Note: Descriptions are shown in the official language in which they were submitted.
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
A SYSTEMS APPROACH TO DISEASE STATE, HEALTH, AND
COMORBIDITY ASSESSMENT
By
Ivan Osorio
This application claims priority from United States patent application
12/816,357,
entitled "A Systems Approach to Disease State and Health Assessment," filed
June 15, 2010;
and United States patent application 12/816,348, entitled "A Systems Approach
to
Comorbidity Assessment," filed June 15, 2010.
1. FIELD OF THE DISCLOSURE
This disclosure relates generally to medical device systems and, more
particularly, to
medical device systems and methods capable of assessing a state of a disease.
2. DESCRIPTION OF THE RELATED ART
Diseases or disorders and their treatments, if available, often have a
deleterious
impact on the patient's overall health and well being. Traditionally, medical
practice focuses
its diagnostic and therapeutic efforts to the specific disorder (primary
diagnosis), an approach
that while necessary and, in at least certain ways, useful, ignores the "spill-
over" effect of the
disease or treatment on the patient's overall health and well being. By
compartmentalizing
diseases, a reductionist practice, medicine as presently practiced ignores the
substantial and
fundamental loss of information inherent to this approach. That is,
fragmenting into their
constituent parts complex, non-linear network systems (of which the human body
is a
paradigm) so as to facilitate their study. This fragmentation will often lead
to imprecise and
distorted findings because the assembly of systems (which determines overall
health) is
greater than the sum of its parts. This viewpoint central to general systems
theory applies to
1
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
various embodiments of the present disclosure. For example, embodiments
disclosed herein
illustrate how the deleterious impact on the patient as a whole is a function
of the disease
type, its rate of progression, severity, and duration, and of the type of
therapy and its dose.
Clearly, the impact is often more severe or complicated when the patient has
multiple disease
types or several therapies.
Various elements of the system interact through interconnected feed-back and
feed-
forward loops. Figure 16, for example, illustrates the complex nature of the
primary disease
of obesity where feed-forward and feed-back loops can accelerate the
progression of the
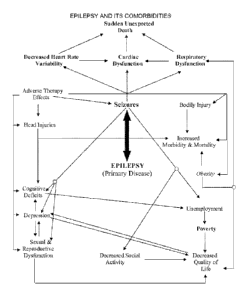
disease. Figure 17 provides a similar example for the primary disease of
epilepsy.
Embodiments of the present disclosure utilize systems theory's tenets by
recognizing and
incorporating a primary disease or disorder with its co-morbidities. Numerous
embodiments
of the present disclosure demonstrate how largely positive feed-forward and
feed-back loops
between diseases or disorders and their expected mutually amplifying effects
result in wide
variations of disease/disorder progression or regression. Further, the
potential for
transmutability and context-dependency of whether a disorder or disease is a
primary one or a
co-morbibity is apparent through the example of how obesity may become a co-
morbidity in
subjects treated for epilepsy.
Obesity and its co-morbidities together provide a non-limiting example that
illustrates
how undesired "spillover" effects from a primary disease or condition under
consideration
can negatively impact the patient's health. Obesity is a disorder of epidemic
proportions in
the US, and substantially increases the patient's risk of developing diabetes
mellitus, arterial
hypertension, hyperlipidemia and obstructive sleep apnea, while shortening
life span and
degrading quality of life. Arterial hypertension, diabetes and hyperlipidemia,
in turn,
accelerate atherosclerosis, which further increases the risks for myocardial
infarction, stroke,
.. congestive heart failure, and avascular gangrene. Similarly, obstructive
sleep apnea causes
2
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
intractable arterial hypertension, atrial fibrillation, cognitive
deterioration, depression, sexual
dysfunction, and chronic headaches.
This exemplary list of a primary disorder (e.g., obesity, Figure 16) and its
"co-
morbidities" illustrates how the human body may be considered as a densely
interconnected
(bidirectional) network of "nodes" (body systems and/or organs), stressing the
dependence of
its complex dynamics on the integrity of each of its component "nodes". The
interactions
among the nodes may be viewed as feed-forward or feedback loops that under
pathological
conditions have an amplifying effect or "positive" (e.g., positive feedback)
effect on
themselves and on the network. A "systems" approach to human disease which
would
acknowledge the anatomical and functional interconnections one organ or body
system's
function has on the others, is lacking in the field of health care, to the
detriment of quality of
care and cost-effectiveness. Such a "systems" approach, exemplified by
embodiments of the
present disclosure, provides for the systematic and reproducible means of
automatically
tracking the evolution of the primary disease or disorder (e.g., obesity) and
of its co-
morbidities (e.g., hypertension, diabetes, sleep apnea, etc.) to identify or
preferably anticipate
their contribution to any change in the patient's overall health. Embodiments
of the
disclosure may be used either to prevent the emergence of co-morbidities, to
ameliorate their
deleterious impact, and/or to improve or stabilize them. As a result, it is
possible to improve
or preserve health and quality of life of the patient while lessening the
upwardly spiraling
costs of health care.
While many diseases, their co-morbidities, or side effects of treatment may
negatively
affect the general health and well-being of a patient, only a handful of other
examples will be
furnished herein. Such examples should not be construed as limiting the scope
of disclosure
but, on the contrary, merely to serve as examples underscoring the widespread
applicability
and usefulness of the disclosure.
3
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
Epilepsy affects approximately 60 million people worldwide of whom roughly 23
million are resistant to multiple medications. Pharmaco-resistant seizures are
associated with
an increase in mortality and morbidity rates (compared to the general
population and to
epileptics whose seizures are controlled by medications) eventual impairment
of cognitive
functions and mental health and with markedly degraded quality of life for
patients and their
families. Seizures may impair motor control, responsiveness to a wide class of
stimuli, and
other cognitive functions. The sudden onset of a patient's impairment of motor
control,
responsiveness, and other cognitive functions precludes the performance of
necessary and
even simple daily life tasks such as driving a vehicle, cooking, or operating
machinery, as
well as more complex tasks such as acquiring knowledge and socializing. In the
USA alone,
the annual cost of epilepsy care is over USD 15 billion (in 1995 dollars),
most of which is
attributable to subjects with pharmaco-resistant seizures. Certain
pharmacological agents
used for treatment of epilepsy cause osteoporosis, reproductive dysfunction,
liver, bone
marrow, kidney, and skin damage, neurologic and psychiatric dysfunction,
weight gain and in
rare cases, death. Various epilepsy therapies, such as thermal manipulation of
epileptogenic
tissue or local/direct delivery of drugs to it, may also cause adverse
effects, such as
neurologic, autonomic, psychiatric, sleep, appetite, sex drive, and other
disturbances.
Diabetes mellitus, a highly prevalent disease, causes autonomic dysfunction,
accelerates atherosclerosis and with it the incidence of heart attacks,
strokes and avascular
gangrene and has a negative impact on quality of life and mental health. In
patients with
juvenile or Type I diabetes, the physiological responses to counteract
hypoglycemia (a
common adverse effect of insulin) become blunted or absent making this serious
condition
asymptomatic. That is, the symptoms that make a person aware that blood sugar
is low¨ and
which motivate or compel the person to eat¨ do not occur in these patients,
considerably
increasing the risks of brain damage and death due to hypoglycemia.
4
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
Parkinson's is a disease of the brain's basal ganglia, whose main function is
modulation of posture and movements. Parkinson's is associated with autonomic
dysfunction
and increased risks of injury, dementia, and head and bodily injuries caused
by falls.
Autonomic dysfunction, in turn, increases the risk of cardiac arrhythmias,
syncope, and
death, with falls enhancing this risk. These selected examples underscore the
importance of
developing and implementing what is referred herein as a systems approach to
diseases,
where the disease in question and its impact on the other body organs and
functions (co-
morbidities), are assessed as a function of time and space-state and this
information is used
for early intervention so as to prevent deterioration and further
disabilities.
Co-morbidities are common with many other disorders or diseases, further
imposing
psycho-social and/or financial burdens on the patient, the patient's family,
and society in
general, and worsening quality of life. Other costly and highly burdensome
diseases or
disorders include cardiovascular disorders (such as congestive heart failure
and atrial
fibrillation), respiratory disorders such as chronic obstructive pulmonary
disease, depression
and other mood disorders, schizophrenia, anxiety disorders and other
neuropsychiatric
disorders, neuro-degenerative diseases such as Alzheimer's, traumatic brain
injury, migraine
headache, eating disorders (such as obesity, anorexia nervosa, and bulimia),
sleep disorders,
hypertension, and pain (including neuropathic pain and fibromyalgia).
Regardless of the disease in question, it would be useful to assess the
evolution of the
patient's primary disease and comorbidities through characterization of their
direction
(progression, regression, or stabilization), magnitude, and rate of change and
comorbidities,
since disease progression is usually correlated with emergence of new or
worsening of
existing comorbidities, further degrading the patient's health and well-being.
For example,
certain types and severities of epilepsy are associated with changes (compared
to the general
population) in the occurrence of sudden unexpected death (SUDEP), serious
accidents, or
5
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
other fatal events, such as suicide. It would be useful to assess the clinical
evolution of a
patient's epilepsy and its co-morbidities to determine the type(s) of risk(s)
and their
probability of occurrence to either revert the trend, if possible, and if not,
to institute
measures to minimize and manage those risks. No automated system for making
this
assessment, determination, and risk management is known to this inventor at
this time.
Although treatment options for many diseases exist, the efficacy of a
particular
treatment option for a particular disease in a particular patient may be
unpredictable. Further,
the efficacy of a particular treatment option for a particular disease in a
particular patient may
be difficult to gauge, and further, such gauging may be subjectively
determined by the
patient, the physician, or a combination thereof Also, a particular treatment
option for a
particular disease in a particular patient may lead to various side effects,
some of which may
be difficult to gauge.
It would be desirable to have methods and apparatus to reproducibly and in a
clinically useful and cost-effective manner: a) assess a state of a disease of
a patient, such as a
disease that impacts, directly or indirectly, a neurological, autonomic,
endocrinologic, or
psychiatric disease and extent to which they impair overall health and well
being via the
emergence of "co-morbidities"; b) assess the therapies' side effects; c)
improve or stabilize a
existing disease and prevent the emergence of co-morbidities; d) prevent or
ameliorate
adverse treatment effects. The direct clinical and psychosocial benefits to
patients and the
ensuing decrease in the financial burden to the health care system and society
of successfully
implementing said automated comprehensive assessment are readily apparent. The
state of
the art lacks an efficient, systematic, and user-friendly automated system for
making this
assessment. Desirably, an apparatus used in assessment would be implantable or
portable
and operate in real-time or off-line. It would also be desirable for such an
apparatus to
incorporate and analyze, for purposes of assessing disease state, data
obtained using other
6
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
diagnostic devices including those that are not portable, implantable, or
implementable into
hardware or software. The assessment may be made by an apparatus automatically
and this
either contingently (e.g., triggered by a large or sudden change in an index,
or by a patient's
or caregiver command) or at predetermined times; the assessment may be also
made based on
clinical judgment. The assessment may be quantitative (magnitude and rate),
semiquantitative
(questionnaires, subjective scales), or qualititative (e.g. small, slow, etc).
SUMMARY OF THE DISCLOSURE
In one embodiment, the present disclosure provides a method for assessing a
primary
disease state. In one embodiment, the method comprises receiving at least one
autonomic
index; receiving at least one neurologic index; comparing the at least one
autonomic index to
at least a first reference value associated with the at least one autonomic
index; comparing the
at least one neurologic index to at least a second reference value associated
with the at least
one neurologic index; assessing a state of a primary disease of a patient
based on the
comparing; and providing an output relating to the assessment, the output
comprising at least
one of disease stability, disease progression, disease regression, or a
finding that a disease
state cannot be determined.
In one embodiment, the present disclosure provides a method for assessing a
patient's
health. In one embodiment, the method comprises receiving at least one
assessment of at
least one of a patient's primary disease state, a quality of life, or a
physical fitness; comparing
the at least one assessment to at least one reference value associated with at
least one
previous assessment from the patient or with normative data; assessing at
least one of the
patient's primary disease state, quality of life, or physical fitness based on
the comparing; and
providing an output relating to an assessment of the patient's health, wherein
the output
comprises at least one of a disease state stability, disease state
progression, disease state
regression, a finding that the disease state cannot be determined, quality of
life stability,
7
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
quality of life improvement, quality of life decline, a finding that the
quality of life cannot be
determined, physical fitness stability, physical fitness improvement, physical
fitness decline,
or a finding that physical fitness cannot be determined.
In one embodiment, the present disclosure provides a method for assessing a
comorbidity associated with a primary disease. In one embodiment, the method
comprises
receiving at least one of an autonomic index, a neurologic index, a stress
marker index, a
psychiatric index, an endocrine index, a physical fitness index, or a quality
of life index of a
patient; comparing the at least one index to at least one reference value
associated with the at
least one index; assessing a state of a body system of the patient based on
the comparing,
wherein the body system comprises at least one of an autonomic system, a
neurologic system,
a psychiatric system, an endocrine system, or subsystems of the foregoing; and
providing an
output relating to the assessment, wherein the output comprises at least one
of body system
stability, body system improvement, body system regression, or a finding that
a state of the
body system cannot be determined, wherein the body system is a site of the
comorbidity.
In one embodiment, the present disclosure provides a computer readable program
storage medium encoded with instructions that, when executed by a computer,
perform a
method as described above.
BRIEF DESCRIPTION OF THE DRAWINGS
The disclosure may be understood by reference to the following description
taken in
conjunction with the accompanying drawings, in which like reference numerals
identify like
elements, and in which:
Figure 1 illustrates a flowchart depiction of a method for assessing a state
of a
patient's disease, in accordance with an illustrative embodiment of the
present disclosure;
8
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
Figure 2 illustrates a flowchart depiction of a method for assessing a state
of a
patient's disease, in accordance with an illustrative embodiment of the
present disclosure;
Figure 3A provides a stylized diagram of a medical device implanted into a
patient's
body, in accordance with one illustrative embodiment of the present
disclosure;
Figure 3B provides a stylized diagram of a medical device implanted into a
patient's
body, in accordance with one illustrative embodiment of the present
disclosure;
Figure 3C provides a stylized diagram of a medical device implanted into a
patient's
body, in accordance with one illustrative embodiment of the present
disclosure;
Figure 4 is a block diagram of an implantable medical device system, in
accordance
with one illustrative embodiment of the present disclosure;
Figure 5 is a block diagram of a medical device system that includes a medical
device
and an external unit, in accordance with one illustrative embodiment of the
present
disclosure;
Figure 6 is a stylized block diagram of an autonomic index unit of a medical
device or
medical device system, in accordance with one illustrative embodiment of the
present
disclosure;
Figure 7 is a stylized block diagram of a neurologic index unit of a medical
device or
medical device system, in accordance with one illustrative embodiment of the
present
disclosure;
Figure 8 is a stylized block diagram of a kinetic capability determination
unit of a
medical device or medical device system, in accordance with one illustrative
embodiment of
the present disclosure;
Figure 9 is a stylized block diagram of a stress marker index unit of a
medical device
or medical device system, in accordance with one illustrative embodiment of
the present
disclosure;
9
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
Figure 10 is a stylized block diagram of a psychiatric index unit of a medical
device
or medical device system, in accordance with one illustrative embodiment of
the present
disclosure;
Figure 11 is a stylized block diagram of an endocrine index unit of a medical
device
or medical device system, in accordance with one illustrative embodiment of
the present
disclosure;
Figure 12 is a stylized block diagram of an adverse effect of therapy index
unit of a
medical device or medical device system, in accordance with one illustrative
embodiment of
the present disclosure;
Figure 13 is a stylized block diagram of an index comparison unit of a medical
device
or medical device system, in accordance with one illustrative embodiment of
the present
disclosure;
Figure 14 is a stylized block diagram of an assessment unit of a medical
device or
medical device system, in accordance with one illustrative embodiment of the
present
disclosure;
Figure 15 is a stylized block diagram of a forecast unit of a medical device
or medical
device system, in accordance with one illustrative embodiment of the present
disclosure;
Figure 16 is a stylized diagram of relationships between obesity and some of
its
comorbidities, with the direction of an arrow showing a source's tendency to
increase or make
more likely a target; and
Figure 17 is a stylized diagram of relationships between epilepsy and some of
its
comorbidities, with the direction of an arrow showing a source's tendency to
increase or make
more likely a target.
