Note: Descriptions are shown in the official language in which they were submitted.
1
ENTERIC COATED, LOW-STRENGTH PANCRELIPASE FORMULATIONS
FIELD OF THE INVENTION
In various embodiments, the present invention is directed to pharmaceutical
compositions having a
stable, low (diluted) digestive enzyme content comprising at least one
digestive enzyme and at least
one carrier, or a dosage form thereof, In other embodiments, the invention is
also directed to
processes of preparation of the composition or the dosage form. In additional
embodiments, the
invention is directed to the treatment and prevention of disorders associated
with a digestive
enzyme deficiency in a patient in need thereof, comprising administering to
said patient a
pharmaceutically acceptable amount of the composition having a stable low
digestive enzyme
content or dosage form thereof.
BACKGROUND OF THE INVENTION
The proper dosing of medications for patients is an important concern within
the medical field. For
infants or smaller children, or geriatric patients in particular, and
sometimes also for adult
populations, the administration of medications and dosing methods often
present substantial issues.
As is well known in the art, medications are provided in many forms (e.g.,
liquid, solid, and
combinations of solids in liquids) and are delivered to patients in many ways
(e.g., orally, via
injection, transdermally).
The FDA estimates that more than 200,000 Americans suffer from exocrine
pancreatic
insufficiency (EPI). EPI involves a physiological disorder wherein individuals
are incapable of
properly digesting food due to a lack of digestive enzymes made by their
pancreas. That lack of
digestive enzymes leads to disorders such as the maldigestion and
malabsorption of nutrients, which
lead to malnutrition and other consequent undesirable physiological conditions
associated
therewith. These disorders are common for those suffering from cystic fibrosis
(CF) and other
conditions compromising the exocrine function of the pancreas, such as
pancreatic cancer,
pancreatectomy, and pancreatitis. The malnutrition can be life threatening if
left untreated,
particularly in the case of infants and CF patients, and the disorders can
lead to impaired growth,
compromised immune response, and shortened life expectancy.
CA 2812862 2018-05-25
CA 02812862 2013-03-27
WO 2012/042372 2 PCT/IB2011/002419
Digestive enzymes, such as pancrelipase and other pancreatic enzymes products
(PEPs), can be
administered to at least partially remedy EPI. The administered digestive
enzymes provide for
patients to be able to more effectively digest their food. Enzyme therapy is a
critical aspect of
clinical management of nutrition and digestion in the CF population. Recently
published infant
guidelines recommend immediate initiation of PERT (pancreatic Enzyme
Replacement Therapy) in
CF newborns with symptomatic or confirmed pancreatic insufficiency. Within
this framework an
optimal dosing regimen has to be identified. It is believed that the use of
PERT in infants may
improve short and long term growth and nutritional outcomes, and subsequently
increase lung
function and ultimately survival.
Pancreatic enzymes, which have been used in the treatment of EPI to compensate
for lost of
digestive function, have been in use for more than 60 years. Their use until
recently was not subject
modern regulatory guidelines governing drug approvals based on safety, and
efficacy, and
manufacturing controls. Recently, pancreatic enzyme replacement therapies have
become the
subject of US and European regulatory authority initiatives that require that
marketed pancreatic
enzyme products go through the current drug approval process in order to
remain in commerce.
Zenpep , Creon and Pancreaze are three products that successfully went
through the process set
by the FDA and are approved for marketing in the United States. In other
territories/countries
where similar initiatives are still proceeding or have not been implemented as
yet, a variety of
pancreatic enzyme products are still available.
Capsules containing digestive enzymes such as pancrelipase have been developed
for oral
administration. However, if a patient is unable to swallow the capsules, each
capsule can be opened
and the contents sprinkled on a small amount of food, usually a soft, acidic
food (such as
commercially available applesauce) and administered orally to the patient with
a spoon.
Alternatively such medications may be administered orally for infants and
children, using a syringe
device containing the contents suspended in a medium amenable to
administration thereby.
The pancrelipase products are generally labeled as containing three enzyme
classes: lipase,
amylase, and protease, and the levels or potency of which are listed. These
enzymes catalyze the
hydrolysis of fats into glycerol and fatty acids, starch into dextrin and
sugars, and proteins into
amino acids and derived substances. Digestion is, however, a complex process
involving many
other enzymes and substrates that contribute to correct digestive functioning
and producing the full
range of digestive products. Other enzymes contained in pancrelipase include
trypsin,
carboxypeptidases, elastases, phospholipases, and cholesterases amongst other
and various co-
factors and coenzymes. These substances are produced naturally in the pancreas
and also contribute
3
to correct digestive functioning.
Pancrelipase is typically prepared from porcine pancreatic glands, although
other sources can also
be used, for example those described in U.S. 6,051,220, U.S. 2004/0057944,
2001/0046493, and
WO 2006044529.
Pancreatic enzymes show optimal activity under near neutral and slightly
alkaline conditions.
Under gastric conditions, pancreatic enzymes may be inactivated with a
resulting loss in biological
activity. Therefore, exogenously administered enzymes are generally protected
against gastric
inactivation and remain intact during their transit through the stomach and
into the duodenum.
Therefore it is desirable to coat pancreatic enzymes. Pancreatic lipases are
the most sensitive to
gastric inactivation and are the most important class of enzymes in the
treatment of malabsorption.
Lipase activity is typically monitored to determine the stability of an enzyme
composition
containing lipase.
U.S. 7,658,918 describes stable digestive enzymes compositions and explains
that certain
particulate medications, administered orally, are designed to pass through the
stomach of the patient
and thereafter to release within the intestines; the total amount of
pancrelipase (by weight) in the
cores of the particles comprised in the compositions or oral dosage forms
disclosed in said patent is
68-90%.
Aptalis Pharma markets at least some multiparticulate enterically coated
pancrelipase enzymes
beads medications. For example, Aptalis Pharma markets delayed-release
capsules for the
treatment of exocrine pancreatic insufficiency (EPI) in patients under the
designation EUR-1008
and the registered trademark Zenpep . Each Zenpep capsule for oral
administration contains
enteric coated beads with high pancrelipase content (1.8-1.9 mm for 3,000,
5,000 USP units of
lipase, 2.2-2.5 mm for 10,000, 15,000 and 20,000 and 25,000 USP units of
lipase).
All of the marketed pancrelipase products have very high pancrelipase content.
Some commercially available digestive enzyme compositions show a loss of
lipase activity over
time of up to about 35% or more. In order to compensate for the loss of
enzymatic activity during
storage and to ensure that the product provides the label-claimed potency at
the end of the shelf life,
manufacturers typically overfill the dosage forms from 5% to 60% and current
USP specifications
for Pancrelipase Delayed-Release Capsules allow for Pancrelipase equivalent to
not less than 90%
and not more than 165% of the labeled lipase activity. In practice this means
that patients and
CA 2812862 2018-01-18
CA 02812862 2013-03-27
WO 2012/042372 4 PCT/IB2011/002419
prescribers are sometimes unable to judge the dosage strength with accuracy,
with the practical
result that the appropriate dosage needs to be determined empirically for each
new prescription.
Patients with exocrine pancreatic insufficiency disorders rely on these drugs
to provide the enzymes
they need to digest food properly. If the label contains an inaccurate
statement about a particular
product's potency, then the patient is at risk for receiving too much or too
little of the medicine.
In addition, there exist several situations in which a low dosage is needed
and proper dosing of the
medication cannot be achieved using the existing high dosage formulations.
This becomes
particularly relevant when pancrelipase should be a administered in infants
with a dose ranging
from 500 units lipase per meal per kg body weight to 2,000 units lipase per
meal per kg then, low
dosage or diluted pancrelipase dosage form should be available for
administration.
It is generally known that the preparation of low dosage forms having a
uniform drug content faces
several problems. In addition to that, in case of pancrelipase, both the
composition and the process
of preparation of a final diluted formulation should be as such as to ensure
the proper stability upon
storage of the labile enzymes.
Accordingly, it would be desirable to provide a stable low dosage or diluted
digestive enzymes
composition having high content uniformity and capable of maintaining the
necessary activity for
the expected shelf life of the enzymes preparation.
BRIEF SUMMARY OF THE INVENTION
To achieve these and other objects, and to meet these and other needs, and in
view of its purposes,
the present invention relates to a stable low dosage digestive enzyme
composition, and dosage form
comprised thereof.
More particularly, in various embodiments, the present invention relates to a
stable, highly diluted
enzyme composition and dosage form that comprise a plurality of digestive
enzymes beads, more
particularly enterically coated beads. The diluted digestive enzyme beads have
high content
uniformity and exhibit minimal loss of enzymatic activity upon storage.
The present invention provides for a suitable package comprising a sealed
container made of
moisture resistant material, a desiccant, and at least one dosage form
according to the invention.
Moreover, the present invention provides a method of preparing the stable, low
dosage digestive
enzyme composition and dosage form thereof. The method comprises preparing a
suitable diluted
CA 02812862 2013-03-27
WO 2012/042372 5 PCT/IB2011/002419
digestive enzyme blend with at least one carrier to ensure the high uniformity
in digestive enzyme
content, and then coating the beads with a solution comprising an enteric
polymer, thereby forming
a plurality of stable enterically coated diluted digestive enzyme-containing
beads.
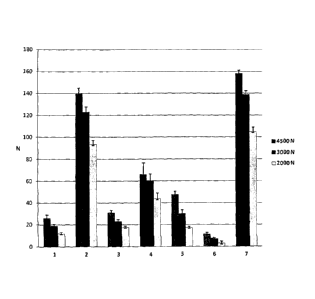
BRIEF DESCRIPTION OF THE FIGURE
Figure 1. Hardness of tablets consisting of pancrelipase and a carrier (blend
1: pancrelipase; blend
2: pancrelipase and microcrystalline cellulose B; blend 3: pancrelipase and
trehalose; blend 4:
pancrelipase and isomalt; blend 5: pancrelipase and calcium bibasic; blend 6:
pancrelipase and
inostol; blend 7: pancrelipase and microcrystalline cellulose A).
