Note: Descriptions are shown in the official language in which they were submitted.
CA 02817065 2013 05 06
WO 2012/064466 PCT/US2011/056627
SELECTIVE DESULFURIZATION OF FCC GASOLINE
FIELD OF THE DISCLOSURE
[0001] Embodiments disclosed herein generally relate to processes for the
desulfurization of gasoline fractions, such as FCC naphtha, having a high ASTM
D86
end point. More particularly, embodiments disclosed herein relate to processes
for the
desulfurization of high end point naphthas to produce gasoline fractions
having a total
sulfur content of less than 20 ppm, by weight. In some embodiments, the total
sulfur
content of the gasoline fraction may be less than 10 ppm, by weight. Other
embodiments disclosed herein may additionally provide for control of the end
point of
the gasoline product.
BACKGROUND
100021 Petroleum distillate streams contain a variety of organic chemical
components.
Generally the streams are defined by their boiling ranges, which determines
the
composition. The processing of the streams also affects the composition. For
instance, products from either catalytic cracking or thermal cracking
processes
contain high concentrations of olefinic hydrocarbons (alkenes, alkynes, and
polyunsaturated compounds such as diolefins) as well as saturated hydrocarbons
(alkanes). Additionally, these components may be any of the various isomers of
the
compounds.
[0003] The composition of untreated naphtha as it comes from the crude
still, or
straight run naphtha, is primarily influenced by the crude source. Naphthas
from
paraffinic crude sources have more saturated straight chain or cyclic
compounds. As
a general rule most of the "sweet" (low sulfur) crudes and naphthas are
paraffinic.
The naphthenic crudes contain more unsaturated and cyclic and polycylic
compounds.
The higher sulfur content crudes tend to be naphthenic. Treatment of the
different
straight run naphthas may be slightly different depending upon their
composition due
to crude source. FCC gasoline is the product of catalytic cracking and is also
referred
to as catalytically cracked naphtha, which may be further processed. Cracked
gasolines, especially catalytically cracked gasolines, ordinarily have a
sufficiently
high octane, and one of the most important objectives in refining these
involves the
removal of sulfur compounds.
1
CA 02817065 2013 05 06
WO 2012/064466 PCT/US2011/056627
[0004] Reformed naphtha or reformate generally requires no further
treatment except
perhaps distillation or solvent extraction for valuable aromatic product
removal.
Reformed naphthas have essentially no sulfur contaminants due to the severity
of their
pretreatment for the process and the process itself.
[0005] Cracked naphtha as it comes from the catalytic cracker has a
relatively high
octane number as a result of the olefinic and aromatic compounds contained
therein.
In some cases this fraction may contribute as much as half of the gasoline in
the
refinery pool together with a significant portion of the octane. Although
olefin
concentration in gasoline increases the octane number, olefins are often
limited in
their concentration in gasoline as they are a known contributor to smog
formation.
An attractive alternative to increased olefin content is the addition of
alcohols to the
gasoline product to raise the octane number. Alcohols such as methanol and
ethanol
can be used as additives.
[0006] Catalytically cracked naphtha (gasoline boiling range material)
currently
forms a significant part (> 1/3) of the gasoline product pool in the United
States and it
provides the largest portion of the sulfur. The sulfur impurities may require
removal,
usually by hydrotreating, in order to comply with product specifications or to
ensure
compliance with environmental regulations. Some users require the sulfur of
the final
product to be below 50 ppm or at or below 10 ppm.
[0007] Various processes for the desulfurization of gasoline boiling
range
hydrocarbon fractions may include U.S. Patent Nos. 5,510,568, 5,595,634,
5,779,883,
5,597,476, 5,837,130, 6,083,378, 6,946,068, 6,592,750, 6,303,020, 6,413,413,
6,338,793, 6,503,864, 6,495,030, 6,444,118, 6,824,676, 7,351,327, 7,291,258,
7,153,415, 6,984,312, and 7,431,827, among others.
[0008] High end point FCC gasoline typically has a higher sulfur
concentration than
normal boiling range catalytically cracked gasoline, requiring a higher
conversion of
the sulfur compounds to meet the sulfur requirements. However, due to a higher
concentration of multi-substituted benzothiophenes (versus
methylbenzothiophenes in
notinal boiling range catalytically cracked gasoline), hydrotreating high end
point
naphthas becomes more challenging. This is due to the fact that sulfur atoms
in multi-
2
CA 02817065 2015-01-21
substituted benzothiophenes are more hindered and slower to react with
hydrogen
than the sulfur atoms in methylbenzothiophenes.
[0009] In addition to supplying high octane blending components, the
cracked
naphthas are often used as sources of olefins in other processes such as
etherifications,
oligomerizations and alkylations. The conditions of hydrotreating of the
naphtha
fraction to remove sulfur will also saturate some of the olefinic compounds in
the
fraction, reducing the octane and causing a loss of source olefins. Severe
operating
conditions typically used to remove sulfur from high end point fractions may
cause an
excessive loss of olefins.
[0010] Accordingly, there exists a need for processes for the
hydrodesulfurization of
high end point FCC gasoline, including processes which preserve, to an extent,
the
olefinic content of the naphtha, minimizing olefins lost to hydrogenation and
recombinant mercaptan formation during the processing of the naphtha.
SUMMARY OF CLAIMED EMBODIMENTS
[0010a] Certain exemplary embodiments provide a process for the
desulfurization of a
high end point sulfur-containing petroleum fraction comprising the steps of:
(a) feeding (1) a sulfur-containing petroleum fraction containing C5
hydrocarbons,
olefins, diolefins, mercaptans, other organic sulfur compounds, and hindered
sulfur
compounds and having an ASTM D86 end boiling point of at least 450 F, and
(2) hydrogen to a first distillation column reactor, wherein the hydrogen is
fed to the
first distillation column reactor at a rate of 10 to 1000 scf/bbl of the
sulfur-containing
petroleum fraction; (b) concurrently in the first distillation column reactor,
(i) contacting the diolefins and the mercaptans in the presence of a Group
VIII metal
catalyst in the rectification section of the first distillation column
reactor, at a
temperature in the range of 260 F to 400 F, a pressure in the range of 75 to
300 psig,
and a WHSV in the range of 1 to 5, thereby reacting: (A) a portion of the
mercaptans
with a portion of the diolefins to form thioethers, and/or (B) a portion of
the dienes
with a portion of the hydrogen to form olefins; and (ii) fractionating the
sulfur-
containing petroleum fraction into (A) a distillate product containing C5
hydrocarbons
having a total sulfur content of less than 10 ppm S, by weight and (B) a first
heavy
3
CA 02817065 2015-01-21
fraction containing C7+ hydrocarbons, sulfur compounds, and hindered sulfur
compounds; (c) recovering the first heavy fraction from the first distillation
column
reactor as a first bottoms; (d) feeding the first bottoms and hydrogen to a
second
distillation column reactor, wherein the hydrogen is fed to the second
distillation
column reactor at a rate of 10 to 1000 scf/bbl of the sulfur-containing
petroleum
fraction; (e) concurrently in the second distillation column reactor, (i)
reacting at least
a portion of the organic sulfur compounds in the first bottoms with hydrogen
in the
presence of a hydrodesulfurization catalyst in the rectification section of
the second
distillation column reactor at a temperature in the range of 300 F to 700 F, a
pressure
in the range of 75 to 300 psig, and a WHSV in the range of Ito 5, to convert a
portion
of the other organic sulfur compounds to hydrogen sulfide, and (ii) separating
the first
heavy fraction into a first intermediate fraction having an ASTM D86 end point
in the
range from 270 F to 400 F and a second heavy fraction; (f) recovering the
first
intermediate fraction, unreacted hydrogen, and hydrogen sulfide from the
second
distillation column reactor as a second overheads, where a total sulfur
content in the
first intermediate fraction is in the range from about 50 ppm S to about 100
ppm S, by
weight; (g) recovering the second heavy fraction containing the hindered
organic
sulfur compounds from the second distillation column reactor as a second
bottoms;
(h) feeding the second bottoms and hydrogen to a first fixed bed reactor
containing a
hydrodesulfurization catalyst; (i) contacting the second bottoms and hydrogen
with
the hydrodrodesulfurization catalyst in the first fixed bed reactor at a
temperature in
the range of 500 F to 700 F, a pressure in the range of 50 to 500 psig, a
hydrogen
partial pressure in the range of 25 psi to about 350 psi, and a WHSV in the
range of
0.5 to 10 to convert at least a portion of the hindered organic sulfur
compounds to
hydrogen sulfide; (j) recovering an effluent from the first fixed bed; (k)
separating
unreacted hydrogen and hydrogen sulfide from the effluent from the first fixed
bed
reactor to recover a desulfurized heavy fraction having a total sulfur content
of less
than 50 ppm S, by weight; (1) separating unreacted hydrogen and hydrogen
sulfide
from the second overheads; (m) feeding at least a portion of the second
overheads and
hydrogen to a second fixed bed reactor containing a hydrodesulfurization
catalyst at a
temperature in the range of 400 F to 600 F, a pressure in the range of 50 to
350 psig,
and a WHSV in the range of 5 to 10 to convert at least a portion of the sulfur
3a
CA 02817065 2015-01-21
compounds in the second overheads to hydrogen sulfide; (n) recovering an
effluent
from the second fixed bed reactor; (o) separating at least a portion of the
hydrogen
sulfide from the effluent from the second fixed bed reactor to form a
desulfurized
intermediate fraction having a total sulfur content of less than 50 ppm S, by
weight;
wherein a combined olefin content of the distillate product, the desulfurized
heavy
fraction, and the desulfurized intermediate fraction is at least 60% of the
olefin
content of the sulfur-containing petroleum fraction.
