Note: Descriptions are shown in the official language in which they were submitted.
CA 02810258 2015-07-30
FUEL ADDITIVES FOR TREATING
INTERNAL DEPOSITS OF FUEL INJECTORS
[0001]
TECHNICAL FIELD:
[0002] The disclosure is directed to certain diesel fuel additives and to
method for
cleaning and/or preventing internal deposits in injectors for diesel fuel
operated engines. In
particular the disclosure is directed to methods that are effective against
internal deposits in
injectors for engines operating on ultra low sulfur diesel fuels.
BACKGROUND AND SUMMARY:
[0003] Recent changes in diesel fuels and diesel fuel additives have
resulted in new
injector performance concerns with deposits, including a new type of deposit
not experienced
with older diesel fuel formulations. The injector performance concerns run
across all segments;
on-road fleets, mining equipment, farming equipment, railroad and inland
marine engines.
[0004] Vehicle operators, fuel marketers, and engine manufacturers are
now seeing
deposits forming on the internal parts of fuel injectors. If left untreated,
these deposits may lead
to significant power loss, reduced fuel economy, and, in extreme cases,
increased downtime and
higher maintenance costs due to premature replacement of "stuck injectors."
The new deposits
are believed to be a result of certain common corrosion inhibitors, biofuel
components and acidic
friction modifier, or other carboxylic components used in the fuel reacting
with trace amounts of
transition metals, alkali metal and alkaline earth metals causing salts that
are less soluble in ultra
low sulfur diesel (ULSD) fuels than in the higher sulfur fuels of the past.
When such salts are
present in fuel that is used in a High Pressure Common Rail (HPCR) engine
design, the salts may
tend to deposit in the very tight tolerance areas of the injectors. Such
deposits may lead to poor
fuel injection, which in turn may lead to lost power, lost fuel economy, rough
running engines,
and eventually excessive vehicle downtime and maintenance expense.
1
CA 02810258 2013-03-25
[0005] ULSD now represents about 79% of all distillate fuel supplied in
the United
States. Also, the Renewable Fuel Standard minimum for biodiesel was raised to
1 billion gallons
in 2012. There are indications that the amount of biodiesel required to be
used in fuel will be
even higher in the future. Accordingly, the changing fuel slate continues to
move toward more
ULSD (with less solubility for salts that can form) and more biodiesel in the
marketplace
(another potential source of deposit causing materials in the fuel system).
[0006] In accordance with the disclosure, exemplary embodiments provide a
method
cleaning up internal components of a fuel injector for a diesel engine. The
method includes
operating a fuel injected diesel engine on a fuel composition that contains a
major amount of
diesel fuel having a sulfur content of 50 ppm by weight or less and from about
5 to about 500
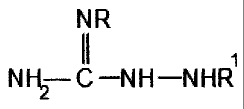
ppm by weight of a reaction product derived from (a) a hydrocarbyl substituted
dicarboxylic
acid, anhydride, or ester and (b) an amine compound or salt thereof of the
formula
NR
1
N H2¨ C ¨N H ¨N HR
wherein R is selected from the group consisting of hydrogen and a hydrocarbyl
group containing
from about 1 to about 15 carbon atoms, and RI is selected from the group
consisting of hydrogen
and a hydrocarbyl group containing from about 1 to about 20 carbon atoms. The
reaction
product is characterized by an FTIR spectrum having a peak intensity in a
region of from about
1630 cm-I to about 1645 cm-I that ranges from about 5 to about 45% of peak
intensities of other
peaks in a region of from about 1500 cm-1 to about 1800 cm-I.
[0007] Another embodiment of the disclosure provides a method for reducing
an amount
of salt deposits on internal components of a fuel injector for a fuel injected
diesel engine. The
method includes operating the diesel engine on a fuel composition that
contains a major amount
of fuel and a minor amount of a reaction product derived from (a) a
hydrocarbyl substituted
dicarboxylic acid, anhydride, or ester and (b) an amine compound or salt
thereof of the formula
NR
II 1
N H2¨ C ¨N H¨NHR
wherein R is selected from the group consisting of hydrogen and a hydrocarbyl
group containing
from about 1 to about 15 carbon atoms, and RI is selected from the group
consisting of hydrogen
and a hydrocarbyl group containing from about 1 to about 20 carbon atoms. The
reaction
2
CA 02810258 2013-03-25
product contains less than one equivalent of an amino triazole group per
molecule of reaction
product.