While the disclosure is susceptible to various modifications and alternative
forms,
specific embodiments thereof have been shown by way of example in the drawings
and are
CA 02802694 2016-02-22
WO 2011/159592
PCT/US2011 /040130
herein described in detail. It should be understood, however, that the
description herein of
specific embodiments is not intended to limit the disclosure to the particular
forms disclosed, but
on the contrary, the intention is to cover all modifications, equivalents, and
alternatives falling
within the scope of the disclosure as defined by the appended claims, which
are construed
following a purposive construction according to Canadian Law.
DETAILED DESCRIPTION OF SPECIFIC EMBODIMENTS
Illustrative embodiments of the disclosure are described herein. In the
interest of clarity,
not all features of an actual implementation are described in this
specification. In the
development of any such actual embodiment, numerous implementation-specific
decisions must
be made to achieve the design-specific goals, which will vary from one
implementation to
another. It will be appreciated that such a development effort, while possibly
complex and time-
consuming, would nevertheless be a routine undertaking for persons of ordinary
skill in the art
having the benefit of this disclosure.
This document does not intend to distinguish between components that differ in
name
but not function. In the following discussion and in the claims, the terms
"including" and
"includes" are used in an open-ended fashion, and thus should be interpreted
to mean
"including, but not limited to." Also, the term "couple" or "couples" is
intended to mean either a
direct or an indirect electrical connection. "Direct contact," "direct
attachment," or providing a
"direct coupling" indicates that a surface of a first element contacts the
surface of a second
element with no substantial attenuating medium there between. The presence of
small
quantities of substances, such as bodily fluids, that do not substantially
attenuate electrical
connections does not vitiate direct contact. The word "or" is used in the
inclusive sense (i.e.,
"and/or") unless a specific use to the contrary is explicitly stated.
The term "electrode" or "electrodes" described herein may refer to one or more
stimulation electrodes (i.e., electrodes for delivering a therapeutic signal
generated by an
11
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
IMD to a tissue), sensing electrodes (i.e., electrodes for sensing a
physiological indication of
a patient's body), and/or electrodes that are capable of delivering a
therapeutic signal, as well
as performing a sensing function.
This disclosure makes uni-variate (e.g., neurological index only) or
multivariate (e.g.,
neurological, autonomic, psychiatric, etc.) comparisons of the effects of the
patient's disease
state (and, in some embodiments, a treatment for the patient's disease) on one
or more body
systems. Comparisons may include one or more index parameters associated with
body
systems. Index parameters may involve measures of central tendency, measures
of
dimensionality including fractal dimensionality, measures of non-linearity,
measures of non-
.. stationarity, measures of long-range dependence or correlation, and
measures of clustering,
such as the pseudo F statistic, including size, shape number and distance
between clusters.
The index parameters may also comprise distributions of any of the foregoing
measures.
The index measures may be treated separately or as a composite (i.e., a single
measure of multivariate indices). Where a composite measure is used, the
component indices
may be weighted differently based on its impact on subject's well-being or
safety, and may
be derived from indices obtained from either implanted or external devices, or
from historical
data.
Comparisons may be intra-subject or inter-subject, including to tables of
normative
values from healthy or special populations, and may be performed as a function
of time or
state space. Comparisons may be quantitative and associated with a statistical
significance
value or qualitative or based on clinical judgment. The analyses performed as
part of
embodiments of this disclosure will yield four quantitative or qualitative
outcomes: disease
progression (deterioration in the index) expressed as one of a numerical
difference in relation
to past values, a rate of change of the index as a function of time from the
previous
measurements, or a qualitative change (e.g., minimal and slow or marked and
rapid); disease
12
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
stabilization (no change in the index); disease regression (improvement in the
index); or
insufficient information/undecidable. The outcomes will be automatically time-
stamped,
reported and stored for comparisons with past and future measurements, along
with the index
values and comparison results leading to the quantitative, semi-quantitative
or qualitative
outcome reported for the index. Models of disease state may be built to issue
forecasts or
prognoses of the patient's future condition or disease state, using various
tools or methods
(e.g., Kalman filtering, Bayesian statistics, etc.). Changes in index values
relative to past or
normative data may also be used to stratify patients into risk categories.
Automated warnings
may be issued for either large or rapid deterioration (indicative of disease
progression) in any
of the index value at the time it is observed or detected or forecast so that
treatment or
preventive measures may be instituted.
Embodiments of the present disclosure provide for assessing a state of a
disease of a
patient. In some embodiments, one or more of an autonomic index, a neurologic
index, a
stress marker index, a psychiatric index, an endocrine index, an adverse
effect of therapy
.. index, a physical fitness index, and/or a quality of life index may be
compared to
predetermined or corresponding reference values. The assessment of the disease
may be
performed based upon the comparisons of the indices to respective reference
values.
Any disease may be considered herein. As discussed above, it should be
apparent that
various diseases may coexist in a positive feedback loop. The term "primary
disease" may be
used herein to refer to a disease which is the subject of medical attention.
For example, for a
patient having both epilepsy and congestive heart failure, a neurologist may
consider epilepsy
a primary disease and congestive heart failure a secondary disease or
comorbidity; whereas a
cardiologist may consider the opposite.
As should be apparent, the methods and systems of the present disclosure may
be
applied in situations where one or more diseases are the subject of medical
attention. For
13
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
example, the methods of the present disclosure may be used by a patient
suffering from
epilepsy to monitor the state of this disease and any comorbidities associated
with it. For
another example, the methods of the present disclosure may be used by a
patient suffering
from both epilepsy and diabetes mellitus to monitor the state of each disease
and any
comorbidities associated with either disease. The states of any one or more
diseases,
including but not limited to the exemplary ones referred to above, and their
associated
comorbidities may be monitored.
As will be described in further details below, Figure 1 shows one exemplary
embodiment of a method according to the present disclosure. One or more of an
autonomic
index, a neurologic index, a stress marker index, a psychiatric index, an
endocrine index, an
adverse effect of therapy index, a physical fitness index, and/or a quality of
life (QOL) index
are determined during a first time period at 112, 114, 115, 116, 117, 118,
and/or 119.
Similarly, one or more of an autonomic index, a neurologic index, a stress
marker index, a
psychiatric index, an endocrine index, an adverse effect of therapy index, a
physical fitness
index, and/or a quality of life (QOL) index are determined during a second
time period at
122, 124, 125, 126, 127, 128, and/or 129. Thereafter, at least two indices are
compared to
respective reference values across the first and second time periods at 130.
In addition to time, "space states" can be used to define conditions in which
the index
values are determined. Herein, "space states" refer to index values as
measured at different
.. locations in or on the patient's body, or in the same location but during
different states (e.g,
wakefulness vs. sleep; sedentary or resting conditions vs. exercise). For
example, the
patient's oxygen saturation can be measured by a pulse ox device, such as
fingertip-
mountable oxygen saturation sensor, and simultaneously the patient's heart
rate can be
measured by an appropriate device, such as an implantable medical device. For
another
example, the patient's oxygen saturation can be measured during a resting
condition and
14
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
during physical exertion. An index can comprise data from one or more
locations in or on the
body collected at one or more times.
Assessments of disease state or comorbidity based on tests, scales, or
questionnaires
may be administered automatically on-line or off-line. Adaptation and
validation of said tests,
scales or questionnaires may be performed as needed to ensure reproducibility
and proper
interpretation.
Once the comparison may be made, a determination at 140 may be made whether
the
comparison can support the conclusion that the patient's disease and/or a
state of a body
system of the patient has changed or remained stable. If not, an indication at
145 may be
made that no conclusion about the patient's disease and/or a body system of
the patient can be
made.
Optionally, the indication at 145 of no conclusion can further comprise
reporting one
or more reasons for the indication; e.g., the sample size may be too small,
the data may be too
noisy, or the analysis may be too complex to be performed.
If the comparison can support the conclusion that the patient's disease and/or
a state of
a body system of the patient has changed or remained stable, a determination
at 146 may be
made whether the patient's disease and/or a state of a body system of the
patient has changed.
If it has not, an indication at 147 may be made that the patient's disease
and/or a body system
of the patient is stable.
However, if the comparison indicates the patient's disease and/or a state of a
body
system of the patient has changed, then a determination at 150 may be made
whether the
patient's disease has progressed or regressed relative to a previous
assessment and/or whether
the patient's body system has improved or worsened relative to a previous
assessment.
As will be described in further details below, Figure 2 shows another
embodiment of
a method according to the present disclosure. In this embodiment, one or more
index values
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
may be acquired at 210, and values derived from measure(s) of central tendency
may be
computed at 215 and/or other features (e.g., dimensionality, stationarity,
clustering, long
range dependencies, fractality, etc.) may be determined at 220. Thereafter,
the features are
compared to a reference value, in a quantitative manner at 230 (such as
statistically or non-
statistically) and/or a semi-quantitative or qualitative (e.g., reflective of
clinical judgment)
manner at 235.
A determination at 240 may then be made as to whether the comparison shows a
change. If the determination is "no," the disease or body system may be
considered stable (at
box 250). If the determination is "yes," the magnitude, direction, and/or rate
of change may
be determined at 260, from which the state of the disease or body system can
be assessed at
265. If desired, the patient's overall health may be assessed at 270, in light
of the disease
state, body system state, and/or other parameters. Alternatively or in
addition, a risk of a
negative outcome, such as risk of death, disease progression, new comorbidity,
or the like can
be reported at 280.
From the comparisons, parametric or non-parametric statistical tests may be
applied to
measures of central tendency or to distributions thereof Such tests include,
but are not
limited to, Student's t-test, Fisher's test, ANOVA, the Kolmogorov-Smimoff, or
the
Mahalanobis, among others. If the measure(s) of central tendency or the
distribution(s) are
statistically significantly different, the magnitude and direction of the
deviation may be
estimated using appropriate tests or measures. Additionally or alternatively,
clinical
judgment may be applied to determine if the changes (whether or not
statistically significant)
are worthy of attention and merit intervention. Analyses may be performed on
the mean,
median, standard deviation, coefficient of variation, nth percentile, or any
other measure of
central tendency of a statistical distribution of index values. Analyses may
be performed,
when applicable, using spectral analysis, high order spectral analysis,
detrended fluctuation
16
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
analysis, fractal analysis, multifractal analysis, correlation dimension, and
combinations
thereof
Other techniques, such as regression analyses, eigen methods (e.g. principal
component and canonical correlation analysis), co-variance analyses,
bootstrapping, or Monte
Carlo may be also used, among others.
Generally, biological data is non-stationary (the statistical properties
change as
function of time) and has long-range dependence (the values or characteristics
of the present
datum depend on previous data values). A variable or observable may be
considered
"stationary" if all of its statistical parameters are independent of time.
Most statistical
techniques are well suited for data that is stationary, but certain variables
or observables (e.g.
heart rate and heart rate variability) that can be used in this disclosure to
assess disease state,
are to some degree nonstationary or cyclo-stationary. The
"identically distributed"
assumption is violated when the sampled process is non-stationary, which, for
example, can
cause the sample mean to be under- or over-estimated.
A two prong approach can be used to maximize the information content extracted
from the analyses and of non-stationary data to better assess disease state:
1. Minimization of
non-stationarity and of long-range dependencies to obtain information devoid
of certain
external influences or perturbations that best reflect the body system's
"static" behavior; 2.
Utilization of methods that address non-stationarity and long-range dependence
to gain
insight into the body system's behavior under external influences. Approaches
to manage or
minimize non-stationarity (e.g., stratification) so that the data may be
analyzed using
conventional statistics and can be applied to extract valuable insights into
disease state.
Certain data (e.g., EKG, EEG) used in this disclosure to assess disease state
have
"memory" which manifests as long range dependences. That is, values are not
independent
from each other such that present values are influenced by past ones.
Estimation of the Hurst
17
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
exponent and Detrended Fluctuation analysis may be used, among other methods,
to
characterize these type of data.
On the assumption a healthy subject's body is continually responding to
changes in
the environment, changes in the degree of non-stationarity and in long-range
dependence in a
biological time series, especially decreases in non-stationarity and/or long-
range dependence,
are usually indicative of dysfunction.
Time-frequency methods may be also applied to the analyses and processing
(including detection, estimation, filtering, etc) of nonstationary processes
that are some of the
subject matter of this disclosure. The methods include but are not limited to
the time-
frequency autoregressive moving-average (TFARMA) model, as well as its special
cases, the
TFAR and TFMA models as they are computationally efficient and stable,
retaining the
simplicity and intuitiveness of the power spectral density. The coherent and
incoherent
statistics based in the sample coherence statistic (that apply a measure of
fitness) and other
methods based on the theory of periodically correlated (cycloperiodic)
processes are
particularly useful for analyses of biological data (e.g., heart rate
variability, cortisol levels)
subject to circadian variations.
Other methods that can be used include the heterogeneous autoregressive (HAR)
models, the autoregressive moving average process of order (p,q) (ARMA(p,q)
process), the
autoregressive integrated moving average process of order (p,d,q) (ARIMA
(p,d,q), the
autoregressive fractionally integrated moving average process (ARFIMA(p,d,q)
process), and
the vector fractionally integrated autoregressive moving-average (VARFIMA)
model.
The smooth localized complex exponentials (SLEX) model may be also applied and
the best model can be selected using the penalized log energy criterion, which
may be derived
to be the Kullback¨Leibler distance between a model and the SLEX principal
components of
the multivariate time series.
18
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
Certain data (e.g., EKG, EEG) used in this disclosure to assess disease state
have
"memory" which manifests as long range dependences. Estimation of the Hurst
exponent and
Detrended Fluctuation analysis may be used, among other methods, to
characterize these type
of data.
