Note: Descriptions are shown in the official language in which they were submitted.
CA 02787374 2012-07-17
WO 2011/091431 PCT/US2011/022444
HOME-USE APPLICATORS FOR NON-INVASIVELY REMOVING
HEAT FROM SUBCUTANEOUS LIPID-RICH CELLS VIA PHASE
CHANGE COOLANTS, AND ASSOCIATED DEVICES, SYSTEMS
AND METHODS
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] The present application claims priority to the following co-pending
U.S.
Provisional Patent Applications, each of which is incorporated herein by
reference:
61/298,175, filed January 25, 2010 and 61/354,615, filed June 14, 2010. To the
extent
that the materials in the foregoing references and/or any other references
incorporated
herein by reference conflict with the present disclosure, the present
disclosure controls.
TECHNICAL FIELD
[0002] The present application relates generally to home-use applicators for
non-
invasively removing heat from subcutaneous lipid-rich cells via phase change
coolants,
and associated devices, systems and methods. In particular, several
embodiments are
directed to devices that a user may easily recharge or regenerate using a
conventional
commercial, clinical, institutional or domestic freezer.
BACKGROUND
[0003] Excess body fat, or adipose tissue, may be present in various locations
of
the body, including, for example, the thighs, buttocks, abdomen, knees, back,
face,
arms, chin, and other areas. Moreover, excess adipose tissue is thought to
magnify
the unattractive appearance of cellulite, which forms when subcutaneous fat
protrudes
into the dermis and creates dimples where the skin is attached to underlying
structural
fibrous strands. Cellulite and excessive amounts of adipose tissue are often
considered to be unappealing. Moreover, significant health risks may be
associated
with higher amounts of excess body fat.
[0004] A variety of methods have been used to treat individuals having excess
body fat and, in many instances, non-invasive removal of excess subcutaneous
CA 02787374 2012-07-17
WO 2011/091431 PCT/US2011/022444
adipose tissue can eliminate unnecessary recovery time and discomfort
associated
with invasive procedures such as liposuction. Conventional non-invasive
treatments for
removing excess body fat typically include topical agents, weight-loss drugs,
regular
exercise, dieting or a combination of these treatments. One drawback of these
treatments is that they may not be effective or even possible under certain
circumstances. For example, when a person is physically injured or ill,
regular exercise
may not be an option. Similarly, weight-loss drugs or topical agents are not
an option
when they cause an allergic or other negative reaction. Furthermore, fat loss
in
selective areas of a person's body often cannot be achieved using general or
systemic
weight-loss methods.
[0005] Other methods designed to reduce subcutaneous adipose tissue include
laser-assisted liposuction and mesotherapy. Newer non-invasive methods include
applying radiant energy to subcutaneous lipid-rich cells via, e.g., radio
frequency and/or
light energy, such as is described in U.S. Patent Publication No. 2006/0036300
and
U.S. Patent No. 5,143,063, or via, e.g., high intensity focused ultrasound
(HIFU)
radiation such as is described in U.S. Patent Nos. 7,258,674 and 7,347,855. In
contrast, methods and devices for non-invasively reducing subcutaneous adipose
tissue by cooling are disclosed in U.S. Patent No. 7,367,341 entitled "METHODS
AND
DEVICES FOR SELECTIVE DISRUPTION OF FATTY TISSUE BY CONTROLLED
COOLING" to Anderson et al. and U.S. Patent Publication No. 2005/0251120
entitled
"METHODS AND DEVICES FOR DETECTION AND CONTROL OF SELECTIVE
DISRUPTION OF FATTY TISSUE BY CONTROLLED COOLING" to Anderson et al.,
the entire disclosures of which are incorporated herein by reference.
BRIEF DESCRIPTION OF THE DRAWINGS
[0006] Many features of the present technology are illustrated in simplified,
schematic and/or partially schematic formats in the following Figures to avoid
obscuring
significant technology features. Many features are not drawn to scale so as to
more
clearly illustrate these features.
[0007] Figure 1 is a partially schematic, partially cut-away illustration of a
cooling
device having a coolant vessel and heat exchanger configured in accordance
with an
embodiment of the disclosure.
-2-
CA 02787374 2012-07-17
WO 2011/091431 PCT/US2011/022444
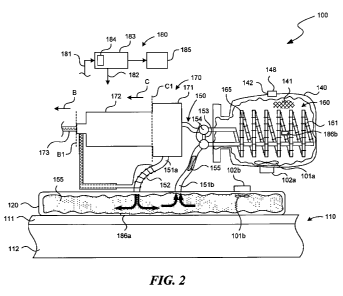
[0008] Figure 2 is a partially schematic, partially cut-away illustration of a
particular
embodiment of the device shown in Figure 1.
[0009] Figure 3 is a partially schematic illustration of a device having an
overall
arrangement generally similar to that shown in Figure 1, configured in
accordance with
still another embodiment of the disclosure.
[0010] Figure 4 is a partially schematic illustration of a device having a
heat
exchanger and a removable coolant vessel configured in accordance with another
embodiment of the disclosure.
[0011] Figure 5A is a partially schematic, enlarged illustration of an
embodiment of
the coolant vessel and heat exchanger shown in Figure 4.
[0012] Figure 5B is a partially schematic, cross-sectional illustration of the
heat
exchanger and coolant vessel taken substantially along line 5B-5B of Figure
5A.
[0013] Figure 6A is a partially schematic, partially cut-away illustration of
a coolant
vessel and heat exchanger configured in accordance with another embodiment of
the
disclosure.
[0014] Figure 6B is a partially schematic, cross-sectional illustration of an
embodiment of the heat exchanger and coolant vessel, taken substantially along
line
6B-6B of Figure 6A.
[0015] Figure 7 is a partially schematic illustration of a device having a
coolant
vessel and heat exchanger that are separable from an applicator in accordance
with
yet another embodiment of the disclosure.
[0016] Figure 8 is a partially schematic illustration of a portion of the
coolant
vessel and heat exchanger, taken substantially along line 8-8 of Figure 7.
[0017] Figure 9 is a partially schematic, cross-sectional illustration of an
applicator
having non-elastic and elastic materials arranged in accordance with an
embodiment of
the disclosure.
[0018] Figure 10 is a partially schematic, cross-sectional illustration of an
applicator having an internal support structure in accordance with an
embodiment of
the disclosure.
-3-
CA 02787374 2012-07-17
WO 2011/091431 PCT/US2011/022444
[0019] Figure 11 is an enlarged illustration of a portion of the applicator
shown in
Figure 10.
DETAILED DESCRIPTION
1. Overview
[0020] Several examples of devices, systems and methods for cooling
subcutaneous adipose tissue in accordance with the presently disclosed
technology
are described below. Although the following description provides many specific
details
of the following examples in a manner sufficient to enable a person skilled in
the
relevant art to practice, make and use them, several of the details and
advantages
described below may not be necessary to practice certain examples and methods
of
the technology. Additionally, the technology may include other examples and
methods
that are within the scope of the claims but are not described here in detail.
[0021] References throughout this specification to "one example," "an
example,"
"one embodiment" or "an embodiment" mean that a particular feature, structure,
or
characteristic described in connection with the example is included in at
least one
example of the present technology. Thus, the occurrences of the phrases "in
one
example," "in an example," "one embodiment" or "an embodiment" in various
places
throughout this specification are not necessarily all referring to the same
example.
Furthermore, the particular features, structures, routines, steps or
characteristics may
be combined in any suitable manner in one or more examples of the technology.
