Note: Descriptions are shown in the official language in which they were submitted.
CA 02805404 2013-01-14
WO 2012/009396 PCMJS2011/043793
USE OF ALPHA-AMINO ETHERS FOR THE REMOVAL OF HYDROGEN SULFIDE
FROM HYDROCARBONS
Cross-Reference to Related Applications
None.
Statement Regarding Federally Sponsored Research or Development
Not Applicable.
Background of the Invention
This invention relates generally to the treatment of sour gas and liquid
hydrocarbon to remove or reduce the levels of hydrogen sulfide therein. The
toxicity of hydrogen
im sulfide in hydrocarbon fluids is well known in the industry. This has
caused considerable
expense and efforts to be expended annually to reduce its content to a safe
level.
In large production facilities, it is generally more economical to install a
regenerative system for treating sour gas streams. These systems typically
employ a compound
used in an absorption tower to contact the produced fluids and selectively
absorb the hydrogen
sulfide and possibly other toxic materials such as carbon dioxide and
mercaptans. The absorption
compound is then regenerated and reused in the system. Typical hydrogen
sulfide absorption
materials include alkanolamines, PEG, hindered amines, and other species that
can be
regenerated.
Nonregenerative scavengers for small plant hydrogen sulfide removal fall into
four
general categories: 1) aldehyde based, 2) metallic oxide based, 3) caustic
based, and 4) other
processes. In the removal of hydrogen sulfide by nonregenerative compounds,
the scavenger
reacts with the hydrogen sulfide to form a nonlethal compound or a compound,
which can be
removed from the hydrocarbon. For example, when formaldehyde reacts with
hydrogen sulfide a
chemical compound known as forinthionals (e.g., trithiane) is formed.
CA 02805404 2013-01-14
WO 2012/009396 PCT/US2011/043793
Prior Art aldehyde scavengers typically include low molecular weight aldehydes
and ketones and adducts thereof. The low molecular weight aldehydes may also
be combined
with an alkyl or alkanolarnine as disclosed in US Patent 4,748,011. Other
aldehyde derived
scavengers include the reaction product of low molecular weight alkanolamines
and aldehydes as
disclosed in US Patent 4,978,512. PCT Application WO 92/01481 discloses a
method of
reducing sulfides in different applications using certain tri-substituted-
hexahydro-s-triazines.
German reference DE4027300 discloses a regenerative solvent for removing H2S
and
mercaptans. US Patent 5,347,004 discloses the use of 1,3,5 alkoxyalkylene
hexahydro triazines.
PCT Application WO 91 US 5232 discloses hydroxyalkyl triazine scavengers,
specifically an
To N,1\r,N"-tris(2-hydroxyethyl)hexahydro-s-triazine. US Patent 5,774,024
discloses the
combination of an alkyl triazine scavenger and quaternary ammonium salt, where
the quaternary
ammonium salt enhances the effectiveness of the alkyl-triazine.. These prior
art attempts
however are frequently water-based chemicals and require significant mixing to
allow the
scavenger to effectively contact the hydrocarbon fluid and remove the hydrogen
sulfide.
Thus there is clear need and utility for an improved method of scavenging
hydrogen sulfide from hydrocarbon fluids using scavengers that are soluble in
the fluid that is
being treated. The art described in this section is not intended to constitute
an admission that any
patent, publication or other information referred to herein is "prior art"
with respect to this
invention, unless specifically designated as such. In addition, this section
should not be construed
to mean that a search has been made or that no other pertinent information as
defined in 37 CFR
1.56(a) exists.
Brief Summary of the Invention
At least one embodiment of the invention is directed towards a method for
2
CA 02805404 2013-01-14
WO 2012/009396 PCT/US2011/043793
removing hydrogen sulfide from a hydrocarbon fluid. The method comprises
contacting the fluid
with an effective amount of a composition comprising a hydrogen sulfide
scavenger. The amount
of hydrogen sulfide scavenger is sufficient to react with the hydrogen sulfide
to reduce the
amount hydrogen sulfide released into the vapor space. The reaction product of
the hydrogen
sulfide scavenger and the hydrogen sulfide remain soluble in the hydrocarbon
fluid. The
hydrogen sulfide scavenger contains at least one alpha-amino ether.
The composition may include one item selected from the list consisting of:
N,N'-oxybis(rnethylene)bis(N,N-dibutylamine),
N,N'-(methylenebis(oxy)bis(methylene))bis(N,N-dibutylamine),
and any combination thereof.
The reaction product of the sulfide scavenging formulation and the hydrogen
sulfide may not form a separate fluid layer. The method may also further
comprise the step of
reacting a secondary amine with a formaldehyde equivalent to form at least
some of the
scavenging formulation. The hydrocarbon fluid may be a liquid.
Detailed Description of the Invention
For purposes of this application the definition of these terms is as follows:
"Alpha-amino ether" means a molecule according to the formula:
RI 3
R2 "4
Where: RI, R2, R3,R1,are carbon containing side chains containing 1 ¨20 carbon
atoms and
includes cyclic and acyclic compounds. The cyclic compounds can be aromatic or
non-aromatic.
