Note: Descriptions are shown in the official language in which they were submitted.
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
EXTRACTION SOLVENTS DERIVED FROM OIL FOR ALCOHOL REMOVAL IN
EXTRACTIVE FERMENTATION
[0001] This application claims the benefit of U.S. Provisional Application No.
61/356,290, filed on June 18, 2010; U.S. Provisional Application No.
61/368,451,
filed on July 28, 2010; U.S. Provisional Application No. 61/368,436, filed on
July
28, 2010; U.S. Provisional Application No. 61/368,444, filed on July 28, 2010;
U.S. Provisional Application No. 61/368,429, filed on July 28, 2010; U.S.
Provisional Application No. 61/379,546, filed on September 2, 2010; and U.S.
Provisional Application No. 61/440,034, filed on February 7, 2011; U.S. Patent
Application No. 13/160,766, filed on June 15, 2011; the entire contents of
which
are all herein incorporated by reference.
[0002] The Sequence Listing associated with this application is filed in
electronic
form via EFS-Web and hereby incorporated by reference into the specification
in
its entirety.
FIELD OF THE INVENTION
[0003] The present invention relates the production of fermentative alcohols
such
as butanol and in particular, to extraction solvents for extractive
fermentation, and
processes for converting oil derived from biomass into the extraction
solvents.
BACKGROUND OF THE INVENTION
[0004] Butanol is an important industrial chemical with a variety of
applications
including use as a fuel additive, as a feedstock chemical in the plastics
industry,
and as a food-grade extractant in the food and flavor industry. Accordingly,
there
is a high demand for butanol as well as for efficient and environmentally
friendly
production methods.
[0005] Production of butanol utilizing fermentation by microorganisms is one
environmentally friendly production method. Some microorganisms that produce
butanol in high yields also have low butanol toxicity thresholds, such that
butanol
needs to be removed from the fermentation vessel as it is being produced. In
situ
product removal (ISPR) (also referred to as extractive fermentation) removes
-1-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
butanol from the fermentation vessel as it is produced, thereby allowing the
microorganism to produce butanol at high yields. One method for ISPR that has
been described in the art is liquid-liquid extraction (U.S. Patent Application
Publication No. 2009/0305370). In order to be technically and economically
viable, ideally, liquid-liquid extraction calls for good contact between the
extractant and the fermentation broth for efficient mass transfer of the
product
alcohol into the extractant; good phase separation of the extractant from the
fermentation broth (during and after fermentation); efficient recovery and
recycle
of the extractant; minimal degradation of the capacity of the extractant to
extract
the product alcohol (by, e.g., preventing the lowering of the partition
coefficient for
the product alcohol into the extractant) and contamination of the extractant
by
lipids that lower the partition coefficient over a long-term operation.
[0006] In particular, the extractant can become contaminated with lipid over
time
with each recycle, for example, by the build-up of lipids present in the
biomass
that is fed to the fermentation vessel as feedstock of hydrolysable starch. As
an
example, a liquified corn mash loaded to a fermentation vessel at 30 wt% dry
corn solids can result in a fermentation broth that contains about 1.2 wt%
corn oil
during conversion of glucose to butanol by simultaneous saccharification and
fermentation (SSF) (with saccharification of the liquified mash occurring
during
fermentation by the addition of glucoamylase to produce glucose). The
dissolution of the corn oil lipids in oleyl alcohol (OA) serving as an
extractant
during ISPR can result in build-up of lipid concentration with each OA
recycle,
decreasing the partition coefficient for the product alcohol in OA as the
lipid
concentration in OA increases with each recycle of OA.
[0007] In addition, the presence of the undissolved solids during extractive
fermentation can negatively affect the efficiency of the alcohol production.
For
example, the presence of the undissolved solids may lower the mass transfer
coefficient inside the fermentation vessel, impede phase separation in the
fermentation vessel, result in the accumulation of corn oil from the
undissolved
solids in the extractant leading to reduced extraction efficiency over time,
increase the loss of solvent because it becomes trapped in solids ultimately
removed as Dried Distillers' Grains with Solubles (DDGS), slow the
-2-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
disengagement of extractant drops from the fermentation broth, and/or result
in a
lower fermentation vessel volume efficiency.
[0008] Several approaches for reducing the degradation of the partition
coefficient
of the extractant used in extractive fermentation have included wet milling,
fractionation, and removal of solids. Wet milling is an expensive, multi-step
process that separates a biomass (e.g., corn) into all of its key components
(germ, pericarp fiber, starch, and gluten) in order to capture value from each
co-
product separately. This process gives a purified starch stream; however, it
is
costly and includes the separation of the biomass into its non-starch
components,
which is unnecessary for fermentative alcohol production. Fractionation
removes
fiber and germ, which contains a majority of the lipids present in ground
whole
corn, resulting in corn that has a higher starch (endosperm) content. Dry
fractionation does not separate the germ and fiber, and therefore, it is less
expensive than wet milling. However, fractionation does not remove the
entirety
of the fiber or germ, and does not result in total elimination of solids.
Furthermore, there is some loss of starch in fractionation. Wet milling of
corn is
more expensive than dry fractionation, but dry fractionation is more expensive
than dry grinding of unfractionated corn. Removal of solids, including germ
containing lipids, from liquified mash prior to use in fermentation can
substantially
eliminate undissolved solids, as described for example in co-pending, commonly
owned U.S. Provisional Patent Application No. 61/356,290, filed June 18, 2010.
However, it would be advantageous if the degradation of the partition
coefficient
of the extractant can be reduced even without fractionation or removal of
undissolved solids. Thus, there is a continuing need to develop more efficient
methods and systems for producing product alcohols such as butanol, through
extractive fermentation in which the degradation of the partition coefficient
of the
extractant is reduced.
[0009] Moreover, the extractant (e.g., oleyl alcohol) is typically added to
the
process, rather than produced at a step in the process and therefore, the
extractant is a raw material expense. Since the extractant can be lost to
adsorption on non-fermentable solids and diluted with lipids introduced into
the
process, the economy of the alcohol production process can be affected by the
-3-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
efficiency of the extractant recovery and recycle. Thus, there exists a
continuing
need for alternative extractants for ISPR that can result in a more economical
process by reducing capital and/or operating costs.
BRIEF SUMMARY OF THE INVENTION
[0010] The present invention satisfies the above needs by providing methods
for
producing product alcohols, such as butanol, in which the lipids in a biomass
are
converted into an extractant that can be used in ISPR, and in which the amount
of lipids that are fed to the fermentation vessel, with the feedstock and/or
upon
extractant recycle, are decreased. The present invention provides further
related
advantages, as will be made apparent by the description of the embodiments
that
follow.
[0011] Chemical conversion of lipids derived from biomass to extractants
including fatty acids, fatty alcohols, fatty amides, fatty acid esters, fatty
acid glycol
esters, and triglycerides, and mixtures thereof (collectively referred to
herein as
"fatty acid extractants") can decrease the amount of lipids present in the
ISPR
extractant. The triglycerides may be hydroxylated or alkoxylated (e.g.,
methoxylated, ethoxylated). Fatty acid extractants would not be expected to
decrease the partition coefficient of the product alcohol, such as isobutanol,
into
the extractant phase as much as lipids. Moreover, the fatty acid extractants
can
be used as the ISPR extractant. The fatty acid extractants can be derived from
the biomass supplying fermentable carbon fed to the fermentation vessel. The
fatty acid extractants can therefore be produced at a step in the alcohol
production process and be used in place of, or in addition to, a supplied,
exogenous ISPR extractant that is not produced in the process (such as
externally supplied oleyl alcohol), thereby reducing or even eliminating the
raw
material expense for the ISPR extractant.
[0012] In addition, extractants may also be produced by converting oil derived
from biomass or feedstock into fatty acids using high temperature and/or high
pressure conditions. Furthermore, oil derived from biomass or feedstock may be
treated with one or more lipases to produce fatty acids or subjected to
hydrogenation to produce fatty alcohols.
-4-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
[0013] The present invention is directed to a composition comprising a
recombinant microorganism capable of producing alcohol from a feedstock;
alcohol; and at least one extractant selected from the group consisting of
fatty
acid, fatty alcohol, fatty amide, fatty ester, triglycerides, and mixtures
thereof;
wherein the extractant is produced from the feedstock. In one embodiment, the
extractant is a mixture of fatty amides, and in a further embodiment, the
mixture
of fatty amides comprises linoleamide, oleamide, palmitamide, or stearamide.
In
another embodiment, the extractant is a mixture of fatty amides and fatty
acids,
and in a further embodiment, the mixture of fatty amides and fatty acids
comprises linoleamide, linoleic acid, oleamide, oleic acid, palmitamide,
palmitic
acid, stearamide, or stearic acid. In one embodiment, the extractant is
selected
from hydroxylated triglycerides, alkoxylated triglycerides, hydroxylated fatty
acids,
alkoxylated fatty acids, hydroxylated fatty alcohols, and alkoxylated fatty
alcohols.
In one embodiment, the triglycerides may be methoxylated or ethoxylated. In
another embodiment, the extractant is selected from saturated fatty acids,
unsaturated fatty acids, saturated fatty alcohols, unsaturated fatty alcohols,
saturated fatty amides, unsaturated fatty amides, saturated fatty esters,
unsaturated fatty esters, and mixtures thereof. In one embodiment, the
extractant may be a liquid or solid. In a further embodiment, the extractant
may
be in the form of beads. In one embodiment, the alcohol is C1 to C8 alkyl
alcohols. In another embodiment, the feedstock comprises rye, wheat, corn,
cane, barley, cellulosic material, lignocellulosic material, or mixtures
thereof.
[0014] In one embodiment, the extractant comprises one or more fatty amides of
the formula R(C=O)N(R')(R"), wherein
R is independently selected from the group consisting of C3 to C27 alkyl
groups
optionally interrupted with one or more double bonds, and
R' and R" are independently selected from the group consisting of hydrogen and
C1-C6 alkyl groups optionally containing one or more hydroxyl groups.
[0015] In another embodiment, the extractant comprises one or more fatty
esters
of the formula R-(C=O)-OCHR'CHR"-OH, wherein
R is independently selected from the group consisting of C3 to C27 alkyl
groups
optionally interrupted with one or more double bonds, and
-5-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
R' and R" are independently selected from the group consisting of hydrogen and
Cl-C4 alkyl groups.
[0016] In one embodiment, the extractant comprises one or more fatty esters of
the formula R-(C=O)-OR', wherein
R is independently selected from the group consisting of C3 to C27 alkyl
groups
optionally interrupted with one or more double bonds, and
R' is an alkyl group of 8 carbons or less.
[0017] The present invention is directed to a method for producing an
extractant
comprising providing a biomass comprising oil; contacting the oil with one or
more substances capable of chemically converting the oil into an extractant
selected from the group consisting of fatty acids, fatty alcohols, fatty
amides, fatty
esters, triglycerides, and mixtures thereof, whereby at least a portion of the
oil is
converted to the extractant. In one embodiment, the triglycerides may be
hydroxylated or alkoxylated (e.g., methoxylated, ethoxylated). In one
embodiment, the extractant may be a liquid or solid. In a further embodiment,
the
extractant may be in the form of beads. In one embodiment, the method further
comprises the step of separating the oil from the biomass prior to contacting
the
oil with the one or more substances. In one embodiment, the one or more
substances is selected from aqueous ammonium hydroxide, anhydrous
ammonia, ammonium acetate, ammonia in water, hydrogen peroxide, toluene,
glacial acetic acid, lipase, and cation exchange resin. In another embodiment,
the extractant has a partition coefficient for a product alcohol greater than
the
partition coefficient of the oil for the product alcohol prior to the oil
being
converted to extractant. In one embodiment, the product alcohol is C1 to C8
alkyl
alcohols. In another embodiment, the biomass comprises corn grain, corn cobs,
crop residues such as corn husks, corn stover, grasses, wheat, rye, wheat
straw,
barley, barley straw, hay, rice straw, switchgrass, waste paper, sugar cane
bagasse, sorghum, sugar cane, soy, components obtained from milling of grains,
cellulosic material, lignocellulosic material, trees, branches, roots, leaves,
wood
chips, sawdust, shrubs and bushes, vegetables, fruits, flowers, animal manure,
or
mixtures thereof.
-6-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
[0018] The present invention is also directed to a method for producing a
product
alcohol comprising: (a) providing biomass comprising oligosaccharides and oil;
(b) contacting the biomass with a saccharification enzyme capable of
converting
oligosaccharides into monosaccharides; (c) separating the oil from the biomass
of (a) or (b); (d) contacting the separated oil with one or more reactants or
solvents to form an extractant; (e) contacting the biomass with a fermentation
broth comprising a recombinant microorganism capable of converting the
monosaccharides to a product alcohol and whereby a product alcohol is
produced; and (f) contacting the product alcohol with the extractant, wherein
the
extractant has a partition coefficient for the product alcohol that is greater
than
the partition coefficient of the oil of the biomass for the product alcohol.
In one
embodiment, the one or more reactants or solvents is selected from aqueous
ammonium hydroxide, anhydrous ammonia, ammonium acetate, ammonia in
water, hydrogen peroxide, toluene, glacial acetic acid, lipase, and cation
exchange resin. In one embodiment, the extractant is selected from fatty acid,
fatty alcohol, fatty amide, fatty ester, triglycerides, and mixtures thereof.
In one
embodiment, the extractant is selected from hydroxylated triglycerides,
alkoxylated triglycerides (e.g., methoxylated, ethoxylated), hydroxylated
fatty
acids, alkoxylated fatty acids, hydroxylated fatty alcohols, and alkoxylated
fatty
alcohols. In another embodiment, the extractant is selected from saturated
fatty
acids, unsaturated fatty acids, saturated fatty alcohols, unsaturated fatty
alcohols,
saturated fatty amides, unsaturated fatty amides, saturated fatty esters,
unsaturated fatty esters, and mixtures thereof. In one embodiment, the
extractant may be a liquid or solid. In a further embodiment, the extractant
may
be in the form of beads. In one embodiment, the alcohol is C1 to C8 alkyl
alcohols. In another embodiment, the oil comprises one or more oils selected
from tallow oil, corn oil, canola oil, capric/caprylic triglycerides, castor
oil, coconut
oil, cottonseed oil, fish oil, jojoba oil, lard, linseed oil, neetsfoot oil,
oiticica oil,
palm oil, peanut oil, rapeseed oil, rice oil, safflower oil, soya oil,
sunflower oil,
tung oil, jatropha oil, wheat oil, rye oil, barley oil, and vegetable oil
blends.
[0019] In one embodiment, the extractant comprises one or more fatty amides of
the formula R(C=O)N(R')(R"), wherein
-7-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
R is independently selected from the group consisting of C3 to C27 alkyl
groups
optionally interrupted with one or more double bonds, and
R' and R" are independently selected from the group consisting of hydrogen and
Cl-C6 alkyl groups optionally containing one or more hydroxyl groups.
[0020] In another embodiment, the extractant comprises one or more fatty
esters
of the formula R-(C=O)-OCHR'CHR"-OH, wherein
R is independently selected from the group consisting of C3 to C27 alkyl
groups
optionally interrupted with one or more double bonds, and
R' and R" are independently selected from the group consisting of hydrogen and
Cl-C4 alkyl groups.
[0021] In one embodiment, the extractant comprises one or more fatty esters of
the formula R-(C=O)-OR', wherein
R is independently selected from the group consisting of C3 to C27 alkyl
groups
optionally interrupted with one or more double bonds, and
R' is an alkyl group of 8 carbons or less.
[0022] The present invention is directed to a method for producing a product
alcohol comprising: (a) providing a fermentation broth comprising a
recombinant
microorganism capable of producing a product alcohol in a fermentation vessel
and whereby a product alcohol is produced; (b) contacting the fermentation
broth
with an extractant to form a two-phase mixture comprising an aqueous phase and
an organic phase, wherein the product alcohol and the oil partition into the
organic phase such that the organic phase comprises the product alcohol and
the
oil; (c) separating the organic phase from the aqueous phase; (d) separating
the
product alcohol from the organic phase; and optionally steps (b) and (c) occur
concurrently. In one embodiment, the method further comprises the step of
producing a feedstock slurry; separating the feedstock slurry to produce (i)
an
aqueous layer, (ii) oil layer, and (iii) a solids layer; and feeding the
aqueous layer
to the fermentation vessel. In another embodiment, the method further
comprises the step of further comprising: contacting the oil of the oil layer
with
one or more substances capable of chemically converting the oil into an
extractant selected from the group consisting of fatty acids, fatty alcohols,
fatty
-8-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
amides, fatty esters, triglycerides, and mixtures thereof, whereby at least a
portion of the oil is converted to the extractant.
[0023] In one embodiment, the extractant is selected from fatty acid, fatty
alcohol,
fatty amide, fatty ester, triglycerides, and mixtures thereof. In another
embodiment, the extractant is selected from hydroxylated triglycerides,
alkoxylated triglycerides (e.g., methoxylated, ethoxylated), hydroxylated,
fatty
acids, alkoxylated fatty acids hydroxylated fatty alcohols, and alkoxylated
fatty
alcohols. In one embodiment, the extractant is selected from saturated fatty
acids, unsaturated fatty acids, saturated fatty alcohols, unsaturated fatty
alcohols,
saturated fatty amides, unsaturated fatty amides, saturated fatty esters,
unsaturated fatty esters, and mixtures thereof. In one embodiment, the
extractant may be a liquid or solid. In a further embodiment, the extractant
may
be in the form of beads. In one embodiment, the product alcohol is C1 to C8
alkyl
alcohols. In another embodiment, the extractant has a partition coefficient
for the
product alcohol that is greater than the partition coefficient of the oil of
the oil
layer for the product alcohol. In one embodiment, the product alcohol is C1 to
C8
alkyl alcohols. In another embodiment, the oil comprises one or more oils
selected from tallow oil, corn oil, canola oil, capric/caprylic triglycerides,
castor oil,
coconut oil, cottonseed oil, fish oil, jojoba oil, lard, linseed oil,
neetsfoot oil,
oiticica oil, palm oil, peanut oil, rapeseed oil, rice oil, safflower oil,
soya oil,
sunflower oil, tung oil, jatropha oil, wheat oil, rye oil, barley oil, and
vegetable oil
blends.
[0024] In some embodiments, a method of removing oil derived from biomass
from a fermentation process includes: contacting a biomass feedstream
including
an amount of oil with one or more substances capable of chemically converting
the oil into an extractant selected from the group consisting of fatty acids,
fatty
alcohols, fatty amides, fatty acid methyl esters, fatty acid glycol esters,
triglycerides and mixtures thereof, whereby at least a portion of the oil is
converted to the extractant. The triglycerides may be hydroxylated or
alkoxylated
(e.g., methoxylated, ethoxylated). The extractant has a partition coefficient
for a
fermentative alcohol greater than the partition coefficient of the oil for the
fermentative alcohol. In some embodiments the biomass feedstream is milled
-9-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
corn and the oil is corn oil. In some embodiments, the method also includes
contacting the biomass feedstream having the extractant with a fermentation
broth, the fermentation broth including the fermentative alcohol, wherein the
fermentative alcohol partitions into the extractant.
[0025] The present invention is directed to a method for producing an
extractant
comprising providing a biomass comprising oil; and converting at least a
portion
of the oil into an extractant selected from the group consisting of fatty
acids, fatty
alcohols, fatty amides, fatty esters, triglycerides, and mixtures thereof. In
one
embodiment, the step of converting the oil into an extractant comprises one or
more of the steps of incubating the oil in the presence of tetrahydrofuran and
lithium aluminum hydride; incubating the oil with sodium hydroxide; incubating
the
oil with sulfuric acid and methanol; incubating the oil with anhydrous ammonia
in
the presence of ammonium acetate; incubating the oil with ammonia in water;
contacting the oil with toluene, cation exchange resin, glacial acetic acid,
lipase,
and hydrogen peroxide; incubating the oil under high temperature conditions,
or
incubating the oil under high pressure conditions.
[0026] In some embodiments, an in situ method of producing an extractant for
in
situ removal of a product alcohol includes: (a) providing biomass including
fermentable sugars and oil, the oil including triglycerides; (b) separating
the oil of
(a) from the biomass; and (c) contacting the separated oil with one or more
reactants or solvents capable of chemically reacting the triglycerides to
obtain a
reaction product selected from the group consisting of fatty acids, fatty
alcohols,
fatty amides, a mixture of fatty amides and fatty acids, fatty acid methyl
esters,
fatty acid glycol esters, triglycerides, and mixtures thereof, whereby the
triglycerides in the oil are converted into the reaction product. The
triglycerides
may be hydroxylated or alkoxylated (e.g., methoxylated, ethoxylated). The
reaction product forms a fermentation product extractant having a partition
coefficient for the product alcohol greater than a partition coefficient of
the oil of
the biomass for the product alcohol.
[0027] In some embodiments, a method for producing butanol includes: (a)
providing biomass including oligosaccharides and oil, the oil including
glycerides;
(b) contacting the biomass with a saccharification enzyme capable of
converting
-10-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
oligosaccharides into monosaccharides; (c) separating the oil from the biomass
of (a) or (b); (d) contacting the separated oil with a composition including
one or
more reactants or solvents whereby the glycerides in the oil form an
extractant;
(e) contacting the biomass with a recombinant microorganism capable of
converting the monosaccharides to butanol whereby a fermentation product
comprising butanol is produced; and (f) contacting the fermentation product
with
the extractant of (d) whereby the butanol is separated from the fermentation
product. The extractant has a partition coefficient for the butanol greater
than the
partition coefficient of the oil of the biomass for the butanol. In some
embodiments, the extractant of step (d) is selected from the group consisting
of
fatty acids, fatty alcohols, fatty amides, a mixture of fatty amides and fatty
acids,
fatty acid methyl esters, fatty acid glycol esters, triglycerides, and
mixtures
thereof. The triglycerides may be hydroxylated or alkoxylated (e.g.,
methoxylated, ethoxylated).
[0028] In some embodiments, a method includes, at a step during a process to
produce a product alcohol from a feedstock, converting at least of portion of
a
plant-derived oil to an extractant having a extractant partition coefficient
for the
product alcohol greater than a partition coefficient of the plant-derived oil
for the
product alcohol. In some embodiments, the plant-derived oil is derived from
the
feedstock.
[0029] In some embodiments, the product alcohol is isobutanol and the
extractant
partition coefficient is at least about 0.28. In some embodiments, the
extractant
partition coefficient for isobutanol is at least about 1. In some embodiments,
the
extractant partition coefficient for isobutanol is at least about 2.
