken or thin over the length of the wall). The distal section
of a pipette tip generally
terminates in an aperture through which fluid passes into or out of the distal
portion. In some
embodiments, the interior surface of the distal region is substantially
smooth. In certain
embodiments, the exterior surface of the distal region comprises a step. In
some embodiments, a
distal section of a pipette tip may contain a filter, insert or other
material.
Many features of the pipette tip embodiments described herein are shared
between the pipette tip
embodiments of different sizes. Therefore, the features will be described in
detail for one pipette
tip size and related to the similar features of the pipette tip embodiments of
other sizes.
Pipette Tip Embodiments Comprising Proximal Flange Feature
Certain pipette tip embodiments can include a flared lead-in surface at the
end of the proximal
region. Certain pipette tip embodiments may include a flange (e.g., annular
flange) at the end of
each pipette tip in the proximal region. In such embodiments, the flange may
be flared, and the
16
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
lead-in diameter of the flange can allow for dispenser engagement tolerance,
which is relevant for
multi-dispenser applications, for example. Such a flange can provide a larger
contact zone for
engaging a pipettor nozzle, and can increase the probability of a sealing
engagement between the
dispenser nozzle not coaxially aligned with a pipette tip by guiding the axial
center of the pipette tip
to axial center of the dispenser nozzle. An annular flange also can provide
pipette tip rigidity in
addition to facilitating dispenser alignment. In some embodiments, pipette
tips described herein
include an annular flange at the proximal terminus of the proximal region. An
example of a flared
lead-in surface and flange is illustrated in FIGS. 1A and 1B (e.g., 60, 65 and
70).
Pipette Tip Embodiments Comprising Blade Feature
Some pipette tip embodiments can include a distal region having a tapered wall
thickness and
terminating with a "knife edge" thickness. The term "knife edge" or "blade,"
as used herein refers
to an edge resulting from a continuous taper of a pipette wall surface. The
taper can be
established by the inner surface disposed at a different angle than the outer
surface along all or a
portion of the axial length of the distal region. In certain embodiments, the
surfaces form a sharply
defined single contiguous edge or boundary of minimal thickness. This feature
can reduce the
area of the surface to which liquid droplets can adhere, and also may reduce
the surface tension
between the tip and the droplets, thereby reducing the probability and
frequency with which
droplets may adhere to the discharge aperture of the pipette tips. This
feature also can reduce the
number of times a user needs to touch a pipette tip to a surface to remove a
droplet adhered to the
pipette tip, which sometimes is referred to as "touching off." This feature
also may increase
precision and accuracy in manual or automated applications ("precision" and
"accuracy" are
described in further detail below).
The term "minimal thickness" as used herein refers to a value representative
of the limits of current
and future manufacturing and molding capabilities. Factors such as plastic
viscosity and flow
characteristics, as well as plastic hardeners (e.g., currently available
plasticizers or hardeners, or
plasticizers yet to be formulated) also may contribute to the minimal
thickness attainable for pipette
tips described herein. Therefore, thicknesses described herein for pipette tip
walls of the distal
opening (e.g. the edge or blade walls of the opening) sometimes are at the
current limit of molding
and manufacturing technology, and it is possible that future molding,
manufacturing and plastics
technology will result in lesser thicknesses.
17
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
In some embodiments, the lower (or distal) about one-quarter of the distance
40 from the distal
region terminus 50 to the junction 30, may comprise a distal terminus 50
featuring a knife or blade
edge wall thickness 53 in the range of about 0.0040 inches to about 0.0055
inches thick. In some
embodiments, the wall thickness 53 at distal terminus 50 can resemble a blade
or knife edge and
can be about 0.0040 inches, 0.0041 inches, 0.0042 inches, 0.0043 inches,
Ø0044 inches, 0.0045
inches, 0.0046 inches, 0.0047 inches, 0.0048 inches, 0.0049 inches, 0.0050
inches, 0.0051
inches, 0.0052 inches, 0.0053 inches, 0.0054 inches, or about 0.0055 inches
thick, in certain
embodiments. In some embodiments, the wall thickness at the distal region
terminus is about
0.0043 inches to about 0.0050 inches. In certain embodiments, the wall
thickness at the distal
region terminus is about 0.0044 inches to about 0.0049 inches. In certain
embodiments, the distal
region comprises a wall thickness that tapers from (a) a point at or between
(i) about the junction of
the proximal region and distal region 30 to (ii) about one quarter of the
axial distance 40 from the
terminus of the distal region to the junction 30, to (b) the distal region
terminus 50, as illustrated in
FIG. 1A.
Without being limited by theory, a knife edge or blade feature (e.g., distal
region terminus wall
thickness 53) may reduce the area of the surface to which liquid droplets can
adhere, and also
may reduce the surface tension between the tip and the droplets, thereby
reducing the probability
and frequency with which droplets may adhere to the discharge aperture of the
pipette tips.
Without being limited by theory, the "inverse taper' (e.g., the taper of the
inner surface caused by
the thinning of the distal terminus, while the outer surface taper remains
constant) of the blade
feature may cause drops of liquid to become less likely to adhere to the
pipette tip while being
dispelled from the pipette tip due to the combination of increased drop
surface area and surface
tension (e.g., the drop is stretched due to the internal inverse taper) and
decreased pipette tip inner
surface area, in some embodiments. Without being limited by theory, the
combination of increased
drop surface area and surface tension combined with the decreased pipette tip
surface area
enables the efficient release of liquid droplets from the surfaces of the
pipette tip. This feature also
may lessen the number of times a user needs to touch a pipette tip to a
surface to remove a
droplet adhered to the pipette tip, and also may increase precision and
accuracy in manual or
automated applications. Reducing the number of times a user needs to touch off
may help
increase throughput of samples (e.g., time savings), increase accuracy of
sample delivery (e.g.,
delivery of entire sample or reagent), and decrease costs (e.g., fewer
repetitive injury claims,
higher sample throughput, and fewer repeated samples due to pipetting error or
inaccuracy). An
example of the time savings associated with the combination of blade feature,
flange feature and
18
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
flexible region feature is described in the Examples section herein. The term
"user" as used herein
refers to a person or extension under the direct or indirect control of a
person (e.g., a pipettor, an
automated device, an automated device controlled by a computer).
.. Pipette Tip Embodiments Comprising Flexible Feature(s)
Some pipette tip embodiments can comprise one or more flexible features. In
certain
embodiments, a pipette tip includes a section of flexible thickness (e.g.,
proximal region) that
sometimes also can include axially oriented alternating regions of increased
thickness (e.g., axially
oriented ribs or sets of ribs). In some embodiments, the ribs comprise a first
set and a second set
of axially oriented ribs. In certain embodiments, the axially oriented ribs
can be alternately spaced
and circumferentially spaced around the external surface of the proximal
region of the pipette tip.
A terminus of a dispenser often sealingly engages an inner portion of a
pipette tip at a sealing
zone, which generally is located a particular distance from the proximal
terminus of the pipette tip.
Thus, a sealing zone in certain embodiments is disposed a particular distance
below the terminal
opening of the pipette tip (e.g., the sealing zone is offset from the edge of
the pipette tip). A
sealing zone often is a point at which a fluid tight, frictional and/or
sealing engagement occurs
between a pipette tip and a dispenser. A sealing zone is axially coextensive
with a region of
flexible thickness and/or increased thickness (e.g., ribs) in some
embodiments. In certain
embodiments, the proximal region comprises a sealing zone. In some
embodiments, a sealing
zone provides a continuous contact zone for frictional and/or sealing
engagement between a
pipette tip and a dispenser.
Incorporating a flexible region (e.g., flexible thickness) in a pipette tip
proximal region (e.g., at a
sealing zone) can reduce the amount of axial force required to engage and/or
disengage a pipette
tip from a dispenser. A pipette tip sometimes includes a flexible proximal
region where the
softness or flexibility allows deflection of the proximal region when a
deflecting force is applied.
The softness or flexibility sometimes is referred to as a "softness rating" or
a "flexibility rating."
Any suitable method can be used to measure pipette tip flexibility in the
flexible region of a pipette
tip. Non-limiting examples of tests that can be utilized to measure pipette
tip flexibility include a
deformation test, a pipette tip engagement test, a pipette tip ejection test,
the like and combinations
thereof. A pipette tip deformation test sometimes includes the use of a force
gauge to press down
on an outer surface (e.g., proximal outer surface, distal outer surface,
proximal and distal outer
19
81625301
surfaces) of the pipette tip, and the force necessary to cause deformation of
the normal pipette tip
shape by a predetermined amount, is recorded. Often the measurement is
presented as pounds of
force necessary to deform the pipette tip, and sometimes the measurement can
be presented in
grams of force necessary to deform a pipette tip, attach a pipette tip to a
pipettor, and/or eject a
pipette tip from a pipettor. An example of a deformation flexibility
experiment is shown in FIG. 9,
and the results of the deformation experiment are presented graphically in
FIG. 10 and in table
form in the examples herein. Pipette tip engagement and ejection experiments
sometimes
includes the use of digital force gauges to measure the amount of force
exerted during
pipette/pipette tip engagement and pipette tip ejection. Examples of
experiments performed to
measure pipette tip deflection (softness of tip), engagement force and
ejection force are presented
in the Examples.
As noted above, a pipette tip generally is affixed to a dispensing device by
inserting a portion of the
dispenser (e.g., dispenser barrel, tip or nozzle) into the proximal or
receiving end of a pipette tip
with a downward or axial force. The downward force applied to the dispenser
that can securely
engage the pipette tip may be less than pipette tips currently manufactured. A
proximal region
having flexible thickness (e.g., in the sealing zone) can reduce the amount of
axial force required to
engage and/or disengage a pipette tip to a dispenser. Non-limiting examples of
reduced axial
forces include an average, mean or nominal axial force reduction of about 20%
to about 80% of the
force required to engage standard inflexible pipette tips (e.g., about 25%,
30%, 35%, 400/c, 45%,
50%, 55%, 60%, 65%, 70%, or 75% of the force required to engage pipette tips
currently
manufactured). A non-limiting example of a manufactured inflexible pipette tip
that can be used as
a standard against which to compare mean or nominal axial force reduction, is
manufactured by
Eppendolinternational (e.g., Eppendorf Dualfilter 100 microliter tip, USA/CDN
Catalog No.
022491237).
Without being limited by theory, circumferentially spaced regions of increased
thickness (e.g.,
axially oriented ribs or sets of ribs) disposed on or protruding from a
flexible thickness at or near a
sealing zone can allow, and can limit, a certain degree of radial expansion of
a circumference
around the proximal region of the pipette tip, and/or segmental expansion of
the proximal region of
the pipette tip. Radial expansion and segmental expansion can allow for a
secure, fluid tight
sealing engagement of a pipette tip with different dispensers having disparate
nozzle or barrel
diameters. Radial and segmental expansion properties can be a result of
circumferentially spaced
alternating regions of thicker and thinner ribs, in some embodiments.
CA 2787274 2017-07-26
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
Certain flexible features described herein can reduce costs and injuries
associated with repetitive
motions, and increase efficiency, precision and accuracy of pipette tip use.
For example, reducing
the axial force required for engagement and/or disengagement of a pipette tip
with a dispenser.
Also, reducing the frequency of "touching off" can reduce the number of
repetitive motions
associated with using pipette tips.
In some embodiments, a proximal region comprises a wall thickness of about
0.005 inches to
about 0.015 inches at or near the sealing zone (e.g., about 0.006, 0.007,
0.008, 0.009, 0.010,
0.011, 0.012, 0.013, 0.014 inches). In some embodiments, the proximal region
comprises a wall
thickness of about 0.008 inches to about 0.012 inches or about 0.009 inches to
about 0.011
inches. The latter-referenced wall thickness is measured at a point of the
proximal region where
there are no ribs (e.g., a point between ribs). Such a thickness measurement
sometimes is
measured at or near where callout 70 in FIG. 2 meets the pipette tip proximal
region, for example.
In some embodiments, the thickness of proximal region 15 gradually increases
below the sealing
zone towards the proximal region/distal region junction. Without being limited
by theory, the
increased thickness below the sealing zone may limit the travel of a dispenser
past the sealing
zone, due to the larger force required to insert the dispenser past the
sealing zone as a result of a
thicker, less flexible area in the proximal region.
In some embodiments, the wall thickness at the junction of the proximal region
and the distal
region, measured from the interior surface to the exterior surface of the
pipette tip, is about 0.017
inches to about 0.030 inches thick (e.g., about 0.018, 0.019, 0.020, 0.021,
0.022, 0.023, 0.024,
0.025, 0.026, 0.027, 0.028, 0.029). In some embodiments, the wall thickness at
this junction is
about 0.022 to about 0.027 inches thick, or about 0.023 to about 0.026 inches
thick. In certain
embodiments, the step from the exterior surface of the distal region to the
exterior surface of the
proximal region at the proximal region/distal region junction is about 0.003
inches to about 0.008
inches thick (e.g., about 0.004, 0.005, 0.006, 0.007 inches thick). This step
is located at about the
position in FIG. 2 where callout 72 meets the pipette tip.
In certain embodiments, the proximal region comprises a first set of axially
extended ribs (e.g., 80)
and a second set of axially extended ribs (e.g., 85). Axially extended ribs,
which also are referred
to herein as "axially oriented ribs," are longer in the direction of the
pipette tip axis, where the axis
extends from the center of the proximal region terminus cross section to the
center of the distal
21
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
region terminus cross section. Axially extended ribs are shorter in the
radial, circumferential
direction around the pipette tip. In certain embodiments, the longer length of
axially extended ribs
is parallel to the pipette tip axis. In some embodiments, the longer length of
axially extended ribs is
at an angle with respect to the pipette tip axis, which angle sometimes is
between about zero to ten
.. degrees from such axis.
In some embodiments, one or more ribs are longer than other ribs on a pipette
tip. Ribs of the first
set sometimes are longer than ribs of the second set, and in certain
embodiments, ribs of the first
set are shorter than ribs of the second set. In certain embodiments, the axial
length of one or more
ribs (e.g., all ribs) is substantially equal to the axial length of the
proximal region (e.g., proximal
region 15, illustrated in FIG. 2 and FIG. 3).
In some embodiments, a pipette tip comprises a set of axially extended ribs
circumferentially
spaced around the external surface of the proximal region of the pipette tip.
The term
.. "circumferentially spaced," "circumferentially configured,"
"circumferentially disposed" and the like
as used herein, refer to axially extended ribs disposed around a circumference
of the proximal
region of a pipette tip.
In certain embodiments, ribs of a first set and a second set are
circumferentially spaced and
alternately spaced around the external surface of the proximal region. The
terms "alternately
spaced", "spaced alternately," "alternates" and grammatical equivalents
thereof, when used to
describe spacing between ribs, or sets of ribs, can refer to one or more ribs
of the first set or first
type between two ribs of the second set or second type, or one or more ribs of
the second set or
second type between two ribs of the first set or first type, and combinations
of the foregoing. In
some embodiments, there can be one or more circumferential spacing distances
between ribs
(e.g., ribs may be spaced equidistant from one another or may be spaced with
different distances).
Ribs may be patterned around the proximal region of a pipette tip in a regular
pattern (e.g., all ribs
are equidistantly spaced, some ribs are equidistantly spaced) in some
embodiments, and in certain
embodiments, ribs are spaced in an irregular pattern. In some embodiments, all
ribs are
equidistant from one another along a circumference of the pipette tip, and
thereby are spaced
regularly along the circumference.
A pipette tip may include any suitable number of ribs that confer proximal
region flexibility. In some
embodiments, pipette tips comprise about 4 or more ribs, and sometimes about 6
to about 60 ribs
22
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
(e.g., about 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23.
24, 25, 26, 27, 28, 29, 30,
31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49,
50, 51, 52, 53, 54, 55, 56,
57, 58, 59 ribs). In certain embodiments, a pipette tip includes a total of
about 8 to about 16 ribs.
In some embodiments, a pipette tip comprises a number of ribs in a first set
equal to the number of
ribs in a second set. In some embodiments, a pipette tip includes about 3 to
about 20 ribs of a first
set and about 3 to about 20 ribs of a second set.
Ribs on a pipette tip have a particular thickness (e.g., height measured from
the exterior surface of
the pipette tip proximal region; height measured from the surface to which
callout 70 in FIG. 2
connects) and a particular width (e.g., the width of the face to which callout
85 in FIG.2 connects).