[0011] In one
aspect, embodiments disclosed herein relate to a process for the
desulfurization of a full boiling range catalytically cracked naphtha
including the
steps of: (a) feeding (1) a full boiling range naphtha containing olefins,
diolefins,
mercaptans and other organic sulfur compounds and having an ASTM D86 end
boiling point of at least 350 F, and (2) hydrogen to a first distillation
column reactor;
(b) concurrently in the first distillation column reactor, (i) contacting the
diolefins and
the mercaptans in the full boiling range naphtha in the presence of a Group
VIII metal
catalyst in the rectification section of the first distillation column reactor
thereby
reacting: (A) a portion of the mercaptans with a portion of the diolefins to
form
thioethers, and/or (B) a portion of the dienes with a portion of the hydrogen
to form
olefins; and (ii) fractionating the full boiling range cracked naphtha into a
distillate
product containing C5 hydrocarbons and a first heavy naphtha containing sulfur
compounds; (c) recovering the first heavy naphtha from the first distillation
column
reactor as a first bottoms; (d) feeding the first bottoms and hydrogen to a
second
distillation column reactor; (e) concurrently in the second distillation
column reactor,
(i) reacting at least a portion of the organic sulfur compounds in the first
bottoms with
hydrogen in the presence of a hydrodesulfurization catalyst in the
rectification section
3b
CA 02817065 2013 05 06
WO 2012/064466 PCT/US2011/056627
,
of the second distillation column reactor to convert a portion of the other
ofganic
sulfur compounds to hydrogen sulfide, and (ii) separating the first heavy
naphtha into
a first intermediate naphtha having an ASTM D86 end point in the range from
270 F
to 400 F and a second heavy naphtha; (f) recovering the first intermediate
naphtha,
unreacted hydrogen, and hydrogen sulfide from the second distillation column
reactor
as a second overheads; (g) recovering the second heavy naphtha containing
hindered
organic sulfur compounds from the second distillation column reactor as a
second
bottoms; (h) feeding the second bottoms and hydrogen to a first fixed bed
reactor
containing a hydrodesulfurization catalyst; (i) contacting the hindered
organic sulfur
compounds and hydrogen with the hydrodrodesulfurization catalyst in the first
fixed
bed reactor to convert at least a portion of the hindered organic sulfur
compounds to
hydrogen sulfide; and (j) recovering an effluent from the first fixed bed
reactor. In
some embodiments, the second bottoms may be combined with a diesel hydrocarbon
fraction for processing in the first fixed bed reactor.
[0012] In another aspect, embodiments disclosed herein relate to a process
for the
desulfurization of a full boiling range catalytically cracked naphtha
including the
steps of:
(a) feeding (1) a full boiling range naphtha containing olefins, diolefins,
mercaptans
and other organic sulfur compounds and having an ASTM D86 end boiling point
of at least 350 F, and (2) hydrogen to a first distillation column reactor;
(b) concurrently in the first distillation column reactor,
(i) contacting the diolefins and the mercaptans in the full boiling range
naphtha in the presence of a Group VIII metal catalyst in the rectification
section of the first distillation column reactor thereby reacting:
(A) a portion of the mercaptans with a portion of the diolefins to form
thioethers, and/or
(B) a portion of the dienes with a portion of the hydrogen to form
olefins; and
(ii) fractionating the full boiling range cracked naphtha into a distillate
product containing C5 hydrocarbons and a first heavy naphtha containing
sulfur compounds;
4
CA 02817065 2013 05 06
WO 2012/064466 PCT/US2011/056627
(c) recovering the first heavy naphtha from the first distillation column
reactor as a
first bottoms;
(d) feeding the first bottoms and hydrogen to a second distillation column
reactor;
(e) concurrently in the second distillation column reactor,
(i) reacting at least a portion of the organic sulfur compounds in the first
bottoms with hydrogen in the presence of a hydrodesulfurization catalyst
in the rectification section of the second distillation column reactor to
convert a portion of the other organic sulfur compounds to hydrogen
sulfide, and
(ii) separating the first heavy naphtha into a first intermediate naphtha
having
an ASTM D86 end point in the range from 270 F to 400 F and a
second heavy naphtha;
(f) recovering the first intermediate naphtha, unreacted hydrogen, and
hydrogen
sulfide from the second distillation column reactor as a second overheads;
(g) recovering the second heavy naphtha containing hindered organic sulfur
compounds from the second distillation column reactor as a second bottoms;
(h) feeding the second bottoms and hydrogen to a first fixed bed reactor
containing a
hydrodesulfurization catalyst;
(i) contacting the hindered organic sulfur compounds and hydrogen with the
hydrodrodesulfurization catalyst in the first fixed bed reactor to convert at
least a portion of the hindered organic sulfur compounds to hydrogen sulfide;
(j) recovering an effluent from the first fixed bed reactor;
(k) separating unreacted hydrogen and hydrogen sulfide from the effluent from
the
first fixed bed reactor;
(1) separating unreacted hydrogen and hydrogen sulfide from the second
overheads;
(m) feeding at least a portion of the second overheads and hydrogen to a
second fixed
bed reactor containing a hydrodesulfurization catalyst to convert at least a
portion of the sulfur compounds in the second overheads to hydrogen sulfide;
(n) recovering an effluent from the second fixed bed reactor;
(o) separating at least a portion of the hydrogen sulfide from the effluent
from the
second fixed bed reactor to form a naphtha fraction having a reduced sulfur
content.
CA 02817065 2013 05 06
WO 2012/064466 PCT/US2011/056627
[0013] In
another aspect, embodiments disclosed herein relate to a process for the
desulfurization of a full boiling range catalytically cracked naphtha
including the
steps of:
(a) feeding (1) a full boiling range naphtha containing olefins, diolefins,
mercaptans
and other organic sulfur compounds and having an ASTM D86 end boiling point
of at least 350 F, and (2) hydrogen to a first distillation column reactor;
(b) concurrently in the first distillation column reactor,
(i) contacting the diolefins and the mercaptans in the full boiling range
naphtha in the presence of a Group VIII metal catalyst in the rectification
section of the first distillation column reactor thereby reacting:
(A) a portion of the mercaptans with a portion of the diolefins to form
thioethers, and/or
(B) a portion of the dienes with a portion of the hydrogen to fat ________ in
olefins; and
(ii) fractionating the full boiling range cracked naphtha into a distillate
product containing C5 hydrocarbons and a first heavy naphtha containing
sulfur compounds;
(c) recovering the first heavy naphtha from the first distillation column
reactor as a
first bottoms;
(d) feeding the first bottoms and hydrogen to a second distillation column
reactor;
(e) concurrently in the second distillation column reactor,
(i) reacting at least a portion of the organic sulfur compounds in the first
bottoms with hydrogen in the presence of a hydrodesulfurization catalyst
in the rectification section of the second distillation column reactor to
convert a portion of the other organic sulfur compounds to hydrogen
sulfide, and
(ii) separating the first heavy naphtha into a first intermediate naphtha
having
an ASTM D86 end point in the range from 270 F to 400 F and a
second heavy naphtha;
(f) recovering the first intermediate naphtha, unreacted hydrogen, and
hydrogen
sulfide from the second distillation column reactor as a second overheads;
(g) recovering the second heavy naphtha containing hindered organic sulfur
compounds from the second distillation column reactor as a second bottoms;
6
CA 02817065 2013 05 06
WO 2012/064466 PCT/US2011/056627
(h) feeding the second bottoms and hydrogen to a first fixed bed reactor
containing a
hydrodesulfurization catalyst;
(i) contacting the hindered organic sulfur compounds and hydrogen with the
hydrodrodesulfurization catalyst in the first fixed bed reactor to convert at
least a portion of the hindered organic sulfur compounds to hydrogen sulfide;
(j) recovering an effluent from the first fixed bed reactor;
(k) separating unreacted hydrogen and hydrogen sulfide from the effluent from
the
first fixed bed reactor;
(1) partially condensing the second overheads and separating the uncondensed
portion
of the second overheads including unreacted hydrogen and hydrogen sulfide
from the condensed portion of the second overheads;
(m) feeding at least a portion of the condensed portion of the second
overheads to the
second distillation column reactor as reflux;
(n) feeding the separated effluent (k), the uncondensed portion of the second
overheads, and at least a portion of the condensed second overheads to a
fractionation column for separating unreacted hydrogen and hydrogen sulfide
and to recover a bottoms hydrocarbon fraction;
(o) feeding the bottoms hydrocarbon fraction and hydrogen to a second fixed
bed
reactor containing a hydrodesulfurization catalyst to convert at least a
portion
of the sulfur compounds in the bottoms hydrocarbon fraction to hydrogen
sulfide;
(p) recovering an effluent from the second fixed bed reactor;
(q) separating at least a portion of the hydrogen sulfide from the effluent
from the
second fixed bed reactor to form a naphtha fraction having a reduced sulfur
content; and
(r) forming a gasoline from one or more of (i) at least a portion of the
naphtha fraction
and (ii) at least a portion of the distillate fraction, wherein the gasoline
has a
total sulfur content of less than about 20 ppm S, by weight.
