Note: Descriptions are shown in the official language in which they were submitted.
METHOD OF IDENTIFYING AND TREATING CHRONIC PAIN OF PERIPHERAL
ORIGIN RELATED TO PERIPHERAL NERVE DAMAGE
BACKGROUND
More than 11 million Americans report chronic pain as a significant
disability. The
financial burden of chronic pain in the United States alone is estimated at
higher than $100
billion a year, including lost productivity and medical expenses. Viewed
globally, there is a
large underserved population for pain management medications and/or therapies.
Chronic pain is typically classified as pain lasting more than 6 months and
generally
divided into three main types: nociceptive, psychogenic or neuropathic (e.g.,
due to nerve injury)
although the distinction between these types can be blurred. Especially true
for chronic
neuropathic pain, current treatment options including opioids and nonsteroidal
anti-inflammatory
drugs (e.g. COX inhibitors) are often ineffective, contraindicated or
associated with significant
gastrointestinal and cardiac side effects, sedation, respiratory depression,
addiction and drug
abuse. It is widely believed that pharrnacotherapy, surgical ablation, and
externally applied non-
drug therapies (e.g. transcutaneous electrical nerve stimulation and
acupuncture) have all reached
a ceiling well below the desired level of patients and clinicians. Novel ideas
are thus needed in
pain research.
SUMMARY
The invention provides a solution to the long-standing problem of accurate
identification
of clinical pain and reliable therapeutic intervention to alleviate such pain.
Chronic clinical pain
includes neuropathic pain such as that associated with direct nerve damage,
amputation,
chemotherapy, diabetes, HIV infection or AIDS, Multiple Sclerosis, shingles,
sciatic nerve
compression or injury, as well as spine surgery.
Accordingly, a method of identifying a subject characterized as suffering from
chronic
pain, e.g., chronic neuropathic pain, is carried out by detecting a pain
signature comprising an
pattern of neuronal firing compared to a normal pattern of neuronal firing,
e.g., a pattern
obtained from a subject or a cohort of subjects that have been characterized
as not suffering from
1
Date Recue/Date Received 2021-04-07
CA 02797701 2012-10-26
WO 2011/137193 PCT/US2011/034203
pain. The pattern of firing is obtained from a single neuron or a plurality of
neurons. The brain
of a subject afflicted with chronic pain has stored a pain signature. The
pattern comprises an
elevated evoked response to stimuli, rhythmic after-discharge signaling,
and/or increased
spontaneous background firing. The pain and neuronal firing pattern subsists
after an injury
heals or is completely unrelated to a stimulus, e.g., an injury, or the degree
of a stimulus. In one
example, the pain signature comprises a pattern of neuronal burst-firing, each
burst of the burst
firing comprising at least 10 times, 50 times, 100 times or more, the number
of spikes compared
to a control non-pain pattern. An exemplary pain signature comprises a pattern
of burst-firing
that is characterized by one or more of the following measurable parameters:
(a) a maximum
interval signifying burst onset (6 ms); (b) a maximum interspike interval (9
ms); (c) longest
increase in interspike interval within a burst (2 ms); (d) a minimum number of
spikes within a
burst (2). An aberrant pattern or pain signature is further characterized by a
spontaneous high
frequency rhythmic oscillation of long epoch.
A method of preventing or reducing pain perception involves identifying a
subject using
the criteria described above and administering to the subject at least one
electrical pulse to the
subject, the electrical pulse being at least about 100, 150, or 200 Hz,
between about 1 and about
3 volts, between about 1 and about 3 milliampere, and between about 0.25 and
about 1 second in
duration. Rather than stimulating the aberrantly firing neurons back to a
recovery pattern, the
electrical pulse of at least about 100 Hz jams or halts the pain circuitry at
the level of the source.
Subjects to be diagnosed and/or treated include human patients as well as
animals such as
companion animals (e.g., dogs, cats) as well as livestock and performance
animals (e.g., horses,
cattle, and the like).
The invention includes an anatomically-based and neurotechnology-oriented pain
therapy
system to achieve neuromodulation of specific brain regions, for example using
transcutaneous
magnetic fields or chronically implanted electrodes. In general, in an aspect,
the invention
provides a system including a processor configured to be coupled to an
electrical lead that is
configured to sense electrical activity in a patient, a memory coupled to the
processor, the
memory containing computer readable instructions that, when executed by the
processor, cause
the processor to detect a pain signature in the sensed electrical activity,
determine a treatment
2
CA 02797701 2012-10-26
WO 2011/137193 PCT/US2011/034203
protocol in response to the detected pain signature, and cause the treatment
protocol to be
delivered to the patient via the electrical lead.
In general, in another aspect, the invention provides a system including a
processor
configured to provide an electrical treatment protocol to a patient, the
electrical treatment
protocol being configured to treat chronic pain in the patient, the treatment
protocol including
providing at least one electrical pulse to the patient, the electrical pulse
being at least about 150
Hz, between about 1 and about 3 volts, between about 1 and about 3
milliampere, and between
about 0.25 and about 1 second in duration.
In general, in a further aspect, the invention provides a method of
identifying a subject
comprising chronic pain, including detecting a pain signature comprising a
pattern of neuronal
firing, said pattern comprising an elevated evoked response to stimuli,
rhythmic after-discharge
signaling, and increased spontaneous background firing.
In general, in still another aspect, the invention provides a method of
identifying a subject
comprising chronic pain, comprising detecting a pain signature comprising a
pattern of burst-
firing, each burst of said burst firing comprising at least 10 times the
number of spikes compared
to a control non-pain pattern.
In general, in yet another aspect, the invention provides a method of
identifying a subject
comprising chronic pain, comprising detecting a pain signature including a
pattern of burst-
firing, wherein said pattern comprises burst-firing, said burst firing
including (a) a maximum
interval signifying burst onset (6 ms), (b) a maximum interspike interval (9
ms), (c) longest
increase in interspike interval within a burst (2 ms), or (d) a minimum number
of spikes within a
burst (2).
In general, in an even further aspect, the invention provides a method of
preventing or
reducing pain perception, comprising identifying a subject, and administering
to said subject at
least one electrical pulse to the subject, the electrical pulse being at least
about 150 Hz, between
about 1 and about 3 volts, between about 1 and about 3 milliampere, and
between about 0.25 and
about 1 second in duration.
Various aspects of the invention may provide one or more of the following
capabilities.
The efficiency, battery life, and device life of devices used for
neurostimulation can be improved
3
over prior techniques. More physiologically relevant brain structures can be
targeted compared
with prior techniques. Side effects can be reduced compared with prior
techniques. The
temporal and overall amount of delivered current can be reduced compared with
prior
techniques. The likelihood of excessive tissue exposure, which has been
thought to cause long-
term changes and side effects, can be reduced compared with prior techniques.
The necessity for
combined pharmacologic intervention can be reduced, or possibly eliminated
compared prior
techniques. Chronic pain can be empirically diagnosed.
These and other capabilities of the invention, along with the invention
itself, will be more
fully understood after a review of the following figures, detailed
description, and claims. Other
features and advantages of the invention will be apparent from the following
description of the
preferred embodiments thereof, and from the claims.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 is a diagram of a classic 'pain pathway' illustrating the spinothalamic
tract (STT)
and how nociceptive information is transmitted from the periphery via the
dorsal root ganglion
(DRG) to central structures including the ventropostetior lateral (VPL)
nucleus of the thalamus
to the cerebral cortex (Cc).
Fig. 2A is a diagram of a deep brain stimulation (DBS) system,
Fig. 2B is a flow diagram showing a diagnostic protocol for pain.
Fig. 3 is a graphical representation of a representative example of a single
unit recording
comparison between CCI and Naive subjects. Each graph displays the firing (mV)
of a single
WDR neuron over time and corresponding peristimulus time histograms of the
data. Both VPL
neurons used for recording possessed receptive fields at the hind-paw. CCI
model rats display an
increase in spontaneous background firing (shown in yellow), elevated pressure
and pinch
responses (green), and rhythmic after-discharge signaling (blue).
Fig. 4 is a bar graph showing a comparison of the mean evoked response
frequency over
all group 1 (CCI) and group 2 (naive) rats. Statistically significant
increases in spontaneous
4
CA 2797701 2017-09-28
CA 02797701 2012-10-26
WO 2011/137193 PCT/US2011/034203
activity and after discharge, as well as evoked pressure and pinch responses
are shown (*P
<0.05).
Fig. 5 is a line graph showing a comparison of rat withdrawal latency between
the
hindpaw ipsilateral and contralateral to the sciatic nerve injury as measured
during behavioral
testing. The withdrawal latency of the injured hindpaw (pink) is significantly
lower than
baseline by days 6 and 7 (* P<0Ø5).
Fig. 6 is a graph showing a representative example of a comparison between
Isoflurane
and Pentobarbital anesthesia. Activity in a single VPL unit is recorded under
conditions of
Isoflurane (2%) followed by Pentobarbital 20 minutes later (i.v. 40 mg/kg/hr)
in a single animal.
No major difference in spontaneous or evoked activity is apparent.
Fig. 7 is a bar graph showing an electrophysiological response to mechanical
stimuli in
pre- and post-HFS conditions. VPL neurons of group 5 rats post HFS are
significantly less
reactive to mechanical stimuli via brush, Von Frey, pressure, and pinch than
during initial
baseline recording (P<0.05).
Fig. 8 is a bar graph showing a background electrophysiological comparison in
pre- and
post- HFS conditions. VPL neurons of group 5 rats post HFS display
significantly lower levels
of afterdischarge (20 seconds post mechanical stimulation) while levels of
background firing
remain relatively constant (* P<0.05).
Fig. 9 is a line graph showing withdrawal Latency of the ipsilateral hindpaw
in deep brain
stimulation (DBS) group 6 rats. Within 5 minutes of neurostimulation,
withdrawal latency
significantly increases, with effects lasting 0.5- 2 hrs.
Fig. 10 is a bar graph showing OX-42 antibody staining comparison between CCI
and
CCI+DBS animals in VPL contralateral to CCI (i.e. receiving pain input from
the injured leg and
the implant), DBS subjects displayed significantly higher levels of OX-42
antibody staining as
compared with untreated CCI subjects (*P<0.05). Reflected differences were
measured as
CA 02797701 2012-10-26
WO 2011/137193 PCT/US2011/034203
changes in the mean grayscale value of photographed microscope images of the
VPL, indicating
a physiologic local effect for HFS.
Fig. 11 is a bar graph GFAP antibody staining comparison between CCI and
CCI+DBS
animals in VPL contralateral to CCI (i.e. receiving pain input from the
injured leg and the
implant). Measured differences in GFAP staining were not statistically
significant, suggesting
lack of astrogliosis, tissue scarring or prominent neuroinflammatory reaction
to HFS.
Fig. 12 is a series of bar graph showing burst characterization in response to
various
stimuli, e.g., brush, Von Frey, pressure, and pinch.
Fig. 13 is a graphical representation showing neuronal activity from a single
unit in the
thalamus (VPL) with receptive field in the injured hindpaw one week after CCI.
Note distinct
firing pattern associated with CCI and emergence of spontaneous high frequency
rhythmic
oscillation of long epoch (-) after pinch. The high spontaneous rate of firing
is only briefly
interrupted by application of lidocaine (4%) directly on the dorsal surface of
the spinal cord at
upper thoracic level, whereas evoked responses to brush and pinch disappear.
Note sustained
and more elevated spontaneous firing within seconds after lidocaine
application, indicating a
phenomenon independent of peripheral or caudal input from the spinal cord
(i.e. inherent within
the brain).
Fig. 14 is a drawing and a photograph of rodent into which microelectrodes
were
implanted in the brain with tips located in the VPL nucleus of the thalamus.
Signal generated
from these electrodes (RE) was relayed to a data acquisition system via a head
stage fixed to the
skull of the animal. Neuropathic pain was measured behaviorally and induced by
chronic
constriction injury (CCI) of the sciatic nerve, a 'mixed' nerve that receives
sensory input from
the leg and connects with central nervous system (CNS) circuitry projecting
into the VPL. To
evoke thermal nociception, a laser beam was focused on the plantar hindlimb to
illicit a
withdrawal behavior of measurable latency.
Fig. 15 is a photograph of a modified microelectrode design. Electrode
segments were
fused together with Epoxy resin in order to form a respective cathode and
anode during DBS
6
CA 02797701 2012-10-26
WO 2011/137193 PCT/US2011/034203
trials. The Teflon coating was cut to reveal 0.5 mm of silver wire on each
electrode. Cathode
and Anode are separated by lmm from tip to tip as shown.
Fig. 16 is a photograph of a recording setup. Following craniotomy, the
microelectrode
was lowered to a depth of 5-6 mm until an appropriate VPL unit was isolated
for recording.
Fig. 17 is an illustration of the relative position of the bipolar stimulating
electrodes in
relation to the VPL, and the area directly affected by stimulation (shaded)
based on several
modeling studies (refer to text).