[0008] A further embodiment of the disclosure provides a method for
preventing
plugging of a fuel filter for fuel injectors of a fuel injected diesel engine.
The method includes
providing a major amount of fuel and a minor amount of a reaction product
derived from (a) a
hydrocarbyl substituted dicarboxylic acid, anhydride, or ester and (b) an
amine compound or salt
thereof of the formula
NR
II 1
N F12¨ C ¨N H ¨N HR
wherein R is selected from the group consisting of hydrogen and a hydrocarbyl
group containing
from about 1 to about 15 carbon atoms, and R1 is selected from the group
consisting of hydrogen
and a hydrocarbyl group containing from about 1 to about 20 carbon atoms. The
reaction
product is characterized by an FTIR spectrum having a peak intensity in a
region of from about
1630 cm-1 to about 1645 cm-1 that ranges from about 5 to about 45% of peak
intensities of other
peaks in a region of from about 1500 cm-1 to about 1800 cm-1, and wherein the
reaction product
contains less than one equivalent of an amino triazole group per molecule of
reaction product.
[0009] An advantage of the fuel additive described herein is that the
additive may not
only reduce the amount of internal deposits forming on direct and/or indirect
diesel fuel
injectors, but the additive may also be effective to clean up dirty fuel
injectors and may prevent
the plugging of fuel filters in the fuel supply to the fuel injectors.
[00010] Additional embodiments and advantages of the disclosure may be set
forth in part
in the detailed description which follows, and/or may be learned by practice
of the disclosure. It
is to be understood that both the foregoing general description and the
following detailed
description are exemplary and explanatory only and are not restrictive of the
disclosure, as
claimed.
BRIEF DESCRIPTION OF THE DRAWINGS:
[00011] FIG. 1 is a portion of an FTIR spectrum of a prior art product.
[00012] FIG. 2 is a portion of an FTIR spectrum of a reaction product
according to the
disclosure.
3
CA 02810258 2013-03-25
[00013] FIG. 3 is a graphical representation of exhaust gas cylinder
temperatures over
time for a four cylinder diesel engine at the beginning of a test for fuel
additives.
[00014] FIG. 4 is a graphical representation of exhaust gas cylinder
temperatures over
time for a four cylinder diesel engine after eight hours of testing using no
fuel detergent.
[00015] FIGs. 5 and 6 are graphical representations of exhaust gas cylinder
temperatures
over time for a four cylinder diesel engine using conventional fuel
detergents.
[00016] FIG. 7 is graphical representations of exhaust gas cylinder
temperatures over
time for a four cylinder diesel engine using a fuel detergent according to an
embodiment of the
disclosure.
[00017] FIG. 8 is a graphical representation of exhaust gas cylinder
temperatures over
time for a four cylinder diesel engine at the end of a dirty up test cycle.
[00018] FIG. 9 is a graphical representation of exhaust gas cylinder
temperatures over
time for a four cylinder diesel engine using a fuel detergent according to an
embodiment of the
disclosure to clean-up dirty fuel injectors of FIG. 6.
DETAILED DESCRIPTION OF EXEMPLARY EMBODIMENTS
[00019] The compositions of the present application may be used in a minor
amount in a
major amount of diesel fuel and may be made by reacting an amine compound or
salt thereof of
the formula
NR
II 1
N H2¨ C ¨N H ¨N HR
wherein R is selected from the group consisting of hydrogen and a hydrocarbyl
group containing
from about 1 to about 15 carbon atoms, and R1 is selected from the group
consisting of hydrogen
and a hydrocarbyl group containing from about 1 to about 20 carbon atoms with
a hydrocarbyl
substituted dicarboxylic acid, anhydride, or ester, wherein the reaction
product contains less than
one equivalent of amino triazole group per molecule of reaction product. The
reaction product is
characterized by an FTIR spectrum having a peak intensity in a region of from
about 1630 cm-1
to about 1645 cm-1 that ranges from about 5 to about 45% of peak intensities
of other peaks in a
region of from about 1500 cm-1 to about 1800 cm-1.
