Note: Descriptions are shown in the official language in which they were submitted.
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
METHODS AND COMPOSITIONS FOR PROTECTING
AGAINST NEUROTOXIC AGENTS
FIELD OF THE INVENTION
Particular aspects relate generally to methods for protecting against or
reducing
neurotoxicity of exposure to a neurotoxic agent, comprising administering an
electrokinetically
altered aqueous fluid as provided herein, and preferably wherein protecting
against or reducing
loss of motor coordination in the subject exposed to the neurotoxin is
afforded. Particular
aspects relate to protecting or reducing neurotoxin-mediated neuronal
apoptosis and/or
activating or inducing at least one of PI-3 kinase and Akt phosphorylation in
neurons. Particular
aspects relate generally to methods for preserving or improving motor
coordination in a subject
having a neurodegenerative condition or disease, comprising administering an
electrokinetically
altered aqueous fluid as provided herein.
CROSS-REFERENCE TO RELATED APPLICATIONS
The Application claims the benefit of priority to United States Patent
Application No.
12/771,476, filed April 30, 2010, and entitled "COMPOSITIONS AND METHODS FOR
TREATMENT OF NEURODEGENERATIVE DISEASES," United States Provisional Patent
Application Nos. 61/413,899 filed 15 November 2010 and entitled "METHODS AND
COMPOSITIONS FOR PROTECTING AGAINST NEUROTOXICITY OF A NEUROTOXIC
AGENT, AND IMPROVING MOTOR COORDINATION ASSOCIATED WITH A
NEURODEGENERATIVE CONDITION OR DISEASE," and 61/454,409 filed 18 March 2011
of same title, all of which are incorporated herein by reference in their
entirety.
BACKGROUND OF THE INVENTION
Neurodegenerative diseases are a group of diseases typified by deterioration
of neurons
or their myelin sheath. This destruction of neurons eventually leads to
dysfunction and
disabilities. Often times inflammation is found to be a component of
neurodegenerative diseases
and adds to the pathogenesis of the neurodegeneration (Minagar, et al. (2002)
J. Neurological
Sci. 202:13-23; Antel and Owens (1999) J. Neuroimmunol. 100: 181-189; Elliott
(2001) Mol.
Brain. Res. 95:172-178; Nakamura (2002) Biol. Pharm. Bull. 25:945-953; Whitton
PS. (2007)
Br J Pharmacol. 150:963-76). Collectively, these diseases comprise the art-
recognized
inflammatory neurodegenerative diseases. Neuroinflammation may occur years
prior to any
1
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
considerable loss of neurons in some neurodegenerative disorders (Tansey et.
al., Fron
Bioscience 13:709-717, 2008). Many different types of immune cells, including
macrophages,
neutrophils, T cells, astrocytes, and microglia, can contributed to the
pathology of immune-
related diseases, like Multiple Sclerosis (M.S.), Parkinson's disease,
amyloidosis (e.g.,
Alzheimer's disease), amyotrophic lateral sclerosis (ALS), prion diseases, and
HIV-associated
dementia. More specifically, research groups have noted that in MS the injury
to myelin is
mediated by an inflammatory response (Ruffini et. al. (2004) Am J Pathol
164:1519-1522) and
that M.S. pathogensis is exacerbated when leukocytes infiltrate the CNS (Dos
Santos et. al.
(2008) J Neuroinflammation 5:49). One research group has developed genetic
models to test
CNS inflammation and its effects in MS (through the animal model experimental
autoimmune
encephalomyelitis (EAE). In addition, pro-inflammatory cytokines (specifically
TNF-alpha)
were found to be elevated in Alzheimer's disease, Parkinson's disease, and
amyotrophic lateral
sclerosis (ALS). (Greig et al (2006) Ann NY Acad of Sci 1035:290-315). These
inflammatory
neurodegenerative diseases may, therefore, be effectively treated by anti-
inflammatory drugs.
Inflammatory neurodegenerative diseases include but are not limited to:
multiple
sclerosis (MS), Parkinson's disease, amyloidosis (e.g., Alzheimer's disease),
amyotrophic lateral
sclerosis (ALS), HIV-associated dementia, stroke/cerebral ischemia, head
trauma, spinal cord
injury, Huntington's disease, migraine, cerebral amyloid angiopathy, AIDS, age-
related
cognitive decline; mild cognitive impairment and prion diseases in a mammal.
Multiple sclerosis (MS) is a chronic inflammatory neurodegenerative disease of
the
central nervous system (CNS) that affects approximately 1,100,000 people all
over the world, in
particular affects young adults (Pugliatti et al. (2002) Clin. Neurol. Neuros.
104:182-191). MS
is characterized pathologically by demyelination of neural tissue, which
results clinically in one
of many forms of the disease, ranging from benign to chronic-progressive
patterns of the disease
state. More specifically, five main forms of multiple sclerosis have been
described: 1) benign
multiple sclerosis; 2) relapsing-remitting multiple sclerosis (RRMS); 3)
secondary progressive
multiple sclerosis (SPMS); 4) primary progressive multiple sclerosis (PPMS);
and 5)
progressive-relapsing multiple sclerosis (PRMS). Chronic progressive multiple
sclerosis is a
term used to collectively refer to SPMS, PPMS, and PRMS. The relapsing forms
of multiple
sclerosis are SPMS with superimposed relapses, RRMS and PRMS.
Throughout the course of the disease there is a progressive destruction of the
myelin
sheath surrounding axons. Since intact myelin is essential in the preservation
of axonal integrity
(Dubois-Dalcq et al., Neuron. 48, 9-12 (2005)) systematic destruction
eventually leads,
2
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
clinically, to various neurological dysfunctions including numbness and pain,
problems with
coordination and balance, blindness, and general cognitive impairment.
Interestingly, MS
progression can differ considerably in patients with some having slight
disability even after
several decades of living with the disease, while others becoming dependent
upon a wheelchair
only a few years after being diagnosis.
The etiology of MS currently is unknown, but studies examining genetic
evidence, the
molecular basis, and immunology factors are beginning to elucidate the course
of the disease
and the mechanism by which demylination occurs. In genetic analyses, some
reports have
indicated that related individuals have higher incidence of MS when compared
to normal
population (0.1% prevalence of MS): an identical twin having a 30% chance of
developing the
disease if the other twin has MS and fraternal twins and siblings have a 1-2%
chance if a another
sibling is affected by MS. Several groups have utilized linkage and
association studies to
discover the genes responsible for this heritability and found that the
relative risk of being
affected by MS is 3-4 fold higher to those carrying a the major
histocompatibility complex
(MHC) class II allele of the human leukocyte antigen (HLA)-DR2 allele. Other
genes have been
identified that associate with MS, but a much lower risk. The link between MS
susceptibility
and MHC Class II strongly suggests a role for CD4+ T-cells in the pathogenesis
of MS
(Oksenberg et al., JAMA 270:2363-2369 (1993); Olerup et al., Tissue Antigens
38:1-3 (1991)).
In addition, identification of genes that are differentially expressed in MS
patients
suffering from MS compared to healthy individuals has been attempted. Gene
microarrays have
been used 1) to examine transcription from MS plaque types (acute verses
chronic) and plaque
regions (active verses inactive) (Lock and Heller (2003)); 2) to compare
peripheral blood
mononucleocytes (PBMC) in RRMS patients verses controls, from patients both
with and
without interferon-(3 treatment (Sturzebecher et al. (2003)); and 3) to
examine CNS cells in
stages of experimental allergic encephalomyelitis (EAE) in mice, an animal
model of MS (Lock
et al. (2002)). Much of what these experiments discovered was expected,
including the finding
that anti-inflammatory, anti-apoptotic genes are down-regulated and pro-
inflammatory,
proliferation genes are up-regulated. Surprising results include
identification of potential novel
targets for therapeutic application such as osteopontin (Chabas et al. 2001)
and TRAIL
(Wandinger et al. 2003)). However, many of the genes that have differential
regulation when
comparing expression from MS patients with healthy individuals have unknown
significance in
MS development, because any genes that may affect MS susceptibility and/or
progression are
still unknown.
3
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
Further research has determined that inflammatory responses initiated by
autoreactive
CD4+ T-cells can mediate injury to myelin (Bruck et al., J. Neurol. Sci.
206:181-185 (2003)).
In general, it is believed that much of the damage occurring to myelin sheaths
and axons during
an episode of MS happens through autoreactive T cell response which produces
an inflammatory
response including the secretion of proinflammatory (e.g. Thl and Th17)
cytokines (Prat et al.,
J. Rehabil. Res. Dev. 39:187-199 (2002); Hemmer et al., Nat. Rev. Neurosci.
3:291-301 (2002)).
Treatments that currently are available for MS include glatiramer acetate,
interferon-0,
natalizumab, and mitoxanthrone. In general, these drugs suppress the immune
system in a
nonspecific fashion and only marginally limit the overall progression of
disease. (Lubetzki et al.
(2005), Curr. Opin. Neurol. 18:237-244). Thus, there exists a need for
developing therapeutic
strategies to better treat MS.
Glatiramer acetate is composed of glutamic acid, lysine, alanine, and tyrosine
as a
random polymer. Glatiramer acetate has limited effectiveness and significant
side effects, for
example, lump at the site of injection, chills, fever, aches, shortness of
breath, rapid heartbeat
and anxiety. In an important clinical study using 943 patients with primary
progressive MS,
glatiramer acetate failed to halt the progression of disability and the
disease (Wolinsky, et al
(2007) Ann Neurol 61:13-24).
Interferon-0 is a naturally occurring protein produced by fibroblasts and part
of the
innate immune response. As a drug for MS, interferon-0 is about 18-38%
effective in reducing
the rate of MS episodes. Side effects include mild ones flu-like symptoms and
reactions at the
site of injection and more serious (e.g., depression, seizures, and liver
problems)
Mitoxantrone is a treatment for MS. It was developed as a chemotherapy
treatment for
use in combating cancer-working by interfering with DNA repair and synthesis
and is not
specific to cancer cells. Side effects from mitoxantrone can be quite severe
and include nausea,
vomiting, hair loss, heart damage, and immunosuppression.
Natalizumab is a humanized monoclonal antibody that targets alpha4-integren,
which is
a cellular adhesion molecule. Natalizumab is believed to work by keeping
immune cells that
cause inflammation from crossing the blood brain barrier (BBB). Side effects
include fatigue,
headache, nausea, colds, and allergic reactions.
Parkinson's disease
Parkinson's disease (PD), another inflammatory neurodegeneration disease, is
characterized by movement disorders, including muscle rigidity and slow
physical movements.
4
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
PD is the second most frequent neurodegenerative disorder, affecting up to 1
million people in
the US alonel. PD prevalence increases with age, from 0.3% in the general US
population to
1% to 2% in persons aged 65 years or older, and 4% to 5% in individuals aged
85 years or
olderl. With an overall increasing life expectancy, numbers of PD patients in
the US and other
countries are expected to double by 20302.
PD is a progressive disease characterized by motor symptoms that include
tremor,
rigidity, bradykinesia (slowness of movement), gait impairment, and postural
change. The
disease also involves non-motor symptoms such as cognitive deficits,
depression, and sleep
disorders. Like Alzheimer's disease, PD is a proteinopathy. Misfolded a-
synuclein accumulates
inside neurons and forms so-called Lewy bodies, one of the neuropathological
hallmarks of PD.
Initially thought to be caused exclusively by the loss of dopaminergic neurons
in the substantia
nigra, PD recently has been recognized to have an inflammatory component that
activates brain
microglial cells and is involved in the progression of neuronal cell death. A
perceived
pathophysiological cause of Parkinson's disease is progressive destruction of
dopamine
producing cells in the basal ganglia which comprise the pars compartum of the
substantia nigra,
basal nuclei located in the brain stern. Loss of dopamineric neurons results
in a relative excess
of acetylcholine. Jellinger, K. A., Post Mortem Studies in Parkinson's Disease-
-Is It Possible to
Detect Brain Areas For Specific Symptoms?, J Neural Transm 56 (Supp); 1-
29:1999. In
addition, recent research into Parkinson's disease has observed that due to
enhanced expression
of cytokines and HLA-DR antigens it is likely that the immune response
contributes to the
neuronal damage (Czlonkowska et. al. (2002) Med Sci Monit 8:RA165-77).
Effective treatment at an early stage represents an unmet clinical need in the
care of PD
patients. Levodopa (L-DOPA) is the most efficacious pharmacologic treatment
for PD, but is
usually prescribed late in the course of the disease due to severe side
effects. Dopamine receptor
agonists and monoamine oxidase type B inhibitors have shown an inverse
correlation between
efficacy and the occurrence and severity of side effects, and trials exploring
other treatment
options including coenzyme Q10, tocopherol (Vitamin E), amantidine, and beta-
blockers have
either failed to demonstrate benefits or have not produced sufficient data for
a thorough risk vs.
benefit evaluation. Neuroprotection in particular has been a key, yet elusive,
goal in PD
treatment.
Amyloidosis develops when certain proteins have altered structure and tend to
bind to
each building up in particular tissue and blocking the normal tissue
functioning. These altered
structured proteins are called amyloids. Often amyloidoses is split into two
categories: primary
5
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
or secondary. Primary amyloidoses occur from an illness with improper immune
cell function.
Secondary amyloidoses usually arise from a complication of some other chronic
infectious or
inflammatory diseases. Examples of such include Alzheimer's disease and
rheumatoid arthritis.
Since the underlying problem in secondary amyloidosis is inflammation,
treating inflammation
likely will be beneficial.
Alzheimer's disease is another type of inflammatory neurodegenerative disease.
It is
exemplified by the increasing impairment of learning and memory, although the
disease may
manifest itself in other ways indicating altered cognitive ability. Throughout
the disease the
progressive loss of neurons and synapses in the cerebral cortex leads to gross
atrophy of the
neural tissue. Although the cause of Alzheimer's is unknown, many believe that
inflammation
plays an important role and clinical studies have shown that inflammation
considerably
contributes to the pathogenesis of the disease (Akiyama, et. al. (2000)
Neurobiol Aging. 21:383-
421.
In amyotrophic lateral sclerosis, a link between inflammation and the disease
has been
suggested (Centonze, et. al. (2007) Trends Pharm Sci 28:180-7). In addition,
TNF-alpha mRNA
has been found to be expressed in spinal cords of a transgenic mouse model for
amyotrophic
lateral sclerosis. Interestingly, the transcript was detected as early as
prior to onset motor
difficulties until death caused by ALS (Elliot (2001) Brain Res Mol Brain Res
95:172-8).
Neurotoxins
Neurotoxins are toxins that specifically act upon neurons, their synapses, or
the nervous
system in its entirety. They are substances which cause damage to the
structures of the brain
which in turn leads to chronic disease. Neurotoxins include, for example,
adrenergic
neurotoxins, cholinergic neurotoxins, dopaminergic neurotoxins, excitotoxins,
and other
neurotoxins. Examples of adrenergic neurotoxins include N-(2-chloroethyl)-N-
ethyl-2-
bromobenzylamine hydrochloride. Examples of cholinergic neurotoxins include
acetylethylcholine mustard hydrochloride. Examples of dopaminergic neurotoxins
include 6-
hydroxydopamine HBr (6-OHDA), 1-methyl-4-(2-methylphenyl)-1,2,3,6-tetrahydro-
pyridine
hydrochloride, 1-methyl-4-phenyl-2,3-dihydropyridinium perchlorate, N-methyl-4-
phenyl-
1,2,5,6tetrahydropyridine HC1 (MPTP), 1-methyl-4-phenylpyridinium iodide
(MPP+), paraquat,
and rotenone. Examples of excitotoxins include NMDA and kainic acid.
MPTP, MPP+, paraquat, rotenone and 6-OHDA have been been shown to induce PD
like
symptoms in animal models. (See, K. Ossowska, et al., (2006). "Degeneration of
dopaminergic
6
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
mesocortical neurons and activation of compensatory processes induced by a
long-term paraquat
administration in rats: Implications for Parkinson's disease". Neuroscience
141 (4): 2155-2165;
and Caboni P, et al., (2004). "Rotenone, deguelin, their metabolites, and the
rat model of
Parkinson's disease". Chem Res Toxicol 17 (11): 1540-8; Simon et al., Exp
Brain Res, 1974, 20:
375-384; Langston et al., Science, 1983, 219: 979-980; Tanner, Occup Med,
1992, 7: 503-513;
Lion et al., Neurology, 1997, 48: 1583-1588).
SUMMARY OF THE INVENTION
Particular aspects provide methods for protecting against or reducing
neurotoxicity of
exposure to a neurotoxic agent, comprising administering to a subject in need
thereof a
therapeutically effective amount of an electrokinetically altered aqueous
fluid comprising an
ionic aqueous solution of charge- stabilized oxygen-containing nanostructures
substantially
having an average diameter of less than about 100 nanometers and stably
configured in the ionic
aqueous fluid in an amount sufficient to provide for neuroprotection against
the neurotoxic
agent, wherein an method for protecting against or reducing neurotoxicity of
exposure to a
neurotoxic agent is afforded. In certain aspects, the methods comprise
protecting against or
reducing loss of motor coordination in the subject exposed to the neurotoxin.
In particular
aspects, protecting or reducing neurotoxin-mediated neuronal apoptosis is
afforded, and/or
activating or inducing at least one of PI-3 kinase and Akt phosphorylation in
neurons (e.g., of a
subject) is afforded.
In particular aspects, the charge-stabilized oxygen-containing nanostructures
are stably
configured in the ionic aqueous fluid in an amount sufficient to provide, upon
contact of a living
cell by the fluid, modulation of at least one of cellular membrane potential
and cellular
membrane conductivity.
In particular embodiments, administering the fluid comprises administering the
fluid
prior to exposure to the neurotoxic agent.
In certain aspects, the charge-stabilized oxygen-containing nanostructures are
the major
charge-stabilized gas-containing nanostructure species in the fluid. In
particular aspects, the
percentage of dissolved oxygen molecules present in the fluid as the charge-
stabilized oxygen-
containing nanostructures is a percentage selected from the group consisting
of greater than:
0.01%, 0.1%, 1%, 5%; 10%; 15%; 20%; 25%; 30%; 35%; 40%; 45%; 50%; 55%; 60%;
65%;
70%; 75%; 80%; 85%; 90%; and 95%. In certain aspects, the total dissolved
oxygen is
7
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
substantially present in the charge-stabilized oxygen-containing
nanostructures. In certain
embodiments, the charge- stabilized oxygen-containing nanostructures
substantially have an
average diameter of less than a size selected from the group consisting of: 90
nm; 80 nm; 70 nm;
60 nm; 50 nm; 40 nm; 30 nm; 20 nm; 10 nm; and less than 5 nm.
In certain aspects, the ionic aqueous solution comprises a saline solution,
and/or is
superoxygenated. In certain aspects, the fluid comprises a form of solvated
electrons.
In particular aspects, alteration of the electrokinetically altered aqueous
fluid comprises
exposure of the fluid to hydrodynamically-induced, localized electrokinetic
effects. In certain
embodiments, exposure to the localized electrokinetic effects comprises
exposure to at least one
of voltage pulses and current pulses. In certain embodiments, exposure of the
fluid to
hydrodynamically-induced, localized electrokinetic effects, comprises exposure
of the fluid to
electrokinetic effect-inducing structural features of a device used to
generate the fluid.
In certain aspects, the electrokinetically altered aqueous fluid modulates
localized or
cellular levels of nitric oxide.
In particular aspects, the electrokinetically altered aqueous fluid promotes a
localized
decrease at the site of administration of at least one cytokine selected from
the group consisting
of: IL-lbeta, IL-8, TNF-alpha, and TNF-beta.
Particular aspects of the methods comprise combination therapy, wherein at
least one
additional therapeutic agent is administered to the patient. In certain
embodiments, the at least
one additional therapeutic agent is selected from the group consisting of:
adrenergic neurotoxins,
cholinergic neurotoxins, dopaminergic neurotoxins, excitotoxins and
chemotherapeutic agents.
In particular aspects, modulation of at least one of cellular membrane
potential and
cellular membrane conductivity comprises modulating at least one of cellular
membrane
structure or function comprising modulation of at least one of a conformation,
ligand binding
activity, or a catalytic activity of a membrane associated protein. In
particular aspects, the
membrane associated protein comprises at least one selected from the group
consisting of
receptors, transmembrane receptors, ion channel proteins, intracellular
attachment proteins,
cellular adhesion proteins, and integrins. In particular aspects, the
transmembrane receptor
comprises a G-Protein Coupled Receptor (GPCR). In particular aspects, the G-
Protein Coupled
Receptor (GPCR) interacts with a G protein a subunit. In particular aspects,
the G protein a
subunit comprises at least one selected from the group consisting of Gas , Gai
, Gaq , and Gait.
In particular aspects, the at least one G protein a subunit is Gaq.
8
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
In certain aspects, modulating cellular membrane conductivity, comprises
modulating
whole-cell conductance. In particular embodiments, modulating whole-cell
conductance,
comprises modulating at least one voltage-dependent contribution of the whole-
cell
conductance.
In particular aspects, modulation of at least one of cellular membrane
potential and
cellular membrane conductivity comprises modulating intracellular signal
transduction
comprising modulation of a calcium dependant cellular messaging pathway or
system. In
particular aspects, modulation of at least one of cellular membrane potential
and cellular
membrane conductivity comprises modulating intracellular signal transduction
comprising
modulation of phospholipase C activity. In particular aspects, modulation of
at least one of
cellular membrane potential and cellular membrane conductivity comprises
modulating
intracellular signal transduction comprising modulation of adenylate cyclase
(AC) activity. In
particular aspects, modulation of at least one of cellular membrane potential
and cellular
membrane conductivity comprises modulating intracellular signal transduction
associated with
at least one condition or symptom selected from the group consisting of:
chronic inflammation
in the central nervous and brain, and acute inflammation in the central
nervous and brain.
Certain aspects of the methods comprise administration to a cell network or
layer, and
further comprising modulation of an intercellular junction therein. In
particular aspects, the
intracellular junction comprises at least one selected from the group
consisting of tight junctions,
gap junctions, zona adherins and desmasomes. In certain embodiments, the cell
network or
layers comprises at least one selected from the group consisting of
endothelial cell and
endothelial-astrocyte tight junctions in CNS vessels, blood-cerebrospinal
fluid tight junctions or
barrier, pulmonary epithelium-type junctions, bronchial epithelium-type
junctions, and intestinal
epithelium-type junctions.
In particular aspects, the electrokinetically altered aqueous fluid is
oxygenated, and the
oxygen in the fluid is present in an amount of at least 8 ppm, at least 15,
ppm, at least 25 ppm, at
least 30 ppm, at least 40 ppm, at least 50 ppm, or at least 60 ppm oxygen at
atmospheric
pressure. In certain aspects, the amount of oxygen present in charge-
stabilized oxygen-
containing nanostructures of the electrokinetically-altered fluid is at least
8 ppm, at least 15,
ppm, at least 20 ppm, at least 25 ppm, at least 30 ppm, at least 40 ppm, at
least 50 ppm, or at
least 60 ppm oxygen at atmospheric pressure.
9
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
In certain aspects, the electrokinetically altered aqueous fluid comprises at
least one of a
form of solvated electrons, and electrokinetically modified or charged oxygen
species. In
particular embodiments, the form of solvated electrons or electrokinetically
modified or charged
oxygen species are present in an amount of at least 0.01 ppm, at least 0.1
ppm, at least 0.5 ppm,
at least 1 ppm, at least 3 ppm, at least 5 ppm, at least 7 ppm, at least 10
ppm, at least 15 ppm, or
at least 20 ppm. In certain aspects, the electrokinetically altered oxygenated
aqueous fluid
comprises solvated electrons stabilized, at least in part, by molecular
oxygen.
In particular aspects, the ability to modulate of at least one of cellular
membrane
potential and cellular membrane conductivity persists for at least two, at
least three, at least four,
at least five, at least 6, at least 12 months, or longer periods, in a closed
gas-tight container.
In certain aspects, the membrane associated protein comprises CCR3.
In particular aspecxts treating or administrating comprises administration by
at least one
of topical, inhalation, intranasal, oral and intravenous.
In certain embodiments, the charge-stabilized oxygen-containing nanostructures
of the
electrokinetically-alterd fluid comprise at least one salt or ion from Tables
1 and 2 disclosed
herein.
Additional aspects provide a pharmaceutical composition, comprising an amount
of an
electrokinetically altered aqueous fluid comprising an ionic aqueous solution
of charge-
stabilized oxygen-containing nanostructures substantially having an average
diameter of less
than about 100 nanometers and stably configured in the ionic aqueous fluid in
an amount
sufficient for protecting against or reducing neurotoxicity of exposure to a
neurotoxic agent.
Yet further aspects provide methods for preserving or improving motor
coordination in a
subject, having a neurodegenerative condition or disease, comprising
administering to a subject
having a neurodegenerative condition or disease characterized by loss of motor
coordination, a
therapeutically effective amount of an electrokinetically altered aqueous
fluid comprising an
ionic aqueous solution of charge- stabilized oxygen-containing nanostructures
substantially
having an average diameter of less than about 100 nanometers and stably
configured in the ionic
aqueous fluid in an amount sufficient to provide for preserving or improving
motor coordination
in the subject, wherein a method for preserving or improving motor
coordination in a subject
having a neurodegenerative condition or disease is afforded. In certain
aspects, activation or
induction of at least one of PI-3 kinase and Akt phosphorylation is afforded.
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
In particular aspects, the neurodegenerative condition or disease comprises at
least one
inflammatory neurrodegenerative condition or disease selected from the group
consisting of
multiple sclerosis, amyotrophic lateral sclerosis, Alzheimer's disease,
Parkinson's disease,
stroke/cerebral ischemia, head trauma, spinal cord injury, Huntington's
disease, migraine,
cerebral amyloid angiopathy, inflammatory neurodegenerative condition
associated with AIDS,
age-related cognitive decline; mild cognitive impairment and prion diseases in
a mammal.
Preferably, the inflammatory neurodegenerative condition or disease comprises
at least one of
multiple sclerosis, amyotrophic lateral sclerosis, Alzheimer's disease,
Parkinson's disease.
Certain aspects of the methods comprise a synergistic or non-synergistic
inhibition or
reduction in inflammation by simultaneously or adjunctively treating the
subject with another
anti-inflammatory agent, for example, wherein said other anti-inflammatory
agent comprises a
steroid or glucocorticoid steroid. In certain aspects, the glucocorticoid
steroid comprises
Budesonide or an active derivative thereof.
Certain aspects of the methods comprise combination therapy, wherein at least
one
additional therapeutic agent is administered to the patient. In particular
embodiments, the at
least one additional therapeutic agent is selected from the group consisting
of: glatiramer acetate,
interferon-0, mitoxantrone, natalizumab, inhibitors of MMPs including
inhibitor of MMP-9 and
MMP-2, short-acting (32-agonists, long-acting (32-agonists, anticholinergics,
corticosteroids,
systemic corticosteroids, mast cell stabilizers, leukotriene modifiers,
methylxanthines, (32-
agonists, albuterol, levalbuterol, pirbuterol, artformoterol, formoterol,
salmeterol,
anticholinergics including ipratropium and tiotropium; corticosteroids
including
beclomethasone, budesonide, flunisolide, fluticasone, mometasone,
triamcinolone,
methyprednisolone, prednisolone, prednisone; leukotriene modifiers including
montelukast,
zafirlukast, and zileuton; mast cell stabilizers including cromolyn and
nedocromil;
methylxanthines including theophylline; combination drugs including
ipratropium and albuterol,
fluticasone and salmeterol, budesonide and formoterol; antihistamines
including hydroxyzine,
diphenhydramine, loratadine, cetirizine, and hydrocortisone; immune system
modulating drugs
including tacrolimus and pimecrolimus; cyclosporine; azathioprine;
mycophenolatemofetil; and
combinations thereof.
In certain aspects, the at least one additional therapeutic agent is a TSLP
and/or TSLPR
antagonist. In particular embodiments, the TSLP and/or TSLPR antagonist is
selected from the
group consisting of neutralizing antibodies specific for TSLP and the TSLP
receptor, soluble
11
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
TSLP receptor molecules, and TSLP receptor fusion proteins, including TSLPR-
immunoglobulin Fc molecules or polypeptides that encode components of more
than one
receptor chain.
In particular aspects, the charge-stabilized oxygen-containing nanostructures
of the
electrokinetically-alterd fluid comprise at least one salt or ion from Tables
1 and 2 disclosed
herein.
BRIEF DESCRIPTION OF THE DRAWINGS
Figures 1 A-C demonstrate the results of a series of patch clamping
experiments that
assessed the effects of the electrokinetically generated fluid (e.g., RNS-60
and Solas) on
epithelial cell membrane polarity and ion channel activity at two time-points
(15 min (left
panels) and 2 hours (right panels)) and at different voltage protocols.
Figures 2 A-C show, in relation to the experiments relating to Figures 1 A-C,
the graphs
resulting from the subtraction of the Solas current data from the RNS-60
current data at three
voltage protocols (A. stepping from zero mV; B. stepping from -60 mV; C.
stepping from -120
mV) and the two time-points (15 mins (open circles) and 2 hours (closed
circles)).
Figures 3 A-D demonstrate the results of a series of patch clamping
experiments that
assessed the effects of the electrokinetically generated fluid (e.g., Solas
(panels A. and B.) and
RNS-60 (panels C. and D.)) on epithelial cell membrane polarity and ion
channel activity using
different external salt solutions and at different voltage protocols (panels
A. and C. show
stepping from zero mV; panels B. and D. show stepping from -120 mV).
Figures 4 A-D show, in relation to the experiments relating to Figures 3 A-D,
the graphs
resulting from the subtraction of the CsCl current data (shown in Figure 3)
from the 20 mM
CaC12 (diamonds) and 40 mM CaC12 (filled squares) current data at two voltage
protocols
(panels A. and C. stepping from zero mV; B. and D. stepping from -120 mV) for
Solas (panels
A. and B.) and Revera 60 (panels C. and D.).
Figure 5 shows that the inventive electrokinetic fluid (RNS-60) was
substantially
efficacious in an art-recognized Experimental Autoimmune Encephalomyelitis
(EAE) rat model
of Multiple Sclerosis (MS).
Figure 6 shows a schematic depiction of the EAE induction and treatment
regimens used
in the experiment shown in Figure 7.
Figure 7A is a graphical representation of the body weight (in grams) of the
animals
subjected to the EAE treatment regimen used in the experiment shown in Figures
5 and 6.
12
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
Figure 7B shows the calculated change in body weight (in percentage) of the
animals subjected
to the EAE treatment regimen.
Figures 8 A-D show that the inventive electrokinetic fluid (RNS-60) had little
affect on
the level of total white blood cells (WBC), neutrophils, and lymphocytes when
compared to the
vehicle control during the EAE treatment regimen as used in the experiment
shown in Figures 5
and 6. Panels A, B, C, and D show the results at study day 0, 7, 14, and 21,
respectively.
