Note: Descriptions are shown in the official language in which they were submitted.
CA 02801144 2012-11-29
WO 2011/150486 PCT/CA2010/000814
METHODS OF FRACTURING WITH AND PROCESSING LPG BASED TREATMENT
FLUIDS
TECHNICAL FIELD
100011 This document relates to the processing of liquefied petroleum gas
(LPG)-
containing-treatment fluids, and further to the recycle and re-use of
recovered LPG frac
fluid.
BACKGROUND
[0001] In the conventional fracturing of wells, producing formations, new
wells or low
producing wells that have been taken out of production, a formation can be-
fractured to
attempt to achieve higher production rates. Proppant and fracturing fluid are
mixed in a
blender and then pumped into a well that penetrates an oil or gas bearing
formation. High
pressure is applied to the well, the formation fractures and proppant carried
by the fracturing
fluid flows into the fractures. The proppant in the fractures holds the
fractures open after the
pressure is relaxed and production is resumed. Various fluids have been
disclosed for use as
the fracturing fluid, including liquefied petroleum gas (LPG).
[0002] LPG has been advantageously used as a fracturing fluid to simplify the
recovery and clean-up of frac fluids after a frac. Exemplary LPG frac systems
are disclosed
in WO2007098606. Some of these systems send recovered fracturing fluid
straight to a flare
stack for disposal. This method of disposal, while sometimes economical,
results in the loss
of potentially valuable fluids. Other of these systems produce the recovered
LPG frac fluid to
a sales line, since the recovered fluid almost always contains natural gases
that have salable
value. However, delivery to a sales line requires the recovered fluids to be
pressurized.
During pressurization the LPG may condense out, triggering closure of the gas
compressor.
One way to resolve this issue is to add more natural gas to the recovered frac
fluid to lower
the dew point. Since the recovered LPG frac fluid may contain gelling
chemicals, the
requirements and costs of processing the recovery stream are increased.
[0003] Based on the prior references, it is not obvious that LPG from
recovered LPG
frac fluid can be economically processed on-site. This is especially true when
the LPG frac
fluids contain gelling chemicals.
CA 02801144 2012-11-29
WO 2011/150486 PCT/CA2010/000814
2
SUMMARY
[0004] A method of processing liquefied petroleum gas used in a treatment
fluid
previously injected into a hydrocarbon reservoir is disclosed, the method
comprising:
recovering at least a portion of the treatment fluid from the hydrocarbon
reservoir to produce
recovered treatment fluid; and separating liquefied petroleum gas in the form
of a gas or
liquid from the recovered treatment fluid using a separator.
[0005] An apparatus for processing liquefied petroleum gas used in a treatment
fluid
previously injected into a hydrocarbon reservoir is also disclosed, the
apparatus comprising:
a separator; a recovery line for recovering treatment fluid from the
hydrocarbon reservoir,
the recovery line connected to supply recovered treatment fluid to the
separator, the separator
being to separate a liquefied petroleum gas portion in gas or liquid form from
the recovered
treatment fluid.
[0006] An apparatus is also disclosed for processing liquefied petroleum gas
previously injected as at least a portion of a treatment fluid into a
hydrocarbon reservoir
through a well penetrating the hydrocarbon reservoir, the apparatus
comprising: a separator
connected to a recovery line to receive fluids flowed back from the well for
separating the
fluids into at least a liquefied petroleum gas portion, and a natural gas
portion; and the
separator further being connected to supply the liquefied petroleum gas
portion to at least
one of a storage tank for storing liquefied petroleum gas, a flare, and a
pipeline.
[0007] A method is also disclosed of processing liquefied petroleum gas used
in the
treatment of a hydrocarbon reservoir penetrated by a well, the method
comprising: flowing
fluid from the well, the fluid comprising liquefied petroleum gas that has
been previously
injected into the well; providing the fluid to a separator, the liquefied
petroleum gas being
provided at least partially as a gas; and with the separator, separating the
fluid into at least a
liquefied petroleum gas portion, and a natural gas portion, for at least one
of further
processing, sale, disposal, delivery, storage, or re-use of each respective
portion.
[0008] A method of recycling liquefied petroleum gas used in the treatment of
a
hydrocarbon reservoir penetrated by a well is also disclosed. Fluid is flowed
from the well,
the fluid comprising liquefied petroleum gas that has been previously injected
into the well.
CA 02801144 2012-11-29
WO 2011/150486 PCT/CA2010/000814
3
The fluid is provided to a separator, the liquefied petroleum gas being
provided at least
partially as a gas. With the separator, the fluid is separated into at least a
liquefied petroleum
gas portion, a natural gas portion, an aqueous portion, a liquid hydrocarbon
portion and a
solids portion for at least one of sale, disposal, delivery, storage, or re-
use of each respective
portion.
[0009] An apparatus is also provided for recycling liquefied petroleum gas
previously injected as at least a portion of a treatment fluid into a
hydrocarbon reservoir
through a well penetrating the hydrocarbon reservoir, the apparatus comprising
one or more
storage tanks and a separator. The one or more storage tanks are for storing
liquefied
petroleum gas. The separator is connected to a recovery line to receive fluids
flowed back
from the well for separating the fluids into at least a liquefied petroleum
gas portion, a
natural gas portion, an aqueous portion, a liquid hydrocarbon portion and a
solids portion.
The separator is further connected to supply the liquefied petroleum gas
portion to at least
one of the one or more storage tanks.
[0010] A method of recycling liquefied petroleum gas used in the treatment of
a
hydrocarbon reservoir is disclosed. Liquefied petroleum gas is injected as at
least a portion
of a treatment fluid into the hydrocarbon reservoir. At least a portion of the
treatment fluid
injected into the hydrocarbon reservoir is recovered. At least a portion of
the liquefied
petroleum gas is separated from the recovered treatment fluid. Liquefied
petroleum gas
separated from the recovered treatment fluid is re-used by injecting it into
at least one
subsequent hydrocarbon reservoir.
[0011] A method of recycling liquefied petroleum gas used in the treatment of
a
hydrocarbon reservoir is also disclosed. Treatment fluid previously injected
into the
hydrocarbon reservoir is recovered, the treatment fluid comprising liquefied
petroleum gas.
At least a portion of the liquefied petroleum gas is separated from the
recovered treatment
fluid. The separated liquefied petroleum gas is then stored.
[0012] An apparatus for recycling liquefied petroleum gas previously injected
as at
least a portion of a, treatment fluid into a hydrocarbon reservoir through a
well penetrating
the hydrocarbon reservoir is also disclosed. The apparatus comprises one or
more storage
tanks, and a separator. The one or more storage tanks are configured to store
liquefied
CA 02801144 2012-11-29
WO 2011/150486 PCT/CA2010/000814
4
petroleum gas, at least one of the one or more storage tanks containing
liquefied petroleum
gas and being connected to supply the liquefied petroleum gas to the well. The
separator is
connected to receive recovered treatment fluids from the well and further
adapted to separate
and supply at least a portion of the liquefied petroleum gas from the
recovered treatment
fluid to at least one of the one or more storage tanks.
[00131 An apparatus for recycling liquefied petroleum gas previously injected
as at
least a portion of a treatment fluid into a hydrocarbon reservoir through a
well penetrating
the hydrocarbon reservoir is also disclosed. The apparatus comprises a
separator. The
separator is connected to receive recovered treatment fluids comprising
liquefied petroleum
gas previously injected into the well. The separator is also adapted to
separate at least a
portion of the liquefied petroleum gas from the recovered treatment fluid. The
separator is
further connected to supply the separated at least a portion of the liquefied
petroleum gas to
at least one storage tank adapted to store liquefied petroleum gas.
[0014] A method of processing fluid used in the treatment of a hydrocarbon
reservoir
penetrated by a well is also disclosed, the method comprising: flowing fluid
from the well,
the fluid comprising gaseous liquefied petroleum gas that has been previously
injected into
the well; liquefying the gaseous liquefied petroleum gas with the fluid and
providing the
fluid to a separator; and with the separator, separating the fluid into at
least a natural gas
portion and a liquefied petroleum gas portion for at least one of further
processing, sale,
disposal, delivery, storage, or re-use of each respective portion.
[0015] A method of treating a subterranean formation is also disclosed, the
method
comprising: introducing a hydrocarbon fracturing fluid into the subterranean
formation, the
hydrocarbon fracturing fluid comprising a gel of at least liquefied petroleum
gas and a
gelling agent; subjecting the hydrocarbon fracturing fluid to pressures above
the formation
pressure; and at least partially vaporizing the liquefied petroleum gas in
order to break the
gel.
