Note: Descriptions are shown in the official language in which they were submitted.
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
SUPPLEMENTATION OF FATTY ACIDS
FOR IMPROVING ALCOHOL PRODUCTIVITY
[0001] This application claims the benefit of U.S. Provisional Application No.
61/356,290, filed on June 18, 2010; U.S. Provisional Application No.
61/368,451,
filed on July 28, 2010; U.S. Provisional Application No. 61/368,436, filed on
July
28, 2010; U.S. Provisional Application No. 61/368,444, filed on July 28, 2010;
U.S. Provisional Application No. 61/368,429, filed on July 28, 2010; U.S.
Provisional Application No. 61/379,546, filed on September 2, 2010; and U.S.
Provisional Application No. 61/440,034, filed on February 7, 2011; U.S. Patent
Application No. 13/160,766, filed on June 15, 2011; the entire contents of
which
are all herein incorporated by reference.
[0002] The Sequence Listing associated with this application is filed in
electronic
form via EFS-Web and hereby incorporated by reference into the specification
in
its entirety.
FIELD OF THE INVENTION
[0003] The present invention relates to the production of fermentative
alcohols,
such as butanol, and in particular to alcohol fermentation processes for
achieving
improved alcohol productivity in which the fermentative growth of recombinant
microorganisms is in the presence of fatty acids derived from biomass at a
step in
the fermentation process.
BACKGROUND OF THE INVENTION
[0004] Alcohols have a variety of applications in industry and science such as
a
beverage (i.e., ethanol), fuel, reagents, solvents, and antiseptics. For
example,
butanol is an alcohol that is an important industrial chemical with a variety
of
applications including use as a fuel additive, as a feedstock chemical in the
plastics industry, and as a food-grade extractant in the food and flavor
industry.
-1-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
Accordingly, there is a high demand for alcohols such as butanol, as well as
for
efficient and environmentally-friendly production methods.
[0005] Production of alcohol such as butanol utilizing fermentation by
microorganisms is one environmentally friendly production method.
Microorganisms such as yeasts have been used for the production of alcohol
products where naturally produced pyruvate is used as a starting substrate in
their biosynthetic pathways. Butanol can be produced biologically as a by-
product of yeast fermentation, but the yield can be typically very low. To
enhance
production of desired products such as butanol, yeasts have been engineered to
express enzymes that alter endogenous biosynthetic pathways or introduce new
pathways, and/or by disrupting expression of endogenous enzymes to alter
metabolite flow. Introduced pathways that use cellular pyruvate as a substrate
include pathways for production of, for example, isomers of butanol.
Disruption
of pyruvate decarboxylase has been used to increase pyruvate availability for
pathways that produce desired products such as butanol. Additionally,
recombinant microbial production hosts expressing a 1-butanol biosynthetic
pathway (U.S. Patent Application Publication No. 2008/0182308A1), a 2-butanol
biosynthetic pathway (U.S. Patent Application Publication Nos. 2007/025941OA1
and 2007/0292927), and an isobutanol biosynthetic pathway (U.S. Patent
Application Publication No. 2007/0092957) have been described.
[0006] For example, Saccharomyces cerevisiae yeast can be metabolically
engineered with disruptive mutations in the two primary pyruvate decarboxylase
(PDC) genes. These genes, commonly referenced as PDC1 and PDC5, produce
enzymes that are directly involved with ethanol production, and disruption of
these genes has a negative impact on growth. Knock-out of pyruvate
decarboxylase and alcohol dehydrogenase (ADH) alters the biosynthetic pathway
resulting in the production of less fatty acid. Fatty acids are needed for
cell wall
formation and thus, necessary for cell growth. The importance of fatty acids
for
cell growth is demonstrated, for example, in Otoguro, et al., (J. Biochem.
89:523-
529, 1981), which describes the effect of the antibiotic cerulenin, a known
inhibitor of fatty acid synthesis, on cell growth. Cerulenin was added to a S.
cerevisiae culture causing inhibition of the growth, but the growth was
restored
-2-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
when oleic acid with certain saturated fatty acids (specifically, myristic
acid,
palmitic acid or pentadecanoic acid) was added.
[0007] Glucose metabolism in yeast generally follows a pathway of converting
glucose to pyruvate to acetyl-CoA to cell mass. Correspondingly, there can be
conversion of pyruvate to acetaldehyde to ethanol or a conversion of
acetaldehyde to acetyl-CoA to fatty acid synthesis. With regard to recombinant
microorganisms, a single PDC deletion reduces maximum growth but to a much
lower extent. When only one PDC gene is disrupted, the other PDC gene is
active enough to allow carbon flux to acetaldehyde and subsequently, ethanol
and acetate. However, when butanol product is desired, ethanol production
reduces butanol product yield on the substrate. The PDC genes are responsible
for taking pyruvate to acetaldehyde, and the PDC1 and PDC5 double mutation
prevents the production of acetaldehyde, altering the pathway to fatty acid
biosynthesis and thereby inhibiting cell growth.
[0008] Thus, there exists a continuing need for methods for fermentative
alcohol
production using recombinant microorganisms in which growth rate and/or
biomass production of the microorganisms can be improved despite reduction or
elimination of fatty acid biosynthesis by the microorgansim. The present
invention provides further related advantages, as will be made apparent by the
description of the embodiments that follow.
BRIEF SUMMARY OF THE INVENTION
[0009] The present invention is directed to a method comprising: (a) providing
a
fermentation broth comprising a recombinant microorganism that produces a
product alcohol from a fermentable carbon source, wherein the recombinant
microorganism comprises a reduction or elimination of pyruvate decarboxylase
activity; (b) contacting the fermentation broth with a fermentable carbon
source
whereby the recombinant microorganism consumes the fermentable carbon
source and produces the product alcohol; and (c) contacting the fermentation
broth with fatty acids derived from biomass at a step in the fermentation
process,
wherein at least one of (i) growth rate and (ii) fermentable carbon
consumption of
the recombinant microorganism is greater in the presence of the fatty acids
than
-3-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
the growth rate and/or the fermentable carbon consumption of the recombinant
microorganism is in the absence of the fatty acids. In a further embodiment,
steps (b) and (c) occur substantially simultaneously. In one embodiment, the
fatty acids are selected from oleic acid, palmitic acid, myristic acid, and
mixtures
thereof and in another embodiment, the biomass is derived from corn grain,
corn
cobs, crop residues such as corn husks, corn stover, grasses, wheat, rye,
wheat
straw, barley, barley straw, hay, rice straw, switchgrass, waste paper, sugar
cane
bagasse, sorghum, sugar cane, soy, components obtained from milling of grains,
cellulosic material, lignocellulosic material, trees, branches, roots, leaves,
wood
chips, sawdust, shrubs and bushes, vegetables, fruits, flowers, animal manure,
and mixtures thereof. In a further embodiment, the fermentable carbon source
is
derived from the biomass. In one embodiment, the product alcohol is butanol
and in another embodiment, the fermentation broth further comprises ethanol.
In
another embodiment, the recombinant microorganism has a one or more
pyruvate decarboxylase (PDC) gene deletions.
[0010] The present invention is also directed to a method for producing a
product
alcohol comprising: (a) providing biomass comprising a fermentable carbon
source and oil; (b) converting at least a portion of the oil into fatty acids
to form a
biomass comprising the fatty acids; (c) contacting the biomass with a
fermentation broth comprising a recombinant microorganism capable of
producing a product alcohol from a fermentable carbon source, and wherein the
recombinant microorganism comprises a reduction or elimination of pyruvate
decarboxylase activity; (d) contacting the fatty acids with the fermentation
broth,
wherein at least one of (i) growth rate and (ii) fermentable carbon
consumption of
the recombinant microorganism is greater in the presence of the fatty acids
than
the growth rate and/or the fermentable carbon consumption of the recombinant
microorganism is in the absence of the fatty acids. In a further embodiment,
the
step (b) of converting at least a portion of the oil into fatty acids
comprises
contacting the oil with one or more substances capable of hydrolyzing the
portion
of the oil into fatty acids. In one embodiment, the one or more substances
comprise one or more enzymes and in another embodiment, the one or more
enzymes comprise lipase enzymes. In a further embodiment, prior to step (c),
-4-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
the one or more enzymes may be inactivated after at least a portion of the oil
is
hydrolyzed. In one embodiment, one or more of steps (b), (c), and (d) occurs
in
the fermentation vessel and in another embodiment, one or more of steps (b),
(c),
and (d) occurs substantially simultaneously. In one embodiment, the method
further comprises the step separating the oil from the biomass prior to the
step
(b) of converting at least a portion of the oil into fatty acids. In another
embodiment, the method further comprises: liquefying the biomass to produce a
liquefied biomass, wherein the liquefied biomass comprises oligosaccharides;
and contacting the liquefied biomass with a saccharification enzyme capable of
converting oligosaccharides into fermentable sugar to form a saccharified
biomass, and wherein step (c) comprises contacting the saccharified biomass
with the fermentation broth comprising a recombinant microorganism. In one
embodiment, the fatty acids are selected from oleic acid, palmitic acid,
myristic
acid, and mixtures thereof and in another embodiment, the biomass is derived
from corn grain, corn cobs, crop residues such as corn husks, corn stover,
grasses, wheat, rye, wheat straw, barley, barley straw, hay, rice straw,
switchgrass, waste paper, sugar cane bagasse, sorghum, sugar cane, soy,
components obtained from milling of grains, cellulosic material,
lignocellulosic
material, trees, branches, roots, leaves, wood chips, sawdust, shrubs and
bushes, vegetables, fruits, flowers, animal manure, and mixtures thereof. In
one
embodiment, the method further comprises the step of fermenting the
fermentable carbon source to produce product alcohol. In one embodiment, the
product alcohol is butanol and in another embodiment, the fermentation broth
further comprises ethanol. In another embodiment, the recombinant
microorganism has a one or more pyruvate decarboxylase (PDC) gene deletions.
[0011] Another method of the present invention includes a method for producing
a product alcohol comprising: (a) providing a feedstock; (b) liquefying said
feedstock to create a feedstock slurry; (c) separating the feedstock slurry to
produce a product comprising (i) an aqueous layer comprising a fermentable
carbon source, (ii) an oil layer, and (iii) a solids layer; (d) obtaining an
oil from the
oil layer and converting at least a portion of the oil into fatty acids; (e)
feeding the
aqueous layer of (c) to a fermentation vessel containing a fermentation broth
-5-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
comprising a recombinant microorganism capable of producing a product alcohol
from a fermentable carbon source, wherein the recombinant microorganism
comprises a reduction or elimination of pyruvate decarboxylase activity; (f)
fermenting the fermentable carbon source of the aqueous layer to produce the
product alcohol; and (g) contacting the fermentation broth with the fatty
acids,
wherein at least one of (i) growth rate and (ii) fermentable carbon
consumption of
the recombinant microorganism is greater in the presence of the fatty acids
than
the growth rate and/or the fermentable carbon consumption of the recombinant
microorganism is in the absence of the fatty acids. In a further embodiment,
the
step (d) of converting at least a portion of the oil into fatty acids
comprises
contacting the oil with one or more substances capable of hydrolyzing the
portion
of the oil into fatty acids. In one embodiment, the one or more substances
comprise one or more enzymes and in another embodiment, the one or more
enzymes comprise lipase enzymes. In one embodiment, the fatty acids are
selected from oleic acid, palmitic acid, myristic acid, and mixtures thereof
and in
another embodiment, the biomass is derived from corn grain, corn cobs, crop
residues such as corn husks, corn stover, grasses, wheat, rye, wheat straw,
barley, barley straw, hay, rice straw, switchgrass, waste paper, sugar cane
bagasse, sorghum, sugar cane, soy, components obtained from milling of grains,
cellulosic material, lignocellulosic material, trees, branches, roots, leaves,
wood
chips, sawdust, shrubs and bushes, vegetables, fruits, flowers, animal manure,
and mixtures thereof. In one embodiment, the product alcohol is butanol. In
another embodiment, the recombinant microorganism has a one or more
pyruvate decarboxylase (PDC) gene deletions.
[0012] The present invention is also directed to a composition comprising a
recombinant microorganism comprising a reduction or elimination of pyruvate
decarboxylase activity and fatty acids.
[0013] Fatty acids (e.g., oleic acid, palmitic acid, and mixtures thereof)
derived
from biomass at a step in a fermentation process, can be added to a
fermentation
medium comprising a recombinant microorganism that produces a product
alcohol. The microorganism can be yeast or other alcohol-producing
microorganism. Also, the microorganism can have one or more PDC gene
-6-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
deletions and/or have reduced or eliminated pyruvate decarboxylase activity.
The addition of fatty acids can increase glucose consumption, and can improve
microorganism biomass production (cell growth) and growth rate. Improving
growth rate can reduce production time and thereby increase productivity of
the
alcohol fermentation process.
[0014] In some embodiments, a method for producing a product alcohol in a
fermentation process includes (a) providing a fermentation broth including a
recombinant microorganism that produces a product alcohol from a fermentable
carbon source; (b) contacting the fermentation broth with a fermentable carbon
source whereby the microorganism consumes the fermentable carbon source
and produces the product alcohol; and (c) contacting the fermentation broth
with
fatty acids derived from biomass at a step in the fermentation process,
wherein at
least one of (i) growth rate and (ii) fermentable carbon consumption of the
microorganism is greater in the presence of the fatty acids than the growth
rate
and/or the fermentable carbon consumption of the microorganism in the absence
of the fatty acids.
[0015] In some embodiments, the fatty acids are free fatty acids (FFA). In
some
embodiments, the fatty acids include oleic acid. In some embodiments, the
fatty
acids include saturated fatty acids. In some embodiments, the fatty acids
include
palmitic acid. In some embodiments, the fatty acids include myristic acid.
[0016] In some embodiments, the product alcohol is butanol.
[0017] In some embodiments, the fermentable carbon source is derived from the
biomass. In some embodiments, the biomass includes corn and the fatty acids
are corn oil fatty acids.
[0018] In some embodiments, the fermentation broth further includes ethanol.
In
some embodiments, the method further includes contacting the fermentation
broth with ethanol.
[0019] In some embodiments, the step of contacting the fermentation broth with
fatty acids includes contacting triglycerides derived from biomass with one or
more enzymes capable of hydrolyzing triglycerides into free fatty acids,
whereby
the triglycerides are hydrolyzed into free fatty acids; and contacting the
fermentation broth with the free fatty acids, wherein at least one of (i) the
growth
-7-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
rate and (ii) the fermentable carbon consumption of the microorganism is
greater
in the presence of the free fatty acids than in the absence of the free fatty
acids.
In some embodiments, the one or more enzymes include lipase enzymes.
[0020] In some embodiments, the recombinant microorganism has a single
pyruvate decarboxylase (PDC) gene deletion. In some embodiments, the
recombinant microorganism has a double PDC gene deletion. In some
embodiments, the recombinant microorganism has reduced or eliminated
pyruvate decarboxylase activity.
[0021] In some embodiments, the concentration of the fatty acids in the
fermentation broth is not greater than about 0.8 g/L.
[0022] In some embodiments, a method for producing a product alcohol from
fermenting biomass includes (a) providing an aqueous biomass feedstream
including water, fermentable carbon source, and an amount of oil, wherein the
fermentable carbon source and the oil are both derived from said biomass; (b)
hydrolyzing at least a portion of the oil into free fatty acids to form a
biomass
feedstream including the free fatty acids; (c) contacting a fermentation
medium
with the biomass feedstream in a fermentation vessel, the fermentation medium
including a recombinant microorganism that produces a product alcohol; and (d)
fermenting the fermentable carbon source in the fermentation vessel to produce
said product alcohol, wherein at least one of (i) growth rate and (ii)
fermentable
carbon consumption of the microorganism is greater in the presence of the free
fatty acids than the growth rate and/or the fermentable carbon consumption of
the
microorganism in the absence of the free fatty acids.
[0023] In some embodiments, the step (b) of hydrolyzing at least a portion of
the
oil into free fatty acids includes contacting the oil with a composition
including one
or more enzymes capable of hydrolyzing the portion of the oil into free fatty
acids.
In some embodiments, the method further includes, prior to step (c),
inactivating
the one or more enzymes after at least a portion of the oil is hydrolyzed.
[0024] In some embodiments, the aqueous biomass feedstream is a liquefied
mash formed from a milled, unfractionated grain. In some embodiments, the
milled, unfractionated grain is corn and the oil is corn oil.
-8-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
[0025] In some embodiments, a method of producing a product alcohol includes
(a) providing biomass including glucose and oil including an amount of
triglycerides; (b) contacting the oil with a composition including one or more
substances capable of converting the triglycerides into free fatty acids,
whereby
at least a portion of the triglycerides in the oil are converted into free
fatty acids;
(c) contacting the biomass with a fermentation broth including a microorganism
capable of converting the glucose to a product alcohol, whereby a product
alcohol is produced; and (d) contacting the free fatty acids with the
fermentation
broth, wherein at least one of (i) growth rate and (ii) glucose consumption of
the
microorganism is greater in the presence of the free fatty acids than the
growth
rate and/or the glucose consumption of the microorganism in the absence of the
free fatty acids.
[0026] In some embodiments, the method further includes separating the oil of
(a)
from the biomass prior to the step (b) of contacting the oil with the one or
more
substances.
[0027] In some embodiments, step (b) of contacting the oil with a composition
including one or more substances includes contacting the oil with one or more
catalysts capable of hydrolyzing triglycerides into free fatty acids.
[0028] In some embodiments, step (b) of contacting the oil with a composition
including one or more substances includes contacting the oil with one or more
reactants or solvents capable of chemically reacting the triglycerides to
obtain a
reaction product including the free fatty acids.
[0029] In some embodiments, a method for producing butanol includes (a)
providing biomass including starch and oil, wherein the oil includes an amount
of
glycerides; (b) liquefying the biomass to produce a liquefied biomass, wherein
the
liquefied biomass includes oligosaccharides hydrolyzed from the starch; (c)
contacting the biomass of step (a) or the liquefied biomass of step (b) with a
composition including one or more enzymes capable of converting the glycerides
into free fatty acids, whereby at least a portion of the glycerides in the oil
are
converted into free fatty acids; (d) contacting the liquefied biomass with a
saccharification enzyme capable of converting oligosaccharides into
fermentable
sugar including monomeric glucose; (e) contacting the liquefied biomass with a
-9-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
recombinant microorganism capable of converting the fermentable sugar to
butanol whereby butanol is produced; and (f) contacting the free fatty acids
with
the recombinant microorganism, wherein at least one of (i) growth rate and
(ii)
glucose consumption of the recombinant microorganism is greater in the
presence of the free fatty acids than the growth rate and/or the glucose
consumption of the recombinant microorganism in the absence of the free fatty
acids.
[0030] In some embodiments, a fermentation process to produce a product
alcohol from a feedstock includes: (a) liquefying said feedstock to create a
feedstock slurry; (b) centrifuging the feedstock slurry to produce a
centrifuge
product including (i) an aqueous layer including glucose, (ii) an oil layer
including
glycerides, and (iii) a solids layer; (c) hydrolyzing at least a portion of
the
glycerides into free fatty acids; (d) feeding the aqueous layer of (b) to a
fermentation vessel containing a fermentation broth including a recombinant
microorganism capable of producing a product alcohol from glucose; (e)
fermenting the glucose of the aqueous layer to produce the product alcohol;
and
(f) contacting the fermentation broth with the free fatty acids, wherein at
least one
of (i) growth rate and (ii) glucose consumption of the microorganism is
greater in
the presence of the free fatty acids than the growth rate and/or the glucose
consumption of the microorganism in the absence of the free fatty acids.
[0031] In some embodiments, the process to produce a product alcohol from a
feedstock further includes, prior to the step of hydrolyzing the glycerides,
feeding
the glycerides to the fermentation vessel.
[0032] In some embodiments, a fermentation process includes (a) providing a
fermentation broth including a recombinant microorganism that produces a
product alcohol from a fermentable carbon source, a fermentable carbon source,
a product alcohol, and oil derived from biomass, wherein the oil includes
glycerides; (b) contacting the fermentation broth with a first extractant to
form a
two-phase mixture including an aqueous phase and an organic phase, wherein
the product alcohol and the oil partition into the organic phase to form a
product
alcohol-containing organic phase; (c) separating the product alcohol-
containing
organic phase from the aqueous phase; (d) separating the product alcohol from
-10-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
the organic phase to produce a lean organic phase; (e) contacting the lean
organic phase with a composition including one or more catalysts capable of
hydrolyzing the glycerides into free fatty acids to produce a second
extractant
including at least a portion of the first extractant and free fatty acids; and
(f)
repeating step (b) by contacting the fermentation broth with the second
extractant
of step (e), wherein at least one of (i) growth rate and (ii) fermentable
carbon
consumption of the microorganism is greater in the presence of the free fatty
acids than the growth rate and/or the fermentable carbon consumption of the
microorganism in the absence of the free fatty acids.
BRIEF DESCRIPTION OF THE DRAWINGS/FIGURES
[0033] The accompanying drawings, which are incorporated herein and form a
part of the specification, illustrate the present invention and, together with
the
description, further serve to explain the principles of the invention and to
enable a
person skilled in the pertinent art to make and use the invention.
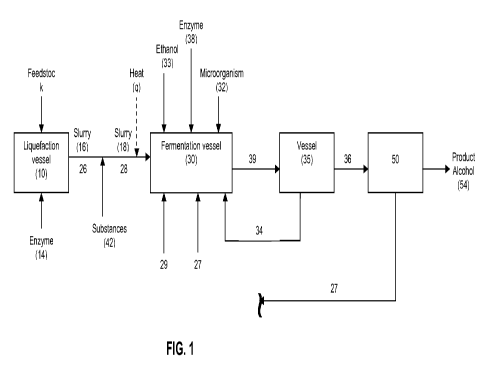
[0034] FIG. 1 schematically illustrates an exemplary method and system of the
present invention, in which a liquefied biomass is contacted with one or more
substances for lipid hydrolysis and fed to a fermentation vessel.
[0035] FIG. 2 schematically illustrates an exemplary method and system of the
present invention, in which a liquefied and saccharified biomass is contacted
with
one or more substances for lipid hydrolysis and fed to a fermentation vessel.
[0036] FIG. 3 schematically illustrates an exemplary method and system of the
present invention, in which undissolved solids and lipids are removed from a
liquefied biomass before fermentation, and in which the removed lipids are
hydrolyzed into free fatty acids using one or more substances, and the free
fatty
acids are fed to a fermentation vessel.
[0037] FIG. 4 schematically illustrates an exemplary method and system of the
present invention, in which lipids derived from native oil are hydrolyzed into
free
fatty acids using one or more substances, and the free fatty acids are fed to
a
fermentation vessel.
-11-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
[0038] FIG. 5 schematically illustrates an exemplary method and system of the
present invention, in which biomass lipids present in an extractant exiting a
fermentation vessel are converted into free fatty acids that are fed to a
fermentation vessel.
[0039] FIG. 6 is a graph illustrating the effect that the presence of fatty
acids in a
fermentation vessel has on glucose consumption for butanologen strain NGCI-
047.
[0040] FIG. 7 is a graph illustrating the effect that the presence of fatty
acids in a
fermentation vessel has on glucose consumption for butanologen strain NGCI-
049.
[0041] FIG. 8 is a graph illustrating the effect that the presence of fatty
acids in a
fermentation vessel has on glucose consumption for butanologen strain NYLA84.
DETAILED DESCRIPTION OF THE INVENTION
[0042] Unless defined otherwise, all technical and scientific terms used
herein
have the same meaning as commonly understood by one of ordinary skill in the
art to which this invention belongs. In case of conflict, the present
application
including the definitions will control. Also, unless otherwise required by
context,
singular terms shall include pluralities and plural terms shall include the
singular.
All publications, patents and other references mentioned herein are
incorporated
by reference in their entireties for all purposes.
[0043] In order to further define this invention, the following terms and
definitions
are herein provided.
[0044] As used herein, the terms "comprises," "comprising," "includes,"
"including," "has," "having," "contains," or "containing," or any other
variation
thereof, will be understood to imply the inclusion of a stated integer or
group of
integers but not the exclusion of any other integer or group of integers. For
example, a composition, a mixture, a process, a method, an article, or an
apparatus that comprises a list of elements is not necessarily limited to only
those
elements but can include other elements not expressly listed or inherent to
such
composition, mixture, process, method, article, or apparatus. Further, unless
expressly stated to the contrary, "or" refers to an inclusive or and not to an
-12-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
exclusive or. For example, a condition A or B is satisfied by any one of the
following: A is true (or present) and B is false (or not present), A is false
(or not
present) and B is true (or present), and both A and B are true (or present).
[0045] Also, the indefinite articles "a" and "an" preceding an element or
component of the invention are intended to be nonrestrictive regarding the
number of instances, that is, occurrences of the element or component.
Therefore "a" or "an" should be read to include one or at least one, and the
singular word form of the element or component also includes the plural unless
the number is obviously meant to be singular.
[0046] The term "invention" or "present invention" as used herein is a non-
limiting
term and is not intended to refer to any single embodiment of the particular
invention but encompasses all possible embodiments as described in the
application.
[0047] As used herein, the term "about" modifying the quantity of an
ingredient or
reactant of the invention employed refers to variation in the numerical
quantity
that can occur, for example, through typical measuring and liquid handling
procedures used for making concentrates or solutions in the real world;
through
inadvertent error in these procedures; through differences in the manufacture,
source, or purity of the ingredients employed to make the compositions or to
carry out the methods; and the like. The term "about" also encompasses
amounts that differ due to different equilibrium conditions for a composition
resulting from a particular initial mixture. Whether or not modified by the
term
"about," the claims include equivalents to the quantities. In one embodiment,
the
term "about" means within 10% of the reported numerical value, alternatively
within 5% of the reported numerical value.