Figures 9 A-H (A-D) show the effect that the inventive electrokinetic fluid
(RNS-60) had
on cytokine levels 7 days (A-D) and 18 days (E-H) after the EAE treatment
regimen as used in
experiment shown in Figures 5 and 6 was initiated. Panels A and E show the
levels of IL-17
after treatment. Panels B and F show the levels of IL-la after treatment.
Panels C and G show
the levels of IL-1(3 after treatment. Panels D and H show the levels of IL-4
after treatment.
Figure 10 shows that the inventive electrokinetic fluid (RNS-60), but not
control normal
saline (NS), attenuates MPP+-induced expression of inducible nitric oxide
synthase (iNOS) and
interleukin-1(3 (IL-1(3) in activated mouse microglial cells (BV-2 microglial
cells).
Figures 11A and B show that RNS60, but not normal saline control (NS),
suppresses
fibrillar A(3(1-42)-mediated apoptosis of human SHSYSY neuronal cells (Figure
11A) and
primary human neurons (Figure 11B). After differentiation, SHSYSY cells were
incubated with
different concentrations of either RNS60 or NS for 1 h followed by insult with
1 M fibrillar
A(3(1-42) peptides. After 18 h of treatment, apoptosis was monitored by TUNEL
(Calbiochem).
AP(42-1) peptides were also incubated as control. Results in each figure
represent three
independent experiments. DAPI staining was used to visualize the nucleus of
cells.
Figure 12 shows that RNS60, but not Vehicle control (Vehicle), is
substantially
efficacious in suppressing clinical score in a dose-responsive manner in an
art-recognized
experimental allergic encephalomyelitis (EAE) mouse MOG model of Multiple
Sclerosis(MS).
Both high and low dose therapeutic daily administration of RNS-60, as well as
the high dose
administration of RNS-60 every three days (administration or RNS-60 in all
instances beginning
concomitant with first clinical signs), showed a marked decrease of clinical
score (open
diamonds = Vehicle control; open squares = dexamethasone positive control;
light "x"s = low
dose (0.09 ml RNS60) daily administration from onset of clinical signs; dark
"x"s = high dose
(0.2 ml RNS60) administration every three days from onset of clinical signs;
and open triangles
= high dose (0.2 ml RNS60) daily administration from onset of clinical signs).
Figures 13 A-C show the results from two gel shift experiments (panels A and
B) and a
luciferase activity (reporter gene) assay (panel C) that examined the effects
of RNS60 on the
activation of NFKB in MBP-primed T cells.
13
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
Figures 14 A-C are graphical representations scoring the coordinated movements
of mice
in a mouse model of PD, wherein the coordinated movements of mice improve when
pre-treated
with RNS60. Panels A and B show the total movement time and distance,
respectively. Panel C
shows the ability of the mice to keep their balance on a rotating rod.
Figures 15 A and B are graphical representations scoring the striatum-
dependent
behaviors of mice in a mouse model of PD, wherein RNS60 treatment prevents the
loss of
striatum-dependent behaviors, stereotypy (grooming, Panel A) and rearing
(vertical movements,
Panel B).
Figures 16 A-C show immunostaining with an anti-tyrosine hydroxylase antibody,
tyrosine hydroxylase is the rate-limiting enzyme involved in dopamine
synthesis, in the
substantia nigra pars compacta. Panel A shows the normal staining of the anti-
tyrosine
hydroxylase antibody in the substantia nigra pars compacta. Panel B shows the
effect that
MPTP has on substantia nigra pars compacta, wherein staining of the substantia
nigra pars
compacta is reduced to approximately one-third. Panel C shows that RNS60
treatment
rescues dopaminergic neurons in mice intoxicated with MPTP.
Figures 17 A and B show the immunofluorescence analysis of phosphor-Akt in
human
neurons. The left, middle and right panels of Figure 17 A show the results
from an experiment
examining the effects of control, RNS60 (RIS60; 10%) and isotonic saline
(10%), respectively,
on Akt phosphorylation in primary neurons. Akt phosphorylation was monitored
by double-
label immunofluorescence using antibodies against (3-tubulin and phospho-Akt.
Beta-tubulin
was used as a marker for neurons and DAPI staining was used to visualize the
nucleus of cells.
Figure 17B shows that RNS60 suppresses fibrillar A(3(1-42)-mediated apoptosis
of human
primary neurons and that this RNS60-mediated suppression can be blocked by the
specific Akt
inhibitor, Aktl. Neurons preincubated with different concentrations of Aktl
for 30 min were
treated with RNS60. After 1 h of incubation, cells were challenged with
fibrillar A(31-42. After
12 h, neuronal apoptosis was monitored by TUNEL. Results represent three
independent
experiments. DAPI staining was used to visualize the nucleus of cells.
Figure 18 is a graphical representation of the ratio between the amount of
phosphorylated
Akt to the total amount of Akt present in astrocytes when treated with either
RNS60 or normal
saline.
Figures 19 A-B show the results from an experiment examining the effects of
RNS60 on
fibrillar A(3(1-42)-mediated tau phosphorylation in primary neurons. Tau
phosphorylation was
monitored by double-label immunofluorescence using antibodies against (3-
tubulin and phospho-
14
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
tau. Beta-tubulin was used as a marker for neurons and DAPI staining was used
to visualize the
nucleus of cells.
Figure 20 shows that RNS60 suppresses fibrillar Ap (1-42)-mediated apoptosis
of human
primary neurons and that this RNS60-mediated suppression can be blocked by an
PI-3 kinase
inhibitor (LY). Neurons preincubated with different concentrations of the PI-3
kinase inhibitor
(LY) for 30 min were treated with RNS60. After 1 h of incubation, cells were
challenged with
fibrillar A(31-42. After 12 h, neuronal apoptosis was monitored by TUNEL.
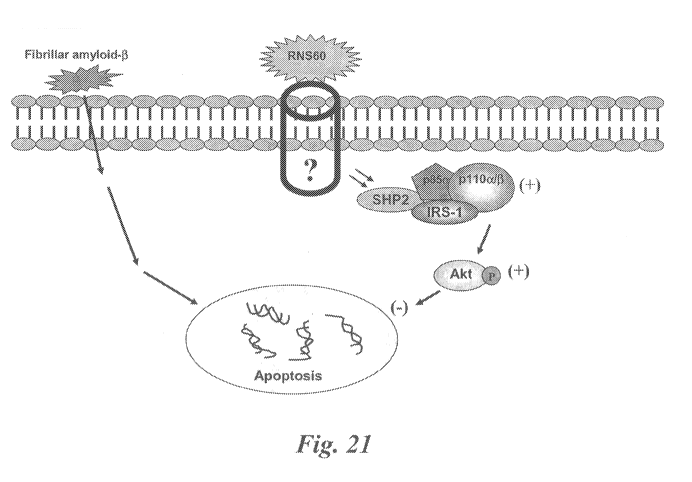
Figure 21 is, according to particular aspects, a schematic diagram of a
signally pathway
for the RNS60-mediated suppressive effect of fibrillar A(31-42-mediated
apoptosis in neurons.
Without being bound by mechanism, the schematic pathway shows an RNS60-
mediated
activation of PI-3 kinase, which in turns activates Akt via phosphorylation.
According to further
aspects, phosphorylated Akt mediates suppression of apoptosis.
DETAILED DESCRIPTION OF THE INVENTION
Certain embodiments disclosed herein relate to providing compositions and
methods of
treatment of at least one symptom of an inflammatory neurodegenerative disease
and/or multiple
sclerosis by contacting the site or administering to a subject, a therapeutic
composition
comprising a novel electrokinetically-generated fluid. In certain specific
embodiments, the
electrokinetically-generated fluids comprise gas-enriched electrokinetically-
generated fluid
comprising oxygen-enriched water.
Neuroprotective compositions and methods
Certain embodiments herein relate to therapeutic compositions and methods of
treatment
for a subject by preventing or alleviating at least one symptom associated
with exposure to a
neurotoxin or neurotoxic agent.
Parkinson's Disease and conditions
Certain embodiments herein relate to therapeutic compositions and methods of
treatment
for a subject by preventing or alleviating at least one symptom of Parkinson's
Disease and/or an
associated condition or disease.
In further embodiments herein relate to the therapeutic compositions and
methods of
treatment for preventing or alleviating complications related to Parkinson's
Disease and/or an
associated condition, including alleviating the symptoms of motor symptoms
(e.g. tremor,
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
rigidity, bradykinesia (slowness of movement) and gait impairment) and non-
motor symptoms
(e.g., such as cognitive deficits, depression, and sleep disorders).
Electrokinetically-generated fluids:
"Electrokinetically generated fluid," as used herein, refers to Applicants'
inventive
electrokinetically-generated fluids generated, for purposes of the working
Examples herein, by
the exemplary Mixing Device described in detail herein (see also
US200802190088 and
W02008/052143, both incorporated herein by reference in their entirety). The
electrokinetic
fluids, as demonstrated by the data disclosed and presented herein, represent
novel and
fundamentally distinct fluids relative to prior art non-electrokinetic fluids,
including relative to
prior art oxygenated non-electrokinetic fluids (e.g., pressure pot oxygenated
fluids and the like).
As disclosed in various aspects herein, the electrokinetically-generated
fluids have unique and
novel physical and biological properties including, but not limited to the
following:
In particular aspects, the electrokinetically altered aqueous fluid comprise
an ionic
aqueous solution of charge-stabilized oxygen-containing nanostructures
substantially having an
average diameter of less than about 100 nanometers and stably configured in
the ionic aqueous
fluid in an amount sufficient to provide, upon contact of a living cell by the
fluid, modulation of
at least one of cellular membrane potential and cellular membrane
conductivity.
In particular aspects, electrokinetically-generated fluids refers to fluids
generated in the
presence of hydrodynamically-induced, localized (e.g., non-uniform with
respect to the overall
fluid volume) electrokinetic effects (e.g., voltage/current pulses), such as
device feature-
localized effects as described herein. In particular aspects said
hydrodynamically -induced,
localized electrokinetic effects are in combination with surface-related
double layer and/or
streaming current effects as disclosed and discussed herein.
In particular aspects the administered inventive electrokinetically-altered
fluids comprise
charge-stabilized oxygen-containing nanostructures in an amount sufficient to
provide
modulation of at least one of cellular membrane potential and cellular
membrane conductivity.
In certain embodiments, the electrokinetically-altered fluids are
superoxygenated (e.g., RNS-20,
RNS-40 and RNS-60, comprising 20 ppm, 40 ppm and 60 ppm dissolved oxygen,
respectively,
in standard saline). In particular embodiments, the electrokinetically-altered
fluids are not-
superoxygenated (e.g., RNS-10 or Solas, comprising 10 ppm (e.g., approx.
ambient levels of
dissolved oxygen in standard saline). In certain aspects, the salinity,
sterility, pH, etc., of the
16
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
inventive electrokinetically-altered fluids is established at the time of
electrokinetic production
of the fluid, and the sterile fluids are administered by an appropriate route.
Alternatively, at least
one of the salinity, sterility, pH, etc., of the fluids is appropriately
adjusted (e.g., using sterile
saline or appropriate diluents) to be physiologically compatible with the
route of administration
prior to administration of the fluid. Preferably, and diluents and/or saline
solutions and/or buffer
compositions used to adjust at least one of the salinity, sterility, pH, etc.,
of the fluids are also
electrokinetic fluids, or are otherwise compatible.
In particular aspects, the inventive electrokinetically-altered fluids
comprise saline (e.g.,
one or more dissolved salt(s); e.g., alkali metal based salts (Li+, Na+, K+,
Rb+, Cs+, etc.),
alkaline earth based salts (e.g., Mg++, Ca++), etc., or transition metal-based
positive ions (e.g.,
Cr, Fe, Co, Ni, Cu, Zn, etc.,), in each case along with any suitable anion
components, including,
but not limited to F-, Cl-, Br-, I-, P04-, S04-, and nitrogen-based anions. .
Particular aspects
comprise mixed salt based electrokinetic fluids (e.g., Na+, K+, Ca++, Mg++,
transition metal
ion(s), etc.) in various combinations and concentrations, and optionally with
mixtures of
couterions. In particular aspects, the inventive electrokinetically-altered
fluids comprise
standard saline (e.g., approx. 0.9% NaCl, or about 0.15 M NaC1). In particular
aspects, the
inventive electrokinetically-altered fluids comprise saline at a concentration
of at least 0.0002
M, at least 0.0003 M, at least 0.001 M, at least 0.005 M, at least 0.01 M, at
least 0.015 M, at
least 0.1 M, at least 0.15 M, or at least 0.2 M. In particular aspects, the
conductivity of the
inventive electrokinetically-altered fluids is at least 10 S/cm, at least 40
S/cm, at least 80
gS/cm, at least 100 S/cm, at least 150 S/cm, at least 200 S/cm, at least
300 gS/cm, or at least
500 S/cm, at least 1 mS/cm, at least 5, mS/cm, 10 mS/cm, at least 40 mS/cm,
at least 80
mS/cm, at least 100 mS/cm, at least 150 mS/cm, at least 200 mS/cm, at least
300 mS/cm, or at
least 500 mS/cm. In particular aspects, any salt may be used in preparing the
inventive
electrokinetically-altered fluids, provided that they allow for formation of
biologically active
salt-stabilized nanostructures (e.g., salt-stabilized oxygen-containing
nanostructures) as
disclosed herein.
According to particular aspects, the biological effects of the inventive fluid
compositions
comprising charge-stabilized gas-containing nanostructures can be modulated
(e.g., increased,
decreased, tuned, etc.) by altering the ionic components of the fluids, and/or
by altering the gas
component of the fluid.
17
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
According to particular aspects, the biological effects of the inventive fluid
compositions
comprising charge-stabilized gas-containing nanostructures can be modulated
(e.g., increased,
decreased, tuned, etc.) by altering the gas component of the fluid. In
preferred aspects, oxygen
is used in preparing the inventive electrokinetic fluids. In additional
aspects mixtures of oxygen
along with at least one other gas selected from Nitrogen, Oxygen, Argon,
Carbon dioxide, Neon,
Helium, krypton, hydrogen and Xenon. As described above, the ions may also be
varied,
including along with varying the gas constitutent(s).
Given the teachings and assay systems disclosed herein (e.g., cell-based
cytokine assays,
patch-clamp assays, etc.) one of skill in the art will readily be able to
select appropriate salts and
concentrations thereof to achieve the biological activities disclosed herein.
TABLE 1. Exemplary cations and anions.
Common Cations:
Name Formula Other name(s)
Aluminum Al+3
Ammonium NH4+
Barium Ba+2
Calcium Ca +2
Chromium(II) Cr+2 Chromous
Chromium(III) Cr+3 Chromic
Copper(I) Cu+ Cuprous
Copper(II) Cu+2 Cupric
Iron(II) Fe +2 Ferrous
Iron(III) Fe +3 Ferric
Hydrogen H+
Hydronium H3O+
Lead(II) Pb+2
Lithium Li+
Magnesium Mg +2
Manganese(II) Mn+2 Manganous
Manganese(III) Mn+3 Manganic
Mercury(I) Hg2+2 Mercurous
Mercury(II) Hg +2 Mercuric
Nitronium NO2+
Potassium K+
Silver Ag+
S odium Na+
18
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
Strontium Sr +2
Tin(II) Sn+2 Stannous
Tin(IV) Sn+4 Stannic
Zinc Zn+2
Common Anions:
Simple ions:
Hydride if Oxide O2-
Fluoride F- Sulfide S2-
Chloride CY Nitride N3-
Bromide Br
Iodide I-
Oxoanions:
Arsenate As043- Phosphate P043
Arsenite As033- Hydrogen phosphate HP042-
Dihydrogen phosphate H2PO4
Sulfate S042- Nitrate N03-
Hydrogen sulfate HS04 Nitrite N02
Thiosulfate 52032"
Sulfite S03 2-
Perchlorate C104 Iodate I03-
Chlorate C103- Bromate Br03-
Chlorite C102
Hypochlorite OC1 Hypobromite OBr
Carbonate C032- Chromate Cr042-
Hydrogen carbonate HCO3- Dichromate Cr2072-
or Bicarbonate
Anions from Organic Acids:
Acetate CH3000 formate HCOO
Others:
Cyanide CN Amide NH2
Cyanate OCN Peroxide 022-
Thiocyanate SCN Oxalate C2042-
Hydroxide Off Permanganate Mn04
19
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
TABLE 2. Exemplary cations and anions.
Monoatomic Cations
Formula Charge Name
........ ......... ......... ......... ......... ......... ......... ........
......... ......... ......... ......... ......... ......... ...
H+ 1+ :.hydrogen ion
...............................................................................
...............................................................................
...............................................................................
.........
Li+ 1+ lithium ion
...............................................................................
...............................................................................
...............................................................................
........ .
Na+ 1+ sodium ion
...............................................................................
...............................................................................
...............................................................................
.......
K+ 1+ potassium ion
...............................................................................
...............................................................................
...............................................................................
........
Cs+ 1+ cesium ion
...............................................................................
...............................................................................
...............................................................................
........ .
Ag 1+ silver ion
::...................................................................::........
...................................................::..........................
...............................................................................
...........::
Mg2 2+ magnesium ion
...............................................................................
...............................................................................
...............................................................................
........
Ca2 2+ calcium ion
...............................................................................
...............................................................................
...............................................................................
....... .
Sr2+ 2+ strontium ion
...............................................................................
...............................................................................
...............................................................................
........ .
Ba2 2+ barium ion
...............................................................................
...............................................................................
...............................................................................
........
Zn2 2+ zinc ion
...............................................................................
...............................................................................
...............................................................................
...... .
Cd2 2+ cadmium ion
...............................................................................
...............................................................................
...............................................................................
........ .
A13 3+ aluminum ion
...............................................................................
...............................................................................
...............................................................................
.........
Polyatomic Cations
........ ......... ......... ......... ......... ......... ......... .........
......... ......... ......... ......... .................
Formula Charge Name
...............................................................................
...............................................................................
...............................................................................
....
1+ ammonium ion
NH4
...............................................................................
...............................................................................
...............................................................................
.........
H3O+ 1+ hydronium ion
...............................................................................
...............................................................................
...............................................................................
........ .
Multivalent Cations
........ ......... ........ ......... ......... ......... ......... .........
......... ......... ......... ......... .................
Formula Charge Name
...............................................................................
...............................................................................
...............................................................................
....... .
Cr2+ 2 chromium(II) or chromous ion
...............................................................................
...............................................................................
...............................................................................
........ .
Cr3 3 chromium(III)or chromic ion
...............................................................................
...............................................................................
...............................................................................
........
Mn2 2 manganese(II) or manganous ion
...............................................................................
...............................................................................
...............................................................................
........
Mn4 4 manganese(IV) ion
...............................................................................
...............................................................................
...............................................................................
....... .
Fe2 2 iron(II) or ferrous ion
...............................................................................
...............................................................................
...............................................................................
........
Fe3+ 3 iron(III) or ferric ion
...............................................................................
...............................................................................
...............................................................................
........ .
Co2+ 2 cobalt(II) or cobaltous ion
...............................................................................
...............................................................................
...............................................................................
....... .
Co3 3 cobalt(II) or cobaltic ion
...............................................................................
...............................................................................
...............................................................................
......... .
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
Ni2 2 nickel(II) or nickelous ion
...............................................................................
...............................................................................
...............................................................................
........ .
Ni3+ 3 nickel(III) or nickelic ion
...............................................................................
...............................................................................
...............................................................................
........
Cu 1 copper(I) or cuprous ion
...............................................................................
...............................................................................
...............................................................................
......... .
Cu2 2 copper(II) or cupric ion
...............................................................................
...............................................................................
...............................................................................
....... .
Sn2+ 2 tin(II) or atannous ion
...............................................................................
...............................................................................
...............................................................................
........
Sn4 4 tin(IV) or atannic ion
...............................................................................
...............................................................................
...............................................................................
........
Pb2 2 lead(II) or plumbous ion
...............................................................................
...............................................................................
...............................................................................
....... .
Pb4+ 4 lead(IV) or plumbic ion
...............................................................................
...............................................................................
...............................................................................
........ .
Monoatomic Anions
...............................................................................
...............................................................................
...............................................................................
......... .
...............................................................................
...............................................................................
...............................................................................
....... .
Formula Charge Name
...............................................................................
...............................................................................
...............................................................................
........ .
H 1 hydride ion
...............................................................................
...............................................................................
...............................................................................
........
F- 1- fluoride ion
...............................................................................
...............................................................................
...............................................................................
........
CY 1- chloride ion
...............................................................................
...............................................................................
...............................................................................
....... .
Br 1 bromide ion
...............................................................................
...............................................................................
...............................................................................
....... .
I 1 iodide ion
...............................................................................
...............................................................................
...............................................................................
........
02_ 2- oxide ion
...............................................................................
...............................................................................
...............................................................................
....... .
S2_ 2- sulfide ion
...............................................................................
...............................................................................
...............................................................................
....... .
N3 3- nitride ion
...............................................................................
...............................................................................
...............................................................................
.........
Polyatomic Anions
Formula Charge Name
...............................................................................
...............................................................................
...............................................................................
.........
Off 1- hydroxide ion
CN Ã1- cyanide ion
...............................................................................
...............................................................................
...............................................................................
...... .
SCN 1 thiocyanate ion
C2H302 1 acetate ion
...............................................................................
...............................................................................
...............................................................................
......... .
C10- 1- hypochlorite ion
...............................................................................
...............................................................................
...............................................................................
...... .
C102 Ã1- chlorite ion
...............................................................................
...............................................................................
...............................................................................
.....
C103 1 chlorate ion
...............................................................................
...............................................................................
...............................................................................
.........
C104 1- Perchlorate ion
...............................................................................
...............................................................................
...............................................................................
....... .
N02 1- nitrite ion
N03 1 nitrate ion
...............................................................................
...............................................................................
...............................................................................
.........
Mn042 2- permanganate ion
...............................................................................
...............................................................................
...............................................................................
..... .
C032 2- carbonate ion
...............................................................................
...............................................................................
...............................................................................
........ .
C2042 2- oxalate ion
...............................................................................
...............................................................................
...............................................................................
......... .
21
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
Cr042- 2 chromate ion
...............................................................................
...............................................................................
...............................................................................
....... .
Cr2072 2- dichromate ion
...............................................................................
...............................................................................
...............................................................................
......
5032 Ã 2- sulfite ion
...............................................................................
...............................................................................
...............................................................................
......... .
5042 2- sulfate ion
...............................................................................
...............................................................................
...............................................................................
...... .
P033 3- phosphite ion
...............................................................................
...............................................................................
...............................................................................
.......
P043 3- phosphate ion
...............................................................................
...............................................................................
...............................................................................
......... .
The present disclosure sets forth novel gas-enriched fluids, including, but
not limited to
gas-enriched ionic aqueous solutions, aqueous saline solutions (e.g., standard
aqueous saline
solutions, and other saline solutions as discussed herein and as would be
recognized in the art,
including any physiological compatible saline solutions), cell culture media
(e.g., minimal
medium, and other culture media) useful in the treatment of diabetes or
diabetes related
disorders. A medium, or media, is termed "minimal" if it only contains the
nutrients essential
for growth. For prokaryotic host cells, a minimal media typically includes a
source of carbon,
nitrogen, phosphorus, magnesium, and trace amounts of iron and calcium.
(Gunsalus and
Stanter, The Bacteria, V. 1, Ch. 1 Acad. Press Inc., N.Y. (1960)). Most
minimal media use
glucose as a carbon source, ammonia as a nitrogen source, and orthophosphate
(e.g., P04) as the
phosphorus source. The media components can be varied or supplemented
according to the
specific prokaryotic or eukaryotic organism(s) grown, in order to encourage
optimal growth
without inhibiting target protein production. (Thompson et al., Biotech. and
Bioeng. 27: 818-
824 (1985)).
In particular aspects, the electrokinetically altered aqueous fluids are
suitable to modulate
13C-NMR line-widths of reporter solutes (e.g., Trehelose) dissolved therein.
NMR line-width
effects are in indirect method of measuring, for example, solute `tumbling' in
a test fluid as
described herein in particular working Examples.
In particular aspects, the electrokinetically altered aqueous fluids are
characterized by at
least one of: distinctive square wave voltametry peak differences at any one
of -0.14V, -0.47V, -
1.02V and -1.36V; polarographic peaks at -0.9 volts; and an absence of
polarographic peaks at -
0.19 and -0.3 volts, which are unique to the electrokinetically generated
fluids as disclosed
herein in particular working Examples.
In particular aspects, the electrokinetically altered aqueous fluids are
suitable to alter
cellular membrane conductivity (e.g., a voltage-dependent contribution of the
whole-cell
conductance as measure in patch clamp studies disclosed herein).
22
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
In particular aspects, the electrokinetically altered aqueous fluids are
oxygenated,
wherein the oxygen in the fluid is present in an amount of at least 15, ppm,
at least 25 ppm, at
least 30 ppm, at least 40 ppm, at least 50 ppm, or at least 60 ppm dissolved
oxygen at
atmospheric pressure. In particular aspects, the electrokinetically altered
aqueous fluids have
less than 15 ppm, less that 10 ppm of dissolved oxygen at atmospheric
pressure, or
approximately ambient oxygen levels.
In particular aspects, the electrokinetically altered aqueous fluids are
oxygenated,
wherein the oxygen in the fluid is present in an amount between approximately
8 ppm and
approximately 15 ppm, and in this case is sometimes referred to herein as
"Solas."
In particular aspects, the electrokinetically altered aqueous fluid comprises
at least one of
solvated electrons (e.g., stabilized by molecular oxygen), and
electrokinetically modified and/or
charged oxygen species, and wherein in certain embodiments the solvated
electrons and/or
electrokinetically modified or charged oxygen species are present in an amount
of at least 0.01
ppm, at least 0.1 ppm, at least 0.5 ppm, at least 1 ppm, at least 3 ppm, at
least 5 ppm, at least 7
ppm, at least 10 ppm, at least 15 ppm, or at least 20 ppm.
In particular aspects, the electrokinetically altered aqueous fluids are
suitable to alter
cellular membrane structure or function (e.g., altering of a conformation,
ligand binding activity,
or a catalytic activity of a membrane associated protein) sufficient to
provide for modulation of
intracellular signal transduction, wherein in particular aspects, the membrane
associated protein
comprises at least one selected from the group consisting of receptors,
transmembrane receptors
(e.g., G-Protein Coupled Receptor (GPCR), TSLP receptor, beta 2 adrenergic
receptor,
bradykinin receptor, etc.), ion channel proteins, intracellular attachment
proteins, cellular
adhesion proteins, and integrins. In certain aspects, the effected G-Protein
Coupled Receptor
(GPCR) interacts with a G protein a subunit (e.g., Gas , Gai, Gaq, and Ga12).
In particular aspects, the electrokinetically altered aqueous fluids are
suitable to modulate
intracellular signal transduction, comprising modulation of a calcium
dependant cellular
messaging pathway or system (e.g., modulation of phospholipase C activity, or
modulation of
adenylate cyclase (AC) activity).
In particular aspects, the electrokinetically altered aqueous fluids are
characterized by
various biological activities (e.g., regulation of cytokines, receptors,
enzymes and other proteins
and intracellular signaling pathways) described in the working Examples and
elsewhere herein.
23
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
In particular aspects, the electrokinetically altered aqueous fluids display
synergy with
any one of erythropoietin, anti-apoptotics (TCH346, CEP-1347),
antiglutamatergics, monoamine
oxidase inhibitors (selegiline, rasagiline), promitochondrials (coenzyme Q10,
creatine), calcium
channel blockers (isradipine), alpha- synuclein, and growth factors (GDNF)..
In particular
aspects, the electrokinetically altered aqueous fluids reduce DEP-induced TSLP
receptor
expression in bronchial epithelial cells (BEC) as shown in working Examples
herein.
In particular aspects, the electrokinetically altered aqueous fluids inhibit
the DEP-
induced cell surface-bound MMP9 levels in bronchial epithelial cells (BEC) as
shown in
working Examples herein.
In particular aspects, the biological effects of the electrokinetically
altered aqueous fluids
are inhibited by diphtheria toxin, indicating that beta blockade, GPCR
blockade and Ca channel
blockade affects the activity of the electrokinetically altered aqueous fluids
(e.g., on regulatory T
cell function) as shown in working Examples herein.
In particular aspects, the physical and biological effects (e.g., the ability
to alter cellular
membrane structure or function sufficient to provide for modulation of
intracellular signal
transduction) of the electrokinetically altered aqueous fluids persists for at
least two, at least
three, at least four, at least five, at least 6 months, or longer periods, in
a closed container (e.g.,
closed gas-tight container).
Therefore, further aspects provide said electrokinetically-generated solutions
and
methods of producing an electrokinetically altered oxygenated aqueous fluid or
solution,
comprising: providing a flow of a fluid material between two spaced surfaces
in relative motion
and defining a mixing volume therebetween, wherein the dwell time of a single
pass of the
flowing fluid material within and through the mixing volume is greater than
0.06 seconds or
greater than 0.1 seconds; and introducing oxygen (02) into the flowing fluid
material within the
mixing volume under conditions suitable to dissolve at least 20 ppm, at least
25 ppm, at least 30,
at least 40, at least 50, or at least 60 ppm oxygen into the material, and
electrokinetically alter
the fluid or solution. In certain aspects, the oxygen is infused into the
material in less than 100
milliseconds, less than 200 milliseconds, less than 300 milliseconds, or less
than 400
milliseconds. In particular embodiments, the ratio of surface area to the
volume is at least 12, at
least 20, at least 30, at least 40, or at least 50.
Yet further aspects, provide a method of producing an electrokinetically
altered
oxygenated aqueous fluid or solution, comprising: providing a flow of a fluid
material between
24
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
two spaced surfaces defining a mixing volume therebetween; and introducing
oxygen into the
flowing material within the mixing volume under conditions suitable to infuse
at least 20 ppm,
at least 25 ppm, at least 30, at least 40, at least 50, or at least 60 ppm
oxygen into the material in
less than 100 milliseconds, less than 200 milliseconds, less than 300
milliseconds, or less than
400 milliseconds. In certain aspects, the dwell time of the flowing material
within the mixing
volume is greater than 0.06 seconds or greater than 0.1 seconds. In particular
embodiments, the
ratio of surface area to the volume is at least 12, at least 20, at least 30,
at least 40, or at least 50.