DETAILED DESCRIPTION OF THE INVENTION
The present invention is directed to a stable composition comprising at least
one digestive enzyme,
and at least one carrier wherein:
a) the total amount of digestive enzymes in the composition is from about 4 to
about 20% by
weight; or
b) at least one carrier of the composition has a large particle size; or
c) the total amount of digestive enzymes in the composition is from about 4 to
about 20% by
weight, and at least one carrier of the composition has a large particle size.
In another embodiment, the total amount of the digestive enzymes in the
composition ranges from
about 5 to about 19% by weight.
In another embodiment, the total amount of the digestive enzymes in the
composition ranges from
about 10 to about 15% by weight. In another embodiment of the invention, the
total amount of the
digestive enzymes in the composition ranges from about 4%, or about 5%, or
about 10%, or about
15%, or about 19% by weight, inclusive of all ranges and sub-ranges there
between.
In the composition of the invention, the digestive enzymes are in form of
beadsõ , preferably in the
form of enterically coated pancrelipase beads.
In various embodimentsof the invention, the diluted digestive enzymes beads
comprise: from about
4 to about 20 wt. % of pancrelipase and from about 70 to about 96% of at least
one carrier; or from
about 5 to about 19 wt. % of pancrelipase and from about 71 to about 95% of at
least one carrier, or
CA 02812862 2013-03-27
WO 2012/042372 6 PCT/IB2011/002419
from about 10 to about 15 wt. % of pancrelipase and from about 75 to about 90%
of at least one
carrier, wherein each said wt. % is based on the total weight of the uncoated
beads.
In one further embodiment of the invention, the beads that are enterically
coated comprise: from
about 10 to about 15 wt. % of pancrelipase and from about 80 to about 85% of
at least one carrier,
wherein each said wt. % is based on the total weight of the uncoated beads.
In the present invention, low pancrelipase content powder blends are disclosed
that have high
content uniformity and very low segregation while also showing excellent
flowability. These
blends are particularly suitable for producing the low or diluted pancrelipase
beads.
For the present invention, the digestive enzyme beads include any kind of
particulate. The term
"bead" includes granules, tablets, spheres, minitablets, microtablets,
microparticles, microspheres,
minimicrospheres, microcapsules, micropellets, as well as particles up to
about 5 mm in diameter.
The bead may be any suitable particle size or shape. For example, the beads
can have a particle size
range of about 50 gm to about 5,000 pm, or of about 50 gm to about 2,000 p.m,
they can have a
nominal (e.g,, mean) particle diameter in the range of about 2 to about 5 nun,
or of less than about 2
mm for example of about 0.5 to about 2 mm. Beads may have diameters for
example of about 0.7 to
about 1.6 mm, or of about 0.7 to about 1.25 mm, or of about 0.7 to about 1.25
mm.
"Minimicrospheres" having the smallest median particle size of about 1.15 mm
or "microtablets"
having highest median particle size at about 2.63 mm are also suitable for the
present process. The
beads can have an average particle size of less than about 800 pm, preferably
less than about 500
p.m, preferably of about 400 pm to about 600 gm or of about 250 1.tm to about
500 p.m. These beads
may have a volume diameter (d(v,0.1) (defined as the diameter where 10% of the
volume
distribution is below this value and 90% is above this value) of not less than
400 pm and a volume
diameter d(v,0.9) (defined as the diameter where 90% of the volume
distribution is below this value
and 10% is above this value) of not more than 900 pm.
All these diluted digestive enzymes beads, more particularly pancrelipase
enzymes beads, suitable
for the preparation of pharmaceutical products may be enterically coated
beads. In embodiments
where there is an enteric coating, this coating acts as a barrier, protecting
the drug substance from
the acidic environment of the stomach and substantially prevents the release
of the medication
before it reaches the small intestine (i.e., the release of enzyme in the
stomach is less than about 10
to about 20% of the total amount of enzyme in the composition). Suitable
combinations of enteric
coating compositions with other coating compositions can be used to provide
the desired type of
control over drug release or therapeutic effects. The enteric coating includes
at least one enteric
CA 02812862 2013-03-27
WO 2012/042372 7 PCT/IB2011/002419
polymer and further excipients. The phrase "enteric polymer" means a polymer
that protects the
digestive enzymes from gastric contents, for example a polymer that is stable
at acidic pH, but can
break down rapidly at higher pH or a polymer whose rate of hydration or
erosion is slow enough to
ensure that contact of gastric contents with the digestive enzymes is
relatively minor while it is in
the stomach, as opposed to the remainder of the gastro-intestinal tract.
The compositions and dosages forms of the invention comprise at least one
digestive enzyme.
The term "digestive enzyme" used herein denotes an enzyme in the alimentary
tract which breaks
down the components of food so that they can be taken or absorbed by the
organism. Non-limiting
examples of digestive enzymes include pancrelipase (also referred to as
pancreatin), lipase, co-
lipase, trypsin, chymotrypsin, chymotrypsin B, pancreatopeptidase,
carboxypeptidase A,
carboxypeptidase B, glycerol ester hydrolase, phospholipase, sterol ester
hydrolase, elastase,
kininogenase, ribonuclease, deoxyribonuclease, a-amylase, papain, chymopapain,
glutenase,
bromelain, ficin, (3-amylase, cellulase, f3-galactosidase, isomaltase, and
mixtures thereof. They are
obtained through extraction from the pancreas or pancreatic juices or produced
artificially or
obtained from sources other than pancreas such as from microorganisms,
bacteria, mold, fungi,
plants or other animal tissues, genetically modified microorganisms, fungi or
plants.
The terms "pancrelipase" or "pancrelipase enzymes" or "pancreatin" denotes a
mixture of several
types of enzymes, including amylase, lipase, and protease enzymes, or mixtures
thereof having
pancreatic origin. Pancrelipase is commercially available, for example from
Nordmark Arzneimittel
GmbH, Scientific Protein Laboratories LLC or Sigma Aldrich; and similar
extracts from porcine,
bovine or other mammalian sources may be used.
The term "lipase" denotes an enzyme that catalyzes the hydrolysis of lipids to
glycerol and simple
fatty acids. Examples of lipases suitable for the present invention include,
but are not limited to
animal lipases (e.g., porcine lipases), bacterial lipases (e.g., Pseudomonas
lipase ancUor
Burkholderia lipase), fungal lipases, plant lipases, recombinant lipases
(e.g., produced via
recombinant DNA technology by a suitable host cell, selected from any one of
microorganisms,
bacteria, yeast, fungi, plants, insects or mammalian host cells in culture, or
recombinant lipases
which include an amino acid sequence that is homologous or substantially
identical to a naturally
occurring sequence, lipases encoded by a nucleic acid that is homologous or
substantially identical
to a naturally occurring lipase-encoding nucleic acid, etc.), synthetic
lipase, chemically-modified
lipase, and mixtures thereof. The term "lipids" broadly includes naturally
occurring molecules
including fats, waxes, sterols, fat-soluble vitamins (such as vitamins A, D, E
and K),
CA 02812862 2013-03-27
WO 2012/042372 8 PCT/IB2011/002419
monoglycerides, diglycerides, triglycerides, phospholipids, etc.
The term "amylase" refers to glycoside hydrolase enzymes that break down
starch, for example a -
amylases, p-amylases, y-amylases, acid a-glucosidases, salivary amylases such
as ptyalin, etc.
Amylases suitable for use in the present invention include, but are not
limited to animal amylases,
bacterial amylases, fungal amylases (e.g., Aspergillus amylase, for example,
Aspergillus oryzae
amylase), plant amylases, recombinant amylases (e.g., produced via recombinant
DNA technology
by a suitable host cell, selected from any one of microorganisms bacteria,
yeast, fungi, plants,
insects or mammalian host cells in culture, or recombinant amylases which
include an amino acid
sequence that is homologous or substantially identical to a naturally
occurring sequence, amylases
encoded by a nucleic acid that is homologous or substantially identical to a
naturally occurring
amylase-encoding nucleic acid, etc.), chemically modified amylases, and
mixtures thereof.
The term "protease" refers generally to enzymes (e.g., proteinases,
peptidases, or proteolytic
enzymes) that break peptide bonds between amino acids of proteins. Proteases
are generally
identified by their catalytic type, e.g., aspartic acid peptidases, cysteine
(thiol) peptidases,
metallopeptidases, serine peptidases, threonine peptidases, alkaline or semi-
alkaline proteases,
neutral and peptidases of unknown catalytic mechanism. Non-limiting examples
of proteases
suitable for use in the present invention include serine proteases, threonine
proteases, cysteine
proteases, aspartic acid proteases (e.g., plasmepsin) metalloproteases and
glutamic acid proteases.
In addition, proteases suitable for use in the present invention include, but
are not limited to animal
proteases, microbial proteases, bacterial proteases, fungal proteases (e.g.,
an Aspergillus melleus
protease), plant proteases, recombinant proteases (e.g., produced via
recombinant DNA technology
by a suitable host cell, selected from any one of bacteria, yeast, fungi,
plant, insect or mammalian
host cells in culture, or recombinant proteases, which include an amino acid
sequence that is
homologous or substantially identical to a naturally occurring sequence,
proteases encoded by a
nucleic acid that is homologous or substantially identical to a naturally
occurring protease-encoding
nucleic acid, etc.), chemically modified proteases, and mixtures thereof.
The pancrelipase enzymes of the compositions or oral dosage forms of the
compositions of the
present invention can include one or more lipases (i.e., one lipase, or two or
more lipases), one or
more amylases (i.e., one amylase, or two or more amylases), one or more
proteases (i.e., one
protease, or two or more proteases), as well as mixtures of these enzymes in
different combinations
and ratios. In certain embodiments, the ratio of amylase/lipase activities in
the compositions can
range from about 1 to about 10, such as from about 2.38 to about 8.75 (e.g.,
determined by
enzymatic assays performed according to USP protocols). In yet another
embodiment, the ratio of
CA 02812862 2013-03-27
WO 2012/042372 9 PCT/IB2011/002419
protease/lipase can range from about 1 to about 8, such as from about 1.86 to
about 5.13
(determined by enzymatic assays performed according to USP protocols). In
still other
embodiments, the ratio of amylase/lipase activities is about 1, about 2, about
3, about 4, about 5,
about 6, about 7, about 8, about 9, or about 10.