[0048] "Biomass" as used herein refers to a natural product containing
hydrolyzable polysaccharides that provide fermentable sugars including any
sugars and starch derived from natural resources such as corn, cane, wheat,
cellulosic or lignocellulosic material and materials comprising cellulose,
hemicellulose, lignin, starch, oligosaccharides, disaccharides and/or
monosaccharides, and mixtures thereof. Biomass may also comprise additional
components such as protein and/or lipids. Biomass may be derived from a single
-13-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
source or biomass can comprise a mixture derived from more than one source.
For example, biomass may comprise a mixture of corn cobs and corn stover, or a
mixture of grass and leaves. Biomass includes, but is not limited to,
bioenergy
crops, agricultural residues, municipal solid waste, industrial solid waste,
sludge
from paper manufacture, yard waste, wood and forestry waste. Examples of
biomass include, but are not limited to, corn grain, corn cobs, crop residues
such
as corn husks, corn stover, grasses, wheat, rye, wheat straw, barley, barley
straw, hay, rice straw, switchgrass, waste paper, sugar cane bagasse, sorghum,
sugar cane, soy, components obtained from milling of grains, trees, branches,
roots, leaves, wood chips, sawdust, shrubs and bushes, vegetables, fruits,
flowers, animal manure, and mixtures thereof. For example, mash, juice,
molasses, or hydrolysate may be formed from biomass by any processing known
in the art for processing the biomass for purposes of fermentation such as by
milling, treating, and/or liquefying and comprises fermentable sugar and may
comprise water. For example, cellulosic and/or lignocellulosic biomass may be
processed to obtain a hydrolysate containing fermentable sugars by any method
known to one skilled in the art. A low ammonia pretreatment is disclosed in
U.S.
Patent Application Publication No. 2007/0031918A1, which is herein
incorporated
by reference. Enzymatic saccharification of cellulosic and/or lignocellulosic
biomass typically makes use of an enzyme consortium for breaking down
cellulose and hemicellulose to produce a hydrolysate containing sugars
including
glucose, xylose, and arabinose. (Saccharification enzymes suitable for
cellulosic
and/or lignocellulosic biomass are reviewed in Lynd, et al. (Microbiol. Mol.
Biol.
Rev. 66:506-577, 2002).
[0049] Mash, juice, molasses, or hydrolysate may include feedstock 12 and
feedstock slurry 16 as described herein. An aqueous feedstream may be derived
or formed from biomass by any processing known in the art for processing the
biomass for purposes of fermentation such as by milling, treating, and/or
liquefying and comprises fermentable carbon substrate (e.g., sugar) and may
comprise water. An aqueous feedstream may include feedstock 12 and
feedstock slurry 16 as described herein.
-14-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
[0050] "Biomass production" as used herein refers to microorganism biomass
production (i.e., cell biomass production or cell growth) such as during
cultivation
of microorganisms pre-fermentation or during fermentative growth of
microorganisms.
[0051] "Feedstock" as used herein means a feed in a fermentation process, the
feed containing a fermentable carbon source with or without undissolved
solids,
and where applicable, the feed containing the fermentable carbon source before
or after the fermentable carbon source has been liberated from starch or
obtained
from the break down of complex sugars by further processing such as by
liquefaction, saccharification, or other process. Feedstock includes or is
derived
from a biomass. Suitable feedstocks include, but are not limited to, rye,
wheat,
corn, cane, barley, cellulosic material, lignocellulosic material, or mixtures
thereof.
[0052] "Fermentation broth" as used herein means the mixture of water, sugars,
dissolved solids, optionally microorganisms producing alcohol, product
alcohol,
and all other constituents of the material held in the fermentation vessel in
which
product alcohol is being made by the reaction of sugars to alcohol, water, and
carbon dioxide (C02) by the microorganisms present. From time to time, as used
herein the term "fermentation medium" and "fermented mixture" can be used
synonymously with "fermentation broth."
[0053] "Fermentable carbon source" or "fermentable carbon substrate" as used
herein means a carbon source capable of being metabolized (or "consumed") by
the microorganisms disclosed herein for the production of fermentative
alcohol.
Suitable fermentable carbon sources include, but are not limited to,
monosaccharides such as glucose or fructose; disaccharides such as lactose or
sucrose; oligosaccharides; polysaccharides such as starch or cellulose; C5
sugars such as xylose and arabinose; one carbon substrates including methane;
and mixtures thereof. The term "consumed" as used herein includes processes
by which compounds, for example, organic compounds such as glucose are
broken down by the action of enzymes from a cell which results in the
production
of energy that may be used by the cell.
-15-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
[0054] "Fermentable sugar" as used herein refers to one or more sugars capable
of being metabolized (or "consumed") by the microorganisms disclosed herein
for
the production of fermentative alcohol.
[0055] "Fermentation vessel" as used herein means the vessel in which the
fermentation reaction is carried out whereby product alcohol such as butanol
is
made from sugars.
[0056] "Liquefaction vessel" as used herein means the vessel in which
liquefaction is carried out. Liquefaction is the process in which
oligosaccharides
are liberated from the feedstock. In some embodiments where the feedstock is
corn, oligosaccharides are liberated from the corn starch content during
liquefaction.
[0057] "Saccharification vessel" as used herein means the vessel in which
saccharification (i.e., the break down of oligosaccharides into
monosaccharides)
is carried out. Where fermentation and saccharification occur simultaneously,
the
saccharification vessel and the fermentation vessel may be one in the same
vessel.
[0058] "Sugar" as used herein refers to oligosaccharides, disaccharides,
monosaccharides, and/or mixtures thereof. The term "saccharide" also includes
carbohydrates including starches, dextrans, glycogens, cellulose, pentosans,
as
well as sugars.
[0059] As used herein, "saccharification enzyme" means one or more enzymes
that are capable of hydrolyzing polysaccharides and/or oligosaccharides, for
example, alpha-1,4-glucosidic bonds of glycogen, or starch. Saccharification
enzymes may include enzymes capable of hydrolyzing cellulosic or
lignocellulosic
materials as well.
[0060] "Undissolved solids" as used herein means non-fermentable portions of
feedstock, for example, germ, fiber, and gluten.
[0061] "Product alcohol" as used herein refers to any alcohol that can be
produced by a microorganism in a fermentation process that utilizes biomass as
a source of fermentable carbon substrate. Product alcohols include, but are
not
limited to, Ci to C8 alkyl alcohols. In some embodiments, the product alcohols
are C2 to C8 alkyl alcohols. In other embodiments, the product alcohols are C2
to
-16-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
C5 alkyl alcohols. It will be appreciated that Ci to C8 alkyl alcohols
include, but
are not limited to, methanol, ethanol, propanol, butanol, and pentanol.
Likewise
C2 to C8 alkyl alcohols include, but are not limited to, ethanol, propanol,
butanol,
and pentanol. "Alcohol" is also used herein with reference to a product
alcohol.
[0062] "Butanol" as used herein refers with specificity to the butanol isomers
1-
butanol (1-BuOH), 2-butanol (2-BuOH), tert-butanol, and/or isobutanol (iBuOH
or
i-BuOH or I-BUOH, also known as 2-methyl-1-propanol), either individually or
as
mixtures thereof. From time to time, when referring to esters of butanol, the
terms "butyl esters" and "butanol esters" may be used interchangeably.
[0063] "Propanol" as used herein refers to the propanol isomers isopropanol or
1-
propanol.
[0064] "Pentanol" as used herein refers to the pentanol isomers 1-pentanol, 3-
methyl-1-butanol, 2-methyl-1-butanol, 2,2-dimethyl-1-propanol, 3-pentanol, 2-
pentanol, 3-methyl-2-butanol, or 2-methyl-2-butanol.
[0065] The term "alcohol equivalent" as used herein refers to the weight of
alcohol that would be obtained by a perfect hydrolysis of an alcohol ester and
the
subsequent recovery of the alcohol from an amount of alcohol ester.
[0066] The term "aqueous phase titer" as used herein refers to the
concentration
of a particular alcohol (e.g., butanol) in the fermentation broth.
[0067] The term "effective titer" as used herein refers to the total amount of
a
particular alcohol (e.g., butanol) produced by fermentation or alcohol
equivalent
of the alcohol ester produced by alcohol esterification per liter of
fermentation
medium. For example, the effective titer of butanol in a unit volume of a
fermentation includes: (i) the amount of butanol in the fermentation medium;
(ii)
the amount of butanol recovered from the organic extractant; (iii) the amount
of
butanol recovered from the gas phase, if gas stripping is used; and (iv) the
alcohol equivalent of the butyl ester in either the organic or aqueous phase.
[0068] "In Situ Product Removal (ISPR)" as used herein means the selective
removal of a specific fermentation product from a biological process such as
fermentation, to control the product concentration in the biological process
as the
product is produced.
-17-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
[0069] "Extractant" or "ISPR extractant" as used herein means an organic
solvent
used to extract any product alcohol such as butanol or used to extract any
product alcohol ester produced by a catalyst from a product alcohol and a
carboxylic acid or lipid. From time to time, as used herein the term "solvent"
may
be used synonymously with "extractant." For the processes described herein,
extractants are water-immiscible.
[0070] The terms "water-immiscible" or "insoluble" refer to a chemical
component
such as an extractant or solvent, which is incapable of mixing with an aqueous
solution such as a fermentation broth, in such a manner as to form one liquid
phase.
[0071] The term "aqueous phase" as used herein refers to the aqueous phase of
a biphasic mixture obtained by contacting a fermentation broth with a water-
immiscible organic extractant. In an embodiment of a process described herein
that includes fermentative extraction, the term "fermentation broth" then
specifically refers to the aqueous phase in biphasic fermentative extraction.
[0072] The term "organic phase" as used herein refers to the non-aqueous phase
of a biphasic mixture obtained by contacting a fermentation broth with a water-
immiscible organic extractant.
[0073] The term "fatty acid" as used herein refers to a carboxylic acid (e.g.,
aliphatic monocarboxylic acid) having C4 to C28 carbon atoms (most commonly
C12 to C24 carbon atoms), which is either saturated or unsaturated. Fatty
acids
may also be branched or unbranched. Fatty acids may be derived from, or
contained in esterified form, in an animal or vegetable fat, oil, or wax.
Fatty acids
may occur naturally in the form of glycerides in fats and fatty oils or may be
obtained by hydrolysis of fats or by synthesis. The term fatty acid may
describe a
single chemical species or a mixture of fatty acids. In addition, the term
fatty acid
also encompasses free fatty acids.
[0074] The term "fatty alcohol" as used herein refers to an alcohol having an
aliphatic chain of C4 to C22 carbon atoms, which is either saturated or
unsaturated.
-18-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
[0075] The term "fatty aldehyde" as used herein refers to an aldehyde having
an
aliphatic chain of C4 to C22 carbon atoms, which is either saturated or
unsaturated.
[0076] The term "carboxylic acid" as used herein refers to any organic
compound
with the general chemical formula -000H in which a carbon atom is bonded to
an oxygen atom by a double bond to make a carbonyl group (-C=O) and to a
hydroxyl group (-OH) by a single bond. A carboxylic acid may be in the form of
the protonated carboxylic acid, in the form of a salt of a carboxylic acid
(e.g., an
ammonium, sodium, or potassium salt), or as a mixture of protonated carboxylic
acid and salt of a carboxylic acid. The term carboxylic acid may describe a
single
chemical species (e.g., oleic acid) or a mixture of carboxylic acids as can be
produced, for example, by the hydrolysis of biomass-derived fatty acid esters
or
triglycerides, diglycerides, monoglycerides, and phospholipids.
[0077] "Native oil" as used herein refers to lipids obtained from plants
(e.g., biomass) or animals. "Plant-derived oil" as used herein refers to
lipids
obtain from plants in particular. From time to time, "lipids" may be used
synonymously with "oil" and "acyl glycerides." Native oils include, but are
not
limited to, tallow, corn, canola, capric/caprylic triglycerides, castor,
coconut,
cottonseed, fish, jojoba, lard, linseed, neetsfoot, oiticica, palm, peanut,
rapeseed,
rice, safflower, soya, sunflower, tung, jatropha, and vegetable oil blends.
[0078] The term "separation" as used herein is synonymous with "recovery" and
refers to removing a chemical compound from an initial mixture to obtain the
compound in greater purity or at a higher concentration than the purity or
concentration of the compound in the initial mixture.
[0079] As used herein, "recombinant microorganism" refers to microorganisms
such as bacteria or yeast, that are modified by use of recombinant DNA
techniques, for example, by engineering a host cell to comprise a biosynthetic
pathway such as a biosynthetic pathway to produce an alcohol such as butanol.
[0080] The present invention provides methods for producing product alcohol
(e.g., fermentative alcohol) in which alcohol-producing microorganisms in a
fermentation vessel are contacted with fatty acids which were derived from
native
oil such as biomass lipids at a step in a fermentation process. This fatty
acid
-19-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
supplementation during the fermentative growth of the microorganism can
increase the fermentable carbon consumption of the microorganisms, growth
rate, and biomass production, particularly with regard to recombinant
microorganisms that have reduced or eliminated pyruvate decarboxylase
activity.
[0081] In some embodiments, glycerides in the oil can be chemically converted
into fatty acids which are contacted with a fermentation broth including a
recombinant microorganism that produces a product alcohol from fermentable
carbon source. In other embodiments, the glycerides in the oil can be
catalytically (e.g., enzymatically) hydrolyzed into fatty acids which are
contacted
with a fermentation broth including a recombinant microorganism. In some
embodiments, the fatty acids can be obtained from hydrolysis of lipids found
in
the biomass which supplies the fermentable carbon source for fermentation. The
fatty acids can also be used as an ISPR extractant to remove the product
alcohol
from the fermentation broth.
[0082] The fatty acids can be saturated, mono-unsaturated, poly-unsaturated,
and mixtures thereof. For example, oil having a naturally-occurring fatty acid
composition including a mixture of palmitic acid and oleic acid (e.g., corn
oil) can
be hydrolyzed to produce a mixture of free oleic acid and free palmitic acid
which
can be contacted with a fermentation broth in a fermentation vessel. In some
embodiments, the concentration of the carboxylic acid (such as fatty acid) in
the
fermentation vessel exceeds the solubility limit in the aqueous phase and
results
in the production a two-phase fermentation mixture comprising an organic phase
and an aqueous phase. In some embodiments, the concentration of carboxylic
acids in the fermentation broth is typically not greater than about 0.8 g/L
and is
limited by the solubility of the carboxylic acid in the broth.
[0083] Growth rate and/or fermentable carbon consumption of the microorganism
is greater in the presence of fatty acids than the growth rate and the
fermentable
carbon consumption of the microorganism in the absence of fatty acids.
Correspondingly, fatty acid supplementation according to the methods of the
present invention can achieve increased cell concentration and increased
alcohol
production than could be achieved in the absence of such fatty acid
supplementation. In some embodiments, the microorganism may be a butanol-
-20-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
producing microorganism or other microorganism that typically requires
supplementation of a 2-carbon substrate, for example, ethanol, to survive and
grow. In such embodiments, the fatty acid supplementation according to
methods of the present invention can allow such 2-carbon dependent
microorganisms to survive and grow in the absence of ethanol supplementation.
In some embodiments, the microorganisms can be deficient in production of
acetyl-CoA from pyruvate. In some embodiments, the microorganism is
metabolically engineered with disruptive mutations in one or more pyruvate
decarboxylase (PDC) genes such that the pathway to fatty acid biosynthesis is
modified. In some embodiments, the microorganism is metabolically engineered
with disruptive mutations in two PDC genes such as genes PDC1 and PDC5,
resulting in an altered pathway to fatty acid biosyntheses. Thus, the methods
of
the present invention can attain improved alcohol productivity by providing an
optimal environment for fermentative growth of recombinant microorganisms.
[0084] The present invention will be described with reference to the Figures.
FIG. 1 illustrates an exemplary process flow diagram for production of
fermentative alcohol according to an embodiment of the present invention. As
shown, a feedstock 12 can be introduced to an inlet in a liquefaction vessel
10
and liquefied to produce a feedstock slurry 16. Feedstock 12 contains
hydrolysable starch that supplies a fermentable carbon source (e.g.,
fermentable
sugar such as glucose), and can be a biomass such as, but not limited to, rye,
wheat, corn, cane, barley, cellulosic material, lignocellulosic material, or
mixtures
thereof, or can otherwise be derived from a biomass. In some embodiments,
feedstock 12 can be one or more components of a fractionated biomass and in
other embodiments, feedstock 12 can be a milled, unfractionated biomass. In
some embodiments, feedstock 12 can be corn such as dry milled, unfractionated
corn kernels, and the undissolved solids can include germ, fiber, and gluten.
The
undissolved solids are non-fermentable portions of feedstock 12. For purposes
of the discussion herein with reference to the embodiments shown in the
Figures,
feedstock 12 will often be described as constituting milled, unfractionated
corn, in
which the undissolved solids have not been separated therefrom. However, it
should be understood that the exemplary methods and systems described herein
-21 -
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
can be modified for different feedstocks whether fractionated or not, as
apparent
to one of skill in the art. In some embodiments, feedstock 12 can be high-
oleic
corn, such that corn oil derived therefrom is a high-oleic corn oil having an
oleic
acid content of at least about 55 wt% oleic acid. In some embodiments, the
oleic
acid content in high-oleic corn oil can be up to about 65 wt% as compared with
the oleic acid content in normal corn oil which is about 24 wt%. High-oleic
oil can
provide some advantages for use in the methods of the present invention, as
hydrolysis of the oil provides free fatty acids having a high oleic acid
content for
contacting with a fermentation broth. In some embodiments, the fatty acids or
mixtures thereof comprise unsaturated fatty acids. The presence of unsaturated
fatty acids decreases the melting point providing advantages for handling. Of
the
unsaturated fatty acids, those which are monounsaturated, that is, possessing
a
single carbon-carbon double bond, may provide advantages with respect to
melting point without sacrificing suitable thermal and oxidative stability for
process considerations.
[0085] The process of liquefying feedstock 12 involves hydrolysis of
polysaccharides in feedstock 12 into sugars including, for example, dextrins
and
oligosaccharides, and is a conventional process. Any known liquefying
processes as well as the corresponding liquefaction vessel, normally utilized
by
the industry can be used including, but not limited to, the acid process, the
acid-
enzyme process, or the enzyme process. Such processes can be used alone or
in combination. In some embodiments, the enzyme process can be utilized and
an appropriate enzyme 14, for example, alpha-amylase, is introduced to an
inlet
in liquefaction vessel 10. Water can also be introduced to liquefaction vessel
10.
In some embodiments, a saccharification enzyme, for example, glucoamylase,
may also be introduced to liquefaction vessel 10. In additional embodiments, a
lipase may also be introduced to liquefaction vessel 10 to catalyze the
conversion
of one or more components of the oil to free fatty acids.
[0086] Feedstock slurry 16 produced from liquefying feedstock 12 includes
sugar, oil 26, and undissolved solids derived from the biomass from which
feedstock 12 was formed. In some embodiments, the oil is in an amount of about
0 wt% to at least about 2 wt% of the fermentation broth composition. In some
-22-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
embodiments, the oil is in an amount of at least about 0.5 wt% of the
feedstock.
Feedstock slurry 16 can be discharged from an outlet of liquefaction vessel
10.
In some embodiments, feedstock 12 is corn or corn kernels and therefore,
feedstock slurry 16 is a corn mash slurry.
[0087] One or more substances 42 can be added to feedstock slurry 16.
Substances 42 are capable of hydrolyzing glycerides in oil 26 to free fatty
acids
(FFA) 28. For example, when feedstock 12 is corn, then oil 26 is the
feedstock's
constituent corn oil and the free fatty acids 28 are corn oil fatty acids
(COFA).
Thus, after introduction of substances 42 to feedstock slurry 16, at least a
portion
of the glycerides in oil 26 are hydrolyzed to FFA 28 resulting in a feedstock
slurry
18 having FFA 28.
[0088] In some embodiments, one or more substances 42 are one or more
catalysts 42 capable of catalytically hydrolyzing glycerides in oil 26 to free
fatty
acids 28 (FFA). Thus, after introduction of catalyst 42 to feedstock slurry
16, at
least a portion of the glycerides in oil 26 are hydrolyzed to FFA 28 resulting
in a
feedstock slurry 18 having FFA 28 and catalyst 42.
[0089] The resulting acid/oil composition from hydrolyzing oil 26 is typically
at
least about 17 wt% FFA. In some embodiments, the resulting acid/oil
composition from hydrolyzing oil 26 is at least about 20 wt% FFA, at least
about
25 wt% FFA, at least about 30 wt% FFA, at least about 35 wt% FFA, at least
about 40 wt% FFA, at least about 45 wt% FFA, at least about 50 wt% FFA, at
least about 55 wt% FFA, at least about 60 wt% FFA, at least about 65 wt% FFA,
at least about 70 wt% FFA, at least about 75 wt% FFA, at least about 80 wt%
FFA, at least about 85 wt% FFA, at least about 90 wt% FFA, at least about 95
wt% FFA, or at least about 99 wt% FFA.
[0090] Alternatively, in some embodiments, substance(s) 42 can alternatively
constitute one or more reactants or solvents capable of chemically reacting
oil 26
to FFA 28 for contacting with recombinant microorganism 32. For example, corn
oil fatty acids can be synthesized from corn oil as oil 26 by base hydrolysis
using
NaOH and water as substances 42, as further described in co-pending,
commonly owned U.S. Provisional Application Serial No. 61/368,436, filed on
July 28, 2010, and incorporated herein in its entirety by reference thereto.
Also,
-23-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
for example, corn oil triglycerides as oil 26 can be reacted with aqueous
ammonium hydroxide as reactant 42 to obtain fatty acid (and fatty amide) as
further described in Roe, et al., (Am. Oil Chem. Soc. 29:18-22, 1952), which
is
incorporated herein in its entirety by reference thereto. For purposes of the
discussion herein with reference to the embodiments shown in the Figures,
substance(s) 42 will often be described as constituting one or more catalysts
as
substance(s) 42 for the hydrolysis of biomass lipids to FFA 28 supplemented
during fermentative growth of recombinant microorganism 32. However, it should
be understood that the exemplary methods and systems described herein can be
modified such that substance(s) 42 are reactant(s) and/or solvent(s) that are
capable of chemically converting the biomass lipids into FFA 28.
[0091] In some embodiments, catalyst 42 can be one or more enzymes, for
example, hydrolase enzymes such as lipase enzymes. Lipase enzymes used
may be derived from any source including, for example, Absidia, Achromobacter,
Aeromonas, Alcaligenes, Alternaria, Aspergillus, Achromobacter, Aureobasidium,
Bacillus, Beauveria, Brochothrix, Candida, Chromobacter, Coprinus, Fusarium,
Geotricum, Hansenula, Humicola, Hyphozyma, Lactobacillus, Metarhizium,
Mucor, Nectria, Neurospora, Paecilomyces, Penicillium, Pseudomonas,
Rhizoctonia, Rhizomucor, Rhizopus, Rhodosporidium, Rhodotorula,
Saccharomyces, Sus, Sporobolomyces, Thermomyces, Thiarosporella,
Trichoderma, Verticillium, and/or a strain of Yarrowia. In a preferred aspect,
the
source of the lipase is selected from the group consisting of Absidia
blakesleena,
Absidia corymbifera, Achromobacter iophagus, Alcaligenes sp., Alternaria
brassiciola, Aspergillus flavus, Aspergillus niger, Aureobasidium pullulans,
Bacillus pumilus, Bacillus strearothermophilus, Bacillus subtilis, Brochothrix
thermosohata, Candida cylindracea (Candida rugosa), Candida paralipolytica,
Candida Antarctica lipase A, Candida antartica lipase B, Candida ernobii,
Candida deformans, Chromobacter viscosum, Coprinus cinerius, Fusarium
oxysporum, Fusarium solani, Fusarium solani pisi, Fusarium roseum culmorum,
Geotricum penicillatum, Hansenula anomala, Humicola brevispora, Humicola
brevis var. thermoidea, Humicola insolens, Lactobacillus curvatus, Rhizopus
oryzae, Penicillium cyclopium, Penicillium crustosum, Penicillium expansum,
-24-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
Penicillium sp. I, Penicillium sp. II, Pseudomonas aeruginosa, Pseudomonas
alcaligenes, Pseudomonas cepacia (syn. Burkholderia cepacia), Pseudomonas
fluorescens, Pseudomonas fragi, Pseudomonas maltophilia, Pseudomonas
mendocina, Pseudomonas mephitica lipolytica, Pseudomonas alcaligenes,
Pseudomonas plantari, Pseudomonas pseudoalcaligenes, Pseudomonas putida,
Pseudomonas stutzeri, and Pseudomonas wisconsinensis, Rhizoctonia solani,
Rhizomucor miehei, Rhizopus japonicus, Rhizopus microsporus, Rhizopus
nodosus, Rhodosporidium toruloides, Rhodotorula glutinis, Saccharomyces
cerevisiae, Sporobolomyces shibatanus, Sus scrofa, Thermomyces lanuginosus
(formerly Humicola lanuginose), Thiarosporella phaseolina, Trichoderma
harzianum, Trichoderma reesei, and Yarrowia lipolytica. In a further preferred
aspect, the lipase is selected from the group consisting of Thermomcyces
lanuginosus, Aspergillus sp. lipase, Aspergillus niger lipase, Candida
antartica
lipase B, Pseudomonas sp. lipase, Penicillium roqueforti lipase, Penicillium
camembertii lipase, Mucor javanicus lipase, Burkholderia cepacia lipase,
Alcaligenes sp. lipase, Candida rugosa lipase, Candida parapsilosis lipase,
Candida deformans lipase, lipases A and B from Geotrichum candidum,
Neurospora crassa lipase, Nectria haematococca lipase, Fusarium heterosporum
lipase Rhizopus delemar lipase, Rhizomucor miehei lipase, Rhizopus arrhizus
lipase, and Rhizopus oryzae lipase. Suitable commercial lipase preparations
suitable as enzyme catalyst 42 include, but are not limited to, Lipolase 100
L,
Lipex 100L, Lipoclean 2000T, Lipozyme CALB L, Novozym CALA L, and
Palatase 20000L, available from Novozymes, or from Pseudomonas fluorescens,
Pseudomonas cepacia, Mucor miehei, hog pancreas, Candida cylindracea,
Rhizopus niveus, Candida antarctica, Rhizopus arrhizus or Aspergillus
available
from SigmaAldrich.
