Note: Descriptions are shown in the official language in which they were submitted.
CA 02797270 2012-10-23
WO 2011/139849 PCT/US2011/034365
THERMAL SIGNALING OR MARKING DEVICE
[001] The present application claims priority to U.S. Provisional Patent
Application No. 61/343,469, filed April 28, 2010, which is incorporated herein
by
reference.
[002] The present disclosure relates to the field of portable, self-contained
thermal signaling or marking devices and in particular, thermal signaling or
marking
devices that derive their energy from a controlled chemical reaction which is
not
pyrotechnic in nature.
[003] There are many situations where a reliable heat source is required in
which it is not possible or practical to employ electricity or flammable
fuels. For
example, a camper may wish to have a hot meal or beverage but may not be close
to a source of electricity to power a resistance-type heating device or
microwave
oven. The surrounding environment, for example a petroleum tank farm, may
prohibit the use of burning fuels such as charcoal or stove fuel. In these
situations a
non-electric, non-pyrotechnic heating system would be desired. A variety of
such
systems have been developed which because of their nature do not require a
source
of electricity and are not a source of ignition. Examples of these systems may
be
found in the prior art and have been variously employed as hand warmers, food
and
beverage heaters and therapeutic heaters.
[004] Another application for a non-electric, non-pyrotechnic heating device
is as a signaling device. Law enforcement and military personnel often employ
thermal imaging equipment to observe individuals and other objects covertly.
This
thermal imaging equipment is able to discern very small differences in the
apparent
temperature of objects. A person hiding in a field of thick brush may not be
visible to
the naked eye, particularly at night when there is little or no light. A
thermal imaging
device, however, will be able to detect the difference in the temperature of
the brush
as compared to the temperature of the individual's skin or the clothing the
individual
is wearing. A "heat image" of the individual is then displayed which makes the
individual highly visible.
[005] With the increased use of thermal imaging equipment, there is also a
parallel need for improved thermal sources. For example, it may be desirable
to
-1-
CA 02797270 2012-10-23
WO 2011/139849 PCT/US2011/034365
mark a trail or a turn in the road in a manner that can only be seen by those
with
thermal imagers. A potential target might be marked with a thermal "beacon" so
that
it can be seen by others using thermal imagers. In a combat situation, a
soldier may
want to covertly signal allies who are using thermal imaging equipment by
marking
the soldier's location with a thermal beacon so that the soldier may be
rescued or at
least be identified as a "friendly."
[006] While a variety of devices have been developed for the purpose
previously described, there is still a need for a heat generating device which
has
many, if not all, of the following characteristics: low-cost, reliable,
compact, safe,
easy to deploy, highly efficient, not a source of ignition, non-toxic, bio-
degradable,
and environmentally friendly.
[007] It is accordingly an object of the disclosure to provide a multiple-part
electrochemical thermal signaling or marking system comprising at least one
first
part comprising at least one super-corroding alloy and at least one absorbent
material, and at least one second part. The at least one first part and the at
least
one second part must be kept separate until use, at which time they are
combined
and heat generation commences.
[008] In another aspect of the present disclosure, a method of water or
aqueous moisture detection is provided wherein a multiple-part electrochemical
thermal signaling or marking system comprising at least one first part
comprising at
least one super-corroding alloy and at least one absorbent material, and at
least one
second part is deployed over an area of interest. In some embodiments, thermal
energy is emitted upon deployment due to the presence of water or aqueous
moisture in the area of interest. In other embodiments, thermal energy is
emitted at
a time after deployment when water or aqueous moisture enters the area of
interest.
[009] In a further embodiment, the dry components of the thermal signaling
or marking system can be mixed and deployed over an area of interest. As
above,
thermal energy is emitted in certain embodiments upon deployment due to the
presence of water or aqueous moisture in the area of interest. In other
embodiments, thermal energy is emitted at a time after deployment when water
or
aqueous moisture enters the area of interest.
[010] In another aspect of the present disclosure, at least one of the parts
may be contained inside a housing which keeps the at least one first part of
the
-2-
CA 02797270 2012-10-23
WO 2011/139849 PCT/US2011/034365
multi-part electrochemical thermal signaling or marking system separate from
the at
least one second part, until such time as mixing is desired.
[011] In a further embodiment, the present disclosure is directed to a
containment device comprising at least one first containment part, at least
one
second containment part, at least one vent member in fluid communication with
the
outside of the containment device and at least one of the at least one first
containment member and the at least one second containment member, and at
least
one breakable barrier separating the at least one first containment part from
the at
least one second containment part.
[012] In yet another embodiment, the present disclosure is directed to a
containment device comprising at least one first member comprising a sealable
opening, at least one second member bound to the at least one first member to
provide at least one space between the at least one first member and the at
least
one second member wherein the at least one space comprises at least one super-
corroding alloy and at least one absorbent material. The opening is in fluid
communication with the at least one space and the outside of the containment
device through which one of water, an aqueous solution, an electrolyte, or
mixtures
of any of the foregoing may be introduced into the at least one space at which
time
heat generation commences.
[013] In a further embodiment, the present disclosure is directed to a
containment device comprising at least one first containment part comprising
at least
one super-corroding alloy and at least one absorbent material, at least one
breakable containment part disposed within the at least one first containment
part,
and at least one vent member in fluid communication with the at least one
first
containment member and the outside of the containment device. The parts of the
electrochemical thermal signaling or marking system disclosed in the present
disclosure are contemplated to be contained in such housing and/or containment
devices.
[014] Additional objects and advantages of the disclosure will be set forth in
part in the description which follows, and in part will be obvious from the
description,
or may be learned by practice of the disclosure. The objects and advantages of
the
present disclosure will be realized and attained by means of the elements and
combinations particularly pointed out in the appended claims.
-3-
CA 02797270 2012-10-23
WO 2011/139849 PCT/US2011/034365
[015] It is to be understood that both the foregoing general description and
the following detailed description are exemplary and explanatory only and are
not
restrictive of the disclosure, as claimed.
[016] The accompanying drawings, which are incorporated in and constitute
a part of this specification, illustrate embodiments of the disclosure and
together with
the description, serve to explain the principles of the disclosure.
BRIEF DESCRIPTION OF THE DRAWINGS
[017] FIG. 1 depicts a device for producing non-electric, non-pyrotechnic
heat.