Additional embodiments provide a method of producing an electrokinetically
altered
oxygenated aqueous fluid or solution, comprising use of a mixing device for
creating an output
mixture by mixing a first material and a second material, the device
comprising: a first chamber
configured to receive the first material from a source of the first material;
a stator; a rotor having
an axis of rotation, the rotor being disposed inside the stator and configured
to rotate about the
axis of rotation therein, at least one of the rotor and stator having a
plurality of through-holes; a
mixing chamber defined between the rotor and the stator, the mixing chamber
being in fluid
communication with the first chamber and configured to receive the first
material therefrom, and
the second material being provided to the mixing chamber via the plurality of
through-holes
formed in the one of the rotor and stator; a second chamber in fluid
communication with the
mixing chamber and configured to receive the output material therefrom; and a
first internal
pump housed inside the first chamber, the first internal pump being configured
to pump the first
material from the first chamber into the mixing chamber. In certain aspects,
the first internal
pump is configured to impart a circumferential velocity into the first
material before it enters the
mixing chamber.
Further embodiments provide a method of producing an electrokinetically
altered
oxygenated aqueous fluid or solution, comprising use of a mixing device for
creating an output
mixture by mixing a first material and a second material, the device
comprising: a stator; a rotor
having an axis of rotation, the rotor being disposed inside the stator and
configured to rotate
about the axis of rotation therein; a mixing chamber defined between the rotor
and the stator, the
mixing chamber having an open first end through which the first material
enters the mixing
chamber and an open second end through which the output material exits the
mixing chamber,
the second material entering the mixing chamber through at least one of the
rotor and the stator;
a first chamber in communication with at least a majority portion of the open
first end of the
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
mixing chamber; and a second chamber in communication with the open second end
of the
mixing chamber.
Additional aspects provide an electrokinetically altered oxygenated aqueous
fluid or
solution made according to any of the above methods. In particular aspects the
administered
inventive electrokinetically-altered fluids comprise charge-stabilized oxygen-
containing
nanostructures in an amount sufficient to provide modulation of at least one
of cellular
membrane potential and cellular membrane conductivity. In certain embodiments,
the
electrokinetically-altered fluids are superoxygenated (e.g., RNS-20, RNS-40
and RNS-60,
comprising 20 ppm, 40 ppm and 60 ppm dissolved oxygen, respectively, in
standard saline). In
particular embodiments, the electrokinetically-altered fluids are not-
superoxygenated (e.g.,
RNS-10 or Solas, comprising 10 ppm (e.g., approx. ambient levels of dissolved
oxygen in
standard saline). In certain aspects, the salinity, sterility, pH, etc., of
the inventive
electrokinetically-altered fluids is established at the time of electrokinetic
production of the
fluid, and the sterile fluids are administered by an appropriate route.
Alternatively, at least one of
the salinity, sterility, pH, etc., of the fluids is appropriately adjusted
(e.g., using sterile saline or
appropriate diluents) to be physiologically compatible with the route of
administration prior to
administration of the fluid. Preferably, and diluents and/or saline solutions
and/or buffer
compositions used to adjust at least one of the salinity, sterility, pH, etc.,
of the fluids are also
electrokinetic fluids, or are otherwise compatible therewith.
The present disclosure sets forth novel gas-enriched fluids, including, but
not limited to
gas-enriched ionic aqueous solutions, aqueous saline solutions (e.g., standard
aqueous saline
solutions, and other saline solutions as discussed herein and as would be
recognized in the art,
including any physiological compatible saline solutions), cell culture media
(e.g., minimal
medium, and other culture media).
Neurotoxins:
By "toxic agent" or "neurotoxic agent" (neurotoxin) is meant a substance that
through its
chemical action injures, impairs, or inhibits the activity of a component of
the nervous system.
The list of neurotoxic agents that cause neuropathies is lengthy (see a list
of exemplary
neurotoxic agents provided in Table 3 below). Such neurotoxic agents include,
but are not
limited to, neoplastic agents such as vincristine, vinblastine, cisplatin,
taxol, or dideoxy-
compounds, e.g., dideoxyinosine; alcohol; metals; industrial toxins involved
in occupational or
26
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
environmental exposure; contaminants in food or medicinals; or over doses of
vitamins or
therapeutic drugs, e.g., antibiotics such as penicillin or chloramphenicol, or
mega-doses of
vitamins A, D, or B6.
Neurotoxicity may occur upon exposure to natural or artificial toxic
substances
(neurotoxins) that alters the normal activity of the nervous system in such a
way as to cause
damage to nervous tissue, and can eventually disrupt or kill neurons.
Neurotoxicity can result
from exposure to substances used in chemotherapy, radiation treatment, drug
therapies, certain
drug abuse, and organ transplants, as well as exposure to heavy metals,
certain foods and food
additives, pesticides, industrial and/or cleaning solvents, cosmetics, and
some naturally
occurring substances. Symptoms may appear immediately after exposure or be
delayed. They
may include limb weakness or numbness, loss of memory, vision, and/or
intellect,
uncontrollable obsessive and/or compulsive behaviors, delusions, headache,
cognitive and
behavioral problems and sexual dysfunction. Individuals with certain disorders
may be
especially vulnerable to neurotoxins.
According to particular embodiments, the compositions disclosed herein are
used to
prevent or ameliorate neurotoxicity caused by exposure to a variety of agents
as discussed
herein.
Certain toxins can cause peripheral neuropathy. Lead toxicity is associated
with a motor
neuropathy. Arsenic and mercury cause a sensory neuropathy. Thallium can cause
a sensory
and autonomic neuropathy. Several organic solvents and insecticides can also
cause
polyneuropathy. Alcohol is directly toxic to nerves and alcohol abuse is a
major cause of
neuropathy. The subject method can be used, in certain embodiments, as part of
a broader
detoxification program.
In still another embodiment, the methods and compositions of the present
invention can
be used for the treatment of neuropathies caused by drugs. Several drugs are
known to cause
neuropathy. They include, among others, vincristine and cisplatinum in cancer,
nitrofurantoin,
which is used in pyelonephritis, amiodarone in cardiac arrhythmias, disulfuram
in alcoholism,
ddC and ddl in AIDS, and dapsone which is used to treat leprosy. As above, the
subject method
can be used, in certain embodiments, as part of a broader detoxification
program.
Another aspect of the invention provides a conjoint therapy wherein one or
more other
therapeutic agents are administered with the subject compound. Such conjoint
treatment may be
achieved by way of the simultaneous, sequential or separate dosing of the
individual
27
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
components of the treatment. Conjoint administration thus includes
administration as part of the
same pharmaceutical preparation, simultaneous administration of separate
pharmaceutical
preparations, as well as administration of separate pharmaceutical
preparations at different times
on the same day, adjacent days, or otherwise as part of a single therapeutic
regimen. For
example, the subject method can be carried out conjointly with other
neuroprotective agents.
The dosages recited herein would be adjusted to compensate for such additional
components in
the therapeutic composition. Progress of the treated patient can be monitored
by conventional
methods.
In yet other embodiments, the subject method can be carried out conjointly
with the
administration of growth and/or trophic factors. For instance, the
combinatorial therapy can
include a trophic factor such as glial cell line-derived neurotrophic factor,
nerve growth factor,
cilliary neurotrophic factor, schwanoma-derived growth factor, glial growth
factor, striatal-
derived neuronotrophic factor, platelet-derived growth factor, brain-derived
neurotrophic factor
(BDNF), and scatter factor (HGF-SF). Antimitogenic agents can also be used, as
for example,
cytosine, arabinoside, 5-fluorouracil, hydroxyurea, and methotrexate.
Determination of a therapeutically effective amount and/or a prophylactically
effective
amount of administered composition of the invention, e.g., to be adequately
neuroprotective, can
be readily made one skilled in the art by the use of known techniques. The
dosages may be
varied depending upon the requirements of the patient in the judgment of the
attending clinician,
the severity of the condition being treated, the risk of further degeneration
to the CNS, and the
particular neurotoxin. In determining the therapeutically effective trophic
amount or dose,
and/or the prophylactically effective amount or dose, a number of factors are
considered by the
attending clinician, including, but not limited to: the specific cause of the
degenerative state and
its likelihood of recurring or worsening; pharmacodynamic characteristics of
the particular
neurotoxic agent; the desired time course of treatment; the species of mammal;
its size, age, and
general health; the response of the individual patient; the particular
compound administered; the
bioavailability characteristics of the preparation administered; the dose
regimen selected; the
kind of concurrent treatment; and other relevant circumstances.
Treatment can be initiated with smaller dosages that are less than the optimum
dose.
Thereafter, the dosage may be increased by small increments until the optimum
effect under the
circumstances is reached. For convenience, the total daily dosage may be
divided and
administered in portions during the day if desired. A therapeutically
effective trophic amount
28
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
and a prophylactically effective neuroprotective amount of therapeutic
composition, for instance,
is expected to vary depending on the route of administration, and other
factors as discussed
above.
Compositions effective for the prevention or treatment of degeneration of
neurons (e.g.,
dopaminergic neurons and motoneurons and the like) in animals, e.g., dogs,
rodents, may also be
useful in treatment of disorders in humans. Those skilled in the art of
treating in such disorders
in humans will be guided, from the data obtained in animal studies, to the
correct dosage and
route of administration of the compound to humans. In general, the
determination of dosage and
route of administration in humans is expected to be similar to that used to
determine
administration in animals.
The identification of those patients who are in need of prophylactic treatment
for
disorders marked by degeneration of neurons (e.g. dopaminergic neurons and/or
motoneurons
and the like) is well within the ability and knowledge of one skilled in the
art. Certain of the
methods for identification of patients that are at risk and that can be
treated by the subject
method are appreciated in the medical arts, such as family history of the
development of a
particular disease state and the presence of risk factors associated with the
development of that
disease state in the subject patient. Risk of environmental (e.g., chemical)
exposure. A clinician
skilled in the art can readily identify such candidate patients, by the use
of, for example, clinical
tests, physical examination, medical/family history, vocation/occupation, etc.
Protecting soldiers against any kind of threat and preserving their ability to
fight has
become a major concern of armies. Nerve gas (e.g., sarin, soman or Vx) is one
such threat. One
class of nerve agents (also known as nerve gases) are phosphorus-containing
organic chemicals
(organophosphates) that block acetylcholinesterase, an enzyme that normally
relaxes the activity
of acetylcholine, a neurotransmitter. There are two main classes of nerve
agents, G agents (e.g.,
GA, tabun or ethyl N,N-dimethylphosphoramidocyanidate; GB, sarin or O-
isopropyl
methylphosphonofluoridate; GD, soman or O-pinacolyl methylphosphonofluoridate;
GF,
cyclosarin or cyclohexyl methylphosphonofluoridate; GV, P-[2-
(dimethylamino)ethyl]-N,N-
dimethylphosphonamidic fluoride)) and V agents (VE, S-(diethylamino)ethyl O-
ethyl
ethylphosphonothioate; VG, Amiton or Tetram or 0,0-diethyl-S-[2-
(diethylamino)ethyl]phosphorothioate; VM, phosphonothioic acid, methyl-, S-(2-
(diethylamino)ethyl) O-ethyl ester); VX, O-ethyl-S-
[2(diisopropylamino)ethyl]methylphosphonothiolate). A third group of agents,
the Novichok
29
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
agents, are organophosphate compounds that inhibit the enzyme cholinesterase,
preventing the
normal breakdown of acetylcholine.
Insecticides, the organophosphates, such as dichlorvos, malathion and
parathion, are
nerve agents.
TABLE 3- Neurotoxic Agents
AGENT ACTIVITY AGENT ACTIVITY
actazolimide Diuretic imipramine antidepressant
Acrylamide flocculant, grouting agent indolmethacin anti-inflammatory
adriamycin Antineoplastic inorganic lead toxic metal in
paint, etc.
alcohol (i.e. ethanol) solvent, recreational drug iso-niazid antituberculousis
almitine respiratory stimulant lithium antidepressant
amiodarone Antiarrthymic meth lmercur industrial waste
amphotericin Antimicrobial metformin antidiabetic
arsenic herbicide, insecticide methylhydrazine synthetic
intermediate
aurothioglucose Antirheumatic metronidazole anti protozoal
barbiturates anticonvulsive, sedative misonidazole radiosensitizer
buckthorn toxic berry nitrofurantoin urinary antiseptic
antineoplastic,
carbimates Insecticide nitrogen mustard
nerve gas
carbon disulfide industrial applications nitous oxide anesthetic
chlorarnphenicol Antibacterial or ano hos hates insecticides
chloro uine Antimalarial ospolot anticonvulsant
chlorestyramine Antih erli o roteinemic penicillin antibacterial
cisplatin Antineoplastic perhexiline antiarrhythmic
clioquinol amebicide, antibacterial perhexiline antiarrythmic =
maleate
colestipol Antih erlio roteinemic phenytoin anticonvulsant
colchicine gout suppressant platnim drug component
colistin Antimicrobial primidone anticonvulsant
cycloserine Antibacterial procarbazine antineoplastic
cytarabine Antineoplastic pyridoxine vitamin B6
dapsone dermatological ie- sodium cyanate antisickling
leprosy
dideox c tidine Anatineoplastic streptomycin antimicrobial
dideoxyinosine Antineoplastic sulphonamides antimicrobial
dideox th midine Antiviral suramin anteneoplastic
disulfiram Antialcohol tamoxifen antineoplastic
doxorubicin Antineoplastic taxol antineoplastic
ethambutol Antibacterial thalidomide antileprous
ethionamide Antibacterial thallium rat poison
glutethimide sedative, hypnotic triamterene diuretic
gold Antirheumatic trimethyltin toxic metal
hexacarbons Solvents L-t o han health food
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
AGENT ACTIVITY AGENT ACTIVITY
additive
hormonal contraceptives vincristine Antineoplastic
hexamethylolmelamine fireproofing, crease vinblastine Antineoplastic
roofin
hydralazine Antihypertensive vindesine Antineoplastic
hydroxychloroquine Antirheumatic vitamine A or D mega doses
In particular embodiments, the methods and compositions of the present
invention can be
used for the prevention or amelioration of chemotheraphy induced neurotoxicity
(see, e.g,
United States Patent Number 7,129,250, (published as 2004/0220202), which is
incorporated by
reference herein in its entirety, and in particular for its teachings of
exemplary neurotoxins).
For example, in particular embodiments, the methods and compositions of the
present
invention can be used with an anti-cancer agent such as an anti-cancer drug, a
cytokine, and/or
supplementary potentiating agent(s). The use of cocktails in the treatment of
cancer is routine.
In this embodiment, a common administration vehicle (e.g., orally available or
injectable
solution, etc.) could contain both a compositions of the present invention and
the anti-cancer
drug and/or supplementary potentiating agent. Thus, cocktails comprising
compositions of the
present invention as well as other compounds are within the scope of the
invention.
Compounds having anti-neoplastic properties include, but are not limited to:
Acivicin;
Aclarubicin; Acodazole Hydrochloride; Acronine; Adozelesin; Aldesleukin;
Altretamine;
Ambomycin; Ametantrone Acetate; Aminoglutethimide; Amsacrine; Anastrozole;
Anthramycin;
Asparaginase; Asperlin; Azacitidine; Azetepa; Azotomycin; Batimastat;
Benzodepa;
Bicalutamide; Bisantrene Hydrochloride; Bisnafide Dimesylate; Bizelesin;
Bleomycin Sulfate;
Brequinar Sodium; Bropirimine; Busulfan; Cactinomycin; Calusterone;
Caracemide;
Carbetimer; Carboplatin; Carmustine; Carubicin Hydrochloride; Carzelesin;
Cedefingol;
Chlorambucil; Cirolemycin; Cisplatin; Cladribine; Crisnatol Mesylate;
Cyclophosphamide;
Cytarabine; Dacarbazine; Dactinomycin; Daunorubicin Hydrochloride; Decitabine;
Dexormaplatin; Dezaguanin; Dezaguanine Mesylate; Diaziquone; Docetaxel;
Doxorubicin;
Doxorubicin Hydrochloride; Droloxifene; Droloxifene Citrate; Dromostanolone
Propionate;
Duazomycin; Edatrexate; Eflornithine Hydrochloride; Elsamitrucin; Enloplatin;
Enpromate;
Epipropidine; Epirubicin Hydrochloride; Erbulozole; Esorubicin Hydrochloride;
Estramustine;
Estramustine Phosphate Sodium; Etanidazole; Ethiodized Oil I 131; Etoposide;
Etoposide
Phosphate; Etoprine; Fadrozole Hydrochloride; Fazarabine; Fenretinide;
Floxuridine;
Fludarabine Phosphate; Fluorouracil; Flurocitabine; Fosquidone; Fostriecin
Sodium;
31
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
Gemcitabine; Gemcitabine Hydrochloride; Gold An 198; Hydroxyurea; Idarubicin
Hydrochloride; Ifosfamide; Imofosine; Interferon Alfa-2a; Interferon Alfa-2b;
Interferon Alfa-
nl; Interferon Alfa-n3; Interferon Beta-la; Interferon Gamma-lb; Iproplatin;
Irinotecan
Hydrochloride; Lanreotide Acetate; Letrozole; Leuprolide Acetate; Liarozole
Hydrochloride;
Lometrexol Sodium; Lomustine; Losoxantrone Hydrochloride; Masoprocol;
Maytansine;
Mechlorethamine Hydrochloride; Megestrol Acetate; Melengestrol Acetate;
Melphalan;
Menogaril; Mercaptopurine; Methotrexate; Methotrexate Sodium; Metoprine;
Meturedepa;
Mitindomide; Mitocarcin; Mitocromin; Mitogillin; Mitomalcin; Mitomycin;
Mitosper; Mitotane;
Mitoxantrone Hydrochloride; Mycophenolic Acid; Nocodazole; Nogalamycin;
Ormaplatin;
Oxisuran; Paclitaxel; Pegaspargase; Peliomycin; Pentamustine; Peplomycin
Sulfate;
Perfosfamide; Pipobroman; Piposulfan; Piroxantrone Hydrochloride; Plicamycin;
Plomestane;
Porfimer Sodium; Porfiromycin; Prednimustine; Procarbazine Hydrochloride;
Puromycin;
Puromycin Hydrochloride; Pyrazofurin; Riboprine; Rogletimide; Safingol;
Safingol
Hydrochloride; Semustine; Simtrazene; Sparfosate Sodium; Sparsomycinl,
Spirogermanium
Hydrochloride; Spiromustine; Spiroplatin; Streptonigrin; Streptozocin;
Strontium Chloride Sr
89; Sulofenur; Talisomycin; Taxane; Taxoid; Tecogalan Sodium; Tegafur;
Teloxantrone
Hydrochloride; Temoporfin; Teniposide; Teroxirone; Testolactone; Thiamiprine;
Thioguanine;
Thiotepa; Tiazofurin; Tirapazamine; Topotecan Hydrochloride; Toremifene
Citrate; Trestolone
Acetate; Triciribine Phosphate; Trimetrexate; Trimetrexate Glucuronate;
Triptorelin; Tubulozole
Hydrochloride; Uracil Mustard; Uredepa; Vapreotide; Verteporfin; Vinblastine
Sulfate;
Vincristine Sulfate; Vindesine; Vindesine Sulfate; Vinepidine Sulfate;
Vinglycinate Sulfate;
Vinleurosine Sulfate; Vinorelbine Tartrate; Vinrosidine Sulfate; Vinzolidine
Sulfate; Vorozole;
Zeniplatin; Zinostatin; Zorubicin Hydrochloride.
Other anti-neoplastic compounds include: 20epi-1,25 dihydroxyvitamin D3; 5-
ethynyluracil; abiraterone; aclarubicin; acylfulvene; adecypenol; adozelesin;
aldesleukin; ALL-
TK antagonists; altretamine; ambamustine; amidox; amifostine; aminolevulinic
acid; amrubicin;
amsacrine; anagrelide; anastrozole; andrographolide; angiogenesis inhibitors;
antagonist D;
antagonist G; antarelix; anti-dorsalizing morphogenetic protein-1;
antiandrogen, prostatic
carcinoma; antiestrogen; antineoplaston; antisense oligonucleotides;
aphidicolin glycinate;
apoptosis gene modulators; apoptosis regulators; apurinic acid; ara-CDP-DL-
PTBA; arginine
deaminase; asulacrine; atamestane; atrimustine; axinastatin 1; axinastatin 2;
axinastatin 3;
azasetron; azatoxin; azatyrosine; baccatin III derivatives; balanol;
batimastat; BCR/ABL
32
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
antagonists; benzochlorins; benzoylstaurosporine; beta lactam derivatives;
beta-alethine;
betaclamycin B; betulinic acid; bFGF inhibitor; bicalutamide; bisantrene;
bisaziridinylspermine;
bisnafide; bistratene A; bizelesin; breflate; bropirimine; budotitane;
buthionine sulfoximine;
calcipotriol; calphostin C; camptothecin derivatives; canarypox IL-2;
capecitabine;
carboxamide-amino-triazole; carboxyamidotriazole; CaRest M3; CARN 700;
cartilage derived
inhibitor; carzelesin; casein kinase inhibitors (ICOS); castanospermine;
cecropin B; cetrorelix;
chlorins; chloroquinoxaline sulfonamide; cicaprost; cis-porphyrin; cladribine;
clomifene
analogues; clotrimazole; collismycin A; collismycin B; combretastatin A4;
combretastatin
analogue; conagenin; crambescidin 816; crisnatol; cryptophycin 8; cryptophycin
A derivatives;
curacin A; cyclopentanthraquinones; cycloplatam; cypemycin; cytarabine
ocfosfate; cytolytic
factor; cytostatin; dacliximab; decitabine; dehydrodidemnin B; deslorelin;
dexifosfamide;
dexrazoxane; dexverapamil; diaziquone; didemnin B; didox; diethylnorspermine;
dihydro-5-
azacytidine; dihydrotaxol, 9-; dioxamycin; diphenyl spiromustine; docosanol;
dolasetron;
doxifluridine; droloxifene; dronabinol; duocarmycin SA; ebselen; ecomustine;
edelfosine;
edrecolomab; eflomithine; elemene; emiteftir; epirubicin; epristeride;
estramustine analogue;
estrogen agonists; estrogen antagonists; etanidazole; etoposide phosphate;
exemestane;
fadrozole; fazarabine; fenretinide; filgrastim; finasteride; flavopiridol;
flezelastine; fluasterone;
fludarabine; fluorodaunorunicin hydrochloride; forfenimex; formestane;
fostriecin; fotemustine;
gadolinium texaphyrin; gallium nitrate; galocitabine; ganirelix; gelatinase
inhibitors;
gemcitabine; glutathione inhibitors; hepsulfam; heregulin; hexamethylene
bisacetamide;
hypericin; ibandronic acid; idarubicin; idoxifene; idramantone; ilmofosine;
ilomastat;
imidazoacridones; imiquimod; immunostimulant peptides; insulin-like growth
factor-1 receptor
inhibitor; interferon agonists; interferons; interleukins; iobenguane;
iododoxorubicin;
ipomeanol, 4-; irinotecan; iroplact; irsogladine; isobengazole;
isohomohalicondrin B; itasetron;
jasplakinolide; kahalalide F; lamellarin-N triacetate; lanreotide; leinamycin;
lenograstim;
lentinan sulfate; leptolstatin; letrozole; leukemia inhibiting factor;
leukocyte alpha interferon;
leuprolide+estrogen+progesterone; leuprorelin; levamisole; liarozole; linear
polyamine
analogue; lipophilic disaccharide peptide; lipophilic platinum compounds;
lissoclinamide 7;
lobaplatin; lombricine; lometrexol; lonidamine; losoxantrone; lovastatin;
loxoribine; lurtotecan;
lutetium texaphyrin; lysofylline; lytic peptides; maitansine; mannostatin A;
marimastat;
masoprocol; maspin; matrilysin inhibitors; matrix metalloproteinase
inhibitors; menogaril;
merbarone; meterelin; methioninase; metoclopramide; MIF inhibitor;
mifepristone; miltefosine;
33
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
mirimostim; mismatched double stranded RNA; mitoguazone; mitolactol; mitomycin
analogues;
mitonafide; mitotoxin fibroblast growth factor-saporin; mitoxantrone;
mofarotene;
molgramostim; monoclonal antibody, human chorionic gonadotrophin;
monophosphoryl lipid
A+myobacterium cell wall sk; mopidamol; multiple drug resistance gene
inhibitor; multiple
tumor suppressor 1-based therapy; mustard anticancer agent; mycaperoxide B;
mycobacterial
cell wall extract; myriaporone; N-acetyldinaline; N-substituted benzamides;
nafarelin; nagrestip;
naloxone+pentazocine; napavin; naphterpin; nartograstim; nedaplatin;
nemorubicin; neridronic
acid; neutral endopeptidase; nilutamide; nisamycin; nitric oxide modulators;
nitroxide
antioxidant; nitrullyn; 06-benzylguanine; octreotide; okicenone;
oligonucleotides; onapristone;
ondansetron; ondansetron; oracin; oral cytokine inducer; ormiaplatin;
osaterone; oxaliplatin;
oxaunomycin; paclitaxel analogues; paclitaxel derivatives; palauamine;
palmitoylrhizoxin;
pamidronic acid; panaxytriol; panomifene; parabactin; pazelliptine;
pegaspargase; peldesine;
pentosan polysulfate sodium; pentostatin; pentrozole; perflubron;
perfosfamide; perillyl alcohol;
phenazinomycin; phenylacetate; phosphatase inhibitors; picibanil; pilocarpine
hydrochloride;
pirarubicin; piritrexim; placetin A; placetin B; plasminogen activator
inhibitor; platinum
complex; platinum compounds; platinum-triamine complex; porfimer sodium;
porfiromycin;
propyl bis-acridone; prostaglandin J2; proteasome inhibitors; protein A-based
inmmune
modulator; protein kinase C inhibitor; protein kinase C inhibitors,
microalgal; protein tyrosine
phosphatase inhibitors; purine nucleoside phosphorylase inhibitors; purpurins;
pyrazoloacridine;
pyridoxylated hemoglobin polyoxyethylene conjugate; raf antagonists;
raltitrexed; ramosetron;
ras farnesyl protein transferase inhibitors; ras inhibitors; ras-GAP
inhibitor; retelliptine
demethylated; rhenium Re 186 etidronate; rhizoxin; ribozymes; RII retinamide;
rogletimide;
rohitukine; romurtide; roquinimex; rubiginone B 1; ruboxyl; safingol;
saintopin; SarCNU;
sarcophytol A; sargramostim; Sdi 1 mimetics; semustine; senescence derived
inhibitor 1; sense
oligonucleotides; signal transduction inhibitors; signal transduction
modulators; single chain
antigen binding protein; sizofiran; sobuzoxane; sodium borocaptate; sodium
phenylacetate;
solverol; somatomedin binding protein; sonermin; sparfosic acid; spicamycin D;
spiromustine;
splenopentin; spongistatin 1; squalamine; stem cell inhibitor; stem-cell
division inhibitors;
stipiamide; stromelysin inhibitors; sulfinosine; superactive vasoactive
intestinal peptide
antagonist; suradista; suramin; swainsonine; synthetic glycosaminoglycans;
tallimustine;
tamoxifen methiodide; tauromustine; tazarotene; tecogalan sodium; tegafur;
tellurapyrylium;
telomerase inhibitors; temoporfin; temozolomide; teniposide;
tetrachlorodecaoxide; tetrazomine;
34
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
thaliblastine; thalidomide; thiocoraline; thrombopoietin; thrombopoietin
mimetic; thymalfasin;
thymopoietin receptor agonist; thymotrinan; thyroid stimulating hormone; tin
ethyl etiopurpurin;
tirapazamine; titanocene dichloride; topotecan; topsentin; toremifene;
totipotent stem cell factor;
translation inhibitors; tretinoin; triacetyluridine; triciribine;
trimetrexate; triptorelin; tropisetron;
turosteride; tyrosine kinase inhibitors; tyrphostins; UBC inhibitors;
ubenimex; urogenital sinus-
derived growth inhibitory factor; urokinase receptor antagonists; vapreotide;
variolin B; vector
system, erythrocyte gene therapy; velaresol; veramine; verdins; verteporfin;
vinorelbine;
vinxaltine; vitaxin; vorozole; zanoterone; zeniplatin; zilascorb; zinostatin
stimalamer.
Anti-cancer supplementary potentiating agents include, but are not limited to:
tricyclic
anti-depressant drugs (e.g., imipramine, desipramine, amitryptyline,
clomipramine,
trimipramine, doxepin, nortriptyline, protriptyline, amoxapine and
maprotiline); non-tricyclic
anti-depressant drugs (e.g., sertraline, trazodone and citalopram); Ca"
antagonists (e.g.,
yerapamil, nifedipine, nitrendipine and caroverine); calmodulin inhibitors
(e.g., prenylamine,
trifluoroperazine and clomipramine); Amphotericin B; Triparanol analogues
(e.g., tamoxifen);
antiarrhythmic drugs (e.g., quinidine); antihypertensive drugs (e.g.,
reserpine); Thiol depleters
(e.g., buthionine and sulfoximine) and Multiple Drug Resistance reducing
agents such as
Cremaphor EL
Inflammation
Inflammation may occur as a defensive response to invasion of the subject by
foreign
material, particularly of microbial origin. Additionally, mechanical trauma,
toxins, and
neoplasia may induce inflammatory responses. The accumulation and subsequent
activation of
leukocytes are central events in the pathogenesis of most forms of
inflammation. Inflammation
deficiencies can compromise the host, leaving it susceptible to worsening
infection or trauma.
Excessive inflammation, such as prolonged inflammatory responses, may lead to
inflammatory
diseases including but not limited to diabetes, arteriosclerosis, cataracts,
chronic skin disorders,
reperfusion injury, and cancer, to post-infectious syndromes such as in
infectious meningitis,
rheumatic fever, and to rheumatic diseases such as systemic lupus
erythematosus and
rheumatoid arthritis. These diseases affect millions of people worldwide every
year, and lead to
increased mortality and morbidity. The commonality of the inflammatory
response in these
varied disease processes makes its regulation a major element in the
prevention, or treatment of
human disease.
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
Overproduction of pro-inflammatory cytokines has been implicated in the
pathogenesis
of numerous inflammatory and autoimmune diseases. Secretion of TNFa is a
primary event in
the initiation of the inflammatory cascade (Brennan F. M., et. al. Lancet,
1989, 2:244-7;
Haworth C, et. al. Eur. J. Immunol. 1991, 21:2575-2579) and directly
contributes to the
initiation and maintenance of these diseases. Other cytokines also play a
role, including
interleukin 1(3 (IL-10), IL-6, IL-8, IL-12 nitric oxide (NO), IFN-y,
granulocyte colony
stimulating factor (G-CSF), granulocyte macrophage-colony stimulating factor
(GM-CSF), and
IL-10. Certain of these cytokines (e.g. IL-8) may increase or exacerbate an
inflammatory
response, while others (e.g. IL-10) may decrease or alleviate the inflammatory
response.
Cells of the immune system, macrophages in particular, secrete many of these
cytokines
in response to activating stimuli. Target cells of the cytokines may be
localized in any body
compartment and may act via long-distance mechanisms, or may act on
neighboring cells. Thus,
cytokines may regulate inflammation in a localized or systemic manner.