[0092] After at least a portion of the glycerides are hydrolyzed, in some
embodiments, catalyst 42 can be inactivated. Any method known in the art can
be used to render catalyst 42 inactive. For example, in some embodiments,
catalyst 42 can be inactivated by the application of heat, and/or by adjusting
the
pH of the reaction mass to a pH where catalyst 42 is irreversibly inactivated,
and/or by adding a chemical or biochemical species capable of selectively
-25-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
inactivating the catalyst activity. As shown, for example, in the embodiment
of
FIG. 1, heat q is applied to feedstock slurry 18, whereby catalyst 42 becomes
inactive. The application of heat q can be applied to feedstock slurry 18
before
feedstock slurry 18 is fed to a fermentation vessel 30. Heat-treated feedstock
slurry 18 (with inactive catalyst 42) is then introduced into a fermentation
vessel
30 along with a microorganism 32 to be included in a fermentation broth held
in
fermentation vessel 30. Alternatively, feedstock slurry 18 can be fed to
fermentation vessel 30 and subjected to heat q while in the fermentation
vessel,
before fermentation vessel inoculation of microorganism 32. For example, in
some embodiments, catalyst inactivation treatment can be achieved by heating
feedstock slurry 18 with heat q to temperature of at least about 75 C for at
least
about 5 minutes, at least about 75 C for at least about 10 minutes, at least
about
75 C for at least about 15 minutes, at least about 80 C for at least about 5
minutes, at least about 80 C for at least about 10 minutes, at least about 80
C for
at least about 15 minutes, at least about 85 C for at least about 5 minutes,
at
least about 85 C for at least about 10 minutes, or at least about 85 C for at
least
about 15 minutes. In some embodiments, after being subject to heat q,
feedstock
slurry 18 is cooled to an appropriate temperature for fermentation prior to
introduction to fermentation vessel 30 (or prior to fermentation vessel
inoculation
in the case that the application of heat q is conducted in the fermentation
vessel).
For example, in some embodiments, the temperature of feedstock slurry 18 is
about 30 C prior to contacting with a fermentation broth.
[0093] Inactivation of catalyst 42 is preferred when it is desirable to
prevent
catalyst 42 from esterifying alcohol with fatty acids 28 in the fermentation
vessel.
In some embodiments, production of an alcohol ester by esterification of
product
alcohol in a fermentation medium with an organic acid (e.g., fatty acid) and a
catalyst (e.g., lipase) is desirable, as further described in co-pending,
commonly
owned U.S. Provisional Application Serial No. 61/368,429, filed on July 28,
2010;
U.S. Provisional Application Serial No. 61/379,546, filed on September 2,
2010;
and U.S. Provisional Application Serial No. 61/440,034, filed on February 7,
2011; all incorporated herein in its entirety by reference thereto. For
example, for
butanol production, active catalyst 42 in fermentation vessel (introduced via
slurry
-26-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
18) can catalyze the esterification of the butanol with fatty acids 28
(introduced
via slurry 18) to form fatty acid butyl esters (FABE) in situ. In such
embodiments
in which alcohol esters of fatty acids are desirable, the methods described
herein
can be modified so as to omit inactivated of catalyst 42 prior to contacting a
fermentation broth including product alcohol. Thus, with reference to the
exemplary process flow diagrams of Figure 1-5, these alternative embodiments
can be achieved by omitting the application of heat q to the process stream
containing catalyst 42, such that catalyst 42 esterifies the product alcohol
with
fatty acids 28 in fermentation vessel 30. Moreover, in some embodiments, the
exemplary process flow diagrams of Figure 1-5 can be modified so as to omit
heat q if unneeded for chemical conversion of oil 26 to FFA 28 using one or
more
reactants or solvents as the substance(s) 42 instead of catalysts 42.
[0094] Fermentation vessel 30 is configured to ferment slurry 18 to produce a
product alcohol such as butanol. In particular, microorganism 32 metabolizes
the
fermentable sugar in slurry 18 and excretes a product alcohol. Microorganism
32
is selected from the group of bacteria, cyanobacteria, filamentous fungi, and
yeast. In some embodiments, microorganism 32 can be a bacteria such as
E.coli. In some embodiments, microorganism 32 can be a fermentative
recombinant microorganism. The slurry can include sugar, for example, in the
form of oligosaccharides and water, and can comprise less than about 20 g/L of
monomeric glucose, more preferably less than about 10 g/L or less than about
g/L of monomeric glucose. Suitable methodology to determine the amount of
monomeric glucose is well known in the art. Such suitable methods known in the
art include HPLC.
[0095] In some embodiments, slurry 18 is subjected to a saccharification
process
in order to break the complex sugars (e.g., oligosaccharides) in slurry 18
into
monosaccharides that can be readily metabolized by microorganism 32. Any
known saccharification process that is routinely utilized by the industry, can
be
used including, but not limited to, the acid process, the acid-enzyme process,
or
the enzyme process. In some embodiments, simultaneous saccharification and
fermentation (SSF) can occur inside fermentation vessel 30 as shown in FIG. 1.
In some embodiments, an enzyme 38, such as glucoamylase, can be introduced
-27-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
to an inlet in fermentation vessel 30 in order to breakdown the starch or
oligosaccharides to glucose capable of being metabolized by microorganism 32.
[0096] Optionally, ethanol 33 may be supplied to fermentation vessel 30 to be
included in the fermentation broth. In some embodiments, when a recombinant
microorganism having a butanol biosynthetic pathway is used as microorganism
32 for butanol production, microorganism 32 may require supplementation of a 2-
carbon substrate (e.g., ethanol) to survive and grow. Thus, in some
embodiments, ethanol 33 may be supplied to fermentation vessel 30.
[0097] However, it has been surprisingly found that methods of the present
invention, in which free fatty acid (e.g., FFA 28) is present in the
fermentation
vessel, can allow reduction of the amount of ethanol 33 typically supplied for
a
given recombinant microorganism without detriment to the vitality of the
recombinant microorganism. Further, in some embodiments, the methods of the
present invention provide that the alcohol (e.g., butanol) production rate
without
ethanol supplementation to be comparable with the production rate that can be
realized when ethanol 33 is supplemented. As further demonstrated by the
comparative examples presented in the Examples 1-14 below, the butanol
production rate when fatty acid but not ethanol is in the fermentation vessel
can
be greater than the butanol production rate when neither fatty acid nor
ethanol is
in the fermentation vessel. Thus, in some embodiments, the amount of ethanol
33 supplementation is reduced compared to conventional processes. For
example, a typical amount of ethanol added to a fermentation vessel for
microorganisms requiring supplementation of a 2-carbon substrate is about 5
g/L
anhydrous ethanol (i.e., 5 g anhydrous ethanol per liter of fermentation
medium).
In some embodiments, the fermentation is not supplemented with any ethanol 33.
In the latter case, the stream of ethanol 33 is entirely omitted from the
fermentation vessel. Thus, in some embodiments of the present invention, it is
possible to reduce or eliminate the cost associated with supplemental ethanol
33,
as well as the inconvenience associated with storing vats of ethanol 33 and
supplying it to the fermentation vessel during butanol fermentation. Moreover,
regardless of ethanol supplementation, in some embodiments, the methods of
the present invention can provide a higher rate of glucose uptake by
-28-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
microorganism 32 by virtue of the presence of fatty acids during the
fermentation.
According to the methods described herein, the fatty acids can be introduced
into
fermentation vessel 30 as FFA 28, hydrolyzed from feedstock oil 26 of slurry
16,
or otherwise hydrolyzed from native oil such as biomass lipids at a step in
the
fermentation process. Fatty acids can also be introduced into fermentation
vessel as an ISPR extractant 29.
[0098] In fermentation vessel 30, alcohol is produced by microorganism 32. In
situ product removal (ISPR) can be utilized to remove the product alcohol from
the fermentation broth. In some embodiments, ISPR includes liquid-liquid
extraction. Liquid-liquid extraction can be performed according to the
processes
described in U.S. Patent Application Publication No. 2009/0305370, the
disclosure of which is hereby incorporated in its entirety. U.S. Patent
Application
Publication No. 2009/0305370 describes methods for producing and recovering
butanol from a fermentation broth using liquid-liquid extraction, the methods
comprising the step of contacting the fermentation broth with a water-
immiscible
extractant to form a two-phase mixture comprising an aqueous phase and an
organic phase. Typically, the extractant can be an organic extractant selected
from the group consisting of saturated, mono-unsaturated, poly-unsaturated
(and
mixtures thereof) C12 to C22 fatty alcohols, C12 to C22 fatty acids, esters of
C12 to
C22 fatty acids, C12 to C22 fatty aldehydes, and mixtures thereof, which
contacts a
fermentation broth and forms a two-phase mixture comprising an aqueous phase
and an organic phase. The extractant may also be an organic extractant
selected from the group consisting of saturated, mono-unsaturated, poly-
unsaturated (and mixtures thereof) C4 to C22 fatty alcohols, C4 to C28 fatty
acids,
esters of C4 to C28 fatty acids, C4 to C22 fatty aldehydes, and mixtures
thereof,
which contacts a fermentation broth and to form a two-phase mixture comprising
an aqueous phase and an organic phase. Free fatty acids 28 from slurry 18 can
also serve as an ISPR extractant 28. For example, when free fatty acids 28 are
corn oil fatty acids (COFA), ISPR extractant 28 is COFA. ISPR extractant (FFA)
28 contacts the fermentation broth and forms a two-phase mixture comprising an
aqueous phase 34 and an organic phase. The product alcohol present in the
fermentation broth preferentially partitions into the organic phase to form an
-29-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
alcohol-containing organic phase 36. In some embodiments, fermentation vessel
30 has one or more inlets for receiving one or more additional ISPR
extractants
29 which form a two-phase mixture comprising an aqueous phase and an organic
phase, with the product alcohol partitioning into the organic phase.
[0099] The biphasic mixture can be removed from fermentation vessel 30 as
stream 39 and introduced into a vessel 35, in which the alcohol-containing
organic phase 36 is separated from the aqueous phase 34. The alcohol-
containing organic phase 36 is separated from the aqueous phase 34 of the
biphasic stream 39 using methods known in the art including, but not limited
to,
siphoning, decantation, aspiration, centrifugation, using a gravity settler,
membrane-assisted phase splitting, and the like. All or part of the aqueous
phase 34 can be recycled into fermentation vessel 30 as fermentation medium
(as shown), or otherwise discarded and replaced with fresh medium, or treated
for the removal of any remaining product alcohol and then recycled to
fermentation vessel 30. Then, the alcohol-containing organic phase 36 is
treated
in a separator 50 to recover product alcohol 54, and the resulting alcohol-
lean
extractant 27 can then be recycled back into fermentation vessel 30, usually
in
combination with fresh FFA 28 from slurry 18 and/or with fresh extractant 29,
for
further extraction of the product alcohol. Alternatively, fresh FFA 28 (from
slurry
18) and/or extractant 29 can be continuously added to the fermentation vessel
to
replace the ISPR extractant(s) removed in biphasic mixture stream 39.
[00100] In some embodiments, any additional ISPR extractant 29 can be an
exogenous organic extractant such as oleyl alcohol, behenyl alcohol, cetyl
alcohol, lauryl alcohol, myristyl alcohol, stearyl alcohol, 1-undecanol, oleic
acid,
lauric acid, myristic acid, stearic acid, methyl myristate, methyl oleate,
undecanal,
lauric aldehyde, 20-methylundecanal, and mixtures thereof. In some
embodiments, ISPR extractant 29 can be a carboxylic acid or free fatty acid
and
in some embodiments, the carboxylic acid or free fatty acid have a chain of 4
to
28 carbons, 4 to 22 carbons in other embodiments, 8 to 22 carbons in other
embodiments, 10 to 28 carbons in other embodiments, 7 to 22 carbons in other
embodiments, 12 to 22 carbons in other embodiments, 4 to 18 carbons in other
embodiments, 12 to 22 carbons in other embodiments, and 12 to 18 carbons in
-30-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
still other embodiments. In some embodiments, ISPR extractant 29 is one or
more of the following fatty acids: azaleic, capric, caprylic, castor, coconut
(i.e., as
a naturally-occurring combination of fatty acids including lauric, myrisitic,
palmitic,
caprylic, capric, stearic, caproic, arachidic, oleic, and linoleic), dimer,
isostearic,
lauric, linseed, myristic, oleic, olive, palm oil, palmitic, palm kernel,
peanut,
pelargonic, ricinoleic, sebacic, soya, stearic acid, tall oil, tallow, #12
hydroxy
stearic, or any seed oil. In some embodiments, ISPR extractant 29 is one or
more of diacids, for example, azelaic acid and sebacic acid. Thus, in some
embodiments, ISPR extractant 29 can be a mixture of two or more different
fatty
acids. In some embodiments, ISPR extractant 29 can be a free fatty acid
derived
from chemical or enzymatic hydrolysis of glycerides derived from native oil.
For
example, in some embodiments, ISPR extractant 29 can be free fatty acids 28'
obtained by enzymatic hydrolysis of native oil such as biomass lipids as later
described with reference to the embodiment of FIG. 4. In some embodiments,
ISPR extractant 29 can be a fatty acid extractant selected from the group
consisting of fatty acids, fatty alcohols, fatty amides, fatty acid methyl
esters,
lower alcohol esters of fatty acids, fatty acid glycol esters, hydroxylated
triglycerides, and mixtures thereof, obtained from chemical conversion of
native
oil such as biomass lipids as described for example in co-pending, commonly
owned U.S. Provisional Application Serial No. 61/368,436, filed on July 28,
2010.
In such embodiments, the biomass lipids for producing extractant 29 can be
from
a same or different biomass source from which feedstock 12 is obtained. For
example, in some embodiments, the biomass lipids for producing extractant 29
can be derived from soya, whereas the biomass source of feedstock 12 is corn.
Any possible combination of different biomass sources for extractant 29 versus
feedstock 12 can be used, as should be apparent to one of skill in the art. In
some embodiments, additional ISPR extractant 29 includes COFA.
[00101] In situ extractive fermentation can be carried out in a batch mode or
a
continuous mode in fermentation vessel 30. For in situ extractive
fermentation,
the organic extractant can contact the fermentation medium at the start of the
fermentation forming a biphasic fermentation medium. Alternatively, the
organic
extractant can contact the fermentation medium after the microorganism has
-31-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
achieved a desired amount of growth, which can be determined by measuring the
optical density of the culture. Further, the organic extractant can contact
the
fermentation medium at a time at which the product alcohol level in the
fermentation medium reaches a preselected level. In the case of butanol
production, for example, the ISPR extractant can contact the fermentation
medium at a time before the butanol concentration reaches a level which would
be toxic to the microorganism. After contacting the fermentation medium with
the
ISPR extractant, the butanol product partitions into the extractant,
decreasing the
concentration in the aqueous phase containing the microorganism, thereby
limiting the exposure of the production microorganism to the inhibitory
butanol
product.
[00102] The volume of the ISPR extractant to be used depends on a number of
factors including the volume of the fermentation medium, the size of the
fermentation vessel, the partition coefficient of the extractant for the
butanol
product, and the fermentation mode chosen, as described below. The volume of
the extractant can be about 3% to about 60% of the fermentation vessel working
volume. Depending on the efficiency of the extraction, the aqueous phase titer
of
butanol in the fermentation medium can be, for example, from about 1 g/L to
about 85 g/L, from about 10 g/L to about 40 g/L, from about 10 g/L to about
20 g/L, from about 15 g/L to about 50 g/L or from about 20 g/L to about 60
g/L. In
some embodiments, the resulting fermentation broth after alcohol
esterification
can comprise free (i.e., unesterified) alcohol and in some embodiments, the
concentration of free alcohol in the fermentation broth after alcohol
esterification
is not greater than 1, 3, 6, 10, 15, 20, 25, 30 25, 40, 45, 50, 55, or 60 g/L
when
the product alcohol is butanol, or when the product alcohol is ethanol, the
concentration of free alcohol in the fermentation broth after alcohol
esterification
is not greater than 15, 20, 25, 30 25, 40, 45, 50, 55, 60, 65, 70, 75, 80, 85,
90,
95, or 100 g/L. Without being held to theory, it is believed that higher
butanol titer
may obtained with the extractive fermentation method, in part, from the
removal
of the toxic butanol product from the fermentation medium, thereby keeping the
level below that which is toxic to the microorganism.
-32-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
[00103] In a batchwise mode of in situ extractive fermentation, a volume of
organic
extractant is added to the fermentation vessel and the extractant is not
removed
during the process. This mode requires a larger volume of organic extractant
to
minimize the concentration of the inhibitory butanol product in the
fermentation
medium. Consequently, the volume of the fermentation medium is less and the
amount of product produced is less than that obtained using the continuous
mode. For example, the volume of the extractant in the batchwise mode can be
20% to about 60% of the fermentation vessel working volume in one
embodiment, and about 30% to about 60% in another embodiment.
[00104] Gas stripping (not shown) can be used concurrently with the ISPR
extractant to remove the product alcohol from the fermentation medium.
[00105] In the embodiment of FIG. 1, the product alcohol is extracted from the
fermentation broth in situ, with the separation of the biphasic mixture 39
occurring
in a separate vessel 35. In some embodiments, separation of the biphasic
mixture 39 can occur in the fermentation vessel, as shown in the embodiments
of
later described FIGs. 2 and 3 in which the alcohol-containing organic phase
stream 36 exits directly from fermentation vessel 30. Aqueous phase stream 34
can also exit directly from fermentation vessel 30, be treated for the removal
of
any remaining product alcohol and recycled, or discarded and replaced with
fresh
fermentation medium. The extraction of the product alcohol by the organic
extractant(s) can be done with or without the removal of the microorganism
from
the fermentation broth. The microorganism can be removed from the
fermentation broth by means known in the art including, but not limited to,
filtration or centrifugation. For example, aqueous phase stream 34 can include
microorganism 32 such as yeast. Microorganism 32 can be easily separated
from the aqueous phase stream, for example, in a centrifuge (not shown).
Microorganism 32 can then be recycled to fermentation vessel 30 which over
time can increase the production rate of alcohol production, thereby resulting
in
an increase in the efficiency of the alcohol production.
[00106] In a continuous mode of in situ extractive fermentation, the volume of
the
extractant can be about 3% to about 50% of the fermentation vessel working
volume in one embodiment, about 3% to about 30% in another embodiment, 3%
-33-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
to about 20% in another embodiment; and 3% to about 10% in another
embodiment. Because the product is continually removed from the reactor, a
smaller volume of extractant is required enabling a larger volume of the
fermentation medium to be used.
[00107] As an alternative to in situ extractive fermentation, the product
alcohol can
be extracted from the fermentation broth downstream of fermentation vessel 30.
In such an instance, the fermentation broth can be removed from fermentation
vessel 30 and introduced into vessel 35 for contacting with the ISPR
extractant to
obtain biphasic mixture 39 in vessel 35, which is then separated into the
organic
36 and aqueous 34 phases. Alternatively, the ISPR extractant can be added to
the fermentation broth in a separate vessel (not shown) prior to introduction
to
vessel 35.
[00108] As a non-limiting prophetic example, with reference to the embodiment
of
FIG. 1, an aqueous suspension of ground whole corn (as feedstock 12) which
can nominally contain about 4 wt% corn oil, can be treated with amylase (as
liquefaction enzyme 14) at about 85 C to 120 C for 30 minutes to 2 hours, and
the resulting liquefied mash 16 cooled to between 65 C and 30 C and treated
with 0.1 ppm to 10 ppm (in some embodiments, 0.5 ppm to 1.0 ppm) of lipase (as
catalyst 42) at pH 4.5 to 7.5 (in some embodiments, between pH 5.5 and 6.5)
for
sufficient time to produce from at least 30% to as high as at least 99%
conversion
of the available fatty acid content in lipids to free fatty acids. Optionally,
the
liquefied and lipase-treated mash 18 can be heated to inactivate lipase 42
prior to
fermentation. Mash 18 can be cooled to about 30 C (e.g., using a heat-
exchanger) and loaded to fermentation vessel 30 at about 25% to 30 wt% dry
corn solids. Saccharification of the liquefied mash 18 during fermentation by
the
addition of glucoamylase (as saccharification enzyme 38) can result in the
production of glucose. The resulting fermentation broth can contain
significantly
less than the amount of corn oil (e.g., about 1.2 wt% corn oil) that can be
present
in a fermentation broth using a liquefied mash that has not been treated with
lipase 42. In particular, the lipase 42 treatment can result in the conversion
of
corn oil lipids 26 (triglycerides (TG)) into COFA as FFA 28 (and some
diglycerides (DG) or monoglycerides (MG)) that contact with the fermentation
-34-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
broth. The growth rate and/or glucose consumption of the microorganism in the
fermentation broth can be greater in the presence of the COFA than the growth
rate and the fermentable carbon consumption of the microorganism in the
absence of the fatty acids. For example, as later described below in the
Examples, FIGs. 6-8 illustrate an increased glucose consumption by butanol-
producing microorganisms in the presence of supplemented fatty acids.
[00109] In some embodiments, the system and processes of FIG. 1 can be
modified such that feedstock slurry 16 (having oil 26) and catalyst 42 are
introduced and contacted in fermentation vessel 30 so as to produce slurry 18
(having FFA 28). The fermentation vessel temperature can then be raised to
heat inactivate catalyst 42. The fermentation vessel temperature can then be
reduced, and the fermentation vessel can be inoculated with microorganism 32,
whereby the sugars of slurry 18 can be fermented to produce a product alcohol.
[00110] In some embodiments, the system and processes of FIG. 1 can be
modified such that simultaneous saccharification and fermentation (SSF) in
fermentation vessel 30 is replaced with a separate saccharification vessel 60
(see FIG. 2) prior to fermentation vessel 30, as should be apparent to one of
skill
in the art. Thus, slurry 18 can be saccharified either before fermentation or
during fermentation in an SSF process. It should also be apparent that
catalyst
42 for hydrolysis of feedstock oil 26 can be introduced before, after, or
contemporaneously with saccharification enzyme 38. Thus, in some
embodiments, addition of enzyme 38 and catalyst 42 can be stepwise (e.g.,
catalyst 42, then enzyme 38, or vice versa), or substantially simultaneous
(i.e., at
exactly the same time as in the time it takes for a person or a machine to
perform
the addition in one stroke, or one enzyme/catalyst immediately following the
other
catalyst/enzyme as in the time it takes for a person or a machine to perform
the
addition in two strokes).
[00111] For example, as shown in the embodiment of FIG. 2, the system and
processes of FIG. 1 can be modified such that simultaneous saccharification
and
fermentation (SSF) in fermentation vessel 30 is replaced with a separate
saccharification vessel 60 prior to fermentation vessel 30. FIG. 2 is
substantially
identical to FIG. 1 except for the inclusion of a separate saccharification
vessel
-35-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
60 receiving enzyme 38, with catalyst 42 being introduced to a liquefied,
saccharified feedstock stream 62. Feedstock slurry 16 is introduced into
saccharification vessel 60 along with enzyme 38 such as glucoamylase, whereby
sugars in the form of oligosaccharides in slurry 16 can be broken down into
monosaccharides. A liquefied, saccharified feedstock stream 62 exits
saccharification vessel 60 to which catalyst 42 is introduced. Feedstock
stream
62 includes monosaccharides, oil 26, and undissolved solids derived from the
feedstock. Oil 26 is hydrolyzed by the introduction of catalyst 42, resulting
in a
liquefied, saccharified feedstock slurry 64 having free fatty acids 28 and
catalyst
42.
[00112] Alternatively, in some embodiments, catalyst 42 can be added with
saccharification enzyme 38 to simultaneously produce glucose and hydrolyze oil
lipids 26 to free fatty acids 28. The addition of enzyme 38 and catalyst 42
can be
stepwise (e.g., catalyst 42, then enzyme 38, or vice versa) or simultaneous.
Alternatively, in some embodiments, slurry 62 can be introduced to
fermentation
vessel, with catalyst 42 being added directly to the fermentation vessel 30.
[00113] In the embodiment of FIG. 2, heat q is applied to feedstock slurry 64,
whereby catalyst 42 becomes inactive, and heat-treated slurry 64 is then
introduced to fermentation vessel 30 along with alcohol-producing
microorganism
32 which metabolizes monosaccharides to produce a product alcohol (e.g.,
butanol). Alternatively, slurry 64 can be fed to fermentation vessel 30 and
subjected to heat q while in the fermentation vessel before inoculation of
microorganism 32.
[00114] As described above with reference to FIG. 1, free fatty acids 28 can
also
serve as an ISPR extractant for preferentially partitioning the product
alcohol from
the aqueous phase. In some embodiments, one or more additional ISPR
extractants 29 can also be introduced into fermentation vessel 30. Separation
of
the biphasic mixture occurs in fermentation vessel 30, whereby alcohol-
containing organic phase stream 36 and aqueous phase stream 34 exit directly
from fermentation vessel 30. Alternatively, separation of the biphasic mixture
can
be conducted in a separate vessel 35 as provided in the embodiments of FIG. 1.