The
headings provided herein are for convenience only and are not intended to
limit or
interpret the scope or meaning of the claimed technology.
[0022] Certain embodiments of the technology described below may take the form
of computer-executable instructions, including routines executed by a
programmable
computer or controller. Those skilled in the relevant art will appreciate that
the
technology can be practiced on computer or controller systems other than those
shown
and described below. The technology can be embodied in a special-purpose
computer, controller, or data processor that is specifically programmed,
configured or
constructed to perform one or more of the computer-executable instructions
described
below. Accordingly, the terms "computer" and "controller" as generally used
herein
refer to any data processor and can include internet appliances, hand-held
devices,
-4-
CA 02787374 2012-07-17
WO 2011/091431 PCT/US2011/022444
multi-processor systems, programmable consumer electronics, network computers,
mini computers, and the like. The technology can also be practiced in
distributed
environments where tasks or modules are performed by remote processing devices
that are linked through a communications network. Aspects of the technology
described below may be stored or distributed on computer-readable media,
including
magnetic or optically readable or removable computer discs as well was media
distributed electronically over networks. In particular embodiments, data
structures and
transmissions of data particular to aspects of the technology are also
encompassed
within the scope of the present technology. The present technology encompasses
both
methods of programming computer-readable media to perform particular steps, as
well
as executing the steps.
[0023] One embodiment of a cooling device for cooling subcutaneous lipid-rich
cells in a human includes an applicator that is releasably positionable in
thermal
communication with human skin. The device further includes a coolant vessel
having a
coolant and a heat transfer conduit having a heat transfer fluid that is
isolated from fluid
contact with the coolant. A heat exchanger is operatively coupled between the
coolant
vessel and heat transfer conduit to transfer heat between the heat transfer
fluid and the
coolant, and a fluid driver is operatively coupled to the heat transfer
conduit to direct
the heat transfer fluid between the applicator and the heat exchanger.
[0024] In a further particular embodiment, the coolant has a liquid/solid
phase
transition temperature greater than the liquid/solid phase transition
temperature of the
heat transfer fluid. The heat exchanger is positioned within the coolant
vessel and
includes a heat exchanger conduit that, together with the heat transfer
conduit and the
applicator, form a sealed, closed-loop path for the heat transfer fluid.
Accordingly, the
entire device can be placed in a freezer (e.g., a domestic freezer) to freeze
the coolant
in preparation for treating lipid-rich cells in a human. In other embodiments,
only
selected components of the device are removable to freeze or otherwise cool
the
coolant.
(0025] A method for cooling human tissue in accordance with a particular
embodiment of the disclosure includes releaseably attaching an applicator to a
human,
and removing heat from subcutaneous lipid-rich tissue of the human via the
applicator
to selectively reduce lipid-rich cells of the tissue (e.g., via the body's
reaction to
-5-
CA 02787374 2012-07-17
WO 2011/091431 PCT/US2011/022444
cooling). The heat is removed by directing a chilled heat transfer fluid to
applicator and
transferring absorbed heat from the heat transfer fluid to a coolant. In
particular
embodiments, the coolant can remain solid, remain liquid or change phase from
a solid
to a liquid as it receives heat from the heat transfer fluid. The method still
further
includes re-cooling the coolant. Selected methods in accordance with another
embodiment of the disclosure include removing the heat by directing a chilled
heat
transfer fluid into a flexible envelope and through a porous internal support
structure
within the envelope, while the porous internal structure at least restricts
fluid pressure
in the envelope from (a) bulging the envelope outwardly, or (b) collapsing the
internal
structure, or (c) both (a) and (b). Still another method includes directing
the chilled
heat transfer fluid into an applicator, between two flexible portions of the
applicator,
each having a different elasticity.
[0026] Without being bound by theory, the selective effect of cooling on lipid-
rich
cells is believed to result in, for example, membrane disruption, cell
shrinkage,
disabling, damaging, destroying, removing, killing or other methods of lipid-
rich cell
alteration. Such alteration is believed to stem from one or more mechanisms
acting
alone or in combination. It is thought that such mechanism(s) trigger an
apoptotic
cascade, which is believed to be the dominant form of lipid-rich cell death by
non-
invasive cooling. In any of these embodiments, the effect of tissue cooling is
to
selectively reduce lipid-rich cells.
[0027] Apoptosis, also referred to as "programmed cell death", is a
genetically-
induced death mechanism by which cells self-destruct without incurring damage
to
surrounding tissues. An ordered series of biochemical events induce cells to
morphologically change. These changes include cellular blebbing, loss of cell
membrane asymmetry and attachment, cell shrinkage, chromatin condensation and
chromosomal DNA fragmentation. Injury via an external stimulus, such as cold
exposure, is one mechanism that can induce cellular apoptosis in cells. Nagle,
W.A.,
Soloff, B.L., Moss, A.J. Jr., Henle, K.J. "Cultured Chinese Hamster Cells
Undergo
Apoptosis After Exposure to Cold but Nonfreezing Temperatures" Cryobiology 27,
439-451 (1990).
[0028] One aspect of apoptosis, in contrast to cellular necrosis (a traumatic
form
of cell death causing local inflammation), is that apoptotic cells express and
display
-6-
CA 02787374 2012-07-17
WO 2011/091431 PCT/US2011/022444
phagocytic markers on the surface of the cell membrane, thus marking the cells
for
phagocytosis by macrophages. As a result, phagocytes can engulf and remove the
dying cells (e.g., the lipid-rich cells) without eliciting an immune response.
Temperatures that elicit these apoptotic events in lipid-rich cells may
contribute to long-
lasting and/or permanent reduction and reshaping of subcutaneous adipose
tissue.
[0029] One mechanism of apoptotic lipid-rich cell death by cooling is believed
to
involve localized crystallization of lipids within the adipocytes at
temperatures that do
not induce crystallization in non-lipid-rich cells. The crystallized lipids
selectively may
injure these cells, inducing apoptosis (and may also induce necrotic death if
the
crystallized lipids damage or rupture the bi-lipid membrane of the adipocyte).
Another
mechanism of injury involves the lipid phase transition of those lipids within
the cell's bi-
lipid membrane, which results in membrane disruption or disfunction, thereby
inducing
apoptosis. This mechanism is well-documented for many cell types and may be
active
when adipocytes, or lipid-rich cells, are cooled. Mazur, P., "Cryobiology: the
Freezing
of Biological Systems" Science, 68: 939-949 (1970); Quinn, P.J., "A Lipid
Phase
Separation Model of Low Temperature Damage to Biological Membranes"
Cryobiology,
22: 128-147 (1985); Rubinsky, B., "Principles of Low Temperature Preservation"
Heart
Failure Reviews, 8, 277-284 (2003). Another mechanism of injury may involve a
disfunction of ion transfer pumps across the cellular membrane to maintain
desired
concentrations of ions such as potassium (K+) or sodium (Na+). An ion
imbalance
across the cell membrane may result from lipid phase transition of lipids
within the
cell's bi-lipid membrane or by another mechanism, thereby inducing apoptosis.
Other
yet-to-be-understood apoptotic mechanisms may exist, based on the relative
sensitivity
to cooling of lipid-rich cells compared to non-lipid rich cells.
