Note: Descriptions are shown in the official language in which they were submitted.
WO 2012/010563 CA 02805502 2013-01-15 PCT/EP2011/062277
1
Description
MEDICAMENT CARTRIDGES WITH NON-STANDARD DIMENSIONS
FIELD OF DISCLOSURE
The present disclosure is generally directed to drug delivery devices and
reservoirs (i.e.,
ampoules and cartridges), particularly reservoirs containing a medicament.
More
particularly, the present application is generally directed to cartridges
having non-
standard dimensions that can provide a coding system for drug delivery device
components to prevent unwanted cross use. As just one example, such medicament
cartridges may comprise an ampoule having a distal head portion having
multiple
outside diameters, preferably having at least two different diameters. The
disclosure
also includes an improved method of manufacturing a finished cartridge using
the
improved ampoule. Exemplary medical delivery devices that accept the
cartridges of
this disclosure include, but are not limited to, syringes, pen type injection
syringes,
pumps, inhalers, or other similar injection or infusing devices that require
at least one
reservoir containing at least one medicament.
BACKGROUND
Medicament reservoirs such as ampoules, cartridges, or vials are generally
known.
Such reservoirs are especially used for medicaments that may be self
administered by a
patient. For example, with respect to insulin, a patient suffering from
diabetes may
require a certain amount of insulin to either be injected via a pen type
injection syringe
or infused via a pump. With respect to certain known reusable pen type drug
delivery
devices, a patient loads a cartridge containing the insulin into a proximal
end of a
cartridge holder. After the cartridge has been correctly loaded, the user may
then be
called upon to select a dose of medicament. Multiple doses may be dosed from
the
cartridge. Where the drug delivery device comprises a reusable device, once
the
cartridge is empty, the cartridge holder is disconnected from the drug
delivery device
and the empty cartridge is removed and replaced with a new cartridge. Most
suppliers
WO 2012/010563 CA 02805502 2013-01-15 PCT/EP2011/062277
2
of such cartridges recommend that the user dispose of the empty cartridges
properly.
Where the drug delivery device comprises a disposable device, once the
cartridge is
empty, the user is recommended to dispose of the entire device.
Such known self-administration systems requiring the removal and reloading of
empty
cartridges have certain limitations. For example, in certain generally known
systems, a
user simply loads a new cartridge into the delivery system without the drug
delivery
device or without the cartridge having a mechanism of preventing cross use of
an
incorrect cartridge. That is, the drug delivery device does not have a
mechanism for
determining if the medicament contained in the cartridge is indeed the correct
type of
medicament to be administered by the patient. Alternatively, certain known
drug delivery
devices do not present a mechanism for determining if the correct type of
medicament
within the cartridge should be used with that particular drug delivery system.
This
potential problem could be exacerbated given that certain elderly patients,
such as
those suffering from diabetes, may have limited manual dexterity. Identifying
an
incorrect medicament is quite important, since the administration of a
potentially
incorrect dose of a medicament such as a short acting insulin in lieu of a
long insulin
could result in injury or even death.
Some drug delivery devices or systems may use a color coding scheme to assist
a user
or care giver in selecting the correct cartridge to be used with a drug
delivery device.
However, such color coding schemes pose challenges to certain users,
especially those
users suffering from poor eyesight or color blindness: a situation that can be
quite
prevalent in patients suffering from diabetes.
Another concern that may arise with such disposable cartridges is that these
cartridges
are manufactured in essentially standard sizes and manufactured to comply with
certain
recognized local and international standards, for example ISO Standard 11608-3
2001.
Consequently, such cartridges are typically supplied in standard sized
cartridges (e.g., 3
ml cartridges). Therefore, there may be a variety of cartridges supplied by a
number of
different suppliers and containing a different medicament but they may fit a
single drug
delivery device. As just one example, a first cartridge containing a first
medicament from
WO 2012/010563 CA 02805502 2013-01-15 PCT/EP2011/062277
3
a first supplier may fit a medical delivery device provided by a second
supplier. As such,
a user might be able to load and then dispense an incorrect medicament (such
as a
rapid or basal type of insulin) into a drug delivery device without being
aware that the
medical delivery device was perhaps neither designed nor intended to be used
with
such a cartridge.
As such, there is a growing desire from users, health care providers, care
givers,
regulatory entities, and medical device suppliers to reduce the potential risk
of a user
loading an incorrect drug type into a drug delivery device. There is also,
therefore, a
desire to reduce the risk of dispensing an incorrect medicament (or the wrong
concentration of the medicament) from such a drug delivery device.
There is, therefore, a general need to physically dedicate or mechanically
code a
cartridge to its drug type and design an injection device that accepts or
works with the
dedication or coded features provided on or with the cartridge so as to
prevent
unwanted cartridge cross use. Similarly, there is also a general need for a
dedicated
cartridge that allows the medical delivery device to be used with an
authorized cartridge
containing a specific medicament while also preventing undesired cartridge
cross use.
There is also a general need to provide a dedicated cartridge that is
difficult to tamper
with so that the cartridge may not be compromised in that the cartridge can be
used
with an unauthorized drug or drug delivery device. Because such cartridges may
be
difficult to tamper with, they may also reduce the risk of counterfeiting:
i.e., making it
more difficult for counterfeiters to provide unregulated counterfeit
medicament carrying
products.
