Note: Descriptions are shown in the official language in which they were submitted.
CA 02806820 2013-01-28
WO 2012/014149 PCT/1B2011/053318
1
N-METHYLFORMAMIDE SOLVATE OF DASATINIB
Field of the Invention
The present invention relates to a N-Methylformamide solvate of dasatinib and
a process for its preparation.
Background of the Invention
Dasatinib monohydrate of Formula A, chemically described as N-(2-chloro-6-
methylpheny1)-2-R644-(2-hydroxyethyl)-1-piperazinyll-2-methyl-4-
pyrimidinyljamino]-
5-thiazole carboxamide monohydrate, is a cyclic protein tyrosine kinase
inhibitor.
Dasatinib monohydrate, marketed under the brand name Sprycel , is indicated
for the
treatment of adults with chronic, accelerated, or myeloid or lymphoid blast
phase chronic
myeloid leukemia (CML) with resistance or intolerance to prior therapy
including
imatinib. Sprycele is also indicated for the treatment of adults with
Philadelphia
chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) with resistance or
intolerance to prior therapy.
CH3
CH3 0 NN
41, NHS / NHN H20
CI OH
Formula A
U.S. Patent No. 6,596,746 provides a process for the preparation of dasatinib.
U.S. Patent No. 7,491,725 provides for the crystalline monohydrate, butanol
solvate form, crystalline ethanol solvates and neat forms of dasatinib. It
also provides a
process for the preparation of crystalline monohydrate, butanol solvate form,
crystalline
ethanol solvate and neat form of dasatinib.
U.S. Publication No. 2009/0118297 provides for the anhydrous form, amorphous
form, iso-propanol solvate, a n-propanol-dimethylsulfoxide ("DMSO") solvate, a
DMSO
solvate, a hemi tetrahydrofuran ("THF") solvate, a 2-methyl-tetrahydrofuran
("2-methyl
THF") solvate, a hemi 1,4-dioxane solvate, a pyridine solvate, a toluene
solvate, a methyl
CA 02806820 2013-01-28
WO 2012/014149 PCT/1B2011/053318
2
isobutyl ketone ("MIBK") solvate, a mono acetone solvate, an iso-propanol
("IPA")-
DMS0 solvate, a 2-butanol-DMS0 solvate, an IPA-DMF solvate, an IPA solvate, an
n-
propanol-DMF solvate, an n-propanol solvate, a 2-butanol-DMF solvate, a 2-
butanol
solvate, an n-butanol-DMSO solvate, a DMF-water solvate, a DMF solvate, a
methyl
isopropyl ketone ("MIPK") solvate, a dimethoxyethane solvate, a cellosolve
solvate, a
methylacetate solvate, a methanol solvate, an ethylacetate solvate, a 2-
pentanole solvate, a
dimethyl carbonate solvate, an isopropylacetate solvate, an ethyleneglycol
solvate, a
dichloromethane solvate, a methylformate solvate, a tert-butanol solvate, a
dimethoxyethane solvate, a methylethylketone ("MEK") solvate, a
monochlorobenzene
solvate, a propylene glycol monoethyl ether ("PGME") solvate, a glycerol
solvate, a
cyclopentyl methyl ether solvate, a methyl tert butyl ether ("MTBE") solvate,
an
amylalcohol solvate, a glycerol formal solvate of dasatinib and processes for
their
preparation.
WO 2010/062715 discloses an isosorbide dimethyl ether solvate of dasatinib, a
N,N'-dimethylethylene urea solvate of dasatinib, and a N,N'-dimethyl-N,N'-
propylene urea
solvate of dasatinib and processes for their preparation.
WO 2010/067374 discloses a dimethylformamide solvate, dimethyl sulfoxide
solvate, toluene solvate, isopropyl acetate solvate, crystalline Form I of
dasatinib
characterized by their X-ray powder diffractogram and processes for their
preparation.
Polymorphism is defined as the ability of a substance to exist as two or more
crystalline phases that have different arrangements and/or conformations of
the molecule
in the crystal lattice. Different polymorphs may differ in their physical
properties, such as,
melting point, solubility, X-ray diffraction patterns, and the like. Although
these
differences disappear once the compound is dissolved, they can appreciably
influence the
pharmaceutically relevant properties of the solid form, such as its handling
properties,
dissolution rate and stability. Such properties can significantly influence
the processing,
the shelf life, and the commercial acceptance of a polymorph. Polymorphic
forms of a
compound can be distinguished in the laboratory by analytical methods such as
X-ray
Diffraction (XRD) powder as well as single crystal, DSC, IR, Solid state NMR,
or Raman
spectroscopy.
