Note: Descriptions are shown in the official language in which they were submitted.
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
1
PEPTIDOMIMETICS FOR MODULATING INTERLEUKIN-1 RECEPTOR
REFERENCE TO RELATED APPLICATION
[0001] This application claims the benefit of U.S. Provisional Patent
Application No.
61/162,070, filed March 20, 2009, the contents of which are hereby
incorporated by reference
in their entireties.
BACKGROUND OF THE INVENTION
[0002] The Interleukin-1 (IL-1) family of polypeptide hormones represents an
important class of cytokines which are expressed by a variety of cell types
including
monocytes (which are the predominant source of IL-1), fibroblasts, endothelial
cells, smooth
muscle cells, osteoclasts, astrocytes, epithelial cells, T-cells, B-cells and
numerous cancer
cells. This family of cytokines includes more than 7 distinct but structurally
related
molecules including IL-la and IL-1(3. Receptors for IL-1 recognize both a and
(3 forms and
both forms have similar biological properties. The biological properties of IL-
1 are numerous
and include mediating many immunological and inflammatory responses to
infection and
injury.
[0003] Two distinct receptor proteins of IL-1 have been cloned and
characterized: IL-
1RI (Sims, et at. 1989), which generates the biological effects of IL-1; and
IL-1RII. In
addition, a receptor accessory protein (IL-1RAcP), which is the putative
signal-transducing
subunit of the receptor complex, has been identified. Generally, one of the
first events in
signal transduction, following IL-1 binding, is the formation of an IL-1R/IL-
1RacP complex
which leads to IRAK (IL-1 receptor associated kinase) recruitment to the
complex and to a
cascade of phosphorylation by kinases, causing the activation of
transcriptional factors
including NFKB and AP- 1. The IL-1 R/IL-1 RacP complex can also recruit and
activate
kinases like P13K and Akt and can also lead to the activation of the PLC/PKC
pathway of
signalization (Daun and Fenton, 2000).
[0004] Despite its normally beneficial effects on an organism response to
infection
and injury, actions of IL-1 can be harmful in some instances. For example,
inappropriate
production or response to IL-1 have been shown in many acute and chronic
inflammatory
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
2
diseases such as rheumatoid arthritis, inflammatory bowel disease (IBD),
osteoarthritis,
psoriasis, septic shock, encephalitis and respiratory distress syndrome. IL-1
has also been
shown to play a role in several other illnesses including Alzheimer's disease,
periventricular
leukomalacia, meningitis, stroke, and a number of autoimmune diseases.
[0005] Generally, Interleukin-1 (IL-1) plays a role in the regulation of
inflammation
by stimulating generation of inflammatory mediators like IL-6, prostaglandin
E2 (PGE2; via
the induction the COX-2 and PGE synthase (mPGES) expression) and itself,
therefore
enhancing the process of inflammation. Another biological activity of IL-1 is
to induce
proliferation and activation of numerous cell types like T-cells (Cullinan, et
at. 1998; Dunne
and O'Neill 2003). IL-1 may also increase the level of collagenase in an
arthritic joint and
has been implicated in the acute and chronic stages of immunopathology in
rheumatoid
arthritis. IL-1 may be responsible for altering endothelial cell function,
directing the
chemotaxis of lymphocytes and leucocytes into synovial tissue and inducing the
secretion of
latent collagenase by chondrocytes and fibroblasts. IL-1 is considered, along
with TNF, as
the prototype of inflammatory cytokines. However, the effects of IL-1 are not
limited to
inflammation and this cytokine also plays a role in bone formation and
remodeling, insulin
secretion and fever induction.
[0006] As a major pro-inflammatory cytokine, IL-1 is a potentially powerful
target
for therapeutic intervention in diseases like articular cartilage injury such
as in arthritis.
Osteoarthritis and rheumatoid arthritis are only second to heart disease for
causing work
disabilities in North America and their prevalence increase dramatically with
age (Hallegua
and Weisman 2002).
[0007] Current approaches for treating IL-1 related diseases include the
development
of soluble receptors, monoclonal antibodies, mimetics of cytokines, antisense
techniques and
kinase inhibitors. Recently, short peptides known as AllosteramersTM that
specifically target
the IL-1 receptor activity have been developed. See, U.S. Patent No.
7,432,341, entitled
"Cytokine receptor modulators and method of modulating cytokine receptor
activity," and
U.S. Pub. No. 20060094663 entitled "Interleukin-1 Receptor Antagonists,
Compositions, and
Methods of Treatment," the entire disclosure of both of which are hereby
incorporated by
reference.
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
3
SUMMARY OF THE INVENTION
[0008] The present invention encompasses the discovery that IL-1R modulatory
peptides (e.g., IL-1R AllosteramersTM) can be optimized by incorporating one
or more
lactams and/or Bab residues into the peptide. Thus, the present invention
provides, among
other things, peptidomimetics that have improved ability to modulate (e.g.,
inhibit or activate)
IL-1 receptor activity.
[0009] In one aspect, the present invention provides an IL-1R modulatory
peptidomimetic comprising one or more lactams and/or Bab residues. In some
embodiments,
the present invention provides a peptidomimetic comprising one or more lactams
and/or Bab
residues and at least three (e.g., at least four, five, or six) out of seven
contiguous amino acids
that appear in an extracellular region of the IL-1 receptor (SEQ ID NO: 1) or
IL-1 receptor
accessory protein (IL-1RacP) (SEQ ID NO:2), wherein the at least three (e.g.,
at least four,
five, or six) amino acids maintain their relative positions as they appear in
the extracellular
region of the IL-1 receptor or IL-1 receptor accessory protein (IL-1RacP). In
other
embodiments, the present invention provides a peptidomimetic comprising one or
more
lactams and/or Bab residues and at least three (e.g., at least four, five, or
six) out of seven
contiguous amino acids that appear in an extracellular region of the IL-1
receptor (SEQ ID
NO: l) or IL-1 receptor accessory protein (IL-1RacP) (SEQ ID NO:2), wherein
the at least
three (e.g., at least four, five, or six) amino acids maintain their relative
positions, but in the
inverse configuration, as they appear in the extracellular region of the IL-1
receptor or IL-1
receptor accessory protein (IL-1RacP).
[0010] In some embodiments, the present invention provides a peptidomimetic
comprising one or more lactams and/or Bab residues and at least three (e.g.,
at least four,
five, or six) amino acids from any one peptide selected from SEQ ID NO:3-40,
wherein the at
least three (e.g., at least four, five, or six) amino acids maintain their
relative positions as they
appear in said peptide sequence. In some embodiments, the present invention
provides a
peptidomimetic comprising one or more lactams and/or Bab residues and at least
three (e.g.,
at least four, five, or six) amino acids from RYTVELA (SEQ ID NO: 12) wherein
the at least
three (e.g., at least four, five, or six) amino acids maintain their relative
positions as they
appear in RYTVELA (SEQ ID NO:12).
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
4
[0011] In some embodiments, lactams suitable for the present invention have
the
structure of formula (I) or (II), as defined and described herein. In some
embodiments,
lactams suitable for the present invention are selected from the group
consisting of alpha-
amino-gamma-lactam (Agl), beta-amino-gamma-lactam (Bgl), beta-hydroxy-alpha-
amino-
gamma-lactam (Agl(4-OH)), and combination thereof In some embodiments, lactams
suitable for the present invention contain haa, I9aa, or Qaa.
[0012] In some embodiments, the present invention provides a peptidomimetic
modified from any one of SEQ ID NO:3-40, wherein the peptidomimetic comprises
at least
three (e.g., at least four, five or six) amino acids from the corresponding
peptide sequence
and a lactam and/or Bab residue replacement or insertion. In some embodiments,
the at least
three (e.g., at least four, five or six) amino acids maintain their relative
positions as they
appear in the corresponding peptide sequence. In some embodiments, the
invention provides
peptidomimetics modified from any one of peptide as shown in Tables 1 and 2
(e.g., SEQ ID
NOs:3-60), wherein the modification comprises a lactam replacement or
insertion. In some
embodiments, a lactam suitable for the present invention is selected from the
group consisting
of alpha-amino-gamma-lactam (Agl), beta-amino-gamma-lactam (Bgl), beta-hydroxy-
alpha-
amino-gamma-lactam (Agl(4-OH)), and combination thereof In some embodiments, a
lactam
suitable for the present invention contains haa, I9aa, or Qaa.
[0013] In some embodiments, a peptidomimetic of the present invention
comprises at
least one D-amino acid. In some embodiments, all of the amino acids present in
a
peptidomimetic of the present invention are D-amino acids.
[0014] In some embodiments, a peptidomimetic of the present invention
comprises
one or more modifications to increase protease resistance, serum stability
and/or
bioavailability, such as N- and/or C-terminal acetylation, glycosylation,
biotinylation,
amidation, substitution with D-amino acid or unnatural amino acid, and/or
cyclization of the
peptide.
[0015] In another aspect, the present invention provides a method of modifying
an IL-
1R modulatory peptide (e.g., AllosteramerTM). In some embodiments, a method
according to
the invention includes steps of. (a) providing a peptide that modulates the IL-
1R activity; (b)
modifying the peptide by introducing one or more lactams and/or Bab residues
into the
peptide; and (c) testing the IL-1R modulatory activity of the modified
peptide. In some
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
embodiments, the peptide modulates the IL-1R activity non-competitively. In
some
embodiments, the peptide is a Negative Allosteric Modulator (NAM) of the IL-1R
activity.
In some embodiments, the peptide is a Positive Allosteric Modulator (PAM) of
the IL-1R
activity. In some embodiments, the peptide is both an NAM and a PAM of the IL-
1R
activity. In some embodiments, a method according to the invention further
includes a step
of identifying a modified peptide having improved ability to modulate (e.g.,
inhibit or
activate) the IL-1R activity as compared to a control (e.g., the corresponding
unmodified
parent peptide).
[0016] The present invention also encompasses any IL-1R modulatory peptide and
modified IL-1R modulatory peptide according to various methods described
herein. In some
embodiments, the invention provides a peptidomimetic having a sequence of any
one of SEQ
ID NO:61-105.
[0017] The invention further relates to pharmaceutical compositions containing
various peptides or peptidomimetics described herein and their uses. For
example, the
present invention provides a method of inhibiting the activity of an IL-1
receptor in a cell by
contacting the cell with a peptide or peptidomimetic described herein. The
present invention
further provides a method of treating an IL-1 related disease, disorder or
condition (e.g., an
inflammatory disease, disorder or condition) by administering to a subject in
need of
treatment a peptide or peptidomimetic described herein.
[0018] In this application, the use of "or" means "and/or" unless stated
otherwise. As
used in this application, the term "comprise" and variations of the term, such
as "comprising"
and "comprises," are not intended to exclude other additives, components,
integers or steps.
[0019] Other features, objects, and advantages of the present invention are
apparent in
the detailed description, drawings and claims that follow. It should be
understood, however,
that the detailed description, the drawings, and the claims, while indicating
embodiments of
the present invention, are given by way of illustration only, not limitation.
Various changes
and modifications within the scope of the invention will become apparent to
those skilled in
the art.
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
6
DEFINITIONS
[0020] In order to facilitate the understanding of certain terms used in the
specification and claims, a number of definitions are first provided herein
below. Additional
definitions of these terms and other terms are provided throughout the
specification.
[0021] Unless defined otherwise, the scientific and technological terms and
nomenclature used herein have the same meaning as commonly understood by a
person of
ordinary skill to which this invention pertains. Generally, the procedures of
cell cultures,
infection, molecular biology methods and the like are common methods used in
the art. Such
standard techniques can be found in reference manuals such as, for example,
Ausubel et at.,
Current Protocols in Molecular Biology, Wiley Interscience, New York, 2001;
and
Sambrook et al., Molecular Cloning: A Laboratory Manual, 3rd edition, Cold
Spring Harbor
Laboratory Press, N.Y., 2001.
[0022] In order for the present invention to be more readily understood,
certain terms
are first defined. Additional definitions for the following terms and other
terms are set forth
throughout the specification.
[0023] About or approximately: As used herein, the term "approximately" or
"about," as applied to one or more values of interest, refers to a value that
is similar to a
stated reference value. In certain embodiments, the term "approximately" or
"about" refers
to a range of values that fall within 25%, 20%, 19%, 18%, 17%, 16%, 15%, 14%,
13%, 12%,
11%, 10%, 9%, 8%, 7%, 6%, 5%, 4%, 3%, 2%, 1%, or less in either direction
(greater than or
less than) of the stated reference value unless otherwise stated or otherwise
evident from the
context (except where such number would exceed 100% of a possible value). The
terms
"about" and "approximately" are used as equivalents.
[0024] Agonist: As used herein, the term "agonist" refers to a compound that
directly
or indirectly stimulates, activates or enhances a biological activity of a
target protein. For
example, an IL-1 agonist is a compound that directly or indirectly stimulates,
activates or
enhances a biological activity of IL-1 or IL-1R/IL-1RacP. The term "agonist"
also includes
potentiators of known compounds with agonist properties. In some embodiments,
an agonist
can be a peptide or peptidomimetic. In some embodiments, an agonist is a
peptide or
peptidomimetic that stimulates, activates or enhances an activity of IL-1 or
IL-1R/IL-1RacP
without competing with a natural ligand of the IL-1 receptor. Such an agonist
is also referred
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
7
to as an allosteric agonist or a Positive Allosteric Modulator (PAM). In some
embodiments,
an IL-1 agonist binds to IL-1 or IL-1 R/IL-1 RacP without competing with the
binding of a
natural ligand.
[0025] Antagonist: As used herein, the term "antagonist" refers to any
molecule that
directly or indirectly inhibits, suppresses, inactivates and/or decreases an
activity of a target
protein. For example, an IL-1 antagonist is a compound that directly or
indirectly stimulates,
activates or enhances an activity of IL-1 or IL-1R/IL-lRacP. The term
"antagonist" also
includes potentiators of known compounds with antagonistic properties. In some
embodiments, an antagonist can be a peptide or peptidomimetic. In some
embodiments, an
antagonist is a peptide or peptidomimetic that inhibits, suppresses,
inactivates and/or
decreases an activity of IL-1 or IL-1R/IL-lRacP without competing with a
natural ligand of
the IL-1 receptor. Such an antagonist is also referred to as an allosteric
antagonist or a
Negative Allosteric Modulator (NAM). In some embodiments, an IL-1 antagonist
binds to
IL-1 or IL-1R/IL-1RacP without competing with the binding of a natural ligand.
[0026] Amino acid: As used herein, the term "amino acid," in its broadest
sense,
refers to any compound and/or substance that can be incorporated into a
polypeptide chain.
In some embodiments, "amino acid" refers to a molecule containing both an
amino group and
a carboxyl group. In certain embodiments, an amino acid is an alpha amino
acid. Suitable
amino acids include, without limitation, natural alpha-amino acids such as L-
isomers of the
20 common naturally occurring alpha-amino acids, D-isomers of naturally
occurring alpha-
amino acids, unnatural alpha-amino acids, natural beta-amino acids (e.g., beta-
alanine), and
unnatural beta-amino acids. a,a-Disubstituted amino acids, N-alkyl amino
acids, lactic acid
and other unconventional amino acids may also be suitable components for the
peptides or
peptidomimetics of the present invention. Examples of unconventional amino
acids include
but are not limited to citrulline, ornithine, norvaline, 4-(E)-butenyl-4(R) -
methyl-N-
methylthreonine (MeBmt), N-methyl-leucine (MeLeu), aminoisobutyric acid,
statine, N-
methyl-alanine (MeAla). As used herein, the twenty natural amino acids and
their
abbreviations follow conventional usage. However, unless specifically noted,
an amino acid
abbreviation should cover both L- and D-isomers.
[0027] Animal: As used herein, the term "animal" refers to any member of the
animal kingdom. In some embodiments, "animal" refers to humans, at any stage
of
development. In some embodiments, "animal" refers to non-human animals, at any
stage of
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
8
development. In certain embodiments, the non-human animal is a mammal (e.g., a
rodent, a
mouse, a rat, a rabbit, a monkey, a dog, a cat, a sheep, cattle, a primate,
and/or a pig). In
some embodiments, animals include, but are not limited to, mammals, birds,
reptiles,
amphibians, fish, insects, and/or worms. In some embodiments, an animal may be
a
transgenic animal, genetically-engineered animal, and/or a clone.
[0028] Biological activity: As used herein, the term "biological activity,"
when used
in the context of IL-1 receptor, or "IL-1R/IL-lRacP activity" or "receptor
activity," refers to
any detectable biological activity of IL-1 or IL-1R/IL-lRacP gene or protein.
It can include
specific biological activity of IL-1R/IL-lRacP proteins in cell signaling. A
suitable
biological activity may be measured by PGE2 production, proliferation assays
and changes in
down stream gene and protein expression (e.g., IL-6, IL-1, COX enzymes). A
suitable
biological activity may also include for example, binding ability to, e.g.,
substrates,
interacting proteins and the like. For example, measuring the effect of a test
compound on its
ability to inhibit or increase (e.g., modulate) IL-1 response or IL-1R binding
or interaction, is
considered herein as measuring a biological activity of IL-1R according to the
present
invention. Broadly intra-or inter-molecular binding of the receptor subunits
(e.g., IL-1R and
IL-1RacP) in the absence vs the presence of the peptide, peptide derivative or
peptidomimetic
of the invention is yet another example of a biological activity according to
the invention.
IL-1R/IL-lRacP biological activity also includes any biochemical measurement
of this
receptor, conformational changes, phosphorylation status, any downstream
effect of the
receptor's signaling such as protein phosphorylation (or any other
posttranslational
modification e.g. ubiquitination, sumolylation, palmytoylation, prenylation
etc), kinase effect
or any other feature of the protein that can be measured with techniques known
in the art.
Finally, IL-1R/IL-1RacP biological activity includes a detectable change in
cell architecture,
cell proliferation or other cell phenotype that is modulated by the action of
a ligand (i.e., 1L-
1) on the predetermined receptor.
[0029] Comprising: As used in this specification and claim(s), the words
"comprising" (and any form of comprising, such as "comprise" and "comprises"),
"having"
(and any form of having, such as "have" and "has"), "including" (and any form
of including,
such as "includes" and "include") or "containing" (and any form of containing,
such as
"contains" and "contain") are inclusive or open-ended and do not exclude
additional, un-
recited elements or method steps. The use of the word "a" or "an" when used in
conjunction
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
9
with the term "comprising" in the claims and/or the specification may mean
"one" but it is
also consistent with the meaning of "one or more," "at least one," and "one or
more than
one."
[0030] Control: As used herein, the term "control" has its art-understood
meaning of
being a standard against which results are compared. Typically, controls are
used to augment
integrity in experiments by isolating variables in order to make a conclusion
about such
variables. In some embodiments, a control is a reaction or assay that is
performed
simultaneously with a test reaction or assay to provide a comparator. In one
experiment, the
"test" (i.e., the variable being tested) is applied. In the second experiment,
the "control," the
variable being tested is not applied. In some embodiments, a control is a
historical control
(i.e., of a test or assay performed previously, or an amount or result that is
previously
known). In some embodiments, a control is or comprises a printed or otherwise
saved record.
A control may be a positive control or a negative control.
[0031] Functional: As used herein, a "functional" biological molecule is a
biological
molecule in a form in which it exhibits a property and/or activity by which it
is characterized.
[0032] Functional derivative: As used herein, the term "functional derivative"
denotes, when used in the context of a functional derivative of an amino acid
sequence, refers
to a molecule that shares structural similarity and retains a biological
activity that is
substantially similar to that of the original sequence. A functional
derivative or equivalent
may be a natural derivative or may be prepared synthetically. Such derivatives
include amino
acid sequences having substitutions, deletions, or additions of one or more
amino acids,
provided that the biological activity of the protein is conserved (e.g., it
acts as a non-
competitive antagonist of the IL-1 receptor). The substituting amino acid
generally has
chemico-physical properties, which are similar to that of the substituted
amino acid. The
similar chemico-physical properties include, similarities in charge,
bulkiness, hydrophobicity,
hydrophylicity and the like. The term "functional derivatives" is intended to
include
"segments", "variants", "analogs" or "chemical derivatives" of the subject
matter of the
present invention. In some embodiments, a functional derivative shares at
least 70%, 75%,
80%, 85%, 90%, 95%, 96%, 97%, 98%, 99% identity to the original amino acid
sequence.
[0033] IL-1: Unless otherwise noted, "IL-1" refers to either or both IL-l a
and IL-1(3.
The term "IL" refers to the broad family of interleukins.
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
[0034] Inhibiting: The terms "inhibiting," "reducing" or "prevention," or any
variation of these terms, when used in the claims and/or the specification
include any
measurable decrease as compared to a baseline control or complete inhibition
of the receptor
activity to achieve a desired result. For example, a peptide is said to be
inhibiting IL-I
activity when a decrease in PGE2 production is measured following a treatment
with the
peptides, peptide derivatives or peptidomimetics of the present invention as
compared to in
the absence of these peptides.
[0035] In vitro: As used herein, the term "in vitro" refers to events that
occur in an
artificial environment, e.g., in a test tube or reaction vessel, in cell
culture, etc., rather than
within a multi-cellular organism.
[0036] In vivo: As used herein, the term "in vivo" refers to events that occur
within a
multi-cellular organism such as a non-human animal.
[0037] Inflammatory disease, disorder or condition: As used herein, the term
an
"inflammatory disease, disorder or condition" refers to any disease, disorder,
or condition in
which the immune system abnormally activated or suppressed. In some
embodiments, an
inflammatory disease, disorder, or condition that can be treated according to
the invention is
inflammation of the upper and lower respiratory tract, for example, bronchial
asthma, allergic
asthma, non-allergic asthma, lymphomatous tracheobronchitis, allergic
hypersensitivity or a
hypersecretion condition, such as chronic bronchitis and cystic fibrosis;
pulmonary fibrosis of
various aetiologies (e.g., idiopathic pulmonary fibrosis), chronic obstructive
pulmonary
disease (COPD), sarcoidosis, allergic and non-allergic rhinitis; allergic or
non-allergic
urticaria; a skin-related diseases characterized by deregulated inflammation,
tissue
remodeling, angiogenesis, and neoplasm, a disease of the gastrointestinal
tract, such as
Crohn's disease, Hirschsprung's disease, diarrhea, malabsorption conditions,
and
inflammatory conditions; a disorder of the central and peripheral nervous
system, such as
depression, anxiety states, Parkinson's disease, migraine and other forms of
cranial pain,
strokes, emesis; a disease of the immune system, such as in the splenic and
lymphatic tissues,
an autoimmune disease or other immune-related diseases; a disease of the
cardiovascular
system, such as pulmonary edema, hypertension, atherosclerosis, pre-eclampsia,
complex
regional pain syndrome type 2, stroke and chronic inflammatory diseases such
as arthritis, a
bone-related diseases such as rheumatoid arthritis, as well as pain, chronic
pain such as
fibromyalgia, and other disorders in which the action of neurokinins,
tachykinins or other
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
11
related substances (e.g., hemokinins) are involved in the pathogenesis,
pathology, and
aetiology.
[0038] Additional examples of inflammatory disorders include acne vulgaris;
acute
respiratory distress syndrome; Addison's disease; allergic intraocular
inflammatory diseases,
ANCA-associated small-vessel vasculitis; ankylosing spondylitis; atopic
dermatitis;
autoimmune hemolytic anemia; autoimmune hepatitis; Behcet's disease; Bell's
palsy; bullous
pemphigoid; cerebral ischaemia; cirrhosis; Cogan's syndrome; contact
dermatitis; Cushing's
syndrome; dermatomyositis; diabetes mellitus; discoid lupus erythematosus;
lupus nephritis;
eosinophilic fasciitis; erythema nodosum; exfoliative dermatitis; focal
glomerulosclerosis;
focal segmental glomerulosclerosis; segmental glomerulosclerosis; giant cell
arteritis; gout;
gouty arthritis; graft-versus-host disease; hand eczema; Henoch-Schonlein
purpura; herpes
gestationis; hirsutism; idiopathic cerato- scleritis; idiopathic
thrombocytopenic purpura;
immune thrombocytopenic purpura inflammatory bowel or gastrointestinal
disorders,
inflammatory dermatoses; lichen planus; lymphomatous tracheobronchitis;
macular edema;
multiple sclerosis; myasthenia gravis; myositis; nonspecific fibrosing lung
disease;
osteoarthritis; pancreatitis; pemphigoid gestationis; pemphigus vulgaris;
periodontitis;
polyarteritis nodosa; polymyalgia rheumatica; pruritus scroti;
pruritis/inflammation, psoriasis;
psoriatic arthritis; pulmonary histoplasmosis; relapsing polychondritis;
rosacea caused by
sarcoidosis; rosacea caused by scleroderma; rosacea caused by Sweet's
syndrome; rosacea
caused by systemic lupus erythematosus; rosacea caused by urticaria; rosacea
caused by
zoster-associated pain; sarcoidosis; scleroderma; septic shock syndrome;
shoulder tendinitis
or bursitis; Sjogren's syndrome; Still's disease; Sweet's disease; systemic
lupus
erythematosus; systemic sclerosis; Takayasu's arteritis; temporal arteritis;
toxic epidermal
necrolysis; transplant-rejection and transplant-rejection-related syndromes;
tuberculosis;
type-1 diabetes; ulcerative colitis; uveitis; vasculitis; and Wegener's
granulomatosis.
Desirably the autoimmune disorder is inflammatory bowel disease, an
inflammatory skin
disorder such as psoriasis, or multiple sclerosis.
[0039] Isolated: As used herein, the term "isolated" refers to a substance
and/or
entity that has been (1) separated from at least some of the components with
which it was
associated when initially produced (whether in nature and/or in an
experimental setting),
and/or (2) produced, prepared, and/or manufactured by the hand of man.
Isolated substances
and/or entities may be separated from at least about 10%, about 20%, about
30%, about 40%,
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
12
about 50%, about 60%, about 70%, about 80%, about 90%, about 95%, about 98%,
about
99%, substantially 100%, or 100% of the other components with which they were
initially
associated. In some embodiments, isolated agents are more than about 80%,
about 85%,
about 90%, about 91%, about 92%, about 93%, about 94%, about 95%, about 96%,
about
97%, about 98%, about 99%, substantially 100%, or 100% pure. As used herein, a
substance
is "pure" if it is substantially free of other components. As used herein, the
term "isolated
cell" refers to a cell not contained in a multi-cellular organism.
[0040] Modulator: As used herein, the term "modulator" refers to a compound
that
alters or elicits an activity. For example, the presence of a modulator may
result in an
increase or decrease in the magnitude of a certain activity compared to the
magnitude of the
activity in the absence of the modulator. In certain embodiments, a modulator
is an inhibitor
or antagonist, which decreases the magnitude of one or more activities. In
certain
embodiments, an inhibitor completely prevents one or more biological
activities. In certain
embodiments, a modulator is an activator or agonist, which increases the
magnitude of at
least one activity. In certain embodiments the presence of a modulator results
in an activity
that does not occur in the absence of the modulator. As used herein, the terms
"inhibiting,"
"reducing," "preventing," or "antagonizing," or any variations of these terms
as used herein,
refer to a measurable decrease of a biological activity. In some embodiments,
the decrease is
a 10%, 15%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%, or 95% reduction in the
biological activity relative to a control. As used herein, the terms
"stimulating," "increasing,"
or "agonizing," or any variations of these terms as used herein, refer to a
measurable increase
of a biological activity. In some embodiments, the increase is a 10%, 15%,
20%, 30%, 40%,
50%, 60%, 70%, 80%, 90%, or 95% increase in the biological activity relative
to a control.
[0041] Molecule: As used herein, the terms "molecule", "compound", "agent" or
"ligand" are used interchangeably and broadly to refer to natural, synthetic
or semi-synthetic
molecules or compounds. The term "molecule" therefore denotes for example
chemicals,
macromolecules, cell or tissue extracts (from plants or animals) and the like.
Non-limiting
examples of molecules include peptides, antibodies, carbohydrates and
pharmaceutical
agents. The agents can be selected and screened by a variety of means
including random
screening, rational selection and by rational design using for example protein
or ligand
modeling methods such as computer modeling. The terms "rationally selected" or
"rationally
designed" are meant to define compounds which have been chosen based on the
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
13
configuration of interacting domains of the present invention or on the
configuration of
antagonist peptides and/or peptidomimetics of the present invention. As will
be understood
by the person of ordinary skill, macromolecules having non-naturally occurring
modifications
are also within the scope of the term "molecule." For example,
peptidomimetics, well known
in the pharmaceutical industry and generally referred to as peptide analogs
can be generated
by modeling as mentioned above. Similarly, in a preferred embodiment, the
polypeptides of
the present invention are modified to enhance their stability. In some cases
this modification
enhances the biological activity of the peptide. The molecules identified in
accordance with
the teachings of the present invention have a therapeutic value in diseases or
conditions in
which the physiology or homeostasis of the cell and/or tissue is compromised
by a defect in
IL-1 production or response. Non-limiting examples of such diseases or
conditions include
acute and chronic inflammatory diseases such as rheumatoid arthritis,
inflammatory bowel
disease (IBD), osteoarthritis, psoriasis, septic shock, encephalitis and
respiratory distress
syndrome, Alzheimer's disease, periventricular leukomalacia, meningitis,
stroke, and a
number of autoimmune diseases. It will be understood that the compounds are
herein
described interchangeably as "API-X" "TTI-X" or simply by the number of the
compound
(for example: "101.10", "API-101.10" or "TTI-101.10").
[0042] Peptides: As used herein, the term "peptides" refers to macromolecules
which
comprise a multiplicity of amino or imino acids (or their equivalents) in
peptide linkage. In
the polypeptide or peptide notation used herein, the left-hand direction is
the amino-terminal
direction and the right-hand direction is the carboxy-terminal direction, in
accordance with
standard usage and convention. Peptides may include moieties other than amino
acids (e.g.,
may be glycoproteins, proteoglycans, etc.) and/or may be otherwise processed
or modified.
