Note: Descriptions are shown in the official language in which they were submitted.
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
ANTI-HER3 ANTIBODIES AND COMPOSITIONS
Field of the Invention
The present invention relates to novel recombinant antibodies targeting the
HER3 receptor and
compositions comprising two or more of these antibodies for use in human
cancer therapy.
Background of the Invention
The EGFR receptor family
The epidermal growth factor receptor (EGFR) family (also known as the ErbB
family) is a
subgroup of the receptor tyrosine kinases (RTKs) and consists of four members:
HER1/EGFR/ErbB, HER2/ErbB2, HER3/ErbB3 and HER4/ErbB4. The members of the EGFR
to family are closely related single-chain modular glycoproteins with an
extracellular ligand
binding region, a single transmembrane domain and an intracellular tyrosine
kinase (reviewed
in Ferguson (2008) Annu Rev Biophys. 37: 353-373). In normal physiological
settings the
ErbB family regulates key events in coordination of cell growth,
differentiation and migration
(Citri et al. (2006) Nat Rev Mol Cell Biol. 7: 505-516). EGFR, HER2 and HER3
are believed to
play crucial roles in the malignant transformation of normal cells and in the
continued growth
of cancer cells (pro-survival pathway). EGFR and HER2 have been found to be
overexpressed
by many epithelial cancers (Slamon et al. (1987) Science, 235: 177-182;
Arteaga (2002)
Oncologist 7 Suppl 4: 31-39; Bodey et al. (1997) Anticancer Res. 17: 1319-
1330; Rajkumar et
al. (1996) J Pathol. 179: 381-385). Overexpression of EGFR and HER2 has
furthermore been
linked to disease progression, reduced survival, poor response and
chemotherapy resistance in
several human epithelial cancers (Slamon et al. (1987) supra; Baselga et al.
(2002) Oncologist
7 Suppl 4: 2-8).
HER3 structure
The third member of the ErbB family, known as human epidermal growth factor
receptor 3
(HER3, ErbB3) was identified in 1989 by Kraus et al. (Proc Natl Acad Sci USA
1989; 86: 9193-
9197). The HER3 gene encodes a protein of 1342 amino acids with striking
structural
similarities to EGFR and HER2. Features such as overall size, four
extracellular subdomains (I-
IV) with two cysteine clusters (domains II and IV), and a tyrosine kinase
domain show
structural similarities to EGFR and HER2 (Cho and Leahy (2002) Science, 297:
1330-1333).
The tyrosine kinase domain of HER3 shows 59% sequence homology to the tyrosine
kinase
domain of EGFR (Brennan et al. (2000) Oncogene, 19: 6093-6101).
Regulation of HER3 activation
Neu differentiation factor (NDF), heregulin (HRG) and neuregulin 1 (NRG1) are
synonyms for
the glycoprotein which is a ligand for HER3 (Peles et al. (1992) Cell, 69: 205-
216; Wen et al.
1
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
(1992) Cell, 69: 559-572). At least 15 isoforms of the NRG1 protein have been
identified. The
isoforms are produced from the single NRG1 gene through alternative splicing
and multiple
promoters (Falls et al. (2003) Exp Cell Res, 284: 14-30). Three structural
characteristics apply
for the functional differences of the isoforms. These structural
characteristics are the type of
EGF-like domain (a or 13), the N-terminal sequence (type I, II or III) and
whether the isoform
is initially synthesized as a transmembrane or non-membrane protein (Falls et
al. (2003)
supra). The type I sub-group of NRG1 isoforms have a unique N-terminal
sequence followed
by an immunoglobulin-like domain and then an EGF-like domain. Type II variants
contain an
N-terminal kringle-like sequence, the immunoglobulin domain and the EGF-like
domain. The
to type III variants contain an N-terminal hydrophobic domain within a
cysteine-rich region, omit
the immunoglobulin domain and then continue into the EGF-like domain and
various
downstream alternative exons. Downstream from the EGF-like domain the NRG1
isoform may
contain a linker sequence, a transmembrane domain and a cytoplasmic tail
(Falls et al. (2003)
supra). Some of the NRG1 isoforms are subject to glycosylation in the spacer
region between
the immunoglobulin-like domain and the EGF-like domain (Hayes et al. (2008) J
Mammary
Gland Biol Neoplasia, 13: 205-214).
As is the case for EGFR, HER3 exists in a tethered conformation and in an
extended
conformation. In the tethered conformation the dimerization arm is buried by
interactions with
domain IV, leaving domains I and III too far apart for efficient ligand
binding (Cho and Leahy
et al. (2002) supra). Ligand binding to the extracellular domains I and III
occurs in the
extended conformation of HER3 and leads to heterodimerization with other
members of the
ErbB family (or other RTK members, e.g. MET), the extended and ligand-bound
HER3 molecule
preferentially heterodimerizing with HER2 (Pinkas-Kramarski et al. (1996) EMBO
J, 15: 2452-
2467). No HER3 homodimers are formed upon ligand binding (Ferguson et al.
(2000) EMBO J,
19: 4632-4643).
In contrast to EGFR and HER2, the tyrosine kinase of HER3 has impaired
catalytic activity,
insufficient for any detectable biological response (Pinkas-Kramarski et al.
(1996) supra; Guy
et al. (1994) Proc Natl Acad Sci USA, 91: 8132-8136). Two amino acid residues
which are
highly conserved in the catalytic domains of protein kinases (Hanks et al.
(1988) Science, 241:
42-52) are altered in the catalytic domain of HER3. These are the substitution
of aspargine for
aspartic acid at residue 815 and substitution of histamine for glutamate at
residue 740. The
two amino acid substitutions may be the reason why HER3 lacks catalytic
activity of its
tyrosine kinase domain (Plowman et al. (1990) Proc Natl Acad Sci USA, 87: 4905-
4909).
Because of the impaired intrinsic kinase activity of HER3, the receptor needs
to heterodimerize
with another ErbB family member in order to respond to its own ligand binding
(Berger et al.
(2004) FEBS Lett, 569: 332-336).
2
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
Termination of HER3 signaling
Little is known about endocytosis of HER3. Moreover, different studies have
suggested that
HER3 is endocytosis impaired to the same extent as HER2 (Baulida et al. (1996)
J Biol Chem,
271: 5251-5257). In agreement with this the HER3-NRG1 complex was found to be
internalized less efficiently and slower than the EGFR-EGF complex, supporting
that HER3 is
not endocytosed as efficiently as EGFR (Baulida et al. (1997) Exp Cell Res,
232: 167-172;
Waterman et al. (1999) EMBO J, 18: 3348-3358). However, when the C-terminal
tail of EGFR
was replaced with the C-terminal tail of HER3, EGFR became endocytosis
impaired, suggesting
that a region in the C-terminus of HER3 protects the receptor against
internalization
to (Waterman et al. (1999) supra). It has also been suggested that NRG1
does not efficiently
target HER3 to degradation due to the dissociation of the ligand-receptor
complexes in
endosomes, as it is observed when EGF is activated by TGFa (Waterman et al.
(1999) supra).
Expression and physiological role of HER3
HER3 has like EGFR and HER2 been shown to be of importance in the mammary
gland
development (Schroeder et al. (1998) Cell Growth Differ, 9: 451-464). While
EGFR and HER2
are highly expressed and co-localized in the pubscent mouse mammary gland,
HER3 is only
expressed at low levels in postpubscent mammary glands from virgin mice, but
is expressed at
higher levels during pregnancy and lactation (Schroeder et al. (1998) supra).
The higher
expression levels of HER3 during pregnancy and lactation implies the
importance of HER3 in
the later stages of mammary gland development and differentiation (Jackson-
Fisher et al.
(2008) Breast Cancer Res, 10: R96). Studies with HER3-deficient mice further
indicated the
regulatory role of HER3 in morphogenesis of mammary epithelium through the
PI3K/AKT
signaling pathway (Jackson-Fisher et al. (2008) supra). Other studies showed
high levels of
HER3 expression by ductal epithelial cells in rats by day 14-16 of pregnancy,
also
demonstrating the regulatory role of HER3 in morphogenesis of mammary
epithelium (Darcy
et al. (2000) J Histochem Cytochem, 48: 63-80).
Targeted knockout of the HER3 gene in mice resulted in embryonic lethality at
day 13.5 due to
underdeveloped cardiac valves which were unable to support proper cardiac
function due to
blood reflux (Erickson et al. (1997) Development, 124: 4999-5011). Other
defects include
abnormalities in brain development, especially in the midbrain region
including the cerebellum,
and severe defects in Schwann cells of peripheral axons of sensory and motor
neurons
(Erickson et al. (1997) supra; Riethmacher et al. (1997) Nature, 389: 725-
730).
In vitro studies have also implicated HER3, in combination with HER2, in the
development of
keratinocytes (Marikovsky et al. (1995) Oncogene, 10: 1403-1411), Schwann cell
precursors
(Syroid et al. (1996) Proc Natl Acad Sci USA, 93: 9229-9234), oligodendrocytes
(Vartanian et
al. (1997) J Cell Biol, 137: 211-220) and the neuromuscular synapse (Zhu et
al. (1995) EMBO
J, 14: 5842-5848).
3
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
The tissue distribution of HER3 is not much different from EGFR
(www.proteinatlas.org).
Despite the impaired kinase activity of HER3, the receptor plays an essential
role in the ErbB
network through the PI3K/AKT signaling (Citri et al. (2003) Exp Cell Res, 284:
54-65). Due to
the requirement of heterodimerization for initiation of signaling, the
physiological role of HER3
may overall resemble those identified for EGFR and HER2. The precise role of
HER3 in the
human adults is unknown, however, due to the embryonic lethality of HER3
knockout in mice
and the sparse data on HER3 inhibition.
to HER3 and cancer
HER3 is unique in its ability to channel ErbB signaling to the PI3K/AKT
signaling pathway,
which favors tumor growth and progression (Prigent et al. (1994) EMBO J, 13:
2831-2841).
The critical role of HER3 in regulation of tumor growth is also supported by
the observation
that HER2 overexpression in human breast cancer often is associated with
higher levels of
HER3 expression (Naidu et al. (1998) Br ] Cancer, 78: 1385-1390). Moreover,
overexpression
of HRG results in increased transformation and tumorigenicity (Atlas et al.
(2003) Mol Cancer
Res, 1: 165-175), while blockade of NRG inhibits tumorigenicity and metastasis
(Tsai et al.
(2003) Oncogene, 22: 761-768), indicating the importance of the presence of a
HER3 ligand
for cancer development.
The presence of HER2 homodimers on the cell surface and thereby exaggeration
of HER2
signaling causes transformation of epithelial cells (reviewed in Yarden and
Sliwkowski (2001)
Nat Rev Mol Cell Biol, 2: 127-137). However the HER2-HER3 dimer has the
ability to induce
signal transduction through both the mitogen-activated protein kinase (MAPK)
and the AKT
pathway. Activation of both the MAPK pathway and the AKT pathway implies the
additional
oncogenic potential of the HER2-HER3 heterodimer compared to the HER2
homodimer
(reviewed in Citri et al. (2003) supra).
High expression of HER3 is found in many of the same tumor types that
overexpress HER2,
including bladder and colorectal cancer in addition to breast cancer (Bodey et
al. (1997)
Anticancer Res, 17: 1319-1330; Rajkumar et al. (1996) J Pathol, 179: 381-385;
Lemoine et
al. (1992) Br J Cancer, 66: 1116-1121; Maurer et al. (1998) Hum Pathol, 29:
771-777). While
more studies are needed to establish the association between HER3
overexpression and
clinical outcome, the clinical indications support the results from in vitro
studies that neither
HER2 nor HER3 can be considered as stand-alone receptors in relation to
cancer.
4
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
Anti-HER3 antibodies
A number of anti-HER3 antibodies have been described in the literature. See,
for example, WO
2011/060206, WO 2011/044311, WO 2011/022727, WO 2010/127181, WO 2008/100624,
WO
2007/077028, WO 03/013602 and WO 97/35885.
AMG 888 (Amgen/Daiichi Sankyo) is a fully human monoclonal antibody that is
said to inhibit
human HER3 oncogenic signaling. AMG 888 is currently being investigated in
clinical trials for
treatment of cancer.
to MM-121 (Merrimack Pharmaceuticals) is an anti-HER3 antibody that is said
to block heregulin
binding to and hence activation of HER3; see WO 2010/019952 and Schoeberl et
al., Cancer
Res.70(6):2485-94, March 2010. MM-121 is also currently being investigated in
clinical trials
for treatment of cancer.
Pertuzumab is an anti-HER2 antibody that functions as a HER dimerization
inhibitor which
inhibits dimerization of HER2 to HER3 and the other EGFR receptors. Franklin
et al. (Cancer
Cell 2004, 5(4):317-28) disclose that pertuzumab binds HER2 near the center of
domain II,
sterically blocking a binding pocket necessary for HER2-HER3
heterodimerization and
signaling. The amino acid sequence of pertuzumab is disclosed in WO
2006/033700 and US
2006/0121044 A1.
In spite of the fact that certain anti-HER3 antibodies are known and in some
cases being
investigated in clinical trials, no anti-HER3 antibodies are currently
approved for therapeutic
use. In view of the critical role of HER3 in regulation of tumor growth as
outlined above, there
is therefore a need for new antibodies that target the HER3 receptor as well
as mixtures of
such anti-HER3 antibodies.
Summary of the invention
The present invention is directed to novel recombinant antibodies targeting
the HER3 receptor
as well as compositions comprising two or more of these antibodies and use of
the antibodies
and compositions for human cancer therapy, e.g. for the treatment of breast
cancer, ovarian
cancer, gastric cancer and other cancers that express or overexpress HER3, or
that have a
signature of HER3 pathway activation (e.g. NSCLC, glioblastoma). Compared to
the currently
available treatments for such cancers, including available monoclonal
antibodies directed
against other receptors of the EGFR family, it is contemplated that the
antibodies of the
invention may provide a superior clinical response either alone or,
preferably, in a composition
comprising two or more such antibodies, and optionally in combination with
other treatments
such as chemotherapy.
5
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
In one aspect, the invention relates to novel recombinant anti-HER3 antibodies
based on the
antibodies referred to herein as antibodies 4785, 4889, 4935, 5038, 5082,
5101, 5106, 5143,
5144 and 5259, as well as humanized and/or affinity matured variants thereof.
In one
embodiment, this aspect of the invention relates to a recombinant anti-HER3
antibody
molecule comprising the heavy chain CDR3 sequence of any one of the antibodies
referred to
herein as antibodies antibodies 4785, 4889, 4935, 5038, 5082, 5101, 5106,
5143, 5144 and
5259.
Further embodiments of this aspect of the invention include: a recombinant
anti-HER3
to antibody molecule comprising the heavy chain CDR3 sequence and the light
chain CDR3
sequence of any one of antibodies 4785, 4889, 4935, 5038, 5082, 5101, 5106,
5143, 5144
and 5259, and which competes for binding with said antibody; a recombinant
anti-HER3
antibody molecule comprising the heavy chain CDR1, CDR2 and CDR3 sequences and
light
chain CDR1, CDR2 and CDR3 sequences of any one of these antibodies; and a
recombinant
anti-HER3 antibody comprising the heavy chain variable region sequence and the
light chain
variable region sequence of any one of these antibodies, or comprising a heavy
chain variable
region sequence and a light chain variable region sequence each having at
least 90%
sequence identity, preferably at least 95% sequence identity, such as at least
96%, at least
97%, at least 98% or at least 99% sequence identity, with the heavy chain
variable region
and light chain variable region sequences, respectively, of any one of these
antibodies, and
which competes for binding with said antibody.
Another aspect of the invention relates to a recombinant antibody composition,
comprising at
least first and second recombinant anti-HER3 antibodies, wherein the first and
second
antibodies bind distinct epitopes of HER3, and wherein one or both of the
first and second
antibodies are selected from the group of antibodies outlined above.
A further aspect of the invention relates to an immunoconjugate comprising a
recombinant
anti-HER3 antibody of the invention conjugated to an anti-cancer agent. A
related aspect
relates to compositions comprising at least first and second recombinant anti-
HER3 antibodies
of the invention, wherein at least one anti-HER3 antibody in said composition
is an
immunoconjugate.
A further aspect of the invention relates to a nucleic acid molecule having a
nucleotide
sequence that encodes an anti-HER3 antibody of the invention, as well as
expression vectors
comprising such a polynucleotide and host cells that have been transfected
with such an
expression vector.
A still further aspect of the invention relates to methods for producing
antibodies and
polyclonal antibody compositions of the invention.
6
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
A still further aspect of the invention relates to methods for treating a
disease in a human or
animal subject, in particular treatment of cancer in humans, by administering
an anti-HER3
antibody or composition of the invention to said subject. A related aspect is
the use of one or
more anti-HER3 antibodies of the invention for preparation of a medicament for
use in treating
a disease in a human or animal, in particular for the treatment of cancer in
humans.
A still further aspect of the invention relates to a method for inducing
internalization of HER3
on the surface of cells that express or overexpress HER3, the method
comprising contacting
to the cells with a recombinant anti-HER3 antibody or immunoconjugate or a
recombinant anti-
HER3 antibody composition of the invention.
Additional aspects of the invention and particular embodiments will be
apparent from the
description and examples below.
Drawing description
Figures 1-10 show the metabolic activity of MDA-MB-175 cells treated with
different
concentrations of the indicated anti-HER3 antibodies for 96 hours.
Figure 11 shows the results of western blot analyses of phospho-HER3 levels in
the cell lines
MDA-MB-175 and MCF7 after 1 hour of pre-treatment with the indicated
antibodies, followed
by stimulation with 10 nM heregulin beta.
Figures 12-15 show the metabolic activity of selected mixtures of two anti-
HER3 antibodies in
four cancer cell lines.
Figures 16-19 show the metabolic activity of selected mixtures of three anti-
HER3 antibodies
in four cancer cell lines.
Figures 20 and 21 show the growth inhibition activity of two different
mixtures of two anti-
HER3 antibodies compared to the individual antibodies in the two mixtures in
the cancer cell
line MDA-MB-175.
Figures 22 and 23 show the growth inhibition activity of a mixture of two anti-
HER3 antibodies
of the invention compared to the reference antibodies MM-121 (anti-HER3) and
pertuzumab
(anti-HER2) in the two cancer cell lines MDA-MB-175 and MCF7.
