Note: Descriptions are shown in the official language in which they were submitted.
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
OSMOTICALLY DRIVEN MEMBRANE PROCESSES AND SYSTEMS AND
METHODS FOR DRAW SOLUTE RECOVERY
FIELD OF THE TECHNOLOGY
[0001] Generally, the invention relates to osmotically driven membrane
processes and
more particularly to draw solute recovery techniques for osmotically driven
membrane
processes.
BACKGROUND
[0002] In general, osmotically driven membrane processes involve two
solutions
separated by a semi-permeable membrane. One solution may be, for example,
seawater, while
the other solution is a concentrated solution that generates a concentration
gradient between the
seawater and the concentrated solution. This gradient draws water from the
seawater across the
membrane, which selectively permits water to pass, but not salts, into the
concentrated solution.
Gradually, the water entering the concentrated solution dilutes the solution.
The solutes then
need to be removed from the dilute solution to generate potable water.
Traditionally, the potable
water was obtained, for example, via distillation; however, the solutes were
typically not
recovered and recycled.
SUMMARY
[0003] The invention generally relates to osmotically driven membrane
systems and
methods, for example, forward osmosis (FO), pressure retarded osmosis (PRO),
osmotic dilution
(OD), direct osmotic concentration (DOC), and the like, and to systems and
methods for draw
solute recovery in the osmotically driven membrane systems/processes.
[0004] In one aspect, the invention relates to an osmotically driven
membrane process in
the manner of a forward osmosis separation process. The process includes the
steps of
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
introducing a first solution on a first side of a semi-permeable membrane,
detecting at least one
characteristic of the first solution, selecting a molar ratio for a
concentrated draw solution
comprising ammonia and carbon dioxide based on the at least one detected
characteristic,
introducing the concentrated draw solution comprising ammonia and carbon
dioxide at the
selected molar ratio on a second side of the semi-permeable membrane to
maintain a desired
osmotic concentration gradient across the semi-permeable membrane, promoting
flow of at least
a portion of the first solution across the semi-permeable membrane to form a
second solution on
the first side of the semi-permeable membrane and a dilute draw solution on
the second side of
the semi-permeable membrane, introducing at least a portion of the dilute draw
solution to a
separation operation to recover draw solutes and a solvent stream,
reintroducing the draw solutes
to the second side of the semi-permeable membrane to maintain the selected
molar ratio of
ammonia to carbon dioxide in the concentrated draw solution, and collecting
the solvent stream.
[0005] In various embodiments, the separation operation includes using an
absorber
configured to condense the draw solutes into the concentrated draw solution.
The solvent
stream, dilute draw solution, or concentrated draw solution may be used as an
absorbent in the
absorber. Cooling may be used with the absorber. In some embodiments, the
process may
further include the step of compressing a gas stream resulting from separation
of the draw solutes
from the dilute draw solution using a gas compressor or a steam eductor driven
by hydraulic
pressure on an absorbing liquid stream to promote reabsorption of draw solutes
into the
concentrated draw solution. The process may further include the step of
applying pressure on the
first solution to enhance or cause flux through the semi-permeable membrane
into the
concentrated draw solution. The process may further include the step of
selecting a concentrated
draw solution having a draw solute characterized by an ability to have its
removal from solution
2
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
or introduction into solution enhanced by the use of a catalyst, characterized
by an ability to have
its removal from solution or introduction into solution enhanced by a reusable
physical or
chemical agent, or characterized by an ability to have its removal from
solution or introduction
into solution enhanced by an electric energy field, magnetic energy field or
other change of
environment such that susceptibility of the draw solute to separation is
increased to enhance
draw solute removal and reuse.
[0006] In additional embodiments, the process may further include the step
of detecting a
volumetric change with respect to at least one of the first solution and the
concentration draw
solution, and modifying a flow channel relating to the semi-permeable membrane
in response to
the detected change to maintain a desired flow characteristic. In other
embodiments, the process
may further include the step of enhancing draw solute removal or absorption
using a catalyst,
reagent, consumable, reusable material, electric energy field or magnetic
energy field. In still
other embodiments, the process may further include the step reducing process
energy by using at
least one of mechanical vapor recompression, thermal vapor recompression,
vacuum distillation,
sweep gas distillation, pervaporation and/or a closed cycle heat pump. The
process may further
include the step of using carbon dioxide to precipitate the draw solutes and
using ammonia to
reabsorb precipitate for pressure retarded osmosis. The process may further
include the step of
introducing a seeded slurry to the first solution. In at least one embodiment,
the process may
further include the step of using a super-saturation of salts in the first
solution during a first
operation and desaturation of salts during a second operation before return to
the first operation
for resaturation.
[0007] In another aspect, the invention relates to a system for osmotic
extraction of a
solvent from a first solution. The system includes a first chamber having an
inlet fluidly
3
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
connected to a source of the first solution, a second chamber having an inlet
fluidly connected to
a source of a concentrated draw solution comprising ammonia and carbon dioxide
in a molar
ratio of at least 1 to 1, a semi-permeable membrane system separating the
first chamber from the
second chamber, a separation system fluidly connected downstream of the second
chamber and
configured to receive a dilute draw solution from the second chamber and to
recover draw
solutes and a solvent stream, and a recycling system having an absorber
configured to facilitate
reintroduction of the draw solutes to the second chamber to maintain the molar
ratio of ammonia
to carbon dioxide in the concentrated draw solution. In one embodiment, the
separation system
includes a distillation column.
[0008] In some embodiments, the absorber may include a packed column. In
at least one
embodiment, the absorber includes a membrane contactor. The membrane contactor
may be
constructed and arranged to facilitate parallel flow of a cooled absorbent and
series flow of draw
solute gases in the membrane contactor. In some embodiments, the distillation
column may
include a membrane distillation apparatus. In some embodiments, the recycling
system may
further include a compression operation downstream of the absorber to enhance
condensation of
draw solute gases. In at least one embodiment, the compression operation
includes a gas
compressor, a steam eductor, or a liquid stream eductor. The separation
operation may further
include a carbon sequestration loop to absorb and desorb draw solutes, the
carbon sequestration
loop configured to complete absorption of draw solute gases and increase their
pressure to
promote their absorption into the concentrated draw solution.
[0009] In some embodiments, the semi-permeable membrane system may include
a
membrane module immersed in the first solution, where the concentrated draw
solution flows
through an interior of the membrane module. In at least one embodiment, the
semi-permeable
4
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
membrane system has a first flow channel associated with the first solution
from which solvent is
extracted, the first flow channel having a tapering geometry or a
configuration of flow spacers
such that an effective volume of the first flow channel decreases along a
length of the first flow
channel, and a second flow channel associated with the concentrated draw
solution into which
solvent is extracted from the first solution, the second flow channel having
an expanding
geometry or a configuration of flow spacers such that an effective volume of
the second flow
channel increases along a length of the second flow channel. In some
embodiments, the semi-
permeable membrane system includes a pyramidal membrane module array to
accommodate a
decrease in a volume of the first solution and an increase in a volume of the
concentrated draw
solution, the pyramidal membrane module array configured such that in a
counter flow
arrangement there are fewer membrane modules in a direction of a draw solution
inlet and a feed
solution outlet, and more membrane modules in a direction of a feed solution
inlet and a draw
solution outlet.
[0010] Furthermore, the system may include a downstream zero liquid
discharge or other
reduced discharge stream operation having an inlet configured to receive the
concentrated
solution. The system may further include a vacuum or air scouring/stripping
system configured
to assist in stripping draw solutes. The system may further include a
pervaporation system
configured to strip draw solutes. In some embodiments, the pervaporation
system may have a
membrane that is selective for draw solute gases relative to water vapor. The
system may further
include a membrane distillation system to strip draw solutes. In at least some
embodiments,
membranes may be used for both separation of draw solutes and heat exchange in
a module. In
some embodiments, the recovered draw solutes can be delivered to one or more
additional
downstream operations.
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
[00111 In another aspect, the invention relates to a method for osmotic
generation of
power. The method may include the steps of performing a membrane separation
operation to
form a first solution, precipitating draw solutes from the first solution,
separating precipitated
draw solutes to form a second solution, promoting production of gases from the
second solution,
separating the gases with a technique such as kinetic based differential
absorption, and using at
least one separated gas to redissolve precipitated solutes to form a third
solution for reuse in the
membrane separation step.
[0012] In another aspect of the invention, a forward osmosis separation
process may
include introducing a first solution on a first side of a semi-permeable
membrane, introducing a
concentrated draw solution on a second side of the semi-permeable membrane to
maintain a
desired osmotic concentration gradient across the semi-permeable membrane,
promoting flow of
at least a portion of the first solution across the semi-permeable membrane to
form a second
solution on the first side of the semi-permeable membrane and a dilute draw
solution on the
second side of the semi-permeable membrane, introducing at least a portion of
the dilute draw
solution to a separation operation to recover draw solutes and a solvent
stream, reintroducing the
draw solutes to the second side of the semi-permeable membrane to maintain a
desired molar
ratio in the concentrated draw solution, and collecting the solvent stream. In
one or more
embodiments, the recovered draw solutes from the separation operation are
brought into contact
with an absorbing solution.
[0013] In another aspect of the invention, an apparatus for osmotic
extraction of a solvent
from a first solution may include a first chamber having an inlet fluidly
connected to a source of
a first solution, a second chamber having an inlet fluidly connected to a
source of a concentrated
draw solution, a semi-permeable membrane separating the first chamber from the
second
6
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
chamber, and a separation system fluidly connected downstream of the second
chamber and
configured to receive a dilute draw solution from the second chamber and to
recover draw
solutes and a solvent stream. The apparatus may also include a recovery system
that includes an
absorber configured to facilitate reintroduction of the draw solutes to the
second chamber to
maintain a desired concentration of solutes in the concentrated draw solution.
[0014] In various embodiments, at least one of the separation system and
the recovery
system may include a membrane device. The membrane device may be constructed
and
arranged to bring recovered draw solute(s) into contact with an absorbing
solution. The
membrane device may be further constructed and arranged to strip draw solutes
from the dilute
draw solution. In some embodiments, at least one of the separation and
recovery systems may
include a suspended liquid membrane. The separation operation may include a
multi-stage
solute recovery operation using, for example, column or membrane distillation.
In the case of
multi-stage solute recovery, the system(s) may be constructed and arranged
such that material
and energy streams both flow in series. In at least one embodiment, the multi-
stage solute
recovery operation may include at least one heat pump.
[0015] In another aspect, a method for separating solute and product
solvent from a draw
solution using a plurality of distillation columns may include introducing
draw solution to each
of at least a first distillation column and at least a second distillation
column, applying thermal
energy from a source of thermal energy to the first distillation column to
vaporize at least a
portion of the draw solution in the first distillation column, directing the
vaporized portion of the
draw solution from the first distillation column to the second distillation
column as an energy
stream such that the vaporized portion of the draw solution from the first
distillation column acts
as a source of thermal energy for the second distillation column to vaporize
at least a portion of
7
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
the draw solution in the second distillation column, and flowing both the draw
solution and
energy streams in series within at least the first and second distillation
columns, whereby draw
solution solutes and product solvent contained in the draw solution are
separated in the at least
first and second distillation columns. In some embodiments, the method may
include assisting at
least one of the first and second distillation columns with a heat pump.
[0016] In another aspect, the invention relates to an apparatus (and
related method) for
recovering draw solution solutes from a dilute draw solution. The apparatus
includes an
osmotically driven membrane system having a source of dilute draw solution
that includes
thermally removable solutes and a separation system in fluid communication
with the
osmotically driven membrane system. The separation system includes at least
one membrane
contactor having a first side and a second side, wherein the first side is in
fluid communication
with the source of dilute draw solution. In one or more embodiments, the
separation system
further includes a source of thermal energy in communication with the dilute
draw solution for
vaporizing at least a portion of draw solutes out of the dilute draw solution
and a source of
absorbing solution in fluid communication with the second side of the membrane
contactor. At
least a portion of the vaporized draw solutes pass from the first side to the
second side of the
membrane contactor and are absorbed by the absorbing solution.
