Note: Descriptions are shown in the official language in which they were submitted.
CA 02791134 2012-09-06
PF0000071528/PP
1
Process for producing mineral oil from underground mineral oil deposits
Description
The present invention relates to a process for producing mineral oil from
mineral oil
deposits, in which the mineral oil yield is increased by blocking highly
permeable regions
of the mineral oil formation by separate injection of at least two different
formulations into
the deposit, said formulations not mixing with one another until within the
deposit, and the
mixture forming highly viscous gels under the influence of the deposit
temperature. The
process can be used especially in the final stage of deposit development, when
watering
out in production increases, and particularly after the water flooding of the
deposits.
In natural mineral oil deposits, mineral oil occurs in cavities of porous
reservoir rocks which
are closed off from the surface of the earth by impervious overlying strata.
In addition to
mineral oil, including proportions of natural gas, a deposit further comprises
water with a
higher or lower salt content. The cavities may be very fine cavities,
capillaries, pores or the
like, for example those having a diameter of only approx. 1 m; the formation
may
additionally also have regions with pores of greater diameter and/or natural
fractures.
After the well has been sunk into the oil-bearing strata, the oil at first
flows to the
production wells owing to the natural deposit pressure, and erupts from the
surface of the
earth. This phase of mineral oil production is referred to by the person
skilled in the art as
primary production. In the case of poor deposit conditions, for example a high
oil viscosity,
rapidly declining deposit pressure or high flow resistances in the oil-bearing
strata, eruptive
production rapidly ceases. With primary production, it is possible on average
to produce
only 2 to 10% of the oil originally present in the deposit. In the case of
higher-viscosity
mineral oils, eruptive production is generally completely impossible.
In order to enhance the yield, what are known as secondary production
processes are
therefore used.
The most commonly used process in secondary mineral oil production is water
flooding.
This involves injecting water through what are known as injection wells into
the oil-bearing
strata. This artificially increases the deposit pressure and forces the oil
out of the injection
wells to the production wells. By water flooding, it is possible to
substantially increase the
yield level under particular conditions.
EK11-0528CA
CA 02791134 2012-09-06
PF0000071528/PP
2
In the ideal case of water flooding, a water front proceeding from the
injection well should
force the oil homogeneously over the entire mineral oil formation to the
production well. In
40 practice, a mineral oil formation, however, has regions with different
levels of flow
resistance. In addition to oil-saturated reservoir rocks which have fine
porosity and a high
flow resistance for water, there also exist regions with low flow resistance
for water, for
example natural or synthetic fractures or very permeable regions in the
reservoir rock.
Such permeable regions may also be regions from which oil has already been
recovered.
45 In the course of water flooding, the flooding water injected naturally
flows principally
through flow paths with low flow resistance from the injection well to the
production well.
The consequences of this are that the oil-saturated deposit regions with fine
porosity and
high flow resistance are no longer flooded, and that increasingly more water
and less
mineral oil is produced via the production well. In this context, the person
skilled in the art
50 refers to "watering out of production". The effects mentioned are
particularly marked in the
case of heavy or viscous mineral oils. The higher the mineral oil viscosity,
the more
probable is rapid watering out of production.
For production of mineral oil from deposits with high mineral oil viscosity,
the mineral oil
55 can also be heated by injecting steam in the deposit, thus reducing the oil
viscosity. As in
the case of water flooding, however, steam and steam condensate can also
strike
undesirably rapidly through highly permeable zones from the injection wells to
the
production wells, thus reducing the efficiency of the tertiary production.
60 The prior art discloses measures for closing such highly permeable zones
between
injection wells and production wells by means of suitable measures. As a
result of these,
highly permeable zones with low flow resistance are blocked and the flood
water or the
flood steam flows again through the oil-saturated, low-permeability strata.
Such measures
are also known as "conformance control". An overview of measures for
conformance
65 control is given by Borling et al. "Pushing out the oil with Conformance
Control" in Oilfield
Review (1994), pages 44 if.
For conformance control, it is possible to use comparatively low-viscosity
formulations of
particular chemical substances which can be injected easily into the
formation, and the
70 viscosity of which rises significantly only after injection into the
formation under the
conditions which exist in the formation. To enhance the viscosity, such
formulations
comprise suitable inorganic, organic or polymeric components. The rise in
viscosity of the
injected formulation can firstly occur with a simple time delay. However,
there are also
known formulations in which the rise in viscosity is triggered essentially by
the temperature
75 rise when the injected formulation is gradually heated to the deposit
temperature in the
deposit. Formulations whose viscosity rises only under formation conditions
are known, for
example, as "thermogels" or "delayed gelling systems". However, these
formulations are
EK11-0528CA
CA 02791134 2012-09-06
PF0000071528/PP
3
usable efficienctly only for deposits whose temperature is above 60 C. For
"cold" deposits
whose temperature is less than 60 C,
80 formulations which are partly mixed before injection are used, the
viscosity of which at first
remains low and which do not form components which increase the viscosity of
the
formulation through chemical reactions until after injection into the deposit.
This group includes formulations comprising urotropin, urea and aluminum salts
or other
85 metal salts. Processes for mineral oil production with this group of
formulations are
disclosed, for example, in RU2066743, RU2250367 and RU2382174. One
disadvantage of
this process is that the chemical reaction between urotropin, urea and metal
salt also sets
in at low temperatures above ground even in the course of mixing, and another
is that the
reaction time within which the formulation forms a viscous gel is
comparatively short. The
90 result of this is that a so-called gel bank forms only in the zone close to
the injection, but
not in zones somewhat further away.