[018] FIG. 2 depicts an exploded view of a device for producing non-
electric, non-pyrotechnic heat.
[019] FIG. 3 depicts an exploded view of another device for producing non-
electric, non-pyrotechnic heat.
[020] FIG. 4 depicts another device for producing non-electric, non-
pyrotechnic heat.
[021] FIG. 5 depicts yet another device for producing non-electric, non-
pyrotechnic heat.
[022] FIG. 6 depicts a further device for producing non-electric, non-
pyrotechnic heat.
DESCRIPTION OF THE EMBODIMENTS
[023] The heating devices and systems according to the present disclosure
derive their energy from a controlled chemical reaction which is not
pyrotechnic in
nature. As used herein, the term "pyrotechnic" refers to any process whereby a
fuel
undergoes rapid oxidation such as in the burning or catalytic decomposition of
a fuel
with an oxidizer. The term is also defined to include non-oxidizing reactions
such as
those between dissimilar metals or other materials known as "thermites" or
materials
which undergo "gasless" combustion. The term does not include galvanic
reactions.
[024] As used herein, the term "non-electric" refers to devices and systems
that do not utilize electricity outside of the electric generating system
itself. The term
does not include galvanic reactions.
[025] An embodiment of the present disclosure is directed to a multi-part
electrochemical thermal signaling or marking system comprising at least one
first
-4-
CA 02797270 2012-10-23
WO 2011/139849 PCT/US2011/034365
part comprising at least one super-corroding alloy and at least one absorbent
material, and at least one second part comprising at least one component. In
certain
embodiments, the at least one second part comprises a component chosen from
water, an aqueous solution, at least one electrolyte, and mixtures of any of
the
foregoing. As used herein, the term "aqueous solution" refers to a solution
comprising water and at least one additional substance, which may be a solid,
a
liquid, a gas, or a combination of any of the foregoing. Also as used herein,
the term
"electrolyte" refers to any chemical compound that ionizes when dissolved or
molten
to produce an electrically conductive medium.
[026] In accordance with the present disclosure, the at least one absorbent
material can be chosen from, for example, natural and synthetic polymeric
absorbents, hydrocolloid/polysaccharide absorbents, cellulosic absorbents, gum
and
resin absorbents, inorganic absorbents, gel-forming fluid-interactive adhesive
dressings, wool, cotton, lint, at least one super-absorbent polymer, and
mixtures of
any of the foregoing.
[027] In certain embodiments, the at least one absorbent material
comprises at least one super-absorbent polymer. Examples of the at least one
super-absorbent polymer useful in the present disclosure include, for example,
solid
water-swellable, water-insoluble polymeric sorbents which are lightly cross-
linked
polymers, such as polyvinylpyrrolidones, sulfonated polystyrenes, sulfonated
polyvinyltoluenes, poly-sulfoethyl acrylates, poly-2-hydroxyethyl acrylates,
polyacrylates, hydrolyzed polyacrylamides and copolymers of acrylamide with
acrylic
acid described in U.S. Patent No. 3,669,103, hydrocolloid absorbent material
described in U.S. Patent No. 3,670,731, sodium polyacrylate, potassium
polyacrylate, lithium polyacrylate, ammonium polyacrylate, and mixtures of any
of the
foregoing. In certain embodiments, the at least one super-absorbent polymer
comprises sodium polyacrylate. A suitable super-absorbent polymer for this
purpose
is produced by Emerging Technologies, Inc., Greensboro, North Carolina,
currently
sold under the trade name 2G-70.
[028] In some embodiments in accordance with the present disclosure, at
least a portion of the at least one super-absorbent polymer comprises a
coating. In
other embodiments, a substantial amount of the super-absorbent polymer
comprises
a coating. As used herein, the term "substantial amount" refers to an amount
greater
than about 50% of the total amount. Non-limiting examples include an amount
-5-
CA 02797270 2012-10-23
WO 2011/139849 PCT/US2011/034365
greater than 60% of the total amount, an amount greater than 70% of the total
amount, an amount greater than 75% of the total amount, an amount greater than
80% of the total amount, an amount greater than 90% of the total amount, and
an
amount greater than 95% of the total amount. Examples of coating materials
useful
in the present disclosure include coatings comprising at least one hydrophobic
component. In certain embodiments, the at least one hydrophobic component is
chosen from water insoluble thermoplastic organic materials including
hydrocarbons
and naturally occurring resins from petroleum, asphalt and coal tar; organic
silicon
compounds including polyorganosiloxanes, polysiloxanes containing halogens
including fluorine, halohydrocarbons, including polymers containing chlorine
and
fluorine; various polymers in the form of natural or synthetic emulsions;
hydrophobic
pyrogenic silica; and mixtures of any of the foregoing. In certain other
embodiments,
the hydrophobic component comprises pyrogenic silica. A suitable pyrogenic
silica
for this purpose is produced by Wacker Chemical Corporation, Adrian, Michigan,
currently sold under the trade name HDK H15.