From this information, a prognosis formulated on the degree and rate of, e.g.,
cognitive changes may be determined at 260, the prognosis or a calculated
probability of a
serious outcome (e.g. mortality or extreme morbidity) may be estimated at 265,
and/or a
probability of an accident or injury may be estimated at 270, and provided to
a predesignated
person(s) so that appropriate and timely action(s), including preventive ones,
may be taken.
Models may built to simulate the temporal evolution of the various indices
and, using these
models, the probability estimates of various outcomes may be optimized.
In one embodiment, the present disclosure provides a method, apparatus and
system
to perform assessment of a state of a primary disease and/or co-morbidities of
a patient and
use these data to assess the patient's state of health and well-being.
Embodiments disclosed
herein call for receiving at least one first autonomic index, neurologic
index, stress marker
index, psychiatric index, an endocrine index, a physical fitness index, or
quality of life index
of a patient. The embodiments disclosed herein may also comprise receiving at
least one
second autonomic index, neurologic index, stress marker index, psychiatric
index, endocrine
index, physical fitness index, or quality of life index of the patient.
As used herein, the term "signals" includes (i) the "raw" signal (e.g., EKG)
as
recorded with a device, or parts or components of said signal (e.g., R-wave
peaks), (ii)
features derived from the "raw" signal using statistical, mathematical, or
spectral tools (e.g.,
heart rate variability derived from R wave intervals, heart rate variability
triangular index,
SDNN, pNN50, LF, HF, LF/HF, etc.), (iii) the relationship the signal has with
other signals
or events, such as changes in heart rate as a function of respiratory
frequency and/or tidal
19
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
volume; and (iv) any recordable output such as cognitive functions,
electrical, chemical,
mechanical, thermal, or acoustic signals or any other information that may be
used on any
time scale to infer or extract useful information, about the state of a
subject or of any of his
organs. The signals may be analog or digital and can be converted
therebetween.
For example, in the case of EKG, this signal can be compared to itself (e.g.,
compiling
a master EKG signal by combining parallel EKG signals detected from
electrodes), compared
to another body signal (e.g., aligning R waves of the EKG to respiration
patterns or EEG
patterns), or compared to events or non-body signals (e.g., EKG variations due
to circadian
rhythms, stress, exercise, or emotions). Similarly, other body signals
discussed herein
contain a myriad of information packed into the signal. The term "signal" is
used herein to
include all of this additional information that can be extracted or inferred
from the signal and
not simply the raw signal alone.
In another embodiment, one or more windows vary in window length as a function
of
time, disease state, another variable, or parameter.
In one embodiment, three or more windows are used to allow the comparison of
isolated periodic events in the patient's life. For example, the system can
compare indices by
stratifying data and comparing one night to previous night(s) or designated
times (e.g, 12:00
AM - 06:00AM, to the same time interval. Similarly, the system can compare day
to
previous day(s), REM sleep to prior REM sleep, morning to prior morning(s),
cardiovascular
exercise to previous cardiovascular exercise session(s), etc. as a function of
ultradian,
circadian, "lunar" or other rhythms. Comparisons may be made as a function of
ultradian,
circadian, "lunar" or other rhythms and between similar strata or different
strata.
In one embodiment, the first index has two or more windows of different
lengths
associated with it. The second index can have just one window, or it can also
have two,
three, or more windows associated with it. Monitoring the entropy of the HRV
signal, for
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
example, could track the entropy in a 30 second window, a 24 hour window, and
a 6 month
window.
In one embodiment, the first index may be a first autonomic index and the
second
index may be a second autonomic index. In a further embodiment, the first
autonomic index
may be a cardiovascular index or a respiratory index, and the second autonomic
index may be
a cardiovascular index or a respiratory index.
In one embodiment, the first index may be an autonomic index and the second
index
may be a neurologic index. Exemplary autonomic indices include, but are not
limited to,
those derivable from cardiovascular signals, breathing signals, pupillary
signals, skin signals,
or blood pressure, among others. In one particular embodiment, the autonomic
index may be
heart rate, values calculable from heart rate (such as magnitude and/or rate
of change of heart
rate, and heart rate variability, among others), heart beat wave morphology,
and heart beat
complex morphology (including measures of premature ventricular contractions
(PVCs)),
among others. Signals relating to these particular indices can be detected by
any appropriate
sensor, such as an R-wave detector or an electrocardiography (EKG) device,
among others.
The definition of various indices as autonomic or neurologic, or various
autonomic
indices as cardiovascular or respiratory, is to some extent arbitrary, though
the present
disclosure uses these terms in a consistent manner. Specifically, the
autonomic system,
which is under brain (neurological) control, exerts (in a normal body)
powerful influences on
the cardiovascular, respiratory, and gastrointestinal systems. The integrity
of these body
systems is a condition for optimal brain (neurological) function. For
simplicity and clarity,
the term "autonomic" system or index will encompass cardiovascular,
respiratory, dermal
(skin), pupillary, gastrointestinal systems or their indices.
Brain neurological signals may be derived from EEG, ECoG,
magnetoencephalography, brain imaging methods and modalities, chemical
methods, EMG,
21
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
or accelerometry, among others. Body kinetic neurological signals can be
detected by
electromyography, accelerometry, and/or inclinometry, or means for measuring
force, among
others. Cognitive signals may be obtained via manual or automated tests and
questionnaires.
Exemplary neurological indices include, but are not limited to, those
derivable from
electrical signals (EEG, evoked responses), chemical-metabolic signals,
cognitive (relating to
functional and cognitive decline, as well as risk of future decline) signals,
and kinetic signals
(relating to gait, posture, accessory movements, falls), hippocampus and
entorhinal cortex
volumes, basal forebrain nuclei volumes, and cortical thickness to determine
the pattern and
rate of atrophy. Other techniques, such as deformation-based and voxel-based
morphometry,
structural and effective connectivity by using diffusion tensor imaging,
tractography,
functional magnetic resonance imaging and positron emission tomography, may be
employed
to assess the state of disease. Other neurological signals (e.g. rate,
amplitude and pattern of
spikes) such as those generated by the cranial nerves spinal cord, spinal
roots or nerves, may
be used to assess disease state.
Through the application of appropriate techniques to EEG, ECoG, EKG, or other
biological signals recorded from epileptic brains, maximal seizure intensity
(Si), duration
(Sd), and extent of spread (Sc) may be computed. These measures may be used to
compute
seizure severity (SS) by transforming them (Si, Sd, Sc) into their
corresponding percentiles
Pi, Pd, and Pc and calculating their average: SS = (Pi + Pd + Pc)/3. These
measures (Si, Sd,
Sc) may be used without transformation or they may be transformed using for
example
natural logarithms. Seizure severity and the time elapsed between seizures
(interseizure
interval) defined as the time between the end of a seizure and the onset of
the next one
provide insight into the status of a patient's epilepsy. For example,
increases in mean or
median seizure severity and/or decreases in mean or median interseizure
interval would
indicate the disease has progressed.
22
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
Cognitive neurological indices may comprise measures of attention, simple or
complex reaction time, verbal or spatial memory, executive functions,
calculation, language,
reasoning, visuo-spatial functions, evoked responses, EEG, MEG, or brain
imaging (e.g.,
static (MRI) or functional (e.g., PET or fMRI)) among others. In one
embodiment, the
neurologic index may include one of more tests such as, a structured clinical
interview,
Wechsler Adult Intelligence Scale (WAIS-III) but will be updating to WAIS-IV
Wechsler
Memory Scale-Third Edition (WMS-III: Logical Memory I & II, Faces I & II,
Spatial Span),
California Verbal Learning Test-21 Edition (CVLT-II), Rey-Osterreith Complex
Figure Test
(ROCF), Continuous Visual Memory Test, Sentence Repetition Test (Multilingual
Aphasia
.. Examination), Complex Ideational Material (Boston Diagnostic Aphasia
Examination),
Category Fluency Test, Controlled Oral Word Association (COWA), Boston Naming
Test
(BNT), Wide Range Achievement Test-4th Edition (WRAT-IV: Reading), Trail
Making Test
(Part A & B) , Wisconsin Card Sort Test (WCST), Ruff Figural Fluency Test
(RFFT),
Sensory Imperception Test, Finger-Tip Number Writing Test, Benton Facial
Recognition
Test, Grooved Pegboard Test, Finger Tapping Test, Thumb-Finger Sequencing
Test,
Edinburgh Handedness Inventory, Minnesota Multiphasic Personality Inventory-21
Edition
(If reading level _> 6th grade; if not use Personality Assessment Inventory).
Responsiveness tests based on measurement of simple or complex reaction times,
are
the subject of copending patent application 12/756,065, filed April 7, 2010.
In one particular embodiment, the neurological index may be a measure of
responsiveness or a measure of physical (in)stability, such as number,
frequency, or severity
of falls. Signals relating to these particular indices can be detected by any
appropriate sensor,
such as an external responsiveness testing device or an internal
responsiveness testing device
(such as one making use of an implantable sound device to generate a tone or
sound in
23
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
proximity to the ear, an implantable accelerometer to detect a physical
motion, such as the
movement of a limb or a tap on the skin at the accelerometer implant site),
among others.
Psychiatric indices (including, but not limited to, mood, thoughts, and
hallucinations),
and quality of life indices are also subject to measurement in the present
disclosure. Those
skilled in the art know of the existence of clinically validated tools to
assess quality of life
and psychiatric status. The measurements provided by these tools may be used
to
automatically warn the patient or the caregivers of impending psychiatric
decompensation or
of high risk of suicide, so that an adverse or fatal outcome may be averted
through timely
intervention.
In one embodiment, the quality of life (QOL) factors may include one or more
of
scales such as, but are not limited to: the generic scale for quality of life;
the Psychological
General Well-Being Scale (PGWB); the WHO-Five Well-Being Index (WHO-5); the
Quality
of Life in Depression Scale (QLDS); the Social Functioning Scale-36 (SF-36);
the Social
Functioning Scale-12 (SF-12); the Quality of Life Enjoyment and Satisfaction
Questionnaire-
Short Form (Q-LES-Q-SF); and the Streamlined Longitudinal Interval
Continuation
Evaluation-Condensed Version (SLICE-C).
Alternatively or in addition, the QOL index may be a health-related QOL index
indicative of the patient's morbidity or any comorbidities.
Psychiatric assessment may entail administration of one or more of the
following tests
or scales: mini-mental state examination or Folstein test, abbreviated mental
test score,
Millon Clinical Multiaxial Inventory-III, psychometric tests such as the WISC
or WAIS,
Minnesota Multiphasic Personality Inventory, child Behavior Checklist, the
Beck Depression
Inventory. Other tests are the NEO-PI, the 16PF, the OPQ (Occupational
Personality
Questionnaire), the Five Factor Personality Inventory - Children (FFPI-C),
which are based
on the Big Five taxonomy. Additional tests include but are not limited to the
Behaviour and
24
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
Symptom Identification Scale (BASIS-32), the Beck Hopelessness Scale, the
Bipolar
Affective Disorder Dimension, the Scale Composite International Diagnostic
Interview, the
Depression-Anxiety stress scale, the General Health Questionnaire, the
InterSePT Scale for
Suicidal Thinking, the Kessler Psychological Distress scale, the Major (ICD-
10) Depression
Inventory Psychotic symptoms rating scale, the Psychological general well-
being index, and
the suicide intent scale.
Cardiovascular autonomic indices may be tested with the so called Ewing's
battery,
which consists of three tests reflecting cardiovascular parasympathetic and
two tests
reflecting cardiovascular sympathetic function: Heart rate response to forced
breathing; heart
rate response to the Valsalva maneuver; heart rate response to standing erect;
blood pressure
response to standing, and blood pressure response to handgrip. Other tests
include baroreflex
sensitivity, sympathetic skin response, heart rate and blood pressure
variation during normal
and deep breathing, maximum systolic blood pressure increase in isometric
work, the
Valsalva maneuver, or postural change, blood pressure response to postural
changes
including tilting, the 30:15 ratio of heart rate response to standing, and
time domain
parameters such as SDNN, PNN50, rMSDD, EKG morphology, EKG rhythm pattern,
heart
sounds, blood pressure, chest wall deflection, ejection fraction, heart size,
ventricular wall
thickness, and heart contractility, among others. These indices can be derived
from signals
detected by electrocardiography, blood pressure monitors, a microphone,
apexcardiography,
or echocardiography, among other techniques.
Respiratory autonomic signals, skin autonomic signals, temperature autonomic
signals, and the like can be detected, and indices (e.g., rate, pattern, tidal
volume, vital
capacity, forced vital capacity, skin resistance, sweat production, tympanic
temperature,
rectal temperature, and core temperature, among others) derived therefrom, by
various
.. techniques and apparatus.
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
Changes in catecholamines (e.g, epinephrine, serotonin) and in metabolites
(vanillyl
mandelic acid, metanephrine) in blood, urine, or other bodily fluids, during
resting conditions
or exercise, or as functions of time of day may be also be measured to assess
the integrity of
the autonomic nervous system. Direct recording of efferent postganglionic
muscle
sympathetic nerve traffic via microneurography and application of the regional
norepinephrine spillover technique may be also used as autonomic indices. A
technique
using electrodes positioned on the abdominal skin to record stomach
contractions
(electrogastrography) provides information about autonomic function through
measurement
of the dominant frequency (power) of contractions and classify them as normal
(eugastria) or
abnormal (bradygastria, tachygastria).
Changes in hormone levels in blood or other bodily fluids, during resting
conditions
or exercise, or as functions of time of day may be also be measured to assess
the integrity of
the endocrine system. For example, the human body has its highest melatonin
levels between
about midnight and eight a.m.; variations in the time of highest melatonin
levels, or increases
or decreases in the levels themselves, may reflect impairment of the endocrine
system.
Therapies may adversely impact any body system. The tests or measurements
described above for assessment of autonomic, neurologic, psychiatric, or
endocrine function
described in multiple parts of this specification, may be used for identifying
and quantifying
adverse effects of any type of therapy for any disease. Assessment of liver,
bone marrow,
kidney, or skin may be performed with any of the existing blood (e.g liver
enzymes, blood
hemogram, creatinine, BUN, etc.), radiologic/imaging (liver or kidney
ultrasound), or
histologic (e.g. biopsies of bone marrow, skin, liver or kidney) tests that
are common practice
in medicine.
In this disclosure, "adverse effects of therapy" encompasses any and all body
systems
(e.g., neurological, autonomic, psychiatric, hepatic, renal, dermal, etc.)
that may be
26
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
negatively affected by any form of therapy (e.g., electrical, pharmacologic,
thermal,
cognitive, etc). It is remarked that autonomic and adverse effect of therapy
indices share in
common several dermal (skin) indices (e.g. temperature; color, texture), the
only difference
being that skin resistance is exclusively an autonomic index.
Changes in a person's physical fitness encompasses long-term and/or global
measures
of activity, such as measures of physical fitness (e.g., VO2max, etc.).