100141 In another aspect, embodiments disclosed herein relate to a
process for the
desulfurization of a full boiling range naphtha including the steps of:
(a) feeding (1) a full boiling range naphtha containing olefins, diolefins,
mercaptans
and other organic sulfur compounds and having an ASTM D86 end boiling point
of at least 350 F, and (2) hydrogen to a first distillation column reactor;
7
CA 02817065 2013 05 06
WO 2012/064466 PCT/US2011/056627
(b) concurrently in the first distillation column reactor,
(i) contacting the diolefins and the mercaptans in the full boiling range
naphtha in the presence of a Group VIII metal catalyst in the rectification
section of the first distillation column reactor thereby reacting:
(A) a portion of the mercaptans with a portion of the diolefins to form
thioethers, and/or
(C) a portion of the dienes with a portion of the hydrogen to form
olefins; and
(ii) fractionating the full boiling range cracked naphtha into a distillate
product containing C5 hydrocarbons and a first heavy naphtha containing
sulfur compounds;
(c) recovering the first heavy naphtha from the first distillation column
reactor as a
first bottoms;
(d) feeding the first bottoms and hydrogen to a second distillation column
reactor;
(e) concurrently in the second distillation column reactor,
(i) reacting at least a portion of the organic sulfur compounds in the first
bottoms with hydrogen in the presence of a hydrodesulfurization catalyst
in the rectification section of the second distillation column reactor to
convert a portion of the other organic sulfur compounds to hydrogen
sulfide, and
(ii) separating the first heavy naphtha into a first intermediate naphtha
having
an ASTM D86 end point in the range from 270 F to 400 F and a
second heavy naphtha;
(f) recovering the first intermediate naphtha, unreacted hydrogen, and
hydrogen
sulfide from the second distillation column reactor as a second overheads;
(g) recovering the second heavy naphtha containing hindered organic sulfur
compounds from the second distillation column reactor as a second bottoms;
(h) feeding the second bottoms and hydrogen to a first fixed bed reactor
containing a
hydrodesulfurization catalyst;
(i) contacting the hindered organic sulfur compounds and hydrogen with the
hydrodrodesulfurization catalyst in the first fixed bed reactor to convert at
least a portion of the hindered organic sulfur compounds to hydrogen sulfide;
(j) recovering an effluent from the first fixed bed reactor;
8
CA 02817065 2013 05 06
WO 2012/064466 PCT/US2011/056627
(k) separating unreacted hydrogen and hydrogen sulfide from the effluent from
the
first fixed bed reactor;
(1) separating unreacted hydrogen and hydrogen sulfide from the second
overheads;
(m) feeding at least a portion of the second overheads and hydrogen to a
second fixed
bed reactor containing a hydrodesulfurization catalyst to convert at least a
portion of the sulfur compounds in the second overheads to hydrogen sulfide;
(n) recovering an effluent from the second fixed bed reactor;
(o) separating at least a portion of the hydrogen sulfide from the effluent
from the
second fixed bed reactor to form a H2S separated naphtha fraction;
(p) fractionating the H2S separated naphtha fraction to form a heavy naphtha
fraction
and a mid-range gasoline fraction; and
(q) recycling at least a portion of the heavy naphtha fraction to the second
fixed bed
reactor; and
(r) forming a gasoline from one or more of (i) at least a portion of the
distillate
product, (ii) at least a portion of the naphtha fraction, and (iii) at least a
portion
of the effluent from the first fixed bed reactor, wherein the gasoline has a
total
sulfur content of less than about 20 ppm S, by weight..
[0015] In some embodiments, the high end point naphtha being treated may
have an
ASTM endpoint of greater than about 470 F; greater than about 470 F in other
embodiments; greater than about 500 F in other embodiments; greater than about
525 F in other embodiments; and greater than about 550 F in yet other
embodiments.
[0016] Other aspects and advantages of the invention will be apparent from
the
following description and the appended claims.
BRIEF DESCRIPTION OF DRAWINGS
[0017] Figure 1 is a simplified flow diagram in schematic form of one
embodiment of
processes for hydrodesulfurization of naphtha fractions according to
embodiments
disclosed herein.
[0018] Figure 2 is a simplified flow diagram in schematic foini of one
embodiment of
processes for hydrodesulfurization of naphtha fractions according to
embodiments
disclosed herein.
9
CA 02817065 2013 05 06
WO 2012/064466 PCT/US2011/056627
[0019] Figure 3 is a simplified flow diagram in schematic Bolin of one
embodiment of
processes for hydrodesulfurization of naphtha fractions according to
embodiments
disclosed herein.
DETAILED DESCRIPTION
100201 In one aspect, embodiments disclosed herein relate to a process for
the
desulfurization of a high end point FCC gasoline. Embodiments disclosed herein
generally relate to processes for the desulfurization of FCC naphtha having a
high
ASTM D86 end point, such as greater than about 350 F, greater than 400 F,
greater
than 450 F, greater than 470 F, greater than 500 F, greater than 525 F, or
greater than
550 F. More particularly, embodiments disclosed herein relate to processes for
the
desulfurization of high end point naphthas to produce gasoline fractions
having a total
sulfur content of less than 20 ppm, by weight. In some embodiments, the total
sulfur
content of the resulting gasoline fraction may be less than 10 ppm, by weight.
Other
embodiments disclosed herein may additionally provide for control of the end
point of
the gasoline product.
[0021] "Recombinant mercaptans," as used herein, refers to mercaptans that
are not in
the feed to the present process but are the reaction products of the H2S
generated by
the hydrogenation of sulfur-containing compounds in the present process and
alkenes
in the feed. Thus, the recombinant mercaptans are not necessarily the same as
those
destroyed by the hydrodesulfurization of a first portion of the present
process,
although they may be.
[0022] Within the scope of this application, the expression "catalytic
distillation
reactor system" denotes an apparatus in which the catalytic reaction and the
separation of the products take place at least partially simultaneously. The
apparatus
may comprise a conventional catalytic distillation column reactor, where the
reaction
and distillation are concurrently taking place at boiling point conditions, or
a
distillation column combined with at least one side reactor, where the side
reactor
may be operated as a liquid phase reactor or a boiling point reactor. While
both
catalytic distillation reactor systems described may be preferred over
conventional
liquid phase reaction followed by separations, a catalytic distillation column
reactor
may have the advantages of decreased piece count, reduced capital cost,
increased
CA 02817065 2013 05 06
WO 2012/064466 PCT/US2011/056627
catalyst productivity per pound of catalyst, efficient heat removal (heat of
reaction
may be absorbed into the heat of vaporization of the mixture), and a potential
for
shifting equilibrium. Divided wall distillation columns, where at least one
section of
the divided wall column contains a catalytic distillation structure, may also
be used,
and are considered "catalytic distillation reactor systems" herein.
[0023] The hydrocarbon feed to the processes disclosed herein may be a
sulfur-
containing petroleum fraction which boils in the gasoline boiling range,
including
FCC gasoline, coker pentane/hexane, coker naphtha, FCC naphtha, straight run
gasoline, pyrolysis gasoline, and mixtures containing two or more of these
streams.
Such gasoline blending streams typically have a normal boiling point within
the range
of 0 F and 470 F, as determined by an ASTM D86 distillation. Feeds of this
type
include light naphthas typically having a boiling range of about C6 to 330 F;
full
range naphthas, typically having a boiling range of about C5 to 420 F, heavier
naphtha fractions boiling in the range of about 260 F to 412 F, or heavy
gasoline
fractions with high end points boiling in the range of about 330 F to 470 F or
higher.