Lipase activities in the compositions or oral dosage forms of the present
invention can be from
about 500 to about 5,000 USP units, preferably from about 750 and about 3,000
USP units. In one
embodiment of the invention, the lipase activity can range from about 675 to
about 825 USP units,
the amylase activity from about 1,600 to about 6,575 USP units, and the
protease activity from
about 1,250 to about 3,850 USP units.
The carrier(s) is/are used in tabletting to increase the bulk of the tablet to
a practical size for
compression. These ingredients used in the beads of the present invention have
the characteristics
of excellent carriers for dry blends providing blend flowability and
workability and preventing
segregation, and provide pancrelipase content uniformity. A well defined
particle size distribution
is relevant to provide outstanding flow and mixing properties. Moreover the
carrier must have low
residual moisture content (low "free water" content).
The carrier may be selected from the group consisting of polyols, sugars,
sugar alcohols, cellulose,
calcium phosphate salts, and amino acids. More specifically, in certain
embodiments of the
invention the carrier is selected from the group consisting of
microcrystalline cellulose, trehalose,
inositol, L-proline in anhydrous form, anhydrous dibasic calcium phosphate,
lactose anhydrous,
lactose monohydrate, isomalt, mannitol and mixtures thereof, as well as other
carriers known in the
art.
In a particular embodiment of the invention, the carrier has a large particle
size. The term "large
size" is defined to be greater than 100 pm; particularly from about 100 gm to
about 300 um, and
more particularly from about 160 um, about 180 um, about 280 um, inclusive of
all ranges and
subranges therebetween, e.g., about 160 um to about 280 pm, about 160 um to
about 180 p.m, about
180 p.m, to about 280 um.
Microcrystalline cellulose is a form of cellulose obtained by spray-drying
washed, acid-treated
cellulose. It is available in several grades that range in average particle
size from 20-100 pm. In
addition, microcrystalline cellulose having a mean particle size greater than
100 um (large particle
size microcrystalline cellulose) is also available; e.g., large particle size
microcrystalline cellulose
of about 160 p.m or about 180 pm.
CA 02812862 2013-03-27
WO 2012/042372 10 PCT/IB2011/002419
In one particular embodiment of the invention the carrier is large particle
size microcrystalline
cellulose.
The large particle size microcrystalline cellulose may have a moisture content
equal to or less than
5%, nominal mean particle size of about 160 gm, mesh size 38: amount retained
< 1.0%, mesh size
of 94: amount retained <50.0%, mesh size 300: amount retained < 70.0%. It has
preferably a LoD
(loss on drying) of not more than 3.8%.
In another embodiment, the large particle size microcrystalline cellulose may
have a moisture
content equal to or less than 5%, nominal mean particle size of about 180 p.m,
mesh size 60: amount
retained?: 10.0%, mesh size of 100: amount retained >50.0%. It has preferably
LoD not more than
1.5%.
In a further embodiment, the microcrystalline cellulose having a moisture
content equal to or less
than 5%, nominal mean particle size of about 50 p.m, mesh size 60: amount
retained < 1.0%, mesh
size of 200: amount retained <30.0% is used in very low amount (such as about
5.8% by weight of
total carrier weight) in admixture with the microcrystalline cellulose having
a larger particle size.
Another suitable carrier may be hydrated or anhydrous trehalose (a-D-
glucopyranosyl-a-D-
glucopyranoside, which is a naturally occurring, non-reducing disaccharide. It
is found, for
example, in the blood of insects, in fungi, in certain yeasts, and in certain
drought-resistant plants. It
can be manufactured by fermentation of certain strains of yeast. Trehalose is
sweet tasting, and has
been suggested for use as a sweetener having reduced cariogenicity in chewing
gum and the like.
Trehalose is normally manufactured and used as the crystalline dehydrate.
Amorphous particulate
trehalose may have particle size in the range of about 180 gm to about 280 gm.
A particular
trehalose used according to the invention is trehalose in its 9.5% dihydrate
form, having a low
hygroscopic profile. The marketed trehalose used in one embodiment of the
present invention is
Trehalose G.
Other examples of carriers suitable for use in the present invention are
inositol, L-proline in
anhydrous form, anhydrous dibasic calcium phosphate (LoD of 0.1-0.2%),
lactose, anhydrous
lactose (monohydrate with LoD: 4.5-5.5 %), and isomalt (LoD of 0.12%).
In the compositions of present invention one single carrier may be used but
also a combination of
two or more different carriers may be used.
In one embodiment of the invention, only large particle size microcrystalline
cellulose is used.
CA 02812862 2013-03-27
WO 2012/042372 11 PCT/IB2011/002419
In another embodiment, a binary blend of microcrystalline cellulose and
trehalose is used.
In a further embodiment, a blend of two celluloses is used.
In another embodiment of the present invention, the carrier is a mixture of
1:1 w/w of
microcrystalline cellulose having a moisture content equal to or less than 5%,
nominal mean
particle size of about 160 pm, mesh size 38: amount retained < 1.0%, mesh size
94: amount
retained <50.0%, mesh size 300: amount retained < 70.0%, and trehalose.
In another embodiment of the present invention, the carrier is a mixture of
1:1 w/w of
microcrystalline cellulose having a moisture content equal to or less than 5%,
nominal mean
particle size of about 180 um, mesh size 60: amount retained? 10.0%, mesh size
of 100: amount
retained 250.0%, and trehalose.
In another embodiment, the carrier is a mixture of 1:1 w/w of two
microcrystalline celluloses (MC);
one MC having a moisture content less than 5%, nominal mean particle size of
about 160 um, mesh
size 38 amount retained < 1.0%, mesh size of 94, amount retained <50.0%, mesh
size 300: amount
retained < 70.0%, and the other MC having a moisture content equal to or less
than 5%, nominal
mean particle size of about 180 um, mesh size 60 amount retained? 10.0%, mesh
size of 100,
amount retained 250.0%.
In another embodiment the carrier is a mixture of 16:1 w/w of two
microcrystalline celluloses;
respectively the first MC having moisture content less than 5%, nominal mean
particle size of about
160 um, mesh size 38: amount retained < 1.0%, mesh size of 94: amount retained
<50.0%, mesh
size 300: amount retained < 70.0%, and the other MC having a moisture content
equal to or less
than 5%, nominal mean particle size of about 50 um, mesh size 60: amount
retained < 1.0%, mesh
size of 200: amount retained <30.0%.
The blends comprising pancrelipase and carrier/s and optionally further
excipients must have
excellent flow properties and consistent particle size. The flow
characteristics should enable the
loading of the tablet die without difficulty. A sieving procedure can be
incorporated to ensure a
more controlled even particle size. This is important to guarantee thorough
mixing of the
components and final homogeneity of the blend.
In addition to the digestive enzymes and the carrier, the beads of the
compositions or oral dosage
forms of the present invention can further comprise one or more
pharmaceutically acceptable
excipients. In one embodiment of the invention the amount of excipient is
about 5% w/w of the
CA 02812862 2013-03-27
WO 2012/042372 12 PCT/IB2011/002419
blend. The term "excipients" includes other pharmaceutically acceptable
ingredients added to the
active component(s) of a composition (e.g., the diluted digestive enzymes) in
order to improve
processing, stability, palatability, etc. Non-limiting examples of suitable
excipients include
pharmaceutically acceptable binders, stabilizers, disintegrants, lubricants,
glidants, diluents, dyes
(coloring agents), stabilizers and mixtures thereof etc. It will be
appreciated by those skilled in the
art of pharmaceutical formulations that a particular excipient may carry out
multiple functions in
the composition. The excipients can have a low moisture content, in particular
the excipients should
have very low "free water" content (less than 15%, less than 10%, about 3% or
less ).The "free
water" is the unbound water.
Non-limiting examples of suitable binders and diluents include starches,
modified celluloses (e.g.,
hydroxypropylcellulose, carboxymethylcellulose sodium), alginic acid,
polyvinyl pyrrolidone
(povidone), amino acids (proline) and mixtures thereof.
Non-limiting examples of suitable disintegrants include dibasic calcium
phosphate, dibasic calcium
phosphate dihydrate, tribasic calcium phosphate, alginic acid,
hydroxypropylcellulose (such as L-
HPC), carboxymethylcellulose calcium, carboxymethylcellulose sodium, cross-
linked
carboxymethylcellulose sodium, swellable ion exchange resins, alginates,
formaldehyde-casein,
cellulose, croscarmellose sodium (e.g., Ac-Di-Sole), crospovidone (e.g., cross-
linked polyvinyl
pyrrolidone) (e.g., Kollidon , CL, Polyplasdone XL, Polyplasdone XL-10),
sodium
carboxymethyl starch, sodium starch glycolate (e.g., Explotab , Explotab CV),
starches (corn
starch, rice starch, maize starch), and mixtures thereof. These disintegrants
have low amount of
moisture content (LoD), preferably less than 15%, even more preferably less
than 10%, for example
croscarrnellose sodium may have LoD of less than 15%, sodium starch glycolate
may have LoD of
about 7-10%, maize starch may have LoD of less than 15%.
Non-limiting examples of suitable lubricants include calcium stearate,
magnesium stearate, sodium
stearyl fumarate, stearic acid, zinc stearate, talc, waxes, Sterotex ,
Stearowet , and mixtures
thereof.
Non-limiting examples of suitable glidants include colloidal silicon dioxide,
talc, and mixtures
thereof.
Non-limiting examples of suitable stabilizers include trehalose, proline,
dextran, maltose, sucrose,
mannitol, polyols, silica gel, aminoguanidine, pyridoxamine, and mixtures
thereof.