The table below shows, by way of example, the dependence of the gel formation
time of
an aqueous composition comprising 4% by weight of urotropin, 20% by weight of
urea and
95 17.6% by weight of polyhydroxyaluminum chloride ((AInOH)m C13n-m, AluStar
), 58.4% by
weight of water, based on the total weight of the solution, at different
temperatures.
Table 1:
Temperature [ C] Gel formation time measured by rheology [h]
50% 90%
20 20 55
30 5 17
40 2 6
50 1 2
100 50% and 90% mean, respectively, that 50% and 90% of the solution is
present as a gel.
Therefore, it is impossible in the case of deposit temperatures of only about
20 C to pump
the mixture based on urotropin, urea and gel-forming metal salt deep into the
deposit,
since even solutions with gel content 50% are no longer sufficiently mobile,
and it typically
105 takes more than one day for the mixture to reach the site in the formation
where it is to act.
Thus, the formulations injected do not reach the highly permeable zones that
they are
actually supposed to block at all; instead, viscous gels are formed actually
at the injection
well or in the zone close to the borehole. The gels can hinder the further
pumping of the
E K 11-0528CA
CA 02791134 2012-09-06
PF0000071528/PP
4
gel-forming formulation, and can naturally also prevent subsequent water or
steam
110 flooding.
It was therefore an object of the present invention to provide a process for
producing
mineral oil from mineral oil formations, in which watering out of production
is reduced and
the level of oil recovery rises, and which is also suitable for deposits with
relatively low
115 temperature.
This object is achieved by the following process for producing mineral oil
from
underground mineral oil deposits into which at least one injection well and at
least one
production well have been sunk, comprising at least the following process
steps:
120
(1) injecting one or more flooding media into at least one injection well and
withdrawing
mineral oil through at least one production well,
(2) blocking highly permeable zones in the mineral oil deposit in the region
between
125 the at least one injection well and the at least one production well, by
injecting at
least one aqueous formulation F, and at least one aqueous formulation F2 each
separately in succession through the at least one injection well into the
deposit, the
formulations mixing with one another in the formation after injection to form
viscous
gels,
130
(3) continuing the injection of one or more flooding media into the injection
well,
wherein
135 formulation F, comprises water and urotropin,
formulation F2 comprises water and urea, and
F, and/or F2 comprise(s) at least one further compound M which is selected
from metal
compounds and semimetal compounds and is able form gels when admixed with
bases,
the injection well temperature before process step (2) being not more than 60
C.
140
In a preferred embodiment, this object is achieved by the following process
for producing
mineral oil from underground mineral oil deposits into which at least one
injection well and
at least one production well have been sunk, comprising at least the following
process
steps:
145
(1) injecting one or more flooding media into at least one injection well and
withdrawing
mineral oil through at least one production well,
EK11-0528CA
CA 02791134 2012-09-06
PF0000071528/PP
(2) blocking highly permeable zones in the mineral oil deposit in the region
between
150 the at least one injection well and the at least one production well, by
injecting at
least one aqueous formulation F, and at least one aqueous formulation F2 each
separately in succession through the at least one injection well into the
deposit, the
formulations mixing with one another in the formation in a mixing zone after
injection to form viscous gels,
155
(3) continuing the injection of one or more flooding media into the injection
well,
wherein
160 formulation F, comprises water and urotropin,
formulation F2 comprises water and urea, and F, and/or F2 comprise(s) at least
one further
compound M which is selected from metal compounds and semimetal compounds and
is
able form gels when admixed with bases, the metal compounds and semimetal
165 compounds being selected from Fe(II) and Fe(III) salts, vanadium salts,
zirconium salts,
aluminum(Ill) salts and colloidal silicon compounds,
and wherein the concentration of urotropin is at least 1% by weight, the
concentration of
urea at least 5.75% by weight and the concentration of the at least one
compound M at
170 least 5% by weight in the mixing zone of the formulations after step (2),
based on the sum
of water, urotropin, urea and the at least one compound M present in the
mixing zone,
the injection well temperature before process step (2) being not more than 60
C.
175 The process according to the invention has the advantage that it is also
possible to block
high-permeability zones in a controlled manner by means of inorganic gels even
in
deposits with low temperature. The process enables blockage even of cooled
(for example
by water flooding), washed-out rock zones in the deposit. The distance between
the
borehole and the gel bank in the process according to the invention can be
regulated
180 irrespective of temperature. This achieves efficient blockage of high-
permeability zones,
reduces watering out of production and increases the level of oil recovery.
Index of figures:
185 Figure 1 Illustration of the results of a simulation calculation of the
mixing zone after
inventive injection of a portion F1, of a portion F2 and of a portion F1;
EK11-0528CA
CA 02791134 2012-09-06
PF0000071528/PP
6
Figure 2 Illustration of the pressure plot of the core flooding test according
to
example 1;
190
Figure 3 Illustration of the pressure plot of the core flooding test according
to
example 2.
With regard to the invention, the following specific details are given:
195
The process according to the invention for producing mineral oil is a process
for secondary
or tertiary mineral oil production, which means that it is employed after
primary mineral oil
production due to the autogenous pressure of the deposit has stopped, and the
pressure
in the deposit has to be maintained by injecting water and/or steam.
200
Deposits
The deposits may be deposits for all kinds of oil, for example those for light
or heavy oil. In
one embodiment of the invention, the deposits are heavy oil deposits, i.e.
deposits which
205 comprise mineral oil with an API gravity of less than 22.3 API.
To execute the process, at least one production well and at least one
injection well are
sunk into the mineral oil deposits. In general, one deposit is provided with
several injection
wells and with several production wells.
