Note: Descriptions are shown in the official language in which they were submitted.
CA 02794658 2012-09-26
Title of the Invention
PRODUCTION METHOD OF HOT ROLLED STEEL SHEET AND PRODUCTION METHOD
OF HOT-DIP GALVANIZED STEEL SHEET
Field of the Invention
[0001]
The present invention relates to a method for producing
a hot rolled steel sheet having a good external appearance by
restraining the generation of mill scale on a steel surface at
the time of hot-rolling a steel slab. The present invention also
relates to a method for producing a Si-containing hot rolled steel
sheet suitable for hot-dip galvanizing. Furthermore, the
present invention relates to a method for producing a hot-dip
galvanized steel sheet by use of a high-strength Si-containing
steel sheet as a base material.
Description of the Related Arts
[0002]
In general, a hot rolled steel sheet is produced by
hot-rolling a steel slab and then coiling the resultant by a coiler.
On the surfaces of the hot rolled steel sheet coiled by the coiler,
there exists mill scale generated in the hot rolling process from
the slab heating step to the coiling step.
[0003]
Hot rolled steel sheets are classified into steel sheets
shipped in the state that oxide scale generated in the hot rolling
process are present (so-called steel sheets as hot rolled) , and
sheets shipped in the state that oxide scale are removed
1
,
CA 02794658 2012-09-26
(so-called hot rolled steel sheets as pickled) . The hot rolled
steel sheets as pickled are shipped after hot rolled steel sheets
are pickled to remove mill scale on their surfaces.
[0004]
If the generation of the mill scale is restrained in the
hot rolling process, any pickling step for removing the mill scale
becomes unnecessary. However, a suggestion has not been made
so far about technique for restraining the generation of the mill
scale in the hot rolling process.
[0005]
In recent years, from the viewpoint of the preservation
of the global environment, an improvement in the fuel-efficiency
of cars has become an important theme. Thus, there becomes a
trend to try to make the strength of the material of car body
higher to reduce the wall thickness and thereby to try to reduce
the car body weight. When Si is added to steel, a high-strength
steel sheet excellent in workability may be produced. However,
the use of a steel slab wherein Si is added to steel causes a
problem that in a hot rolling process there frequently generated
surface defects called red scale (hereinafter referred to as red
scale defects) . The problem causes deterioration in the quality
of the external appearance of hot rolled steel sheets.
[0006]
In recent years, in the fields of cars, household electrical
appliances, building materials and others, surface-treated steel
sheets to which rust-prevention is given, in particular, hot-dip
galvanized steel sheets excellent in rust prevention, have been
2
CA 02794658 2012-09-26
used. Hot rolled steel sheets are also used for application of
hot-dip galvanizing. When a hot rolled steel sheet is used for
application of hot-dip galvanizing, a thin steel sheet having
the surfaces from which mill scale are removed by pickling the
hot rolled steel sheet, or a thin steel sheet further subjected
to cold rolling after the pickling is used as a base steel sheet
for galvanizing. This base steel sheet is degreased in a
pre-treatment step, recrystallization-annealed, and then
subjected to hot-dip galvanizing, or the resultant sheet is
further subjected to alloying treatment in a CGL (continuous
galvanizing line), thereby to produce a hot-dip galvanized steel
sheet.
[0007]
When a high-strength Si-containing steel sheet is
subjected to hot-dip galvanizing, there is a problem not only
causing external appearance defects attributable to red scale
defects but also hindering the galvanizability due to a Si oxide
generating on a surface layer of the steel sheet.
[0008]
Examples of the type of a heating furnace in the CGL include
a DFF (direct firing type), an NOF (non-oxidizing type), and an
all-radiant-tube type, and the like. In recent years, the
construction of all-radiant-tube type CGLs has been increasing
since this type furnace is easily operated and pickups are hardly
generated in a roll inside this furnace. All-radiant-tube type
CGLs are different from DFF, (direct firing type) and NOF
(non-oxidizing type) and do not require any oxidizing step in
3
CA 02794658 2012-09-26
advance. For this reason, in high-strength steel sheets
containing an easily oxidizable element such as Si or Mn, a Si
oxide or a Mn oxide is generated on the surface layers of the
steel sheets. Thus, the all-radiant-tube type CGLs have
disadvantage for securing a good galvanizing performance.
[0009]
Patent Document 1 relates to a technique of using a
high-strength steel sheet containing easily oxidizable elements
such as Si and Mn in a large amount as a base steel sheet for
galvanizing, to keep a good galvanizing performance surely in
an all-radiant-tube type CGL. This Patent Document 1 discloses
a technique that at the time of producing a hot-dip galvanized
steel sheet (GI) having a galvanization layer in which hot-dip
galvanizing is performed but no subsequent treatment is conducted,
the temperature for heating in a reducing furnace is specified
by a relationship with the partial pressure of water vapor in
the atmosphere and further the dew point is raised to enhance
the potential of oxygen, thereby Si, Mn and others are internally
oxidized.
Patent Document 2 discloses a technique that at the time
of producing a hot-dip galvanized steel sheet (GA) by performing
hot-dip galvanizing and then subjecting the resultant
galvanization layer to alloying treatment, the temperature for
heating in a reducing furnace is specified by a relationship with
the partial pressure of water vapor in the atmosphere, and further
the dew point is raised to enhance the potential of oxygen, thereby
Si, Mn and others are internally oxidized. However, according
4
CA 02794658 2012-09-26
to these techniques, the furnace body is violently damaged to
make it impossible to produce a high-strength Si-containing
hot-dip galvanized steel sheet having a good external appearance.