[029] In certain embodiments, the at least one super-absorbent polymer is
present in an amount ranging from about 0.5 percent to about 90 percent by
weight,
based on the dry weight of the at least one first part. For example, the at
least one
super-absorbent polymer can be present in an amount ranging from about 0.5
percent to about 80 percent by weight, based on the dry weight of the at least
one
first part, such as from about 0.5 percent to about 75 percent by weight, from
about
0.5 percent to about 70 percent by weight, from about 0.5 percent to about 65
percent by weight, from about 0.5 percent to about 60 percent by weight, about
0.5
percent to about 55 percent by weight, from about 0.5 percent to about 50
percent by
weight, from about 0.5 percent to about 45 percent by weight, from about 0.5
percent
to about 40 percent by weight, about 0.5 percent to about 35 percent by
weight, from
about 0.5 percent to about 30 percent by weight, from about 0.5 percent to
about 25
percent by weight, from about 0.5 percent to about 20 percent by weight, from
about
1 percent to about 80 percent by weight, from about 1 percent to about 75
percent by
weight, from about 1 percent to about 70 percent by weight, from about 1
percent to
about 65 percent by weight, from about 1 percent to about 60 percent by
weight,
about 1 percent to about 55 percent by weight, from about 1 percent to about
50
percent by weight, from about 1 percent to about 45 percent by weight, from
about 1
percent to about 40 percent by weight, from about 1 percent to about 35
percent by
-6-
CA 02797270 2012-10-23
WO 2011/139849 PCT/US2011/034365
weight, from about 1 percent to about 30 percent by weight, from about 1
percent to
about 25 percent by weight, from about 1 percent to about 20 percent by
weight,
from about 2 percent to about 80 percent by weight, from about 2 percent to
about
75 percent by weight, from about 2 percent to about 70 percent by weight, from
about 2 percent to about 65 percent by weight, from about 2 percent to about
60
percent by weight, about 2 percent to about 55 percent by weight, from about 2
percent to about 50 percent by weight, from about 2 percent to about 45
percent by
weight, from about 2 percent to about 40 percent by weight, from about 2
percent to
about 35 percent by weight, from about 2 percent to about 30 percent by
weight,
from about 2 percent to about 25 percent by weight, from about 2 percent to
about
20 percent by weight, from about 5 percent to about 80 percent by weight, from
about 5 percent to about 75 percent by weight, from about 5 percent to about
70
percent by weight, from about 5 percent to about 65 percent by weight, from
about 5
percent to about 60 percent by weight, about 5 percent to about 55 percent by
weight, from about 5 percent to about 50 percent by weight, from about 5
percent to
about 45 percent by weight, from about 5 percent to about 40 percent by
weight,
from about 5 percent to about 35 percent by weight, from about 5 percent to
about
30 percent by weight, from about 5 percent to about 25 percent by weight, and
from
about 5 percent to about 20 percent by weight. In certain embodiments, the at
least
one super-absorbent polymer is present in an amount of about 1 percent, about
3
percent, about 5 percent, about 7 percent, about 10 percent, about 15 percent,
about
20 percent, about 25 percent, about 30 percent, about 35 percent, about 40
percent,
about 45 percent, about 50 percent by weight, about 55 percent, about 60
percent,
about 65 percent, about 70 percent, about 75 percent, about 80 percent, about
85
percent, and about 90 percent by weight, based on the dry weight of the at
least one
first part. It is also intended that the amount of the at least one super-
absorbent
polymer can range between any of the numerical values listed above.
[030] The multi-part electrochemical thermal signaling or marking system of
the present disclosure also comprises at least one super-corroding alloy. In
certain
embodiments, the at least one super-corroding alloy comprises at least one
first alloy
component chosen from aluminum, magnesium, zinc, and mixtures of any of the
foregoing and at least one second alloy component chosen from iron, copper,
nickel,
titanium, chromium, carbon, and mixtures of any of the foregoing.
-7-
CA 02797270 2012-10-23
WO 2011/139849 PCT/US2011/034365
[031] For example, in certain embodiments, the at least one super-
corroding alloy comprises magnesium and iron. A representative example of a
suitable super-corroding alloy is an alloy with an atomic weight ratio of
about 5% iron
to about 95% magnesium, although other ratios may be suitably employed. A
suitable alloy for this purpose is produced by Dymatron, Inc., Cincinnati,
Ohio
currently sold under the trade name C-5 . The alloy, when reacted with a
liquid
electrolyte, such as salt water, can liberate a significant amount of heat.
The
reaction continues either until all of the at least one first alloy component
has been
consumed or the heat of the reaction has caused the water to evaporate,
thereby
removing the electrolyte from the system. Without wishing to be bound by any
particular theory, the rate of consumption of the at least one super-corroding
alloy
can be modulated by varying the atomic weight ratio of the at least one second
alloy
component to the at least one first alloy component present such that varying
the
atomic weight percent of the at least one second alloy component prolongs the
thermal output of the electrochemical thermal signaling or marking system by
slowing the reaction rate.
[032] In some embodiments, the at least one first alloy component may be
present in an atomic weight percent ranging from about 80 percent to about
99.5
percent and the at least one second alloy component may be present in an
atomic
weight percent ranging from about 0.5 percent to about 20 percent. For
example,
the at least one first alloy component may be present in an atomic weight
percent
ranging from about 80 percent to about 99 percent, such as from about 80
percent to
about 98 percent, from about 80 percent to about 97 percent, from about 80
percent
to about 96 percent, from about 80 percent to about 95 percent, from about 80
percent to about 93 percent, from about 82 percent to about 93 percent, from
about
86 percent to about 93 percent, from about 88 percent to about 93 percent, and
from
about 90 percent to about 93 percent. In certain embodiments, the at least one
first
alloy component is present in an anomic weight percent of about 80 percent,
about
82 percent, about 86 percent, about 88 percent, about 90 percent, about 93
percent,
about 95 percent, about 97 percent, about 98 percent, about 99 percent, and
about
99.5 percent. The at least one second alloy component, for example, can be
present
in an atomic weight percent ranging from 0.5 percent to 18 percent, such as
from
about 0.5 percent to about 14 percent, from about 0.5 percent to about 12
percent,
from about 0.5 percent to about 10 percent, from about 1 percent to about 10
-8-
CA 02797270 2012-10-23
WO 2011/139849 PCT/US2011/034365
percent, from about 2 percent to about 10 percent, from about 3 percent to
about 10
percent, from about 4 percent to about 10 percent, from about 5 percent to
about 10
percent, and from about 7 percent to about 10 percent. In some embodiments,
the
at least one second alloy component is present in an atomic weight percent of
about
0.5 percent, about 1 percent, about 2 percent, about 3 percent, about 4
percent,
about 5 percent, about 7 percent, about 10 percent, about 12 percent, about 14
percent, about 18 percent, and about 20 percent. It is aldo intended that the
atomic
weight percent of the at least one first alloy component and the atomic weight
percent of the at least one second alloy component can range between any of
the
numerical values listed above.
[033] Hydrogen gas can also be a product of the reaction described above.
To account for the reactions that produce hydrogen gas, certain embodiments of
the
present disclosure provide a suitable venting means to relieve the hydrogen
gas.
According to these embodiments, it is possible to employ a plurality of vents
in a
manner so that regardless of device orientation, one vent will always be above
the
fluid level. In some embodiments, the venting means includes at least one vent
which relieves hydrogen and retains the fluid. In other embodiments, the
containment device comprises a porous material that allows hydrogen gas
release
while maintaining the fluid therein. In some embodiments, a substantial amount
of
the containment device comprises the porous material. In other embodiments,
the
containment device is itself made from the porous material. An example of a
porous
material useful in the present disclosure includes hydrophobic spun-bonded
polyethylene, such as Tyvek manufactured by DuPont.
