Note: Descriptions are shown in the official language in which they were submitted.
CA 02794066 2016-05-11
. 1
GALACTOSIDE INHIBITOR OF GALECTINS
Technical field
The present invention relates to novel compounds, the use of said
compounds as medicament and for the manufacture of a medicament for the
treatment of pulmonary fibrosis, such as Idiopathic pulmonary fibrosis in
mammals. The invention also relates to pharmaceutical compositions com-
prising said novel compounds.
Background Art
Idiopathic pulmonary fibrosis (IPF) represents a massive worldwide
health burden. It is a chronic condition of unknown etiology in which repeated
acute lung injury causes progressive fibrosis resulting in destruction of lung
architecture, deteriorating lung function with consequent respiratory failure
and death. Although idiopathic pulmonary fibrosis (IPF) is the archetypal and
most common cause of lung fibrosis, numerous respiratory diseases can pro-
gress to pulmonary fibrosis, and this usually signifies a worse prognosis. The
median time to death from diagnosis is 2.5 years and the incidence and prev-
alence of IPF continues to rise. It remains one of the few respiratory condi-
tions for which there are no effective therapies, and there are no reliable
bio-
markers to predict disease progression. The mechanisms resulting in pulmo-
nary fibrosis are unclear but centre around aberrant wound healing as a con-
sequence of repetitive epithelial injury from an as yet unknown cause. IPF is
characterized by fibroblastic foci containing fibroblasts/ myofibroblasts
which
show increased activation response to fibrogenic cytokines such as trans-
forming growth factor-131 (TGF-131). Given the non-responsiveness of many
cases of IPF to current anti-inflammatory treatments the myofibroblasts within
fibroblastic foci represent a potential novel therapeutic target.
The bleomycin model of pulmonary fibrosis is the best characterised
rodent model and is the industry standard model. It causes oxidant-mediated
DNA damage and induces initial lung inflammation followed by progressive
fibrosis over 2 - 4 weeks. When administered during the later phase of the
CA 02794066 2012-10-31
2
injury the anti-fibrotic potential of novel compounds can be assessed.
There is a big unmet need for drugs for treatment of Idiopathic pulmo-
nary fibrosis.
Galectin inhibitors, in particular Gal-3 inhibitors have been described
by the some of the present inventors in earlier published patent applications.
None of these galectin inhibitors have been tested in a bleomycin model.
Some of the prior art galectin inhibitors have the following general formulas
HO OH
..&...\____
R11_ RI
RvO Rv 0
-,Z ORvii
RH'
µRiv
as described in WO/2005/113568,
and
Rii-Y HO OH
N Nõ X,
'N HO RI
as described in WO/2005/113569, in which RI can be a D-galactose,
and
OH OH
_y_......Ø..\__x
Z
RI'
as described in WO/2010/126435.
Summary of the invention
Galectin-3 is a 8-galactoside binding lectin that is highly expressed in
fibrotic tissue of diverse etiologies. The present inventors have examined the
role of galectin-3 in bleomycin and TGF-81-induced lung fibrosis in mice, and
have established its relevance in human IPF. In particular it is shown that
galectin-3 inhibition may represent a novel therapeutic strategy for treatment
of
CA 02794066 2012-10-31
3
lung fibrosis. A novel compound has been tested and shown to be an inhibitor
of galectin-3, in particular the compound of the present invention blocked
TGF-13-induced 13-catenin activation in vitro and attenuated the late stage
pro-
gression of lung fibrosis following bleomycin in vivo.
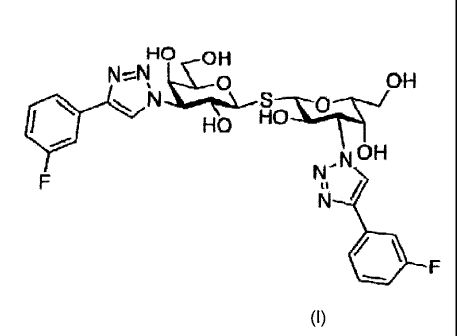
Accordingly, the present invention relates to a compound of the gen-
eral formula (I):
HO OH
N I, OH
N S
HO I-I
N OH
N
L-1 /
N
F
(I).
In a further aspect the present invention relates to a pharmaceutical
composition comprising the compound of formula (I) and optionally a pharma-
ceutically acceptable additive, such as carrier or excipient.
The compound of formula (I) of the present invention is suitable for use
in a method for treating pulmonary fibrosis, such as Idiopathic pulmonary fi-
brosis in a mammal. Typically, such mammal is a human subject.
Moreover, in a still further aspect the present invention relates to a
method for treatment of pulmonary fibrosis, such as Idiopathic pulmonary fi-
brosis comprising administering to a mammal a therapeutically effective
amount of the compound of formula (I).
In another aspect the present invention concerns a process of prepar-
ing a compound of formula I comprising the step of reacting bis-(3-deoxy-3-
azido-p-D-galactopyranosyl) sulfane with 3-fluorophenylacetylene and an
amine, such as triethylamine, optionally in the presence of a catalyst, such
as
Cu(I), in a solvent, such as N,N-dimethylformamide (DMF), resulting in the
compound of formula I.