-36-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
The remaining process operations of the embodiment of FIG. 2 are identical to
FIG. 1 and therefore, will not be described in detail again.
[00115] In still other embodiments, oil 26 derived from feedstock 12 can be
catalytically hydrolyzed into FFA 28 either prior to or during liquefaction,
such that
feedstock slurry 18 having FFA 28 exits directly from liquefaction vessel 10
and
can be fed to fermentation vessel 30. For example, feedstock 12 having oil 26
can be fed to liquefaction vessel 10 along with catalyst 42 for hydrolysis of
at
least a portion of the glycerides in oil 26 into FFA 28. Enzyme 14 (e.g.,
alpha-
amylase) which hydrolyzes the starch in feedstock 12 can also be introduced to
vessel 10 to produce a liquefied feedstock. The addition of enzyme 14 and
catalyst 42 can be stepwise or simultaneous. For example, catalyst 42 can be
introduced, and then enzyme 14 can be introduced after at least a portion of
oil
26 has been hydrolyzed. Alternatively, enzyme 14 can be introduced, and then
catalyst 42 can be introduced. The liquefaction process can involve the
application of heat q. In such embodiments, catalyst 42 can be introduced
prior
to or during liquefaction when the process temperature is below that which
inactivates catalyst 42, so that oil 26 can be hydrolyzed. Thereafter,
application
of heat q can provide a two-fold purpose of liquefaction and inactivation of
catalyst 42, if inactivation is desired.
[00116] In some embodiments including any of the earlier described embodiments
with respect to FIGs. 1 and 2, undissolved solids can be removed from the
feedstock slurry prior to introduction into fermentation vessel 30. For
example, as
shown in the embodiment of FIG. 3, feedstock slurry 16 is introduced into an
inlet
of a separator 20 which is configured to discharge the undissolved solids as a
solid phase or wet cake 24. For example, in some embodiments, separator 20
may include a filter press, vacuum filtration, or a centrifuge for separating
the
undissolved solids from feedstock slurry 16. Optionally, in some embodiments,
separator 20 can also be configured to remove some or substantially all of oil
26
present in feedstock slurry 16. In such embodiments, separator 20 can be any
suitable separator known in the art for removing oil from an aqueous
feedstream
including, but not limited to, siphoning, aspiration, decantation,
centrifugation,
using a gravity settler, membrane-assisted phase splitting, and the like. The
-37-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
remaining feedstock including the sugar and water is discharged as an aqueous
stream 22 to fermentation vessel 30.
[00117] In some embodiments, separator 20 removes oil 26 but not undissolved
solids. Thus, aqueous stream 22 fed to fermentation vessel 30 includes
undissolved solids. In some embodiments, separator 20 includes a tricanter
centrifuge 20 that agitates or spins feedstock slurry 16 to produces a
centrifuge
product comprising an aqueous layer containing the sugar and water
(i.e., stream 22), a solids layer containing the undissolved solids (i.e., wet
cake
24), and an oil layer (i.e., oil stream 26). Methods and systems for removing
undissolved solids from feedstock slurry 16 via centrifugation are described
in
detail in co-pending, commonly owned U.S. Provisional Application Serial No.
61/356,290, filed June 18, 2010, which is incorporated herein in its entirety
by
reference thereto.
[00118] In any case, catalyst 42 can be contacted with the removed oil 26 to
produce a stream of FFA 28 including catalyst 42, as shown in FIG. 3. Heat q
can then be applied to the stream of FFA 28, whereby catalyst 42 becomes
inactive. The stream of FFA 28 and inactive catalyst 42 can then be introduced
into fermentation vessel 30 along with stream 22 and microorganism 32.
Alternatively, FFA 28 and active catalyst 42 can be fed to fermentation vessel
30
from vessel 40, and active catalyst 42 can thereafter be subjected to heat q
and
inactivated while in the fermentation vessel before inoculation of
microorganism
32.
[00119] FFA 28 can serve as ISPR extractant 28 and forms a biphasic mixture in
fermentation vessel 30. Product alcohol produced by SSF partitions into
organic
phase 36 constituted by FFA 28. In some embodiments, one or more additional
ISPR extractants 29 can also be introduced into fermentation vessel 30. Thus,
oil
26 (e.g., from feedstock) can be catalytically hydrolyzed to FFA 28, thereby
decreasing the rate of build-up of lipids in an ISPR extractant while also
producing an ISPR extractant. The organic phase 36 can be separated from the
aqueous phase 34 of the biphasic mixture 39 at vessel 35. In some
embodiments, separation of the biphasic mixture 39 can occur in the
fermentation
vessel, as shown in the embodiments of described in FIGs. 2 and 3 in which the
-38-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
alcohol-containing organic phase stream 36 exits directly from fermentation
vessel 30. Organic phase 36 can be introduced to separator 50 for recovery of
product alcohol 54 and optional recycle of recovered extractant 27 as shown in
FIG. 1. The remaining process operations of the embodiment of FIG. 3 are
identical to FIG. 1 and therefore, will not be described in detail again.
[00120] When wet cake 24 is removed via centrifuge 20, in some embodiments, a
portion of the oil from feedstock 12, such as corn oil when the feedstock is
corn,
remains in wet cake 24. Wet cake 24 can be washed with additional water in the
centrifuge once aqueous solution 22 has been discharged from the centrifuge
20.
Washing wet cake 24 will recover the sugar (e.g., oligosaccharides) present in
the wet cake and the recovered sugar and water can be recycled to the
liquefaction vessel 10. After washing, wet cake 20 can be dried to form Dried
Distillers' Grains with Solubles (DDGS) through any suitable known process.
The
formation of the DDGS from wet cake 24 formed in centrifuge 20 has several
benefits. Since the undissolved solids do not go to the fermentation vessel,
DDGS does not have trapped extractant and/or product alcohol such as butanol,
it is not subjected to the conditions of the fermentation vessel, and it does
not
contact the microorganisms present in the fermentation vessel. All these
benefits
make it easier to process and sell DDGS, for example, as animal feed. In some
embodiments, oil 26 is not discharged separately from wet cake 24, but rather
oil
26 is included as part of wet cake 24 and is ultimately present in the DDGS.
In
such instances, the oil can be separated from the DDGS and converted to an
ISPR extractant 29 for subsequent use in the same or different alcohol
fermentation process. Methods and systems for removing undissolved solids
from feedstock 16 via centrifugation are described in detail in co-pending,
commonly owned U.S. Patent Application No. 61/356,290, filed June 18, 2010,
which is incorporated herein in its entirety by reference thereto.
[00121] In still other embodiments (not shown), saccharification can occur in
a
separate saccharification vessel 60 (see FIG. 2) which is located between
separator 20 and liquefaction vessel 10, as should be apparent to one of skill
in
the art.
-39-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
[00122] In still other embodiments as shown, for example, in the embodiment of
FIG. 4, a native oil 26' is supplied to a vessel 40 to which catalyst 42 is
also
supplied, whereby at least a portion of glycerides in oil 26' are hydrolyzed
to form
FFA 28'. Catalyst 42 can be subsequently inactivated, such as by the
application
of heat q. A product stream from vessel 40 containing FFA 28' and inactive
catalyst 42 are then introduced into fermentation vessel 30, along with
aqueous
feedstock stream 22 in which feedstock oil 26 and in some embodiments, the
undissolved solids have been previously removed by means of separator 20
(see, e.g., the embodiment of FIG. 3). Saccharification enzyme 38 and
microorganism 32 are also introduced into fermentation vessel 30, whereby a
product alcohol is produced by SSF.
[00123] Alternatively, oil 26' and catalyst 42 can be fed directly to
fermentation
vessel 30 in which oil 26' is hydrolyzed to FFA 28', rather than using vessel
40.
Thereafter, active catalyst 42 can be subjected to heat q and inactivated
while in
the fermentation vessel before inoculation of microorganism 32. Alternatively,
FFA 28' and active catalyst 42 can be fed to fermentation vessel 30 from
vessel
40, and active catalyst 42 can thereafter be subjected to heat q and
inactivated
while in the fermentation vessel before inoculation of microorganism 32. In
such
embodiments, feedstock slurry 16 including oil 26, rather than stream 22 in
which
oil 26 was removed, can be fed to fermentation vessel 30 and contacted with
active catalyst 42. Active catalyst 42 can therefore be used to hydrolyze oil
26
into FFA 28, thereby reducing the loss and/or degradation of the partition
coefficient of the extractant over time that is attributable to the presence
of the oil
in the fermentation vessel.
[00124] In some embodiments, the system and processes of FIG. 4 can be
modified such that simultaneous saccharification and fermentation in
fermentation vessel 30 is replaced with a separate saccharification vessel 60
prior to fermentation vessel 30, as should be apparent to one of skill in the
art.
[00125] In some embodiments, native oil 26' can be tallow, corn, canola,
capric/caprylic triglycerides, castor, coconut, cottonseed, fish, jojoba,
lard,
linseed, neetsfoot, oiticica, palm, peanut, rapeseed, rice, safflower, soya,
sunflower, tung, jatropha, vegetable oil blends, and mixtures thereof. In some
-40-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
embodiments, native oil 26' is a mixture of two or more native oils, for
example, a
mixture of palm and soybean oils. In some embodiments, native oil 26' is a
plant-
derived oil. In some embodiments, the plant-derived oil can be, though not
necessarily, derived from biomass that can be used in a fermentation process.
The biomass can be the same or different source from which feedstock 12
(shown in FIG. 4 as stream 22) is obtained. Thus, for example, in some
embodiments, oil 26' can be derived from corn, whereas feedstock 12 can be
cane. For example, in some embodiments, oil 26' can be derived from corn and
the biomass source of feedstock 12 is also corn. Any possible combination of
different biomass sources for oil 26' versus feedstock 12 can be used as
should
be apparent to one of skill in the art. The remaining process operations of
the
embodiment of FIG. 4 are identical to FIG. 1 and therefore, will not be
described
in detail again.
[00126] In some embodiments of the present invention, biomass oil present in
feedstock 12 can be converted to FFA 28 at a step following alcoholic
fermentation. FFA 28 can then be introduced in the fermentation vessel, and
contacted with fermentation broth for achieving improved growth rate and/or
fermentable carbon consumption of the alcohol-producing microorganism. FFA
28 can as also serve as ISPR extractant 28. For example, in the embodiment of
FIG. 5, feedstock 12 is liquefied to produced feedstock slurry 16 which
includes
oil 26 derived from the feedstock. Feedstock slurry 16 can also include
undissolved solids from the feedstock. Alternatively, the undissolved solids
can
be separated from slurry 16 via a separator such as a centrifuge (not shown).
Feedstock slurry 16 containing oil 26 is introduced directly to fermentation
vessel
30 containing a fermentation broth including saccharification enzyme 38 and
microorganism 32. A product alcohol is produced by SSF in fermentation vessel
30. Alternatively, in some embodiments, the process can be modified to include
a separate saccharification vessel as discussed in connection with FIG. 2.
[00127] ISPR extractant 29 is introduced to fermentation vessel 30 to form a
biphasic mixture, and the product alcohol is removed by partitioning into the
organic phase of the ISPR extractant 29. Oil 26 also partitions into the
organic
phase. Separation of the biphasic mixture occurs in fermentation vessel 30,
-41 -
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
whereby alcohol-containing organic phase stream 36 and aqueous phase stream
34 exit directly from fermentation vessel 30. Alternatively, separation of the
biphasic mixture can be conducted in a separate vessel 35 as provided in the
embodiments of FIG. 1. Organic phase stream 36 including oil 26 is introduced
into separator 50 to recover product alcohol 54 from extractant 29. The
resulting
alcohol-lean extractant 27 includes recovered extractant 29 and oil 26.
Extractant 27 is contacted with catalyst 42, whereby at least a portion of
glycerides in oil 26 are hydrolyzed to form FFA 28. Heat q can then be applied
to
extractant 27 including FFA 28 so as to inactivate catalyst 42 before being
recycled back into fermentation vessel 30. Such recycled extractant stream 27
can be a separate stream or a combined stream with fresh, make-up extractant
stream 29. The subsequent withdrawal of alcohol-containing organic phase 36
from fermentation vessel 30 can then include FFA 28 and ISPR extractant 29 (as
fresh extractant 29 and recycled extractant 27), in addition to the product
alcohol
and additional oil 26 from newly introduced feedstock slurry 16. Organic phase
36 can then be treated to recover the product alcohol, and recycled back into
fermentation vessel 30 after contacting with catalyst 42 for hydrolysis of
additional oil 26, in the same manner as just described. In some embodiments,
use of make-up ISPR extractant 29 can be phased out as the fermentation
process is operated over time because the process itself can produce FFA 28 as
a make-up ISPR extractant for extracting the product alcohol. Thus, the ISPR
extractant can be the stream of recycled extractant 27 with FFA 28.
[00128] Thus, FIGs. 1-5 provide various non-limiting embodiments of methods
and
systems involving fermentation processes and FFAs 28 produced from hydrolysis
of biomass derived oil 26, and FFAs 28' produced from catalytic hydrolysis of
native oil 26' such as plant-derived oil that can be used for contacting with
a
microorganism during fermentative growth, whereby growth rate and/or
fermentable carbon consumption of the microorganism is greater in the presence
of the free fatty acids, allowing for improved alcohol productivity.
[00129] From the above discussion and the Examples, one skilled in the art can
ascertain essential characteristics of the present invention and can make
various
changes and modifications of the invention to adapt to various uses and
-42-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
conditions without departing from the present invention. For example, in some
embodiments, fatty acid supplementation according to the present invention can
be employed pre-fermentation, that is, during seed culturing of microorganisms
32 prior to fermentation in fermentation vessel 30. Typically, microorganisms
32
such as yeast can be grown from a seed culture to a desired cell concentration
before being harvested and inoculated into fermentation vessel 30, as known in
the art. Thus, according to some embodiments, the seed culture medium can be
contacted with FFA 28 whereby improved growth rates and microorganism
biomass production can be achieved, which can reduce the pre-fermentation time
associated with the seed culturing phase of an alcohol fermentation process.
Thus, it should be apparent that fatty acid supplementation according to the
present invention can be employed at various stages in an alcohol fermentation
process, for example, during pre-fermentation culturing and fermentation, for
improving overall process efficiency without departing from the present
invention.
[00130] In some embodiments, including any of the aforementioned embodiments
described with reference to FIGs. 1-5, the fermentation broth in fermentation
vessel 30 includes at least one recombinant microorganism 32 which is
genetically modified (that is, genetically engineered) to produce butanol via
a
biosynthetic pathway from at least one fermentable carbon source into butanol.
In particular, recombinant microorganisms can be grown in a fermentation broth
which contains suitable carbon substrates. Additional carbon substrates may
include, but are not limited to, monosaccharides such as fructose;
oligosaccharides such as lactose maltose, or sucrose; polysaccharides such as
starch or cellulose; or mixtures thereof and unpurified mixtures from
renewable
feedstocks such as cheese whey permeate, cornsteep liquor, sugar beet
molasses, and barley malt. Other carbon substrates may include ethanol,
lactate, succinate, or glycerol.
[00131] Additionally, the carbon substrate may also be one-carbon substrates
such
as carbon dioxide or methanol for which metabolic conversion into key
biochemical intermediates has been demonstrated. In addition to one and two
carbon substrates, methylotrophic organisms are also known to utilize a number
of other carbon containing compounds such as methylamine, glucosamine, and a
-43-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
variety of amino acids for metabolic activity. For example, methylotrophic
yeasts
are known to utilize the carbon from methylamine to form trehalose or glycerol
(Bellion, et al., Microb. Growth C1 Compd., [Int. Symp.], 7th (1993), 415-32,
Editor(s): Murrell, J. Collin; Kelly, Don P. Publisher: Intercept, Andover,
UK).
Similarly, various species of Candida will metabolize alanine or oleic acid
(Sulter,
et al., Arch. Microbiol. 153:485-489, 1990). Hence it is contemplated that the
source of carbon utilized in the present invention may encompass a wide
variety
of carbon containing substrates and will only be limited by the choice of
organism.
[00132] Although it is contemplated that all of the above mentioned carbon
substrates and mixtures thereof are suitable, in some embodiments, the carbon
substrates are glucose, fructose, and sucrose, or mixtures of these substrates
with C5 sugars such as xylose and/or arabinose for yeast modified to use C5
sugars. Sucrose may be derived from renewable sugar sources such as sugar
cane, sugar beets, cassava, sweet sorghum, and mixtures thereof. Glucose and
dextrose may be derived from renewable grain sources through saccharification
of starch based feedstocks including grains such as corn, wheat, rye, barley,
oats, and mixtures thereof. In addition, fermentable sugars may be derived
from
renewable cellulosic or lignocellulosic biomass through processes of
pretreatment and saccharification, as described in, for example, U.S. Patent
Application Publication No. 2007/0031918 Al, which is herein incorporated by
reference. In addition to an appropriate carbon source (from aqueous stream
22), fermentation broth must contain suitable minerals, salts, cofactors,
buffers
and other components, known to those skilled in the art, suitable for the
growth of
the cultures and promotion of an enzymatic pathway comprising a dihydroxyacid
dehydratase (DHAD).
[00133] Recombinant microorganisms that produce butanol via a biosynthetic
pathway can include a member of the genera Clostridium, Zymomonas,
Escherichia, Salmonella, Serratia, Erwinia, Klebsiella, Shigella, Rhodococcus,
Pseudomonas, Bacillus, Lactobacillus, Enterococcus, Alcaligenes, Klebsiella,
Paenibacillus, Arthrobacter, Corynebacterium, Brevibacterium,
Schizosaccharomyces, Kluyveromyces, Yarrowia, Pichia, Candida, Hansenula,
-44-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
or Saccharomyces. In one embodiment, recombinant microorganisms can be
selected from the group consisting of Escherichia coli, Lactobacillus
plantarum,
and Saccharomyces cerevisiae. In one embodiment, the recombinant
microorganism is a crabtree-positive yeast selected from Saccharomyces,
Zygosaccharomyces, Schizosaccharomyces, Dekkera, Torulopsis,
Brettanomyces, and some species of Candida. Species of crabtree-positive
yeast include, but are not limited to, Saccharomyces cerevisiae, Saccharomyces
kluyveri, Schizosaccharomyces pombe, Saccharomyces bayanus,
Saccharomyces mikitae, Saccharomyces paradoxus, Zygosaccharomyces rouxii,
and Candida glabrata. For example, the production of butanol utilizing
fermentation by a microorganism, as well as which microorganisms produce
butanol, is known and is disclosed, for example, in U.S. Patent Application
Publication No. 2009/0305370, herein incorporated by reference. In some
embodiments, microorganisms comprise a butanol biosynthetic pathway.
Suitable isobutanol biosynthetic pathways are known in the art (see, e.g.,
U.S.
Patent Application Publication No. 2007/0092957, herein incorporated by
reference). In some embodiments, at least one, at least two, at least three,
or at
least four polypeptides catalyzing substrate to product conversions of a
pathway
are encoded by heterologous polynucleotides in the microorganism. In some
embodiments, all polypeptides catalyzing substrate to product conversions of a
pathway are encoded by heterologous polynucleotides in the microorganism. In
some embodiments, the microorganism comprises a reduction or elimination of
pyruvate decarboxylase activity. Microorganisms substantially free of pyruvate
decarboxylase activity are described in U.S. Patent Application Publication
No.
2009/0305363, herein incorporated by reference.
[00134] Construction of certain strains, including those used in the Examples,
is
provided herein.
Construction of Saccharomyces cerevisiae strain BP1083 ("NGCI-070")
[00135] The strain BP1064 was derived from CEN.PK 113-7D (CBS 8340;
Centraalbureau voor Schimmelcultures (CBS) Fungal Biodiversity Centre,
Netherlands) and contains deletions of the following genes: URA3, HIS3, PDC1,
PDC5, PDC6, and GPD2. BP1064 was transformed with plasmids pYZ090 (SEQ
-45-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
ID NO: 1, described in U.S. Provisional Application Serial No. 61/246,844) and
pLH468 (SEQ ID NO: 2) to create strain NGCI-070 (BP1 083, PNY1 504).
[00136] Deletions, which completely removed the entire coding sequence, were
created by homologous recombination with PCR fragments containing regions of
homology upstream and downstream of the target gene and either a G418
resistance marker or URA3 gene for selection of transformants. The G418
resistance marker, flanked by loxP sites, was removed using Cre recombinase.
The URA3 gene was removed by homologous recombination to create a scarless
deletion or if flanked by loxP sites, was removed using Cre recombinase.
[00137] The scarless deletion procedure was adapted from Akada, et al., (Yeast
23:399-405, 2006). In general, the PCR cassette for each scarless deletion was
made by combining four fragments, A-B-U-C, by overlapping PCR. The PCR
cassette contained a selectable/counter-selectable marker, URA3 (Fragment U),
consisting of the native CEN.PK 113-7D URA3 gene, along with the promoter
(250 bp upstream of the URA3 gene) and terminator (150 bp downstream of the
URA3 gene). Fragments A and C, each 500 bp long, corresponded to the 500 bp
immediately upstream of the target gene (Fragment A) and the 3' 500 bp of the
target gene (Fragment C). Fragments A and C were used for integration of the
cassette into the chromosome by homologous recombination. Fragment B (500
bp long) corresponded to the 500 bp immediately downstream of the target gene
and was used for excision of the URA3 marker and Fragment C from the
chromosome by homologous recombination, as a direct repeat of the sequence
corresponding to Fragment B was created upon integration of the cassette into
the chromosome. Using the PCR product ABUC cassette, the URA3 marker was
first integrated into and then excised from the chromosome by homologous
recombination. The initial integration deleted the gene, excluding the 3' 500
bp.
Upon excision, the 3' 500 bp region of the gene was also deleted. For
integration
of genes using this method, the gene to be integrated was included in the PCR
cassette between fragments A and B.
URA3 Deletion
[00138] To delete the endogenous URA3 coding region, a ura3::loxP-kanMX-loxP
cassette was PCR-amplified from pLA54 template DNA (SEQ ID NO: 3). pLA54
-46-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
contains the K. lactis TEF1 promoter and kanMX marker, and is flanked by loxP
sites to allow recombination with Cre recombinase and removal of the marker.
PCR was done using Phusion DNA polymerase (New England BioLabs Inc.,
Ipswich, MA) and primers BK505 and BK506 (SEQ ID NOs: 4 and 5). The URA3
portion of each primer was derived from the 5' region upstream of the URA3
promoter and 3' region downstream of the coding region such that integration
of
the loxP-kanMX-loxP marker resulted in replacement of the URA3 coding region.
The PCR product was transformed into CEN.PK 113-7D using standard genetic
techniques (Methods in Yeast Genetics, 2005, Cold Spring Harbor Laboratory
Press, Cold Spring Harbor, NY, pp. 201-202) and transformants were selected on
YPD containing G418 (100 pg/mL) at 30 C. Transformants were screened to
verify correct integration by PCR using primers LA468 and LA492 (SEQ ID NOs:
6 and 7) and designated CEN.PK 113-7D Aura3::kanMX.
HIS3 Deletion
[00139] The four fragments for the PCR cassette for the scarless HIS3 deletion
were amplified using Phusion High Fidelity PCR Master Mix (New England
BioLabs Inc., Ipswich, MA) and CEN.PK 113-7D genomic DNA as template,
prepared with a Gentra Puregene Yeast/Bact, kit (Qiagen, Valencia, CA).
HIS3 Fragment A was amplified with primer oBP452 (SEQ ID NO: 14) and primer
oBP453 (SEQ ID NO: 15) containing a 5' tail with homology to the 5' end of
HIS3
Fragment B. HIS3 Fragment B was amplified with primer oBP454 (SEQ ID NO:
16) containing a 5' tail with homology to the 3' end of HIS3 Fragment A, and
primer oBP455 (SEQ ID NO: 17) containing a 5' tail with homology to the 5' end
of HIS3 Fragment U. HIS3 Fragment U was amplified with primer oBP456 (SEQ
ID NO: 18) containing a 5' tail with homology to the 3' end of HIS3 Fragment
B,
and primer oBP457 (SEQ ID NO: 19) containing a 5' tail with homology to the 5'
end of HIS3 Fragment C. HIS3 Fragment C was amplified with primer oBP458
(SEQ ID NO: 20) containing a 5' tail with homology to the 3' end of HIS3
Fragment U, and primer oBP459 (SEQ ID NO: 21). PCR products were purified
with a PCR Purification kit (Qiagen, Valencia, CA). HIS3 Fragment AB was
created by overlapping PCR by mixing HIS3 Fragment A and HIS3 Fragment B
and amplifying with primers oBP452 (SEQ ID NO: 14) and oBP455 (SEQ ID NO:
-47-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
17). HIS3 Fragment UC was created by overlapping PCR by mixing HIS3
Fragment U and HIS3 Fragment C and amplifying with primers oBP456 (SEQ ID
NO: 18) and oBP459 (SEQ ID NO: 21). The resulting PCR products were
purified on an agarose gel followed by a Gel Extraction kit (Qiagen, Valencia,
CA). The HIS3 ABUC cassette was created by overlapping PCR by mixing HIS3
Fragment AB and HIS3 Fragment UC and amplifying with primers oBP452 (SEQ
ID NO: 14) and oBP459 (SEQ ID NO: 21). The PCR product was purified with a
PCR Purification kit (Qiagen, Valencia, CA).