Peptides may contain L-amino acids, D-amino acids, or both and may contain any
of a variety
of amino acid modifications or analogs known in the art. Useful modifications
include, e.g.,
terminal acetylation, amidation, glycosylation, biotinylation, substitution
with D-amino acid
or unnatural amino acid, and/or cyclization of the peptide. In some
embodiments, peptides
may comprise natural amino acids, non-natural amino acids, synthetic amino
acids, and
combinations thereof. As used herein, a "short peptide" refers to any peptide
containing up
to 25 amino acids (e.g., up to 20, 15, 12, 10, 9, 8, 7, 6, 5, 4, or 3). In
some embodiments, a
short peptide contains 5-25 amino acids. As used in this application, peptides
also include
peptidomimetics unless indicated otherwise.
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
14
[0043] Peptidomimetic: Herein the terminologies "mimic," "mimetic,"
peptidomimetic" and the like are used herein interchangeably.
[0044] Pharmaceutically acceptable carrier: As used herein, the term
"pharmaceutically acceptable carrier" refers to a non-toxic carrier medium
that does not
destroy the pharmacological activity of the compound with which it is
formulated.
[0045] Purified: As used herein, the term "purified" refers to a molecule
(e.g. IL-1
receptor, peptides, peptide derivatives, peptidomimetics, nucleic acids,
proteins etc.) having
been separated from a component of the composition in which it was originally
present.
Thus, for example, a "purified IL-1 receptor" has been purified to a level not
found in nature.
A "substantially pure" molecule is a molecule that is lacking in most other
components (e.g.,
30, 40, 50, 60, 70, 75, 80, 85, 90, 95, 96, 97, 98, 99, 100% free of
contaminants). By
opposition, the term "crude" means molecules that have not been separated from
the
components of the original composition in which it was present. Therefore, the
term
"separating" or "purifying" refers to methods by which one or more components
of the
biological sample are removed from one or more other components of the sample.
Sample
components include nucleic acids in a generally aqueous solution that may
include other
components, such as proteins, carbohydrates, or lipids. A separating or
purifying step
preferably removes at least about 70% (e.g., 70, 75, 80, 85, 90, 95, 96, 97,
98, 99, 100%),
more preferably at least about 90% (e.g., 90, 91, 92, 93, 94, 95, 96, 97, 98,
99, 100%) and,
even more preferably, at least about 95% (e.g., 95, 96, 97, 98, 99, 100%) of
the other
components present in the sample from the desired component. For the sake of
brevity, the
units (e.g. 66, 67...81, 82,...91, 92%....) have not systematically been
recited but are
considered, nevertheless, within the scope of the present invention.
[0046] Reverse peptide: As used herein, the term "reverse peptide" refers to
peptides
arranged in a reverse sequence relative to a corresponding region of the IL-1R
or IL-lRacP
protein. Likewise, the term "reverse-D peptide" refers herein to peptides
containing D-amino
acids, arranged in a reverse sequence relative to a corresponding peptide
containing L-amino
acids. For example, the C-terminal residue of an L-peptide becomes N-terminal
for the
reverse D-peptide, and so forth. Without wishing to be bound by theory, it is
contemplated
that reverse D-peptides may often retain the same tertiary conformation and
therefore the
same activity, as the L-amino acid peptides. It is also contemplated that a D-
peptide may be
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
more stable and resistant to enzymatic degradation in vitro and in vivo.
Therefore, a D-
peptide may have greater therapeutic efficacy than the corresponding L-
peptide.
[0047] Subject: As used herein, the term "subject" or "patient" refers to an
animal,
preferably a mammal, most preferably a human who is the object of treatment,
observation or
experiment.
[0048] Substantially: As used herein, the term "substantially" refers to the
qualitative
condition of exhibiting total or near-total extent or degree of a
characteristic or property of
interest. One of ordinary skill in the biological arts will understand that
biological and
chemical phenomena rarely, if ever, go to completion and/or proceed to
completeness or
achieve or avoid an absolute result. The term "substantially" is therefore
used herein to
capture the potential lack of completeness inherent in many biological and
chemical
phenomena.
[0049] Suffering from: An individual who is "suffering from" a disease,
disorder,
and/or condition has been diagnosed with or displays one or more symptoms of
the disease,
disorder, and/or condition.
[0050] Susceptible to: An individual who is "susceptible to" a disease,
disorder,
and/or condition has not been diagnosed with the disease, disorder, and/or
condition. In some
embodiments, an individual who is susceptible to a disease, disorder, and/or
condition may
not exhibit symptoms of the disease, disorder, and/or condition. In some
embodiments, an
individual who is susceptible to a disease, disorder, and/or condition will
develop the disease,
disorder, and/or condition. In some embodiments, an individual who is
susceptible to a
disease, disorder, and/or condition will not develop the disease, disorder,
and/or condition.
[0051] Therapeutically effective amount: As used herein, the term
"therapeutically
effective amount" of a therapeutic agent means an amount that is sufficient,
when
administered to a subject suffering from or susceptible to a disease,
disorder, and/or
condition, to treat, diagnose, prevent, and/or delay the onset of the
symptom(s) of the disease,
disorder, and/or condition. It will be appreciated by those of ordinary skill
in the art that a
therapeutically effective amount is typically administered via a dosing
regimen comprising at
least one unit dose.
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
16
[0052] Therapeutic agent: As used herein, the phrase "therapeutic agent"
refers to
any agent that, when administered to a subject, has a therapeutic effect
and/or elicits a desired
biological and/or pharmacological effect. In some embodiments, a therapeutic
agent of the
invention refers to a peptide inhibitor or derivatives thereof according to
the invention.
[0053] Treating: As used herein, the term "treat," "treatment," or "treating"
refers to
any method used to partially or completely alleviate, ameliorate, relieve,
inhibit, prevent,
delay onset of, reduce severity of and/or reduce incidence of one or more
symptoms or
features of a particular disease, disorder, and/or condition. Treatment may be
administered to
a subject who does not exhibit signs of a disease and/or exhibits only early
signs of the
disease for the purpose of decreasing the risk of developing pathology
associated with the
disease.
[0054] Definitions of specific functional groups and chemical terms are
described in
more detail below. For purposes of this invention, the chemical elements are
identified in
accordance with the Periodic Table of the Elements, CAS version, Handbook of
Chemistry
and Physics, 75th Ed., inside cover, and specific functional groups are
generally defined as
described therein. Additionally, general principles of organic chemistry, as
well as specific
functional moieties and reactivity, are described in Organic Chemistry, Thomas
Sorrell,
University Science Books, Sausalito, 1999; Smith and March March's Advanced
Organic
Chemistry, 5th Edition, John Wiley & Sons, Inc., New York, 2001; Larock,
Comprehensive
Organic Transformations, VCH Publishers, Inc., New York, 1989; Carruthers,
Some Modern
Methods of Organic Synthesis, 3rd Edition, Cambridge University Press,
Cambridge, 1987;
the entire contents of each of which are incorporated herein by reference.
[0055] Unless otherwise stated, structures depicted herein are also meant to
include
all isomeric (e.g., enantiomeric, diastereomeric, and geometric (or
conformational)) forms of
the structure; for example, the R and S configurations for each asymmetric
center, Z and E
double bond isomers, and Z and E conformational isomers. Therefore, single
stereochemical
isomers as well as enantiomeric, diastereomeric, and geometric (or
conformational) mixtures
of the present compounds are within the scope of the invention. Unless
otherwise stated, all
tautomeric forms of the compounds of the invention are within the scope of the
invention.
Additionally, unless otherwise stated, structures depicted herein are also
meant to include
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
17
compounds that differ only in the presence of one or more isotopically
enriched atoms. For
example, compounds having the present structures including the replacement of
hydrogen by
deuterium or tritium, or the replacement of a carbon by a 13C- or 14C-enriched
carbon are
within the scope of this invention. Such compounds are useful, for example, as
analytical
tools, as probes in biological assays, or as therapeutic agents in accordance
with the present
invention.
[0056] Where a particular enantiomer is preferred, it may, in some embodiments
be
provided substantially free of the corresponding enantiomer, and may also be
referred to as
"optically enriched." "Optically-enriched," as used herein, means that the
compound is
made up of a significantly greater proportion of one enantiomer. In certain
embodiments the
compound is made up of at least about 90% by weight of a preferred enantiomer.
In other
embodiments the compound is made up of at least about 95%, 98%, or 99% by
weight of a
preferred enantiomer. Preferred enantiomers may be isolated from racemic
mixtures by any
method known to those skilled in the art, including chiral high pressure
liquid
chromatography (HPLC) and the formation and crystallization of chiral salts or
prepared by
asymmetric syntheses. See, for example, Jacques et al., Enantiomers, Racemates
and
Resolutions (Wiley Interscience, New York, 1981); Wilen, et al., Tetrahedron
33:2725
(1977); Eliel, E.L. Stereochemistry of Carbon Compounds (McGraw-Hill, NY,
1962); Wilen,
S.H. Tables of Resolving Agents and Optical Resolutions p. 268 (E.L. Eliel,
Ed., Univ. of
Notre Dame Press, Notre Dame, IN 1972).
[0057] Alkenyl: The term "alkenyl," as used herein, denotes a monovalent group
derived from a straight- or branched-chain aliphatic moiety having at least
one carbon-
carbon double bond by the removal of a single hydrogen atom. In certain
embodiments,
alkenyl contains 2-6 carbon atoms. In certain embodiments, alkenyl contains 2-
5 carbon
atoms. In some embodiments, alkenyl contains 2-4 carbon atoms. In another
embodiment,
alkenyl contains 2-3 carbon atoms. Alkenyl groups include, for example,
ethenyl ("vinyl"),
propenyl ("allyl"), butenyl, 1-methyl-2-buten-1-yl, and the like.
[0058] Alkyl: The term "alkyl," as used herein, refers to a monovalent
saturated,
straight- or branched-chain hydrocarbon radical derived from an aliphatic
moiety containing
between one and six carbon atoms by removal of a single hydrogen atom. In some
embodiments, alkyl contains 1-5 carbon atoms. In another embodiment, alkyl
contains 1-4
carbon atoms. In still other embodiments, alkyl contains 1-3 carbon atoms. In
yet another
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
18
embodiment, alkyl contains 1-2 carbons. Examples of alkyl radicals include,
but are not
limited to, methyl, ethyl, n-propyl, isopropyl, n-butyl, iso-butyl, sec-butyl,
sec-pentyl, iso-
pentyl, tert-butyl, n-pentyl, neopentyl, n-hexyl, sec-hexyl, n-heptyl, n-
octyl, n-decyl, n-
undecyl, dodecyl, and the like.
[0059] Alkylene: The term "alkylene" refers to a bivalent alkyl group. An
"alkylene
chain" is a polymethylene group, i.e., -(CH2)õ-, wherein n is a positive
integer, preferably
from 1 to 6, from 1 to 4, from 1 to 3, from 1 to 2, or from 2 to 3. A
substituted alkylene chain
is a polymethylene group in which one or more methylene hydrogen atoms are
replaced with
a substituent. Suitable substituents include those described below for a
substituted aliphatic
group.
[0060] Alkynyl: The term "alkynyl," as used herein, refers to a monovalent
group
derived from a straight- or branched-chain aliphatic moiety having at least
one carbon-
carbon triple bond by the removal of a single hydrogen atom. In certain
embodiments,
alkynyl contains 2-6 carbon atoms. In certain embodiments, alkynyl contains 2-
5 carbon
atoms. In some embodiments, alkynyl contains 2-4 carbon atoms. In another
embodiment,
alkynyl contains 2-3 carbon atoms. Representative alkynyl groups include, but
are not
limited to, ethynyl, 2-propynyl ("propargyl"), 1-propynyl, and the like.
[0061] Aliphatic: The term "aliphatic" or "aliphatic group", as used herein,
denotes a
hydrocarbon moiety that may be straight-chain (i.e., unbranched), branched, or
cyclic
(including fused, bridging, and spiro-fused polycyclic) and may be completely
saturated or
may contain one or more units of unsaturation, but which is not aromatic.
Unless otherwise
specified, aliphatic groups contain 1-6 carbon atoms. In some embodiments,
aliphatic groups
contain 1-4 carbon atoms, and in yet other embodiments aliphatic groups
contain 1-3 carbon
atoms. Suitable aliphatic groups include, but are not limited to, linear or
branched, alkyl,
alkenyl, and alkynyl groups, and hybrids thereof such as (cycloalkyl)alkyl,
(cycloalkenyl)alkyl or (cycloalkyl)alkenyl.
[0062] Aryl: The term "aryl" used alone or as part of a larger moiety as in
"aralkyl",
"aralkoxy", or "aryloxyalkyl", refers to monocyclic and bicyclic ring systems
having a total
of five to 10 ring members, wherein at least one ring in the system is
aromatic and wherein
each ring in the system contains three to seven ring members. The term "aryl"
may be used
interchangeably with the term "aryl ring". In certain embodiments of the
present invention,
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
19
"aryl" refers to an aromatic ring system which includes, but not limited to,
phenyl, biphenyl,
naphthyl, anthracyl and the like, which may bear one or more substituents.
Also included
within the scope of the term "aryl", as it is used herein, is a group in which
an aromatic ring is
fused to one or more non-aromatic rings, such as indanyl, phthalimidyl,
naphthimidyl,
phenantriidinyl, or tetrahydronaphthyl, and the like.
[0063] Cycloalkylene: As used herein, the term "cycloalkylene" refers to a
bivalent
cycloalkyl group. In certain embodiments, a cycloalkylene group is a 1,1-
cycloalkylene
group (i.e., a spiro-fused ring). Exemplary 1,1-cycloalkylene groups include .
In
other embodiments, a cycloalkylene group is a 1,2-cycloalkylene group or a 1,3-
X,
d
cycloalkylene group. Exemplary 1,2-cycloalkylene groups include and
[0064] Cycloaliphatic: The terms "cycloaliphatic", "carbocycle",
"carbocyclyl",
"carbocyclo", or "carbocyclic", used alone or as part of a larger moiety,
refer to a saturated or
partially unsaturated cyclic aliphatic monocyclic or bicyclic ring systems, as
described
herein, having from 3 to 10 members, wherein the aliphatic ring system is
optionally
substituted as defined above and described herein. Cycloaliphatic groups
include, without
limitation, cyclopropyl, cyclobutyl, cyclopentyl, cyclopentenyl, cyclohexyl,
cyclohexenyl,
cycloheptyl, cycloheptenyl, cyclooctyl, cyclooctenyl, and cyclooctadienyl. In
some
embodiments, the cycloalkyl has 3-6 carbons. The terms "cycloaliphatic",
"carbocycle",
"carbocyclyl", "carbocyclo", or "carbocyclic" also include aliphatic rings
that are fused to
one or more aromatic or nonaromatic rings, such as decahydronaphthyl,
tetrahydronaphthyl,
decalin, or bicyclo[2.2.2]octane, where the radical or point of attachment is
on an aliphatic
ring.
[0065] Halogen: The terms "halo" and "halogen" as used herein refer to an atom
selected from fluorine (fluoro, -F), chlorine (chloro, -Cl), bromine (bromo, -
Br), and iodine
(iodo, -I).
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
[0066] Heteroaryl: The terms "heteroaryl" and "heteroar-", used alone or as
part of a
larger moiety, e.g., "heteroaralkyl", or "heteroaralkoxy", refer to groups
having 5 to 10 ring
atoms, preferably 5, 6, or 9 ring atoms; having 6, 10, or 14 it electrons
shared in a cyclic
array; and having, in addition to carbon atoms, from one to five heteroatoms.
The term
"heteroatom" refers to nitrogen, oxygen, or sulfur, and includes any oxidized
form of
nitrogen or sulfur, and any quaternized form of a basic nitrogen. Heteroaryl
groups include,
without limitation, thienyl, furanyl, pyrrolyl, imidazolyl, pyrazolyl,
triazolyl, tetrazolyl,
oxazolyl, isoxazolyl, oxadiazolyl, thiazolyl, isothiazolyl, thiadiazolyl,
pyridyl, pyridazinyl,
pyrimidinyl, pyrazinyl, indolizinyl, purinyl, naphthyridinyl, and pteridinyl.
The terms
"heteroaryl" and "heteroar-", as used herein, also include groups in which a
heteroaromatic
ring is fused to one or more aryl, cycloaliphatic, or heterocyclyl rings,
where the radical or
point of attachment is on the heteroaromatic ring. Nonlimiting examples
include indolyl,
isoindolyl, benzothienyl, benzofuranyl, dibenzofuranyl, indazolyl,
benzimidazolyl,
benzthiazolyl, quinolyl, isoquinolyl, cinnolinyl, phthalazinyl, quinazolinyl,
quinoxalinyl, 4H-
quinolizinyl, carbazolyl, acridinyl, phenazinyl, phenothiazinyl, phenoxazinyl,
tetrahydroquinolinyl, tetrahydroisoquinolinyl, and pyrido[2,3-b]-1,4-oxazin-
3(4H)-one. A
heteroaryl group may be mono- or bicyclic. The term "heteroaryl" may be used
interchangeably with the terms "heteroaryl ring", "heteroaryl group", or
"heteroaromatic",
any of which terms include rings that are optionally substituted. The term
"heteroaralkyl"
refers to an alkyl group substituted by a heteroaryl, wherein the alkyl and
heteroaryl portions
independently are optionally substituted.
[0067] Heteroatom: The term "heteroatom" means one or more of oxygen, sulfur,
nitrogen, phosphorus, or silicon (including, any oxidized form of nitrogen,
sulfur,
phosphorus, or silicon; the quaternized form of any basic nitrogen or; a
substitutable nitrogen
of a heterocyclic ring, for example N (as in 3,4-dihydro-2H-pyrrolyl), NH (as
in pyrrolidinyl)
or NR-'- (as in N-substituted pyrrolidinyl)).
[0068] Heterocycle: As used herein, the terms "heterocycle", "heterocyclyl",
"heterocyclic radical", and "heterocyclic ring" are used interchangeably and
refer to a stable
4- to 7-membered monocyclic or 7-10-membered bicyclic heterocyclic moiety that
is either
saturated or partially unsaturated, and having, in addition to carbon atoms,
one or more,
preferably one to four, heteroatoms, as defined above. When used in reference
to a ring atom
of a heterocycle, the term "nitrogen" includes a substituted nitrogen. As an
example, in a
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
21
saturated or partially unsaturated ring having 0-3 heteroatoms selected from
oxygen, sulfur
or nitrogen, the nitrogen may be N (as in 3,4-dihydro-2H-pyrrolyl), NH (as in
pyrrolidinyl),
or +NR (as in N-substituted pyrrolidinyl).
[0069] A heterocyclic ring can be attached to its pendant group at any
heteroatom or
carbon atom that results in a stable structure and any of the ring atoms can
be optionally
substituted. Examples of such saturated or partially unsaturated heterocyclic
radicals include,
without limitation, tetrahydrofuranyl, tetrahydrothienyl, pyrrolidinyl,
pyrrolidonyl,
piperidinyl, pyrrolinyl, tetrahydroquinolinyl, tetrahydroisoquinolinyl,
decahydroquinolinyl,
oxazolidinyl, piperazinyl, dioxanyl, dioxolanyl, diazepinyl, oxazepinyl,
thiazepinyl,
morpholinyl, and quinuclidinyl. The terms "heterocycle", "heterocyclyl",
"heterocyclyl
ring", "heterocyclic group", "heterocyclic moiety", and "heterocyclic
radical", are used
interchangeably herein, and also include groups in which a heterocyclyl ring
is fused to one
or more aryl, heteroaryl, or cycloaliphatic rings, such as indolinyl, 3H-
indolyl, chromanyl,
phenanthridinyl, 2-azabicyclo [2.2. 1 ]heptanyl, octahydroindolyl, or
tetrahydroquinolinyl,
where the radical or point of attachment is on the heterocyclyl ring. A
heterocyclyl group
may be mono- or bicyclic. The term "heterocyclylalkyl" refers to an alkyl
group substituted
by a heterocyclyl, wherein the alkyl and heterocyclyl portions independently
are optionally
substituted.
[0070] Optionally substituted: As described herein, compounds of the invention
may
contain "optionally substituted" moieties. In general, the term "substituted",
whether
preceded by the term "optionally" or not, means that one or more hydrogens of
the designated
moiety are replaced with a suitable substituent. Unless otherwise indicated,
an "optionally
substituted" group may have a suitable substituent at each substitutable
position of the group,
and when more than one position in any given structure may be substituted with
more than
one substituent selected from a specified group, the substituent may be either
the same or
different at each position. Combinations of substituents envisioned under this
invention are
preferably those that result in the formation of stable or chemically feasible
compounds. The
term "stable", as used herein, refers to compounds that are not substantially
altered when
subjected to conditions to allow for their production, detection, and, in
certain embodiments,
their recovery, purification, and use for one or more of the purposes
disclosed herein.
[0071] Suitable monovalent substituents on a substitutable carbon atom of an
"optionally substituted" group are independently halogen; -(CH2)o-4R ; -(CH2)0-
40R ; -0-
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
22
(CH2)0_4C(O)OR ; -(CH2)0-4CHOR )2; -(CH2)0_4SR ; -(CH2)0-4Ph, which may be
substituted
with R ; -(CH2)0 40(CH2)0_1Ph which may be substituted with R ; -CH=CHPh,
which may
be substituted with R ; -NO2; -CN; -N3; -(CH2)o-4N(R )2; -(CH2)o-4N(R )C(O)R ;
-N(R )C(S)R ; -(CH2)o-4N(R )C(O)NR 2; -N(R )C(S)NR 2; -(CH2)o-4N(R )C(O)OR ;
-N(R )N(R )C(O)R ; -N(R )N(R )C(O)NR 2; -N(R )N(R )C(O)OR ; -(CH2)0 4C(O)R ;
-C(S)R ; -(CH2)0_4C(O)OR ; -(CH2)0C(O)SR ; -(CH2)0C(O)OSiR 3; -(CH2)0_40C(O)R
; -
OC(O)(CH2)0_4SR-, SC(S)SR ; -(CH2)0SC(O)R ; -(CH2)0_4C(O)NR 2; -C(S)NR 2; -
C(S)SR ; -SC(S)SR , -(CH2)0_40C(O)NR 2; -C(O)N(OR )R ; -C(O)C(O)R ;
-C(O)CH2C(O)R ; -C(NOR )R ; -(CH2)0SSR ; -(CH2)0S(0)2R ; -(CH2)0S(0)20R ;
-(CH2)00S(0)2R ; -S(0)2NR 2; -(CH2)0_4S(O)R ; -N(R )S(0)2NR 2; -N(R )S(0)2R ;
-N(OR )R ; -C(NH)NR 2; -P(0)2R ; -P(O)R 2; -OP(O)R 2; -OP(O)(OR )2; -SiR 3; -
(C1_4
straight or branched alkylene)O-N(R )2; or -(C1_4 straight or branched
alkylene)C(O)O-N(R )2, wherein each R may be substituted as defined below and
is
independently hydrogen, C1-6 aliphatic, -CH2Ph, -O(CH2)o-IPh, or a 5-6-
membered saturated,
partially unsaturated, or aryl ring having 0-4 heteroatoms independently
selected from
nitrogen, oxygen, or sulfur, or, notwithstanding the definition above, two
independent
occurrences of R , taken together with their intervening atom(s), form a 3-12-
membered
saturated, partially unsaturated, or aryl mono- or bicyclic ring having 0-4
heteroatoms
independently selected from nitrogen, oxygen, or sulfur, which may be
substituted as defined
below.
[0072] Suitable monovalent substituents on R (or the ring formed by taking
two
independent occurrences of R together with their intervening atoms), are
independently
halogen, -(CH2)0-2R', -(haloR'), -(CH2)0 20H, -(CH2)0_20R', -(CH2)0-2CH(OR')2;
-O(haloR'), -CN, -N3, -(CH2)02C(O)R -(CH2)02C(O)OH, -(CH2)02C(O)OR', -(CH2)0_
2SR', -(CH2)0_2SH, -(CH2)02NH2, -(CH2)0_2NHR', -(CH2)02NR'2, -NO2, -SiR'3, -
OSiR'3,
-C(O)SR', -(C1.4 straight or branched alkylene)C(O)OR', or -SSR' wherein each
R' is
unsubstituted or where preceded by "halo" is substituted only with one or more
halogens, and
is independently selected from C1-4 aliphatic, -CH2Ph, -O(CH2)o-IPh, or a 5-6-
membered
saturated, partially unsaturated, or aryl ring having 0-4 heteroatoms
independently selected
from nitrogen, oxygen, or sulfur. Suitable divalent substituents on a
saturated carbon atom of
R include =0 and =S.
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
23
[0073] Suitable divalent substituents on a saturated carbon atom of an
"optionally
substituted" group include the following: =O, =S, =NNR*2, =NNHC(O)R*,
=NNHC(O)OR*,
=NNHS(0)2R*, =NR*, =NOR*, -O(C(R*2))2_30-, or -S(C(R*2))2_3S-, wherein each
independent occurrence of R* is selected from hydrogen, Ci_6 aliphatic which
may be
substituted as defined below, or an unsubstituted 5-6-membered saturated,
partially
unsaturated, or aryl ring having 0-4 heteroatoms independently selected from
nitrogen,
oxygen, or sulfur. Suitable divalent substituents that are bound to vicinal
substitutable
carbons of an "optionally substituted" group include: -O(CR*2)2_30-, wherein
each
independent occurrence of R* is selected from hydrogen, Ci_6 aliphatic which
may be
substituted as defined below, or an unsubstituted 5-6-membered saturated,
partially
unsaturated, or aryl ring having 0-4 heteroatoms independently selected from
nitrogen,
oxygen, or sulfur.
[0074] Suitable substituents on the aliphatic group of R* include halogen, -
R',
-(haloR'), -OH, -OR', -O(haloR'), -CN, -C(O)OH, -C(O)OR', -NH2, -NHR', -NR'2,
or
-NO2, each R' is unsubstituted or where preceded by "halo" is substituted only
with
one or more halogens, and is independently Ci_4 aliphatic, -CH2Ph, -
O(CH2)o_1Ph, or a 5-6-
membered saturated, partially unsaturated, or aryl ring having 0-4 heteroatoms
independently
selected from nitrogen, oxygen, or sulfur.
[0075] Suitable substituents on a substitutable nitrogen of an "optionally
substituted"
group include -Rt, -NRt2, -C(O)Rt, -C(O)ORt, -C(O)C(O)Rt, -C(O)CH2C(O)Rt, -
S(O)2Rt,
-S(O)2NRt2, -C(S)NRt2, -C(NH)NRt2, or -N(R)S(O)2Rt; wherein each Rt is
independently
hydrogen, Ci_6 aliphatic which may be substituted as defined below,
unsubstituted -OPh, or
an unsubstituted 5-6-membered saturated, partially unsaturated, or aryl ring
having 0-4
heteroatoms independently selected from nitrogen, oxygen, or sulfur, or,
notwithstanding the
definition above, two independent occurrences of Rt, taken together with their
intervening
atom(s) form an unsubstituted 3-12-membered saturated, partially unsaturated,
or aryl mono-
or bicyclic ring having 0-4 heteroatoms independently selected from nitrogen,
oxygen, or
sulfur.
[0076] Suitable substituents on the aliphatic group of Rt are independently
halogen,
-R', -(haloR'), -OH, -OR', -O(haloR'), -CN, -C(O)OH, -C(O)OR', -NH2, -NHR', -
NR'2, or
-NO2, each R' is unsubstituted or where preceded by "halo" is substituted only
with
one or more halogens, and is independently Ci_4aliphatic, -CH2Ph, -
O(CH2)o_1Ph, or a 5-6-
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
24
membered saturated, partially unsaturated, or aryl ring having 0-4 heteroatoms
independently
selected from nitrogen, oxygen, or sulfur.
[0077] Partially unsaturated: As used herein, the term "partially unsaturated"
refers
to a ring moiety that includes at least one double or triple bond between ring
atoms but is not
aromatic. The term "partially unsaturated" is intended to encompass rings
having multiple
sites of unsaturation, but is not intended to include aryl or heteroaryl
moieties, as herein
defined.
[0078] Unsaturated: The term "unsaturated", as used herein, means that a
moiety has
one or more units of unsaturation.
BRIEF DESCRIPTION OF THE DRAWINGS
[0079] The drawings are for illustration purposes only, not for limitation.
[0080] Figure 1 is an exemplary graph showing the inhibition of IL-1 induced
TF-1
cell proliferation by peptides 54-59.
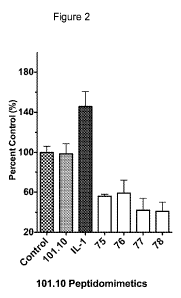
[0081] Figure 2 is an exemplary graph showing the inhibition of IL-1 induced
TF-1
cell proliferation by compounds 75-78.
[0082] Figure 3 illustrates activity of exemplary peptidomimetics 101.164-
101.167.
DETAILED DESCRIPTION OF CERTAIN EMBODIMENTS
[0083] The present invention provides, among other things, peptidomimetics
containing one or more lactams and/or Bab residues that can effectively
modulate (e.g.,
inhibit or activate) the biological activity of IL-1 R. In some embodiments,
peptidomimetics
of the present invention are modified from IL-1R modulatory peptides (such as
AllosteramersTM) by lactam and/or Bab replacement or insertion.
Peptidomimetics according
to the present invention can be used to treat various IL-1 related diseases,
disorders or
conditions.
[0084] Various aspects of the invention are described in detail in the
following
sections. The use of sections is not meant to limit the invention. Each
section can apply to
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
any aspect of the invention. In this application, the use of "or" means
"and/or" unless stated
otherwise.