CA 02794066 2012-10-31
4
Detailed description of the invention
In a broad aspect the present invention relates to a compound of the
general formula (I):
1-10
0 OH
S
HO 1-1(31'1.14(
N OH
N
N
F
0)
The compound of formula (I) has the chemical name (IUPAC) bis (3-
deoxy-3-(3-fluoropheny1-1H-1,2,3-triazol-1-y1)-(3-D-galactopyranosyl) sulfane,
and as used herein is intended to cover the compound of formula (I) in any
possible form, such as solid or liquid, a salt, a solvate, or in free form.
Typically, the compound of formula (I) is bis (3-deoxy-3-(3-
fluoropheny1-1H-1,2,3-triazol-1-y1)-(3-D-galactopyranosyl) sulfane as the free
form. In a further embodiment the compound of formula (I) is bis (3-deoxy-3-
(3-fluoropheny1-1H-1,2,3-triazol-1-y1)-p-D-galactopyranosyl) sulfane as the
free form without any solvate, such as anhydrated.
In a still further embodiment the compound of formula (l) is useful for
treating pulmonary fibrosis, and therefore is suitable for use as a
medicament.
In a further aspect the present invention relates to a compound of for-
mula (I) for use in a method for treating pulmonary fibrosis, such as
Idiopathic
pulmonary fibrosis in a mammal. Such a mammal is typically a human sub-
ject, preferably a human subject diagnosed with IPF.
In a still further aspect the present invention relates to a method for
treatment of pulmonary fibrosis, such as Idiopathic pulmonary fibrosis com-
prising administering to a mammal a therapeutically effective amount of a
compound of formula (I).
CA 02794066 2012-10-31
When the compounds and pharmaceutical compositions herein dis-
closed are used for the above treatment, a therapeutically effective amount of
at least one compound is administered to a mammal in need of said treat-
ment.
5 The term "treatment" and "treating" as used herein means the man-
agement and care of a patient for the purpose of combating a condition, such
as a disease or a disorder. The term is intended to include the full spectrum
of
treatments for a given condition from which the patient is suffering, such as
administration of the active compound to alleviate the symptoms or complica-
tions, to delay the progression of the disease, disorder or condition, to
allevi-
ate or relief the symptoms and complications, and/or to cure or eliminate the
disease, disorder or condition as well as to prevent the condition, wherein
prevention is to be understood as the management and care of a patient for
the purpose of combating the disease, condition, or disorder and includes the
administration of the active compounds to prevent the onset of the symptoms
or complications. The treatment may either be performed in an acute or in a
chronic way. The patient to be treated is preferably a mammal; in particular a
human being, but it may also include animals, such as dogs, cats, cows,
sheep and pigs.
The term "a therapeutically effective amount" of a compound of formula
(I) of the present invention as used herein means an amount sufficient to
cure, alleviate or partially arrest the clinical manifestations of a given
disease
and its complications. An amount adequate to accomplish this is defined as
"therapeutically effective amount". Effective amounts for each purpose will
depend on the severity of the disease or injury as well as the weight and gen-
eral state of the subject. It will be understood that determining an
appropriate
dosage may be achieved using routine experimentation, by constructing a
matrix of values and testing different points in the matrix, which is all
within
the ordinary skills of a trained physician or veterinary.
In a still further aspect the present invention relates to a pharmaceuti-
cal composition comprising the compound of formula (I) and optionally a
pharmaceutically acceptable additive, such as a carrier or an excipient.
CA 02794066 2012-10-31
6
As used herein "pharmaceutically acceptable additive" is intended
without limitation to include carriers, excipients, diluents, adjuvans, colour-
ings, aroma, preservatives etc. that the skilled person would consider using
when formulating a compound of the present invention in order to make a
pharmaceutical composition.
The adjuvants, diluents, excipients and/or carriers that may be used in
the composition of the invention must be pharmaceutically acceptable in the
sense of being compatible with the compound of formula (I) and the other in-
gredients of the pharmaceutical composition, and not deleterious to the re-
cipient thereof. It is preferred that the compositions shall not contain any
ma-
terial that may cause an adverse reaction, such as an allergic reaction. The
adjuvants, diluents, excipients and carriers that may be used in the pharma-
ceutical composition of the invention are well known to a person within the
art.
As mentioned above, the pharmaceutical compositions as herein dis-
closed may, in addition to the compounds herein disclosed, further comprise
at least one pharmaceutically acceptable adjuvant, diluent, excipient and/or
carrier. In some embodiments, the pharmaceutical compositions comprise
from 1 to 99 weight % of said at least one pharmaceutically acceptable adju-
vant, diluent, excipient and/or carrier and from 1 to 99 weight % of a com-
pound as herein disclosed. The combined amount of the active ingredient and
of the pharmaceutically acceptable adjuvant, diluent, excipient and/or carrier
may not constitute more than 100 A by weight of the pharmaceutical compo-
sition.
In some embodiments only one compound as herein disclosed is used
for the purposes discussed above.
In some embodiments two or more of the compound as herein dis-
closed are used in combination for the purposes discussed above.
The pharmaceutical composition according to the present invention
comprising a compound of the invention may be adapted for oral, intrave-
nous, topical, intraperitoneal, nasal, buccal, sublingual, or subcutaneous ad-
ministration, or for administration via the respiratory tract in the form of,
for
example, an aerosol or an air-suspended fine powder. Therefore, the phar-
maceutical composition of the present invention may be in the form of, for
CA 02794066 2012-10-31
7
example, tablets, capsules, powders, nanoparticles, crystals, amorphous
substances, solutions, transdermal patches or suppositories.