CA 02812862 2013-03-27
WO 2012/042372 13 PCT/IB2011/002419
Dyes and coloring compounds such as inorganic or organic pigments may be also
added to the
blend. Non limiting examples are metal oxides, such as TiO2, Fe2O3 /Fez.
3H20, caramel, malt
extract (Corocon ), sugar cane (brown sugar). The LoD of metal oxides is less
than 1%.
One or more of the excipients used in the present invention can function as a
dessicant to further
stabilized the composition. Suitable excipients useful as desiccants include
any pharmaceutically
acceptable excipient that binds water tightly, or reduces the water activity
of a composition. For
example, the composition of the present invention can include about 1-4%
silica gel, or about 2.5%
silica gel, anhydrous proline or trehalose.
In one embodiment of the present invention the enterically coated beads
comprise about 15 wt. % of
pancrelipase, about 80% of the carrier and about 5% of further excipients,
wherein each said wt. %
is based on the total weight of the uncoated beads.
In another embodiment the enterically coated beads comprise about 10 wt. % of
pancrelipase, about
85% of the carrier and about 5% of further excipients, wherein each said wt. %
is based on the total
weight of the uncoated beads.
The diluted pancrelipase beads of the invention may have an enteric coating
comprising about 10 to
about 20 wt. % of at least one enteric polymer wt. % based on the total weight
of the coated beads.
Non-limiting examples of gastro-resistant ¨ enteric polymers are cellulose
acetate phthalate,
hydroxypropylmethylcellulose phthalate, hydroxypropylmethylcellulose acetate
succinate,
polyvinylacetate phthalate, copolymers of methacrylic acid, esters of
methylmethacrylate, and
shellac. These polymers are commercially available with different brand names,
such as:
Cellacefate (cellulose acetate phthalate), Eudragit L100, S100, L30D, FS30D,
L100-55
(copolymers of methacrylic acid), Aquateric (cellulose acetate phthalate),
Aqoat (hydroxypropyl
methylcelluloacetate succinate), HP55 (hydroxypropyl methylcellulose
phthalate).
The coating may further comprise stabilizing agents. Other optional
ingredients of the coating are
plasticizers, anti-sticking agents, inorganic compound (such as talc,
magnesium stearate, colloidal
silicon dioxide and combinations thereof); further optionally a low viscosity
ethylcellulose). Non-
limiting examples of suitable plasticizers include triacetin, tributyl
citrate, triethyl citrate, acetyl tri-
n-butyl citrate, diethyl phthalate, dibutyl sebacate, polyethylene glycol,
polypropylene glycol, castor
oil, acetylated mono- and di-glycerides, cetyl/myristil alcohol, and mixtures
thereof. The preferred
plasticizer is a non-phthalate plasticizer or mixtures thereof of two or more
(preferably two) of the
listed plasticizers in any combinations.
CA 02812862 2013-03-27
WO 2012/042372 14 PCT/IB2011/002419
The inorganic material can include, for example, silicon dioxide, sodium
salts, calcium salts,
magnesium salts, aluminum salts, aluminum hydroxides, calcium hydroxides
magnesium
hydroxides, talc, and combinations thereof. In one embodiment, this material
is talc.
Depending on the intended use of the composition, the ratio of the enteric
polymer and the at least
one inorganic material may be in a range of from about 10:1 to about 1:60 by
weight. In another
embodiment, the ratio of the enteric polymer and the at least one inorganic
material ranges from
about 8:1 to about 1:50 by weight. In another embodiment, the ratio of the
enteric polymer and the
at least one inorganic material ranges from about 6:1 to about 1:40 by weight.
The ratio of the
enteric polymer and the at least one inorganic material may range from about
5:1 to about 1:30 by
weight, preferably the ratio of the enteric polymer and the at least one
inorganic material ranges
from about 4:1 to about 1:25 by weight or from about 4:1 to about 1:9 by
weight. The ratio of the
enteric polymer and the at least one inorganic material may range from about
10:4 to about 10:7 by
weight. The inorganic material of the enteric coating comprises about 1 to
about 10% by weight of
the weight of the total weight of the particles. In another embodiment the
inorganic material
comprises about 3, about 5, about 7, or about 10% by weight of the particles.
When the inorganic
material is talc, it comprises about 20 to about 60% of the dry coating
weight, for example about
20%, about 25%, about 30%, about 35%, about 40%, about 45%, about 50%, about
55%, or about
60% of the dry coating weight (inclusive of all ranges, sub-ranges, and values
therebetween). In a
preferred embodiment, the inorganic compound is talc. In still another
particular embodiment, the
dry coating of the particles comprises about 31% talc.
In one embodiment of the invention, the coating comprises about 10 to about
20% of a least one
enteric polymer, about 4 to about 10% of a least one inorganic compound, and
about 1 about 2% of
at least one plasticizer (based on the total weight of the particles). For
example, the coating can
comprise about 10 to about 20% of hydroxypropylmethylcellulose phthalate,
about 4 to about 10%
of talc, and about 1 to about 2% of triethyl citrate (based on the total
weight of the particles).
The coating can be applied to the diluted digestive enzyme-containing beads as
a solution of the
enteric polymer (and optionally a suspended inorganic material) in an organic
solvent such as an
alcohol (e.g. ethanol, isopropyl alcohol), a ketone (e.g. acetone), methylene
chloride, or mixtures
thereof (e.g. mixtures of acetone and ethanol). In a preferred embodiment the
hydroxypropylmethylcellulose phthalate is the enteric polymer and acetone is
the solvent.
The coated diluted digestive enzyme-containing beads can then be formulated
into any suitable oral
dosage form. The preferred dosage forms of the present invention are the
capsules. The capsules
CA 02812862 2013-03-27
WO 2012/042372 15 PCT/IB2011/002419
themselves can be comprised of any conventional biodegradable material known
in the art, for
example, gelatin, polysaccharides such as pullulan, or modified cellulosic
materials such as
hydroxypropylmethylcellulose. In order to improve the stability of the
stabilized digestive enzymes,
the capsule can be dried prior to filling, or a capsule comprised of a low
moisture content material
can be selected. In a preferred embodiment, the capsule shell is comprised of
hydroxypropylmethylcellulose and has a water content of about 5% or less, for
example about any
of 4% or less, 2% or less, or 2-5%, or 3-5%, preferably having a water content
of less than about
3% even preferably less than 2%.
The term "moisture content", also referred to as "water content", means the
amount of water that a
composition contains. For compositions that do not change volume with changing
moisture content,
the moisture content can be expressed volumetrically (i.e., by volume) as the
ratio of the mass of
moisture to the dry volume of the material. For compositions that change
volume with changing
moisture content, the moisture content can be expressed gravimetrically (i.e.,
by weight) as the
mass of water removed upon drying per unit dry mass of the specimen.
Determination of moisture
content can be achieved by any of the conventional methods known in the art.
For example, the
moisture content can be determined by chemical titration, such as Karl Fischer
titration, in which a
sample is dissolved in an electrochemical titration cell. Water from the
sample is consumed in an
electrochemical reaction whose endpoint is measured potentiometrically,
thereby providing a direct
measure of the amount of water in the sample. Alternatively, relatively simple
thermogravimetric
methods may be used such as "Loss on Drying" (LoD), in which the mass of a
sample is measured
prior to, and after controlled drying. The loss of mass after drying is
attributed to loss of moisture.
Commercially available moisture analyzers (e.g., available from Mettler
Toledo, Sartorius AG, etc.)
can also be used to determine moisture content.
The moisture content of the ingredients and of the compositions or oral dosage
forms of the present
invention can be measured by any suitable method known in the art, for example
LoD, or
thermogravimetric analysis. LoD is the preferred method.
The compositions or oral dosage forms of the present invention, comprising at
least one digestive
enzyme, may have a water activity of about 0.6 or less, about 0.5 or less,
about 0.4 or less, about 0.3
or less, about 0.2 or less, or about 0.1 or less, inclusive of all ranges and
subranges therebetween
(i.e., any of about 0.5 to about 0.6, about 0.4 to about 0.6, about 0.3 to
about 0.6, about 0.2 to about
0.6, about 0.1 to about 0.6, about 0.4 to about 0.5, about 0.3 to about 0.5,
about 0.2 to about 0.5,
about 0.1 to about 0.5, about 0.3 to about 0.4, about 0.2 to about 0.4, about
0.1 to about 0.4, about
0.2 to about 0.3, about 0.1 to about 0.3, about 0.1 to about 0.2, etc.).
Compositions or oral dosage
CA 02812862 2013-03-27
WO 2012/042372 16 PCT/IB2011/002419
forms of the present invention, maintained at a low water activity, have been
found to be
substantially more stable compared to conventional digestive enzymes
compositions maintained at
higher water activity levels.
Water activity, also referred to as "aw", is the relative availability of
water in a substance. As used
herein, the term "water activity" is defined as the vapor pressure of water in
a sample divided by the
vapor pressure of pure water at the same temperature. Pure distilled water has
a water activity of
exactly one. Water activity is temperature dependent. That is, water activity
changes as the
temperature changes. In the present invention, water activity is measured at a
temperature ranging
from about 0 C to about 50 C, preferably from about 10 C to about 40 C.
The water activity of a product can be determined by measuring the relative
humidity of the air
surrounding the sample at equilibrium. Accordingly, measurement of water
activity in a sample is
typically carried out in an enclosed (usually insulated) space where this
equilibrium can take place.
At equilibrium, the water activity of the sample and the relative humidity of
the air are equal, and
therefore a measurement of the equilibrium relative humidity (ERH) of the air
in the chamber
provides a measure of the water activity of the sample. At least two different
types of water activity
instruments are commercially available. One type of water activity instruments
uses chilled-mirror
dew point technology (e.g., AquaLab water activity meters available from
Decagon Devices, Inc.)
while others measure relative humidity with sensors that change electrical
resistance or capacitance
(e.g., water activity meters available from Rotronic ). The water activity of
the compositions or oral
dosage forms of the present invention can be measured by any suitable method
known in the art
The compositions or dosage forms of the present invention, comprising at least
one stabilized
digestive enzyme show no loss of enzymatic activity after three months of
accelerated stability
testing. The composition or dosage form may exhibit a loss of enzyme activity
of no more than
about 25%, no more than about 20%, no more than about 15%, no more than about
12%, no more
than about 10%, no more than about 8%, or no more than about 5%, after six
months of accelerated
stability testing.