4
CA 02810258 2013-03-25
[00020] For comparison purposes, FIG. 1 shows an FTIR spectrum of a
compound made
with from about mole ratio of hydrocarbyl carbonyl to amine ranging from about
1:1 to about
1:2.5. The peak at about 1636 cm-1 is believed to be an aminotriazole peak. By
comparison, the
reaction product made according to the disclosed embodiments has an FTIR
spectrum as shown
in FIG. 2, wherein the peak intensity at about 1636 cm-1 is substantially
smaller than the peak
intensity of other peaks in a region of from about 1500 cm-1 to about 1800 cm-
1. For example,
the reaction product according to the disclosure has a peak intensity in the
region of froml 630
cm-1 to about 1645 cm-1 that ranges from about 5 to about 45% of peak
intensities of other peaks
in a region of from about 1500 cm-1 to about 1800 cm-1. In other embodiments,
the reaction
product has a characteristic peak intensity in the range of from1630 cm-1 to
about 1645 cm-1 that
is no more than 30 %, for example no more than 25 %, and typically no more
than 10 % of the
intensity of other peaks in the range of from about 1500 cm-1 to about 1800 cm-
1.
[00021] As used herein, the term "hydrocarbyl group" or "hydrocarbyl" is
used in its
ordinary sense, which is well-known to those skilled in the art. Specifically,
it refers to a group
having a carbon atom directly attached to the remainder of a molecule and
having a
predominantly hydrocarbon character. Examples of hydrocarbyl groups include:
(1) hydrocarbon substituents, that is, aliphatic (e.g., alkyl or alkenyl),
alicyclic (e.g.,
cycloalkyl, cycloalkenyl) substituents, and aromatic-, aliphatic-, and
alicyclic-substituted
aromatic substituents, as well as cyclic substituents wherein the ring is
completed through
another portion of the molecule (e.g., two substituents together form an
alicyclic radical);
(2) substituted hydrocarbon substituents, that is, substituents containing non-
hydrocarbon
groups which, in the context of the description herein, do not alter the
predominantly
hydrocarbon substituent (e.g., halo (especially chloro and fluoro), hydroxy,
alkoxy,
mercapto, alkylmercapto, nitro, nitroso, amino, alkylamino, and sulfoxy);
(3) hetero-substituents, that is, substituents which, while having a
predominantly
hydrocarbon character, in the context of this description, contain other than
carbon in a
ring or chain otherwise composed of carbon atoms. Hetero-atoms include sulfur,
oxygen,
nitrogen, and encompass substituents such as pyridyl, furyl, thienyl, and
imidazolyl. In
general, no more than two, or as a further example, no more than one, non-
hydrocarbon
substituent will be present for every ten carbon atoms in the hydrocarbyl
group; in some
embodiments, there will be no non-hydrocarbon substituent in the hydrocarbyl
group.
CA 02810258 2013-03-25
[00022] "Biorenewable fuels" and "biodiesel fuels" as used herein is
understood to mean
any fuel which is derived from resources other than petroleum. Such resources
include, but are
not limited to, corn, maize, soybeans and other crops; grasses, such as
switchgrass, miscanthus,
and hybrid grasses; algae, seaweed, vegetable oils; natural fats; and mixtures
thereof. In an
aspect, the biorenewable fuel may include monohydroxy alcohols, such as those
having from 1 to
about 5 carbon atoms. Non-limiting examples of suitable monohydroxy alcohols
include
methanol, ethanol, propanol, n-butanol, isobutanol, t-butyl alcohol, amyl
alcohol, and isoamyl
alcohol.
[00023] As used herein, the term "major amount" is understood to mean an
amount greater
than or equal to 50 wt. %, for example from about 80 to about 98 wt .%
relative to the total
weight of the composition. Moreover, as used herein, the term "minor amount"
is understood to
mean an amount less than 50 wt. % relative to the total weight of the
composition.
[00024] As used herein, the term "salts or salt deposits" are understood to
mean transition
metal, alkali metal or alkaline earth metal carboxylates.
Amine Compound
[00025] Suitable amine compounds of the formula
NR
II 1
N H2¨ C ¨N H ¨N HR
may be chosen from guanidines and aminoguanidines or salts thereof wherein R
and R1 are as
defined above. Accordingly, the amine compound may be chosen from the
inorganic salts of
guanidines, such as the halide, carbonate, nitrate, phosphate, and
orthophosphate salts of
guanidines. The term "guanidines" refers to guanidine and guanidine
derivatives, such as
aminoguanidine. In one embodiment, the guanidine compound for the preparation
of the
additive is aminoguanidine bicarbonate. Aminoguanidine bicarbonates are
readily obtainable
from commercial sources, or can be prepared in a well-known marmer.
