Note: Descriptions are shown in the official language in which they were submitted.
CA 02812309 2013-03-15
WO 2012/065253
PCT/CA2011/001271
PROCESSES FOR EXTRACTING ALUMINUM FROM
ALUMINOUS ORES
TECHNICAL FIELD
[0001] The present disclosure relates to improvements in the field of
chemistry applied to extraction of aluminum from aluminous ores. For
example, such processes are useful for extracting aluminum from aluminous
ores comprising various types of metals such as Fe, K, Mg, Na, Ca, Mn, Ba,
Zn, Li, Sr, V, Ni, Cr, Pb, Cu, Co, Sb, As, B, Sn, Be, Mo, or mixtures thereof.
BACKGROUND OF THE DISCLOSURE
[0002] More than 96 % of the alumina which is produced worldwide is
obtained from bauxite, which is a mineral that is particularly rich in alumina
(40 ¨ 60 %) and whose main suppliers are from Jamaica, Australia, Brazil,
Africa and Russia. In certain areas of the world there are large quantities of
aluminous ores, which are aluminosilicates (for example argillite, nepheline,
etc.) that are relatively rich in alumina (20 ¨ 28 (3/0). However such areas
have
received little attention up to now because the production costs for
extracting
aluminum from such ores remained too high. In these aluminous materials,
and contrary to bauxite, aluminum oxide is associated with silicated or
sulfated phases. Thus, the Bayer process cannot be used, which means that
alternative treatments for the production of alumina must be used or
developed. Various processes have been proposed so far in order to extract
aluminum from such aluminous ores comprising aluminosilicates but there is
still room for improvement or for alternative routes.
SUMMARY OF THE DISCLOSURE
[0003] According to one aspect, there is provided a process for
extracting
aluminum ions from argillite, the process comprising :
leaching the argilite with HCI;
1
CA 02812309 2013-03-15
WO 2012/065253
PCT/CA2011/001271
at least partially removing iron from the argillite by
substantially selectively precipitating at least a portion of the iron ions by
reacting the iron ions with a base so as to obtain an Al-rich composition or
by
substantially complexing the iron ions with an extracting agent; and
optionally purifying said Al-rich composition by:
- substantially selectively precipitating said
aluminum ions;
- by means of a hollow fiber membrane; or
- by means of a liquid-liquid extraction.
[0004] According to another aspect, there is provided a process for
extracting aluminum ions from argillite, the process comprising :
leaching the argillite with HCI so as to obtain a
composition comprising the aluminum ions and iron ions;
at least partially removing the iron ions from the
composition by substantially selectively precipitating at least a portion of
the
iron ions by reacting the composition with a base and at least partially
removing the precipitated iron ions so as to obtain an Al-rich composition;
and
optionally purifying said Al-rich composition by:
- substantially selectively precipitating said
aluminum ions;
- by means of a hollow fiber membrane; or
- by means of a liquid-liquid extraction.
2
CA 02812309 2013-03-15
WO 2012/065253
PCT/CA2011/001271
[0005] According
to another aspect, there is provided a process for
extracting aluminum ions from an aluminous ore, the process comprising:
leaching the aluminous ore with HCI;
at least partially removing iron from the aluminous ore by
substantially selectively precipitating at least a portion of the iron ions by
reacting the iron ions with a base so as to obtain an Al-rich composition or
by
substantially complexing the iron ions with an extracting agent; and
optionally purifying said Al-rich composition by:
- substantially selectively precipitating said
aluminum ions;
- by means of a hollow fiber membrane; or
- by means of a liquid-liquid extraction.
[0006] According
to another aspect, there is provided a process for
extracting aluminum ions from an aluminous ore, the process comprising :
leaching the aluminous ore with an acid so as to obtain a
composition comprising the aluminum ions and iron ions;
at least partially removing the iron ions from the
composition by substantially selectively precipitating at least a portion of
the
iron ions by reacting the composition with a base and at least partially
removing the precipitated iron ions so as to obtain an Al-rich composition;
and
optionally purifying said Al-rich composition by:
- substantially selectively precipitating said
aluminum ions;
3
CA 02812309 2013-03-15
WO 2012/065253
PCT/CA2011/001271
- by means of a hollow fiber membrane; or
- by means of a liquid-liquid extraction.
