Note: Descriptions are shown in the official language in which they were submitted.
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
METHODS OF MITIGATING EFFECTS OF RADIATION AND REDUCING THE
RISK OF SYSTEMIC INFECTION
RELATED APPLICATIONS
This application claims priority to US Provisional Patent Application No.
61/354067,
filed June 11, 2010, the contents of which are incorporated herein in their
entirety by reference.
BACKGROUND OF THE INVENTION
With increasing threat of a nuclear detonation, it is essential to develop new
countermeasures that can be delivered post-exposure to protect civilians and
immediate care
providers. Further urgency is mandated by the realization that a combination
of radiation with
traumatic injury, dermal injury or burns can be up to ten times more lethal
than radiation alone.
A detonation will injure thousands of people, who, without an effective
countermeasure for
combined radiation injury, will likely die from what should have been a sub-
lethal dose of
radiation.
In addition, more than 50% of all cancer patients undergo some degree of
radiation
therapy. It is well known that radiation therapy affects adjacent normal
tissue, often preventing
closure of surgical wounds and leading to later breakdown of skin or formation
of chronic ulcers
that fail to heal.
Currently, there are no products that have been approved for mitigating
effects of
radiation on individuals after radiation exposure. A number of potential
products that are being
evaluated only target a particular aspect of radiation combined injury, and
therefore, have limited
efficacies. For radiotherapy related injuries, most treatments are largely
based on good wound
1
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
care with the use of standard antibiotics and wound dressings with surgical
repair of larger
ulcerated or non-healing areas. The only FDA approved radiotherapy protective
agent,
Amifostin, however, is required to be injected into adjacent tissues prior to
fractionated
radiotherapy. Therefore, a need exists for new methods for preventing and
treating radiation
30 induced injuries resulted from accidental radiation exposure or
radiotherapy.
SUMMARY OF THE INVENTION
Applicants have discovered that post-exposure injection of the thrombin
peptide
derivative TP508 can increase survival time and delay onset of septic
bacterial growth in mice
35 that were exposed to a lethal dose of gamma irradiation (Examples 3 and 4).
In addition, either
topical treatment or systemic injection of the thrombin peptide derivative
TP508 can promote
healing of an open dermal wound in mice that were exposed to radiation
(Example 5).
The present invention is directed to a method of reducing the risk of
mortality or
extending the life expectancy (by, e.g., at least 5%, 10%, 20%, 25%, 50%, 75%
or 100%) in a
40 subject exposed to a lethal dose of radiation, or to a dose of radiation
that when combined with
injury would be lethal, comprising administering to the subject an effective
amount of a
thrombin peptide derivative comprising Asp-Ala-R, wherein R is a serine
esterase conserved
sequence.
In another embodiment, the present invention is directed to a method of
reducing the risk
45 of developing systemic bacterial, fungal or viral infection in a subject
exposed to radiation. The
method comprises administering to the subject an effective amount of a
thrombin peptide
2
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
derivative comprising Asp-Ala-R, wherein R is a serine esterase conserved
sequence.
In another embodiment, the present invention is directed to a method of
treating a subject
with traumatic injury, dermal injury and/or burn injury who is also exposed to
radiation,
50 comprising administering to the subject an effective amount of a thrombin
peptide derivative,
wherein the thrombin peptide derivative comprises Asp-Ala-R, wherein R is a
serine esterase
conserved sequence.
The present invention is also directed to a method of reducing radiation
related injury in a
subject undergoing radiation therapy, comprising administering to the subject
an effective
55 amount of a thrombin peptide derivative, wherein the thrombin peptide
derivative comprises
Asp-Ala-R, wherein R is a serine esterase conserved sequence.
In another embodiment, the present invention is directed to a method of
reducing the risk
of developing a radiation induced illness in a subject undergoing radiation
therapy, comprising
administering to the subject an effective amount of a thrombin peptide
derivative, wherein the
60 thrombin peptide derivative comprises Asp-Ala-R, wherein R is a serine
esterase conserved
sequence.
In another embodiment, the present invention is directed to a method of
promoting
healing of a wound on a subject that was caused by radiation exposure and or
has been exposed
to radiation, comprising administering to the wound an effective amount of a
thrombin peptide
65 derivative, or comprising administering an effective amount of a thrombin
peptide derivative
systemically post radiation exposure, wherein the thrombin peptide derivative
comprises Asp-
Ala-R, wherein R is a serine esterase conserved sequence.
3
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
In yet another embodiment, the present invention is directed to a method of
reducing the
risk of developing bacterial, fungal or viral infection in the blood of a
subject that has not been
70 exposed to radiation and that may be at risk of developing bacterial,
fungal or viral infection in
the blood. The method comprises administering to the subject an effective
amount of a thrombin
peptide derivative comprising Asp-Ala-R, wherein R is a serine esterase
conserved sequence.
The present invention is also directed to the use of a thrombin peptide
derivative for
reducing the risk of mortality in a subject exposed to a lethal dose of
radiation, reducing the risk
75 of developing bacterial, fungal or viral infection in a subject exposed to
radiation, treating a
subject with traumatic injury, dermal injury and/or burn injury who is also
exposed to radiation,
reducing radiation related injury in a subject undergoing radiation therapy,
reducing the risk of
developing a radiation induced illness in a subject undergoing radiation
therapy; promoting
healing of a wound on a subject that was caused by radiation exposure and/or
has been exposed
80 to radiation, wherein the thrombin peptide derivative comprises Asp-Ala-R,
wherein R is a serine
esterase conserved sequence. For the use for promoting healing of a wound on a
subject that was
caused by radiation exposure and/or has been exposed to radiation.
BRIEF DESCRIPTION OF THE DRAWINGS
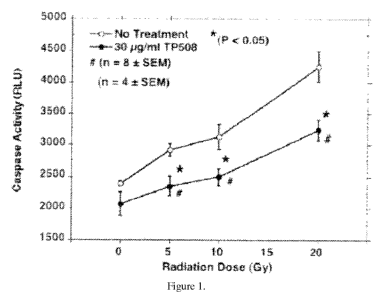
Figure 1 is a graph that shows caspase activity measured in human
microvascular
85 endothelial cells (HMVEC) exposed to 0, 5, 10 or 20 Gy of radiation and
treated with saline or
30 ug/ml TP508.
Figure 2 is a graph that shows percent of mice surviving on different days
after sustaining
8 Gy radiation exposure and a dermal excision, who have also received
treatment with saline
placebo or TP508 delivered topically or intravenously.
4
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
90 Figure 3 is a graph that shows percent of mice surviving at different days
after receiving
12 Gy radiation exposure and a single post-exposure bolus dose of either
saline or TP508.
Figure 4 is a bar graph that shows the number of live bacteria (CFU) in the
blood of mice
6 and 7 days after exposure to 0 or 12 Gy radiation and either a saline
placebo or TP508
injection.
95 Figure 5 is a bar graph that shows the rate of linear wound healing
(measured as mm/day)
in mice at days 0-5 and 5-16 post-exposure to 0 or 8 Gy irradiation and either
saline placebo or
TP508 applied topically or by intravenous injection.
Figure 6 is a panel with two bar graphs. The bar graph in panel A shows serum
IL-6
levels (ng/ml) measured 11 days post-irradiation in mice irradiated at 0 and 8
Gy and treated
100 with either saline placebo (P) or TP508 administered either topically
(TPt) or intravenously
(TPiv). The bar graph in panel B shows serum IL-6 levels (ng/ml) measured 7
days post-
irradiation in mice irradiated at 0 and 12 Gy and treated with either saline
placebo (P) or TP508
administered either topically (TPt) or intravenously (TPiv).
Figure 7 is a bar graph showing fold increase in the sprouting area after 5
days of aortic
105 explant culture. The aortas were isolated from mice 24 hours after
exposure to 0, 3, 8 or 10 Gy
radiation and treatment with either saline placebo or TP508 administered
intravenously.
DETAILED DESCRIPTION OF THE EMBODIMENTS
The present invention is directed to methods of reducing the adverse effects
of radiation
exposure in a subject comprising administering to the subject an effective
amount of a thrombin
110 peptide derivative described therein. Radiation exposure can, for example,
result from nuclear
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
detonation, nuclear weaponry, accidental radiation exposure (such as due to
accidents at nuclear
reactors or inadequate protection from radiation source) or radiation therapy.
Low-dose
radiation exposure can be an occupational hazard affecting airline workers and
astronauts;
workers at nuclear power and nuclear fuel processing plants; research
laboratory workers; and
115 uranium miners. In addition, medical diagnostic tests, such as X-rays, can
be a source of low-
level radiation exposure for the general public.
The present invention is also directed to methods of reducing the risk of
developing
bacterial, fungal or viral infection in the blood in the subjects who have not
been exposed to
radiation, comprising administering to the subject an effective amount of a
thrombin peptide
120 derivative described herein. The infection can enter the bloodstream as a
complication of
diseases, such as pneumonia or meningitis, during surgery (especially when it
involves mucous
membranes such as the gastrointestinal tract), or due to catheters and other
foreign bodies
entering the arteries or veins (including intravenous drug abuse). In
addition, individuals
suffering from pulmonary conditions, inflammatory bowel disease and systemic
inflammatory
125 response syndrome (SIRS), or individuals who are immunocompromised are
also at the risk of
blood infection. In the hospital, indwelling catheters are a frequent cause of
blood infections
because they provide a means by which bacteria normally found on the skin can
enter the
bloodstream. Other causes of blood infections include dental procedures
(occasionally including
simple tooth brushing), herpes (including herpetic whitlow), urinary tract
infections, peritonitis,
130 Clostridium difficile colitis, intravenous drug use, and colorectal
cancer. Blood infections may
also be a consequence of oropharyngeal, gastrointestinal or genitourinary
surgery or exploration.