[0030] In addition to the apoptotic mechanisms involved in lipid-rich cell
death,
local cold exposure is also believed to induce lipolysis (i.e., fat
metabolism) of lipid-rich
cells and has been shown to enhance existing lipolysis which serves to further
increase
the reduction in subcutaneous lipid-rich cells. Vallerand, A.L., Zamecnik. J.,
Jones,
P.J.H., Jacobs, I. "Cold Stress Increases Lipolysis, FFA Ra and TG/FFA Cycling
in
Humans" Aviation, Space and Environmental Medicine 70, 42-50 (1999).
[0031] One expected advantage of the foregoing techniques is that the
subcutaneous lipid-rich cells can be reduced generally without collateral
damage to
-7-
CA 02787374 2012-07-17
WO 2011/091431 PCT/US2011/022444
non-lipid-rich cells in the same region. In general, lipid-rich cells can be
affected at low
temperatures that do not affect non-lipid-rich cells. As a result, lipid-rich
cells, such as
those associated with cellulite, can be affected while other cells in the same
region are
generally not damaged even though the non-lipid-rich cells at the surface may
be
subjected to even lower temperatures than those to which the lipid-rich cells
are
exposed.
2. Representative Devices and Methods that Include Applicators, Coolant
Vessels,
and Heat Exchangers Arranged as a Single Unit
[0032] Figure 1 is a partially schematic, partially cut-away illustration of a
device
100 having an applicator 120 operatively coupled to a coolant vessel 140 to
cool
human tissue 110. In particular, the device 100 is configured to cool a
subcutaneous,
lipid-rich tissue 112, without damaging the overlying dermis 111, generally in
the
manner described above. The applicator 120 is coupled to the coolant vessel
140 by a
heat transfer conduit 150 that carries a heat transfer fluid 155. Accordingly,
the heat
transfer conduit 150 includes a supply portion 151a that directs the heat
transfer fluid
155 to the applicator 120, and a return portion 151b that receives heat
transfer fluid
155 exiting the applicator 120. The heat transfer fluid 155 is propelled
through the heat
transfer conduit 150 by a fluid driver 170, e.g., a pump or other suitable
device. The
heat transfer conduit 150 is typically insulated to prevent the ambient
environment from
heating the heat transfer fluid 155. Other elements of the device (aside from
the
cooling surface of the applicator 120 in contact with the tissue 110) are also
insulated
from the ambient environment to prevent heat loss and frost formation.
[0033] The heat transfer conduit 150 is connected to a heat exchanger 160
having
a heat exchanger conduit (e.g., tubing) 161 that is positioned within or at
least partially
within the coolant vessel 140. The coolant vessel 140 contains a coolant 141
that is in
close thermal contact with the heat exchanger 160, but is isolated from direct
fluid
contact with the heat transfer fluid 155 contained within the heat exchanger
tubing 161.
Accordingly, the heat exchanger 160 facilitates heat transfer between the heat
transfer
fluid 155 and the coolant 141, while preventing these fluids from mixing. As a
result,
the coolant 141 can be selected to have a composition different than that of
the heat
transfer fluid 155. In particular embodiments, the coolant 141 can be selected
to have
a phase transition temperature (from liquid/gel to solid) that is less than
normal body
-8-
CA 02787374 2012-07-17
WO 2011/091431 PCT/US2011/022444
temperature (about 37 C) and in particular embodiments, in the range of from
about
37 C to about -20 C, or about 25 C to about -20 C, or about 0 C to about -12
C, or
about -3 C to about -6 C, to present a constant temperature environment to the
heat
transfer fluid 155 as the coolant 141 transitions from a solid to a
liquid/gel. The heat
transfer fluid 155 in such embodiments has a phase transition temperature that
is less
than that of the coolant 141. Accordingly, the heat transfer fluid 155 remains
in a fluid
state even when the coolant 141 or a portion of the coolant 141 is in a solid
state. As a
result, the heat transfer fluid 155 can flow within the heat transfer conduit
150 to
convey heat away from the human tissue 110 even when the coolant 141 is frozen
or
at least partially frozen.
[0034] In operation, the device 100 can be prepared for use by placing the
major
components (e.g., the applicator 120, the heat transfer conduit 150, the heat
exchanger 160 and the coolant vessel 140), as a unit, in a suitably cold
environment.
In a particular embodiment, the cold environment includes a freezer (e.g., a
domestic
freezer), in which the temperature typically ranges from about -10 C to about -
20 C,
sufficient to freeze the coolant 141. After the coolant 141 is frozen, the
device 100 can
be removed from the freezer or other cold environment, as a unit, and the
applicator
120 can be attached to the human tissue 110 using a cuff or other suitable
attachment
device (e.g., having a Velcro closure, a buckle, or other releasable
feature).
Optionally, the user can apply a lotion between the applicator 120 and the
skin to
facilitate heat transfer and/or provide a moisturizing or other cosmetic
effect. Whether
or not the user applies a lotion or another intermediate constituent, the
applicator 120 is
positioned in thermal communication with the user's skin, so as to effectively
remove
heat from the lipid-rich tissue 112. The fluid driver 170 is then activated to
drive the
heat transfer fluid 155 through the heat transfer conduit 150, thus
transferring heat
from the subcutaneous lipid-rich tissue 112 to the frozen coolant 141 via the
heat
exchanger 160. As the coolant 141 melts, the temperature within the coolant
vessel
140 remains approximately constant so as to provide a constant or nearly
constant
heat transfer fluid temperature to the human tissue 110. After the human
tissue 110
has been cooled for an appropriate period of time, causing some or all of the
coolant
141 to melt, the device 100 can be removed as a unit from the human tissue
110, as
indicated by arrow A, and the coolant 141 can be re-frozen by placing the
device 100 in
the freezer. Accordingly, the cooling capacity of the coolant vessel 140 can
be readily
-9-
CA 02787374 2012-07-17
WO 2011/091431 PCT/US2011/022444
recharged or regenerated prior to a subsequent treatment process. The
appropriate
tissue-cooling period of time can be controlled by properly selecting the
cooling
capacity of the coolant 141, or via a controller and/or sensor, as described
in further
detail later with reference to Figure 2.
[0035] In particular embodiments described above with reference to Figure 1
and
below with reference to Figures 2-8, the coolant 141 changes phase as it is
heated by
the heat transfer fluid 155, and then changes back again when it is cooled. In
other
embodiments, the coolant 141 can be heated and cooled without undergoing phase
changes. For example, the coolant 141 can remain in a solid phase throughout
both
the heating and cooling processes, or can remain in a liquid phase throughout
both
processes. In such cases, the cooling process (whether it takes place in a
freezer or
other environment) does not freeze the coolant. When the coolant 141 remains a
solid,
its phase transition temperature is above that of the heat transfer fluid.
When the
coolant 141 remains a liquid, its phase transition temperature can be above,
below, or
equal to that of the heat transfer fluid 155. In such cases, the heat transfer
fluid 155
and the coolant 141 can have different or identical compositions, while
remaining
isolated from direct fluid contact with each other.
[0036] Figure 2 is a partially schematic, partially cut-away illustration of
an
embodiment of the device 100 that operates in accordance with the general
principles
described above with reference to Figure 1. Accordingly, the device 100 shown
in
Figure 2 includes an applicator 120 and a coolant vessel 140 thermally
connected to
the applicator 120 via a heat exchanger 160 and a heat transfer conduit 150.
[0037] One characteristic of the device 100 shown in both Figure 1 and Figure
2 is
that the when the applicator 120 is first placed against the human tissue 110,
the heat
transfer fluid 155 in the heat transfer conduit 150 and the applicator 120
will be at or
approximately at the temperature of the cold environment in which the device
100 was
placed. In at least some cases, this temperature may be uncomfortably low.