[0007] According another aspect, there is provided a process for
extracting
aluminum ions from a mixture comprising iron ions and the aluminum ions.
The process comprises recovering the aluminum ions from a composition
comprising the aluminum ions, the iron ions, an organic solvent and an
extracting agent adapted to form an organometallic complex substantially
selectively with the iron ions or with the aluminum ions which is soluble in
the
organic solvent.
[0008] It was found that the processes of the present disclosure are
effective for extracting aluminum from various aluminous ores. More
particularly, it was found that such processes were efficient for extracting
aluminum from ores having a considerable amount of iron such as argillite.
Such processes were thus found to be an interesting alternative to the Bayer
process. In fact, the Bayer process was found not to be efficient for
extracting
aluminum from certain ores such as ores having a high iron content (for
example argillite).
BRIEF DESCRIPTION OF DRAWINGS
[0009] In the following drawings, which represent by way of example
only,
various embodiments of the disclosure:
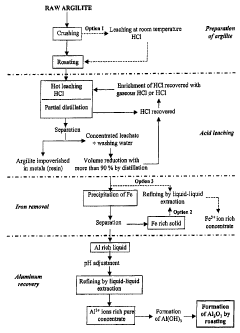
[0010] Fig. 1 shows a bloc diagram of a process according to one
embodiment of a process for extracting aluminum from an aluminous ore.
DETAILLED DESCRIPTION OF VARIOUS EMBODIMENTS
[0011] The acid used for leaching the aluminous ore can be HCI, H2SO4,
HNO3 or mixtures thereof. More than one acid can be used as a mixture or
separately. Solutions made with these acids can be used at various
concentration. For example, concentrated solutions can be used. For
example, 6 M or 12 M HCI can be used. For example, up to 100 % wt H2SO4
can be used.
4
CA 02812309 2013-03-15
WO 2012/065253
PCT/CA2011/001271
[0012] The processes of the present disclosure can be effective for
treating
various aluminous ores or aluminum-bearing ores. For example, clays,
argillite, mudstone, beryl, cryolite, garnet, spine!, bauxite, or mixtures
thereof
can be used as starting material.
[0013] The leaching can be carried out under pressure into an
autoclave.
For example, it can be carried out at a pressure of about 5 KPa to about 850
KPa, about 50 KPa to about 800 KPa, about 100 KPa to about 750 KPa,
about 150 KPa to about 700 KPa, about 200 KPa to about 600 KPa, or about
250 KPa to about 500 KPa. The leaching can be carried out at a temperature
of at least 80 C, at least 90 C, or about 100 C to about 110 C. In certain
cases, it can be done at higher temperatures.
[0014] The leaching can also be carried out under pressure. For
example,
the pressure can be about 100 to about 300 or about 150 to about 200 psig.
The leaching can be carried out for about 30 minutes to about 5 hours. It can
be carried out at a temperature of about 60 C to about 200 C.
[0015] According to one embodiment, the process can comprise:
leaching the argillite with HCI so as to obtain a leachate
comprising the aluminum ions and the iron ions, and a solid residue;
separating the leachate from the solid residue;
at least partially removing the iron ions from the leachate
by substantially selectively precipitating at least a portion of the iron ions
by
reacting the base with the leachate and removing a so-formed precipitate, so
as to obtain an Al-rich aqueous composition; and
purifying the Al-rich aqueous composition by substantially
selectively precipitating the aluminum ions by reacting the composition with
an
acid or base, and by recovering the precipitated aluminum ions.
[0016] According to another embodiment, the process can comprise:
CA 02812309 2013-03-15
WO 2012/065253
PCT/CA2011/001271
leaching the argillite with HCI so as to obtain a leachate
comprising the aluminum ions and the iron ions, and a solid residue;
separating the leachate from the solid residue;
at least partially removing the iron ions from the leachate
by substantially selectively precipitating at least a portion of the iron ions
by
reacting the base with the leachate and removing a so-formed precipitate, so
as to obtain the Al-rich aqueous composition; and
purifying the Al-rich aqueous composition by means of a
hollow fiber membrane membrane, or by a liquid-liquid extraction.