3
Examples include but are not limited to, methyl, ethyl, propyl, tert-butyl,
cyclopentyl,
cyclohexyl, morpholino, and phenyl, and they all can be the same group or one
or more
different groups. B is an ether group, which is either an oxygen atom or a
group having an
oxygen atom at both ends (such as -OCH20- or-OCsUt0-).
"Formaldehyde equivalent" means a composition of matter containing at least
one
group according to the formula: (CH20),1 in which n is an integer greater than
or equal to 1,
and/or a composition of matter including formaldehyde or related molecules
such as
paraformaldehyde, and/or s-trioxane.
"Hydrocarbon fluid" means a liquid or gas predominantly comprising organic
material
including but not limited to kerosene, crude oil, crude oil emulsions,
oilfield condensate,
petroleum residua, refined fuels, distillate fuels, fuel oil, heating oils,
diesel fuel, gasoline, jet
fuel, bunker fuel oils, and any combination thereof.
"Non-Regenerative Scavenger'' means a scavenger, which is consumed by the
process
of scavenging.
"Regenerative Scavenger" means a scavenger, which is not consumed by the
process
of scavenging.
"Scavenger" means a composition of matter, such as but not limited to alpha-
amino
ethers, useful in reducing the amount of or mitigating the effects of some
other composition of
matter, such as but not limited to hydrogen sulfide, in a fluid medium.
In the event that the above definitions or a description stated elsewhere in
this
application is inconsistent with a meaning (explicit or implicit) which is
commonly used, in a
dictionary, or stated in a source referenced herein, the application and the
claim terms in
particular are understood to be construed according to the definition or
description in this
application, and not according to the common definition, or dictionary
definition.
4
CA 2805404 2018-03-21
In light of the above, in the event that a term can only be understood if it
is construed by a
dictionary, if the term is defined by the Kirk-Othmer Encyclopedia of Chemical
Technology,
5th Edition, (2005), (Published by Wiley, John & Sons, Inc.) this definition
shall control how
the term is to be defined in the claims.
In at least one embodiment, the hydrogen sulfide in a hydrocarbon fluid is
reduced by
the introduction of an alpha-amino ether scavenger into the fluid.
In at least one embodiment the alpha-amino ether is a portion of a scavenging
formulation is used in a hydrocarbon fluid. The formulation comprises alpha-
amino ether and
can consist of a carrier liquid as well. The formulation can be introduced
into the hydrocarbon
fluid by mechanical means including but not limited to injection pumps or any
mechanism
disclosed in US Patents 5,744,024 and 5,840,177. In the context of gaseous
hydrocarbon
fluids, the gas may be passed through an absorption tower containing a
scavenging
formulation.
One advantage of the use of the alpha-amino ether scavenger over other
scavengers is
that the alpha-amino ether scavenger is soluble in hydrocarbon fluids, since
it is not a water-
based product.
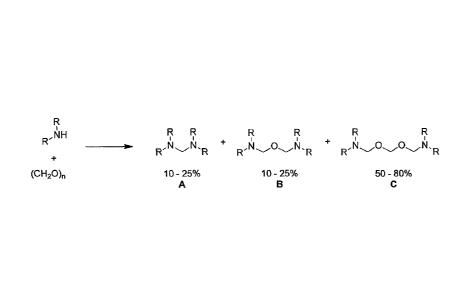
As illustrated in FIG. 1, in at least one embodiment the hydrogen sulfide
scavenger is
produced by reacting a secondary amine with formaldehyde or other formaldehyde
equivalents such as paraformaldehyde or s-trioxane. This produces a
composition of matter
that predominantly comprises two forms of alpha-amino ethers (compounds B and
C). 10%-
25% of the composition is a mono oxygen ether (compound B) and 50%-80% of the
composition is a bis oxygen ether with a single carbon atom between the ether
oxygens
(compound C). The reaction product also comprises 10-25% of the non-ether
diamine
(compound A). Compound A is an unavoidable side product of the reaction
mixture. The
reaction can be performed by mixing the reactants in solvents such as but not
limited to
Naphtha (Petroleum) Heavy Aromatic Solvents
CA 2805404 2018-03-21
CA 02805404 2013-01-14
WO 2012/009396 PCT/US2011/043793
(such as Aromatic 150 and Solvesso by ExxonMobil) or Naphtha Light Aromatic
Solvents (such
as (Aromatic 100 by Americhem Sales Corporation).
In at least one embodiment the ratio of amine to formaldehyde in the reaction
mixture is inclusively within the range of L5:1 to 1:1.5 and is preferably
between 1.2:1 and 1:1.2.
In at least one embodiment any of the R and R' groups correspond to any of the
RI, R2, R3, and R4, groups described in the definition of "alpha-amino ether".
In at least one embodiment when R is n-butyl and R' is H then:
Compound A is N,N,N',N'-tetrabutylmethanediamine,
Compound B is N,N'-oxybis(methylene)bis(N,N-dibutylene), and
Compound C is N,N'-(methylenebis(oxy)bis(methylene))bis(N,N'-dibutylamine).
