Note: Descriptions are shown in the official language in which they were submitted.
CA 02790560 2012-08-20
WO 2011/103626 PCT/AU2011/000196
1
Hydrogen Storage Unit
Field of the invention
The invention relates to a hydrogen storage unit and particularly a unit
which. can be
used for solid metal hydride absorption/adsorption and desorption of hydrogen.
Background of the invention
Hydrogen may be stored for use as a fuel or for other purposes. Some hydrogen
storage units include an enclosed volume carrying a bed of hydrogen storage
material
such as catalysed MgH2 or other high temperature metal hydride (and various
alloys).
There are a number of thermal challenges associated with such storage units.
Typical
hydrogen storage materials must be . held within a narrow band of operating
temperatures in the vicinity of 365 C to operate effectively.
Typically, a temperature gradient of less, than 20 C is required within the
bed of a
hydrogen storage material during absorption/adsorption in order to ensure all
the
material absorbs/adsorbs the full amount of hydrogen it is chemically capable
of. If the
temperature of the coldest material is more than 20 C below the temperature of
the
hottest material in the bed, the catalyst will chemically react with the
hydrogen to.form a
hydride, thereby decreasing its effectiveness. If this temperature difference
is
significantly more than 20 C, as commonly occurs with the material, closest
the outer
wall, the resulting kinetics will be substantially slowed and the
absorption/adsorption will
not proceed to completion in a practical time.
As the storage unit is filled, hydrogen is absorbed/adsorbed by the hydrogen
storage
material. This reaction is exothermic, i.e. gives off heat. The heat of
reaction adds.to the
heat of compression and must be dissipated in order to hold the hydrogen
storage
material within the desired operating temperature range.
As the storage unit is emptied, i.e. when delivering hydrogen, hydrogen is
desorbed
from the hydrogen storage material. This reaction is endothermic, i.e. absorbs
heat.
CA 02790560 2012-08-20
WO 2011/103626 PCT/AU2011/000196
2
Some storage units incorporate a heater to maintain the hydrogen storage
material
within the desired operating range of temperatures while the storage unit is
delivering
hydrogen. As the desirable operating temperature is typically Swell above the
ambient
temperature, it. is desirable to minimise heat losses from the storage unit to
the ambient
environment to minimise the energy input required from the heater, i.e.
maximise the
thermal efficiency.
Hydrogen storage materials produce stress and can deform or. destroy the
vessel
because the material expands when it absorbs/adsorbs hydrogen. Stress
accumulation
can also occur in hydride beds at high packing densities as a result of the
finely
pulverised particles falling in to gaps at the bottom of the vessel; thus
causing the
hydride packing fraction at the bottom of the vessel to gradually increase.
Figure 1 schematically illustrates a cross section of a prior art hydrogen
storage unit 10
in the form of a tank including an outer wall 14 containing a bed 12 of
hydrogen storage
material in the form of hydride. The outer wall 14 is mechanically stressed by
a net
outward force FR. The net outward. force FR is equal to. the force of
expansion (i.e.
mechanical stress generated by the hydride) plus the hydrostatic force (i:e.
the pressure
of the hydrogen).
Figure 2 illustrates an approach of the prior art to minimising the
temperature gradient in
the metal hydride bed. Figure 2 is an axial cross section view illustrating
an. outer vessel
20 thermally insulated by layer 30 about its exterior.
The outer vessel,20 carries aluminium foam 18, dividers 32 and metal hydride
particles
34. The metal hydride particles fill the void spaces of the aluminium foam 18.
The
mixture of the aluminium foam 18 and the metal hydride particles.34 desirably
has a
higher thermal conductivity than a solid bed of metal hydride particles. The
dividers 32
extend transversely across, and are spaced longitudinally along, the outer
vessel 20 to
minimise the movement of the metal hydride particles 34 (which is typically in
the form
of a pulverised powder) to eliminate dense spots. The dividers 32 minimise the
distance
the metal hydride particles 34 can travel.