WO 2012/014149
CA 02806820 2013-01-28
PCT/1B2011/053318
3
The solvent medium and/or mode of isolation play a very important role in
obtaining one polymorphic form over another.
The discovery of new solid state forms and solvates of a pharmaceutical
compound
provides a new opportunity to improve the pharmacokinetic properties of a
pharmaceutical
product. Therefore, there is a need for additional solid state forms of
dasatinib.
The present inventors have now surprisingly found a N-Methylformamide solvate
of dasatinib. The novel N-Methylformamide solvate of dasatinib of the present
invention
is suitable for preparing pharmaceutical compositions.
Summary of the Invention
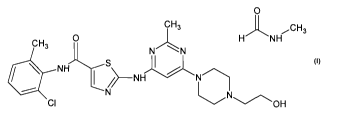
In one general aspect, the present invention provides for N-Methylformamide
solvate of dasatinib of Formula B.
0 CH3
HNH ...-- CH 3
CH3 jNH CI 1
I
OH
Formula B
In another general aspect, the present invention provides for crystalline N-
Methylformamide solvate of dasatinib characterized by an X-Ray Powder
Diffractogram
(XRPD) substantially as depicted in Figure 1.
In another general aspect, the present invention provides for crystalline N-
Methylformamide solvate of dasatinib characterized by an X-ray Powder
Diffractogram
which includes interplanar distances at approximately 14.21, 7.11, 5.80, 4.74,
and 3.65 A.
Embodiments of this aspect may include one or more of the following features.
For example, the crystalline N-Methylformamide solvate of dasatinib may be
further
characterized by an X-ray Powder Diffractogram which includes interplanar
distance at
approximately 7.45, 4.83, 4.29, 3.82, 3.73 and 3.49 A.
The crystalline N-Methylformamide solvate of dasatinib may further be
characterized by a DSC thermogram substantially as depicted in Figure 2. For
example,
CA 02806820 2013-01-28
WO 2012/014149 PCT/1B2011/053318
4
the crystalline N-Methylformamide solvate of dasatinib may have characteristic
DSC
endothermic peaks at about 160 C and about 287 C.
The crystalline N-Methylformamide solvate of dasatinib may also be further
characterized by a TGA substantially as depicted in Figure 3.
In yet another general aspect, the present invention provides for a process
for the
preparation of N-Methylformamide solvate of dasatinib. The process includes:
a) treating dasatinib with N-Methylformamide; and
b) isolating N-Methylformamide solvate of dasatinib.
Embodiments of this aspect may include one or more of the following features.
For example, step a) is carried out at a temperature of 20 C to 50 C and the
amount of N-
Methylformamide is 3 to 10 times the amount of dasatinib.
In another general aspect, the present invention provides for the use of N-
Methylformamide solvate of dasatinib for the preparation of other polymorphic
forms or
solvates of dasatinib.
In yet another general aspect, the present invention provides for a
pharmaceutical
composition which includes a therapeutically effective amount of N-
Methylformamide
solvate of dasatinib and one or more pharmaceutically acceptable excipients.
In another general aspect, the present invention provides for a method for the
treatment of adults with chronic, accelerated, or myeloid or lymphoid blast
phase chronic
myeloid leukemia (CML) with resistance or intolerance to prior therapy
including imatinib
and for the treatment of adults with Philadelphia chromosome-positive acute
lymphoblastic leukemia (Ph+ ALL) with resistance or intolerance to prior
therapy. The
method includes administering to a mammal in need thereof a therapeutically
effective
amount of N-Methylformamide solvate of dasatinib.
Brief Description of the Drawings
Figure 1 and Figure la depicts X-Ray Powder Diffractogram (XRPD) of N-
Methylformamide solvate of dasatinib and the associated values, respectively.
Figure 2 depicts Differential Scanning Calorimetry (DSC) thermogram of N-
Methylformamide solvate of dasatinib.
CA 02806820 2013-01-28
WO 2012/014149
PCT/1B2011/053318
5
Figure 3 depicts Thermogravimetric Analysis (TGA) of N-Methylformamide
solvate of dasatinib.
The XRPD was determined by using PANalytical X' Pert Pro X-Ray Powder
Diffractometer in the range 0 C to 40 C 20 and under tube voltage and current
of 45 Kv
and 40 mA, respectively. Copper radiation of wavelength 1.54 angstrom and
Xceletor
detector was used.
The TGA was recorded on TA (Q500) (Rate of heating = 10 C/minute).
The DSC was recorded on Mettler Toledo (DSC 821) (Rate of heating =
C/minute).