It is an aim of the invention to reduce the risk of a user dispensing the
wrong
medicament from the drug delivery device.
SUMMARY
WO 2012/010563 CA 02805502 2013-01-15 PCT/EP2011/062277
4
The aim is achieved by an ampoule for use in a cartridge containing a
medicament for
use in a drug delivery device, the ampoule having non-standard dimensions to
provide a
coding system to reduce the risk of a user dispensing the wrong medicament
from the
drug delivery device. The cartridge contains an ampoule having a head portion
with
variable diameters.
One embodiment of the ampoule for containing a medicament comprises a constant
diameter body, a neck portion and a head portion located distally of the neck
portion
and having a non-constant, variable diameter. In other words, the head portion
is not a
tube having a constant diameter. Rather the head portion has a section having
a
diameter which differs from the diameter of another section of the head
portion. The
non-constant or variable diameter serves as coding feature. The ampoule may
have a
proximal and distal end, where the distal end of the ampoule has the head
portion that
defines an opening where a medicament, such as insulin, can be dispensed
through a
needle cannula, for example. The transition between the head portion and the
neck
portion may be an inwardly converging shoulder. The neck portion may enlarge
into a
constant diameter reservoir or body that comprises the major portion of the
ampoule
volume. The ampoule may be a part of a cartridge, which in addition to the
ampoule,
may also contains a septum mounted across the distal opening of the ampoule by
a
ferrule and a piston that is slidably positioned inside the proximal end of
the ampoule
and forms a seal to contain the medicament within the ampoule.
One embodiment of the ampoule for containing a medicament comprises a constant
diameter body having a proximal end and a distal end; a neck portion having a
diameter
DND at the distal end; and a head portion located distally of the neck portion
and having
a variable, non-constant diameter.
In one embodiment the head portion has a first diameter and a second diameter,
where
the first diameter is less than the second diameter. The diameter DND of the
neck
portion may be less than the first and second diameters.
CA 02805502 2013-01-15
WO 2012/010563 PCT/EP2011/062277
5
According to exemplary arrangements, both a starting ampoule and a finished
cartridge
are provided having "non-standard" dimensions (i.e., non-ISO standard), where
the
finished cartridge is intended for use with a matched reservoir holder of a
drug delivery
device. A system comprised of such non-standard ampoules and finished
cartridges
allows for a coding system that distinguishes cartridges containing the same
medicament at different concentration levels and/or cartridges containing
different
medicaments. A matching cartridge holder accepts only the non-standard
finished
cartridge.
A standard cartridge is comprised of ampoule having a proximal and distal end,
where
the distal end of the ampoule has a head portion that defines an opening where
a
medicament, such as insulin, can be dispensed through a needle cannula. The
head
portion has a pierceable septum fixed to the ampoule by a ferrule that is
typically crimp
fitted to the head portion. Importantly, it is noted that the head portion of
a standard ISO
ampoule is "bottle shaped" and has a constant outside diameter that terminates
in a
neck portion before enlarging into a constant diameter reservoir that
comprises the
major portion of the ampoule volume. The finished cartridge, in addition to
the ampoule,
also contains a bung, stopper, or piston that is slidably position inside the
proximal end
of the ampoule and forms a seal to contain the medicament within the ampoule.
Typically, the ampoule is formed from glass, but will work with any known
materials of
construction, such as, plastics or like materials.
A "standard cartridge" is known to those skilled in the art of drug delivery
devices to be
one that comports with the dimensions set by International Standard ISO 11608-
3:2000A. For a cartridge nominally holding 3 ml, the ISO standard specifies
the
following standard dimensions:
L (total length) 63.90 + 0.30
NL (length of distal neck) 6.30 max.
D (distal diameter) 8.0 max.
WO 2012/010563 CA 02805502 2013-01-15PCT/EP2011/062277
6
The 8 mm max. dimension shown above for the distal diameter of the cartridge
is
measured as a cross-section of the head portion of the distal end of ampoule
and
includes the thickness of the ferrule. Thus, this dimension D is a function of
the wall
thickness of the ampoule, the thickness of ferrule, and the size of the distal
opening of
the ampoule. As mentioned, this dimension D is a uniform diameter (non-
variable) that
begins at the very distal end of the ampoule and continues until termination
at the neck
portion. Figure 2 shows a cross-sectional view of a "standard" ampoule as part
of a
finished ISO standard cartridge. Although the ISO standards provide dimensions
and
shapes of the starting ampoules, manufacturing tolerances of + 0.20 mm are
industry
normal. In order to use such a standard cartridge to administer medicament by
injection,
the drug delivery device typically has a cartridge holder with an internal
diameter of 8.2
mm or greater to ensure the standard cartridge will fit into the cartridge
holder portion of
the injection device. Like the ampoule, the distal end of a standard cartridge
holder has
single, constant diameter cavity designed to accept the constant diameter
distal head
portion of the finished cartridge.
In one aspect, a medicament ampoule is provided comprising a constant diameter
body
having a proximal end and a distal end. There is also a neck portion having a
diameter
DND at the distal end of the ampoule, which defines a transition point between
the
CA 02805502 2013-01-15
WO 2012/010563 PCT/EP2011/062277
7
constant diameter body and the distal end of the ampoule. At the very distal
end of the
ampoule is a head portion located distally of the neck portion. This head
portion is
unique in that is has a variable, non-constant diameter. The ampoule also has
a total
length L and an outside diameter OD that defines the constant diameter body
portion of
the ampoule. Preferably, the head portion comprises at least two different
diameters
that are separate from the diameter of the neck portion DND. This variable
diameter
head portion is distinguishable from the single or uniform head diameter found
on an
ISO standard ampoule. Preferably, the head portion of my ampoule has a first
and a
second head diameter, where the first head diameter is smaller than the second
head
diameter, and both the first and second diameters are larger than the neck
diameter
DND.
