Note: Descriptions are shown in the official language in which they were submitted.
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
TREATMENT OF MCI AND ALZHEIMER'S DISEASE
BACKGROUND OF THE INVENTION
[0001] Alzheimer's disease (AD) is a well-known but incompletely understood
progressive neurodegenerative disease affecting ever-larger numbers of
individuals in the
aging population. Currently Alzheimer's disease affects 4 million Americans.
Statistics from
the National Institute on Aging estimate that there may be 14 million
Americans with
Alzheimer's disease by 2040 unless preventative strategies are developed.
[0002] The earliest clinical manifestation of Alzheimer's disease is described
as a
syndrome called Mild Cognitive Impairment (MCI). While detection of MCI may
permit
necessary lifestyle modifications to be planned and implemented, no therapies
are currently
available that forestall the progression of MCI to Alzheimer's disease or to
treat Alzheimer's
disease.
[0003] In 2007 testimony before the US Senate, FDA Commissioner Dr. Andrew C.
von Eschenbach stated that "the estimated 4.5 million cases of Alzheimer's
today can be
expected to rise to about 16 million by 2050." Dr. Eschenbach explained that
five drugs were
approved for AD treatment -- tacrine, rivastigmine, galantamine, donepezil,
and memantine --
the first four of which act by elevating acetylcholine levels in the brain,
and the last of which
is an antagonist of the N-methyl-D-aspartate receptor. Thus, Dr. Eschenbach
pointed out that
none of the five approved drugs have been shown to prevent or slow the
underlying nerve
degeneration in [AD] patients. He continued: "We await, together with the rest
of the world,
[] new drugs that may some day be able to treat the underlying cause of this
insidious disease
as well as other neurological diseases ..."
SUMMARY OF THE INVENTION
[0004] The present invention encompasses the discovery that nifedipine and its
oxidized or nitroso derivatives can effectively inhibit A[31-40 generation,
reduce A[3
processing enzymes and inactivate related biochemical pathways, both in vitro
and in vivo.
1
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
More surprisingly, the present inventors discovered that a lactam (e.g., a
compound of
formula (Ic) or (Ic-i) such as NFD-L1) can also effectively inhibit AP1-40
generation, reduce
A(3 processing enzymes and inactivate related biochemical pathways, both in
vitro and in
vivo. Without wishing to be bound by any theory, it is contemplated that
nitroso-nifedipine
may likely be a pro-drug that converts stoichiometrically into lactam once
administered in
vivo. Thus, the present invention provides, among other things, novel
therapeutic methods
and compositions, based on nifedipine and its oxidized or nitroso derivatives,
and/or lactam
and its derivatives (e.g., a compound of formula (Ic) or (Ic-i), e.g., NFD-
L1), that can
effectively treat, slow or prevent Mild Cognitive Impairment (MCI) and/or
Alzheimer's
disease, as well as delaying the progression from MCI to AD.
[0005] In one aspect, the present invention provides a pharmaceutical
composition
suitable for treating, slowing, or preventing a neurological disease in a
human subject
comprising a therapeutically effective amount of one or more therapeutic
agents and a
pharmaceutically acceptable carrier. In some embodiments, the neurological
disease is a
neurodegenerative disease. In some embodiments, the neurodegenerative disease
is Mild
Cognitive Impairment (MCI) and/or Alzheimer's disease. In some embodiments, a
therapeutic agent is of formula (Ia) as defined and described herein. In some
embodiments, a
therapeutic agent is of formula (lb) as defined and described herein. In some
embodiments, a
therapeutic agent is of formula (Ic) as defined and described herein. In some
embodiments, a
therapeutic agent suitable for the invention is selected from the group
consisting of
nifedipine, oxidized nifedipine, nitroso-nifedipine, lactam (e.g., a compound
of formula (Ic)
or (Ic-i), e.g., NFD-L1), thyroxine (T4), triiodothyronine (T3) and
combinations thereof In
some embodiments, a therapeutic agent suitable for the invention is a calcium
channel
blocker. In some embodiments, a therapeutic agent suitable for the invention
is not a calcium
channel blocker. In some embodiments, a therapeutic agent suitable for the
invention
increases calcium influx.
[0006] In some embodiments, a therapeutic agent suitable for the invention
comprises
nifedipine. In some embodiments, a therapeutic agent suitable for the
invention comprises
oxidized nifedipine. In some embodiments, a therapeutic agent suitable for the
invention
comprises nitroso-nifedipine. In some embodiments, a therapeutic agent
suitable for the
invention comprises lactam (e.g., a compound of formula (Ic) or (Ic-i), e.g.,
NFD-L1). In
some embodiments, a therapeutic agent suitable for the invention comprises a
mixture of
2
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
nitroso-nifedipine, oxidized nifedipine, and nifedipine. In some embodiments,
a therapeutic
agent suitable for the invention comprises a mixture of nitroso-nifedipine and
lactam (e.g., a
compound of formula (Ic) or (Ic-i), e.g., NFD-L1). In some embodiments, a
therapeutic
agent suitable for the invention comprises a mixture of lactam (e.g., a
compound of formula
(Ic) or (Ic-i), e.g., NFD-L1), oxidized nifedipine, and nifedipine. In some
embodiments, a
therapeutic agent suitable for the invention comprises 55% nitroso-nifedipine,
11% oxidized
nifedipine, and 34% nifedipine. In some embodiments, a therapeutic agent
suitable for the
invention comprises one or more (e.g., two, three, four) of lactam (e.g., a
compound of
formula (Ic) or (Ic-i), e.g., NFD-L1), nitroso-nifedipine, oxidized
nifedipine, and nifedipine.
In some embodiments, various therapeutic agents described herein further
comprises
thyroxine (T4) and/or triiodothyronine (T3). In some embodiments, a
therapeutic agent
suitable for the invention comprises nifedipine, oxidized nifedipine, nitroso-
nifedipine,
thyroxine (T4) and/or triiodothyronine (T3).
[0007] In some embodiments, a pharmaceutical composition according to the
present
invention comprises a therapeutic agent in a therapeutically effective amount
of about 0.01 to
about 1000 mg (e.g., about 0.01 to about 200 mg, about 0.01 to about 100 mg,
about 0.1 to
about 50 mg, about 0.01 to about 10 mg, about 0.01 to about 5 mg, about 0.01
to about 2.5
mg, about 0.01 to about 2.0 mg, about 0.01 to about 1.5 mg, about 0.01 to
about 1.0 mg,
about 0.01 to about 0.5 mg, about 0.01 to about 0.1 mg) per dose. In some
embodiments, a
pharmaceutical composition according to the present invention comprises
nitroso-nifedipine
in a therapeutically effective amount of about 10 mg to 2.5 g (e.g., about 10
mg to 2.0 g,
about 10 mg to 1.5 g, about 10 to about 1000 mg, about 10 mg to about 500 mg)
per dose.
[0008] In some embodiments, a pharmaceutical composition according to the
present
invention comprises a therapeutic agent in a therapeutically effective amount,
wherein the
therapeutically effective amount is insufficient to induce an adverse event in
a human subject.
In some embodiments, an adverse event is liver toxicity. In some embodiments,
a
pharmaceutical composition according to the present invention comprises a
therapeutic agent
in a therapeutically effective amount, wherein the therapeutically effective
amount is
insufficient to induce an adverse event in a human subject, wherein the agent
is nitroso-
nifedipine and the adverse event is liver toxicity.
3
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
[0009] In some embodiments, a pharmaceutical composition according to the
present
invention is formulated for oral, subcutaneous, intravenous, transdermal,
intraperitoneal,
intramuscular, intracerebroventricular, intraparenchymal, intrathecal,
intracranial, buccal,
mucosal, nasal, or rectal administration. In certain embodiments, a
pharmaceutical
composition according to the present invention is formulated for oral
administration. In some
embodiments, a pharmaceutical composition according to the invention is
formulated for
immediate or extended release.
[0010] In another aspect, the present invention provides a method for
treating,
slowing, or preventing a neurological disease in a human subject, the method
comprising
administering to the subject who is suffering from or susceptible to a
neurological disease a
therapeutic agent, such that at least one symptom or feature associated with
the neurological
disease is reduced in abundance, intensity, severity, or frequency, or has
delayed onset. In
some embodiments, a neurological disease is a neurodegenerative disorder. In
some
embodiments, the present invention provides a method for treating, slowing, or
preventing
Mild Cognitive Impairment (MCI) and/or Alzheimer's disease in a human subject,
the
method comprising administering to a subject who is suffering from or
susceptible to MCI or
Alzheimer's disease a therapeutically effective amount of one or more
therapeutic agents,
such that at least one symptom or feature associated with the MCI or
Alzheimer's disease is
reduced in abundance, intensity, severity, or frequency, or has delayed onset.
In some
embodiments, a symptom or feature is cognitive decline, production of amyloid
beta protein,
beta-secretase activity, gamma-secretase activity, paired helical filaments,
phosphorylated tau
protein in the brain, and/or an immune or inflammatory condition in the
central nervous
system. In some embodiments, an immune or inflammatory condition in the
central nervous
system is viral meningitis, viral encephalitis, fungal meningitis, fungal
encephalitis, multiple
sclerosis, schizophrenia, myasthenia gravis, or charcot joint. In some
embodiments,
production of amyloid beta protein comprises production of AP1-40. In some
embodiments,
production of amyloid beta protein comprises production of AP1-42. In some
embodiments,
production of amyloid beta protein is reduced by increasing an alpha-secretase
activity. In
some embodiments, alpha-secretase activity is ADAM-10 activity. In some
embodiments,
the gamma-secretase activity is reduced by inhibiting presenilin-1 (PS-1),
nicastrin, APH-I
and/or PEN-2 activity. In some embodiments, the gamma-secretase activity is
reduced by
inhibiting orphan G-coupled receptor 3 (GPCR-3) activity. In some embodiments,
an
4
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
immune or inflammatory condition is reduced by decreasing the level of one or
more
cytokines (e.g., IL-1, IL-6, TNF-a) in the central nervous system.
[0011] In some embodiments, a therapeutically effective amount of an agent
according to the present invention is sufficient to increase a glutamate
transporter level in the
brain of a human subject. In some embodiments, a glutamate transporter level
is a glial
glutamate transporter EAAT2 level. In some embodiments, a therapeutically
effective
amount of an agent according to the present invention is insufficient to
induce an adverse
event in a human subject. In some embodiments, an adverse event is liver
toxicity.
[0012] In some embodiments, a therapeutic agent used in a method according to
the
present invention is of formula (Ia) as defined and described herein. In some
embodiments, a
therapeutic agent used in a method according to the invention is of formula
(lb) as defined
and described herein. In some embodiments, a therapeutic agent used in a
method according
to the invention is of formula (Ic) as defined and described herein. In some
embodiments, a
suitable therapeutic agent is selected from the group consisting of
nifedipine, oxidized
nifedipine, nitroso-nifedipine, lactam (e.g., a compound of formula (Ic) or
(Ic-i), e.g., NFD-
L1), thyroxine (T4), triiodothyronine (T3) and combinations thereof. In some
embodiments,
a suitable therapeutic agent is a calcium channel blocker. In some
embodiments, a suitable
therapeutic agent is not a calcium channel blocker. In some embodiments, a
suitable
therapeutic agent increases calcium influx.
[0013] In some embodiments, a suitable therapeutic agent comprises nifedipine.
In
some embodiments, a suitable therapeutic agent comprises oxidized nifedipine.
In some
embodiments, a suitable therapeutic agent comprises nitroso-nifedipine. In
some
embodiments, a suitable therapeutic agent comprises lactam (e.g., a compound
of formula
(Ic) or (Ic-i), e.g., NFD-L1). In some embodiments, a suitable therapeutic
agent used in a
method according to the present invention comprises a mixture of nitroso-
nifedipine,
oxidized nifedipine, and nifedipine. In some embodiments, a suitable
therapeutic agent
comprises a mixture of nitroso-nifedipine and lactam (e.g., a compound of
formula (Ic) or
(Ic-i), e.g., NFD-L1). In some embodiments, a suitable therapeutic agent
comprises a
mixture of lactam (e.g., a compound of formula (Ic) or (Ic-i, e.g., NFD-Li),
oxidized
nifedipine, and nifedipine. In some embodiments, a suitable therapeutic agent
used in a
method according to the present invention comprises 55% nitroso-nifedipine,
11% oxidized
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
nifedipine, and 34% nifedipine. In some embodiments, a suitable therapeutic
agent
comprises one or more (e.g., two, three, four) of lactam (e.g., a compound of
formula (Ic) or
(Ic-i), e.g., NFD-L1), nitroso-nifedipine, oxidized nifedipine, and
nifedipine. In some
embodiments, suitable agents described herein further comprises T3/T4. In some
embodiments, an suitable agent used in a method of the present invention
comprising
nifedipine, oxidized nifedipine, and/or nitroso-nifedipine further comprises
thyroxine (T4)
and/or triiodothyronine (T3).
[0014] In some embodiments, a method according to the present invention
administers to a subject in need of treatment a therapeutic agent in a
therapeutically effective
amount of about 0.01 to about 1000 mg (e.g., about 0.01 to about 200 mg, about
0.01 to about
100 mg, about 0.1 to about 50 mg, about 0.01 to about 10 mg, about 0.01 to
about 5 mg,
about 0.01 to about 2.5 mg, about 0.01 to about 2.0 mg, about 0.01 to about
1.5 mg, about
0.01 to about 1.0 mg, about 0.01 to about 0.5 mg, about 0.01 to about 0.1 mg)
per dose. In
some embodiments, a method according to the present invention administers to a
subject in
need of treatment a therapeutic agent comprising nitroso-nifedipine in a
therapeutically
effective amount of about 10 mg to about 2.5 g (e.g., about 10 mg to about 2.0
g, about 10 mg
to about 1.5 g, about 10 mg to about 1000 mg, or about 10 mg to about 500 mg)
per dose. In
some embodiments, an agent used in a method according to the present invention
is
administered by oral, subcutaneous, intravenous, transdermal, intraperitoneal,
intramuscular,
intracerebroventricular, intraparenchymal, intrathecal, intracranial, buccal,
mucosal, nasal, or
rectal administration. In certain embodiments, an agent used in a method
according to the
present invention is administered orally.
[0015] In some embodiments, according to a method of the present invention, an
agent is administered monthly, bi-weekly, or weekly. In some embodiments,
according to a
method of the present invention, an agent is administered daily. In some
embodiments,
according to a method of the present invention, an agent is administered twice
daily, three
times daily, or four times daily.
[0016] In some embodiments, a subject treated by a method of the present
invention
has a diminished or elevated level of a biomarker (e.g., a protein biomarker
complex) as
compared to a control. In some embodiments, a suitable biomarker is a protein
biomarker
complex comprising at least one of a transthyretin protein and/or a
prostaglandin-H2 D-
6
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
isomerase protein, and at least one second, different protein selected from a
transthyretin,
prostaglandin-H2 D-isomerase, beta-2-microglobulin, cystatin C, superoxide
dismutase [Cu--
Zn], plasma retinol-binding protein, phosphatidylethanolamine-binding protein,
carbonic
anhydrase 2, and/or serotransferrin protein. In some embodiments, a suitable
protein
biomarker complex comprises prostaglandin-D2-synthase and transthyretin
(PDS/TTR
complex). In some embodiments, a suitable biomarker comprises one or more of
(i) beta
amyloid 40 (A[340), (ii) beta amyloid 42 (A[342), (iii) the ratio of A[340 to
A[342, and (iv) the
ratio of phosphorylated tau to total tau. In some embodiments, a biomarker is
determined in a
fluid sample (e.g., CSF, serum, whole blood, blood plasma, urine, ascitic
fluid, saliva, tissue
effusion, lavage, and combinations thereof) obtained from the subject. In some
embodiments, a suitable control is indicative of a level of the biomarker in a
subject selected
from the group consisting of a healthy individual, a patient suffering from
Alzheimer's
disease with a pre-determined stage, the subject before the treatment, and
combinations
thereof.
[0017] In some embodiments, a subject to be treated has a test score
indicative of
cognitive impairment. In some embodiments, a test score indicative of
cognitive impairment
is an MMSE score (e.g., lower than 27, e.g., 21-26). In some embodiments, a
test score
indicative of cognitive impairment is a CDR score (e.g., above 0, e.g., 0.5,
e.g., 1).
[0018] In some embodiments, a method according to the invention further
includes a
step of first determining the therapeutically effective amount of the
therapeutic agent based
on the level of a biomarker and/or a cognitive test score.
[0019] In yet another aspect, the invention provides a solid oral dosage form
comprising nitroso-nifedipine and nifedipine, and wherein the mass ratio of
nitroso-
nifedipine to nifedipine is at least about 1:1 (e.g., at least about 2:1, at
least about 4:1, at least
about 8:1, at least about 16:1, at least about 32:1, at least about 64:1, at
least about 100:1, at
least about 200:1, at least about 500:1, or at least about 1000:1). In some
embodiments, a
solid oral dosage form according to the present invention further comprises
one or more
pharmaceutically acceptable excipients (e.g., a binder, a buffer, a diluent, a
dispersant, an
emollient, a film-forming agent, a glidant, a light-blocking agent, a
preservative, a solvent, a
stabilizing agent, a surfactant, a suspending agent, and/or a tonicity agent).
In some
7
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
embodiments, a solid dosage form is for controlled or extended release. In
some
embodiments, a solid dosage form is for immediate release.
[0020] In yet another aspect, the invention provides benzo[c]
[2,7]naphthyridine-
5(6H)-one compounds. In some embodiments, provided compounds are of the
general
formula (Ic):
O R1
R3
%~O N
2
R
(R5)
N O
H
(Ic)
or a pharmaceutically acceptable salt thereof, wherein:
R1 and R2 are independently an optionally substituted group selected from Ci_6
aliphatic, Ci_6 heteroaliphatic, aryl, heteroaryl, or cyano;
R3 is an optionally substituted group selected from Ci_6 aliphatic, Ci_6
heteroaliphatic
or aryl;
R5 is halogen, optionally substituted Ci_6 aliphatic, hydroxyl, alkoxy, amino,
alkylamino, cyano, nitro, or nitroso; and
n is 0, 1, 2, or 3.
[0021] In certain embodiments, provided compounds are of formula (Ic-i):
0 R1
R3
~O N
R2
(R5)
N O
H
(Ic-i)
or a pharmaceutically acceptable salt thereof, wherein:
8
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
RI and R2 are independently Ci_6 aliphatic or cyano;
R3 is Ci_6 aliphatic;
R5 is halogen, C1_6 aliphatic, hydroxyl, alkoxy, amino, alkylamino, cyano,
nitro, or
nitroso; and
n is 0, 1, 2, or 3.
[0022] In certain embodiments, an inventive compound is NFD-L1.
[0023] Among other things, the present invention also provides pharmaceutical
compositions containing a compound described herein (e.g., a compound of
formula Ic or Ic-
i) and methods of use. In some embodiments, the present invention provides a
method of
treating, slowing, or preventing a neurological disease in a human subject by
administering to
a subject who is suffering from or susceptible to a neurological disease a
compound described
herein (e.g., such as a compound of formula Ic or Ic-i). In some embodiments,
the present
invention provides a method of treating, slowing, or preventing a
neurodegenerative disease
in a human subject by administering to a subject who is suffering from or
susceptible to a
neurodegenerative disease a compound described herein (e.g., such as a
compound of
formula Ic or Ic-i). In some embodiments, the present invention provides a
method of
treating, slowing, or preventing Mild Cognitive Impairment (MCI) and/or
Alzheimer's
disease in a human subject by administering to a subject who is suffering from
or susceptible
to MCI or Alzheimer's disease a compound described herein (e.g., such as a
compound of
formula Ic or Ic-i). In some embodiments, the invention provides a method of
inhibiting beta
secretase (BACE) in a human subject comprising administering to the human
subject a
compound described herein (e.g., such as a compound of formula Ic or Ic-i). In
some
embdoiments, the invention provides a method of modulating an inflammatory
condition in
the central nervous system of a human subject by administering to the human
subject a
compound described herein (e.g., such as a compound of formula Ic or Ic-i).