Metalloproteinases
Metalloproteinases are a superfamily of proteinases (enzymes) classified into
families
and subfamilies as described, for example, in N. M. Hooper FEBS Letters 354:1-
6, 1994.
Examples of metalloproteinases include the matrix metalloproteinases (MMPs)
such as the
collagenases (MMP1, MMP8, MMP13), the gelatinases (MMP2, MMP9), the
stromelysins
(MMP3, MMP10, MMP II), matrilysin (MMP7), metalloelastase (MMP12), enamelysin
(MMP19), the MT-MMPs (MMP14, MMP15, MMP16, MMP17); the reprolysin or
adamalysin
or MDC family which includes the secretases and sheddases such as TNF
converting enzymes
(ADAM10 and TACE); the astacin family which include enzymes such as
procollagen
processing proteinase (PCP); and other metalloproteinases such as aggrecanase,
the endothelin
converting enzyme family and the angiotensin converting enzyme family.
Collectively, the
metalloproteinases are known to cleave a broad range of matrix substrates such
as collagen,
proteoglycan and fibronectin. Metalloproteinases are implicated in the
processing, or secretion,
of biological important cell mediators, such as tumour necrosis factor (TNF);
and the post
translational proteolysis processing, or shedding, of biologically important
membrane proteins,
such as the low affinity IgE receptor CD23 (see, e.g., N. M. Hooper et al.,
Biochem. J. 321:265-
279, 1997).
36
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
Not surprisingly, therefore, metalloproteinases are believed to be important
in many
physiological disease processes that involve tissue remodeling (e.g.,
embryonic development,
bone formation, uterine remodelling during menstruation, etc.). Moreover,
inhibition of the
activity of one or more metalloproteinases may well be of benefit in these
diseases or conditions,
for example: various inflammatory and allergic diseases such as, inflammation
of the joint
(especially rheumatoid arthritis, osteoarthritis and gout), inflammation of
the gastro-intestinal
tract (especially inflammatory bowel disease, ulcerative colitis and
gastritis), inflammation of
the skin (especially psoriasis, eczema, dermatitis); in tumour metastasis or
invasion; in disease
associated with uncontrolled degradation of the extracellular matrix such as
osteoarthritis; in
bone resorptive disease (such as osteoporosis and Paget's disease); in
diseases associated with
aberrant angiogenesis; the enhanced collagen remodelling associated with
diabetes, periodontal
disease (such as gingivitis), corneal ulceration, ulceration of the skin, post-
operative conditions
(such as colonic anastomosis) and dermal wound healing; demyelinating diseases
of the central
and peripheral nervous systems (such as multiple sclerosis); Alzheimer's
disease; extracellular
matrix remodelling observed in cardiovascular diseases such as restenosis and
atherosclerosis;
asthma; rhinitis; and chronic obstructive pulmonary diseases (COPED).
MMP12, also known as macrophage elastase or metalloelastase, was initially
cloned in
the mouse (Shapiro et al., Journal of Biological Chemistry 267: 4664, 1992)
and has also been
cloned in man by the same group in 1995. MMP12 is preferentially expressed in
activated
macrophages, and has been shown to be secreted from alveolar macrophages from
smokers
(Shapiro et al, 1993, Journal of Biological Chemistry, 268: 23824) as well as
in foam cells in
atherosclerotic lesions (Matsumoto et al, Am. J. Pathol. 153: 109, 1998). A
mouse model of
COPD is based on challenge of mice with cigarette smoke for six months, two
cigarettes a day
six days a week. Wild-type mice developed pulmonary emphysema after this
treatment. When
MMP12 knock-out mice were tested in this model they developed no significant
emphysema,
strongly indicating that MMP12 is a key enzyme in the COPD pathogenesis. The
role of MMPs
such as MMP12 in COPD (emphysema and bronchitis) is discussed in Anderson and
Shinagawa, 1999, Current Opinion in Anti-inflammatory and Immunomodulatory
Investigational Drugs 1(1): 29-38. It was recently discovered that smoking
increases
macrophage infiltration and macrophage-derived MMP-12 expression in human
carotid artery
plaques (Matetzky S, Fishbein M C et al., Circulation 102:(18), 36-39 Suppl.
S, Oct. 31, 2000).
37
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
MMP9-(Gelatinase B; 92 kDa-TypeIV Collagenase; 92 kDa Gelatinase) is a
secreted
protein which was first purified, then cloned and sequenced, in 1989 (S. M.
Wilhelm et al., J.
Biol. Chem. 264 (29): 17213-17221, 1989; published erratum in J. Biol. Chem.
265 (36): 22570,
1990) (for review of detailed information and references on this protease see
T. H. Vu & Z.
Werb (1998) (In: Matrix Metalloproteinases, 1998, edited by W. C. Parks & R.
P. Mecham, pp.
115-148, Academic Press. ISBN 0-12-545090-7). The expression of MMP9 is
restricted
normally to a few cell types, including trophoblasts, osteoclasts, neutrophils
and macrophages
(Vu & Werb, supra). However, the expression can be induced in these same cells
and in other
cell types by several mediators, including exposure of the cells to growth
factors or cytokines.
These are the same mediators often implicated in initiating an inflammatory
response. As with
other secreted MMPs, MMP9 is released as an inactive Pro-enzyme, which is
subsequently
cleaved to form the enzymatically active enzyme. The proteases required for
this activation in
vivo are not known. The balance of active MMP9 versus inactive enzyme is
further regulated in
vivo by interaction with TIMP-1 (Tissue Inhibitor of Metalloproteinases-1), a
naturally-
occurring protein. TIMP-1 binds to the C-terminal region of MMP9, leading to
inhibition of the
catalytic domain of MMP9. The balance of induced expression of ProMMP9,
cleavage of Pro-
to active MMP9 and the presence of TIMP-1 combine to determine the amount of
catalytically
active MMP9 which is present at a local site. Proteolytically active MMP9
attacks substrates
which include gelatin, elastin, and native Type IV and Type V collagens; it
has no activity
against native Type I collagen, proteoglycans or laminins. There has been a
growing body of
data implicating roles for MMP9 in various physiological and pathological
processes.
Physiological roles include the invasion of embryonic trophoblasts through the
uterine
epithelium in the early stages of embryonic implantation; some role in the
growth and
development of bones; and migration of inflammatory cells from the vasculature
into tissues.
MMP9 release, measured using enzyme immunoassay, was significantly enhanced in
fluids and in AM supernatants from untreated asthmatics compared with those
from other
populations (Am. J. Resp. Cell & Mol. Biol., 5:583-591, 1997). Also, increased
MMP9
expression has been observed in certain other pathological conditions, thereby
implicating
MMP9 in disease processes such as COPD, arthritis, tumour metastasis,
Alzheimer's disease,
multiple sclerosis, and plaque rupture in atherosclerosis leading to acute
coronary conditions
such as myocardial infarction (see also W007087637A3, incorporated herein by
reference).
38
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
Recently, it has been demonstrated that the levels of MMP-9 are significantly
increased
in patients with stable asthma and even higher in patients with acute
asthmatic patients
compared with healthy control subjects. MMP-9 plays a crucial role in the
infiltration of airway
inflammatory cells and the induction of airway hyperresponsiveness indicating
that MMP-9 may
have an important role in inducing and maintaining asthma (Vignola et al.,
Sputum
metalloproteinase- 9/tis sue inhibitor of metalloproteinase-1 ratio correlates
with airflow
obstruction in asthma and chronic bronchitis, Am J Respir Crit Care Med
158:1945-1950, 1998;
Hoshino et al., Inhaled corticosteroids decrease subepithelial collagen
deposition by modulation
of the balance between matrix metalloproteinase-9 and tissue inhibitor of
metalloproteinase-1
expression in asthma, J Allergy Clin Immunol 104:356-363, 1999; Simpson et
al., Differential
proteolytic enzyme activity in eosinophilic and neutrophilic asthma, Am J
Respir Crit Care Med
172:559-565,2005; Lee et al., A murine model of toluene diisocyanate-induced
asthma can be
treated with matrix metalloproteinase inhibitor, J Allergy Clin Immunol
108:1021-1026, 2001;
and Lee et al., Matrix metalloproteinase inhibitor regulates inflammatory cell
migration by
reducing ICAM-1 and VCAM-1 expression in a murine model of toluene
diisocyanate-induced
asthma, J Allergy Clin Immunol 2003;111:1278-1284).
MMP inhibitors:
A number of metalloproteinase inhibitors are known (see, for example, the
reviews of
MMP inhibitors by Beckett R. P. and Whittaker M., 1998, Exp. Opin. Ther.
Patents, 8(3):259-
282; and by Whittaker M. et al, 1999, Chemical Reviews 99(9):2735-2776). WO
02/074767
discloses hydantoin derivatives of formula that are useful as MMP inhibitors,
particularly as
potent MMP12 inhibitors. U.S. Patent Application Serial No. 11/721,590
(published as
20080032997) discloses a further group of hydantoin derivatives that are
inhibitors of
metalloproteinases and are of particular interest in inhibiting MMPs such as
MMP12 and
MMP9. Novel triazolone derivatives for inhibiting MMPs such as MMP12 and MMP9
are
disclosed in U.S. Patent Application Serial No. 10/593543 (published as
20070219217).
Additional MMP12 and MMP9 inhibitors are disclosed in 11/509,490 (published as
20060287338) (see also 10/831265 (published as 20040259896)).
Additionally, two compounds, 4-(4-phenoxyphenylsulfonyl)butane-1,2-dithiol (1)
and 5-
(4-phenoxyphenylsulfonyl)pentane-1,2-dithiol (2), have been shown to bind
selectively and
inhibit potently MMP-2 and MMP-9 (Bernardo, et. al (2002) J. Biol. Chem.
277:11201-11207).
These two compounds may have significant use in the clinic to inhibit MMP-2
and -9 and
39
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
therefore lessen inflammation. In addition, the use of certain tetracycline
antibiotics (e.g.,
Minocycline and Doxycycline) at sub-antibiotic levels has been shown to
effectively inhibit
MMP activity. Certain aspects of this invention include using the inventive
fluids in
combination with sub-antibiotic levels useful to inhibit MMP.
Methods of Treatment
The term "treating" refers to, and includes, reversing, alleviating,
inhibiting the progress
of, or preventing a disease, disorder or condition, or one or more symptoms
thereof; and
"treatment" and "therapeutically" refer to the act of treating, as defined
herein.
A "therapeutically effective amount" is any amount of any of the compounds
utilized in
the course of practicing the invention provided herein that is sufficient to
reverse, alleviate,
inhibit the progress of, or prevent a disease, disorder or condition, or one
or more symptoms
thereof.
Certain embodiments herein relate to therapeutic compositions and methods of
treatment
for a subject by preventing or alleviating at least one symptom associated
with exposure to a
neurotoxin. For example, the therapeutic compositions and/or methods disclosed
herein may be
useful for treating or preventing one or more condition or disease selected
from the group
consisting multiple sclerosis (MS), Parkinson's disease, amyloidosis (e.g.
Alzheimer's disease),
amyotrophic lateral sclerosis (ALS), prion diseases, and HIV-associated
dementia.
Many conditions or diseases associated with inflammation have been treated
with
steroids, methotrexate, immunosuppressive drugs including cyclophosphamide,
cyclosporine,
azathioprine and leflunomide, nonsteroidal anti-inflammatory agents such as
aspirin,
acetaminophen and COX-2 inhibitors, gold agents and anti-malarial treatments.
These drugs
have a variety of disadvantages, and adverse reactions including injection
site reactions, rash,
upper respiratory infections, autoimmune disorders and increased
susceptibility to infections. In
addition, many anti-inflammatory pharmaceutical drugs require intravenous (IV)
or
subcutaneous (SC) administration, as opposed to more convenient and compliant
oral or topical
dermal routes. Accordingly, a need still exists for the development of novel
medicaments and
treatment methods for conditions and diseases relating to inflammation.
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
Combination therapy:
Additional aspects provide the herein disclosed inventive methods, further
comprising
combination therapy, wherein at least one additional therapeutic agent is
administered to the
patient. In certain aspects, the at least one additional therapeutic agent is
selected from the
group consisting of any one of erythropoietin, anti-apoptotics (TCH346, CEP-
1347),
antiglutamatergics, monoamine oxidase inhibitors (selegiline, rasagiline),
promitochondrials
(coenzyme Q10, creatine), calcium channel blockers (isradipine), alpha-
synuclein, and/or
growth factors (GDNF).
Anti-inflammatory Activity of the Electrokineticall-generated Gas-Enriched
Fluids and
Solutions:
According to certain aspects of the present invention, the gas-enriched fluids
and/or
solutions disclosed herein have anti-inflammatory properties and effects, and
can be used as
anti-inflammatory agents for the treatment of subjects afflicted by diseases
or disorders relating
to inflammatory neurodegeneration. Previous results showed that the inventive
oxygen-enriched
fluid (water) affected a down regulation of particular cytokines, especially
IL-6, IL-8, and IL-1 (3
in cytokine profiles in stimulated lymphocytes from a healthy blood donor.
Increased production of pro-inflammatory cytokines has been implicated in the
pathogenesis of numerous inflammatory and autoimmune diseases. Secretion of
TNFa is a
primary event in the initiation of the inflammatory cascade (Brennan F. M.,
et. al. Lancet, 1989,
2:244-7; Haworth C, et. al. Eur. J. Immunol. 1991, 21:2575-2579) and directly
contributes to the
initiation and maintenance of inflammatory and autoimmune diseases. Other pro-
inflammatory
cytokines also play a role, including interleukin 10 (IL-1(3), IL-6, IL-8, IL-
12 nitric oxide, IFN-y
and GM-CSF, while anti-inflammatory cytokines such as IL-10 may reduce
disease. Cells of
the immune system, macrophages in particular, secrete many of these cytokines
in response to
activating stimuli.
A variety of cell types are involved in the inflammatory process.
Overproduction of
TNFa by monocytes, macrophages and other immune cells is a key element in the
pathogenesis
of a multitude of diseases. Macrophages and T-cells in particular play a
central role in the
initiation and maintenance of the immune response. Once activated by
pathological or
immunogenic stimuli, macrophages respond by releasing a host of cytokines,
including TNF-a,
IL-10, IL-8, IL-12, nitric oxide (NO), IL-6, GM-CSF, G-CSF, M-CSF and others.
T-cells release
41
)O
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
IL-2, IL-4, INF-y, and other inflammatory cytokines. These cytokines activate
other immune
cells and some can also act as independent cytotoxic agents. Excessive release
of macrophage
and T-cell derived inflammatory mediators can particularly lead to damage of
normal cells and
surrounding tissues.
Pro-inflammatory cytokines have been implicated in HIV-AIDS, and other viral
infections including the cytomegalovirus, influenza virus and the herpes
family of viruses.
TNFa enhances the basal activity of the major immediate early
enhancer/promoter of human
cytomegalovirus and may play a role in reactivation of latent HCMV infection
in premonocytic
cells (Prosch S., et. al. Virology 1995, 208:197-206).
Additionally, a number of inflammatory cytokines contribute to mortality in
patients
suffering from sepsis or endotoxic shock. For example, TNFa and IL-1(3 have a
well-established
central role in sepsis, septic shock and endotoxic shock. Increased levels of
these cytokines are
associated with fever, hypotension and shock (Smith J. W. et. al. J. Clin.
Oncol. 1992, 10:1141-
1152; Chapman P. B., et. al. J. Clin. Oncol. 1987, 5:1942-1951) together with
the induction of
gene expression for phospholipase A2 (Gronich J., et. al. J. Clin. Invest.
1994, 93:1224-1233)
and NO synthase.
The induction of NO from smooth muscle cells mediates decreased mean arterial
pressure and systemic vascular resistance during septic shock, suggesting a
fundamental role for
NO. Thus, therapies that target downregulatory effects on IL-8, IL-10, and NO
could be
beneficial in the treatment of inflammatory diseases or disorders, including
sepsis, septic shock,
and endotoxic shock.
Overproduction of TNFa contributes to the clinical features of numerous
autoimmune
diseases such as diabetes and rheumatoid arthritis. Systemic lupus
erythematosus (SLE) is also
precipitated by increased IL-10 and TNFa levels. Within lupus patients, serum
C-reactive
protein, IL-l.beta and TNFa levels were higher than in controls, suggesting
that an increased
inflammatory response plays a role in the disease (Lion L. B. Clin. Exp.
Rheumatol. 2001,
19:515-523). A study of patients with one form of SLE, neuropsychiatric lupus
erythematosus
(NPLE), showed that the number of peripheral blood mononuclear cells
expressing mRNA for
TNFa as well as the cerebrospinal fluid level of NO metabolites correlated
with NPLE disease
severity (Svenungsson E., et al. Ann. Rheum. Dis. 2001, 60:372-9).
IL-1 and TNFa play a central role in various acute as well as chronic
responses in animal
models. Additionally, IL-11, IFNa and IFN(3 may also up-regulate inflammatory
reactions.
42
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
Conversely, several cytokines may be involved in down-regulation of
inflammatory responses
(i.e. IL-4, IL-10, IL-13, among others). As set forth in Example 1, cells
contacted with the
inventive gas-enriched fluid showed an increase in IFN-y levels with T3
antigen than in the
control culture media with T3 antigen, while IL-8 was lower in the inventive
gas-enriched
culture media with T3 antigen than in the control culture media with T3
antigen. Additionally,
IL-6, IL-8, and TNF-a levels were lower in the inventive gas-enriched media
with PHA, than in
the control media with PHA, while IL-1(3 levels were lower in the inventive
gas-enriched fluid
with PHA when compared with control media with PHA. In the inventive gas-
enriched media
alone, IFN-y levels were higher than in control media. These results are
consistent with an anti-
inflammatory microenvironment.
NO is recognized as a mediator and regulator of inflammatory responses. It
possesses
cytotoxic properties toward pathogens, but can also have deleterious effects
on the subject's own
tissues. (Korhonen et al., Curr Drug Targets Inflamm Allergy 4(4): 471-9,
2005). NO reacts
with soluble guanylate cyclase to form cyclic guanosine monophosphate (cGMP),
which
mediates many of the effects of NO. NO can also interact with molecular oxygen
and
superoxide anion to produce reactive oxygen species that can modify various
cellular functions.
These indirect effects of NO have a significant role in inflammation, where NO
is produce in
high amounts by inducible NO synthase (iNOS) and reactive oxygen species are
synthesized by
activated inflammatory cells.
NO can be produced by keratinocytes, fibroblasts, endothelial cells, and
possibly others.
Some of the vascular actions of NO include vasodilation, inhibiting platelet
adhesion to the
vascular endothelium, inhibiting leukocyte adhesion to the vascular
endothelium, and
scavenging superoxides. (Shah et al., Env. Health Persp. v. 106 (5): 1139-
1143.)
Furthermore, inhibition of NO synthesis has been shown to delay wound
contraction,
alter collagen organization, and alter neoepidermis thickness. (Amadeu and
Costa, J. Cutan.
Pathol. 33: 465-473, 2006.) Mast cell migration and angiogenesis in wounds is
also affected by
inhibition of NO. (Id.) Without being bound to any particular theory of
mechanism, in certain
embodiments the inventive gas-enriched fluids may be modulating localized
and/or cellular NO
production, or degradation, consistent with the spectrum of wound healing
effects illustrated in
the Examples section disclosed herein. Due to variable pathways of regulation,
in certain
embodiments, the inventive gas-enriched fluid may increase NO production
and/or retard NO
43
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
degradation, whereas in other certain embodiments, the inventive gas-enriched
fluid may
decrease NO production and/or hasten NO degradation.
Specifically, wounds treated with oxygen-enriched saline solution showed an
increase in
wound healing at days 4 through 11, and between days 3 and 11, the new
epidermis in wounds
treated with the oxygen-enriched saline solution migrated at two to four times
as fast as the
epidermis of the wounds treated with the normal saline solution, as set forth
in Example 9
herein. The study also showed that between 15 and 22 days, wounds treated by
the oxygen-
enriched saline solution differentiated at a more rapid rate as evidenced by
the earlier formation
of more mature epidermal layers. At all stages, the thickening that occurs in
the epidermis
associated with normal healing did not occur within the wounds treated by the
oxygen-enriched
saline solution.
Thus, in accordance with this spectrum of wound healing effects, but without
wishing to
be bound by any particular theory, it is believed that the oxygen-enriched
saline solution may
modulate the localized and/or cellular level of NO within the wounds. NO
modulates growth
factors, collagen deposition, inflammation, mast cell migration, epidermal
thickening, and
neovascularization in wound healing. Furthermore, nitric oxide is produced by
an inducible
enzyme that is regulated by oxygen.
In the case of mast cell migration, differences also occurred in early and
late migration
for the oxygen-enriched solution. This is consistent with what is known in the
art regarding
inhibition of NO synthesis (Amadeu and Costa, J. Cutan Pathol 33: 465-473,
2006).
In the first two phases of the inflammatory process, the foreign body is
either destroyed,
for example, if the foreign body is an organism, or the tissue around it is
loosened, for example,
if it is a splinter. In the healing phase, the inflammation begins to subside;
individual blood
vessels and vascular patterns become normal once again; and repair of the
wound commences.
The three main events in the repair process are (1) formation of new
connective tissue by
proliferating fibroblasts; (2) regeneration of epithelium; and (3) outgrowth
of new capillaries.
Even before the inflammation subsides, fibroblasts begin moving into the
injured area
from the surrounding normal tissue, where they usually exist in a dormant
state. They migrate
by an amoeboid movement along strands of fibrin and distribute themselves
throughout the
healing area. Once fixed into position in the injured tissue, they begin to
synthesize collagen
and secrete this protein, which arranges itself into fibers. The fibers orient
themselves with their
44
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
longitudinal axes in the direction of the greatest stress. As the collagen
bundles grow in
firmness, the fibroblasts gradually degenerate and attach closely to the
bundles, and the injured
area transforms into scar tissue.
Simultaneously with scar tissue formation, the intact epidermal cells on the
edge of the
wound begin to proliferate and move, as one sheet, toward the center of the
injured area. As the
inflammation subsides, a need for a direct supply of blood arises, and
angiogenesis occurs at the
wound site.
Inflammation is a complex process that involves multiple cell types. For
example, mast
cells release mediators that trigger an early phase of vasodilation,
accompanied by the separation
of endothelial cells and exposure of collagen fibers in the subendothelial
layer. Fibers in the
intercellular gaps that form in blood vessels trap platelets and trigger the
release of mediators
from these cells.
In addition to platelets, the exposed collagen fibers also interact with
proteins of the
plasma that filter through the pores of the dilated vessel wall, including the
triggering factor of
the blood-clotting cascade, increased vasodilation, increased blood vessel
permeability, and
chemotaxis.
Additionally, the complement cascade can be activated by several stimuli: the
injured
blood vessels, the proteolytic enzymes released by the damaged cells, the
membrane
components of any participating bacteria, and antigen-antibody complexes. Some
of the
activated complement components act as chemotactic factors, responsible for
the influx of
leukocytes into the inflamed area, while others facilitate phagocytosis and
participate in cell
lysis.
In addition, it is believed that the inventive gas-enriched fluids or
solutions may also
regulate at least one cytokine involved in at least one aspect of
inflammation, the cytokine(s)
including, but not limited to MAF (macrophage activating factor), MMIF
(macrophage
migration inhibition factor), MCF (macrophage chemotactic factor), LMIF
(leukocyte migration
inhibition factor), HRFs (histamine releasing factors), TF (transfer factors),
interleukins (IL-1,
IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-11, IL-12, IL-13, IL-
14, IL-15, etc.),
TNF-a, TNF-(3, interferons (IFN-a, IFN-(3, IFN-y, IFN-~, IFN-S, etc.), G-CSF
(granulocyte
colony stimulating factor), GM-CSF (granulocyte-macrophage CSF), M-CSF
(macrophage
CSF), multi-CSF (IL-3), fibroblast growth factor (aFGF, bFGF), EGF (epidermal
growth factor),
NGF (nerve growth factor), PDGF (platelet-derived growth factor), VEGF
(vascular endothelial
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
growth factor), transforming growth factors (TGF-a, TGF-0, etc.), NAP-2
(neutrophil-activating
protein 2), PF-4 (platelet factor 4), thromboglobulin, MCP-1 (monocyte
chemoattractant protein
1), MCP-3, MIP-1 a, MIP-1(3-+ (macrophage inflammatory proteins), RANTES
(regulated upon
activation normal T expressed and presumably secreted chemokine), HSPs (heat
shock proteins),
GRPs (glucose-regulated proteins), ubiquitin, and others.
Thus, in certain embodiments, the gas-enriched fluids and/or therapeutic
compositions
may increase production and/or secretion of anti-inflammatory molecules or
cytokines or
decrease the degradation of anti-inflammatory molecules or cytokines, thereby
alleviating or
preventing at least one symptom of inflammation and/or inflammatory
neurodegeneration. In
other embodiments, the gas-enriched fluids and/or therapeutic compositions of
the present
invention may decrease production and/or secretion of pro-inflammatory
molecules or cytokines
or increase the degradation of pro-inflammatory molecules or cytokines,
thereby alleviating or
preventing at least one symptom of inflammation and/or inflammatory
neurodegeneration.
Previous studies had shown a critical role of anti-MOG antibodies in
augmentation of
demyelination and worsening of EAE (experimental autoimmune
encephalomyelitis), an animal
model system for the human autoimmune disorder of rheumatoid arthritis.
(Linington, et al.
1992. J. Neuroimmunol. 40:219-224). Additionally, antibodies against MOG have
been
implicated in the pathogenesis of multiple sclerosis. (Berger et al. N. Engl.
J. Med. 2003 Jul
10;349(2):139-45).
As set forth in previous experiments the inventive gas-enriched fluid of the
present
invention amplifies the lymphocyte response to an antigen for which an animal
was previously
primed. As indicated in previous experiments, lymphocyte proliferation was
greater for
response to MOG challenge when cultured in fluid reconstituted with the
inventive gas-enriched
fluid comprising solvated electrons, when compared with pressurized,
oxygenated fluid
(pressure pot) or control deionized fluid.
Exemplary relevant Molecular Interactions:
Conventionally, quantum properties are thought to belong to elementary
particles of less
than 10-10 meters, while the macroscopic world of our everyday life is
referred to as classical, in
that it behaves according to Newton's laws of motion.
Recently, molecules have been described as forming clusters that increase in
size with
dilution. These clusters measure several micrometers in diameter, and have
been reported to
46
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
increase in size non-linearly with dilution. Quantum coherent domains
measuring 100
nanometers in diameter have been postulated to arise in pure water, and
collective vibrations of
water molecules in the coherent domain may eventually become phase locked to
electromagnetic
field fluctuations, providing for stable oscillations in water, providing a
form of `memory' in the
form of excitation of long lasting coherent oscillations specific to dissolved
substances in the
water that change the collective structure of the water, which may in turn
determine the specific
coherent oscillations that develop. Where these oscillations become stabilized
by magnetic field
phase coupling, the water, upon dilution may still carry `seed' coherent
oscillations. As a cluster
of molecules increases in size, its electromagnetic signature is
correspondingly amplified,
reinforcing the coherent oscillations carried by the water.
Despite variations in the cluster size of dissolved molecules and detailed
microscopic
structure of the water, a specificity of coherent oscillations may nonetheless
exist. One model
for considering changes in properties of water is based on considerations
involved in
crystallization.
A simplified protonated water cluster forming a nanoscale cage is shown in
Applicants'
previous patent application: WO 2009/055729. A protonated water cluster
typically takes the
form of H+(H20),,. Some protonated water clusters occur naturally, such as in
the ionosphere.
Without being bound by any particular theory, and according to particular
aspects, other types of
water clusters or structures (clusters, nanocages, etc) are possible,
including structures
comprising oxygen and stabilized electrons imparted to the inventive output
materials. Oxygen
atoms may be caught in the resulting structures. The chemistry of the semi-
bound nanocage
allows the oxygen and/or stabilized electrons to remain dissolved for extended
periods of time.
Other atoms or molecules, such as medicinal compounds, can be caged for
sustained delivery
purposes. The specific chemistry of the solution material and dissolved
compounds depend on
the interactions of those materials.
Fluids processed by the mixing device have been shown previously via
experiments to
exhibit different structural characteristics that are consistent with an
analysis of the fluid in the
context of a cluster structure. See, for example, WO 2009/055729.
Charge-stabilized nanostructures (e.¾ , charge stabilized oxygen-containing
nano structures):
As described previously in Applicants' WO 2009/055729, "Double Layer Effect,"
"Dwell Time," "Rate of Infusion," and "Bubble size Measurements," the
electrokinetic mixing
device creates, in a matter of milliseconds, a unique non-linear fluid dynamic
interaction of the
47
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
first material and the second material with complex, dynamic turbulence
providing complex
mixing in contact with an effectively enormous surface area (including those
of the device and
of the exceptionally small gas bubbles of less that 100 nm) that provides for
the novel
electrokinetic effects described herein. Additionally, feature-localized
electrokinetic effects
(voltage/current) were demonstrated using a specially designed mixing device
comprising
insulated rotor and stator features.
As well-recognized in the art, charge redistributions and/or solvated
electrons are known
to be highly unstable in aqueous solution. According to particular aspects,
Applicants'
electrokinetic effects (e.g., charge redistributions, including, in particular
aspects, solvated
electrons) are surprisingly stabilized within the output material (e.g.,
saline solutions, ionic
solutions). In fact, as described herein, the stability of the properties and
biological activity of
the inventive electrokinetic fluids (e.g., RNS-60 or Solas) can be maintained
for months in a
gas-tight container, indicating involvement of dissolved gas (e.g., oxygen) in
helping to generate
and/or maintain, and/or mediate the properties and activities of the inventive
solutions.
Significantly, the charge redistributions and/or solvated electrons are stably
configured in the
inventive electrokinetic ionic aqueous fluids in an amount sufficient to
provide, upon contact
with a living cell (e.g., mammalian cell) by the fluid, modulation of at least
one of cellular
membrane potential and cellular membrane conductivity (see, e.g., cellular
patch clamp working
Example 23 from WO 2009/055729 and as disclosed herein).
As described herein under "Molecular Interactions," to account for the
stability and
biological compatibility of the inventive electrokinetic fluids (e.g.,
electrokinetic saline
solutions), Applicants have proposed that interactions between the water
molecules and the
molecules of the substances (e.g., oxygen) dissolved in the water change the
collective structure
of the water and provide for nanoscale cage clusters, including nanostructures
comprising
oxygen and/or stabilized electrons imparted to the inventive output materials.