Accordingly, the device 100 and associated methods can include features for
reducing
the likelihood that the user will encounter a potentially detrimental effect
or
uncomfortably cold sensation when first using the device 100. In a particular
embodiment, the device 100 can include a heater 152 positioned to heat the
heat
transfer fluid 155 entering the applicator 120 via the supply portion 151a.
This
-10-
CA 02787374 2012-07-17
WO 2011/091431 PCT/US2011/022444
arrangement can increase the temperature of the heat transfer fluid 155 by at
least an
amount sufficient to reduce the user's discomfort and/or provide a safe and
efficacious
treatment. In a further particular aspect of this embodiment, the device 100
can be
configured to shunt the heat transfer fluid 155 away from the heat exchanger
160 while
the heat transfer fluid temperature is initially elevated. This arrangement
can avoid
unnecessarily melting the coolant 141 before treatment begins. Accordingly,
the device
100 can include a shunt channel 153 connected between the supply portion 151a
and
the return portion 151b in parallel with the heat exchanger 160 to bypass the
heat
exchanger 160. One or more shunt valves 154 (two are shown in Figure 2) are
positioned to regulate flow through the shunt channel 153, e.g., to open or
partially
open the shunt channel 153 during initial startup, and then close or partially
close the
shunt channel 153 after the temperature of the applicator 120 has been
elevated by a
sufficient amount.
[0038] The device 100 can include a controller 180 to control the heater 152,
the
shunt valves 154, and/or other features of the device 100. For example, in a
particular
embodiment, the controller 180 includes a microprocessor 183 having a timer
component 184. When the device 100 is initially powered (e.g., by activating
the fluid
driver 170), the microprocessor 183 can automatically open the shunt channel
153 via
the shunt valves 154, and activate the heater 152. The heater 152 and the
shunt
channel 153 can remain in this configuration for a predetermined time, after
which the
microprocessor 153 automatically issues control signals deactivating the
heater 152
and closing the shunt channel 153. Accordingly, the timer component 184
operates as
a sensor by sensing the passage of time during which the heater 152 is
actively heating
the heat transfer fluid 155. In other embodiments described further below, one
or more
sensors can detect other characteristics associated with the device 100.
[0039] In a particular embodiment, the microprocessor 183 can direct the
control
signals 182 based on inputs 181 received from one or more temperature sensors
186.
For example, the device 100 can include a first temperature sensor 186a
positioned at
the applicator 120. The microprocessor 183 can automatically activate the
heater 182
and the shunt channel 183 until the first temperature sensor 186a indicates a
temperature suitable for placing the applicator 120 against the human tissue
110. The
device 100 can include a second temperature sensor 186b located at the coolant
-11-
CA 02787374 2012-07-17
WO 2011/091431 PCT/US2011/022444
vessel 140 (e.g., the center of the coolant 141). The microprocessor 183 can
accordingly direct control signals 182 that activate the fluid driver 170 for
as long as the
second temperature sensor 186b indicates a constant and/or suitably low
temperature.
When the second temperature sensor 186b identifies a temperature rise
(indicating
that the coolant 141 has completely melted), the microprocessor 183 can
automatically
deactivate the fluid driver 170. If the coolant 141 is not selected to change
phase
during heating and cooling, the micro-processor 183 can deactivate the fluid
driver 170
when the temperature of the coolant 141 exceeds a threshold temperature. The
controller 180 can include an output device 185 that indicates the operational
modes or
states of the device 100. For example, the output device 185 can display
visual signals
(e.g., via different colored LEDs) and/or aural signals (e.g., via an audio
speaker) to
signify when the applicator 120 is ready to be applied to the human tissue
110, when
the treatment program is over, and/or when temperatures or other
characteristics of
any of the device components are outside pre-selected bounds.
[0040] In yet another embodiment, the controller 180 can direct a simplified
process for handling the initial temperature of the heat transfer fluid 155.
In particular,
the controller 180 can monitor the temperature signal provided by the first
temperature
sensor 186a, without activating the fluid driver 170, and without the need for
the heater
152 or the shunt channel 153. Instead, the controller 180 can generate an
output
(presented by the output device 185) when the ambient conditions cause the
heat
transfer fluid 155 to rise to an acceptable temperature, as detected by the
first
temperature sensor 186a. The user can optionally accelerate this process by
applying
heat to the applicator 120 and/or the heat transfer conduit 150 via an
external heat
source. An advantage of this approach is that it can be simpler than the
integrated
heater 152 described above. Conversely, the heater 152 (under the direction of
the
controller 180) can be more reliable and quicker, at least in part because the
heater
152 is positioned within the insulation provided around the heat transfer
conduit 150
and other device components.
[0041] The device 100 can include a variety of features configured to enhance
uniform heat distribution and heat transfer. For example, the heat exchanger
160 can
include fins 165 on the heat exchanger tubing 161 to increase the surface area
available to transfer heat between the heat transfer fluid 155 and the coolant
141. The
-12-
CA 02787374 2012-07-17
WO 2011/091431 PCT/US2011/022444
coolant vessel 140 can also include a first agitator 101a that distributes the
melting
coolant 141 within the coolant vessel 140 to provide for a more uniform
temperature
and heat transfer rate within the vessel 140. In one embodiment, the first
agitator 101 a
can include a magnetically driven device, and can be magnetically coupled to a
first
actuator motor 102a positioned outside the coolant vessel 140. Accordingly,
the
agitator 101 a can operate without the need for a sealed drive shaft
penetrating into the
coolant vessel 140. A similar arrangement can be used at the applicator 120.
In
particular, the applicator 120 can include a second agitator 101b driven by a
second
actuator motor 102b to distribute the heat transfer fluid 155 uniformly within
the
applicator 120. Suitably positioned internal fluid channels can be used in
addition to or
in lieu of the second agitator 101 b to uniformly distribute the heat transfer
fluid 155 in
the applicator 120. A representative device that includes such features is a
Model No.
10240 pad, available from Breg Polar Care (bregpolarcare.com). The actuator
motors
102a, 102b can be operatively coupled to a power cord 173, which also provides
power
to the fluid driver 170 and the heater 152. In other embodiments, the device
100 can
include other elements that agitate and/or distribute the fluid in the
applicator 120
and/or the coolant vessel 140. Such elements can include liquid jets, shaft-
driven
stirrers, pistons and/or other devices that move the solid and/or liquid
portion of the
coolant 141 within the coolant vessel 140, and/or actuators that vibrate,
shake, tip or
otherwise move the coolant vessel 140 itself or heat exchanger 160 within the
coolant
vessel.
[0042] As noted above, the applicator 120, the heat transfer conduit 150, the
heat
exchanger 160, and the coolant vessel 140 can be moved as a unit between the
target
tissue 110 and a freezer or other cold environment prior to and after
treatment. In a
particular embodiment, the remaining components or elements of the device 100
shown in Figure 2 can also be placed in the freezer. For example, when the
fluid driver
170 includes a pump 171 driven by a pump motor 172, these components (along
with
the controller 180) can also be placed in the freezer. In other embodiments,
one or
more of these components may be removed prior to placing the rest of the
device 100
in the freezer. For example, the power cord 173 can be removed from the motor
172
and other system components at a junction 131 as indicated by arrow B. In
another
embodiment, the pump motor 172 can be removed from the device 100 at a
junction
C1 as indicated by arrow C. For example, the pump motor 172 can be
magnetically
-13-
CA 02787374 2012-07-17
WO 2011/091431 PCT/US2011/022444
coupled to the pump 171, generally in the manner of the stirrers described
above to
make connecting and disconnecting the motor 172 easier. In still another
aspect of this
embodiment, the controller 180 and/or components of the controller 180 can be
carried
by the motor 172 and can accordingly be removed from the device 100 along with
the
motor 172.
