Note: Descriptions are shown in the official language in which they were submitted.
CA 02792401 2012-09-06
WO 2011/114000 PCT/F12011/050222
1
METHOD OF PROCESSING NICKEL BEARING RAW MATERIAL
FIELD OF THE INVENTION
The invention relates to a hydrometallurgical method of processing nickel
containing raw material such as sulphidic nickel concentrate or sulphidic
nickel ore where the raw material is leached in chloride-based leaching
media in a process integrated with solvent extraction and electrowinning
process stages for obtaining metallic nickel. The solvent extraction stage
comprises a nickel solvent extraction stage, where nickel is extracted from
io an aqueous solution containing high concentration of chloride.
BACKGROUND OF THE INVENTION
The world nickel resources are divided into two major categories, sulphide
ore and oxidized ore (laterite ore). The conventional exploitation of nickel
is sulphide ore essentially comprises a pyrometallurgical process step
followed
by hydrometallurgical process steps, where the ore is first finely ground, and
then the nickel sulphide minerals are concentrated by froth flotation into a
nickel concentrate. The concentrate is treated further by smelting and
reduction to produce a nickel bearing matte, which also contains copper,
20 cobalt, and iron. The matte is then refined by known hydrometallurgical
processes, which might include oxidative leaching or pressure leaching,
followed by impurity removal and hydrogen reduction or electrowinning.
A drawback of the smelting process is the generation of sulphur dioxide,
25 which has to be treated in an acid plant to produce sulphuric acid, a
product
that is not always easy to dispose of from the smelter location. Losses of
nickel and cobalt into smelter slag are significant, and there can be problems
in dealing with some of the minor elements in concentrates, such as
magnesium and arsenic.
Lean ores set challenges in the concentration process. Poor quality
concentrates are difficult and expensive to refine in pyrometallurgical
CA 02792401 2012-09-06
WO 2011/114000 PCT/F12011/050222
2
processes. Pyrometallurgical treatment of such concentrates is especially
difficult, when the magnesium content in the concentrate is high and
respectively the iron content is low. In such a case, the iron/magnesium ratio
of the concentrate becomes low causing further difficulties. For example
when the MgO content of the slag created in the smelting process is over
11%, the viscosity of the slag rises so high that it disturbs the removal of
the
slag from the furnace. As the viscosity rises part of the nickel matte remain
in
the slag. In arid areas, saline water must be used for wet concentration, in
which case the concentrate contains halides that are hazardous in
io pyrometallurgical processes.
A large number of hydrometallurgical routes for processing nickel sulphide
concentrates are disclosed in literature. In general the prior art processes
include grinding or fine grinding of the concentrate, where after the sulphide
is is treated in oxidative pressure leaching for processing sulphuric acid
for the
leach process.
The Activox process that is described for example in the EP patent
1 303 641 comprises the grinding of nickel concentrate into a very fine
20 ground material, where after it is subjected to oxidative leaching at a
high
pressure for separating nickel into the sulphate solution, and subsequently
impurities are removed by known methods, and metallic nickel is recovered.
A drawback of the above described hydrometallurgical processes is that a
25 large part of the sulphur contained in the sulphide is oxidized into
sulphuric
acid, which results in high expenses caused by neutralization reagents, and
the creation of large waste quantities that must be removed, such as
ammonium sulphate and gypsum. It can be estimated that the high expenses
resulting from these two factors when combined make said processes
30 commercially less attractive.
CA 02792401 2012-09-06
WO 2011/114000 PCT/F12011/050222
3
The WO patent application 96/41029, "Chloride assisted hydrometallurgical
extraction of nickel and cobalt from sulphide ores", describes oxidative
pressure leaching of nickel and cobalt sulphide ores in the presence of
oxygen and likewise an acidic leaching containing halide, copper and
sulphate ions. The obtained solution is subjected to solids separation and
solution purification, precipitation of the mixed nickel and cobalt hydroxide,
re-leaching of the precipitate in an ammoniacal solution, where after the
metals are separated by solvent extraction and recovered by electrowinning.
The process suffers similar limitations as the sulphate-based
io hydrometallurgical processes described above.
US patent 3,880,653 describes the recovery of metallic nickel from a nickel
matte containing copper and precious metals. The leaching of the nickel
matte is realized as a concurrent process, where the nickel matte is first
is suspended to a chloride solution obtained from nickel electrowinning and
containing monovalent copper. The chloride solution is conducted to a
leaching step, where also chlorine created in electrolysis is fed. Chlorine
oxidizes the monovalent copper, which in turn dissolves the nickel and is at
the same time reduced back to monovalent form and precipitated as copper
20 sulphide. The sulphur contained in the dissolving sulphides is
precipitated as
element sulphur. The precious metals remain undissolved in the leaching.