[0030] In some embodiments, the process to produce a product alcohol from a
feedstock includes (a) producing a feedstock slurry; (b) separating the
feedstock
slurry of (a) to produce a product including (i) an aqueous layer, (ii) a oil
layer,
and (iii) a solids layer; and (c) feeding the aqueous layer of (b) to a
fermentation
vessel. In some embodiments, the step of separating the feedstock slurry
occurs
by centrifugation. In some embodiments, the oil layer is plant-derived oil
layer.
In some embodiments, the process further includes obtaining at least a portion
of
plant-derived oil from the plant-derived oil layer.
-11-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
[0031] In some embodiments, the process further includes adding the extractant
to the fermentation vessel to form a two-phase mixture including an aqueous
phase and a product alcohol-containing organic phase, whereby the product
alcohol partitions into the product alcohol-containing organic phase.
[0032] In some embodiments, the process further includes fermenting sugar of
the aqueous phase to produce the product alcohol, whereby the product alcohol
partitions into the product alcohol-containing organic phase.
[0033] In some embodiments, a method of removing oil derived from biomass
from a fermentation process includes (a) providing a fermentation broth
including
a product alcohol and oil derived from biomass, the oil including glycerides;
(b)
contacting the fermentation broth with an extractant to form a two-phase
mixture
comprising an aqueous phase and an organic phase, the product alcohol and the
oil partitioning into the organic phase such that the organic phase comprises
the
product alcohol and the oil; (c) separating the organic phase from the aqueous
phase; (d) separating the product alcohol from the organic phase; and (e)
contacting the organic phase with a composition comprising one or more
reactants or solvents whereby the glycerides in the oil form additional
extractant;
and (f) repeat step (b) by contacting the fermentation broth with the
additional
extractant of step (e).
[0034] In some embodiments, the additional extractant is selected from the
group
consisting of fatty acid, fatty alcohol, fatty amide, fatty acid methyl ester,
fatty acid
glycol ester, triglyceride, and mixtures thereof. The triglycerides may be
hydroxylated or alkoxylated (e.g., methoxylated, ethoxylated).
[0035] In some embodiments, the product alcohol is butanol.
[0036] In some embodiments, an in situ fermentation extractant-forming
composition includes (a) oil derived from biomass; (b) one or more substances
capable of chemically converting the oil into one or more products selected
from
the group consisting of fatty acids, fatty alcohols, fatty amides, fatty acid
methyl
esters, fatty acid glycol esters, and triglycerides; and (c) the one or more
products, wherein the one or more products are in an amount from about 50 wt%
to about 99 wt% of the composition. The triglycerides may be hydroxylated or
alkoxylated (e.g., methoxylated, ethoxylated).
-12-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
[0037] In some embodiments, a composition includes a recombinant
microorganism capable of producing butanol, butanol, and at least one solvent
selected from the group consisting of fatty acid, fatty alcohol, fatty amide,
fatty
acid methyl ester, fatty acid glycol ester, triglyceride, and mixtures
thereof. The
triglycerides may be hydroxylated or alkoxylated (e.g., methoxylated,
ethoxylated).
[0038] In some embodiments, a composition includes a recombinant
microorganism capable of producing butanol, butanol, and fatty alcohols.
[0039] In some embodiments, a composition includes a recombinant
microorganism capable of producing butanol, butanol, and a mixture of fatty
amides, wherein the mixture of fatty amides comprises linoleamide, oleamide,
palmitamide, and stearamide.
[0040] In some embodiments, a composition includes a composition comprising a
recombinant microorganism capable of producing butanol, butanol, and corn oil,
wherein the corn oil is from about 28% to about 67% hydroxylated.
BRIEF DESCRIPTION OF THE DRAWINGS/FIGURES
[0041] The accompanying drawings, which are incorporated herein and form a
part of the specification, illustrate the present invention and, together with
the
description, further serve to explain the principles of the invention and to
enable a
person skilled in the pertinent art to make and use the invention.
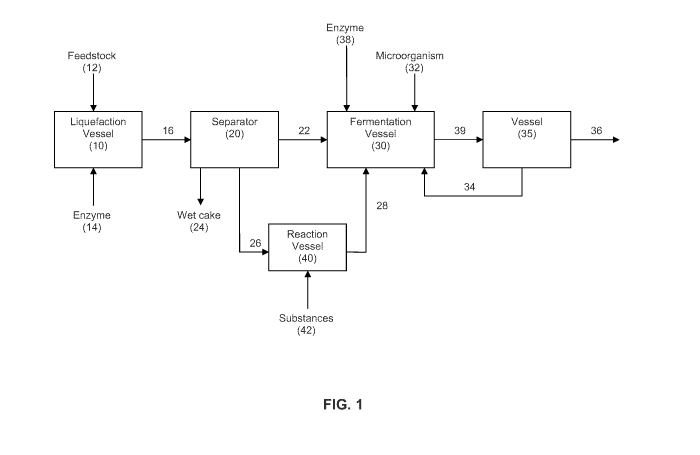
[0042] FIG. 1 schematically illustrates an exemplary method and system of the
present invention, in which lipids are removed from a liquefied biomass before
fermentation, and in which the removed lipids are converted into an extractant
and supplied to a fermentation vessel.
[0043] FIG. 2 schematically illustrates an exemplary method and system of the
present invention, in which lipids are removed from a liquefied and
saccharified
biomass before fermentation, and in which the removed lipids are converted
into
an extractant and supplied to a fermentation vessel.
[0044] FIG. 3 schematically illustrates an exemplary method and system of the
present invention, in which lipids are removed from a biomass and converted
into
an extractant that is supplied to a fermentation vessel.
-13-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
[0045] FIG. 4 schematically illustrates an exemplary method and system of the
present invention, in which lipids in a biomass feedstream are converted into
an
extractant and supplied to a fermentation vessel.
[0046] FIG. 5 schematically illustrates an exemplary method and system of the
present invention, in which lipids present in a first extractant exiting a
fermentation vessel are separated from the first extractant and converted into
a
second extractant that is supplied to a fermentation vessel.
DETAILED DESCRIPTION OF THE INVENTION
[0047] Unless defined otherwise, all technical and scientific terms used
herein
have the same meaning as commonly understood by one of ordinary skill in the
art to which this invention belongs. In case of conflict, the present
application
including the definitions will control. Also, unless otherwise required by
context,
singular terms shall include pluralities and plural terms shall include the
singular.
All publications, patents and other references mentioned herein are
incorporated
by reference in their entireties for all purposes.
[0048] In order to further define this invention, the following terms and
definitions
are herein provided.
[0049] As used herein, the terms "comprises," "comprising," "includes,"
"including," "has," "having," "contains," or "containing," or any other
variation
thereof, will be understood to imply the inclusion of a stated integer or
group of
integers but not the exclusion of any other integer or group of integers. For
example, a composition, a mixture, a process, a method, an article, or an
apparatus that comprises a list of elements is not necessarily limited to only
those
elements but can include other elements not expressly listed or inherent to
such
composition, mixture, process, method, article, or apparatus. Further, unless
expressly stated to the contrary, "or" refers to an inclusive or and not to an
exclusive or. For example, a condition A or B is satisfied by any one of the
following: A is true (or present) and B is false (or not present), A is false
(or not
present) and B is true (or present), and both A and B are true (or present).
[0050] Also, the indefinite articles "a" and "an" preceding an element or
component of the invention are intended to be nonrestrictive regarding the
-14-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
number of instances, i.e., occurrences of the element or component. Therefore,
"a" or "an" should be read to include one or at least one, and the singular
word
form of the element or component also includes the plural unless the number is
obviously meant to be singular.
[0051] The term "invention" or "present invention" as used herein is a non-
limiting
term and is not intended to refer to any single embodiment of the particular
invention but encompasses all possible embodiments as described in the
application.
[0052] As used herein, the term "about" modifying the quantity of an
ingredient or
reactant of the invention employed refers to variation in the numerical
quantity
that can occur, for example, through typical measuring and liquid handling
procedures used for making concentrates or solutions in the real world;
through
inadvertent error in these procedures; through differences in the manufacture,
source, or purity of the ingredients employed to make the compositions or to
carry out the methods; and the like. The term "about" also encompasses
amounts that differ due to different equilibrium conditions for a composition
resulting from a particular initial mixture. Whether or not modified by the
term
"about," the claims include equivalents to the quantities. In one embodiment,
the
term "about" means within 10% of the reported numerical value, alternatively
within 5% of the reported numerical value.
[0053] "Biomass" as used herein refers to a natural product containing
hydrolysable polysaccharides that provide fermentable sugars including any
sugars and starch derived from natural resources such as corn, cane, wheat,
cellulosic or lignocellulosic material and materials comprising cellulose,
hemicellulose, lignin, starch, oligosaccharides, disaccharides and/or
monosaccharides, and mixtures thereof. Biomass may also comprise additional
components such as protein and/or lipids. Biomass may be derived from a single
source or biomass can comprise a mixture derived from more than one source.
For example, biomass may comprise a mixture of corn cobs and corn stover, or a
mixture of grass and leaves. Biomass includes, but is not limited to,
bioenergy
crops, agricultural residues, municipal solid waste, industrial solid waste,
sludge
from paper manufacture, yard waste, wood and forestry waste. Examples of
-15-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
biomass include, but are not limited to, corn grain, corn cobs, crop residues
such
as corn husks, corn stover, grasses, wheat, rye, wheat straw, barley, barley
straw, hay, rice straw, switchgrass, waste paper, sugar cane bagasse, sorghum,
sugar cane, soy, components obtained from milling of grains, trees, branches,
roots, leaves, wood chips, sawdust, shrubs and bushes, vegetables, fruits,
flowers, animal manure, and mixtures thereof. For example, mash, juice,
molasses, or hydrolysate may be formed from biomass by any processing known
in the art for processing the biomass for purposes of fermentation such as by
milling, treating, and/or liquefying and comprises fermentable sugar and may
comprise water. For example, cellulosic and/or lignocellulosic biomass may be
processed to obtain a hydrolysate containing fermentable sugars by any method
known to one skilled in the art. A low ammonia pretreatment is disclosed in
U.S.
Patent Application Publication No. 2007/0031918A1, which is herein
incorporated
by reference. Enzymatic saccharification of cellulosic and/or lignocellulosic
biomass typically makes use of an enzyme consortium for breaking down
cellulose and hemicellulose to produce a hydrolysate containing sugars
including
glucose, xylose, and arabinose. (Saccharification enzymes suitable for
cellulosic
and/or lignocellulosic biomass are reviewed in Lynd, et al. (Microbiol. Mol.
Biol.
Rev. 66:506-577, 2002).
[0054] Mash, juice, molasses, or hydrolysate may include feedstock 12 and
feedstock slurry 16 as described herein. An aqueous feedstream may be derived
or formed from biomass by any processing known in the art for processing the
biomass for purposes of fermentation such as by milling, treating, and/or
liquefying and comprises fermentable carbon substrate (e.g., sugar) and may
comprise water. An aqueous feedstream may include feedstock 12 and
feedstock slurry 16 as described herein.
[0055] "Feedstock" as used herein means a feed in a fermentation process, the
feed containing a fermentable carbon source with or without undissolved
solids,
and where applicable, the feed containing the fermentable carbon source before
or after the fermentable carbon source has been liberated from starch or
obtained
from the break down of complex sugars by further processing such as by
liquefaction, saccharification, or other process. Feedstock includes or is
derived
-16-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
from a biomass. Suitable feedstock include, but are not limited to, rye,
wheat,
corn, cane, barley, cellulosic material, lignocellulosic material, or mixtures
thereof. Where reference is made to "corn oil," it will be appreciated that
the term
encompasses the oil produced from a given feedstock in other embodiments of
the present invention.
[0056] "Fermentation broth" as used herein means the mixture of water, sugars,
dissolved solids, optionally microorganisms producing alcohol, product
alcohol,
and all other constituents of the material held in the fermentation vessel in
which
product alcohol is being made by the reaction of sugars to alcohol, water, and
carbon dioxide (C02) by the microorganisms present. From time to time, as used
herein the term "fermentation medium" and "fermented mixture" can be used
synonymously with "fermentation broth."
[0057] "Fermentable carbon source" or "fermentable carbon substrate" as used
herein means a carbon source capable of being metabolized by the
microorganisms disclosed herein for the production of fermentative alcohol.
Suitable fermentable carbon sources include, but are not limited to,
monosaccharides such as glucose or fructose; disaccharides such as lactose or
sucrose; oligosaccharides; polysaccharides such as starch or cellulose; one
carbon substrates; and mixtures thereof.
[0058] "Fermentable sugar" as used herein refers to sugar capable of being
metabolized by the microorganisms disclosed herein for the production of
fermentative alcohol.
[0059] "Fermentation vessel" as used herein means the vessel in which the
fermentation reaction by which product alcohol such as butanol is made from
sugars is carried out.
[0060] "Liquefaction vessel" as used herein means the vessel in which
liquefaction is carried out. Liquefaction is the process in which
oligosaccharides
are liberated from the feedstock. In some embodiments where the feedstock is
corn, oligosaccharides are liberated from the corn starch content during
liquefaction.
[0061] "Saccharification vessel" as used herein means the vessel in which
saccharification (i.e., the break down of oligosaccharides into
monosaccharides)
-17-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
is carried out. Where fermentation and saccharification occur simultaneously,
the
saccharification vessel and the fermentation vessels may be one in the same
vessel.
[0062] "Sugar" as used herein refers to oligosaccharides, disaccharides,
and/or
monosaccharides.
[0063] As used herein, "saccharification enzyme" means one or more enzymes
that are capable of hydrolyzing polysaccharides and/or oligosaccharides, for
example, alpha-1,4-glucosidic bonds of glycogen, or starch. Saccharification
enzymes may include enzymes capable of hydrolyzing cellulosic or
lignocellulosic
materials as well.
[0064] "Undissolved solids" as used herein means non-fermentable portions of
feedstock, for example, germ, fiber, and gluten.
[0065] "Product alcohol" as used herein refers to any alcohol that can be
produced by a microorganism in a fermentation process that utilizes biomass as
a source of fermentable carbon substrate. Product alcohols include, but are
not
limited to, Ci to C8 alkyl alcohols. In some embodiments, the product alcohols
are C2 to C8 alkyl alcohols. In other embodiments, the product alcohols are C2
to
C5 alkyl alcohols. It will be appreciated that Ci to C8 alkyl alcohols
include, but
are not limited to, methanol, ethanol, propanol, butanol, and pentanol.
Likewise
C2 to C8 alkyl alcohols include, but are not limited to, ethanol, propanol,
butanol,
and pentanol. "Alcohol" is also used herein with reference to a product
alcohol.
[0066] "Butanol" as used herein refers with specificity to the butanol isomers
1-
butanol (1-BuOH), 2-butanol (2-BuOH), and/or isobutanol (iBuOH or I-BUGH,
also known as 2-methyl-1 -propanol), either individually or as mixtures
thereof.
[0067] "Propanol" as used herein refers to the propanol isomers isopropanol or
1-
propanol.
[0068] "Pentanol" as used herein refers to the pentanol isomers 1-pentanol, 3-
methyl-1-butanol, 2-methyl-1-butanol, 2,2-dimethyl-1-propanol, 3-pentanol, 2-
pentanol, 3-methyl-2-butanol, or 2-methyl-2-butanol.
[0069] The term "alcohol equivalent" as used herein refers to the weight of
alcohol that would be obtained by a perfect hydrolysis and recovery of an
amount
of alcohol ester.
-18-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
[0070] The term "aqueous phase titer" as used herein refers to the
concentration
of a particular alcohol (e.g., butanol) in the fermentation broth.
[0071] The term "effective titer" as used herein refers to the total amount of
a
particular alcohol (e.g., butanol) produced by fermentation or alcohol
equivalent
of the alcohol ester produced by alcohol esterification per liter of
fermentation
medium. For example, the effective titer of butanol in a unit volume of a
fermentation includes: (i) the amount of butanol in the fermentation medium;
(ii)
the amount of butanol recovered from the organic extractant; (iii) the amount
of
butanol recovered from the gas phase, if gas stripping is used, and (iv) the
alcohol equivalent of the butanol ester in either the organic or aqueous
phase.
[0072] "In Situ Product Removal (ISPR)" as used herein means the selective
removal of a specific fermentation product from a biological process such as
fermentation to control the product concentration in the biological process as
the
product is produced.
[0073] "Extractant" or "ISPR extractant" as used herein means an organic
solvent
used to extract any product alcohol such as butanol isomer. The extractant may
be a solid or liquid at fermentation temperature. From time to time, as used
herein the term "solvent" may be used synonymously with "extractant."
[0074] "Fatty acid extractants" as used herein means extractants derived from
native oil by chemically reacting the glycerides in the native oil with one or
more
solvents or reactants to obtain one or more reaction products selected from
the
group consisting of fatty acids, fatty alcohols, fatty amides, fatty acid
methyl
esters, fatty acid glycol esters, triglycerides, and mixtures thereof. The
triglycerides may be hydroxylated or alkoxylated (e.g., methoxylated,
ethoxylated).
[0075] "Native oil" as used herein refers to lipids obtained from plants
(e.g.,
biomass) or animals. "Plant-derived oil" as used herein refers to lipids
obtain
from plants, in particular. From time to time, "lipids" may be used
synonymously
with "oil" and "glycerides." Native oils include, but are not limited to,
tallow, corn,
canola, capric/caprylic triglycerides, castor, coconut, cottonseed, fish,
jojoba, lard,
linseed, neetsfoot, oiticica, palm, peanut, rapeseed, rice, safflower, soya,
sunflower, tung, jatropha, and vegetable oil blends.
-19-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
[0076] The term "fatty acid" as used herein refers to a carboxylic acid (e.g.,
aliphatic monocarboxylic acid) having C4 to C28 carbon atoms (most commonly
C12 to C24 carbon atoms), which is either saturated or unsaturated. Fatty
acids
may also be branched or unbranched. Fatty acids may be derived from, or
contained in esterified form, in an animal or vegetable fat, oil, or wax.
Fatty acids
may occur naturally in the form of glycerides in fats and fatty oils or may be
obtained by hydrolysis of fats or by synthesis. The term fatty acid may
describe a
single chemical species or a mixture of fatty acids. In addition, the term
fatty acid
also encompasses free fatty acids.
[0077] The term "fatty alcohol" as used herein refers to an alcohol having a
long,
aliphatic chain of C4 to C22 carbon atoms, which is either saturated or
unsaturated.
[0078] The term "fatty aldehyde" as used herein refers to an aldehyde having a
long, aliphatic chain of C4 to C22 carbon atoms, which is either saturated or
unsaturated.
[0079] The term "fatty amide" as used herein refers to an amide having a long,
aliphatic chain of C4 to C22 carbon atoms, which is either saturated or
unsaturated
[0080] The term "fatty ester" as used herein refers to an ester having a long
aliphatic chain of C4 to C22 carbon atoms, which is either saturated or
unsaturated.
[0081] The term "water-immiscible" refers to a chemical component such as an
extractant or solvent, which is incapable of mixing with an aqueous solution
such
as a fermentation broth, in such a manner as to form one liquid phase.
[0082] The term "aqueous phase" as used herein refers to the aqueous phase of
a biphasic mixture obtained by contacting a fermentation broth with a water-
immiscible organic extractant. In an embodiment of a process described herein
that includes fermentative extraction, the term "fermentation broth" then
specifically refers to the aqueous phase in biphasic fermentative extraction.
[0083] The term "organic phase" as used herein refers to the non-aqueous phase
of a biphasic mixture obtained by contacting a fermentation broth with a water-
immiscible organic extractant.
-20-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
[0084] The term "separation" as used herein is synonymous with "recovery" and
refers to removing a chemical compound from an initial mixture to obtain the
compound in greater purity or at a higher concentration than the purity or
concentration of the compound in the initial mixture.
[0085] As used herein, "recombinant microorganism" refers to microorganisms
such as bacteria or yeast, that are modified by use of recombinant DNA
techniques, for example, by engineering a host cell to comprise a biosynthetic
pathway such as a biosynthetic pathway to produce an alcohol such as butanol.
[0086] The present invention provides extractants obtained by chemical
conversion of oil derived from biomass and methods of producing the
extractants.
In particular, the glycerides in the oil can be chemically converted into one
or
more products including fatty acids, fatty alcohols, fatty amides, fatty acid
methyl
esters, fatty acid glycol esters, and triglycerides, and mixtures thereof,
collectively
referred to herein as fatty acid extractants. The triglycerides may be
hydroxylated or alkoxylated (e.g., methoxylated, ethoxylated). Fatty acid
extractants can serve as extractants for in situ removal of a product alcohol
such
as butanol from a fermentation broth. Thus, the present invention also
provides
methods for producing a product alcohol such as butanol through extractive
fermentation using the extractants that were produced from the biomass oil.
The
present invention also provides methods for removing oil from an alcohol
fermentation process by separating the oil derived from a feedstock. The
feedstock can be liquefied to create a slurry prior to oil removal. Thus, the
slurry
includes a fermentable carbon source, oil, and undissolved solids. The oil,
and in
some embodiments, the undissolved solids, can be removed from the slurry prior
to the slurry being fed to the fermentation vessel. Removal of the oil and in
some
embodiments, the undissolved solids, can reduce the loss, degradation of the
partition coefficient of the extractant over time that is attributable to the
presence
of the oil (and in some embodiments the solids) in the fermentation vessel.
Moreover, the separated oil can be chemically converted into a fatty acid
extractant which can be fed to the fermentation vessel. The fatty acid
extractant
can have a partition coefficient for a fermentative alcohol greater than a
partition
coefficient of the oil for the fermentative alcohol. Further, the fatty acid
extractant
-21 -
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
can be used in place of or in addition to a commercial exogenous extractant
such
as oleyl alcohol that was not chemically converted from the feedstock
according
to the methods of the present invention. Thus, the methods of the present
invention can reduce the raw material expense associated with the exogenous
extractant by producing an extractant at a step in a fermentation process via
chemical conversion of oil derived from a feedstock.
[0087] The present invention will be described with reference to the Figures.
FIG. 1 illustrates an exemplary process flow diagram for production of
fermentative alcohol according to an embodiment of the present invention. As
shown, a feedstock 12 can be introduced to an inlet in a liquefaction vessel
10
and liquefied to produce a feedstock slurry 16. Feedstock 12 contains
hydrolysable starch that supplies a fermentable carbon source (e.g.,
fermentable
sugar such as glucose), and can be a biomass such as, but not limited to rye,
wheat, corn, cane, barley, cellulosic material, lignocellulosic material, or
mixtures
thereof, or can otherwise be derived from a biomass. In some embodiments,
feedstock 12 can be one or more components of a fractionated biomass and in
other embodiments, feedstock 12 can be a milled, unfractionated biomass. In
some embodiments, feedstock 12 can be corn such as dry milled, unfractionated
corn kernels, and the undissolved particles can include germ, fiber, and
gluten.
