Note: Descriptions are shown in the official language in which they were submitted.
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
METHOD AND APPARATUS FOR TRANSDERMAL STIMULATION OVER
THE PALMAR AND PLANTAR SURFACES
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] The present application claims benefit of priority to U.S.
Provisional
Patent Application Serial No. 61/403,680 filed September 20, 2010, which is
incorporated by reference herein in its entirety for all purposes. The
following
applications are also incorporated herein by reference in their entirety for
all purposes:
PCT Application Serial No. PCT/US10/54167 filed October 26, 2010; PCT
Application
Serial No. PCT/US10/054353 filed October 27, 2010; U.S. Patent Application
Serial No.
12/508,529 filed July 23, 2009, which is a continuation in part of U.S. Patent
Application
Serial No. 11/866,329 filed October 2, 2007, which claims priority to U.S.
Provisional
Patent Application Serial No. 60/848,720 filed October 2, 2006; U.S. Patent
Application
Serial No. 12/695,087 filed January 27, 2010, which is a continuation of U.S.
Patent
Application Serial No. 11/332,797 filed January 17, 2006; U.S. Patent
Application Serial
Nos. 12/509,362 filed July 24, 2009; 12/469,365 filed May 20, 2009 which is a
continuation of U.S. Patent Application Serial No. 11/866,329 filed October 2,
2007
which claims priority to U.S. Provisional Patent Application Serial No.
60/848,720 filed
October 2, 2006, and 12/469,625 filed May 20, 2009 which is a continuation of
U.S.
Patent Application Serial No. 11/866,329 filed October 2, 2007 which claims
priority to
U.S. Provisional Patent Application Serial No. 60/848,720 filed October 2,
2006; and
12/509,304 filed July 24, 2009 which is a continuation of U.S. Patent
Application Serial
No. 12/508,529 filed July 23, 2009 which is a continuation-in-part of U.S.
Patent
Application Serial No. 11/866,329 filed October 2, 2007 which claims priority
to U.S.
Provisional Patent Application Serial No. 60/848,720 filed October 2, 2006;
and
12/509,345 filed July 24, 2009 which is a continuation of U.S. Patent
Application Serial
No. 12/508,529 filed July 23, 2009 which is a continuation-in-part of U.S.
Patent
Application Serial No. 11/866,329 filed October 2, 2007 which claims priority
to U.S.
Provisional Patent Application Serial No. 60/848,720 filed October 2, 2006.
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
FIELD OF THE INVENTION
100021 The present apparatus and methods relate generally to energy
emitting
apparatus and methods for providing a medical therapy. The apparatus and
methods may
provide for central and peripheral nerve and other tissue modulation or
stimulation
therapies
BACKGROUND
[0003] The OAB and UI market in the United States is well over a $12
billion a
year industry. It affects over 16% of all Americans, for a total U.S. market
of
approximately 34 million men and women each year. Due to social stigmas
attached to
OAB and UI, as well as misunderstanding of the signs and symptoms associated
with
OAB and UI, only 40% of those affected (13.6M) seek treatment. Of those 13.6
million
individuals, nearly 30% are unsatisfied with their current therapy.
[0004] The use of pulsed electromagnetic stimulation (PES) has been
well
established as a beneficial therapy in a variety of medical applications. The
scientific
principle behind this technology is that an electric current passed through a
coil will
generate an electromagnetic field. These fields, in turn, have been shown to
induce
current within conductive materials placed within the field. When applied to
the human
body, pulsed electromagnetic stimulation has been found to be an effective
method of
stimulating nerves resting within the electromagnetic field. Recent data
highlights the
beneficial effects of invasive, needle-based electrostimulation (ES) of the
posterior tibial
nerve in individuals with OAB and UI. ES has been found to modulate bladder
dysfunction through its action on the pudendal nerve and the sacral plexus
which
provides the major excitatory input to the bladder.
[0005] Current treatment options for OAB and UI are exercise and behavioral
modifications, pharmacological therapies, surgical intervention, and
neuromodulation.
Although each of these treatment options targets the UI and OAB populations,
each has
severe limitations.
[0006] Exercise and behavioral modifications often require patients
to adhere to
stringent routines, including scheduled voiding, maintenance of a bladder
diary, and
intense exercise regiments. While this may be a viable option for a small
group of highly
dedicated individuals, its daily impact on one's life makes it an unattractive
option for
most individuals.
2
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
[0007] Pharmacological intervention is the most widely prescribed
therapy for
OAB and UI. Unfortunately, as with the ingestion of any chemical, patients are
often
subject to side effects from their drug therapy. This is especially
detrimental in older and
elderly patient populations where interaction with other prescribed
medications can have
adverse effects. Further, there is a high rate of dissatisfaction,
approximately 30%,
amongst individuals using pharmacological treatment.
[0008] Surgical intervention is an extremely invasive treatment and
often results
in the long-term, and in some cases permanent, requirement for
catheterization. The high
expense of these procedures, coupled with the negative impact the procedures
have on
the patients quality of life, make this an option only when all other
treatment options
have been exhausted.
[0009] Neuromodulation is another treatment alternative for OAB and
UI
patients. Sacral nerve stimulation (SNS) has shown itself to be an effective
treatment
option for those with OAB or UI. However, the procedure requires the permanent
implantation of an electrical stimulation device in the patient. One estimate
puts the cost
at nearly $14,000 with additional routine care costs of $593 per patient per
year.
Additionally, SNS's risk of battery failure, implant infection, and electrode
migration,
lead to a high reoperation rate and make this procedure unattractive.
[0010] More recently, the introduction of a posterior tibial nerve
stimulator, often
referred to as SANS, has shown itself to be another neuromodulation
alternative. Yet as
is the case with other forms of neuromodulation, this system is invasive in
its nature. It
requires the insertion of a needle two inches into the patient's ankle region
in order to
stimulate the posterior tibial nerve. As well, it requires a minimum of 12
sessions for
initial treatment, with the possibility of additional sessions needed for
maintenance.
Despite its high cost and invasive nature, though, an abundance of published
peer-
reviewed clinical trials demonstrate the safety and efficacy of the SANS
therapy.
SUMMARY
[0011] In certain variations, a method for providing transdermal
electrical
stimulation therapy to a patient is provided. The method may include
positioning a
stimulator electrode over a glabrous skin surface overlying a target nerve of
a subject.
Electrical stimulation may be delivered through or across the glabrous skin
surface to the
3
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
target nerve to stimulate the target nerve, while remaining safe and tolerable
to the
patient. Electrical stimulation may be delivered at frequencies that may be
painful or
intolerable when applied over non-glabrous surfaces of the body. The
electrical
stimulation may be utilized to treat various conditions, e.g., urinary
incontinence and
overactive bladder.
100121 In certain variations, an applicator, e.g., an ergonomic
applicator, for
providing transdermal electrical stimulation therapy to a patient is provided.
The
applicator may be configured to position a stimulator electrode over a
glabrous skin
surface of the subject to deliver transdermal electrical stimulation through
or across the
glabrous skin surface to an underlying target nerve, resulting in stimulation
of the target
nerve.
100131 In certain variations, a method for providing an energy based
stimulation
therapy to a subject is provided. The method may include positioning an energy
emitting
device in proximity to a glabrous surface overlying a target tissue. Energy
may be
delivered through the glabrous skin surface to the target tissue to stimulate
the target
tissue.
100141 In certain variations, another method for providing an energy
based
stimulation therapy to a subject is proved. The method may include positioning
an
energy emitting device in proximity to a skin surface overlying a target
nerve. Energy
may be delivered at a frequency of about 1 Hz to about 30 Hz through the skin
surface to
the target nerve, thereby generating motor and/or sensory nerve conduction of
the target
nerve while remaining safe and tolerable to the subject. Optionally, energy
may be
delivered at less than 10 Hz to generate nerve conduction.
100151 In certain variations, systems for electromagnetic induction
therapy may
include one or more conductive coils disposed within or along an applicator.
The coils
may be configured to generate a magnetic field focused on a target nerve,
muscle or
other body tissues in proximity to the coil. One or more sensors may be
utilized to detect
electrical conduction in the target nerve, to detect a muscular response
caused by an
electrical conduction in the target nerve, or to detect stimulation of a
nerve, muscle or
other body tissues and to provide feedback about the efficacy of the applied
electromagnetic induction therapy. A controller in communication with the
sensor may
be adjustable to vary a current through the at least one coil so as to adjust
the magnetic
field focused upon the target nerve, muscle or other body tissues. Optionally,
a user or
4
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
patient may detect stimulation of a nerve, muscle or body tissue and the
therapy may be
adjusted based on feedback from the user or patient.
[0016] In certain variations, the applicator may be configured to
intermittently
apply or deliver pulsed magnetic fields to a target nerve, muscle or tissue
without
causing habituation of the target nerve, muscle or tissue.
[0017] In certain variations, methods of electromagnetic induction
therapy may
include one or more of the following steps. A first portion of a patient's
body may be
positioned relative to or in proximity to an applicator or an applicator may
be positioned
relative to or in proximity to a first portion of a patient's body, such that
a target nerve,
muscle or tissue within the first portion of the body is in proximity to one
or more
conductive coils disposed within or along the applicator. A current may be
passed
through a coil to generate a magnetic field focused on the target nerve,
muscle or tissue.
An electrical conduction through the target nerve, a muscular response caused
by an
electrical conduction through the target nerve or stimulation of a nerve,
muscle, or body
tissue may be detected by a sensor positioned along a second portion of the
body. A
signal from the sensor indicative of the electrical conduction or stimulation
may be
received, which provides feedback about the efficacy of the applied
electromagnetic
induction therapy. The current may be adjusted by a controller in
communication with
the conductive coils based on the feedback.
[0018] Optionally, a user may detect stimulation of a nerve, muscle or body
tissue and the therapy may be adjusted based on feedback from the user. In
certain
variations, pulsed magnetic fields may be intermittently applied or delivered
a target
nerve, muscle or tissue without causing habituation of the target nerve,
muscle or tissue.
Such intermittent magnetic fields may be used to treat chronic conditions,
e.g., chronic
pain, without causing habituation.
[0019] In certain variations, applicators may be ergonomic or may be
designed or
configured to accommodate, approximate or be positioned relative to or in
proximity to
specific regions of the body or anatomy. The specific regions of the body or
anatomy
may be positioned relative to the applicators, or the applicators may be
positioned
relative to the specific regions of the body or anatomy to treat various
conditions, for
example, osteoarthritis, arthritis, back or neck pain, atrophy or paralysis,
chronic pain,
phantom or neuropathic pain, neuralgia, migraines, orthopedic conditions.
5
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
[0020] Other features and advantages will appear hereinafter. The
features and
elements described herein can be used separately or together, or in various
combinations
of one or more of them.
BRIEF DESCRIPTION OF THE DRAWINGS
[0021] The drawings constitute a part of this specification and
include exemplary
embodiments of the invention, which may be embodied in various forms. It is to
be
understood that in some instances various aspects of the embodiments may be
shown
exaggerated or enlarged to facilitate an understanding of the embodiments.
[0022] FIG. 1 is a schematic view of an apparatus for magnetic induction
therapy
according to a first variation.
[0023] FIG. 2 is a schematic view of an apparatus for magnetic
induction therapy
according to a second variation.
[0024] FIG. 3 is a schematic view of an apparatus for magnetic
induction therapy
according to a third variation.
[0025] FIG. 4 is a schematic view of an apparatus for magnetic
induction therapy
according to a fourth variation.
[0026] FIG. 5 is a schematic view of an apparatus for magnetic
induction therapy
according to a fifth variation.
[0027] FIGS. 6A-6D are schematic illustrations depicting a first method of
use of
an apparatus for magnetic induction therapy. This method is based on adjusting
the
position of the conductive coils so to optimize a magnetic flow applied to a
target nerve.
[0028] FIGS. 7A-7D are schematic illustrations of a second method of
use of an
apparatus for magnetic induction therapy. This method is based on locking the
conductive coils in position once electrical conduction in a target nerve has
been
detected.
[0029] FIG. 8 is a schematic view of a variation that includes a
plurality of
sensors.
[0030] FIGS. 9A-9D are schematic representations of different
garments adapted
to operate as apparatus for magnetic induction therapy.
[0031] FIG. 10 is a schematic view of an apparatus for providing
electrical
stimulation.
[0032] FIG. 11 is a schematic view of another variation of an
apparatus for
providing electrical stimulation.
6
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
[0033] FIG. 12 shows a schematic view of an energy emitting system
including a
microneedle patch sensor.
[0034] FIG. 13-15 shows magnified bottom views of variations of
microneedle
patches.
[0035] FIGS. 16-17 shows magnified side views of variations of a
microneedle
patch.
[0036] FIG. 18 shows a magnified bottom perspective view of a
microneedle
patch.
[0037] FIG. 19 shows a representative cross sectional view of the
skin composed
of an outer stratum corneum covering the epidermal and dermal layers of skin
and the
underlying subcutaneous tissue, with a variation of a microneedle patch
attached thereto.
[0038] FIG. 20a shows a magnified side view of a variation of a
microneedle
patch including multiple electrodes.
[0039] FIG. 20b-20D show variations of a microneedle patches
including
multiple electrodes.
[0040] FIG. 21 shows a schematic view of an energy emitting system
including a
microneedle patch sensor placed behind a subject's knee.
[0041] FIGS. 22-23 show schematic views of energy emitting systems
including
an electrode needle and sensor.
[0042] FIGS. 24-25 show schematic views of energy emitting systems
including
an electrode needle without a sensor.
[0043] FIG. 26 shows a schematic view of an energy emitting system
including a
microneedle patch for providing stimulation.
[0044] FIGS. 27-28 show schematic views of energy emitting systems
including
an electrode needle and microneedle patch for providing stimulation.
[0045] FIG. 29a-29d show a prospective, side, top and rear views of
an energy
emitting device in the form of a foot cradle.
[0046] FIGS 30a-30b show schematic views of an energy emitting device
in the
form of a knee support.
[0047] FIGs. 31a-3 lb shows a schematic view of a variation of an arm
applicator
and a foot, knee or leg applicator.
[0048] FIG. 32 shows a schematic view of a variation of a back
applicator.
[0049] FIG. 33 shows a schematic view of a variation of a system
including a
back applicator, a sensor and logic controller.
7
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
[0050] FIG. 34 shows a schematic view of system including multiple
back
applicators, a sensor and logic controller.
[0051] FIG. 35 shows a schematic view of a variation of a system
including a
back applicator held on a patient's body by an ergonomic positioning element
in the
form of a belt and a logic controller.
[0052] FIG. 36 shows a schematic view of a variation of an applicator
designed
to stimulate a nerve responsible for phantom or neuropathic pain.
[0053] FIG. 37 shows a schematic view of a variation of a facial
neuralgia
applicator.
[0054] FIG. 38 shows a schematic view of a variation of an applicator which
may
be placed over the occipital nerve for the treatment of migraines.
[0055] FIG. 39 shows a schematic view of a variation of an applicator
which may
be placed over the frontal cortex for the treatment of depression.
[0056] FIG. 40 shows a schematic view of a variation an applicator in
the form of
a stimulator coil platform for positioning one or more coils in proximity to a
knee or
popliteal nerve.
[0057] FIG. 41 shows a schematic view of a system including a
variation of a
back applicator held on a patient's body by an ergonomic positioning element
in the
form of a shoulder harness, a sensor, and a logic controller.
[0058] Figures 42A and 42B show an example of how the amount of stimulator
power required to achieve a desired stimulus may be automatically adjusted as
a result of
fibroses.
[0059] Figures 43A and 43B show variations of a coil device
positioned on a
skull.
[0060] Figure 44 shows a view of the underside or glabrous surface of the
foot
and exemplary sites for delivering electrical stimulation.
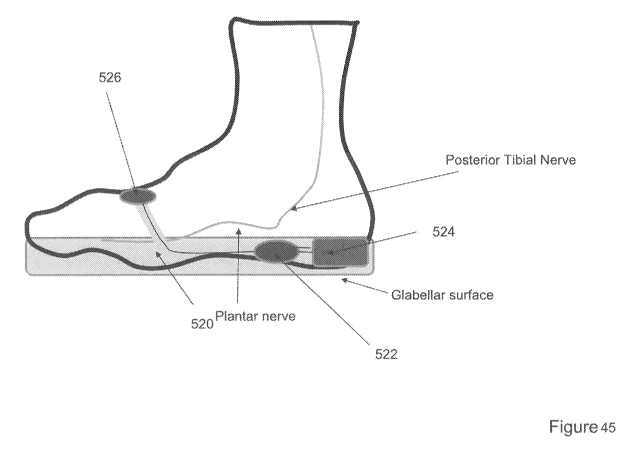
[0061] Figure 45 shows a perspective view of one variation of an
insole for
delivering electrical stimulation over a glabrous surface of the foot.
[0062] Figure 46 shows a perspective view of a variation of an insole
for
delivering electrical stimulation over a glabrous surface of the foot,
including a sensor
feedback feature.
[0063] Figure 47 shows a perspective view of one variation of
electrodes for
delivering electrical stimulation over a glabrous surface of a foot.
8
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
[0064] Figure 48 shows a perspective view of one variation of a hand
applicator
for delivering electrical stimulation over a glabrous surface of a hand.
[0065] Figure 49 shows a perspective view of one variation of
electrodes for
delivering electrical stimulation over a glabrous surface of a hand.
DETAILED DESCRIPTION
[0066] In certain variations, various apparatus and methods for
providing
magnetic induction therapy or electrical stimulation therapy are provided. In
certain
variations, various apparatus and methods may provide for central and
peripheral nerve
and other tissue modulation or stimulation therapies, including both
excitation and
blocking of nerve impulses. In certain variations, a low frequency induction
therapy may
be performed. In certain variations, these apparatus and methods may be useful
in the
treatment and prevention of urinary incontinence (UI), overactive bladder
(OAB) and
other conditions.
[0067] In certain variations, apparatus and methods for magnetic induction
therapy, in which dosage of magnetic energy can be regulated according to
conduction in
a target nerve exposed to the magnetic field are provided.
[0068] In certain variations, apparatus and methods for magnetic
induction
therapy, in which the flow of magnetic energy can be adjusted directionally by
the
patient or a healthcare provider without altering the position of a housing
containing
conductive coils that produce the magnetic field are provided.
[0069] In certain variations, apparatus and methods for treating a
variety of
ailments by providing energy to a target nerve, for example magnetic energy,
electrical
energy or ultrasound energy, at a location and in an amount optimized by
detecting
conduction in the target nerve are provided.
[0070] In certain variations, an energy emitting apparatus for
delivering a
medical therapy that includes one or more energy generators, a logic
controller
electrically connected to the one or more energy generators, and one or more
sensors for
detecting electric conduction in a target nerve, which are connected to the
logic
controller is provided. The one or more energy generators produce energy
focused on the
target nerve upon receiving a signal from the logic controller, and the
applied energy is
varied by the logic controller according to an input provided by the one or
more sensors
based on electric conduction in the target nerve. The feedback provided by the
sensors to
9
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
the logic controller about the efficacy of the applied treatment causes the
logic controller
to modulate the current transmitted to the coils.
100711 The applied energy may be a magnetic field, an electrical
field, an
ultrasound, a visible light, or an infrared or an ultraviolet energy. When a
magnetic field
is applied, the energy-emitting device is an apparatus that provides a
magnetic induction
therapy and that includes one or more conductive coils disposed in an
ergonomic
housing. A logic controller is electrically connected to the one or more
coils, and one or
more sensors detect electric conduction in the target nerve and are connected
to the logic
controller so to provide a feedback to the logic controller. The conductive
coils receive
an electric current from the logic controller and produce a magnetic field
focused on a
target nerve, and the electric current fed by the logic controller is varied
by the logic
controller according to an input provided by the sensors, thereby causing
amplitude,
frequency or direction of the magnetic field, or the firing sequence of the
one or more
coils, to be varied according to the efficiency of the treatment provided to
the target
nerve. In certain variations, the housing containing the conductive coils may
be a flexible
wrap, a cradle or a garment, and the coils may be overlapping and/or be
disposed in
different positions within the housing, so to generate a magnetic field on
different body
parts with the desired direction and amplitude.
[0072] The one or more coils may be stationary or movable within the
housing,
making it possible to optimize the direction of magnetic flow to the target
nerve by
disposing the coils in the most effective direction. In different variations,
the coils may
be movable manually by acting on a knob, lever, or similar type of actuator,
or may be
translated automatically by the logic controller in response to the input
provided by the
sensors. When a preferred position for the coils has been established, the
coils may be
locked in position and maintain that position during successive therapy
sessions. In other
variations, the sensors may be incorporated within the housing, or instead may
be
disposed on a body part of interest independently of the housing.
[0073] In still other variations, the inductive coils are disposed in
a housing that
is situated externally to a patient's body, and additional inductive coils are
implanted into
the body of the patient and are magnetically coupled to the external inductive
coils. With
this coil arrangement, energy may be transmitted from the external coils to
the internal
coils either to recharge or to activate an implantable device. In yet other
variations, the
electric current may varied by the logic controller both on the basis of an
input provided
by the one or more sensors and also an input provided by the patient according
to a
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
muscular response she has perceived, for example, the twitching of a toe after
application
of the magnetic field.