In another aspect, a finished medicament cartridge is provided comprising an
ampoule
having the characteristics described above with the addition of a ferrule that
partially
encloses a septum. The ferrule is fixed to the head portion at the distal end
of the
ampoule preferably by crimping the ferrule around both the first and second
head
portion diameters. A medicament is sealed inside the ampoule by a piston that
is
slideably positioned within the proximal portion of the ampoule. Most
preferably, the
ferrule conforms exactly to the dimensions of the head portion of the ampoule
and
includes both diameters, but not the neck portion. The at least two outside
distal
diameters, D1 and D2, of the distal end of the finished cartridge are measured
in same
manner as defined by the ISO standards (i.e., the cross-section of the distal
end of the
ampoule including the ferrule). Preferably, D1 is in the range from about 7.50
to about
8.00 mm, and D2 is in the range from about 5.7 to about 6.5 mm. Although
either D1 or
D2 could equal that specified in the ISO Standards, the ampoule and/or
cartridge would
still be considered "non-standard" because the ampoule was manufactured with a
variable diameter head portion having at least two distinct diameters in the
head portion,
exclusive of the neck diameter. Likewise, even though the total length L of
such an
ampoule and/or cartridge could be equal to the ISO standard set forth above,
the
cartridge would still be considered "non-standard" because the head portion
would have
at least two different diameters. The ISO standard, contrary to the
disclosure, specifies
only a single uniform diameter for the head portion of the finished cartridge.
WO 2012/010563 CA 02805502 2013-01-15 PCT/EP2011/062277
8
Manufacturing or simply providing a drug delivery device, either disposable or
refillable,
that has a cartridge holder with an internal distal cavity that matches the
contour (i.e.,
the variable head portion diameters) of the finished cartridges of this
disclosure (i.e.,
"non-standard"), but will not accept a "standard" 3 ml cartridge as defined by
ISO, will
provide a needed coding feature. This exclusion of standard cartridges
provides a way
to prevent or reduce the potential risk of a user loading an incorrect drug
type into a
drug delivery device. Likewise, this prevents undesired cartridge cross use.
The disclosure also concerns a system of medicament cartridges. The system may
comprise a first cartridge which comprises a first ampoule and contains a
first
medicament; and a second cartridge comprising a second ampoule and containing
a
second medicament. The second diameter of the first and second ampoules is the
same dimension and the first diameter of the first and second ampoules is a
different
dimension. In one embodiment the first medicament has a first concentration
and the
second medicament has a second concentration, where the second concentration
is not
equal to the first concentration. In an alternative embodiment the first
medicament in the
first cartridge is different than the second medicament in the second
cartridge
One embodiment of a system of medicament cartridges comprises at least two
cartridges. A first cartridge contains a first concentration of medicament. A
second
cartridge contains a second concentration of medicament. The first and second
cartridges each comprise an ampoule having a constant diameter body having a
proximal end and a distal end, a neck portion having a diameter DND at the
distal end,
and a head portion located distally of the neck portion and having at least
two diameters,
D1 and D2; where D2 of each ampoule of each cartridge is the same dimension
and D1
of each ampoule of each cartridge is a different dimension. The second
concentration
may be not equal to the first concentration. The medicament in the first
cartridge may be
different than the medicament in the second cartridge.
The first and second cartridges may further comprises a septum mounted across
the
distal opening of the ampoule by a ferrule which conforms to the dimensions of
the head
WO 2012/010563 CA 02805502 2013-01-15 PCT/EP2011/062277
9
portion. The ferrule may include the first and second diameters of the
respective
ampoule.
Accordingly, the disclosure includes a system of cartridges, defined as two or
more
cartridges, where a first cartridge can contain a first concentration of
medicament and a
second cartridge can contain a second concentration of medicament. The first
and
second cartridges in the system each comprise an ampoule having a proximal
portion, a
total length L, and a head portion having at least two measurable diameters,
exclusive
of the neck diameter. A ferrule partially enclosing a septum is fixed to the
ampoule and
preferably surrounds both head diameters. The distal head diameters can be
varied to
distinguish the different concentrations and/or different medicaments
contained in the
cartridges. Preferably, the second concentration of medicament is not equal to
the first
concentration. Alternatively, the medicament in the first cartridge could be
different than
the medicament in the second cartridge. Of course, the system could include a
number
of cartridges, where each cartridge has the same D1, but a different D2. In
such a
system, different medicament can be coded or matched to different D2
diameters.
Likewise, the system could include a number of cartridges, where each
cartridge has
the same D2, but a different D1. In such a system, different medicament can be
coded
or matched to different D1 diameters. Improper cartridge use can be avoided by
providing drug delivery devices with cartridge holders that only accept one or
more
cartridges with the matching head portion diameters. This is accomplished by
varying
the internal dimensions and/or design at the distal end of the cartridge
holders.
The disclosure also relates to an improved method of assembling the finished
cartridge.