[0017] According to another embodiment the process can comprise:
leaching the argillite with HCI so as to obtain a leachate
comprising the aluminum ions and the iron ions, and a solid residue;
separating the leachate from the solid residue;
at least partially removing the iron ions from the leachate
by substantially selectively precipitating at least a portion of the iron ions
by
reacting the base with the leachate and removing a so-formed precipitate, so
as to obtain the Al-rich aqueous composition; and
purifying the Al-rich aqueous composition by substantially
selectively precipitating the aluminum ions and recovering the precipitated
aluminum ions.
[0018] For example, the Al-rich aqueous composition can be purified by
complexing the aluminum ions with an extracting agent so as to obtain a
complex, separating the complex form the composition and precipitating the
aluminum ions. For example, the extracting agent can be bis(2,4,4-
trimethylpentyl) phosphinic acid.
6
CA 02812309 2013-03-15
WO 2012/065253
PCT/CA2011/001271
[0019] For example, the Al-rich aqueous composition can be purified by
complexing impurities contained in Al-rich aqueous composition with an
extracting agent, at least partially removing the complexed impurities from
the
composition and precipitating the aluminum ions. For example, the extracting
agent can be chosen from di-2-ethylhexyl phosphoric acid (HDEHP),
bis(2,4,4-trimethylpentyl) phosphinic acid and 2-ethylhexyl phosphonic acid
mono-2-ethylhexyl ester.
[0020] The base that can be used for substantially selectively
precipitating
the iron ions can be KOH, NaOH, or a mixture thereof.
[0021] The base that can be used for substantially selectively
precipitating
the aluminum ions can be KOH, NaOH, or a mixture thereof.
[0022] For example, in an acidic medium, the precipitation of iron
ions can
be carried out at a pH of about 3 to about 6, about 3.0 to about 5.5, about 3
to
about 5, about 3 to about 4, about 3.0 to about 3.5, about 3.5 to about 4.0,
about 4.0 to about 5.0, about 4.0 to about 4.5, or about 4.5 to about 5Ø
[0023] For example, the Al-rich composition can be purified by
reacting the
Al-rich composition with a base for substantially selectively precipitating
the
aluminum ions at a pH of about 5 to about 6, about 5.0 to about 5.5, or about
5.5 to about 6Ø
[0024] According to another aspect, there is provided a process for
producing alumina comprising :
obtaining aluminum ions by means of a process as defined in
the present disclosure; and
converting the aluminum ions into alumina.
[0025] For example, tha aluminum ions can be converted into alumina by
heating Al(OH)3 at a temperature of about 800 C to about 1200 C.
[0026] According to another aspect, there is provided a process for
producing alumina comprising:
7
CA 02812309 2013-03-15
WO 2012/065253
PCT/CA2011/001271
converting the alumina into aluminum.
[0027]
According to another aspect, there is provided a process for
producing aluminum comprising :
obtaining alumina by means of a process as defined in the
present disclosure; and
converting the alumina into aluminum.
[0028]
According to another aspect, there is provided a process for
extracting aluminum from an aluminous ore, the process comprising:
- leaching
the aluminous ore with an acid so as to
obtain a leachate and a solid residue;
removing at least a portion of iron ions contained
in the leachate by:
(i) substantially selectively precipitating the at
least portion of the iron ions in basic conditions in which the pH is of at
least
10, so as to obtain an aluminum enriched composition; or
(ii) substantially selectively complexing the at
least portion of the iron ions with an extracting agent adapted to form an
organometallic complex substantially selectively with the iron ions so as to
obtain an aluminum enriched composition.
[0029] In the
processes of the present disclosure, the acid can be HCI. The
aluminuous ore can be leached with HCI at a temperature of at least 80 C, at
least 90 C, or about 100 C to about 110 C. HCI can have a concentration of
about 6 M. The alunimuous ore / acid ratio can be about 1 / 10 in weight by
volume.
8
CA 02812309 2013-03-15
WO 2012/065253
PCT/CA2011/001271
[0030] For example, the removal of the at least portion of iron ions
can be
carried out by precipitating the iron ions from a basic aqueous composition.
The composition can comprise comprising NaOH or KOH.
[0031] For example, the removal of the at least portion of iron ions
can be
carried out by reacting the leachate with a base in order to obtain a pH of at
least 10 and precipitating the iron ions.