Fig 18A is a line graph, and Fig. 18B is a bar graph. Fig. 18A shows
representative
examples of tonic firing in two units under naive and CCI conditions. Note
increased rate of
spontaneous firing and firing evoked by pressure (Pr), pinch (Pi) and
afterdischarge (AD) in CCI
rat compared to naïve. Fig. 2B shows mean rate of firing in two groups of VPL
neurons in naïve
and CCI rats (n=9-1 l/gr).
Figs. 19A-B are line graphs, and Fig. 19C is a bar graph. Fig. 19A shows a
representative example of spontaneously rhythmic oscillation (grey shade),
which was abolished
after complete spinal transection (arrow, asterisk indicates absence of a
response to brush after
transection). Rhythmic oscillation was observed in 3/9 (33%) neurons in rats
with CCI. Fig. 19B
shows phase histograms during rhythmic oscillation fitted with a sine wave
curve (before spinal
transection, left panel). Note elimination of oscillation after transaction
reflected by a much
lower amplitude sine wave (right panel). Fig. 19C shows sine wave parameters
in neurons with
oscillation fitted to phase histograms.
Figs 20A-B are line graphs showing representative examples of the effect of
HFS (100
Hz) and LFS (25 Hz) on the firing rate of two VPL neurons in two rats with
CCI. HFS attenuated
all evoked activity and afterdischarge, whereas spontaneous firing remained
unchanged. Note
increasing effects with incremental increases in voltage, compared to lack of
prominent effect
using LFS. Figs. 20C-D are graphs showing mean percent change in firing rates
after HFS (n=9
units) or LFS (n=5 units) compared to pre-stimulation baselines for each unit.
HFS significantly
inhibited all evoked responses and after discharge in units recorded from CCI
rats, except
spontaneous activity, in contrast to LFS which had no significant effect.
7
CA 02797701 2012-10-26
WO 2011/137193 PCT/US2011/034203
Fig. 21 is a graphical representation showing examples of burst firing under
naive and
CCI conditions during peripheral brush stimulation. Upper traces in each panel
represent burst
activity with corresponding spiking activity in lower traces (shaded insets
represent expanded
time scales of activity periods in grey boxes). Note increased burst events
and number of spikes
per burst after CCI.
Fig. 22 is a series graphical representations showing a detailed analysis of
burst firing
showing significantly different patterns in rats with CCI compared to naïve.
Evoked burst
activity occurred in naïve and CCI rats (although spontaneous burst was very
rare in naïve rats).
Burst parameters were different in CCI rats, showing a consistent increase in
the number of
bursts and % spikes for all firing modalities, whereas inter-burst periods
were consistently
decreased. HFS reversed these changes to near 'normal' or naïve values.
Fig. 23 is a series graphical representations showing local field potentials
(upper traces)
before, during and after HFS with corresponding power spectra (lower panels)
showing a
prominent peak between 10-20 Hz (corresponding to f3 activity; arrow). HFS had
no effect on
this peak or the overall power distribution up to 500 Hz during spontaneous
firing (note
emergence of peaks at 200 Hz matching stimulation frequency and a harmonic
thereof at 400 Hz;
arrowheads).
Fig. 24A is a bar graph, and Figs. 24B-C are line graphs. Fig. 24A shows mean
withdrawal latencies before and after CCI (n=5 rats). Pre-CCI values represent
average
withdrawal latencies in both hindpaws which were not significantly different,
whereas post-CCI
latency was significantly decreased in ipsilateral (injured) hindpaws compared
to contralateral
(uninjured) hindpaws, indicating thermal hyperalgesia. Fig. 24B shows the
effect of HFS
(arrows) on withdrawal latencies in ipsilateral and contralateral hindpaws
(arrowhead denotes
sham condition, i.e. connecting the stimulating electrodes to the stimulator
without applying
voltage). Fig. 24C shows mean withdrawal latencies 5 min before HFS and at 5
and 10 min after
HFS showing significant increase in latency 5 min after HFS (n=4 rats),
suggesting attenuation
of hyperalgesia.
8
CA 02797701 2012-10-26
WO 2011/137193 PCT/US2011/034203
Fig. 25 is a bar graph showing mean rate of firing in VPL neurons with
receptive fields
excluding the hindpaws from two groups of naive and CCI rats (n=9-11/gr),
showing no
difference in firing rates except for firing evoked by brush.
Fig. 26A-B are graphical representations showing mean percent change in firing
rates in
two groups of VPL neurons under CCI and naive conditions. Fig. 26A shows that
relatively
'moderate' microstimulation (100 HZ, 0.5 V, 1 s duration pulse) resulted in a
significantly
decreased firing evoked by pressure and pinch as well as afterdischarge (n=4
out of 9 neurons).
Fig. 26B shows that HFS decreased the firing rate in naïve rats, reaching
significant levels for all
firing modalities except for the weakest von Frey filament stimulation (0.6 g)
and afterdischarge
(n=6 units).
Fig. 27 is a graphical representation showing Mean withdrawal latencies in
hindpaws
contralateral to injury in CCI rats 5 min before HFS and at 5 and 10 min after
HFS showing non-
significant change in latency (n=4 rats).
Fig. 28 is a series of photographs and a bar graph. Chronic microelectrode
implant had
no effect on the mean ratio of OX-42 or GFAP immunofluorescence intensity in
the vicinity of
stimulating electrode tips, suggesting limited or absent glial activation or
reactive gliosis (n=4
rats).
Fig. 29 is a series of tables (Table 1, Table 2, Table 3, Table 4).
Fig. 30 is a graph showing a representative record of firing of neurons in the
thalamus in
an awake patient with chronic pain. Pain was repeatedly induced during
continuous recording of
the neural activity (from upper left to lower right). Dotted lines indicate
tapping of the hand for
activation of touch-evoked pain. Solid lines indicate pain was verbally
reported by the patient.
Fig. 31 is a graph of a representative record of a unit in the thalamus of a
rat with spinal
cord injury pain to illustrate burst events (compare with Fig. 30). During 60
sec of firing
activity, 2 burst epochs alternated with unique periods identified as 'a' and
'b'. These 2 epochs
spontaneously alternated in a repeated manner separated by interepoch
intervals of low firing
activity, exhibiting a rhythmic oscillatory firing pattern.
9
CA 02797701 2012-10-26
WO 2011/137193 PCT/US2011/034203
Fig. 32 is a line graph showing a spectral analysis of spontaneous activity.
Mean power
spectra for patients with Complex Regional Pain Syndrome (CRPS) (red) and
normal subjects
(black) showing shifting of brain activity to a lower frequency in pain
patients.
Fig. 33 is a graphical representation of local field potential (LFP) recorded
from the VPL
contralateral to CCI; Spontaneous activity is followed by increased activity
evoked by brushing
of the receptive filed in the injured paw (t=19-40s).
Fig. 34 is a series of graphs showing spontaneous activity and activity in
response to
brushing of the contralateral paw. Spectral power was computed using FFT
analysis of the
recorded signal from the VPL bilaterally and normalized for each VPL. A broad
peak (1-15 Hz)
was observed under spontaneous conditions in CT, which more prominently
shifted leftwards (1-
3 Hz) in CCI. During evoked responses, the peak at around 5 Hz was more
prominent in CCI
compared to CT, with a broader power distribution in the higher-frequency (5-
15 Hz) region.
Overall power was increased bilaterally during evoked responses to brush;
however, this increase
was almost 3 folds higher in CCI (note increased evoked/spontaneous ratio of
area power from
1.16 in CT to 3.05 in CCI). Red plots represent Gaussian data fit.
Fig. 35 is a series of illustrations depicting LFP recordings of the six
groups (Gr, n=8/Gr.
total 48 rats; Exp: Experimental, Ct: Control):
- Grl: Record LFP in somatosensory cortex (SI) first ipsilateral (Ct) then
contralateral (Exp) to
CCI before/after SCS.
- Gr 2: Record LFP in SI first contralateral (Exp) then ipsilateral (Ct) to
CCI before/after SCS.
- Gr 3: Record LFP in VPL first ipsilateral (Ct) then contralateral (Exp) to
CCI before/after SCS.
- Gr 4: Record LFP in VPL first contralateral (Exp) then ipsilateral (Ct) to
CCI before/after SCS.
- Gr 5: Record LFP in SI in naïve before/after SCS.
- Gr 6: Record LFP in VPL in naïve before/after SCS.
DETAILED DESCRIPTION
Chronic pain is a serious challenge in terms of pathophysiology, diagnosis,
therapy and
social burden. Studies in humans and laboratory animals suggest a relationship
between
intractable pain and ectopic neuronal activity in thalamic and cortical areas,
leading to
CA 02797701 2012-10-26
WO 2011/137193 PCT/US2011/034203
dysfunctional connectivity in the brain's 'pain network'. Contributing to this
network are dense
interconnections between thalamic and cortical modules whose interactions are
being
investigated in terms of directionality and temporal dynamics. In humans,
intracranial electrode
recordings demonstrate altered neuronal activity within these networks in
patients with chronic
pain. Single-cell electrophysiology and magneto-encephalographic (MEG) studies
further
support the hypothesis of thalamo-cortical dysrhythmia (TCD) in patients with
complex regional
pain syndrome, whereas, interestingly, imaging studies show cortical thinning
under chronic pain
conditions. Similar physiological results were found using animal models of
pain, thus allowing
for more detailed mechanistic analysis, whereby a series of studies have
validated the
pathophysiology of thalamo-cortico-thalamic circuitry.
Rather than being the symptom of a disease process, chronic pain is itself a
disease
process. Chronic pain is unrelenting and not self¨limiting and as stated
earlier, can persist for
years and even decades after the initial injury. If not treated, chronic pain
can lead to anxiety,
fear, depression, sleeplessness and impairment of social interaction. Chronic,
non¨malignant
pain is predominately neuropathic in nature and involves damage either to the
peripheral or
central nervous systems.
Nociceptive and neuropathic pain are caused by different neurophysiological
processes,
and therefore respond to different treatment modalities. Nociceptive pain is
mediated by
receptors on A-delta and C-fibers which are located in skin, bone, connective
tissue, muscle and
viscera. These receptors serve a biologically useful role at localizing
noxious chemical, thermal
and mechanical stimuli. Nociceptive pain can be somatic or visceral in nature.
Somatic pain
tends to be well localized, constant pain that is described as sharp, aching,
throbbing, or
gnawing. Visceral pain, on the other hand, tends to be vague in distribution,
paroxysmal in
nature and is usually described as deep, aching, squeezing and colicky in
nature. Examples of
nociceptive pain include: post-operative pain, pain associated with trauma,
and the chronic pain
of arthritis. Nociceptive pain often responds to opioids and non-steroidal
anti-inflammatories
(NSAIDS). Neuropathic pain, in contrast to nociceptive pain, is described as
"burning",
"electric", "tingling", and "shooting" in nature and can be unrelated to a
stimulus such as an
injury. Examples of neuropathic pain include: monoradiculopathies, trigeminal
neuralgia,
11
CA 02797701 2012-10-26
WO 2011/137193 PCT/US2011/034203
postherpetic neuralgia, phantom limb pain, complex regional pain syndromes and
the various
peripheral neuropathies. Neuropathic pain tends to be only partially
responsive to opioid
therapy.
As is discussed above, chronic pain is a significant clinical problem. Most
potent
treatment is opiate derivatives; however, these drugs are associated with
serious side effects.
Moreover, one type of chronic pain, neuropathic pain (due to direct damage to
the nervous
system (peripheral nerves, spinal cord or brain)) is usually resistant to
treatment. During
peripheral neuropathic pain, the degree of pain is often unrelated to the
degree of tissue damage
at the site of nerve injury. Clinical data indicate abnormal activity pattern
in patients with
chronic pain, particularly in the sensory thalamus (ventroposterior lateral;
VPL), a major nuclear
relay for sensory information.
Chronic abnormal sensations (sensory neuropathies) following peripheral nerve
injury are
caused by long-term changes in brain activity patterns. Sensory neuropathies
and abnormal brain
patterns are reversible with direct intervention in the brain by deep brain
stimulation (DBS).
The data described herein was generated using an art-recognized pre-clinical
rat model of
peripheral neuropathy (chronic constriction injury, CCI). The brain area
studied was in the
sensory thalamus (ventroposterior lateral; VPL), a major nuclear relay for
sensory information.
The VPL on one side of the brain receives sensory input from the contralateral
side of the body
(neurons in the VPL contralateral to CCI were studied). Neuronal activity from
single neurons in
the VPL was recorded in live animals under deep anesthesia as extracellular
action potentials.
Neuronal patterns recorded were either evoked by stimuli on the corresponding
receptive field in
the body or spontaneous (no stimulation of the receptive field). Sensory
neuropathies were
tested using standard behavioral measurement of thermal sensitivity to a heat
stimulus (latency
of withdrawal to moderately noxious heat) in awake, unrestrained, non-
anesthetized rats. For
reversal of abnormal activity patterns, DBS was delivered in the VPL under
deep anesthesia
during recording. For reversal of thermal hypersensitivity. DBS was delivered
in the VPL in
awake, unrestrained, non-anesthetized rats.