Thus, in a still further aspect the present invention relates to a pharma-
ceutical composition for intrapulmonary administration. Typically, such com-
position is delivered by a nebulizer or inhaler, preferably a nebulizer.
The following characteristics are required for the delivery device: It should
be
able to provide a specific dose accurately and repeatedly. It should be able
to
provide 2 or more different dose levels, for instance through repeated dosing
or by adjusting the dose provide to the patient. The device should ensure that
the drug is delivered to the bronchiolar space or preferably to the
bronchiolar
and the alveolar space of the lung preferably uniformly over the lung tissue.
Hence, the device should generate aerosols or dry powder of an adequately
small size to ensure this delivery.
Inhalation nebulizers deliver therapeutically effective amounts of phar-
maceuticals by forming an aerosol which includes particles of a size that can
easily be inhaled. The aerosol can be used, for example, by a patient within
the bounds of an inhalation therapy, whereby the therapeutically effective
pharmaceutical or drug reaches the patient's respiratory tract upon
inhalation.
A variety of inhalation nebulizers are known. EP 0 170 715 A1 uses a
compressed gas flow to form an aerosol. A nozzle is arranged as an aerosol
generator in an atomizer chamber of the inhalation nebulizer and has two
suction ducts arranged adjacent a compressed-gas channel. When com-
pressed air flows through the compressed-gas channel, the liquid to be nebu-
lized is drawn in through the suction ducts from a liquid storage container.
EP 0 432 992 A discloses a nebulizer comprising an aerosol generator
having a liquid storage container, a perforate mebrane and a vibrator. The
vibrator is operable to vibrate the membrane such that it dispenses an aerosol
from a liquid through holes provided in the membrane.
US 5.918.593 relates to ultrasonic nebulizers generating an aerosol by
interaction between an amount of liquid and a piezo electric element. Droplets
of various sizes are expelled from a surface of a liquid bulk when vibrational
energy is transferred from the piezo element to the liquid. The droplets thus
generated are filtered in an atomizer chamber since oversized droplets have
CA 02794066 2012-10-31
8
to be removed from the droplets expelled from the surface in order to gener-
ate an aerosol for inhalation by a patient. This nebulizer is representative
of
continuously operating inhalation nebulizers, in which the aerosol generator
produces an aerosol not only during inhalation but also while the patient ex-
hales. The aerosol produced by the aerosol generator is actually inhaled by
the patient only in the inhalation phase, while any aerosol produced at other
times is lost.
Dry powder inhalers, such as metered dose medicament inhalers are
well known for dispensing medicament to the lungs of a patient. Some previ-
ous inhalers have comprised a pressurized aerosol dispensing container,
wherein the aerosols contain gas propellants in which the powdered medica-
ment is suspended. Upon actuation, the aerosol contents are expelled,
through a metering valve, and into the lungs of the patient.
Several types of non-aerosol, breath actuated dry powder inhalers
have therefore been provided. For example, U.S. Patent No. 5,503,144 to
Baconõ shows a breath-actuated dry-powder inhaler. The device includes a
dry powder reservoir for containing a dry powdered medicament, a metering
chamber for removal of the powdered medicament from the reservoir in dis-
crete amounts, and an air inlet for entraining the removed powdered medica-
ment through a mouth piece upon patient inhalation.
US5458135 discloses a method and apparatus for producing an aero-
solised dose of a medicament for subsequent inhalation by a patient. The
method comprises first dispersing a preselected amount of the medicament in
a predetermined volume of gas, usually air. The dispersion may be formed
from a liquid or a dry powder. The method relies on flowing substantially the
entire aerosolised dose into a chamber which is initially filled with air and
open through a mouthpiece to the ambient. After the aerosolised medicament
has been transferred to the chamber, the patient will inhale the entire dose
in
a single breath.
US 6 065 472 discloses a powder inhalation device comprising a hous-
ing containing a pharmacologically active compound, a conduit with an outlet
extending into the housing through which a user can inhale to create an air-
flow through the conduit, a dosing unit for delivering a dose of the compound
CA 02794066 2012-10-31
9
to the conduit and baffles arranged within the said conduit to aid disintegra-
tion of powder agglomerates entrained in said airflow.
Regardless of whether an aerosol or non-aerosol inhaler is used, it is
of utmost importance that particles of the dispensed dry powder medicament
be small enough to ensure the adequate penetration of the medicament into
the bronchial region of a patient's lungs during inhalation. However, because
the dry powder medicament is composed of very small particles, and often
provided in a composition including a carrier such as lactose, non-defined
agglomerates or aggregates of the medicament form at random prior to being
dispensed. It has therefore been found preferably to provide breath-actuated
dry powder inhalers with means for breaking down the agglomerates of me-
dicament or medicament and carrier before inhalation of the medicament.
The pharmaceutical composition of the present invention may option-
ally comprise two or more compounds of the present invention. The composi-
tion may also be used together with other medicaments within the art for the
treatment of related disorders.
The typical dosages of the compounds of the present invention vary
within a wide range and depend on many factors, such as the route of ad-
ministration, the requirement of the individual in need of treatment, the indi-
vidual's body weight, age and general condition.
The compound of formula (I) may be prepared as described in the ex-
perimental section below.