[034] Without wishing to be bound by any particular theory, the thermal "life"
of the electrochemical thermal signaling or marking system can depend on such
aspects as the surface area of the reactive materials, electrolyte
conductivity, and
availability of water. One method for improving the duration of thermal
output, is to
delay reaction of some of the at least one super-corroding alloy. One method
of
accomplishing this delay is by coating particles of the at least one super-
corroding
alloy to prevent water access to the particles. In certain embodiments, the at
least
one super-corroding alloy comprises a mixture of particles comprising
particles that
are at least partially coated with a coating and particles that are
substantially
uncoated. As used herein, "partially coated" means that the particles comprise
a
-9-
CA 02797270 2012-10-23
WO 2011/139849 PCT/US2011/034365
coating on at least 5 percent of the particle surface. "Substantially
uncoated" as
used herein indicates that the particles are uncoated on at least 95 percent
of the
surface of the particles. Without wishing to be bound by any particular
theory, a
coating can be used to slow the reaction between the super-corroding alloy and
water or an aqueous solution in the presence of at least one electrolyte
allowing for
control of the thermal life span of the thermal signaling or marking system.
In some
embodiments, the at least one super-corroding alloy comprises particles chosen
from uncoated particles, substantially uncoated particles, and at least
partially coated
particles and mixtures of any of the foregoing. In a further embodiment, the
particles
comprise a mixture of uncoated particles and at least partially coated
particles.
[035] In certain embodiments, the quantity of substantially uncoated
particles is greater than the quantity of at least partially coated particles.
In certain
other embodiments, the quantity of substantially uncoated particles is less
than the
quantity of at least partially coated particles. In yet another embodiment,
the quantity
of substantially uncoated particles is substantially the same as the quantity
of at least
partially coated particles. As used in this context, "substantially the same"
means
that the first value is within 1 percent of the second value. In other
embodiments,
particles are coated in aggregate thereby forming semi-rigid structures
comprising
the particles.
[036] In some embodiments, the coating comprises a water-soluble coating.
In other embodiments, the coating comprises a water-permeable coating. In
further
embodiments, the coating comprises a water-degradable coating. In other
embodiments, the coating comprises at least one ethoxylated long chain
alcohol. A
representative example of a suitable at least one ethoxylated long-chain
alcohol
comprises aliphatic alcohols of between 20 and 50 carbon atoms, although
others
may be suitably employed. Suitable ethoxylated long-chain alcohols for this
purpose
are produced by Baker Hughes, Sugar Land, Texas, currently sold under the
trade
name Unithox Ethoxylates, 700 series. In yet other embodiments, the coating
comprising at least one ethoxylated long chain alcohol further comprises at
least one
water insoluble material. Suitable water insoluble materials include aliphatic
alcohols
of between 20 and 50 carbon atoms, although others may be suitably employed.
In
still other embodiments, the at least one super-corroding alloy comprises
particles
chosen from uncoated particles, substantially uncoated particles, and at least
partially coated particles, and mixtures of any of the foregoing, and the
coating is
-10-
CA 02797270 2012-10-23
WO 2011/139849 PCT/US2011/034365
chosen from a water-soluble coating, a water-permeable coating, a water-
degradable coating, a coating comprising at least one ethoxylated long-chain
alcohol, and mixtures of any of the foregoing.
[037] In some embodiments, coating of the at least one super-corroding
alloy may be applied by "panning," "spray-coating," or similar processes. In
other
embodiments, a coating is applied by a process referred to as the "Wurster"
process
as described in U.S. Patent No. 3,196,827. In this process, the particles to
be
coated are maintained in a fluidized bed. The coating material is introduced
as a fine
spray that adheres to the particles. Process time and application rate
determine
overall coating thickness. The Wurster process is well-suited to coating small
and
irregular particles. Without wishing to be bound by any particular theory,
coating
materials may be applied in a non-aqueous form to avoid wetting the super-
corroding
alloy and initiating the reaction (even in the absence of salt) and thereby
degrading
the alloy.
[038] Without wishing to be bound by any particular theory, another method
of delaying the reaction of some of the at least one super-corroding alloy is
by
varying the particle size of the at least one super-corroding alloy. The
reaction tends
to occur at the particle surface such that smaller particles tend to react at
a higher
reaction rate than larger particles. In accordance with one embodiment of the
present disclosure, the at least one super-corroding alloy may comprise
particles
ranging in size from about U.S. Standard Sieve 14 to about U.S. Standard Sieve
200. In another embodiment, the at least one super-corroding alloy may
comprise a
mixture of particles comprising particles ranging in size from about U.S.
Standard
Sieve 16 to about U.S. Standard Sieve 20 and particles ranging in size from
about
U.S. Standard Sieve 45 to about U.S. Standard Sieve 140. Additionally, in
other
embodiments, the quantity of particles ranging in size from about U.S.
Standard
Sieve 45 to about U.S. Standard Sieve 140 is substantially the same as the
quantity
of particles ranging in size from about Standard Sieve 16 to about U.S.
Standard
Sieve 20. As used in this context, "substantially the same" means that the
first value
is within 1 percent of the second value. In still other embodiments, the at
least one
super-corroding alloy may comprise particles chosen from particles ranging in
size
from about U.S. Standard Sieve 14 to about U.S. Standard Sieve 200, particles
ranging in size from about U.S. Standard Sieve 16 to about U.S. Standard Sieve
20,
-11-
CA 02797270 2012-10-23
WO 2011/139849 PCT/US2011/034365
particles ranging in size from about U.S. Standard Sieve 45 to about U.S.
Standard
Sieve 140, and mixtures of any of the foregoing.
[039] In other embodiments, the quantity of particles ranging in size from
about U.S. Standard Sieve 45 to about U.S. Standard Sieve 140 is greater than
the
quantity of particles ranging in size from about U.S. Standard Sieve 16 to
about U.S.
Standard Sieve 20. And in still other embodiments, the quantity of particles
ranging
in size from about U.S. Standard Sieve 45 to about U.S. Standard Sieve 140 is
less
than the quantity of particles ranging in size from about Standard Sieve 16 to
about
U.S. Standard Sieve 20.