[0017] In various embodiments, the absorbing solution is also the source
of thermal
energy, for example, in the form of steam. The separation system can also
include a condenser
in communication with the absorbing solution as it exits the at least one
membrane contactor, a
heat pump in communication with the membrane contactor, and/or a reboiler in
communication
with the heat pump and a source of water discharged by the membrane contactor.
In one or more
embodiments, the membrane contactor is a selective membrane that substantially
inhibits the
8
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
transfer of water vapor, but facilitates the transfer of vaporized draw
solutes. In one
embodiment, the selective membrane can be a suspended liquid membrane.
[0018] In another aspect, the invention relates to an apparatus for
recovering draw
solution solutes from an osmotically driven membrane system. The apparatus
includes a
membrane module configured for receiving a dilute draw solution from the
osmotically driven
membrane system and a heat pump module in fluid communication with the
membrane module
for providing (or assisting) a source of thermal energy to the membrane
module. The membrane
module can include at least one membrane system, which can be disposed in a
housing. In one
or more embodiments, the at least one membrane system includes at least one
membrane system
for stripping solutes out of the dilute draw solution (i.e., a stripping
membrane) and at least one
membrane system for bringing draw solution solutes into contact with an
absorbing solution (i.e.,
an absorbing membrane). In one embodiment, the membrane system is a multi-
stage solute
recovery system, where multiple membrane modules are utilized with material
and energy flows
either in series or in parallel to suit a particular application. The membrane
system(s) can
include a selective membrane such as, for example, a suspended liquid
membrane. In one or
more embodiments, the heat pump module includes a heat pump in fluid
communication with a
source of vaporized draw solutes discharged by the membrane module and a
reboiler in fluid
communication with a source of water discharged by the membrane module.
[0019] In another aspect, the invention relates to an apparatus for
recovering draw
solution solutes from an osmotically driven membrane system that uses multi-
stage solute
recovery with multiple distillation columns and/or membrane modules. In one
embodiment, the
apparatus includes a first distillation column (or membrane module), a heat
pump, and a second
distillation column (or membrane module). The first distillation column
includes a first inlet
9
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335
PCT/US2011/052565
coupled to a first source of dilute draw solution (e.g., the osmotically
driven membrane system)
for introducing a portion of dilute draw solution into a first end of the
first distillation column; a
first heat transfer means coupled to the first distillation column at a second
end, where the first
heat transfer means has an inlet coupled to a first source of thermal energy
and an outlet coupled
to the first distillation column for directing thermal energy to the first
distillation column to
cause the dilute draw solution solutes in the first distillation column to
vaporize; a first outlet for
removing the vaporized dilute draw solution solutes from the first
distillation column; and a
second outlet for removing a bottoms product from the first distillation
column. Alternatively,
the first source of thermal energy can be introduced directly to the first
distillation column. The
heat pump is coupled to the first outlet of the first distillation column. The
second distillation
column includes a first inlet coupled to a second source of dilute draw
solution for introducing a
portion of dilute draw solution into a first end of the second distillation
column; a second heat
transfer means coupled to the second distillation column at a second end,
where the second heat
transfer means has an inlet coupled to the heat pump for receiving the
vaporized dilute draw
solution solutes for use as a second source of thermal energy, a first outlet
coupled to the second
distillation column for directing the second source of thermal energy to the
second distillation
column to cause the dilute draw solution solutes in the second distillation
column to vaporize,
and a second outlet configured to return the vaporized dilute draw solution
solutes from the first
distillation column condensed within the second heat transfer means to the
osmotically driven
membrane system; a first outlet for removing the vaporized dilute draw
solution solutes from the
second distillation column; and a second outlet for removing a bottoms product
from the second
distillation column.
[0020] In
various embodiments, the apparatus includes a second heat pump coupled to
1()
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
the first outlet of the second distillation column and at least a third
distillation column. The third
distillation column includes a first inlet coupled to a third source of dilute
draw solution (e.g., the
osmotically driven membrane system) for introducing a portion of dilute draw
solution into a
first end of the third distillation column; a third heat transfer means
coupled to the third
distillation column at a second end, where the third heat transfer means has
an inlet coupled to
the second heat pump for receiving the vaporized dilute draw solution solutes
for use as a third
source of thermal energy for use with the third distillation column, a first
outlet coupled to the
third distillation column for directing the third source of thermal energy to
the third distillation
column to cause the dilute draw solution solutes in the third distillation
chamber to vaporize, and
a second outlet configured to return the vaporized dilute draw solution
solutes from the second
distillation column condensed within the third heat transfer means to the
osmotically driven
membrane system; a first outlet for removing the vaporized draw solution
solutes from the third
distillation column; and a second outlet for removing a bottoms product from
the third
distillation column. In one or more embodiments, the first outlet of the third
distillation column
is configured to return the vaporized dilute draw solution solutes from the
third distillation
column to the osmotically driven membrane system. In one embodiment, the first
and second
distillation columns are configured for parallel operation and the first and
second sources of
dilute draw solution are the osmotically driven membrane system. In another
embodiment, the
first and second distillation columns are configured for series operation and
the second source of
dilute draw solution is the bottoms product of the first distillation column.
[0021] In another aspect, the invention relates to an apparatus for
recovering draw solutes
from an osmotically driven membrane process. The apparatus includes an
osmotically driven
membrane system including a source of dilute draw solution having thermally
removable solutes
11
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
and a separation module in fluid communication with the osmotically driven
membrane system.
The separation module includes at least one of a membrane device or a
distillation apparatus in
fluid communication with the source of dilute draw solution and at least one
heat pump coupled
to the at least one of a membrane device or a distillation apparatus. The heat
pump is configured
to provide a source of thermal energy (or assist an existing source of thermal
energy) to the at
least one of a membrane device or a distillation apparatus to vaporize the
thermally removable
solutes.
[0022] In various embodiments, the membrane device includes at least one
membrane
contactor configured to at least one of bring vaporized draw solutes in
contact with an absorbing
solution or strip draw solutes from the dilute draw solution. In one or more
embodiments, the
membrane contactor is a suspended liquid membrane. In one or more embodiments,
the
distillation apparatus includes a multi-stage solute recovery apparatus, for
example, multi-stage
column and/or membrane distillation apparatus. In one embodiment, the multi-
stage recovery
apparatus is constructed and arranged such that material and energy streams
both flow in series,
for example, through a first distillation column and a second distillation
column.
[0023] In another aspect, the invention relates to a method of recovering
draw solutes
from an osmotically driven membrane system. The method includes the steps of
providing a
source of dilute draw solution from the osmotically driven membrane system,
where the dilute
draw solution comprises thermally removable solutes, introducing at least a
portion of the dilute
draw solution to a separation system, introducing a source of thermal energy
to the separation
system, vaporizing the dilute draw solution solutes out of the dilute draw
solution, recovering the
vaporized dilute draw solution solutes, and recycling the draw solution
solutes from the
separation system to the osmotically driven membrane system.
12
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
[0024] In one or more embodiments, the step of vaporizing the dilute draw
solution
solutes includes exposing the dilute draw solution solutes to the source of
thermal energy via a
membrane contactor to strip the solutes from the dilute draw solution. The
step of recovering the
vaporized draw solution solutes can include exposing the vaporized draw
solution solutes to an
absorbing solution via a membrane contactor. In yet other embodiments, the
step of vaporizing
the dilute draw solution solutes includes exposing the dilute draw solution to
a multi-stage solute
recovery process, for example, multi-stage column distillation. In one
embodiment, the dilute
draw solution and source of thermal energy flow in series through the multi-
stage solute recovery
process, for example, through at least a first distillation column (or
membrane module) and a
second distillation column (or membrane module). Additionally, the step of
vaporizing the
dilute draw solution solutes includes assisting the source of thermal energy
with a heat pump.
[0025] These and other objects, along with advantages and features of the
present
invention herein disclosed, will become apparent through reference to the
following description
and the accompanying drawings. Furthermore, it is to be understood that the
features of the
various embodiments described herein are not mutually exclusive and can exist
in various
combinations and permutations.
BRIEF DESCRIPTION OF THE DRAWINGS
[0026] In the drawings, like reference characters generally refer to the
same parts
throughout the different views. Also, the drawings are not necessarily to
scale, emphasis instead
generally being placed upon illustrating the principles of the invention and
are not intended as a
definition of the limits of the invention. For purposes of clarity, not every
component may be
labeled in every drawing. In the following description, various embodiments of
the present
invention are described with reference to the following drawings, in which:
13
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
[0027] FIG. 1 is a schematic representation of an exemplary osmotically
driven
membrane system/process using a solute recovery system in accordance with one
or more
embodiments of the invention;
[0028] FIG. 2 is a schematic representation of an osmotically driven
membrane
system/process in accordance with one or more embodiments of the invention;
[0029] FIG. 3 is a schematic representation of an alternative osmotically
driven
membrane system/process in accordance with one or more embodiments of the
invention;
[0030] FIG. 4 is a schematic representation of another alternative
osmotically driven
membrane system/process in accordance with one or more embodiments of the
invention;
[0031] FIG. 5 is a schematic representation of another alternative
osmotically driven
membrane system/process in accordance with one or more embodiments of the
invention;
[0032] FIG. 6 is a schematic representation of a portion of a draw solute
recovery system
using a membrane contactor to facilitate absorption of draw solution vapors in
accordance with
one or more embodiments of the invention;
[0033] FIG. 7 is a schematic representation of a portion of a draw solute
recovery system
using a membrane contactor to facilitate stripping of draw solutes in
accordance with one or
more embodiments of the invention;
[0034] FIG. 8 is a schematic representation of a portion of a draw solute
recovery system,
where dilute draw solution stripping and absorbing functions are integrated
into a single module;
[0035] FIG. 9 is a schematic representation of a portion of a draw solute
recovery system
using membrane distillation integrated with a closed cycle heat pump in
accordance with one or
more embodiments of the invention;
[0036] FIG. 10 is a schematic representation of a portion of a draw solute
recovery
14
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
system using membrane distillation integrated with an open cycle heat pump in
accordance with
one or more embodiments of the invention;
[0037] FIG. 11 is a schematic representation of a selective membrane for
use in a draw
solute recovery system in accordance with one or more embodiments of the
invention;
[0038] FIG. 12 is a schematic representation of a portion of a draw solute
recovery
system using a selective membrane for simultaneous stripping and absorption of
draw solutes in
accordance with one or more embodiments of the invention;
[0039] FIG. 13 is a schematic representation of a portion of a draw solute
recovery
system in accordance with one or more embodiments of the invention;
[0040] FIG. 14 is a schematic representation of a portion of a solute
recovery system
using multi-stage solute recovery in accordance with one or more embodiments
of the invention;
[0041] FIG. 15 is a schematic representation of a portion of a solute
recovery system
using heat pump assisted multi-stage solute recovery in accordance with one or
more
embodiments of the invention;
[0042] FIG. 16 is a schematic representation of a portion of a draw solute
recovery
system using column distillation and a heat pump in accordance with one or
more embodiments
of the invention;
[0043] FIG. 17 is a schematic representation of a portion of a draw solute
recovery
system using column distillation and a heat pump in accordance with one or
more embodiments
of the invention; and
[0044] FIG. 18 is a schematic representation of a portion of a draw solute
recovery
system using an eductor in accordance with one or more embodiments of the
invention.
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335
PCT/US2011/052565
DETAILED DESCRIPTION
[0045] Various embodiments of the invention may be used in any osmotically
driven
membrane process, such as FO, PRO, OD, DOC, etc. An osmotically driven
membrane process
for extracting a solvent from solution may generally involve exposing the
solution to a first
surface of a forward osmosis membrane. In some embodiments, the first solution
(known as a
process or feed solution) may be seawater, brackish water, wastewater,
contaminated water, a
process stream, or other aqueous solution. In at least one embodiment, the
solvent is water;
however, other embodiments may use non-aqueous solvents. A second solution
(known as a
draw solution) with an increased concentration of solute(s) relative to that
of the first solution
may be exposed to a second opposed surface of the forward osmosis membrane.
Solvent, for
example water, may then be drawn from the first solution through the forward
osmosis
membrane and into the second solution generating a solvent-enriched solution
via forward
osmosis.
[0046] Forward osmosis generally utilizes fluid transfer properties
involving movement
of solvent from a less concentrated solution to a more concentrated solution.
