Note: Descriptions are shown in the official language in which they were submitted.
CA 02808172 2013-02-12
PF 0000070949/SD
BASF SE 1
Device for storing hot, corrosively active liquids and use of the device
6
Description
The invention is based on a device for receiving hot, corrosively active
liquids, compri-
sing a space enclosed by a wall for receiving the liquid. Furthermore, the
invention also
relates to a use of the device.
The device for receiving hot, corrosively active liquids is, for example, a
tank which is
used for receiving the heat storage medium in a solar power plant. In a solar
power
plant, heat is generated during the day, as long as the sun shines, by means
of the
solar energy. The heat is used for generating electricity. Generally, the heat
is used to
vaporize water and to drive a generator for generating electricity by the
steam pro-
duced.
To allow a solar power plant to be operated continuously, a heat storage
medium is
heated up by means of the solar energy. This heat storage medium is stored in
a well
insulated tank. To extract heat, for example when the sun is not shining, the
heated
heat storage medium is removed and used for example far vaporizing water. The
heat
storage medium thereby gives off heat and is cooled. The cold heat storage
medium is
then, for example, made to pass into a second tank, for cold heat storage
medium. To
make uninterrupted operation possible in a solar power plant, large solar
power plants
require very large heat reservoirs.
To vaporize the water in a solar power plant and heat the steam to a
temperature ap-
propriate for operation, it is necessary to heat the heat storage medium to
correspon-
dingly high temperatures. At present, a heat reservoir in a solar power plant
is operated
at a working temperature in the range between 290 and 390 C. Moreover, it is
currently
being attempted to extend the temperature range to 550 C, or even to
temperatures
above that.
Molten salts, for example, are used as heat storage media. On account of the
large
amount of heat storage medium that is required to operate a large solar power
plant,
here, too, alternatives are being sought. Alternative heat storage media are,
for examp-
le, also those comprising sulfur. Both in the case of molten salts and in the
case of sul-
fur-comprising heat storage media, at high temperatures corrosion occurs on
the tanks,
which are usually produced from steel. For example, some molten nitrates may
cause
the embrittlement of various high-grade steels at temperatures over 550 C.
Although
the high-grade steels remain stable, they become sensitive to impact. In the
case of
storage materials comprising large amounts of sulfur, for example sulfur
comprising 1%
= CA 02808172 2013-02-12
PF 0000070949/SD
BASF SE 2
by weight potassium sulfide, notable corrosion occurs at temperatures above
350 C,
= leading to rapid penetrative destruction of typical iron- and nickel-
based high-grade
steels as the temperature increases above 400 C. Chloride-comprising molten
salts
are also highly corrosive at high temperatures. At lower temperatures, the
corrosivity is
much less, in some cases even non-existent.
Materials which withstand corrosive substances even at high temperatures are,
for
example, ceramics and glasses. However, these materials generally cannot be
joined
together without seals to form large structures, such as are necessary for
heat storage
tanks. Sealing material that is used can be corrosively attacked at high
temperatures.
In addition, these materials are generally brittle and, when joined together
to form a
structure, cannot withstand high internal pressures.
It is therefore an object of the present invention to provide a device for
receiving hot,
corrosively acting liquids which is corrosion-resistant and sealed and has
sufficient me-
chanical stability to be able to receive even large amounts of liquid.
The object is achieved by a device for receiving hot, corrosively acting
liquids which
comprises a space enclosed by a wall for receiving the liquid, the space
having an in-
ner insulation.
In this case either the inner insulation may lie directly against the wall of
the tank or
there may be a gap formed between the inner insulation and the wall.
The inner insulation avoids the hot liquid comprisesed in the space coming
into contact
with the wall. As a result of the insulating effect of the inner insulation,
the temperature
of the side facing the wall is much lower than the temperature of the hot
liquid. This
achieves the effect that the temperature of the wall bounding the space can be
kept
below the temperature at which corrosion occurs.
To protect the inner insulation from inadmissible forces acting on it, in
particular when
there is a gap between the inner insulation and the wall of the space in which
the liquid
is kept, the inner insulation preferably has passages through which the liquid
can flow.
As a result, a pressure equalization is established on the inside of the
insulation and
the outside of the insulation. The inner insulation consequently does not need
to be
stable with respect to a pressure acting from the inside. In particular if the
gap between
the inner insulation and the wall is not uniform, or else in some places the
inner insula-
tion lies against the wall and in some places a gap is produced, liquid flows
through the
passages into the gap until pressure equalization is established. This avoids
deforming
of the inner insulation, which could possibly lead to destruction.
CA 02808172 2013-02-12
PF 0000070949/SD
BASF SE 3
= The passages are in this case designed in such a way that liquid can
flow into the pas-
sages but no convection occurs. This makes it possible for liquid to flow out
of the tank
through the passages, but no mass transfer to take place in the passages once
they
have been filled. In particular when the liquid to be filled has a low thermal
conductivity,
for example in the case of a molten sulfur, the liquid comprisesed in the
passages then
also has an insulating effect. Although the liquid comes into contact with the
wall of the
space as a result of passing through the passages, it has a lower temperature
than the
liquid in the reservoir, the thickness of the insulation being chosen such
that the tempe-
rature in the region of the wall is so low that no corrosion occurs, or at
least only mini-
mal corrosion.
To compensate for a different thermal expansion of the material of the inner
insulation
and the wall by which the space is enclosed, it is preferred if the inner
insulation has
expansion joints. The expansion joints are preferably likewise dimensioned
such that
no convection occurs in them. In a preferred embodiment, the passages in the
inner
insulation for pressure equalization serve at the same time as expansion
joints, which
are used to prevent the inner insulation from being destroyed by thermal
expansion. In
this way it is possible to even withstand loads caused by exposure to changing
tempe-
ratures without the inner insulation being destroyed.
The size of the passages and/or the expansion joints is dependent here on the
viscosi-
ty of the liquid comprisesed in the space.