A method of closing the distal end of an ampoule may comprise providing an
ampoule
having a variable diameter head portion having a locating surface and an
opening;
positioning a septum on the opening; and securing the septum to the head
portion with
a ferrule using a plunger, where the plunger exerts a force in a proximal
direction to
press the ferrule on the head portion until the press causes the ferrule to
contact the
locating surface. A rolling plate may exert a force in a distal direction to
crimp the ferrule
to the head portion.
CA 02805502 2013-01-15
WO 2012/010563 PCT/EP2011/062277
10
In the production process for closing the distal end of a conventional ampoule
there is
no locating surface because the head portion is cylindrical in shape and is
uniform in
cross-section dimension until it tapers into the neck portion. As such, the
sealing of the
ferrule to head portion and fixation of the septum is exclusively controlled
by force,
which must be large enough in order to sufficiently compress the septum in
order to
thus ensure leak-tightness, but it cannot be so large that the overhang of the
ferrule
material (typically aluminum foil) has a larger surface than the opposite
surface that is
available on the glass body. This would lead to an edge of the ferrule that is
unclean,
"frayed" or sticks out. In the situations where there are changes in the
assembly process,
for example, where the material of the septum or ferrule is changed because of
alternative suppliers or where there is a wear of the assembly tools, the
required force
must be determined anew by an iterative trial and error process that leads to
production
waste.
The method makes use of a locating surface formed by a horizontal or
substantially
horizontal surface associated with either the first or second diameters in the
head
portion of the cartridge. The machine used to seal the ferrule to the ampoule
employs a
plunger tool that is forced downwardly around the head portion of the ampoule
until the
surface of the aluminum cap reaches the locating surface of the diameter
defined by
either D1 or D2 on the head portion of the ampoule. In this manner the
production or
assembly process using a locating surface is dependent on the position of the
locating
surface and on the wear and tear of the tool, which simplifies the production
process.
The drug delivery device and the non-standard cartridges described herein can
be
considered a drug delivery system. Such a drug delivery system can be
reusable,
meaning the cartridge can be replaced when empty, or the system can be non-
reusable
(disposable), meaning the cartridge cannot be replaced and the entire system
is thrown
away when the cartridge is empty.
In any of the arrangements described above, it is possible to add mechanical
coding
features to the non-standard cartridges, for example, coded labels or
ring/bands/collars
attached to the proximal end of the ampoule. These as well as other advantages
of
WO 2012/010563 CA 02805502 2013-01-15PCT/EP2011/062277
11
various aspects will become apparent to those of ordinary skill in the art by
reading the
following detailed description, with appropriate reference to the accompanying
drawings.
The scope of the invention is defined by the content of the claims. The
invention is not
limited to specific embodiments but comprises any combination of elements of
different
embodiments. Moreover, the invention comprises any combination of claims and
any
combination of features disclosed by the claims.
BRIEF DESCRIPTION OF THE DRAWINGS
Exemplary embodiments are described herein with reference to the drawings, in
which:
Figure 1 illustrates an exemplary pen type drug delivery device;
Figure 2 illustrates a cross-sectional view of an ISO standard drug cartridge;
Figure 3 is a cross-sectional view of exemplary drug cartridge in accordance
with our
proposed concept;
Figure 4 is a cross-sectional view of another exemplary drug cartridge in
accordance
with our proposed concept;
Figure 5 is a cross-sectional view of the manufacturing process for an ISO
standard
cartridge; and
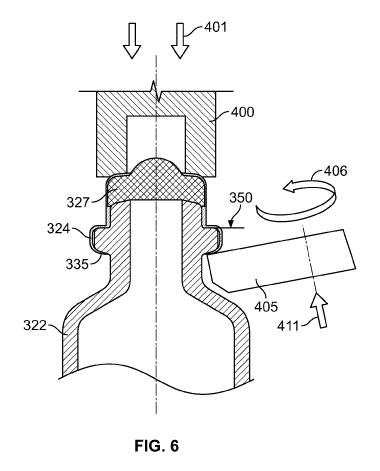
Figure 6 is a cross-sectional view of the manufacturing process for exemplary
drug
cartridge in accordance with our proposed concept.
DETAILED DESCRIPTION
Figure 1 illustrates a drug delivery device 100 in the form of a pen type
syringe. This
drug delivery device 100 comprises a dose setting mechanism 102, a cartridge
holder
CA 02805502 2013-01-15
WO 2012/010563 PCT/EP2011/062277
12
104, and a removable cap 106. A proximal end 105 of the cartridge holder 104
and a
distal end 103 of the dose setting mechanism 102 are removably secured
together. The
pen type syringe may comprise a re-usable or a disposable pen type syringe.
Where the
syringe comprises a reusable device, the cartridge holder 104 and the dose
setting
mechanism 102 are removably coupled together. In a disposable device, they may
be
permanently coupled together. In Figure 1, the dose setting mechanism 102
comprises
a spindle 109, such as a threaded spindle that rotates when a dose is
injected.
To inject a previously set dose, a double ended needle assembly (not shown) is
attached to a distal end 108 of the cartridge holder 104. Preferably, the
distal end 108 of
the cartridge holder 104 comprises a thread 121 (or other suitable connecting
mechanism such as a snap lock, snap fit, form fit, or bayonet lock mechanism)
so that
the needle assembly may be removably attached to the distal end 108 of the
cartridge
holder 104. When the drug delivery device 100 is not in use, the removable cap
106 can
be releasably retained over the cartridge holder 104.