An immune response to blood infection can lead to sepsis and septic shock,
which have a
relatively high mortality rate. In the methods described herein, the peptide
of the present
6
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
invention can be administered in combination with an antibiotic.
135 In one aspect, the present invention is directed to a method of reducing
the risk of
mortality in a subject exposed to a lethal dose of radiation, comprising
administering to the
subject an effective amount of a thrombin peptide derivative described herein.
A lethal dose of radiation is a dose that would cause death in half of the
tested subjects in
days, i.e. LD50 in 10 days. The lethal dose depends on the identity of the
subject. For example,
140 a lethal dose for a human is about 3.5 Gy or greater and a lethal dose for
a mouse is about 12 Gy
or greater.
The subject who is exposed to a lethal dose of radiation may also have
additionally
sustained traumatic injury, dermal injury and/or burn injury.
A "burn injury" is a type of skin injury caused by heat, electricity,
chemicals, light,
145 radiation, friction or heat. Burn injury caused by radiation exposure,
such as in the event of
nuclear detonation, includes, for example, thermal burns from infrared heat
radiation, beta burns
from shallow ionizing beta radiation, and gamma burns from highly penetrating
gamma
radiation. The burn injury can be first-degree, second-degree or third-degree
burn. Various
percentage of total body surface area (TBSA) may be affected by the burn
injury. For example,
150 less than 1%, greater than 1%, greater than 5%, greater than 10%, greater
than 15%, greater than
20%, greater than 30%, greater than 40%, greater than 50%, or 1-10%, 10-20%,
20-30%, 30-
40%, 40-50% or 50-70% of TBSA was affected by the burn injury.
A "traumatic injury" is a physical injury produced by force or shock. A
traumatic injury
is often associated with secondary complications, such as shock, respiratory
failure and death.
7
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
155 For example, a traumatic injury can be caused by force of explosions or
force of falling and
flying objects. A traumatic injury also includes an injury to an internal
organ resulting in
hemorrhaging from the organ and/or at least partial loss of function. For
example, an injury to an
internal organ can be caused by penetration, such as from a bullet or flying
projectile. A
traumatic injury can also include injuries to musculoskeletal system, such as
bones, muscles,
160 cartilages, tendons, ligaments, joints and connective tissues. In one
embodiment, a traumatic
injury is a bone fracture.
Dermal injury is an injury to the dermis layer of the skin. Severe dermal
injury is a
dermal injury that results in dermal wound that covers at least 100 mm2 and/or
is full-thickness
wound (i.e., wound that penetrates through both the epidermis and dermis layer
of the skin).
165 In one embodiment, the burn injury, traumatic injury or dermal injury
sustained by the
subject expose the subject to systemic infection. "Systemic infection" is an
infection that has
entered the blood stream and may affect multiple organs and/or tissues or the
body as a whole.
In another embodiment, the thrombin peptide derivatives described herein
reduce
leucocytopenia and/or neutropenia in the subject being treated by the methods
of the present
170 invention. Alternatively, the thrombin peptide derivatives described
herein reduces the decrease
in population of bone marrow progenitor cells from radiation damage in the
subject being treated
by the methods of the present invention.
In another aspect, the present invention is a method of reducing the risk of
developing
systemic bacterial, fungal or viral infection in a subject, comprising
administering to the subject
175 an effective amount of a thrombin peptide derivative described herein. In
one embodiment, the
subject has been exposed to a bacterial, fungal or viral infection. In one
example, a subject who
8
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
is at risk of developing systemic bacterial, viral or fungal infection has
been exposed to radiation.
For example, in the event of nuclear detonation, health workers, hospital
patients, rescue
workers, sanitarian workers and people who are in an environment or a place
that is likely to
180 have an outbreak of bacterial, fungal or viral infection, such as in a
hospital. Alternatively, a
subject who suffers from burn injury, traumatic injury or dermal injury
resulting from radiation
exposure, for example, from nuclear detonation, is more susceptible to
bacterial, fungal or viral
infection than a subject who does not suffer such injuries. In another
alternative, a subject who
has an unhealed wound prior to radiation exposure is more likely to develop
bacterial, fungal or
185 viral infection. In yet another alternative, a patient who is undergoing
radiotherapy and has a pre-
existing wound that is exposed to the radiation, for example from a surgery,
would have higher
risk for developing bacterial, fungal or viral infection than a patient who
does not have a pre-
existing wound. The thrombin peptide derivatives described herein are
effective in reducing the
risk of developing systemic bacterial, fungal or viral infection in the
subjects described above
190 upon radiation exposure.
In one embodiment, the method reduces the risk of developing bacterial, fungal
or viral
infection in the blood of the subject. High doses of radiation exposure can
result in acute illness,
such as breakdown of intestinal walls, which would render the subject more
susceptible to septic
infection. In one embodiment, the present invention is directed to a method of
delaying the onset
195 of septic systemic infection in a subject who is exposed to radiation
comprising administering to
the subject an effective amount of a thrombin peptide derivative described
herein. The subject
may be exposed to a lethal dose of radiation. Alternatively, the subject is
exposed to a sub-lethal
dose of radiation.
In another alternative, the subject is at risk of developing bacterial, fungal
or viral
9
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
200 infection and has not been exposed to radiation. These subjects include
one or more of the
following: a) a subject has sustained traumatic injury, severe dermal injury
and/or burn; b) a
subject that underwent an invasive medical or dental procedure; c) a subject
that underwent
insertion of an invasive medical device; d) a subject who has pneumonia or
other pulmonary
conditions that could lead to acute respiratory distress syndrome or systemic
infections; e) a
205 subject who is immunocompromised; f) a subject who is an infant or is
older than 60 years old,
or g) subject from one or more of the categories a-d, who is an infant or
older than 60 years old.
An invasive medical procedure that exposes a subject to infection is any
procedure that
involves either making a surgical cut in the skin or inserting an instrument,
such as a needle or a
tube, into the body of a subject. An invasive medical procedure increases a
risk of introducing
210 foreign organisms, such as bacteria or fungi, into the body of a subject,
leading to an increased
risk of bacterial, fungal or viral infection in the blood of the subject. An
example of an invasive
medical procedure is a surgery for any indication. Another example of an
invasive medical
procedure is a procedure wherein an invasive medical devise is introduced into
a subject.
Examples of invasive medical devises can include an intravenous or an arterial
line, a breathing
215 tube, a urinary catheter, a surgical drain, an artificial joint, or a
feeding tube. Examples of
feeding tubes can include G-tube/PEG tube, J-tube (jejunostomy tube) and NG-
tube (nasogastric
tube).
In another embodiment, the present invention is directed to a method of
reducing the risk
of developing a bacterial, fungal or viral infection in the blood of a subject
who has pneumonia.
220 Pneumonia is an inflammatory condition of the lung, especially of the
alveoli. Infection is the
most common cause of pneumonia. Infecting agents can be bacteria, viruses,
fungi, or parasites.
Chemical burns or physical injury to the lungs can also produce pneumonia.
Bacteria are the
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
most common cause of pneumonia, with Streptococcus pneumoniae the most
commonly isolated
bacteria in the cases of community-acquired pneumonia. Another important Gram-
positive cause
225 of pneumonia is Staphylococcus aureus, with Streptococcus agalactiae being
an important cause
of pneumonia in newborn infants. Gram-negative bacteria cause pneumonia less
frequently than
gram-positive bacteria. Some of the gram-negative bacteria that cause
pneumonia include
Haemophilus influenzae, Klebsiella pneumoniae, Escherichia coli, Pseudomonas
aeruginosa and
Moraxella catarrhalis. These bacteria often live in the stomach or intestines
and may enter the
230 lungs if vomit is inhaled. "Atypical" bacteria which cause pneumonia
include Chlamydophila
pneumoniae, Mycoplasma pneumoniae, and Legionella pneumophila.
In another embodiment, the present invention is directed to a method of
reducing the risk
of developing a bacterial, fungal or viral infection in the blood of a subject
who is
immunocompromised. An immunocompromised subject is a subject whose immune
system is
235 weakened or absent. Subjects who are inimunocon-npromised are less capable
of battling
infections because of an immune response that is not properly functioning.
Examples of
immunocompror ised subjects can include: a) subjects who have genetic defects
that can affect
functioning of their immune svslems; b) subjects who have diseases such as
AIDS or canc(:rs,
including leukem-nia, lymphoma or multiple mn_yeloma.; c) subjects who have
chronic diseases,
240 such as end stage renal disease requiring dialysis. diabetes, or
cirrhosis; d) subjects who receive
treatrn_ents that can include steroids, ch(mlm aerapy, radiation,
immuunosuppressive post-
transplant medications; and c) subjects who are pregnant.
