Note: Descriptions are shown in the official language in which they were submitted.
WO 2012/004769 CA 02804880 2013-01-091
PCT/1B2011/053050
METHOD AND APPARATUS FOR PRODUCING GAS
FIELD OF THE INVENTION
This invention relates to a method and apparatus for producing gas. More
particularly, but not exclusively, this invention relates to an electrolysis
cell
and method in which combustible gasses, such as hydrogen gas and oxygen
gas are produced through the electrolysis of an aqueous electrolytic solution
and are kept separate upon production.
BACKGROUND TO THE INVENTION
An electrolysis cell uses electricity to convert water to hydrogen and oxygen
in gas phase. A known electrolysis cell includes a proton exchange
membrane in order to separate the hydrogen and oxygen gases produced
through the electrolysis process. The electrolysis cell further includes an
anode positioned along a first face of the proton exchange membrane and a
cathode positioned along a second opposite face of the proton exchange
membrane.
A known proton exchange membrane is a semi-permeable membrane
generally made from ionomers and designed to conduct protons while being
impermeable to gases, such as oxygen and hydrogen. Proton exchange
membranes can be made from either pure polymer membranes or from
WO 2012/004769 CA 02804880 2013-01-092
PCT/1B2011/053050
composite membranes where other materials are embedded in a polymer
matrix.
A first disadvantage of the known proton exchange membrane is the high
cost of the membrane, since it requires that a noble-metal catalyst (typically
platinum) be used to separate the hydrogen's electrons and protons. The
platinum catalyst is also extremely sensitive to carbon monoxide poisoning,
making it necessary to employ an additional reactor to reduce carbon
monoxide in the fuel gas if the hydrogen is derived from an alcohol or
hydrocarbon fuel. This again adds to the cost of using the known proton
exchange membrane.
Further disadvantages of the know proton exchange membranes are their
poor conductivity at lower relative humidity and their poor mechanical
properties at temperatures above approximately 100 C. The operating
temperature of these membranes is relatively low and temperatures near 100
C are not high enough to perform useful cogeneration.
Another disadvantage of the known proton exchange membranes is that their
efficiency goes down as the voltage applied across the cell goes up, due to
poor gas removal from the membrane. Also, the electrodes cannot be
stacked too close together, as this will inhibit gas removal from the
membrane.
WO 2012/004769 CA 02804880 2013-01-093
PCT/1B2011/053050
In this specification, the term "combustible fluid" includes within its scope
combustible gas containing predominantly hydrogen and/or oxygen in gas
phase.
OBJECT OF THE INVENTION
It is accordingly an object of the present invention to provide a method and
apparatus for producing gas, with which the above disadvantages may be
overcome and which are useful alternatives to known electrolysis cells and
methods for producing gas.
SUMMARY OF THE INVENTION
According to a first aspect of the invention there is provided a method for
producing combustible fluid from an electrolytic solution during a process of
electrolysis including the steps of:
- providing an electrolytic solution;
- providing an electrolysing apparatus having first and second
spaced apart permeable electrodes, defining a chamber between
them, having at least one inlet;
- passing the solution into the chamber via the inlet; and
- applying a voltage to the apparatus across the electrodes to
electrolyse the solution in the chamber, so that a first combustible
fluid forms on the first electrode and a second combustible fluid
forms on the second electrode, and the first combustible fluid
WO 2012/004769 CA 02804880 2013-01-094
PCT/1B2011/053050
passes out of the chamber via the first electrode and the second
combustible fluid passes out of the chamber via the second
electrode.
The combustible fluid may be hydrogenated and oxygenated fluid and more
specifically the combustible fluid may be hydrogen and oxygen gas.
The permeable electrodes may each be perforated or foraminous.
Each permeable electrode may further be of a mesh or foam material.
Each permeable electrode may be made of a 316 stainless steel or nickel
material.
The first and second electrodes may be provided in relative close proximity to
one another and may be substantially parallel.
The first and second permeable electrodes may have a correct and
predetermined ratio of open to closed area (also known as the PPI (pores per
square inch)), which may be influenced by the size of the inlet and the
pressure of the solution being provided to the apparatus.
CA 02804880 2013-01-09
WO 2012/004769 PCT/1B2011/053050
5
The first and second permeable electrodes may be one set of permeable
electrodes and the apparatus may include a plurality of sets of permeable
electrodes, all having a similar configuration.
The electrolysing apparatus may define at least one inlet passage in fluid
flow communication with all of the inlets and the method may include the step
of passing the solution into the chambers of all of the sets of permeable
electrodes via the inlet passage.