Figure 24 is a western blot showing HER3 levels at various times in whole cell
lysates of
OVCAR-8 cells treated with the individual anti-HER3 antibodies 5082 or 5038 or
a mixture of
the two antibodies.
7
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
Figure 25 is a western blot performed on whole cell lysates of MDA-MB-175
cells, showing
inhibition of phosphorylation of HER3 and AKT at various times by the
individual anti-HER3
antibodies 5082 or 5038 or a mixture of the two antibodies.
Figure 26 shows the in vivo efficacy of the individual anti-HER3 antibodies
5038 and 5082 and
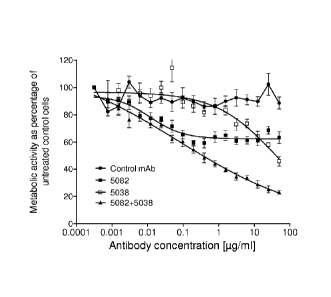
the mixture of 5038+5082 in the A549 lung cancer xenograft model.
Figures 27-32 show the results of a domain mapping of anti-HER3 antibodies by
titration of
to the antibodies and negative controls against coated HER3 antigens.
Figure 33 shows a table with the results of epitope binning of anti-HER3
antibodies by
antibody cross-competition analysis.
Figure 34 is a graphic illustration of the relationship between assigned
epitope bins for anti-
HER3 antibodies, where overlapping circles represent antibodies with
overlapping epitopes.
Figure 35 shows the in vivo efficacy of the anti-HER3 monoclonal antibodies
5038 and 5082
and the mixture of 5038+5082 in the BxPC3 pancreatic cancer xenograft model,
expressed as
tumor volume.
Figure 36 shows the in vivo efficacy of the anti-HER3 monoclonal antibodies
5038 and 5082
and the mixture of 5038+5082 in the BxPC3 pancreatic cancer xenograft model,
expressed as
percent survival.
Detailed description of the invention
Definitions
The term "antibody" or "antibody molecule" describes a functional component of
serum and is
often referred to either as a collection of molecules (antibodies or
immunoglobulin) or as one
molecule (the antibody molecule or immunoglobulin molecule). An antibody is
capable of
binding to or reacting with a specific antigenic determinant (the antigen or
the antigenic
epitope), which in turn may lead to induction of immunological effector
mechanisms. An
individual antibody is usually regarded as monospecific, and a composition of
antibodies may
be monoclonal (i.e., consisting of identical antibody molecules) or polyclonal
(i.e., consisting of
two or more different antibodies reacting with the same or different epitopes
on the same an-
tigen or even on distinct, different antigens). Each antibody has a unique
structure that
enables it to bind specifically to its corresponding antigen, and all natural
antibodies have the
same overall basic structure of two identical light chains and two identical
heavy chains.
Antibodies are also known collectively as immunoglobulins.
8
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
The terms "antibody" or "antibodies" as used herein are also intended to
include chimeric and
single chain antibodies, as well as binding fragments of antibodies, such as
Fab, Fv fragments
or single chain Fv (scFv) fragments, as well as multimeric forms such as
dimeric IgA molecules
or pentavalent IgM. An antibody may be of human or non-human origin, for
example a murine
or other rodent-derived antibody, or a chimeric, humanized or reshaped
antibody based e.g.
on a murine antibody.
Each heavy chain of an antibody typically includes a heavy chain variable
region (VH) and a
to heavy chain constant region. The heavy chain constant region typically
includes three
domains, referred to as CH1, CH2 and CH3. Each antibody light chain typically
includes a light
chain variable region (VL) and a light chain constant region. The light chain
constant region
typically includes a single domain, referred to as CL. The VH and VL regions
may be further
subdivided into regions of hypervariability ("hypervariable regions", which
may be
hypervariable in sequence and/or in structurally defined loops). These are
also referred to as
complementarity determining regions (CDRs), which are interspersed with
regions that are
more conserved, termed framework regions (FRs). Each VH and VL typically
includes three
CDRs and four FRS, arranged from the amino terminus to the carboxy terminus in
the
following order: FR1, CDR1, FR2, CDR2, FR3, CDR3, FR4. The amino acid residues
in the
variable regions are often numbered using a standardized numbering method
known as the
Kabat numbering scheme (Kabat et al. (1991) Sequences of Proteins of
Immunological
Interest, 5th Ed. Public Health Service, National Institutes of Health,
Bethesda, MD, USA).
In the appended sequence listing, the light chain (LC) DNA and amino acid
sequences include
both the light chain variable region (VL) sequence and the human kappa
constant region
sequence. As mentioned below in Example 1, the human kappa constant region
starts with the
amino acids -TVAAP- (Thr Val Ala Ala Pro) and ends at the C-terminal with the
amino acids -
NRGEC (Asn Arg Gly Glu Cys). Therefore, as used herein, the terms "light chain
variable region
sequence" or "VL" are understood to refer to the N-terminal part of a light
chain sequence in
the sequence listing before the start of the human kappa constant region (i.e.
before the
amino acids TVAAP).
The antibody numbers used herein in the context of whole antibodies, e.g.
"antibody 5082",
refer to the specific antibodies described in the examples and defined in the
appended
sequence listing. For example, antibody 5082 is an antibody with a heavy chain
comprising the
heavy chain variable region sequence set forth in SEQ ID NO:18 and the IGHG1
heavy chain
constant region sequence set forth in SEQ ID NO:44, and a light chain with the
amino acid
sequence set forth in SEQ ID NO:20, where the light chain sequence as
explained above
includes both the light chain variable region sequence (residues 1-108 in SEQ
ID NO:20) and
the human kappa constant region sequence (residues 109-214 in SEQ ID NO:20).
9
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
The invention is also intended to encompass antibodies that are "derived from"
or "based on"
a specified antibody described herein, where such an antibody comprises,
depending on the
particular context, one of the following: the heavy chain CDR3 sequence of
said specified
antibody; the heavy chain CDR3 sequence and the light chain CDR3 sequence of
said specified
antibody; the heavy chain CDR1, CDR2 and CDR3 sequences and light chain CDR1,
CDR2 and
CDR3 sequences of said specified antibody; or the heavy chain variable region
sequence and
the light chain variable region sequence of said specified antibody, or a
humanized and/or
affinity matured variant of said heavy chain variable region sequence and/or
light chain
to variable region sequence, or a heavy chain and/or light chain variable
region sequence having
at least 90% sequence identity, preferably at least 95% sequence identity,
such as at least
96%, at least 97%, at least 98% or at least 99% sequence identity, with the
respective heavy
chain variable region and light chain variable region sequences. An antibody
that is derived
from or based on a specified antibody described herein will generally bind the
same HER3
epitope as said specified antibody and will preferably exhibit substantially
the same activity as
said specified antibody. An antibody is considered to bind the same HER3
epitope as the
specified antibody if it competes for binding with said specified antibody.
The specificity of an antibody's interaction with a target antigen resides
primarily in the amino
acid residues located in the six CDRs of the heavy and light chain. The amino
acid sequences
within CDRs are therefore much more variable between individual antibodies
than sequences
outside of CDRs. Because CDR sequences are responsible for most antibody-
antigen
interactions, it is possible to express recombinant antibodies that mimic the
properties of a
specific naturally occurring antibody, or more generally any specific antibody
with a given
amino acid sequence, by constructing expression vectors that express CDR
sequences from
the specific antibody grafted into framework sequences from a different
antibody. As a result,
it is possible to "humanize" a non-human antibody and still substantially
maintain the binding
specificity and affinity of the original antibody. A more detailed discussion
of humanization is
provided below.
A "chimeric antibody" refers in its broadest sense to an antibody that
contains one or more
regions from one antibody and one or more regions from one or more other
antibodies. As
used herein, a "chimeric antibody" is generally an antibody that is partially
of human origin
and partially of non-human origin, i.e. derived in part from a non-human
animal, for example
a mouse or other rodent, or an avian such as a chicken. Chimeric antibodies
are preferred
over non-human antibodies in order to reduce the risk of a human anti-antibody
response,
e.g. a human anti-mouse antibody response in the case of a murine antibody. An
example of a
typical chimeric antibody is one in which the variable region sequences are
murine sequences
derived from immunization of a mouse, while the constant region sequences are
human. In
the case of a chimeric antibody, the non-human parts, i.e. typically the
framework regions of
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
the variable region sequences, may be subjected to further alteration in order
to humanize the
antibody.
The term "humanize" refers to the fact that where an antibody is wholly or
partially of non-
human origin, for example a murine antibody obtained from immunization of mice
with an
antigen of interest or a chimeric antibody based on such a murine antibody, it
is possible to
replace certain amino acids, in particular in the framework regions and
constant domains of
the heavy and light chains, in order to avoid or minimize an immune response
in humans. It is
known that all antibodies have the potential for eliciting a human anti-
antibody response,
to which correlates to some extent with the degree of "humanness" of the
antibody in question.
Although it is not possible to precisely predict the immunogenicity and
thereby the human
anti-antibody response of a particular antibody, non-human antibodies tend to
be more
immunogenic than human antibodies. Chimeric antibodies, where the foreign
(usually rodent)
constant regions have been replaced with sequences of human origin, have been
shown to be
generally less immunogenic than antibodies of fully foreign origin, and the
trend in therapeutic
antibodies is towards humanized or fully human antibodies. For chimeric
antibodies or other
antibodies of non-human origin, it is therefore preferred that they be
humanized to reduce the
risk of a human anti-antibody response.
For chimeric antibodies, humanization typically involves modification of the
framework regions
of the variable region sequences. Amino acid residues that are part of a
complementarity
determining region (CDR) will typically not be altered in connection with
humanization,
although in certain cases it may be desirable to alter individual CDR amino
acid residues, for
example to remove a glycosylation site, a deamidation site or an undesired
cysteine residue.
N-linked glycosylation occurs by attachment of an oligosaccharide chain to an
asparagine
residue in the tripeptide sequence Asn-X-Ser or Asn-X-Thr, where X may be any
amino acid
except Pro. Removal of an N-glycosylation site may be achieved by mutating
either the Asn or
the Ser/Thr residue to a different residue, preferably by way of conservative
substitution.
Deamidation of asparagine and glutamine residues can occur depending on
factors such as pH
and surface exposure. Asparagine residues are particularly susceptible to
deamidation,
primarily when present in the sequence Asn-Gly, and to a lesser extent in
other dipeptide
sequences such as Asn-Ala. When such a deamidation site, in particular Asn-
Gly, is present in
a CDR sequence, it may therefore be desirable to remove the site, typically by
conservative
substitution to remove one of the implicated residues.
Numerous methods for humanization of an antibody sequence are known in the
art; see e.g.
the review by Almagro & Fransson (2008) Front Biosci. 13: 1619-1633. One
commonly used
method is CDR grafting, which for e.g. a murine-derived chimeric antibody
involves
identification of human germline gene counterparts to the murine variable
region genes and
grafting of the murine CDR sequences into this framework. CDR grafting may be
based on the
11
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
Kabat CDR definitions, although a recent publication (Magdelaine-Beuzelin et
al. (2007) Crit
Rev.Oncol Hematol. 64: 210-225) has suggested that the IMGT definition
(www.imgt.org) may
improve the result of the humanization. Since CDR grafting may reduce the
binding specificity
and affinity, and thus the biological activity, of a CDR grafted non-human
antibody, back
mutations may be introduced at selected positions of the CDR grafted antibody
in order to
retain the binding specificity and affinity of the parent antibody.
Identification of positions for
possible back mutations can be performed using information available in the
literature and in
antibody databases. Amino acid residues that are candidates for back mutations
are typically
those that are located at the surface of an antibody molecule, while residues
that are buried or
to that have a low degree of surface exposure will not normally be altered.
An alternative
humanization technique to CDR grafting and back mutation is resurfacing, in
which non-
surface exposed residues of non-human origin are retained, while surface
residues are altered
to human residues.
In certain cases, it may also be desirable to alter one or more CDR amino acid
residues in
order to improve binding affinity to the target epitope. This is known as
"affinity maturation"
and may optionally be performed in connection with humanization, for example
in situations
where humanization of an antibody leads to reduced binding specificity or
affinity and it is not
possible to sufficiently improve the binding specificity or affinity by back
mutations alone.
Various affinity maturation methods are known in the art, for example the in
vitro scanning
saturation mutagenesis method described by Burks et al. (1997) PNAS USA, vol.
94, pp. 412-
417 and the stepwise in vitro affinity maturation method of Wu et al. (1998)
PNAS USA, vol.
95, pp. 6037-6042.
As noted above, the present invention encompasses humanized antibodies, i.e.
antibodies as
otherwise described that have been subjected to humanization. These may also
be referred to
as "humanized variants" of an antibody of the invention. In particular, the
terms "heavy chain
variable region sequence" and "light chain variable region sequence" as used
herein with
reference to any specific amino acid sequence are intended to encompass not
only that specific
sequence but also any humanized variant thereof. Affinity matured variants of
the anti-HER3
antibodies described herein are also intended to by encompassed by the present
invention.
As used herein, a reference to a heavy chain variable region sequence or a
light chain variable
region sequence with a particular minimum level of sequence identity compared
to a specified
heavy chain variable region or light chain variable region sequence, e.g.
having at least 90%
or at least 95% sequence identity with the reference sequence, such as at
least 96%, at least
97%, at least 98% or at least 99% sequence identity, is intended to include,
but not to be
limited to, humanized and/or affinity matured variants of such reference
sequence.
12
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
The term "recombinant antibody" refers to an antibody that is expressed from a
cell or cell line
transfected with an expression vector (or possibly more than one expression
vector, typically
two expression vectors) comprising the coding sequence of the antibody, where
said coding
sequence is not naturally associated with the cell.
The term "vector" refers to a nucleic acid molecule into which a nucleic acid
sequence can be
inserted for transport between different genetic environments and/or for
expression in a host
cell. A vector that carries regulatory elements for transcription of the
nucleic acid sequence (at
least a suitable promoter) is referred to as an "an expression vector". The
terms "plasmid" and
to "vector" may be used interchangeably. Expression vectors used in the
context of the present
invention may be of any suitable type known in the art, e.g. a plasmid or a
viral vector.
The terms "polyclonal antibody" or "mixture of [monoclonal] antibodies" refer
to a
composition of two or more different antibody molecules which are capable of
binding to or
reacting with different specific antigenic determinants on the same or on
different antigens. In
the context of the present invention, the individual antibodies of a
polyclonal antibody bind to
different antigenic determinants of HER3. Preferably the individual antibodies
of a polyclonal
antibody of the invention bind to different epitopes of HER3, more preferably
distinct and
substantially non-overlapping epitopes. The variability of a polyclonal
antibody is generally
thought to be located in the variable regions of the antibody molecules. A
"recombinant
polyclonal anti-HER3 antibody composition" is a composition comprising a
mixture of two or
more recombinant monoclonal antibodies that bind HER3.
It is well-known in the art that antibodies exist as different isotypes, such
as the human
isotypes IgG1, IgG2, IgG3, IgG4, IgA1 and IgA2, or the murine isotypes IgG1,
IgG2a, IgG2b,
IgG3 and IgA. An antibody of the invention may be of any isotype. Although it
is possible for
the individual antibodies of a polyclonal antibody composition of the
invention to include
antibodies of more than one isotype, they are preferably all of the same
isotype.
A recombinant antibody composition comprising "at least first and second
recombinant anti-
HER3 antibodies" will comprise at least two of the specified antibodies, but
may include more
than two of the anti-HER3 antibodies described herein. In certain cases such a
recombinant
antibody composition may include a relatively large number of individual anti-
HER3 antibodies,
e.g. up to 10 or more, such as up to 15 or 20, but will normally include less
than 10 different
anti-HER3 antibodies, i.e. 2, 3, 4, 5, 6, 7, 8 or 9 antibodies. Recombinant
antibody
compositions of the invention will more typically include not more than about
6 different anti-
HER3 antibodies, and in many cases they will include not more than 4 different
anti-HER3
antibodies. In preferred embodiments, a recombinant antibody composition of
the invention
will therefore include 2, 3 or 4 different anti-HER3 antibodies, typically 2
or 3 different anti-
HER3 antibodies.
13
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
The term "CDR" or "complementarity determining region" refers to the
"hypervariable" regions
found in the variable domains of an antibody that are primarily responsible
for determining the
antibody's binding specificity. See the definition in Lefranc et al (2003),
!MGT unique
numbering for immunoglobulin and T cell receptor variable domains and Ig
superfamily V-like
domains, Dev. Comp Immunol. 27, 55-77. Each of the heavy and light chains of
an antibody
contain three CDR regions, referred to as CDR1, CDR2 and CDR3, of which CDR3
shows the
greatest variability.
to The term "epitope" is used to describe a part of a larger molecule (e.g.
antigen or antigenic
site) having antigenic or immunogenic activity in an animal. An epitope having
immunogenic
activity is a portion of a larger molecule that elicits an antibody response
in an animal. An
epitope having antigenic activity is a portion of a larger molecule to which
an antibody
immunospecifically binds as determined by any method known in the art.
Antigenic epitopes
are not necessarily immunogenic. An antigen is a substance to which an
antibody or antibody
fragment immunospecifically binds, e.g. a toxin, virus, bacteria, protein or
DNA. An antigen or
antigenic site often has more than one epitope, unless it is very small, and
is often capable of
stimulating an immune response. Epitopes may be linear or conformational. A
linear epitope
generally consists of about 6 to 10 adjacent amino acids on a protein molecule
that are
recognized by an antibody. In contrast, a conformational epitope consists of
amino acids that
are not arranged sequentially, but where an antibody recognizes a particular
three-
dimensional structure. When a protein molecule folds into a three-dimensional
structure, the
amino acids forming the epitope are juxtaposed, enabling the antibody to
recognize the
conformational epitope. In a denatured protein only linear epitopes are
recognized. A
conformational epitope, by definition, must be on the outside of the folded
protein.
The term "distinct epitopes" refers to the fact that when two different
antibodies of the
invention bind distinct epitopes, there is less than 100% competition for
antigen binding,
preferably less than 80% competition for antigen binding, more preferably less
than 50%
competition for antigen binding, and most preferably as little competition as
possible, such as
less than about 25% competition for antigen binding. Antibodies capable of
competing with
each other for binding to the same antigen may bind the same or overlapping
epitopes or may
have a binding site in the close vicinity of one another, so that competition
is mainly caused by
steric hindrance. An analysis for "distinct epitopes" of antibody pairs may be
performed by
methods known in the art, for example by way of binding experiments under
saturating
antibody conditions using either FACS (fluorescence activated cell sorting) or
other flow
cytometry analysis on cells expressing HER3 and individual fluorescent labeled
antibodies, or
by Surface Plasmon Resonance (SPR) using HER3 antigen captured or conjugated
to a flow cell
surface. A method for determining competition between antibodies using SPR is
described in
Example 12 below.