In rats with neuropathic injury, abnormal neuronal activity was recorded in
the VPL
contralateral to CCI (similar results were confirmed in another model of
peripheral neuropathy
12
CA 02797701 2012-10-26
WO 2011/137193 PCT/US2011/034203
by spinal nerve ligation; SNL). The neuropathy-induced abnormal activity in
all rats included
hyperexcitability of evoked responses, emergence of high spontaneous firing
and aberrant
evoked burst (in addition to occasional spontaneous rhythmic firing in some
rats). Abnormal
neuronal activity occurred exclusively in neurons with receptive fields in the
leg (supplied by the
injured sciatic nerve). Neuronal activity recorded from the VPL with receptive
fields beyond the
injured leg, and those from the ventrolateral medial (VPM) nuclear group
(which receives major
input from the face), were not different from those in naïve rats. Tissue
collected from
neuropathic rats (postmortem) showed local neuroinflammation in the VPL
contralateral to CCI.
DBS reversed all abnormal patterns of neuronal activity in the VPL (except
spontaneous
discharge, which remained high in neuropathic rats), with no side effects. DBS
reversed thermal
hyperalgesia in neuropathic rats, with no side effects.
Pain signature
The neuronal activity patterns that make up the pain signature can be divided
into two
major categories: spontaneous and evoked. Spontaneous activity is further
divided between
baseline activity (on-going spontaneous discharge in the absence of overt
bodily stimuli) and
afterdischarge (on-going spontaneous discharge immediately following the
cessation of a
noxious bodily stimulus). Evoked activity is further divided between activity
in response to
noxious (e.g. painful high pressure or pinch) or non-noxious stimuli (e.g.
gentle touch or brush).
To make use of the 'signal' (i.e. for the sensory to detect it reliably), the
signal could be either
detected in an autonomous 'rigid' manner (device with pre-programmed fixed set
of parameters),
an autonomous 'flexible' manner (device capable to 'learning', i.e. with
capacity to correct for
errors to improve reliability of accurate detection), or recognized by outside
observer
(experimenter, healthcare practitioner or self in the form of Biofeedback).
One way of objectively or empirically quantifying the signature is by
computing the rate
of firing (i.e. number of action potentials in time) for individual neurons.
The data show, for
example, that the firing rate during pinch under pain conditions is higher
compared to normal.
One point to consider is that, for example, the firing rate during pressure
under pain conditions is
lower compared to that during pinch under normal conditions. Thus relying on
firing rates
exclusively to distinguish normal from pain states will not suffice to program
an automated
13
CA 02797701 2012-10-26
WO 2011/137193 PCT/US2011/034203
detector, unless advance or real-time knowledge of the stimulation state is
obtained. Though
burst characteristics are based on parameters different than firing rate (e.g.
number of bursts,
mean spikes/burst), the same argument also applies (overlapping data between
spontaneous and
evoked activities).
In spite of these apparent limitations, one alternative is to consider a real-
life example.
Evoked noxious events are rare throughout an individual's daily activities,
including those with
chronic pain. Such events are usually the result of infrequent injuries
sustained from falling or
projectiles. Therefore, the category of activity evoked by noxious stimuli
could be ignored
(including noxious heat, and consequently, including afterdischarge). A major
category of
activity throughout daytime is predominately evoked by light touch, secondary
to gentle touch
such as clothing, tapping. 'feeling', etc. A second major category is
spontaneous activity. Of
note, pressure and pinch-evoked activities under pain conditions are
exceedingly higher than any
other type of activity under normal or pain condition, constituting a 'safety
margin' for
programming. If noxious events do occur, they would be interpreted as
exceeding a set limit for
'pain touch' anyhow, and the period of afterdischarge would fall within the
therapeutic time
window and would therefore be prevented. Furthermore, the difference between
spontaneous and
pressure or pinch-evoked activities is exceedingly high, therefore allowing
for the setting of 2
distinct zones of activities termed 'normal' or 'pain', respectively.
Another option for the design of a closed-loop device to detect pain
signature, based on
firing rate, is to couple the device to a mechanical sensing probe on the
affected area of the body
(superficially on the skin) capable of detecting mechanical energies such as
touch, pressure and
pinch stimuli, as well as thermal energies such as hot/cold surfaces, and
relay this information to
the closed-loop device in parallel to the main neuronal detector of the brain
signature. Such a
design would enable an automated response while lessening the need for an
observer or feedback
from the subject to classify the type of neuronal activity (i.e. spontaneous
or evoked).
Other types of neuronal activities are also envisioned for the detection of
the signature.
These include neuronal activity recorded directly or indirectly at the level
of Local Field
Potential (LFP: i.e., sampling from a neuronal population) detecting shifts in
power spectra using
Fourier type analysis, absence or emergence of new spectral peaks),
electroencephalogram
14
CA 02797701 2012-10-26
WO 2011/137193 PCT/US2011/034203
(EEG), magnetoencephalogram (MEG), in addition to other types of imaging
techniques and
brain scans (for example Magnetic Resonance Imaging, MRI and fMRI and Positron
Emission
Tomography or PET, etc.)
Sensor design
The sensor part of the closed-loop device for pain management, or an open loop
sensor
device for pain diagnosis, depends on the capability of the sensor to record
neuronal activity
(from single neurons or a population of neurons, directly or indirectly using
surrogate
measurements such as blood flow or volume). Such pain signature manifests high
temporal and
special resolutions, as the said neuronal activity is generated by a specific
population of neurons
in the brain (hence close proximity of the probe is needed for specific
detection of the electric
signal), and that the activity pattern or changes thereof occurs mostly in the
order of milliseconds
or seconds. While current technology allows such high temporal and special
resolutions using
implantable microelectrodes, the use of other 'sensor' technologies, in
particular non-invasive
EEG functional imaging is useful.
More importantly, design strategy considers the possibility of not only
recording from a
single area or structure in the brain, or looking at multiple areas or
structures in the brain
individually, but also studying the interaction between these regions under
normal and
pathological or pain conditions, as it is known that network connectivity in
the brain is altered in
chronic pain patients. Dysfunctional network connectivity will manifest by
combined temporal
and spatial analysis of neuronal activity among more than one brain area or
structure at any of
the recording or detection levels discussed above.
Stimulation design
A closed-loop device is programmed to detect the pain signature and operate
upon
detection of such signals to send a command to an operator that would deliver
therapy with the
aims of reversing the signature. For example, this device would be turned ON
in wakeful states
and OFF during sleep, depending on condition severity and need. Furthermore,
the device is
optionally set to deliver a therapeutic pulse periodically or intermittently
(e.g., every 2 hrs).
Clinical application
CA 02797701 2012-10-26
WO 2011/137193 PCT/US2011/034203
In addition to being used for analgesia (decreasing existent pain), the device
is used for
anesthesia during invasive or surgical procedures, in particular if
anesthetics or sedatives are
contraindicated. More importantly, the diagnostic aspects of the device are
useful in cases where
subjects or patients are non-cooperative, unable to respond, cognitively
impaired, facing
language barrier, or where simply verbal reporting is unreliable (e.g., in the
pediatric population
or with adult drug-seekers).
Deep brain Stimulation
Embodiments of the invention provide techniques for developing a safe,
effective and
long-term treatment strategy for persistent pain using, for example, deep
brain stimulation (DBS)
for the relief of chronic pain. The techniques can include measuring
electrical activity in a
patient's brain to determine if a certain pain signature exists. This can
involve the use of, for
example, electrodes implanted into a patient's brain. The technique can also
include providing
therapeutic electrical stimulation to, for example, the brain of the patient
at predefined times,
frequencies, voltages, periodicities, and currents. The electrical stimulation
can be provided in
response to detecting the presence of the predefined pain signature in the
patient in a closed-loop
design, or can be provided on a periodic basis in a open loop system (e.g.,
every 1-2 hours).
Other embodiments are within the scope of the invention.
One embodiment includes the use of a closed-loop design that can enable
neurostimulation to be triggered upon detection of, for example, abnormal
neuronal activity
linked to (or immediately preceding) pain episodes, thus reversing aberrant
neuronal activity and
attenuating (or even preventing) pain, without interfering with 'normal' brain
activity. An
additional benefit can also be the delivery of high frequency current (e.g.,
>150 Hz) that blocks
(or 'jams') neuronal firing with no reported side effects.
Anatomically, a major relay station to ascending sensory information is
preferably
targeted in the thalamus, based on empirical evidence showing a characteristic
burst firing
pattern recorded from the thalamus of awake patients with neuropathic pain,
which closely
resembles that recorded from the thalamus of animal models of neuropathic
pain. To this end, it
has been 1) identified a thalamic neuronal activity pattern associated with
neuropathic pain in
16
CA 02797701 2012-10-26
WO 2011/137193 PCT/US2011/034203
anesthetized rats ('pain signature'); 2) determined an optimal stimulation
protocol that reverses
pain-related thalamic firing; 3) achieved reversal of pain-related behavior by
neurostimulation.
A series of stimulation protocols have been tested and several have been
identified that
best achieve reversal of pain-related neuronal activity with the least amount
of current delivered
in duration and intensity. High frequency stimulation (e.g., >150 Hz) can
'jam' neuronal
circuitry, resulting in 'lesion' effects that are reversible. The brain
circuitry targeted would
preferably be the pain circuitry directly, mainly the sensory thalamus. The
data show that a brief
pulse train at high frequency typically effectively attenuates neuronal
hyperexcitability in
thalamic neurons associated with chronic pain, and attenuates pain behavior.
A neuronal activity pattern has been characterized in thalamic neurons that is
associated
with chronic pain. The rationale for choosing thalamic neurons is at least
partially based on tests
showing that thalamic sensory neurons typically undergo distinctive plasticity
changes under
conditions of spinal cord injury-pain, and that reversal of these plastic
changes by pharrnacologic
treatment is linked to reversal of pain behavior. In support of this, data
suggest that thalamic
sensory neurons with receptive field in the dermatome of the injured sciatic
nerve (a model of
neuropathic pain) undergo distinct changes, including hyper-responsiveness to
peripheral stimuli,
increased spontaneous firing and increased probability of afterdischarge. In
addition, these
experiments have been validated in awake un-anesthetized rats, and tested the
anti-nociceptive
effects of neurostimulation on nociceptive behavior in a rat model of chronic
pain.
During normal nociception (e.g., Fig. 1), information about stimulus location
and
intensity is encoded in precise patterns of action potential firing.
Individual neurons produce,
and dynamically switch between, a multitude of discrete firing modes such as
single spikes,
bursts (e.g., which can be configured in a variety of ways given differences
in timing and
patterning), spindle waves and spike motif trains termed 'epochs.' Firing
patterns are a product
of (and influence) neurons in directly wired local circuits and in widely
distributed circuits. One
such circuit element, the thalamus, serves as an important sensory relay to
higher cortical
circuits.
An overall increase in thalamic gain is associated with an increased transfer
ratio at the
thalamocortical synapse that serves to more potently activate cortical
circuits involved in pain
17
CA 02797701 2012-10-26
WO 2011/137193 PCT/US2011/034203
sensation. In other brain areas, for example in the visual system, the
information content of
bursts is typically higher than single spikes. In the hippocampus, the
probability of generating at
least one postsynaptic spike is higher for bursts than for single spikes.
Thalamic nociceptive
neurons undergo spontaneous firing activity in normal human subjects and rats,
conferring
distinct neuronal rhythmicity (oscillations) at defined resonant frequencies.
Temporal
coincidence of such activity patterns with cortical activity mediates
functional states that
characterize sensory experiences. Several neurological conditions can upset
this temporal
coincidence, and abnormal thalamic activity has been linked to chronic painful
conditions. For
example, spinal cord injury-induced pain behavior is associated with a higher
prevalence of
spontaneous burst firing in the ventroposterior lateral (VPL) nucleus of the
thalamus, in addition
to an increased number of neurons with oscillatory firing pattern; burst
intervals are more
regular, between-event intervals are longer and burst events contain more
spikes.
Rhythmic network oscillation in the thalamus is modifiable by thalamic events
and
external synaptic input. En passant axons of thalamocortical, in addition to
corticothalamic,
relay neurons receive tuning from the surrounding nucleus reticularis feedback
circuit that could
be reconfigured after injury to the nervous system. Unstable or aberrantly
processed nociceptive
inputs lead to abnormal generation or amplification of nociceptive
information. Therefore,
neuromodulation by neurostimulation is an effective strategy to treat and/or
manage chronic
pain.
Thus, in view of the foregoing, a therapeutic system comprises the following:
1) a
detector linked to a stimulator in a closed-loop device to detect and reduce
abnormal brain
activity, thus attenuating pain in an automated way; 2) a biocompatible closed-
loop
neurostimulation device specific for chronic pain, 3) surgical brain implant
and testing of the
device, and 4) clinical application for chronic pain treatment.