Without being
bound by mechanism, the configuration of the nanostructures in particular
aspects is such that
they: comprise (at least for formation and/or stability and/or biological
activity) dissolved gas
(e.g., oxygen); enable the electrokinetic fluids (e.g., RNS-60 or Solas saline
fluids) to modulate
(e.g., impart or receive) charges and/or charge effects upon contact with a
cell membrane or
related constituent thereof; and in particular aspects provide for
stabilization (e.g., carrying,
harboring, trapping) solvated electrons in a biologically-relevant form.
48
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
According to particular aspects, and as supported by the present disclosure,
in ionic or
saline (e.g., standard saline, NaC1) solutions, the inventive nanostructures
comprise charge
stabilized nanostrutures (e.g., average diameter less that 100 nm) that may
comprise at least one
dissolved gas molecule (e.g., oxygen) within a charge-stabilized hydration
shell. According to
additional aspects, the charge-stabilized hydration shell may comprise a cage
or void harboring
the at least one dissolved gas molecule (e.g., oxygen). According to further
aspects, by virtue of
the provision of suitable charge-stabilized hydration shells, the charge-
stabilized nanostructure
and/or charge-stabilized oxygen containing nano-structures may additionally
comprise a
solvated electron (e.g., stabilized solvated electron).
Without being bound by mechanism or particular theory, after the present
priority date,
charge-stabilized microbubbles stabilized by ions in aqueous liquid in
equilibrium with ambient
(atmospheric) gas have been proposed (Bunkin et al., Journal of Experimental
and Theoretical
Physics, 104:486-498, 2007; incorporated herein by reference in its entirety).
According to
particular aspects of the present invention, Applicants' novel electrokinetic
fluids comprise a
novel, biologically active form of charge-stabilized oxygen-containing
nanostructures, and may
further comprise novel arrays, clusters or associations of such structures.
According to the charge-stabilized microbubble model, the short-range
molecular order
of the water structure is destroyed by the presence of a gas molecule (e.g., a
dissolved gas
molecule initially complexed with a nonadsorptive ion provides a short-range
order defect),
providing for condensation of ionic droplets, wherein the defect is surrounded
by first and
second coordination spheres of water molecules, which are alternately filled
by adsorptive ions
(e.g., acquisition of a `screening shell of Na' ions to form an electrical
double layer) and
nonadsorptive ions (e.g., CY ions occupying the second coordination sphere)
occupying six and
12 vacancies, respectively, in the coordination spheres. In under-saturated
ionic solutions (e.g.,
undersaturated saline solutions), this hydrated `nucleus' remains stable until
the first and second
spheres are filled by six adsorptive and five nonadsorptive ions,
respectively, and then
undergoes Coulomb explosion creating an internal void containing the gas
molecule, wherein
the adsorptive ions (e.g., Na' ions) are adsorbed to the surface of the
resulting void, while the
nonadsorptive ions (or some portion thereof) diffuse into the solution (Bunkin
et al., supra). In
this model, the void in the nanostructure is prevented from collapsing by
Coulombic repulsion
between the ions (e.g., Na' ions) adsorbed to its surface. The stability of
the void-containing
nanostrutures is postulated to be due to the selective adsorption of dissolved
ions with like
49
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
charges onto the void/bubble surface and diffusive equilibrium between the
dissolved gas and
the gas inside the bubble, where the negative (outward electrostatic pressure
exerted by the
resulting electrical double layer provides stable compensation for surface
tension, and the gas
pressure inside the bubble is balanced by the ambient pressure. According to
the model,
formation of such microbubbles requires an ionic component, and in certain
aspects collision-
mediated associations between particles may provide for formation of larger
order clusters
(arrays) (Id).
The charge-stabilized microbubble model suggests that the particles can be gas
microbubbles, but contemplates only spontaneous formation of such structures
in ionic solution
in equilibrium with ambient air, is uncharacterized and silent as to whether
oxygen is capable of
forming such structures, and is likewise silent as to whether solvated
electrons might be
associated and/or stabilized by such structures.
According to particular aspects, the inventive electrokinetic fluids
comprising charge-
stabilized nanostructures and/or charge-stabilized oxygen-containing
nanostructures are novel
and fundamentally distinct from the postulated non-electrokinetic, atmospheric
charge-stabilized
microbubble structures according to the microbubble model. Significantly, this
conclusion is
unavoidable, deriving, at least in part, from the fact that control saline
solutions do not have the
biological properties disclosed herein, whereas Applicants' charge-stabilized
nanostructures
provide a novel, biologically active form of charge-stabilized oxygen-
containing nanostructures.
According to particular aspects of the present invention, Applicants' novel
electrokinetic
device and methods provide for novel electrokinetically-altered fluids
comprising significant
quantities of charge-stabilized nanostructures in excess of any amount that
may or may not
spontaneously occur in ionic fluids in equilibrium with air, or in any non-
electrokinetically
generated fluids. In particular aspects, the charge-stabilized nanostructures
comprise charge-
stabilized oxygen-containing nanostructures. In additional aspects, the charge-
stabilized
nanostrutures are all, or substantially all charge-stabilized oxygen-
containing nanostructures, or
the charge- stabilized oxygen-containing nanostructures the major charge-
stabilized gas-
containing nanostructure species in the electrokinetic fluid.
According to yet further aspects, the charge-stabilized nanostructures and/or
the charge-
stabilized oxygen-containing nanostructures may comprise or harbor a solvated
electron, and
thereby provide a novel stabilized solvated electron carrier. In particular
aspects, the charge-
stabilized nanostructures and/or the charge- stabilized oxygen-containing
nanostructures provide
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
a novel type of electride (or inverted electride), which in contrast to
conventional solute
electrides having a single organically coordinated cation, rather have a
plurality of cations stably
arrayed about a void or a void containing an oxygen atom, wherein the arrayed
sodium ions are
coordinated by water hydration shells, rather than by organic molecules.
According to particular
aspects, a solvated electron may be accommodated by the hydration shell of
water molecules, or
preferably accommodated within the nanostructure void distributed over all the
cations. In
certain aspects, the inventive nanostructures provide a novel `super
electride' structure in
solution by not only providing for distribution/stabilization of the solvated
electron over
multiple arrayed sodium cations, but also providing for association or partial
association of the
solvated electron with the caged oxygen molecule(s) in the void-the solvated
electron
distributing over an array of sodium atoms and at least one oxygen atom.
According to
particular aspects, therefore, 'solvated electrons' as presently disclosed in
association with the
inventive electrokinetic fluids, may not be solvated in the traditional model
comprising direct
hydration by water molecules. Alternatively, in limited analogy with dried
electride salts,
solvated electrons in the inventive electrokinetic fluids may be distributed
over multiple charge-
stabilized nanostructures to provide a `lattice glue' to stabilize higher
order arrays in aqueous
solution.
In particular aspects, the inventive charge-stabilized nanostructures and/or
the charge-
stabilized oxygen-containing nanostructures are capable of interacting with
cellular membranes
or constituents thereof, or proteins, etc., to mediate biological activities.
In particular aspects,
the inventive charge- stabilized nanostructures and/or the charge- stabilized
oxygen-containing
nanostructures harboring a solvated electron are capable of interacting with
cellular membranes
or constituents thereof, or proteins, etc., to mediate biological activities.
In particular aspects, the inventive charge-stabilized nanostructures and/or
the charge-
stabilized oxygen-containing nanostructures interact with cellular membranes
or constituents
thereof, or proteins, etc., as a charge and/or charge effect donor (delivery)
and/or as a charge
and/or charge effect recipient to mediate biological activities. In particular
aspects, the inventive
charge- stabilized nanostructures and/or the charge- stabilized oxygen-
containing nanostructures
harboring a solvated electron interact with cellular membranes as a charge
and/or charge effect
donor and/or as a charge and/or charge effect recipient to mediate biological
activities.
In particular aspects, the inventive charge-stabilized nanostructures and/or
the charge-
stabilized oxygen-containing nanostructures are consistent with, and account
for the observed
51
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
stability and biological properties of the inventive electrokinetic fluids,
and further provide a
novel electride (or inverted electride) that provides for stabilized solvated
electrons in aqueous
ionic solutions (e.g., saline solutions, NaCl, etc.).
In particular aspects, the charge- stabilized oxygen-containing nanostructures
substantially comprise, take the form of, or can give rise to, charge-
stabilized oxygen-containing
nanobubbles. In particular aspects, charge- stabilized oxygen-containing
clusters provide for
formation of relatively larger arrays of charge- stabilized oxygen-containing
nanostructures,
and/or charge-stabilized oxygen-containing nanobubbles or arrays thereof. In
particular aspects,
the charge-stabilized oxygen-containing nanostructures can provide for
formation of
hydrophobic nanobubbles upon contact with a hydrophobic surface.
In particular aspects, the charge- stabilized oxygen-containing nanostructures
substantially comprise at least one oxygen molecule. In certain aspects, the
charge-stabilized
oxygen-containing nanostructures substantially comprise at least 1, at least
2, at least 3, at least
4, at least 5, at least 10 at least 15, at least 20, at least 50, at least
100, or greater oxygen
molecules. In particular aspects, charge-stabilized oxygen-containing
nanostructures comprise
or give rise to nanobubles (e.g., hydrophobid nanobubbles) of about 20 nm x
1.5 nm, comprise
about 12 oxygen molecules (e.g., based on the size of an oxygen molecule
(approx 0.3 nm by
0.4 nm), assumption of an ideal gas and application of n=PV/RT, where P=1 atm,
R=0.082^057^l.atm/mol.K; T=295K; V=pr2h=4.7x10-22 L, where r=10x10-9 m,
h=1.5x10-9
m, and n=1.95x10-22 moles).
In certain aspects, the percentage of oxygen molecules present in the fluid
that are in
such nanostructures, or arrays thereof, having a charge-stabilized
configuration in the ionic
aqueous fluid is a percentage amount selected from the group consisting of
greater than: 0.1%,
1%; 2%; 5%; 10%; 15%; 20%; 25%; 30%; 35%; 40%; 45%; 50%; 55%; 60%; 65%; 70%;
75%;
80%; 85%; 90%; and greater than 95%. Preferably, this percentage is greater
than about 5%,
greater than about 10%, greater than about 15%f, or greater than about 20%. In
additional
aspects, the substantial size of the charge-stabilized oxygen-containing
nanostructures, or arrays
thereof, having a charge-stabilized configuration in the ionic aqueous fluid
is a size selected
from the group consisting of less than: 100 nm; 90 nm; 80 nm; 70 nm; 60 nm; 50
nm; 40 nm; 30
nm; 20 nm; 10 nm; 5 nm; 4 nm; 3 nm; 2 nm; and 1 nm. Preferably, this size is
less than about
50 nm, less than about 40 nm, less than about 30 nm, less than about 20 nm, or
less than about
10 nm.
52
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
In certain aspects, the inventive electrokinetic fluids comprise solvated
electrons. In
further aspects, the inventive electrokinetic fluids comprises charge-
stabilized nanostructures
and/or charge-stabilized oxygen-containing nanostructures, and/or arrays
thereof, which
comprise at least one of: solvated electron(s); and unique charge
distributions (polar, symmetric,
asymmetric charge distribution). In certain aspects, the charge-stabilized
nanostructures and/or
charge- stabilized oxygen-containing nanostructures, and/or arrays thereof,
have paramagnetic
properties.
By contrast, relative to the inventive electrokinetic fluids, control pressure
pot
oxygenated fluids (non-electrokinetic fluids) and the like do not comprise
such electrokinetically
generated charge-stabilized biologically-active nanostructures and/or
biologically-active charge-
stabilized oxygen-containing nanostructures and/or arrays thereof, capable of
modulation of at
least one of cellular membrane potential and cellular membrane conductivity.
Systems for Making Gas-Enriched Fluids
The system and methods as previously disclosed in Applicants' WO 2009/055729
patent application allow gas (e.g. oxygen) to be enriched stably at a high
concentration with
minimal passive loss. This system and methods can be effectively used to
enrich a wide variety
of gases at heightened percentages into a wide variety of fluids. By way of
example only,
deionized water at room temperature that typically has levels of about 2-3 ppm
(parts per
million) of dissolved oxygen can achieve levels of dissolved oxygen ranging
from at least about
5 ppm, at least about 10 ppm, at least about 15 ppm, at least about 20 ppm, at
least about 25
ppm, at least about 30 ppm, at least about 35 ppm, at least about 40 ppm, at
least about 45 ppm,
at least about 50 ppm, at least about 55 ppm, at least about 60 ppm, at least
about 65 ppm, at
least about 70 ppm, at least about 75 ppm, at least about 80 ppm, at least
about 85 ppm, at least
about 90 ppm, at least about 95 ppm, at least about 100 ppm, or any value
greater or
therebetween using the disclosed systems and/or methods. In accordance with a
particular
exemplary embodiment, oxygen-enriched water may be generated with levels of
about 30-60
ppm of dissolved oxygen.
Table 3 illustrates various partial pressure measurements taken in a healing
wound
treated with an oxygen-enriched saline solution (Table 3) and in samples of
the gas-enriched
oxygen-enriched saline solution of the present invention.
53
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
TABLE 3
TISSUE OXYGEN MEASUREMENTS
Probe Z082BO
In air: 171 mmHg 23 C
Column Partial Pressure (mmHg)
BI 32-36
B2 169-200
B3 20-180*
B4 40-60
*wound depth minimal, majority >150, occasional 20 s
Routes and Forms of Administration
In particular exemplary embodiments, the gas-enriched fluid of the present
invention
may function as a therapeutic composition alone or in combination with another
therapeutic
agent such that the therapeutic composition prevents or alleviates at least
one symptom of
inflammation. The therapeutic compositions of the present invention include
compositions that
are able to be administered to a subject in need thereof. In certain
embodiments, the therapeutic
composition formulation may also comprise at least one additional agent
selected from the group
consisting of: carriers, adjuvants, emulsifying agents, suspending agents,
sweeteners,
flavorings, perfumes, and binding agents.
As used herein, "pharmaceutically acceptable carrier" and "carrier" generally
refer to a
non-toxic, inert solid, semi-solid or liquid filler, diluent, encapsulating
material or formulation
auxiliary of any type. Some non-limiting examples of materials which can serve
as
pharmaceutically acceptable carriers are sugars such as lactose, glucose and
sucrose; starches
such as corn starch and potato starch; cellulose and its derivatives such as
sodium
carboxymethyl cellulose, ethyl cellulose and cellulose acetate; powdered
tragacanth; malt;
gelatin; talc; excipients such as cocoa butter and suppository waxes; oils
such as peanut oil,
cottonseed oil; safflower oil; sesame oil; olive oil; corn oil and soybean
oil; glycols; such as
propylene glycol; esters such as ethyl oleate and ethyl laurate; agar;
buffering agents such as
magnesium hydroxide and aluminum hydroxide; alginic acid; pyrogen-free water;
isotonic
saline; Ringer's solution; ethyl alcohol, and phosphate buffer solutions, as
well as other non-
toxic compatible lubricants such as sodium lauryl sulfate and magnesium
stearate, as well as
coloring agents, releasing agents, coating agents, sweetening, flavoring and
perfuming agents,
preservatives and antioxidants can also be present in the composition,
according to the judgment
54
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
of the formulator. In particular aspects, such carriers and excipients may be
gas-enriched fluids
or solutions of the present invention.
The pharmaceutically acceptable carriers described herein, for example,
vehicles,
adjuvants, excipients, or diluents, are well known to those who are skilled in
the art. Typically,
the pharmaceutically acceptable carrier is chemically inert to the therapeutic
agents and has no
detrimental side effects or toxicity under the conditions of use. The
pharmaceutically acceptable
carriers can include polymers and polymer matrices, nanoparticles,
microbubbles, and the like.
In addition to the therapeutic gas-enriched fluid of the present invention,
the therapeutic
composition may further comprise inert diluents such as additional non-gas-
enriched water or
other solvents, solubilizing agents and emulsifiers such as ethyl alcohol,
isopropyl alcohol, ethyl
carbonate, ethyl acetate, benzyl alcohol, benzyl benzoate, propylene glycol,
1,3-butylene glycol,
dimethylformamide, oils (in particular, cottonseed, groundnut, corn, germ,
olive, castor, and
sesame oils), glycerol, tetrahydrofurfuryl alcohol, polyethylene glycols and
fatty acid esters of
sorbitan, and mixtures thereof. As is appreciated by those of ordinary skill,
a novel and
improved formulation of a particular therapeutic composition, a novel gas-
enriched therapeutic
fluid, and a novel method of delivering the novel gas-enriched therapeutic
fluid may be obtained
by replacing one or more inert diluents with a gas-enriched fluid of
identical, similar, or
different composition. For example, conventional water may be replaced or
supplemented by a
gas-enriched fluid produced by mixing oxygen into water or deionized water to
provide gas-
enriched fluid.
In certain embodiments, the inventive gas-enriched fluid may be combined with
one or
more therapeutic agents and/or used alone. In particular embodiments,
incorporating the gas-
enriched fluid may include replacing one or more solutions known in the art,
such as deionized
water, saline solution, and the like with one or more gas-enriched fluid,
thereby providing an
improved therapeutic composition for delivery to the subject.
Certain embodiments provide for therapeutic compositions comprising a gas-
enriched
fluid of the present invention, a pharmaceutical composition or other
therapeutic agent or a
pharmaceutically acceptable salt or solvate thereof, and at least one
pharmaceutical carrier or
diluent. These pharmaceutical compositions may be used in the prophylaxis and
treatment of
the foregoing diseases or conditions and in therapies as mentioned above.
Preferably, the carrier
must be pharmaceutically acceptable and must be compatible with, i.e. not have
a deleterious
effect upon, the other ingredients in the composition. The carrier may be a
solid or liquid and is
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
preferably formulated as a unit dose formulation, for example, a tablet that
may contain from
0.05 to 95% by weight of the active ingredient.
Possible administration routes include oral, sublingual, buccal, parenteral
(for example
subcutaneous, intramuscular, intra-arterial, intraperitoneally,
intracisternally, intravesically,
intrathecally, or intravenous), rectal, topical including transdermal,
intravaginal, intraoccular,
intraotical, intranasal, inhalation, and injection or insertion of implantable
devices or materials.
Administration Routes
Most suitable means of administration for a particular subject will depend on
the nature
and severity of the disease or condition being treated or the nature of the
therapy being used, as
well as the nature of the therapeutic composition or additional therapeutic
agent. In certain
embodiments, oral or topical administration is preferred.
Formulations suitable for oral administration may be provided as discrete
units, such as
tablets, capsules, cachets, syrups, elixirs, chewing gum, "lollipop"
formulations,
microemulsions, solutions, suspensions, lozenges, or gel-coated ampules, each
containing a
predetermined amount of the active compound; as powders or granules; as
solutions or
suspensions in aqueous or non-aqueous liquids; or as oil-in-water or water-in-
oil emulsions.
Additional formulations suitable for oral administration may be provided to
include fine
particle dusts or mists which may be generated by means of various types of
metered dose
pressurized aerosols, atomizers, nebulisers, or insufflators. In particular,
powders or other
compounds of therapeutic agents may be dissolved or suspended in a gas-
enriched fluid of the
present invention.
Formulations suitable for transmucosal methods, such as by sublingual or
buccal
administration include lozenges patches, tablets, and the like comprising the
active compound
and, typically a flavored base, such as sugar and acacia or tragacanth and
pastilles comprising
the active compound in an inert base, such as gelatin and glycerine or sucrose
acacia.
Formulations suitable for parenteral administration typically comprise sterile
aqueous
solutions containing a predetermined concentration of the active gas-enriched
fluid and possibly
another therapeutic agent; the solution is preferably isotonic with the blood
of the intended
recipient. Additional formulations suitable for parenteral administration
include formulations
containing physiologically suitable co-solvents and/or complexing agents such
as surfactants
and cyclodextrins. Oil-in-water emulsions may also be suitable for
formulations for parenteral
56
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
administration of the gas-enriched fluid. Although such solutions are
preferably administered
intravenously, they may also be administered by subcutaneous or intramuscular
injection.
Formulations suitable for urethral, rectal or vaginal administration include
gels, creams,
lotions, aqueous or oily suspensions, dispersible powders or granules,
emulsions, dissolvable
solid materials, douches, and the like. The formulations are preferably
provided as unit-dose
suppositories comprising the active ingredient in one or more solid carriers
forming the
suppository base, for example, cocoa butter. Alternatively, colonic washes
with the gas-
enriched fluids of the present invention may be formulated for colonic or
rectal administration.
Formulations suitable for topical, intraoccular, intraotic, or intranasal
application include
ointments, creams, pastes, lotions, pastes, gels (such as hydrogels), sprays,
dispersible powders
and granules, emulsions, sprays or aerosols using flowing propellants (such as
liposomal sprays,
nasal drops, nasal sprays, and the like) and oils. Suitable carriers for such
formulations include
petroleum jelly, lanolin, polyethyleneglycols, alcohols, and combinations
thereof. Nasal or
intranasal delivery may include metered doses of any of these formulations or
others. Likewise,
intraotic or intraocular may include drops, ointments, irritation fluids and
the like.
Formulations of the invention may be prepared by any suitable method,
typically by
uniformly and intimately admixing the gas-enriched fluid optionally with an
active compound
with liquids or finely divided solid carriers or both, in the required
proportions and then, if
necessary, shaping the resulting mixture into the desired shape.
For example a tablet may be prepared by compressing an intimate mixture
comprising a
powder or granules of the active ingredient and one or more optional
ingredients, such as a
binder, lubricant, inert diluent, or surface active dispersing agent, or by
molding an intimate
mixture of powdered active ingredient and a gas-enriched fluid of the present
invention.
Suitable formulations for administration by inhalation include fine particle
dusts or mists
which may be generated by means of various types of metered dose pressurized
aerosols,
atomizers, nebulisers, or insufflators. In particular, powders or other
compounds of therapeutic
agents may be dissolved or suspended in a gas-enriched fluid of the present
invention.
For pulmonary administration via the mouth, the particle size of the powder or
droplets is
typically in the range 0.5-10 M, preferably 1-5 M, to ensure delivery into
the bronchial tree.
For nasal administration, a particle size in the range 10-500 M is preferred
to ensure retention
in the nasal cavity.
Metered dose inhalers are pressurized aerosol dispensers, typically containing
a
57
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
suspension or solution formulation of a therapeutic agent in a liquefied
propellant. In certain
embodiments, as disclosed herein, the gas-enriched fluids of the present
invention may be used
in addition to or instead of the standard liquefied propellant. During use,
these devices
discharge the formulation through a valve adapted to deliver a metered volume,
typically from
10 to 150 L, to produce a fine particle spray containing the therapeutic
agent and the gas-
enriched fluid. Suitable propellants include certain chlorofluorocarbon
compounds, for
example, dichlorodifluoromethane, trichlorofluoromethane,
dichlorotetrafluoroethane and
mixtures thereof.
The formulation may additionally contain one or more co-solvents, for example,
ethanol
surfactants, such as oleic acid or sorbitan trioleate, anti-oxidants and
suitable flavoring agents.
Nebulisers are commercially available devices that transform solutions or
suspensions of the
active ingredient into a therapeutic aerosol mist either by means of
acceleration of a compressed
gas (typically air or oxygen) through a narrow venturi orifice, or by means of
ultrasonic
agitation. Suitable formulations for use in nebulisers consist of another
therapeutic agent in a
gas-enriched fluid and comprising up to 40% w/w of the formulation, preferably
less than 20%
w/w. In addition, other carriers may be utilized, such as distilled water,
sterile water, or a dilute
aqueous alcohol solution, preferably made isotonic with body fluids by the
addition of salts,
such as sodium chloride. Optional additives include preservatives, especially
if the formulation
is not prepared sterile, and may include methyl hydroxy-benzoate, anti-
oxidants, flavoring
agents, volatile oils, buffering agents and surfactants.
Suitable formulations for administration by insufflation include finely
comminuted
powders that may be delivered by means of an insufflator or taken into the
nasal cavity in the
manner of a snuff. In the insufflator, the powder is contained in capsules or
cartridges, typically
made of gelatin or plastic, which are either pierced or opened in situ and the
powder delivered
by air drawn through the device upon inhalation or by means of a manually-
operated pump. The
powder employed in the insufflator consists either solely of the active
ingredient or of a powder
blend comprising the active ingredient, a suitable powder diluent, such as
lactose, and an
optional surfactant. The active ingredient typically comprises from 0.1 to 100
w/w of the
formulation.
In addition to the ingredients specifically mentioned above, the formulations
of the
present invention may include other agents known to those skilled in the art,
having regard for
the type of formulation in issue. For example, formulations suitable for oral
administration may
58
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
include flavoring agents and formulations suitable for intranasal
administration may include
perfumes.
The therapeutic compositions of the invention can be administered by any
conventional
method available for use in conjunction with pharmaceutical drugs, either as
individual
therapeutic agents or in a combination of therapeutic agents.
The dosage administered will, of course, vary depending upon known factors,
such as the
pharmacodynamic characteristics of the particular agent and its mode and route
of
administration; the age, health and weight of the recipient; the nature and
extent of the
symptoms; the kind of concurrent treatment; the frequency of treatment; and
the effect desired.
A daily dosage of active ingredient can be expected to be about 0.001 to 1000
milligrams (mg)
per kilogram (kg) of body weight, with the preferred dose being 0.1 to about
30 mg/kg.
According to certain aspects daily dosage of active ingredient may be .001
liters to 10 liters,
with the preferred dose being from about .01 liters to 1 liter.
Dosage forms (compositions suitable for administration) contain from about 1
mg to
about 500 mg of active ingredient per unit. In these pharmaceutical
compositions, the active
ingredient will ordinarily be present in an amount of about 0.5-95% weight
based on the total
weight of the composition.
Ointments, pastes, foams, occlusions, creams and gels also can contain
excipients, such
as starch, tragacanth, cellulose derivatives, silicones, bentonites, silica
acid, and talc, or mixtures
thereof. Powders and sprays also can contain excipients such as lactose, talc,
silica acid,
aluminum hydroxide, and calcium silicates, or mixtures of these substances.
Solutions of
nanocrystalline antimicrobial metals can be converted into aerosols or sprays
by any of the
known means routinely used for making aerosol pharmaceuticals. In general,
such methods
comprise pressurizing or providing a means for pressurizing a container of the
solution, usually
with an inert carrier gas, and passing the pressurized gas through a small
orifice. Sprays can
additionally contain customary propellants, such as nitrogen, carbon dioxide,
and other inert
gases. In addition, microspheres or nanoparticles may be employed with the gas-
enriched
therapeutic compositions or fluids of the present invention in any of the
routes required to
administer the therapeutic compounds to a subject.
The injection-use formulations can be presented in unit-dose or multi-dose
sealed
containers, such as ampules and vials, and can be stored in a freeze-dried
(lyophilized) condition
requiring only the addition of the sterile liquid excipient, or gas-enriched
fluid, immediately
59
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
prior to use. Extemporaneous injection solutions and suspensions can be
prepared from sterile
powders, granules, and tablets. The requirements for effective pharmaceutical
carriers for
injectable compositions are well known to those of ordinary skill in the art.
See, for example,
Pharmaceutics and Pharmacy Practice, J. B. Lippincott Co., Philadelphia, Pa.,
Banker and
Chalmers, Eds., 238-250 (1982) and ASHP Handbook on Injectable Drugs, Toissel,
4th ed.,
622-630 (1986).
Formulations suitable for topical administration include lozenges comprising a
gas-
enriched fluid of the invention and optionally, an additional therapeutic and
a flavor, usually
sucrose and acacia or tragacanth; pastilles comprising a gas-enriched fluid
and optional
additional therapeutic agent in an inert base, such as gelatin and glycerin,
or sucrose and acacia;
and mouth washes or oral rinses comprising a gas-enriched fluid and optional
additional
therapeutic agent in a suitable liquid carrier; as well as creams, emulsions,
gels and the like.
Additionally, formulations suitable for rectal administration may be presented
as
suppositories by mixing with a variety of bases such as emulsifying bases or
water-soluble
bases. Formulations suitable for vaginal administration may be presented as
pessaries, tampons,
creams, gels, pastes, foams, or spray formulas containing, in addition to the
active ingredient,
such carriers as are known in the art to be appropriate.
Suitable pharmaceutical carriers are described in Remington's Pharmaceutical
Sciences,
Mack Publishing Company, a standard reference text in this field.
The dose administered to a subject, especially an animal, particularly a
human, in the
context of the present invention should be sufficient to affect a therapeutic
response in the
animal over a reasonable time frame. One skilled in the art will recognize
that dosage will
depend upon a variety of factors including the condition of the animal, the
body weight of the
animal, as well as the condition being treated. A suitable dose is that which
will result in a
concentration of the therapeutic composition in a subject that is known to
affect the desired
response.
The size of the dose also will be determined by the route, timing and
frequency of
administration as well as the existence, nature, and extent of any adverse
side effects that might
accompany the administration of the therapeutic composition and the desired
physiological
effect.
It will be appreciated that the compounds of the combination may be
administered: (1)
simultaneously by combination of the compounds in a co-formulation or (2) by
alternation, i.e.
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
delivering the compounds serially, sequentially, in parallel or simultaneously
in separate
pharmaceutical formulations. In alternation therapy, the delay in
administering the second, and
optionally a third active ingredient, should not be such as to lose the
benefit of a synergistic
therapeutic effect of the combination of the active ingredients. According to
certain
embodiments by either method of administration (1) or (2), ideally the
combination should be
administered to achieve the most efficacious results. In certain embodiments
by either method of
administration (1) or (2), ideally the combination should be administered to
achieve peak plasma
concentrations of each of the active ingredients. A one pill once-per-day
regimen by
administration of a combination co-formulation may be feasible for some
patients expected to be
exposed to a neurotoxin. According to certain embodiments effective peak
plasma
concentrations of the active ingredients of the combination will be in the
range of approximately
0.001 to 100 M. Optimal peak plasma concentrations may be achieved by a
formulation and
dosing regimen prescribed for a particular patient. It will also be understood
that the inventive
fluids and any one of erythropoietin, anti-apoptotics (TCH346, CEP-1347),
antiglutamatergics,
monoamine oxidase inhibitors (selegiline, rasagiline), promitochondrials
(coenzyme Q10,
creatine), calcium channel blockers (isradipine), alpha-synuclein, and/or
growth factors (GDNF)
or the physiologically functional derivatives of any thereof, whether
presented simultaneously or
sequentially, may be administered individually, in multiples, or in any
combination thereof. In
general, during alternation therapy (2), an effective dosage of each compound
is administered
serially, where in co-formulation therapy (1), effective dosages of two or
more compounds are
administered together.