[0024] In this application, the use of "or" means "and/or" unless stated
otherwise. As
used in this application, the term "comprise" and variations of the term, such
as "comprising"
and "comprises," are not intended to exclude other additives, components,
integers or steps.
As used in this application, the terms "about" and "approximately" are used as
equivalents.
9
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
Any numerals used in this application with or without about/approximately are
meant to
cover any normal fluctuations appreciated by one of ordinary skill in the
relevant art.
BRIEF DESCRIPTION OF THE DRAWINGS
[0025] The drawings are for illustration purposes only and not for limitation.
[0026] Figure 1 illustrates an exemplary Western blot analysis of the PDS/TTR
complex expressed in cell culture medium by control epithelial cells, control
epithelial cells
treated with acrolein, and late stage AD epithelial cells.
[0027] Figure 2 illustrates exemplary survival data for cortical neurons
treated with
medium from control epithelial cells or AD epithelial cells.
[0028] Figure 3 illustrates exemplary results indicating that PHF 1
immunopositivity
was detected in SYSY cells resulting from exposure to the PDS/TTR protein
complex.
[0029] Figure 4 illustrates exemplary Western blot data showing reduction of
the
PDS/TTR complex expressed by control epithelial cells treated with acrolein,
acrolein plus
T3/T4, acrolein plus nifedipine mixture (nitroso nifedipine 55%, oxidized
nifedipine 11% and
nifedipine 34%) and acrolein plus nifedipine mix and T3/T4.
[0030] Figure 5 summarizes the numbers of PDS/TTR-positive cells determined by
immunostaining in cultures treated with acrolein, acrolein plus T3/T4,
acrolein plus
nifedipine mixture (nitroso nifedipine 55%, oxidized nifedipine 11% and
nifedipine 34%) and
acrolein plus nifedipine mix and T3/T4.
[0031] Figure 6 illustrates that nifedipine mix does not function as a calcium
channel
blocker compared to fresh nifedipine as determined by confocal microscopy and
a calcium
fluorescent dye.
[0032] Figure 7 illustrates exemplary results indicating that inflammatory
cytokine
production was inhibited by nifedipine mix.
[0033] Figure 8 illustrates exemplary results indicating that inflammatory
cytokine
production was inhibited by NFD-Ll.
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
[0034] Figure 9 illustrates quantification of PHF-1 immunostaining for SYSY
cultures treated with medium from epithelial cells treated with acrolein and
combinations of
nifedipine, analogs, mixtures and T3/T4.
[0035] Figure 10 illustrates exemplary results indicating that AB1_42
generation is
inhibited by nifedipine, oxidized nifedipine, nitroso nifedipine and T3/T4.
[0036] Figure 11 illustrates exemplary results indicating effect of
nifedipine,
nifedipine analogs and nifedipine mix, with and without T3/T4 on A(3 1-42
production from
H4 cells.
[0037] Figure 12 illustrates exemplary results indicating effects of known
calcium
channel blockers such as Amilodpine, Dilitiazem, Felodipine, Isradipine,
Nicardipine, and
Nimodipine on A(3 1-42 generation in H4 neuroglioma cultures.
[0038] Figure 13 illustrates exemplary results indicating effects of NFD-L1 on
A(3 1-
42 generation in H4 neuroglioma cultures.
[0039] Figure 14 illustrates exemplary results indicating that nitroso-
nifedipine
significantly inhibits BACE activity.
[0040] Figure 15 illustrates exemplary results indicating that NFD-L1
significantly
inhibits BACE activity.
[0041] Figure 16 illustrates exemplary results indicating the effect of
nifedipine mix
on PS-11 PEN-2, BACE-1 and Nicastrin, with and without T3/T4.
[0042] Figure 17 illustrates exemplary results indicating the effect of
nifedipine,
nifedipine mix and/or T3/T4 on AB1-40 generation and certain AB1-40 processing
enzymes
in a mouse model.
[0043] Figure 18 illustrates exemplary results indicating that treatment with
nitroso-
nifedipine leads to a decrease in levels of AB1-40 in a mouse model.
[0044] Figure 19 illustrates exemplary results indicating that nifedipine,
nifedipine
mix and/or T3/T4 reduced GPCR-3 levels in H4 cultures or in mice treated
acutely with
drugs. The GPCR-3 levels were determined using Western blot analysis.
11
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
[0045] Figure 20 illustrates exemplary results indicating the effect of other
classes of
blood pressure drugs on the levels of GPCR-3 in H4 cultures with and without
T3/T4.
[0046] Figure 21 illustrates exemplary results showing survival of H4 cells
after
treatment with increasing concentrations of nitroso-nifedipine.
[0047] Figure 22 summarizes exemplary effects of nitroso-nifedipine on levels
of
enzymes involved in AB processing.
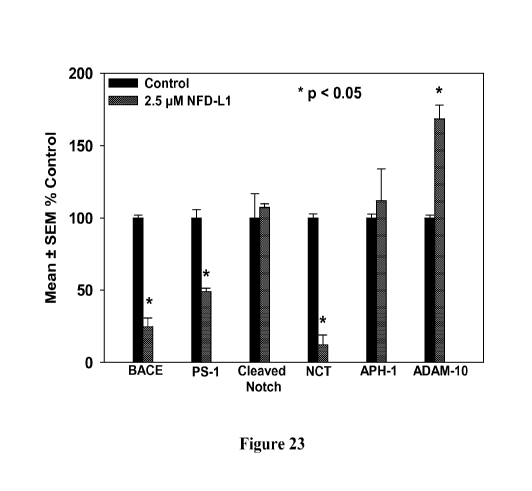
[0048] Figure 23 summarizes exemplary effects of NFD-L1 on levels of enzymes
involved in AB processing.
[0049] Figure 24 summarizes exemplary effects of nitroso-nifedipine on levels
of
enzymes involved in AB processing in a mouse model.
[0050] Figure 25 summarizes exemplary effects of NFD-L1 on levels of enzymes
involved in AB processing in a mouse model.
[0051] Figure 26 illustrates exemplary results indicating the effect of
nifedipine,
nifedipine mix and/or T3/T4 on the levels of enzymes involved in Tau
phosphorylation
measured in the mouse brains treated with corresponding compounds.
[0052] Figure 27 illustrates exemplary effects of nifedipine and nitroso-
nifedipine on
glutamate transporter levels.
[0053] Figure 28 illustrates exemplary results indicating that nitroso-
nifedipine does
not induce liver damage in mice.
[0054] Figure 29 illustrates exemplary trajectories fitted according to the
NLMIXED
model of MMSE verse age based on a human association study.
[0055] Figure 30 illustrates exemplary results indicating levels of A(31-42
and A(3
processing enzymes such as PS-1, Nicas, BACE, APH-1 and PEN-2 in front lobe
specimens
of subjects from a neuropsychological test score association study who came to
autopsy. 4
subjects were on calcium channel blockers, including nifedipine and 4 subjects
were not on
any calcium channel blocker. AB levels determined using Invitrogen ELISAs.
Protein levels
determined using Western blot analysis and antibodies specific to each
protein.
12
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
[0056] Figure 31 illustrates exemplary results indicating enzyme levels
involved in
Tau phosphorylation in frontal lobe specimens from the same subjects shown in
Figure 18.
[0057] Figure 32 illustrates exemplary results indicating that treatment of H4
neuroglioma cultures with nitroso-nifedipine leads to a significant increase
in calcium influx
as compared to control.
[0058] Figure 33 illustrates exemplary results from a photochemical synthesis
of
nitroso-nifedipine.
[0059] Figure 34 illustrates exemplary results from a synthesis of NFD-L1.
DEFINITIONS
[0060] Unless defined otherwise, the scientific and technological terms and
nomenclature used herein have the same meaning as commonly understood by a
person of
ordinary skill to which this invention pertains. Generally, the procedures of
cell cultures,
infection, molecular biology methods and the like are common methods used in
the art. Such
standard techniques can be found in reference manuals such as, for example,
Ausubel et al.,
Current Protocols in Molecular Biology, Wiley Interscience, New York, 2001;
and
Sambrook et al., Molecular Cloning: A Laboratory Manual, 3rd edition, Cold
Spring Harbor
Laboratory Press, N.Y., 2001.
[0061] In order for the present invention to be more readily understood,
certain terms
are first defined. Additional definitions for the following terms and other
terms are set forth
throughout the specification.
[0062] Alzheimer's patient: As used herein, the terms "Alzheimer's patient,"
"AD
patient," and "individual diagnosed with AD" all refer to an individual who
has been
diagnosed with AD or has been given a probable diagnosis of Alzheimer's
Disease (AD).
[0063] Animal: As used herein, the term "animal" refers to any member of the
animal kingdom. In some embodiments, "animal" refers to humans, at any stage
of
development. In some embodiments, "animal" refers to non-human animals, at any
stage of
development. In certain embodiments, the non-human animal is a mammal (e.g., a
rodent, a
13
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
mouse, a rat, a rabbit, a monkey, a dog, a cat, a sheep, cattle, a primate,
and/or a pig). In
some embodiments, animals include, but are not limited to, mammals, birds,
reptiles,
amphibians, fish, insects, and/or worms. In some embodiments, an animal may be
a
transgenic animal, genetically-engineered animal, and/or a clone.
[0064] Approximately: As used herein, the term "approximately" or "about," as
applied to one or more values of interest, refers to a value that is similar
to a stated reference
value. In certain embodiments, the term "approximately" or "about" refers to a
range of
values that fall within 25%,20%,19%,18%,17%,16%,15%,14%,13%,12%, 11%, 10%
,
9%, 8%, 7%, 6%, 5%, 4%, 3%, 2%, 1%, or less in either direction (greater than
or less than)
of the stated reference value unless otherwise stated or otherwise evident
from the context
(except where such number would exceed 100% of a possible value).
[0065] Biological fluid sample: As used herein, the term "biological fluid
sample"
encompasses a variety of fluid sample types obtained from an individual and
can be used in a
diagnostic or monitoring assay. The term encompasses whole blood, blood serum
or blood
plasma, cerebrospinal fluid (CSF), urine and other liquid samples of
biological origin. The
term also includes samples that have been manipulated in any way after their
procurement,
such as by treatment with reagents, solubilization, or enrichment for certain
components,
such as proteins or polynucleotides.
[0066] Combination therapy: The term "combination therapy", as used herein,
refers
to those situations in which two or more different pharmaceutical agents are
administered in
overlapping regimens so that the subject is simultaneously exposed to both
agents.
[0067] Control: As used herein, the term "control" has its art-understood
meaning of
being a standard against which results are compared. Typically, controls are
used to augment
integrity in experiments by isolating variables in order to make a conclusion
about such
variables. In some embodiments, a control is a reaction or assay that is
performed
simultaneously with a test reaction or assay to provide a comparator. In one
experiment, the
"test" (i.e., the variable being tested) is applied. In the second experiment,
the "control," the
variable being tested is not applied. In some embodiments, a control is a
historical control
(i.e., of a test or assay performed previously, or an amount or result that is
previously
known). In some embodiments, a control is or comprises a printed or otherwise
saved record.
A control may be a positive control or a negative control.
14
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
[0068] Dosing regimen: A "dosing regimen", as that term is used herein, refers
to a
set of unit doses (at least one and often more than one) that are administered
individually
separated by periods of time. The recommended set of doses (i.e., amounts,
timing, route of
administration, etc.) for a particular therapeutic agent constitutes its
dosing regimen.
[0069] Functional: As used herein, a "functional" biological molecule is a
biological
molecule in a form in which it exhibits a property and/or activity by which it
is characterized.
[0070] Inhibition: As used herein, the terms "inhibition," "inhibit" and
"inhibiting"
refer to processes or methods of decreasing or reducing activity and/or
expression of a protein
or a gene of interest. Typically, inhibiting a protein or a gene refers to
reducing expression or
a relevant activity of the protein or gene by at least 10% or more, for
example, 20%, 30%,
40%, or 50%, 60%, 70%, 80%, 90% or more, or a decrease in expression or the
relevant
activity of greater than 1-fold, 2-fold, 3-fold, 4-fold, 5-fold, 10-fold, 50-
fold, 100-fold or
more as measured by one or more methods described herein or recognized in the
art.
[0071] In vitro: As used herein, the term "in vitro" refers to events that
occur in an
artificial environment, e.g., in a test tube or reaction vessel, in cell
culture, etc., rather than
within a multi-cellular organism.
[0072] In vivo: As used herein, the term "in vivo" refers to events that occur
within a
multi-cellular organism such as a non-human animal.
[0073] Isolated: As used herein, the term "isolated" refers to a substance
and/or
entity that has been (1) separated from at least some of the components with
which it was
associated when initially produced (whether in nature and/or in an
experimental setting),
and/or (2) produced, prepared, and/or manufactured by the hand of man.
Isolated substances
and/or entities may be separated from at least about 10%, about 20%, about
30%, about 40%,
about 50%, about 60%, about 70%, about 80%, about 90%, about 95%, about 98%,
about
99%, substantially 100%, or 100% of the other components with which they were
initially
associated. In some embodiments, isolated agents are more than about 80%,
about 85%,
about 90%, about 91%, about 92%, about 93%, about 94%, about 95%, about 96%,
about
97%, about 98%, about 99%, substantially 100%, or 100% pure. As used herein, a
substance
is "pure" if it is substantially free of other components. As used herein, the
term "isolated
cell" refers to a cell not contained in a multi-cellular organism.
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
[0074] Individual with MCI: As used herein, "an individual with MCI (mild
cognitive impairment)" is typically an individual who meets the following
clinical criteria of
amnestic MCI (Petersen et al. Arch Neurol 56:303-308 (1999): 1) memory
complaints
corroborated by an informant, 2) objective memory impairment for age and
education, 3)
normal general cognitive function, 4) intact activities of daily living, and
5) the subject does
not meet criteria for dementia.
[0075] Individual with EAD: As used herein, an "individual with EAD (early or
moderate Alzheimer's disease)" is an individual who demonstrate the following
criteria: 1) a
decline in cognitive function for a previous higher level, 2) declines in one
or more areas of
cognition in addition to memory, 3) a clinical dementia rating scale score of
0.5 to 1, and 4) a
clinical examination that excluded other causes of dementia.
[0076] Individual with LAD: As used herein, an "individual with LAD (severe or
late
stage Alzheimer's disease)" is an individual who meets the standard clinical
diagnostic
criteria for probable AD (McKhann et al. Neurology 34:939-48 (1984).
[0077] Lactam: As used herein, a "lactam" is a cyclic amide. Typically,
prefixes
indicate how many carbon atoms (apart from the carbonyl moiety) are present in
the ring: (3-
lactam (2 carbon atoms outside the carbonyl, 4 ring atoms in total), y-lactam
(3 and 5), 6-
lactam (4 and 6). In some embodiment, a lactam suitable for the invention is
defined by
formula (Ic) or formula (Ic-i). In some embodiments, a lactam suitable for the
invention is
NFD-L1.
[0078] Reference value: As used herein, a "reference value" can be an absolute
value; a relative value; a value that has an upper and/or lower limit; a range
of values; an
average value; a median value, a mean value, or a value as compared to a
particular control or
baseline value. A reference value can be based on an individual sample value,
such as for
example, a value obtained from a sample from the individual with AD, MCI or
cognitive
impairment, but at an earlier point in time, or a value obtained from a sample
from an AD
patient other than the individual being tested, or a "normal" individual, that
is an individual
not diagnosed with AD. The reference value can be based on a large number of
samples,
such as from AD patients or normal individuals or based on a pool of samples
including or
excluding the sample to be tested.
16
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
[0079] Neurological disease: As used herein, the phrase "neurological disease"
refers
to a disease or disorder of the central nervous system. Neurological diseases
include multiple
sclerosis, neuropathies, and neurodegenerative disorders such as AD,
Parkinson's disease,
amyotrophic lateral sclerosis (ALS), Huntington's disease, mild cognitive
impairment (MCI)
and frontotemporal dementia. Additional exemplary neurological diseases
include epilepsy,
convulsive disorder, pain, anxiety, depression, schizophrenia, post-anesthesia
cognitive
decline, opioid tolerance, drug abuse, alcohol abuse, schizophrenia,
neuroleptic malignant
syndrome, Tourette's syndrome, Pick's Disease, dementia, delirium,
neurodegeneration in
Down Syndrome, Familial British Dementia, Familial Danish Dementia, Korsakoffs
disease,
olivopontocerebellar atrophy, HIV-induced dementia and blindness, multi-
infarct dementia,
hereditary motor and sensory neuropathies (HMSN, also known as peroneal
muscular atrophy
or Charcot-Marie-Tooth disease), diabetic polyneuropathy, olivopontocerebellar
atrophy,
age-onset neurological deterioration, alcoholic polyneuropathy, tinnitus, and
pathophysiologically symptomology.
[0080] Normal individual: As used herein, a "Normal" individual or "healthy"
individual refers to an individual who has or would be assessed by a physician
as not having
AD or MCI, and has an Mini-Mental State Examination (MMSE) (referenced in
Folstein et
al., J. Psychiatr. Res 1975; 12:1289-198) score or would achieve a MMSE score
in the range
of 25-30. A "Normal" individual is generally age-matched within a range of 5
to 10 years,
including but not limited to an individual that is age-matched, with the
individual to be
assessed.
[0081] Protein: As used herein, the term "protein" refers to a polypeptide
(i.e., a
string of at least two amino acids linked to one another by peptide bonds).
Proteins may
include moieties other than amino acids (e.g., may be glycoproteins,
proteoglycans, etc.)
and/or may be otherwise processed or modified. Those of ordinary skill in the
art will
appreciate that a "protein" can be a complete polypeptide chain as produced by
a cell (with or
without a signal sequence), or can be a characteristic portion thereof. Those
of ordinary skill
will appreciate that a protein can sometimes include more than one polypeptide
chain, for
example linked by one or more disulfide bonds or associated by other means.
Polypeptides
may contain L-amino acids, D-amino acids, or both and may contain any of a
variety of amino
acid modifications or analogs known in the art. Useful modifications include,
e.g., terminal
acetylation, amidation, etc. In some embodiments, proteins may comprise
natural amino
17
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
acids, non-natural amino acids, synthetic amino acids, and combinations
thereof. The term
"peptide" is generally used to refer to a polypeptide having a length of less
than about 100
amino acids.
[0082] Subject: As used herein, the term "subject" or "patient" refers to any
organism to which compositions in accordance with the invention may be
administered, e.g.,
for experimental, diagnostic, prophylactic, and/or therapeutic purposes.
Typical subjects
include animals (e.g., mammals such as mice, rats, rabbits, non-human
primates, and humans;
insects; worms; etc.).
[0083] Substantially: As used herein, the term "substantially" refers to the
qualitative
condition of exhibiting total or near-total extent or degree of a
characteristic or property of
interest. One of ordinary skill in the biological arts will understand that
biological and
chemical phenomena rarely, if ever, go to completion and/or proceed to
completeness or
achieve or avoid an absolute result. The term "substantially" is therefore
used herein to
capture the potential lack of completeness inherent in many biological and
chemical
phenomena.
[0084] Suffering from: An individual who is "suffering from" a disease,
disorder,
and/or condition has been diagnosed with or displays one or more symptoms of
the disease,
disorder, and/or condition.
[0085] Susceptible to: An individual who is "susceptible to" a disease,
disorder,
and/or condition has not been diagnosed with the disease, disorder, and/or
condition. In some
embodiments, an individual who is susceptible to a disease, disorder, and/or
condition may
not exhibit symptoms of the disease, disorder, and/or condition. In some
embodiments, an
individual who is susceptible to a disease, disorder, and/or condition will
develop the disease,
disorder, and/or condition. In some embodiments, an individual who is
susceptible to a
disease, disorder, and/or condition will not develop the disease, disorder,
and/or condition.