[0032] For example, the precipitated iron ions can be separated from
the
rest of the leachate by carrying out a filtration, a decantation, a
centrifugation,
or mixtures thereof.
[0033] The processes can further comprise rinsing the obtained
precipitated iron ions with a basic solution. The basic solution can have a
concentration of about 0.01 M to about 0.02 M. The pH can be at least 11, at
least 12, about 10.8 to about 11.2, or about 11.5 to about 12.5. The process
can further comprise purifying the precipitated iron ions by means of a hollow
fiber membrane.
[0034] The removal of the at least portion of iron ions can be carried
out by
reacting the leachate, under acidic conditions, with the extracting agent and
an organic solvent in order to obtain a composition comprising an acidic
aqueous phase comprising aluminum ions and an organic phase comprising
iron ions complexed with the extracting agent. The aluminum enriched
composition can be obtained by separating the aqueous phase from the
organic phase. The aqueous phase can have a pH of about 1 to about 2.5, or
about 2. The extracting agent can be chosen from di-2-ethylhexyl phosphoric
acid (HDEHP), bis(2,4,4-trimethylpentyl) phosphinic acid and 2-ethylhexyl
phosphonic acid mono-2-ethylhexyl ester). The extracting agent can have a
concentration of about 0.5 M to about 1.5 M in the organic phase or about 1 M
in the organic phase.
[0035] For example, the organic solvent can be chosen from C5-C12
alkanes and mixtures thereof. The organic solvent can be heptane. The
9
CA 02812309 2013-03-15
WO 2012/065253
PCT/CA2011/001271
composition can have a volumic ratio organic phase: aqueous phase of about
1:1. The organic phase and the aqueous phase can be separated by means
of a filtration membrane. The membrane can be a hollow fiber membrane.
The membrane can comprise polypropylene, polyvinylidene difluoride, or a
mixture thereof.
[0036] After passing the composition through the membrane, the aqueous
phase can separated from the organic phase. The aluminum ions can be
recovered in the aqueous phase and the aqueous phase is treated with a
base (such as NaOH or KOH). The aqueous phase can be treated with the
base so as to obtain a pH of at least about 4. The process can further
comprise a separation by filtration to obtain Al(OH)3, which can be eventually
washed.
[0037] For example, the aluminous ore can be crushed and roasted before
being leached.
[0038] For example, before removal of the iron ions, the leachate is
treated
with a base.
[0039] For example, before removal of the iron ions, the leachate can
be
distilled so as to reduce its volume.
[0040] For example, the process can further comprise at least partially
recovering the aluminum ions present in the aluminum enriched composition.
[0041] For example, the aluminum enriched composition can be treated
with an extracting agent adapted to form an organometallic complex
substantially selectively with the aluminum ions in the presence of an organic
solvent and an acid solution in order to form a composition comprising an
acidic aqueous phase comprising impurities and an organic phase comprising
aluminum ions complexed with the extracting agent. The aluminum ions can
be recovered by separating the aqueous phase from the organic phase. For
example, the aqueous phase can have a pH of about 2.5 to about 3.5. The
extracting agent can be a phosphinic acid or a derivative thereof. The
CA 02812309 2013-03-15
WO 2012/065253
PCT/CA2011/001271
extracting agent can be bis(2,4,4-trimethylpentyl) phosphinic acid. The
extracting agent can have a concentration of about 10 % to about 25 A) v/v or
about 20 % v/v with respect to the organic solvent. The organic solvent can be
chosen from C5-C12 alkanes and mixtures thereof. The organic solvent can be
heptane. The composition can have a volumic ratio aqueous phase: organic
phase of about 1:1 to about 1:3. The organic phase and the aqueous phase
can be separated by means of a membrane (for example a hollow fiber
membrane). The membrane can comprise polypropylene, polyvinylidene
difluoride, or a mixture thereof. The composition can be at a temperature of
about 30 C to about 50 C, or about 35 C to about 45 C. After passing the
composition through the membrane, the aqueous phase can be separated
from the organic phase. The complexed aluminum ions can be recovered in
the organic phase. The organic phase can then be treated with HCI so as to
obtain an aqueous composition comprising the aluminum ions. The aluminum
ions can be converted into Al(OH)3 by contacting it with a base. Al(OH)3 can
then be converted into A1203. Such a conversion of Al(OH)3 into A1203 can be
carried out at a temperature of about 800 C to about 1200 C.