Accordingly, the present invention relates to a process of preparing a
compound of formula I comprising the step of reacting bis-(3-deoxy-3-azido-
p-D-galactopyranosyl) sulfane with 3-fluorophenylacetylene and an amine,
such as triethylamine, optionally in the presence of a catalyst, such as
Cu(I),
in a solvent, such as N,N-dimethylformamide (DMF), resulting in the com-
pound of formula I. In a particular embodiment, the present invention relates
to a process of preparing a compound of formula I comprising the steps as
described in the scheme 1 in the experimental section. Moreover, the present
invention relates to a compound of formula (I) obtainable by the step of react-
ing bis-(3-deoxy-3-azido-3-D-galactopyranosyl) sulfane with 3-
fluorophenylacetylene and an amine, such as triethylamine, optionally in the
CA 02794066 2015-11-16
presence of a catalyst, such as Cu(I), in a solvent, such as N,N-
dimethylformamide (DMF), resulting in the compound of formula I, such as
obtainable by the steps as described in the scheme 1 in the experimental sec-
tion.
5 Further embodiments of the process of the present invention are de-
scribed in the experimental section herein, and each individual process as
well as each starting material constitutes embodiments that may form part of
claims.
The above embodiments should be seen as referring to any one of the
1 0 aspects (such as 'method for treatment', 'pharmaceutical composition',
'com-
pound for use as a medicament', or 'compound for use in a method') described
herein as well as any one of the embodiments described herein unless it is
specified that an embodiment relates to a certain aspect or aspects of the pre-
sent invention.
All headings and sub-headings are used herein for convenience only
and should not be construed as limiting the invention in any way.
Any combination of the above-described elements in all possible varia-
tions thereof is encompassed by the invention unless otherwise indicated
herein or otherwise clearly contradicted by context.
The terms "a" and "an" and "the" and similar referents as used in the
context of describing the invention are to be construed to cover both the sin-
gular and the plural, unless otherwise indicated herein or clearly
contradicted
by context.
Recitation of ranges of values herein are merely intended to serve as a
shorthand method of referring individually to each separate value falling
within
the range, unless other-wise indicated herein, and each separate value is in-
corporated into the specification as if it were individually recited herein.
Un-
less otherwise stated, all exact values provided herein are representative of
corresponding approximate values (e.g., all exact exemplary values provided
CA 02794066 2016-05-11
11
with respect to a particular factor or measurement can be considered to also
pro-vide a corresponding approximate measurement, modified by "about,"
where appropriate).
All methods described herein can be performed in any suitable order
unless other-wise indicated herein or otherwise clearly contradicted by con-
text.
The use of any and all examples, or exemplary language (e.g., "such
as") provided herein, is intended merely to better illuminate the invention
and
does not pose a limitation on the scope of the invention unless otherwise indi-
cated. No language in the specification should be construed as indicating any
element is essential to the practice of the invention unless as much is explic-
itly stated.
The citation of patent documents herein is done for convenience only
and does not reflect any view of the validity, patentability and/or
enforceability
of such patent documents.
The description herein of any aspect or embodiment of the invention
using terms such as "comprising", "having", "including" or "containing" with
reference to an element or elements is intended to provide support for a simi-
lar aspect or embodiment of the invention that "consists of', "consists essen-
tially of", or "substantially comprises" that particular element or elements,
un-
less otherwise stated or clearly contradicted by context (e.g., a composition
described herein as comprising a particular element should be understood as
also describing a composition consisting of that element, unless otherwise
stated or clearly contradicted by context).
This invention includes all modifications and equivalents of the subject
matter recited in the aspects or claims presented herein to the maximum ex-
tent permitted by applicable law.
The present invention is further illustrated by the following examples
which, however, are not to be construed as limiting the scope of protection.
The features disclosed in the foregoing description and in the following exam-
ples may, both separately and in any combination thereof, be material for re-
alizing the invention in diverse forms thereof.
CA 02794066 2015-11-16
12
Experimental
Synthesis of bis (3-deoxy-3-(3-fluoropheny1-1H-1,2,3-triazol-1-y1)-13-D-
galactopyranosyl) sulfane.
General Methods.
Melting points were recorded on a Kofler apparatus (Reichert) and are
uncorrected. Proton nuclear magnetic resonance (1H) spectra were recorded
on a Bruker DRX 400 (400 MHz) or a Bruker ARX 300 (300 MHz) spectrome-
ter; multiplicities are quoted as singlet (s), doublet (d), doublet of
doublets
(dd), triplet (t), apparent triplet (at) or apparent triplet of doublets
(atd). Carbon
nuclear magnetic resonance (13C) spectra were recorded on a Bruker DRX
400 (100.6 MHz) spectrometer. Spectra were assigned using COSY, HMQC
and DEPT experiments. All chemical shifts are quoted on the 6-scale in parts
per million (ppm). Low- and high-resolution (FAB-HRMS) fast atom bom-
bardment mass spectra were recorded using a JEOL SX-120 instrument and
low- and high- resolution (ES-HRMS) were recorded on a Micromass TM Q-
TOFTm instrument. Optical rotations were measured on a Perkin-Elmer 341
polarimeter with a path length of 1 dm; concentrations are given in g per 100
mL. Thin layer chromatography (TLC) was carried out on Merck Kieselgel
sheets, pre-coated with 60F254 silica. Plates were developed using 10%
sulfuric acid. Flash column chromatography was carried out on silica
(Matrex TM 60A, 35- 70pm, Grace Amicon). Acetonitrile was distilled from
calcium hydride and stored over 4A molecular sieves. DMF was distilled from
4A molecular sieves and stored over 4A molecular sieves.