In one embodiment, the bacterial, fungal or viral infection includes (in the
presence or
absence of radiation exposure), but is not limited to, infection of
staphylococci (e.g.,
245 staphylococcus aureus), enterococci, streptococci (e.g., streptococcus
pneumoniae),
11
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
pseudomonas aeruginosa, burkholderia cenocepacia, mycobacterium avium,
enterobacter,
bacteroidesfragilis, streptococcus pyogenes, enterococcus sp., haemophilus
influenzae,
legionella sp., chlamydia pneumoniae, escherichia coli, clostridium sp.,
staphylococcus sp.,
enterobacter sp., proteus sp., neiserria meningitidis, listeria monocytogenes,
Candida sp. (e.g.,
250 Candida albicans), enterococcus sp., klebsiella, s. agalactiae, and
aspergillus. The bacterial,
fungal or viral infection also includes systemic bacterial, systemic fungal
infections and systemic
viral infections.
In the event of nuclear detonation, people often suffer open dermal wounds in
addition to
radiation exposure. Additionally, radiation exposure can often cause skin
injuries, such as skin
255 ulceration. In the case of radiation therapy, cancer patients often
undergo radiation therapy
following surgical removal of the tumor and consequently have surgical wounds
that are exposed
to radiation when undergoing radiation therapy. A subject may also have a pre-
existing wound
before radiation exposure, including nuclear detonation, accidental radiation
exposure or
radiation therapy. The thrombin peptide derivatives described herein can
promote healing of
260 these wounds described above.
In one embodiment, the radiation exposure is sub-lethal. For example, the
radiation
exposure is less than 3.5 Gy when the subject is a human.
In yet another aspect, the present invention is directed to a method of
reducing radiation
related injury in a subject who is undergoing a radiation therapy. The method
comprises
265 administering to the subject an effective amount of a thrombin peptide
derivative described
herein.
A "radiation related injury" is an injury due to radiation exposure resulting
from nuclear
12
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
detonation, nuclear weaponry, accidental radiation exposure or a radiation
therapy. For example,
when a subject is exposed to a high dose of radiation, such as in the event of
nuclear detonation,
270 the radiation exposure often causes acute illness in the subject,
including hematopoietic
syndrome as a result of effects of radiation on the bone marrow, spleen and
lymph nodes,
gastrointestinal syndrome (such as breakdown of the intestinal wall) due to
the effects of
radiation on the cells lining the digestive tract, and brain damage. Radiation
exposure, such as
radiation therapy, can also cause various skin injuries, such as intense
reddening, blistering and
275 ulceration of the skin at the irradiated site and late stage skin
breakdown, and injury to hair
follicles causing hair loss. Large dose of radiation exposure can cause
permanent hair loss,
damaged sebaceous and sweat glands, atrophy, fibrosis (e.g., subcutaneous
fibrosis), decreased
or increased skin pigmentation, and ulceration or necrosis of the exposed
tissue. In one
embodiment, the radiation related injury is skin ulceration or late stage
breakdown. Radiation
280 exposure, such as radiation therapy, can also damage musculoskeletal
system, such as bones,
muscles, cartilages, tendons, ligaments, joints and connective tissues. In one
embodiment, the
present invention is directed to a method of promoting healing of a bone in a
subject who is
exposed and/or has been exposed to radiation exposure, wherein the bone is a
fractured bone or
has been surgically treated, for example, to remove tumor in the bone.
285 In one embodiment, for methods of reducing radiation related injury in a
subject who is
undergoing a radiation therapy, the thrombin peptide derivative is
administered to normal tissue
of the subject that is exposed or is to be exposed to the radiation. For
example, the thrombin
peptide derivative is topically administered to the normal skin that is
exposed to the radiation.
During a radiation therapy, radiation often causes damage to underlying
tissues surrounding the
290 target site of radiation, often because radiation exposure cannot be
limited to the target site. To
13
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
reduce the radiation related injury to these normal tissues, the thrombin
peptide derivative can be
directly applied to the underlying tissues, locally (e.g., by injection or
implantation of a sustained
release device and the like or through a catheter) or systemically, e.g.,
before, during or after the
radiation therapy. Alternatively, the thrombin peptide derivative can be
applied or delivered
295 locally or systemically during the radiation therapy or after the
radiation therapy.
In yet another aspect, the present invention is directed to a method of
reducing the risk of
developing a radiation induced illness in a subject undergoing radiation
therapy, comprising
administering to the subject an effective amount of a thrombin peptide
derivative described
herein.
300 A "radiation induced illness" refers to a disorder, disease or condition
that resulted from
cellular damages caused by radiation exposure. For example, exposure to
radiation can result in
various cellular damages, such as damages to hematopeotic cells, decreased
availability, viability
and function of progenitor cells, delayed angiogensis and revascularization,
apoptosis of
intestinal microvascular endothelial cells, epithelial cells, crypt cells,
neuronal cells in the brain
305 and other tissues, and myocardium. As such, radiation induced illnesses
include diseases,
disorders or conditions resulting from the above-described cellular damages
caused by radiation
exposure. Exemplary radiation induced illness include, but is not limited to,
leucocytopenia,
neutropenia, infections, systemic inflammatory response syndrome (SIRS),
sepsis, multiple
organ dysfunction syndrome (MODS), lung damage, lung/airway disease, brain
microvascular
310 damage, brain cerebrovascular damage, stroke, atherosclerosis, peripheral
vascular damage,
peripheral artery disease (PAD), diabetic neuropathy and angiopathy and
cancer.
In yet another aspect, the present invention is directed to a method of
promoting healing
14
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
of a wound on a subject that was caused by radiation exposure and/or has been
exposed to
radiation. The method comprises administering to the wound (e.g., topically or
systemically,
315 such as by I.V.) an effective amount of a thrombin peptide derivative
described herein.
A wound is a type of injury in which skin is torn, cut or punctured (an open
wound). An
open wound can include incisions or incised wounds (caused by a clean, sharp-
edged object such
as a knife, a razor or a glass splinter); lacerations (irregular tear-like
wounds caused by blunt
trauma); abrasions; puncture wounds; penetration wounds or gunshot wounds
(caused by a bullet
320 or similar projectile driving into or through the body).
As used herein, the thrombin derivative peptides, the modified thrombin
peptide
derivatives and the thrombin peptide derivative dimers described below can be
collectively
referred to as "thrombin peptide derivatives." The thrombin derivative
peptides, the modified
thrombin peptide derivatives and each polypeptide in the thrombin peptide
derivative dimers
325 have 19 to 23 amino acids (i.e., 19-23 amino acids in length).
Thrombin Derivative Peptides
Thrombin peptide derivatives (also: "thrombin derivative peptides") are
analogs of
thrombin that have an amino acid sequence derived at least in part from that
of thrombin and are
330 active at the non-proteolytically activated thrombin receptor (NPAR).
Thrombin peptide
derivatives can include, for example, peptides that are produced by
recombinant DNA methods,
peptides produced by enzymatic digestion of thrombin, and peptides produced
synthetically,
which can comprise amino acid substitutions compared to thrombin and/or
modified amino
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
acids, especially at the termini.
335 Thrombin peptide derivatives of the present invention include thrombin
derivative
peptides described in U.S. Patent Nos. 5,352,664 and 5,500,412. In one
embodiment, the
thrombin peptide derivatives of the present invention is a thrombin peptide
derivative or a
physiologically functional equivalent, i.e., a polypeptide with no more than
about fifty amino
acids, preferably no more than about thirty amino acids and having sufficient
homology to the
340 fragment of human thrombin corresponding to thrombin amino acids 508-530
(Ala-Gly-Tyr-Lys-
Pro-Asp-Glu-Gly-Lys-Arg-Gly-Asp-Ala-Cys-Glu-Gly-Asp-Ser-Gly-Gly-Pro-Phe-Val;
SEQ ID
NO:6) that the polypeptide activates NPAR.
In another embodiment, the thrombin peptide derivatives of the present
invention is a
thrombin peptide derivative comprising a moiety represented by Structural
Formula (I):
345 Asp-Ala-R (I).
R is a serine esterase conserved domain. Serine esterases, e.g., trypsin,
thrombin, chymotrypsin
and the like, have a region that is highly conserved. "Serine esterase
conserved domain" refers to
a polypeptide having the amino acid sequence of one of these conserved regions
or is sufficiently
homologous to one of these conserved regions such that the thrombin peptide
derivative retains
350 NPAR activating ability.
A physiologically functional equivalent of a thrombin derivative encompasses
molecules
which differ from thrombin derivatives in aspects which do not affect the
function of the
thrombin receptor binding domain or the serine esterase conserved amino acid
sequence. Such
aspects may include, but are not limited to, conservative amino acid
substitutions (as defined
16
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
355 below) and modifications, for example, amidation of the carboxyl terminus,
acetylation of the
amino terminus, conjugation of the polypeptide to a physiologically inert
carrier molecule, or
sequence alterations in accordance with the serine esterase conserved
sequences.
A domain having a serine esterase conserved sequence can comprise a
polypeptide
sequence containing at least 4-12 of the N-terminal amino acids of the
dodecapeptide previously
360 shown to be highly conserved among serine proteases (Asp-Xi-Cys-X2-Gly-Asp-
Ser-Gly-Gly-
Pro-X3-Val; SEQ ID NO: 13); wherein Xi, is either Ala or Ser; X2 is either Glu
or Gln; and X3 is
Phe, Met, Leu, His, or Val).
In one embodiment, the serine esterase conserved sequence comprises the amino
acid
sequence of SEQ ID NO:14 (Cys-Glu-Gly-Asp-Ser-Gly-Gly-Pro-Phe-Val) or a C-
terminal
365 truncated fragment of a polypeptide having the amino acid sequence of SEQ
ID NO: 14. It is
understood, however, that zero, one, two or three amino acids in the serine
esterase conserved
sequence can differ from the corresponding amino acid in SEQ ID NO:14.