Hydrocarbyl Carbonyl Compound
[00026] The hydrocarbyl carbonyl reactant compound of the additive may be
any suitable
compound having a hydrocarbyl moiety and a carbonyl moiety, and that is
capable of bonding
6
CA 02810258 2013-03-25
with the amine compound to form the additives of the disclosure. Non-limiting
examples of
suitable hydrocarbyl carbonyl compounds include, but are not limited to,
hydrocarbyl substituted
succinic anhydrides, hydrocarbyl substituted succinic acids, and esters of
hydrocarbyl substituted
succinic acids.
[00027] In some aspects, the hydrocarbyl carbonyl compound may be a
polyalkylene
succinic anhydride reactant having the following formula:
0
2
0
0
wherein R2 is a hydrocarbyl moiety, such as for example, a polyalkenyl radical
having a number
average molecular weight of from about 100 to about 5,000 daltons. For
example, the number
average molecular weight of R2 may range from about 200 to about 3,000
daltons, as measured
by GPC. Unless indicated otherwise, molecular weights in the present
specification are number
average molecular weights.
[00028] In the above formula, the R2 hydrocarbyl moiety may comprise one or
more
polymer units chosen from linear or branched alkenyl units. In some aspects,
the alkenyl units
may have from about 2 to about 10 carbon atoms. For example, the polyalkenyl
radical may
comprise one or more linear or branched polymer units chosen from ethylene
radicals, propylene
radicals, butylene radicals, pentene radicals, hexene radicals, octene
radicals and decene radicals.
In some aspects, the R2 polyalkenyl radical may be in the form of, for
example, a homopolymer,
copolymer or terpolymer. In one aspect, the polyalkenyl radical is
isobutylene. For example, the
polyalkenyl radical may be a homopolymer of polyisobutylene comprising from
about 10 to
about 60 isobutylene groups, such as from about 20 to about 30 isobutylene
groups. The
polyalkenyl compounds used to form the R2 polyalkenyl radicals may be formed
by any suitable
methods, such as by conventional catalytic oligomerization of alkenes.
[00029] In an additional aspect, the hydrocarbyl moiety R2 may be derived
from a linear
alpha olefin or an acid-isomerized alpha olefin made by the oligomerization of
ethylene by
methods well known in the art. These hydrocarbyl moieties can range from about
8 carbon
7
CA 02810258 2015-07-30
atoms to over 40 carbon atoms. For example, alkenyl moieties of this type may
be derived from
a linear C18 or a mixture of C20_24 alpha olefins or from acid-isomerized CI6
alpha olefins.
[00030] In some aspects, high reactivity polyisobutenes having relatively
high proportions
of polymer molecules with a terminal vinylidene group may be used to form the
R2 group. In
one example, at least about 60%, such as about 70% to about 90%, of the
polyisobutenes
comprise terminal olefinic double bonds. There is a general trend in the
industry to convert to
high reactivity polyisobutenes, and well known high reactivity polyisobutenes
are disclosed, for
example, in U.S. Pat. No. 4,152,499.
[00031] Specific examples of hydrocarbyl carbonyl compounds include such
compounds
as dodecenylsuccinic anhydrides, C16-18 alkenyl succinic anhydride, and
polyisobutenyl succinic
anhydride (PIBSA), and acid and ester compounds derived therefrom. In some
embodiments,
the PIBSA may have a polyisobutylene portion with a vinylidene content ranging
from about 4%
to greater than about 90%. In some embodiments, the molar ratio of the number
of carbonyl
groups to the number of hydrocarbyl moieties in the hydrocarbyl carbonyl
compound may range
from about 0.5:1 to about 5:1.
[00032] In some aspects, approximately one mole of maleic anhydride may be
reacted per
mole of polyalkylene, such that the resulting polyalkenyl succinic anhydride
has about 0.8 to
about 1 succinic anhydride group per polyalkylene substituent. In other
aspects, the molar ratio
of succinic anhydride groups to alkylene groups may range from about 0.5 to
about 3.5, such as
from about 1 to about 1.1.
[00033] The hydrocarbyl carbonyl compounds may be made using any suitable
method.
Methods for forming hydrocarbyl carbonyl compounds are well known in the art.