The term "accelerated stability testing" or "accelerated storage testing"
refers to test methods used
to simulate the effects of relatively long-term storage conditions on enzyme
activity, which can be
carried out in a relatively short time. Accelerated stability testing methods
are known in the art to be
a reliable alternative to real-time stability testing, and can accurately
predict the shelf life of
biological products. Such "accelerated stability testing" conditions are known
in the art and are in
accordance with the International Conference for Harmonization of Technical
Requirements for
CA 02812862 2013-03-27
WO 2012/042372 17 PCT/IB2011/002419
Registration Of Pharmaceuticals for Human Use: Stability Testing of New Drug
Substances and
Products Q1A, herein incorporated by reference in its entirety.
After storage (or periodically during storage) the enzyme activity of the
samples can be tested using
conventional methods for assaying digestive enzyme activity (e.g., U.S.
Pharmacopoeia,
Pancrelipase: Assay for lipase activity; herein incorporated by reference in
its entirety).
The compositions of the present invention, and dosage forms comprising the
compositions of the
present invention, have high stability compared to conventional digestive
enzymes (e.g.,
pancrelipase) compositions and dosage forms and deliver the clinically useful
amount of digestive
enzyme to a patient, comprising infants or newborns.
The composition or dosage form (e.g., tablet or capsule) of the present
invention can be stored in
any suitable package. For example, the package can be a glass or plastic jar
with a threaded or
press-fit closure. Alternatively, the compositions or dosage forms of the
present invention can be
packaged as a unit dosage form in "blister packs". Improved stability of the
digestive enzyme
compositions or dosage forms can be provided by providing a moisture-proof
seal, and/or a
moisture-proof package. Non-limiting examples of suitable moisture-proof
packages include glass
jars, plastic jars incorporating moisture barrier resins or coatings,
aluminized plastic (e.g., Mylar)
packaging, etc. The term "moisture-proof' refers to a package which has
permeability to water of
less than about 0.5 mg water per cm3 of container volume per day.
Containers (e.g., bottles) can be closed with any suitable closure, especially
closures which
minimize the ingress of moisture during storage. For example, the compositions
or dosage forms of
the present invention can be thermosealed aluminum liners and polyethylene
foam cap liners. In
order to ensure package integrity and minimize moisture ingress during
storage, sealed packages
containing the compositions or dosage forms of the present invention can be
leak-tested after
dispensing the composition or dosage form of the present invention and sealing
the package. For
example, the sealed packages can be tested by applying a controlled vacuum to
the closure, and
detecting the decrease in vacuum over time. Suitable leak-testing equipment
includes those
manufactured by Bonfiglioli (e.g., model LF-01-PKV or model PKV 516).
Packages containing the compositions or dosage forms of the present invention
can also contain a
desiccant (i.e., a substance which absorbs, reacts with, or adsorbs water)
capable of reducing the
humidity inside the package, for example a desiccant, capable of "scavenging"
moisture from the
atmosphere sealed inside the package. Non-limiting examples of suitable
desiccants that can be
CA 02812862 2013-03-27
WO 2012/042372 18 PCT/IB2011/002419
placed inside such packages include zeolites (e.g., molecular sieves such as 4
A molecular sieves),
clay (e.g., montmorillonite clay), silica gel, or combinations thereof. In one
embodiment, the
desiccant comprises molecular sieves.
In addition, it is common practice when packaging oral pharmaceutical unit
doses to add a ''plug" of
a cellulosic material, such as cotton, into the top of the container to fill
the empty space at the top of
the container, thereby minimizing movement of the contents. Cellulosic
materials are somewhat
hygroscopic, and can act as a "reservoir" of moisture inside the package.
Accordingly, in the present
invention, no cellulosic or cotton "plug" is added. One embodiment of the
present invention is the
process of preparation of the composition and dosage form with low and uniform
pancrelipase
content that comprises the following steps:
a) mixing the at least one digestive enzyme and at least one carrier or
mixture thereof and optional
further excipients to form a mixture; the mixing is carried out under mild
condition (such as manual
grinding in mortar); high energy milling should be avoided to reduce the risk
of lipase activity
reduction;
b) direct compressing the mixture into beads;
c) coating the beads with a solution comprising at least one enteric polymer.
The process further comprises the following steps:
d) preparing the dosage forms with the coated beads, such as filling capsules
with the coated beads;
e) packaging the dosage forms.
It is highly relevant that all process steps are conducted under strict
control of environmental
moisture, which should be kept at a very low level; for examples the absolute
moisture of incoming
air during coating should be kept at values of about 2 to about 3 g/kg,
relative humidity during step
d) should be less than 40%. Moreover, all ingredients of the blend and of the
coating should also
have very low moisture content (or preferably less than about 15, or less than
about10%, or less
than about 5%, or less than about 3%). The capsule shell should also have low
moisture content
(less than about 5%, preferably less than about 3%) to minimize water transfer
to the product. The
packaging configuration should also be carefully chosen in order minimize
water permeability.
Only under these circumstances do the final diluted digestive enzyme
compositions or dosage forms
have prolonged storage stability.
CA 02812862 2013-03-27
WO 2012/042372 19 PCT/IB2011/002419
Several dosage formulations can be made in which different dimensions of the
tablets are obtained.
A pancrelipase blend containing the pancrelipase, the carrier(s) and the
additional excipients can be
tabletted for example using round 2 mm diameter beveled punches, or with round
1.5 mm diameter,
1.2 mm radius of curvature punches to produce microtablets with different
dimensions. The blend
may be tabletted using compression parameters suitable to obtain pancrelipase
minitablets or
microtablets. For example, diluted pancrelipase microtablets can be produced
according to this
invention with weight of about 2 mg to about 4 mg, preferably from about 2.6
to about 3.64 mg,
with friability lower than about 2.5 p/p (USP method) and with thickness from
about 1.5 to about
2.0 mm.
One embodiment of the present invention provides a method of treating or
preventing a condition or
disorder associated with digestive enzyme deficiency in a patient, comprising
administering the
pharmaceutical composition or dosage form of the present invention to a
patient (e.g., a mammal
such as a human) in need thereof.
In another embodiment, the invention provides a method of treating or
preventing a disorder or
condition associated with digestive enzyme deficiency, comprising
administering the composition
or dosage form of the present invention to a patient in need thereof, wherein
the composition or
dosage form comprises, in addition to the digestive enzymes, at least one
proton pump inhibitor, or
one antacid, or other medicament which increases GI pH. In still another
embodiment, the present
invention provides a method of treating or preventing a disorder or condition
associated with
digestive enzyme deficiency, comprising administering a composition or dosage
form of the present
invention, in combination with a dosage form comprising at least one proton
pump inhibitor, one
antacid, or other medicament which increases GI pH.
Disorders or conditions that can be treated with the composition or dosage
forms of the present
invention include conditions in which the patient has no or low levels of
digestive enzymes or in
which patients require digestive enzyme supplementation. For example, such
conditions can include
exocrine pancreatic insufficiency, cystic fibrosis, chronic pancreatitis,
other pancreatic diseases
(e.g., hereditary, post-traumatic and allograft pancreatitis, hemochromatosis,
Shwachman
syndrome, lipomatosis, or hyperparathyroidism), side-effects of cancer or
cancer treatment, side-
effects of surgery (e.g., gastrointestinal bypass surgery, Whipple procedure,
total pancreatectomy,
etc.) or other conditions in which pancreatic enzymes cannot reach the
intestine, poor mixing (e.g.,
Billroth II gastrectomy, other types of gastric bypass surgery, gastrinoma,
etc.), side effects of drug
treatments such as treatment with metformin or those drugs used to treat the
symptoms of HIV and
autoimmune diseases such as diabetes in which the pancreas may be compromised,
obstruction
CA 02812862 2013-03-27
WO 2012/042372 20 PCT/IB2011/002419
(e.g., pancreatic and biliary duct lithiasis, pancreatic and duodenal
neoplasms, ductal stenosis),
malabsorption associated with celiac disease, food allergies and aging.
Particularly relevant for the purpose of present invention is the treatment of
newborns and infants in
need of treatment thereof with the diluted composition or dosage form of the
present invention.
The term "pharmaceutically effective amount" refers to an amount of
composition of the invention
or dosage form thereof, as disclosed herein, effective in reducing or
ameliorating conditions or
symptoms associated with pancreatic enzyme insufficiency in a patient.
In one embodiment of the invention, an effective amount of the compositions or
dosages forms
herein disclosed are administered in the treatment of pancreatic enzyme
replacement therapy
(PERT) in CF (cystic fibrosis) newborns or infants with symptomatic or
confirmed pancreatic
insufficiency or exocrine pancreatic insufficiency. The compositions or dosage
forms are
administered for improving coefficient of fat absorption (CFA).
From the foregoing description and the experiments disclosed herein, it can be
seen that the present
invention provides several important advantages. The described invention
provides diluted
pancrelipase compositions and dosage forms characterized by high content
uniformity and stability,
and the composition and dosage forms herein are therefore suitable for use
with infants and
newborns who need low doses of pancreatic enzymes.
It is to be understood that both the general description and the detailed
description herein are
exemplary, but not restrictive of the invention, and that all embodiments can
naturally be combined
with one another.
EXAMPLES
Methods
Dissolution test. a) Acid stage medium (pH 1.2): Place 2.00 g of sodium
chloride in 800 mL,
purified water and stir until complete solubilization. Add 7 mL 37% HCl and
mix. Adjust the pH of
the solution to 1.20 0.05 with 1 N HC1 or 1 N NaOH. Dilute to 1000 mL with
purified water;
check the pH and adjust to 1.20 0.05 with 1 N HC1 or 1 N NaOH, if needed. b)
Enteric stage
medium (pH 6.0): Place 9.20 g monobasic potassium phosphate and 2.00 g sodium
chloride in 800
mL purified water and stir until complete solubilization. Adjust the pH of the
solution to 6.00
0.05 with 1 N NaOH. Dilute to 1,000 mL with purified water; check the pH and
adjust to 6.00
0.05 with 1 N HC1 or 1 N NaOH, if needed.
