Note: Descriptions are shown in the official language in which they were submitted.
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
PROTEIN COMPOSITIONS AND METHODS OF MAKING AND USING
THEREOF
CROSS REFERENCE TO RELATED APPLICATION
This application claims priority upon U.S. provisional application Serial No.
61/181,807, filed May 28, 2009. This application is hereby incorporated by
reference
in its entirety for all of its teachings.
BACKGROUND
In recent years, consumption of poultry meat and further processed poultry
products has greatly increased worldwide. One of the reasons for the increased
consumer preference for poultry products is the greater availability of choice
poultry
cuts, such as wings, thighs and breast. Consequently, in the U.S., the
consumption of
chicken and turkey from 1950 to 2007 increased from 12 to 52 kg per capita. In
Canada, per capita consumption of chicken was 21.5 kg in 1989 and reached 31.8
kg
in 2008. As a result, considerable quantity of poultry carcass parts, such as
necks,
backs, and drumsticks, have become available. This is referred to as "low
value"
poultry meat. Utilization of these less desirable parts can be achieved
through
mechanical deboning to produce mechanically separated poultry meat (MSPM) for
the manufacture of variety meats, canned meats and emulsified-type products.
The main problem encountered with MSPM is due to its method of
production, which includes grinding meat and bones together and forcing the
mixture
through a perforated drum with consequent separation into two fractions, such
as
mechanically separated meat paste and bone residue. This causes the release of
a
considerable amount of fat and heme components from the bone marrow which
becomes incorporated into the meat product, and thorough disruption of muscle
cells.
Hence, the fundamental problems with proper utilization of MSPM are the high
content of lipids, pigments and connective tissue, which lead to dark meat
color,
susceptibility to lipid oxidation, undesired textural properties and sometimes
unpleasant odor due to the rancidity of fat. These properties may result in
problems
1
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
with further processing and consumer acceptance.
The meat industry often loses vast amounts of revenue each year due to the
inability to efficiently recover and use "low value" meat. Even though it may
have
viable industrial applications, this meat is often discarded as waste post-
meat
processing and is generally viewed as a useless by-product of meat processing.
Therefore, it would be desirable to find a use for "low value" poultry meat.
SUMMARY
Described herein are the isolation and use of meat proteins and their
applications thereof. In one aspect, meat proteins such as, for example, fish,
poultry,
bovine, or porcine can be used to make films, meat binders or extenders, and
extrudable food articles. The advantages of the invention will be set forth in
part in
the description which follows, and in part will be obvious from the
description, or
may be learned by practice of the aspects described below. The advantages
described
below will be realized and attained by means of the elements and combinations
particularly pointed out in the appended claims. It is to be understood that
both the
foregoing general description and the following detailed description are
exemplary
and explanatory only and are not restrictive.
BRIEF DESCRIPTION OF THE DRAWINGS
The accompanying drawings, which are incorporated in and constitute a part
of this specification, illustrate several aspects described below.
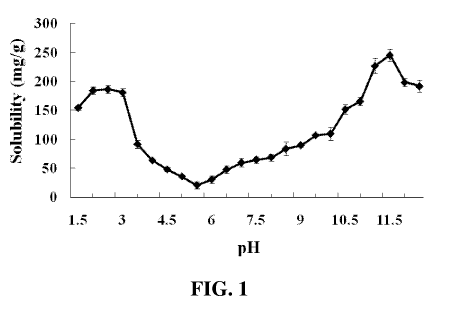
Figure 1 shows the solubility (mg/g) profile of mechanically separated turkey
meat (MSTM) proteins at pH values from 1.5 to 12Ø Muscle tissue was
homogenized in 50 volumes of deionized water and pH was adjusted by using 0.2
M
and 1 M HCl or NaOH. Results are presented as mean (n=4) standard deviation.
Figure 2 shows the extractability of proteins recovered from MSTM by acid-
and alkaline-aided extractions. Sarcoplasmic proteins were solubilized in
phosphate
buffer, while total proteins were solubilized in phosphate buffer (pH 7.4)
containing
potassium iodide. Results are presented as mean (n=4) standard deviation.
Different
2
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
letters for respective parameters in the figure represent significant (P <
0.05)
difference.
Figure 3 shows the surface hydrophobicity of myofibrillar and sarcoplasmic
proteins at different extraction pH values. Hydrophobicity is expressed as
initial
slopes of relative fluorescence intensity versus protein concentration in the
presence
of 1-anilino-8-napthalenesulfonate. Results are presented as mean (n=4)
standard
deviation. Different letters for respective parameters in the figure represent
significant
(P < 0.05) difference.
Figure 4 shows the total and reactive (free) sulfhydryl content of proteins
recovered from MSTM at different extraction pH values. Analyses were performed
by
using Ellman's reagent. Results are presented as mean (n=4) standard
deviation.
Figure 5 shows the sodium dodecyl sulfate-polyacrylamide gel electrophoresis
patterns of different samples from acid and alkaline extraction processes.
Lane 1 is the
molecular weight standard. Lanes 2, 3, 4 and 5 refer to the protein after
isoelectric
precipitation for extraction at pH values of 2.5; 3.5; 10.5 and 11.5,
respectively. Lane
6 represents MSTM. Lanes 7, 8, 9 and 10 refer to the final protein isolate for
extraction at pH values of 2.5; 3.5; 10.5 and 11.5, respectively.
*MHC - myosin heavy chains.
**MLC - myosin light chains.
Figure 6 shows the effect of time and extraction pH on oxidative stability of
protein recovered from MSTM as determined by induced thiobarbituric acid
relative
substances (TBARs). Results are presented as mean (n=4) standard deviation.
Figure 7 shows cooking loss of proteins recovered from MSTM at different
extraction pH. No statistical differences were observed. Data were
statistically
analyzed by one-way ANOVA. Results are presented as mean (n=4) standard
deviation.
Figure 8 shows expressible moisture (expressed as a water loss) of proteins
recovered from MSTM at different extraction pH. Results are presented as mean
3
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
(n=6) standard deviation. Different alphabetical letters in the figure
represent
significant (P < 0.05) difference between means.
Figure 9 shows the total heme pigments content of proteins recovered from
MSTM as different extraction pH. The total original material (raw MSTM)
contained
3.77 mg of total heme pigments per lg of meat. Results are presented as mean
(n=3)
standard deviation. Different alphabetical letters in the figure represent
significant (P
< 0.05) difference between means.
Figure 10 shows the hardness, chewiness, springiness and cohesiveness of
proteins recovered from MSTM at different extraction pH. No statistical
differences
were observed. Data were statistically analyzed by one-way ANOVA. Results are
presented as mean (n=3) standard deviation.
Figure 11 shows the changes in dynamic viscoelastic behaviour (DVB) of
proteins recovered from MSTM at different extraction pH. The samples were
prepared with 2.5% of NACI additon. The rheograms show storage modulus (G),
loss
modulus (G") and tan delta (8) development during heating from 4 to 80 C at 2
C/min.
Figure 12 shows the average storage modulus (G', kPa) at 5, 56.6 and 80 C
for proteins recovered from MSTM at different extraction pH. Results are
presented
as mean (n=4) standard deviation. Different alphabetical letters in the
figure
represent significant (P < 0.05) difference between means.
Figure 13 shows the changes in dynamic viscoelastic behaviour (DVB) of
proteins recovered from MSTM at different extraction pH. The samples were
prepared with 2.5% of NACI addition. The rheograms show storage modulus (G),
loss modulus (G") and tan delta (8) development during cooling from 80 to 4 C
at 2
C/min.
Figure 14 shows an exemplary method for solubilizing and isolating a
poultry protein.
Figure 15 shows a poultry meat slurry mixed with an acidic or a basic
4
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
solution prior to centrifugation.
Figure 16 shows a solubilized protein layer after contacting the poultry
meat slurry with an acidic or a basic solution followed by centrifugation.
Figure 17 shows a biopolymer film made by the methods described
herein.
DETAILED DESCRIPTION
Before the present compounds, compositions, and/or methods are disclosed
and described, it is to be understood that the aspects described below are not
limited
to specific compounds, synthetic methods, or uses as such may, of course,
vary. It is
also to be understood that the terminology used herein is for the purpose of
describing
particular aspects only and is not intended to be limiting.
In this specification and in the claims that follow, reference will be made to
a
number of terms that shall be defined to have the following meanings:
It must be noted that, as used in the specification and the appended claims,
the
singular forms "a," "an" and "the" include plural referents unless the context
clearly
dictates otherwise. Thus, for example, reference to "a protein" includes
mixtures of
two or more such proteins, and the like.
"Optional" or "optionally" means that the subsequently described event or
circumstance can or cannot occur, and that the description includes instances
where
the event or circumstance occurs and instances where it does not. For example,
the
phrase "optionally a flaxseed oil" means that the flaxseed oil can or can not
be
included.
Described herein are the isolation and use of meat proteins and their
applications thereof. Using the techniques described herein, meat such as, for
example, fish, poultry, bovine, or porcine can be used to make films, meat
binders or
extenders, and extrudable food articles.
Proteins as defined herein may include either a non-post-translationally
modified protein, a post-translationally modified protein, or a combination
thereof. In
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
one aspect, the protein may include a full-length protein, full-length
proteins, or
peptide fragments of a full-length protein(s). In one aspect, the protein can
be derived
from an animal such as a fish, bird, cow, or pig, and in another aspect, the
protein can
be derived utilizing recombinant DNA technology. If derived from an animal,
the
protein can be obtained, for example, during meat processing where meat is
separated
from the bone of the animal. During meat processing, what is termed "low
value"
meat may adhere to the bone after processing. In one aspect, this meat is
skeletal
muscle, which is composed of myofibrillar protein.
In certain aspects, the protein may include, for example, a poultry protein.
Although poultry protein can be derived from "low value" meat, poultry protein
can
be isolated from any poultry meat. Examples of poultry meat include, but are
not
limited to, chicken, turkey, duck, ostrich, quails, pigeons, geese, guinea
fowls, and
swans. In one aspect, poultry protein includes, but is not limited to, poultry
myofibrillar protein. In one aspect, the poultry myofibrillar protein includes
a chicken
myofibrillar protein, a turkey myofibrillar protein, a duck myofibrillar
protein, an
ostrich myofibrillar protein, quail myofibrillar protein, pigeons myofibrillar
protein,
geese myofibrillar protein, guinea fowl myofibrillar protein, swan
myofibrillar
protein, or any combination thereof. In another aspect, the poultry meat is
mechanically separated poultry meat (MSPM) having a 20-30% fat content and
poor
shelf stability. Methods for isolating proteins such as poultry protein are
discussed in
detail below.
The amount of protein present in the article can vary depending upon the
article or application selected and the source of the protein and isolation
techniques.
In this aspect, the protein can include 15 wt%, 16 wt%, 17 wt%, 18 wt%, 19
wt%, 20
wt%, 21 wt%, 22 wt%, 23 wt%, 24 wt%, 25 wt%, 26 wt%, 27 wt%, 28 wt%, 29 wt%,
30 wt%, 31 wt%, 32 wt%, 33 wt%, 34 wt%, 35 wt%, 36 wt%, 37 wt%, 38 wt%, 39
wt%, 40 wt%, 41 wt%, 42 wt%, 43 wt%, 44 wt%, 45 wt%, 46 wt%, 47 wt%, 48 wt%,
49 wt%, 50 wt%, 51 wt%, 52 wt%, 53 wt%, 54 wt%, 55 wt%, 56 wt%, 57 wt%, 58
wt%, 59 wt%, 60 wt%, 61 wt%, 62 wt%, 63 wt%, 64 wt%, 65 wt%, 66 wt%, 67 wt%,
68 wt%, 69 wt%, and 70 wt% of the total weight of the article or composition,
6
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
wherein any one wt% can serve either as an upper or lower end point of a wt%
range.
In one aspect, the protein compositions described herein can be used to make
films. When making films, the protein composition also includes a plasticizer.
Plasticizers increase the plasticity, elasticity, or fluidity of films
described herein. In
one aspect, the protein compositions produced herein can be used to make
edible
films and coatings. Edible plasticizers include glycerol, acetylated
monoglycerides,
trioctyl citrate, trihexyl citrate, sorbitol, and polyethylene glycol 400. In
one aspect,
the plasticizer can include 15 wt%, 16 wt%, 17 wt%, 18 wt%, 19 wt%, 20 wt%, 21
wt%, 22 wt%, 23 wt%, 24 wt%, 25 wt%, 26 wt%, 27 wt%, 28 wt%, 29 wt%, 30 wt%,
31 wt%, 32 wt%, 33 wt%, 34 wt%, 35 wt%, 36 wt%, 37 wt%, 38 wt%, 39 wt%, 40
wt%, 41 wt%, 42 wt%, 43 wt%, 44 wt%, 45 wt%, 46 wt%, 47 wt%, 48 wt%, 49 wt%,
50 wt%, 51 wt%, 52 wt%, 53 wt%, 54 wt%, 55 wt%, 56 wt%, 57 wt%, 58 wt%, 59
wt%, 60 wt%, 61 wt%, 62 wt%, 63 wt%, 64 wt%, 65 wt%, 66 wt%, 67 wt%, 68 wt%,
69 wt%, and 70 wt% of the total weight of the edible films and coatings,
wherein any
one wt% can serve either as an upper or lower end point of a wt% range.