10 Detailed Description of the Invention
The present invention provides for the N-Methylformamide solvate of dasatinib
of Formula B
CH3 0 11NcH3 H NH õcH3
NHS I NHN
CI OH
Formula B
The present invention may be in the form of crystalline N-Methylformamide
solvate of dasatinib.
The crystalline N-Methylformamide solvate of dasatinib has an X-Ray Powder
Diffractogram (XRPD) substantially as depicted in Figure 1 of the accompanied
drawings. The crystalline N-Methylformamide solvate of dasatinib has an X-ray
Powder Diffractogram which shows characteristic peaks expressed as interplanar
distance at approximately 14.21, 7.11, 5.80, 4.74, and 3.65 A. The crystalline
N-
Methylformamide solvate of dasatinib may be further characterized by peaks
expressed as interplanar distance at approximately 7.45, 4.83, 4.29, 3.82,
3.73 and 3.49
A.
WO 2012/014149 CA 02806820 2013-01-28PCT/1B2011/053318
6
The crystalline N-Methylformamide solvate of dasatinib has a DSC
thermogram substantially as depicted in Figure 2 of the accompanied drawings.
The
DSC thermogram shows characteristic endothermic peaks at about 160 C and about
287 C.
The crystalline N-Methylformamide solvate of dasatinib has a TGA
substantially as depicted in Figure 3 of the accompanied drawings. The TGA of
the
crystalline N-Methylformamide solvate of dasatinib shows a weight loss of
about
10.6%.
The present invention also provides for a process for the preparation of N-
Methylformamide solvate of dasatinib. The process includes:
a) treating dasatinib with N-Methylformamide; and
b) isolating N-Methylformamide solvate of dasatinib.
Dasatinib in any previously known crystalline or amorphous form prepared by
methods known in the art can be used as the starting material.
Step a) of the process involves adding dasatinib to N-Methylformamide or
adding
N-Methylformamide to dasatinib in optional order of succession at a suitable
temperature
of between 20 C to 50 C; preferably under stirring. For example, the addition
may be
performed at a temperature of between 20 C to 35 C.
N-Methylformamide may be used in an amount of 3 to 10 times the amount of
dasatinib.
After the completion of addition, the resultant mixture is heated to a
temperature of
between about 50 C to 80 C for about 15 minutes to 3 hours. For example, it
may be
heated at about 60 C to 70 C.
Step b) of the process involves isolating N-Methylformamide solvate of
dasatinib
through common isolation techniques, such as one or more of washing,
crystallization,
precipitation, cooling, filtration, filtration under vacuum, decantation and
centrifugation,
or a combination thereof
For example, step b) involves filtration of the reaction mass obtained in step
a) to
remove foreign particulate matter or treated with activated charcoal to remove
coloring
CA 02806820 2013-01-28
WO 2012/014149 PCT/1B2011/053318
7
and other related impurities. The filtrate is maintained at about 10 C to 40 C
for a time
period of 2 hours to 24 hours to allow the N-Methylformamide solvate of
dasatinib to
crystallize.
The isolated crystalline N-Methylformamide solvate of dasatinib may be dried
at
40 C to 60 C under vacuum for about 12 hours to 28 hours.
The N-Methylformamide solvate of dasatinib may also be used for the
preparation
of other polymorphic forms or solvates of dasatinib.
The present invention also provides for a pharmaceutical composition that
includes
a therapeutically effective amount of N-Methylformamide solvate of dasatinib
and one or
more pharmaceutically acceptable excipient.
The present invention also provides for a method for the treatment of adults
with
chronic, accelerated, or myeloid or lymphoid blast phase chronic myeloid
leukemia
(CML) with resistance or intolerance to prior therapy including imatinib and
for the
treatment of adults with Philadelphia chromosome-positive acute lymphoblastic
leukemia
(Ph+ ALL) with resistance or intolerance to prior therapy. The method includes
administering to a mammal in need thereof a therapeutically effective amount
of N-
Methylformamide solvate of dasatinib.
While the present invention has been described in terms of its specific
embodiments, certain modifications and equivalents will be apparent to those
skilled in the
art and are intended to be included within the scope of the present invention.
EXAMPLE
Preparation of N-Methylformamide Solvate of Dasatinib
Dasatinib (3.24 g) was charged in a round bottom flask. N-methyl formamide (20
ml) was added to it. The reaction mixture was heated at 70 C for 30 minutes
and filtered.
The filtrate was collected in a beaker and kept at a temperature of 20 C to 35
C overnight
for crystallization. The solid was filtered and suck dried for 30 minutes.
Solid was
unloaded and dried under a vacuum at 55 C to 60 C for 24 hours to obtain the
title
compound.
Yield: 1.95 g