14
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
The distinct epitopes are preferably "non-overlapping" in the sense that two
different anti-
HER3 antibodies in a composition of the invention have a sufficiently low
competition for
antigen binding that the two antibodies are able to bind their respective
epitopes
simultaneously. It will be understood by persons skilled in the that there can
be different
degrees of overlap, and that distinct epitopes can be considered to be "non-
overlapping" in
spite of the presence of some degree of overlap, as long as the respective
antibodies are able
to substantially bind their epitopes. This is generally considered to be the
case when the
competition for antigen binding between two antibodies is less than about 50%.
Similarly, an antibody that "competes for binding" with an anti-HER3 antibody
of the invention
may be defined as one that exhibits competition for antigen binding of about
50% or more.
Antibodies binding to different epitopes on the same antigen can have varying
effects on the
activity of the antigen to which they bind, depending on the location of the
epitope. An
antibody binding to an epitope in an active site of the antigen may block the
function of the
antigen completely, whereas another antibody binding at a different epitope
may have no or
little effect on the activity of the antigen alone. Such antibodies may,
however, still activate
complement and thereby result in the elimination of the antigen, and may
result in synergistic
effects when combined with one or more antibodies binding at different
epitopes on the same
antigen. In the context of the present invention, the epitope is preferably a
portion of the
extracellular domain of HER3. Antigens of the present invention are preferably
extracellular
domain HER3 proteins, polypeptides or fragments thereof to which an antibody
or antibody
fragment immunospecifically binds. A HER3 associated antigen may also be an
analog or
derivative of the extracellular domain of HER3 polypeptide or fragment thereof
to which an
antibody or antibody fragment immunospecifically binds.
The term "immunoglobulin" is commonly used as a collective designation of the
mixture of
antibodies found in blood or serum, but may also be used to designate a
mixture of antibodies
derived from other sources.
The term "cognate VH and VL coding pair" describes an original pair of VH and
VL coding
sequences contained within or derived from the same antibody-producing cell.
Thus, a cognate
VH and VL pair represents the VH and VL pairing originally present in the
donor from which such
a cell is derived. The term "an antibody expressed from a VH and VL coding
pair" indicates that
an antibody or an antibody fragment is produced from a vector, plasmid or
other
polynucleotide containing the VH and VL coding sequence. When a cognate VH and
VL coding
pair is expressed, either as a complete antibody or as a stable fragment
thereof, they preserve
the binding affinity and specificity of the antibody originally expressed from
the cell they are
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
derived from. A library of cognate pairs is also termed a repertoire or
collection of cognate
pairs, and may be kept individually or pooled.
By "protein" or "polypeptide" is meant any chain of amino acids, regardless of
length or post-
s translational modification. Proteins can exist as monomers or multimers,
comprising two or
more assembled polypeptide chains, fragments of proteins, polypeptides,
oligopeptides, or
peptides.
The term "head-to-head promoters" (also known as "bi-directional promoters")
refers to a
to promoter pair being placed in close proximity so that transcription of
two gene fragments
driven by the promoters occurs in opposite directions.
The term "transfection" is herein used as a broad term for introducing foreign
DNA into a cell.
The term is also meant to cover other functional equivalent methods for
introducing foreign
15 DNA into a cell, such as e.g., transformation, infection, transduction
or fusion of a donor cell
and an acceptor cell.
The term "HER3" (also known as ErbB-3) stands for "Human Epidermal growth
factor Receptor
3" as described above in the "Background of the invention" section. As used
herein, it is
20 intended to include variants, isoforms and species homologs of HER3.
Preferably, binding of an
antibody of the invention to HER3 inhibits the growth of cells expressing HER3
(i.e. typically
tumor cells) by inhibiting formation of heteromeric complexes between HER3 and
other ErbB
family members, e.g. heterodimerization with HER2.
25 As used herein, the term "inhibits growth" (e.g., referring to cells) is
intended to include any
measurable decrease in the proliferation (increase in number of cells) or
metabolism of a cell
when contacted with an anti-HER3 antibody as compared to the growth of the
same cells in
the absence of an anti-HER3 antibody, e.g. inhibition of growth of a cell
culture by at least
about 100/0, and preferably more, such as at least about 20% or 30%, more
preferably at least
30 about 40% or 50%, such as at least about 60%, 70%, 80%, 90%, 99% or even
100%.
Growth inhibition can e.g. be determined in relevant cancer cell lines as
described in the
examples below.
As used herein, the terms "inhibits dimerization" or "inhibits dimer
formation" refer to any
35 measurable reduction in the ability of HER3 to form dimers with other
receptors, in particular
HER2, but also EGFR or HER4, as a result of binding of an anti-HER3 antibody
compared to
dimer formation in the absence of an anti-HER3 antibody.
The term "treatment" as used herein refers to administration of an anti-HER3
antibody or
40 antibody composition of the invention in a sufficient amount to ease,
reduce, ameliorate or
16
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
eradicate (cure) symptoms or disease states. Administration of two or more
anti-HER3
antibodies of the invention will generally be by way of simultaneous
administration of the
antibodies, preferably in the form of a composition containing all of the anti-
HER3 antibodies
to be used for treatment. However, it is also possible to administer two or
more anti-HER3
antibodies of the invention separately. References herein to e.g.
administration of a
recombinant antibody composition comprising at least two anti-HER3 antibodies
should
therefore be understood as encompassing not only administration of a
composition comprising
the at least two antibodies as such, but also separate administration of the
antibodies.
Combinations of two or more anti-HER3 antibodies of the invention can thus be
administered
to simultaneously, sequentially or separately.
The percent identity between two sequences, e.g. variable region sequences,
refers to the
number of identical positions shared by the sequences (calculated as # of
identical
positions/total # of positions x 100), taking into account gaps that must be
introduced for
optimal alignment of the two sequences. The comparison of sequences and
determination of
percent identity between two sequences may be accomplished using readily
available
software. Suitable software programs are available from various sources, both
for online use
and for download, and for alignment of both protein and nucleotide sequences.
One suitable
program is ClustalW (Thompson et al. (1994) Nucleic Acids Res. 11;22(22):4673-
80),
available from www.clustal.org, or alternatively e.g. from the European
Bioinformatics
Institute (www.ebi.ac.uk), which also provides various other protein and
nucleotide informatics
tools.
Particular embodiments
One aspect of the invention relates to various novel anti-HER3 antibodies. In
one embodiment,
the invention thus relates to a recombinant anti-HER3 antibody comprising the
heavy chain
CDR3 sequence of any one of the antibodies referred to herein as antibodies
4785, 4889,
4935, 5038, 5082, 5101, 5106, 5143, 5144 and 5259.
In another embodiment, the invention relates to a recombinant anti-HER3
antibody comprising
the heavy chain CDR3 sequence and the light chain CDR3 sequence of any one of
antibodies
4785, 4889, 4935, 5038, 5082, 5101, 5106, 5143, 5144 and 5259.
In another embodiment, the invention relates to a recombinant anti-HER3
antibody comprising
the heavy chain CDR1, CDR2 and CDR3 sequences and light chain CDR1, CDR2 and
CDR3
sequences of any one of antibodies 4785, 4889, 4935, 5038, 5082, 5101, 5106,
5143, 5144
and 5259.
In a further embodiment, the invention relates to a recombinant anti-HER3
antibody
ao comprising the heavy chain variable region sequence and the light chain
variable region
17
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
sequence of any one of antibodies 4785, 4889, 4935, 5038, 5082, 5101, 5106,
5143, 5144
and 5259, or comprising a humanized and/or affinity matured variant of said
heavy chain
and/or light chain variable region sequence, or comprising a heavy chain
variable region
sequence and a light chain variable region sequence each having at least 90%
or at least 95%
sequence identity with said heavy chain variable region and light chain
variable region
sequences, such as at least 96%, at least 97%, at least 98% or at least 99%
sequence
identity with said sequences, and which competes for binding with said
antibody.
In a further embodiment, the invention relates to a recombinant anti-HER3
antibody that binds
to the same epitope as and which competes for binding with any of the
antibodies defined above,
as well as antibody compositions comprising one or more of such antibodies,
preferably
comprising at least two such antibodies, e.g. two or three such antibodies as
described
elsewhere herein.
Table 1 below shows the sequence ID numbers, as set forth in the appended
sequence listing,
for the DNA and amino acid sequences of the heavy chain variable regions (VH)
and the light
chains (LC) of antibodies 4785, 4889, 4935, 5038, 5082, 5101, 5106, 5143, 5144
and 5259
(where, as explained above, the light chain sequence includes both the light
chain variable
region (VL) sequence and the human kappa constant region sequence).
Table 1: Sequence ID numbers for the DNA and amino acid sequences of the heavy
chain
variable regions and the light chains of selected anti-HER3 antibodies
Antibody No. VH DNA seq. VH protein seq. LC DNA seq. LC protein
seq.
4785 1 2 3 4
4889 5 6 7 8
4935 9 10 11 12
5038 13 14 15 16
5082 17 18 19 20
5101 21 22 23 24
5106 25 26 27 28
5143 29 30 31 32
5144 33 34 35 36
5259 37 38 39 40
In another aspect, the invention relates to a recombinant antibody composition
comprising at
least first and second recombinant anti-HER3 antibodies, wherein the first and
second
antibodies bind distinct epitopes of HER3, and wherein at least one of the
first and second
antibodies are selected from the group of antibodies outlined above, for
example wherein both
or all of the anti-HER3 antibodies in the composition are selected from the
group of antibodies
outlined above.
18
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
One embodiment of this aspect of the invention thus relates to a recombinant
antibody
composition comprising at least first and second recombinant anti-HER3
antibodies, wherein
the first and second antibodies bind distinct epitopes of HER3, and wherein
each of the first
and second antibodies comprise the heavy chain CDR3 sequence of an antibody
selected from
the group consisting of antibodies 4785, 4889, 4935, 5038, 5082, 5101, 5106,
5143, 5144
and 5259.
Another embodiment of this aspect of the invention relates to a recombinant
antibody
composition comprising at least first and second recombinant anti-HER3
antibodies, wherein
to the first and second antibodies bind distinct epitopes of HER3, and
wherein each of the first
and second antibodies comprise the heavy chain and light chain CDR3 sequences
of an
antibody selected from the group consisting of antibodies 4785, 4889, 4935,
5038, 5082,
5101, 5106, 5143, 5144 and 5259.
A further embodiment of this aspect of the invention relates to a recombinant
antibody
composition comprising at least first and second recombinant anti-HER3
antibodies, wherein
the first and second antibodies bind distinct epitopes of HER3, and wherein
each of the first
and second antibodies comprise the heavy chain CDR1, CDR2 and CDR3 sequences
and light
chain CDR1, CDR2 and CDR3 sequences of an antibody selected from the group
consisting of
antibodies 4785, 4889, 4935, 5038, 5082, 5101, 5106, 5143, 5144 and 5259.
A further embodiment of this aspect of the invention is a recombinant antibody
composition
comprising at least first and second recombinant anti-HER3 antibodies, wherein
the first and
second antibodies bind distinct epitopes of HER3, and wherein each of the
first and second
antibodies comprise the heavy chain variable region sequence or a humanized
and/or affinity
matured variant thereof and the light chain variable region sequence or a
humanized and/or
affinity matured variant thereof of an antibody selected from the group
consisting of
antibodies 4785, 4889, 4935, 5038, 5082, 5101, 5106, 5143, 5144 and 5259; or
wherein
each of the first and second antibodies comprise a heavy chain variable region
sequence and a
light chain variable region sequence each having at least 90% sequence
identity, preferably at
least 95% sequence identity, such as at least 96%, at least 97%, at least 98%
or at least 99%
sequence identity, with the heavy chain variable region and light chain
variable region
sequences, respectively, of an antibody selected from the group consisting of
antibodies 4785,
4889, 4935, 5038, 5082, 5101, 5106, 5143, 5144 and 5259, and wherein the first
and second
antibodies compete for binding with the respective antibodies from which they
are derived.
A particular embodiment is an antibody composition comprising at least first
and second
recombinant anti-HER3 antibodies that bind distinct epitopes of HER3; wherein
a) the first recombinant antibody comprises a heavy chain variable region
sequence
and a light chain variable region sequence having at least 90% sequence
identity, preferably
19
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
at least 95% sequence identity, such as at least 96%, at least 97%, at least
98% or at least
99% sequence identity, with the heavy chain variable region and light chain
variable region
sequences, respectively, of any one reference antibody selected from
antibodies 4785, 4889,
4935, 5038, 5082, 5101, 5106, 5143, 5144 and 5259, and wherein the first
recombinant
antibody binds the same epitope as and competes for binding with said
reference antibody;
and
b) the second recombinant antibody comprises a heavy chain variable region
sequence
and a light chain variable region sequence having at least 90% sequence
identity, preferably
at least 95% sequence identity, such as at least 96%, at least 97%, at least
98% or at least
to 99% sequence identity, with the heavy chain variable region and light
chain variable region
sequences, respectively, of any one reference antibody selected from
antibodies 4785, 4889,
4935, 5038, 5082, 5101, 5106, 5143, 5144 and 5259, wherein said reference
antibody is
different from the reference antibody of a), and wherein the second
recombinant antibody
binds the same epitope as and competes for binding with said reference
antibody.
A still further embodiment of this aspect of the invention is a recombinant
antibody
composition comprising at least first and second recombinant anti-HER3
antibodies, wherein
the first and second antibodies bind distinct epitopes of HER3, and wherein
the first and
second antibodies are selected from the group consisting of antibodies 4785,
4889, 4935,
5038, 5082, 5101, 5106, 5143, 5144 and 5259, or humanized and/or affinity
matured variants
thereof.
A still further embodiment of this aspect of the invention is a recombinant
antibody
composition comprising at least first and second recombinant anti-HER3
antibodies, wherein
the first and second antibodies bind distinct epitopes of HER3, and wherein
the first and
second antibodies are selected from the group consisting of antibodies that
bind to the same
epitope as and compete for binding with antibodies 4785, 4889, 4935, 5038,
5082, 5101,
5106, 5143, 5144 and 5259.
A further embodiment of this aspect of the invention is an antibody
composition comprising at
least first and second recombinant anti-HER3 antibodies that bind distinct
epitopes of HER3,
wherein at least one of said antibodies is selected from the group consisting
of:
(a) an antibody comprising the heavy chain CDR3 sequence and the light
chain
CDR3 sequence of antibody 4785;
(b) an antibody comprising the heavy chain CDR3 sequence and the light
chain
CDR3 sequence of antibody 4889;
(c) an antibody comprising the heavy chain CDR3 sequence and the light
chain
CDR3 sequence of antibody 4935;
CA 02816520 2013-04-30
WO 2012/059858
PCT/1B2011/054835
(d) an antibody comprising the heavy chain CDR3 sequence and the light
chain
CDR3 sequence of antibody 5038;
(e) an antibody comprising the heavy chain CDR3 sequence and the light
chain
CDR3 sequence of antibody 5082;
(f) an antibody
comprising the heavy chain CDR3 sequence and the light chain
CDR3 sequence of antibody 5101;
(9) an
antibody comprising the heavy chain CDR3 sequence and the light chain
CDR3 sequence of antibody 5106;
(h) an antibody comprising the heavy chain CDR3 sequence and the light
chain
to CDR3 sequence of antibody 5143;
(i) an antibody comprising the heavy chain CDR3 sequence and the light
chain
CDR3 sequence of antibody 5144; and
an antibody comprising the heavy chain CDR3 sequence and the light chain
CDR3 sequence of antibody 5259.
Preferably, both of said first and second recombinant anti-HER3 antibodies are
selected from
antibodies (a) - (j) set forth above. The composition may also comprise at
least a third
recombinant anti-HER3 antibody, preferably an antibody selected from
antibodies (a) - (j)
above. In another embodiment, the antibody composition may comprise at least
first and
second recombinant anti-HER3 antibodies that bind distinct epitopes of HER3,
wherein each of
said first and second antibodies binds the same epitope as and competes for
binding with one
of antibodies (a) - (j) set forth above.
A still further embodiment of this aspect of the invention is an antibody
composition
comprising at least first and second recombinant anti-HER3 antibodies that
bind distinct
epitopes of HER3, wherein at least one of said antibodies is selected from the
group consisting
of:
(A) an
antibody comprising CDR1, CDR2 and CDR3 of the heavy chain variable
region and CDR1, CDR2 and CDR3 of the light chain variable region of antibody
4785;
(B) an antibody
comprising CDR1, CDR2 and CDR3 of the heavy chain variable
region and CDR1, CDR2 and CDR3 of the light chain variable region of antibody
4889;
(C) an antibody comprising CDR1, CDR2 and CDR3 of the heavy chain variable
region and CDR1, CDR2 and CDR3 of the light chain variable region of antibody
4935;
(D) an antibody comprising CDR1, CDR2 and CDR3 of the heavy chain variable
region and CDR1, CDR2 and CDR3 of the light chain variable region of antibody
5038;
(E) an antibody comprising CDR1, CDR2 and CDR3 of the heavy chain variable
region and CDR1, CDR2 and CDR3 of the light chain variable region of antibody
5082;
(F) an antibody comprising CDR1, CDR2 and CDR3 of the heavy chain variable
region and CDR1, CDR2 and CDR3 of the light chain variable region of antibody
5101;
21
CA 02816520 2013-04-30
WO 2012/059858
PCT/1B2011/054835
(G) an antibody comprising CDR1, CDR2 and CDR3 of the heavy chain variable
region and CDR1, CDR2 and CDR3 of the light chain variable region of antibody
5106;
(H) an antibody comprising CDR1, CDR2 and CDR3 of the heavy chain variable
region and CDR1, CDR2 and CDR3 of the light chain variable region of antibody
5143;
(I) an antibody
comprising CDR1, CDR2 and CDR3 of the heavy chain variable
region and CDR1, CDR2 and CDR3 of the light chain variable region of antibody
5144; and
(3) an
antibody comprising CDR1, CDR2 and CDR3 of the heavy chain variable
region and CDR1, CDR2 and CDR3 of the light chain variable region of antibody
5259.
to In this embodiment, both of said first and second recombinant anti-HER3
antibodies are
preferably selected from antibodies (A) - (3) set forth above. The composition
may also
comprise at least a third recombinant anti-HER3 antibody, preferably an
antibody selected
from antibodies (A) - (3) above. In another embodiment, the antibody
composition may
comprise at least first and second recombinant anti-HER3 antibodies that bind
distinct epitopes
of HER3, wherein each of said first and second antibodies binds the same
epitope as and
competes for binding with one of antibodies (A) - (3) set forth above.