Osmotic pressure
generally promotes transport of solvent across a forward osmosis membrane from
feed to draw
solutions. The solvent-enriched solution, also referred to as a dilute draw
solution, may be
collected at a first outlet and undergo a further separation process. In some
non-limiting
embodiments, purified water may be produced as a product from the solvent-
enriched solution.
A second product stream, i.e., a depleted or concentrated process solution,
may be collected at a
second outlet for discharge or further treatment. The concentrated process
solution may contain
one or more target compounds which it may be desirable to concentrate or
otherwise isolate for
16
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335
PCT/US2011/052565
downstream use.
[0047] FIG.
1 depicts one exemplary osmotically driven membrane system/process 10
utilizing a draw solute recovery system 22 in accordance with one or more
embodiments of the
invention. As shown in FIG. 1, the system/process 10 includes a forward
osmosis module 12,
such as those described in U.S. Patent Nos. 6,391,205 and 7,560,029; and PCT
Publication Nos.
W02009/155596, and W02011/053794; the disclosures of which are hereby
incorporated by
reference herein in their entireties. The module 12 is in fluid communication
with a feed
solution source or stream 14 and a draw solution source or stream 16. The draw
solution source
16 can include, for example, a saline stream, such as sea water, or another
solution as described
herein that can act as an osmotic agent to dewater the feed source 14 by
osmosis through a
forward osmosis membrane within the module 12. The module 12 outputs a stream
of
concentrated solution 18 from the feed stream 14 that can be further
processed. The module 12
also outputs a dilute draw solution 20 that can be further processed via the
recovery system 22,
as described herein, where draw solutes and a target solvent can be recovered.
In accordance
with one or more embodiments of the invention, the draw solutes are recovered
for reuse.
Various osmotically driven membrane systems/processes are described with
respect to FIGS. 2-
5.
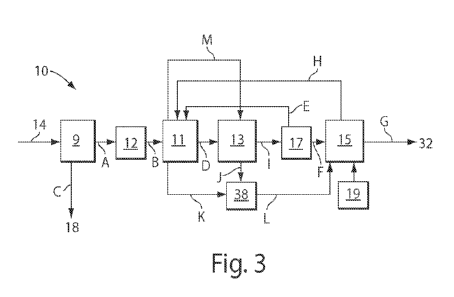
[0048] FIGS.
2 and 3 depict osmotically driven membrane processes utilizing forward
osmosis in accordance with one or more embodiments of the invention. As shown
in FIG. 2, a
solution 14, for example, seawater, brackish water, wastewater, contaminated
water or other
solution, referred to as the first solution, is introduced to or disposed in a
first chamber 9. The
first chamber 9 is in fluid communication with a semi-permeable membrane 12,
as illustrated by
arrow A. A second solution having a concentration greater than the first
solution is introduced to
17
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
or disposed in a second chamber 11. The higher concentration solution enables
the solvent, e.g.,
water, from the first solution in the first chamber 9 to osmose across the
semi-permeable
membrane 12 into the more concentrated second solution located within the
second chamber 11,
as illustrated by arrow B. Having lost much of its solvent, the remaining
first solution in the first
chamber 9 is concentrated in solute. The solute may be discarded as
illustrated by arrow C if
considered a waste product. Alternatively, the solute may be a target compound
and may be
collected for further processing or downstream use as a desired product. The
resulting solvent-
enriched second solution in the second chamber ills then introduced, as
illustrated by arrow D,
into a third chamber 13. In the third chamber 13, solutes in the solvent-
enriched second solution
may be separated out and recycled back into second chamber 11, as illustrated
by arrow E, to
maintain the concentration of the second solution. The third chamber 13 and
recycling operation
(arrow E) are optional in one or more embodiments of the invention. The
remaining solvent-
enriched second solution in the third chamber 13 may then be introduced, as
illustrated by arrow
F, into a fourth chamber 15. In the fourth chamber 15 the remaining solvent-
enriched second
solution may be heated to remove any remaining solutes to produce a solvent
stream, illustrated
by arrow G. In some embodiments, such as those involving treatment of
wastewater, the solvent
stream may be purified water. In the fourth chamber 15, heat may remove any
remaining solutes
by breaking them down into their constituent gases, the gases may be returned
to the second
chamber 11, as illustrated by arrow H, to maintain the concentration gradient
of the second
solution in chamber 11 and act as reagent.
[0049] The forward osmosis membranes may generally be semi-permeable, for
example,
allowing the passage of solvent such as water, but excluding dissolved solutes
therein, such as
sodium chloride, ammonium carbonate, ammonium bicarbonate, ammonium carbamate,
other
18
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/(14(1335 PCT/US2011/052565
salts, sugars, drugs or other compounds. Many types of semi-permeable
membranes are suitable
for this purpose provided that they are capable of allowing the passage of the
solvent (e.g.,
water), while blocking the passage of the solutes and not reacting with the
solutes in the solution.
The membrane can have a variety of configurations, including thin films,
hollow fiber
membranes, spiral wound membranes, monofilaments and disk tubes. There are
numerous well-
known, commercially available semi-permeable membranes that are characterized
by having
pores small enough to allow water to pass while screening out solute molecules
such as sodium.
chloride and their ionic molecular species such as chloride. Such semi-
permeable membranes
can be made of organic or inorganic materials. In some embodiments, membranes
made of
materials such as cellulose acetate, cellulose nitrate, polysulfone,
polyvinylidene fluoride,
polyamide and acrylonitrile co-polymers may be used. Other membranes may be
mineral
membranes or ceramic membranes made of materials such as ZrO2 and TiO2.
[0050] Generally, the material selected for use as the semi-permeable
membrane should
be able to withstand various process conditions to which the membrane may be
subjected. For
example, it may be desirable that the membrane be able to withstand elevated
temperatures, such
as those associated with sterilization or other high temperature processes. In
some embodiments,
a forward osmosis membrane module may be operated at a temperature in the
range of about 0
degrees Celsius to about 100 degrees Celsius. In som.e non-limiting
embodiments, process
temperatures may range from. about 40 degrees Celsius to about 50 degrees
Celsius. Likewise, it
may be desirable for the membrane to be able to maintain integrity under
various pH conditions.
For example, one or more solutions in the membrane environment, such as the
draw solution,
may be more or less acidic or basic. In some non-limiting embodiments, a
forward osmosis
membrane module may be operated at a pH level of between about 2 and about
1.1. In certain
19
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
non-limiting embodiments, the pH level may be about 7 to about 10. The
membranes used need
not be made out of one of these materials and they can be composites of
various materials. In at
least one embodiment, the membrane may be an asymmetric membrane, such as with
an active
layer on a first surface, and a supporting layer on a second surface. In some
embodiments, an
active layer may generally be a rejecting layer. For example, a rejecting
layer may block passage
of salts in sonic non-limiting embodiments. In some embodiments, a supporting
layer, such as a
backing layer, may generally be inactive.
[0051] In accordance with one or more embodiments, at least one forward
osmosis
membrane may be positioned within a housing or casing. The housing may
generally be sized
and shaped to accommodate the membranes positioned therein. For example, the
housing may
be substantially cylindrical if housing spirally wound forward osmosis
membranes. The housing
of the module may contain inlets to provide feed and draw solutions to the
module as well as
outlets for withdrawal of product streams from the module. In some
embodiments, the housing
may provide at least one reservoir or chamber for holding or storing a fluid
to be introduced to or
withdrawn from the module. In at least one embodiment, the housing may be
insulated.
[0052] In accordance with one or more embodiments, draw solutes may be
recovered for
reuse. Solutes may be stripped from the dilute draw solution to produce
product water
substantially free of the solutes. Gaseous solutes may then be condensed or
absorbed to form a
concentrated draw solution. An absorber may use dilute draw solution as an
absorbent. In other
embodiments, product water may be used as an absorbent, for all or a portion
of the absorbing of
the gas streams from a solute recycle system.
[0053] In accordance with one or more embodiments, a portion of the dilute
draw
solution may be used to absorb draw solute gases from, for example, a
distillation column. In at
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
least one embodiment, both cooling and mixing with an absorbent may occur in
an absorption
column or membrane module, as discussed herein. The mixing of the gases with a
portion of the
dilute draw solution acting as an absorbent (to then become the concentrated
draw solution) may
occur in a vessel. The vessel may generally be sized to provide an area large
enough to facilitate
interaction between the absorbent and the gases. In some embodiments, a packed
column may
be used as an absorber. In one or more embodiments, a stripping distillation
column and an
absorbing column may be used in conjunction. Heating may occur in the
distillation column,
while cooling and contact with the dilute draw solution absorbent may occur in
the absorbing
column. In one embodiment, approximately 25% of the dilute draw solution
stream may be
directed to an absorber to serve as an absorbent fluid, with the remaining
approximately 75% of
the dilute stream being directed to the stripper as its feed stream. The
balance between these two
streams will dictate the concentration of the reconcentrated draw solution
returned to the
membrane system, as well as the size of the absorber and/or stripper, as well
as the quantity of
heating required in the stripper and cooling required before, after, and/or
within the absorber or
stages of the absorber.
[0054] In accordance with one or more embodiments, it may be desirable to
use low
temperatures for stripping solutes in view of low temperature heat sources
having low cost and
few or no alternative uses. The lower the temperature of the stripping,
however, the lower its
pressure, and lower pressure condensation and absorption has slower kinetics,
in some cases
making the absorption of certain compounds, such as carbon dioxide, quite
difficult. Various
methods may be used to absorb remaining gases after solutes have been
stripped, and some
portion (typically between about 60-80%) of these have been condensed, with
the remaining
gases having a low tendency to continue to absorb in a short time frame.
21
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
[0055] In some embodiments, the remaining vapor stream may be compressed
to raise its
pressure and thereby increase the absorption kinetics. A compressor may be
used. In other
embodiments, a steam jet may be used in which a small portion of steam may be
mixed with
vapors to increase pressure to an intermediate pressure between the two
streams. In still other
embodiments, an absorbing solution may be pressurized and introduced into an
eductor jet to
entrain and compress the solute vapor (see, for example, FIGS. 16-18).
[0056] In one or more embodiments, an absorber with series flow of vapors
and series or
parallel flow of absorbent may be used in various configurations, using
membrane contactors,
packing within a column, or similar equipment. (See, for example, FIGS. 6-18).
In one
embodiment, series flow of vapor may be coupled with parallel flow of
absorbent that has been
cooled, such that no cooling need take place within the absorbing device. In
other embodiments,
cooling may take place in the device. A heat exchange area as well as a mass
interface area may
both be in a single device. Absorbent may be used to form a mixture that may
be directed to join
a concentrated draw solution stream. Absorbents may include dilute draw
solution, product
water, water with added ammonia, liquid ammonia and non-volatile carbon
dioxide sequestrate
which would then exit in the product water or be removed or destroyed.
[0057] In accordance with one or more embodiments, a carbon dioxide
absorbing/desorbing loop may be implemented such that a solution is used as
the absorbent at a
low pressure to absorb carbon dioxide. The solution may then be pressurized in
liquid form, and
heated to desorb the carbon dioxide at a higher pressure, allowing the carbon
dioxide to be
absorbed in a condenser or other manner described above. In this way, some
embodiments may
resemble a carbon dioxide sequestration system. In some embodiments, the
absorbing solution
may include ammonia in water. In other embodiments, the absorbing solution may
include a
22
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
non-volatile solute that may complex with the carbon dioxide and then may be
induced to release
it, such that the solute is recycled in the absorbing system. In some
embodiments, heat may be
used. Catalysts and/or reagents may be used in some embodiments. The use of a
catalyst or
reagent in one or more processes, or in a condenser, may increase the kinetics
of draw solute
condensation or reabsorption.
[0058] In accordance with one or more embodiments, dilute draw solution
may be
directed to a stripper (see, e.g.. FIG. 7), where low temperature heat causes
the draw solutes to
evaporate leaving a product water substantially without said solutes. A heat
exchanger may be
used to condense a portion of the vapors. In at least one embodiment, about
70% of the vapors
may be condensed. An absorber system (see, for example, FIG. 6) may be used to
introduce a
portion of the remaining vapors to absorb into a dilute draw solution stream.