210
The initial deposit temperature - i.e. the temperature before step (2) of the
process
according to the invention - is typically 8 to 60 C, preferably 8 to 50 C,
more preferably 8
to 40 C, even more preferably 8 to 30 C and especially 8 to 25 C, measured at
the
injection well. The deposit temperature changes as a result of the application
of the
215 process according to the invention typically at least within the region
between the injection
wells and the production wells.
Process
220 According to the invention, the process comprises at least three process
steps (1), (2) and
(3), which are executed in this sequence, but not necessarily in immediate
succession.
The process may of course also comprise further process steps which can be
executed
before, during or after steps (1), (2) and (3).
225 Process step (1)
EK11-0528CA
CA 02791134 2012-09-06
PF0000071528/PP
7
In a first process step, (1), one or more flooding media such as nitrogen,
carbon dioxide,
water, and water comprising customary additives known to those skilled in the
art, such as
thickeners and surfactants, preferably water or water comprising additives,
are injected
230 into the at least one injection well, and mineral oil is withdrawn through
at least one
production well. The present invention also provides a process wherein the
flooding
medium is selected from water optionally comprising additives. The term
"mineral oil" in
this context does not of course mean single-phase oil; what is meant is
instead the typical
emulsions which comprise oil and formation water and which are produced from
mineral oil
235 deposits.
The water injected typically has a temperature of 5 to 60 C, preferably of 5
to 50 C and
more preferably of 5 to 40 C.
240 As a result of the injection of water, a zone forms in the region between
the injection well
and the production well, in which oil is displaced by water.
As a result of the injection of flooding media such as water, the original
deposit
temperature can change, i.e. it can be increased or lowered according to
whether the
245 flooding medium injected has a higher or lower temperature than the
original deposit
temperature.
As a result of the injection of a flooding medium such as water, the pressure
in the deposit
rises, and zones form in the region between the injection well and the
production well, in
250 which oil is displaced by the flooding medium.
As a result of the natural inhomogeneity of the permeability of the deposit,
the "washed-
out" zones having high permeability form within a certain time between the
injector and
producers. These zones may have very different geometries and dimensions and
are very
255 difficult to predict. These zones are often arranged at the small
geological faults which
cannot be discovered by conventional test methods and test instruments, or at
particular
rock strata.
When watering out of production rises relatively rapidly after commencement of
water
260 flooding, this is a clear indication of water breakthrough.
Process step (2)
Process step (2) can be employed as soon as production becomes excessively
watered
265 out, or what is called a water breakthrough is registered. In the event of
water
breakthrough, water flows through high-permeability zones from the injection
well to the
EK11-0528CA
CA 02791134 2012-09-06
PF0000071528/PP
8
production well. High-permeability zones need not, however, necessarily be
produced by
the water flooding, but may also be present naturally in a formation. In
addition, it is
possible that permeable zones have already been created in a process step
preceding the
270 process according to the invention.
For preparation for process step (2), it may be advantageous to measure the
temperature
in the region of the injection well and to determine the temperature range of
the deposit in
the region under the influence of flooding. Methods of determining the
temperature range
275 of a mineral oil deposit are known in principle to those skilled in the
art. The temperature
distribution is generally determined by temperature measurements at particular
sites in the
formation in combination with simulation calculations, the simulation
calculations taking
account of factors including amounts of heat introduced into the formation and
the
amounts of heat removed from the formation. Alternatively, each of the regions
can also
280 be characterized by its average temperature. It is clear to the person
skilled in the art that
the analysis of the temperature range outlined constitutes merely an
approximation of the
actual conditions in the formation.
Process step (2) can be performed immediately after process step (1).
285
In the course of process step (2), high-permeability zones of the mineral oil
deposit in the
region between the injection wells and the production wells are blocked by
injecting
aqueous formulations through the at least one injection well.
290 According to the invention, at least two different aqueous formulations F,
and F2 are used
for this purpose. Formulation F, comprises water and urotropin
(hexamethylenetetramine).
The different formulation F2 comprises water and urea. In addition, either F,
or F2, or both
F, and F2, comprise(s) at least one compound M selected from metal compounds
and
semimetal compounds which can form gels when admixed with bases.
295
To execute the process, the at least two formulations F, and F2 are each
injected
separately into the deposit through one or more injection wells. The injection
wells are
typically the same as were used in process step (1) for Injection of water or
steam.
300 The injection is undertaken in such a way that the two formulations mix in
the formation
after injection.
Formulations F, and F2
305 According to the invention, formulations F, and F2 are composed in terms
of their
components such that they form viscous gels after mixing underground under the
influence
EK11-0528CA
CA 02791134 2012-09-06
PF0000071528/PP
9
of the deposit, even at temperatures of at most 60 C, whereas the separate,
unmixed
formulations F, and F2 cannot form gels even at relatively high temperatures
(for example
50 C). The viscous gels formed after mixing block cavities in the mineral oil
formation and
310 thus block flow paths for water and/or steam. According to the invention,
the gels are
inorganic gels, especially hydroxides or oxide hydrates of metals or
semimetals.
The at least one further compound M can form gels when admixed with base. It
is selected
from metal compounds and semimetal compounds which can form gels when admixed
315 with base. These may be selected, for example, from Fe(II) and Fe(Ill)
salts, vanadium
salts, zirconium salts, aluminum(III) salts and colloidal Si compounds. In a
preferred
embodiment of the invention, the at least one further compound M is selected
from water-
soluble aluminum(III) salts.
320 The water-soluble aluminum(III) salts are preferably selected from
aluminum nitrate,
aluminum chloride, aluminum sulfate, aluminum acetate, aluminum
acetylacetonate, partly
hydrolyzed derivatives thereof and mixtures thereof. Partly hydrolyzed
derivatives of the
aluminum(III) salts mentioned above include, for example, aluminum
hydroxychloride.