Bis (3-deoxy-3-(3-fluoropheny1-1H-1,2,3-triazol-1-y1)-(3-D-
galactopyranosyl) sulfane (TD139) was prepared in accordance with the
reaction scheme 1 below:
CA 027 94066 2012-10-31
13
Ph PhPh Ph
Bu4N+NO2-
iTf20 DMF
Tf20
'C) pyridine 0 60% over 2 ci pyridine -()
0 -20 C o steps when 0 -20 C 0
___&\0Ø....\____ "AcCI
1:_nii_1_30._
0
HO SPh Tf0 _SPh SPh
0,00.=\.....\__--SPh
OH OAc OAc OAc
OH OTf
(1) (2) (3) (4)
Ph
Bu4N+N3-
DMF
.\(:) AcOH HO ,OH Ac20
Ac0 OAc
59% over pyridine
0 (80%)
2 steps". 60 C \--0 ---11"
_&70....\_ _
NA0.07,,,,..\_.-SPh N3 _ SPh
N3__ SPh
OAc OAc
OAc (6) (7)
(5)
Br2
CH2Cl2 Ac0 OAc
68% over
---S- MeCN
3 steps
__.&\..Ø,. Et3N Ac0 OAc
N3 50-60% Ac0
Ac0 over 2 steps
....&\6Ø.._\___
Br
(8)
Ac0
OAc
ONH
Thiourea a0Me
MeCN
reflux
HO Me0H (10)
75% NbAc
OH
Ac0 OAc
N S
0 3
N3 S OH
OAc
)r.-NH2 F (11) N3 OH
(9) H2N Br 3-fluorophenylacetylene
e
+ HO , tCriuelthylamine
DMF
OH
76%
¨ \ 0 HO
N ,NS-"Xa0...\
NJ' HO
HO
F
/ \NO
N H
TD139 41, N
(Scheme 1)
Compound (1) (cf. reaction scheme above) is commercial from Carbo-
synth Limited 8 & 9 Old Station Business Park - Compton - Berkshire - RG20
6NE ¨ UK or synthesized in three near-quantitative steps from D-galactose,
CA 02794066 2012-10-31
14
(cf e.g. Li, Z. and Gildersleeve, J. C. J. Am. Chem. Soc. 2006, 128, 11612-
11619)
Phenyl 2-0-acetyl-4,6-0-benzylidene-1-thio-3-0-
trifluoromethanesulfonyl-p-D-galactopyranoside (2)
Compound 1 (10.5 g, 29.2 mmol) was dissolved in dried pyridine (4.73
mL , 58.4 mmol) and dried CH2Cl2 (132 mL ). The reaction mixture was
cooled, under stirring, until -20 C (Ice and NaCI bath 3:1). Slowly and under
N, atmosphere, Tf20 (5.68 mL, 33.6 mmol) was added. The reaction mixture
was monitored by TLC (heptane:Et0Ac, 1:1 and toluene:acetone, 10:1).
When the reaction was complete, AcCI (2.29 ml , 32.1 mmol) was added and
keeping stirring, the temperature was increased to room temperature. This
mixture was monitored by TLC too (heptane:Et0Ac, 1:1 and toluene:acetone,
10:1). When it was complete, it was quenched with CH2Cl2 and washed with 5
% HCI, NaHCO3 (saturated ¨ hereafter sat) and NaCI (sat). The organic layer
was dried over MgSO4, filtered and concentrated under reduced pressure.
Phenyl 2-0-acetyl-4,6-0-benzyliden-1-thio-p-D-gulopyranoside (3)
Tetrabutylammonium nitrite (25.3 g, 87.7 mmol) was added to a solu-
tion of compound 2 (15.6 g, 29.2 mmol) in DMF (110 mL ) and was kept stir-
ring, under N2 atmosphere, at 50 C. (The reaction started being purple and
turned garnet). The reaction was monitored by TLC (heptane:Et0Ac, 1:1 and
toluene:acetone, 10:1) and quenched with CH2Cl2 . The mixture was washed
with 5 % HCI, NaHCO, (sat) and NaCI (sat). The organic layer was dried over
MgSO4, filtered and concentrated under reduced pressure followed by purifi-
cation by flash chromatography (Eluent heptane:Et0Ac, 1:1 and hep-
tane:Et0Ac, 1:2) and recrystallized from a mixture of Et0Ac and Heptane
(1:3).1H NMR in CDCI, 6 7.60-7.57 (m, 2H, Ar), 7.43-7.40 (m, 2H, Ar), 7.37-
7.34 (m, 3H, Ar), 7.29-7.25 (m, 3H, Ar), 5.50 (s, 1H, PhCH), 5.15 (d, 1H,
J=10.29 Hz, H-1), 5.10 (dd, 1H, J=10.27 Hz, 2.85 Hz, H-2), 4.36 (dd, 1H, J=
12.49 Hz,1.4 Hz, H-6), 4.18 (br s, 1H, H-3), 4.08 (dd, 1H, J= 3.59 Hz, 1.04
Hz, H-6), 4.03 (dd, 1H, J= 12.53 Hz, 1.75 Hz, H-4), 3.88 (s, 2H, H-5 + OH),
CA 02794066 2012-10-31
2.12 ( s, 3H, OAc).