CA 02790560 2012-08-20
WO 2011/103626 PCT/AU2011/000196
3
A line 22 extends longitudinally within the interior of the outer vessel 20.
The line 22 is a
tubular structure with gas permeable walls forming a porous metal filter.,
Line 22 thereby fluidly communicates with the interior of the outer vessel 20
to define
reversible flow path 24 through which hydrogen is received into, and delivered
from, the
interior of the outer vessel 20. Desirably the line 22 is positioned above an
upper extent
of the metal hydride particles 34.
The storage unit 16 includes a cooling tube 28 in the form of a U shaped loop
extending
within the interior of the outer vessel 20 along the full length of the
storage unit 16. The
cooling tube 28 includes an inlet 26 and an. outlet 25. Coolant is received
into the tube
28 via the inlet 26 and absorbs heat from the aluminium foam 18 and metal
hydride
particles 34 as it traverses the interior of the outer vessel 20 before
emerging from the
outlet 25.
Figure 3 illustrates a transverse cross section view of the prior art storage
unit 16 of
Figure 2.
These various constructions of the prior art have significant drawbacks.
Aluminium foam is very expensive. It typically costs at least three times as.
much as the
metal hydride. Moreover, the utilisation of the aluminium foam requires the
metal
hydride. be in fine powder form so as to effectively fill the pores of the
foam and achieve
high packing densities. Production. of fine powder significantly increases the
material
production cost due to increased tooling demands.
Dividers can reduce the stress 'on the wall of the cylinder but do not
eliminate it. The
resulting stress on the wall of the cylinder with dividers can still far
exceed the
hydrostatic stress exerted by the gaseous hydrogen. Therefore, the cylinder
walls need
to be much thicker than other storage tanks to resist such force.
The drawback, of using a heavily insulated cylinder with internal heat
exchanger for
removing heat is the cost of safe heat transfer fluids for extracting the heat
at, say,
350 C. The available fluids are typically expensive, auto ignitable in air and
highly toxic.
CA 02790560 2012-08-20
WO 2011/103626 PCT/AU2011/000196
4
On top of this, the working temperature of the available fluids is typically
limited to
400 C which relates to a limited desorption pressure of 3 to 5bar which in
turn limits the
rate at which hydrogen may be delivered from the. unit. To achieve higher
pressure
desorption, in high temp hydrides, significantly higher temperatures are
required.
Temperatures up to 600 C and beyond are achievable with electric heater
elements.
The above reference to the prior art is not intended to be an admission that
the
information forms part of the common general knowledge of a person skilled in
the art.
Summary of the, invention
One aspect of the invention. provides a hydrogen storage unit including:
a fluid communication port ;
an outer vessel wall; and
an inner compartment for hydrogen storage material in fluid, communication
with
the fluid communication port, the inner compartment being spaced from the
outer vessel
wall to define a peripheral volume between the inner compartment and outer
vessel
wall.
In a preferred form of. the invention, the inner compartment is in fluid
communication
with the peripheral volume and the fluid communication port includes one or
more flow
paths for fluidly communicating the inner compartment with the exterior of the
storage
unit to deliver hydrogen from, and receive hydrogen in to, the hydrogen
storage unit.
The flow communication port is configured to at least substantially bypass the
peripheral
volume. during desorption of hydrogen. During delivery, the hydrogen in the
peripheral
volume is preferably static or slow moving to insulate the hydrogen storage
material
.from the exterior.
It is desirable that the outer vessel wall be substantially mechanically
isolated from
mechanical stresses generated by hydrogen storage material contained within
the inner
compartment. Further it is desirable to isolate the vessel from thermal
expansion or
CA 02790560 2012-08-20
WO 2011/103626 PCT/AU2011/000196
contraction stresses that may be induced by temperature differentials that
occur during
cycling of the hydride.