One particular embodiment of this aspect of the invention relates to a
recombinant antibody
composition comprising at least first and second recombinant anti-HER3
antibodies, wherein
the first and second antibodies compete for binding with antibodies 5082 and
5106,
respectively, and are:
= antibodies 5082 and 5106, or humanized and/or affinity matured variants
thereof;
= an antibody comprising the heavy chain CDR3 sequence of antibody 5082,
and an
antibody comprising the heavy chain CDR3 sequence of antibody 5106;
= an antibody comprising the heavy and light chain CDR3 sequences of antibody
5082,
and an antibody comprising the heavy and light chain CDR3 sequences of
antibody
5106;
= an antibody comprising the heavy and light chain CDR1, CDR2 and CDR3
sequences of
antibody 5082, and an antibody comprising the heavy and light chain CDR1, CDR2
and
CDR3 sequences of antibody 5106;
= an antibody comprising the heavy and light chain variable region
sequences of
antibody 5082, and an antibody comprising the heavy and light chain variable
region
sequences of antibody 5106; or
= an antibody comprising heavy and light chain variable region sequences
each having at
least 90% sequence identity, preferably at least 95%, 96%, 97%, 98% or 99%
sequence identity with the heavy and light chain variable region sequences,
respectively, of antibody 5082, and an antibody comprising heavy and light
chain
variable region sequences each having at least 90% sequence identity,
preferably at
least 95%, 96%, 97%, 98% or 99% sequence identity with the heavy and light
chain
variable region sequences, respectively, of antibody 5106.
22
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
Another such embodiment relates to a recombinant antibody composition
comprising at least
first and second recombinant antibodies, wherein the first and second
antibodies compete for
binding with antibodies 5082 and 4785, respectively, and are:
= antibodies 5082 and 4785, or humanized and/or affinity matured variants
thereof;
= an antibody comprising the heavy chain CDR3 sequence of antibody 5082,
and an
antibody comprising the heavy chain CDR3 sequence of antibody 4785;
= an antibody comprising the heavy and light chain CDR3 sequences of
antibody 5082,
and an antibody comprising the heavy and light chain CDR3 sequences of
antibody
4785;
= an antibody comprising the heavy and light chain CDR1, CDR2 and CDR3
sequences of
antibody 5082, and an antibody comprising the heavy and light chain CDR1, CDR2
and
CDR3 sequences of antibody 4785;
= an antibody comprising the heavy and light chain variable region
sequences of
antibody 5082, and an antibody comprising the heavy and light chain variable
region
sequences of antibody 4785; or
= an antibody comprising heavy and light chain variable region sequences
each having at
least 90% sequence identity, preferably at least 95%, 96%, 97%, 98% or 99%
sequence identity with the heavy and light chain variable region sequences,
respectively, of antibody 5082, and an antibody comprising heavy and light
chain
variable region sequences each having at least 90% sequence identity,
preferably at
least 95%, 96%, 97%, 98% or 99% sequence identity with the heavy and light
chain
variable region sequences, respectively, of antibody 4785.
Another such embodiment relates to a recombinant antibody composition
comprising at least
first and second recombinant antibodies, wherein the first and second
antibodies compete for
binding with antibodies 5082 and 5038, respectively, and are:
= antibodies 5082 and 5038, or humanized and/or affinity matured variants
thereof;
= an antibody comprising the heavy chain CDR3 sequence of antibody 5082,
and an
antibody comprising the heavy chain CDR3 sequence of antibody 5038;
= an antibody comprising the heavy and light chain CDR3 sequences of
antibody 5082,
and an antibody comprising the heavy and light chain CDR3 sequences of
antibody
5038;
= an antibody comprising the heavy and light chain CDR1, CDR2 and CDR3
sequences of
antibody 5082, and an antibody comprising the heavy and light chain CDR1, CDR2
and
CDR3 sequences of antibody 5038;
= an antibody comprising the heavy and light chain variable region sequences
of
antibody 5082, and an antibody comprising the heavy and light chain variable
region
sequences of antibody 5038; or
= an antibody comprising heavy and light chain variable region sequences
each having at
least 90% sequence identity, preferably at least 95%, 96%, 97%, 98% or 99%
23
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
sequence identity with the heavy and light chain variable region sequences,
respectively, of antibody 5082, and an antibody comprising heavy and light
chain
variable region sequences each having at least 90% sequence identity,
preferably at
least 95%, 96%, 97%, 98% or 99% sequence identity with the heavy and light
chain
variable region sequences, respectively, of antibody 5038.
Another such embodiment relates to a recombinant antibody composition
comprising at least
first and second recombinant antibodies, wherein the first and second
antibodies compete for
binding with antibodies 5082 and 5144, respectively, and are:
= antibodies 5082 and 5144, or humanized and/or affinity matured variants
thereof;
= an antibody comprising the heavy chain CDR3 sequence of antibody 5082, and
an
antibody comprising the heavy chain CDR3 sequence of antibody 5144;
= an antibody comprising the heavy and light chain CDR3 sequences of
antibody 5082,
and an antibody comprising the heavy and light chain CDR3 sequences of
antibody
5144;
= an antibody comprising the heavy and light chain CDR1, CDR2 and CDR3
sequences of
antibody 5082, and an antibody comprising the heavy and light chain CDR1, CDR2
and
CDR3 sequences of antibody 5144;
= an antibody comprising the heavy and light chain variable region
sequences of
antibody 5082, and an antibody comprising the heavy and light chain variable
region
sequences of antibody 5144; or
= an antibody comprising heavy and light chain variable region sequences
each having at
least 90% sequence identity, preferably at least 95%, 96%, 97%, 98% or 99%
sequence identity with the heavy and light chain variable region sequences,
respectively, of antibody 5082, and an antibody comprising heavy and light
chain
variable region sequences each having at least 90% sequence identity,
preferably at
least 95%, 96%, 97%, 98% or 99% sequence identity with the heavy and light
chain
variable region sequences, respectively, of antibody 5144.
Another such embodiment relates to a recombinant antibody composition
comprising at least
first and second recombinant antibodies, wherein the first and second
antibodies compete for
binding with antibodies 4889 and 5143, respectively, and are:
= antibodies 4889 and 5143, or humanized and/or affinity matured variants
thereof;
= an antibody comprising the heavy chain CDR3 sequence of antibody 4889,
and an
antibody comprising the heavy chain CDR3 sequence of antibody 5143;
= an antibody comprising the heavy and light chain CDR3 sequences of
antibody 4889,
and an antibody comprising the heavy and light chain CDR3 sequences of
antibody
5143;
= an antibody comprising the heavy and light chain CDR1, CDR2 and CDR3
sequences of
antibody 4889, and an antibody comprising the heavy and light chain CDR1, CDR2
and
CDR3 sequences of antibody 5143;
24
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
= an antibody comprising the heavy and light chain variable region
sequences of
antibody 4889, and an antibody comprising the heavy and light chain variable
region
sequences of antibody 5143; or
= an antibody comprising heavy and light chain variable region sequences
each having at
least 90% sequence identity, preferably at least 95%, 96%, 97%, 98% or 99%
sequence identity with the heavy and light chain variable region sequences,
respectively, of antibody 4889, and an antibody comprising heavy and light
chain
variable region sequences each having at least 90% sequence identity,
preferably at
least 95%, 96%, 97%, 98% or 99% sequence identity with the heavy and light
chain
to variable region sequences, respectively, of antibody 5143.
Another such embodiment relates to a recombinant antibody composition
comprising at least
first and second recombinant antibodies, wherein the first and second
antibodies compete for
binding with antibodies 4785 and 5038, respectively, and are:
= antibodies 4785 and 5038, or humanized and/or affinity matured variants
thereof;
= an antibody comprising the heavy chain CDR3 sequence of antibody 4785, and
an
antibody comprising the heavy chain CDR3 sequence of antibody 5038;
= an antibody comprising the heavy and light chain CDR3 sequences of
antibody 4785,
and an antibody comprising the heavy and light chain CDR3 sequences of
antibody
5038;
= an antibody comprising the heavy and light chain CDR1, CDR2 and CDR3
sequences of
antibody 4785, and an antibody comprising the heavy and light chain CDR1, CDR2
and
CDR3 sequences of antibody 5038;
= an antibody comprising the heavy and light chain variable region
sequences of
antibody 4785, and an antibody comprising the heavy and light chain variable
region
sequences of antibody 5038; or
= an antibody comprising heavy and light chain variable region sequences
each having at
least 90% sequence identity, preferably at least 95%, 96%, 97%, 98% or 99%
sequence identity with the heavy and light chain variable region sequences,
respectively, of antibody 4785, and an antibody comprising heavy and light
chain
variable region sequences each having at least 90% sequence identity,
preferably at
least 95%, 96%, 97%, 98% or 99% sequence identity with the heavy and light
chain
variable region sequences, respectively, of antibody 5038.
Another such embodiment relates to a recombinant antibody composition
comprising at least
first and second recombinant antibodies, wherein the first and second
antibodies compete for
binding with antibodies 4785 and 5259, respectively, and are:
= antibodies 4785 and 5259, or humanized and/or affinity matured variants
thereof;
= an antibody comprising the heavy chain CDR3 sequence of antibody 4785,
and an
antibody comprising the heavy chain CDR3 sequence of antibody 5259;
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
= an antibody comprising the heavy and light chain CDR3 sequences of
antibody 4785,
and an antibody comprising the heavy and light chain CDR3 sequences of
antibody
5259;
= an antibody comprising the heavy and light chain CDR1, CDR2 and CDR3
sequences of
antibody 4785, and an antibody comprising the heavy and light chain CDR1, CDR2
and
CDR3 sequences of antibody 5259;
= an antibody comprising the heavy and light chain variable region
sequences of
antibody 4785, and an antibody comprising the heavy and light chain variable
region
sequences of antibody 5259; or
= an antibody comprising heavy and light chain variable region sequences each
having at
least 90% sequence identity, preferably at least 95%, 96%, 97%, 98% or 99%
sequence identity with the heavy and light chain variable region sequences,
respectively, of antibody 4785, and an antibody comprising heavy and light
chain
variable region sequences each having at least 90% sequence identity,
preferably at
least 95%, 96%, 97%, 98% or 99% sequence identity with the heavy and light
chain
variable region sequences, respectively, of antibody 5259.
Another such embodiment relates to a recombinant antibody composition
comprising at least
first and second recombinant antibodies, wherein the first and second
antibodies compete for
binding with antibodies 5106 and 4889, respectively, and are:
= antibodies 5106 and 4889, or humanized and/or affinity matured variants
thereof;
= an antibody comprising the heavy chain CDR3 sequence of antibody 5106,
and an
antibody comprising the heavy chain CDR3 sequence of antibody 4889;
= an antibody comprising the heavy and light chain CDR3 sequences of
antibody 5106,
and an antibody comprising the heavy and light chain CDR3 sequences of
antibody
4889;
= an antibody comprising the heavy and light chain CDR1, CDR2 and CDR3
sequences of
antibody 5106, and an antibody comprising the heavy and light chain CDR1, CDR2
and
CDR3 sequences of antibody 4889;
= an antibody comprising the heavy and light chain variable region
sequences of
antibody 5106, and an antibody comprising the heavy and light chain variable
region
sequences of antibody 4889; or
= an antibody comprising heavy and light chain variable region sequences
each having at
least 90% sequence identity, preferably at least 95%, 96%, 97%, 98% or 99%
sequence identity with the heavy and light chain variable region sequences,
respectively, of antibody 5106, and an antibody comprising heavy and light
chain
variable region sequences each having at least 90% sequence identity,
preferably at
least 95%, 96%, 97%, 98% or 99% sequence identity with the heavy and light
chain
variable region sequences, respectively, of antibody 4889.
26
CA 02816520 2013-04-30
WO 2012/059858
PCT/1B2011/054835
Tables 2 and 3 below show the CDR1, CDR2 and CDR3 amino acid sequences of the
heavy
chain (Table 2) and the light chain (Table 3) of various anti-HER3 antibodies
according to the
invention. The amino acid sequences of the heavy chain variable region and the
light chain,
including the light chain variable region, of these antibodies, as well as the
encoding DNA
sequences (optimized for expression in CHO cells) are provided in the appended
sequence
listing. See Table 1 above for an overview of the SEQ ID numbers for these
sequences.
Table 2: Heavy chain CDR1, CDR2 and CDR3 sequences of selected anti-HER3
antibodies
SEQ ID NOs
Antibody Number H CDR1 H CDR2 H CDR3
(CDR1/2/3)
4785 GYSFTSYY IYPGSGHT CARPPYYSNYADVW 45-47
4889 GYSITSAYY VSYDGSN CAREGDYGYSDYW 48-50
4935 GYTFTSYY IYPGNVHT CVRRYGYDGDWFAYW 51-53
5038 GYSITSGFY ISYDGSN CARGGGYYGNLFDYW 54-56
5082 GYSITSAYY IGYDGRN CSREGDYGYSDYW 48, 57-58
5101 GFTFSSYG IRDGGGYT CARGILDYW 59-61
5106 GFTFSSFA ISDGGSHL CARGILDYW 62-63, 61
5143 GYSFTSYY IYPGSGHT CARPPYYSNYADVW 45-47
5144 GFSLSRYS IWGGGST CVRKGITTTGFDYW 64-66
5259 GFSLSRYT IWGGGST CARKGITTTGFDYW 67,
65, 68
Table 3: Light chain CDR1, CDR2 and CDR3 sequences of selected anti-HER3
antibodies
SEQ ID NOs
Antibody number L CDR1 L CDR2 L CDR3
(CDR1/3)
4785 QSLLNSGNQKNY WAS CQSDYSYPYTF 69, 70
4889 QDISNY YTS CQQSNTLPWTF 71, 72
4935 ESVDSYGNTF RAS CQQSNEDPWTF 73, 74
5038 QDISNY HTS CQQGITLPWTF 71, 75
5082 QDINNY YTS CQQSETLPWTF 76, 77
5101 QDISNY YTS CQQGNTLPYTF 71, 78
5106 QDINNY YTS CQQYSRIPYTF 76, 79
5143 QSLLNSGNQKNY WAS CQNDYSYPYTF 69, 80
5144 SSVSY DTS CQQLSSYPPTF 81, 82
5259 SSVSY DTS CQQLNSYPPTF 81, 83
Another aspect of the invention relates to nucleic acid molecules comprising a
nucleotide
sequence that encodes an antibody of the invention, i.e. an antibody selected
from the group
consisting of antibodies 4785, 4889, 4935, 5038, 5082, 5101, 5106, 5143, 5144
and 5259, or
a humanized and/or affinity matured variant thereof; or encoding a heavy
and/or light chain
variable region sequence of such an antibody, or a heavy and/or light chain
sequence having
at least 90% sequence identity, preferably at least 95% sequence identity,
such as at least
96%, at least 97%, at least 98% or at least 99% sequence identity, with such a
heavy and/or
light chain variable region sequence.
27
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
In one embodiment of this aspect of the invention, the nucleic acid molecule
comprises a
nucleotide sequence selected from the group consisting of SEQ ID NOS 1, 3, 5,
7, 9, 11, 13,
15, 17, 19, 21, 23, 25, 27, 29, 31, 33, 35, 37 or 39, or sequences that encode
the same
amino acid sequence as any one of said nucleotide sequences.
A further aspect of the invention relates to an expression vector comprising a
nucleic acid
molecule as defined above. As noted above, expression vectors for use in the
context of the
present invention may be of any suitable type known in the art, e.g. a plasmid
or a viral
to vector.
A still further aspect of the invention relates to a host cell comprising a
nucleic acid molecule
as defined above, wherein said host cell is capable of expressing an anti-HER3
antibody
encoded by said nucleic acid molecule.
In a further aspect the binding specificities of any two individual antibodies
disclosed herein
may be combined in one bispecific binding molecule. Such a bispecific binding
molecule
preferably comprises the heavy and light chain CDR1, CDR2 and CDR3 sequences
of the two
selected antibodies. The bispecific binding molecule may be a dual variable
domain antibody,
i.e. wherein the two arms of the antibody comprise two different variable
domains, or may be
in the form of an antibody fragment such as a bispecific Fab fragment or a
bispecific scFv.
Production of anti-HER3 antibodies and antibody compositions
An additional aspect of the invention relates to methods for producing an anti-
HER3 antibody
or a mixture of anti-HER3 antibodies of the invention. One embodiment of this
aspect of the
invention relates to a method for producing an anti-HER3 antibody as defined
herein,
comprising providing a host cell as defined above capable of expressing an
anti-HER3
antibody, cultivating said host cell under conditions suitable for expression
of the antibody,
and isolating the resulting antibody.
In another embodiment, the invention relates to method for producing a mixture
of
recombinant anti-HER3 antibodies comprising at least first and second
recombinant anti-HER3
antibodies as described herein, the method comprising providing at least a
first host cell and a
second host cell, wherein the first and second host cells each are capable of
expressing a
recombinant anti-HER3 antibody, cultivating the first and second host cells
under conditions
suitable for expression of the first and second antibodies, and isolating the
resulting first and
second antibodies.
An antibody or antibody composition of the present invention may be produced
by methods
generally known in the art for production of recombinant monoclonal or
polyclonal antibodies.