IL-IR Modulatory Peptides
[0085] Various IL-1R modulatory peptides may be optimized according to the
present
invention. Suitable IL-1R modulatory peptides include antagonistic peptides or
agonistic
peptides. As used herein, an IL-1R antagonistic peptide refers to any peptide
that can directly
or indirectly inhibit, suppress, inactivate and/or decrease the receptor
activity of IL-1R and/or
IL-lRacP. As used herein, an IL-1R agonistic peptide refers to any peptide
that can directly
or indirectly activates, enhance, stimulate and/or increase the receptor
activity of IL-1R
and/or IL-lRacP. In particular, suitable IL-1R modulatory peptides may be
AllosteramersTM
(short peptides composed of 5-25 amino acids derived from body's own proteins
that can
interact with the protein from which it is derived, or with its associated
proteins, without
competing with a natural ligand) that interact with a region of the
extracellular domain of the
IL-1R/IL-lRacP receptor complex. Typically, suitable peptides for the
invention modulate
the IL-1R receptor activity non-competitively (i.e., without competing with a
natural ligand
of the receptor). In some embodiments, a suitable peptides for the invention
is a Negative
Allosteric Modulator (NAM) of the IL-1R activity. In some embodiments, a
suitable peptide
for the invention is a Positive Allosteric Modulator (PAM) of the IL-1R
activity. In some
embodiments, a suitable peptide is both an NAM and a PAM of the IL-1R
activity.
[0086] In some embodiments, suitable IL-1R modulatory peptides may be
strategically designed to interact with at least one of an extracellular
flexible region of the IL-
1R/IL-1RacP complex that are responsible for oligomerization, and that are
important for the
appropriate conformation of the IL-1 receptor which enables signaling. Such
flexible regions
include, but are not limited to, juxtamembranous regions, regions containing a
helix, R sheet,
loops and/or R turns, regions between domains, regions between two R chains,
and
combinations thereof. Thus, in some embodiments, IL-1R modulatory peptides are
designed
based on amino acid sequences that appear within such flexible regions. This
approach is
known as Module X technology and is further described in U.S. Patent No.
7,432,341,
entitled "Cytokine receptor modulators and method of modulating cytokine
receptor
activity," and in U.S. Pub. No. 20060094663 entitled "Interleukin-1 Receptor
Antagonists,
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
26
Compositions, and Methods of Treatment," the entire disclosure of both of
which are hereby
incorporated by reference.
[0087] In some embodiments, suitable IL-1R modulatory peptides may be designed
simply based on the primary linear amino acid sequence of extracellular or
transmembrane
regions of the IL-1R and IL-1RacP protein without first characterizing any
tertiary or
secondary structure of the receptor. This method is also referred to as the
Module X
"walking" method and is further described in U.S. Provisional Application
Serial No.
61/172,533, entitled "TNF receptor antagonists and uses thereof," the entire
disclosure of
which is hereby incorporated by reference.
[0088] For example, suitable IL-1R modulatory peptides may be designed based
on
contiguous amino acids that appear in the extracellular and/or transmembrane
regions of the
IL-1 receptor. The amino acid sequence of the IL-1R is shown below. The
projected
transmembrane domain is underlined and bolded. The extracellular region is N-
terminal to
the transmembrane domain.
MKVLLRLICFIALLISSLEADKCKEREEKIILVSSANEIDVRPCPLNPNEHKGTITWYKD
DSKTPVSTEQASRIHQHKEKLWFVPAKVEDSGHYYCVVRNSSYCLRIKISAKFVENE
PNLCYNAQAIFKQKLPVAGDGGLV CPYMEFFKNENNELPKLQ WYKDCKPLLLDNIH
FSGVKDRLIVMNVAEKHRGNYTCHASYTYLGKQYPITRVIEFITLEENKPTRPVIVSP
ANETMEVDLGSQIQLICNVTGQLSDIAYWKWNGSVIDEDDPVLGEDYYSVENPANK
RRSTLITVLNI SEIE SRFYKHPFTCFAKNTHGIDAAYIQLIYPVTNFQKHMIGIC VTLT
VIIVCSVFIYKIFKIDIVLWYRDSCYDFLPIKASDGKTYDAYILYPKTVGEGSTSDCDIF
VFKVLPEVLEKQCGYKLFIYGRDDYVGEDIVEVINENVKKSRRLIIILVRETSGFSWLG
GSSEEQIAMYNALVQDGIKVVLLELEKIQDYEKMPESIKFIKQKHGAIRWSGDFTQGP
QSAKTRFWKNVRYHMPVQRRSPSSKHQLLSPATKEKLQREAHVPLG (SEQ ID NO: 1)
[0089] In some embodiments, suitable IL-1R modulatory peptides may be designed
based on contiguous amino acids that appear in the extracellular regions of
the IL-1RacP.
The amino acid sequence of an exemplary isoform of the IL-1RacP protein is
shown below.
The sequences of other isoforms are known in the art. The projected
transmembrane domain
is underlined and bolded. The extracellular region is N-terminal to the
transmembrane
domain.
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
27
MTLLWCVV SLYFYGILQ SDASERCDDWGLDTMRQIQVFEDEPARIKCPLFEHFLKFN
YSTAHSAGLTLIWYWTRQDRDLEEPINFRLPENRISKEKDVLWFRPTLLNDTGNYTC
MLRNTTYCSKVAFPLEVVQKDSCFNSPMKLPVHKLYIEYGIQRITCPNVDGYFPSSV
KPTITWYMGCYKIQNFNNVIPEGMNLSFLIALISNNGNYTCVVTYPENGRTFHLTRTL
TVKVVGSPKNAVPPVIHSPNDHVVYEKEPGEELLIPCTVYFSFLMDSRNEVWWTIDG
KKPDDITIDVTINESISHSRTEDETRTQILSIKKVTSEDLKRSYVCHARSAKGEVAKAA
KVKQKVPAPRYTVELACGFGATVLLVVILIVVYHVYWLEMVLFYRAHFGTDETIL
DGKEYDIYVSYARNAEEEEFVLLTLRGVLENEFGYKLCIFDRDSLPGGIVTDETLSFIQ
KSRRLLV VLSPNYVLQGTQALLELKAGLENMASRGNINVILV QYKAVKETKVKELK
RAKTVLTVIKWKGEKSKYPQGRFWKQLQVAMPVKKSPRRSSSDEQGLSYSSLKN
(SEQ ID NO:2)
[0090] Typically, a suitable peptide is a short peptide. As used herein, a
short peptide
includes any peptide that contains up to 25 amino acids (e.g., up to 5, 6, 7,
8, 9, 10, 11, 12,
13, 14, 15, 20, or 25 amino acids) or equivalents thereof. In some
embodiments, a suitable
peptide contains 5-25 amino acids (e.g., 5-20, 5-15, 5-12, 5-10, 6-25, 6-20, 6-
15, 6-12, 6-10,
7-25, 7-20, 7-15, 7-12, or 7-10 amino acids) or equivalents thereof. In some
embodiments, a
suitable peptide is 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 20, or 25 amino
acids long.
[0091] In some embodiments, candidate peptides are designed to contain a
sequence
corresponding to at least 5 (e.g., at least 6, 7, 8, 9, 10, 11, 12, 13, 14,
15, 18, 20, or 25)
contiguous amino acids that appear in an extracellular or transmembrane region
of the IL-
l RacP or IL-1 R protein.
[0092] In some embodiments, a sequence corresponding to at least 5 (e.g., at
least 6,
7, 8, 9, 10, 11, 12, 13, 14, 15, 18, 20, or 25) contiguous amino acids that
appear in an
extracellular or transmembrane region of a receptor includes any sequence
having at least
70% (e.g., at least 75%, 80%, 85%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%,
98%, 99%)
identical to the sequence of at least 5 (e.g., at least 6, 7, 8, 9, 10, 11,
12, 13, 14, 15, 18, 20, or
25) contiguous amino acids. Percentage of amino acid sequence identity can be
determined
by alignment of amino acid sequences. Alignment of amino acid sequences can be
achieved
in various ways that are within the skill in the art, for instance, using
publicly available
computer software such as BLAST, ALIGN or Megalign (DNASTAR) software. Those
skilled in the art can determine appropriate parameters for measuring
alignment, including
any algorithms needed to achieve maximal alignment over the full length of the
sequences
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
28
being compared. Preferably, the WU-BLAST-2 software is used to determine amino
acid
sequence identity (Altschul et at., Methods in Enzymology 266, 460-480 (1996);
http://hg.wustl.edu/blast/README.html). WU-BLAST-2 uses several search
parameters,
most of which are set to the default values. The adjustable parameters are set
with the
following values: overlap span=1, overlap fraction=0.125, word threshold
(T)=11. HSP score
(S) and HSP S2 parameters are dynamic values and are established by the
program itself,
depending upon the composition of the particular sequence, however, the
minimum values
may be adjusted and are set as indicated above.
[0093] In some embodiments, a sequence corresponding to at least 5 (e.g., at
least 6,
7, 8, 9, 10, 11, 12, 13, 14, 15, 18, 20, or 25) contiguous amino acids that
appear in an
extracellular or transmembrane region of a receptor includes any sequence
otherwise
identical to the sequence of the at least 5 (e.g., at least 6, 7, 8, 9, 10,
11, 12, 13, 14, 15, 18, 20,
or 25) contiguous amino acids but incorporating one or more D-amino acid
substitutions for
corresponding L-amino acids. Peptides containing such a sequence are also
known as D-
isomers. In some embodiments, a sequence corresponding to at least 5 (e.g., at
least 6, 7, 8,
9, 10, 11, 12, 13, 14, 15, 18, 20, or 25) contiguous amino acids that appear
in an extracellular
or transmembrane region of a receptor includes any sequence that is an inverse
of the at least
(e.g., at least 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 18, 20, or 25) contiguous
amino acids.
Peptides containing such a sequence are also known as reversed L-peptides. In
some
embodiments, a sequence corresponding to at least 5 (e.g., at least 6, 7, 8,
9, 10, 11, 12, 13,
14, 15, 18, 20, or 25) contiguous amino acids that appear in an extracellular
or
transmembrane region of a receptor includes any sequence that is otherwise an
inverse of the
at least 5 (e.g., at least 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 18, 20, or 25)
contiguous amino acids
but incorporating one or more D-amino acid substitutions for corresponding L-
amino acids.
Peptides containing such a sequence are also known as reversed D-peptides.
[0094] In some embodiments, a peptide suitable for the invention contains a
sequence
that includes at least 4 (e.g., at least 5, 6, or 7) from at least 7 (e.g., at
least 8, 9, 10, 11, 12)
contiguous amino acids that appear in an extracellular or transmembrane region
of the IL-
lRacP or IL-1R protein, wherein the at least 4 (e.g., at least 5, 6, or 7)
amino acids maintain
their relative positions and/or spacing as they appear in the extracellular or
transmembrane
region of the IL-lRacP or IL-1R protein. In some embodiments, a peptide
suitable for the
invention contains a sequence that includes at least 4 (e.g., at least 5, 6,
or 7) from at least 7
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
29
(e.g., at least 8, 9, 10, 11, 12) contiguous amino acids that appear in an
extracellular or
transmembrane region of the IL-lRacP or IL-1R protein, the at least 4 (e.g.,
at least 5, 6, or 7)
amino acids maintain their relative positions and/or spacing, but in the
inverse configuration,
as they appear in the extracellular or transmembrane region of the IL-1RacP or
IL-1R protein.
[0095] Exemplary IL-1R antagonistic peptides suitable for the present
invention are
shown in Table 1.
Table 1: Exemplary Peptides
LIST OF SEQUENCE NUMBERS
SEQ ID NO:3 API-101 APRYTVELA
SEQ ID NO:4 API-101.1 AARYTVELA
SEQ ID NO:5 API-101.2 APAYTVELA
SEQ ID NO:6 API-101.3 APRATVELA
SEQ ID NO:7 API-101.4 APRYAVELA
SEQ ID NO:8 API-101.5 APRYTAELA
SEQ ID NO:9 API-101.6 APRYTVALA
SEQ ID NO:10 API-101.7 APRYTVELA
SEQ ID NO:11 API-101.9 PRYTVELA
SEQ ID NO: 12 API-101.10 RYTVELA
SEQ ID NO: 13 API-101.11 YTVELA
SEQ ID NO: 14 API-101.12 TVELA
SEQ ID NO:15 API-101.101 XYTVELA (X = Citrulline)
SEQ ID NO:16 API-101.102 XYTVQLA (X = Citrulline)
SEQ ID NO: 17 API-101.103 RYTVQLA
SEQ ID NO: 18 API-101.104 RFTVELA
SEQ ID NO: 19 API-101.105 RYSVELA
SEQ ID NO:20 API-101.106 RYVVELA
SEQ ID NO:21 API-101.107 RYTPELA
SEQ ID NO:22 API-101.108 RYTVEL
SEQ ID NO:23 API-101.113 RYTPEL
SEQ ID NO:24 API-101.114 KYTPELA
SEQ ID NO:25 API-101.115 XYTPELA (X = Omithine
SEQ ID NO:26 API-101.116 RWTPELA
SEQ ID NO:27 API-101.117 RYTPDLA
SEQ ID NO:28 API-101.118 RYTPQLA
SEQ ID NO:29 API-101.119 RYTPEFA
SEQ ID NO:30 API-101.120 RYTPEMA
SEQ ID NO:31 API-101.121 XRYTPELA (X = Acetyl)
SEQ ID NO:32 API-101.122 RYTPEPA
SEQ ID NO:33 API-101.123 RYTPALA
SEQ ID NO:34 API-101.126 XYTPEL (X = Ornithine
SEQ ID NO:35 API-101.127 RFVPELA
SEQ ID NO:36 API-101.128 RWTPEL
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
SEQ ID NO:37 API-101.129 RYTPEV
SEQ ID NO:38 API-101.132 RFTPEL
SEQ ID NO:39 API-101.133 KYTPEL
SEQ ID NO:40 API-101.134 XYTPEL (X = Citrulline)
[0096] Suitable peptides may include naturally-occurring amino acids and/or
unnatural amino acids. For example, suitable peptides may be composed of all L-
amino
acids, all D-amino acids, a combination of L- and D-amino acids, or a
combination of
naturally-occurring and unnatural amino acids. Exemplary peptides containing a
combination of L-amino acids, D-amino acids and unnatural amino acids are
shown in Table
2.
Table 2: Additional Exemplary Peptides and Peptidomimetics
Unless otherwise noted, lower cases represent D-amino acids and upper cases
represent L-
amino acids.
LIST OF SEQUENCE NUMBERS
SEQ ID NO:41 101.135 Rytpel
SEQ ID NO:42 101.145 Tel
SEQ ID NO:43 101.146 rytPel
SEQ ID NO:44 101.147 rytpEl
SEQ ID NO:45 101.148 rytpeL
SEQ ID NO:46 101.149 rtppel
SEQ ID NO:47 101.150 ryPpel
SEQ ID NO:48 101.151 rypPel
SEQ ID NO:49 101.152 ryPPel
SEQ ID NO:50 101.153 ryxpel x=D- i ecolate
SEQ ID NO:51 101.154 ryXpel X=L- i ecolate
SEQ ID NO:52 101.155 rytxel x=D- i ecolate
SEQ ID NO:53 101.156 rytXel X=L- i ecolate
SEQ ID NO:54 101.157 rhtpel (d-amino acids
SEQ ID NO:55 101.158 rztpel z=D-Do a
SEQ ID NO:56 101.159 r(cha)tpel
cha=D-c clohex alanine
SEQ ID NO:57 101.160 rytp('D-alpha-
aminoadiis acid )l
SEQ ID NO:58 101.161 rytpe(cha)
cha=D-c clohex alanine
SEQ ID NO:59 101.162 HN=C H2 NH CH2 4CO- el
SEQ ID NO:60 101.163 Rytphl
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
31
[0097] Additional examples of IL-1R antagonistic peptides are disclosed in
U.S.
Application Publication No. US 2006/0094663, entitled "Interleukin-1 Receptor
Antagonists,
Compositions, and Methods of Treatment," the entire disclosure of which is
hereby
incorporated by reference.
Peptidomimetics containing lactam and/or Bab residues
[0098] One useful modification in optimizing a peptide structure is the
addition of
conformational constraints. Without wishing to be bound by theory, it is
contemplated that
conformational constraints can lock the secondary structure of a peptide in a
bioactive
conformation, thus enhancing biological potency by reducing the entropic cost
of binding. If
the conformation constraint stabilizes the bioactive conformation, the
stabilization often
results in a significant increase in biological activity. For example, alpha-
amino-gamma-
lactam (Agl) and beta-amino-gamma-lactam (Bgl) can be utilized to constrain
the backbone
conformation of linear peptides to give beta-turn mimics. In Agl, the gamma-
lactam may
force the C-terminal amide into the trans-orientation and restricts the psi-
torsion angle to the
range -125 100, favoring a type II' beta-turn geometry contingent on
configuration.
Similarly, Bgl too can stabilize a type II' beta-turn conformation contingent
on configuration.
H2N
HZN, 4,q NH NH NH NH
HZN HZN,
0 0 0 0
(S)-Agl (R)-Agl (R)-Bgl (S)-Bgl
[0099] In some embodiments, the present invention provides a peptidomimetic
comprising one or more lactams of formula (I):
~ Rx ) n
m j N f
H
0
(I)
wherein:
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
32
each RX is independently -R, halogen, -OR, -SR, -N(R)2, -CN, -NO2, -C(O)R, -
CO2R, -
C(O)N(R)2, -C(O)C(O)R, -C(O)CH2C(O)R, -S(O)R, -SO2R, -SO2N(R)2, -NRC(O)R, -
NRC(O)N(R)2, -NRSO2R, -NRSO2N(R)2, -N(R)N(R)2, -C=NN(R)2, -C=NOR, -OC(O)R,
or -OC(O)N(R)2;
each R is independently hydrogen or an optionally substituted group selected
from C1-6
aliphatic; phenyl; a 3- to 7-membered saturated or partially unsaturated
carbocyclic ring;
a 5- to 6-membered monocyclic heteroaryl ring having 1-3 heteroatoms
independently
selected from nitrogen, oxygen, and sulfur; or a 4- to 7-membered saturated or
partially
unsaturated heterocyclic ring having 1-3 heteroatoms independently selected
from
nitrogen, oxygen, and sulfur; or
two R groups attached to the same nitrogen atom may be taken together with
their
intervening atoms to form a 4- to 7- membered saturated or partially
unsaturated
heterocyclic ring having 1-2 heteroatoms independent selected from nitrogen,
oxygen,
and sulfur;
m is 0, 1, or 2; and
nis0, 1, 2, or 3.
[0100] In some embodiments, m of formula (I) is 0. In other embodiments, m is
1 or
2.
[0101] In some embodiments, n of formula (I) is 1. In other embodiments, n is
0 or 2.
[0102] In some embodiments, a lactam of formula (I) is alpha-amino-gamma-
lactam
(Agl), beta-amino-gamma-lactam (Bgl), or beta-hydroxy-alpha-amino-gamma-lactam
(Agl(4-
OH)).
[0103] In some embodiments, a lactam of formula (I) will take up the space of
approximately one amino acid residue in a peptidomimetic.
[0104] In some embodiments, the present invention provides a peptidomimetic
comprising one or more lactams of formula (II):
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
33
H
q
HN
N TOP_
O (I
I)
wherein:
each R3' is independently -R, halogen, -OR, -SR, -N(R)2, -CN, -NO2, -C(O)R, -
CO2R, -
C(O)N(R)2, -C(O)C(O)R, -C(O)CH2C(O)R, -S(O)R, -SO2R, -SO2N(R)2, -NRC(O)R, -
NRC(O)N(R)2, -NRSO2R, -NRSO2N(R)2, -N(R)N(R)2, -C=NN(R)2, -C=NOR, -OC(O)R,
or -OC(O)N(R)2;
each R is independently hydrogen or an optionally substituted group selected
from C1-6
aliphatic; phenyl; a 3- to 7-membered saturated or partially unsaturated
carbocyclic ring;
a 5- to 6-membered monocyclic heteroaryl ring having 1-3 heteroatoms
independently
selected from nitrogen, oxygen, and sulfur; or a 4- to 7-membered saturated or
partially
unsaturated heterocyclic ring having 1-3 heteroatoms independently selected
from
nitrogen, oxygen, and sulfur; or
two R groups attached to the same nitrogen atom may be taken together with
their
intervening atoms to form a 4- to 7- membered saturated or partially
unsaturated
heterocyclic ring having 1-2 heteroatoms independent selected from nitrogen,
oxygen,
and sulfur;
p is 0, 1, or 2; and
each q is independently 0, 1, 2, or 3.
[0105] In some embodiments, p of formula (II) is 0. In other embodiments, p is
1 or
2.
[0106] In some embodiments, each q of formula (II) is 1 or 2.
[0107] In some embodiments, a lactam of formula (II) is indolizidin-2-one
amino acid
(haa), indolizidin-9-one amino acid (I9aa), or quinolizidinone amino acid
(Qaa).
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
34
[0108] In some embodiments, a lactam of formula (II) will take up upto two
amino
acid residue space in a peptidomimetic.
[0109] In some embodiments, lactams suitable for the invention are (3-, y- or
6-
lactams. In some embodiments, lactams suitable for the invention are four-,
five-, six-,
seven- or eight-membered lactams.
[0110] In addition, benzylaminobutyryl "Bab" residue may be used to optimize a
peptide according to the present invention. In some embodiments, a
peptidomimetic
according to the present invention includes one ore more Bab residues as shown
below:
H
Benzylaminobutyryl "Bab" residue
[0111] In some embodiments, the present invention provides derivatives
containing
lactams and/or Bab residues of various peptides described herein (e.g., in
Tables 1 and 2).
Suitable derivatives may contain one or more (e.g., one, two or three) lactam
and/or Bab
replacements or insertions. In some embodiments, derivatives according to the
invention
comprise a lactam and/or Bab insertion or replacement at position 1, 2, 3, 4,
5, 6, 7, 8 or 9
from the N-terminus of any one of the peptides described herein, for example
as shown in
Tables 1 (SEQ ID NO:3-40) and 2 (SEQ ID NO:41-60). In particular embodiments,
derivatives according to the invention comprise a lactam and/or Bab insertion
or replacement
at position 1, 2, 3, 4, 5, 6, or 7 from the N-terminus of peptide 101.10
RYTVELA (SEQ ID
NO:12).
[0112] In some embodiments, the present invention provides a peptidomimetic
comprising one or more lactams and/or Bab residues and at least three (e.g.,
at least four, at
least five, or at least six) out of seven contiguous amino acids that appear
in an extracellular
or transmembrane region of the IL-1 receptor (SEQ ID NO: 1) or IL-1 receptor
accessory
protein (IL-1RacP) (SEQ ID NO:2), wherein the at least three (e.g., at least
four, at least five,
or at least six) amino acids maintain their relative positions as they appear
in the extracellular
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
or transmembrane region of the IL-lR or IL-lRacP protein. In other
embodiments, the
present invention provides a peptidomimetic comprising one or more lactams
and/or Bab
residues and at least three (e.g., at least four, at least five, or at least
six) out of seven
contiguous amino acids that appear in an extracellular or transmembrane region
of the IL-1R
or IL-lRacP protein, wherein the at least three (e.g., at least four, at least
five, or at least six)
amino acids maintain their relative positions, but in the inverse
configuration, as they appear
in the extracellular or transmembrane region of the IL-1R or IL-1 RacP
protein. In some
embodiments, the one or more lactams and/or Bab residues replace one or more
amino acids
of the seven contiguous amino acids. In some embodiments, the one or more
lactams and/or
Bab residues are inserted at the N-terminus, C-terminus, or internally.
[0113] In some embodiments, the present invention provides a peptidomimetic
comprising one or more lactams and/or Bab residues and at least three (e.g.,
at least four, at
least five, at least six) amino acids from any one of SEQ ID NOS:3-40, wherein
the at least
three (e.g., at least four, at least five, or at least six) amino acids
maintain their relevant
positions as they appear in the corresponding sequence. In some embodiments,
the present
invention provides a peptidomimetic comprising one or more lactams and/or Bab
residues
and at least three (e.g., at least four, at least five, or at least six) amino
acids from RYTVELA
(SEQ ID NO: 12), wherein the at least three (e.g., at least four, at least
five, or at least six)
amino aicds maintain their relevant positions as they appear in RYTVELA (SEQ
ID NO: 12).
In some embodiments, one or more lactams and/or Bab residues replace one or
more amino
acids of RYTVELA (SEQ ID NO:12). In some embodiments, the one or more lactams
are
inserted at the N-terminus, C-terminus, or internally.
[0114] Various methods can be used to introduce lactam and/or Bab residue into
the
peptides described herein. In some embodiments, systematic scans of a peptide
described
herein with lactam and/or Bab can be used. Various synthetic strategies for
the synthesis of
Agl peptide mimics are known in the art and can be used for the present
invention. See, for
example, Toniolo, C. Int. J. Pept. Protein Res. 1990, 35, 287, and references
therein; Wolf, J-
P.; Rapoport, H. J. Org. Chem. 1989, 54, 3164. Schuster, M.; Blechert, S.
Angew. Chem. Int.
Ed. 1997, 36, 2036. Wolfe, M. S.; Dutta, D.; Aube, J. J. Org. Chem. 1997, 62,
654. (b) Noth,
J.; Frankowski, K. J.; Neuenwander, B.; Aube, J. J. Comb. Chem. 2008,10,456;
Piscopio, A.
P. D.; Miller J. F.; Koch K. Tetrahedron Letters. 1998, 39, 2667; Armstrong,
S.K. J. Chem.
Soc., Perkin Trans. 1 1998, 1, 371; Piscopio, A. D.; Miller, J. F.; Koch K.
Tetrahedron 1999,
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
36
55, 8189; Galaud, F.; Lubell, W. D. Biopolymers (Peptide Science) 2005, 80,
665; Bhooma,
R.; Rodney, J. J. Org. Chem. 2006, 71, 2151, and references therein, the
teachings of all of
which are incorporated herein by reference.
[0115] Exemplary methods are described in the Examples section. Additional
methods are described in co-owned U.S. Patent Application entitled "Processes
for Preparing
Amino-substituted gamma-lactams" filed on even date, the entire disclosure of
which is
hereby incorporated by reference. In addition, various methods have been used
to
incorporate various lactams to other proteins and peptides. Those methods and
lactams are
suitable for the present invention and can be found in the following:
Freidinger et al, Science
210:656-8 (1980) (alpha-amino-gamma-lactam incorporated into luteinizing
hormone-
releasing hormone (LRHH); Mishra et al, Prog, Neuropsychopharmacol. Biol.
Psych. 14:821-
827 (1990) (amino-gamma-lactam analogs of the Pro-Gly-Leu-NHe sequence for D2
dopamine receptor modulation); United States Publication No. 2006/0229240
(parathyroid
hormone (PTH) derivatives containing lactam bridges); United States
Publication No.
2009/0209491 (lactam-containing inhibitors of post-proline cleaving enzymes
(PPCE));
Gomez et al, Bioorg. Med. Chem. Lett. 19:1733-1736 (2009) (lactam-containing
peptidomimetic inhibitors of STAT3).
[0116] Although exemplary methods described in the Example sections are based
on
IL-1R antagonistic peptide 101.10 RYTVELA (SEQ ID NO: 12), similar methods can
be used
to introduce various lactams and/or Bab residues into any one of the peptides
described
herein (e.g., peptides having an amino acid sequence as shown in Tables 1 and
2) or variants
thereof. As used herein, variants of a peptide include peptides that have an
amino acid
sequence at least 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%, 98%, 99%,
identical to the sequence of the parent peptide. In some embodiments, variants
of peptides as
shown in Tables 1 and 2 (e.g., SEQ ID NOs:3-60) include peptides having at
least three, four,
five, six, or seven amino acids from any one of peptides as shown in Tables 1
and 2 (e.g.,
SEQ ID NOs:3-60), wherein the at least three, four, five, six, or seven amino
acids maintain
their relative positions and/or spacing as they appear in the corresponding
parent peptide.
[0117] The biological activity of peptidomimetics synthesized according to the
invention can be tested using various functional assays known in the art and
as described
herein. For example, the lactam peptidomimetics synthesized according to the
invention can
be tested for its ability to inhibit or enhance the activity of the IL-1
receptor using various IL-
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
37
1 functional assays (e.g., thymocyte TF-1 proliferation assay). Exemplary
methods are
described in the Examples section. Additional assays are described in U.S.
Application
Publication No. US 2006/0094663, entitled "Interleukin-1 Receptor Antagonists,
Compositions, and Methods of Treatment," the entire disclosure of which is
hereby
incorporated by reference.
[0118] The present invention further relates to methods of improving the
biological
activity of a peptide by imposing conformational constraints. In some
embodiments, the
methods according to the invention include introducing a lactam and/or Bab
residue
replacement or insertion into a peptide such that the lactam/Bab structure
locks the secondary
structure of a peptide in a bioactive conformation. In some embodiments, a
lactam/Bab
residue is introduced at N-terminus (also referred to as position 1 from the N-
terminus) of a
peptide. In some embodiments, a lactam/Bab residue is introduced at position
2, 3, 4, 5, 6, 7,
8 or 9, from the N-terminus of the peptide. In some embodiments, the methods
include a step
of testing the activity of modified peptide as compared to a control to
identify modified
peptides that have improved biological activity (e.g., the IL-1R antagonistic
or agonistic
activity). In some embodiments, the unmodified parent peptide is used as the
control. In
some embodiments, a modified peptide according to the invention has 2-fold,
2.5-fold, 3-
fold, 3.5-fold, 4-fold, 4.5-fold, 5-fold, 5.5-fold, 6-fold, 6.5-fold, 7-fold,
7.5-fold, 8-fold, 8.5-
fold, 9-fold, 9.5-fold, or 10-fold increased IL-1R modulatory (e.g.,
antagonistic or agonistic)
activity as compared to a control (e.g., an unmodified parent peptide).
[0119] Exemplary lactam peptidomimetics are shown in Tables 3, 4, and 6-8, and
in
the Examples.