[00140] Competent cells of CEN.PK 113-7D Aura3::kanMX were made and
transformed with the HIS3 ABUC PCR cassette using a Frozen-EZ Yeast
Transformation IITM kit (Zymo Research Corporation, Irvine, CA).
Transformation
mixtures were plated on synthetic complete media lacking uracil supplemented
with 2% glucose at 30 C. Transformants with a his3 knockout were screened for
by PCR with primers oBP460 (SEQ ID NO: 22) and oBP461 (SEQ ID NO: 23)
using genomic DNA prepared with a Gentra Puregene Yeast/Bact. kit
(Qiagen, Valencia, CA). A correct transformant was selected as strain CEN.PK
113-7D Aura3::kanMX Ahis3::URA3.
KanMX Marker Removal from the Aura3 Site and URA3 Marker Removal from
the Ahis3 Site
[00141] The KanMX marker was removed by transforming CEN.PK 113-7D
Aura3::kanMX Ahis3::URA3 with pRS423::PGAL1-cre (SEQ ID NO: 66,
described in U.S. Provisional Application No. 61/290,639) using a Frozen-EZ
Yeast Transformation IITM kit (Zymo Research Corporation, Irvine, CA) and
plating on synthetic complete medium lacking histidine and uracil supplemented
with 2% glucose at 30 C. Transformants were grown in YP supplemented with
1% galactose at 30 C for -6 hours to induce the Cre recombinase and KanMX
marker excision and plated onto YPD (2% glucose) plates at 30 C for recovery.
An isolate was grown overnight in YPD and plated on synthetic complete medium
containing 5-fluoro-orotic acid (5-FOA, 0.1%) at 30 C to select for isolates
that
lost the URA3 marker. 5-FOA resistant isolates were grown in and plated on
YPD for removal of the pRS423::PGAL1-cre plasmid. Isolates were checked for
loss of the KanMX marker, URA3 marker, and pRS423::PGAL1-cre plasmid by
-48-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
assaying growth on YPD+G418 plates, synthetic complete medium lacking uracil
plates, and synthetic complete medium lacking histidine plates. A correct
isolate
that was sensitive to G418 and auxotrophic for uracil and histidine was
selected
as strain CEN.PK 113-7D Aura3::loxP Ahis3 and designated as BP857. The
deletions and marker removal were confirmed by PCR and sequencing with
primers oBP450 (SEQ ID NO: 24) and oBP451 (SEQ ID NO: 25) for Aura3 and
primers oBP460 (SEQ ID NO: 22) and oBP461 (SEQ ID NO: 23) for Ahis3 using
genomic DNA prepared with a Gentra Puregene Yeast/Bact. kit (Qiagen,
Valencia, CA).
PDC6 Deletion
[00142] The four fragments for the PCR cassette for the scarless PDC6 deletion
were amplified using Phusion High Fidelity PCR Master Mix (New England
BioLabs Inc., Ipswich, MA) and CEN.PK 113-7D genomic DNA as template,
prepared with a Gentra Puregene Yeast/Bact. kit (Qiagen, Valencia, CA).
PDC6 Fragment A was amplified with primer oBP440 (SEQ ID NO: 26) and
primer oBP441 (SEQ ID NO: 27) containing a 5' tail with homology to the 5' end
of PDC6 Fragment B. PDC6 Fragment B was amplified with primer oBP442 (SEQ
ID NO: 28), containing a 5' tail with homology to the 3' end of PDC6 Fragment
A,
and primer oBP443 (SEQ ID NO: 29) containing a 5' tail with homology to the 5'
end of PDC6 Fragment U. PDC6 Fragment U was amplified with primer oBP444
(SEQ ID NO: 30) containing a 5' tail with homology to the 3' end of PDC6
Fragment B, and primer oBP445 (SEQ ID NO: 31) containing a 5' tail with
homology to the 5' end of PDC6 Fragment C. PDC6 Fragment C was amplified
with primer oBP446 (SEQ ID NO: 32) containing a 5' tail with homology to the
3'
end of PDC6 Fragment U, and primer oBP447 (SEQ ID NO: 33). PCR products
were purified with a PCR Purification kit (Qiagen, Valencia, CA). PDC6
Fragment
AB was created by overlapping PCR by mixing PDC6 Fragment A and PDC6
Fragment B and amplifying with primers oBP440 (SEQ ID NO: 26) and oBP443
(SEQ ID NO: 29). PDC6 Fragment UC was created by overlapping PCR by
mixing PDC6 Fragment U and PDC6 Fragment C and amplifying with primers
oBP444 (SEQ ID NO: 30) and oBP447 (SEQ ID NO: 33). The resulting PCR
products were purified on an agarose gel followed by a Gel Extraction kit
-49-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
(Qiagen, Valencia, CA). The PDC6 ABUC cassette was created by overlapping
PCR by mixing PDC6 Fragment AB and PDC6 Fragment UC and amplifying with
primers oBP440 (SEQ ID NO: 26) and oBP447 (SEQ ID NO: 33). The PCR
product was purified with a PCR Purification kit (Qiagen, Valencia, CA).
[00143] Competent cells of CEN.PK 113-7D Aura3::IoxP Ahis3 were made and
transformed with the PDC6 ABUC PCR cassette using a Frozen-EZ Yeast
Transformation IITM kit (Zymo Research Corporation, Irvine, CA).
Transformation
mixtures were plated on synthetic complete media lacking uracil supplemented
with 2% glucose at 30 C. Transformants with a pdc6 knockout were screened for
by PCR with primers oBP448 (SEQ ID NO: 34) and oBP449 (SEQ ID NO: 35)
using genomic DNA prepared with a Gentra Puregene Yeast/Bact. kit
(Qiagen, Valencia, CA). A correct transformant was selected as strain CEN.PK
113-7D Aura3::IoxP Ahis3 Apdc6::URA3.
[00144] CEN.PK 113-7D Aura3::IoxP Ahis3 Apdc6::URA3 was grown overnight in
YPD and plated on synthetic complete medium containing 5-fluoro-orotic acid
(0.1 %) at 30 C to select for isolates that lost the URA3 marker. The deletion
and
marker removal were confirmed by PCR and sequencing with primers oBP448
(SEQ ID NO: 34) and oBP449 (SEQ ID NO: 35) using genomic DNA prepared
with a Gentra Puregene Yeast/Bact. kit (Qiagen, Valencia, CA). The absence
of the PDC6 gene from the isolate was demonstrated by a negative PCR result
using primers specific for the coding sequence of PDC6, oBP554 (SEQ ID NO:
36) and oBP555 (SEQ ID NO: 37). The correct isolate was selected as strain
CEN.PK 113-7D Aura3::IoxP Ahis3 Apdc6 and designated as BP891.
PDC1 Deletion ilvDSm Integration
[00145] The PDC1 gene was deleted and replaced with the ilvD coding region
from
Streptococcus mutans ATCC No. 700610. The A fragment followed by the ilvD
coding region from Streptococcus mutans for the PCR cassette for the PDC1
deletion-ilvDSm integration was amplified using Phusion High Fidelity PCR
Master Mix (New England BioLabs Inc., Ipswich, MA) and NYLA83 (described
herein and in U.S. Provisional Application No. 61/246,709) genomic DNA as
template, prepared with a Gentra Puregene Yeast/Bact. kit (Qiagen, Valencia,
CA). PDC1 Fragment A-ilvDSm (SEQ ID NO: 141) was amplified with primer
-50-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
oBP513 (SEQ ID NO: 38) and primer oBP515 (SEQ ID NO: 39) containing a 5'
tail with homology to the 5' end of PDC1 Fragment B. The B, U, and C fragments
for the PCR cassette for the PDC1 deletion-ilvDSm integration were amplified
using Phusion High Fidelity PCR Master Mix (New England BioLabs Inc.,
Ipswich, MA) and CEN.PK 113-7D genomic DNA as template, prepared with a
Gentra Puregene Yeast/Bact. kit (Qiagen, Valencia, CA). PDC1 Fragment B
was amplified with primer oBP516 (SEQ ID NO: 40) containing a 5' tail with
homology to the 3' end of PDC1 Fragment A-ilvDSm, and primer oBP517 (SEQ
ID NO: 41) containing a 5' tail with homology to the 5' end of PDC1 Fragment
U.
PDC1 Fragment U was amplified with primer oBP518 (SEQ ID NO: 42)
containing a 5' tail with homology to the 3' end of PDC1 Fragment B, and
primer
oBP519 (SEQ ID NO: 43) containing a 5' tail with homology to the 5' end of
PDC1 Fragment C. PDC1 Fragment C was amplified with primer oBP520 (SEQ
ID NO: 44), containing a 5' tail with homology to the 3' end of PDC1 Fragment
U,
and primer oBP521 (SEQ ID NO: 45). PCR products were purified with a PCR
Purification kit (Qiagen, Valencia, CA. PDC1 Fragment A-ilvDSm-B was created
by overlapping PCR by mixing PDC1 Fragment A-ilvDSm and PDC1 Fragment B
and amplifying with primers oBP513 (SEQ ID NO: 38) and oBP517 (SEQ ID NO:
41). PDC1 Fragment UC was created by overlapping PCR by mixing PDC1
Fragment U and PDC1 Fragment C and amplifying with primers oBP518 (SEQ ID
NO: 42) and oBP521 (SEQ ID NO: 45). The resulting PCR products were
purified on an agarose gel followed by a Gel Extraction kit (Qiagen, Valencia,
CA). The PDC1 A-ilvDSm-BUC cassette (SEQ ID NO: 142) was created by
overlapping PCR by mixing PDC1 Fragment A-ilvDSm-B and PDC1 Fragment
UC and amplifying with primers oBP513 (SEQ ID NO: 38) and oBP521 (SEQ ID
NO: 45). The PCR product was purified with a PCR Purification kit (Qiagen,
Valencia, CA).
[00146] Competent cells of CEN.PK 113-7D Aura3::IoxP Ahis3 Apdc6 were made
and transformed with the PDC1 A-ilvDSm-BUC PCR cassette using a Frozen-EZ
Yeast Transformation IITM kit (Zymo Research Corporation, Irvine, CA).
Transformation mixtures were plated on synthetic complete media lacking uracil
supplemented with 2% glucose at 30 C. Transformants with a pdcl knockout
-51-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
ilvDSm integration were screened for by PCR with primers oBP511 (SEQ ID NO:
46) and oBP512 (SEQ ID NO: 47) using genomic DNA prepared with a Gentra
Puregene Yeast/Bact. kit (Qiagen, Valencia, CA). The absence of the PDC1
gene from the isolate was demonstrated by a negative PCR result using primers
specific for the coding sequence of PDC1, oBP550 (SEQ ID NO: 48) and oBP551
(SEQ ID NO: 49). A correct transformant was selected as strain CEN.PK 113-7D
Eura3::IoxP Ehis3 Epdc6 Epdcl ::ilvDSm-URA3.
[00147] CEN.PK 113-7D Eura3::IoxP Ehis3 Epdc6 Epdcl::ilvDSm-URA3 was
grown overnight in YPD and plated on synthetic complete medium containing 5-
fluoro-orotic acid (0.1 %) at 30 C to select for isolates that lost the URA3
marker.
The deletion of PDC1, integration of ilvDSm, and marker removal were confirmed
by PCR and sequencing with primers oBP511 (SEQ ID NO: 46) and oBP512
(SEQ ID NO: 47) using genomic DNA prepared with a Gentra Puregene
Yeast/Bact. kit (Qiagen, Valencia, CA). The correct isolate was selected as
strain
CEN.PK 113-7D Eura3::IoxP Ehis3 Epdc6 Epdcl::ilvDSm and designated as
BP907.
PDC5 Deletion sadB Integration
[00148] The PDC5 gene was deleted and replaced with the sadB coding region
from Achromobacter xylosoxidans. A segment of the PCR cassette for the PDC5
deletion-sadB integration was first cloned into plasmid pUC19-URA3MCS.
[00149] pUC19-URA3MCS is pUC19 based and contains the sequence of the
URA3 gene from Saccaromyces cerevisiae situated within a multiple cloning site
(MCS). pUC19 contains the pMB1 replicon and a gene coding for beta-
lactamase for replication and selection in Escherichia coli. In addition to
the
coding sequence for URA3, the sequences from upstream and downstream of
this gene were included for expression of the URA3 gene in yeast. The vector
can be used for cloning purposes and can be used as a yeast integration
vector.
[00150] The DNA encompassing the URA3 coding region along with 250 bp
upstream and 150 bp downstream of the URA3 coding region from
Saccaromyces cerevisiae CEN.PK 113-7D genomic DNA was amplified with
primers oBP438 (SEQ ID NO: 12) containing BamHI, Ascl, Pmel, and Fsel
restriction sites, and oBP439 (SEQ ID NO: 13) containing Xbal, Pacl, and Notl
-52-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
restriction sites, using Phusion High Fidelity PCR Master Mix (New England
BioLabs Inc., Ipswich, MA). Genomic DNA was prepared using a Gentra
Puregene Yeast/Bact. kit (Qiagen, Valencia, CA). The PCR product and
pUC19 (SEQ ID NO: 144) were ligated with T4 DNA ligase after digestion with
BamHI and Xbal to create vector pUC19-URA3MCS. The vector was confirmed
by PCR and sequencing with primers oBP264 (SEQ ID NO: 10) and oBP265
(SEQ ID NO: 11).
[00151] The coding sequence of sadB and PDC5 Fragment B were cloned into
pUC19-URA3MCS to create the sadB-BU portion of the PDC5 A-sadB-BUC PCR
cassette. The coding sequence of sadB was amplified using pLH468-sadB (SEQ
ID NO: 67) as template with primer oBP530 (SEQ ID NO: 50) containing an Ascl
restriction site, and primer oBP531 (SEQ ID NO: 51) containing a 5' tail with
homology to the 5' end of PDC5 Fragment B. PDC5 Fragment B was amplified
with primer oBP532 (SEQ ID NO: 52) containing a 5' tail with homology to the
3'
end of sadB, and primer oBP533 (SEQ ID NO: 53) containing a Pmel restriction
site. PCR products were purified with a PCR Purification kit (Qiagen,
Valencia,
CA). sadB-PDC5 Fragment B was created by overlapping PCR by mixing the
sadB and PDC5 Fragment B PCR products and amplifying with primers oBP530
(SEQ ID NO: 50) and oBP533 (SEQ ID NO: 53). The resulting PCR product was
digested with Ascl and Pmel and ligated with T4 DNA ligase into the
corresponding sites of pUC19-URA3MCS after digestion with the appropriate
enzymes. The resulting plasmid was used as a template for amplification of
sadB-Fragment B-Fragment U using primers oBP536 (SEQ ID NO: 54) and
oBP546 (SEQ ID NO: 55) containing a 5' tail with homology to the 5' end of
PDC5 Fragment C. PDC5 Fragment C was amplified with primer oBP547 (SEQ
ID NO: 56) containing a 5' tail with homology to the 3' end of PDC5 sadB-
Fragment B-Fragment U, and primer oBP539 (SEQ ID NO: 57). PCR products
were purified with a PCR Purification kit (Qiagen, Valencia, CA). PDC5 sadB-
Fragment B-Fragment U-Fragment C was created by overlapping PCR by mixing
PDC5 sadB-Fragment B-Fragment U and PDC5 Fragment C and amplifying with
primers oBP536 (SEQ ID NO: 54) and oBP539 (SEQ ID NO: 57). The resulting
PCR product was purified on an agarose gel followed by a Gel Extraction kit
-53-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
(Qiagen, Valencia, CA). The PDC5 A-sadB-BUC cassette (SEQ ID NO: 143)
was created by amplifying PDC5 sadB-Fragment B-Fragment U-Fragment C with
primers oBP542 (SEQ ID NO: 58) containing a 5' tail with homology to the 50
nucleotides immediately upstream of the native PDC5 coding sequence, and
oBP539 (SEQ ID NO: 57). The PCR product was purified with a PCR Purification
kit (Qiagen, Valencia, CA).
[00152] Competent cells of CEN.PK 113-7D Aura3::loxP Ahis3 Apdc6
Apdcl::ilvDSm were made and transformed with the PDC5 A-sadB-BUC PCR
cassette using a Frozen-EZ Yeast Transformation IITM kit (Zymo Research
Corporation, Irvine, CA). Transformation mixtures were plated on synthetic
complete media lacking uracil supplemented with 1% ethanol (no glucose) at
30 C. Transformants with a pdc5 knockout sadB integration were screened for
by PCR with primers oBP540 (SEQ ID NO: 59) and oBP541 (SEQ ID NO: 60)
using genomic DNA prepared with a Gentra Puregene Yeast/Bact. kit
(Qiagen, Valencia, CA). The absence of the PDC5 gene from the isolate was
demonstrated by a negative PCR result using primers specific for the coding
sequence of PDC5, oBP552 (SEQ ID NO: 61) and oBP553 (SEQ ID NO: 62). A
correct transformant was selected as strain CEN.PK 113-7D Aura3::IoxP Ahis3
Apdc6 Apdcl ::ilvDSm Apdc5::sadB-URA3.
[00153] CEN.PK 113-7D Aura3::IoxP Ahis3 Apdc6 Apdcl::ilvDSm Apdc5::sadB-
URA3 was grown overnight in YPE (1% ethanol) and plated on synthetic
complete medium supplemented with ethanol (no glucose) and containing 5-
fluoro-orotic acid (0.1 %) at 30 C to select for isolates that lost the URA3
marker.
The deletion of PDC5, integration of sadB, and marker removal were confirmed
by PCR with primers oBP540 (SEQ ID NO: 59) and oBP541 (SEQ ID NO: 60)
using genomic DNA prepared with a Gentra Puregene Yeast/Bact. kit
(Qiagen, Valencia, CA). The correct isolate was selected as strain CEN.PK 113-
7D Aura3::IoxP Ahis3 Apdc6 Apdcl::ilvDSm Apdc5::sadB and designated as
BP913.
GPD2 Deletion
[00154] To delete the endogenous GPD2 coding region, a gpd2::IoxP-URA3-loxP
cassette (SEQ ID NO: 145) was PCR-amplified using IoxP-URA3-IoxP (SEQ ID
-54-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
NO: 68) as template DNA. loxP-URA3-loxP contains the URA3 marker from
(ATCC No. 77107) flanked by IoxP recombinase sites. PCR was done using
Phusion DNA polymerase (New England BioLabs Inc., Ipswich, MA) and
primers LA512 and LA513 (SEQ ID NOs: 8 and 9). The GPD2 portion of each
primer was derived from the 5' region upstream of the GPD2 coding region and
3'
region downstream of the coding region such that integration of the IoxP-URA3-
IoxP marker resulted in replacement of the GPD2 coding region. The PCR
product was transformed into BP913 and transformants were selected on
synthetic complete media lacking uracil supplemented with 1% ethanol (no
glucose). Transformants were screened to verify correct integration by PCR
using primers oBP582 and AA270 (SEQ ID NOs: 63 and 64).
[00155] The URA3 marker was recycled by transformation with pRS423::PGAL1-
cre (SEQ ID NO: 66) and plating on synthetic complete media lacking histidine
supplemented with 1 % ethanol at 30 C. Transformants were streaked on
synthetic complete medium supplemented with 1% ethanol and containing 5-
fluoro-orotic acid (0.1 %) and incubated at 30 C to select for isolates that
lost the
URA3 marker. 5-FOA resistant isolates were grown in YPE (1% ethanol) for
removal of the pRS423::PGAL1-cre plasmid. The deletion and marker removal
were confirmed by PCR with primers oBP582 (SEQ ID NO: 63) and oBP591
(SEQ ID NO: 65). The correct isolate was selected as strain CEN.PK 113-7D
Aura3::IoxP Ahis3 Apdc6 Apdcl::ilvDSm Apdc5::sadB Agpd2::loxP and
designated as PNY1503 (BP1064).
[00156] BP1064 was transformed with plasmids pYZ090 (SEQ ID NO: 1) and
pLH468 (SEQ ID NO: 2) to create strain NGCI-070 (BP1 083; PNY1 504).
Construction of Strains NYLA74, NYLA83, and NYLA84
[00157] Insertion-inactivation of endogenous PDC1 and PDC6 genes of S.
cerevisiae. PDC1, PDC5, and PDC6 genes encode the three major isozymes of
pyruvate decarboxylase is described as follows:
-55-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
Construction of pRS425::GPM-sadB
[00158] A DNA fragment encoding a butanol dehydrogenase (SEQ ID NO: 70)
from Achromobacter xylosoxidans (disclosed in U.S. Patent Application
Publication No. 2009/0269823) was cloned. The coding region of this gene
called sadB for secondary alcohol dehydrogenase (SEQ ID NO: 69) was
amplified using standard conditions from A. xylosoxidans genomic DNA,
prepared using a Gentra Puregene kit (Qiagen, Valencia, CA) following the
recommended protocol for gram negative organisms using forward and reverse
primers N473 and N469 (SEQ ID NOs: 74 and 75), respectively. The PCR
product was TOPO -Blunt cloned into pCR 4 BLUNT (InvitrogenTM, Carlsbad,
CA) to produce pCR4Blunt::sadB, which was transformed into E. coli Mach-1
cells. Plasmid was subsequently isolated from four clones, and the sequence
verified.
[00159] The sadB coding region was PCR amplified from pCR4Blunt::sadB. PCR
primers contained additional 5' sequences that would overlap with the yeast
GPM1 promoter and the ADH1 terminator (N583 and N584, provided as SEQ ID
NOs: 76 and 77). The PCR product was then cloned using "gap repair"
methodology in Saccharomyces cerevisiae (Ma, et al., Gene 58:201-216, 1987)
as follows. The yeast-E. coli shuttle vector pRS425::GPM::kivD::ADH which
contains the GPM1 promoter (SEQ ID NO: 72), kivD coding region from
Lactococcus lactis (SEQ ID NO: 71), and ADH1 terminator (SEQ ID NO: 73)
(described in U.S. Patent Application Publication No. 2007/0092957 Al, Example
17) was digested with BbvCI and Pacl restriction enzymes to release the kivD
coding region. Approximately 1 g of the remaining vector fragment was
transformed into S. cerevisiae strain BY4741 along with 1 g of sadB PCR
product. Transformants were selected on synthetic complete medium lacking
leucine. The proper recombination event, generating pRS425::GPM-sadB, was
confirmed by PCR using primers N142 and N459 (SEQ ID NOs: 108 and 109).
Construction of pdc6:: PGPM1-sadB integration cassette and PDC6 deletion:
[00160] A pdc6::PGPM1-sadB-ADH1t-URA3r integration cassette was made by
joining the GPM-sadB-ADHt segment (SEQ ID NO: 79) from pRS425::GPM-sadB
-56-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
(SEQ ID NO: 78) to the URA3r gene from pUC19-URA3r. pUC19-URA3r (SEQ
ID NO:80) contains the URA3 marker from pRS426 (ATCC No. 77107) flanked by
75 bp homologous repeat sequences to allow homologous recombination in vivo
and removal of the URA3 marker. The two DNA segments were joined by SOE
PCR (as described by Horton, et al., Gene 77:61-68, 1989) using as template
pRS425::GPM-sadB and pUC19-URA3r plasmid DNAs, with Phusion DNA
polymerase (New England BioLabs Inc., Ipswich, MA) and primers 114117-11A
through 114117-11D (SEQ ID NOs: 81, 82, 83, and 84), and 114117-13A and
114117-13B (SEQ ID NOs: 85 and 86).
[00161] The outer primers for the SOE PCR (114117-13A and 114117-13B)
contained 5' and 3' -50 bp regions homologous to regions upstream and
downstream of the PDC6 promoter and terminator, respectively. The completed
cassette PCR fragment was transformed into BY4700 (ATCC No. 200866) and
transformants were maintained on synthetic complete media lacking uracil and
supplemented with 2% glucose at 30 C using standard genetic techniques
(Methods in Yeast Genetics, 2005, Cold Spring Harbor Laboratory Press, Cold
Spring Harbor, NY, pp. 201-202). Transformants were screened by PCR using
primers 112590-34G and 112590-34H (SEQ ID NOs: 87 and 88), and 112590-
34F and 11 2590-49E (SEQ ID NOs: 89 and 90) to verify integration at the PDC6
locus with deletion of the PDC6 coding region. The URA3r marker was recycled
by plating on synthetic complete media supplemented with 2% glucose and 5-
FOA at 30 C following standard protocols. Marker removal was confirmed by
patching colonies from the 5-FOA plates onto SD-URA media to verify the
absence of growth. The resulting identified strain has the genotype: BY4700
pdc6::PGPM1-sadB-ADH1 t.
Construction of pdcl :: PPDC1-ilvD integration cassette and PDC1 deletion:
[00162] A pdcl:: PPDC1-ilvD-FBA1t-URA3r integration cassette was made by
joining the ilvD-FBA1t segment (SEQ ID NO: 91) from pLH468 (SEQ ID NO: 2) to
the URA3r gene from pUC19-URA3r by SOE PCR (as described by Horton, et
al., Gene 77:61-68, 1989) using as template pLH468 and pUC19-URA3r plasmid
DNAs, with Phusion DNA polymerase (New England BioLabs Inc., Ipswich, MA)
-57-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
and primers 114117-27A through 114117-27D (SEQ ID NOs: 111, 112, 113, and
114).