In at least one
embodiment, a second absorber system may use a concentrated ammonia solution
to absorb the
remaining draw solute vapors. Liquid streams exiting the condenser, and the
first and second
absorbers, may be mixed and used as all or part of the concentrated draw
solution.
[0059] As noted above, a separation process in accordance with one or more
embodiments may start with the first solution contained within the first
container 12. The first
solution may be an aqueous or non-aqueous solution that is being treated,
either for the purpose
of purified water recovery, for the removal of undesirable solutes, or for the
concentration and
recovery of desired solutes. Included among undesirable solutes are undesired
chemically
precipitable soluble salts such as sodium chloride (NaCe. Typical examples of
the first solution
include aqueous solutions such as seawater, brine and other saline solutions,
brackish water,
mineralized water, industrial waste water, and product streams associated with
high purity
applications, such as those affiliated with the food and pharmaceutical
industries. In general, any
23
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
type of solvent compatible with the draw solution may be used, for example,
any solvent capable
of dissolving the draw solutes. The first solution may be filtered and pre-
treated in accordance
with known techniques in order to remove solid and chemical wastes, biological
contaminants,
and otherwise prevent membrane fouling, prior to osmotic separation and is
then supplied to the
first chamber 9, as indicated by arrow 10.
[0060] Additionally, the first solution may be any solution containing
solvent and one or
more solutes for which separation, purification or other treatment is desired.
In some
embodiments, the first solution may be non-potable water such as seawater,
salt water, brackish
water, gray water, and some industrial water. It may be desired to produce
purified or potable
water from such a stream for downstream use. A process stream to be treated
may include salts
and other ionic species such as chloride, sulfate, bromide, silicate, iodide,
phosphate, sodium,
magnesium, calcium, potassium, nitrate, arsenic, lithium, boron, strontium,
molybdenum,
manganese, aluminum, cadmium, chromium, cobalt, copper, iron, lead, nickel,
selenium, silver,
and zinc. In some examples, the first solution may be brine, such as salt
water or seawater,
wastewater or other contaminated water. In other embodiments, the first
solution may be a
process stream containing one or more solutes, such as target species, which
it is desirable to
concentrate, isolate, or recover. Such streams may be from an industrial
process such as a
pharmaceutical or food grade application. Target species may include
pharmaceuticals, salts,
enzymes, proteins, catalysts, microorganisms, organic compounds, inorganic
compounds,
chemical precursors, chemical products, colloids, food products, or
contaminants. The first
solution may be delivered to a forward osmosis membrane treatment system from
an upstream
unit operation such as industrial facility, or any other source such as the
ocean.
[0061] Like the first solution, the second solution may be an aqueous
solution, i.e., the
24
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
solvent is water. In other embodiments, non-aqueous solutions such as organic
solvents may be
used for the second solution. The second solution may be a draw solution
containing a higher
concentration of solute relative to the first solution. The draw solution may
generally be capable
of generating osmotic pressure within an osmotically driven membrane system.
The osmotic
pressure may be used for a variety of purposes, including desalination, water
treatment, solute
concentration, power generation, and other applications. A wide variety of
draw solutions may
be used. In some embodiments, the draw solution may include one or more
removable solutes.
In at least some embodiments, thermally removable (thermolytic) solutes may be
used. For
example, the draw solution may comprise a thermolytic salt solution. In some
embodiments, an
ammonia and carbon dioxide draw solution may be used, such as those disclosed
in U.S. Patent
No. 7,560,029. In one embodiment, the second solution may be a concentrated
solution of
ammonia and carbon dioxide.
[0062] In accordance with one or more embodiments, the ratio of ammonia to
carbon
dioxide should substantially allow for the full absorption of the draw
solution gases into the
absorbing fluid, i.e., a portion of the dilute draw solution as described
above, based on the
highest concentration of the draw solution in the system. The concentration,
volume, and flow
rate of the draw solution should generally be matched to the concentration,
volume, and flow rate
of the feed solution, such that the desired difference in osmotic pressure
between the two
solutions is maintained throughout the membrane system and range of feedwater
recovery. This
may be calculated in accordance with one or more embodiments taking into
consideration both
internal and external concentration polarization phenomena in the membrane and
at its surface.
In one non-limiting desalination embodiment, a concentrated draw solution
inlet flow rate may
be used which is approximately 33% of the saline feedwater flow rate,
typically in the range of
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
about 25% to 75% for a seawater desalination system. A lower salinity feed may
require draw
solution inlet rates of about 5% to 25% of the feedwater flow. The dilute draw
solution outlet
rate may typically be about 50% to 100% of the feedwater inlet rate, and about
three to four
times the volume of the brine discharge.
[0063] In accordance with one or more embodiments, the ratio of ammonia to
carbon
dioxide should generally be matched to the concentrations of the draw solution
and the
temperatures used in the draw solute removal and recovery process. If the
ratios are not
sufficiently high, it will not be possible to completely absorb the draw
solute gases into salts for
reuse in the concentrated solution, and if the ratio is too high, there will
be an excess of ammonia
in the draw solution which will not properly condense in a desired temperature
range, such as
that necessary for the use of waste heat to drive the process. For example, in
some embodiments
a distillation column may strip gases at about 50 C and an absorbing column
may operate at
about 20 C. The ratio of ammonia to carbon dioxide should further be
considered to prevent the
passage of ammonia into the feed solution through the membrane. If the ratio
is too high, this
may cause unionized ammonia to be present in higher concentrations in the draw
solution
(normally primarily ammonium) than are necessary or desirable. Other
parameters, such as
feedwater type, desired osmotic pressure, desired flux, membrane type and draw
solution
concentration may impact the preferred draw solution molar ratio. The ratio of
ammonia to
carbon dioxide may be monitored and controlled in an osmotically driven
membrane process. In
at least one embodiment, the draw solution may comprise ammonia and carbon
dioxide in a
molar ratio of greater than 1 to 1. In some non-limiting embodiments, the
ratio for a draw
solution at approximately 50 C, and with the molarity of the draw solution
specified as the
molarity of the carbon dioxide within that solution, may be at least about 1.1
to 1 for up to 1
26
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
molar draw solution, about 1.2 to 1 for up to 1.5 molar draw solution, about
1.3 to 1 for up to 3
molar draw solution, about 1.4 to 1 for up to 4 molar draw solution, about 1.5
to 1 for up to 4.5
molar draw solution, about 1.6 to 1 for up to 5 molar draw solution, about 1.7
to 1 for up to 5.5
molar draw solution, about 1.8 to 1 for up to 7 molar draw solution, about 2.0
to 1 for up to 8
molar draw solution and about 2.2 to 1 for up to 10 molar draw solution.
Experiments indicate
that these are approximately the minimum ratios needed for stable solubility
of solutions of these
concentrations at this approximate temperature. At lower temperatures, higher
ratios of
ammonia to carbon dioxide are required for the same concentrations. At higher
temperatures,
lower ratios may be required, but some pressurization of the solution may also
be required to
prevent decomposition of the solutes into gases. Ratios greater than 1 to 1,
even at overall
concentrations of less than 2 molar greatly increase the stability of the
solutions and prevent
evolution of carbon dioxide gas and in general thermolytic splitting of the
draw solutions in
response to even moderate amounts of heat and/or reduction of pressure. The
draw solution
generally has a concentration of solute greater than that of the feed
solution. This may be
achieved using solutes that are soluble enough to produce a solution that has
a higher
concentration than the feed solution. One or more characteristics of the draw
solution may be
adjusted based on the process stream supplied to the separation system for
treatment. For
example, the volume, flow rate, or concentration of solutes in the feed
solution may impact one
or more parameters selected for the draw solution. Requirements pertaining to
discharge streams
associated with the system may also impact one or more operational parameters.
Other
operational parameters may also be varied based on an intended application of
the forward
osmosis separation system. Preferably, the solute within the second solution
should be easily
removable from solution through a separation process, wherein said separation
process separates
27
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
the solute into at least one species that is more readily dissolved in the
solvent of the solution,
i.e., the soluble species, and one species that is not readily dissolved
within the solvent, i.e., the
less-soluble species, and the solute should pose no health risk if trace
amounts remain in the
resulting solvent. The existence of the soluble and less-soluble species of
solutes allows for the
solutions to be adjusted or manipulated as needed. Typically, the soluble and
less soluble solute
species reach a point in solution in which, under the particular condition
temperature, pressure,
pH, etc., neither species of solute is either increasing or decreasing with
respect to the other, i.e.,
the ratio of the soluble to insoluble species of solute is static. This is
referred to as equilibrium.
Given the particular conditions of the solution, the species of solute need
not be present in a one
to one ratio at equilibrium. Through the addition of a chemical, referred to
herein as a reagent,
the balance between the species of solutes can be shifted. Using a first
reagent, the equilibrium
of the solution can be shifted to increase the amount of the soluble species
of solute. Likewise,
using a second reagent, the equilibrium of the solution may be shifted to
increase the amount of
the less-soluble solute species. After the addition of the reagents, the ratio
of species of solutes
may stabilize at a new level which is favored by the conditions of the
solution. By manipulating
the equilibrium in favor of the soluble species of solute, a second solution
with a concentration
near saturation can be achieved, a state in which the solutions solvent cannot
dissolve anymore
of the solute.
[0064] Preferred solutes for the second (draw) solution may be ammonia and
carbon
dioxide gases and their products, ammonium carbonate, ammonium bicarbonate,
and ammonium
carbamate. Ammonia and carbon dioxide, when dissolved in water at a molar
ratio of about 1,
form a solution comprised primarily of ammonium bicarbonate and to a lesser
extent the related
products ammonium carbonate and ammonium carbamate. The equilibrium in this
solution
28
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
favors the less-soluble species of solute, ammonium bicarbonate, over the
soluble species of
solute, ammonium carbamate and to a lesser extent ammonium carbonate.
Buffering a solution
comprised primarily of ammonium bicarbonate with an excess of ammonia so that
the molar
ratio of ammonia to carbon dioxide is greater than 1 will shift the
equilibrium of the solution
towards the more soluble species of the solute, ammonium carbamate. The
ammonia is more
soluble in water and is preferentially adsorbed by the solution. Because
ammonium carbamate is
more readily adsorbed by the solvent of the second solution, its concentration
can be increased to
the point where the solvent cannot adsorb anymore of the solute, i.e.,
saturation. In some non-
limiting embodiments, the concentration of solutes within this second solution
achieved by this
manipulation is greater than about 2 molal, more than about 6 molal, or about
6 molal to about
12 molal.
[0065] Ammonia may be a preferred first reagent for ammonium carbamate
since it is
one of the chemical elements that results when the solute ammonium carbamate
is decomposed,
otherwise referred to as a constituent element. In general, it is preferred
that the reagent for the
solvent be a constituent element of the solute, as any excess reagent can
easily be removed from
the solution when the solvent is removed and, in a preferred embodiment, the
constituent element
can be recycled as the first reagent. However, other reagents that can
manipulate the equilibrium
of the solute species in solution are contemplated so long as the reagent is
easily removed from
the solution and the reagent posses no health risk if trace elements of the
reagent remain within
the final solvent.
[0066] In accordance with one or more embodiments, a draw solution should
generally
create osmotic pressure and be removable, such as for regeneration and
recycle. In some
embodiments, a draw solution may be characterized by an ability to undergo a
catalyzed phase
29
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335
PCT/US2011/052565
change in which a draw solute is changed to a gas or solid which can be
precipitated from an
aqueous solution using a catalyst. In some embodiments, the mechanism may be
coupled with
some other means, such as heating, cooling, addition of a reactant, or
introduction of an electrical
or magnetic field. In other embodiments, a chemical may be introduced to react
with a draw
solute reversibly or irreversibly to reduce its concentration, change its
rejection characteristics by
the membrane, or in other ways make it easier to remove. In at least one
embodiment,
introduction of an electrical filed may cause a change in the draw solute,
such as a phase change,
change in degree of ionization, or other electrically induced changes that
make the solute easier
to remove. In some embodiments, solute passage and/or rejection may be
manipulated, such as
by adjusting a pH level, adjusting the ionic nature of a solute, modifying the
physical size of a
solute or promoting another change that causes the draw solute to readily pass
through a
membrane where previously it had been rejected. For example, an ionic species
may be rendered
nonionic, or a large species may be made relatively smaller. In some
embodiments, separation
techniques not using heating, such as electrodialysis (ED), cooling, vacuum or
pressurization
may be implemented. In at least one embodiment, an electrical gradient may be
implemented in
accordance with one or more known separation techniques. In some embodiments,
certain
separation techniques, such as ED, may be used to reduce species to be
separated such as to
lower electrical requirements. In at least one embodiment, the solubility of
organic species may
be manipulated, such as by changing temperature, pressure, pH or other
characteristic of the
solution. In at least some embodiments, ion exchange separation may be
implemented, such as
sodium recharge ion exchange techniques, or acid and base recharged ion
exchange to recycle
draw solutes, including, for example, ammonium salts.