Even in the case of open-pored refractory insulating bricks, from which the
inner insula-
tion may be produced for example, although liquid penetrating into the pores
reduces
the insulating quality of the insulating bricks, the low thermal conductivity
of the liquid,
for example of sulfur, is sufficient to build up a sufficiently strong
insulation.
The inner insulation may, for example, be built up from substantially cuboidal
elements.
Substantially cuboidal elements also comprise elements in which the width
increases
outwardly to match a tank with a circular cross section, so that the expansion
joints
between the elements have a uniform width, and elements which are designed in
the
form of circular segments which match the diameter of the tank. The passages
or ex-
pansion joints are, for example, gaps between the cuboidal elements. Further
preventi-
on of convection is possible by the cuboidal elements being laid in rows in an
offset
manner to build up the inner insulation. A gap between two cuboidal elements
is then
only as high in each case as such a cuboidal element and is interrupted by a
cuboidal
element of the next row.
CA 02808172 2013-02-12
PF 0000070949/SD
BASF SE
4
The inner insulation may be both self-supporting and formed by securing
insulating
= elements to the wall. In the case of self-supporting insulation,
insulating elements are,
for example, laid in rows to form an inner wall, it being possible for this
wall to be free-
standing or lying against the wall of the space. This is particularly
preferred if the self-
supporting inner insulation has expansion joints.
To improve the insulation further, it is possible to form a second insulating
layer
between the wall and the inner insulation. The second insulating layer may in
this case
be formed from the same material as the inner insulation. It is also possible
to use two
different materials.
If a second insulating layer is included between the wall and the inner
insulation, it is
possible, for example, for the inner insulation, which is preferably self-
supporting, to be
formed from an abrasion-resistant material, for example refractory brick of
alumina, and
the second insulating layer to comprise a highly insulating material, for
example glass
foam.
The inner insulation may also be built up from more than two layers. In this
case, at
least one layer is preferably a self-supporting inner insulation, while the
other layers
may or may not be self-supporting. It is also possible, for example, to build
up alter-
nating self-supporting insulating layers and highly insulating material in
multiple layers.
Furthermore, however, it is also possible for all the layers of the insulation
to be self-
supporting.
In particular if the second insulating layer is not self-supporting, it is
advantageous if it
is bounded on the inside and on the outside by a self-supporting inner
insulation. It is
preferred, however, if each layer of the insulation is self-supporting.
In a preferred embodiment, a seal of a corrosion-stable material is included
between
the inner insulation and the wall. The seal of corrosion-stable material may
be, for
example, an inliner, for example in the form of a corrugated metal sheet. The
use of a
seal of a corrosion-stable material makes it possible to use a non-corrosion-
stable me-
tal for the wall. Corrosion-stable materials, for example corrosion-stable
high-grade
steels, are generally expensive and also have lower strength values than
steels that
are not corrosion-stable with respect to the liquid comprisesed in the space.
Use of the
seal of the corrosion-stable material makes it possible to produce the wall of
the en-
closed space, for example a tank, from a steel which is not stable with
respect to the
liquid comprisesed in the space. The seal of the corrosion-stable material
helps to
avoid the liquid that is comprisesed in the space coming into contact with the
wall.
CA 02808172 2013-02-12
PF 0000070949/SD
BASF SE 5
The device for storing the hot, corrosively active liquid is, for example, a
tank. This ge-
nerally has a wall and a cover, so as to produce a closed space in which the
hot, corro-
sively active liquid is comprisesed. The wall of the tank may be made, for
example,
from materials typical for tank construction, for example steel or high-grade
steel. In
particular if a seal of a corrosion-stable material is used, it is possible
also to use mate-
rials which are not corrosion-stable with respect to the liquid comprisesed in
the tank
for the wall of the tank.
Suitable corrosion-stable materials from which the seal may be produced are,
for exa-
mple, graphite or aluminum.
If the device for storing hot, corrosively active liquids is a tank, it is
usually closed by a
tank cover. Insulating elements are then likewise provided on the tank cover.
The insu-
lation of the tank cover also avoids in the region of the tank cover -
especially when the
tank is completely filled - the tank cover coming into contact with the hot,
corrosively
active liquid. Moreover, it also avoids heat being given off to the
surroundings via the
tank cover.
Apart from a tank, the storage space enclosed by a wall may also be a cavity
in the
ground. In this case it is possible on the one hand for the cavity to be a
natural cavity,
while alternatively it is also possible for example to produce a cavity
artificially. The
advantage of a cavity in the ground is that greater heights of the reservoir
can be reali-
zed, since it can be subjected to a higher hydrostatic pressure than
conventional tanks
because the forces occurring on the wall as a result of the hydrostatic
pressure are
absorbed by the ground. A great height for the space is appropriate in
particular
whenever the corrosively active liquid comprisesed in the space is a heat
reservoir in-
tended to be operated as a thermocline reservoir. In a heat reservoir operated
as a
thermocline reservoir there is cold liquid at the bottom and hot liquid at the
top. A great
height increases the time it takes for the temperature to be equalized by heat
conduc-
tion. In this way it is possible to realize very large heat reservoirs, for
example for solar
power plants, which can be used, for example, as daily, weekly and in
principle also
monthly or even yearly reservoirs. This is helpful in particular because
natural energy
sources such as wind and the sun fluctuate.
A further advantage of a cavity in the ground is that a heat reservoir for a
solar power
plant can also be operated under pressure and at a maximum temperature well
above
440 C, since a system pressure of more than 1 bar can be applied even in the
case of
large reservoirs. A further advantage is that the hot, corrosively active
liquid can be
kept in a cavity in the ground with the exclusion of air, allowing the risk of
fire to be gre-
atly reduced.
