Note: Descriptions are shown in the official language in which they were submitted.
CA 02817678 2013-05-10
Process for producing expandable thermoplastic beads with improved
expandability
Description
The invention relates to a process for producing expandable thermoplastic
polymer beads
comprising cavities via extrusion of a polymer melt comprising blowing agent
through a
die plate and pelletization in a chamber comprising liquid under a pressure in
the range
from 1.5 to 15 bar.
Expandable moldable foams can be produced by the suspension process, via
postimpregnation of polymer pellets or by the melt extrusion process. The melt
extrusion
process is particularly versatile in respect of possible starting materials
and additives.
Methods for producing expandable polymers by the melt impregnation process are
known. Application to various polymer systems has now been demonstrated for a
number
of materials, for example for acrylonitrile-containing styrene copolymers (WO
2009/000872) and for elastified expandable moldable foams (WO 2009/112549).
The properties of moldable foams are markedly dependent on the cell structure,
e.g. cell
size or cell size distribution. By way of example, therefore, thermal,
mechanical and
optical properties, and feel, can be altered by influencing the cell size.
Because of the
process-technology parameters that apply, only limited control of the cell
structure can be
achieved without changing the constitution of the material.
Control of the cell size is therefore often achieved by using nucleating
agents, examples
being inorganic additives, and organic nucleating agents such as waxes, where
these
provide an interface to the system and thus reduce the energy barrier for
heterogeneous
nucleation at the phase boundary between polymer and nucleating agent.
However, nucleating agents of this type have only limited suitability, because
they
sometimes have low efficiency and they have an adverse effect on the
mechanical
properties or fire properties of the foam. By way of example, addition of
inorganic,
particulate nucleating agents such as talc can reduce toughness, characterized
for
example via bending energy or resistance to cracking. Although it is possible
to use
compatibilizers or surface-modified fillers to improve the coupling of the
inorganic
nucleating agents thus to improve mechanical properties, these in turn exhibit
lower
nucleating efficiency.
The inorganic nucleating agents should moreover have low solubility in the
polymer
CA 02817678 2013-05-10
,
..
2
requiring nucleation, so as to allow phase separation. By way of example,
therefore,
olefinic waxes cannot be used in olefinic polymers. Organic nucleating agents,
such as
olefinic waxes, e.g. Luwax0, are not suitable for all materials. Again,
toughened styrene
foams cannot use olefinic nucleating agents, since they do not provide phase
separation
within the material but instead migrate into the polyolefin phases. It is then
impossible to
achieve effective nucleation.
An increase in cell density can likewise be achieved via use of low-solubility
blowing
agents. However, blowing agents of this type require very high solution
pressures and
exhibit a long residence time in the prefoamed beads or fully foamed moldings,
i.e. they
have disadvantages specific to the process and to the application.
The use of organic blowing agents and of inert gases for control of the foam
structure and
foam properties of expandable polymers has not hitherto been widely described.
Some
relevant patent specifications are collated below, and encompass not only foam
extrusion
but also expandable and expanded beads.
WO 2004/022636 describes the production of foam beads using water as blowing
agent.
In order to improve water-compatibility, solubilizers such as ethanol and
acetone are
used. Further blowing agents are moreover used, for example pentane, butane,
and/or
CO2. A primary aim of water addition is to reduce the amounts of the organic
blowing
agents in the expandable pellets, and no effect on cell size is described.
DE 198 190 58 describes the production and use of expandable styrene polymers
with a
low level of incipient foaming. Incipient foaming to bulk densities which are
below the bulk
density of the unfoamed materials by from 0.1 to 20% can be achieved by
variation of the
process parameters in the suspension polymerization process and in the melt
impregnation process, using pentane as blowing agent. The intention of the
incipient
foaming is to permit production of foams with relatively coarse cells.
However,
achievement of homogeneous incipient foaming of the material is very highly
dependent
on the process conditions selected and there is restricted scope for
variation.
WO 2005/092959 describes the use of a plurality of blowing agents in
multiphase polymer
systems. An aim here is to produce nanoporous foams via selective impregnation
of a
nanoscale structure with a blowing agent and subsequent foaming. A necessary
precondition for achieving the fine-cell structure here is a multiphase blend
structure.
By way of example, EP-A 846 141 describes the use of blowing agent
combinations. The
continuous conduct of a process for producing expandable styrene polymers adds
C3 ¨ C7
hydrocarbons individually or in a mixture with CO2 as blowing agent. The
process
CA 02817678 2013-05-10
=
3
comprises bulk polymerization of the styrene polymer as far as a certain
conversion, the
dispersion of the resulting prepolymer in liquid form in an aqueous phase with
suspension
stabilizer, and subsequent polymerization to completion. Blowing agents can be
added
during any of the steps of the process. When CO2 is used it is moreover
advantageous to
use CO2 absorbers. There is no description of any specific function of the CO2
during or
after the production process.