In certain embodiments, the maximum thickness of a rib is about 0.060 inches,
and sometimes the
maximum thickness of a rib is about 0.037 inches to about 0.060 inches (e.g.,
about 0.038, 0.039,
0.040, 0.041, 0.042, 0.043, 0.044, 0.045, 0.046, 0.047, 0.048, 0.049, 0.050,
0.051, 0.052, 0.053,
0.054, 0.055, 0.056, 0.057, 0.058, 0.059 inches thick). Sometimes the maximum
thickness of a rib
is about 0.016 inches to about 0.027 inches thick (e.g., about 0.017, 0.018,
0.019, 0.020, 0.021,
0.022, 0.023, 0.024, 0.025, 0.026 inches thick), and sometimes the maximum
thickness of a rib is
about 0.011 to about 0.021 inches thick (e.g., about 0.012, 0.013, 0.014,
0.015, 0.016, 0.017,
0.018, 0.019, 0.020 inches thick). The foregoing thickness can be applicable
to a first set of ribs,
and if a second set of ribs is present on a pipette tip, the second set of
ribs often have a smaller
maximum thickness. For a second set of ribs, the maximum thickness sometimes
is about 0.003
inches to about 0.009 inches thick (e.g., about 0.004, 0.005, 0.006, 0.007,
0.008, 0.009 inches
thick). In some embodiments, the first set of ribs have a maximum thickness
about 2-fold to about
10-fold greater than the maximum thickness of the second set of ribs (e.g.,
about 3-fold, 4-fold, 5-
fold, 6-fold, 7-fold, 8-fold, 9-fold greater). The width of ribs on a pipette
tip sometimes is about
0.015 inches to about 0.025 inches (e.g., about 0.016, 0.017, 0.018, 0.019,
0.020, 0.021, 0.022,
0.023, 0.024 inches). In some embodiments, the maximum thickness of a rib is
about 1.2-fold to
about 7-fold greater than the wall thickness of the pipette tip at or near the
sealing zone (e.g.,
about 2-fold, 3-fold, 4-fold, 5-fold, 6-fold greater). Where a second set of
thinner ribs are present
on a pipette tip, the pipette tip wall thickness at or near the sealing zone
sometimes is about 1.2-
fold to about 2.0-fold thicker than the maximum thickness of the ribs in the
second set (e.g., about
1.2, 1.4, 1.5, 1.6, 1.7, 1.8, 1.9-fold thicker).
In certain embodiments where there are different types of ribs on a pipette
tip, ribs of a first set
have a maximum thickness greater than the maximum thickness of ribs of a
second set. In some
23
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
embodiments, ribs of a first set (e.g., 80) have a mean thickness greater than
the mean thickness
of ribs of a second set (e.g., 85). In certain embodiments, ribs of the first
set have a nominal
thickness greater than the nominal thickness of ribs of the second set, and in
some embodiments,
ribs of the first set have an average thickness greater than the average
thickness of ribs of the
second set. In certain embodiments, the thickness at or near the proximal
terminus of the distal
region is substantially similar to the thickness at or near the distal
terminus of the proximal region.
Ribs can have any useful profile shape, as seen from the side or the end, with
the proviso the
shape is suitable for adding rigidity to proximal region 15 flexible thickness
70. Non-limiting
examples of profile shapes that can be utilized for ribs in pipette tips
described herein include arc,
pyramid, flat, rectangle, semi-circular, stepped, rhombus, parallelogram,
trapezoid and the like, and
combinations of the foregoing. In some embodiments, the size and shape of the
distal terminus of
ribs 80 and 85 also provides additional surface area for seating engagement
with a pipette tip rack
or a nested pipette tip. In some embodiments, ribs can be configured to have
additional termini 83
(e.g., ribs with a stepped shape or profile, not shown in FIG. 2, but see
termini 283 and 383 in
FIGS. 5 and 6, respectively).
In some embodiments, one end of ribs in the first set, one end of ribs in the
second set, or one end
of ribs in the first and the second set is co-extensive with, or terminates
at, the flange. In some
.. embodiments, one end of ribs in the first set, one end of ribs in the
second set, or one end of ribs in
the first and the second set is co-extensive with, or terminates at the
junction between the flange
and proximal region. In certain embodiments, one end of ribs in the first set,
one end of ribs in the
second set, or one end of ribs in the first and the second set is co-extensive
with, or terminates at
the junction between the proximal region and the distal region. In some
embodiments, one end of
ribs in the first set, of ribs in the second set, or of ribs in the first set
and the second set extend
from the junction of the flange and proximal region to the junction of the
proximal and distal
regions. In some embodiments, one or more (e.g., all) ribs on a pipette tip
extend over the sealing
zone.
FIG. 1A and FIG. 2 show certain rib embodiments. Extending axially from near
the base of flange
60 to junction 30, and spaced circumferentially around the external surface of
proximal region 15,
are alternating ribs 80 and 85 or rib sets, in some embodiments. Alternating
ribs 80, 85 often have
different maximum, mean, average or nominal thicknesses. In some embodiments,
proximal
region 15 may comprise flexible thickness 70 with alternating regions of first
rib thickness (e.g., ribs
24
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
of the first set) 80 and second rib thickness (e.g., ribs of the second set)
85 on the exterior surface
of proximal region 15. In certain embodiments, the circumferential and axial
midpoint of the
alternating ribs are spaced around a circumference of the pipette tip proximal
region.
In some embodiments, the thickness of proximal region 15 flexible thickness 70
can vary. In
certain embodiments, the thickness can taper from a from a less flexible to a
more flexible
thickness (e.g., to about 0.008 inches to about 0.012 inches or about 0.009
inches to about 0.011
inches). In some embodiments, the thickness of proximal region 15 can
gradually increase from a
more flexible thickness to a less flexible thickness (e.g., about 0.022 to
about 0.027 inches thick, or
about 0.023 to about 0.026 inches thick) towards the distal end of proximal
region 15, at or near
junction 30. In certain embodiments, the thickness of proximal region 15
flexible thickness 70 can
taper towards the sealing zone and gradually increase towards junction 30. In
some embodiments,
the thickness of proximal region 15 flexible thickness 70 can remain constant.
In certain
embodiments, the thickness of proximal region 15 flexible thickness 70 does
not have a continuous
axial thickness in the region of the sealing zone.
Without being limited by theory, the combination of proximal region 15
flexible thickness 70 and the
regions of increased thickness in ribs 80 and 85 may allow some radial and/or
segmental
expansion to accommodate, and sealingly engage, the leading edge of an
inserted pipette nozzle
or barrel, while also reducing the axial force required to achieve said
sealing engagement.
Illustrated in FIGS. 4B-4D are the heights and widths of alternating ribs 80,
85 at each lower
successive cross-section in proximal region 15. The increase in the height
(e.g., protrusion above
proximal region 15 flexible thickness 70) and width along the axial length of
the ribs provides for an
increase in rigidity towards the distal portion of the proximal region near
junction 30, thereby
providing a lower zone, in the proximal region, past which an engaging
pipettor nozzle cannot be
inserted, without the application of excessive downward axial forces. The term
"excessive
downward axial forces" as used herein refers to the application of sufficient
force to cause physical
damage or deformation of the pipette tip, such that the pipette tip is no
longer capable of
functioning for its intended purpose.
The radial and/or segmental expansion that accommodates, and sealingly engages
the leading
edge of an inserted pipette nozzle, can be attributed to the flexible
thickness of the proximal region.
The flexible thickness can be rated in terms of its softness or flexibility.
In some embodiments, the
softness or flexibility can be measured as pounds of force required for
deflection, and in certain
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
embodiments, the softness or flexibility can be measured as grams of force
required for deflection,
tip insertion or tip ejection. A non-limiting method of measuring softness or
flexibility is
determining the amount of force required to cause a predetermined amount of
deflection in the
proximal region of the pipette tip, using a digital force gauge, and is
described in further detail in
Example 1.
In some embodiments, pipette tips described herein sometimes have a mean,
nominal or average
deflection force to deflect a pipette tip a given (e.g., defined) amount from
the resting position of
below about 1.75 pounds of force, below about 1.70 pounds of force, below
about 1.65 pounds of
force, below about 1.60 pounds of force, below about 1.55 pounds of force,
below about 1.50
pounds of force, below about 1.45 pounds of force, below about 1.40 pounds of
force, below about
1.35 pounds of force, below about 1.30 pounds of force, below about 1.25
pounds of force, below
about 1.20 pounds of force, below about 1.15 pounds of force, and below about
1.10 pounds of
force required for deflection of the pipette tip proximal region. In some
embodiments, a pipette tip
proximal region has a minimal deflection force of about 1.07 pounds. In
certain embodiments, a
pipette tip proximal region has a maximal deflection force of about 1.75
pounds. In some
embodiments, a pipette tip has a deflection force in the range of between
about 1.07 pounds and
about 1.26 pounds (e.g., about 1.07 pounds, about 1.08 pounds, about 1.09
pounds, about 1.10
pounds, about 1.11 pounds, about 1.12 pounds, about 1.13 pounds, about 1.14
pounds, about
__ 1.15 pounds, about 1.16 pounds, about 1.17 pounds, about 1.18 pounds, about
1.19 pounds,
about 1.20 pounds, about 1.21 pounds, about 1.22 pounds, about 1.23 pounds,
about 1.24
pounds, about 1.25 pounds, and about 1.26 pounds of force).
Without being limited by theory, regions of increased wall thickness (e.g.,
ribs 80, 85) may help
retain tip integrity under circumstances where excess downward axial forces
are applied, for
example. Additionally, alternating ribs may aid in providing a better sealing
engagement by
ensuring the correct longitudinal axis alignment of the pipettor barrel and
the sealing zone in
proximal region 15. In some embodiments, the additional rigidity offered by
ribs 80, 85 may direct
the advancing pipettor barrel into the correct alignment to ensure a fluid
tight, sealing engagement
of pipette tip embodiment 10 and a pipettor nozzle or barrel.
In some embodiments, the co-extensive bottom or terminus surfaces of proximal
region 15 flexible
thickness 70 and ribs 80, 85 (e.g., rib termini 82 and 90, respectively), near
junction 30, can
provide a seating support surface 72. In some embodiments, the terminus
surfaces are configured
26
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
to have a width sufficient to overlap the diameter of the openings commonly
found in many
commercially available pipette tip storage units, and can therefore interact
with pipette tip rack,
pipette card or pipette box, support surfaces to provide seating engagement.
Thus, the pipette tip
embodiments described herein are configured in a manner compatible with many
commercially
available pipette tip storage systems, in some embodiments.
Advantageous Benefits of Flange, Flexible and Blade features
The advantageous benefits of features described herein (e.g., flange feature,
blade feature, flexible
features, or combinations thereof) sometimes is a cumulative effect realized
over the course of
repeated cycles of pipetting. A pipette cycle frequently includes the steps of
(a) applying a pipette
tip to a pipettor, (b) aspirating a solution, (c) dispensing the solution into
a receptacle, and (d)
ejecting the pipette tip from the pipettor. In certain embodiments, dispensing
optionally includes
one or more of (i) touching the distal terminus of the pipette tip to a wall
of the receptacle after the
fluid is dispensed from the interior of the tip, (ii) visual inspection of the
tip to determine if any liquid
adhered to the tip, or (iii) touching the distal terminus of the pipette tip
to a wall of the receptacle
after the fluid is dispensed from the interior of the tip and visual
inspection of the tip to determine if
any liquid adhered to the tip. Pipetting efficiency sometimes can be measured
by the time required
to complete one, two, three, four, five or more pipetting cycles involving
steps (a) to (d). In some
embodiments, pipetting efficiency is measured by determining the average time
required to
complete three full cycles of steps (a) to (d). In some embodiments, step (c)
includes touching the
distal terminus of the pipette tip to a wall of the receptacle after the fluid
is dispensed from the
interior of the tip.
In certain embodiments, the average time to compete three cycles of steps (a)
to (d) is 20.88
seconds or less (e.g., about 20.88 seconds or less, about 20.80 seconds or
less, about 20.75
seconds or less, about 20.70 seconds or less, about 20.65 seconds or less,
about 20.60 seconds
or less, about 20.55 seconds or less, about 20.50 seconds or less, about 20.45
seconds or less,
about 20.40 seconds or less, about 20.35 seconds or less, about 20.30 seconds
or less, about
20.25 seconds or less, about 20.20 seconds or less, about 20.15 seconds or
less, about 20.10
seconds or less, or about 20.00 seconds or less). In some embodiments, the
average time to
complete a single cycle of steps (a) to (d) is about 6.7 seconds or less
(e.g., about 6.7 seconds or
less, about 6.6 seconds or less, or about 6.5 seconds or less). The average
time to complete a
single cycle of steps (a) to (d) can be determined by taking the average time
to complete 3 cycles
27
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
of steps (a) to (d) and dividing by 3, to arrive at the average time required
to complete a single
cycle. Similarly, the average time to complete a single cycle of steps (a) to
(d) can be determined
by taking the average time to complete any number of cycles and diving the
time by the number of
cycles.
Provided also herein is a method for manipulating a solution using pipette
tips described herein,
comprising: (a) applying a pipette tip to a pipettor, (b) aspirating a
solution, (c) dispensing the
solution into a receptacle, and (d) ejecting the pipette tip from the
pipettor, where the average time
to complete 3 cycles of steps (a) to (d) is about 20.88 seconds or less. In
some embodiments the
average time to complete a single cycle of steps (a) to (d) is about 6.7
seconds or less. In certain
embodiments, dispensing includes touching the distal terminus of the pipette
tip to a wall of the
receptacle after the fluid is dispensed from the interior of the tip.
Measurements of pipetting efficiency can provide data allowing the results of
modifications to
pipette tip shape, features or materials to be quantified. Pipetting
efficiency can be measured
using the pipetting cycle tests described herein or using other methods of
measurement known to
a user. Accordingly, also provided herein is a method for measuring improved
pipetting efficiency,
comprising: (a) applying a pipette tip to a pipettor, (b) aspirating a
solution, (c) dispensing the
solution into a receptacle, and (d) ejecting the pipette tip from the
pipettor, wherein achieving an
average time to complete 3 cycles of steps (a) to (d) in about 20.88 seconds
or less is indicative of
improved pipetting efficiency. In some embodiments the average time to
complete a single cycle
of steps (a) to (d) is about 6.7 seconds or less. In certain embodiments,
dispensing includes
touching the distal terminus of the pipette tip to a wall of the receptacle
and/or visually inspecting
the pipette tip for liquid, after the fluid is dispensed from the interior of
the tip.
Example 5 and FIGS. 16 and 17 present data indicative of the average time to
complete 3 pipette
cycles for pipette tips described herein, as compared to custom and generic
pipette tips. Tips
described herein provide time savings advantages that, when scaled to the
number of pipette tip
cycles performed by a user on a daily, weekly, monthly and/or yearly basis,
can provide significant
time and cost savings. Custom and generic pipette tips are further described
in the Examples.
In some embodiments the combination of features of pipette tips described
herein contributes to a
reduction in the average time required to complete one or more pipetting
cycles of between about
20% and about 90%. In certain embodiments the reduction in time is due, in
whole or in part, to a
28
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
reduction in the amount of fluid that remains with the pipette tip. In some
embodiments the pipette
tip blade tip feature contributes to the reduction in liquid retained by the
pipette tip. In certain
embodiments the fluid retained by the pipette tip is less than about 0.065% of
the liquid drawn into
the tip after the liquid is dispensed. In some embodiments, the fluid retained
by the pipette tip is no
more than 0.00012% of the liquid drawn into the tip after the liquid is
dispensed. In certain
embodiments, less than 3.72% of the pipette tips described herein, utilized in
a pipetting cycle,
retain a portion of the liquid drawn into the pipette tips after the liquid is
dispelled. In some
embodiments, no more than 0.00012% of the pipette tips described herein,
utilized in a pipetting
cycle, retain a portion of the liquid drawn into the pipette tips after the
liquid is dispelled. In certain
embodiments, about 3.72% or less of the pipette tips described herein,
utilized in a pipetting cycle,
retain less than about 0.065% of the liquid drawn into the tips after the
liquid is dispensed.
Other Features of Certain Pipette Tip Embodiments
Pipette tip embodiments also may comprise one or more of the following
features illustrated in
FIGS. 1A-D and FIG. 2: step(s) 55 along the outer surface of the distal region
20; region of inner
surface where wall taper of the inner and outer surfaces reaches 0 degrees and
the wall surfaces
become parallel 57; flange 60; flange rim 65; flange lead-in surface 67;
proximal region flexible
thickness 70 that extends from the junction 75 of flange 60 and proximal
region 15 to the junction
30 of proximal region 15 and distal region 20.