100741 In yet other variations, the source of energy for nerve
stimulation may be
electrical energy and nerve conduction may be detected at a site sufficiently
distant from
the site of stimulation, so to enable detection of nerve conduction despite
any
interference from the direct electrical stimuli. In these variations, direct
electrical
stimulation of nerve and muscle may be tailored to provide optimal therapy
and, in the
case of electrode migration or other electrode malfunction, to report lack of
stimulation
of the bodily tissues. Furthermore, these variations enable a reduction in
power
requirement, because control of the signal is provided by the sensor to the
signal
generator loop.
[0075] In other variations, an energy emitting system for providing a
medical
therapy is provided. The system may include one or more conductive coils
disposed
within or along a housing and configured to generate a magnetic field focused
on a target
nerve in proximity to coils; one or more sensors in the form of microneedle
patch
configured to detect electrical conduction in the target nerve; and a
controller coupled to
the conductive coils and optionally in communication with the sensor.
[0076] In other variations, an energy emitting system for providing a
medical
therapy is provided. The system may include one or more microneedle patches
having
one or more microneedle arrays deposited on a surface of one or more
electrodes and
configured to generate or deliver an electrical or magnetic stimulus or field
focused on a
target nerve in proximity to the microneedle patch; one or more sensors
configured to
detect electrical conduction in the target nerve; and a controller coupled to
the
conductive coils and optionally in communication with the sensor. Optionally,
the above
variations may incorporate an electrode needle. Optionally, the above
variations or
systems may be utilized without a sensor or mechanism for detecting conduction
or
stimulation.
[0077] Methods of use of the above apparatus, systems and variations
thereof for
treating various conditions are also described herein.
[0078] Referring first to FIG. 1, a first variation includes a coil wrap
20, which is
depicted as disposed over ankle 22 circumferentially to surround a portion of
tibial nerve
24. Because tibial nerve 24 is targeted, this variation is particularly suited
for the
treatment of OAB and VI. In other variations, coil wrap 20 may be configured
to
surround other body parts that contain a portion of tibial nerve 24 or of
other nerves
11
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
branching from or connected to tibial nerve 24, still making these variations
suitable for
treating OAB and VI. In still other variations, coil wrap 20 may be configured
for
surrounding body parts that contain other nerves when treatments of other
ailments are
intended.
[0079] Coil wrap 20 may be manufactured from a variety of materials
suitable
for wearing over ankle 22. Preferably, coil wrap is produced from a soft, body-
compatible material, natural or synthetic, for example, cotton, wool,
polyester, rayon,
Gore-Tex , or other fibers or materials known to a person skilled in the art
as non-
irritating and preferably breathable when tailored into a garment. Coil wrap
22 may even
be manufactured from a molded or cast synthetic material, such as a urethane
gel, to add
extra comfort to the patient by providing a soft and drapable feel.
Additionally, coil wrap
may be produced from a single layer of material or from multiple material
layers and
may include padding or other filling between the layers.
[0080] Coil wrap 20 contains one or more conductive coils 26 arranged
to
15 produce a pulsed magnetic field that will flow across tibial nerve 24
and generate a
current that will flow along tibial nerve 24 and spread along the length of
tibial nerve 24
all the way to its sacral or pudendal nerve root origins. Coils 26 may be a
single coil
shaped in a simple helical pattern or as a figure eight coil, a four leaf
clover coil, a
Helmholtz coil, a modified Helmholtz coil, or may be shaped as a combination
of the
20 aforementioned coils patterns. Additionally, other coil designs beyond
those mentioned
hereinabove might be utilized as long as a magnetic field is developed that
will
encompass tibial nerve 24 or any other target nerve. When a plurality of coils
is utilized,
such coils may be disposed on a single side of ankle 22, or may be disposed on
more
than one side, for example, on opposing sides, strengthening and
directionalizing the
flow of the magnetic field through tibial nerve 24 or other peripheral nerves
of interest.
100811 Coil wrap 20 is preferably configured as an ergonomic wrap,
for example,
as an essentially cylindrical band that can be pulled over ankle 22, or as an
open band
that can be wrapped around ankle 22 and have its ends connected with a buckle,
a hoop
and loop system, or any other closing system known to a person skilled in the
art. By
properly adjusting the position of coil wrap 20 over ankle 22, a patient or a
health care
provider may optimize the flow of the magnetic field through tibial nerve 24,
based on
system feedback or on sensory perceptions of the patient, as described in
greater detail
below.
12
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
[0082] The electric current that produces the magnetic field by
flowing through
coils 26 is supplied by a programmable logic controller 28, which is connected
to coils
26, for example, with a power cord 32. A sensor 30 that feeds information to
logic
controller 28 is also provided, in order to tailor the strength of the
magnetic field and
[0083] In this variation, as well as in the other variations described
hereinafter,
sensor 30 may include one or more sensor patches and may be placed at
different
distances from the region of direct exposure to the magnetic field. For
example, sensor
30 may be configured as a voltage or current detector in the form of an EKG
patch and
may be placed anywhere in the vicinity of the target nerve to detect its
activation. For
[0084] By virtue of the above described arrangement, coil wrap 20
provides a
reproducibly correct level of stimulation during an initial therapy session
and during
successive therapy sessions, because the presence or absence of nerve
conduction is
[0085] If the magnetic pulse does not substantially interfere with
sensor 30,
sensor 30 may be placed directly within the field of stimulation, so that
power supplied
to the system may be conserved. This is particularly important for battery-
powered
[0086] In a method of use of coil wrap 20, the amplitude and/or
firing sequence
of coils 26 may be ramped up progressively, so that the magnetic field is
increased in
strength and/or breadth until nerve conduction is detected, after which the
applied
13
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
stimulus is adjusted or maintained at its current level for the remainder of
the therapy.
The level of stimulation may be also controlled through a combination of
feedback from
sensor 30 and feedback based on perceptions of the patient. For example, the
patient may
activate a switch once she perceives an excessive stimulation, in particular,
an excessive
level of muscular stimulation. In one instance, the patient may be asked to
push a button
or turn a knob when she feels her toe twitching or when she experiences
paresthesia over
the sole of her foot. The patient will then continue pressing the button or
keep the knob
in the rotated position until she can no longer feel her toe twitching or
paresthesia in her
foot, indicating that that level of applied stimulation corresponds to an
optimal therapy
level. From that point on, the patient may be instructed to simply retain her
foot, knee, or
other limb within coil wrap 20 until therapy has been terminated while the
system is kept
at the optimal level. Adding patient input enables control of coil wrap 20
during
outpatient treatments, because the patient is now able to adjust the intensity
of the
magnetic field herself beyond the signals provided to logic controller 28 by
sensor 30.
[0087] Detecting and, if the case, measuring conduction in one or more
nerves
along the conduction pathways of the stimulated nerve confirms that the target
nerve has
been stimulated, providing an accurate assessment of the efficiency of the
applied
therapy on the patient. A concomitant detection of muscle contraction may also
confirm
that the target nerve is being stimulated and provide an indication to the
patient or to a
healthcare provider as to whether stimulation has been applied at an excessive
level in
view of the anatomical and physiological characteristics of the patient.
[0088] Based on the foregoing, coil wrap 20 allows for a consistent,
user-friendly
targeting and modulation of the peripheral nerves via the posterior tibial
nerve on an
outpatient basis, in particular, the targeting and modulation of the pudendal
nerve and of
the sacral plexus. When multiple coils 26 are present, coils 26 may be
activated
simultaneously or differentially to generate the desired magnetic field. The
direction and
location of each of coils 26 may be reversibly or irreversibly adjusted by the
healthcare
provider or by the patient, customizing the location of the applied
stimulation to the
anatomy and therapy needs of each patient. After a healthcare provider has
optimized
position and firing sequence for each of coils 26, the patient may be sent
home with coil
wrap 20 adjusted to consistently target the desired nerve. In one variant of
the present
variation, an automatic feedback system adjusts one or more of firing
sequence, firing
strength or position of coils 26 within coil wrap 20 during the initial setup
and also
during successive therapy sessions.
14
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
[0089] In summary, certain variations include the creation of a loop
consisting of
feeding information on nerve conduction to logic controller 28 and on logic
controller 28
tailoring the electrical current sent to coil wrap 20 according to the
information received
from sensor 26 based on whether or not the nerve is receiving the desired
stimulation
and, in some variations, the desired amount of stimulation. This arrangement
offers an
unparalleled level of therapy control and flexibility within a home care
setting, because a
consistent, repeatable stimulation of the target nerve can be attained. Aside
from
adjusting the position of coils 26 in accordance with the patient's anatomy
and
physiological variations, controlling pulse amplitude is also of great
importance even
during different therapy sessions with the same patient. For example, a
patient with leg
edema will encounter difficulties in properly adjusting coil wrap 20 based on
whether her
legs and ankles are swollen or not swollen, and the power required to
penetrate to
posterior tibial nerve 24 (in the case of a VI therapy) will vary greatly due
to the variable
depth of the nerve. Thus, having feedback provided by sensor 26 becomes a
necessity for
achieving an accurate dosage of the treatment rather than an option. Benchtop
testing has
demonstrated that a system constructed as described herein is capable of non-
invasively
generating electrical currents similar to those found in therapeutic electro-
stimulation and
to do so in different settings.
[0090] Referring now to FIG. 2, a second variation will be described
with
reference to a coil wrap 34 disposed over ankle 36 for the purpose of treating
VI by
targeting tibial nerve 38. In this second variation, one or more Helmholtz
coils 40 are
disposed within coil wrap 34 to create a more narrowly directed magnetic field
over
tibial nerve 38. Like in the all other variations described herein, more than
one coil (in
the present variation, more than one Helmholtz coil 40) may be placed within
coil wrap
34 and be disposed in different positions within coil wrap 34, in order to
optimize
magnetic flux over tibial nerve. For example, two Helmholtz coils may be
disposed one
opposite to the other within coil wrap 34.
[0091] Having coil windings arranged along a common longitudinal
axis, as
required in a Helmholtz coil configuration, generates a more focused magnetic
field and
a more accurate targeting of tibial nerve 38 or of any other nerve. Like in
the previous
variation, the operation of coils 40 is controlled by a logic controller 42,
which is in turn
connected to sensor 44 that monitors conduction in tibial nerve 44 and that
generates a
feedback to logic controller 42 about the efficiency of the therapy in
progress. Therefore,
like in the previous variation, the coupling of sensor 44 with logic
controller 42
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
optimizes operation of coil wrap 34 according to results measured at the level
of tibial
nerve 38. Also like in the previous variation, manual adjustments to the
parameters of
electric current provided by logic controller 42 to Helmholtz coil 40 may also
be made
manually by the patient or by a healthcare provider, and coil wrap 34 may be
structured
so that the position of Helmholtz coil 40 within coil wrap 34 is adjusted as
desired either
manually by the patient or by a healthcare provider, or automatically by logic
controller
42.
100921 Referring now to FIG. 3, a third variation includes a coil
wrap 46
configured for wrapping over the popliteal fossa of a patient, in the region
of the knee, to
stimulate the posterior tibial nerve (not shown). The configuration and
structure of coil
wrap 46 reflect the body portion covered by coil wrap 46, but the key system
components of coil wrap 46, such as the type, number and disposition of the
coils (for
example, the use of overlapping coils); the connections of the coils with a
logic
controller; and the use of one or more sensors (also not shown) to detect
nerve
conduction are all comparable to those in the previously described variations.
[0093] Referring now to FIG. 4, a fourth variation includes a
footrest or foot
cradle 48, which is structured to contain at least a portion of a foot 50. One
or more coils
52 are enclosed within cradle 48, and a sensor 54 is disposed along the
pathway of tibial
nerve 55, sensing conduction in tibial nerve 55, and is also connected to a
logic
controller 56. Coils 52, sensor 54 and logic controller 56 may be arranged in
different
configurations, in the same manner as in the preceding variations.
[0094] Cradle 48 may be made from a variety of materials and may be
monolithic, or have a hollow or semi-hollow structure to enable the movement
of coils
52 within cradle 48, as described in greater detail below. Preferably, cradle
48 has an
ergonomically design allowing the ankle and heel of the patient to be retained
within
cradle 48, in a position that matches the positions of stimulating coils 52 to
the area of
stimulation. The design of cradle 48 provides for a particularly comfortable
delivery of
therapy to patients that prefer to remain seated during their therapy, and
enables the
patient to perform the required therapy within a health care facility, or to
take cradle 48
home, typically after an initial session and appropriate training in a health
care facility. In
that event, the patient will be trained to apply sensor 54 autonomously and to
adjust
stimulation to a comfortable level.
[0095] FIG. 4 shows coils 52 disposed as overlapping and the use of a
single
sensor patch 54 positioned proximally to the stimulation site. However, coil
52 may be
16
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
configured as a single coil, a figure eight coil, a four leaf clover coil, a
Helmholtz coil, a
modified Helmholtz coil or a any combination of the aforementioned coils, or
as any
other coil design providing an effective stimulation to the target nerve. In
addition, coils
52 may be fired individually, sequentially or simultaneously according to the
feedback
provided by sensor 54.
[0096] In one variant of this variation, sensor 54 may include a
conductive
electrode patch that provides a feedback to logic controller 56 for adjusting,
if necessary,
the stimulation parameters of coils 52. Alternatively, sensor 54 may be a
sensor patch
that is either applied to the skin of the patient or is incorporated within
the structure of
cradle 48.
[0097] Referring now to FIG. 5, a fifth variation includes a knee
rest or knee
cradle 58 that contains one or more conductive coils 60, one or more sensors
62 and a
logic controller 64. The components of this variation are similar to those
described with
reference to the preceding variations, as regards the structure and materials
of cradle 58,
the nature and disposition of coils 60, the type and operation of sensor 62,
and the
function and operation of logic controller 64. Cradle 58 is configured to
target the
popliteal fossa of the patient, thus to target tibial nerve 66. In that
respect, the present
variation is similar to the variation illustrated in FIG. 3, but while the
variation of FIG. 3
is configured as a wrap that may be worn while the patient is standing, the
present
variation is configured as a cradle that is more suited for treatment while
the patient is
sitting or laying down.
[0098] A method of use of the foot cradle depicted in FIG. 4 is
described with
reference to FIGS. 6A-6D. During a first step illustrated in FIG. 6A, foot 68
is disposed
in cradle 70 that contains one or more conductive coils 72, which are
connected to a
logic controller (not shown) that manages the flow of electric power to coils
72.
[0099] During a second step illustrated in FIG. 6B, a sensor 74 is
disposed on
foot 68 or on ankle 76 or on another appropriate portion of the patient's
body, in order to
detect conductivity in tibial nerve 78 or in another target nerve.
[0100] During a third step illustrated in FIG. 6C, a healthcare provider
analyzes
conductivity measurements provided by sensor 74 (for example, by reading gauge
77)
and first adjusts the positioning of coils 72 until conduction in nerve 78 is
detected. For
example, the healthcare provider may rotate a knob 80, slide a lever or
actuate any other
displacement system for coils 72 that is known in the art, so that coils 72
are translated
until a magnetic field of the proper amplitude and intensity is applied to
cause
17
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
conduction in nerve 78. The position of coils 72 is then fine-tuned manually
until an
optimal level of conduction in nerve 78 is attained, and the therapy is
continued for a
length of time as prescribed by the attending healthcare provider.
[0101] During a fourth, optional step illustrated in FIG. 6D, settings
for successive
therapy sessions are set, for example by locking knob 80 (in one variation,
with a pin 81)
so that the healthcare provider or the patient repeat the therapy using the
predetermined
settings. Alternatively, the patient may be trained to adjust the amplitude
and/or strength
of the applied magnetic field, as each therapy session requires.
[0102] While the present method has been described with regard to foot
cradle 70,
the same method steps may be envisioned for coil wraps or cradles of different
configurations, for example, for the coil wraps and cradles described with
reference to
the previous figures.
[0103] In an alternative variation, the logic controller (not shown) may
automatically
adjust coil positioning to optimize therapy during the initial and successive
sessions.
While this set-up may be more difficult to implement, it also provides for an
accurate
targeting of the target nerve during each therapy session, regardless of
alterations in
patient positioning or changes to the anatomy of the patient (for example,
when a foot is
swollen). In this variation, the device simply varies the orientation of coils
84 until
stimulation has been sensed.
[0104] Further, coils 84 may be translated along a single direction (for
example,
horizontally) or along a plurality of directions, to provide for the most
accurate
positioning of coils 84 with respect to the target nerve.
[0105] A second method of use of the foot cradle depicted in FIG. 4 is
described now
with reference to FIG. 7. While this second method is also described with
reference to a
foot cradle 82 employing one or more coils 84 that have a reversibly lockable,
adjustable
orientation, the present method may be equally implemented with a body-worn
coil
wrap, such as those described with reference to the previous figures, or to
other
variations. In this method, the patient or the healthcare provider adjusts the
positioning of
coils 84 to detect conductivity in target nerve 89.
[0106] The position of coils 84 may be translated in different directions
(in the
illustrated variation, may be translated horizontally) and may be locked in an
initial
position once conduction in nerve 89 is detected by a sensor (for example,
sensing patch
86).
18
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
[0107] More particularly, FIG. 7A illustrates the initial positioning of
foot 88 into
cradle 82 and of sensor patch 86 on ankle 90 or other appropriate body part of
the
patient. After proper positioning of foot 88 is attained, a knob 92 (or other
equivalent
device) may be employed to adjust the position of coils 84, based on the
signals (for
example, nerve conduction signals) provided by sensor patch 86, as shown in
FIG. 7B.
[0108] With reference to FIG. 7C, after neural conduction is detected,
coils 84 are
locked in place, and, with further reference to FIG. 7D, foot cradle 82
retains coils 84
locked in position for further use in a home or healthcare office environment.
Therefore,
in the present method, the patient or a healthcare provider simply adjusts
coil position by
sliding coils 84 back and along one axis until electric conduction in the
target nerve is
detected, although adjustments along all three axes may be possible in
different variants
of the present variation.
[0109] Referring now to FIG. 8, a sixth variation relates to the use of
multiple
sensors. While FIG. 8 depicts a variation shaped as a foot cradle 98, it
should be
understood that the following description also relates to any other design,
whether
shaped as a cradle or a wrap or otherwise. The plurality of sensors 94
described herein
may detect a variety of physiologic changes, including neural impulses,
muscular
contraction, twitching, etc. that may occur with neural or muscular
stimulation.
[0110] One or more of the illustrated sensors 94 may be employed over
body regions
being stimulated (for example, back, leg, arm, neck, head, torso, etc.) and
may be either
incorporated within an actual cradle or wrap or, otherwise, be applied
separately from the
cradle or the wrap.
[0111] Sensors 94 may be structured as disposable, single-use, EKG-type
patches
that are attached to the body outside of cradle 98 along the nerve conduction
pathway
and are then connected to the logic controller (not shown) before beginning
therapy. This
arrangement provides for an intimate body contact of sensors 94 without the
risk of
infection or other detrimental side effects that may be present with
transcutaneous
devices. Sensors 94 may be employed both for beginning and for monitoring the
stimulation therapy; more specifically, sensors 94 may be employed during the
beginning of the therapy to optimize the strength of the magnetic field and/or
to adjust
the positioning of coils 96 within the cradle 98. Once therapy has begun,
sensors 94
continue to monitor nerve conduction to ensure that the correct level of
stimulation is
being provided. In the event that for some reason nerve conduction decays
during
19
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
therapy, the logic controller can automatically adjust the magnetic field,
ensuring that the
appropriate therapy is delivered for the appropriate amount of time.
[0112] One or more of sensors 94 in this variation, or any of the
variations described
herein, may take the form of an inductive coil designed to receive impulses
from the
underlying nerves, so that inductive technologies may be used to both
stimulate the nerve
or tissues as well as to record the effect of the stimulation on nerves or
tissues. Any of
sensors 94 may be connected to the logic controller through one or more
connection
modes, including, but not limited to, wireless signals, wired signals, radio
frequencies,
Bluetooth, infrared, ultrasound, direct switching of the current circuit,
etc., so long as
communication between the sensor and the device is effective.
[0113] During implementation of the present method, a healthcare
provider may
simply elect to use sensors 94 to adjust the device, for example, to lock
coils 96 into
position, during the first therapy session and not require the use of sensors
94 during
each successive therapy session.
[0114] Referring now to FIGS. 9A-9D, there are shown different, non-
limiting
variations shaped as body worn ergonomic applicator garments. Each of these
variations
is shown with overlapping coils, although coils of any configurations may be
used. Each
of the wraps of FIGS. 9A-9D corresponds to a coil wrap, into which a body part
may be
placed. These garments contain one or more sensors (not shown) that provide
feedback
to a logic controller (also not shown), or sensors may be applied separately
from those
garments. Systems may also be included for reversibly or irreversibly locking
the coils
within the applicator.