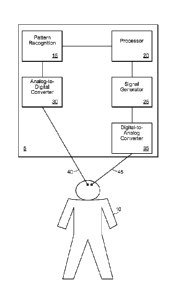
Referring to Fig. 2A, a deep brain stimulation (DBS) system 5 for use with a
patient 10 is
shown. Preferably, the DBS system 5 includes a pattern recognition system 15,
a processor 20, a
signal generator 25, an analog-to-digital converter 30, and a digital to
analog converter 35.
While the DBS system 5 is shown in Fig. 2A as including separate discrete
blocks (e.g.. 15, 20,
25, 30, and 35), other configurations are possible. For example, the
functionality of one or more
18
CA 02797701 2012-10-26
WO 2011/137193 PCT/US2011/034203
of the blocks 15, 20, 25, 30, and 35 can be combined into a single device
and/or routine.
Furthermore, while Fig. 2A includes a number of discrete blocks (e.g., 15. 20,
25, 30, and 35),
certain blocks may be omitted in some configurations (e.g., pattern
recognition system 15 and
analog-to-digital converter 30 can be omitted in non-closed loop systems).
The analog-to-digital converter 30 is configured to receive signals from the
brain of the
patient 10 via an electrical lead 40, and/or any other device that can measure
neuronal activity
(e.g., functional scanners). The electrical lead 40 can be configured to be
implanted
intracranially in the brain of the patient 10, although, the electrical lead
40 can be configured to
measure electrical activity of the patient 10 in other areas (e.g., the VPL,
hippocampus, and/or
brain stem). Preferably, the electrical lead 40 is configured to be attached
and/or in close
proximity to a wide dynamic range (WDR) neuron in the brain of the patent 10,
although other
neurons can be used. Preferably, the WDR neuron is chosen as a function or
psychological
correlate of chronic pain being felt by the patient 10. For example, the WDR
neuron chosen can
correspond to the portion of the body which the patient 10 feels chronic pain
(e.g., a neuron
corresponding to the right leg of a patient suffering from chronic pain in
their right leg,
technically defined as a "receptive field"). The electrical lead 40 is
configured to detect
electrical activity in the brain of the patient 10, and to relay the sensed
information to the system
5. Preferably, upon receiving sensed information from the electrical lead 40,
the analog-to-
digital converter converts the signal into a form desired by the pattern
recognition system 15.
The pattern recognition system 15 is configured to monitor the signal provided
by the
electrical lead 40 to determine the presence of specific neuronal activity
associated with chronic
pain (e.g., the pain signatures identified in the exemplary data described
herein). For example,
the pattern recognition system 15 can be configured to detect an increase in
spontaneous
background firing, an increase in rate of firing evoked by external stimulus
(e.g., pressure or
pinch), rhythmic after-discharge signaling, rhythmic oscillation, abnormal
bursting, etc.
Preferably, electrical lead 40 is configured to detect neuronal activity
(e.g., a pain signature) in
the sensory thalamus (ventral posterolateral, VPL) of the brain of the patient
10. The pattern
recognition system 15 can be configured to detect at least two different major
types of neuronal
activity spontaneous and evoked. Spontaneous activity is typically independent
or temporally
19
CA 02797701 2012-10-26
WO 2011/137193 PCT/US2011/034203
not associated with the presentation of an overt stimulus or identifiable
cause. Spontaneous
activity can best be described as an increase in the rate of spontaneous
activity in pain subjects
compared to naive/normal. Evoked activity is typically activity correlated
with an overt stimulus
or identifiable cause. Evoked activity can best be described as an increase in
the rate of evoked
activity in pain subjects in response to peripherally applied noxious and non-
noxious cutaneous
stimuli compared to naive/normal. In addition, abnormal bursting activity can
occur during both
spontaneous and evoked firing in pain compared to naive/normal.
The pattern recognition system 15 is configured to communicate with the
processor 20,
and is configured to provide information to the processor 20 in a
predetermined format over a
network connection (e.g., a bus or network connection in embodiments where the
pattern
recognition system 15 is separate from the processor 20). The pattern
recognition system 15 can
be configured to perform various signal processing functions on the signals
sensed from the
patient 10 (e.g., frequency analysis, Fourier transform, inverse Fourier
transform, filtering, de-
noising, threshold analysis, analysis of interspike intervals, analysis of
burst cycle periods,
analysis of spikes within bursts, etc.).
The processor 20 can be configured to examine information provided by the
pattern
recognition system 15 to determine the appropriate response. For example, the
processor 20 is
configured to differentiate between various patterns that can be recognized by
the pattern
recognition system 15 and to determine an appropriate response. The processor
20 can
differentiate between multiple recognized patterns, and determine an
appropriate response
strategy using, for example, a look-up table. The appropriate response can be
nothing at all, or,
for example, can be to cause an electrical signal to be provided to the brain
of the patient 10 via
an electrical lead 45.
The processor 20 can be configured to reverse the pain signature in the brain
of the
patient 10 using neurostimulation (or more accurately, neuromodulation). For
example,
neuromodulation can include the application of electricity of a predefined
voltage, frequency,
current, and duration to the brain of a patient 10. Preferably, the
neuromodulation applied to the
brain of the patient 10 is configured to "jam" the neuronal activity of the
patient 10 (i.e., rather
than further stimulating it). Preferably, this neuromodulation is achieved by
providing high
CA 02797701 2012-10-26
WO 2011/137193 PCT/US2011/034203
frequency current (e.g., between 150-200 Hz, 1-3 volts, 1-3 mA, and
substantially of 0.25-0.75
ms rectangular pulses of 1 second duration (assuming tissue impedance of 1000
S2). Preferably,
by delivering a low voltage, brief, and high frequency pulse to neuronal
structures that
preferentially respond to pain stimuli, the pain signature can transiently be
reversed back to
"normal." In addition, the neuromodulation protocol can be configured to
transiently attenuate
pain behavior in pain subjects to the level of that in naive/normal, while
otherwise retaining
tactile sensitivity. Electrical treatment is provided to the deep brain, the
VPL, and/or WDR
neurons.
One exemplary treatment protocol includes electrical stimulation of the brain
of the
patient 10 using periodic pulses of electricity. For example, intermittent
pulses (e.g., 1 pulse,
every 1-3 hours) can be provided anywhere along the pain circuitry of the
patient 10, but
preferably in the brain VPL nucleus. Preferably, each of the pulses has a
brief (e.g., 1 sec)
duration, high frequency (e.g., 150-200 Hz), and a low voltage (e.g., 1.5-2
V). Preferably, each
of these electrical pulses can "jam" the overactive circuitry in the brain of
the patient 10, based
on the temporal profile characterized herein. For example, for 2-3 hours, pain
symptoms can be
temporarily relieved after providing an electrical pulse.
The system 5 can be open-loop and/or closed-loop. In an open-loop embodiment,
the
system 5 can be programmed to provide electrical therapy according to a
predetermined protocol
(e.g., frequency, duration, voltage, amperage) without the use of the pattern
recognition system
15 and the analog-to-digital converter 30. In an open loop-embodiment, the
treatment protocol
can be stored in a memory that is connected to the processor 20. In a closed-
loop embodiment,
the system 5 preferably uses information received via, for example, the
pattern recognition
system 15 and the analog-to-digital converter 30 to treat cognitive,
affective, and emotive
neurological conditions, owing to the characterization of the pain signature
described herein.
While the closed-loop system described herein discusses the use of an
electrical lead 40
implanted in the brain of the patient 10, other configurations are possible
(e.g., receiving
diagnostic information from fMRI, or PET scanning). Additionally, while
separate leads (e.g..
leads 40, 45 are discussed, a single lead could instead be used for sensing
and provision of the
21
CA 02797701 2012-10-26
WO 2011/137193 PCT/US2011/034203
electrical signals. Additionally, the system 5 can be controlled manually
(e.g., by actuating a
button, or via a remote connection.
Electrophysiological measurements for pain signature:
Electrophysiological measurements of wide dynamic range (WDR) thalamic neurons
in
chronic constriction injury (CCI) rats indicate elevated evoked response to
pressure and pinch
stimuli in addition to rhythmic after-discharge signaling and an increase in
spontaneous
background firing (Figs. 3 and 4), in addition to abnormal burst. The group of
rats that
underwent CCI followed by thermal behavioral testing (n=10) display a
statistically significant
(P<0.05) decrease of the ipsilateral hindpaw withdrawal reflex over the course
of one week, with
a marked separation in the withdrawal latency of the ipsilateral and
contralateral hindpaws (Fig.
5). Treatment efficacy was assessed in part based on the reversal of this
known effect.
Anesthesia:
No significant difference resulted in neuronal activity under intravenously
administered
pentobarbital sodium as compared with isoflurane gas anesthesia (Fig. 6). For
this reason,
animals were tested exclusively with isoflurane gas and generalizations may be
applied across
experiments with a variety of anesthetization methods.
Deep Brain Stimulation:
Deep Brain Stimulation of CCI animals resulted in an attenuation of mean
firing rate in
response to all forms of mechanical stimuli, in addition to a statistically
significant decrease in
afterdischarge (Figs. 7 and 8). Furthermore, behavioral testing of awake DBS
rats in group 6
revealed a corresponding increase in withdrawal latency following high
frequency DBS (Fig. 9;
data represent values normalized to pre-FHS or Baseline 100%).
Histology:
Postmortem histological analysis of these rats as compared with CCI control
animals is
indicative of a statistically significant bilateral increase in VPL Ox 42
antibody staining (P<0.05)
while levels of GFAP antibody staining remain constant (Figs. 10 and 11).
Fig. 12 shows characterization of bursts in response to various stimuli. The
Requirements for defining bursts were:
= Maximum interval signifying burst onset (6 ms)
22
CA 02797701 2012-10-26
WO 2011/137193 PCT/US2011/034203
= Maximum interspike interval (9 ms)
= Longest increase in interspike interval within a burst (2 ms)
= Minimum number of spikes within a burst (2).
The following methods were used to generate the data described herein.
CCI: Chronic Constrictive Injury (CCI) was induced 7-9 days prior to data
acquisition.
Animals were anesthetized with isoflurane (2.5%). The surgical procedure
consisted of a
modification of the original loose ligation model designed by Bennett and Xie.
The process
involved isolation of the sciatic nerve via blunt dissection of the biceps
femoris followed by a
unilateral loose ligation with 5-0 gauge chromic gut ligature at three sites
above the branching of
the nerve, lmm apart. The ligation initiates an inflammatory response that
results in chromic gut
constriction of the nerve. Following the surgery, overlying muscles and skin
were closed with 4-
0 nylon sutures and the rodents were allowed time for recovery. Thermal
hyperalgesia resulting
from CCI has been found to remain relatively constant for a period of 5-27
days following the
injury.
Electrophysiology: Single unit firing -unit recording (i.e. sampling neuronal
activity one
at a time) was recorded under deep anesthesia (1.5% Isoflurane). Extracellular
single-unit
recordings in and were made with a 0.005" 5M I Teflon-coated silver
microelectrode (A-M
Systems, Carlsborg, WA). DBS animals were implanted with a modified electrode
as shown
below (Figs. 14, 15, 16). Each subject was placed in a stereotaxic frame, and
a limited
craniotomy exposed the brain surface vertical to the recording sites within
the VPL [Bregma
(-3.3; -2.5); lateral (2.8; 3.6); vertical (5.4; 6.4)] (Fig. 16). Electrical
signals were amplified and
filtered at 3000 Hz and processed with a CED micro 1401 data acquisition
system and SPIKE-2
software (Cambridge Electronic Design, Cambridge, England).
Waveforms were sorted to extract activity of a single neuron using automated
template-
matching. A hydraulic micropositioning device (Kopf Instruments, Tajunga,
California) was
employed in all vertical electrode penetrations through nervous tissue. As the
microelectrode is
lowered into the estimated region, a single "unit" or neuron can be isolated
by stimulating the
suspected somatosensory receptive field via tapping, brushing, pinching the
skin, or
manipulating the limbs of the anesthetized subject until excitation at the
location of the electrode
23
CA 02797701 2012-10-26
WO 2011/137193 PCT/US2011/034203
tip is measured via changes in current. This process was used in order to
identify VPL units
innervated by the sciatic nerve. Spontaneous activity was then measured,
followed by evoked
responses to mechanical stimulation within the receptive field. Six mechanical
stimuli were
applied during each recording session: (i) brush (BR); (ii-iv) increasing
intensity von Frey
filaments (0.6g, 8g. and 15g forces); (v) pressure (PR); (vi) pinch (PI). Wide
Dynamic Range
(WDR) thalamic neurons were specifically targeted based on their response to
each of the
mechanical stimuli.
Alternative Anesthesia Preparation: During preliminary trials, an additional
cohort of
animals underwent either tracheal intubation for the administration of 1.2%-2%
isoflurane, or IV
cannulation for the administration of pentobarbital sodium (40 mg/kg/hr) prior
to
electrophysiological recording. The purpose of these groups was to establish
the minimal effect
of anesthesia type and level on VPL firing activity.