After the first leaching step, the whole batch of slurry is conducted to a
second step, where the dissolved divalent copper is precipitated by means of
nickel matte. The solution and the solid material are separated, and the
25 solution is conducted to nickel electrowinning.
JP patent application 10-140257 describes the recovery of nickel by means
of chlorine leaching and electrolysis from materials, such as nickel matte,
containing nickel, cobalt, copper and sulphur. Nickel matte is leached in
30 concurrent leaching into a chloride solution that contains monovalent
copper,
and chlorine is supplied into the solution for leaching nickel and other
metals.
When chlorine is supplied into the first steps of the leaching process, the
CA 02792401 2012-09-06
WO 2011/114000 PCT/F12011/050222
4
sulphur contained in the nickel matte also is partly dissolved and forms
sulphuric acid in the solution. In the final step of the leaching process,
there
is no more supplied chlorine but air, which means that in the final step, the
leaching is carried out by means of the oxygen contained in the air and
sulphuric acid. The nickel chloride bearing solution is conducted to
electrolysis for recovering the metallic nickel, and the chlorine created in
the
electrolysis is used for leaching the raw material. Also the recycled solution
obtained from electrolysis is used for leaching the raw material.
io Among the methods described above, the latter two deal with nickel
mattes
that are produced by first processing the nickel concentrate
pyrometallurgically. The large quantity of sulphur dioxide created in the
process, which sulphur dioxide is generally processed further into sulphuric
acid, can be regarded as a drawback in the pyrometallurgical treatment. The
is use and commercial marketing of sulphuric acid is difficult,
particularly when
the smelter location is far from the location where sulphuric acid should be
used.
WO publication W02007/039665 discloses a method of producing a nickel
20 product from nickel-bearing sulphide raw materials, such as nickel
sulphide
concentrate or ore or scrap. In the method, the raw material is leached in
atmospheric conditions to an aqueous solution of sodium chloride and
copper (II) chloride. Chlorine, hydrogen and sodium hydroxide needed in the
process are produced in a chlorine-alkali electrolysis cell that is integrated
as
25 a process stage in the overall process. The nickel-enriched pregnant
leach
solution is subjected to a precipitation of dissolved iron and sulphates, and
the precipitate is fed into the final step of the leaching process. Nickel is
precipitated as nickel hydroxide Ni(OH)2 from the pregnant leach solution by
means of sodium hydroxide.
WO publication W02007/039664 discloses a method of recovering nickel
from a nickel sulphide containing material including the steps of: providing a
CA 02792401 2012-09-06
WO 2011/114000 PCT/F12011/050222
nickel sulphide containing material; oxidative leaching of the nickel sulphide
containing material with a sodium chloride leach solution containing cupric
chloride and hydrochloric acid in an oxidising atmosphere at atmospheric
pressure and temperature to form a pregnant leach solution containing
5 dissolved nickel; treating the pregnant leach solution containing
dissolved
nickel to separate copper and recycle it at least partly back to the leaching
stage (b); purification of the nickel pregnant leach solution by the use of
solvent extraction to remove cobalt, zinc and residual copper; recovering
nickel from the pregnant leach solution to form a nickel depleted leach
io solution; and electrolytically treating the depleted leach solution in
chlorine
alkali electrolysis to recover chlorine, hydrogen, and sodium hydroxide.
W02007/039664 does not refer to a possibility of recovering nickel from a
sulphate solution in electrowinning after nickel solvent extraction stage.
is A cobalt solvent extraction from chloride environment is known where
cobalt
is extracted from very high chloride environment using an anionic extractant
to extract a cobalt-chloride complex. Also a cationic extractant for cobalt
extraction from chloride containing sulphate solutions is known. In the known
applications the chloride concentrations relatively low.
OBJECT OF THE INVENTION
The object of the present invention is to overcome disadvantages of the prior
art processes and to provide a new and advantageous hydrometallurgical
process for producing nickel metal.
The invention provides method where the advantages of atmospheric
leaching are utilized and recycling and regeneration of leaching chemicals
are arranged through chlorine-alkali electrolysis. The method is particularly
suitable for recovering metals from poor nickel concentrates and ores. The
recycling of reagents within the process makes the method particularly
advantageous.