[0024] Processes disclosed herein are additionally suitable for the
desulfurization of
"high end point" petroleum fractions, which is herein defined as a naphtha
fraction
having an ASTM D86 end point of at least 450 F. Increasing the end point of
the
naphtha changes the behavior of the gasoline toward hydrodesulfurization, as
the
sulfur content of the gasoline increases dramatically with an increase in end
point,
rendering a significant number of prior processes unsuitable. Further, higher
end
point fractions typically include multi-substituted sulfur compounds, as
described
above, including multi-substituted benzothiophenes. These high end point
sulfur-
containing compounds are referred to herein as "hindered sulfur compounds" as
these
compounds are much less reactive during hydrodesulfurization processes. In
some
embodiments, high end point gasoline fractions that may be processed according
to
processes disclosed herein may have an ASTM D86 end point of at least 450 F,
at
least 470 F in other embodiments; at least 500 F in other embodiments; at
least 510 F
in other embodiments; at least 520 F in other embodiments; at least 525 F in
other
embodiments; and at least 550 F in yet other embodiments. In other
embodiments,
high end point gasoline fractions that may be processed according to
embodiments
disclosed herein may have an ASTM D86 end point in the range from about 450 F
to
11
CA 02817065 2013 05 06
WO 2012/064466 PCT/US2011/056627
about 550 F; from about 470 F to about 550 F in other embodiments; and from
about
500 F to about 520 F in yet other embodiments.
[0025] Organic sulfur compounds present in these gasoline fractions occur
principally
as mercaptans, aromatic heterocyclic compounds, and disulfides. Relative
amounts of
each depend on a number of factors, many of which are refinery, process and
feed
specific. In general, heavier fractions contain a larger amount of sulfur
compounds,
and a larger fraction of these sulfur compounds are in the form of aromatic
heterocyclic compounds. In addition, certain streams commonly blended for
gasoline,
such as FCC naphthas, contain high amounts of the heterocyclic compounds.
Gasoline streams containing significant amounts of these heterocyclic
compounds are
often difficult to process using many of the prior art processes. Very severe
operating
conditions have been conventionally specified for hydrotreating processes to
desulfurize gasoline streams, resulting in loss of olefinic content and a
large octane
penalty. Prior methods of catalytic distillation for high-end point gasolines
have not
been successful in removing the required amount of sulfur due to the
difficulty of
breaking the hindered sulfur bonds in high-end point naphtha. Adsorption
processes,
used as an alternative to hydrogen processing, have very low removal
efficiencies, as
the aromatic heterocyclic sulfur compounds have adsorptive properties similar
to the
aromatic compounds in the hydrocarbon matrix.
[0026] Aromatic heterocyclic compounds that may be removed by the
processes
disclosed herein include alkyl substituted thiophene, thiophenol,
alkylthiophene,
benzothiophene, and multi-substituted benzothiophenes. Among the aromatic
heterocyclic compounds of particular interest are thiophene, 2-
methylthiophene, 3-
methylthiophene, 2-ethylthiophene, benzothiophene and dimethylbenzothiophene.
Mercaptans that may be removed by the processes described herein often contain
from 2-10 carbon atoms, and are illustrated by materials such as 1-ethanthiol,
2-
propanethiol, 2-butanethiol, 2-methyl-2-propanethiol, pentanethiol,
hexanethiol,
heptanethiol, octanethiol, nonanethiol, and thiophenol.
[0027] Sulfur in these gasoline streams may be in one of several
molecular forms,
including thiophenes, mercaptans and disulfides. For a given gasoline stream,
the
sulfur compounds tend to be concentrated in the higher boiling portions of the
stream
(i.e., the heavier fractions of the stream), with hindered sulfur compounds
being
12
CA 02817065 2013 05 06
WO 2012/064466 PCT/US2011/056627
present in higher concentrations at elevated boiling points, such as above
about 350 F,
and especially above about 450 F, and even more especially above about 500 F.
The
sulfur within the higher boiling portions of the stream may be more difficult
to
remove due to increased concentration of multi-substituted benzothiophenes.
High
end point naphtha streams that are particularly rich in hindered sulfur
compounds may
be suitably treated according to embodiments disclosed herein to produce a
gasoline
range product meeting desired sulfur specifications.
[0028] The total sulfur content of gasoline streams to be treated using
the processes
disclosed herein will generally exceed 50 ppm by weight, and typically range
from
about 150 ppm to as much as several thousand ppm sulfur. For fractions
containing at
least 5 volume percent boiling over about 520 F, the sulfur content may exceed
about
1000 ppm by weight, and may be as high as 5000 to 10000 ppm by weight or even
higher.
100291 In addition to the sulfur compounds, naphtha feeds, including FCC
naphtha,
may include paraffins, naphthenes, and aromatics, as well as open-chain and
cyclic
olefins, dienes, and cyclic hydrocarbons with olefinic side chains. A cracked
naphtha
feed useful in the processes described herein may have an overall olefins
concentration ranging from about 5 to 60 weight percent in some embodiments;
from
about 25 to 50 weight percent in other embodiments.
[0030] In general, processes described herein may treat a naphtha or
gasoline fraction
in one or more catalytic distillation reactor systems. Each catalytic
distillation reactor
system may have one or more reaction zones containing one or more of a
hydrogenation catalyst, a thioetherification catalyst, and/or a
hydrodesulfurization
catalyst. For example, reactive distillation zones may be contained within the
stripping section, hydrodesulfurizing the heavier compounds in the feed, or
within the
rectification section, hydrodesulfurizing the lighter compounds in the feed,
or both.
Hydrogen may also be fed to the catalytic distillation reactor system, and in
some
embodiments, a portion of the hydrogen may be fed below each respective
reaction
zone.
100311 In each catalytic distillation reactor system, the steps to
catalytically react the
naphtha feed with hydrogen may be carried out at a temperature in the range of
100 F
to 1000 F, at pressures in the range from about 0.1 to 500 psig, with hydrogen
partial
13
CA 02817065 2013 05 06
WO 2012/064466 PCT/US2011/056627
pressures in the range from 0.01 to 100 psi at 2 to 2000 scf/bbl at weight
hourly space
velocities (WHSV) in the range of 0.1 to 10 hr-1 based on feed rate and a
particulate
catalyst packaged in structures. If advanced specialty catalytic structures
are used
(where catalyst is one with the structure rather than a form of packaged
pellets to be
held in place by structure), the liquid hourly space velocity (LHSV) for such
systems
should be about in the same range as those of particulate or granular-based
catalytic
distillation catalyst systems as just referenced. In other embodiments,
conditions in a
reaction distillation zone of a naphtha hydrodesulfurization distillation
column reactor
system are: temperatures in the range from 450 F to 700 F, total pressure in
the range
from 75 to 300 psig, hydrogen partial pressure in the range from 6 to 75 psia,
WHSV
of naphtha in the range from about 1 to 5, and hydrogen feed rates in the
range from
10-1000 scf/bbl.
[0032] The operation of a distillation column reactor results in both a
liquid and a
vapor phase within the distillation reaction zone. A considerable portion of
the vapor
is hydrogen, while a portion of the vapor is hydrocarbons from the hydrocarbon
feed.
In catalytic distillation it has been proposed that the mechanism that
produces the
effectiveness of the process is the condensation of a portion of the vapors in
the
reaction system, which occludes sufficient hydrogen in the condensed liquid to
obtain
the requisite intimate contact between the hydrogen and the sulfur compounds
in the
presence of the catalyst to result in their hydrogenation. In particular,
sulfur species
concentrate in the liquid while the olefins and H2S concentrate in the vapor,
allowing
for high conversion of the sulfur compounds with low conversion of the olefin
species.
[0033] As in any distillation, there is a temperature gradient within the
catalytic
distillation reactor system. The lower end of the column contains higher
boiling
material and thus is at a higher temperature than the upper end of the column.
The
lower boiling fraction, which contains more easily removable sulfur compounds,
is
subjected to lower temperatures at the top of the column, which may provide
for
greater selectivity, that is, no hydrocracking or less saturation of desirable
olefinic
compounds. The higher boiling portion is subjected to higher temperatures in
the
lower end of the distillation column reactor to crack open the sulfur
containing ring
compounds and hydrogenate the sulfur. The heat of reaction simply creates more
boil
up, but no increase in temperature at a given pressure. As a result, a great
deal of
14
CA 02817065 2013 05 06
WO 2012/064466 PCT/US2011/056627
control over the rate of reaction and distribution of products can be achieved
by
regulating the system pressure.
