Note: Descriptions are shown in the official language in which they were submitted.
CA 02817351 2013-05-08
WO 2012/066299
PCT/GB2011/001631
- 1 -
METHOD AND SYSTEM FOR ELECTROLYTICALLY REDUCING A SOLID
FEEDSTOCK
The invention relates to an electrolysis method using apparatus comprising an
electrode module or assembly.
Background
The present invention concerns a method for the reduction of a solid feedstock
comprising a metal compound or compounds, such as a metal oxide, to form a
reduced product. As is known from the prior art, electrolytic processes may be
io used, for example, to reduce metal compounds or semi-metal compounds to
metals, semi-metals, or partially-reduced compounds, or to reduce mixtures of
metal compounds to form alloys. In order to avoid repetition, the term metal
will
be used in this document to encompass all such products, such as metals,
semi-metals, alloys, intermetallics, and partially-reduced products.
In recent years there has been great interest in the direct production of
metal by
reduction of a solid feedstock, for example, a solid metal-oxide feedstock.
One
such direct reduction process is the Cambridge FFC electro-decomposition
process (as described in WO 99/64638). In the FFC process a solid
compound, for example a solid metal oxide, is arranged in contact with a
cathode in an electrolysis cell comprising a fused salt. A potential is
applied
between the cathode and an anode of the cell such that the compound is
reduced. In the FFC process, the potential that produces the solid compound
is lower than a deposition potential for a cation from the fused salt. For
example, if the fused salt is calcium chloride, then the cathode potential at
which the solid compound is reduced is lower than a deposition potential for
depositing metallic calcium from the salt.
Other reduction processes for reducing feedstock in the form of a cathodically-
connected solid metal compound have been proposed, such as the polar
process described in WO 03/076690 and the process described in
WO 03/048399.
Conventional implementations of the FFC process and other electrolytic
reduction processes typically involve the production of a feedstock in the
form
CA 02817351 2013-05-08
WO 2012/066299
PCT/GB2011/001631
- 2 -
of a preform or precursor, fabricated from a powder of the solid compound to
be reduced. This preform is then painstakingly coupled to a cathode to enable
the reduction to take place. Once a number of preforms have been coupled to
the cathode, then the cathode can be lowered into the molten salt and the
preforms can be reduced. It can be highly labour intensive to produce the
preforms and then attach them to the cathode. Although this methodology
works well on a laboratory scale, it does not lend itself to the mass
productions
of metal on an industrial scale.
It is an aim of the invention to provide an electrolysis method that is more
suitable for the reduction of a solid feedstock on an industrial scale.
Summary of Invention
The invention provides a method of electrolytically reducing a solid feedstock
as defined in the appended independent claim to which reference should now
be made. Preferred or advantageous features of the invention are set out in
dependent subclaims.
Thus, a method of electrolytically reducing a solid feedstock may comprise the
zo steps of: positioning an electrode module comprising at least one
electrode in a
first position for loading of feedstock, loading solid feedstock onto the
electrode
module, moving the electrode module from the first position and engaging the
electrode module with an electrolysis chamber such that the feedstock is in
contact with a molten salt within the electrolysis chamber, and applying a
voltage to the electrode module such that the solid feedstock is reduced.
Preferably the electrode module comprises at least a cathode and an anode
coupleable to a power supply such that a potential can be generated between
the anode and the cathode. The electrode module may comprise one or more
bipolar electrodes.
Preferably the solid feedstock is loaded such that it contacts a cathode or a
cathodic surface of a bipolar electrode.
CA 02817351 2013-05-08
WO 2012/066299
PCT/GB2011/001631
-3 -
it may be advantageous that the electrode module is transferred from the first
position within a transfer module. The transfer module may take the form of a
housing defining a chamber into which the electrode module may be raised.
Preferably the transfer module is sealable such that the electrode module may
be transferred in controlled conditions, for example under an inert
atmosphere.
It may be particularly preferable that the electrode module is heated to a
predetermined temperature prior to engaging with the electrolysis chamber.
In a preferable embodiment the electrolysis chamber contains a molten salt
when the electrode module is engaged. If the electrode module is not at an
appropriate temperature then thermal distortion or thermal shock of
components of the electrode module may occur and may result in failure of
components of the electrode module. Thus it is preferable that the electrode
module is heated to a temperature close to the temperature of the molten salt.
The predetermined temperature may therefore be within the range of about
500 C to 1200 C, depending on the temperature of the molten salt. Particularly
preferable temperatures are in the range of 700 C to 1000 C, for example
about 800 C or 850 C.
Advantageously, the electrode module may be heated under an inert
atmosphere within the transfer module. The transfer module may comprise
heating elements that raise the temperature within the module to a
predetermined temperature in order to heat the electrode module.
Alternatively,
the transfer module may comprise means for allowing a heated gas to be
introduced into the transfer module to heat the electrode module.
It may be preferable that the electrode module is transferred from the
transfer
module to the heating station in order to be heated to the predetermined
temperature. For example, the transfer module may engage with a heating
station and transfer the electrode module into a discrete heating station to
allow
the electrode module to be heated. In this embodiment the transfer module
need not comprise heating elements itself.
Thus it may be preferable that the electrode module is transferred from the
loading station within the transfer module, lowered into the heating station,
CA 02817351 2013-05-08
WO 2012/066299
PCT/GB2011/001631
- 4 -
heated to the predetermined temperature, and raised back into the transfer
module to be transferred to the electrolysis chamber.
The electrode module is preferably sealed within the transfer chamber of the
transfer module by the closing of a closure. A preferable closure is a gate
valve
in which the gate is slideable to seal the transfer chamber within the
transfer
module.
An opening of the electrolysis chamber, i.e. an opening through which the
electrode module may be passed to engage with the electrolysis chamber, is
preferably closed by an openable closure. A particularly preferable closure is
a
gate valve that is openable to allow the electrode module to pass to the
electrolysis chamber.
is It may be desirable to remove the electrode module from the electrolysis
chamber after electrolysis in order to recover the reduced feedstock.
Preferably the removal of the electrode module occurs at or near to the
working
temperature of the electrolysis chamber and under conditions in which a molten
salt contained within the electrolysis chamber is still in the molten state.
Preferably the electrode module is lifted from the electrolysis chamber into a
transfer module.
It may be advantageous that the electrode module is cooled under an inert
atmosphere within the transfer module after being removed from the
electrolysis chamber. If the electrode module is at a high temperature, for
example 800 C, it is important that the module does not come into contact with
oxygen or air until the temperature is reduced sufficiently to avoid auto-
ignition
of any carbon components of the electrode module or the rapid oxidation of any
reduced feedstock located on the electrode module.