One example
of a known method for forming a hydrocarbyl carbonyl compound comprises
blending a
polyolefin and maleic anhydride. The polyolefin and maleic anhydride reactants
are heated to
temperatures of, for example, about 150 C. to about 250 C., optionally, with
the use of a
catalyst, such as chlorine or peroxide. Another exemplary method of making the
polyalkylene
succinic anhydrides is described in U.S. Pat. No. 4,234,435
[00034] The hydrocarbyl carbonyl and amine compounds described above may
be mixed
together under suitable conditions to provide the desired reaction product of
the present
8
CA 02810258 2015-07-30
disclosure. In one aspect of the present disclosure, the reactant compounds
may be mixed
together in a mole ratio of hydrocarbyl carbonyl compound to amine ranging
from about 1:0.5 to
about 1:1.5. For example, the mole ratio of the reactants may range from about
1:0.5 to about
1:0.95.
[000351 Suitable reaction temperatures may range from about 130 C. to
less than about
200 C. at atmospheric pressure. For example, reaction temperatures may range
from about 140
C. to about 160 C. Any suitable reaction pressures may be used, such as,
including
subatmospheric pressures or superatmospheric pressures. However, the range of
temperatures
may be different from those listed where the reaction is carried out at other
than atmospheric
pressure. The reaction may be carried out for a period of time within the
range of about 1 hour
to about 8 hours, preferably, within the range of about 2 hours to about 6
hours.
[00036] In some aspects of the present application, the dispersant
products of this
application may be used in combination with a diesel fuel soluble carrier.
Such carriers may be
of various types, such as liquids or solids, e.g., waxes. Examples of liquid
carriers include, but
are not limited to, mineral oil and oxygenates, such as liquid polyalkoxylated
ethers (also known
as polyalkylene glycols or polyalkylene ethers), liquid polyalkoxylated
phenols, liquid
polyalkoxylated esters, liquid polyalkoxylated amines, and mixtures thereof
Examples of the
oxygenate carriers may be found in U.S. Pat. No. 5,752,989, issued May 19,
1998 to Henly et. al.
Additional examples of oxygenate carriers include alkyl-substituted aryl
polyalkoxylates
described in U.S. Patent Publication No. 2003/0131527, published Jul. 17, 2003
to Colucci et. al.
[00037] In other aspects, compositions of the present application may not
contain a carrier.
For example, some compositions of the present application may not contain
mineral oil or
oxygenates, such as those oxygenates described above.
[00038] One or more additional optional compounds may be present in the
fuel
compositions of the disclosed embodiments. For example, the fuels may contain
conventional
quantities of cetane improvers, corrosion inhibitors, cold flow improvers
(CFPP additive), pour
point depressants, detergents, solvents, demulsifiers, lubricity additives,
friction modifiers, amine
stabilizers, combustion improvers, dispersants, antioxidants, heat
stabilizers, conductivity
improvers, metal deactivators, marker dyes, organic nitrate ignition
accelerators, cyclomatic
9
CA 02810258 2015-07-30
manganese tricarbonyl compounds, and the like. In some aspects, the
compositions described
herein may contain about 10 weight percent or less, or in other aspects, about
5 weight percent or
less, based on the total weight of the additive concentrate, of one or more of
the above additives.
Similarly, the fuels may contain suitable amounts of conventional fuel
blending components
such as methanol, ethanol, dialkyl ethers, and the like.
1000391 In some aspects of the disclosed embodiments, organic nitrate
ignition
accelerators that include aliphatic or cycloaliphatic nitrates in which the
aliphatic or
cycloaliphatic group is saturated, and that contain up to about 12 carbons may
be used.
Examples of organic nitrate ignition accelerators that may be used are methyl
nitrate, ethyl
nitrate, propyl nitrate, isopropyl nitrate, allyl nitrate, butyl nitrate,
isobutyl nitrate, sec-butyl
nitrate, tert-butyl nitrate, amyl nitrate, isoamyl nitrate, 2-amyl nitrate, 3-
amyl nitrate, hexyl
nitrate, heptyl nitrate, 2-heptyl nitrate, octyl nitrate, isooctyl nitrate, 2-
ethylhexyl nitrate, nonyl
nitrate, decyl nitrate, undecyl nitrate, dodecyl nitrate, cyclopentyl nitrate,
cyclohexyl nitrate,
methylcyclohexyl nitrate, cyclododecyl nitrate, 2-ethoxyethyl nitrate, 2-(2-
ethoxyethoxy)ethyl
nitrate, tetrahydrofuranyl nitrate, and the like. Mixtures of such materials
may also be used.