325 The pH of that formulation F, and/or F2 which comprises the at least one
further compound
M selected from water-soluble aluminum(III) salts is typically s 5, preferably
<_ 4.5 and
more preferably <_ 4.
The colloidal Si compounds are preferably colloidal SiO2, which should
advantageously
330 have an average particle size of 4 nm to 300 nm. The specific surface area
of the SiO2
may, for example, be 100 to 300 m2/g.
When the formulations are mixed, the following chemical reactions take place:
335 (a) (CH2)6N4 + 10 H2O F 6 CH2O + 4 NH4OH
(b) CO(NH2)2 + CH2O -) urea-formaldehyde resin + H2O
(c) compound M (for example Aln(OH)mCl3n-m) + NH4OH 4
340 inorganic gel (for example AI(OH)3) + NH4CI
The chemical reaction between formaldehyde and urea (reaction equation (b))
causes the
equilibrium of the urotropin hydrolysis (reaction equation (a)) to shift in
the direction of
formaldehyde/ammonium hydroxide. This means that the hydrolysis level of the
urotropin
345 rises in the presence of urea and, as a result, also the amount of
ammonium hydroxide.
Ammonium hydroxide reacts with compound M (for example polyhydroxyl- chloride
EK11-0528CA
CA 02791134 2012-09-06
PF0000071528/PP
aluminum (Alustar 1010L)) and forms a gel (aluminum hydroxide) and water-
soluble salt
(ammonium chloride) (reaction equation (c)). If only urotropin and aluminum
salt are
present, and not urea, no gel formation takes place, as shown in example 1.
The same
350 applies when urea and aluminum salt are present, but urotropin is absent.
As well as water, the formulations may optionally also comprise further water-
miscible
organic solvents. Examples of such solvents comprise alcohols. In general,
formulations F,
and F2 (F) should, however, comprise at least 80% by weight of water based on
the sum of
355 all solvents in the formulation, preferably at least 90% by weight and
more preferably at
least 95% by weight. Most preferably, only water should be present.
Formulations F, and/or F2 may additionally comprise further components which
can
accelerate or slow gel formation. Examples thereof comprise further salts or
naphthenic
360 acids. In addition, formulations F, and/or F2 may also comprise thickening
additives, for
example thickening polymers.
After formulations F, and F2 have been mixed, the increase in the pH results
in formation
of high-viscosity, water-insoluble gels which comprise metal ions, hydroxide
ions and
365 possibly further components. In the case of use of aluminum compounds, an
aluminum
hydroxide or aluminum oxide hydrate gel may form, which may of course also
comprise
further components, for example the anions of the aluminum salt(s) used.
Preferably, in formulation F1, the urotropin is used in an amount of 6 to 32%
by weight,
370 preferably 15 to 25% by weight, based on the sum of all components of
formulation F,.
Likewise preferably, the urea is used in formulation F2 in an amount of 16 to
36% by
weight, preferably 20 to 30% by weight, based on the sum of all components of
formulation
F2-
375
If the at least one further compound M is added only to formulation F, or only
to
formulation F2, the concentration of the at least one compound M is typically
4 to 8% by
weight, based on the total weight of formulation F, or F2 and based on the
anhydrous
compound.
380
When the at least one compound M is present both in formulation F, and in F2,
the
concentration of the at least one compound F, in the two formulations is
typically selected
such that the total concentration of M, based on F, and F2, is 2 to 4% by
weight.
385 The concentration of urea and of urotropin should be such that a
sufficient amount of base
can form to lower the pH to such an extent that a gel can indeed precipitate
out. In the
EK11-0528CA
CA 02791134 2012-09-06
PF0000071528/PP
11
case of aluminum, the amount of urea and urotropin should therefore at least
be such that
3 mol of base are released per mole of AI(III).
390 Via the concentration of the components, it is in principle also possible
to determine the
time until gel formation after mixing, though it should be considered that the
mixing of
formulations F, and F2 in the formation is not necessarily complete, and that
a certain
inaccuracy correspondingly exists in the setting of gel formation times. The
higher the
concentration of the urea and of the urotropin, the greater - for a given
concentration of
395 the metal compound - the rate of gel formation. This relationship can be
used by the
person skilled in the art to extend or to shorten the gel formation time in a
controlled
manner.
Performance of process step (2)
400
According to the invention, the at least two formulations F, and F2 are each
injected
separately into the deposit through one or more injection wells, and the
formulations do not
mix until underground. The injection of formulations F, and F2 is generally
followed by
subsequent flooding with water in order to displace formulations F, and F2
further into the
405 deposit. In the context of the present invention, subsequent flooding
refers to the volume
of water which is injected directly after the injection of formulations F, and
F2 in order to
bring formulations F, and F2 to the desired sites underground, and to achieve
maximum
mixing of formulations F, and F2. If subsequent flooding is stopped too early,
it may be the
case that F, and F2 do not come into contact sufficiently with one another, if
at all. If
410 subsequent flooding proceeds for too long, gel formation in the mixing
zone is disrupted.
After subsequent flooding, flooding is typically stopped for 1-3 days.
Frequently, a portion of water is injected between an injection of
formulations F, and F2 or
F2 and F,. This portion of water injected between two portions of formulations
F, and F2 is
415 also referred to hereinafter as buffer water. The volume of water injected
should not be
greater here, and should preferably be less, than the volume of the
subsequently injected
portion of F, or F2. The volume of the formulation F, is abbreviated to VF1,
the volume of
the formulation F2 to VF2. In general, the volume of formulation F, or of the
portion of F,
injected in each case is less than the volume of formulation F2 or of the
injected portion of
420 F2. The volume of the buffer water injected between formulations F, and F2
or F2 and F, is
guided typically by the smaller volume. The volume of the buffer water is
preferably at
least 1 m3, especially 1 m3 to VF1, where VF1 is the volume of formulation F,
which has just
been injected beforehand. The volume of such a portion of buffer water may
especially be
40% to 100% of the injected portion comprising urotropin (formulation F,),
preferably 40 to
425 80% and more preferably 40 to 60%.