Physical fitness may be
defined as the capacity to carry out the day's activities, pursue recreational
activities, and
have the physical capability to handle emergency situations. Physical fitness
can be measured
using strength tests (e.g., One repetition max), speed and power tests (e.g.,
30m sprint;
standing vertical jump), endurance Tests (e.g., Balke 15 minute run), and
flexibility tests
(e.g., sit and reach test).
In another embodiment, the index value may be derivable from one or more
cranial
nerve signals (e.g, spike frequency, amplitude, or pattern, among others). In
yet another
embodiment, the index value may be derivable from one or more autonomic nerve
or ganglia
signals (e.g, spike frequency, amplitude, or pattern, among others).
Seizures are powerful biological stressors and inductors of stress marker
indices and
deplete the body of certain anti-oxidants, such as glutathione peroxidase.
Exemplary stress
marker indices comprise changes (direction, rate, and magnitude) in glucose,
prolactin,
cortisol, catecholamines, chromogranin A, free radicals or reactive oxygen
species, lactic
acid, blood gases, N-acetylaspartate, in the expression of heat shock
proteins, and in
metabolites of any or all thereof For example, a "cortisol parameter" refers
to a stress
marker index relating to cortisol or a metabolite thereof, and a
"catecholamine parameter"
refers to a stress marker index relating to a catecholamine or a metabolite
thereof The
concentration of certain compounds that protect from biological stress (e.g.,
dehydroepiandrosterone or its sulfate conjugate, glutathione peroxidase) or
the body's total
27
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
antioxidant capacity may be also measured to determine if it is adequate and
if not to increase
it using commercially or naturally available antioxidants to stall disease
progression. Stress
marker index indices and antioxidants may be measured in brain (invasively and
non-
invasively) , CSF, plasma, serum, erythrocytes, urine, and saliva, (e.g. alpha
amylase).
Corticotropin-releasing factor (CRF) and the related urocortin peptides are
other examples of
stress markers.
The time window or space-state over which the various indices are quantified
can
have any desired duration, and if a second time window or space-state may be
used, it can
have any desired second duration. The first time window or space-state and the
second time
window or space-state can have any relationship. The two time windows may be
overlapping, partially overlapping, contiguous, or non-contiguous, and the
second time
window or space-state may be a subwindow of the first time window or space-
state. In other
words, the second time window or space-state may be fully overlapped by the
first time
window or space-state.
In another embodiment, the first time window or space-state and the second
time
window or space-state are non-contiguous. In other words, the first and second
time window
or space-states do not overlap and there exists a temporal gap between them.
Embodiments of the present disclosure also comprise comparing the at least one
index
to at least one reference value associated with the at least one index. In
addition, if at least
one second index has been determined as described above, the method can
comprise
comparing the at least one second index or marker to at least one second
reference value
associated with the at least one second index.
The reference values can be preselected, selected from a finite set of
predetermined
options, or can be dynamically recalculated during performance of the method.
They may be
determined from the patient's history or from a set of normative data. For
example, the
28
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
reference values can be prior values of the index. For example, if the index
value is heart rate
variability (HRV), the corresponding reference value can be a single value
defined by a
physician in view of the patient's age, sex, fitness level, body mass index,
physical fitness
level at the time of the measurementõ initial disease state, or other values;
it can be a value
.. chosen from a set of predetermined options relating to different typical
initial disease states or
the like; or it can be dynamically recalculated, such as from an indicator of
central tendency
(e.g., a mean, a median, or a percentile value) of HRV data over one or more
timescales, such
as the past hour, day, week, month, or year, among others, to account for
ultradian, circadian,
catamenial, lunar, and seasonal variations.
In one embodiment, comparing the at least one first index to the at least one
first
reference value, the at least one second index to the at least one second
reference value, or
both comprises determining a statistical relationship (which may be linear or
non-linear,
positive or negative) between the index or indices and the reference value or
values. For
example, the statistical relationship may be a number of standard deviations,
percentile ranks,
or the like between the reference value and the corresponding index.
The comparison may involve, when applicable, using spectral analysis, high
order
spectral analysis, detrended fluctuation analysis, fractal analysis,
multifractal analysis,
correlation dimension, and combinations thereof For example, the comparison
may involve
determining the slope of a trendline of data points, the shape of a curve of
data points, the
smoothness of a set of data points, or an autocorrelation of a set of data
points, among others.
The terms "index" refers to values, quantities, classes, categories or items
derived
from signals (raw or processed). The term "reference" corresponds to
quantities, classes,
categories or items derived from signals (raw or processed) recorded from a
subject in the
past (recent or distant) or obtained from tables of comparable normative
values obtained from
other normal or diseased subjects. For example, the HRV calculated from data
collected over
29
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
a period time ending in the present day (the index) may be compared to HRV
calculated from
data collected over an identical period of time 1 year earlier (the
reference). The index as
specified above may be also compared to HRV calculated from subjects that
match the
demographic and clinical characteristics (the other reference) of the subject
in question.
Determination about the state of the subject's disease may be made by using
either or both of
the references. For example, a cognitive (e.g. verbal memory) or EEG index
(e.g., power in
the alpha band) calculated from data collected from a subjects over a period
time ending in
the present day (the index), may be compared to verbal memory or alpha band
power
calculated from data collected from over an identical period of time 1 year
earlier (the
reference) and to a third period of time, three years earlier. These indices
as specified above
may be also compared to verbal memory and alpha band power obtained from
subjects that
match the demographic and clinical characteristics (the other reference) of
the subject in
question. Determination about the state of the subject's disease may be made
by using either
or both of the references.
In one embodiment, comparing the at least one index to the at least one
reference
value comprises determining a non-linear relationship between the index or
indices and the
reference value or values. For example, the non-linear relationship may be
related to a
pattern matching relationship or a non-linear mathematic relationship between
the reference
value and the corresponding index.
Also, embodiments of the present disclosure comprises assessing a state of a
disease
or a body system of the patient based on comparing the at least one index to
the at least one
reference value. If at least one second index is received, assessing can be
further based on
comparing the at least one second index to the at least one second reference
value. For
example, a second index and a third index can be received, and assessing can
further based on
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
comparing the second index to a second reference value and comparing the third
index to a
third reference value.
In a further embodiment, embodiments of the present disclosure further
comprise
receiving at least one third autonomic index, neurologic index, stress marker
index,
psychiatric index, an endocrine index, an adverse effect of therapy index, a
physical fitness
index, or quality of life index of a patient over a third time window. In this
further
embodiment, the method also further comprises comparing the at least one third
index to at
least one third reference value. In this further embodiment, assessing the
state of the disease
of the patient may be based on comparing the at least one first index to the
at least one first
reference value associated with the at least one first index, the at least one
second index to the
at least one second reference value associated with the at least one second
index, and the at
least one third index to the at least one third reference value associated
with the at least one
third index.
Although not limited to the following, exemplary systems capable of
implementing
embodiments of the present disclosure are described below. Figure 3A depicts a
stylized
implantable medical system (IMD) 300 for implementing one or more embodiments
of the
present disclosure. An electrical signal generator 310 may be provided, having
a main body
312 comprising a case or shell with a header 316 for connecting to an
insulated, electrically
conductive lead assembly 322. The generator 310 may be implanted in the
patient's chest in a
pocket or cavity formed by the implanting surgeon just below the skin
(indicated by a dotted
line 345), similar to the implantation procedure for a pacemaker pulse
generator.
A nerve electrode assembly 325, preferably comprising a plurality of
electrodes
having at least an electrode pair, may be conductively connected to the distal
end of the lead
assembly 322, which preferably comprises a plurality of lead wires (one wire
for each
electrode). Each electrode in the electrode assembly 325 may operate
independently or
31
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
alternatively, may operate in conjunction with the other electrodes. In one
embodiment, the
electrode assembly 325 comprises at least a cathode and an anode. In another
embodiment,
the electrode assembly comprises one or more unipolar electrodes.
Lead assembly 322 may be attached at its proximal end to connectors on the
header
316 of generator 310. The electrode assembly 325 may be surgically coupled to
the vagus
nerve 327 in the patient's neck or at another location, e.g., near the
patient's diaphragm or at
the esophagus/stomach junction. Other (or additional) cranial nerves such as
the trigeminal
and/or glossopharyngeal nerves may also be used to deliver the electrical
signal in particular
alternative embodiments. In one embodiment, the electrode assembly 325
comprises a
bipolar stimulating electrode pair 326, 328 (i.e., a cathode and an anode).
Suitable electrode
assemblies are available from Cyberonics, Inc., Houston, Texas, USA as the
Model 302
electrode assembly. However, persons of skill in the art will appreciate that
many electrode
designs could be used in the present disclosure. In one embodiment, the two
electrodes are
wrapped about the vagus nerve, and the electrode assembly 325 may be secured
to the vagus
nerve 327 by a spiral anchoring tether 330 such as that disclosed in U.S. Pat.
No. 4,979,511
issued Dec. 25, 1990 to Reese S. Terry, Jr.. Lead assembly 322 may be secured,
while
retaining the ability to flex with movement of the chest and neck, by a suture
connection to
nearby tissue (not shown).
In alternative embodiments, the electrode assembly 325 may comprise
temperature
.. sensing elements, blood pressure sensing elements, and/or heart rate sensor
elements. Other
sensors for other body parameters may also be employed. Both passive and
active
stimulation may be combined or delivered by a single IMD according to the
present
disclosure. Either or both modes may be appropriate to treat a specific
patient under
observation.
32
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
In alternative embodiments, the implantable medical device system further
comprises
an electrical stimulator comprising an autonomic signal sensor 360a (not to
scale) adapted to
be coupled to a body part, such as an internal organ 380 (Figure 3B) or a
neurologic signal
sensor 360n (also not to scale) adapted to record (either non-invasively or
invasively) from a
portion of the nervous system, such as the frontal cortex 390 (Figure 3C) or
another region of
the brain. The physician can select precise locations for coupling to the
internal organ 380 or
frontal cortex 390 (or other portion of the nervous system) based on his or
her observations of
the patient's medical condition, among other values. In
various embodiments, the
implantable medical device system may comprise one, two, or three of the IMD
300, the
autonomic signal sensor 360a, and the neurologic signal sensor 360n.
The electrical pulse generator 310 may be programmed with an external device
(ED)
such as computer 350 using programming software. A programming wand 355 may be
coupled to the computer 350 as part of the ED to facilitate radio frequency
(RF)
communication between the computer 350 and the pulse generator 310. The
programming
wand 355 and computer 350 permit non-invasive communication with the generator
310 after
the latter may be implanted. In systems where the computer 350 uses one or
more channels
in the Medical Implant Communications Service (MICS) bandwidths, the
programming wand
355 may be omitted to permit more convenient communication directly between
the
computer 350 and the pulse generator 310.
Turning now to Figure 4, a block diagram depiction of a medical device 400 is
provided, in accordance with one illustrative embodiment of the present
disclosure.
In some embodiments, the medical device 400 may be implantable (such as
implantable electrical signal generator 310 from Figure 3), while in other
embodiments the
medical device 400 may be completely external to the body of the patient.
33
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
The medical device 400 (such as generator 310 from Figure 3) may comprise a
controller 410 capable of controlling various aspects of the operation of the
medical device
400. The controller 410 may be capable of receiving internal data or external
data, and in one
embodiment, may be capable of causing a therapy unit 420 (Figure 4) to
generate and deliver
an electrical signal to target tissues of the patient's body for treating a
medical condition. For
example, the controller 410 may receive manual instructions from an operator
externally, or
may cause the electrical signal to be generated and delivered based on
internal calculations
and programming. The controller 410 may be capable of affecting substantially
all functions
of the medical device 400.
The controller 410 may comprise various components, such as a processor 415, a
memory 417, etc. The processor 415 may comprise one or more microcontrollers,
microprocessors, etc., capable of performing various executions of software
components.
The memory 417 may comprise various memory portions where a number of types of
data
(e.g., internal data, external data instructions, software codes, status data,
diagnostic data,
etc.) may be stored. The memory 417 may comprise one or more of random access
memory
(RAM), dynamic random access memory (DRAM), electrically erasable programmable
read-
only memory (EEPROM), flash memory, etc.
As stated above, in one embodiment, the medical device 400 may also comprise a
therapy unit 420 capable of generating and delivering electrical signals to
one or more
electrodes 326, 328 via leads 401 (Figure 4). A lead assembly such as lead
assembly 322
(Figure 3) may be coupled to the medical device 400. Therapy may be delivered
to the leads
401 comprising the lead assembly 322 by the therapy unit 420 based upon
instructions from
the controller 410. The therapy unit 420 may comprise various circuitry, such
as electrical
signal generators, impedance control circuitry to control the impedance "seen"
by the leads,
and other circuitry that receives instructions relating to the delivery of the
electrical signal to
34
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
tissue. The therapy unit 420 may be capable of delivering electrical signals
over the leads
401 comprising the lead assembly 322. As should be apparent, in certain
embodiments, the
medical device 400 does not comprise a therapy unit 420, lead assembly 322, or
leads 401.
In other embodiments, a lead 401 may be operatively coupled to an electrode,
wherein
the electrode may be adapted to couple to at least one of a portion of a brain
structure of the
patient, a cranial nerve of a patient, a spinal cord of a patient, a
sympathetic nerve structure of
the patient, or a peripheral nerve of the patient.
The medical device 400 may also comprise a power supply 430. The power supply
430 may comprise a battery, voltage regulators, capacitors, etc., to provide
power for the
operation of the medical device 400, including delivering the therapeutic
electrical signal.
The power supply 430 comprises a power source that in some embodiments may be
rechargeable. In other embodiments, a non-rechargeable power source may be
used. The
power supply 430 provides power for the operation of the medical device 400,
including
electronic operations and the electrical signal generation and delivery
functions. The power
supply 430 may comprise a lithium/thionyl chloride cell or a lithium/carbon
monofluoride
(LiCFx) cell if the medical device 400 is implantable, or may comprise
conventional watch or
2V batteries for external (i.e., non-implantable) embodiments. Other battery
types may also
be used.
The medical device 400 may also comprise a communication unit 460 capable of
facilitating communications between the medical device 400 and various
devices. In
particular, the communication unit 460 may be capable of providing
transmission and
reception of electronic signals to and from an monitoring unit 470, such as a
handheld
computer or PDA that can communicate with the medical device 400 wirelessely
or by cable.
The communication unit 460 may include hardware, software, firmware, or any
combination
thereof
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
The medical device 400 may also comprise one or more sensor(s) 360a, 360n
coupled
via sensor lead(s) 411 to the medical device 400. Sensor(s) 360a are capable
of receiving
signals related to an autonomic index, such as the patient's heart beat, blood
pressure, and/or
temperature, among others, and delivering the signals to the medical device
400. Sensor(s)
360n are capable of receiving signals related to a neurologic index, and
delivering the signals
to the medical device 400. In one embodiment, the sensor(s) 360a, 360n may be
the same as
implanted electrode(s) 326, 328 (Figure 3). In other embodiments, the
sensor(s) 360a, 360n
are separate structures that may be placed on the patient's skin, such as over
the patient's heart
or elsewhere on the patient's body. The sensor(s) 360a, 360n and accompanying
leads may
be considered an interface for the medical device 400 to receive, e.g., at
least one of
autonomic data, neurologic data, or stress data, such as from the sensors
360a, 360n.