[0034] Processes disclosed herein may additionally treat a naphtha or
gasoline
fraction, or a select portion thereof, in one or more fixed bed reactor
systems. Each
fixed bed reactor system may include one or more reactors in series or
parallel, each
reactor having one or more reaction zones containing one or more
hydrodesulfurization catalysts. Such fixed bed reactors may be operated as a
vapor
phase reactor, a liquid phase reactor, or a mixed phase (V/L) reactor and may
include
traditional fixed bed reactors, trickle bed reactors, pulse flow reactors, and
other
reactor types known to those skilled in the art. The operating conditions used
in the
fixed bed reactor systems may depend upon the reaction phase(s), the boiling
range of
the naphtha fraction being treated, catalyst activity, selectivity, and age,
and the
desired sulfur removal per reaction stage, among other factors.
[0035] The flow of components through processes disclosed herein provides
for
efficient processing of high end point naphtha streams to reduce the total
sulfur
content of the streams to meet specifications and regulations. Further, the
process
flow schemes provide for the processing of high olefin-content portions of the
naphtha at less severe conditions, maintaining a significant portion of the
olefin
content, and thus preserving high octane value components.
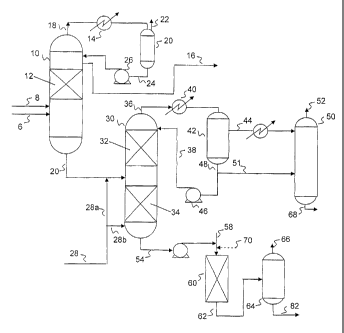
[0036] Referring now to Figure 1, a simplified process flow diagram of an
embodiment of the hydrodesulfurization processes disclosed herein is
illustrated.
Hydrogen and a naphtha or other organic sulfur-containing hydrocarbon feed,
which
may include hindered sulfur compounds, may be fed via flow lines 6 and 8,
respectively, to a first catalytic distillation reactor system 10 having one
or more
reactive distillation zones 12 for hydrotreating the hydrocarbon feed. As
illustrated,
catalytic distillation reactor system 10 includes at least one reactive
distillation zone
12, located in an upper portion of the column, above the feed inlet, for
treating the
light hydrocarbon components in the feed.
[0037] Reaction zone 12 may include one or more catalysts for the
hydrogenation of
dienes, reaction of mercaptans and dienes (thioetherification), and/or
hydrodesulfurization. For example, conditions in the first catalytic
distillation reactor
system 10 may provide for thioetherification and/or hydrogenation of dienes
and
CA 02817065 2013 05 06
WO 2012/064466 PCT/US2011/056627
removal of mercaptan sulfur from the C5/C6 portion of the hydrocarbon feed.
The
C5/C6 portion of the naphtha, having a reduced sulfur content as compared to
the
C5/C6 portion of the feed, may be recovered from catalytic distillation
reactor system
as a side draw product 16.
100381 An overheads fraction may be recovered from catalytic distillation
reactor
system 10 via flow line 18, and may contain light hydrocarbons, unreacted
hydrogen
and hydrogen sulfide.. The first overheads 18 may be cooled, such as using a
heat
exchanger 14, and fed to a stripper 20. In stripper 20, hydrogen sulfide and
unreacted
hydrogen may be separated from the hydrocarbons contained in the overhead
fraction,
with unreacted hydrogen and hydrogen sulfide withdrawn from stripper 20 via
flow
line 22. Condensed hydrocarbons may be withdrawn from stripper 20 and fed to
first
catalytic distillation reactor system 10 as a total or partial reflux via flow
line 24 and
pump 26.
100391 The C5/C6 side draw product withdrawn from catalytic distillation
reactor
system 10 via flow line 16 may contain many of the olefins present in the
hydrocarbon feed. Additionally, dienes in the C5/C6 cut may be hydrogenated
during
treatment in catalytic distillation reactor system 10. This hydrogenated,
desulfurized
C5/C6 side draw product may thus be recovered for use in various processes. In
various embodiments, the C5/C6 side draw product may be used as a gasoline
blending fraction, hydrogenated and used as a gasoline blending feedstock, and
as a
feedstock for ethers production, among other possible uses. The particular
processing
or end use of the C5/C6 fraction may depend upon various factors, including
availability of alcohols as a raw material, and the allowable olefin
concentration in
gasoline for a particular jurisdiction, among others
100401 The heavy naphtha, e.g. C7+ boiling range components, including any
thioethers formed in reaction zone 12 and various other sulfur and hindered
sulfur
compounds in the hydrocarbon feed, may be recovered as a bottoms fraction from
catalytic distillation reactor system 10 via flow line 20. Where catalytic
distillation
reactor system 10 includes a reaction zone in the stripping section of the
column, or
where boil-up of C7+ components into reaction zone 12 occurs, the recovered
bottoms fraction may be at least partially desulfurized.
[0041] The bottoms fraction recovered via flow line 20 is then fed to a
second
catalytic distillation reactor system 30 containing one or more reactions
zones
16
CA 02817065 2013 05 06
WO 2012/064466 PCT/US2011/056627
containing one or more hydrodesulfurization catalysts. Hydrogen may be fed to
catalytic distillation reactor system 30 via flow line 28.
[0042] In some embodiments, catalytic distillation reactor system 30
may contain a
reaction zone 32 in the rectification section reacting at least a portion of
the organic
sulfur compounds in the hydrocarbon feed with hydrogen, converting at least a
portion of the organic sulfur compounds to hydrogen sulfide. Catalytic
distillation
reactor system 30 may be operated at conditions to facilitate the
aforementioned
reaction and to concurrently separate the hydrocarbon feed into a first
intermediate
naphtha fraction having an ASTM D86 end point in the range from about 270 F to
about 400 F, recovered as an overheads via flow line 36, and a heavy naphtha
fraction, recovered as a bottoms fraction via flow line 54.
[0043] If desired, catalytic distillation reactor system 30 may include
distillation
reaction zones 32, 34, in each of the rectification and stripping sections of
the column,
such that the heavy fraction may be at least partially hydrodesulfurized as it
traverses
downward through catalytic distillation reactor system 30. In such a case,
hydrogen
may be fed below the lowermost reaction zone via flow line 28b, or
alternatively may
be fed below each reactive distillation zone 32 and 34, such as via flow lines
28a and
28b, respectively.
[0044] The overheads product recovered from catalytic distillation
reactor system 30
via flow line 36 may contain the intermediate fraction hydrocarbons as well as
hydrogen sulfide and unreacted hydrogen. The overheads fraction may then be
processed to separate the hydrogen and hydrogen sulfide. For example, the
overheads
fraction may be partially condensed via indirect heat exchange using a heat
exchanger
40 and fed to a "hot drum" 42 for separation of the condensate from the
uncondensed
vapors, which include hydrocarbons, hydrogen sulfide, and hydrogen. The
condensate may be recovered from drum 42 via flow line 48, a portion of which
may
be fed as reflux to catalytic distillation reactor 30 via pump 46 and flow
line 38. The
remainder of the condensate may be fed via flow line 51, and the uncondensed
vapors
may be fed via flow line 44, to "cold drum" 50. Cold drum 50 may then separate
hydrogen and hydrogen sulfide, recovered via flow line 52, from the
intermediate and
heavy hydrocarbon components, recovered via flow line 68.
[0045] The bottoms product recovered via flow line 54 may have a fairly
high
concentration of sulfur. However, it is actually beneficial to the process to
have a
17
CA 02817065 2013 05 06
WO 2012/064466 PCT/US2011/056627
minimal amount of catalyst in reaction zone 34, leaving a high concentration
of sulfur
in the bottoms product, as this minimizes the concentration of hydrogen
sulfide
available for recombinant mercaptan formation in the upper portion of
catalytic
distillation column reactor 30 and the associated overheads recovery system.
[0046] The bottoms fraction recovered via flow line 54 from catalytic
distillation
reactor system 30 is hot (at reboil temperature) and does not contain a
significant
amount of hydrogen sulfide due to the counter-current flow pattern of the
reactive
distillation process. The bottoms fraction recovered via flow line 54 is then
fed to a
fixed bed reactor 60 for additional hydrotreating. Additional hydrogen, over
that
dissolved in the bottoms, may be fed to fixed bed reactor 60 via flow line 58,
if
necessary or desired. The partial pressure of hydrogen in the fixed be unit is
typically
greater than about 20 psi, such as between about 25 psi and about 350 psi,
providing
additional driving force for the removal of sulfur from any hindered sulfur
compounds
in the heavy end of the hydrocarbon feed 8. High hydrogen concentrations may
be
used in the fixed bed reactor 60 as most olefins have been separated and
recovered via
flow lines 16 and 36. Additionally, use of select hydrodesulfurization
catalysts, such
as Co / Mo catalysts, in fixed bed reactor 60 may prevent saturation of
aromatic
compounds, thus avoiding the accompanying octane loss. Fixed bed reactor 60
and
the resulting hydrodesulfurization of hindered sulfur compounds allows for the
processing of very high endpoint feedstocks, even those having an endpoint in
excess
of 550 F in some embodiments.