The electrode module may be cooled under an inert atmosphere within the
transfer module after being removed from the electrolysis chamber. Thus, the
transfer module may comprise cooling means such as water cooling tubes or
CA 02817351 2013-05-08
WO 2012/066299
PCT/GB2011/001631
-5 -
may comprise means for passing a cooling gas through the transfer module in
order to reduce the temperature of the electrode module.
Alternatively, the electrode module may be transferred within the transfer
module to a discrete cooling station to be cooled to a predetermined
temperature under an inert atmosphere.
It is preferable that the cooling station comprises a cooling chamber within
which the electrode module may be engaged to effect cooling. The method
may therefore comprise the steps of transferring the electrode module to the
cooling station within a transfer module, lowering the electrode module into
the
cooling station, cooling the electrode module to the predetermined
temperature,
and raising the electrode module back into the transfer module to be
transferred away from the cooling station.
The molten salt remaining on the electrode module will solidify as the
electrode
module is cooled. Thus, the electrode module, once cooled, will be coated in a
film of solidified salt. It may be advantageous, therefore, for the electrode
module to be transferred to a washing station for washing salt from the
reduced
feedstock. The washing station may comprise washing apparatus suitable for
directing jets of water towards the electrode module in order to wash salt
from
the feedstock. The washing station may further comprise means for collecting
used water from the washing process.
It may be advantageous that the electrode module is transferred to a discrete
unloading station to facilitate access to the electrode module for unloading
the
reduced feedstock. It is particularly preferable that the electrode module has
removable trays that can be decoupled from the electrode module. Thus, it
may be preferable that the solid feedstock is loaded onto removable trays
separate from the electrode module and then the removable trays are coupled
to the electrode module to load feedstock onto the electrode module. It may
also be advantageous that the trays may be removed from the electrode
module in order to facilitate unloading of reduced feedstock.
CA 02817351 2013-05-08
WO 2012/066299
PCT/GB2011/001631
- 6 -
Preferably the feedstock is loaded such that it contacts a cathode structure
of
the electrode module for example the surface of a cathode or a cathodic
surface of a bipolar electrode. This is essential if the reaction to reduce
the
feedstock is to occur using the FFC process. Other reduction process may be
used, however
It is particularly preferable that the electrolysis reaction in the method
proceeds
by electro-deoxidation of the solid feedstock, for example electro-deoxidation
by means of the FFC process.
The method may be applicable for use with any electrode module that is
capable of being loaded with feedstock and engaged within an electrolysis
chamber for electrolysis of the feedstock. There may be a number of specific
embodiments of electrode module that may be preferably used in the method.
In a preferred embodiment of the invention, it may be advantageous that the
electrode module is a removable electrode module for engagement with an
electrolysis chamber, the removable electrode module comprising, a first
electrode, a second electrode, and
a suspension structure comprising a suspension rod coupled, preferably at one
end of the rod, to the first electrode, in which the second electrode is
suspended by, or supported by, the suspension structure and in which the
suspension structure comprises at least one electrically-insulating spacer
element for retaining the second electrode in spatial separation from the
first
electrode.
In a further preferred embodiment of the invention, it may be advantageous
that
the electrode module is a removable electrode module for engagement with an
electrolysis chamber, the removable electrode module comprising, an anode,
and a cathode for supporting a portion of solid feedstock for reduction by
electrolysis in a molten salt electrolyte, the feedstock being retained in
contact
with the cathode.
In a further preferred embodiment of the invention, it may be advantageous
that
the electrode module is a removable electrode module for engagement with an
CA 02817351 2013-05-08
WO 2012/066299
PCT/GB2011/001631
- 7 -
electrolysis chamber, the removable electrode module comprising, a first
electrode, and a cover, in which, when the removable electrode is engaged with
the electrolysis apparatus, the first electrode is located within the
electrolysis
chamber such that it may be used for electrolysis, and the cover spans an
s opening of the electrolysis chamber.
In a further preferred embodiment of the invention, it may be advantageous
that
the electrode module is a removable electrode module for engagement with an
electrolysis chamber, the removable electrode module comprising, a lifting
element to enable the module to be lifted, a first electrode coupled to a
lower
end of a suspension rod, and a resilient means disposed between the lifting
element and an upper end of the suspension rod.
The various aspects and embodiments of the invention described herein are
particularly suitable for the production of metal by the reduction of a solid
feedstock comprising a solid metal oxide. Pure metals may be formed by
reducing a pure metal oxide and alloys and intermetallics may be formed by
reducing feedstocks comprising mixed metal oxides or mixtures of pure metal
oxides.
Some reduction processes may only operate when the molten salt or electrolyte
used in the process comprises a metallic species (a reactive metal) that forms
a more stable oxide than the metallic oxide or compound being reduced. Such
information is readily available in the form of thermodynamic data,
specifically
Gibbs free energy data, and may be conveniently determined from a standard
Ellingham diagram or predominance diagram or Gibbs free energy diagram.
Thermodynamic data on oxide stability and Ellingham diagrams are available
to, and understood by, electrochemists and extractive metallurgists (the
skilled
person in this case would be well aware of such data and information).
Thus, a preferred electrolyte for a reduction process may comprise a calcium
salt. Calcium forms a more stable oxide than most other metals and may
therefore act to facilitate reduction of any metal oxide that is less stable
than
calcium oxide. In other cases, salts containing other reactive metals may be
used. For example, a reduction process according to any aspect of the
CA 02817351 2013-05-08
WO 2012/066299
PCT/GB2011/001631
- 8 -
invention described herein may be performed using a salt comprising lithium,
sodium, potassium, rubidium, caesium, magnesium, calcium, strontium, barium,
or yttrium. Chlorides or other salts may be used, including mixture of
chlorides
or other salts.
By selecting an appropriate electrolyte, almost any metal oxide may be capable
of reduction using the methods and apparatuses described herein. In
particular,
oxides of beryllium, boron, magnesium, aluminium, silicon, scandium, titanium,
vanadium, chromium, manganese, iron, cobalt, nickel, copper, zinc,
io germanium, yttrium, zirconium, niobium, molybdenum, hafnium, tantalum,
tungsten, and the lanthanides including lanthanum, cerium, praseodymium,
neodymium, samarium, and the actinides including actinium, thorium,
protactinium, uranium, neptunium and plutonium may be reduced, preferably
using a molten salt comprising calcium chloride.
The skilled person would be capable of selecting an appropriate electrolyte in
which to reduce a particular metal oxide, and in the majority of cases an
electrolyte comprising calcium chloride will be suitable.