Preferably, the amino
acids in the serine esterase conserved sequence which differ from the
corresponding amino acid
in SEQ ID NO: 14 are conservative substitutions as defined below, and are more
preferably
370 highly conservative substitutions. A "C-terminal truncated fragment"
refers to a fragment
remaining after removing an amino acid or block of amino acids from the C-
terminus, said
fragment having at least six and more preferably at least nine amino acids.
In another embodiment, the serine esterase conserved sequence comprises the
amino acid
sequence of SEQ ID NO:15 (Cys-Xi-Gly-Asp-Ser-Gly-Gly-Pro-X2-Val; Xi is Glu or
Gln and X2
375 is Phe, Met, Leu, His or Val) or a C-terminal truncated fragment thereof
having at least six
amino acids, preferably at least nine amino acids.
17
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
In a preferred embodiment, the thrombin peptide derivative comprises a serine
esterase
conserved sequence and a polypeptide having a more specific thrombin amino
acid sequence
Arg-Gly-Asp-Ala (SEQ ID NO: 16). One example of a thrombin peptide derivative
of this type
380 comprises Arg-Gly-Asp-Ala-Cys-Xi-Gly-Asp-Ser-Gly-Gly-Pro-X2-Val (SEQ ID
NO: 1). X1 and
X2 are as defined above. The thrombin peptide derivative can comprise the
amino acid sequence
of SEQ ID NO:6 (Ala-Gly-Tyr-Lys-Pro-Asp-Glu-Gly-Lys-Arg-Gly-Asp-Ala-Cys-Glu-
Gly-Asp-
Ser-Gly-Gly-Pro-Phe-Val) or an N-terminal truncated fragment thereof, provided
that zero, one,
two or three amino acids at positions 1-9 in the thrombin peptide derivative
differ from the
385 amino acid at the corresponding position of SEQ ID NO:6. Preferably, the
amino acid residues in
the thrombin peptide derivative which differ from the corresponding amino acid
residues in SEQ
ID NO:6 are conservative substitutions as defined below, and are more
preferably highly
conservative substitutions. An "N-terminal truncated fragment" refers to a
fragment remaining
after removing an amino acid or block of amino acids from the N-terminus,
preferably a block of
390 no more than six amino acids, more preferably a block of no more than
three amino acids.
Optionally, the thrombin peptide derivatives described herein can be amidated
at the C-
terminus and/or acylated at the N-terminus. In a specific embodiment, the
thrombin peptide
derivatives comprise a C-terminal amide and optionally comprise an acylated N-
terminus,
wherein said C-terminal amide is represented by -C(O)NRaRb, wherein Ra and Rb
are
395 independently hydrogen, a C1-C1o substituted or unsubstituted aliphatic
group, or Ra and Rb,
taken together with the nitrogen to which they are bonded, form a C1-C10 non-
aromatic
heterocyclic group, and said N-terminal acyl group is represented by R,C(0)-,
wherein RL. is
hydrogen, a C1-C1o substituted or unsubstituted aromatic group, or a C1-C1o
substituted or
unsubstituted aromatic group. In another specific embodiment, the N-terminus
of the thrombin
18
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
400 peptide derivative is free (i.e., unsubstituted) and the C-terminus is
free (i.e., unsubstituted) or
amidated, preferably as a carboxamide (i.e., -C(O)NH2). In a specific
embodiment, the thrombin
peptide derivative comprises the following amino acid sequence: Ala-Gly-Tyr-
Lys-Pro-Asp-Glu-
Gly-Lys-Arg-Gly-Asp-Ala-Cys-Glu-Gly-Asp-Ser-Gly-Gly-Pro-Phe-Val (SEQ ID NO:6).
In
another specific embodiment, the thrombin peptide derivative comprises the
amino sequence of
405 Arg-Gly-Asp-Ala-Cys-Glu-Gly-Asp-Ser-Gly-Gly-Pro-Phe-Val (SEQ ID NO: 17).
Alternatively,
the thrombin peptide derivative comprises the amino acid sequence of SEQ ID
NO: 18: Asp-Asn-
Met-Phe-Cys-Ala-Gly-Tyr-Lys-Pro-Asp-Glu-Gly-Lys-Arg-Gly-Asp-Ala-Cys-Glu-Gly-
Asp-Ser-
Gly-Gly-Pro-Phe-Val-Met-Lys-Ser-Pro-Phe. The thrombin peptide derivates
comprising the
amino acids of SEQ ID NO:6, 17, or 18 can optionally be amidated at the C-
terminus and/or
410 acylated at the N-terminus. Preferably, the N-terminus is free (i.e.,
unsubstituted) and the C-
terminus is free (i.e., unsubstituted) or amidated, preferably a carboxamide
(i.e., -C(O)NH2). It is
understood, however, that zero, one, two or three amino acids at positions 1-9
and 14-23 in the
thrombin peptide derivative can differ from the corresponding amino acid in
SEQ ID NO:6. It is
also understood that zero, one, two or three amino acids at positions 1-14 and
19-33 in the
415 thrombin peptide derivative can differ from the corresponding amino acid
in SEQ ID NO:18.
Preferably, the amino acids in the thrombin peptide derivative which differ
from the
corresponding amino acid in SEQ ID NO:6 or SEQ ID NO:18 are conservative
substitutions as
defined below, and are more preferably highly conservative substitutions.
Alternatively, an N-
terminal truncated fragment of the thrombin peptide derivative having at least
fourteen amino
420 acids or a C-terminal truncated fragment of the thrombin peptide
derivative having at least
eighteen amino acids can be used in the methods of the present invention.
A "C-terminal truncated fragment" refers to a fragment remaining after
removing an
19
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
amino acid or block of amino acids from the C-terminus. An "N-terminal
truncated fragment"
refers to a fragment remaining after removing an amino acid or block of amino
acids from the N-
425 terminus. It is to be understood that the terms "C-terminal truncated
fragment" and "N-terminal
truncated fragment" encompass acylation at the N-terminus and/or amidation at
the C-terminus,
as described above.
A preferred thrombin peptide derivative for use in the disclosed method
comprises the
amino acid sequence SEQ ID NO:2: Ala-Gly-Tyr-Lys-Pro-Asp-Glu-GIy-Lys-Arg-Gly-
Asp-Ala-
430 Cys-Xi-Gly-Asp-Ser-Gly-Gly-Pro-X2-Val. Another preferred thrombin peptide
derivative for use
in the disclosed method comprises the amino acid sequence of SEQ ID NO: 19:
Asp-Asn-Met-
Phe-Cys-Ala-Gly-Tyr-Lys-Pro-Asp-Glu-Gly-Lys-Arg-Gly-Asp-Ala-Cys-X 1-Gly-Asp-
Ser-Gly-
Gly-Pro-X2-Val-Met-Lys-Ser-Pro-Phe. Xi is Glu or Gln; X2 is Phe, Met, Leu, His
or Val. The
thrombin peptide derivatives of SEQ ID NO:2 and SEQ ID NO: 19 can optionally
comprise a C-
435 terminal amide and/or acylated N-terminus, as defined above. Preferably,
the N-terminus is free
(i.e., unsubstituted) and the C-terminus is free (i.e., unsubstituted) or
amidated, preferably as a
carboxamide (i.e., -C(O)NH2). Alternatively, N-terminal truncated fragments of
these preferred
thrombin peptide derivatives, the N-terminal truncated fragments having at
least fourteen amino
acids, or C-terminal truncated fragments of these preferred thrombin peptide
derivatives, the C-
440 terminal truncated fragments having at least eighteen amino acids, can
also be used in the
disclosed method.
TP508 is an example of a thrombin peptide derivative and is 23 amino acid
residues long,
wherein the N-terminal amino acid residue Ala is unsubstituted and the COOH of
the C-terminal
amino acid Val is modified to an amide represented by -C(O)NH2 (SEQ ID NO:3).
Another
445 example of a thrombin peptide derivative comprises the amino acid sequence
of SEQ ID NO:6,
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
wherein both N- and C-termini are unsubstituted ("deamide TP508"). Other
examples of
thrombin peptide derivatives which can be used in the disclosed method include
N-terminal
truncated fragments of TP508 (or deamide TP508), the N-terminal truncated
fragments having at
least fourteen amino acids, or C-terminal truncated fragments of TP508 (or
deamide TP508), the
450 C-terminal truncated fragments having at least eighteen amino acids.
As used herein, a "conservative substitution" in a polypeptide is the
replacement of an
amino acid with another amino acid that has the same net electronic charge and
approximately
the same size and shape. Amino acids with aliphatic or substituted aliphatic
amino acid side
chains have approximately the same size when the total number of carbon and
heteroatoms in
455 their side chains differs by no more than about four. They have
approximately the same shape
when the number of branches in their side chains differs by no more than one.
Amino acids with
phenyl or substituted phenyl groups in their side chains are considered to
have about the same
size and shape. Listed below are five groups of amino acids. Replacing an
amino acid in a
polypeptide with another amino acid from the same group results in a
conservative substitution:
460 Group I: glycine, alanine, valine, leucine, isoleucine, serine, threonine,
cysteine, and non-
naturally occurring amino acids with Cl-C4 aliphatic or Cl-C4 hydroxyl
substituted
aliphatic side chains (straight chained or monobranched).
Group II: glutamic acid, aspartic acid and non-naturally occurring amino acids
with
carboxylic acid substituted C1-C4 aliphatic side chains (unbranched or one
branch point).