[0016] In various embodiments, there may be included any one or more of the
following features: Separating may comprise separating an aqueous portion and
a liquid
hydrocarbon portion from the recovered treatment fluid. Separating may
comprise separating
a solids portion from the recovered treatment fluid. Separating may comprise
vaporizing the
CA 02801144 2012-11-29
WO 2011/150486 PCT/CA2010/000814
liquefied petroleum gas in the recovered treatment fluid. Separating may
comprise separating
a natural gas portion from the recovered treatment fluid. Separating may
comprise a first
stage comprising separating gases from the recovered treatment fluid, the
gases comprising
gaseous liquefied petroleum gas and natural gas, and a second stage comprising
separating
the gases into a liquefied petroleum gas portion and the natural gas portion.
The second
stage may comprise liquefying the gaseous liquefied petroleum gas to separate
the gases into
the liquefied petroleum gas portion and the natural gas portion. The liquefied
petroleum gas
portion and the natural gas portion may be separated as a cooled stream of LPG
and a cooled
stream of natural gas, respectively, and in which liquefying comprises cooling
a stream of
the gases by transferring heat from the stream of the gases to one or more of
the cooled
stream of LPG and the cooled stream of natural gas. The separator may be a
multi phase
separator, such as a five-phase separator having as output liquefied petroleum
gas in gas or
liquid form, natural gas, an aqueous portion, a liquid hydrocarbon portion,
and a solids
portion. At least part of the natural gas portion may be supplied to a sales
line. Liquefied
petroleum gas may be separated from the recovered treatment fluid in the form
of a liquid.
The liquefied petroleum gas separated from the recovered treatment fluid may
be re-used as
a well treatment fluid. The separator may be connected to supply the liquefied
petroleum gas
separated from the recovered treatment fluid to at least one of a storage tank
for storing
liquefied petroleum gas, a flare, and a pipeline. A heater may be on the
recovery line. The
separator may be adapted to separate a natural gas portion from the recovered
treatment
fluid. The separator may comprise a first separator stage adapted to at least
separate gases
from the fluids, the gases comprising gaseous liquefied petroleum gas and
natural gas; and a
second separator stage connected to receive the gases from the first separator
stage and
adapted to separate the gases into the liquefied petroleum gas portion and the
natural gas
portion. The second separator stage may comprise a liquefier connected to
liquefy the
gaseous liquefied petroleum gas from the gases. At least partially vaporizing
may comprise
reducing the pressure the hydrocarbon fracturing fluid is subjected to. The
hydrocarbon
fracturing fluid may exclude a breaker. Separating may comprise separating the
fluid into
and a liquids portion comprising at least one of water and liquid
hydrocarbons. Separating
the fluid into a liquids portion may comprise separating the fluid into an
aqueous portion and
CA 02801144 2012-11-29
WO 2011/150486 PCT/CA2010/000814
6
a liquid hydrocarbon portion. The liquefied petroleum gas portion may be
stored. Providing
may comprise vaporizing the liquefied petroleum gas in the fluid flowed from
the well.
Vaporizing may comprise heating the fluid. Separating may comprise a first
stage
comprising separating gases from the fluid flowed back from the well, the
gases comprising
gaseous liquefied petroleum gas and natural gas, and a second stage of
separating the
gaseous liquefied petroleum gas from the natural gas. The gaseous liquefied
petroleum gas
may be liquefied to create the liquefied petroleum gas portion. The liquefied
petroleum gas
portion may be re-injected into at least one of the well and another well as
part of a process
of treating the well and the other well, respectively. Separating may comprise
separating the
gases, the aqueous portion, the liquid hydrocarbon portion, and the solids
portion using a
four phase separator. At least part of the natural gas portion may be supplied
to a sales line.
The separator may comprise a first separator stage adapted to at least
separate gases from the
recovered treatment fluid, the gases comprising gaseous liquefied petroleum
gas and natural
gas; and a second separator stage connected to receive the gases from the
first separator stage
and adapted to separate and supply liquefied petroleum gas from the natural
gas. The second
separator stage may comprise a liquefier connected to liquefy the gaseous
liquefied
petroleum gas separated from the first separator stage. Liquefying may
comprise at least one
of pressurization and cooling. Liquefying may comprise cooling with a
refrigeration unit.
[0017] These and other aspects of the device and method are set out in the
claims,
which are incorporated here by reference.
BRIEF DESCRIPTION OF THE FIGURES
[0018] Embodiments will now be described with reference to the figures, in
which
like reference characters denote like elements, by way of example, and in
which:
[0019] Fig. I is a side elevation view, of a treatment system designed to
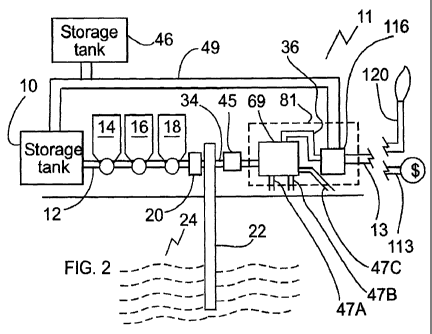
process LPG
treatment fluids.
[0020] Fig. 2 is a side elevation view, of a further treatment system designed
to
process LPG treatment fluids.
[00211 Fig. 3 is a flow diagram of a method of processing liquefied petroleum
gas
used in the treatment of a hydrocarbon reservoir penetrated by a well.
CA 02801144 2012-11-29
WO 2011/150486 PCT/CA2010/000814
7
[0022] Fig. 4 is a side elevation view, partially in section, of a further
treatment
system designed to recycle LPG treatment fluids.
[0023] Fig. 5 is a flow diagram of a further method of recycling liquefied
petroleum
gas used in the treatment of a hydrocarbon reservoir.
[0024] Fig. 6 is a flow diagram of a method of processing liquefied petroleum
gas
used in a treatment fluid previously injected into a hydrocarbon reservoir.
[0025] Figs. 7-9 are schematics of various embodiments of treatment systems
for
processing LPG treatment fluids.
[0026] Fig. 10 is a flow diagram of a further method of processing liquefied
petroleum gas used in the treatment of a hydrocarbon reservoir penetrated by a
well.
[0027] Fig. II is a flow diagram of a method of treating a subterranean
formation.
[0028] Figs. 12A-C together form a schematic that illustrates a further
embodiment
of an apparatus for processing liquefied petroleum gas used in a treatment
fluid.
DETAILED DESCRIPTION
[0029] Immaterial modifications may be made to the embodiments described here
without departing from what is covered by the claims.
[0030] LPG may include a variety of petroleum and natural gases existing in a
liquid
state at ambient temperatures and moderate pressures. In some cases, LPG
refers to a
mixture of such fluids. These mixes are generally more affordable and easier
to obtain than
any one individual LPG, since they are hard to separate and purify
individually. Unlike
conventional hydrocarbon based fracturing fluids, common LPGs are tightly
fractionated
products resulting in a high degree of purity and very predictable
performance. Exemplary
LPGs include ethane, propane, butane, or various mixtures thereof. As well,
exemplary
LPGS also include isomers of propane and butane, such as iso-butane. Further
LPG
examples include HD-5 propane, commercial butane, and n-butane. The LPG
mixture may
be controlled to gain the desired hydraulic fracturing and clean-up
performance. LPG fluids
used may also include minor amounts of pentane (such as i-pentane or n-
pentane), and
higher weight hydrocarbons.
CA 02801144 2012-11-29
WO 2011/150486 PCT/CA2010/000814
8
[00311 LPGs tend to produce excellent fracturing fluids. LPG is readily
available,
cost effective and is easily and safely handled on surface as a liquid under
moderate pressure.
LPG is completely compatible with formations and formation fluids, is highly
soluble in
formation hydrocarbons and eliminates phase trapping - resulting in increased
well
production. LPG may be readily and predictably viscosified to generate a fluid
capable of
efficient fracture creation and excellent proppant transport. After
fracturing, LPG may be
recovered very rapidly, allowing savings on clean up costs. In some
embodiments, LPG may
be predominantly propane, butane, or a mixture of propane and butane. In some
embodiments, LPG may comprise more than 80%, 90%, or 95% propane, butane, or a
mixture of propane and butane.