After formation of the protein/ plasticizer composition, the composition is
cast
to produce a film. In one aspect, the casting step involves applying the
protein/
plasticizer composition onto a substrate (e.g. Teflon coated trays or silicone
resin
plates) set on a level surface followed by drying the protein/ plasticizer
composition.
In one aspect, the cast composition can be dried at from 20 C to 50 C for 5
to 15
hours. In another aspect, the cast composition can be dried at room
temperature by air
blowing for 2 to 24 hours followed by further drying in an environmental
chamber
from 25 C to 80 C at a relative humidity of 10 % to 30 % for a period of 24
hours to
48 hours. The thickness of the film can vary depending on the amount of
protein/
plasticizer mixture that is cast. In one aspect the film can be 0.03 mm, 0.035
mm,
0.04 mm, 0.045 mm, 0.05 mm, 0.055 mm, 0.06 mm, 0.065 mm, 0.07 mm, 0.075 mm,
0.08 mm wherein any one mm can serve either as an upper of lower end point of
a
mm range.
In certain aspects, additional components can be added to the
7
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
protein/plasticizer composition to enhance the mechanical properties of the
edible
film. For example, a lipid can be added to the composition in order to
increase water
barrier capacity. Suitable lipids include, but are not limited to, beeswax,
stearic acid,
palmitic acid, myristic acid, lauric acid, stearyl alcohol, hexadecanol,
tetradecanol, or
any combination thereof. In one aspect, the amount of lipid used is 0.5 % by
weight.
In certain aspects, the protein compositions described herein can be used to
produce an edible film. The edible film may be used as either a coating, a
casing, or
packaging. For example, the film can be applied as a casing or coating to
edible foods
including, but not limited to, meat products such as poultry, beef, bison,
pork, lamb,
goat, fruits, eggs, cheese, or any combination thereof. The edible films and
coatings
provide numerous benefits in the meat industry including, but not limited to:
1. Application of edible coatings prior to vacuum-packaging of meat may
prevent moisture loss, thereby maintaining saleable weight and alleviating
texture,
flavor, and color changes.
2. Edible coatings on meat cuts may hold in juices, prevent dripping, enhance
product presentation, and eliminate the need for placing absorbent pads at the
bottom
of plastic retail trays.
3. Lipid and myoglobin oxidation in meats may be reduced by using edible
coatings of low oxygen permeability.
4. Edible coating solutions that have been heated just prior to application
may
reduce the loads of spoilage and pathogenic microorganisms and partially
inactivate
proteolytic enzymes at the surface of coated meat cuts.
5. Volatile flavor loss from and foreign odor pick-up by meat may be
restricted
with edible coatings.
6. Seasonings and/or browning agents may be imbedded into coatings and
applied to meat products prior to cooking.
7. Used as an active packaging, edible coatings carrying antioxidants (e.g.,
tocopherols) and/or antimicrobials (e.g., organic acids) may be used for
direct
treatment of meat surfaces, thereby delaying meat rancidity and discoloration
and
reducing microbial loads.
8
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
8. Oil uptake by meat products during deep-fat frying may be reduced through
application of coatings prior to battering and breading.
9. Coatings may reduce meat charring and stickiness to the cooking surface
during broiling/grilling
In other aspects, the protein compositions described herein can be used to
produce biodegradable packaging films. With the increasing concern over
environmental safety of non-degradable synthetic products from traditional
petroleum
resource, there is interest in natural degradable products from renewable
sources as
alternatives to synthetic polymers. In one aspect, poultry protein such as
chicken
myofibrillar protein is a viable renewable resource for producing
environmentally safe
industrial products. Modification of the myofibrillar protein can be performed
in
order enhance the mechanical properties of the film. For example, the use of
crosslinkers that possess two or more groups capable of reacting with amino
groups
present on the protein can be used herein. In one aspect, compounds possessing
two
or more aldehyde groups such as, for example, glyoxal, can form a strong gel
network
suitable for use in biodegradable packaging films. In one aspect, the amount
of
crosslinker that can be used is from 0.25% to 2.0 % by weight of the
composition.
In other aspects, the protein compositions described herein can be used to
produce surimi. Surimi is a much-enjoyed food product in many Asian cultures
and is
available in many shapes, forms, and textures. Surimi is a useful ingredient
for
producing various kinds of processed foods. It allows a manufacturer to
imitate the
texture and taste of a more expensive product such as, for example, a lobster
tail,
using a relatively low-cost material. In one aspect, the surimi is poultry
surimi.
In another aspect, the protein compositions described herein can be used to
make extruded food articles. For example, the extruded article can be a
noodle. In
order to address the growing obesity and overweight, as well as diabetes
problems,
low calorie and fiber enriched foods are getting momentum in their
development. In
many parts of the world, consumption of pasta or noodles is significant. Fiber
enriched pasta or noodle with whole grain; hydrocolloids and other fiber
source have
not made much progress. In one aspect, poultry proteins mixed with a
plasticizer
9
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
(e.g., glycerol) and an alginate (e.g., sodium alginate) can be used to make
noodles.
Other materials suitable for making noodles besides alginates include, but are
not
limited to, (3-glucans, konjac glucomannan, and pectins. An exemplary
procedure for
making noodles is provided in the Examples. In the preparation of the noodle
forming solution, various flavors and colorants can be introduced as needed.
In yet another aspect, the protein compositions described herein can be used
as
meat binders or extenders used in processed meat products. In one aspect, the
compositions described herein can be used in processed poultry meat products
such
as, for example, white meat chicken nuggets. In other aspects, the meat binder
can be
added to restructured meat products, comminuted meat products, emulsified meat
products, marinated meat products, or any combination thereof. In one aspect,
the
restructured meat product includes chicken nuggets, cordon blue, or a
combination
thereof; the comminuted meat product includes sausages, hamburgers, or a
combination thereof; and the emulsified meat products includes bologna, hot
dogs, or
a combination thereof. In one aspect, when the meat binder is added to
marinated
meat products, the meat binder is mixed with water and added to a meat
products by,
for example, injection of the meat binder into the meat products. In another
aspect,
the protein composition is a dry powder that is mixed with one or more spices
to
produce protein-fortified spice composition.
The use of the protein compositions described herein as meat extenders or
binders have numerous benefits including:
1. Increase raw and cooked yields dramatically with cleaner label.
2. Restore succulence to frozen meats.
3. Reduce or eliminate phosphates and soybean protein isolates.
4. Pre-cook yields equal to or better than phosphates and soybean proteins
isolates.
5. Cook yields superior to phosphates and soybean protein isolate.
6. Recover meat from trimmings as a soluble protein marinade.
7. Establish a new control of quality/consistency in intact meat cuts.
In other aspects, the protein composition can be used as a nutritional
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
supplement. For example, the protein composition can be a dry powder that can
be
added to sports drinks. Alternatively, the protein composition can be added to
foods
such as energy bars and gels.
The protein compositions described herein can be optionally supplemented
with additional ingredients depending upon the application and nature of the
proteins.
In one aspect, these additional ingredients optionally include fish oil,
flaxseed oil, an
essential oil including citrus or various spices, or any combination thereof;
these
spices can include, but are not limited to, black pepper, white pepper,
nutmeg, celery
seed, glow, fennel, coriander, ginger, and tumeric. Fish oil and flaxseed oil
both
contain essential fatty acids. Essential fatty acids include the omega-3 and
omega-6
families. Essential fatty acids are fatty acids that cannot be constructed
within an
organism from other components by any known chemical pathways and must
therefore be obtained directly via ones diet. Essential fatty acids are
crucial to make
eicosanoids, endocannabinoids, lipoxins, resolvins, isofurans, neurofurans,
isoprostanes, hepoxilins, epoxyeicosatrienoic acids (EETs) and neuroprotectin
D.
When present in the biopolymer film, the fish oil, flaxseed oil, an essential
oil
including citrus or various spices, or any combination thereof can include 0
wt%, 0.5
wt%, 1 wt%, 1.5 wt%, 2 wt%, 2.5 wt%, 3 wt%, 3.5 wt%, 4 wt%, 4.5 wt%, 5 wt%,
5.5
wt%, 6 wt%, 6.5 wt%, 7 wt%, 7.5 wt%, 8 wt%, 8.5 wt%, 9 wt%, 9.5 wt%, and 10
wt% of the total weight of the biopolymer film wherein any one wt% can serve
either
as an upper of lower end point of a wt% range.
In other aspects, the protein compositions can be optionally supplemented
with anti-microbial agents, anti-viral agents, anti-oxidants, release agents,
time-
release agents, colorants, flavors, cross-linking agents, or any combination
thereof.
In one aspect, anti-microbial agents prevent the growth of bacteria and
further
aid in preserving a film. If the film is used as a casing or coating for
edible food, anti-
microbial agents in the edible film may further help to preserve the shelf-
life of that
edible food. Anti-microbial agents include, for example, bactriocines, (i.e.
Pediocin
PA-1, nisin, etc.) and enzymes including, but not limited to, lysozyme,
butyric acid,
and lauric acid.
11
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
Anti-viral agents can include, for example lauric acid.
Anti-oxidants can be added to the protein compositions to further enhance the
nutritional value of the article (e.g., film) and to further preserve the
article. Anti-
oxidants include, for example, vitamins such as vitamin E, alpha-lipoic acid,
rosemary
extract, oregano extract, green tea extract, blueberry extract, BHT (butylated
hydroxytoluene), BHA (butylated hydroxyanisole), beta-carotenes, licopenes, or
any
combination thereof.
In one aspect, time-release agents can be incorporated in the protein
composition. Time-release agents include, for example, gelatin, collagen,
alginate,
beta-glucans, guar gum, or any combination thereof.
In one aspect, colorants can be added to the protein composition. For
example, ff the film is used as a casing or coating for edible food, these
colorants may
enhance the edible food's appearance thus making the edible food appear fresh.
Colorants include, for example, beet root extract, carminic acid, beta-
carotenes, or any
combination thereof.
In certain aspects, the protein composition includes a flavor or flavoring
agent.
Flavors include, for example, chicken, beef, turkey, ostrich, bison, goat,
protein
hydrosilate, or any combination thereof.
In one aspect, cross-linking agents can be used in the protein compositions
described herein to further aid in strengthening or solidifying the biopolymer
films.
Crosslinking agents include, for example, transglutaminases, ferulic acid,
glyoxal,
glutaraldehyde, or any combination thereof. As discussed above, crosslinking
agents
can enhance the mechanical properties of a biodegradable film produced by the
protein compositions.
When present in the protein composition, the anti-microbial agents, the anti-
viral agents, the anti-oxidants, the release agents, the time-release agents,
the
colorants, the flavors, cross-linking agents, or any combination thereof can
include 0
wt %, 0.5 wt%, 1 wt%, 2 wt%, 3 wt%, 4 wt%, 5 wt%, 6 wt%, 7 wt%, 8 wt%, 9 wt%,
wt%, 11 wt%, 12 wt%, 13 wt%, 14 wt%, 15 wt%, 16 wt%, 17 wt%, 18 wt%, 19
wt%, 20 wt%, 21 wt%, 22 wt%, 23 wt%, 24 wt%, 25 wt%, 26 wt%, 27 wt%, 28 wt%,
12
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
29 wt%, and 30 wt% of the total weight of the composition wherein any one wt%
can
serve either as an upper of lower end point of a wt% range.
In one aspect, the protein compositions can be produced by first isolating a
poultry protein. In one aspect, the poultry protein can be isolated by the
following
steps:
(a) contacting a low value poultry meat with an acidic solution or basic
solution;
(b) centrifuging the low value poultry meat produced in step (a);
(c) isolating one or more proteins from the low value poultry meat produced in
step (b).
In one aspect, if the protein composition is used to make a meat binder or
extender, after step (c), the isolated protein may be dried by air drying or
lyophilization to produce a dry powder. Lyophilization can include freeze
drying the
isolated protein at 50 C to 55 C for 8 to 12 hours.
In certain aspects, the compositions, including the biopolymer films are
desired. In one aspect, when the protein composition is used to make a film,
the
method involves:
(a) isolating one or more proteins from a poultry meat to produce a first
composition;
(b) admixing a plasticizer with the first composition to produce a second
composition; and
(c) casting the second composition to produce the film.