[0010]
Patent Document 3 discloses a technique of specifying, for
the atmosphere of a reducing zone, the concentrations of H20 and
02, which are oxidizing gases and further specifying the
concentration of CO2 to enhance the potential of oxygen, thereby
Si, Mn and others are internally oxidized so as to restrain
external oxidation to improve the external appearance of a
galvanization. However, this technique has fears, such as
deterioration in the galvanization-external-appearance by
in-furnace pollution attributable to CO2, and a change in
mechanical properties by carburization into the steel sheet
surface layer.
[0011]
For this reason, in the case of using, as abase steel sheet
for galvanizing, a high-strength steel sheet containing easily
oxidizable elements such as Si and Mn in a large amount, any
all-radiant-tube type'CGL makes it impossible to produce a
hot-dip galvanized steel sheet having a good galvanizing
property.
Prior Art Documents
Patent Documents
[0012]
[Patent Document 1] Japanese Patent Application
CA 02794658 2014-05-15
73461-115
Laid-Open No. 2004-323970
[Patent Document 2] Japanese Patent Application
Laid-Open No. 2004-315960
[Patent Document 3] Japanese Patent Application
Laid-Open No. 2006-233333
Summary of the Invention
[0013]
In light of this situation, the present invention has been
made. It is an object of the present invention to provide a
production method for producing a hot rolled steel sheet which
makes it possible to restrain the generation of mill scale on
steel sheet surfaces in a hot rolling process. It is another
object of the present invention to provide a method for producing
a hot rolled steel sheet having a beautiful external appearance
by preventing, for a Si-containing hot rolled steel sheet, the
generation of red scale defects.
[0014]
It is still another object of the present invention to
provide a method for producing a hot rolled steel sheet which
prevents the generation of external appearance defects resulting
from a galvanization omission or red scale defects and is suitable
for producing a hot-dip galvanized steel sheet having a beautiful
external appearance. It is a further object of the present
invention to provide a method for producing a hot-dip galvanized
steel sheet having a beautiful external appearance which
6
CA 02794658 2014-05-15
73461-115
generates no external appearance defects resulting from
galvanization omission or red scale defects regardless of the
type of a heating furnace in a CGL.
[0015]
The subject matter of the present invention relates to the
following.
[0016]
[1] A method for producing a hot rolled steel sheet, comprising:
a slab heating step of heating a steel slab in a slab heating
furnace,
a step of hot-rolling the heated steel slab in a rough rolling
mill and a finishing rolling machine to form a strip, and
a coiling step of coiling the strip in a coiler,
characterized by performing the steps from the slab heating
step to the coiling step in a non-oxidizing atmosphere.
[0017]
[2] The method for producing a hot rolled steel sheet according
to [1], wherein the non-oxidizing atmosphere is a N2 atmosphere.
[0018]
[3] The method for producing a hot rolled steel sheet according
to [2], wherein the non-oxidizing atmosphere is the N2 atmosphere
containing H2 in an amount of 1 to 10% by volume, and further
has a dew point of -40 C to +20 C.
[0019]
[4] The method for producing a hot rolled steel sheet according
to [1], [2] or [3], wherein the steel slab contains:
C: 0.01-0.15%, Si: 0.1-1.8%, Mn: 1.0-2.7%, Al: 0.01-1.5%, P:
7
CA 02794658 2014-05-15
73461-115
0.005-0.025%, and S: 0.01% or less, by mass.
[0020]
[5] The method for producing a hot rolled steel sheet according
to [4], wherein the steel slab further contains at least one
element selected from the group consisting of: Cr: 0.05-1.0%,
Mo: 0.05-1.0%, Nb: 0.005-0.05%, Ti: 0.005-0.05%, Cu: 0.05-1.0%,
Ni: 0.05-1.0%, and B: 0.001-0.005%, by mass.
[0021]
[6] A method for producing a hot-dip galvanized steel sheet,
comprising:
removing mill scale by pickling the hot rolled steel sheet
produced by the method according to [4] or [5],
or
removing mill scale by pickling the hot rolled steel sheet
and further cold-rolling the hot rolled steel sheet; and
subsequently hot-dip galvanizing the hot rolled steel sheet.
[0022]
[7] A method for producing a hot-dip galvanized steel sheet,
further comprising subjecting alloying treatment to the hot-dip
galvanized steel sheet produced by the method according to [6].
[0023]
According to the present invention, the atmosphere in steps
of from the slab heating through the hot rolling to the coiling
is controlled to a non-oxidizing atmosphere, thereby making it
possible to restrain the generation of mill scale on the steel
8
CA 02794658 2012-09-26
sheet surfaces. Thus, a hot rolled steel sheet having no scale
on the surfaces can be produced. This hot rolled steel sheet
can be shipped as a hot rolled steel sheet of a "hot rolled steel
sheets as pickled" without performing any pickling step for
removing mill scale. In addition, according to the present
invention, any pickling is omitted and a material-reduction with
an acid is not caused, so that the yield can be improved.
[0024]
In a Si-containing hot rolled steel sheet, easily
oxidizable elements such as Si, Mn and Al are internally oxidized.
Thus, the generation of red scale defects and the generation of
a temper color are prevented, so that a hot rolled steel sheet
having a beautiful external appearance can be produced. In a
case where this Si-containing hot rolled steel sheet is used as
abase steel sheet for a hot-dip galvanized steel sheet, the easily
oxidizable elements such as Si, Mn and Al do not undergo any
selective external oxidation when the base steel sheet is
annealed in a CGL. Thus, the generation of galvanization
omission caused by selective external oxidization of the easily
oxidizable elements such as Si, Mn and Al can be prevented.