[0047] The heavy gasoline effluent from fixed bed reactor 60 may be
recovered via
flow line 62. The effluent may then be fed via flow line 62 to drum 64,
separating
hydrogen sulfide and unreacted hydrogen from the liquid hydrocarbon effluent.
The
hydrogen and hydrogen sulfide may be withdrawn from drum 64 via flow line 66.
The hydrocarbon effluent, having a reduced sulfur concentration, may be
recovered
via flow line 82.
[0048] In some embodiments, the hydrocarbon effluent recovered via flow
line 82
may be combined with one or more of the lighter fractions, recovered via flow
lines
16 and 68, for use as a 'gasoline blend stock or for further processing, as
will be
described below. In other embodiments, the heavy hydrocarbon fraction may be
processed along with a heavy hydrocarbon fraction, such as a diesel
hydrocarbon
18
CA 02817065 2013 05 06
WO 2012/064466 PCT/US2011/056627
fraction, fed via flow line 70, for further reducing the sulfur content of the
heavy
fraction and the diesel fraction.
[0049] Referring now to Figures 2 and 3, simplified process flow diagrams
of
embodiments of the hydrodesulfurization processes disclosed herein is
illustrated,
where like numerals represent like parts. Hydrogen and a naphtha or other
organic
sulfur-containing hydrocarbon feed, which may include hindered sulfur
compounds,
may be fed via flow lines 6 and 8, respectively, to a first catalytic
distillation reactor
system 10 having one or more reactive distillation zones 12 for hydrotreating
the
hydrocarbon feed. As illustrated, catalytic distillation reactor system 10
includes at
least one reactive distillation zone 12, located in an upper portion of the
column,
above the feed inlet, for treating the light hydrocarbon components in the
feed.
[0050] Reaction zone 12 may include one or more catalysts for the
hydrogenation of
dienes, reaction of mercaptans and dienes (thioetherification), and/or
hydrodesulfurization. For example, conditions in the first catalytic
distillation reactor
system 10 may provide for thioetherification and/or hydrogenation of dienes
and
removal of mercaptan sulfur from the C5/C6 portion of the hydrocarbon feed.
The
C5/C6 portion of the naphtha, having a reduced sulfur content as compared to
the
C5/C6 portion of the feed, may be recovered from catalytic distillation
reactor system
as a side draw product 16.
[0051] An overheads fraction may be recovered from catalytic distillation
reactor
system 10 via flow line 18, and may contain light hydrocarbons, unreacted
hydrogen
and hydrogen sulfide.. The first overheads 18 may be cooled, such as using a
heat
exchanger 14, and fed to a stripper 20. In stripper 20, hydrogen sulfide and
unreacted
hydrogen may be separated from the hydrocarbons contained in the overhead
fraction,
with unreacted hydrogen and hydrogen sulfide withdrawn from stripper 20 via
flow
line 22. Condensed hydrocarbons may be withdrawn from stripper 20 and fed to
first
catalytic distillation reactor system 10 as a total or partial reflux via flow
line 24 and
pump 26.
[0052] The C5/C6 side draw product withdrawn from catalytic distillation
reactor
system 10 via flow line 16 may contain many of the olefins present in the
hydrocarbon feed. Additionally, dienes in the C5/C6 cut may be hydrogenated
during
treatment in catalytic distillation reactor system 10. This hydrogenated,
desulfurized
C5/C6 side draw product may thus be recovered for use in various processes. In
19
CA 02817065 2013 05 06
WO 2012/064466 PCT/US2011/056627
various embodiments, the C5/C6 side draw product may be used as a gasoline
blending fraction, hydrogenated and used as a gasoline blending feedstock, and
as a
feedstock for ethers production, among other possible uses. The particular
processing
or end use of the C5/C6 fraction may depend upon various factors, including
availability of alcohols as a raw material, and the allowable olefin
concentration in
gasoline for a particular jurisdiction, among others
[0053] The heavy naphtha, e.g. C7+ boiling range components, including any
thio ethers formed in reaction zone 12 and various other sulfur and hindered
sulfur
compounds in the hydrocarbon feed, may be recovered as a bottoms fraction from
catalytic distillation reactor system 10 via flow line 20. Where catalytic
distillation
reactor system 10 includes a reaction zone in the stripping section of the
column, or
where boil-up of C7+ components into reaction zone 12 occurs, the recovered
bottoms fraction may be at least partially desulfurized.
[0054] The bottoms fraction recovered via flow line 20 is then fed to a
second
catalytic distillation reactor system 30 containing one or more reactions
zones
containing one or more hydrodesulfurization catalysts. Hydrogen may be fed to
catalytic distillation reactor system 30 via flow line 28.
[0055] In some embodiments, catalytic distillation reactor system 30 may
contain a
reaction zone 32 in the rectification section reacting at least a portion of
the organic
sulfur compounds in the hydrocarbon feed with hydrogen, converting at least a
portion of the organic sulfur compounds to hydrogen sulfide. Catalytic
distillation
reactor system 30 may be operated at conditions to facilitate the
aforementioned
reaction and to concurrently separate the hydrocarbon feed into a first
intermediate
naphtha fraction having an ASTM D86 end point in the range from about 270 F to
about 400 F, recovered as an overheads via flow line 36, and a heavy naphtha
fraction, recovered as a bottoms fraction via flow line 54.
[0056] If desired, catalytic distillation reactor system 30 may include
distillation
reaction zones 32, 34, in each of the rectification and stripping sections of
the column,
such that the heavy fraction may be at least partially hydrodesulfurized as it
traverses
downward through catalytic distillation reactor system 30. In such a case,
hydrogen
may be fed below the lowennost reaction zone via flow line 28b, or
alternatively may
be fed below each reactive distillation zone 32 and 34, such as via flow lines
28a and
28b, respectively.
CA 02817065 2013 05 06
WO 2012/064466 PCT/US2011/056627
[0057] The
bottoms product recovered via flow line 54 may have a fairly high
concentration of sulfur. However, it is actually beneficial to the process to
have a
minimal amount of catalyst in reaction zone 34, leaving a high concentration
of sulfur
in the bottoms product, as this minimizes the concentration of hydrogen
sulfide
available for recombinant mercaptan formation in the upper portion of
catalytic
distillation column reactor 30 and the associated overheads recovery system.
[0058] The bottoms fraction recovered via flow line 54 from catalytic
distillation
reactor system 30 is hot (at reboil temperature) and does not contain a
significant
amount of hydrogen sulfide due to the counter-current flow pattern of the
reactive
distillation process. The bottoms fraction recovered via flow line 54 is then
fed to a
fixed bed reactor 60 for additional hydrotreating. Additional hydrogen, over
that
dissolved in the bottoms, may be fed to fixed bed reactor 60 via flow line 58.
The
partial pressure of hydrogen in the fixed be unit is greater than about 20
psi, such as
between about 25 psi and about 350 psi, providing additional driving force for
the
removal of sulfur from any hindered sulfur compounds in the heavy end of the
hydrocarbon feed 8. High hydrogen concentrations may be used in the fixed bed
reactor 60 as most olefins have been separated and recovered via flow lines 16
and 36.
Additionally, use of select hydrodesulfurization catalysts, such as Co / Mo
catalysts,
in fixed bed reactor 60 may prevent saturation of aromatic compounds, thus
avoiding
the accompanying octane loss.
Fixed bed reactor 60 and the resulting
hydrodesulfurization of hindered sulfur compounds allows for the processing of
very
high endpoint feedstocks, even those having an endpoint in excess of 550 F in
some
embodiments.
[0059] The heavy gasoline effluent from fixed bed reactor 60 may be
recovered via
flow line 62. In some embodiments, a portion of the effluent in flow line 62
may be
recycled to the inlet of reactor 60, such as via flow line 61. The effluent
may then be
fed via flow line 62 to drum 64, separating hydrogen sulfide and unreacted
hydrogen
from the liquid hydrocarbon effluent. The hydrogen and hydrogen sulfide may be
withdrawn from drum 64 via flow line 66. The hydrocarbon effluent, having a
reduced sulfur concentration, may be recovered via flow line 82.