In one aspect, the one or more proteins are derived from a poultry meat. In
certain aspects, the poultry meat is prepared as a meat slurry. The meat
slurry may
include poultry meat that is tempered from 0 C to 4 C overnight and then
minced in
a commercial-grade food processor with ice water. Next, the meat slurry
containing
the one or more proteins is contacted with either an acidic solution or a
basic solution
for a sufficient time and concentration to remove or isolate the desired
proteins from
the poultry meat. Not wishing to be bound by theory, the acidic and the basic
solutions solubilize the poultry protein present in the poultry meat (i.e.,
meat slurry).
In one aspect, the poultry meat is contacted with an acidic or basic solution
13
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
from 30 minutes to 1 hour. The acidic and basic solutions used herein can have
various molarities ranging from 0.05 M to 2 M. In one aspect, the one or more
proteins derived from a poultry meat are contacted with an acidic solution at
a pH
from 2.0 to 4Ø In another aspect, the one or more proteins derived from a
poultry
meat are contacted with a basic solution at a pH from 10.5 to 12Ø In each of
these
aspects, a protein layer can be formed after contacting with either the acidic
or basic
solution. In one aspect, the acidic solution includes HCl, citric acid, acetic
acid, or
any combination thereof. In another aspect, the basic solution includes NaOH,
NaHCO3 and phosphate buffers, or any combination thereof. Although it is
desirable
to use an aqueous solution of acid or base, it is also possible to use other
organic
solvents as well.
In one aspect, before contacting the meat slurry with either an acidic or
basic
solution, 2 to 10 mmole/L of citric acid can be added to promote the removal
of polar
lipids (i.e. phospholipids and cholesterol). In a further aspect, before
contacting the
meat slurry with either an acidic or basic solution, 8 to 16 mmol/L of calcium
chloride
(CaC12) can be added to promote the removal of polar lipids (i.e.
phospholipids and
cholesterol). In yet another aspect, before contacting the meat slurry with
either an
acidic or basic solution, 8 to 16 mmol/L of calcium chloride (CaC12) and 2 to
10
mmol/L of citric acid can be added to promote the removal of polar lipids
(i.e.
phospholipids and cholesterol). By removing the polar lipids such as
phospholipids,
lipid oxidation can be reduced or prevented.
After solubilizing the protein with an acidic solution or a basic solution, a
protein layer is generally produced. The protein layer is next separated or
isolated
from the fat and unwanted sediment, which is also present within the meat
slurry. In
one aspect, in order to further enhance separation and isolation of the
protein layer,
the mixture can be centrifuged. In one aspect, the meat slurry that has been
contacted
with either an acidic or basic solution is centrifuged between 6,000 rpm to
20,000 rpm
for a period of 5 minutes to 1 hour. In certain aspects, the meat slurry that
has been
contacted with either an acidic or basic solution is centrifuged between 6,000
rpm to
20,000 rpm for a period of 5 minutes to 20 minutes. In this aspect and upon
14
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
completing the centrifugation step, three layers are formed - an upper layer,
a middle
layer, and a bottom layer. The upper layer is a fat layer. The middle layer
contains
the solubilized protein (i.e. the protein layer), and the bottom layer
contains unwanted
sediment. In this aspect, the solubilized protein is carefully removed from
the fat and
sediment layers. In one aspect, the protein layer is an isolated protein layer
that
includes, for example, an aqueous layer of protein, a precipitated layer of
protein, or a
combination thereof. As defined herein, "isolate" means removing a desired
protein
or proteins from a mixture of proteins, fats, and carbohydrates or sugars. In
this
aspect, the isolated protein layer is 40%, 41%, 42%, 43%, 44%, 45%, 46%, 47%,
48%, 49%, 50%, 51%, 52%, 53%, 54%, 55%, 56%, 57%, 58%, 59%, 60%, 61%,
62%, 63%, 64%, 65%, 66%, 67%, 68%, 69%, 70%, 71%, 72%, 73%, 74%, 75%,
76%, 77%, 78%, 79%, 80%, 81%, 82%, 83%, 84%, 85%, 86%, 87%, 88%, 89%,
90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99%, or 100% poultry
myofibrillar protein. In another aspect, the isolated protein layer is
essentially free of
connective tissue or connective tissue protein.
In certain aspects, when the protein composition is used to make a meat binder
or extender, the protein layer can be isolated and the pH can be adjusted by
contacting
the protein layer with a second acidic or basic solution. In this aspect, the
pH can be
adjusted to the isoelectric point of the desired protein and subsequently
precipitated
and recovered. For example, the pH may be precipitated at a pH 5.2, which can
be
desired protein's isoelectric point. Next, the precipitated protein can be
adjusted to
pH values ranging from 6.2 to 7.0 and subjected to a second centrifugation
step to
further isolate the protein of choice. After the second centrifugation step,
the isolated
protein may be dried by air drying at 50 C to 55 C for 12 to 20 hours or by
lyophilization to produce a meat binder.
In a further aspect, the protein may be separated using a chromatography step.
This chromatography step can include, for example, high-performance liquid
chromatography (HPLC), affinity chromatography, size exclusion chromatography,
ion exchange chromatography, or any combination thereof.
In one aspect, the isolated protein layer can be mixed with 2 to 10 wt%
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
sucrose, 2 to 10 wt% sorbitol, and 0 to 1 wt% sodium tripolyphosphate. In
certain
aspects, the isolated protein layer mixed with 2 to 10 wt% sucrose, 2 to 10
wt%
sorbitol, and 0 to 1 wt% sodium tripolyphosphate can be dried and subsequently
used
as a meat binder.
In another aspect, the protein solution is contacted and admixed with a
plasticizer to produce a film. In one aspect, the protein is 1 wt% to 3 wt%
and the
plasticizer is added at a range from 0.5 wt% to 1.5 wt%. In another aspect,
fish oil,
flaxseed oil, anti-microbial agents, anti-viral agents, anti-oxidants, release
agents,
time-release agents, colorants, flavors, cross-linking agents, or any
combination
thereof can be optionally added to the protein/ plasticizer mixture. In one
aspect, the
protein and plasticizer are mixed together for 1 to 3 hours by stirring, and
then
allowed to dry.
EXAMPLES
The following examples are put forth so as to provide those of ordinary skill
in
the art with a complete disclosure and description of how the compounds,
compositions, and methods described and claimed herein are made and evaluated,
and
are intended to be purely exemplary and are not intended to limit the scope of
what
the inventors regard as their invention. Efforts have been made to ensure
accuracy
with respect to numbers (e.g., amounts, temperature, etc.) but some errors and
deviations should be accounted for. Unless indicated otherwise, parts are
parts by
weight, temperature is in C or is at ambient temperature, and pressure is at
or near
atmospheric. There are numerous variations and combinations of reaction
conditions,
e.g., component concentrations, desired solvents, solvent mixtures,
temperatures,
pressures and other reaction ranges and conditions that can be used to
optimize the
product purity and yield obtained from the described process. Only reasonable
and
routine experimentation will be required to optimize such process conditions.
16
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
1. Chemical and Functional Properties of Recovered Proteins from Acid-
and Alkaline-Aided Extractions
Material and Methods
1. Materials
Mechanically separated turkey meat (MSTM) obtained from Lilydale Inc.
(Edmonton, AB, Canada). MSTM (250 g) was filled into polyethylene bags and
kept
at -20 C until use. Before extraction, samples were thawed overnight at 4 C.
All the
reagents and chemicals used in the study were of analytical grade.
2. Methods
Protein solubility
In order to find the effect of different pH on the solubility of proteins in
raw
MSTM, a solubility curve was created, as described by Kim YS, Park JW, Choi
YJ.
New approaches for the effective recovery of fish proteins and their
physicochemical
characteristics. Fish Sci 2003;69(6):1231-1239. Six grams of raw MSTM was
mixed
with 300 mL of refrigerated, deionized water in a homogenizer (Fisher
Scientific,
Power Gen 1000 S1, Schwerte, Germany) at a setting of 3 for 1 minute. The pH
of the
homogenate (30 mL) was adjusted from pH 1.5 to 12.0 in 0.5 intervals, using
0.2 M
and 1 M HCl or NaOH, with the aid of a pH meter (Denver Instrument, Ultra
Basic,
UP-10, Colorado, USA). The homogenate was centrifuged at 25,900 x g at 4 C for
20
min. The protein concentration of the supernatant was determined by Biuret
method.
The protein solubility of the middle layer was expressed as milligram per gram
of
meat. Four replications were carried out for each measurement.
Extraction procedures
Preparation of protein isolate by acid-aided process
The acid-aided protein recovery from MSTM was done as per the methods of
Liang and Hultin (Liang Y, Hultin HO. Functional protein isolates from
mechanically
deboned turkey by alkaline solubilization with isoelectric precipitation. J
Muscle
17
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
Food 2003;14(3):195-205) and Betti and Fletcher (Betti M, Fletcher DL. The
influence of extraction and precipitation pH on the dry matter yield of
broiler dark
meat. Poult Sci 2005;84(8):1303-1307) with some modifications. Protein
extractions
were carried out under low temperature conditions (4 C) in order to maintain
the
functionality of the final product. For each test, 200 g of MSTM were
homogenized
with cold (1-3 C) distilled water/ice mixture at 1:5 ratio (meat: water/ice,
wt/vol)
using a 900-Watt Food Processor (Wolfgang Puck WPMFP15, W.P. Appliances Inc.,
Hollywood, FL, USA) for 15 min. After homogenization, 1200 mL of the meat
slurry
was transferred to a beaker and placed at 4 C for 30 min. Further, the
proteins in the
homogenate were solubilized by drop-wise addition of 2 M HC1 to reach the
maximum solubility points at acid conditions with pH values of 2.5 and 3.5, as
determined from the solubility profile. The protein suspension was centrifuged
using
an Avanti J-E refrigerated centrifuge (Beckman Coulter Inc., Palo Alto, CA,
USA)
at 25,900 x g for 20 min at 4 C. After centrifugation, three layers were
formed: an
upper layer of MSTM neutral lipids; a middle layer of water-soluble proteins
and a
bottom layer of water-insoluble proteins and membrane lipids (this layer is
termed the
sediment fraction in the text). The middle layer of soluble proteins was
collected and
pH was adjusted to 5.2 by 2 M NaOH in order to isoelectrically precipitate
proteins.
The precipitated proteins were thereafter centrifuged at 25,900 x g for 20 min
at 4 C.
The precipitate was re-suspended in water/ice mixture (water/ice, 350mL/350g)
by
homogenization for 10 min and pH of the homogenate was then adjusted to 6.2.
The
proteins were finally collected via centrifugation at 25,900 x g for 20 min at
4 C.
After complete extraction the moisture content of the resulting protein
isolates was
adjusted to 80%. Cryoprotectants (5% sorbitol, 4% sucrose, 0.3%
tripolyphosphate,
0.4% sodium bicarbonate and 0.03% sodium nitrite) were mixed with protein
isolates
in a pre-chilled Wolfgang Puck WPMFP15 900-Watt Food Processor (W.P.
Appliances Inc., Hollywood, FL, USA). The isolated proteins were stored in the
freezer at -20 C until analysis.
18
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
Preparation of protein isolate by alkaline-aided process
The extraction process was carried out in the same sequence as acid-aided
extractions, and differs just in the first solubilization step. Here, the MSTM
proteins
were initially solubilized at pH values of 10.5 or 11.5.
Total protein content and recovery yield
The total protein contents of both the raw material and final protein isolates
from different solubilization methods was estimated by the Biuret procedure.
Meat
sample (1 g) was dispersed in 20 mL of 0.5 M NaOH, heated in the boiling water
for
min and cooling, in an ice-water bath. After cooling the solution was filtered
through Whatman No. 1 filter paper. Then, 15 mL of the filtrate was
centrifuged with
mL of anhydrous ether (J-6B/P Beckman, Beckman Instruments, Inc, CA, USA)
at 2278 x g for 10 min. After centrifugation, 1 mL of the lower phase was
taken and
mixed with 4 mL of Biuret reagent and the absorbance was measured at 540 nm (V-
530, Jasco Corporation, Tokyo, Japan). Bovine serum albumin (Hy-Clone, UT,
USA)
was used as a standard.
Protein recovery of acid- and alkaline-aided treatments was determined
according to the method described by Omana et al. (Omana D, Xu Y, Moayedi V,
Betti M. Alkali aided protein extraction from chicken dark meat: chemical and
functional properties of recovered proteins. Process Biochem 2010;45(3):375-
381).
The recovery yield was expressed as a difference in total protein content of
isolates
(after isoelectric precipitation or final) and raw MSTM.