Additionally, external appearance defects attributable to red
scale defects are not generated, either. Thus, a hot-dip
galvanized steel sheet having a beautiful external appearance
can be yielded.
Brief Description of the Drawing
[0025]
9
CA 02794658 2012-09-26
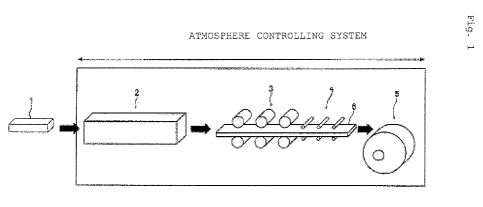
Fig. 1 is a schematic perspective view for describing an
atmosphere controlling system.
Embodiments for Carrying Out the Invention
[0026]
Hereinafter, the present invention will be specifically
described.
[0027]
Fig. 1 is a schematic perspective view for describing an
embodiment of an atmosphere controlling system used at the time
of carrying out the present invention. In Fig. 1, reference
number 1 represents a steel slab; 2, a slab heating furnace; 3,
a rough rolling mill; 4, a finish rolling mill; 5, a coiler; and
6, a hot rolled steel sheet (strip). The steel slab 1 is heated
to a predetermined temperature in the slab heating furnace 2,
and then hot-rolled in the rough rolling mill 3 and the finish
rolling mill 4 so as to be made to the hot rolled steel sheet
6 having a predetermined thickness. The sheet 6 is then coiled
by the coiler 5.
[0028]
In the conventional art, in steps of from the slab heating
to the coiling by a coiler, oxide scales are inevitably generated
on the surfaces of the steel sheet by atmospheric oxidation.
Moreover, when a Si-containing steel slab is used, there arises
a problem that red scale defects are generated. Red scale defects
are surface defects peculiar to Si-containing steel sheets, and
are surface defects which are a scale pattern, in the form of
CA 02794658 2012-09-26
stripes, that is generated by a matter that when a slab is heated,
regions where Fe oxide scale are locally generated for some causes,
and a region where Fe2SiO4 (fayalite) is generated on an interface
of the base iron so as to restrain the generation of Fe oxide
scale and then the Fe oxide scale are extended by hot rolling.
[0029]
As illustrated in Fig. 1, in the present invention, an
enclosure is placed from the slab heating furnace 2 to the coiler
for blocking the outside air to inhibit the incorporation of
oxygen thereinto. The atmosphere in the enclosure is controlled
into a non-oxidizing atmosphere in which iron is not oxidized.
[0030]
The non-oxidizing atmosphere, in which iron is not oxidized,
is a N2 atmosphere, a He atmosphere or an Ar atmosphere.
Considering costs, a N2 atmosphere is preferred.
[0031]
Furthermore, it is preferred to incorporate H2 into a N2
atmosphere in an amount of 1 to 10% by volume and to set the dew
point in the range of -40 to +20 C. Even when the outside air
is incorporated into the atmosphere to oxidize steel surfaces,
the produced iron oxide can be reduced in a case where H2 is
incorporated in an amount of 1% or more by volume and further
the dew point is set to +20 C or lower. Thus, a beautiful external
appearance having no temper color can be obtained. If the dew
point is higher than 20 C, iron is oxidized. If the dew point
is lower than -40 C, the control is difficult and the costs
increase. Thus, the dew point is preferably from -40 to 20 C.
11
CA 02794658 2012-09-26
If the amount of H2 is less than 1% volume, the iron oxide generated
in the steel surfaces cannot be reduced. As the content by
percentage of H2 is higher, a more advantageous result can be
obtained from the viewpoint of the reduction of the iron oxide.
However, if the content by percentage is more than 10%, the costs
increase. Accordingly, the H2 content by percentage is
preferably from 1 to 10% by volume. When the dew point of the
atmosphere is raised, the control of the dew point can be attained
by blowing humidified gas. When the dew point is lowered, the
control can be attained by introducing dry N2 wherein the water
content is decreased, or by absorbing and removing water in the
atmosphere.
[0032]
Expect that the atmosphere is controlled as described above,
conditions for production from the step of heating a slab to the
step for coiling may be ordinary manners.
[0033]
Mill scale formed in the step of producing the slab need
to be removed by a technique such as polishing before the slab
is put into a slab heating furnace.
[0034]
In the present invention, the atmosphere from a slab heating
step to a coiling step is controlled to a non-oxidizing atmosphere,
in which iron is not oxidized, thereby the generation of mill
scale on the surfaces of the steel sheet is restrained. Since
no mill scale are present on the surfaces of the steel sheet coiled
by the coiler, a surface state possible to ship the steel sheet
12
CA 02794658 2012-09-26
as it is, is obtained as a hot rolled steel sheets as pickled
without conducing pickling for removing any mill scale. The
composition of components of the steel slab that produces an
effect of restraining the generation of any mill scale is not
particularly limited.
[0035]
In Si-containing steel, to which Si is added, no Fe2S104
(fayalite) is generated on any interface of the base iron by
controlling the heating atmosphere at the time of heating a slab
to a non-oxidizing atmosphere not to generate any Fe oxidized
scale, at the same time, by internally oxidizing Si which creates
a solid solution in surface layers of the slab. Therefore, red
scale defects are not generated in the (resultant) hot rolled
steel sheet. In a case where easily oxidizable elements such
as Mn and Al are added, the easily oxidizable elements such as
Mn and Al are internally oxidized when the slab is heated.