465 Group III: lysine, ornithine, arginine and non-naturally occurring amino
acids with amine
or guanidino substituted C1-C4 aliphatic side chains (unbranched or one branch
point).
21
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
Group IV: glutamine, asparagine and non-naturally occurring amino acids with
amide
substituted Cl -C4 aliphatic side chains (unbranched or one branch point).
Group V: phenylalanine, phenylglycine, tyrosine and tryptophan.
470 As used herein, a "highly conservative substitution" in a polypeptide is
the replacement
of an amino acid with another amino acid that has the same functional group in
the side chain
and nearly the same size and shape. Amino acids with aliphatic or substituted
aliphatic amino
acid side chains have nearly the same size when the total number of carbon and
heteroatoms in
their side chains differs by no more than two. They have nearly the same shape
when they have
475 the same number of branches in the their side chains. Examples of highly
conservative
substitutions include valine for leucine, threonine for serine, aspartic acid
for glutamic acid and
phenylglycine for phenylalanine. Examples of substitutions which are not
highly conservative
include alanine for valine, alanine for serine and aspartic acid for serine.
480 Modified Thrombin Peptide Derivatives
In one embodiment of the invention, the thrombin peptide derivatives are
modified
relative to the thrombin peptide derivatives described above, wherein cysteine
residues of
aforementioned thrombin peptide derivatives are replaced with amino acids
having similar size
and charge properties to minimize dimerization of the peptides. Examples of
suitable amino
485 acids include alanine, glycine, serine, or an S'-protected cysteine.
Preferably, cysteine is replaced
with alanine. The modified thrombin peptide derivatives have about the same
biological activity
as the unmodified thrombin peptide derivatives. See Publication No. US
2005/0158301 Al,
22
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
which is hereby incorporated by reference.
It will be understood that the modified thrombin peptide derivatives disclosed
herein can
490 optionally comprise C-terminal amides and/or N-terminal acyl groups, as
described above.
Preferably, the N-terminus of a thrombin peptide derivative is free (i.e.,
unsubstituted) and the C-
terminus is free (i.e., unsubstituted) or amidated, preferably as a
carboxamide (i.e., -C(O)NH2).
In a specific embodiment, the modified thrombin peptide derivative comprises a
polypeptide having the amino acid sequence of SEQ ID NO:4: Arg-Gly-Asp-Ala-Xaa-
Xi-Gly-
495 Asp-Ser-Gly-Gly-Pro-X2-Val, or a C-terminal truncated fragment thereof
having at least six
amino acids. More specifically, the thrombin peptide derivative comprises the
amino acid
sequence of SEQ ID NO:20: Ala-Gly-Tyr-Lys-Pro-Asp-Glu-Gly-Lys-Arg-Gly-Asp-Ala-
Xaa-
Glu-Gly-Asp-Ser-Gly-Gly-Pro-Phe-Val or a fragment thereof comprising amino
acids 10-18 of
SEQ ID NO:20. Even more specifically, the thrombin peptide derivative
comprises the amino
500 acid sequence SEQ ID NO:5: Ala-Gly-Tyr-Lys-Pro-Asp-Glu-Gly-Lys-Arg-Gly-Asp-
Ala-Xaa-
Xi-Gly-Asp-Ser-Gly-Gly-Pro-X2-Val, or a fragment thereof comprising amino
acids 10-18 of
SEQ ID NO:5. Xaa is alanine, glycine, serine or an S-protected cysteine. Xi is
Glu or Gln and
X2 is Phe, Met, Leu, His or Val. Preferably Xi is Glu, X2 is Phe, and Xaa is
alanine. One
example of a thrombin peptide derivative of this type is a polypeptide having
the amino acid
505 sequence Ala-Gly-Tyr-Lys-Pro-Asp-Glu-Gly-Lys-Arg-Gly-Asp-Ala-Ala-Glu-Gly-
Asp-Ser-Gly-
Gly-Pro-Phe-Val (SEQ ID NO:21). A further example of a thrombin peptide
derivative of this
type is the polypeptide H-Ala-Gly-Tyr-Lys-Pro-Asp-Glu-Gly-Lys-Arg-Gly-Asp-Ala-
Ala-Glu-
Gly-Asp-Ser-Gly-Gly-Pro-Phe-Val-NH2 (SEQ ID NO:22). Another example of a
thrombin
peptide derivative of this type is the polypeptide H-Ala-Gly-Tyr-Lys-Pro-Asp-
Glu-Gly-Lys-Arg-
510 Gly-Asp-Ala-Ser-Glu-Gly-Asp-Ser-Gly-Gly-Pro-Phe-Val-NH2 (SEQ ID NO:30)
Zero, one, two
23
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
or three amino acids in the thrombin peptide derivative differ from the amino
acid at the
corresponding position of SEQ ID NO:4, 20, 5, 21 or 22, provided that Xaa is
alanine, glycine,
serine or an S-protected cysteine. Preferably, the difference is conservative
as defined below.
In another specific embodiment, the thrombin peptide derivative comprises a
polypeptide
515 having the amino acid sequence SEQ ID NO:23: Asp-Asn-Met-Phe-Xbb-Ala-Gly-
Tyr-Lys-Pro-
Asp-Glu-Gly-Lys-Arg-Gly-Asp-Ala-Xaa-Glu-Gly-Asp-S er-Gly-Gly-Pro-Phe-V al-Met-
Lys-Ser-
Pro-Phe, or a fragment thereof comprising amino acids 6-28. More preferably,
the thrombin
peptide derivative comprises a polypeptide having the amino acid sequence SEQ
ID NO:24:
Asp-Asn-Met-Phe-Xbb-Ala-Gly-Tyr-Lys-Pro-Asp-Glu-Gly-Lys-Arg-Gly-Asp-Ala-Xaa-X
i -Gly-
520 Asp-Ser-Gly-Gly-Pro-X2-Val-Met-Lys-Ser-Pro-Phe, or a fragment thereof
comprising amino
acids 6-28. Xaa and Xbb are independently alanine, glycine, serine or an S-
protected cysteine. Xi
is Glu or Gln and X2 is Phe, Met, Leu, His or Val. Preferably Xi is Glu, X2 is
Phe, and Xaa and
Xbb are alanine. One example of a thrombin peptide derivative of this type is
a polypeptide
comprising the amino acid sequence Asp-Asn-Met-Phe-Ala-Ala-Gly-Tyr-Lys-Pro-Asp-
Glu-Gly-
525 Lys-Arg-Gly-Asp-Ala-Ala-Glu-Gly-Asp-Ser-Gly-Gly-Pro-Phe-Val-Met-Lys-Ser-
Pro-Phe(SEQ
ID NO:25). A further example of a thrombin peptide derivative of this type is
the polypeptide H-
Asp-Asn-Met-Phe-Ala-Ala-Gly-Tyr-Lys-Pro-Asp-Glu-Gly-Lys-Arg-Gly-Asp-Ala-Ala-
Glu-Gly-
Asp-Ser-Gly-Gly-Pro-Phe-Val-Met-Lys-Ser-Pro-Phe-NH2 (SEQ ID NO:26). Zero, one,
two or
three amino acids in the thrombin peptide derivative can differ from the amino
acid at the
530 corresponding position of SEQ ID NO:23, 24, 25 or 26. Xaa and Xbb are
independently alanine,
glycine, serine or an S-protected cysteine. Preferably, the difference is
conservative as in
conservative substitutions of the thrombin peptide derivatives.
An "S-protected cysteine" is a cysteine residue in which the reactivity of the
thiol moiety,
24
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
-SH, is blocked with a protecting group. Suitable protecting groups are known
in the art and arc
535 disclosed, for example, in T. W. Greene and P. G. M. Wuts, Protective
Groups in Organic
Synthesis, 3`d Edition, John Wiley & Sons, (1999), pp. 454-493, the teachings
of which are
incorporated herein by reference in their entirety. Suitable protecting groups
should be non-
toxic, stable in pharmaceutical formulations and have minimum additional
functionality to
maintain the activity of the thrombin peptide derivative. A free thiol can be
protected as a
540 thioether, a thioester, or can be oxidized to an unsymmetrical disulfide.
Preferably the thiol is
protected as a thioether. Suitable thioethers include, but are not limited to,
S-alkyl thioethers
(e.g., CI-C5 alkyl), and S-benzyl thioethers (e.g, cysteine-S-S-t-Bu).
Preferably the protective
group is an alkyl thioether. More preferably, the S-protected cysteine is an S-
methyl cysteine.
Alternatively, the protecting group can be: 1) a cysteine or a cysteine-
containing peptide (the
545 "protecting peptide") attached to the cysteine thiol group of the thrombin
peptide derivative by a
disulfide bond; or 2) an amino acid or peptide ("protecting peptide") attached
by a thioamide
bond between the cysteine thiol group of the thrombin peptide derivative and a
carboxylic acid in
the protecting peptide (e.g., at the C-terminus or side chain of aspartic acid
or glutamic acid).
The protecting peptide can be physiologically inert (e.g., a polyglycine or
polyalanine of no more
550 than about fifty amino acids optionally interrupted by a cysteine), or can
have a desirable
biological activity.
