Note: Descriptions are shown in the official language in which they were submitted.
CA 02794932 2012-09-28
WO 2011/123145 PCT/US2010/040219
SYSTEMS AND METHODS FOR RECYCLING PLASTIC
TECHNICAL FIELD
[0001] The present disclosure relates generally to the recycling of plastic.
Certain
embodiments relate more specifically to systems and methods for vaporizing
plastic
and recovering organic molecules from the resultant vapor.
BRIEF DESCRIPTION OF THE DRAWINGS
[0002] The written disclosure herein describes illustrative embodiments that
are
non-limiting and non-exhaustive. Reference is made to certain of such
illustrative
embodiments that are depicted in the figures, in which:
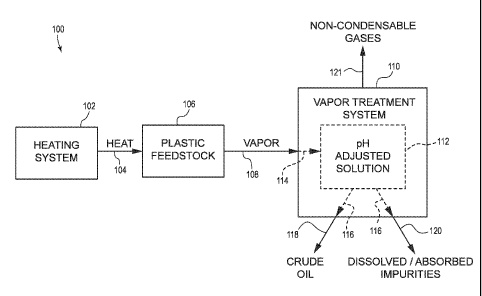
[0003] FIG. 1 is a schematic flow diagram of an embodiment of a plastic
recycling
system;
[0004] FIG. 2 is a schematic flow diagram of another embodiment of a plastic
recycling system;
[0005] FIG. 3 is a schematic flow diagram of another embodiment of a plastic
recycling system;
[0006] FIG. 4 is a cross-sectional view of an embodiment of a baffle that is
compatible with an embodiment of a condenser that may be used in the system of
FIG. 3;
[0007] FIG. 5 is a schematic flow diagram of an embodiment of a vacuum system
that is compatible with the plastic recycling system of FIG. 3;
[0008] FIG. 6 is a schematic flow diagram of an embodiment of a series of
heating systems and containers in which the containers are commonly joined to
a
gas transfer line, wherein plastic feedstock within the containers is being
vaporized
and removed from the containers in a continuous batch process;
[0009] FIG. 7 is a schematic flow diagram of another embodiment of a plastic
recycling system;
[0010] FIG. 8 is a schematic flow diagram of another embodiment of a plastic
recycling system; and
[0011] FIG. 9 is a schematic flow diagram of a cleaning system and a reservoir
that can be used in another embodiment of a plastic recycling system such as
the
recycling system of FIG. 3.
1
CA 02794932 2012-09-28
WO 2011/123145 PCT/US2010/040219
DETAILED DESCRIPTION
[0012] Certain embodiments of systems and methods described herein are
configured for efficient recycling of plastic. Some systems and methods can
quickly
and simply convert waste plastics into one or more purified organic molecular
species, which can be considered as a crude hydrocarbon material or crude oil.
The
crude oil may be readily stored, transported, and/or refined into fuel or
other
commercially relevant materials.
[0013] In some embodiments, a quantity of waste plastic feedstock can be
introduced into a sealable cartridge or container. The container can be heated
under
vacuum conditions such that the plastic feedstock transitions into a vapor
(e.g., one
or more gases), which can be removed from the cartridge for further
processing. For
example, the vapor can be introduced into a condenser and directly contacted
with a
pH adjusted solution, which can, in some instances, absorb a portion of the
vapor
and condense another portion thereof. The condensed material can comprise one
or
more organic molecular species that can be termed herein as a crude oil. The
crude
oil can be separated from the other portions of the vapor that are absorbed
into the
pH adjusted solution, and thus the crude oil can be of a clean or purified
quality such
that it may be readily refined from its crude state.
[0014] Various illustrative embodiments of inventive systems and methods will
now be described. Advantages of the systems and methods, as well as features
and
steps thereof, respectively, will be apparent from the disclosure that
follows.
[0015] FIG. 1 depicts a process flow diagram of an embodiment of a plastic
recycling system 100. The plastic recycling system 100 includes a heating
system
102 that is configured to deliver heat 104 to a plastic feedstock 106. The
heating
system 102 can comprise any suitable heating mechanism, such as, for example,
a
combustion burner, a fluidized bed burner, a retort, or any other such heating
system. In some applications, the heating system 102 operates at a high and
steady
temperature.
[0016] The plastic feedstock 106 can comprise waste plastics of one or more
varieties (e.g., mixed plastics), and may include trace amounts of non-plastic
contamination or impurities. For example, the impurities may be of an external
nature (e.g., water, foodstuffs, labeling, soil, paper, or cellulose waste) or
may result
from internal amendments (e.g., glass, metal, iron, bromine, and/or chlorine).
The
2
CA 02794932 2012-09-28
WO 2011/123145 PCT/US2010/040219
plastic feedstock 106 may be provided in a ground, chipped, or other form that
can
promote the transfer of heat thereto.
[0017]The heat 104 provided by the heating system 102 can be sufficient to
crack or
depolymerize the plastic feedstock 106 and convert at least a portion thereof
into a
vapor 108. The vapor 108 can include one or more gaseous organic species, one
or
more gaseous inorganic species, and/or one or more varieties of entrained
particles.
In particular, the vapor 108 can include depolymerized non-polar organic
gases,
which may be desirable for collection and refinement, and which can be mixed
with
impurities. The organic gases can include, for example, one or more paraffins,
olefins, naphthenes, aromatics, and/or other classes of hydrocarbon materials.
The
mixed-in impurities can include, for example, inorganic acids (e.g.,
hydrochloric acid,
hydrobromic acid), entrained metals or metalloids (e.g., cadmium, iron,
antimony);
and/or organic acids (e.g., terephthalic acid). In some embodiments, the vapor
108
may include additional molecular species, such as polar organic molecules,
which
may or may not be collected with the non-polar organic molecules. For example,
the
vapor 108 can include one or more alcohols, ketones, ethers, phenols,
carboxylic
acids, or other polar organic molecules.
[0018] In some embodiments, the plastic feedstock 106 may be heated under
vacuum conditions, or under negative pressure. In other embodiments, the
plastic
feedstock 106 may be heated under positive pressure. In still other or further
embodiments, the plastic feedstock 106 may be heated under atmospheric
pressure
conditions, or under any suitable combination of the foregoing (e.g., the
pressure
may be varied during a heating event).
[0019] The vapor 108 can be delivered to a vapor treatment system 110 that
effects a phase change of at least a portion of the vapor 108 such that
certain
molecules transition from a gaseous state to a liquid state. The vapor
treatment
system 110 may also be referred to as a vapor treatment unit or a vapor
treatment
vessel. The illustrated vapor treatment system 110 includes a pH adjusted
solution
112 that is used to effect the condensation. Moreover, the pH adjusted
solution 112
can be configured to absorb at least a portion of the impurities from the
vapor 108.