CA 02792401 2012-09-06
WO 2011/114000 PCT/F12011/050222
6
A special advantage of the present invention is that it provides a
hydrometallurgical method for recovering metallic nickel where the metallic
nickel is won on a cathode of an electrowinning cell from a sulphate
electrolyte.
Further, the present invention provides a method of handling and recovering
impurities of the raw material. Such impurities are for example magnesium
and halides which, when treated according to the present invention, can be
treated without disturbing the recovery of nickel.
SUMMARY OF THE INVENTION
The invention relates to a method for producing nickel metal from nickel-
bearing sulphide raw materials, such as nickel sulphide concentrate or ore or
scrap.
Nickel bearing feed material such as nickel sulphide concentrate or ore or
scrap, particularly poor nickel concentrates and ores can be successfully
treated in according to the method of the present invention. Even low-grade
nickel feed materials (Ni < 1-10%) can be processed and number of
impurities, such as cobalt, iron, magnesium, zinc, copper and arsenic, can be
treated without disturbing the recovery of nickel. Generally nickel sulphide
bearing raw material always contains a certain amount of copper, iron, cobalt
and magnesium
A method according to the present invention comprises the steps of:
(a) providing a nickel sulphide containing material;
(b) leaching the nickel sulphide containing material with a chloride
leach solution at atmospheric pressure in a leaching stage to form
a pregnant leach solution containing dissolved nickel and a
concentration of chloride;
CA 02792401 2012-09-06
WO 2011/114000 PCT/F12011/050222
7
(C)
extracting the dissolved nickel from the leach solution with solvent
extraction to produce a nickel sulphate containing electrolyte;
(d) recovering nickel from the electrolyte at a nickel electrowinning
stage;
(e) regenerating
depleted chloride containing process solutions from
process steps c) to d) in chlorine-alkali electrolysis stage to
recover chlorine, hydrogen and sodium hydroxide back to the
process.
io
The leaching of the nickel raw material is carried out in chloride-based
aqueous solution. The leaching step preformed in the leaching stage
produces a process liquid containing very high amount of chlorides. In a
minor metal removal stage cobalt and other minor metals are separated from
is the very high chloride content solution in a solvent extraction
process using a
cationic extractant. The raffinate from the minor metal removal stage (MM
SX) is fed to a nickel solvent extraction stage (Ni SX) where nickel is
selectively extracted from a very high chloride solution and stripped to a
sulphate liquid to form a nickel sulphate electrolyte. The nickel electrolyte
is
20 conducted to a nickel electrowinning stage. The reagents, such as
chlorine,
hydrogen and sodium hydroxide, needed in the leaching stage and in other
processing steps, are obtained from chlorine-alkali electrolysis that is
integrated as a step in the process.
25 According to the method, the raw material is preferably leached in
atmospheric conditions to an aqueous solution containing sodium chloride
and copper (II) chloride. The pregnant leach solution (PLS) from the leaching
stage is lead to solvent extraction stages. The chloride content of the PLS is
over 100 g/I.
According to one embodiment of the invention the production method of a
nickel product contains the following steps:
CA 02792401 2012-09-06
WO 2011/114000 PCT/F12011/050222
8
a) nickel bearing raw material is leached in two or more steps by a
solution containing sodium chloride and copper (II) chloride as a
countercurrent leach in atmospheric conditions, so that the first
leaching step when observed in the proceeding direction of the
concentrate is non-oxidative, and the next steps are oxidative with
respect to forming a nickel enriched nickel chloride - sodium chloride
pregnant leach solution (PLS) and leach residue,
b) the nickel-enriched pregnant leach solution is subjected to a liquid
purification for a precipitation of dissolved iron and sulphates, and the
precipitate is fed into the final step of the leaching process,
c) cobalt and other minor metals (such as zinc and copper) are
separated from the very high chloride containing liquid with cationic
extractants in a cobalt solvent extraction unit. The cobalt depleted
raffinate is conducted to a nickel solvent extraction (Ni SX) unit,
d) cobalt is recovered from cobalt solvent extraction unit in an aqueous
solution and precipitated,
e) nickel is extracted from the cobalt solvent extraction raffinate in a
nickel solvent extraction unit with cationic extractants,
f) nickel sulphate electrolyte from the nickel solvent extraction stage is
conducted to a nickel electrowinning plant, where nickel is won to
cathodes,
g) the sodium chloride solution, depleted in nickel, is conducted to
magnesium precipitation, where magnesium is precipitated from the
solution, by means of sodium hydroxide, as magnesium hydroxide
Mg(01-1)2,
h) other divalent dissolved impurity residues are removed from the NaCI
solution by means of ion exchange,
i) the NaCI solution is subjected to concentration,
j) the concentrated NaCI solution is conducted to chlorine-alkali
electrolysis, where part of the solution is by electrolysis processed into
chlorine, hydrogen and sodium hydroxide that are used as reagents in
the method,
CA 02792401 2012-09-06
WO 2011/114000 PCT/F12011/050222
9
k) the NaCI solution that was depleted in the electrolysis is fed to the
final leaching step of the concentrate and/or ore.