[00040] Examples of suitable optional metal deactivators useful in the
compositions of the
present application are disclosed in U.S. Pat. No. 4,482,357, issued Nov. 13,
1984. Such metal
deactivators include, for example, salicylidene-o-aminophenol, disalicylidene
ethylenediamine,
disalicylidene propylenediamine, and N,N'-disalicylidene-1,2-diaminopropane.
[00041] Suitable optional cyclomatic manganese tricarbonyl compounds which
may be
employed in the compositions of the present application include, for example,
cyclopentadienyl
manganese tricarbonyl, methylcyclopentadienyl manganese tricarbonyl, indenyl
manganese
tricarbonyl, and ethylcyclopentadienyl manganese tricarbonyl. Yet other
examples of suitable
cyclomatic manganese tricarbonyl compounds are disclosed in U.S. Pat. No.
5,575,823, issued
Nov. 19, 1996, and U.S. Pat. No. 3,015,668, issued Jan. 2, 1962.
[00042] When formulating the fuel compositions of this application, the
additives may be
employed in amounts sufficient to reduce or inhibit deposit formation in a
diesel engine. In
some aspects, the fuels may contain minor amounts of the above described
reaction product that
controls or reduces the formation of engine deposits, for example injector
deposits in diesel
CA 02810258 2013-03-25
engines. For example, the diesel fuels of this application may contain, on an
active ingredient
basis, an amount of the reaction product in the range of about 5 mg to about
500 mg of reaction
product per Kg of fuel, such as in the range of about 20 mg to about 120 mg of
reaction product
per Kg of fuel. In aspects, where a carrier is employed, the fuel compositions
may contain, on an
active ingredients basis, an amount of the carrier in the range of about 1 mg
to about 100 mg of
carrier per Kg of fuel, such as about 5 mg to about 50 mg of carrier per Kg of
fuel. The active
ingredient basis excludes the weight of (i) unreacted components such as
polyalkylene
compounds associated with and remaining in the product as produced and used,
and (ii)
solvent(s), if any, used in the manufacture of the reaction product either
during or after its
formation but before addition of a carrier, if a carrier is employed.
1000431 The additives of the present application, including the reaction
product described
above, and optional additives used in formulating the fuels of this invention
may be blended into
the base diesel fuel individually or in various sub-combinations. In some
embodiments, the
additive components of the present application may be blended into the diesel
fuel concurrently
using an additive concentrate, as this takes advantage of the mutual
compatibility and
convenience afforded by the combination of ingredients when in the form of an
additive
concentrate. Also, use of a concentrate may reduce blending time and lessen
the possibility of
blending errors.
[00044] The diesel fuels of the present application may be applicable to
the operation of
both stationary diesel engines (e.g., engines used in electrical power
generation installations, in
pumping stations, etc.) and ambulatory diesel engines (e.g., engines used as
prime movers in
automobiles, trucks, road-grading equipment, military vehicles, etc.). For
example, the fuels
may include any and all middle distillate fuels, diesel fuels, biorenewable
fuels, biodiesel fuel,
gas-to-liquid (GTL) fuels, jet fuel, alcohols, ethers, kerosene, low sulfur
fuels, synthetic fuels,
such as Fischer-Tropsch fuels, liquid petroleum gas, bunker oils, coal to
liquid (CTL) fuels,
biomass to liquid (BTL) fuels, high asphaltene fuels, fuels derived from coal
(natural, cleaned,
and petcoke), genetically engineered biofuels and crops and extracts
therefrom, and natural gas.
"Biorenewable fuels" as used herein is understood to mean any fuel which is
derived from
resources other than petroleum. Such resources include, but are not limited
to, corn, maize,
soybeans and other crops; grasses, such as switchgrass, miscanthus, and hybrid
grasses; algae,
seaweed, vegetable oils; natural fats; and mixtures thereof. In an aspect, the
biorenewable fuel
11
CA 02810258 2013-03-25
can comprise monohydroxy alcohols, such as those comprising from 1 to about 5
carbon atoms.
Non-limiting examples of suitable monohydroxy alcohols include methanol,
ethanol, propanol,
n-butanol, isobutanol, t-butyl alcohol, amyl alcohol, and isoamyl alcohol.