The inner compartment of the-storage unit is constructed to contain hydrogen
storage
material. According to such units, the outer vessel may surround at least a
substantial
portion or totally contain the inner compartment and be spaced therefrom to
define the
peripheral volume around the inner compartment. The inner compartment may
include a
compartment wall and end pieces, the end pieces engaging the outer vessel wall
to
support the inner compartment within the outer vessel. The compartment wall is
preferably cylindrical and the end pieces conical,. frusto-conical. or
'hemispherical in
shape. By supporting the inner compartment at the end pieces, intermediate
structural
components between the inner compartment and the outer vessel can be avoided
thus
reducing or eliminating heat conduction pathways between the hydrogen storage
material in the inner compartment, the outer vessel and the ambient outer
environment.
Optionally, the inner compartment wall is substantially cylindrical and the
outer vessel
wall totally encloses the inner compartment. The outer vessel may include a.
substantially cylindrical interior wall concentrically surrounding the inner
compartment
defining the peripheral volume which is an annular space. Preferably the inner
compartment is supported within the outer vessel at the end pieces only. Thus
there
are no structural supports between the outer vessel wall and the inner
compartment wall
and hence no direct path through structural supports for heat conduction. In
one form of
the invention, the inner compartment fluidly communicates with the peripheral
volume
around the inner compartment during receipt of hydrogen so that the peripheral
volume
is pressurised. The inner compartment fluidly communicates with the
peripheral. volume
at a location proximal to the fluid communication port and preferably through
the end
pieces. The fluid communication may be provided by gas equalisation ports in
the end
pieces of the inner compartment.
According to another form of the invention, the peripheral volume is fluidly
isolated from
the inner compartment. A fluid pressure device may be provided to control the
pressure
within the peripheral. volume. By way of example, the fluid pressure device
may be
CA 02790560 2012-08-20
WO 2011/103626 PCT/AU2011/000196
6
configured or controlled to evacuate the peripheral volume during said
delivery of
hydrogen.
The inner compartment may be predominantly formed of sheet metal such as 0.75
millimetre thick stainless steel (or other hydrogen compatible materials i.e.
copper,
aluminium etc)..
The hydrogen storage material may include one or more high temperature metal
hydrides.
A heating element may be provided to.heat the hydrogen storage material during
said
delivery of hydrogen.
A second aspect of the invention provides a hydrogen storage unit including:
a fluid communication port;
an inner compartment for containing a hydrogen storage material;
an outer vessel wall surrounding the inner compartment to define a peripheral.
volume about the inner compartment isolated from the inner compartment;
a fluid pressure device in communication with the peripheral volume; and
a controller for controlling the fluid pressure device;
wherein the controller is configured to:
i) reduce the pressure in the peripheral volume during desorption of
hydrogen to insulate the inner compartment; and-
ii) increase the pressure in the peripheral volume during
absorption/adsorption of hydrogen to conduct heat from the inner compartment
to
the outer vessel wall.
CA 02790560 2012-08-20
WO 2011/103626 PCT/AU2011/000196
7
A third aspect of the invention provides a hydrogen storage unit consistent
with the first
aspect wherein:
the inner, compartment is connected to the peripheral volume by at least one
pressure equalising port; and
the fluid communication port fluidly communicates with the inner compartment
bypassing the peripheral volume;
As with the first aspect, in the second and third aspects, the inner
compartment may
include a compartment wall and end pieces, the end pieces engaging the outer
vessel
wall to support the inner compartment within the outer vessel. The compartment
wall is
preferably cylindrical and the end pieces conical or frusto-conical in shape.
By
supporting the inner compartment at the end pieces, intermediate. structural
components between the inner compartment and the outer vessel can be avoided
thus
reducing or eliminating heat conduction pathways between the hydrogen storage
material in the inner compartment, the outer vessel and the ambient outer
environment.
The fluid communication port enters the outer vessel at the top of the
hydrogen storage
vessel and passes through an aperture in the top end piece of the inner
compartment.