Preferably the leaching of nickel sulphide bearing concentrate and/or ore is
carried out in two steps.
In the first leaching step, raw material is leached in non-oxidative
conditions
by means of copper (II) chloride, so that part of the sulphides contained in
the concentrate are dissolved, and copper is precipitated as copper sulphide.
io In the non-oxidative leaching step of raw material, the pH is within the
range
0.5 ¨ 3Ø
In the oxidative leaching steps of raw material, the raw material is
advantageously leached by means of copper (II) chloride for dissolving
is sulphides, and in the first leaching step the precipitated copper
sulphide is
made to be dissolved into copper (II) chloride. In the oxidative leaching
steps, the pH is adjusted within the range 1.7 ¨ 2.8, preferably within the
range 2.0 ¨ 2.5.
20 According to one embodiment of the method, the oxidative reagent
employed
in the oxidative leaching steps of concentrate and/or ore is oxygen-bearing
gas, which is oxygen, oxygen-enriched air or air, and hydrochloric acid is fed
into the leaching process.
25 According to another embodiment of the method, the oxidative reagent
employed in the oxidative leaching steps of concentrate and/or ore is
chlorine formed in chlorine-alkali electrolysis.
According to one embodiment of the method, into a nickel-enriched pregnant
30 leach solution there is fed a calcium compound and sodium hydroxide for
removing the dissolved sulphates and iron. The created iron/gypsum
precipitate is conducted to the final leaching step.
CA 02792401 2012-09-06
WO 2011/114000 PCT/F12011/050222
According to a preferred embodiment of the method, cobalt is removed from
the pregnant leach solution by a cationic solvent extraction reagent, the
raffinate from the cobalt extraction is fed to a nickel solvent extraction,
where
s nickel is extracted by a cationic organic extractant and sulphate based
nickel
solution suitable as an electrolyte for nickel electrowinning stage is
produced.
The nickel electrolyte from the nickel solvent extraction plant is advanced to
a nickel electrowinning plant, where nickel is won to cathodes in a
conventional manner.
Depending on the impurity levels in the nickel electrolyte and Ni EW
electrolyte quality requirements further purification of the nickel
electrolyte
can be done for example with an ionic exchange process, where small
amounts for example copper, cadmium, iron and zinc can be removed from
is the produced advance electrolyte.
According to a preferred embodiment of the method, the nickel depleted
NaCI solution, the raffinate from the nickel solvent extraction, which has
become poor in nickel, is subjected to Mg precipitation in order to remove
dissolved magnesium prior to the electrowinning stage. The magnesium
precipitation is advantageously carried out at the pH value 9-10.
The hydrochloric acid that is used as the leach reagent of raw material is
advantageously made of the hydrogen and chlorine created in the chlorine-
alkali electrolysis.
A nickel bearing raw material may contain gold and/or other precious metals
(PGM). In that case the gold is dissolved in the final leaching step and
recovered from the solution of the final leaching step; the gold-free solution
is
conducted to the preceding leach step, when observed in the proceeding
direction of the concentrate. Other precious metals are recovered from the
leach residue.
CA 02792401 2012-09-06
WO 2011/114000 PCT/F12011/050222
11
According to one embodiment of the method, part of the depleted solution
created in the concentration of sodium chloride is conducted to a concentrate
leaching process.
LIST OF DRAWINGS
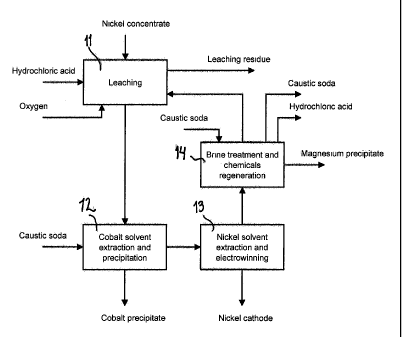
Figure 1 represents a schematic flow sheet of one preferred embodiment of
the invention, and
figure 2 represents a schematic drawing of another embodiment of the
invention.