[0036]
In the case where the hot rolled steel sheet in which the
easily oxidizable elements such as Si, Mn and Al are internally
oxidized, is pickled, or is further cold-rolled after the
pickling, and then using the hot rolled steel sheet which has
been pickled, or the cold rolled steel sheet which has been
cold-rolled, as a base steel sheet for galvanizing, the
, internally oxidized easily oxidizable elements such as Si, Mn
and Al do not shift onto the steel sheet surfaces in an annealing
step in a CGL. Therefore, a galvanization defect attributable
to the external oxidization of the easily oxidizable elements
13
CA 02794658 2012-09-26
such as Si, Mn and Al is not generated, and further a poor external
appearance resulting from red scale defects is not generated,
either.
[0037]
In order to prevent the generation of red scale defects
and a temper color, and also prevent the galvanizability from
being hindered by the easily oxidizable elements such as Si which
are externally oxidized in a CGL, it is preferred that the steel
slab in which Si is added into steel has a composition described
below. Any symbol "%" in connection with each component denotes %
by mass as far as the symbol is not particularly otherwise
specified.
[0038]
C: 0.01-0.15%
C is preferably incorporated in an amount of 0.01% or more
in order to make the strength of the steel high. When the amount
is 0.15% or less, the steel can surely keep weldability.
[0039]
Si: 0.1-1.8%
Si is an element effective for making the strength of the
steel high. If the Si amount is less than 0.1%, red scale defects
are not generated even when the present invention is even not
used. If the Si amount is more than 1.8%, Si cannot be
sufficiently internally oxidized in the slab heating step even
according to the present invention. Thus, Si remains in the form
of a solid solution so that Si is selectively oxidized on the
surface layers to generate a temper color. Moreover, the
14
CA 02794658 2012-09-26
solid-solution-form Si remaining in the annealing step in a CGL
is externally oxidized with selectivity, so that a galvanization
defect is caused. Thus, the amount thereof is preferably 1.8%
or less.
[0040]
Mn: 1.0-2.7%
In order to make the strength of the steel high, adding
Mn is more effective. If the Mn amount is less than 1.0%, a poor
external appearance is not generated when the present invention
is even not used. If the Mn amount is more than 2.7%, Mn cannot
be internally oxidized sufficiently in the slab heating step so
that Mn dissolved in a solid form remains. As a result, Mn is
selectively oxidized on the surface layers to generate a temper
color. Moreover, the solid-form-dissolved Mn remaining in the
annealing step in a CGL is externally oxidized selectively, so
that a galvanization defect is caused. Thus, the amount thereof
is preferably 2.7% or less.
[0041]
Al: 0.01-1.5%
The lower limit is an amount at which Al is inevitably
incorporated. Al has a remaining-y-phase-stabilizing effect.
Thus, Al may be added to improve the mechanical properties. For
the purpose, it is preferred to incorporate Al in an amount of
0.1% or more. If the Al amount is more than 1.5%, Al is not
sufficiently internally oxidized in the slab heating step so that
Al dissolved in a solid form remains so that Al is selectively
oxidized on the surface layers to generate a temper color.
CA 02794658 2012-09-26
Moreover, the solid-form-dissolved Al remained in the annealing
step in a CGL is externally oxidized selectively, so that a
galvanization defect is caused. Thus, the amount thereof is
preferably 1.5% or less.
[0042]
P: 0.005-0.025%
Pis an element which is inevitably incorporated. In order
to make the precipitation of cementite delay to retard the advance
of the transformation, P is incorporated in an amount of 0.005%
or more. If the amount is more than 0.025%, the weldability
deteriorates and further the steel is not sufficiently internally
oxidized in the slab heating step. Thus, the steel is oxidized
in the annealing step in a CGL so that the surface quality
deteriorates. Thus, the amount thereof is preferably 0.025% or
less.
[0043]
S: 0.01% or less
S is an element which is inevitably incorporated. The
lower limit is not specified. However, when S is incorporated
in a large amount, the weldability deteriorates. Further, when
the steel is annealed, S precipitates on the surfaces so that
the external appearance deteriorates. Thus, the amount thereof
is preferably 0.01% or less.
[0044]
The balance is Fe and inevitable impurities. Besides these
elements, one or more elements selected from the following may
be optionally added in order to raise mechanical properties of
16
CA 02794658 2012-09-26
the steel sheet: Cr: 0.05-1.0%, Mo: 0.05-1.0%, Nb: 0.005-0.05%,
Ti: 0.005-0.05%, Cu: 0.05-1.0%, Ni: 0.05-1.0%, and B:
0.001-0.005%. Cr, Mo, Nb, Cu and Ni have an advantageous effect
of promoting the internal oxidation of Si and restraining
selective external oxidation when these elements are added alone
or in a multiple form of two or more thereof. These elements
may be added not to improve the mechanical properties but to
promote the internal oxidation of Si.
[0045]
When the above-mentioned elements are added, desired
ranges of the components will be described hereinafter.
[0046]
Cr does not easily give an effect of promoting the
hardenability, or the internal oxidation of Si if the amount
thereof is less than 0.05%. If the amount is more than 1.0%,
Cr is externally oxidized selectively so that the galvanizability
deteriorates. Thus, the Cr amount is desirably from 0.05 to 1.0%.
[0047]
Mo does not easily gives an effect of adjusting the strength
nor an effect of promoting the internal oxidation of Si at the
time of adding Mo together with Nb, Ni or Cu if the Mo amount
is less than 0.05%. If the amount is more than 1.0%, the cost
increases. Thus, the Mo amount is desirably from 0.05 to 1.0%.