Specific embodiments of the invention
Specific embodiments of the invention will now be described with reference to
the figures in which;
Figure 1 is a perspective-view of a removable electrode module embodying one
or more aspects of the invention;
Figure 2 is a side-view of the removable electrode module of Figure 1;
Figure 3 is a plan-view of the removable electrode module of Figure 1;
Figure 4 is a cross-sectional side-view of the removable electrode module of
Figure 1 illustrating the structure of the various electrodes and supporting
components of the removable electrode module;
CA 02817351 2013-05-08
WO 2012/066299
PCT/GB2011/001631
- 9 -
Figure 5 is a schematic cross-sectional illustration of an electrolysis
apparatus
having an electrolysis chamber suitable for receiving the removable electrode
module embodiment illustrated in Figure 1;
Figure 6 is a schematic cross-sectional illustration showing the removable
electrode module of Figure 1 in engagement with the electrolysis apparatus
illustrated in Figure 5;
Figure 7 is a schematic cross-sectional illustration showing the removable
io electrode module of Figure 1 housed within a transfer module seated on
the
electrolysis apparatus of Figure 5, in preparation for engaging the electrode
module with the electrolysis chamber of the electrolysis apparatus;
Figure 8 is a schematic cross-sectional illustration showing the removable
electrode module of Figure 1 after it has been passed from a transfer module
and engaged with the electrolysis apparatus of Figure 5;
Figure 9 is a perspective-view of a removable cathode-tray structure suitable
for use as a cathode-tray in the removable electrode module of Figure 1;
Figure 10 is a plan-view of the cathode-tray structure of Figure 9;
Figure 11 is a side-view of the cathode-tray structure of Figure 9;
Figure 12 is a cross-sectional illustration of a second embodiment of a
removable electrode module according to one or more aspects of the invention;
Figure 13 is a cross-sectional illustration of a third embodiment of a
removable
electrode module according to one or more aspects of the invention.
Figure 14 is a schematic cross-sectional illustration of an alternative method
of
coupling a removable electrode module according to an embodiment of the
invention to a lifting means;
CA 02817351 2013-05-08
WO 2012/066299
PCT/GB2011/001631
- 10 -
Figure 15 is a schematic illustration of an electrode module situated at a
loading station with a transfer module located above the electrode module;
Figure 16 is a schematic illustration of an electrode module held within a
transfer module above a heating station;
Figure 17 is a schematic illustration showing a transfer module engaged with a
heating station;
Figure 18 is a schematic illustration of an electrode module held within a
transfer module above a cooling station;
Figure 19 is a schematic illustration showing a transfer module engaged with a
cooling station; and
Figure 20 is a schematic illustration of an electrode module undergoing
washing at a washing station.
A removable electrode module according to a first embodiment of the invention
zo - will now be described with reference to Figures 1 to 4. The electrode
module 10 comprises a terminal anode 20, a terminal cathode 30, and seven
bipolar electrodes 40, 41, 42, 43, 44, 45, 46 distributed in spatial
separation
from each other above the terminal cathode 30 and below the terminal
anode 20. The terminal cathode 30, the terminal anode 20, and each of the
intermediate bipolar electrodes 40, 41, 42, 43, 44, 45, 46, are substantially
circular in shape and have a diameter of about 550 mm.
The terminal cathode 30 has a composite structure consisting of a lower
portion
and an upper portion. The lower portion is a substantially cathode base
element 30a formed from a disc of grade 310 stainless steel having a diameter
of 550 mm and a thickness of 60 mm. The upper portion is provided by a
removable tray-assembly 30b seated on an upper surface of the base
element 30a. The removable tray-assembly 30b is illustrated in Figures 9, 10
and 11 and will be described in more detail below. A central hole having a
CA 02817351 2013-05-08
WO 2012/066299
PCT/GB2011/001631
- 11 -
diameter of about 130 mm is defined through the central portion of the
assembled tray-assembly 30b.
Each of the seven bipolar electrodes 40, 41, 42, 43, 44, 45, 46, has a
composite structure comprising a lower portion 40a, 41a, 42a, 43a, 44a,
45a, 46a and an upper, or tray-assembly, portion 40b, 41b, 42b, 43b, 44b,
45b, 46b. The upper, tray-assembly, portions of each of the bipolar electrodes
are identical to the upper, tray-assembly, portion 30b of the terminal
cathode 30.
The lower portions 40a, 41a, 42a, 43a, 44a, 45a, 46a of each of the bipolar
electrodes are formed from discs of carbon, for example graphite, having a
diameter of 550 mm and a thickness of 60 mm. A hole having a diameter of
about 130 mm is defined through the central portion of each of the bipolar
electrodes 40, 41, 42, 43, 44, 45, 46.
On a lower surface of each bipolar electrode a plurality of channels 50 of
approximately 10 mm in width are defined in order to aid the channelling of
gas
evolved on the lower surface of each bipolar electrode to the outer
zo circumference of each bipolar electrode.
A first bipolar electrode 40 is supported directly above the terminal cathode
30
by a first electrically-insulating spacer element 60. The first electrically-
insulating spacer element 60 is a tubular spacer formed from alumina. The
first
electrically-insulating spacer element may alternatively be formed from other
electrically-insulating ceramic materials such as yttria or boron nitride.
In some embodiments the first electrically-insulating spacer element 60 is
seated directly on the cathode base element 30a. In other embodiments, a
ceramic insert 70, formed from a ceramic material that will not reduce under
the
cell operating conditions, is disposed between the terminal cathode base
element 30a and the first electrically-insulating spacer element 60.
A lower surface of the lower portion 40a of the first bipolar electrode 40 is
seated on the first electrically-insulating spacer element 60 such that the
first
CA 02817351 2013-05-08
WO 2012/066299
PCT/GB2011/001631
- 12 -
bipolar electrode 40 is supported, through the first electrically-insulating
spacer
element 60, by the terminal cathode base element 30a.
The second bipolar electrode 41 is supported directly above the first bipolar
electrode 40 by means of a second electrically-insulating spacer element 61.
The second electrically-insulating spacer element 61 is a tubular alumina
element that is substantially identical to the first electrically-insulating
spacer
element 60. The second electrically-insulating spacer element is seated on an
upper surface of the lower portion 40a of the first bipolar electrode 40. A
lower
surface of the lower portion 41a of the second bipolar electrode is, in turn,
seated on the second electrically-insulating spacer element such that the
second bipolar electrode 41 is supported, by means of the second electrically-
insulating spacer element 61, by the first bipolar electrode.