[0067] In
accordance with one or more embodiments, disclosed draw solutions may be
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
used in any osmotically driven membrane process, for example, applications
involving pressure
retarded osmosis, forward osmosis, or pressure assisted forward osmosis. In
some embodiments,
disclosed draw solutions may be used in an osmotic heat engine, such as that
described in PCT
Publication No. W02008/060435, the disclosure of which is hereby incorporated
by reference
herein in its entirety. An osmotic heat engine may convert thermal energy into
mechanical work
using a semi-permeable membrane to convert osmotic pressure into electrical
power. A
concentrated ammonia-carbon dioxide draw solution may create high osmotic
pressures which
generate water flux through a semi-permeable membrane against a hydraulic
pressure gradient.
Depressurization of the increased draw solution volume in a turbine may
produce electrical
power. The process may be maintained in steady state operation through the
separation of
diluted draw solution into a re-concentrated draw solution and deionized water
working fluid,
both for reuse in the osmotic heat engine. In some embodiments involving use
of disclosed draw
solutions in an osmotic heat engine, efficiency may be enhanced by
precipitating the draw solute.
In at least one embodiment, disclosed draw solutions may be used in systems
and methods for
grid energy storage in which use of salinity gradients involving osmotic
pressure gradients or
differences between two solutions may be used to produce hydraulic pressure in
a concentrated
solution, allowing for the generation of power. In accordance with one or more
embodiments
involving distillation columns, such as the multi-stage distillation columns
described in PCT
Publication No. W02007/1146094, the disclosure of which is hereby incorporated
by reference
herein in its entirety; dilute draw solution may be used as an absorbing fluid
in a heat exchanger
or absorber for heat transfer to each stage. In accordance with one or more
embodiments,
disclosed draw solutions may also be used in various direct osmosis
concentration (DOC)
applications.
31
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335
PCT/US2011/052565
[0068]
Referring back to FIGS. 2-3, in accordance with one or more embodiments, the
osmotically driven membrane process may begin by bringing the first solution
and the second
solution into contact with the first and second sides of the semi-permeable
membrane 12,
respectively. Although the first and second solutions can remain stagnant, it
is preferred that
both the first and second solutions are introduced by cross flow, i.e., flows
parallel to the surface
of the semi-permeable membrane 12. This increases the amount of surface area
of the semi-
permeable membrane 12 a given amount of the solutions comes into contact with,
thereby
increasing the efficiency of the forward osmosis. Since the second solution in
the second
chamber 11 has a higher solute concentration than the first solution in the
first chamber 9, the
solvent in the first solution diffuses to the second solution by forward
osmosis. In some
embodiments, the difference in concentration between the two solutions is so
great that the
solvent passes through the semi-permeable membrane 12 without the addition of
pressure to the
first solution. Overall, this process may result in the removal of about 50%
to about 99.9% of
the solvent contained within the first solution. During the separation
process, the first solution
becomes more concentrated as it loses solvent and the second solution becomes
more diluted as
it gains solvent. Despite this occurrence, the concentration gradient between
the two solutions
remains significant. The depleted solution on the first side of the membrane,
and the diluted
solution on the second side of the membrane may each be further processed for
the recovery of
one or more desired products. For example, the depleted solution on the first
side of the
membrane may contain solutes which are target species whose concentration and
recovery is
desired. Alternatively, the depleted solution on the first side of the
membrane may be discarded
as waste. Likewise, the diluted solution on the second side of the membrane
may be rich in
solvent which may be a desired product.
32
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
[0069] The discharge 18, i.e., the concentrated first solution, has a
greater concentration
of solutes. Thus, the excess solutes can be removed from the concentrated
first solution prior to
returning the first solution to its source or recirculating the first solution
through the present
method. This can be done, for example, by contacting the concentrated first
solution with a solar
evaporator, a mechanism of simple screen filtration, hydrocyclone, or a
precipitation mass or
other nucleation point to precipitate the solute. This precipitated solute may
be further processed
to make it suitable for consumer or industrial purposes.
[0070] Having extracted the solvent of the first solution into the second
solution by
forward osmosis, thereby forming a solvent-enriched second solution, it may
then be desirable to
remove the solutes from the solvent-enriched second solution to isolate the
solvent. In some
non-limiting embodiments, this can be accomplished by precipitating the
solutes out of the
solution, decomposing the solutes into their constituent gases that vaporize
out of solution,
distilling the solvent out of the solution or absorption of the solutes onto a
surface. In at least
one embodiment, removing a portion of the solutes by precipitation decreases
the amount of
energy required to heat the solution to decompose the remaining solutes, and
decomposition
results in the complete removal of the solutes. Potential precipitation and
decomposition steps
are described with reference to the third and fourth chamber 13 and 15,
respectively.
[0071] The solvent-enriched second solution in the second chamber 11 may
be
withdrawn to a third chamber 13, as shown by arrow D. The solvent-enriched
second solution
may then be treated to remove a portion of the solutes from the solvent-
enriched solution by
precipitation. A second reagent may be introduced to adjust the equilibrium of
the soluble and
less-soluble solute species in favor of the less-soluble solute species. As
with the first reagent,
any chemical capable of adjusting the equilibrium is suitable, so long as it
is easily removed
33
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
from the solvent-enriched second solution and posses no health risk.
Preferably the reagent is a
constituent element of the solute, and in the case of the preferred solute
ammonium carbamate is
carbon dioxide gas. In some non-limiting embodiments, when the solvent-
enriched second
solution is diffused with carbon dioxide, the ratio of ammonia to carbon
dioxide in solution may
be reduced to around between 1 and 1.5 and the equilibrium in the solvent-
enriched second
solution shifts back towards the less-soluble species of solute, ammonium
bicarbonate. The less-
soluble species of solute may then precipitate out of solution. The
precipitation of the
ammonium bicarbonate may result in a substantial reduction in the
concentration of solutes
within the solvent-enriched second solution to about 2 to 3 molar. Preferably,
the temperature of
the solvent-enriched second solution in the third chamber 13 is lowered to
about 18 to 25 C,
preferably about 20 to 25 C to assist in the precipitation of the solute. The
precipitated solute
may then be filtered from the solution.
[0072] In some embodiments, the precipitated solute may be filtered within
the third
chamber 13; however, in the embodiment shown in FIG. 3, the solution is
directed to a filtration
chamber 17, as shown by arrow I. Using well known methods, such as a
hydrocyclone, a
sedimentation tank, column filtration, or a simple screen filtration, the
precipitated solute may be
removed from the solvent-enriched solution. For example, the precipitate may
be allowed to
settle out of solution by gravity at which time the remaining solution may
then be siphoned off.
The remaining solvent-enriched second solution may be transferred from the
filter chamber 17 to
a fourth chamber 15, as shown by arrow F, where it is then heated to decompose
the solutes into
their constituent gases. In one embodiment, these constituent gases may be
ammonia and carbon
dioxide. The energy required for the separation process is the heat required
to raise the
temperature of the solution to a temperature which results in the complete
removal of the
34
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
ammonium carbamate solute. Additional heat is also required to make up for the
inefficiency of
heat transfer of the enthalpies of vaporization and solution of the solutes
recycled within the
process. Specifically, heating causes the remaining solutes in the solvent-
enriched second
solution to decompose into their constituent gases, which leave the solution.
In some
embodiments, a vacuum or air flow may be maintained over the solvent-enriched
second solution
while it is being heated in order to improve the efficiency and or lower the
temperature at which
the decomposition gases vaporize out of solution. By generating the air flow
over the fourth
chamber it may be possible to remove all of the solutes at a lower temperature
than typically
used. This decomposition may result in a solvent product, such as a potable
water product,
which may be further treated for end use. In general, a potable water product
should have a pH of
about 7, and further pH adjustments and or additions of desirable
constituents, such as salts and
or residual disinfectants, may be necessary to make the water suitable for its
intended purpose.
[0073] The solvent-enriched second solution may be heated using a
combination of
external heat sources 19 and heat pumped through a heat exchanger 38 from the
exothermic
introduction of gases and solutes (arrows J and K). The external heat source
19 may be supplied
by any thermal source including solar and geothermal energy. The sources may
be similar to
those of distillation. In some embodiments, the sources may be primarily from
cogeneration
environments, making use of waste heat from power generation or industrial
processes.
Furthermore, the process efficiency may be maintained by using a heat
exchanger 38 to capture
the heat released during the previous steps in the present method of
desalination. As shown by
the arrows J and K in FIG. 3, heat released from the chemical reactions within
the second and
third chambers 11, 13 may be pumped to the heat exchanger 38 that then pumps
this heat to the
fourth chamber 15 to assist in heating the solvent-enriched second solution,
as shown by arrow
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335
PCT/US2011/052565
L. In an alternative embodiment, additional heat is generated by allowing the
constituent gases
released to condense on the exterior of the chamber 15 in which the solvent-
enriched second
solution is being heated, thus, transferring the energy from this exothermic
reaction to the fourth
chamber 15. The condensate, which in one embodiment is ammonium carbamate, may
then be
recycled to the second solution in the second chamber 11.
[0074] It is
also preferable to recycle the solutes and solute constituents removed from
the second solution to limit the environmental impact and cost of the present
method of forward
osmosis separation. The precipitated solute discarded from a filtration
chamber may be recycled
to the second chamber 11, where it can dissolve in the second solution and
thereby maintain the
high concentration of the second solution, as shown by arrow E. Additionally,
the constituent
gases removed from the solvent-enriched second solution in the fourth chamber
15 can be
recycled back to the second or third chambers 11, 13 as shown by arrows H and
M, respectively,
where they act as reagents. hl one embodiment, the solute is ammonium
carbamate, which is
decomposed into its constituent gases: ammonia and carbon dioxide. These gases
are then
recycled to the second chamber 11, as shown by arrow H. Since the ammonia is
more soluble
than the carbon dioxide, the ammonia is preferentially adsorbed by the second
solution and acts
as a reagent by adjusting the equilibrium of the solute species in favor of
ammonia carbamate.
The remaining carbon dioxide is withdrawn from the second chamber 11, as shown
by arrow M,
and transferred to the third chamber 13, where it acts as a reagent and alters
the equilibrium of
the second solution in favor ammonium bicarbonate. Since some embodiments
contemplate
recycling the constituent gases derived from the decomposition of the solutes,
it may be
necessary to precipitate less than optimal amounts of the solutes to ensure
that enough gas is
recycled to maintain the efficiency of the present process. Typically,
removing about half of the
36
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335
PCT/US2011/052565
solutes from solution by precipitation should assure that sufficient amounts
of the constituent
gases will be generated to maintain the present process. The process described
herein may be
conducted continuously, or in batches, to better isolate the solutes from
solvent throughout the
process.
[0075] One non-limiting embodiment of an apparatus for conducting the
present method
is shown in FIG. 4. The apparatus has a first chamber 9 that has an inlet 21
and an outlet 23.
The inlet 21 for the first chamber 9 is in communication with a source for the
first solution, such
as a holding tank for a solution having undergone pre-treatment or being
introduced from an
upstream operation, or to a natural source for the first solution, such as the
sea, a lake, a stream,
or other bodies of water and waterways. The inlet 21 for the first chamber 9
may incorporate a
pump in order to siphon the first solution from its source and or a screen or
filter to remove
particulates. It also may optionally include heating or cooling devices in
order to adjust the
temperature of the first solution. Similarly, the outlet 23 for the first
chamber 9 may incorporate
a pump in order to extract the first solution from the first chamber 9. The
outlet 23 may be used
to recirculate the first solution directly to the source for the first
solution, although preferably,
the first solution will be pumped into or across a precipitation device prior
to being returned to
the source of the first solution. Such a precipitation device may include a
solar evaporation bed,
a mechanism of simple screen filtration, a hydrocyclone, or a precipitation
mass or other
nucleation point operation, or other types known to those skilled in the art.