Embodiments of the solution 112 can readily absorb organic acids, inorganic
acids,
metals, metalloids, and/or certain polar organic molecules. The term "pH
adjusted
solution" is used in a broad sense and includes solutions that are not pH
neutral and
that exhibit any or all of the various properties described herein. For
example, a pH
3
CA 02794932 2012-09-28
WO 2011/123145 PCT/US2010/040219
adjusted solution can be formulated to remove impurities from the vapor 108,
and in
further embodiments, can be immiscible with condensed oils so as to be readily
separated therefrom. For example, in some embodiments, the pH adjusted
solution
112 can comprise an acidic solution, which may, in some cases, be strongly
acidic.
In further embodiments, the pH adjusted solution 112 can comprise a buffered
aqueous solution adjusted to a desired pH value. In various embodiment, the pH
adjusted solution 112 can have a pH value that is less than 7, less than about
6.5,
less than about 6, less than about 5.5, less than about 5, less than about 4,
or less
than about 3.
[0020] The pH adjusted solution 112 can include one or more chemical
amendments of any suitable variety to achieve the desired properties of the
solution.
Such properties can include, for example, the ability to remove one or more
impurities from the vapor 108 and/or a high immiscibility with oil. Adjustment
or
optimization of one or more of foregoing properties may be achieved by
altering the
concentration of the one or more chemical amendments within the pH adjusted
solution 112. For example, the presence, combination, and/or concentration of
one
or more materials within the pH adjusted solution 112 can optimize removal of
contaminants from the vapor 108 as it interacts with the pH adjusted solution
112. In
various embodiments, the pH adjusted solution can include strong and/or weak
inorganic acids (e.g. hydrochloric acid, acetic acid), one or more pH buffer
solutions
(e.g., acetic acid + sodium acetate), one or more chelating agents (e.g.,
ethylenediaminetetraacetic acid (EDTA)), and/or one or more coagulants and/or
flocculants (e.g. calcium hydroxide, polyacrylamide).
[0021] The vapor treatment system 110 can be configured to effect direct
contact between the vapor 108 received therein and the pH adjusted solution
112, as
depicted at the broken arrow 114. For example, as further discussed below, in
some
embodiments, the pH adjusted solution 112 may be sprayed into contact with the
vapor 108, whereas in other embodiments, the vapor 108 may be bubbled through
the solution 112. The pH adjusted solution 112 can absorb or dissolve portions
of
the vapor 112 (e.g., organic acids, inorganic acids, metals, metalloids,
and/or certain
polar organic molecules). The pH adjusted solution 112 also can be provided at
a
lower temperature than that of the vapor 108 such that the solution 112
condenses
at least those portions of the vapor 112 that are immiscible therein (e.g.,
non-polar
organic molecules).
4
CA 02794932 2012-09-28
WO 2011/123145 PCT/US2010/040219
[0022] Those portions of the condensed vapor 108 that are immiscible in the pH
adjusted solution 112 (i.e., the hydrophobic portions) can be readily
separated from
the solution 112, as indicated at the broken arrows 116. In some embodiments,
the
separation (or at least one or more stages thereof) takes place within the
vapor
treatment system 110, whereas in other embodiments, the separation (or at
least
one or more stages thereof) takes place within a separator that is independent
of the
vapor treatment system 110 (see, e.g., FIG. 2).
[0023] In some embodiments, the immiscible portions are removed from the
vapor treatment system 110 as a form of crude oil 118. The crude oil 118 thus
can
have few or no impurities, as the impurities that were present in the plastic
feedstock
106 are dissolved or absorbed into the pH adjusted solution 112. In some
embodiments, at least some of the dissolved or absorbed impurities can remain
within the pH adjusted solution 112 within the vapor treatment system 110. For
example, in some instances, after the pH adjusted solution 112 has amassed the
impurities, it may continue to be used within the vapor treatment system 110,
such
that the impurities are not removed (at least not immediately) from the vapor
treatment system 110. In other or further embodiments, dissolved or absorbed
impurities are removed from the vapor treatment system 110 separately from the
oil
118, as shown at the arrow 120.
[0024] Certain classes of polar organic molecules may only partially (or at
least
partially) partition into the pH adjusted solution 112. For example, a portion
of
certain alcohols, ketones, ethers, phenols, carboxylic acids, and/or other
polar
organic molecules may partition into the pH adjusted solution 112 and another
portion thereof may partition into the crude oil 118. Accordingly, in some
embodiments, crude oil 118 that includes a portion of a species of polar
organic
molecules may be separated from a pH adjusted solution 112 that contains
another
portion of the species of polar organic molecules.
[0025] The vapor 108 may include portions that do not condense within the
vapor
treatment system 110 and are not absorbed by the pH adjusted solution 112.
Such
non-condensable gases 121 can be removed separately from the vapor treatment
system 110, and may be combusted or disposed of in any other suitable manner.
[0026] In various embodiments, the vapor treatment system 110 may operate
under vacuum conditions, or under negative pressure. In other embodiments, the
vapor treatment system 110 may operate under positive pressure. In still other
or
CA 02794932 2012-09-28
WO 2011/123145 PCT/US2010/040219
further embodiments, the vapor treatment system 110 may operate under
atmospheric pressure conditions, or under any suitable combination of the
foregoing
(e.g., the pressure may be varied during a condensing event).
[0027] The system 100 can be well suited for quickly cracking or
depolymerizing
the plastic feedstock 106. For example, in some embodiments, heating of the
plastic
feedstock 106 and conversion thereof into the vapor 108 can be performed at
high
temperatures at which a variety of different molecular species may be gasified
simultaneously. Such different molecular species might have different
vaporization
temperatures at a given pressure, and a temperature at which the plastic
feedstock
106 is heated can exceed this temperature for some or all of the molecular
species.
The molecular species can then be separated from each other when the vapor 108
is
delivered to the vapor treatment system 110, as previously described.
Accordingly,
the system 100 can operate without the heating system 102 slowly heating up
and
occasionally holding steady at various discreet temperature levels along the
way so
as to allow for individual molecular species to be gasified sequentially. It
is to be
appreciated, however, the system 100 may also be used in an operational mode
in
which the heating system 102 and the plastic feedstock 106 progress through a
series of sequential heating steps or levels, as just described.
[0028] FIG. 2 depicts a process flow diagram of another embodiment of a
plastic
recycling system 100, which resembles the system 100. The system 100 includes
a
heating system 102 that provides heat 104 to a plastic feedstock 106. The
plastic
recycling system 100 further comprises a sealable container 122 that retains
the
plastic feedstock 106 during the heating. The container 122 can be configured
to
hold a negative pressure therein.
