Note: Descriptions are shown in the official language in which they were submitted.
CA 02814454 2013-04-11
WO 2011/105989
PCT/US2010/002729
Patent Application of
Wayne T. Bliesner
For
Title: Reversible hydride thermal energy storage cell optimize . for solar
applications
Field of Invention
This invention relates to a solar energy collection and storage system and
more
particularly to a solar energy collection and storage system which converts
solar energy
into thermal energy storing the energy in the heat of formation of calcium
hydride.
Background
Solar energy provides a thermal power source which can supply a significant
fraction of
the world energy needs. A complete system which can absorb the sunlight during
the day
and produce continuous electricity provides a benefit to the society. Large
scale solar
power production, in GWatt quantities, is the focus of this patent. A power
tower
concept provides a solution where a central receiver absorbs sunlight from a
field of
mirrors focused on the tower. The Barstow facility, Solar 1 and 2, provides an
example
of this type of facility. For extended operation a thermal storage medium of
potassium
nitrate/ sodium nitrate salts allow several hours of extended run time be: ond
the daylight
hours. These systems use the heat of fusion w lin the salt, ie when a ma,
erial transitions
from a liquid to a solid, as the storage medium. The energy density of 'he
salt mixture
requires significant quantities of salts to provide a few extra hours of ope;
ation due to the
low energy density.
Summary
Providing continuous electricity from solar significantly increases he benefit
and
usefulness of this energy source. This invention utilizes the heat of
formation between
calcium and hydrogen to form calcium hydride as the energy storage medium.
Calcium
hydride provides up to 20 times the energy density of the solar 1 and 2 salt
systems.
Calcium hydride is chosen due to its ability to be broken apart using a
thermal heat
source; such as the sun. The process is endothermic absorbing the suns e-ergy
during the
day and storing it chemically. The process is reversed at night with e calcium
and
hydrogen recombining to form calcium hydride in an exothermic reactiu The
direction
of the reaction is controlled by the temperature and pressure in the reaction
chamber.
Calcium hydride is one of the more stable hydrides and will operate reversibly
in the
1200 F to 2200 F temperature range. At these temperatures a Stirling engine or
supercritical CO2 Brayton cycle can be used, integrated with a heat pipe, to
produce
electricity from the thermal storage.
CA 02814454 2013-04-11
WO 2011/105989
PCT/US2010/002729
The hydrogen, separated during the daytime, is stored in a separate low
temperature
hydride tank which allows a low cost bulk storage technique for the hydrogen.
Titanium
iron hydride provides a low cost storage solution for the hydrogen.
Heat exchangers with thermal storage can be lased to store the heat when the
hydrogen is
cooled from temperatures of approximately 2000 F to 100 F. \ method fot owing
the
thermal energy from the hydrogen is to use a -thermo-cline filled with boron
oxide and
dispersed graphite fibers placed perpendicular to the hydrogen flow heat
exchanger. The
top of the thermo-cline remains at high temperature while the hi atom remains
at a lower
temperature. Hydrogen flows between two manifolds located within the thermo-
cline
within a series of tubes dispersed within the boron oxide to transfer thermal
energy to and
from the hydrogen. When the hydrogen flows from the reactor to the storage it
is cooled
. by the boron oxide. When the hydrogen flows from the storage to the reactor
it is heated
by the boron oxide. A second method for stotring the hydrogen thermal energy
uses a
high temperature boron oxide liquid counter¨flow heat exchanger which provides
an
efficient solution to recover the majority of th .e heat from the hydrogen. A
second low
temperature counter flow heat exchanger, u .sing a nitrate salt mixture, can
also be
integrated to extract more of the energy from t' he hydrogen.
The advantage of this system is that it is 21 completely reversible closed
cycle. The
intermittent sunlight can be chemically stored and released at a controlled
rate for electric
power production. The system uses materials which are low cost and provide a
competitive electrical production facility for both small and very large scale
application.