The first chamber 9
is separated from a second chamber 11 by a semi-permeable membrane 12.
[0076] The second chamber 11 has an inlet 25 and first and second outlets
27, 29. The
inlet 25 provides a source for the second solution and may incorporate a pump
as well as a
heating device. The first outlet 27 for the second chamber 11 is in
communication with a third
37
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
chamber 13, and provides a conduit for transferring the solvent-enriched
second solution to the
third chamber 13. This first outlet 27 for the second chamber 11 can
incorporate a pump to
withdraw the water-enriched second solution from the second chamber 11. In
another
embodiment of the present invention, the first outlet 27 for the second
chamber 11 may
incorporate a cooling device to cool the solvent-enriched second solution as
discussed above.
The second outlet 29 for the second chamber 11 provides a conduit for any gas
remaining when
gases from fourth chamber 15 are introduced into the second solution through
inlet 25, which in
one embodiment would be primarily carbon dioxide gas, as ammonia is expected
to
preferentially absorb into this solution, to be transferred to the third
chamber 13.
[0077] In some embodiments, the third chamber 13 is where a portion of the
solute is
precipitated out of the solvent-enriched second solution. The third chamber
13, in addition to the
inlets for connecting to outlets 27, 29, has an outlet 31 in communication
with a filtration device
17 for separating the precipitate from the solvent-enriched second solution.
The filtration device
17 is of any of the types disclosed above, and in one embodiment is a
sedimentation tank. The
filtration device 17 has two outlets 33, 35. The first outlet 33 may be used
to dispose of the
precipitated solute or return it to the second chamber 11 through the second
chamber inlet 25,
and the second outlet 35 may be used to transfer the remaining solvent-
enriched second solution
to the fourth chamber 15. In an alternate embodiment, the filtration device 17
may be
incorporated into the third chamber 13, in which case the third chamber 13
will have an
additional outlet, one outlet to transfer the remaining solvent-enriched
second solution to the
fourth chamber 15 and another outlet to dispose of the precipitated solute, or
in an alternative
embodiment, return the precipitated solute to the second chamber 11 through
the second chamber
inlet 25.
38
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
[0078] The fourth chamber 15 may incorporate a heating device for heating
the
remaining solvent-enriched second solution. The fourth chamber 15 also
incorporates a first
outlet 37, which may incorporate a vacuum, fan, or other device for generating
airflow for
venting the constituent gases. Preferably, the first outlet 37 for the fourth
chamber 15 is in
communication with the inlet 25 for the second chamber 11 to recycle the
constituent gases as
the second solute. The second outlet 39 acts as a conduit for withdrawing the
final solvent
product, such as potable or purified water.
[0079] Any materials may be used to construct the various holding and/or
storage devices
(chambers, vessels, and receptacles), conduits, piping, and related equipment,
as long as they
will withstand the weight of the solutions, and be non-reactive with any
solutes within the
solutions. Typical materials are non-corrosive, non-reactive materials such as
stainless steel,
plastic, polyvinyl chloride (PVC), fiberglass, and so forth. The vessels can
take any suitable
configuration, but are typically cylindrical tanks, contoured or fitted tanks,
and so forth. The
receptacles are typically water towers, cylindrical tanks, contoured or fitted
tanks, and so forth.
As discussed above, it is important to note that the chambers are shown as
separate units, but the
invention is not limited to that configuration, and where appropriate, any
number of chambers
can be contained within a single vessel, for example, partitioned into two
chambers separated by
the semi-permeable membrane 12.
[0080] The heating and cooling devices can be electrical heaters,
refrigeration units, solar
collectors, and heat exchangers such as steam condensers, circulators and so
forth, such as are
well known in the art, but preferably heat exchangers. The heating and cooling
devices, along
with any other equipment used within the process that may have power
requirements, can derive
their energy from any variety of commonly used sources, including, for
example, waste steam,
39
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
solar energy, wind or geothermal energy, and conventional sources.
[0081] With reference to FIG. 5, a concentration process in accordance
with one or more
embodiments is disclosed. A first solution 14 is exposed to one side of a
forward osmosis
membrane 12. In embodiments where the first solution 14 comprises a waste
stream to be
treated, the first solution 14 is typically aqueous and contains a solution of
species, such as salts,
proteins, catalysts, microorganisms, organic or inorganic chemicals, chemical
precursors or
products, colloids, or other constituents. In embodiments where the first
solution 14 contains
desired target species to be concentrated and recovered, the first solution
may comprise a
pharmaceutical, salt, enzyme, protein, catalyst, microorganism, organic
compound, inorganic
compound, chemical precursor, chemical product, colloid, food product or
contaminant. The
exposure of the first solution 14 to one side of the membrane 12 may be
achieved in many
configurations, two of which are immersion of the membrane 12 in the solution
or direction of
the solution past the membrane 12. This solution may be introduced
continuously, in batch, once
or many times, to a vessel or direction means. This input stream of the first
solution 14 is not
shown in the schematic.
[0082] A second solution 16 comprised, for example, of species including
water,
ammonia, and carbon dioxide, which is capable of generating an osmotic
pressure that is higher
than that of the first solution 14, is exposed to the side of the membrane
opposite that exposed to
the first solution 14. This exposure may be achieved by many techniques, but
may include
immersion of the membrane 12 in the second solution (though not if immersion
is used for the
first solution) or the direction of the second solution past the membrane
surface. The membrane
12, being impermeable to all or some of the species of the first solution 14,
such as salts, charged
and/or large molecules, microorganisms, and particulate matter, but allowing
the passage of the
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335
PCT/US2011/052565
solvent, such as water, allows the difference in osmotic pressure between the
first and second
solutions to induce a flux of water through the membrane from the first to the
second solution.
This flux may be allowed to significantly, partly, or largely not dilute the
second solution 16
and/or concentration the first solution 14. Some, none, few, or one of the
select or target species
of the first solution may also be expected to pass through the membrane 12,
depending on the
membrane type and/or the intention of the process use.
[0083] A
portion of the solvent-enriched second solution is directed (as stream 20) to
a
draw solute separation operation 22, such as a distillation column, membrane
distillation
operation, or pervaporation operation, which causes the solutes in the solvent-
enriched second
solution, for example ammonia and carbon dioxide solutes, including species of
ammonium
salts, to be removed by adding heat to the draw solute separation operation 22
and/or applying a
pressure difference to the gases above and/or produced by the draw solute
separation operation.
In accordance with one or more embodiments, a membrane separation method, such
as
pervaporation, may allow for the separation of the draw solution gases from
the dilute draw
solution with significant restrictions on the flow of water vapor which may
increase the
efficiency of the separation process. Pervaporation materials may include
natural or synthetic
polymers such as polyurethane or natural rubber, or suspended liquid membranes
that act as
passive or active selective membranes for ammonia and carbon dioxide relative
to water vapor.
In some embodiments, a pervaporation or similar membrane separation method may
be used in
place of or in conjunction with a distillation column. In at least one
embodiment, an absorber
column may also be implemented. In another embodiment, the gases of stream 26
are
compressed to raise the temperature at which they may be reabsorbed into the
draw solution
completely or near completely. In accordance with one or more embodiments, an
absorber may
41
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
be used with cooling prior to or during reabsorption of draw solutes. In at
least some
embodiments, a portion of the dilute draw stream may be diverted to the
absorber as an
absorbent flow.
[0084] The draw solute separation operation produces a solvent stream,
designated
stream 32, which is reduced in concentration of the species of stream 20,
either partially,
substantially, or completely, and a gas stream, designated stream 26,
containing the removed
species from stream 20. Stream 26 is directed to an operation 41 designated to
reconstitute the
second solution 16, which will be used to augment, replace, or maintain the
characteristics of the
second solution 16, such as volume or concentration. This operation may
include dissolving the
species in water, a portion of the second solution, precipitation and mixing
with the second
solution or some other method, such that the species removed in operation 22
are reintroduced to
the second solution. This reintroduction is shown as the dashed stream 45.
Rejected
components of solution 14 may be removed from solution 14, periodically or
continuously, as
water is removed from this solution. This operation may include settling,
hydrocyclone
separation, precipitation, force gradient (such as electrical or magnetic),
blowdown, or other unit
operation. This stream of components removed from solution 14 is shown as
stream 18. In
some embodiments, stream 18 may be a desired product stream or may be
discarded as waste.
By these techniques, osmotic pressure is used to remove solvent from a
solution by osmotically
driven flux through a semi-permeable membrane, for example separating solvent
from a
pharmaceutical compound, food product, or other desired species in solution,
or treating a
process stream by the removal of undesired solutes to produce a purified
product stream. Stream
18 may additionally be treated to remove any of the draw solutes from second
solution 16 that
have migrated through the membrane into the first solution 14. This treatment
may include
42
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
distillation, air stripping, pervaporation, breakpoint chlorination, ion
exchange or other means.
The draw solutes removed from stream 18 may be reintroduced to the second
solution 16
through mixing with stream 45 or by other means.
[0085] FIGS. 6-18 depict a variety of draw solute recovery systems 22, as
introduced
hereinabove, that may be used with the osmotically driven membrane systems
also described
herein. In accordance with one or more embodiments, the recovery system 22 can
include the
use of a membrane contactor, for example as described with respect to FIGS. 6-
10. Using a
membrane for the exchange of mass and energy between the liquid and gas
streams generally
performs the functions of column distillation in a membrane device. One
benefit of this
approach is that liquid and gas volume flows and velocities are largely
independent, insofar as
flooding, entrainment, foaming, and the like do not occur. In the rare case
where liquid
penetrates the porous membrane into the gas stream, this does not occur
substantially.
Additionally, density differences are not used to cause the two streams to
interact, as is done with
conventional distillation columns. Instead, pressure is used to cause the
liquid and gas streams to
flow, as would be done in pipes with liquid or gas only. For this reason,
membrane based
distillation devices need not be placed vertically, as is necessary for
conventional distillation
columns. Thus, compact, horizontal membrane arrays such as parallel modules in
trains, for
example, may be used to serve the function of large diameter, tall
conventional distillation
columns. This allows for the significant reduction in footprint and height
requirements.
[0086] Moreover, the mixing of gas and liquid phases conventionally
provided for by
packing inside of a distillation column may now be provided by membrane area.
A liquid stream
may flow on one side of the membrane and the gas stream on the other side with
free gas
exchange between the two phases through the pores in the membrane. Coating the
pores is
43
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
possible if it does not inhibit gas transfer to a degree that is not
compensated for by the benefits
provided by the coating. The membrane may be designed to be dry or wet. In the
dry membrane
design, the pores of the membrane and the material of the membrane may be such
that surface
tension prevents water from penetrating through the pores to the gas stream.
In the wet design,
water may fill the pores but may not flow beyond these pores into the gas
stream in significant
amounts. In either case, gas exchange is substantially uninhibited.
[0087] A further benefit of using the disclosed membrane contactors for
distillation is
that no metal is necessary, which leads to excellent longevity for contactors
in comparison to
alloys, which may be very expensive and corrode over time. For instance, the
presence of a few
thousand ppm of NaC1 in a stream that boils at 100 C would require titanium
or Hastelloy
alloys (such as those available from Haynes International, Inc. of Kokomo,
Indiana), but could
be served easily with a polymer (e.g., polyetheretherketone) contactor module.
An additional
benefit of using the disclosed membrane contactors for draw solution recovery
is that the
temperatures necessary for such recovery are well within the temperature
tolerances of polymers
that may be used in such contactors. An additional benefit is that HETPs (flow
path length per
theoretical equilibrium stage) may be quite small compared to conventional
packing, leading to
compact and less expensive contactor arrays for the same function as a much
larger column.