[0032] Referring to Fig. 1, an embodiment of an apparatus 11 for processing
liquefied petroleum gas used in a treatment fluid previously injected into a
hydrocarbon
reservoir 24, for example through a well 22 penetrating the hydrocarbon
reservoir 24, is
illustrated. Referring to Figs. 7, 8, and 9, various embodiments of apparatus
11 are
illustrated. Referring to Fig. 7, apparatus l 1 has a separator 81 and may
have a recovery line
34, recovery line 34 being for recovering treatment fluid from the hydrocarbon
reservoir 24
(shown in Fig. 1), the recovery line 34 being connected to supply recovered
treatment fluid
to the separator 81, the separator 81 being to separate a liquefied petroleum
gas portion in
gas or liquid form from the recovered treatment fluid Thus, the separator 81
may be
connected to receive fluid, for example recovered treatment fluids, flowed
back from the
well 22. Recovered treatment fluids maybe stored before sending to separator
81 through
recovery line 34. Separator 81 may be provided for separating the recovered
treatment fluids
into one or more of a liquefied petroleum gas portion (line 49), and a natural
gas portion
(line 13). Separator 81, which may include for example one or more devices,
for further
example primary and secondary separators 83 and 85, respectively, may be
further connected
to supply separated liquefied petroleum gas to at least one of a storage tank
(for example
tank 10 in Fig. 1) for storing liquefied petroleum gas, a flare (for example
flare 120), and a
pipeline (for example sales line 113 in Fig. 1).
[0033] Referring to Fig. 1, in some embodiments, apparatus 11 comprises one or
more storage tanks 10 for storing liquefied petroleum gas. Storage tank 10 is
configured to
CA 02801144 2012-11-29
WO 2011/150486 PCT/CA2010/000814
9
store liquefied petroleum gas. Referring to Fig. 2, in some embodiments at
least one of the
one or more storage tank 10 contains liquefied petroleum gas and is connected
to supply the
liquefied petroleum gas to the well 22. Referring to Fig. 7, in some
embodiments, the
separator 81 is provided for separating the recovered treatment fluids into a
liquids portion
(line 87), for example comprising at least one of water and liquid
hydrocarbons. In the
embodiment illustrated, liquids, for example C6+ and water, are removed from
primary
separator 83 via line 87, where they are sent to pressure'tank 93, and
optionally vented to
flare 120. The liquids portion may include various dissolved chemicals, such
as gelling
chemicals. The LPG portion separated into line 49 may be combined with line
87. The
contents of the pressure tank 93 may be processed, for example after transport
to the nearest
LPG facility. Referring to Fig. 1, in some embodiments, separator 81 may
separate the
recovered treatment fluid into an aqueous portion (line 47B) and a liquid
hydrocarbon
portion (line 47A). Referring to Fig. 7, in some embodiments a solids portion
may be
separated out, using for example a sand catcher/separator 89 or satellite
tank. The solids
portion may be removed by at least one of separator 81 as shown and a separate
device.
[0034] Referring to Fig. 1, in the embodiment shown separator 81 is provided
for
separating the fluids into at least a liquefied petroleum gas portion (line
49), a natural gas
portion (line 13), a liquid hydrocarbon portion (line 47A), an aqueous portion
(line 47B), and
a solids portion (47C). Separator 81 may be further connected to supply the
liquefied
petroleum gas portion to at least one of the storage tanks 10. The recycled
LPG portion can
then be for example further processed, re-used, stored, sent to a sales line,
disposed of, or
delivered to another user. Where the LPG portion is sent to a sales line, the
flow of LPG may
require careful control to ensure that the minimum standards for pipe line
contents are met,
for example the dew point is not exceeded. Most natural gas sales lines allow
methane
streams with a maximum of 10% propane volume in the total gas stream. If the
sales line is
directed to a facility, flow of the LPG portion into the pipeline should be
controlled to ensure
that the facility capacity is not exceeded.
[0035] Referring to Fig. 2, LPG may be initially contained within a storage
tank 10,
as for example LPG or LPG and other frac fluids. Tank 10 may comprise, for
example, a
tanker truck or a large vessel. The LPG may be pumped from reservoir 10 down
line 12,
CA 02801144 2012-11-29
WO 2011/150486 PCT/CA2010/000814
where various chemicals may be added to the fluid, for example via a chemical
addition
system 14. Other components may be added as well, such as gelling agent and
proppant,
from addition systems 16 and 18, respectively. The addition systems may be,
for example,
hoppers. Once the LPG is prepared and ready, a frac pressure pump 20 injects
the LPG down
a well 22 and into hydrocarbon reservoir 24. The liquefied petroleum gas may
be initially
injected as at least part of a treatment fluid containing for example lower
vapor pressure
hydrocarbons. The concept of reservoir treatment is well known, and the
details need not be
described here. In fracturing treatments, pressure may be applied to the LPG
injected into the
hydrocarbon reservoir 24. The pressure may be sufficient to cause fracturing
of the
hydrocarbon reservoir.
[0036) Referring to Fig. 3, a method of recycling liquefied petroleum gas used
in the
treatment of a hydrocarbon reservoir penetrated by a well is illustrated.
Referring to Fig. 7,
in a stage 100 (shown in Fig. 3), fluid is flowed from the well 22, the fluid
comprising LPG
that has been previously injected into the well 22, for example using the
system illustrated in
Fig. 2. When LPG frac fluid is recovered out of well 22, it is almost always a
combination of
solids, liquids (including LPG), and gas (including LPG and reservoir gases).
[0037] In a stage 102 (shown in Fig. 3), the fluid is provided to a separator
81, the
LPG being provided at least partially as a gas. In some embodiments,
separating or providing
comprises vaporizing the LPG in the fluid flowed from the well 22, for example
by heating
the fluid. Referring to Fig. 2, beating the fluid may be accomplished using
heater 45, which
may be a line heater. It is advantageous that the heating step be carved out
on a moving
stream of fluids, since this results in a more efficient and quick
vaporization than merely
heating the fluids in separator 69 for example. Referring to Fig. 7,
vaporization of all LPG in
the fluid also allows higher boiling liquids and gelling chemicals to be
removed via
deposition in for example primary separator 83. Referring to Fig. 2, in some
embodiments, a
vacuum source may be used to vaporize the LPG in the fluids. Where a vacuum
source is
used, additional heat may need to be supplied to fluids in separator 81 to
offset the cooling
effects of vaporizing LPG. Referring to Fig. 1, in some embodiments a heater
may not be
required, for example if the recovered fluid with the present LPG returned
largely, and
CA 02801144 2012-11-29
WO 2011/150486 PCT/CA2010/000814
11
preferably entirely, as a gas, or in some cases where no gelling agents are
present in the
treatment fluid
[0038] Referring to Fig. 7, in a stage 104 (shown in Fig. 3), the fluid is
separated
with the separator 81 into at least a liquefied petroleum gas portion and a
natural gas portion
for at least one of further processing, sale, disposal, delivery, storage, or
re-use of each
respective portion. Referring to Fig. 1, as discussed herein, the fluid may be
separated within
the separator 81 further into at least one of a solids portion, and a liquids
portion for further
example a liquid hydrocarbon portion, and an aqueous portion, for at least one
of further
processing, sale, disposal, delivery, storage, or re-use of each respective
portion. Further
processing may be achieved for example at a processing facility. Sale may be
achieved by
for example the transfer of liquid hydrocarbons into a sales line 113.
Disposal may be
achieved for example by flaring natural gases from a flare stack 120, or by
dumping the
solids portion at a dump site according to applicable environmental standards.
Delivery may
be achieved by for example loading the aqueous portion into a tanker truck for
transport to a
processing facility. Storage may be achieved by for example storing the liquid
hydrocarbon
portion in a storage tank. Re-use may be achieved by for example re-using the
LPG portion
in a subsequent fracturing operation. The liquefied petroleum gas portion,
natural gas
portion, liquid hydrocarbon portion, aqueous portion, and solids portion are
illustrated as
being separated into exemplary lines 49, 13, 47A, 47B, and 47C, respectively.
[0039] Separating may comprise a first stage comprising separating gases from
the
recovered treatment fluid flowed back from the well, the separated gases
comprising gaseous
liquefied petroleum gas and natural gas, and a second stage of separating the
gases into a
liquefied petroleum gas portion and the natural gas portion. This effectively
separates the
gaseous liquefied petroleum gas from the natural gas. Referring to Fig. 2, the
first separator
stage may be adapted to at least separate gases from the fluids and may be a
multi phase
separator, for example a four phase separator 69. The four phase separator 69
may be used to
separate the aqueous portion, the liquid hydrocarbon portion, the gas portion,
and the solids.