Detailed Description
The storage system consists of three tank systems: The first is the calcium
and calcium
hydride storage cell (1) with reaction chamber. Thc second is the hydrogen
storage tanks
(2) using a low temperature titanium iron hydride material. The third tank
provides
thermal storage used for extracting the energy fr. . the hydrogen prior to the
hydrogen
being stored in the low temperatuic hydride tanks
single thermo-cline tank is used
to store the hydrogen energy as it cools from 2000 J to near room temperature.
This
embodiment uses a high temperature double tank (17) to cool the hydrogen from
2000 F
to 100 F. This thermal storage tank combination is also used to heat the
hydrogen prior to
it entering the calcium cell (1).
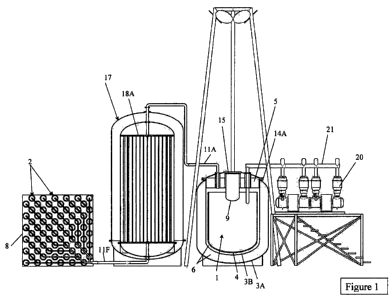
Figure 1 shows an embodiment of a three tank system. The calcium/ calcium
hydride
tank (1) consists of an outer tank (3a), a mid tank (3b), and inner tank (4)
with an
insulation layer (6) between the three tanks. The inner tank (4) is maintained
at
temperatures of 1500 F to 2000 F while the outer tank (3a) is slightly above
room
temperature. The insulation (6) maintains a minimal heat loss between the
thiec tanks.
The inner tank (4) contains the two materials calcium and calcium hydri le.
The calcium
floats above the calcium hydride. Above 1800 F both materials are liquid. The
insulation layer (6) extends completely around both inner tank (4) and mid
tank (3b)
including the top and bottom.
2
CA 02814454 2013-04-11
WO 2011/105989 PCT/US2010/002729
The mid shell serves as a containment shell for the liquids during operation.
The
temperature drop through the insulation is sufficient so that the mid tank
(3b) operates
below the melting point of Calcium. If a leak occurs the liquid Calcium
freezes prior to
reaching the mid tank (3b). The use of silicon diokide insulation is due to
the reaction
which occurs with the liquid Calcium. Free silicon will form which solidifies
stopping
the reaction. Calcium oxide powder provides an inert layer bet ween the mid
tank (3b)
and inner tank (4).
The reaction between calcium and hydrogen to produce calcium hydride is
exothermic.
This provides the heat source to drive a thermal engine such as a Stirling
engine, Brayton
turbine, or steam turbine. Calcium, calcium hydride, and hydrogen gas are in
equilibrium
with the hydrogen pressure a function of the reaction temperature. At
approximately
1800 F this equilibrium is 1 atmosphere pressure. At 2000 F this is
approximately 3
atmospheres pressure. The system pressure and temperature combination is used
to
create either an exothermic or endothermic reaction between the 3 components.
The level of calcium and calcium hydride, within the inner tank (4), varies
depending on
the state of charge of the thermal cell. A fully charged cell is all calcium
with hydrogen
stored separately. A fully discharged cell is all calcium hydride. Hydrogen is
used to
pressurize the three tank regions within cell (1); including the lid (5).
A hydrogen inlet and outlet line (11a) is located in the top of the calcium/
calcium
hydride tank (1). A bolt flange (14a), at the top of tanks (3a), and (3b) join
together to
seal the two tanks. A water cooled lid (5) maintains o-ring seal temperatures.
A quartz
window (15) is located on the top of the calcium/ calcium hydride tank (1), in
lid (5), so
that sunlight can project onto the reaction chamber (9). The reaction chamber
(9) is
fabricated of a molybdenum lanthanum oxide material. The inner surface of the
reaction
chamber (9) is chromium plated to protect the molybdenum from oxidation if air
enters
into the reaction chamber (9) while at high temperature and to provide a low
emissivity
surface for the sunlight absorption after the chromium plating is oxidized. A
series of
helio-stat mirrors located on the ground around the solar thermal reaction
tower are
focused on a down facing mirror, which is attached to the top of a down mirror
tower,
and which is located above the calcium/calcium hydride tank (1) and focuses
sunlight
through the quartz window (15). The area between the quartz window (15) and
the
reaction chamber (9) does not have insulation. Argon, nitrogen gas or a vacuum
fills the
reaction chamber (9).