[0043] Certain features described above in the context of a processor-based
automatic control system can, in other embodiments, operate without a
processor, or
can operate manually. For example, the shunt valves 154 can include
thermostatic
radiator values, or similar valves that have an integrated temperature sensor
(e.g., a
mechanical thermostat) that autonomously drives the valve without the need for
a
processor. In other embodiments, the coolant 141 can change color as it
undergoes its
phase change, which can eliminate the need for the second temperature sensor
186b.
In one aspect of this embodiment, the coolant vessel 140 is transparent,
allowing the
user to readily see both when the coolant 141 is frozen and when the coolant
141 has
melted. In the event the device 100 loses coolant 141 over the course of time,
the
coolant vessel 140 can include a fill/drain port 142. In a particular aspect
of this
embodiment, the fill/drain port 142 can have a removable plug 148 that is
transparent,
in addition to or in lieu of the coolant vessel 140 being transparent.
Similarly, the heat
transfer fluid 155 can include constituents that change color when the heat
transfer
fluid attains a temperature that is no longer suitable for properly chilling
the tissue 110.
The applicator 120 and/or the heat transfer conduit 150 (or portions thereof)
can be
made transparent to allow the user to easily determine when this temperature
threshold
has been exceeded.
[0044] Both the coolant 141 and the heat transfer fluid 155 are selected to be
highly thermally conductive. Suitable constituents for the coolant 141 include
water in
combination with propylene glycol, ethylene glycol, glycerin, ethanol,
isopropyl alcohol,
hydroxyethyl cellulose, salt, and/or other constituents. In at least some
embodiments,
the same constituents can be used for the heat transfer fluid 155, but the
ratios of the
constituents (and therefore the overall composition of the heat transfer
fluid) are
selected to produce a lower liquid/solid phase transition temperature. Both
the heat
transfer fluid 155 and the coolant 141 can be selected to have high heat
conductivity
and low toxicity in case of a leak. Both can include an anti-microbial agent
to restrict or
-14-
CA 02787374 2012-07-17
WO 2011/091431 PCT/US2011/022444
prevent algae formulation and/or propagation of other undesirable life forms.
The
coolant 141 can be selected to have a high heat capacity to better absorb heat
from
the heat transfer fluid 155. The heat transfer fluid 155 can have a relatively
low heat
capacity so that it readily heats up when the heater 152 is activated. The
heat transfer
fluid 155 can also be selected to have a low viscosity at operating
temperatures to
facilitate flow through the heat transfer conduit 150, the heat exchanger 160
and the
applicator 120. In any of these embodiments the coolant vessel 140 in which
the
coolant 141 is disposed can be flexible and elastic, and/or can include a vent
or other
feature to accommodate volume changes as the coolant 141 changes phase.
[0045] Figure 3 is a partially schematic, isometric illustration of an
embodiment of
the device 100 described above with reference to Figure 2. As shown in Figure
3, the
applicator 120 has a generally flexible configuration, allowing it to conform
to the shape
of the tissue to which it is applied. An attachment device 123 releaseably
attaches the
applicator 120 to the tissue and can accordingly include a strap (e.g.
Velcro), a cuff
(e.g., generally similar to a blood pressure cuff) or another suitable device.
The coolant
vessel 140 is housed in a coolant vessel housing 143 that is in turn attached
to or
otherwise includes the support structure 121. The support structure 121 can be
at
least partially flexible so that when it is attached to the applicator 120, it
does not overly
inhibit the ability of the applicator 120 to conform to the human tissue. In
one
embodiment, the support structure 121 and the coolant vessel housing 143 can
be
supported relative to the applicator 120 with standoffs. In another
embodiment, an
optional foam or other flexible layer (e.g. an inflatable air bladder) 122 can
be
positioned between the support structure 121 and the applicator 120 to further
facilitate
the ability of the applicator 120 to flex relative to the coolant vessel
housing 143.
[0046] In one aspect of an embodiment shown in Figure 3, the power cord 173
can be releaseably attached directly to the pump motor 172, thus allowing the
power
cord 173 to be removed before the device 100 is placed in the freezer. The
power cord
173 can be connected directly to an AC outlet, and can include a DC converter
if the
pump motor 172 is a DC motor. If the pump motor 172 is coupled to a
rechargeable
battery located within the housing 143, the power cord 173 can be used to
recharge the
battery.
-15-
CA 02787374 2012-07-17
WO 2011/091431 PCT/US2011/022444
[0047] In another aspect of this embodiment, the pump motor 172 itself can be
removed from the coolant vessel housing 143, along with the power cord 173,
generally
in the manner described above with reference to Figure 2. In still a further
particular
aspect of this embodiment, the controller 180 (not visible in Figure 3) and
associated
output device 185 can be carried by the pump motor 172 and can accordingly be
readily removed from the coolant vessel housing 143 along with the pump motor
172.
[0048] One feature of particular embodiments of the device 100 described above
with reference to the Figures 1-3 is that the applicator 120, the coolant
vessel 140, the
heat exchanger 160, and the heat transfer conduit 150 can be configured as an
inseparable unit (at least during normal use - components may be separated by
an
authorized servicer if necessary during a maintenance or repair process).
Accordingly,
these components form a sealed, closed-loop path for the heat transfer fluid
155. An
advantage of this feature is that it is simple to use. In particular, the user
can place the
entire device 100 (or at least the above components) in the freezer or other
cold
environment until the coolant 141 is frozen, and can remove the entire device
100 as a
unit from the freezer or other cold environment prior to cooling the target
tissue.
Because the arrangement is simple to use, it can be particularly suitable for
home use.
Because it does not include removable components (in certain embodiments) or
separable fluid connections, it is expected to be more robust than systems
that do
include such features. Because the coolant 141 has a fixed liquid/solid phase
transition temperature, the device 100 can easily control the temperature of
the heat
transfer fluid 155 with a reduced level of active control, and the device 100
can be
thermally recharged in any environment having a temperature less than the
phase
transition temperature.
[0049] Another feature of particular embodiments of the device 100 described
above is that the volume of heat transfer fluid 155 contained in the system
can be
made relatively low by using short lengths and/or small diameters for the heat
transfer
conduit 150 and the heat exchanger tubing 161, and a low (e.g., thin) profile
for the
applicator 120. Accordingly, the coolant 141 can more quickly cool the heat
transfer
fluid 155 and the entirety of the effective heat transfer surface of the
applicator 120.
Having a low thermal mass for the heat transfer fluid 155 will also reduce the
amount of
-16-
CA 02787374 2012-07-17
WO 2011/091431 PCT/US2011/022444
time and/or energy required to elevate the temperature of the applicator 120
to a
comfortable level after the device 100 has been removed from the freezer.
[0050] Still another feature of particular embodiments of the device 100
described
above is that the unitary arrangement of the device is expected to produce a
compact
size and therefore low mass. These features in turn can make it easier to
position the
device in a freezer (e.g., a domestic freezer), and can make the device more
comfortable and convenient to wear during use.