The combinations of the invention may conveniently be presented as a
pharmaceutical
formulation in a unitary dosage form. A convenient unitary dosage formulation
contains the
active ingredients in any amount from 1 mg to 1 g each, for example but not
limited to, 10 mg to
300 mg. The synergistic effects of the inventive fluid in combination with any
one of
erythropoietin, anti-apoptotics (TCH346, CEP-1347), antiglutamatergics,
monoamine oxidase
inhibitors (selegiline, rasagiline), promitochondrials (coenzyme Q10,
creatine), calcium channel
blockers (isradipine), alpha-synuclein, and/or growth factors (GDNF) may be
realized over a
wide ratio, for example 1:50 to 50:1 (inventive fluid: erythropoietin, anti-
apoptotics (TCH346,
CEP-1347), antiglutamatergics, monoamine oxidase inhibitors (selegiline,
rasagiline),
promitochondrials (coenzyme Q10, creatine), calcium channel blockers
(isradipine), alpha-
synuclein, and/or growth factors (GDNF)). In one embodiment the ratio may
range from about
61
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
1:10 to 10:1. In another embodiment, the weight/weight ratio of inventive
fluid to any one of
erythropoietin, anti-apoptotics (TCH346, CEP-1347), antiglutamatergics,
monoamine oxidase
inhibitors (selegiline, rasagiline), promitochondrials (coenzyme Q10,
creatine), calcium channel
blockers (isradipine), alpha-synuclein, and/or growth factors (GDNF) in a co-
formulated
combination dosage form, such as a pill, tablet, caplet or capsule will be
about 1, i.e. an
approximately equal amount of inventive fluid and any one of erythropoietin,
anti-apoptotics
(TCH346, CEP- 1347), antiglutamatergics, monoamine oxidase inhibitors
(selegiline, rasagiline),
promitochondrials (coenzyme Q10, creatine), calcium channel blockers
(isradipine), alpha-
synuclein, and/or growth factors (GDNF). In other exemplary co-formulations,
there may be
more or less inventive fluid and any one of erythropoietin, anti-apoptotics
(TCH346, CEP-
1347), antiglutamatergics, monoamine oxidase inhibitors (selegiline,
rasagiline),
promitochondrials (coenzyme Q10, creatine), calcium channel blockers
(isradipine), alpha-
synuclein, and/or growth factors (GDNF). In one embodiment, each compound will
be
employed in the combination in an amount at which it exhibits anti-
inflammatory activity when
used alone. Other ratios and amounts of the compounds of said combinations are
contemplated
within the scope of the invention.
A unitary dosage form may further comprise inventive fluid and any one of
erythropoietin, anti-apoptotics (TCH346, CEP-1347), antiglutamatergics,
monoamine oxidase
inhibitors (selegiline, rasagiline), promitochondrials (coenzyme Q10,
creatine), calcium channel
blockers (isradipine), alpha-synuclein, and/or growth factors (GDNF), or
physiologically
functional derivatives of either thereof, and a pharmaceutically acceptable
carrier.
It will be appreciated by those skilled in the art that the amount of active
ingredients in
the combinations of the invention required for use in treatment will vary
according to a variety
of factors, including the nature of the condition being treated and the age
and condition of the
patient, and will ultimately be at the discretion of the attending physician
or health care
practitioner. The factors to be considered include the route of administration
and nature of the
formulation, the animal's body weight, age and general condition and the
nature and severity of
the disease to be treated.
It is also possible to combine any two of the active ingredients in a unitary
dosage form
for simultaneous or sequential administration with a third active ingredient.
The three-part
combination may be administered simultaneously or sequentially. When
administered
sequentially, the combination may be administered in two or three
administrations. According to
62
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
certain embodiments the three-part combination of inventive fluid and any one
of erythropoietin,
anti-apoptotics (TCH346, CEP-1347), antiglutamatergics, monoamine oxidase
inhibitors
(selegiline, rasagiline), promitochondrials (coenzyme Q10, creatine), calcium
channel blockers
(isradipine), alpha-synuclein, and/or growth factors (GDNF) may be
administered in any order.
Neurotoxic agents:
Neurotoxic agents are toxins that specifically act upon neurons, their
synapses, or the
nervous system in its entirety. They are substances which cause damage to the
structures of the
brain which in turn leads to chronic disease. Neurotoxins include adrenergic
neurotoxins,
cholinergic neurotoxins, dopaminergic neurotoxins, excitotoxins, and other
neurotoxins.
Examples of adrenergic neurotoxins include N-(2-chloroethyl)-N-ethyl-2-
bromobenzylamine
hydrochloride. Examples of cholinergic neurotoxins include acetylethylcholine
mustard
hydrochloride. Examples of dopaminergic neurotoxins include 6-hydroxydopamine
HBr (6-
OHDA), 1-methyl-4-(2-methylphenyl)-1,2,3,6-tetrahydro-pyridine hydrochloride,
1-methyl-4-
phenyl-2,3-dihydropyridinium perchlorate, N-methyl-4-phenyl-
1,2,5,6tetrahydropyridine HC1
(MPTP), 1-methyl-4-phenylpyridinium iodide (MPP+), paraquat, and rotenone.
Examples of
excitotoxins include NMDA and kainic acid.
MPTP, MPP+, paraquat, rotenone and 6-OHDA have been been shown to induce PD
like
symptoms in animal models. (See, K. Ossowska, et al., (2006). "Degeneration of
dopaminergic
mesocortical neurons and activation of compensatory processes induced by a
long-term paraquat
administration in rats: Implications for Parkinson's disease". Neuroscience
141 (4): 2155-2165;
and Caboni P, et al., (2004). "Rotenone, deguelin, their metabolites, and the
rat model of
Parkinson's disease". Chem Res Toxicol 17 (11): 1540-8; Simon et al., Exp
Brain Res, 1974, 20:
375-384; Langston et al., Science, 1983, 219: 979-980; Tanner, Occup Med,
1992, 7: 503-513;
Lion et al., Neurology, 1997, 48: 1583-1588).
Neuroprotective
Neuroprotection within the nervous system protects neurons from apoptosis or
degeneration, for example following a brain injury or as a result of chronic
neurodegenerative
diseases. A "neuroprotective effect" is aimed to prevent and treat
complications that might
result in central nervous system (CNS) damage. Neuroprotection can be
estimated by
parameters of cell survival or cell death delay, arrest or slowing of the
disease progression,
disease onset and disease mortality delay.
63
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
Examples, as described herein, show that the electrokinetically altered
aqueous fluids
have neuro protective properties, wherein the electrokinetically altered
aqueous fluids was
shown to protect neurocells from MPTP-induced PD symptoms. According to
certain
embodiments, the electrokinetically altered aqueous fluids have substantial
utility in protecting
against and/or reducing the effects related to being exposed to neurotoxins.
Neuroprotective agents include but are not limited to erythropoietin, anti-
apoptotics
(TCH346, CEP-1347), antiglutamatergics, monoamine oxidase inhibitors
(selegiline, rasagiline),
promitochondrials (coenzyme Q10, creatine), calcium channel blockers
(isradipine), alpha-
synuclein, and growth factors (GDNF).
The following examples are meant to be illustrative only and not limiting in
any way.
EXAMPLES
EXAMPLE I
Microbubble Size
Experiments were performed with a gas-enriched fluid by using the diffuser of
the
present invention in order to determine a gas microbubble size limit. The
microbubble size limit
was established by passing the gas enriched fluid through 0.22 and 0.1 micron
filters. In
performing these tests, a volume of fluid passed through the diffuser of the
present invention and
generated a gas-enriched fluid. Sixty milliliters of this fluid was drained
into a 60 ml syringe.
The dissolved oxygen level of the fluid within the syringe was then measured
by Winkler
titration. The fluid within the syringe was injected through a 0.22 micron
Millipore Millex
GP50 filter and into a 50 ml beaker. The dissolved oxygen rate of the material
in the 50 ml
beaker was then measured. The experiment was performed three times to achieve
the results
illustrated in Table 4 below.
Table 4
DO AFTER 0.22 MICRON
DO IN SYRINGE FILTER
42.1 ppm 39.7 ppm
43.4 ppm 42.0 ppm
43.5 ppm 39.5 ppm
64
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
As can be seen, the dissolved oxygen levels that were measured within the
syringe and
the dissolved oxygen levels within the 50 ml beaker were not significantly
changed by passing
the diffused material through a 0.22 micron filter, which implies that the
microbubbles of
dissolved gas within the fluid are not larger than 0.22 microns.
A second test was performed in which a batch of saline solution was enriched
with the
diffuser of the present invention and a sample of the output solution was
collected in an
unfiltered state. The dissolved oxygen level of the unfiltered sample was 44.7
ppm. A 0.1
micron filter was used to filter the oxygen-enriched solution from the
diffuser of the present
invention and two additional samples were taken. For the first sample, the
dissolved oxygen
level was 43.4 ppm. For the second sample, the dissolved oxygen level was 41.4
ppm. Finally,
the filter was removed and a final sample was taken from the unfiltered
solution. In this case,
the final sample had a dissolved oxygen level of 45.4 ppm. These results were
consistent with
those in which the Millipore 0.22 micron filter was used. Thus, the majority
of the gas bubbles
or microbubbles within the saline solution are approximately less than 0.1
microns in size.
EXAMPLE 2
(Patch clamp analysis conducted on Calu-3 cells perfused with inventive
electrokinetically
generated fluids (RNS-60 and Solas) revealed that (i) exposure to RNS-60 and
Solas resulted in
increases in whole cell conductance, (ii) that exposure of cells to the RNS-60
produced an
increase in a non-linear conductance, evident at 15 min incubation times, and
(iii) that exposure
of cells to the RNS-60 produced an effect of RNS-60 saline on calcium
permeable channels)
Overview. In this Example, patch clamp studies were performed to further
confirm the
utilities, as described herein, of the inventive electrokinetically generated
saline fluids (RNS-60
and Solas), including the utility to modulate whole-cell currents. Two sets of
experiments were
conducted.
The summary of the data of the first set of experiments indicates that the
whole cell
conductance (current-to-voltage relationship) obtained with Solas saline is
highly linear for both
incubation times (15 min, 2 hours), and for all voltage protocols. It is
however evident, that
longer incubation (2 hours) with Solas increased the whole cell conductance.
Exposure of cells
to the RNS-60 produced an increase in a non-linear conductance, as shown in
the delta currents
(Rev-Sol subtraction), which is only evident at 15 min incubation time. The
effect of the RNS-
60 on this non-linear current disappears, and is instead highly linear at the
two-hour incubation
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
time. The contribution of the non-linear whole cell conductance, as previously
observed, was
voltage sensitive, although present at all voltage protocols.
The summary of data of the second set of experiments indicates that there is
an effect of
the RNS-60 saline on a non-linear current, which was made evident in high
calcium in the
external solution. The contribution of the non-linear whole cell conductance,
although voltage
sensitive, was present in both voltage protocols, and indicates an effect of
RNS-60 saline on
calcium permeable channels.
First set of experiments (increase of conductance; and activation of a non-
linear voltage
regulated conductance)
Materials and Methods:
The Bronchial Epithelial line Calu-3 was used in Patch clamp studies. Calu-3
Bronchial
Epithelial cells (ATCC #HTB-55) were grown in a 1:1 mixture of Ham's F12 and
DMEM
medium that was supplemented with 10% FBS onto glass coverslips until the time
of the
experiments. In brief, a whole cell voltage clamp device was used to measure
effects on Calu-3
cells exposed to the inventive electrokinetically generated fluids (e.g., RNS-
60;
electrokinetically treated normal saline comprising 60 ppm dissolved oxygen;
sometimes
referred to as "drug" in this Example).
Patch clamping techniques were utilized to assess the effects of the test
material (RNS-
60) on epithelial cell membrane polarity and ion channel activity.
Specifically, whole cell
voltage clamp was performed upon the Bronchial Epithelial line Calu-3 in a
bathing solution
consisting of: 135mM NaCl, 5mM KC1, 1.2mM CaC12, 0.8mM MgC12, and 10mM HEPES
(pH
adjusted to 7.4 with N-methyl D-Glucamine). Basal currents were measured after
which RNS-
60 was perfused onto the cells.
More specifically, patch pipettes were pulled from borosilicate glass (Garner
Glass Co,
Claremont, CA) with a two-stage Narishige PB-7 vertical puller and then fire-
polished to a
resistance between 6-12 Mohms with a Narishige MF-9 microforge (Narishige
International
USA, East Meadow, NY). The pipettes were filled with an intracellular solution
containing (in
mM): 135 KC1, 10 NaCl, 5 EGTA, 10 Hepes, pH was adjusted to 7.4 with NMDG (N-
Methyl-
D-Glucamine).
66
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
The cultured Calu-3 cells were placed in a chamber containing the following
extracellular solution (in mM): 135 NaCl, 5 KC1, 1.2 CaC12, 0.5 MgC12 and 10
Hepes (free
acid), pH was adjusted to 7.4 with NMDG.
Cells were viewed using the 40X DIC objective of an Olympus IX71 microscope
(Olympus Inc., Tokyo, Japan). After a cell-attached gigaseal was established,
a gentle suction
was applied to break in, and to attain the whole-cell configuration.
Immediately upon breaking
in, the cell was voltage clamped at -120, -60, -40 and 0 mV, and was
stimulated with voltage
steps between 100 mV (500 ms/step). After collecting the whole-cell currents
at the control
condition, the same cell was perfused through bath with the test fluid
comprising same
extracellular solutes and pH as for the above control fluid, and whole-cell
currents at different
holding potentials were recorded with the same protocols.
Electrophysiological data were acquired with an Axon Patch 200B amplifier, low-
pass
filtered at 10 kHz, and digitized with 1400A Digidata (Axon Instruments, Union
City, CA). The
pCLAMP 10.0 software (Axon Instruments) was used to acquire and to analyze the
data.
Current (I)-to-voltage (V)
relationships (whole cell conductance) were obtained by plotting the actual
current value at
approximately 400 msec into the step, versus the holding potential (V). The
slope of the UV
relationship is the whole cell conductance.
Drugs and Chemicals. Whenever indicated, cells were stimulated with a cAMP
stimulatory cocktail containing 8-Br-cAMP (500 mM), IBMX (isobutyl-l-
methylxanthie, 200
mM) and forskolin (10 mM). The cAMP analog 8-Br-cAMP (Sigma Chem. Co.) was
used from
a 25 mM stock in H2O solution. Forskolin (Sigma) and IBMX (Sigma) were used
from a DMSO
solution containing both 10 mM Forskolin and 200 mM IBMX stock solution. The
data
obtained are expressed as the mean SEM whole cell current for 5-9 cells.
Results:
Figures 1 A-C show the results of a series of patch clamping experiments that
assessed
the effects of the electrokinetically generated fluid (e.g., RNS-60 and Solas)
on epithelial cell
membrane polarity and ion channel activity at two time-points (15 min (left
panels) and 2 hours
(right panels)) and at different voltage protocols (A, stepping from zero mV;
B, stepping from -
60 mV; and C, stepping from -120 mV). The results indicate that the RNS-60
(filled circles)
has a larger effect on whole-cell conductance than Solas (open circles). In
the experiment
67
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
similar results were seen in the three voltage protocols and at both the 15
minute and two-hour
incubation time points.
Figures 2 A-C show graphs resulting from the subtraction of the Solas current
data from
the RNS-60 current data at three voltage protocols ("Delta currents") (A,
stepping from zero
mV; B, stepping from -60 mV; and C, stepping from -120 mV) and the two time-
points (15
mins (open circles) and 2 hours (filled circles)). These data indicated that
at the 15 minute time-
point with RNS-60, there is a non-linear voltage-dependent component that is
absent at the 2
hour time point.
As in previous experiments, data with "Normal" saline gave a very consistent
and time-
independent conductance used as a reference. The present results were obtained
by matching
groups with either Solas or RNS-60 saline, and indicate that exposure of Calu-
3 cells to the
RNS-60 saline under basal conditions (without cAMP, or any other stimulation),
produces time-
dependent effect(s), consistent with the activation of a voltage-regulated
conductance at shorter
incubation times (15 min). This phenomenon was not as apparent at the two-hour
incubation
point. As described elsewhere herein, the linear component is more evident
when the
conductance is increased by stimulation with the cAMP "cocktail". Nonetheless,
the two-hour
incubation time showed higher linear conductance for both the RNS-60 and the
Solas saline, and
in this case, the RNS-60 saline doubled the whole cell conductance as compared
to Solas alone.
This evidence indicates that at least two contributions to the whole cell
conductance are affected
by the RNS-60 saline, namely the activation of a non-linear voltage regulated
conductance, and
a linear conductance, which is more evident at longer incubation times.
Second set of experiments (effect on calcium permeable channels)
Methods for second set of experiments:
See above for general patch clamp methods. In the following second set of
experiments,
yet additional patch clamp studies were performed to further confirm the
utility of the inventive
electrokinetically generated saline fluids (RNS-60 and Solas) to modulate
whole-cell currents,
using Calu-3 cells under basal conditions, with protocols stepping from either
zero mV or -120
mV holding potentials.
The whole-cell conductance in each case was obtained from the current-to-
voltage
relationships obtained from cells incubated for 15 min with either saline. To
determine whether
there is a contribution of calcium permeable channels to the whole cell
conductance, and
68
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
whether this part of the whole cell conductance is affected by incubation with
RNS-60 saline,
cells were patched in normal saline after the incubation period (entails a
high NaCl external
solution, while the internal solution contains high KCI). The external saline
was then replaced
with a solution where NaCl was replaced by CsCI to determine whether there is
a change in
conductance by replacing the main external cation. Under these conditions, the
same cell was
then exposed to increasing concentrations of calcium, such that a calcium
entry step is made
more evident.
Results:
Figures 3 A-D show the results of a series of patch clamping experiments that
assessed
the effects of the electrokinetically generated fluid (e.g., Solas (panels A
and B) and RNS-60
(panels C and D)) on epithelial cell membrane polarity and ion channel
activity using different
external salt solutions and at different voltage protocols (panels A and C
show stepping from
zero mV, whereas panels B and D show stepping from -120 mV). In these
experiments one
time-point of 15 minutes was used. For Solas (panels A and B) the results
indicate that: 1) using
CsC1 (square symbols) instead of NaCl as the external solution, increased
whole cell
conductance with a linear behavior when compared to the control (diamond
symbols); and 2)
CaC12 at both 20 mM CaC12 (circle symbols) and 40 mM CaC12 (triangle symbols)
increased
whole cell conductance in a non-linear manner. For RNS-60 (panels C and D),
the results
indicate that: 1) using CsCI (square symbols) instead of NaCl as the external
solution had little
effect on whole cell conductance when compared to the control (diamond
symbols); and 2)
CaC12 at 40 mM (triangle symbols) increased whole cell conductance in a non-
linear manner.
Figures 4 A-D show the graphs resulting from the subtraction of the CsC1
current data
(shown in Figure 3) from the 20 mM CaC12 (diamond symbols) and 40 mM CaC12
(square
symbols) current data at two voltage protocols (panels A and C, stepping from
zero mV; and B
and D, stepping from -120 mV) for Solas (panels A and B) and RNS-60 (panels C
and D). The
results indicate that both Solas and RNS-60 solutions activated a calcium-
induced non-linear
whole cell conductance. The effect was greater with RNS-60 (indicating a
dosage
responsiveness), and with RNS-60 was only increased at higher calcium
concentrations.
Moreover, the non-linear calcium dependent conductance at higher calcium
concentration was
also increased by the voltage protocol.
The data of this second set of experiments further indicates an effect of RNS-
60 saline
and Solas saline for whole cell conductance data obtained in Calu-3 cells. The
data indicate that
69
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
15-min incubation with either saline produces a distinct effect on the whole
cell conductance,
which is most evident with RNS-60, and when external calcium is increased, and
further
indicates that the RNS-60 saline increases a calcium-dependent non-linear
component of the
whole cell conductance.
The accumulated evidence suggests activation by Revalesio saline of ion
channels,
which make different contributions to the basal cell conductance.
Taken together with Applicants' other data (e.g., the data of Applicants other
working
Examples) particular aspects of the present invention provide compositions and
methods for
modulating intracellular signal transduction, including modulation of at least
one of membrane
structure, membrane potential or membrane conductivity, membrane proteins or
receptors, ion
channels, lipid components, or intracellular components with are exchangeable
by the cell (e.g.,
signaling pathways, such as calcium dependant cellular signaling systems,
comprising use of the
inventive electrokinetically generated solutions to impart electrochemical
and/or conformational
changes in membranous structures (e.g., membrane and/or membrane proteins,
receptors or
other membrane components) including but not limited to GPCRs and/or g-
proteins. According
to additional aspects, these effects modulate gene expression, and may
persist, dependant, for
example, on the half lives of the individual messaging components, etc.
EXAMPLE 3
(The inventive electrokinetic fluid was shown to be substantially efficacious
in a dose-responsive
manner in an art-recognized acute Experimental Allergic (Autoimmune)
Encephalomyelitis
(EAE) rat MBP model of Multiple Sclerosis(MS))
Overview:
In this working EXAMPLE, the inventive electrokinetic fluid RNS-60 was
evaluated at
two doses, in both prophylactic and therapeutic administration regimens, in an
art-recognized
Myelin Basic Protein MBP induced acute Experimental Allergic Encephalomyelitis
(EAE) rat
model. The inventive electrokinetic fluid RNS-60 was shown to be substantially
efficacious in a
dose-responsive manner. Both the therapeutic (daily administration of RNS-60
beginning
concomitant with MBP injection) and prophylactic (daily administration of RNS-
60 beginning
seven days prior to MBP injection) RNS-60 dosage regimens showed a marked
decrease, as well
as a delayed onset (in the high dose groups) of clinical score. According to
particular aspects of
the present invention, therefore, the inventive electrokinetic compositions
have substantial utility
for treating, including alleviating and preventing, the symptoms of EAE in an
art-recognized rat
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
model of human MS. According to further aspects of the present invention,
therefore, the
inventive electrokinetic compositions have substantial utility for treating,
including alleviating
and preventing, the symptoms of MS in afflicted mammals (preferably humans).
In yet further
aspects, the inventive electrokinetic compositions cross the Blood Brain
Barrier (BBB), and thus
provided a novel method for treating inflammatory conditions of the central
nervous system.
Multiple Sclerosis (MS). Multiple Sclerosis (MS) is a demyelinating disease of
the
central nervous system (CNS), and is one of the most common disabling
neurological diseases in
young adults. The main characteristics of this disease are focal areas of
demyelination and
inflammation. The disease course is unpredictable and life-long, and affects
women more
commonly than men. The etiology of the disease appears to be dependent on
genetic and
environmental factors. In the periphery, antigen is bound by antigen
presenting cells (APC) via
MCH II. ThO cells bind to the antigen and undergo activation and
differentiation. Adhesion
molecules and matrix metalloproteases (MMPs) help the Thl cells to bind and
penetrate the
Blood Brain Barrier (BBB). Upon crossing the BBB into the CNS, Thl cells
engage antigen-
MHC complexes and produce pro-inflammatory cytokines leading to damage in the
CNS. The
autoimmune system recognizes myelin proteins as foreign and begins to attack.
Historically,
while Thl cells are thought to play a predominant role in the pathology of the
disease, recent
evidence indicates that a proinflammatory cascade of Th17 cells, IL-6 and TGF-
(3 plays a critical
role in the pathogenesis of EAE and MS.
Experimental Autoimmune Encephalomyelitis (EAE). Experimental Autoimmune
Encephalomyelitis (EAE), also called Experimental Allergic Encephalomyelitis,
is a non-human
animal model of Multiple Sclerosis (MS). While not MS, the different forms and
stages of EAE
resemble the various forms and stages of MS very closely in a large number of
ways. More
specifically, EAE is an acute or chronic-relapsing, acquired, inflammatory and
demyelinating
autoimmune disease. The animals are injected with the whole or parts of
various proteins (e.g.,
Myelin Basic Protein (MBP), Proteolipid Protein (PLP), and Myelin
Oligodendrocyte
Glycoprotein (MOG)) that make up myelin, the insulating sheath that surrounds
nerve cells
(neurons), to induce an autoimmune response against the animal's own myelin
that closely
resembles MS in humans. EAE has been induced in a number of different animal
species
including mice, rats, guinea pigs, rabbits, macaques, rhesus monkeys and
marmosets. For
various reasons including the number of immunological tools, the availability,
lifespan and
fecundity of the animals and the resemblance of the induced disease to MS,
mice and rats are the
most commonly used species. The acute rat EAE model has a strong inflammatory
component
71
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
and is therefore an appropriate model in which to investigate the therapeutic
potential of an
agent that targets immune events in MS.
MBP-induced EAE. MPB in Lewis rats following one dose will lead to relapse
that is
characterized mainly by hind paw paralysis. Lewis rats are subjected to MBP
injection on day
0. Disease develops between day 12-16, with full disease recovery occurring
between days 18-
21. The model is self limiting and does not show demyelination.
Materials and Methods:
Production and Characterization of the test fluid (RNS-60). Filter sterilized
RNS-60 was
prepared by Applicants according to methods described in US2008/0219088
(published on 11
September 2008), US2008/0281001 (published on 11 November 2008) and
W02008/052143
(published on 02 May 2008), all of which are incorporated herein by reference
in their entirety
and particularly for all aspects relating to the apparatus and/or methods for
preparing
Applicants' inventive electrokinetic fluids. The dissolved oxygen (DO) content
of the RNS-60
used was 59 ppm, as determined by the Winkler Titration assay (Y.C. Wong &
C.T. Wong. New
Way Chemistry for Hong Kong A-Level Volume 4, Page 248. Or Standard Methods
for the
Examination of Water and Wastewater - 20th Edition ISBN 0-87553-235-7). RNS-60
fluid was
labeled with a test item (TI) number, receipt date, storage conditions and
expiry date. The
storage conditions and handling of the RNS-60 was per Applicants'
specification to ensure
stability at the Testing Facility during testing. Fluid was kept refrigerated
at 2-8 C when not in
use. Vials containing fluid were used as single use containers.
Vehicle control fluid. Vehicle control fluid was Normal Saline for injection
(0.9%) from
Hospira.
Dexamethasone. Dexamethasone was purchased from Sigma (Cat. No. D1756; Lot No.
096K1805). For administration, Dexamethasone (white powder) was diluted in
ethanol to
achieve a concentration of 1 mg/ml and then diluted again in distilled water
to achieve a dose
concentration of 0.1 mg/ml.
EAE Induction Items:
MBP antigenic agent. MBP was Myelin Basic Protein from guinea pig (Des-Gly-77,
Des-His-78)-MBP (68-84); Cat. No. H-6875; provided by MD Bioscience). MBP was
dissolved
in physiological saline at a concentration of 2 mg/ml;
CFA sensitizing agent. Complete Freund's Adjuvant (CFA) was from MD
Biosciences
Division of Morwell Diagnostics GmbH (Cat. No. IMAD-4). CFA suspension,
containing heat
72
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
killed Mycobacterium Tuberculosis H37 Ra at a concentration of 4 mg/ml, was
used as supplied;
and
MBP/CFA Emulsion (Antigenic/Sensitizing agents). Prior to the single
inoculations
carried out on study day 0, one volume of MBP solution was mixed with an equal
volume of
CFA 4 mg/ml by employing two syringes connected by a Luer fitting to
thoroughly mix the
emulsive mixture to equal a total dose volume of 100 l/animal. The dose was
delivered as 2x
50 l subcutaneous (SC) bilateral injections into the intraplantar paw
regions.
Test animals; Rats. Sixty (60) female Lewis rats (6-7 weeks of age at study
initiation)
were obtained from Harlan Laboratories Israel, Ltd. Weight variation of
animals at the time of
treatment initiation should not exceed 20% of the mean weight. The health
status of the animals
used in this study is examined upon their arrival. Only animals in good health
were acclimatized
to laboratory conditions and used in the study. Prior to entry in the study,
the animals were
acclimated for at least 5 days. During acclimation and throughout the study
duration, animals
were housed within a limited access rodent facility and kept in groups of
maximum 5 rats in
polypropylene cages fitted with solid bottoms and filled with sterile wood
shavings as bedding
material. Animals were provided ad libitum with a commercial rodent diet and
had free access
to drinking water, which was supplied to each cage via polyethylene bottles
with stainless steel
sipper tubes. A feed lot analysis of the diet batch used in the study was
included in the archives
with the study data. Water was monitored periodically. Automatically
controlled environmental
conditions were set to maintain temperature at 20-24 C with a relative
humidity (RH) of 30-
70%, a 12:12 hour light:dark cycle and 15-30 air changes/hr in the study room.
Temperature and
RH were monitored daily. The light cycle was monitored by the control clock.
Animals were
given a unique animal identification using tail marks. This number also
appeared on a cage
card, visible on the front of each cage. The cage card also contained the
study and group
numbers, route of administration, gender, strain and all other relevant
details as to treatment
group.
TABLE 5. Constitution of Test Groups and Dose Levels, listing the 6
experimental
groups comprising the study:
Volume
Group Group Dose Level Dosage
Number Size Test Material Route (mg/kg/admin) (nil/kg) Regime
IF n=10 Vehicle Control IV 0 2 ml for 7 days prior to
350 g rat disease
induction until
the end of the
study
73
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
Volume
Group Group Dose Level Dosage
Number Size Test Material Route (mg/kg/admin) (ml/kg) Regime
2F n=10 Dexamethasone IP 1 10 Once daily
beginning on
study day 0
3F n=10 RNS-60 IV 1 ml for 7 days prior to
350 g rat disease
induction until
the end of the
study
4F n=10 RNS-60 IV 2 ml for 7 days prior to
350 g rat disease
induction until
the end of the
study
5F n=10 RNS-60 IV 1 ml for Once daily
350 g rat beginning on
study day 0
6F n=10 RNS-60 IV 2 ml for Once daily
350 g rat beginning on
study day 0
Test procedures and Principles of the Acute EAE Murine Model. Experimental
Allergic
Encephalomyelitis (EAE) is a central nervous system (CNS) autoimmune
demyelinating disease
that mimics many of the clinical and pathologic features of Multiple Sclerosis
(MS). The acute
rat model consists of a sensitization period, induced by the single
subcutaneous (SC) injection of
Myelin basic protein (MBP) emulsified in Complete Freund's Adjuvant (CFA) on
day 0 of the
study.
A schematic depiction of EAE induction and treatment regimens is shown in
FIGURE
6).
EAE Induction:
MBP/CF A. As shown in the schematic description in FIGURE 6), all animals were
subjected on study day 0 (study commencement) to a single inoculum injection
consisting of a
homogenate emulsive mixture of MBP and CFA (MBP/CFA encephalitogenic emulsive
inoculum (100 g MBP/200 g CFA) was injected at a total dose volume of 100
l/animal and
delivered as 2 x 50 l subcutaneous (SC) bilateral injections into the
intraplantar paw regions).
Treatment:
Treatment Regimen and Procedure. All compounds were prepared fresh each day by
a
person different than the one scoring the animals. The person that scored the
animals received
74
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
vials marked only with group numbers and was unaware of the treatment.
Route of Administration: (i) RNS-60 (IV); (ii) Vehicle Controls: (IV); and
(iii) Positive
Controls: (IP).