[0115] More particularly, FIG. 9A illustrates a variation, in which
coils 100 are
embedded in a knee wrap 102 and are connected to a logic controller (not
shown) by a
connector 104. FIG. 9B instead illustrates a variation, in which coils 106 are
disposed
within an abdominal garment, for example shorts 108 and in which coils 106 are
also
connected to a logic controller (not shown) by a connector 110. A marking 112
may be
added on one side of shorts 108 to indicate wrap orientation. FIG. 9C
illustrates a coil
wrap shaped like a band 114, in which coils 116 are connected to a logic
controller (not
shown) by a connector 118. When this variation is employed, band 114 may be
wrapped
around a body portion (for example, an arm) and be retained in place by a
system known
in the art, for example, a hook and loop system, a strap and buckle system, or
simply a
hook disposed at one end of band 114 for engaging fabric or other material in
another
portion of band 114. FIG. 9D illustrates a variation shaped as a shoulder
strap 120, the
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
length of which may be adjusted by a buckle 122 and which has coils 124
disposed in
one or more points, for example, at the joint between an arm and a shoulder as
shown.
Each of these variations includes one or ore sensors (not shown) that may be
coupled to
the garment, or that may be applied separately from the garment.
[0116] Other variations that are not illustrated include, bur are not
limited to: a head
worn garment, such as a cap; a neck worn garment, such as a neck brace; and a
lower-
back garment. Each garment and applicator may also utilize the locking,
targeting coil
feature described previously, without requiring the use of the any sensing
components
after a proper positioning of the coils in relation to the target nerve or
nerves has been
established.
[0117] Still other variations are depicted in FIGS. 10 and 11. In these
variations, the
source of energy for nerve stimulation is electrical energy that is dispensed
through a
percutaneous stimulator, such as a percutaneous needle 124, or a
transcutaneous
stimulator, such as an electrode 126. As shown in FIG. 10, an electrical pulse
controller
128 is electrically connected both to percutaneous needle 124 and to sensor
134, to
provide the desired feedback and modulate the power to percutaneous needle
134. In the
variation of FIG. 11, electrical pulse controller 130 is connected both to
electrode 126
and to sensor 136, and performs a function similar to that of electrical pulse
controller
128. With these variations, nerve conduction may be detected at a site
sufficiently distant
from the site of stimulation, so to enable detection of nerve conduction
despite the
confounding interference from the direct electrical stimuli. Further, direct
electrical
stimulation of nerve and muscle may be tailored to provide optimal therapy
and, in the
case of electrode migration or other electrode malfunction, to report lack of
stimulation
of the bodily tissues. Still further, these variations enable a reduction in
power
requirement, because control of the signal is provided by the sensor to the
signal
generator loop.
[0118] As shown, a device constructed according to the principles
described herein
provides a targeted and precise stimulation of the posterior tibial nerve, or
of other
peripheral nerves, in a non-invasive manner by employing an ergonomic wrap or
cradle
that specifically targets the posterior tibial nerve in a consistent and
repeatable manner.
For example, in patients with OAB or VI, the novel, reversibly lockable
movement of the
coils and the use of a logic controller -sensor loop enables the application
of a magnetic
field that can be varied in location, amplitude and strength according to the
amount of
stimulation actually induced in one or more target nerves and of the response
of the
21
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
patient to the therapy. An apparatus according to the variations described
herein may
deliver any frequency of stimulation, including low frequencies, high
frequencies, mid
frequencies and ultrahigh frequencies, and overlapping and non-overlapping
coils may
be used to generate the desired field, although overlapping or Helmholtz coils
are
preferred due to their ability to target a broader region and achieve more
thorough
stimulation.
[0119] Ailments that may be treated through the use of apparatus and
methods as
described herein include not only OAB and VI, but also obesity, depression,
urinary
incontinence, fecal incontinence, hypertension, pain, back pain, restless leg
syndrome,
Guillain Barre syndrome, quadriplegia, paraplegia, diabetic polyneuropthy,
dyskinesias,
paresthesias, dental procedure pain, knee osteoarthritis, anesthesia (pain
relief during
surgery), Alzheimer's disease, angina (chest pain from heart disease),
ankylosing
spondylitis, back pain, bum pain, cancer pain, chronic pain, dysmenorrhea
(painful
menstruation), headache, hemiplegia, hemiparesis (paralysis on one side of the
body),
labor pain, local anesthesia during gallstone lithotripsy, facial pain,
trigeminal neuralgia,
bruxism (tooth grinding) pain, myofascial pain, pregnancy-related nausea or
vomiting,
neck and shoulder pain, pain from broken bones, rib fracture or acute trauma,
diabetic
peripheral neuropathy, phantom limb pain, post-herpetic neuralgia (pain after
shingles),
postoperative ileus (bowel obstruction), irritable bowel syndrome,
postoperative nausea
or vomiting, postoperative pain, post-stroke rehabilitation, rheumatoid
arthritis, skin
ulcers, spinal cord injury, temporomandibular joint pain, detrusor
instability, spinal
muscular atrophy (in children), pain during hysteroscopy, gastroparesis,
chronic
obstructive pulmonary disease rehabilitation, carpal tunnel syndrome, soft
tissue injury,
multiple sclerosis, intermittent claudication, attention-deficit hyperactivity
disorder
(ADHD), cognitive impairment, knee replacement pain, achalasia, atopic eczema,
bursitis, carpal tunnel syndrome, dementia, depression, dry mouth, dystonia,
enhanced
blood flow in the brain, enhanced blood perfusion of the uterus and placenta,
esophageal
spasm, fibromyalgia, fracture pain, Guillain-Barre syndrome, hemophilia,
herpes, hip
pain, interstitial cystitis, irritable bowel syndrome, pruritis, joint pain,
labor induction,
local anesthesia, menstrual cramps, muscle cramps, muscle spasticity, muscle
strain or
pain, musculoskeletal trauma, myofascial pain dysfunction syndrome, nerve
damage,
osteoarthritis, pain medication adjunct, pancreatitis, Raynaud's phenomenon,
repetitive
strain injuries, sacral pain, schizophrenia, shingles, shoulder subluxation,
sickle cell
anemia pain, Skin flap ischemia (during plastic surgery), sphincter of Oddi
disorders,
22
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
sports injuries, thrombophlebitis, tinnitus (ringing in the ear), restless
legs, tremor,
whiplash and neuralgias. In contrast to implantable nerve stimulators, this
therapy is
completely non-invasive and does not require a major surgery to implant a
permanent
nerve stimulation device. Moreover, this therapy can be controlled to optimize
the level
of therapy delivered according to power consumption and nerve stimulation
requirements and need not be delivered by a professional healthcare provider.
[0120] In other variations, neural stimulation may be applied as
electrical
transcutaneous stimulation, for example, by inserting an invasive electrical
needle into a
target body part and by modulating stimulation is modulated on the basis of
information
sent back to the logic controller from the one or more sensors that are used
to detect
and/or maintain the correct level of stimulation. The transcutaneous
electrical stimulation
sensor may be placed in the body independently or be incorporated within the
wrap and
may provide, among other things, feedback as to the quality of the electrical
connection
to the skin, which is directly related to the burn risk inherently associated
with this type
of therapy. In fact, these methods of stimulation may not be optimal due to
the resulting
skin irritation and risk of potential burns, a very serious issue in the large
percentage of
patients that have neuropathies. Even when patches are applied to monitor
transcutaneous stimulation very closely, the patches may still become
displaced and
allow a burn to occur. Moreover, potentially interfering electrical impulses
may develop
at the treatment site, creating a noisy environment for the detection of nerve
conduction.
[0121] In still other variations, an external coil or coils may be
inductively connected
to an implanted coil or coils may be utilized. In these variations, an
ergonomic applicator
may be adjusted by the user or by a healthcare provider such to optimize
inductive power
transmission between the external and implanted coils. One or more sensors may
be
utilized to provide a feedback that the relative coil positions have been
optimized, and
the external coil may then be reversibly locked into position within the
ergonomic
applicator. Two applications of this variation relate to the transfer of power
to recharge
an implantable device, and to the transfer of power to activate an implantable
device.
[0122] In the first application, when an implantable rechargeable device
is utilized,
the external coils may be used for recharging the implanted device by means of
inductive
fields generated by the external coils. The external coils may include
circuitry that
determines the amount of resistance encountered by the magnetic field or other
electrical
properties related to the quality and degree of the magnetic coupling that is
being
established. Based on this feedback, the position of the external coils may be
adjusted
23
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
manually or automatically to optimize the coupling achieved with during each
recharging
session. Alternatively, a sensor may be incorporated into the implantable
device and may
communicate the degree and quality of the magnetic coupling to the external
coils and/or
the connected circuitry via wireless communication, providing a feedback for
the
automatic or manual adjustment of the external recharging coils.
[0123] The coils within the ergonomic applicator may be reversibly
locked into place
for the duration of the recharge session, and the implantable device may also
communicate to the external recharging unit that the implantable device has
been fully
recharged, terminating the recharging session has been completed. By providing
for an
intermittent recharging of an implanted device, an apparatus according as
described
herein enables the implantable device to devote more power to performing its
intended
function optimally and with a lesser concern about protecting or extending
battery life.
[0124] In the second application, the powering coils may contain
circuitry to
determine the amount of resistance encountered by the applied magnetic field,
or other
electrical properties that may reflect the quality and degree of the magnetic
coupling that
is being achieved. Based on this feedback, the powering coils in the
applicator may be
adjusted manually or automatically to activate and optimize the coil coupling
at the
beginning of each therapy session. Alternatively, a sensor may be incorporated
into the
implantable device and communicate the degree and quality of the magnetic
coupling
externally via wireless communication, which may in turn provide feedback for
the
automatic or manual adjustment of the powering coil. In one variant of the
present
variation, the inductive coils may be magnetically coupled to a needle
targeting the
posterior tibial nerve.
[0125] An exemplary method of use of an apparatus as described herein on
a patient
suffering from VI and/or OAB includes the following steps:
[0126] The patient places a conductive wrap contained within a flexible
material
over a region of the ankle (or alternatively over the knee) to provide the
required pulsed
magnetic field. Alternatively, the patient may use an ergonomic foot/leg rest
or cradle
having embedded coils.
[0127] A sensor (for example, a sensor patch) is placed on the patient's
body along
the path of the nerve, ideally proximal to the stimulation site to ensure
afferent nerve
stimulation, and is connected to a logic controller.
[0128] A physician or healthcare provider adjusts the coils in the wrap
or cradle
until nerve conduction is achieved based on patient and sensor feedback. An
optimal
24
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
position is sought, and the coils may be reversibly locked into position
within the
conductive wrap or ergonomic cradle and remain in this position during
subsequent use.
[0129] During the therapy session, the logic controller provides an
electric current to
the coils, generating an inductive magnetic field. In one variation, this
field begins at low
amplitude and slowly ramps up until nerve conduction exceeds a threshold
level, as
signaled by the sensor and possibly by the patient, who may feel motor
conduction.
Alternatively, one or more coils may also be activated to increase the covered
area of
stimulation in the event that stimulation does not occur with the initial coil
configuration
or is inadequate
[0130] The optimal stimulation may be determined in a variety of manners,
for
example, by measuring exposure to electromagnetic fields capable of generating
a square
wave electric signal at a frequency of 10-30 Hz at the targeted tissue depth.
The square
wave configuration of the signal may be generated via Fourier transformation
or may be
a ramped current generated in any manner.
[0131] The inductive magnetic pulses continue for an appropriate duration
of use, for
example, for 15-30 minutes. The sensor may remain in place during the entire
therapy
session to ensure that stimulation occurs consistently and to provide for
appropriate
corrections if nerve conduction deteriorated. The logic controller may be
powered either
by a portable power source such as a battery, or by or a fixed power source
such as a
traditional wall outlet.
[0132] The conductive wrap and/or ergonomic cradle is removed from the
body
when therapeutic stimulation is not being delivered, typically at the end of
the therapy
session.
[0133] The conductive wrap and/or ergonomic cradle is reapplied along
with the
sensor patch (ideally disposable) from time to time as indicated, for example,
on a daily
basis, and steps 4-8 are repeated.
[0134] The devices and methods described herein may be applied to any
body
tissues, including nerve, muscle, skin, vasculature, or any other organ or
tissue within the
human body. Further, the devices and methods described herein may be used to
treat any
conditions suited for neuromodulation regardless of whether the stimulation
source is an
electromagnetic field, a direct electric current, a RF field, infrared energy,
visible light,
ultraviolet light, ultrasound, or other energy dispensing device.
[0135] In other variations, as shown in FIG. 12, an energy emitting
system 210 for
providing a medical therapy includes one or more conductive coils 212 disposed
within
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
or along a housing 214, one or more sensors 216 configured to detect
electrical
conduction in a target nerve or to detect muscle stimulation, and a controller
218
connected or coupled to the conductive coils 212 and optionally in
communication with
the sensor 216. In certain variations (as shown in Fig. 12), the controller
218 can be
integral with the housing 214). The coils 212 are configured such that an
electrical
current generated by the controller 218 is passed through the coils 212
generating a
magnetic field which will stimulate a target nerve, e.g., the tibial nerve
220, a muscle or
other body part containing a portion of a target nerve, or any nerves
branching off of a
target nerve, located in proximity to the coils 212. In this particular
variation, the
housing 214 is in the form of a foot cradle, as shown in FIG. 4, however, the
housing
could also be in the form of a flexible wrap, garment or other design suitable
for use with
a subject. In various variations described herein, sensors may detect voltage
or current
and may be connected, coupled, wirelessly connected or coupled or otherwise in
communication with the housing and/or controller using a variety of methods or
techniques known in the art. The sensor may be placed over a muscle to detect
muscle
stimulation resulting from stimulating the target nerve (as shown in Fig. 12)
or over any
other portion of the subject's body suitable for detecting conduction of the
target nerve.
[0136] Referring to FIG 13 and 16, the sensor may be in the form of a
microneedle
patch 228, which can be removably attached to the skin surface of a subject.
The
microneedle patch 228 may include a housing 231, having one or more electrodes
232
and one or more microneedles 235 deposited or arrayed on a surface of the
electrode 232,
forming one or more microneedle arrays 234. In FIG 13, microneedle patch 228
has the
shape of a square, and the microneedles 235 are arrayed on the bottom surface
236 of the
electrode 232 in a 16 X 16 configuration. However, as shown in FIGS 14-15,
microneedle patches may be designed in a variety of shapes, e.g., round, oval
229,
rectangular 230, hexagonal, and a variety of sizes. The microneedles may be
arrayed in a
variety of arrangements and patterns (e.g., 14 X 14, 12 X 12, etc.) depending
on the
particular use and needles.
[0137] Additionally, microneedles may be attached, deposited, or arrayed
on an
electrode surface or patch in a variety of configurations and arrangements,
depending on
where the particular microneedle patch will be utilized and the treatment to
be delivered.
The number of microneedles included in an array can vary. For example, the
number of
microneedles may range from about 5 to 500 or 100 to 400 or about 200 to 300
or about
256. In certain variations where microneedles are composed of strong, highly
26
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
conductive material, the number of microneedles necessary may be less and may
range
from about 5 to 100 or 10 to 50 or 5 to 50. However, where microneedles are
composed
of higher resistance metal, a greater number of needles may be needed, e.g.,
about 100 to
500 or about 200 to 300 or greater than 500.
[0138] Referring to FIG. 18, a magnified view of a microneedle array 234
composed
of one or more microneedles 235 is shown. Microneedles 235 may include a base
portion 238 and an upper portion 239. Microneedles 235 may have lengths in the
range
of about 1 to 400 microns or 10 to 400 microns or preferably about 100 to 150
microns,
and a diameter in the range of about 1 to 100 microns. A microneedle 235 may
be
-- tapered in diameter, going from a larger to smaller diameter from the base
portion 238 to
the upper portion 239 where the distal tip 240 of the microneedle is
preferably pointed or
sharp. The upper portion 239 of the microneedle 235 may have a diameter in the
range
of about 10-30 microns or about 15 to 25 microns. Optionally, for ease of
production,
the base portion 238 of the microneedle 235 may be thicker than the distal tip
240 or
-- upper portion 239 of the microneedle 235. In certain variations, as shown
in FIG 17, a
bulb 237 may be provided at the distal tip 240 of a microneedle 235 to provide
for
effective anchoring of the microneedle 235 in the skin of a patient or
subject.
Microneedles 335 can include any number of friction or grip increasing
features. For
example, they may include projections, barbs, bulbs or a roughened surface or
tip.
-- Microneedles 235 may take on various configurations, e.g., straight, bent,
filtered,
hollow or a combination of the above.
[0139] In other variations, microneedles may have lengths that range
from about 480
to 1450 microns, widths from about 160 to 470 microns, thicknesses from about
30 to
100 microns and tip angles from about 20 to 90 degrees, and arrays can contain
from 5 to
-- 50 microneedles. For example, microneedles having these dimensions have
been shown
to be less painful than hypodermic needles. Length and number of microneedles
can
affect the level of pain experienced. Decreasing microneedle length and/or the
number
of microneedles may be beneficial and act to further reduce pain and provide
comfort.
[0140] In certain variations, the one or more microneedles may include
an
-- electrically conductive material such that the microneedles may transmit an
electrical
signal to an overlying electrode or other surface. Microneedles may be
constructed of an
electrically conductive material and/or coated with an electrically conductive
material.
Optionally, microneedles may be coated with an electrically conductive
material and
constructed of a non-conductive material. Microneedles may be fabricated using
a
27
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
variety of materials, e.g., metals, stainless steel, solid or coat of gold
over NI, Pd or Pd-
Co, Pt, silicon, silicon dioxide, polymers, glass, biocompatible polymers,
titanium, silver,
or suture materials. Biodegradable polymers may also be used such that if a
tip of a
microneedle were to snap or break off during insertion, it would easily
biodegrade.
[0141] A microneedle array 234 may be constructed or fabricated using any
variety
of manufacturing methods known to persons of ordinary skill in the art.
Microneedles
may be arrayed, attached, etched or deposited onto a surface of an electrode.
In another
variation, microneedles may be etched from or deposited onto a silicon
electrode, such
that the microneedle patch, including electrode and microneedles, are made
from one
material creating a durable and stable microneedle patch.
[0142] As shown in FIG 18, microneedles may be fabricated by creating
micron
sized holes on a silicon substrate and by using a KOH solution to create the
needle shape.
In other variations, the microneedles may be made of non-conductive material
but may
still be utilized to provide superior anchoring properties such that a
microneedle patch
may effectively adhere or attaché to a subject's skin.
[0143] In certain variations, microneedle arrays are fabricated by
patterning SU-8
onto glass substrates and defining needle shapes by lithography. The tips of
the needles
can be sharpened using reactive ion etching. Optionally, holes may be drilled,
e.g., by
laser, through the microneedles and base substrate. Holes may be drilled off-
center, but
parallel to the microneedle axis, terminating in side-opening holes along the
needle shaft
below the needle tip. If desired, the holes can serve as micro fluidic needle
bores for
injection or infusion of drugs, medicines, insulin, proteins, nanoparticles
that would
encapsulate a drug or demonstrate the ability to deliver a virus for
vaccinations, etc. to be
used separately or in combination with electrical or magnetic therapy. The
microneedles
may also be coated with nickel by electroplating, which can increase their
mechanical
strength.
[0144] In certain variations, microneedle patches or microneedle
electrode arrays are
made by fabricating master structures from which replicates are molded and
then made
electrically active. For example, SU-8 may be spun on a glass substrate
bearing an array
mask pattern, baked, and then exposed from the backside to from a tapered
needle
structure. Microneedles may be sharpened by RIE etching. A PDMS
(polydimethylsiloxane) or similar material mold can then be copied from the
master. A
PMMA (polymethylmethacrylate) microneedle array is formed by solvent-casting
and
then released from the mold.
28
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
[0145] To provide the arrays with electrical functionality, a Ti/Cu seed
layer may be
deposited on the PMMA array and patterned by excimer laser to electrically
isolate
adjacent rows. A Ni layer (e.g., about 15 to 30 microns or 20 to 25 microns
thick) may
be electroplated on the patterned seed layer to enhance structural rigidity. A
backside
electrical connection to the array may be formed by backside etching of a hole
and
forming an electrical connection through the hole.
[0146] In another variation, the microneedle array is arranged in a 16 X
16 array (i.e.,
256 needles). Each needle has a height of about 400 microns and the base
diameter is
about 100 microns. The pitch between microneedles can be about 250 microns.
The
microneedle arrays are then coated with metal and laser-etched to provide
electrical
functionality. Optionally, rows of microneedles can be electrically isolated
from each
other so that alternating rows can provide alternating electrical polarity.
The arrays are
also interfaced with a power source. Microneedles may be made of polymer,
coated with
a metal, and etched to act as alternating electrodes. In certain variations,
the firing
sequence of the microneedles by rows or groups may be varied or configured to
alternate.
[0147] In certain variations, a microneedle array may include one or
more
microneedles having multiple channels. For example, a multichannel silicon
microneedle may be constructed to deliver bioactive compounds into neural or
other
tissue while simultaneously monitoring and stimulating neurons and nerves.
[0148] FIG. 19 shows a cross sectional view of the skin 10 composed of
an outer
stratum corneum 15 covering the epidermis 16. The skin also includes the
dermis 18,
subcutaneous tissue/fat 12, and these layers cover muscle tissue 14. As shown
in FIG
19, when a microneedle patch 228 is attached to a subject's skin, the
microneedles 235
pierce the outer insulating stratum corneum layer 15. The microneedle patch
228 can
detect current passing through a stimulated nerve, and provide a superior
signal as the
current detected is conducted through the microneedles 235, thereby bypassing
the
poorly conductive stratum corneum layer 15 which generally encompasses the
outer 10
to 15 microns of skin. In other variations, microneedles 235 may be fabricated
to be
long enough to penetrate the stratum corneum 15, but short enough not to
puncture nerve
endings, thus reducing the risk of pain, infection or injury.