[0029] The system 100 can include a vacuum system 124 that is configured to
maintain a negative pressure within the container 122 and within a vapor
treatment
system 110. The vacuum system 124 can continuously evacuate gases from the
container 122 such that depolymerization of the plastic feedstock 106 occurs
in an
oxygen-deprived or oxygen-free environment. The vacuum system 124 draws the
vapor 108 into the vapor treatment system 110, where it is contacted by the pH
adjusted solution 112. The vacuum system 124 draws the non-condensable gases
121 from the vapor treatment system 110, and may distribute them to a
combustion
unit or other suitable disposal device.
6
CA 02794932 2012-09-28
WO 2011/123145 PCT/US2010/040219
[0030] The system 100 includes a separator 128 that receives an emulsion 126
of condensed material from the vapor treatment system 110. The emulsion 126
can
comprise crude oil 118 that includes a small amount of the pH adjusted
solution 112
entrained therein. The separator 128 can be configured to separate the crude
oil
118 from the pH adjusted solution 112 based on the difference in relative
density
between these materials. For example, the separator 128 can comprise a
settling
tank that allows gravitational separation of the solution 112 from the crude
oil 112. In
other embodiments, the separator 128 may comprise a centrifuge.
[0031] FIG. 3 illustrates another embodiment of a plastic recycling system
200,
which can resemble the plastic recycling systems 100, 100 described above in
certain respects. Accordingly, like features are designated with like
reference
numerals, with the leading digits incremented to "2." For example, the plastic
recycling system 200 includes a heating system 202, a sealable container 222,
a
vapor treatment system 210, a vacuum system 224, and a separator 228.
Likewise,
the vapor treatment system 210 includes a pH adjusted solution 212 such as the
pH
adjusted solution 112 described above. Relevant disclosure set forth above
regarding similarly identified features thus may not be repeated hereafter.
[0032] The illustrated heating system 202 includes a burner 230 and a heating
plenum 232. The burner 230 can comprise any suitable combustion burner, which
may be configured to run on any suitable fuel. The fuel may be supplied by a
fuel
train 234, such as natural gas or propane piping. The fuel train 234 can
include any
suitable combination of flow switches and valves (not shown) to allow for the
desired
amount of fuel to be delivered to the burner 230. Fuel from the fuel train 234
can be
delivered via any suitable fuel delivery line 235, such as conduit or piping.
[0033] The burner 230 can be in fluid communication with a combustion blower
236 and a circulation fan 237, each of which may have variable speed
capabilities.
The combustion blower 236 supplies fresh air to the burner 230, whereas the
circulation fan 237 circulates heated exhaust from the heating plenum 232 back
to
the burner 230. The circulation fan 237 also can selectively draw in fresh air
to
provide a desired exhaust/air mixture to the burner 230. The heating system
202
can comprise any suitable arrangement of ducts 238 for transporting air from
one
portion of the heating system 202 to another.
[0034] The heating plenum 232 is configured to selectively receive therein the
sealable container 222, which may also be referred to as a cartridge. When
situated
7
CA 02794932 2012-09-28
WO 2011/123145 PCT/US2010/040219
within the heating plenum 232, the container 222 can seal, or substantially
seal, the
plenum such that heated air may circulate within the heated plenum but not
escape
into the surrounding atmosphere. Illustrative examples of heating plenums 232
and
containers 222 that may be used with the plastic recycling system 200 are
disclosed
in U.S. Patent Application No. 12/751,911, filed March 31, 2010, titled
DEVICES,
SYSTEMS, AND METHODS FOR RECYCLING PLASTIC.
[0035] The container 222 can retain a quantity of plastic feedstock 206
therein,
which can be melted and vaporized as a result heat delivered thereto by the
heating
plenum 232, as further discussed hereafter. In the illustrated embodiment, the
heating plenum 232 includes a sidewall 240 and an inner conduit 242, which
rises
upwardly at an interior of the sidewall 240. The sidewall 240 can include a
series of
ports (not shown) through which heated air can be delivered inwardly (e.g.,
toward
the inner conduit 242), and the inner conduit 242 can include a series of
ports 243
through which heated air can be delivered outwardly (e.g., toward the sidewall
240).
[0036] The sidewall 240 can be in fluid communication with a first duct line
238
from the burner 230, and the inner conduit 242 can be in fluid communication
with a
second duct line 238 from the burner 230. A damper 244 can be situated
relative to
the first and second duct lines 238 so as to control the relative amount of
heated air
that is delivered to the sidewall 240 and to the inner conduit 242.
[0037] Heated exhaust that has been used to heat the container 222 can be
removed from the heating plenum 232 via a separate duct line 238 and
circulated to
the burner 230 by the fan 237. At least a portion of the heated exhaust may be
diverted and delivered to a heat exchanger 246 by opening a valve 248 and
activating a venting fan 249. The heat exchanger 248 is described further
below.
[0038] Although the illustrated embodiment of the heating system 202 uses
heated air to heat the container 222 and its contents, it is to be appreciated
that any
other or further suitable mechanisms for heating the container 222 and its
contents
are also possible. For example, a heated fluid other than air (e.g., a heated
liquid)
may be circulated through the heating plenum 232. In other or further
embodiments,
heating mechanisms may include electrical direct contact heating, induction
heating,
or radiant heating.
[0039] The heating system 202 can further include a variety of sensor and
control
components. For example, the heating system 202 can include one or more
pressure sensors 250 and/or temperature sensors 252, which can provide to a
8
CA 02794932 2012-09-28
WO 2011/123145 PCT/US2010/040219
subsystem controller 254 various data regarding the operation of the burner
230 and
the amount of heat being delivered to the container 222. The sensors 250, 252
can
communicate with the subsystem controller 254 in any suitable manner, such as
by
wired or wireless connection. In the illustrated embodiment, communication
lines
(e.g., electrical wires) connect the sensors 250, 252 to the subsystem
controller 254.
Likewise, communication lines can connect other components of the heating
system
202 (e.g., the blower 236 and the fan 237) to the subsystem controller 254.
The
communication lines are not shown in FIG. 3 so as not to obscure the drawing.
[0040] The subsystem controller 254 can alter operational parameters of the
heating system 202 in response to data received from the sensors 250, 252,
and/or
as a result of other programming. For example, if it is determined from
temperature
sensors 252 that are associated with the inner conduit 242 and the sidewall
240 that
the temperature of heated air being delivered to an inner portion of the
container 222
is deficient, the control system 254 can compensate by changing a setting of
the
damper 244 so that more heat is delivered to the inner conduit 242 and less is
delivered to the sidewall 240. In some applications, it may be desirable to
selectively
alter the relative amounts of heat delivered to a peripheral region and a
central
region of the container 222 over the course of a heating cycle. In some
instances, if
it is determined that all temperatures throughout the heating plenum 232 are
too low,
the control system 254 may increase a speed of the blower 236 and/or the fan
237.