[0086] Therapeutically effective amount: As used herein, the term
"therapeutically
effective amount" of a therapeutic agent means an amount that is sufficient,
when
administered to a subject suffering from or susceptible to a disease,
disorder, and/or
condition, to treat, diagnose, prevent, and/or delay the onset of the
symptom(s) of the disease,
disorder, and/or condition.
18
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
[0087] Therapeutic agent: As used herein, the phrase "therapeutic agent"
refers to
any agent that, when administered to a subject, has a therapeutic effect
and/or elicits a desired
biological and/or pharmacological effect. As used herein, the terms
"therapeutic agent" and
"agent" are used inter-changeably.
[0088] Treating: As used herein, the term "treat," "treatment," or "treating"
refers to
any method used to partially or completely alleviate, ameliorate, relieve,
inhibit, prevent,
delay onset of, reduce severity of and/or reduce incidence of one or more
symptoms or
features of a particular disease, disorder, and/or condition. Treatment may be
administered to
a subject who does not exhibit signs of a disease and/or exhibits only early
signs of the
disease for the purpose of decreasing the risk of developing pathology
associated with the
disease.
[0089] Definitions of specific functional groups and chemical terms are
described in
more detail below. For purposes of this invention, the chemical elements are
identified in
accordance with the Periodic Table of the Elements, CAS version, Handbook of
Chemistry
and Physics, 75th Ed., inside cover, and specific functional groups are
generally defined as
described therein. Additionally, general principles of organic chemistry, as
well as specific
functional moieties and reactivity, are described in Organic Chemistry, Thomas
Sorrell,
University Science Books, Sausalito, 1999; Smith and March March's Advanced
Organic
Chemistry, 5th Edition, John Wiley & Sons, Inc., New York, 2001; Larock,
Comprehensive
Organic Transformations, VCH Publishers, Inc., New York, 1989; Carruthers,
Some Modern
Methods of Organic Synthesis, 3rd Edition, Cambridge University Press,
Cambridge, 1987.
[0090] The compounds of the present invention may exist in particular
geometric or
stereoisomeric forms. The present invention contemplates all such compounds,
including
cis- and trans-isomers, R- and S-enantiomers, diastereomers, (D)-isomers, (L)-
isomers, the
racemic mixtures thereof, and other mixtures thereof, as falling within the
scope of the
invention.
[0091] Aliphatic: The term "aliphatic" or "aliphatic group", as used herein,
denotes a
hydrocarbon moiety that may be straight-chain (i.e., unbranched), branched, or
cycloaliphatic
(including fused, bridging, and spiro-fused polycyclic) and may be completely
saturated or
19
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
may contain one or more units of unsaturation, but which is not aromatic.
Unless otherwise
specified, aliphatic groups contain 1-6 carbon atoms. In some embodiments,
aliphatic groups
contain 1-4 carbon atoms, and in yet other embodiments aliphatic groups
contain 1-3 carbon
atoms. Suitable aliphatic groups include, but are not limited to, linear or
branched, alkyl,
alkenyl, and alkynyl groups, and hybrids thereof such as (cycloalkyl)alkyl,
(cycloalkenyl)alkyl or (cycloalkyl)alkenyl.
[0092] Alkenyl: The term "alkenyl," as used herein, denotes a monovalent group
derived from a straight- or branched-chain aliphatic moiety having at least
one carbon-
carbon double bond by the removal of a single hydrogen atom. In certain
embodiments,
alkenyl contains 2-6 carbon atoms. In certain embodiments, alkenyl contains 2-
5 carbon
atoms. In some embodiments, alkenyl contains 2-4 carbon atoms. In another
embodiment,
alkenyl contains 2-3 carbon atoms. Alkenyl groups include, for example,
ethenyl ("vinyl"),
propenyl ("allyl"), butenyl, 1-methyl-2-buten-1-yl, and the like.
[0093] Alkyl: The term "alkyl," as used herein, refers to a monovalent
saturated,
straight- or branched-chain hydrocarbon radical derived from an aliphatic
moiety containing
between one and six carbon atoms by removal of a single hydrogen atom. In some
embodiments, alkyl contains 1-5 carbon atoms. In another embodiment, alkyl
contains 1-4
carbon atoms. In still other embodiments, alkyl contains 1-3 carbon atoms. In
yet another
embodiment, alkyl contains 1-2 carbons. Examples of alkyl radicals include,
but are not
limited to, methyl, ethyl, n-propyl, isopropyl, n-butyl, iso-butyl, sec-butyl,
sec-pentyl, iso-
pentyl, tert-butyl, n-pentyl, neopentyl, n hexyl, sec-hexyl, n heptyl, n-
octyl, n-decyl, n-
undecyl, dodecyl, and the like.
[0094] Alkynyl: The term "alkynyl," as used herein, refers to a monovalent
group
derived from a straight- or branched-chain aliphatic moiety having at least
one carbon-
carbon triple bond by the removal of a single hydrogen atom. In certain
embodiments,
alkynyl contains 2-6 carbon atoms. In certain embodiments, alkynyl contains 2-
5 carbon
atoms. In some embodiments, alkynyl contains 2-4 carbon atoms. In another
embodiment,
alkynyl contains 2-3 carbon atoms. Representative alkynyl groups include, but
are not
limited to, ethynyl, 2-propynyl ("propargyl"), 1-propynyl, and the like.
[0095] Amino: The term "amino," as used herein, refers to a group of the
formula (-
NH2).
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
[0096] Alkoxy: The term "alkoxy" refers to a "substituted hydroxyl" of the
formula
(-OR'), wherein R' is an alkyl group, as defined herein, and the oxygen moiety
is directly
attached to the parent molecule.
[0097] Alkylamino: The term "alkylamino" refers to a "substituted amino" of
the
formula (-NRh2), wherein Rh is, independently, a hydrogen or an alkyl group,
as defined
herein, and the nitrogen moiety is directly attached to the parent molecule.
[0098] Aryl - As used herein, the term "aryl" refers to an optionally
substituted
monocyclic and bicyclic ring systems having a total of five to 10 ring
members, wherein at
least one ring in the system is aromatic and wherein each ring in the system
contains three to
seven ring members. The term "aryl" may be used interchangeably with the term
"aryl ring".
In certain embodiments of the present invention, "aryl" refers to an aromatic
ring system
which includes, but not limited to, phenyl, biphenyl, naphthyl, anthracyl and
the like, which
may bear one or more substituents.
[0099] Cycloaliphatic: The terms "cycloaliphatic", "carbocycle",
"carbocyclyl",
"carbocyclo", or "carbocyclic", used alone or as part of a larger moiety,
refer to a saturated or
partially unsaturated cyclic aliphatic monocyclic or bicyclic ring systems, as
described
herein, having from 3 to 10 members, wherein the aliphatic ring system is
optionally
substituted as defined above and described herein. Cycloaliphatic groups
include, without
limitation, cyclopropyl, cyclobutyl, cyclopentyl, cyclopentenyl, cyclohexyl,
cyclohexenyl,
cycloheptyl, cycloheptenyl, cyclooctyl, cyclooctenyl, and cyclooctadienyl. In
some
embodiments, the cycloalkyl has 3-6 carbons. The terms "cycloaliphatic",
"carbocycle",
"carbocyclyl", "carbocyclo", or "carbocyclic" also include aliphatic rings
that are fused to
one or more aromatic or nonaromatic rings, such as decahydronaphthyl,
tetrahydronaphthyl,
decalin, or bicyclo[2.2.2]octane, where the radical or point of attachment is
on an aliphatic
ring.
[0100] Cyano: The term "cyano," as used herein, refers to a group of the
formula (-
CN).
[0101] Halogen: The terms "halo" and "halogen" as used herein refer to an atom
selected from fluorine (fluoro, -F), chlorine (chloro, -Cl), bromine (bromo, -
Br), and iodine
(iodo, -I).
21
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
[0102] Heteroaliphatic - As used herein, the terms "heteroaliphatic" or
"heteroaliphatic group", denote an optionally substituted hydrocarbon moiety
having, in
addition to carbon atoms, from one to five heteroatoms, that may be straight-
chain (i.e.,
unbranched), branched, or cyclic ("heterocyclic") and may be completely
saturated or may
contain one or more units of unsaturation, but which is not aromatic. Unless
otherwise
specified, heteroaliphatic groups contain 1-6 carbon atoms wherein 1-3 carbon
atoms are
optionally and independently replaced with heteroatoms selected from oxygen,
nitrogen and
sulfur. In some embodiments, heteroaliphatic groups contain 1-4 carbon atoms,
wherein 1-2
carbon atoms are optionally and independently replaced with heteroatoms
selected from
oxygen, nitrogen and sulfur. In yet other embodiments, heteroaliphatic groups
contain 1-3
carbon atoms, wherein 1 carbon atom is optionally and independently replaced
with a
heteroatom selected from oxygen, nitrogen and sulfur. Suitable heteroaliphatic
groups
include, but are not limited to, linear or branched, heteroalkyl,
heteroalkenyl, and
heteroalkynyl groups.
[0103] Heteroaryl - As used herein, the term "heteroaryl" used alone or as
part of a
larger moiety, e.g., "heteroaralkyl", or "heteroaralkoxy", refers to an
optionally substituted
group having 5 to 10 ring atoms, preferably 5, 6, or 9 ring atoms; having 6,
10, or 1471
electrons shared in a cyclic array; and having, in addition to carbon atoms,
from one to five
heteroatoms. Heteroaryl groups include, without limitation, thienyl, furanyl,
pyrrolyl,
imidazolyl, pyrazolyl, triazolyl, tetrazolyl, oxazolyl, isoxazolyl,
oxadiazolyl, thiazolyl,
isothiazolyl, thiadiazolyl, pyridyl, pyridazinyl, pyrimidinyl, pyrazinyl,
indolizinyl, purinyl,
naphthyridinyl, and pteridinyl. The terms "heteroaryl" and "heteroar-", as
used herein, also
include groups in which a heteroaromatic ring is fused to one or more aryl,
carbocyclic, or
heterocyclic rings, where the radical or point of attachment is on the
heteroaromatic ring. Non
limiting examples include indolyl, isoindolyl, benzothienyl, benzofuranyl,
dibenzofuranyl,
indazolyl, benzimidazolyl, benzthiazolyl, quinolyl, isoquinolyl, cinnolinyl,
phthalazinyl,
quinazolinyl, quinoxalinyl, 4H-quinolizinyl, carbazolyl, acridinyl,
phenazinyl,
phenothiazinyl, phenoxazinyl, tetrahydroquinolinyl, and
tetrahydroisoquinolinyl. A
heteroaryl group may be mono- or bicyclic. The term "heteroaryl" may be used
interchangeably with the terms "heteroaryl ring", "heteroaryl group", or
"heteroaromatic",
any of which terms include rings that are optionally substituted.
22
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
[0104] Heteroatom - As used herein, the term "heteroatom" refers to nitrogen,
oxygen, or sulfur, and includes any oxidized form of nitrogen or sulfur, and
any quaternized
form of a basic nitrogen. The term "nitrogen" also includes a substituted
nitrogen.
[0105] Heterocyclic - As used herein, the terms "heterocycle", "heterocyclyl",
"heterocyclic radical", and "heterocyclic ring" are used interchangeably and
refer to a stable
optionally substituted 5- to 7-membered monocyclic or 7- to 10-membered
bicyclic
heterocyclic moiety that is either saturated or partially unsaturated, and
having, in addition to
carbon atoms, one or more heteroatoms, as defined above. A heterocyclic ring
can be
attached to its pendant group at any heteroatom or carbon atom that results in
a stable
structure and any of the ring atoms can be optionally substituted. Examples of
such saturated
or partially unsaturated heterocyclic radicals include, without limitation,
tetrahydrofuranyl,
tetrahydrothienyl, pyrrolidinyl, pyrrolidonyl, piperidinyl, pyrrolinyl,
tetrahydroquinolinyl,
tetrahydroisoquinolinyl, decahydroquinolinyl, oxazolidinyl, piperazinyl,
dioxanyl,
dioxolanyl, diazepinyl, oxazepinyl, thiazepinyl, morpholinyl, and
quinuclidinyl. The terms
"heterocycle", "heterocyclyl", "heterocyclyl ring", "heterocyclic group",
"heterocyclic
moiety", and "heterocyclic radical", are used interchangeably herein, and also
include groups
in which a heterocyclyl ring is fused to one or more aryl, heteroaryl, or
carbocyclic rings,
such as indolinyl, 3H-indolyl, chromanyl, phenanthridinyl, or
tetrahydroquinolinyl, where
the radical or point of attachment is on the heterocyclyl ring. A heterocyclyl
group may be
mono- or bicyclic. The term "heterocyclylalkyl" refers to an alkyl group
substituted by a
heterocyclyl, wherein the alkyl and heterocyclyl portions independently are
optionally
substituted.
[0106] Nitro: The term "nitro," as used herein, refers to a group of the
formula (-
NO2).
[0107] Nitroso: The term "nitroso," as used herein, refers to a group of the
formula
(-NO).
[0108] Partially unsaturated: As used herein, the term "partially unsaturated"
refers
to a ring moiety that includes at least one double or triple bond between ring
atoms but is not
aromatic. The term "partially unsaturated" is intended to encompass rings
having multiple
sites of unsaturation, but is not intended to include aryl or heteroaryl
moieties, as herein
defined.
23
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
[0109] Unsaturated: The term "unsaturated", as used herein, means that a
moiety has
one or more units of unsaturation.
[0110] Optionally substituted - As described herein, compounds of the
invention may
contain "optionally substituted" moieties. In general, the term "substituted",
whether
preceded by the term "optionally" or not, means that one or more hydrogens of
the designated
moiety are replaced with a suitable substituent. Unless otherwise indicated,
an "optionally
substituted" group may have a suitable substituent at each substitutable
position of the group,
and when more than one position in any given structure may be substituted with
more than
one substituent selected from a specified group, the substituent may be either
the same or
different at every position. Combinations of substituents envisioned by this
invention are
preferably those that result in the formation of stable or chemically feasible
compounds. The
term "stable", as used herein, refers to compounds that are not substantially
altered when
subjected to conditions to allow for their production, detection, and, in
certain embodiments,
their recovery, purification, and use for one or more of the purposes
disclosed herein.
[0111] Suitable monovalent substituents on a substitutable carbon atom of an
"optionally substituted" group are independently halogen; -(CH2)0-4R ; -(CH2)0-
40R ; -0-
(CH2)0-4C(O)OR ; -(CH2)o-4CH(OR )2; -(CH2)0-4SR ; -(CH2)o-4Ph, which may be
substituted with R ; -(CH2)0_4O(CH2)0_1Ph which may be substituted with R ; -
CH=CHPh,
which may be substituted with R ; -NO2; -CN; -N3; -(CH2)o-4N(R )2; -(CH2)0_
4N(R )C(O)R ; -N(R )C(S)R ; -(CH2)o-4N(R )C(O)NR 2; -N(R )C(S)NR 2; -(CH2)0_
4N(R )C(O)OR ; -N(R )N(R )C(O)R ; N(R )N(R )C(O)NR 2; N(R )N(R )C(O)OR ; -
(CH2)0 4C(O)R ; -C(S)R ; -(CH2)0 4C(O)OR ; -(CH2)0-4C(O)SR ; -(CH2)0 4C(O)OSiR
3; -
(CH2)0-40C(O)R ; -OC(O)(CH2)o-4SR-, SC(S)SR ; -(CH2)0-4SC(O)R ; -(CH2)0_
4C(O)NR 2; -C(S)NR 2; -C(S)SR ; -SC(S)SR , -(CH2)0-40C(O)NR 2; -C(O)N(OR )R ; -
C(O)C(O)R ; -C(O)CH2C(O)R ; -C(NOR )R ; -(CH2)0-4SSR ; -(CH2)0 4S(0)2R ; -
(CH2)0_
4S(0)20R ; -(CH2)0 4OS(0)2R ; -S(0)2NR 2; -(CH2)0-4S(O)R ; -N(R )S(0)2NR 2; -
N(R )S(0)2R ; -N(OR )R ; -C(NH)NR 2; -P(0)2R ; -P(O)R 2; -OP(O)R 2; -OP(O)(OR
)2;
SiR 3; -(C1 straight or branched alkylene)O-N(R )2; or -(C1-4straight or
branched
alkylene)C(O)ON(R )2, wherein each R may be substituted as defined below and
is
independently hydrogen, Ci_6 aliphatic, -CH2Ph, -O(CH2)0_1Ph, or a 5-6-
membered
saturated, partially unsaturated, or aryl ring having 0-4 heteroatoms
independently selected
24
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
from nitrogen, oxygen, or sulfur, or, notwithstanding the definition above,
two independent
occurrences of R , taken together with their intervening atom(s), form a 3-12-
membered
saturated, partially unsaturated, or aryl mono- or bicyclic ring having 0-4
heteroatoms
independently selected from nitrogen, oxygen, or sulfur, which may be
substituted as defined
below.
[0112] Suitable monovalent substituents on R (or the ring formed by taking
two
independent occurrences of R together with their intervening atoms), are
independently
halogen, -(CH2)0_2R', -(haloR'), -(CH2)0 2OH, -(CH2)0_2OR', -(CH2)0 2CH(OR')2;
-
O(haloR'), -CN, -N3, -(CH2)0_2C(O)R', -(CH2)0_2C(O)OH, -(CH2)0_2C(O)OR', -
(CH2)0_
2SR', -(CH2)0~2SH, -(CH2)0_2NH2, -(CH2)0_2NHR', -(CH2)0_2NR'2 -NO2, -SiR-3, -
OSiR-3,
-C(O)SR', -(C1 straight or branched alkylene)C(O)OR', or -SSR' wherein each R'
is
unsubstituted or where preceded by "halo" is substituted only with one or more
halogens, and
is independently selected from Ci4 aliphatic, -CH2Ph, -O(CH2)0 1Ph, or a 5-6-
membered
saturated, partially unsaturated, or aryl ring having 0-4 heteroatoms
independently selected
from nitrogen, oxygen, or sulfur. Suitable divalent substituents on a
saturated carbon atom of
R include =0 and =S.
[0113] Suitable divalent substituents on a saturated carbon atom of an
"optionally
substituted" group include the following: =O, =S, =NNR*2, =NNHC(O)R*,
=NNHC(O)OR*,
=NNHS(O)2R*, =NR*, =NOR*, -O(C(R*2))2_3O-, or -S(C(R*2))2_3S-, wherein each
independent occurrence of R* is selected from hydrogen, Ci_6 aliphatic which
may be
substituted as defined below, or an unsubstituted 5-6-membered saturated,
partially
unsaturated, or aryl ring having 0-4 heteroatoms independently selected from
nitrogen,
oxygen, or sulfur. Suitable divalent substituents that are bound to vicinal
substitutable
carbons of an "optionally substituted" group include: -O(CR*2)2_30-, wherein
each
independent occurrence of R* is selected from hydrogen, Ci_6 aliphatic which
may be
substituted as defined below, or an unsubstituted 5-6-membered saturated,
partially
unsaturated, or aryl ring having 0-4 heteroatoms independently selected from
nitrogen,
oxygen, or sulfur.
[0114] Suitable substituents on the aliphatic group of R* include halogen, -
R', -
(haloR'), -OH, -OR', -O(haloR'), -CN, -C(O)OH, -C(O)OR', NH2, -NHR', -NR'2, or
-
NO2, each R' is unsubstituted or where preceded by "halo" is substituted only
with
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
one or more halogens, and is independently C1 aliphatic, -CH2Ph, -O(CH2)0_1Ph,
or a 5-6-
membered saturated, partially unsaturated, or aryl ring having 0-4 heteroatoms
independently
selected from nitrogen, oxygen, or sulfur.