[0051] Yet another feature of at least some of the foregoing embodiments is
that
the simplicity of the device can reduce manufacturing costs and therefore the
costs to
the user. In at least some instances, the device need not include the
serviceable
component features described above because the device may be cheaper to
replace
than repair. The device can include an automated lock-out or shut-down feature
that
activates after a pre-determined number of uses to prevent use beyond an
expected
period of threshold efficacy or useful life.
3. Representative Devices and Methods that Include Separable Coolant Vessels
[0052] Figure 4 is a partially schematic, partially cut-away illustration of
an
embodiment of a device 400 having a user-removable or separable coolant vessel
440,
unlike the configurations described above with reference to Figures 1-3. In
particular,
the device 400 can include a heat exchanger 460 having a heat exchanger
conduit
(e.g., tubing) 461 positioned external to the coolant vessel 440, allowing the
coolant
vessel 440 to be removed from the device 400 (as indicated by arrow D) for
thermal
recharging or regeneration. Accordingly, the coolant vessel 440 can be placed
in a
cold environment (e.g., a freezer) to re-cool (e.g., re-freeze) the coolant
141, without
placing the entire device 400 in the cold environment. This arrangement may be
suitable for applications in which freezer space is limited and thus placing
only the
coolant vessel 440 in the freezer is advantageous. As a result, certain
aspects of the
device 400 can be simpler than the device 100 described above with reference
to
Figures 1-3. For example, the heat transfer conduit 150 is not cooled along
with the
coolant vessel 440 and accordingly the need for the heater 152 and/or shunt
channel
153 and shunt valves 154 described above with reference to Figure 2 can be
eliminated. Conversely, an advantage of the arrangement described above with
reference to Figures 1-3 is that the interface between heat exchanger tubing
161 and
-17-
CA 02787374 2012-07-17
WO 2011/091431 PCT/US2011/022444
the coolant vessel 140 need not be disturbed when the coolant vessel 140 is
chilled.
As described further below with reference to Figures 5A-6B, certain aspects of
the
device 400 are designed to mitigate the potential impact of detaching and
reattaching
the heat exchanger 460 and the coolant vessel 440.
[0053] Figure 5A is an enlarged, partially schematic illustration of an
embodiment
of the coolant vessel 440 and the heat exchanger 460 in which the heat
exchanger
tubing 461 is positioned around the outside of the coolant vessel 440. In
particular, the
heat exchanger tubing 461 can have a serpentine shape extending upwardly and
downwardly along the longitudinal axis of the coolant vessel 440. The heat
transfer
fluid 155 passes through the heat transfer tubing 461 as indicated by arrows
E. To
remove the coolant vessel 440 from the heat exchanger 460, the user pulls the
coolant
vessel 440 upwardly as indicated by arrow D in Figure 5A. The heat exchanger
tubing
461 can be "springy" and can accordingly be resiliently biased inwardly toward
the
coolant vessel 440 to releasably secure the coolant vessel 440 in position,
and to
provide intimate thermal contact between the heat exchanger tubing 461 and the
exterior surface of the coolant vessel 440. This feature can also promote a
"scrubbing"
mechanical contact between the heat exchanger tubing 461 and the exterior
surface of
the coolant vessel 440 to remove frost build-up or other residue to ensure
good thermal
contact as these components are connected. Further details of the foregoing
arrangement are described below with reference to Figure 5B.
[0054] Figure 5B is a partially schematic, cross-sectional illustration of the
heat
exchanger 460 and the coolant vessel 440, taken substantially along line 5B-5B
of
Figure 5A. As shown in Figure 5B, the coolant vessel 440 can have an outer
surface
with a series of recesses 449, each of which is sized and positioned to
receive a
portion of the heat exchanger tubing 461. The exterior surface of the coolant
vessel
440 can include a first thermally conductive surface 462a that is in intimate
thermal and
physical contact with a corresponding second thermally conductive surface 462b
of the
heat exchanger tubing 461. Accordingly, this arrangement can readily transfer
heat
between the heat transfer fluid 155 within the heat exchanger tubing 461, and
the
coolant 141 within the coolant vessel 440. The coolant vessel 440 can include
features
for uniformly distributing the liquid portion of the coolant 141 (e.g.,
agitators) in a
manner generally similar to that described above with reference to Figure 2.
-18-
CA 02787374 2012-07-17
WO 2011/091431 PCT/US2011/022444
[0055] Figures 6A and 6B illustrate another arrangement of a coolant vessel
640
that is removably attached to a corresponding heat exchanger 660 in accordance
with
another embodiment of the technology. In one aspect of this embodiment, the
coolant
vessel 640 includes multiple vertically extending blind channels 644 defined
at least in
part by a thermally conductive channel wall 645. The heat exchanger 660
includes
thermally conductive heat exchanger tubing 661 that directs the heat transfer
fluid 155
into and out of the blind channels 644. In particular, the heat exchanger
tubing 661
can include supply sections 664a that extend into the blind channels 644 and
are
coupled to a supply manifold 663a. The heat exchanger tubing 661 can further
include
corresponding return sections 664b that also extend into each of the blind
channels
644 and are coupled to a return manifold 663b. In a particular embodiment, the
return
sections 664b are located annularly inwardly within the corresponding supply
sections
664a. Accordingly, the heat transfer fluid enters the supply sections 664a,
rises within
the blind channels 664 and then descends through the return sections 664b, as
indicated by arrows E. The coolant vessel 640 is removed from the heat
exchanger
660 by pulling it upwardly away from the heat exchanger 660 as indicated by
arrow D,
and is replaced by placing it downwardly over the heat exchanger 660, with the
blind
channels 644 aligned with the corresponding supply sections 664a. The blind
channels
644 and the corresponding supply sections 664a can be tapered and/or otherwise
biased into contact with each other to promote thermal contact and to
facilitate
mechanically scraping frost from surfaces of either element.
[0056] Figure 6B is a partially schematic, cross-sectional illustration of the
coolant
vessel 640 and the heat exchanger 660, taken substantially along line 6B-6B of
Figure
6A. As shown in Figure 6B, the blind channels 664 include thermally conductive
channel walls 665 that are in intimate thermal contact with the outer surfaces
of the
supply sections 664a. Arrows E indicate the radially inward path of the heat
transfer
fluid 155 as it moves from the supply sections 664a to the return sections
664b.
4. Representative Devices and Methods that Include Separable Coolant Vessels
and Heat Exchangers
[0057] Figure 7 is a partially schematic, partially cut-away illustration of a
device
700 having a releasable coupling 756 between a heat exchanger 760 and a
coolant
vessel 740 on one hand, and the heat transfer conduit 150 on the other.
Accordingly,
the releasable coupling 756 can include a supply coupling 757a at the supply
portion
-19-
CA 02787374 2012-07-17
WO 2011/091431 PCT/US2011/022444
151a of the heat transfer conduit 150, and a return coupling 757b at the
return portion
151b of the heat transfer conduit 150. The couplings 757a, 757b can include
any
suitable fluid-tight, easily releasable and reattachable elements. For
example, the
couplings 757a, 757b can include quick-release couplings generally similar to
those
used for intravenous fluid connections.