[0048]
Nb does not easily give an effect of adjusting the strength
nor an effect of promoting the internal oxidation of Si at the
time of adding Nb together with Mo if the Nb amount is less than
17
CA 02794658 2012-09-26
0.005%. If the amount is more than 0.05%, the cost increases.
Thus, the Nb amount is desirably from 0.005 to 0.05%.
[0049]
Ti does not give an effect of adjusting the strength if
the Ti amount is less than 0.005%. If the amount is more than
0.05%, the galvanizability deteriorates. Thus, the Ti amount
is desirably from 0.005 to 0.05%.
[0050]
If the amount of Cu is less than 0.05%, the following effect
is not easily obtained: the effect of promoting the formation
of a remaining y-phase, or the effect of promoting the internal
oxidation of Si when Cu is added together with Ni or Mo. If the
amount is more than 1.0%, the cost increases. Thus, the Cu amount
is desirably from 0.05 to 1.0%.
[0051]
If the amount of Ni is less than 0.05%, the following effect
is not easily obtained: the effect of promoting the formation
of a remaining y-phase, or the effect of promoting the internal
oxidation of Si when Ni is added together with Cu or Mo. If the
amount is more than 1.0%, the cost increases. Thus, the Ni amount
is desirably from 0.05 to 1.0%.
[0052]
If the B amount is less than 0.001%, the effect of promoting
the hardenability is not easily obtained. If the amount is more
than 0.005%, the galvanizability deteriorates. Thus, the B
amount is desirably from 0.001 to 0.005%.
It goes without saying that B does not need to be added
18
CA 02794658 2012-09-26
when the addition thereof is unnecessary for an improvement in
mechanical properties.
[0053]
When the steel slab having the above-mentioned component
composition is used to produce a hot rolled steel sheet, the easily
oxidizable elements such as Si, Mn and Al on the steel sheet
surface layers can be internally oxidized by shutting out the
outside air and by keeping the steps from the slab heating step
to the coiling step in a non-oxidizing controlled atmosphere
which does not incorporate oxygen. In other words, when oxygen
is incorporated, the easily oxidizable elements such as Si, Mn
and Al which are more easily oxidized than Fe, are externally
oxidized with selectivity but not internally oxidized. However,
in the non-oxidizing atmosphere which does not incorporate oxygen,
0 supplied from H20 in the atmosphere becomes an oxygen-supplying
source, so that the easily oxidizable elements such as Si, Mn
and Al that are dissolved in a solid form in the steel are
internally oxidized though Fe is not oxidized. As a result, the
generation of red scale defects and a temper color can be
prevented.
[0054]
On the surface of the hot rolled steel sheet wound around
the coiler, there exists a very thin oxidized coat generated
during the hot rolling process. Thus, when the hot rolled steel
sheet is used as a base steel sheet for galvanizing, the rolled
steel sheet is pickled by an ordinary pickling treatment after
the hot rolling process, so that the oxidized coat on the surface
19
CA 02794658 2012-09-26
is completely removed. The pickled hot rolled steel sheet, or
a cold rolled steel sheet obtained by cold-rolling the pickled
hot rolled steel sheet in the usual way is used as a base steel
sheet for galvanizing. This base steel sheet is charged into
a CGL.
[0055]
In the base steel sheet (high-strength Si-containing steel
sheet), the easily oxidizable elements such as Si, Mn and Al are
internally oxidized in the hot rolling process, and red scale
defects are not generated. Accordingly, even when the steel
sheet is heated in a heating furnace of any one of the types
selected from a DFF (direct firing type), an NOF (non-oxidizing
type) and an all-radiant-tube type in the CGL, oxides of the easily
oxidizable elements such as Si, Mn and Al do not diffuse onto
the steel sheet surfaces, regardless of the heating furnace type.
Thus, a good galvanizability is surely kept and further a poor
external appearance attributable to red scale defects is not
generated, so that a good external appearance is obtained.
Conditions for the heating furnace in a CGL may be ordinary
conditions.
[0056]
The galvanization coating weight is preferably from 20 to
120 g/m2 for each of the surfaces. If the weight is less than
20 g/m2, it is difficult to surely keep the corrosion resistance.
If the weight is more than 120 g/m2, the galvanization peeling
resistance deteriorates. In the hot-dip galvanized steel sheet
that has been alloyed, the Fe content by percentage of the
CA 02794658 2012-09-26
galvanization layer is preferably from 7 to 15%. If the content
by percentage is less than 7%, alloying unevenness is generated
and the flaking resistance deteriorates. If the content by
percentage is more than 15%, the galvanization peeling resistance
deteriorates.
[0057]
Conditions for the hot-dip galvanizing and conditions for
the alloying treatment may be ordinary manners.