28
CA 2816520 2017-04-28
Thus, in the case of production of a single antibody of the invention, any
method known in
the art for production of recombinant monoclonal antibodies may be used. For
production of
an antibody composition comprising two or more anti-HER3 antibodies of the
invention, the
individual antibodies may be produced separately, i.e. each antibody being
produced in a
separate bioreactor, or the individual antibodies may be produced together in
single
bioreactor. When the number of different antibodies in a composition is more
than e.g. two or
three, it will generally be preferably for reasons of cost efficiency to
produce the antibodies
together in a single bioreactor. On the other hand, when the composition only
contains a
small number of different antibodies, e.g. two, three or possibly four
different antibodies, a
decision to produce them separately in different bioreactors or together in a
single bioreactor
will have to be made based on the individual circumstances. If the antibody
composition is
produced in more than one bioreactor, the purified anti-HER3 antibody
composition can be
obtained by pooling the antibodies obtained from individually purified
supernatants from each
bioreactor. Various approaches for production of a polyclonal antibody
composition in
multiple bioreactors, where the cell lines or antibody preparations are
combined at a later
point upstream or prior to or during downstream processing, are described in
WO
2009/129814.
In the case of production of two or more individual antibodies in a single
bioreactor, this may
be performed e.g. as described in WO 2004/061104 or WO 2008/145133. The method
described in WO 2004/061104 is based on site-specific integration of the
antibody coding
sequence into the genome of the individual host cells, ensuring that the VH
and VL protein
chains are maintained in their original pairing during production.
Furthermore, the site-
specific integration minimizes position effects, and therefore the growth and
expression
properties of the individual cells in the polyclonal cell line are expected to
be very similar.
Generally, the method involves the following: i) a host cell with one or more
recombinase
recognition sites; ii) an expression vector with at least one recombinase
recognition site
compatible with that of the host cell; iii) generation of a collection of
expression vectors by
transferring the selected VH and VL coding pairs from the screening vector to
an expression
vector such that a full-length antibody or antibody fragment can be expressed
from the vector
(such a transfer may not be necessary if the screening vector is identical to
the expression
vector); iv) transfection of the host cell with the collection of expression
vectors and a vector
coding for a recombinase capable of combining the recombinase recognition
sites in the
genome of the host cell with that in the vector; v) obtaining/generating a
polyclonal cell line
from the transfected host cell and vi) expressing and collecting the antibody
composition
from the polyclonal cell line.
WO 2008/145133 describes an alternative approach to production of two or more
different
antibodies in a single bioreactor. This method involves generation of a
polyclonal cell line
capable of expressing a polyclonal antibody or other polyclonal protein
comprising two or
more distinct members by a) providing a set of expression vectors, wherein
each of said
vectors comprises at least one copy of a distinct nucleic acid encoding a
distinct member of
the polyclonal protein, separately transfecting host cells with each of the
expression vectors
29
CA 2816520 2017-04-28
under conditions avoiding site-specific integration of the expression vectors
into the genome
of the cells, thereby obtaining two or more compositions of cells, each
composition
expressing one distinct member of the polyclonal protein, and c) mixing the at
least two
compositions of cells to obtain a polyclonal cell line. The methods of WO
2004/061104 and
WO 2008/145133 both have the advantage of allowing all of the members
constituting the
recombinant polyclonal antibody to be produced in a single bioreactor and to
be purified in a
single process, thereby avoiding the need for separate production and
purification processes
for each antibody, while at the same time resulting in a surprisingly uniform
production of the
different antibodies. The method of WO 2008/145133 has the further advantage
of providing
an increased yield, since each production cell can carry multiple copies of
the polynucleotide
encoding a particular antibody.
The antibodies of the invention may be produced in various types of cells,
including
mammalian cells as well as non-mammalian eukaryotic or prokaryotic cells, such
as plant
cells, insect cells, yeast cells, fungi, E. coli etc. However, the antibodies
are preferably
produced in mammalian cells, for example CHO cells, COS cells, BHK cells,
myeloma cells
(e.g. Sp2/0 or NSO cells), fibroblasts such as NIH 3T3, or immortalized human
cells such as
HeLa cells, HEK 293 cells or PER.C6 cells.
Methods for transfecting a nucleic acid sequence into a host cell are well-
known in the art
(see, e.g., Sambrook et al., Molecular Cloning: A Laboratory Manual, Cold
Spring Harbor
Laboratory Press, 3rd Edition, 2001). For site-specific integration e.g. as
described in WO
2004/061104, a suitable host cell will comprise one or more recombinase
recognition sites in
its genome. In this case, a suitable expression vector comprises a
recombination recognition
site matching the recombinase recognition site(s) of the host cell. Further
details regarding
e.g. transfer of selected VH and VL coding pairs from a screening vector using
the site-
specific integration approach may be found in WO 2004/061104.
When an antibody composition of the invention comprising two or more anti-HER3
antibodies
is to be produced in a single bioreactor, cell lines with similar
proliferation rates and
preferably similar antibody expression levels may be selected to generate a
polyclonal cell
line. The polyclonal cell line is then generated by mixing the individual cell
lines in a
predefined ratio. See WO 2009/129814, WO 2004/061104 and WO 2008/145133 for
further
information and examples relating to generating polyclonal cell lines
expressing a polyclonal
antibodies as well as production of polyclonal antibodies using such cell
lines.
One embodiment of the present invention is thus a polyclonal cell line capable
of expressing
two or more anti-HER3 antibodies of the present invention. A further
embodiment is a
polyclonal cell line wherein each individual cell is capable of expressing a
single VH and VL
pair, and the polyclonal cell line as a whole is capable of expressing a
collection of VH and VL
pairs, where each VH and VL pair encodes an anti-HER3 antibody.
CA 2816520 2017-04-28
A recombinant antibody composition of the present invention may be
manufactured in a
single bioreactor by culturing one ampoule from a polyclonal working cell bank
(pWCB) in an
appropriate medium for a period of time to allow for a sufficient level of
antibody expression
while maintaining substantial uniformity in the relative expression levels of
the individual
antibodies expressed by the polyclonal cell line. A production time of between
approximately
15 and 50 days will normally be suitable. Culturing methods known in the art
such as fed
batch or perfusion culturing may be used. The culture medium is preferably a
serum-free
medium, more preferably a serum-free and protein free medium, e.g. a
chemically defined
medium. Such culture media are typically designed for growth of the particular
cell type being
used for production, and numerous suitable media formulations are commercially
available.
The recombinant antibody composition is obtained from the culture medium and
purified by
conventional purification techniques. These may include, for example, affinity
chromatography combined with subsequent purification steps such as ion-
exchange
chromatography, hydrophobic interaction chromatography and gel filtration, as
these
purification techniques have frequently been used for the purification of
recombinant
antibodies. When two or more antibodies are produced by a polyclonal cell line
in a single
bioreactor, the presence of all the individual members in the polyclonal
antibody composition
is typically assessed subsequent to purification, for example by ion-exchange
chromatography. Characterization of a polyclonal antibody composition may be
performed
e.g. as described in WO 2006/007853, WO 2009/065414, WO 2011/042024 and WO
2011/042027.
Therapeutic compositions
Another aspect of the invention is a pharmaceutical composition comprising as
an active
ingredient at least one anti-HER3 antibody of the invention, or an anti-HER3
recombinant
Fab or another anti-HER3 recombinant antibody fragment composition.
Preferably, the active
ingredient of such a pharmaceutical composition is an anti-HER3 recombinant
antibody
composition as described above comprising two or more anti-HER3 antibodies.
Such
compositions are intended for amelioration, prevention and/or treatment of
cancer. The
pharmaceutical composition may be administered to a human or to a domestic
animal or pet,
but will typically be administered to humans.
The ratio between the individual antibodies in a therapeutic composition of
the invention, or,
in the case of individual antibodies of the invention being administered
simultaneously,
sequentially or separately, the ratio between the antibodies to be
administered, will often be
such that the antibodies are administered in equal amounts, but this need not
necessarily be
the case. Thus, a composition of the invention comprising two anti-HER3
antibodies will often
contain them in a 1:1 ratio, and a composition comprising three anti-HER3
antibodies will
often contain them in a 1:1:1 ratio. Depending on the characteristics of the
individual
antibodies, however, it may be desirable to use non-equal amounts of the
different
antibodies. Suitable ratios for the different anti-HER3 antibodies in
compositions of the
invention may be determined as described in WO 2010/040356, which describes
methods for
31
CA 2816520 2017-04-28
identifying and selecting the optimal stoichiometric ratio between chemical
entities in a
combinatorial drug product, e.g. a polyclonal antibody composition, to obtain
a combinatorial
drug with optimal potency and efficacy.
In addition to at least one antibody of the invention or fragment thereof, the
pharmaceutical
composition will further comprise at least one pharmaceutically acceptable
diluent, carrier or
excipient. These may for example include preservatives, stabilizers,
surfactants/wetting
agents, emulsifying agents, solubilizers, salts for regulating the osmotic
pressure and/or
buffers. Solutions or suspensions may further comprise viscosity-increasing
substances,
such as sodium carboxymethylcellulose, carboxymethylcellulose, dextran,
polyvinylpyrrolidone or gelatin. A suitable pH value for the pharmaceutical
composition will
generally be in the range of about 5.5 to 8.5, such as about 6 to 8, e.g.
about 7, maintained
where appropriate by use of a buffer.
Conventional pharmaceutical practice may be employed to provide suitable
formulations or
compositions to administer to e.g. cancer patients. The administration will
typically be
therapeutic, meaning that it is administered after a cancer condition has been
diagnosed.
Any appropriate route of administration may be employed, for example
parenteral,
intravenous, intra-arterial, subcutaneous, intramuscular, intraperitoneal,
intranasal, aerosol,
suppository or oral administration. Pharmaceutical compositions of the
invention will typically
be administered in the form of liquid solutions or suspensions, more typically
aqueous
solutions or suspensions, in particular isotonic aqueous solutions or
suspensions.
The pharmaceutical compositions of the invention are prepared in a manner
known per se,
for example, by means of conventional dissolving, lyophilizing, mixing,
granulating or
confectioning processes. The pharmaceutical compositions may be formulated
according to
conventional pharmaceutical practice (see, for example, Remington: The Science
and
Practice of Pharmacy (21st edition), ed. A.R. Gennaro, 2005, Lippincott
Williams & Wilkins,
Philadelphia, PA, USA; and Encyclopedia of Pharmaceutical Technology, ed. J.
Swarbrick,
3rd edition, 2006, lnforma Healthcare, New York, NY, USA).
32
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
As an alternative to a liquid formulation, the compositions of the invention
may be prepared in
lyophilized form comprising the at least one antibody alone or together with a
carrier, for
example mannitol, in which case the composition is reconstituted with a liquid
such as sterile
water prior to use.
The pharmaceutical compositions comprise from approximately 1% to
approximately 95%,
preferably from approximately 20% to approximately 90%, active ingredient.
Pharmaceutical
compositions according to the invention may e.g. be produced in unit dose
form, such as in
the form of ampoules, vials, suppositories, tablets or capsules. The
formulations can be
to administered to human individuals in therapeutically or prophylactically
effective amounts
(e.g., amounts which prevent, eliminate, or reduce a pathological condition)
to provide
therapy for a cancerous disease or other condition. The preferred dosage of
therapeutic agent
to be administered is likely to depend on such variables as the severity of
the cancer, the
overall health status of the particular patient, the formulation of the
compound excipients, and
its route of administration.
Therapeutic uses of antibodies and compositions according to the invention
The anti-HER3 antibodies and pharmaceutical compositions according to the
present invention
may be used for the treatment or amelioration of a disease in a mammal, in
particular
treatment of cancer in humans. One embodiment of the invention is a method of
preventing,
treating or ameliorating one or more symptoms associated with cancer in a
human or other
mammal, comprising administering an effective amount of an anti-HER3
recombinant antibody
composition of the present invention to said mammal.
A particular embodiment relates to a method for treating a human patient with
a disorder
characterized by expression of HER3, in particular cancer, the method
comprising
administering to said patient a recombinant anti-HER3 antibody as defined
herein or,
preferably, a recombinant antibody composition comprising at least two anti-
HER3 antibodies
as defined herein.
An additional embodiment relates to a method for reducing heterodimer
formation between
HER3 and other ErbB family receptors in cells that express HER3, the method
comprising
contacting said cells with a recombinant anti-HER3 antibody as defined herein
or, preferably, a
recombinant antibody composition comprising at least two anti-HER3 antibodies
as defined
herein.
A further embodiment of the present invention is the use of an anti-HER3
recombinant
antibody or antibody composition of the present invention for the preparation
of a composition
for the treatment, amelioration or prevention of one or more symptoms
associated with cancer
33
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
in a human or other mammal, e.g. for treatment of a human patient with a
disorder
characterized by expression of HER3.
Based upon a number of factors, including HER3 expression levels, the
following tumor types
in particular may be indicated for treatment with an antibody composition of
the invention:
breast, ovarian, gastric, colon, rectum, prostate, bladder, pancreas, head and
neck, and non-
small cell lung cancer. Antibody compositions of the invention are
contemplated to be
particularly applicable to treatment of cancers that express HER3, for example
certain
epithelial cancers such as many breast cancers, ovarian cancers and gastric
(stomach)
to cancers.
In connection with each of these indications, two main clinical pathways are
contemplated,
namely 1) adjunctive therapy in connection with at least one additional
therapeutic treatment
or 2) as a monotherapy. These two options are briefly discussed below.
1) Adjunctive therapy: In adjunctive therapy, also known as combination
therapy, patients will
be treated with antibodies of the present invention in combination with at
least one additional
therapeutic treatment, typically a chemotherapeutic or antineoplastic agent
and/or radiation
therapy. Alternatively or additionally, the anti-HER3 antibodies and
compositions of the
invention may also be used in combination with a different anti-cancer
antibody, e.g. an
antibody targeting EGFR or VEGF. The primary cancer targets listed above may
thus be
treated by administration of an antibody or composition of the invention in
addition to
standard first line and second line therapy. Protocol designs will address
effectiveness as
assessed e.g. by reduction in tumor mass as well as the ability to reduce
usual doses of
standard chemotherapy. Such dosage reductions may allow additional and/or
prolonged
therapy by reducing dose-related toxicity of the chemotherapeutic agent.
By combining the antibody compositions of the invention with agents known to
induce terminal
differentiation of cancer cells, the effect may be improved further. Such
compounds may, for
example, be selected from the group consisting of retinoic acid, trans-
retinoic acids, cis-
retinoic acids, phenylbutyrate, nerve growth factor, dimethyl sulfoxide,
active form vitamin
D3, peroxisome proliferator-activated receptor gamma, 12-0-
tetradecanoylphorbol 13-
acetate, hexamethylene-bis-acetamide, transforming growth factor-beta, butyric
acid, cyclic
AMP, and vesnarinone. Preferably, the compound is selected from the group
consisting of
retinoic acid, phenylbutyrate, all-trans-retinoic acid, active form vitamin D.
Pharmaceutical articles comprising an antibody composition of the invention
and at least one
chemotherapeutic or antineoplastic compound may be used as a combination
treatment for
the simultaneous, separate or successive administration in cancer therapy. The
chemotherapeutic compound may by any chemotherapeutic agent suitable for
treatment of
34
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
the particular cancer in question, for example an agent selected from the
group consisting of
alkylating agents, for example platinum derivatives such as cisplatin,
carboplatin or
oxaliplatin; plant alkoids, for example paclitaxel, docetaxel or irinotecan;
antitumor antibiotics,
for example doxorubicin (adriamycin); topoisomerase inhibitors such as
topotecan; and
antimetabolites, for example fluorouracil or other fluoropyrimidines.
It is also contemplated that antibodies of the invention may be used in
adjunctive therapy in
connection with tyrosine kinase inhibitors (TKIs). These are synthetic, mainly
quinazoline-
derived, low molecular weight molecules that interact with the intracellular
tyrosine kinase
to domain of receptors and inhibiting ligand-induced receptor
phosphorylation by competing for
the intracellular Mg-ATP binding site. Several tyrosine kinase inhibitors that
block EGFR family
receptors are currently in clinical development. For a review of these TKIs
see Spector et al.
(2007) Breast Cancer Res. 9(2): 205. Pharmaceutical articles comprising an
antibody
composition of the invention and at least one TKI targeting HER3 may thus also
be used as a
combination treatment for the simultaneous, separate or successive
administration in cancer
therapy.
In other embodiments, the antibody compositions of the present invention may
be used in
combination with other antibody therapeutics. Examples of these include e.g.
antibodies
against EGFR (Erbitux0 or Vectibix0) or VEGF (AvastinC)), as well as other
anti-RTK
antibodies, for example one or more antibodies against one or more other RTK
targets such as
HER2 or MET. In yet other embodiments, the antibody compositions of the
present invention
may be used in combination with an agent known to stimulate cells of the
immune system,
such combination treatment leading to enhanced immune-mediated enhancement of
the
efficacy of the antibody compositions of the invention. Examples of such
immune-stimulating
agents include recombinant interleukins (e.g. IL-21 and IL-2).
2) Monotherapy: In connection with the use of the antibodies in accordance
with the present
invention in monotherapy of tumors, the antibodies may be administered to
patients without
concurrent use of a chemotherapeutic or antineoplastic agent, i.e. as a stand-
alone therapy.
Immunoconjugates
Another option for therapeutic use of the antibodies and compositions of the
invention is in the
form of immunoconjugates, i.e. antibodies conjugated to one or more anti-
cancer agents. In
particular in the case of compositions comprising two or more individual
antibodies of the
invention that bind distinct HER3 epitopes, it is contemplated that this may
generate a cross-
linked antibody-receptor lattice on the cell surface, thereby potentially
resulting in an
increased level of receptor internalization as compared to the use of a single
monoclonal
antibody. Conjugation of one or more of the individual antibodies of such a
composition to one
ao or more anti-cancer agents therefore has the potential to specifically
and effectively deliver
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
the conjugated anti-cancer agents to the interior of tumor cells, thereby
augmenting the effect
of the anti-HER3 antibodies of the invention to provide an improved tumor cell-
killing activity.
Various types of anti-cancer agents may be conjugated to the antibodies of the
invention,
including cytotoxic agents (including conventional chemotherapy agents and
other small
molecule anti-cancer drugs), cytokines (in which case the conjugate may be
termed an
"immunocytokine"), toxins (in which case the conjugate may be termed an
"immunotoxin")
and radionuclides, and a few immunoconjugates have already been approved for
clinical use.
These include ZevalinC) (a murine anti-CD20 antibody conjugated to 90Y),
BexxarC) (a murine
to anti-CD20 antibody conjugated to 1311) and MylotargC) (a humanized anti-
CD33 antibody
conjugated to calicheamicin). Other immunoconjugates that have been tested in
clinical trials
include antibodies conjugated to e.g. doxorubicin or a maytansinoid compound.