DETAILED DESCRIPTION OF THE INVENTION
In this context, the concept of nickel sulphide bearing raw material means
mainly nickel sulphide concentrate or ore but it can also be scrap type or
is matte. For the sake of simplicity, the text only mentions concentrate.
Each
leaching step typically consists of a series of several reactors, where the
suspension of solution and solids is transferred as overflow from one reactor
to the next. The reactors are provided with agitators. In between the process
steps, there is performed thickening, so that the solution and the solids are
transferred to different steps in the countercurrent leaching. The description
of the invention describes a two-step leaching process, but it may turn out to
be necessary to apply several steps. The notion of atmospheric conditions
means that the operations are carried out at the pressure of the environment
and at a temperature, which is within the range of 90 C ¨ the boiling point
of
the solution ¨ i.e. roughly 110 C. The alkaline chloride can be sodium or
potassium chloride.
A method according to the present invention is described below with
reference to figures.
CA 02792401 2012-09-06
WO 2011/114000 PCT/F12011/050222
12
The leaching of nickel sulphide bearing raw material (nickel concentrate in
Figs. 1 and 2) is carried out in countercurrent leaching 11, 21 in two or more
steps.
In one embodiment of the invention the raw material is fed into the first
leaching step, and the chloride solutions and oxidative reagents used in the
leaching process are fed into the second step. In the first leaching step, the
conditions are adjusted such that part of the nickel and iron contained in the
raw material are dissolved, owing to the influence of divalent copper, and the
monovalent copper created in the leaching process is precipitated as copper
sulphide. Neither oxygen nor other oxidant is fed into the first leaching step
for dissolving copper, but the conditions in the first leaching step are non-
oxidative. The leaching takes place in the pH range 0.5 ¨ 3.0, depending on
the raw material. The principal reaction that takes place in this step can be
illustrated by the leaching reaction of pentlandite:
2(Ni,Fe)9S8 + 18 CuCl2 9 NiCl2 + 9 FeCl2 + 9Cu2Ssi. + 7 S (1)
The reaction (1) can be described as an exchange reaction where divalent
copper is reduced to monovalent and at the same time dissolves the iron and
nickel contained in the concentrate. Other nickel and iron minerals, such as
violarite, millerite and pyrrhotite, are also dissolved according to the same
principle, and respectively copper sulphide and element sulphur are
precipitated. The magnesium contained in the nickel raw material is
dissolved, thus forming magnesium chloride.
The copper contained in the raw material does not dissolve in the first
leaching step conditions, but only in the second step, or if there are several
leaching steps, only in the last step. If the copper content of the raw
material
is not sufficient for efficient leaching, more copper is brought to the
leaching
process in some suitable way, for instance in the form of copper concentrate
or copper sulphate. Advantageously the copper content in the solution is of
CA 02792401 2012-09-06
WO 2011/114000 PCT/F12011/050222
13
the order 5-50 g/I. By adjusting the conditions to be suitable in the way
described above, nearly all of the copper is precipitated in the first step as
sulphide and is conducted, along with the precipitate, to the next leaching
step. It is an advantage of the method that there is not needed a separate
precipitation step for separating copper from the nickel-enriched solution,
but
precipitation is carried out in connection with the leaching of nickel. If the
quantity of the copper contained in the concentrate is more than what is
needed in the leaching process, part of the copper can be removed in a way
suitable for the purpose.
The nickel-enriched NiC12-NaCI solution, PLS, obtained from the leaching
step 11, 21, contains an amount of dissolved iron and sulphates. The
removal of iron and sulphates is carried out in a solution purification step
22.
Sulphates are advantageously precipitated by means of a calcium
is compound, such as limestone, or other calcium compound, so that when
calculated as sulphur, their content left in the solution is at the most 2
g/I. In
order to oxidize iron into trivalent form, it is advantageous to feed lye
(NaOH)
in the solution, in which case iron is precipitated from the solution. The
precipitate can be fed back to the final step of the concentrate leaching
process, from where iron precipitate and gypsum are removed along with the
leach residue.
In case the copper content of the nickel concentrate is higher than what is
needed in the leaching process, the nickel-enriched NaCI solution still
contains divalent copper. The copper can be removed in a separate copper
recovery step, which is advantageously cobalt solvent extraction 12, 23 or a
minor metal removal 23 according to the present invention.