In other or further embodiments, any desired alteration to the operational
parameters
of the heating system 202 may be effected manually.
[0041] A master control system 255 may be configured to communicate with the
subsystem controller 254 (e.g., via an Ethernet cable or other suitable
communication device, whether wired or wireless), and may also be configured
to
communicate with additional subsystem controllers 256, 258, etc. that are each
dedicated to other subsystems of the plastic recycling system 200. For
example,
separate subsystem controllers 256, 258 may be dedicated to the vapor
treatment
system 210 and the vacuum system 224, respectively. In some embodiments, the
subsystem controllers 254, 256, 258 are situated locally (e.g., near the
various
subsystems with which they are associated), whereas the master control system
255
may be situated in a supervisory station in which an operator can monitor the
instantaneous status of the multiple subsystems of the system 200 and can make
changes thereto as desired, whether onsite or offsite.
9
CA 02794932 2012-09-28
WO 2011/123145 PCT/US2010/040219
[0042] For the sake of convenience, the subsystem controller 254, 256, 258
associated with a particular component may not be identified hereafter, nor
will it be
explicitly stated that a particular subsystem controller 254, 256, 258 and/or
the
master control system 255 is able to monitor and/or control the operation of a
particular component of the plastic recycling system 200, although such is
understood. It is also noted that steps or control events discussed herein
which can
be effected the controllers 254, 256, 258 and/or the master control system 255
may
be embodied in machine-executable instructions that are to be executed by a
general-purpose or special-purpose computer (or other electronic device).
Alternatively, the steps or control events may be performed or instigated by
hardware components that include specific logic for performing the steps or
control
events, or by a combination of hardware, software, and/or firmware. Some or
all of
the steps may be performed locally (e.g., via a subsystem controller) or
remotely
(e.g., via the master control system 255).
[0043] As previously discussed, the sealable container 222 may be selectively
coupled with the heating plenum 232 or removed therefrom. In some embodiments,
the container 222 is positioned externally to the heating plenum 232 for
filling. A lid
260 is removed or otherwise opened to permit entry of the plastic feedstock
206.
Once the container 222 has been filled, the lid 260 can then be closed and the
container 222 can be hoisted (e.g., via a crane) into the heating plenum 232.
The
container 222 can cooperate with the heating plenum 232 to prevent heated
exhaust
gases from exiting from the heating plenum 232 at a connection interface
between
the container 222 and the heating plenum 232.
[0044] An evacuation port 261 of the container 222 can be connected to a gas
transfer line 262. The gas transfer line 262 can be in fluid communication
with the
vapor treatment system 210 which, in turn, can be in fluid communication with
the
vacuum system 224. Accordingly, connection of the gas transfer line 262 to the
evacuation port 261 can place the contents of the container 222 under vacuum,
and
the heating of the contents thus may take place in an oxygen-deprived or
oxygen-
free environment.
[0045] As the container 222 is heated, the plastic feedstock 206 can melt and
eventually gasify or vaporize. The resultant vapor 208 is drawn from the
container
222 through the evacuation port 261, through the gas transfer line 262, and
then into
the vapor treatment system 210. In certain embodiments, the gas transfer line
262
CA 02794932 2012-09-28
WO 2011/123145 PCT/US2010/040219
includes a knock out tank 264, which can have a volume greater than that
schematically depicted in FIG. 3. The knock out tank 264 can comprise a
container
that acts as a failsafe in the event that the contents of the container 222
are rapidly
forced through the evacuation port 261, which could result from a large
pressure
fluctuation within the container 222 (e.g., due to an undesired ignition of
gases).
Such expelled contents can be collected in the knock out tank 264 and
prevented
from entering the vapor treatment system 210. In other embodiments, the knock
out
tank 264 is omitted (see FIG. 7).
[0046] A pressure sensor 266 and a temperature sensor 268 can be positioned in
the gas transfer line 262 to monitor the pressure and temperature of the vapor
208
as it exits the container 222. In some embodiments, one or more of the
temperature
and pressure readings can be used to determine when vaporization of the
plastic
feedstock 206 is at or near completion such that the container 222 is ready to
be
removed from the heating plenum 232 and replaced with a filled container 222.
For
example, in some embodiments, a heating temperature within the heating plenum
232 can be maintained at a substantially constant level or set point value
(e.g., at
about 1100 degrees Fahrenheit, in some embodiments). A temperature of the
vapor
208 can rise to a maximum level (e.g., a time-averaged maximum level) during
the
heating, and can steadily remain near the maximum level (e.g., can sustain
only
minor fluctuations) as the vaporization continues. As the process nears
completion
and fewer gases are created, the temperature of the vapor 208 exiting the
container
222 can drop. A size of this drop can signal that the container 222 should be
replaced. In various embodiments, a replacement event may be signaled when the
temperature of vapor 208 drops within a range of from about 10 to about 30
percent
of the maximum level, or drops within a range of from about 15 to about 25
percent
of the maximum level. In some embodiments, a replacement event may be signaled
with the temperature drops by an amount equal to or greater than about 15, 20,
25,
or 30 percent. In other or further embodiments, a replacement event may be
signaled when the temperature drops from the maximum level by no less than
about
80, 90, or 100 degrees Fahrenheit. In still other or further embodiments, a
replacement event may be signaled by the passage of no less than about 1/2,
3/4, or
1 hour after the maximum level is reached.
[0047] With reference to FIGS. 3 and 4, the vapor 208 can be introduced into
the
vapor treatment system 210 in any suitable manner. In the illustrated
embodiment,
11
CA 02794932 2012-09-28
WO 2011/123145 PCT/US2010/040219
the vapor treatment system 210 includes a condenser 270 and a reservoir 286.
As
shown in FIG. 3, in some embodiments, the vapor 208 is introduced into a
condensing tower 271 of the condenser 270 substantially without altering a
trajectory
of the vapor 208. As shown in FIG. 4, in other embodiments, the vapor 208
encounters a baffle 272 upon entering the condensing tower 271. The vapor 208
can initially hit a guard plate 274, and can then be routed downward and
outwardly
about a bottom edge 276 of the baffle 272. The baffle 272 can be configured to
provide the vapor 208 with an even flow pattern. In particular, the baffle 272
can
cause the vapor 208 to rise in a uniform distribution about a periphery of the
bottom
edge 276.
[0048] FIG. 4 also illustrates that the gas transfer line 262 may be angled
upwardly toward the condensing tower 271. Such an arrangement can permit any
condensed material that may collect in the gas transfer line 262 to flow down
the line
262 and into the knock out tank 264 (FIG. 3). This may be of a particular
benefit
when the plastic recycling system 200 is shut down and hot vapor 208 no longer
flows through the gas transfer line 262 so as to keep the line clear. For
embodiments that do not include a knock out tank 264, the condensed material
may
instead flow down the line 262 to any suitable fluid collection point.