Four phase separators are known, and may be purchased commercially, for
example those
units sold by Canadian Sub-Surface or Grant Production Testing Services Ltd. A
4-phase
separator 69 may separate out gas, oil, water, and solids for example through
lines 36, 47A,
CA 02801144 2012-11-29
WO 2011/150486 PCT/CA2010/000814
12
47B, and 47C, respectively. Such multi-phase separators may use augers, weirs,
and
centrifuges for example.
[0040] The second separator stage, which may be accomplished using for example
at
least a liquefier 116, may be connected to receive the gases from the first
separator stage and
adapted to separate and supply liquefied petroleum gas from the natural gas.
Referring to
Fig. 8, the second separator stage may include liquefier 116 and secondary
separator 85, with
liquefier 116 supplying a stream of fluids through line 122 comprising
liquefied petroleum
gas and natural gas to the separator 85, where the liquid and gas streams are
then separated
into lines 49 and 13, respectively. In this and other embodiments, liquefier
116 may be an on
stream refrigeration unit, for example at least one of a chiller unit or Joule-
Thompson unit
made by DPC. A refrigeration unit may cool the gas stream to a suitable dew
point level. In
some embodiments, the stream of fluids is dehydrated in association with the
use of liquefier
116. In other embodiments, suitable water control methods may be used, such as
methanol
injection to prevent freezing in the refrigeration unit. In some embodiments,
the liquefier 116
is connected to liquefy the gaseous liquefied petroleum gas separated from the
natural gas.
Gas liquefaction may be carried out using known techniques. The liquefier may
be carried
out using at least one of high pressure and low temperature, for example using
at least one of
a refrigeration circuit/device, a compressor, a distiller, a cooler, and a
condenser. The natural
gases are generally reservoir gases, and may include methane, carbon dioxide,
nitrogen,
helium and hydrogen sulfide. In one embodiment, natural gas means
predominantly
methane. It should be understood that low boiling gases, for example ethane
and carbon
dioxide, which were used as part of the treatment fluid may also be present,
and hence
removed in this stage as well. The vapors may be at least one of pressurized
to the maximum
and cooled to conditions required to liquefy only the liquefied petroleum gas,
for example
propane or butane, separated as a gas, in order to separate LPG from undesired
higher
boiling gases. This way, lower boiling gas molecules such as nitrogen, carbon
dioxide,
methane, and sometimes ethane for example may be separated as gases and not
liquefied
with the LPG. In some embodiments the liquefying conditions, and indeed the
process as a
whole, is tailored to reduce or exclude the amount of ethane that liquefies
along with the
desired LPG portion to be separated. This may be the case for example if the
LPG portion
CA 02801144 2012-11-29
WO 2011/150486 PCT/CA2010/000814
13
desired to be separated is predominantly propane, butane, or propane and
butane. Referring
to Fig. 1, these gases, particularly methane, may be removed for example to a
flare stack 120
or to a sales line 113 for disposal or sale, respectively. In general it is
advantageous to supply
at least part of the natural gas portion to a sales line. Meanwhile, the LPG
produced can be
transferred to storage tanks 10, 46 via lines 49. A compressor (not shown) may
be required
prior to the sales line. Wherever pressurization is required, the fluid may
require
conditioning such as for example dehydration or dew point control to prevent
freezing and
damage of various pressurization equipment. Most natural gas sales lines
require a maximum
water dew point of -10 C at operating pressure.
[0041) Referring to Fig. 2, in some embodiments the method further comprises
liquefying the gaseous liquefied petroleum gas to create the liquefied
petroleum gas portion
and re-injecting the liquefied petroleum gas portion into at least one of the
well 22 and
another well (not shown) as part of a process of treating the well and the
other well,
respectively. In this way, the LPG is recycled and re-used as an LPG frac
fluid. This
represents an advantage over traditional LPG frac-ing where the recovered
fluid is disposed
of or sold, because up to 90, 95, and 99% of the LPG fluid used in the
treatment can be
recycled into a re-usable form.
[0042] Embodiments of the processes disclosed herein may be used to remove
contaminants from the LPG fluid to produce a re-usable fluid. Exemplary
contaminants may
include those from the injected treatment fluid, for example particulates such
as proppant,
gelling chemicals, and non LPG gases such as CO2. Exemplary contaminants also
include
those introduced from the hydrocarbon reservoir 24 such as particulates,
water, and non-LPG
gases such as formation gases. Removing may further comprise at least one of
settling,
phase-separating, centrifuging, and filtering.
[00431 Other embodiments of the processes disclosed herein may be used to
produce
salable natural gas from the recovered treatment fluids. During the fracturing
process, natural
gases mix with the injected LPG treatment fluids, and the mixed natural gases
may represent
a valuable commodity once they reach the surface. By separating and producing
the natural
gas from the recovered fluids, the natural gas may be used to offset the cost
of the fracturing
treatment.
CA 02801144 2012-11-29
WO 2011/150486 PCT/CA2010/000814
14
[0044] The liquids, including the oil and water portions, may be separated
from the
fluid in a liquid separation unit (not shown). The liquid separation unit may
have several
weirs designed to allow water to collect at the bottom of each compartment
defined by the
weirs, in order that the water may be removed. The solids portion may also be
removed, for
example by at least one of settling and filtration. In some embodiments, the
liquid removal
stage may also comprise one or more of decanting, centrifuging, skimming, and
drying, and
may include other suitable steps. Such a unit may have a gas takeoff for
removing the gases
from the recovered treatment fluid, such as gaseous LPG and lighter weight
gases.
[0045] In some embodiments, separator 81 acts as a five-component separator.
It
should be understood that the function of separator 81 may be achieved in a
variety of ways
for example using a single separator or more than one separators. A five phase
separator may
have as output liquefied petroleum gas in gas or liquid form, natural gas, an
aqueous portion,
a liquid hydrocarbon portion, and a solids portion. For example, the separator
may be three
separator stages, each a different piece of equipment, one for removing gases,
one for
removing LPG from the gases, and another for separating the solids, oil, and
water. In other
examples, the four-phase separator 69 may be composed of smaller separation
units or
systems. The various sub-systems of separator 81 may be combined together in a
single unit,
for example on a mobile unit such as a trailer bed or a skid, or may be made
up by several
smaller distinct systems. In some embodiments, natural gas removal may be the
initial step.
In addition, other treating stages or units may be added as desired, for
example a chemical
treatment stage to remove gelling chemicals. Prior to cleaning, the recovered
fluids may be
stored in a suitable storage unit.
[0046] Referring to Fig. 4, an apparatus 11 for recycling liquefied petroleum
gas
previously injected as at least a portion of a treatment fluid into a
hydrocarbon reservoir 24
through a well 22 penetrating the hydrocarbon reservoir 24 is illustrated
Apparatus 11
comprises one or more storage tanks 10, 46. Storage tanks 10, 46 are
configured to store
liquefied petroleum gas, at least one of the one or more storage tanks, in
this case tank 10,
containing liquefied petroleum gas and being connected to supply the liquefied
petroleum
gas to the well. A recovery line 34 may be connected to recover treatment
fluid from the well
22 and supply recovered treatment fluid. Apparatus 11 further comprises a
separator 81
CA 02801144 2012-11-29
WO 2011/150486 PCT/CA2010/000814
connected to receive recovered treatment fluids from the well 22 and further
adapted to
separate and supply at least a portion of the liquefied petroleum gas from the
recovered
treatment fluid to at least one of the one or more storage tanks 10, 46. In
some embodiments,
separator 81 is connected to receive fluids from the recovery he 34.
[0047] Referring to Fig. 4, a further apparatus 11 is illustrated for
recycling liquefied
petroleum gas previously injected as at least a portion of a treatment fluid
into a hydrocarbon
reservoir 24 through a well 22 penetrating the hydrocarbon reservoir 24. The
apparatus 11
comprises a separator, for example separator 81. The separator is connected to
receive
recovered treatment fluids comprising liquefied petroleum gas previously
injected into the
well 22, for example via line 34. The separator is also adapted to separate at
least a portion
of the liquefied petroleum gas from the recovered treatment fluid, and further
connected, via
for example line 49, to supply the separated at least a portion of the
liquefied petroleum gas
to at least one storage tank 10, 46, adapted to store liquefied petroleum gas.
Apparatus 11
may have a heater 45 for vaporizing LPG in the recovered fluid.