[0060] The overheads product recovered from catalytic distillation
reactor system 30
via flow line 36 may contain the intermediate fraction hydrocarbons as well as
hydrogen sulfide and unreacted hydrogen. The overheads fraction may then be
21
CA 02817065 2015-01-21
processed to separate the hydrogen and hydrogen sulfide. For example, the
overheads
fraction may be partially condensed via indirect heat exchange using a heat
exchanger
40 and fed to a "hot drum" 42 for separation of the condensate from the
uncondensed
vapors, which include hydrocarbons, hydrogen sulfide, and hydrogen. The
condensate may be recovered from drum 42 via flow line 48, a portion of which
may
be fed as reflux to catalytic distillation reactor 30 via pump 46 and flow
line 38. The
remainder of the condensate may be fed via flow line 51, and the uncondensed
vapors
may be fed via flow line 44, to "cold drum" 50. Cold drum 50 may then separate
hydrogen and hydrogen sulfide, recovered via flow line 52, from the
intermediate and
heavy hydrocarbon components, recovered via flow line 68.
[0061] In some embodiments of the hydrodesulfurization processes disclosed
herein,
it may be desired to recover a desulfurized hydrocarbon stream inclusive of
both the
intermediate fraction and the heavy fraction. Referring now to Figure 1, the
separated
heavy gasoline effluent recovered from drum 64 via flow line 82 may be fed to
cold
drum 50 for additional removal of hydrogen and hydrogen sulfide, if necessary,
and
recovered for further processing along with the intermediate fraction via flow
line 68.
The heavy gasoline effluent may alternatively be combined with the
intermediate
fraction downstream of drum 50.
[0062] The combined heavy and intermediate fractions may then be fed via
flow line
68 and hydrogen via flow line 72 to a second fixed bed reactor 74 containing a
hydrodesulfurization catalyst. The desulfurized heavy gasoline fraction may
thus act
as a heavy, inert diluent for the hydrodesulfurization of the intermediate
fraction in
second fixed bed reactor 74. Second fixed bed reactor 74 may be especially
useful for
removing mercaptan and recombinant mercaptan sulfur formed in the overhead
system and present in the intermediate fraction. The effluent from second
fixed bed
reactor 74 may then be fed via flow line 76 to stripper 80 for the separation
of
hydrogen and hydrogen sulfide, recovered via flow line 78, from the
hydrodesulfurized intermediate and heavy gasoline fractions, recovered via
flow line
84.
[0063] In some embodiments, processes disclosed herein may provide control
over
the end point of the intermediate gasoline fractions recovered as a product.
Referring
now to Figure 2, the intermediate fraction may be fed via flow line 68 and
hydrogen
via flow line 72 to a second fixed be reactor 74 containing a
hydrodesulfurization
22
CA 02817065 2013 05 06
WO 2012/064466 PCT/US2011/056627
catalyst. The effluent from second fixed bed reactor 74 may then be fed via
flow line
76 to stripper 80 for the separation of hydrogen and hydrogen sulfide,
recovered via
flow line 78, from the hydrodesulfurized intermediate gasoline fraction,
recovered via
flow line 84.
[0064] The inteimediate gasoline fraction may then be fed to separator 92
for
fractionation of the hydrodesulfurized intermediate gasoline fraction to
recover a light
intermediate naphtha fraction via flow line 94 and a heavy naphtha fraction
via flow
line 86. Control of the end point of the intermediate naphtha fraction may be
controlled by the operating conditions used in separator 92. An intermediate
naphtha
fraction having a higher end point may be achieved using higher temperatures
and/or
lower pressures in separator 92.
[0065] In some embodiments, the heavy naphtha fraction recovered via flow
line 86
may be recycled to fixed bed reactor 74 to act as a heavy, inert diluent, as
described
above. A portion of the heavy gasoline recovered from drum 64 via flow line 82
may
also be fed via flow line 90 to second fixed bed reactor 74 to act as a
diluent, to
provide heavy hydrocarbons for additional control of the end point of the
intermediate
naphtha recovered via flow line 94, and to provide heavy material for control
of reboil
temperature in separator 92. As necessary, heavy hydrocarbons recirculating
from
separator 92 to fixed bed reactor 74 may be withdrawn via flow line 98 and
recovered
with the heavy gasoline fraction in flow line 82.
[0066] In the configuration as illustrated in Figure 2, the heavy
fraction recovered via
flow line 82 may be useful as a diesel gasoline fraction. In such instances,
it may be
desired to saturate aromatics in the heavy gasoline fraction. Thus, a refiner
may opt
to load a Ni/Mo catalyst, a Co/Mo catalyst, a Ni/W catalyst, or a mixture
thereof in
fixed bed reactor 60 to meet the local diesel specifications.
[0067] To result in high end point gasoline products having a low sulfur
content, such
as less than 50 ppm sulfur, by weight, in some embodiments, and less than 20
ppm or
ppm sulfur in other embodiments, the hydrocarbons recovered from drum 50 via
flow line 68 may have a target sulfur concentration of less than about 150 ppm
sulfur,
by weight. In some embodiments, the target sulfur concentration of the
hydrocarbons
recovered via flow line 68 may be less than 125 ppm sulfur, by weight; less
than 100
ppm sulfur, by weight in other embodiments; and from 50 ppm to 100 ppm sulfur,
by
weight, in yet other embodiments.
23
CA 02817065 2015-01-21
[0068] Operating conditions useful in each of catalytic distillation
reactor systems 10,
30 and fixed bed reactor systems 60, 74 are provided in Table 1 below. Such
conditions are useful in attaining the target product sulfur concentrations as
detailed
above.
Table 1
Reactor / Reaction Zone 10 30 60 74
Temperature ( F) 260-400 300-700 500-700 400-600
Pressure (psig) 75-300 75-300 50-500 50-350
WHSV 1-5 1-5 0.5-10 5-10
Hydrogen partial pressure (psi) 5-75 5-75 20-400
Hydrogen feed rates (scf/bbl) 10-1000 10-1000
[0069] Catalysts useful in the first catalytic distillation reactor column
may be
characterized as thioetherification catalysts or alternatively hydrogenation
catalysts.
In the first catalytic distillation reactor column, reaction of the diolefins
with the
sulfur compounds is selective over the reaction of hydrogen with olefinic
bonds. The
preferred catalysts are palladium and/or nickel or a Ni / Pd dual bed as shown
in U.S.
Pat. No. 5,595,643, since in the first catalytic distillation reactor column
the sulfur
removal is carried out with the intention to preserve the olefins. Although
the metals
are normally deposited as oxides, other forms may be used. The nickel is
believed to
be in the sulfide form during the hydrogenation.
[0070] Another suitable catalyst for the thioetherification reaction may be
0.34 wt %
Pd on 7 to 14 mesh alumina spheres, supplied by Sud-Chemie, designated as G-
68C.
The catalyst also may be in the form of spheres having similar diameters. They
may
be directly loaded into standard single pass fixed bed reactors which include
supports
and reactant distribution structures. However, in their regular form they form
too
compact a mass for operation in a catalytic distillation reactor system column
and
must then be prepared in the form of a catalytic distillation structure. The
catalytic
distillation structure must be able to function as catalyst and as mass
transfer medium.
The catalyst must be suitably supported and spaced within the column to act as
a
catalytic distillation structure.
[0071] Without being bound to any specific theory, the catalyst is believed
to be the
hydride of palladium which is produced during operation. The hydrogen rate to
the
24
CA 02817065 2013 05 06
WO 2012/064466 PCT/US2011/056627
catalytic reactor must be sufficient to maintain the catalyst in the active
form because
hydrogen is lost from the catalyst by hydrogenation, but kept below that which
would
cause flooding of the column which is understood to be the "effectuating
amount of
hydrogen" as that term is used herein. Generally the mole ratio of hydrogen to
diolefins and acetylenes in the feed is at least 1.0 to 1.0 and preferably 2.0
to 1Ø
[0072] In second and subsequent catalytic distillation reactor columns and
catalytic
fixed bed reaction zones used in embodiments disclosed herein, it may be the
purpose
of the catalyst to destroy the sulfur compounds to produce a hydrocarbon
stream
containing hydrogen sulfide, which is easily separated from the heavier
components
therein. Hydrogen and hydrogen sulfide may be separated from heavy hydrocarbon
components in a stripping column, as described above. The focus of these
catalytic
reactions that occur after the first catalytic distillation reactor column is
to carry out
destructive hydrogenation of the sulfides and other organic sulfur compounds.
[0073] Catalysts useful as the hydrodesulfurization catalyst in the
reaction zones of
the catalytic distillation reactor systems may include Group VIII metals, such
as
cobalt, nickel, palladium, alone or in combination with other metals, such as
molybdenum or tungsten, on a suitable support, which may be alumina, silica-
alumina, titania-zirconia or the like. Normally the metals are provided as the
oxides
of the metals supported on extrudates or spheres and as such are not generally
useful
as distillation structures. Alternatively, catalyst may be packaged in a
suitable
catalytic distillation structure, which characteristically can accommodate a
wide range
of typically manufactured fixed-bed catalyst sizes.