Minor Metals Solvent Extraction (MM SX)
Feed solution from solution purification stage is fed to the minor metals
solvent extraction process 12 ,23 (MM SX). In the MM SX process minor
CA 02792401 2012-09-06
WO 2011/114000 PCT/F12011/050222
14
impurity metals like Co, Cu, Zn and Mn are extracted from the feed solution
with a cationic extractant in a very high chloride environment. Multiple
extraction stages are used.
Before the extraction stages a separate organic and/or crud removal circuit
can be installed if needed. pH control can be done in the mixer-settlers or in
the organic tank (pre-neutralization) or a combination of these neutralization
systems can be used.
io Loaded organic from the extraction stages can be scrubbed (chemical
impurity removal) or washed (physical impurity removal) from the entrained
nickel, chloride or some other impurity component. Need for the scrubbing
and/or washing stages is determined by the feed quality to the MM SX
process and the desired product quality from the MM SX process. One or
is more scrubbing/washing stages can be used.
Loaded organic from the extraction or scrubbing and/or washing stages is
fed to the stripping stages, where metals from the organic phase are stripped
with an acidic solution to the aqueous phase. Metals from the organic phase
20 can be stripped in one or more solutions. Selective strip, where two or
more
stripping solutions are produced, are normally preferred solution, because
selective strip increases the stripping liquid qualities. However, metals from
a
single strip solution can also be separated after the MM SX process for
example with a selective precipitation process as carbonates, hydroxides or
25 sulphides. One or more stripping stages can be used.
Nickel Solvent Extraction
Nickel solvent extraction (Ni SX) feed solution from the MM SX process, MM
30 SX raffinate, is pumped to the nickel solvent extraction process 13, 24.
Before solution from the MM SX is fed to the Ni SX extraction stages some
organic entrainment removal processes and equipment must be installed
CA 02792401 2012-09-06
WO 2011/114000 PCT/F12011/050222
between MM SX and Ni SX. This equipment usually includes diluent wash,
after settler, carbon filters and storage tanks. All or any combination of
this
equipment can be used.
5 After the organic removal Ni SX feed solution is fed to the nickel
solvent
extraction stages. In the nickel solvent extraction nickel is extracted
selectively from the feed stream with a cationic extractant in a very high
chloride environment. Multiple extraction stages are used. pH control can be
done in the mixer-settlers or in the organic tank (pre-neutralization) or a
io combination of these neutralization systems can be used.
Loaded organic from the extraction stages can be scrubbed (chemical
impurity removal) or washed (physical impurity removal) from the entrained
impurities like chloride, magnesium and calcium. One or more scrubbing
is and/or washing stages are used.
Loaded organic from the scrubbing and/or washing stages is fed to the
stripping stages, where nickel from the organic phase is stripped with an
acidic solution to the aqueous phase. Produced stripping liquid, advance
electrolyte, is used as a feed solution for a nickel electrowinning (Ni EW)
process 13, 26. Used acid can be sulphuric or hydrochloride acid. Normally
at this stage chloride based process is converted to a sulphate phase
process and thus sulphuric acid is used for stripping.
Depending on the impurity levels in the advance electrolyte and Ni EW
electrolyte quality requirements further purification of the advance
electrolyte
can be done for example with an ionic exchange process, where small
amounts for example copper, cadmium, iron and zinc can be removed from
the produced advance electrolyte.
Magnesium removal
CA 02792401 2014-06-12
16
Magnesium is a harmful substance in the nickel electrowinning 13, 26.
Magnesium is removed from the nickel electrolyte in a magnesium removal stage
14, 25. Magnesium would be precipitated (process step 25 in Fig 2) by lye from
the product solution, obtained from nickel solvent extraction, by raising the
solution pH value to roughly 9, so that Mg was precipitated as magnesium
hydroxide which also is a commercial product.
The quantity of magnesium is generally largest, and the dimensioning of the
final
solution purification such as ion exchange according to the Mg quantity
becomes
fairly expensive. One advantageous method according to the invention is to
remove the magnesium from the solution in the Mg precipitation step 25 by
using
sodium hydroxide NaOH, formed in the chlorine- alkali electrolysis 14, 29, as
the
precipitation reagent. The solution pH is raised to within the range 9-10, in
which
case Mg is precipitated as magnesium hydroxide Mg(OH)2. The hydroxide
precipitate is subjected to thickening, and the underflow of the thickening is
recycled to the precipitation step advantageously for improving the quality of
the
precipitate. When the hydrometallurgical treatment is completed with a
separate
magnesium recovery, it is possible thereby to treat concentrates where the
magnesium quantity is hazardously high for treating the concentrate
pyrometallurgically.