[0049] With continued reference to FIG. 3, the condenser 270 can include a
lower
sprayer 280 and an upper sprayer 282 that are separated from each other by one
or
more sections of column packing 284. For example, three sections of column
packing 284 may separate the upper and lower sprayers 282, 280. Each of the
upper and lower sprayers 282, 280 provides a spray or shower of the pH
adjusted
solution 212, but at different temperatures. The lower sprayer 282 can provide
a
spray at a higher temperature (e.g., a warm temperature, such as, for example,
within a range of from about 120 to about 150 degrees Fahrenheit), whereas the
upper sprayer 280 can provide a spray at a lower temperature (e.g., a cool
temperature, such as, for example, within a range of from about 70 to about 80
degrees Fahrenheit).
[0050] The lower sprayer 282 may be used primarily as a cleaning device for
removing impurities from the vapor 208. A temperature of the lower sprayer 282
may be sufficiently high to permit the pH adjusted solution 212 to dissolve or
absorb
portions of the vapor 208 substantially without condensing any other portion
of the
vapor 208. The sprayed pH adjusted solution 212 can drop into a reservoir 286,
12
CA 02794932 2012-09-28
WO 2011/123145 PCT/US2010/040219
which is discussed further below. Accordingly, the lower sprayer 282 and/or
the
reservoir 286 may be used to separate impurities from the vapor 208, and thus
may
be referred to individually or collectively herein as a washing system or
cleaning
system 287.
[0051] After passing through the lower sprayer 282, the remaining portion of
the
vapor 208 passes upwardly through the column packing 284 and loses energy
thereto. The vapor 208 then encounters the lower temperature pH adjusted
solution
212 that is sprayed from the upper sprayer 282. At least a portion of the
remaining
vapor 208 is thus condensed and drops into the reservoir 286. The pH adjusted
solution 212 that is sprayed from the upper sprayer 282 thus may also be
referred to
as a condensing liquid.
[0052] Those portions of the vapor 208 that are not condensed (i.e., non-
condensable gases) are then passed to a caustic scrubber 290, which passes the
remaining vapor 208 through a caustic solution so as to chemically scrub the
vapor
(e.g., remove mercaptan sulfur species therefrom) and so as to neutralize
trace
levels of free inorganic acids. The remainder of the vapor 208 passes from the
caustic scrubber 290 through the vacuum system 224, and is then pushed to an
environmental control device 292.
[0053] Any suitable vacuum system 224 may be used with the plastic recycling
system 200. One illustrative embodiment is depicted in FIG. 5. The vacuum
system
224 includes a first blower 294 and a second blower 296 that are in parallel
with
each other. One or more valves 298 may be included in parallel with the
blowers
294, 296. In such an arrangement, both blowers 294, 296 may be used at startup
of
the plastic recycling system 200 in order to place the vapor treatment system
210
and the container 222 under vacuum, whereas only one blower 294, 296 may be
used once the recycling system 200 is operational. The vacuum system 224 may
cycle between use of the blowers 294, 296 to keep their usage times
approximately
equal. Moreover, the valve 298 may be maintained in a slightly open or vented
configuration, which can result in a relatively uniform vacuum during a
transition
between blowers 294, 296, as well as during sustained operation of either
blower
294, 296.
[0054] In some instances, it can be desirable to maintain a vacuum in the
container 222, the vapor treatment system 210, and the caustic scrubber 290
during
operation of the system 200. For example, in some embodiments, the vacuum
13
CA 02794932 2012-09-28
WO 2011/123145 PCT/US2010/040219
system 224 (which may more generally be referred to as a pressure system)
provides a magnitude of negative pressure that is sufficiently great to
maintain a
vacuum within the container 222, as well as other portions of the system 200
that are
in fluid communication therewith, even if spikes of positive pressure occur as
the
plastic feedstock 206 is being vaporized. In some embodiments, the vacuum
system
224 maintains a negative pressure that has a magnitude of no less than about
8, 10,
or 12 inches of water column.
[0055] In other embodiments, a pressure system may provide a positive pressure
within the container 222, the vapor treatment system 210, and the caustic
scrubber
290. In still other embodiments, a pressure system (e.g., the vacuum system
224)
may be omitted from or not used in the plastic recycling system 200. For
example,
atmospheric pressure conditions may be maintained within the container 222,
the
vapor treatment system 210, and the caustic scrubber 290. Providing higher
pressures to the plastic recycling system 200 can cause the vapor 208 to be
heated
for longer periods within the container 222, which can result in greater
depyrolization
and lighter organic molecules within the vapor 208. Under such conditions,
more
fuel from the fuel train 234 may be consumed to provide the vapor 208 with
sufficient
energy to pass through the system 210.
[0056] Any suitable environmental control device 292 can be used with the
plastic
recycling system 200. In some embodiments, the environmental control device
292
can comprise a burner or other combustion device. For example, in some
embodiments, the environmental control device 292 can comprise a CEB clean
enclosed burner, which is available from N.V. Bekaert S.A., of Kortrijk,
Belgium. In
the illustrated embodiment, exhaust from the environmental control device 292
is
shown as being vented to atmosphere. In other embodiments, the hot exhaust may
instead be transferred to other portions of the plastic recycling system 200.
For
example, in some embodiments, exhaust from the environmental control device
292
can be delivered to the heating system 202 and may be used to heat the
container
222.
[0057] Referring again to the reservoir 286, the absorbed and condensed
portions
of the vapor 208 drop into a tank 300 that includes a weir 302 at an upper
edge
thereof. The pH adjusted solution 212, which retains the absorbed impurities,
may
facilitate coagulation of some contaminants which have a greater relative
density
than the condensed crude oil material, and may settle to the bottom of the
tank 300.
14
CA 02794932 2012-09-28
WO 2011/123145 PCT/US2010/040219
Accordingly, the condensed crude oil rises to the top of the tank 300 and
flows over
the weir 302 into a temporary containment region 304. At this stage, the crude
oil
may be slightly emulsified with the pH adjusted solution 212 (e.g., may have a
small
quantity of pH adjusted solution 212 entrained therein), and thus this
material that
consists primarily of crude oil may be referred to as an emulsion 226. As
discussed
further below, the emulsion 226 can be removed from the containment region 304
and delivered to a separator or settling tank 228.