[00045] Accordingly, aspects of the present application are directed to
methods for
reducing the amount of injector deposits of a diesel engine having at least
one combustion
chamber and one or more direct fuel injectors in fluid connection with the
combustion chamber.
In another aspect, the improvements may also be observed in indirect diesel
fuel injectors. In
some aspects, the methods comprise injecting a hydrocarbon-based compression
ignition fuel
comprising the reaction product additive of the present disclosure, through
the injectors of the
diesel engine into the combustion chamber, and igniting the compression
ignition fuel. In some
aspects, the method may also comprise mixing into the diesel fuel at least one
of the optional
additional ingredients described above.
[00046] In one embodiment, the diesel fuels of the present application may
be essentially
free, such as devoid, of conventional succinimide dispersant compounds. The
term "essentially
free" is defined for purposes of this application to be concentrations having
substantially no
measurable effect on injector cleanliness or deposit formation.
[00047] In yet other aspects of the present application, the fuel additive
may be free or
substantially free of 1,2,4-triazoles. For example, the compositions may be
substantially free of
triazoles of formula II,
R4
NR
H2C
\N
N
wherein R4 and R5 are independently chosen from hydrogen and hydrocarbyl
groups, with the
proviso that at least one of R4 and R5 is not hydrogen. Examples of
hydrocarbyl groups include
C2 to C50 linear, branched or cyclic alkyl groups; C2 to C50 linear, branched
or cyclic alkenyl
groups; and substituted or unsubstituted aryl groups, such as phenyl groups,
tolyl groups and
xylyl groups.
12
CA 02810258 2013-03-25
EXAMPLES
[00048] The following examples are illustrative of exemplary embodiments
of the
disclosure. In these examples as well as elsewhere in this application, all
parts and percentages
are by weight unless otherwise indicated. It is intended that these examples
are being presented
for the purpose of illustration only and are not intended to limit the scope
of the invention
disclosed herein.
[00049] In the following examples, the effect the detergent additive had
on diesel fuel
contaminated with carboxylate salts for high pressure common rail diesel fuel
systems was
evaluated. An engine test was used to demonstrate the propensity of fuels to
provoke fuel
injector sticking and was also used to demonstrate the ability of certain fuel
additives to prevent
or reduce the amount of internal deposit in the injectors. An engine
dynamometer test stand was
used for the installation of the Peugeot DW10 diesel engine for running the
injector sticking
tests. The engine was a 2.0 liter engine having four cylinders. Each
combustion chamber had
four valves and the fuel injectors were DI piezo injectors have a Euro V
classification.
[00050] The core protocol procedure consisted of running the engine
through a cycle for
8-hours and allowing the engine to soak (engine off) for a prescribed amount
of time. The
injector performance was then characterized by measuring the cylinder exhaust
temperature for
each cylinder. A test was stopped and considered to have failed (one or more
injectors sticking)
if the exhaust temperature of any cylinder was more than 65 C. above any other
cylinder exhaust
temperature at any point in time. A test was also considered to have failed if
after allowing the
engine to cool to ambient temperature, a cold start showed a temperature
difference of 45 C. or
more in cylinder exhaust temperatures. Sticking of the needle and thus failure
could also be
confirmed by disassembling the injector and subjectively determining the force
required to
remove the needle from the nozzle housing. Cleanliness tests were run for keep-
clean
performance as well as clean-up performance.
[00051] Test preparation involved flushing the previous test's fuel from
the engine prior to
removing the injectors. The test injectors were inspected, cleaned, and
reinstalled in the engine.
If new injectors were selected, the new injectors were put through a 16-hour
break-in cycle.
Next, the engine was started using the desired test cycle program. Once the
engine was warmed
up, power was measured at 4000 RPM and full load to check for full power
restoration after
cleaning the injectors. If the power measurements were within specification,
the test cycle was
13
CA 02810258 2013-03-25
initiated. The following Table 1 provides a representation of the DW10
sticking test cycle that
was used to evaluate the fuel additives according to the disclosure.
Table 1 - One hour representation of DW10 sticking test cycle.