Exemplary sensor(s) 360a include electrocardiography (EKG) devices,
accelerometers, inclinometers, pupillometers, face or body temperature
monitors, skin
resistance monitors, and/or sound and pressure sensors, among others.
Alternatively or in addition to sensors 360, the medical device 400 can
comprise at
least one interface (not shown) capable of receiving data relating to at least
one index. For
example, the interface can receive data comprising index values. Alternatively
or in addition,
the interface can receive data further processible by other components of the
medical device
400.
The medical device may also comprise at least one of an autonomic index
determination unit 465 capable of determining at least one autonomic index, a
neurologic
index determination unit 475 capable of determining at least one neurologic
index, a stress
marker index determination unit 477 capable of determining at least one stress
marker index,
a psychiatric index determination unit 479 capable of determining at least one
psychiatric
index, an endocrine index determination unit 481 capable of determining at
least one
36
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
endocrine index, an adverse effect of therapy index determination unit 482
capable of
determining at least one adverse effect of therapy index, or a quality of life
(QOL) index
determination unit 485 capable of determining at least one quality of life
index. If present,
the autonomic index determination unit 465 can receive signals related to an
autonomic index
delivered to the medical device 400 and, from them, determine an autonomic
index. The
autonomic index determination unit 465 will be discussed in more detail below.
Similarly, if
present, the neurologic index determination unit 475 can receive signals
related to a
neurologic index delivered to the medical device 400 and, from them, determine
a neurologic
index. The neurologic index determination unit 475 will be discussed in more
detail below.
.. Similarly, if present, the QOL index determination unit 485 can receive
signals related to a
QOL index delivered to the medical device 400, such as through the
communication unit 460
and, from them, determine a QOL index. In one embodiment, the signals related
to a QOL
index are sent from a remote device 492 that may be capable of gathering such
signals. In
another embodiment, the QOL index determination unit 485 may be located in
remote device
492.
Also, similarly, if present, the stress marker index determination unit 477
can receive
signals related to a stress marker index delivered to the medical device 400
and, from them,
determine a stress marker index. Also, if present, the psychiatric index
determination unit
479 can receive signals related to an psychiatric index delivered to the
medical device 400
and, from them, determine an psychiatric index. Similarly, if present, the
endocrine index
determination unit 481 can determine at least one endocrine index and/or the
adverse effect of
therapy index determination unit 482 can determine at least one adverse effect
of therapy
index. The stress marker index determination unit 477, psychiatric index
determination unit
479, endocrine index determination unit 481, and one adverse effect of therapy
index
determination unit 482 are discussed in more detail elsewhere herein.
37
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
The medical device 400 can also comprise an index comparison unit 495 capable
of
comparing at least one index with at least one reference value. The reference
value may be
stored in the memory 417, in a local database unit 455, a database unit 450,
or a remote
device 492. The index comparison unit 495 will be discussed in more detail
below.
The medical device 400 can also comprise a assessment unit 487 capable of
assessing
a state of a disease of a patient based on at least one output of the index
comparison unit.
The medical device 400 can also comprise a warning unit 489 capable of
providing a
warning signal to the patient, a physician, or a caregiver if assessing
indicates disease
progression. In one embodiment, the warning signal may be proportional to at
least one of a
magnitude of a change of a progression, a rate of change of a disease
progression, or a
correlation of the index value to life span.
The medical device 400 can also comprise a therapy unit adapted to deliver a
therapy
for the disease to a patient. For example, the medical device 400 can comprise
a therapy unit
420 and related hardware, such as lead(s) 401 and electrode(s) 326, 328.
Although not shown, the medical device 400 can comprise an acute disease state
detection unit adapted to detect an acute disease state in the patient. For
example, the acute
disease state detection unit can be adapted to detect an epileptic event.
Common epileptic
events of concern are, for example, clinical seizures, subclinical seizures,
loss of
consciousness, falls to the ground, cognitive impairment during and following
a seizure,
prolonged confusional states, bodily injuries, alterations in heart,
respiratory and in other
functions under autonomic control, sudden unexpected death, psychosis,
depression, suicide,
osteoporosis, obesity, and reproductive dysfunction. Exemplary acute disease
state detection
units can be included in the medical device 400 as a matter of routine
experimentation.
Figure 5 shows a medical device system similar to that shown in Figure 4, but
with
several elements located in an external device not implanted in the patient.
Specifically,
38
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
Figure 5 depicts a medical device system with the following elements located
in an external
disease state unit: the autonomic index determination unit 465, the neurologic
index
determination unit 475, the stress marker index determination unit 477, the
psychiatric index
determination unit 479, the endocrine index determination unit 481, the
adverse effect of
therapy index determination unit 482, the QOL index determination unit 485,
the index
comparison unit 495, and the assessment unit 487 are located in an external
disease state unit.
Housing these elements in an external disease state unit may permit use of
more complex
analysis tools and algorithms because an externally unit generally can have a
faster
microprocessor (which consume more power and generate more heat), more memory,
and
AC power or larger batteries than an implanted device. For example, this
embodiment of
Figure 5 may be useful if the calculations performable by the determination
modules 465,
475, 477, 479, 485 or the units 495, 487 are so complex and/or dependent on
such a large
number of data lookups (e.g., in a local database unit 455 or a database unit
450) that
performing the calculations in the medical device 400 would consume too much
power or
generate too much heat.
Turning to Figure 6, an autonomic index determination unit 465 is shown in
more
detail. The autonomic index determination unit 465 can comprise a
cardiovascular signal unit
612 capable of processing at least one cardiovascular indication received from
sensor(s)
360a. Alternatively or in addition, the autonomic index determination unit 465
can comprise
a respiratory signal unit 614 capable of processing at least one respiratory
indication received
from sensor(s) 360a. Alternatively or in addition, the autonomic index
determination unit
465 can comprise a blood parameter signal unit 623 capable of processing at
least one blood
parameter indication (e.g., blood glucose, blood pH, etc). Alternatively or in
addition, the
autonomic index determination unit 465 can comprise a temperature signal unit
616 capable
of processing at least one temperature indication received from sensor(s)
360a. Alternatively
39
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
or in addition, the autonomic index determination unit 465 can comprise an
optic signal unit
618 capable of processing at least one optic indication received from
sensor(s) 360a.
Alternatively or in addition, the autonomic index determination unit 465 can
comprise a
chemical signal unit 620 capable of processing at least one body chemical
indication received
.. from sensor(s) 360a. Alternatively or in addition, the autonomic index
determination unit
465 can comprise a hormone signal unit 622 capable of processing at least one
hormone
indication received from sensor(s) 360a. Alternatively or in addition, the
autonomic index
determination unit 465 can comprise one or more other autonomic signal unit(s)
624, such as
a skin resistance signal unit.
The autonomic index determination unit 465 can also comprise an autonomic data
processing unit 630. The autonomic data processing unit 630 can perform any
filtering, noise
reduction, amplification, or other appropriate processing of the data received
by the signal
units 612-624 prior to calculation of the autonomic index.
The autonomic index determination unit 465 can also comprise an autonomic
index
calculation unit 640. The autonomic index calculation unit 640 can calculate
an autonomic
index from the data passed by the autonomic data processing unit 630.
For example, the autonomic index calculation unit 640 may calculate the heart
rate, a
change in the heart rate, the speed of change in heart rate, blood pressure,
heart sounds, heart
rhythm, heartbeat wave morphology, heartbeat complex morphology, or the shape
of the
deflection of the thoracic wall as the heart apex beats against it, among
others, from
cardiovascular data received by cardiovascular signal unit 612.
For another example, the autonomic index calculation unit 640 may calculate
the
respiration (breath) rate, respiration pattern, airflow velocity, respiration
amplitude (tidal
volume), oxygen saturation, arterial gas concentrations, or blood pH, among
others, from
respiratory data received by respiratory signal unit 614.
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
For still another example, the autonomic index calculation unit 640 may
calculate a
change in the skin temperature or skin resistance of a part of the patient's
face or a change in
the core temperature of the patient, from temperature data received by
temperature signal unit
616.
Turning to Figure 7, an exemplary embodiment of a neurologic index
determination
unit 475 is shown. The neurologic index determination unit 475 can comprise at
least one of
a neuro-electrical signal unit 712 capable of processing at least one neuro-
electrical signal
received from a sensor 360n; a neuro-chemical signal unit 714 capable of
processing at least
one neuro-chemical signal received from a sensor 360n; a neuro-electrochemical
signal unit
716 capable of processing at least one neuro-electrochemical signal received
from a sensor
360n; or a cognitive signal unit 720 capable of processing at least one
cognitive indication
received from a sensor 360n or another device, such as a remote device 492.
In one embodiment, the cognitive signal unit comprises at least one of a
cognitive
aptitude determination unit 720a capable of processing at least one cognitive
aptitude
indication; an attention aptitude determination unit 720b capable of
processing at least one
attention aptitude indication; a memory aptitude determination unit 720c
capable of
processing at least one memory indication; a language aptitude determination
unit 720d
capable of processing at least one language indication; a visual/spatial
aptitude determination
unit 720e capable of processing at least one visual/spatial indication; a
kinetic capability
determination unit 720f capable of processing at least one kinetic indication;
one or more
other neurologic factor determination unit(s) 720g; or a responsiveness
determination unit
720h.
The neurologic index determination unit 475 can also comprise a neurologic
data
processing unit 730. The neurologic data processing unit 730 can perform any
filtering, noise
41
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
reduction, amplification, or other appropriate processing of the data received
by the signal
units 712-720g prior to calculation of the neurologic index.
The neurologic index determination unit 475 can also comprise a neurologic
index
calculation unit 740. The neurologic index calculation unit 740 can calculate
a neurologic
index from the data passed by the neurologic data processing unit 730.
For example, the neurologic index calculation unit 740 may calculate a brain
index,
such as those determinable from signals yielded by an EEG, ECoG, or depth
electrode (i.e., a
deep brain electrode) as sensor(s) 360n, as received by neuro-electrical
signal unit 712,
neuro-chemical signal unit 714, and/or neuro-electrochemical signal unit 716
and, optionally,
further processed by neurologic data processing unit 730.
The brain index can also be calculated using other neurological signals. For
example,
sensor(s) 360n can detect spikes in neurons or axons in the brain and spinal
cord including
central structures and pathways with autonomic control or modulatory
capabilities, cranial
nerves (e.g., vagus nerve), autonomic ganglia or nerves and peripheral nerves.
Sensor(s) 360n
can also detect neural imaging or brain imaging signals including, for
example: Functional
Magnetic Resonance Imaging (fMRI), Magnetoencephalography (MEG), Positron
Emission
Tomography (PET), Event-Related Optical Signal (EROS), and Diffuse Optical
Imaging
(DOT)). Other imaging techniques such as voltage-senstive dyes, ultrasound,
infra-red, near
infra-red and other forms of thermography. Qualitative or descriptive and
quantitative (e.g.
volumetrics) data obtained from devices that are not part of this system
(e.g., MRI
equipment) may be uploaded and stored into this system for assessing disease
state.
For another example, the neurologic index calculation unit 740 may calculate a
body
kinetic index, such as the body's (or of a portion thereof such as an arm or a
leg) acceleration,
direction, position, smoothness, amplitude, or force of movements, and whether
there are
extraneous or abnormal body oscillations during resting conditions or
movement. The body
42
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
kinetic index may be determinable by electromyography, a mechanogram, an
accelerometer,
and/or an inclinometer as sensor(s) 360n, as received by kinetic capability
determination unit
720f, and, optionally, further processed by neurologic data processing unit
730.
Kinetic indices are voluntary or involuntary motor acts that provide insight
into the
functional state of the nervous system and are thus classified as a neurologic
index. The
ability to perform movements: a) in any direction; b) do it smoothly and with
precision so
that for example, a target (e.g. putting a key into its hole) may be met in
the first attempt or
handwriting is legible; c) changing direction to avoid colliding with an
object interposed on
its path to a target and re-adjusting the trajectory to reach the original
target; and d) with
adaptive force and discriminations so to be able to pick a penny off a flat
surface and also lift
heavy objects. The acceleration and velocity speed, direction and smoothness
may be
quantified using tools such as 3-D accelerometers among others.
Even though physical fitness indices depend to some extent on the integrity of
kinetic
indices, in this disclosure they are considered as distinct from kinetic
indices. Physical fitness
indices are used to assess physical fitness through certain measures as
described herein. A
person who leads a sedentary life may be physically unfit but may have normal
kinetic
indices.
Figure 8 shows a kinetic capability determination unit 720f in more detail.
The
kinetic capability determination unit 720f can be capable of receiving at
least one of an
accelerometer signal, an inclinometer signal, a movement signal, or a
dynamometer (force)
signal via accelerometer signal unit 812, inclinometer signal unit 814,
movement signal unit
816, or dynamometer signal unit 818, respectively. From the at least one of
the
accelerometer signal, the inclinometer signal, the movement signal, or a
dynamometer signal,
one or more of movement data or falls data can be calculated by movement data
calculation
unit 822 or falls data calculation unit 824, respectively. The calculated
movement data and/or
43
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
falls data can be processed by kinetic signal processing unit 830, and
thereafter, kinetic data
calculated by kinetic data calculation unit 840.
Turning to Figure 9, an exemplary embodiment of a stress marker index
determination unit 477 is shown. The stress marker index determination unit
477 can
comprise at least one signal unit 912, capable of receiving a signal as
described above from
which a stress marker index can be derived, such as a cortisol parameter unit
920a, a
catecholamine parameter signal unit 920b, and/or another stress marker
parameter unit 920c.
The stress marker index determination unit 477 can also comprise a stress
marker
index data processing unit 930. The stress marker index data processing unit
930 can
perform any filtering, noise reduction, amplification, or other appropriate
processing of the
data received by the signal unit 912 prior to calculation of the stress marker
index.
The stress marker index determination unit 477 can also comprise a stress
marker
index calculation unit 940. The stress marker index calculation unit 940 can
calculate a stress
marker index from the data passed by the stress marker index processing unit
930. For
example, the stress marker index calculation unit 940 may calculate a stress
marker index as
received by signal unit 912 and, optionally, further processed by stress
marker index data
processing unit 930.
Turning to Figure 10, an exemplary embodiment of a psychiatric index
determination
unit 479 is shown. The psychiatric index determination unit 479 can comprise
at least one
signal unit 1012, capable of receiving a signal as described above from which
a psychiatric
index can be derived. For example, the psychiatric index determination unit
479 can
comprise a psychiatric signal unit 1020, comprising one or more of a thought
determination
unit 1020a, a mood determination unit 1020b, or a judgment determination unit
1020c. The
various determination units 1020a-c can use tests or scales discussed above to
make a
44
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
determination, e.g., by administering a test to provide information regarding
the subject's
thought, mood, or judgment, or receiving the results of such a test from an
external source.
The psychiatric index determination unit 479 can also comprise a psychiatric
index
data processing unit 1030. The psychiatric index data processing unit 1030 can
perform any
filtering, noise reduction, amplification, or other appropriate processing of
the data received
by the signal unit 1012 prior to calculation of the psychiatric index.