Phenyl 2-0-acetyl-4,6-0-benzylidene-1-thio-3-0-
trifluoromethanesulfonyl-p-D-gulopyranoside (4)
5 Compound 3 (1.00 g, 2.48 mmol) was dissolved in dried CH2Cl2 (12.5
mL) and dried pyridine (0.40 mL , 4.96 mmol). The reaction mixture was
cooled, under stirring, until -20 C (Ice and NaCI bath 3:1). Slowly and under
N2 atmosphere, Tf20 (0.48 mL, 2.85 mmol) was added. The reaction mixture
was monitored by TLC (heptane:Et0Ac, 1:1 and toluene:acetone, 10:1) and
10 when it was complete, it was quenched with CH2Cl2 and washed with 5 %
HCI, NaHCO, (sat) and NaCI (sat). The organic layer was dried over MgSO4,
filtered and concentrated under reduced pressure until being dry.
Phenyl 2-0-acetyl-3-azido-4,6-0-benzylidene-3-deoxy-1-thio-p-D-
15 galactopyranoside (5)
Tetrabutylammonium azide (2.12 g, 7.44 mmol) was added carefully to
a solution of compound 4 (1.3256 g, 2.48 mmol) in DMF (10 mL) and was
kept stirring, under N2 atmosphere, at 50 C. The reaction was monitored by
TLC (E:H, 1:1) and concentrated under reduced pressure followed by purifi-
cation by flash chromatography (Eluent heptane:Et0Ac, 2:1 and hep-
tane:Et0Ac, 1:1).1H NMR in CDCI, 6 7.61-7.58 (m, 2H, Ar), 7.44-7.41 (m, 2H,
Ar), 7.39-7.36 (m, 3H, Ar), 7.30-7.24 (m, 3H, Ar), 5.59 (s, 1H, PhCH), 5.35
(t,
1H, J= 9.95 Hz, H-2), 4.73 (d, 1H, J= 9.63 Hz, H-1), 4.44 (dd, 1H, J= 6.24 Hz,
1.60 Hz, H-6), 4.35-4.34 (dd, 1H, J= 3.33 Hz, 0.88 Hz, H-4), 4.11 (dd, 1H, J=
12.48 Hz, 1.67 Hz, H-6), 3.57 (d, 1H, J= 1.15 Hz, H-5), 3.44 (dd, 1H, J= 10.21
Hz, 3.29 Hz, H-3), 2.17 (s, 3H, OAc).
Phenyl 2-0-acetyl-3-azido-3-deoxy-1-thio-13-D-galactopyranoside (6)
Compound 5 (470 mg, 1.1 mmol) was dissolved in 80% acetic acid (75
mL) and the mixture was heated at 60 C. The reaction was monitored by
TLC (heptane:Et0Ac, 1:1). When the reaction was complete, the mixture was
concentrated under reduced pressure and heating.
CA 02794066 2012-10-31
16
Phenyl 2,4,6-tri-O-acetyl-3-azido-3-deoxy-1-thio-p-D-galactopyranoside
(7)
Acetic anhydride (30 mL) was added to a solution of compound 6 (373
mg, 1.1mmol) in dry pyridine (30 mL). The reaction was monitored by TLC
(heptane:Et0Ac, 1:1) and when it was complete, it was concentrated under
reduced pressure. 1H NMR in CDCI3 6 7.54-7.51 (m, 2H, Ar), 7.35-7.30 (m,
3H, Ar), 5.46 (dd, 1H, H-4), 5.23 (t, 1H, H-2), 4.73 (d, 1H, H-1), 4.15 (d,
2H,
H-6, H-6), 3.94 (dt, 1H, H-5), 3.68 (dd, 1H, H-3), 2.18 (s, 3H, OAc), 2.15 (s,
3H, OAc), 2.06 (s, 3H, OAc).
2,4,6-tri-O-acety1-3-azido-3-deoxy-a-D-galactopyranosyl bromide (8)
Compound 7 (237.4 mg, 560 pmol) was dissolved in dry CH2Cl2 (2 mL),
and bromine (32 pl, 620 pmol) was added. The reaction was monitored by
TLC (heptane:Et0Ac, 1:1). When the reaction was complete, a small amount
of cyclopentene was added to the reaction mixture to remove the rests of Br2.
The mixture was concentrated under reduced pressure and purified by quick
Flash chromatography (Eluent: 500mL heptane:Et0Ac, 2:1).
2,4,6-tri-O-acetyl-3-azido-3-deoxy-a-D-galactopyranose-1-
isothiouronium bromide (9)
The sensitive bromide 8 (70.6 mg, 180 pmol) was immediately dis-
solved in dry acetonitrile (1.7 mL) and refluxed with thiourea (13.7 mg, 180
pmol) under N2 for 4 hours. The reaction was monitored by TLC (hep-
tane:Et0Ac, 1:1) and when it was complete, the mixture was cooled.
Bis-(2,4,6-tri-O-acetyl-3-azido-3-deoxy-p-D-galactopyranosyl)-sulfane
(10)
The sensitive bromide 8 (77.0 mg, 196 pmol) and Eth (60 pl, 430
pmol) was added to the last mixture (9). The reaction was monitored by TLC
(heptane:Et0Ac, 1:1). When it was complete, the reaction mixture was con-
centrated under reduced pressure and without heating. The residue was puri-
CA 02794066 2015-11-16
17
fied by flash chromatography (Eluent: heptane:Et0Ac, 1:1).1H NMR in CDCI3
6 5.50 (dd, 2H, H-4,), 5.23 (t, 2H, H-2, H-2'), 4.83 (d, 2H, H-1, H-1'), 4.15
(dd,
4H, H-6, H-6, H-6', H-6'), 3.89 (dt, 2H, H-5, H-5'), 3.70 (dd, 2H, H-3, H-3'),
2.19 (s, 6H, 20Ac), 2.15 (s, 6H, 20Ac), 2.18 (s, 6H, 20Ac).
Bis-(3-azido-3-deoxy-3-D-galactopyranosyl)-sulfane (11)
Compound 10 (160 mg, 0.00024 mol) was dissolved in dry Me0H (2.6
mL) and dry CH2Cl2 (1.6 mL), and Na0Me (1M, 24 pL, 24 pmol) was added.
The reaction was monitored by TLC (heptane:Et0Ac 1:1 and D:M 5:1). When
the reaction was complete, the mixture was neutralized with DuoliteTm C436
until pH 7, filtered and washed with Me0H. The filtered solution was concen-
trated under reduced pressure. The residue was purified by flash chromatog-
raphy (Eluent: CH2C12:Me0H, 5:1) to give pure 11 (74.1 mg, 75%). 1H NMR in
CDCI3 6 4.72 (d, 2H, J=9.7 Hz, H-1, H-1'), 3.95 (br s, 2H, H-4, H-4'), 3.84
(t,
2H, J= 9.8 Hz, H-2, H-2'), 3.74 (dd, 2H, J= 11.47 Hz, 7.23 Hz, H-6, H-6'),
3.64
(dd, 2H, J= 11.48 Hz, 4.72 Hz, H-6, H-6'), 3.60-3.55 (ddd, 2H, 7.15 Hz, 4.67
Hz, 0.93 Hz, H-5, H-5'), 3.36 (dd, 2H, J= 10 Hz, 3.05 Hz, H-3, H-3').
Bis-{3-deoxy-3[4-(3-fluoropheny1)-1H-1,2,3-triazol-1-y1]-3-D-
galactopyranosyl} sulfane (Named TD139)
TD139 was synthesized at ambient temperature by Cu(I)-catalyzed
cycloaddition between bis-(3-azido-3-deoxy-13-D-galactopyranosy1)-sulfane
(11) and 3-fluorophenylacetylene (3 eq.) with Cu(I) (0.2 eq), triethylamine (2
eq.) in N,N-dimethylformamide (DMF, 100 mUmmol sulfane). The reaction
was monitored with tic until complete, concentrated and first purified by
flash
chromatography (Eluent: CH2C12:Me0H, 8:1), followed by final purification by
preparative hplc to give TD139 in 76% yield as a white amorphous solid. 1H-
NMR (CD30D, 400 MHz) 6 8.59 (s, 2H, triazole-H), 7.63 (br d, 2H, 7.6 Hz, Ar-
H), 7.57 (br d, 2H, 8.4 Hz, Ar-H), 7.41 (dt, 2H, 6,0 and 8.0 Hz, Ar-H), 7.05
(br
dt, 2H, 2.4 and 6.4 Hz, Ar-H), 4.93 (dd, 2H, 2,4 and 10.4 Hz, H3), 4.92 (d,
2H,
10.4 Hz, H1), 4.84 (2H, 10.4 Hz, H2), 4.18 (d, 2H, 2.4 Hz, H4), 3.92 (dd, 2H,
4.2 and 7.6 Hz, H5), 3.84 (dd, 2H, 7.6 and 11.4 Hz, H6), 3.73 (dd, 2H, 4.2 and
CA 02794066 2012-10-31
18
11.4 Hz, H6); FAB-HRMS m/z calcd for C28H30F2N6Na08S (M+Na+),
671.1712; found, 671.1705.
Model of bleomycin-induced lung fibrosis
Female C57/B16 mice (10-14 weeks old) were anaesthetized with halo-
thane, and bleomycin or saline was administered intratracheally (33 pg in 50
pl of saline) and lungs were harvested on day 26. TD139 was instilled into the
lungs of mice on days 18, 20, 22 and 24 of bleomycin induced lung injury.
Fibrosis was assessed by histological score of collagen stained lung sections
and by total collagen content by Sircol assay.
Mice were treated with bleomycin (bleo) or saline (control) and bleo-
mycin treated mice were treated with 200 mg/kg pirfenidone twice daily on
days 18-24. TD139 was administered intratracheally on days 18, 20, 22 and
24. Lungs were harvested on day 26.
Figure 1 shows (A) Total lung collagen measured by Sircol assay; (B)
Fibrosis score; and (C) Inflammatory score. Results represent the mean and
SEM (A) or box and whiskers (median, interquartile range, minimum to maxi-
mum, B and C) of n=8 mice per group (n=7 bleo). ***P<0.005, **P<0.01,
*P<0.05. Figure 1E) Beta-catenin activation in vivo was assessed by scoring
sections of bleomycin treated mouse lung (control and 10 ug TD139 treated)
stained with an anti-active beta catenin.
Effect on alveolar epithelial cells
Primary alveolar epithelial cells from WT mice were plated and treated with
TGF-p1 in the presence or absence of 10 1.1M TD139. Figure 1D) Cells were
lysed and analyzed for active p-catenin, total p-catenin and f3-actin by
western
blot.