CA 02808172 2013-02-12
PF 0000070949/SD
BASF SE
6
The inner insulation of the cavity in the ground avoids the hot, corrosively
acting liquid
coming into contact with the ground and releasing substances from the ground
or reac-
ting with them and entraining the substances released or the reaction
products. The
substances or reaction products released from the ground may, for example,
cause
damage by increased corrosion or by leaving deposits on further components of
a plant
in which the device for storing hot, corrosively active liquids is used.
A cavity in the ground may, for example, be artificially produced completely
above
ground, for example by artificially building up a hill in which such a cavity
is formed.
Furthermore, a cavity in the ground may be partially below ground, it being
possible to
use both cavities that have already been caused naturally and artificial
cavities. It is
also possible for the cavity to be created completely underground. In this
case, natural
cavities are used in particular. According to the invention, an inner
insulation is intro-
duced into the cavity in the ground. As already described above, this inner
insulation
serves in particular for avoiding liquid that is stored in the cavity
releasing substances
from the ground or reacting with substances from the ground.
Alumina, silicon carbide, silica, aluminum foam, glass foam or mixtures
thereof are sui-
table, for example, as the material of the inner insulation, both for use in a
tank and for
use in a cavity. It is also possible to provide multiple layers, it being
possible for the
layers to be produced from different materials.
In particular if the device for storing the hot, corrosively acting liquid is
a tank, in parti-
cular a tank with a metal wall, for example a steel wall, it is possible in
spite of the inner
insulation for the tank wall to be at a temperature which can, for example if
touched,
cause injuries. In this case it is preferred if the tank wall is additionally
surrounded by
an outer insulation. Suitable for the outer insulation are, for example,
mineral fiber mats
or standard glass foam panels. With an additional covering of a metal sheet,
for exa-
mple zinc sheet, ingress of moisture into the insulation can be avoided.
The device according to the invention for receiving hot, corrosively acting
liquids is sui-
table in particular for receiving a heat storage medium in a solar power
plant, for exa-
mple a parabolic-trough solar power plant. Heat storage media which can be
used are,
for example, molten salts or sulfur-comprising heat storage media. Suitable in
particular
as a sulfur-comprising heat storage medium is elementary sulfur. To adapt the
vapor
pressure and the melting pressure, it is advantageous to add at least one
anion-
comprising additive to the sulfur.
CA 02808172 2013-02-12
PF 0000070949/SD
BASF SE 7
Suitable in particular as anion-comprising additives are those which, at the
operating
temperature, do not oxidize the sulfur into corresponding oxidation products,
for exa-
mple sulfur oxides, sulfur halides or sulfur oxide halides. Furthermore, it is
advantage-
ous if the anion-comprising additives dissolve well in the sulfur.
Preferred anion-comprising additives are ionic compounds of a metal of the
periodic
table of elements with monoatomic or polyatomic singly or multiply negatively
charged
anions.
Metals of ionic compounds are, for example, alkali metals, preferably sodium,
potassi-
um; alkaline earth metals, preferably magnesium, calcium, barium; metals of
the 13th
group of the periodic table of elements, preferably aluminum; transition
metals, prefe-
rably manganese, iron, cobalt, nickel, copper, zinc.
Examples of such anions are: halides and polyhalides, for example fluoride,
chloride,
bromide, iodide, triiodide; chalcogenides and polychalcogenides, for example
oxide,
hydroxide, sulfide, hydrogen sulfide, disulfide, trisulfide, tetrasulfide,
pentasulfide, he-
xasulfide, selenide, telluride; pnicogens, for example amide, imide, nitride,
phosphide,
arsenide, pseudohalides, for example cyanide, cyanate, thiocyanate; complex
anions,
for example phosphate, hydrogen phosphate, dihydrogen phosphate, sulfate,
hydrogen
sulfate, sulfite, hydrogen sulfite, thiosulfate, hexacyanoferrates,
tetrachloroaluminate,
tetrachloroferrate.
Examples of anion-comprising additives are: aluminum(III)chloride,
iron(III)chloride,
iron(11) sulfide, sodium bromide, potassium bromide, sodium iodide, potassium
iodide,
potassium thiocyanate, sodium thiocyanate, disodium sulfide (Na2S), disodium
tetrasul-
fide (Na2S4), disodium pentasulfide (Na2S5), dipotassium pentasulfide (K2S5),
dipotassi-
urn hexasulfide (K2S6), calcium tetrasulfide (CaS4), barium trisulfide (BaS3),
dipotassi-
urn selenide (K2Se), tripotassium phosphide (K3P), potassium hexacyanoferrate
(II),
potassium hexacyanoferrate (I11), copper (I) thiocyanate, potassium triiodide,
cesium
triiodide, sodium hydroxide, potassium hydroxide, cesium hydroxide, sodium
oxide,
potassium oxide, cesium oxide, potassium cyanide, potassium cyanate, sodium
tetra-
aluminate, manganese(I1)sulfide, cobalt(I1)sulfide, nickel(11)sulfide,
copper(II) sulfide,
zinc sulfide, trisodium phosphate, disodium hydrogen phosphate, sodium
dihydrogen
phosphate, disodium sulfate, sodium hydrogen sulfate, disodium sulfite, sodium
hydro-
gen sulfite, sodium thiosulfate, tripotassium phosphate, dipotassium hydrogen
phos-
phate, potassium dihydrogen phosphate, dipotassium sulfate, potassium hydrogen
sulfate, dipotassium sulfite, potassium hydrogen sulfite, potassium
thiosulfate.
= CA 02808172 2013-02-12
PF 0000070949/SD
BASF SE 8
For the purposes of this application, anion-comprising additives are,
furthermore, mix-
tures of two or more compounds of a metal of the periodic table of elements
with mo-
noatomic or polyatomic formally singly or multiply negatively charged anions,
preferably
anions based on non-metal atoms. According to the current state of knowledge,
the
quantitative ratio of the individual components is not critical here.
The mixture according to the invention preferably comprises elementary sulfur
in the
range from 50 to 99.999% by weight, preferably in the range from 80 to 99.99%
by
weight, particularly preferably 90 to 99.9% by weight, in each case with
respect to the
total mass of the mixture according to the invention.