[0058] One feature of an embodiment shown in Figure 7 is that, like the
embodiments described above with reference to Figures 4-6B, the entire device
700
need not be placed in the freezer or other cold environment to re-solidify or
otherwise
re-cool the coolant 141. In addition, the device 700 does not require that the
thermal
connection between the heat exchanger 760 and the coolant vessel 740 be
disturbed
in order to recharge the coolant vessel 740. Conversely, an advantage of the
arrangements described above with reference to Figures 1-6B is that they do
not
require connecting and disconnecting fluid conduits.
[0059] Figure 8 is a partially schematic, cross-sectional illustration of a
portion of
the heat exchanger 760 and the coolant vessel 740, taken substantially along
line 8-8
of Figure 7. As shown in Figure 8, the coolant vessel 740 can include a vessel
wall 746
having an insulative portion 747a over a portion of its surface, and a
conductive portion
747b in areas adjacent to the heat exchanger tubing 761. For example, the
insulative
portion 747a can include a material such as a plastic that has a low thermal
conductivity to prevent or at least restrict heat transfer to the coolant
vessel 740 except
as it is received from the heat exchanger tubing 761. The conductive portion
747b can
include copper or another highly thermally conductive material that readily
transfers
heat between the coolant 141 and the heat exchanger tubing 761, which can also
include copper or another highly thermally conductive material. The heat
exchanger
tubing 761 can be welded to or otherwise intimately bonded to the conductive
portion
747b in a way that provides high thermal conductivity between the two. In
other
embodiments, the heat exchanger tubing 761 can take the form of a channel that
is
integrally formed with the conductive portion 747b, e.g., in a casting
process.
[0060] When the coolant 141 is selected to undergo a phase change during
operation, it can include a solid component 141 a generally positioned away
from the
vessel wall 746 once the coolant 141 begins to melt, and a liquid component
141b
generally in contact with the inner surface of the vessel wall 746 and
conductive portion
-20-
CA 02787374 2012-07-17
WO 2011/091431 PCT/US2011/022444
o the vessel wall 747b. As described above, the coolant vessel 740 can include
an
agitator or other device to enhance the uniform distribution of heat transfer
within the
coolant vessel 740 by circulating the liquid component 141b, moving the solid
component 141 a, and/or vibrating or otherwise moving the coolant vessel 740.
5. Representative Applicators and Associated Methods
[0061] Figures 9-11 illustrate particular features of applicators that may
form a
portion of any of the devices described above with reference to Figures 1-8.
In other
embodiments, these applicators may be used with devices other than those
expressly
shown and described above with reference to Figures 1-8. The size and shape of
the
applicator can be selected based on the user's physiology and the location on
the
user's body to which the applicator will be attached.
[0062] Figure 9 illustrates an applicator 920 that includes an envelope 924
having
an entry port 928a coupled to a heat transfer fluid supply portion 151 a, and
an exit port
928b coupled to a return portion 151b. The envelope 924 can include a flexible
first
portion 925 in contact with the human tissue 110, and a flexible second
portion 926
facing away from the human tissue 110. The flexible first portion 925 can be
attached
to the flexible second portion 926 at corresponding bonds 927 formed by an
adhesive,
thermal welding, or other suitable process. The first portion 925 has a first
elasticity,
and the second portion 926 has a second elasticity less than the first
elasticity.
Accordingly, the second portion 926 can, in at least some embodiments, be non-
elastic. As used herein, the term "non-elastic" applies to a material that
does not
stretch, or stretches by only an insignificant amount when the applicator 920
is
subjected to normal operating pressures. The term "elastic" as used herein
applies to
a material that does stretch when the applicator is subjected to normal
operating
pressures provided by the attachment of the device to the patient and/or heat
transfer
fluid 155. Because the first portion 925 is more elastic than the second
portion 926, it
can readily conform to the local shape of the human tissue 110. In particular,
the first
portion 925 can conform to the underlying tissue 110 without forming creases
930
(shown in dotted lines in Figure 9), which form in some existing devices and
can
interfere with skin/applicator thermal contact and/or internal flow within the
applicator
920. As a result, the first portion 925 is more likely to remain in close
thermal contact
with the human tissue 110 and can therefore more efficiently transfer heat
away from
-21-
CA 02787374 2012-07-17
WO 2011/091431 PCT/US2011/022444
the tissue 110. The second portion 926 can flex in a manner that accommodates
the
contour of the human tissue 110, without stretching at all, or without
stretching in a
manner that might cause the envelope to bulge outwardly away from the tissue
110
(e.g., at the ends of the applicator 920) and thereby reduce the degree of
thermal
contact between the envelope 924 (and more particularly, the heat transfer
fluid 155)
and the tissue 110.
[0063] In particular embodiments, the second portion 926 can include
polyethylene, polypropylene, nylon, vinyl, and/or another suitable plastic
film. The first
portion 925 can include latex rubber, nitrile, polyisoprene and/or urethane,
and/or
another suitable elastomeric material. An optional elastic mesh 929 can be
positioned
adjacent to the first portion 925 (or the entire envelope 924), and can
include an elastic
nylon, rubber and/or other suitable elastic material. The mesh 929 can prevent
the first
portion 925 from undergoing excessive wear and/or bulging during handling. It
can
accordingly be strong, but thin enough to avoid significantly interfering with
the heat
transfer process between the applicator 920 and the tissue 110.
[0064] In a particular embodiment, the applicator 920 can also include a
flexible
support structure 921 that provides additional support for the envelope 924,
without
inhibiting the ability of the envelope 924 to conform to the tissue 110. The
support
structure 921 can also function as the releasable coupling (e.g., a cuff)
securing the
applicator 920 to the tissue 110. In any of these embodiments, the support
structure
921 can have a pre-formed shape (e.g., a downwardly-facing concave shape) and
can
be resiliently biased toward the pre-formed shape. Accordingly, the applicator
920 can
more readily conform to a convex tissue surface. In particular embodiments, a
family
of applicators having different shapes can be coupled to a similar type of
overall
cooling device to provide for system commonality and interchangeability.
[0065] Figure 10 is a partially schematic, cross-sectional illustration of an
applicator 1020 having an envelope 1024, an external support structure 1021a
generally similar to that described above with reference to Figure 9, and an
internal
support structure 1021b located within the envelope 1024. The internal support
structure 1021 b can be porous, e.g., 50% porosity or higher in some
embodiments, and
in particular embodiments, in the range of from about 75% porosity to about
95%
porosity. Accordingly, the internal support structure 1021 b can diffuse the
heat transfer
-22-
CA 02787374 2012-07-17
WO 2011/091431 PCT/US2011/022444
fluid 155 throughout the envelope 1024 from an entry port 1028a to an exit
port 1028b,
without overly restricting the flow of the heat transfer fluid 155. The
particular porosity
value selected for the internal support structure 1021 b can depend on factors
that
include the viscosity and/or flow rate of the heat transfer fluid 155. In a
particular
embodiment, the internal support structure 1021 b can include a porous matrix
material
having one or multiple layers 1031 (three are shown in Figure 10 for purposes
of
illustration) that can slide relative to each other, as indicated by arrows F.
In a further
particular embodiment, the internal support structure 1021b is attached to the
inner
surfaces of the envelope 1024 to prevent the envelope from overly stretching.
The
envelope 1024 can also include spaced-apart connections 1035 (e.g., stitches
or
perforated panels) that extend from the envelope upper surface through the
internal
support structure 1021b to the envelope lower surface to prevent or restrict
the
envelope 1024 from ballooning when pressurized with the heat transfer fluid
155 while
allowing the layers 1031 to slide laterally relative to each other.