[00163] The outer primers for the SOE PCR (114117-27A and 114117-27D)
contained 5' and 3' -50 bp regions homologous to regions downstream of the
PDC1 promoter and downstream of the PDC1 coding sequence. The completed
cassette PCR fragment was transformed into BY4700 pdc6::PGPM1-sadB-
ADH1t and transformants were maintained on synthetic complete media lacking
uracil and supplemented with 2% glucose at 30 C using standard genetic
techniques (Methods in Yeast Genetics, 2005, Cold Spring Harbor Laboratory
Press, Cold Spring Harbor, NY, pp. 201-202). Transformants were screened by
PCR using primers 114117-36D and 135 (SEQ ID NOs: 92 and 93), and primers
112590-49E and 112590-30F (SEQ ID NOs: 90 and 94) to verify integration at
the PDC1 locus with deletion of the PDC1 coding sequence. The URA3r marker
was recycled by plating on synthetic complete media supplemented with 2%
glucose and 5-FOA at 30 C following standard protocols. Marker removal was
confirmed by patching colonies from the 5-FOA plates onto SD-URA media to
verify the absence of growth. The resulting identified strain "NYLA67" has the
genotype: BY4700 pdc6:: PGPM1-sadB-ADH1t pdcl:: PPDC1-iIvD-FBA1t.
HIS3 deletion
[00164] To delete the endogenous HIS3 coding region, a his3::URA3r2 cassette
was PCR-amplified from URA3r2 template DNA (SEQ ID NO: 95). URA3r2
contains the URA3 marker from pRS426 (ATCC No. 77107) flanked by 500 bp
homologous repeat sequences to allow homologous recombination in vivo and
removal of the URA3 marker. PCR was done using Phusion DNA polymerase
(New England BioLabs Inc., Ipswich, MA) and primers 114117-45A and 114117-
45B (SEQ ID NOs: 96 and 97) which generated a -2.3 kb PCR product. The
HIS3 portion of each primer was derived from the 5' region upstream of the
HIS3
promoter and 3' region downstream of the coding region such that integration
of
the URA3r2 marker results in replacement of the HIS3 coding region. The PCR
product was transformed into NYLA67 using standard genetic techniques
(Methods in Yeast Genetics, 2005, Cold Spring Harbor Laboratory Press, Cold
Spring Harbor, NY, pp. 201-202) and transformants were selected on synthetic
-58-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
complete media lacking uracil and supplemented with 2% glucose at 30 C.
Transformants were screened to verify correct integration by replica plating
of
transformants onto synthetic complete media lacking histidine and supplemented
with 2% glucose at 30 C. The URA3r marker was recycled by plating on
synthetic complete media supplemented with 2% glucose and 5-FOA at 300C
following standard protocols. Marker removal was confirmed by patching
colonies from the 5-FOA plates onto SD-URA media to verify the absence of
growth. The resulting identified strain, called NYLA73, has the genotype:
BY4700 pdc6:: PGPM1-sadB-ADH1 t pdcl:: PPDC1-iIvD-FBA1 t Ahis3.
Construction of pdc5::kanMX integration cassette and PDC5 deletion:
[00165] A pdc5::kanMX4 cassette was PCR-amplified from strain YLR134W
chromosomal DNA (ATCC No. 4034091) using Phusion DNA polymerase (New
England BioLabs Inc., Ipswich, MA) and primers PDC5::KanMXF and
PDC5::KanMXR (SEQ ID NOs: 98 and 99) which generated a -2.2 kb PCR
product. The PDC5 portion of each primer was derived from the 5' region
upstream of the PDC5 promoter and 3' region downstream of the coding region
such that integration of the kanMX4 marker results in replacement of the PDC5
coding region. The PCR product was transformed into NYLA73 using standard
genetic techniques (Methods in Yeast Genetics, 2005, Cold Spring Harbor
Laboratory Press, Cold Spring Harbor, NY, pp. 201-202) and transformants were
selected on YP media supplemented with 1% ethanol and geneticin (200 g/mL)
at 30 C. Transformants were screened by PCR to verify correct integration at
the
PDC locus with replacement of the PDC5 coding region using primers PDC5kofor
and N175 (SEQ ID NOs: 100 and 101). The identified correct transformants have
the genotype: BY4700 pdc6:: PGPM1-sadB-ADH1t pdcl:: PPDC1-iIvD-FBA1t
Ahis3 pdc5::kanMX4. The strain was named NYLA74.
[00166] Plasmid vectors pRS423::CUP1-alsS+FBA-budA and pRS426::FBA-
budC+GPM-sadB were transformed into NYLA74 to create a butanediol
producing strain (NGCI-047).
[00167] Plasmid vectors pLH475-Z4B8 (SEQ ID NO: 140) and pLH468 were
transformed into NYLA74 to create an isobutanol producing strain (NGCI-049).
-59-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
Deletion of HXK2 (hexokinase II):
[00168] A hxk2::URA3r cassette was PCR-amplified from URA3r2 template
(described above) using Phusion DNA polymerase (New England BioLabs Inc.,
Ipswich, MA) and primers 384 and 385 (SEQ ID NOs: 102 and 103) which
generated a -2.3 kb PCR product. The HXK2 portion of each primer was derived
from the 5' region upstream of the HXK2 promoter and 3' region downstream of
the coding region such that integration of the URA3r2 marker results in
replacement of the HXK2 coding region. The PCR product was transformed into
NYLA73 using standard genetic techniques (Methods in Yeast Genetics, 2005,
Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 201-202) and
transformants were selected on synthetic complete media lacking uracil and
supplemented with 2% glucose at 30 C. Transformants were screened by PCR
to verify correct integration at the HXK2 locus with replacement of the HXK2
coding region using primers N869 and N871 (SEQ ID NOs: 104 and 105). The
URA3r2 marker was recycled by plating on synthetic complete media
supplemented with 2% glucose and 5-FOA at 30 C following standard protocols.
Marker removal was confirmed by patching colonies from the 5-FOA plates onto
SD-URA media to verify the absence of growth, and by PCR to verify correct
marker removal using primers N946 and N947 (SEQ ID NOs: 106 and 107). The
resulting identified strain named NYLA83 has the genotype: BY4700 pdc6::
PGPM1-sadB-ADH1 t pdcl:: PPDC1-iIvD-FBA1 t Ahis3 Ahxk2.
Construction of pdc5::kanMX integration cassette and PDC5 deletion:
[00169] A pdc5::kanMX4 cassette was PCR-amplified as described above. The
PCR fragment was transformed into NYLA83, and transformants were selected
and screened as described above. The identified correct transformants named
NYLA84 have the genotype: BY4700 pdc6:: PGPM1-sadB-ADH1t pdcl:: PPDC1-
ilvD-FBA1t Ahis3 Ahxk2 pdc5::kanMX4.
[00170] Plasmid vectors pLH468 and pLH532 were simultaneously transformed
into strain NYLA84 (BY4700 pdc6::PGPM1-sadB-ADH1t pdcl::PPDC1-ilvD-
FBA1t Ahis3 Ahxk2 pdc5::kanMX4) using standard genetic techniques (Methods
in Yeast Genetics, 2005, Cold Spring Harbor Laboratory Press, Cold Spring
-60-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
Harbor, NY) and the resulting "butanologen NYLA84" was maintained on
synthetic complete media lacking histidine and uracil, and supplemented with
1%
ethanol at 30 C.
Expression Vector pLH468
[00171] The pLH468 plasmid (SEQ ID NO:2) was constructed for expression of
DHAD, KivD and HADH in yeast and is described in U.S. Patent Application
Publication No. 2009/0305363, herein incorporated by reference. pLH486 was
constructed to contain: a chimeric gene having the coding region of the ilvD
gene
from Streptococcus mutans (nt position 3313-4849) expressed from the S.
cerevisiae FBA1 promoter (nt 2109 - 3105) followed by the FBA1 terminator (nt
4858 - 5857) for expression of DHAD; a chimeric gene having the coding region
of codon optimized horse liver alcohol dehydrogenase (nt 6286-7413) expressed
from the S. cerevisiae GPM1 promoter (nt 7425-8181) followed by the ADH1
terminator (nt 5962-6277) for expression of ADH; and a chimeric gene having
the
coding region of the codon-optimized kivD gene from Lactococcus lactis (nt
9249-
10895) expressed from the TDH3 promoter (nt 10896-11918) followed by the
TDH3 terminator (nt 8237-9235) for expression of KivD.
[00172] Coding regions for Lactococcus lactis ketoisovalerate decarboxylase
(KivD) and horse liver alcohol dehydrogenase (HADH) were synthesized by
DNA2.0, Inc. (Menlo Park, CA) based on codons that were optimized for
expression in Saccharomyces cerevisiae (SEQ ID NO: 71 and 118, respectively)
and provided in plasmids pKivDy-DNA2.0 and pHadhy-DNA2Ø The encoded
proteins are SEQ ID NOs:117 and 119, respectively. Individual expression
vectors for KivD and HADH were constructed. To assemble pLH467
(pRS426::PTDH3-kivDy-TDH3t), vector pNY8 (SEQ ID NO: 121; also named
pRS426.GPD-ald-GPDt, described in U.S. Patent Application Publication No.
2008/0182308, Example 17, which is herein incorporated by reference) was
digested with Ascl and Sfil enzymes, thus excising the GPD promoter and the
ald
coding region. A TDH3 promoter fragment (SEQ ID NO: 122) from pNY8 was
PCR amplified to add an Ascl site at the 5' end and an Spel site at the 3'
end,
using 5' primer OT1068 and 3' primer OT1067 (SEQ ID NOs: 123 and 124). The
Ascl/Sfil digested pNY8 vector fragment was ligated with the TDH3 promoter
-61-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
PCR product digested with Ascl and Spel and the Spel-Sfil fragment containing
the codon optimized kivD coding region isolated from the vector pKivD-DNA2Ø
The triple ligation generated vector pLH467 (pRS426::PTDH3-kivDy-TDH3t).
pLH467 was verified by restriction mapping and sequencing.
[00173] pLH435 (pRS425::PGPM1-Hadhy-ADH1t) was derived from vector
pRS425::GPM-sadB (SEQ ID NO: 78) which is described in U.S. Provisional
Application Serial No. 61/058,970, Example 3, which is herein incorporated by
reference. pRS425::GPM-sadB is the pRS425 vector (ATCC No. 77106) with a
chimeric gene containing the GPM1 promoter (SEQ ID NO:72), coding region
from a butanol dehydrogenase of Achromobacter xylosoxidans (sad B; DNA SEQ
ID NO: 69; protein SEQ ID NO:70: disclosed in U.S. Patent Application
Publication No. 2009/0269823), and ADH1 terminator (SEQ ID NO: 73).
pRS425::GPMp-sadB contains Bbvl and Pacl sites at the 5' and 3' ends of the
sadB coding region, respectively. A Nhel site was added at the 5' end of the
sadB coding region by site-directed mutagenesis using primers OT1074 and
OT1075 (SEQ ID NOs: 126 and 127) to generate vector pRS425-GPMp-sadB-
Nhel, which was verified by sequencing. pRS425::PGPM1-sadB-Nhel was
digested with Nhel and Pacl to drop out the sadB coding region, and ligated
with
the Nhel-Pacl fragment containing the codon optimized HADH coding region from
vector pHadhy-DNA2.0 to create pLH435.
[00174] To combine KivD and HADH expression cassettes in a single vector,
yeast
vector pRS411 (ATCC No. 87474) was digested with Sacl and Noti, and ligated
with the Sacl-Sall fragment from pLH467 that contains the PTDH3-kivDy-TDH3t
cassette together with the Sall-Notl fragment from pLH435 that contains the
PGPM1-Hadhy-ADH1t cassette in a triple ligation reaction. This yielded the
vector pRS411::PTDH3-kivDy-PGPM1-Hadhy (pLH441) which was verified by
restriction mapping.
[00175] In order to generate a co-expression vector for all three genes in the
lower
isobutanol pathway: ilvD, kivDy, and Hadhy, pRS423 FBA ilvD(Strep) (SEQ ID
NO: 128) which is described in U.S. Patent Application Publication No.
2010/0081154 as the source of the IIvD gene, was used. This shuttle vector
contains an F1 origin of replication (nt 1423 to 1879) for maintenance in E.
coli
-62-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
and a 2 micron origin (nt 8082 to 9426) for replication in yeast. The vector
has an
FBA1 promoter (nt 2111 to 3108; SEQ ID NO: 120) and FBA terminator (nt 4861
to 5860; SEQ ID NO: 129). In addition, it carries the His marker (nt 504 to
1163)
for selection in yeast and ampicillin resistance marker (nt 7092 to 7949) for
selection in E. coli. The ilvD coding region (nt 3116 to 4828; SEQ ID NO: 115;
protein SEQ ID NO: 116) from Streptococcus mutans UA159 (ATCC No. 700610)
is between the FBA promoter and FBA terminator forming a chimeric gene for
expression. In addition, there is a lumio tag fused to the ilvD coding region
(nt
4829-4849).
[00176] The first step was to linearize pRS423 FBA ilvD(Strep) (also called
pRS423-FBA(Spel)-IIvD(Streptococcus mutans)-Lumio) with Sacl and Sacll (with
Sacll site blunt ended using T4 DNA polymerase), to give a vector with total
length of 9,482 bp. The second step was to isolate the kivDy-hADHy cassette
from pLH441 with Sacl and KpnI (with KpnI site blunt ended using T4 DNA
polymerase), which gives a 6,063 bp fragment. This fragment was ligated with
the 9,482 bp vector fragment from pRS423-FBA(Spel)-IIvD(Streptococcus
mutans)-Lumio. This generated vector pLH468 (pRS423::PFBA1-ilvD(Strep)
Lumio-FBA1 t-PTDH3-kivDy-TDH3t-PGPM1-had hy-ADH1 t) which was confirmed
by restriction mapping and sequencing.
pLH532 construction
[00177] The pLH532 plasmid (SEQ ID NO: 130) was constructed for expression of
ALS and KART in yeast. pLH532 is a pHR81 vector (ATCC No. 87541)
containing the following chimeric genes: 1) the CUP1 promoter (SEQ ID NO:
139), acetolactate synthase coding region from Bacillus subtilis (AIsS; SEQ ID
NO: 137; protein SEQ ID NO: 138) and CYC1 terminator2 (SEQ ID NO: 133); 2)
an ILV5 promoter (SEQ ID NO: 134), Pf5.IIvC coding region (SEQ ID NO: 132)
and ILV5 terminator (SEQ ID NO: 135); and 3) the FBA1 promoter (SEQ ID NO:
136), S. cerevisiae KART coding region (ILV5; SEQ ID NO: 131); and CYC1
terminator.
-63-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
[00178] The Pf5.IIvC coding region is a sequence encoding KART derived from
Pseudomonas fluorescens that was described in U.S. Patent Application
Publication No. 2009/0163376, which is herein incorporated by reference.
[00179] The Pf5.IIvC coding region was synthesized by DNA2.0, Inc. (Menlo
Park,
CA; SEQ ID NO: 132) based on codons that were optimized for expression in
Saccharomyces cerevisiae.
pYZ090 construction
[00180] pYZ090 (SEQ ID NO: 1) is based on the pHR81 (ATCC No. 87541)
backbone and was constructed to contain a chimeric gene having the coding
region of the alsS gene from Bacillus subtilis (nt position 457-2172)
expressed
from the yeast CUP1 promoter (nt 2-449) and followed by the CYC1 terminator
(nt 2181-2430) for expression of ALS, and a chimeric gene having the coding
region of the ilvC gene from Lactococcus lactis (nt 3634-4656) expressed from
the yeast ILV5 promoter (2433-3626) and followed by the ILV5 terminator (nt
4682-5304) for expression of KART.
pYZ067 construction
[00181] pYZ067 was constructed to contain the following chimeric genes: 1) the
coding region of the ilvD gene from S. mutans UA159 (nt position 2260-3971)
expressed from the yeast FBA1 promoter (nt 1161-2250) followed by the FBA
terminator (nt 4005-4317) for expression of dihydroxy acid dehydratase (DHAD),
2) the coding region for horse liver ADH (nt 4680-5807) expressed from the
yeast
GPM promoter (nt 5819-6575) followed by the ADH1 terminator (nt 4356-4671)
for expression of alcohol dehydrogenase, and 3) the coding region of the KivD
gene from Lacrococcus lactis (nt 7175-8821) expressed from the yeast TDH3
promoter (nt 8830-9493) followed by the TDH3 terminator (nt 5682-7161) for
expression of ketoisovalerate decarboxylase.
pRS423::CUP1-aIsS+FBA-budA and pRS426::FBA-budC+GPM-sadB and
pLH475-Z4B8 construction
[00182] Construction of pRS423::CUP1-alsS+FBA-budA and pRS426::FBA-
budC+GPM-sadB and pLH475-Z4B8 is described in U.S. Patent Application
Publication No. 2009/0305363, incorporated herein by reference.
-64-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
Examples
[00183] The following nonlimiting examples will further illustrate the
invention. It
should be understood that, while the following examples involve corn as
feedstock and COFA as ISPR extractant obtained from enzymatic hydrolysis of
corn lipids, other biomass sources can be used for feedstock and enzymatic
hydrolysis of biomass oil, without departing from the present invention.
[00184] As used herein, the meaning of abbreviations used was as follows: "g"
means gram(s), "kg" means kilogram(s), "L" means liter(s), "mL" means
milliliter(s), "pL" means microliter(s), "mL/L" means milliliter(s) per liter,
"mL/min"
means milliliter(s) per min, "DI" means deionized, "uM" means micrometer(s),
"nm" means nanometer(s), "w/v" means weight/volume, "OD" means optical
density, "OD600" means optical density at a wavelength of 600 nM, "dcw" means
dry cell weight, "rpm" means revolutions per minute, " C" means degree(s)
Celsius, " C/min" means degrees Celsius per minute, "slpm" means standard
liter(s) per minute, "ppm" means part per million, "pdc" means pyruvate
decarboxylase enzyme followed by the enzyme number.
GENERAL METHODS
Seed Flask Growth
[00185] A Saccharomyces cerevisiae strain that was engineered to produce
isobutanol from a carbohydrate source, with pdcl deleted, pdc5 deleted, and
pdc6 deleted, was grown to 0.55-1.1 g/L dcw (OD600 1.3-2.6 - Thermo Helios a
Thermo Fisher Scientific Inc., Waltham, Massachusetts) in seed flasks from a
frozen culture. The culture was grown at 26 C in an incubator rotating at
300 rpm. The frozen culture was previously stored at - 80 C. The composition
of the first seed flask medium was:
3.0 g/L dextrose
3.0 g/L ethanol, anhydrous
3.7 g/L ForMediumTM Synthetic Complete Amino Acid (Kaiser) Drop-Out:
without HIS, without URA (Reference No. DSCK162CK)
6.7 g/L Difco Yeast Nitrogen Base without amino acids (No. 291920)
-65-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
[00186] Twelve milliliters from the first seed flask culture was transferred
to a 2 L
flask and grown at 300C in an incubator rotating at 300 rpm. The second seed
flask has 220 mL of the following medium:
30.0 g/L dextrose
5.0 g/L ethanol, anhydrous
3.7 g/L ForMediumTM Synthetic Complete Amino Acid (Kaiser) Drop-Out:
without HIS, without URA (Reference No. DSCK162CK)
6.7 g/L Difco Yeast Nitrogen Base without amino acids (No.291920)
0.2 M MES Buffer titrated to pH 5.5-6.0
[00187] The culture was grown to 0.55-1.1 g/L dcw (OD600 1.3-2.6). An addition
of
30 mL of a solution containing 200 g/L peptone and 100 g/L yeast extract was
added at this cell concentration. Then, an addition of 300 mL of 0.2 uM filter
sterilized Cognis, 90-95% oleyl alcohol was added to the flask. The culture
continues to grow to > 4 g/L dcw (OD600 > 10) before being harvested and added
to the fermentation.
Fermentation Preparation
Initial Fermentation Vessel Preparation
[00188] A glass jacked, 2 L fermentation vessel (Sartorius AG, Goettingen,
Germany) was charged with house water to 66% of the liquefaction weight. A pH
probe (Hamilton Easyferm Plus K8, part number: 238627, Hamilton Bonaduz AG,
Bonaduz, Switzerland) was calibrated through the Sartorius DCU-3 Control
Tower Calibration menu. The zero was calibrated at pH=7. The span was
calibrated at pH=4. The probe was then placed into the fermentation vessel
through the stainless steel head plate. A dissolved oxygen probe (p02 probe)
was also placed into the fermentation vessel through the head plate. Tubing
used for delivering nutrients, seed culture, extracting solvent, and base were
attached to the head plate and the ends were foiled. The entire fermentation
vessel was placed into a Steris (Steris Corporation, Mentor, Ohio) autoclave
and
sterilized in a liquid cycle for 30 minutes.
[00189] The fermentation vessel was removed from the autoclave and placed on a
load cell. The jacket water supply and return line was connected to the house
-66-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
water and clean drain, respectively. The condenser cooling water in and water
out lines were connected to a 6-L recirculating temperature bath running at 7
C.
The vent line that transfers the gas from the fermentation vessel was
connected
to a transfer line that was connected to a Thermo mass spectrometer (Prima dB,
Thermo Fisher Scientific Inc., Waltham, Massachusetts). The sparger line was
connected to the gas supply line. The tubing for adding nutrients, extract
solvent,
seed culture, and base was plumbed through pumps or clamped closed.
[00190] The fermentation vessel temperature was controlled at 55 C with a
thermocouple and house water circulation loop. Wet corn kernels (#2 yellow
dent) were ground using a hammer mill with a 1.0 mm screen, and the resulting
ground whole corn kernels were then added to the fermentation vessel at a
charge that was 29-30% (dry corn solids weight) of the liquefaction reaction
mass.
Lipase Treatment Pre-Liquefaction
[00191] A lipase enzyme stock solution was added to the fermentation vessel to
a
final lipase concentration of 10 ppm. The fermentation vessel was held at 55
C,
300 rpm, and 0.3 slpm N2 overlay for >6 hrs. After the lipase treatment was
complete, liquefaction was performed as described below (Liquefaction).
Liquefaction
[00192] An alpha-amylase was added to the fermentation vessel per its
specification sheet while the fermentation vessel was mixing at 300-1200 rpm,
with sterile, house N2 being added at 0.3 slpm through the sparger. The
temperature set-point was changed from 55 C to 85 C. When the temperature
was > 80 C, the liquefaction cook time was started and the liquefaction cycle
was
held at > 80 C for 90-120 minutes. The fermentation vessel temperature set-
point was set to the fermentation temperature of 30 C after the liquefaction
cycle
was complete. N2 was redirected from the sparger to the head space to prevent
foaming without the addition of a chemical antifoaming agent.
-67-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
Lipase Treatment Post-Liquefaction
[00193] The fermentation vessel temperature was set to 55 C instead of 30 C
after
the liquefaction cycle was complete (Liquefaction). The pH was manually
controlled at pH=5.8 by making bolus additions of acid or base when needed. A
lipase enzyme stock solution was added to the fermentation vessel to a final
lipase concentration of 10 ppm. The fermentation vessel was held at 55 C,
300 rpm, and 0.3 slpm N2 overlay for >6 hrs. After the Lipase Treatment was
complete, the fermentation vessel temperature was set to 30 C.
Lipase Heat Inactivation Treatment (Heat Kill Treatment Method)
[00194] The fermentation vessel temperature was held at > 80 C for > 15
minutes
to inactivate the lipase. After the Heat Inactivation Treatment was complete,
the
fermentation vessel temperature was set to 30 C.
Nutrient Addition Prior to Inoculation
[00195] Ethanol (6.36 mL/L, post-inoculation volume, 200 proof, anhydrous) was
added to the fermentation vessel just prior to inoculation. Thiamine was added
to
a final concentration of 20 mg/L and 100 mg/L nicotinic acid was also added
just
prior to inoculation.
Oleyl Alcohol or Corn Oil Fatty Acids Addition Prior to Inoculation
[00196] Added 1 L/L (post-inoculation volume) of oleyl alcohol or corn oil
fatty
acids immediately after inoculation.
Fermentation Vessel Inoculation
[00197] The fermentation vessels p02 probe was calibrated to zero while N2 was
being added to the fermentation vessel. The fermentation vessels p02 probe was
calibrated to its span with sterile air sparging at 300 rpm. The fermentation
vessel was inoculated after the second seed flask with > 4 g/L dcw. The shake
flask was removed from the incubator/shaker for 5 minutes allowing a phase
separation of the oleyl alcohol phase and the aqueous phase. The aqueous
-68-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
phase (110 mL) was transferred to a sterile, inoculation bottle. The inoculum
was
pumped into the fermentation vessel through a peristaltic pump.
Fermentation Vessel Operating Conditions
[00198] The fermentation vessel was operated at 30 C for the entire growth and
production stages. The pH was allowed to drop from a pH between 5.7-5.9 to a
control set-point of 5.2 without adding any acid. The pH was controlled for
the
remainder of the growth and production stage at a pH=5.2 with ammonium
hydroxide. Sterile air was added to the fermentation vessel, through the
sparger,
at 0.3 slpm for the remainder of the growth and production stages. The p02 was
set to be controlled at 3.0% by the Sartorius DCU-3 Control Box PID control
loop,
using stir control only, with the stirrer minimum being set to 300 rpm and the
maximum being set to 2000 rpm. The glucose was supplied through
simultaneous saccharification and fermentation of the liquified corn mash by
adding a a-amylase (glucoamylase). The glucose was kept excess (1-50 g/L)
for as long as starch was available for saccharification.
Analytical
Gas Analysis
[00199] Process air was analyzed on a Thermo Prima (Thermo Fisher Scientific
Inc., Waltham, Massachusetts) mass spectrometer. This was the same process
air that was sterilized and then added to each fermentation vessel. Each
fermentation vessel's off-gas was analyzed on the same mass spectrometer.