The undissolved solids are non-fermentable portions of feedstock 12. For
purposes of the discussion herein with reference to the embodiments shown in
the Figures, feedstock 12 will often be described as constituting milled,
unfractionated corn, in which the undissolved solids have not been separated
therefrom. However, it should be understood that the exemplary methods and
systems described herein can be modified for different feedstocks whether
fractionated or not, as apparent to one of skill in the art. In some
embodiments,
feedstock 12 can be high-oleic corn, such that corn oil derived therefrom is a
high-oleic corn oil having an oleic acid content of at least about 55 wt%
oleic acid.
In some embodiments, the oleic acid content in high-oleic corn oil can be up
to
about 65 wt%, as compared with the oleic acid content in normal corn oil which
is
about 24 wt%. High-oleic oil can provide some advantages for use in the
methods of the present invention, as hydrolysis of the oil can provide a fatty
acid
-22-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
extractant having a high oleic acid content for contacting with a fermentation
broth. In some embodiments, the fatty acids or mixtures thereof comprise
unsaturated fatty acids. The presence of unsaturated fatty acids decreases the
melting point, providing advantages for handling. Of the unsaturated fatty
acids,
those which are monounsaturated, that is, possessing a single carbon-carbon
double bond may provide advantages with respect to melting point without
sacrificing suitable thermal and oxidative stability for process
considerations.
[0088] The process of liquefying feedstock 12 involves hydrolysis of starch in
feedstock 12 into sugars including, for example, dextrins and
oligosaccharides,
and is a conventional process. Any known liquefying processes, as well as the
corresponding liquefaction vessel, normally utilized by the industry can be
used
including, but not limited to, the acid process, the acid-enzyme process, or
the
enzyme process. Such processes can be used alone or in combination. In some
embodiments, the enzyme process can be utilized and an appropriate enzyme
14, for example, alpha-amylase, is introduced to an inlet in liquefaction
vessel 10.
Water can also be introduced to liquefaction vessel 10.
[0089] Feedstock slurry 16 produced from liquefying feedstock 12 includes
sugar, oil, and undissolved solids derived from the feedstock. Feedstock
slurry
16 can be discharged from an outlet of liquefaction vessel 10. In some
embodiments, feedstock 12 is corn or corn kernels and therefore, feedstock
slurry 16 is a corn mash slurry.
[0090] Feedstock slurry 16 is introduced into an inlet of a separator 20 which
is
configured to remove some, or preferably substantially all, of the oil present
in the
feedstock slurry 16. The removed oil is provided as a stream 26 to a reaction
vessel 40, and the remaining feedstock including the sugar and water is
discharged as an aqueous stream 22 to a fermentation vessel 30. Aqueous
stream 22 can include the undissolved solids of the slurry 16, but since the
oil 26
was removed via separator 20, the fermentation broth in fermentation vessel 30
still has a reduced amount of oil. The oil stream 26 discharged from separator
20
has an amount of glycerides, particularly triglycerides, which are contacted
with
one or more substances 42 in reaction vessel 40. Substances 42 are reactants
or solvents capable of chemically converting at least a portion of the
glycerides in
-23-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
oil 26 into a fatty acid extractant 28. In some embodiments, the amount of
fatty
acid extractant 28 in the oil from chemical conversion of the glycerides via
substances 42 can be at least about 17 wt%, at least about 20 wt%, at least
about 30 wt%, at least about 40 wt%, at least about 50 wt%, at least about 60
wt%, at least about 65 wt%, at least about 70 wt%, at least about 75 wt%, at
least
about 80 wt%, at least about 85 wt%, at least about 90 wt%, at least about 95
wt%, or at least about 99 wt%.
[0091] Separator 20 can be any suitable separator known in the art for
removing
oil from an aqueous feedstream, including but not limited to, siphoning,
aspiration
decantation, centrifugation, using a gravity settler, membrane-assisted phase
splitting, and the like. In some embodiments, separator 20 can also remove the
undissolved solids in feedstock slurry 16 and discharge the undissolved solids
as
a solid phase or wet cake 24. For example, in some embodiments, separator 20
can includes a filter press, vacuum filtration, or a centrifuge for separating
the
undissolved solids from feedstock slurry 16. For example, in some embodiments,
separator 20 includes a tricanter centrifuge 20 that agitates or spins
feedstock
slurry 16 to produces a centrifuge product comprising an aqueous layer
containing the sugar and water (i.e., stream 22), a solids layer containing
the
undissolved solids (i.e., wet cake 24), and an oil layer (i.e., oil stream
26). When
slurry 16 is a corn mash slurry, then oil 26 is free corn oil. The term free
corn oil
as used herein means corn oil that is freed from the corn germ. For a corn
mash
slurry as feedstock slurry 16, wet cake 24 includes at least about 50% by
weight
of the undissolved particles present in the feedstock slurry, at least about
55% by
weight of the undissolved particles present in the feedstock slurry, at least
about
60% by weight of the undissolved particles present in the feedstock slurry, at
least about 65% by weight of the undissolved particles present in the
feedstock
slurry, at least about 70% by weight of the undissolved particles present in
the
feedstock slurry, at least about 75% by weight of the undissolved particles
present in the feedstock slurry, at least about 80% by weight of the
undissolved
particles present in the feedstock slurry, at least about 85% by weight of the
undissolved particles present in the feedstock slurry, at least about 90% by
weight of the undissolved particles present in the feedstock slurry, at least
about
-24-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
95% by weight of the undissolved particles present in the feedstock slurry, or
about 99% by weight of the undissolved particles present in the feedstock
slurry.
[0092] When wet cake 24 is removed via centrifuge 20, in some embodiments, a
portion of the oil from feedstock 12, such as corn oil when the feedstock is
corn,
remains in wet cake 24. In such instances, wet cake 24 includes corn oil in an
amount of less than about 20% by weight of dry solids content of wet cake 24.
Wet cake 24 can be discharged out an outlet located near the bottom of
centrifuge 20. Wet cake 24 can also include a portion of the fermentable
carbon
and water. Wet cake 24 can be washed with additional water in the centrifuge
once aqueous solution 22 has been discharged from the centrifuge 20. Washing
wet cake 24 will recover the sugar (e.g., oligosaccharides) present in the wet
cake and the recovered sugar and water can be recycled to the liquefaction
vessel 10. After washing, wet cake 24 can be dried to form Dried Distillers'
Grains with Solubles (DDGS) through any suitable known process. The
formation of the DDGS from wet cake 24 formed in centrifuge 20 has several
benefits. Because the undissolved solids do not go to the fermentation vessel,
the DDGS does not have trapped extractant and/or product alcohol such as
butanol, it is not subjected to the conditions of the fermentation vessel, and
it
does not contact the microorganisms present in the fermentation vessel. These
benefits make it easier to process and sell DDGS, for example as animal feed.
Methods and systems for removing undissolved solids from feedstock 16 via
centrifugation are described in detail in co-pending, commonly owned U.S.
Provisional Patent Application No. 61/356,290, filed on June 18, 2010, which
is
incorporated herein in its entirety by reference thereto.
[0093] In some embodiments, oil 26 is not discharged separately from wet cake
24, but rather oil 26 is included as part of wet cake 24 and is ultimately
present in
the DDGS. In such instances, the oil can be separated from the DDGS and
converted to a fatty acid extractant for subsequent use in the same or
different
alcohol fermentation process. In any case, removal of the oil component of the
feedstock is advantageous to alcohol production such as butanol production
because oil present in the fermentation vessel can dilute the ISPR extractant
and
can reduce the partition coefficient of the fermentative alcohol into the
organic
-25-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
phase. Also, the oil can break down into fatty acids and glycerin, which can
accumulate in the water and reduce the amount of water that is available for
recycling throughout the system. Thus, removal of the oil component of the
feedstock can also increase the efficiency of the product alcohol production
by
increasing the amount of water that can be recycled through the system.
[0094] Aqueous stream 22 and a microorganism 32 are introduced to a
fermentation vessel 30 to be included in a fermentation broth held in
fermentation
vessel 30. Fermentation vessel 30 is configured to ferment aqueous stream 22
to produce a product alcohol such as butanol. In particular, microorganism 32
metabolizes the fermentable sugar in slurry 16 and excretes a product alcohol.
Microorganism 32 is selected from the group of bacteria, cyanobacteria,
filamentous fungi, and yeasts. In some embodiments, microorganism 32 can be a
bacteria, such as E. coli. In some embodiments, microorganism 32 can be a
fermentative recombinant microorganism. Aqueous solution 22 can include the
sugar, for example, in the form of oligosaccharides, and water, and can
comprise
less than about 20 g/L of monomeric glucose, more preferably less than about
10
g/L or less than about 5 g/L of monomeric glucose. Suitable methodology to
determine the amount of monomeric glucose is well known in the art. Such
suitable methods known in the art include HPLC.
[0095] In some embodiments, aqueous stream 22 is subjected to a
saccharification process in order to break the complex sugars (e.g.,
oligosaccharides) in stream 22 into monosaccharides that can be readily
metabolized by microorganism 32. Any known saccharification process, normally
utilized by the industry can be used including, but not limited to, the acid
process,
the acid-enzyme process, or the enzyme process. In some embodiments,
simultaneous saccharification and fermentation (SSF) can occur inside
fermentation vessel 30. In some embodiments, an enzyme 38 such as
glucoamylase, can be introduced to an inlet in fermentation vessel 30 in order
to
breakdown the starch to glucose which can be metabolized by microorganism 32.
[0096] In situ product removal (ISPR) can be utilized to remove the product
alcohol from fermentation vessel 30 as the product alcohol is produced by
microorganism 32. For extractive fermentation, such ISPR includes liquid-
liquid
-26-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
extraction. Liquid-liquid extraction can be performed according to the
processes
described in U.S. Patent Application Publication No. 2009/0305370, the
disclosure of which is hereby incorporated in its entirety. U.S. Patent
Application
Publication No. 2009/030537 describes methods for producing and recovering
butanol from a fermentation broth using extractive fermentation, the methods
comprising the step of contacting the fermentation broth with a water
immiscible
extractant. Typically, the extractant can be an organic extractant selected
from
the group consisting of fatty acids, fatty alcohols, fatty amides, a mixture
of fatty
amides and fatty acids, esters of fatty acids, fatty aldehydes, fatty acid
methyl
esters, fatty acid glycol esters, triglycerides and mixtures thereof, to form
a two-
phase mixture comprising an aqueous phase and an organic phase. With
reference to the embodiment of FIG. 1, fermentation vessel 30 has one or more
inlets for receiving one or more water immiscible ISPR extractants, including
fatty
acid extractant 28 from vessel 40. Fatty acid extractant 28 contacts the
fermentation broth and forms a two-phase mixture comprising an aqueous phase
and an organic phase. The product alcohol present in the fermentation broth
partitions into the organic phase. The biphasic mixture can be removed from
fermentation vessel 30 as stream 39 and introduced into a vessel 35, in which
separation of the aqueous and organic phases is performed to produce an
alcohol-containing organic phase 36 and an aqueous phase 34. The alcohol-
containing organic phase 36 is separated from the aqueous phase 34 of the
biphasic mixture 39 using methods known in the art, including but not limited
to,
siphoning, decantation, centrifugation, using a gravity settler, membrane-
assisted
phase splitting, and the like. All or part of the aqueous phase 34 can be
recycled
into fermentation vessel 30 as fermentation medium (as shown), or otherwise
discarded and replaced with fresh medium, or treated for the removal of any
remaining product alcohol and then recycled to fermentation vessel 30. The
alcohol-containing organic phase 36 is treated to recover the product alcohol,
and
the resulting alcohol-lean extractant can then be recycled back (not shown)
into
fermentation vessel 30, usually in combination with fresh make-up extractant
28,
for further extraction of the product alcohol. Alternatively, fresh extractant
28 can
-27-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
be continuously added to the fermentation vessel to replace the extractant
removed in biphasic mixture stream 39.
[0097] In some embodiments, one or more additional ISPR extractants can be
introduced into fermentation vessel 30, such as extractant 29 illustrated in
the
embodiments of FIGs. 3-5, to form a two-phase mixture comprising an aqueous
phase and an organic phase, with the product alcohol partitioning into the
organic
phase. Such one or more additional extractants 29 can be another fatty acid
extractant and/or an exogenous organic extractant such as oleyl alcohol,
behenyl
alcohol, cetyl alcohol, lauryl alcohol, myristyl alcohol, stearyl alcohol, 1-
undecanol, oleic acid, lauric acid, myristic acid, stearic acid, methyl
myristate,
methyl oleate, undecanal, lauric aldehyde, 20-methylundecanal, and mixtures
thereof. In some embodiments, ISPR extractant 29 can be a carboxylic acid, and
in some embodiments, ISPR extractant 29 can be a free fatty acid. In some
embodiments, the carboxylic acid or free fatty acid can have a chain of 4 to
28
carbons, 4 to 22 carbons in other embodiments, 8 to 22 carbons in other
embodiments, 10 to 28 carbons in other embodiments, 7 to 22 carbons in other
embodiments, 12 to 22 carbons in other embodiments, 4 to 18 carbons in other
embodiments, 12 to 22 carbons in other embodiments, and 12 to 18 carbons in
still other embodiments.
[0098] In some embodiments, ISPR extractant 29 is one or more of the following
fatty acids: azaleic, capric, caprylic, castor, coconut (i.e., as a naturally-
occurring
combination of fatty acids including lauric, myrisitic, palmitic, caprylic,
capric,
stearic, caproic, arachidic, oleic, and linoleic, for example), dimer,
isostearic,
lauric, linseed, myristic, oleic, palm oil, palmitic, palm kernel, pelargonic,
ricinoleic, sebacic, soya, stearic acid, tall oil, tallow, and #12 hydroxy
stearic. In
some embodiments, ISPR extractant 29 is one or more of diacids, for example,
azelaic acid and sebacic acid. Thus, in some embodiments, ISPR extractant 29
can be a mixture of two or more different fatty acids. In some embodiments,
ISPR extractant 29 can be free fatty acids produced from enzymatic hydrolysis
of
native oil such as biomass lipids as described, for example, in co-pending,
commonly owned U.S. Provisional Patent Application No. 61/368,444, filed on
July 28, 2010. In such embodiments, the biomass lipids for producing
extractant
-28-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
29 can be from a same or different biomass source from which feedstock 12 is
obtained. For example, in some embodiments, the biomass lipids for producing
extractant 29 can be derived from soya, whereas the biomass source of
feedstock 12 is corn. Any possible combination of different biomass sources
for
extractant 29 versus feedstock 12 can be used, as should be apparent to one of
skill in the art.
[0099] In the embodiment of FIG. 1, the product alcohol is extracted from the
fermentation broth in situ, with the separation of the biphasic mixture 39
occurring
in a separate vessel 35. In situ extractive fermentation can be carried out in
a
batch mode or a continuous mode in fermentation vessel 30. For in situ
extractive fermentation, the organic extractant can contact the fermentation
medium at the start of the fermentation forming a biphasic fermentation
medium.
Alternatively, the organic extractant can contact the fermentation medium
after
the microorganism has achieved a desired amount of growth, which can be
determined by measuring the optical density of the culture. Further, the
organic
extractant can contact the fermentation medium at a time at which the product
alcohol level in the fermentation medium reaches a preselected level. In the
case
of butanol production, for example, the ISPR extractant can contact the
fermentation medium at a time before the butanol concentration reaches a toxic
level. After contacting the fermentation medium with the ISPR extractant, the
butanol product partitions into the extractant, decreasing the concentration
of
butanol in the aqueous phase containing the microorganism, thereby limiting
the
exposure of the production microorganism to the inhibitory butanol product.
[00100] The volume of the ISPR extractant to be used depends on a number of
factors including the volume of the fermentation medium, the size of the
fermentation vessel, the partition coefficient of the extractant for the
butanol
product, and the fermentation mode chosen, as described below. The volume of
the extractant can be about 3% to about 60% of the fermentation vessel working
volume. Depending on the efficiency of the extraction, the aqueous phase titer
of
butanol in the fermentation medium can be, for example, from about 5 g/L to
about 85 g/L, from about 10 g/L to about 40 g/L, from about 10 g/L to about 20
g/L, from about 15 g/L to about 50 g/L, or from about 20 g/L to about 60 g/L.
-29-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
Without being held to theory, it is believed that higher butanol titer may
obtained
with the extractive fermentation method, in part, from the removal of the
toxic
butanol product from the fermentation medium, thereby keeping the level below
that which is toxic to the microorganism.
[00101] In a batchwise mode of in situ extractive fermentation, a volume of
organic
extractant is added to the fermentation vessel and the extractant is not
removed
during the process. This mode requires a larger volume of organic extractant
to
minimize the concentration of the inhibitory butanol product in the
fermentation
medium. Consequently, the volume of the fermentation medium is less and the
amount of product produced is less than that obtained using the continuous
mode. For example, the volume of the extractant in the batchwise mode can be
20% to about 60% of the fermentation vessel working volume in one
embodiment, and about 30% to about 60% in another embodiment.
[00102] Gas stripping (not shown) can be used concurrently with the organic
extractant to remove the product alcohol from the fermentation medium.
[00103] In some embodiments, separation of the biphasic mixture can occur in
the
fermentation vessel, as shown in the embodiments of later described FIGs. 4
and
5. In particular, in a continuous mode of in situ extractive fermentation, in
one
embodiment, extractant 28 may be introduced into fermentation vessel 30 to
obtain the biphasic mixture therein, with the alcohol-containing organic-phase
stream 36 exiting directly from fermentation vessel 30. Aqueous phase stream
34 can also exit directly from fermentation vessel 30, be treated for the
removal
of any remaining product alcohol and recycled, or discarded and replaced with
fresh fermentation medium. The extraction of the alcohol product by the ISPR
extractant can be done with or without the removal of microorganism 32 from
the
fermentation broth. Microorganism 32 can be removed from the fermentation
broth by means known in the art including, but not limited to, filtration or
centrifugation. For example, aqueous phase stream 34 can include
microorganism 32 such as a yeast. Microorganism 32 can be easily separated
from the aqueous phase stream, for example, in a centrifuge (not shown).
Microorganism 32 can then be recycled to fermentation vessel 30 which over
-30-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
time can increase the production rate of alcohol production, thereby resulting
in
an increase in the efficiency of the alcohol production.
[00104] In a continuous mode of in situ extractive fermentation, the volume of
the
extractant can be about 3% to about 50% of the fermentation vessel working
volume in one embodiment, about 3% to about 30% in another embodiment, 3%
to about 20% in another embodiment; and 3% to about 10% in another
embodiment. Because the product is continually removed from the reactor, a
smaller volume of extractant is required enabling a larger volume of the
fermentation medium to be used.
[00105] As an alternative to in situ extractive fermentation, the product
alcohol can
be extracted from the fermentation broth downstream of fermentation vessel 30.
In such an instance, the fermentation broth can be removed from fermentation
vessel 30 and introduced into vessel 35. Extractant 28 can then be introduced
in
vessel 35 and contacted with the fermentation broth to obtain biphasic mixture
39
in vessel 35, which is then separated into the organic 36 and aqueous 34
phases.
Alternatively, extractant 28 can be added to the fermentation broth in a
separate
vessel (not shown) prior to introduction to vessel 35.
[00106] Fatty acid extractant 28 has a partition coefficient for the product
alcohol
greater than the partition coefficient of oil 26 for the product alcohol. For
example, where the feedstock 12 is corn, corn oil 26, if present in the
fermentation broth, can have a partition coefficient for the product alcohol
of less
than about 0.28, whereas fatty acid extractant 28 derived from corn oil 26 can
have a partition coefficient of about 0.28 and greater. In one embodiment,
fatty
acid extractant 28 has a partition coefficient for the product alcohol such as
butanol of at least about 1, at least about 2 in another embodiment, at least
about
2.5 in another embodiment, at least about 2.75 in another embodiment, and at
least about 3 in another embodiment. Thus, removal of the oil component of the
feedstock increases the efficiency of the product alcohol production in
extractive
fermentation by not only reducing the threat to degradation of the partition
coefficient of the ISPR extractant, but also by serving as a raw material for
the
production of a fatty acid extractant that can partition the product alcohol
from the
aqueous phase more so than the oil itself. Moreover, fatty acid extractant 28
-31-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
derived from oil 26 in feedstock 12 can be used alone or in combination with
an
exogenous extractant (e.g., externally supplied oleyl alcohol), thereby
reducing or
eliminating the cost associated with the exogenous extractant.
[00107] Moreover, in the instance that fatty acid extractant 28 includes free
fatty
acids, a rate of glucose consumption by microorganism 32 in fermentation
vessel
30 can be higher in the presence of such free fatty acids than in the absence
of
such free fatty acids. Thus, in some embodiments of the present invention, the
fermentation broth can be contacted with a fatty acid extractant having free
fatty
acids, whereby the free fatty acids can increase glucose uptake by
microorganism 32 compared to the glucose uptake when an ISPR extractant
without free fatty acids (e.g., oleyl alcohol) is used in extractive
fermentation. For
example, as illustrated in Table 1 of Example 2 described below, fatty
amide/fatty
acid mixtures used as fatty acid extractants in extractive fermentation can
provide
a higher rate of glucose uptake by a Saccharomyces butanologen than when
using oleyl alcohol as an extractant. Methods for producing a product alcohol
from a fermentation process in which free fatty acids are produced at a step
in
the process and are contacted with microorganism cultures in a fermentation
vessel for improving microorganism growth rate and glucose consumption are
described in co-pending, commonly owned U.S. Provisional Patent Application
No. 61/368,451, filed on July 28, 2010, which is incorporated herein in its
entirety
by reference thereto.
[00108] In some embodiments, the system and processes of FIG. 1 can be
modified such that simultaneous saccharification and fermentation in
fermentation vessel 30 is replaced with a separate saccharification vessel 60
between separator 20 and fermentation vessel 30, as should be apparent to one
of skill in the art.