The psychiatric index determination unit 479 can also comprise a psychiatric
index
calculation unit 1040. The psychiatric index calculation unit 1040 can
calculate a psychiatric
index from the data passed by the psychiatric index processing unit 1030.
For example, the psychiatric index calculation unit 1040 may calculate a
psychiatric
index as received by signal unit 1012 and, optionally, further processed by
psychiatric index
data processing unit 1030.
Turning to Figure 11, an exemplary embodiment of an endocrine index
determination
unit 481 is shown. The endocrine index determination unit 481 can comprise at
least one
signal unit 1112, capable of receiving a signal as described above from which
an endocrine
index can be derived. In a particular embodiment, as depicted, the endocrine
index
determination unit 481 can comprise a hormone signal unit 722, as described
above.
The endocrine index determination unit 481 can also comprise an endocrine
index
data processing unit 1130. The endocrine index data processing unit 1130 can
perform any
filtering, noise reduction, amplification, or other appropriate processing of
the data received
by the signal unit(s) 1112, 722 prior to calculation of the endocrine index.
The endocrine index determination unit 481 can also comprise an endocrine
index
calculation unit 1140. The endocrine index calculation unit 1140 can calculate
an endocrine
index from the data passed by the endocrine index processing unit 1130.
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
For example, the endocrine index calculation unit 1140 may calculate an
endocrine
index as received by signal unit(s) 1112, 722 and, optionally, further
processed by endocrine
index data processing unit 1130.
Turning to Figure 12 an exemplary embodiment of an adverse effect of therapy
index
.. determination unit 482 is shown. The adverse effect of therapy index
determination unit 482
can comprise at least one signal unit 1212, capable of receiving a signal as
described above
from which an adverse effect of therapy index can be derived. In a particular
embodiment, as
depicted, the adverse effect of therapy determination unit 482 can comprise
one or more of a
liver signal unit 1220, a bone marrow signal unit 1222, a kidney signal unit
1224, or a skin
signal unit signal unit 1226.
The adverse effect of therapy index determination unit 482 can also comprise
an
adverse effect of therapy index data processing unit 1230. The adverse effect
of therapy
index data processing unit 1230 can perform any filtering, noise reduction,
amplification, or
other appropriate processing of the data received by the signal unit(s) 1212-
1226 prior to
calculation of the adverse effect of therapy index.
The adverse effect of therapy index determination unit 482 can also comprise
an
adverse effect of therapy index calculation unit 1240. The adverse effect of
therapy index
calculation unit 1240 can calculate an adverse effect of therapy index from
the data passed by
the adverse effect of therapy index processing unit 1230.
For example, the adverse effect of therapy index calculation unit 1240 may
calculate
an adverse effect of therapy index as received by signal unit(s) 1212-1226
and, optionally,
further processed by adverse effect of therapy index data processing unit
1230.
Turning to Figure 13, a block diagram of an index comparison unit 495 is
depicted.
The index comparison unit 495 comprises an index data interface 1310, which
receives index
information from one or more of the index determination units 465, 475, 485,
477, and 479;
46
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
an index data lookup unit 1320, which looks up a reference value for the index
from a lookup
table; and a reference value to index comparison unit 1330, which compares the
determined
index value from units 465-485 to the reference index value returned by index
data lookup
unit 1320.
Turning to Figure 14, a block diagram of an assessment unit 487 is depicted.
The
assessment unit 487 receives comparison information from index comparison unit
495 and
performs an assessment of at least one disease state, at least one
comorbidity, or both. The
assessment unit 487 contains an assessment processing unit 1410 that processes
the
comparison information in view of one or more stored or otherwise accessible
assessment
criteria returned by assessment criteria unit 1420. The assessment can be
performed in real-
time (without substantial delay between performing the assessment and taking
any prior
action referred to herein) or off-line (involving calculations making use of
data stored for
some length of time). The assessment processing unit 1410 yields an
assessment. If the
assessment unit 487 assesses a disease state, the output can comprise at least
one of disease
stability, disease progression, disease regression, or a finding that a
disease state cannot be
determined with a sufficient degree of confidence. If the assessment unit 487
assesses a state
of a body system of a patient, the output can comprise at least one of body
system stability,
body system improvement, body system decline, or a finding that a body system
of the
system cannot be determined with a sufficient degree of confidence. Exemplary
body
systems include, but are not limited to, an autonomic system, a neurologic
system, a
psychiatric system, an endocrine system, a hepatic system, a bone marrow
system, a renal
system, and a skin system. The assessment unit 487 can then store or log its
assessment in a
memory, provide an output to the patient, a caregiver, or a physician, or the
like.
Alternatively or in addition, one or more of the indices considered in making
the assessment
can also be stored or logged.
47
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
In a particular embodiment, the output of the assessment can comprise
quantitative
data relating to the state of the disease or the body system. For example, in
one embodiment,
the output can comprise at least one of a magnitude of a change of a
progression, a magnitude
of a change of a regression, a rate of change of a progression, or a rate of
change of a
regression, a magnitude of a change of an improvement, a magnitude of a change
of a
decline, a rate of change of an improvement, or a rate of change of a decline.
Alternatively or
in addition, an output of an assessment of a disease state can comprise
identifying new
comorbidities not previously identified. These data can be used to assess the
overall state of
health of the subject and, when analyzed in the context of quality of life
index value, provide
an assessment of the patient's well being.
Regardless of its content, the output can be any form of audio, visual, or
other
communication. Exemplary outputs include, but are not limited to, text,
graphics, video,
animation, a sound tone or tones, and melodies, among others. The outputs can
be sent to
any device capable of presenting them to a user, such as a telephone, a
handheld device, a
computer, a television, or a loudspeaker, among others.
Turning to Figure 15, a block diagram of a forecast (or prognosis) unit 1500
is
depicted. The forecast (or prognosis) unit comprises a forecast data receiving
module 1510
capable of receiving information from one or more of the assessment unit 487,
one or more
index determination units 465, 475, 477, 479, or 485, one or more index
comparison units
495, or a memory 417 storing prior outputs of such a unit, and forecast (or
prognosis)
estimation and issuance module 1520 capable of estimating from the received
data a future
state of the disease, wherein the forecast comprises a disease stability, a
disease progression,
a disease regression, and/or a state of the body system, wherein the forecast
comprises a body
system stability, a body system improvement, a body system decline, or a
finding that no
forecast can be made. In a further embodiment, wherein the forecast is of
disease
48
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
progression, the forecast comprises at least one of a risk of an increased
magnitude of change
of progression, a risk of an increased rate of change of progression, or a
risk of emergence of
one or more comorbidities associated with the disease. These data can be used
to issue a
prognosis for a state of health of patient. Forecasts or prognoses may be
based on
qualitative, semiquantitative, or qualitative measures obtained using
conventional forecasting
methods, such as those used in the fields of geophysics, finance, population
dynamics
including epidemics, material sciences (e.g. predicting material fatigue), and
dynamical
control systems. When appropriate, clinical judgment may be applied alone or
in the frame
of Bayesian statistics.
In one embodiment, a medical device system is provided. The medical device
system
can comprise an interface to receive at least one of autonomic data,
neurologic data, and
quality of life data. The interface can be similar to that described above.
The medical device system can comprise at least one of an autonomic index
determination unit capable of determining at least one autonomic index, a
neurologic index
determination capable of determining at least one neurologic index unit, or a
quality of life
index determination unit capable of determining at least one quality of life
index. The index
determination unit(s) can be similar to those described above.
The medical device system can comprise an index comparison unit capable of
comparing at least one index with at least one reference value. The index
comparison unit
can be similar to those described above.
The medical device system can comprise a assessment unit capable of assessing
a
state of a disease or a body system of a patient based on at least one output
of the index
comparison unit. The assessment unit can be similar to those described above.
In one embodiment, the medical device system further comprises a therapy unit
adapted to deliver a therapy for the disease to a patient. The therapy unit
may administer
49
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
therapy in a contingent ("closed-loop") manner in response to a particular
manifestation of
the disease, or in a non-contingent ("open-loop") manner without reference to
at least one
particular manifestation of the disease. A combination of contingent and non-
contingent
therapies may be also administered. Further, when therapy is administered in a
contingent
manner, a particular therapy regimen may be selected in response to particular
findings
output by the assessment unit. For example, if the assessment unit finds the
patient's disease
state is worsening, one or more therapy parameters may be modified to provide
a more
intensive therapy. For example, if the therapy is electrical stimulation of a
neural tissue of a
patient, the on-time, amplitude, and/or frequency of stimulation may be
increased, and/or the
off-time decreased, to provide a more intensive therapy. Therapy type (e.g.,
electrical,
pharmacological, thermal, cognitive, etc.), and parameters including time or
timing of
delivery may be tailored to state variations in the probability of occurrence
or severity of a
manifestation of the disease.
In another embodiment, the medical device system further comprises an acute
disease
state detection unit adapted to detect an acute disease state in the patient.
By "acute disease
state" is meant a particular manifestation of the disease that is more intense
or debilitating
than the patient's baseline presentation. For example, if the disease is
epilepsy, the "acute
disease state" can be an epileptic event, such as a seizure. In this example,
the acute disease
state detection unit may be adapted to detect an epileptic event.
In another embodiment, the medical device system further comprises a disease
warning unit adapted to provide a warning signal of change in a disease state,
of an
impending acute disease state, or a change in a body system parameter. For
example, a
warning signal may be provided if there is a change in a cardiovascular index
that may be
deemed serious, exceeds a predetermined value, or if there may be an impending
seizure.
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
This warning signal may be delivered to the subject, a caregiver, or to an
emergency medical
unit.
In one embodiment, the warning signal may be proportional to at least one of a
magnitude of a change of a disease progression, a rate of change of a disease
progression, or
a correlation of the index value to life span (deterioration in an autonomic
cardiac parameter
may be more likely to negatively impact life span than in a neurologic index,
such as
cognitive decline). Alternatively or in addition, in one embodiment, the
warning signal may
be proportional to at least one of a magnitude of a change of a body system
decline or a rate
of change of a body system decline. For example, the warning signal may
comprise a tone
characterized by a pitch and/or a volume, and the warning signal may become
higher in pitch
and/or louder in proportion to a magnitude or rate of change of a disease
state or a body
system decline.
In addition to components of the medical device 400 described above, an
implantable
medical system may comprise a storage unit to store an indication of at least
one of seizure or
an increased risk of a seizure. The storage unit may be the memory 417 of the
medical
device 400, another storage unit of the medical device 400, or an external
database, such as
the local database unit 455 or a remote database unit 450. The storage unit
can allow
retention of a history of index values and/or assessments of disease state
and/or comorbidity.
For example, the output from this storage unit can be sent to the assessment
unit 487 to
quantify disease state and determine if there is progression, stabilization,
or regression of the
disease, or if a determination cannot be made. Alternatively or in addition,
the output from
this storage unit can be sent to the assessment unit 487 to quantify a state
of a body system of
a patient. The medical device 400 may communicate the indication via the
communications
unit 460. Alternatively or in addition to an external database, the medical
device 400 may be
51
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
adapted to communicate the indication to at least one of a patient, a
caregiver, or a healthcare
provider.
In various embodiments, one or more of the units or modules described above
may be
located in a monitoring unit 470 or a remote device 492, with communications
between that
unit or module and a unit or module located in the medical device 400 taking
place via
communication unit 460. For example, in one embodiment, one or more of the
interface, the
autonomic index determination unit 465, the neurologic index determination
unit 475, the
stress marker index determination unit 477, the psychiatric index
determination unit 479, an
endocrine index determination unit 481, an adverse effect of therapy index
determination unit
482, a physical fitness index determination unit 483, the quality of life
index determination
unit 485, the index comparison unit 495, and/or the assessment unit 487 may be
external to
the medical device 400, e.g., in a monitoring unit 470. Locating one or more
of the foregoing
units outside the medical device 400 may be advantageous if the calculation(s)
is/are
computationally intensive, in order to reduce energy expenditure and heat
generation in the
medical device 400 or to expedite calculation.
The monitoring unit 470 may be a device capable of transmitting and receiving
data to
and from the medical device 400. In one embodiment, the monitoring unit 470
may be a
computer system capable of executing a data-acquisition program. The
monitoring unit 470
may be controlled by a healthcare provider, such as a physician, at a base
station in, for
example, a doctor's office. In alternative embodiments, the monitoring unit
470 may be
controlled by a patient in a system providing less interactive communication
with the medical
device 400 than another monitoring unit 470 controlled by a healthcare
provider. Whether
controlled by the patient or by a healthcare provider, the monitoring unit 470
may be a
computer, preferably a handheld computer or PDA, but may alternatively
comprise any other
.. device that may be capable of electronic communications and programming,
e.g., hand-held
52
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
computer system, a PC computer system, a laptop computer system, a server, a
personal
digital assistant (PDA), an Apple-based computer system, a cellular telephone,
etc. The
monitoring unit 470 may download various parameters and program software into
the
medical device 400 for programming the operation of the medical device, and
may also
.. receive and upload various status conditions and other data from the
medical device 400.
Communications between the monitoring unit 470 and the communication unit 460
in the
medical device 400 may occur via a wireless or other type of communication,
represented
generally by line 477 in Figure 4. This may occur using, e.g., wand 355
(Figure 3) to
communicate by RF energy with an implantable signal generator 310.
Alternatively, the
wand may be omitted in some systems, e.g., systems in which the MD 400 may be
non-
implantable, or implantable systems in which monitoring unit 470 and MD 400
operate in the
MICS bandwidths.
In one embodiment, the monitoring unit 470 may comprise a local database unit
455.
Optionally or alternatively, the monitoring unit 470 may also be coupled to a
database unit
450, which may be separate from monitoring unit 470 (e.g., a centralized
database wirelessly
linked to a handheld monitoring unit 470). The database unit 450 and/or the
local database
unit 455 are capable of storing various patient data. These data may comprise
patient
parameter data acquired from a patient's body, therapy parameter data, seizure
severity data,
therapeutic efficacy data, and/or disease state assessment data. The database
unit 450 and/or
the local database unit 455 may comprise data for a plurality of patients, and
may be
organized and stored in a variety of manners, such as in date format, severity
of disease
format, etc. The database unit 450 and/or the local database unit 455 may be
relational
databases in one embodiment. A physician may perform various patient
management
functions (e.g., programming parameters for a responsive therapy and/or
setting thresholds
for one or more detection parameters) using the monitoring unit 470, which may
include
53
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
obtaining and/or analyzing data from the medical device 400 and/or data from
the database
unit 450 and/or the local database unit 455. The database unit 450 and/or the
local database
unit 455 may store various patient data.
One or more of the blocks illustrated in the block diagrams of the medical
device 400
in Figures 4-12 may comprise hardware units, software units, firmware units,
or any
combination thereof Additionally, one or more blocks illustrated in Figures 4-
12 may be
combined with other blocks, which may represent circuit hardware units,
software
algorithms, etc. Additionally, any number of the circuitry or software units
associated with
the various blocks illustrated in Figures 4-12 may be combined into a
programmable device,
such as a field programmable gate array (FPGA), an ASIC device, etc.