Accordingly, when the
applicator 1020 is coupled to an upstream fluid driver 1070a, the pressure
exerted by
the incoming heat transfer fluid 155 on the envelope 1024 will be less likely
to expand
the envelope 1024.
[0066] The internal support structure 1021b can resist buckling, in addition
to or in
lieu of resisting bulging or ballooning. For example, the internal support
structure
1021 b can have a high enough buckling strength so that when the applicator
1020 is
coupled to a downstream fluid driver 1070b, the envelope 1024 will not
collapse upon
itself due to external, ambient pressure (e.g., to the point that it inhibits
the flow of heat
transfer fluid 155) when the heat transfer fluid 155 is withdrawn through the
exit port
1028b. In particular embodiments, the heat transfer fluid 155 may be withdrawn
via a
pressure that is up to about 2 psi below the pressure outside the envelope
1024. In
other embodiments, the foregoing pressure differential can be up to about 5
psi or 10
psi without the envelope 1024 collapsing on itself. This will help keep the
envelope
from ballooning due to positive internal pressure. Another advantage of the
downstream fluid driver 1070b is that if the envelope 1024 is inadvertently
punctured,
the downstream fluid driver 1070b will suck air through the puncture, while
the
upstream fluid driver 1070a will continue to pump heat transfer fluid 155
through such a
puncture.
-23-
CA 02787374 2012-07-17
WO 2011/091431 PCT/US2011/022444
[0067] Figure 11 is a partially schematic, enlarged illustration of a portion
of the
applicator 1020 circled in Figure 10. As shown in Figure 11, the internal
support
structure 1021b can include small pores 1034 distributed throughout the
structure. At
the interface with the tissue 110, the pores can form a distributed
arrangement of
generally hemispherical dimples. When the envelope 1024 includes a material
that is
not elastic, the material will tend to crease when folded over a convex
portion of the
tissue 110. The pores 1034 are small enough so that they accommodate or
receive
small "microcreases" 1033 that can form along the surface of the envelope
1024.
Unlike the creases 930 described above with reference to Figure 9, the
microcreases
1033 are very small and accordingly do not significantly inhibit the internal
flow within
the applicator and do not significantly disrupt the uniformity of the heat
transfer
between the heat transfer fluid 155 within the envelope 1024, and the tissue
110
outside the envelope 1024. In effect, the microcreases 1033 can distribute the
creasing effect of the envelope material over a larger area that reduces the
overall
impact of the effect on fluid flow and heat transfer. In particular
embodiments, the
microcreases 1033 can have a generally hemispherical shape that is pre-set
into the
envelope material using a thermoset process. In other embodiments, the shape
and/or
formation process of the microcreases 1033 can be different. In still another
embodiment, the entire portion of the envelope 1024 in contact with the
patient tissue
can have a pre-set or pre-formed shape (e.g., a hemispherical or other concave
shape)
that is maintained as the envelope is placed in contact with the patient
tissue;
[0068] In a particular embodiment, the internal support structure 1021b can
include a TN Blue non-abrasive non-woven polyester pad available from
Glit/Microtron.
This material can be made in multiple layers (e.g., two layers, each 0.35 of
an inch
thick) encased in a polyether-polyurethane film envelope 1024 having a
thickness of
0.006 -0.012 inches. The internal support structure 1021b, which is already
porous
due to the fibrous make-up of the material, can be even further perforated
with a hole
pattern, producing small diameter holes spaced uniformly spaced apart, and
oriented
generally perpendicular to the major surfaces of the envelope 1024. These
holes can
facilitate bending the internal support structure 1021b to conform to convex
and/or
concave shapes. It is expected that the relatively thin overall dimensions of
the
resulting applicator 1020 (e.g., from about 0.25 inch to about 0.50 inch) will
allow the
applicator 1020 to readily conform to the human anatomy. The low flow
impedance of
-24-
CA 02787374 2012-07-17
WO 2011/091431 PCT/US2011/022444
the internal support structure 1021 b is expected to allow flow rates of
approximately 0.1
to 5 liters per minute, suitable for adequately cooling the adjacent tissue.
In addition,
the three-dimensional nature of the fibrous, porous structure can facilitate a
uniform
distribution of the heat transfer fluid 155 within the applicator 1020,
producing a more
uniform treatment of the adjacent tissue 110.
[0069] The porosity of the internal support structure 1021b can vary from one
portion of the applicator 1020 to another, and/or can vary depending upon the
local
flow direction desired for the heat transfer fluid 155. For example, the
porosity of the
internal support structure 1021b can be selected to enhance heat transfer from
the
tissue in the peripheral areas of the applicator 1020, e.g., to account for
the expected
increase in heat transfer losses to the ambient environment in these areas.
The
porosity can be altered by adjusting the number and/or size of the pores
within the
internal support structure 1021 b, as well as the spatial orientation of the
pores.
[0070] From the foregoing, it will be appreciated that specific embodiments of
the
technology have been described herein for purposes of illustration, but that
various
modifications can be made without deviating from the technology. For example,
the
devices described above can include components that provide mechanical energy
to
create a vibratory, massage and/or pulsatile effect in addition to cooling the
subcutaneous tissue. Representative components are described in U.S. Patent
No.
7,367,341 and in commonly assigned U.S. Patent Publication No. 2008/0287839,
both
of which are incorporated herein by reference. While certain features of the
devices
described above make them particularly suitable for home use, such features do
not
preclude the devices from being used in hospital or clinical office settings.
In such
embodiments, the devices or portions of the devices can be cooled in
commercial,
clinical or institutional freezers and/or coolers. The shapes, sizes and
compositions of
many of the components described above can be different than those disclosed
above
so long as they provide the same or generally similar functionalities. For
example, the
conduits and tubing described above can have other shapes or arrangements that
nevertheless effectively convey fluid. The fluid driver can be operatively
coupled to the
heat transfer conduit without being directly connected to the heat transfer
conduit, e.g.,
by being connected to the heat exchanger that conveys the heat transfer fluid,
or by
being connected to the applicator. The controller can implement control
schemes other
-25-
CA 02787374 2012-07-17
WO 2011/091431 PCT/US2011/022444
than those specifically described above, and/or can be coupled to sensors
other than
those specifically described above (e.g., pressure sensors) in addition to or
in lieu of
temperature and time sensors, to detect changes associated with the cooling
device.
The controller can in some cases accept user inputs, though in most cases, the
controller can operate autonomously to simplify the use of the device. As
discussed
above, the coolant in some embodiments can go through a phase change during
heating and cooling, so that the cooling process freezes or solidifies the
coolant. In
other embodiments for which no phase change occurs, the cooling process does
not
freeze or solidify the coolant.
[0071] Certain aspects of the technology described in the context of
particular
embodiments may be combined or eliminated in other embodiments. For example,
the
applicators described above in the content of Figures 9-11 can be used with
any of the
devices described above with reference to Figures 1-8. The thermal connections
between the heat exchanger tubing and the coolant vessel described in the
content of
Figure 8 can be applied to the arrangement shown and described in the content
of
Figures 1-3. The heaters and flow agitators described in the context of
certain
embodiments can be eliminated in other embodiments. Further, while advantages
associated with certain embodiments of the technology have been described
within the
context of those embodiments, other embodiments may also exhibit such
advantages,
and not all embodiments need necessarily exhibit such advantages to fall
within the
scope of the present disclosure. Accordingly, the present disclosure and
associated
technology can encompass other embodiments not expressly shown or described
herein.
-26-