[00109] In still other embodiments, as shown, for example, in the embodiment
of
FIG. 2, saccharification can occur in a separate saccharification vessel 60
which
is located between separator 20 and liquefaction vessel 10. FIG. 2 is
substantially identical to FIG. 1 except for the inclusion of separate
saccharification vessel 60 receiving enzyme 38, with oil stream 26 being
separated from a liquefied, saccharified feedstock stream 62. Feedstock slurry
-32-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
16 is introduced into saccharification vessel 60 along with enzyme 38 such as
glucoamylase, whereby sugars in the form of oligosaccharides in slurry 16 can
be
broken down into monosaccharides. A liquefied, saccharified feedstock stream
62 exits saccharification vessel 60 and is introduced into separator 20.
Feedstock stream 62 includes monosaccharides, oil, and undissolved solids
derived from the feedstock. In separator 20, feedstock stream 62 is separated
into oil stream 26 and a substantially aqueous stream 23, which is fed to
fermentation vessel 30. In the embodiment shown, aqueous stream 23 includes
undissolved solids. Alternatively, the solids can be removed in separator 20
as a
wet cake 24, as described with reference to the embodiment of FIG. 1. The oil
stream 26 discharged from separator 20 has an amount of glycerides,
particularly
triglycerides, which are contacted with one or more substances 42 in reaction
vessel 40. Substances 42 chemically converts at least a portion of the
glycerides
from oil 26 into fatty acid extractant 28 which is fed to fermentation vessel
30.
The remaining process operations of the embodiment of FIG. 2 are identical to
FIG. 1 and therefore, will not be described in detail again.
[00110] In some embodiments of the present invention, as shown, for example,
in
the embodiment of FIG. 3, extractive fermentation can employ a fatty acid
extractant 28' that is derived from a biomass source that is the same or
different
from the biomass source of feedstock 12, but that is not derived from the
actual
oil contained in feedstock 12. For example, in the instance when feedstock 12
is
corn, fatty acid extractant 28' can be derived from corn oil, but the corn oil
producing extractant 28' is not the corn oil contained in feedstock 12 and
separated to reaction vessel 40 as provided in the embodiment of FIG. 1. As
another example, fatty acid extractant 28' can be derived from soybeans (or
soya) as the biomass source, whereas the biomass source of feedstock 12 is
corn. Any possible combination of different biomass sources for extractant 28'
versus feedstock 12 can be used, as should be apparent to one of skill in the
art.
[00111] In some embodiments, fatty acid extractant 28' is derived from any
native
oil and therefore, can be derived from either a biomass source or
alternatively, an
animal source.
-33-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
[00112] In the embodiment of FIG. 3, liquefied aqueous stream 22 is fed to
fermentation vessel 30 along with saccharification enzyme 38 and microorganism
32, whereby a product alcohol is produced by simultaneous saccharification and
fermentation (SSF). In some embodiments, saccharification can occur in a
separate vessel such as described with reference to the embodiment of FIG. 2,
for example. In some embodiments, preferably, liquefied aqueous stream 22 has
had at least oil 26 removed via separator 20 (see FIG. 1) and in some
embodiments, also has the undissolved solids removed as wet cake 24, prior to
introduction to fermentation vessel 30 (see FIG. 1). A native oil, such as a
plant-
derived oil, is introduced into reaction vessel 40 as stream 26' along with
substance(s) 42 for chemically converting at least a portion of oil 26' to
fatty acid
extractant 28'. Oil stream 26' is not oil 26 derived from feedstock slurry 16
upstream (see FIG. 1). Any plant-derived oil or other native oil that can be
chemically converted to fatty acid extractant 28' for ISPR can be the source
of oil
stream 26'. Fatty acid extractant 28' from reaction vessel 40 is then
introduced to
fermentation vessel 30, whereby the product alcohol partitions into the fatty
acid
extractant 28' to a greater extent that the product alcohol would partition
into oil
26' if present in the fermentation vessel.
[00113] Thus, in some embodiments, the product alcohol is extracted using
fatty
acid extractant 28' obtained from a plant-derived oil 26' that that is not the
same
oil 26 originally introduced in the process via feedstock 12. Optionally, one
or
more additional extractants 29 can be introduced into fermentation vessel 30
for
preferentially partitioning the product alcohol from the aqueous phase. The
one
or more additional extractants 29 can be an exogenous extractant such as
exogenously supplied oleyl alcohol, that was not produced in the process
and/or
can be another fatty acid extractant. In some embodiments, such other fatty
acid
extractant 29 can be produced from an oil that is derived from a biomass
source
that is the same or different from either of the biomass sources of
fermentation
vessel feed stream 22 and oil stream 26'.
[00114] The remaining process operations of the embodiment of FIG. 3 are
identical to FIGs. 1 and 2, except for aqueous phase 34 not shown as being fed
back to fermentation vessel 30 and therefore, will not be described in detail
-34-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
again. It should be understood, however, that in any of the embodiments
presented herein, that all or part of aqueous phase 34 can be recycled,
discarded, and/or further treated to remove product alcohol as described above
with reference to FIG. 1.
[00115] In some embodiments of the present invention, the oil derived from
feedstock 12 is not separated in separator 20, but rather is chemically
converted
into a fatty acid extractant in situ, for example, in feedstock 12 either
prior to or
during liquefaction, in slurry 16, or in saccharified stream 62 (see FIG. 2).
For
example, in the embodiment of FIG. 4, feedstock 12 is fed to liquefaction
vessel
along with appropriate enzyme 14, for example, alpha-amylase, for
hydrolyzing the starch in feedstock 12 to produce a liquefied feedstock. Also
introduced into liquefaction vessel 10, either before, during, or after
liquefaction of
feedstock 12, are one or more substances 42 for chemically converting the oil
present in feedstock 12 to fatty acid extractant 28. Substances 42 can be
introduced to liquefaction vessel 10 either before or after the addition of
enzyme
14, and the oil in feedstock 12 can be converted to extractant 28 either
before,
during, or after liquefaction of feedstock 12. In any case, oil in feedstock
12 is
converted to fatty acid extractant 28, such that a biphasic stream 18 exits
liquefaction vessel 10. Biphasic stream 18 includes both fatty acid extractant
28
as well as the sugar, water, and undissolved solids forming liquefied aqueous
phase 22. In some embodiments, where fatty acid extractant includes fatty
acids,
aqueous phase 22 of biphasic stream 18 can include glycerol (glycerin) from
converting the glycerides in the oil to fatty acids. In some embodiments, such
glycerol, if present, can be removed from the stream 18 prior to introduction
into
fermentation vessel 30.
[00116] With reference to FIG. 4, biphasic stream 18 (i.e., streams 22, 28) is
contacted with the fermentation broth in fermentation vessel 30 to form a
biphasic
mixture. In fermentation vessel 30, product alcohol produced by SSF partitions
into an organic phase including fatty acid extractant 28. Alternatively, in
some
embodiments, the process can be modified to include a separate
saccharification
vessel as discussed in connection with FIG. 2. Separation of the biphasic
mixture occurs in fermentation vessel 30, whereby alcohol-containing organic
-35-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
phase stream 36 and aqueous phase stream 34 exit directly from fermentation
vessel 30. Alternatively, separation of the biphasic mixture can be conducted
in a
separate vessel 35 as provided in the embodiments of FIGs. 1-3. Optionally,
one
or more additional extractants 29 can be introduced into fermentation vessel
30
to form an organic phase that preferentially partitions the product alcohol
from the
aqueous phase. The remaining process operations of the embodiment of FIG. 4
are identical to the previously described figures and therefore, will not be
described in detail again.
[00117] In some embodiments of the present invention, biomass oil present in
feedstock 12 can be separated from the process streams at a step following
alcoholic fermentation. The post-fermentation separated oil can then be
converted to a fatty acid extractant and introduced as an ISPR extractant in
the
fermentation vessel. For example, in the embodiment of FIG. 5, feedstock 12 is
liquefied to produced feedstock slurry 16 which includes oil 26 derived from
the
feedstock. Feedstock slurry 16 can also include undissolved solids from the
feedstock. Alternatively, the undissolved solids can be separated from slurry
16
via a separator such as a centrifuge (not shown). Feedstock slurry 16
containing
oil 26 is introduced directly to fermentation vessel 30 containing a
fermentation
broth including saccharification enzyme 38 and microorganism 32. A product
alcohol is produced by SSF in fermentation vessel 30. Alternatively, in some
embodiments, the process can be modified to include a separate
saccharification
vessel as discussed in connection with FIG. 2.
[00118] ISPR extractant 29 is introduced to fermentation vessel 30 to form a
biphasic mixture, and the product alcohol is removed by partitioning into the
organic phase of the ISPR extractant 29. Oil 26 also partitions into the
organic
phase. ISPR extractant 29 can be one or more fatty acid extractants and/or
exogenous organic extractants not derived from native oil (e.g., oleyl
alcohol). If
extractant 29 is a fatty acid extractant, the extractant 29 can be fatty acid
extractant 28' (see FIG. 3) produced from a native oil such as a plant-derived
oil
that is not the same oil originally introduced in the process via feedstock
12.
[00119] Separation of the biphasic mixture occurs in fermentation vessel 30,
whereby alcohol-containing organic phase stream 36 and aqueous phase stream
-36-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
34 exit directly from fermentation vessel 30. Alternatively, separation of the
biphasic mixture can be conducted in a separate vessel 35 as provided in the
embodiments of FIGs. 1-3. Organic phase stream 36 including oil 26 is
introduced into a separator 50 to recover product alcohol 54 from extractant
29.
The resulting alcohol-lean extractant 27 includes recovered extractant 29 and
oil
26. Extractant 27 including oil 26 is introduced into reaction vessel 40 and
contacted with one or more substances 42 (e.g., reactants and/or solvents)
which
chemically convert at least a portion of oil 26 into fatty acid extractant 28.
[00120] Extractant 27 including into fatty acid extractant 28 can then be
recycled
back into fermentation vessel 30. Such recycled extractant stream 27 can be a
separate stream or a combined stream with fresh, make-up extractant stream 29.
The subsequent withdrawal of alcohol-containing organic phase 36 can then
include fatty acid extractant 28 and ISPR extractant 29, in addition to oil 26
and
the product alcohol. Organic phase 36 can then be treated to recover the
product
alcohol, react the oil to form a fatty acid extractant, and be recycled back
into
fermentation vessel 30 as the resulting alcohol-lean fatty acid extractant 28
and
alcohol-lean extractant 29. In some embodiments, use of extractant 29 can be
phased out as the fermentation process is operated over time, because the
process itself can produce a sufficient amount of fatty acid extractant 28 for
extracting the product alcohol. Thus, the ISPR extractant can be recycled
extractant 27 and fatty acid extractant 28 as a make up ISPR extractant via
reaction vessel 40.
[00121] Alternatively, in some embodiments, organic phase stream 36 including
oil
26 can be introduced into reaction vessel 40 prior to product alcohol recovery
54
in separator 50. In such embodiments, organic phase stream 36 can be
introduced in reaction vessel 50 and contacted with one or more substances 42
for producing fatty acid extractant 28. The resulting organic phase stream 36
including fatty acid extractant 28 can then be introduced into separator 50 to
recover product alcohol 54, and the resulting alcohol-lean extractant can then
be
recycled back into fermentation vessel 30 as extractant stream 27 including
fatty
acid extractant 28. In still other embodiments, oil 26 can be separated from
organic phase stream 36 or extractant stream 27 prior to contacting oil 26
with
-37-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
substance(s) 42 for producing fatty acid extractant 28. Fatty acid extractant
28
can then be used as an ISPR extractant fed to fermentation vessel 30, a
different
fermentation vessel (e.g., operating in parallel or in series with
fermentation
vessel 30 in an alcohol manufacturing plant), or stored for later use.
[00122] Thus, FIGs. 1-5 provide various non-limiting embodiments of methods
and
systems involving fermentation processes and fatty acid extractants 28
produced
from biomass-derived oil 26, and fatty acid extractants 28' produced from
native
oil such as plant-derived oil 26' that can be used to remove product alcohol
in
extractive fermentation. These fatty acid extractants 28 and 28' may be
selected
from the group consisting of fatty acids, fatty alcohols, fatty amides, fatty
acid
methyl esters, fatty acid glycol esters, triglycerides, and mixtures thereof.
The
triglycerides may be hydroxylated or alkoxylated (e.g., methoxylated,
ethoxylated). Chemical conversion of glycerides from native oil to the fatty
acid
extractants described herein can be conducted using any reaction scheme known
in the art. With reference to plant-derived oil such as corn oil, for example,
in
some embodiments, hydroxylated triglycerides as a fatty acid extractant 28 or
28'
can be produced by contacting corn oil as oil 26 or 26' with various reactants
and
solvents 42 (see Example 1 below for details) to achieve hydroxylation shown
in
Equation I:
(I)
o
00
0
OH
O OH
O O
OH
O
OH
-38-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
[00123] In some embodiments, corn oil triglycerides as oil 26 or 26' can be
reacted with aqueous ammonium hydroxide as reactant 42 to obtain fatty amide
and fatty acid, which together or separately can be used as fatty acid
extractants
28 or 28', as described in Roe, et al., Am. Oil Chem. Soc. 29:18-22, 1952, and
shown in Equation II, for example:
(II)
Aq. NH4OH
Corn oil triglycerides fatty amide + fatty acid + glycerol
160 C
[00124] In some embodiments, aqueous ammonium hydroxide is about 28 wt%
ammonia in water. In some embodiments, the mixture of corn oil fatty amides
and corn oil fatty acids produced according to Equation (II) can be used to
produce a single fatty acid extractant 28 or 28'. In some embodiments, the
mixture of fatty amides and fatty acids can include linoleamide, linoleic
acid,
oleamide, oleic acid, palmitamide, palmitic acid, stearamide, and stearic
acid. In
such embodiments, such mixture can be composed of about 37 wt% linoleamide,
about 18% linoleic acid, about 19 wt% oleamide, about 9 wt% oleic acid, about
8.7 wt% palmitamide, about 4.3 wt% palmitic acid, about 1.2 wt% stearamide,
and about 0.7 wt% stearic acid. It should be understood that other composition
amounts are possible and can depend on the naturally-occurring amounts of
linoleic acid, oleic acid, palmitic acid, and stearic acid in the corn oil
used. For
example, it would be expected that high-oleic corn oil as oil 26 or 26', which
can
have, for example, up to about 65 wt% oleic acid content, would produce a
mixture that is higher in oleamide and oleic acid pursuant to the reaction of
Equation (II) than would be produced when using normal corn oil which is about
24 wt% oleic acid content. In some embodiments, corn oil fatty amides and corn
oil fatty acids produced according to Equation (II) can be mixed with fatty
acids to
vary the ratio of fatty amide to fatty acid in the fatty acid extractant 28 or
28'. In
some embodiments, a mixture of fatty amide to fatty acid can be in a ratio of
about 2:1 to about 1:2 mixture.
-39-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
[00125] In some embodiments, pure corn oil fatty amides as fatty acid
extractant
28 or 28' can be synthesized from corn oil as oil 26 or 26' using as
substances 42
anhydrous ammonia as reactant with ammonium acetate as a catalyst, as
described in Kohlhase, et al., J. Am. Oil Chem. Soc. 48:265-270, 1971, for
example. In some embodiments, the pure corn oil fatty amides can include
linoleamide, oleamide, palmitamide, and stearamide. In such embodiments, the
pure corn oil fatty amides can be composed of about 55 wt% linoleamide, about
28 wt% oleamide, about 13 wt% palmitamide, and about 2 wt% stearamide. As
noted above, it should be understood that other composition amounts are
possible and can depend on the naturally-occurring amounts of linoleic acid,
oleic
acid, palmitic acid, and stearic acid in the corn oil used.
[00126] In some embodiment, fatty acid extractant 28 or 28' can include fatty
amide of the formula R(C=O)N(R')(R"), wherein R is independently selected from
the group consisting of C3 to C27 alkyl groups optionally interrupted with one
or
more double bonds, and R' and R" are independently selected from the group
consisting of hydrogen and Cl-C6 alkyl groups optionally containing one or
more
hydroxyl groups.
[00127] In some embodiments, corn oil fatty acids as a fatty acid extractant
28 or
28' can be synthesized from corn oil as oil 26 or 26' by base hydrolysis using
NaOH and water as substances 42 (see, e.g., Example 4 below), according to
the reaction of Equation III, for example:
(III)
O
pO___'~R1
O
0___U_1R2 1)NaOLH 3 R-C(=O)OH
0 2) H+
O--ILIR3
[00128] In some embodiments, pure corn oil fatty amides and pure corn oil
fatty
acids can be mixed to produce a single fatty acid extractant 28 or 28'. Such
mixture of fatty amide to fatty acid can be a 2:1 mixture, and a 1:2 in other
embodiments, for example.
-40-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
[00129] In some embodiments, fatty alcohols as fatty acid extractant 28 or 28'
can
be produced from corn oil as oil 26 or 26' by reduction using tetrahydrofuran
(THF) and LiAIH4 as substances 42 (see, e.g., Example 3 below), according to
the reaction of Equation IV, for example:
-41 -
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
(IV)
R1COCH2
I LiAIH4, THE
R2COICH 0-25 C No- 3 RCH2OH + HOCH2CHOHCH2OH
R3CO2C H 2
[00130] In some embodiments, corn oil as oil 26 or 26' can be contacted with
methanol and an acid catalyst as substances 42 to produce fatty acid esters as
fatty acid extractant 28 or 28'. For example, corn oil can be reacted with an
alcohol including, but not limited to, an alcohol of eight carbons or less, in
the
presence of sulfuric acid to yield fatty acid esters (see, e.g., Example 4
below),
according to the reaction of Equation V:
(V)
uO
O/\R1
O
0 R2 + R'OH R-C(=O)-OR'
O
O--I~-R3
[00131] In some embodiments, corn oil as oil 26 or 26' can be converted to
corn oil
ethylene glycol ester (FAGE) as fatty acid extractant 28 or 28' by producing
fatty
acid methyl esters (FAME) (see Equation V, above) and further reacting FAME
with ethylene glycol as an additional substance 42 (see, e.g., Example 5
below),
according to the reaction of Equation VI, for example:
(VI)
R-(C=O)-OMe + HO-CH2CH2-OH -- R-(C=O)-O-CH2CH2-OH
[00132] In some embodiments, fatty alcohols may be hydroxylated and may used
as extractants. For example, fatty alcohols may be reacted with peracetic acid
and then with an aqueous acid to hydroxylate the double bonds along the chain
(see, e.g., Example 8) as shown in Equation VII:
-42-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
HO
HO111 OH
HO
[00133] In some embodiments, the extractant may be a liquid or solid such as
beads. An extractant that would be available in a solid form such as beads
could
be easily handled during the manufacturing process. In addition, the product
alcohol (e.g., butanol) could be recovered from this extractant, for example,
by
gas stripping, dissolving in another solvent, or any other applicable method
known to one skilled in the art.
[00134] In some embodiments, including any of the aforementioned embodiments
described with reference to FIGs. 1-5, the fermentation broth in fermentation
vessel 30 includes at least one recombinant microorganism 32 which is
genetically modified (that is, genetically engineered) to produce butanol via
a
biosynthetic pathway from at least one fermentable carbon source into butanol.
In particular, recombinant microorganisms can be grown in a fermentation broth
which contains suitable carbon substrates. Additional carbon substrates may
include, but are not limited to, monosaccharides such as fructose;
oligosaccharides such as lactose maltose, or sucrose; polysaccharides such as
starch or cellulose; or mixtures thereof and unpurified mixtures from
renewable
feedstocks such as cheese whey permeate, cornsteep liquor, sugar beet
molasses, and barley malt. Other carbon substrates may include ethanol,
lactate, succinate, or glycerol.
[00135] Additionally the carbon substrate may also be one-carbon substrates
such
as carbon dioxide or methanol for which metabolic conversion into key
biochemical intermediates has been demonstrated. In addition to one and two
carbon substrates, methylotrophic organisms are also known to utilize a number
-43-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
of other carbon containing compounds such as methylamine, glucosamine, and a
variety of amino acids for metabolic activity. For example, methylotrophic
yeasts
are known to utilize the carbon from methylamine to form trehalose or glycerol
(Bellion, et al., Microb. Growth C1 Compd., [Int. Symp.], 7th (1993), 415-32,
Editor(s): Murrell, J. Collin; Kelly, Don P. Publisher: Intercept, Andover,
UK).
Similarly, various species of Candida will metabolize alanine or oleic acid
(Sulter,
et al., Arch. Microbiol. 153:485-489, 1990). Hence it is contemplated that the
source of carbon utilized in the present invention may encompass a wide
variety
of carbon containing substrates and will only be limited by the choice of
organism.
[00136] Although it is contemplated that all of the above mentioned carbon
substrates and mixtures thereof are suitable, in some embodiments, the carbon
substrates are glucose, fructose, and sucrose, or mixtures of these with C5
sugars such as xylose and/or arabinose for yeasts cells modified to use C5
sugars. Sucrose may be derived from renewable sugar sources such as sugar
cane, sugar beets, cassava, sweet sorghum, and mixtures thereof. Glucose and
dextrose may be derived from renewable grain sources through saccharification
of starch based feedstocks including grains such as corn, wheat, rye, barley,
oats, and mixtures thereof. In addition, fermentable sugars may be derived
from
renewable cellulosic or lignocellulosic biomass through processes of
pretreatment and saccharification, as described, for example, in U.S. Patent
Application Publication No. 2007/0031918 Al, which is herein incorporated by
reference. In addition to an appropriate carbon source (from aqueous stream
22), fermentation broth must contain suitable minerals, salts, cofactors,
buffers,
and other components, known to those skilled in the art, suitable for the
growth of
the cultures and promotion of an enzymatic pathway comprising a dihydroxyacid
dehydratase (DHAD).
[00137] Recombinant microorganisms to produce butanol via a biosynthetic
pathway can include a member of the genera Clostridium, Zymomonas,
Escherichia, Salmonella, Serratia, Erwinia, Klebsiella, Shigella, Rhodococcus,
Pseudomonas, Bacillus, Lactobacillus, Enterococcus, Alcaligenes, Klebsiella,
Paenibacillus, Arthrobacter, Corynebacterium, Brevibacterium,
-44-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
Schizosaccharomyces, Kluyveromyces, Yarrowia, Pichia, Candida, Hansenula,
or Saccharomyces. In one embodiment, recombinant microorganisms can be
selected from the group consisting of Escherichia coli, Lactobacillus
plantarum,
and Saccharomyces cerevisiae. In one embodiment, the recombinant
microorganism is a crabtree-positive yeast selected from Saccharomyces,
Zygosaccharomyces, Schizosaccharomyces, Dekkera, Torulopsis,
Brettanomyces, and some species of Candida. Species of crabtree-positive
yeast include, but are not limited to, Saccharomyces cerevisiae, Saccharomyces
kluyveri, Schizosaccharomyces pombe, Saccharomyces bayanus,
Saccharomyces mikitae, Saccharomyces paradoxus, Zygosaccharomyces rouxii,
and Candida glabrata. For example, the production of butanol utilizing
fermentation with a microorganism, as well as which microorganisms produce
butanol, is known and is disclosed, for example, in U.S. Patent Application
Publication No. 2009/0305370, herein incorporated by reference. In some
embodiments, microorganisms comprise a butanol biosynthetic pathway.