In one embodiment, the present disclosure may include coupling of at least one
electrode to each of two or more cranial nerves. (In this context, two or more
cranial nerves
mean two or more nerves having different names or numerical designations, and
do not refer
to the left and right versions of a particular nerve). In one embodiment, at
least one electrode
may be coupled to either or both vagus nerves or a branch of either or both
vagus nerves.
The term "operatively" coupled may include directly or indirectly coupling.
Each of the
nerves in this embodiment or others involving two or more cranial nerves may
be stimulated
according to particular activation modalities that may be independent between
the two
nerves.
Although not so limited, in one embodiment, the method further comprises
applying a
therapy to a neural tissue of the patient, in response to the assessing. In a
further
embodiment, the therapy may be an electrical therapy. In a further embodiment,
the neural
tissue may be a cranial nerve, such as the vagus nerve.
Therapies using electrical currents or fields to provide a therapy to a
patient
(electrotherapy) are beneficial for certain neurological disorders, such as
epilepsy.
54
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
Implantable medical devices have been effectively used to deliver therapeutic
electrical
stimulation to various portions of the human body (e.g., the vagus nerve) for
treating
epilepsy. As used herein, "stimulation," "neurostimulation," "stimulation
signal,"
"therapeutic signal," or "neurostimulation signal" refers to the direct or
indirect application of
an electrical, mechanical, magnetic, electro-magnetic, photonic, acoustic,
cognitive, and/or
chemical signal to a neural structure in the patient's body. The signal may be
an exogenous
signal that is distinct from the endogenous electro-chemical,activity inherent
to the patient's
body and the environment. In other words, the stimulation signal (whether
electrical,
mechanical, magnetic, electro-magnetic, photonic, acoustic, cognitive, and/or
chemical in
nature) applied to a cranial nerve or to other nervous tissue structure in the
present disclosure
may be a signal applied from a medical device, e.g., a neurostimulator.
Alternatively or in
addition, electrochemical activity inherent to the patient's body or brain may
be tapped,
harnessed or modified (as in the case of cognitive therapy) to treat a disease
manifestation or
the disease itself
A "therapeutic signal" refers to a stimulation signal delivered to a patient's
body with
the intent of treating a medical condition through a suppressing (blocking) or
modulating
effect to neural tissue. The effect of a stimulation signal on neuronal
activity may be
suppressing or modulating; however, for simplicity, the terms "stimulating",
suppressing and
modulating, and variants thereof, are sometimes used interchangeably herein.
In general,
however, the delivery of an exogenous signal itself refers to "stimulation" of
the neural
structure, while the effects of that signal, if any, on the electrical
activity of the neural
structure are properly referred to as suppression or modulation.
Depending upon myriad factors such as the history (recent and distant) of the
nervous
system, stimulation parameters and time of day, to name a few, the effects of
stimulation
upon the neural tissue may be excitatory or inhibitory, facilitatory or
disfacilitatory and may
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
suppress, enhance or leave unaltered, neuronal activity. One can witness a
suppressing effect
when, for example, a stimulation signal applied to neural tissue prevents or
ameliorates
abnormal neurological activity (e.g., epileptic seizures). This suppressing
effect takes place
through multiple mechanisms, as described in the foregeoing articles.
Suppression of
abnormal neural activity is a threshold or suprathreshold process and the
temporal scale over
which it may occur is usually on the order of tens or hundreds of
milliseconds. Modulation of
abnormal or undesirable neural activity, unlike suppression is a "sub-
threshold" process in
the spatio-temporal domain that may summate and result under certain
conditions, in
threshold or suprathreshold neural events. The temporal scale of modulation is
much longer
.. than that of suppression, encompassing seconds to months or even years. In
addition to
inhibition or dysfacilitation, modification of neural activity may occur by
wave annihilation
(a concept borrowed from wave mechanics) or through phase resetting.
Electrotherapy may be provided by implanting an electrical device, i.e., an
implantable medical device (IMD), inside a patient's body stimulation of a
nervous tissue,
such as a cranial nerve. Generally, electrotherapy signals that suppress or
modulate neural
activity are delivered by the IMD via one or more leads or wirelessly. When
applicable, the
leads generally terminate at their distal ends in one or more electrodes, and
the electrodes, in
turn, are coupled to tissue in the patient's body. For example, a number of
electrodes may be
attached to various points of a nerve or other tissue inside a human body for
delivery of a
neurostimulation signal.
While contingent (also referred to as "closed-loop," "active," or "feedback"
stimulation (i.e., electrotherapy applied in response to sensed information,
such as heart rate))
stimulation schemes have been proposed, non-contingent, programmed periodic
stimulation
is the prevailing modality. For example, vagus nerve stimulation for the
treatment of
epilepsy usually involves a series of grouped electrical pulses defined by an
"on-time" (such
56
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
as 30 sec) and an "off-time" (such as 5 min). This type of stimulation may be
also referred to
as "open-loop," "passive," or "non-feedback" stimulation. Each sequence of
pulses during an
on-time may be referred to as a "pulse burst." The burst may be followed by
the off-time
period in which no signals are applied to the nerve. During the on-time,
electrical pulses of a
defined electrical current (e.g., 0.5 - 2.0 milliamps) and pulse width (e.g.,
0.25 ¨ 1.0
milliseconds) are delivered at a defined frequency (e.g., 20 ¨ 30 Hz) for a
certain duration
(e.g., 10 - 60 seconds). The on-time and off-time parameters together define a
duty cycle,
which is the ratio of the on-time to the combination of the on-time and off-
time, and which
describes the percentage of time that the electrical signal is applied to the
nerve.
In open-loop VNS, the on-time and off-time may be programmed to define an
intermittent pattern in which a repeating series of electrical pulse bursts
are generated and
applied to a cranial nerve such as the vagus nerve. The off-time may be
provided to
minimize adverse effects and conserve power. If the off-time is set at zero,
the electrical
signal in open-loop VNS may provide continuous stimulation to the vagus nerve.
Alternatively, the off time may be as long as one day or more, in which case
the pulse bursts
are provided only once per day or at even longer intervals. Typically,
however, the ratio of
"off-time" to "on-time" may range from about 0.5 to about 10.
In addition to the on-time and off-time, the other parameters defining the
electrical
signal in VNS may be programmed over a range of values. The pulse width for
the pulses in
a pulse burst of open-loop VNS may be set to a value not greater than about 1
msec, such as
about 250-500 sec, and the number of pulses in a pulse burst may be typically
set by
programming a frequency in a range of about 20-300 Hz (i.e., 20 pulses per
second to 300
pulses per second). A non-uniform frequency may also be used. Frequency may be
altered
during a pulse burst by either a frequency sweep from a low frequency to a
high frequency, or
vice versa. Alternatively, the timing between adjacent individual signals
within a burst may
57
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
be randomly changed such that two adjacent signals may be generated at any
frequency
within a range of frequencies.
Returning to systems for providing cranial nerve stimulation, such as that
shown in
Figure 3, and as stated above, alternatively or in addition to a responsive
treatment, if any,
cranial nerve stimulation may be provided on a continuous basis to alleviate
chronic aspects
of the patient's medical disorder. Where cranial nerve stimulation may be
provided based
solely on programmed off-times and on-times, the stimulation may be referred
to as passive,
inactive, open-loop, non-feedback, or non-contingent stimulation. In contrast,
stimulation
may be triggered by one or more feedback loops according to changes in the
body or brain of
.. the patient. This stimulation may be referred to as active, closed-loop,
feedback-loop, or
contingent stimulation. In
one embodiment, feedback-loop stimulation may be
manually-triggered stimulation, in which the patient manually causes the
activation of a pulse
burst outside of the programmed on-time/off-time cycle at a time of the
patient's choosing,
for example, in response to a sensation of an impending seizure. The patient
may manually
activate an implantable signal generator 310 to stimulate the cranial nerve,
such as vagus
nerve 327, to treat an acute episode of a medical condition, e.g., a seizure.
The patient may
also be permitted to alter the intensity of the signals applied to the cranial
nerve within limits
established by the physician.
Patient activation of a medical device 300 may involve use of an external
control
magnet for operating a reed switch in an implanted device, for example.
Certain other
techniques of manual and automatic activation of implantable medical devices
are disclosed
in U.S. Pat. No. 5,304,206 to Baker, Jr., et al. ("the '206 patent").
According to the '206
patent, means for manually activating or deactivating the electrical signal
generator 310 may
include a sensor such as piezoelectric element mounted to the inner surface of
the generator
.. case and adapted to detect light taps by the patient on the implant site.
One or more taps
58
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
applied in fast sequence to the skin above the location of the electrical
signal generator 310 in
the patient's body may be programmed into the implanted medical device 300 as
a signal for
intensification of the electrical signal. Two taps spaced apart by a slightly
longer duration of
time may be programmed into the medical device 300 to indicate a desire to de-
intensify the
electrical signal. The patient may be given limited control over operation of
the device to an
extent which may be determined by the program or entered by the attending
physician. The
patient may also activate the medical device 300 using other suitable
techniques or apparatus.
In one embodiment, the medical device 400 may also be capable of detecting a
manual input from the patient. The manual input may include a magnetic signal
input, a tap
input, a wireless data input to the medical device 400, etc.
The above methods may be performed by a computer readable program storage
device encoded with instructions that, when executed by a computer, perform
the method
described herein.
In one embodiment, the disease is obesity. As depicted in Figure 16, with the
directionality of each arrow indicating an amplifying effect, obesity
substantially increases
the patient's risk of developing diabetes mellitus, arterial hypertension,
hyperlipidemia and
obstructive sleep apnea, while shortening life span and degrading quality of
life. Arterial
hypertension, diabetes and hyperlipidemia, in turn, accelerate
atherosclerosis, further
increasing the risks for myocardial infarction, stroke, congestive heart
failure, and avascular
gangrene. Similarly, obstructive sleep apnea causes intractable arterial
hypertension, atrial
fibrillation, cognitive deterioration, depression, sexual dysfunction, and
chronic headaches.
In one embodiment, the disease is epilepsy. Pharmaco-resistant seizures are
associated with an increase in mortality and morbidity rates (compared to the
general
population and to epileptics whose seizures are controlled by medications),
eventual
impairment of cognitive functions and mental health, and markedly degraded
quality of life
59
- CA 02802694 2016-02-22
W02011/159592
PCT/US2011/040130
,
for patients and their families. Seizures may impair motor control,
responsiveness to a wide
class of stimuli, and other cognitive functions. Certain pharmacological
agents used for
treatment of epilepsy cause osteoporosis, reproductive dysfunction, liver and
bone marrow
damage, and in rare cases, death. Figure 17 depicts relationships between
epilepsy and some
of its comorbidities, with the directionality of each arrow indicating an
amplifying effect.
All of the methods and apparatuses disclosed and claimed herein may be made
and
executed without undue experimentation in light of the present disclosure.
While the methods
and apparatus of this disclosure have been described in terms of particular
embodiments, it will
be apparent to those skilled in the art that variations may be applied to the
methods and
apparatus and in the steps, or in the sequence of steps, of the method
described herein without
departing from the concept and scope of the disclosure, as defined by the
appended claims,
which are construed following a purposive construction according to Canadian
Law. It should be
especially apparent that the principles of the disclosure may be applied to
selected cranial
nerves other than, or in addition to, the vagus nerve to achieve particular
results in treating
patients having epilepsy, depression, or other medical conditions.
In various embodiments, the present disclosure relates to the subject matter
of the
following numbered paragraphs:
1. A medical device system, comprising:
at least one of an autonomic index determination unit capable of determining
at least
one autonomic index, a neurologic index determination unit capable of
determining at least one
neurologic index, a stress marker index determination unit capable of
determining at least one
stress marker index, a psychiatric index determination unit capable of
determining at least one
psychiatric index, an endocrine index determination unit capable of
determining at least one
endocrine index, an adverse effect of therapy index determination unit capable
of determining at
least one adverse effect of therapy index, a physical fitness index
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
determination unit capable of determining at least one physical fitness index,
or a quality of
life index determination unit capable of determining at least one quality of
life index;
an index comparison unit capable of comparing at least one index with at least
one
reference value;
a body system state assessment unit capable of assessing a state of a body
system of a
patient, wherein the body system comprises at least one of an autonomic
system, a neurologic
system, a psychiatric system, an endocrine system, an hepatic system, a renal
system, a bone
marrow system, a skin system, or subsystems of the foregoing; and
an output unit capable of providing an output relating to the assessment,
wherein the
output comprises at least one of body system stability, body system
improvement, body
system decline, or a finding that a state of the body system cannot be
determined.
2. The medical device system of numbered paragraph 1, further comprising a
disease state assessment unit capable of assessing a state of a disease,
wherein the output
comprises disease stability, disease progression, disease regression, or a
finding that a disease
.. state cannot be determined.
3. The medical device system of numbered paragraph 2, further comprising:
a comorbidity identification unit adapted to identify a comorbidity associated
with the
disease.
4. The medical device system of numbered paragraph 1, further comprising:
at least one interface capable of receiving at least one of an autonomic
signal, a
neurologic signal, a stress marker signal, a psychiatric signal, an endocrine
signal, an adverse
effect of therapy signal, a physical fitness signal, or a quality of life
signal of a patient.
5. The medical device system of numbered paragraph 1, further comprising:
61
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
a forecast unit capable of forecasting a state of the body system, wherein the
forecast
comprises a body system stability, a body system improvement, a body system
decline, or a
finding that no forecast can be made.
6. The medical device system of numbered paragraph 1, further comprising:
a logging unit capable of logging one or more of the assessments or indices.
7. The medical device system of numbered paragraph 1, wherein the autonomic
index determination unit comprises at least one of:
a cardiovascular indication processing unit,
a respiration indication processing unit,
a blood parameter indication processing unit,
a pupillary response indication processing unit,
a body temperature indication processing unit, or
a skin resistance indication processing unit; and
the neurologic index determination unit comprises at least one of:
an attention aptitude indication processing unit,
a responsiveness indication processing unit,
a memory indication processing unit,
a kinetic indication processing unit, or
a cognitive aptitude indication processing unit; and
the stress marker index determination unit comprises at least one of:
a cortisol parameter indication processing unit, or
a catecholamine parameter indication processing unit.
8. A medical device system, comprising:
an autonomic index determination unit capable of determining at least one
autonomic
index;
62
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
a neurologic index determination unit capable of determining at least one
neurologic
index;
an index comparison unit capable of comparing the at least one autonomic index
with
at least one first reference value and the at least one neurologic index with
at least one second
reference value;
an epilepsy disease state assessment unit capable of assessing a state of an
epilepsy
disease; and
an output unit capable of providing an output relating to the assessment,
wherein the
output comprises at least one of disease stability, disease progression,
disease regression, or a
finding that a disease state cannot be determined.
9. The medical device system of numbered paragraph 8, further comprising:
a warning unit adapted to provide a warning if the epilepsy disease state
assessment
unit yields an assessment of disease progression.