A means of filling the inside of the reaction chamber (9) with insulation
reduces heat loss
at night. A movable set of plates, fitted within the reaction chamber (9),
could be setup to
close the region with insulation at night. During the day the plates would
move in an
outward direction to allow a clear space between the quartz window (15) and
the reaction
chamber (9). An alternative would be to have a hinged insulated or reflective
lid which
closes over the quartz window (15) during the night.
The inner tank (4) is made of molybdenum with Lanthanum oxide dispersed within
the
metal which raises the recrystallization temperature above the metal operating
3
CA 02814454 2013-04-11
WO 2011/105989 PCT/US2010/002729
temperature. Remaining below the recrystallization temperature is very
important to the
strength and toughness of the molybdenum. The outer tank (3a) is made of a low
temperature stainless such as 304 or 316. The mid tank (3b) is made of a high
temperature stainless such as RA330 or Haynes 230. A Rhenium layer (16) is
coated on
the inside of the inner tank (4). This Rhenium layer (16) provides a barrier
between the
Calcium or calcium hydride and the braze junctions used to fabricate the
molybdenum
tank (4). If the molybdenum parts can be fabricated without seams, or with e-
beam
welds, then the Rhenium coating layer is not required. The inner tank (4) is
fabricated
from sheet stock and is brazed together at the side, top, and bottom
locations. The
molybdenum is machined to provide a tapered overlapping joint for brazing. A
chromium layer is electroplated at the joint locations. The parts are brazed
with a Cobalt-
Palladium alloy to provide sealed junctions. Once the brazes are complete the
Rhenium
is applied locally over the braze junction with a thickness of approximately
0.004 inches
on the inner tank surface. In order to enhance the e-beam welding
characteristics of the
molybdenum an addition of 10% to 40% Rhenium can be added. As an alternative
the
inner tank (4) could be fabricated from a ceramic material such as calcium
aluminate or
calcium oxide. The material could have a molybdenum lanthanum oxide wire mesh
distributed within the ceramic wall to increase the inner tank strength and
prevent large
cracks from forming. The insulation between tank (4) and (3b) can be a powder
fill of
calcium oxide or it can be a rigid insulation such as silicon dioxide or
aluminum oxide.
The hydride tanks (2) are used to hold the hydrogen at ambient temperature.
The tanks
contain a solid porous material which allows rapid hydrogen transport in and
out of the
porous material. A chemical bond is created within the material which creates
a metal
hydride. Titanium iron hydride is chosen for this application as ambient
temperature low
cost material. Over 1% hydrogen by weight is absorbed when the hydride is
created.
Hydrogen is absorbed and released when maintained between OC and 50 C. The
hydrogen pressure varies with temperature. As the temperature is raised the
pressure
rises to several atmospheres. A flowing water heat exchanger tank (8)
surrounds the
hydride tanks (2) and maintains the hydride powder temperature during the
hydrogen
absorption and desorption processes. The hydrogen movement results in either
an
exothermic or endothermic reaction. When hydrogen is added to the titanium
iron
powder thermal energy is released into the water bath (8). When hydrogen is
removed
from the titanium iron hydride thermal energy is added from the water bath
into the
hydride powder to maintain the hydrogen pressure.
Thp thermo-cline double tank (17) is used to store the hydrogen thermal energy
when it is
being cooled from approximately 2000F to 100F. The thermo-cline tank uses the
specific
heat of liquid boron oxide to store the hydrogen energy. The high temperature
dual tanks
(17) operate between approximately 2000F at the top of the tank and 100 F at
the bottom
using boron oxide as a heat transfer fluid. The thermo-cline tank (17) is used
to cool the
hydrogen during charging and heat the hydrogen during discharge.
A Stirling engine (20) is located next to the calcium/ calcium hydride storage
tank (1). A
metal vapor boiler and heat pipe/ metal boiler (21) connects from the Stirling
engine (20)
4
CA 02814454 2013-04-11
WO 2011/105989 PCT/US2010/002729
into the inner tank (4). Magnesium vapor is used as a heat transfer fluid
within the boiler
and heat pipe (21).