Protein extractability
Frozen MSTM protein isolates were thawed overnight at 4 C. Sarcoplasmic
and total protein extractability was determined by homogenizing (Fisher
Scientific,
Power Gen 100051) 2 g of sample at speed setting of 1 for 45 sec in 20 mL of
30 mM
phosphate buffer (pH 7.4) and 50 mM phosphate buffer containing 0.55 M
potassium
iodide (pH 7.4), respectively. The homogenate was centrifuged in Avanti J-E
refrigerated centrifuge (Beckman Coulter Inc., Palo Alto, CA, USA) at 15,300 x
g for
19
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
15 min at 4 C. The supernatant was filtered through Whatman No. 1 filter
paper and
the protein content of the clear filtrate was determined by Biuret method.
Surface hydrophobicity
Sarcoplasmic and myofibrillar protein surface hydrophobicity were
determined using 1-anilino-8-naphthalenesulfonate fluorescent probes (ANS; 8
mM in
0.1 M phosphate buffer, pH 7.0) according to the method described by Kim et
al.
Sarcoplasmic proteins were extracted by homogenizing 2 grams of meat sample in
20
mL of 30 mM phosphate buffer (pH 7.4) for 45 sec, followed by centrifugation
at
15,300 x g for 15 min at 4 C. The supernatant was used as sarcoplasmic
protein
solution. The pellet obtained after centrifugation was re-suspended in 50 mM
phosphate buffer containing 0.55 M potassium iodide (pH 7.4), homogenized and
centrifuged as described above. The supernatant was filtered through Whatman
No. 1
filter paper and protein concentration was determined using Biuret method. The
protein solutions were serially diluted with the same buffer to the final
volume of 4
mL to obtain concentrations ranging from 0.008% to 0.03%. After mixing with 20
tl
of ANS solution, fluorescence was measured using a fluorescence plate reader
(Fluoroscan Ascent FL; Thermo Electron Corp., Vantaa, Finland) at an
excitation
wavelength of 355 and emission wavelength of 460 nm. The net relative
fluorescence
intensity (RFI) was obtained by subtracting the RFI of each sample measured
without
ANS from that with ANS. The initial slope of the RFI versus protein
concentration
(expressed in percents) was calculated by linear regression analysis and used
as an
index of the protein surface hydrophobicity.
Total and reactive suljhydryl content
The estimation of total (T-SH) and reactive (R-SH) sulfhydryl groups were
performed using protocols of Choi and Park (Choi YJ, Park JW. Acid-aided
protein
recovery from enzyme-rich Pacific whiting. J Food Sci 2002;67(8):2962-2967)
and
Kim et al., respectively. Protein extracts were prepared by homogenizing
(setting 3
for 1 min) 2.5 gram of recovered protein in 25 mL of tris-glycine buffer (pH
8.0)
containing 5 mM of EDTA. The homogenate was filtered before use. For T-SH
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
estimation, to 1 mL of the filtrate, 4 mL of 10 M Urea and 50 l of Ellman's
reagent
((10 mM 5,5'-dithiobis (2-nitrobenzoic acid)) were added and mixed well by
vortex
mixer (Fisher, Scientific, On, Canada). In case of R-SH, 1 mL of filtrate was
mixed
with 4 mL of tris-glycine buffer (pH 8.0) and 50 l of Ellman's reagent. The
mixture
was kept for 1 h at 4 C with intermittent stirring. The absorbance of the
solutions was
measured at 412 nm against a blank of Ellman's reagent at the same
concentration
without proteins using a spectrophotometer (V-530, Jasco Corporation, Tokyo,
Japan). The SH content was calculated by using molar extinction coefficient of
13,
600 M-1 cm -1 and was expressed as mol/g of protein. The protein content of
the
filtrate was determined by Biuret method.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
Proteins were separated according to the procedure described by Laemmli
(Laemmli UK. Cleavage of structural protein during the assembly of the head of
bacteriophage T4. Nature 1970;277:680-685). Precast 10-20% ready gels (Bio-Rad
Laboratories Inc., Hercules, CA) were used to separate proteins in a Mini-
PROTEAN
tetra cell attached to a PowerPack Basic electrophoresis apparatus (Bio-Rad
Laboratories Inc., 1000 Alfred Nobel Drive, Hercules, CA, USA). For each
sample,
20 g of protein was loaded and the run was carried out at constant voltage of
200 V.
After staining and destaining, gels were scanned using an Alpha Innotech gel
scanner
(Alpha Innotech Corp., San Leandro, CA) with FluorChem SP software. Standard
protein marker from Bio-Rad (Bio-Rad Laboratories Inc., Hercules, CA, USA) was
loaded into a separate well.
Amino acid analysis
Amino acid analysis was carried out on a Beckman System 6300 High
Performance Analyzer by post-column ninhydrin methodology after hydrolysis of
proteins in 6 N HCl and 0.1% phenol for 1 h at 160 C. Pickering Laboratories
15 cm
sodium column and Pickering's sodium eluent buffers were used in the study.
Data
was collected and analyzed using Beckman System Gold software.
21
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
Total lipid extraction
Total lipid content was determined using the method of Folch et al. (Folch J,
Lees M, Stanley GHS. A simple method for the isolation and purification of
total
lipids from animal tissues. J Biol Chem 1957;226(l):467-509). Accordingly,
10.0 g
of processed meat and 5.0 grams of raw meat were separately extracted with 120
mL
of Folch solution (chloroform: methanol solution, 2: 1, vol/vol) by
homogenization
for 10 min. After 30 min, the homogenates were filtered through Whatman No. 1
filter paper. To allow clear phase separation, 40 mL of 0.88% (vol/vol) sodium
chloride solution was added and the mixture was carefully transferred to a
separating
funnel. After separation, the chloroform phase was filtered through anhydrous
sodium
sulphate (Fisher Scientific, NJ, USA) and transferred into a pre-weighed round-
bottom flask, while the upper phase was discarded as it was rich in non-lipid
components. Thereafter, the chloroform was evaporated at 40 C using a rotary
evaporator (Rotavapor, RE 121, Buchi, Switzerland). The flasks were then
placed in a
hot air oven for drying at 60 C for 30 min and weighed accurately after
desiccation
for 30 min. For further analysis of lipid classes, the total lipid extract was
washed
with 10 mL of chloroform and dissolved lipids were transferred into pre-
weighed
vials and frozen at -20 C. Lipid reduction was calculated from the difference
in lipid
content between raw and treated materials and expressed as percentage.
Fractionation of the main lipid classes
The method of Ramadan and Morsel (Ramadan MF, Morsel JT. Determination
of the lipid classes and fatty acid profile of Niger (Guizotia abyssinica
Cass.) seed oil.
Phytochem Anal 2003; 14(6):3 66-3 70) was used to separate the triglycerides
(neutral
lipids) and phospholipid (polar lipids) fractions in total lipid extracts. The
separation
of two lipid classes was accomplished using a glass column (30 cm x 2 cm;
height x
diameter) (Chemiglass Life Sciences, NJ, USA) packed with silica gel (70-230
mesh;
Whatman, NJ, USA) by applying the slurry of the adsorbent in chloroform (1:5,
wt/vol). Lipid solution (9 mL) obtained from the total fat extraction was
applied to the
column. Neutral lipids were eluted first using 60 mL of chloroform. After the
22
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
triglycerides were removed, 60 mL of methanol was applied to the column, which
resulted in elution of polar lipids. The obtained fractions were completely
evaporated
to dryness and kept in a hot air oven at 60 C for 30 min. The final weight of
the
flasks was taken after desiccating for 30 min. Both neutral and polar lipid
parts were
determined gravimetrically and expressed as percentage.
TBARs measurement
Lipid susceptibility to oxidation was measured by the induced thiobarbituric
acid reactive substances (TBARs) method as a modification of the procedure of
Kornburst and Mavis (Kornburst DJ, Mavis RD. Relative susceptibility of
microsomes from lung, heart, liver, kidney, brain and testes to lipid
peroxidation:
correction with vitamin E content. Lipids 1980;15:315-322). Briefly, 3 g of
sample
was homogenized in 27 mL of 1.15% KC1 with a Power Gen 1000 Si homogenizer
(Schwerte, Germany) for 1 min at setting 3. A 200 l aliquot of the homogenate
was
mixed with 1000 l of 80 mM Tris-maleate buffer (pH 7.4), 400 l of 2.5 mM
ascorbic acid and 400 l of 50 mM ferrous sulphate and incubated for 0, 30,
60, 100
and 150 min in a 37 C water bath. After incubation, 4 mL of TBA-TCA-HCl
mixture
(26 mM TBA (thiobarbituric acid), 0.92 M TCA (trichloroacetic acid) and 0.8 mM
HC1) was added to the sample and further the test tubes were placed in boiling
water
for 15 min. After cooling to room temperature, the absorbance was recorded at
532
nm against the blank containing all the reagents except homogenate. TBARs
concentration was calculated using the extinction coefficient of
F532 = 1.56 x 105 M-i cm i. The extent of lipid oxidation was expressed as
nanomoles
of malonaldehyde (MDA) per gram of meat.
Analysis of connective tissue components
Collagen and glycosaminoglycan concentration in raw meat, sediment after
first centrifugation, and final isolates were estimated by analyzing
hydroxyproline and
uronic acid content, respectively. Samples were dehydrated and defatted with
several
changes of acetone and then with chloroform: methanol (2:1, vol/vol) solution.
For
hydroxyproline analysis, dry-defatted samples (50-100 mg) were hydrolyzed in 6
N
23
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
HCl in the presence of nitrogen at 110 C for 20 h. Then, hydrochloric acid
was
removed by evaporation in a hot water bath (80 C) with nitrogen flushing. The
dried
preparation was cooled to room temperature, dissolved in water and filtered
(Whatman No. 1). The clear filtrate was subjected to the colorimetric method
of
hydroxyproline analysis as reported by Stegemann and Stalder (Stegemann H,
Stalder
K. Determination of hydroxyproline. Clin Chim Acta 1967;18:267-273).
For uronic acid determination, dry-defatted samples (50-200 mg) were
digested with twice crystallized papain (4 g/mg of tissue) in 20 volumes of
0.1 M
sodium acetate buffer (pH 5.5) containing 0.005 M EDTA and 0.005 M cysteine
hydrochloride at 65 C overnight. After proteolysis, trichloroacetic acid was
added to
each digest to a final concentration of 7% (wt/vol) and the mixture was held
at 4 C
overnight. After the removal of precipitated proteins by filtration (Whatman
No. 1),
the filtrate was dialyzed in running tap water for 24 h and then for another
24 h in
double deionized water at 4 C. The uronic acid content in glycosaminoglycan
containing fraction retained in the dialysis tube was determined by the
carbazole
reaction with glucuronolactone as a standard. The reaction mixture consisted
of 0.5
mL of solution containing glycosaminoglycan or glucuronolactone standard, 3.0
mL
of sulfuric acid reagent (0.2 M sodium tetraborate decahydrate in sulfuric
acid) and
0.1 mL of 0.5% (wt/vol) carbazole in methanol.
Statistical analysis
The entire experiment, from MSTM through final protein isolate was
replicated at least three times. The results were expressed as mean value
standard
deviation. Data were subjected to one-way-analysis of variance (ANOVA) using
the
General Linear Model procedure of the Statistical System Software of SAS
institute
(SAS user's guide. Statistics, Version 9.0, SAS Institute. Inc., Cary, NC. USA
2006).
To identify significant differences among mean values within the evaluated
parameters at various pH treatments, HSD Tukey's adjustment with a 95%
confidence
level (P <w 0.05) was performed.
Results and Discussion
24
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
Protein solubility
The basis for using pH-shifting processing on MSTM utilization is the fact
that solubilization of muscle proteins is maximum at low and high pH values.
Solubility is not only significant for the determination of the optimum
conditions for
protein extraction, but also of great importance in food industry
applications. The high
solubility at certain pH values is required for efficient separation of the
soluble
proteins from undesirable meat constituents (lipids, connective tissue,
impurities,
etc.). However, low solubility is needed to precipitate the solubilized
proteins at
isoelectric point for better recovery. In order to investigate the effect of
different pHs
on MSTM proteins, a solubility curve was constructed with pH range from 1.5 to
12.0
in 0.5 increments (Figure 1). The lowest solubility (or highest precipitation)
in
deionized water occurred at pH 5.5, which is in the range of isoelectric
points for the
majority of muscle proteins. At the isoelectric point, the negative and
positive charges
are equal, thus association among protein molecules is strong due to the ionic
linkages. As a consequence, protein-water interactions are replaced by protein-
protein
interactions and precipitation occurs. An increase in solubility was observed
with
either acidification or alkalization, when the proteins become positively or
negatively
charged, respectively. These net charges provide more binding sites for water,
resulting in electrostatic repulsion among molecules, hydration of charged
residues
and increased protein-solvent interactions contributing to the increased
solubility. The
highest protein solubility in acidic conditions, (186.2 mg/g) was attained at
pH 2.5,
while for alkaline conditions a maximum value of 245.3 mg/g was found with pH
11.5. The rapid increase in solubility on the acidic side compared to the
alkaline might
be attributed to more ionizable groups with pKa values between 2.5 and 7.0
than
between 7.0 and 11Ø The protein solubility profile showed a U-shaped
pattern;
however, unlike the typical solubility curve for fish muscle protein
homogenates, the
solubility was found to be maximum at pH 11.5 and decreased at pH 12Ø
Therefore,
additional pH points 11.25 and 11.75 were added to the MSTM protein solubility
analysis. The results confirmed the decreasing solubility with increasing pH
from 11.5
to 12Ø This finding indicated that poultry meat proteins are likely to
behave
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
differently when exposed to the extreme alkaline conditions compared to fish
muscle
proteins.