[0149] In certain variations, microneedles are formed such that they are
in direct
contact with their corresponding or overlying electrodes. For example, a
microneedle
patch may include an adhesive electrode pad and may utilize a conductive gel
to help
29
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
hold the microneedles in place to prevent shear forces from breaking or
bending the
microneedles.
[0150] In certain variation, as shown in FIGs. 20a-20d, a microneedle
patch or
applicator may include multiple electrodes on a single patch or applicator,
e.g., positive,
negative, and/or control or ground electrodes, where the microneedles will be
grouped in
multiple arrays such that they conduct to the appropriate electrode. For
example, FIGS
20a and 20b show a single patch having positive, negative and control
electrodes where a
separate array of electrodes is in contact with each respective electrode.
This
arrangement can be created using a single patch. Alternatively, as shown in
FIG. 20c,
two patches may be implemented, one including the control electrode with
corresponding microneedle array and the other including the positive and
negative
electrodes with corresponding microneedle arrays. The various electrodes could
be
interchanged. Alternatively, as shown in FIG. 20d, three patches may be
implemented,
each having a separate electrode (control, positive, or negative) with a
corresponding
microneedle array. In use, in certain variations, the control may be attached
above or
near bone, while the positive and/or negative electrodes may be attached above
nerve or
muscle.
[0151] Referring again to FIG. 12, the energy emitting system 210 can be
used to
treat or prevent various conditions, e.g., urinary incontinence, restless leg
syndrome and
fecal incontinence, among others. Energy emitting system 210 includes one or
more
conductive coils 212 disposed within or along a housing 214, one or more
sensors 216
configured to detect electrical conduction in the target nerve or to detect
muscle
stimulation, and a controller 218 coupled to the conductive coils 212 and
optionally in
communication with the sensor 216. The coils 212 are configured such that an
electrical
current generated by the controller 218 is passed through the coils 212
generating a
magnetic field which will stimulate a target nerve, e.g., the tibial nerve
220, a muscle or
other body part containing a portion of a target nerve, or any nerves
branching off of a
target nerve, located in proximity to the coils 212. In this particular
variation, the
housing 214 is in the form of a foot cradle, as shown in FIG. 4, however, the
housing
could also be in the form of a flexible wrap, garment or other design suitable
for use with
a subject.
[0152] Referring again to FIG. 12, energy emitting system 210 may be
used to treat
or prevent various conditions, e.g., urinary incontinence, restless leg
syndrome or fecal
incontinence. In certain variations, a method of using the energy emitting
system 210
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
includes positioning a first portion of a patient's body, for example a foot,
ankle, or leg,
relative to housing 214 such that a posterior tibial nerve 220 within the
first portion of
the patient's body is in proximity to one or more conductive coils 212
disposed within or
along the housing. In this particular variation, a patient's foot is
positioned in a housing
which is in the form of a foot cradle 215. A sensor in the form of a
microneedle patch
228 may optionally be positioned along a second portion of the patient's body
in
proximity to the posterior tibial nerve 220. In this particular variation,
microneedle patch
228 is attached to the patient's foot over a muscle to detect muscle
stimulation.
Alternatively, a patch could be placed elsewhere on the patient, for example,
on the leg
in proximity to the posterior tibial nerve 220, proximal to and up-stream from
coils 212.
Microneedle patch 228 may be composed of one or more microneedle arrays and
one or
more electrodes, as described supra.
[0153] Once the patient's foot is in position and the microneedle patch
228 (e.g.,
conductive microneedle patch) is in place, a current is passed from controller
218
through coils 212, and as a result, the coils 212 generate a magnetic field
which is
focused on the posterior tibial nerve 220. The magnetic field stimulates
tibial nerve 220,
generating a current that will flow along the tibial nerve 220 and spread
along its length,
to its sacral or pudendal nerve roots. Microneedle patch 228 detects
corresponding
muscle stimulation or twitching or electrical conduction through the
stimulated posterior
tibial nerve. Upon detection, the microneedle array may conduct and transmit
an
electrical signal to the overlying electrode of microneedle patch 228. The
signal may be
transmitted to controller 218, which can be integral or a separate controller
or device, or
a separate controller coupled to controller 218. The controller can then be
varied or
adjusted (to adjust the current or magnetic field) based on the signal
received from
microneedle patch 228 to ensure that adequate conduction of the posterior
tibial nerve
220 occurs and an adequate and accurate dosage of treatment is being received.
Although shown utilizing a sensor, it is also contemplated that the system
could be used
without a sensor.
[0154] Referring to FIG. 21, the method of using energy emitting system
210
described above with respect to FIG. 12 may be varied such that a conductive
microneedle patch 228 is placed in proximity to or proximally over the
afferent posterior
tibial nerve 220, i.e., behind the patient's knee. In this position, a
conductive
microneedle patch 228 detects electrical conduction through the afferent
posterior tibial
nerve, i.e., it detects the electrical signal traveling through the posterior
tibial nerve back
31
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
up to the brain and spinal cord or it may detect corresponding muscle
stimulation. The
microneedle patch 228 sends the signal to controller 218 or to a separate
controller
coupled to controller 218. The controller can then be varied or adjusted based
on the
signal received from microneedle patch 228 to ensure that adequate conduction
or
stimulation of the posterior tibial nerve 220 occurs and an adequate and
accurate dosage
of treatment is being received.
[0155] A sensor utilized in the energy emitting system 210 may be a
microneedle
patch 228 as described above or optionally the sensor may a sensor type known
in the art
(e.g., EKG sensor) or as described in any of the variations herein. It is also
contemplated
that energy emitting system 250 can be utilized without a sensor. Optionally,
the sensor
may be positioned within or along the housing, e.g., the foot cradle, along
with the one or
more conductive coils, or positioned at a site distant from the housing or
conductive
coils.
[0156] In certain variations, energy emitting system 210 my optionally
include one
or more conductive microneedle patches which can be positioned in proximity to
the
target nerve or muscle and provide an additional or supplemental electrical or
magnetic
stimulus to the target nerve or muscle.
[0157] Referring to FIG. 22, the energy emitting system 210 described
above with
respect to FIG. 12 may be varied to create energy emitting system 260. Energy
emitting
system 260 further includes one or more percutaneous electrode needles 262 or
other
needles or other percutaneous electrodes coupled to a controller 218 and
having an end
insertable into a subject's body in proximity to said target nerve or
stimulation site. The
percutaneous electrode needle 262 is inductively coupled to one or more
conductive coils
212. In use, a first portion of a patient's body, for example a foot, ankle,
or leg, is
positioned relative to housing 214, e.g., foot cradle 215, such that a target
nerve, e.g.,
posterior tibial nerve 220, located within the first portion of the patient's
body is in
proximity to one or more conductive coils 212 disposed within or along the
housing 214.
Conductive coils 212 are positioned proximate, optionally down-stream or
distal to, a
selected stimulation site 261. The percutaneous electrode needle 262 is
inserted through
the skin at a location and to a depth that brings the tip in close proximity
to the
stimulation site or target nerve to be stimulated. The controller 218 is
activated and a
current passes through conductive coils 212. The resulting magnetic field
generates a
current that traverses the internal stimulation site 261 by passing from
conductive coils
212 to the internal percutaneous electrode needle 262, as indicated by arrow
i. Also, the
32
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
percutaneous electrode needle may be positioned within the generated magnetic
field,
whereby the magnetic field itself generates a current in the percutaneous
electrode which
stimulates a target nerve or traverses an internal stimulation site.
Optionally, a current
may be passed from the controller 218 through conductive coils 212 and/or from
the
controller 218 through percutaneous electrode needle 262, traversing the
internal
stimulation site as the current passes between the coils and needle.
[0158] In energy emitting system 260, current density and subsequent
electric field
intensity generated between conductive coils 212 and percutaneous electrode
needle 262
is greater than that generated by traditional percutaneous stimulators. A
greater electric
field intensity makes site location for conductive coils 212 and percutaneous
electrode
needle 262 easier. Furthermore, the load impedance through the surface of the
skin is
much higher than the internal impedance, and as such, the relatively high load
impedance
lessens the likelihood of damage to tissue and nerves due to high current
pulses.
[0159] Referring again to FIG. 22, a percutaneous electrode needle for
use in any of
the energy emitting systems described herein may include a variety of designs.
For
example, percutaneous electrode needle 262 may include a metal or plastic
handle 263 to
provide a secure grip for the user, while minimizing the risk of shock to the
user. The
needle tip can have a terminal portion 264 which may extend between about 0.5
and 10
mm or about 2.0 mm from the needle tip and may be constructed out of medical
grade
stainless steel or other biocompatible metals. The diameter of the needle can
be small
(less than about 0.25 mm) which minimizes trauma during insertion. Optionally,
needle
262 can be coated with Teflon or similar insulative material 265 except for an
exposed
tip area 264. This allows for a higher field density at the tip for more
precise operation.
The exposed needle tip area 264 should have a sufficiently large surface area
so as not to
create too high a local current field that may cause irritation or pain.
[0160] In another variation, as shown in FIG. 23, percutaneous electrode
needle 272
may be used in energy emitting system 260. Percutaneous electrode needle 272
may be
constructed out of medical grade stainless steel or other biocompatible
electrically
conductive metal. Percutaneous electrode needle 272 includes a first end 276
for
insertion into the patient's body in proximity to the preselected internal
stimulation site or
target nerve to be stimulated, and a second end 277. The size of the needle
electrode 272
is preferably small, for example 34G needle electrode (0.22x10 mm), to
minimize trauma
during insertion. Percutaneous electrode needle 272 may also include an
electrically
conductive adaptor, e.g., an electrically conductive tape member 273. The tape
member
33
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
273 includes an electrically conductive adhesive portion 274 and an
electrically
conductive non-adhesive portion 275. Alternatively, the adaptor may include an
electrically conductive clip. The second end 277 of the needle electrode 272
preferably
includes an enlarged portion to enable the electrically conductive tape member
273 to be
more easily adhered thereto. Once it is determined that the percutaneous
needle
electrode 272 is properly positioned, the needle is fixedly adhered to the
electrically
conductive tape member 273 by folding the ends of the adhesive portion 274 of
the
electrically conductive tape member 272 over the second end 277 of the needle
electrode
thereby forming an electrical connection there between. The percutaneous
needle
electrode 272 is electrically coupled to controller 218 via electrically
conductive tape
member 273. Various other implantable or insertable electrode needles known to
persons of skill in the art may also be utilized in the above described
systems.
[0161] In certain variations of energy emitting system 260 as described
above and
shown in FIGS. 22-23, a sensor 216, such as a conductive microneedle patch
228, may
be utilized to detect electrical conduction through the stimulated posterior
tibial nerve
220 or to detect muscle stimulation, and transmit the signal to controller
218. The signal
may be transmitted to controller 218, a separate controller or device, or a
separate
controller coupled to controller 218. The controller can then be varied or
adjusted based
on the signal from microneedle patch 228 to ensure that adequate conduction of
the
posterior tibial nerve 220 occurs and an adequate and accurate dosage of
treatment is
being received. It is also contemplated that energy system 260 may be utilized
without a
sensor 216, see for example FIGS. 24-25. Optionally, other types of sensors
may be
used in place of a microneedle patch sensor, such as other sensors described
herein and
sensors known to persons of ordinary skill in the art. The sensor may be
placed over a
portion of the subject's body suitable for detecting conduction of the target
nerve (e.g.,
on the leg as shown) or over a muscle to detect muscle stimulation resulting
from
stimulating the target nerve.
[0162] In certain variations, as shown in FIG. 26, an energy emitting
system 250 for
providing a medical therapy includes a microneedle patch 252 (e.g., conductive
microneedle patch) having one or more microneedle arrays deposited on a
surface of one
or more electrodes; one or more sensors 221 configured to detect electrical
conduction in
the target nerve or to detect muscle stimulation; and a controller 218 coupled
to
microneedle patch 252 and in communication with sensor 221. The microneedle
patch
252 is configured such that an electrical current generated by the controller
218 is passed
34
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
through the microneedle patch 252, generating a magnetic field or delivering
or
generating an electrical or magnetic stimulus to a target nerve, e.g., the
tibial nerve 220, a
muscle or other body part containing a portion of a target nerve, or any
nerves branching
off of a target nerve, located in proximity to microneedle patch 252.
[0163] Referring to FIG. 26, a method of using the energy emitting system
250 may
include placing a conductive microneedle patch 252 on a first portion of a
patient's body,
for example a foot, ankle, or leg, in proximity to posterior tibial nerve 220
within the first
portion of the patient's body. Sensor 221 is positioned along a second portion
of the
patient's body in proximity to the posterior tibial nerve 220. In this
particular variation,
sensor 216 is attached to the patient's leg in proximity to the posterior
tibial nerve 220,
proximal to and up-stream from conductive microneedle patch 252. Conductive
microneedle patch 252 is composed of one or more microneedle arrays and one or
more
electrodes, as described in the variations above.
[0164] Once conductive microneedle patch 252 and sensor 221 are in
position, a
current is passed from controller 218 through conductive microneedle patch
252,
resulting in an electrical stimulus of the posterior tibial nerve 220.
Alternatively, the
microneedle array may be insulated or constructed of non conductive material
such that
the microneedle patch 252 generates a magnetic field that stimulates tibial
nerve 220 in a
manner similar to the one or more coils described in the variations above,
without an
electrical stimulus. Whether the stimulus is electrical or magnetic, either
stimulus will
generate a current that will flow along the tibial nerve 220 and spread along
its length, to
its sacral or pudendal nerve roots. Sensor 221 detects electrical conduction
through the
stimulated posterior tibial nerve 220, and then transmits the signal to
controller 218. In
certain variations, the sensor may be in the form of a microneedle patch
sensor. The
signal may be transmitted to controller 218, a separate controller or device,
or a separate
controller coupled to controller 218. The controller can then be varied or
adjusted based
on the signal from sensor 221 to ensure that adequate conduction of the
posterior tibial
nerve 220 occurs and an adequate and accurate dosage of treatment is being
received.
[0165] The sensor utilized in the energy emitting system 250 may be a
sensor of the
type described above, with respect to other variations. Optionally, for
example, the
sensor may be a microneedle patch. It is also contemplated that energy
emitting system
250 can be utilized without a sensor. The sensor may be placed over a portion
of the
subject's body suitable for detecting conduction of the target nerve (e.g., on
the leg as
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
shown) or over a muscle to detect muscle stimulation resulting from
stimulating the
target nerve.
[0166] In certain variations, energy emitting system 250 my optionally
include one
or more conductive coils disposed within or along a housing which can be
positioned in
proximity to the target nerve or muscle and provide an additional or
supplemental
stimulation of the target nerve or muscle.
[0167] Referring to FIG. 27, the energy emitting system 250 described
above with
respect to FIG. 26 may be varied to create energy emitting system 280. Energy
emitting
system 280 further includes one or more percutaneous electrode needles 262
coupled to a
controller 218 and having an end insertable into a subject's body in proximity
to said
target nerve. Optionally, the electrode needle may be non-percutaneous, such
that it is
insertable in an orifice or opening in the subject, such as a natural orifice.
The
percutaneous electrode needle 262 is inductively coupled to conductive
microneedle
patch 252. In use, a microneedle patch 252 is placed on a first portion of a
patient's
body, for example a foot, ankle, or leg, in proximity to posterior tibial
nerve 220 within
the first portion of the patient's body and down-stream or distal to a
selected stimulation
site 261. The percutaneous electrode needle 262 is inserted through the skin
at a location
and to a depth that brings the tip in close proximity to the target nerve to
be stimulated.
[0168] The controller 218 is activated and a current passes through
microneedle
patch 252 and traverses the internal stimulation site 261 by passing from
microneedle
patch 252 to the internal percutaneous electrode needle 262, as indicated by
arrow i. The
current passing through microneedle patch 252 may also generate a magnetic
field which
can generate a current that traverses the internal stimulation site 261 by
passing from
microneedle patch 252 to the internal percutaneous electrode needle 262. Also,
the
percutaneous electrode needle may be positioned within the generated magnetic
field,
whereby the magnetic field generates a current in the percutaneous electrode
which
stimulates a target nerve and traverses an internal stimulation site.
Optionally, a current
may be passed from the controller 218 through microneedle patch 252 and/or
from the
controller 218 through percutaneous electrode needle 262, traversing the
internal
stimulation site as the current passes between the patch and needle.
[0169] Referring to FIG. 28, energy emitting system 280 may be modified
by using
percutaneous electrode needle 272 in place of percutaneous electrode needle
262.
Percutaneous electrode needle 272 would be constructed and function as
described above
with respect to FIG. 23. Various other implantable or insertable electrode
needles known
36
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
to persons of skill in the art may also be utilized in the above described
systems.
Additionally, energy emitting system 280 may utilize a sensor to detect
electrical
conduction through the stimulated posterior tibial nerve 220 and send a
corresponding
signal indicative of the detected conduction to controller 218 or other device
such that
the electrical or magnetic stimulus can be adjusted as necessary. The sensor
may be a
sensor 221, or optionally the sensor may be a microneedle patch. It is also
contemplated
that energy emitting system 280 can be utilized without a sensor. The sensor
may be
placed over a portion of the subject's body suitable for detecting conduction
of the target
nerve (e.g., on the leg as shown) or over a muscle to detect muscle
stimulation resulting
from stimulating the target nerve.
[0170] In any of the above systems, variations are contemplated where
the sensors
are also coupled or connected to or otherwise in communication with energy
emitting
devices, e.g., the conductive coils or conductive microneedle patches.
[0171] In certain variations, the one or more microneedles of the
microneedle patch
may include an electrically conductive material such that the microneedles may
transmit
an electrical signal to an overlying electrode or other surface. Microneedles
may be
constructed of an electrically conductive material and/or coated with an
electrically
conductive material. Optionally, microneedles may be coated with an
electrically
conductive material and constructed of a non-conductive material. Microneedles
may be
fabricated using a variety of materials, e.g., metals, stainless steel, solid
or coat of gold
over NI, Pd or Pd-Co, Pt, silicon, silicon dioxide, polymers, glass,
biocompatible
polymers, titanium, silver, or suture materials. Biodegradable polymers may
also be
used such that if a tip of a microneedle were to snap or break off during
insertion, it
would easily biodegrade. Optionally, the microneedle patch may be non-
conductive.
[0172] In certain variations, an electrode patch for improved conductance
or
conduction is provided. The patch can include at least one electrode having a
first
surface and/or a second surface. The electrode may optionally be attached to
various
other materials or adhesive materials. An array of microneedles may be
deposited on a
surface of the electrode, or attached to a patch or other material and
indirectly or directly
connected to the electrode. The array of microneedles may include a conductive
material. Such patches may be used as a sensor to detect muscle stimulation or
electrical
conduction, or to provide or deliver an electrical stimulus or magnetic field,
e.g., to a
target nerve, and may optionally be used in any of the variations described
herein or in
any application where improved conductance or conduction is desired.
Microneedles
37
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
yield improved reduction in impedance compared to simple abrasion and other
techniques, and are less painful and more comfortable for the patient.
[0173] In certain variations, typical voltage sensed at the skin and
detectable or
conductable by a microneedle patch or microneedle array may range from about 1
to 400
microvolts or about 10 to 300 microvolts.
101741 In certain variations, methods of treating a subject with urinary
incontinence
or various pelvic floor disorders utilizing the energy emitting systems
described herein
are contemplated. Symptoms associated with urinary incontinence may be
observed,
detected, or diagnosed. An energy emitting device having one or more energy
generators, e.g., one or more conductive coils or one or more microneedle
patches, may
be positioned in proximity to a target nerve, e.g., the tibial or posterior
tibial nerve or
popliteal or sacral nerve or branches thereof of a subject or patient along a
first portion of
a subject's or patient's body. The subject may or may not be exhibiting
symptoms
associated with urinary incontinence. In the case of the conductive coils, the
coils may
be positioned within or along a housing, such as a foot or knee cradle, and a
foot or leg
may be positioned therein. In the case of a microneedle patch, the patch may
be attached
to a subject's skin. Optionally, the method involves positioning a first
portion of a
subject's body, the subject exhibiting symptoms associated with urinary
incontinence,
relative to an energy emitting device such that a target nerve within the
first portion of
the body is in proximity to at least one energy generator disposed within or
along the
energy emitting device.
[0175] A current is then passed through the energy generator to produce,
generate or
deliver energy, e.g., a magnetic or electromagnetic field or electrical or
magnetic energy
or stimulus, focused on the tibial or posterior tibial nerve or branches
thereof. This in
turn may cause the stimulation of a pudendal nerve, sacral plexus, or other
nerves in the
pelvic floor. Various nerves innervating the various muscles, sphincters,
nerves, organs
and conduits of the urinary tract and bladder may be stimulated directly or
indirectly. In
certain variations, a current is passed through one more coils, which generate
a magnetic
or electromagnetic field which stimulates the posterior tibial nerve. In
certain
variations, the positioning of the coils relative to the first portion of the
subject's body
may be adjusted to re-focus the magnetic field on the posterior tibial nerve
as needed. In
certain variations, a current is passed through a microneedle patch generating
or
delivering an electrical or magnetic stimulus or field. The positioning of the
microneedle
38
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
patch relative to the first portion of the subject's body may be adjusted to
re-focus the
electrical or magnetic stimulus or field on the posterior tibial nerve as
needed.