DBS: After measurements of at least 2 consecutive series of recording
spontaneous and
evoked activities, the electrode was disconnected from the recording
equipment. The cathode of
an isolated pulse stimulator was connected to the electrode and the anode was
connected to the
skin of the rat at the base of the head, acting as a body ground. Preliminary
rectangular pulses
0.5 ms in width were applied at a frequency of 100 Hz at 0.5V for is.
Immediately after
stimulation, the electrode was disconnected from the stimulator and
reconnected to the recording
equipment and recording resumed. Background activity was recorded for 40s and
progressive
mechanical stimuli as described above were then applied for a duration of 20s
each with 40s rest
in between each. For any given unit, when there was no apparent change in
activity, electrical
stimulation was applied again with an increase in intensity (to 1.0 V or 1.5
V), frequency (from
100 Hz to 200 Hz), or number (5 times every 3s) of stimuli. The maximum
stimulation was
1.5V at 200Hz for is repeated every 3s for a total of 5 pulse events. When
there was an apparent
inhibition of the responses to at least one mechanical stimulus, DBS was
stopped and
consecutive electrophysiological recordings of a series of spontaneous and
evoked activities
were tested every 10 min.
Behavioral Testing: Behavioral tests of the CCI rats were performed with
respect to
thermal and mechanical stimulation in order to verify the presence of
allodynia and hyperalgesia.
24
CA 02797701 2012-10-26
WO 2011/137193 PCT/US2011/034203
Each animal was placed in a Plexiglas chamber situated on an elevated glass
plate 30 minutes
prior to testing for acclimatization. The thermal behavioral test consists of
focusing a radiant heat
source (4.7 amps) through the glass floor onto the plantar surface of the
rat's hind limb, resulting
in withdrawal behavior. The measured withdrawal latency is defined to begin at
the onset of
laser beam exposure and end upon movement of the rat hind paw from the floor
surface. Five
stimulation pulse events separated by 5 min were averaged for each hindpaw and
reported as the
withdrawal latency for a given session. In order to test the effectiveness of
HFS therapy on
awake rodents, one group of animals underwent behavioral testing throughout
the DBS treatment
regimen. DBS electrodes were held in place with orthodontic resin and
microelectrode leads
were stored in a small plastic container surgically implanted at the base of
the skull during
behavioral trials. All DBS behavioral testing animals received initial
stimulation at 1.5V and
200 Hz within 6-8 days post surgery. Baseline pre-operative behavioral data
was recorded for
analysis beginning one day prior to initial neurostimulation. Following the
DBS event,
behavioral tests were repeated 5 minutes and 30 minutes post treatment.
Histology and Image Analysis: In addition to verifying electrode placement,
supplementary postmortem tissue analysis was used to identify the activation
levels of glial cells
in the region of interest. This provided the opportunity to assess
microgliosis and astrogliosis
associated with glial scarring. In order to obtain images for subsequent
analysis, animals are
anesthetized (5% isoflurane) and transcardially perfused with ice cold
phosphate buffered saline
(PBS) supplemented with 10 USP units of anticoagulant Heparin Sulfate for 5
minutes
(10m1/min) followed by cooled 4% paraformaldehyde (PFA) in PBS for 5 minutes
(10 ml/min).
This fixation process was used in order to preserve nervous tissue form
degradation. Following
decapitation with a small animal guillotine, the head was stored in PFA over
night. The brains
were then removed and stored in cold 30% sucrose until fully impregnated. The
formalin-fixed
brains were blocked in the desired orientation and placed in tissue-embedding
media (0.C.T.
Compound 4583, Tissue-Tek). Brains were stored at -80 degrees Fahrenheit and
cut into 30 [tm
sections with a microtome. These sections (ranging from Bregma -2.12mm to -
4.16mm) were
mounted on slides, dried, and stained with OX-42 or GFAP for further analysis
of microglia or
actrocytes, respectively. All histological images were captured via
fluorescent microscope
CA 02797701 2012-10-26
WO 2011/137193 PCT/US2011/034203
(Eclipse 80i. Nikon with X-cite 120 EXFO fluorescent illumination).
Photographs were taken
via a high sensitivity digital camera (Retiga Exi Fast 1394, Q Imaging), and
were then uploaded
and digitally analyzed using IP LAB software (version 3.94r4, Scanalytics
Inc). For quantitative
comparison, the mean grayscale value of a 500x500 pixel region of interest for
each image was
used as an approximate measure of cell density.
The subject matter described herein can be implemented in digital electronic
circuitry, or
in computer software, firmware, or hardware, including the structural means
disclosed in this
specification and structural equivalents thereof, or in combinations of them.
The subject matter
described herein can be implemented as one or more computer program products,
such as one or
more computer programs tangibly embodied in an information carrier (e.g., in a
machine-
readable storage device), or embodied in a propagated signal, for execution
by, or to control the
operation of, data processing apparatus (e.g., a programmable processor, a
computer, or multiple
computers). A computer program (also known as a program, software, software
application, or
code) can be written in any form of programming language, including compiled
or interpreted
languages, and it can be deployed in any form, including as a stand-alone
program or as a
module, component, subroutine, or other unit suitable for use in a computing
environment. A
computer program does not necessarily correspond to a file. A program can be
stored in a
portion of a file that holds other programs or data, in a single file
dedicated to the program in
question, or in multiple coordinated files (e.g., files that store one or more
modules,
sub-programs, or portions of code). A computer program can be deployed to be
executed on one
computer or on multiple computers at one site or distributed across multiple
sites and
interconnected by a communication network.
The processes and logic flows described in this specification, including the
method steps
of the subject matter described herein, can be performed by one or more
programmable
processors executing one or more computer programs to perform functions of the
subject matter
described herein by operating on input data and generating output. The
processes and logic
flows can also be performed by, and apparatus of the subject matter described
herein can be
implemented as, special purpose logic circuitry, e.g., an FPGA (field
programmable gate array)
or an ASIC (application specific integrated circuit).
26
CA 02797701 2012-10-26
WO 2011/137193 PCT/US2011/034203
Processors suitable for the execution of a computer program include, by way of
example,
both general and special purpose microprocessors, and any one or more
processor of any kind of
digital computer. Generally, a processor will receive instructions and data
from a read-only
memory or a random access memory or both. The essential elements of a computer
are a
processor for executing instructions and one or more memory devices for
storing instructions and
data. Generally, a computer will also include, or be operatively coupled to
receive data from or
transfer data to, or both, one or more mass storage devices for storing data,
e.g., magnetic,
magneto optical disks, or optical disks. Information carriers suitable for
embodying computer
program instructions and data include all forms of non-volatile memory,
including by way of
example semiconductor memory devices, (e.g., EPROM. EEPROM, and flash memory
devices);
magnetic disks, (e.g., internal hard disks or removable disks); magneto
optical disks; and optical
disks (e.g., CD and DVD disks). The processor and the memory can be
supplemented by, or
incorporated in, special purpose logic circuitry.
To provide for interaction with a user, the subject matter described herein
can be
implemented on a computer having a display device, e.g., a CRT (cathode ray
tube) or LCD
(liquid crystal display) monitor, for displaying information to the user and a
keyboard and a
pointing device, (e.g., a mouse or a trackball), by which the user can provide
input to the
computer. Other kinds of devices can be used to provide for interaction with a
user as well. For
example, feedback provided to the user can be any form of sensory feedback,
(e.g., visual
feedback, auditory feedback, or tactile feedback), and input from the user can
be received in any
form, including acoustic, speech, or tactile input.
The subject matter described herein can be implemented in a computing system
that
includes a back-end component (e.g., a data server), a middleware component
(e.g., an
application server), or a front-end component (e.g., a client computer having
a graphical user
interface or a web browser through which a user can interact with an
implementation of the
subject matter described herein), or any combination of such back-end,
middleware, and
front-end components. The components of the system can be interconnected by
any form or
medium of digital data communication, e.g., a communication network. Examples
of
27
CA 02797701 2012-10-26
WO 2011/137193 PCT/US2011/034203
communication networks include a local area network ("LAN") and a wide area
network
("WAN"), e.g., the Internet.
Example 1: Single-unit physiology in the ventral posterolateral nucleus of the
thalamus
in neuropathic rats
Neuropathic pain secondary to nerve injury is often chronic and accompanied by
dysesthesias. It is linked to long-term changes in neuronal physiology, known
as neuroplasticity,
which is well described in peripheral nerves and the spinal cord but
relatively less understood in
the brain. In spite of recent advances in pharmacotherapy, neuropathic pain
remains poorly
managed.
An early clinical account of aberrant thalamic physiology was documented,
which was
later localized to the intralaminar, medial and ventral thalamic nuclear
groups of patients with
neurogenic pain, central deafferentation pain, as well as peripheral
neuropathic pain. Under these
painful conditions, single unit activity is generally described in terms of
higher probability of
spontaneous firing, increased rate of evoked firing, ectopic bursting, and
dysrhythmic activity.
Clinical evidence of aberrantly firing thalamic neurons in chronic pain is
corroborated by
data from animal models. In rats with central pain following spinal cord
injury, neurons in the
ventral posterolateral (VPL) nucleus of the thalamus manifest higher
probability of spontaneous
firing, afterdischarge, increased evoked responses and characteristic bursting
patterns. In
comparison, little is known about changes in tonic or burst firing of VPL
neurons following
peripheral neuropathic injury without central lesion.
Nociceptive neurons in the VPL receive ascending projections mainly from
spinothalamic tract neurons and project to several cortical areas including
the primary
somatosensory cortex. Within the VPL, a group of neurons responds to a wide
dynamic range
(WDR) of mechanical stimuli, phenotypically homologous to WDR neurons at
spinal cord level
whose role in central sensitization and chronic pain is well documented.
In addition to the correlation between pain and neuroplasticity, the
therapeutic effects of
neuromodulation by deep brain stimulation (DBS) further suggests that brain
plasticity is likely
to have functional significance. For example, DBS in the periaqueductal gray
and motor cortex
effectively relieves pain symptoms and decreases the requirement for pain
medication. More
28
than 1000 clinical cases of DBS for chronic pain were preformed in the
seventies and eighties.
Although the Food and Drug Administration (FDA) rescinded its approval in the
late eighties,
there has been resurgence of interest in this medical procedure in the last
decade with an
emphasis on patient selectivity and, more importantly, understanding basic
mechanisms.
Regarding DBS in the VPL, information related to the effects of
microstimulation on
neuroplasticity and sensory phenomena is limited, with clinical studies
reporting mixed results.
Although the mechanisms of DBS are not well understood, stimulation frequency
represents a key factor, with high frequency stimulation (HFS, >100 Hz)
mimicking the
functional effects of ablation, also referred to as 'jamming' of local
circuitry. HFS in the VPL
reduces mechanical allodynia in rats with peripheral neuropathy.
HFS can be used to inhibit hyperactive VPL neurons, thus reversing
neuroplasticity and,
consequently, behavioral hypersensitivity. The firing of single units
extracellularly from VPL
neurons in naïve rats was recorded. Firing from neuropathic rats after chronic
constriction injury
(CCI) of the sciatic nerve was also recorded. The data show that tonic and
burst firing patterns
in rats with CCI were significantly different from those in naïve rats and
were partially reversed
after microstimulation in the VPL at high (but not low) frequency, with
subsequent attenuation
of hyperalgesia.
The following materials and methods were used to generate the data described
in this
example.
Adult Male Sprague-Dawley rats (250-300g) were used in this study.
Chronic constriction injury (CCI). As previously described (Owolabi SA, Saab
CY
(2006) Fractalkine and minocycline alter neuronal activity in the spinal cord
dorsal horn. FEBS
Lett 580:4306-4310; LeBlanc BW, Iwata M, Mallon AP, Rupasinghe CN, Goebel DJ,
Marshall
J, Spaller MR, Saab CY (2010) A cyclic peptide targeted against PSD-95 blocks
central
sensitization and attenuates thermal hyperalgesia. Neuroscience 167:490-500),
a modified CCI from the originally described model (Bennett
GJ, Xie YK (1988) A peripheral mononeuropathy in rat that produces disorders
of pain sensation
like those seen in man. Pain 33:87-107
was performed. The
sciatic nerve was exposed after skin incision at the mid-thigh level and blunt
dissection of the
29
Date Recue/Date Received 2021-04-07
biceps femoris under deep anesthesia (isoflurane 3-4 %). Three chromic gut (5-
0) ligatures were
tied loosely around the nerve 1 mm apart, proximal to its trifurcation. After
CCI, the overlying
muscles and skin were closed in layers with 4-0 nylon sutures and the animal
was allowed to
recover. Rats were then maintained under the same pre-operative conditions and
fed ad libitum.
At day 7 after CCI, neuropathic manifestations are persistent for several
weeks thereafter.
Single-unit extracellular recording. Animals from naive and CCI groups
underwent
extracellular single unit recording from VPL neurons according to established
methods (Hains
BC, Saab CY, Waxman SG (2006) Alterations in burst firing of thalamic VPL
neurons and
reversal by Na(v)1.3 antisense after spinal cord injury. J Neurophysiol
95:3343-3352).