EK11-0528CA
CA 02791134 2012-09-06
PF0000071528/PP
12
The present invention also provides a process in which the viscosity of the
buffer water is
increased by adding one or more additives before injection.
430 In addition, the present invention also provides a process in which the
total volume of the
buffer water injected is less than the total volume of the formulations F, and
F2 injected.
In addition, the present invention provides a process in which subsequent
flooding is
effected using water whose viscosity has been increased by adding one or more
additives.
435
In addition, the present invention provides a process in which the densities
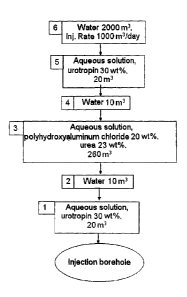
of the aqueous
formulations F, and F2, of any buffer water injected and of the water for
subsequent
flooding are balanced out using glycerol.
440 The present invention thus also provides a process in which VF1 s VF2.
In addition, the present invention thus also provides a process in which the
volume ratio
VF2: VF1 lies in the range from 10 000: 1 to 1 : 1, preferably in the range
from 1000: 1 to
1 : 1, more preferably a range from 100: 1 to 1 : 1 and especially in the
range from 10 : 1.
445
The total volume of formulations F, and F2 injected (VF1 + VF2) depends on the
geological
properties of the mineral oil deposit and may vary within wide ranges.
The total volume (VF1 + VF2) is generally in the range from 500 to 5 m3,
preferably in the
450 range from 200 to 10 m3 and especially in the range from 100 to 20 m3.
The formulations should not mix with one another until after they have flowed
through the
zone close to the injection well, in order that they actually reach the high-
permeability
zones in the mineral oil formation and do not form gels at too early a stage.
455
Use of analytical models and simulation models known to those skilled in the
art
determines the desired distance of the mixing zone from the injector. This
distance can be
regulated within a certain range by the variation of the volumes of the
portions of
formulations F, and F2, and optional injection of different volumes of buffer
water.
460
On injection of the portions of formulations F, and F2, and optionally of
buffer water, and on
subsequent flooding with water, the concentration of urotropin, urea and
compound(s) M in
the formulations decreases to an increasing extent as a result of addition of
formation
water and flooding water. The degree to which the concentration is reduced can
be
465 predicted on the basis of laboratory studies and mathematical models.
Thus, the
concentration of the chemical components before the injection of formulations
F, and F2 is
EK11-0528CA
CA 02791134 2012-09-06
PF0000071528/PP
13
adjusted such that the concentration of urotropin in the deposit zone in which
the
formulations mix is at least 1% by weight, the concentration of the
compound(s) M is at
least 5% by weight and the concentration of the urea is at least 5.75% by
weight, based on
470 the total amount of the solutions present in the mixing zone and of the
water.
Preferably in accordance with the invention, before the injection of
formulations F, and F2,
the three-dimensional position of the mixing zone of the formulation portions
in the deposit
is determined, and the volume for the subsequent flooding is determined on
this basis. In
475 most cases, the calculation of the optimal volumes of F, and F2 and the
determination of
the three-dimensional position of the mixing zone of the formulation portions
is possible
only as an approximation. Consequently, in the case of serial pumping of the
portions, the
subsequent flooding (water injected before flooding is stopped) is conducted
at low rates
(volume of flooding medium per unit time) and, after occurrence of the first
signs of an
480 increase in flooding pressure, flooding is stopped. The "mixing zone of
the formulation
portions" refers to the deposit zone in which the formulations mix. The three-
dimensional
position of the mixing zone is understood to mean the extent of the deposit
zone in which
the formulations mix, and the distance of this zone from the injection well
through which
subsequent flooding is effected. The mixing of formulations F, and F2 will
typically take
485 place not far from the injector (max. 10-15 meters), since the dilution of
F, and F2 has a
logarithmic dependence on the distance from injector. An important advantage
of the
process is that it is virtually ruled out even at low injection rates that
gelation commences
directly in the injector. Known processes in which urotropin solutions, which
gelate very
rapidly, are used do not have this advantage.
490
Formulations F, and F2 can be injected in different sequences. It is possible
first to inject a
formulation F, and then a formulation F2. It is also possible to inject at
least two portions of
formulation F, and at least two portions of formulation F2 alternately into
the deposit. It is
also possible first to inject a portion of formulation F1, then a portion of
formulation F2 and
495 subsequently a further portion of formulation F,.
The measures which follow have been found to be useful for achieving
substantially
complete mixing of formulations F, and F2-
500 Typically, the volume of the buffer solution injected is at least 1 m3 and
at most 50 m3, but
should not exceed the volume VF, where VF is the volume of formulation F, or
F2 which has
just been injected beforehand.
Preferably, the total volume of the buffer water injected is less than the
total volume of the
505 formulations F, and F2 injected.
EK11-0528CA
CA 02791134 2012-09-06
PF0000071528/PP
14
Preferably in accordance with the invention, the buffer water viscosity is
increased by
adding additives before injection into the deposit. The additives used may be
different
organic and inorganic compounds, for example water-soluble PAM
(polyacrylamide),
510 biopolymers such as xanthan, inter alia.
These additives can also be used to thicken formulations F, and F2. The
increase in
viscosity avoids rapid mixing of formulations F, and F2.