[0058] The pH adjusted solution 212 within the tank 300 can be cycled to the
lower and upper sprayers 280, 282. In particular, a circulation pump 310 can
move
solution 212 from the tank 300 through a fluid line 312 to each of the
sprayers 280,
282. The pressure and temperature at the lower and upper sprayers 280, 282 can
be monitored by separate pressure sensors 314, 318 and separate temperature
sensors 316, 320, respectively. A portion of the fluid line 312 can pass
through a
heat exchanger 322, which is coupled to a cooling system 324 through a cooling
loop 326. The cooling system 324 can be of any suitable variety, and may
include a
cooling tower and/or a chiller. A cooling line pump 328 can control a flow of
cooling
fluid through the cooling loop 326. Pressure and temperature at each of the
sprayers 280, 282 can be controlled by adjusting one or more settings of the
circulation pump 310, the cooling line pump 328, the cooling system 324,
and/or a
valve 330 associated with the lower sprayer 280 and a valve 332 associated
with the
upper sprayer 332.
[0059] As previously discussed, in some embodiments, it is desirable to
maintain
the lower sprayer 280 at a temperature that is within a range of from about
120 to
about 150 degrees Fahrenheit. Such a temperature range can be too high to
effect
condensation of organic molecular species that are within the vapor 208, and
may
also facilitate absorption of impurities from the vapor 208. Additionally,
crude oil that
collects in the tank 300 and in the containment region 304 can be in a liquid
or free
flowing state within it is within such a temperature range, or when it is at a
temperature slightly above such a range. Accordingly, when the solution 212
that is
ejected from the lower sprayer 280 is within such a temperature range, the
solution
212 and the emulsion 226 that are in the reservoir 286 can be maintained at a
temperature that is within this temperature range, or that is higher than this
range,
due to absorption of heat from the incoming vapor 208.
CA 02794932 2012-09-28
WO 2011/123145 PCT/US2010/040219
[0060] In some embodiments, it is desirable to maintain the upper sprayer 282
at
a temperature that is within a range of from about 70 to about 80 degrees
Fahrenheit. For example, such a temperature range can be well suited for
condensing organic molecules. Additionally, it can be desirable for certain
gases
exiting the vapor treatment system 210 (e.g., methane, ethane, propane, and/or
butane) to remain in a gaseous state so that they may be burned more readily
in the
environmental control device 292. Accordingly, data obtained by a temperature
sensor 340 that is at an exit port of the vapor treatment system 210 can be
used in
adjusting a temperature of the upper sprayer 282 to a desired value.
[0061] In other embodiments, the vapor treatment system 210 may include a
single sprayer, such as the upper sprayer 282. The single sprayer 282 can
simultaneously effect both absorption of impurities and condensation of the
crude oil.
In certain of such embodiments, it may be desirable to add to or alter certain
separation steps discussed below, since the resultant emulsion 226 may include
a
greater number of impurities (e.g., due to a greater degree of entrainment of
the pH
adjusted solution 212 and/or dissolved or free impurities within the crude oil
218).
For example, it may be desirable to permit the emulsion 226 to settle within
the
settling tank 228 for a longer period of time.
[0062] As the pH adjusted solution 212 absorbs impurities from the vapor 208,
a
composition of the solution can change. For example, in some embodiments, the
pH
adjusted solution 212 may become more or less acidic. Accordingly, in some
embodiments, a pH sensor 342 can be included within the tank 300 to monitor
the
composition of the solution 212. The fluid line 312 can include an pH
adjustment
port 344 through which an acid or other suitable material may be added to the
solution 212 that is circulated from the reservoir 286. For example, an acid
may be
introduced into the fluid line via the pH adjustment port 344 when it is
determined
from the pH sensor 342 that the acidity of the solution 212 has dropped. Water
from
a makeup water source 345 may be added to the fluid line 312. For example, if
an
acidic level of the pH adjusted solution 212 that is within the reservoir 286
has
increased beyond an upper threshold, water from the makeup water source 345
may
be added to the fluid line 312.
[0063] In some embodiments, a temperature sensor 350 is included in the
reservoir 286 (e.g., within the tank 300) to ensure that the temperature of
the
reservoir 286 does not drop below a desired level. For example, as previously
16
CA 02794932 2012-09-28
WO 2011/123145 PCT/US2010/040219
discussed, it may be desirable to maintain the temperature within the
reservoir 286
at a level at which the oil or emulsion 226 is in a liquid or flowable state.
A heating
element 352 may be used in conjunction with the temperature sensor 350 to keep
the oil and water within the reservoir 286 warm, such as during periods of
shutdown.
[0064] In certain embodiments, the reservoir 286 includes a level sensor 354
to
monitor a level of the oil or emulsion 226 and includes another level sensor
356 to
monitor a level of the pH adjusted solution 212. Data obtained from the level
sensor
354 can be used in controlling a pump 360, which is configured to pump
emulsion
226 from the temporary containment region 354 to the settling tank 228. The
pump
360 may comprise any suitable variety of pump, and may be well suited for
pumping
thick material which may be highly viscous. For example, the pump 360 may
comprise a positive displacement pump.
[0065] The emulsion 226 can be pumped through a heat traced line 361 into a
separation tank 362 contained within the settling tank 228. The separation
tank 362
can include angled sidewalls 363 and an entry baffle 364. The separation tank
362
can encourage further separation of the crude oil 218 from the pH adjusted
solution
212, such as by separation due to relative densities. The crude oil 218 can
flow over
an upper edge of the separation tank 362 into a holding area 366. The crude
oil 218
can removed from the holding area 366 and stored or transported as desired.
For
example, the oil 218 can be moved to a storage tank or transported to a
refinery
(e.g., via an oil tanker).
[0066] The crude oil 218 may be relatively waxy and solid at room temperature.
Accordingly, it may be desirable to maintain the crude oil 218 in a liquid
form to
facilitate separation of the solution 212 therefrom and/or removal of the oil
218 from
the settling tank 228. The settling tank thus may include a temperature sensor
369,
which can be used to selectively activate a heating fluid loop 370 that is in
communication with the heat exchanger 248. A heating line pump 372 can control
the flow of heating fluid through the heating fluid loop 370.
[0067] Removal of the crude oil 218 from the settling tank 228 is illustrated
by the
arrow 374. The pH adjusted solution 212 likewise can be removed from the
settling
tank 228, as indicated by the arrow 376. In some embodiments, the pH adjusted
solution 212 is returned to the reservoir 286, and may thereafter be cycled
through
the sprayers 280, 282.
17
CA 02794932 2012-09-28
WO 2011/123145 PCT/US2010/040219
[0068] With continued reference to FIG. 3, it is again noted that during
operation
of the heating system 202 and the container 222, heated air is circulated
within the
heating plenum 232 so as to melt the plastic feedstock 206 and convert it into
one or
more gases within the vapor 208. A vacuum is applied via the evacuation port
261
so as to remove the vapor 208 from the container 222. The removed gases can
then
be processed as desired.