Immunotoxins
that have been tested in clinical trials include several antibodies conjugated
to a truncated
Pseudomonas exotoxin A. An immunocytokine comprising a humanized EpCAM
antibody
conjugated to IL-2 has also been tested.
In the case of antibodies of the invention conjugated to cytotoxic agents,
these may e.g.
belong to any of the major classes of chemotherapy drugs, including alkylating
agents (e.g.
carboplatin, cisplatin, oxaliplatin), antimetabolites (e.g. methotrexate,
capecitabine,
gemcitabine), anthracyclines (e.g. bleomycin, doxorubicin, mitomycin-C) and
plant alkaloids
(e.g. taxanes such as docetaxel and paclitaxel, and vinca alkaloids such as
vinblastine,
vincristine and vinorelbine). Since the use of immunoconjugates specifically
directs the anti-
cancer agent to the tumors, and in particular to the interior of the tumor
cells subsequent to
internalization, immunoconjugates based on the anti-HER3 antibodies of the
invention may
advantageously be based on highly cytotoxic agents such as calicheamicin or
maytansine
derivatives, or on toxins such as bacterial toxins (e.g. Pseudomonas exotoxin
A, diphtheria
toxin) or plant toxins (e.g. ricin).
The conjugated anti-cancer agent in an immunoconjugate is generally linked to
the antibody
by means of a labile linker that is relatively stable in serum but which
allows release of the
agent when the immunoconjugate is internalized into the target cell. Suitable
linkers include,
for example, chemical linkers that are stable at neutral pH in serum but are
subjected to acid
hydrolysis in the mildly acidic conditions within the lysosomes subsequent to
internalization,
disulfide linkers that are cleaved by intracellular thiols, and peptide
linkers that are stable in
serum but which are subjected to enzymatic cleavage in intracellular
compartments.
Various conjugation arrangements can be envisioned in compositions containing
two or more
antibodies of the invention. For example, with two antibodies it would be
possible to conjugate
the antibodies to two or more different anti-cancer drugs or to conjugate one
antibody to a
prodrug which is activated by an agent such as an enzyme conjugated to the
other antibody.
36
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
The general concept of antibody-directed enzyme prodrug therapy (ADEPT) has
been
described for monoclonal antibodies, where a prodrug is activated by an enzyme
targeted to
the tumor by a mAB-enzyme conjugate, but the present invention may provide an
opportunity
for tailoring this approach to particular conditions. It may thus be possible
to specifically
increase tumor cell killing while sparing or reducing damage to normal
tissues.
For further information on anti-cancer immunoconjugates, see Wu et al. (2005)
Nature
Biotechnology 23(9):1137-1146; Schrama et al. (2006) Nature Reviews/Drug
Discovery
5:147-159; and Rohrer (2009) chimica oggi/Chemistry Today 27(5):56-60.
Dose and Route of Administration
The antibodies and compositions of the invention will be administered in an
effective amount
for treatment of the condition in question, i.e. at dosages and for periods of
time necessary to
achieve a desired result. A therapeutically effective amount may vary
according to factors such
as the particular condition being treated, the age, sex and weight of the
patient, and whether
the anti-HER3 antibodies are being administered as a stand-alone treatment or
in combination
with one or more additional anti-cancer treatments.
An effective amount for tumor therapy may be measured by its ability to
stabilize disease
progression and/or ameliorate symptoms in a patient, and preferably to reverse
disease
progression, e.g. by reducing tumor size. The ability of an antibody or
composition of the
invention to inhibit cancer may be evaluated by in vitro assays, e.g. as
described in the
examples, as well as in suitable animal models that are predictive of the
efficacy in human
tumors. Suitable dosage regimens will be selected in order to provide an
optimum therapeutic
response in each particular situation, for example, administered as a single
bolus or as a
continuous infusion, and with possible adjustment of the dosage as indicated
by the exigencies
of each case.
While specific dosing for antibodies in accordance with the invention has not
yet been
determined, certain dosing considerations can be determined through comparison
with a
similar product (e.g. a monoclonal antibody directed against HER2 or EGFR)
that has been
approved for therapeutic use. It is thus contemplated that an appropriate
dosage of an
antibody composition of the invention will be similar to the recommended
dosage for the anti-
HER2 monoclonal antibody trastuzumab (HerceptinC)) or the anti-EGFR monoclonal
antibody
panitumumab (VectibixC)). Depending on the particular condition, HerceptinC)
is administered
(by way of infusion) for treatment of breast cancer at either an initial dose
of 4 mg/kg and
subsequent weekly doses of 2 mg/kg, or an initial dose of 8 mg/kg and
subsequent doses of 6
mg/kg every three weeks, while Vectibix is administered at a dose of 6 mg/kg
every 14
days.
37
CA 02816520 2016-10-26
It is contemplated that a suitable dose of an antibody composition of the
invention will be in the
range of 0.1-100 mg/kg, such as about 0.5-50 mg/kg, e.g. about 1-20 mg/kg. The
antibody
composition may for example be administered in a dosage of at least 0.25
mg/kg, e.g. at least
0.5 mg/kg, such as at least 1 mg/kg, e.g. at least 1.5 mg/kg, such as at least
2 mg/kg, e.g. at
least 3 mg/kg, such as at least 4 mg/kg, e.g. at least 5 mg/kg; and e.g. up to
at most 50
mg/kg, such as up to at the most 30 mg/kg, e.g. up to at the most 20 mg/kg,
such as up to at
the most 15 mg/kg. Administration will normally be repeated at suitable
intervals, e.g. once
every week, once every two weeks, once every three weeks, or once every four
weeks, and for
as long as deemed appropriate by the responsible doctor, who may optionally
increase or
decrease the dosage as necessary.
Three distinct delivery approaches are contemplated for delivery of the
antibodies of the
invention. Conventional intravenous delivery will presumably be the standard
delivery technique
for the majority of tumors. However, in connection with tumors in the
peritoneal cavity, such as
tumors of the ovaries, biliary duct, other ducts, and the like,
intraperitoneal administration may
prove favorable for obtaining high dose of antibody at the tumor and to
minimize antibody
clearance. Similarly, certain solid tumors possess vasculature that is
appropriate for regional
perfusion. Regional perfusion may allow the obtainment of a high dose of the
antibody at the
site of a tumor and minimize short term clearance of the antibody.
As with any protein or antibody infusion-based therapeutic product, safety
concerns are related
primarily to (i) cytokine release syndrome, i.e. hypotension, fever, shaking,
chills, (ii) the
development of an immunogenic response to the protein (i.e. development of
human antibodies
.by the patient to the recombinant antibody product), and (iii) toxicity to
normal cells that
express the HER3 receptor. Standard tests and follow-up procedures are
utilized to monitor any
such safety concerns.
The invention will be further described in the following non-limiting
examples.
EXAMPLES
Example 1: Cloning of anti-HER3 antibodies
Immunization
Three female mice, one BALB/cJ mouse, one C57BL/6 mouse and one C3H mouse (8-
10 weeks
old), were used for the immunizations. The mice were immunized with
commercially available
HER3 protein (R&D Systems cat. #348-RB). For the first four immunizations,
HER3 protein
38
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
was diluted in PBS and mixed 1:1 (v/v) with Freund's adjuvant. The fifth and
final
immunization was given without adjuvant with the HER3 protein in PBS.
Adjuvant is used to enhance and modulate the immune response. In the first
immunization
Complete Freund's adjuvant (CFA) was used, whereas Incomplete Freund's
adjuvant (IFA) was
used for the second, third and fourth immunizations. IFA is an oil-in-water
emulsion composed
of mineral oils, and CFA is IFA with added heat-killed, dried Mycobacterium
species. Both
adjuvants have a depot effect. The mycobacterium in CFA results in a strong
activation of the
immune system, which leads to long-term persistence of the immune response.
Only stable
to emulsions were administered to mice.
Ten pg recombinant HER3 protein was used for each immunization. In total, the
mice received
five injections. All mice were injected subcutaneously (s.c.) with 200 pl
antigen-adjuvant
emulsion for the first four injections and intraperitoneally (i.p.) with 100
pl antigen in PBS for
the fifth injection. A summary of the immunizations, adjuvants, injection
routes etc. is found in
Table 4.
The mice were sacrificed by cervical dislocation, and the spleens and inguinal
lymph nodes
were harvested. Single cell suspensions were prepared by macerating through a
70 pm cell
strainer (Falcon, BD Biosciences, Cat. No.352350). Cells from the three mice
were pooled, re-
suspended in cold RPMI-1640 with 10% FBS and spun down.
Table 4: Immunization summary.
Day Immuni- Adjuvant Antigen Antigen Dose Route of
zation pg/dose conc. volume administration
pg/mL
0 1st CFA 10 50 200 pl s.c.
21 2nd IFA 10 50 200 pl s.c.
42 3rd IFA 10 50 200 pl s.c.
69 4th IFA 10 50 200 pl s.c.
86 5th PBS 10 100 100 pl i.p.
89 Organ
harvest
FACS sorting of murine plasma cells
To remove red blood cells the pooled cell suspension was lysed in 0.17 M
NH4CI. Following
lysis the cells were washed twice in 2% FBS/PBS. Cells were re-suspended in 1
ml 2%
FBS/PBS, incubated with Fc-block (anti-mouse CD16/CD32, BD Biosciences, Cat.
No.553141)
and washed once. Following re-suspension in 2% FBS/PBS, the cells were stained
with anti-
mouse CD43-FITC (BD Biosciences, Cat. No.553270), anti-mouse CD138-PE (BD
Biosciences,
Cat. No. 553714), anti-mouse IgM-Horizon (BD Biosciences, Cat. No. 560575),
anti-mouse
IgG1-APC (BD Biosciences, Cat. No. 550874), anti-mouse MHC II (I-A/I-Ed)-
biotin (BD
Biosciences, Cat. No. 553622) and anti-mouse B220/CD45R-PerCP (BD Biosciences,
Cat. No.
553093) for 20 min in the dark. Cells were washed, incubated with Streptavidin-
APC-Cy7 (BD
39
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
Blosciences, Cat. No. 554063) for 20 min and washed. Cells were FACS sorted on
a FACSArIaTM
cell sorter. Cells that were B22010wMHCIIIntCD43+CD138+IgM- were single cell
sorted into 384-
well microtiter plates containing PCR reaction buffer. Plates were
centrifuged, frozen and
stored at -80 C.
Linkage of cognate VH and VL pairs
Linkage of VH and VL coding sequences was performed on the single cells gated
as plasma
cells, facilitating cognate pairing of the VH and VL coding sequences. The
procedure utilized a
two-step PCR procedure based on a one-step multiplex overlap-extension RT-PCR
followed by
to a nested PCR. The primer mixes used in the present example only amplify
kappa light chains.
Primers capable of amplifying lambda light chains could, however, be added to
the multiplex
primer mix and nested PCR primer mix if desired. If lambda primers are added,
the sorting
procedure should be adapted such that lambda positive cells are not excluded.
The principle
for linkage of cognate VH and VL sequences is described in detail in WO
2005/042774 and in
Meijer et al. (2006) J Mol Biol. 358(3):764-72.
96-well PCR plates were thawed and the sorted cells served as template for the
multiplex
overlap-extension RT-PCR. The sorting buffer added to each well before the
single-cell sorting
contained reaction buffer (OneStep RT-PCR Buffer; Qiagen), primers for RT-PCR
and RNase
inhibitor (RNasin, Promega). The primers used for the overlap extension RT-PCR
as well as the
primer concentrations were the same as those listed in Table 3 of WO
2008/104183. This was
supplemented with OneStep RT-PCR5Enzyme Mix (25x dilution; Qiagen) and dNTP
mix (200
pM each) to obtain the given final concentration in a 20 pl reaction volume.
The plates were
incubated for 30 min at 55 C to allow for reverse transcription (RT) of the
RNA from each cell.
Following the RT, the plates were subjected to the following PCR cycle: 10 min
at 94 C,
35x(40 sec at 94 C, 40 sec at 60 C, 5 min at 72 C), 10 min at 72 C.
The PCR reactions were performed in a H2OBIT Thermal Cycler with a Peel Seal
Basket for 24
96-well plates (ABgene) to facilitate a high throughput. The PCR plates were
stored at -20 C
after cycling.
For the nested PCR step, 96-well PCR plates were prepared with the following
mixture in each
well (20 pl reactions) to obtain the given final concentration: lx FastStart
buffer (Roche),
dNTP mix (200 pM each), nested primer mix, Phusion DNA Polymerase (0.08 U;
Finnzymes)
and FastStart High Fidelity Enzyme Blend (0.8 U; Roche). The primers used for
the nested PCR
as well as the primer concentrations were the same as those listed in Table 4
of WO
2008/104183. As template for the nested PCR, 1 pl was transferred from the
multiplex
overlap-extension PCR reactions. The nested PCR plates were subjected to the
following
thermocyling: 35x(30 sec at 95 C, 30 sec at 60 C, 90 sec at 72 C), 10 min at
72 C.
Randomly selected reactions were analyzed on a 1% agarose gel to verify the
presence of an
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
overlap-extension fragment of approximately 890 basepairs (bp). The plates
were stored at
-20 C until further processing of the PCR fragments.
The repertoires of linked VH and VL coding pairs from the nested PCR were
pooled, without
mixing pairs from different donors, and were purified by preparative 1%
agarose gel
electrophoresis. The human kappa constant light chain encoding sequence was
spliced by
overlap extension to the VL coding region of the pooled PCR products of linked
VH and VL
coding pairs as described in WO 2008/104183. The human kappa constant light
chain
encoding sequence was amplified from a plasmid containing the coding sequence
of a human
to antibody with a kappa light chain in a reaction containing: Phusion
Enzyme (2 U; Finnzymes),
lx Phusion buffer, dNTP mix (200 pM each), hKCforw-v2 primer and Kappa3'
primer (see
Table 5 of WO 2008/104183 for primers and concentrations used), and plasmid
template
pLL138 (10 ng/pl) in a total volume of 50 pl. The reaction was subjected to
the following
thermocycling: 25x(30 sec at 95 C, 30 sec at 55 C, 45 sec at 72 C), 10 min at
72 C. The
resulting PCR fragment was purified by preparative 1% agarose gel
electrophoresis.
The purified pooled PCR fragments from each repertoire were spliced to the
amplified and
purified PCR fragment of the human kappa constant encoding region (SEQ ID
NO:42) by the
following splicing by overlap extension PCR (50 pl total volume) containing:
human kappa
constant encoding region fragment (1.4 ng/pl), purified pooled PCR fragment
(1.4 ng/pl),
Phusion DNA Polymerase (0.5 U; Finnzymes) and FastStart High Fidelity Enzyme
Blend (0.2 U;
Roche), lx FastStart buffer (Roche), dNTP mix (200 pM each), mhKCrev primer
and m31-I set
primers (see Table 5 of WO 2008/104183 for primers and concentrations used).
The reaction
was subjected to the following thermocycling: 2 min at 95 C, 25x(30 sec at 95
C, 30 sec at
55 C, 1 min at 72 C), 10 min at 72 C. The resulting PCR fragment (approx. 4518
bp) was
purified by preparative 1% agarose gel electrophoresis.
Insertion of cognate VH and VL coding pairs into a screening vector
In order to identify antibodies with binding specificity to HER3, the VH and
VL coding sequences
obtained were expressed as full-length antibodies. This involved insertion of
the repertoire of
VH and VL coding pairs into an expression vector and transfection into a host
cell.
A two-step cloning procedure was employed for generation of a repertoire of
expression
vectors containing the linked VH and VL coding pairs. Statistically, if the
repertoire of
expression vectors contains ten times as many recombinant plasmids as the
number of
cognate paired VH and VL PCR products used for generation of the screening
repertoire, there
is a 99% likelihood that all unique gene pairs will be represented. Thus, if
400 overlap-
extension V-gene fragments were obtained, a repertoire of at least 4000 clones
would be
generated for screening to have a 99% likelihood of obtaining all unique gene
pairs.
41
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
Briefly, the purified PCR products of the repertoires of linked VH and VL
coding pairs, spliced to
the human kappa constant coding region, were cleaved with Xhol and Notl DNA
endonucleases at the recognition sites introduced into the termini of PCR
products. The
cleaved and purified fragments were ligated into an XhollNotl digested
mammalian IgG
expression vector, 00-VP-002 (described in WO 2008/104183), by standard
ligation
procedures. The ligation mix was electroporated into E. coli and added to 2xYT
plates
containing the appropriate antibiotic and incubated at 37 C overnight. The
amplified repertoire
of vectors was purified from cells recovered from the plates using standard
DNA purification
methods (Qiagen). The plasmids were prepared for insertion of promoter-leader
fragments by
to cleavage using Ascl and Nhel endonucleases. The restriction sites for
these enzymes were
located between the VH and VL coding gene pairs. Following purification of the
vector, an Ascl-
Nhel digested bi-directional mammalian promoter-leader fragment was inserted
into the Ascl
and Nhel restriction sites by standard ligation procedures. The ligated vector
was amplified in
E. coli and the plasmid was purified using standard methods. The generated
repertoire of
screening vectors was transformed into E. coli by conventional procedures.
Colonies obtained
were consolidated into 384-well master plates and stored.
A two-step procedure was employed for amplification of mammalian expression
plasmids.
First, bacteria were lysed and DNA was denatured by incubation in sodium
hydroxide.
Subsequently, the TempliPhi amplification was performed (GE Amersham). This
method
utilizes bacteriophage (1)29 DNA polymerase to exponentially amplify double-
stranded circular
DNA templates by rolling circle amplification. For antibody expression in
mammalian cells, the
293FreestyleTM expression system (Invitrogen) was applied using standard
transfection
conditions as recommended by the manufacturer. The cells were supplemented
with valproate
to 50mM prior to transfection and the next day Tryptone N1 was added to a
final concentration
of 1.5% (w/v) of the transfection volume. Supernatants containing antibodies
were harvested
six days post transfection. Expression levels were estimated with standard
anti-IgG ELISA.
Screening for binding to recombinant HER3 protein (ELISA)
Antibody specificity was determined by ELISA using recombinant HER3-protein as
antigen.