At least some contemplated scavenging compositions include formulations
comprising:
(Compound A, B, and C), (A and B), (A and C), (B and C), (C alone), and (B
alone).
EXAMPLES
The foregoing may be better understood by reference to the following
example, which is presented for purposes of illustration and is not intended
to limit the
scope of the invention.
Samples of hydrocarbon fluids were tested to determine the efficiency of
the scavenger. Table 1 compares the inventive composition in naphtha at 22
degrees C,
Table 2 kerosene at 22 degrees C, and Table 3 slurry oil (such as carbon black
oil, decant
oil, and clarified slurry oil produced in a refinery) at 97 degrees C. The
samples
contained variable levels of hydrogen sulfide and were comparatively treated
with various
6
CA 02805404 2013-01-14
WO 2012/009396
PCT/US2011/043793
dosages of alpha-amino ether scavenger or left untreated, and the amounts H2S
reduced in
each sample was recorded.
Table 1: Naphtha 22 it
1-125
inks ppm 1125 Percent Dose
Treatment (ppm) Reduced
Reduction Ratio Time
Untreated 800
Compounds A- C 20 780 97.5 0.2 2 h
Compounds A- C 6 794 99.2 03 2 h
Compounds A- C 3 797 99.6 0_2 24 h
Compounds A.- C <1 >799 99.9 03 24h
2h
Compounds A- C 12 (4) 788 (796) 98.5 (99_5) 0.2 (24h)
Table &wing 22 C
H2S
kyjg ppm H25 Percent Dose
Treatment (ppm) Reduced
Reduction Ratio Time
Untreated 1400
Compounds A -C 400 1000 71 0,1 2 h
Compounds A -C 220 1180 84 02 2 h
Compounds A - C 95 1305 93 0.3 2 h
Table 3: Slurry oil 97
H2S
litylo ppm 1125 Percent Dose
Treatment (ppm) Reduced
Reduction Ratio Time
Untreated 1300
Compounds A - C 450 850 65 0_1 2.5 h
Compounds A - C 240 1060 82 0,2 2.5 h
Compounds A - C 180 1120 86 0.3 2.5 h
Compounds A - C 140 1160 89 0.4 2.5 h
Compounds A - C 90 1210 93 0.5 2_5 h
The amount of H2S present in the vapor space was determined by
measuring the vapor space hydrogen sulfide levels according to ASTM D5705-03.
The
test procedure was modified by running at temperatures other than 60 C. A one-
gallon
sample was divided into multiple 500 milliliter samples for testing. The
treated containers
were pre-dosed with Compounds A-C and then the fluid being tested was poured
into the
container.
In each example, the dose ratio was the number used to determine the ppm
7
treat rate for the sample. For table 1 the untreated sample resulted in a
vapor space hydrogen
sulfide measurement of 800 ppm. A dose ratio of 0.2 indicates the sample was
treated with
160 ppm of additive. A dose ratio of 0.3 indicates that the sample was treated
with 240 ppm
of additive.
This data demonstrates that the presence of the alpha-amino ether scavenger
reduced
the H2S in the hydrocarbon fluids in a relatively short amount of time and
continued to reduce
the H2S the longer the sample was exposed to the alpha-amino ether prior to
testing.
While this invention may be embodied in many different forms, there are shown
in the
drawings and described in detail herein specific preferred embodiments of the
invention. The
present disclosure is an exemplification of the principles of the invention
and is not intended
to limit the invention to the particular embodiments illustrated. Furthermore,
the invention
encompasses any possible combination of some or all of the various embodiments
described
herein and incorporated herein.
The above disclosure is intended to be illustrative and not exhaustive. This
description
will suggest many variations and alternatives to one of ordinary skill in this
art. All these
alternatives and variations are intended to be included within the scope of
the claims where
the term "comprising" means "including, but not limited to". Those familiar
with the art may
recognize other equivalents to the specific embodiments described herein which
equivalents
are also intended to be encompassed by the claims.
All ranges and parameters disclosed herein are understood to encompass any and
all
subranges subsumed therein, and every number between the endpoints. For
example, a stated
8
CA 2805404 2018-03-21
CA 02805404 2013-01-14
WO 2012/009396 PCT/US2011/043793
range of "1 to 10" should be considered to include any and all subranges
between (and inclusive
of) the minimum value of 1 and the maximum value of 10; that is, all subranges
beginning with a
minimum value of 1 or more, (e.g. 1 to 6.1), and ending with a maximum value
of 10 or less,
(e.g. 2.3 to 9.4,3 to 8,4 to 7), and finally to each number 1, 2, 3, 4, 5, 6,
7, 8, 9, and 10 contained
within the range.
This completes the description of the preferred and alternate embodiments of
the
invention. Those skilled in the art may recognize other equivalents to the
specific embodiment
described herein which equivalents are intended to be encompassed by the
claims attached hereto.
9