[0048] Referring to Fig. 5, a further method of recycling liquefied petroleum
gas
used in the treatment of a hydrocarbon reservoir 24 is illustrated. Referring
to Fig. 2, in a
stage 200 liquefied petroleum gas is injected as at least a portion of a
treatment fluid into the
hydrocarbon reservoir24. In a stage 202 at least a portion of the treatment
fluid injected into
the hydrocarbon reservoir 24 is recovered. In a stage 204 at least a portion
of the liquefied
petroleum gas is separated from the recovered treatment fluid. In a stage 206
liquefied
petroleum gas separated from the recovered treatment fluid is re-used by
injecting it into at
least one subsequent hydrocarbon reservoir.
[0049] Referring to Fig. 6, a method of processing, for example recycling,
liquefied
petroleum gas used in a treatment fluid previously injected into a hydrocarbon
reservoir is
illustrated. Referring to Fig. 1, in stage 208 (shown in Fig. 6) at least a
portion of the
treatment fluid is recovered from the hydrocarbon reservoir 24 to produce
recovered
treatment fluid, for example via line 34. In stage 210 (shown in Fig. 6),
liquefied petroleum
gas is then separated from the recovered treatment fluid in the form of a gas
or liquid, or a
gas and a liquid, for example using separator 81. The separated liquefied
petroleum gas may
be stored, for example in at least one of storage tanks 10, 46. In other
embodiments, the
CA 02801144 2012-11-29
WO 2011/150486 PCT/CA2010/000814
16
separated LPG may be transported via a sales line (shown in Fig. 5 as line 113
for example)
instead of, or in addition to being stored. The method may further comprise re-
using at least
a portion of the liquefied petroleum gas as a well treatment fluid, for
example through
storage, addition of chemicals, and then injection of the resulting fluid into
at least one
subsequent hydrocarbon reservoir. This further stage may be similar to stage
206.
[0050] Referring to Fig. 10, a further method of recycling liquefied petroleum
gas
used in the treatment of a hydrocarbon reservoir is illustrated. Referring to
Fig. 9, in a stage
300 (shown in Fig. 10) fluid is flowed from the well 22, the fluid comprising
gaseous
liquefied petroleum gas that has been previously injected into the well 22.
The fluid may
flow for example through a recovery line 34, past for example sand catcher 89
and choke 91.
In a stage 302, the gaseous liquefied petroleum gas is liquefied, for example
with liquefier
116, with the fluid and provided to a separator, for example primary separator
83. In a stage
304, the fluid is separated with the separator 83 into at least a natural gas
portion (line 13)
and a liquefied petroleum gas portion (line 49) for at least one of further
processing, sale,
disposal, delivery, storage, or re-use of each respective portion. This method
may be used for
quick separation of the natural gas portion. The LPG portion may include other
species, such
as for example heavier hydrocarbons and water.
[00511 Referring to Figs. 1 - 10, exemplary systems are illustrated for
carrying out
the embodiments of the methods disclosed herein. These systems are general
schematics, and
a skilled worker will understand that additional components that are not shown
may be
required to implement the system. The illustrated components are for
illustration only, and
therefore are not to scale.
[0052] Referring to Fig. 11, a method of treating a subterranean formation,
for
example hydrocarbon reservoir 24 in Figs. 1 and 2, is illustrated. In a first
stage 306, a
hydrocarbon fracturing fluid is introduced into the subterranean formation,
the hydrocarbon
fracturing fluid comprising a gel of at least liquefied petroleum gas and a
gelling agent. In a
stage 308, the hydrocarbon fracturing fluid is subjected to, for example using
at least one
frac pressure pump (not shown), to pressures above the formation pressure, for
example
pressures at or above fracturing pressures. In a stage 310, the liquefied
petroleum gas is at
least partially vaporized, for example reduced sufficiently in density, in
order to break the
CA 02801144 2012-11-29
WO 2011/150486 PCT/CA2010/000814
17
gel. Stage 310 may be accomplished in numerous ways, for example by at least
one of
allowing the frac fluid to mix with natural gases downhole to lower the
critical temperature
of the frac fluid, allowing the temperature of the frac fluid in the formation
to rise by
equalization with the formation temperature, and reducing the pressure the
frac fluid is
subjected to. Such methods are advantageous in that they do not require, and
in some cases
may exclude altogether, the use of a breaker to break the gel. Thus, the cost
of preparing
such a fracturing fluid is reduced, and the cost of recycling or processing
flowback from
such a fluid may be reduced as well.
[0053] Referring to Fig. 2, a further method of treating a subterranean
formation, for
example reservoir 24, is also disclosed. A first fluid part of a hydrocarbon
fracturing fluid is
introduced into the subterranean formation, the first fluid part comprising
liquefied
petroleum gas, the first fluid part for example comprising predominantly
liquefied petroleum
gas. The first fluid part may be for example a pad of for further example 50
cubic meters of
LPG. A second fluid part of the hydrocarbon fracturing fluid is then
introduced into the
subterranean formation after the first fluid part, the second fluid part
comprising one or more
of liquid hydrocarbons with at least six carbons or LPG. In one embodiment the
second fluid
part comprises predominantly liquid hydrocarbons with at least six carbons.
The second fluid
part may be used to carry proppant. The second fluid part may comprise a
gelling agent. The
first part and second part may at least partially mix together downhole. The
hydrocarbon
fracturing fluid is then subjected to pressures above the formation pressure,
for example
above fracturing pressures. This method may allow cheaper heavier liquid
hydrocarbon
fracturing fluids to be used in a frac, while still taking advantage of the
ease of removal of
LPG from the formation. After squeezing the LPG into the formation, the second
fluid part
acts like a plunger to press the injected LPG pad further into the formation
to frac. Upon
flowback, the volatile LPG pad actively aids to push the second fluid part out
of the
formation, thus simplifying clean-up. In addition, using a pad of ungelled LPG
is
advantageous particularly in oil reservoirs because the LPG pad mixes with and
dilutes the
oil, allowing the following injection of gelled frac fluid to more effectively
penetrate the
reservoir.
CA 02801144 2012-11-29
WO 2011/150486 PCT/CA2010/000814
18
[0054] Figs. 12A-C illustrate a further embodiment of a process of processing
liquefied petroleum gas used in a treatment fluid. In addition, Table 1 is
provided below to
indicate the composition of fluids at various stages in the process scheme. As
LPG treatment
fluids are recovered from the well, the feed mole fraction of propane drops
from around 0.90
for initial recovery to zero at full recovery. Table I illustrate statistics
for a feed recovery
stream (Feed) at 0.67 mole fraction propane, eventually recovering purified
LPG
(Product LPG) with a propane mole fraction of 0.9, and a butane mole fraction
of 0.07, with
around 0.01 mole fraction of methane. The LPG produced as Product LPG is
suitable for
use in a further treatment, sale, or storage, as may be desired. The operation
of the illustrated
schematic will now be described Starting at the stream referenced as "Feed",
recovered
treatment fluids are fed into the system, and pass through a satellite tank
(Sat 1) to remove
solids, before heading as stream Si through a heater H2, in order to fully
vaporize any LPG
in the fluid. Exit stream S2 is then combined in mixer M5 with stream 24,
which will be
discussed in greater detail below. The resulting stream S6 then passes into
one or more
separator tanks Sep3 and Sep4. Generally, if the recovered treatment fluids
contain water or
heavier hydrocarbons, such components would be removed from the fluid stream
from the
base of Sep3 and Sep4 in streams S2 B and S 6B, respectively. Should the
fluids that are
removed as S2_B and S6 -B contain high mole fractions of LPG components, these
streams
may be rerouted back into stream S24, or into the De-ethanizer Dl as desired.
[0055] The gaseous stream S3, which contains mainly methane, ethane, propane,
and
butane all as gases, is taken from the top of Sep3 and passed through a
compressor CPl in
order to pressurize the fluids. Thus begins a series of pressurizations and
temperature
reductions that will eventually liquefy the LPG components and separate out
methane gas.
The gas stream is then passed through a forced flow air cooler AC I, and sent
as stream S5
into the next separator tank Sep4, where gas stream S7 is taken off of the top
of for further
processing. Because of the subsequent pressurization and cooling of the gas
stream, at this
stage a hydrate reducing agent may be added to the stream S7. In the
illustration provided,
the hydrate reducing agent combined with stream S7 in mixer MI is supplied via
stream S22
as a mixture of Ethylene glycol (0.82 mole fraction) and water (0.18 mole
fraction). In this
case, the hydrate reducing agent is added in about 1% VN, although other
concentrations
CA 02801144 2012-11-29
WO 2011/150486 PCT/CA2010/000814
19
may be used as desired. In addition, the hydrate reducing agent may be added
at more than
one point in the stream, such as after each heat exchanger. Another example of
a hydrate
reducing agent is methanol. In addition, the hydrate reducing agent may be
supplied as part
of a hydrate reducing agent regeneration system aimed to regenerate and re-use
the agent. An
example of such a regeneration system is further detailed below.