This support structure is repeated for each of the bipolar electrodes. Thus, a
third bipolar electrode 42 is supported by the second bipolar electrode 41 by
means of a third electrically-insulating spacer element 62. A fourth bipolar
electrode 43 is supported by the third bipolar electrode 42 by means of a
fourth
electrically-insulating spacer element 63. A fifth bipolar electrode 44 is
supported by the fourth bipolar electrode 43 by means of a fifth electrically-
insulating spacer element 64. A sixth bipolar electrode 45 is supported by the
fifth bipolar electrode 44 by means of a sixth electrically-insulating spacer
element 65. A seventh bipolar electrode 46 is supported by the sixth bipolar
electrode 45 by means of seventh electrically-insulting spacer element 46.
The terminal anode 20 is formed from a disc of graphite having a diameter of
550 mm and a thickness of 60 mm. Channels are defined on the lower surface
of the anode the same way as defined above in relation to the bipolar
electrodes. One purpose of these channels is to assist the removal of gas
evolved at the lower surface of the terminal anode 20. A hole is defined
through a central portion of the terminal anode 20 having a diameter of about
130 mm. The terminal anode is supported directly above the seventh bipolar
electrode 46 by means of an eighth electrically-insulating spacer element 67.
CA 02817351 2013-05-08
WO 2012/066299
PCT/GB2011/001631
- 13 -
The removable electrode module 10 further comprises an insulating ceramic
cover 100 disposed directly above the terminal anode 20. The cover 100 is
formed from alumina, although any thermally-insulating ceramic material could
be used, and is designed to cover an electrolysis chamber of an electrolysis
apparatus during an electrolysis reaction. The cover 100 is supported by an
upper surface of the terminal anode 20 by means of a ninth electrically-
insulating supporting element 68. The ninth electrically-insulating support 68
is
similar to the electrically-insulating support elements previously described,
but
has greater length.
io
A central hole is defined through the cover 100. Thus, a hole or cavity is
defined that extends downwardly through the removable electrode module from
an upper surface 101 of the cover 100 through the tubular electrically-
insulating
spacer 68, through the centre of the anode, and through each of the bipolar
electrodes and their associated spacer elements. A suspension rod 110
extends through this hole or cavity and is coupled to the cathode base
element 30a of the terminal cathode 30 by means of a thread that engages with
a threaded hole defined in the cathode base element 30a. The suspension
rod 110 does not contact any other electrode or spacing element. At the point
zo that the suspension rod 110 passes through the central hole defined
through
the cover 100, a seal is formed by means of a graphite gland packing, for
example braided graphite rope or other similar gland packing materials 120.
At its upper portion, the suspension rod 110 is coupled to a j-slot type
connector 130. A j-slot connector is a bayonet connector that is well known
for
coupling sections of pipe in the oil industry. The coupling between the
suspension rod and the j-slot connector is achieved by means of washers and
nuts 111.
The suspension rod 110 may be used to lift the entire removable electrode
module 10, for example when raising or lowering the electrode module. In use,
the suspension rod may need to function at high temperatures. Therefore, the
rod 110 and associated nuts and washers 111 that couple the rod 110 to the
j-slot connector 130 are formed from a high nickel alloy suitable for
operation at
high temperatures.
CA 02817351 2013-05-08
WO 2012/066299
PCT/GB2011/001631
- 14 -
The anode 20 is coupled to two graphite risers 21, 22 to enable an electrical
connection to be made between a power supply (not shown) and the terminal
anode 20. The graphite risers 21, 22 are coupled to the terminal anode 20 by
s means of graphite studs 23, 24. The graphite risers 21, 22 extend
vertically
above the terminal anode 20 through holes defined in the cover 100, such that
an electrical connection can be made with an uppermost portion of the risers
when the removable electrode module is located in engagement with an
electrolysis chamber of an electrolysis apparatus. A gap between the
to risers 21, 22 and the associated holes defined through the cover 100
for the
risers to pass through is sealed by means of braided graphite rope or other
similar gland packing materials 25.
The removable electrode module 10 is designed to have three loading or
is support conditions.
In the first of these three conditions, the removable electrode module is
seated
on a lower surface of the cathode base element 30a. In this condition the
weight of all of the bipolar elements, the anode, and the cover are
transferred
zo through the cathode base element 30a and the suspension rod 110 is not
in
tension.
In a second loading condition, the j-slot connector 130 is coupled to a
lifting
mechanism, and the entire weight of the module is supported through the
25 suspension rod 110, which is coupled to the cathode base element 30a.
In a third loading condition, the removable electrode module 10 may be
supported at multiple points on a lower surface 102 of the cover 100. In this
condition the weight of the module is supported by the cover 100 and
30 transferred through the suspension rod 110, which is coupled to the
cathode
base element 30a.
Thus, the module may be free-standing on its cathode base element 30a, it
may be suspended by the j-slot coupling 130 at an upper end of the suspension
35 rod 110, or it may be suspended by the underside 102 of the cover 100.
CA 02817351 2013-05-08
WO 2012/066299
PCT/GB2011/001631
- 15 -
The suspension rod 110 is coated or clad with an electrically-insulating
material
115 throughout its length from the point of coupling to the cathode base
element 30a to the point of sealing with the braided graphite rope 120 as the
suspension rod 110 passes through the cover 100. This electrically-insulating
material is an alumina coating 115, but may be any high temperature
electrically-insulating material. For example, the coating 115 may be boron
nitride. The coating may be applied by any known method, for example by dip
coating or by spray coating.
The removable tray-assembly that forms part of the terminal cathode 30 and
each of the seven bipolar electrodes 40, 41, 42, 43, 44, 45, 46 is illustrated
in
Figures 9, 10 and 11. The tray assembly 30b, 40b, 41b, 42b, 43b, 44b, 45b,
46b, is formed of two couplable portions 151, 152. When coupled together, the
entire tray-assembly is substantially circular and has a diameter of about
542 mm at room temperature. The tray-assembly is metallic and so the
diameter may increase to about 550 mm at the working temperature of the
removable electrode module (usually between about 500 C and 1200 C when
used in an electrolysis reaction in a molten salt) due to thermal expansion.
A base 153, 156 of each of the tray-assembly portions 151, 152 is formed from
a mesh suitable for supporting a solid feedstock. Around the circumference of
the assembled tray-assembly a circumferential lip is raised extending about
mm above the level of the mesh 153, 156. A plurality of downwardly
25 extending feet 155 extend downwards from the circumferential lip 154 by
a
distance of about 10 mm below the level of the mesh 153,156.
The entire tray-assembly may be seated on an upper surface of an associated
electrode portion to form an electrode of the electrode module. For example,
30 a tray assembly 30b may be seated on an upper surface of the terminal
cathode base plate 30a to form a terminal cathode 30, or a tray assembly 40b,
41b, 42b, 43b, 44b, 45b, 46b may be seated on an upper surface of the lower
portion of a bipolar electrode 40a, 41a, 42a, 43a, 44a, 45a, or 46a to form a
bipolar electrode. Electrical contact is made between the tray-assembly and
its
associated electrode portion through the downwardly extending feet 155.