Suitable isobutanol biosynthetic pathways are known in the art (see, e.g.,
U.S.
Patent Application Publication No. 2007/0092957, herein incorporated by
reference). In some embodiments, at least one, at least two, at least three,
or at
least four polypeptides catalyzing substrate to product conversions of a
pathway
are encoded by heterologous polynucleotides in the microorganism. In some
embodiments, all polypeptides catalyzing substrate to product conversions of a
pathway are encoded by heterologous polynucleotides in the microorganism. In
some embodiments, the microorganism comprises a reduction or elimination of
pyruvate decarboxylase activity. Microorganisms substantially free of pyruvate
decarboxylase activity are described in U.S. Patent Application Publication
No.
2009/0305363, herein incorporated by reference.
[00138] Construction of certain strains, including those used in the Examples,
is
provided herein.
Construction of Saccharomyces cerevisiae strain BP1064 and
isobutanologen BP1083 (NGCI-070)
[00139] The strain BP1064 was derived from CEN.PK 113-7D (CBS 8340;
Centraalbureau voor Schimmelcultures (CBS) Fungal Biodiversity Centre,
-45-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
Netherlands) and contains deletions of the following genes: URA3, HIS3, PDC1,
PDC5, PDC6, and GPD2. BP1064 was transformed with plasmids pYZ090 (SEQ
ID NO: 1, the construction of which is described in US Patent Application No.
61/246,844, filed September 29, 2009, herein incorporated by reference.) and
pLH468 (SEQ ID NO: 2) to create isobutanologen strain NGCI-070 (BP1 083).
[00140] Deletions, which completely removed the entire coding sequence, were
created by homologous recombination with PCR fragments containing regions of
homology upstream and downstream of the target gene and either a G418
resistance marker or URA3 gene for selection of transformants. The G418
resistance marker, flanked by loxP sites, was removed using Cre recombinase.
The URA3 gene was removed by homologous recombination to create a scarless
deletion or if flanked by loxP sites, was removed using Cre recombinase.
[00141] The scarless deletion procedure was adapted from Akada, et al., (Yeast
23:399-405, 2006). In general, the PCR cassette for each scarless deletion was
made by combining four fragments, A-B-U-C, by overlapping PCR. The PCR
cassette contained a selectable/counter-selectable marker, URA3 (Fragment U),
consisting of the native CEN.PK 113-7D URA3 gene, along with the promoter
(250 bp upstream of the URA3 gene) and terminator (150 bp downstream of the
URA3 gene). Fragments A and C, each 500 bp long, corresponded to the 500 bp
immediately upstream of the target gene (Fragment A) and the 3' 500 bp of the
target gene (Fragment C). Fragments A and C were used for integration of the
cassette into the chromosome by homologous recombination. Fragment B (500
bp long) corresponded to the 500 bp immediately downstream of the target gene
and was used for excision of the URA3 marker and Fragment C from the
chromosome by homologous recombination, as a direct repeat of the sequence
corresponding to Fragment B was created upon integration of the cassette into
the chromosome. Using the PCR product ABUC cassette, the URA3 marker was
first integrated into and then excised from the chromosome by homologous
recombination. The initial integration deleted the gene, excluding the 3' 500
bp.
Upon excision, the 3' 500 bp region of the gene was also deleted. For
integration
of genes using this method, the gene to be integrated was included in the PCR
cassette between fragments A and B.
-46-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
URA3 Deletion
[00142] To delete the endogenous URA3 coding region, a ura3::loxP-kanMX-loxP
cassette was PCR-amplified from pLA54 template DNA (SEQ ID NO: 3). pLA54
contains the K. lactis TEF1 promoter and kanMX marker, and is flanked by loxP
sites to allow recombination with Cre recombinase and removal of the marker.
PCR was done using Phusion DNA polymerase (New England BioLabs Inc.,
Ipswich, MA) and primers BK505 and BK506 (SEQ ID NOs: 4 and 5). The URA3
portion of each primer was derived from the 5' region upstream of the URA3
promoter and 3' region downstream of the coding region such that integration
of
the loxP-kanMX-loxP marker resulted in replacement of the URA3 coding region.
The PCR product was transformed into CEN.PK 113-7D using standard genetic
techniques (Methods in Yeast Genetics, 2005, Cold Spring Harbor Laboratory
Press, Cold Spring Harbor, NY, pp. 201-202) and transformants were selected on
YPD containing G418 (100 pg/mL) at 30 C. Transformants were screened to
verify correct integration by PCR using primers LA468 and LA492 (SEQ ID NOs:
6 and 7) and designated CEN.PK 113-7D Aura3::kanMX.
HIS3 Deletion
[00143] The four fragments for the PCR cassette for the scarless HIS3 deletion
were amplified using Phusion High Fidelity PCR Master Mix (New England
BioLabs Inc., Ipswich, MA) and CEN.PK 113-7D genomic DNA as template,
prepared with a Gentra Puregene Yeast/Bact, kit (Qiagen, Valencia, CA).
HIS3 Fragment A was amplified with primer oBP452 (SEQ ID NO: 14) and primer
oBP453 (SEQ ID NO: 15) containing a 5' tail with homology to the 5' end of
HIS3
Fragment B. HIS3 Fragment B was amplified with primer oBP454 (SEQ ID NO:
16) containing a 5' tail with homology to the 3' end of HIS3 Fragment A, and
primer oBP455 (SEQ ID NO: 17) containing a 5' tail with homology to the 5' end
of HIS3 Fragment U. HIS3 Fragment U was amplified with primer oBP456 (SEQ
ID NO: 18) containing a 5' tail with homology to the 3' end of HIS3 Fragment
B,
and primer oBP457 (SEQ ID NO: 19) containing a 5' tail with homology to the 5'
end of HIS3 Fragment C. HIS3 Fragment C was amplified with primer oBP458
(SEQ ID NO: 20) containing a 5' tail with homology to the 3' end of HIS3
-47-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
Fragment U, and primer oBP459 (SEQ ID NO: 21). PCR products were purified
with a PCR Purification kit (Qiagen, Valencia, CA). HIS3 Fragment AB was
created by overlapping PCR by mixing HIS3 Fragment A and HIS3 Fragment B
and amplifying with primers oBP452 (SEQ ID NO: 14) and oBP455 (SEQ ID NO:
17). HIS3 Fragment UC was created by overlapping PCR by mixing HIS3
Fragment U and HIS3 Fragment C and amplifying with primers oBP456 (SEQ ID
NO: 18) and oBP459 (SEQ ID NO: 21). The resulting PCR products were
purified on an agarose gel followed by a Gel Extraction kit (Qiagen, Valencia,
CA). The HIS3 ABUC cassette was created by overlapping PCR by mixing HIS3
Fragment AB and HIS3 Fragment UC and amplifying with primers oBP452 (SEQ
ID NO: 14) and oBP459 (SEQ ID NO: 21). The PCR product was purified with a
PCR Purification kit (Qiagen, Valencia, CA).
[00144] Competent cells of CEN.PK 113-7D Aura3::kanMX were made and
transformed with the HIS3 ABUC PCR cassette using a Frozen-EZ Yeast
Transformation IITM kit (Zymo Research Corporation, Irvine, CA).
Transformation
mixtures were plated on synthetic complete media lacking uracil supplemented
with 2% glucose at 30 C. Transformants with a his3 knockout were screened for
by PCR with primers oBP460 (SEQ ID NO: 22) and oBP461 (SEQ ID NO: 23)
using genomic DNA prepared with a Gentra Puregene Yeast/Bact. kit
(Qiagen, Valencia, CA). A correct transformant was selected as strain CEN.PK
113-7D Aura3::kanMX Ahis3::URA3.
KanMX Marker Removal from the Aura3 Site and URA3 Marker Removal from
the Ahis3 Site
[00145] The KanMX marker was removed by transforming CEN.PK 113-7D
Aura3::kanMX Ahis3::URA3 with pRS423::PGAL1-cre (SEQ ID NO: 66,
described in U.S. Provisional Application No. 61/290,639) using a Frozen-EZ
Yeast Transformation IITM kit (Zymo Research Corporation, Irvine, CA) and
plating on synthetic complete medium lacking histidine and uracil supplemented
with 2% glucose at 30 C. Transformants were grown in YP supplemented with
1% galactose at 30 C for -6 hours to induce the Cre recombinase and KanMX
marker excision and plated onto YPD (2% glucose) plates at 30 C for recovery.
An isolate was grown overnight in YPD and plated on synthetic complete medium
-48-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
containing 5-fluoro-orotic acid (5-FOA, 0.1%) at 30 C to select for isolates
that
lost the URA3 marker. 5-FOA resistant isolates were grown in and plated on
YPD for removal of the pRS423::PGAL1-cre plasmid. Isolates were checked for
loss of the KanMX marker, URA3 marker, and pRS423::PGAL1-cre plasmid by
assaying growth on YPD+G418 plates, synthetic complete medium lacking uracil
plates, and synthetic complete medium lacking histidine plates. A correct
isolate
that was sensitive to G418 and auxotrophic for uracil and histidine was
selected
as strain CEN.PK 113-7D Aura3::loxP Ahis3 and designated as BP857. The
deletions and marker removal were confirmed by PCR and sequencing with
primers oBP450 (SEQ ID NO: 24) and oBP451 (SEQ ID NO: 25) for Aura3 and
primers oBP460 (SEQ ID NO: 22) and oBP461 (SEQ ID NO: 23) for Ahis3 using
genomic DNA prepared with a Gentra Puregene Yeast/Bact. kit (Qiagen,
Valencia, CA).
PDC6 Deletion
[00146] The four fragments for the PCR cassette for the scarless PDC6 deletion
were amplified using Phusion High Fidelity PCR Master Mix (New England
BioLabs Inc., Ipswich, MA) and CEN.PK 113-7D genomic DNA as template,
prepared with a Gentra Puregene Yeast/Bact. kit (Qiagen, Valencia, CA).
PDC6 Fragment A was amplified with primer oBP440 (SEQ ID NO: 26) and
primer oBP441 (SEQ ID NO: 27) containing a 5' tail with homology to the 5' end
of PDC6 Fragment B. PDC6 Fragment B was amplified with primer oBP442 (SEQ
ID NO: 28), containing a 5' tail with homology to the 3' end of PDC6 Fragment
A,
and primer oBP443 (SEQ ID NO: 29) containing a 5' tail with homology to the 5'
end of PDC6 Fragment U. PDC6 Fragment U was amplified with primer oBP444
(SEQ ID NO: 30) containing a 5' tail with homology to the 3' end of PDC6
Fragment B, and primer oBP445 (SEQ ID NO: 31) containing a 5' tail with
homology to the 5' end of PDC6 Fragment C. PDC6 Fragment C was amplified
with primer oBP446 (SEQ ID NO: 32) containing a 5' tail with homology to the
3'
end of PDC6 Fragment U, and primer oBP447 (SEQ ID NO: 33). PCR products
were purified with a PCR Purification kit (Qiagen, Valencia, CA). PDC6
Fragment
-49-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
AB was created by overlapping PCR by mixing PDC6 Fragment A and PDC6
Fragment B and amplifying with primers oBP440 (SEQ ID NO: 26) and oBP443
(SEQ ID NO: 29). PDC6 Fragment UC was created by overlapping PCR by
mixing PDC6 Fragment U and PDC6 Fragment C and amplifying with primers
oBP444 (SEQ ID NO: 30) and oBP447 (SEQ ID NO: 33). The resulting PCR
products were purified on an agarose gel followed by a Gel Extraction kit
(Qiagen, Valencia, CA). The PDC6 ABUC cassette was created by overlapping
PCR by mixing PDC6 Fragment AB and PDC6 Fragment UC and amplifying with
primers oBP440 (SEQ ID NO: 26) and oBP447 (SEQ ID NO: 33). The PCR
product was purified with a PCR Purification kit (Qiagen, Valencia, CA).
[00147] Competent cells of CEN.PK 113-7D Aura3::IoxP Ahis3 were made and
transformed with the PDC6 ABUC PCR cassette using a Frozen-EZ Yeast
Transformation IITM kit (Zymo Research Corporation, Irvine, CA).
Transformation
mixtures were plated on synthetic complete media lacking uracil supplemented
with 2% glucose at 30 C. Transformants with a pdc6 knockout were screened for
by PCR with primers oBP448 (SEQ ID NO: 34) and oBP449 (SEQ ID NO: 35)
using genomic DNA prepared with a Gentra Puregene Yeast/Bact. kit
(Qiagen, Valencia, CA). A correct transformant was selected as strain CEN.PK
113-7D Aura3::IoxP Ahis3 Apdc6::URA3.
[00148] CEN.PK 113-7D Aura3::IoxP Ahis3 Apdc6::URA3 was grown overnight in
YPD and plated on synthetic complete medium containing 5-fluoro-orotic acid
(0.1 %) at 30 C to select for isolates that lost the URA3 marker. The deletion
and
marker removal were confirmed by PCR and sequencing with primers oBP448
(SEQ ID NO: 34) and oBP449 (SEQ ID NO: 35) using genomic DNA prepared
with a Gentra Puregene Yeast/Bact. kit (Qiagen, Valencia, CA). The absence
of the PDC6 gene from the isolate was demonstrated by a negative PCR result
using primers specific for the coding sequence of PDC6, oBP554 (SEQ ID NO:
36) and oBP555 (SEQ ID NO: 37). The correct isolate was selected as strain
CEN.PK 113-7D Aura3::IoxP Ahis3 Apdc6 and designated as BP891.
PDC1 Deletion ilvDSm Integration
-50-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
[00149] The PDC1 gene was deleted and replaced with the ilvD coding region
from
Streptococcus mutans ATCC No. 700610. The A fragment followed by the ilvD
coding region from Streptococcus mutans for the PCR cassette for the PDC1
deletion-ilvDSm integration was amplified using Phusion High Fidelity PCR
Master Mix (New England BioLabs Inc., Ipswich, MA) and NYLA83 (described
herein and in U.S. Provisional Application No. 61/246,709) genomic DNA as
template, prepared with a Gentra Puregene Yeast/Bact. kit (Qiagen, Valencia,
CA). PDC1 Fragment A-ilvDSm (SEQ ID NO: 138) was amplified with primer
oBP513 (SEQ ID NO: 38) and primer oBP515 (SEQ ID NO: 39) containing a 5'
tail with homology to the 5' end of PDC1 Fragment B. The B, U, and C fragments
for the PCR cassette for the PDC1 deletion-ilvDSm integration were amplified
using Phusion High Fidelity PCR Master Mix (New England BioLabs Inc.,
Ipswich, MA) and CEN.PK 113-7D genomic DNA as template, prepared with a
Gentra Puregene Yeast/Bact. kit (Qiagen, Valencia, CA). PDC1 Fragment B
was amplified with primer oBP516 (SEQ ID NO: 40) containing a 5' tail with
homology to the 3' end of PDC1 Fragment A-ilvDSm, and primer oBP517 (SEQ
ID NO: 41) containing a 5' tail with homology to the 5' end of PDC1 Fragment
U.
PDC1 Fragment U was amplified with primer oBP518 (SEQ ID NO: 42)
containing a 5' tail with homology to the 3' end of PDC1 Fragment B, and
primer
oBP519 (SEQ ID NO: 43) containing a 5' tail with homology to the 5' end of
PDC1 Fragment C. PDC1 Fragment C was amplified with primer oBP520 (SEQ
ID NO: 44), containing a 5' tail with homology to the 3' end of PDC1 Fragment
U,
and primer oBP521 (SEQ ID NO: 45). PCR products were purified with a PCR
Purification kit (Qiagen, Valencia, CA. PDC1 Fragment A-ilvDSm-B was created
by overlapping PCR by mixing PDC1 Fragment A-ilvDSm and PDC1 Fragment B
and amplifying with primers oBP513 (SEQ ID NO: 38) and oBP517 (SEQ ID NO:
41). PDC1 Fragment UC was created by overlapping PCR by mixing PDC1
Fragment U and PDC1 Fragment C and amplifying with primers oBP518 (SEQ ID
NO: 42) and oBP521 (SEQ ID NO: 45). The resulting PCR products were
purified on an agarose gel followed by a Gel Extraction kit (Qiagen, Valencia,
CA). The PDC1 A-ilvDSm-BUC cassette (SEQ ID NO: 139) was created by
overlapping PCR by mixing PDC1 Fragment A-ilvDSm-B and PDC1 Fragment
-51-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
UC and amplifying with primers oBP513 (SEQ ID NO: 38) and oBP521 (SEQ ID
NO: 45). The PCR product was purified with a PCR Purification kit (Qiagen,
Valencia, CA).
[00150] Competent cells of CEN.PK 113-7D Aura3::IoxP Ahis3 Apdc6 were made
and transformed with the PDC1 A-ilvDSm-BUC PCR cassette using a Frozen-EZ
Yeast Transformation IITM kit (Zymo Research Corporation, Irvine, CA).
Transformation mixtures were plated on synthetic complete media lacking uracil
supplemented with 2% glucose at 30 C. Transformants with a pdcl knockout
ilvDSm integration were screened for by PCR with primers oBP511 (SEQ ID NO:
46) and oBP512 (SEQ ID NO: 47) using genomic DNA prepared with a Gentra
Puregene Yeast/Bact. kit (Qiagen, Valencia, CA). The absence of the PDC1
gene from the isolate was demonstrated by a negative PCR result using primers
specific for the coding sequence of PDC1, oBP550 (SEQ ID NO: 48) and oBP551
(SEQ ID NO: 49). A correct transformant was selected as strain CEN.PK 113-7D
Aura3::IoxP Ahis3 Apdc6 Apdcl ::ilvDSm-URA3.
[00151] CEN.PK 113-7D Aura3::IoxP Ahis3 Apdc6 Apdcl::ilvDSm-URA3 was
grown overnight in YPD and plated on synthetic complete medium containing 5-
fluoro-orotic acid (0.1 %) at 30 C to select for isolates that lost the URA3
marker.
The deletion of PDC1, integration of ilvDSm, and marker removal were confirmed
by PCR and sequencing with primers oBP511 (SEQ ID NO: 46) and oBP512
(SEQ ID NO: 47) using genomic DNA prepared with a Gentra Puregene
Yeast/Bact. kit (Qiagen, Valencia, CA). The correct isolate was selected as
strain
CEN.PK 113-7D Aura3::IoxP Ahis3 Apdc6 Apdcl::ilvDSm and designated as
BP907.
PDC5 Deletion sadB Integration
[00152] The PDC5 gene was deleted and replaced with the sadB coding region
from Achromobacter xylosoxidans. A segment of the PCR cassette for the PDC5
deletion-sadB integration was first cloned into plasmid pUC19-URA3MCS.
[00153] pUC19-URA3MCS is pUC19 based and contains the sequence of the
URA3 gene from Saccharomyces cerevisiae situated within a multiple cloning
site
(MCS). pUC19 contains the pMB1 replicon and a gene coding for beta-
-52-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
lactamase for replication and selection in Escherichia coli. In addition to
the
coding sequence for URA3, the sequences from upstream and downstream of
this gene were included for expression of the URA3 gene in yeast. The vector
can be used for cloning purposes and can be used as a yeast integration
vector.
[00154] The DNA encompassing the URA3 coding region along with 250 bp
upstream and 150 bp downstream of the URA3 coding region from
Saccharomyces cerevisiae CEN.PK 113-7D genomic DNA was amplified with
primers oBP438 (SEQ ID NO: 12) containing BamHI, Ascl, Pmel, and Fsel
restriction sites, and oBP439 (SEQ ID NO: 13) containing Xbal, Pacl, and Notl
restriction sites, using Phusion High Fidelity PCR Master Mix (New England
BioLabs Inc., Ipswich, MA). Genomic DNA was prepared using a Gentra
Puregene Yeast/Bact. kit (Qiagen, Valencia, CA). The PCR product and
pUC19 (SEQ ID NO: 140) were ligated with T4 DNA ligase after digestion with
BamHI and Xbal to create vector pUC19-URA3MCS. The vector was confirmed
by PCR and sequencing with primers oBP264 (SEQ ID NO: 10) and oBP265
(SEQ ID NO: 11).
[00155] The coding sequence of sadB and PDC5 Fragment B were cloned into
pUC19-URA3MCS to create the sadB-BU portion of the PDC5 A-sadB-BUC PCR
cassette. The coding sequence of sadB was amplified using pLH468-sadB (SEQ
ID NO: 67) as template with primer oBP530 (SEQ ID NO: 50) containing an Ascl
restriction site, and primer oBP531 (SEQ ID NO: 51) containing a 5' tail with
homology to the 5' end of PDC5 Fragment B. PDC5 Fragment B was amplified
with primer oBP532 (SEQ ID NO: 52) containing a 5' tail with homology to the
3'
end of sadB, and primer oBP533 (SEQ ID NO: 53) containing a Pmel restriction
site. PCR products were purified with a PCR Purification kit (Qiagen,
Valencia,
CA). sadB-PDC5 Fragment B was created by overlapping PCR by mixing the
sadB and PDC5 Fragment B PCR products and amplifying with primers oBP530
(SEQ ID NO: 50) and oBP533 (SEQ ID NO: 53). The resulting PCR product was
digested with Ascl and Pmel and ligated with T4 DNA ligase into the
corresponding sites of pUC19-URA3MCS after digestion with the appropriate
enzymes. The resulting plasmid was used as a template for amplification of
sadB-Fragment B-Fragment U using primers oBP536 (SEQ ID NO: 54) and
-53-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
oBP546 (SEQ ID NO: 55) containing a 5' tail with homology to the 5' end of
PDC5 Fragment C. PDC5 Fragment C was amplified with primer oBP547 (SEQ
ID NO: 56) containing a 5' tail with homology to the 3' end of PDC5 sadB-
Fragment B-Fragment U, and primer oBP539 (SEQ ID NO: 57). PCR products
were purified with a PCR Purification kit (Qiagen, Valencia, CA). PDC5 sadB-
Fragment B-Fragment U-Fragment C was created by overlapping PCR by mixing
PDC5 sadB-Fragment B-Fragment U and PDC5 Fragment C and amplifying with
primers oBP536 (SEQ ID NO: 54) and oBP539 (SEQ ID NO: 57). The resulting
PCR product was purified on an agarose gel followed by a Gel Extraction kit
(Qiagen, Valencia, CA). The PDC5 A-sadB-BUC cassette (SEQ ID NO: 141)
was created by amplifying PDC5 sadB-Fragment B-Fragment U-Fragment C with
primers oBP542 (SEQ ID NO: 58) containing a 5' tail with homology to the 50
nucleotides immediately upstream of the native PDC5 coding sequence, and
oBP539 (SEQ ID NO: 57). The PCR product was purified with a PCR Purification
kit (Qiagen, Valencia, CA).