[0056] The stream S9 is then sent through one or more heat exchangers Hx3,
Hxl,
and Hx2 in series in order to drop the temperature of the stream. Heat
exchangers used
herein may use recycled heat in order to make the process more economical. In
general, the
system shown in Figs. 12A-C may be operated using only one gas or diesel
powered
generator to provide electrical energy to power all the LPG recycling
equipment. Stream 13
is then sent through a chiller C4, of a refrigeration unit, where the
temperature is reduced
further to liquefy LPG components in the stream. Finally, the stream S16 is
sent into a low
temperature separator LTS, which in the example shown is a 3 phase separator.
At the base
of the LTS is removed stream LTS Hvy, which is mostly a stream of hydrate
reducing agent.
LTS Hvy is then fed back into the hydrate reducing regeneration system,
discussed further
below.
[0057] As shown, the liquefied petroleum gas portion and the natural gas
portion
may be separated as a cooled stream of LPG (LTS_HC Liq) and a cooled stream of
natural
gas (LTSVap), respectively. Tracking the path of the natural gas, stream
LTSVap is removed
from the top of separator LTS, and passed through heat exchanger Hx2 in order
to warm up
and cool the stream of gases S 12 that are in the process of being cooled. In
general,
liquefying the gaseous LPG may further comprise cooling a stream of the gases,
such as
streams S9, 510, S12 and S13, by transferring heat from the stream of the
gases to one or
more of the cooled stream of LPG and the cooled stream of natural gas. This
recycles heat,
reducing the operating costs of the systems and increasing efficiency. It also
allows the LPG
portion and natural gas portions to be heated to desired temperatures. It
should be understood
that heat exhange in any embodiment disclosed herein may recycle heat from
another part of
the process, or may even exchange heat with the environment. An example of the
latter is
shown with heater C 1, which is a representation of heat transfer from the
environment to the
hydrate reducing agent in stream S26. Referring back to the path of natural
gas in the stream
CA 02801144 2012-11-29
WO 2011/150486 PCT/CA2010/000814
LTSVap, after passing through two heat exchangers Hx2 and Hx3, the gas may be
suitably
heated and ready for production as sales gas. Additional processing may be
carried out on
the stream of Sales Gas as desired, for example to tailor the dew point or
composition.
Alternatively, the natural gas produced here or at any stage of the
embodiments disclosed
herein could be used to power the recycling process.
[00581 Tracking the path of purified LPG from separator LTS, stream LTS HC Liq
has thus far had the mole fraction of propane raised from the Feed stream from
0.66 to 0.85.
Additional processing may be carried out at this stage, such as further
purification achievable
by passing the LPG stream through a De-ethanizer D 1. Stream LTS HC Liq is
first passed
through De-Ethanizer Feed Pump D2, and heat exchanger Hxl, before being sent
into the
De-ethanizer DI. From the base of De-ethanizer DI, a stream of purified LPG
(Product LPG) is removed with a combined propane/butane mole fraction of 0.97.
Various
components such as a re-boiler (not referenced) may be used with De-Ethanizer
as desired.
Gases from the top of De-ethanizer D1 may still have relatively high mole-
fractions of
methane or propane, and may be re-circulated back into the system through
stream S24.
[0059] The Hydrate reducing agent regeneration system will now be described,
with
reference to the exemplary hydrate reducing agent of ethylene glycol and
water. Various
mole ratios of ethylene glycol and water may be used, such as 4:1 or 1:1, as
examples. From
separator LTS, stream LTS_Hvy, which is largely water and ethylene glycol, is
heated by the
environment at heater Cl, before being passed as stream S25 through heat
exchanger Hx4.
Stream 30_1 is then passed through a flash separator tank EF FlasbSep, where a
relatively
small amount of gases is removed as stream S31M1, and liquids are removed as
stream S27
and passed into an ethylene glycol regenerator EG-Regen. EG-Regen may have,
for example
condensers and reboilers as desired. Vapors are removed from EG-Regen as
stream EG-
RegenVap, and may largely consist of water vapor in the example shown. The
liquid leaving
EG-Regen as stream EGRegenLiq has roughly the same Ethylene Glycol/Water
composition
as the input stream "Makeup", and is thus combined with stream "Makeup" and
cycled again
through the system.
[0060] Chiller C4 may be part of a suitable refrigeration system such as the
one
shown. The exemplary refrigeration system shown here is a propane system, with
a Chiller
CA 02801144 2012-11-29
WO 2011/150486 PCT/CA2010/000814
21
C4, a Suction Scrubber (RefSnScrbr), a heater HI, a compressor RefCompr, an
air-cooler
AC3, an accumulator RefAccum, and other components as required and known in
the art,
such as valve V 11. Other components used in the system as a whole, such as
lines, valves
(example V 1-4, 6, 7, and 11) are understood to be conventional components.
[00611 Table 1: Properties of fluid at various stages of Recycling. PENN Name
EGRegenLiq EG_RegenVap Feed LT5-Hvy LTSVap LTS HC_LIq
VapF rac 0 1 1 0. 1 ....... ......... ...0õ
....._. ............ .._..- . ....... ........ .... __..................
......... _._......... ..._..................................
_......................... ....... ........................ .... .
T iC) 134 110.9 16 -38.5 -38.5 -3&5
P [kPa) 135 132 45 800 1135_53855 1135.53855 1135 53855
MoleFluw [k8male/111 - 7 85 O 23 389 95 3.08 56.05 7309
.. ... ......... ..... ........
StdLigVolumeFlow [m3Jhrj 0 112 0.004 15.53 0-116 3.74 6.232
stcGasV0lumeFlow ISCMDJ j52E+03 1 30E+02 1 08E+OS 1 75E+03 3.13E+04 4.16E404
MoleFraction [Fraction)
HYDROGEN 0 0 0 0 0 0
HELIUM-4 0 0 0 0 0 0
0
...OXYGEN_ 0 0......._.....~..._._..... ...... _ ..... 0 0
NITIOGEN 0 0 0.07 0 0.24 0.01
........... ......... _. ....................
..........................................................
CARBON DIOXIDE 0 0 0 0 0 0
HYDROGEN SULFIDE 0 0 0 0 0 0
METHANE 0 0 0.19 0 0.61 0.07
_...._ ......................................... _................
_..................... ...._....__......_..... .... .
ETHANE 0 0.03 0 0.03 0.04
._....... . .. ........... . 0 ........... ....
PROPANE 0 0 0.67 ........................ 0_... 0.11 0.85
.. .......................... ....._............................ ..
ISOBUTANE 0 0 0.02 0 0 ..,,.,..Ø01
n-BUTANE 0 0 OA3 O 0 0.02
...... .... ............................ ...._._...._._._._..._...-....
_.._.._.... ._... ....._._...._...._.._.._._...
ISOPENTANE 0 0 0 0 0 0
n.PENTANE 0 0 ...............0 0 0 0
0 0 0
n-HEXANE 0.......__...._....., ~......:.::... ,....__.d....
........................ ...............................Ø..
,........ _
C7+.
..........................................p............................_......
........................ ........................_...... _
MassFraction [Fraction]
WATER 0.1838 ...................Ø9796......._...__._
..................._...:Ø21............4..10E-06............5,24EA7
ETHYLENE GLYCOL 0.8162 0.0102 0 0.7893 9.63E-09 1.97E-06
CA 02801144 2012-11-29
WO 2011/150486 PCT/CA2010/000814
22
Name Makeup Product LPG Si 52 52,8 53 54
VapFrac... 0 0 .............. 1............ 0 1 1
T (C1. --- --- -.-_...Z~._..._..............__..._-29.8..._.._.._
56.__............57;4 60 60 112.7
P[kPaJ 150 1245 800 765 765 765 1835
MolPFlow (k8rnole/h) 0 134-09 190.17 190-37 0 214.67 214.67
StdUgVolumeFlow (m3/hr] 0 11.793 15.537 15.537 0 17.385 17.385
------------------------
StdGasVoiumeFlow (SCMD) 8.00E-01 7.62E+04 1.08E+05 1.08E+05 5.69E-38 1.22E+05
1.22E+05
Molefraction [Fraction]
- --------------------------------.._.