Example 1
[0058]
Soft steel slabs containing chemical composition shown in
Table 1 and the balance being Fe and inevitable impurities were
prepared with a thickness of 200 mm, and then a laboratory test
was performed for the production of hot rolled steel sheets from
a slab heating step to a coiling step under the following
condition: The slab was heated in a heating furnace, and then
the slab was rolled into a strip with a thickness of 3 mm by a
rough rolling mill and a finish rolling mill. The strip was
coiled around a coiler. The slab heating temperature of the
heating furnace was set to 1250 C, and the finishing temperature
in the finishing rolling and the coiling temperature were set
to 900 C and 550 C, respectively. The atmosphere from the heating
furnace to the coiler was controlled into an atmosphere shown
in Table 2. The resultant coil was cooled and then uncoiled to
be evaluated about the external appearance. For the external
appearance, the color tone thereof was observed with the naked
eyes. The uncoiled steel in which no temper color was generated
21
CA 02794658 2012-09-26
to give an external appearance equivalent to that of a
conventional hot rolled steel sheet as pickled was judged to be
"white", the uncoiled steel sheet having a black external
appearance equivalent to that of a conventional steel sheet as
hot rolled was judged to be "black", and the uncoiled steel sheet
in which a temper color was generated to give an external
appearance discolored into a light brown color was judged to be
"light brown". Any hot rolled steel sheet that is judged to be
"white" has an external appearance possible to be shipped the
steel sheet without the pickling for removing scale. For any
steel sheet that is judged to be "light brown" or "black",
performing the pickling is necessary for removing scale in order
to ship the steel sheet as a hot rolled steel sheet as pickled.
[0059]
[Table 1]
(% by mass)
Si Mn Al P S Ti
0.002 0.01 0.1 0.03 0.01 0.004 0.02
22
[0060]
[Table 2]
Hot rolling atmosphere External
No
Controlling Atmosphere Dew point ( C)Atmosphere type appearance
1 Not made Atmospheric airNot controlled Oxidizing Black
Comparative example
2 Made N2 Not controlled
Non-oxidizing White Inventive example
3 Made N2+3%H2 -40 Non-oxidizing
White Inventive example
4 Made N2+3%H2 0 Non-oxidizing
White Inventive example
Made N2+3%H2 20 Non-oxidizing White
Inventive example
0
6 Made N2+3%H2 60 Oxidizing
Light BrownComparative example 1.)
co
0
0
CA 02794658 2012-09-26
[0061]
The results are shown in Table 2. As is evident from Table
2, the hot rolled steel sheets of the inventive examples in which
of each the atmosphere is controlled within the scope of the
present invention, give the beautiful external appearances
possible to be shipped as they are, as hot rolled steel sheet
as pickled. By contrast, the hot rolled steel sheets of the
comparative examples in which the atmosphere is out of the scope
of the present invention, do not give the beautiful external
appearances possible to be shipped as they are, as hot rolled
steel sheets as pickled.
Example 2
[0062]
The results obtained by performing an experiment for
producing a hot rolled steel sheet by use of a Si-containing steel
slab is explained.
A steel slab with a thickness of 300 mm containing chemical
composition shown in Table 3 and the balance being Fe and
inevitable impurities, was prepared.
Steels B, C and D were each a steel slab containing the
following: C: 0.01-0.15%, Si: 0.1-1.8%, Mn: 1.0-2.7%, Al:
0.01-1.5%, P: 0.005-0.025%, and S: 0.01% or less, and each of
these percentages (%s) represents % by mass.
Steels E, F, G, H, I and J were each a steel slab further
containing one or more elements selected from the following: Cr:
0.05-1.0%, Mo: 0.05-1.0%, Nb: 0.005-0.05%, Ti: 0.005-0.05%, Cu:
0.05-1.0%, Ni: 0.05-1.0%, and B: 0.001-0.005%, and each of these
24
CA 02794658 2012-09-26
percentages (%s) represents % by mass.
Steel K was a steel slab in which the amount of Si was out
of the range of 0.1-1.8%.
Steel L was a steel slab in which the amount of Mn was out
of the range of 1.0-2.7%.
Steel M was a steel slab in which the amount of P was out
of the range of 0.005-0.025%.
Steel N was a steel slab in which the amount of S was out
of the range of 0.01% or less.
A laboratory test of the production of hot rolled steel
sheets from a slab heating step to a coiling step was performed
under the following condition: The slab was heated in a heating
furnace, and then the slab was rolled into a strip with a thickness
of 3 mm by a rough rolling mill and a finish rolling mill. The
strip was coiled around a coiler. The slab heating temperature
of the heating furnace was set to 1250 C, and the finishing
temperature in the finish rolling and the coiling temperature
were set to 900 C and 550 C, respectively. The atmosphere from
the heating furnace to the coiler was controlled into an
atmosphere shown in Table 4. The resultant coil was cooled and
then uncoiled, and the external appearance was observed with the
naked eyes to be evaluated about the color tone thereof and as
to whether or not red scale defects were generated. The color
tone was judged in the same way as in Example 1. The criterion
for judging whether or not any one of the uncoiled steel sheets
had an external appearance possible to be shipped as a hot rolled
steel sheet as pickled was the same as in Example 1.
[0063]
[Table 3]
(% by
mass)
Steel symbol C Si Mn Al P S Cr Mo
B Nb Cu Ni Ti Classification
B 0.03 1.0 2.0 0.03 0.010 0.004
- - - - - - - Inventive steel
C 0.08 0.1 2.0 0.03 0.010 0.004 - -
- - - - - Inventive steel
D 0.10 0.1 1.6 1.20 0.010 0.004
- - - - - - - Inventive steel
E 0.05 1.6 1.9 0.02 0.010 0.004
0.3 - - - - - - Inventive steel n
_
0
F 0.05 1.5 1.9 0.03 0.010 0.004 - 0.1
- - - - - Inventive steel I.)
-.3
q3.
G 0.05 1.6 2.2 0.03 0.010 0.004
- - 0.003 - - - - Inventive steel
m
ul
N.) H 0.05 1.5 2.0 0.05 0.010 0.004 - -
0.001 0.03 - - - Inventive steel co
m
1.)
0
I 0.05 1.7 1.9 0.03 0.010 0.004 - 0.1
- - 0.1 0.2 - Inventive steel
1
0
J 0.05 1.6 1.9 0.04 0.010 0.004
- - 0.001 - - - 0.02 Inventive steel
q3.