Table 3. Exemplary Compounds and TF-1 Cells Proliferation Assay
SEQ ID NO Compound Compound Compound Structure Percentage
Number Name Control (/o)
- - Control - 100
(Proliferation)
12 101.10 101.10 vela-NHz 69
90 94 LRM 17 ryt-(S)-Agl-ela-NH2 132
89 93 LRM 18 ry- -A l-vela-NHz 150
Amino pentanoic-y-
99 3 WC125 (3R,6R,9R)-I2aa-ql- 88
NHZ
100 4 WC126 Omega amino 165
hexanoic-y-
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
38
(3R,6R,9R)-I2aa-gl-
NH2
101 5 WC127 oy-(3R,6R,9R)-I2 aa-ql- 95
NH2
96 6 WC115 oy-(3R,6R,9R)-I2 aa-el- 94
NH2
97 7 WC116 Amino pentanoic-y-
66
3R,6R,9R -I as-el-NH2
98 8 WC117 Amino hexanoic-y-
41
3R,6R,9R -I as-el-NHz
66 59 AJ-303-A ve- R -A l-a-NH2 93
65 58 AJ-350-A rytv-(R)-Agl-la-NH2 76
64 57 AJ-328-A ryt-(R)-Agl-ela-NH2 78
63 56 AJ-351-A ry- R -A l-vela-NH2 122
62 55 AJ-366-A r- R -A l-tvela-NH2 74
61 54 AJ-333-A R -A l- vela-NH2 44
70 74 AJ-328-B ryt-(R)-Bab-ela-NH2 41
69 73 AJ-351-B ry- R -Bab-vela-NH2 42
68 72 AJ-366-B r- R -Bab-tvela-NH2 59
67 71 AJ-333-B R -Bab- vela-NH2 56
*Results are shown as percentage of control.
Table 4. Exemplary peptidomimetics
Crude Purity Yield HRMS
Peptide' Purity % b %C %a m/z m/z
(calcd) (obsd)
...............................................................................
..........................
(R)-Agl-D-Tyr-D-Thr-D-Val-D-Glu-
D-Leu-D-Ala-NH2 (SEQ ID NO:61) 55 >99 19 777.4141 777.4138
54 .......................................... .....
....... ....... .......
D-Arg-(R)-Agl-D-Thr-D-Val-D-Glu-
D-Leu-D-Ala-NH2 (SEQ ID NO:62) 41 >99 15 770.4519 770.4514
55 .......................
...... .................. ....... ..........
D-Arg-D-Tyr-(R)-Agl-D-Val-D-Glu-
D-Leu-D-Ala-NH2 (SEQ ID NO:63) 58 >99 7 832.4675 832.4676
56 ............................~.................. .~.........................
D-Arg-D `Tyr-D-Thr-(R) `Agl-D `Glu-
D-Leu-D-Ala-NH2 (SEQ ID NO:64) 50 >99 11 833.4468 833.4458
57
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
D-Arg-D-Tyr-D-Thr-D-Val-(R)-Agl-
D-Leu-D-Ala-NH2 (SEQ ID NO:65) 36 >99 21 803.4726 803.4724
58
......................................
...............................................................................
...................................................
D-Arg-D-Tyr-D-Thr-D-Val-D-Glu-
(R)-Agl-D-Ala-NH2 (SEQ ID
N0:66) 81 ; >99 7 820.4311 820.4306
59
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
39
'Bold lettering indicates lactam residue. bRP-HPLC purity at 214 nm of the
crude peptide.
'RP-HPLC purity at 214 nm of the purified peptide dYields after purification
by RP-HPLC
are based on Fmoc
Table 5. Exemplary peptidomimetics
HRMS
Crude Purity Yield :.............................
%C %d m/z m/z
Peptide' Purity
y (calcd) obsd)
(R)-Bab-D-Tyr-D-Thr-D-Val-D-Glu-
D-Leu-D-Ala-NH2 (SEQ ID NO:67) 28 >99 10 885.4714 885.4716
71
D-Arg-(R)-Bab-D-Thr-D-Val-D-
Glu-D-Leu-D-Ala-NH2 (SEQ ID 45 >99 15 878.5092 878.5094
NO:68)
72
D-Arg-D-Tyr-(R)-Bab-D-Val-D-
Glu-D-Leu-D-Ala-NH2 (SEQ ID 27 >99 3 940.5240 940.5251
NO:69)
73
D-Arg-D-Tyr-D-Thr-(R)-Bab-D-
Glu-D-Leu-D-Ala-NH2 (SEQ ID 6 >99 4 942.5027 942.5043
NO:70)
74
'Bold lettering indicates lactam residues. ARP-HPLC % at 214 nm of the crude
mixture of the
parent lactam peptide. ARP-HPLC purity at 214 nm of the purified peptide
dYields after
purification by RP-HPLC are based on Fmoc loading test for Rink resin.
Table 6. Exemplary peptidomimetics
Crude HRMS
Yiel
Purity %b Punt
Peptide' (lactamizati Y%' %d m/z m/z
(calcd) (obsd)
on time
(R)-Bgl-D-Tyr-D-Thr-D-Val-D-Glu- 777,414
D-Leu-D-Ala-NH2 (SEQ ID NO:71) 71(4 h) >99 22 1 777.4145
D-Arg-(R)-Bgl-D-Thr-D-Val-D-Glu- 770.451
D-Leu-D-Ala-NH2 (SEQ ID NO:72) 87(6 h) >99 24 770.4521
76
D-Arg-D-Tyr-(R)-B gl-D-Val-D-Glu-
832.467
D-Leu-D-Ala-NH2 (SEQ ID NO:73) 86(4 h) >99 23 6 832.4677
77
D-Arg-D-Tyr-D-Thr-(R)-Bgl-D-Glu- 833.446
79(7 h)
4~ 8 833.4470
D-Leu-D-Ala-NH2(SEQIDNO`7 >99 17
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
78
D-Arg-D-Tyr-D-Thr-D-Val-(R)-Bgl- 826.454 826.4544
D-Leu-D-Ala-NH2 (SEQ ID NO:75) 87(4 h) >99 7 6e e
79
D-Arg-D-Tyr-D-Thr-D-Val-D-Glu- 820.431
(R)-Bgl-D-Ala-NH2 (SEQ ID NO:76) 50(4 h) >99 14 2 820.4313
(S)-BgI-D-Tyr-D-Thr-D-Val-D-Glu- 777,414
D-Leu-D-Ala-NH2 (SEQ ID NO:77) 70(10 h) >99 13 1 777.4138
81
D-Arg-(S)-Bgl-D-Thr-D-Val-D-Glu- 770.451
D-Leu-D-Ala-NH2 (SEQ ID NO:78) 82(10 h) >99 16 9 770.4518
82
D-Arg-D-Tyr-(S)-Bgl-D-Val-D -Glu-
832.467
D-Leu-D-Ala-NH2 (SEQ ID NO:79) 60(8 h) >99 17 6 832.4674
83
D-Arg-D-Tyr-D-Thr-(S)-Bgl-D-Glu- 833.446
D-Leu-D-Ala-NH2 (SEQ ID NO:80) 87(7 h) >99 14 8 833.4469
84
D-Arg-D-Tyr-D-Thr-D-Val-(S)-Bgl- 803.472
D-Leu-D-Ala-NH2 (SEQ ID NO:81) 90(4 h) >99 13 6 803.4724
D-Arg-D-Tyr-D-Thr-D-Val-D-Glu- 820.413
(S)-Bgl-D-Ala-NH2 (SEQ ID NO:82) 96(4 h) >99 11 1 820.4136
86
....... 6
...............................................................................
......................................
'Bold lettering indicates lactam residues. RP-HPLC purity at 214 nm of the
crude peptide.
CRP-HPLC purity at 214 nm of the purified peptide dYields after purification
by RP-HPLC
are based on Fmoc loading test for Rink resin. eThe cation [M+Na]+ was
observed, theoretical
mass was calculated accordingly.
Table 7. Exemplary peptidomimetics
Crude HRMS
Purity Yield \`
Peptide' Purity o e 0/ d m/z m/z
o b ~o o
~o calcd) (obsd)
D-Arg-(S)-Bgl-D-Tyr-D-Thr-D-Val-
D-Glu-D-Leu-D-Ala-NH2 (SEQ ID 56 >99 18 933.5152 933.5152
NO:83)
87
D-Arg- D-Tyr-(S)-Bgl-D-Thr-D-Val-
D-Glu-D-Leu-D-Ala-NH2 (SEQ ID
N0:84) 55 >99 6 933.5152 933.5137
88
D-Arg-D-Tyr-D-Thr-(S)-Bgl-D-Val-
71 >99 10 933.5152 933.5152
D-Glu-D-Leu-D-Ala-NH2 (SEQ ID
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
41
NO:85)
89
D-Arg `D-Tyr `D-Thr-D-Val-(S)-Bgl-
D-Glu-D-Leu-D-Ala-NH2 (SEQ ID 68 >99 6 933.5152 933.5147
NO:86)
D-Arg-D `Tyr-D-Thr `D-Val-D-Glu-
(S)-Bgl-D-Leu-D-Ala-NH2 (SEQ ID
N0:87) 37 >99 3 933.5152 933.5154
D-Arg-D-Tyr-D-Thr-D-Val-D-Glu-D-
Leu-(S)-BgI-D-Ala-NH2 (SEQ ID
N0:88) 97 >99 18 933.5152 933.5150
92
Bold lettering indicates lactam residues. RP-HPLC purity at 214 nm of the
crude peptide.
'RP-HPLC purity at 214 nm of the purified peptide dYields after purification
by RP-HPLC
are based on Fmoc loading test for Rink resin. eThe cation [M+Na]+ was
observed, theoretical
mass was calculated accordingly.
Table 8. Exemplary peptidomimetics
Purity Yield HRMS
Peptide Purity
a (%)b m/z calcd m/z obsd
93 D-Arg-D-Tyr-(S)-Agl-D-Val-D-Glu-
D-Leu-D-Ala-NH2 (SEQ ID NO:89) 32 >99 15 832.4676 832.4678
94 D-Arg-D-Tyr-D-Thr-(S)-Agl-D-Glu-
D-Leu-D-Ala-NH2 (SEQ ID NO:90) 18 >99 5 834.4468 834.4459
(R)-Agl-D-Tyr-D-Thr-D-Pro-D-Glu-
D-Leu-D-Ala-NH2 (SEQ ID NO:91) 55 >95 10 775.3985 775.3986
96 (R)-Agl-D-Tyr-D-Thr-(R)-Agl -D-
Glu-D-Leu-D-Ala-NH2 (SEQ ID
NO:92) 30 >99 5 761.3828 761.3832
97 (R)-Agl-(R)-Agl -D-Thr-D-Val -D-
Glu-D-Leu-D-Ala-NH2 (SEQ ID
NO:93) 45 >65 5 697.3880 697.3901
aRP-HPLC purity at 214 nm of the crude peptide. RP-HPLC purity at 214 nm of
the purified
peptide 'Yields after purification by RP-HPLC are based on resin and lantern
loading. Bold
lettering indicates lactam residues.
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
42
Peptide preparation
[0120] Peptides (including the peptide portions of peptidomimetics) of the
present
invention are obtained by any method of peptide synthesis known to those
skilled in the art,
including synthetic (e.g., exclusive solid phase synthesis, partial solid
phase synthesis,
fragment condensation, classical solution synthesis) and recombinant
techniques. For
example, the peptides or peptide derivatives can be obtained by solid phase
peptide synthesis,
which in brief, consists of coupling the carboxyl group of the C-terminal
amino acid to a
resin (e.g., benzhydrylamine resin, chloromethylated resin, hydroxymethyl
resin) and
successively adding N-alpha protected amino acids. The protecting groups maybe
any such
groups known in the art. Before each new amino acid is added to the growing
chain, the
protecting group of the previous amino acid added to the chain is removed.
Such solid phase
synthesis has been described, for example, by Merrifield, 1964, J. Am. Chem.
Soc. 85: 2149;
Vale et al., 1981, Science, 213: 1394-1397, in US Patent Nos. 4,305,872 and
4,316,891,
Bodonsky et at., 1966, Chem. Ind. (London), 38:1597; Pietta and Marshall,
1970, Chem.
Comm. 650. The coupling of amino acids to appropriate resins is also well
known in the art
and has been described in US Patent No. 4,244,946. (Reviewed in Houver-Weyl,
Methods of
Organic Chemistry. Vol E22a. Synthesis of Peptides and peptidomimetics, Murray
Goodman,
Editor-in-Chief, Thieme. Stuttgart. New York 2002).
[0121] During any process of the preparation of a compound of the present
invention,
it may desirable to protect sensitive reactive groups on any of the molecule
concerned. This
may be achieved by means of conventional protecting groups such as those
described in
Protective Groups In Organic Synthesis by T.W. Greene & P.G.M. Wuts, 1991,
John Wiley
and Sons, New-York; and Peptides: chemistry and Biology by Sewald and Jakubke,
2002,
Wiley-VCH, Wheinheim p.142. For example, alpha amino protecting groups include
acyl
type protecting groups (e.g., trifluoroacetyl, formyl, acetyl), aliphatic
urethane protecting
groups (e.g., t-butyloxycarbonyl (BOC), cyclohexyloxycarbonyl), aromatic
urethane type
protecting groups (e.g., fluorenyl-9-methoxy-carbonyl (Fmoc),
benzyloxycarbonyl (Cbz),
Cbz derivatives) and alkyl type protecting groups (e.g., triphenyl methyl,
benzyl). The amino
acids side chain protecting groups include benzyl (For Thr and Ser), Cbz (Tyr,
Thr, Ser, Arg,
Lys), methyl ethyl, cyclohexyl (Asp, His), Boc (Arg, His, Cys) etc. The
protecting groups
may be removed at a convenient subsequent stage using methods known in the
art.
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
43
[0122] In one embodiment, the peptides of this invention, including the
analogs and
other modified variants, may generally be synthesized according to the FMOC
protocol in an
organic phase with protective groups. They can be purified with a yield of 70%
with HPLC
on a C18 column and eluted with an acetonitrile gradient of 10-60%. Their
molecular weight
can then be verified by mass spectrometry (Reviewed in Fields, G.B. "Solid-
Phase Peptide
Synthesis". Methods in Enzymology. Vol. 289, Academic Press, 1997).
[0123] Alternatively, peptides of this invention that consist of genetically
encoded
amino acids may be prepared in recombinant systems using polynucleotide
sequences
encoding the peptides. It is understood that a peptide of this invention may
contain more than
one of the above-described modifications within the same peptide.
[0124] Purification of the synthesized peptide or peptide derivatives is
carried out by
standard methods, including chromatography (e.g., ion exchange, size
exclusion, affinity),
centrifugation, precipitation or any standard technique for the purification
of peptides and
peptide derivatives. In one embodiment, thin-layered chromatography is
employed. In
another embodiment, reverse phase HPLC is employed. Other purification
techniques well
known in the art and suitable for peptide isolation and purification may be
used in the present
invention.
[0125] Where the processes for the preparation of the compounds according to
the
present invention give rise to mixtures of stereoisomers, these isomers may be
separated by
conventional techniques such as preparative chromatography. The compounds may
be
prepared in racemic form, or individual enantiomers may be prepared either by
enantiospecific synthesis or by resolution. The compounds may, for example, be
resolved
into their components enantiomers by standard techniques such as the formation
of
diastereoisomeric pairs by salt formation with an optically active acid
followed by fractional
crystallization and regeneration of the free base. The compounds may also be
resolved by
formation of diastereomeric esters or amides, followed by removal of the
chiral auxiliary.
Alternatively, the compounds may be resolved using chiral HPLC column.
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
44
Further peptidomimetic modifications
[0126] Peptide mimetics that are structurally related to therapeutically
useful peptides
may be used to produce an equivalent or enhanced therapeutic or prophylactic
effect.
Generally, peptidomimetics are structurally similar to paradigm polypeptide
(i.e., a
polypeptide that has a biological or pharmacological activity) such as
naturally occurring
receptor-binding polypeptides but have one or more peptide linkages optionally
replaced by
linkages like -CH2NH-, -CH2S-, -CH2-CH2-, -CH=CH- (cis and trans), -CH2SO-, -
CH(OH)CH2-, -COCH2- etc., by methods well known in the art (Spatola A.F.,
Peptide
Backbone Modifications, Vega Data, March 1983, 1(3): 267; Spatola et at., Life
Sci., 1986,
38:1243-1249; Hudson D. et at., Int. J. Pept. Res. 1979., 14: 177-185;
Weinstein. B., 1983,
Chemistry and Biochemistry, of Amino Acids, Peptides and Proteins, Weinstein
Eds, Marcel
Dekker, New-York). Such peptide mimetics may have significant advantages over
natural
polypeptides including more economical production, greater chemical stability,
enhanced
pharmacological properties (e.g., half-life, absorption, potency, efficiency
etc), reduced
antigenicity and others.
[0127] While peptides are effective in inhibiting wild-type IL-1 in vitro,
their
effectiveness in vivo might be compromised by the presence of proteases. Serum
proteases
have specific substrate requirements. The substrate must have both L-amino
acids and
peptide bonds for cleavage. Furthermore, exopeptidases, which represent the
most prominent
component of the protease activity in serum, usually act on the first peptide
bond of the
peptide and require a free N-terminus (Powell et at. 1993). In light of this,
it is often
advantageous to utilize modified versions of peptides also termed peptide
analogs or
derivatives. The modified peptides retain the structural characteristics of
the original L-
amino acid peptides that confer biological activity with regard to IL-1, but
are
advantageously not readily susceptible to cleavage by protease and/or
exopeptidases.
[0128] Systematic substitution of one or more amino acids of a consensus
sequence
with D-amino acid of the same type (e.g., D-lysine in place of L-lysine) may
be used to
generate more stable peptides. Thus, a peptide derivative or peptidomimetic of
the present
invention may be all L, all D or mixed D, L peptide. In some embodiments,
peptides or
peptidomimetics contain all D-amino acids. In some embodiments, peptides or
peptidomimetics contain a D-amino acid at N-terminus or C-terminus. Reverse-D
peptides
are peptides containing D-amino acids, arranged in a reverse sequence relative
to a peptide
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
containing L-amino acids. Thus, the C-terminal residue of an L-amino acid
peptide becomes
N-terminal for the D-amino acid peptide, and so forth. It is thought that a
reverse D-peptide
retains the same tertiary conformation and therefore the same activity, as the
corresponding
L-amino acid peptide, but is more stable to enzymatic degradation in vitro and
in vivo, and
thus have greater therapeutic efficacy than the original peptide. In addition
to reverse-D-
peptide, constrained peptides comprising a consensus sequence or a
substantially identical
consensus sequence variation may be generated by methods well known in the art
(Rizo et
Gierasch, Ann. Rev. Biochem., 1992., 61: 387), for example, by adding cysteine
residues
capable of forming disulfide bridges which cyclize the peptide. Cyclic
peptides have no free
N- or C-termini.
[0129] A cyclic derivative containing intramolecular disulfide bond may be
prepared
by conventional solid phase synthesis while incorporating suitable S-protected
cysteine or
homocysteine residues at the positions selected for cyclization such as the
amino and carboxy
termini (SahM et at., 1996., J. Pharm. Pharmacol. 48: 197). Following
completion of the
chain assembly, cyclization can be performed either (1) by selective removal
of the S-
protecting group with a consequent on-support oxidation of the corresponding
two free SH-
functions, to form a S-S bonds, followed by conventional removal of the
product from the
support and appropriate purification procedure; or (2) by removal of the
peptide from the
support along with complete side chain deprotection, followed by oxidation of
the free SH-
functions in highly dilute aqueous solution.
[0130] A cyclic derivative containing an intramolecular amide bond may be
prepared
by conventional solid phase synthesis while incorporating suitable amino and
carboxyl side
chain protected amino acid derivatives, at the position selected for
cyclization. The cyclic
derivatives containing intramolecular -S-alkyl bonds can be prepared by
conventional solid
phases while incorporating an amino acid residue with a suitable amino-
protected side chain,
and a suitable S-protected cysteine or homocysteine residue at the position
selected for
cyclization.
[0131] Substitution of unnatural amino acids for natural amino acids in a
subsequence
of the peptides can also confer resistance to proteolysis. Such a substitution
can, for instance,
confer resistance to proteolysis by exopeptidases acting on the N-terminus.
Such substitutions
have been described and these substitutions do not affect biological activity.
Examples of
non-naturally occurring amino acids include a,a -disubstituted amino acids, N-
alkyl amino
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
46
acids, lactic acids, C-a-methyl amino acids, and (3-methyl amino acids. Amino
acids analogs
useful in the present invention may include but are not limited to (3-alanine,
norvaline,
norleucine, 4-aminobutyric acid, orithine, hydroxyproline, sarcosine,
citrulline, cysteic acid,
cyclohexylalanine, 2-aminoisobutyric acid, 6-aminohexanoic acid, t-
butylglycine,
phenylglycine, o-phosphoserine, N-acetyl serine, N-formylmethionine, 3-
methylhistidine and
other unconventional amino acids. Furthermore, the synthesis of peptides with
unnatural
amino acids is routine and known in the art.
[0132] One other effective approach to confer resistance to peptidases acting
on the
N-terminal or C-terminal residues of a peptide is to add chemical groups at
the peptide
termini, such that the modified peptide is no longer a substrate for the
peptidase. One such
chemical modification is glycosylation of the peptides at either or both
termini. Certain
chemical modifications, in particular N-terminal glycosylation, have been
shown to increase
the stability of peptides in human serum (Powell et at. 1993). Other chemical
modifications
which enhance serum stability include, but are not limited to, the addition of
an N-terminal
alkyl group, consisting of a lower alkyl of from 1 to 20 carbons, such as an
acetyl group,
and/or the addition of a C-terminal amide or substituted amide group. In
particular the present
invention includes modified peptides consisting of peptides bearing an N-
terminal acetyl
group and/or a C-terminal amide group.
[0133] Also included by the present invention are other types of peptide
derivatives
containing additional chemical moieties not normally part of the peptide,
provided that the
derivative retains the desired functional activity of the peptide. Examples of
such derivatives
include (i) N-acyl derivatives of the amino terminal or of another free amino
group, wherein
the acyl group may be an alkanoyl group (e.g., acetyl, hexanoyl, octanoyl), an
aroyl group
(e.g., benzoyl) or a blocking group such as Fmoc (fluorenylmethyl-O-CO-); (ii)
esters of the
carboxy terminal or of another free carboxy or hydroxyl group; (iii) amide of
the
carboxyterminal or of another free carboxyl group produced by reaction with
ammonia or
with a suitable amine; (iv) phosphorylated derivatives; (v) derivatives
conjugated to an
antibody or other biological ligand and other types of derivatives.
[0134] Longer peptide sequences which result from the addition of extra amino
acid
residues to the peptides of the invention are encompassed by the present
invention.
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
47
[0135] Other derivatives included in the present invention are dual peptides
consisting
of two of the same, or two different peptides of the present invention
covalently linked to one
another either directly or through a spacer, such as by a short stretch of
alanine residues or by
a putative site for proteolysis (e.g., by cathepsin, see US Patent No.
5,126,249 and European
Patent No. 495,049). Multimers of the peptides of the present invention
consist of polymer of
molecules formed from the same or different peptides or derivatives thereof.
[0136] In another embodiment, the peptide derivatives of the present invention
are
chimeric or fusion proteins comprising a peptide of the present invention or
fragment thereof
linked at its amino or carboxy terminal end, or both, to an amino acid
sequence of a different
protein. Such a chimeric or fusion protein may be produced by recombinant
expression of a
nucleic acid encoding the protein. In one embodiment such a chimeric or fusion
protein
contains at least 6 amino acids of a peptide of the present invention and has
a functional
activity equivalent or greater than that of a peptide of the invention.
[0137] Peptide derivatives of the present invention can be made by altering
the amino
acid sequences by substitutions, additions or deletions that provide for
functionally equivalent
molecules, or functionally enhanced or diminished molecules, as desired. The
derivative of
the present invention include, but are not limited to those containing, as
primary amino acid
sequence, all or part of the amino acid sequence of the peptides of the
present invention
including altered sequences in which functionally equivalent amino acid
residues are
substituted for an equivalent in the sequence. For example, one or more amino
acid residues
within the sequence can be substituted by another amino acid of a similar
polarity, which act
as a functional equivalent, resulting in a silent alteration. Substitution for
an amino acid
within the sequence may be selected from other members of the class to which
the amino acid
belongs. For example, the positively charged (basic) amino acids include,
arginine, lysine and
histidine. The nonpolar (hydrophobic) amino acids include, leucine,
isoleucine, alanine,
phenylalanine, valine, proline, tryptophan and methionine. The uncharged polar
amino acids
include serine, threonine, cysteine, tyrosine, asparagine and glutamine. The
negatively
charged (acid) amino acids include glutamic acid and aspartic acid. The amino
acid glycine is
sometimes included in the nonpolar amino acids family and sometimes in the
uncharged
(neutral) polar amino acids family. Substitutions that are done within a
family of amino acids
are generally understood to be conservative substitutions.
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
48
[0138] Once a peptidomimetic of the present invention is identified, it may be
isolated and purified by any number of standard methods including but not
limited to
differential solubility (i.e., precipitation), centrifugation, chromatography
(affinity, ion
exchange, size exclusion and the like) or by any other standard techniques
used for the
purification of peptides, peptidomimetics or proteins. The functional
properties of an
identified peptide of interest may be evaluated using any functional assay
known in the art. In
one embodiment assays for evaluating downstream receptor function in
intracellular signaling
are used (e.g., PGE2 synthesis).
[0139] In one embodiment, peptidomimetic compounds of the present invention
are
obtained with the following three-phase process: 1) scanning the peptides of
the present
invention to identify regions of secondary structure involved in the binding
of the IL-1
receptor; 2) using conformationally constrained dipeptide surrogates to refine
the backbone
geometry and provide organic platforms corresponding to these surrogates; and
3) using the
best organic platforms to display organic pharmocophores in libraries of
candidates designed
to mimic the desired activity of the native peptide. In more details the three
phases are as
follows. In phase 1, the peptide leads are scanned and their structure
abridged to identify the
requirements for their activity. A series of peptide analogs of the original
are synthesized. In
phase 2, the best peptide analogs are investigated using the conformationally
constrained
dipeptide surrogates. Indolizidin-2-one, indolizidin-9-one and quinolizidinone
amino acids
(haa, I9aa and Qaa respectively) are used as platforms for studying backbone
geometry of the
best peptide candidates. These and related platforms (Reviewed in Halab, Li;
Gosselin, F;
Lubell, WD; Biopolymers (Peptide Science) Vol 55, 101-122. 2000; Hanessian,
S.J.
McNaughton-Smith G; Lombart, H-G.; Lubell,W.D. Tetrahedron Vol. 53, 12789-
12854,
1997) may be introduced at specific regions of the peptide in order to orient
the
pharmacophores in different directions. Biological evaluation of these analogs
identifies
improved leads that mimic the geometric requirements for activity. In phase 3,
the platforms
from the most active leads are used to display organic surrogates of the
pharmacophores
responsible for activity of the native peptide. The pharmacophores and
scaffolds are
combined in a parallel synthesis format. Derivation of peptides and the
different phases can
be done by other means and methods known in the art.
[0140] Structure function relationships determined from peptidomimetics of the
present invention may be used to refine and prepare analogous molecular
structures having
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
49
similar or better properties. Thus, also within the scope of the present
invention are
molecules, in addition to those specifically disclosed, that share the
structure, polarity, charge
characteristics and side chain properties of the specific embodiments
exemplified herein.
[0141] Peptidomimetics of the present invention are functionally active (i.e.,
capable
of exhibiting one or more of the identified functional activities associated
with a peptide of
the present invention). For example, such peptides, peptide derivatives,
peptidomimetics or
analogs that inhibit a desired property (e.g., binding of the IL-lRacP to a
protein partner or
ligand) can be used as inhibitors of such property and its physiological
correlates. Peptides,
derivatives, peptidomimetics or analogs of the peptides of the present
invention can be tested
for the inhibition of cell signaling through the IL-1 R/IL-1 RacP receptor by
any functional
assay known in the art (e.g., PGE2 synthesis).
Assays
[0142] Methods for testing the ability of candidate compounds to inhibit or
activate
IL-1 receptor activity are presented herein. It will be understood that the
invention is not so
limited. Indeed, other assays well known in the art can be used in order to
identify non-
competitive, extracellular agonists or antagonists of the present invention.
[0143] Generally, screens of an IL-1R/IL-lRacP antagonist or agonist may be
based
on assays that measure a biological activity of IL-1R/IL-lRacP. Assays of the
present
invention employ either a natural or recombinant IL-1 receptor. A cell
fraction or cell free
screening assays for antagonists of IL-1 activity can use in situ purified, or
purified
recombinant IL-1 receptor. Cell-based assays can employ cells which express IL-
1 receptor
naturally, or which contain recombinant IL-1 receptor. In all cases, the
biological activity of
IL-1 receptor can be directly or indirectly measured; thus inhibitors or
activators of IL-1
receptor activity can be identified.
[0144] In some embodiments, an assay is a cell-based assay in which a cell
which
expresses a IL-1R/IL-lRacP receptor complex or biologically active portion
thereof, either
natural or recombinant in origin, is contacted with a test compound, and the
ability of the test
compound to modulate IL-1R/IL-lRacP receptor biological activity, e.g.,
modulation of
PGEz production, proliferation assays, binding of IL-1R to a binding partner
(IL-1RacP) or
any other measurable biological activity of the IL-1 receptor is determined.
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
[0145] In some embodiments, determining the ability of the test compound to
modulate the activity of IL-1R/IL-1RacP receptor complex can be accomplished
by
determining the ability of the test compound to modulate the activity of a
downstream
effector of an IL-1R/IL-1RacP receptor target molecule. For example, the
activity of the test
compound on the effector molecule can be determined. Non-limiting examples of
such
downstream effector, include interleukin receptor activated kinase (IRAK);
TRAF, activation
of NI-KB (e.g. p65), mutagenic activated protein kinases (MAPK). Other
examples of
effector molecules which could be assayed to define the modulatory (agonist or
antagonist)
activity of the compounds of the present invention are described in Sims et
at. 2002; and
Kashiwamura et at. 2002.