The mixture according to the invention preferably comprises anion-comprising
additives
in the range from 0.001 to 50% by weight, preferably in the range from 0.01 to
20% by
weight, particularly preferably 0.1 to 10% by weight, in each case with
reference to the
total mass of the mixture according to the invention.
The mixture according to the invention may comprise further additives, for
example
additives which reduce the melting point of the mixture. The proportion of
further additi-
ves generally lies in the range from 0.01 to 50% by weight, in each case with
respect to
the total mass of the mixture.
Furthermore, mixtures of alkali polysulfides of the general formula
(M1xM20_02Sy
may also be used, where M1, M2= Li, Na, K, Rb, Cs and M1 is not the same as M2
and
0.05 x 0.95 and 2.0 y 6Ø
In a preferred embodiment of the invention, M1 = K and M2= Na.
In a further preferred embodiment of the invention, 0.20 x 5_ 0.95. In a
particularly
preferred embodiment of the invention, 0.50 x 0.90.
In a further preferred embodiment of the invention, 3.0 y 6Ø In a
particularly prefer-
red embodiment of the invention, y = 4.0, 5.0 or 6Ø
In a particularly preferred embodiment of the invention, M1= K, M2 = Na, 0.20
< x <
0.95 and 3.0 y 6Ø
CA 02808172 2013-02-12
PF 0000070949/SD
BASF SE
9
In a most particularly preferred embodiment of the invention, M1 = K, M2 = Na,
0.50 x
0.90 and y = 4.0, 5.0 or 6Ø
Likewise suitable are mixtures of alkali polysulfides and alkali thiocyanates
according to
the general formula
((M1xM2(1-x))2Sy)m (M3zM4(1-z)SCN)(1-m)
where M', M2, M3, M4 = Li, Na, K, Rb, Cs and M1 is not the same as M2, M3 is
not the
same as M4 and 0.05 5_ X 1, 0.05 z 1, 2.0 y 6.0 and m is the quantitative pro-
portion of substance with 0.05 5_ n 0.95.
In a preferred embodiment of the invention, M1 and M3 = K and M2 and M4 = Na.
In a further preferred embodiment of the invention, 0.20 x 1. In a
particularly prefer-
red embodiment of the invention, 0.50 x 1.
In a further preferred embodiment of the invention, 3.0 y 1. In a particularly
prefer-
red embodiment of the invention, y = 4.0, 5.0 or 6Ø
In a further preferred embodiment of the invention, 0.20 z 1. In a
particularly prefer-
red embodiment of the invention, 0.50 z 1.
In a further preferred embodiment of the invention, 0.20 m 0.80. In a
particularly
preferred embodiment of the invention, 0.33 m 0.80.
In a particularly preferred embodiment of the invention, M' and M3 = K, M2 and
M4 =
Na, 0.20 x 1, 0.20 z 0.95, 3.0 y 6.0 and 0.20 m 0.95.
In a most particularly preferred embodiment of the invention, M1 and M3 = K
and M2
and M4 = Na, 0.50 x 1, 0.50 z 0.95, y = 4.0, 5.0 or 6.0 and 0.33 m < 0.80.
In a further particularly preferred embodiment of the invention, M1 and M3 =
K, x = 1, z
= 1, y = 4.0, 5.0 or 6.0 and 0.33 m 0.80.
In a further particularly preferred embodiment of the invention, M1 and M3 =
K, x = 1, z
= 1, y = 4 and m = 0.5.
CA 02808172 2013-02-12
PF 0000070949/SD
BASF SE 10
In a further particularly preferred embodiment of the invention, M1 and M3 =
K, x = 1, z
= 1, y = 5 and m = 0.5.
In a further particularly preferred embodiment of the invention, M1 and M3 =
K, x = 1, z
= 1, y = 6 and m = 0.5.
Apart from the use for receiving a heat storage medium in a solar power plant,
the de-
vice according to the invention may, however, also be used as tanks or
reactors that
are exposed to high temperature corrosion and are always operated with the
same
medium. The device according to the invention is unsuitable for operation with
different
media, since the space enclosed by the wall can only be cleaned with
difficulty. Una-
voidable chinks and gaps retain remains of media which cannot be removed, or
only
with great difficulty.
Embodiments of the invention are explained in more detail in the description
which
follows and are represented in the figures, in which:
Figure 1 shows a device formed as a thermocline reservoir for receiving hot,
corrosi-
vely acting liquids,
Figure 2 shows a detail of a self-supporting inner insulation,
Figure 3 shows an example of the structure of an inner insulation with
insulating
panels,
Figure 4 shows a structure of a tank cover with insulating elements,
Figure 5 shows a structure of a tank wall with self-supporting inner
insulation,
Figure 6 shows a schematic representation of a device for receiving hot,
corrosively
acting liquids as a cavity in the ground,
Figure 7 shows the structure of the self-supporting inner insulation in a
cavity in the
ground,
Figure 8 shows the device designed as a composite reservoir for receiving hot,
cor-
rosively acting liquids,
Figure 9 shows a flange connection with inner insulation,
CA 02808172 2013-02-12
PF 0000070949/SD
BASF SE 11
Figure 10 shows a flap with inner insulation.
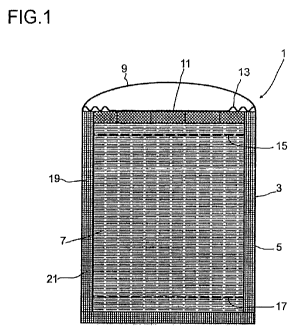
In Figure 1, a device formed as a thermocline reservoir for receiving a hot,
corrosively
acting liquid is represented.
A thermocline reservoir 1, as represented in Figure 1, may be used, for
example, as a
heat reservoir in a solar power plant.