[0042] According to one embodiment, the composition can comprise an
acidic aqueous phase comprising aluminum ions and an organic phase
comprising iron ions complexed with the extracting agent and wherein the
aluminum ions are recovered by separating the aqueous phase from the
organic phase. The aqueous phase can have a pH of about 1 to about 2.5 or
of about 2. The extracting agent can be chosen from phosphoric acids and
derivatives thereof, and phosphinic acids and derivatives thereof. For
example, the extracting agent can be chosen from di-2-ethylhexyl phosphoric
acid (HDEHP), bis(2,4,4-trimethylpentyl) phosphinic acid and 2-ethylhexyl
phosphonic acid mono-2-ethylhexyl ester. The extracting agent can have a
concentration of about 0.5 M to about 1.5 M in the organic phase or of about 1
M in the organic phase. The composition can have a volumic ratio organic
phase: aqueous phase of about 1:1. After extraction (passing the composition
through the membrane), the aqueous phase can be separated from the
organic phase, and the aluminum ions can recovered in the aqueous phase
11
CA 02812309 2013-03-15
WO 2012/065253
PCT/CA2011/001271
and the aqueous phase can be treated with a base (for example NaOH, KOH,
or a mixture thereof). The aqueous phase can be treated with the base so as
to obtain a pH of at least about 4. The process can further comprise treating
the organic phase with HCI and isolating the iron ions in the form of Fe3+.
[0043] According to another embodiment, the composition can comprise
an acidic aqueous phase comprising iron ions and an organic phase
comprising aluminum ions complexed with the extracting agent, and wherein
the aluminum ions are recovered by separating the aqueous phase from the
organic phase. The aqueous phase can have a pH of about 2.5 to about 3.5.
The extracting agent can be a phosphinic acid or a derivative thereof. For
example, the extracting agent can be bis(2,4,4-trimethylpentyl) phosphinic
acid. The extracting agent can have a concentration of about 10 % to about
25 `)/0 v/v with respect to the organic solvent or of about 20 % v/v with
respect
to the organic solvent. The composition can have a volumic ratio aqueous
phase : organic phase of about 1:1 to about 1:3. During the process, the
composition can be at a temperature of about 30 C to about 50 C or at a
temperature of about 35 C to about 45 C. After extraction through the
membrane, the aqueous phase can be separated from the organic phase.
The complexed aluminum ions can be recovered in the organic phase. The
organic phase can then be treated with HC1 so as to obtain an aqueous
composition comprising the aluminum ions.
[0044] For example, the organic solvent can be chosen from
hydrocarbons. For example, the organic solvent can be chosen from 05-012
alkanes and mixtures thereof. The organic solvent can also be hexane or
heptane. The organic phase and the aqueous phase can be separated by
means of a filtration membrane, for example a hollow fiber membrane. Such
membrane can comprise polypropylene, polyvinylidene difluoride, or a mixture
thereof. The aqueous phase can be treated with the base so as to obtain a pH
of at least about 4. The process can also further comprise a separation by
filtration so as to obtain Al(OH)3. The process can also comprise washing the
Al(OH)3. The process can also comprise converting Al(OH)3 into A1203.
12
CA 02812309 2013-03-15
WO 2012/065253
PCT/CA2011/001271
Conversion of Al(OH)3 into A1203 can be carried out at a temperature of about
800 C to about 1200 C.
[0045] According to another aspect there is provided a composition
comprising aluminum ions, iron ions, an organic solvent and an extracting
agent adapted to form an organometallic complex substantially selectively
with the iron ions or with the aluminum ions which is soluble in the organic
solvent.
[0046] According to another aspect, there is provided a composition
comprising an acidic aqueous phase comprising aluminum ions and an
organic phase comprising iron ions complexed with an extracting agent.
[0047] According to another aspect, there is provided a composition
comprising an acidic aqueous phase comprising iron ions and an organic
phase comprising aluminum ions complexed with an extracting agent.