[0069] Upon removal of all or substantially all of the desired gases from the
container 222, the container 222 can be removed from the heating plenum 232
and
replaced with an additional container 222 that has been charged with a
quantity of
the plastic feedstock 206. The foregoing heating a vapor removal processes can
then be repeated, and the removed container 222 can be cleaned and charged
with
plastic feedstock 206 for future use. The successive coupling, heating,
removal, and
replacement of a series of charged containers 222 for a single heating plenum
232
can be referred to as a batch process.
[0070] FIG. 6 illustrates another embodiment of the plastic recycling system
200
in which plastic feedstock may be vaporized and processed in a manner that is
referred to herein as a continuous batch process. The plastic recycling system
200
can be identical to the embodiments described above with respect to FIG. 3,
except
that the system 200 includes four separate heating systems 202A, 2026, 202C,
202D, each with a separate burner 230A, 2306, 230C, 230D that is configured to
heat a separate heating plenum 232A, 2326, 232C, 232D. Although not shown in
FIG. 6, an optional exhaust path from each of the heating plenums 232A, 2326,
232C, 232D can be provided to the heat exchanger 248 (see FIG. 3).
[0071] Each heating plenum 232A, 2326, 232C, 232D is configured to receive a
separate container 222A, 2226, 222C, 222D therein, and each container can be
filled with a separate quantity of plastic feedstock 206A, 2066, 206C, 206D.
As
illustrated in FIG. 6, a first container 222A can be inserted in a first
heating plenum
232A and heated for a first period of time; a second container 222B can be
inserted
in a second heating plenum 232B at the end of the first period, and both the
first and
second containers 222A, 222B can be heated for a second period; a third
container
222C can be inserted in a third heating plenum 232C at the end of the second
period, and the first, second, and third containers 222A, 2226, 222C can be
heated
for a third period; and a fourth container 222D can be inserted in a fourth
heating
18
CA 02794932 2012-09-28
WO 2011/123145 PCT/US2010/040219
plenum 232D at the end of the third period, and the first, second, third, and
fourth
containers 222A, 222B, 222C, 222D can be heated for a fourth period.
[0072] FIG. 6 illustrates a point in time at the end of the fourth period of
heating
time. As shown, nearly all of the plastic feedstock 206A has been vaporized
and
removed from the container 222A, such that only solid carbon material (e.g.,
non-
hydrocarbon product) remains. By comparison, all of the plastic feedstock 206B
has
been melted within the second container 222B, and a portion thereof has been
vaporized and removed; all of the plastic feedstock 206C has been melted
within the
third container 222C, but relatively little has been vaporized and removed;
and only
some of the plastic feedstock 206D has been melted within the fourth container
222D.
[0073] At this point in time, a filled fifth container 222E can be positioned
near the
first heating plenum 232A. The first container 222A can then be removed from
the
first heating plenum 232A and the fifth container 222E can be introduced into
the first
heating plenum 232A in its place. The fifth, second, third, and fourth
containers
222E, 222B, 222C, 222D can then be heated for a fifth period of time.
Replacement
of a single container 222 at the end of a heating period can continue in
series for
each of the second, third, and fourth containers 222B, 222C, 222D,
respectively, and
can cycle through to the fifth heating plenum 222E.
[0074] As shown in FIG. 6, each of the containers 222A, 222B, 222C, 222D can
be connected to the gas transfer line 262, which can supply a negative
pressure via
the vacuum system 224 (FIG. 3), as described above. Vapors, such as the vapors
208C, 208D, thus can be mixed within the gas transfer line 262 as they are
delivered
to the vapor treatment system 210. Such an arrangement thus can be relatively
insensitive to the species of molecules that are contained within a vapor 208
from
any given container 222. This insensitivity to molecular species can be
particularly
useful for a continuous batch mode operation, since the vaporization process
can
proceed without careful coordination among the various heating systems 202A,
202B, 202C, 202D to ensure that only a single molecular species is drawn
simultaneously from the containers 222A, 222B, 222C, 222D. Stated otherwise,
the
recycling system 200 can operate properly, even when a variety of different
molecular species are introduced into the vapor 206 of any given container 222
and/or even when a variety of different molecular species are introduced into
the
common gas transfer line 262 from multiple containers 222.
19
CA 02794932 2012-09-28
WO 2011/123145 PCT/US2010/040219
[0075] The gas transfer line 262 can include a series of pressure sensors
266A,
2666, 266C, 266D and/or temperature sensors 268A, 2686, 268C, 268D, which can
be used in determining whether a container 222 is ready to be replaced, as
discussed above. In other or further embodiments, the gas transfer line 262
can
include a series of valves 384, which may be manipulated so as to permit
removal of
a container 222 without interrupting vapor collection from the remaining
containers
222. In some embodiments, the recycling system 200 includes an inert gas purge
system 380 that can be used to flush any portion of the gas transfer line 262.
The
inert gas purge system 380 can include a series of valves 382, which may be
manipulated appropriately to introduce an inert gas where desired. In some
instances, nitrogen or some other inert gas may be used to purge a full
section of the
gas transfer line 262 from process gases before removal of a spent container
222
and/or may be used to purge oxygen from (or to dilute oxygen within) a portion
of the
gas transfer line 262 after a new cartridge 222 has been connected thereto.
[0076] Measures may be taken to prevent or reduce heat losses when a
container 222 is removed from its respective plenum 232 and replaced. For
example, in some embodiments, a burner 230 is turned off just prior to removal
of a
container 222 from the associated heating plenum 232, and air can be drawn
down
into the heating plenum 232 as the container 222 is removed and replaced. The
burner 230 can then be activated again once the new container 222 is in place.
[0077] The illustrated embodiment of the recycling system 200 includes four
heating plenums 232. In some instances, a total of eight containers 222 may be
used effectively with such a system, as some spent containers may be cleaned
and
filled while the remaining containers are in use. However, more or fewer
containers
may be used with such a system. Likewise, more or fewer heating plenums may be
used in continuous batch processes.
[0078] FIG. 7 illustrates another embodiment of a plastic recycling system
200,
which is similar to the system 200 illustrated in FIG. 3. Like the system 200,
the
system 200 includes a vacuum system 224 and a caustic scrubber 290. However,
the vacuum system 224 is situated in line with the scrubber 290 between the
vapor
treatment system 210 and the scrubber 290, rather than between the scrubber
290
and the environmental control device 292. The output of the scrubber 290 thus
may
be delivered directly to the environmental control device 292.