In certain embodiments, the interior surface 130 of the distal region 20 is
substantially smooth, as
illustrated in FIGS. 1C-1D. FIG. 1B provides a side view of 200 microliter
pipette tip embodiment
10, highlighted with line 1C-1C that denotes the cross-section presented in
FIG. 1C. FIG. 1B
features are labeled identically to the features presented in FIG. 1A. FIG. 1C
illustrates the
substantially smooth interior surface 130 of the distal region 20, and also
highlights detail area 1D,
which is presented in FIG. 1D. Pipette embodiment 10 may comprise annular
groove 120 on the
interior surface of proximal region 15 (see FIG. 1C). Annular groove 120 may
provide a region of
increased surface area for interaction with a mold core pin, as described
below in further detail.
FIG. 1D is an enlarged view of the detail area highlighted in FIG. 1C.
Illustrated in FIG. 1D is a
gradually decreasing taper. The decreasing taper is denoted by the change in
taper from about
4.2 degrees to about 2.7 degrees. The decrease in taper continues until the
taper angle reaches 0
at or near region 57, in the range of about 0.008 to about 0.012 inches from
distal region terminus
50. In some embodiments, the region of 0 degree taper 57 (e.g., the region
where the inner and
29
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
outer walls become essentially parallel, for example) can be about 0.008
inches, about 0.009
inches, about 0.010 inches, about 0.011 inches or about 0.012 inches from
distal region terminus
50. This region, starting approximately 0.01 inches from distal terminus 50
and ending at distal
terminus 50, defines the knife edge or blade region of pipette tip embodiment
10. The region
where the taper ends is highlighted as a line 57 denoting the point where the
inner and outer walls
become essentially parallel (e.g., taper angle becomes 0 degrees). The distal
terminus region wall
53 thickness in this area was described above, and in the embodiment
illustrated in FIG. 1D is
about 0.0044 inches thick.
In some embodiments, the exterior surface of the distal region may comprise a
step. In certain
embodiments, the exterior surface of the distal region may comprise more than
one step. Exterior
surface step(s) 55 can aid in visual assessment of the uptake or delivery of
sample or reagent by
providing external visual volumetric gradations, which allow the user to
determine if sample has
been successfully acquired or expelled, and can allow the user to visually
determine how much
sample has been delivered, in reverse pipetting applications for example.
Reverse pipetting is the
process whereby a pipettor plunger is depressed to its fully depressed
position, and sample is
taken up. Taking up sample in this manner allows more than the preset volume
to be taken up.
The preset volume of sample is then delivered by depressing the plunger to the
first stop. This
ensures delivery of the correct volume to more than one sample, since the
pipette tip has actually
taken up more than one volume of sample to be delivered. This technique can be
useful for
delivering a sample or reagent to many tubes, where the possibility of cross
contamination is
minimal (e.g., when pipetting the initial reagent or liquid into a tube,
during reaction set up).
Proximal region 15 also may comprise a frustum-shaped cavity within the
interior of proximal
region 15, in certain embodiments, as illustrated in FIGS. 4A-4D. FIGS. 4A-4D
illustrate a view
looking down the top of various cross-sections of pipette tip embodiment 10.
The areas, in
proximal region 15, in which the cross-sections are taken, are illustrated in
FIG. 3 as lines; A-A, B-
B, C-C, and D-D. Also illustrated in FIG. 4A-4D (and not previously described)
are proximal region
inner surface 100, flange tapered inner surface 110, and annular groove 120.
In some
embodiments, the frustum-shaped cavity is substantially smooth.
In certain embodiments, the frustum-shaped cavity comprises an optional
annular groove 120. As
described above, annular groove 120 is an area of increased surface area
formed during the
molding process that corresponds to a portion of the mold core pin. The core
pin often forms the
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
internal surfaces of the object to be molded, for example the pipette tips
described herein. The
distance between the core pin and the mold cavity (e.g., the part of the mold
that forms the outer
surface of the object) determines the thickness of the object to be molded
(e.g., pipette tip). The
shape of the core pin can offer an increased surface area upon which the
cooling pipette tip (e.g.,
specifically annular groove 120) may find purchase and therefore remain in
contact with the core
pin during cooling and separation from the portion of the mold that forms the
pipette tip outer
surface, which in turn may facilitate release and ejection of the pipette tip
from the mold core after
cooling of the pipette tip. Annular groove 120 resides on the interior surface
100 of proximal region
15. The sealing zone, which is located in the proximal region of a pipette
tip, sometimes is located
.. at a position in the pipette tip interior proximal of the annular groove
120, sometimes is located at a
position distal to annular groove 120, and sometimes is located in the same
region as annular
groove 120.
In some embodiments, the proximal region also may be in connection with an
annular flange 60 at
the proximal terminus of proximal region 15. Flange 60 at the proximal
terminus of pipette tip 10 in
proximal region 15 may be flared, and the lead-in surface 67 (see FIG. 4A)
diameter of the flange
60 can allow for pipettor engagement tolerance in multi-pipettor applications.
Flange 60 can
provide a larger contact zone for engaging a pipettor nozzle, and can increase
the probability of a
sealing engagement between a pipettor nozzle not coaxially aligned with a
pipette tip by guiding
.. the axial center of the pipette tip to the axial center of the pipettor
nozzle. Without being limited by
theory, it is expected that the edge of the flange 60 also may provide pipette
tip rigidity, in some
embodiments, and also may facilitate pipette entry and seating, in certain
embodiments.
As noted above, the pipette tip embodiments described herein can be configured
in any volume.
.. Multiple features and properties described for 200 microliter pipette tip
embodiment 10 are also
common to the pipette tips configured in different sizes, such as 10
microliter, 300 microliter and
1250 microliter pipette tips, for example (referred to herein after as 10
microliter pipette tip, 300
microliter pipette tip and 1250 microliter pipette tip, respectively).
Therefore, while FIGS. 1A-1D, 2,
3 and 4A-40 often pertain to 200 microliter pipette tips, certain features
illustrated in FIGS. 1A-1D,
2, 3 and 4A-4D are related to features of 10 microliter, 300 microliter and
1250 microliter pipette tip
embodiments, and similar reference characters are utilized in FIGS. 5-8. For
example, the distal
region terminus is referenced as 50 in FIGS. 1A-1D, and 10 microliter pipette
tip embodiment 200
has distal region terminus 250, in FIG. 5.
31
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
microliter and 10 microliter extra long pipette tip embodiments 200 and 300,
respectively, may
comprise one or more of the following features illustrated in FIG. 5 and FIG.
6: proximal region 215,
315; distal region 220, 320; junction between distal region and proximal
region 230, 330; tapered
junction surface 232; region 240, 340 that is about one-quarter of the
distance from the distal
5 region terminus to the junction; distal region terminus 250, 350; blade
or knife edge wall thickness
253, 353 at distal region terminus; step(s) 355; flange 260, 360; flange rim
265, 365; proximal
region flexible thickness 270, 370 that extends from the junction 275, 375 of
flange 260, 360 and
proximal region 215, 315 to junction 230, 330 of proximal region 215, 315 and
distal region 220,
320. Proximal region 215, 315, between junctions 275, 375 and 230, 330
sometimes can include
10 alternating ribs 280, 380 and 285, 385, which can end in rib termini.
Illustrated in FIGS. 5 and 6
are ribs ending in termini 282, 283, and 290.
300 microliter pipette tip embodiment 400, may comprise one or more of the
following features
illustrated in FIG. 7: proximal region 415; distal region 420; junction
between distal region and
proximal region 430; tapered junction surface 432 (not shown); region 440 that
is about one-
quarter of the distance from the distal region terminus to the junction;
distal region terminus 450;
blade or knife edge wall thickness 453 at distal region terminus; step(s) 455;
flange 460; flange rim
465; proximal region flexible thickness 470 that extends from the junction 475
of flange 460 and
proximal region 415 to junction 430 of proximal region 415 and distal region
420. Proximal region
415, between junctions 475 and 430 sometimes can include alternating ribs 480
and 485, which
can end in rib termini. Illustrated in FIG. 7 are ribs ending in termini 482
and 490.
1250 microliter pipette tip embodiment 500, may comprise one or more of the
following features
illustrated in FIG. 8: proximal region 515; distal region 520; junction
between distal region and
proximal region 530; tapered junction surface 532 (not shown); region 540 that
is about one-
quarter of the distance from the distal region terminus to the junction;
distal region terminus 550;
blade or knife edge wall thickness 553 at distal region terminus; step(s) 555;
flange 560; flange rim
565; proximal region flexible thickness 570 that extends from the junction 575
of flange 560 and
proximal region 515 to junction 530 of proximal region 515 and distal region
520. Proximal region
.. 515, between junctions 575 and 530 sometimes can include alternating ribs
580 and 585, which
can end in rib termini. Illustrated in FIG. 8 are ribs ending in termini 582
and 590.
The 10 microliter, 300 microliter and 1250 microliter pipette tip embodiments
also may comprise
features and properties illustrated or described for 200 microliter pipette
tip embodiment 10, but not
illustrated in FIGS. 5-8. For example, 10 microliter pipette tip embodiment
200 may also comprise,
32
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
a smooth inner distal or proximal surface, as illustrated in FIGS. 1C and 1D.
The 10 microliter, 300
microliter and 1250 microliter pipette tip embodiments also may comprise a
smooth distal inner
surface. The 10 microliter, 300 microliter and 1250 microliter pipette tip
embodiments also may
comprise; a region of 0 degree taper about 0.01 inches above the distal region
terminus 20;
flexibility contributed by proximal wall thickness 70; rigidity contributed by
alternating regions of
increased thickness in rib 80, 85 regions and the lower portion of the
proximal region, co-extensive
rib and proximal region termini that provide for seating engagement with
pipette tip storage units
and the like. In some embodiments, all features and properties described for
the 200 microliter
pipette tip embodiment, and applicable to the 10 microliter, 300 microliter
and 1250 microliter
pipette tip embodiments are understood to be incorporated into the 10
microliter, 300 microliter and
1250 microliter pipette tip embodiments. Additionally, in some embodiments,
features such as the
smooth inner surface (e.g., 100 and 130) or annular groove 120, which are not
shown in certain
embodiments, are understood to be adaptable, and can be included in certain
embodiments where
they are not shown. Therefore, it will be understood, all features shown and
described for 200
microliter pipette tip embodiment 10, but not shown or described for the other
pipette tip
embodiments described herein, can be included in the 10 microliter, 10
microliter extra long, 300
microliter and 1250 microliter pipette tip embodiments.
Pipette Tip Filters
In certain embodiments, pipette tips may comprise one or more of a filter
component and/or an
insert component. A filter component and/or insert component may be located in
any suitable
portion of a pipette tip, and sometimes is located in a proximal portion of a
pipette tip near a pipette
tip aperture that can engage a dispensing device. A filter component and/or
insert component
sometimes also can be located in a distal portion of the pipette tip near a
pipette tip aperture that
can engage a fluid. A filter can be of any shape (e.g., plug, disk; U.S.
Patent Nos. 5156811 and
7335337) and can be manufactured from any material that impedes or blocks
migration of aerosol
through the pipette tip to the proximal section terminus, including without
limitation, polyester, cork,
plastic, silica, gels, and the like, and combinations thereof. In some
embodiments a filter may be
porous, non-porous, hydrophobic, hydrophilic or a combination thereof. A
filter in some
embodiments may include vertically oriented pores, and the pore size may be
regular or irregular.
Pores of a filter may include a material (e.g., granular material) that can
expand and plug pores
when contacted with aerosol (e.g., U.S. Patent No. 5,156,811). In certain
embodiments, a filter
may include nominal, average or mean pore sizes of about 30, 25, 20, 15, 10,
9, 8, 7, 6, 5, 4, 3, 2,
33
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
1, 0.5, or 0.05 micrometers, for example. A section of a pipette tip also may
include an insert or
material that can interact with a molecule of interest, such as a biomolecule.
The insert or material
may be located in any suitable location for interaction with a molecule of
interest, and sometimes is
located in the distal section of a pipette tip (e.g., a material or a terminus
of an insert may be
located at or near the terminal aperture of the distal section). An insert may
comprises one or
more components that include, without limitation, multicapillaries (e.g., US
2007/0017870), fibers
(e.g., randomly oriented or stacked, parallel orientation), and beads (e.g.,
silica gel, glass (e.g.
controlled-pore glass (CPG)), nylon, Sephadex , Sepharose , cellulose, a metal
surface (e.g.
steel, gold, silver, aluminum, silicon and copper), a magnetic material, a
plastic material (e.g.,
polyethylene, polypropylene, polyamide, polyester, polyvinylidenedifluoride
(PVDF)), Wang resin,
Merrifield resin or Dynabeads0). Beads may be sintered (e.g., sintered glass
beads) or may be
free (e.g., between one or two barriers (e.g., filter, frit)). Each insert may
be coated or derivitized
(e.g., covalently or non-covalently modified) with a molecule that can
interact with (e.g., bind to) a
molecule of interest (e.g., 018, nickel, affinity substrate).
Pipette Tip Materials
Each pipette tip can be manufactured from a commercially suitable material.
Pipette tips often are
manufactured from one or more moldable materials, independently selected from
those that
include, without limitation, polypropylene (PP), polyethylene (PE), high-
density polyethylene
(HDPE), low-density polyethylene (LDPE), polyethylene teraphthalate (PET),
polyvinyl chloride
(PVC), polytetrafluoroethylene (PTFE), polystyrene (PS), high-density
polystyrene, acrylnitrile
butadiene styrene copolymers, crosslinked polysiloxanes, polyurethanes,
(meth)acrylate-based
polymers, cellulose and cellulose derivatives, polycarbonates, ABS,
tetrafluoroethylene polymers,
corresponding copolymers, plastics with higher flow and lower viscosity or a
combination of two or
more of the foregoing, and the like.
Non-limiting examples of plastics with higher flow and lower viscosity
include, any suitable material
having a hardness characterized by one or more of the following properties, in
certain
embodiments: a melt flow rate (230 degrees Celsius at 2.16 kg) of about 30 to
about 75 grams per
10 minutes using an ASTM D 1238 test method; a tensile strength at yield of
about 3900 to about
5000 pounds per square inch using an ASTM D 638 test method; a tensile
elongation at yield of
about 7 to about 14% using an ASTM D 638 test method; a flexural modulus at 1%
sectant of
about 110,000 to about 240,000 pounds per square inch using an ASTM D 790 test
method; a
34
81625301
notched lzod impact strength (23 degrees Celsius) of about 0.4 to about 4.0
foot pounds per inch
using an ASTM D 256 test method; and/or a heat deflection temperature (at
0.455 MPa) of about
160 degrees to about 250 degrees Fahrenheit using an ASTM D 648 test method. A
material used
to construct the distal section and/or axial projections include moldable
materials in some
embodiments. Non-limiting examples of materials that can be used to
manufacture the distal
section and/or axial projections include polypropylene, polystyrene,
polyethylene, polycarbonate,
and the like, and mixtures thereof. In certain embodiments, pipette tips
described herein are not
made from an elastomer.
Materials suitable for use in embodiments described herein, and methods for
manufacture using
those materials have been described in United States Provisional Patent
Application No.
61/144,031, filed on January 12, 2009, and entitled "FLEXIBLE PIPETTE TIPS",
having attorney
docket number PEL-1007-PV.
Anti-Microbial Materials
A pipette tip also may include one or more antimicrobial materials. An
antimicrobial material may
be coated on a surface (e.g., inner and/or outer surface) or impregnated in a
moldable material, in
some embodiments. One or more portions or sections, or all portions and
sections, of a pipette tip
or other pipette tip tray component may include one or more antimicrobial
materials. In some
embodiments anti-microbial agents or substances may be added to the moldable
plastic during the
manufacture process. In some embodiments, the anti-microbial agent or
substance can be an
anti-microbial metal. The addition of anti-microbial agents may be useful in
(i) decreasing the
amount of microbes present in or on a device, (ii) decreasing the probability
that microbes reside in
or on a device, and/or (iii) decreasing the probability that microbes form a
biofilm in or on a device,
for example. Antimicrobial materials include, without limitation, metals,
halogenated hydrocarbons,
quaternary salts and sulfur compounds.