[00156] Competent cells of CEN.PK 113-7D Aura3::IoxP Ahis3 Apdc6
Apdcl::ilvDSm were made and transformed with the PDC5 A-sadB-BUC PCR
cassette using a Frozen-EZ Yeast Transformation IITM kit (Zymo Research
Corporation, Irvine, CA). Transformation mixtures were plated on synthetic
complete media lacking uracil supplemented with 1% ethanol (no glucose) at
30 C. Transformants with a pdc5 knockout sadB integration were screened for
by PCR with primers oBP540 (SEQ ID NO: 59) and oBP541 (SEQ ID NO: 60)
using genomic DNA prepared with a Gentra Puregene Yeast/Bact. kit
(Qiagen, Valencia, CA). The absence of the PDC5 gene from the isolate was
demonstrated by a negative PCR result using primers specific for the coding
sequence of PDC5, oBP552 (SEQ ID NO: 61) and oBP553 (SEQ ID NO: 62). A
correct transformant was selected as strain CEN.PK 113-7D Aura3::IoxP Ahis3
Apdc6 Apdcl ::ilvDSm Apdc5::sadB-URA3.
[00157] CEN.PK 113-7D Aura3::IoxP Ahis3 Apdc6 Apdcl::ilvDSm Apdc5::sadB-
URA3 was grown overnight in YPE (1% ethanol) and plated on synthetic
complete medium supplemented with ethanol (no glucose) and containing 5-
fluoro-orotic acid (0.1 %) at 30 C to select for isolates that lost the URA3
marker.
-54-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
The deletion of PDC5, integration of sadB, and marker removal were confirmed
by PCR with primers oBP540 (SEQ ID NO: 59) and oBP541 (SEQ ID NO: 60)
using genomic DNA prepared with a Gentra Puregene Yeast/Bact. kit
(Qiagen, Valencia, CA). The correct isolate was selected as strain CEN.PK 113-
7D Aura3::IoxP Ahis3 Apdc6 Apdcl::ilvDSm Apdc5::sadB and designated as
BP913.
GPD2 Deletion
[00158] To delete the endogenous GPD2 coding region, a gpd2::loxP-URA3-loxP
cassette (SEQ ID NO: 142) was PCR-amplified using loxP-URA3-loxP (SEQ ID
NO: 68) as template DNA. loxP-URA3-loxP contains the URA3 marker from
(ATCC No. 77107) flanked by IoxP recombinase sites. PCR was done using
Phusion DNA polymerase (New England BioLabs Inc., Ipswich, MA) and
primers LA512 and LA513 (SEQ ID NOs: 8 and 9). The GPD2 portion of each
primer was derived from the 5' region upstream of the GPD2 coding region and
3'
region downstream of the coding region such that integration of the IoxP-URA3-
IoxP marker resulted in replacement of the GPD2 coding region. The PCR
product was transformed into BP913 and transformants were selected on
synthetic complete media lacking uracil supplemented with 1% ethanol (no
glucose). Transformants were screened to verify correct integration by PCR
using primers oBP582 and AA270 (SEQ ID NOs: 63 and 64).
[00159] The URA3 marker was recycled by transformation with pRS423::PGAL1-
cre (SEQ ID NO: 66) and plating on synthetic complete media lacking histidine
supplemented with 1 % ethanol at 30 C. Transformants were streaked on
synthetic complete medium supplemented with 1% ethanol and containing 5-
fluoro-orotic acid (0.1 %) and incubated at 30 C to select for isolates that
lost the
URA3 marker. 5-FOA resistant isolates were grown in YPE (1% ethanol) for
removal of the pRS423::PGAL1-cre plasmid. The deletion and marker removal
were confirmed by PCR with primers oBP582 (SEQ ID NO: 63) and oBP591
(SEQ ID NO: 65). The correct isolate was selected as strain CEN.PK 113-7D
Aura3::IoxP Ahis3 Apdc6 Apdcl::ilvDSm Apdc5::sadB Agpd2::IoxP and
designated as PNY1503 (BP1064).
- 55 -
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
[00160] BP1064 was transformed with plasmids pYZ090 (SEQ ID NO: 1) and
pLH468 (SEQ ID NO: 2) to create strain NGCI-070 (BP1083; PNY1504).
Construction of Strains NYLA74 and NYLA83
[00161] Insertion-inactivation of endogenous PDC1 and PDC6 genes of S.
cerevisiae. PDC1, PDC5, and PDC6 genes encode the three major isozymes of
pyruvate decarboxylase is described as follows:
Construction of pRS425::GPM-sadB
[00162] A DNA fragment encoding a butanol dehydrogenase (SEQ ID NO: 70)
from Achromobacter xylosoxidans (disclosed in U.S. Patent Application
Publication No. 2009/0269823) was cloned. The coding region of this gene
called sadB for secondary alcohol dehydrogenase (SEQ ID NO: 69) was
amplified using standard conditions from A. xylosoxidans genomic DNA,
prepared using a Gentra Puregene kit (Qiagen, Valencia, CA) following the
recommended protocol for gram negative organisms using forward and reverse
primers N473 and N469 (SEQ ID NOs: 74 and 75), respectively. The PCR
product was TOPO -Blunt cloned into pCR 4 BLUNT (InvitrogenTM, Carlsbad,
CA) to produce pCR4Blunt::sadB, which was transformed into E. coli Mach-1
cells. Plasmid was subsequently isolated from four clones, and the sequence
verified.
[00163] The sadB coding region was PCR amplified from pCR4Blunt::sadB. PCR
primers contained additional 5' sequences that would overlap with the yeast
GPM1 promoter and the ADH1 terminator (N583 and N584, provided as SEQ ID
NOs: 76 and 77). The PCR product was then cloned using "gap repair"
methodology in Saccharomyces cerevisiae (Ma, et al., Gene 58:201-216, 1987)
as follows. The yeast-E. coli shuttle vector pRS425::GPM::kivD::ADH which
contains the GPM1 promoter (SEQ ID NO: 72), kivD coding region from
Lactococcus lactis (SEQ ID NO: 71), and ADH1 terminator (SEQ ID NO: 73)
(described in U.S. Patent Application Publication No. 2007/0092957 Al, Example
17) was digested with BbvCI and Pacl restriction enzymes to release the kivD
coding region. Approximately 1 g of the remaining vector fragment was
-56-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
transformed into S. cerevisiae strain BY4741 along with 1 g of sadB PCR
product. Transformants were selected on synthetic complete medium lacking
leucine. The proper recombination event, generating pRS425::GPM-sadB (SEQ
ID NO: 124), was confirmed by PCR using primers N142 and N459 (SEQ ID
NOs: 108 and 109).
Construction of pdc6:: PGPMI-sadB integration cassette and PDC6 deletion
[00164] A pdc6::PGPM1-sadB-ADH1t-URA3r integration cassette was made by
joining the GPM-sadB-ADHt segment (SEQ ID NO: 79) from pRS425::GPM-sadB
(SEQ ID NO: 78) to the URA3r gene from pUC19-URA3r. pUC19-URA3r (SEQ
ID NO:80) contains the URA3 marker from pRS426 (ATCC No. 77107) flanked by
75 bp homologous repeat sequences to allow homologous recombination in vivo
and removal of the URA3 marker. The two DNA segments were joined by SOE
PCR (as described by Horton, et al., Gene 77:61-68, 1989) using as template
pRS425::GPM-sadB and pUC19-URA3r plasmid DNAs, with Phusion DNA
polymerase (New England BioLabs Inc., Ipswich, MA) and primers 114117-11A
through 114117-11D (SEQ ID NOs: 81, 82, 83, and 84), and 114117-13A and
114117-13B (SEQ ID NOs: 85 and 86).
[00165] The outer primers for the SOE PCR (114117-13A and 114117-13B)
contained 5' and 3' -50 bp regions homologous to regions upstream and
downstream of the PDC6 promoter and terminator, respectively. The completed
cassette PCR fragment was transformed into BY4700 (ATCC No. 200866) and
transformants were maintained on synthetic complete media lacking uracil and
supplemented with 2% glucose at 30 C using standard genetic techniques
(Methods in Yeast Genetics, 2005, Cold Spring Harbor Laboratory Press, Cold
Spring Harbor, NY, pp. 201-202). Transformants were screened by PCR using
primers 112590-34G and 112590-34H (SEQ ID NOs: 87 and 88), and 112590-
34F and 11 2590-49E (SEQ ID NOs: 89 and 90) to verify integration at the PDC6
locus with deletion of the PDC6 coding region. The URA3r marker was recycled
by plating on synthetic complete media supplemented with 2% glucose and 5-
FOA at 30 C following standard protocols. Marker removal was confirmed by
patching colonies from the 5-FOA plates onto SD-URA media to verify the
-57-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
absence of growth. The resulting identified strain has the genotype: BY4700
pdc6::PGPM1-sadB-ADH1 t.
Construction of pdc1:: PPDC1-ilvD integration cassette and PDC1 deletion
[00166] A pdc1:: PPDC1-ilvD-FBA1t-URA3r integration cassette was made by
joining the ilvD-FBA1t segment (SEQ ID NO: 91) from pLH468 (SEQ ID NO: 2) to
the URA3r gene from pUC19-URA3r by SOE PCR (as described by Horton, et
al., Gene 77:61-68, 1989) using as template pLH468 and pUC19-URA3r plasmid
DNAs, with Phusion DNA polymerase (New England BioLabs Inc., Ipswich, MA)
and primers 114117-27A through 114117-27D (SEQ ID NOs: 110, 111, 112, and
113).
[00167] The outer primers for the SOE PCR (114117-27A and 114117-27D)
contained 5' and 3' -50 bp regions homologous to regions downstream of the
PDC1 promoter and downstream of the PDC1 coding sequence. The completed
cassette PCR fragment was transformed into BY4700 pdc6::PGPM1-sadB-
ADH1t and transformants were maintained on synthetic complete media lacking
uracil and supplemented with 2% glucose at 30 C using standard genetic
techniques (Methods in Yeast Genetics, 2005, Cold Spring Harbor Laboratory
Press, Cold Spring Harbor, NY, pp. 201-202). Transformants were screened by
PCR using primers 114117-36D and 135 (SEQ ID NOs: 92 and 93), and primers
112590-49E and 112590-30F (SEQ ID NOs: 90 and 94) to verify integration at
the PDC1 locus with deletion of the PDC1 coding sequence. The URA3r marker
was recycled by plating on synthetic complete media supplemented with 2%
glucose and 5-FOA at 30 C following standard protocols. Marker removal was
confirmed by patching colonies from the 5-FOA plates onto SD-URA media to
verify the absence of growth. The resulting identified strain "NYLA67" has the
genotype: BY4700 pdc6:: PGPM1-sadB-ADH1t pdc1:: PPDC1-iIvD-FBA1t.
HIS3 deletion
[00168] To delete the endogenous HIS3 coding region, a his3::URA3r2 cassette
was PCR-amplified from URA3r2 template DNA (SEQ ID NO: 95). URA3r2
contains the URA3 marker from pRS426 (ATCC No. 77107) flanked by 500 bp
-58-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
homologous repeat sequences to allow homologous recombination in vivo and
removal of the URA3 marker. PCR was done using Phusion DNA polymerase
(New England BioLabs Inc., Ipswich, MA) and primers 114117-45A and 114117-
45B (SEQ ID NOs: 96 and 97) which generated a -2.3 kb PCR product. The
HIS3 portion of each primer was derived from the 5' region upstream of the
HIS3
promoter and 3' region downstream of the coding region such that integration
of
the URA3r2 marker results in replacement of the HIS3 coding region. The PCR
product was transformed into NYLA67 using standard genetic techniques
(Methods in Yeast Genetics, 2005, Cold Spring Harbor Laboratory Press, Cold
Spring Harbor, NY, pp. 201-202) and transformants were selected on synthetic
complete media lacking uracil and supplemented with 2% glucose at 30 C.
Transformants were screened to verify correct integration by replica plating
of
transformants onto synthetic complete media lacking histidine and supplemented
with 2% glucose at 30 C. The URA3r marker was recycled by plating on
synthetic complete media supplemented with 2% glucose and 5-FOA at 30 C
following standard protocols. Marker removal was confirmed by patching
colonies from the 5-FOA plates onto SD-URA media to verify the absence of
growth. The resulting identified strain, called NYLA73, has the genotype:
BY4700 pdc6:: PGPM1-sadB-ADH1 t pdcl:: PPDC1-iIvD-FBA1 t Ahis3.
Construction of pdc5::kanMX integration cassette and PDC5 deletion
[00169] A pdc5::kanMX4 cassette was PCR-amplified from strain YLR134W
chromosomal DNA (ATCC No. 4034091) using Phusion DNA polymerase (New
England BioLabs Inc., Ipswich, MA) and primers PDC5::KanMXF and
PDC5::KanMXR (SEQ ID NOs: 98 and 99) which generated a -2.2 kb PCR
product. The PDC5 portion of each primer was derived from the 5' region
upstream of the PDC5 promoter and 3' region downstream of the coding region
such that integration of the kanMX4 marker results in replacement of the PDC5
coding region. The PCR product was transformed into NYLA73 using standard
genetic techniques (Methods in Yeast Genetics, 2005, Cold Spring Harbor
Laboratory Press, Cold Spring Harbor, NY, pp. 201-202) and transformants were
selected on YP media supplemented with 1% ethanol and geneticin (200 g/mL)
-59-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
at 30 C. Transformants were screened by PCR to verify correct integration at
the
PDC locus with replacement of the PDC5 coding region using primers PDC5kofor
and N175 (SEQ ID NOs: 100 and 101). The identified correct transformants have
the genotype: BY4700 pdc6:: PGPM1-sadB-ADH1t pdcl::PPDC1-ilvD-FBA1t
Ahis3 pdc5::kanMX4. The strain was named NYLA74.
Deletion of HXK2 (hexokinase II)
[00170] A hxk2::URA3r cassette was PCR-amplified from URA3r2 template
(described above) using Phusion DNA polymerase (New England BioLabs Inc.,
Ipswich, MA) and primers 384 and 385 (SEQ ID NOs: 102 and 103) which
generated a -2.3 kb PCR product. The HXK2 portion of each primer was derived
from the 5' region upstream of the HXK2 promoter and 3' region downstream of
the coding region such that integration of the URA3r2 marker results in
replacement of the HXK2 coding region. The PCR product was transformed into
NYLA73 using standard genetic techniques (Methods in Yeast Genetics, 2005,
Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 201-202) and
transformants were selected on synthetic complete media lacking uracil and
supplemented with 2% glucose at 30 C. Transformants were screened by PCR
to verify correct integration at the HXK2 locus with replacement of the HXK2
coding region using primers N869 and N871 (SEQ ID NOs: 104 and 105). The
URA3r2 marker was recycled by plating on synthetic complete media
supplemented with 2% glucose and 5-FOA at 30 C following standard protocols.
Marker removal was confirmed by patching colonies from the 5-FOA plates onto
SD-URA media to verify the absence of growth, and by PCR to verify correct
marker removal using primers N946 and N947 (SEQ ID NOs: 106 and 107). The
resulting identified strain named NYLA83 has the genotype: BY4700
pdc6::PGPM1 -sadB-ADH1 t pdcl:: PPDC1-iIvD-FBA1 t Ahis3 Ahxk2.
Construction of NYLA93
[00171] Described below is insertion-inactivation of endogenous PDC1, PDC5,
and
PDC6 genes of S. cerevisiae. PDC1, PDC5, and PDC6 genes encode the three
major isozymes of pyruvate decarboxylase. The resulting PDC inactivation
strain
-60-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
was used as a host for expression vectors pYZ067 (SEQ ID NO: 129) and
pYZ090 (SEQ ID NO: 1), the construction of which is described in U.S.
Provisional Patent Application No. 61/246,844, filed September 29, 2009,
herein
incorporated by reference.
Deletion of NAD-dependent glycerol 3-phosphate dehydrogenase
[00172] A gpd2::loxP-URA3-loxP cassette was PCR-amplified from pUC19::loxP-
URA3-IoxP plasmid template using Phusion DNA polymerase (New England
BioLabs Inc., Ipswich, MA) and primers LA512 and LA513 (SEQ ID NOs: 8 and
9) which generated a -1.6 kb PCR product. pUC19::loxP-URA3-IoxP (SEQ ID
NO: 130) contains the URA3 marker from (ATCC No. 77107) flanked by loxP
recombinase sites. The GPD2 portion of each primer was derived from the 5'
region upstream of the GPD2 promoter and 3' region downstream of the coding
region such that integration of the loxP-URA3-loxP marker results in
replacement
of the GPD2 coding region. The PCR product was transformed into NYLA83
using standard genetic techniques (Methods in Yeast Genetics, 2005, Cold
Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 201-202) and
transformants were selected on synthetic complete media lacking uracil and
supplemented with 2% glucose at 30 C. Transformants were screened by PCR
to verify correct integration at the GPD2 locus with replacement of the HXK2
coding region using primers LA516 and N175 (SEQ ID NO: 132 and 101). The
URA3 marker is recycled by transformation with pRS423::PGAL1-cre (SEQ ID NO:
131) and plating on synthetic complete media lacking histidine supplemented
with
2% glucose at 30 C. Colonies are patched onto YP (1% galactose) plates at
30 C to induce URA3 marker excision and are transferred onto YPD plates at
30 C for recovery. Removal of the URA3 marker is confirmed by patching
colonies from the YPD plates onto synthetic complete media lacking uracil to
verify the absence of growth. The identified correct clones have the genotype:
BY4700 pdc6:: PGPM1-sadB-ADH1t pdc1:: PP0C1-i1vD-FBA1t Ahis3 Ahxk2
dgpd2::loxP. The strain was named NYLA92.
Construction of pdc5::loxP-kanMX-IoxP integration cassette and PDC5 deletion
-61-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
[00173] A pdc5::loxP-kanMX-IoxP cassette was PCR-amplified from plasmid
pUC19::loxP-kanMX-IoxP (SEQ ID NO: 135) using Phusion DNA polymerase
(New England BioLabs Inc., Ipswich, MA) and primers LA249 and LA397 (SEQ
ID NOs: 136 and 137) which generated a -2.2 kb PCR product. pUC19::loxP-
kanMX-IoxP (SEQ ID NO: 135) contains the kanMX gene from pFA6 (Wach, et
al., Yeast 10:1793-1808, 1994) and K. lactis TEF1 promoter and terminator
flanked by loxP recombinase sites. The PDC5 portion of each primer was
derived from the 5' region upstream of the PDC5 promoter and 3' region
downstream of the coding region such that integration of the loxP-kanMX-loxP
marker results in replacement of the PDC5 coding region. The PCR product was
transformed into NYLA92 using standard genetic techniques (Methods in Yeast
Genetics, 2005, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY,
pp. 201-202) and transformants were selected on YP media supplemented with
1 % ethanol and geneticin (200 g/ml) at 30 C. Transformants were screened by
PCR to verify correct integration at the PDC5 locus with replacement of the
PDC5
coding region using primers LA363 and LA364 (SEQ ID NOs: 133 and 134). The
identified correct transformants have the genotype: BY4700 pdc6:: PGPMI-sadB-
ADH1t pdc1::PPpc1-i1vD-FBA1t Ahis3 Ahxk2 Agpd2::IoxP Apdc5:loxP-kanMX-
loxP. The strain was named NYLA93.
NYLA93 (pYZ067/pYZ090)
[00174] Plasmid vectors pYZ067 and pYZ090 were simultaneously transformed
into strain NYLA93 (BY4700 pdc6:: PGPM1-sad8 ADH1t pdcl:: PPpcj-i1vD-FBA1t
Ahis3 Ahxk2 Agpd2::IoxP Apdc5:loxP-kanMX-loxP) using standard genetic
techniques (Methods in Yeast Genetics, 2005, Cold Spring Harbor Laboratory
Press, Cold Spring Harbor, NY) and the resulting strain (isobutanologen
NYLA93,
also referred to as NGCI-065) was maintained on synthetic complete media
lacking histidine and uracil, and supplemented with 1 % ethanol at 30 C.
Expression Vector pLH468
-62-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
[00175] The pLH468 plasmid (SEQ ID NO: 2) was constructed for expression of
DHAD, ketoisovalerate decarboxylase (KivD) and horse liver alcohol
dehydrogenase (HADH) in yeast.
[00176] Coding regions for Lactococcus lactis ketoisovalerate decarboxylase
(KivD) and horse liver alcohol dehydrogenase (HADH) were synthesized by
DNA2.0 based on codons that were optimized for expression in Saccharomyces
cerevisiae (SEQ ID NO: 71 and 117, respectively) and provided in plasmids
pKivDy-DNA2.0 and pHadhy-DNA2Ø The encoded proteins are SEQ ID NOs:
116 and 118, respectively. Individual expression vectors for KivD and HADH
were constructed. To assemble pLH467 (pRS426::PTDH3-kivDy-TDH3t), vector
pNY8 (SEQ ID NO: 120; also named pRS426.GPD-ald-GPDt, described in U.S.
Patent Application Publication No. 2008/0182308, Example 17, which is herein
incorporated by reference) was digested with Ascl and Sfil enzymes, thus
excising the GPD promoter and the ald coding region. A TDH3 promoter
fragment (SEQ ID NO: 121) from pNY8 was PCR amplified to add an Ascl site at
the 5' end, and an Spel site at the 3' end, using 5' primer OT1068 and 3'
primer
OT1067 (SEQ ID NOs: 122 and 123). The Ascl/Sfil digested pNY8 vector
fragment was ligated with the TDH3 promoter PCR product digested with Ascl
and Spel, and the Spel-Sfil fragment containing the codon optimized kivD
coding
region isolated from the vector pKivD-DNA2Ø The triple ligation generated
vector pLH467 (pRS426::PTDH3-kivDy-TDH3t). pLH467 was verified by restriction
mapping and sequencing.