The pH-shifting process, which is widely used for extraction of proteins from
fish sources, was found to be possible to apply for the recovery of poultry
meat
proteins, MSTM in particular. Based on the solubility study, four pH values
(2.5; 3.5;
10.5 and 11.5) were selected as solubilization pHs for the protein extraction
from
MSTM.
Protein content and recovery yield
A high recovery yield is important for economic reasons. The yield of protein
achieved by acid or alkaline treatments is predominantly driven by three major
factors: the solubility of the protein during exposure to low or high pH, the
size of the
sediments after centrifugation and the solubility at precipitation pH. The
results
obtained for different extraction stages are shown in Table 1.
Table 1. Protein content (%) and recovery yield (%) during different stages of
protein
extraction from MSTM'
Extraction Protein yield Final protein Final protein
pH after pI, % content, % yield, %
pH 2.5 70.6 1.7 18.5b 0.6 66.4a 5.4
pH 3.5 69.1 2.2 18.2b 1.3 57.1b 4.7
pH 10.5 67.3 6.9 19.6a 0.2 63.6ab 6.3
pH 11.5 68.7 1.4 19.0ab0.2 64.8a 2.5
'Results are presented as mean (n = 4) standard deviation.
Different letters within a column indicate significant differences; P < 0.05.
pI refers to the isoelectric precipitation.
The yield of the proteins recovered by isoelectric precipitation indicated no
significant difference (P = 0.7972) due to the extraction pH. The final
protein content
was found to be different between acid and alkaline treatments, with a
tendency to
increase from low to high pH values. Final protein content was found to be
maximum
(19.6%) when MSTM was solubilized at pH 10.5, and minimum when solubilized pH
2.5 and 3.5. The highest final recovery yields were found at extraction pH of
2.5 and
26
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
11.5 (66.4% and 64.8% respectively), while the lowest was observed at pH 3.5
(57.1%) (P = 0.0097). The increase in recovery yield for pH of 2.5 and 11.5 is
highly
associated with the solubility profile (Figure 1), which showed the highest
solubility
at these pH values. Slight decrease of recovery yield at pH 10.5 resulted
mainly from
decreased amount of solubilized proteins as indicated by the MSTM solubility
profile.
In general, the per cent of loss in recovery yield between precipitation (pH
5.2) and
re-adjusting to pH 6.2 was found to be around 6%. The results indicated that
optimizing pH during solubilization is the prerequisite step to achieve the
maximum
protein recovery from MSTM.
Extractability of recovered proteins
Extractability is an important property since the amount of protein available
in
the solution affects the functional properties expected from proteins. The
conformation of proteins, which is related to the environment, plays a
significant role
in determination of protein functionality. Also protein extractability relates
to the
surface hydrophobic (protein-protein) and hydrophilic (protein-solvent)
interactions.
The highest total protein extractability (Figure 2) was observed at pH 10.5,
with a value of 73.7 mg/g. The difference in extractability between
solubilization pHs
can be explained by the different degrees of denaturation and the consequences
of
different degree of protein refolding after pH readjustment to 6.2. The
results
indicated that protein isolates prepared at pH 10.5 were less denatured
compared to
those prepared at pH 2.5, 3.5 and 11.5. SDS-PAGE profiles of protein after
isoelectric
precipitation also revealed that protein hydrolysis was comparatively less at
pH 10.5,
which may be the reason for less denaturation (Figure 5). The lowest amount of
solubilized total proteins (62.3 mg/g) was found at extraction pH of 2.5.
Sarcoplasmic
protein extractability from recovered proteins as a function of pH was not
significantly (P = 0.0563) different among treatments (Figure 2). The
sarcoplasmic
protein fraction comprised around 58% of total soluble proteins, which
confirms the
fundamental theory of the pH-shifting method, that a sizeable amount of
sarcoplasmic
proteins are recovered during acid- and alkali-aided processes.
27
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
Protein surface hydrophobicity
Hydrophobic interactions play a major role in defining the conformation and
interactions of protein molecules in solution, thereby affecting the stability
of native
protein structures. Surface hydrophobicity of proteins helps to determine the
rate of
protein unfolding due to different processing methods.
Myofibrillar protein hydrophobicity (Figure 3) was shown to be significantly
different (P < 0.0001) between treatments and the trend was similar to that
observed
in protein extractability (Figure 2). Extraction at pH 10.5 showed highest
myofibrillar
hydrophobicity (Ho = 465). Similar values were observed for extractions
conducted at
pH values of 3.5 and 11.5 while at pH 2.5 extracted samples represented the
lowest
value. The myofibrillar hydrophobicity was found to increase with an increase
in total
protein extractability (Figures 2 and 3). Even though the observed results
appear to be
in contradiction, it is important to point out that protein extractability
depends not
only on the amount of hydrophobic groups exposed to the protein surface, but
also on
the intrinsic factors such as protein conformation and surface
polarity/hydrophobicity
ratio. In these circumstances, although proteins isolated at pH 10.5 showed
the highest
surface hydrophobicity and protein extractability, it might be possible that
after the
readjustment to pH 6.2, the amount of polar and ionic groups were still
predominant
over the non-polar groups even if these latter were exposed to the surface.
Therefore,
exploring the surface polarity/hydrophobicity ratio could be a better
indicator of
protein denaturation than surface hydrophobicity by itself.
Sarcoplasmic protein hydrophobicity of the extracted proteins was
significantly higher (P < 0.0001) for the alkali processed samples compared to
acidic
treatments (Figure 3). The cause of increased hydrophobicity might be due to
the
change in protein conformation, particularly due to partial protein unfolding.
As a
result, the intramolecular bonds which stabilize protein structure are
ruptured, thus
facilitating the exposure of hydrophobic groups to the surface.
Sulfhydryl content
Sulfhydryl groups are considered to be the most reactive functional group in
28
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
proteins. The total and reactive sulfhydryl content of proteins extracted at
different pH
values indicated no significant difference between treatments (P = 0.5825 and
P =
0.9841, respectively), even though hydrophobicity was higher at pH 10.5.
However,
an increase in total and reactive sulfhydryl group content was found for pH
treated
samples compared to raw MSTM (data not shown), which is probably related to
the
protein unfolding, resulting in exposure of sulfhydryl groups to the protein
surface.
The ratio T-SH/R-SH for raw and processed meat was also characterized. For raw
MSTM, the ratio was equal to 1.42. A slight decrease of T-SH/R-SH ratio for
protein
isolates (1.32 and 1.36 for acid and alkaline extractions, respectively) was
observed,
which may be the result of increasing the amount of disulfide bond formation.
SDS-PAGE profile
Protein bands corresponding to myosin heavy chains (MHC), actin,
tropomyosin a and (3 were most abundant after isoelectric precipitation
fractions and
in the final protein isolate (Figure 5). The electrophoretic profile of the pI
precipitated
proteins showed less hydrolysis of myosin heavy chains (MHC) for pH 10.5
treated
samples, suggesting lower level of denaturation extension. No difference in
protein
profile was observed among different pH treatments for the final protein
isolates. The
presence of myosin light chains of low molecular weight showed the degradation
of
myosin into its subunits. The intensity of bands corresponding to myosin heavy
chain,
actin, tropomyosin a and (3 were increased in the extracted samples suggesting
that
the concentration of these proteins increased in the final protein isolates.
Hence, this
may have effects on the improved functionality of proteins in the final
isolates
compared to that of raw material. Unpublished results showed appreciable
gelation,
emulsion and foaming capacities of the MSTM protein isolates. With improved
functionalities of protein isolates, MSTM can be better utilized for value-
added
processing.
Amino acid composition
The amino acids composition of raw MSTM and protein isolates obtained by
extractions at different pH are shown in Table 2. Glutamic acid was found to
be the
29
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
predominant amino acid and was significantly (P = 0.0143) higher in acid
treated
samples compared to raw meat. Lysine, an essential dietary amino acid, was
found to
be significantly (P = 0.0023) increased for the acid treated samples compared
to the
raw meat. No significant difference (P > 0.05) was found between raw and
processed
meat for alanine, glycine, isoleucine, leucine, phenylalanine, proline,
threonine,
tyrosine and valine. A significant (P < 0.0001) loss of methionine for the pH
treated
samples was observed. The reason for the methionine loss might be due to its
oxidation during the extraction process, where the proteins are exposed to
acidic or
alkali environment. It was reported that methionine can be oxidized to
methionine
sulfoxide and methionine sulfone during processing. The amino acid histidine
was
found to decrease 82% on average for all extraction pH values, excluding pH
11.5
where it was not detected. The ratio of total essential amino acids to total
amino acids
showed no statistically significant (P = 0.1575) difference, suggesting no
effect of
acid and alkaline extractions on amino acid concentrations.
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
ti
7
o0 0o c, `n oc N o0
Z M p op oc 00 O M N
p O O p O N 0 ~n Oo O
W =ay +I +I +I ~ I +I ~ O O +I ~ O O +I O O O +I
oc N Z +I +I Q +I +I +I +I +I oc
V Z OD d,
O~ N Oo Oo M m m c i kn M
z w
O
H M GO
}, pp O N Oo OO CA
v7 \O \O N
'" - O M- O p N 0 O O O O O O
^~ ^~ +I I I I +I I +I +I I Q +I +I I +I +I +I pl
O
cd U O O \O O N N N
GO Vj O GO O M M M --~ V7
C/1 W
mm m O N O M N oc N
y O p 0 O D O O N p 0 ~n oc M ~I ~I I ~I ~I ocI N I O 3C I O O O
oc O N M M N O a1 a1 00
o cr ~o o; M N r a1 a1
cc-1 o a; o M m m v~ M
~. W
coo
U s_ N m N OO a1 O00 N N C
D D O O N 0 'r N N N O
yr +I +I +I +I +I O O O O O O O O +I
kr)
y N r p O I +I +I ~I Z +I +I I +I +I +I
+ O `O O O O `O \O
O~ v~ - ~`? M M M O Oo ~o
N O Oo M M m N ~n M
O
N M O ~o ,n ao Oo O O p
N 0 O N M N N O O N N O
O ~% O O O +I 0 0 0 0 0 0 0 O O O O
" +I +I +I - +I +I +I +I +I I +I +I +I +I +I +I +I
O m N N O M a, M O p
M N M M N N N C a,
coo
R-i 01 \O 01 l~ M 00 ~p N c n N M
O >
7~ ro Q
*U fU *U
sue-" s}- ~- U *U 0
0 fU. U p con
0 C)
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
ti
H
z
H
O
z
O
H
coo
H
o an
O
o O
un coo
7~ coo
O M
con
U ~ U
4~ U cd
con
O
> as
7~ C) p as
U p
U
con c!
U `n z )
O ~I H O
75 C) t!~ cn
00 ICI U (- )
cd is c
as as 0
OUVEO=
E E as C)
cl~
0 0 as .' as as
O O U - .=
as as
as ) as
cd u ~". U .
cl~ -- cn as
En ;4
as uW Q W
as >x ~HZ- ~N
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
Lipid reduction and TBARs
Lipid reduction is the principal factor for producing functional protein
isolates
from MSTM since the raw material is highly rich in triacyglycerols and
membrane
phospholipids. The latter contribute greatly to the oxidative reactions due to
the high
content of unsaturated fatty acids. The amount of lipids that can be removed
is linked
to the fat content of the starting material. The total, neutral and polar
lipids content of
raw MSTM were 23.5, 14.3 and 7.5%, respectively. Acid and alkaline extractions
of
MSTM resulted in protein isolates with significantly (P < 0.0001) reduced
lipid
content compared to the initial material (Table 3). However, no significant (P
> 0.05)
difference was found between pH treatments for removal of total, neutral and
polar
lipids, which on average were equal to 92.3, 93.0 and 90.7%, respectively. A
large
reduction of lipids from MSTM by the pH-shifting technique was expected, as at
extreme pH values, the proteins are solubilized, favoring the release of the
storage and
membrane lipids. During the centrifugation step the lipids are released to the
aqueous
environment due to the differences in solubility and particle density. The
meat: water
ratio (1:5, wt/vol) used in the study also contributed to the high removal of
lipids from
MSTM. Several studies have showed that a pH-shifting process is effective for
lipids
removal.