Briefly, Nunc Maxisorb plates (cat.# 464718) were coated with 1 pg/m1 HER3
protein (R&D
Systems cat.#348-RB), diluted in PBS at 4 C overnight. Prior to blocking in 50
pl 2% milk-
PBS + 0.05% Tween 20 the plates were washed once with PBS-T. The plates were
washed
once with PBS-T and 20 pl of 2% milk-PBS-T, and 10 pl supernatants from
FreeStyle293
transfectants were added and incubated for 1 hour at room temperature, after
which the
plates were washed once with PBS-T, 20 pl per well. Secondary antibody (HRP-
Goat-anti-
human kappa light chain, Serotec, cat.# STAR 100P) diluted 1:25000 in 2% milk-
PBS-T was
added to detect the antibodies bound to the wells and incubated for 1 hour at
room
temperature. The plates were washed once in PBS-T before addition of 25 pl
substrate (Kem-
42
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
En-Tec Diagnostics, cat.# 4518) that was incubated for 5 min. 25 pl 1M
sulphuric acid was
added after the incubation to stop the reaction. Specific signal was detected
on an ELISA
reader at 450 nm. From the ELISA data 480 positive antibody clones were
identified and
selected for sequence analysis and validation of binding to HER3.
Sequence analysis and clone selection
The clones identified as binding to HER3 by ELISA were retrieved from the
original master
plates (384-well format) and streaked on agar plates to generate single
colonies, which were
picked to LB-medium cultures and incubated at 37 C overnight with vigorous
shaking. Plasmid
to DNA was isolated from the clones using Qiaprep 96 turbo mini-prep kit
(Qiagen, cat. # 27193)
and submitted for DNA sequencing of the V-genes. The sequences were aligned
and all the
unique clones were selected. Multiple alignments of obtained sequences
revealed the
uniqueness of each particular clone and allowed for identification of unique
antibodies.
Following sequence analysis of the sequenced clones, 33 clusters of related
sequences with
two to over 40 members as well as over 20 clonotypes that were only
represented once were
identified. Each cluster of related sequences has probably been derived
through somatic
hypermutations of a common precursor clone. Overall, one to two clones from
each cluster
were chosen for validation of sequence and specificity. Based on the cluster
analysis, 119
clones were selected for small-scale expression and further characterization.
Sequences of
selected antibody variable regions are shown in the accompanying sequence
listing. As
explained above, the light chain sequences shown in the sequence listing all
include the same
human kappa constant region, which starts with amino acids -TVAAP- and ends at
the C-
terminal -NRGEC. In order to validate the antibody encoding clones, DNA
plasmid was
prepared and transfection of FreeStyle CHO-S cells (Invitrogen) at 2 ml scale
was performed
for expression. The supernatants were harvested 6 days after transfection.
Expression levels
were estimated with standard anti-IgG ELISA, and the specificity was
determined by HER3
specific ELISA as described above in "Screening for binding to recombinant
HER3 protein" and
by high throughput screening confocal microscopy of antibody binding to HER3
overexpressing
cells (see below).
Screening for binding to HER3 overexpressing cells (OPERA)
The 119 clones were screened for binding to the HER3-overexpressing breast
cancer cell line
(MCF-7) using confocal microscopy. 10,000 MCF-7 cells were seeded into each
well of 384-well
cell carrier plates (Perkin Elmer, cat.# 6007439) and allowed to attach
overnight. The media
was again discarded and the cells were washed and fixed with 2% formaldehyde
solution
(Aldrich cat.# 533998). After washing, 40 pl of antibody supernatant was
transferred to each
well and plates were incubated for 2 hours, after which the media in the wells
was discarded
and 30 pl new media containing 2 pg/ml of Alexa-488 labelled goat anti-human
IgG (H+L,
Invitrogen cat.# A11013), 2 pg/ml CellMask Blue (Invitrogen cat.# H34558) and
1 pM
Hoechst 33342 (Invitrogen cat.# H3570) was added to each well and plates were
incubated
43
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
for another 30 minutes. The level of fluorescence was then measured using an
OPERA high
throughput confocal microscope (Perkin Elmer).
From the binding data obtained by ELISA and OPERA validation screens, 64
clones were
selected for medium scale expression.
Example 2: Functional characterization of selected anti-HER3 antibodies
67 unique antibodies were selected for functional testing using a viability
assay. Cellular
to damage will inevitably result in loss of the ability of the cell to
maintain and provide energy for
metabolic cell function and growth. Metabolic activity assays are based on
this premise,
usually measuring mitochondrial activity. The cell proliferation reagent WST-1
(Roche Cat. No.
11 644 807 001) is a ready-to-use substrate which measures the metabolic
activity of viable
cells. In this example the WST-1 assay was used to measure the number of
metabolically
active cells after treatment of cancer cells with 2 pg/ml of different anti-
HER3 antibodies for
96 hours.
The cancer cell lines MDA-MB-175 (ATCC cat.# HTB-25), A431NS (ATCC cat.# CRL-
2592),
MCF-7 (ATCC cat.# HTB-22) and MDA-MB-453 (ATCC cat.# HTB-130) were seeded into
96-
well plates at a concentration of 1000 cells/well in media containing 2 pg/ml
of anti-HER3
antibody. The plates were incubated for 4 days in a humidified incubator at 37
C. 20 pl of
WST-1 reagent was then added per well and the plates were incubated for one
hour at 37 C.
Plates were then transferred to a orbital plate shaker and left for another
hour. The
absorbance was measured at 450 nm and 620 nm (reference wavelength) on an
ELISA reader.
The difference in the levels of metabolically active cells (MAC) was
calculated as percent of the
control supernatants as follows:
(OD exp ¨ 0Dmedia)
%MAC ¨ 1 lx 00
(ODuntreat.¨ ODinedia)
It is assumed that the metabolic activity correlates with the number of viable
cells, a lower
%MAC corresponding to a higher level of cell growth inhibition by the
antibodies.
The results of this analysis for selected antibodies are shown in Table 5
below, where data is
provided for the individual cancer cell lines as well as the median level of
inhibition across the
four cell lines. It is evident from these results that HER3 antibodies with a
range of functional
activities have been identified and that the antibodies in the repertoire
exhibit an inhibitory
effect on all or most of the tested cancer cell lines.
44
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
Table 5. Percent metabolically active cells (MAC) in the presence of anti-HER3
antibodies
Antibody No. MDA-MB-175 MCF-7 + MDA-
453 A431N5 Median
1 nM Heregulin
4785 60 80 53 70 65
4889 50 76 52 80 64
4935 74 95 67 86 80
5038 78 76 65 83 77
5082 43 60 66 67 63
5101 65 88 80 82 81
5106 57 87 78 87 82
5143 73 87 69 78 75
5144 79 89 73 79 79
5259 72 90 81 77 79
Dose-response curves were generated for the ten antibodies in Table 5 using
the cell line
MDA-MB-175, which is the most sensitive to HER3 inhibition; see Figures 1-10,
which show
metabolic activity of MDA-MB-175 cells treated with different concentrations
of the indicated
antibodies for 96 hours. All tested antibodies block proliferation of MDA-MB-
175 cells, but on
the basis of the in vivo data shown in Figures 1-10 the antibodies 5101 and
5106 appear to be
the most efficacious.
to
Example 3: Inhibition of HER3 phosphorylation by anti-HER3 antibodies
This example demonstrates that anti-HER3 antibodies are able to inhibit ligand-
induced
phosphorylation of HER3.
Methods
In order to investigate the level of HER3 phosphorylation in cell lines
treated with anti-HER3
antibodies, western blot analyses were performed on whole cell lysates of MDA-
MB-175 and
MCF7 cells that were pre-treated with the antibodies for 1 hour and then
stimulated with
10 nM of heregulin beta. Cells were grown in T-75 culture flasks and at 80%
confluency the
culture media were removed, and the cells were washed in 1xPBS and treated
with 10 pg/ml
of the antibodies diluted in 5 ml medium containing 0.5% FBS. Cells were
treated for one
hour, after which whole cell lysates were prepared using standard RIPA buffer.
The total
protein concentration was determined in each sample and 10 pg protein was
analyzed by
western blotting using primary antibody against phosphorylated HER3 (pHER3).
Results
The results of western blot analyses of phospho-HER3 levels in the cell lines
MDA-MB-175 and
MCF7 after 1 hour of pre-treatment with the indicated antibodies, followed by
stimulation with
10 nM heregulin beta, are shown in Figure 11. The different anti-HER3
antibodies inhibited
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
ligand-induced HER3 phosphorylation to various degrees, with the best
antibodies in this assay
being 5101, 5106, 5259 and 4889. Antibodies 5038 and 5143 had only limited
effect on the
levels of phosphorylated HER3 in these cell lines.
Example 4: Functional characterization of mixtures of two anti-HER3 antibodies
This example describes in vitro testing of all possible mixtures of two
antibodies among ten
selected anti-HER3 antibodies of the invention with confirmed binding to human
HER3. The
antibody mixtures were evaluated for their ability to inhibit the growth of
four different cancer
to cell lines: MDA-MB-175, MCF7 (+ 1 nM Heregulin beta), H1437 (+ 1 nM
Heregulin beta) and
A431NS (1 pg/ml of anti-EGFR mixture 992+1024). The anti-EGFR mixture 992+1024
is
contains equal amounts of the two anti-EGFR antibodies referred to as 992 and
1024 as
described in WO 2008/104183.
Methods
Antibodies 4785, 4889, 4935, 5038, 5082, 5101, 5106, 5143, 5144 and 5259, each
of which
had confirmed binding to the human HER3 receptor, were tested in all possible
mixtures of two
antibodies in order to identify antibody mixtures with optimal efficacy. The
methods used, e.g.
for preparing the different antibody combinations in the 384-well plates, were
those generally
described in WO 2010/040356. Further details are provided below.
Mixtures of two antibodies
The ten antibodies were diluted to a concentration of 25 pg/ml in 1xPBS, and
100 pl of
antibody solution was added to the wells of 384-well feeder plates for use in
preparing
mixtures of two antibodies for testing.
For each of the four cell lines tested, two separate 384-well plates were
used, with 46 pl of
media containing cells being added to the wells. A Biomek 3000 laboratory
automation
workstation (Beckman Coulter) was used to add 2 pl of each of two different
antibodies from
the feeder plates to the wells of the 384-well plates containing media +
cells, such that all
combinations of two different antibodies were represented. In addition, the
plates included
media control wells (50 pl 1xPBS media; no cells), untreated control wells (50
pl 1xPBS media
+ cells; no antibody) , and wells containing (in addition to 46 pl of media +
cells) 4 pl of
media with only one of the ten antibodies of the invention as an additional
control.
The plates with wells containing mixtures of two antibodies, as well as media
and untreated
control wells or a single antibody of the invention, were incubated for 4 days
in a humidified
incubator at 37 C, after which 5 pl of the cell proliferation reagent WST-1
diluted 1:1 in 1xPBS
was added to all relevant wells on the plates. The plates were then incubated
for 1 hour at
37 C and subsequently transferred to orbital shakers and incubated for another
hour. The
46
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
absorbance was measured at 450 nm and 620 nm (reference wavelength) on an
ELISA reader.
The amount of metabolically active cells (MAC) was calculated as described in
Example 2.
Dose-response curves were generated for selected mixtures (highlighted in bold
in Table 6
below) using the cell lines MDA-MB-175 and MCF-7. Prior to performing the WST-
1 assay, the
appropriate antibodies and antibody mixes were diluted to a final total
antibody concentration
of 100 pg/ml in appropriate media supplemented with 2% of FBS and 1%
Penicillin/Streptomycin (P/S), yielding a final total antibody concentration
of 50 pg/ml in the
well containing the highest antibody concentration. A two-fold serial dilution
of the antibodies
to was then performed. Relevant numbers of cells were then added to the
experimental wells in a
384-well plate. The plates were incubated for 4 days in a humidified incubator
at 37 C. The
amount of metabolically active cells (MAC) was calculated as the percent of
the untreated
control as described in Example 2.
Results
The individual antibodies and mixtures of two antibodies were ranked according
to their
median effect on cell growth, calculated as %MAC. The results are shown below
in Table 6.
The results show that the level of growth inhibition by the various mixtures
varies considerably
between the different cell lines, while the difference in the median %MAC is
less pronounced.
The monoclonal antibody 5082 was found to have the highest level of median
growth inhibition
(lowest %MAC), while several antibody mixtures are superior at inhibiting the
cell line MDA-
MB-175. It should be noted that although the antibodies and antibody mixtures
in Table 6 are
ranked based on the median %MAC for the four cell lines, it is contemplated
that individual
antibody mixtures may be of interest based on an effect demonstrated in any
one or more cell
lines, and that a high level of inhibition (low %MAC) in just a single cell
line may translate into
a highly useful antibody combination in vivo against certain types of cancers.
Dose-response curves were generated for mixtures of two antibodies that bind
non-
overlapping epitopes of HER3 and unique epitope bin combinations (highlighted
in bold). The
results show that all the mixtures inhibit all four cell lines although with
different potency.
Table 6. Level of cancer cell growth inhibition by mixtures of two anti-HER3
antibodies in the
four cancer cell lines MDA-MB-175, MCF71 H1437 and A431NS. The level of
inhibition is shown
as % metabolically active cells (%MAC).
MDA-MB-175 MCF7 +1nM H1437 + A431N5 +
Median
Heregulin beta 1 nM Heregulin 1
pg/ml
beta Sym004*
%MAC SD %MAC SD %MAC SD %MAC SD %MAC
5082 42.7 4.6 55.2 3.5 69.4 5.2 74.5 5.1
62.3
5082+5106 37.1 5.9 55.2 0.9 77.1 3.9 82.9 6.1 66.1
47
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
MDA-MB-175 MCF7 +1nM H1437 + A431N5 + Median
Heregulin beta 1 nM Heregulin 1 pg/m1
beta Syrn004*
%MAC SD %MAC SD %MAC SD %MAC SD %MAC
5082+4889 47.5 7.3 65.8 4.4 72.3 4.7 77.7 6.7
69.0
5082+5101 43.7 11.3 61.6 7.7 76.8 9.8 84.4 8.3
69.2
5082+5259 52.7 3.2 59.3 10.4 79.1 4.5 90.0 8.4
69.2
5082+4935 45.9 5.1 58.7 7.8 79.8 7.2 90.0 8.8
69.2
5082+4785 45.5 10.9 61.9 8.6 77.1 8.5 87.7 10.3 69.5
5082+5143 51.5 4.3 65.4 10.3 75.1 4.2 84.2 5.0
70.2
5082+5144 49.3 5.1 60.8 9.8 80.0 4.2 86.1 6.6 70.4
5082+5038 32.2 7.7 62.7 11.2 79.0 5.6 90.2 8.8 70.9
5038+4889 39.9 6.9 69.2 8.6 77.2 10.2 90.4 13.1 73.2
4785+4889 37.0 1.0 65.2 2.4 81.8 5.1 96.5 14.4
73.5
4935+4889 47.2 9.9 73.3 6.4 82.7 7.3 86.6 11.8
78.0
4889+5143 44.5 2.3 76.1 1.9 82.3 2.4 89.9 8.1 79.2
5038+5143 67.3 6.5 75.3 2.7 84.0 6.4 90.1 9.5
79.6
4889+5259 53.6 4.9 78.2 2.7 81.2 4.3 87.0 8.3
79.7
4785+5038 47.9 6.0 74.2 10.8 86.7 4.8 100.4 2.3 80.5
4785+5259 63.1 10.6 77.1 9.6 83.9 11.3 98.9 4.2 80.5
5106+4889 47.1 3.0 78.4 3.5 83.7 4.6 90.0 8.0 81.1
5101+4889 51.3 5.2 82.3 5.7 84.7 6.0 82.3 14.1
82.3
5038+5259 61.8 5.1 77.7 7.5 86.9 2.9 104.0 5.2 82.3
4785+5106 52.3 6.7 80.9 11.1 84.0 3.2 92.5 7.1
82.4
4889 54.0 18.1 90.8 7.4 83.3 4.2 82.7 5.5
83.0
5143+5259 74.2 9.5 79.6 3.8 87.7 4.7 96.9 3.3
83.6
5101 54.6 6.6 83.7 6.6 100.7 2.5 84.6 8.5
84.1
4785 60.7 11.1 81.2 7.8 88.3 11.1 91.4 6.5 84.8
5259 69.2 10.8 79.4 6.5 90.3 4.2 96.0 7.4
84.8
5038 80.3 19.1 86.3 9.6 84.1 8.9 93.4 2.8
85.2
5144+4889 58.1 5.4 87.0 5.4 86.2 5.4 89.7 11.8
86.6
5038+4935 63.9 4.6 87.7 5.2 85.8 8.6 103.4 7.3 86.8
5038+5101 63.8 8.8 87.9 2.9 86.0 4.4 89.5 8.7
86.9
4785+5143 72.1 11.6 92.3 4.5 82.8 5.1 92.5 5.6
87.6
5038+5106 65.3 4.0 90.7 8.2 84.9 5.2 94.6 7.6
87.8
5101+5106 59.3 4.2 84.6 4.6 102.8 5.5 91.2 6.9
87.9
5143 69.0 13.9 92.7 7.7 84.0 3.8 93.8 3.3
88.4
5101+5259 61.6 12.1 85.0 5.1 99.5 8.8 92.2 4.0
88.6
4935+5259 74.9 10.5 88.4 6.7 89.2 0.7 102.0 2.9 88.8
5038+5144 73.6 9.7 85.1 7.1 93.3 5.2 97.0 6.5
89.2
4785+5101 64.7 13.8 87.8 7.9 91.1 5.1 92.3 7.3
89.5
4785+5144 69.5 6.9 87.1 2.0 93.8 7.2 97.8 3.9
90.5
5106 47.6 10.7 84.3 14.0 97.8 4.9 98.1 3.4
91.0
5106+5143 70.8 5.2 91.0 5.6 91.7 6.4 94.5 3.7
91.4
5101+5143 66.2 10.0 93.0 7.6 93.1 6.3 89.9 5.4
91.4
4785+4935 72.2 6.8 90.6 3.8 93.2 6.8 102.7 5.5 91.9
5106+5259 63.1 4.0 87.9 2.4 98.6 4.4 99.2 3.4
93.2
48
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
MDA-MB-175 MCF7 +1nM H1437 + A431N5 +
Median
Heregulin beta 1 nM Heregulin 1
pg/ml
beta Sym004*
%MAC SD %MAC SD %MAC SD %MAC SD %MAC
4935+5106 65.6 5.6 95.3 14.7 92.1 6.3 99.7
11.1 93.7
5144+5143
80.0 11.1 91.8 5.0 96.2 10.5 98.9 7.5 94.0
4935 78.1 15.0 98.6 12.8 91.1 6.7 99.6
15.0 94.8
4935+5143 87.3 6.4 101.6 11.3 91.6 9.7 99.6
9.4 95.6
5144+5106
62.2 10.4 92.0 3.9 100.1 7.6 101.3 5.4 96.0
4935+5101 72.8 14.8 96.3 5.7 96.2 7.1 100.6
11.3 96.3
5144 72.6 16.4 94.8 11.5 99.2 8.9 99.0
10.9 96.9
5144+5101 62.6 9.9 98.6 1.7 105.6 2.3 97.8 5.1
98.2
5144+5259
79.0 2.0 98.0 7.1 98.8 3.5 102.4 5.4 98.4
4935+5144 81.4 4.8 104.6 16.3 99.5 5.4 99.5
8.7 99.5
* Sym004 is a mixture of two recombinant anti-EGFR antibodies directed against
non-
overlapping EGFR epitopes; see WO 2008/104183 and Pedersen et al. (2010)
Cancer Res.