The thermocline reservoir 1 comprises a tank 3, which is constructed for
example from
a metallic material, for example steel. For this purpose, a tank wall 5 is
produced from
the metallic material, the wall thickness of the tank wall 5 being chosen such
that it is
mechanically stable with respect to the pressures occurring in the tank. To be
taken
into consideration in particular here is the downwardly increasing hydrostatic
pressure
of a liquid 7 comprisesed in the tank. The tank 3 is closed by a tank cover 9.
In additi-
on, a further cover 11 may be provided, resting on the liquid 7 comprisesed in
the tank
3 when the tank 3 is completely filled, so that no gas is comprisesed in the
tank 3. To
compensate for fluctuations in the liquid level, it is possible for
compensating regions
13 to be provided on the further cover 11. These may, for example, take the
form of a
bellows. The compensating region 13 allows the further cover 11 to be
positively
connected to the tank wall 5, for example by a welding process. This makes a
gastight
connection possible. When there is an increase in the liquid level or a
decrease in the
liquid level, the further cover 11 is then raised or lowered, so that it
always closes off
the tank in such a way that no gas is comprisesed in the tank. Alternatively
or in additi-
on, it is also possible to compensate for changes in the volume of the storage
material
in response to a change in temperature by supplying or removing liquid, for
example
from a buffer tank.
If the tank 3 is used as a thermocline reservoir 1, there is a first manifold
15 in the up-
per region of the tank 3. By way of the first manifold 15, hot liquid can be
uniformly fed
into the tank. At the same time, to keep the level of the liquid in the tank
constant, col-
der liquid is removed by way of a second manifold 17 at the bottom of the tank
3. Cold
liquid is uniformly removed through the first manifold 15 and the second
manifold 17,
so that preferably no convective flow occurs, and consequently a very small
vertical
heat conduction arises in the tank. In this way it is possible to store liquid
in the tank
such that generally colder liquid of higher density is comprisesed in the
lower region
and warmer liquid of lower density is comprisesed in the upper region. In the
ideal
case, the liquid in the tank has two temperatures, that is a higher
temperature in the
upper region and a lower temperature in the lower region. Between the hot
region and
the cold region, a temperature boundary layer forms. Since heat conduction in
the Ii-
quid cannot be prevented, in an actual case it is not possible however for
there to be a
CA 02808172 2013-02-12
PF 0000070949/SD
BASF SE 12
sharp delineation between hot and cold liquid, but instead there forms a
temperature
transition from the hot liquid to the colder liquid. The longer the storage
continues, the
more and more indistinct the transition becomes, as a result of heat
conduction.
In a solar power plant, the supply of hot liquid takes place by way of the
first manifold
and the removal of colder liquid takes place by way of the second manifold 17,
when the liquid used as the heat storage medium is heated up by solar energy.
If the
sun does not shine, but electricity is to continue being generated in the
solar power
plant, the heat stored in the heat storage medium is used for vaporizing water
to drive
10 the turbines driving the generators. For this purpose, the hot heat
storage medium is
removed from the tank 3 by way of the first manifold 15, gives off heat into a
heat
exchanger, in which the water used as an operating fluid is vaporized and
superheated,
and the cold heat storage medium is then returned by way of the second
manifold 17 in
the lower region of the tank. By removing the hot heat storage medium from the
tank 3
15 and by removing cold heat storage medium during heating up, the temperature
boundary layer in the tank 3 shifts in each case. During heating up of the
heat storage
medium, i.e. when hot heat storage medium is supplied by way of the first
manifold 15
and colder liquid is removed by way of the second manifold 7, the temperature
bounda-
ry layer shifts downward, whereas, when the heat stored in the liquid 7 is
used, the
temperature boundary layer is shifted upward, since the amount of hot heat
storage
medium in the tank 3 decreases and the amount of cold heat storage medium, the
heat
of which has already been used, increases.
Used, for example, as the liquid 7 which serves as the heat storage medium is
a mol-
ten salt or a sulfur-comprising heat storage medium. Suitable in particular as
the sulfur-
comprising heat storage medium is elementary sulfur, which however may be
contami-
nated or comprise further additives. Both molten salts and sulfur are highly
corrosive at
relatively high temperatures with respect to iron- or nickel-comprising
materials. For
example, molten nitrates cause the embrittlement of high-grade steels at
temperatures
over 550 C. Although the high-grade steels remain stable, they become
sensitive to
impact. Sulfur-comprising heat storage media, for example sulfur with 1%
potassium
sulfide, produce notable corrosion on typical iron/nickel high-grade steels at
tempera-
tures above 350 C, leading in a short time to penetrative destruction of the
high-grade
steels as the temperature increases from 500 C.
Chloride-comprising molten salts are also highly corrosive at high
temperatures.
To prevent the corrosion, according to the invention an inner insulation 19 is
included in
the tank 3. The inner insulation 19 avoids the liquid 7 comprisesed in the
tank 3 coming
into contact with the wall 21, which encloses the space receiving the liquid
7. Moreover,
CA 02808172 2013-02-12
PF 0000070949/SD
BASF SE 13
on account of the insulation, the temperature at the wall 21 is much lower
than the
temperature of the liquid 7 in the tank 3.
An example of the structure of the inner insulation 19 is represented in
Figure 2. The
inner insulation, as it is represented in Figure 2, is self-supporting. For
this purpose,
substantially cuboidal elements or - to correspond to the rounding of the tank
- optional-
ly also slightly trapezoidal elements 23 or else elements 23 in the form of
circular seg-
ments are arranged offset in two rows. Between every two cuboidal elements 23
of a
row there is a gap 25. The gaps 25 serve to compensate for different thermal
expansi-
ons of the materials of the inner insulation 19 and the tank wall 5. To build
up the inner
insulation as it is represented in Figure 2, the cuboidal elements 23 are laid
in rows of
layers one above the other, it being preferred for cuboidal elements 23 that
are lying
one above the other likewise to be arranged offset in relation to one another.
The offset
arrangement has the effect of limiting the geometrical extent of the gaps 25.