The hydrstgan Foes are attached as follows:
I) Hydrogen inlet and outlet (Ira) connects; from the reactor (1) to the top
of the
thermo-cline heat exchanger (17). A floating seal at the thermo-ciine junction
provides for thermal expansion.
2) The bottom of the thermo-cline heat exchanger (17) connects to the hydride
tanks
(2). The common hydride tank feed line is (I IF).
Water flowing through an underground heat exchanger is used It) maintain the
hydride
tank (2) temperature by circulating through a reservoir surround inv, the
hydride tanks (2)
Figure 2 shows an embodiment of the system where a heat pipe (10) is
integrated at the
top of the hydride storage cell (1). In this configuration the ,vstem behavior
for the
hydrogen flow and energy flow is identical to figure 1 when ,he solar heat is
applied
directly to the bottom of the reaction chamber (9). The differen t. is in the
location of the
solar heat target. For figure 1 the solar energy passes throug;= the quartz
window (15)
and projects directly onto the bottom of the reaction chamber' This
This system requires
that the solar input beam be substantially vertical passing thri-igh the
quartz window
(15). To accomplish the solar heat input in figure 1 the side focusing helio-
stats first
create an approximately constant diameter sunlight beam of 12 inches in
diameter for the
100 kW system. Multiple beams, from the side focusing 'who ctats, are
reflected from
individual down beam mirrors located directly above the quart? ;ndow (15). The
design
in figure 2 eliminates the need for the down beam mirrors ane Mows the side
focusing
hello-stat beam to project directly on the side of the heat pipe. (10) which
is projecting
from the top of the reactor (1). A quartz bell (7) is added over the heat pipe
(10) to
minimize thermal losses and protect it from atmospheric corrosion. At night an
insulation cover is folded over the quartz bell (7) 1;, minimize thermal
losses.
Operation
The system operates in a charging mode and a discharging mode. System
operation can
be maintained by controlling temperatures in both the calcium reactor and
titanium iron
hydride storage tanks. The total system can operate without valves so that the
pressure is
constant throughout the system. Hydrogen pressure in the hydride storage (2),
thermo-
cline (17), and reactor (1) are constant as long as the flow rates are slow or
stopped.
Piping is sized to minimize pressure drop through the system while the
hydrogen is
flowing during normal operation. The equilibrium hydrogen pressure is
approximately 1
atmosphere at 1800 F and 5 atmospheres at 2000 F in the calcium reactor (1)
between the
calcium, hydrogen, and calcium hydride. Below equilibrium temperature the
reaction is
exothermic creating calcium hydride. Above equilibrium temperature the
reaction is
endothermic creating calcium and hydrogen. As an example of system operation:
if the
pressure is approximately constant at 3 atmospheres then the equilibrium
temperature is
approximately 1900 F. If the reactor (1) is heated by the sun then the
temperature rises
slightly and hydrogen is formed. The rate of hydrogen produced from the
reactor (1) is a
CA 02814454 2013-04-11
WO 2011/105989
PCT/US2010/002729
function of the temperature above equilibrium. The hydrogen generation rate
will
increase until a new equilibrium is reached in solar energy absorbed and the
energy
required for the endothermic reaction. When the solar energy is reduced, at
night or
wito at ' vi PIOUS overhead, then the temperature drops within the calcium
reactor (1)
untit a. new equilibrium is reached in hydrogen flow. As the solar energy goes
to zero
then the temperature drops to a point where hydrogen wants to flow back into
the calcium
reactor (1). The hydrogen reacts with the calcium and creates an exothermic
reaction
which produces a new equilibrium temperature. The system is designed so that
all
processes operate in the 1 to 5 atmospheres and 1800 F to 2000 F reactor (1)
temperature
ranges. One of the features of this system is the automatic stability based on
heat input to
the calcium reactor which allows the system to operate without hydrogen flow
rate
controls.