[0115] Suitable substituents on a substitutable nitrogen of an "optionally
substituted"
group include -Rt, -NRt2, -C(O)Rt, -C(O)ORt, -C(O)C(O)Rt, -C(O)CH2C(O)Rt, -
S(O)2Rt, -S(O)2NRt2, -C(S)NRt2, -C(NH)NRt2, or -N(R)S(O)2Rt; wherein each Rt
is
independently hydrogen, Ci_6 aliphatic which may be substituted as defined
below,
unsubstituted -OPh, or an unsubstituted 5-6-membered saturated, partially
unsaturated, or
aryl ring having 0-4 heteroatoms independently selected from nitrogen, oxygen,
or sulfur, or,
notwithstanding the definition above, two independent occurrences of Rt, taken
together with
their intervening atom(s) form an unsubstituted 3-12-membered saturated,
partially
unsaturated, or aryl mono- or bicyclic ring having 0-4 heteroatoms
independently selected
from nitrogen, oxygen, or sulfur.
[0116] Suitable substituents on the aliphatic group of Rt are independently
halogen, -
R', -(haloR'), -OH, -OR', -O(haloR'), -CN, -C(O)OH, -C(O)OR', -NH2 NHR' NR'2,
or -NO2, each R' is unsubstituted or where preceded by "halo" is substituted
only
with one or more halogens, and is independently C1 aliphatic, -CH2Ph, -
O(CH2)0_1Ph, or a
5-6-membered saturated, partially unsaturated, or aryl ring having 0-4
heteroatoms
independently selected from nitrogen, oxygen, or sulfur.
DETAILED DESCRIPTION
[0117] The present invention provides, among other things, therapeutic
compositions
and methods that can effectively treat, slow or prevent mild cognitive
impairment (MCI) or
Alzheimer's disease (AD).
[0118] As described in the Examples section, the present invention is, in
part, based
on the following unexpected discoveries: (1) a protein complex PDS/TTR, known
as a
biomarker for early diagnosis of MCI or Alzheimer's disease, is neurotoxic and
induces
characteristic symptoms and features of Alzheimer's disease in cell cultures;
(2)
dihydropyridine calcium channel blockers (like nifedipine), their oxidized,
nitroso derivatives
26
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
and mixtures (which no longer function as calcium channel blockers), and/or
T3/T4
effectively reduce or eliminate the ability of the PDS/TTR complex to induce
AD-like
symptoms and underlying enzymes and biochemical pathways in cell cultures and
reduce
endogenous levels of AP1-40 peptide in animal models; and (3) human
association studies
demonstrated that the use of dihydropyridine calcium channel blockers
significantly delays
the onset of cognitive decline thus indicating that these compounds may be
used to
effectively treat Alzheimer's disease. Surprisingly, the inventors found that
oxidized, nitroso
nifedipine derivatives and mixtures no longer function as calcium channel
blockers. In some
embodiments, nitroso nifedipine or a derivative thereof increases calcium
influx. Without
wishing to be bound by any theory, it is contemplated that the ability of
these compounds to
treat MCI or Alzheimer's disease may be independent of their ability to block
calcium
channels.
[0119] More surprisingly, the present inventors discovered that lactam such as
NFD-
Ll can also effectively inhibit A(31-40 generation, reduce A(3 processing
enzymes and
inactivate related biochemical pathways, both in vitro and in vivo, similar to
nitroso-
nifedipine. Without wishing to be bound by any theory, it is contemplated that
nitroso-
nifedipine may likely be a pro-drug that converts stoichiometrically into
lactam once
administered in vivo.
[0120] Thus, the present invention contemplates methods and compositions that
can
effectively treat Alzheimer's disease based on therapeutically effective
amount of nifedipine,
oxidized or nitroso nifedipine derivatives, lactam (e.g., a compound of
formula (1c) or (Ic-i),
e.g., NFD-L1), thyroxine (T4), triiodothyronine (T3) and combinations thereof.
In some
embodiments, the present invention provides methods for treating, slowing, or
preventing
Mild Cognitive Impairment (MCI) and/or Alzheimer's disease in a human subject,
comprising administering to a subject who is suffering from or susceptible to
MCI or
Alzheimer's disease a therapeutically effective amount of an agent selected
from the group
consisting of nifedipine, oxidized nifedipine, nitroso-nifedipine, lactam
(e.g., a compound of
formula (Ic) or (Ic-i), e.g., NFD-L1), thyroxine (T4), triiodothyronine (T3)
and combinations
thereof, such that at least one symptom or feature associated with the MCI or
Alzheimer's
disease is reduced in abundance, intensity, severity, or frequency, or has
delayed onset. In
some embodiments, the present invention contemplates methods and compositions
that can
effectively treat Alzheimer's disease based on therapeutically effective
amount of a
27
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
compound of formula (Ia), (lb), (Ic), (II) and combinations thereof In some
embodiments,
an agent suitable for the invention does not function as a calcium channel
blocker. In some
embodiments, an agent suitable for the invention increases calcium influx.
[0121] It is further contemplated that inventive methods according to the
invention
can be combined with sensitive biomarkers and/or cognitive test scores to
identify patents,
including those at an early stage of the disease, for treatment and to monitor
efficacy of the
treatment. Thus, the present invention is particularly useful to treat early
stage patients,
especially, those patients having symptoms described as Mild Cognitive
Impairment (MCI)
and/or to prevent progression of MCI to Alzheimer's disease.
[0122] Various aspects of the invention are described in detail in the
following
sections. The use of sections is not meant to limit the invention. Each
section can apply to
any aspect of the invention. In this application, the use of "or" means
"and/or" unless stated
otherwise.
Therapeutic Agents
[0123] Therapeutic agents suitable for the present invention include both
calcium
channel blockers (e.g., dihydropyridine calcium channel blockers such as
nifedipine) and
non-calcium channel blockers (e.g., oxidized nifedipine, nitroso-nifedipine,
mixture of
nifedipine and its derivatives, thyroxine (T4), triiodothyronine (T3)).
[0124] In some embodiments, a therapeutic agent suitable for the present
invention is
of formula (Ia) or (lb):
R1 H
R2 R1 N R2
R3'0 I I 011 R4 R3'0 01~ R4
O O O O
R9 n R9 n
(Ia) (Ib)
28
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
or a pharmaceutically acceptable salt thereof, wherein:
Ri and R2 are independently Ci_6 aliphatic or cyano;
R3 and R4 are independently Ci_6 aliphatic;
R5 is halogen, Ci_6 aliphatic, hydroxyl, alkoxy, amino, alkylamino, cyano,
nitro, or nitroso;
and
nis0, 1, 2,or3.
[0125] In some embodiments, compounds of formula (Ia) are referred to as
"reduced"
or "dihydropyridines". In some embodiments, compounds of formula (lb) are
referred to as
"oxidized" or "dehydro".
[0126] In some embodiments, R1 and R2 are independently Ci_3 alkyl. In some
embodiments, R3 and R4 are independently Ci_4 alkyl. In some embodiments, R1
and R2 are
methyl. In some embodiments, R3 and R4 are methyl.
[0127] In some embodiments, a therapeutic agent suitable for the present
invention is
nifedipine, oxidized nifedipine, or nitroso-nifedipine. As used herein,
"nitroso-nifedipine" is
an oxidized analog of nifedipine, as shown below.
H
N N I N
0 0~ 0 0~ 0 0
0 I 0 0 O 0 I O
N02 NO2 N=O
nifedipine oxidized nifedipine nitroso-nifedipine
[0128] In some embodiments, therapeutic agents suitable for the present
invention
include, but are not limited to, dihyropyridine compounds such as amlodipine,
aranidipine,
azelnidipine, barnidipine, benidipine, cilnidipine, clevidipine, efonidipine,
felodipine,
isradipine, lacidipine, manidipine, lercanidipine, nicardipine, nifedipine,
nilvadipine,
nimodipine, nisoldipine, nitrendipine, and pranidipine. In some embodiments,
therapeutic
agents suitable for the present invention include, but are not limited to,
oxidized amlodipine,
oxidized aranidipine, oxidized azelnidipine, oxidized barnidipine, oxidized
benidipine,
oxidized cilnidipine, oxidized clevidipine, oxidized efonidipine, oxidized
felodipine, oxidized
29
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
isradipine, oxidized lacidipine, oxidized manidipine, oxidized lercanidipine,
oxidized
nicardipine, oxidized nifedipine, oxidized nilvadipine, oxidized nimodipine,
oxidized
nisoldipine, oxidized nitrendipine, and oxidized pranidipine. It will be
understood by one of
ordinary skill in the art that an "oxidized" dihydropyridine compound (e.g.,
oxidized
amlodipine, oxidized nimodipine, oxidized nivaldipine) is the pyridine version
of said
compound.
H
N N
O I I O~\0/ ~O
O O O O
N02 N02
nimodipine oxidized nimodipine
H
N O /NH2 N O /NH2
0 I I 0\/ 0 0,,-,,,-
0 O O O
I I
amlodipine oxidized amlodipine
H
N CN N CN
O I I O O O
O O O O
N02 N02
nilvadipine oxidized nilvadipine
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
[0129] Further exemplary therapeutic agents include the following:
H
N
H
N N
o\ I I O 0
O O 0 0
\ I \ N02 NO2
H
N
H H
N N o ~ I I 0
O 0
O O
/ I \
F F
H
N
H H
N N o ~ I I 0
F F F
O O 0 0
\I \I
F F
H
N
o N
~ I I 0
O O
O O
F F
31
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
H H H
N N N
O O\ O O\ O O
O O O O O O
F
OH OH OH
H H H
N N N
O I O\ O llhIIIIIo\ /O O
O O O O O O
F F F F
OH OH OH
H H H
N N N
O O\ O O\ O O
O O O O O O
F F \ \
I I I
OH OH OH
H H
H N N
O N O\ O O\ O O
/
O O O O O O
Z Z Z Z
Z Z
O O
O Z
HO HO
HO Z Z
wherein Z is H, F, Cl, Br, or I;
32
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
H
N N N
Too Too Too
Z
z z
O
O o'Q OH z z
OH OH Z OH
wherein Z is H, F, Cl, Br, or I.
[01301 In some embodiments, a therapeutic agent suitable for the present
invention is
of formula (Ic):
0 R1
R3
O N
2
R
(R5)
N O
H
(Ic)
or a pharmaceutically acceptable salt thereof, wherein:
R1 and R2 are independently an optionally substituted group selected from Ci_6
aliphatic, Ci_6 heteroaliphatic, aryl, heteroaryl, or cyano;
R3 is an optionally substituted group selected from Ci_6 aliphatic, Ci_6
heteroaliphatic
or aryl;
R5 is halogen, optionally substituted Ci_6 aliphatic, optionally substituted
Ci_6
heteroaliphatic, hydroxyl, alkoxy, amino, alkylamino, cyano, nitro, or
nitroso; and
n is 0, 1, 2, or 3.
33
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
[0131] In some embodiments, a therapeutic agent suitable for the present
invention is
of formula (Ic-i):
0 R1
R3
O N
2
R
(R5)
N O
H
(Ic-i)
or a pharmaceutically acceptable salt thereof, wherein:
Ri and R2 are independently Ci_6 aliphatic or cyano;
R3 is C1-6 aliphatic;
R5 is halogen, Ci_6 aliphatic, hydroxyl, alkoxy, amino, alkylamino, cyano,
nitro, or
nitroso; and
n is 0, 1, 2, or 3.
[0132] As defined generally above, R1 of formula (Ic) is an optionally
substituted
group selected from Ci_6 aliphatic, Ci_6 heteroaliphatic, aryl, heteroaryl, or
cyano. In some
embodiments, R1 is substituted. In some embodiments, R1 is unsubstituted. In
some
embodiments, R1 is C1-6 aliphatic. In some embodiments, R1 is Ci_4 alkyl. In
some
embodiments, R1 is methyl, ethyl, propyl, butyl, or isopropyl. In some
embodiments, R1 is
methyl. In some embodiments, R1 is isopropyl. In some embodiments, R1 is
cyano. In some
embodiments, R1 is Ci_6 heteroaliphatic. In some embodiments, R1 is -
OCH2CH2NH2. In
some embodiments, R1 is aryl. In some embodiments, R1 is heteroaryl.
[0133] As defined generally above, R2 of formula (Ic) is an optionally
substituted
group selected from Ci_6 aliphatic, Ci_6 heteroaliphatic, aryl, heteroaryl, or
cyano. In some
embodiments, R2 is substituted. In some embodiments, R2 is unsubstituted. In
some
embodiments, R2 is C1-6 aliphatic. In some embodiments, R2 is Ci_4 alkyl. In
some
embodiments, R2 is methyl, ethyl, propyl, butyl, or isopropyl. In some
embodiments, R2 is
methyl. In some embodiments, R2 is isopropyl. In some embodiments, R2 is
cyano. In some
34
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
embodiments, R2 is Ci_6 heteroaliphatic. In some embodiments, R2 is -
OCH2CH2NH2. In
some embodiments, R2 is aryl. In some embodiments, R2 is heteroaryl.
[0134] In some embodiments, R1 and R2 are independently Ci_3 alkyl. In some
embodiments, at least one of R1 and R2 is methyl. In some embodiments, R1 and
R2 are
methyl.
[0135] As defined generally above, R3 of formula (Ic) is an optionally
substituted
group selected from Ci_6 aliphatic, Ci_6 heteroaliphatic, or aryl. In some
embodiments, R1 is
substituted. In some embodiments, R1 is unsubstituted. In some embodiments, R1
is Ci_6
aliphatic. In some embodiments, R1 is Ci_4 alkyl. In some embodiments, R1 is
methyl, ethyl,
propyl, butyl, or isopropyl. In some embodiments, R1 is methyl. In some
embodiments, R1 is
isopropyl. In some embodiments, R1 is ethyl. In some embodiments, R1 is C1_6
heteroaliphatic. In some embodiments, R1 is -CH2CH2OCH3. In some embodiments,
R1 is
aryl.
[0136] As defined generally above, R5 of formula (Ic) is halogen, optionally
substituted Ci_6 aliphatic, optionally substituted Ci_6 heteroaliphatic,
hydroxyl, alkoxy, amino,
alkylamino, cyano, nitro, or nitroso. In some embodiments, R5 is substituted.
In some
embodiments, R5 is unsubstituted. In some embodiments, R5 is Ci_6 aliphatic.
In some
embodiments, R5 is Ci_4 alkyl. In some embodiments, R5 is methyl, ethyl,
propyl, butyl, or
isopropyl. In some embodiments, R5 is methyl. In some embodiments, R5 is
cyano. In some
embodiments, R5 is halogen. In some embodiments, R5 is Ci_6 heteroaliphatic.
In some
embodiments, R5 is hydroxyl. In some embodiments, R5 is alkoxy. In some
embodiments,
R5 is amino. In some embodiments, R5 is alkylamino. In some embodiments, R5 is
nitro. In
some embodiments, R5 is nitroso.
[0137] As defined generally above, n of formula (Ic) is 0, 1, 2, or 3. In some
embodiments, n is 0. In some embodiments, n is 1. In some embodiments, n is 2.
In some
embodiments, n is 3.
[0138] In some embodiments, a therapeutic agent suitable for the present
invention is
NFD-L1.
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
0 CH3
H3C,, 0 N
CH3
N O
H
NFD-L1
[0139] Further exemplary therapeutic agents include the following:
0 CH3 0 CH3 0 O~~NH2
"~O N N /~O N
CH3 CH3 CH3
N O N O N O
H H H
O CN O CH3
O N O N
/ I \ CH3 / I \ CN
N O N O
H H
[0140] In some embodiments, a therapeutic agent suitable for the present
invention is of
formula (II):
O OH
NH2
I I
I X
HO
Y
(II)
36
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
or a pharmaceutically acceptable salt thereof, wherein:
X is -CHz-, -0-, or -NH-; and
Yis -Hor -I.
[0141] In some embodiments, X is -CH2-. In some embodiments, X is -0-. In some
embodiments, X is -NH-.
[0142] In some embodiments, a therapeutic agent suitable for the present
invention is
thyroxine (T4) or triiodothyronine (T3):
O OH
O OH
NH2
NH2
I I
I I I O
HO
DaO
HO
triiodothyronine (T3) thyroxine (T4)
[0143] In some embodiments, a therapeutic agent suitable for the present
invention is
a mixture of various compounds described herein. For example, two or more
compounds of
formula (Ia) or (lb) can be combined to form a therapeutic agent. In some
embodiments, two
or more of nifedipine, oxidized nifedipine, and nitroso-nifedipine are
combined. In some
embodiments, T3 and/or T4 are combined with one or more of nifedipine,
oxidized
nifedipine, and nitroso-nifedipine. In certain embodiments, nifedipine,
oxidized nifedipine,
and nitroso-nifedipine are combined to form a nifedipine mix or mixture.
[0144] Compounds can be mixed at various mass or molar ratios. For example, a
therapeutic agent according to the invention can be a mixture of two or more
of nifedipine,
oxidized nifedipine, nitroso-nifedipine, NFD-L1, thyroxine (T4), and
triiodothyronine (T3) at
pre-determined mass or molar ratios. In some embodiments, a therapeutic agent
suitable for
the invention contains a mixture of nitroso-nifedipine and nifedipine. In some
embodiments,
nitroso-nifedipine and nifedipine can be mixed at a mass or molar ratio of
about 1000:1,
37
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
about 500:1, about 200:1, about 100:1, about 64:1, about 32:1, about 16:1,
about 10:1, about
8:1, about 5:1, about 4:1, about 3:1, about 2:1, about 1:1, about 1:2, about
1:3, about 1:4,
about 1:5, about 1:8, about 1:10, about 1:16, about 1:32, about 1:64, about
1:100, about
1:200, about 1:500, or about 1:1000. In some embodiments, nitroso-nefidipine
and nifedipine
can be mixed at a mass or molar ratio ranging from about 1:1000 to about
1000:1 (e.g., about
1:500 to about 500:1, about 1:200 to about 200:1, about 1:100 to about 100:1,
about 1:10 to
about 10:1, about 1:16 to about 16:1, about 1:32 to about 32:1, about 1:64 to
about 64:1,
about 1:1 to about 32:1, about 1:1 to about 10:1, about 100:1 to about 1000:1,
about 10:1 to
about 100:1, about 1:1000 to 1:1, about 1:1 to about 1000:1, or about 1:100 to
about 1:10). In
some embodiments, a therapeutic agent suitable for the invention contains a
mixture of
oxidized-nifedipine and nifedipine. In some embodiments, oxidized-nifedipine
and
nifedipine can be mixed at a mass or molar ratio of about 1000:1, about 500:1,
about 200:1,
about 100:1, about 64:1, about 32:1, about 16:1, about 10:1, about 8:1, about
5:1, about 4:1,
about 3:1, about 2:1, about 1:1, about 1:2, about 1:3, about 1:4, about 1:5,
about 1:8, about
1:10, about 1:16, about 1:32, about 1:64, about 1:100, about 1:200, about
1:500, or about
1:1000. In some embodiments, oxidized-nefidipine and nifedipine can be mixed
at a mass or
molar ratio ranging from about 1:1000 to about 1000:1 (e.g., about 1:500 to
about 500:1,
about 1:200 to about 200:1, about 1:100 to about 100:1, about 1:10 to about
10:1, about 1:16
to about 16:1, about 1:32 to about 32:1, about 1:64 to about 64:1, about 1:1
to about 32:1,
about 1:1 to about 10:1, about 100:1 to about 1000:1, about 10:1 to about
100:1, about 1:1000
to 1:1, about 1:1 to about 1000:1, or about 1:100 to about 1:10). In some
embodiments, a
therapeutic agent suitable for the invention contains a mixture of nitroso-
nifedipine and
oxidized nifedipine. In some embodiments, nitroso-nifedipine and oxidized-
nifedipine can be
mixed at a mass or molar ratio of about 1000:1, about 500:1, about 200:1,
about 100:1, about
64:1, about 32:1, about 16:1, about 10:1, about 8:1, about 5:1, about 4:1,
about 3:1, about 2:1,
about 1:1, about 1:2, about 1:3, about 1:4, about 1:5, about 1:8, about 1:10,
about 1:16, about
1:32, about 1:64, about 1:100, about 1:200, about 1:500, or about 1:1000. In
some
embodiments, nitroso-nefidipine and oxidized nifedipine can be mixed at a mass
or molar
ratio ranging from about 1:1000 to about 1000:1 (e.g., about 1:500 to about
500:1, about
1:200 to about 200:1, about 1:100 to about 100:1, about 1:10 to about 10:1,
about 1:16 to
about 16:1, about 1:32 to about 32:1, about 1:64 to about 64:1, about 1:1 to
about 32:1, about
1:1 to about 10:1, about 100:1 to about 1000:1, about 10:1 to about 100:1,
about 1:1000 to
1:1, about 1:1 to about 1000:1, or about 1:100 to about 1:10). In some
embodiments, a
38
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
therapeutic agent suitable for the invention contains a mixture of nitroso-
nifedipine, oxidized
nifedipine, and nifedipine. In some embodiments, nitroso-nifedipine, oxidized
nifedipine,
and nifedipine are mixed at a mass or molar ratio of about 5:1:3, 5:2:2,
6:3:1, 10:4:1, 3:1:5,
2:5:5, or 1:1:1. In some embodiments, a therapeutic agent contains a mixture
of T3 and T4.