[0146] In some embodiments, it may be desirable to immobilize either IL-1, IL-
1R,
IL-1RacP or an interacting peptide or peptidomimetic of the present invention
to facilitate
separation of complexed from uncomplexed forms of one or both of the
interacting proteins,
as well as to accommodate automation of the assay. Binding of a test compound
to IL-1R
protein or interaction of IL-1R protein with a target molecule (e.g., IL-
1RacP) in the presence
and absence of a candidate compound can be accomplished in any vessel suitable
for
containing the reactants. Examples of such vessels include microtiter plates,
test tubes and
micro-centrifuge tubes. In one embodiment a fusion protein can be provided
which adds a
domain that allows one or both of the proteins to be bound to a matrix. For
example,
glutathione-S-transferase/IL-1R fusion proteins or glutathione-S-
transferase/IL-1RacP fusion
proteins can be adsorbed onto glutathione sepharose beads (Sigma Chemical, St.
Louis, MO),
or glutathione derivatized microtiter plates, which are then combined with the
test compound
or the test compound and either the non-adsorbed target protein or IL-1R
protein and the
mixture incubated under conditions conducive to complex formation (e.g. at
physiological
conditions for salt and pH). Following incubation the beads or microtiter
plate wells are
washed to remove any unbound components, and complex formation determined
either
directly or indirectly, for example, as described above. Alternatively, the
complexes can be
dissociated from the matrix, and the level of IL-1R binding or activity
determined using
standard techniques.
[0147] It will be understood that the in vivo experimental models such as
described
and exemplified herein can also be used to carry out an in vitro assay.
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
51
In vitro assays
[0148] In some embodiments, candidate peptides or peptidometics are tested for
their
ability to activate or inhibit ability of an IL-1 receptor to modulate
cellular proliferation with
the incorporated tritiated thymidine method. In some embodiments, candidate
peptides are
tested for their ability to inhibit ability of an IL-1 receptor to modulate
cellular proliferation,
using for example, the assays described in (Baker et at. 1995; Cheviron,
Grillon et at. 1996);
(Elliott et at. 1999; Hu et at. 1999).
[0149] In some embodiments, candidate peptides or peptidomimetics are tested
for
their ability to modulate the phosphorylation state of IL-1R or portion
thereof, or an upstream
or downstream target protein in the IL-1R/IL-1RacP pathway, using for example
an in vitro
kinase assay.
[0150] In some embodiments, candidate peptides targeting IL-1R are tested for
PGE2
levels, IL-6 or collagenase expression or any other molecule having a level
which is modified
following IL-1 stimulation in IL-1R/IL1RacP expressing cells, such as
chondrocytes and
fibroblasts.
[0151] Additonal assays are known in the art and examples are described in
In vivo
[0152] The assays described above may be used as initial or primary screens to
detect
promising lead compounds for further development. Lead peptides will be
further assessed in
additional, different screens. Therefore, this invention also includes
secondary IL-1R screens
that may involve various assays utilizing mammalian cell lines expressing
these receptors or
other assays.
[0153] Tertiary screens may involve the study of the identified inhibitors in
animal
models for clinical symptoms. Accordingly, it is within the scope of this
invention to further
use an agent (peptide or peptidomimetic) identified as described herein in an
appropriate
animal model such as a rat or a mouse. For example, a peptide can be used in
an animal
model to determine the efficacy, toxicity, or side effects of treatment with
such an agent.
Alternatively, an agent identified as described herein can be used in an
animal model to
determine the mechanism of action of such an agent. Furthermore, this
invention pertains to
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
52
uses of novel agents identified by the above-described screening assays for
treatment (e.g.
treatments of different types of disorders associated with a deregulation or
malfunction of IL-
1 receptor), as described herein. Non-limiting animal models which can be used
in such
assays include: collagen-induced arthritis in rat, animal model of acute IBD,
tumor growth in
immunosuppressed mouse, sensitization of the airways in newborn mice and any
other
known animal model including transgenic animals.
[0154] Additional assays for testing and identifying IL-1R antagonists are
described
in U.S. Application Publication No. US 2006/0094663, entitled "Interleukin-1
Receptor
Antagonists, Compositions, and Methods of Treatment," the disclosure of which
is
incorporated by reference.
Pharmaceutical compositions
[0155] The present invention relates to a method for modulating (e.g.,
inhibiting or
activating) IL-1 receptor activity through its interaction with the peptides,
peptide derivatives
and peptidomimetics of the present invention. In view of the importance of IL-
1 and or IL-
1 R/IL I RacP receptor function in numerous pathways and conditions in
animals, the peptides,
peptide derivatives and peptidomimetics of the present invention are useful in
the treatment
of IL-1 related diseases, disorders or conditions.
[0156] Therefore, methods of the present invention comprise administering to a
subject in need thereof or at risk of being in need thereof an effective
amount of a peptide,
peptide derivative or peptidomimetic, or a composition comprising a peptide,
peptide
derivative or peptidomimetic to a subject, to modulate (e.g., inhibit) IL-
1R/RacP biological
activity. In one embodiment, an effective amount of a therapeutic composition
comprising a
peptide or peptide derivative thereof and a suitable pharmaceutical carrier is
administered to a
subject to inhibit IL-1 R/IL-1 RacP biological activity to prevent, ameliorate
symptoms or treat
a disorder, disease or condition related to abnormal signaling through IL-
1R/IL-lRacP (e.g.,
overstimulation of the IL-1/IL-1RacP receptor via an overproduction of IL-1/IL-
1RacP ligand
or via a constitutively active receptor or any other defect). In one
embodiment, the subject is
an animal. In another embodiment, the subject is a mammal, and preferably a
human.
[0157] The peptides, peptide derivatives and peptidomimetics of the present
invention
are used in the treatment, prophylaxis or amelioration of symptoms in any
disease condition
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
53
or disorder where the inhibition of IL-1R/IL-lRacP biological activity might
be beneficial.
Diseases, conditions or disorders to which the peptides, peptide derivatives
or
peptidomimetics of the present invention may be beneficial include, but are
not limited to the
following examples: chronic and acute inflammation diseases like rheumatoid
arthritis,
inflammatory bowel disease, septic shock, osteoarthritis, psoriasis,
encephalitis,
glomerulonephritis, respiratory distress syndrome and Reiter's syndrome. Other
conditions
include, systemic lupus erythematosus, scleroderma, Crohn's disease,
ulcerative colitis,
inflammatory joint disease, cachexia in certain leukemias, Alzheimer's
disease, numerous
types of cancers, diabetes mellitus (type I), pulmonary hypertension, stroke,
periventricular
leucopenia and meningitis.
[0158] The present invention can also be used to treat other inflammatory
diseases,
disorders and conditions including, but not limited to, CNS demyelinating
diseases, multiple
sclerosis, acute disseminated encephalomyelitis (ADEM), idiopathic
inflammatory
demyelinating disease, transverse myelitis, Devic's disease, progressive
multifocal
leukoencephaly, Guillain-Barre syndrome, chronic inflammatory demyelinating
polyneuropathy, anti-MAG neuropathy, inflammatory bowel disease, sepsis,
septic shock,
adult respiratory distress syndrome, pancreatitis, trauma-induced shock,
asthma, bronchial
asthma, allergic rhinitis, cystic fibrosis, stroke, acute bronchitis, chronic
bronchitis, acute
bronchiolitis, chronic bronchiolitis, gout, spondylarthropathris, ankylosing
spondylitis,
Reiter's syndrome, psoriatic arthropathy, enterapathric spondylitis, juvenile
arthropathy or
juvenile ankylosing spondylitis, reactive arthropathy, infectious or post-
infectious arthritis,
gonoccocal arthritis, tuberculous arthritis, viral arthritis, fungal
arthritis, syphilitic arthritis,
Lyme disease, arthritis associated with "vasculitic syndromes," polyarteritis
nodosa,
hypersensitivity vasculitis, Luegenec's granulomatosis, polymyalgin
rheumatica, joint cell
arteritis, calcium crystal deposition arthropathris, pseudo gout, non-
articular rheumatism,
bursitis, tenosynomitis, epicondylitis (tennis elbow), carpal tunnel syndrome,
repetitive use
injury (typing), miscellaneous forms of arthritis, neuropathic joint disease
(charco and joint),
hemarthrosis (hemarthrosic), Henoch-Schonlein purpura, hypertrophic
osteoarthropathy,
multicentric reticulohistiocytosis, arthritis associated with certain
diseases, surcoilosis,
hemochromatosis, sickle cell disease and other hemoglobinopathries,
hyperlipoproteineimia,
hypogammaglobulinemia, hyperparathyroidism, acromegaly, familial Mediterranean
fever,
Behat's Disease, systemic lupus erythrematosis, and relapsing polychondritis,
inflammatory
conditions resulting from harmful stimuli, such as pathogens, damaged cells,
or irritants,
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
54
sarcoidosis, disseminated intravascular coagulation, atherosclerosis,
Kawasaki's disease,
macrophage activation syndrome (MAS), HIV, graft-versus-host disease,
Sjogren's
syndrome, vasculitis, autoimmune thyroiditis, dermatitis, atopic dermatitis,
myasthenia
gravis, inflammatory conditions of the skin, cardiovascular system, nervous
system, liver,
kidney and pancreas, cirrhosis, eosinophilic esophagitis, cardiovascular
disorders, disorders
associated with wound healing, respiratory disorders, chronic obstructive
pulmonary disease,
emphysema, acute inflammatory conditions, atopic inflammatory disorders,
bacterial, viral,
fungal or protozoan infections, pulmonary diseases, systemic inflammatory
response
syndrome (SIRS), hemophagocytic lymphohistiocytosis (HLH), juvenile rheumatoid
arthritis,
osteoarthritis, psoriatic arthritis, lupus nephritis, lupus-associated
arthritis, ankylosing
spondylitis, autoimmune diseases and related diseases or conditions.
[0159] Compositions within the scope of the present invention should contain
the
active agent (e.g. peptide, peptide derivative or peptidomimetic) in an amount
effective to
achieve the desired therapeutic effect while minimizing adverse side effects.
Pharmaceutically acceptable preparations and salts of the active agent are
within the scope of
the present invention and are well known in the art. For the administration of
polypeptide
antagonists and the like, the amount administered should be chosen so as to
minimize adverse
side effects. The amount of the therapeutic or pharmaceutical composition
which is effective
in the treatment of a particular disease, disorder or condition will depend on
the nature and
severity of the disease, the target site of action, the patient's weight,
special diets being
followed by the patient, concurrent medications being used, the administration
route and
other factors that will be recognized by those skilled in the art. The dosage
will be adapted by
the clinician in accordance with conventional factors such as the extent of
the disease and
different parameters from the patient. Typically, 0.001 to 100 mg/kg/day will
be administered
to the subject. Effective doses may be extrapolated from dose response curves
derived from
in vitro or animal model test systems. For example, in order to obtain an
effective mg/kg dose
for humans based on data generated from rat studies, the effective mg/kg
dosage in rat is
divided by six.
[0160] Various delivery systems are known and can be used to administer
peptides,
peptide derivatives or peptidomimetics or a pharmaceutical composition of the
present
invention. The pharmaceutical composition of the present invention can be
administered by
any suitable route including, intravenous or intramuscular injection,
intraventricular or
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
intrathecal injection (for central nervous system administration), orally,
topically,
subcutaneously, subconjunctivally, or via intranasal, intradermal, sublingual,
vaginal, rectal
or epidural routes.
[0161] Other delivery system well known in the art can be used for delivery of
the
pharmaceutical compositions of the present invention, for example via aqueous
solutions,
encapsulation in microparticles, or microcapsules.
[0162] In yet another embodiment, the pharmaceutical compositions of the
present
invention can be delivered in a controlled release system. In some
embodiments, polymeric
materials are used (see Smolen and Ball, Controlled Drug Bioavailability, Drug
product
design and performance, 1984, John Wiley & Sons; Ranade and Hollinger, Drug
Delivery
Systems, pharmacology and toxicology series, 2003, 2"d edition, CRRC Press);
in other
embodiments, a pump is used (Saudek et al., 1989, N. Engl. J. Med. 321: 574).
[0163] Compounds of the present invention may also be delivered by the use of
monoclonal antibodies as individual carriers to which the compound molecules
are coupled.
The compounds of the present invention may also be coupled to a class of
biodegradable
polymers useful in achieving controlled release of the drug, non-limiting
examples, include:
polylactic acid, polyorthoesters, cross-linked amphipathic block copolymers
and hydrogels,
polyhydroxy butyric acid and polydihydropyrans.
[0164] As mentioned above, pharmaceutical compositions of the present
invention
comprise a peptide, peptide derivative or peptidomimetic combined with a
pharmaceutically
acceptable carrier. The term carrier refers to diluents, adjuvants, excipients
such as a filler or
a binder, a disintegrating agent, a lubricant a silica flow conditioner a
stabilizing agent or
vehicles with which the peptide, peptide derivative or peptidomimetic is
administered. Such
pharmaceutical carriers include sterile liquids such as water and oils
including mineral oil,
vegetable oil (e.g., peanut oil, soybean oil, sesame oil, canola oil), animal
oil or oil of
synthetic origin. Aqueous glycerol and dextrose solutions as well as saline
solutions may also
be employed as liquid carriers of the pharmaceutical compositions of the
present invention.
Of course, the choice of the carrier depends on the nature of the peptide,
peptide derivative or
peptidomimetic, its solubility and other physiological properties as well as
the target site of
delivery and application. For example, carriers that can penetrate the blood
brain barrier are
used for treatment, prophylaxis or amelioration of symptoms of diseases or
conditions (e.g.
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
56
inflammation) in the central nervous system. Examples of suitable
pharmaceutical carriers are
described in Remington: The Science and Practice of Pharmacy by Alfonso R.
Gennaro,
2003, 21th edition, Mack Publishing Company.
[0165] Further pharmaceutically suitable materials that may be incorporated in
pharmaceutical preparations of the present invention include absorption
enhancers, pH
regulators and buffers, osmolarity adjusters, preservatives, stabilizers,
antioxidants,
surfactants, thickeners, emollient, dispersing agents, flavoring agents,
coloring agents and
wetting agents.
[0166] Examples of suitable pharmaceutical excipients include water, glucose,
sucrose, lactose, glycol, ethanol, glycerol monostearate, gelatin, rice,
starch flour, chalk,
sodium stearate, malt, sodium chloride and the like. The pharmaceutical
compositions of the
present invention can take the form of solutions, capsules, tablets, creams,
gels, powders
sustained release formulations and the like. The composition can be formulated
as a
suppository, with traditional binders and carriers such as triglycerides (see
Remington: The
Science and Practice of Pharmacy by Alfonso R. Gennaro, 2003, 21th edition,
Mack
Publishing Company). Such compositions contain a therapeutically effective
amount of the
therapeutic composition, together with a suitable amount of carrier so as to
provide the form
for proper administration to the subject. The formulations are designed so as
to suit the mode
of administration and the target site of action (e.g., a particular organ or
cell type).
[0167] The pharmaceutical compositions of the present invention can be
formulated
as neutral or salt forms. Pharmaceutically acceptable salts include those that
form with free
amino groups and those that react with free carboxyl groups. Non-toxic alkali
metal, alkaline
earth metal and ammonium salts commonly used in the pharmaceutical industry
include
sodium, potassium, lithium, calcium, magnesium, barium, ammonium, and
protamine zinc
salts, which are prepared by methods well known in the art. The term also
includes non-toxic
acid addition salts, which are generally prepared by reacting the compounds of
the present
invention with suitable organic or inorganic acid. Representative salts
include the
hydrobromide, hydrochloride, valerate, oxalate, oleate, laureate, borate,
benzoate, sulfate,
bisulfate, acetate, phosphate, tysolate, citrate, maleate, fumarate, tartrate,
succinate, napsylate
salts and the like.
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
57
[0168] Examples of fillers or binders that may be used in accordance with the
present
invention include acacia, alginic acid, calcium phosphate (dibasic),
carboxymethylcellulose,
carboxymethylcellulose sodium, hydroxyethylcellulose, hydroxypropylcellulose,
hydroxypropylmethylcellulose, dextrin, dextrates, sucrose, tylose,
pregelatinized starch,
calcium sulfate, amylose, glycine, bentonite, maltose, sorbitol,
ethylcellulose, disodium
hydrogen phosphate, disodium phosphate, disodium pyrosulfite, polyvinyl
alcohol, gelatin,
glucose, guar gum, liquid glucose, compressible sugar, magnesium aluminum
silicate,
maltodextrin, polyethylene oxide, polymethacrylates, povidone, sodium
alginate, tragacanth
micro crystalline cellulose, starch, and zein. In certain embodiments, a
filler or binder is
microcrystalline cellulose.
[0169] Examples of disintegrating agents that may be used include alginic
acid,
carboxymethylcellulose, carboxymethylcellulose sodium, hydroxypropylcellulose
(low
substituted), microcrystalline cellulose, powdered cellulose, colloidal
silicon dioxide, sodium
croscarmellose, crospovidone, methylcellulose, polacrilin potassium, povidone,
sodium
alginate, sodium starch glycolate, starch, disodium disulfite, disodium
edathamil, disodium
edetate, disodiumethylenediaminetetraacetate (EDTA) crosslinked
polyvinylpyrollidines,
pregelatinized starch, carboxymethyl starch, sodium carboxymethyl starch,
microcrystalline
cellulose.
[0170] Examples of lubricants include calcium stearate, canola oil, glyceryl
palmitostearate, hydrogenated vegetable oil (type I), magnesium oxide,
magnesium stearate,
mineral oil, poloxamer, polyethylene glycol, sodium lauryl sulfate, sodium
stearate fumarate,
stearic acid, talc and, zinc stearate, glyceryl behapate, magnesium lauryl
sulfate, boric acid,
sodium benzoate, sodium acetate, sodium benzoate/sodium acetate (in
combination), DL-
leucine.
[0171] Examples of silica flow conditioners include colloidal silicon dioxide,
magnesium aluminum silicate and guar gum. Another most preferred silica flow
conditioner
consists of silicon dioxide.
[0172] Examples of stabilizing agents include acacia, albumin, polyvinyl
alcohol,
alginic acid, bentonite, dicalcium phosphate, carboxymethylcellulose,
hydroxypropylcellulose, colloidal silicon dioxide, cyclodextrins, glyceryl
monostearate,
hydroxypropyl methylcellulose, magnesium trisilicate, magnesium aluminum
silicate,
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
58
propylene glycol, propylene glycol alginate, sodium alginate, carnauba wax,
xanthan gum,
starch, stearate(s), stearic acid, stearic monoglyceride and stearyl alcohol.
[0173] The present invention also provides for modifications of peptides or
peptide
derivatives such that they are more stable once administered to a subject
(i.e., once
administered it has a longer half-life or longer period of effectiveness as
compared to the
unmodified form). Such modifications are well known to those skilled in the
art to which this
invention pertain (e.g., polyethylene glycol derivatization a.k.a. PEGylation,
microencapsulation, etc).
[0174] The IL-1R/IL-lRacP antagonists or agonists of the present invention may
be
administered alone or in combination with other active agents useful for the
treatment,
prophylaxis or amelioration of symptoms of a IL-1, IL-1R/IL-1RacP associated
disease or
condition. Thus, the compositions and methods of the present invention can be
used in
combination with other agents exhibiting the ability to modulate IL-1 activity
(e.g., synthesis,
release and/or binding to IL-1R/IL-lRacP) or to reduce the symptoms of an IL-1
associated
disease (e.g., rheumatoid arthritis and inflammatory bowel disease). Example
of such agents
include but are not limited to antirheumatic drugs such as chloroquine,
auranofin
(RidauraTM), dexamethasone, sodium aurothiomalate, methotrexate (see Lee et
at., 1988,
Proc. Int. Acad. Sci, 85:1204), probucol (see Ku et at., 1988, Am. J. Cardiol.
62:778),
pentoxyfylline (e.g., Sullivan et at., 1988, Infect. Immun. 56 :1722),
disulfiram (see Marx
1988, Science, 239:257), antioxidants such as nordihydroguaiaretic acid (lee
et at., 1988, Int
J. Immunopharm., 10:385), IL-1 Trap (see e.g., 2003, Curr. Opin. Inv. Drugs,
4(5): 593-597),
Anakinra (KineretTM, PCT Application W000236152), leflunomide, corticosteroids
(MedrolTM, DeltasoneTM, OrasoneTM) as well as other agents such as those
described in
Bender and Lee (1989) Annual Reports in Medicinal Chemistry, chapter 20:
Pharmacological
Modulation of IL-1: 185-193). Other drugs may also be used in combination with
the
compounds of the present invention like anti-inflammatory drugs such as Non
Steroidal
Antiinflammatory Drugs (NSAIDS, e.g., Rofecoxib (VIOXXTM), Celecoxib
(CelebrexTM),
Valdecoxib (BextraTM), AspirinTM, advilTM), anti TNF-a drugs (Infliximab,
etanercept,
adalimumab), collagenase inhibitors and others. Of course a combination of two
or more
peptides, derivatives and peptide mimetics and their combination with one or
more drug can
also be used, in all combinations (e.g. one or more peptide with one or more
mimetic, one or
more mimetic with one or more derivative, one ore more peptide with one or
more drug etc.).
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
59
[0175] The present invention is illustrated in further details by the
following non-
limiting examples. The examples are provided for illustration only and should
not be
construed as limiting the scope of the invention.
EXEMPLIFICATION
[0176] As depicted in the Examples below, in certain exemplary embodiments,
compounds are prepared according to the following general procedures. It will
be
appreciated that, although the general methods depict the synthesis of certain
compounds of
the present invention, the following general methods, and other methods known
to one of
ordinary skill in the art, can be applied to all compounds and subclasses and
species of each
of these compounds, as described herein.
EXAMPLE 1
Synthesis of IL-1R antagonistic peptide 101.10 D-Agl analogs
(a) (R)-Agl-D-Tyr-D-Thr-D-Val-D-Glu-D-Leu-D-Ala-NHz (54) (SEQ ID NO:61)
HO 0
O H O H O
H2N, -
OH
N N,.= N
) ,r N N N NH2
O O H O H O
OH
[0177] (R)-Agl-D-Tyr-D-Thr-D-Val-D-Glu-D-Leu-D-Ala-NH2 (54) (SEQ ID NO:61)
was prepared as described above, using microwave assisted annulation
conditions B over 8 h,
to give the desired lactam peptide TFA salt 59 (57 mg, 55% crude purity as
analyzed by
analytical RP-HPLC (UV 214), 2-40% MeCN in H20, 0.1 % FA, 15 min gradient).
Purification was then carried out by preparatory RP-HPLC (2-20% MeCN in H20,
0.1 % FA,
20 min gradient) to give the desired formic acid salt 54 (16 mg, 20%) as a
white foam. The
purified product was analyzed by analytical RP-HPLC (UV 214) using both MeCN
tR 10.98
(0-60% MeCN in H20, 0.1% FA, 30min gradient) and MeOH tR 15.30 (0-60% MeOH in
H20, 0.1 % FA, 30min gradient) and revealed >99% purity. HRMS Calcd. for
C36H57011Ns
[M+H]+ 777.4141, found 777.4138.
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
(b) D-Arg-(R)-Agl-D-Thr-D-Val-D-Glu-D-Leu-D-Ala-NHz (55) (SEQ ID NO:62)
H2N O
rOHO1O
NH
HN=<
0 OH
NH2
[0178] D-Arg-(R)-Agl-D-Thr-D-Val-D-Glu-D-Leu-D-Ala-NH2 (55) (SEQ ID NO:62)
was prepared as described above, using microwave assisted annulation
conditions B over 10
h, to give the desired lactam peptide TFA salt 55 (99 mg, 41 % crude purity as
analyzed by
analytical RP-HPLC (UV 214), 0-20% MeCN in H20, 0.1 % FA, 15 min gradient).
Purification was then carried out by preparatory RP-HPLC (0-20% MeCN in H20,
0.1 % FA,
30 min gradient) to give the desired formic acid salt 55 (22 mg, 15%) as a
white foam. The
purified product was analyzed by analytical RP-HPLC (UV 214) using both MeCN
tR 8.26
(0-60% MeCN in H20, 0.1% FA, 30 min gradient) and MeOH tR 9.36 (0-60% MeOH in
H20,
0.1% FA, 30min gradient) and revealed >99% purity. HRMS Calcd. for
C33H600i0Nii
[M+H]+ 770.4519, found 770.4514.
(c) D-Arg-D-Tyr-(R)-Agl-D-Val-D-Glu-D-Leu-D-Ala-NHz (56) (SEQ ID NO:63)
H2N
NH
_j-NH
HO O
H2NNH O ~ -
O H O
HW- N NN NH2
O H O O
OH
[0179] D-Arg-D-Tyr-(R)-Agl-D-Val-D-Glu-D-Leu-D-Ala-NH2 (56) (SEQ ID NO:63)
was prepared as described above, using microwave assisted annulation
conditions B over 6 h,
to give the desired lactam peptide TFA salt 56 (89 mg, 58% crude purity as
analyzed by
analytical RP-HPLC (UV 214), 0-40% MeCN in H20, 0.1 % FA, 20 min gradient).
Purification was then carried out by preparatory RP-HPLC (0-40% MeCN in H2O,
0.1 % FA,
30min gradient) to give the desired formic acid salt 56 (13 mg, 7%) as a white
foam. The
purified product was analyzed by analytical RP-HPLC (UV 214) using both MeCN
tR 9.84
(0-60% MeCN in H2O, 0.1% FA, 3 0min gradient) and MeOH tR 12.65 (0-60% MeOH in
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
61
H20, 0.1% FA, 30min gradient) and revealed >99% purity. HRMS Calcd. for
C3sH620i0Nii
[M+H]+ 832.4676, found 832.4676.
(d) D-Arg-D-Tyr-D-Thr-(R)-Agl-D-Glu-D-Leu-D-Ala-NHz (57) (SEQ ID NO:64)
0
OH H{NNH2
0
O H O N NH O
H2N N N N
H O ,H ~ A
O O
HO
HNNH
NH2
[0180] D-Arg-D-Tyr-D-Thr-(R)-Agl-D-Glu-D-Leu-D-Ala-NH2 (57) (SEQ ID NO:64)
was prepared as described above, using microwave assisted annulation
conditions B over 4 h,
to give the desired lactam peptide TFA salt 57 (79 mg, 50% crude purity as
analyzed by
analytical RP-HPLC (UV 214), 2-40% MeCN in H2O, 0.1 % FA, 15 min gradient).
Purification was then carried out by preparatory RP-HPLC (2-20% MeCN in H2O,
0.1 % FA,
20 min gradient) to give the desired formic acid salt 57 (21 mg, 11 %) as a
white foam. The
purified product was analyzed by analytical RP-HPLC (UV 214) using both MeCN
tR 9.19
(0-60% MeCN in H2O, 0.1% FA, 30 min gradient) and MeOH tR 11.15 (0-60% MeOH in
H2O, 0.1 % FA, 30 min gradient) and revealed >99% purity. HRMS Calcd. for
C37H6001IN1I
[M+H]+ 833.4468, found 833.4459.
(e) D-Arg-D-Tyr-D-Thr-D-Val- (R)-AgI-D-Leu-D-Ala-NHz (58) (SEQ ID NO:65)
H2NYNH
NH2
NH
H O H O O NH O
N
H2NN N N"
O O H O
OH
[0181] D-Arg-D-Tyr-D-Thr-D-Val-(R)-Agl-D-Leu-D-Ala-NH2 (58) (SEQ ID NO:65)
was prepared as described above, using microwave assisted annulation
conditions B over 4 h,
to give the desired lactam peptide TFA salt 58 (157 mg, 36% crude purity as
analyzed by
analytical RP-HPLC (UV 214), 0-40% MeCN in H2O, 0.1% FA, 30 min gradient).
Purification was then carried out by preparatory RP-HPLC (0-40% MeCN in H2O,
0.1 % FA,
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
62
30 min gradient) to give the desired formic acid salt 58 (37 mg, 21%) as a
white foam. The
purified product was analyzed by analytical RP-HPLC (UV 214) using both MeCN
tR 9.93
(0-60% MeCN in H20, 0.1% FA, 30 min gradient) and MeOH tR 12.57 (0-60% MeOH in
H20, 0.1% FA, 30 min gradient) and revealed >99% purity. HRMS Calcd. for
C37H6209Ni i
[M+H]+ 803.4727, found 803.4725.
69 D-Arg-D-Tyr-D-Thr-D-Val-D-Glu-(R)-Agl-D-Ala-NH2 (59) (SEQ ID NO:66)
OH
H
O Fi O O
H2N HN HN H`,. N ~NH2
O "OH O O O
NH 0 OH
HN1~1 NH2
[0182] D-Arg-D-Tyr-D-Thr-D-Val-D-Glu-(R)-Agl-D-Ala-NH2 (59) (SEQ ID NO:66)
was prepared as described above, using microwave assisted annulation
conditions B over 3 h,
to give the desired lactam peptide TFA salt 59 (70 mg, 81 % crude purity as
analyzed by
analytical RP-HPLC (UV 214), 0-40% MeCN in H2O, 0.1 % FA, 4 min gradient).
Purification
was then carried out by preparatory RP-HPLC (0-40% MeCN in H2O, 0.1 % FA, 20
min
gradient) to give the desired formic acid salt 59 (2.6 mg, 7%) as a white
foam. The purified
product was analyzed by analytical RP-HPLC (UV 214) using both MeCN tR 8.52 (0-
60%
MeCN in H2O, 0.1 % FA, 30 min gradient) and MeOH tR 9.91 (0-60% MeOH in H2O,
0.1 %
FA, 30 min gradient) and revealed >99% purity. HRMS Calcd. for C36H58011Nii
[M+H]+
820.4312, found 820.4307.
EXAMPLE 2
Optimized protocol for (R)-Bgl lactam peptide synthesis
[0183] (S)- and (R)-Bgl 101.10 analogs were synthesized using optimized
conditions.