Brief description of the drawings
Figure 1 is a schematic transverse cross section view of a hydrogen storage
unit;
Figure 2 is an axial cross section view of a hydrogen. storage unit;
Figure 3 is a transverse cross section view of the hydrogen storage unit of
Figure 2;
Figure 4 is a side view of a hydrogen storage unit in accordance with an
embodiment of
the invention;
Figure 5 is an axial cross section view of the hydrogen storage unit of Figure
4;
CA 02790560 2012-08-20
WO 2011/103626 PCT/AU2011/000196
8
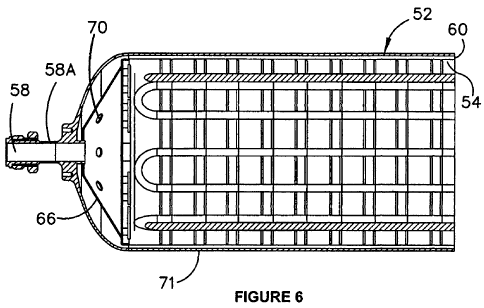
Figure 6 is a close up view of an end portion of the axial cross section
illustrated in
Figure 5;
Figure 7 is a schematic transverse cross, section view of a hydrogen storage
unit in
accordance with another embodiment of the invention;
Figure 8 is a FEA simulation of hydrogen storage unit (a) without and (b) with
hydrogen
gap; and
Figure 9 is a FEA simulation of hydrogen storage unit showing heat flux with
(a) 7 bar
hydrogen in the gap for an absorption/adsorption, and (b) 1 bar hydrogen in
the gap for
a desorption.
Detailed description of the embodiments
It will be understood -that the invention disclosed and defined in this
specification
extends to all alternative combinations of two or more of the individual
features
mentioned or evident from the text or drawings. All of these different
combinations
constitute various alternative aspects of the invention.
Figures 4, 5 and 6 illustrate a preferred embodiment of the invention. The
hydrogen
storage unit 50 includes an outer. vessel wall 52A carrying an inner
compartment 54A
which in turn carries a body of hydrogen storage material 56 preferably in the
form of
MgH2. A tubular neck 58 defines a fluid communication port for an inlet/outlet
flow path
communicating with hydrogen storage material 56 carried in the inner
compartment
54A.
The outer vessel wall 52A is,substantially cylindrical and terminates in
outwardly domed
ends 62 and 64. An electrical junction box 51 is carried by domed end 64. The
hydrogen
storage vessels generally stand upright on base 57 including supports 72 and
junction
.box 51. for the connection of electrical power to electrical heating elements
55. The
heating elements extend. from the junction.box 51 but only that part of the
elements.
contained within the inner compartment is active and provides heat.
CA 02790560 2012-08-20
WO 2011/103626 PCT/AU2011/000196
9
The inner compartment 54A is also cylindrical and sits concentrically within
the outer,
vessel 52A. The exterior of the inner compartment 54A is about 3mm smaller (in
radius)
than the inner surface of the outer vessel wall 52A to define a peripheral
volume 60 in
the form of an annular gas space which is about 3mm thick. In this embodiment,
the
peripheral volume 60 is filled with hydrogen. and thus forms a hydrogen gap.
The inner compartment allows for the thermal expansion and contraction of the
hydrogen storage material independent of the outer vessel wall. This reduces
the levels
of thermally induced stresses experienced by the outer vessel and reduces the
likelihood of structural failure of the outer vessel.
Figure 7 schematically illustrates some of the advantages of this
construction. The
peripheral volume 60' mechanically isolates the inner wall 54' from the outer
wall 52'
such that the inner wall 54' experiences a net outward expansion force equal
to the
mechanical forces generated by expansion of the hydrogen storage material.
This
stress is not transferred to the outer wall 52' so that the outer wall 52'
experiences a net
outward force equal to the hydrostatic pressure.
The inner compartment 54A includes an inner compartment wall 54 and end pieces
66,
68. The end pieces 66, 68 engage the outer vessel wall to support the inner
compartment within the outer vessel. The engagement.can be either directly
onto the
inner dome end piece 62 or via a' connection to the fluid communication port
which in
turn is secured to the domed end piece 62.