Non-limiting examples of metals with anti-microbial properties are silver,
gold, platinum, palladium,
copper, iridium (i.e. the noble metals), tin, antimony, bismuth, zinc cadmium,
chromium, and
thallium. The afore-mentioned metal ions are believed to exert their effects
by disrupting
respiration and electron transport systems upon absorption into bacterial or
fungal cells. A
commercially accessible form of silver that can be utilized in devices
described herein is
SMARTSILVER NovaResin. SMARTSILVER NovaResin is a brand of antimicrobial
master batch
CA 2787274 2017-07-26
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
additives designed for use in a wide range of polymer application. Billions of
silver nanoparticles
can easily be impregnated into PET, PP, PE and nylon using standard extrusion
or injection
molding equipment. SMARTSILVER NovaResin additives may be delivered as
concentrated
silver-containing master batch pellets to facilitate handling and processing.
NovaResin is designed
to provide optimum productivity in a wide range of processes, including fiber
extrusion, injection
molding, film extrusion and foaming.
Further non-limiting examples of anti-microbial substances or agents include,
without limitation,
inorganic particles such as barium sulfate, calcium sulfate, strontium
sulfate, titanium oxide,
aluminum oxide, silicon oxide, zeolites, mica, talcum, and kaolin.
Halogenated hydrocarbons, include, without limitation, halogenated derivatives
of salicylanilides
(e.g., 5-bromo-salicylanilide; 4',5-dibromo-salicylanilide; 3,4',5-tribromo-
salicylanilide; 6-chloro-
salicylanilide; 4'5-dichloro-salicylanilide; 3,4'5-trichloro-salicylanilide;
4',5-diiodo-salicylanilide;
3,4',5-triiodo-salicylanilide; 5-chloro-3'-trifluoromethyl-salicylanilide; 5-
chloro-2'-trifluoromethyl-
salicylanilide; 3,5-dibromo-3'-trifluoromethyl-salicylanilide; 3-chloro-4-
bromo-4'-trifluoromethyl-
salicylanilide; 2',5-dichloro-3-phenyl-salicylanilide; 3',5-dichloro-4'-methyl-
3-phenyl-salicylanilide;
3',5-dichloro-4'-phenyl-3-phenyl-salicylanilide; 3,3',5-trichloro-6'-(p-
chlorophenoxy)-salicylanilide;
3',5-dichloro-5'-(p-bromophenoxy)-salicylanilide; 3,5-dichloro-6'-phenoxy-
salicylanilide; 3,5-
dichloro-6'-(o-chlorophenoxy)-salicylanilide; 5-chloro-6'-(o-chlorophenoxy)-
salicylanilide; 5-chloro-
6'-beta-naphthyloxy-salicylanilide; 5-chloro-6'-alpha-na phthyloxy-
salicylanilide; 3,3',4-trichloro-5,6'-
beta-naphthyloxy-salicylalide and the like).
Halogenated hydrocarbons also can include, without limitation, carbanilides
(e.g., 3,4,4'-trichloro-
carbanilide (TRICLOCARBAN); 3,3',4-trichloro derivatives; 3-trifluoromethy1-
4,4'-dichlorocarbanilide
and the like). Halogenated hydrocarbons include also, without limitation,
bisphenols (e.g., 2,2'-
methylenebis(4-chlorophenol); 2,2'-methylenebis(4,5-dichlorophenol); 2,2'-
methylenebis(3,4,6-
trichlorophenol); 2,2'-thiobis(4,6-dichlorophenol); 2,2'-diketobis(4-
bromophenol); 2,2'-
methylenebis(4-chloro-6-isopropylphenol); 2,2'-isopropylidenebis(6-sec-butyl-4-
chlorophenol) and
the like).
Also included within hydrogenated hydrocarbons are halogenated mono-and poly-
alkyl and aralkyl
phenols (e.g., methyl-p-chlorophenol; ethyl-p-chlorophenol; n-propyl-p-
chlorophenol; n-butyl-p-
chlorophenol; n-amyl-p-chlorophenol; sec-amyl-p-chlorophenol; n-hexyl-p-
chlorophenol;
36
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
cyclohexyl-p-chlorophenol; n-heptyl-p- chlorophenol; n-octyl-p-chlorophenol; o-
chlorophenol;
methyl-o-chlorophenol; ethyl-o-chlorophenol; n-propyl-o-chlorophenol; n-butyl-
o-chlorophenol; n-
amyl-o-chlorophenol; tert-amyl-o-chlorophenol; n-hexyl-o-chlorophenol; n-
heptyl-o-chlorophenol; p-
chlorophenol; o- benzyl-p-chlorophenol; o-benzyl-m-methyl-p-chlorophenol; o-
benzyl-m, m-
di methyl-p- chlorophenol; o-phenylethyl-p-chlorophenol ; o-phenylethyl-m-
methyl-p-chlorophenol ;
3- methyl-p-chlorophenol; 3,5-dimethyl-p-chlorophenol; 6-ethyl-3-methyl-p-
chlorophenol; 6-n-
propy1-3-methyl-p-chlorophenol; 6-iso-propy1-3-methyl-p-chlorophenol; 2-ethyl-
3, 5-dimethyl-p-
chlorophenol; 6-sec butyl-3-methyl-p-chlorophenol; 6-diethylmethy1-3-methyl-p-
chlorophenol; 6-
iso-propy1-2-ethy1-3-methyl-p-chlorophenol; 2-sec amyl-3, 5-dimethyl-p-
chlorophenol; 2-
diethylmethy1-3, 5-dimethyl-p-chlorophenol; 6-sec octy1-3-methyl-p-
chlorophenol; p-bromophenol;
methyl-p-brdmophenol; ethyl-p-bromophenol; n-propyl-p- bromophenol; n-butyl-p-
bromophenol; n-
amyl-p-bromophenol; sec-amyl-p-bromophenol; n- hexyl-p-bromophenol; cyclohexyl-
p-
bromophenol; o-bromophenol; tert-amyl-o-bromophenol; n-hexyl-o-bromophenol; n-
propyl-m, m-
dimethyl-o-bromophenol; 2-phenyl phenol; 4-chloro- 2-methyl phenol; 4-chloro-3-
methyl phenol; 4-
chloro-3, 5-dimethyl phenol; 2. 4-dichloro-3, 5- dimethylphenol; 3,4, 5, 6-
terabromo-2-
methylphenol; 5-methyl-2-pentylphenol; 4-isopropyl-3- methylphenol; 5-chloro-2-
hydroxydiphenylemethane).
Halogenated hydrocarbons also include, without limitation, chlorinated phenols
(e.g.,
parachlorometaxylenol, p-chloro-o-benzylphenol and dichlorophenol); cresols
(e.g., p-chloro-m-
cresol), pyrocatechol; p-chlorothymol; hexachlorophene; tetrachlorophene;
dichlorophene; 2,3-
dihydroxy-5,5'-dichlorophenyl sulfide; 2,2'-dihydroxy-3,3',5,5'-
tetrachlorodiphenyl sulfide; 2,2'-
dihydroxy-3,3',5,5',6,6'-hexachlorodiphenyl sulfide and 3,3'-dibromo-5,5'-
dichloro-2,2'-
dihydroxydiphenylamine). Halogenated hydrocarbons also may include, without
limitation,
.. resorcinol derivatives (e.g., p-chlorobenzyl-resorcinol; 5-chloro-2, 4-
dihydroxy-di-phenyl methane;
4'-chloro-2, 4-dihydroxydiphenyl methane; 5-bromo-2,4- dihydroxydiphenyl
methane; 4'-bromo-2,
4-dihydroxydiphenyl methane), diphenyl ethers, anilides of thiophene
carboxylic acids,
chlorhexidines, and the like.
Quaternary salts include, without limitation, ammonium compounds that include
alkyl ammonium,
pyridinum, and isoquinolinium salts (e.g., 2,2'-methylenebis(4-chlorophenol);
2,2'-methylenebis(4,5-
dichlorophenol); 2,2'-methylenebis(3,4,6-trichlorophenol); 2,2'-thiobis(4,6-
dichlorophenol); 2,2'-
diketobis(4-bromophenol); 2,2'-methylenebis(4-chloro-6-isopropylphenol); 2,2'-
isopropylidenebis(6-
sec-buty1-4-chlorophenol); cetyl pyridinium chloride;
diisobutylphenoxyethoxyethyldimethylbenzyl
37
81625301
ammonium chloride; N-methyl-N- (2- hydroxyethyl)-N-(2-hydroxydodecy1)-N-benzyl
ammonium
chloride; cetyl trimethylammonium bromide; stearyl trimethylammonium bromide;
()ley'
dimethylethylammonium bromide; lauryidimethylchlorethoxyethylammonium
chloride;
lauryidimethylbenzyl-ammonium chloride; alkyl (Cg-Cig) dimethyl (3, 4-
dichlorobenzyI)-ammonium
chloride; lauryl pyridinium bromide; lauryl iso-quinolinium bromide; N
(lauroyloxyethylaminoformylmethyl) pyridinium chloride, and the like).
Sulfur active compounds include, without limitation, thiuram sulfides and
dithiocarbamates, for
example (e.g., disodium ethylene bis-dithiocarbamate (Nabam); diammonium
ethylene bis-
dithiocarbamate (amabam); Zn ethylene bis-dithiocarbamate (ziram); Fe ethylene
bis-
dithiocarbamate (ferbam); Mn ethylene bis-dithiocarbamate (manzate);
tetramrethyl thiuram
disulfide; tetrabenzyl thiuram disulfide; tetraethyl thiuram disulfide;
tetramethyl thiuram sulfide, and
the like).
In certain embodiments, an antimicrobial material comprises one or more of
4',5-
dibromosalicylanilide; 3,4',5-tribromosalicylanilide; 3,4',5-
trichlorosalicylanilide; 3,4,4'-
trichlorocarbanilide; 3-trifluoromethyI4,4'-dichlorocarbanilide; 2,2'-
methylenebis(3,4,6-
trichlorophenol); 2,4,4'-trichloro-2'-hydroxydiphenyl ether; Tyrothricin; N-
methyl-N-(2-hydroxyethyl-
N-(2-hydroxydodecy1)-N-benzylammonium chloride; cetyl pyridinium chloride;
2,3,5-
tribromosalicylanilide; chlorohexidine digluconate; chlorohexidine diacetate;
4',5-
dibromosalicylanilide; 3,4,4'-trichlorocarbanilide; 2,4,4'-trichloro-2-
hydroxydiphenyl ether
(TRICLOSAN; 5-chloro-2-(2,4-dichlorophenoxy)phenol); 2,2'-dihydroxy-5, 5'-
dibromo-diphenyl
ether) and the like. Methods of manufacture of anti-microbial containing
plastics, and amounts of
anti-microbial substances used in manufacture of anti-microbial containing
plastics have been
described in United States Provisional Patent Application No. 61/144,029,
filed on January 12,
2009, and entitled "ANTIMICIROBIAL FLUID HANDLING DEVICES AND METHODS OF
MANUFACTURE", having attorney docket number PEL-1004-PV2.
38
CA 2787274 2017-07-26
81625301
Anti-Static Materials
In certain embodiments anti-static agents can be incorporated into the
moldable plastic during the
manufacture process of pipette tips described herein. A pipette tip may
comprise any type of
electrically conductive material, such as a conductive metal for example. Non-
limiting examples of
electrically conductive metals include platinum (Pt), palladium (Pd), copper
(Cu), nickel (Ni), silver
(Ag) and gold (Au). The metals may be in any form in or on a pipette tip, for
example, such as
metal flakes, metal powder, metal strands or coating of metal.
Electrically conductive materials, or portions thereof, may be any material
that can contain movable
electric charges, such as carbon for example. In some embodiments, a pipette
tip comprises
about 5% to about 40% or more carbon by weight (e.g., 7-10%, 9-12%, 11-14%, 13-
16%, 15-18%,
17-20%, 19-22%, 21-24%, 23-26%, 25-28%, 27-30%, 29-32%, 32-34%, 33-36%, or 35-
38%
carbon by weight). Methods for manufacturing components comprising an anti-
static member have
been described in United States Provisional Patent Application No. 61/147,065,
filed on January
23, 2009, and entitled "ANTI-STATIC PIPETTE TIP TRAYS", having attorney docket
number PEL-
1009-PV.
Precision and Accuracy
Pipette tip "precision" refers to the ability of a plurality of pipette tips
to deliver about the same
volume of fluid, with a relatively small standard deviation, for a given
dispenser (e.g., pipette tips
stated to deliver 200 microliters of fluid consistently deliver about 197
microliters of fluid). Pipette
tip "accuracy" refers to the ability of a plurality of pipette tips to deliver
a particular volume of fluid
.. (e.g., pipette tips stated to deliver 200 microliters of fluid deliver, in
practice, about 200 microliters
of fluid). One measure of pipette tip precision is a calculated percent
"coefficient of variation,"
which also is referred to herein as "CV" and discussed in greater detail
hereafter.
Coefficient of variation (CV) can be calculated for a pipette tip lot in a
variety of manners. In
general, percent CV equals (a) the quotient of (i) standard deviation in
volume dispensed from the
pipette tips, divided by (ii) the average volume dispensed from the pipette
tips, (b) multiplied by
100. A CV value often is calculated for a particular lot of pipette tips. One
of many protocols can
be selected for collecting pipette tips in the lot to calculate a CV value.
Random pipette tips may
be selected from a lot after a manufacturing run is completed in some
embodiments, and in certain
39
CA 2787274 2017-07-26
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
embodiments, pipette tips are collected at different time points during the
manufacturing run of the
lot (e.g., pipette tips are collected at time points during the manufacture
run at regular intervals).
In certain embodiments pertaining to CV measurements, water is dispensed from
pipette tips of a
particular lot using one dispensing device, and volume of each dispensed
amount is weighed. The
average and standard deviation of all weighed aliquots of water then can be
calculated in such
embodiments.
In some embodiments pertaining to CV measurements, liquid containing a dye is
dispensed from
each pipette tip into a well of a tray having an array of wells. The average
volume can be
determined from the weight of the plate containing the dispensed liquid less
the weight of the plate
before liquid was dispensed. The standard deviation in volume dispensed into
each well can be
determined by optically determining the volume in each well by the amount of
dye in each well
(e.g., using a light, fluorescence, luminescence or absorbance detector in a
plate reader).
In some embodiments, pipette tip embodiments described herein can deliver a
volume of double
distilled water with a CV of 10% or less, when the pipettor is set at a low or
minimum volume. In
certain embodiments, pipette tips described herein can deliver a volume of
double distilled water
with a CV of 5% or less, when the pipettor is set at a high or maximum volume.
The precision and
accuracy measurements of the pipette tips is dependent on the condition and
calibration of the
pipettor being tested with the tips described herein. In general, accuracy and
CV values for the
pipette tip embodiments described herein can range between 1% and 10%
depending on the
volume at which the pipettor is tested, and the condition and calibration of
the pipettor (e.g., CV of
9%, 8%, 7%, 6%, 5%, 4%, 3%, 2% or less).
Pipette tips ¨ Methods of Use
Pipette tips frequently are used in conjunction with a pipetting device
(manual or automated) to
take up, transport or deliver precise volumes of liquids or reagents. In some
embodiments, suitably
configured pipette tips also can be used to prepare or isolate biomolecules of
interest (e.g., nucleic
acids, proteins, antibodies and the like). In certain embodiments a
biomolecule of interest can be
contained in a biological fluid or biological preparation with a fluid
component.
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
Provided herein is a method of using a pipette tip comprising (a) inserting a
pipettor into a pipette
tip, and (b) contacting the pipette tip with a fluid, where the pipette tip
comprises a proximal region
and a distal region, and further where the proximal region comprises a first
set of axially oriented
ribs and a second set of axially oriented ribs, the ribs of the first set and
the second set are
circumferentially spaced and alternately spaced around the proximal region,
and ribs of the first set
have a maximum thickness greater than the maximum thickness of ribs of the
second set.
Provided also herein in some embodiments, is method of using a pipette tip
comprising, (a)
inserting a pipettor into a pipette tip, and (b) contacting the pipette tip
with a fluid, where the pipette
tip comprises a proximal region and a distal region, and further where the
distal region wall
thickness tapers from (a) a point at or between (i) about the junction of the
proximal region and
distal region to (ii) about one-quarter of the axial distance from the
terminus of the distal region to
the junction, to (b) the distal region terminus, and the wall thickness at the
distal region terminus is
about 0.0040 inches to about 0.0055 inches.
In certain embodiments, the wall thickness of the tip at the distal region
terminus is 0.0055 or less.