This Thermo Prima dB has a calibration check run every Monday morning at
6:00 am. The calibration check was scheduled through the Gas Works v1.0
(Thermo Fisher Scientific Inc., Waltham, Massachusetts) software associated
with the mass spectrometer. The gas calibrated for were:
-69-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
GAS Calibration Concentration mole % Cal Frequency
Nitrogen 78 % weekly
Oxygen 21 % weekly
Isobutanol 0.2 % yearly
Argon 1 % weekly
Carbon Dioxide 0.03 % weekly
[00200] Carbon dioxide was checked at 5% and 15% during calibration cycle with
other known bottled gases. Oxygen was checked at 15% with other known
bottled gases. Based on the analysis of the off-gas of each fermentation
vessel,
the amount of isobutanol stripped, oxygen consumed, and carbon dioxide
respired into the off-gas was measured by using the mass spectrometer's mole
fraction analysis and gas flow rates (mass flow controller) into the
fermentation
vessel. Calculate the gassing rate per hour and then integrating that rate
over
the course of the fermentation.
Biomass Measurement
[00201] A 0.08% Trypan Blue solution was prepared from a 1:5 dilution of 0.4%
Trypan Blue in NaCl (VWR BDH8721-0) with 1X PBS. A 1.0 mL sample was
pulled from a fermentation vessel and placed in a 1.5 mL Eppendorf centrifuge
tube and centrifuged in an Eppendorf, 5415C at 14,000 rpm for 5 minutes. After
centrifugation, the top solvent layer was removed with an m200 Variable
Channel
BioHit pipette with 20-200 pL BioHit pipette tips. Care was made not to remove
the layer between the solvent and aqueous layers. Once the solvent layer was
removed, the sample was re-suspended using a Vortex-Genie set at 2700 rpm.
[00202] A series of dilutions was required to prepare the ideal concentration
for
hemacytometer counts. If the OD was 10, a 1:20 dilution would be performed to
achieve 0.5 OD which would give the ideal amount of cells to be counted per
square, 20-30. In order to reduce inaccuracy in the dilution due to corn
solids,
multiple dilutions with cut 100-1000 pL BioHit pipette tips were required.
Approximately, 1 cm was cut off the tips to increase the opening which
prevented
the tip from clogging. For a 1:20 final dilution, an initial 1:1 dilution of
fermentation sample and 0.9% NaCl solution was prepared. Then, a 1:1 dilution
-70-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
of the previous solution (i.e., the initial 1:1 dilution) and 0.9% NaCl
solution (the
second dilution) was generated followed by a 1:5 dilution of the second
dilution
and Trypan Blue Solution. Samples were vortexed between each dilution and cut
tips were rinsed into the 0.9% NaCl and Trypan Blue solutions.
[00203] The cover slip was carefully placed on top of the hemacytometer
(Hausser
Scientific Bright-Line 1492). An aliquot (10 pL) was drawn of the final Trypan
Blue dilution with an m20 Variable Channel BioHit pipette with 2-20 pL BioHit
pipette tips and injected into the hemacytometer. The hemacytometer was
placed on the Zeis Axioskop 40 microscope at 40x magnification. The center
quadrant was broken into 25 squares and the four corner and center squares in
both chambers were then counted and recorded. After both chambers were
counted, the average was taken and multiplied by the dilution factor (20),
then by
25 for the number for squares in the quadrant in the hemacytometer, and then
divided by 0.0001 mL which is the volume of the quadrant that was counted. The
sum of this calculation is the number cells per mL.
LC Analysis of Fermentation Products in the Aqueous Phase
[00204] Samples were refrigerated until ready for processing. Samples were
removed from refrigeration and allowed to reach room temperature (about one
hour). Approximately 300 pL of sample was transferred with a m1000 Variable
Channel BioHit pipette with 100-1000 pL BioHit pipette tips into a 0.2 um
centrifuge filter (Nanosep MF modified nylon centrifuge filter), then
centrifuged
using a Eppendorf, 5415C for five minutes at 14,000 rpm. Approximately 200 pL
of filtered sample was transferred into a 1.8 auto sampler vial with a 250 pL
glass
vial insert with polymer feet. A screw cap with PTFE septa was used to cap the
vial before vortexing the sample with a Vortex-Genie set at 2700 rpm.
[00205] Sample was then run on Agilent 1200 series LC equipped with binary,
isocratic pumps, vacuum degasser, heated column compartment, sampler
cooling system, UV DAD detector and RI detector. The column used was an
Aminex HPX-87H, 300 X 7.8 with a Bio-Rad Cation H refill, 30X4.6 guard column.
Column temperature was 40 C, with a mobile phase of 0.01 N sulfuric acid at a
flow rate of 0.6 mL/min for 40 minutes. Results are shown in Table 1.
-71-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
Table 1: Retention times of fermentation products in aqueous phase
HPLC 302/310 FW RID Retention Range of UV
Normalized to 10 pL Time, min Standards, g/L Retention
injections Time, min
citric acid 192.12 8.025 0.3-17 7.616
glucose 180.16 8.83 0.5-71
pyruvic acid (Na) 110.04 9.388 0.1-5.2 8.5
A-Kiv (Na) 138.1 9.91 0.07-5.0 8.55
2,3-dihydroxyisovaleric 156.1 10.972 0.2-8.8 10.529
acid (Na)
succinic acid 118.09 11.561 0.3-16 11.216
lactic acid (Li) 96.01 12.343 0.3-17 11.948
glycerol 92.09 12.974 0.8-39
formic acid 46.03 13.686 0.2-13 13.232
acetate (Na) 82.03 14.914 0.5-16 14.563
meso-butanediol 90.12 17.583 0.1-19
(+/-)-2,3-butanediol 90.12 18.4 0.2-19
isobutyric acid 88.11 19.685 0.1-8.0 19.277
ethanol 46.07 21.401 0.5-34
isobut raldeh de 72.11 27.64 0.01-0.11
isobutanol 74.12 32.276 0.2-15
3-OH-2-butanone (acetoin) 88.11 0.1-11 17.151
GC Analysis of Fermentation Products in the Solvent Phase
[00206] Samples were refrigerated until ready for processing. Samples were
removed from refrigeration and allowed to reach room temperature (about one
hour). Approximately 150 pL of sample was transferred using a m1000 Variable
Channel BioHit pipette with 100-1000 pL BioHit pipette tips into a 1.8 auto
sampler vial with a 250 pL glass vial insert with polymer feet. A screw cap
with
PTFE septa was used to cap the vial.
[00207] Sample was then run on Agilent 7890A GC with a 7683B injector and a
G2614A auto sampler. The column was a HP-InnoWax column (30 m x 0.32 mm
ID, 0.25 pm film). The carrier gas was helium at a flow rate of 1.5 mL/min
measured at 45 C with constant head pressure; injector split was 1:50 at 225
C;
oven temperature was 45 C for 1.5 minutes, 45 C to 160 C at 10 C/min for
0 minutes, then 230 C at 35 C/min for 14 minutes for a run time of 29 minutes.
Flame ionization detection was used at 260 C with 40 mL/min helium makeup
gas. Results are shown in Table 2.
-72-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
Table 2: Retention times of fermentation products in solvent phase
GC 302/310 FW Solvent Range of Standards,
Normalized to 10 lJL Retention g/L
injections Time, min
isobut raldeh de 72.11 2.75 0.7-10.4
ethanol 46.07 3.62 0.5-34
isobutanol 74.12 5.53 0.2-16
3-OH-2-butanone (acetoin) 88.11 8,29 0.1-11
+/- -2,3-butanediol 90,12 10.94 0.1-19
isobut ric acid 88.11 11.907 0.1-7.9
meso-butanediol 90.12 11.26 0.1-6.5
glycerol 92.09 16.99 0.8-9
[00208] Samples analyzed for fatty acid butyl esters were run on Agilent 6890
GC
with a 7683B injector and a G2614A auto sampler. The column was a HP-DB-
FFAP column (15 meters x 0.53 mm ID (Megabore), 1-micron film thickness
column (30 m x 0.32 mm ID, 0.25 pm film). The carrier gas was helium at a flow
rate of 3.7 mL/min measured at 45 C with constant head pressure; injector
split
was 1:50 at 225 C; oven temperature was 100 C for 2.0 minutes, 100 C to 250 C
at 10 C/min, then 250 C for 9 minutes for a run time of 26 minutes. Flame
ionization detection was used at 300 C with 40 mL/min helium makeup gas. The
following GC standards (Nu-Chek Prep; Elysian, MN) were used to confirm the
identity of fatty acid isobutyl ester products: iso-butyl palmitate, iso-butyl
stearate,
iso-butyl oleate, iso-butyl linoleate, iso-butyl linolenate, iso-butyl
arachidate.
[00209] Examples 1-14 describe various fermentation conditions that may be
used
for the claimed methods. As an example, some fermentations were subjected to
Lipase Treatment pre-liquefaction and others were subjected to Lipase
Treatment
post-liquefaction. In other examples, the fermentation was subjected to Heat
inactivation Treatment. Following fermentation, the effective isobutanol titer
(Eff
Iso Titer) was measured, that is, the total grams of isobutanol produced per
liter
aqueous volume. Results are shown in Table 3.
Example 1 - (control)
[00210] Experiment identifier 2010YO14 included: Seed Flask Growth method,
Initial Fermentation Vessel Preparation method, Liquefaction method, Nutrient
Addition Prior to Inoculation method, Fermentation Vessel Inoculation method,
-73-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
Fermentation Vessel Operating Conditions method, and all of the Analytical
methods. Oleyl alcohol was added in a single batch between 0.1- 1.0 hr after
inoculation. The butanologen was NGCI-070.
Example 2
[00211] Experiment identifier 2010YO15 included: Seed Flask Growth method,
Initial Fermentation Vessel Preparation method, Liquefaction method, Lipase
Treatment Post-Liquefaction method, Nutrient Addition Prior to Inoculation
method, Fermentation Vessel Inoculation method, Fermentation Vessel
Operating Conditions method, and all of the Analytical methods. Oleyl alcohol
was added in a single batch between 0.1- 1.0 hr after inoculation. The
butanologen was NGCI-070.
Example 3
[00212] Experiment identifier 2010YO16 included: Seed Flask Growth method,
Initial Fermentation vessel Preparation method, Liquefaction method, Lipase
Treatment Post-Liquefaction method, Nutrient Addition Prior to Inoculation
method with the exception of the exclusion of ethanol, Fermentation Vessel
Inoculation method, Fermentation Vessel Operating Conditions method, and all
of
the Analytical methods. Oleyl alcohol was added in a single batch between 0.1-
1.0 hr after inoculation. The butanologen was NGCI-070.
Example 4
[00213] Experiment identifier 2010YO17 included: Seed Flask Growth method,
Initial Fermentation Vessel Preparation method, Liquefaction method, Heat Kill
Treatment method Post-Liquefaction, Nutrient Addition Prior to Inoculation
method with the exception of the exclusion of ethanol, Fermentation Vessel
Inoculation method, Fermentation Vessel Operating Conditions method, and all
of
the Analytical methods. Oleyl alcohol was added in a single batch between 0.1-
1.0 hr after inoculation. The butanologen was NGCI-070.
-74-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
Example 5
[00214] Experiment identifier 2010YO18 included: Seed Flask Growth method,
Initial Fermentation Vessel Preparation method, Liquefaction method, Lipase
Treatment Post-Liquefaction method with the exception of only adding 7.2 ppm
lipase after liquefaction, Heat Kill Treatment method post-liquefaction,
Nutrient
Addition Prior to Inoculation method, Fermentation Vessel Inoculation method,
Fermentation Vessel Operating Conditions method, and all of the Analytical
methods. Oleyl alcohol was added in a single batch between 0.1- 1.0 hr after
inoculation. The butanologen was NGCI-070.
Example 6 - (control)
[00215] Experiment identifier 2010YO19 included: Seed Flask Growth method,
Initial Fermentation Vessel Preparation method, Liquefaction method, Heat Kill
Treatment method Post-Liquefaction, Nutrient Addition Prior to Inoculation
method, Fermentation Vessel Inoculation method, Fermentation Vessel
Operating Conditions method, and all of the Analytical methods. Oleyl alcohol
was added in a single batch between 0.1- 1.0 hr after inoculation. The
butanologen was NGCI-070.
Example 7 - (control)
[00216] Experiment identifier 2010YO21 included: Seed Flask Growth method,
Initial Fermentation Vessel Preparation method, Lipase Treatment Pre-
Liquefaction method, the Liquefaction method, Heat Kill Treatment during
liquefaction, Nutrient Addition Prior to Inoculation method, Fermentation
Vessel
Inoculation method, Fermentation Vessel Operating Conditions method, and all
of
the Analytical methods. Oleyl alcohol was added in a single batch between 0.1-
1.0 hr after inoculation. The butanologen was NGCI-070.
Example 8
[00217] Experiment identifier 2010YO22 included: Seed Flask Growth method,
Initial Fermentation Vessel Preparation method, Liquefaction method, Nutrient
Addition Prior to Inoculation method, Fermentation Vessel Inoculation method,
-75-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
Fermentation Vessel Operating Conditions method, and all of the Analytical
methods. Oleyl alcohol was added in a single batch between 0.1- 1.0 hr after
inoculation. The butanologen was NGCI-070.
Example 9
[00218] Experiment identifier 2010YO23 included: Seed Flask Growth method,
Initial Fermentation Vessel Preparation method, Liquefaction method, Lipase
Treatment Post-Liquefaction method, no Heat Kill Treatment, Nutrient Addition
Prior to Inoculation method, Fermentation Vessel Inoculation method,
Fermentation Vessel Operating Conditions method, and all of the Analytical
methods. Corn oil fatty acids made from crude corn oil was added in a single
batch between 0.1- 1.0 hr after inoculation. The butanologen was NGCI-070.
Example 10
[00219] Experiment identifier 2010YO24 included: Seed Flask Growth method,
Initial Fermentation Vessel Preparation method, Lipase Treatment Pre-
Liquefaction method, Liquefaction method, Heat Kill Treatment during
liquefaction, Nutrient Addition Prior to Inoculation method with the exception
of
there being no addition of ethanol, Fermentation Vessel Inoculation method,
Fermentation Vessel Operating Conditions method, and all of the Analytical
methods. Oleyl alcohol was added in a single batch between 0.1- 1.0 hr after
inoculation. The butanologen was NGCI-070.
Example 11
[00220] Experiment identifier 2010YO29 included: Seed Flask Growth method,
Initial Fermentation Vessel Preparation method, Lipase Treatment Pre-
Liquefaction method, Liquefaction method, Heat Kill Treatment during
liquefaction, the Nutrient Addition Prior to Inoculation method, Fermentation
Vessel Inoculation method, Fermentation Vessel Operating Conditions method,
and all of the Analytical methods. Corn oil fatty acids made from crude corn
oil
-76-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
was added in a single batch between 0.1-1.0 hr after inoculation. The
butanologen was NGCI-070.
Example 12
[00221] Experiment identifier 2010YO30 included: Seed Flask Growth method,
Initial Fermentation Vessel Preparation method, Lipase Treatment Pre-
Liquefaction method, Liquefaction method, Heat Kill Treatment during
liquefaction, Nutrient Addition Prior to Inoculation method with the exception
of
there being no addition of ethanol, Fermentation Vessel Inoculation method,
Fermentation Vessel Operating Conditions method, and all of the Analytical
methods. Corn oil fatty acids made from crude corn oil was added in a single
batch between 0.1- 1.0 hr after inoculation. The butanologen was NGCI-070.
Example 13 - (control)
[00222] Experiment identifier 2010YO31 included: Seed Flask Growth method,
Initial Fermentation Vessel Preparation method, Liquefaction method, Lipase
Treatment Post Liquefaction method, no Heat Kill Treatment, Nutrient Addition
Prior to Inoculation method with the exception of there being no addition of
ethanol, Fermentation Vessel Inoculation method, Fermentation Vessel
Operating Conditions method, and all of the Analytical methods. Corn oil fatty
acids made from crude corn oil was added in a single batch between 0.1- 1.0 hr
after inoculation. The butanologen was NGCI-070.
Example 14
[00223] Experiment identifier 2010YO32 included: Seed Flask Growth method,
Initial Fermentation Vessel Preparation method, Liquefaction method, Lipase
Treatment Post-Liquefaction method, no Heat Kill Treatment, Nutrient Addition
Prior to Inoculation method, Fermentation Vessel Inoculation method,
Fermentation Vessel Operating Conditions method, and all of the Analytical
methods. Corn oil fatty acids made from crude corn oil was added in a single
batch between 0.1- 1.0 hr after inoculation. The butanologen was NGCI-070.
-77-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
Table 3: Fermentation conditions for Examples 1-14
Example Experimental Lipase Max cell Ethanol Solvent Heat Kill EffIso max Eff
# Identifier Count x g/L Lipase Titer Iso rate
10' /L* /L/h
1 2010YO14 none 27.2 5 Oleyl none 56.0 0.79
alcohol
2 2010YO15 10 ppm 31.5 5 Oleyl none 52.4 0.74
alcohol
3 2010YO16 10 ppm 6.7 0 Oleyl none 25.9 0.36
alcohol
4 2010YO17 none 7.9 0 Oleyl post - 17.2 0.25
alcohol liquefaction
2010YO18 7.2 ppm 16.2 5 Oleyl post - 45.8 0.66
alcohol liquefaction
6 2010YO19 none 17.5 5 Oleyl post - 48.1 0.69
alcohol liquefaction
7 2010YO21 10 ppm 21.2 5 Oleyl during 46.8 0.82
alcohol liquefaction
8 2010YO22 none 9 5 Oleyl during 56.2 0.87
alcohol liquefaction
9 2010YO23 10 ppm 12.8 5 Corn Oil none 60.3 1.3
Fatty
Acids
2010YO24 10 ppm 25.3 0 Oleyl during 19.8 0.33
alcohol liquefaction
11 2010YO29 10 ppm 21.2 5 Corn Oil during 28.36 0.52
Fatty liquefaction
Acids
12 2010YO30 10 ppm 9 0 Corn Oil during 12.71 0.24
Fatty liquefaction
Acids
13 2010YO31 10 ppm 12.8 0 Corn Oil none 18.86 0.35
Fatty
Acids
14 2010YO32 10 ppm 25.3 5 Corn Oil none 53.36 0.92
Fatty
Acids
The "Eff Iso Titer g/L" = total grams of isobutanol produced per liter aqueous
volume
Example 15
[00224] The experimental identifier was GLNOR432A. NGCI-047 (a butanediol
producer) was grown in 25 mL medium in a 250 mL flask from a frozen vial to
1 OD. The pre-seed culture was transferred to a 2 L flask and grown to 1.7-1.8
OD. The medium for both flasks was:
3.0 g/L dextrose
3.0 g/L ethanol, anhydrous
6.7 g/L Difco Yeast Nitrogen Base without amino acids (No. 291920)
-78-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
1.4 g/L Yeast Dropout Mix (Sigma Y2001)
mL/L 1 % w/v L-Leucine stock solution
2 mL/L 1 % w/v L-Tryptophan stock solution
[00225] A 1 L, Applikon fermentation vessel was inoculated with 60 mL of the
seed
flask. The fermentation vessel contained 700 mL of the following sterile
medium:
20.0 g/L dextrose
8.0 mL/L ethanol, anhydrous
6.7 g/L Difco Yeast Nitrogen Base without amino acids (No. 291920)
2.8 g/L Yeast Dropout Mix (Sigma Y2001)
mL/L 1 % w/v L-Leucine stock solution
4 mL/L 1 % w/v L-Tryptophan stock solution
0.5 mL Sigma 204 Antifoam
0.8 mL/L 1 % w/v Ergesterol solution in 1:1::Tween 80:Ethanol
[00226] The residual glucose was kept excess with a 50% w/w glucose solution.
The dissolved oxygen concentration of the fermentation vessel was controlled
at
30% with stir control. The pH was controlled at pH=5.5. The fermentation
vessel
was sparged with 0.3 slpm of sterile, house air. The temperature was
controlled
at 30 C.
Example 16
[00227] The experimental identifier was GLNOR434A. This example is the same
as example 15 with the exception of the addition of 3 g of oleic acid and the
addition of 3 g of palmitic acid prior to inoculation. NGCI-047 (a butanediol
producer) was the biocatalyst.
[00228] FIG. 6 shows that there were more grams per liter of glucose consumed
in
the fermentation vessel that received the fatty acids. The squares represent
the
fermentation vessel that received oleic acid and palmitic acid. The circles
represent the fermentation vessel that did not receive any extra fatty acids.
-79-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
Example 17
[00229] The experimental identifier was GLNOR435A. This example was the
same as example 15 except it was inoculated with NGCI-049 (an isobutanol
producer).
Example 18
[00230] The experimental identifier was GLNOR437A. This example was the
same as Example 16 except it was inoculated with NGCI-049 (an isobutanol
producer).
[00231] FIG. 7 shows that there were more grams per liter of glucose consumed
in
the fermentation vessel that received the fatty acids. The squares represent
the
fermentation vessel that received oleic acid and palmitic acid. The circles
represent the fermentation vessel that did not receive any extra fatty acids.
Example 19
[00232] The experimental identifier was 090420_3212. This example was run
similarly to Example 15 except it was inoculated with butanologen NYLA84 (an
isobutanol producer). This fermentation was run in a 1 L Sartorius
fermentation
vessel.
Example 20
[00233] The experimental identifier was 2009Y047. This example was run
similarly to Example 16 except it was inoculated with butanologen NYLA84 (an
isobutanol producer). This fermentation was run in a 1 L Sartorius
fermentation.
[00234] FIG. 8 shows that there were more grams per liter of glucose consumed
in
the fermentation vessel that received the fatty acids. The squares represent
the
fermentation vessel that received oleic acid and palmitic acid. The circles
represent the fermentation vessel that did not receive any extra fatty acids.
The
results of Examples 15 to 20 are shown in Table 4 which shows +/- fatty acid
addition, maximum optical density, and g/L glucose consumed
-80-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
Table 4
69 hours
Fatty g/L
Experimental Acids 69 hours glucose
Example # Identifier Strain Added Product OD600 consumed
15 GLNOR432A NYLA74 - butanediol 12.8 86.0
16 GLNOR434A NYLA74 + butanediol 23.1 95.9
17 GLNOR435A NYLA74 - isobutanol 2.4 16.9
18 GLNOR437A NYLA74 + isobutanol 4.5 18.3
19 0904203212 NYLA84 - isobutanol 9.6 39.3
20 2009YO47 NYLA84 + isobutanol 20.2 49.1
EXAMPLE 21
Lipase treatment of Liquefied Corn Mash for Simultaneous Saccharification and
Fermentation with In-situ Product Removal Using Oleyl Alcohol
[00235] Samples of broth and oleyl alcohol taken from fermentations run as
described above in Examples 1, 2, and 3 were analyzed for wt% lipid
(derivatized
as fatty acid methyl esters, FAME) and for wt% free fatty acid (FFA,
derivatized
as fatty acid methyl esters, FAME) according to the method described by E. G.
Bligh and W. J. Dyer (Canadian Journal of Biochemistry and Physiology, 37:911-
17, 1959, hereafter Reference 1). The liquefied corn mash that was prepared
for
each of the three fermentations was also analyzed for wt% lipid and for wt%
FFA
after treatment with Lipolase 100 L (Novozymes) (10 ppm of Lipolase total
soluble protein (BCA protein analysis, Sigma Aldrich)) per kg of liquefaction
reaction mass containing 30 wt% ground corn kernels). No lipase was added to
the liquefied corn mash in Example 1 (control), and the fermentations
described
in Examples 2 and 3 containing liquefied corn mash treated with lipase (no
heat
inactivation of lipase) were identical except that no ethanol was added to the
fermentation described in Example 3.
[00236] The % FFA in lipase-treated liquefied corn mash prepared for
fermentations run as described in Examples 2 and 3 was 88% and 89%,
respectively, compared to 31 % without lipase treatment (Example 1). At 70 h
(end of run (EOR)), the concentration of FFA in the OA phase of fermentations
run as described in Examples 2 and 3 (containing active lipase) was 14% and
20%, respectively, and the corresponding increase in lipids (measured as corn
oil
-81-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
fatty acid methyl ester derivatives) was determined by GC/MS to be due to the
lipase-catalyzed esterification of COFA by OA, where COFA was first produced
by lipase-catalyzed hydrolysis of corn oil in the liquefied corn mash. Results
are
shown in Table 5.