The effect of different extraction pHs on TBARs development in the MSTM
protein isolates is shown in Figure 6. Analysis on lipid oxidation showed no
significant difference among different pH treatments (P > 0.05). However,
there was a
significant (P < 0.001) decrease in the amount of malonaldehyde (MDA) for
recovered meat compared to the raw meat (data not shown). The reason for
decreased
lipid oxidation is probably due to the higher removal of membrane lipids. No
difference in the amount of MDA in the samples processed at different pH
refers to
the analysis of polar lipids content (Table 3), wherein no differences were
found
among the various extraction pH conditions.
33
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
ti
~ o m o 0
N
+I +I +I +I
con
z 0 0 0
x a
+Iao+I a1 +Ik+I00
0 Q U 0 0 0 0 0 0 0 0
0 0
z
U
y U
0 +I +I +I +I
as 7~
}' m N N
as
as M M N N N
as
as 7~
as
z r" as
p U
0 N O
y O
> ~ p p O y as
+I +I +I +I y y
o
a; U o o - - Oo as
o as
O >
+I +I +I +I
II
~n M N M C)
7r N N N N y~
O
O O O N ON U cn
O H +~ O O O O
E y +I +I +I +I y
.., cd
i Z
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
Analysis of connective tissue fractions
The extracellular matrix of connective tissue is composed of collagen fibers
embedded in an amorphous ground substance containing glycosaminoglycans.
Glycosaminoglycans are linear unbranched polymers of repeating disaccharide
units
of hexosamine and uronic acid. Thus, the amount of uronic acid residues is
important
for quantitative analysis of glycosaminoglycans. Collagen is the major protein
of
connective tissue, with relatively small amounts of glycosaminoglycans.
Determination of the amino acid hydroxyproline is an accurate way of
measurement
of collagen, since there are no other known animal proteins containing any
appreciable amounts of this amino acid. The collagen and glycosaminoglycans
concentrations were estimated by determining hydroxyproline and uronic acid,
respectively, in MSTM sediment obtained after the first centrifugation and in
the final
protein isolate (Table 4). The hydroxyproline concentration in the final
isolates (< 1
g/mg) was on average 23 and 82 times lower compared to MSTM (z 12 g/mg) and
the sediment fraction (23.3-59.1 g/mg), respectively, with no significant
difference
among pH treatments (P = 0.5026). This indicates that the myofibrillar and
sarcoplasmic proteins were the major part of the extracted proteins, while
most of the
connective tissue (collagen) present in the sediment. At acidic extraction
conditions,
the concentration of hydroxyproline in the sediment was 1.6 times higher at pH
2.5
compared to pH 3.5.
A similar trend between these pH values was observed for the uronic acid
concentration. The ratio of uronic acid to hydroxyproline, which represents
the
estimation of amorphous ground substance to collagen fiber, was similar
between pH
2.5 and 3.5 and was almost identical to the value (0.14) corresponding to
MSTM.
When the proteins were extracted in alkaline conditions, it was found that
hydroxyproline and uronic acid concentrations in the sediment fractions were
more
than two times higher (P = 0.0005 and P < 0.0001, respectively) at pH 11.5
than pH
10.5. However, the uronic acid values were lower compared to the corresponding
values observed at acidic pH, which resulted in the lower (> 3 times) uronic
acid to
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
hydroxyproline ratio.
The results give an insight into the possibility of extracting proteoglycans
from
the sediment fraction during the co-extraction of valuable components from
MSTM.
Table 4. Hydroxyproline and uronic acid contents ( g/mg of dry-defatted
weight) in
mechanically separated turkey meat (MSTM) and its protein fractions obtained
during
extraction process'
Hydroxyproline Hydroxyproline Uronic acid Uronic acid
content in the content in the content in the content in the
Treatment sediment after
1sr final protein sediment after ls` final protein
centrifugation isolate centrifugation isolate
Raw
MSTM 11.6 0.8 - 1.7 0.1 -
pH 2.5 50.6a 4.0 0.5 0.1 7.6a 0.1 0.5' 0.1
pH 3.5 31.7b 5.2 0.6 0.2 4.3b 0.0 0.4b 0.0
pH 10.5 23.36 4.5 0.6 0.3 1.6 0.2 0.9a 0.0
pH 11.5 59.la 5.5 0.3 0.1 3.5b 0.5 0.8a 0.1
'Results are presented as mean (n = 2) standard deviation. Different letters
within a
column indicate significant differences; P < 0.05.
Conclusions
The study demonstrated that at pH 2.5 and 11.5, the proteins from MSTM
were most soluble, leading to the highest protein yields at these pH values.
Among the
different extraction pH values, the highest total extractability was achieved
at pH
10.5. Acid- and alkaline-aided extractions were equally effective in removing
total,
neutral and polar lipids from MSTM. Consequently, TBARs analysis showed no
difference between acid and alkaline treatments; however the values were
significantly lower compared to raw MSTM (P < 0.0001). SDS-PAGE profiles for
both acid and alkaline extractions indicated higher concentration of myosin
heavy
chains, actin and tropomyosin compared to MSTM indicating concentration of
myofibrillar proteins. No statistical difference in the ratio of total
essential amino
acids to total amino acids between MSTM and extracted proteins indicated that
there
36
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
was no substantial effect of acid and alkaline treatments. Analysis of uronic
acid
content revealed that most of the proteoglycans accumulated in the sediment
fractions,
hence paving the way for a co-extraction technology of the valuable components
from
MSTM.
II. Effect of Acid- and Alkaline-Aided Extractions on Functional and
rheological properties of proteins recovered
Material and Methods
Raw material and chemicals
Mechanically separated turkey meat (MSTM) was obtained from Lilydale Inc.
(Edmonton, AB, Canada). MSTM (250 g) was filled into polyethylene bags and
kept
at -20 C until use. Before extraction, samples were thawed overnight at 4 C.
All the
reagents and chemicals used in the study were of analytical grade.
Preparation of protein isolate by acid-aided process
The acid-aided protein recovery from MSTM was accomplished by the
method described by Liang and Hultin and Betti and Fletcher with slight
modifications. Protein extractions were conducted under low temperature
conditions
(4 C) in order to maintain the functional properties of the final product.
To prepare
the protein isolate, 200 g of MSTM was homogenized with cold (1-3 C)
distilled
water/ice mixture at 1:5 ratio (meat:water/ice, wt/vol) using a 900-Watt Food
Processor (Wolfgang Puck WPMFP15, W.P. Appliances Inc., Hollywood, FL, USA)
for 15 min. Homogenate (1200 mL) was transferred to a beaker and allowed to
stand
for 30 min at 4 C. The proteins in the homogenate were then solubilized by
drop
wise addition of 2 M HCl to reach the maximum solubility at pH values of 2.5
and
3.5, as determined from the solubility profile, as reported by Hrynets and
others
(2010). Acidic or alkaline homogenates were centrifuged in Avanti R J-E
refrigerated
centrifuge (Beckman Coulter Inc., Palo Alto, CA, USA) at 25,900 x g for 20 min
at 4
C. Three phases were formed after centrifugation: a top phase of MSTM neutral
lipids; a middle aqueous phase of water-soluble proteins and a sediment phase,
37
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
containing a water-insoluble protein fraction and membrane lipids. The middle
layer
of soluble proteins was collected and pH was adjusted to isoelectric point (pH
5.2)
with 2 M NaOH. The precipitated proteins were recovered by centrifugation at
25,900
x g for 20 min at 4 C. The precipitate was re-suspended in water/ice mixture
(water/ice, 350mL/350g) by homogenization for 10 min. The pH of the resultant
homogenate was adjusted to 6.2 by drop wise addition of 2 M NaOH. Proteins
were
finally collected by centrifugation at 25,900 x g for 20 min at 4 C. The
moisture
content of the recovered protein isolates was adjusted to 80%. Cryoprotectants
(5%
sorbitol, 4% sucrose, 0.3% tripolyphosphate, 0.4% sodium bicarbonate and 0.03%
sodium nitrite) were mixed with protein isolates in a pre-chilled Wolfgang
Puck
WPMFP15 900-Watt Food Processor (W.P. Appliances Inc., Hollywood, FL, USA).
The isolated proteins were stored in the freezer at -20 C until analysis.
Preparation of protein isolate by alkaline-aided process
For the alkali extraction process the procedures described above were
followed except for the first solubilization step. Here the MSTM proteins were
initially solubilized at pH values of 10.5 or 11.5.
Cooking loss
Raw samples were prepared by manually grinding protein isolate samples (12
g) with 2.5% NaCl in a pestle and mortar for 10 min. The paste was packed in
polypropylene capped tubes (1.7 cm x 10 cm, Simport, QC, Canada) without air
pockets. The stuffed tubes were then heated in the water bath at 95 C until
the
internal temperature reached 75 O C. The internal temperature was measured
using
thermocouples, inserted in the centre of the sample. After cooking, the gel
was
removed from the tubes and accurately weighed individually. The samples were
then
stored in polyethylene bags at 4 C overnight prior to texture profile
analysis. The
cooking loss was calculated as follows:
Cooking loss (Original sample weight - weight after cooking) x 100
Original weight
38
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
Determination of expressible moisture (EM)
The expressible moisture of the protein isolates, as an estimation of water
loss,
was evaluated by using a texture profile analyzer (TA-XT Express, Stable micro
systems, Ltd., Surrey, England), which was set to the adhesive test mode prior
to the
measurements. Three grams of meat sample were placed on the pre-weighed filter
paper (Whatman No. 1), and pressed between two glass plates, with a target
force of
1000 g for 2 min. After squeezing, the filter paper along with the absorbed
water was
immediately weighed. Expressible moisture was expressed as percentage. Six
press
tests were performed for each treatment. The following formula was used to
calculate
the expressible moisture:
Expressible moisture (Wet paper - Dry paper) x 100
Meat weight
Emulsifying activity index (EAI) and emulsion stability index (ESI)
The measurements of emulsifying activity index and emulsion stability index
were conducted according to the method described by Moure and others (2002)
with
slight modifications. Oil-in-water emulsion was prepared by mixing corn oil
with
protein solution (myofibrillar or sarcoplasmic at the concentration of 0.4
mg/ml) at
1:3 ratio (vol/vol) in an homogenizer (Fisher Scientific, Power Gen 1000 S1,
Schwerte, Germany) operated for 1 min at setting 3. Immediately after
homogenization, 0.05 mL of emulsion was diluted to 5 mL with 0.1 % sodium
dodecyl
sulphate (SDS) solution and the absorbance was measured at 500 nm in a 1-cm
path
cuvette using a spectrophotometer (V-530, Jasco Corporation, Tokyo, Japan).
The
EAI was calculated from the following equation:
EAI = 2.33 x AO
where AO is the absorbance estimated just after emulsion preparation. The
emulsion
stability index was determined by measuring the absorbance of these emulsions
after
min of standing. The ESI was deduced as follows:
39
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
An
ESI = 10 x Ao - Aio
where A10 is the absorbance determined after 10 min.
Foam expansion (FE) and foam volume stability (FVS)
The measurement of foamability was performed as the method described by
Wilde and Clark (1996). Known volumes of proteins (myofibrillar and
sarcoplasmic)
were whipped using a vortex mixer (Fisher Scientific, On, Canada) at speed 10
for 2
min.
Foamability or foam expansion was expressed as percentage volume increase
after
mixing using the following equation:
Foam expansion (%) = Foam volume (mL) x 100
Initial liquid volume
The stability of the foam volume was calculated as percentage of foam
remaining
after 30 min at 25 C.
Foam volume stability (%) = Volume of foam (mL) retained after 30 minx 100
Volume of foam after whipping
Total pigment determination
The total pigment content was evaluated by direct spectrophotometric
measurement according to the method of Fraqueza and others (Fraqueza MJ,
Cardoso
AS, Ferreira MC, Barreto AS. 2006 "Incidence of pectoralis major turkey
muscles
with light and dark color in a Portuguese slaughterhouse" Poult Sci 85
(11):1992-
2000), with slight modifications. For each run, 10 g of the sample was weighed
into
50 mL capped glass tubes and 40 mL of acetone, 1 mL of HC1, and 1 mL of water,
were added. The mixture was vortexed for 3 min and allowed to stand for 1 hour
at
room temperature. The extract was filtered through Whatman No. 1 filter paper,
and
the absorbance was read at 640 nm against an acid-acetone blank using a UV/VIS
spectrophotometer (V-530, Jasco Corporation, Tokyo, Japan). The absorbance
value
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
was multiplied by a coefficient of 17.18 and the concentration of total heme
pigments
was expressed in milligrams of myoglobin per gram of meat.