[0074] The catalysts may contain components from Group V, VIB, VIII metals
of the
Periodic Table or mixtures thereof The incorporation of the distillation
column
reactor systems may reduce the deactivation of catalysts and may provide for
longer
runs than the fixed bed hydrogenation reactors of the prior art. The Group
VIII metal
may also provide increased overall average activity. Catalysts containing a
Group
VIB metal, such as molybdenum, and a Group VIII metal, such as cobalt or
nickel,
are preferred. Catalysts suitable for the hydrodesulfurization reaction
include cobalt-
molybdenum, nickel-molybdenum and nickel-tungsten. The metals are generally
present as oxides supported on a neutral base such as alumina, silica-alumina
or the
CA 02817065 2015-01-21
like. The metals are converted to the sulfide either in use or prior to use by
exposure
to sulfur compound containing streams and hydrogen.
[0075] The catalyst may also catalyze the hydrogenation of the olefins and
dienes
contained within the light cracked naphtha and to a lesser degree the
isomerization of
some of the mono-olefins. The hydrogenation, especially of the mono-olefins in
the
lighter fraction, may not be desirable.
[0076] The catalyst typically is in the form of extrudates having a
diameter of 1/8,
1/16 or 1/32 inches and an L/D of 1.5 to 10. The catalyst also may be in the
form of
spheres having similar diameters. They may be directly loaded into standard
single
pass fixed bed reactors which include supports and reactant distribution
structures.
However, in their regular form they form too compact a mass for operation in
the
catalytic distillation reactor system column and must then be prepared in the
form of a
catalytic distillation structure. As described above, the catalytic
distillation structure
must be able to function as catalyst and as mass transfer medium. The catalyst
must
be suitably supported and spaced within the column to act as a catalytic
distillation
structure.
[0077] In some embodiments, the catalyst is contained in a structure as
disclosed in
U.S. Patent No. 5,730,843. In other embodiments, catalyst is contained in a
plurality
of wire mesh tubes closed at either end and laid across a sheet of wire mesh
fabric
such as demister wire. The sheet and tubes are then rolled into a bale for
loading into
the distillation column reactor. This embodiment is described, for example, in
U.S.
Patent No. 5,431,890. Other useful catalytic distillation structures are
disclosed in
U.S. Patent Nos. 4,731,229, 5,073,236, 5,431,890 and 5,266,546.
10078] Hydrodesulfurization catalysts described above with relation to the
operation
of the catalytic distillation reactor systems may also be used in the fixed
bed reactors.
In selected embodiments, catalysts used in the fixed bed reactors may include
hydrodesulfurization catalysts that only promote the desulfurization of
mercaptan
species, which are among the easiest to convert to hydrogen sulfide.
Conditions in the
fixed bed reactors, including high temperature and high hydrogen mole
fractions, are
conducive to olefin saturation. For preservation of olefin content and
conversion of
26
CA 02817065 2013 05 06
WO 2012/064466 PCT/US2011/056627
mercaptans to hydrogen sulfide at these conditions, suitable catalysts may
include
nickel catalysts with very low molybdenum promotion, or no promoters at all,
and
molybdenum catalysts with very low copper promotion, or no promoters at all.
Such
catalysts may have lower hydrogenation activity, promoting the desulfurization
of the
mercaptan species without significant loss of olefins.
[0079] The effluent streams from the catalytic distillation reactor
systems may be
condensed in one or more stages, separating the hydrocarbons from the hydrogen
sulfide and the hydrogen. As described above, it may be advantageous to use a
hot
drum ¨ cold drum system to limit the formation of recombinant mercaptans. The
recovered hydrogen may be compressed and recycled to various portions of the
hydrodesulfurization systems described herein.
[0080] As mentioned above, heavy hydrocarbons may act as a diluent in
fixed bed
reactor 74 in embodiments disclosed herein. Dilution may result in a decreased
driving force for the reverse reaction (recombinant mercaptan formation) as
well as
aid in olefin preservation. The heavy gasoline fraction recycle may dilute the
olefins
and hydrogen sulfide in the overhead fraction fed to the fixed bed reactor.
This may
reduce the amount of hydrogen required to provide dilution in the fixed bed
reactors,
and may also reduce the pressure drop across the associated control valve.
This non-
hydrogen dilution of the fixed bed reactor feed may in turn reduce the power
required
to run compressors, due to decreased hydrogen traffic.
[0081] As described above, embodiments disclosed herein may provide for
the
production of a high end point gasoline, such as may be recovered by one or
more of
flow lines 94, 84, and 82, having a total sulfur content of less than 50, 20,
or even 10
ppm by weight.
[0082] After treatment according to the processes described herein, the
sulfur content
of the C5/C6 side draw product recovered via flow line 16 may be less than
about 50
ppm in some embodiments; less than 40 ppm in other embodiments; less than 30
ppm
in other embodiments; less than 20 ppm in other embodiments; less than 10 ppm
in
other embodiments; less than 5 ppm in other embodiments; and less than 1 ppm
in yet
other embodiments, where each of the above are based on weight.
[0083] After treatment according to the processes described herein, the
sulfur content
of the hydrocarbon fraction recovered via flow line 82 may be less than about
50 ppm
27
CA 02817065 2013 05 06
WO 2012/064466 PCT/US2011/056627
in some embodiments; less than 40 ppm in other embodiments; less than 30 ppm
in
other embodiments; less than 20 ppm in other embodiments; less than 10 ppm in
other
embodiments; less than 5 ppm in other embodiments; and less than 1 ppm in yet
other
embodiments, where each of the above are based on weight.
100841 After treatment according to the processes described herein, the
sulfur content
of the intermediate hydrocarbon fraction recovered via flow line 94 may be
less than
about 50 ppm in some embodiments; less than 40 ppm in other embodiments; less
than 30 ppm in other embodiments; less than 20 ppm in other embodiments; less
than
ppm in other embodiments; less than 5 ppm in other embodiments; and less than
1
ppm in yet other embodiments, where each of the above are based on weight.
[0085] After treatment according to the processes described herein, the
sulfur content
of the heavy hydrocarbon fraction recovered via flow line 82 may be less than
about
50 ppm in some embodiments; less than 40 ppm in other embodiments; less than
30
ppm in other embodiments; less than 20 ppm in other embodiments; less than 10
ppm
in other embodiments; less than 5 ppm in other embodiments; and less than 1
ppm in
yet other embodiments, where each of the above are based on weight.
100861 In contrast to typical hydrodesulfurization processes, which often
use harsh
operating conditions resulting in significant loss of olefins, desulfurized
products
resulting from the processes disclosed herein may retain a significant portion
of the
olefins, resulting in a higher value end product. In some embodiments,
products
resulting from the processes described herein may have an overall olefins
concentration ranging from 5 to 55 weight percent; from about 10 to about 50
weight
percent in other embodiments; and from about 20 to about 45 weight percent in
other
embodiments. As compared to the initial hydrocarbon feed (flow line 8) the
overall
product streams recovered from embodiments disclosed herein (including flow
lines
16, 94, 82, and 84 as appropriate for the respective embodiments) may retain
at least
25% of the olefins in the initial hydrocarbon feed; at least 30% of the
olefins in the
initial hydrocarbon feed in other embodiments; at least 35% of the olefins in
the initial
hydrocarbon feed in other embodiments; at least 40% of the olefins in the
initial
hydrocarbon feed in other embodiments; at least 45% of the olefins in the
initial
hydrocarbon feed in other embodiments; at least 50% of the olefins in the
initial
hydrocarbon feed in other embodiments; and at least 60% of the olefins in the
initial
hydrocarbon feed in other embodiments.
28
CA 02817065 2013 05 06
WO 2012/064466 PCT/US2011/056627
100871 Advantageously, embodiments disclosed herein may provide for the
production of a low sulfur content gasoline fraction (having <10 ppm S, by
weight in
some embodiments) from a hydrocarbon feedstock having an ASTM D-86 end point
of at least 350 F, and even from a high end point hydrocarbon feedstock (e.g.,
having
an end point of greater than 450 F, 470 F, 500 F, 525 F, or 550 F and
containing
hindered sulfur compounds). Additionally, due to the treatment at varying
severities
and selected operating conditions, including dilution with heavy hydrocarbons
or use
of appropriate catalysts, embodiments disclosed herein may provide for one or
more a
high retention of olefins, select saturation of olefins and/or aromatics, and
reduced
recombinant mercaptan formation. A further benefit of processes according to
embodiments disclosed herein is the ability to control the end point of the
intermediate gasoline fraction produced.
100881 While the invention has been described with respect to a limited
number of
embodiments, those skilled in the art, having benefit of this disclosure, will
appreciate
that other embodiments can be devised which do not depart from the scope of
the
invention as disclosed herein. Accordingly, the scope of the invention should
be
limited only by the attached claims.
29