[0048] According to another aspect, there is provided a process for at
least
partially separating aluminum ions from iron ions comprised in a composition,
the process comprising substantially selectively precipitating at least a
portion
of the iron ions in basic conditions in which the pH is of at least 10. The
iron
ions can be precipitated from a basic aqueous composition comprising NaOH
or KOH. For example, the base can be reacted with the composition so as to
obtain a mixture in which the pH is of at least 10, and then, the at least
portion
of precipitated iron ions can be separated from the rest of the mixture. For
example, the precipitated iron ions can be separated from the rest of the
mixture by carrying out a filtration, a decantation, a centrifugation, or
combinations thereof. The process can further comprise rinsing the obtained
precipitated iron ions with a basic solution. The basic solution can have a
concentration of about 0.01 M to about 0.02 M. The pH can be at least 11, at
least 12, about 10.8 to about 11.2, or about 11.5 to about 12.5. The process
can further comprise purifying the precipitated iron ions by means of a hollow
fiber membrane.
13
CA 02812309 2013-03-15
WO 2012/065253 PCT/CA2011/001271
[0049] The various
parameters, embodiments and examples previously
described concerning the processes can also be applied, when possible, to
these compositions.
[0050] Further features and
advantages will become more readily apparent
from the following description of various embodiments as illustrated by way of
examples only in the appended drawings wherein:
[0051] As it can be seen
from Fig. 1, such a process can comprise
various steps, and each of these steps can eventually be individually
considered has being a process.
Preparation of argillite sample
[0052] Argillite can be
finely crushed in order to help along during the
following steps. For example, micronization can shorten the reaction time by
few hours (about 2 to 3 hours). In order to remove most of the iron, a
leaching
step at room temperature is optionally carried out between the crushing step
and the roasting step (see option 1). This operation is, for ex ample, carried
out with hydrochloric acid HCI (12 M) and an argillite / acid ratio (weight /
volume) of 1:5 is used. Depending on experimental conditions (sizes of the
particles, time of treatment, agitation system), about 65 % to about 93 % of
the iron can then be removed. However, this leaching step can also bring in a
certain percentage of the aluminum (0 - 5 A)). The last step of the
preparation
of argillite comprises roasting the pretreated argillite. This can be
accomplished at a temperature greater than 550 C for a period of about 1 to
2 hours. For example, a heat treatment makes it possible to increase the
quantity of extracted aluminum by about 30 % to about 40 % for the same
period of time. In others words, the quantity of extracted aluminum is
doubled.
When leaching at room temperature is carried out, a phase separation before
roasting can be made in order to recover the acid and reduce heating costs.
14
CA 02812309 2013-03-15
WO 2012/065253
PCT/CA2011/001271
Acid leaching
[0053] Acid leaching comprises reacting the crushed and roasted
argillite with a hydrochloric acid solution at elevated temperature during a
given period of time. For example, the argillite / acid ratio can be of about
of
1:10 (weight / volume), the HCI concentration can be of about 6 M, the
temperature can be of about 100 C to about 110 C, and the reaction time
can be of about 5 to about 7 hours. Under such conditions, more than about
90 % of the aluminum and about 100 % of the iron can be extracted in
addition to impurities.
[0054] During the second half of such a treatment (for example the
last
2 or 3 hours), a portion of the acid can be recovered by condensation. Once
the extraction is terminated, the solid (argillite impoverished in metals) can
be
separated from the liquid by decantation or by filtration, after which it is
washed. The residual leachate and the washing water may be completely
evaporated. The corresponding residue can thereafter be washed many times
with water so as to decrease acidity and to lower the quantities of sodium
hydroxide (NaOH) that are required to adjust the pH during iron removal. Final
volume accounts for 10 % to 20 % of initial volume. The acid recovered will
can be re-utilized after having adjusted its titer either by adding gaseous
HCI,
or by adding concentrated HCI (12 M). After the reaction, the titer of the
acid
can vary from about 4 M to about 6 M depending on experimental conditions.
With respect to the solid, it represents about 65 % to about 75 % of the
initial
mass of argillite, it can be valorized and be used again either as an ion
exchange resin, or as an adsorbent.