[0176] Optionally, electrical conduction through the target nerve, e.g.,
the posterior
tibial nerve, or muscle stimulation can be detected via at least one sensor. A
conductive
sensor may be positioned in proximity to the posterior tibial nerve along a
second portion
of the subject's body. Optionally, a sensor may be positioned over a
corresponding
muscle to detect muscle stimulation or twitching resulting from nerve
stimulation.
Optionally, the electrical conduction is detected along a second portion of
the subject's
body which is different from the first portion of the body. Optionally, the
sensor in the
form of a microneedle patch. In certain variations, the sensor may be
positioned behind
a subject's knee to detect the electrical conduction along the afferent
posterior tibial
nerve or on another portion of a patient's leg or foot. In other variations,
the sensor may
be positioned within or along a housing along with the one or more conductive
coils.
[0177] Where a sensor is used, a signal is received from the sensors and
the signal is
indicative of the electrical conduction of the target nerve, e.g., posterior
tibial nerve. The
current may be adjusted or varied using a controller which is in communication
with the
energy generator. Adjustments may be made in response to the nerve or muscle
stimulation detected by the conductive sensor, in order to optimize or ensure
adequate
treatment of urinary incontinence by achieving the appropriate level of
conductance and
appropriate level of nerve or muscle stimulation. Appropriate levels or
parameters for
current, frequency, magnetic field, treatment duration, etc., are those that
result in an
observed or detected reduction or prevention of symptoms associated with
urinary
incontinence. Treatment could also be administered and the appropriate levels
and
parameters achieved through observing or detecting reduction or prevention of
symptoms where a sensor is not used. Examples of these symptoms include but
are not
limited to the inability to control urinary function, urinary leakage, and
loss of bladder
control.
[0178] In certain variations, the amplitude, frequency, direction of a
generated
magnetic field, electrical or magnetic stimulus, or firing sequence of the
coils or
microneedles making up the microneedle array may be adjusted. Optionally, the
current
may be varied according to a muscular response in the patient. Thus, to treat
urinary
incontinence, the magnetic field or electrical stimulus is applied to a
subject or patient
until the desired effects (e.g., reduction of symptoms) are achieved.
39
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
[0179] In certain variations, methods of treating a subject with fecal
incontinence
utilizing the energy emitting systems described herein are contemplated.
Symptoms
associated with fecal incontinence may be observed, detected, or diagnosed. An
energy
emitting device having one or more energy generators, e.g., one or more
conductive coils
or one or more microneedle patches, may be positioned in proximity to a target
nerve,
e.g., the tibial or posterior tibial nerve, or popliteal or sacral nerve or
branches thereof, of
a subject along a first portion of a subject's body. The subject may or may
not be
exhibiting symptoms associated with fecal incontinence. In the case of the
conductive
coils, the coils may be positioned within or along a housing, such as a foot
or knee
cradle, and a foot or leg may be positioned therein. In the case of a
microneedle patch,
the patch may be attached to a subject's skin. Optionally, the method involves
positioning a first portion of a subject's body, the subject exhibiting
symptoms
associated with fecal incontinence, relative to an energy emitting device such
that a
target nerve within the first portion of the body is in proximity to at least
one energy
generator disposed within or along the energy emitting device.
[0180] A current is then passed through the energy generator to produce,
generate or
deliver energy, e.g., a magnetic or electromagnetic field or electrical or
magnetic energy
or stimulus, focused on the tibial or posterior tibial nerve or branches
thereof. This in
turn causes the stimulation of a pudendal nerve, sacral plexus, or nerves in
the pelvic
floor. Various nerves innervating the various muscles, sphincters, rectum,
nerves, organs
and conduits associated with bowel movements, fecal control, and the
intestines may be
stimulated directly or indirectly. Optionally, a current is passed through one
more coils,
which generate a magnetic or electromagnetic field which stimulates the
posterior tibial
nerve. In certain variations, the positioning of the coils relative to the
first portion of the
subject's body may be adjusted to re-focus the magnetic field on the posterior
tibial
nerve as needed. In certain variations, a current is passed through a
microneedle patch
generating or delivering an electrical or magnetic stimulus or field. The
positioning of
the microneedle patch relative to the first portion of the subject's body may
be adjusted
to re-focus the electrical or magnetic stimulus or field on the posterior
tibial nerve as
needed.
[0181] Optionally, electrical conduction through the target nerve, e.g.,
the posterior
tibial nerve, or muscle stimulation can be detected via at least one sensor. A
conductive
sensor may be positioned in proximity to the posterior tibial nerve along a
second portion
of the subject's body. Optionally, a sensor may be positioned over a
corresponding
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
muscle to detect muscle stimulation or twitching resulting from nerve
stimulation.
Optionally, the electrical conduction is detected along a second portion of
the subject's
body which is different from the first portion of the body. Optionally, the
sensor is in the
form a of a microneedle patch. In certain variations, the sensor may be
positioned
behind a subject's knee to detect the electrical conduction along the afferent
posterior
tibial nerve or on another portion of a patient's leg or foot. In other
variations, the sensor
may be positioned within or along a housing along with the one or more
conductive
coils.
[0182] Where a sensor is used, a signal is received from the sensors and
the signal is
indicative of the electrical conduction of the target nerve, e.g., posterior
tibial nerve. The
current may be adjusted or varied using a controller which is in communication
with the
energy generator. Adjustments may be made in response to the nerve or muscle
stimulation detected by the conductive sensor, in order to optimize or ensure
adequate
treatment of fecal incontinence by achieving the appropriate level of
conductance and
appropriate level of nerve or muscle stimulation. Appropriate levels or
parameters for
current, frequency, magnetic field, treatment duration, etc., are those that
result in an
observed or detected reduction or prevention of symptoms associated with fecal
incontinence. Treatment could also be administered and the appropriate levels
and
parameters achieved through observing or detecting reduction or prevention of
symptoms where a sensor is not used. Examples of these symptoms include but
are not
limited: the loss of voluntary control to retain stool in the rectum; loss of
fecal control;
inability to control bowel movements, and fecal leaking:
[0183] In certain variations, the amplitude, frequency, direction of a
generated
magnetic field, electrical or magnetic stimulus, or firing sequence of the
coils or
microneedles making up the microneedle array may be adjusted. Optionally, the
current
may be varied according to a muscular response in the patient. Thus, to treat
fecal
incontinence, the magnetic field or electrical stimulus is applied to a
subject or patient
until the desired effects (e.g., reduction of symptoms) are achieved.
[0184] In certain variations, methods of treating a subject with
restless leg syndrome
utilizing the energy emitting systems described herein are contemplated.
Victims
afflicted with Restless Leg Syndrome (RLS or Ekbom's syndrome), are unable to
remain
seated or to stand still. Activities that require maintaining motor rest and
limited
cognitive stimulation, such as transportation, e.g., in a car, plane, train,
etc., or attending
longer meetings, lectures, movies or other performances, become difficult if
not
41
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
impossible. These sensations become more severe at night and RLS patients find
sleep to
be virtually impossible, adding to the diminishing quality of their lives. The
urge to
move, which increases over periods of rest, can be completely dissipated by
movement,
such as walking. However, once movement ceases, symptoms return with increased
intensity. If an RLS patient is forced to lie still, symptoms will continue to
build like a
loaded spring and, eventually, the legs will involuntary move, relieving
symptoms
immediately.
[0185] Thus, symptoms associated with restless leg syndrome may be
observed,
detected, or diagnosed. An energy emitting device having one or more energy
generators, e.g., one or more conductive coils or one or more microneedle
patches, may
be positioned in proximity to a target nerve, e.g., the tibial or posterior
tibial nerve, or
popliteal or sacral nerve or branches thereof or other nerves associated with
restless leg
syndrome, of a subject along a first portion of a subject's body. The subject
may or may
not be exhibiting symptoms associated with restless leg syndrome. In the case
of the
conductive coils, the coils may be positioned within or along a housing, such
as a foot or
knee cradle, and a foot or leg may be positioned therein. In the case of a
microneedle
patch, the patch may be attached to a subject's skin. Optionally, the method
involves
positioning a first portion of a subject's body, the subject exhibiting
symptoms
associated with restless leg syndrome, relative to an energy emitting device
such that a
target nerve within the first portion of the body is in proximity to at least
one energy
generator disposed within or along the energy emitting device.
[0186] A current is then passed through the energy generator to produce,
generate or
deliver energy, e.g., a magnetic field or electrical or magnetic energy or
stimulus,
focused on the tibial or posterior tibial nerve or branches thereof or other
nerves
associated with restless leg syndrome. This in turn causes the stimulation of
a pudendal
nerve, sacral plexus or other nerves innervating the pelvic floor or various
muscles,
nerves, or organs associated with restless leg syndrome. The various nerves
may
stimulated directly or indirectly. Optionally, a current is passed through one
more coils,
which generates a magnetic or electromagnetic field which stimulates the
posterior tibial
nerve. In certain variations, the positioning of the coils relative to the
first portion of the
subject's body may be adjusted to re-focus the magnetic field on the posterior
tibial
nerve as needed. In certain variations, a current is passed through a
microneedle patch
generating or delivering an electrical or magnetic stimulus or field. The
positioning of
the microneedle patch relative to the first portion of the subject's body may
be adjusted
42
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
to re-focus the electrical or magnetic stimulus or field on the posterior
tibial nerve as
needed.
[0187] Optionally, electrical conduction through the target nerve, e.g.,
the posterior
tibial nerve, or muscle stimulation can be detected via at least one sensor. A
conductive
sensor may be positioned in proximity to the posterior tibial nerve along a
second portion
of the subject's body. Optionally, a sensor may be positioned over a
corresponding
muscle to detect muscle stimulation or twitching resulting from nerve
stimulation.
Optionally, the electrical conduction is detected along a second portion of
the subject's
body which is different from the first portion of the body. Optionally, the
sensor in the
form a of a microneedle patch. In certain variations, the sensor may be
positioned
behind a subject's knee to detect the electrical conduction along the afferent
posterior
tibial nerve or on another portion of a patient's leg or foot. In other
variations, the sensor
may be positioned within or along a housing along with the one or more
conductive
coils.
[0188] Where a sensor is used, a signal is received from the sensors and
the signal is
indicative of the electrical conduction of the target nerve, e.g., posterior
tibial nerve. The
current may be adjusted or varied using a controller which is in communication
with the
energy generator. Adjustments may be made in response to the nerve or muscle
stimulation detected by the conductive sensor, in order to optimize or ensure
adequate
treatment of restless leg syndrome by achieving the appropriate level of
conductance and
appropriate level of nerve or muscle stimulation. Appropriate levels or
parameters for
current, frequency, magnetic field, treatment duration, etc., are those that
result in an
observed or detected reduction or prevention of symptoms associated with
restless leg
syndrome. Treatment could also be administered and the appropriate levels and
parameters achieved through observing or detecting reduction or prevention of
symptoms where a sensor is not used. Examples of these symptoms include but
are not
limited to: uncomfortable sensations in the limbs, irresistible urges to move,
usually the
legs; motor restlessness; when at rest, symptoms return or worsen; and
symptoms worsen
in the evening and at night.
[0189] In certain variations, the amplitude, frequency, direction of a
generated
magnetic field, electrical or magnetic stimulus, or firing sequence of the
coils or
microneedles making up the microneedle array may be adjusted. Optionally, the
current
may be varied according to a muscular response in the patient. Thus, to treat
restless leg
43
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
syndrome, the magnetic field or electrical stimulus is applied to a subject or
patient until
the desired effects (e.g., reduction of symptoms) are achieved.
[0190] In certain variations, methods of treating a subject suffering
from premature
ejaculation or various pelvic floor disorders utilizing the energy emitting
systems
described herein are contemplated. Symptoms associated with premature
ejaculation
may be observed, detected, or diagnosed. An energy emitting device having one
or more
energy generators, e.g., one or more conductive coils or one or more
microneedle
patches, may be positioned in proximity to a target nerve, e.g., the tibial or
posterior
tibial nerve or popliteal or sacral nerve or branches thereof of a subject
along a first
portion of a subject's body. The subject may or may not be exhibiting symptoms
associated with premature ejaculation. In the case of the conductive coils,
the coils may
be positioned within or along a housing, such as a foot or knee cradle, and a
foot or leg
may be positioned therein. In the case of a microneedle patch, the patch may
be
attached to a subject's skin. Optionally, the method involves positioning a
first portion
of a subject's body, the subject exhibiting symptoms associated with premature
ejaculation, relative to an energy emitting device such that a target nerve
within the first
portion of the body is in proximity to at least one energy generator disposed
within or
along the energy emitting device.
[0191] A current is then passed through the energy generator to produce,
generate or
deliver energy, e.g., a magnetic or electromagnetic field or electrical or
magnetic energy
or stimulus, focused on the tibial or posterior tibial nerve or branches
thereof. This in
turn may cause the stimulation of a pudendal nerve, sacral plexus, or other
nerves in the
pelvic floor or nerves associated with the control of ejaculation. Various
nerves
innervating the various muscles, sphincters, nerves, organs and conduits of
the urinary
tract, bladder or reproductive system, or pelvic floor may be stimulated
directly or
indirectly. Optionally, a current is passed through one more coils, which
generates a
magnetic or electromagnetic field which stimulates the posterior tibial nerve.
In certain
variations, the positioning of the coils relative to the first portion of the
subject's body
may be adjusted to re-focus the magnetic field on the posterior tibial nerve
as needed. In
certain variations, a current is passed through a microneedle patch generating
or
delivering an electrical or magnetic stimulus or field. The positioning of the
microneedle
patch relative to the first portion of the subject's body may be adjusted to
re-focus the
electrical or magnetic stimulus or field on the posterior tibial nerve as
needed.
44
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
[0192] Optionally, electrical conduction through the target nerve, e.g.,
the posterior
tibial nerve, or muscle stimulation can be detected via at least one sensor. A
conductive
sensor may be positioned in proximity to the posterior tibial nerve along a
second portion
of the subject's body. Optionally, a sensor may be positioned over a
corresponding
muscle to detect muscle stimulation or twitching resulting from nerve
stimulation.
Optionally, the electrical conduction is detected along a second portion of
the subject's
body which is different from the first portion of the body. Optionally, the
sensor in the
form of a microneedle patch. In certain variations, the sensor may be
positioned behind
a subject's knee to detect the electrical conduction along the afferent
posterior tibial
nerve or on another portion of a patient's leg or foot. In other variations,
the sensor may
be positioned within or along a housing along with the one or more conductive
coils.
[0193] Where a sensor is used, a signal is received from the sensors and
the signal is
indicative of the electrical conduction of the posterior tibial nerve. The
current may be
adjusted or varied using a controller which is in communication with the
energy
generator. Adjustments may be made in response to the nerve or muscle
stimulation
detected by the conductive sensor, in order to optimize or ensure adequate
treatment of
premature ejaculation by achieving the appropriate level of conductance and
appropriate
level of nerve or muscle stimulation. Appropriate levels for current,
frequency, magnetic
field, treatment duration, etc., are levels that result in an observed or
detected reduction
or prevention of symptoms associated with premature ejaculation. Treatment
could also
be administered and the appropriate levels and parameters achieved through
observing or
detecting reduction or prevention of symptoms where a sensor is not used.
Examples of
these symptoms include but are not limited to: ejaculation that frequently
occurs within
one minute or less of penetration; the inability to delay ejaculation on
penetrations; or
persistent or recurrent ejaculation with minimal stimulation before, on or
shortly after
penetration.
[0194] In certain variations, the amplitude, frequency, direction of a
generated
magnetic field, electrical or magnetic stimulus, or firing sequence of the
coils or
microneedles making up the microneedle array may be adjusted. Optionally, the
current
may be varied according to a muscular response in the patient. Thus, to treat
premature
ejaculation, the magnetic field or electrical stimulus is applied to a subject
or patient until
the desired effects (e.g., reduction of symptoms) are achieved.
[0195] Exemplary treatment parameters for treating various conditions,
e.g., urinary
incontinence, using the systems and methods described herein may include the
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
following. Operation of a conductive coil at about 10 to 20 hertz generating a
magnetic
field of about .25 to 1.5 tesla, where the coil is administered to a patient
for a duration of
about 30 minutes/day or 30 minutes per week, depending on the severity of the
symptoms, until the symptoms subside. The above treatment parameters or
variations on
the parameters may be used for treatment of urinary incontinence, fecal
incontinence,
restless leg syndrome, or premature ejaculation or other conditions. For
example, the
coil may be operated at various parameter ranges falling with the following
ranges: about
5 to 100 hertz, about 1 to 10 tesla, for about 15 minutes to 2 hours per day
or week. In
treating premature ejaculation, a patient may receive treatment about 4 to 10
hours prior
to intercourse. A maintenance phase of treatment, after the initial treatment,
may vary
for various conditions. For example, the maintenance phase may require
application of
the systems and methods described herein at the parameters described herein
for 30
minutes/week or 30 minutes/month. Any treatment parameter may be varied or
modified
based on the effect on the patient or sensor or patient feedback regarding
stimulation,
until the desired result of treating or preventing a condition is achieved.
[0196] In certain variations, as shown in FIGs. 29a-29d, energy emitting
device may
include a controller 289 and a foot cradle 290. Foot cradle 290 may include
vertical foot
plate 291, and horizontal foot plate 292, where each plate can be adjusted
using vertical
foot plate knob 293 and horizontal foot plate knob 294. One or more EMG plugs
295 are
provided. An air core coil 297 or other type of coil is provided. A display
screen 296
may also be provided along with power cord 298. The display screen 296 can
display a
variety of information to the user and/or practitioner such as the level of
power or current
applied, treatment time, temperature of the cradle device, detected current
levels and/or
physiological parameters, etc., to facilitate effective and efficient
therapeutic treatment.
The information can be used to vary or adjust the controller to ensure that
adequate
conduction of a target nerve, e.g., posterior tibial nerve 220 or muscle
stimulation occurs
and an adequate and accurate dosage of treatment is being received. Controls
may also
be included to affect the following: power, field strength, frequency, pulse,
start/pause
and cancelation of therapy (as shown) or other parameters one of skill in the
art would
find necessary or useful to control or monitor. In certain variations, a
sensor may be
connected, connected or in communication with the foot cradle or other energy
emitting
apparatus, controller, housing , conductive coils, or microneedle patch.
[0197] In certain variations, as shown in FIGS 30A-30B, an energy
emitting device
may include a controller and a knee support or knee cradle. The cradle may be
46
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
configured to provide the conductive coil in proximity to the popliteal fossa
or area
directly behind the knee. In certain variations, the knee cradle may be
configured to
cradle or surround at least a portion of the knee or substantially the entire
knee without
placing direct pressure on the popliteal fossa, thereby minimizing or avoiding
venous
thrombosis. In one variation, the device may be utilized while the knee is in
the flexed
position (Fig. 30A). In another variation, the device may be utilized while
the knee is in
a non-flexed position (Fig. 30B).
[0198] In certain variations, the energy emitting device, e.g., foot
support or cradle,
knee support or cradle, etc., includes a conductive coil positioned such that
a target nerve
is automatically targeted. The conductive coil is configured, sized and
positioned within
the device such that the generated magnetic field may encompass and stimulate
the target
nerve in any patient based on the target nerve's anatomical location, thus
providing
automatic targeting of the nerve in any patient once the patient positions a
particular
body portion in the device.
[0199] In certain variations described herein, sensors may detect voltage
or current
and may be connected, coupled, wirelessly connected or coupled or otherwise in
communication with housing, conductive coils, microneedle patch, energy
emitting
apparatus, energy generators, or electrode needles and/or controller using a
variety of
methods or techniques known in the art. In various variations described
herein,
housings, conductive coils, microneedle patches, energy emitting apparatus,
energy
generators, or electrode needles may be connected, coupled, wirelessly
connected or
coupled or otherwise in communication with each other, controllers or sensors,
using a
variety of methods or techniques known in the art.
[0200] Coils used in any of the variations described herein and
illustrated in the
corresponding figures may take on a variety of shapes, sizes, and
configurations. For
example, a coil may be shaped as a spiral (as shown) or have a simple helical
pattern or
be a figure eight coil, a four leaf clover coil, a Helmholtz coil, a modified
Helmholtz coil,
or may be shaped as a combination of the aforementioned coil patterns.
Additionally,
other coil designs beyond those mentioned hereinabove might be utilized as
long as a
magnetic field is developed that will encompass a target nerve.