CA 02817351 2013-05-08
WO 2012/066299
PCT/GB2011/001631
- 16 -
When a removable electrode module comprising the removable tray-
assemblies 30b, 40b, 41b, 42b, 43b, 44b, 45b, 46b is located in an
electrolysis
chamber containing a molten salt, molten salt is able to flow into a gap
created
between the upper surface of an electrode portion on which the tray
assembly is seated and the mesh base 153, 156. The molten salt is therefore
able to flow upwardly through the mesh base 153, 156 of the tray-
assembly and, therefore, over any solid feedstock supported on the base
153, 156.
The tray-assembly is formed having a central hole for surrounding an
electrically-insulating spacer element, for example the electrically-
insulating
spacer element 60 that supports the first bipolar electrode 40.
The tray-assembly is formed in two couplable portions, i.e. the first portion
151
and the second portion 152, each portion being substantially semicircular. The
two portions 151,152 are coupleable by means of a stud and slot arrangement.
Studs 160 extend from a mating surface or mating edge 162 of the second
portion and slots 161 for receiving the studs 160 are defined in a
corresponding
zo mating surface 163 of the first portion 151.
In use, each half or each portion 151, 152 of the tray-assembly may be
separately removed from the removable electrode module 10 in order to load
feedstock or unload reduced product.
The removable tray-assemblies form the uppermost portion of the terminal
cathode and each of the bipolar electrodes. These portions of the respective
electrodes become cathodic when the removable electrode module is used for
electrolysis.
The removable tray-assemblies 30b, 40b, 41b, 42b, 43b, 44b, 45b, 46b are
manufactured from 310-grade stainless steel. The removable tray-
assemblies may be made from many other materials, and the choice of material
may depend on the nature of the feedstock to be reduced. For example, it may
be desirable to use a tray-assembly formed from a metal that will not
CA 02817351 2013-05-08
WO 2012/066299
PCT/GB2011/001631
- 17 -
contaminate the reduced product. For example, it may be desirable to form the
cathode tray assembly from tantalum, or tantalum coated metal, where the
removable electrode module is to be used for the reduction of a tantalum oxide
to tantalum metal.
A removable electrode module according to the first specific embodiment
described above may be of particular advantage when used for the reduction of
a solid feedstock in a molten salt electrolyte. The removable tray-assemblies
allow a solid feedstock to be conveniently loaded onto each separate
io removable tray-assembly portion 151, 152 and loaded into the removable
electrode module by seating the loaded tray-assembly portions in an
appropriate position in the electrode module.
At room temperature, the removable electrode module 10 has a total height
from the lower surface of the cathode base plate 30a to the lower surface of
the
cover 100 of 1645 mm. The height from the lower surface of the cathode base
plate 30a to the top of the j-slot connector 130 is 2097 mm. As stated above,
the diameter of the electrodes 30, 40-46 is 550 mm. The maximum diameter of
the cover 100 is 830 mm. Some of these dimensions will be subject to change
zo as the temperature varies. In particular, the height values may be
increased by
5 to 10 mm at the working temperature of the electrode module.
The removable electrode module 10 according to the first embodiment of the
invention described above may be advantageously used with any electrolysis
apparatus having an electrolysis chamber suitable for receiving the module 10
in engagement. A schematic illustration of such an electrolysis apparatus 200
is provided by Figure 5.
The electrolysis apparatus 200 comprises a housing 210 containing an
electrolysis chamber 220 defined within a graphite crucible 230, an upper
rim 231 of the graphite crucible 230 defining an opening into the electrolysis
chamber 220. An upper surface of the rim 231 is coated with a 15 mm thick
section of a resilient graphite material for sealing the rim 231 against an
underside of the cover 100 of the removable electrode module 10. The sealing
CA 02817351 2013-05-08
WO 2012/066299
PCT/GB2011/001631
- 18 -
material seated on the upper rim 231 is a braided graphite gland packing
material that may be deformed and regain its shape.
The housing 210 furthermore contains furnace heating elements 240 for
maintaining the temperature of the graphite crucible 230, a molten salt inlet
250
and a molten salt outlet 260 for allowing a flow of molten salt through the
electrolysis chamber 220. A gas vent line 270 is provided towards an upper
portion of the electrolysis chamber 220 to allow the escape of gases evolved
during any electrolysis reaction taking place within the electrolysis chamber.
A DC supply cathode bus bar 280 is coupled to the graphite crucible 230 and
enables the entire graphite crucible 230 to directly couple the graphite
crucible
to a power supply.
The graphite crucible 230 is lined with an alumina liner 290. The alumina
is liner 290 provides an electrical insulation between side-walls of the
graphite
crucible 230 and any removable electrode module 10 engaged within the
electrolysis chamber 220. Although made from alumina, the liner may be made
from any suitable electrically insulating ceramic material that is
substantially
inert under the processing conditions within the electrolysis chamber 220.
An upper portion of the electrolysis apparatus comprises a gate-valve type
closure 300 that enables external access to be provided to the electrolysis
chamber 220. The gate-valve closure 300 comprises a gate 310 formed from a
thermal barrier material, for example a ceramic material. An actuation
device 320 allows the gate 310 to slide back-and-forth to open and close the
gate valve 300, thereby allowing access to the electrolysis chamber 220 within
the electrolysis apparatus 200.
Figure 6 illustrates a removable electrode module, according to the first
embodiment described above in relation to Figures 1 to 4, engaged with an
electrolysis apparatus of the type illustrated in Figure 5.
A lower internal surface of the graphite crucible 230 is raised forming a
pedestal 232. When engaged with the electrolysis chamber 220, the
removable electrode module 10 is seated on this raised pedestal 232 within the
CA 02817351 2013-05-08
WO 2012/066299
PCT/GB2011/001631
- 19 -
graphite crucible 230. Thus, the lower surface of the terminal cathode 30 of
the
removable electrode module is in physical and electrical contact with an
internal
surface of the graphite crucible 230.
The bipolar electrodes 40-46 and the anode 20 of the removable electrode
module 10 are situated within a portion of the electrolysis chamber that is
electrically-insulated from the side-wall of the crucible 230 by the ceramic
liner 290. A lower surface 102 of the cover 100 of the removable electrode
module 10 makes contact with the upper rim 231 of the graphite crucible 230.
As the cover comes into contact with the rim 231 the flexible graphite sealing
material seated on the upper rim deforms to enable a seal to be made. It is
noted that the graphite sealing material could alternatively or additionally
be
located on the lower surface 102 of the cover 100.