In some embodiments, T3 and T4 can be mixed at a mass or molar ratio of about
1000:1,
about 500:1, about 200:1, about 100:1, about 64:1, about 32:1, about 16:1,
about 10:1, about
8:1, about 5:1, about 4:1, about 3:1, about 2:1, about 1:1, about 1:2, about
1:3, about 1:4,
about 1:5, about 1:8, about 1:10, about 1:16, about 1:32, about 1:64, about
1:100, about
1:200, about 1:500, or about 1:1000. In some embodiments, T3 and T4 can be
mixed at a
mass or molar ratio ranging from about 1:1000 to about 1000:1 (e.g., about
1:500 to about
500:1, about 1:200 to about 200:1, about 1:100 to about 100:1, about 1:10 to
about 10:1,
about 1:16 to about 16:1, about 1:32 to about 32:1, about 1:64 to about 64:1,
about 1:1 to
about 32:1, about 1:1 to about 10:1, about 100:1 to about 1000:1, about 10:1
to about 100:1,
about 1:1000 to 1:1, about 1:1 to about 1000:1, or about 1:100 to about 1:10).
In some
embodiments, various compounds and mixtures described herein can be further
combined to
generate desirable therapeutic agents for the invention. For example, a T3/T4
mix can be
combined with any of the nifedipine, nifedipine derivatives (e.g., oxidized or
nitroso-
nifedipine) or nifedipine mixtures described herein.
[0145] In some embodiments, a therapeutic agent suitable for the invention
comprises
a mixture of nitroso-nifedipine and lactam (e.g., a compound of formula (Ic)
or (Ic-i) such as
NFD-L1). In some embodiments, nitroso-nifedipine and lactam (e.g., a compound
of formula
(Ic) or (Ic-i) such as NFD-L1) can be mixed at a mass or molar ratio of about
1000:1, about
500:1, about 200:1, about 100:1, about 64:1, about 32:1, about 16:1, about
10:1, about 8:1,
about 5:1, about 4:1, about 3:1, about 2:1, about 1:1, about 1:2, about 1:3,
about 1:4, about
1:5, about 1:8, about 1:10, about 1:16, about 1:32, about 1:64, about 1:100,
about 1:200,
about 1:500, or about 1:1000. In some embodiments, nitroso-nefidipine and
lactam (e.g., a
compound of formula (Ic) or (Ic-i) such as NFD-L1) can be mixed at a mass or
molar ratio
ranging from about 1:1000 to about 1000:1 (e.g., about 1:500 to about 500:1,
about 1:200 to
about 200:1, about 1:100 to about 100:1, about 1:10 to about 10:1, about 1:16
to about 16:1,
about 1:32 to about 32:1, about 1:64 to about 64:1, about 1:1 to about 32:1,
about 1:1 to about
10:1, about 100:1 to about 1000:1, about 10:1 to about 100:1, about 1:1000 to
1:1, about 1:1
to about 1000:1, or about 1:100 to about 1:10). In some embodiments, a
therapeutic agent
39
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
suitable for the invention contains a mixture of lactam (e.g., a compound of
formula (Ic) or
(Ic-i) such as NFD-L1) and oxidized nifedipine. In some embodiments, lactam
(e.g., a
compound of formula (Ic) or (Ic-i) such as NFD-L1) and oxidized-nifedipine can
be mixed at
a mass or molar ratio of about 1000:1, about 500:1, about 200:1, about 100:1,
about 64:1,
about 32:1, about 16:1, about 10:1, about 8:1, about 5:1, about 4:1, about
3:1, about 2:1,
about 1:1, about 1:2, about 1:3, about 1:4, about 1:5, about 1:8, about 1:10,
about 1:16, about
1:32, about 1:64, about 1:100, about 1:200, about 1:500, or about 1:1000. In
some
embodiments, lactam (e.g., a compound of formula (Ic) or (Ic-i) such as NFD-
L1) and
oxidized nifedipine can be mixed at a mass or molar ratio ranging from about
1:1000 to about
1000:1 (e.g., about 1:500 to about 500:1, about 1:200 to about 200:1, about
1:100 to about
100:1, about 1:10 to about 10:1, about 1:16 to about 16:1, about 1:32 to about
32:1, about
1:64 to about 64:1, about 1:1 to about 32:1, about 1:1 to about 10:1, about
100:1 to about
1000:1, about 10:1 to about 100:1, about 1:1000 to 1:1, about 1:1 to about
1000:1, or about
1:100 to about 1:10). In some embodiments, a therapeutic agent suitable for
the invention
contains a mixture of lactam (e.g., a compound of formula (Ic) or (Ic-i) such
as NFD-L1),
oxidized nifedipine, and nifedipine. In some embodiments, lactam (e.g., a
compound of
formula (Ic) or (Ic-i) such as NFD-L1), oxidized nifedipine, and nifedipine
are mixed at a
mass or molar ratio of about 5:1:3, 5:2:2, 6:3:1, 10:4:1, 3:1:5, 2:5:5, or
1:1:1. In some
embodiments, a therapeutic agent contains a mixture of T3 and T4. In some
embodiments,
T3 and T4 can be mixed at a mass or molar ratio of about 1000:1, about 500:1,
about 200:1,
about 100:1, about 64:1, about 32:1, about 16:1, about 10:1, about 8:1, about
5:1, about 4:1,
about 3:1, about 2:1, about 1:1, about 1:2, about 1:3, about 1:4, about 1:5,
about 1:8, about
1:10, about 1:16, about 1:32, about 1:64, about 1:100, about 1:200, about
1:500, or about
1:1000. In some embodiments, T3 and T4 can be mixed at a mass or molar ratio
ranging
from about 1:1000 to about 1000:1 (e.g., about 1:500 to about 500:1, about
1:200 to about
200:1, about 1:100 to about 100:1, about 1:10 to about 10:1, about 1:16 to
about 16:1, about
1:32 to about 32:1, about 1:64 to about 64:1, about 1:1 to about 32:1, about
1:1 to about 10:1,
about 100:1 to about 1000:1, about 10:1 to about 100:1, about 1:1000 to 1:1,
about 1:1 to
about 1000:1, or about 1:100 to about 1:10). In some embodiments, various
compounds and
mixtures described herein can be further combined to generate desirable
therapeutic agents
for the invention. For example, a T3/T4 mix can be combined with any of the
nifedipine,
nifedipine derivatives (e.g., oxidized or nitroso-nifedipine), compound of
formula (I-c) (e.g.,
NFD-L1, or nifedipine mixtures described herein.
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
Biomarkers for Identifying Patients or Monitoring Treatment
[0146] Various biomarkers can be used to identify subject or patient who is
suffering
from, susceptible to or at risk of MCI or Alzheimer's disease. As used herein,
a biomarker is
a characteristic bio-molecule which is differentially present in a sample
taken from a subject
of one phenotypic status (e.g., having a disease) as compared with another
phenotypic status
(e.g., not having a disease). A biomarker is differentially present between
different
phenotypic statuses if the mean or median expression level of the biomarker in
the different
groups is calculated to be statistically significant. Biomarkers, alone or in
combination,
provide measures of relative risk that a subject belongs to one phenotypic
status or another.
Therefore, they are useful as markers for disease (diagnostics), therapeutic
effectiveness of a
drug (theranostics) and drug toxicity.
[0147] For example, the inventors have recently shown that levels of a -55 kDa
proteinaceous complex containing prostaglandin-D2-synthase and transthyretin
(PDS/TTR
complex) may serve as a sensitive and specific diagnostic biomarker of MCI and
AD, as
detailed in US Pat. Pub. No. 2008/0026405, which is incorporated herein by
reference.
[0148] Typically, the PDS/TTR complex presents in cerebrospinal fluid and
appears
to be a sensitive and specific biomarker of the disease. Formation of the
PDS/TTR complex
was been localized to the choroid plexus, an assembly of epithelial cells
located adjacent to
the lateral ventricles. The choroid plexus functions as the blood-CSF barrier.
The choroid
plexus passes water, salts and selected small molecules from the blood to the
CSF but
effectively prevents blood proteins form entering the CSF. Proteins required
for CSF are
synthesized by the choroid plexus. Thus, the choroid plexus also functions as
the source of
CSF. Epithelial cells isolated from choroid plexus obtained fresh from short
post mortem
autopsies of late stage AD patients have been grown and expanded in culture.
Examination
of cell culture medium obtained from AD epithelial cells showed elevated
levels of the
PDS/TTR complex compared to control cells. Thus, an elevated PDS/TTR complex
level as
compared to a normal control can be used to identify subjects or patients
suffering from,
susceptible to or at risk of developing MCI or Alzheimer's disease.
[0149] In some embodiments, a biomarker suitable for the present invention
comprises at least one of transthyretin and/or a prostaglandin-H2 D-isomerase,
and at least
41
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
one second protein selected from tranthyretin, prostaglandin-H2 D-isomerase,
beta-2-
microglobulin, cystatin C, superoxide dismutase [Cu-Zn], plasma retinol-
binding protein,
phosphatidylethanolamine-binding protein, carbonic anhydrase 2 and/or
serotransferrin. Mild
cognitive impairment or Alzheimer's disease status is determined by
correlating the obtained
measurement with standards.
[0150] In some embodiments, neuronal thread protein, tau (total; T-tau and
various
phosphorylated forms; P-tau), and/or derivatives of amyloid precursor protein
(APP)
including A[340 and A(342, may be used as biomarkers to identify patient
population for
treatment with compositions and methods of the present invention. In some
embodiments, a
subject in need of treatment has an abnormal level of a protein biomarker
complex as
compared to a control, wherein the protein biomarker complex comprises one or
more of (i)
beta amyloid 40 (A[340), (ii) beta amyloid 42 (A[342), (iii) the ratio of
A[340 to A[342, and (iv)
the ratio of phosphorylated tau to total tau.
[0151] Additional biomarkers have been reported in the literature and may be
used to
identify patients for treatment according to the invention including, but not
limited to, those
described in Fahnestock et al, J. Neural. Transm. Suppl. 2002(62):241-52
(2002); Masliah et
al, Neurobiol. Aging 16(4):549-56 (1995); Power et al, Dement. Geriatr. Cong.
Disord.
12(2):167-170 (2001); Burbach et al, J. Neurosci. 24(10):2421-30 (2004), Li et
al,
Neuroscience 113(3):607-15 (2002), and Sanna et al, J. Clin. Invest.
111(2):241-50 (2003),
each of which is incorporated herein by reference.
[0152] In some embodiments, a biomarker is determined in a fluid sample
obtained
from the subject. In some embodiments, a fluid sample is selected from the
group consisting
of CSF, serum, whole blood, blood plasma, urine, ascitic fluid, saliva, tissue
effusion, lavage,
and combinations thereof.
[0153] Various methods can be used to measure biomarkers qualitatively and
quantitatively. For example, to detect a protein complex (such as PDS/TTR), a
sandwich
enzyme linked immunoassay (ELISA) cane be utilized that traps a first
component of the
complex (e.g., PDS) and probes for a second component (e.g. TTR). Additional
exemplary
methods are described in US Pat. Pub. No. 2008/0026405, which is incorporated
herein by
42
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
reference. Other methods are well known in the art and can be used to practice
the present
invention.
[0154] Typically, the measured level of a biomarker is compared to one or more
controls or reference levels. Suitable reference level used for comparison
with the measured
level for a AD biomarker may vary, depending on aspect of the invention being
practiced, as
will be understood by one of ordinary skill in the art. To identify subjects
suffering from or
susceptible to AD or MCI, a suitable "reference level" is typically a level
indicative of
healthy individuals, in particular, age-matched healthy individuals. A
reference level can be
determined in parallel with patient sample. A reference level can also be a
pre-determined
level or based on historical data. For example, a suitable reference level can
be an average of
levels obtained from a population that is not afflicted with AD or MCI.
Typically, a suitable
reference level is derived from (e.g., is the mean or median of) levels
obtained from an age-
matched population.
[0155] Typically, a subject in need of treatment has an greater or elevated
level of a
biomarker described herein as compared to a control or reference level
indicative of a healthy
individual or population.
[0156] For treatment monitoring purposes, a suitable reference level is
typically a
level indicative of healthy individuals or individuals suffering from
Alzheimer's disease (e.g.,
with a pre-determined stage, such as MCI, EAD, or LAD). A reference level can
be
determined in parallel with patient sample. A reference level can also be a
pre-determined
level or based on historical data. For example, a suitable reference level can
be an average of
levels obtained from a population that is not afflicted with AD or MCI, or a
population that
has been diagnosed with MCI or AD (e.g., EAD or LAD). Alternately, a suitable
reference
level may be a historical reference level for a particular patient, for
example, a level that was
obtained from a sample derived from the same individual, but at an earlier
point in time (e.g.,
before the treatment or an earlier point in the treatment). Typically, a
suitable reference level
is derived from (e.g., is the mean or median of) levels obtained from an age-
matched
population.
[0157] For AD patient stratification (i.e., methods of stratifying AD patients
into
mild, moderate and severe stages of AD), suitable reference levels are
normally derived from
43
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
(e.g., is the mean or median of) levels obtained from a population which has
been diagnosed
with a particular stage of AD (e.g., EAD or LAD) or MCI.
[0158] In some embodiments, the level of a suitable biomarker (such as the -55
kDa
PDS/TTR complex) can be used to monitor the efficacy of the treatment.
Typically, the goal
of a therapy would be, ideally, to decrease, lower or diminish the level of
the PDS/TTR
complex in a subject so that a fluid sample taken from the subject would
contain no
detectable complex. A more conservative, subsidiary, goal of therapy would be
to forestall
any increase in the level of the -55 kDa PDS/TTR complex. Accordingly, a
person of
ordinary skill in the medical therapeutic arts would be able to determine
whether a given
therapeutic regime is accomplishing the chosen therapeutic goal based on the
level of an
appropriate biomarker. In this way, a person of ordinary skill in the medical
therapeutic arts
would also be able to determine the effective amount of a therapeutic agent
described herein
based on the measured level of a suitable biomarker as compared to appropriate
controls or
reference levels.
[0159] Typically, aged-matched populations are used to derive various
reference
levels. Age-matched populations are ideally the same age as the individual
being tested, but
approximately age-matched populations are also acceptable. Approximately age-
matched
populations may be within 1, 2, 3, 4, or 5 years of the age of the individual
tested, or may be
groups of different ages which encompass the age of the individual being
tested.
Approximately age-matched populations may be in 2, 3, 4, 5, 6, 7, 8, 9, or 10
year increments
(e.g. a "5 year increment" group which serves as the source for reference
values for a 62 year
old individual might include 58-62 year old individuals, 59-63 year old
individuals, 60-64
year old individuals, 61-65 year old individuals, or 62-66 year old
individuals).
[0160] The process of comparing a measured value and a reference value can be
carried out in any convenient manner appropriate to the type of measured value
and reference
value for the AD biomarker at issue. For example, "measuring" can be performed
using
quantitative or qualitative measurement techniques, and the mode of comparing
a measured
value and a reference value can vary depending on the measurement technology
employed.
For example, the measured values used in the methods of the invention will
most commonly
be quantitative values (e.g., quantitative measurements of concentration, such
as nanograms
of AD biomarker per milliliter of sample, or absolute amount). As with
qualitative
44
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
measurements, the comparison can be made by inspecting the numerical data, by
inspecting
representations of the data (e.g., inspecting graphical representations such
as bar or line
graphs). As a non-limiting example, a measured value is generally considered
to be
substantially equal to or greater than a reference value if it is at least
about 95% of the value
of the reference value (e.g., a measured value of 1.71 can be considered
substantially equal to
a reference value of 1.80). A measured value is considered less or lower than
a reference
value if the measured value is less than 95% of the reference value (e.g., a
measured value of
1.7 can be considered less than a reference value of 1.80).
Tests of cognitive function
[0161] Various cognitive tests may also be used to identify subject or patient
who is
suffering from, susceptible to or at risk of MCI or Alzheimer's disease. Two
exemplary
cognitive tests are the Mini Mental Status Examination (MMSE) and the Clinical
Dementia
Rating (CDR).
[0162] In some embodiments, an MMSE score is used to identify a subject in
need of
treatment with the compositions and methods described herein. An MMSE score is
a
composite score representing multiple tests of cognitive function. The maximum
possible
total MMSE score is 30 points. The MMSE can be used to classify the severity
of cognitive
impairment in patients with dementia or other medical conditions. Table 1
shows how
MMSE scores generally represent degrees of cognitive function.
Table 1
MMSE Score Cognitive Function
27-30 normal cognitive function
21-26 mild cognitive impairment
11-20 moderate cognitive impairment
0-10 severe cognitive impairment
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
[0163] In some embodiments, a subject in need of treatment has an MMSE score
of
21-26 (mild cognitive impairment), 11-20 (moderate cognitive impairment), or 0-
10 (severe
cognitive impairment).
[0164] In some embodiments, a CDR score is used to identify a subject in need
of
treatment with the compositions and methods described herein. An CDR score is
constructed
from six domains that are scored individually: memory, orientation, judgment
and problem
solving, community affairs, home and hobbies, and personal care. Table 2 shows
how CDR
scores generally represent degrees of cognitive function.
Table 2
MMSE Score Cognitive Function
0 no cognitive impairment
0.5 very mild dementia
1 mild dementia
2 moderate dementia
3 severe dementia
[0165] In some embodiments, a CDR score above 0 indicates that a subject may
be
suffering from, susceptible to or at risk of MCI or Alzheimer's disease. In
some
embodiments, a subject in need of treatment may have a CDR score of 0.5 (very
mild
dementia), 1 (mild dementia), 2 (moderate dementia), or 3 (severe dementia).
[0166] In some embodiments, a cognitive test score (such as an MMSE score or
CDR
score) can be used to monitor the efficacy of the treatment. Typically, an
effective therapy
should improve the cognitive test score. Therefore, by comparing the cognitive
test scores
before and after the treatment or from different time points of a treatment
regimen, a person
of ordinary skill in the medical therapeutic arts can determine whether a
given therapeutic
regime is effective. For example, a person of ordinary skill in the medical
therapeutic arts
would be able to determine or adjust the effective amount of a therapeutic
agent described
herein by based on relative cognitive test scores determined before the
treatment or from
different time points of a treatment regimen.
46
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
Pharmaceutical Compositions and Administration
[0167] The present invention encompasses pharmaceutical compositions
comprising
therapeutic agents such as those disclosed herein. In some embodiments, a
pharmaceutical
composition of the invention contain a therapeutically effective amount of a
therapeutic agent
and a pharmaceutically acceptable carrier.
[0168] As used herein, the term "pharmaceutically acceptable carrier" means a
non-
toxic, inert solid, semi-solid liquid filler, diluent, encapsulating material,
formulation
auxiliary of any type, or simply a sterile aqueous medium, such as saline.