Color characteristics
The color characteristics of samples were measured on the surface of raw
MSTM and freshly prepared protein isolates using a Minolta CR-400 (Konica
Minolta
Sensing Americas, Inc, Ramsey, NJ 07446). A white standard plate was used to
calibrate the colorimeter. Tristimulus color coordinates L*, a* and b* were
recorded.
The L* value on a 0 to 100 scale denotes the color from black (0) to white
(100). The
a* value denotes redness (+) or greenness (-), and the b* value denotes
yellowness (+)
or blueness (-). Three readings per treatment sample were taken and the
average
reading was recorded. The intensity of the red, saturation, Hue and whiteness
were
calculated as follows:
Intensity of the red = a* / b*
Saturation = (a2 + b2)v2
Hue = arctan b*/a*
Whiteness = 100 - [(100-L* )2 + a*2 + b*2]12
Texture profile analysis
Texture profile analysis was carried out on cooked samples by employing a
texture profile analyzer (TA-XT Express, Stable micro systems, Ltd., Surrey,
England). The samples were cut into cylinders (17 mm diameter, 10 mm height)
and
subjected to the TPA mode analysis. Three samples per treatment were
compressed to
50% of their original height for 2 cycles with the aluminum cylinder probe (d
= 5 cm).
The time between two compressions was set as 1 s. Determination of texture
attributes
were performed at the trigger force of 5 g with the speed of 5 mm/s.
Attributes were
calculated as follows. Hardness: the maximum force required for the first
compression. Chewiness: the work needed to chew a solid sample to a steady
state of
swallowing. Springiness: the ability of the sample to recover to its original
shape after
the first compression. Cohesiveness: represents how well the product
withstands a
41
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
second deformation relative to how it behaved under the first deformation.
Measurements of samples were carried out at room temperature. Data were
recorded
and analyzed automatically by software provided with the instrument.
Dynamic viscoelastic behavior of isolated proteins
The dynamic viscoelastic (DVB) behavior of isolated proteins during heating
and cooling was monitored using a Physica MCR Rheometer (Anton Paar GmbH,
Virginia, USA) under oscillatory mode, employing a 2.5 cm parallel plate
measuring
geometry. Four grams of protein isolate were mixed thoroughly with 2.5% of
sodium
chloride (w/w) in a pestle and mortar to obtain a fine ground paste. The paste
was
subjected to DVB measurements. The gap between measuring geometry and peltier
plates was adjusted to 1.0 mm. Approximately 2 g of paste was placed on the
peltier
plate at 4 C. Once the sample was pressed by lowering the measuring geometry
plate, excess sample was removed with a stainless steel spatula. The samples
were
heated from 4 to 80 C at a rate of 2 C/min and cooled from 80 to 4 C
at the
same rate. To determine the linear viscoelastic region (LVR) an amplitude
sweep was
carried out in a range of deformation from 0.1 to 10%. After determining LVR,
measurements of the samples were conducted by applying a controlled strain
(0.5%)
with a constant frequency set at 1 Hz. The two sine waves had a phase
difference tan
= , which gave elastic (storage modulus G') and viscous (loss modulus G' ' )
elements of gel. These two values along with tan = were recorded
simultaneously
throughout the heating and cooling processes by the instrument. Four
replications
were performed, each using a fresh paste preparation and the average values
were
plotted.
Statistical analysis
All data were analyzed by one-way-analysis of variance (ANOVA) using
General Linear Model procedure of the Statistical System Software of SAS
institute
(Version 9.0, SAS Institute. USA. 2006) and reported as means and standard
deviation among means. The entire experiment, from MSTM through final protein
isolate was replicated at least three times. Comparison of means within the
evaluated
42
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
parameters at various pH treatments was carried out by HSD Tukey's adjustment
with
a 95% confidence level. Significance of difference was established at P <
0.05.
Results and discussion
Cooking loss and expressible moisture
The ability of meat proteins to retain water is one of the most important
quality attributes influencing product yield and it also has an impact on
eating quality
of the product. Cooking loss provides an insight into the tenderness of a meat
product,
which is related to the ability of proteins to bind water and fat. Expressible
moisture is
a measure of the water holding capacity (WHC) of meat proteins and changes in
WHC indicate the changes in the charge and structure of myofibrillar proteins.
In the
present study the effect of different pH of extraction on WHC was assessed by
estimation of cooking and water loss. No significant (P = 0.5699) difference
was
found for cooking loss (Figure 7) between different treatments. However,
cooking
loss of protein isolates was significantly lower (6.23% on average; P <
0.0001)
compared to raw MSTM (29.27%; Table 5). Such a significant difference in
cooking
loss between raw and processed meat is probably due to the difference in
composition
of those two materials. Total lipid content of raw meat and isolated proteins
was
23.50% and 1.81%, respectively. Therefore, while subjected to heat treatment
raw
meat will be loosing more fat in addition to the water loss resulting in
higher cooking
loss.
The results obtained from the analysis of expressible moisture, as an
evaluation of water loss, are presented in Figure 8. Expressible moisture
varied
between treatments and the highest (14.26%: P = 0.0249) was obtained for
samples
processed with pH 2.5. Proteins extracted with pH 10.5 represented the lowest
water
loss of 12.86%. This decrease in water loss, which refers to the higher
ability to retain
water, is probably the result of higher protein content of samples extracted
with pH
10.5. Extraction of proteins at this pH also resulted in the highest surface
hydrophobicity of myofibrillar proteins. The exposure of hydrophobic amino
acids to
the protein surface may increase the number of hydrophobic interactions,
leading to
43
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
the formation of a gel network with higher ability to entrap water. Water loss
was
found to be significantly (P < 0.0001) higher for raw MSTM (46.70%) compared
to
the processed meat. These results suggest that WHC of MSTM could be greatly
improved by the extraction treatments.
Table 5. Characteristics of raw mechanically separated turkey meat (MSTM)l
Parameter Value
Cooking loss (%) 29.27 5.1
Expressible moisture (%) 46.70 0.61
EAI (myofibrillar proteins) 2.37 0.18
EAI (sarcoplasmic proteins) 1.01 0.07
ESI (myofibrillar proteins) 56.67 3.19
ESI (sarcoplasmic proteins) 7.80 0.53
FE (myofibrillar proteins) (%) 93.30 20.82
FE (sarcoplasmic proteins) (%) 10.0 0
FVS (myofibrillar proteins) (%) 61.69 10.85
FVS (sarcoplasmic proteins) (%) 0
Total heme pigments (mg/g of meat) 3.77 0.69
Hardness (gram force) 142.82 20.33
Springiness 0.66 0.03
Chewiness 43.63 11.27
Cohesiveness 0.46 0.02
'Results are presented as mean standard deviation.
Emulsion activity index (EAI) and emulsion stability index (ESI)
Emulsion is a heterogeneous system consisting of at least two immiscible
liquid
phases, one of which is dispersed in the other in the form of droplets.
Emulsion is
stabilized through physical entrapment of fat globules within protein matrix
followed
by formation of an interfacial protein film around the small fat globules. The
ability of
protein to adsorb at the water-oil interface during the formation of emulsion
avoiding
flocculation and coalescence is indicated by EAI. On the other hand, ESI
estimates
the rate of decrease of the emulsion turbidity due to droplet coalescence and
creaming, leading to emulsion destabilization. Therefore, EAI and ESI increase
when
proteins favor emulsion formation and stabilization, respectively. The
emulsification
44
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
properties of acid and alkaline extracted proteins were evaluated by their
ability to
form and stabilize emulsion with oil and the results are presented in Table 6.
EAI of myofibrillar proteins was significantly different (P = 0.0184) between
treatments, with the highest value obtained at pH 11.5. In general, alkali
extracted
protein showed slightly higher EAI compared to acid extracted protein. Among
the
myofibrillar and sarcoplasmic proteins, the latter showed significantly lower
emulsification ability (P = 0.0010).
Among treatments, there was a tendency to be significantly higher for (P =
0.0592) ESI of myofibrillar proteins for alkali extracted samples. The latter
values
were also around 7 times higher compared to the ESI of sarcoplasmic protein
fraction.
This effectiveness of myofibrillar proteins is probably due to the ability of
myosin to
display both hydrophobic affinity for fat and hydrophilic affinity for water.
Myosin
provides a distribution of polar and non-polar amino acids thus enhancing the
orientation between two unlike phases. High length to diameter ratio of the
myosin
molecule also contributes to the molecular flexibility and rearrangement at
the protein
film interface. ESI of sarcoplasmic proteins showed significant (P = 0.0039)
difference in stability indexes with alkali extracted samples representing the
highest
values (6.42 and 6.60%).
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
N O -
kn
>
z Al
o c
coo
> coo
coo z
to N M
O cd pq O O O O
O O E
n O O? N ao N \O }~
coo
w \O d, O O
p +I +I +I +I
c O O -~ 0
~ ~ ~ O O O O =~
coo 0
-
W a N N N N
coo
DC =0 'r '
U U 'r '
cd N (n _ _ ~I
o
u
con
u
O con
con
U U
c, con
kr)
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
Foam expansion (FE) and foam volume stability (FVS)
Food foams are dispersions of gas bubbles in a continuous liquid or semisolid
phase. Foaming is responsible for the desirable rheological properties of many
foods.
The behavior of the proteins at the liquid/air interface is important since
the formation
of protein film around air bubbles is essential for foam capacity and
stability. The
foam capacity and foam volume stability of protein isolates from MSTM prepared
at
different extraction pH are presented in Table 6. The foaming properties
between the
pH treatments were found to have no significant differences (P > 0.05), except
foam
expansion of sarcoplasmic proteins. The latter was significantly (P = 0.0014)
lower
when proteins were extracted at pH 2.5. Therefore, reduced foam expansion of
sarcoplasmic proteins at pH 2.5 might be associated with the low
hydrophobicity at
this pH. FE of sarcoplasmic proteins were found to be significantly (P =
0.0019)
lower compared to that of myofibrillar proteins. However there was no
significant (P
= 0.8550) difference between the FVS of these two protein fractions. This
suggests
that even though myofibrillar proteins have higher ability to form the foam,
the
stability might be maintained at the same level by both myofibrillar and
sarcoplasmic
protein fractions. FE (myofibrillar and sarcoplasmic) and FVS of sarcoplasmic
proteins were found to be significantly (P < 0.0001) lower for raw MSTM (Table
5)
compared to processed samples. This denotes that conformational changes of
proteins
during acid and alkaline treatments lead to improvement of foaming properties.
Total pigments and color characteristics
Color is an important factor for determining consumers' perception of product
quality and significantly influences purchasing decisions. Color is also a
principal
characteristic when different processing treatments are compared, especially
considering increased interest of current markets in isolates as white as
possible. The
two pigments which are mainly responsible for the color of MSTM are myoglobin
and hemoglobin, thus their effective removal could greatly improve color
characteristics of recovered meat. The total pigment analysis showed
significantly (P
< 0.0079) higher content in alkali extracted samples (0.56 mg/g of meat)
compared to
47
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
that of acid treatments (0.44 mg/g of meat) (Figure 9). The highest total
pigment
removal was found when MSTM proteins were extracted at pH 2.5 (88.96%), while
the lowest was for extractions at pH 10.5 and 11.5. Stronger protein-protein
interactions at alkaline pH probably result in higher aggregation of
sarcoplasmic
proteins leading to precipitation into sediments after isoelectric
precipitation. In
general, extractions showed 86.72% removal of total pigments, resulting in
around 0.5
mg of heme pigments per 1.0 g of meat.
Color characteristics (L*, a*, b*, a*/b*, saturation, Hue and whiteness) of
the
recovered proteins by the pH-shifting process are presented in Table 7. The
results are
shown in comparison to the initial material (raw MSTM). In general acidic and
alkaline isolates greatly (P < 0.0001) decreased in the redness (a*), with no
difference
being found within pH treatments. The decrease in redness is due to the
removal of
pigments during extraction (Figure 9). Yellowness (b*) values remained
constant (P =
0.0984) for raw MSTM and different extraction treatments. Lightness (L*) was
significantly increased (P = 0.0035) for the samples processed with extraction
pH of
2.5. The concentration of total pigments is the influential factor for the L*
values.
Thus, the extension of total pigment removal, which was observed to be higher
for
acid treated samples contributed to the increased lightness. Whiteness
increased
significantly (P = 0.021) compared to the raw meat, with the highest value
(64.82)
observed at pH 2.5. This is expected, because the whiteness values are mainly
influenced by the lightness, which was the highest for samples extracted with
pH 2.5.
Both lightness and whiteness values are in agreement with the results obtained
from
the analysis of total pigment content, which indicated the highest removal at
extraction pH of 2.5. A significant decrease (P = 0.0036) was observed for
a*/b*,
which indicates a decrease in intensity of the redness value. The ratio
decreased from
the original value of 0.45 found for raw MSTM to 0.24 in general for processed
meat.