515 In a preferred embodiment, first a portion of formulation F, and then a
portion of
formulation F2 are injected. Between these portions, buffer water is injected
into the
deposit. The volume of the buffer water may vary between 1 m3 and 50 m3, but
it should
not exceed the volume of a portion of the urotropin-water solution (F,). This
is followed by
subsequent flooding with water, the volume of the water used for subsequent
flood being
520 Vn. After the injection of Vn, the two portions of formulations F, and F2
reach the zone in
which they mix to the maximum degree.
In order not to disrupt gel formation by shear stresses, the subsequent water
flooding is
stopped for 1-3 days and then restarted. This measure is suitable in
accordance with the
525 invention for all embodiments, in order to promote gel formation.
In a further preferred embodiment, three portions of formulations selected
from F, and F2
are injected into the deposit, the first and last portion being selected from
F, and a portion
of formulation F2 being injected inbetween. Between the individual portions,
buffer water
530 can be injected into the deposit.
In a further preferred embodiment, more than three portions of formulations
selected from
F, and F2 are injected alternately into the deposit, the first and last
portions of formulation
being selected from F,. Between the individual portions, buffer water can be
injected into
535 the deposit.
It is generally advantageous, after injection of formulations F1, F2 and the
subsequent
flooding water, to inject no flooding medium into the at least one injection
well for one to
three days, in order not to disrupt gel formation in the deposit. It is
especially preferred, in
540 the course of subsequent flooding, to continuously measure the pressure on
injection of
the water used for subsequent flooding and, after the pressure has risen by
about 2 to 5%,
not to inject any flooding medium into the at least one injection well for one
to three days.
The pressure rise shows the commencement of gel formation, which can then
proceed
without disruption.
545
EK11-0528CA
CA 02791134 2012-09-06
PF0000071528/PP
In a further preferred embodiment, first a formulation F, and then at least
one formulation
F2 is injected. In this embodiment, formulation F, comprises a viscosity-
increasing additive,
for example a water-soluble thickening polymer, in such an amount that the
viscosity of
formulation F, under deposit conditions is somewhat greater than that of
formulation F2
550 injected thereafter. Examples of such polymers comprise polyacrylamide,
microgels based
on polyacrylamide, or biopolymers. The slightly higher viscosity makes the
flow rate of the
first injected portion of formulation F, in the formation somewhat lower than
that of the
subsequently injected formulation F2. Formulation F2 can accordingly penetrate
particularly
well into the flowing front of formulation F, and mix therewith. In general,
the viscosity of
555 the injected formulation F, should not be more than 30%, for example 10%
to 30%, higher
than the viscosity of formulation F2 injected later.
In a further preferred embodiment, the portions of aqueous formulations F, and
F2, buffer
water and subsequent flooding water (water for displacement of formulations F,
and F2)
560 are prepared with the same density. This prevents flooding portions having
different
densities from taking different routes when pumped into the productive
geological stratum
and not coming into proper contact with one another. When, for example, the
urotropin
solution (10% by weight) is prepared using fresh water, the solution density
is 1.03 g/cm3,
the density of the urea solution with aluminum salt being 1.10 g/cm3. When the
buffer
565 water, for example, has a higher density (formation water density up to
1.2-1.3 g/cm3) than
formulations F, and F2, temporal separation of the portions of formulations F,
and F2 in the
productive geological stratum is more difficult. This is also true of the
subsequent flooding
water. The density of the flooding portions is balanced out by addition of
salts, preferably
selected from NaCl and CaC12, salt-containing formation water (accompanying
water), or
570 glycerol. It is possible to use crude glycerol, which is a by-product in
biodiesel production
and is readily soluble in water. Salt-containing formation water can also be
used for
production of formulations F, and F2.
In the production of biodiesel from rapeseed oil, the by-product obtained is
glycerol (crude
575 glycerol). For instance, 10 I of oil and 1 1 of methanol with addition of
reagents give about
10 I of biodiesel and 1 I of glycerol. Glycerol (C3H5(OH)3) is a trihydric
alcohol. The slightly
viscous, water-soluble, odorless liquid comprises approx. 85 to 90% dry mass.
Depending
on the water content and temperature, the density of crude glycerol is 1.1 to
1.3 g/cm3.
580
Data and properties of crude glycerol:
glycerol content 80 - 82%
water content 10 - 15%
585 NaCl 5 - 7%
EK11-0528CA
CA 02791134 2012-09-06
PF0000071528/PP
16
methanol 0.01 - 0.5%
density 1.23 - 1.27 g/cm3 at 20 C
ecological information - biodegradable.
590 Crude glycerol is miscible with urotropin solution and with urea solution.
As mentioned above, the density of formulations F, and F2, of the buffer water
and of the
subsequent flooding water can also be modified by addition of salt. For
example, for an
aqueous solution with 24% by weight of NaCl the density of the solution is
1.184 g/l, and
595 for an aqueous solution of 20% by weight of CaC12 the density of the
solution is 1.179 g/l.
Typically, injection of the last portion of formulation, which may be selected
from F, and F2,
is followed by subsequent flooding with water before performance of step (3).
The water
used here may have increased viscosity by virtue of addition of one or more
additives.
600 Suitable additives are the compounds already mentioned above for this
purpose for
formulations F1, F2 and the buffer water.
Process step (3)
605 After process step (2), oil production is continued in process step (3)
through at least one
production well. This can be done immediately thereafter, or else optionally
after a short
break, for example a break of 1 to 3 days.