When the quantity of other impurities, such as zinc and nickel left in the
solution
after magnesium removal 14, 25, is of the order milligrams per litre, the most
advantageous method for removing them is ion exchange (brine purification 28
in
Fig 2). Ion exchange is preferably carried out by means of a chelating ion
exchange resin. Ion exchange functions according to known technique, so that
the impurities bound in the resin are removed by means of hydrochloric acid,
and
the resin is regenerated with a NaOH solution. Consequently, the reagents
needed in ion exchange are advantageously obtained from the chlorine-alkali
electrolysis that forms part of the process.
CA 02792401 2012-09-06
WO 2011/114000 PCT/F12011/050222
17
The content of the NaCI solution, obtained from the brine treatment 14, 28,
can be of the order 150-240 g/L NaCI, preferably 200 g/L. However, for the
chlorine-alkali electrolysis it is necessary to raise the NaCI content in the
solution up to the order of 160-300 g/L, preferably 280-300 g/L. The content
increase is carried out in some suitable way, for example by means of a
vacuum evaporator or immersion evaporator in the evaporation step. When
necessary, the depleted NaCI solution created in the evaporation step can be
conducted to the concentrate leaching process (not in the figure).
io The purified and concentrated sodium chloride solution is conducted to
chlorine-alkali electrolysis 14, 29 for producing the chlorine, hydrogen and
sodium hydroxide needed in different steps of the nickel product process.
The chlorine-alkali electrolysis functions in a known fashion. The NaCI saline
solution is in the electrolysis conducted to the anode side, where the
electric
is current disintegrates it, thus forming chlorine gas. Sodium ions proceed
through a membrane placed in between the anode and cathode sides to the
cathode side, where the electric current disintegrates water to hydrogen gas,
thus forming sodium hydroxide. The NaCI solution conducted to electrolysis
is depleted in the electrolysis in the proportion of the gases and lye
produced
20 there from. The NaCI content of the solution removed from electrolysis
is of
the order 150 - 240 g/L, preferably 200 g/L, and it is recycled back to the
raw
material leaching process.
As was maintained above, the sodium hydroxide formed in the chlorine-alkali
25 electrolysis 14, 29 is used at least in the precipitation of magnesium
hydroxide. Sodium hydroxide is also needed in the ion exchange
regeneration, and when necessary, it can also be used in the removal of
sulphates.
30 An advantageous method for leaching nickel bearing raw material is to
feed
oxygen to the final leaching step and to adjust the leach conditions by
feeding hydrochloric acid therein according to reaction 3. The required
CA 02792401 2012-09-06
WO 2011/114000 PCT/F12011/050222
18
hydrochloric acid is advantageously made of the hydrogen and chlorine
created in the electrolysis of the hydrochloric acid production process.
EXAMPLE 1
Nickel bearing feed material (e.g. nickel sulphide concentrate or ore or bulk
concentrate containing mainly nickel and copper sulphides, scrap or matte) is
leached in sodium chloride solution (50-200 g/I NaCI) containing divalent
io copper ions. Leaching is conducted in two or more counter-current
leaching
stages. The first leaching stage is operated in non-oxidative conditions and
the subsequent stages in oxidative conditions.
In the first stage feed material is mixed with the process solution from the
is second leaching stage. Nickel sulphides are leached according to the
reaction (1).
2(Ni,Fe)9S8+ 18 CuCl2 4 9 NiCl2 + 9 FeCl2 + 9Cu2S.1, + 7 S (1)
20 Nickel and iron are transferred in solution while copper is precipitated
in solid
phase. Sulphide sulphur is converted mainly to elemental sulphur. Slurry
from the first leaching stage is treated in a solid-liquid separation step.
Solids
are transferred to the subsequent oxidative leaching stage(s) and liquid is
recovered as PLS, which is sent to solution purification stages. Temperature
25 and pH in the first leaching stage is 80-110 and 0.5-3.0, respectively.
Solids from the first leaching stage are mixed with depleted brine from the
chlor-alkali electrolysis and further leached in oxidative leaching stages.
Leach is oxidised with hydrochloric acid and oxidising gas such as oxygen,
30 air, oxygen enriched air or chlorine. As a result copper sulphide
precipitated
in the first leaching stage is leached. Copper is released in solution as
divalent copper ions, which in turn are consumed in nickel and iron sulphide
leaching according to reaction (2).