The first combustible fluid outlet passage may be in fluid flow communication
with all of the first combustible fluid outlets of all of the sets of
permeable
electrodes and the second combustible fluid outlet passage may be in fluid
flow communication with all of the second combustible fluid outlets of all of
the sets of permeable electrodes, the arrangement being such that the first
combustible fluid formed on the first electrode passes out of the apparatus
via the first combustible fluid outlet passage and the second combustible
fluid
formed on the second electrode passes out of the apparatus via the second
combustible fluid outlet passage.
According to a second aspect of the invention there is provided an
electrolysing apparatus in which combustible fluid is produced from an
electrolytic solution in a process of electrolysis comprising:
WO 2012/004769 CA 02804880 2013-01-096
PCT/1B2011/053050
first and second spaced apart permeable electrodes defining an
inlet chamber between them;
at least one inlet into the inlet chamber for passing the
electrolytic solution into said inlet chamber;
a first combustible fluid chamber on a first side of the set of
electrodes and a second combustible fluid chamber on a
second side of the set of electrodes; and
a first combustible fluid outlet from the first combustible fluid
chamber and a second combustible fluid outlet from the second
combustible fluid chamber;
the arrangement being such that the electrolytic solution passes into
the inlet chamber via the inlet where electrolysis takes place; and such
that a first combustible fluid forms on the first electrode; and such that
a second combustible fluid forms on the second electrode; and further
such that the first combustible fluid passes through the first electrode
into the first combustible fluid chamber; and such that the second
combustible fluid passes through the second electrode into the second
combustible fluid chamber; and such that the first combustible fluid
passes out the first combustible fluid chamber via the first combustible
fluid outlet; and the second combustible fluid passes out the second
combustible fluid chamber via the second combustible fluid outlet.
WO 2012/004769 CA 02804880 2013-01-09 PCT/1B2011/053050
7
The combustible fluid may be hydrogenated and oxygenated fluid and more
specifically the combustible fluid may be hydrogen and oxygen gas.
The permeable electrodes may each be perforated or foraminous.
Each permeable electrode may further be of a mesh or foam material,
Each permeable electrode may be made of a 316 stainless steel or nickel
material.
The first and second electrodes may be provided in relative close proximity to
one another and may be substantially parallel.
The first and second electrodes may each include at least one connector tab
for connecting to a power supply to supply a voltage over the electrolysing
apparatus to electrolyse the electrolytic solution.
The first and second electrodes may incorporate a solid outer ring for the
purpose of fluid sealing, attachment of the connection tab, and distribution
of
current around the electrode.
The first and second permeable electrodes may have a correct and
predetermined ratio of open to closed area (also known as the PPI (pores per
WO 2012/004769 CA 02804880 2013-01-098
PCT/1B2011/053050
square inch)), which may be influenced by the size of the inlet and the
pressure of the solution being provided to the apparatus.
The electrolysing apparatus may include a gasket positioned in the peripheral
region between the two electrodes forming the set of electrodes.
The gasket may be a first gasket and the electrolysing apparatus may include
a plurality of second gaskets, each positioned in the peripheral region
between adjacent sets of electrodes.
The apparatus may include first and second outer end members, each being
of polyethylene.
The apparatus may be cylindrical or multi-agonal in shape.
The apparatus may include circulating means, such as a pump, to circulate
the solution through the apparatus and to force the solution into the first
chamber.
The first combustible fluid outlets may be aligned to define a first
combustible
fluid outlet passage, so that first combustible fluid produced in all of the
first
combustible fluid chambers passes out of the apparatus via the first
combustible fluid outlet passage.
WO 2012/004769 CA 02804880 2013-01-099
PCT/1B2011/053050
The second combustible fluid outlets may be aligned to define a second
combustible fluid outlet passage, so that second combustible fluid produced
in all of the second combustible fluid chambers passes out of the apparatus
via the second combustible fluid outlet passage.
The apparatus may include a first combustible fluid collection container
connected to the first combustible fluid outlet passage and a second
combustible fluid collection container connected to the second combustible
fluid outlet passage.