1
1.)
K 0.14 1.9 1.9 0.03 0.010 0.004
- - - - - - 0.05 Comparative
steel '
L 0.14 1.2 3.2 0.03 0.010 0.004
- - - - - - - Comparative steel
M 0.14 1.5 1.6 0.03 0.035 0.004 - -
- - - - - Comparative steel
N 0.14 1.5 1.6 0.03 0.010 0.02
- - - - - - - Comparative steel
_
[0064]
[Table 4]
Hot
roll ed Hot rolling atmosphere External
appearance
Steel
Notes
steel
sheet No. Controlling Atmosphere Dew point ( C) Atmosphere type
Color tone Red scale defects
1 B Not made Atmospheric air Not controlled Oxidizing
Black Generated Comparative example
2 B Made N2 Not controlled Non-oxidizing White
Not generated Inventive example
3 B Made N2+3%H2 -40 Non-oxidizing White
Not generated Inventive example
4 B Made N2+3%H2 o Non-oxidizing White
Not generated Inventive example
0
B Made N2+3%1-12 20 Non-oxidizing White Not
generated Inventive example o
K.)
-3
6 B Made N2+3%H2 60 Oxidizing Light
Brown Not generated Comparative example
ko
Fl.
m
NJ 7 C Made N2+3%H2 20 Non-oxidizing White
Not generated Inventive example m
m
--1
K.)
8 D Made N2+3%H2 20 Non-oxidizing White
Not generated Inventive example 0
H
..
K.)
(1)
9 E Made N2+3%H2 20 Non-oxidizing White
Not generated Inventive example
ko
1
F Made N2+3%H2 20 Non-oxidizing White Not
generated Inventive example N.)
m
11 G Made N2+3%H2 20 Non-oxidizing White
Not generated Inventive example
12 H Made N2+3%H2 20 Non-oxidizing White
Not generated Inventive example
13 I Made N2+3%H2 20 Non-oxidizing White
Not generated Inventive example
14 J Made N2+3%H2 20 Non-oxidizing White
Not generated Inventive example
K Made N2+3%H2 20 Non-oxidizing Light Brown Not
generated Comparative example
16 L Made N2+3%H2 20 Non-oxidizing Light Brown
Not generated Comparative example
17 14 Made N2+3%H2 20 Non-oxidizing Light Brown
Not generated Comparative example
18 N Made N2+3%H2 20 Non-oxidizing Light Brown
Not generated Comparative example
CA 02794658 2012-09-26
[00651
The results are shown in Table 4. As is evident from Table
4, the hot rolled steel sheets of the inventive examples in which
a steel slab having components described below is used and
further the atmosphere is controlled to produce the hot rolled
steel sheet, give such a beautiful external appearance possible
to be shipped as they are, as a hot rolled steel sheet as pickled
is obtained. Further, red scale defects are not generated.
Steel slabs containing the following: C: 0.01-0.15%, Si:
0.1-1.8%, Mn: 1.0-2.7%, Al: 0.01-1.5%, P: 0.005-0.025%, and S:
0.01% or less, and each of these percentages (%s) represents %
by mass.
Steel slabs containing the following: C: 0.01-0.15%, Si:
0.1-1.8%, Mn: 1.0-2.7%, Al: 0.01-1.5%, P: 0.005-0.025%, and S:
0.01% or less, and further containing one or more elements
selected from the following: Cr: 0.05-1.0%, Mo: 0.05-1.0%, Nb:
0.005-0.05%, Ti: 0.005-0.05%, Cu: 0.05-1.0%, Ni: 0.05-1.0%, and
B: 0.001-0.005%, and each of these percentages (%s) represents %
by mass.
By contrast, the hot rolled steel sheets of the
comparative examples, in which the atmosphere is out of the
scope of the present invention, do not give such a beautiful
external appearance possible to be shipped as they are, as a
hot rolled steel sheet as pickled.
Example 3
[0066]
The hot rolled steel sheets produced in Example 2 were
28
CA 02794658 2012-09-26
=
pickled to remove the oxide film generated by the hot rolling.
Some of the hot rolled steel sheets were used as pickled hot
rolled steel sheets without being subjected to any other
treatment, and some of the others were subjected further to cold
rolling at a rolling reduction ratio of 50% after the pickling
so as to be made to cold rolled steel sheets. The thus-produced
hot rolled steel sheets and cold rolled steel sheets were each
annealed at 850 C and then subjected to hot-dip galvanizing in
an all-radiant type CGL simulator. Some thereof were further
subjected to alloying treatment. For the hot-dip galvanized
steel sheets (GA) subjected to the alloying treatment after the
hot-dip galvanizing, a 0.14% Al-containing Zn bath was used.
For the hot-dip galvanized steel sheet (GI) subjected to no
alloying treatment after the hot-dip galvanizing, a 0.18%
Al-containing Zn bath was used. The galvanization adhesion
amount was adjusted to 50 g/m2 for each of the surfaces (of each
of the steel sheets) by gas-wiping. The method for the hot-dip
galvanizing and the method for the alloying treatment were used
with the usual methods.
[0067]
The external appearances of the thus-produced galvanized
steel sheets were observed, and the following were observed:
whether or not the defects attributable to red scale defects
were generated; and whether or not a galvanization omission was
generated. Any steel sheet in which at least one of the defect
attributable to the red scale defects and the galvanization
omission was recognized was judged to be poor in external
29
CA 02794658 2012-09-26
appearance, and any steel sheet in which any one of the defect
attributable to the red scale defects and the galvanization
omission was not recognized was judged to be beautiful in
external appearance.