Thrombin Peptide Derivative Dimers
In some aspects of the present invention, the thrombin peptide derivatives of
the methods
are thrombin peptide derivative dimers. See Publication No. US 2005/0153893,
which is hereby
555 incorporated by reference. The dimers essentially do not revert to
monomers and still have about
the same biological activity as the thrombin peptide derivatives monomer
described above. A
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
"thrombin peptide derivative dimer" is a molecule comprising two thrombin
peptide derivatives
linked by a covalent bond, preferably a disulfide bond between cysteine
residues. Thrombin
peptide derivative dimers are typically essentially free of the corresponding
monomer, e.g.,
560 greater than 95% free by weight and preferably greater than 99% free by
weight. Preferably the
polypeptides are the same and covalently linked through a disulfide bond.
The thrombin peptide derivative dimers of the present invention comprises the
thrombin
peptide derivatives described above. Specifically, thrombin peptide
derivatives have less than
about fifty amino acids, preferably less than about thirty-three amino acids.
Thrombin peptide
565 derivatives also have sufficient homology to the fragment of human
thrombin corresponding to
thrombin amino acid residues 508-530: Ala-Gly-Tyr-Lys-Pro-Asp-Glu-Gly-Lys-Arg-
Gly-Asp-
Ala-Cys-Glu-Gly-Asp-Ser-Gly-Gly-Pro-Phe-Val (SEQ ID NO:6) so that the
polypeptide
activates NPAR.
In a specific embodiment, each thrombin peptide derivative comprising a dimer
570 comprises a polypeptide having the amino acid sequence SEQ ID NO:1: Arg-
Gly-Asp-Ala-Cys-
Xi-Gly-Asp-Ser-Gly-Gly-Pro-X2-Val, or a C-terminal truncated fragment thereof
comprising at
least six amino acids. More specifically, each thrombin peptide derivative
comprises the amino
acid sequence of SEQ ID NO:6: Ala-Gly-Tyr-Lys-Pro-Asp-Glu-Gly-Lys-Arg-Gly-Asp-
Ala-Cys-
Glu-Gly-Asp-Ser-Gly-Gly-Pro-Phe-Val, or a fragment thereof comprising amino
acids 10-18 of
575 SEQ ID NO. 5. Even more specifically, the thrombin peptide derivative
comprises the amino
acid sequence SEQ ID NO:2: Ala-Gly-Tyr-Lys-Pro-Asp-Glu-Gly-Lys-Arg-Gly-Asp-Ala-
Cys-
Xi-Gly-Asp-Ser-Gly-Gly-Pro-X2-Val, or a fragment thereof comprising amino
acids 10-18 of
SEQ ID NO:2. Xi is Glu or Gln and X2 is Phe, Met, Leu, His or Val. Preferably
Xi is Glu, and
X2 is Phe. One example of a thrombin peptide derivative of this type is a
polypeptide comprising
26
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
580 the amino acid sequence Ala-Gly-Tyr-Lys-Pro-Asp-Glu-Gly-Lys-Arg-Gly-Asp-
Ala-Cys-Glu-
Gly-Asp-Ser-Gly-Gly-Pro-Phe-Val (SEQ ID NO:6). A further example of a thrombin
peptide
derivative of this type is a polypeptide having the amino acid sequence H-Ala-
Gly-Tyr-Lys-Pro-
Asp-Glu-Gly-Lys-Arg-Gly-Asp-Ala-Cys-Glu-Gly-Asp-Ser-Gly-Gly-Pro-Phe-Val-NH2
(SEQ ID
NO:3). Zero, one, two or three amino acids in the thrombin peptide derivative
differ from the
585 amino acid at the corresponding position of SEQ ID NO:6, 1, 2, or 3.
Preferably, the difference is
conservative as for conservative substitutions of the thrombin peptide
derivatives.
One example of a thrombin peptide derivative dimer of the present invention is
represented by Formula (IV):
gipp,
590 In another specific embodiment, each thrombin peptide derivative
comprising a dimer
comprises a polypeptide comprising the amino acid sequence SEQ ID NO:27: Ala-
Gly-Tyr-Lys-
Pro-Asp-Glu-Gly-Lys-Arg-Gly-Asp-Ala-Cys-Glu-Gly-Asp-Ser-Gly-Gly-Pro-Phe-V al-
Met-Lys-
Ser-Pro-Phe-Asn-Asn-Arg-Trp-Tyr, or a C-terminal truncated fragment thereof
having at least
twenty-three amino acids. More preferably, each thrombin peptide derivative
comprises the
595 amino acid sequence SEQ ID NO:28: Ala-Gly-Tyr-Lys-Pro-Asp-Glu-Gly-Lys-Arg-
Gly-Asp-
Ala-Cys-X1-Gly-Asp-Ser-Gly-Gly-Pro-X2-Val-Met-Lys-Ser-Pro-Phe-Asn-Asn-Arg-Trp-
Tyr, or
a C-terminal truncated fragment thereof comprising at least twenty-three amino
acids. Xi is Glu
27
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
or Gln and X2 is Phe, Met, Leu, His or Val. Preferably Xi is Glu, and X2 is
Phe. One example of
a thrombin peptide derivative of this type is a polypeptide comprising the
amino acid sequence
600 Ala-Gly-Tyr-Lys-Pro-Asp-Glu-Gly-Lys-Arg-Gly-Asp-Ala-Cys-Glu-Gly-Asp-Ser-
Gly-Gly-Pro-
Phe-Val-Met-Lys-Ser-Pro-Phe-Asn-Asn-Arg-Trp-Tyr (SEQ ID NO:27). A further
example of a
thrombin peptide derivative of this type is a polypeptide comprising the amino
acid sequence H-
Ala-Gly-Tyr-Lys-Pro-Asp-Glu-Gly-Lys-Arg-Gly-Asp-Ala-Cys-Glu-Gly-Asp-Ser-Gly-
Gly-Pro-
Phe-Val-Met-Lys-Ser-Pro-Phe-Asn-Asn-Arg-Trp-Tyr-NH2 (SEQ ID NO:29). Zero, one,
two or
605 three amino acids in the thrombin peptide derivative differ from the amino
acid at the
corresponding position of SEQ ID NO:27, 28 or 29. Preferably, the difference
is conservative as
defined for conservative substitutions of the thrombin peptide derivatives.
A "subject" is preferably a human, but can also be an animal in need of
treatment with a
thrombin peptide derivative disclosed herein, e.g., companion animals (e.g.,
dogs, cats, and the
610 like), farm animals (e.g., cows, pigs, horses and the like) and laboratory
animals (e.g., rats, mice,
guinea pigs and the like).
An "effective amount" is the quantity of the thrombin peptide derivative
described herein
that results in an improved clinical outcome of the condition being treated
with the thrombin
peptide derivative compared with the absence of treatment. The amount of the
thrombin peptide
615 derivative administered will depend on the degree, severity, and type of
the disease or condition,
the amount of therapy desired, and the release characteristics of the
pharmaceutical formulation.
It will also depend on the subject's health, size, weight, age, sex and
tolerance to drugs.
Typically, the thrombin peptide derivative is administered for a sufficient
period of time to
achieve the desired therapeutic effect. Typically, from about 1 g per day to
about 1 mg per day
620 of the thrombin peptide derivatives (preferably from about 5 g per day to
about 100 g per day)
28
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
is administered to the subject in need of treatment, especially for a local
means of administration.
The thrombin peptide derivatives can also be administered at a dose of from
about 0.1 mg/kg/day
to about 15 mg/kg/day, with from about 0.2 mg/kg/day to about 3 mg/kg/day
being preferred,
especially for systemic means of administration. Typical dosages for the
thrombin peptide
625 derivative of the invention are also 5-500 mg/day, preferably 25-250
mg/day, especially for
systemic means of administration.
In the methods described herein, the thrombin peptide derivative or
composition can be
administered before, during or after the radiation exposure. In the methods
described herein, the
peptide of the present invention can be administered in combination with an
angiogenic growth
630 factor. An "angiogenic growth factor" is a polypeptide which stimulates
the development of
blood vessels, e.g., promotes angiogenesis, endothelial cell growth, stability
of blood vessels,
and/or vasculogenesis. For example, angiogenic factors, include, but are not
limited to, e.g.,
VEGF-A and members of the VEGF family, P1GF, PDGF family, fibroblast growth
factor family
(FGFs), TIE ligands (Angiopoietins), ephrins, ANGPTL3, ANGPTL4, etc.
Angiogenic factors
635 also include polypeptides, such as growth hormone, insulin-like growth
factor-I (IGF-I), VIGF,
epidermal growth factor (EGF), CTGF and members of its family, and TGF-a and
TGF-(3.
"Treating" means that following a period of administering the thrombin peptide
derivative or composition comprising a thrombin peptide derivative, a
beneficial therapeutic
and/or prophylactic result is achieved, which can include a decrease in the
severity of symptoms
640 or delay in or inhibition of the onset of symptoms, increased longevity
and/or more rapid or more
complete resolution of the disease or condition, or other improved clinical
outcome as measured
according to the site that is being observed or the parameters measured for a
particular disease or
disorder.
29
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
"Reducing the risk" refers to decreasing the probability of developing a
disease, disorder
645 or medical condition, in a subject, wherein the subject is, for example, a
subject who is at risk for
developing the disease, disorder or condition.
"Reducing radiation related injury" refers to a decrease in the severity of
injuries induced
by radiation exposure.
The disclosed thrombin peptide derivative can be administered by any suitable
route,
650 locally (e.g., topically) or systemically, including, for example, by
parenteral administration.