The' second part of the system is the hydrogen storage using an ambient
temperature
hydride. Titanium iron hydride provides an ideal solution for the storage of
hydrogen
due to its ability to combine and release hydrogen near ambient temperature
conditions.
The process is exothermic when hydrogen is 'combining to form the titanium
iron hydride
and endothermic when hydrogen is being released. Operating near ambient
conditions
allows this heat, to and from the titanium iron hydride reaction, to come from
the
surroundings so that extra energy is not required by the system. The movement
of
hydrogen in and out of the titanium iron hydride is a function of the titanium
iron hydride
temperature which also operates in an equilibrium condition. The equilibrium
pressure is
approximately 1.5 atmospheres at 32 F and 5.5 atmospheres at 86 F. To keep the
hydrogen pressure below 5 atmospheres the titanium iron hydride needs to be
cooled
during the day while hydrogen is flowing into the titanium iron hydride powder
as this
process is exothermic and releases heat. To maintain an approximately constant
temperature a flowing water bath (8) is used which surrounds multiple tanks
(2). The
tanks (2) are positioned sideways, with the hydride powder filling
approximately 70% of
the volume, to allow for expansion of the titanium iron hydride during the
absorption
process. Using the earth as a heat sink allows an almost constant temperature
for both
day and night for the water bath (8). A temperature of approximately 60 F,
based on an
average ground temperature, allows the hydrogen equilibrium to stay near 3
atmospheres
during the system operation.
Charging mode: During the daylight hours solar energy is available for thermal
storage.
The reaction of the calcium hydride breaking apart to form calcium and
hydrogen is
endothermic and absorbs the focused heat from the sunlight. A group of side
focusing
helio-stats direct the suns energy through the quartz window (15) and onto the
reaction
chamber (9). The equilibrium pressure for calcium hydride varies with
temr:.rature. The
calcium and calcium hydride located in tank (4) is maintained at approximately
1900 F
by controlling the hydrogen pressure in storage cell (1) using the temperature
of the
titanium iron hydride tanks as an automatic control system. Maintaining the
same
pressure in tanks (3A), (3B) and (4) eliminates the stress on the wall of
tanks (4) and (3B)
due to the pressure. The tank (3A) is at low temperature and provides the
structure for
the pressure. When solar energy is available to heat the calcium reactor the
temperature
rises above equilibrium, for a given pressure, which causes hydrogen to be
released. The
6
CA 02814454 2013-04-11
WO 2011/105989 PCT/US2010/002729
calcium temperature is maintained using the energy balance between the
incoming solar
energy, the calcium, and the hydrogen reaction rate and direction. The
temperature
increment above equilibrium increases the hydrogen flow rate until an energy
balance
occurs between the incoming solar energy and the endothermic reaction rate of
the
calcium and hydrogen evolution. The process stabilizes automatically so that
no valves
or control system is required for the hydrogen flow. The hydrogen exits
through the
hydrogen outlet (11a).
The hydrogen exiting the hydrogen line (11a) is at approximately 1900 F and
contains a
substantial amount of thermal energy. The hydrogen flows through the thermo-
cline heat
exchanger (18a) and is cooled by the boron oxide in the thermal storage (17).
The
hydrogen temperature drops from 2000 F to 100 F while the boron oxide is
heated from
100 F to 2000 F. Graphite fiber oriented perpendicular to the hydrogen flow
increases
the heat transfer within the boron oxide in the radial direction. Graphite has
a much
lower thermal conductivity perpendicular to the fiber direction. This allows
the boron
oxide to operate with the 1900 F temperature gradient within the tank with
minimal
thermal losses.
The temperature of the calcium/ calcium hydride, is maintained at
approximately 1900 F
in tank (4) by maintaining the hydrogen pressure above the calcium to
approximately 3
atmospheres. The system flow chart is shown in figure 4.
Discharge mode: The Stirling engine (20) extracts heat directly from tank (4)
which
contains the calcium and calcium hydride. A heat pipe (21) connects to the hot
side of
the Stirling engine and passes through the outer tank lid (5) and into tank
(4). Sufficient
heat pipe area is extended within tank (4), or on the external surface of tank
(4), to
prevent the calcium hydride from solidifying around the heat pipe while the
engine is
extracting thermal energy.