Further-
more, it is preferred that the gaps 25 are dimensioned such that no convective
flow
occurs. Although liquid 7 can flow into the gaps 25, a constant mass transfer
in the
gaps should be avoided once they are filled with the liquid 7. In particular
in the case of
poorly heat conducting liquids, as is the case for example with a molten
sulfur, the li-
quid comprisesed in the gaps 25 then also has an insulating effect. The design
in two
offset rows, such as that represented in Figure 2, avoids liquid getting
through the inner
insulation 19 to the wall 21.
In an alternative embodiment, it is also possible for the inner insulation to
be built up
from one row of cuboidal elements 23. In this case, the liquid passes through
the gaps
25 to the wall 21. On account of the insulating effect of the insulation 19
and as a result
of the gaps 25 being designed in such a way that no convective flow occurs,
the tem-
perature of the liquid that has flowed through the gaps 25 is also reduced, so
that the
temperature of the liquid coming into contact with the wall 211s lower than
the tempera-
ture of the liquid 7 in the tank 3. The thickness of the insulation 19 is in
this case cho-
sen such that the temperature of the liquid 25 passing through the gaps is
such that the
temperature lies below the temperature at which the liquid has a highly
corrosive effect
on the material of the wall 21.
In Figure 3, an example of the structure of an inner insulation of insulating
panels is
represented. As a difference from the embodiment of an inner insulation 19 re-
presented in Figure 2, the inner insulation 19 that is represented in Figure 3
is not self-
supporting. The inner insulation 19 comprises individual insulating panels 27,
which are
mounted on the wall 21. The wall thickness of the wall 21, which forms the
tank wall 5,
is chosen such that the wall 21 is stable with respect to forces acting on it,
for example
as a result of the hydrodynamic pressure of the liquid comprisesed in the
tank.
CA 02808172 2013-02-12
PF 0000070949/SD
BASF SE 14
= To provide the insulation, the insulating panels 27 are, for example,
secured to the wall
21 by suitable wall hooks 29. The advantage of using wall hooks 29 is that the
indivi-
dual insulating panels 27 of the inner insulation 19 can be mounted in a
simple way
and, if need be, can also be taken down again. Apart from securing with wall
hooks 29,
however, it is also possible to secure the insulating panels 27 to the wall 21
in any
other desired way known to a person skilled in the art. For example, it is
also possible
to adhesively attach the insulating panels to the wall 21. This has the
disadvantage,
however, that it is no longer easily possible to take them down.
To compensate for stresses occurring, the insulating panels 27 are also
mounted such
that a gap 25 is respectively produced between two insulating panels 27. The
dimensi-
ons of the gaps 25 should also be chosen in the embodiment represented in
Figure 3 in
such a way that no convective flow occurs in the gap 25. As a result, during
filling, the
gap 25 is filled by liquid running in, but this then remains in the gap 25 and
thus likewi-
se serves to provide insulation. Since the insulating panels 26 do not
generally lie flush
against the wall 21, liquid also flows behind the insulating panels 27.
However, the in-
sulation with the insulating panels 27 has the effect that the liquid that
comes into
contact with the wall 21 has already cooled down to such an extent that it no
longer has
a corrosive effect on the wall 21.
To protect the insulating panels 27 from corrosion, it is possible to provide
them additi-
onally with a corrosion-resistant coating 31. Suitable here as the corrosion-
resistant
coating is any desired coating known to a person skilled in the art. Suitable
coatings
are, for example, coatings with enamel or an A1203 coating.
A coating 31 of the insulating panels 27 is particularly appropriate whenever
a material
which is not stable with respect to the liquid 7 comprisesed in the tank is
used as the
material for the insulating panels 27.
A possible structure of a tank cover with insulating elements is represented
in Figure 4.
The structure represented in Figure 4 for a tank cover corresponds
substantially to the
structure represented in Figure 3 of a tank wall with insulating panels
mounted on it.
To ensure an insulation also in the upward direction, insulating elements 35
are provi-
ded on the tank cover 33. In a way analogous to that represented in Figure 3,
the insu-
lating elements 35 may, for example, be secured with the aid of hooks 37.
However,
securement by screw connections or adhesive bonding is also possible, for
example.
Depending on the material used for the insulating elements 35 and the liquid 7
to be
CA 02808172 2013-02-12
PF 0000070949/SD
= BASF SE
15
stored in the tank, it is possible to coat the insulating elements 35 with a
corrosion-
resistant coating 31. If gaps 25 are formed between the individual insulating
elements
35, it is also preferred on the tank cover 33 to be able to compensate for
different ther-
mal expansions of the insulating material of the insulating elements 35 and
the material
of the tank cover 33.
In Figure 5, a structure of a tank wall with self-supporting inner insulation
is re-
presented.
The tank wall 5 is formed by a load-bearing steel shell. This is designed so
as to be
mechanically stable and able to absorb the forces acting, for example as a
result of
pressures occurring, without deforming. On the inside, the tank wall 5 is
adjoined by a
corrosion-resistant seal 39. The corrosion-resistant seal 39 is, for example,
a high-
grade steel inliner. This may, for example, take the form of a corrugated
metal sheet.
The use of the corrosion-resistant seal 39 makes it possible to use as
material for the
tank wall 5 a steel which is not corrosion-stable with respect to the liquid
comprisesed
in the tank. The corrosion-resistant seal 39 avoids the liquid being able to
come into
contact with the material of the tank wall 5.
The corrosion-resistant seal 39 is adjoined on the inside by a first
insulating layer 41.