In some embodiments, the wall thickness at the distal region terminus is about
0.0043 inches to
about 0.0050 inches. In certain embodiments, the wall thickness at the distal
region terminus is
about 0.0044 inches to about 0.0049 inches.
Pipette Tips ¨ Methods of Manufacture
Pipette tips may be manufactured by injection molding. In some embodiments,
pipette tips
described herein are injection molded as a unitary construct. Injection
molding is a manufacturing
process for producing objects (e.g., pipette tips, for example) from
thermoplastic (e.g., nylon,
polypropylene, polyethylene, polystyrene and the like, for example) and
thermosetting plastic (e.g.,
epoxy and phenolics, for example) materials. The plastic material of choice
often is fed into a
heated barrel, mixed, and forced into a mold cavity where it cools and hardens
to the configuration
of the mold cavity. The melted material sometimes is forced or injected into
the mold cavity,
through openings (e.g., a sprue), under pressure. A pressure injection method
ensures the
complete filling of the mold with the melted plastic. After the mold cools,
the mold portions are
separated, and the molded object is ejected. In some embodiments, additional
additives can be
included in the plastic or heated barrel to give the final product additional
properties (e.g., anti-
microbial, or anti-static properties, for example).
41
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
The mold is configured to hold the molten plastic in the correct geometry to
yield the desired
product upon cooling of the plastic. Injection molds sometimes are made of two
or more parts, and
comprise a core pin. The core pin sometimes can determine the thickness of the
object wall, as
the distance between the core pin and the outer mold portion is the wall
thickness. Molds are
__ typically designed so that the molded part reliably remains on the core pin
when the mold opens,
after cooling. The core pin sometimes can be referred to as the ejector side
of the mold. The part
can then fall freely away from the mold when ejected from the core pin, or
ejector side of the mold.
In some embodiments, ejector pins and/or an ejector sleeve push the pipette
tip from the core pin.
Also provided herein is a mold for manufacturing a device by an injection mold
process, which
comprises a body that forms an exterior portion of the device and a member
that forms an inner
surface of the device, where the member comprises an irregular surface that
results in a portion of
the inner surface that is irregular (e.g., annular groove 120). In some
embodiments, the member is
a core pin for forming the inner surface of a pipette tip.
Provided also herein is a method for manufacturing a pipette tip comprising
(a) contacting a pipette
tip mold with a molten polymer, and releasing the formed pipette tip from the
mold after cooling,
where the pipette tip comprises a proximal region and a distal region, and
further where the
proximal region comprises an exterior surface and an annular flange at the
proximal terminus of
the proximal region, the proximal region comprises a first set of axially
oriented ribs and a second
set of axially oriented ribs, the ribs of the first set and the second set are
circumferentially spaced
and alternately spaced around the exterior surface of the proximal region, and
ribs of the first set
have a maximum thickness greater than the maximum thickness of ribs of the
second set.
Also provided herein in some embodiments, is method of manufacturing a pipette
tip comprising,
(a) contacting a pipette tip mold with a molten polymer, and releasing the
formed pipette tip from
the mold after cooling, where the pipette tip comprises a proximal region and
a distal region, and
further where the proximal region comprises an exterior surface and an annular
flange at the
proximal terminus of the proximal region, the distal region wall thickness
tapers from (a) a point at
or between (i) about the junction of the proximal region and distal region to
(ii) about one-quarter of
the axial distance from the terminus of the distal region to the junction, to
(b) the distal region
terminus, and the wall thickness at the distal region terminus is about 0.0040
inches to about
0.0055 inches.
42
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
Provided also herein is a method for manufacturing a device having an inner
surface and an
exterior surface, which comprises: (a) injecting a liquid polymer mixture into
a mold that comprises
a body that forms the exterior surface of the device and a member that forms
the inner surface of
the device, (b) curing the device in the mold (e.g., partially curing or fully
curing), and (c) ejecting
the device from the mold, where the member comprises an irregular surface
(e.g., annular groove
120) that results in a portion of the inner surface of the device that is
irregular. The polymer
mixture comprises a polymer and a material that can provide one or more of the
following
properties; anti-microbial activity, anti-static function, anti-foaming
function and combinations
thereof.
Examples
Example 1: Pipette tip deflection
A "soft" or flexible pipette tip often will be easier to mount onto a pipettor
than a "hard" or reduced
flexibility pipette tip, thus offering several benefits, such as better fit,
reduced insertion and/or
ejection forces and the ability to fit a larger variety of pipettor nozzles
(e.g., a more universal fit).
The flexibility or "softness" of pipette tips described herein was quantified
and compared to
competitors commercially available pipette tips.
To conduct the experiment, a force gauge (Imada model DS2-44 force gauge) was
mounted to a
fixed aluminum base plate on a table top stand, and a lever with a handle was
mounted to the
force gauge, as shown in FIG. 9. The depth that the gauge can travel was fixed
by incorporating a
travel stop on the stand. The travel stop was configured such that the depth
the gauge could travel
was fixed throughout the experiment so the only change measurable was the
force required to
depress each tip that same depth or travel distance. Each tip was placed under
the force gauge
and the handle depressed. The force reading, in pounds, was then recorded. Six
different tip
styles were used and five independent, randomly chosen, tips per style were
tested. The tips were
placed on top of the aluminum plate to ensure that the force used on the tip
was not bending the
tip. The force required for deformation would therefore only change due to the
stiffness or pliability
of the individual tip. The competitors tips tested included (designated as Tip
1, Tip 2, and the like);
tip 1,200 microliter with filter; tip 2, 100 microliter with filter; tip 3,200
microliter with filter; tip 4, 100
microliter with filter; tip 5, 300 microliter without filter; and a 300
microliter non-filter pipette tip
43
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
embodiment as described herein. The results are presented graphically in FIG.
10 and in the table
below. Results are presented as pounds of force.
Sample 1 Sample 2 Sample 3 Sample 4 Sample 5 Average
Tip 1 1.71 1.61 1.91 1.73 2.04 1.8
Tip 2 2.16 2.4 2.17 2.87 2.31 2.38
Tip 3 4.67 4.98 5.54 4.51 3.9 4.72
Tip 4 6.94 5.94 5.51 7.75 8.4 6.91
Tip 5 7.66 8.49 9.46 9.86 9.89 9.07
Pipette tip described 1.26 1.13 1.09 1.07 1.1 1.13
herein
The results presented herein indicate that 300 microliter non-filter pipette
tips described herein are,
on average, up to about 8 fold (e.g., between about 1.5 and about 8 fold;
about 1.5 fold, about 2
fold, about 2.5 fold, about 3 fold, about 3.5 fold, about 4 fold, about 4.5
fold, about 5 fold, about 5.5
fold, about 6 fold, about 6.5 fold, about 7 fold, about 7.5 fold and about 8
fold) more flexible than
some currently available competitor pipette tips.
Example 2: Ergonomic Testing - Materials and Methods
Ergonomic testing of pipette tips was performed to quantify the ergonomic
performance of tips
described herein. Popular, commercially available pipettors were utilized to
conduct these
experiments. Tips described herein were compared to custom tips manufactured
by market
leading pipette companies (e.g., for their brand of pipettor) and also to a
popular generic pipette tip
brand. Pipettors utilized in these experiments were designated pipette 1,
pipette 2, pipette 3,
pipette 4 and pipette 5, and corresponding custom tips for specific pipettors
were similarly
designated (e.g., pipette tip 1 was a custom tip for pipettor 1, pipette tip 2
was a custom tip for
pipettor 2, etc). The generic pipette tip was designated as "generic". Pipette
tips described herein
were designated "TDH".
Controlled laboratory testing was conducted by Certified Professional
Ergonomists utilizing 11
subjects whose occupations routinely utilize pipetting. Pipette tip
performance was measured in
terms of reduced tipping and de-tipping forces, enhanced user comfort, reduced
muscle effort
44
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
levels and reduced fatigue potential. The tips were tested in comparison to
published guidelines
and generally accepted biomechanics and physiologic criteria. Experiments
described herein were
designed to quantify the ergonomics performance of the tips with regard to the
appropriate
categories of ergonomics comfort and risk.
Ergonomic testing was accomplished using a combination of objective and
subjective
measurement techniques. The primary measurements included:
(a) Tip Application Effort & Force
(b) Tip Ejection Forces Effort & Force
(c) Aspiration & Dispense Muscle Effort Levels
(d) Comfort and Performance Surveys
(e) Pipetting cycle time
(f) Ranking Surveys
(g) Anthropometric Measurements
(h) Video documentation
The experimental design included appropriate sampling methods (e.g., multiple
trials, pipettor and
pipette tip randomization, and the like) to allow a valid statistical analysis
of product performance.
Video and photographic documentation of the testing also was collected.
Prior to the start of testing, participants completed a background survey
regarding pipetting
experience and a musculoskeletal stress survey of aches, pains or discomfort
experienced at work.
Anthropometric measurements also were collected. The test subjects included 3
women and 8
men with pipetting experience (11 total). The participants included
scientists, research technicians,
biologists, a chemist and graduate students. The average age of the
participants was 25.9 years
and participants had been using pipettes for an average of 4.0 years, for an
average of up to 3.3
hrs/day.
Each test participant completed a series of pipetting tasks using each of the
following tip types; (a)
a tip as described herein, (b) a custom tip for a specific brand of pipettor,
and (c) a generic tip,
selected for its popularity. Each tip was tested using the 5 different
pipettor brands. Each
participant also was monitored using electomyographic (EMG) data collection,
as shown in FIG.
11. Standardized calibration routines were utilized to ensure accuracy of
sampling.
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
Pipetting task tests
Several tests were completed on each pipette and tip combination. These
included (i) full cycle
testing, (ii) on/off testing, and (iii) step by step sequence testing. In full
cycle testing, participants
completed a series of three full pipetting cycles that included pipette tip
application, pipette tip use
(e.g., liquid aspiration, followed by liquid dispensing) and pipette tip
ejection. During on/off testing,
participants completed a series of 12 applications of a pipette tip followed
by tip ejection. The step
by step sequence testing included tip application, aspiration, dispensing and
tip ejection, in
consecutive order.
Two trials were performed for each test. Following the completion of each test
sequence, the
participants were asked to rate their perceived level of physical exertion. At
the completion of tip
testing for a pipette, the participants completed a survey of tip performance.
Anthropometric measurements
Anthropometric measurements were taken for all participants in the study. The
participants
represented the anthropometric range of the general population (5th percentile
female to 95th
percentile male). The results of anthropometric measurements are presented in
the table below.
Measurements presented in the table are in inches where not otherwise
indicated.
46
Arm
0
Hand Length. Standing
Standing t-)
o
,-.
Weight Hand Breadth (Acrom- Shoulder
Elbow Power Bench
O
o
,...
Gender (lbs) Height Length (Metacarpal) Fngrtip) Height height
Grip Ht c.4
o
oe
Female 135 63 6.825 3.125 25.5 52.5 39.675 50
38
Female 108 63 6.625 3 25.25 51.25 38.75 60
35
Female 150 69 6.875 3.125 29 59
44.625 85 36
Average 131.00 65.00 6.78 3.08 26.58 54.25 41.02
65.00 36.33
C)
,
0
NJ
-.3
CO
--3
NJ
-.3
.6. Arm
Hand Length. Standing
Standing 0
1--,
i.,
i
Weight Hand Breadth (Acrom- Shoulder
Elbow Power Bench 0
-_,
i
Gender (lbs) Height Length (Metacarpal) Fngrtip) Height height
Grip Ht
01
Male 185 74 7.8 4.5 30.3 61.4 45
104 41.6
Male 320 74 7.6 3.7 31.1 62 46
155 4.1
Male 175 73 7.9 3.7 31.6 62.5
45.4 72.5 38
Male 200 71 8 4 28.8 58
45.6 130 38 od
(-)
Male 185 70 7.8 3.8 30.1 58
42.4 125 38
cA
Male 195 65 7.4 3.8 27.5 54.6 41
85 37 t..)
o
,...
Male 148 66
,--.
O
t.4
Male 200 73 8.1 3.8 31.5 61.5
45.6 125 38.3 t.)
,-.
i.)
o
Average 201.00 70.75 7.78 3.88 30.13 59.71
44.43 113.79 33.56
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
Musculoskeletal stress survey
Participants were surveyed regarding the presence of aches, pains or
discomfort during their
normal work activities, as shown graphically in FIG. 12. Among those working
in laboratories (10 of
the 11 participants), 50% experienced discomfort in their fingers,
forearms/elbows and legs/feet.
Some of the participants indicated that extended durations of pipetting
contributed to their
discomfort and fatigue. The majority of those reporting discomfort indicated
that the frequency of
discomfort ranged between "Rarely" to "Sometimes" and the severity of the
discomfort was in the
"Mild" to "Moderate" range.
Measurement of muscle effort levels during pipette use
Measurements of muscle effort during pipette use were monitored using
electromyography (EMG).
EMG was used to assess the potential for fatigue and the overall exertion
associated with the
various tips. Reductions in muscle effort, measured in terms of percent
maximum voluntary
contraction (%MVC), can provide an improved opportunity for blood flow,
lactate resorption and
fatigue relief. Research indicates that static muscle contractions below 10%
MVC do not restrict
blood flow and the physiological equilibrium of muscle is maintained at an
aerobic level. At muscle
tensions of 20-30% of MVC a "blood flow dept" can occur, limiting oxygen
supply and removal of
waste products from muscle. Static contractions exceeding 30% MVC result in a
decrease in blood
flow and total blood flow occlusion occurs at approximately 50-60% MVC. Lower
muscle exertions
following physical activity can provide a greater recovery potential.
Five muscle groups from the pipetting arm were monitored by EMG.
Representative EMG tracings
are shown in FIG. 13. The muscle groups monitored included the major muscles
involved in
hand/finger exertions (e.g., forearm flexor and extensor muscles), the
interosseous muscles of the
thumb, the bicep, and trapezius muscles.
A calibration routine was conducted at the start of testing to obtain the MVC
for each participants'
muscles. The corresponding EMG signals were scaled using the MVC to obtain the
percent of
muscle exertion associated with each subsequent test (%MVC). The applied
muscle effort levels
were analyzed to determine the physical requirements associated with each
pipette and tip
combination. In addition, cycle time to complete the task was measured as a
gauge of product
efficiency, ease of use and productivity. The results were statistically
analyzed to determine
48
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
differences in performance between the products. The primary measurements
included; cycle time
(e.g., productivity rate in seconds); muscle work (e.g., sum of the average
exertion across the 5-
muscle groups tested, % maximum voluntary contraction); average exertion
(e.g., the average level
of muscle effort among the 5-muscle groups tested (%MVC)); peak (e.g., the
average peak level of
exertion among the muscle groups tested (%MVC)); total work done (e.g., the
sum of the total
exertions across all 5-muscle groups tested (%MVC)).
Example 3: Measurement of Overall Performance
Pipette tip effort across tasks was used as a measure of overall pipette tip
performance. All
pipettors with the exception of pipettor 3 were tested with all pipette tips.
Pipettor 3 could not
accept the generic tips, or tips as described herein.
FIG. 14 graphically illustrates the total muscle work done as a measure of tip
performance. The
measurements were taken for each of the 4 aspects of pipette tip usage (e.g.,
apply tip, aspirate
liquid, dispense liquid and de-tip or eject tip). The results shown in FIG. 14
indicate that the tips as
described herein, perform as well if not better than the generic and custom
tips for each of the
pipettors tested.
FIG. 15 graphically illustrates the total muscle work during a pipetting cycle
as a measure of tip
performance. The results presented are the average of the muscle work
measurements taken for
full cycle testing and on/off testing. The results shown in FIG. 15 indicate
that tips described
herein perform substantially better than generic tips and custom tips designed
for a specific
pipettor application.
The results presented in FIGS. 14 and 15 are summarized in the tables below,
respectively. The
term "TDH" in columns labeled 'Tips" in tables presented throughout the
disclosure refer to "tips
described herein (TDH)".