The activity of 1-2 units/animal was recorded. Rats were initially
anaesthetized with isoflurane (4 % in induction chamber), and maintained by
tracheal intubation
(1.5 %; interestingly, no difference was noted in the firing rate under
isoflurane or pentobarbital
sodium (60 mg/kg) anesthesia). The head was fixed in a stereotaxic apparatus
(Kopf
Instruments, Tujunga, CA, USA) and skin incision and a limited craniotomy
exposed the brain
surface vertical to the recording sites within the thalamus. Neuronal units
were isolated from the
VPL nuclei of the thalamus [respective stereotaxic coordinates in mm: bregma (-
3.3, -2.5);
lateral (2.8, 3.6); vertical (5.4, 6.4)]. Extracellular single-unit recordings
were made with a 5
1\41-2 Teflon-coated tungsten microelectrode (A-M Systems, Carlsborg, WA,
USA). Electrical
signals were amplified and filtered at 300-3000 Hz (DAM80. World Precision
Instruments,
Sarasota, FL, USA), processed by a data collection system (CED micro1401mkII;
Cambridge
Instruments, Cambridge, UK) to construct peristimulus time histograms. The
stored digital
record of individual unit activity was retrieved and analysed off-line with
Spike2 software
(Cambridge Electronic Design, CED, Cambridge, UK). Once a unit was identified
by a gentle
probing of the body surface, its receptive field was mapped and stimulated by
an experimenter.
For testing evoked activity, six routine natural mechanical stimuli were
applied in the
following order: brush, by a cotton applicator to the skin; three von Frey
filaments (0.6, 8 and 15
g) with enough force to cause buckling of the filament at a regular frequency
of 1 application per
sec; pressure, by attaching a large arterial clip with a weak grip to a fold
of skin (144 g/mm2) and
pinch, by applying a small arterial clip with a strong grip to a fold of skin
(583 e/mm2).
CA 2797701 2017-09-28
Multireceptive units were identified by their responsiveness to brush,
pressure and pinch, and
with increasing responsiveness to incrementing strength von Frey stimuli. Care
was taken to
ensure that each stimulus was applied to the primary receptive field, and that
isolated units
displayed action potentials that remained stable for the duration of each
experiment using Spike2
template matching. Firing activity was computed as mean frequency of spikes/20
s, and evoked
responses and after discharges were calculated by subtracting the pre-stimulus
baseline activity
to yield a net increase in discharge rate. Afterdischarge was defined as
continuous discharge
after noxious pinch stimulus removal for 20 s. Cursors were set at the
beginning and the end of
the stimulus, and all of the spikes occurring between the cursors were summed.
Cursors were
also set at the beginning of the trace and after 40 s (baseline or un-evoked
firing), and the spikes
occurring during this period were summed to provide a measure of the
background activity. The
two sums were divided by the respective duration and the resulting averages
(spikes/s) subtracted
to yield the value attributed to the response (total number of spikes/s in
excess of the background
activity during the stimulus). One to two neurons with individually mapped
receptive field were
recorded from each rat. Neuronal activity was analyzed off-line using Spike2.
In some rats, the spinal cord was also exposed by laminectomy at thoracic (T4-
T6) level
and topical 2% lidocaine was applied using a cotton pledget to the dorsal and
lateral surfaces of
the spinal cord, followed 10 min later by complete cord transection using fine
scissor while
recording from the VPL neuron continued.
Estimated charge density. A theoretical limit of 30 u.C/cm2 has been proposed
for the
maximal allowable charge density above which tissue damage occurs (Medtronic
(1998) DES
TM technical manual. Minneapolis: Medtronic; Kuncel AM, Grill WM (2004)
Selection of
stimulus parameters for deep brain stimulation. Clin Neurophysiol 115:2431-
2441; Shimojima
Y, Morita H, Nishikawa N, Kodaira M, Hashimoto T, Ikeda S (2010) The safety of
transcranial
magnetic stimulation with deep brain stimulation instruments. Parkinsonism
Relat Disord
16:127-131, = based on the following formula:
Voltage (V) x Pulse width (its)
Impedance (0) x electrode SA (cm2)
31
CA 2797701 2017-09-28
CA 02797701 2012-10-26
WO 2011/137193 PCT/US2011/034203
Accordingly, given the following approximations of stimulation parameters at 1
V, 500
s width, 1500 S-2 and 0.02 cm2 electrode tip surface area, the charge density
within the vicinity
of the silver microelectrode tip used in our behavioral experiments is roughly
16 C/cm2, i.e.
below maximal density (charge density for Tungsten microelectrodes used in the
acute
electrophysiology experiments is much lower due to higher electrode
impedance). In addition,
modeling studies suggest the possibility of 'current steering' using bipolar
stimulating electrodes
so that the shape of the area subjected to stimulation can more closely
overlap with a particular
region of interest in the brain, therefore improving stimulation efficacy and
minimizing side
effects. Referring to modeling studies (e.g., Butson CR, Maks CB, McIntyre CC
(2006) Sources
and effects of electrode impedance during deep brain stimulation. Clin
Neurophysiol 117:447-
454; Butson CR, McIntyre CC (2006) Role of electrode design on the volume of
tissue activated
during deep brain stimulation. J Neural Eng 3:1-8; Butson CR, McIntyre CC
(2008) Current
steering to control the volume of tissue activated during deep brain
stimulation. Brain Stimul
1:7-15), the stimulation parameters with a monopolar electrode would
theoretically result in a
spherical electric field with a radius of approximately 2 mm, whereas
considering the bipolar
electrode design and their orientation in the brain, an optimal overlap was
predicted between the
electric field potential and the VPL nucleus according approximations
illustrated in Fig 17.
Burst analysis. Burst events were identified using Spike 2 Burst script using
the
following criteria: 6 ms of the maximum interval between two events that
signifies the start of a
burst, 9 ms of the longest interval between two events within a burst, and 2
of the minimum
number of events in a burst. The following parameters were calculated during
the recording
periods of background activity, six natural mechanical stimuli evoked
discharges and after
discharge: Number of burst events, mean inter burst time (ms), and % spikes in
burst:
Mean spikes/burst x Number of bursts x 100
Total spikes
Micro-stimulation in the VPL. To test the effect of microstimulation on
neuronal firing
in anesthetized rats, the same recording electrode was used for
microstimulation within the VPL.
After identifying single units and recording pre-stimulation (baseline) firing
rates, the electrode
was disconnected from the recording equipment and connected to a stimulator (A-
M Systems
32
CA 02797701 2012-10-26
WO 2011/137193 PCT/US2011/034203
Isolated Pulse Stimulator). Rectangular pulses 0.5 ms width were applied at a
frequency of 100
Hz at 0.5 V for 1 s. Immediately after stimulation, the electrode was
disconnected from the
stimulator and reconnected to the recording equipment and data acquisition
resumed within 2-3
min after baseline measurements. For any given unit, if no change in firing
was apparent after
stimulation, electrical stimulation was applied again with an increase in
intensity (1.0 V and 1.5
V). frequency (100 Hz and 200 Hz), respectively, up to 5 microstimulation
pulses every 3 s.
When at least one evoked response was modulated by microstimulation, DBS was
stopped and
consecutive electrophysiological recordings of firing rates were tested every
10 min. Therefore,
for any given unit, "minimum" stimulation consisted of a single 0.5 V, is
pulse at 100Hz, and
"maximum" stimulation consisted of 5 x 1.5 V, 1 s rectangular pulse at 200 Hz.
To test the effect of microstimulation on behavior in non-anesthetized rats,
bipolar silver
wires (-1.5 Kfl) were chronically implanted on the day of CCI. Electrode
segments were fused
together with Epoxy resin forming cathode and anode. Teflon coating was
removed exposing
0.5 mm on each electrode tip, which were separated by 1 mm from tip-to-tip
vertically. Initially,
the longer tip was connected to the recording equipment and local field
potentials were recorded
to localize the VPL area with a receptive field in the contralateral hindpaw
(see description
below for additional details on the orientation of the electrodes relative to
the VPL for optimal
steering of electric field and overlap with VPL nuclear structure). When a
distinct increase in
activity was recorded in response to mechanical probing of the hindpaw, the
wires were fixed to
the skull permanently using a screw and orthodontic resin while microelectrode
leads were
encased in a small plastic container fixed to the base of the skull.
Withdrawal latencies were
measured 5 min before (baseline) and up to 2 hr after microstimulation (3 x
1.5 V, 1 s
rectangular pulse at 200 Hz; A-M Systems Isolated Pulse Stimulator).
Behavioral analysis. Thermal sensitivity of the paw was assessed by measuring
the
latency of the withdrawal reflex in response to a radiant heat source. Animals
were placed in
Plexiglas boxes on an elevated glass plate under which a radiant heat source
(4.7 amps) was
applied to the plantar surface of the hindpaw. Paw withdrawal latencies (PWLs)
of five
stimulations, separated by 5 min rest, were averaged for each paw. For
'baseline' pre-operative
values, data were averaged for both paws as no difference was observed in PWLs
between paws.
33
CA 02797701 2012-10-26
WO 2011/137193 PCT/US2011/034203
OX-42 and GFAP immunoreactivity. Rats were anesthetized with pentobarbital (60
mg/kg, i.p.) then perfused intracardially with ice-cold PBS followed by
buffered 4%
paraformaldehyde. Brains were post-fixed with buffered 4% paraformaldehyde
overnight,
equilibrated in 30% sucrose, and frozen to -80 C in OCT cryogenic compound
(TissueTek
Sakura). Coronal sections (30 pm) were adhered to glass slides and blocked
with goat serum.
Sections were stained for OX-42 (Santa Cruz Biotech, mouse IgG, 1:50), or GFAP
(Chemicon
International, mouse IgG, 1:100) overnight at 4 C. Slides were washed with PBS
and probed
with goat anti-mouse IgG (VectorLab 1:2000), visualized with a Nikon Eclipse
Fluorescent
microscope, and images were captured using a Qiacam CCD camera. Mean
fluorescent intensity
was measured using ImageJ (NIH v1.43n) in 3 predetermined non-redundant (160
um)2 boxes
within the VPL bilateral to CCI per slide in each animal.
Statistical analysis. All statistical tests were performed at the alpha level
of significance
of 0.05 using parametric tests. Data were tested for significance using one-
way ANOVA to
determine degree of variability within a sample and whether there was a
difference between
groups among the obtained means. Tests of factors including pairwise
comparisons were carried
out where appropriate, with either the paired Student's t-test for
before¨after comparisons or the
two sample Student's t-test to compare two groups. Data management and
statistical analyses
were performed using Excel and presented as mean standard deviation.
Extracellularly recorded action potentials were isolated from single units in
the VPL
nucleus of the thalamus under deep anesthesia using template matching
techniques. All units
discharged action potentials at relatively constant voltage amplitudes and
responded with
increased firing rates to contralateral mechanical stimuli. In a
representative example from a
naïve rat, peristimulus time histogram shows increased firing rate when
noxious and non-noxious
stimuli are applied to the receptive field in the contralateral hindpaw,
noting a graded response to
increased strengths of von Frey filaments, compared to almost absent
spontaneous firing (Fig
18A. upper panel). Seven days after CCI, evoked responses to pressure and
pinch stimuli are
increased, while spontaneous activity and afterdischarge are elevated (Fig
18A, lower panel).
Compared to naive rats, the mean firing rates of VPL neurons with receptive
fields in the
contralateral hindpaw in rats with CCI increased significantly in response to
brush and pinch to
34
CA 02797701 2012-10-26
WO 2011/137193 PCT/US2011/034203
246% and 137%, respectively, with emergence of ectopic spontaneous activity
and significant
elevation of afterdischarge to 243% (Fig 18B and Table 1 (Fig. 29)). In
contrast, the mean
frequency of evoked responses to brush and von Frey filaments (0.6, 8 and 15
g) in rats with CCI
were not significantly different from corresponding values in naïve rats.
Thus, sciatic
neuropathy is associated with plasticity of WDR neurons in the VPL with
receptive fields in the
contralateral injured hindpaw. Plasticity manifests as selective
hyperexcitability in response to
non-noxious pressure and noxious pinch, in addition to an emergence of un-
evoked firing at a
high rate. Of note, evoked responses to von Frey filaments (0.6, 8 and 15 g),
pressure and pinch
of VPL neurons with somatic receptive fields excluding the hindpaws in rats
with CCI were not
significantly different from corresponding values in naïve rats, except for a
significant increase
in brush-evoked responses after CCI, whereas spontaneous activity and
afterdischarge were less
than 1 Hz in both naïve and CCI rats (Fig. 25).