The first insulating layer 41 is preferably self-supporting and built up from
cuboidal
elements which are laid one on top of the other in layers. It is advantageous
if gaps are
formed between the individual cuboidal elements of the first insulating layer
41, as also
represented for example in Figure 2. The first insulating layer 41 is, for
example, of a
highly heat-insulating material. Good insulation is achieved as a result. The
first insula-
ting layer 41 is adjoined by a second insulating layer 43. The second
insulating layer 43
is, for example, produced from an abrasion-resistant material, so that it also
serves in
particular for the purpose that the inner insulation 19 is not damaged by
movement of
the liquid in the tank. The second insulating layer 43 is also preferably self-
supporting
and laid in layers of cuboidal elements. Here, too, it is advantageous if gaps
are formed
between the individual elements of the second insulating layer 43, to be able
to com-
pensate for different thermal expansions of the materials of the first
insulating layer 41,
the second insulating layer 43 and the tank wall 5.
Liquid can flow through the gaps 25 between the individual elements of the
first insula-
ting layer 41 and the second insulating layer 43 in the direction of the wall
21. The li-
quid then collects at the corrosion-resistant seal 39. The fact that on each
of both sides
of the insulation 19 there is liquid at the same pressure, as a result of
pressure equali-
zation, avoids the occurrence of a high internal pressure acting on the
insulation 19
CA 02808172 2013-02-12
PF 0000070949/SD
BASF SE
16
and not compensated from the outside. This largely avoids deformation of the
inner
insulation 19.
Since, in spite of the inner insulation 19, the temperature at the tank wall 5
may be so
high that there is the risk of injuries, for example if the tank wall 5 is
touched, it is also
possible for the tank wall 5 to be adjoined on the outside by an outer
insulation 45. The
outer insulation 45 may, for example, be formed from conventional insulating
materials,
for example mineral fibers or glass fibers. To make the tank weatherproof, the
outer
insulation 45 is then covered, for example, with metal sheets 47. The metal
sheets 47
that are used are, for example, commercially available zinc sheets, which are
particu-
larly weather-resistant.
In Figure 6, a device for receiving hot, corrosively active liquids is
schematically re-
presented in the form of a cavity in the ground.
As a difference from the structure represented in Figures 1 to 5, it is
alternatively also
possible to design the device for receiving the hot, corrosively active liquid
as a cavity
49 in the ground 51. This has the advantage that there is no need for a tank
confine-
ment in the form of a tank wall 5 which is stable with respect to high
pressures. The
forces acting on the wall 21 are absorbed by the ground 51. The device may,
for exa-
mple, likewise be a thermocline reservoir. If the device for receiving the
liquid is a
thermocline reservoir, a first inflow 53 is provided in the upper region,
allowing the hot
heat storage medium to be fed into the cavity 49 or removed from the cavity
49, and a
second inflow 55 is provided, opening out into the lower region of the cavity
49 and
allowing cold heat storage medium to be removed or fed in. The function
otherwise
corresponds to the thermocline reservoir represented in Figure 1.
As a difference from a thermocline reservoir in the form of a tank, in the
case of a cavi-
ty 49 in the ground Slit is possible to realize much greater heights of the
reservoir. As
a result, the diameter can be reduced for the same amount of heat storage
medium, so
that the temperature boundary layer is made smaller. This makes it possible to
operate
the thermocline reservoir over a longer period of time without complete
temperature
equalization taking place by heat conduction. This is possible in particular
because the
ground can absorb very much greater compressive forces than a conventional
tank
wall 5 of steel.
To avoid substances being released from the ground 51 by the liquid that is
compri-
sesed in the cavity 49 and serves as the heat storage medium, and possibly
reacting
with the liquid to form undesired products, the cavity 49 is lined with an
inner insulation
CA 02808172 2013-02-12
PF 0000070949/SD
BASF SE 17
19 in the same way as the tank represented in Figure 1. The structure of the
inner insu-
lation 19 is in this case substantially the same as that represented in
Figures 3 and 5.
A further possibility for a structure of an inner insulation 19 in a cavity in
the ground 51
is represented in Figure 7. Also in the embodiment represented in Figure 7,
the inner
insulation 19 comprises a first insulating layer 41 and a second insulating
layer 43. The
second insulating layer 43 is preferably self-supporting and comes into
contact with the
liquid 7 comprisesed in the cavity 49. For this purpose, the second insulation
43 is, for
example, laid in layers of cuboidal elements. The first insulating layer 41
serves as ad-
ditional insulation and is, for example, produced from a material which can
bear pres-
sure, so that the second insulating layer 43 is pressed against the first
insulating layer
41 on account of the pressure acting on it of the liquid comprisesed in the
cavity 49,
and the forces acting as a result are borne by the first insulating layer 41.
The first insulating layer 41 may, for example, be formed from glass foam or
insulating
bricks.
It is preferred if passages 57 are formed in the inner insulation 19. The
passages 57
serve in this case as relief outlets, through which liquid can flow behind the
inner insu-
lation 19.
The passages 57 are in this case designed such that a convective flow is
avoided, so
that liquid flows once through the passages 57 and, for example, flows into
voids 59
that are located behind the inner insulation 19. If the liquid comprisesed in
the cavity 49
is sulfur, it cools down in the voids 59 and solidifies, whereby the inner
insulation 19 is
supported by pressure from behind. To ensure continuous pressure equalization,
it is
advantageous if the temperature at the passages 57 always remains so high that
the
sulfur does not solidify but continues to be in a molten state. For this
purpose it is pos-
sible, for example, to provide temperature sensors with which the temperature
is mea-
sured. If the temperature decreases too much, it is then possible, for
example, to melt
the solidified sulfur again by the use of suitable heating elements.
The same also applies correspondingly to the use of molten salts, for example,
which
should likewise be kept in the liquid state in the region of the passages 57
and, if the
temperature decreases too much, be able to be heated, for example, in order to
liquefy
them again.
To be able to realize very large thermocline reservoirs with a correspondingly
large
cross-sectional area, it is possible to provide composite reservoirs with
masonry inner
walls. Such a reservoir is represented by way of example in Figure 8. The use
of a
= CA 02808172 2013-02-12
PF 0000070949/SD
BASF SE
18
composite reservoir makes it possible to keep the span of a tank roof within
limits that
are feasible in static design. To produce the composite reservoir, the cavity
49 is divi-
ded into discrete individual reservoirs 61 by inner insulations 19. The same
liquid, for
example a molten sulfur, is contained in each of the individual reservoirs.