HYDROGEN 0 0 0 0 0 0 0
HELIUM-4 0 0 0 0 0 0 0
..._.__ ..... .......... ...........................-.-..-
....._.v.........__..__.......... -
___,.............,.....,.,.._............................__. .._. _ _....._..
,._ _
OXYGEN 0 0 0 0 0 0 0
NITROGEN 0 0 0.07 0.07 0 0.07 0.07
............. ..... ........ ..... ...... . ........... .... .... ........
..... ......... -._ ...- - ..-_ . ...... ....... CARBON DIOXIDE 0 0 0 0 0 0 0
HYDROGEN SULFIDE 0 0 0 0 0 =..=., .,.,,,.. _~ .._........_...-0
METHANE 0 0.01 0.19 0.19 0.03 0.2 0.2
...- _.......... .......... .._.._ . .... .... __- . .... ..... .... ......
..... . _-.-...._._.................. __.._..._.._...-. ...-,._
ETHANE 0 0.02 0.03 0.03 0.01 0.03 0.03
_........... .._....... --..---..................... ..._....-......
........... _.-,_....._............ __
PROPANE 0 0.9 0.67 0.67 0.8 0.66 0.66
ISORUTANE 0 0.03 0.02 0.02 . _. 0.04 0.02_.___._ 0.02
n-BUTANE 0 0.04 0.03 0.03 0.07 0.02 0.02
_.............,_...__..__... ..----..._------ ....... -------- .-._..-_----- --
--._....
ISOPENTANE 0 0 0 0 0 0 0
n-PENTANE 0 0 0 0 0 0 0
_..._
......... ....... _............ ...... ....... _......_....._....
..._...._..... _._........ ................ ....... .........._-.__-_
...................._.__......---- ....._........ _...... ........ .... ----
......
n-HEXANE 0 0 0 0 0.01 0 0
-- - __......._.._.................... _........_....... -..__-------
........_.._........_.__.
C7+~ 0 0 0 0 0.03 0 0
_ . .. . ... .... __=- --.......
MassFraction [Fraction]
WATER 0.2 5.77E-04 0.001 0.001 3.02E-04 0.0018 0.0018
__._..
_._.--.._._..__..... _ ................. ....._............ ...__.....
__.._....... ..'--.......... ....... -.-.._.......
.._._.........._._..........__..._..... .._....... _._.........
..__..,..__..._. ..... _....
ETHYLENE GLYCOL 0.8 1.01E-06 0 0 4.77E-08 6.77E-09 6.77E-09
Name S5 56 S6_0 57 S9 810 S12
VapFrac 0.60261 1 0 1 0.97656 0.96768 0.75397
T [C).--_.. .. 25 60 25..... .. __ 25 25 24 13.5
..... ...... .............. _. .............
P[kPa} - 1800 765 1800 1800 1800 1765___ 1730
MaleFluw (k8mule/1i) 274.87 714.67 85.31 129.36 137.21 732.21 132.21
StdUIgVovumeFlow (m3/hr) 17.385 17.385 7.408 9.976 _ ._.=._ , _._10.088 10-099-
-.10.088
.. .._.
StdGasV0lumeFl0w (SCMD] 1 22E+05 1.22E+05 4.85E+04 7 36E404 7.52E404 7.52E+04
7.52E404
MOIeFractlon [Fraction)
_ .. ... __ .
HYDROGEN 0 0 0 0 0 0 0
HELIUM-4 0 0 0 0 0 0 0
- - - -- --------- -
OXYGEN 0 0 0 0 0 0 0
........_...... ......._._..._ .............................._.....
NITROGEN 0.07 0.07 0.01 0.11 0.11 0.11 0.11
CARBON DIOXIDE 0 0 0....... 0 O 0 0
HYDROGEN SULFIDE 0 0 0 0 ..................... ...... ......... ... 0
0.................... 0
........._..........._..... ....... __ _.. _. .......... .............
............. ....... ...... ........ ......_-.-------
METHANE 0.2 0.2 0.04 0.3 0.3 0.3 0.3
._...._
_...__..... _.........._ ...................... ...... ..._.............- -
.............._.................... .... ................
.._..__.................. ................ -....-._----- ............
ETHANE 0.03 0.03 0.02 0.04 0.03 0.03 0.03
...._-..._.._... _. ..... .. ...._.... .=..__._.........__.._......... ..
_,.._.
PROPANE 0.66 0.66 0.85 0.53 0 52 0.52 0..52
ISOBUTANE 0.02 0.02 0.03 0.01 0.01õ 0.02 ., .. - 0.01
.... ........... ........... ..... ........ ........ . ._...._.__....
n-BU1ANE 0.02 0.02 0 04 0.01 0.01 0.01 0.01
---- ....... .. ....... .-- --- - ----- .... .... .... .. _.. ---------------
ISOPENTANE - 0 0 O 0 0 0 --- 0
n-PENTANE 0 0 0 0 0 0 0
n-HEXANE 0 0 0 0 0 0 0
C7+ 0 0 0 0 0 0 0
MassFraction [Fraction)
WAFER 0.0018 0.0018 0.0028 9.39E-04 0.0059 00059 00059
ETHYLENE GLYCOL 6.77E-09 6.77E-09 1.46F-09 1.48E-11 0.0223 0.0223 0.0223
CA 02801144 2012-11-29
WO 2011/150486 PCT/CA2010/000814
23
Name Si3 S14 Si5 516 S17 518 S19
Vapfrac - y- 0,71206 0 0.3915 0.42392 0 1 0
F (CJ 10.3 4 2 -34 -38 5 4 2 8.5 -38.1
............... --...-...---
P (kPaJ 1695 .104:999 1660 1135.53855 104.999 1100.53855 IS50
MoleFlaw (kBmole/ti] ~_ 32.21 2_85 132.2] 132.21 . 2.85 96.05 73.09
StdU ValumeFlow (m3/hr 10.088 0 112 10.088 10.088 0.112 3.74 6.232
StdGasVolumeFiow SCMD 7-52E+Oa 1.62E+03 7.52E+04 7.52E+04 1.62E+03 3-19E+04
4.16E+04
Molefraction [Fraction)
HYDROGEN _ 0 0 0 0 0 0 0
HELIUM-4 0 0 _.--o0 0 0 0
-.._..-_.._._.__...._ ...................,_..._............___--------------- -
------- ---'------------.
OXYGEN 0 0 0 0 0 0 0
NITROGEN 0.11 0 0,11 0.11 0 0.24 0.01
.
- ...... _ ....... . ....... .._.... ..... ............ . _......._ .... -- .
. . ... .
CARBON DIOXIDE 0 0 0 0 0 0 0
.... .........._....._ ......_ ....__..,. _ ........ ..... _ ... _. _..._
..__...... ............ _._._....._..-=--
HYDROGEN SULFIDE 0 0 0 0 0 0 0
METHANE 0.3 0 0.3 0.3 0 0.61 0.07
.._............. _ ...----........------
ETHANE 0.03 0 0.03 0.03 0 0.03 0.04
PROPANE 0.52 0 0.52 0.52 0 0.11 0.85
IS686TANE 0.01 0 0.01 0.01 0 0 OA2
n-BUTANE 0.01 0 0.01 0.01 0 0 0.02
........ ....__.... ...._........ .........
.__..........~..........................- ..................... ..
ISOPENTANE 0 0 0 0 0 0 0
----- ---- ---
n-PENTANE 0 0 0 0 0 0 0
........._...._...._
.............._....._......_...._........_.........__..__...
....._.._.................... ................ ........
...................._......... ......._.......... .........._....__... --'----
_.......
n-HEXANE 0 0 0 0 0 0 0
.......__ ................ ........ ... _.......... ..... ...... ...........
....................
C7+t 0 0 0 0 0 0 0
MassFraction (Fraction)
_.....__-__........... ..................._.._.................--
,......_......._...... _....._....._......_.~_........ __.._._-
.....___._...._...__.--__._._...._...__......
WATER 0.0059 0.1838 0.0059 0.0059 0.1838 4.10E-06 5.24E-07
ETHYLENE GLYCOL 0.0223 0.8162 0.0223 0.0223 0.8162 9.63E-09 1.97E-06
Name 520 S21 S22 S24 525 526 527
Vapfrac 0.09838 0.09838 0 0.96676 0.00045 0,00035 0
............ .... _........_............._....................... __._..--
._............ ............. ...........