The compartment wall is preferably cylindrical and the compartment end pieces
conical
or frusto-conical in shape. By supporting the inner compartment at the
compartment end
pieces, intermediate structural components between the inner compartment and
the
outer vessel are not required thus reducing or eliminating heat conduction
pathways
between the hydrogen storage material in the inner compartment 54A, the outer
vessel
71 and the ambient outer environment.
As illustrated the outer vessel wall 52A encapsulates substantially all of the
compartment 54A.
CA 02790560 2012-08-20
WO 2011/103626 PCT/AU2011/000196
The fluid communication conduit 58A from fluid communication port 58 enters
the outer
vessel at the top of the hydrogen 'storage vessel and passes through an
aperture in the
top. end piece 62 of the, inner compartment. The fluid communication conduit
58A
communicates directly with an interior of the compartment 54A whereby hydrogen
can
be received.into or delivered from the storage unit 50 with only minimal
disturbance of
the hydrogen carried in the peripheral volume 60.
In this embodiment the top 'compartment end piece 66, which defines an end of
the
inner compartment 54A to which the fluid communication conduit 58A is
communicated,
includes pressure equalisation ports in the form of 'six small apertures 70
equally
spaced on a pitch circle concentric to conduit 58A. As illustrated, the
apertures 70 -are
proximal to the neck 58.
During the removal of hydrogen from the hydrogen storage unit 50.
(desorption), the
hydrogen storage material is heated by heating elements in the inner
compartment.
Thus when the fluid communication port 58 is opened, hydrogen flows out of
unit 50,
from the' inner compartment 54A. The peripheral volume 60 communicates with
the
inner compartment 54A via the apertures 70 so that pressure in the peripheral
volume
60 is reduced.
The skilled person will appreciate that when the. fluid communication. port 58
is first
opened to remove hydrogen, and the hydrogen storage material heated to desorb
hydrogen, there may be an initial flow of hydrogen from the peripheral volume
60,
through the aperture 70, towards the outlet 50 but thereafter during
removal/desorption,
the hydrogen in the peripheral volume is more'or.less static. This peripheral
volume of
low pressure hydrogen has been found to be an effective insulator useful for
insulating
the hydrogen storage material 56 from the outer vessel wall 52 and the
exterior of the
unit. This is useful for reducing heat loss during hydrogen removal/desorption
and
reducing the amount of heat which needs to be supplied by heating elements 55.
The unit 50 is filled by supplying hydrogen. at pressure to the fluid
communication port
58 whereby the hydrogen is received into the inner compartment 54A. The
received
hydrogen its absorbed/adsorbed by the hydrogen storage material 56. As the
CA 02790560 2012-08-20
WO 2011/103626 PCT/AU2011/000196
11
absorption/adsorption of hydrogen is exothermic, during
receiving/absorption/adsorption ,
the unit 50 is heated by the.heat of reaction as the hydrogen storage material
56
absorbs/adsorbs the hydrogen. As discussed, it is important that this heat is
dissipated
so that the hydrogen storage material 56 is maintained within its effective
operating
temperate range.
According to the illustrated embodiment, as hydrogen is provided at pressure
into the
vessel 50 through the fluid communication port 58, hydrogen flows from the
inner
compartment 54A and into the peripheral volume 60 via the apertures 70. As
such,
during receiving/absorption/adsorption the peripheral volume 60 is occupied by
dense
pressurised hydrogen which forms an effective. heat conductor. Thus during
receiving/absorption/adsorption, the peripheral ' volume effectively:
conducts. heat from
the hydrogen storage material 56 to the outer vessel 52 and in turn its
exterior to
dissipate heat.