The respecti-
ve individual reservoirs, which are separated from each other by the inner
insulation
19, are advantageously hydrostatically connected by lead-throughs. This makes
it pos-
sible to keep the liquid level in the discrete individual reservoirs 61
uniform.
In Figure 9, a flange connection with inner insulation is represented.
To be able to feed liquid into the tank or remove it, it is necessary to
connect lines to
the tank. The connection to lines usually takes place by suitable flanges.
Such a flange
connection is represented by way of example in Figure 9. For this purpose, a
flange 63
is formed on the tank 3. A line 65 is connected to a second flange 67. The
second flan-
ge 67 is in this case designed to be partially concentric about the line 65,
insulating
material 69 being included between the flange 67 and the line 65. At the same
time,
between the first flange 63 on the tank 3 and the second flange 67 there is
the inner.
insulation 19. This design also achieves uniform insulation in the region of
the flange.
The connection of the first flange 62 and the second flange 67 takes place by
conventi-
onal connecting measures, for example by means of screws 71. In addition, a
sealing
element is usually positioned between the first flange 63 and the second
flange 67.
A flap in a line which is provided with an inner insulation is represented in
Figure 10.
To provide corrosion protection in particular for lines through which hot,
corrosively
acting liquid flows, it is also possible likewise to provide the lines with an
inner insulati-
on 19. To control the through-flow, it is possible, for example, to use
fittings. Such fit-
tings are, for example, flaps 73. In the region of the flap 73, the inner
insulation 19 is
interrupted, a stop 75 being located in the region of the interruption. To
close the line
65, the flap 73 may be positioned such that it strikes against the stop 75. By
pivoting
the flap 73, the line 65 can be opened. Use of the inner insulation 19
prevents the hot
material that flows through the line 65 from coming into direct contact with
the material
of the line 65. To protect the stop 75 and the flap 73, they are preferably
provided with
a high-temperature and corrosion-resistant coating 77.
The inner insulation 19, not only in lines and fittings but also in tanks,
makes it possible
to design an installation which is operated with hot, corrosively acting
liquids. Such an
installation is, for example, a solar power plant, for example a parabolic-
trough solar
power plant.
CA 02808172 2013-02-12
PF 0000070949/SD
BASF SE
Examples 19
Example 1
A tank with inner insulation contains sulfur at a temperature of 390 C. The
tank has an
inner insulation 19 of refractory bricks. The tank wall is formed from steel.
On the outsi-
de, the steel is enclosed by an outer insulation of mineral wool.
Table 1 shows the temperatures which respectively occur at the transitions
from brick
to steel, steel to mineral wool and mineral wool to the surroundings.
Table 1: Temperature profile in a device according to the invention with inner
insula-
tion of refractory bricks
Layer Thickness Thermal Apparent Heat capacity Temperature
[cm] conductivity density [KJ/kg K] [001
[W/m K] [kg/m3]
Inner tempera- 390
ture
Refractory 25 0.112 2100 1 236
bricks
Steel 0.5 50 7850 0.47 236
Mineral wool 12 0.04 20 0.85 30
It can be seen from the temperature profile that the temperature decreases
from the
inside of the refractory bricks to the outside of the refractory bricks by 154
C. The tern-
perature at which molten material possibly passing through the refractory
bricks comes
into contact with the tank wall of steel is consequently 236.41 C. This is a
temperature
at which most steels are corrosion-resistant to sulfur and additives comprised
by the
sulfur. Corrosion consequently does not occur.
Example 2
A structure in which hot sulfur at a temperature of 390 C comes into contact
with the
inner insulation is considered. The inner insulation is built up from a layer
of refractory
bricks and a glass foam layer, which adjoins the refractory bricks. Between
the glass
foam layer and the tank wall of steel there is a gap, into which sulfur has
flowed.
CA 02808172 2013-02-12
PF 0000070949/SD
BASF SE 20
The temperatures respectively on the outside of the individual layers are
listed in Table
2.
Table 2: Temperature profile in a device according to the invention with two
insula-
ting layers
Layer Thickness Thermal Apparent Heat capacity Temperature
[cm] conductivity density [KJ/kgK] [ C]
[W/m K] [kg/m3]
Inner tempera- 390
ture
Refractory 25 0.112 2100 1 245
bricks
Glass foam 20 0.06 140 0.85 30.2
Sulfur 0.1 0.269 1960 0.71 30.0
Steel 0.5 50 7850 0.47 30.0
The additional layer of glass foam, preferably of borosilicate glass or quartz
glass,
which has been introduced between the tank wall of steel and the refractory
bricks, has
the effect of reducing the temperature at the wall of steel to such an extent
that it is
only 30 C. At this temperature, corrosion on the steel shell is no longer
likely. The sul-
fur that is between the glass foam and the tank wall of steel is solid.
Moreover, no outer insulation is necessary, since the temperature of the tank
wall of
steel is already so low that it does not present any risk if touched.
CA 02808172 2013-02-12
PF 0000070949/SD
BASF SE 21
List of designations
1 thermocline reservoir 41 first insulating layer
3 tank 25 43 second insulating layer
5 tank wall 45 outer insulation
7 liquid 47 metal sheet
9 tank cover 49 cavity
11 further cover 51 ground
13 compensating region 30 53 first inflow
15 first manifold 55 second inflow
17 second manifold 57 passage
19 inner insulation 59 void
21 wall 61 individual reservoir
23 cuboidal element 35 63 flange
25 gap 65 line
27 insulating panel 67 second flange
29 wall hook 69 insulating material
31 corrosion-resistant coating 71 screw
33 tank cover 40 73 flap
35 insulating element 75 stop
37 hook 77 high-temperature and corrosion-
39 corrosion-resistant seal resistant coating