_..._
7 ( . _ . . . . _ . . . . . . . . 16 16 4.6 14.8 =24 -38.3 100
P (kPaJ ^ _y _.... __......._.....,_..... 1515 1515 1800 - 1241 480.01042
515.01042 445.01042
MoleFlow niple/li] 73.09 73.09 2.85 24.3 3.08 3,08 3,08
j_.-.. -._...._-_.._._....__. ................ ..... ............ .....
...__...__.__.....:_...... ..............._....._............ .....
......_..._..._.___-_-... _...___.._.._...__.._...__.._............... _-
StdLigVoiumeFlow [m3/1 6,232 6.232 0.112 1.847 0.116 0.116 0,116
StdGaWolumeFlowSCMD 416E+04 416E+04 162EW3 1.38E+04 1.75E+03 1.75E+03_
.175E+03
MoleFraction, -Fraction}
HYDROGEN 0 0 0 0 0 0 0
....... ......... . ... ___......_.._....._..._._...._..._
....................._.....__.._..._........_.__.._......_....._..__........,..
..._...._._........ ..._ .~
14ELIUM-4 0 0 0 0 0 0 0
OXYGEN 0 0 0 0 0 0 0
NITROGEN 0.01 0.01 0 0.04 0 0 0
CARBON DIOXIDE 0 0 0 0 0 0 0
HYDROGEN SULFIDE 0 0 0 0 0 0 0
METHANE 0.07 0.07 0 0.3 0 0 0
_ ..._.._... -T--
ETHANE 0704 0.04 0 0.06 0 0 O
PROPANE 0.85 0.85 O 0.57 0 0 0
I5OBUTANE 0.01 0.01 0 0.01 0 0 0
n-BUTANE 0.02 0.02 0 0.01 0 0 0
.... ..... _.... ...... .............................................
......... ...... .._...... _._.._...__.._....._,..,.,.,.....,,_.,.,,,,__._....
ISOPENfANE 0 0 0 0 0 0 0
. ... ..... .._............ ..... -
n-PENTANE 0 0._.... 0 0 0 0 0
..__..._
--........ .......__..... ........ ......_..........._.__.....
_..._._._.......... .......
n-HEXANE 0 0 0 0 0 0 0
_........._... -...._..,..........._..... ......... .... ..........-
._........_..__..._.._.. ......._....---...._._._.. .....
C7+= O -.- 0 0 0 0 0 0
MassFraction (Fraion ._...,..... ..... ..............
...._.............................._........_........._...._
_......_......................._......_ ......................_...
.ipn ........ct......] .......................................
WATER 5.24E-07 5.24E-07 0.1838 0.0087 0.21 0.21 0.71
.......... .._...... .._.._._ ._.._.,...._,...._.:._...._..... _....... .....--
..... ........ ....-----....... .._.._.- --------.-
ETHYLENE GLYCOL 1.97E-06 1.97E-06 0.8162 6.60E-08 0.7893 0.7893 0.7896
CA 02801144 2012-11-29
WO 2011/150486 PCT/CA2010/000814
24
Name S30 S301 S31 S31 1 S34 $42 S43
Va Frac 0 0.00088 0 1 0 1 1
- ---.^-`. -~~.- _......_.._.......__...._..- ^~._,__..__.........._..__._.
_._....__...._-.__..__.----------------- ----.
T [C) 134 100 4.2 100 4.6 -37 -37
P ~kPaJ 134 999 445 01042 104 999 445 01042 2000 126.69348 126.69348
MOIeFIow , kSmrtle i 2.AS oA 2.A5 0 2.SS 86.85 PA aS
5tdligVOlpmeFlow [m3/hr) ..... 0_112 0116 0.112 0 0.112 7.592 7.592
StdGas'OlumeFiow [SCMD .1.62E+03 1.75E+03 1.62E+03 1.55E+00 1.62E+03 4.94E+04
4.94E+04
......._.._.._ ...... ....... ------------------- _.__._..........._._.._
MoleFraction [Fraction)
HYDROGEN 0 0 0 O 0 0 0
HELIUM-4 0 0 0 0 0 0 0
OXYGEN 0 0 0 0 0 0 0
....... ..-_........... ._._.._.._..._.._..... .__
_........_.....__._._........
.._........_..........._.._...._......-_.._..
NITROGEN 0 0 0 0.02 0 0 0
....._..... .... _..... ............-._.._.._._....._..._ ......._..__........
__................ ....... __...... _......__..... _.........__...... .......
._._......._....... .._.......--
CARBON DIOXIDE .0 ......_..__._._ 0 0 0.07 0 0 0
HYDROGEN SULFIDE 0 0 0 0 0 O 0
METHANE ........_._._._..._..._._._....._.....Ø79......... 0 0 0
ETHANE 0 0 0 0.02 0 O 0
PROPANE 0 0 0 0 0 1 1
.............._._-..... .... ....._..__...__..... _........... ........
....... ._................. _........
_....
ISOBUTANE 0 0 0 0 0 0 0
n-BUTANE 0 0 0 0 0 0 0
............... ............................
ISOPENTANE 0 0 0 0 0 0 0
n-PENTANE 0 0 0 0 0 0 0
n-HEXANE 0 0 0 0 0 0 0
C7+ O O 0 0 0 0
MassFraction (Fraction)
....... ...._.._ ................................. ..............
....._...._.._........ ...... _..... _....... ......_...... .... ---
._............. .._...__...........__._.._...._-,._...._..__..._...._.._.... _
-------- _.....__..._........... ..
WATER 0.1838 0.21 0.1838 0.1013 0.1838 0 0
ETHYLENE GLYCOL 0,8162 0.7893 0.8162 0.0085 0.8162 0 0
Name $44 S45 $46 547 548 549 Sales Gas
Vap.rac 0 i...._...... _. 1 0- 0 0.36977 1
..,.._..._...__.,_....._.._.. ...._.__.._...... _._..._.._...... ..
[CJ -37 34 51.5 25 25 37 18
............................
................ ._....... ............................_.....................
........._.,.._............ ......... ............................
P )kPa] 126.69348 126,69348 987.63929 952,63929 952.63929 126.79348 1065.53855
_....__..._.......... ..__..__..__.._....._..._ ....... .......
................_....._....,._......_...... ...........
__.............._._._......._...._.......... -_ - _ -~ ._....__..._..-....
M"ffluwikBmWe/Irl.._._..._..._.......:
.................n_........_...._..._*6.85 .___......__86.95 86.85 86.85 PA.83
56.05
StdLigVolurn ...w (m3Jhr] 0 7 592 7 592 7 592 .592 7.592 3.74
...
SttlGasVolumeflow ISCMD 5.69E-38 4.94E+04 4.94E+04 4.94E404 4.949404 4.94E+04
3.19E+04
MoleFraction [Fraction]
HYDROGEN _! 0 _ 0 0 0 0 0 0
HELIUM-4 0 0 0 0 0 0 0
OXYGEN . `O O O O 0 O O
NITROGEN P .......................... 0 0. 0
.........................._0._._.._......._.._.. ..........
........................ ...__..__....... 0 24
CARBON DIOXIDE .....__0 0 .. .....0 .....~ ................. 0......... 0 0
HYDROGEN SULFIDE 0 0
....................b........................._....._..__.._..._..__Q.
_._0.............-........_..0
METHANE 0 0 0 0 0 0 0.61
ETHANE 0 0 0 0 0 0 0.03
PROPANE 1 1 1 1 1 1 0.11
.. ..._._.._ ._ ... .... _.._...................
ISOBUTAN 0 0 0 ........ 0 0 0
n- BUTANE 0 0 0 0 0 0 0
...- ._ .... ........ ........ ...... . .
ISOPENTANE - - - 0 0 0 0 0 0 0
n.PENTANE O~ 0 0 0 0 0 0
............. ....... __............._.................. .-.......... ........
........... ........ _.........
......................................_..................... .... .....
........ ..._...... .............. _.._.........
n-HEXANE 0 0 0 0 0 0 0
. .... .......... ......... .... ....... ..........,,............._
C7+* 0 0 0 0 . 0 0
MassFraction [Fraction]
WATER O 0 0 0 0 0 4.10E-06
ETHYLENE GLYCOL 0 0 0 0 0 0 9.63E-09
[0062] In the claims, the word "comprising" is used in its inclusive sense and
does
not exclude other elements being present. The indefinite article "a" before a
claim feature
CA 02801144 2012-11-29
WO 2011/150486 PCT/CA2010/000814
does not exclude more than one of the feature being present. Each one of the
individual
features described here may be used in one or more embodiments and is not, by
virtue only
of being described here, to be construed as essential to all embodiments as
defined by the
claims.