In addition, spontaneous rhythmic oscillation was observed in 33% of VPL
neurons
recorded from rats with CCI. In one representative example, the firing rate
during oscillatory
epochs reached that of the pinch-evoked response. However, rhythmic
oscillation and brush-
evoked responses were abolished by complete transection of the spinal cord at
thoracic level,
whereas spontaneous firing remained high (Fig. 19A). One example (Fig. 19B)
shows a
peristimulus rate histogram of the same unit superimposed over a sinusoidal
curve during the
period of rhythmic oscillation (before transection) compared to nearly flat
rate histogram after
transection. Mean amplitude and frequency of sinusoidal curves were 24.4 8.2
spikes/s (peak-
to-peak) and 0.0095 0.00035 cycle/s, respectively (Fig 19C). These data
indicated that, in
addition to the high spontaneous firing rate, sciatic neuropathy is correlated
with a rhythmically
ectopic firing pattern in a group of VPL neurons dependant on on-going
peripheral and/or
ascending input below thoracic level.
To test whether microstimulation within the VPL modulates the firing of single
units,
HFS was delivered through the recording electrode and the rate of firing was
measured before
and after micro stimulation. In rats with CCI, graded attenuation of evoked
firing was achieved
with incremental increase in voltage amplitude at 100 Hz and a prominent
effect was noted at 1.5
V. whereas spontaneous activity was not affected (Fig. 20A). Results with 100
Hz and 200 Hz
CA 02797701 2012-10-26
WO 2011/137193 PCT/US2011/034203
were comparable and therefore pooled together collectively. All mean firing
rates were
significantly decreased up to approximately -50% within 10-15 min after HFS
for all firing
modes except spontaneous activity (Fig. 20C and Table 2). By comparison, LFS
at 25 Hz had no
significant effect on neuronal firing in rats with CCI at 1.5V, the same
voltage amplitude which
was otherwise effective with HFS (Fig. 20B, D and Table 2). Results with 25 Hz
and 40 Hz
were comparable and therefore pooled together. Interestingly, even 'moderate'
HFS (mFHS; 1 x
100 Hz, 0.5 V, 1s) resulted in significant per cent decreases in the firing
rates of pressure and
pinch-evoked responses and afterdischarge in rats with CCI. In naive rats, HFS
also attenuated
all evoked responses except for von Frey 8 g filament (Fig. 26 and Table 4).
Therefore,
micro stimulation within the VPL at high (>100 Hz), but not low (>40 Hz)
frequency effectively
reverses neuroplasticity induced by sciatic neuropathy by attenuating evoked
firing and
afterdischarge.
Because tonic and burst firing contribute to signal processing, several burst
parameters
were measured for single units in the VPL with contralateral hindpaw receptive
fields in naive
rats and in those with CCI. Fig. 21 shows examples of burst firing during
brush-evoked
responses from a naive rat and another rat seven days after CCI. In naive
rats, spontaneous bursts
events were almost absent, whereas those in rats with CCI were increased in
number (Fig. 22 and
Table 3). Spontaneous bursts were detected in only 2 out of 14 units from
naive rats (mean burst
events 0.4 0.3). Therefore burst parameters during spontaneous activity in
naive rats were not
analyzed. In contrast, burst events were increased significantly for all
firing modes in rats with
CCI compared to those in naïve rats, which were attenuated after HFS with
significant changes
for pressure and pinch-evoked responses (Fig. 22 and Table 3). Similar trends
were observed
between groups for per cent spikes in burst values, whereas changes in the
opposite directions
were noted for mean interburst time values (i.e. values decreased for all
firing modes in rats with
CCI compared to those in naive rats and were increased after HFS). In general,
therefore,
bursting patterns consistently deviated from normal after sciatic neuropathy
reaching significant
levels. These changes were consistently reversed in the normal direction after
HFS, indicating
that HFS reverses several aberrant features of neuroplasticity including tonic
and burst firing
properties.
36
Although spontaneous tonic and burst firing at the single unit level in rats
with CCI was
not affected by microstimulation, studies were carried out to determine
whether HFS modulates
the local field potential within the VPL. Normalized power spectrum (computed
by fast Fourrier
Transfer and plotted using Spike2) of local field potential recorded from the
VPL of a rat with
CCI shows a dominant peak in the low i3 frequency range (10-20 Hz) which does
not vary
significantly in amplitude or frequency before and after HFS (Fig. 23),
suggesting HFS causes
minimal or no modulation of spontaneous neuronal activity in the VPL at a
population level.
Since HFS parameters comparable to those used in this study have been
demonstrated to
attenuate CCI-induced allodynia (Kupers RC, Gybels TM (1993) Electrical
stimulation of the
ventroposterolateral thalamic nucleus (VPL) reduces mechanical allodynia in a
rat model of
neuropathic pain. Neurosci Lett 150:95-98,
experiments were
carried out to determine whether HFS also modulates thermal hyperalgesia in
rats with CCI.
Consistent with prior observations using this procedure (Saab CY, HaMs BC
(2009) Remote
neuroimmune signaling: a long-range mechanism of nociceptive network
plasticity. Trends
Neurosci 32:110-117; Saab CY, Shamaa F, El Sabban ME, Safieh-Garabedian B,
Jabbur SJ,
Saade NE (2009) Transient increase in cytoldnes and nerve growth factor in the
rat dorsal root
ganglia after nerve lesion and peripheral inflammation. J Neuroimmunol 208:94-
103; LeBlanc
BW, Iwata M, Mallon AP, Rupasinghe CN, Goebel DJ, Marshall J, Spaller MR, Saab
CY (2010)
A cyclic peptide targeted against PSD-95 blocks central sensitization and
attenuates thermal
hyperalgesia. Neuroscience 167:490-500;
PWL in the injured hindpaw was significantly decreased to 6.8 + 1.1 s compared
to 10.6 1.9 s
in the non-injured hindpaw seven days after CCI, and relative to 11.1 1.6 s
for the mean
latency of both hindpaws pre-operatively (Fig. 24A), indicating that sciatic
neuropathy reliably
resulted in thermal hyperalgesia. Using bipolar stimulating electrodes
chronically implanted
contralateral to sciatic injury on the day of CCI, HFS voltage at 1.5 V was
delivered within the
VPL while testing the thermal withdrawal reflex. Under sham conditions
(connecting the
electrode to the stimulator without voltage stimulation), withdrawal latency
in the injured
hindpaw remained decreased compared to the non-injured hindpaw (Fig. 24B).
However,
withdrawal latency in the injured hindpaw was transiently reversed 5 min after
HFS with
37
CA 2797701 2017-09-28
CA 02797701 2012-10-26
WO 2011/137193 PCT/US2011/034203
reproducible effects after 2 hr. The mean withdrawal latency was transiently
and significantly
reversed from 6.3 0.9 s to 9.1 0.1 sand 7.3 0.1 sat 5 min and 10 min
after HFS,
respectively, in the injured hindpaw. Of note, the withdrawal latency in the
non-injured hindpaw
also increased after HFS, however, this change was not significant (Fig. 27).
Lastly. GFAP and OX-42 were quantified in tissue sections in or around the tip
of the
chronically implanted electrodes in the VPL, which revealed no significant
changes in
expression compared to the contralateral VPL (Fig. 28), indicating limited
glial reactivity to
electrode implantation and HFS.
Peripheral neuropathic injury is associated with neuroplasticity of VPL
neurons with
receptive fields in the contralateral injured hindpaw. Abnormal physiologic
properties include
higher rate of spontaneous firing, increased rates of afterdischarge and
evoked firing in response
to non-noxious and noxious mechanical stimuli. In addition, significant
changes in burst firing
and rhythmic oscillations were observed. Otherwise, the firing of neurons with
receptive fields
elsewhere in the body was not changed, except for increased responses to brush
stimuli.
The data confirmed enhanced spontaneous firing and increased activity evoked
by brush,
pressure and pinch stimuli in rats ten days after CCI. Noting minor
modifications to the CCI
model used in this study, these observations were exended to include hyper-
responsiveness to
von Frey stimulation and afterdischarge at an earlier (day 7) time point
concomitant with
neuropathic behavior, in addition to other abnormal patterns of tonic and
burst firing.
Hyper-responsiveness, rhythmic oscillations and bursting in VPL neurons are
observedin
a rat model of central neuropathic pain following spinal cord contusion.
Relative to neuronal
firing in normal rats, firing in rats with spinal lesion alternated between
simple, burst and spindle
epochs, whereas the number of spikes per burst decreased and interburst
interval was not
changed. Compared to this study, different lesion sites (sciatic versus
spinal) likely account for
the incompletely matching patterns of aberrant firing in the VPL. However,
spontaneous
neuronal discharge in rats with peripheral or central neuropathy remained high
after complete
transection of the spinal cord, suggesting these changes in specific are
likely 'intrinsic' to VPL
neurons, while rhythmic oscillations described in this study depend on on-
going ascending input.
38
In addition to firing rate, spike timing is a key factor in determining
information content
in spike trains. Burst spikes and isolated spikes both contribute to the
coding of stimulus-related
information (Middleton JW, Yu N, Longtin A, Maier L (2011) Routing the flow of
sensory
signals using plastic responses to bursts and isolated spikes: experiment and
theory. J Neurosci
31:2461-2473.
Therefore, burst firing is an important feature
of the neural code and can effectively impact postsynaptic neurons, including
upstream from the
VPL in the somatosensory cortex. Bursting can be modulated by the temporal
frequency content
of incoming signals, which in the case of peripheral neuropathy is
significantly altered at
peripheral and spinal cord levels. The duration of bursts and the relative
timing of burst spikes
can encode specific stimulus features. Neurons in the lateral geniculate
nucleus discharge bursts
and isolated spikes in response to visual stimuli, with different stimulus
conditions preferentially
evoking different bursting patterns. Bursting has also been reported in
thalamocortical
projection cells, including the auditory and the somatosensory systems.
Although the functional
significance of bursting in VPL neurons and modulation thereof under chronic
pain conditions
are unknown, interestingly, short-term plasticity of thalamocortical synapses
is thought to
accentuate the sensitivity of cortical responses to ascending sensory input.
Burst firing has been documented in the ventral thalamus in a patient with
root injury and
occurred during episodes of self-reported touch-evoked allodynia. Direct
microstimulation
within a thalamic nucleus that contains abnormally active neurons produces
burning dysesthesia
in patients with spinal deafferentation pain, as well as with root injury
pain, whereas
microstimulation in the same area might also produce pain relief. These
observations suggest
that the aberrant physiology of thalamic cells may be directly related to the
dysesthesias, whereas
the functional outcome depends on the pain condition and the neurostimulation
protocol. It's
worth noting that changes in the patterns of burst firing, rather than burst
firing pre se, might be a
reliable marker for pain-related plasticity. For example, burst firing can
occur in deafferented
subjects without pain.
HFS in CCI rats caused a 'reversal' effect on abnormal bursting patterns in
the direction
of normal condition, showing consistent results across all firing modalities.
This is in agreement
with the premise that HFS in the therapeutic range for motor disorders works
mainly by
39
CA 2797701 2017-09-28
CA 02797701 2012-10-26
WO 2011/137193 PCT/US2011/034203
inhibiting neuronal firing or neuronal 'jamming'. The data described herein
relates to
hyperactive neurons in the VPL and a comparison of the effects of HFS and LFS
on neuronal
firing at the single unit level. Whereas HFS effectively reversed neuronal
hyper-responsiveness,
spontaneous activity did not change and overall responsiveness to mechanical
stimuli was
diminished but not abolished. Prior to the invention, no study compared the
effects of varying
stimulation frequency on analgesia. It is interesting to note that low
frequency (50 Hz)
stimulation in the VPL produces little or no analgesia in naïve rats. Data
using in vivo and
computational models suggest that high frequency signals suppress bursting by
transiently
interrupting the depolarizations caused by low-frequency signals, for example
those evoked by
peripheral mechanical stimuli. In a clinical setting, single units in the
intralaminar thalamic
nuclei of patients with chronic deafferentati on pain discharge action
potentials spontaneously at a
high rate, often rhythmically. In these patients, bursts are described as
short (2-6 spikes/burst)
with a frequency of 1-4 burst/s, or long (30-80 spikes/burst) with a similar
frequency. As
described herein, spontaneous burst firing matched well predominantly with the
short bursting
mode (2.4 0.1 spikes/s and 0.6 0.4 burst/s; values derived from data in Fig.
22 and Table 4).
In rats with central pain, the firing of neurons in the somatosensory cortex
is also changed
in ways similar to those described for VPL neurons. Dysfunctional
thalamocortical
communication may underlie cognitive disorders and sensory disturbances
resembling tactile
allodynia and thermal hyperalgesia, common symptoms of chronic pain. Analysis
of magnetic
encephalography activity in patients with complex regional pain syndrome shows
a distinct shift
in power spectra relative to normal subjects, suggesting that the
physiological changes in the
brain associated with chronic pain may manifest as significant disturbances in
network
connectivity. Corroborating these electrophysiological data, imaging studies
reported several
brain regions with decreased grey matter, most commonly in the cingulate,
orbitofrontal, and
insular cortices. Some studies also show changes in primary and secondary
somatosensory
cortices and the thalamus. This somewhat diffuse anatomical distribution
reflects the co-
morbidity of chronic pain with several affective disorders, as well as
cofounding variables
caused by various medications in clinical studies involving pain patients.