An inner cartridge cavity 111 defined by the cartridge holder 104 is
dimensioned and
configured to securely receive and retain a non-standard cartridge 120. Figure
2
illustrates a partial cross-sectional view of the distal end of a standard ISO
cartridge 120
having a uniform, non-variable, diameter D for the head portion 131 that may
be used
with the drug delivery device 100 illustrated in Figure 1 provided the inner
cavity 111 is
contoured and/or conformed to matched the uniform shape of the head portion
131 of
the standard ISO cartridge 120. The cartridge 120 includes an ampoule 122
extending
from a distal end 130 to a proximal end 132. The distal end 130 is defined by
the
combination of the head portion 131 and a neck portion 133, where the
transition is an
inwardly converging shoulder 135.
At the distal end 130, the ampoule 122 includes a constant and uniform
diameter head
portion 131 having diameter D located distally of the neck portion 133. An
annular bead
134 extends circumferentially thereabout at the extreme distal end of shoulder
135. A
pierceable seal or septum 127 is securely mounted across the open distal end
of the
ampoule 122. The septum 127 may be held in place by a metallic sleeve or
ferrule 124.
WO 2012/010563 CA 02805502 2013-01-15PCT/EP2011/062277
13
This ferrule 124 may be crimped around the circumferential bead 134 at the
distal end
of the neck portion 133. The medicament 125 is pre-filled into the cartridge
120 and is
retained within the cartridge 120, in part, by the pierceable seal or septum
127, the
ferrule 124, and a piston 128. The piston 128 is in sliding fluid-tight
engagement with the
inner tubular wall of the ampoule 122. Axially directed forces acting upon the
piston 128
during dose injection or dose administration urges the medication 125 from the
cartridge
120 though a double ended needle (not shown) mounted onto the distal end 108
of the
cartridge holder 104 and into the injection site. Such axial forces may be
provided by
the spindle 109.
Turning to Figures 3 and 4, which show examples of the ampoule 322 and
finished
cartridges 320 of this disclosure, each is characterized in that the head
portion 331 has
a variable diameter separate and apart from the diameter DNP of neck portion
333. At
the distal end 330, the ampoule 322 includes a non-uniform head portion 331
having, at
a minimum at least two different diameters, shown as D1 and D2 located
distally of the
neck portion 333. In each of the embodiments shown in Figs. 3 and 4 an outside
distal
diameter D1 is smaller than D2. As with the standard ISO cartridge, the
cartridges of the
disclosure, as depicted in these figures, has an annular bead 334 extending
circumferentially at the extreme distal end of shoulder 335, a pierceable seal
or septum
327 securely mounted across the open distal end of the ampoule 322 by a
metallic
sleeve or ferrule 324, which may be crimped around the circumferential bead
334 at the
distal end of the neck portion 333. The ampoule 322 contains a medicament 325
pre-
filled into the cartridge 320 that is retained within the cartridge 320, in
part, by the
pierceable seal or septum 327, the ferrule 324, and a slidable piston (not
shown), but
which can be the same as piston 128 shown in Figure 2.
The disclosure also covers an improved manufacturing or filling process that
is possible
as a result of the variable diameter head portion 331 of ampoule 322. The
improved
manufacturing process makes use of a locating surface 350 located on the head
portion
331, preferably on a horizontal or nearly horizontal surface, such as locating
surface
350 shown in Fig. 6. In contrast, Fig. 5 illustrates a manufacturing process
for an ISO
standard cartridge 120 where there is no locating surface because of the
uniform
WO 2012/010563 CA 02805502 2013-01-15PCT/EP2011/062277
14
constant diameter of the head portion 131 of the ampoule 122. In the
manufacturing
process a press 400 exerts a force in direction 401 to secure the ferrule 124
to head
portion 131. The plunger is spring-loaded and is driven down until the counter
pressure
of septum 127 that is compressed by this process reaches a force 410. Rolling
plate
405 exerts an upward force 411 and moves in direction 406 to attach the
overhang of
ferrule 124 that is created by the compression of the septum 127 to the head
portion
131 of ampoule 122. In the improved process, as shown in Fig. 6, the press 400
is
forced downward until the press 400 causes the ferrule 324 to encounter the
locating
surface 350 of ampoule 322. The same rolling plate 405 is used to secure
ferrule 324 to
the shoulder 335. This improved production process is only dependent on the
position
of the locating surface 350 and the wear and tear of the tool, but not the
counter
pressure exerted by the septum 327, which simplifies the production process.
A portion of the cartridge holder 104 defining the cartridge holder cavity 111
is of
substantially uniform diameter. This inner diameter is preferably slightly
greater than the
outer diameter OD at the proximal end of the main body of cartridge 320. The
distal
interior of the cartridge holder 104 is configured, molded, formed or
otherwise designed
to conform to the variable diameter head portion 331 of the cartridges of the
disclosure.
In this manner, when the cartridge 320 is loaded into the cavity 111 of the
cartridge
holder 104 and the cartridge holder 104 is then connected to the dose setting
member
102, the cartridge 320 will be securely held within the cartridge cavity 111.