[040] In some embodiments, the at least one super-corroding alloy
comprises pellets formed from compressing particles of the least one super-
corroding alloy. For example, such pellets can range in size from U.S.
Standard
Sieve 200 to pellets comprising a diameter of about 5 mm and a length of about
6
mm. Without wishing to be bound by any particular theory, the reaction of the
at
least one super-corroding alloy to produce thermal signaling or marking takes
place
at the surface of the pellet and slowly consumes the pellet. The geometries of
the
pellets, surface area (over which the reaction is occurring), and volume (or
mass)
can be selected to provide a desired reaction time in order to optimize the
duration of
thermal output. In certain embodiments, a pellet of at least one super-
corroding alloy
is produced by compressing particles of the at least one super-corroding alloy
under
a pressure of about 100,000 Kg/cm2 (approx. 1.5 million PSI). The compressed
pellets can form any desirable shape, and nonlimiting examples include, for
example, shapes chosen from cylindrical, spherical, wedge, and star.
[041] The at least one super-corroding alloy described in the present
disclosure may be present in an amount ranging from about 1 percent to about
99.5
percent by weight, based on the dry weight of the at least one first part. For
example, the at least one super-corroding alloy can be present in an amount
ranging
from about 1 percent to about 90 percent by weight, based on the dry weight of
the
at least one first part, such as from about 1 percent to about 80 percent,
from about
1 percent to about 75 percent by weight, from about 1 percent to about 70
percent by
weight, from about 1 percent to about 60 percent by weight, from about 1
percent to
about 50 percent by weight, from about 1 percent to about 40 percent by
weight,
from about 1 percent to about 30 percent by weight, from about 1 percent to
about
25 percent by weight, from about 1 percent to about 20 percent by weight, from
-12-
CA 02797270 2012-10-23
WO 2011/139849 PCT/US2011/034365
about 3 percent to about 80 percent, from about 3 percent to about 75 percent
by
weight, from about 3 percent to about 70 percent by weight, from about 3
percent to
about 60 percent by weight, from about 3 percent to about 50 percent by
weight,
from about 3 percent to about 40 percent by weight, from about 3 percent to
about
30 percent by weight, from about 3 percent to about 25 percent by weight, from
about 3 percent to about 20 percent by weight, from about 4 percent to about
75
percent by weight, from about 4 percent to about 70 percent by weight, from
about 4
percent to about 60 percent by weight, from about 4 percent to about 50
percent by
weight, from about 4 percent to about 40 percent by weight, from about 4
percent to
about 30 percent by weight, from about 4 percent to about 25 percent by
weight,
from about 4 percent to about 20 percent by weight, from about 5 percent to
about
75 percent by weight, from about 5 percent to about 70 percent by weight, from
about 5 percent to about 65 percent by weight, from about 5 percent to about
60
percent by weight, from about 5 percent to about 50 percent by weight, from
about 5
percent to about 40 percent by weight, from about 5 percent to about 30
percent by
weight, from about 5 percent to about 25 percent by weight, from about 5
percent to
about 20 percent by weight, from about 10 percent to about 65 percent by
weight,
from about 10 percent to about 60 percent by weight, from about 10 percent to
about
50 percent by weight, from about 10 percent to about 40 percent by weight,
from
about 10 percent to about 30 percent by weight, from about 10 percent to about
25
percent by weight, and from about 10 percent to about 20 percent by weight. In
other embodiments, the at least one super-corroding alloy is present in an
amount of
about 1 percent, about 4 percent, about 8 percent, about 10 percent, about 20
percent, about 25 percent, about 30 percent, about 35 percent, about 40
percent,
about 45 percent, about 50 percent, about 55 percent, about 60 percent, about
65
percent, about 70 percent, about 75 percent, about 80 percent, about 85
percent,
about 90 percent, about 95 percent, and about 99.5 percent by weight, based on
the
dry weight of the at least one first part. It is also intended that the amount
of the at
least one super-corroding alloy can range between any of the numerical values
listed
above.
[042] The multi-part electrochemical thermal signaling or marking system of
the present disclosure further comprises a component of the at least one
second part
wherein the component is chosen from water, an aqueous solution, at least one
electrolyte, and mixtures of any of the foregoing. In some embodiments, the
-13-
CA 02797270 2012-10-23
WO 2011/139849 PCT/US2011/034365
component is chosen from water and an aqueous solution, and the at least one
first
part further comprises at least one electrolyte. In other embodiments, the
component comprises at least one electrolyte. In still other embodiments, the
component comprises at least one electrolyte and at least one of water and an
aqueous solution. As used herein, "aqueous solution" refers to a solution
comprising
water and at least one other material.
[043] In some embodiments, the at least one electrolyte comprises at least
one salt comprising a cation chosen from Li+, Na', K+, Mgt+, Ca2+, and
combinations
of any of the foregoing; and an anion chosen from Cl-, Br, I-, C104, BF4 , PF6
, ASF6 ,
SbF6 , CH3CO2, CF3SO3 , N(CF3SO2)2 , C(CF3SO2)2, C032 and combinations of
any of the foregoing.
[044] In certain embodiments, the at least one electrolyte is present in an
amount ranging from about 0.01 percent to about 36 percent by weight, based on
the
weight of the at least one second part. For example, the at least one
electrolyte can
be present in an amount ranging from about 0.01 percent to about 30 percent by
weight, based on the weight of the at least one second part, such as from
about 0.01
percent to about 25 percent, from about 0.01 percent to about 20 percent by
weight,
from about 0.01 percent to about 15 percent, from about 0.01 percent to about
10
percent by weight, from about 0.01 percent to about 5 percent, from about 0.01
percent to about 1 percent by weight, from about 0.1 percent to about 30
percent by
weight, from about 0.1 percent to about 25 percent, from about 0.1 percent to
about
20 percent by weight, from about 0.1 percent to about 15 percent, from about
0.1
percent to about 10 percent by weight, from about 0.1 percent to about 5
percent,
from about 0.1 percent to about 1 percent by weight, from about 0.5 percent to
about
30 percent by weight, based on the weight of the at least one second part,
such as
from about 0.5 percent to about 25 percent, from about 0.5 percent to about 20
percent by weight, from about 0.5 percent to about 15 percent, from about 0.5
percent to about 10 percent by weight, from about 0.5 percent to about 5
percent,
from about 0.5 percent to about 1 percent by weight, from about 1 percent to
about
30 percent by weight, from about 1 percent to about 25 percent, from about 1
percent to about 20 percent by weight, from about 1 percent to about 15
percent,
from about 1 percent to about 10 percent by weight, from about 1 percent to
about 5
percent, from about 4 percent to about 25 percent, from about 4 percent to
about 20
percent by weight, from about 4 percent to about 15 percent, and from about 4
-14-
CA 02797270 2012-10-23
WO 2011/139849 PCT/US2011/034365
percent to about 10 percent by weight. In other embodiments, the at least one
electrolyte is present in an amount of about 0.01 percent, 0.1 percent, 0.5
percent, 1
percent, about 2 percent, about 4 percent, about 6 percent, about 8 percent,
about
percent, about 15 percent, about 20 percent, about 25 percent, about 30
percent,
and about 36 percent by weight, based on the weight of the at least one second
part.