[0201] The coils may have a variety of dimensions and configurations. In
certain
variations, a coil may have a central aperture. The diameter of the aperture
may range
from about 0.5 inch to 2 inches or 1 inch to 1.5 inches or the aperture may
have a
diameter of about 1 inch. The diameter of the coil body may vary. For example,
the
47
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
diameter may range from about 3.0 to about 7 inches or from about 4 to about 5
inches or
the diameter may about 4.5 inches. The coil body may include any suitable
number of
turns. For example, the coil body may include from about 2 to about 25 turns
or from
about 10 to about 20 turns or 14 to 17 turns. The adjacent turns may be spaced
apart
from each other, providing a gap there between. An end or cross section of a
turn may
have various dimensions. For example, the end or cross section may have a
height that is
greater than its width. An end or cross section of a turn may have a height
ranging from
about 1 to 5 cm or from about 10 mm to 51 mm (about .3 inches to 2 inches) or
about 25
mm to 40 mm (about 1 inch to 1.5 inches) or about 12 mm to 40 mm (about .5
inch to
1.5 inch) or about .5 inch to 2 inch. The end or cross section of the turn may
have a
width ranging from about 0.5 mm to about 5mm (about .019 inch to .19 inch) or
from
about lmm to about 2 mm (about .03 inch to .07 inch) or about .2 mm to about
1.6 mm
(about .01 inch to .06 inch). The above are all exemplary dimensions, where
other
dimensions are also contemplated depending on the use and configuration of a
device.
[0202] In certain variations, a system or device for electromagnetic or
magnetic
induction therapy may include one or more conductive coils disposed within or
along an
applicator. The coil may be configured to generate an electromagnetic or
magnetic field
focused on a target nerve, muscle or other body tissue positioned in proximity
to the coil.
The system may also include one or more sensors. The sensor may be configured
to
detect electrical conduction in the target nerve or to detect stimulation of a
muscle or
other body tissue. The sensor may also detect a muscular response caused by an
electrical conduction in a target nerve. The sensor provides feedback about
the efficacy
of the applied electromagnetic or magnetic induction therapy. Optionally, a
user may
provide such feedback based on detection by the user, with or without the use
of a
sensor. The system may also include a controller which is in communication
with the
sensor. The controller may be adjustable to vary a current through the coil in
order to
adjust the magnetic field focused upon the target nerve based on feedback from
the
sensor or user. The various systems or devices described herein may be
utilized with or
without a sensor.
[0203] A variety of electromagnetic or magnetic induction applicators
designed or
configured to stimulate various portions of a patient's body for treating
various
conditions are contemplated herein.
[0204] Figure 31A illustrates a variation of a hand or arm applicator
310. The hand
or arm applicator 310 may be ergonomic or contoured to a hand or arm to be
positioned
48
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
relative to or in proximity to a hand or arm to generate an electromagnetic or
magnetic
field focused on a target nerve, muscle or other tissue within the hand or
arm.
Optionally, a hand or arm applicator 310 may be designed to stimulate the
entire hand or
arm of a patient, for example, where the patient has limited or reduced nerve
innervation
to those portions of the body.
[0205] Figure 31B also illustrates a variation of a foot, knee or leg
applicator 320.
The foot, knee or leg applicator 320 may be ergonomic or contoured to a foot,
knee or
leg to be positioned relative to or in proximity to a foot, knee or leg to
generate an
electromagnetic or magnetic field focused on a target nerve, muscle or other
tissue within
the foot, knee or leg. Optionally, a foot, knee or leg applicator 320 may be
designed to
stimulate the entire foot, knee or leg of a patient, for example, where the
patient has
limited or reduced nerve innervation to those portions of the body.
[0206] Figure 32 illustrates a variation of a stand alone back
applicator 330. The
back applicator 330 may be ergonomic or contoured to the back or to a specific
area of
the back to be positioned relative to or in proximity to the back to generate
an
electromagnetic or magnetic field focused on a target nerve, muscle or other
tissue within
the back. A back applicator 330 may be aligned along the spine or positionable
in
proximity to the spine. The back applicator 330 may be utilized to stimulate
nerve
offshoots, dorsal ganglion, the spinal cord itself or any other nerve in the
body, to treat
various conditions, for example, to treat atrophy or paralysis.
[0207] The back applicator 330 may include several coils, which may be
pulsed
intermittently. In certain variations, a sensor may be placed on muscle in
dermatome to
provide feedback to ensure stimulation of the proper dorsal root ganglion or
vertebral
body. The sensor may provide feedback to channel energy or current to the
proper or
effective coil in an applicator, e.g., in an applicator having multiple coils.
[0208] Figure 33 shows a system including a corded back applicator 340,
a sensor
342 and a logic controller 344. Various sensors may be utilized, e.g., a three
lead EMG,
other EMG electrode, a microneedle electrode, or any sensor for detecting
physiologic
changes associated with nerve firing and/or muscle contraction. The sensor 342
provides
feedback which may be used to monitor and/or control therapy. The sensor 342
may be
used to position or optimize therapy in a clinic or home healthcare setting.
The
applicator 340 may or may not contain a pulse generator and/or logic
controller circuitry.
Figure 33 shows the logic controller 344 and pulse generator as a separate
unit. The
logic controller may optimize therapy and minimize energy usage or overheating
based
49
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
on feedback from sensor 342. Optionally, the logic controller 344 may be
incorporated
into an applicator. The logic controller 344, whether separate from the
applicator or
incorporated in the applicator, may be controlled based on feedback from the
sensor.
[0209] Figure 34 shows a system including a whole back applicator 350, a
sensor
352 and a logic controller 354. One or more back applicators 350 may be
provided. One
or more applicators 350 may include automated therapy targeting. The
applicators 350
may include multiple coils, which can be fired sequentially to stimulate the
entire spine
or chain of dorsal root ganglion (with or without user or sensor feedback) for
osteoarthritis therapy, back or neck pain therapy, prevention of muscular
atrophy and/or
nerve recovery after paralysis, stroke, or after suffering other nerve
damaging conditions.
In one variation, one or more applicators 350 may include multiple coils fired
sequentially in order to determine the optimal coil for stimulation based on
user or sensor
feedback. Once the optimal coil is determined, that coil may be selected and
used for the
remainder of the therapy. In another variation, one or more applicators may
include one
or more coils that are slidable, adjustable or movable within the applicator
housing. The
coils may be moved within the applicator housing to treat a large area and/or
to be
focused on the optimal treatment zone based on feedback from the user and/or
feedback
from the sensor.
[0210] Figure 35 shows a variation of a back applicator 360 which may be
positioned in proximity to or aligned along a spine. The back applicator 360
may have
ergonomic features or may be placed in proximity to a spine or a spine may be
positioned in proximity to the applicator 360. The applicator 360 may include
several
coils that are pulsed intermittently. As shown in Figure 35, the back
applicator 360 or
focused back applicator may me held on a patient by an ergonomic positioning
element
361 (e.g., a belt) and may be fit such the cervical, thoracic, lumbar, sacral
and/or
lumbosacral curvatures hold the back applicator 360 in the optimal position.
The
applicator 360 may be located anywhere along the positioning element 361
depending on
the individual and area to be stimulated. Optionally, a sensor lead 362 may be
placed
over musculature or along a nerve excited by activation of the applicator 360.
In one
variation, a coil power line 365 for supplying power or current from the logic
controller
364 to coils positioned in the applicator 360 may include fluid cooling, e.g.,
air or liquid
cooling.
[0211] Figure 36 shows an applicator 366 designed or configured to
generate a
magnetic field focused on a target nerve responsible for phantom or
neuropathic pain.
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
The applicator 366 or phantom pain therapeutic stimulator unit may be utilized
to treat
phantom pain or neuropathic pain, to provide phantom pain or neuropathic pain
therapy.
The applicator 366 may be ergonomic or contoured to be positioned relative to
or in
proximity to a nerve responsible for phantom or neuropathic pain.
[0212] Figure 37 shows a facial neuralgia applicator 380. Facial neuralgia
applicator
380 may be may be ergonomic or contoured to a face or head to be positioned
relative to
a face or head to stimulate a nerve responsible for facial neuralgias. The
applicator 380
may be designed or configured to be positioned relative to, in proximity to or
on a
patient's face or head and to generate a magnetic field focused on nerves
responsible for
facial neuralgias, e.g., the trigeminal nerve, to treat facial neuralgia.
Optionally, a
sensor may be positioned along a facial nerve to ensure adequate therapy and
to provide
feedback, e.g., to a logic controller, regarding nerve conduction or body
stimulation.
[0213] In certain variations, an applicator may be designed to
ergonomically target
common nerves responsible for common neuralgias in order to treat such
neuralgias. In
other variations, an applicator may be used for treating neuralgias virtually
anywhere on
a patient's body, including in deep nerves due to the ability of magnetic
fields generated
by the applicator to penetrate painlessly. In certain variations, an
applicator may be
designed to generate a magnetic field focused on a target nerve to treat
central or
peripheral neuralgias.
[0214] Figure 38 shows a depression applicator 386 which is designed or
configured
to be positioned relative to, in proximity to or over a frontal cortex. The
applicator 386
may be ergonomic or contoured to a head to be positioned relative to a head to
stimulate
the frontal cortex. The applicator 386 may generate an electromagnetic or
magnetic field
focused on the frontal cortex to treat depression. A sensor may be positioned
in the
offshoots of the motor cortex. The sensor may provide feedback to ensure
appropriate
placement of the applicator 386 or coil. In one variation, the applicator 386
may include
a therapeutic coil and a targeting coil (e.g., a small non-treatment coil),
which may be
positioned a certain distance behind the therapeutic coil, e.g., about 5 cm
behind the
therapeutic coil. When firing of the targeting coil is sensed by the sensor
(or user-
feedback), the therapeutic coil may be positioned in the correct or optimal
position over
the frontal cortex for depressive therapy.
[0215] Figure 39 shows a migraine applicator 390 which is designed or
configured to
be positioned relative to, in proximity to or over an occipital nerve. The
applicator 390
may be ergonomic or contoured to a face or head to be positioned relative to a
face or
51
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
head to stimulate the occipital nerve. The applicator 390 may generate an
electromagnetic or magnetic field focused on the occipital nerve to halt,
prevent or treat
migraines. The applicator 390 may have an ergonomic design to ensure
appropriate
placement over the occipital nerve. In one variation, the applicator 390 may
be a single
(or few) pulse device. The applicator 390 may be in a portable format. The
applicator
390 may also be without any significant cooling features. Optionally, the
applicator 390
may have cooling features. In another variation, an applicator may be a
multiple pulse,
higher frequency device. Such an applicator may include cooling features,
where
cooling is provided by utilizing liquids or airflow, such as rapid airflow to
cool the coils
or applicator.
[0216] Figure 40 shows a variation of an applicator 396 in the form of a
stimulator
coil platform which may be ergonomic and contoured to a knee. The applicator
396 is
configured to be positioned relative to or in proximity to a knee or the
applicator 396 is
configured such that a knee may be positioned relative to or in proximity to
the
applicator 396. The applicator 396 may be configured to generate an
electromagnetic or
magnetic field focused on the popliteal nerve for peripheral nerve stimulation
to treat
various conditions, e.g., overactive bladder, neuropathic pain or restless
legs. In one
variation, a stimulator coil may target an area behind a patient's knee or the
popliteal
fossa, and the knee may be rested on a stimulator coil platform applicator in
any position.
[0217] In certain variations, an applicator may include one or two
(bilateral)
magnetic field generating coils, which may be positioned around the knee when
the
patient is in a sitting, standing or prostrate position. In certain
variations, a pulse
generator or logic controller 397 may send energy through one or more coils to
create an
electromagnetic or magnetic field. The applicator or coils may generate
stimulator or
non-stimulator fields. Sensor or user feedback may provide feedback to logic
controller
to optimize therapy, e.g., with the stimulator fields. An applicator may be
utilized for
generating magnetic fields focused on an area of a patient's body, e.g., the
knee, to treat
various orthopedic indications, e.g., knee pain or osteoarthritis. An
applicator may be
utilized for generating magnetic fields focused on a area of a patient's body
to treat
various non-orthopedic indications, via, e.g., peripheral nerve stimulation.
[0218] Figures 30A-30B, show a variation of an applicator 400 which may
be
utilized for popliteal nerve stimulation and/or treatment of the knee. The
applicator may
be designed or configured to generate an electromagnetic or magnetic field
focused on
the popliteal nerve for popliteal nerve stimulation or on the knee for
treating
52
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
osteoarthritis. The applicator is configured to be positioned relative to or
in proximity to
a knee or the applicator is configured such that a knee may be positioned
relative to or in
proximity to the applicator. A leg may rest on the applicator coil or be
positioned above
it. Optionally, as shown in Figure 30B, a foot rest 104 may be provided for
holding up a
foot.
[0219] Figure 41 shows a system including a variation of an ergonomic
back
applicator 410 held on a patient's body by an ergonomic positioning element
411 in the
form of a shoulder harness. A sensor 412, and a logic controller 414 are also
provided.
The applicator 410 may include various positioning elements 411, e.g., a
shoulder
harness, an upper torso garment, or an ergonomic back-countered plate. The
applicator
410 may be stimulator or non-stimulator. In another variation, an applicator
may be
rested on a seat or chair such that a stimulator coil reliably overlies the
area of the
patient's body requiring stimulation. In certain variations, one or more coils
may be
fixed on the applicator (requiring prior targeting by a healthcare provider or
patient) or
one or more coils may move freely within or along the applicator and may be
locked into
position when the desired or optimal position is located. Coils may also move
automatically in order to optimize targeting of the coil based on sensor or
user feedback.
The system may be incorporated into a single unit or, as illustrated, have at
least two
components including a separate logic controller.
[0220] For any of the applicators described herein, such applicators may
include one
or more of the following features. The applicators may be ergonomic or
contoured to the
specific region of the body or anatomy to which the applicator will be
delivering
stimulation. The applicators may be configured or designed to be positioned
relative to,
on, around, or in proximity to a specific region of the body or the
applicators may be
configured or designed such that the targeted region of the body may be
positioned
relative to, on, around or in proximity to the applicator. The applicators may
be
openable or adjustable to allow for insertion or entrance of the targeted body
part or
anatomy into the applicator or to allow for placement of the applicator onto
or around the
targeted body part or anatomy. The applicators may be flexible or ergonomic to
accommodate nearly any type of body habitus. In certain variations, a solenoid-
type coil
may incorporated into an applicator for delivering PEMF stimulation directly
to the
targeted areas or regions of a body. In certain variations, any of the
applicators
described herein may approximate the respective targeted body area or anatomy
or the
53
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
applicators may be designed such that the body region or targeted anatomy may
approximate the applicator.
[0221] In certain variations, any of the applicators or systems
described herein may
be used to provide electromagnetic or magnetic induction therapy with or
without a
sensor.
[0222] In certain variations, electromagnetic stimulating devices or
applicators for
providing stimulation to tissues of the human body, including nerves, muscles
(including
superficial and deep muscles), and/or other body tissues for the treatment of
various
conditions, including, e.g., chronic and acute pain, are provided.
[0223] The devices may utilize an inductive coil encased within an
ergonomic, body-
contoured applicator to target specific regions of the body. The coils may be
designed to
target peripheral nerves throughout the body that have been implicated or
involved in
pain syndromes.
[0224] The various designs and configuration of the devices described
herein allow
for easier application, more consistent therapy and home use while targeting
anatomic
regions with therapeutic pulsed electromagnetic fields. The fields may also be
delivered
or applied in an intermittent manner to allow for convenience and ease of use
while
providing a durable benefit. With intermittent external stimulation by pulsed
electromagnetic or magnetic fields, a nerve or other tissues may be stimulated
in manner
that provides a continued and lasting effect on nerve, muscle or tissue
function, without
habituation.
[0225] The electromagnetic or magnetic induction stimulation devices
described
herein substantially improve the state of the art electromagnetic stimulation
technology
and may incorporate the delivery of PEMF therapy into a user friendly, body
contoured
applicator. In certain variations, a delivery system for PEMF therapy may
include
elements such as, e.g., (1) an ergonomic, body contoured applicator which
provides for
repetitive application and consistent therapy onto the same body area. The
applicator
may be coded with clear markings to facilitate repetitive and consistent
therapy onto the
same body area; (2) the use of a sensor to provide feedback that stimulation
is occurring
effectively; and/or (3) the use of intermittent stimulation to effectively
treat various
conditions, e.g., chronic pain, without habituation. These elements
individually or the
various combinations of these elements have provided for an easy to use,
ergonomically
designed system that has applications within a host of clinical and home ease
of use
health applications.
54
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
[0226] In certain variations, an electromagnetic or magnetic induction
stimulation
device able to provide stimulation to tissues of the human body, including
nerves,
muscles (including superficial and deep muscles), and/or other body tissues
without
significant discomfort to the patient is provided. Conductive stimulating
coils may be
encased in an ergonomic, body-contoured applicator that is coded with clear
markings to
provide for repetitive application and consistent therapy onto the same body
area. The
design of the applicator allows for ease of use and also for the targeting of
anatomic
regions to be exposed to the impulses of the PEMFs. The electromagnetic
stimulating
device may provide PEMF in a manner that is patient user friendly and the
device may
be portable. The device may be utilized in a hospital, an outpatient clinic, a
therapist's
office, or at a patient's home.
[0227] In certain variations, an electromagnetic or magnetic induction
stimulation
device may stimulate regions of the body to treat conditions requiring both
maximal
stimulation (i.e., sufficient to cause contraction of muscle fibers and firing
of nerves) as
well as submaximal stimulation (which will be sufficient to provide therapy
but not to
cause contraction of muscle fibers).
[0228] The electromagnetic or magnetic induction or stimulating devices
described
herein may be utilized for various indications. The indications may be divided
into
maximal and submaximal categories, in which the former requires significantly
higher
levels of inducting current than the latter. The maximal applications of the
device
include, but are not limited to: Non-invasive stimulation (intermittent or
continuous) of
the peripheral nervous system for treating chronic pain; stimulation of a
nerve for the up-
or down-regulation of hormones or cellular proliferation; treatment and/or
prevention of
atrophy, which would be therapeutic during recovery after an individual
sustains a
fracture, experiences paralysis of a limb or other body part, or undergoes
surgery, such as
ACL repair in the knee; treatment of neurogenic or overactive bladder and
bowel; and
stimulation of the central nervous system to alter neural pathways or up/down-
regulate
the aforementioned factors.
[0229] Additional applications of the devices include but are not
limited to: treatment
of neuropathic pain (e.g., phantom pain in limbs or other neurologic pain) or
orthopedic
pain (back and neck pain or skeletal related pain); treatment of overactive
bladder and
bowel; and treatment of arthritis and/or orthopedic conditions.
[0230] In certain variations, a device is provided for delivering PEMF
stimulation to
selective anatomic regions of the body, utilizing an ergonomic applicator
designed to
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
facilitate accurate and targeted delivery of therapy. The applicator may be
coded with
clear or solid markings to provide for repetitive application and consistent
therapy onto
the same body area of the body. This design may facilitate the placement of
the device
for the stimulation of key nerves, muscles, and/or body tissues.
[0231] In certain variations, a device is provided which may be utilized to
electromagnetically stimulate selective nerves, muscles, and/or body tissues,
where the
device is user friendly and capable of being used even by an unskilled patient
in a home
healthcare setting.
[0232] In certain variations, a device is provided to
electromagnetically stimulate
selective nerves, muscles, and body tissues to provide consistent therapy,
with an
ergonomic applicator targeting key nerves and eliminating the requirement for
a highly
trained operator to manipulate the device.
[0233] In certain variations, an electromagnetic or magnetic induction
system or
device may be configured or designed to provide intermittently applied pulsed
magnetic
fields in the treatment of chronic conditions, such as pain. For example, a
device as
described herein may provide shorter, intermittent stimulation to treat
chronic pain or
other chronic conditions. The delivery of pulsed magnetic fields may have a
continued
and lasting effect on nerve function in treating conditions, such as,
overactive bladder as
well as other chronic neurological and orthopedic conditions such as
neuropathic pain,
restless legs and orthopedic pain (e.g., spinal pain, back pain, etc.)
[0234] In certain variations, intermittent pulsed magnetic fields may be
utilized for
the treatment of chronic and acute non-orthopedic conditions such as
neuropathic pain,
phantom pain and chronic neuralgias, as well chronic and acute orthopedic
conditions,
such as back pain and neck pain. The therapeutic magnetic fields may be
applied
frequently (e.g., several times a day) or less frequently (e.g., once a week
or once a
month) depending on the durability of the effect for the individual patient.
Treatment
involving the use of magnetic fields does not require surgery or needles to
stimulate a
nerve. Also, the deliver of intermittent pulsed magnetic fields prevents the
nerve from
becoming habituated to the stimulator signal by ensuring that there are
periods during
which the nerve is not subjected to the stimulator signal. Accordingly, the
electromagnetic or magnetic induction systems or devices described herein may
provide
unparalleled ease of use, non-invasiveness, reliability of therapy based on
sensor
feedback and/or ergonomic targeting, and/or a lack of habituation due to
intermittent
stimulation provided by certain systems and devices.
56
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
[0235] In certain variations, the electromagnetic or magnetic induction
systems or
devices described herein may incorporate an air-cooled coil wherein the air
coolant, e.g.,
liquid or air, is drawn through and/or in between the turns of the inductive
coil, in direct
contact with conductive surfaces of the coil. Drawing air or other fluid
through the coil
prevents the coil from heating up to the degree that could damage the coil and
the
electronics of a device, or expose the patient to excessive temperatures.