In use, the temperature within the electrolysis chamber may vary considerably.
Thus, the dimensions of some components of the removable electrode module,
for example the suspension rod 110, may change by several millimetres. The
resilient material seated on the upper rim of the graphite crucible 230
preferably
has sufficient resilience and deformability to accommodate any such thermal
distortion and maintain a viable seal with the underside 102 of the cover 100.
The anode risers 21, 22 of the removable electrode module extend upwardly
through the cover 100. Electrical contact may be made with these risers by
actuatable DC anode bus bars 250, which may be actuated to contact the
anode risers and thus provide an electrical connection between the anode and
the power supply.
In use, the electrolysis chamber 220 is filled with a molten salt and a
removable
electrode module loaded with a reduceable feedstock is engaged with the
electrolysis chamber. The anode bus bars are actuated to contact the anode
risers 21, 22 and a potential is applied between the anode 20 (by way of the
anode risers and the actuatable anodic bus bars 250) and the terminal
cathode 30 (by way of the graphite crucible 230 and the cathodic DC bus
bar 280). The potential applied is sufficient to reduce the feedstock. The
CA 02817351 2013-05-08
WO 2012/066299
PCT/GB2011/001631
- 20 -
required potential may vary dependent upon the type of feedstock and the
composition of the molten salt.
In many situations, in particular for the reduction of a solid feedstock in a
molten salt electrolyte, it may be advantageous to be able to engage a
removable electrode module with an electrolysis chamber of an electrolysis
apparatus that is at or near to its working temperature. For many molten salt
electrolytes this means that the electrolysis chamber contains a molten salt
at a
temperature of between 500 C and 1200 C. If a removable electrode module
at room temperature was to be inserted into an electrolysis chamber containing
a molten salt at a temperature of, for example, 1000 C, then the components of
the removable electrode module would be likely to undergo severe and rapid
thermal distortion. In particular, the ceramic components of the removable
electrode module may undergo severe thermal shock and, thus, fail. As a
complication, if the removable electrode module as described above in relation
to the first embodiment of a removable electrode module were pre-heated to a
temperature of 1000 C in air, the graphite components of the removable
electrode module would combust.
It may be particularly desirable to be able to remove a removable electrode
module from an electrolysis chamber of an electrolysis apparatus immediately
after electrolysis has taken place and without waiting for the electrolysis
chamber to cool. Care would need to be taken to ensure that oxygen
containing atmosphere such as air did not come into contact with the
removable electrode module at high temperatures. Failure to safeguard
against this could result in the graphite components of the electrode module
combusting, reduced metallic product located within the removable electrode
module combusting or oxidising and severe thermal deformations and failures
occurring due to rapid cooling of the module.
In order to allow the removable electrode module to be engaged with the
electrolysis chamber of the electrolysis apparatus at temperature near to
working temperature, and in order to allow the removable electrode module to
be disengaged from the electrolysis chamber at a temperature close to working
temperature, it is desirable that the removable electrode module can be
CA 02817351 2013-05-08
WO 2012/066299
PCT/GB2011/001631
-21 -
withdrawn into a transfer module before being transferred or transported to
the
electrolysis apparatus. A transfer module may include heating and/or cooling
elements. A transfer module may simply be a shroud within which an inert
atmosphere can be maintained that insulates a preheated electrode module
prior to loading into the electrolysis chamber or insulates an electrode
module
recently disengaged from an electrolysis chamber prior to being transported to
a separate location for a controlled cooling.
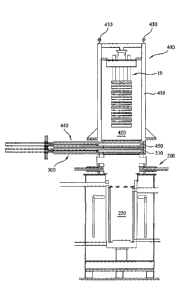
Figure 7 illustrates a removable electrode module as described above in
io relation to Figures 1 to 4 located within an embodiment of a removable
transfer
module 400. The removable transfer module 400 comprises a housing 410
formed from 310-grade stainless steel and lined with a refractory lining. The
refractory lining may be a ceramic brick lining or any other suitable
material,
such as fibreboard, that thermally insulates the interior of the transfer
module.
The interior of the transfer module comprises a transfer cavity 420 within
which
a removable electrode module 10 may be located.
A transfer module may comprise a means for coupling to the j-slot connector at
the top of the removable transfer module and means for withdrawing the
removable transfer module into the transfer chamber 420. For example, the
transfer module 400 may comprise a winch for lifting the removable electrode
module.
An upper portion of the transfer module 400 comprises means for lifting the
transfer module such as a hook or hooks 430. Such lifting means enable the
entire transfer module to be lifted and moved to and from an electrolysis
apparatus 200.
A lower portion of the transfer module 400 is closed by a gate-valve 440. This
gate-valve comprises a thermally resistant gate 450 that is actuable to open
and close an opening into the transfer module chamber 420. The transfer
module, including the gate-valve, may conveniently be seated atop the gate-
valve of an electrolysis apparatus 200, as described above in relation to
Figure 5. By opening the gate-valves associated with both the transfer
module 440 and the electrolysis apparatus 200, access can be provided to the
CA 02817351 2013-05-08
WO 2012/066299
PCT/GB2011/001631
- 22 -
opening of the electrolysis chamber 220. The removable electrode module 10
can then be lowered from the transfer chamber 420, through the openings of
both the gate-valve associated with the transfer module and the gate-valve
associated with the electrolysis apparatus, to enable the electrode module to
be
located within the electrolysis chamber 220. The respective gate-valves can
then be closed, as illustrated in Figure 8, and the transfer module 400 may
then
be removed.
The first embodiment of a removable transfer module, as described above and
io illustrated in Figures 1 to 4, comprised eight effective working
electrodes on
which solid feedstock could be reduced (i.e. the upper portion of the terminal
cathode 30 and the upper portions of each of the bipolar electrodes 40-46).
For some reactions it may be desired to reduce a lower volume of a solid
feedstock. For such purposes, it may be desirable that a removable electrode
module has a lower area of cathodic-electrode surface. A second embodiment
of a removable electrode module according to one or more aspects of the
invention is illustrated by Figure 12.
The overall dimensions of the removable electrode module as illustrated in
Figure 12 are the same as the removable electrode module illustrated in
Figures 1 to 4 and, thus, this second embodiment of a removable electrode
module may be used in conjunction with the same electrolysis apparatus as the
first embodiment. However, the removable electrode module of the second
embodiment of the invention 1200 comprises a terminal cathode 1230 and a
terminal anode 1220, with only a single bipolar electrode 1240 disposed
between the terminal anode 1220 and the terminal cathode 1230. The terminal
anode terminal cathode and the bipolar electrode are identical in construction
to the equivalent structures described above in relation to the first
embodiment
of the invention. As there are fewer bipolar electrodes disposed between the
terminal anode 1220 and the terminal cathode 1230, the graphite electrode
risers 1221 and 1222 are substantially longer than those described above in
relation to the first aspect of the invention. If needed, several sections of
graphite risers may be joined by internal threaded studs 1226. The cover 1201
is supported directly above the upper surface of the anode 1220 by means of a
plurality of electrically insulating ceramic spacers 1268.