Dose Levels and Volume Dosages: (i) RNS-60: Low dose 2 ml for 350 g; High dose
4 ml
for 350 g; (ii) Vehicle Controls: 0; and (iii) Positive Control
(Dexamethasone): 1 mg/kg.
Supportive Care. Unless determined during the course of the study, once EAE
experimental effects were expected and/or observed (approximately 8-12 days
post the single
encephalitogenic inoculation), or when the animals were showing a decrease is
body weight
greater than 15% from their previous determination or a decrease greater than
20% of their
initial body weight measurement, appropriate supportive care was carried out
on a case-by-case
basis.
Feeding and Watering. An additional water source consisting of chipped pellets
or
mealy rodent diet, soaked in drinking water is placed on the cage bottom and
in front of the
crawling/non-mobile animals.
Dehydration. Animals may be subjected to subcutaneous (SC) supplemental fluid
therapy with Dextrose 5% solution at least twice daily and up to 2
ml/animal/day until body
weight returns to be within 10% of the initial determination.
Urination. Palpation of the animals' abdomen is carried out in order to assist
with
voiding and to observe whether the animals can empty their bladder.
Other Special Care. Animals' perianal areas and hind legs were cleaned as
needed with
a moistened gauze pad.
OBSERVATIONS AND EXAMINATIONS:
Clinical Signs. Throughout the entire 21-day study, careful clinical
examinations were
carried out and recorded at least once daily in addition to the EAE clinical
scoring and
assessment (see below). Observations included changes in skin, fur, eyes,
mucous membranes,
occurrence of secretions and excretions (e.g. diarrhea) and autonomic activity
(e.g., lacrimation,
salivation, piloerection, pupil size, unusual respiratory pattern), gait,
posture and response to
handling, as well as the presence of unusual behavior, tremors, convulsions,
sleep and coma.
Body Weights. Body weight loss can be the first sign of disease initiation,
while a
sudden marked weight gain tends to accompany remission of EAE symptoms.
Therefore,
determination of individual body weights of animals was made shortly before
EAE induction on
study day 0 (study commencement) and thereafter on a daily basis throughout
the entire 21-day
observation period.
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
EAE Clinical Scoring and Assessments. Initially, all animals were examined for
signs of
any neurological responses and symptoms prior to EAE induction (study day 0)
and thereafter
examined on a daily basis throughout the entire 21-day observation period. To
avoid
experimental bias, EAE reactions are determined in a blinded fashion, as much
as possible, by a
staff member unaware of the specific treatment applied. EAE reactions were
scored and
recorded according to a classical, art-recognized conventional 0-5 scale in
ascending order of
severity as shown below in Table 6:
TABLE 6. EAE reactions were scored and recorded according to a classical, art-
recognized conventional 0-5 scale in ascending order of severity.
Grade Si,gns/Symptoms
0 No abnormalities
0.5 Tail weakness distal half
1 Tail weakness proximal half
1.5 Hind paw weakness one paw
2 Hind paw weakness two paws
2.5 Fore paw paralysis one paw
3 Fore paw paralysis two paws
4 Full paralysis
5 Death
Blood Samples. On the day of study termination (day 21), all animals were bled
1 hour
post injection. Samples were collected on study days 0 (prophylactic groups
only), 7, 14, and
21. Plasma was collected in heparinized vials and kept at -20 C. A volume of
300 l was stored
for the blood count analysis and 100pl was stored and used for further
cytokine analysis via
Luminex Technology. Blood counts were analyzed for days 0, 7, 14, and 21.
Tissue Collection. At study termination, the animals were perfused with 4%
PFA.
Brains and spinal cords were collected and kept in 4% PFA.
Humane Endpoints. Animals found in a moribund condition and/or animals showing
severe pain and enduring signs of severe distress were humanely euthanized.
STATISTICS / DATA EVALUATION:
Evaluation was primarily based on the relative recorded changes in both
neurological
symptoms and body weights, expressed as absolute values, percentage (%) change
and mean
group values obtained in all treated groups vs. those of the Vehicle Control.
Analysis of the data
by appropriate statistical methods was applied to determine significance of
treatment effects.
76
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
ANIMAL CARE AND USE STATEMENT:
This study was performed following approval of an application form submitted
to the
appropriate Committee for Ethical Conduct in the Care and Use of Laboratory
Animals that the
study complied with the rules and regulations set forth.
RESULTS:
Results of the study are shown in FIGURE 5, where time (days after MBP
injection) is
shown on the X-axis, and "Clinical scores" (see above under "Materials and
Methods") are
shown on the Y-axis.
Figure 5 shows that the inventive electrokinetic fluid (RHS-60) was
substantially
efficacious in an art-recognized Experimental Autoimmune Encephalomyelitis
(EAE) rat model
of Multiple Sclerosis (MS) (see above under "Materials and Methods").
Specifically, compared to the vehicle control group (filled diamonds) over a
17 day
period, both the therapeutic (daily administration of RNS-60 beginning
concomitant with MBP
injection) and prophylactic (daily administration of RNS-60 beginning seven
days prior to MBP
injection) RNS-60 dosage regimens showed a marked decrease, as well as a
delayed onset (in
the high dose groups) of clinical score.
The clinical score of the low dose (daily one cc injection) RNS-60 therapeutic
group was
approximately one-half (1/2) that of the vehicle control group, while the
clinical score of the
high dose (daily two cc injection) RNS-60 therapeutic group was not only
approximately one-
fifth (1/5) to one-tenth (1/10) that of the vehicle control group, but also
displayed delayed onset.
The clinical score of the low dose (daily one cc injection) RNS-60
prophylactic group
was approximately one-third (1/3) that of the vehicle control group, while the
clinical score of
the high dose (daily two cc injection) RNS-60 prophylactic group was not only
zero (no
detectable clinical score) through day 16, thereby displaying substantially
delayed onset, but
when observable at day 17 was less than one-tenth (1/10) that of the vehicle
control group at the
same time point.
According to particular aspects of the present invention, therefore, the
inventive
electrokinetic compositions have substantial utility for treating, including
alleviating and
preventing, the symptoms of EAE in art-recognized rat models of human MS.
77
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
EXAMPLE 4
(The inventive electrokinetic fluid was shown to be effective in sustaining
the weight of rats in an
art-recognized acute Experimental Allergic (Autoimmune) Encephalomyelitis
(EAE) rat MBP
model of Multiple Sclerosis(MS))
Overview:
This working EXAMPLE discloses the weight change of rats subjected to the
experiment
described in Example 7. Body weight loss can be the first sign of disease
initiation, while a
sudden marked weight gain tends to accompany remission of EAE symptoms.
Therefore,
determination of individual body weights of animals was made shortly before
EAE induction on
study day 0 (study commencement) and on a daily basis throughout the 21-day
observation
period. The effect of the inventive electrokinetic fluid RNS-60 on body weight
was shown to be
effective in sustaining the weight of rats subjected to the EAE rat model
(Figure 7).
Body weight data:
Figure 7 shows the body weight in grams (panel A) and as a percentage (panel
B) based
on 100 grams. After a slight reduction of the mean body weight of in the
animals treated in this
Example, the mean body weight began to increase until study termination. At
study termination,
the mean body weight gain was 20% in the Vehicle treated animals (Group 1F).
Throughout the
study, the Dexamethasone treatment group (Group 2F) which was administered
starting on study
day 0 had 10% mean body weight loss during the study. At study termination,
the
Dexamethasone treated animals lost 2% of mean body weight. The prophylactic,
low dose
treated group (Group 3F) showed up to 4% mean body weight loss on study days 1-
3, and then
gained 23% of the mean body weight by the day of study termination. The
prophylactic, high
dose treated group (Group 4F) showed up to 5% mean body weight loss on study
days 1-3, and
then gained 28% of the mean body weight by the day of study termination. The
therapeutic, low
dosed treated group (Group 5F) showed up to 4% mean body weight loss on study
days 1-3, and
then gained 21% of the mean body weight by the day of study termination. The
therapeutic,
high dose treated group (Group 6F) showed up to 4% mean body weight loss on
Study Days 1-3,
then gained 19% of the mean body weight by the day of study termination.
Thus the inventive electrokinetic fluid RNS-60 was found to be effective in
sustaining
the weight of rats subjected to the EAE rat model.
According to particular aspects of the present invention, therefore, the
inventive
electrokinetic compositions have substantial utility for treating, including
alleviating and
preventing, the symptoms of EAE in art-recognized rat models of human MS.
78
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
EXAMPLE 5
(The inventive electrokinetic fluid was shown to have little effect on the
level of white blood
cells, neutrophils, and lymphocytes in blood samples taken from rat subjected
to the art-
recognized acute Experimental Allergic (Autoimmune) Encephalomyelitis (EAE)
rat MBP model
of Multiple Sclerosis(MS))
Overview:
This working EXAMPLE discloses the level of white blood cells, neutrophils,
and
lymphocytes in blood samples taken from rats during the experiment as
described in Example 7.
To determine whether the change in cytokine levels was due to an overall
change in white blood
cells, Applicants' took blood samples, throughout the experiment, from rats
subjected to the
EAE experiment.
Level of white blood cells, neutrophils, and lymphocytes:
Figures 8 A-D show the levels of white blood cells, neutrophils, and
lymphocytes in
blood samples that were collected throughout the EAE experiment.
White blood cells (WBC), neutrophils and lymphocytes were counted one hour
after the
Test Item was administered on study days 0 (panel A), 7 (panel B), 14 (panel
C) and 21 (panel
D). The maximum WBC count one hour after the animals were treated with Vehicle
on Study
Day 7 was 8.23 0.36 points. Treatment with Dexamethasone significantly reduced
the average
WBC count vs. Vehicle to 2.46 0.38 points (p<0.05). Therapeutic treatment with
the Test Item
at a low dose (Group 5F) significantly increased the average WBC count vs.
Vehicle to
9.59 0.46 points (p<0.1). Therapeutic treatment with the Test Item at a high
dose (Group 6F)
significantly increased the average WBC count vs. Vehicle to 10.84 0.88 points
(p<0.05).
The maximum WBC count one hour after animals were treated with Vehicle on
study
day 14 was 6.34 0.28 points. Treatment with Dexamethasone significantly
reduced the average
WBC count vs. Vehicle to 3.79 0.69 points (p<0.05). Prophylactic treatment
with the Test Item
at the high dose (Group 4F) significantly increased the average WBC count vs.
Vehicle to
7.83 0.51 points (p<0.05). Therapeutic treatment with the Test Item at the low
dose (Group 5F)
significantly increased the average WBC count vs. Vehicle to 7.65 0.52 points
(p<0.05).
Therapeutic treatment with the Test Item at the high dose (Group 6F)
significantly increased the
average WBC count vs. Vehicle to 8.05 0.43 points (p<0.05). The maximum WBC
count one
hour after animals were treated with Vehicle on study day 21 was 9.09 0.75
points. Treatment
79
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
with Dexamethasone significantly reduced the average WBC count vs. Vehicle to
5.12 0.57
points (p<0.05).
The maximum neutrophils count one hour after animals were treated with the
Vehicle on
study day 7 was 26.20 1.62 points. Treatment with Dexamethasone significantly
increased the
average neutrophils count versus vehicle to 65.38 4.62 points (p<0.05).
Prophylactic treatment
with the Test Item at the high dose (Group 4F) significantly increased the
average neutrophils
count versus vehicle to 31.90 0.96 points (p<0.05). Therapeutic treatment with
the Test Item at
the high dose (Group 6F) significantly increased the average neutrophils count
versus vehicle to
33.90 2.79 points (p<0.05).
The maximum Neutrophils count one hour after animals were treated with Vehicle
on
study day 14 was 33.00 2.58 points. Treatment with Dexamethasone significantly
increased the
average neutrophils count vs. Vehicle to 73.10 3.15 points (p<0.05).
The maximum neutrophils count one hour after animals were treated with Vehicle
on
study day 21 was 41.40 2.32 points. Treatment with Dexamethasone significantly
increased the
average neutrophils count vs. Vehicle to 89.33 1.97 points (p<0.05).
Therapeutic treatment
with the Test Item at the high dose (Group 6F) significantly decreased the
average neutrophils
count vs. Vehicle to 34.60 3.08 points (p<O.1).
The maximum lymphocytes count one hour after treated with Vehicle on study day
7
was 73.20 1.95 points. Treatment with Dexamethasone significantly reduced the
average
lymphocytes count vs. Vehicle to 30.63 1.31 points (p<0.05). Prophylactic
treatment with the
Test Item at the high dose (Group 4F) significantly reduced the mean
lymphocytes count vs.
Vehicle to 68.30 1.42 points (p<O. 1). Therapeutic treatment with the Test
Item at the high dose
(Group 6F) significantly reduced the average lymphocytes count vs. Vehicle to
64.80. 3.00
points (p<0.05).
The maximum lymphocytes count one hour after treated with Vehicle on study day
14
was 66.10 2.53 points. Treatment with Dexamethasone significantly reduced the
average
lymphocytes count vs. Vehicle to 26.80 3.23 points (p<0.05).
The maximum lymphocytes count one hour after treated with Vehicle on study day
21
was 57.50 2.09 points. Treatment with Dexamethasone significantly reduced the
average
lymphocytes count vs. Vehicle to 10.11 2.08 points (p<0.05). Therapeutic
treatment with the
Test Item at the high dose (Group 6F) significantly increased the average
lymphocytes count vs.
Vehicle to 66.20 2.74 points (p<0.05).
Thus the inventive electrokinetic fluid RNS-60 administered prophylactically
and
therapeutically at the high dose significantly increased the neutrophils count
and significantly
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
decreased the lymphocytes count versus the Vehicle at study day 7. The
inventive electrokinetic
fluid RNS-60 administered prophylactically at the high dose, and
therapeutically at both doses,
significantly increased the WBC count versus the Vehicle at study day 14. The
Test Item
RNS60 administered therapeutically at the high dose, significantly decreased
the neutrophils
count and increased the Lymphocytes count versus the Vehicle at study day 21.
Thus the
inventive electrokinetic fluid RNS-60 was found to have little effect on the
overall levels of
WBC, neutrophils, and lymphocytes.
EXAMPLE 6
(The inventive electrokinetic fluid was shown to effect the level of certain
cytokines in blood
samples taken from rat subjected to the art-recognized acute Experimental
Allergic
(Autoimmune) Encephalomyelitis (EAE) rat MBP model of Multiple Sclerosis(MS))
Overview:
This working EXAMPLE discloses the level of cytokines as discovered in blood
samples
taken from rats during the experiment as described in Example 7. The inventive
electrokinetic
fluid RNS-60 was evaluated in the therapeutic administration regimens, as
described in Example
7. The inventive electrokinetic fluid RNS-60 was shown to affect the level of
certain cytokines
in blood samples taken from rat subjected to the EAE rat model.
Certain cytokines have been shown to have a role in Multiple Sclerosis. In
particular
interleukin 17 (IL-17), also known as CTLA-8 or IL-17A, has been demonstrated
to have
elevated levels in the central nervous system in acute and chronic EAE
(Hofstetter, H. H., et al.,
Cellular Immunology (2005), 237:123-130). IL-17 is a pro-inflammatory cytokine
which
stimulates the secretion of a wide range of other cytokines from various non-
immune cells. IL-
17 is capable of inducing the secretion of IL-6, IL-8, PGE2, MCP-1 and G-CSF
by adherent
cells like fibroblasts, keratinocytes, epithelial and endothelial cells and is
also able to induce
ICAM-1 surface expression, proliferation of T cells, and growth and
differentiation of CD34+
human progenitors into neutrophils when cocultured in presence of irradiated
fibroblasts
(Fossiez et al., 1998, Int.Rev.Immunol. 16, 541-551). IL-17 is predominantly
produced by
activated memory T cells and acts by binding to a ubiquitously distributed
cell surface receptor
(IL-17R) (Yao et al., 1997, Cytokine, 9, 794-800 ). A number of homologues of
IL-17 have
been identified which have both similar and distinct roles in regulating
inflammatory responses.
For a review of IL- 17 cytokine/receptor families see Dumont, 2003, Expert
Opin. Ther. Patents,
13, 287-303.
81
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
IL-17 may contribute to a number of diseases mediated by abnormal immune
responses,
such as rheumatoid arthritis and air-way inflammation, as well as organ
transplant rejection and
antitumour immunity. Inhibitors of IL- 17 activity are well known in the art,
for example an IL-
17R:Fc fusion protein was used to demonstrate the role of IL-17 in collagen-
induced arthritis
(Lubberts et al., J.Immunol. 2001,167, 1004-1013) and neutralising polyclonal
antibodies have
been used to reduce peritoneal adhesion formation (Chung et al., 2002,
J.Exp.Med., 195, 1471-
1478). Neutralising monoclonal antibodies are commercially available (R&D
Systems UK).
Thus based on the role IL-17 plays in the pathogenesis of MS, Applicants'
examined the
effect that inventive electrokinetic fluid RNS-60 had on levels of IL-17 in
blood samples taken
from rats in the EAE study.
Cytokine data:
Levels of various cytokines in the blood were analyzed during the study. In
brief, all
animals were bled 1-hour post injection and plasma was collected in
heparinized vials. 100 l
samples were analyzed for various inflammatory cytokines by Luminex technology
(using
Procarta rat cytokine assay kit PC4127 from Panomics) which enables
measurement of multiple
cytokines from the same sample, simultaneously. Due the non-Gaussian
distributed data and
occasional results below the assay detection threshold, the nonparametric Cox
regression model
for censored data was adapted to compare the different fluids. As show in
Figures 9 A-H, levels
of ILla, ILlb, and IL17 were most notably reduced by both therapeutic
treatment doses (high
and low) of RNS60. Clinical manifestation of MBP induced EAE starts around day
10 and
peaks around day 18. Hence, we considered the day 7 (just prior to disease
manifestation) and
day 18 (around the peak of the disease) to be the most important time points
for cytokine
analysis. Systemic levels of ILla, ILlb and IL17 on days 7 and 18, from 10
animals/group are
presented in Figures 9 A-H. ...
IL-1 is one of the major pro-inflammatory cytokines and is an upstream
mediator of the
innate immune responses. IL-1 induces the production of various growth and
trophic factors,
inflammatory mediators, adhesion molecules and other cytokines directly and
indirectly, as well
as using a positive feedback loop (A. Basu et al., The type 1 interleukin-1
receptor is essential
for the efficient activation of microglia and the induction of multiple
proinflammatory mediators
in response to brain injury, J. Neurosci. 22 (2002), pp. 6071-6082; P.N.
Moynagh, The
interleukin-1 signaling pathway in astrocytes: a key contributor to
inflammation in the brain, J.
Anat. 207 (2005), pp. 265-269). These include important modulators such as
NGF, ICAM 1,
IL6, TNFa, CSF etc. The progression of MS involves the activation of auto-
antigen-reactive T
82
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
cells in the periphery, followed by invasion into the CNS. IL-1 is crucial in
the development of
MS as they participate not only in myelin-specific T cell activation but also
represent the main
mediator of macrophage activation in the periphery [R. Furlan et al., HSV-1-
mediated IL-1
receptor antagonist gene therapy ameliorates MOG(35-55)-induced experimental
autoimmune
encephalomyelitis in C57BL/6 mice, Gene 7-her. 14 (2007), pp. 93-98)). In EAE
models for
MS, both IL-la and IL-10 have been shown to be mediators of the inflammatory
process.
Peripheral levels of IL-1 P correlate with the clinical course and IL-10
reactivity has been shown
during EAE in CNS-infiltrating macrophages and in resident microglial cells
((C.A. Jacobs et
al., Experimental autoimmune encephalomyelitis is exacerbated by IL-1 alpha
and suppressed
by soluble IL-1 receptor, J. Immunol. 146 (1991), pp. 2983-2989)). Therefore,
IL-1 is a suitable
therapeutic target in EAE and MS. A non-selective inhibitory mechanism of IL-1
has been
shown in existing therapeutic agents for MS; that is interferon beta, anti-
inflammatory
glucocorticoids, immunosuppressants, atorvastatin and omega-3 polyunsaturated
fatty acids [
F.L. Sciacca et al., Induction of IL-1 receptor antagonist by interferon beta:
implication for the
treatment of multiple sclerosis, J. Neurovirol. 6 (Suppl. 2) (2000), pp. S33-
S37. ; R. Pannu et
al., Attenuation of acute inflammatory response by atorvastatin after spinal
cord injury in rats, J.
Neurosci. Res. 79 (2005), pp. 340-350; A.P. Simopoulos, Omega-3 fatty acids in
inflammation
and autoimmune diseases, J. Am. Coll. Nutr. 21 (2002), pp. 495-505)). As
demonstrated in
Figure 9 C-F, IV administration of RNS60 effectively lowers the systemic
levels of both ILla
and IL1(3. For ILla, RNS60treatment lowered the blood level significantly
compared to the
vehicle treated group, and was as effective as dexamethasone at this time
point. However at the
18 day time point, the treatment has no significant effect on the ILla
systemic level. Systemic
levels of IL1(3 were also reduced significantly after 7 days of IV treatment
of RNS60, to the
levels comparable to the dexamethsone treatment groups, without any sign of
toxic side effects.
Although the same trend was noted at the 18 day time point, the differences
were not statistically
significant when compared to the control group.IL-17 is a also crucial
effector cytokine with
potent proinflammatory effects. It induces the expression of other
proinflammatory cytokines
such as tumor necrosis factor-a and chemokines, attracts neutrophilic
leukocytes, and enhances
the maturation of dendritic cells (Kolls JK, Linden A.Interleukin-17 family
members and
inflammation.Immunity. 2004 Oct;21(4):467-76). IL-17-producing cells are
thought to be
essential inflammatory mediators in autoimmune diseases such as collagen-
induced arthritis,
colitis, psoriasis, and EAE. T helper17 cells in EAE are CD4+ cells and they
are present both in
the immune periphery and in the inflamed central nervous system in EAE.
Moreover,
neutralization of IL-17 ameliorates clinical disease, a finding that is
paralleled by reduced EAE
83
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
severity in IL- 17-deficient animals ((from Gold and Liihder, Interleukin-17-
Extended Features
of a Key Player in Multiple Sclerosis Am J Pathol. 2008 January; 172(1): 8-
10.). 7 day IV
treatment with RNS60 caused a significant reduction in IL17 levels in blood,
once again to a
level similar to dexamethasone treated animals. The same was followed even
after 18 days of
treatment although the results were not statistically significant. It is
important to note that
RNS60 is effective not only in lowering the IL1 levels but the combination of
the two key
cytokines in EAE, IL1 and IL17 with no notable toxic side effects even after
21 days of IV
injections.
In addition to IL1 and IL17, a number of other molecules that play critical
role in
inflammation of the nervous system are also modulated by RIS60. These include
Rantes, KC,
NGF and ICAM (data not shown).
Thus the inventive electrokinetic fluid RNS-60 had a significant effect on
levels of IL-17
in blood samples taken from rats in the EAE study. In addition, since IL- 17
stimulates the
secretion of IL-6, IL-8, PGE2, MCP-1 and G-CSF, it seems likely that the
inventive
electrokinetic fluid RNS-60 would have a significant effect on the level of
these cytokines in
blood. According to particular aspects of the present invention, therefore,
the inventive
electrokinetic compositions have substantial utility for treating, including
alleviating and
preventing, the symptoms of EAE in art-recognized rat models of human MS.
EXAMPLE 7
(The inventive electrokinetic fluid (e.g., RNS60) was shown to inhibit the
expression of both
iNOS and IL-1/i in a dose-dependent manner in microglial cells)
Overview:
According to particular aspects as described herein, the inventive
electrokinetic fluids
have substantial utility for treating Parkinson's disease (PD).
Parkinson's disease (PD) is one of the most devastating neurodegenerative
disorders in
humans. PD may appear at any age, but it is uncommon in people younger than
30. Clinically,
PD is characterized by tremor, bradykinesia, rigidity and postural
instability. Pathologically, it
is indicated by gliosis and progressive degeneration of the dopaminergic
neurons associated with
the presence of intracytoplasmic inclusions (Lewy bodies) in the substantia
nigra pars compacta
(SNpc). In postmortem PD brain, dying neurons have been reported to display
morphological
characteristics of apoptosis, including cell shrinkage, chromatin
condensation, and DNA
fragmentation. Therefore, development of effective neuroprotective therapeutic
approacheso
84
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
halt the disease progression is of paramount importance. The MPTP mouse model
has
substantial utility for testing and validating therapeutic approaches against
PD.
Microglial activation plays an important role in the pathogenesis of
Parkinson's disease
(PD) as well as other neurodegenerative disorders. Particular features of PD
are modeled in 1-
methyl-4- phenyl- 1,2,3,6-tetrahydropyridine (MPTP)-intoxicated animals. The
neurotoxic effect
of MPTP depends on its conversion into MPP+. In glial cells, monoamine oxidase
B (MAO-B)
converts MPTP to MPP+, which then activates glial cells, and recently, it has
been shown that
MPP+ induces the expression of proinflammatory molecules in microglia. In
addition, MPP+
causes apoptosis of dopaminergic neurons.
In this working EXAMPLE, the ability of RNS60 to modulate the expression of
proinflammatory molecules in MPP+-stimulated microglial cells was confirmed.
Materials and Methods:
Briefly, mouse BV-2 microglial cells were incubated with different
concentrations of
RNS60 and normal saline (NS) for 1 hour followed by stimulation with 2 gM MPP+
under
serum-free conditions. After 6 hours, total RNA was isolated and mRNA of iNOS
and IL-1(3
was measured by semi-quantitative RT-PCR. Data are representative of three
independent
experiments.
RESULTS:
As evidenced by semi-quantitative RT-PCR analysis in Figure 10, MPP+ alone
induced
the expression of inducible nitric oxide synthase (iNOS) and interleukin-1P(IL-
lei) mRNAs in
mouse BV-2 microglial cells. Signficantly, RNS60 inhibited the expression of
both iNOS and
IL-10 in a dose-dependent manner in microglial cells (Figure 10). By contrast,
under similar
experimental condition, the normal saline control (NS) had no effect on the
expression of these
two proinflammatory genes (Figure 10) indicating the specificity of the
effect.
Specifically, Figure 10 shows that the inventive electrokinetic fluid (RNS-
60), but not
control normal saline (NS), attenuates MPP+-induced expression of inducible
nitric oxide
synthase (iNOS) and interleukin-1(3 (IL-10) in mouse microglial cells. BV-2
microglial cells
preincubated with different concentrations of RNS60 and normal saline (NS) in
serum-free
media for 1 h were stimlated with MPP+(a Parkinsonian toxin). After 6 h of
stimulation, total
RNA was isolated and the mRNA expression of iNOS and IL-10 was analyzed by
semi-
quantitative RT-PCR. Results represent three independent experiments.
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
According to particular aspects therefore, because MPP+ is a Parkinsonian
toxin, these
results indicate that RNS60 has a protective effect in an art-recognized MPTP-
induced mouse
model of Parkinson's disease.
According to particular aspects, the inventive electrokinetic fluids have
substantial utility
for treating Parkinson's disease (PD).
EXAMPLE 8
(The inventive electrokinetic fluid (e.g., RNS60) was shown to protect nerve
cells and primary
human neurons from amyloid-fi toxicity)
Overview:
According to particular aspects as described herein, the inventive
electrokinetic fluids
have substantial utility for treating Alzheimer's disease (AD).
Alzheimer's disease (AD) is a neurodegenerative disorder resulting in
progressive
neuronal death and memory loss. Increased TUNEL staining in postmortem AD
brains indicates
that neurons in the brains of AD patients die through apoptosis. Fibrillar
amyloid-(3 peptides
participate in the pathophysiology of AD. Neuropathologically, the disease is
characterized by
neurofibrillary tangles and neuritic plaques composed of aggregates of 0-
amyloid (A(3) protein, a
40-43 amino acid proteolytic fragment derived from the amyloid precursor
protein, and
phosphorylated tau. It has been found that over-expression of the A(3 peptides
intracellularly in
transgenic mice causes chromatin segmentation, condensation, and increased
TUNEL staining.
Cell culture studies have also shown that A(3 peptides are apoptotic and
cytotoxic to neuronal
cells, and It has been shown that fibrillar A(31-42 peptides are capable of
inducing apoptosis in
neuronal cells.
Additionally, studies are increasingly being directed at characterizing the
link between
inflammation and AD, and widespread glial activation has been found around
plaques and
tangles.
In this EXAMPLE, the effect of RNS60 in blocking A(3(1-42)-induced apoptosis
in
human SHSYSY nerve cells and primary human neurons was confirmed.
Materials and Methods:
Fragmented DNA of SHSSY human neuronal cells was detected in situ by the
terminal
deoxynucleotidyltransferase (TdT)- mediated binding of 3'-OH ends of DNA
fragments
generated in response to fibrillar A(31-42, using a commercially available kit
(TdT FragELTM)
from Calbiochem. Briefly, cover slips were treated with 20 g/ml proteinase K
for 15 min at
86
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
room temperature and washed prior to TdT staining. Neurons were isolated as
described
previously and cultured (1,2).
RESULTS:
As demonstrated in Figures 11A and B, fibrillar A(31-42 peptides markedly
induced the
formation of apoptotic bodies in neuronal cells. We also observed loss of
neuronal processing
after A(31-42 treatment (2"d row; Figure 11A). In contrast, reverse peptides
A042-1 were unable
to induce neuronal apoptosis and loss ofprocesses (3rd row; Figure 11A).
Significantly, RNS60
at different doses tested markedly blocked AP (1-42)-induced apoptosis and
preserved processes
in neuronal cells (4`h, 5th & 6th rows; Figures 11 A and B). By contrast,
normal saline control
fluid (NS) had no effect on A(3(1-42)-induced apoptosis and loss of processes
(7th & 8th rows;
Figure 11A).
Specifically, Figure 11A shows that RNS60, but not normal saline control (NS),
suppresses fibrillar A(3(1-42)-mediated apoptosis of human SHSYSY neuronal
cells. After
differentiation, SHSYSY cells were incubated with different concentrations of
either RNS60 or
NS for 1 h followed by insult with 1 pM fibrillar A(3(1-42) peptides. After 18
h of treatment,
apoptosis was monitored by TUNEL (Calbiochem). A(3(42-1) peptides were also
incubated as
control. Results represent three independent experiments.
In addition, Figure 11B, 2"d and 3rd row shows that RNS60 suppresses fibrillar
Af3(1-42)-
mediated apoptosis of primary human neurons. Neurons were incubated with RNS60
for 1 h
followed by insult with 1 pM fibrillar Af3(1-42) peptides. After 18 h of
treatment, apoptosis was
monitored by TUNEL (Calbiochem). A(3(42-1) peptides were also incubated as
control. Results
represent three independent experiments.
These results indicate that the etiological reagent of AD (fibrillar A(31-42)
induces
apoptosis in neurons via an RNS60-sensitive pathway and that RNS60 can
strongly inhibit
fibrillar induced apoptosis in both cultured and primary neurons.