[0236] In certain variations, the systems and devices described therein
may be
utilized to stimulate nerves for a variety of conditions, including, e.g.,
atrophy
prevention, nerve repair/regeneration, neuromodulation, chronic pain, up or
down
regulation of hormones, restless legs, phantom pain, etc. The systems and
devices may
also be used to stimulate muscles and/or other body tissues to accelerate
tissue healing,
regeneration and/or growth.
[0237] In certain variations, the electromagnetic or magnetic induction
systems or
devices described herein and other implantable or extracorporeal devices may
allow for
the automatic adjusting of nerve stimulation based on feedback.
[0238] In one variation, an extracorporeal or implantable device, e.g.,
any of the
electromagnetic or magnetic induction devices described herein, a pacemaker,
defibrillator, or nerve stimulator, may include a feature that allows for
automatic
adjustment of nerve stimulation based on feedback provided by a sensor or
user. This
feature may minimize pain and power usage while ensuring optimal therapy
delivery. A
device may include a stimulator and a sensing component. The stimulator may be
automatically adjustable based on feedback from the sensor up to a maximal
(safe)
threshold. Each therapy may start with lower powered pulses, followed by
increasing
power pulses until the sensor detects stimulation. The algorithm allows for
the minimal
amount of power to be used and allows for automatic adjustment of power
settings as
conditions change.
[0239] In one variation, an implantable device may include a sensor,
such that the
device can stimulate tissue or nerves and sense stimulation at the same site.
For
example, the sensor may provide feedback to the implantable device regarding
nerve
conduction at the site of stimulation. As fibroses develops around an implant,
at the site
of stimulation, the feedback will indicate whether a target nerve is no longer
being
effectively stimulated due to the fibroses, which will cause the power or
level of
stimulation to increase or decrease, as is necessary, to effectively stimulate
the target site
and overcome any obstruction due to fibroses. As fibroses occurs around an
implant, a
57
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
patient need not report back to a physician or other operator for adjustment
of the
stimulator power of the device. The device will automatically adjust the
stimulator
power or level based on sensing stimulation of the target nerve or tissue.
This eliminate
the guesswork involved by the user in monitoring their therapy one day at a
time on their
own, as they notice the effect of the therapy wear off. This also eliminates
the risk of the
user being exposed to unnecessarily high power levels that might otherwise by
set in
order to minimize frequent return visits to a physician or operator for
adjustments.
[0240] Figures 42A and 42 show an example of how the amount of
stimulator power
required to achieve a desired stimulus may be automatically adjusted as a
result of
fibroses, according to the above described feature. According to Figure 42A,
after the
initial implant of the device, the level of stimulator power is increased
until stimulation
of the target nerve or tissue is sensed (indicated by square box at, e.g.,
about 10mV). An
effective stimulator therapy may then be delivered. According to Figure 42B,
after
fibroses sets in, in order to maintain the desired level of stimulation to
provide an
effective stimulator therapy, the level of stimulator power is increased until
stimulation
of the target nerve or tissue is sensed (indicated by square box, e.g., at 20
mV).
According to the example in Figure 42B, the presence of fibroses required an
increase in
the stimulator power level to deliver an effective stimulator therapy.
[0241] The automatic adjustment feature based on sensor feedback may be
utilized in
any stimulator or non-stimulator implant or extracorperal device, where the
device
incorporates a sensor capable of detecting the desired stimulus and a feedback
loop
capable of automatically adjusting parameters (e.g., power, frequency, etc.)
to ensure
appropriate stimulation.
[0242] In certain variations, the electromagnetic or magnetic induction
systems or
devices described herein and other implantable or extracorporeal devices may
include a
feature that allows for automatic targeting of coils.
[0243] A device may include multiple inductive coils or one or more
movable
inductive coils. The device may also include a sensor based feedback
algorithm. In one
variation, the device includes a targeting or movable coil which may be
positioned over
or in proximity to a patient's body at a site that elicits a response that can
be sensed
automatically or detected by a user. Once this response is detected, the coil
may either
move to its stimulation position, or in the event that a small targeting coil
is used, the
therapeutic coil may already overlie the treatment area. Once the response is
detected,
the therapy may automatically begin.
58
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
[0244] In one example, relating to the treatment of depression, the
motor cortex is
stimulated until the thumb is seen to move. The coil may then be advanced,
e.g., about 5
cm. to about 5 inches, forward to a position over the frontal cortex. This
feature
eliminates the guesswork that may otherwise be involved in moving or
positioning a coil,
and automates therapy based on user feedback or EMG senor or other sensor
feedback,
e.g., over a thumb.
[0245] Figure 43A shows an example of a device 420 positioned on a
skull. The
device includes a treatment coil 422 and a targeting coil 423. The treatment
coil 422
may be positioned by EMG detection with targeting coil 423 stimulation, where
the
targeting coil may not move.
[0246] In another variation shown in Figure 43B, an ergonomic fixture or
applicator
430 (e.g., a helmet) may be worn and a coil 432 positioned on the applicator
may slide or
move from its targeting position to its therapeutic position automatically or
by user
intervention.
[0247] The feature that allows for automatic targeting of coils may be
utilized in any
device designed to stimulate nerve, body or other tissues with stimulator or
sub-
stimulator fields in which the device may be targeted based on a detectable
signal or
response.
[0248] Other conditions that may be treated utilizing the various
electromagnetic or
magnetic induction stimulation systems and methods described herein include
but are not
limited to: pelvic pain, interstitial cystitis, fibromyalgia, chronic fatigue
and preterm
labor, pain syndromes, Irritable Bowel Syndrome, Vulvodynia, Herpetiuc
neuralgia,
trigeminal neuralgia and Myofascial pain.
[0249] EXAMPLES
[0250] The following Examples are provided for illustration, not
limitation. One with
skill in the art would be able to use these examples as guidelines for making
and using
comparable devices.
[0251] In each example, intermittent therapy should be applied and
symptoms/scores
tracked for a minimum of 6 weeks in order to determine the full extent of the
therapies
effect.
[0252] Example 1: Empirical Testing of Efficacy in the Treatment of
Neuropathic
Pain: The optimal stimulus intensity for neuropathic pain treatment; the
optimal
application parameters, i.e. frequency of stimulation, duration of treatment,
location of
stimulator coils in each disposable array of coils; and the optimal coil
59
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
diameter/placement within the strays can be determined using the following
experimental protocol: Before, during and after treatment, patients will
report scores of
neuropathic pain after weekly stimulation over a minimum of 6 weeks.
[0253] Example 2: Empirical Testing of Efficacy in the Treatment of
Neuromuscular
Pain: The efficacy of neuromuscular pain treatment can be tested by monitoring
patient
reported pain scores. A standardized scale may be utilized and, when feasible,
local
biopsy and blood tests can be useful in determining the impact of the
therapeutic fields
on circulating factors and local mediators. The optimal pulse amplitude,
duration, site of
stimulation will be assessed based on reported pain scores and diagnostic
tests.
[0254] Example 3: Empirical Testing of Efficacy in the Treatment of
Orthopedic
conditions (i.e., Arthritis, Back pain and neck pain): The efficacy of
arthritis treatment
can be tested by monitoring patient reported functionality scores. A
standardized
subjective functionality scale may be utilized and, when feasible, local
biopsy may be
useful in determining the impact of the therapeutic fields on the cartilage
and arthritic
regions treated. As cartilage destruction is a well-studied side-effect of
arthritis,
reduction of this degeneration will be a valuable marker for efficacy of
therapeutic
treatments. The optimal pulse amplitude, duration, site of stimulation will be
assessed
based on reported functionality scores and diagnostic tests. Pain scores may
also be
measured to determine the device's impact on orthopedic conditions such as
back pain,
neck pain, etc. A standardized pain scale may be used before and after
treatment to
determine potential benefit.
[0255] It is also contemplated that any of the energy emitting systems
or devices
described herein can be used with or without a sensor for detecting conduction
of a
stimulated nerve or muscle of tissue stimulation resulting from the
electromagnetic or
magnetic field generated by the conductive coil and delivered to a patient or
an electrical
stimulus delivered to a patient. Also, in any of the above variations, a
controller may
optionally be connected, coupled, integral to or otherwise in communication
with the
conductive coils and/or the sensor. Optionally, the sensor may be connected,
coupled,
integral to or otherwise in communication with the conductive coil.
[0256] In certain variations, transdermal electrical stimulation therapy
may be
provided to a patient or subject. One or more stimulator electrodes may be
positioned
over a glabrous skin surface overlying a target nerve of the patient.
Electrical
stimulation or an electrical stimulus may then be delivered via the stimulator
electrode
through or across the glabrous skin surface to the target nerve in the
patient, to stimulate
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
the target nerve. The electrical stimulation may be delivered at a level and
in a manner
sufficient to generate motor and/or sensory nerve conduction. For example, the
electrical
stimulation may be delivered at a frequency of about 5 Hz to about 80 Hz or
about 20
HZ or 30 Hz to about 60Hz or greater than 30 Hz, while remaining safe and
tolerable to
the patient. The stimulation may be delivered in a non-invasive manner.
[0257] The stimulator electrode may be positioned over or on various
regions of the
body. In certain variations, the stimulator electrode may be positioned over a
palm or
plantar skin surface. For example, the stimulator electrode may be positioned
over a
glabrous skin surface overlying a plantar nerve of the foot, where the
electrical
stimulation may stimulate the plantar nerve. Stimulation of the plantar nerve
may result
in stimulation of the tibial nerve to treat various conditions, such as
urinary incontinence
or overactive bladder.
[0258] Various types of electrodes may be utilized as a stimulator
electrode, e.g., a
surface electrode, microneedle electrode, a TENS (transcutaneous electrical
nerve
stimulation) patch or other electrode that may be positioned over or on the
skin surface.
Optionally, a conductive substance may be injected or implanted near the
target nerve to
improve electrical conductivity to the target nerve from the stimulatory
electrode.
Electrical stimulation may be delivered intermittently or on a chronic basis
and may
include one or more electrical signals designed to be constructive and/or
destructive in
order to improve tissue penetration and/or signal tolerance.
[0259] In certain variations, nerve stimulation resulting from the
electrical
stimulation therapy may be detected via at least one sensor positioned on or
near the
subject. The sensor may provide a signal indicative of the detected electrical
stimulation
thereby providing feedback about the efficacy of the applied electrical
stimulation
therapy such that the therapy may be adjusted or optimized. The feedback loop
may be
queried such that the electrical stimulation therapy may be adjusted to ensure
that the
minimum amount of energy is being applied to stimulate the target nerve while
reducing
the risk of burns or intolerance. Optionally, the feedback loop may be queried
such that
the positioning of the stimulator electrode may be adjusted to optimize the
electrical
stimulation therapy. Various sensors may be utilized, including but not
limited to a
surface electrode, a microneedle electrode, or motion sensor. In certain
variations, the
sensor may detect conduction of motor and/or sensory nerves. For example, a
sensor
may detect afferent or efferent nerve stimulation of the target nerve or other
nerve or
related nerve.
61
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
[0260] In certain variations, a ground electrode may be utilized. For
example, the
ground electrode may be positioned on the subject to facilitate penetration of
an
electrical current from the stimulatory electrode through the glabrous surface
to stimulate
the target nerve.
[0261] In certain variations, a stimulator electrode may be positioned over
or
attached to a glabrous skin surface with an adhesive or other form of
attachment or
fastener. In other variations, a stimulator electrode may be positioned over
or held in
contact with a glabrous skin surface with an ergonomic applicator.
[0262] Various applicators for positioning electrodes over various
regions of the
body to deliver electrical stimulation, e.g., transdermal electrical
stimulation, may be
utilized. For example, an applicator may be in the form of an insole
configured to be
positioned against, over, or in contact with the plantar surface or a glabrous
surface of
the foot such that an electrode positioned on the insole may deliver
electrical stimulation
through or across the glabrous skin surface, to a target nerve or other tissue
within the
foot. The insole may be configured to be positioned in an orthotic or a shoe
such that
electrical stimulation may be delivered to the subject while the subject is
walking. In
other variations, an applicator may be a foot plate or foot rest or cradle on
which a foot
or other portion of the leg or body may be positioned to receive electrical
stimulation
through or across a glabrous skin surface, from the plate, rest or cradle or
from electrodes
of the plate, rest or cradle.
[0263] In another variation, an applicator may be a glove, brace or
other hand wrap
which is configured to be positioned against, in contact with, or over the
palmar surface
or a glabrous surface of a hand. An electrode of the glove, brace or wrap may
deliver
electrical stimulation through or across the glabrous skin surface to a target
nerve or
tissue within the hand.
[0264] Various applicators or ergonomic applicators may be utilized to
provide
transdermal electrical stimulation therapy to a subject. In certain
variations, an
applicator may include one or more stimulator electrodes and one or more
electrical
pulse generators or controllers. The electrical pulse generator may be coupled
to the
stimulator electrode. The electrical pulse generator may be incorporated into
the
applicator. Optionally, the electrical pulse generator may be separate from
the applicator
or located remotely from the applicator or stimulator electrode. An applicator
may
include one or more sensor electrodes configured to detect nerve stimulation
and/or
provide feedback about the efficacy of the applied electrical stimulation
therapy. Such
62
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
feedback may allow the therapy to be adjusted, modulated and/or optimized. A
sensor
may detect motor and/or sensory nerve stimulation. For example, a sensor may
detect
afferent or efferent nerve stimulation. In certain variations, the positioning
of the
stimulator electrode may be adjusted based on feedback from the sensor in
order to
optimize the electrical stimulation therapy.
[0265] The applicator may also include one or more ground or ground
electrodes.
The ground electrode may be a component of the applicator or may be attached
to the
subject separately via a strap or other attachment. The ground electrode may
be
positioned on the subject at a location that facilitates penetration of an
electrical current
through a glabrous surface to stimulate the target nerve, e.g., on the
opposite surface of a
body portion relative to the stimulator electrode.
[0266] As stated supra, an applicator may be designed for various
portions of the
body. An ergonomic applicator may be in the form of an insole which can be
positioned
against or over the plantar surface or a glabrous surface of the foot such
that an electrode
of the insole may deliver electrical stimulation through or across a glabrous
surface to a
target nerve within the foot. The insole may be positioned in an orthotic or a
shoe. An
applicator may also be in the form of a foot plate, cradle or foot rest. In
other variations,
the applicator may be designed to stimulate a portion of a hand or upper
extremity. For
example, an ergonomic applicator in the form of a glove or brace may be
configured to
be positioned against or over the palmar surface of a hand such that the
electrode of the
glove or brace may deliver electrical stimulation across a glabrous surface to
a target
nerve within the hand.
[0267] Any of the various applicators or ergonomic applicators described
herein may
be designed or configured to position or locate one or more stimulator
electrodes over or
on a glabrous skin surface of a patient to deliver transdermal electrical
stimulation
through or across the glabrous skin surface to an underlying target nerve or
other tissue
to stimulate the target nerve or tissue. In certain variations, an electrical
stimulation or
stimulus may be delivered at various frequencies, e.g., at a frequency of
about 5 Hz to
about 60 Hz, or greater than 30 Hz, while remaining safe and tolerable to the
subject.
[0268] In other variations, one or more stimulatory electrodes may be
otherwise
attached to a skin surface, e.g., as a wired or wireless patch, adhesive or
microneedle
electrode in the absence of an ergonomic applicator, to deliver electrical
stimulation
across or through a glabrous skin surface to stimulate an underlying target
nerve or
tissue. Such electrodes may be used in combination with one or more ground or
sensor
63
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
electrodes. Such stimulatory electrodes may deliver electrical stimulation or
an electrical
stimulus at various frequencies, e.g., at a frequency of about 5 Hz to about
60 Hz, or
greater than 30 Hz, while remaining safe and tolerable to the subject.
[0269] In certain variations an applicator may include two or more
stimulator
electrodes or two more stimulator electrodes may be utilized without an
applicator.
[0270] In certain variations, methods, systems and/or applicators for
providing an
energy based stimulation therapy to a subject are provided. An energy emitting
device
may be positioned in proximity to a glabrous surface overlying a target
tissue. Energy
may be delivered from the energy emitting device through or across the
glabrous skin
surface to the target tissue to stimulate the target tissue, such as a target
nerve. Various
energy sources or forms of energy may be delivered by the energy emitting
device,
including but not limited to, an electric current, an electromagnetic or
magnetic field or
electromagnetic or magnetic induction stimulation, ultrasound, or RF fields.
Energy may
be delivered at various frequencies. For example, the energy may be delivered
at a
frequency of about 5 Hz to about 60 Hz, or greater than 30 Hz, while remaining
safe and
tolerable to the subject.
[0271] The energy based stimulation therapy may be utilized to treat
various
conditions, including but not limited to, overactive bladder, urinary
incontinence, fecal
incontinence, chronic pain, depression, migraine, epilepsy, obesity, restless
leg
syndrome, or foot drop. Energy may be delivered through a glabrous skin
surface to
provide neuromodulation to stimulate other tissue. Tissues that may be
stimulated
include but are not limited a central nerve, peripheral nerve, muscle, skin,
or vasculature.
Optionally, conductive substance may be implanted or injected near a target
tissue to
improve conductivity to the target tissue.
[0272] In one variation, a method for treating urinary incontinence or
overactive
bladder in a subject may include one or more of the following steps, a
stimulator
electrode, (e.g., in an applicator or as an adhesive or attachment electrode
as described
above) may be positioned over a glabrous skin surface overlying a plantar
nerve or other
nerve in the foot. An electrical stimulation may then be delivered through or
across the
glabrous skin surface to the plantar nerve to stimulate the plantar nerve
which results in
stimulation of the tibial nerve to treat urinary incontinence or overactive
bladder, e.g., via
stimulation of the sacral plexus or pudendal nerve.
64
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
[0273] In other variations, any of the various electrical stimulation
methods and
electrical stimulator applicators or electrodes may be utilized to treat any
of the
conditions described herein.
[0274] In certain variations, methods, systems and/or applicators for
providing an
energy based stimulation therapy to a subject are provided. An energy emitting
device
may be positioned in proximity to or over a skin surface overlying a target
nerve or
tissue. The energy emitting device may include any of the devices, systems or
applicators described herein and/or illustrated in any of the various figures
1-49, e.g., an
electrode or applicator for delivering electrical stimulation or an applicator
for providing
electromagnetic or magnetic stimulation or induction therapy. Energy may be
delivered
at a frequency of about 1 Hz to about 30 Hz through or across a skin surface
(e.g., a
glabrous skin surface or any other skin surface or non-glabrous skin surface)
to the a
target nerve to generate motor and/or sensory nerve conduction while remaining
safe and
tolerable to the subject. In certain variations, energy may be delivered at a
frequency of
less than 10 Hz to generate motor and/or sensory nerve conduction.
[0275] Energy delivered transdermally, through, or across a patient's
skin at a
frequency from about 1 Hz to about 30 Hz, or at a frequency of less than 10 Hz
has
unexpectedly been found to stimulate or generate motor and/or sensory
conduction in a
target nerve. For example, energy delivered transdermally, through, or across
a patients
skin at about 1 Hz to about 30 Hz, or at less than 10 Hz has unexpectedly been
found to
stimulate or generate motor and/or sensory nerve conduction of a tibial nerve,
where
such level of stimulation may be sufficient to treat a patient suffering from
urinary
incontinence, overactive bladder, fecal incontinence or other conditions. The
energy
may be delivered through or across a glabrous skin surface or non-glabrous
skin surface
or any other skin surface (e.g., any skin surface overlying a tibial nerve).
[0276] Various energy sources or forms of energy may be delivered by the
energy
emitting device, including but not limited to, an electric current, an
electromagnetic or
magnetic field, ultrasound, or RF fields. The energy based stimulation therapy
may be
utilized to treat various conditions, including but not limited to, overactive
bladder,
urinary incontinence, fecal incontinence, chronic pain, depression, migraine,
epilepsy,
obesity, restless leg syndrome, or foot drop. Energy may be delivered through
a glabrous
skin surface or any other skin surface to provide neuromodulation to stimulate
other
tissue. Tissues that may be stimulated include but are not limited a central
nerve,
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
peripheral nerve, muscle, skin, or vasculature. Optionally, conductive
substance may
implanted or injected near a target tissue to improve conductivity to the
target tissue.
[0277] Figure 44 shows the anatomy of a foot, providing a view of the
underside,
sole or plantar or glabrous surface of the foot. The medial and lateral
plantar nerves
innervate the sole of the foot and arise from the posterior branch of the
tibial nerve.
Various stimulator sites for stimulating the plantar nerves or branches
thereof may be
located near, in proximity to or along the plantar nerves. Figure 44 shows one
example
of a stimulator site 510 located over the glabrous surface of the foot, over
the plantar
nerves. A second exemplary stimulator site 512 is also depicted at a location
over the
glabrous surface of the foot, over the plantar nerves, distal to the first
stimulator site 510.
An energy based stimulus, e.g., an electrical or electromagnetic stimulus, may
be
delivered transdermally, through or across a glabrous surface of the foot at
one or more
of the stimulator sites to stimulate the plantar nerves or other nerves in the
foot.