10. The medical device system of numbered paragraph 8, further comprising:
a therapy unit adapted to deliver a therapy for epilepsy to a patient.
11. The medical device system of numbered paragraph 8, further comprising:
at least one interface capable of receiving at least one of autonomic data or
neurologic
data.
12. The medical device system of numbered paragraph 8, further comprising:
a forecast unit capable of forecasting a state of the disease, wherein the
forecast
comprises a disease stability, a disease progression, a disease regression, or
a finding that no
forecast can be made.
13. The medical device system of numbered paragraph 8, further comprising:
a logging unit capable of logging one or more of the assessments or indices.
63
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
14. The medical device system of numbered paragraph 8, wherein the
autonomic
index determination unit comprises at least one of:
a cardiovascular indication processing unit,
a respiration indication processing unit,
a blood parameter indication processing unit,
a pupillary response indication processing unit,
a body temperature indication processing unit, or
a skin resistance indication processing unit; and
the neurologic index determination unit comprises at least one of:
an attention aptitude indication processing unit,
a responsiveness indication processing unit,
a memory indication processing unit,
a kinetic indication processing unit, or
a cognitive aptitude indication processing unit.
15. The medical device system of numbered paragraph 8, further comprising:
a comorbidity identification unit adapted to identify a comorbidity associated
with the
disease.
16. A medical device system, comprising:
at least one assessment unit capable of assessing at least one of a patient's
disease
state, a quality of life, or a physical fitness,
at least one determination unit capable of determining at least one of a
disease state
assessment, a quality of life assessment, or a physical fitness assessment;
64
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
at least one comparison unit capable of comparing the at least one disease
state
assessment, quality of life assessment, or physical fitness assessment to at
least one reference
value,
a disease state assessment unit capable of assessing at least one of disease
state,
quality of life, or physical fitness; and
an output unit capable of providing an output relating to an assessment of the
patient's
health, wherein the output comprises disease stability, disease progression,
disease
regression, or a finding that a disease state cannot be determined.
101. A computer readable program storage unit encoded with instructions that,
when executed by a computer, perform a method for assessing a primary disease
state and a
body system impacted by the primary disease, comprising:
receiving at least a first index and a second index, each index relating to at
least one
of an autonomic index, a neurologic index, a stress marker index, a
psychiatric index, an
endocrine index, an adverse effect of therapy index, a physical fitness index,
or a quality of
life index of a patient,
comparing the at least one first index to at least one first reference value
associated
with the at least one first index;
comparing the at least one second index to at least one second reference value
associated with the at least one second index;
assessing a state of said primary disease of the patient based on the
comparing;
assessing a state of a body system of the patient based on the assessing the
state of the
disease, wherein the body system comprises at least one of an autonomic
system, a
neurologic system, a psychiatric system, an endocrine system, a hepatic
system, a renal
system, a bone marrow system, a skin system, or subsystems of the foregoing;
and
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
providing an output relating to the assessment of the state of the primary
disease and
the assessment of the state of the body system, wherein the output comprises
at least one of
disease stability, disease progression, disease regression, or a finding that
a disease state
cannot be determined, and the output further comprises at least one of body
system stability,
body system improvement, body system decline, or a finding that a state of the
body system
cannot be determined.
102. The computer readable program storage unit of numbered paragraph 101,
wherein the method further comprises:
assessing a state of a second disease of the patient based on the comparing,
wherein
the output further comprises at least one of disease stability, disease
progression, disease
regression, or a finding that a disease state cannot be determined; and
assessing a state of a second body system of the patient based on the
comparing,
wherein the body system comprises at least one of an autonomic system, a
neurologic system,
a psychiatric system, an endocrine system, a hepatic system, a renal system, a
bone marrow
.. system, a skin system, or subsystems of the foregoing, and the output
further comprises at
least one of body system stability, body system improvement, body system
decline, or a
finding that a state of the body system cannot be determined.
103. The computer readable program storage unit of numbered paragraph 101,
wherein the output comprises at least one of a magnitude of a change of a
progression, a
magnitude of a change of a regression, a rate of change of a progression, or a
rate of change
of a regression, a magnitude of a change of an improvement, a magnitude of a
change of a
regression, a rate of change of an improvement, or a rate of change of a
regression.
104. The computer readable program storage unit of numbered paragraph 101,
wherein the at least one first index is at least one autonomic index and the
at least one second
index is at least one neurologic index.
66
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
105. The computer readable program storage unit of numbered paragraph 104,
wherein the at least one autonomic index comprises a cardiovascular parameter,
a respiration
parameter, a body temperature parameter, a skin resistance parameter, or two
or more thereof;
and
the at least one neurologic index comprises an attention aptitude parameter, a
responsiveness parameter, a memory parameter, a kinetic parameter, a cognitive
aptitude
parameter, or two or more thereof
106. The computer readable program storage unit of numbered paragraph 101,
wherein the disease is epilepsy.
107. The computer readable program storage unit of numbered paragraph 101,
wherein the method further comprises providing a warning signal to the
patient, a physician,
or a caregiver if assessing indicates at least one of disease progression or
body system
decline.
108. The computer readable program storage unit of numbered paragraph 107,
wherein the warning signal is proportional to at least one of a magnitude of a
change of a
progression, a rate of change of a progression, a magnitude of a change of a
body system
decline, or a rate of change of a body system decline.
109. The computer readable program storage unit of numbered paragraph 101,
wherein the first index comprises a weighted composite of a first plurality of
autonomic
indices, neurologic indices, stress marker indices, psychiatric indices,
endocrine indices,
adverse effect of therapy indices, physical fitness indices, quality of life
indices, or two or
more thereof;
the second index comprises a weighted composite of a second plurality of
autonomic
indices, neurologic indices, stress marker indices, psychiatric indices,
endocrine indices,
67
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
adverse effect of therapy indices, physical fitness indices, quality of life
indices, or two or
more thereof;
or both.
110. The computer readable program storage unit of numbered paragraph 101,
wherein at least one of the first reference value or the second reference
value is based on the
patient's history or on normative data.
111. The computer readable program storage unit of numbered paragraph 101,
wherein at least one of the first index or the second index comprises a
measure of central
tendency, a measure of dimensionality, a measure of fractality, a measure of
stationarity, a
measure of long-range dependency, a measure of clustering, a distribution of
measures of
central tendency, a distribution of measures of dimensionality, a distribution
of measures of
fractality, a distribution of measures of stationarity, a distribution of
measures of long-range
dependency, a distribution of measures of clustering, or two or more thereof
112. The computer readable program storage unit of numbered paragraph 101,
wherein the method further comprises forecasting a state of the disease,
wherein the forecast
comprises a disease stability, a disease progression, a disease regression, or
a finding that no
forecast can be made.
113. The computer readable program storage unit of numbered paragraph 112,
wherein the forecast is of disease progression and the forecast comprises at
least one of a risk
of an increased magnitude of change of progression, a risk of an increased
rate of change of
progression, or a risk of emergence of one or more comorbidities associated
with the disease.
114. The computer readable program storage unit of numbered paragraph 101,
wherein the method further comprises forecasting a state of the body system,
wherein the
forecast comprises a body system stability, a body system improvement, a body
system
decline, or a finding that no forecast can be made.
68
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
115. The computer readable program storage unit of numbered paragraph 101,
wherein the assessment of the state of the disease comprises identifying one
or more
comorbidities associated with the disease.
116. A computer readable program storage unit encoded with instructions that,
when executed by a computer, perform a method for assessing a patient's
health, comprising:
receiving at least one assessment of at least one of a patient's disease
state, a quality of
life, or a physical fitness,
comparing the at least one assessment to at least one reference value
associated with
at least one previous assessment from the patient or with normative data,
assessing at least one of disease state, quality of life, or physical fitness
based on the
comparing; and
providing an output relating to an assessment of the patient's health, wherein
the
output comprises at least one of disease state stability, disease state
progression, disease state
regression, a finding that the disease state cannot be determined, quality of
life stability,
quality of life improvement, quality of life decline, a finding that the
quality of life cannot be
determined, physical fitness stability, physical fitness improvement, physical
fitness decline,
or a finding that physical fitness cannot be determined.
201. A medical device system, comprising:
at least one of an autonomic index determination unit capable of determining
at least
one autonomic index, a neurologic index determination unit capable of
determining at least
one neurologic index, a stress marker index determination unit capable of
determining at least
one stress marker index, a psychiatric index determination unit capable of
determining at
least one psychiatric index, an endocrine index determination unit capable of
determining at
least one endocrine index, an adverse effect of therapy index determination
unit capable of
determining at least one adverse effect of therapy index, a physical fitness
index
69
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
determination unit capable of determining at least one physical fitness index,
or a quality of
life index determination unit capable of determining at least one quality of
life index;
an index comparison unit capable of comparing at least one first index with at
least
one first reference value associated with the at least one first index and
comparing at least one
second index with at least one second reference value associated with the at
least one second
index;
a body system state assessment unit capable of assessing a state of a body
system of a
patient, wherein the body system comprises at least one of an autonomic
system, a neurologic
system, a psychiatric system, an endocrine system, a hepatic system, a renal
system, a bone
marrow system, a skin system, or subsystems of the foregoing;
a disease state assessment unit capable of assessing a state of a disease; and
an output unit capable of providing an output relating to the assessment,
wherein the
output comprises at least one of body system stability, body system
improvement, body
system decline, or a finding that a state of the body system cannot be
determined, and the
output further comprises disease stability, disease progression, disease
regression, or a
finding that a disease state cannot be determined.
202. The medical device system of numbered paragraph 201, further comprising:
at least one interface capable of receiving at least one of an autonomic
index, a
neurologic index, a stress marker index, a psychiatric index, an endocrine
index, an adverse
effect of therapy index, a physical fitness index, or a quality of life index
of a patient.
203. The medical device system of numbered paragraph 201, further comprising:
a forecast unit capable of forecasting at least one of a state of the disease
or a state of
the body system, wherein the forecast comprises a disease stability, a disease
progression, a
disease regression, a body system stability, a body system improvement, a body
system
decline, or a finding that no forecast can be made.
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
204. The medical device system of numbered paragraph 201, further comprising:
a logging unit capable of logging one or more of the assessments or indices.
205. The medical device system of numbered paragraph 201, wherein the
autonomic index determination unit comprises at least one of:
a cardiovascular indication processing unit,
a respiration indication processing unit,
a blood parameter indication processing unit,
a pupillary response indication processing unit,
a body temperature indication processing unit, or
a skin resistance indication processing unit; and
the neurologic index determination unit comprises at least one of:
an attention aptitude indication processing unit,
a responsiveness indication processing unit,
a memory indication processing unit,
a kinetic indication processing unit, or
a cognitive aptitude indication processing unit; and
the stress marker index determination unit comprises at least one of:
a cortisol parameter indication processing unit, or
a catecholamine parameter indication processing unit.
206. The medical device system of numbered paragraph 201, further comprising:
a comorbidity identification unit adapted to identify a comorbidity associated
with the
disease.
207. A medical device system, comprising:
at least one of an autonomic index determination unit capable of determining
at least
one of a cardiovascular parameter, a respiratory parameter, or an autonomic
parameter of a
71
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
patient; or a neurologic index determination unit capable of determining at
least one of a
responsiveness parameter or a kinetic parameter of the patient;
an index comparison unit capable of comparing the cardiovascular parameter
with at
least one first reference value, the respiratory parameter with at least one
second reference
value, and the kinetic parameter with at least one third reference value;
a real-time body system state assessment unit capable of assessing in real-
time a state
of a body system of the patient, wherein the body system comprises at least
one of an
autonomic system, a neurologic system, a psychiatric system, an endocrine
system, a hepatic
system, a renal system, a bone marrow system, a skin system, or subsystems of
the foregoing;
a communication unit capable of sending at least one of the real-time
assessment, the
at least one autonomic parameter, or the at least one neurologic parameter to
an off-line body
system state assessment unit;
an off-line body system state assessment unit capable of (a) receiving the at
least one
of the real-time assessment, the at least one autonomic parameter, or the at
least one
neurologic parameter; (b) receiving at least one second index comprising at
least one of an
autonomic index, a neurologic index, a stress marker index, a psychiatric
index, an endocrine
index, an adverse effect of therapy index, a physical fitness index, or a
quality of life index of
a patient; and (c) assessing off-line a state of a body system of the patient,
wherein the body
system comprises at least one of an autonomic system, a neurologic system, a
psychiatric
system, an endocrine system, a hepatic system, a renal system, a bone marrow
system, a skin
system, or subsystems of the foregoing; and
an output unit capable of providing an output relating to at least one of the
real-time
assessment or the off-line assessment, wherein the output relating to the real-
time assessment
comprises at least one of body system stability, body system improvement, body
system
decline, or a finding that a state of the body system cannot be determined,
and the output
72
CA 02802694 2012-12-13
WO 2011/159592
PCT/US2011/040130
relating to the off-line assessment comprises at least one of body system
stability, body
system improvement, body system decline, or a finding that a state of the body
system cannot
be determined.
208. The medical device system of numbered paragraph 207, wherein the
communication unit is further capable of providing a warning signal to the
patient, a
physician, or a caregiver if assessing in real-time indicates body system
decline.
209. The medical device system of numbered paragraph 208, wherein the warning
signal is proportional to at least one of a magnitude of a change in body
system decline or a
rate of change in body system decline.
210. The medical device system of numbered paragraph 208, further comprising a
storage unit capable of storing at least one of the real-time assessment, the
off-line
assessment, the at least one autonomic index, the at least neurologic index,
or the at least one
second index.
211. A medical device system, comprising:
at least one assessment unit capable of assessing at least one of a patient's
disease
state, a quality of life, or a physical fitness,
at least one determination unit capable of determining at least one of a
disease state
assessment, a quality of life assessment, or a physical fitness assessment;
at least one comparison unit capable of comparing the at least one disease
state
assessment, quality of life assessment, or physical fitness assessment to at
least one reference
value,
a disease state assessment unit capable of assessing at least one of disease
state,
quality of life, or physical fitness; and
an output unit capable of providing an output relating to an assessment of the
patient's
health, wherein the output comprises disease state stability, disease state
progression, disease
73
CA 02802694 2016-02-22
W02011/159592
PCT/US2011/040130
state regression, a finding that the disease state cannot be determined,
quality of life stability,
quality of life improvement, quality of life decline, a finding that the
quality of life cannot be
determined, physical fitness stability, physical fitness improvement, physical
fitness decline, or a
finding that physical fitness cannot be determined.
The particular embodiments disclosed above are illustrative only as the
disclosure may
be modified and practiced in different but equivalent manners apparent to
those skilled in the art
having the benefit of the teachings herein. Furthermore, no limitations are
intended to the details
of construction or design herein shown other than as described in the claims
below. It is,
therefore, evident that the particular embodiments disclosed above may be
altered or modified
and all such variations are considered within the scope of the disclosure and
the claims, which
are construed following a purposive construction according to Canadian Law.
Accordingly, the
protection sought herein is as set forth in the claims below.
74