CA 02812862 2013-03-27
WO 2012/042372 21 PCT/IB2011/002419
The measurement of lipolytic activity is carried out using a method based on
the compendia
procedure of lipase assay described in the pancrelipase USP monograph, which
is based on the
titration, by means of pH-stat method, of the free fatty acids formed from the
hydrolysis of
esterified fatty acids in the substrate used (olive oil). It is based on the
following principle: lipase
catalyses the hydrolysis of the triglycerides which leads to the formation of
free fatty acids (FFA).
The titration of the formed FFA according to time provides for the
determination of the enzymatic
activity of lipase, which can be expressed in units: 1 U = 1 mole of formed
FFA per minute. The
reaction occurs by maintaining a steady pH value through an experimental
system that provides for
the addition of NaOH (titrant) when the pH value changes compared to a fixed
value (pHstat
method). The quantity of added titrant according to time corresponds to the
quantity of FFA formed
by the lipase action on the triglycerides. Provided that the procedure is
carried out with a suitable
quantity of substrate and under experimental conditions where the enzyme is
stable, linear kinetics
for the FFA formation according to time can be obtained. The curve slope
{added titrant = f
(volume (mL)/ time (minutes))) gives the lipase enzymatic activity.
The measurement of nroteolytic activity is carried out according to the
compendia procedure
described in the pancrelipase USP monograph.
Example 1. Pancrelipase - carrier blends compatibility
Binary blends of pancrelipase and carrier are prepared by mixing to ascertain
the stability of the
pancrelipase in presence of said ingredients. This binary blend contains
pancrelipase in amount of
60 mg and the carrier in amount of 324 mg; the tested carriers are:
microcrystalline cellulose
(microcrystalline cellulose C: moisture content equal or less than 5%, nominal
mean particle size of
50 [tm, mesh size 60: amount retained < 1.0%, mesh size of 200: amount
retained <30.0%;
marketed as Avicel PH101), trehalose, lactose monohydrate, isomalt, proline
inositol. The
samples are packaged in 10 mL PET and glass vials in the absence of desiccant.
They are stored
under two different conditions: mild storage condition (25 C, 65% relative
humidity (RH)) and
aggravated storage conditions (40 C, 75% relative humidity). Lipase activity
is tested after
different periods of storage according to the compendia method described
herein.
Table 1. Lipase activity of the blends stored at 25 C/65 RH, PET vials (lipase
activity of the blends
is calculated as % of the lipase activity of the pancrelipase sample)
Storage time
Carrier 0 1 week 2 weeks 4 weeks
Pancrelipase none 95 94 90 80
CA 02812862 2013-03-27
WO 2012/042372 22 PCT/1B2011/002419
Storage time
Carrier 0 1 week 2 weeks 4 weeks
(USP units
lipasq)
Pancrelipas Cellulose 98 100 99 103
microcrystalline C
Pancrelipase Trehalose 100 99 99 98
Pancrelipase Lactose 99 100 98 98
monohydrate
Pancrelipase Anhydrous dibasic 93 98 96 100
calcium phosphate
Pancrelipase% Isomalt 100 98 94 99
Pancrelipase% Proline 100 101 99 87
Pancrelipase% Inositol 100 97 96 103
Table 2. Lipase activity of the blends stored at 25 C/65 RH, glass vials
(lipase activity of the blends
is calculated as % of the lipase activity of the pancrelipase sample)
Storage time _
Carrier 0 1 week
Pancrelipase None 95 94
(USP units
lipase)
Pancrelipase Cellulose 98 98
microcrystalline C
Pancrelipase Trehalose 100 99
Pancrelipase Lactose 99 97
monohydrate
Pancrelipase Anhydrous dibasic 93 102
calcium phosphate
Pancrelipase Isomalt 100 97
Table 3. Lipase activity of the blends stored at 40 C / 75 RH, PET vials
(lipase activity of the
blends is calculated as % of the lipase activity of the pancrelipase sample)
Storage time
Carrier 0 1 week 2 weeks 4 weeks
Pancrelipase None 95 96 48 31
CA 02812862 2013-03-27
WO 2012/042372 23 PCT/IB2011/002419
(USP units
lipase)
Pancrelipase% Cellulose 98 103 113 113
microc_gstalline C
Pancrelipase% Trehalose _ 100 97 100 106
Pancrelipase% Lactose 99 99 106 113
monohydrate
Pancrelipase% Anhydrous dibasic 93 99 106 113
calcium phosphate
Pancrelipase% Isomalt , 100 96 102 100
Pancrelipase% Inositol 100 101 110 106
Example 2. Physical characterization of the pancrelipase - carrier blends.
Pancrelipase is blended with one or more carriers and the physical
characterization of these mixture
is carried out by measuring the density (both bulk and tapped), the Can index
(compactability
index), the flowability (flow rate through an orifice is measured as the mass
per time flowing from
funnel, USP method), the LoD. The summary of the results is reported in Table
4.
Table 4.
Density Mass flow g/sec (100g) LoD %
________________________ Carr
index 24W
24h/ 72h/ 72h/
Batch 0 10 0 15 0 20 0 30 room
room room room
untapped tapped mm mm mm mm T---0 temp; temp; temp; temp;
g/s g/s g/s g/s closed
open closed open
vial vial vial vial
Reference sampleA 0.657 0.781 15.88 7.1 / / /
0.96 1.51 2.69 / /
Microcrystalline No No No
0.438 0.561 21.93 20.8 3.68 4.05 4.04
/ /
cellulose C3 flow flow flow
Microcrystalline
0.423 0.500 15.40 10,4 / / / 1.09 1.59 2.12 /
/
cellulose B2
Trehalose G 0.757 0.892 15.13 5.9 / / / 6.45 6.7 6.52
/ /
2
Lactose
0.549 0.632 13.13 7.1 / / / 0.43 0.71 0.87 /
/
Monohydrate _ _
L-Proline 0.512 0.581 11.88 4.5 / / / 0.43 / / 0.52
0.97
Calcium Bibasic 0.694 0.806 13.90 9.1 / / / 0.70 / /
0.89 1.25
Isomalt 0.434 0.500 13.20 5.5 / / / 2.55 / / 2.57
2.84
CA 02812862 2013-03-27
WO 2012/042372 24 PCT/1B2011/002419
Density Mass flow gisec (100g) LoD %
____________________________ Carr
index 24h/ 24W 72h/ 72h/
Batch 0 10 0 15 0 20 0 30 room
room room room
untappedtapped mm mm mm mm T---0 temp; temp; temp; temp;
g/s g/s g/s g/s closed
open closed open
vial vial vial
vial
Anhydrous lactose 0.769 0.833 7.68 4.34 / / /
0.43 / / 0.51 0.86
Microcrystalline
0.434 0.515 15.731 4.34 / / / 3.89 3.92 3.96 / /
cellulose Al _
Inositol 0.609 0.781 22.02 6.66 / / / 0.61 / /
0.45 0.93
Microcrystalline
No
cellulose 82+C3 0.442 0.549 19.49 5.88 / / 2.72 2.8 2.91
/ /
blends (1:1) flow
Microcrystalline
No
cellulose Al +C3 0.454 0.568 20.07 14.28 / /
3.36 4.17 4.17 / /
(1:1) flow
Microcrystalline
No
cellulose C3 0.561 0.724 22.51 8.33 / / 2.78 5.64 5.86
/ /
flow
+Trehalose G (1:1) _ _
_
Microcrystalline
No
cellulose C3 + L- 0.515 0.649 20.65 12.5 / /
2.39 2.19 2.18 / /
flow
Proline (1:1)
. _
Microcrystalline
cellulose C3 No
M55 0.684 18.86 3.33 / / 2.07 2.27 2.15
/ /
+Lactose anhydrous flow
(1:1)
Microcrystalline
cellulose C3 No
0.521 0.657 20.70 10.0 / / 1.94 2.18 2.36
/ /
Lactose flow
Monohydrate (1:1) _
Microcrystalline
cellulose C3 + No
0.561 0.704 20.31 10.5 / / 2.1 2.5
2.65 /
Calcium bibasic flow
(1:1)
Microcrystalline
No
cellulose C3 + 0.510 0.609 16.26 12.5 / / 338 3.37 3.4 /
/
flow
Isomalt (1:1)
Microcrystalline
No
cellulose C3 + 0.531 0.675 21.33 6.66 / / 2.29 / /
2.06 2.27
flow
Inositol (1:1)
A Reference sample: pancrelipase (90%), croscarmellose sodium (3.0%),
hydrogenated castor oil
(1.0%), colloidal silicone dioxide (0.5%), microcrystalline cellulose (5%)
(Avicele PH101);
magnesium stearate (0.5%)
Table 5. Types of microcrystalline celluloses
Nominal Particle size analysis: LoD
mean particle Mesh size Amount
CA 02812862 2013-03-27
WO 2012/042372 25 PCT/IB2011/002419
- size (pm) ____________________________ retained %
- 38 <1 <5%
IMicrocrystalline
<
cellulose A 160 94 50 300
2Microcrystalline 180 60 > 10 0 <5%
_¨ =
cellulose B 100 > 50
3-Microcrystalline 50 60 <1
cellulose C 200 < 30
Crystalline cellulose A is marketed as Vivapure12; crystalline cellulose B is
marketed as Avicel
LM200; crystalline cellulose C is marketed as Avicel PH101.