[0278] In certain variations, electrical stimulation may be delivered
transdermally,
through or across a glabrous surface on a subject, e.g., through or across a
glabrous
surface of the foot or hand. The electrical stimulation may be sufficient to
generate
conduction of motor and/or sensory nerves. An electrical stimulation may be
delivered
by placing one or more electrodes anywhere over or on a glabrous skin surface
overlying
one or more target nerves or other target tissue. Stimulator and/or ground
electrodes may
be utilized. Exemplary electrodes may include but are not limited to surface
electrodes,
dry electrodes, gel electrodes, microneedle electrodes or any other suitable
electrode for
delivering an electrical stimulus. An electrode may be adhered or otherwise
attached to a
glabrous surface. Optionally, an electrode may be held or positioned in
contact with, in
proximity to, or over a glabrous surface with a wearable garment, cradle,
applicator or
body portion rest or support (as described in further detail herein).
[0279] In one variation, electrical stimulation may be delivered to one
or more of the
stimulator sites or similar sites depicted in Figure 44, by positioning one or
more
electrodes over a glabrous surface of the foot, over an underlying plantar
nerve or other
target nerve. A ground electrode (not shown) may be positioned anywhere on the
foot.
For example, the ground electrode may be positioned over a posterior or upper
surface of
the foot to encourage or facilitate deeper penetration of the electrical
current or
stimulation, through the glabrous surface of the foot, to a plantar nerve or
other target
nerve within the foot.
66
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
[0280] Delivery of electrical stimulation through or across a glabrous
surface of the
body via an electrode positioned over a glabrous surface, e.g., a glabrous
surface on a
palmar or plantar surface, unexpectedly allows for the use of a higher
frequency and/or
higher amplitude electrical pulsation or electrical stimulus to deliver the
electrical
stimulation than would otherwise be safe and/or tolerable to deliver
electrical stimulation
through a non-glabrous surface of the body. For example, an electrical
stimulus having a
frequency of about 5 Hz to about 60 Hz (a range found to be effective for
generating
motor and/or sensory nerve conduction of the posterior tibial nerve) may be
utilized to
stimulate a target nerve (to generate motor and/or sensory nerve conduction
therein) or
tissue through or across a glabrous skin surface (via an electrode positioned
over the
glabrous skin surface) in a manner that remains safe and tolerable to the
patient and
avoids burns or injury. Optionally, an electrical stimulus having a frequency
of about 5
Hz to about 60 Hz, or greater than 30 Hz, may be utilized.
[0281] In contrast, utilizing an electrical stimulus having a frequency
of about 5 Hz
to about 60 Hz or greater to stimulate a target nerve or tissue through a non-
glabrous skin
surface (via an electrode positioned over the non-glabrous skin surface) is
intolerable and
painful, resulting in burns or injury, and thus making such a procedure
impractical and
not feasible.
[0282] For example, delivering electrical stimulation through a non-
glabrous surface
of the body, for example, by stimulating a site overlying a nerve near the
medial
malleolus to elicit a motor response of the abductor hallucis longus,
generates a painful
shock to the patient. While at a single pulse, such as in the use for EMG
diagnostics,
such electrical stimulation may be tolerable, as the frequency increases, the
shocking
sensation builds and quickly becomes painful and intolerable.
[0283] It is contemplated that other energy sources, for example, an
electromagnetic
or magnetic stimulus having a frequency of about 5 Hz to about 60 Hz, or
greater than 30
Hz, may be utilized to simulate a target nerve or tissue through a glabrous
surface in a
manner that remains safe and tolerable to the patient.
[0284] Various electrodes and/or applicators for applying an electrical
or other
energy based stimulation to a patient are described herein.
[0285] Figure 45 shows one variation of an ergonomic insole or shoe
applicator for
delivering electrical stimulation over the glabrous surface of a foot. The
ergonomic
insole 520 may include one or more stimulator electrodes 522 for delivering
electrical
stimulation to a user. The electrode 522 may be attached to or positioned in
the
67
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
ergonomic insole 520. The ergonomic insole 520 may hold or position the
stimulator
electrode 522 against, in contact with, or in proximity to a glabrous surface
of the foot, to
deliver the electrical stimulation through the glabrous surface to an
underlying target
nerve or tissue. Optionally, the electrodes may be utilized for providing
stimulation,
sensing and/or grounding. In certain variations, the electrode may be attached
to one or
more wires or may be wireless and/or coupled to an electrical pulse generator
or other
generator or controller and/or may not be attached to or positioned in an
ergonomic
insole. Any of the electrodes described herein may be durable, reusable,
and/or
disposable.
[0286] An electrical pulse generator 524 or other generator or controller
may be
coupled to the stimulator electrode 522. The electrical pulse generator 524
may be
incorporated into or attached to the ergonomic insole 520. A ground electrode
526 or
other ground may also be provided. The ground electrode 526 may be attached to
a strap
or band extending from or attached to the ergonomic insole, an orthotic, shoe,
or shoe
applicator or elsewhere on the user's body. The ground electrode may be
attached to one
or more wires or may be wireless and/or may be coupled to the stimulator
electrode 522
and/or the pulse generator or controller. The ground electrode 526 may be
positioned
anywhere on the foot. For example, the ground electrode 526 may be positioned
over a
posterior or upper surface of the foot to encourage or facilitate deeper
penetration of the
electrical current or stimulation, through the glabrous surface of the foot,
to a plantar
nerve or other target nerve within the foot.
[0287] In certain variations, the electrical pulse generator or
controller may be
located distant or remotely from an ergonomic insole and the electrode
positioned
therein. For example, the electrical pulse generator may attached elsewhere on
the body,
e.g., attached to a belt, inside a pocket or strapped to the calf or other
region of the body.
The pulse generator may communicate with or be coupled to the stimulator
electrode or
other electrodes via a wire or wirelessly.
[0288] In certain variations, the ergonomic insole may be custom built
into an
orthotic, shoe applicator or other support for the foot. For example, the
ergonomic insole
may be built into an orthotic or shoe applicator providing the user with the
freedom to
walk around while receiving electrical stimulation therapy.
[0289] Other ergonomic applicators, designed for other regions of the
body, may be
utilized for delivering electrical stimulation over various regions of the
body. For
example, Figure 48 shows a glove, brace or other hand wrap or glove like
applicator 550.
68
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
The applicator 550 may include one or more stimulator electrodes 552, such
that the
applicator 550 may hold or position an electrode 552 over or in contact with a
glabrous
surface of the hand or palmar region of the hand to deliver electrical
stimulation through
or across the glabrous surface, to an underlying target nerve or tissue within
the hand. A
ground electrode 556 or other ground may be attached to the hand at another
location
and/or may be attached to a strap of the applicator. The stimulator and/or
ground
electrodes 552, 556 may be coupled to an electrical pulse generator 554, which
may be
positioned in the applicator 550 or at various locations on or away from the
patient.
Optionally, a sensor electrode (not shown) may be attached to the patient to
detect nerve
or other tissue stimulation and to provide feedback regarding the efficacy of
the therapy
in order to optimize the therapy. The sensor may be coupled to the electrical
pulse
generator 554.
[0290] In other variations, an applicator may be in the form of a foot
or hand plate,
cradle or support. For example, a user may rest or position their bare foot on
a foot plate
to receive electrical stimulation therapy from the plate or from one or more
electrodes
attached to the plate. In another variation, a user may rest or position the
palm of their
hand on a hand plate or support to receive electrical stimulation therapy from
the plate or
from one or more electrodes attached to the plate to provide upper extremity
stimulation.
[0291] Figure 46 shows another variation of an ergonomic insole or shoe
applicator
for delivering electrical stimulation over the glabrous surface of a foot. The
ergonomic
insole 530 may include one or more electrodes 532 for delivering electrical
stimulation
to a user. The electrode 532 may be attached to or positioned in the ergonomic
insole
530. The ergonomic insole 20 may hold or position the electrode 532 against,
in contact
with, or in proximity to or over a glabrous surface or plantar surface of the
foot, to
deliver the electrical stimulation through the glabrous surface to an
underlying target
nerve or tissue. In certain variations, the electrode may be attached to one
or more wires
or may be wireless and/or may be coupled to an electrical pulse generator or
other
generator or controller and/or may not be attached to or positioned in an
ergonomic
insole.
[0292] An electrical pulse generator 534 or other generator or controller
may be
coupled to the stimulator electrode 532. The electrical pulse generator 534
may be
incorporated into or attached to the ergonomic insole 530. A ground electrode
536 or
other ground may also be provided. The ground electrode 536 may be attached to
a strap
or band extending from or attached to the ergonomic insole, an orthotic, shoe,
or shoe
69
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
applicator or elsewhere on the user's body. The ground electrode may be
attached to one
or more wires or may be wireless and/or may be coupled to the stimulator
electrode 532
and/or the pulse generator or controller. The ground electrode 536 may be
positioned
anywhere on the foot. For example, the ground electrode 534 may be positioned
over a
posterior or upper surface of the foot to encourage or facilitate deeper
penetration of the
electrical current or stimulation, through the glabrous surface of the foot,
to a plantar
nerve or other target nerve within the foot.
[0293] In certain variations, the electrical pulse generator or
controller may be
located distant or remotely from an ergonomic insole and the electrode
positioned
therein. For example, the electrical pulse generator may attached elsewhere on
the body,
e.g., attached to a belt, inside a pocket or strapped to the calf or other
region of the body.
The pulse generator may communicate with or be coupled to the stimulator
electrode or
other electrodes via a wire or wirelessly.
[0294] The ergonomic insole 530 may include one or more sensor
electrodes. For
example, in one variation, as shown in Figure 46, the stimulator electrode 532
may also
act as a sensor electrode. The electrode 532 may detect stimulation of the
underlying
target nerve, to provide feedback regarding the efficacy of the applied
electrical
stimulation therapy. For example, the electrode 532 may detect motor and/or
sensory
nerve conduction. Detection of and feedback regarding nerve stimulation via
the
electrode 532 may provide for automatic adjustment of the treatment parameters
or may
guide or allow for manual adjustment of the treatment parameters in order to
optimize
stimulation therapy. Optionally, an additional sensor electrode 533 may be
provided as
well.
[0295] In certain variations, one or more separate or dedicated sensor
electrodes may
be attached to or positioned in the ergonomic insole to continuously and/or
intermittently
sense the stimulation of a nerve. The ergonomic insole 530 may hold or
position the
sensor electrode against, in contact with, or in proximity to a glabrous
surface of the foot,
to detect stimulation of the underlying target nerve, to provide feedback
regarding the
efficacy of the applied electrical stimulation therapy. For example, the
sensor electrode
may detect motor and/or sensory nerve conduction. Detection of and feedback
regarding
nerve stimulation via the sensor electrode may provide for automatic
adjustment of the
treatment parameters or may guide or allow for manual adjustment of the
treatment
parameters in order to optimize stimulation therapy. The sensor electrode may
be
coupled or connected to the electrical pulse generator. In certain variations,
the sensor
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
electrode may be attached to one or more wires, may be wireless and/or may be
coupled
to a pulse generator and/or may not be attached to or positioned in an
ergonomic insole.
[0296] A sensor or applicator with a sensor may be capable of sensing
stimulation of
a nerve underlying a glabrous surface allowing for manual or automatic
feedback to
adjust the parameters of the stimulation or the position of the area being
stimulated in
order to optimize the stimulator therapy.
[0297] In certain variation, as sensor electrode may be positioned or
placed along the
path of the nerve conduction or motor or sensory impulse. Optionally, the
sensor
electrode may be positioned proximal to a stimulator electrode, along the
nerve
conduction path
[0298] In certain variations, the electrodes may be attached to one or
more wires,
may be wireless and/or may be coupled to an electrical pulse generator or
other generator
or controller and/or may not be positioned in an applicator or support. For
example, one
or more stimulator electrodes may be adhered to or otherwise attached to a
glabrous skin
surface of a patient. One or more ground electrodes and/or one or more sensor
electrodes
may also be adhered to or otherwise attached to the patient. The stimulator
electrode,
sensor electrode and/or ground may be coupled to an electrical pulse generator
and/or
each other using wires or wirelessly. The electrical pulse generator may
positioned in
various locations or located anywhere on a patient. For example, the
electrical pulse
generator or controller may be held by a patient, located on a belt or strap
worn by the
patient, or positioned in a pocket or pouch on the patient.
[0299] For example, Figure 47 shows one variation of a stimulator
electrode 542
attached to a glabrous skin surface of the foot or sole of the foot. A ground
electrode 546
may be attached to the foot at another location. The stimulator and/or ground
electrodes
542, 546 may be coupled to an electrical pulse generator 544, which may be
positioned
at various locations on or away from the patient. Optionally, a sensor
electrode (not
shown) may be attached to the patient to detect nerve or other tissue
stimulation and to
provide feedback regarding the efficacy of the therapy to optimize the
therapy. The
sensor may be coupled to the electrical pulse generator 544.
[0300] Figure 49 shows another variation of a stimulator electrode 562
attached to a
glabrous skin surface of the hand or palm of the hand. A ground electrode 566
may be
attached to the hand at another location. The stimulator and/or ground
electrodes 562,
566 may be coupled to an electrical pulse generator 564, which may be
positioned at
various locations on or away from the patient. Optionally, a sensor electrode
(not
71
CA 02812086 2013-03-19
WO 2012/040243 PCT/US2011/052415
shown) may be attached to the patient to detect nerve or other tissue
stimulation and to
provide feedback regarding the efficacy of the therapy to optimize the
therapy. The
sensor may be coupled to the electrical pulse generator 564.
103011 In certain variations, systems and methods for providing
electrostimulation
(ES) of the posterior tibial nerve in individuals with OAB and UI, via
transdermal
electrical fields, are provided. Various electrodes for delivering the
electrical stimulation
may be utilized, including but not limited to surface electrodes with and
without gel,
microneedle electrodes, and electrodes under strong pressure.
[0302] Electrical stimulation may be directed to nerves underlying
glabrous skin
surfaces of the body (e.g., the palms and soles). Higher levels of power (than
what
would be utilized and tolerated on non-glabrous skin surfaces) may be utilized
when
delivering electrical stimulation transdermally, through or across a glabrous
skin surface,
while remaining safe and tolerable. For example, stimulation of the plantar
nerves or
other nerves of the foot via a surface electrode positioned over the glabrous
surface of
the plantar surface is highly tolerable and results in sensation similar to
that found with
needle-based, invasive stimulation of the posterior tibial nerve, but in a non-
invasive
manner.
[0303] Various devices or applicators may be applied to the feet and/or
hand or other
body portions in order to stimulate nerves underlying glabrous surfaces on an
intermittent or continuous basis. Electrical stimulation may be delivered via
surface or
microneedle electrodes. Various devices may be used in conjunction with
implantable or
injectable substances in order to improve electrical conductivity to a target
nerve. As
described supra, the various methods and devices for providing transdermal
electrical
stimulation over a glabrous skin surface may be used to treat any disorder
that is
impacted by neuromodulation including, but not limited to: overactive bladder,
urinary
incontinence, fecal incontinence, chronic pain, depression, migraine,
epilepsy, obesity,
restless leg, or foot drop. The methods may be applied either intermittently
or, if
necessary, on a chronic basis. A stimulator electrode surface may be held in
contact with
a glabrous surface via an adhesive or an ergonomic applicator.
[0304] Feedback may be provided to the stimulator to indicate that
stimulation is
occurring as intended. This may involve an eMG type measurement device, a
motion
sensor or other sensing device. This feedback loop may be intermittently
queried so that
72
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
the stimulation may be adjusted to ensure that the minimum amount of energy is
being
used to stimulate to reduce the risk of burns or intolerance.
[0305] In certain variations, the device may include an ergonomic wrap
or cradle.
The sensor feedback may allow for optimization of the positioning of
electrodes based
on sensor feedback further reducing the risk of burns or intolerance while
increasing the
efficacy of the neuromodulation.
[0306] The methods and devices described herein may be utilized to
stimulate
various body tissues, including nerve, muscle, skin, vasculature, or any other
organ or
tissue within the human body. The methods and devices may be used to treat any
suitable condition or perform any suitable function via neuromodulation
regardless of
whether the stimulation source is electromagnetic fields, direct electric
current,
ultrasound, or RF fields.
[0307] In certain variations, methods, systems and/or devices for
performing
neuromodulation and/or low frequency induction therapy through glabrous skin
surfaces,
e.g., through palmar or plantar surfaces, are provided. The methods and system
may be
utilized for treating or preventing various conditions, such as urinary
incontinence (UI)
and/or overactive bladder (OAB).
[0308] For example, a patient suffering from UI or OAB may place the
glabrous
surface of their foot over an insole, foot rest or foot plate applicator to
provide contact
between the glabrous surface of their foot and a stimulator electrode of the
insole, foot
rest, or foot plate applicator. Alternatively, a tethered or wired electrode
may be used
without an applicator or separate from an applicator. The electrode may be
attached to or
held in contact with the glabrous surface with an adhesive or as a cutaneous
patch.
[0309] The stimulator electrode may be positioned over the glabrous
surface along
the course of a target nerve. The stimulator electrode may be positioned
proximal to a
stimulation site to ensure that afferent nerve stimulation occurs. A ground
electrode may
be placed on the body as well. One or more sensing electrodes may be placed
along the
path of the nerve conduction or motor or sensory impulse. Stimulator and/or
sensing
electrodes may be connected or coupled to a pulse generator. The pulse
generator and
electrodes may be incorporated or integrated into an insole, foot plate or
other applicator.
Alternatively, the electrodes may be connected or coupled to a pulse generator
where the
electrodes are not incorporated or integrated into the insole, foot plate or
applicator.
73
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
[0310] Electrical stimulation via the stimulator electrodes may begin at
a low
amplitude and may slowly be ramped up or increased until nerve conduction is
detected
by the sensor electrode and/or detected by the patient who may signal that
motor
conduction has occurred, e.g., by pressing a button or other indicator.
[0311] Once stimulation is detected, the electrical pulses or electrical
stimulation
may continue for the directed duration of use or therapy. Electrical
stimulation may be
delivered intermittently to provide intermittent therapy.
For example, electrical
stimulation may be delivered for 15-30 minute intervals. In another example,
electrical
stimulation may be delivered continuously to provide continuous therapy.
[0312] A sensor may remain in place for the duration of the electrical
stimulation
therapy to ensure that stimulation occurs the entire time or substantially the
entire time,
and to allow for correction or adjustment if the signal deteriorates. A
controller for
operating a pulse generator or sensor may be powered by a portable power
source (e.g., a
battery) or a fixed power source (e.g., a traditional wall outlet).
[0313] In certain variations, the electrical stimulation may have of a
square wave
electric signal at a frequency of about 5 Hz to about 60 Hz at the targeted
tissue depth.
The square wave configuration of the signal may be generated via Fourier
transformation
or may be a ramped current generated in any manner.
103141 The insole, foot rest, or foot plate may be removed from the body
when
therapeutic stimulation is not being delivered. The insole, foot rest, or foot
plate may be
reapplied along with a sensor patch (which may be disposable) as indicated.
For
example, the electrical stimulation therapy my be administered on a daily
basis, where
one or more of the above steps are repeated.
[0315] Electrical stimulation may be delivered according to any of the
variations
described herein, in a manner such that the stimulation provides motor,
sensory and/or
subthreshhold stimulation. Any of the various energy based stimulation systems
described herein may be utilized to provide therapy in various settings, e.g.,
in home use
or to provide ambulatory type therapies.
[0316] Each of the individual variations described and illustrated
herein has discrete
components and features which may be readily separated from or combined with
the
features of any of the other variations. Modifications may be made to adapt a
particular
situation, material, composition of matter, process, process act(s) or step(s)
to the
objective(s), spirit or scope of the present invention.
74
CA 02812086 2013-03-19
WO 2012/040243
PCT/US2011/052415
[0317] Methods recited herein may be carried out in any order of the
recited events
which is logically possible, as well as the recited order of events.
Furthermore, where a
range of values is provided, every intervening value between the upper and
lower limit of
that range and any other stated or intervening value in that stated range is
encompassed
within the invention. Also, any optional feature of the inventive variations
described
may be set forth and claimed independently, or in combination with any one or
more of
the features described herein.
[0318] All existing subject matter mentioned herein (e.g., publications,
patents,
patent applications and hardware) is incorporated by reference herein in its
entirety
except insofar as the subject matter may conflict with that of the present
invention (in
which case what is present herein shall prevail). The referenced items are
provided
solely for their disclosure prior to the filing date of the present
application. Nothing
herein is to be construed as an admission that the present invention is not
entitled to
antedate such material by virtue of prior invention.
[0319] Reference to a singular item, includes the possibility that there
are plural of
the same items present. More specifically, as used herein and in the appended
claims,
the singular forms "a," "an," "said" and "the" include plural referents unless
the context
clearly dictates otherwise. It is further noted that the claims may be drafted
to exclude
any optional element. As such, this statement is intended to serve as
antecedent basis for
use of such exclusive terminology as "solely," "only" and the like in
connection with the
recitation of claim elements, or use of a "negative" limitation. Unless
defined otherwise,
all technical and scientific terms used herein have the same meaning as
commonly
understood by one of ordinary skill in the art to which this invention
belongs.
[0320] This disclosure is not intended to be limited to the scope of the
particular
forms set forth, but is intended to cover alternatives, modifications, and
equivalents of
the variations described herein. Further, the scope of the disclosure fully
encompasses
other variations that may become obvious to those skilled in the art in view
of this
disclosure. The scope of the present invention is limited only by the appended
claims.