Step Duration(minutes) Engine speed Load
Torque(Nm) Boost air after
(rpm) (%)
Intercooler ( C)
1 2 1750 20 62 45
2 7 3000 60 173 50
3 2 1750 20 62 45
4 7 3500 _ 80 212 50
2 1750 20 62 45
6 10 4000 100 50
7 2 1250 10 25 43
8 7 3000 100 50
9 2 1250 10 25 43
10 2000 100 50
11 2 1250 10 25 43
12 7 4000 100 50
Example 1 (Injector Sticking Engine Test)
[00052] Diesel engine nozzle sticking tests were conducted using the
Peugeot DW10
engine following the protocol of Table 1. For keep-clean testing, the engine
was run with diesel
fuel doped with metal carboxylate salts and with the detergent additive
indicated in the example.
For clean-up testing, the engine was first run with diesel fuel doped with
metal carboxylate salts
without a detergent additive to establish a baseline of stuck fuel injectors.
Next, the engine was
run with the same fuel containing the detergent additive indicated. In all of
the tests, the fuels
tested contained 200 ppmv lubricity modifier and 1600 ppmv cetane improver, 20
ppmw of
dodecyl succinic acid, 3 ppmw of NaOH, and 25 ppmwv of water. At the beginning
of the test,
no injector sticking was indicated by a uniform exhaust gas temperature for
all 4-cylinders as
shown in FIG. 3. However, a cold start of the engine after 8 hours showed
injector sticking as
shown in FIG. 4. In all of the figures, curve A is cylinder 1, curve B is
cylinder 2, curve C is
cylinder 3 and curve D is cylinder 4.
Comparative Example 2
[00053] In this example, a conventional succinimide dispersant additive was
added to the
fuel at a treat rate of 75 ppmw. FIG. 5 shows the injectors sticking after a
16 hour test with the
fuel containing the conventional detergent.
14
CA 02810258 2013-03-25
Comparative Example 3
[00054] In this example a quaternary ammonium salt Diesel fuel additive
package was
added to the fuel at a treat rate of 75 ppmw. FIG. 6 shows the injector
sticking after a 7 hour test
with this fuel.
Example 4
[00055] The detergent additive of the disclosure was added to the fuel at a
treat rate of 75
ppmw. After a 16 hour test, FIG. 7 shows that none of the injectors were
stuck. Physical
inspection of the injectors upon completion of the test confirmed that none of
the injectors were
stuck.
Example 5
[00056] In this test, a base fuel containing the metal salts described
above was run in the
engine for 8 hours to dirty-up the fuel injectors. FIG. 8 shows that after a
cold start of the
engine, the injectors were stuck.
Example 6
[00057] In this test, the ability of the detergent additive of the
disclosure to the clean-up
dirty fuel injectors of FIG. 8 was demonstrated. In this example, 30 ppmw of
the detergent
additive of the disclosure was combined with 120 ppmw of a conventional
succinimide
dispersant and this mixture was added to the fuel. FIG. 9 shows that after a
16 hour test, none of
the injectors were stuck.
[00058] As indicated by the foregoing examples, fuel additives containing
detergent
additive of the disclosure provides a significant reduction in internal
deposits in diesel fuel
injectors when engines are operated on ULSD fuels as compared to conventional
fuel detergent
additives and that the detergent additive was effective for cleaning up dirty
fuel injectors.
[00059] It is noted that, as used in this specification and the appended
claims, the singular
forms "a," "an," and "the," include plural referents unless expressly and
unequivocally limited to
one referent. As used herein, the term "include" and its grammatical variants
are intended to be
non-limiting, such that recitation of items in a list is not to the exclusion
of other like items that
can be substituted or added to the listed items
[00060] For the purposes of this specification and appended claims, unless
otherwise
indicated, all numbers expressing quantities, percentages or proportions, and
other numerical
values used in the specification and claims, are to be understood as being
modified in all
CA 02810258 2015-07-30
instances by the term "about." Accordingly, unless indicated to the contrary,
the numerical
parameters set forth in the following specification and attached claims are
approximations that
can vary depending upon the desired properties sought to be obtained by the
present disclosure.
At the very least, and not as an attempt to limit the application of the
doctrine of equivalents to
the scope of the claims, each numerical parameter should at least be construed
in light of the
number of reported significant digits and by applying ordinary rounding
techniques.
1000611
While particular embodiments have been described, alternatives, modifications,
variations, improvements, and substantial equivalents that are or can be
presently unforeseen can
arise to applicants or others skilled in the art. The scope of the claims
should not be limited by
the preferred embodiments set forth in the examples, but should be given the
broadest
interpretation consistent with the description as a whole.
16