49
CA 02787274 2012-07-16
WO 2011/091308
PCT/US2011/022129
Total Work Muscle Average Peak
Test Tips Done Work Exertion Exertion
Apply Tip TDH 144.91 28.98 14.80 26.98
Custom* 149.00 29.80 14.19 25.10
Generic 161.92 32.38 15.93 29.45
Aspirate TDH 143.08 28.62 11.25 17.02
Custom* 149.84 29.97 10.88 16.68
Generic 160.53 32.11 12.16 18.44
Dispense TDH 111.49 22.30 10.60 18.54
Custom* 113.96 22.79 10.31 17.88
Generic 119.04 23.81 11.01 18.80
De-tip TDH 90.42 18.08 13.15 21.50
Custom* 95.73 19.15 12.76 21.99
Generic 102.64 20.53 13.63 23.76
Total Work Muscle Average Peak
Test Tips Done Work Exertion Exertion
Full Cycle TDH 1438.57 287.71 15.11 43.29
Custom* 1502.03 300.41 14.72 43.88
Generic 1601.45 320.29 15.45 47.38
On Off TDH 2503.00 500.60 17.85 49.85
Custom* 2593.77 518.75 17.37 49.33
Generic 2878.00 575.60 18.29 55.11
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
*Due to tip fit limitations, the Custom tip results do not include Pipettor 3
for Overall Performance.
Pipettor 3 results are presented in Example 4, Performance Across Pipette
Tips.
Example 4: Performance Across Pipette Tips
Tip performance was examined using full cycle and on/off tests for each
pipette. Statistical
analysis was performed at either p<0.05 or p<0.1 confidence intervals. The
results are
summarized in the table below.
Total Muscle Muscle Average Peak
Product Test Tips Time Work Work Exertion Exertion
Full
Pipettor 1 Cycle TDH 19.48 1336.08 267.22 14.22 38.30
Full
Pipettor 1 Cycle Custom 21.39 1516.00 303.20 14.27
40.67
Full
Pipettor 1 Cycle Generic 20.59 1506.08 301.22
14.59 43.49
Pipettor 1 On Off TDH 26.79 2284.29 456.86 17.38 48.83
Pipettor 1 On Off Custom 29.02 2317.99 463.60 16.58 45.90
Pipettor 1 On Off Generic 30.43 2785.79 557.16 18.73 54.32
Full
Pipettor 2 Cycle TDH 20.39 1420.92 284.18 15.26 44.18
Full
Pipettor 2 Cycle Custom 20.33 1498.06 299.61 15.06 45.80
Full
Pipettor 2 Cycle Generic 21.37 1690.77 338.15 16.19
53.78
Pipettor 2 On Off TDH 28.25 2568.33 513.67 18.83 50.01
Pipettor 2 On Off Custom 32.41 3187.52 637.50 19.70 58.74
Pipettor 2 On Off Generic 32.25 2822.47 564.49 17.40 53.56
51
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
Full
Pipettor 3 Cycle Custom 21.43 1494.65 298.93 14.05
37.73
Pipettor 3 On Off Custom 29.87 2516.26 503.25 16.90
46.17
Full
Pipettor 4 Cycle TDH 20.01 1477.72 295.54 15.02
46.54
Full
Pipettor 4 Cycle Custom 21.20 1502.86 300.57 14.60
43.53
Full
Pipettor 4 Cycle Generic 21.98 1607.65 321.53 15.02
48.33
Pipettor 4 On Off TDH 30.68 2709.46 541.89 17.63
51.68
Pipettor 4 On Off Custom 32.70 2449.55 489.91 15.21 46.42
Pipettor 4 On Off Generic 33.75 3286.21 657.24 19.12 65.92
Full
Pipettor 5 Cycle TDH 19.43 1527.66 305.53 16.03
44.22
Full
Pipettor 5 Cycle Custom 20.07 1491.20 298.24 14.95
45.50
Full
Pipettor 5 Cycle Generic 20.86 1596.84 319.37 15.61
42.91
Pipettor 5 On Off TDH 27.90 2449.94 489.99 17.57
48.89
Pipettor 5 On Off Custom 28.54 2266.01 453.20 17.03 43.33
Pipettor 5 On Off Generic 29.92 2662.90 532.58 18.00 47.85
The results summarized in the table above illustrate that, on average, the
tips described herein
consistently resulted in shorter cycle times and frequently required less
total and average muscle
work than the competitors tips.
52
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
Example 5: Productivity measurements
Speed of task completion was used to measure the overall contribution to
productivity for each
pipette tip. Full cycle testing and on/off testing were used to determine time
to complete pipetting
tasks. The results presented in the tables below and FIGS. 16 and 17 indicate
that on average the
custom and generic tips were 5.25% and 6.83%, respectively, slower than tips
described herein
during the completion of the pipetting cycle. The on/off test results
indicated that the custom and
generic tips were 7.57 and 10.98% slower, respectively, than tips described
herein.
Speed advantages of tips described herein can be attributed to the following
factors; (i) flared tip
opening (e.g., enables the user to more easily align the pipettor and pipette
tip), (ii) reduced effort
to apply and eject the tips described herein (e.g., contributes to faster
cycling times), and (iii) color
contrast between tips and pipette tip rack (e.g., tips described herein are
packaged in a black rack
which can improve visibility when applying a tip to the pipettor barrel).
Due to the repetitive nature of pipette use, improvements in speed performance
translate to a
reduction in the overall exposure to the stressors that contribute to
ergonomic risk.
The results of the productivity measurements are presented in the table below
and in FIGS.16 and
.. 17.
% Diff Compared
Test Tips Time to TDH
Full Cycle TDH 19.84
Full Cycle Custom 20.88 5.25%
Full Cycle Generic 21.19 6.83%
On Off TDH 28.41
On Off Custom 30.56 7.57%
On Off Generic 31.53 10.98%
53
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
Example 6: Product Performance, Comfort and Ranking Surveys
Subjects evaluated while performing pipetting tasks were surveyed at various
points in the test to
obtain feedback and their opinions regarding product performance and perceived
exertion levels.
The methods involved standardized, numerically based ratings survey
techniques. A summary of
the surveys and results are presented in the following sections.
Perceived exertion ratings
.. The participants were asked to rate their overall perceived exertion at the
completion of the on/off
and full cycle tests for each pipette tip. The survey used was based on
standardized perceptions
of effort using a modified Borg scale, shown in the table below. Borg ratings
below three (e.g.,
"Moderate") generally are considered to be acceptable levels of exertion for
tasks that have
extended durations. The Borg scale can be used as a subjective determination
of the physical
requirements associated with a task, and a relative comparison of products
used to perform a
given task.
Borg CR-10 Scale (rating of perceived exertion [RPE])
0 Nothing at all
0.5 Extremely weak (hardly noticeable)
1 Very weak
2 Weak (light)
3 Moderate
4
5 Strong (heavy)
6
7 Very Strong
8
9
10 Extremely Strong (almost maximal)
Maximal
The results of perceived exertion testing are presented graphically in FIGS.
18-22. Generally, the
results suggested that testing participants perceived the tips described
herein as requiring the
54
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
lowest, or next to lowest, physical effort among the tips tested. FIG. 18
graphically represents the
average overall ratings of perceived exertion for all pipette tips. FIG. 19
graphically illustrated the
perceived exertion ratings for all pipette tips tested using pipettor 2. FIG.
20 graphically illustrated
the perceived exertion ratings for all pipette tips tested using pipettor 4.
FIG. 21 graphically
illustrated the perceived exertion ratings for all pipette tips tested using
pipettor 5. FIG. 22
graphically illustrated the perceived exertion ratings for all pipette tips
tested using pipettor 1.
Pipettor 3 was not tested in these experiments due to pipette tip fitment
problems as noted herein.
Pipette tip performance ratings
A product performance survey was administered to each participant at the
completion of each
pipette/tip combination test. The survey included six questions pertaining to
the participants'
perceptions of tip performance and ease of use and comfort. A 10-point scale
was utilized where
10 indicated the best response (e.g., exceptional performance) and 1 indicated
the worst response
(e.g., extremely poor performance). The survey questions included; (1) effort
to apply tip; (2) ease
of aligning pipette on tip; (3) confidence that tip is sealed on pipettor; (4)
effort to eject tip; (5)
performance during "touch off"; and (6) overall comfort during use. "Touching
off' is the act of
touching the dispensing end of the pipette tip against the bottom or sidewall
of the liquid receptacle
in order to remove the last drop of liquid that may adhere to the outer
surface of the pipette tip.
Generally, the tips described herein received the highest (e.g., best) ratings
by participants across
each of the survey criteria. The results of the subjective surveys are
presented graphically in
FIGS. 23-28, and also are summarized in the table below.
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
OVERALL RATINGS BY PIPETTE
Effort Effort
Applying Ease to Confidence Tip Ejecting "Touch-
Overall
Pipette Tips Tip Align Sealed Tip Off Comfort
Pipettor 1 TDH 9.00 9.23 8.07 8.63 8.63 8.92
Pipettor 1 Custom 8.37 8.22 7.69 8.38 8.01 8.34
Pipettor 1 Generic 7.55 8.08 8.35 7.59 8.19 7.79
Pipettor 2 TDH 8.07 8.33 8.06 7.40 7.96 8.12
Pipettor 2 Custom 7.48 7.34 7.92 6.56 7.81 7.05
Pipettor 2 Generic 6.77 6.85 7.77 6.44 7.58 6.49
Pipettor 3 Custom 8.23 7.60 9.00 8.30 8.02 8.48
Pipettor 4 TDH 8.35 7.82 7.73 8.56 8.27 8.05
Pipettor 4 Custom 8.05 7.05 7.55 7.85 7.66 7.52
Pipettor 4 Generic 7.16 6.41 7.14 7.32 7.39 6.75
Pipettor 5 TDH 8.59 8.77 8.14 8.61 8.54 8.75
Pipettor 5 Custom 8.89 8.26 7.95 8.71 8.58 8.58
Pipettor 5 Generic 7.28 6.94 7.79 6.87 7.87 7.34
Example 7: Pipette Tip Ratings
The participants were asked to rank each of the tips from "most preferred'' to
"least preferred at the
completion of all phases of testing. The ranking categories for the pipette
tip ratings were based
on the following criteria; (1) effort to apply pipette tip to pipettor; (2)
effort to eject pipette tip from
pipettor; (3) ease of aligning pipette tip with pipettor barrel; (4) overall
comfort of a particular tip; (5)
overall speed and efficiency of task completion with a particular pipette tip;
and (6) overall
preference of use.
56
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
Each pipette tip was awarded points base on the ranking received for each of
the criteria. The
product ranked as "most preferred" received a ranking value of "1", and the
least preferred
received a ranked value of "3". The results are presented graphically in FIGS.
29 and 30. FIG. 29
shows the results for effort to apply pipette tip to pipettor (e.g., "tip
application effort" panel), effort
to eject pipette tip from pipettor (e.g., "tip ejection effort" panel), and
ease of aligning pipette tip with
pipettor barrel (e.g., "ease of alignment" panel) for each pipette tip tested.
FIG. 30 shows the
results for overall comfort of a particular tip (e.g., "overall comfort"
panel), overall speed and
efficiency of task completion with a particular pipette tip (e.g.,
"speed/efficiency" panel), and overall
preference of use (e.g., "overall preference panel") of a particular tip. The
results shown in FIGS.
29 and 30 indicate that the tips described herein were ranked as the most
preferred in nearly all
categories and was ranked similarly to the custom (e.g., brand specific)
pipette tips in overall
performance. The popular generic tip selected due to is popularity ranked as
least preferred in all
categories used in pipette tip ranking.
Example 8: Pipette Tip Application and Ejection Forces.
Pipette tip application and ejection forces were measured using a digital
force gauge. The forces
were measured on the 200 microliter and 1000 microliter capacities for each
brand of pipette tip
tested. The pipette tips tested were (i) the tips described herein, (ii)
custom tips (e.g., brand
specific), and (ii) the popular generic pipette tip. The test results for
pipette tips on each brand of
pipettor are shown graphically in FIGS. 31-39. The results shown for pipettor
3 only reflect the
brand specific custom tip due to fitment of pipette tips as noted herein.
FIG. 31 shows the results of pipettor 1 with tips of the 200 microliter
capacity. FIG. 32 shows the
results of pipettor 1 with tips of the 1000 microliter capacity. FIG. 33 shows
the results of pipettor 2
with tips of the 200 microliter capacity. FIG 34 shows the results of pipettor
2 with tips of the 1000
microliter capacity. FIG. 35 shows the results of pipettor 3 using only brand
specific custom pipette
tips in the 200 microliter and 1000 microliter capacities. FIG. 36 shows the
results of pipettor 4 with
tips of the 200 microliter capacity. FIG. 37 shows the results of pipettor 4
with tips of the 1000
microliter capacity. FIG. 38 shows the results of pipettor 5 with tips of the
200 microliter capacity.
FIG. 39 shows the results of pipettor 5 with tips of the 1000 microliter
capacity.
57
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
The magnitude of the difference between the applied forces for the tips
described herein as
compared to the generic and customs tips varied with both the size of the tip
being tested and the
pipettor being used, however, the results presented in FIGS. 31-39 indicate
that the tips described
herein outperformed the custom and generic tips in a substantial majority of
the tests..
Example 9: Conclusions of Independent Ergonomic Testing Facility
Ergonomic testing of pipette tips described herein against other manufacturers
pipette tips
indicated significant measureable differences in the factors associated with
user effort, measured
forces, user perceptions, fatigue potential and comfort. The overall ergonomic
performance of the
tips described herein was equal to or better than the other commercial
products tested in
substantially all categories. A brief summary of some of the measureable
differences is presented
below.
Productivity
On average the Custom and Generic tips were 5.25% and 6.83%, respectively,
slower during the
completion of the pipetting cycle, than the tips described herein.
Additionally, the on/off test
indicated that the Custom and Generic tips were 7.57% and 10.98%,
respectively, slower than the
tips described herein.
Reductions in muscle effort
On average, the tips described herein consistently resulted in shorter cycle
times and often
required less total and average muscle work. Tips described herein were
significantly faster and/or
required less effort than the custom and generic tips in the majority of all
full cycle and on/off tests,
performed with all 5 pipettors tested, measured at confidence intervals of
either (p<0.05) and
(p<0.1).
Lowest measured forces
The forces measured during application of the generic 200 microliter and 1000
microliter pipette
tips were considerably higher than forces measured for the tips described
herein when used in
58
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
conjunction with pipettor 5 (e.g., 39.6% to 56.4% higher), pipettor 4 (e.g.,
30.1% to 63.9% higher),
pipettor 2 (e.g., 82.9 % to 18.3% higher) and pipettor 1 (e.g., 20% higher,
for the 200 microliter tip).
The forces measured during application of the 200 microliter and 1000
microliter pipettes were
higher than the tips described herein when used in conjunction with pipettor 5
(e.g., 4.0% to 45.4%
higher), pipettor 4 (e.g., 54.3% for the 1000 microliter tip), pipettor 2
(e.g., 31.6% to 11.9% higher)
and pipettor 1(e.g., 8.0% to 11.0% higher). Additionally, tip ejection forces
associated with the tips
described herein were lower than the forces measured for the generic and
custom tips for the 200
microliter and 1000 microliter applications, with the exception of the custom
(e.g., brand specific)
1000 microliter pipette tip for pipettor 1. For the 200 microliter version of
pipettor 1, the generic tip
.. required 138% more effort for tip ejection than tips described herein.
Lowest perceived effort for use
Tips described herein were perceived as requiring the lightest effort when
used with pipettors 1, 2
and 4. For pipettor 5, tips described herein were also perceived as requiring
a lighter effort than
the generic tips and similar level of effort when compared to the custom or
brand specific tip for
pipettor 5. In general, the overall perceived level of effort associated with
tips described herein
corresponded to a "Very weak" to "Weak" level of exertion.
Consistently earned the highest ratings or were ranked equally with custom
application tips
by experienced users
Generally, the over all ratings of product performance for tips described
herein were consistently
better than the other tips tested, when compared across all pipettor models.
Additionally, tips
described herein were consistently rated better than the custom and generic
tips when used with
pipettors 1, 2 and 4. Tips described herein were also rated better than
generic tips when used in
conjunction with pipettor 5 and were rated similarly to the custom tips for
pipettor 5. Experienced
pipette and pipette tip users ranked tips described herein as the "most
preferred" in 5 of the 6
categories tested (e.g., tip application effort, tip ejection effort, ease of
aligning pipette on tip,
overall comfort to use and overall speed and efficiency).
59
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
Example 10: Comparison of pipetting accuracy and task productivity as measured
by liquid
retention and time required for task completion where minimizing sample loss
is a factor
Many types of medical and scientific analysis require handling of samples that
are available in
limiting quantities and/or often involve reagents that are difficult, and/or
expensive, to prepare. In
these circumstances, users of the samples or reagents must ensure that samples
are accurately
and substantially completely dispensed to ensure assay consistency and to
minimize waste of
reagents. Ensuring that a sample is substantially completely dispensed may add
time and
therefore costs to the productivity of clinical or laboratory personnel
performing analysis that
involve limiting or expensive reagents.