Peptide synthesis was carried out in syringe tubes as described earlier. When
the residue was
reached for lactam synthesis, Fmoc deprotection was performed as usual and the
resin was
transferred into a 5 mL glass microwave vessel. A solution of cyclic
sulfamidate (R)-17 (5
eq.) and DIEA (1 eq.) in THE (2 mL) was added, the reactor was sealed and the
mixture was
irradiated under microwave at 60 C for 1 h. The resin was then transferred
into a 6 mL
syringe tube, washed with DMF (3 times), MeOH (3 times), DCM (3 times), and
with three
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
63
cycles comprising washing with 2% AcOH in DMSO for 30 min and washing with
MeCN
for 5 minutes. The resin was transferred back to a 5 mL glass microwave
vessel, a
DMSO:H20:AcOH 75:23:2 (2 mL) was added, the reactor sealed and the mixture was
irradiated under microwave at 100 C for 3 h. The resin was transferred back
into a syringe
tube and underwent an acetyl-capping step by treatment with a solution of
acetic anhydride (5
eq.) in DMF (4 mL) in the presence of DIEA (5 eq.) for 1.5 h. The peptide
synthesis was then
continued as previously described.
(R)-Bab 101.10 analogs
EXAMPLE 3
(R)-Bab-D-Tyr-D-Thr-D-Val-D-Glu-D-Leu-D-Ala-NH2 (71) (SEQ ID NO:67)
HO 0
H O OHH O H O
H2N~,N N". N N N N NH2
OHO O H O H O
OH
[0184] (R)-Bab-D-Tyr-D-Thr-D-Val-D-Glu-D-Leu-D-Ala-NH2 (71) (SEQ ID NO:67)
was isolated while purifying compound 54, prepared as described above (28% of
54 crude
mixture as analyzed by analytical RP-HPLC (column a, UV: 1= 214 nm, 2-40% MeCN
in
H2O, 0.1 % FA, 15 min gradient). Purification was carried out by preparatory
RP-HPLC
(column A, 2-20% MeCN in H2O, 0.1 % FA, 20 min gradient) to give the desired
formic acid
salt 71 (10 mg, 10%) as a white fluffy solid. The purified product was
analyzed by analytical
RP-HPLC (column a, UV: 1= 214 nm) using both MeCN tR 14.11 (5-40% MeCN in H2O,
0.1 % FA, 15 min gradient) and MeOH tR 12.69 (20-80% MeOH in H2O, 0.1 % FA, 15
min)
and revealed >99% purity. HRMS Calcd. for C43H64012Ns [M+H]+ 885.4714, found
885.4716.
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
64
EXAMPLE 4
D-Arg-(R)-Bab-D-Thr-D-Val-D-Glu-D-Leu-D-Ala-NH2 (72) (SEQ ID NO:68)
NH2
HNNH
H H O = H O = H O
H2N~NN N~N NTN(NH2
O OHO ,,OHH O H 0
0 OH
[0185] D-Arg-(R)-Bab-D-Thr-D-Val-D-Glu-D-Leu-D-Ala-NH2 (72) (SEQ ID NO:68)
was isolated while purifying compound 55, prepared as described above (45% of
55 crude
mixture as analyzed by analytical RP-HPLC (column a, UV: 1= 214 nm, 0-20% MeCN
in
H20, 0.1 % FA, 15 min gradient). Purification was carried out by preparatory
RP-HPLC
(column A, 0-20% MeCN in H20, 0.1% FA, 30 min gradient) to give the desired
formic acid
salt 72 (27 mg, 15%) as a white fluffy solid. The purified product was
analyzed by analytical
RP-HPLC (column a, UV: 1= 214 nm) using both MeCN tR 9.04 (0-60% MeCN in H20,
0.1% FA, 30 min gradient) and MeOH tR 11.00 (0-60% MeOH in H20, 0.1% FA, 30
min)
and revealed >99% purity. HRMS Calcd. for C40H68011Nii [M+H]+ 878.5092, found
878.5094.
EXAMPLE 5
D-Arg-D-Tyr-(R)-Bab-D-Val-D-Glu-D-Leu-D-Ala-NH2 (73) (SEQ ID NO:69)
OH HO 0
/ Yi
O = H H O = H O =
HZN HNN HN H NH2
O oho O O
NH
H2N"ill NH
[0186] D-Arg-D-Tyr-D-Thr-(R)-Bab-D-Glu-D-Leu-D-Ala-NH2 (73) (SEQ ID NO:69)
was isolated while purifying compound 56, prepared as described above (27% of
56 crude
mixture as analyzed by analytical RP-HPLC (column a, UV: 1= 214 nm, 0-40% MeCN
in
H20, 0.1 % FA, 15 min gradient). Purification was carried out by preparatory
RP-HPLC
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
(column A, 0-40% MeCN in H20, 0.1% FA, 30 min gradient) to give the desired
formic acid
salt 73 (7 mg, 3%) as a white fluffy solid. The purified product was analyzed
by analytical
RP-HPLC (column a, UV: 1= 214 nm) using both MeCN tR 9.12 (0-60% MeCN in H20,
0.1 % FA, 15 min gradient) and MeOH tR 9.45 (0-60% MeOH in H20, 0.1 % FA, 15
min) and
revealed >99% purity. HRMS Calcd. for C45H7001IN1I [M+H]+ 940.5240, found
940.5251.
EXAMPLE 6
D-Arg-D-Tyr-D-Thr-(R)-Bab-D-Glu-D-Leu-D-Ala-NH2 (74) (SEQ ID NO:70)
NH2
0 OHH H H
NH HN N N N^,~,N NH2
H = H
H2NAN OHO O
HO N~Z HO 0
[0187] D-Arg-D-Tyr-D-Thr-(R)-Bab-D-Glu-D-Leu-D-Ala-NH2 (74) (SEQ ID NO:70)
was isolated while purifying compound 57, prepared as described above (6% of
57 crude
mixture as analyzed by analytical RP-HPLC (column a, UV: 1= 214 nm, 2-40% MeCN
in
H20, 0.1 % FA, 15 min gradient). Purification was carried out by preparatory
RP-HPLC
(column A, 2-20% MeCN in H20, 0.1 % FA, 20 min gradient) to give the desired
formic acid
salt 74 (8.5 mg, 4%) as a white fluffy solid. The purified product was
analyzed by analytical
RP-HPLC (column a, UV: 1= 214 nm) using both MeCN tR 12.27 (5-40% MeCN in H20,
0.1 % FA, 15 min gradient) and MeOH tR 17.09 (5-40% MeOH in H20, 0.1% FA, 15
min)
and revealed >99% purity. HRMS Calcd. for C44H68012Nii [M+H]+ 942.5027, found
942.5043.
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
66
(R)-Bgl 101.10 scan compounds
EXAMPLE 7
(R)-Bgl-D-Tyr-D-Thr-D-Val-D-Glu-D-Leu-D-Ala-NH2 (75) (SEQ ID NO:71)
HO 0
0 O OHH O ) O =
HZN ,. =
N N,= N NN NNHZ ':)~ O H H O H O
OH
[0188] (R)-Bgl-D-Tyr-D-Thr-D-Val-D-Glu-D-Leu-D-Ala-NH2 (75) (SEQ ID NO:71)
was prepared on a 0.072 mmol, in a syringe tube following the optimized
protocol as
described above, using microwave assisted annulation over 240 min, to give the
desired
lactam peptide TFA salt 75 (34 mg) in 71 % crude purity as determined by
analytical RP-
HPLC (column b, UV: 1= 214 nm, 0-80% MeCN in H20, 0.1% FA, 30 min gradient).
Purification was carried out by preparative RP-HPLC (column B, 10-30% MeCN in
H2O,
0.1 % FA, 30 min gradient) to give the desired FA salt product 75 (13 mg, 22%)
as a white
fluffy solid. The purified product was analyzed by analytical RP-HPLC (column
b, UV: 1=
214 nm) using both MeCN tR 11,8 (5-60% MeCN in H2O, 0.1% FA, 25 min gradient)
and
MeOH tR 16.41 (0-80% MeOH in H2O, 0.1 % FA, 25 min gradient) and revealed >99%
purity. HRMS Calcd. m/z for C36H57011Ns [M+H]+ 777.4141, found 777.4145.
EXAMPLE 8
D-Arg-(R)-Bgl-D-Thr-D-Val-D-Glu-D-Leu-D-Ala-NH2 (76) (SEQ ID NO:72)
HO 0
OHH O O
O - _
NH2
N N HEN H--Y
~` O O O
HNNH HN
O
H2N
NH2
[0189] D-Arg-(R)-Bgl-D-Thr-D-Val-D-Glu-D-Leu-D-Ala-NH2 (76) (SEQ ID NO:72)
was prepared on a 0.078 mmol scale, in a syringe tube following the optimized
protocol as
described above, using microwave assisted annulation over 360 min, to give the
desired
lactam peptide TFA salt 76 (35 mg) in 87% crude purity as determined by
analytical RP-
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
67
HPLC (column a, UV: 1= 214 nm, 0-20% MeCN in H20, 0.1% FA, 30 min gradient).
Purification was carried out by preparative RP-HPLC (column A, 2-20% MeCN in
H20,
0.1 % FA, 30 min gradient) to give the desired FA salt product 76 (16 mg, 24%)
as a white
fluffy solid. The purified product was analyzed by analytical RP-HPLC (column
a, UV: 1=
214 nm) using both MeCN tR 7.79 (5-60% MeCN in H20, 0.1% FA, 25 min gradient)
and
MeOH tR 10.28 (0-80% MeOH in H20, 0.1 % FA, 25 min gradient) and revealed >99%
purity. HRMS Calcd. m/z for C33H600i0Nii [M+H]+ 770.4519, found 770.4521.
EXAMPLE 9
D-Arg-D-Tyr-(R)-Bgl-D-Val-D-Glu-D-Leu-D-Ala-NH2 (77) (SEQ ID NO:73)
HZN
NH
NH
HO O
HZN--~- NH O
O O
O HN N H N H NHZ
O O
OH
[0190] D-Arg-D-Tyr-(R)-Bgl-D-Val-D-Glu-D-Leu-D-Ala-NH2 (77) (SEQ ID NO:73)
was prepared on a 0.082 mmol scale, in a syringe tube following the optimized
protocol as
described above, using microwave assisted annulation over 240 min, to give the
desired
lactam peptide TFA salt 77 (34 mg) in 86% crude purity as determined by
analytical RP-
HPLC (column b, UV: 1= 214 nm, 0-20% MeCN in H20, 0.1% FA, 30 min gradient).
Purification was carried out by preparative RP-HPLC (column B, 0-20% MeCN in
H2O,
0.1 % FA, 30 min gradient) to give the desired FA salt product 77 (17 mg, 23%)
as a white
fluffy solid. The purified product was analyzed by analytical RP-HPLC (column
b, UV: 1=
214 nm) using both MeCN tR 9.55 (5-60% MeCN in H2O, 0.1% FA, 25 min gradient)
and
MeOH tR 12.99 (0-80% MeOH in H2O, 0.1 % FA, 25 min gradient) and revealed >99%
purity. HRMS Calcd. m/z for C3sH62010N11 [M+H]+ 832.4676, found 832.4677.
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
68
EXAMPLE 10
D-Arg-D-Tyr-D-Thr-(R)-Bgl-D-Glu-D-Leu-D-Ala-NH2 (78) (SEQ ID NO:74)
0 HO
N
NHOHH H ON, N O
HZNANH p O p-~NH
NH O
\1 O H
HN
HN
H2N 0 NH2
[0191] D-Arg-D-Tyr-D-Thr-(R)-Bgl-D-Glu-D-Leu-D-Ala-NH2 (78) (SEQ ID NO:74)
was prepared on a 0.087 mmol scale, in a syringe tube following the optimized
protocol as
described above, using microwave assisted annulation over 420 min, to give the
desired
lactam peptide TFA salt 78 (62 mg) in 79% crude purity as determined by
analytical RP-
HPLC (column b, UV: 1= 214 nm, 0-30% MeCN in H20, 0.1% FA, 30 min gradient).
Purification was carried out by preparative RP-HPLC (column B, 18% MeCN in
H20, 0.1 %
FA, isocratic) to give the desired FA salt product 78 (16 mg, 17%) as a white
fluffy solid.
The purified product was analyzed by analytical RP-HPLC (column b, UV: 1= 214
nm) using
both MeCN tR 8.00 (5-60% MeCN in H20, 0.1% FA, 25 min gradient) and MeOH tR
9.93 (0-
80% MeOH in H20, 0.1 % FA, 25 min gradient) and revealed >99% purity. HRMS
Calcd.
m/z for C37H60011Nii [M+H]+ 834.4468, found 834.4470.
EXAMPLE 11
D-Arg-D-Tyr-D-Thr-D-Val-(R)-Bgl-D-Leu-D-Ala-NH2 (79) (SEQ ID NO:75)
H2NYNH
NH NH2
H O OHH O O p NH p
HZNN H,. N H
OH
[0192] D-Arg-D-Tyr-D-Thr-D-Val-(R)-Bgl-D-Leu-D-Ala-NH2 (79) (SEQ ID NO:75)
was prepared on a 0.098 mmol scale, in a syringe tube following the optimized
protocol as
described above, using microwave assisted annulation over 240 min, to give the
desired
lactam peptide TFA salt 79 (35 mg) in 87% crude purity as determined by
analytical RP-
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
69
HPLC (column a, UV: 1= 214 nm, 0-80% MeCN in H20, 0.1% FA, 30 min gradient).
Purification was carried out by preparative RP-HPLC (column A, 0-30% MeCN in
H20,
0.1 % FA, 30 min gradient) to give the desired FA salt product 79 (6 mg, 7%)
as a white
fluffy solid. The purified product was analyzed by analytical RP-HPLC (column
a, UV: 1=
214 nm) using both MeCN tR 9.67 (5-60% MeCN in H20, 0.1% FA, 25 min gradient)
and
MeOH tR 13.18 (0-80% MeOH in H20, 0.1 % FA, 25 min gradient) and revealed >99%
purity. HRMS Calcd. m/z for C37H6109N11 [M+Na]+ 826.4546, found 826.4544.
EXAMPLE 12
D-Arg-D-Tyr-D-Thr-D-Val-D-Glu-(R)-Bgl-D-Ala-NH2 (80) (SEQ ID NO:76)
HZN'r NH
NH HO 0
OH
H O H O = H
HzN qH1 N~N O
O O H O N
OH O
NH2
[0193] D-Arg-D-Tyr-D-Thr-D-Val-D-Glu-(R)-Bgl-D-Ala-NH2 (80) (SEQ ID NO:76)
was prepared on a 0.105 mmol scale, in a syringe tube following the optimized
protocol as
described above, using microwave assisted annulation over 240 min, to give the
desired
lactam peptide TFA salt 80 (35 mg) in 50% crude purity as determined by
analytical RP-
HPLC (column b, UV: 1= 214 nm, 0-60% MeCN in H20, 0.1% FA, 25 min gradient).
Purification was carried out by preparative RP-HPLC (column B, 0-20% MeCN in
H20,
0.1 % FA, 30 min gradient) to give the desired FA salt product 80 (13 mg, 14%)
as a white
fluffy solid. The purified product was analyzed by analytical RP-HPLC (column
b, UV: 1=
214 nm) using both MeCN tR 7.27 (5-60% MeCN in H20, 0.1% FA, 25 min gradient)
and
MeOH tR 9.04 (0-80% MeOH in H20, 0.1 % FA, 25 min gradient) and revealed >99%
purity.
HRMS Calcd. m/z for C36H58011N11 [M+H]+ 820.4312, found 820.4313.
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
(S)-Bgl 101.10 scan compounds
EXAMPLE 13
(S)-Bgl-D-Tyr-D-Thr-D-Val-D-Glu-D-Leu-D-Ala-NH2 (81) (SEQ ID NO:77)
HO 0
0 O OHH OH
H2N
N N~,= N :)~ NN N NH2
O H O H O
OH
[0194] (S)-Bgl-D-Tyr-D-Thr-D-Val-D-Glu-D-Leu-D-Ala-NH2 (81) (SEQ ID NO:77)
was prepared on a 0.102 mmol scale, in a syringe tube following the optimized
protocol as
described above, using microwave assisted annulation over 600 min, to give the
desired
lactam peptide TFA salt 81 (34 mg) in 70% crude purity as determined by
analytical RP-
HPLC (column a, UV: 1= 214 nm, 0-80% MeCN in H20, 0.1% FA, 30 min gradient).
Purification was carried out by preparative RP-HPLC (column A, 10-30% MeCN in
H2O,
0.1% FA, 30 min gradient) to give the desired FA salt product 81 (11 mg, 13%)
as a white
fluffy solid. The purified product was analyzed by analytical RP-HPLC (column
a, UV: 1=
214 nm) using both MeCN tR 12.00 (5-60% MeCN in H2O, 0.1% FA, 25 min gradient)
and
MeOH tR 19.49 (0-60% MeOH in H2O, 0.1 % FA, 25 min gradient) and revealed >99%
purity. HRMS Calcd. m/z for C36H57011Ns [M+H]+ 777.4141, found 777.4138.
EXAMPLE 14
D-Arg-(S)-Bgl-D-Thr-D-Val-D-Glu-D-Leu-D-Ala-NH2 (82) (SEQ ID NO:78)
HO 0
OHH O H O
H
N N N HNHZ
O O O O
HN HN
NH O
H2N -t
NH2
[0195] D-Arg-(S)-Bgl-D-Thr-D-Val-D-Glu-D-Leu-D-Ala-NH2 (82) (SEQ ID NO:78)
was prepared on a 0.105 mmol scale, in a syringe tube following the optimized
protocol as
described above, using microwave assisted annulation over 600 min, to give the
desired
lactam peptide TFA salt 82 (33 mg) in 82% crude purity as determined by
analytical RP-
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
71
HPLC (column a, UV: 1= 214 nm, 0-20% MeCN in H20, 0.1% FA, 30 min gradient).
Purification was carried out by preparative RP-HPLC (column A, 0-20% MeCN in
H20,
0.1 % FA, 30 min gradient) to give the desired FA salt product 82 (13 mg, 16%)
as a white
fluffy solid. The purified product was analyzed by analytical RP-HPLC (column
a, UV: 1=
214 nm) using both MeCN tR 7.14 (5-60% MeCN in H20, 0.1% FA, 25 min gradient)
and
MeOH tR 8.62 (0-80% MeOH in H20, 0.1 % FA, 25 min gradient) and revealed >99%
purity.
HRMS Calcd. m/z for C33H600ioNii [M+H]+ 770.4519, found 770.4518.
EXAMPLE 15
D-Arg-D-Tyr-(S)-Bgl-D-Val-D-Glu-D-Leu-D-Ala-NH2 (83) (SEQ ID NO:79)
HZN
NH
NH
HO O
HZN--~- NH O
O O
O HN -CfN' H N NH2
O H O
OH
[0196] D-Arg-D-Tyr-(S)-Bgl-D-Val-D-Glu-D-Leu-D-Ala-NH2 (83) (SEQ ID NO:79)
was prepared on a 0.105 mmol scale, in a syringe tube following the optimized
protocol as
described above, using microwave assisted annulation over 480 min, to give the
desired
lactam peptide TFA salt 83 (52 mg) in 60% crude purity as determined by
analytical RP-
HPLC (column a, UV: 1= 214 nm, 0-80% MeCN in H20, 0.1% FA, 30 min gradient).
Purification was carried out by preparative RP-HPLC (column A, 5-15% MeCN in
H20,
0.1 % FA, 50 min gradient) to give the desired FA salt product 83 (15 mg, 17%)
as a white
fluffy solid. The purified product was analyzed by analytical RP-HPLC (column
a, UV: 1=
214 nm) using both MeCN tR 9.43 (5-60% MeCN in H20, 0.1% FA, 25 min gradient)
and
MeOH tR 14.71 (0-60% MeOH in H20, 0.1 % FA, 25 min gradient) and revealed >99%
purity. HRMS Calcd. m/z for C3sH620i0Nii [M+H]+ 832.4676, found 832.4674.
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
72
EXAMPLE 16
D-Arg-D-Tyr-D-Thr-(S)-Bgl-D-Glu-D-Leu-D-Ala-NH2 (84) (SEQ ID NO:80)
0 HO
NH OH HN,
HNA
2 O NH O O ~-NH O
OH
HN
HN
H2N 0 NH2
[0197] D-Arg-D-Tyr-D-Thr-(S)-Bgl-D-Glu-D-Leu-D-Ala-NH2 (84) (SEQ ID NO:80)
was prepared on a 0.105 mmol scale, in a syringe tube following the optimized
protocol as
described above, using microwave assisted annulation over 420 min, to give the
desired
lactam peptide TFA salt 84 (28 mg) in 87% crude purity as determined by
analytical RP-
HPLC (column a, UV: 1= 214 nm, 0-80% MeCN in H20, 0.1% FA, 30 min gradient).
Purification was carried out by preparative RP-HPLC (column A, 0-20% MeCN in
H20,
0.1 % FA, 20 min gradient) to give the desired FA salt product 84 (12 mg, 14%)
as a white
fluffy solid. The purified product was analyzed by analytical RP-HPLC (column
a, UV: 1=
214 nm) using both MeCN tR 7.56 (5-60% MeCN in H20, 0.1% FA, 25 min gradient)
and
MeOH tR 8.91 (0-80% MeOH in H20, 0.1 % FA, 25 min gradient) and revealed >99%
purity.
HRMS Calcd. m/z for C37H6001IN11 [M+H]+ 834.4468, found 834.4469.
EXAMPLE 17
D-Arg-D-Tyr-D-Thr-D-Val-(S)-Bgl-D-Leu-D-Ala-NH2 (85) (SEQ ID NO:81)
H2NYNH
NH NH2
H O OHH O O O NH p
H2N~N H` N H
OH
[0198] D-Arg-D-Tyr-D-Thr-D-Val-(S)-Bgl-D-Leu-D-Ala-NH2 (85) (SEQ ID NO:81)
was prepared on a 0.105 mmol scale, in a syringe tube following the optimized
protocol as
described above, using microwave assisted annulation over 240 min, to give the
desired
lactam peptide TFA salt 85 (40 mg) in 90% crude purity as determined by
analytical RP-
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
73
HPLC (column a, UV: 1= 214 nm, 0-80% MeCN in H20, 0.1% FA, 30 min gradient).
Purification was carried out by preparative RP-HPLC (column A, 0-20% MeCN in
H20,
0.1% FA, 30 min gradient) to give the desired FA salt product 85 (11 mg, 13%)
as a white
fluffy solid. The purified product was analyzed by analytical RP-HPLC (column
a, UV: 1=
214 nm) using both MeCN tR 9.48 (5-60% MeCN in H20, 0.1% FA, 25 min gradient)
and
MeOH tR 10.47 (0-80% MeOH in H20, 0.1 % FA, 25 min gradient) and revealed >99%
purity. HRMS Calcd. m/z for C37H6109N11 [M+H]+ 804.4726, found 804.4724.
EXAMPLE 18
D-Arg-D-Tyr-D-Thr-D-Val-D-Glu-(S)-Bgl-D-Ala-NH2 (86) (SEQ ID NO:82)
HZN'r NH
NH HO 0
OH
H O H O = H
HzNN qH1 N N~NO
O O H O N
OH O
NH2
[0199] D-Arg-D-Tyr-D-Thr-D-Val-D-Glu-(S)-Bgl-D-Ala-NH2 (86) (SEQ ID NO:82)
was prepared on a 0.105 mmol scale, in a syringe tube following the optimized
protocol as
described above, using microwave assisted annulation over 240 min, to give the
desired
lactam peptide TFA salt 86 (29 mg) in 96% crude purity as determined by
analytical RP-
HPLC (column a, UV: 1= 214 nm, 0-20% MeCN in H20, 0.1% FA, 30 min gradient).
Purification was carried out by preparative RP-HPLC (column A, 0-20% MeCN in
H20,
0.1 % FA, 30 min gradient) to give the desired FA salt product 86 (10 mg, 11
%) as a white
fluffy solid. The purified product was analyzed by analytical RP-HPLC (column
a, UV: 1=
214 nm) using both MeCN tR 7.6 (5-60% MeCN in H20, 0.1% FA, 25 min gradient)
and
MeOH tR 8.9 (0-80% MeOH in H20, 0.1 % FA, 25 min gradient) and revealed >99%
purity.
HRMS Calcd. m/z for C36H58011N11 [M+H]+ 820.4131, found 820.4136.
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
74
(R)-Bgl insertion 101.10 compounds
EXAMPLE 19
D-Arg-(S)-Bgl-D-Tyr-D-Thr-D-Val-D-Glu-D-Leu-D-Ala-NH2 (87) (SEQ ID NO:83)
NH
HZN-~ NH HO O
O O H O H 0
N N N NH2
HN N N ~~[H ---r
HZN O O H V H
OH
[0200] D-Arg-(S)-Bgl-D-Tyr-D-Thr-D-Val-D-Glu-D-Leu-D-Ala-NH2 (87) (SEQ ID
NO:83) was prepared on a 0.048 mmol scale, in a syringe tube following the
optimized
protocol as described above, using microwave assisted annulation over 600 min,
to give the
desired lactam peptide TFA salt 87 (27 mg) in 56% crude purity as determined
by analytical
RP-HPLC (column a, UV: 1= 214 nm, 0-40% MeCN in H20, 0.1% FA, 30 min
gradient).
Purification was carried out by preparative RP-HPLC (column A, 2-20% MeCN in
H20,
0.1 % FA, 25 min gradient) to give the desired FA salt product 87 (9 mg, 18%)
as a white
fluffy solid. The purified product was analyzed by analytical RP-HPLC (column
a, UV: 1=
214 nm) using both MeCN tR 10.23 (5-60% MeCN in H20, 0.1% FA, 25 min gradient)
and
MeOH tR 15.99 (0-60% MeOH in H20, 0.1 % FA, 25 min gradient) and revealed >99%
purity. HRMS Calcd. m/z for C42H69012N12 [M+H]+ 933.5152, found 933.5152.
EXAMPLE 20
D-Arg-D-Tyr-D-(S)-Bgl-Thr-D-Val-D-Glu-D-Leu-D-Ala-NH2 (88) (SEQ ID NO: 84)
HO 0
OHH O H O
NH
H2N4 N'- N N N N NH2
NH H H
NH
HN OH
H2N 0
[0201] D-Arg-D-Tyr-D-(S)-Bgl-Thr-D-Val-D-Glu-D-Leu-D-Ala-NH2 (88) (SEQ ID
NO:84) was prepared on a 0.052 mmol scale, in a syringe tube following the
optimized
protocol as described above, using microwave assisted annulation over 600 min,
to give the
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
desired lactam peptide TFA salt 88 (6 mg) in 55% crude purity as determined by
analytical
RP-HPLC (column b, UV: 1= 214 nm, 0-60% MeCN in H20, 0.1 % FA, 25 min
gradient).
Purification was carried out by preparative RP-HPLC (column B, 2-15% MeCN in
H20,
0.1 % FA, 30 min gradient) to give the desired FA salt product 88 (3 mg, 6%)
as a white
fluffy solid. The purified product was analyzed by analytical RP-HPLC (column
b, UV: 1=
214 nm) using both MeCN tR 9.22 (0-60% MeCN in H20, 0.1% FA, 25 min gradient)
and
MeOH tR 14.01 (0-60% MeOH in H20, 0.1 % FA, 25 min gradient) and revealed >99%
purity. HRMS Calcd. m/z for C42H69012N12 [M+H]+ 933.5152, found 933.5137.
EXAMPLE 21
D-Arg-D-Tyr-D-Thr-(S)-Bgl-D-Val-D-Glu-D-Leu-D-Ala-NH2 (89) (SEQ ID NO:85)
HO O
NH OH O O O
--~H
N H NH,
HZN4 NH H H
O N
NH 0 O O
HN OH
H2N 0
[0202] D-Arg-D-Tyr-D-Thr-(S)-Bgl-D-Val-D-Glu-D-Leu-D-Ala-NH2 (89) (SEQ ID
NO:85) was prepared on a 0.055 mmol scale, in a syringe tube following the
optimized
protocol as described above, using microwave assisted annulation over 480 min,
to give the
desired lactam peptide TFA salt 89 (16 mg) in 71 % crude purity as determined
by analytical
RP-HPLC (column a, UV: 1= 214 nm, 0-60% MeCN in H2O, 0.1% FA, 25 min
gradient).
Purification was carried out by preparative RP-HPLC (column A, 2-20% MeCN in
H2O,
0.1 % FA, 20 min gradient) to give the desired FA salt product 89 (6 mg, 10%)
as a white
fluffy solid. The purified product was analyzed by analytical RP-HPLC (column
a, UV: 1=
214 nm) using both MeCN tR 10.38 (5-60% MeCN in H2O, 0.1% FA, 25 min gradient)
and
MeOH tR 16.25 (0-60% MeOH in H2O, 0.1 % FA, 25 min gradient) and revealed >99%
purity. HRMS Calcd. m/z for C42H69012N12 [M+H]+ 933.5152, found 933.5152.
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
76
EXAMPLE 22
D-Arg-D-Tyr-D-Thr-D-Val-(S)-Bgl-D-Glu-D-Leu-D-Ala-NH2 (90) (SEQ ID NO:86)
H2NYNH
NH HO
O
= H O O Hr' O .~
HZN~-N H"
0 0 N N NH O
':~ H 0
HOH O
[0203] D-Arg-D-Tyr-D-Thr-D-Val-(S)-Bgl-D-Glu-D-Leu-D-Ala-NH2 (90) (SEQ ID
NO:86) was prepared on a 0.058mmol scale, in a syringe tube following the
optimized
protocol as described above, using microwave assisted annulation over 420 min,
to give the
desired lactam peptide TFA salt 90 (7 mg) in 68% crude purity as determined by
analytical
RP-HPLC (column b, UV: 1= 214 nm, 5-30% MeCN in H20, 0.1% FA, 30 min
gradient).
Purification was carried out by preparative RP-HPLC (column B, 5-15% MeCN in
H20,
0.1 % FA, 30 min gradient) to give the desired FA salt product 90 (3 mg, 6%)
as a white
fluffy solid. The purified product was analyzed by analytical RP-HPLC (column
b, UV: 1=
214 nm) using both MeCN tR 9.90 (5-60% MeCN in H20, 0.1% FA, 25 min gradient)
and
MeOH tR 15.20 (0-60% MeOH in H20, 0.1 % FA, 25 min gradient) and revealed >99%
purity. HRMS Calcd. m/z for C42H69012N12 [M+H]+ 933.5152, found 933.5147.