Preferably, the oil production can be effected by customary methods, by
injection of one or
610 more flooding media through at least one injection well into the mineral
oil deposit, and
withdrawal of crude oil through at least one production well. The flooding
medium may
especially be selected from the flooding media listed as suitable for step
(1). Preference is
given to using water and/or water comprising additives as the flooding medium.
The at
least one injection well may be the injection wells already used for injection
of formulations
615 F, and F2, or else other injection wells in suitable arrangement.
The oil production can, however, of course also be continued by means of other
methods
known to those skilled in the art. For example, the flooding media used may
also be
viscous solutions of silicate-containing products or thickening polymers.
These may be
620 synthetic polymers, for example copolymers comprising polyacrylamide or
acrylamide. In
addition, they may also be biopolymers, for example particular
polysaccharides.
It is of course possible, after process step (3), to perform process steps (2)
and (3) once
again. This can be done at regular intervals, for example once per year or as
soon as
625 water breakthrough is registered. More particularly, step (2) is repeated
when critical
EK11-0528CA
CA 02791134 2012-09-06
PF0000071528/PP
17
watering out of production is attained in the mineral oil production in step
(3). This stage is
typically reached when watering out of production is more than 70-90%.
According to the invention, "critical watering out of production" is
understood to mean a
630 water content of the mineral oil withdrawn from the at least one
production well of more
than 70% by weight, preferably more than 90% by weight, based in each case on
the total
weight of the mineral oil withdrawn from the at least one production well.
As already explained above, the term "mineral oil" is understood to mean not
single-phase
635 oil but typically an emulsion composed of oil and formation water.
Critical watering out of
production has occurred when the mineral oil produced comprises more than 70%
by
weight, preferably more than 90% by weight, of formation water, based in each
case on
the total weight of the emulsion withdrawn from the at least one production
well.
640 Advantages
The process according to the invention for oil production has the following
advantages
compared to known technologies:
645 = It is possible even in a deposit with a temperature of at most 60 C to
reduce the
permeability of the high-permeability zones.
= It is possible to operatively block the high-permeability channels in the
deposit on
water breakthrough, even at a relatively great distance from the injection
well.
650
= The process according to the invention prevents gel formation in the
injection well
and thus increases reliability in oil production.
= The process according to the invention is inexpensive, does not need any new
655 chemical products for the implementation, is based on the use of
conventional
technical means, and allows efficient profile modification even in cold or
cooled
carriers/deposits.
The invention is illustrated in detail hereinafter by the working examples
which follow:
660
Example I
EK11-0528CA
CA 02791134 2012-09-06
=
PF0000071528/PP
18
For one example, the rheological properties of formulations F1, F2 and of the
mixture of
665 formulations F, and F2 were determined. The samples were equilibrated at
20 C and at
50 C in a heating cabinet. The concentration figures are each based on the
overall
formulation/mixture. The AIC13 was used in the form of a solution of
polyaluminum chloride
in water which is available under the Alustar 1010 product name from Applied
Chemicals.
The results are shown in table 2.
670
Table 2:
Com- % by Viscosity Temperature
ponents wt. ri before
equilibra- 20 C 50 C
tion
[mPa.s] Gel n tgel -1
formation [mPa.s] [days] [mPa.s]
time tgel
[days]
F1 urotropin 16.0 1.3 100 no gel 27 no gel
water 84.0 formation formation
F2 urea 32.0 2.5 100 no gel 27 no gel
AIC13 8.0 formation formation
water 60.0
ca
urea 16.0
o AICI3 4.0 2.0 3 6960 1 4980
N O
LL- urotropin 8.0
water 72.0
Example 2: Employment of the process in an oil field
675
The deposit is a typical deposit containing mineral oil having a viscosity of
150-180 cP. A
section of the deposit is provided with an injection well and several
production wells and is
EK11-0528CA
CA 02791134 2012-09-06
PF0000071528/PP
19
flooded with water for several years. In some production wells which
communicate with the
injection well, watering out of production is registered. The deposit is
fissured by geological
680 faults and has inhomogeneous permeability.
Pumping scheme
In order to conduct the profile modification and to block the high-
permeability zones in the
685 oil-bearing stratum, a first portion of formulation F, (30% by weight of
urotropin in water) is
made up in a vessel above ground. It is possible to use fresh water, salt
water or formation
water. Using customary equipment, 20 m3 of formulation F, are injected and
pressed into
the deposit through the injection well. The first portion of formulation F,
has a low viscosity
and flows predominantly through the high-permeability regions of the deposit.
690
Subsequently, 10 m3 of water are injected into the deposit. The injection of
the 10 m3 of
water mobilizes the first portion of formulation F, and forces it from the
injection well into
the mineral oil deposit.
695 After the water, 260 m3 of a formulation F2 (20% by weight of
aluminum(III) chloride
(ALUSTAR ) and 23% by weight of urea in water) are injected through the
injection well.
Subsequently, approx. 10 m3 of water are injected and, immediately thereafter,
another 20
m3 of the abovementioned formulation F,. Thus, a total of 260 m3 of F2 and 40
m3 of F, are
pumped in.
700
The sequence of the individual injections is shown schematically in figure 1.
As a result of these measures, the mixing of formulations F, and F2 forms at
least one
"bank" composed of formulations F, and F2 and displacing water in the oil-
bearing stratum.
705 In the mixed formulations F, and F2, the chemical reactions shown above
take place and
lead to gel formation. On displacement of the formulation banks, there is
firstly retention
(adsorption) of urotropin, urea and aluminum salt in the rock, and secondly
dilution, which
leads to a reduction in concentration of the gel-forming substances in the
formulation
banks.
710
Calculation/simulation of gel formation
The simulation was conducted using the pumping scheme described above.