70(2):588-597.
Figures 12-15 show the metabolic activity of selected mixtures of anti-HER3
antibodies (the
mixtures highlighted in bold in the table above) in the four different cell
lines. Figure 12 shows
the metabolic activity in the MDA-MB-175 cell line, Figure 13 shows activity
in the A431NS cell
line in the presence of 1 pg/ml Sym004, Figure 14 shows the activity in the
MCF7 cell line in
the presence of nM Heregulin beta, and Figure 15 shows the activity in the
H1437 cell line in
to the presence of 1nM Heregulin beta.
Example 5: Functional characterization of mixtures of three anti-HER3
antibodies
This example describes in vitro testing of mixtures of three antibodies with
non-overlapping
epitopes and unique bin combinations among selected anti-HER3 antibodies of
the invention
with confirmed binding to human HER3. The antibody mixtures were evaluated for
their ability
to inhibit the growth of four different cancer cell lines: MDA-MB-175, MCF7 (+
1 nM Heregulin
beta), H1437 ((+ 1 nM Heregulin beta) and A431NS (1 pg/ml of anti-EGFR mixture
992+1024).
Methods
Antibodies 4785, 4889, 5038, 5082, 5106, 5143 and 5259, each of which had
confirmed
binding to the human HER3 receptor, were tested in mixtures of three
antibodies in order to
identify antibody mixtures with optimal efficacy. The selected antibodies and
antibody
mixtures were tested for ability to inhibit the growth and proliferation of
the cancer cell lines
MDA-MB-175, MCF7 (+ 1 nM Heregulin beta), H1437 (+ 1 nM Heregulin beta) and
A431NS (1
pg/ml of anti-EGFR mixture 992+1024) using the WST-1 viability assay as
described in
Example 4.
49
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
Results
All the tested mixtures of three antibodies were found to inhibit all four
cell lines, although
with different potency.
Figures 16-19 show the metabolic activity of different mixtures of three anti-
HER3 antibodies
in the four cancer cell lines. Figure 16 shows the metabolic activity in the
MDA-MB-175 cell
line, Figure 17 shows activity in the A431NS cell line in the presence of 1
pg/ml Sym004,
Figure 18 shows the activity in the MCF7 cell line in the presence of nM
Heregulin beta, and
Figure 19 shows the activity in the H1437 cell line in the presence of 1nM
Heregulin beta.
Example 6: Synergistic inhibition of cancer growth by anti-HER3 2 mixtures
This example demonstrates that certain anti-HER3 antibody mixtures
synergistically inhibit
growth of cancer cells.
Methods
Antibodies 5038, 5082 and 5144, each of which had confirmed binding to the
human HER3
receptor, were tested as mixtures of two antibodies, 5038+5082 and 5082+5144,
in each case
comparing the mixture of two antibodies to the two individual antibodies in
the mixture, in
order to investigate synergistic inhibition of cell growth. The selected
antibodies and antibody
mixtures were tested for their ability to inhibit the growth and proliferation
of the cancer cell
line MDA-MB-175 using the WST-1 viability assay as described in Example 4.
Results
The results show that a mixture of antibodies 5038+5082 or 5082+5144
synergistically inhibit
growth of the cancer cell line MDA-MB-175 (Figures 20 and 21).
Example 7: Comparison of anti-HER3 antibody mixture and reference monoclonal
antibodies
This example describes an in vitro comparison of an anti-HER3 antibody mixture
(5038+5082) and analogues of the reference antibodies pertuzumab and MM-121 in
the cell
lines MDA-MB-175 and MCF-7. Pertuzumab is an anti-HER2 antibody that binds to
the
dimerization arm of HER2 and blocks HER2/HER3 heterodimerization. The
pertuzumab
analogue has the light chain and heavy chain amino acid sequences of
pertuzumab as
disclosed in WO 2006/033700 and US 2006/0121044 A1. MM-121 is an anti-HER3
antibody
that blocks heregulin binding to and hence activation of HER3 (see WO
2010/019952 and
Schoeberl et al., Cancer Res.70(6):2485-94, March 2010).
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
Methods
Dose-response curves were generated as described in Example 4. Analogues of
the reference
anti-HER3 monoclonal antibody MM-121 (Merrimack; sequence disclosed in WO
2008/100624)
and the reference anti-HER2 antibody pertuzumab (also known as OmnitargTM, 2C4
and R-
1273; sequence disclosed in US 2006/121044 A1) were generated by synthesizing
the whole
lambda light chain (MM-121) or kappa light chain (pertuzumab) of the
respective antibodies
without signal peptide, adding flanking NheI and NotI restriction sites, and
cloning into an
expression vector for transient expression in HEK 293 cells. The heavy chain
VH regions of the
respective antibodies were synthesized without signal peptide, after which
AscI and XhoI sites
to were added, and the resulting sequences were cloned into the same
expression vectors used
for light chain expression, which also contained sequences encoding the three
constant IgG
heavy chain domains CH1, CH2 and CH3. The vectors included a pair of CMV
promoters in
head-to-head orientation for expression of the two chains of each antibody.
Results
The results show that the antibody mixture 5038+5082 is superior to the
reference antibody
MM-121 at inhibiting the growth of the two cancer cell lines MDA-MB-175 and
MCF7. No
apparent superiority of 5038+5082 over pertuzumab was found using these cell
lines. The
metabolic activity in MDA-MB-175 cells is shown in Figure 22, and the
metabolic activity in
MCF7 cells in the presence of 1 nm Heregulin beta is shown in Figure 23.
Example 8: Anti-HER3 Antibody Mixture Induces HER3 Degradation
This example demonstrates that a mixture of two anti-HER3 antibodies is able
to induce more
efficacious HER3 degradation compared to individual anti-HER3 antibodies. A
pertuzumab
analogue was also tested in this study.
Methods
In order to investigate the levels of HER3 in cell lines treated with anti-
HER3 antibodies,
western blot analyses were performed on whole cell lysates of OVCAR-8 cells
treated with
antibodies for various times. Cells were grown in T-75 culture flasks and at
80% confluency
the culture media was removed, the cells washed in 1xPBS and treated with 20
pg/ml of the
antibodies diluted in 5 ml medium containing 0.5% FBS. Cells were treated for
1, 2, 4, 16 or
48 hours after which whole cell lysates were prepared using standard RIPA
buffer. The total
protein concentration was determined in each sample and 10 pg protein analyzed
by western
blotting using primary antibody against HER3.
Results
Results from the investigation of HER3 levels (Figure 24) demonstrated that
both the
ao individual antibodies and the mixture induced rapid HER3 degradation.
However, the anti-
51
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
HER3 mixture induced a higher level of HER3 degradation compared to the
individual
antibodies. The pertuzumab analogue was not able to induce HER3 degradation,
which was
expected given that it binds to HER2 and not to HER3.
Example 9: Anti-HER3 Antibody Mixture Inhibits HER3 Signaling
This example demonstrates that a mixture of two anti-HER3 antibodies is able
to induce more
efficacious suppression of HER3 phosphorylation and downstream signaling than
individual
antibodies.
Methods
In order to investigate the levels of HER3 phosphorylation and downstream
signaling, cell lines
were treated with anti-HER3 antibodies for various times, and western blot
analyses were
performed on whole cell lysates. MDA-MB-175 cells were grown in T-75 culture
flasks and at
80% confluency the culture media was removed, after which the cells were
washed in 1xPBS
and treated with 10 pg/ml of the antibodies diluted in 5 ml medium containing
0.5% FBS.
Cells were treated for 2, 4, 16 or 48 hours after which whole cell lysates
were prepared using
standard RIPA buffer. The total protein concentration was determined in each
sample and
10 pg of protein was analyzed by western blotting using primary antibodies
against pHER3,
pAKT(Ser465), AKT and Actin respectively.
Results
Results from the investigation of HER3 signaling (Figure 25) demonstrated that
both the
individual antibodies and the mixture induced rapid inhibition of HER3 and AKT
phosphorylation. However, the anti-HER3 mixture was superior to the individual
antibodies at
inhibiting HER3 and AKT phosphorylation.
Example 10: In vivo efficacy of anti-HER3 mixtures
To evaluate the in vivo efficacy of the anti-HER3 monoclonal antibodies 5038
and 5082 and
the mixture of 5038+5082 the compounds were tested in the A549 lung cancer
xenograft
model.
Methods
2x106 A549 cells were inoculated subcutaneously into the left flank of eight
to ten week old
female athymic nude mice. Tumors were measured twice weekly with calipers and
tumor
volume in mm3 was calculated according to the formula: (width)2x length x 0.5.
At an average
tumor size of 115 mm3 the mice were randomized and treatment was initiated.
The mice were
treated with twice weekly intraperitoneal injections of 50 mg/kg 5038, 5082 or
5038+5082 for
ao five weeks (10 injections in total) followed by an observation period.
The experiment included
52
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
the anti-EGFR monoclonal antibody cetuximab (as an isotype control) and a
vehicle control
that were each dosed and administered following the same schedule as for the
anti-HER3
antibodies.
Results
The results of this study are shown in Figure 26, where it is seen that in
mice treated with
5038 or the combination of 5038+5082 tumor growth was controlled in response
to treatment
from day 26 (treatment start) until day 37. After this point, the tumors in
the two groups
started growing, although growth was slower compared to tumors in the
cetuximab or vehicle
to control groups. While the results after this point are not statistically
significant, there is a clear
tendency that tumors in the 5038 and 5038+5082 treated groups grow slower
compared to
tumors in the control group as well as groups receiving cetuximab or 5082.
Mice treated with
5082 alone or with cetuximab did not respond to treatment and showed tumor
growth kinetics
similar to the vehicle control group.
In summary, the results suggest that 5038 and the combination of 5038+5082
were better at
controlling the growth of the A549 tumor xenografts compared to treatment with
5082 alone,
cetuximab or the vehicle control.
Example 11: Mapping of anti-HER3 antibodies to individual HER3 domains
This example demonstrates that the generated panel of anti-HER3 antibodies
with functional
activity is directed against HER3 extracellular domain (ECD) domain I or
domain II as shown
by the binding profile to human/mouse chimeric receptor constructs.
Methods
Because the anti-HER3 antibody panel was raised in mice, antibodies are not
expected to
recognize epitopes present on murine HER3 due to the concept of "self
tolerance".
Consequently, chimeric receptor constructs in which the human sequences coding
for
individual domains are replaced by murine sequences can be employed for
epitope mapping
purposes, since the antibodies are not expected to bind the murines sequence
inserted in the
human HER3 construct. Human and murine HER3 mRNA sequences (accession number
M29366 and NM_010153.1 respectively) were downloaded from NCBI, and
extracellular
domains I-Iv were assigned as described by Kani et al. (J. Biol. Chem. (2005)
280:8238-
8247). Chimeric human/murine domain exchange variants, in which each of the
four human
extracellular HER3 domains were replaced by the respective murine DNA
sequence, were
provided with an N-terminal histidine tag, gene synthesized and transiently
expressed in Hek
293 cells. A fully human and a murine ECD construct were used as positive and
negative
controls, respectively. Supernatants from the transiently expressed receptor
constructs were
purified by histidine tag affinity chromatography using nickel NTA columns,
and purified
proteins were coated in ELISA plates at 1 pg/ml in carbonate buffer overnight.
The next day
53
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
wells were blocked with 1% BSA/PBS-T and titrations of antibodies diluted in
blocking buffer
were added. Finally, wells were washed, and the ELISA was developed by
addition of mouse
anti-human IgG Fe conjugated to HRP followed by wash and addition of TMB
substrate.
Results
Antibodies 5101 and 5106 were found to have broken tolerance in the course of
immunization
and reacted against murine HER3 (Figures 27-32). But because 5101 and 5106
have lower
ELISA optical density (OD) values on the murine DI - human DII-IV HER3
construct (i.e. a
construct with murine domain I and human domains II-IV) than on other chimeric
constructs
to in which domain I has a human sequence, it can be concluded that these
two antibodies bind
epitopes located within domain I (DI) of human HER3. Antibodies 5144 and 5259
did not
recognize murine DI and bound weakly to murine DII exchange variants. These
two antibodies
are consequently directed against epitopes overlapping with domain I and II on
human HER3.
The rest of the antibodies did not recognize the murine DI exchange variant
but did bind the
other variants and are therefore directed against epitopes on domain I of
Human HER3.
The domain mapping of anti-HER3 antibodies is shown in Figures 27-32, which is
a titration of
anti-HER3 antibodies and negative controls against coated HER3 antigens. Bound
antibody was
detected with an anti-human Fc antibody. High background is observed against
human HER3
Fc, due to cross reactivity between the anti-Fc conjugate and the HER3 Fc
fusion protein.
However, the negative controls clearly demonstrate the difference between the
specific and
nonspecific binding. Table 7 below provides an overview of the HER3 domains
targeted by the
different anti-HER3 antibodies.
Table 7: Domains targeted by different anti-HER3 antibodies
mAb HER3 Domain
4785 DI
4889 DI
4935 DI
5038 DI
5082 DI
5101* DI
5106* DI
5143 DI
5144 DI/DII *Antibodies cross-reacting between
5259 DI/DII human and mouse HER3 ECD
Example 12: Epitope binning of anti-HER3 antibodies by Surface Plasmon
Resonance
This example demonstrates how pairs of anti-HER3 antibodies with functional
activity target at
least five non-overlapping epitope bins on domain I or II of HER3 ECD.
54
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
Methods
Antibody cross-competition analysis was performed by testing antibody pairs
with Surface
Plasmon Resonance (SPR) analysis on a Biacore 2000 instrument (GE Healthcare,
Denmark). A
CM5 sensor chip (GE Healthcare, Denmark) was conjugated with 10,000 Resonance
units (RU)
of an anti-tetra histidine antibody (Qiagen, Germany) according to the
manufacturer's
instructions. Histidine-tagged HER3-Fc fusion protein (R&D Systems) was
diluted in HBS buffer
and captured on an anti-histidine surface at a flow rate of 5 pl/minute.
Antibody combinations
were evaluated in competitive binding experiments at a saturating
concentration of 40 pg/ml
by recording the maximum response levels with and without competition. The
chip surface was
to regenerated by injection of 10 mM Glycine-HCI, pH 2.
Results
The 10 anti-HER3 antibodies tested were found to cluster to 5 different non-
overlapping
epitope bins (Figures 33+34). Epitope bin 1 contained the three antibodies
4785, 4935 and
5143. Epitope bin II contained antibodies 5082 and 4889, of which mAb 5082
also was found
to cross-compete with mAb 5143 from epitope bin 1. Epitope bin III was unique
and contained
only mAb 5038. Epitope bin IV contained mAbs 5101 and 5106, which also cross-
reacted with
mouse HER3 protein (Example 11). Finally, epitope bin V contained antibodies
5144 and 5259,
which were found to bind similar epitopes present on both DI and DII (Example
11).
Figure 33 shows a table with the results of epitope binning by antibody cross-
competition
analysis. The analysis was performed by first saturating HER3 antigen with the
antibodies
listed at the top, followed by injection the antibodies listed on the left.
The numbers in the
cells refer to percent competition, calculated as:
(1- (maximal response with competition/maximal response without competition) x
100
The boxed cells represent antibody pairs that inhibit each other by at least
50%. Antibodies
are assigned into epitope bins according to the competition profile; see
Figure 34, which
provides a graphic illustration of the relationship between assigned epitope
bins, where
overlapping circles represent antibodies with overlapping epitopes.
Example 13: In vivo efficacy of anti-HER3 mixtures
To evaluate the in vivo efficacy of the anti-HER3 monoclonal antibodies 5038
and 5082 and
the mixture of 5038+5082 we tested the compounds in the BxPC3 pancreatic
cancer xenograft
model.
Methods
5x106 BxPC3 cells were inoculated subcutaneously into the left flank of eight
to ten week old
female athymic nude mice. Tumors were measured twice weekly with calipers and
tumor
CA 02816520 2013-04-30
WO 2012/059858 PCT/1B2011/054835
volume in mm3 was calculated according to the formula: (width)2x length x 0.5.
At an average
tumor size of 165 mm3 the mice were randomized and treatment was initiated.
The mice were
treated with twice weekly intraperitoneal injections of 50 mg/kg 5038, 5082 or
a mixture of
5038+5082 for 3 weeks (6 injections in total) followed by an observation
period. The
experiment included a vehicle control, which was dosed and administered
following the same
schedule as described for the anti-HER3 antibodies above.
Results
In mice treated with 5038 or the combination of 5038+5082 the tumor growth
were inhibited
to significantly better compared to mice in the vehicle control group
(Figure 35). In mice treated
with 5038+5082 a significant difference compared to the vehicle control group
was observed
as early as five days after the first treatment and lasted throughout the
study period. Mice
treated with 5038 were significantly better at controlling tumor growth
compared to vehicle
control treated animals from day 57 and, with the exception of one day (day
64), this was
observed throughout the study period.
The effective inhibition of tumor growth by 5038 and the combination of
5038+5082 could
also be observed by looking at survival. Both of these treatments were
significantly better
compared to the vehicle control group as calculated by a Log rank (Mantel Cox)
test with a p-
value of 0.003 for 5038 and 0.001 for 5038+5082 (Figure 36).
In summary, 5038 and the combination of 5038+5082 were significantly better at
inhibiting
tumor growth and improve survival of animals with BxPC3 tumor xenografts
compared to
vehicle control.
56