More
particularly, because the distal interior of the cartridge holder 104 is
designed to match
the variable diameter of the neck portion 331 of the cartridge 320, only
matching
cartridges 320 and cartridge holders 104 will allow compatible fit and
attachment to the
dose setting mechanism 102 of the drug delivery device 100.
A number of doses of a medicament 325 may be dispensed from the cartridge 320.
It
will be understood that the cartridge 320 may contain a type of medicament 325
that
must be administered often, such as one or more times a day. One such
medicament
325 is insulin. The dose setting mechanism 102 comprises a dose setter 117 at
the
proximal end 107 of the drug delivery device 100. In one preferred
arrangement, the
WO 2012/010563 CA 02805502 2013-01-15PCT/EP2011/062277
15
dose setter 117 may extend along the entire length of the dose setting
mechanism. The
dose setter 117 may be rotated by a user to set a dose.
To administer a dose that may be set by rotating the dose setter 117, the user
attaches
the needle assembly comprising a double ended needle on the distal end 108 of
the
cartridge holder 104. In this manner, the needle assembly pierces the seal 127
of the
cartridge 120 and is therefore in liquid communication with the medicament
125. The
user pushes on the dose setter 117 to inject the set dose. The same dose
setting and
dose administration procedure is followed until the medicament 125 in the
cartridge is
expended and then a new cartridge 120 must be loaded in the device. To
exchange an
empty cartridge 120, the user is called upon to remove the cartridge holder
104 from the
dose setting mechanism 102.
A coding system comprising the non-standard cartridges 320 of the disclosure
for use
with a drug delivery system, such as drug delivery device 100, is provided. In
an
example, a system of cartridges 320 are manufactured where the head portion
331 of
the cartridges 320 are variable in diameter having at least two measurable
diameters
D1 and D2, this being contrary to the constant and uniform distal diameter D
of a
"standard cartridge" 120 as required by the ISO standards. As such, each
cartridge 320
could have the same D1, but a different D2, or vice versa, with either or both
of D1 or
D2 coded or matched to a different medicament 325 or concentration of
medicament
325. Because the cartridge holder 104 is manufactured to fit the non-standard
distal
diameters D1, D2 for each cartridge system, an attempt to insert or use a
standard
cartridge 120 will fail and as such it will not be possible to accidentally
use a standard
cartridge 120 in place of the non-standard cartridge 320.
Although aimed primarily at the insulin market, the proposed non-standard
cartridge
schemes may apply to other drugs. Likewise, the coding system may apply to
various
drug delivery devices 100.
The proposed cartridge system results in a number of advantages. For example,
the
proposed system help to assist a user to ensure that a given drug delivery
device
CA 02805502 2013-01-15
WO 2012/010563 PCT/EP2011/062277
16
component is only attached to a drug delivery device component for which it is
intended.
The system also results in a low cost coding mechanism since the manufacture
of
cartridges 320 with varying distal diameters D1, D2 and matching holders 104
does not
require a large number of parts and can be manufactured in a cost effective
manner.
Moreover, there are quite a large number of different possible dimensions for
the
variable diameter head portion 331 that can be used. Consequently, with the
proposed
non-standard cartridge schemes, a large number of medicaments 325 can be
distinguished from one another.
In given embodiments, the coding may be designed to block all incorrect
reservoirs from
being inserted into an incorrect cartridge holder 104. In alternative
embodiments, the
coding may be designed to block reservoirs of a given type, but not all types
of
reservoirs. For example, in an embodiment, the coding may block only
reservoirs not
intended for the housing and that comprise dangerous drugs. For instance, a
short-
acting drug could be fitted into a device intended for long-acting drugs, but
not vice
versa. As another example, a low concentration drug could be fitted into a
device
intended for high concentration drugs, but not vice versa.
Exemplary embodiments have been described. However, as those of skill in the
art will
recognize certain changes or modifications to such arrangements may be made.
Those
skilled in the art will understand, however, that further changes,
modifications, revisions
and/or additions may be made to the presently disclosed arrangements without
departing from the true scope and spirit of the present disclosure, which is
defined by
the claims.
The term "drug" or "medicament", as used herein, means a pharmaceutical
formulation
containing at least one pharmaceutically active compound,
wherein in one embodiment the pharmaceutically active compound has a molecular
weight up to 1500 Da and/or is a peptide, a proteine, a polysaccharide, a
vaccine, a
DNA, a RNA, a antibody, an enzyme, an antibody, a hormone or an
oligonucleotide, or
a mixture of the above-mentioned pharmaceutically active compound,
WO 2012/010563 CA 02805502 2013-01-15PCT/EP2011/062277
17
wherein in a further embodiment the pharmaceutically active compound is useful
for the
treatment and/or prophylaxis of diabetes mellitus or complications associated
with
diabetes mellitus such as diabetic retinopathy, thromboembolism disorders such
as
deep vein or pulmonary thromboembolism, acute coronary syndrome (ACS), angina,
myocardial infarction, cancer, macular degeneration, inflammation, hay fever,
atherosclerosis and/or rheumatoid arthritis,
wherein in a further embodiment the pharmaceutically active compound comprises
at
least one peptide for the treatment and/or prophylaxis of diabetes mellitus or
complications associated with diabetes mellitus such as diabetic retinopathy,
wherein in a further embodiment the pharmaceutically active compound comprises
at
least one human insulin or a human insulin analogue or derivative, glucagon-
like
peptide (GLP-1) or an analogue or derivative thereof, or exedin-3 or exedin-4
or an
analogue or derivative of exedin-3 or exedin-4.