EXAMPLE 23
D-Arg-D-Tyr-D-Thr-D-Val-D-Glu-(S)-Bgl-D-Leu-D-Ala-NH2 (91) (SEQ ID NO:87)
H2NYNH
NH HO 0
OH
H O H O = H
H2NN qHI N N~NO
O O H O N O
OH HN
~-NH2
O
[0204] D-Arg-D-Tyr-D-Thr-D-Val-D-Glu-(S)-Bgl-D-Leu-D-Ala-NH2 (91) (SEQ ID
NO:87) was prepared on a 0.065 mmol scale, in a syringe tube following the
optimized
protocol as described above, using microwave assisted annulation over 240 min,
to give the
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
77
desired lactam peptide TFA salt 91 (20 mg) in 37% crude purity as determined
by analytical
RP-HPLC (column b, UV: 1= 214 nm, 2-30% MeCN in H20, 0.1% FA, 25 min
gradient).
Purification was carried out by preparative RP-HPLC (column B, 5-15% MeCN in
H20,
0.1 % FA, 25 min gradient) to give the desired FA salt product 91 (2 mg, 3%)
as a white
fluffy solid. The purified product was analyzed by analytical RP-HPLC (column
b, UV: 1=
214 nm) using both MeCN tR 9.96 (5-60% MeCN in H20, 0.1% FA, 25 min gradient)
and
MeOH tR 15.43 (0-60% MeOH in H20, 0.1 % FA, 25 min gradient) and revealed >99%
purity. HRMS Calcd. m/z for C42H69012N12 [M+H]+ 933.5152, found 933.5154.
EXAMPLE 24
D-Arg-D-Tyr-D-Thr-D-Val-D-Glu-D-Leu-(S)-Bgl-D-Ala-NH2 (92) (SEQ ID NO:88)
H2NYNH
NH HO O
0
H O O H O = H O
H2N^ H H N HNH2
o O O I
OH
[0205] D-Arg-D-Tyr-D-Thr-D-Val-D-Glu-D-Leu-(S)-Bgl-D-Ala-NH2 (92) (SEQ ID
NO:88) was prepared on a 0.070 mmol scale, in a syringe tube following the
optimized
protocol as described above, using microwave assisted annulation over 240 min,
to give the
desired lactam peptide TFA salt 92 (27 mg) in 97% crude purity as determined
by analytical
RP-HPLC (column a, UV: 1= 214 nm, 5-30% MeCN in H2O, 0.1% FA, 30 min
gradient).
Purification was carried out by preparative RP-HPLC (column A, 2-30% MeCN in
H2O,
0.1 % FA, 30 min gradient) to give the desired FA salt product 92 (13 mg, 18%)
as a white
fluffy solid. The purified product was analyzed by analytical RP-HPLC (column
a, UV: 1=
214 nm) using both MeCN tR 10.04 (5-60% MeCN in H2O, 0.1% FA, 25 min gradient)
and
MeOH tR 15.69 (0-60% MeOH in H2O, 0.1 % FA, 25 min gradient) and revealed >99%
purity. HRMS Calcd. m/z for C42H69012N12 [M+H]+ 933.5152, found 933.5150.
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
78
EXAMPLE 25
[3H]thymidine incorporation for TF-1 cell proliferation measurements
[0206] Human TF-1 cells (5x104 cells/well) were cultured in complete RPMI
medium
(GIBCO RPMI Medium 1640, Invitrogen) supplemented with GM-CSF (Granulocyte
Macrophage Colony Stimulating Factor, 2 ng/ml; BD Biosource). Cells were
deprived of
growth factors for 18 h before preincubation with lactam peptide (1 M)
followed by
treatment with IL-1(3 (10 or 25 ng/mL). After 24 h incubation at 37 C,
[3H]thymidine (1
gCi/mL; Amersham) was added and the cells were incubated for another 24 h.
Cells were
harvested, washed two times with PBS (10 mM Na2HPO4, 2 mM KH2PO4, 137 mM NaCl,
2.7 mM KC1; pH 7.4) and lysed with a 0.1 N NaOH/ 0.1% Triton X-100 solution.
Scintillation cocktail (Fisher Scientific, 8 mL/sample) was added to the
lysate, and after 3 h,
radioactivity was measured (Beckman Multi-Purpose Scintillation Coulter
Counter LS6500).
Results were analyzed by one- or two-way ANOVA factoring for concentration or
treatments. Postanova comparisons among means were performed using the Tukey-
Framer
method. Statistical significance was set at p < 0.05. Data are presented as
mean SEM.
Inhibition of thymocytes TF-1 proliferation by 101.10 lactam analogs 54-59
[0207] The efficacy of Agl peptides 54-59 was ascertained by measuring their
influence on IL-1 induced human thymocyte TF-1 proliferation as assessed by
incorporation
of [3H]thymidine as previously described.
[0208] Among the analogs tested, five maintained some inhibitory effect on TF-
1
proliferation (Figure 1). Peptide 56 failed to block proliferation of TF-1
cells treated with IL-
1. Relative to 101.10, lactams 59, 58, 57, and 55, all exhibited similar
efficacy, suggesting
that the balance between side chain removal and conformational constraint at
positions 2 and
4-6 does not perturb activity. Replacement of the N-terminal D-Arg residue by
(R)-Agl in
compound 54 led to 2.2 fold increase in efficacy compared to 101.10,
suggesting that the
Argi side chain may not be necessary for activity.
[0209] The efficacy of Agl peptides 75-78 as allosteric negative modulators of
the IL-
1 receptor I was also ascertained by measuring their influence on IL-1 induced
human
thymocyte TF-1 proliferation as assessed by incorporation of [3H]thymidine
(Figure 2).
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
79
[0210] Results show that all (R)-Bab analogs tested exhibited an improvement
in
efficacy compared to 101.10, from a 2.4 fold (compound 78) to a 1.7 fold
(compound 76)
increase. Inhibition of TF-1 proliferation obtained with compound 75 confirms
that the Arg'
side chain does not seem to be necessary for activity. Reasons for the
increase of efficacy
observed with compounds 76-78 remains unclear and requires further
investigation regarding
the influence of features brought by a Bab residue, which, while not wishing
to be limited by
theory, may include the character of the aromatic ester and the increased
conformational
flexibility from insertion of an amino alkyl chain into the peptide backbone.
EXAMPLE 26
Synthesis of D-Arg-D-Tyr-(S)-Agl-D-Val-D-Glu-D-Leu-D-Ala-NH2 (93) (SEQ ID
NO:89): lantern-resin comparison and synthesis of multiple lactam residues on
SynPhase Lanterns
(a) Rink Amide-MBHA resin
Swelling and Fmoc-deprotection
[0211] A 12 mL plastic filtration tube with polyethylene frit was charged with
Rink
Amide-MBHA resin (200 mg, 0.134 mmol, 0.67 mmol/g) and DCM (7 mL). The tube
was
sealed and shaken for 0.5 h. The resin was then filtered and taken up in
freshly prepared 20%
piperidine in DMF solution (7 mL), shaken for 30 min, filtered, retreated with
20%
piperidine/DMF solution (7 mL) and shaken for 30 min. A positive Kaiser colour
test
indicated qualitatively the presence of free amine.
Washing
[0212] Washing steps after coupling or deprotection steps were performed by
successive agitations for 1 min and filtration from DMF (3 x 7 mL), MeOH (3 x
7 mL) and
DCM (3 x 7 mL).
Amino Acid couplings
[0213] A solution of N-(Fmoc)amino acid (3 equiv.), HBTU (3 equiv.) and DIEA
(6
equiv.) in DMF (7 mL) was prepared in a small sample vial, stirred for 3 min
and then added
to the resin. The reaction mixture was shaken for 1 h with Fmoc-D-Ala, for 3 h
with Fmoc-D-
Val, Fmoc-D-Thr(tBu), Fmoc-D-Tyr(tBu) and Fmoc-D-Arg(Pbf) and for 4 h with
Fmoc-D-
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
Leu, Fmoc-D-Glu(tBu), at room temperature. The completeness of each coupling
was
verified by the Kaiser test on few resin beads. (Kaiser, E.; Colescott, R. L.;
Bossinger C. D.;
Cook P. I. Anal. Biochem. 1970, 34, 595.)
Silylation and Alkylation
[0214] After Fmoc protecting group removal, resin was dried in vacuo for at
least 3 h.
The anhydrous resin, in a 12 mL plastic filtration tube with polyethylene
frit, was flushed
with argon, swollen in THE (7 mL), treated with BSA (5 equiv.), shaken for 6
h, filtered
under argon and treated with a solution of sulfamidate (5 equiv.) in THE (7
mL). After
shaking for 18 h at room temperature, the resin was filtered and washed under
argon with
THE (3 x 7 mL).
Microwave Assisted Annulation
[0215] A 2 mL glass microwave vial was charged with resin and a freshly
prepared
I% AcOH/DMSO solution (2 mL). The vial was sealed, heated in the microwave at
110 C
(pressure 1 bar) for 6 h. The resin was then washed from the microwave vessel
into a 12 mL
plastic filtration tube with polyethylene frit.
Cleavage test
[0216] Monitoring of reaction progress was performed by LC-MS analyses of
material cleaved from resin. Typically, a small resin sample (3-5 mg) was
treated with a
mixture of TFA/H20/TES (1 mL, 95/2.5/2.5, v/v/v) for lh and filtered. The
filtrate was
evaporated, dissolved in water and acetonitrile and examined by LC-MS.
Final Cleavage
[0217] The peptide was cleaved from the resin by shaking in TFA/H20/TES (7 mL,
95/2.5/2.5, v/v/v) for 3 h. The resin was filtered and washed with TFA. The
combined filtrate
and washings were concentrated in vacuo. The resulting residue was dissolved
in a minimum
volume of TFA (-1 mL), transferred to a centrifuge tube and precipitated by
the addition of
ice-cold diethyl ether (40 mL). The peptide was separated by centrifugation
and the diethyl
ether was carefully decanted from the tube. The precipitated peptide was
washed twice with
cold diethyl ether. The resulting white solid was dissolved in water and
freeze-dried to give a
white powder that was purified by preparative RP-HPLC, using the specified
conditions.
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
81
(b) Polystyrene Rink amide lanterns
[0218] D-Sized polystyrene Rink amide lanterns with a 35-gmol loading were
used
for the synthesis of 93 and 94 and A-sized polystyrene Rink amide lanterns
with a 75-gmol
loading were used for the synthesis of 95-97. A 20-mL sample vials with a
cover in which
seven 0,3 mm diameter holes were drilled was used and solutions were removed
simply by
reversing the flask.
Swelling and Fmoc-Deprotection
[0219] Swelling and Fmoc-deprotection steps were respectively performed by
immersing lanterns for 30 min in DCM and in DMF/Pip (80/20, v/v) solution. A
positive
Kaiser colour test on a sliver of lantern indicated qualitatively the presence
of free amine.
Washing
[0220] Washing steps after coupling and deprotection steps were performed by
dipping the lanterns in DMF (3 x 3 min), MeOH (1 x 3 min) and DCM (3 x 3 min),
successively.
Amino acid coupling
[0221] A DMF solution containing the Fmoc-protected amino acid (3 equiv.),
HBTU
(3 equiv.), and DIEA (6 equiv.) were freshly prepared in a 20-mL flask and
lanterns were
immersed in the coupling solution for 3 h with Fmoc-D-Ala, Fmoc-D-Leu, Fmoc-D-
Val,
Fmoc-D-Tyr(tBu), Fmoc-D-Pro and Fmoc-D-Arg(Pbf) and for 4 h with Fmoc-D-
Glu(tBu)
and Fmoc-D-Thr(tBu), at room temperature. The completeness of each coupling
was verified
by a Kaiser test on a sliver of lantern.
Silylation and Alkylation
[0222] After Fmoc protecting group deprotection, lanterns were then dried in
vacuo
for at least 3 h. The anhydrous lanterns, in 2- or 5- mL glass microwave
vials, were flushed
with argon, suspended in THF, treated with a solution of sulfamidate (4
equiv.) and DIEA (0-
1.1 equiv) in THF and heated in the microwave at 60-70 C for 1-2h. The
lanterns were
washed under argon with THE Alternately, the alkylation with sulfamidate was
preceded by
a treatment with BSA (5 equiv) in THF for 1-6 h.
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
82
Microwave Assisted Annulation
[0223] A 2- or 5-mL glass microwave vial was charged with lanterns and a
freshly
prepared solution of DMSO/AcOH (99:1, v/v) or DMSO/H20/AcOH (75:23:2, v/v/v).
The
vial was sealed, heated in the microwave at 80 C for 5-10 h. The lanterns
were then washed
as previously described.
Capping
[0224] After lactam annulation, a capping of the deletion sequence from
incomplete
sulfamidate alkylation was performed by immersing the lantern in a DMF
solution of acetic
anhydride (10 equiv.) and DIEA (10 equiv) for 1 h.
Cleavage test
[0225] Monitoring of reaction progress was performed by LC-MS analyses of
material cleaved from lantern. Typically, a slice of lantern was treated with
a mixture of
TFA/H20/TES (1 mL, 95/2.5/2.5, v/v/v) for lh and filtered. The filtrate was
evaporated,
dissolved in water and acetonitrile and examined by LC-MS.
Peptide cleavage
[0226] The peptide was cleaved by immersing the lantern in TFA/H20/TES (3 mL,
95/2.5/2.5, v/v/v) for 3 h. The cleavage cocktail was removed directly from
the tubes,
peptides were precipitated with ice-cold diethyl ether, centrifuged, and
decanted.
Precipitation, centrifugation, and decantation operations were repeated twice.
The resulting
white solid was dissolved in water (10 mL) and freeze-dried to give a white
powder that was
analysed for purity and purified by preparative RP-HPLC, using the specified
conditions.
Discussion for Example 26
A comparative study was first made between Rink amide MBHA resin and Rink-
amide
SynPhase lantern as supports in the synthesis of lactam peptide 93, in which
the D-Thr3
residue was replaced by (S)-Agl (Scheme 10). Assembly of the C-terminal
peptide fragment
(vela) was done by standard SPPS protocols. The amino terminal of the peptide
was then
alkylated with (4S)-(Fmoc)oxathiazinane ester (S)-8, which along with its
enantiomer (R)-8,
were synthesized in solution from L- and D-Met as described above. Peptide
alkylation was
preceded by N-silylation of the peptide amine with N,O-
bis(trimethylsilyl)acetamide (BSA)
to minimize bis-alkylation. Lactam annulation was performed by microwave
irradiation, the
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
83
Fmoc group was removed and the sequence was elongated to provide the
supported,
protected Agl peptide 93. Lactam peptide 93 was obtained by side-chain
deprotection and
cleavage from the resin and lantern upon treatment with TFA. The crude
peptides were
analyzed by RP-HPLC and comparable crude purity was obtained using resin and
lantern
(33% and 32%, respectively) indicating that Agl peptide synthesis was
unaffected by the
support. After cleavage from the resin, the main impurities in crude material
93 were the
deletion sequence from incomplete sulfamidate alkylation (30%) and uncyclized
product
(17%). After cleavage from lantern, the main impurity was the acetylated (S)-
Agl-vela
peptide (39%) probably due to an undesired partial Agl Fmoc deprotection prior
to treatment
with the acetic anhydride. The residual amine capping was performed on lantern
after lactam
annulation to avoid complications from a deletion sequence due to incomplete
sulfamidate
alkylation.
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
84
Scheme 10
o OtBu
sPPS 0 _ 0
Fmoc-HN SS = H
H2N H^~N HNH SS
SS = Rink amide resin or lantern
0 0
O OtBu
1) BSA, THF, RT O O
BnO2C HN N
2) p THE HN ~ H/~NH SS
I
f0
N" CO2Bn NHFmoc 0
Fmoc (8)
O OtBu
MW, 1%AcOH/DMSO
O O
H
FmocHN N HeN HNH SS
O IOI
O
HNYNH2
HN
O OH
1) SPPS O
O O
2) TFA/TES/H20 = H = H =
(95:2.5:2.5, v/v/v) H2N N H N H N H
NH2
O O
O
O
OH
D-Arg-D-Tyr-(S)-Agl-D-Val-D-Glu-D-Leu-D-Ala-NH2
93
SEQ ID NO: 89
[0227] The rytvela analog 93 was synthesized in parallel on color-tagged
lanterns
with the analog 94, in which the D-Va14 residue was replaced by (S)-Agl. The C-
and N-
terminal peptide fragments were assembled by standard SPPS protocols. The
alkylation with
sulfamidate (S)-8 was performed after treatment of the peptide lanterns with
BSA for 1 h, and
comparable results were obtained by overnight alkylation at room temperature
and heating at
60 C with microwave-irradiation for 1 h. Lactam annulation was accomplished in
I% AcOH
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
in DMSO with heating at 110 C under microwave irradiation for 6 h, or in an
oil bath for
longer times. As previously reported on resin conversion to lactam was cleaner
using the
microwave; however, irradiation at 110 C caused the lanterns to change
morphology and
degrade. Although lactam product could be recovered after cleavage from the
altered lantern,
it was observed that at 80 C, annulation could be effected without melting
lantern and good
conversion was achieved after 10 h.
[0228] In light of the improved activity of the (R)-Agli and maintained
potency of
(R)-Ag12 and (R)-Ag14 analogs of rytvela, the combinations of (R)-Agli-(R)-
Ag12 and (R)-
Agli-(R)-Ag14 in rytvela analogs 96 and 97 were explored. For comparison, the
(R)-Agl'-D-
Pro4 analog 95 was also synthesized. The three analogs were constructed using
a split-and-
pool approach on SynPhase-lanterns equipped with a Rink amide linker. The
lanterns were
equipped with coloured spindles as a visual tagging system. (R)-Cyclic
sulfamidate (R)-8 was
used to alkylate peptides bound to the lanterns suspended in THE at 60 C for
lh of
microwave irradiation. Monitoring the reaction progress by LC-MS analyses
after TFA-
mediated cleavage of a lantern slice, it was found that, in contrast to
synthesis on resin, the
silylation with BSA had little effect on the amount of bis-alkylation product
formed on
lantern and comparable percentages of alkylated peptides 100 and 101 (27-30%
and 50-52%,
respectively) were achieved. This prompted the investigation of peptide
alkylation without a
prior BSA treatment. Longer heating in the microwave (2h) at higher
temperature (70 C) and
the addition of 1.1 equiv of DIEA to the sulfamidate solution increased the
amount of 100
and 101 (>40% and >90%, respectively); however bis-alkylation of 98 was
significant (50%).
[0229] Subsequently, the condition for lactam annulation on both 100 and 101
were
optimized, and it was found that the addition of water to the DMSO (I% AcOH)
solution
gave, in both cases, quantitative lactam cyclization at 80 C under microwave
irradiation.
[0230] Investigating the feasibility of introducing a second lactam motif into
the same
peptide, alkylation and lactam annulation were performed using conditions
previously
effective for installing Agl residues: alkylation with (R)-8 (4 equiv) in THE
(0.1 M) with
microwave irradiation for lh at 70 C; lactam formation in a solution of
DMSO/AcOH/H20
(75 : 2 : 23, v/v/v) with microwave irradiation for 10 h at 80 C. These
conditions gave
respectively 50, 8 and 26% conversions to 107, 108, 109. The addition of a
second (R)-Agl
residue presented a more difficult challenge than the first (R)-Agl residue.
Different
conditions were pursued to improve conversion in the second alkylation step
including
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
86
solvent, temperature and microwave irradiation time, without much success. The
addition of
0.5 equiv of DIEA to the sulfamidate solution favoured alkylation. This amount
of DIEA was
chosen to avoid bis-alkylation, which was promoted with more base (1.1 equiv).
Lactam
formation by microwave irradiation at 80 C, Fmoc-deprotection and cleavage
gave the crude
peptides, which were analyzed by RP-HPLC to have 55, 30 and 45% crude purity
for 95, 96
and 97, respectively (Table 9 and Scheme 11).
[0231] The efficacy of Agl peptides 93 and 94 was ascertained by measuring
their
influence on IL-1 induced human thymocyte TF-1 proliferation as assessed by
incorporation
of [3H]thymidine as in Example 25. The percentages of proliferation of TF-1
cells (150% and
132%, respectively) pre-treated with peptides 93 and 94 demonstrated that both
these analogs
lost inhibitory activity on TF-1 proliferation exhibited by rytvela (69%).
Synthesis of (R)-Agl-D-Tyr-D-Thr-D-Val-(R)-Agl-D-Leu-D-Ala-NH2 (98) (SEQ ID
NO:94)
OH O
YO
N H =
H2Nl" N\\\**' N Ni"'" N ,-YNH2
H H
O O O O
OH
[0232] (R)-Agl-D-Tyr-D-Thr-D-Val-(R)-Agl-D-Leu-D-Ala-NH2 (98) (SEQ ID
NO:94) was prepared on an A-sized polystyrene Rink amide lantern as outlined
in Example
26. The crude peptide purity (30%) was assessed by RP-HPLC-MS (UV 214), 5-90%
MeOH
in H20, 0.1% FA, 20 min gradient, tR 13.76 min., MS calcd. for C35H54Ng09
[M+H]+ 731.4,
found 731.2).
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
87
EXAMPLE 27
alpha-Amino-beta-hydroxy-gamma-lactams, constrained Serine and Threonine
dipeptide mimics
Synthesis
(a) Synthesis of N-(Fmoc) Oxiranylglycine reagent 106
CI Me -q-
O
~S,,,,'-,S,,,,'i CI ^yCOZMe m-CPBA O~COZMe
191 C, 2h 1,2-DCE NFmoc
I
H NFmoc HFmoc 40 C, 17h H
106 107
[0233] From known compound 107, an adapted literature procedure was followed:
N-(Fmoc)Vinylglycine methyl ester
[0234] A 2,4-dichlorotoluene (30 mL) solution of N-(Fmoc)Met(S=O)-OMe 107
(4.01 g, 10.0 mmol) was heated to 191 C for 2h under Argon. The
unconcentrated crude was
loaded directly onto a 6.5 cm diameter X 13 cm high pad of silica, which was
eluted using a
step gradient of 3--->6--->9--->12--->20--->35--->60% ethyl acetate: hexanes,
400mL per step)
yielding 2.81 g (84%) of N(Fmoc)Vinylglycine methyl ester.
N-(Fmoc)Oxiranylglycine methyl ester (106)
[0235] N-(Fmoc)Vinylglycine methyl ester (1.69g, 5.00 mmol) and m-CPBA
(commercial <77%, 5.6g, 25 mmol) were heated in 1,2-dichloroethane at 40 C
for 17h. The
crude was filtered over a frit, concentrated and purified by flash
chromatography (7.5 cm
wide X 8.5 cm high silica pad, eluded by step gradient of 3->6->9->15->20->25%
ethyl
acetate: toluene, 400 mL per step) yielding 833 mg (47%) of N-
(Fmoc)oxiranylglycine
methyl ester.
Representative procedure for the synthesis ofl3-hydroxy-a-amino-y-lactams
[0236] D-Phe-OMe=HCl (77.6 mg, 360 mmol) in dilute Na2CO3 was extrated with
CHC13 (3 X 1 mL), dried over MgSO4, concentrated, and dried under high vacuum
for a few
hours to yield 40.8 mg (78%) of D-Phe-OMe. The free base (26.1 mg, 0.180 mmol)
was
immediately used in a reaction with N-(Fmoc)oxiranylglycine methyl ester 106
(18.4 mg,
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
88
0.052 mmol) at 76 C in 2,2,2-trifluoroethanol (0.3 mL) for 15h. The crude was
concentrated
in vacuo, and purified by flash chromatography (2.2 cm wide X 2 cm high silica
pad, step
gradient of 28-*31-*34-*37-*50-*70% ethyl acetate: hexanes, 20 mL per step)
yielding
21.9 mg (91%) product (Rfproduct = 0.2 with 60% ethyl acetate: hexanes eluent,
Rf oxiranylglycine
= 0.55).
Spectroscopic Data
(a) (2S)-[(9H-Fluoren-9-ylmethoxycarbonylamino)]-oxiranyl-acetic acid methyl
ester (106)
Oj~ ~CO2Me
~NFmoc
H
[0237] 1H NMR (400 MHz, CDC13) 6 7.77 (d, J= 7.4 Hz, 2H), 7.60 (t, J= 6.7 Hz,
2H), 7.41 (t, J = 7.4 Hz, 2H), 7.32 (td, J = 7.2, 2.5 Hz, 2H), 5.35 (d, J =
9.0 Hz, 1 H), 4.74 (d,
J= 8.9 Hz, 1H), 4.43 (d, J= 7.1 Hz, 2H), 4.22 (t, J= 6.8 Hz, 1H), 3.83 (s,
3H), 3.51-3.47 (m,
1H), 2.79 (t, J= 4.3 Hz, 1H), 2.63 (dd, J= 4.6, 2.6 Hz, 1H). 13C NMR (75 MHz,
CDC13)
170.3, 156.3, 143.9, 143.7, 141.51, 141.47, 127.9, 127.27, 127.26, 125.2,
120.2, 76.8, 67.4,
53.2, 51.3, 47.3, 44Ø HRMS(ESI+) for MH+ = C2oH2oNO5+; calculated: 354.1336,
found:
354.1331 (dif m/z = 1.3 ppm).
(b) 2-[3-(9H-Fluoren-9ylmethoxycarbonylamino)-4-hydroxy-2-oxo pyrrolidin-l-
ylJ propionic acid benzyl ester (108)
HO
FmocNH N-_/C02Bn
O Me
[0238] 1H NMR (300 MHz, CDC13) 6 7.78 (d, J= 7.3 Hz, 2H), 7.59 (d, J= 7.3 Hz,
2H), 7.46-7.29 (m, 9H), 5.70 (br s, 1 H), 5.16 (dd, J = 15.3, 12.2, 2H), 5.00-
4.82 (m, 2H),
4.58-4.32 (m, 3H), 4.23 (t, J= 7.0 Hz, 1H), 4.13 (pseudo t, J= 7.2 Hz, 1 H),
3.69 (t, J= 3.69
Hz, 1H), 3.24 (t, J= 8.7 Hz, 1H), 1.47 (d, J= 7.2 Hz, 3H). 13C NMR (75 MHz,
CDC13): 6
(ppm) 170.6, 169.3, 158.2, 143.7, 141.5, 135.3, 128.9, 128.8, 128.4, 128.1,
127.3, 125.2,
120.3, 73.7, 67.9, 67.5, 60.7, 49.7, 47.6, 47.1, 15.1. HRMS (ESI+) for MH+ =
C29H29N2O6+;
calculated: 501.2020, found: 501.2032 (error m/z = 2.4 ppm).
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
89
2-[3-(9H-Fluoren-9 ylmethoxycarbonylamino)-4-hydroxy-2-oxo pyrrolidin-1 ylJ-4-
methyl-
pentanoic acid methyl ester (109)
HO
FmocNH N C02Me
O
[0239] iH NMR (400 MHz, CDC13): 6 (ppm) 7.77 (d, J= 7.5 Hz, 2H), 7.59 (d, J=
7.5
Hz, 2H), 7.41 (t, J= 7.5 Hz, 2H), 7.32 (t, J= 7.4 Hz), 5.77 (br s, 1H), 4.88
(pseudo t, J= 8.2
Hz, 1 H), 4.44 (d, J = 7.1 Hz, 2H), 4.29 (q, J = 8.2 Hz, 1 H), 4.22 (t, J =
7.0 Hz, 1 H), 4.13 (d, J
= 8.0 Hz, 1H), 3.72 (s, 1H), 3.56 (t, J= 8.8 Hz, 1H), 3.38 (t. J= 9.0 Hz, 1H),
1.83-1.35 (m,
3H), 1.01-0.87 (m, 6H). ESI+ for MH+ = C26H3,N206+ calculated and found:
467.2; for MNa+
= C26H30N206Na+ calculated and found: 489.2.
(d) [3-(9H-Fluoren-9 ylmethoxycarbonylamino)-4-hydroxy-2-oxo pyrrolidin-1-
ylJ-acetic acid benzyl ester (110)
HO
FmocNH N-_/C02Bn
0
[0240] iH NMR (300 MHz, CDC13): 6 (ppm) 7.78 (d, J= 7.5 Hz, 2H), 7.59 (d, J=
7.4
Hz, 2H), 7.45-7.30 (m, 9H), 5.73 (s, 1H), 5.18 (s, 2H), 4.97 (very br), 4.48-
4.36 (m, 3H),
4.28-4.07 (m, 4H), 3.63 (dd, J= 9.4, 8.3 Hz, 1H), 3.44 (dd, J= 9.3, 8.1 Hz,
1H). ESI+ for
MH+= C2gH27N206+ calculated and found: 487.2. For MNa+ = C28H26N2O6Na+
calculated and
found: 509.2.
(e) 2-[3-(9H-Fluoren-9 ylmethoxycarbonylamino)-4-hydroxy-2-oxo pyrrolidin-l -
ylJ-3phenylpropionic acid methyl ester (111)
HO
FmocNH N-_/C02Me
0 ~-Ph
[0241] iH NMR (300 MHz, CDC13): 6 (ppm) 7.74 (d, J= 7.5 Hz, 2H), 7.55 (d, J=
7.2
Hz, 2H),7.39 (t, J= 7.3 Hz, 2H), 7.33-7.13 (m, 7H), 5.68 (s, 1H), 5.06 (dd, J=
11.4, 4.9 Hz,
1H), 4.57-4.41 (m, 1H), 4.37 (d, J= 7.1 Hz, 2H), 4.30 (q, J= 8.1 Hz, 1H), 4.18
(t, J= 6.9 Hz,
1 H), 3.82 (dd, J = 8.0, 1.8 Hz, 1 H), 3.75 (s, 3 H), 3.69 (dd, J = 9.0, 8.0
Hz, 1 H), 3.3 9 (dd, J =
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
14.7, 5.0 Hz, I H), 3.14 (t, J = 3.4 Hz, I H), 2.96 (dd, J = 14.7, 11.5, I H).