In yet another embodiment, the at least one electrolyte is present in an
amount of
about 4 percent by weight, based on the weight of the at least one second
part. It is
also intended that the amount of the at least one electrolyte can range
between any
of the numerical values listed above.
[045] In some embodiments, the at least one first part further comprises at
least one hydrophobic component. In certain embodiments, the at least one
hydrophobic component is chosen from water insoluble thermoplastic organic
materials including hydrocarbons and naturally occurring resins from
petroleum,
asphalt and coal tar, organic silicon compounds including polyorganosiloxanes,
polysiloxanes containing halogens including fluorine, halohydrocarbons,
including
polymers containing chlorine and fluorine, various polymers in the form of
natural or
synthetic emulsions, hydrophobic pyrogenic silica, and mixtures of any of the
foregoing. In certain other embodiments, the hydrophobic component comprises
pyrogenic silica. A suitable pyrogenic silica for this purpose is produced by
Wacker
Chemical Corporation, Adrian, Michigan, currently sold under the trade name
HDK
H15.
[046] In some embodiments, the hydrophobic component is present in an
amount ranging from about 0.1 percent to about 10 percent by weight, based on
the
dry weight of the at least one first part. For example, the at least one
hydrophobic
component can be present in an amount ranging from about 0.1 percent to about
9
percent by weight, based on the dry weight of the at least one first part,
such as from
about 0.1 percent to about 8 percent, from about 0.1 percent to about 6
percent by
weight, from about 0.1 percent to about 4 percent by weight, from about 0.1
percent
to about 2 percent by weight, from about 0.5 percent to about 8 percent by
weight,
from about 0.5 percent to about 6 percent by weight, from about 0.5 percent to
about
4 percent by weight, from about 0.5 percent to about 2 percent by weight, from
about
1 percent to about 6 percent by weight, from about 1 percent to about 4
percent by
weight, from about 1 percent to about 2 percent by weight, from about 2
percent to
about 6 percent by weight, and from about 2 percent to about 4 percent by
weight.
-15-
CA 02797270 2012-10-23
WO 2011/139849 PCT/US2011/034365
In other embodiments, the at least one hydrophobic component is present in an
amount of about 0.5 percent, about 1 percent, about 2 percent, about 4
percent,
about 6 percent, about 8 percent, about 9 percent, and about 10 percent by
weight,
based on the dry weight of the at least one first part. In yet another
embodiment, the
at least one hydrophobic component is present in an amount of less than about
1
percent by weight, based on the dry weight of the at least one first part. It
is also
intended that the amount of the at least one hydrophobic component can range
between any of the numerical values listed above.
[047] In certain embodiments of the present disclosure, the at least one first
part of the electrochemical thermal signaling or marking system further
comprises at
least one binder. The at least one binder may be a water soluble binder with a
melting point ranging from about 80 C to about 650 C. For example, the melting
point of the water soluble binder can range from about 80 C to about 160 C,
from
about 100 C to about 180 C, from about 120 C to about 200 C, from about 140 C
to
about 220 C, from about 160 C to about 260 C, from about 180 C to about 280 C,
from about 200 C to about 325 C, from about 250 C to about 400 C, from about
300 C to about 500 C, and from about 400 C to about 650 C. In certain
embodiments, the at least one binder is chosen from polyacrylates,
polymethacrylates, methacrylic acid-ethyl acrylate copolymers, polyvinyl
pyrrolidones, polysaccharides, substituted polysaccharides, cellulose ethers,
polyvinyl alcohols, polyethylene glycols, ethoxylated polyvinyl alcohols,
ethoxylated
long chain alcohols, and mixtures of any of the foregoing. In further
embodiments,
the at least one binder comprises at least one ethoxylated long-chain alcohol.
A
representative example of a suitable at least one ethoxylated long-chain
alcohol
comprises aliphatic alcohols of between 20 and 50 carbon atoms, although
others
may be suitably employed. Suitable ethoxylated long-chain alcohols for this
purpose are produced by Baker Hughes, Sugar Land, Texas, currently sold under
the trade name Unithox Ethoxylates, 700 series.
[048] In other embodiments, at least one of the at least one first part and
the at least one second part may comprise additional components. In some
embodiments, an additional component includes at least one color changing dye
wherein the at least one color changing dye may indicate the stage of the
thermal
emitting reaction. In certain embodiments, the at least one color changing dye
may
change color with respect to temperature, pH or other chemical
characteristics.
-16-
CA 02797270 2012-10-23
WO 2011/139849 PCT/US2011/034365
[049] The thermal signaling or marking devices and systems according to
the present disclosure have many, if not all, of the following
characteristics: low-cost,
reliability, compact, safe, easy to deploy, highly efficient, not a source of
ignition,
non-toxic, bio-degradable, and environmentally friendly. Certain embodiments
of the
present disclosure provide a relatively uniform heat signature over the
surface of the
device; other embodiments of the present disclosure provide a device with a
temporally tailored heat output; further embodiments of the present disclosure
provide a heat generator that will not leak during operation; other
embodiments of
the present disclosure provide a heat generator which recovers heat from
gasses
and vapors before venting; and still other embodiments of the present
disclosure
provide for the recovery of liquid water from water vapor which may be used to
further the reaction of the heating chemistry.