High a*/b* obtained for the raw meat is primarily because of the high total
pigment
content. Saturation values determine how different the color is from gray and
expressed as depth, vividness and purity. There was a significant decrease in
saturation (P = 0.0036) observed between raw MSTM and acid extracted meat. The
48
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
samples having a dominant red color would give a higher saturation value than
the
samples with a more homogenous structure. In this study the lower purity of
alkali
extracted meat compared to acid treatments might be the result of higher total
pigment
content. Hue angle shows the degree of departure from the true red axis to the
CIE
color space. The Hue values were found to be significantly (P = 0.0046) higher
for
proteins recovered at pH 2.5; 3.5 and 11.5 compared to raw MSTM. This is
expected,
since increased Hue angle indicates a decrease in perceived redness. As the
result of
extraction procedures, the red color was decreased due to the removal of heme
pigments. Consequently samples decreased in darkness with the dominance of
Hue,
which is an indication that the color shifted slightly to the yellowish
spectrum.
Table 7. Color characteristics of proteins recovered from MSTM at different
extraction pH'
Parameter Raw MSTM 2.5 3.5 10.5 11.5
a* 7.45 0.24a 3.66 1.12b 2.84 0.76b 4.51 0.21b 3.58 1.19b
b* 16.49 0.36 14.43 2.25 14.44 0.98 16.05 0.25 15.82 0.39
L* 58.94 1.55b 69.84 1.75a 61.59 b b
4.74ab 59.90 2.75 57.40 4.08
a*/b * 0.45 0.02a 0.27 0.13b 0.20 0.07b 0.28 0.02ab 0.22 0.07b
Saturation 18.10 0.31a 14.95 1.88b 14.74 0.81b 16.67 0.20ab 16.24
0.64ab
Hue 65.67 0.95b 75.23 6.67a 78.75 3.68a 74.30 0.89ab 77.36 3.81a
Whiteness 52.46 1.27b 64.82 1.99a 57.85 4.38ab 54.93 2.47b 53.19 4.36b
'Results are presented as mean (n=3) standard deviation.
Least square means in each row corresponding to respective parameters with
different superscripts are significantly different (P < 0.05) from one
another.
Texture profile analysis and dynamic viscoelastic behavior of isolated
proteins
49
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
Complimentary information on textural properties of protein isolates was
obtained using small and large deformation tests. A small deformation test was
applied to investigate elastic and viscoelastic properties of gels, which is
related to el
quality and strength. Uniaxial compression of a gel sample between two flat
parallel
plates (large deformation test) was used to determine textural properties,
such as
hardness, chewiness, springiness and cohesiveness.
Texture profile analysis (TPA) of the MSTM protein gels is summarized in
Figure 10. No significant differences (P > 0.05) were found for any of the
parameters.
Generally, the higher hardness of the gels developed from MSTM protein was
observed at pH 10.5, with the value of 1773 gram force. The lowest value for
chewiness (934) was observed at pH 3.5. The lower value for this parameter is
associated with the higher ability to form a viscoelastic network (Figure 11
A, B), as
chewiness represent the ability of the sample to regain its shape after
compression.
Chewiness is also one of the important characteristics, which associates with
meat
tenderness. No significant difference found for springiness value is probably
due to
the same water content between samples, as the extraction process was followed
by
adjustment of water content to 80%. While no difference among treatments was
found
for cohesiveness, the samples extracted at pH 11.5 appeared to be higher.
Gelation of muscle protein is a multi-step thermodynamic process which
involves protein unfolding and aggregation prior to the formation of three-
dimensional network structures indicated that rheological parameters could be
used to
predict sensory, texture and functionality of comminuted meat products. The
dynamic
rheological technique is widely used for the evaluation of gelation of
myofibrillar
proteins. Viscoelastic properties of storage (G' ), loss (G' ') modulus and
tan delta
(=) between acid and alkaline extractions were determined upon heating and
cooling.
Changes in storage modulus, loss modulus and tan = during heating is given in
Figure
11 A, B and C. Proteins isolated at different pH values showed a similar trend
for both
G' and G' ' values. However, the G' values were considerably higher in
magnitude
than the G' ' values indicating the formation of more elastic gels. The G' '
value is
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
an estimation of energy dissipated as heat per sinusoidal cycle and is used to
evaluate
the gel viscosity.
During the heating phase, the G' showed only marginal change, until the
temperature reached 36 C, where onset of gelation occurred (Figure 11A).
Storage
modulus (G') is a measure of the energy stored in material and recovered from
it per
cycle of sinusoidal shear deformation and indicates solid or elastic
characteristics. The
increase in G' has been attributed to the ordered protein aggregation and
formation of
three-dimensional network with water entrapment in the matrix. The gelation
starts
with unfolding of myosin molecules at 35 - 40 C. The same increasing
pattern was
observed in loss modulus for heat induced gelation of dark chicken meat
protein
isolates indicating the formation of a viscoelastic network. G' values
increased until
temperature reached 56 - 58 C; further increase in temperature caused
weakening
of the gels as shown by decrease in G' values. This decrease might be due to
the
result of denaturation of light meromyosin, leading to increased fluidity. The
maximum increase in G' value was in the temperature range of 40 to 56.6 C.
The
forces which are responsible for the formation of the gel network include
hydrophobic
interactions, disulphide cross bridges and hydrogen bonds. Overall, the
patterns of
slopes for acid and alkaline extracted MSTM proteins were similar, excluding
pH
10.5.
Tan = values indicated a major transition point at temperature of 47.3 C for
proteins extracted at pH 2.5, 3.5, 11.5 and 51.9 C for proteins extracted
with pH 10.5
(Figure 11 Q. This transition point refers to the denaturation of the myosin
molecule.
This is consistent with rheological analysis of alkali-extracted proteins from
dark
chicken meat. This may be attributed to the transition temperature at 50.1 O C
to the
denaturation of myosin. One minor transition point was observed for acid
extracted
samples at around 65 C, which corresponds to the denaturation point of
collagen.
Above 35 C tan = values were found to be decreasing until the temperature
reached
47 C for pH 2.5, 3.5, 11.5 and 52 C for the pH 10.5. In general, a decrease
in tan
indicates the formation of an ordered gel network. The use of tan = to
estimate the gel
51
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
characteristics has the advantage of incorporating the contributions of both
G' and
G' ' into a single parameter to evaluate the final network.
Storage modulus values of different protein isolates at various temperatures
(5
0, 56.6 0 and 80 C) is given in Figure 12. The highest (P < 0.0001) G '
value (at 5 0,
56.6 0 and 80 C) was obtained for the sample extracted with pH 3.5. The
lowest was
observed with pH 10.5 extracted samples, while G' for proteins extracted at
more
extreme pH of 2.5 and 11.5 was not significantly different from each other.
The same
trend was observed with increasing temperature to 56.6 C (the peak value for
storage
modulus). At 80 C protein extracted with pH 3.5 possessed significantly (P =
0.0005) higher G' compared to pH 2.5 and 11.5.
On cooling from 80 0 to 5 C, all samples showed an increase in G' and G'
as interactions between the proteins become stronger with the decrease in
temperature
(Figure 13). However, a notable difference was observed for pH 10.5 extracted
proteins, where G' and G' ' showed the lowest values. During cooling the
highest
value was reached at the end of the gelation process. The increase in storage
and loss
modulus is attributed to the formation of hydrogen bonds during cooling. High
G'
value during cooling is also an indication of the formation of a firm gel
structure.
Conclusion
The present study indicated that functional properties and rheological
characteristics of MSTM could be greatly improved by extraction procedures.
Emulsion activity index of myofibrillar proteins was better at extraction pH
of 11.5.
Proteins extracted at pH 3.5 showed higher ability to form a viscoelastic gel
network.
Acid extractions were more efficient in heme pigment removal, which resulted
in
better color characteristics than alkali treated samples. The study revealed
that acid
and alkaline processing can be the alternatives for recovering functional
proteins from
MSTM. In conclusion, proteins extracted at pH 3.5 were found to be the most
suitable considering the rheological characteristics as well as pigment
removal.
III. Preparation of Films
52
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
Protein Solubilization and Extraction
To purify myofibrillar protein isolates, certain amounts of ground chicken
thigh meat or mechanically separated poultry meat was tempered at 4 C
overnight
and then minced in a commercial-grade food processor with ice water. Figure 14
shows an exemplary method for solubilizing and isolating a poultry protein.
The
resulting meat slurry was placed in a large mixing beaker and the pH was
adjusted to
pH 10.5 or 2.5 for protein solubilization using alkali (NaOH) or acids (HC1
and citric
acid) with constant mixing (Figure 15). After 30 minutes, the entire contents
of the
mixing beaker were centrifuged at 13,000 rpm for 20 minutes. Three layers were
formed after centrifugation: an upper fat layer, a middle aqueous layer of
soluble
myofibrillar protein and a bottom sediment layer (Figure 16). The middle
protein
supernatant layer was carefully removed and without further dewatering, the
protein
solution was used in the protein film formation.
Protein Film Formation
Biopolymer films were prepared from casting a film-forming solution based
on mechanically separated and thigh meat protein extracts resulting from the
solubilization and extraction steps described above. The protein concentration
of the
film-forming solution was 1.0% and glycerol as a plasticizer was added at 50%
(w/w)
of protein. The film forming solution was cast on to Teflon coated trays or
silicone
resin plates set on a level surface and air blown for 12 hours at room
temperature prior
to further drying in an environmental chamber at 25 C and 50% relative
humidity for
24 hours. The resulting film was manually pealed off of the Teflon coated
tray.
Figure 17 shows the resulting biopolymer film.
IV. Crosslinking of Protein Compositions
With mechanical stirrer, 5.0% myofibrillar protein solution produced using
the techniques described herein was prepared at pH 11.5. Into the sticky
protein
solutions, 1.0% of the following crosslinkers was added: formaldehyde,
glutaraldehyde or glyoxal. After vigorous stirring, the gelation processes
were
observed. The use of glyoxal provided the best physical properties. The pH
used to
53
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
form the protein gels were change from pH 11.5 to pH 7.5-8.5. Glyoxal
crosslinked
the myofibrillar protein to form strong gel network, which has applications
for
making films.
V. Temperature Effects on Film Production
In order to improve the film performance, the temperature effect was
evaluated on the formulation of 1.6% myofibrillar protein and 0.7% glycerol at
pH
11.5. The results are shown in Tables 8-10. With the increase of temperature,
water
vapor permeability (WVP) increased and tensile strength (TS) decreased (Tables
8
and 9). High temperatures did not promote protein crosslinking, but degraded
the
larger protein molecules to small ones. Therefore, a weaker film structure was
formed
and water vapor can readily pass through the film. Regarding the temperature
influence on elongation at break of the films, no obvious pattern was seen
(Table 10).
Table 8 Temperature effect on Water Vapor Permeability
(WVP)
T ( C) WVP (g/m per s per Pa) Stdev
22 8.97E-11 4.55E-12
45 8.80E-11 2.22E-12
70 9.16E-11 4.13E-12
90 1.09E-10 5.90E-12
Table 9 Temperature effect on Tensile Strength (TS)
T ( C) TS (MPa) Stdev
22 3.46 0.82
45 3.32 0.58
70 2.85 0.70
90 1.96 0.52
Table 10 Temperature effect on Elongation at Break (EAB)
T ( C) EAB (%) Stdev
22 132.6 43.3
45 116.4 49.8
70 125.3 52.0
90 87.3 47.1
VI. Preparation of Noodles using Protein Formulations
54
CA 02801040 2012-11-28
WO 2010/136894 PCT/IB2010/001355
An aqueous solution of 1.6% myofibrillar protein extracted from chicken thigh
meat with 1.0% sodium alginate and 0.7% glycerol was prepared. Next, a 5.0%
calcium chloride solution was prepared. With the 30 ml syringe without needle,
the
homogenous thick protein solution was extruded into the calcium chloride
solution to
produce a high protein and alginate fiber enriched noodle. After water rinse,
the
noodle could be consumed wet or oven-dried depending on the end-use. The
protein
solution can contain various flavors and colorants.
It is to be understood that the above-described compositions and modes of
application are only illustrative of preferred embodiments of the present
invention.
Numerous modifications and alternative arrangements may be devised by those
skilled in the art without departing from the spirit and scope of the present
invention
and the appended claims are intended to cover such modifications and
arrangements.
Thus, while the present invention has been described above with particularity
and
detail in connection with what is presently deemed to be the most practical
and
preferred embodiments of the invention, it will be apparent to those of
ordinary skill
in the art that numerous modifications, including, but not limited to,
variations in size,
materials, shape, form, function and manner of operation, assembly and use may
be
made without departing from the principles and concepts set forth herein.