Removal of iron
[0055] Removal of iron can be carried out by precipitation of the
iron
ions in (i) basic medium or (ii) an acidic medium. For example, in a basic
medium, precipitation can be carried out at a pH of at least 10 or at a pH of
about 11.5 to about 12.5. For example, in an acidic medium, the precipitation
can be carried out at a pH of about 3 to about 6, about 3 to about 5, about 3
to
CA 02812309 2013-03-15
WO 2012/065253 PCT/CA2011/001271
about 4, about 3.0 to about 3.5, about 3.5 to about 4.0, about 4.0 to about
5.0,
about 4.0 to about 4.5, or about 4.5 to about 5.0, by adding the base. Such a
step under basic or acidic conditions can be made by adding NaOH or KOH
for example at a concentration of about 0.1 M to about 18 M. For examples, a
concentration of 0.1 M, 1 M, 6 M or 10 M can be used. Then, all that is
required is to separate the solid portion from the liquid portion by
filtration,
decantation or centrifugation and to rinse the solid by means of a diluted
base, such as a solution of NaOH (for example NaOH at a concentration of
0.01 M to 0.02 M). Then, the solid is washed with distilled water. The liquid
portion comprises aluminum and alkaline-earths A substantially complete
removal of the iron and of nearly all the impurities (other metals) can thus
be
achieved. Optionally, it is possible to recover iron by using a refining step
by
liquid-liquid extraction through a hollow fiber membrane (see option 2).
[0056] Alternatively (see
option 3), removal of iron can be carried out
by using an extracting agent and a hollow fiber membrane. Various extracting
agents that could substantially selectively complex iron ions over aluminum
ions (or aluminum ions over iron ions) could be used in such a step depending
an Al / Fe ratio. For example, extraction can be carried out by using HDEHP
(diethylhexylphosphoric acid) as an extracting agent adapted to complex iron
ions. A concentration of about 1 M of HDEHP can be used in an organic
solvent, such as heptane or any hydrocarbon solvent. Such an extraction can
require relatively short contact times (few minutes). For example, the pH of
the order of 2 can be used and aqueous phase / organic phase ratio can be of
about 1:1. It was observed that is possible to extract from 86% to 98 % iron
under such conditions. It will be understood that in the present case, iron is
trapped in the organic phase. To recover iron in an aqueous phase, a reverse
extraction with hydrochloric acid (2 M or 6 M) and organic phase / acidic
phase ratio of about 1:0.5 can then be carried out. In such a case, the
resulting aqueous phase is rich in Fe3+ ions.
16
CA 02812309 2013-03-15
WO 2012/065253 PCT/CA2011/001271
Aluminum recovery
[0057] The solution
obtained from the previous step using either the
precipitation or the extraction technique is relatively clean and mainly
contains
aluminum for example about 90 % to 95 % (without the alkaline-earths in the
case of precipitation). Recovery of the latter can be carried out by liquid-
liquid
extraction for example by using a same hollow fiber membrane and an
extracting agent that is adapted to complex at least substantially selectively
aluminum over other metals or residues.
[0058] For example,
bis(2,4,4-trimethylpentyl) phosphinic acid (such as the
one sold under the name CyanexTM 272) can be used as an extracting agent
specific to aluminum. For example, this extracting agent can be used at a
concentration of about 20 % v/v in an organic solvent such as heptane. The
ratios between the aqueous phase and the organic phase can be of about 1:1
to about 1:3. For example, the extraction temperatures can be of about 40 C
and the pH can be maintained at about 2.5 to about 3.5. It was observed that
such a technique makes it possible to extract more than 70 - 90 % of the
aluminum.
[0059] After the aluminum
has been trapped in the organic phase, it can
berecovered in the form of a concentrate of Al3+ ions by using a back
extraction. For example, the reverse extraction can be carried out at a
temperature of about 40 C with hydrochloric acid (for example at a
concentration of 6 M). Under this condition, more than 90 % of aluminum can
be recovered. Then, Al3+ can be converted into aluminum hydroxide Al(OH)3
by addition of NaOH. Finally, Al(OH)3 can be converted into alumina (alumina
A1203) by roasting Al(OH)3 for example at a temperature of about 800 C
to1200 C.
17
CA 02812309 2013-03-15
WO 2012/065253
PCT/CA2011/001271
The following non-limiting examples further illustrate the disclosure.
Examples
Example 1
Preparation of argillite sample
[0060] Crushing of mudstone : The resulting micronization average
employed for the tests ranges between 10 and 50 microns.