[050] In another aspect of the present disclosure, at least one of the parts
may be contained inside a housing which keeps the at least one first part of
the
multi-part electrochemical thermal signaling or marking system separate from
the at
least one second part, until such time as mixing is desired.
[051] In an additional embodiment, the present disclosure is directed to a
containment device comprising at least one first containment part, at least
one
second containment part, at least one vent member in fluid communication with
the
outside of the containment device and at least one of the at least one first
containment member and the at least one second containment member, and at
least
one breakable barrier separating the at least one first containment part from
the at
least one second containment part.
[052] In yet another embodiment, the present disclosure is directed to a
containment device comprising at least one first member comprising a sealable
opening, at least one second member bound to the at least one first member to
provide at least one space between the at least one first member and the at
least
one second member wherein the at least one space comprises at least one super-
corroding alloy and at least one absorbent material. The opening is in fluid
communication with the at least one space and the outside of the containment
device through which one of water, an aqueous solution, an electrolyte, or
mixtures
of any of the foregoing thereof may be introduced into the at least one space
at
which time heat generation commences.
-17-
CA 02797270 2012-10-23
WO 2011/139849 PCT/US2011/034365
[053] n a further embodiment, the present disclosure is directed to a
containment device comprising at least one first containment part comprising
at least
one super-corroding alloy and at least one absorbent material, at least one
breakable containment part disposed within the at least one first containment
part,
and at least one vent member in fluid communication with the at least one
first
containment member and the outside of the containment device. The parts of the
electrochemical thermal signaling or marking system according to the present
disclosure are contemplated to be contained in such housing and/or containment
devices.
[054] n further embodiments, the containment device comprises at least
one insulating element which minimizes undesirable heat loss. For example, the
containment device can comprise at least one insulating element that is at
least
partially transparent to thermal radiation (e.g., infrared) thereby allowing a
thermal
imager to detect the temperature of the contents inside the containment device
rather than just detecting the cooler outer surface of the containment device.
In
further embodiments, at least one insulating element prevents thermal energy
losses
due to undesirable conduction to the air while maintaining desirable radiation
of
thermal energy. Suitable examples for use as the at least one insulating
element
include, for example, polyolefins such as polyethylenes and polypropylenes. In
additional embodiments, the containment device can be covered with a structure
commonly referred to as "bubble wrap" to minimize conductive and convective
heat
loss while still maintaining high levels of thermal radiation. In certain
embodiments,
containment devices comprising at least one insulating element can possess a
40 C
temperature difference between the contents of the containment device and the
outer surface of the containment device.
[055] n some embodiments, the device is comprised of rigid materials
which provide a rigid construct to the device. In other embodiments, the
device is
comprised of flexible materials thereby imparting flexibility to the device
and
conformity to the surface onto which the device may be disposed.
[056] Without wishing to be bound by any particular theory, the thermal
energy generated by the disclosed electrochemical thermal signaling or marking
systems emanate in multiple directions. As a result, the electrochemical
thermal
signaling or marking systems may be designed to direct energy in a desired
direction. In some embodiments, the containment device comprises at least one
-18-
CA 02797270 2012-10-23
WO 2011/139849 PCT/US2011/034365
reflecting element which reflects at least a portion of the emitted thermal
energy in a
desired direction.
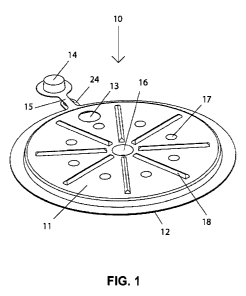
[057] FIG. 1 depicts a thermal signaling or marking device, 10, comprising a
container with an interior space contained between first member, 11, and
second
member, 12. These members may be joined together by heat sealing, sonic
welding, RF welding, vibratory welding or any other suitable means. At least
one
vent, 13, is provided to relieve gas pressure generated during operation. This
same
vent may also be employed to introduce water to the device. A plug, 14, is
provided
to seal the container when not in use and to temporarily seal the container
during
mixing. The plug may be constructed so that it forms a hermetic seal when
pressed
fully into the hole but permits venting when not pressed fully into the hole.
The plug
may be designed to snap in place so it will not accidentally dislodge. A
strap, 15,
prevents plug 14 from being lost with respect to the device. At least one bond
point,
16, may be employed to join first member 11 and second member 12 together.
This
serves to maintain a fixed distance between these members, increase rigidity
of the
device and preserve the internal volume of the device. Additional spacing
elements
17, may be incorporated into the device to further stabilize the structure of
the
device. These elements may or may not comprise bonds between the first and
second members. Strengthening ribs, 18, may be incorporated into first and/or
second members as may be desired to improve rigidity of the structure. An
optional
hook or hole, 24, may be provided to facilitate attachment of the device to a
nail or
string.
[058] FIG. 2 depicts an exploded view of the device depicted in FIG. 1.
First member 11, and second member 12, when sealed together peripherally,
define
an interior space which contains first reactive material, 19, which can
comprise the
dry ingredient mixture. A peelable, pressure-sensitive seal, 25, may be used
to
cover the vent hole prior to use; otherwise; plug 14 depicted in FIG 1 can
serve this
purpose. A radiation reflector, 20, is attached to or otherwise part of second
member 12. This reflector comprises a metal foil, metallized film or any other
suitable form of thermal reflector. A thermal insulator, 21, may be employed
to limit
conductive heat losses. If desired, an adhesive layer, 22, may also be
provided. In
certain embodiments, a pressure sensitive adhesive is used so that the entire
device
may be readily adhered to surfaces. In such embodiments, a removable release
-19-
CA 02797270 2012-10-23
WO 2011/139849 PCT/US2011/034365
liner, not shown in the figure, can be used to prevent contamination of the
adhesive
prior to use.
[059] FIG. 3 depicts an exploded view of a thermal signaling or marking
device 10 wherein plug 14 is integral to second member 12. A portion of
radiation
reflector 20, thermal insulator 21, and adhesive layer 22 may be removed so
that
clear access is provided for plug 14 to fit into vent 13.