[00177] pLH435 (pRS425::PGPMI-Hadhy-ADH1 t) was derived from vector
pRS425::GPM-sadB (SEQ ID NO: 78) which is described in U.S. Provisional
Patent Application No. 61/058970, Example 3, which is herein incorporated by
reference. pRS425::GPM-sadB is the pRS425 vector (ATCC No. 77106) with a
chimeric gene containing the GPM1 promoter (SEQ ID NO: 72), coding region
from a butanol dehydrogenase of Achromobacter xylosoxidans (sad B; DNA SEQ
ID NO: 69; protein SEQ ID NO: 70: disclosed in U.S. Patent Application
Publication No. 2009/0269823), and ADH1 terminator (SEQ ID NO: 73).
pRS425::GPMp-sadB contains Bbvl and Pacl sites at the 5' and 3' ends of the
sadB coding region, respectively. A Nhel site was added at the 5' end of the
-63-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
sadB coding region by site-directed mutagenesis using primers OT1074 and
OT1075 (SEQ ID NO: 125 and 126) to generate vector pRS425-GPMp-sadB-
Nhel, which was verified by sequencing. pRS425::PGPM1-sadB-Nhel was
digested with Nhel and Pacl to drop out the sadB coding region, and ligated
with
the Nhel-Pact fragment containing the codon optimized HADH coding region from
vector pHadhy-DNA2.0 to create pLH435.
[00178] To combine KivD and HADH expression cassettes in a single vector,
yeast
vector pRS411 (ATCC No. 87474) was digested with Sacl and Nod, and ligated
with the Sacl-Sall fragment from pLH467 that contains the PTDH3-kivDy-TDH3t
cassette together with the Sall-Notl fragment from pLH435 that contains the
PGPMI-Hadhy-ADH1t cassette in a triple ligation reaction. This yielded the
vector
pRS411::PTDH3-kivDy-PGPMI-Hadhy (pLH441), which was verified by restriction
mapping.
[00179] In order to generate a co-expression vector for all three genes in the
lower
isobutanol pathway: ilvD, kivDy and Hadhy, pRS423 FBA ilvD(Strep) (SEQ ID
NO: 127) was used, which is described in U.S. Provisional Patent Application
No.
61/100,792, as the source of the IIvD gene. This shuttle vector contains an F1
origin of replication (nt 1423 to 1879) for maintenance in E. coli and a 2
micron
origin (nt 8082 to 9426) for replication in yeast. The vector has an FBA1
promoter (nt 2111 to 3108; SEQ ID NO: 119) and FBA terminator (nt 4861 to
5860; SEQ ID NO: 128). In addition, it carries the His marker (nt 504 to 1163)
for
selection in yeast and ampicillin resistance marker (nt 7092 to 7949) for
selection
in E. coli. The ilvD coding region (nt 3116 to 4828; SEQ ID NO: 1154 protein
SEQ ID NO: 115) from Streptococcus mutans UA159 (ATCC No. 700610) is
between the FBA promoter and FBA terminator forming a chimeric gene for
expression. In addition there is a lumio tag fused to the ilvD coding region
(nt
4829-4849).
[00180] The first step was to linearize pRS423 FBA ilvD(Strep) (also called
pRS423-FBA(Spel)-I lvD(Streptococcus mutans)-Lumio) with Sacl and Sacll (with
Sacll site blunt ended using T4 DNA polymerase), to give a vector with total
length of 9,482 bp. The second step was to isolate the kivDy-hADHy cassette
from pLH441 with Sacl and Kpnl (with Kpnl site blunt ended using T4 DNA
-64-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
polymerase) which gives a 6,063 bp fragment. This fragment was ligated with
the 9,482 bp vector fragment from pRS423-FBA(Spel)-IIvD(Streptococcus
mutans)-Lumio. This generated vector pLH468 (pRS423::PFBAI-
ilvD(Strep)Lumio-FBAIt-PTOH3-kivDy-TDH3t-PGPMI-hadhy-ADH1t), which was
confirmed by restriction mapping and sequencing.
pYZ090 and pYZ067
[00181] pYZ090 was constructed to contain a chimeric gene having the coding
region of the alsS gene from Bacillus subtilis (nt position 457-2172)
expressed
from the yeast CUP1 promoter (nt 2-449) and followed by the CYC1 terminator
(nt 2181-2430) for expression of acetolactate synthase (ALS), and a chimeric
gene having the coding region of the ilvC gene from Lactococcus lactis (nt
3634-
4656) expressed from the yeast ILV5 promoter (2433-3626) and followed by the
ILV5 terminator (nt 4670-5292) for expression of keto-acid reductoisomerase
(KART).
[00182] pYZ067 was constructed to contain the following chimeric genes: 1) the
coding region of the ilvD gene from S. mutans UA159 (nt position 2260-3971)
expressed from the yeast FBA1 promoter (nt 1161-2250) followed by the FBA
terminator (nt 4005-4317) for expression of DHAD, 2) the coding region for
HADH
(nt 4680-5807) expressed from the yeast GPM promoter (nt 5819-6575) followed
by the ADH1 terminator (nt 4356-4671) for expression of alcohol dehydrogenase,
and 3) the coding region of the KivD gene from Lacrococcus lactis (nt 7175-
8821)
expressed from the yeast TDH3 promoter (nt 8830-9493) followed by the TDH3
terminator (nt 5682-7161) for expression of ketoisovalerate decarboxylase.
[00183] Further, while various embodiments of the present invention have been
described above, it should be understood that they have been presented by way
of example only, and not limitation. It will be apparent to persons skilled in
the
relevant art that various changes in form and detail can be made therein
without
departing from the spirit and scope of the invention. Thus, the breadth and
scope
of the present invention should not be limited by any of the above-described
exemplary embodiments, but should be defined only in accordance with the
claims and their equivalents.
-65-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
[00184] All publications, patents, and patent applications mentioned in this
specification are indicative of the level of skill of those skilled in the art
to which
this invention pertains, and are herein incorporated by reference to the same
extent as if each individual publication, patent, or patent application was
specifically and individually indicated to be incorporated by reference.
Examples
[00185] The following nonlimiting examples will further illustrate the
invention, in
which partition coefficients of fatty acid extractants for butanol are
demonstrated.
It should be understood that, while the above-mentioned chemical conversions
and the following examples involve corn oil as the plant-derived oil for
producing
fatty acid extractants, other native oils such as plant-derived oils may be
used
without departing from the present invention. From the above discussion and
these Examples, one skilled in the art can ascertain essential characteristics
of
the present invention and can make various changes and modifications of the
invention to adapt to various uses and conditions without departing from the
present invention.
[00186] As used herein, the meaning of abbreviations used was as follows: "g"
means gram(s), "kg" means kilogram(s), "L" means liter(s), "mL" means
milliliter(s), "mL/L" means milliliter(s) per liter, "mL/min" means
milliliter(s) per min,
"pL" means microliter(s), "DI" means deionized, "uM" means micrometer(s), "nM"
means nanometer(s), "w/v" means weight/volume, "GC" means gas
chromatograph, "OD" means optical density, "OD600" means optical density at a
wavelength of 600 nM, "dcw" means dry cell weight, "rpm" means revolutions per
minute, " C" means degree(s) Celsius, " C/min" means degrees Celsius per
minute, "slpm" means standard liter(s) per minute, "ppm" means part per
million,
"pdc" means pyruvate decarboxylase enzyme followed by the enzyme number.
[00187] Examples 1-6 describe exemplary methods for chemically converting corn
oil into the following fatty acid extractants: hydroxylated triglycerides
(Example 1),
fatty amides and mixtures with fatty acids (Example 2), fatty alcohols
(Example
3), fatty acids (Example 4), fatty acid methyl esters (Example 5); and fatty
acid
glycol esters (Example 6). Example 7 provides a series of comparative examples
-66-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
of extractive fermentation experiments that were conducted using the water-
immiscible extractants listed in Tables 3 and 7-10, for which the performance
data are summarized in Table 11.
Example 1
Hydroxylated Triglycerides from Corn Oil
A. Corn oil hydroxylation (63% hydroxylation)
[00188] To a three-neck 500mL flask equipped with a mechanical stirrer and
addition funnel was added corn oil (50.0 g), toluene (25.0 mL), Amberlyte IR-
120
resin (12.5 g), and glacial acetic acid (7.5 g). The resulting mixture was
heated to
60 C, and then hydrogen peroxide (41.8 g of 30% H202 in water) was added
dropwise over one hour. The mixture was stirred at 60 C for two hours, upon
which time the reaction mixture was worked up: resin was removed by
filtration,
and the filtrate partitioned between ethyl acetate (75 mL) and water (50 mL).
After the layers were separated, the organic layer was washed with sat. aq.
NaHCO3 solution (50 mL), and brine (50 mL). The organic layer was dried over
anh. Na2SO4 and concentrated in vacuo to obtain 48.9g of yellow oil. The 1H
NMR analysis of the crude reaction product showed that 63% of double bonds
were epoxidized.
[00189] To a 500 mL round bottom flask was added epoxidized corn oil (20.0 g),
tetrahydrofuran (THF) (100.0 mL), and sulfuric acid (50 mL of 1.7 M aqueous
solution). The cloudy mixture was stirred for two hours at 50 C, and then
worked
up by partitioning between water (100 mL) and ethyl acetate (200 mL). The
organic layer was washed with water (3x50 mL) and then brine (50 mL). The
organic layer was dried over anh. Na2SO4 and concentrated in vacuo to obtain
19.9 g of dark yellow oil (63% hydroxylation corn oil).
B. Corn oil hydroxylation (47% hydroxylation)
[00190] To a three-neck 500 mL flask, equipped with a mechanical stirrer and
addition funnel was added corn oil (50.0 g), toluene (25.0 mL), Amberlyte IR-
120
resin (12.5 g), and glacial acetic acid (7.5 g). The resulting mixture was
heated to
60 C, and then hydrogen peroxide (41.8 g of 30% H202 in water) was added
-67-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
dropwise over one hour. The mixture was stirred at 6000 for one hour, upon
which time the reaction mixture was worked up: the resin was removed by
filtration, and the filtrate partitioned between ethyl acetate (75 mL) and
water (50
mL). After the layers were separated, the organic layer was washed with sat.
aq.
NaHCO3 solution (50 mL), and brine (50 mL). The organic layer was dried over
anh. Na2SO4 and concentrated in vacuo to obtain 49.8g of yellow oil. The 1H
NMR analysis of the crude reaction product showed that 47% of double bonds
were epoxidized.
[00191] To a 500 mL round bottom flask was added epoxidized corn oil (20.0 g),
THE (100.0 mL), and sulfuric acid (50 mL of 1.7M aqueous solution). The cloudy
mixture was stirred for two hours at 50 C, and then worked up by partitioning
between water (100 mL) and ethyl acetate (200 mL). The organic layer was
washed with water (3x50 mL) and then brine (50 mL). The organic layer was
dried over anh. Na2SO4 and concentrated in vacuo to obtain 19.2 g of dark
yellow
oil (47% hydroxylation corn oil).
C. Corn oil hydroxylation (28% hydroxylation)
[00192] To a three-neck 500 mL flask, equipped with a mechanical stirrer and
addition funnel was added corn oil (50.0 g), toluene (25.0 mL), Amberlyte IR-
120
resin (12.5 g), and glacial acetic acid (7.5g). The resulting mixture was
heated to
60 C, and then hydrogen peroxide (41.8 g of 30% H202 in water) was added
dropwise over one hour. The mixture was stirred at 60 C for two hours, upon
which time the reaction mixture was worked up: the resin was removed by
filtration, and the filtrate partitioned between ethyl acetate (75 mL) and
water (50
mL). After the layers were separated, the organic layer was washed with sat.
aq.
NaHCO3 solution (50 mL), and brine (50 mL). The organic layer was dried over
anh. Na2SO4 and concentrated in vacuo to obtain 47.2 g of yellow oil. The 1H
NMR analysis of the crude reaction product showed that 28% of double bonds
were epoxidized.
[00193] To a 500 mL round bottom flask was added epoxidized corn oil (20.0 g),
THE (100.0 mL), and sulfuric acid (50 mL of 1.7M aqueous solution). The cloudy
mixture was stirred for two hours at 50 C, and then worked up by partitioning
-68-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
between water (100 mL) and ethyl acetate (200 mL). The organic layer was
washed with water (3x50 mL) and then brine (50 mL). The organic layer was
dried over anh. Na2SO4 and concentrated in vacuo to obtain 20.3 g of dark
yellow
oil (28% hydroxylation corn oil).
Partition coefficient measurement
[00194] To a 5 mL vial was added 0.910 g of the 67% hydroxylated corn oil, and
0.910 mL of 3wt% iBuOH water solution. The biphasic mixture was vigorously
stirred using Vortex Genie for 10 minutes. Upon mixing, the separation of
layers was aided by centrifuging the mixture using Fisher Scientific Centrific
228
centrifuge (3300 rpm) for 10 minutes. 0.100 g of both layers were taken. The
organic, upper layer was diluted to 1.00 mL with toluene solution of ethylene
glycol diethylether (10.1 mg/mL), and the water layer was diluted to 1.00 mL
with
methanol solution of ethylene glycol diethylether (10.2 mg/mL). The
concentrations of i-BuOH in both phases were measured using a calibrated gas
chromatograph (GC). The same procedure was repeated for 47% and 28%
hydroxylated corn oil. The partition coefficient thus measured was 3.2 for the
67% hydroxylated corn oil, 2.3 for the 47% hydroxylated corn oil, and 2.1 for
the
28% hydroxylated corn oil.
[00195] The above outlined procedure was repeated with 6% i-BuOH water
solution. The partition coefficients for 67% -, 47% -, and 28% -hydroxylated
corn
oils were 2.9, 2.9, and 2.0, respectively.
Example 2
Fatty Amides Plus Fatty Acids, and Pure Fatty Amides from Corn Oil
[00196] Corn oil was reacted with aqueous ammonium hydroxide in a manner
similar to that described by Roe, et al., J. Am. Oil Chem. Soc. 29:18-22,
1952.
Mazola corn oil (0.818 L, 755 g) was placed in a 1 gallon stainless steel
reactor
to which was added 1.71 L (1540 g) of aqueous ammonium hydroxide (28% as
NH3). The reactor was heated with stirring to 160 C and was maintained at that
temperature with stirring for 7 h during which time the pressure reached 400
psi.
The reactor was cooled and the product, a creamy white solid, was removed and
-69-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
the reactor rinsed with ethyl acetate. The product was dissolved in 5 L ethyl
acetate and washed 5 times with 500 mL each of water which was neutralized
with H2SO4. The ethyl acetate was then dried over anhydrous Na2SO4 and the
solvent removed on a rotary evaporator leaving a light brown soft solid.
[00197] 13C NMR in CDC13 indicated that the product contained an approximate
2:1
ratio of fatty amide to fatty acid and that the conversion of the corn oil to
product
was quantitative. The product had a melting point of 57-58 C, but dropped
about
11 C when saturated with water.
[00198] Pure corn oil fatty amide was synthesized from corn oil according to
Kohlhase, et al., J. Am. Oil Chem. Soc. 48:265-270, 1971 using anhydrous
ammonia with ammonium acetate as a catalyst.
[00199] Three grams of ammonium acetate were placed in a 400 mL stainless
steel shaker tube to which was added 51.8 g of corn oil. Anhydrous ammonia
(89.7 g) was then added and the reactor sealed and heated for 7 h at 125 C
during which time the pressure reached 1300 psi. The reactor was cooled, the
light colored solid removed and the reactor rinsed with ethyl acetate. The
product
dissolved in ethyl acetate was then worked up as in the case of the fatty
amide/fatty acid mixture above.
[00200] Fatty acids were synthesized from corn oil by base hydrolysis using
NaOH
as described below in Example 4.
[00201] Three preparations: (1) the 2:1 mixture of corn oil fatty amide and
corn oil
fatty acid from aqueous ammonia, (2) a 2:1 mixture of pure corn oil fatty
amide:pure corn oil fatty acid, and (3) a 1:2 mixture of pure corn oil fatty
amide:corn oil fatty acid, were all tested for their ability to extract
isobutanol from
a 3% solution in water. Seven hundred milligrams of each was added to 2.1 mL
of water containing 3% isobutanol in a 20 mL scintillation vial and placed on
a
rotary shaker overnight at 30 C. In all three cases, the organic phase became
liquid at this temperature, indicating a further lowering of the melting point
with
the uptake of isobutanol. Fifty microliters of the upper phase were diluted
with
either 200 pL of toluene containing ethylene glycol diethylether (10.068
mg/mL)
as a GC standard or 200 pL of isopropanol containing the same concentration of
ethylene glycol diethylether. Fifty microliters of the lower phase was diluted
with
-70-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
150 pL of methanol and 50 pL of isopropanol containing the same concentration
of ethylene glycol diethylether. The concentrations of isobutanol in both
phases
were determined using a calibrated GC. The partition coefficients measured
were as follows: 3.81 for (1), 4.31 for (2), and 3.58 for (3).
[00202] Fatty amide/fatty acid aqueous ammonia preparation (1), and a
preparation (1a) constituted by preparation (1) mixed 1:1 with pure corn oil
fatty
acid (equivalent to 1:2 fatty amide:fatty acid) were incubated in shake flasks
with
fermentation broth containing the Saccharomyces butanologen NGCI-070 at a
ratio of 3 parts broth to 1 part amide/acid mixture. Preparation (1) was a
soft
solid, while preparation (1a) was a liquid at 30 C. Starting at a glucose
concentration of 8.35 g/L, the shake flasks were then incubated for 25 h on an
incubator shaker and the consumption of glucose followed as a function of
time.
Table 1 indicates that the fatty amide/fatty acid mixtures at both ratios were
not
toxic to the butanologen and even showed higher rates of glucose uptake than
with oleyl alcohol.
-71-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
Table 1
Flask Glucose conc. g/L)
Time = 0 18 hrs 25 hrs
Oleyl Alcohol 8.35 4.26 0
Oleyl Alcohol 8.35 4.46 0
2:1 Synthesized Fatty
Amide:Fatty Acid Mix
(Preparation (1)) 8.35 3.06 0
2:1 Synthesized Fatty
Amide:Fatty Acid Mix
(Preparation (1)) 8.35 3.22 0
1:1 Synthesized Fatty Amide
Fatty Acid Mix:Pure Fatty
Acids (Preparation (1a)) 8.35 2.73 0
1:1 Synthesized Fatty Amide
Fatty Acid Mix:Pure Fatty
Acids
(Preparation (1a)) 8.35 2.73 0
Example 3
Fatty Alcohols from Corn Oil
[00203] With reference to the reaction of Equation IV above for producing
fatty
alcohols from corn oil, a 22L, round-bottom flask equipped with a mechanical
stirrer, reflux condenser with N2 source, addition funnel, internal
thermocouple,
and rubber septum was flame-dried under nitrogen. The flask was charged with
132 g (3.30 moles) of 95% lithium aluminum hydride powder that is weighed out
in a dry box and loaded into a solids addition funnel. The 22L flask was
cooled
with an ice bath, and 9.0 liters of anhydrous THE were added into the reactor
via
a cannula. The resulting slurry was cooled to 0-5 C and a solution of 956 g
(1.10
moles) of Wesson corn oil in 1.00 liter of anhydrous THE was added dropwise
over 2-3 hours while holding the reaction temperature at 5-20 C. After adding
the
corn oil, the slurry was stirred overnight at ambient temperature. When the
reaction was done, as verified by TLC chromatography, it was quenched by the
dropwise addition of a solution of 130 g of water dissolved in 370 mL of THF.
Then 130 g of 15% aqueous NaOH solution was added followed by the addition
of 400 g of water. The mixture was vigorously stirred while warming to room
temperature and produced a white granular solid. The solids were filtered off
-72-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
using a fritted-glass filter funnel and washed with additional THF. The THE
was
removed on a rotary evaporator and the residue was taken up in 3.00 liters of
ethyl acetate. The product solution was washed with 2x 1.00 L of water, 1 x
1.00
L of brine, dried over Na2SO4, filtered, and concentrated in vacuo to give 836
g
(97%) of fatty alcohols as yellow oil. The crude fatty alcohol mixture was
then
distilled (140 C/l mmHg), and used in the following partition coefficients
experiments.
Partition coefficient experiments
[00204] To each of the five 5-mL vials were added 1 mL of fatty alcohol
mixture,
and 1 mL of 3 wt% iBuOH water solution. The biphasic mixture was vigorously
stirred using Vortex Genie for 10, 20, 30, 40, and 60 minutes, respectively.
Upon mixing, the separation of layers was aided by centrifuging the mixture
using
Fisher Scientific Centrific 228 centrifuge (3300 rpm) for 10 minutes. 0.100 mL
of
both layers were taken. The organic, upper layer was diluted to 1.00 mL with
toluene solution of ethylene glycol diethylether, and the water layer was
diluted to
1.00 mL with methanol solution of ethylene glycol diethylether. The
concentrations of i-BuOH in both phases were measured using a calibrated GC.
The partition coefficient thus measured was 2.70.
[00205] The same partition coefficient measurement, as described above was run
for 6 wt% i-BuOH concentration. The partition coefficient thus measured was
3.06.
[00206] In the following Examples 4-6, the methods used for determination of
the
partition coefficients of the extractants was the quiescent method. For the
quiescent method, 5 mL of either a 3% or 6% (w/v) solution of isobutanol in
water
was put into a vial and 5 mL of the solvent of interest was carefully added
onto
the top of the water solution so as not to mix the physical phases. After the
indicated period of time, a sample of the clear portion of the solvent phase
and of
the aqueous phase were removed and analyzed for isobutanol content by GC.
Any emulsion layer was ignored for this analysis. For the GC analysis, 100 uL
of
the sample was added to 400 uL of isopropanol. 500 uL of a solution of
diethylene glycol diethyl ether (internal standard) was added and the solution
was
-73-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
shot on a Carbowax column with an FID detector and the concentration of
isobutanol was determined.
[00207] Other methods known in the art can also be used for determining the
partition coefficients of extractants according to the present invention. For
example, the shaking method can be used. As an example for the shaking
method, 5 mL of either a 3% of 6% (w/v) solution of isobutanol in water can be
added to a centrifuge tube along with 5 mL of the solvent of interest. The
tube
can be shaken vigorously for 1 minute. The tube can be then spun in a
centrifuge at approx. 12500G for 15 minutes. Samples of the clear solvent
layer
and the clear aqueous layer can be removed and analyzed for isobutanol content
by the method described above.