Some examples of
the materials that can serve as pharmaceutically acceptable carriers are
sugars, such as
lactose, glucose and sucrose, starches such as corn starch and potato starch,
cellulose and its
derivatives such as sodium carboxymethyl cellulose, ethyl cellulose and
cellulose acetate;
powdered tragacanth; malt, gelatin, talc; excipients such as cocoa butter and
suppository
waxes; oils such as peanut oil, cottonseed oil, safflower oil, sesame oil,
olive oil, corn oil and
soybean oil; glycols, such as propylene glycol, polyols such as glycerin,
sorbitol, mannitol
and polyethylene glycol; esters such as ethyl oleate and ethyl laurate, agar;
buffering agents
such as magnesium hydroxide and aluminum hydroxide; alginic acid; pyrogen-free
water;
isotonic saline, Ringer's solution; ethyl alcohol and phosphate buffer
solutions, as well as
other non-toxic compatible substances used in pharmaceutical formulations.
[0169] Wetting agents, emulsifiers and lubricants such as sodium lauryl
sulfate and
magnesium stearate, as well as coloring agents, releasing agents, coating
agents, sweetening,
flavoring and perfuming agents, preservatives and antioxidants can also be
present in the
composition, according to the judgment of the formulator. Examples of
pharmaceutically
acceptable antioxidants include, but are not limited to, water soluble
antioxidants such as
ascorbic acid, cysteine hydrochloride, sodium bisulfite, sodium metabisulfite,
sodium sulfite,
and the like; oil soluble antioxidants, such as ascorbyl palmitate, butylated
hydroxyanisole
(BHA), butylated hydroxytoluene (BHT), lecithin, propyl gallate, alpha-
tocopherol and the
like; and the metal chelating agents such as citric acid, ethylenediamine
tetraacetic acid
(EDTA), sorbitol, tartaric acid, phosphoric acid and the like.
47
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
[0170] The term "therapeutically effective amount" or simply "effective
amount" of a
therapeutic agent, as used herein, refers to an amount of therapeutic agent
that is sufficient,
when administered to a subject in need of treatment according to an
appropriate regimen, to
alleviate, ameliorate, stabilize, and/or delay the onset of at least one
symptom or feature
associated with MCI or Alzheimer's disease as well as delay in progression of
one or more
symptoms of MCI or Alzheimer's disease (e.g., delay in progression with
respect to
abundance, intensity, severity, or frequency). It will be understood, however,
that the total
daily usage of the therapeutic agents and compositions of the present
invention will be
decided by the attending physician within the scope of sound medical judgment.
The specific
therapeutically effective dose level for any particular patient will depend
upon a variety of
factors including the disorder being treated and the severity of the disorder;
activity of the
specific compound employed; the specific composition employed; the age, body
weight,
general health, sex and diet of the patient; the time of administration, route
of administration,
and rate of excretion of the specific compound employed; the duration of the
treatment; drugs
used in combination or coinciding with the specific compound employed; and
like factors
well known in the medical arts.
[0171] In some embodiments, a therapeutically effective dose of a therapeutic
agent
of the present invention can range, for example, from 0.01 to 100 mg/kg body
weight or
more. In some embodiments, a therapeutically effective dose of a therapeutic
agent of the
present invention ranges from about 0.1 to about 50 mg/kg body weight (e.g.,
about 0.1 to
about 35 mg/kg, about 0.1 to about 15 mg/kg, about 6.25 to about 35 mg/kg,
about 12.5 to
about 35 mg/kg, about 6.25 to about 25 mg/kg, about 35 mg/kg). In some
embodiments, a
therapeutically effective amount of a therapeutic agent ranges from about 0.01
mg to about
2.5 g per dose (e.g., from about 0.01 mg to about 2.0 g, from about 0.01 mg to
about 1.5 g,
from about 0.01 mg to about 1.0 g, per dose). In some embodiments, a
therapeutically
effective amount of a therapeutic agent ranges from about 0.01 to about 1000
mg (e.g., about
0.01 to about 500 mg, about 0.01 to about 250 mg, about 0.01 to about 200 mg,
about 0.01 to
about 150 mg, about 0.01 to about 100 mg, about 0.01 to about 50 mg, about
0.01 to about 10
mg, about 0.01 to about 5 mg, about 0.01 to about 2.5 mg, about 0.01 to about
2.0 mg, about
0.01 to about 1.5 mg, about 0.01 to about 1.0 mg, about 0.01 to about 0.5 mg,
about 0.01 to
about 0.1 mg) per dose. In some embodiments, the therapeutically effective
amount of a
therapeutic agent (in particular, nitroso-nifedipine) ranges from about 100 mg
to about 5 g
48
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
(e.g., about 100 mg to about 3 g, about 100 mg to about 2.5 g, about 100 mg to
about 2 g,
about 100 mg to about 1.5 g, about 100 mg to about 1000 mg, about 100 mg to
about 500 mg,
about 100 mg to about 250 mg) per dose. In some embodiments, a therapeutically
effective
amount of a therapeutic agent can be about 0.01 mg, about 0.05 mg, about 0.1
mg, about 0.5
mg, about 1 mg, about 5 mg, about 10 mg, about 25 mg, about 50 mg, about 100
mg, about
500 mg, about 1000 mg, about 1.5 g, about 2 g, about 2.5 g, about 3 g, or
about 5 g per dose.
Typically, the amount described herein is the total amount of all active
compounds in a
composition. For example, if a composition contains a mix of nifedipine,
nitroso-nifedipine
and oxidized nifedipine, a therapeutically effective amount is the combined
amount of
nifedipine, nitroso-nifedipine and oxidized nifedipine.
[0172] In some embodiments, a therapeutically effective amount of a
therapeutic
agent as described herein is an amount insufficient to induce an adverse event
(e.g., liver
toxicity) in a human subject.
[0173] In some embodiments, a therapeutic agent as described herein is
administered
once daily. In some embodiments, a therapeutic agent as described herein is
administered
multiple times per day, e.g., twice, three times, or four times daily. In some
embodiments, a
total daily dose of a therapeutic agent ranges from about 0.01 mg to about 5 g
per day in
multiple doses or in a single dose (e.g., from about 0.01 mg to about 4.0 g,
from about 0.01
mg to about 3.0 g, from about 0.01 mg to about 2.5 g, from about 0.01 mg to
about 2.0 g,
from about 0.01 mg to about 1.5 g, from about 0.01 mg to about 1.0 g, per day
in multiple
doses or in a single dose). In some embodiments, a total daily dose of a
therapeutic agent
ranges from about 0.01 to about 1000 mg (e.g., about 0.01 to about 500 mg,
about 0.01 to
about 250 mg, about 0.01 to about 200 mg, about 0.01 to about 150 mg, about
0.01 to about
100 mg, about 0.01 to about 50 mg, about 0.01 to about 10 mg, about 0.01 to
about 5 mg,
about 0.01 to about 2.5 mg, about 0.01 to about 2.0 mg, about 0.01 to about
1.5 mg, about
0.01 to about 1.0 mg, about 0.01 to about 0.5 mg, about 0.01 to about 0.1 mg)
per day in a
single dose or in multiple doses. In some embodiments, a total daily dose of a
therapeutic
agent (in particular, nitroso-nifedipine) ranges from about 50 mg to about 5 g
(e.g., about 50
mg to about 4 g, about 100 mg to about 3 g, about 100 mg to about 2.5 g, about
100 mg to
about 2 g, about 100 mg to about 1.5 g, about 100 mg to about 1000 mg, about
100 mg to
about 500 mg, about 100 mg to about 250 mg) per day in a single dose or in
multiple doses.
In some embodiments, a total daily dose of a therapeutic agent can be about
0.01 mg, about
49
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
0.05 mg, about 0.1 mg, about 0.5 mg, about 1 mg, about 5 mg, about 10 mg,
about 25 mg,
about 50 mg, about 100 mg, about 500 mg, about 1000 mg, about 1.5 g, about 2
g, about 2.5
g, about 3 g, about 3.5 g, about 4 g, about 4.5 g, or about 5 g. Typically, an
amount described
herein is the total amount of all active compounds in a composition. For
example, if a
composition contains a mix of nifedipine, nitroso-nifedipine and oxidized
nifedipine, a
therapeutically effective amount is the combined amount of nifedipine, nitroso-
nifedipine and
oxidized nifedipine.
[0174] In some embodiments, a therapeutic agent as described herein is
administered
monthly, bi-weekly, weekly, twice a week, or three times a week. In these
instances, the
daily doses described above reflects the average daily dose.
[0175] In certain situations, it may be important to maintain a fairly high
dose of the
active agent in the blood stream of the patient, particularly early in the
treatment. Hence, at
least initially, it may be important to keep the dose relatively high and/or
at a substantially
constant level for a given period of time, e.g., at least about six or more
hours, e.g., at least
about twelve or more hour, e.g., at least about twenty-four or more hours.
[0176] The compounds of the present invention may be administered alone or in
combination or in concurrent therapy with other agents which affect the
central or peripheral
nervous system, particularly selected areas of the brain.
[0177] Pharmaceutical compositions according to the present invention may be
administered by any route, including oral, subcutaneous, intravenous,
intraperitoneal,
intramuscular, intracerebroventricular, intraparenchymal, intrathecal,
intracranial, buccal,
mucosal, nasal, rectal, auricular, conjunctival, cutaneous, electro-osmosis,
endocervical,
endosinusial, endotracheal, enteral, epidural, extra-amniotic, extracorporeal,
hemodialysis,
infiltration, interstitial, intra-abdominal, intra-amniotic, intra-arterial,
intra-articular,
intrabiliary, introbrochial, intrabursal, intracardiac, intracaritlaginous,
intracavitary,
intracerebral, intracisternal, intracorneal, intracoronal, intracoronary,
intracorporus
cavernosum, intradermal, intradiscal, intraductal, intraduodenal, intradural,
intraepidermal,
intraesophageal, intragastric, intragingival, intraileal, intralesional,
intralymphatic,
intramedullary, intrameningeal, intramuscular, intraocular, intraovarian,
intrapericardial,
intrapleural, intraprostatic, intrapulmonary, intrasinal, intrasynovial,
intratendinous,
intratesticular, intrathecal, intrathroacic, intratubular, intratumor,
intratympanic, intrauterine,
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
intravascular, intravenous bolus, intravenous drip, intraventricular,
intravesical, intravitreal,
iontophoresis, irrigation, laryngeal, nasogastric, occlusive dressing
technique, ophthalmic,
oropharyngeal, parenteral, percutaneous, peridural, perineural, periodontal,
respiratory,
retrobulbar, soft tissue, subarachnoid, subconjunctival, sublingual,
submucosal, topical,
transdermal, transmucosal, transplacental, transtracheal, transtympanic,
ureteral, urethral, and
vaginal. In certain embodiments, a pharmaceutical composition of the present
invention is
administered by a route selected from oral, subcutaneous, intravenous,
transdermal,
intraperitoneal, intramuscular, intracerebroventricular, intraparenchymal,
intrathecal,
intracranial, buccal, mucosal, nasal, and rectal. In certain embodiments, a
pharmaceutical
composition of the present invention is administered orally.
[0178] Liquid dosage forms for oral administration may include
pharmaceutically
acceptable emulsions, microemulsions, solutions, suspensions, syrups and
elixirs containing
inert diluents commonly used in the art, such as water, isotonic solutions, or
saline. Such
compositions may also comprise adjuvants, such as wetting agents; emulsifying
and
suspending agents; sweetening, flavoring and perfuming agents.
[0179] Injectable preparations, for example, sterile injectable aqueous or
oleaginous
suspensions may be formulated according to the known art using suitable
dispersing or
wetting agents and suspending agents. The sterile injectable preparation may
also be a sterile
injectable solution, suspension or emulsion in a nontoxic parenterally
acceptable diluent or
solvent, for example, as a solution in 1,3-butanediol. Among the acceptable
vehicles and
solvents that may be employed are water, Ringer's solution, U.S.P. and
isotonic sodium
chloride solution. In addition, sterile, fixed oils are conventionally
employed as a solvent or
suspending medium. For this purpose any bland fixed oil can be employed
including
synthetic mono- or diglycerides. In addition, fatty acids such as oleic acid
are used in the
preparation of injectables.
[0180] The injectable formulation can be sterilized, for example, by
filtration through
a bacteria-retaining filter, or by incorporating sterilizing agents in the
form of sterile solid
compositions, which can be dissolved or dispersed in sterile water or other
sterile injectable
medium just prior to use.
[0181] In order to prolong the effect of a drug, it is often desirable to slow
the
absorption of a drug from subcutaneous or intramuscular injection. The most
common way
51
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
to accomplish this is to inject a suspension of crystalline or amorphous
material with poor
water solubility. The rate of absorption of the drug becomes dependent on the
rate of
dissolution of the drug, which is, in turn, dependent on the physical state of
the drug, for
example, the crystal size and the crystalline form. Another approach to
delaying absorption
of a drug is to administer the drug as a solution or suspension in oil.
Injectable depot forms
can also be made by forming microcapsule matrices of drugs and biodegradable
polymers,
such as polylactide-polyglycoside. Depending on the ratio of drug to polymer
and the
composition of the polymer, the rate of drug release can be controlled.
Examples of other
biodegradable polymers include polyorthoesters and polyanhydrides. Depot
injectables can
also be made by entrapping the drug in liposomes or microemulsions, which are
compatible
with body tissues.
[0182] Suppositories for rectal administration of the drug can be prepared by
mixing
the drug with a suitable non-irritating excipient, such as cocoa butter and
polyethylene glycol
which are solid at ordinary temperature but liquid at the rectal temperature
and will,
therefore, melt in the rectum and release the drug.
[0183] Solid dosage forms for oral administration include, but are not limited
to,
capsules, tablets, pills, powders, gelcaps and granules. In such solid dosage
forms,
therapeutic agent may be admixed with at least one inert diluent such as
sucrose, lactose or
starch. Such dosage forms may also comprise additional substances other than
inert diluents,
e.g., tableting lubricants and other tableting aids such as magnesium stearate
and
microcrystalline cellulose. In the case of capsules, tablets and pills, the
dosage forms may
also comprise buffering agents. Tablets and pills can additionally be prepared
with enteric
coatings and other release-controlling coatings.
[0184] Solid compositions of a similar type may also be employed as fillers in
soft
and hard-filled gelatin capsules using such excipients as lactose or milk
sugar as well as high
molecular weight polyethylene glycols and the like.
[0185] The active compounds can also be in micro-encapsulated form with one or
more excipients as noted above. The solid dosage forms of tablets, dragees,
capsules, pills,
and granules can be prepared with coatings and shells such as enteric coatings
and other
coatings well known in the pharmaceutical formulating art. They may optionally
contain
opacifying agents and can also be of a composition that they release the
active ingredient(s)
52
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
only, or preferably, in a certain part of the intestinal tract, optionally in
a delayed manner.
Examples of embedding compositions which can be used include polymeric
substances and
waxes.
[0186] Dosage forms for topical or transdermal administration of a compound of
this
invention further include ointments, pastes, creams, lotions, gels, powders,
solutions, sprays,
inhalants or patches. The active component is admixed under sterile conditions
with a
pharmaceutically acceptable carrier and any needed preservatives or buffers as
may be
required. Ophthalmic formulations, ear drops, eye ointments, powders and
solutions are also
contemplated as being within the scope of this invention.
[0187] The ointments, pastes, creams and gels may contain, in addition to an
active
compound of this invention, excipients such as animal and vegetable fats,
oils, waxes,
paraffins, starch, tragacanth, cellulose derivatives, polyethylene glycols,
silicones, bentonites,
silicic acid, talc and zinc oxide, or mixtures thereof.
[0188] Powders and sprays can contain, in addition to the active compounds of
this
invention, excipients such as lactose, talc, silicic acid, aluminum hydroxide,
calcium silicates
and polyamide powder, or mixtures of these substances. Sprays can additionally
contain
customary propellants, such as chlorofluorohydrocarbons.
[0189] Transdermal patches can provide controlled delivery of active compound
to
the body. Such dosage forms can be made by dissolving or dispersing the
compound in the
proper medium. Absorption enhancers can also be used to increase the flux of
the compound
across the skin. The rate can be controlled by either providing a rate
controlling membrane
or by dispersing the compound in a polymer matrix or gel.
[0190] Pharmaceutical compositions described herein can be formulated for
immediate release or controlled release (also referred to as slow, sustained
or extended
release). Various slow or extended release formulations or devices are well
known to those
of ordinary skill in the art. Examples include, but are not limited to, those
described in U.S.
Pat. Nos. 5,674,533, 5,059,595, 5,120,548, 5,073,543, 5,639,476, 5,354,556,
and 5,733,566,
each of which is incorporated herein by reference. Such dosage forms can be
used to provide
slow or controlled-release of one or more active ingredients using, for
example,
hydropropylmethyl cellulose, other polymer matrices, gels, permeable
membranes, osmotic
53
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
systems, multilayer coatings, microparticles, liposomes, microspheres, or a
combination
thereof to provide the desired release profile in varying proportions.
Suitable controlled-
release formulations known to those of ordinary skill in the art can be
readily selected and
adapted for use with therapeutic agents of the invention. For example, the
invention
encompasses solid oral dosage forms such as, but not limited to, tablets,
capsules, gelcaps,
and caplets that are formulated for controlled-release (i.e., slow release,
extended release, or
sustained release).
[0191] Advantages of controlled-release formulations include extended activity
of the
drug, reduced dosage frequency, and increased patient compliance. For example,
controlled
or extended release formulations can keep adequate dose levels constantly
available inside a
patient body to enhance delivery across the blood-brain barrier.
[0192] Most controlled-release formulations are designed to initially release
an
amount of therapeutic agent (active ingredient) that promptly produces the
desired
therapeutic effect, and gradually and continually release of other amounts of
thug to maintain
this level of therapeutic or prophylactic effect over an extended period of
time. In order to
maintain this constant level of drug inside the body, the drug must be
released from the
dosage form at a rate that will replace the amount of drug being metabolized
and excreted
from the body. Controlled-release of an active ingredient can be stimulated by
various
conditions including, but not limited to, pH, temperature, enzymes, water, or
other
physiological conditions or compounds.
[0193] In some embodiments, two or more therapeutic agents may be administered
in
combination. The two or more therapeutic agents may be administered separately
from one
another, as part of a multiple dosage regimen. Alternatively, those agents may
be part of a
single dosage form, mixed together with a compound of this invention in a
single
composition. If administered as part of a multiple dosage regime, the two
therapeutic agents
may be submitted simultaneously, sequentially or within a period of time from
one another
normally within five hours from one another.
[0194] As used herein, the term "combination," "combined," and related terms
refers
to the simultaneous or sequential administration of therapeutic agents in
accordance with this
invention. For example, a compound of the present invention may be
administered with
another therapeutic agent simultaneously or sequentially in separate unit
dosage forms or
54
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
together in a single unit dosage form. Accordingly, the present invention
provides a single
unit dosage form comprising a provided compound, an additional therapeutic
agent, and a
pharmaceutically acceptable carrier, adjuvant, or vehicle.
[0195] The invention is provided in numerous embodiments and can be discerned,
inter alia, in various examples. The following examples provide an
illustrative but non-
limiting description of the breadth and applicability of the invention.
EXAMPLES
Example 1. Neurotoxic effect of PDS/TTR complex
[0196] It has been shown that examination of cell culture medium obtained from
epithelial cells derived from Alzheimer's disease patients showed elevated
levels of the
PDS/TTR complex compared to control cells, indicating that the PDS/TTR complex
can be
used as an effective biomarker for the early diagnosis of Alzheimer's disease.
See, U.S.
Application Pub. No. 20080026405, the disclosure of which is incorporated by
reference
herein. This example shows that in addition to being a biomarker for disease,
the PDS/TTR
complex is also neurotoxic.