CA 02817351 2013-05-08
WO 2012/066299
PCT/GB2011/001631
-23 -
Apart from these specific adaptations required to ensure the external
dimensions of this removable electrode module are the same as the
dimensions of the module of the first embodiment of the invention, all other
elements of the removable electrode module according to the second
embodiment of the invention are the same as described above.
According to certain aspects of the invention, it is not essential that a
removable electrode module comprises a bipolar electrode. Figure 13
io illustrates a third specific embodiment of a removable electrode module
according to one or more aspects of the invention. This third embodiment
comprises a terminal anode 1320 and a terminal cathode 1330, but does not
comprise a bipolar electrode. The terminal cathode 1330 and the terminal
anode 1320 are constructed in the same way as the terminal anode 20 and the
terminal cathode 30 described above in relation to the first embodiment of the
invention. The external dimensions of the removable electrode module 1300 of
the third embodiment are the same as the dimensions of the first and second
embodiments of a removable electrode module. All other details of the third
embodiment of a removable electrode module as illustrated in Figure 13 are as
zo described above in relation to the first embodiment or the second
embodiment
of the removable electrode module.
In the embodiments described above a suspension rod 110 is coupled to a
j-slot connector 130 by clamping an end of the rod 110 to the connector 130 by
means of washers and bolts 111. Any tolerance needed to form a seal
between an underside of the cover 100 and a rim 231 of a crucible 230 forming
an opening into an electrolysis chamber 220 is achieved by the use of a
resilient sealing material on the rim. Figure 14 illustrates an alternative
coupling that may be used in an embodiment of a removable electrode module.
For ease of reference, components that are identical to those present in the
first embodiment described above have been given the same reference
numerals.
In the alternative embodiment illustrated in Figure 14 a suspension rod 110 of
the electrode module is coupled to a j-slot connector 130 by means of a flange
CA 02817351 2013-05-08
WO 2012/066299
PCT/GB2011/001631
- 24 -
1410 which transfers load through a set of Bellville springs 1400 and on to
the
j-slot connector. The flange 1410 is secured against the spring 1400 by means
of nuts 1420.
When the module is lifted, the weight of the module is transferred through the
suspension rod 110 and compresses the spring 1400. The spring urges
upwards against a lower surface of the flange 1410. The spring 1400 may be
any suitable spring means. For example, the spring may comprise a helical
spring.
Io
Coupling an electrode module to a lifting means such as a j-slot connector
with
a resilient spring disposed between may provide advantages in use. For
example, as the electrode module is lowered into an electrolysis chamber as
described above, contact is made between a rim surrounding the opening of
the chamber and a lower surface 102 of the cover 100 in order to form a seal.
In the embodiments described above, the base plate 30a of the module must
be seated in physical contact with the internal wall of the crucible in order
to
provide a cathodic connection. The use of a resilient means such as a
Belleville spring 1400 disposed between the lifting means and the suspension
zo rod may allow additional travel of the electrode module after a seal has
been
formed by the cover 100. Furthermore, such a resilient means may
advantageously accommodate dimensional changes in the suspension rod
caused by thermal fluctuations.
An embodiment of a removable electrode module that includes a resilient
means disposed between a suspension rod or rods supporting the electrodes
and a lifting means may be employed as an alternative to using a resilient
sealing material surrounding the opening of an electrolysis chamber or in
addition to it.
The following description of a method for electrolytically reducing a solid
feedstock according to a specific embodiment of the invention uses a
removable electrode module 10 as described above. The processing method
can be broken down into a number of steps.
CA 02817351 2013-05-08
WO 2012/066299
PCT/GB2011/001631
-25 -
Step 1 ¨The removable electrode module 10 comprises eight layers of
cathodic electrodes (i.e. one cathode and seven bipolar electrodes that act as
both anode and cathode) and an anode. An upper surface of each of the
working electrodes comprises a removable tray-assembly itself formed of two
coupleable parts 151, 152. As a first step in the process a feedstock
consisting
of a plurality of solid oxide preforms is loaded onto a surface of each of the
removable tray-assembly elements 151, 152.
Step 2 ¨ The loaded tray-assemblies are transferred to a removable electrode
io module located at an electrode module loading station. The loaded
tray-assemblies are seated on the removable electrode module 10 and each
pair of tray-assembly portions 151, 152 forms a cathodic portion of a cathodic
electrode (for example electrodes referenced 30, 40, 41, 42, 43, 44, 45, 46).
Step 3 ¨ The loaded removable electrode module 10 is raised up into a transfer
chamber 420 of a transfer module 400. In order to raise the removable
electrode module 10 into the transfer chamber 420, the transfer module 400 is
positioned vertically above the working station. A gate valve 440 of the
transfer
module 400 is actuated causing a gate 450 to slide open and allow access to
the transfer chamber 420. A bayonet coupling (not shown) is lowered by a
winch 465 located on the transfer module 400. The bayonet fitting couples to a
j-slot connector 130 on the removable electrode module 10, thereby allowing
the removable electrode module 10 to be raised into the transfer module
chamber 420.
Step 4 ¨ The entire transfer module 400 containing the removable electrode
module 10 may be lifted and moved by means of a plurality of hooks 430. At
Step 4 the entire transfer module, containing the electrode module, is moved
to
a position vertically above a heating station. This is illustrated in Figure
16.
The transfer module 400 is coupled to the heating station 500 with the
removable electrode module 10 positioned directly above a heating
chamber 510. The heating station comprises a housing 501 containing a
heating chamber 510 surrounded by a plurality of heating elements 520.
CA 02817351 2013-05-08
WO 2012/066299
PCT/GB2011/001631
- 26 -
Step 6 ¨ The electrode module is lowered, by means of the winch 465 until a
lower surface 102 of the cover 100 is seated on a rim 502 of the heating
chamber 510. The bayonet coupling is removed from the j-slot connector 130
and the gate 450 is closed. The removable electrode module 10 is now
s engaged with the heating station and the entire weight of the module is
supported through the lower surface of the cover 100. This arrangement is
illustrated in Figure 17.