Example 4
Corn Oil Fatty Acids
[00208] Round bottom flask (5L) was equipped with a mechanical stirrer,
thermocouple, heating mantle, condenser, and nitrogen tee. Charged with 500 g
of food grade corn oil, 1 L of water and 75 g of sodium hydroxide. Mixture was
heated to 90 C and held for three hours, during which time it became a single
thick, emulsion-like single phase. At the end of this time, TLC shows no
remaining corn oil in the mixture. The mixture was then cooled to 72 C and 500
mL of 25% sulfuric acid was added to acidify the mixture. It was then cooled
to
room temperature and 2 L of diethyl ether was added. The ether layer was
washed 3x1 L with 1% sulfuric acid, 1x1 L with saturated brine, dried over
MgS04, and filtered. The ether was removed by rotovap and then the oil was
purged with nitrogen overnight, obtaining 470 g of a yellow oil that partially
crystallized overnight. Titration for free fatty acids via AOCS method Ca 5a-
40
shows a fatty acid content of 95% expressed as oleic acid. A sample was
silanized by reacting 104 mg with 100 uL of N-methyl-N-
(trim ethylsilyl)trifluoroacetamide in 1 mL of dry pyridine. Gas
chromatography-
mass spectrometry (GCMS) analysis of the silanized product shows the presence
of the TMS derivatives of the 16:0, 18:2, 18:1, 18:0, and 20:0 acids.
-74-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
[00209] The partition coefficient of isobutanol in the COFA/water system at an
initial 6% I-BuOH concentration after 168 hours as determined by the quiescent
method is 2.8.
Example 5
Corn Oil Fatty Acid Methyl Esters (FAME)
[00210] Round bottom flask (5L) equipped with a mechanical stirrer,
thermocouple,
heating mantle, condenser, and nitrogen tee. Charged with 1500 g of food grade
corn oil, 1500 g of methanol, and 30 g of concentrated sulfuric acid. The
mixture
was refluxed for 24 hours and followed by thin layer chromatography. The
reaction was then cooled and the layers were separated. The organic layer was
washed 1x1 L with water, 1x1 L with saturated sodium bicarbonate, 2x1 L with
water, 1x1 L with saturated brine, and then dried over MgSO4. The yield was
1416 g of a pale yellow oil. GCMS analysis shows the presence of the methyl
esters of the acids 16:0, 18:2, 18:1, 18:0, 20:1, and 20:0. Titration for free
fatty
acids via AOCS method Ca 5a-40 shows a fatty acid content of 0.2% expressed
as oleic acid.
[00211] The partition coefficient of isobutanol in the FAME/water system at an
initial 6% I-BuOH concentration after 236 hours as determined by the quiescent
method is 1.06.
Example 6
Corn Oil Ethylene Glycol Ester (FAGE)
[00212] Round bottom flask (3L) was equipped with a mechanical stirrer,
thermocouple, heating mantle, Dean-Stark trap, condenser, nitrogen purge, and
nitrogen tee; and charged with 1000 g of Corn Oil Fatty Acid Methyl Ester
(FAME) and 1000 g of ethylene glycol. 2 g of clean sodium is added to the
mixture and it is heated to 60 C. After 90 minutes, the temperature was
increased to 100 C and nitrogen is slowly sub-surface sparged into the
reaction.
Methanol is collected in the Dean-Stark trap. The temperature is slowly
increased to 160 C over 3 hours and methanol continues to distill from the
reaction. After another two hours, a total of 100 mL of methanol was
collected.
-75-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
The reaction was cooled to room temperature and was neutralized with 20 g of
25% sulfuric acid. The layers were separated and the top layer was washed with
4x200 ml of 10% calcium chloride solution. Emulsions were formed but would
separate with time. The organic layer was washed with 250 mL of saturated
brine, dried over MgSO4, and filtered to give 916 g of a clear yellow oil.
Titration
for free fatty acids via AOCS method Ca 5a-40 shows 2.9% of acid present -
expressed as oleic acid. A sample was silanized by reacting 109 mg with 100 uL
of N-methyl-N-(trimethylsilyl)trifluoroacetamide in 1 ml of dry pyridine. GCMS
analysis of the silanized product shows the presence of the TMS derivatives of
the 16:0, 18:2, and 18:1 acids, along with the 16:0, 18:2, 18:1, and 18:0
ethylene
glycol monoesters.
[00213] The partition coefficient of isobutanol in the FAGE/water system at an
initial 6% I-BuOH concentration after 192 hours as determined by the quiescent
method is 2.3.
Example 7
Comparative Fermentation Examples
[00214] The materials listed in Table 2 were used in the comparative examples
of
Example 7. All commercial reagents were used as received. Solvents
synthesized from corn oil were also used as received.
Table 2
Materials
Seed Flask and Fermentation Media Components
- Yeast Nitrogen Base w/o amino acids, Becton Dickinson and Company
- (291920)
- Yeast Dropout Mix, Sigma Aldrich (Y2001)
- L-Leucine, Sigma Aldrich (L8000)
- L-Tryptophan, Sigma Aldrich (T0254)
- Ethanol >99.5%, Sigma Aldrich (459844)
- 50% w/w glucose solution
- Ergosterol, Fluka (45480)
- Tween 80, Sigma Aldrich (P8074)
- Yeast Extract, Becton Dickinson and Company (212750)
- Peptone, Becton Dickinson and Company (211820)
- Nicotinic Acid, Alfa Aesar (Stock # A12683 or L02659)
- Thiamine Hydrochloride, Sigma Aldrich (T4562)
-76-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
Commercial Solvents
- 90-95% Oleyl Alcohol, Cognis, Lot # CE81210020
- Oleic Acid, Sigma Aldrich (27728)
- IsofolTM 12, Sasol North America, Lot # 65604
Synthesized Solvents (using the methods described in above Examples 2-4
and 6)
- Corn Oil Fatty Acids
- Corn Oil Ethylene Glycol Ester
- Corn Oil Fatty Alcohols, Preparation A
- Corn Oil Fatty Alcohols, Preparation B
- Corn Oil Fatty Amides/ Acids
- Corn Oil Fatty Amides
- Corn Oil Fatty Acid Methyl Ester + 1,2-Propanediol
Stock Solutions
- 1OXYEP
- Add 100 g/L yeast extract and 200 g/L peptone in 500 mL of warm diH2O
(60 C). Continue heating while bringing solution to a final volume of 1 L then
filter sterilize.
- 1% Ergosterol in 50:50 v/v ethanol:tween 80
- Add 10 g/L ergosterol into a warm solution (50 C) of 50:50 ethanol:tween
heat until the ergosterol dissolves and filter sterilize.
- 10OX Nicotinic Acid/ Thiamine Hydrochloride
- Add 10 g/L nicotinic acid and 2 g/L thiamine to diH2O then filter sterilize.
Strains
- NGCI-065
- NGCI-070
General Methods
[00215] Optical density was measured using an Amersham Biosciences Ultrospec
2100 Pro spectrophotometer. Measurements were typically made at a
wavelength of 600 nanometers.
[00216] Glucose concentrations were measured using a YSI Life Sciences 2700
Select Biochemistry Analyzer. Fermentation samples were centrifuged at 13,200
rpm for 2 minutes in a 1.7 mL microcentrifuge tube and the aqueous supernatant
analyzed for glucose concentration.
[00217] Fermentation conditions: 30% p02; temperature: 30 C; pH 5.5 (unless
noted otherwise); initial batch glucose: 20 g/L, maintained during production.
[00218] Both HPLC and GC analyses were used for the quantization of isobutanol
in the aqueous phase and solvent phase respectively. Isobutanol in the aqueous
-77-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
was measured after filtration through a 0.2 um nylon filter with a HPLC
(Agilent
1100, Agilent, Santa Clara, CA) under the following conditions:
Column: Bio-Rad, Aminex HPX-87H, No. 125-0143
Mobile Phase: 0.01 M Na2HPO4, pH=8.0
Injection Volume: 10 uL
Flow Rate: 0.6 mL/min
Run Time: 22.5 minutes
Column Temperature: 65 C
Detectors: Refractive Index
Detector Temperature: 40 C
UV Detection: 210 nm, 4 nm bandwidth, Ref 360 nm, 100 nm bandwidth.
[00219] The solvent phase was measured with a GC (HP6890, Agilent, Santa
Clara, CA) under the following conditions:
Column: J&W Scientific DB Waxter (50 m X 0.32 mm ID, 1 um film)
Gas Carrier: Helium 4 mL/min
Injection Volume: 2 uL
Make Up Flow Rate: 40 mL/min
Run Time: 29 minutes
Oven Temperature: 40 C for 5 min, 40 C to 230 C @10 C/min.,
min 230 C
Injector Split: 1:5 @ 250 C
Flame Ionization Detection: 250 C
Comparative Examples: GLNOR635A-640A of Corn Oil Derived Extractants
and Their Constituents
[00220] A series of comparative examples were conducted using the water-
immiscible extractants listed in Table 3. The extractants were added to the
fermentor broth at time zero exposing the culture to the solvent for the
duration of
the fermentation. Isobutanol concentrations in both the aqueous phase and
organic phase were measured to calculate the partitioning coefficient of the
-78-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
extraction solvent. Glucose utilization was used to determine the
biocompatability of the microorganism to the extractant.
Table 3
Composition of Extractants Used for Fermentation Examples GLNOR635-640A
Example Extractant
GLNOR635A Oleyl Alcohol
GLNOR636A Corn Oil Fatty Acids
(COFA)
GLNOR637A Oleic Acid
GLNOR638A Oleic Acid
GLNOR639A Oleic Acid
GLNOR640A Oleic Acid
[00221] The fermentations were carried out as described with the strain NGCI-
065.
The inoculum was prepared in two stages and incubated at 30 C and 250 rpm in
an incubator shaker (Innova 4200, New Brunswick Scientific, Edison, NJ). The
first stage or pre-seed was inoculated from a frozen glycerol seed stock, two
vials
were placed into a 250 mL flask with 30 mL of filter sterilized pre-seed media
(Table 4) and grown for 24 hours to an OD of approximately 2. 15 mL of the pre-
seed was then transferred to a 2L flask with 270 mL of filter sterilized seed
media
(Table 5) for the second stage which was incubated for 24 hours. 30 mL of
filter
sterilized 1OX YEP and 300 mL of filter sterilized 90-95% oleyl alcohol was
then
added and incubated for an additional 24 hours to a final OD of approximately
5-
in the aqueous phase of the seed culture. For examples GLNOR639 and
GLNOR640, the pH was 4.5.
Table 4
Pre-seed/ Stage 1 Media Composition
Pre-seed Media Components Amount per Liter
Yeast Nitrogen Base w/o Amino 6.7 g
Acids
Yeast Dropout Mix 1.4
L-Leucine (1 % w/v Stock Soln 20 mL
L-Tryptophan (1 % w/v Stock 4 mL
Soln
Ethanol 3.0 mL
50% w/w glucose solution 5.4 mL
-79-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
Table 5
Seed/ Stage 2 Media Composition
Seed Media Components Amount per
Liter
Yeast Nitrogen Base w/o Amino 6.7 g
Acids
Yeast Dropout Mix 2.8
L-Leucine (1 % w/v Stock Soln 20 mL
L-Tryptophan (1 % w/v Stock 4 mL
Soln
Ethanol 3.0 mL
50% w/w glucose solution 50.4 mL
MES Buffer 38.4
Additions after 24hrs of Amount per
incubation Flask
1 OX Yeast Extract Peptone 30 mL
100 /L YE and 200 /L Peptone)
90-95% Oleyl Alcohol 300 mL
[00222] Fermentation vessels (Applikon AD1 010 Bioreactor, Applikon
Biotechnology, Dover, NJ) were sterilized with diH2O for 30 minutes (Amsco
Renaissance 3033 Revas Steam Sterilizer, Steris Corporation, Mentor, OH).
Once the vessels were finished sterilization in the autoclave and cooled to 30
C,
the sterile diH2O was removed and the filter sterilized fermentation media was
added to a volume of 280 mL. 70 mL of the aqueous phase from the second
stage seed flasks was added to the fermentation vessel for a final aqueous
volume of 350 mL. Immediately after inoculation, 100 mL of 1OX YEP media
supplementation was added as well as 450 mL of the respective extraction
solvent for a final solvent to broth ratio of approximately 1:1. Fermentation
set-
point conditions were temperature 30 C, p02 30%, pH 5.5 for GLNOR635A-638A
and pH 4.5 for GLNOR639A-640A. The fermentation was sampled
approximately every 8 hours from the time of inoculation to monitor glucose
concentration, which was maintained between 5-20 g/L through the addition of
50% w/w glucose solution, and analyzed for isobutanol accumulation in both the
aqueous phase and the extractant phase. Enough sample volume was taken at
each time point to obtain a sample from both of the phases, then those samples
were centrifuged in order to ensure a clean cut of each.
-80-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
Table 6
Fermentation Media Composition
Fermentation Media Components Amount per Liter
Yeast Nitrogen Base w/o Amino 6.7 g
Acids
Yeast Dropout Mix 2.8
L-Leucine (1 % w/v Stock Soln 20 mL
L-Tryptophan (1 % w/v Stock Soln 4 mL
Ethanol 4.5 mL
50% w/w glucose solution 40.0 g
1 % Ergosterol in 50:50 (v/v) 1.0 mL
Ethanol:Tween 80
Additions Post Inoculation
1 OX Yeast Extract Peptone (100g/L 100 mL
YE and 200 /L Peptone
90-95% Oleyl Alcohol 450 mL
Comparative Examples: GLNOR661A-666A of Corn Oil Derived Extractants
and Their Constituents
[00223] Comparative examples GLNOR661A-666A were conducted using the
water-immiscible extractants listed in Table 7. The examples in this set were
performed the same as in the previous examples GLNOR635A-640A with the
exception of the strain, pH, and supplement addition at time zero. Strain NGCI-
070 was used, pH set point was 5.5, and 4 mL nicotinic acid/ thiamine media
supplementation was added instead of 100 mL yeast extract peptone for this
series of examples.
Table 7
Composition of Extractants Used for Fermentation Examples GLNOR661-666A
Example Extractant
GLNOR661A Oleyl Alcohol
GLNOR662A No Solvent
GLNOR663A Corn Oil Fatty
Acids
GLNOR664A Corn Oil Fatty
Acids
GLNOR665A Oleic Acid
GLNOR666A Oleic Acid
-81-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
Comparative Examples: GLNOR69OA-695A of Corn Oil Derived Extractants
and Their Constituents
[00224] Comparative examples GLNOR69OA-695A were conducted using the
water-immiscible extractants listed in Table 8. The examples in this set were
performed the same as in the previous examples GLNOR661A-666A. Strain
NGCI-070 was used.
Table 8
Composition of Extractants Used for Fermentation Examples GLNOR690-695A
Example Extractant
GLNOR690A Oleyl Alcohol
GLNOR691A Oleyl Alcohol
GLNOR692A Corn Oil Fatty Alcohols*
GLNOR693A Corn Oil Fatty Alcohols*
GLNOR694A Corn Oil Ethylene Glycol
Ester
GLNOR695A Corn Oil Ethylene Glycol
Ester
*Synthesized Fatty Alcohols, Preparation A
Comparative Examples GLNOR721A-726A of Corn Oil Derived Extractants
and Their Constituents
[00225] Comparative examples GLNOR721A-726A were conducted using the
water-immiscible extractants listed in Table 9. The examples in this set were
performed the same as in the previous examples GLNOR69OA-695A. Strain
NGCI-070 was used.
-82-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
Table 9
Composition of Extractants Used for Fermentation Examples GLNOR721-726A
Example Extractant
GLNOR721A Corn Oil Fatty Acid Methyl Ester + 1,2-
Propanediol
GLNOR722A Corn Oil Fatty Acid Methyl Ester + 1,2-
Propanediol
GLNOR723A Corn Oil Fatty Alcohols**
GLNOR724A Corn Oil Fatty Alcohols**
GLNOR725A Corn Oil Fatty Amides/ Acids*
GLNOR726A Corn Oil Fatty Amides/ Acids*
*2:1 Synthesized Fatty Amide:Fatty Acid Mix (Preparation (1)) of Example 2
**Synthesized Fatty Alcohols, Preparation B
Comparative Examples: GLNOR749A-754A of Corn Oil Derived Extractants
and Their Constituents
[00226] Comparative examples GLNOR749A-754A were conducted using the
water-immiscible extractants listed in Table 10. The examples in this set were
performed the same as in the previous examples GLNOR721A-726A. Strain
NGCI-070 was used.
Table 10
Composition of Extractants Used for Fermentation Examples GLNOR749-754A
Example Extractant
GLNOR749A IsofolTM 12**
GLNOR750A IsofolTM 12
GLNOR751A Corn Oil Fatty Acids
GLNOR752A Corn Oil Fatty Acids
GLNOR753A Corn Oil Fatty Amides/
Acids*
GLNOR754A Corn Oil Fatty Amides/
Acids*
*1:1 Synthesized Fatty Amide Fatty Acid Mix:Pure Fatty Acids
(Preparation (1 a)) of Example 2
**lsofolTM 12: 2-butyl-1-octanol
[00227] The performance data for the fermentation examples GLNOR635A-640A,
GLNOR661A-666A, GLNOR69OA-695A, GLNOR721A-726A, GLNOR749A-754A
are summarized in Table 11, which provides the aqueous isobutanol
-83-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
concentrations (g/L), solvent isobutanol concentrations (g/L), and solvent
partition
coefficients (Kp) for the exemplary corn oil derived extractants and their
constituents as compared with conventional commercial solvents of oleyl
alcohol
and IsofolTMTable 11
Example Solvent Aqueous Kp
(g/L) (g/L)
GLNOR635 21.5 6.4 3.38
GLNOR636 9.8 3.9 2.52
GLNOR637 5.6 2.1 2.64
GLNOR638 5.5 2.1 2.56
GLNOR639 3 1.2 2.54
GLNOR640 3 1.2 2.51
GLNOR661 19 5.7 3.35
GLNOR662 X 5.1 X
GLNOR663 16.3 5.9 2.76
GLNOR664 12.1 4.6 2.65
GLNOR665 12 4.6 2.61
GLNOR666 12.4 4.9 2.51
GLNOR690 14.1 3.8 3.74
GLNOR691 13.6 3.6 3.77
GLNOR692 NA NA NA
GLNOR693 NA NA NA
GLNOR694 13.4 5 2.68
GLNOR695 12.2 4 3.07
GLNOR721 12.7 4.4 2.85
GLNOR722 11.5 4.0 2.89
GLNOR723 14.5 5.0 2.88
GLNOR724 18.0 6.3 2.87
GLNOR725 NA 6.2 NA
-84-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
GLNOR726 NA NA NA
GLNOR749 20.0 5.0 4.05
GLNOR750 20.1 4.8 4.22
GLNOR751 X 1.8 X
GLNOR752 14.3 6.0 2.38
GLNOR753 12.0 4.3 2.81
GLNOR754 19.5 7.2 2.71
Example 8
Fatty alcohol hydroxylation (65% hydroxylation)
[00228] To a three-neck 250 mL flask, equipped with a mechanical stirrer and
addition funnel was added fatty alcohol mixture (43 g, 0.16mmol), toluene
(25.0
mL), Amberlyte IR-120 resin (12.5 g), and glacial acetic acid (7.5g). The
resulting
mixture was heated to 60 C, and then hydrogen peroxide (41.8 g of 30% H202 in
water) was added dropwise over 30 minutes. The mixture was stirred at 60 C for
two hours, upon which time the reaction mixture was worked up: the resin was
removed by filtration, and the filtrate partitioned between ethyl acetate (75
mL)
and water (50 mL). After the layers were separated, the organic layer was
washed with sat. aq. NaHCO3 solution (50 mL), and brine (50 mL). The organic
layer was dried over anh. Na2SO4 and concentrated in vacuo to obtain 50 g of
yellow oil. The 1H NMR analysis of the crude reaction product showed that 65%
of double bonds were epoxidized. The resulting mixture was taken on to the
next
step without purification.
[00229] To a 500 mL round bottom flask was added epoxidized fatty alcohols
(14.5 g), THE (200.0 mL), and sulfuric acid (100 mL of 1.7M aqueous solution).
The cloudy mixture was stirred at 50 C overnight, and then worked up by
partitioning between water (100 mL) and ethyl acetate (200 mL). The organic
layer was washed with water (2xlOOmL), followed by sat. aq. NaHCO3 solution
(100 mL), and then brine (100 mL). The organic layer was dried over anh.
Na2SO4 and concentrated in vacuo to obtain 14.7g of thick clear liquid and
white
solid mixture.
-85-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
Measurement of the partition coefficient
[00230] To a 1 m L solution of 3% i-BuOH solution in water was added 1 mL of
the
hydroxylated fatty alcohol mixture, and the resulting two-phase mixture was
stirred vigorously using a vortex for ten minutes. The experiment was done in
duplicate, as well as on the 6% i-BuOH solution. After the mixing, the layers
were separated, and the samples were taken from both layers to measure i-
BuOH concentration using GC (Table 12). A partition coefficient of 3.7 was
observed.
Table 12. Partition coefficient measurement data for i-BuOH partitioning
between
water and hydroxylated fatty alcohols
Dilution Total
i-BuOH Factor Partition Conc.
Sample (Amt) (20x) Coefficient (mg/mL)
Organic layer i-BuOH, 3% 1.13 22.6 3.53 29
Organic layer i-BuOH, 3% 1 20 3.45 25.8
Water layer i-BuOH, 3% 0.32 6.4
Water layer i-BuOH, 3% 0.29 5.8
Organic layer i-BuOH, 6% 2.15 43 3.91 54
Organic layer i-BuOH, 6% 1.96 39.2 3.84 49.4
Water layer i-BuOH, 6% 0.55 11
Water layer i-BuOH, 6% 0.51 10.2
3.68
[00231] While various embodiments of the present invention have been described
above, it should be understood that they have been presented by way of example
only, and not limitation. It will be apparent to persons skilled in the
relevant art
that various changes in form and detail can be made therein without departing
from the spirit and scope of the invention. Thus, the breadth and scope of the
present invention should not be limited by any of the above-described
exemplary
embodiments, but should be defined only in accordance with the following
claims
and their equivalents.
[00232] All publications, patents and patent applications mentioned in this
specification are indicative of the level of skill of those skilled in the art
to which
this invention pertains, and are herein incorporated by reference to the same
-86-
CA 02801209 2012-11-29
WO 2011/159991 PCT/US2011/040842
extent as if each individual publication, patent or patent application was
specifically and individually indicated to be incorporated by reference.
-87-