From the above Table 4 it can be evinced that the microcrystalline cellulose C
(moisture content
equal to or less than 5%, nominal mean particle size of 50 gm, mesh size 60:
amount retained <
1.0%, mesh size of 200: amount retained <30.0%) has low mass flow which is an
indication of
critical issues during the direct compression process. To avoid such issues
with carriers having low
flowability, an additional treatment step (such as wet-granulation) would
typically be carried out to
increase the mass flow. However, any such additional steps are detrimental to
the enzymatic
activity of the pancrelipase formulation and therefore should be avoided to
reduce the risk of
degradation.
Example 3. Hardness measurements of tablets of the pancrelipase - carrier
blends
Pancrelipase raw material (e.g., received from Nordmark) is mixed with
different carriers to form
the seven different blends: blend 1: pancrelipase; blend 2: pancrelipase and
microcrystalline
cellulose B; blend 3: pancrelipase and trehalose; blend 4: pancrelipase and
isomalt; blend 5:
pancrelipase and calcium bibasic; blend 6: pancrelipase and inositol; blend 7:
pancrelipase and
microcrystalline cellulose A. Theses blends are tabletted by direct
compression and hardness is
measured for each sample. The results are reported in Figure 1.
Suitable hardness values are very crucial since low hardness is critical
during the subsequent step of
coating process.
Example 4. Preparation of 15% diluted pancrelipase microtablets.
Pancrelipase raw material (e.g., received from Nordmark) is mixed with the
carrier(s) and the
further excipients to form the different blends. Three different blends are
prepared.
CA 02812862 2013-03-27
WO 2012/042372 26 PCT/IB2011/002419
The first blend (blend 1) contains: 15% pancrelipase, 80% cellulose
microcrystalline A (moisture
content less than 5%, nominal mean particle size of 160 um, mesh size 38:
amount retained < 1.0%,
mesh size 94: amount retained <50.0%, mesh size 300: amount retained < 70.0%),
and 5%
excipients (croscarmellose sodium, 3.0%; hydrogenated castor oil, 1.0%;
colloidal silicone dioxide,
0.5%; magnesium stearate 0.5%), wherein each said wt % is based on the total
weight of the blend.
The second blend (blend 2) contains: 15% pancrelipase, 40% cellulose
microcrystalline A
(moisture content less than 5%, nominal mean particle size of 160 rn, mesh
size 38: amount
retained ( 1.0%, mesh size 94: amount retained <50.0%, mesh size 300: amount
retained < 70.0%.),
40% trehalose (Trehalose G), 5% excipients (croscarmellose sodium, 3.0%;
hydrogenated castor
oil, 1.0%; colloidal silicone dioxide, 0.5%. magnesium stearate 0.5%), wherein
each said wt % is
based on the total weight of the blend.
The third blend (blend 3) contains: 15% pancrelipase, 40% cellulose
microcrystalline B (moisture
content equal or less than 5%, nominal mean particle size of 180 um, mesh size
60: amount retained
> 10.0%, mesh size 100: amount retained >50.0%.), 40% trehalose (trehalose G),
5% excipients
(croscarmellose sodium, 3.0%; hydrogenated castor oil, 1.0%; colloidal
silicone dioxide, 0.5%;
magnesium stearate 0.5%), wherein each said wt % is based on the total weight
of the blend.
Microcrystalline celluloses A and B are defined in Table 5 of Example 3.
The three blends are then tabletted to produce microtablets (1.5 x 1.5 mm).
The microtablets are
tested for, lipase activity, disintegration time, LoD; their weight, thickness
and friability are also
measured on each batch produced (Table 6).
Table 6.
Test tablet 1 tablet 2 tablet 3
(blend 1) (blend 2) (blend 3)
_Lipase (USP units/mg) 14.6 14.9 15.3
Disintegration (min) 3 4 3
_LoD(%) 2.3 1.8 2.7
Weight (mean value) (g) 0.0034 0.0035 0.0035
Thicicness*(mean value) (mm) 1.51 1.48 1.50
Friability* (mean value) (%) 1.1 1.2 1.3
*USP method (20 g of MT, 30 mm at 25 rpm)
CA 02812862 2013-03-27
WO 2012/042372 27 PCT/IB2011/002419
The above utab has high homogeneity in terms of pancrelipase content (CV%
below 5%).
Example 5. Preparation of 10% diluted pancrelipase minitablets
Pancrelipase raw material (e.g. obtained from Nordmark) and carrier
(microcrystalline cellulose)
and excipients (e.g., croscarmellose sodium, hydrogenated castor oil,
colloidal silicon dioxide,
microcrystalline cellulose, and magnesium stearate) are mixed to form a blend.
The composition of
the blend is reported in the following table (Table 7), and has a density of
0.75 - 0.76 g/ml.
Table 7.
Kg (theoretical)
Component
for 1 batch
Microcrystalline cellulose A 297.6 80
Pancrelipase 37.2 10
Croscarmellose sodium , 11.16 3
Hydrogenated castor oil 3.72 1
Colloidal silicon dioxide 1.86 0.5
Microcrystalline cellulose C 18.6 5
Magnesium stearate 0,3-0,4 g/ml 1.86 0.5
Total 372 100
Microcrystalline celluloses A and C are defined in Table 5 of Example 3.
The above blend is tabletted using round 2 mm diameter beveled punches; the
compression
parameters (Table 8) are set to obtain pancrelipase minitablets (MTs) having
the following physical
characteristics: weight between 0.074 g and 0.086 g, with friability lower
than 2.5 % p/p (USP
method), thickness between 2.0 and 2.4 mm.
Table 8.
Compression parameter 10% pancrelipase MT
Tabletting machine speed (rpm) 20
Forced feeding (rpm) 20
Average compression force (kN) about 10
Average pre compression force (kN) about 10
Dosing chamber parameters (mm) 5 - 5.5
4 batches (A-D) of the 10% diluted pancrelipase MTs are produced with these
blends; they have the
following physical properties (Table 9).
Table 9.
CA 02812862 2013-03-27
WO 2012/042372 28 PCT/IB2011/002419
Batch A Batch B Batch C Batch D
Weight (mean value) (g) 0.079 0.079 0.08 0.081
Thickness*(mean value) (mm) 2.2 2.2 2.2 2.2
Friability* (mean value) (%) 0.7 0.8 0.6 0.7
*USP method (20 g of MT, 30 min at 25 rpm)
Example 6. Content uniformity of 10% pancrelipase MTs
The uniformity of the dosage units is demonstrated by measuring the content
uniformity. Each
batch is prepared as in example 4 and is assayed by measuring the lipase
content according to
compendia methods for assaying digestive enzymes activity (e.g., United States
Pharmacopoeia,
Pancrelipase: assay for lipase activity). The assay is repeated 10 times per
each batch and the CV%
results are reported in Table 10.
Table 10.
Batch Coefficient of variation (%)
A 3.2
2.4
2.0
3.3
The MTs prepared show high homogeneity in terms of pancrelipase content. In
fact, the
requirements for dosage uniformity are met by all assayed batches since the
CV% is below 5%.
Example 7. Coating of diluted pancrelipase tablets ( Ts and MTs)
The 15% diluted pancrelipase Ts and the 10% diluted pancrelipase MTs
(Examples 5 and 6,
respectively) are then coated by fluid bed with a coating formulation (having
the composition of
Table 11) in a coating pan. The coating may be started when the tablets reach
temperature of 15-
32 C. The composition of the coated particles prepared according to the
standard coating method
applied for Zenpep minitabs produces uniform, smooth and homogeneous
particles (as analyzed
by microscopic examination).
Table 11.
CA 02812862 2013-03-27
WO 2012/042372 29 PCT/IB2011/002419
Component % (w/w)
Hypromellose phthalate (HP55) 7.64
Triethyl citrate (TEC) 0.76
Talc 3.82
Acetone 87.78
total 100.00
Hydroxypropylmethylcellulose capsules with very low moisture content are then
filled with the
coated diluted pancrelipase microtablets.
Example 8. Enzymatic activity and dissolution of enterically coated diluted
pancrelipase
formulation
HPMC capsules (size 4 white OP /white OP) are filled with diluted enterically
coated pancrelipase
Ts. The capsules are stored in glass bottle with PP closure- liner, Minipax
desiccants. Enzymatic
activities are measured on the formulations stored under different conditions
(at 25 C and 60%
relative humidity, and at 40 C and 75% relative humidity) (Tables 13-18).
Storage stability of bulk
enterically coated diluted pancrelipase microtablets stored at 40 C and 75%
relative humidity in
glass bottle with PP closure- liner, Minipax desiccants are also tested (Table
12). The dissolution of
the microtablets is also measured.
The enterically coated microtablets 1 ( T1) contains: 15% pancrelipase, 80%
cellulose
microcrystalline A (moisture content less than 5%, nominal mean particle size
of 160 um, mesh
size 38: amount retained < 1.0%, mesh size 94: amount retained <50.0%, mesh
size 300: amount
retained < 70.0% ), and 5% excipients (croscarrnellose sodium, 3.0%;
hydrogenated castor oil,
1.0%; colloidal silicone dioxide, 0.5%; magnesium stearate 0.5%), wherein each
said wt % is based
on the total weight of the uncoated Ts.
The enterically coated microtablets 2 ( T2) contains: 15% pancrelipase, 40%
cellulose
microcrystalline A (moisture content less than 5%, nominal mean particle size
of 160 m, mesh
size 38: amount retained < 1.0%, mesh size 94: amount retained <50.0%, mesh
size 300: amount
retained < 70.0%), 40% trehalose, 5% excipients (croscarmellose sodium, 3.0%;
hydrogenated
castor oil, 1.0%; colloidal silicone dioxide, 0.5%, magnesium stearate 0.5%),
wherein each said wt
% is based on the total weight of the uncoated Ts.
The enterically coated microtablets 3 ( T3) contains: 15% pancrelipase, 40%
cellulose
microcrystalline B (moisture content equal or less than 5%, nominal mean
particle size of 180 jam,
CA 02812862 2013-03-27
WO 2012/042372 30 PCT/IB2011/002419
mesh size 60: amount retained > 10.0%, mesh size 100: amount retained
>50.0%.), 40% trehalose,
5% excipients (croscarrnellose sodium, 3.0%; hydrogenated castor oil, 1.0%;
colloidal silicone
dioxide, 0.5%; magnesium stearate 0.5%), wherein each said wt % is based on
the total weight of
the Ts.