According to particular aspects, the inventive electrokinetic fluids have
substantial utility
for treating Alzheimer's disease (AD), and in preferred aspects, preventing or
slowing
progression of AD.
35
87
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
EXAMPLE 9
(The inventive electrokinetic fluid was shown to be substantially efficacious
in suppressing
clinical score in a dose-responsive manner in an art-recognized mouse MOG
model of Multiple
Sclerosis (MS))
Overview:
In this working EXAMPLE, the inventive electrokinetic fluid RNS-60 was
evaluated at
two doses, in therapeutic administration regimens, in an art-recognized
experimental allergic
encephalomyelitis (EAE) mouse MOG model of Multiple Sclerosis (MS).
Materials and Methods:
Experimental allergic encephalomyelitis (EAE) is a central nervous system
(CNS)
autoimmune demyelinating disease that mimics many of the clinical and
pathologic features of
multiple sclerosis (MS). The MOG murine model consists of a sensitization
period, induced by
the single subcutaneous (SC) injection of MOG emulsified in complete Freund's
adjuvant
(CFA) on study day 0 (200 gg MOG / 300 gg CFA injected at a total dose volume
of 200
l/animal delivered as 2 X 100 l subcutaneous bilateral injections over the
paralumbar region);
followed by intraperitoneal (IP) supplemental immunostimulation with pertussis
toxin (PT) at 20
g/kg (approximately 400 ng/mouse) via intraperitoneal (IP) injection once at
the time of EAE
induction on study day 0 and again, 48 hours later on study day 2 (Gilgun-
Sherki Y. et al.,
Neurosciences Research 47:201-207, 2003). Animals were then treated with RNS60
IV
infusion at indicated in Figure 12. Animals used were Female C57BL/6J mice
from Harlan
Laboratories Israel, Ltd. (10 animals/group); young adults; 8-9 weeks old at
study initiation.
All the animals were examined for signs of neurological responses and symptoms
prior
to EAE induction (study day 0) and thereafter examined on a daily basis
throughout the 35-day
observation period. EAE reactions were scored and recorded according to the
art-recognized 0-
15 scale in ascending order of severity. The clinical score was determined by
summing the
score of each section (see, e.g., Weaver et al., FASEB 2005; The FASEB Journal
express article
10.1096/fj.04-2030fje. Published online August 4, 2005. ).
RESULTS:
Figure 12 shows that RNS60, but not Vehicle control (Vehicle), is
substantially
efficacious in suppressing clinical score in a dose-responsive manner in an
art-recognized mouse
MOG model of Multiple Sclerosis(MS). Both high and low dose therapeutic daily
administration of RNS-60, as well as the high dose administration of RNS-60
every three days
(administration or RNS-60 in all instances beginning concomitant with first
clinical signs),
showed a marked decrease of clinical score (open diamonds = Vehicle control;
open squares =
88
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
dexamethasone positive control; light "x"s = low dose (0.09 ml RNS60) daily
administration
from onset of clinical signs; dark "x"s = high dose (0.2 ml RNS60)
administration every three
days from onset of clinical signs; and open triangles = high dose (0.2 ml
RNS60) daily
administration from onset of clinical signs).
In comparison with the MBP model of Example herein above, this mouse MOG model
is
known in the art for its ability to mimic the characteristic axonal damage of
MS which the MBP
model does not show, and extends the observed therapeutic efficacy over longer
periods (28-30
days compared to 21 days with the MBP model). According to further aspects,
RNS60, but not
Vehicle control (Vehicle), is substantially efficacious in reducing axonal
damage in this mouse
MOG model.
According to particular aspects of the present invention, the inventive
electrokinetic
compositions have substantial utility for treating, including alleviating and
preventing,
symptoms in an art-recognized mouse model of human MS. According to further
aspects of the
present invention, the inventive electrokinetic compositions have substantial
utility for treating,
including alleviating and preventing, the symptoms of MS in afflicted mammals
(preferably
humans).
In yet further aspects, the inventive electrokinetic compositions cross the
Blood Brain
Barrier (BBB), and thus provide a novel method for treating inflammatory
conditions of the
central nervous system.
EXAMPLE 10
(RNS60, but not normal saline (NS), attenuated the activation of NFKB in MBP-
primed T cells)
Overview. NF--KB kinase is a kinase widely recognized in the art as mediating
inflammatory responses in inflammation-mediated conditions and diseases.
This Example shows that RNS60, but not normal saline (NS), attenuated the
activation
of NFxB in MBP-primed T cells. According to particular aspects, therefore, the
present
electrokinetically-generated fluids have substantial utility for treating
inflammation and
inflammation-mediated conditions and diseases, including but not limited to,
diabetes and
related metabolic disorders, insulin resistance, neurodegenerative diseases
(e.g., M.S.,
Parkinson's, Alzheimer's, etc), asthma, cystic fibrosis, vascular/coronary
disease, retinal and/or
macular degeneration, digestive disorders (e.g., inflammatory bowel disease,
ulcerative colitis,
Crohn's, etc.).
89
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
Methods. For the experiments shown in Figures 13 A and 13 B, T cells isolated
from
MBP-immunized mice were re-primed with MBP and after 24 h, cells received
different
concentrations of RNS60 and NS. After 2 h of treatment, DNA-binding activity
of NF-KB was
monitored in nuclear extracts by electrophoretic mobility shift assay (EMSA).
For experiments shown in Figure 13 C, T cells isolated from MBP-immunized mice
were
transfected with PBIIX-Luc, an NF-KB dependent reporter construct, followed by
repriming
with MBP. After 24 h of MBP priming, cells were treated with different
concentrations of
RNS60 and NS for 2 h followed by assay of luciferase activity in total cell
extracts by a
luciferase assay kit (Promega). In other cases, MBP-primed T cells were also
stimulated with 30
nM PMA for 1 h. In these cases, PMA was added after 1 h of pretreatment with
RNS60 and NS.
Results are mean + SD of three different experiments.
Results. Figures 13 A-C show that RNS60, but not normal saline (NS),
attenuated the
activation of NF-xB in MBP-primed T cells. Specifically, Figures 13 A and 13 B
show that
RNS60 (see middle three lanes of Figures 13 A and 124 B), but not NS (see
right-most lane of
Figures 13 A and 13 B), attenuated the activation of NF--KB in MBP-primed T
cells in a dose-
responsive manner.
Likewise, the bar graph of Figure 13 C shows that that RNS60 (see second,
third and
fourth bars of Figures 13 A and 13 B), but not NS (see fifth bar of Figures 13
A and 13 B),
attenuated the activation of NF-KB in MBP-primed T cells, and hence also
attenuated luciferase
activity from the transfected NF-KB-dependent reporter construct (PBIIX-Luc)
in total cell
extracts, in a dose-responsive manner.
According to particular aspects, therefore, the disclosed electrokinetically-
generated
fluids have substantial utility for treating inflammation and inflammation-
mediated conditions
and diseases, including but not limited to, diabetes and related metabolic
disorders, insulin
resistance, neurodegenerative diseases (e.g., M.S., Parkinson's, Alzheimer's,
etc), asthma, cystic
fibrosis, vascular/coronary disease, retinal and/or macular degeneration,
digestive disorders
(e.g., inflammatory bowel disease, ulcerative colitis, Crohn's, etc.).
EXAMPLE 11
(RNS60, but not normal saline (NS), attenuated the MPTP induced pathological
signs of
Parkinson's disease in mice)
Overview:
Mice can be induced to exhibit pathological signs of Parkinson's disease (PD)
(e.g.,
reduction in movement time, reduction in movement distance, lower ability to
balance on a
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
rotation rod, tremors, and loss of the striatum-controlled behavioral patterns
stereotypy and
rearing (vertical movements)) by treating them with 1-methyl-4- phenyl-1,2,3,6-
tetrahydropyridine (MPTP). The neurotoxic effect of MPTP depends on its
conversion into
MPP+. In glial cells, monoamine oxidase B (MAO-B) converts MPTP to MPP+, which
then
activates glial cells, and recently, it has been shown that MPP+ induces the
expression of
proinflammatory molecules in microglia. In addition, MPP+ has been shown to
cause apoptosis
of dopaminergic neurons.
In this working EXAMPLE, the ability of RNS60 to reduce the pathological
symptoms
of PD (e.g., improve the coordinated movements, prevent the loss of straitum
dependent
behaviors, and rescue of dopaminergic neurons) in MPTP-treated mice was
confirmed.
Materials and Methods:
Briefly, C57BL/6 mice received four intraperitoneal injections of MPTP-HC1 (18
mg/kg
of free base) in saline at 2-hour intervals. Control animals received the same
volume of saline.
Treatment with RNS60 or normal saline (NS) started 1 day before the MPTP
intoxication.
Locomotor activity was measured 7 days after the MPTP injections with a
computer-assisted
Digiscan infrared activity monitor (Figures 14 and 15). Data are presented as
mean SEM, P
values were calculated by ANOVA; * = P < .05, ** = P < .01, *** = P < .001, ns
= not
significant.
For the experiments verifying that RNS60 treatment rescues dopaminergic
neurons in
mice intoxicated with MPTP, the striatum was dissected 7 days after the MPTP
intoxication
(Figure 16). The presence of dopaminergic neurons in the substantia nigra pars
compacta was
detected by immunostaining with an antibody to tyrosine hydroxylase, the rate-
limiting enzyme
involved in dopamine synthesis. Panel A shows the striatum from the control
mouse = healthy
control mouse not intoxicated with MPTP, Panel B shows the striatum from the
MPTP = MPTP-
challenged mouse, Panel C shows the striatum from the MPTP + RNS60 = MPTP-
challenged
mouse that was treated with RNS60.
RESULTS:
As evidenced by the locomotion analysis in Figure 14 and 15, MPP+ alone
induced PD-
like symptoms in the subjects, including reducing the movement time (Figure
14A), distance
(Figure 14B), the ability of the mice to keep their balance on a rotating rod
(Figure 14C), loss of
the striatum-controlled behavioral patterns stereotypy (grooming) (Figure
15A), and rearing
91
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
(vertical movements) (Figure 15B). Signficantly, RNS60 substantially
alleviated these
symptoms and in some coordinated movement experiments the mice behavior was
similar to the
control mice. By contrast, under similar experimental conditions, mice pre-
treated with the
normal saline control (NS) and then induced with MPP+ had similar symptoms as
MPP+
treatment alone (Figures 14 and 15). Thus, these data indicate that RNS60 had
a specific
protective effect on the MPP+-intoxicated mice.
Thus, Figures 14 and 15 show that the inventive electrokinetic fluid (RNS-60),
but not
control normal saline (NS), improves coordinated movements and prevents the
loss of striatum-
dependent behaviors of mice in a mouse model of PD.
Furthermore, immunostaining in the substantia nigra pars compacta, the part of
the brain
predominantly affected in PD, revealed a notable rescue of dopaminergic
neurons in mice
treated with RNS60 (Figure 16), confirming the neuroprotective activity of the
treatment. As
can be seen in Figure 16, MPP+ intoxication led to the loss of tyrosine
hydroxylase (TH)-
positive neurons and the pre-treatment of RNS60 protected TH-positive neurons
in the
substantia nigra pars compacta (SNpc).
In addition, quantitation of striatal TH immunostaining of all groups of mice
(n=6 per
group) will be performed as described previously (1, 2). Optical density
measurements will be
obtained by digital image analysis (Scion). Striatal TH optical density
basically reflects
dopaminergic fiber innervation.
According to particular aspects therefore, because MPP+ is a neurotoxin, these
results
indicate that RNS60 has a protective effect from neurotoxins. According to
further particular
aspects, because MPP+ is a dopaminergic neurotoxin, these results indicate
that RNS60 has a
protective effect from dopaminergic neurotoxins.
According to particular aspects, the inventive electrokinetic fluids have
substantial utility
for preventing neurotoxic symptoms resulting from exposure to a neurotoxin.
References cited in the above section:
1. Ghosh, A., Roy, A., Liu, X., Kordower, J.H., Mufson, E.J., Mosely, R.L.,
Ghosh, S.,
Gendelman, H.E. & Pahan, K. 2007. Selective inhibition of NF-KB activation
prevents
dopaminergic neuronal loss in a mouse model of Parkinson's disease. Proc.
Natl. Acad. Sci.
U.S.A. 104: 18754-18759.
92
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
2. Ghosh, A., Roy, A., Matras, J., Brahmachari, S., Gendelman, H.E., & Pahan,
K. 2009.
Simvastatin inhibits the activation of p21ra, and prevents the loss of
dopaminergic neurons in a
mouse model of Parkinson's disease. J. Neurosci. 29: 13543 - 13556.
EXAMPLE 12
(RNS60, but not normal saline (NS), suppresses the MPTP induced expression of
microglial
iNOS in vivo in the substantia nigra pars compacta (SNpc))
Overview:
According to particular aspects as described herein, the inventive
electrokinetic fluids
have substantial utility for protecting neural cells from neurotoxins.
Mice can be induced to exhibit pathological signs of Parkinson's disease (PD)
by treating
them with 1-methyl-4- phenyl-1,2,3,6-tetrahydropyridine (MPTP). The neurotoxic
effect of
MPTP depends on its conversion into MPP+. In glial cells, monoamine oxidase B
(MAO-B)
converts MPTP to MPP+, which then activates glial cells, and recently, it has
been shown that
MPP+ induces the expression of proinflammatory molecules in microglia. In
addition, MPP+
causes apoptosis of dopaminergic neurons.
In the working EXAMPLES 7 and 11, the ability of RNS60 to inhibit MPP+-induced
expression of inducible nitric oxide synthase (iNOS) and IL-lei in microglial
cells and protect
striatal dopaminergic neurons and improve locomotor activities in MPTP mouse
model of PD
was shown. Additional experiments are conducted to a) examine the effect of
RIS60 on
microglial iNOS in vivo in the substantia nigra pars compacta (SNpc) of MPTP-
intoxicated
mice.
Materials and Methods:
Male C57BL/6 mice (n=3 in each group) receiving RIS60 or IS (300 l/d/mouse
via i.p.
injection) one day prior to MPTP intoxication will receive four MPTP
injections every 2 h
interval. Treatment with RIS60/IS will continue and after 1 d of MPTP
intoxication, mice are
killed, and their brains are fixed, embedded, and processed for iNOS
immunostaining as
described previously (1,2). Briefly, ventral midbrain sections of all groups
of mice (Saline,
MPTP, MPTP-RIS60-300 l, MPTP-IS-300 l) undergo free-floating double-
imunolabeling
with antibodies against iNOS and CD1lb (for microglia) as described (1-3).
93
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
CD 1 lb-positive, iNOS-positive, and cells, which are positive for both CD 1
lb and iNOS
will be counted using the "Microsuite Biological Suite" software in Olympus
IX81 fluorescent
microscope to determine whether microglial activation and expression of iNOS
is reduced in
SNpc of RIS60-treated MPTP-intoxicated mice compared to that of control MPTP
mice and IS
(Vehicle) -treated MPTP mice. Six nigral sections of each brain isolated from
each of three
animals are used to determine the effect of RIS60 on protein levels of CDIlb
and iNOS in the
SNpc of MPTP-treated mice.
RESULTS:
According to certain embodiments, RNS60 but not normal saline suppresses the
MPTP
induced expression of microglial iNOS in vivo in the substantia nigra pars
compacta (SNpc)).
Thus these in vivo experiments confirm the results seen in Example 7, wherein
semi-quantitative
PCR showed that RNS60 but not normal saline suppresses the MPTP induced
expression of
iNOS in mouse microglial cells.
References cited in the above section:
1. Ghosh, A., Roy, A., Liu, X., Kordower, J.H., Mufson, E.J., Mosely, R.L.,
Ghosh, S.,
Gendelman, H.E. & Pahan, K. 2007. Selective inhibition of NF-KB activation
prevents
dopaminergic neuronal loss in a mouse model of Parkinson's disease. Proc.
Natl. Acad. Sci.
U.S.A. 104: 18754-18759.
2. Ghosh, A., Roy, A., Matras, J., Brahmachari, S., Gendelman, H.E., & Pahan,
K. 2009.
Simvastatin inhibits the activation of p2lra, and prevents the loss of
dopaminergic neurons in a
mouse model of Parkinson's disease. J. Neurosci. 29: 13543 - 13556.
3. Roy, A. & Pahan, K. 2010. Prospects of statins in Parkinson's disease.
Neuroscientist 16:
000-000.
EXAMPLE 13
(RNS60, but not normal saline (NS), induced the activation of Akt
phosphorylation in primary
neurons and in astrocytes)
Overview. Akt is a serine/threonine protein kinase that plays a key role in
multiple
cellular processes including glucose metabolism, cell proliferation,
apoptosis, transcription and
cell migration. Akt is known to regulate cellular survival. In particular,
phosphorylated Akt has
94
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
been shown to inhibit apoptosis by inactivating BAD (a pro-apoptoic protein).
(See, Song G, et
al., (2005). "The activation of Akt/PKB signaling pathway and cell survival".
J. Cell. Mol. Med.
9 (1): 59-71.) Phosphorylation of Akt, as recognized in the art, is a major
player in protecting
cells, including neural cells, from toxic and pro-apoptotic stimuli.
In this working EXAMPLE, the ability of RNS60 to induce phosphorylation of Akt
in
primary neural cells and astrocytes was confirmed. Further, the role of Akt in
RNS60's ability
to block apoptosis was demonstrated.
Materials and Methods:
Neurons were isolated as described previously and cultured (1,2). Astrocytes
were
isolated and cultured as described previously. Neurons or astrocytes were
treated with 10%
RIS60 or IS (used as a control) for 0', 15', 30', 60', 90', 120', & 180' and
activation of Akt was
monitored by western blot of cell extracts with antibodies against phospho-Akt
and normal Akt
(Cell Signaling). Total Akt was detected by antibodies against normal Akt.
Neurons or
astrocytes were treated with different doses RIS60 (2%, 5%, 10%, & 20%).
Different doses of
IS were used as control. Activation of Akt was monitored as described above.
Figure 17 A shows the results from an experiment examining the effects of
RNS60,
compared with normal saline (NS) control, on inducing the phosphorylation of
Akt in primary
neurons. Akt phosphorylation was monitored by double-label immunofluorescence
using
antibodies against (3-tubulin and phospho-Akt. Beta-tubulin was used as a
marker for neurons
and DAPI staining was used to visualize the nucleus of cells. Panels B and C
shows that Akt
phosphorylation was induced by 10% RNS60, whereas contol normal saline ("NS")
had no
effect.
Figure 17B shows the results from an experiment examining the effects of
inhibiting
Akt in primary neurons in the presence and absence of RNS60. Fibrillar A(31-42
(Bachem
Biosciences) was changed into the fibrillar form as described previously
(1,3). The function of
phosphorylated Akt in neurons was inhibited by AktI (a specific inhibitor of
Akt obtained from
Calbiochem). Neurons preincubated with different concentrations of AktI for 30
min were
treated with RNS60. After 1 h of incubation, cells were challenged with
fibrillar Abl-42. After
12 h, neuronal apoptosis was monitored by TUNEL and after 24 h, cell death was
assessed by
MTT and LDH release as described previously (1,2). The results (Figure 17B)
showed that the
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
Akt inhibitor, AktI, abrogated the protective effect of RNS60 on fibrillar Ab-
challenged
neurons.
The results confirmed, therefore, that RNS60 requires Akt to protect neurons
from Ab
toxicity. Figure 18 shows the results from a time course (0 minutes, 15
minutes, 60 minutes, and
120 minutes) experiment examining the effects of RNS60, compared with normal
saline (NS)
control, on inducing the phosphorylation of Akt in primary neurons. The graph
represents the
ratio between the amount of phosphorylated Akt to the total amount of Akt
present in astrocytes
when treated with either RNS60 or normal saline. As can be seen in figure 18,
RNS60 induces a
four-fold increase in Akt phosphorylation in astrocytes when compared to the
effects of normal
saline (NS). Thus, RNS60 specifically induces Akt phosphorylation.
According to particular aspects as described herein and not being bound by any
particular mechanism, the inventive electrokinetic fluids have substantial
utility for protecting
neural cells from neurotoxins by preventing apoptosis induced by exposure to
toxins.
References cited in the above section:
1. Jana, A. & Pahan, K. 2004. Fibrillar amyloid-(3 peptides kill human primary
neurons via
NADPH oxidase-mediated activation of neutral sphingomyelinase: Implications
for Alzheimer's
disease. J. Biol. Chem. 279: 51451-51459.
2. Jana, A. & Pahan, K. 2004. HIV-1 gp120 induces apoptosis in human primary
neurons
through redox-regulated activation of neutral sphingomyelinase. J. Neurosci.
24: 9531-9540.
EXAMPLE 14
(RNS60, but not normal saline (NS), attenuated fibrillar Af 1-42 peptide
induced Tau
phosphorylation in primary neurons)
Overview. Hyperphosphorylation of Tau is a hallmark of tangles in brain and
neuronal
tissue and can lead to one of several diseases which grouped together are
known as taupathies.
Taupathies include, but are not limited to Alzheimer's disease, argyorphilic
grain disease,
frontotemporal dementia, progressive supranuclear palsy, corticobasal
degeneration,
frontotemporal lobar degeneration (Pick's disease), and Dementia pugilistica
(DP) (a.k.a.,
boxer's dementia, chronic boxer's encephalopathy).
96
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
Figures 19 A-B show the results from an experiment examining the effects of
RNS60
compared with normal saline (NS) control, on fibrillar A(3(1-42)-mediated tau
phosphorylation
in primary neurons. Tau phosphorylation was monitored by double-label
immunofluorescence
using antibodies against 0-tubulin and phospho-tau. Beta-tubulin was used as a
marker for
neurons and DAPI staining was used to visualize the nucleus of cells. The
third and fourth
panels from the top in the column labeled "(p)-Tau", shows that Tau
phosphorylation was
inhibited by RNS60 in a dose-dependent manner, whereas contol normal saline
("NS") had no
effect, even at the high dose of 10% (see bottom panel the column labeled "(p)-
Tau".
EXAMPLE 15
(The protective effect of RNS60 in the presence of a neurotoxin was blocked by
an Akt inhibitor)
Overview. Akt is a serine/threonine protein kinase that plays a key role in
multiple
cellular processes including glucose metabolism, cell proliferation,
apoptosis, transcription and
cell migration. In particular, Akt is known to regulate cellular survival. In
particular,
phosphorylated Akt has bee shown to inhibit apoptosis by inactivating BAD (a
pro-apoptoic
protein). (See, Song G, et al., (2005). "The activation of Akt/PKB signaling
pathway and cell
survival". J. Cell. Mol. Med. 9 (1): 59-71.) Phosphorylation of Akt, as
recognized in the art, is a
major player in protecting cells, including neural cells, from toxic and pro-
apoptotic stimuli.
Working EXAMPLES 13 and 14 showed that a) Akt was phosphoylated in the
presence
of RNS60 in primary neurons and b) RNS60 attenuated fibrillar AB1-42 peptide
induced Tau
phosphorylation in primary neurons. The experiments disclosed in this working
EXAMPLE
confirmed that the protective effect of RNS60 in the presence of a neurotoxin
could be blocked
by an Akt inhibitor. Thus this EXAMPLE confirmed that RNS60 requires Akt to
protect
neurons from A(3 toxicity.
Neurons or astrocytes were isolated and cultured as described previously
(1,2). Neurons
or astrocytes were treated with 10% RIS60 or IS (used as a control) for 0',
15', 30', 60', 90',
120', & 180' and activation of Akt was monitored by western blot of cell
extracts with
antibodies against phospho-Akt and normal Akt (Cell Signaling). Total Akt was
detected by
antibodies against normal Akt. Neurons or astrocytes were treated with
increasing doses of
97
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
RNS60 (2%, 5%, 10%, & 20%). Different doses of NS were used as control.
Activation of Akt
was monitored as described above.
Fibrillar A(31-42 (Bachem Biosciences) was changed into the fibrillar form as
described
previously (1,3). The function of phosphorylated Akt in neurons was inhibited
by Aktl (a
specific inhibitor of Akt obtained from Calbiochem)
Neurons preincubated with different concentrations of Aktl for 30 min were
treated with
RNS60. After 1 h of incubation, cells were challenged with fibrillar A(31-42.
After 12 h,
neuronal apoptosis was monitored by TUNEL and after 24 h, cell death was
assessed by MTT
and LDH release as described previously (1,2).
The results showed that the Akt inhibitor, AktI, abrogated the protective
effect of RNS60
on fibrillar A(3-challenged neurons. Thus, the results confirmed that RNS60
requires Akt to
protect neurons from A(3 toxicity.
References cited in the above section:
1. Jana, A. & Pahan, K. 2004. Fibrillar amyloid-(3 peptides kill human primary
neurons via
NADPH oxidase-mediated activation of neutral sphingomyelinase: Implications
for Alzheimer's
disease. J. Biol. Chem. 279: 51451-51459.
2. Jana, A. & Pahan, K. 2004. HIV-1 gp 120 induces apoptosis in human primary
neurons
through redox-regulated activation of neutral sphingomyelinase. J. Neurosci.
24: 9531-9540.
3. Jana, M. & Pahan, K. 2008. Fibrillar amyloid-(3 peptides activate microglia
via toll-like
receptor 2: Implications for Alzheimer's disease. J. Immunol. 181: 7254-7262.
EXAMPLE 16
(The protective effect of RNS60 in the presence of a neurotoxin was blocked by
a PI-3 kinase
inhibitor)
Overview. PI-3 kinase plays a key role in multiple cellular processes
including cell
growth, proliferation, differentiation, motility, survival and intracellular
trafficking. In addition,
PI 3-kinases are also a key component of the insulin signaling pathway. In
particular, PI-3
kinase is known to phosphorylate, and hence, activate Akt, which is a major
player in protecting
cells, including neural cells, from toxic and pro-apoptotic stimuli.
Working EXAMPLES 13 and 15 herein above showed that: a) Akt was phosphoylated
in
the presence of RNS60 in primary neurons; and b) RNS60-mediated protection
from a
98
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
neurotoxin could be blocked by an Akt inhibitor. This EXAMPLE further
demonstrates that
RNS60-mediated protection from neurotoxin induced apoptosis requires the PI-3
kinase
pathway.
Figure 20 shows the results from an experiment examining the effects of RNS60
on
human primary neurons that have been treated with a PI-3 kinase inhibitor.
Human primary
neurons were isolated and cultured as described previously (1,2). Fibrillar
A(31-42 (Bachem
Biosciences) was changed into the fibrillar form as described previously
(1,3). The function of
PI-3 kinase in neurons was inhibited by LY294002 (a specific inhibitor of PI-3
kinase obtained
from Enogene).
Neurons preincubated with 2 pm LY294002 were treated with RNS60. After 1 h of
incubation, cells were challenged with fibrillar A(31-42. After 12 h, neuronal
apoptosis was
monitored by TUNEL and after 24 h, cell death was assessed by MTT and LDH
release as
described previously (1,2).
The results showed that the PI-3 kinase inhibitor, LY294002, abrogated the
protective
effect of RNS60 on fibrillar A(3-challenged neurons. Thus, the results
demonstrate that RNS60
requires PI-3 kinase to protect neurons from A(3 toxicity.
According to certain embodiments, therefore, and as schematically represented
in Figure
21, in neurons, RNS60 activates PI-3 kinase via membrane effects (e.g., via
modulation of ion
channel(s), which in turn phosphorylates and activates Akt. Phosphorylated Akt
then blocks
neurotoxin-mediated apoptosis of the neuronal cells.
Incorporation by Reference. All of the above U.S. patents, U.S. patent
application publications,
U.S. patent applications, foreign patents, foreign patent applications and non-
patent publications
referred to in this specification and/or listed in the Application Data Sheet,
are incorporated
herein by reference, in their entirety.
It should be understood that the drawings and detailed description herein are
to be
regarded in an illustrative rather than a restrictive manner, and are not
intended to limit the
invention to the particular forms and examples disclosed. On the contrary, the
invention
includes any further modifications, changes, rearrangements, substitutions,
alternatives, design
choices, and embodiments apparent to those of ordinary skill in the art,
without departing from
the spirit and scope of this invention, as defined by the following claims.
Thus, it is intended
99
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
that the following claims be interpreted to embrace all such further
modifications, changes,
rearrangements, substitutions, alternatives, design choices, and embodiments.
The foregoing described embodiments depict different components contained
within, or
connected with, different other components. It is to be understood that such
depicted
architectures are merely exemplary, and that in fact many other architectures
can be
implemented which achieve the same functionality. In a conceptual sense, any
arrangement of
components to achieve the same functionality is effectively "associated" such
that the desired
functionality is achieved. Hence, any two components herein combined to
achieve a particular
functionality can be seen as "associated with" each other such that the
desired functionality is
achieved, irrespective of architectures or intermedial components. Likewise,
any two
components so associated can also be viewed as being "operably connected", or
"operably
coupled", to each other to achieve the desired functionality.
While particular embodiments of the present invention have been shown and
described,
it will be obvious to those skilled in the art that, based upon the teachings
herein, changes and
modifications may be made without departing from this invention and its
broader aspects and,
therefore, the appended claims are to encompass within their scope all such
changes and
modifications as are within the true spirit and scope of this invention.
Furthermore, it is to be
understood that the invention is solely defined by the appended claims. It
will be understood by
those within the art that, in general, terms used herein, and especially in
the appended claims
(e.g., bodies of the appended claims) are generally intended as "open" terms
(e.g., the term
"including" should be interpreted as "including but not limited to," the term
"having" should be
interpreted as "having at least," the term "includes" should be interpreted as
"includes but is not
limited to," etc.). It will be further understood by those within the art that
if a specific number
of an introduced claim recitation is intended, such an intent will be
explicitly recited in the
claim, and in the absence of such recitation no such intent is present. For
example, as an aid to
understanding, the following appended claims may contain usage of the
introductory phrases "at
least one" and "one or more" to introduce claim recitations. However, the use
of such phrases
should not be construed to imply that the introduction of a claim recitation
by the indefinite
articles "a" or "an" limits any particular claim containing such introduced
claim recitation to
inventions containing only one such recitation, even when the same claim
includes the
introductory phrases "one or more" or "at least one" and indefinite articles
such as "a" or "an"
(e.g., "a" and/or "an" should typically be interpreted to mean "at least one"
or "one or more");
100
CA 02798127 2012-10-29
WO 2011/137317 PCT/US2011/034508
the same holds true for the use of definite articles used to introduce claim
recitations. In
addition, even if a specific number of an introduced claim recitation is
explicitly recited, those
skilled in the art will recognize that such recitation should typically be
interpreted to mean at
least the recited number (e.g., the bare recitation of "two recitations,"
without other modifiers,
typically means at least two recitations, or two or more recitations).
Accordingly, the invention
is not limited except as by the appended claims.
101