[060] FIG. 4 provides yet another thermal signaling or marking device 10
comprising a frangible vessel, 26. The frangible vessel comprises water, an
aqueous solution, a liquid electrolyte, or mixtures thereof. As used herein,
the term
"liquid electrolyte" refers to at least one electrolyte combined with one of
water or an
aqueous solution. When the vessel is broken, the liquid contacts the dry
reactants
and heating is initiated. Breaking of the vessel may be accomplished by
application
of force to the device 10 such that at least a portion of this force is
transmitted to the
frangible vessel. Pressure-sensitive seal 25 covers vent 13 until either
manually
removed or until pressure build up in the device forces the seal from first
member 11
at which time venting occurs. At least one vent 13 may be placed at various
and
multiple locations to allow venting during the evolution of gasses by the
device.
Such embodiments may be useful as a self-activating device. For example, such
a
device, in its un-activated state, may be placed on a roadway. Vehicular or
foot
traffic which contacts the device will rupture the frangible vessel and
initiate the
heating reaction.
[061] FIG. 5 depicts a thermal signaling or marking device 10 comprising a
fillable bladder, 27, wherein water, aqueous solution, liquid electrolyte, or
mixtures
thereof may be added by removing closure means, 28 and pouring the water,
aqueous solution, liquid electrolyte, or mixtures thereof into a fillable
bladder, 27.
Said bladder is also frangible, rupturable or otherwise designed to release
its
contents when sufficient force is transmitted to the bladder. The bladder may
be
formed in any number of ways including, but not limited to, blow molding,
thermoforming, rotomolding or heat sealing two, more or less planar, members
together such that a hydraulic reservoir is formed between the members. The
bladder may of course be part of, and integral to, first member 11 and second
member 12 as well. In either case, the liquid contents are retained in the
bladder
and kept separate from first reactive material 19 until the bladder integrity
is violated
at which time the liquid contents of the bladder contact the first reactive
material and
-20-
CA 02797270 2012-10-23
WO 2011/139849 PCT/US2011/034365
heating in commenced. A vent 13 may be employed or a closure means, 28, of a
self-venting type may be used. Such embodiments, like that shown in FIG. 4,
may
also be self-activating. In the case of FIG. 5, it is not necessary to have
the water,
electrolyte or any other liquid components contained within the device until
immediately before deployment. This may be desirable if the device is to be
transported, stored or otherwise handled in a manner in which the device may
become accidentally or prematurely activated.
[062] It is also contemplated that the bladder 27 is a time-release bladder.
In certain embodiments, the bladder is made from water soluble materials that
will
dissolve upon exposure to the liquid components. The rate that the material
dissolves, for example, in a matter of minutes, can be controlled by the
material used
to make the bladder and the thickness of the bladder walls. Suitable materials
for
these embodiments include polyvinyl acetate, poly(alkylmethacrylate),
poly(ethylene)
oxide, alkyl cellulose such as ethyl cellulose and methyl cellulose,
carboxymethyl
cellulose, hydrophilic cellulose derivatives, polyethylene glycol,
polyvinylpyrrolidone,
cellulose acetate, cellulose acetate butyrate, cellulose acetate phthalate,
cellulose
acetate trimellitate, polyvinyl acetate phthalate, hydroxypropylmethyl
cellulose
phthalate, hydroxypropylmethyl cellulose acetate succinate, and polyvinyl
acetaldiethylamino acetate.
[063] FIG. 6 depicts another thermal signaling or marking device 10
comprising a pouched member, 31, which may be fabricated by bonding, sewing or
otherwise joining first member, 32, to second member, 33. Seal zones, 30,
create
pouches of a defined space. Members 32 and 33 may be fabricated from a cloth-
like
material such as a woven or a non-woven fabric, or they may be fabricated from
perforated films which may or may not be porous. In either case, at least a
portion of
each of these aforementioned pouches is porous and will permit the entry of
water
aqueous solution, the liquid electrolyte or mixtures thereof. The pouches
formed
within the pouch member comprise the dry ingredients as may be desired and
serve
to maintain relative position of these dry ingredients with respect to the
overall
device. The pouched member 31 may be staked, bonded or otherwise adhered to at
least a portion of the interior of device 10.
[064] In certain embodiments, the pouches contain an absorbent material
such as a super absorbent polymer; in other embodiments the pouches themselves
can be made of an absorbent material. in some embodiments, the absorbent
-21 -
CA 02797270 2012-10-23
WO 2011/139849 PCT/US2011/034365
material may be intermixed with the first reactive material to create a more
or less
homogeneous mixture. Without wishing to be bound by any particular theory,
when
the second reactive material (for example, water or liquid electrolyte) is
added and
comes into contact with the pouches, it passes though the pouch wall and is
absorbed by the absorbent material. As the absorbent material swells, the
mixture
containing the absorbent material and the first reactive material grows in
volume. As
the mixture volume grows, its more or less homogeneous nature is preserved.
This
prevents grouping of the first reactive material and ensures that sufficient
electrolyte
is available to reach all of the first reactive material.
[065] While the figures illustrate devices that are more or less planar, there
is no limit to the shape of the instant invention. For example, the device may
take
the form of a rod or bar, similar to a "light stick." Additionally, the device
may take
the form of a letter, numbers, arrows or other indicia as may be desired. The
device
may even take the form of a flexible or inflatable structure.
Example 1 Formula for Dry Ingredient Mixture
Super-absorbent Polymer 8.10 g 20%
HDK H15 Silica 0.40 g 1%
Sodium Chloride 1.50 g 4%
FD&C yellow 5/FD&C blue 1 0.004 g 0.0100%
C-5 alloy 20 g 50%
Coated C-5 Alloy 10 g 25%
Total Dry Mass 40.00 g 100%
Table 1
-22-
CA 02797270 2012-10-23
WO 2011/139849 PCT/US2011/034365
Example 2 Formula for Dry Ingredient Mixture
Super-absorbent Polymer
4.048 g 45.0%
HDK H15 Silica
0.200 g 2.2%
Sodium Chloride 0.751 g 8.3%
FD&C yellow 5/FD&C blue 1 0.002 g 0.022%
C5-alloy 4.000 g 44.4%
Total dry mass 9.000 g 100.0%
Table 2
[066] Other embodiments of the disclosure will be apparent to those skilled
in the art from consideration of the specification and practice of the
disclosure
disclosed herein. It is intended that the specification and examples be
considered as
exemplary only, with a true scope and spirit of the disclosure being indicated
by the
following claims.
-23-