[0197] First of all, it was found that acrolein (an alpha, beta unsaturated
three carbon
aldehydic by-product of lipid peroxidation) causes normal control epithelial
cells to express
the PDS/TTR complex into culture medium at comparable levels to the epithelial
cells
derived from Alzheimer's disease patients. For these experiments, primary
cultures of
choroid plexus epithelail cells were established from short post mortem
interval autopsies
using established methods. AD and normal control cultures were grown to
confluence in
MEM growth medium containing 2% fetal bovine serum and 1% epithelial growth
factor
(EGF). Normal control cultures were switched to Opti-MEM containing N2
supplement and
were treated with vehicle (controls) or with 5 M acrolein for 72 hours.
Cultures from AD
subjects were switched to N2 supplemented medium and maintained for 72 hours.
After
treatment, medium was collected from each flask and was desalted using PD-10
columns.
The eluted proteins were then freeze-dried, resuspended in 25 l water and
analyzed using
Western blot analysis and antibodies specific to PDS and TTR. Figure 1 shows
exemplary
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
results illustrating an Western blot analysis of the PDS/TTR complex expressed
in cell
culture medium by control epithelial cells, control epithelial cells treated
with acrolein, and
epithelial cells derived from late stage Alzheimer's disease patients. As
shown in Figure 1,
acrolein increased the expression of the PDS/TTR complex in control epithelial
cells to a
level comparable to that in late stage AD (LAD) epithelial cells.
[0198] Medium from LAD epithelial cells that contains the PDS/TTR complex was
then used to treat cortical neurons. To determine if PDS/TTR complex generated
by LAD
choroid plexus epithelial cultures or normal control cultures treated with
vehicle or with 5
M acrolein negatively impacted primary cortical neurons, normal control
epithelial cultures
were switched to N2 supplemented medium and treated with vehicle alone
(controls) or with
M acrolein for 16 hours. LAD cultures were switched to N2 medium for 16 hours.
After
treatment, medium was collected from each culture type (LAD; normal controls
treated with
vehicle alone or normal controls treated with 5 M acrolein) and was added to
primary rat
neuron cultures (7 days in culture). Primary cultures treated were subjected
to the
conditioned medium for 16 hours and cell viability was measured using MTT
reduction
assays. An exemplary result was shown in Figure 2. As shown in Figure 2, the
survival rate
for cortical neurons treated with LAD epithelial medium is significantly lower
as compared
to the control (Figure 2). This experiment indicates that the PDS/TTR complex
is itself
neurotoxic.
[0199] To further investigate the neurotoxic effect of the PDS/TTR complex,
SYSY
neuroblastoma cells were exposed to conditioned medium from LAD or normal
control
epithelial cells for 16 hours. Following exposure to conditioned medium, cells
were fixed in
70% methanol/30% acetone and were subjected to immunohistochemistry using anti-
PHF-1
antibody. PHF-1 recognizes aberrantly phosphorylated Tau as observed in AD NFT
(neurofibrillary tangles). As shown in Figure 3, close to 25% of SYSY cells
treated with
LAD epithelial cell medium are PHF-1 positive as compared to about 3% of SYSY
cells
treated with control medium. Paired helical filaments are precursors to
neurofibrillary
tangles. Therefore, PHF1 immunopositivity is typically an indicator of the
formation of late-
stage neurofibrillary tangles in Alzheimer's disease. Thus, this experiment
shows that the
PDS/TTR complex promotes the formation of paired helical filaments in SYSY
neuroblastoma cells, indicating the PDS/TTR that complex can promote amyloid
beta peptide
(AB) generation by H4 neuroglioma cells.
56
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
[0200] To determine if the complex generated by epithelial cultures would
impact
inflammatory cytokine pathways, cultures of human astrocytomas were plated at
a density of
2.5 X 105 cells/well and were exposed to conditioned medium for 24 hours.
Following
exposure medium was collected from each well and levels of inflammatory
cytokines (IL-6,
TNF-a, TGF-B and IL-6) were determined using commercially available ELISAs.
Results of
the assays showed the PDS/TTR complex activated 2 inflammatory cytokine
pathways (i.e.,
IL-6, TNF-a) in astrocytoma cultures (data not shown), indicating a role of
the PDS/TTR in
neuroinflammation.
[0201] In summary, the experiments described in this example established that
the
PDS/TTR complex causes various biochemical changes that can directly impact
hallmarks of
Alzheimer's disease.
Example 2. Nifedipine, Nifedipine analog mix and/or T3/T4 inhibit PDS/TTR
expression
[0202] The assays described in Example 1 provide a tool to identify potential
therapeutic agents that can protect neuronal cells against the PDS/TTR
complex. The
inventors observed that compounds such as nifedipine (1,4-dihydro-2,6-dimethyl-
4-(2-
nitrophenyl)-3,5-pyridinydicarboxylic acid dimethyl ester, CAS#21829-25-4
(Sigma
Aldrich)), a calcium channel blocker prescribed for high blood pressure; or
nifedipine
analogs such as oxidized derivative of nifedipine ((2,6-dimethyl-4-(2-
nitrophenyl)- 3,5-
pyridinedicarboxylic acid dimethyl ester, CAS# 67035-22-7 (Sigma Aldrich)) or
a nitroso
derivative of nifedipine (2,6-dimethyl-4-(2-nitrosophenyl)- 3,5-
pyridinedicarboxylic acid
dimethyl ester, CAS#50428-14-3 (Sigma Aldrich)), can effectively inhibit the
expression of
PDS/TTR complex in cell culture, individually or in combination. In addition,
T3 and T4
were also evaluated and found to be effective in inhibiting the expression of
PDS/TTR
complex.
[0203] Specifically, epithelial cells were treated with 5 M acrolein, 5 M
acrolein
plus 0.5 M T3/ 0.5 M T4, 5 M acrolein plus 1 M nifedipine mixture (nitroso-
nifedipine
55%, oxidized nifedipine 11% and nifedipine 34%), or 5 M acrolein plus 1 M
nifedipine
mixture and 0.5 M T3/ 0.5 M T4, as described in Example 1. The amount of
PDS/TTR
secreted into the culture medium by each culture was determined by Western
blot analysis as
described above. Exemplary data was shown in Figure 4. As can be seen, the
amount
57
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
expressed by cells treated with acrolein plus T3/T4, acrolein plus nifedipine
mixture, or
acrolein plus nifedipine mixture and T3/T4, was significantly less than that
expressed by cells
treated only with acrolein.
[0204] In addition, immunostaining was used to determine the PDS/TTR-positive
cells and the numbers of PDS/TTR-positive cells from treated and untreated
cell cultures
were counted and compared. Exemplary results were summarized in Figure 5. As
can be
seen from Figure 5, the number of PDS/TTR-positive cells in acrolein alone-
treat sample is
about 600% of the number of PDS/TTR-positive cells in untreated control. By
contrast, the
numbers of PDS/TTR-positive cells in samples treated with acroline plus
acrolein plus
T3/T4, acrolein plus nifedipine mixture, or acrolein plus nifedipine mixture
and T3/T4 were
significantly reduced as compared to untreated control.
[0205] To determine if the nifedipine mix functions through blocking calcium
channels, we evaluated the impact of the nifedipine mix on the calcium
channels. SYSY
neuroblastoma cultures were pretreated for 16 hours with fresh nifedipine or
the nifedipine
mix and were then switched to calcium free medium and loaded with 5 M Fura-2
fluorescent dye. Cultures were then rinsed with calcium free medium and
exposed to calcium
containing medium and fluorescence resulting from Ca binding to Fura-2 was
measured using
confocal microscopy and excitation at 340 nm. 50 to 100 cells were imaged per
dish for 3
separate dishes. An exemplary result is shown in Figure 6. Interestingly, as
shown in Figure
6, the nifedipine mix had minimal (-30%) activity as a calcium channel blocker
compared to
fresh nifedipine indicating these compounds may act through an alternative,
novel
mechanism.
[0206] Therefore, the experiments described in this example indicate that
nifedipine analog mix and T3/T4, alone or in combination, can effectively
inhibit PDS/TTR
expression in epithelial cells, and this effect is likely to be independent of
calcium channels.
Furthermore, T3/T4 improved the effectiveness of the nifedipine analogs.
Example 3. Nifedipine analogs inhibit inflammatory cytokine production
58
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
[0207] It was reported that inflammatory response elements (cytokines) are
elevated
in Alzheimer's disease patients. The inventors tested the nifedipine mix and
individual
analogs in astrocytoma cultures. Human astrocytoama cells were plated at 2.5 X
105
cells/well in 6 well culture plates and were grown for 24 hours. Cultures were
then switched
to serum free Opti-MEM and treated with the nifedipine mixture and individual
analogs for
24 hours. Three 6-well plates were subjected to each treatment. Following
treatment,
medium was collected from each well and levels of IL-1B, IL-6, TNF-a and TGF-B
were
measured using commercially available ELISAs. Exemplary results are shown in
Figure 7.
As can be seen from Figure 7, IL-1, IL-6 and TNF-a secreted in the medium were
significantly reduced with the treatment of nifedipine mix or oxidized
nifedipine, indicating
these compounds have a direct positive effect on neuroinflammation.
Example 4. NFD-L1 inhibits inflammatory cytokine production
[0208] Using a similar procedure to that described in Example 3, NFD-L1 was
tested
in astrocytoma cultures. As can be seen from Figure 8, IL-1, IL-6, and TNF-a
secreted in the
medium were significantly reduced with the treatment of NFD-L1, indicating
that NFD-L1
has a direct positive effect on neuroinflammation. The results shown in this
example indicate
that a lactam such as NFD-L1 can effectively inhibit inflammatory condition in
the central
nervous system.
Example 5. Nifedipine, Nifedipine mix and/or T3/T4 reduce PHF-1 levels
[0209] As described in Example 1, SYSY neuroblastoma cells exposed to medium
from LAD epithelial cells that contained significantly higher levels of the
PDS/TTR complex
displayed significantly increased PHF-1 immunostaining as compared to those
exposed to
medium from untreated control cultures. In this experiment, SYSY cells were
exposed to
medium from epithelial cells treated with acrolein and combinations of
nifedipine, nifedipine
analogs, mixtures of nifedipine analogs and T3/T4 using procedures described
in Example 1.
59
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
As shown in Figure 9, nifedipine mix and nifedipine/nifedipine analogs plus
T3/T4
significantly reduced PHF-1 levels.
Example 6. Inhibition of A(31-42 production from H4 neuroglioblastoma cells
[0210] In this example, the inventors used H4 neuroglioblastoma cells stably
transfected to overexpress amyloid precursor protein (APP) to further
investigate if
nifedipine, nifedipine analogs (e.g., oxidized nifedipine or nitroso-
nifedipine) and/or T3/T4
can inhibit the production of AP 1-42. H4 neuroglioblastoma cells stably
transfected with a
construct overexpressing amyloid precursor protein (APP) secret AB1_42 into
the culture
medium. These H4 cells were treated with 1 M fresh nifedipine, 1 M oxidized
nifedipine,
1 M nitroso-nifedipine, or 0.5 M T3/0.5 M T4 for 16 hours. The AB levels in
the culture
medium were measured using ELISAs (Invitrogen). As shown in Figure 10,
treatment of
fresh nifedipine, oxidized nifedipine, nitroso-nifedipine, or T3/T4 lead to
significantly
decreased production of AB 1-42.
[0211] Furthermore, the effect of nifedipine, nifedipine analogs and
nifedipine mix on
AB1-42 production from H4 cells were further tested with and without T3/T4.
Exemplary
results are summarized in Figure 11. As can be seen, T3/T4 improves the
inhibitory effect of
nifedipine, nifedipine analogs and nifedipine mix on AB1-42 production.
[0212] We then tested if other calcium channel blockers can inhibit A(3 1-42
generation in H4 neuroglioma cultures. Known calcium channel blockers such as
Amilodpine, Dilitiazem, Felodipine, Isradipine, Nicardipine, and Nimodipine
were used in
this experiment. Specifically, H4 cells were treated with 1 M each drug, with
and without
T3/T4, in opti-MEM (Serum free) for 16 hours and AB1-42 secreted into medium
was
measured using Invitrogen ELISAs. Exemplary results are shown in Figure 12. As
shown in
Figure 12, Nicardipine, showed a trend (p < 0.10) toward a significant
decrease in AB
secretion and Nimodipine led to a significant (p < 0.05) decrease in AB
formation. The other
drugs did not significantly alter levels of AB formation. Combining the
alternate calcium
channel blockers with T3/T4 showed a significant decrease of AB formation when
T3/T4
were combined with Amlodipine and Dilitiazem. Combinations of T3/T4 with the
other
drugs did not provide any significant decrease in AB formation.
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
Example 7. Inhibition of A(31-42 production from H4 neuroglioblastoma cells by
NFD-L1
[0213] Using a procedure similar to that described in Example 6, NFD-L1 was
tested
for inhibition of A(3 1-42 generation in H4 neuroglioma cultures. As shown in
Figure 13, A(3
1-42 generation is inhibited by NFD-L I. The results shown in this example
indicate that a
lactam such as NFD-L1 can effectively inhibit A(31-42 production.
Example 8. Inhibition of beta secretase (BACE) and gamma secretase activity
[0214] This surprising finding that nifedipine, nifedipine analogs and
nifedipine mix
can effectively inhibit API-42 peptide generation prompted further
investigation into possible
mechanism of API-42 peptide reduction. It was contemplated that API-42
production depends
on the activity of beta secretase (BACE), an enzyme that cleaves the amyloid
precursor
protein at the beta secretase cleavage site, and the gamma secretase complex
composed of
presenilin-1 (PS-1), nicastrin, APH-1 and PEN-2 that cleaves at the gamma
secretase
cleavage site. The inventors tested whether the inhibition of API-42
production in our culture
model system was due to inhibition of BACE and/or gamma secretase activities.
[0215] BACE activity was measured using a fluorescent substrate and purified
recombinant BACE as part of a commercial kit from Invitrogen. As shown in
Figure 14,
nifedipine alone or in combination with thyroxine slightly inhibited BACE
activity; however,
nitroso-nifedipine alone and in combination with thyroxine led to significant
inhibition of
BACE activity (Figure 14).
[0216] Examination of protein levels of BACE and individual components of the
gamma secretase complex in H4 cultures treated with the nifedipine mix alone
or in
combination with T3/T4 revealed that the nifedipine mix alone significantly
reduced levels of
PS-1 and PEN-2. Levels of BACE-1 were decreased but not significantly.
However, the
nifedipine mix plus T3/T4 significantly reduced PS-1, PEN-2, BACE-1 and
Nicastrin. APH-
1 was not affected by any treatment. Exemplary results were shown in Figure
16. Protein
levels were determined in individual cells (50 -100 cells/dish; 3
dishes/experiment) using
immunohistochemistry and confocal microscopy and were verified in total cell
homogenate
61
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
using Western blot analysis. Antibodies specific to each protein were
purchased from
commercial vendors.
[0217] Experiments described in this example demonstrated that nifedipine
mixtures,
their oxidized and nitroso derivatives, and/or T3/T4 directly act on the
enzymes responsible
for AB production.
Example 9. Inhibition of beta secretase (BACE) by NFD-L1
[0218] Using a similar procedure as described in Example 8, NFD-L1 was tested
for
inhibition of BACE activity. As shown in Figure 15, NFD-L1 inhibits BACE. This
example
indicates that a lactam such as NFD-L1 can effectively inhibit beta secretase
(BACE)
activity.
Example 10. Inhibition of AB1-40 production in vivo
[0219] Based on the in vitro data described above, the inventors initiated an
acute
exposure study in 3 month old C57-Black-6 (C57BL/6) mice. In this study, six
groups of six
C57BL/6 mice were subjected to intraperitoneal (IP) injections of vehicle
(2%DMSO/98%
polyethylene glycol-3000 (PEG-3000), 25 mg/kg nifedipine or nifedipine mix,
T3/T4 (10
mg/kg T3 and 10 mg/kg T4), nifedipine mix plus T3/T4 and nifedipine plus T3/T4
on three
consecutive days. Animals were euthanatized 1 hour after the third injection.
The brains and
terminal serum were removed and immediately frozen in liquid nitrogen and
stored at -80 C
until used for analysis.
[0220] One hemisphere of brains was homogenized for AB1-40 measurements
(Invitrogen ELISA) and the other homogenized for protein levels. In addition,
levels of PS-1,
BACE, cleaved Notch, an essential substrate for PS-1, Nicast, APH-1 were
measured using
Western blot analysis. As shown in Figure 17, mice treated with both T3/T4 and
nifedipine
mix plus T3/T4 showed a modest (25%) but significant decrease in A(3 1-40
levels compared to
animals treated with vehicle. Levels of PS-1, Nicast, and APH-1 were
significantly
decreased in mice treated with nifedipine, nifedipine mix plus T3/T4. BACE
protein levels
62
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
were significantly decreased in mice treated with nifedipine, nifedipine plus
T3/T4 and T3/T4
alone. In contrast, there were no significant differences in levels of cleaved
Notch with any
treatment (Figure 17).
[0221] Additionally the brains were extracted and analyzed by GC/MS for levels
of
nifedipine and its analogs. Oxidized nifedipine was found in all samples
analyzed indicating
that components of the mixture were passing the blood-brain barrier and thus
available for
neuronal protection. This experiments has shown that these derivatives possess
the brain
permeability desired for treatment of Alzheimer's disease.
[0222] Experiments described in this example demonstrated that nifedipine
mixtures,
their oxidized and nitroso derivatives, and/or T3/T4 reduce endogenous levels
of A(3 1-40
peptide in vivo.
Example 11. Nitroso-nifedipine inhibits production of AB1-40 in vivo
[0223] Additional experiments were conducted to show that nitroso-nifedipine
can
effectively inhibit production of AB1-40 in vivo. Specifically, C57B16 mice (6
per group)
were given IP injections of increasing concentrations of nitroso-nifedipine in
2% DMSO/98%
PEG-300 for 3 days. The animals were sacrificed 15 minutes following the final
injection.
Brains were quickly removed, split into hemispheres, and snap frozen in liquid
nitrogen.
Brains were shipped on dry ice and maintained at -80 C until used for
analysis. One
hemisphere of each brain was homogenized in diethylamine (200 mg wet
weight/mL)
containing complete protease inhibitors using a Dounce homogenizer. Homogenate
was
centrifuged at 16,000 X g for 30 minutes and 50 pL soluble protein subjected
to AB1-40
quantification using a Covance ELISA per manufacturer's instructions. Results
are expressed
as % vehicle treated animals. Results of the analyses showed acute treatment
with 35 mg/kg
nitroso-nifedipine led to a significant decrease in levels of AB1-40 (Figure
18).
[0224] Thus, this example demonstrates that nitroso-nifedipine effectively
inhibits
production of AB 1-40 in vivo.
63
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
Example 12. Inhibition of Orphan G-coupled receptor protein 3 (GPCR-3) in
vitro and in
vivo
[0225] This example was conducted to test if nifedipine, nifedipine mixtures,
and/or
T3/T4 can inhibit the orphan G-coupled receptor protein 3 (GPCR-3), an enzyme
which is
suggested to play a role in maintaining stability of the gamma secretase
complex (which, as
discussed above, is important for cleavage of APP to form A(3).
[0226] H4 neuroglioblastoma cells were treated with 1 M mixed nifedipine, 1
M
mixed nifedipine plus 0.5 M T3/0.5 M T4, 0.5 M T3/0.5 M T4, 1 M fresh
nifedipine, 1
M fresh nifedipine plus 0.5 M T3/0.5 M T4 for 16 hours. Levels of GPCR-3
were
measured using Western blot analysis using a GPCR-3 specific antibody. As
shown in
Figure 19, nifedipine mixtures, fresh nifedipine, and/or T3/T4 significantly
reduced GPCR-3
expression levels in H4 cells.
[0227] In addition, GPCR-3 levels were determined in the C57BL/6 mice
described
above in Example 10 using Western blot analysis. Exemplary results are also
shown in
Figure 19. Nifedipine mixtures and T3/T4, fresh nifedipine, and T3/T4 also
reduced GPCR-3
expression levels in mice.