The rate of hydrogen flowing out of the hydride tanks (2) is controlled by the
temperature
of the reaction chamber (1). As the calcium and calcium hydride temperature
drops the
equilibrium pressure drops until the pressure in the reactor (1) falls below
the hydride
tank (2) pressure.
Hydrogen flows from the hydride tank (2) into the heat exchanger (18A).
Passing
through the heat exchanger (18A) heats the hydrogen to approximately 1900 F
where it
then flows into the hydrogen inlet line (11a). The temperature in the inner
tank (4) is
maintained at approximately 1900 F by the hydrogen flow rate out of the
hydride tank
(2).
The system flowchart is shown in figure 3.
7
CA 02814454 2013-04-11
WO 2011/105989 PCT/US2010/002729
Variations to the baseline system:
Different metals could be used in place of the calcium. These could include:
magnesium,
strolling; thatinua ilithinnl, sodium, potassium, titanium, or zirconium_ Also
boro-
hydrides couhl be used such as lithium. sodium, Off 11,(1)m
The system could operate without either the high temperature storage tank (17)
or the low
temperature storage tank (22). In this case a secondary cooling loop using an
external
heat exchanger with the surroundings could be used such as with air, a solid
heat sink, or
water cooling.
Multiple heat engines or a single engine could be used to extract the heat
from tank (4).
The heat engines could include any variation of brayton, Stirling, or rankine
cycles. A
secondary steam cycle could also be used to supply peaking power utilizing a
steam
turbine.
An inert gas within tank (3A) could be nitrogen or argon instead of hydrogen
to reduce
the heat loss. The gas in (3A) would not have the filter (24) through the (3b)
wall.
The hydride storage material could be a number of hydrides including:
Magnesium
nickel hydride, lithium aluminum hydride, magnesium iron hydride, lanthanum
nickel
aluminum hydride, calcium nickel hydride, titanium iron hydride, or magnesium
hydride.
The hydrogen could also be stored as a compressed gas or liquid.
The system can be operated over a wider temperature range such as 1200 F to
2500 F.
The system can operate with the temperature in tank (4) below 1800 F so that
the
Calcium hydride is a solid. .
A Koval- seal could be used to support reaction chamber (9) at the top of lid
(5). Both the
bolt flange (14a) and the quartz window (15) hold down ring could be water
cooled.
The nitrate salt mixture could use lithium nitrate instead of, or with, the
calcium nitrate as
a eutectic with the potassium and sodium nitrate salts.
For operation during long periods of cloudy weather a 2' heat source is
required. One
solution is to use a burner and air pre-heater assembly where the heat is
directed into an
exhaust heat exchanger to absorb the combustion energy. A closed hydrogen loop
between the calcium tank (1) and the exhaust heat exchanger would provide a
technique
to maintain the calcium temperature. The hydrogen would be pumped in and out
of the
region above the liquid calcium in tank (1) with the additional heat being
extracted from
the exhaust heat exchanger. Any type of fuel could be used for this purpose.
A variation on the quartz window (15) cover would be to use a 2 way shape
memory
alloy attached to an insulated multi-segment door. The segments would be
hinged against
the top wall of the reaction chamber (9). During daylight hours heating from
the sun
8
CA 02814454 2013-04-11
WO 2011/105989 PCT/US2010/002729
would cause the memory metal to flex downward opening the segments by rotating
the
insulation up against the reaction chamber (9) wall so that sun could enter.
At night the
cooling from lack of sunlight would cool the memory metal and it would flex to
rotate the
insulation segments so as to close the lid which reduces thermal loss at
night.
Conclusions
The thermal storage system provides a unique and significant advantage in that
it
provides a continuous low cost high energy density control system. Integ ation
of these
features allows economically viable storage systems over a much broader size
range than
.existing storage systems. The claims provide details of how the unique
features are
,integrated into a complete system.
9