13C NMR (75 MHz,
CDC13): 6 (ppm) 170.3, 169.6, 158.2, 143.64, 143.56, 141.5, 141.4, 135.9,
129.0, 128.6, 128.0,
127.4, 127.25, 127.26, 125.14, 125.12, 120.2, 73.5, 67.8, 60.3, 55.1, 52.8,
48.0, 47.1, 35.4.
ESI+ for C29H29N2O6+ calculated and found: 501.2. For C29H28N2O6Na+ calculated
and found
523.2. HRMS (ESI+) for MH+ = C29H29N2O6+ calculated: 501.2020, found: 501.2027
(diff.
m/z = 1.4 ppm).
(f) 2-[3-(9H-Fluoren-9 ylmethoxycarbonylamino)-4-hydroxy-2-oxo pyrrolidin-1-
ylJ-3-methyl-butyric acid methyl ester (112)
HO
FmocNH N-_/CO2Me
O
[0242] 1H NMR (700 MHz, CDC13): 6 (ppm) 7.77 (d, J= 7.7 Hz, 2H), 7.59 (dd, J=
7.5, 4.0 Hz, 2H), 7.41 (t, J= 7.5 Hz, 2H), 7.33 (tt, J= 7.5, 1.1 Hz, 2H), 5.78
(s, 1H), 5.02 (br
s, 1 H), 4.52 (d, J = 9.5 Hz, 1 H), 4.44 (dd, J = 10.7, 7.1, 1 H), 4.42 (dd, J
= 10.7, 7.1, 1 H), 4.36
(q, J = 8.0 Hz, 1 H), 4.23 (t, J = 7.1 Hz, 1 H), 4.14 (ddd, J = 8.1, 1.9, 1.0
Hz, 1 H), 4.03 (dd, J =
9.5, 8.0 Hz, 1H), 3.73 (s, 3H), 3.25 (dd, J= 9.6, 8.2 Hz, 1H), 2.23 (doublet
of septuplets, J=
9.5, 6.7 Hz, 1H), 0.99 (d, J= 6.7 Hz, 3H), 0.96 (d, J= 6.7 Hz, 3H). 13C NMR
(100 MHz,
CDC13): 6 (ppm) 170.6, 169.8, 158.2, 143.7, 143.6, 141.5, 128.0, 127.3, 125.1,
120.2, 73.6,
67.9, 60.5, 59.9, 52.3, 48.0, 47.1, 28.1, 19.4, 19.3 (diasteriotopic Me). HRMS
(ESI+) for MH+
= C25H29N2O6+; calculated 453.2020, found: 453.2028 (dif m/z = 1.8 ppm).
(g) 2-[3-(9H-Fluoren-9 ylmethoxycarbonylamino)-4-hydroxy-2-oxo pyrrolidin-l -
ylJ-3-(4-hydroxyphenyl) propionic acid methyl ester (113)
HO
FmocNH N-,/CO2Me
O
OH
[0243] 1H NMR (300 MHz, CDC13): 6 (ppm) 7.77 (d, J= 7.6 Hz, 2H), 7.57 (d, J=
7.6
Hz, 2H), 7.41 (t, J= 7.6, 2H), 7.36 (br, 1H), 7.32 (t, J= 6.8, 2H), 7.02 (d,
J= 8.3 Hz, 2H),
6.70 (d, J = 8.4 Hz, 2H), 6.20 (very br s, 1 H), 5.63 (br s, 1 H), 5.09 (dd, J
= 11.7, 4.8 Hz, 1 H),
4.45-2.48 (m, 3H), 4.20 (t, J= 6.9 Hz, 1H), 3.92 (d, J= 8.3 Hz, 1H), 3.77 (s,
3H), 3.83-3.68
(m, 1 H), 3.31 (dd, J = 11.5, 4.7 Hz, 1 H), 3.15 (t, J = 8.6 Hz, 1 H), 2.8 8
(t, J = 11.8 Hz, 1 H).
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
91
ESI+ for MH+ = C29H29N207+ calculated and found: 517.2. For MNa+ =
C29H28N2O7Na+
calculated: 539.2, found: 539.3.
(h) 2-[3-(9H-Fluoren-9 ylmethoxycarbonylamino)-4-hydroxy-2-oxo pyrrolidin-l-
ylJ-3-(JH-indol-3yl)propionic acid methyl ester (114)
HO
FmocNH N-_/CO2Me
O
N
H
[0244] 1H NMR (300 MHz, CDC13): 6 (ppm) 8.17 (br s, 1H), 7.77 (d, J= 7.7 Hz,
2H),
7.62-7.53 (m, 3H), 7.45-7.10 (m, 7H), 7.00 Hz (br s, 1H), 5.65 (br s, 1H),
5.18 (dd, J= 11.2,
4.7 Hz, 1 H), 4.82 (very br s, 1 H), 4.3 8-4.26 (m, 3H), 4.18 (t, J = 6.9 Hz,
1 H), 3.94 (dd, J =
8.2, 2.4 Hz, 1 H), 3.79 (s, 3H), 3.72 (t, J = 8.6 Hz, 1 H), 3.49 (dd, J =
15.5, 4.7 Hz, 1 H), 3.27-
3.14 (m, 2H). 13C NMR (75.5 MHz, CDC13): 6 (ppm) 170.7, 169.7, 158.1, 143.7,
143.6,
141.46,141.44,136.3, 128.0,127.3, 127.1, 125.1,
122.6,122.0,120.2,119.9,118.4,111.5,
110.5, 73.5, 67.8, 60.4, 54.4, 52.8, 47.8, 47.1, 25.5. HRMS (ESI) for MH+ =
C3,H3oN3O6+;
calculated: 540.2129, found: 540.2141 (diff. m/z = 2.2 ppm).
(i) 3-[3-(9H-Fluoren-9 ylmethoxycarbonylamino)-4-hydroxy-2-oxo pyrrolidin-l -
ylJ propionic acid benzyl ester (115)
HO
FmocNH N~~CO2Bn
0
[0245] 1H NMR (300 MHz, CDC13): 6 (ppm) 7.78 (d, J= 7.5 Hz, 2H), 7.59 (d, J=
7.3
Hz, 2H), 7.45-7.29 (m, 9H), 5.68 (br s, I H), 5.13 (s, 2H), 4.87 (br s, I H),
4.44 (d, J= 6.8 Hz,
I H), 4.31-4.19 (m, 2H), 4.00 (d, J= 8.0 Hz, I H), 3.79-3.67 (m, I H), 3.62-
3.46 (m, 2H), 3.28
(t, J= 8.8 Hz, 1H), 2.63 (t, J= 6.6 Hz, 2H). 13C NMR (300 MHz, CDC13): 6 (ppm)
171.1,
168.9, 158.2, 143.7, 143.6, 141.48, 141.47, 135.6, 128.8, 128.6, 128.0,
127.27, 127.26, 125.14,
125.11, 120.24, 120.22, 73.5, 67.8, 67.0, 60.8, 50.9, 47.1, 39.0, 32.5. HRMS
(ESI) for MH+ _
C29H29N2O6 ; calculated: 501.2020, found: 501.2032 (dif m/z = 2.3).
(j) 3-[3-(9H-Fluoren-9 ylmethoxycarbonylamino)-4-hydroxy-2-oxo pyrrolidin-1-
ylJ-benzoic acid methyl ester (116)
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
92
HO
CO2Me
FmocNH N
O I
[0246] 1H NMR (300 MHz, CDC13): 6 (ppm) 8.15 (pseudo t, J= 1.9 Hz, 1H), 7.94
(ddd, J = 8.1, 2.4, 1.0 Hz, 1 H), 7.89 (dt, J = 7.9, 1.3 Hz, 1 H), 7.78 (d, J
= 7.6 Hz, 2H), 7.61
(d, J = 7.5 Hz, 2H), 7.48 (t, J = 8.0 Hz, 1 H), 7,43 (t, J = 7.3 Hz, 2H), 7.34
(td, J = 7.5, 1.2 Hz,
2H), 5.84 (br s, 1H), 5.09 (br s, 1H), 4.54-4.42 (m, 3H), 4.31-4.21 (m, 2H),
4.08 (dd, J= 9.8,
8.1 Hz, 1H), 3.94 (s, 3H), 3.79 (dd, J= 9.6, 8.3 Hz, 1H). ESI+ for MH+=
C27H25N2O6+;
calculated and found: 473.2. For MNa+ = C27H24N2O6Na+; calculated and found:
495.2.
Discussion
[0247] Enantiomerically pure (2S,3R)-N-(Cbz)-oxiranylglycine was prepared from
L-
Met according to literature procedures and examined in reactions with Ala-
013n. In
acetonitrile at 90 C, the desired sequential alkylation / lactam formation
occurred producing
target (3-hydroxy-a-amino-y-lactam, albeit in 30% yield. The utility of Fmoc
protection in
peptide synthesis compelled further examination using N-(Fmoc)oxiranylglycine
106, which
was prepared in an analogous manner from L-Met. Epoxide 106 reacted with Ala-
013n to
produce lactam 108 in 10% yield. Little improvement was obtained in attempts
to yield
lactam 108 using Lewis and Bronsted acid catalysts. In the reaction between N-
(Fmoc)Oxiranylglycine 106 and different amino acid analogs, 2,2,2-
trifluoroethanol (TFE) as
solvent proved optimum.
[0248] For example, substrates with sterically demanding side chains such as
the
methyl esters of Phe, Val and Trp, reacted well with 106. In the case of Gly-
OBn, lower
reaction temperatures mitigated losses from Fmoc deprotection. The
nucleophilic phenol of
unprotected Tyr-OMe was tolerated, however the ester-protected glutamate side
competed for
lactamization producing N-alkyl pyroglutamate. In addition to proteinogenic
examples, the
methyl ester of aminobenzoic acid as well as the benzyl ester of beta-Ala gave
respectively
58 and 67% yield.
[0249] The stereogenic carbons in these dipeptide mimics are derived from the
chiral
pool with diasteroselective induction to set the 3-hydroxy center by way of
selective
epoxidation of vinylglycine. The oxiranylglycine diasteriomers 106 were
separable, allowing
all possible sterioisomers to be obtained by choice of chirality in the
starting material.
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
93
Preparation of (3-hydroxy-a-amino-y-lactam peptides on polystyrene Rink amide
lantern
Synthesis of rytvela /3-hydroxy-a-amino-y-lactam analogue
D-Arg-D-Tyr-Agl(4-OH)-D-Val-D-Glu-D-Leu-D-Ala-NH2 (99) (SEQ ID NO:95)
H2N
NH
NH
HO 0
H2N b-NH O OH
O O = H O
HN N Nib N NH2
O H O O
OH
[0250] D-Arg-D-Tyr-Agl(4-OH)-D-Val-D-Glu-D-Leu-D-Ala-NH2 (99) (SEQ ID
NO:95) was prepared on an A-sized polystyrene Rink amide lantern as outlined
in Example
25 except the sulfamidate alkylation step was substituted by the following:
[0251] After Fmoc protecting group deprotection, the lanterns were treated
with an
oxiranylglycine 106 (3 equiv.) in 2,2,2-trifluoroethanol (0.06 M) and heated
to 80 C under
microwave irradiation for 12h. Lantern washing and subsequent elongation was
done as
described, providing the desired lactam peptide as the TFA salt 99 (50% crude
purity by
analytical RP-HPLC-MS (UV 214), 5-80% MeOH in H2O, 0.1% FA, 20 min gradient,
tR
8.45 min., MS calcd. for C3sH62011Nii [M+H]+ 848.4, found 848.6.).
[0252] Alternatively, the hydroxylactam dipeptidyl esters may be hydrolyzed
and
used as dipeptide building blocks in standard peptide synthesis.
[0253] These scaffolds may find application in medicinal chemistry, especially
because the hydroxy and protected amino groups are ideally suited for
orthogonally
elaborating these structures further. In the context of solid supported
peptide synthesis,
elaboration of the hydroxy group would allow for mimicry of other constrained
amino acid
residues, by the attachment of carbohydrates, phosphonate, sulfate and other
ester and ether
types.
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
94
EXAMPLE 28
Solid-Phase Synthesis of Indolizidinone Analogs of API-101.10
Rink Amide Resin swelling and deprotection
[0254] A 12 mL plastic filtration tube with polyethylene frit was charged with
Rink
resin (300 mg, 0.09 mmol, 0.3 mmol/g) and DMF (7 mL). The tube was sealed and
shaken
for 0.5 h. The resin was then filtered and taken up in freshly prepared 20%
piperidine in
DMF solution (7 mL), shaken for 30 min, filtered, retreated with 20%
piperidine/DMF
solution (7 mL) and shaken for 30 min. The resin was washed by successive
agitations for 1
min and filtered from DMF (3 x 7 mL), MeOH (3 x 7 mL) and DCM (3 X 7 mL). A
positive
Kaiser colour test indicated qualitatively the presence of free amine.
Amino Acid couplings
H
FmocHN\\ , N
O O^OH
Fmoc-I2aa-OH
[0255] The resin was first swollen in DMF (7 mL) for 15 min. Meanwhile, a
solution
of N-(Fmoc)amino acid (Fmoc-Xaa-OH, 3 equiv.), HBTU (3 equiv.) and DIEA (6
equiv.) in
DMF (7 mL) was prepared in a small sample vial, stirred for 10 min and then
added to the
resin. The reaction mixture was shaken for 8 h with Fmoc-D-Leu-OH, 12 h with
Fmoc-D-
Glu(tBu)-OH and Fmoc-D-Gln(Trt)-OH, 18 h with Fmoc-I2aa-OH, 10 h with Fmoc-D-
Tyr(tBu)-OH, 11 h with Fmoc-D-Om(Boc)-OH, HOOC(CH2)5NH(Boc) and
HOOC(CH2)4NH(Fmoc), at room temperature. The resin was then filtered and
respectively
washed by shaking for 1 min with DMF (3 x 7 mL), MeOH (3 x 7 mL) and DCM (3 x
7
mL). A negative Kaiser test response indicated completion of the reaction. The
resin was then
dried in vacuo.
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
Silylation and Alkylation (for peptides WC144 and WC145)
0----
I
O ~/ i N "/CO2Bn
Fmoc
6-membered cyclic D-sulfamidate (N-(Fmoc)oxathiazinane)
O
C02Bn
O0// 'N
1
Fmoc
6-membered cyclic L-sulfamidate (N-(Fmoc)oxathiazinane)
[0256] After swelling the resin, the Fmoc protecting group was removed as
described
above. The resin was then dried in vacuo for at least 3 h. The anhydrous
resin, in a 12 mL
plastic filtration tube with polyethylene frit, was then flushed with argon,
swollen in THE (7
mL), treated with BSA (5 equiv.), shaken for 16 h, filtered under argon and
treated with a
solution of sulfamidate (5 equiv.) in THE (7 mL). After shaking for 24 h, the
resin was
filtered and washed under argon with THE (3 x 7 mL), MeOH (3 x 7 mL) and DCM
(3 X 7
mL) and dried in vacuo.
Microwave Assisted Annulation (for peptides WC144 and WC145)
[0257] A 2 mL glass microwave vial was charged with resin and either DMF (2
mL)
or a freshly prepared 1% acetic acid/DMSO solution (2 mL). The vial was
sealed, heated in
the microwave at 100 C (pressure 1 bar) for 3 h and then cooled using a jet
of air. The resin
was then washed from the microwave vessel into a 12 mL plastic filtration tube
with
polyethylene frit and washed by shaking for 1 min with DMF (3 x 7 mL), MeOH (3
x 7 mL)
and DCM (3 x 7 mL) and then dried in vacuo.
Alkylation (for peptides WC146 and WC147)
CO2Me
FmocN
O1i-O
O
5-membered cyclic D-sulfamidate (N-(Fmoc)oxathiazolidine)
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
96
CO2Me
FmocN
O~:II-O
O
5-membered cyclic L-sulfamidate (N-(Fmoc)oxathiazolidine)
[0258] The resin was swollen in DMF (4 mL) for 20 min and filtered. A 20%
piperidine solution in DMF (4 mL) was added and the suspension was shaken for
20 min and
filtered. This operation was repeated and the resin was washed with DMF (3 x 3
mL), MeOH
(3 x 3 mL) and DCM (3 x 3 mL). At this point the Kaiser test gave a positive
response.
[0259] The peptidyl resin was then transferred into a dry 2 mL microwave
vessel. A
solution of freshly prepared sulfamidate in THE (1.5 mL) was added under
argon, followed
by DIEA. The mixture was then heated under microwave irradiation at 60 C for
2.5 hours.
The resin was washed with DCM (3 x 6 mL), MeOH (3 x 6 mL) and DCM (3 x mL).
Microwave Assisted Annulation (for peptides WC146 and WC147)
[0260] A 2 mL glass microwave vial was charged with resin and a mixture of
DMSO/H20/AcOH (75:23:2, 2 mL). The vial was sealed, heated in the microwave at
100 C
(pressure 1 bar) for 4h and then cooled using a jet of air. The resin was then
washed from the
microwave vessel into a 12 mL plastic filtration tube with polyethylene frit
and washed by
shaking for 1 min with DMF (3 x 7 mL), MeOH (3 x 7 mL) and DCM (3 x 7 mL) and
then
dried in vacuo.
Resin Capping
[0261] The resin was swollen in a solution of di-tent-butyl dicarbonate (5
equiv.) in
DMF (7 mL), treated with DIEA (10 equiv.), shaken for 1 h, filtered and washed
by shaking
for 1 min with DMF (3 x 7 mL), MeOH (3 x 7 mL) and DCM (3 X 7 mL) and then
dried in
vacuo.
Peptide Cleavage
[0262] The resin was first swollen in a plastic filtration tube with
polyethylene frit, as
described for Fmoc removal above, treated with a freshly prepared 20%
piperidine / DMF
solution (7 mL), shaken for 15 min, filtered, treated with a second portion of
20%
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
97
piperidine/DMF solution (7 mL) and shaken for 15 min. The resin was then
filtered and
washed by shaking for 1 min with DMF (3 x 7 mL), MeOH (3 x 7 mL) and DCM (3 x
7
mL). A positive Kaiser colour test indicated qualitatively the presence of
free amine. The
peptide was then cleaved from the resin by shaking in TFA/H20/TES (7 mL,
95/2.5/2.5,
v/v/v) for 2 h. The resin was filtered, washed with TFA (7 mL) and the
combined filtrate and
washings were concentrated in vacuo. The resulting residue was dissolved in a
minimum
volume of TFA (-1 mL), transferred to a centrifuge tube and precipitated by
the addition of
ice-cold diethyl ether (40 mL). The peptide was then centrifuged and the
diethyl ether was
carefully decanted from the tube. The treatment of the precipitated peptide
with cold diethyl
ether wash was repeated twice. The resulting white solid was dissolved in
water (10 mL) and
freeze-dried to give a white foam that was purified by preparatory RP-HPLC,
using the
specified conditions.
D-Orn-D-Tyr-haa-D-Glu-D-Leu-NHz (WC115) (SEQ ID NO:96)
NHZ
H
0 JOZH
H
H NN N\`~N = H O
Z O H N
O Ce-N NHZ
O
OH
[0263] D-Orn-D-Tyr-12 aa-D-Glu-D-Leu-NHz (WC115) (SEQ ID NO:96) was
prepared as described above to give the desired peptide TFA salt (38 mg, 71%
crude purity as
analyzed by analytical RP-HPLC (UV 214), 0-40 MeCN, 8 min gradient).
Purification was
then carried out by preparatory RP-HPLC (5-40 MeCN, 15 min gradient) to give
the desired
formic acid salt WC115 (11.5 mg, 21%) as a white foam. The purified product
was analyzed
by analytical RP-HPLC (UV 214) using both MeCN TR 13.57 (2-40 MeCN, 20 min
gradient)
and MeOH TR 14.99 (10-60 MeOH, 20 min gradient) and revealed >99% purity. HRMS
Calcd. for C34H5209Ns [M+H]+ 717.3930, found 717.3936.
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
98
H2N(CH2)4C0-D-Tyr-haa-D-Glu-D-Leu-NH2 (WC116) (SEQ ID NO:97)
H
CO2H
O
(;N ' H O
H2N N N\`.,
p H O p^HN NH2
O
OH
[0264] H2N(CH2)4CO-D-Tyr-haa-D-Glu-D-Leu-NH2 (WC116) (SEQ ID NO: 97) was
prepared as described above to give the desired peptide TFA salt (15 mg, 47%
crude purity as
analyzed by analytical RP-HPLC (UV 214), 0-40 MeCN, 8 min gradient).
Purification was
then carried out by preparatory RP-HPLC (5-40 MeCN, 25 min gradient) to give
the desired
formic acid salt WC116 (5.3 mg, 10%) as a white foam. The purified product was
analyzed
by analytical RP-HPLC (UV 214) using both MeCN TR 16.43 (2-40 MeCN, 20 min
gradient)
and MeOH TR 19.39 (10-60 MeOH, 20 min gradient) and revealed >99% purity. HRMS
Calcd. for C34H52O9N7 [M+H]+ 702.3821, found 702.3827.
H2N(CH2)5CO -D-Tyr-haa-D-Glu-D-Leu-NHz (WC117) (SEQ ID NO:98)
H
_J02H
H O
H N N N\`~, N H O
2 O H O p^N^ N NH2
\ H CIO
/ OH
[0265] H2N(CH2)5CO-D-Tyr-haa-D-Glu-D-Leu-NH2 (WC117) (SEQ ID NO:98) was
prepared as described above to give the desired peptide TFA salt (23.4 mg, 44%
crude purity
as analyzed by analytical RP-HPLC (UV 214), 0-40 MeCN, 8 min gradient).
Purification was
then carried out by preparatory RP-HPLC (2-30 MeCN, 20 min gradient) to give
the desired
formic acid salt WC117 (9.8 mg, 18%) as a white foam. The purified product was
analyzed
by analytical RP-HPLC (UV 214) using both MeCN TR 16.80 (2-40 MeCN, 20 min
gradient)
and MeOH TR 17.95 (10-60 MeOH, 20 min gradient) and revealed >99% purity. HRMS
Calcd. for C35H54O9N7 [M+H]+ 716.3978, found 716.3980.
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
99
H2N(CH2)4C0-D-Tyr-haa-D-Gln-D-Leu-NH2 (WC125) (SEQ ID NO:99)
H
COZNHZ
O
(;N ' H O
HZN N N\`.,
p H O H N NHZ
O
OH
[0266] H2N(CH2)4CO-D-Tyr-haa-D-Gln-D-Leu-NH2 (WC125) (SEQ ID NO:99) was
prepared as described above to give the desired peptide TFA salt (33.0 mg, 62%
crude purity
as analyzed by analytical RP-HPLC (UV 214), 0-40 MeCN, 8 min gradient).
Purification was
then carried out by preparatory RP-HPLC (5-40 MeCN, 20 min gradient) to give
the desired
formic acid salt WC125 (10.7 mg, 20%) as a white foam. The purified product
was analyzed
by analytical RP-HPLC (UV 214) using both MeCN TR 16.21 (0-40 MeCN, 25min
gradient)
and MeOH TR 17.95 (0-80 MeOH, 25 min gradient) and revealed >97% purity. HRMS
Calcd. for C34H53OsNs [M+H]+ 701.3981, found 701.3979.
H2N(CH2)5CO -D-Tyr-haa-D-Gln-D-Leu-NHz (WC126) (SEQ ID NO: 100)
H
CO2NH2
O
H QN O
H N N N\\\, H
2 O H O OH- NH2
O
OH
[0267] H2N(CH2)5CO-D-Tyr-haa-D-Gln-D-Leu-NH2 (WC 126) (SEQ ID NO: 100)
was prepared as described above to give the desired peptide TFA salt (38.0 mg,
71% crude
purity as analyzed by analytical RP-HPLC (UV 214), 0-40 MeCN, 8 min gradient).
Purification was then carried out by preparatory RP-HPLC (5-40 MeCN, 15 min
gradient) to
give the desired formic acid salt WC126 (11.0 mg, 20%) as a white foam. The
purified
product was analyzed by analytical RP-HPLC (UV 214) using both MeCN TR 15.37
(0-40
MeCN, 25min gradient) and MeOH TR 18.38 (0-60 MeOH, 25 min gradient) and
revealed
>99% purity. HRMS Calcd. for C35H5508Ns [M+H]+ 715.4137, found 715.4156.
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
100
D-Orn-D-Tyr-haa-D-Gln-D-Leu-NHz (WC127) (SEQ ID NO: 101)
NH2
f 0 H CO2NH2
= H
N N\\\, H O
H N
2
,*~'Y ,
O H O HN NH2
O
OH
[0268] D-Orn-D-Tyr-12 aa-D-Gln-D-Leu-NHz (WC127) (SEQ ID NO:101) was
prepared as described above to give the desired peptide TFA salt (45.0 mg, 84%
crude purity
as analyzed by analytical RP-HPLC (UV 214), 0-40 MeCN, 8 min gradient).
Purification was
then carried out by preparatory RP-HPLC (5-40 MeCN, 15 min gradient) to give
the desired
formic acid salt WC127 (8.7 mg, 17%) as a white foam. The purified product was
analyzed
by analytical RP-HPLC (UV 214) using both MeCN TR 14.71 (0-60 MeCN, 20 min
gradient)
and MeOH TR 11.12 (0-60 MeOH, 25 min gradient) and revealed >99% purity. HRMS
Calcd. for C34H54O8N9 [M+H]+ 716.4090, found 716.4089.
D-Agl-D-Tyr-haa-D-Glu-D-Leu-NHz (WC144) (SEQ ID NO: 102)
H
CO2H
H2NW N NN = H O
p H O 0~- N NH2
O
OH
[0269] D-Agl-D-Tyr-I 2aa-D-Glu-D-Leu-NHz (WC144) (SEQ ID NO:102) was
prepared as described above to give the desired peptide TFA salt (34.0 mg, 44%
crude purity
as analyzed by analytical RP-HPLC (UV 214), 0-40 MeCN, 8 min gradient).
Purification was
then carried out by preparatory RP-HPLC (2-40 MeCN, 25 min gradient) to give
the desired
formic acid salt WC144 (4.4 mg, 6%) as a white foam. The purified product was
analyzed
by analytical RP-HPLC (UV 214) using both MeCN TR 16.47 (2-40 MeCN, 25 min
gradient)
and MeOH TR 19.14 (0-60 MeOH, 25 min gradient) and revealed >96% purity. HRMS
Calcd. for C33H48O9N7 [M+H]+ 686.3508, found 686.3512.
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
101
Agl-D-Tyr-haa-D-Glu-D-Leu-NHz (WC145) (SEQ ID NO: 103)
H
CO2H
H2N N = H O
~~~' Q N
O H O J~N NH2
O
OH
[0270] Agl-D-Tyr-haa-D-Glu-D-Leu-NHz (WC145) (SEQ ID NO: 103) was prepared
as described above to give the desired peptide TFA salt (30.0 mg, 39% crude
purity as
analyzed by analytical RP-HPLC (UV 214), 0-40 MeCN, 8 min gradient).
Purification was
then carried out by preparatory RP-HPLC (2-40 MeCN, 25 min gradient) to give
the desired
formic acid salt WC145 (5.0 mg, 6%) as a white foam. The purified product was
analyzed
by analytical RP-HPLC (UV 214) using both MeCN TR 16.18 (2-40 MeCN, 25 min
gradient)
and MeOH TR 18.73 (0-60 MeOH, 25 min gradient) and revealed >99% purity. HRMS
Calcd. for C33H48O9N7 [M+H]+ 686.3508, found 686.3514.
D-Bgl-D-Tyr-12 aa-D-Glu-D-Leu-NHz (WC146) (SEQ ID NO: 104)
O H
O CO2H
H NV C N N\\\, N' = H O
2 H O O-)'-HN NH2
O
OH
[0271] D-Bgl-D-Tyr-I aa-D-Glu-D-Leu-NHz (WC146) (SEQ ID NO: 104) was
prepared as described above to give the desired peptide TFA salt (37.0 mg, 48%
crude purity
as analyzed by analytical RP-HPLC (UV 214), 0-40 MeCN, 8 min gradient).
Purification was
then carried out by preparatory RP-HPLC (2-40 MeCN, 25 min gradient) to give
the desired
formic acid salt WC146 (10.5 mg, 13%) as a white foam. The purified product
was analyzed
by analytical RP-HPLC (UV 214) using both MeCN TR 15.85 (2-40 MeCN, 25 min
gradient)
and MeOH TR 18.80 (0-60 MeOH, 25 min gradient) and revealed >98% purity. HRMS
Calcd. for C33H48O9N7 [M+H]+ 686.3508, found 686.3514.
CA 02793683 2012-09-18
WO 2010/106441 PCT/IB2010/000914
102
Bgl-D-Tyr-haa-D-Glu-D-Leu-NHz (WC147) (SEQ ID NO: 105)
0 H
O CO2H
H N N N\`", N H O
2 H O N
OH NH2
O
OH
[0272] Bgl-D-Tyr-I 2aa-D-Glu-D-Leu-NHz (WC147) (SEQ ID NO:105) was prepared
as described above to give the desired peptide TFA salt (37.0 mg, 48% crude
purity as
analyzed by analytical RP-HPLC (UV 214), 0-40 MeCN, 8 min gradient).
Purification was
then carried out by preparatory RP-HPLC (2-40 MeCN, 25 min gradient) to give
the desired
formic acid salt WC147 (9.8 mg, 13%) as a white foam. The purified product was
analyzed
by analytical RP-HPLC (UV 214) using both MeCN TR 16.04 (2-40 MeCN, 25 min
gradient)
and MeOH TR 18.83 (0-60 MeOH, 25 min gradient) and revealed >99% purity. HRMS
Calcd. for C33H48O9N7 [M+H]+ 686.3508, found 686.3508.
[0273] While we have described a number of embodiments of this invention, it
is
apparent that our basic examples may be altered to provide other embodiments
that utilize the
compounds and methods of this invention. Therefore, it will be appreciated
that the scope of
this invention is to be defined by the appended claims rather than by the
specific
embodiments that have been represented by way of example. The present
application also
refers to a number of publications or other documents, the contents of all of
which are
incorporated by reference herein in their entireties.
[0274] We claim: