Note: Descriptions are shown in the official language in which they were submitted.
CA 02794227 2012-09-24
PF 70006
I
"as originally filed"
PE wax dispersions in the coating of plastics
Description
The invention relates to an aqueous paint formulation comprising at least one
aqueous
basecoat and at least one aqueous dispersion of at least one specific ethylene
copolymer wax, and to the use thereof for coating plastics.
Plastics moldings are used in numerous areas, such as in automobiles, in the
household, etc. The painting of plastics moldings often is difficult, since
typical coating
materials adhere poorly to plastics materials. In particular, the painting of
nonpolar
plastics materials such as polypropylene (PP) and thermoplastic olefins (TPO)
presents
difficulties. Consequently, a primer layer comprising an adhesion-promoter
system is
often applied to the cleaned plastics surface, and this boosts the adhesion
between the
surface and a basecoat. The formulation used for the primer coat frequently
comprises
organic solvents and chlorinated polyolefins (CPO). Over the primer coat, the
basecoat
and, where used, a clearcoat are then applied. In Europe, both basecoat and
clearcoat
are primarily water-based.
US-A-3637428 describes substrates based on copolymers of ethylene and a polar
comonomer, which are coated with a basecoat formulation comprising the film-
forming
resin, a vinyl acetate polymer as adhesion promoter, and an organic solvent.
Disadvantages are the use of an organic solvent and the fact that the coating
is
suitable only for very specific substrates.
US-A 5,585,192 describes a water-based formulation for promoting adhesion,
comprising a maleinized polyolefin and a polyolefin wax. As a primer coat, the
formulation boosts the adhesion between polymer substrates of PP, TPO, and PE,
and
a basecoat.
For enhancing the adhesion of paints to PP and TPO surfaces, the company
Eastman
markets the product Advantis 510W ( = registered trademark), which is an
aqueous
dispersion of a halogen-free polyolefin adhesion promoter. Advantis 510W can
be
mixed directly with aqueous paint formulations based on acrylic or on
polyurethane,
and applied as a mixture to a plastics substrate.
PF 70006 CA 02794227 2012-09-24
2
US-A-20030018139 discloses solventborne and aqueous primer formulations
comprising a carboxylated polyolefin which is modified with polyfunctional
alcohols.
The polyolefin is preferably a propylene-ethylene copolymer grafted with
carboxyl-
containing monomers, preferably maleic anhydride, and modified fully or partly
by
reaction with a polyfunctional alcohol. Aqueous emulsions are obtained by
neutralizing
some of the carboxyl groups with an amine or an inorganic base. The
aforementioned
reaction products are used as primers, and improve the adhesion of paints to
plastics
substrates such as TPO. Also described are mixtures of the basecoat with the
alcohol-
modified, carboxylated polyolefin, which can be applied in this form to a
substrate.
On grounds of costs, attempts are increasingly being made to do without the
primer
coat, while nevertheless ensuring satisfactory adhesion of a waterborne
basecoat to a
plastics surface.
It is an object of the invention, therefore, to provide improved plastics
coatings which
meet the aforementioned requirements.
It has now been found that aqueous basecoat formulations which additionally
comprise
aqueous dispersions based on specific polyethylene copolymer waxes are
distinguished by particularly effective promotion of adhesion in the context
of the
coating of plastics.
The invention accordingly provides an aqueous paint formulation composed of at
least
one aqueous basecoat and at least one aqueous dispersion of at least one at
least
partially neutralized ethylene copolymer wax which is selected from ethylene
copolymer waxes comprising as comonomers in copolymerized form:
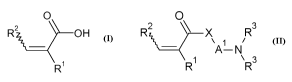
(A) 12% to 40%, preferably 20% to 35%, by weight of at least one ethylenically
unsaturated carboxylic acid of the general formula I,
0
R2 \\ O H
R~ II
where
R' and R2 are selected independently of one another from hydrogen and
unbranched and branched C,-C,o-alkyl;
(B) 60% to 88%, preferably 80% to 65%, by weight of ethylene;
(C) 0% to 10%, preferably 0% to 5%, by weight of at least one further
comonomer;
CA 02794227 2012-09-24
PF 70006
3
or
(A') 5% to 50%, preferably 20% to 40%, by weight of at least one comonomer of
the
general formula II
O
RZ X R3
A-N
R~ R3 II
where
R' and R2 are selected independently of one another from hydrogen and
unbranched and branched C1-Cloalkyl,
R3 is selected independently at each occurrence from hydrogen, unbranched and
branched C1-C10alkyl, and C3-C12cycloalkyl, where two radicals R3 may be
joined
to one another to form a 3- to 10-membered ring,
X is selected from oxygen, sulfur, and N-R4,
R4 is selected from unbranched and branched C1-Cloalkyl and hydrogen, and
A' is a divalent group selected from C1-C10alkylene, C4-C10cycloalkylene, and
phenylene;
(B') 50% to 95% by weight of ethylene, preferably 60% to 80% by weight, and
(C) zero to 20% by weight, preferably zero to 10% by weight, of at least one
further
comonomer;
where the ethylene copolymer wax comprising the comonomers (A), (B), and
optionally
(C) has a molecular weight M, of 10 000 to 150 000 g/mol, and the ethylene
copolymer
wax comprising the comonomers (A'), (B'), and optionally (C') has a molecular
weight
M, of 5000 to 40 000 g/mol.
Comonomers (A), (B), and (C), and (A'), (B'), and (C'), that are comprised in
copolymerized form are those fractions of comonomer which are incorporated
molecularly into the ethylene copolymer waxes used in accordance with the
invention,
and add up to 100% by weight.
The fraction of the aqueous ethylene copolymer wax dispersion in the paint
formulation
of the invention is generally 0.1% to 10%, preferably 0.5% to 5%, more
preferably 1%
to 3%, by weight, based on the aqueous basecoat.
CA 02794227 2012-09-24
PF 70006
4
An ethylene copolymer used in accordance with the invention and comprising
comonomer (A') in copolymerized form may be present with partial protonation
or as
the free amine.
In the formulae I and II the variables are defined as follows:
R' and R2 are the same or different;
R' is selected from hydrogen and
unbranched and branched C1-C10alkyl, such as, for example, methyl, ethyl, n-
propyl,
isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, n-pentyl, isopentyl, sec-
pentyl,
neopentyl, 1,2-dimethylpropyl, isoamyl, n-hexyl, isohexyl, sec-hexyl, n-
heptyl, n-octyl,
2-ethylhexyl, n-nonyl, n-decyl; more preferably C1-C4alkyl such as methyl,
ethyl,
n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, and tert-butyl, more
particularly methyl;
R2 is selected from unbranched and branched C1-C10alkyl such as, for example,
methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl,
n-pentyl,
isopentyl, sec-pentyl, neopentyl, 1,2-dimethylpropyl, isoamyl, n-hexyl,
isohexyl, sec-
hexyl, n-heptyl, n-octyl, 2-ethylhexyl, n-nonyl, n-decyl; more preferably C1-
C4alkyl such
as methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, and tert-
butyl,
particularly methyl,
and very preferably hydrogen;
the radicals R3 are different or preferably the same and are selected from
hydrogen
and branched and preferably unbranched C1-C10alkyl such as, for example,
methyl,
ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, tert-butyl, n-
pentyl, isopentyl, sec-
pentyl, neopentyl, 1,2-dimethylpropyl, isoamyl, n-hexyl, isohexyl, sec-hexyl,
n-heptyl,
n-octyl, 2-ethylhexyl, n-nonyl, n-decyl; preferably methyl, ethyl, n-propyl, n-
butyl,
n-pentyl, isopentyl, n-hexyl, n-heptyl, n-octyl, n-nonyl, n-decyl; more
preferably
C1-C4alkyl such as methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-
butyl, and
tert-butyl, very preferably methyl;
C3-C12cycloalkyl such as, for example, cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl,
cycloheptyl, cyclooctyl, cyclononyl, cyclodecyl, cycloundecyl, and
cyclododecyl;
preferably cyclopentyl, cyclohexyl, and cycloheptyl
where two radicals R3 may be joined to one another to form a 3- to 10-
membered,
preferably 5- to 7-membered, ring which is optionally substituted by C1-
C4alkyl radicals,
with particular preference a group N(R3)2 may be selected from
PF 70006 CA 02794227 2012-09-24
-N -N -N
If the radicals R3 are different, then one of the radicals R3 may be hydrogen.
5 X is selected from sulfur, N-R4, and, in particular, oxygen.
R4 is selected from hydrogen and unbranched and branched C,-C,oalkyl such as,
for
example, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl,
tert-butyl,
n-pentyl, isopentyl, sec-pentyl, neopentyl, 1,2-dimethylpropyl, isoamyl, n-
hexyl,
isohexyl, sec-hexyl, n-heptyl, n-octyl, 2-ethylhexyl, n-nonyl, n-decyl,
preferably
hydrogen and C,-C4alkyl such as methyl, ethyl, n-propyl, isopropyl, n-butyl,
isobutyl,
sec-butyl, and tert-butyl, more preferably methyl, and hydrogen;
A' is selected from divalent groups such as
C1-C,oalkylene, such as, for example, -CH2-, -CH(CH3)-, -(CH2)2-, -CH2-CH(CH3)-
, cis-
and trans-CH(CH3)-CH(CH3)-, -(CH2)3-, -CH2-CH(C2H5)-, -(CH2)4-, -(CH2)5-, -
(CH2)6-,
-(CH2)7-, -(CH2)5-, -(CH2)9-, -(CH2)10-; preferably C2-C4alkylene, such as -
(CH2)2-,
-CH2-CH(CH3)-, -(CH2)3-, -(CH2)4-, and -CH2-CH(C2H5)-, more preferably -(CH2)2-
,
-(CH2)3-, and -(CH2)4-, very preferably -(CH2)2-;
C4-C,ocycloalkylene such as, for example,
preferably
in isomerically pure form or as an isomer mixture,
and
phenylene, as for example ortho-phenylene, meta-phenylene, and, with
particular
preference, para-phenylene.
CA 02794227 2012-09-24
PF 70006
6
In one embodiment of the present invention R' is hydrogen or methyl. Very
preferably
R' is methyl.
In one embodiment of the present invention R' is hydrogen or methyl and R2 is
hydrogen. Comonomer (A) very preferably is methacrylic acid.
In one embodiment of the present invention R1 is hydrogen or methyl and R2 is
hydrogen, both groups R3 are the same and are in each case methyl or ethyl.
In one embodiment of the present invention X-A'-N(R3)2 is O-CH2-CH2-N(CH3)2.
Comonomer (A') very preferably is dimethylaminoethyl methacrylate.
In one embodiment of the present invention X-A'-N(R3)2 is O-CH2-CH2-CH2-
N(CH3)2.
In one embodiment of the present invention comonomer (A') is in protonated
form.
In one embodiment of the present invention the ethylene copolymer comprises no
further comonomers (C) or (C') in copolymerized form.
In another embodiment of the present invention the ethylene copolymer
comprises, in
addition to the comonomers (A) and (B), at least one further comonomer (C) as
well in
copolymerized form, selected from: (c1) ethylenically unsaturated C3-C10
carboxylic
esters, (c2) ethylenically unsaturated C4-C,o dicarboxylic acids or their
anhydrides, and
(c3) epoxide esters of ethylenically unsaturated C3-CIO monocarboxylic acids.
Ethylenically unsaturated C3-C10 carboxylic esters (c1) are, for example,
methyl
(meth)acrylate, ethyl (meth)acrylate, n-butyl (meth)acrylate, n-hexyl
(meth)acrylate,
2-ethylhexyl (meth)acrylate, n-octyl (meth)acrylate, n-decyl (meth)acrylate,
and/or
2-propylheptyl (meth)acrylate.
Used very preferably as ethylenically unsaturated carboxylic esters (c1) are
methyl
acrylate and/or methyl methacrylate.
Ethylenically unsaturated C4-C10 dicarboxylic acids (c2) include, for example,
itaconic
acid, mesaconic acid, citraconic acid, fumaric acid, and, in particular,
maleic acid.
Examples of their anhydrides include itaconic anhydride and, in particular,
maleic
anhydride.
PF 70006 CA 02794227 2012-09-24
7
Examples of epoxide esters of ethylenically unsaturated C3-C10 monocarboxylic
acids
(c3) include, in particular, esters of crotonic acid and/or (meth)acrylic acid
with glycidol,
preferably glycidyl acrylate and especially glycidyl methacrylate.
Used very preferably as ethylenically unsaturated carboxylic esters (c1) are
methyl
acrylate and/or methyl methacrylate.
In another embodiment of the present invention the ethylene copolymer
comprises, in
addition to the comonomers (A') and (B'), at least one further comonomer (C')
as well
in copolymerized form, selected from:
(c'1) C1-C20alkyl esters of ethylenically unsaturated C3-C10 monocarboxylic
acids, also
called, for short, ethylenically unsaturated C3-C20 carboxylic esters,
examples being
methyl (meth)acrylate, ethyl (meth)acrylate, n-butyl (meth)acrylate, n-hexyl
(meth)acrylate, 2-ethylhexyl (meth)acrylate, n-octyl (meth)acrylate, n-decyl
(meth)acrylate, 2-propylheptyl (meth)acrylate;
(c'2) mono- and di-C1-C10alkyl esters of ethylenically unsaturated C4-C10
dicarboxylic
acids, examples being monomethyl and dimethyl maleate, monoethyl and diethyl
maleate, monomethyl and dimethyl fumarate, monoethyl and diethyl fumarate,
monomethyl and dimethyl itaconate, mono-n-butyl and di-n-butyl maleate, and
mono-
2-ethylhexyl and di-2-ethylhexyl maleate;
(c'3) vinyl esters or allyl esters of C1-C10 carboxylic acids, preferably
vinyl esters or
ally) esters of acetic acid or propionic acid, more preferably vinyl
propionate and vinyl
acetate, and very preferably vinyl acetate;
(c'4) epoxide esters of ethylenically unsaturated C3-Ci0 monocarboxylic acids,
more
particularly esters of crotonic acid and/or (meth)acrylic acid with glycidol,
preferably
glycidyl acrylate and especially glycidyl methacrylate; and
(c'5) anhydrides of ethylenically unsaturated dicarboxylic acids such as
itaconic
anhydride and especially maleic anhydride.
The ethylene copolymer formed from the comonomers (A) and (B) or (A') and (B')
preferably comprises, as comonomer (C) or (C'), maleic anhydride and/or
glycidyl
methacrylate.
In one embodiment of the present invention comonomer (A) is in neutralized
form.
CA 02794227 2012-09-24
PF 70006
8
The ethylene copolymer waxes employed in the dispersions used in accordance
with
the invention, and comprising the comonomers (A), (B), and, optionally, (C),
have in
general a melt flow rate (MFR) in the range from 1 to 50 g/10 min, preferably
5 to
20 g/10 min, more preferably 7 to 15 g/10 min, measured at 160 C under a load
of
325 g in accordance with EN ISO 1133. Their acid number is typically 50 to 200
mg
KOH/g copolymer, preferably 100 to 200 mg KOH/g copolymer, determined in
accordance with DIN 53402.
The molecular weight MW of the ethylene copolymer waxes employed in the
dispersions
used in accordance with the invention, and comprising the comonomers (A), (B),
and,
optionally, (C), is in general from 10 000 to 150 000 g/mol, preferably from
20 000 to
120 000 g/mol, more preferably from 50 000 to 100 000 g/mol.
The melting ranges of the ethylene copolymer waxes employed in the dispersions
used
in accordance with the invention, and comprising the comonomers (A), (B), and,
optionally, (C), are situated in general in the range from 60 to 110 C,
preferably in the
range from.70 to 90 C, as determined by DSC in accordance with DIN 51007.
The ethylene copolymer waxes employed in the dispersions used in accordance
with
the invention, and comprising the comonomers (A), (B), and, optionally, (C),
may be
alternating copolymers or block copolymers or, preferably, gradient or random
copolymers.
The molecular weight MN, of the ethylene copolymer waxes employed in the
dispersions
used in accordance with the invention, and comprising the comonomers (A'),
(B'), and,
optionally, (C'), is from 5000 to 40 000 g/mol, preferably from 10 000 to 30
000 g/mol,
more preferably from 15 000 to 25 000 g/mol.
The melting point of the ethylene copolymer waxes employed in the dispersions
used
in accordance with the invention, and comprising the comonomers (A'), (B'),
and,
optionally, (C'), is situated in general in the range from 40 to 100 C,
preferably in the
range from 40 to 80 C, as determined by DSC in accordance with DIN 51007.
The ethylene copolymer waxes used can be prepared by conventional processes
for
copolymerizing ethylene (B) or (B'), comonomer (A) or (A'), and, optionally,
further
comonomers (C) or (C'), in stirred high-pressure autoclaves or in high-
pressure tube
reactors. Preparation in stirred high-pressure autoclaves is preferred.
Stirred high-
pressure autoclaves are known: a description is found in, for example,
Ullmann's
Encyclopedia of Industrial Chemistry, 5th edition, entry heading: Waxes,
volume A 28,
p. 146 ff., Verlag Chemie Weinheim, Basel, Cambridge, New York, Tokyo, 1996.
The
PF 70006 CA 02794227 2012-09-24
9
length/diameter ratio of such autoclaves is predominantly in ranges from 5:1
to 30:1,
preferably 10:1 to 20:1. The high-pressure tube reactors which may likewise be
employed are found likewise in Ullmann's Encyclopedia of Industrial Chemistry,
5th
edition, entry heading: Waxes, volume A 28, p. 146 if., Verlag Chemie
Weinheim,
Basel, Cambridge, New York, Tokyo, 1996. Details on the preparation of
ethylene
copolymer are also given in WO 2008/101937.
The preparation of aqueous dispersions of ethylene copolymer waxes is known
per se.
A preferred procedure is to place one or more ethylene copolymers in a vessel
together
with further substances, the vessel being, for example, a flask, an autoclave
or a tank,
and to heat the contents.
In the case of ethylene copolymer waxes comprising the comonomers (A'), (B'),
and,
optionally, (C'), one or more Bronsted acids such as, for example, organic
acids like
glacial acetic acid, formic acid, lactic acid, butyric acid, benzoic acid,
methanesulfonic
acid, and para-toluenesulfonic acid, or inorganic acids like nitric acid,
hydrochloric acid,
phosphoric acid, and sulfuric acid, and water, and optionally further
substances,
examples being emulsifiers, are added, the sequence of addition of Bronsted
acid or
Bronsted acids and also, where used, further substances being arbitrary.
The amount of Bronsted acid added is such that the ethylene copolymer is in
partially
or, preferably, fully neutralized form. In one embodiment of the present
invention an
excess of Bronsted acid is used.
If comonomer (A') in the ethylene copolymer wax comprising the comonomers
(A'),
(B'), and, optionally, (C') is already in at least partly protonated form, it
may not be
necessary to add Bronsted acid.
In the case of ethylene copolymer waxes synthesized from the comonomers (A),
(B),
and, optionally, (C), it is usual to add one or more basic substances, with
which the
ethylene copolymer waxes are at least partly neutralized, examples being
hydroxides
and/or carbonates and/or hydrogen carbonates of alkali metals, or preferably
amines
such as, for example, ammonia and organic amines such as, for example,
alkylamines,
N-alkylethanolamines, alkanolamines and polyamines. Examples of alkylamines
include the following: triethylamine, diethylamine, ethylamine,
trimethylamine,
dimethylamine, methylamine. Preferred amines are monoalkanolamines,
N,N-dialkylalkanolamines, N-alkylalkanolamines, dialkanolamines, N-
alkyldialkanolamines,
and trialkanolamines having in each case 2 to 18 C atoms in the hydroxyalkyl
radical
and optionally in each case 1 to 6 C atoms in the alkyl radical, preferably 2
to 6 C
atoms in the alkanol radical, and optionally 1 or 2 C atoms in the alkyl
radical.
PF 70006 CA 02794227 2012-09-24
Especially preferred are ethanolamine, diethanolamine, triethanolamine,
methyldiethanolamine, n-butyldiethanolamine, N,N-dimethylethanolamine, and
2-amino-2-methylpropan-1-ol. Especially preferred are ammonia and
N,N-dimethylethanolamine. Examples of polyamines include the following:
5 ethylenediamine, tetramethylethylenediamine (TMEDA), diethylenetriamine, and
triethylenetetramine.
In one embodiment of the present invention dispersions of ethylene copolymer
waxes
used in accordance with the invention and synthesized from the comonomers (A),
(B),
10 and, optionally, (C) comprise an amount of basic substance or basic
substances such
that at least half, preferably at least 60 mol%, of the carboxyl groups in the
ethylene
copolymer wax or waxes are neutralized.
In one embodiment of the present invention dispersions of ethylene copolymer
waxes
used in accordance with the invention and synthesized from the comonomers (A),
(B),
and, optionally, (C) comprise basic substance or basic substances, and more
particularly amine, in an amount such that the carboxyl groups of the ethylene
copolymer wax or waxes are neutralized quantitatively.
In one embodiment of the present invention dispersions of ethylene copolymer
waxes
used in accordance with the invention and synthesized from the comonomers (A),
(B),
and, optionally, (C) may comprise more basic substance or basic substances,
more
particularly amine, than is or are necessary for complete neutralization of
the ethylene
copolymer wax or waxes - for example, an excess of up to 100 mol%, preferably
up to
50 mol%.
If it is desired to prepare the dispersion in question at a temperature above
100 C, it is
advantageous to operate under increased pressure and to select the vessel
accordingly. The dispersion formed is homogenized, by mechanical or pneumatic
stirring or by shaking, for example. It is advantageously heated to a
temperature above
the melting point of the ethylene copolymer wax. Heating takes place
advantageously
to a temperature which is at least 10 C, more advantageous to a temperature
which is
at least 30 C, above the melting point of the ethylene copolymer wax.
The aqueous dispersions used in accordance with the invention have a solids
content
in the range from 5% to 40%, preferably 10% to 30%, by weight. The water used
for
the dispersions is preferably deionized, i.e., purified by distillation or by
means of an ion
exchanger.
= PF 70006 CA 02794227 2012-09-24
11
The pH of the aqueous dispersions comprising ethylene copolymer waxes
synthesized
from comonomers (A), (B), and, optionally, (C) is generally 7 to 14,
preferably 8 to 10.
The pH of the aqueous dispersions comprising ethylene copolymer waxes
synthesized
from comonomers (A'), (B'), and, optionally, (C') is generally 1 to 7,
preferably 3 to 6.
The aforementioned aqueous ethylene copolymer wax dispersions are used in
accordance with the invention, with typical waterborne basecoat materials, to
prepare
the aqueous modified paint formulations of the invention. For this purpose,
the
aforementioned aqueous ethylene copolymer wax dispersions are admixed directly
to
commercial aqueous paint formulations (i.e., basecoat) based, for example, on
polyurethane, polyester, alkyd, melamine and/or polyacrylate resins.
Further provided by the invention is the use of the aqueous modified paint
formulations
of the invention for coating plastics. The plastics may be moldings from many
different
sectors. Examples from the automobile sector include, for example, bumpers,
tank
covers, etc. Examples from the household sector are small appliances,
packaging,
toys, etc. Examples of plastics materials used for this purpose include
polyurethanes,
polyamides, polycarbonates, polyesters, and, in particular, nonpolar plastics
materials.
Examples of the latter are polyolefins such as polyethylene, ethylene
copolymers,
polypropylene, propylene copolymers, polyolefin mixtures with other polymers,
such as
PP/EPDM blends, for example, and PVC.
For coating, the cleaned and dried plastics surfaces are provided with the
modified
basecoat formulation. The substrate may be coated in a variety of ways, for
example
by dipping, spraying or application of the modified basecoat formulation. Over
this
basecoat formulation it is then possible to apply a topcoat, generally a
clearcoat. The
film thickness of the basecoat and of the clearcoat is dependent on the
particular
application and may vary considerably.
The plastics surfaces provided with the modified basecoat formulation are
notable for
particularly good adhesion of the paint.
Examples
Preparation of the ethylene copolymers
In a high-pressure autoclave of the type described in the literature (M.
Buback et al.,
Chem. Ing. Tech. 1994, 66, 510), ethylene and either N,N-dimethylaminoethyl
methacrylate (DMAEMA) or methacrylic acid (MAA) were copolymerized
continuously
CA 02794227 2012-09-24
PF 70006
12
at temperatures of 200 to 250 C. Ethylene was fed continuously into the high-
pressure
autoclave under the reaction pressure. Separately from this, the copolymer,
optionally
diluted with isododecane, was fed continuously into the high-pressure
autoclave.
Separately from this, the initiator solution, consisting of tert-amyl
peroxypivalate
dissolved in isododecane, is fed continuously into the high-pressure
autoclave.
Separately from this, where used, propionaldehyde is fed continuously into the
high-
pressure autoclave. The pressure during polymerization was 1500 to 2500 bar.
This
gave ethylene copolymers having the analytical data evident from table 1.
Table 1: Analytical data of ethylene copolymers used
Ex. Amount Amount of Amount Amount Melting Mw Q
No. of DMAEMA of of MAA point [g/mol] [mPa=s]
ethylene [% by wt.] ethylene [% by wt.] [ C]
[% by wt.] [% by wt.]
1 63 37 - - 49 17 000 2600
2 69 31 - - 41 21 700 7100
3 - - 73 27 80 90 000 > 50 000
By "amount" is meant the fraction of copolymerized MAA or DMAEMA,
respectively, in
the particular ethylene copolymer. The amount of MAA and of N,N-
dimethylaminoethyl
methacrylate in the ethylene copolymers was determined by 1H NMR spectroscopy.
q: Dynamic melt viscosity, measured at 120 C in a plate/cone viscometer (PP 35
Ti)
with a 1.0 mm gap, and D = 10 [1/s] in accordance with DIN 53018-1
Preparation of aqueous dispersions of the ethylene copolymers
A 2-liter autoclave with anchor stirrer was charged in each case with the
amount of
ethylene copolymer from examples 1 to 3 which is indicated in table 2. This
initial
charge was heated to 130 C with stirring and subsequently the amount of acid
or
amine (feed 1) indicated in table 2 was added dropwise over the course of 15
minutes.
Thereafter, over the course of 30 minutes, the remaining amount of water (feed
2) was
added, and stirring was continued at 130 C (external temperature) for 15
minutes. After
that the external temperature was lowered to 100 C, the mixture was stirred at
100 C
for an hour, and was then cooled to room temperature over the course of 15
minutes.
Filtration through a Perlon filter (100 pm) gave the corresponding aqueous
dispersions.
CA 02794227 2012-09-24
PF 70006
13
Table 2: Ethylene copolymer dispersions
No. Copoly- Amount Glacial acetic DMEA Amount pH of Solids
mer of (B) [g] acid Feed 1 of H2O emulsion content
ex. Feed 1 [g], [% by wt.]
Feed 2
D.1 1 225 32 g - 800 4.9 20
in 69 ml H2O
D.2 2 225 28 g - 800 4.6 20
in 72 ml H2O
D.3 3 225 - 38 g 648 9.0 21
in 114 ml H2O
Coating of plastics surfaces with modified basecoat formulation
Plastics plaques measuring 15 x 20 cm made from PP/EPDM were cleaned with
isopropanol and then dried. Each commercial basecoat formulation comprising
PU,
melamine, and polyacrylate resins was admixed with the above-described
ethylene
copolymer dispersions D.1 to D.3 in concentrations of 0.5%/1.0%/5.0% by
weight. The
cleaned plastics surfaces were coated with each modified basecoat formulation.
The
surfaces were flamed and coated with a clearcoat. The completed coated
plastics
plaques were flamed again. For application of the basecoat formulation, a 90
pm box
coater and, for the clearcoat, a 100 pm rod coater were used. This gave an
overall dry
film thickness of 70 pm. As a control, a plastics plaque was coated only with
the
basecoat formulation without addition of the above-described ethylene
copolymer
dispersions and clearcoat.
The plaques were scored with a knife in two parallel lines both horizontally
and
vertically. The quality of the adhesion was tested in accordance with DIN
55662 in a
steam jet test. The results of the steam jet test have been summarized in
figure 1.
Figure 1 shows, in table form, images of the plastics plaques thus treated.
For
purposes of comparison, the aforementioned control (1) is shown above the
table. In
the columns of the table, the respective concentration (conc.) of the ethylene
copolymer dispersions in the basecoat formulation is indicated in % by weight.
In the
rows of the table (K.1 to K.3), the ethylene copolymer dispersion used in each
case
(D.1 to D.3) is indicated.
Figure 1 shows that the modified basecoat formulations used in accordance with
the
invention significantly increase the adhesion to the plastics plaque.
Particularly good
adhesion was obtained with basecoat formulations comprising D. 1.