Of course the apertures 70 are not essential. According to another embodiment
of the
invention, the peripheral volume 60 is fluidly isolated from the inner
compartment 54A
so that the peripheral volume 60 and the inner compartment 54A may be held at
different pressures. According to this 'embodiment, as illustrated in Figure
9, a fluid
pressure device, such as a positive displacement compressor, may be used to
control
the pressure within the peripheral volume 60'. A controller 74 is, configured
to drive the
fluid pressure device 73 to control the pressure within the peripheral volume
60' to
achieve the desired degree of insulation. By way of example, the controller 74
and fluid
pressure device 73 might reduce the pressure in the peripheral volume 60'
during
removal/desorption for improved insulation and reduced heat losses. Indeed the
fluid
pressure devices 73 and controller 74 may be configured to evacuate the
peripheral
volume for improved insulation, i.e. by providing a negative pressure.. The
fluid pressure
device 73 and the controller 74 may be integrated. Alternatively they may be
separated
by some distance. The controller 74 may be mounted within the junction box 51.
During receiving/absorption/adsorption the fluid pressure device 73 and
controller 74
will typically increase the pressure within the peripheral volume 60' for
improved heat
transfer.
CA 02790560 2012-08-20
WO 2011/103626 PCT/AU2011/000196
12
Variants of the invention have application to:
= solid-state, hydrogen,cylinder packs for replacement of compressed gas
MCP's;
= solid state hydrogen storage system packaged in a shipping container;
= solid state hydrogen storage system for interface to high temp fuel cell for
waste
heat recovery;
= hydrogen storage system to interface to internal combustion engine for wast
heat
utilisation;
= hydrogen storage systems for refuelling applications; and
= hydrogen storage systems for PEM,fuel cell applications.
To illustrate the effectiveness of the invention, Figure 8.shows a finite
element analysis
simulation of hydrogen storage unit without (A) and with (B) hydrogen gap. The
chilling
effect is clearly noticeable on the unit without the air gap in the material
closest to the
outer wall. In contrast, the chilling effect in the unit with the hydrogen gap
is significantly
less.
The thin gap also allows a large temperature difference between the edge of
the hydride.
bed and the cylinder wall. This allows the hydride bed to operate with <20degC
temperature gradient from the centre to the perimeter (e.g. Tcenter = 370DegC,
Ti,,ner wall
360 DegC) while the outer wall operates at a much lower temperature of
250DegC.
Lastly, the. hydrogen pressure within the gap can be changed between
absorption/adsorption and desorption to bias the heat transfer to match the
requirements. For absorption/adsorption, where good heat transfer between the
bed
and the wall is required, the hydrogen pressure should be high to increase
conductivity.
In contrast, during desorption where heat loss is undesirable, the pressure
can be much
lower or a vacuum to minimise the thermal conductivity between the bed and the
outer
wall. The effect of hydrogen pressure in.the gap is shown in Figure 9 which is
a, finite
CA 02790560 2012-08-20
WO 2011/103626 PCT/AU2011/000196
13
element analysis simulation of hydrogen storage unit showing the much greater
heat
flux with (a) 7 bar hydrogen in the gap for an absorption/adsorption, and (b)
1 bar
hydrogen in the gap for a desorption
A hydrogen filled gap between the bed and the wall is naturally pressurised
during
absorption/adsorption when higher pressures are desirable. The ' higher
pressure
hydrogen conducts heat well from the bed. to the outer wall of the. unit where
the
external cooling system can extract the heat.
A hydrogen filled gap between the bed and the wall is naturally depressurised
or even
vacuumed during desorption when lower pressures are desirable. The lower
pressure
hydrogen/vacuum insulates heat well from the bed to the wall of the unit
thereby
minimising heat loss:
The pressurisation and depressurisation of the gap can be passive and follow
the
pressure changes of the unit for. absorption/adsorption and desorption.
Alternatively, the
gap can be isolated from the metal hydride bed and therefore have a different
pressure
to the bed. This would allow inducing a vacuum inside the gap while desorbing
at
positive pressure as well as absorbing/adsorbing at low pressure with a much
higher
pressure of hydrogen in the gap.
It will be understood that the invention disclosed and defined in this
specification
extends to all alternative combinations of the individual features mentioned
or evident
from the text or drawings. All of these different combinations constitute
various
alternative aspects of the invention.