Insulin analogues are for example Gly(A21), Arg(B31), Arg(B32) human insulin;
Lys(B3),
Glu(B29) human insulin; Lys(B28), Pro(B29) human insulin; Asp(B28) human
insulin;
human insulin, wherein proline in position B28 is replaced by Asp, Lys, Leu,
Val or Ala
and wherein in position B29 Lys may be replaced by Pro; Ala(B26) human
insulin;
Des(B28-630) human insulin; Des(B27) human insulin and Des(B30) human insulin.
Insulin derivates are for example B29-N-myristoyl-des(B30) human insulin; B29-
N-
palmitoyl-des(B30) human insulin; B29-N-myristoyl human insulin; B29-N-
palmitoyl
human insulin; B28-N-myristoyl LysB28ProB29 human insulin; B28-N-palmitoyl-
LysB28ProB29 human insulin; B30-N-myristoyl-ThrB29LysB30 human insulin; B30-N-
palmitoyl- ThrB29LysB30 human insulin; B29-N-(N-palmitoyl-Y-glutamy1)-des(B30)
human insulin; B29-N-(N-lithocholyl-Y-glutamyI)-des(B30) human insulin; B29-N-
(w-
carboxyheptadecanoyI)-des(B30) human insulin and B29-N-(w-carboxyhepta-
idecanoyl)
human insulin.
CA 02805502 2013-01-15
WO 2012/010563 PCT/EP2011/062277
18
Exendin-4 for example means Exendin-4(1-39), a peptide of the sequence H His-
Gly-
Glu-Gly-Thr-Phe-Thr-Ser-Asp-Leu-Ser-Lys-Gln-Met-Glu-Glu-Glu-Ala-Val-Arg-Leu-
Phe-
Ile-Glu-Trp-Leu-Lys-Asn-Gly-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Pro-Ser-NH2.
Exendin-4 derivatives are for example selected from the following list of
compounds:
H-(Lys)4-des Pro36, des Pro37 Exendin-4(1-39)-NH2,
H-(Lys)5-des Pro36, des Pro37 Exendin-4(1-39)-NH2,
des Pro36 [Asp28] Exendin-4(1-39),
des Pro36 [IsoAsp28] Exendin-4(1-39),
des Pro36 [Met(0)14, Asp28] Exendin-4(1-39),
des Pro36 [Met(0)14, IsoAsp28] Exendin-4(1-39),
des Pro36 [Trp(02)25, Asp28] Exendin-4(1-39),
des Pro36 [Trp(02)25, IsoAsp28] Exendin-4(1-39),
des Pro36 [Met(0)14 Trp(02)25, Asp28] Exendin-4(1-39),
des Pro36 [Met(0)14 Trp(02)25, IsoAsp28] Exendin-4(1-39); or
des Pro36 [Asp28] Exendin-4(1-39),
des Pro36 [IsoAsp28] Exendin-4(1-39),
des Pro36 [Met(0)14, Asp28] Exendin-4(1-39),
des Pro36 [Met(0)14, IsoAsp28] Exendin-4(1-39),
des Pro36 [Trp(02)25, Asp28] Exendin-4(1-39),
des Pro36 [Trp(02)25, IsoAsp28] Exendin-4(1-39),
des Pro36 [Met(0)14 Trp(02)25, Asp28] Exendin-4(1-39),
des Pro36 [Met(0)14 Trp(02)25, IsoAsp28] Exendin-4(1-39),
wherein the group -Lys6-NH2 may be bound to the C-terminus of the Exendin-4
derivative;
or an Exendin-4 derivative of the sequence
H-(Lys)6-des Pro36 [Asp28] Exendin-4(1-39)-Lys6-NH2,
des Asp28 Pro36, Pro37, Pro38Exendin-4(1-39)-NH2,
H-(Lys)6-des Pro36, Pro38 [Asp28] Exendin-4(1-39)-NH2,
CA 02805502 2013-01-15
WO 2012/010563 PCT/EP2011/062277
19
H-Asn-(Glu)5des Pro36, Pro37, Pro38 [Asp28] Exendin-4(1-39)-NH2,
des Pro36, Pro37, Pro38 [Asp28] Exendin-4(1-39)-(Lys)6-NH2,
H-(Lys)6-des Pro36, Pro37, Pro38 [Asp28] Exendin-4(1-39)-(Lys)6-NH2,
H-Asn-(Glu)5-des Pro36, Pro37, Pro38 [Asp28] Exendin-4(1-39)-(Lys)6-NH2,
H-(Lys)6-des Pro36 [Trp(02)25, Asp28] Exendin-4(1-39)-Lys6-NH2,
H-des Asp28 Pro36, Pro37, Pro38 [Trp(02)25] Exendin-4(1-39)-NH2,
H-(Lys)6-des Pro36, Pro37, Pro38 [Trp(02)25, Asp28] Exendin-4(1-39)-NH2,
H-Asn-(Glu)5-des Pro36, Pro37, Pro38 [Trp(02)25, Asp28] Exendin-4(1-39)-NH2,
des Pro36, Pro37, Pro38 [Trp(02)25, Asp28] Exendin-4(1-39)-(Lys)6-NH2,
H-(Lys)6-des Pro36, Pro37, Pro38 [Trp(02)25, Asp28] Exendin-4(1-39)-(Lys)6-
NH2,
H-Asn-(Glu)5-des Pro36, Pro37, Pro38 [Trp(02)25, Asp28] Exendin-4(1-39)-(Lys)6-
NH2,
H-(Lys)6-des Pro36 [Met(0)14, Asp28] Exendin-4(1-39)-Lys6-NH2,
des Met(0)14 Asp28 Pro36, Pro37, Pro38 Exendin-4(1-39)-NH2,
H-(Lys)6-desPro36, Pro37, Pro38 [Met(0)14, Asp28] Exendin-4(1-39)-NH2,