CA 02792401 2012-09-06
WO 2011/114000 PCT/F12011/050222
19
2(Ni,Fe)9S8 + 36 CuCl2 4 9 NiCl2 + 9 FeCl2 + 36 CuCI + 16 S (2)
Again sulphur in metal sulphides in converted mainly into elemental sulphur.
Copper(I) chloride produced in the reaction (2) is re-oxidised according to
reaction (3) back to copper(II) chloride.
4 CuCI + 02 + 4 HCI 4 4 CuCl2 + 2 H20 (3)
Hydrochloric acid used in leaching is produced in chlor-alkali electrolysis.
In the second leaching stage leached iron is oxidised and precipitated as iron
oxides or hydroxides. In reactions (4) and (5) iron oxidation and
precipitation
as goethite is shown as an example.
FeCl2 + 2 CuCl2 4 FeCI3 + CuCI (4)
FeCI3 + 2 H20 4 Fe00H.1, + 3 HCI (5)
After the oxidative leaching stages solid-liquid mixture is subjected to solid-
liquid separation step where iron and sulphur residue is separated from
nickel containing solution. Residue is sent to tailings facility. Additional
residue stabilisation step may be applied. Liquid from the solid-liquid
separation step is transferred to the preceding leaching stage.
If gold is present in the feed material, it is leached in the oxidative
leaching
stages and recovered.
During the leaching process iron and sulphur (as sulphate) are also leached.
Iron and sulphates are removed in solution purification. Oxidising gas (02,
air, oxygen enriched air, chlorine) is injected in PLS in order to oxidise
iron
and the pH of the solution is adjusted with sodium hydroxide and/or calcium
carbonate/hydroxide to precipitate iron as iron hydro-oxides and sulphur as
gypsum. Resulting solids are separated from PLS and recycled to oxidative
leaching stages. Treated PLS is transferred to cobalt solvent extraction.
CA 02792401 2012-09-06
WO 2011/114000 PCT/F12011/050222
EXAMPLE 2
Sulphidic nickel concentrate was leached according to the method of the
5 invention. The major part of the nickel contained in the concentrate was
bound in pentlandite. Other main minerals were antigorite, millerite and
pyrrhotite. The chemical analysis of the concentrate was:
Ni Fe S Co Cu MgO
0/0 0/0 0/0 0/0 0/0 0/0
3.78 29.3 16.3 0.23 0.2 19.23
Leaching step 1
Nickel concentrate (690g) was leached in a solution (2500 ml), the initial
content of which was:
Ni Fe S Co Cu Mg Na
g/I mg/I mg/I mg/I g/I mg/I g/I
7.62 1.2 1680 670 6.8 1680 30.5
Oxidant was not used in the leaching, and the pH value of the solution was
2-3 during the leaching process. Copper was precipitated from the solution
as chalcochite Cu2S, and at the same time iron was dissolved. After leaching
three hours at the temperature of 95 C, there was obtained a solution (leach
product solution, PLS), the composition of which was:
Ni Fe S Ca Co Cu Mg Na Zn
g/I mg/I mg/I mg/I mg/I mg/I mg/I g/I mg/I
10.3 2760 2470 614 670 <5 11200 41.2 37.3
After three hours, the composition of solids was:
Ni Fe S Co Cu Mg
0/ 0/ 0/ 0/ 0/ 0/
0 0 0 0 0 0
3.7 29.4 17 0.24 2.3 11.1
Leaching step 2
CA 02792401 2012-09-06
WO 2011/114000 PCT/F12011/050222
21
Solids, i.e. the leach residue from the first leaching step were leached
oxidatively in a solution containing 130 g/I NaCI and 12 g/I Cu2+. Oxygen and
hydrochloric acid were fed in the leaching process for oxidizing the
sulphides. The pH value was maintained within the range 2.0 ¨ 2.5. The
duration of the leaching process was 8 hours, where after the compositions
of the solution and the solids were:
Solution
Ni Fe S Ca Co Cu Mg Na Zn
g/I mg/I mg/I mg/I mg/I g/I mg/I g/I mg/I
10.2 11.5 1180 372 678 10.7 6710 49 27.9
Solids
Ni Fe S Co Cu Mg
0/0 0/0 0/0 0/0 0/0 0/0
0.09 28.5 14.0 0.01 0.38 9.5
The total yields to the solution, calculated from the iron balance, were
(leaching step 1 + leaching step 2):
Nickel 98.2 %
Cobalt 97.2 %
25