BRIEF DESCRIPTION OF THE DRAWINGS
The invention will now be described further by way of a non-limiting example
with reference to the accompanying drawings wherein:
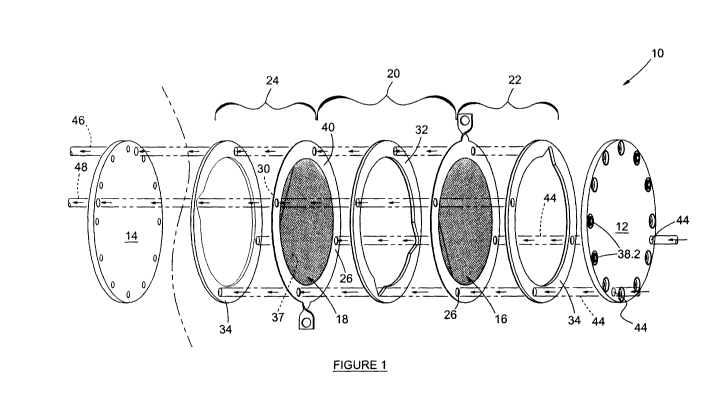
figure 1 is an exploded perspective view of part of an
electrolysis
apparatus according to a preferred embodiment of the
invention; and
figure 2 is a perspective view of the electrolysis apparatus
of figure 1.
WO 2012/004769 CA 02804880 2013-01-09 PCT/1B2011/053050
10
DESCRIPTION OF A PREFERRED EMBODIMENT OF THE INVENTION
Referring to the drawings, an electrolysis apparatus according to a preferred
embodiment of the invention is generally designated by reference numeral
10.
The electrolysis apparatus 10 is adapted to produce oxygenated and
hydrogenated fluid, formed during the electrolysis of an electrolytic solution
passed into the apparatus 10.
The apparatus 10 comprises a first outer end member 12, being of
polyethylene, and a second outer end member 14, also being of
polyethylene. The first and second outer end members 12 and 14 are both
disc shaped and are arranged generally parallel to one another and are
spaced from one another. It is foreseen that the apparatus could be multi-
agonal in shape and not necessarily cylindrical or circular.
The apparatus 10 further includes two spaced apart permeable electrodes, a
first permeable electrode 16 and a second permeable electrode 18. The
permeable electrode 16 and 18 are each of a foraminous or perforated
material. Specifically the permeable electrodes are each of nickel foam
sheet, but could also be 316 stainless steel. The two permeable electrodes
16 and 18 are also arranged generally parallel to one another, are relatively
closely spaced from one another. An inlet chamber 20 is therefore defined
WO 2012/004769 CA 02804880 2013-01-
0911 PCT/1B2011/053050
between the first and second permeable electrodes 16 and 18. A first
oxygenated fluid collection chamber 22 is disposed between the first
permeable electrode 16 and the first end member 12 and a second
hydrogenated fluid collection chamber 24 is disposed between the second
permeable electrode 18 and the second end member 14.
The closer the permeable electrodes 16 and 18 are spaced to each other,
results in a lower resistance between them, which means less voltage needs
to be applied to the apparatus 10, which results in a more efficient apparatus
10.
The inlet chamber 20 has two inlets 26 for allowing electrolytic solution to
pass into said chamber 20. The oxygen and hydrogen collection chambers
22 and 24 are each provided with a fluid outlet. The oxygen collection
chamber 22 is provided with an oxygen outlet 28 and a hydrogen collection
chamber 24 is provided with a hydrogen outlet 30.
The flow of electrolytic solution through the permeable electrodes 16 and 18
will carry with it the oxygen and hydrogen gasses generated on the positive
and negative (first and second) permeable electrodes respectively. There is
thus a natural separation of the hydrogen and oxygen gasses. The close
proximity of the electrodes 16 and 18 also permits hydrolyzing at very low
voltage, permitting high efficiency and high purity hydrogen and oxygen.
WO 2012/004769 CA 02804880 2013-01-
0912 PCT/1B2011/053050
The first and second permeable electrodes 16 and 18 defining first chamber
20 between them forms a set of permeable electrodes. The apparatus 10
could include a plurality of sets of permeable electrodes arranged and
connected to one another in a back-to-front arrangement. Figures 2 and 3
shows the apparatus 10 including 3 sets of permeable members between the
first and second outer electrodes 12 and 14.
The apparatus includes a plurality of intermediate barrier members 42,
positioned between adjacent sets.
The electrolysing apparatus 10 further includes an inlet ring 32 defining the
two inlets 26 and outlet rings 34 defining the oxygen outlet 28 and hydrogen
outlet 30 respectively, located on opposite sides of the two permeable
electrodes 16 and 18. The inlet ring 32 is positioned in the peripheral region
and between the first and second permeable electrodes 16 and 18 to seal the
two electrodes 16 and 18 to one another and the outlet rings 34 are
positioned in the peripheral region on the opposite sides of the permeable
electrodes 16 and 18.