EP-A 987 292 describes the preexpansion of vinylaromatic polymers to give a
bulk
density of from 200 ¨ 600 g/I, and the subsequent postexpansion of the
material after
impregnation with inorganic gases, specifically with 02 - or N2-containing
gases. The
preexpansion and postimpregnation steps here take place at different times.
The
postimpregnation step is required for expansion in all cases.
EP-B 1 000 115 describes a process for producing expandable polymer pellets
specifically based on polystyrene. After impregnation of the polymers with
suitable
blowing agents, an atomization process, and subsequent cooling of the
resultant pellets,
takes place. Possible blowing agents mentioned are inter alia hydrocarbons,
chlorofluorocarbons, CO2, N2, and air, or noble gases. No particular effects
are described
for use of CO2, N2, and air.
US 2,864,778 describes the use of small proportions of CO2 in addition to
aliphatic
hydrocarbons to produce expandable styrene polymers. Addition of CO2 prior to
or during
the polymerization reaction by the suspension process can produce relatively
fine-cell
foams. The proportion needed for this purpose is described as very small. In
the
examples, effects occur even at proportions of 0.4% by weight and less. There
is no
description of other systems for achieving fine-cell products, or of other
processes for
producing expandable styrene polymers.
US 3,328,497 describes the use of gases, specifically of N2 and air, for
producing foams
from expandable styrene polymers and interpolymers. The process described
comprises
the partial expansion of the expandable polymer, which includes an organic
blowing
agent with a boiling point below 80 C, and rapid transfer into an atmosphere
with
increased gas pressure and low temperature. In a final step, the foam is
obtained via
further expansion in a closed mold at increased temperatures. The absorption
of the
gases described by the pellets is achieved by analogy with EP 987 292 after
the
preexpansion of the materials, and no particular effects on cell structure are
described.
US 5,391,581 describes the use of blowing agent mixtures made of aliphatic
hydrocarbons or of alicyclic hydrocarbons and CO2 for producing foam beads
made of
CA 02817678 2013-05-10
4
ethylene-based resins. The cell size of the expanded pellets can be made
homogeneous
by using CO2 and additionally introduced, inorganic nucleating agents, but,
unlike with
expandable beads, no additional expansion prior to production of moldings is
possible.
US 2006/0022366 describes the extrusion of foam sheets (XPS) of styrene-based
polymer systems with use of a plurality of blowing agents. Materials used as
blowing
agents in combination with isobutane, n-pentane, isopentane, or a mixture
thereof are
advantageously water, CO2, ethers, or dialkyl carbonates with a boiling point
below
140 C. The use of the blowing agents mentioned and of the permeation process
which is
more rapid when comparison is made with air generates a reduced pressure after
the
foaming process, and this is advantageous for further shaping processes.
US 2007/0049649 describes a process for producing foamed polymer beads
comprising
microcavities, where the polymer is processed with a gas under high pressure
or a liquid
in the supercritical state in an extruder to give a homogeneous single-phase
mixture, and
is extruded.
EP-A 0 761 729 describes expandable styrene resin beads with fewer than
100 microcavities of diameter about 0.1 to 30 pm, which are obtained via
suspension
polymerization in the presence of a persulfate and of an electrolyte.
It was an object of the present invention to discover a process for producing
expandable
thermoplastic polymer beads with improved expandability which while having
reduced
blowing agent contents blowing agent, where the melt comprises nevertheless
permit
faster prefoaming and controlled adjustment of cell structure.
Accordingly, a process has been discovered for producing expandable,
thermoplastic
polymer beads comprising cavities via extrusion of a polymer melt comprising
blowing
agent through a die plate and pelletization in a chamber comprising liquid
under a
pressure in the range from 1.5 to 15 bar, which comprises using a polymer melt
comprising blowing agent, where the melt comprises
from 0.1 to 5% by weight, preferably from 0.3 to 1.0% by weight, of a
nucleating agent D),
from 1 to 10% by weight, preferably from 2 to 6% by weight, of a blowing
agent E) which in essence remains within the polymer beads,
from 0.01 to 5% by weight, preferably from 0.05 to 1% by weight, of a co-
blowing agent F) forming the cavities,
based in each case on the polymer melt comprising blowing agent.
CA 02817678 2013-05-10
Surprisingly, it has been discovered that the use of volatile, liquid/gaseous
co-blowing
agents F) which form cavities can achieve a cellular structure in the
expandable pellets,
with the result that the subsequent foaming procedure can be improved and the
cell size
can be controlled.