H-Asn-(Glu)5-des Pro36, Pro37, Pro38 [Met(0)14, Asp28] Exendin-4(1-39)-NH2,
des Pro36, Pro37, Pro38 [Met(0)14, Asp28] Exendin-4(1-39)-(Lys)6-NH2,
H-(Lys)6-des Pro36, Pro37, Pro38 [Met(0)14, Asp28] Exendin-4(1-39)-(Lys)6-NH2,
H-Asn-(Glu)5 des Pro36, Pro37, Pro38 [Met(0)14, Asp28] Exendin-4(1-39)-(Lys)6-
NH2,
H-Lys6-des Pro36 [Met(0)14, Trp(02)25, Asp28] Exendin-4(1-39)-Lys6-NH2,
H-des Asp28 Pro36, Pro37, Pro38 [Met(0)14, Trp(02)25] Exendin-4(1-39)-NH2,
H-(Lys)6-des Pro36, Pro37, Pro38 [Met(0)14, Asp28] Exendin-4(1-39)-NH2,
H-Asn-(Glu)5-des Pro36, Pro37, Pro38 [Met(0)14, Trp(02)25, Asp28] Exendin-4(1-
39)-
NH2,
des Pro36, Pro37, Pro38 [Met(0)14, Trp(02)25, Asp28] Exendin-4(1-39)-(Lys)6-
NH2,
H-(Lys)6-des Pro36, Pro37, Pro38 [Met(0)14, Trp(02)25, Asp28] Exendin-4(S1-39)-
(Lys)6-NH2,
H-Asn-(Glu)5-des Pro36, Pro37, Pro38 [Met(0)14, Trp(02)25, Asp28] Exendin-4(1-
39)-
(Lys)6-NH2;
or a pharmaceutically acceptable salt or solvate of any one of the afore-
mentioned
Exedin-4 derivative.
WO 2012/010563 CA 02805502 2013-01-15PCT/EP2011/062277
20
Hormones are for example hypophysis hormones or hypothalamus hormones or
regulatory active peptides and their antagonists as listed in Rote Liste, ed.
2008,
Chapter 50, such as Gonadotropine (Follitropin, Lutropin, Choriongonadotropin,
Menotropin), Somatropine (Somatropin), Desmopressin, Terlipressin,
Gonadorelin,
Triptorelin, Leuprorelin, Buserelin, Nafarelin, Goserelin.
A polysaccharide is for example a glucosaminoglycane, a hyaluronic acid, a
heparin, a
low molecular weight heparin or an ultra low molecular weight heparin or a
derivative
thereof, or a sulphated, e.g. a poly-sulphated form of the above-mentioned
polysaccharides, and/or a pharmaceutically acceptable salt thereof. An example
of a
pharmaceutically acceptable salt of a poly-sulphated low molecular weight
heparin is
enoxaparin sodium.
Pharmaceutically acceptable salts are for example acid addition salts and
basic salts.
Acid addition salts are e.g. HCI or HBr salts. Basic salts are e.g. salts
having a cation
selected from alkali or alkaline, e.g. Na+, or K+, or Ca2+, or an ammonium ion
N+(R1)(R2)(R3)(R4), wherein R1 to R4 independently of each other mean:
hydrogen,
an optionally substituted C1 C6-alkyl group, an optionally substituted C2-C6-
alkenyl
group, an optionally substituted C6-C10-aryl group, or an optionally
substituted C6-C10-
heteroaryl group. Further examples of pharmaceutically acceptable salts are
described
in "Remington's Pharmaceutical Sciences" 17. ed. Alfonso R. Gennaro (Ed.),
Mark
Publishing Company, Easton, Pa., U.S.A., 1985 and in Encyclopedia of
Pharmaceutical
Technology.
Pharmaceutically acceptable solvates are for example hydrates.
WO 2012/010563 CA 02805502 2013-01-15PCT/EP2011/062277
21
Reference numerals
100 drug delivery device
102 dose setting mechanism
103 distal end
104 cartridge holder
105 proximal end
106 removable cap
107 proximal end
108 distal end
109 spindle
111 cartridge cavity
117 dose setter
120 cartridge
121 thread
122 ampoule
124 ferrule
125 medicament
127 septum
128 piston
130 distal end
131 head portion
132 proximal end
133 neck portion
134 bead
135 shoulder
320 cartridge
322 ampoule
324 ferrule
325 medicament
327 septum
WO 2012/010563 CA 02805502 2013-01-15PCT/EP2011/062277
22
330 distal end
331 head portion
333 neck portion
334 bead
335 shoulder
350 surface
400 press
401 direction
405 rolling plate
406 direction
410 force
411 force
D diameter
D1 first diameter
D2 second diameter
OD outer diameter
DND diameter