Parenteral administration can include, for example, intramuscular,
intravenous, subcutaneous, or
intraperitoneal injection or vascular administration, and can also include
transdermal patch and
implanted slow-release devices such as pumps. Topical administration can
include, for example,
creams, gels, ointments or aerosols. Respiratory administration can include,
for example,
655 inhalation or intranasal drops. For certain indications, it is
advantageous to inject or implant the
thrombin peptide derivative directly to the treatment site. The thrombin
peptide derivative can be
advantageously administered in a sustained release formulation. The thrombin
peptide derivative
can be administered chronically, wherein the peptide derivative is
administered over a long
period of time (at least 60 days, but more typically, for at least one year),
at intervals or by a
660 continuous delivery method, to treat a chronic or recurring disease or
condition.
The thrombin peptide derivative can be administered to the subject in
conjunction with an
acceptable pharmaceutical carrier as part of a pharmaceutical composition. The
formulation of
the pharmaceutical composition will vary according to the route of
administration selected.
Suitable pharmaceutical carriers may contain inert ingredients which do not
interact with the
665 compound. The earners should be biocompatible, i.e., non-toxic, non-
inflammatory, non-
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
immunogenic and devoid of other undesired reactions at the administration
site. Examples of
pharmaceutically acceptable carriers include, for example, saline, aerosols,
commercially
available inert gels, or liquids supplemented with albumin, methyl cellulose
or a collagen matrix.
Standard pharmaceutical formulation techniques can be employed, such as those
described in
670 Remington's Pharmaceutical Sciences, Mack Publishing Company, Easton, PA.
Other suitable
pharmaceutical carriers include those described in U.S. Patent No. 7,294,596,
the entire teaching
of which is incorporated herein by reference.
The compositions used in the methods of the present invention can additionally
comprise
a pharmaceutical carrier in which the thrombin peptide derivative is dissolved
or suspended.
675 Examples of pharmaceutically acceptable carriers include, for example,
saline, aerosols,
commercially available inert gels, or liquids supplemented with albumin,
methyl cellulose or a
collagen matrix. Typical of such formulations are gels. Gels are comprised of
a base selected
from an oleaginous base, water, or an emulsion-suspension base, as previously
described. To the
base is added a gelling agent that forms a matrix in the base, increasing its
viscosity to a
680 semisolid consistency. Examples of gelling agents are hydroxypropyl
cellulose, acrylic acid
polymers, and the like. The active ingredients are added to the formulation at
the desired
concentration at a point preceding addition of the gelling agent or can be
mixed after the gelation
process.
Injectable delivery formulations may be administered intravenously or directly
at the site
685 in need of treatment. The injectable carrier may be a viscous solution or
gel.
Delivery formulations include physiological saline, bacteriostatic saline
(saline
containing about 0.9% mg/mL benzyl alcohol), phosphate-buffered saline, Hank's
solution,
31
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
Ringer's-lactate, or liquids supplemented with albumin, methyl cellulose, or
hyaluronic acid.
Injectable matrices include polymers of poly(ethylene oxide) and copolymers of
ethylene and
690 propylene oxide (see Cao et al, J. Biomater. Sci 9:475 (1998) and Sims et
al, Plast Reconstr.
Surg. 98:843 (1996), the entire teachings of which are incorporated herein by
reference).
Methods for encapsulating compositions (such as in a coating of hard gelatin
or
cyclodextran) are known in the art (Baker, et al, "Controlled Release of
Biological Active
Agents", John Wiley and Sons, 1986).
695 Ointments are typically prepared using an oleaginous base, e.g.,
containing fixed oils or
hydrocarbons, such as white petrolatum or mineral oil, or an absorbent base,
e.g., consisting of
an absorbent anhydrous substance or substances, for example anhydrous lanolin.
Following
formation of the base, the active ingredients are added in the desired
concentration.
Creams generally comprise an oil phase (internal phase) containing typically
fixed oils,
700 hydrocarbons, and the like, such as waxes, petrolatum, mineral oil, and
the like, and an aqueous
phase (continuous phase), comprising water and any water-soluble substances,
such as added
salts. The two phases are stabilized by use of an emulsifying agent, for
example, a surface active
agent, such as sodium lauryi sulfate; hydrophilic colloids, such as acacia
colloidal clays, beegum,
and the like. Upon formation of the emulsion, the active ingredients are added
in the desired
705 concentration.
Gels contain a base selected from an oleaginous base, water, or an emulsion-
suspension
base, as previously described. To the base is added a gelling agent which
forms a matrix in the
base, increasing its viscosity to a semisolid consistency. Examples of gelling
agents are
hydroxypropyl cellulose, acrylic acid polymers, and the like. The active
ingredients are added to
32
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
710 the formulation at the desired concentration at a point preceding addition
of the gelling agent.
Diseases and conditions that are treatable with the disclosed thrombin peptide
derivatives
are often accompanied by symptoms and infirmities such as pain and infection.
In certain
instances it may be advantageous to co-administer one or more additional
pharmacologically
active agents along with a thrombin peptide derivative to address such issues.
For example,
715 managing pain and inflammation may require co-administration with
analgesic or an anti-
inflammatory agents. Managing infection may require co-administration with
antimicrobial,
antibiotic or disinfectant agents.
A thrombin peptide derivative can be administered to a subject alone or in
combination
with one or more other therapeutics, for example, a cholesterol-lowering
agent, an anti-
720 hypertensive agent, a beta-blocker, an anti-coagulant, a thrombolytic
agent, an analgesic, an anti-
inflammatory agent, an anti-plaque agent, insulin, a nitric oxide generating
agent, an antiviral
agent or an antibiotic. In one method, a thrombin peptide derivative can be
administered to a
subject in combination with arginine, for example, with arginine as an oral
nutritional
supplement.
725 Thrombin peptide derivatives and modified thrombin peptide derivatives can
be
synthesized by solid phase peptide synthesis (e.g., BOC or FMOC) method, by
solution phase
synthesis, or by other suitable techniques including combinations of the
foregoing methods. The
BOC and FMOC methods, which are established and widely used, are described in
Merrifield, J.
Am. Chem. Sot: 88:2149 (1963); Meienhofer, Hormonal Proteins and Peptides,
C.H. Li, Ed.,
730 Academic Press, 1983, pp. 48-267; and Barany and Merrifield, in The
Peptides, E. Gross and J.
Meienhofer, Eds., Academic Press, New York, 1980, pp. 3-285. Methods of solid
phase peptide
33
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
synthesis are described in Merrifield, R.B., Science, 232: 341 (1986);
Carpino, L.A. and Han,
G.Y., J. Org. Chem., 37: 3404 (1972); and Gauspohl, H. et al, Synthesis, J:
315 (1992)). The
teachings of these six articles are incorporated herein by reference in their
entirety.
735 Thrombin peptide derivative dimers can be prepared by oxidation of the
monomer.
Thrombin peptide derivative dimers can be prepared by reacting the thrombin
peptide derivative
with an excess of oxidizing agent. A well-known suitable oxidizing agent is
iodine.
A "non-aromatic heterocyclic group" as used herein, is a non-aromatic
carbocyclic ring
system that has 3 to 10 atoms and includes at least one heteroatom, such as
nitrogen, oxygen, or
740 sulfur. Examples of non-aromatic heterocyclic groups include piperazinyl,
piperidinyl,
pyrrolidinyl, morpholinyl, thiomorpholinyl.
An "alkyl" is a straight chain or branched saturated hydrocarbon radical.
Typically, an
alkyl group has from 1 to about 10 carbon atoms, preferably from 1 to about 4
carbon atoms.
Exemplary alkyl groups include, but are not limited to, methyl, ethyl, n-
propyl, iso-propyl, n-
745 butyl, sec-butyl, tert-butyl, pentyl, cyclopentyl, hexyl, cyclohexyl,
octyl and cyclooctyl.
The invention is illustrated by the following examples which are not intended
to be
limiting in any way.
Example 1. Effects of TP508 on apoptosis of human microvasular endothelial
cell
(HMVEC) exposed to radiation
750 Human dermal microvascular endothelial cells (HMVEC) were irradiated using
a J.L.,
Shepherd & Associates, Mark 1, 9K, 137Cs Gamma Irradiator to deliver exposures
of 5, 10, and
20 Gy. Following irradiation, cells received 30 a-g/ml TP508 or saline (No
Treatment). After 3h
34
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
cells were assayed for activation of caspase-3 and caspase-7 as a measure of
apoptosis using
Caspase-Glo 3/7 Assay (Promega, Madison, WI). As shown in Figure 1, radiation
caused a
755 dose-dependent increase in Caspase 3/7 activity. TP508 treatment of these
cells, however,
significantly decreased radiation-induced activation of Caspase 3/7. This data
demonstrates that
TP508 attenuates apoptosis in microvascular endothelial cells induced by
radiation.
Example 2. Effect of TP508 on mouse survival following 8 Gy radiation
exposure.
Swiss ICR mice were irradiated (137Cs Gamma Irradiator Mark 30, Shephard and
760 Associates, San Fernando, CA) with exposures of 8 Gy or 3 Gy. After 4
hours or 24 hours, mice
were anesthesized and prepared for surgery. A single 1.5 cm square full dermal
excision was
created and treated topically with saline (25 l) or saline plus TP508 (0.25
g) and covered with
Opsite occlusive dressing. There was a significant decrease in survival in
mice that have
sustained radiation combined injuries (Table 1 below). Significant increase in
survival was
765 observed when mice were injected with TP508 24 hours after radiation
exposure (see Table 1).
Increase in survival was also observed in mice receiving topical treatment of
wounds with TP508
(Table 1 and Figure 2).