CA 02794932 2012-09-28
WO 2011/123145 PCT/US2010/040219
[0079] Other embodiments of the systems 200, 200 may each have multiple
vapor treatment systems 210, which may each receive vapor 208 from one or more
containers 222 within one or more heating systems 202. In arrangements similar
to
that shown in FIG. 7, the outputs of the multiple vapor treatment systems 210
may
be delivered to an input end of the vacuum system 224, and a single output of
the
vacuum 224 may be delivered to the scrubber 290. In arrangements similar to
that
shown in FIG. 3, the outputs of the multiple vapor treatment systems 210 may
each
be delivered to an input end of a separate scrubber 290, such that the plastic
recycling system 200 comprises multiple scrubbers 290. The outputs of the
multiple
scrubbers 290 can be delivered to a single vacuum system 224, and the vacuum
system 224 can have a single output that is in fluid communication with the
environmental control device 292. In still further embodiments, a single
plastic
recycling system 200 or 200 can include multiple vacuum systems 224 and
multiple
scrubbers 290.
[0080] FIG. 8 illustrates another embodiment of a plastic recycling system
400,
which can resemble the plastic recycling systems 200, 200 in many respects.
Accordingly, like features are identified with like reference numerals.
Moreover,
specific features of the recycling system 400 may not be identified by
reference
numerals in the drawings or specifically discussed in the written description
that
follows. However, such features may clearly be the same, or substantially the
same,
as features depicted in other embodiments and/or described with respect to
such
embodiments. Accordingly, the relevant descriptions of such features apply
equally
to the features of the recycling system 400. Any suitable combination of the
features
and variations of the same described with respect to the recycling system 200
can
be employed with the recycling system 400, and vice versa. This pattern of
disclosure applies equally to further embodiments depicted in subsequent
figures
and described hereafter.
[0081] The recycling system 400 includes a heating system 402 that is
configured
to depolymerize a plastic feedstock 206 in a continuous manner. In the
illustrated
embodiment, the heating system 402 comprises a heating unit 431 of any
suitable
variety, such as a fluidized bed burner 432 that is configured to effect rapid
depolymerization of the plastic feedstock 206. Other suitable heating devices
are
also possible. The plastic feedstock 206 can be fed into the heating unit 431
by a
gravity feed hopper 433 or any other suitable feeding mechanism that can
provide a
21
CA 02794932 2012-09-28
WO 2011/123145 PCT/US2010/040219
continuous supply of the plastic feedstock 206 to the fluidized bed burner
432. If
desired, the plastic feedstock may be provided under vacuum or inert gas
conditions.
[0082] FIG. 9 illustrates a portion of another embodiment of a plastic
recycling
system 500, which can be used in such plastic recycling systems as those
described
with respect to FIGS. 3, 7, and 8 (e.g., the recycling systems 200, 200, 400).
Accordingly, like features are identified with like reference numerals. The
system
500 includes a vapor treatment system 510 that includes a washing system or
cleaning system 587 and a condenser 570. The cleaning system 587 includes a
cleaning tank 585 that has a quantity of the pH adjusted solution 212 therein.
The
condenser 570 includes a reservoir 586 that resembles the reservoir 286
described
above and contains a condensing liquid 213.
[0083] A vapor 208 can be introduced into the cleaning system 587 from a gas
transfer line 262. The vapor 208 is placed into direct contact with the pH
adjusted
solution 212 within the cleaning tank 585. For example the vapor 208 can be
bubbled through the pH adjusted solution 212. In this process, impurities may
absorbed from the vapor 208 so as to be extracted therefrom. The solution 212
may
be held at a temperature that is sufficiently high to prevent organic
molecules from
condensing therein.
[0084] After having been bubbled through the solution 212, the remaining vapor
208 can be removed from the cleaning tank 585 and delivered into the reservoir
586.
The condensing liquid 213 can be maintained at a relatively low temperature
and can
be capable of condensing organic molecules from the vapor 208. The vapor 208
thus can be bubbled through the condensing liquid 213, and condensed organic
molecules can collect above the condensing liquid 213 as a crude oil emulsion
226.
Non-condensable gases can be drawn from the reservoir 286 through a caustic
scrubber 290 via a vacuum system 224. Emulsion 226 that has spilled over a
weir
302 can be drawn from the reservoir via a pump 260 for delivery to a settling
tank
228 (see FIG. 3) for further separation.
[0085] The condensing liquid 213 can be maintained at a temperature that is
lower than that of the pH adjusted solution 212. In some embodiments, a
composition of the condensing liquid 213 and the pH adjusted solution 212 are
the
same (e.g., the condensing liquid 213 comprises a quantity of the pH adjusted
solution 212). However, in other embodiments, the condensing liquid 213 may
have
22
CA 02794932 2012-09-28
WO 2011/123145 PCT/US2010/040219
a different composition. For example, the condensing liquid 213 may comprise
neutral water.
[0086] It will be understood by those having skill in the art that changes may
be
made to the details of the above-described embodiments without departing from
the
underlying principles presented herein. For example, any suitable combination
of
various embodiments, or the features thereof, is contemplated. For example,
various embodiments may be configured to operate in one or more of a batch
mode,
a continuous batch mode, or a continuous mode. Other or further embodiments
may
include a condenser system and/or other components (e.g., a container) that
are
configured to operate under one or more of vacuum conditions, atmospheric
pressure conditions, or positive pressure conditions.
[0087] Any methods disclosed herein comprise one or more steps or actions for
performing the described method. The method steps and/or actions may be
interchanged with one another. In other words, unless a specific order of
steps or
actions is required for proper operation of the embodiment, the order and/or
use of
specific steps and/or actions may be modified.
[0088] Throughout this specification, any reference to "one embodiment," "an
embodiment," or "the embodiment" means that a particular feature, structure,
or
characteristic described in connection with that embodiment is included in at
least
one embodiment. Thus, the quoted phrases, or variations thereof, as recited
throughout this specification are not necessarily all referring to the same
embodiment.
[0089] Similarly, it should be appreciated that in the above description of
embodiments, various features are sometimes grouped together in a single
embodiment, figure, or description thereof for the purpose of streamlining the
disclosure. This method of disclosure, however, is not to be interpreted as
reflecting
an intention that any claim require more features than those expressly recited
in that
claim. Rather, inventive aspects lie in a combination of fewer than all
features of any
single foregoing disclosed embodiment. It will be apparent to those having
skill in
the art that changes may be made to the details of the above-described
embodiments without departing from the underlying principles set forth herein.
[0090] The claims following this Detailed Description are hereby expressly
incorporated into this Detailed Description, with each claim standing on its
own as a
separate embodiment. This disclosure includes all permutations of the
independent
23
CA 02794932 2012-09-28
WO 2011/123145 PCT/US2010/040219
claims with their dependent claims. Recitation in the claims of the term
"first" with
respect to a feature or element does not necessarily imply the existence of a
second
or additional such feature or element. Elements specifically recited in means-
plus-
function format, if any, are intended to be construed in accordance with 35
U.S.C.
112 6. Embodiments of the invention in which an exclusive property or
privilege
is claimed are defined as follows.
24