715 The formulae and input data
EK11-0528CA
CA 02791134 2012-09-06
PF0000071528/PP
The dynamic concentration of the aqueous solution in a portion F, or F2 can be
described
by the following formula:
720 K(L) = A, x L x e (-A2 x L)
(1)
L: distance between the front of the solution (a portion) and the injector;
A, and A2: variables which define the nature of the function.
725 In the analytical calculation, the following input parameters were used-
- flooding rate: 1000 m3/day;
- porosity of the deposit stratum: 30%
- max. permissible dilution of formulation F2 in the productive layer: 25%
(four
730 times less than the original concentration);
- minimum concentration after max. permissible dilution of the urotropin
solution F,=
3.3% (original concentration 30%);
- effective thickness of the productive stratum: 15 m;
- volume of the first portion (F1, urotropin): 20 m3;
735 - volume of the second portion (F2, urea and AICI3): 260 m3;
- volume of the third portion (F,, urotropin): 20 m3;
- volume of the buffer water portions (injected in each case between portions
F1, F2
and F, again): 10 m3;
linear coefficient which defines the widening of the zone filled with aqueous
740 solution (portion X of F1, F2): kdiff = 0.1
The widening of the zone filled with aqueous solution (portion X) can be
defined in the
simplest case by the following formula:
745 W= kdiff x L= 0., x L
Calculations and graphs
According to the calculations, the optimal period of subsequent flooding with
water in the
750 present case is 2 days, which corresponds to a volume of water of 2000 m3
at injection
rates of 1000 m3/day. In the case of homogeneous permeability of the flooded
deposit
stratum (ideal case), a ring-like gel bank of width 1 m is formed at a
distance of 11 to 12 m
from the injection well. The proportion of the urea-AICI3 solution mixed with
urotropin (F,) is
89% of the total volume of F2 injected.
755
EK11-0528CA
CA 02791134 2012-09-06
PF0000071528/PP
21
The dependence of the degree of mixing of portions F, and F2 on the period of
subsequent
flooding is defined by the proportion of formulation F2 mixed with F,
(urotropin solution) in
the total volume F2 injected. The limiting parameter is the degree of dilution
of the urea-
AIC13 solution in the mixing zone. The upper limit is the fourfold dilution of
the urea-AIC13
760 solution. According to calculations, the maximum degree of mixing of the
three portions of
aqueous formulations is attained two days after commencement of subsequent
flooding
with water. Even during subsequent flooding with water, the concentration of
urea and
AIC13 in the optimal mixing zone still exceeds the minimum concentration for
gel formation;
therefore, gel formation in the oil-bearing stratum is guaranteed.
765
The reduction in the ring width caused by the radial expansion of the aqueous
formulations
is partly compensated for by the increase in volume of the formulations due to
dilution.
By means of the volumes of the injected portions of formulations F, and F2,
and of the
amount of water in the subsequent flooding, the distance between the injection
well and
770 the gel bank can be regulated.
When gelation commences, the injection pressure also rises. After commencement
of the
rise in injection pressure, the water flooding is stopped for one to three
days. Thereafter,
water flooding is restarted. After high-permeability regions have been
blocked, new flow
775 paths form in further regions of the oil-bearing stratum under the
influence of the flooding
medium, and thus further mineral oil is produced from the formation.
Example 3:
780 Laboratory studies - Core flooding test
The process according to the invention was tested by means of a model test.
For this
purpose, loose deposit material of the oil-bearing stratum of a mineral oil
deposit in North-
Western Germany was pressed in a tube.
785
In the first model test, the permeability of the material was 0.443 darcy
(0.443"10-12 m2).
The filled tube was provided at the ends with devices for injection and
withdrawal of liquids
and was heated to 40 C by means of a heater. As the first step, fresh water
was injected
into the tube and withdrawn at the other end, in an amount of 4 times the pore
volume.
790 Thereafter, a formulation F, was injected (solution of 30% by weight of
urotropin in water,
amount injected corresponds to 0.017 times the pore volume), then a portion of
water
(0.017 times the pore volume), then a formulation F2 (mixture of 20% by weight
of an
aqueous solution of polyaluminum chloride (Aln(OH)mCl3n-m, Al content 9.15% by
weight,
pH < 1, ALUSTAR 1010 L (from Applied Chemicals)) and 23% by weight aqueous
795 solution of urea, remainder : water, amount injected corresponds to 0.243
times the pore
EK11-0528CA
CA 02791134 2012-09-06
PF0000071528/PP
22
volume), then another portion of water (0.017 times the pore volume) and
another portion
of formulation F, (0.017 times the pore volume). After a break for 18 hours at
a constant
temperature of 40 C, water flooding was continued.
800 The results of the test are shown in figure 2. Figure 2 shows the volume
injected, based on
the pore volume of the sample (deposit material), and, as a function thereof,
the mobility
and the pressure gradient. During the water flooding, at the start, the
pressure gradient is
at first low. However, it rises steeply from 0.07 bar/m (0.07*1 05 Pa/m) to
from 20 to 43
bar/m (20 to 43*105 Pa/m) once formulations F, and F2 have each been fully
injected.
805 Thus, the aims of "conformance control" have been achieved.
Example 4:
In the second model test, the permeability of the material was 1.415 darcies
810 (1.415*10-12 m2). The same amounts of F1, F2 and water portions with the
same
concentrations as described above were injected. The results of the test are
shown in
figure 3, analogously to figure 2. The serial injection of formulations F, and
F2 causes the
pressure gradient to rise from 0.05 to from 10 bar/m to 27 bar/m (10 to 27*105
Pa/m) once
formulations F, and F2 have each been fully injected and have mixed in the
core. Thus, the
815 aims of "conformance control" have been achieved.
E K 11-0528CA