Table I. Effect of TP508 on Survival of Mice with Radiation Combined Injuries
Group Treatment Mean St. Median 30-day Significance
Survival Dev. Survival survival
(days) (days) (%)
0 Gy + Saline 30.0 0 Undefined >30 100 1
Wound
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
8 Gy + Saline 13.4 6.0 11 10 2,3
Wound
8 Gy + TP508 19.1 9.1 14 37 3
Wound Topical
8 Gy + TP508 I.V. 25.2 7.5 Undefined >30 67 2
Wound
8 Gy none 21.3 9.3 15 40 1
770
1. Significant decrease in survival when radiation is combined with wounds (p
= 0.0334)
2. Significant increase in survival when mice injected with TP508 24 hr after
radiation exposure (p = 0.0014)
3. Increase in survival after treatment of wounds topically (NS, p = 0.1406))
Example 3. Effect of TP508 on mouse survival following radiation exposure to
the lethal
dose of 12 Gy.
Mice were exposed to a lethal dose of 137Cs gamma irradiation (12 Gy).
Injection of a
775 single bolus dose of TP508 (500 g) within 2 hours after exposure delayed
the mortality of the
first mouse in the treated group by about 3 days and increased the group mean
survival time by
about 15%. (See Figure 3). TP508 has a short half-life and may thus only be
present in blood at
an effective concentration for the first two to three hours. This may explain
why it only extends
survival for a few days.
780 Example 4. Effect of TP508 on bacterial growth in blood of animals post
irradiation
Lethal doses of radiation often cause death due to breakdown of the intestinal
wall and
septic infection leading to death. Therefore, the effect of TP508 to delay the
onset of bacterial
36
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
septic infection in irradiated mice was determined. Blood was drawn from
irradiated mice (see
Example 3) at various days after irradiation. Blood from each mouse (3 mice
per group) was then
785 diluted and cultured to determine the number of live bacteria quantified
as colony forming units
(CPU) per ml of blood. As shown in Figure 4, by day 6 post irradiation (PI),
live bacteria were
present in the blood of irradiated placebo-treated mice, but not from TP508-
treated mice. By day
7 the placebo-treated mice had an average of 1.6 x 106 CFU/ml while those
injected with TP508
were just beginning to show infection, with an average of just over 100
CFU/ml.
790 Example 5. Effect of TP508 on healing of open dermal wounds
Swiss ICR mice were irradiated (137Cs Gamma Irradiator Mark 30, Shephard and
Associates, San Fernando, CA) with exposures of 8 Gy or 3 Gy. After 4 hours or
24 hours, mice
were anesthesized and prepared for surgery. A single 1.5 cm square full dermal
excision was
created and treated topically with saline (25 ul) or saline plus TP508 (0.3
ug) and covered with
795 Opsite occlusive dressing. At 8 Gy, radiation delayed wound healing in
mice receiving dermal
wounds 4 hours after irradiation, but a single topical treatment with TP508
accelerated healing.
The time to 50% wound closure of these wounds was 9.2 days for non-irradiated
control; 13.0
days for 8 GY plus saline; and 8.9 days for 8 Gy plus TP508. Thus, TP508
appears to restore
normal rates of healing to irradiated mice. This was confirmed by calculating
the linear rate of
800 healing in these wounds (See Figure 5). Interestingly, the linear rates of
healing for all groups
was similar during the first 5 days after wounding, perhaps due to contraction
that was not
affected by radiation. From 5 to 16 days, however, radiation significantly
impairs healing, but
TP508 treatment overcomes this impairment.
In a second set of experiments, mice with 3 Gy exposures underwent dermal
wound
37
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
805 surgery 24 hours after irradiation. These wounds also demonstrated delayed
healing relative to
non-irradiated control mice. As with 8 Gy exposure experiments, TP508 topical
treatment
accelerated healing to overcome the effect of radiation. In these experiments,
we also evaluated
effects of post-exposure IV injection of TP508 on wound closure. An IV
injection of TP508
about 20 hours prior to wound injury also accelerated wound closure and tended
to close wounds
810 slightly faster than topical treatment. This slight difference is also
seen in comparisons of the
rates of linear wound healing between non-irradiated control, 3 Gy Saline
Control, 3 Gy topical
TP508 and 4 Gy IV TP508. The combination of IV and topical TP508 treatment did
not appear
to be different than IV treatment alone.
Example 6. Effect of TP508 on apoptosis and proliferation and migration of
intestinal
815 crypt progenitor cells.
At 5 days post-exposure, histological sections of jujenum taken from mice
exposed to 12
Gy whole body irradiation contain a large number of apoptotic cells within the
intestinal cryps,
as determined by tunnel staining. Mice injected with TP508 appear to have
fewer apoptotic
cells. This effect of TP508 was confirmed by measuring EdU incorporation (DNA
synthesis) at
820 2 and 12 days post-exposure, visualizing cells that synthesized DNA during
a 24-hour incubation
period with Click IT . With increasing radiation exposure fewer crypt cells
continue to
proliferate after 2 days. In contrast, with TP508 injection the number of
cells proliferating and
migrating out of the crypt with 3 Gy exposure is equivalent to non-irradiated
(0 Gy) controls. In
the 8 Gy sections, approximately the same number of cells are labeled, but in
the TP508 group,
825 cells tend to migrate farther up into the villi. Even after 15 Gy
exposures, some crypt cells
continue to proliferate in cryps of animals treated with TP508. Even 12 days
after 8 Gy
exposures there is decreased crypt cells proliferation and migration, yet in
animals injected with
38
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
TP508, the proliferation and migration appears to be fully restored to control
levels.
Example 7. Effect of TP508 on radiation and RCI-induced up-regulation of IL-6.
830 ICR white male mice were exposed to 0 Gy (control) or 8 Gy of gamma
radiation and
wounded 24 hours later. Wounds were treated topically with saline placebo (P)
or TP508 in
saline (TPt). A separate group of mice were injected IV with 500 micrograms of
TP508 2 hours
post 8 Gy exposure. After eleven (11) days, serum from mice was analyzed for
amount of IL-6A
using enzyme-linked immunosorbent assay (ELISA) (Figure 6, Panel A).
835 ICR white male mice were exposed to 0 Gy, or 12 Gy nuclear irradiaton
without wounds
and were injected IV with placebo or TP508 post-exposure. Serum was isolated
from mice seven
(7) days later and the amount of IL-6 was determined by enzyme-linked
immunosorbent assay
(ELISA) (Figure 6, Panel B).
The combination of 8Gy radiation exposure and wounding increases IL-6 levels
above
840 wounding alone. Topical TP508 treatment of wounds reduces IL-6 levels by -
75%. Systemic
IV injection of TP508 reduces IL-6 levels by more than 90%. l2Gy exposure
alone without
wounds also increases IL-6 levels. TP508 injection reduces IL-6 production
measured at day 7
by approximately 50%.
Since IL-6 increases correlate with mortality and initiation of systemic
inflammatory
845 response syndrome (SIRS) these results demonstrate that TP508 reduces
systemic inflammatory
response syndrome that was initiated by radiation or radiation combined with
injury.
Example 8. Effect of TP508 on endothelial function as demonstrated by aortic
explant
endothelial cell sprouting assays.
39
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
To determine whether TP508 helped maintain endothelial function, an
established
850 angiogenesis assay was used. In these experiments mice were either non-
irradiated or given
exposures of 3 Gy, 8 Gy or 10 Gy. Approximately 2 hours post-exposure mice
were injected IV
with saline or saline plus TP508 (15 mg/kg). Mice were sacrificed 24 hours
after exposure,
aortas removed and aortic segments were placed on matrigel and cultured in
endothelial
growth medium with growth supplement containing VEGF and FGF2 for 5 days. In
the non-
855 irradiated controls (0 Gy), TP508 more than doubled the amount of
endothelial sprouting from
the aortic segments during 5 day incubations as determined by measuring area
occupied by
sprouts or longest sprout projections. Aortic segment explants from 3 Gy
exposed mice had
some sprouting in the saline injected group, but again this sprouting was more
than doubled in
mice injected with TP508. In the 8 Gy and 10 Gy groups there was virtually no
sprouting from
860 aortic segments isolated from placebo mice, while visible sprouting
continued to be observed at
the edges of explants from T508-treated mice.
Example 9. Effect of TP508 on hematopoietic recovery and increases
proliferation of bone
marrow progenitor cells.
The bone marrow was isolated from non-exposed and mice exposed to 8 Gy with or
865 without TP508 post-exposure injection. The samples were subjected to the
complete blood count
(CBC) analysis, which demonstrated earlier recovery of leukocyte, erythrocyte
and thrombocyte
numbers in mice treated with TP508. This result suggests that TP508 stimulates
hematopoiesis
or protects bone marrow cells (BMCs).
Histology of bone marrow 8 days after exposure to 8 Gy shows depletion of BMCs
in
870 marrow of 8 Gy exposed mice relative to 0 Gy mice. TP508 treatment of
these mice increases
CA 02802176 2012-12-10
WO 2011/156729 PCT/US2011/040006
number and density of BMCS.
This finding was confirmed by EdU incorporation (DNA synthesis). At 12 days
post-
exposure, there is some new proliferation of BMCs representing a limited
degree of recovery in 8
Gy exposed mice. In TP508-treated 8 Gy mice, 3-5 times more proliferating BMCs
were
875 observed. Thus, a single post-exposure injection of TP508 initiates a
cascade of events that has
restorative properties for BMCs.
41