Another benefit is that much higher liquid flow rates are possible in the
disclosed membrane
contactors, without causing difficulties such as entrainment, leading to more
effective use of
membrane area, equivalent conceptually to column diameters as they relate to
liquid loading
rates. Another benefit is that conventional columns need additional height
above and below the
packing to allow for separation of the liquid and gas phases from one another,
which is not
necessary in the disclosed membrane contactors, as the two phases are never
mixed in the same
44
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335
PCT/US2011/052565
channel, and so need not be disengaged.
[0088] FIG.
6 depicts one embodiment of a draw solute recovery system 22 that uses a
membrane device 24 to bring vaporized draw solutes 26 from the draw solute
recovery operation
into contact with an absorbing solution 28. In some embodiments, the membrane
device 24 may
be a membrane contactor. As shown in FIG. 6, the vaporized draw solutes 26
pass on a first side
of the membrane contactor 24 (as represented by arrow 27), while the absorbing
solution 28
passes on the second side of the membrane contactor 24 (as represented by
arrow 29). The
vaporized draw solutes are able to pass through the membrane contactor 24 (as
represented by
arrows 25) and be absorbed by the absorbing solution 28. In one or more
embodiments, the
absorbing solution 28 is a dilute or concentrated draw solution, where the
vaporized draw solutes
are reabsorbed to create a more concentrated source of draw solution for
use/reuse in the
osmotically driven membrane system/process.
[0089] In at
least one embodiment, a draw solute recovery system 122 may include the
use of a stripper, as shown in FIG. 7, to remove substantially all draw
solutes from a dilute draw
solution. As shown in FIG. 7, the dilute draw solution 120 (from, for example,
an osmotically
driven membrane system) passes on one side of a membrane contactor 124, while
a source of
steam 130 (i.e., thermal energy) is introduced to the second side of the
membrane contactor 124.
The dilute draw solution 120 absorbs the heat from the steam 130 and the draw
solutes are
stripped therefrom. Specifically, heat from the steam 130 passes through the
membrane
contactor 124 (arrow 131), heating the dilute draw solution 120 and vaporizing
the draw solutes
therein. The vaporized draw solutes 126 pass through the membrane contactor
124 (arrow 127)
and are absorbed by the steam 130, resulting in a concentrated draw solution
116 (in
substantially vapor form) leaving the system 122. Stripping of the draw
solutes from the dilute
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
draw solution 120 results in a source of water 132 exiting the system 122.
[0090] Additionally or alternatively, a condenser may then be used to
condense all or a
portion of the vapors (see, for example, FIG. 9). A second membrane device may
then be used
with the remaining vapor on the first side of the membrane contactor 124 and
an absorber on the
second side thereof. In some embodiments, distillation with or without
additional absorbing
solution may be present on the second side, such that vapors may be condensed
by passing
through the membrane device. In some embodiments, a condenser may be
positioned upstream
of the membrane device. In other embodiments, the various streams may be
brought into contact
without an intermediate unit operation. Furthermore, a membrane absorber may
fully condense
vapors from a stripper or other draw solute recovery operation. In other
embodiments, the
membrane absorber may be used following a condenser to complete reabsorption
of solutes into
a more concentrated draw solution. This may be particularly useful to reduce
the size of the
solute recovery system. This may also be particularly useful at low
temperatures and pressures.
[0091] Membranes for use in the various embodiments of the solute recovery
systems
can be made of essentially any material suitable for its intended purpose,
including, for example,
polytetrafluoroethylene (PTFE), polyvinylidene fluoride (PVDF),
polyetheretherketone (PEEK),
and polypropylene (PP). In accordance with various embodiments, the membrane
may be
porous or dense. The pores in a porous membrane may be sized so that liquid
(e.g., water) does
not pass through, but gases may pass through. The membrane may be in the form
of hollow
fibers or a flat sheet. In the case of hollow fibers, other fibers intended
for heat transfer may be
mixed with these fibers to enhance the ability to cool a membrane system and
condense gases.
For example, some fibers may be impermeable, but allow for heating or cooling
of the draw
solution through heat transfer at the fiber wall, while other fibers may be
selective and used to
46
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335
PCT/US2011/052565
separate and recycle draw solute. The percentage of each type may be varied,
the longitudinal or
vertical orientation of various fibers may vary, and in some embodiments the
fibers may be
staged in zones. Such arrangements may facilitate reduction of energy
requirements in
applications such as those involving an osmotic heat engine. In some
embodiments, such as
those involving relatively small scale applications, a collapsible mobile
configuration with
integrated mechanics may be implemented. In flat sheet configurations, cooling
features may be
integrated into a housing. In either membrane configuration, cooling may be
used between
membrane stages. Additionally, the membranes may be coated or uncoated to suit
a particular
application and may be asymmetric or symmetric. In some embodiments, hollow
fiber, spiral
wound or plate and frame membrane modules containing the membranes may be
used.
[0092] In
some embodiments, hollow fiber, spiral wound or plate and frame membrane
modules containing coated or uncoated porous membranes may be used for the
distillation of
thermally separable draw solutions used in osmotically driven membrane
processes including,
power generation and/or energy storage. In accordance with one or more
embodiments, heat
exchange area in the form of fibers, membrane sheets, or other heat transfer
materials may be
integrated into membrane modules, or alternated with these modules, to enhance
the ability to
transfer heat, as well as mass, and to condense solute gases. The stripping
and absorption
functions may be integrated into a single module or spread among multiple
membrane units. The
stripping and absorption functions may be carried out across a single
membrane. In such
embodiments, stripping from the dilute draw solution by pervaporation or
membrane distillation
may occur on a first side, with the opposite side of the membrane system
receiving the gases into
solution. This may, for example, be an absorbing fluid such as a dilute or
concentrated draw
solution.
47
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
[0093] FIG. 8 depicts one example of a recovery system 222 with dilute
draw solution
stripping and absorbing functions integrated into a single module or device
224. In accordance
with one or more embodiments, the membrane module 224 may be designed such
that stripping
of draw solutes is carried out with one membrane system and absorption of
gases into a draw
solution to increase its concentration for reuse is carried out with a
condenser and/or second
membrane system. Alternatively or additionally, the stripping may be done by
membrane
distillation, pervaporation, or other similar process, and the absorption may
be done by a
membrane contactor, pervaporation process, or other similar technique.
[0094] As shown in FIG. 8, the solute recovery system 222 includes a
membrane module
224 including at least one absorbing membrane 224a and at least one stripping
membrane 224b.
The module 224 functions similarly to the membranes described with respect to
FIGS. 6 and 7.
Specifically, steam 230 is introduced to one side of the stripper membrane
224b, while a dilute
draw solution 220 is introduced to the other side of the stripper membrane
224b. Heat is
transferred to the dilute draw solution 220 (arrow 231), vaporizing the draw
solutes, which pass
through the membrane 224b (arrow 227), leaving water 232 to exit the module
224. The steam
230, now including the vaporized draw solutes 226, is introduced to one side
of the absorber
membrane 224a, while dilute draw solution 220 is introduced to the other side
of the absorber
membrane, where it acts as an absorbing solution, absorbing the vaporized draw
solutes 226 that
pass through the membrane 224a (arrow 225), resulting in a concentrated draw
solution 216
exiting the module 224. In one embodiment, the module 224 includes a blank
sheet of material
234, for example, an insulator, that serves to keep the mass and/or heat from
the two streams
from interacting with one another.
[0095] In accordance with additional embodiments, the membrane based
solute recovery
48
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
systems can be integrated with a heat pump, offering significant energy
efficiency
improvements, as shown in FIG. 9. In particular, FIG. 9 depicts a solute
recovery system 322
that includes a membrane module 324 coupled to a closed-cycle heat pump 340
with a reboiler
342 and a condenser 344. The operation of the membrane module 324 is similar
to those
previously described, insofar as steam 330 is introduced to the module 324 (in
this case from the
reboiler 342) between two membrane contactors 324a, 324b, while dilute draw
solution 320 is
introduced to the opposite sides of those membrane contactors 324a, 324b. Any
number and
arrangement of membrane contactors can be used to suit a particular
application.
[0096] The steam, now containing the vaporized solutes 326, exits the
module 324 and is
directed to the condenser 344, where at least a portion of the steam and
vaporized draw solutes
326 are condensed and discharged as concentrated draw solution 316. In some
embodiments, the
condenser 344 can be used with an absorbing solution. The heat pump 340, using
the heat
removed at the condenser 344, raises the temperature thereof and directs same
to the reboiler 342
to produce the steam 330. The heat pump 340 may be coupled to a source of
electricity 346 or
other means of power. Water 332 is discharged by the membrane module 324 after
the draw
solutes have been stripped from the dilute draw solution 320. At least a
portion of the water 332
can be used by the reboiler 342 to produce the additional steam 330.
[0097] FIG. 10 depicts an alternative embodiment of a solute recovery
system 422 that
includes a membrane module 424 coupled to an open-cycle heat pump 440 and a
reboiler 442.
The operation of the system 422 is similar to that described with respect to
FIG. 9. For example,
dilute draw solution 420 is introduced to the module 424 on one side of one or
more membrane
contactors 424a, 424b, while steam 430 is introduced to the module 424 from
the reboiler 442 on
the opposite sides of the one or more membrane contactors 424a, 424b. Again,
any number and
49
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
arrangement of membrane contactors can be used to suit a particular
application. The vaporized
draw solutes 426 exit the module 424 and are directed to the heat pump 440,
where their
temperature is raised and directed to the reboiler 442 to produce steam 430
for the stripping
operation. The reboiler 442 discharges a concentrated draw solution 416 that
may be recycled
back to an osmotically driven membrane system. The module 424 also discharges
water 432 that
can be used as is, further processed, and/or have at least a portion thereof
directed to the reboiler
442.
[0098] FIG. 11 depicts a portion of a selective membrane 524, in the form
of a suspended
liquid membrane, that substantially inhibits transport of water vapor, but
facilitates transport of
draw solutes, either with or without carriers within the liquid. In accordance
with one or more
embodiments, the selective material area 536 may not be a liquid, but rather a
gel or a solid, or
comprise most or all of the membrane, rather than being contained within
another material.
Generally, selective membranes may be used for draw solute recovery, for
example, a suspended
liquid membrane may be used for the recycling of draw solution, where the
liquid, gel, polymer
or other material may be largely impermeable to water, but permeable to NH3
and CO2. In other
embodiments, it may contain "carriers" that transport NH3 and/or CO2. One
example would be a
non-polar liquid that allows the permeation of CO2, but not water or water
vapor, and contains
within it a carrier molecule for NH3.
[0099] FIG. 12 depicts the use of a selective membrane 624 to facilitate
simultaneous
stripping and absorption of draw solutes in accordance with one or more
embodiments of the
invention. On one side of the membrane 624, a dilute draw solution stream 620a
may be heated
to thermolytically split, for example ammonium salts, and increase the vapor
pressure of NH3
and CO2. On the other side of the membrane 624, a dilute draw solution 620b is
cooled such that
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
NH3 and CO2 gases 626 are absorbed within it and form ammonium salts. In this
way, a dilute
draw solution may be split into two streams 620a, 620b, one of which is
stripped to become
product water 632, and the other of which is increased in concentration to
become the
concentrated draw solution for reuse 616.
[00100] In accordance with one or more embodiments, a carrier-mediated,
suspended
liquid membrane contactor of this type would substantially decrease the amount
of energy
required to recycle draw solutes, as no water would be transferred as vapor as
part of the
separation, saving the enthalpy of vaporization of the water component of the
heat duty used by
conventional distillation. More broadly, this approach may apply to the
recycling of any
thermally separable draw solute from, for example, FO, DO, DOC, PRO, OGS
(osmotic grid
storage), or similar osmotically driven membrane systems, such that the
suspended barrier would
be relatively impermeable to water and would either transport the draw
solutes, or contain
carriers for the draw solutes, or some combination of the two.
[00101] FIG. 13 depicts a portion of another embodiment of a draw solute
recovery
system 722 for use with an osmotically driven membrane process/system 710. As
shown, a first
portion of dilute draw solution 720a may be directed from chamber 711 of the
system 710 to a
distillation column 750 and a second portion of dilute draw solution 720b may
be directed from
chamber 711 to an absorber module 727. A stream 729 exiting the distillation
column 750 may
be introduced to the absorber module 727, where it is mixed with dilute draw
solution 720b for
return back to chamber 711 so as to reintroduce draw solutes to the draw side
of a forward
osmosis membrane 712.