To measure the benefits of the advantageous features of the pipette tips
described herein (e.g.,
TDH) against commercially available pipette tips, one 200 microliter pipettor
(e.g., the pipettor
previously designated as the 200 microliter version of pipettor 2) was used to
test the accuracy and
time to completion of a specific pipetting cycle. The tests were carried out
by the testing facility
described herein. The pipettor was chosen due to it's performance in other
tests described herein.
Pipettor 2 was tested in conjunction with the tips described herein, the
custom tips for pipettor 2,
and the generic tips also previously tested.
The pipetting cycled used for this analysis included the following steps:
1) aspirate 200 microliters of liquid,
2) dispense the liquid using the pipettor's over-blow feature,
3) visually inspect the tip of the pipette tip to determine if any liquid
remained with the tip,
4) collect any liquid remaining on the tip of those pipette tips with liquid,
and
5) determine (i) time to completion for the total number of each pipette tip
type, (ii) weight
of the collected liquid for each pipette tip type, and (iii) total number and
% pipetted
samples resulting in remaining fluid at the tip.
The term "over-blow feature" as used herein refers to the additional stroke of
a pipettor plunger,
which allows a user to fully dispense liquid by pushing the plunger past the
position normally used
for liquid aspiration. Collecting any remaining liquid on the end of the tips
by touching the tip to a
surface that is subsequently weighed, simulates the action described herein as
"touching-off".
Touching off is a process often used by pipettor users to ensure pipetting
accuracy and
substantially complete delivery of samples. A total of 430 pipette tips (e.g.,
equivalent to about 5
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
racks) of each type were tested using the pipetting cycle described above.
Weight of liquid
collected was measured on a Sartorius G0503 precision scale. The scale was
calibrated prior to
the test (Troemner, Certification number 547366W).
Results
Tips described herein have been designed with features that provide the
advantageous benefits of
substantially complete sample delivery (e.g., blade feature) and ease of tip
engagement and tip
ejection (flexible, ribbed proximal region with flange). The experiments
presented herein
demonstrate the advantageous benefits of the features of tips described
herein. The amount of
liquid and the number of tips that retained liquid were measurements of the
advantages of the
blade tip feature, while the time to completion was a measurement of the
combined benefits of the
blade tip feature (reduced or eliminated the need for touching off) and the
ease of pipette tip
application and ejection. Generally, the results indicate that the tips
described herein (TDH) had
the lowest amount of collectable fluid (e.g., fluid retained on the tip), were
the tips least likely to
retain fluid on the tip, and showed lowest time to completion due to the lack
of fluid retained and
ease of pipette tip engagement and disengagement. The results and further
analysis are
presented in the tables below and in FIGS. 40-41.
Fluid remaining with the tip after dispensing
As noted previously, samples and/or reagents frequently are hard to prepare,
expensive, limiting,
or any combination thereof. Therefore, ensuring substantially complete
delivery of a sample is
advantageous to overall sample processing costs. Increasing the time to allow
substantially
complete delivery of a sample may offset any cost benefits realized by
substantially complete
sample/reagent delivery. Tips described herein were compared to generic and
pipettor 2 custom
tips for fluid retained at the tip of the pipette tip. Tips described herein
feature the "blade tip"
design, whereas the tips of the generic and pipettor 2 custom tips do not
feature the same distal
terminal end. The weights of collected liquid, after completing the pipetting
cycle for 430 of each
pipette tip type, is presented in the table below and graphically in FIG. 40.
Generic Custom TDH
Total Wt. (g) 0.2682 0.0555 0.0001
% Error 0.31186 0.064535 0.000116
61
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
The total weight of the liquid collected was converted to the % error (e.g.,
equivalent to percent
pipetting error) using the following formula;
[W / (X)(N)] * 100 = % pipetting error (Equation 1),
where W = the weight of liquid collected in grams, X = the total weight of
liquid pipetted (e.g., a
constant for this experiment set at 200 microliters which is equivalent to 200
micrograms), and N =
the number of pipette tips sampled (e.g., a constant number for this
experiment, a total of 430 tips
of each type were tested). Using the custom tips as an example,
[0.05551(.2g)(430)]*100=
0.06453% or 0.065%.
The total percent of fluid that remained undelivered to the test samples
(e.g., % error) gives an
indication of pipetting accuracy, and tips described herein resulted in the
least error (e.g.,
0.00012% liquid retained). The generic tips resulted in the greatest error
(e.g., 0.312% liquid
retained). The custom tips performed better than the generic tips, (e.g.,
0.065% liquid retained),
however the custom tips showed a substantially larger liquid retention than
tips described herein.
These results indicate that tips described herein have a higher pipetting
accuracy, with respect to
sample delivery, than other tips described herein.
Number of tips of each type retaining liquid
In addition to determining the weight of the liquid retained for each tip
type, the total number and
percentage of tips of each type, utilized in a pipetting cycle, that retained
liquid also was
determined. The results are presented in the table below and graphically in
FIG. 41.
Generic Custom TDH
# of tips that retained fluid 121 16 1
%Error 28.14 3.72 0.23
The results shown in the table above and in FIG. 41 indicate that the generic
tips had the largest
total number and percentage of tips that retained liquid by significant
margin. Only 1 of the tips
described herein retained any liquid, demonstrating the surprising
advantageous benefit of the
blade tip feature. The percent error value presented in the table above is
calculated by dividing the
number of tips that retained liquid by the number of total samples (e.g., 430
tips), multiplied by 100.
62
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
Productivity
In addition to the benefits of substantially complete delivery of sample as a
benefit of the blade tip
feature, additional design features of pipette tips described herein may
contribute to a general
increase in productivity seen by users of tips described herein, when compared
to identical tasks
performed using other pipette tips (e.g., the generic and/or pipettor specific
custom designed,
pipette tips). Increases in productivity can lead to cost benefits.
The time required to complete the sampling (e.g., utilizing 430 pipette tips)
for each type of tip was
measured during the accuracy test. Each tip was visually inspected following
the dispensing step
to determine if fluid remained on the tip. Samples that had fluid remaining
were subjected to
sample collection and weighing, including data entry at time of measurement,
into a computer
placed adjacent to the scale. The additional time for sample collection,
weighing and data entry
are reflected in the time to complete each pipette tip cycle. The results are
presented graphically
in FIG. 42. Consistent with the other results presented in this example, tips
described herein
substantially outperformed the generic and pipettor specific pipette tips. The
results indicate the
time savings benefit is between about 20% and about 90%, for the pipetting
cycle described.
Different pipetting cycles may yield different time savings benefits, in some
embodiments. The
percent reduction in time was calculated as follows;
[(Time to complete cycle with 430 samples of pipette tip X) ¨ (Time to
complete cycle with 430
samples of pipette tips described herein)/ (Time to complete cycle with 430
samples of pipette tip
X)]*100 = Percent reduction in time to complete pipetting cycle (Equation
2).
Using the custom pipette tips as an example, [14.75 minutes ¨ 11.11
minutes/14.75 minutes]*100
= 24.67%, or about a 24.7% reduction in time to complete the pipetting cycle
as described.
The advantageous benefits of the proximal flexible region and blade tip distal
region features
provide significant reduction in (i) effort of use, (ii) time of pipetting
task completion, and (iii) liquid
retained with tip, all of which can contribute to operational cost savings,
including claims for
repetitive type injuries.
63
CA 02787274 2012-07-16
WO 2011/091308
PCT/US2011/022129
Example 11: Examples of Embodiments
Provided hereafter are certain non-limiting examples of embodiments of the
technology.
1. A pipette tip comprising a proximal region and a distal region, wherein:
the proximal region comprises an exterior surface and an annular flange at the
proximal
terminus of the proximal region;
the proximal region comprises a first set of axially oriented ribs and a
second set of axially
oriented ribs;
the ribs of the first set and the second set are circumferentially spaced and
alternately
spaced around the exterior surface of the proximal region; and
ribs of the first set have a maximum thickness greater than the maximum
thickness of ribs
of the second set.
2. The pipette tip of embodiment 1, wherein the proximal region comprises an
annular flange at
the proximal terminus of the proximal region.
3. The pipette tip of embodiment 1, wherein one end of ribs in the first set,
of ribs in the second
set, or of ribs in the first set and the second set is co-extensive with, or
terminates at, the flange.
4. The pipette tip of embodiment 1, wherein one end of ribs in the first set,
of ribs in the second
set, or of ribs in the first set and the second set is co-extensive with, or
terminates at, the junction
between the flange and the proximal region.
5. The pipette tip of embodiment 1, wherein one end of ribs in the first set,
of ribs in the second
set, or of ribs in the first set and the second set is co-extensive with, or
terminates at, the junction
between the proximal region and the distal region.
6. The pipette tip of embodiment 1, wherein of ribs in the first set, of ribs
in the second set, or of
ribs in the first set and the second set extend from the junction of the
flange and proximal region to
the junction of the proximal and distal regions.
64
CA 02787274 2012-07-16
WO 2011/091308
PCT/US2011/022129
7. The pipette tip of embodiment 1, wherein:
the distal region wall thickness tapers from (a) a point at or between (1)
about the junction of
the proximal region and distal region to (ii) about one-quarter of the axial
distance from the
terminus of the distal region to the junction, to (b) the distal region
terminus, and
the wall thickness at the distal region terminus is about 0.0040 inches to
about 0.0055
inches.
8. The pipette tip of embodiment 7, wherein the wall thickness at the distal
region terminus is
about 0.0043 inches to about 0.0050 inches.
9. The pipette tip of embodiment 8, wherein the wall thickness at the distal
region terminus is
about 0.0044 inches to about 0.0049 inches.
10. The pipette tip of embodiment 1, wherein the interior surface of the
distal region is
substantially smooth.
11. The pipette tip of embodiment 1, wherein the exterior surface of the
distal region comprises a
step.
12. The pipette tip of embodiment 1, wherein the proximal region comprises a
frustum-shaped
cavity within the interior of the proximal region.
13. The pipette tip of embodiment 12, wherein the frustum-shaped cavity is
substantially smooth.
14. The pipette tip of embodiment 12, wherein the frustum-shaped cavity
comprises an annular
groove.
15. The pipette tip of embodiment 1, wherein each rib of the first set
alternates with each rib of the
second set.
16. The pipette tip of embodiment 1, wherein the thickness at or near the
proximal terminus of the
distal region is substantially similar to the thickness at or near the distal
terminus of the proximal
region.
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
17. A pipette tip comprising a proximal region and a distal region, wherein:
the proximal region comprises an exterior surface and an annular flange at the
proximal
terminus of the proximal region;
the distal region wall thickness tapers from (a) a point at or between (I)
about the junction of
the proximal region and distal region to (ii) about one-quarter of the axial
distance from the
terminus of the distal region to the junction, to (b) the distal region
terminus, and
the wall thickness at the distal region terminus is about 0.0040 inches to
about 0.0055
inches.
18. The pipette tip of embodiment 17, wherein the proximal region comprises an
annular flange at
the proximal terminus of the proximal region.
19. The pipette tip of embodiment 17, wherein the proximal region comprises a
first set of axially
oriented ribs and a second set of axially oriented ribs.
20. The pipette tip of embodiment 19, wherein the ribs of the first set and
the second set are
circumferentially spaced and alternately spaced around the proximal region.
21. The pipette tip of embodiment 19, wherein ribs of the first set have a
maximum thickness
greater than the maximum thickness of ribs of the second set.
22. The pipette tip of embodiment 19, wherein one end of ribs in the first
set, of ribs in the second
set, or of ribs in the first set and the second set is co-extensive with, or
terminates at, the flange.
23. The pipette tip of embodiment 19, wherein one end of ribs in the first
set, of ribs in the second
set, or of ribs in the first set and the second set is co-extensive with, or
terminates at, the junction
between the flange and the proximal region.
24. The pipette tip of embodiment 19, wherein one end of ribs in the first
set, of ribs in the second
set, or of ribs in the first set and the second set is co-extensive with, or
terminates at, the junction
between the proximal region and the distal region.
66
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
25. The pipette tip of embodiment 19, wherein one end of ribs in the first
set, of ribs in the second
set, or of ribs in the first set and the second set extend from the junction
of the flange and proximal
region to the junction of the proximal and distal regions.
.. 26. The pipette tip of embodiment 19, wherein each rib of the first set
alternates with each rib of
the second set.
27. The pipette tip of embodiment 19, wherein the thickness at or near the
proximal terminus of
the distal region is substantially similar to the thickness at or near the
distal terminus of the
proximal region.
28. The pipette tip of embodiment 17, wherein the wall thickness at the distal
region terminus is
about 0.0043 inches to about 0.0050 inches.
29. The pipette tip of embodiment 28, wherein the wall thickness at the distal
region terminus is
about 0.0044 inches to about 0.0049 inches.
30. The pipette tip of embodiment 17, wherein the interior surface of the
distal region is
substantially smooth.
31. The pipette tip of embodiment 17, wherein the exterior surface of the
distal region comprises a
step.
32. The pipette tip of embodiment 17, wherein the proximal region comprises a
frustum-shaped
cavity within the interior of the proximal region.
33. The pipette tip of embodiment 32, wherein the frustum-shaped cavity is
substantially smooth.
34. The pipette tip of embodiment 32, wherein the frustum-shaped cavity
comprises an annular
groove.
35. A method of using a pipette tip comprising;
(a) inserting a pipettor into a pipette tip; and
(b) contacting the pipette tip with a fluid;
67
CA 02787274 2012-07-16
WO 2011/091308
PCT/US2011/022129
wherein the pipette tip comprises a proximal region and a distal region, and
further wherein:
the proximal region comprises an exterior surface and an annular flange at the
proximal terminus
of the proximal region;
the proximal region comprises a first set of axially oriented ribs and a
second set of axially oriented
ribs;
the ribs of the first set and the second set are circumferentially spaced and
alternately
spaced around the exterior surface of the proximal region; and
ribs of the first set have a maximum thickness greater than the maximum
thickness of ribs
of the second set.
36. A method of using a pipette tip comprising;
(a) inserting a pipettor into a pipette tip; and
(b) contacting the pipette tip with a fluid;
wherein the pipette tip comprises a proximal region and a distal region, and
further wherein:
the proximal region comprises an exterior surface and an annular flange at the
proximal terminus
of the proximal region, the distal region wall thickness tapers from (a) a
point at or between (i)
about the junction of the proximal region and distal region to (ii) about one-
quarter of the axial
distance from the terminus of the distal region to the junction, to (b) the
distal region terminus, and
the wall thickness at the distal region terminus is about 0.0040 inches to
about 0.0055
inches.
37. A method of manufacturing a pipette tip comprising;
(a) contacting a pipette tip mold with molten polymer; and
(b) releasing the formed pipette tip from the mold after cooling; wherein the
pipette tip has
features imparted by the mold comprising; a proximal region and a distal
region, and further
wherein: the proximal region comprises an exterior surface and an annular
flange at the proximal
terminus of the proximal region;
the proximal region comprises a first set of axially oriented ribs and a
second set of axially
oriented ribs;
the ribs of the first set and the second set are circumferentially spaced and
alternately
spaced around the exterior surface of the proximal region; and
ribs of the first set have a maximum thickness greater than the maximum
thickness of ribs
of the second set.
68
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
38. A method of manufacturing a pipette tip comprising;
(a) contacting a pipette tip mold with molten polymer; and
(b) releasing the formed pipette tip from the mold after cooling; wherein the
pipette tip has
features imparted by the mold comprising; a proximal region and a distal
region, and further
wherein: the proximal region comprises an exterior surface and an annular
flange at the proximal
terminus of the proximal region, the distal region wall thickness tapers from
(a) a point at or
between (i) about the junction of the proximal region and distal region to
(ii) about one-quarter of
the axial distance from the terminus of the distal region to the junction, to
(b) the distal region
terminus, and
the wall thickness at the distal region terminus is about 0.0040 inches to
about 0.0055
inches.
39. A pipette tip comprising a proximal region and a distal region, wherein:
the proximal region comprises an exterior surface and an annular flange at the
proximal
terminus of the proximal region,
the proximal region comprises a plurality of axially oriented ribs;
a thickness of the proximal region is about 0.005 inches to about 0.015
inches;
the thickness is (i) at or near a sealing zone for a dispensing device, and
(ii) at a portion
between the ribs;
the ribs or portion thereof extend over the sealing zone.