In conclusion TD139 is a galectin-3 inhibitor and blocked TGF-p-induced 13-
catenin activation in vitro and bleomycin induced lung fibrosis in vivo and is
believed to represent a novel therapeutic strategy for treatment of lung fibro-
CA 02794066 2012-10-31
19
sis in mammals, in particular humans.
Drug treatment
Mice were divided into the following groups:
Group Induction Treatment Dose Dosing days Administration
1 Control Vehicle N/A
,
2 Bleomycin Vehicle 1820, 22 Intratracheal
and 24
3 Bleomycin TD139 10 ug 18,20, 22 I ntratracheal
and 24
4 Bleomycin TD139 3 ug 18, 20, 22 I ntratracheal
and 24
5 Bleomycin TD139 1 ug 18, 20, 22 Intratracheal
and 24
18, 20,
6 Bleomycin TD139 0.1 ug and 24 22 Intratracheal
200 b..i.d. from
7 oral
Bleomycin Pirfenidone mg/kg
day 18
Immunohistochemistry
Paraffin-embedded sections of mouse tissue were stained with Mas-
son's trichrome and haemotoxylin and eosin (H&E) as per manufacturer's
instructions. Sections were processed for immunohistochemistry and the fol-
lowing primary antibodies used: mouse anti-active (ABC) beta-catenin (Milli-
pore) and sections visualized and quantified.
Determination of lung fibrosis and inflammation
Histological lung inflammation and fibrosis score were carried out in
Masson's trichronne stained sections. Inflammation (peribronchiolar, perivas-
cular, and alveolar wall thickness) scored in > 5 random fields at magnifica-
tion X630 using the following system (peribronchiolar and perivascular, 1 = no
cells, 2 = <20 cells, 3 = 20 ¨ 100 cells, 4 = > 100 cells; alveolar wall thick-
ness, 1 = no cells, 2 = 2 ¨ 3 cells thick, 3 = 4 ¨ 5 cells thick, 4 = > 5
cells
thick). The combined inflammatory score was the sum of these scores. Fi-
brosis score was evaluated as the area of the section positively stained for
collagen (1 = none, 2 = <10%, 3 = < 50%, 4 => 50%). Only fields where the
majority of the field was composed of alveoli were scored.
CA 02794066 2015-11-16
= 20
Determination of lung collagen by sircol TM assay
Collagen content in the left lung lobe was determined by sircol assay
as per manufacturer's instructions. The left lobe was minced in 5 ml of 3
mg/ml pepsin in 0.5 M acetic acid and incubated with shaking at 4oC for 24 h.
Cleared lung extract (0.2 ml) was incubated with 0.8 ml sircol reagent for 1 h
at room temperature and precipitated collagen centrifuged at 10,000g for 5
min at 4oC. Pellets solubilised in 1 ml 1 M NaOH and absorbance measured
at 570 nm alongside collagen standards.
Primary Type II alveolar epithelial cell isolation
Treated and control mouse type 11 lung alveolar epithelial cells (AECs)
were extracted following a standard method. Briefly, 1 ml of 50 U/ml dispase
(BD Biosciences) was administered intratracheally into perfused lungs fol-
lowed by instillation of 0.5 ml of 1% low melting point agarose. The agarose
within the upper airways was allowed to set on ice for 2 minutes and the lungs
were placed in 4 ml 50 U/ml dispase for 45 min at room temperature. The
lung lobes minus the upper airways were then dispersed in DMEM containing
50 pg/ml DNAse 1 (Sigma-Aldrich, UK). The cell suspension was passed
2() through a 100-pm cell strainer and the cells washed in DMEM followed by
resuspension in DMEM containing 10% FCS. The cell suspension was plated
onto tissue culture plastic for 1 h to allow any contaminated fibroblasts and
macrophages to adhere. Non-adherent epithelial cells were counted and cul-
tured for 2 days on tissue culture plastic or cover-slips pre-coated with 5
pg/ml collagen (AMS Biotechnology) and 10 pg/ml fibronectin (Sigma-
Aldrich), Cells were washed three times in PBS before treatment. Epithelial
cells were either incubated in DMEM containing 10% FCS, 50 U/ml penicillin,
50 pg/ml streptomycin and 5 pg/ml L-glutamine or transferred to complete
mouse media (DMEM/F-12 containing 0.25% BSA, 10 nM hydrocortisone, 5
pg/ml Insulin-Transferrin-Sodium-Selenite (ITS) and supplemented with 0.1
mg/ml sodium succinate, 75 pg/ml succinic acid and 1.8 pg/ml choline biter-
trate).
CA 02794066 2012-10-31
21
Western Blotting
Cells were lysed in 25 mM HEPES pH 7.4, 0.3 M NaCI, 1.5 mM MgCl2,
0.2 mM EDTA, 0.5% triton X-100, 0.5 mM dithiothreitol, 1 mM sodium or-
thovanadate and protease inhibitors (Boehringer Mannheim, Sussex, UK; pre-
pared as per manufacturers instructions). Lysates equilibrated for protein
using Pierce BCA protein assay reagent (Pierce) and resolved on 12% SDS-
PAGE gels. Western blot analysis undertaken using the following primary
antibodies; rabbit anti beta-catenin, (BD Biosciences), rabbit polyclonal anti-
beta-actin antibody (Sigma, UK), mouse anti-active (ABC) beta-catenin (Milli-
pore).