[00102] In some embodiments, the solute recovery systems use multi-stage
solute
recovery systems, for example, multi-stage column distillation or membrane
distillation. In
51
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
multi-stage column distillation, two or more columns may receive parallel
streams of dilute draw
solution, each stream being treated entirely to produce product water with the
desired
concentration of remaining draw solutes, for example, less than about lppm
NH3. The heat may
flow between the columns in series, however, the heat consumed by the process
is directed to the
reboiler of the column with the highest temperature and pressure and the tops
vapor from this
column is condensed fully or partially on the external side of the heat
transfer surfaces of the
reboiler of the next column down in the temperature and pressure series. This
condensation may
deliver the necessary heat for the separation, fully or in part, of the second
column, with this
being repeated for each column in the series, until the heat rejected from
cooling the tops vapor
of the column with the lowest temperature and pressure is rejected to a
cooling stream. The
number of stages that may be used between a top and bottom temperature is
generally set by the
differences between condensation temperatures and reboiler temperatures of
columns proximate
to one another in the series, which is related to the composition of the
dilute draw solution. The
desired delta T of the heat exchange equipment is also an important factor.
The foregoing
description is similarly applicable to multi-stage membrane distillation.
[00103] FIG. 14 depicts a solute recovery system 822 that uses a multi-
stage solute
recovery process with both energy and material flows in series in accordance
with one or more
embodiments of the invention. In particular, FIG. 14 depicts a multi-stage
solute recovery
process that uses multi-stage column distillation; however, multi-stage
membrane distillation is
also contemplated and considered within the scope of the invention. As shown
in FIG. 14, the
material (e.g., dilute draw solution 820 from an osmotically driven membrane
process) and
energy (e.g., steam 830) streams may both flow in series. The dilute draw
solution 820 is
introduced to a first column 850a via inlet 801a, as the thermal energy 830 is
introduced via a
52
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
second inlet 802a, at a relatively higher temperature and pressure to reduce
the concentration of
the feed stream 820. The vapor 830' from the first column 850a exits via
outlet 803a and may be
used to provide heat to the next column 750b, which receives the first
column's bottoms product
820' as its feed (via outlet 804a and inlet 801b), which is at a lower
temperature and pressure.
The heated vapor 830' can be introduced to the second column 850b via heat
transfer means
(e.g., a reboiler) 842 and inlet 805, outlet 806, and second inlet 802b. The
condensed vapor is
outputted as a concentrated draw solution 816 via outlet 807, while water 832
is outputted from
the last column 850b via outlet 804b. This may be repeated in any number of
columns until the
desired bottoms composition is achieved.
[00104] This may effectively create a number of heat effects similar to
embodiments
disclosed in U.S. Patent Application Publication No. 2009/0297431 to McGinnis,
the disclosure
of which is hereby incorporated by reference herein in its entirety, where the
material feeds are
in parallel and the energy streams are in series. This method could be used
with membrane
contactors operating at different pressures, in a compact and efficient
arrangement. This
alternative method may be preferable for higher concentration feed streams
and/or higher
temperature heat sources.
[00105] In accordance with additional embodiments, the multi-stage solute
recovery
process may be assisted by a heat pump, offering significant energy efficiency
improvements in
environments where heat sources above, for example, 20 C above ambient
temperature are used.
In some embodiments, a heat pump may be used when fuel combustion, higher
temperature heat
sources, or a back pressure turbine is used to provide heat to the draw solute
recovery process.
[00106] In accordance with one or more embodiments, a heat pump, such as a
thermocompressor (also known as an ejector jet), mechanical compressor (also
known as a
53
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335
PCT/US2011/052565
blower), absorption heat pump, closed-cycle heat pump, refrigeration style
heat pump or other
analogous unit may be used on one or more of the vapor streams from the
columns (or
membrane modules) to raise the temperature at which they partially or fully
condense. By doing
this, the temperature differences between stages may be reduced, allowing more
stages within a
given difference in temperature, or a given number of stages at lower top
pressures and
temperatures and/or higher bottom temperatures and pressures. This may be used
to increase
energy efficiency in the first case, or capital efficiency in the second case,
or some combination
of the two.
[00107] FIG.
15 presents a schematic of one example of heat pump assisted multi-stage
solute recovery in which work is done on a tops stream with a closed or open
cycle heat pump to
allow for the pressures of each column (or membrane module) to be closer
together, allowing for
more stages and greater overall efficiency. As shown in FIG. 15, the recovery
system 822,
which is similar to that described with respect to FIG. 14, includes two or
more distillation
columns 850, where the material streams (dilute draw solution 820) are
introduced (via inlets
801) to the columns 850 in parallel. A source of thermal energy (steam 830) is
introduced (via
inlet 802a) to the first column 850a at a relatively higher temperature and
pressure to reduce the
concentration of the feed stream 820a. The vapor 830' from the first column
850a is directed to
the heat pump 840 (via outlet 803a), where the temperature thereof is raised,
and then forwarded
to the next column 850b to reduce the concentration of the second feed stream
820b. In one or
more embodiments, the heated vapor 830' can be introduced via heat transfer
means (e.g., a
reboiler) 842, as previously described with respect to FIG. 14. The condensed
vapor can be
outputted as a concentrated draw solution 816 or otherwise recycled to an
osmotically driven
membrane system. Each column 850 outputs water 832 for use as is or further
processing
54
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
depending on the quality of the water 832 outputted.
[00108] In another embodiment, heat pumps of the type described above may
be used with
a single column (or membrane module) to fully take advantage of heat pump
thermal efficiencies
in the reduction of latent heat generation, in a compact and capital efficient
implementation. In
at least one embodiment, a heat pump may be used in the solute recovery system
to reduce the
energy required by the process by recycling latent heat. This may be
implemented in single
column/module heat pump embodiments as well.
[00109] Additional techniques for reducing the delta T required in the
solute recovery and
recycling system may be implemented. For example, the draw solution gases may
be
compressed to allow them to form the concentrated solution at a lower delta T.
The desired delta
T for any system might also be achieved by integrating either mechanical or
absorption heat
pumps.
[00110] FIG. 16 depicts one embodiment of a solute recovery system 922 that
uses
column distillation with a semi-open or semi-closed heat pump configuration,
where a portion of
the product water 932 of a column 950, with or without a reboiler, may be
directed wholly or in
part to a reducing valve 956, lowering its pressure, such that the water 932
may be partially or
fully vaporized by heat transfer in a heat exchanger 938 from the condensing
of the tops vapor
930', with or without mixed absorbent. This steam 930" produced by
vaporization of product
water may then be compressed by mechanical or thermojet means 952 to the point
where it may
be used directly as a feed to the bottom of the column 950 (or one of the
previously described
membrane contactor devices) for the stripping of draw solutes. For example, a
column 950
operating at 230 ton may produce a tops vapor 930' that condenses on one side
of a heat
exchanger 938 at approximately 35-50 C, causing water 932 on the opposite
side of the heat
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
exchanger 938, which has been reduced in pressure to approximately 75-90 torr,
to be turned
partially to steam 930". This steam 930" may then be compressed and raised in
pressure to 230
ton and injected directly into the bottom of the column or membrane module.
This arrangement
can be referred to as a semi-open or semi-closed heat pump configuration.
[00111] Other combinations of thermal and/or mechanical heat pumps may be
employed
in conjunction with multiple distillation column staging, either conventional
or membrane based,
so as to best balance the desire to increase the number of stages while
minimizing equipment
costs. These may include, for example, a thermocompressor on one or more
columns, a
mechanical compressor on one or more other columns, and other heat pump types
on other
columns, as needed, as well as integration between heat streams as might
benefit an absorption
heat pump implementation where heat absorbed at a low temperature may deliver
a smaller
quantity of heat at a higher temperature. Thermodynamic advantages of
thermally regenerated,
osmotically driven membrane systems over conventional systems, such as
membrane distillation
or mechanical vapor recompression, which may require the phase change of all
water produced,
may be realized to a fuller extent.
[00112] FIG. 17 depicts an alternative embodiment, similar to FIG. 16,
where a
thermocompressor 954 may be used such that a portion of the product water 932
may be turned
into steam directly (via boiler 942) and introduced as a relatively high
pressure stream 930 used
to upgrade the pressure of the vapor stream 930' from one or more of the
columns 950, thereby
increasing its condensation temperature. In one embodiment, the system 922
utilizes an optional
heat exchanger 938 and valve 956 arrangement, similar to that described with
respect to FIG. 16,
to produce a vapor stream 930" for introduction to the thermocompressor 954.
In this case, a net
reduction in the specific heat duty may be achieved, but a lower quantity of
product water may
56
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335
PCT/US2011/052565
be produced. By way of non-limiting example, a 2-3 stage system (one of the
stages producing a
smaller portion of the separation) requiring 381 MJ/m3 of product water, may
use a
thermocompressor to reduce this duty to potentially as low as 200-250 MJ/m3,
by allowing a 4-5
stage design.
[00113] In
another alternative embodiment, a mechanical compressor may be used, such
that electricity or shaft work is used to compress the vapor from the top of
one or more columns,
thereby increasing the temperature at which this stream condenses, allowing
more stages to be
used. In this case, no reduction in product water quantity would be required,
but electrical
energy would be needed to supplant a portion of the heat required. By way of a
non-limiting
example, a 2-3 stage system requiring 381 MJ/m3 of product water may use
mechanical
compression to reduce this duty to potentially as low as 150-200 MJ/m3, by
allowing a 4-5 stage
design. An additional amount of electrical energy would also be required,
perhaps as much as
12.8 kW for a COP (coefficient of performance, a typical measure of heat pump
efficiency) of 5.
Further substitution of electrical energy for thermal energy is contemplated
and considered
within the scope of the invention.
[00114] In
accordance with one or more embodiments, as illustrated in FIG. 18, vacuum
distillation and/or gas absorption may be integrated with the disclosed
osmotically driven
membrane systems. An eductor 1058, driven by a high pressure water solution
(as may be
assisted by a pump 1060), may be used to draw a vacuum on a distillation
column 1050. The
gas-water mixture exiting the eductor 1058 may flow through a static mixer
1062 to assure that
the gases dissolve in the water. The water solution may then flow to a gas-
liquid separator 1064,
where the gas can be captured and recycled and a portion of the solution 1066
is recycled to
drive the eductor.
57
SUBSTITUTE SHEET (RULE 26)
CA 02811925 2013-03-20
WO 2012/040335 PCT/US2011/052565
[00115] Having now described some illustrative embodiments of the
invention, it should
be apparent to those skilled in the art that the foregoing is merely
illustrative and not limiting,
having been presented by way of example only. Numerous modifications and other
embodiments are within the scope of one of ordinary skill in the art and are
contemplated as
falling within the scope of the invention. In particular, although many of the
examples presented
herein involve specific combinations of method acts or system elements, it
should be understood
that those acts and those elements may be combined in other ways to accomplish
the same
objectives.
[00116] Moreover, it should also be appreciated that the invention is
directed to each
feature, system, subsystem, or technique described herein and any combination
of two or more
features, systems, subsystems, or techniques described herein and any
combination of two or
more features, systems, subsystems, and/or methods, if such features, systems,
subsystems, and
techniques are not mutually inconsistent, is considered to be within the scope
of the invention as
embodied in any claims. Further, acts, elements, and features discussed only
in connection with
one embodiment are not intended to be excluded from a similar role in other
embodiments.
[00117] Furthermore, those skilled in the art should appreciate that the
parameters and
configurations described herein are exemplary and that actual parameters
and/or configurations
will depend on the specific application in which the systems and techniques of
the invention are
used. Those skilled in the art should also recognize or be able to ascertain,
using no more than
routine experimentation, equivalents to the specific embodiments of the
invention. It is,
therefore, to be understood that the embodiments described herein are
presented by way of
example only and that, within the scope of any appended claims and equivalents
thereto; the
invention may be practiced otherwise than as specifically described.
58
SUBSTITUTE SHEET (RULE 26)