Step 7 ¨ The electrode module 10 is heated within the heating chamber 510 of
the heating station 500 to a predetermined temperature. For electrolysis in a
molten salt this predetermined temperature is likely to be somewhere between
500 C and 1200 C. For example, the temperature may be raised to 700 C or
800 C. The rate of heating the module is controlled so that the ceramic
components of the electrode module do not undergo thermal shock. Thus the
is heating may occur at a rate of between 1 C per minute to 10 or 20 C per
minute. For example, heating may occur at a rate of about 5 C per minute.
The heating occurs under an inert atmosphere, for example an argon
atmosphere or a nitrogen atmosphere.
zo Step 8 ¨ Once the electrode module has been heated to the predetermined
temperature the electrode module 10 is once more raised into the transfer
chamber 420 of the transfer module 400.
Step 9 ¨ The gate valve is closed, thereby sealing the transfer chamber 420.
zs An inert atmosphere, for example an atmosphere argon or nitrogen, is
maintained within the transfer module.
Because the walls of the transfer module are insulated, the rate of heat loss
is
low. Thus, once the electrode module 10 has been heated to a predetermined
30 temperature and is sealed within the transfer module 400 the temperature
of
the module 10 reduces slowly.
Step 10 ¨ The transfer module 400 containing the electrode module 10 at a
35 predetermined temperature is moved to a position directly above an
electrolysis
CA 02817351 2013-05-08
WO 2012/066299
PCT/GB2011/001631
- 27 -
chamber. Access to the electrolysis chamber 220 is restricted by the presence
of a gate valve 300 seated above the electrolysis chamber. This is illustrated
in
Figure 7.
Step 11 ¨ The transfer module 400 is coupled to an electrolysis apparatus 200
containing the electrolysis chamber 220. The gate valve 440 associated with
the transfer module is aligned with the gate valve 300 associated with the
electrolysis apparatus such that, when both gate valves are opened the
electrode module 10 is able to gain access to the electrolysis chamber 220.
io
Step 12 ¨ The gate valve 440 on the transfer module is opened.
Step 13 ¨ The gate valve on the electrolysis apparatus 200 is opened.
Step 14 ¨ The electrode module 10 is lowered into engagement with the
electrolysis chamber. The electrolysis chamber contains a molten salt at, or
close to, its desired working temperature. The preheated electrode module 10
is also at, or near, to the working temperature. This working temperature may,
for example, be about 800 C. The electrode module is seated within the
electrolysis chamber 220 such that a lower surface of the terminal cathode 30
of the electrolysis module 10 makes physical contact with the graphite
crucible 230 defining the electrolysis chamber 220. This has been described
above and illustrated in Figure 8.
Step 15 ¨ Anode bus bars 250 are moved into contact with anode risers 21, 22,
of the removable electrode module 10.
A potential is applied between the anode 20 and the cathode 30 of the
electrode module 10. This potential is sufficient to reduce the feedstock that
is
in contact with the cathode and cathodic surfaces of each of the bipolar
electrodes. The potential required to reduce the feedstock will depend on the
nature of the system. Thus, for reduction of the titanium oxide feedstock in a
calcium chloride based molten salt, the potential voltage at each cathodic
surface of the electrode module 10 may be between 2 and 3 Volts.
CA 02817351 2013-05-08
WO 2012/066299
PCT/GB2011/001631
-28 -
The potential is applied for a sufficient period of time to reduce the solid
feedstock.
After electrolysis to reduce the feedstock there may be a number of further
processing steps in order to recover a reduced product from the electrode
module 10. Thus, the following further steps are used in a specific embodiment
of the invention in order to recover a reduced feedstock after electrolysis.
Step 17 - The electrolysis cell current is switched off and, thus, the voltage
at
to the surface of each electrode is removed.
Step 18 -The anode bus bars are withdrawn from contact with the graphite
anode risers 21, 22.
is Step 19 ¨ The electrode module is raised out of the molten salt and into
the
transfer module 400. The electrode module is raised slowly in order to allow
time for molten salt to drip free from the electrode module 10.
Step 20 - The gate valve 300 on the electrolysis apparatus 200 is closed.
Step 21 ¨ the gate valve 440 on the transfer module is closed.
Step 22 ¨ The transfer module containing the electrode module 10 is then
disengaged from the electrolysis apparatus 200 and moved to a position
immediately above a cooling station 600. This is illustrated in Figure 18.
Step 23 ¨ The transfer module 400 is coupled to the cooling station 600.
Step 24 ¨ The gate valve 440 is opened to allow access for the removable
electrode module 10 into a cooling chamber 610.
Step 25 ¨ The electrode module 10 is lowered into the cooling chamber until a
lower surface 102 of the cover 100 rests on an upper rim of the cooling
chamber 610. The cover 100 effectively forms a seal to the cooling
chamber 610. This is illustrated in Figure 19.
CA 02817351 2013-05-08
WO 2012/066299
PCT/GB2011/001631
- 29 -
Step 26 ¨ The cooling chamber comprises walls 620 of a high thermal
conductivity material, for example a metallic material such as a stainless
steel.
The walls contain a water jacket 625, through which a constant supply of cold
water is provided by means of an inlet 630 and outlet 631. The water jacket
allows heat to be removed efficiently from the thermal module 10. The rate of
cooling may be controlled by varying the rate at which water is passed through
the cooling jacket 625.
iip Step 27 ¨ Once the electrode module 10 has been cooled to a
predetermined
temperature it is raised into the transfer module 400.
Step 28 ¨ The cooled electrode module 10 within the transfer module 400 is
moved to a position directly above a wash station.
Step 29 ¨ The cooled electrode module is lowered into the wash station and
seated on its cathode base plate 30a.
Step 30 ¨ Jets of water 700 are emitted from nozzles 705 connected to
piping 710. The jets of water 700 are directed at the electrode module 10 and
in particular directed to the tray-assemblies containing the reduced
feedstock.
The jets of water are preferably directed such that they wash any remaining
salt
encrusting the electrode module and the reduced feedstock away.
Step 31 ¨ After washing, the electrode module 10 is raised into the transfer
module 400.
Step 32 ¨ the washed electrode module and the transfer module are moved to
a position above an unloading station.
Step 33 - The washed electrode module is lowered into position at the
unloading station.
Step 34 ¨ The electrode tray-assemblies containing reduced products are
removed from the electrode module 10.
CA 02817351 2013-05-08
WO 2012/066299
PCT/GB2011/001631
- 30 -
Step 35 ¨ The reduced product is removed from the tray-assemblies and
packaged for further processing.
The specific embodiment of a method described above may not be used in all
cases. For example different steps may be used or some steps may be
omitted.
It is preferred that the reduction of the solid feedstock proceeds by an
to electro-deoxidation reaction such as the FFC process.