Table 5: Lipid and free fatty acid content of fermentations containing oleyl
alcohol as
ISPR solvent and active lipase
fermentation lipase time (h), lipids FFA lipids FFA lipids + % FFA
sample (wt%) (wt%) (g) (g) FFA(g)
Example 1 none liq. mash 0.61 0.28 5.3 2.4 7.7 31
Example 1 none 0.8 h, broth 0.49 0.22 5.5 2.5 8.0 31
Example 1 none 31 h, broth 0.19 0.03 2.1 0.3 2.4 13
Example 1 none 31 h, OA 0.36 0.21 3.4 2.0 5.3 37
Example 1 none 70 h, broth 0.15 0.03 1.7 0.3 2.0 15
Example 1 none 70 h, OA 0.57 0.25 5.3 2.3 7.7 31
Example 2 10 ppm liq. mash 0.13 0.97 1.1 8.5 9.6 88
Example 2 10 ppm 0.8 h, broth 0.15 0.62 1.7 7.0 8.7 81
Example 2 10 ppm 31 h, broth 0.16 0.05 1.8 0.5 2.3 23
Example 2 10 ppm 31 h, OA 0.37 0.23 3.5 2.2 5.7 38
Example 2 10 ppm 70 h, broth 0.17 0.02 1.9 0.3 2.2 13
Example 2 10 ppm 70 h, OA 0.60 0.10 5.7 1.0 6.7 14
Example 3 10 ppm liq. mash 0.12 0.97 1.0 8.5 9.5 89
Example 3 10 ppm 0.8 h, broth 0.32 0.40 3.6 4.5 8.1 56
Example 3 10 ppm 31 h, broth 0.17 0.05 1.9 0.6 2.5 24
Example 3 10 ppm 31 h, OA 0.38 0.22 3.6 2.1 5.7 37
Example 3 10 ppm 70 h, broth 0.15 0.02 1.7 0.2 1.9 13
Example 3 10 ppm 70 h, OA 0.46 0.12 4.4 1.1 5.6 20
EXAMPLE 22
Heat Inactivation of Lipase in Lipase-treated Liquefied Corn Mash to Limit
Production of Oleyl Alcohol Esters of Corn Oil Free Fatty Acids
[00237] Tap water (918.4 g) was added to a jacketed 2-L resin kettle, then
474.6 g
wet weight (417.6 g dry weight) of ground whole corn kernels (1.0 mm screen on
hammer mill) was added with stirring. The mixture was heated to 55 C with
stirring at 300 rpm, and the pH adjusted to 5.8 with 2 N sulfuric acid. To the
mixture was added 14.0 g of an aqueous solution containing 0.672 g of
Spezyme -FRED L (Genencor , Palo Alto, CA), and the temperature of the
mixture increased to 85 C with stirring at 600 rpm and pH 5.8. After 120
minutes
at 85 C, the mixture was cooled to 50 C and 45.0 mL aliquots of the resulting
-82-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
liquefied corn mash were transferred to 50-mL polypropylene centrifuge tubes
and stored frozen at -80 C.
[00238] In a first reaction, 50 g of liquefied corn mash prepared as described
above was mixed with 10 ppm Lipolase 100 L (Novozymes) for 6 h at 55 C and
with no inactivation of lipase at 85 C for 1 h, the mixture was cooled to 30
C. In a
second reaction, 50 g of liquefied corn mash was mixed with 10 ppm Lipolase
for 6 h at 55 C, then heated to 85 C for 1 h (lipase inactivation), then
cooled to
30 C. In a third reaction, 50 g of liquefied corn mash without added lipase
was
mixed for 6 h at 55 C, and with no heating at 85 C for 1 h, the mixture was
cooled to 30 C, 38 g of oleyl alcohol was added, and the resulting mixture
stirred
for 73 h at 30 C. In a fourth reaction, 50 g of liquefied corn mash without
added
lipase was mixed for 6 h at 55 C, then heated to 85 C for 1 h, then cooled to
30 C. Each of the four reaction mixtures was sampled at 6 h, then 38 g of
oleyl
alcohol added, and the resulting mixtures stirred at 30 C and sampled at 25 h
and 73 h. Samples (both liquefied mash and oleyl alcohol (OA)) were analyzed
for wt% lipid (derivatized as fatty acid methyl esters, FAME) and for wt% free
fatty
acid (FFA, derivatized as fatty acid methyl esters, FAME) according to the
method described by Reference 1.
[00239] The % FFA in the OA phase of the second reaction run with heat
inactivation of lipase prior to OA addition was 99% at 25 h and 95% at 73 h,
compared to only 40% FFA and 21% FFA at 25 h and 73 h, respectively, when
the lipase in lipase-treated liquefied corn mash was not heat inactivated
(first
reaction). No significant change in % FFA was observed in the two control
reactions without added lipase. Results are shown in Table 6.
Table 6: Lipid and free fatty acid content of a mixture of liquefied corn mash
and
oleyl alcohol in the presence or absence of active or heat-inactivated lipase
reaction time (h), lipids FFA lipids FFA lipid+FFA % FFA
conditions sample (wt%) (wt%) (mg) (mg) (mg)
ppm active lipase, 6 h, liq. mash 0.08 0.71 41 345 386 89
no 85 C heat treatment 25 h, liq. mash 0.22 0.06 105 27 132 20
25 h, OA 0.58 0.39 212 143 355 40
73 h, liq. mash 0.25 0.05 121 22 143 18
73 h, OA 0.91 0.24 333 88 420 21
-83-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
ppm inactive lipase, 6 h, liq. mash 0.06 0.45 28 224 252 89
85 C heat treatment 25 h, liq. mash 0.10 0.11 49 54 103 53
25 h, OA 0.02 0.96 8 366 374 99
73 h, liq. mash 0.24 0.15 117 72 189 62
73 h, OA 0.06 1.11 23 424 447 95
no lipase, 6 h, liq. mash 0.80 0.40 401 199 599 33
no 85 C heat treatment 25 h, liq. mash 0.30 0.05 147 25 173 15
25 h, OA 0.55 0.36 212 139 351 40
73 h, liq. mash 0.23 0.05 117 26 143 23
73 h, OA 0.79 0.42 305 162 467 34
no lipase, 6 h, liq. mash 0.74 0.36 370 183 553 33
85 C heat treatment 25 h, liq. mash 0.31 0.05 156 27 183 15
25 h, OA 0.60 0.35 233 136 369 37
73 h, liq. mash 0.20 0.05 99 23 121 23
73 h, OA 0.84 0.41 326 159 486 33
EXAMPLE 23
Heat Inactivation of Lipase in Lipase-treated Liquefied Corn Mash for
Simultaneous Saccharification and Fermentation with In-situ Product Removal
Using Oleyl Alcohol
[00240] Three fermentations were run as described above in Examples 4, 5, and
6.
No lipase was added to the liquefied corn mash in Examples 4 and 6 prior to
fermentation, and the Lipase Treatment of the liquefied corn mash in the
fermentation described in Example 5 (using 7.2 ppm of Lipolase total soluble
protein) was followed immediately by Heat Inactivation Treatment (to
completely
inactivate the lipase), and subsequently followed by Nutrient Addition Prior
to
Inoculation and fermentation. The % FFA in liquefied corn mash prepared
without lipase treatment for fermentations run as described in Examples 4 and
6
was 31 % and 34%, respectively, compared to 89% with lipase treatment
(Example 5). Over the course of the fermentations listed in Table 10, the
concentration of FFA in the OA phase did not decrease in any of the three
fermentations, including that containing heat-inactivated lipase. The % FFA in
the OA phase of the fermentation run according to Example 5 (with heat
inactivation of lipase prior to fermentation) was 95% at 70 h (end of run
(EOR)),
compared to only 33% FFA for the remaining two fermentations (Examples 4 and
-84-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
6) where liquefied corn mash was not treated with lipase. Results are shown in
Table 7.
Table 7: Lipid and free fatty acid content of fermentations containing oleyl
alcohol as
ISPR solvent and heat-inactivated lipase (after lipase treatment of liquefied
mash)
fermentation lipase time (h), lipids FFA lipids FFA lipid + % FFA
sample (wt%) (wt%) (g) (g) FFA (g)
Example 4 none liquefied mash 0.65 0.30 7.2 3.3 10.4 31
Example 4 none 0.2 h, broth 0.56 0.28 6.6 3.3 9.9 33
Example 4 none 4.3 h, broth 0.28 0.09 3.3 1.0 4.4 24
Example 4 none 4.3 h, OA 0.45 0.27 4.0 2.4 6.4 37
Example 4 none 30 h, broth 0.17 0.05 2.0 0.6 2.7 24
Example 4 none 30 h, OA 0.63 0.29 5.7 2.6 8.3 32
Example 4 none 53 h, broth 0.13 0.04 1.5 0.5 2.0 23
Example 4 none 53 h, OA 0.67 0.32 6.0 2.9 8.9 32
Example 4 none 70 h, broth 0.13 0.04 1.5 0.4 1.9 23
Example 4 none 70 h, OA 0.64 0.31 5.8 2.8 8.5 33
Example 5 7.2 ppm liquefied mash 0.11 0.89 1.3 9.9 11.2 89
Example 5 7.2 ppm 0.2 h, broth 0.25 0.83 2.9 9.8 12.8 77
Example 5 7.2 ppm 4.3 h, broth 0.14 0.17 1.6 2.1 3.7 56
Example 5 7.2 ppm 4.3 h, OA 0.02 0.84 0.2 7.9 8.1 97
Example 5 7.2 ppm 30 h, broth 0.08 0.18 1.0 2.1 3.1 68
Example 5 7.2 ppm 30 h, OA 0.04 0.92 0.3 8.6 8.9 96
Example 5 7.2 ppm 53 h, broth 0.07 0.11 0.9 1.3 2.2 61
Example 5 7.2 ppm 53 h, OA 0.08 0.95 0.7 8.9 9.6 93
Example 5 7.2 ppm 70 h, broth 0.08 0.10 0.9 1.2 2.1 55
Example 5 7.2 ppm 70 h, OA 0.05 0.94 0.4 8.8 9.2 95
Example 6 none liquefied mash 0.66 0.34 7.3 3.8 11.1 34
Example 6 none 0.2 h, broth 0.63 0.34 7.6 4.0 11.6 34
Example 6 none 4.3 h, broth 0.33 0.10 3.9 1.2 5.1 23
Example 6 none 4.3 h, OA 0.45 0.27 4.0 2.4 6.4 38
Example 6 none 30 h, broth 0.17 0.06 2.1 0.8 2.8 26
Example 6 none 30 h, OA 0.69 0.33 6.2 3.0 9.1 32
Example 6 none 53 h, broth 0.14 0.05 1.6 0.5 2.2 25
Example 6 none 53 h, OA 0.72 0.35 6.4 3.1 9.5 33
Example 6 none 70 h, broth 0.15 0.05 1.8 0.6 2.4 25
Example 6 none 70 h, OA 0.70 0.34 6.2 3.0 9.2 33
EXAMPLE 24
Lipase treatment of Ground Whole Corn Kernels prior to Liquefaction
[00241] Tap water (1377.6 g) was added into each of two jacketed 2-L resin
kettles, then 711.9 g wet weight (625.8 g dry weight) of ground whole corn
kernels (1.0 mm screen on hammer mill) was added to each kettle with stirring.
-85-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
Each mixture was heated to 55 C with stirring at 300 rpm, and the pH adjusted
to
5.8 with 2 N sulfuric acid. To each mixture was added 21.0 g of an aqueous
solution containing 1.008 g of Spezyme -FRED L (Genencor , Palo Alto, CA).
To one mixture was then added 10.5 mL of aqueous solution of Lipolase 100L
Solution (21 mg total soluble protein, 10 ppm lipase final concentration) and
to
the second mixture was added 1.05 mL of aqueous solution of Lipolase 100L
Solution (2.1 mg total soluble protein, 1.0 ppm lipase final concentration).
Samples were withdrawn from each reaction mixture at 1 h, 2 h, 4 h and 6 h at
55 C, then the temperature of the mixture was increased to 85 C with stirring
at
600 rpm and pH 5.8, and a sample was taken when the mixture first reached
85 C. After 120 minutes at 85 C, a sample was taken and the mixtures were
cooled to 50 C and final samples of the resulting liquefied corn mash were
transferred to 50-mL polypropylene centrifuge tubes; all samples were stored
frozen at -80 C.
[00242] In two separate reactions, a 50 g sample of the 10 ppm lipase-treated
liquefied corn mash or a 55 g sample of the 1.0 ppm lipase-treated liquefied
corn
mash prepared as described above was mixed with oleyl alcohol (OA) (38 g) at
30 C for 20 h, then the liquefied mash and OA in each reaction mixture were
separated by centrifugation and each phase analyzed for wt% lipid (derivatized
as fatty acid methyl esters, FAME) and for wt% free fatty acid (FFA,
derivatized
as fatty acid methyl esters, FAME) according to the method described by
Reference 1. The % FFA in the OA phase of the liquefied mash/OA mixture
prepared using heat inactivation of 10 ppm lipase during liquefaction was 98%
at
20 h, compared to only 62% FFA in the OA phase of the liquefied mash/OA
mixture prepared using heat inactivation of 1.0 ppm lipase during
liquefaction.
Results are shown in Table 8.
-86-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
Table 8: Lipid and free fatty acid content of a mixture of liquefied corn mash
and oleyl
alcohol, using lipase treatment of ground corn suspension prior to
liquefaction
(heat inactivation of lipase during liquefaction)
reaction time (h), sample lipids FFA lipids FFA lipid+FFA % FFA
conditions (wt%) (wt%) (mg) (mg) (mg)
ppm lipase 1 h, pre-liquefaction 0.226 0.627 112 311 424 74
at 55 C prior to 2 h, pre-liquefaction 0.199 0.650 99 323 422 77
liquefaction at 4 h, pre-liquefaction 0.151 0.673 75 334 410 82
85 C, mix with 6 h, pre-liquefaction 0.101 0.700 50 348 398 87
OA for 20 h 0 h, 85 C, liq. mash 0.129 0.764 64 380 444 86
2 h, 85 C, liq. mash 0.129 0.751 64 373 437 85
h, 30 C, liq. mash 0.074 0.068 37 34 71 48
20 h, 30 C, OA 0.015 1.035 5.7 394 400 98
1.0 ppm lipase 1 h, pre-liquefaction 0.408 0.480 226 266 492 54
at 55 C prior to 2 h, pre-liquefaction 0.401 0.424 222 235 457 51
liquefaction at 4 h, pre-liquefaction 0.299 0.433 165 240 405 58
85 C, mix with 6 h, pre-liquefaction 0.346 0.453 192 251 442 57
OA for 20 h 0 h, 85 C, liq. mash 0.421 0.407 233 225 458 49
2 h, 85 C, liq. mash 0.424 0.429 235 237 472 50
20 h, 30 C, liq. mash 0.219 0.054 121 30 151 20
20 h, 30 C, OA 0.344 0.573 140 233 373 62
EXAMPLE 25
Lipase Screening for Treatment of Ground Whole Corn Kernels prior to
Liquefaction
[00243] Seven reaction mixtures containing tap water (67.9 g) and ground whole
corn kernels (35.1 g wet wt., ground with 1.0 mm screen using a hammer mill)
at
pH 5.8 were stirred at 55 C in stoppered flasks. A 3-mL sample (t = 0 h) was
removed from each flask and the sample immediately frozen on dry ice, then ca.
0.5 mL of 10 mM sodium phosphate buffer (pH 7.0) containing 1 mg total soluble
protein (10 ppm final concentration in reaction mixture) of one of the
following
lipases (Novozymes) were added to one of each flask: Lipolase 100 L, Lipex
100L, Lipoclean 2000T, Lipozyme CALB L, Novozyme CALA L, and
Palatase 20000L; no lipase was added to the seventh flask. The resulting
mixtures were stirred at 55 C in stoppered flasks, and 3-mL samples were
withdrawn from each reaction mixture at 1 h, 2 h, 4 h and 6 h and immediately
frozen in dry ice until analyzed for wt% lipid (derivatized as fatty acid
methyl
esters, FAME) and for wt% free fatty acid (FFA, derivatized as fatty acid
methyl
-87-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
esters, FAME) according to the method described by Reference 1, and the
percent free fatty acid content was calculated relative to the total combined
concentrations of lipid and free fatty acid was determined for each sample.
Results are shown in Table 9.
Table 9: Percent free fatty acid content (% FFA) of a mixture of ground whole
corn kernels using lipase treatment at 55 C prior to liquefaction
% FFA
time Oh 1 h 2h 4h 6h
Lipolase 100L 33 56 74 76 79
Lipex 100L 34 66 81 83 83
Lipoclean 2000T 38 55 73 69 65
Lipozyme CALB L 39 38 37 43 41
Novozyme CALA L 37 40 44 44 45
Palatase 20000L 37 49 59 62 66
no enzyme 38 33 37 41 42
EXAMPLE 26
Lipase treatment of Ground Whole Corn Kernels prior to Simultaneous
Saccharification and Fermentation with In-situ Product Removal Using Oleyl
Alcohol
[00244] Three fermentations were run as described above in Examples 7, 8, and
10. For fermentations run as described in Examples 7 and 10, lipase (10 ppm of
Lipolase total soluble protein) was added to the suspension of ground corn
and
heated at 55 C for 6 h prior to Liquefaction to produce a liquefied corn mash
containing heat-inactivated lipase. No lipase was added to the suspension of
ground corn used to prepare liquefied corn mash for the fermentation described
in Example 8, but the suspension was subjected to the same heating step at
55 C prior to liquefaction. The % FFA in lipase-treated liquefied corn mash
prepared for fermentations run as described in Examples 7 and 10 was 83% and
86%, respectively, compare to 41% without lipase treatment (Example 8). Over
the course of the fermentations, the concentration of FFA did not decrease in
any
of the fermentations, including that containing heat-inactivated lipase. The %
FFA in the OA phase of the fermentation run according to Examples 7 and 10
-88-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
(with heat inactivation of lipase prior to fermentation) was 97% at 70 h (end
of run
(EOR)), compared to only 49% FFA for the fermentation run according to
Example 8 where ground whole corn kernels had not been treated with lipase
prior to liquefaction. Results are shown in Table 10.
Table 10: Lipid and free fatty acid content of fermentations containing oleyl
alcohol as ISPR solvent and heat-inactivated lipase (lipase treatment of
ground
corn suspension prior to liquefaction)
fermentation lipase time (h), sample lipids FFA lipids FFA lipid + % FFA
(wt%) (wt%) (g) (g) FFA(g)
Example 7 10 ppm pre-lipase/pre-liq. 0.65 0.22 7.1 2.4 9.4 25
Example 7 10 ppm post-lipase/pre-liq. 0.22 0.65 2.4 7.0 9.5 74
Example 7 10 ppm liquefied mash 0.17 0.79 1.8 8.5 10.3 83
Example 7 10 ppm 0.3 h, broth 0.16 0.79 1.8 8.9 10.7 83
Example 7 10 ppm 4.8 h, broth 0.14 0.31 1.6 3.5 5.1 69
Example 7 10 ppm 4.8 h, OA 0.04 0.68 0.3 5.4 5.6 95
Example 7 10 ppm 29 h, broth 0.10 0.12 1.2 1.3 2.5 53
Example 7 10 ppm 29 h, OA 0.03 1.05 0.2 8.2 8.4 98
Example 7 10 ppm 53 h, broth
Example 7 10 ppm 53 h, OA 0.07 1.14 0.5 9.0 9.5 95
Example 7 10 ppm 70 h, broth 0.11 0.07 1.2 0.8 2.0 39
Example 7 10 ppm 70 h, OA 0.03 1.10 0.2 8.7 8.9 97
Example 8 none pre-lipase/pre-liq. 0.62 0.23 6.7 2.5 9.2 27
Example 8 none post-lipase/pre-liq. 0.57 0.26 6.2 2.8 9.0 31
Example 8 none liquefied mash 0.52 0.36 5.6 4.0 9.6 41
Example 8 none 0.3 h, broth 0.50 0.33 5.7 3.8 9.4 40
Example 8 none 4.8 h, broth 0.47 0.14 5.3 1.6 6.9 24
Example 8 none 4.8 h, OA 0.12 0.32 1.0 2.9 3.9 73
Example 8 none 29 h, broth 0.30 0.05 3.4 0.6 4.0 16
Example 8 none 29 h, OA 0.31 0.46 2.7 4.1 6.9 60
Example 8 none 53 h, broth
Example 8 none 53 h, OA 0.47 0.50 4.2 4.4 8.6 51
Example 8 none 70 h, broth 0.22 0.04 2.5 0.5 3.0 17
Example 8 none 70 h, OA 0.40 0.39 3.6 3.5 7.0 49
Example 10 10 ppm pre-lipase/pre-liq. 0.67 0.23 7.4 2.5 9.9 25
Example 10 10 ppm post-lipase/pre-liq. 0.19 0.69 2.1 7.6 9.7 78
Example 10 10 ppm liquefied mash 0.14 0.85 1.6 9.4 11.0 86
Example 10 10 ppm 0.3 h, broth 0.13 0.82 1.5 9.4 10.9 86
Example 10 10 ppm 4.8 h, broth 0.11 0.29 1.3 3.3 4.6 72
Example 10 10 ppm 4.8 h, OA 0.04 0.60 0.3 5.2 5.6 94
Example 10 10 ppm 29 h, broth 0.09 0.14 1.0 1.6 2.6 61
Example 10 10 ppm 29 h, OA 0.01 0.96 0.1 8.4 8.5 99
Example 10 10 ppm 53 h, broth
Example 10 10 ppm 53 h, OA 0.02 0.95 0.2 8.3 8.4 98
Example 10 10 ppm 70 h, broth 0.09 0.08 1.1 0.9 1.9 45
Example 10 10 ppm 70 h, OA 0.03 0.99 0.3 8.7 9.0 97
-89-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
EXAMPLE 27
Lipase treatment of Ground Whole Corn Kernels or Liquefied Corn Mash for
Simultaneous Saccharification and Fermentation with In-situ Product Removal
Using Corn Oil Fatty Acids (COFA)
[00245] Five fermentations were run as described above in Examples 9, 11, 12,
13, and 14. For the fermentations run as described in Examples 9, 13, and 14,
lipase (10 ppm of Lipolase total soluble protein) was added after
Liquefaction
and there was no heat-inactivation of lipase. Fermentations run as described
in
Examples 9 and 14 had 5 g/L of ethanol added prior to inoculation, whereas the
fermentation run as described in Example 13 had no added ethanol. The
fermentations run as described in Examples 11 and 12 employed the addition of
ppm Lipolase total soluble protein to the suspension of ground corn prior to
liquefaction, resulting in heat inactivation of lipase during liquefaction.
The
fermentation run as described in Example 11 had 5 g/L of ethanol added prior
to
inoculation, whereas the fermentation run as described in Example 12 had no
added ethanol. The final total grams of isobutanol (i-BuOH) present in the
COFA
phase of the fermentations containing active lipase was significantly greater
than
the final total grams of i-BuOH present in the COFA phase of the fermentations
containing inactive lipase. The final total grams of isobutanol (i-BuOH)
present in
the fermentation broths containing active lipase were only slightly less than
the
final total grams of i-BuOH present in the fermentation broths containing
inactive
lipase, such that the overall production of i-BuOH (as a combination of free i-
BuOH and isobutyl esters of COFA (FABE)) was significantly greater in the
presence of active lipase when compared to that obtained in the presence of
heat-inactivated lipase. Results are shown in Tables 11 and 12.
-90-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
Table 11: Dependence of the production of free isobutanol (i-BuOH) and
isobutyl
esters of COFA (FABE) in fermentations containing corn oil fatty acids (COFA)
as
ISPR solvent on presence (Examples 9, 13, and 14) or absence (Examples 11 and
12) of active lipase (COFA phase analysis)
fermentation g i-BuOH/ g FABE/ g i-BuOH from FABE/ total g i-BuOH/
fermentation time (h) kg COFA kg COFA kg COFA kg COFA
Example 9 4.5 2.4 0.0 0 2.4
Example 9 28.8 5.4 70.9 16.5 22.0
Example 9 52.4 8.9 199.0 46.4 55.3
Example 9 69.3 4.9 230.9 53.9 69.3
Example 11 6.6 2.3 0.0 0.0 2.3
Example 11 53.5 25.1 2.9 0.6 25.7
Example 11 71.1 24.4 6.3 1.4 25.8
Example 12 6.6 2.3 0.0 0.0 2.3
Example 12 53.5 12.8 1.6 0.4 13.2
Example 12 71.1 12.8 3.0 0.7 13.5
Example 13 6.6 2.3 0.0 0.0 2.3
Example 13 53.5 4.9 72.1 16.0 20.9
Example 13 71.1 4.6 91.4 20.3 24.9
Example 14 6.6 2.1 0.0 0.0 2.1
Example 14 53.5 9.8 197.2 43.8 53.6
Example 14 71.1 4.9 244.5 54.3 59.2
Table 12: Dependence of the production of free isobutanol (i-BuOH) and
isobutyl
esters of COFA (FABE) in fermentations containing corn oil fatty acids (COFA)
as
ISPR solvent on presence (Examples 9, 13, and 14) or absence (Examples 11 and
12) of active lipase (fermentation broth analysis)
fermentation g i-BuOH/ g FABE/ g i-BuOH from FABE/ total g i-BuOH/
sample time (h) kg broth kg broth kg broth kg broth
Example 9 4.5 0.0 0.0 0 0
Example 9 28.8 0.0 12.6 2.9 2.9
Example 9 52.4 0.0 30.3 7.1 7.1
Example 9 69.3 0.0 24.7 5.8 5.8
Example 11 6.6 0.0 0.0 0 0.0
Example 11 53.5 9.8 0.0 0 9.8
Example 11 71.1 9.5 0.0 0 9.5
Example 12 6.6 0.0 0.0 0 0
Example 12 53.5 3.8 0.0 0.0 3.8
Example 12 71.1 5.1 0.0 0.0 5.1
-91-
CA 02800542 2012-11-22
WO 2011/159962 PCT/US2011/040796
Example 13 6.6 0.0 0.0 0 0
Example 13 53.5 2.1 3.0 0.7 2.8
Example 13 71.1 2.1 7.4 1.6 3.7
Example 14 6.6 0.0 0.0 0 0.0
Example 14 53.5 2.9 22.4 5.0 7.9
Example 14 71.1 3.3 19.3 4.3 7.6
[00246] While various embodiments of the present invention have been described
above, it should be understood that they have been presented by way of example
only, and not limitation. It will be apparent to persons skilled in the
relevant art
that various changes in form and detail can be made therein without departing
from the spirit and scope of the invention. Thus, the breadth and scope of the
present invention should not be limited by any of the above-described
exemplary
embodiments, but should be defined only in accordance with the following
claims
and their equivalents.
[00247] All publications, patents and patent applications mentioned in this
specification are indicative of the level of skill of those skilled in the art
to which
this invention pertains, and are herein incorporated by reference to the same
extent as if each individual publication, patent or patent application was
specifically and individually indicated to be incorporated by reference.
-92-