[0228] Therefore, this example demonstrated that nifedipine mixtures, their
oxidized
and nitroso derivatives, and/or T3/T4 reduce GPCR-3 levels in vitro and in
vivo.
[0229] To determine if the reduction of GPCR-3 levels by nifedipine mixtures,
their
oxidized and nitroso derivatives, and/or T3/T4 is through a pathway involved
in blood
pressure regulation, we tested known blood pressure drugs such as atenolol,
captopril, and
enantopirl on H4 cells. Specifically, H4 cells were treated with 1 M each
drug, with and
without T3/T4, in Opti-MEM (Serum free) medium for 16 hours and levels of GPCR-
3 were
measured using confocal microscopy and a specific anti-GPCR-3 antibody.
Exemplary
results are shown in Figure 20. As shown in Figure 20, only captopril + T3/T4
led to a
significant change (decrease) in levels of GPCR-3. This experiment indicates
that the
reduction of GPCR-3 by nifedipine mixtures, their oxidized and nitroso
derivatives, and/or
T3/T4 is independent of blood pressure pathways.
64
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
Example 13. Effects of nitroso-nifedipine on levels of enzymes involved in AB
processing
[0230] This experiment was conducted to determine effects of nitroso-
nifedipine on
levels of enzymes involved in AB processing. Suitable concentration of nitroso-
nifedipine
was first determined based on the survival rate of H4 cells treated with
increasing
concentrations of nitroso-nifedipine. Specifically, H4 neuroglioma cultures
were plated at a
density of 2.5 X 105 cells/well and allowed to attach overnight. Cultures were
switched to
Opti-MEM and treated for 16 hours with increasing concentrations of nitroso-
nifedipine.
Following treatment, MTT was added at a final concentration of 0.5 mg/mL and
cultures
incubated for 30 minutes. Following MTT treatment, medium was removed and the
formazan crystals generated by mitochondrial conversion of MTT were dissolved
in DMSO
and absorbance was measured at 650 nm. Data are reported as mean + SEM %
control MTT
reduction (Figure 21). Results of the studies suggest that H4 cultures are
more resistant to
nitroso-nifedipine compared to nifedipine. Based on survival data, 2.5 pM
nitroso-nifedipine
was chosen for use in additional studies.
[0231] To determine the effects of nitroso-nifedipine on levels of enzymes
involved
in AB processing, H4 neuroglioma cultures that overexpress APP were plated at
a density of
2.5 X 105 cells/dish and allowed to attach overnight. Cultures were switched
to Opti-MEM
and treated with 2.5 pM or 0.5 pM nitroso-nifedipine alone or 2.5 M nitroso-
nifedipine +
nifedipine (0.1 and 0.01 M) or 1 pM T3/T4. Following treatment, cultures were
rinsed
three times in PBS and fixed in 70% methanol/30% acetone for 30 minutes at -20
C.
Cultures were then subjected to immunohistochemistry for BACE, PS-1, GPCR-3,
nicastrin,
and ADAM (the enzyme responsible for alpha secretase cleavage). 30 to 50 cells
were
imaged in 4 -5 fields/dish (3 dishes each treatment) (Figure 22). Similar to
results observed
for nifedipine, nitroso-nifedipine treatment led to a significant decrease in
BACE protein. In
contrast to nifedipine, nitroso-nifedipine did not significantly alter PS-1 or
nicastrin levels.
Also in contrast to nifedipine, nitroso-nifedipine led to a significant
increase in GPCR-3.
Nitroso-nifedipine also led to a significant increase in levels of ADAM-10
which is
responsible for cleavage of AB at the alpha secretase site. Alpha secretase
cleavage leads to
decreased AB1-42.
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
Example 14. Effects of NFD-L1 on levels of enzymes involved in AB processing
[0232] Using a similar procedure as described in Example 13, H4 neuroglioma
cultures were treated with NFD-L1 to determine the effects of NFD-L1 on levels
of enzymes
involved in AB processing. As shown in Figure 23, similar to nitroso-
nifedipine, NFD-L1 led
to a significant decrease in BACE protein and a significant increase in ADAM-
10. NFD-L1
also led to a significant decrease in PS-1 and NCT. This example demonstrated
that
treatment with lactam such as NFD-L1 has significant effect on levels of
enzymes involved in
AB processing.
Example 15. Effects of nitroso-nifedipine on levels of enzymes involved in AB
processing in
vivo
[0233] To determine the effects of nitroso-nifedipine on levels of enzymes
involved
in AB processing in vivo, levels of AB were quantified by ELISA and levels of
proteins
involved in AB processing were quantified by Western blot analysis for mice
treated with
vehicle or 35 mg/kg nitroso-nifedipine (Figure 24). IP injections for 3 days
led to a
significant decrease in AB1-40 and a significant decrease in presenilin-1. The
data also show
that nitroso-nifedipine mediated inhibition of PS-1 did not decrease levels of
cleaved Notch-1
but instead led to a significant increase in levels of cleaved Notch-1. In
addition, treatment
with nitroso-nifedipine led to a significant increase in levels of ADAM-10,
which functions
as an alpha secretase. It is contemplated that increased ADAM-10 levels lead
to increased
cleavage at the alpha secretase position of APP, minimizing generation of AB.
Example 16. Effects of NFD-L1 on levels of enzymes involved in AB processing
in vivo
[0234] The effects of NFD-L1 on levels of enzymes involved in AB processing in
vivo were determined using a similar procedure to that described in Example
15. As shown
in Figure 25, similar to nitroso-nifedipine, IP injections of NFD-Li led to a
significant
decrease in AB1-40 and a significant decrease in presenilin-1. The data also
show that NFD-
Ll mediated inhibition of PS-1 led to a significant increase in levels of
cleaved Notch-1. In
addition, treatment with NFD-L1 led to a significant increase in levels of
ADAM-10. Thus,
66
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
this example demonstrated that treatment with lactam such as NFD-L1 has
significant effect
on levels of enzymes involved in AB processing in vivo.
Example 17. Inhibition of Tau Phospho lr a
[0235] In this example, we tested if nifedipine, nifedipine mixtures, their
oxidized and
nitroso derivatives, and/or T3/T4 can reduce phosphorylated Tau protein.
Phosphorylated tau
protein can result in the self-assembly of tangles of paired helical filaments
and straight
filaments, which are involved in the pathogenesis of Alzheimer's disease.
[0236] Although there are multiple kinases involved in Tau phosphorylation,
glycogen synthase kinase-3B (GSK-33) has received considerable attention as a
major
contributor to Tau hyperphosphorylation in AD. GSK-3 3 is present in -95%
paired helical
filaments identified using specific-phospho-Tau antibodies. GSK-3(3, a
constitutively active
kinase is inactivated by phosphorylation of Ser 9 by protein kinase B (Akt).
Akt, a
serine/threonine kinase is regulated by phosphatidylinositol kinase (P13K)
mediated signaling
and is activated by phosphorylation of a regulatory threonine residue (Thr-
308) by
phosphatidylinositol dependent kinase 1 (PDK1) and by phosphorylation of Ser
473 by
PDKa/TORC2 kinase. In addition, activation of the Akt/GSK-3(3 pathway may be
mediated
by GCPRs coupled to Ga12/13 heterotrimeric G proteins. Activation of Gait has
been shown
to stimulate RhoA and its effector Rho kinase (ROCK). ROCK phosphorylated at
ser160
further transactivates a receptor tyrosine kinase (RTK) that activates the
P13K signaling
pathway leading to phosphorylation/activation of Akt/GSK-3(3 (reviewed by New
et al, "G
protein-coupled receptor-induced Akt activity in cellular proliferation and
apoptosis," FEBS
J, 2007; 274:6025-36.). Phosphoryalted Akt increases phosphorylation and
inactivation of
GSK-3 R therefore reducing Tau phosphorylation.
[0237] We examined levels and the phosphorylation status of proteins involved
in the
tau phorphorylation pathway described above in the mouse brains treated with
nifedipine,
nifedipine mix and/or T3/T4 as described in Example 10. As shown in Figure 26,
the levels
of phosphoryalted ROCK (p-ROCK) and GSK-3 (3 (p-GSK-3 (3) were significantly
increased
in mice treated with nifedipine or nifedipine mix plus T3/T4. T3/T4 alone also
significantly
increased the level of p-GSK-33. The total protein levels of ROCK were not
affected by any
67
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
of the treatment. The level of p-25 was slightly reduced in treated mice.
These results
indicate that nifedipine, nifedipine mix and/or T3/T4 treatment can lead to
reduced Tau
phosphorylation in vivo.
Example 18. Effects of nifedipine and nitroso-nifedipine on glutamate
transport
[0238] To test the effect of nitroso-nifedipine on glutamate transport,
astroglioma
cultures were plated at 2.5 X 105 cells/well and allowed to attach overnight.
The cultures
were switched to Opti-MEM and treated with 2.5 pM nitroso-nifedipine for 16
hours.
Cultures were washed three times with PBS and fixed with 70% methanol/30%
acetone at -20
C for 30 minutes. Following fixation, cultures were immunostained using
antibodies
specific to EAAT1 or EAAT2 (Glut-1), the major glutamate transporters.
Cultures were then
imaged using confocal microscopy and staining intensity quantified using Leica
software. 30
to 50 cells were imaged per field and 5 fields were imaged per culture dish.
Results of the
analyses showed that nitroso-nifedipine led to a significant increase of EAAT2
but no change
in EAAT1 (Figure 27). EAAT2 has been shown to be significantly decreased in AD
brain.
Using tissue specimens from C57/B16 mice treated acutely for 3 days with 25
mg/kg
nifedipine we subjected 20 pg samples of protein to Western blot analysis and
probed for
EAAT2. Results of the analysis showed a significant increase in EAAT2 with
nifedipine.
Together, these data suggest that both nifedipine and nitroso-nifedipine lead
to increased
levels of a key glutamate transporter shown to be altered in AD brain.
Example 19. Liver toxicity study
[0239] To determine if treatment with nitroso-nifedipine leads to liver
toxicity,
alkaline phosphatase levels were quantified in terminal serum samples from
mice treated with
increasing doses of nitroso-nifedipine using an alkaline phosphatase kit
commonly used in
clinical practice (Diagnostic Chemicals Limited). Results of the assays showed
nitroso-
nifedipine did not significantly increase serum alkaline phosphatase levels at
any dose and
actually led to a significant decrease in levels at a dose of 35 mg/kg (Figure
28). These data
suggest that nitroso-nifedipine does not induce liver damage.
68
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
Example 20. Human association studies
[0240] Human association studies were conducted to determine the impact of
calcium
channel blockers on human patients. In the first study, subjects were
segregated into
controls, controls with APOE4 (a gene linked to increased incidence of
Alzheimer's disease),
subjects on dihydropyridine based calcium channel blockers and subjects on
dihydropyridine
based calcium channel blockers and with APOE4. MMSE (Mini Mental Status
Examination)
test was used to measure cognitive function of each subject. A trajectory of
the fitted model
versus age can be determined. Figure 29 shows exemplary results of the NLMIXED
model
of MMSE. This model indicates that subjects on calcium channel blockers show a
4 year lag
in cognitive decline relative to subjects not on calcium channel blockers.
[0241] This human association study demonstrated that the use of
dihydropyridine
calcium channel blockers appears to delay the onset of cognitive decline,
suggesting
dihydropyridine calcium channel blockers can be used to treat
neurodegenerative diseases
such as Alzheimer's disease.
[0242] In the second study, we conducted autopsy on a total of 8 subjects from
the
neuropsychological association study. 4 subjects were on calcium channel
blockers,
including nifedipine and 4 subjects were not on any calcium channel blocker.
Levels of
A131-42 and A(3 processing enzymes such as PS-1, Nicas, BACE, APH-1 and PEN-2
in front
lobe specimens of subjects were determined using standard methods.
Specifically, A(31-42
levels were determined using Invitrogen ELISAs and protein levels were
determined using
Western blot analysis and antibodies specific to each protein. As shown in
Figure 30, the
A(31-42level was significantly reduced in subjects with drugs as compared to
that in subjects
without drugs. Some A(3 processing enzymes including PS-1, Nicas were
significantly
reduced in those subjects with drugs as compared to those without drugs.
Interestingly, the
levels of BACE, APH-1 and PEN-2 were increased in those subjects with drugs as
compared
to subjects without drugs.
[0243] In addition, enzyme levels that are involved in Tau phosphorylation
were also
examined in frontal lobe specimens from the subjects. As shown in Figure 31,
the levels of
phosphorylated p-Akt, p-GSK-3(3 and p-ROCK were all increased in subjects with
drug as
69
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
compared to those in subjects without drug. The total protein levels of Akt,
GSK-33 and
ROCK were comparable in subjects with and without drug. As discussed above,
activated p-
Akt phosphorylates GSK-3(3, which is then inactivated and reduces Tau
phosphorylation.
These results are consistent with the conclusion that the use of calcium
channel blockers can
reduce Tau phosphorylation, useful for treatment of Alzheimer's disease.
[0244] This human association study demonstrated that the use of calcium
channel
blockers appears to reduce A(31-42 level and certain A(31-42 processing
enzymes, and
inactivate enzymes involved in Tau phosphorylation in human patients,
indicating calcium
channel blockers may be effective in treating Alzheimer's disease.
Example 21. Nitroso-nifedipine increases calcium influx
[0245] This experiment was conducted to determine whether or not nitroso-
nifedipine
functions as a calcium channel blocker. H4 neuroglioma cultures were loaded
with 5 M
Fluo-4AM for 30 minutes, washed three times with Locke's with glucose and
treated with
vehicle, 1 pM nifedipine or 2.5 pM nitroso-nifedipine for 1 hour. Cells were
depolarized by
addition of 40 pL 100 mM KC1 and calcium levels quantified 30 seconds
following
depolarization by confocal microscopy (n = 75 - 100 cells/dish for 3
dishes/treatment). As
expected, nifedipine led to a significant decrease in Ca influx following
depolarization. In
contrast, treatment with nitroso-nifedipine led to a significant increase in
Ca influx following
depolarization (Figure 32).
[0246] Therefore, this example demonstrates that, unlike nifedipine, nitroso-
nifedipine does not function as a calcium channel blocker. Surprisingly,
nitroso-nifedipine
increases calcium influx. Without wishing to be bound by any theory, it is
contemplated that
nitroso-nifedipine and its derivatives treat MCI or Alzheimer's disease
through a novel
mechanism independent of blocking calcium channels.
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
Example 22. Synthesis of nitroso-nifedipine
[0247] Photochemical synthesis was used in this example to synthesize
nitrosonifedipine. Specifically, nifedipine (20 mg) was dissolved in 10 mL
acetonitrile in a
pyrex culture tube, capped and photolyzed with a 250W halogen lamp (3M EVW)
for 30
minutes. The product was isolated by solvent removal on a rotary evaporator to
obtain a
blue-green oil (18.1 mg, 94% yield). GC/MS analysis showed greater than 98.5%
conversion
to nitrosonifedipine. An exemplary result is shown in Figure 33.
Example 23. Synthesis of NFD-L1
[0248] Nitroso-nifedipine (10 mg, 30.5 pmol) dissolved in 5 mL ethanol was
mixed
with glutathione (93 mg, 305 pmol) dissolved in 5 mL water and allowed to
react at 37 C for
2 hours. After 2 hours, water was added and the product was extracted with
ethyl acetate.
The solvent was removed in a rotary evaporator to give NFD-L1 as a white solid
in about
85% yield (>95% purity). An exemplary mass spectrum is shown in Figure 34.
Example 24. Treatment of human patients
[0249] A human patient determined to have MCI based on an MMSE score is given
nitroso-nifedipine at a dosage of 1000 mg per day. Nitroso-nifedipine is given
as tablets for
oral administration by patient three times daily.
[0250] Another human patient determined to have early stage Alzheimer's
disease
(EAD) based on a CDR score is given nitroso-nifedipine at a dosage of 800 mg
per day.
Nitroso-nifedipine is given as tablets for oral administration by patient
three times daily.
[0251] A human patient determined to have MCI based on the level of PDS/TTR
complex in a fluid sample obtained from the patient is given nitroso-
nifedipine at a dosage of
1000 mg per day. Nitroso-nifedipine is given as tablets for oral
administration by patient four
times daily.
71
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
EQUIVALENTS
[0252] The foregoing has been a description of certain non-limiting
embodiments of
the invention. Those skilled in the art will recognize, or be able to
ascertain using no more
than routine experimentation, many equivalents to the specific embodiments of
the invention
described herein. Those of ordinary skill in the art will appreciate that
various changes and
modifications to this description may be made without departing from the
spirit or scope of
the present invention, as defined in the following claims.
[0253] In the claims articles such as "a,", "an" and "the" may mean one or
more than
one unless indicated to the contrary or otherwise evident from the context.
Claims or
descriptions that include "or" between one or more members of a group are
considered
satisfied if one, more than one, or all of the group members are present in,
employed in, or
otherwise relevant to a given product or process unless indicated to the
contrary or otherwise
evident from the context. The invention includes embodiments in which exactly
one member
of the group is present in, employed in, or otherwise relevant to a given
product or process.
The invention also includes embodiments in which more than one, or all of the
group
members are present in, employed in, or otherwise relevant to a given product
or process.
Furthermore, it is to be understood that the invention encompasses all
variations,
combinations, and permutations in which one or more limitations, elements,
clauses,
descriptive terms, etc., from one or more of the claims or from relevant
portions of the
description are introduced into another claim. For example, any claim that is
dependent on
another claim can be modified to include one or more limitations found in any
other claim
that is dependent on the same base claim. Furthermore, where the claims recite
a
composition, it is to be understood that methods of using the composition for
any of the
purposes disclosed herein are included, and methods of making the composition
according to
any of the methods of making disclosed herein or other methods known in the
art are
included, unless otherwise indicated or unless it would be evident to one of
ordinary skill in
the art that a contradiction or inconsistency would arise. In addition, the
invention
encompasses compositions made according to any of the methods for preparing
compositions
disclosed herein.
[0254] Where elements are presented as lists, e.g., in Markush group format,
it is to
be understood that each subgroup of the elements is also disclosed, and any
element(s) can be
72
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
removed from the group. It is also noted that the term "comprising" is
intended to be open
and permits the inclusion of additional elements or steps. It should be
understood that, in
general, where the invention, or aspects of the invention, is/are referred to
as comprising
particular elements, features, steps, etc., certain embodiments of the
invention or aspects of
the invention consist, or consist essentially of, such elements, features,
steps, etc. For
purposes of simplicity those embodiments have not been specifically set forth
in haec verba
herein. Thus for each embodiment of the invention that comprises one or more
elements,
features, steps, etc., the invention also provides embodiments that consist or
consist
essentially of those elements, features, steps, etc.
[0255] Where ranges are given, endpoints are included. Furthermore, it is to
be
understood that unless otherwise indicated or otherwise evident from the
context and/or the
understanding of one of ordinary skill in the art, values that are expressed
as ranges can
assume any specific value within the stated ranges in different embodiments of
the invention,
to the tenth of the unit of the lower limit of the range, unless the context
clearly dictates
otherwise. It is also to be understood that unless otherwise indicated or
otherwise evident
from the context and/or the understanding of one of ordinary skill in the art,
values expressed
as ranges can assume any subrange within the given range, wherein the
endpoints of the
subrange are expressed to the same degree of accuracy as the tenth of the unit
of the lower
limit of the range.
[0256] In addition, it is to be understood that any particular embodiment of
the
present invention may be explicitly excluded from any one or more of the
claims. Any
embodiment, element, feature, application, or aspect of the compositions
and/or methods of
the invention can be excluded from any one or more claims. For purposes of
brevity, all of
the embodiments in which one or more elements, features, purposes, or aspects
is excluded
are not set forth explicitly herein.
73
CA 02799162 2012-11-09
WO 2011/142778 PCT/US2010/057287
INCORPORATION OF REFERENCES
[0257] All publications and patent documents cited in this application are
incorporated by reference in their entirety to the same extent as if the
contents of each
individual publication or patent document were incorporated herein.
[0258] What is claimed is:
74