CA 02797701 2012-10-26
WO 2011/137193 PCT/US2011/034203
One important consideration is that of potential tissue damage as a result of
microstimulation. However, the use of an optimal charge density during
microstimulation in
behavioral experiments and absence of glial activation indicate little if any
tissue damage at the
stimulation site using the methods and systems described herein.
The data demonstrated anti-nociceptive effects of HFS on thermal hyperalgesia
in rats
with CCI, complementing previous data showing similar effects in the
mechanical allodynia test
in the same animal model, also using HFS of comparable stimulation parameters.
It has been
demonstrated clinically that electrical stimulation of various sites of the
brain, such as the
periaqueductal gray matter (PAG), median thalamic nuclei, as well as less
commonly targeted
areas such as the pontomesencephalic parabrachial region, are effective in
relieving chronic pain
that is non-responsive to standard pharmacotherapy.
Neurons in the PAG, known to mediate descending anti-nociceptive control unto
lumbar
dorsal horn neurons, could be activated by electrical stimulation in the VPL,
an influence which
is not affected by systemic administration of naloxone, suggesting that the
VPL-PAG analgesic
pathway is unlikely to involve the opioid system. This mechanism provides an
alternative
explanation to the widely held idea that the analgesic effects of subcortical
brain stimulation is
the result of activation of a descending 'pain suppressive system'
concentrated in the
periventricular and periaqueductal regions, which blocks nociceptive
transmission at the level of
the spinal cord. Primate spinothalamic tract (STT) neurons in the spinal cord
are inhibited
bilaterally by low current (<25 A) high frequency (333 Hz) stimulation in the
VPL unilaterally,
in support of data described herein showing increased withdrawal thresholds in
both hindpaws in
response to unilateral microstimulation in the VPL. In addition, VPL
stimulation may cause
sufficient input to the somatosensory cortex to activate corticofugal
inhibitory pathways,
following thalamocortical-corticofugal projections inhibiting spinal cord
nociceptive neurons. It
is also possible that VPL stimulation activates antidromically axon
collaterals of STT neurons
projecting to the medial brain stem, including the PAG and nucleus Raphe
Magnus, thus
resulting in analgesic effects.
Low-voltage stimulation in the VPL at high frequency can locally inhibit VPL
neurons
and subsequently the transmission of nociceptive signals to cortical areas.
The mechanisms
41
CA 02797701 2012-10-26
WO 2011/137193 PCT/US2011/034203
include one or a combination of causes such as 'jamming' of a local
nociceptive circuitry,
activation of inhibitory structures within a wider network, blockade of
membrane ion channels
such as voltage-gated currents, depolarization blockade, synaptic exhaustion,
and induction of
early genes.
Example 2: Objective diagnostic index for pain
Neuropathic pain is a neurological disorder that, prior to the invention,
lacked a reliable
diagnostic. The methods and systems of the invention provide a solution to a
long-standing
problem in pain management. An objective measure for pain leads to more
accurate diagnosis,
monitoring and optimizing therapeutics, thus ameliorating overall clinical
outcome. In addition,
a physiological correlate of pain is useful in the design of closed-loop
neuromodulation systems.
Local field potential (LFP) and multiunit activity are extracellularly
recorded signals
from a local network of neurons. LFP represents the low-frequency (<0.5 kHz)
content of the
raw recording generated by membrane currents of the neurons in a local
neighborhood of the
recording electrode, whereas the high frequency (>1 kHz) content represents
neuronal action
potential spiking. As such, the coordinated activity of cell populations is
reflected by regular
oscillations in LFPs, which has been proposed as a mechanism to optimize the
recording and
analysis of network functions.
LFP is a good candidate for 'pain signature'. Initially studied in great
detail owing to
their ease of recording with non-invasive macroelectrodes, field potentials
contain informative
signals measured out of the pool of a large number of spiking neurons while
relating to sensory
and motor phenomena. Changes in field potentials are triggered by external
events (evoked
potentials) when induced by a stimulus (e.g. noxious mechanical stimulus or
non-noxious tactile
stimulus) or by internal cortical dynamics (intrinsic oscillations or rhythms)
due to thought
processes (e.g. spontaneous pain). Therefore, analysis of LFPs allows for the
study of multiple
neuronal networks simultaneously, for example thalamo-cortical networks
thought to underlie
normal states of consciousness and arousal, as well as neurological disorders
such as pain and
depression. Field potentials are recorded intracortically (LFP, using
penetrating electrodes),
supracortically (EcoG electrodes), on the scalp (EEG), or even with
magnetoencephalograhy
(MEG) which measures tangential fields.
42
CA 02797701 2012-10-26
WO 2011/137193 PCT/US2011/034203
The amount of information contained within a brain signal diminishes as it
passes from
intra- to extra- cellular, from single to multiunit to field potentials, and
from LFP to ECoG to
EEG to MEG. Many have recently argued in favor of balancing optimal
information content
with less invasive recording for translational purposes.
Data showing pain-related brain activity is described below (Figs. 30, 31, 32.
Characteristic burst firing is recorded from the thalamus of patients with
neuropathic pain (Fig.
30). This bursting pattern closely resembles that recorded from the thalamus
of an animal model
of neuropathic deafferentation pain (Fig. 31), whereby reversal of this
neuronal activity was
associated with attenuation of pain behavior.
Additional patterns of brain activity in the VPL nucleus of the thalamus
related to pain
were identified in a rat model of sciatic neuropathy. Recording single-unit
action potentials,
increased evoked firing were demonstrated in response to tactile noxious and
non-noxious
stimuli, increased after-discharge following noxious stimulation, emergence of
spontaneous
firing, as well as characteristic burst events. Furthermore, deep brain
stimulation effectively
reversed 'pain signature' and attenuated pain behavior.
A series of psychiatric disorders, including Tourette and pain, may be
associated with
thalamocortical dysrhythmia (TCD). Synchrony of thalamic and cortical networks
under normal
conditions are thought to underlie consciousness, whereby state-dependant
(sensory or cognitive
state) rhythms of thalamocortical relay neurons are continuously driven by
corticothalamic input
resulting in oscillation, thus binding sensory or cognitive events in a
unified percept. Some
neurological disorders. however, can lead to disregulation of calcium channels
in the brain, thus
causing abnormal calcium spiking and high frequency burst in thalamic
reticular nuclei,
paradoxically driving the thalamocortical network into lower frequency
oscillation (4-10 Hz)
(Fig. 32). The fact that pain-related changes occur at single unit and MEG
levels indicates that
LFP (which is intermediate in terms of information content) can be translated
into less invasive
EEG. Whether it can also be translated into MEG depends to some extent on the
results of this
study.
Neurons at multiple stages of the somatosensory pathway display oscillations
at 7-12 Hz,
including in somatosensory cortex (Si) and the ventral posterior nucleus of
the thalamus.
43
CA 02797701 2012-10-26
WO 2011/137193 PCT/US2011/034203
Therefore, pain is associated with a characteristic pattern of brain activity
embodied in LFPs,
which is useful for objective diagnosis and as well input signal in a closed-
loop technology for
pain therapy. Pain-related LFP can be reversed by spinal cord stimulation
(SCS) or peripheral
nerve stimulation (PNS), thus closing the loop of a hypothetical automated
system.
Example 3: Local Field Potential (LFP) Measurements
Methods for single unit recording are described above, and additional studies
are
described below.
Adult male Sprague Dawley rats (200-250 g) were used. Data were collected and
analyzed by observers blinded to the animal's treatment whenever possible.
The pain model utilized chronic constriction injury of the sciatic nerve. This
is a widely
used model of mononeuropathy that yields reliable pain behavior in rats. Rats
are deeply
anesthetized with isoflurane (1.5-2%) and the sciatic nerve is loosely ligated
with 4 chromic gut
(4-0) sutures. After CCI, the overlying muscles and skin are closed in layers
with 4-0 nylon
sutures.
Pain behavioral is tested daily to determine mechanical and thermal sensory
thresholds.
Pre-operative testing generally begins 2 days prior to CCI and once per day
for 10 days after
CCI. Mechanical sensory thresholds are determined by the standard Dixon up-
down method
utilizing a series of von Frey filaments applied to the glaborous surface of
the paw. Thermal
hyperalgesia is measured by latency of paw withdrawal in response to a radiant
heat source.
Rats are placed in Plexiglas boxes on an elevated glass plate under which a
radiant heat source
(4.7 amps) is applied to the plantar surface of the paw and rats are free to
escape from applied
stimuli. The temperature that the paw can be subjected to can reach a maximum
of 57 C for a
transient time of less than 3 sec. Rats that don't display significant pain
behavior are excluded
from the study.
Electrophysiology evaluations are carried out as follows. After confirming
pain
behavior, a minimal craniotomy at day 7 after CCl/sham under deep anesthesia
(3% isoflurane)
exposes the cortical surface vertically above the VPL or somatosensory cortex
(SI) identified
according to a rat brain atlas. LFP recordings (CED 1401) are made using a
tungsten
microelectrode (125 p.m, 12 Mil) by low-pass filtering of the extracellular
field potential below
44
CA 02797701 2012-10-26
WO 2011/137193 PCT/US2011/034203
300 Hz. Microelectrodes are positioned stereotaxically in areas of the VPL or
SI with
identifiable receptive fields in the injured dermatome (hindpaw on the side of
CCI). Bands
mostly in the low frequency ranges (within the ranges of 0, a, and rhythms),
as well as higher
ranges (100-500 Hz), were studied for 'shifting', spindle wave occurrences and
epochs will also
be identified. Off-line analysis of spectral power was conducted using Spike 2
software (CED
1401). Evoked responses (triggered by tactile noxious and non-noxious stimuli)
and
spontaneous activity was recorded. LFP was recorded contralateral to CCI, as
well as ipsilateral
to CCI in the same animals ('internal' control group), in addition to naïve
animals (Fig. 35).
Since recording from multiple brain structures will subject an animal to
multiple electrode
penetrations, separate groups were used for LFP recordings in VPL versus SI.
Statistical analysis was performed using SigmaStat software. Analysis of
variance
(ANOVA) and parametric tests were used for normally distributed data with
p<0.05 considered
significant. Otherwise, non-parametric tests were used. Paired t-tests will
look at the effect of a
manipulation (e.g. evoked responses or the effect of SCS) within the same
group of animals
(before/after).
Adult male Sprague Dawley rats (220 g) were used to induce chronic pain by
chronic
constriction injury of the sciatic nerve (CCI). Pain behavior was tested on
day 7 post-operatively
before brain recording and thermal hyperalgesia was confirmed, showing
significant decrease of
withdrawal latency in the injured paw compared to the contralateral un-injured
paw, a reflex
triggered by a controlled radiant heat source.
After confirming neuropathic pain behavior, LFP recordings were made under
deep
barbiturate anesthesia from the ventral posterolateral (VPL) nucleus of the
thalamus bilaterally,
with the side ipsilateral to injury serving as control (receiving ascending
information
predominantly from the un-injured paw). The location of the microelectrode was
confirmed by
stereotaxic coordinates (according to a rat brain Atlas) and by increased in
background firing in
response to gentle brushing of the corresponding receptive field in the
contralateral paw
(Fig. 33).
After confirming electrode location in the VPL, spontaneous activity, as well
as activity
in response to brushing of the contralateral paw, was recorded for 20s each.
Off-line analysis
revealed distinct peaks of increased spectral power under different conditions
and major
differences were found between control (normal side) and CCI (injured side)
(Fig. 34).
A reported leftward shift in MEG power spectrum of spontaneous activity in
patients
with chronic pain compared to control is in agreement with Fig. 34 (left
panels). Thalamic LFP
recordings from patients with Tourette Syndrome, a cognitive/movement
disorder, emphasized a
significant correlation between the main LFP frequency and the frequency of
single-unit
interbursts. Data described herein shows that the mean interburst period of
single-unit brush-
evoked activity recorded from the VPL is approximately 400 ms for animals with
CCI and 100
ms for naive, thus predicting a rightward shift in power spectra from 2.5 to
10 Hz after CCI,
exactly matching with what we show in Fig. 34 (right panels). Therefore, the
abnormal bursts
described at single-unit level are contributing to the shift in power spectra
observed at LFP level.
Prominent LFP power spectra was also described in the low frequency (2-7Hz),
as well as in the
a-band (8-13 Hz) but virtually absent 13 activity, also in agreement with Fig.
34 (right panels). A
resemblance in brain LFP and EEG activities in the a-rhythms between awake
humans and
animals under barbiturate anesthesia has been observed, further validating the
approach and
animal models used in these studies.
Further, while the description above refers to the invention, the description
may include
more than one invention. While this invention has been particularly shown and
described with
references to preferred embodiments thereof, it will be understood by those
skilled in the art that
various changes in form and details may be made therein without departing from
the scope of the
invention encompassed by the appended claims.
46
CA 2797701 2017-09-28