Microcrystalline celluloses A and B are defined in Table 5 of Example 3; the
enteric coating
composition is the same as the coating of Example 7 (Table 11)..
Table 12. Stability of bulk enterically coated diluted pancrelipase
microtablets ( Ts); storage
conditions: 40 C+75% relative humidity
Lipase activity USP units /
mg
Batch Time Time Diff time
0 3 mo
T1 11.5 11.2 97
(carrier: microcrystalline cellulose A)
T2 11.7 11.7 100
(carrier: microcrystalline cellulose A and trehalose)
T3 11.5 11.4 99
(carrier, microcrystalline cellulose B and trehalose)
Table 13. Analysis of enterically coated diluted pancrelipase microtablets,
11T1 (carrier:
microcrystalline cellulose A); storage conditions: 25 C, 60% relative humidity
Test Specification Time 0 Time Time Time
1 mo 2 mo 3 mo
Appearance Light small brown corresp corresp corresp
corresp
beads
lipase activity 90-110% of label 735 761 754 774
(USP claim
units/cps) 675-825 USP
units/cps
% label claim 98 101 101 103
Dff TO (%) 104 103 105
protease 1,250-3,850 USP 2,015 2,145 2,145 2,210
activity (USP units/cps
units/cps)
CA 02812862 2013-03-27
WO 2012/042372 31
PCT/1B2011/002419
Test Specification Time 0 Time Time Time
Imo 2 mo 3 mo
_
amylase ' 1,600-6,575 USP 2,600 2,665 2,665 2,795
activity (USP units/cps
units/cps)
Pthalic NMT 1.4% 0.1 0.1 0.1 0.1
Acid(%)
_
LoD (%) NMT 5.0% 2.4 0.5 OA 0.3
dissolution NLT 75% 30 min 84% - 87% 85% 83% _____
(%) (RSD 3.6) (RSD 2.6) (RSD 2.1) (RSD 3.0)
_
dissolution 95% 98% 96% 94%
(%) x1,125 (RSD 2.9) (RSD 2.2) (RSD 1.5) (RSD 3.0)
Weight n=10 ' 65 ' 65 65 65
(mg)
,
Table 14. Analysis of enterically coated diluted pancrelipase microtablets
!ATI. (carrier;
microcrystalline cellulose A); storage conditions: 40 C, 75% relative humidity
_
Test Specification Time 0 Time Time Time
I mo 2 mo 3 mo
_
Appearance Light small brown corresp corresp corresp
corresp
beads
_
lipase activity 90-110% of label 735 754 754 754
(USP unis/cps) claim
675-825 USP
units/cps
% label claim 98 101 101 101
_
Dff TO (%) 103 103 ' 103
_ ____________________________________________________________________
protease 1,250-3,850 USP 2,015 2,080 2,015 2,015 -
activity (USP units/cps
_
_ ____________________________________________________________________
CA 02812862 2013-03-27
WO 2012/042372 32
PCT/1B2011/002419
Test Specification Time 0 Time Time Time
1 mo 2 mo 3 mo
units/cps)
amylase 1,600-6,575 USP 2,600 2,665 2,600 - 2,795
activity (USP units/cps
units/cps)
Pthalic NMT 1.4% 0.1 0.1 0.1 0.1
Acid(%)
LoD (%) NMT 5.0% 2.4 0.4 0.1 0.2
dissolution -NLT 75% 30 min - 84% 84% 83% 82%
(%) (RSD 3.6) (RSD 2.9) (RSD 2.2) RD 1.8)
dissolution - 95% 94% 96% 93%
(%) x1.125 (RSD 2.9) (RSD 2.2) (RSD 2.3) (RSD 1.8)
Weight n---10 65 65 65 65
(mg)
Table 15. Analysis of enterically coated diluted pancrelipase microtablets pi'
2 (carrier:
microcrystalline cellulose A and trehalose); storage conditions: 25 C, 76%
relative humidity
- Test Specification Time 0 Time Time
Time
I mo 2 mo 3 mo
Appearance Light small brown corresp corresp corresp
corresp
beads
lipase activity 90-110% of label 736 755 762 755
(USP claim
units/cps) 675-825 USP
units/cps
% label claim 98 101 102 101
Dff TO (%) 103 104 103
protease 1,250-3,850 USP 1,984 2,048 1,984 2,112
activity (USP units/cps
units/cps)
CA 02812862 2013-03-27
WO 2012/042372 33
PCT/IB2011/002419
Test Specification Time 0 Time Time Time
1 mo 2 mo 3 mo
amylase - 1,600-6,575 U 2,496 - 2,688 2,880 2,816
activity (USP USP/cps
units/cps)
Pthalic NMT 1.4% 0.1 0.1 0.1 0.1
Acid(%)
LoD (%) NMT 5.0% 1.6 0.2 0.2 ' 0.3
dissolution NLT 75% 30 min 84% 91% 87% ' 8= 6%
(%) (RSD 2.0) (RSD 3.6) (RSD 2.4) RD 12.2)
dissolution 94% 103% 98% ' 9= 6%
(%) x1.125 (RSD 2.3) (RSD 3.6) (RSD 2.5) (RSD 1.7)
Weight n=10 64 64 64 ' 6= 4
(mg)
Table 16. Analysis of enterically coated diluted pancrelipase microtablets
1.tT2 (carrier:
microcrystalline cellulose A and trehalose); storage conditions: 40 C, 75%
relative humidity
Test Specification Time 0 Time Time Time
1 mo 2 mo 3 mo
Appearance Light small brown corresp corresp corresp
corresp
beads
lipase activity 90-110% of label 736 755 781 742
(USP claim
units/cps) 675-825 USP
units/cps
% label claim ' 98 101 . 104 199
Dff TO (%) ' 103 ' 106 101
,
protease 1,250-3,850 USP 1,984 - 2,240 2,048 1,984
activity (USP units/cps
units/cps)
_
amylase 1,600-6,575 USP 2,496 - 2,688 2,880 2,688 -
CA 02812862 2013-03-27
WO 2012/042372 34
PCT/IB2011/002419
activity (USP units/cps
units/cps)
Pthalic NMT 1.4% 0.1 0.1 0.1 0.1
Acid(%)
LoD (%) NMT 5.0% 1.6 0.5 0.2 0.3
dissolution NLT 75% 30 min 84% 91% 87% 84%
(%) (RSD 2.0) (RSD 2.6) (RSD 3.7) RD 1.4)
dissolution 94% 103% 98% 94%
(%) x1.125 (RSD 2.3) (RSD 2.7) (RSD 4.0) (RSD 1.7)
Weight n=10 64 64 64 64
(mg)
Table 17. Analysis of enterically coated diluted pancrelipase microtablets T3
(carrier:
microcrystalline cellulose B and trehalose); storage conditions: 25 C, 60%
relative humidity
Test Specification Time 0 Time Time Time
I mo 2 mo 3 mo
Appearance Light small brown ¨ corresp corresp corresp
corresp
beads
lipase activity 90-110% of label 746 770 779 792
(USP claim
units/cps) 675-825 USP
units/cps
% label claim 99 104 104 106
Dff TO (%) 104 104 106
protease 1,250-3,850 USP 1,980 2,112 2,112 2,112
activity (USP units/cps
units/cps)
amylase 1,600-6,575 USP 2,640 2,838 2,772 2,838
activity (USP units/cps
units/cps)
Pthalic NMT 1.4% 0.1 0.1 0.1 0.1
CA 02812862 2013-03-27
WO 2012/042372 35
PCT/IB2011/002419
Test Specification Time 0 Time Time Time
1 mo 2 mo 3 mo
Acid(%)
LoD (%) NMT 5.0% 1.6 0.5 0.2 0.3
dissolution NLT 75% 30 min 87% 91% 89% 91%
(%) (RSD 2.4) (RSD 2.6) (RSD 1.4) RD 3.4)
dissolution 98% 102% 100% 103%
(%) x1.125 (RSD 2.1) (RSD 2.7) (RSD 1.2) (RSD 3.4)
- Weight n=10 66 66 66 66
(mg)
Table 18. Analysis of enterically coated diluted pancrelipase microtablets T3
(carrier:
microcrystalline cellulose B and trehalose); storage conditions: 40 C, 75%
relative humidity
Test Specification Time 0 Time Time Time
1 mo 2 mo 3 mo
Appearance Light small brown corresp corresp corresp
corresp
beads
lipase activity 90-110% of label 746 766 766 766
(USP claim
units/cps) 675-825 USP
units/cps
% label claim 99 102 102 102
Dff TO (%) 103 103 103
protease 1,250-3,850USP 1,980 1,980 2,046 2,046
activity (USP units/cps
units /cps)
amylase 1,600-6,575 USP 2,640 2,838 2,706 2,904
activity (USP units/cps
units/cps)
Pthalic NMT 1.4% 0.1 0.1 0.1 0.1
Acid(%)
CA 02812862 2013-03-27
WO 2012/042372 36 PCT/IB2011/002419
LoD (%) NMT 5.0% 1.6 0.4 0.1 0.3
dissolution NLT 75% 30 min 87% 89% 86% 88%
(%) (RSD 2.4) (RSD 1.0) (RSD 1.4) RD 2.4)
dissolution 98% 100% 97% 99%
(%)x1.125 (RSD 2.1) (RSD 0.9) (RSD 1.2) (RSD 2.1)
Weight n=10 66 66 66 66
(mg)
The results indicate that the diluted pancrelipase of the invention are highly
stable for long period of
time even under aggravated conditions of storage.
Although illustrated and described above with reference to certain specific
embodiments and
examples, the present invention is nevertheless not intended to be limited to
the details shown.
Rather, various modifications may be made in the details within the scope and
range of equivalents
of the claims and without departing from the spirit of the invention. It is
expressly intended, for
example, that all ranges broadly recited in this document include within their
scope all narrower
ranges which fall within the broader ranges.