40. The pipette tip of embodiment 39, wherein the proximal region comprises an
annular flange at
the proximal terminus of the proximal region.
41. The pipette tip of any one of embodiments 39-40, wherein one end of ribs
is co-extensive with,
or terminates at, the flange.
42. The pipette tip of any one of embodiments 39-40, wherein one end of ribs
is co-extensive with,
or terminates at, the junction between the flange and the proximal region.
43. The pipette tip of any one of embodiments 39-40, wherein one end of ribs
is co-extensive with,
or terminates at, the junction between the proximal region and the distal
region.
69
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
44. The pipette tip of any one of embodiments 39-40, wherein the ribs extend
from the junction of
the flange and proximal region to the junction of the proximal and distal
regions.
45. The pipette tip of any one of embodiments 39-44, wherein:
the distal region wall thickness tapers from (a) a point at or between (I)
about the junction of
the proximal region and distal region to (ii) about one-quarter of the axial
distance from the
terminus of the distal region to the junction, to (b) the distal region
terminus, and
the wall thickness at the distal region terminus is about 0.0040 inches to
about 0.0055
inches.
46. The pipette tip of embodiment 45, wherein the wall thickness at the distal
region terminus is
about 0.0043 inches to about 0.0050 inches.
47. The pipette tip of embodiment 46, wherein the wall thickness at the distal
region terminus is
about 0.0044 inches to about 0.0049 inches.
48. The pipette tip of any one of embodiments 39-47, wherein the interior
surface of the distal
region is substantially smooth.
49. The pipette tip of any one of embodiments 39-48, wherein the exterior
surface of the distal
region comprises a step.
50. The pipette tip of any one of embodiments 39-49, wherein the proximal
region comprises a
frustum-shaped cavity within the interior of the proximal region.
51. The pipette tip of embodiment 50, wherein the frustum-shaped cavity is
substantially smooth.
52. The pipette tip of embodiment 51, wherein the frustum-shaped cavity
comprises an annular
groove.
53. The pipette tip of any one of embodiments 39-52, wherein the thickness of
the proximal region
is about 0.007 inches to about 0.0013 inches.
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
54. The pipette tip of any one of embodiments 39-52, wherein the thickness of
the proximal region
is about 0.008 inches to about 0.0012 inches.
55. The pipette tip of any one of embodiments 39-52, wherein the thickness of
the proximal region
is about 0.009 inches to about 0.011 inches.
56. The pipette tip of any one of embodiments 39-52, wherein the thickness of
the proximal region
is about 0.010 inches.
57. The pipette tip of any one of embodiments 39-56, wherein the maximum
thickness of the ribs
is about 0.037 inches to about 0.060 inches.
58. The pipette tip of any one of embodiments 39-56, wherein the maximum
thickness of the ribs
is about 0.016 inches to about 0.027 inches.
59. The pipette tip of any one of embodiments 39-56, wherein the maximum
thickness of the ribs
is about 0.015 inches to about 0.025 inches.
60. The pipette tip of any one of embodiments 39-56, wherein the maximum
thickness of the ribs
is about 0.011 to about 0.021 inches.
61. The pipette tip of any one of embodiments 39-56, wherein the maximum
thickness of the ribs
is about 0.003 inches to about 0.009 inches.
62. The pipette tip of any one of embodiments 1-34 and 39-61, wherein the
proximal region can be
deflected a defined distance from a resting position by a deflection force of
less than 1.75 pounds.
63. The pipette tip of any one of embodiments 1-34 and 39-61, wherein the
proximal region can be
deflected a defined distance from a resting position by a deflection force
between about 1.07
pounds and about 1.26 pounds.
64. A pipette tip comprising a flexible proximal region and a distal region,
wherein the proximal
region can be deflected a defined distance from a resting position by a
deflection force of less than
1.75 pounds.
71
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
65. The pipette tip of embodiment 64, wherein the proximal region is deflected
a defined distance
from the resting position by a deflection force between about 1.07 pounds and
about 1.26 pounds.
66. A pipette tip comprising a proximal region and a distal region, wherein:
the proximal region comprises an exterior surface and an annular flange at the
proximal
terminus of the proximal region;
the proximal region comprises a first set of axially oriented ribs and a
second set of axially
oriented ribs;
the ribs of the first set and the second set are circumferentially spaced and
alternately
spaced around the exterior surface of the proximal region;
ribs of the first set have a maximum thickness greater than the maximum
thickness of ribs
of the second set; and
the proximal region is deflected a defined distance from a resting position by
a deflection
force of less than 1.75 pounds.
67. The pipette tip of embodiment 66, wherein the proximal region is deflected
a defined distance
from the resting position by a deflection force between about 1.07 pounds and
about 1.26 pounds.
68. The pipette tip of embodiments 66 or 67, wherein:
the distal region wall thickness tapers from (a) a point at or between (1)
about the junction of
the proximal region and distal region to (ii) about one-quarter of the axial
distance from the
terminus of the distal region to the junction, to (b) the distal region
terminus, and
the wall thickness at the distal region terminus is about 0.0040 inches to
about 0.0055
inches.
69. A pipette tip comprising a proximal region and a distal region, wherein:
the proximal region comprises an exterior surface and an annular flange at the
proximal
terminus of the proximal region;
the distal region wall thickness tapers from (a) a point at or between (i)
about the junction of the
proximal region and distal region to (ii) about one-quarter of the axial
distance from the terminus of
the distal region to the junction, to (b) the distal region terminus,
the wall thickness at the distal region terminus is about 0.0040 inches to
about 0.0055
inches; and the proximal region is deflected a defined distance from a resting
position by a
deflection force of less than 1.75 pounds.
72
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
70. The pipette tip of embodiment 69, wherein the proximal region is deflected
by the known
distance from the resting position by a deflection force between about 1.07
pounds and about 1.26
pounds.
71. A pipette tip comprising a proximal region and a distal region, wherein:
the proximal region comprises an exterior surface and an annular flange at the
proximal
terminus of the proximal region,
the proximal region comprises a plurality of axially oriented ribs;
a thickness of the proximal region is about 0.005 inches to about 0.015
inches;
the thickness is (i) at or near a sealing zone for a dispensing device, and
(ii) at a portion
between the ribs;
the ribs or portion thereof extend over the sealing zone; and the proximal
region is deflected
a defined distance from a resting position by a deflection force of less than
1.75 pounds.
72. The pipette tip of embodiment 71, wherein the proximal region is deflected
by the defined
distance from the resting position by a deflection force between about 1.07
pounds and about 1.26
pounds.
73. The pipette tip of any one of embodiments 62 to 72, wherein a surface of
the proximal region
is deflected in a direction substantially perpendicular to the axis extending
from the distal portion
terminus to the proximal region terminus.
74. The pipette tip of any one of embodiments 62 to 73, wherein the pipette
tip retains less than
0.065% of the fluid drawn into the pipette tip after the liquid is dispensed.
75. The pipette tip of any one of embodiments 62 to 73, wherein the pipette
tip retains no more
than 0.00012% of the fluid drawn into the pipette tip after the liquid is
dispensed.
76. The pipette tip of any one of embodiments 62 to 75, wherein less than
3.72% of the pipette
tips utilized in a pipette cycle retain a portion of the fluid drawn into the
pipette tips after the liquid is
dispensed.
73
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
77. The pipette tip of any one of embodiments 62 to 75, wherein between 0.05%
to 1.0% of the
pipette tips utilized in a pipette cycle retain a portion of the fluid drawn
into the pipette tips after the
liquid is dispensed.
78. The pipette tip of any one of embodiments 62 to 75, wherein between 0.15%
to 0.3% of the
pipette tips utilized in a pipette cycle retain a portion of the fluid drawn
into the pipette tips after the
liquid is dispensed.
79. The pipette tip of any one of embodiments 62 to 75, wherein between 0.2%
to 0.26% of the
pipette tips utilized in a pipette cycle retain a portion of the fluid drawn
into the pipette tips after the
liquid is dispensed.
80. The pipette tip of any one of embodiments 62 to 79, wherein less than
3.72% of the pipette
tips utilized in a pipette cycle retains less than 0.065% of the fluid drawn
into the pipette tips after
the liquid is dispensed.
81. The pipette tip of embodiment 80, wherein less than 3.72% of the pipette
tips utilized in a
pipette cycle retain no more than 0.00012% of the fluid drawn into the pipette
tips after the liquid is
dispensed.
82. The pipette tip of any one of embodiments 62 to 79, wherein between 0.2%
to 0.26% of the
pipette tips utilized in a pipette cycle retain less than 0.065% of the fluid
drawn into the pipette tips
after the liquid is dispensed.
83. The pipette tip of embodiment 82, wherein between 0.2% to 0.26% of the
pipette tips utilized in
a pipette cycle retain no more than 0.00012% of the fluid drawn into the
pipette tips after the liquid
is dispensed.
84. The pipette tip of any one of embodiments 62 to 83, wherein the pipette
tip contributes to a
reduction of between 20% and 90% in the average time to complete a cycle of
steps in a method
for manipulating a solution.
85. A method for manipulating a solution using a pipette tip, comprising
(a) applying a pipette tip to a pipettor;
74
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
(b) aspirating a solution;
(c) dispensing the solution into a receptacle; and
(d) ejecting the pipette tip from the pipettor,
wherein the average time to complete 3 cycles of steps (a) to (d) is 20.88
seconds or less.
86. The method of embodiment 85, wherein step (c) further comprises touching
the distal terminus
of the pipette tip to a wall of the receptacle after the fluid is dispensed
from the interior of the tip.
87. The method of embodiment 85, wherein step (c) further comprises visually
inspecting the distal
terminus of the pipette tip to determine if any fluid remains associated with
the pipette tip after the
fluid is dispensed.
88. The method of embodiment 85, wherein step (c) further comprises touching
the distal terminus
of the pipette tip to a wall of the receptacle after the fluid is dispensed
from the interior of the tip,
and also further comprises visually inspecting the distal terminus of the
pipette tip to determine if
any fluid remains associated with the pipette tip after the fluid is
dispensed.
89. The method of embodiment 85, wherein the thickness of the tip wall at the
distal terminus is
0.0055 or less.
90. The method of any one of embodiments 85 to 89, wherein the pipette tip
retains less than
0.065% of the fluid drawn into the pipette tip, after the liquid is dispensed.
91. The method of any one of embodiments 85 to 89, wherein the pipette tip
retains no more than
0.00012% of the fluid drawn into the pipette tip, after the liquid is
dispensed.
92. The method of any one of embodiments 85, 74 to 91, wherein less than 3.72%
of the pipette
tips utilized in a pipette cycle retain a portion of the fluid drawn into the
pipette tips after the liquid is
dispensed.
93. The method of any one of embodiments 85 to 91, wherein between 0.05% to
1.0% of the
pipette tips utilized in a pipette cycle retain a portion of the fluid drawn
into the pipette tips after the
liquid is dispensed.
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
94. The method of any one of embodiments 85 to 91, wherein between 0.15% to
0.3% of the
pipette tips utilized in a pipette cycle retain a portion of the fluid drawn
into the pipette tips after the
liquid is dispensed.
95. The method of any one of embodiments 85 to 91, wherein between 0.2% to
0.26% of the
pipette tips utilized in a pipette cycle retain a portion of the fluid drawn
into the pipette tips after the
liquid is dispensed.
96. The method of any one of embodiments 85 to 95, wherein less than 3.72% of
the pipette tips
utilized in a pipette cycle retain less than 0.065% of the fluid drawn into
the pipette tips after the
liquid is dispensed.
97. The pipette tip of embodiment 96, wherein less than 3.72% of the pipette
tips utilized in a
pipette cycle retain no more than 0.00012% of the fluid drawn into the pipette
tip, after the liquid is
dispensed.
98. The pipette tip of any one of embodiments 85 to 95, wherein between 0.2%
to 0.26% of the
pipette tips utilized in a pipette cycle retain less than 0.065% of the fluid
drawn into the pipette tips
after the liquid is dispensed.
99. The pipette tip of embodiment 98, wherein between 0.2% to 0.26% of the
pipette tips utilized in
a pipette cycle retain no more than 0.00012% of the fluid drawn into the
pipette tips after the liquid
is dispensed.
100. The method of any one of embodiments 85 to 99, wherein the pipette tip
contributes to a
reduction of between about 20% and about 90% in the average time to complete a
cycle of steps in
a method for manipulating a solution.
101. A method for dispensing fluid from a pipette tip, comprising,
(a) drawing a volume of fluid into a pipette tip having a wall thickness at
the distal region
terminus of about 0.0040 inches to about 0.0055 inches, and
(b) dispensing the fluid from the pipette tip, wherein the fluid is
substantially completely
dispensed.
76
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
102. The method of claim 101, wherein the method comprises (i) applying a
pipette tip to a
pipettor prior to step (a), (ii) visually inspecting the pipette tip after
step (b), (iii) ejecting the pipette
tip from the pipettor after step (b), and (iv) combinations thereof.
103. The method of claim 101, wherein the pipette tip retains less than 0.065%
of a fluid drawn
into the pipette tip after the liquid is dispensed.
104. The method of claim 103, wherein the pipette tip retains no more than
0.00012% of a fluid
drawn into the pipette tip after the liquid is dispensed.
105. The method of claim 101, wherein the method is performed for a plurality
of pipette tips and
less than 3.72% of the pipette tips retain a portion of the fluid drawn into
the pipette tips after the
liquid is dispensed.
106. The method of claim 105, wherein between 0.2% to 0.26% of the pipette
tips retain a portion
of the fluid drawn into the pipette tips after the liquid is dispensed.
107. The method of claim 101, wherein the pipette tip contributes to a
reduction of between 20%
and 90% in the average time to complete a cycle of steps in a fluid dispensing
procedure.
Selected Features of FIG 1A-1D, FIG 2, FIG 3 and FIG 4A-4D
15 proximal region
20 distal region
junction between distal region and proximal region
about one-quarter of the distance from the distal region terminus to the
junction 30
distal region terminus
53 wall thickness at distal region terminus
30 55 step
57 region where wall taper ends
flange
flange rim
67 flange lead-in surface
77
. .81625301
70 proximal region flexible thickness
72 proximal region flexible thickness terminus
75 junction of flange and proximal region flexible thickness
80 rib (first rib thickness)
82 rib terminus
83 rib terminus
85 rib (second rib thickness)
90 rib terminus
100 inner surface of proximal region
110 flange taper inner surface
120 annular groove
130 inner surface of distal region
Citation of the above patents, patent applications, publications and
documents is not an admission that any of the foregoing is pertinent prior
art, nor does it constitute
any admission as to the contents or date of these publications or documents.
Modifications may be made to the foregoing without departing from the basic
aspects of the
invention. Although the invention has been described in substantial detail
with reference to one or
more specific embodiments, those of ordinary skill in the art will recognize
that changes may be
made to the embodiments specifically disclosed in this application, yet these
modifications and
improvements are within the scope and spirit of the invention.
The invention illustratively described herein suitably may be practiced in the
absence of any
element(s) not specifically disclosed herein. Thus, for example, in each
instance herein any of the
terms "comprising," "consisting essentially of," and "consisting of" may be
replaced with either of
the other two terms. The terms and expressions which have been employed are
used as terms of
description and not of limitation, and use of such terms and expressions do
not exclude any
equivalents of the features shown and described or portions thereof, and
various modifications are
possible within the scope of the invention claimed. The term "a" or "an" can
refer to one of or a
plurality of the elements it modifies (e.g., "a pipette tip" can mean one or
more pipette tips) unless it
78
CA 2787274 2017-07-26
CA 02787274 2012-07-16
WO 2011/091308 PCT/US2011/022129
is contextually clear either one of the elements or more than one of the
elements is described. The
term "about" as used herein refers to a value within 10% of the underlying
parameter (i.e., plus or
minus 10%), and use of the term "about" at the beginning of a string of values
modifies each of the
values (i.e., "about 1, 2 and 3" refers to about 1, about 2 and about 3). For
example, a weight of
"about 100 grams" can include weights between 90 grams and 110 grams. Further,
when a listing
of values is described herein (e.g., about 50%, 60%, 70%, 80%, 85% or 86%) the
listing includes
all intermediate and fractional values thereof (e.g., 54%, 85.4%). Thus, it
should be understood
that although the present invention has been specifically disclosed by
representative embodiments
and optional features, modification and variation of the concepts herein
disclosed may be resorted
to by those skilled in the art, and such modifications and variations are
considered within the scope
of this invention.
Certain embodiments of the invention are set forth in the claims that follow.
79