[0068]
[Table 5]
Hot-dip Hot rolled steel Hot rolling atmosphere
Hot-dip
galvanized
galvanized sheet No. in Steel -
Base steel sheet External appearance Notes
steel sheet
steel sheet No. Table 4 Controlling Atmosphere
Dew point ( C) species
_
Poor external appearance
Comparative
1 1 B Not made Atmospheric
air Not controlled Hot rolled steel sheet GA
(defects attributable to red scale defects)
example
Poor external appearance
Comparative
2 1 B Not made Atmospheric
air Not controlled Cold rolled steel sheet GA
(defects attributable to red scale defects)
example
_
3 3 B Made N2+3%H2 -40 Cold rolled
steel sheet GA Beautiful external appearance Inventive
example
4 4 B Made N2+3%H2 - 0 Cold rolled
steel sheet GA Beautiful external appearance Inventive
example
5 B Made N2+3%H2 . 20 Cold rolled steel
sheet GA Beautiful external appearance
Inventive example n
_ _
6 5 B Made N2+3%H2 20 Hot rolled
steel sheet GA Beautiful external appearance Inventive
example
¨
0
7 5 B MadeN2+3%1-12 20 Hot rolled
steel sheet GI Beautiful external appearance
Inventive example iv
__
-.3
Poor external appearance
Comparative
8 6 B Made N2+3%H2 60 Cold rolled
steel sheet GA q3.
(galvanization omission)
example
0, ,
9 7 C Made N2+3%H2 20 Cold rolled
steel sheet GA Beautiful external appearance
Inventive example in
_ ,
co
8 D Made N2+3%H2 20 Cold rolled steel
sheet GA Beautiful external appearance
Inventive example iv
HA 11 9 E Made N2+3%H2 20
Cold rolled steel sheet GA Beautiful external
appearance Inventive example 0
H
12 10 F Made N2+3%H2 20 Cold rolled
steel sheet GA Beautiful external appearance
Inventive example iv
i
13 11 G Made N2+3%H2 20 Cold rolled
steel sheet GA Beautiful external appearance
Inventive example 0
14 12 _ H Made N2+3%H2 20 Cold rolled
steel sheet GA Beautiful external appearance
Inventive example q3.
1
13 I Made N2+3%H2 20 Cold rolled steel
sheet GA Beautiful external appearance
Inventive example iv
0,
16 14 J Made N2+3%H2 20 Cold rolled
steel sheet GA Beautiful external appearance Inventive
example
Poor external appearance
Comparative
17 15 K Made N2+3%H2 20 Cold rolled
steel sheet GA
(galvanization omission)
example
_
Poor external appearance
Comparative
18 16 L Made N2+3%H2 20 Cold rolled
steel sheet GA
(galvanization omission)
example
. -
Poor external appearance
Comparative
19 17 M Made N2+3%H2 20 Cold rolled
steel sheet GA
(galvanization omission)
example
Poor external appearance
Comparative
18 N Made N2+3%H2 20 Cold rolled steel
sheet GA (galvanization omission) example
,
CA 02794658 2012-09-26
[0069]
The examination results are shown in Table 5. As is
evident from Table 5, the hot-dip galvanized steel sheets of
the inventive examples produced by the method of the present
invention using the steel slab B, C or D wherein the amounts
of C, Si, Mn, Al, P and S are within the specified ranges, give
a galvanized steel sheet good in external appearance even when
the hot-dip galvanized steel sheet is a steel to which Si, Mn
and Al are added. The hot-dip galvanized steel sheets of the
inventive examples produced by the method of the present
invention using the steel slab E, F, G, H, I or J wherein at
least one of Cr, Mo, Nb, Ti, Cu, Ni and B is contained in the
specified amount(s), also give a galvanized steel sheet good
in external appearance. By contrast, for the hot-dip
galvanized steel sheets produced by use of the steel slab
wherein the amount of C, Si, Mn, Al, P and S are out of the
specified range, or the amount of Cr, Mo, Nb, Ti, Cu, Ni and
B are out of the specified range, or the hot-dip galvanized steel
sheets produced in the atmosphere out of the scope of the method
of the present invention, red scale defects or a galvanization
omission is generated so that the external appearance is poor.
Industrial Applicability
[0070]
According to the present invention, a hot rolled steel
sheet possible to be shipped as a "hot rolled steel sheet as
pickled" can be produced even when a pickling step for removing
32
CA 02794658 2012-09-26
mill scale is not conducted. For a Si-containing hot rolled
steel sheet, easily oxidizable elements such as Si, Mn and Al
are internally oxidized so that a Si-containing hot rolled steel
sheet beautiful in the external appearance can be produced in
which red scale defects are not generated and a temper color
is not generated. When this Si-containing hot rolled steel
sheet is used as a base steel sheet for a hot-dip galvanized
steel sheet, easily oxidizable elements such as Si, Mn and Al
are not externally oxidized with selectivity at the time of
annealing in a CGL. Therefore, the generation of a
galvanization omission caused by the selective, external
oxidation of the easily oxidizable elements such as Si, Mn and
Al is prevented, and further a poor external appearance
attributable to red scale defects is not generated, thus a
hot-dip galvanized steel sheet beautiful in external appearance
can be produced.
Description of Reference Numerals
[0071]
1: slab, 2: slab heating furnace, 3: rough rolling mill, 4:
finish rolling mill, 5: coiler, and 6: hot rolled steel sheet
33