[0061] Roasting : Crushed mudstone was roasted at least during 1 hour
at
a temperature of 600 C. Its average composition was:
A1203 21,0 A)
Fe203 8,0 AD
K20 1,5 %
Na20 0,9 %
TiO2 0,9 %
CaO 0,08 %
ZnO 0,06 %
Si02 51,0 %
Acid leaching
[0062] 500 g of argillite crushed and roasted were added to 5 liters
of
hydrochloric acid 6 M. The mixture was then heated at 100 C - 110 C during
7 hours.
[0063] After reaction, the liquid part was separated from the solid
part by
filtration. The solid was washed with distilled water which was added to the
liquid portion. This washing makes it possible to recover part of the aluminum
trapped in the solid. This solid had a dry mass of 345 - 5 g, which
corresponds to a loss of about 30 % - 32 %.
[0064] The remaining liquid part, containing aluminum, iron and a
great
part of the impurities initially present in mudstone, was reduced by
evaporation at a temperature of 100 C to 90 % of its initial volume. Residual
18
CA 02812309 2013-03-15
WO 2012/065253
PCT/CA2011/001271
volume was then 50 mL. The liquid compositions before and after evaporation
were:
Evaporated leaching
Leaching solution
solution
Composition CYO Composition (%)
[concentration (mg/L)] [concentration (mg/L)]
47.63 47.86
Aluminum
[9 250] [59 500]
31.54 31.07
Iron
[6 125] [38 625]
Alkaline-earths 19.30 19.53
(Na, Mg, K, Ca) [3 749] [24 277]
1.53 1.54
Other metals
[297.3] [1 920]
All the ions species seem to remain soluble.
Removal of iron
[0065] The residual volume was slightly diluted (+ 25 /0) and
concentrated
hydroxide sodium (10 M) was added until a pH higher than 11.5 was reached.
The formed precipitate was separated from the solution by standard filtration
and was washed several times with NaOH dilued and hot ultra-pure water.
The precipitate contained all the iron and the majority of the metal
impurities.
The filtrate contained in addition to ions Al3+ mainly alkaline-earths and
some
following impurities:
Major filtrate impurities
(%)
Iron 0.14
Sodium 94.13
Alkaline-earths
5.71
(Mg, K, Ca)
Other metals 0.02
Na+ came from soda and was also the Al(OH)4" counter-ion.
19
CA 02812309 2013-03-15
WO 2012/065253 PCT/CA2011/001271
[0066] Other tests have
been made for precipitating iron ions under acidic
conditions and more particularly at a pH of about 4.5 to about 5.0 with 0.1 M,
1 M and 6M (twice) of NaOH and it was observed that such an embodiment
was efficient. In fact, it was found that around a pH of about 4.6 to about
4.8,
almost all the iron was precipitated.
Aluminum recovery
[0067] The filtrate is
adjusted at a pH of 2.5 to 3.5 by addition of HCI 6 M.
The resulting solution is extracted by means of the complexing agent, Cyanex
272, at a concentration of 20 % volume / volume in an organic solvent with a
volumetric ratio of 1:1. The extraction is carried out at a temperature of 40
C
in a membrane contactor with hollow fibers. In less than about 30 to 60 min,
more than 85 % of aluminum is extracted. The pH adjustment is performed by
a regulation loop controling the NaOH (10 M) addition. Complexed Al3+ in
Cyanex are then recovered by carrying out a back extraction with HCI (6 M) at
40 C and an organic phase / acid phase volumetric ratio of 1:0.5. After the
back extraction, the composition of the recovered acid phase is:
Composition (%)
Aluminum 92.81
Iron 0
Alkaline-earths
7.14
(Na, Mg, K, Ca)
Other metals 0.05
[0068] To increase the
percentage of purity, the Al3+ ions are precipitated
in the form of Al(OH)3 hydroxide, then washed several times with ultra-pure
water. The composition of the hydroxide becomes:
CA 02812309 2013-05-28
Composition (%)
Aluminum 99.09
Iron
Alkaline-earths
0.88
(Na, Mg, K, Ca)
Other metals 0.03
[0069] Further purification can be performed by recrystallization
[0070] The scope of the claims should not be limited by specific
embodiments and examples provided in the disclosure, but should be given
the broadest interpretation consistent with the disclosure as a whole.
21