The first and second electrodes 16 and 18 include conductive connector tabs
(one being the positive terminal and the other being the negative terminal)
for
connecting to a power supply (not shown), such as a battery. The powers
WO 2012/004769 CA 02804880 2013-01-09PCT/1B2011/053050
13
supply thus supplies a voltage of between 1 V and 6 V, over the electrolysing
apparatus 10 to electrolyse the solution. The present apparatus 10 produces
hydrogen and oxygen by applying either a pure DC voltage or pulsed DC
voltage to the apparatus.
Corresponding inlets 26 of the inlet rings of the apparatus 10 are aligned to
define inlet passages 44, so that electrolytic solution is passed into all of
the
chambers 20 of the apparatus 10 via the inlet passages 44. The oxygen
outlets 28 are also aligned to define an oxygen outlet passage 46, so that
oxygenated fluid accumulated in all of the oxygen collection chambers 22
passes out via the oxygen outlet passage 46. Similarly, the hydrogen outlets
30 are also aligned to define a hydrogen outlet passage 48, so that
hydrogenated fluid accumulated in all of the hydrogen collection chambers 24
passes out via the hydrogen outlet passage 48.
The apparatus 10 further includes a circulating means, such as a pump (not
shown) to circulate the solution through the apparatus 10. The electrolytic
solution flowing into the chamber 20 via the inlets 26 is pressurised by being
pumped into the apparatus 10 by the pump, so that the solution is forced
through the permeable electrodes 16 and 18 into the hydrogen and oxygen
collection chambers 22 and 24. The arrangement is such that electrolytic
solution flows into the first chamber 20 via the inlets 26, through the
permeable electrodes 16 and 18 into the oxygen and hydrogen collection
WO 2012/004769 CA 02804880 2013-01-0914
PCT/1B2011/053050
chambers 22 and 24 respectively. Electrolytic action takes place between the
first and second permeable electrodes 16 and 18 respectively. The
oxygenated fluid passes out of the oxygen collection chamber 22 via the
oxygen outlet 28 and the hydrogenated fluid passes out of the hydrogen
collection chamber 24 via the hydrogen outlet 30.
The apparatus 10 could further include a hydrogen collection container (not
shown) connected to the hydrogen outlet passage 48 and an oxygen
collection container (also not shown) connected to the oxygen outlet passage
46. The oxygen and hydrogen collection containers each have a second
electrolytic solution outlet located towards the operatively bottom end of the
containers and oxygen and hydrogen gas outlets located towards the
operatively top end of each of the oxygen and hydrogen collection
containers, respectively. Electrolytic solution passes out of the oxygen and
hydrogen outlets 28 and 30 from the oxygen and hydrogen collection
chambers 22 and 24, together with the respective gases, into the oxygen and
hydrogen collection containers via the outlet passages 46 and 48. The
arrangement is such that hydrogen and oxygen gases within the fluids
passing into the respective containers are released through gravitation and
passed out of the containers via the oxygen and hydrogen gas outlets and
the electrolytic solution passes out of the containers via the second
electrolytic solution outlets. The second electrolytic solution outlets are
connected to the inlet passages 44 and the solution is circulated back to the
WO 2012/004769 CA 02804880 2013-01-0915
PCT/1B2011/053050
apparatus 10 by means of the pump. The gasses are thus stored for later
use.
It is foreseen that there is a positive flow from the first chamber 20 to the
oxygen and hydrogen collection chambers 22 and 24 of the apparatus 10.
The pressurised flow of the electrolytic solution from the first chamber 20 to
the oxygen and hydrogen collection chambers 22 and 24, through the
permeable electrodes, restricts oxygen gas and hydrogen gas, after
formation on the first and second permeable electrodes 16 and 18 to enter
the first chamber 20.
It is further foreseen that the electrolysis apparatus essentially does not
have a
membrane, as in the case of prior art apparatus. This has a number of
advantages, for example, the cost of both a wet or dry membrane is removed,
along with the cost of maintaining the membranes. It also removes the pressure
and temperature limitations that are usually present with the use of
membranes. In the present invention, permeable electrodes are used, which do
not allow for shaded conduction areas to be created by the movement of
gasses across the electrode surface. This increases the effective conduction
area of the electrode, reduces the effective voltage requirement and thereby
improves efficiency.
WO 2012/004769 CA 02804880 2013-01-09PCT/1B2011/053050
16
It will be appreciated that variations in detail are possible with a method
and
apparatus for producing hydrogen and oxygen gasses according to the
invention without departing from the scope of the appended claims.