5
Suitable nucleating agents D) are inorganic or organic nucleating agents.
Examples of
suitable inorganic nucleating agents are talc, silicon dioxide, mica, clay,
zeolites, or
calcium carbonate. Examples of suitable organic nucleating agents are waxes
such as
the polyethylene waxes marketed as Luwax . It is preferable to use talc.
The blowing agent used (component E) comprises from 1 to 10 per cent by
weight,
preferably from 3 to 8 per cent by weight, based on the entirety of components
A) to F) of
the polymer melt comprising blowing agent, of a physical blowing agent. The
blowing
agents can be gaseous or liquid at room temperature (from 20 to 30 C) and
atmospheric
pressure. Their boiling point should be below the softening point of the
polymer mixture,
usually in the range from -40 to 80 C, preferably in the range from -10 to 40
C. Examples
of suitable blowing agents are halogenated or halogen-free blowing agents,
e.g. aliphatic
C3 to Cs-hydrocarbons, alcohols, ketones, or ethers. Examples of suitable
aliphatic
blowing agents are aliphatic C3 to Cs-hydrocarbons such as n-propane, n-
butane,
isobutane, n-pentane, isopentane, n-hexane, neopentane, cycloaliphatic
hydrocarbons
such as cyclobutane and cyclopentane, halogenated hydrocarbons such as methyl
chloride, ethyl chloride, methylene chloride, trichlorofluoromethane,
dichlorofluoromethane, dichlorodifluoromethane, chlorodifluoromethane,
dichlorotetrafluoroethane, and mixtures thereof. Preference is given to the
following
halogen-free blowing agents: isobutane, n-butane, isopentane, n-pentane,
neopentane,
cyclopentane, and mixtures thereof.
Blowing-agent-retention capability after storage can be improved, and lower
minimum
bulk densities can be achieved, if the blowing agent preferably comprises a
proportion of
from 25 to 100 percent by weight, particularly preferably from 35 to 95
percent by weight,
based on the blowing agent, of isopentane or cyclopentane. Particular
preference is given
to use of mixtures made of from 30 to 98% by weight, in particular from 35 to
95% by
weight, of isopentane and from 70 to 2% by weight, in particular from 65 to 5%
by weight,
of n-pentane.
Blowing agents E) preferably used comprise aliphatic C3 ¨ C7-hydrocarbons or a
mixture
thereof, particularly preferably isobutane, isopentane, n-pentane and mixtures
thereof. It
is preferable that the polymer melt comprising blowing agent comprises less
than 0.5% by
weight of water.
CA 02817678 2013-05-10
6
The process for adjusting to said cavity morphology can also be termed
prenucleation,
and the cavities are in essence formed via the co-blowing agent F).
The co-blowing agent F) forming the cavities differs from the actual blowing
agent E) in its
solubility in the polymer. In the production process, blowing agent E) and co-
blowing
agent F) are initially dissolved completely in the polymer at sufficiently
high pressure. The
pressure is then reduced, preferably within a short period, and the solubility
of the co-
blowing agent F) is thus reduced. This causes onset of phase separation within
the
polymeric matrix, and a prenucleated structure is produced. Because the actual
blowing
agent E) has relatively high solubility and/or has low diffusion rate, it
remains
predominantly dissolved in the polymer. A temperature reduction is preferably
carried out
simultaneously with the pressure reduction, in order to inhibit excessive
nucleation of the
system and to reduce diffusion of the actual blowing agent E) out the
material. This is
achieved by using co-blowing agent F) in conjunction with ideal pelletization
conditions.
It is preferable that at least 80% by weight of the co-blowing agent F)
escapes within the
period of 24 h from the expandable thermoplastic beads on storage at 25 C,
atmospheric
pressure, and 50% relative humidity. The solubility of the co-blowing agent F)
in the
expandable thermoplastic beads is preferably below 0.1% by weight.
It is preferable to use co-blowing agents F) which moreover have a higher
diffusion rate
and/or increased permeability and/or an increased vapor pressure when
comparison is
made with the actual blowing agent E); it is particularly preferable that the
co-blowing
agents F) exhibit a plurality of these characteristics. In order to provide
additional support
for the nucleation process, small amounts of conventional nucleating agents
can be used,
examples being inorganic particles, such as talc.
In all cases, the amount added of the co-blowing agent F) used during the
prenucleation
process should exceed the maximum solubility under the prevailing process
conditions. It
is therefore preferable to use co-blowing agents F) which have low, but
adequate,
solubility in the polymer. Among these are in particular gases, such as
nitrogen, carbon
dioxide, and air, or noble gases, and particular preference is given to
nitrogen, the
solubility of which in many polymers decreases at low temperatures and
pressures.
However, it is also possible to use other, liquid additives.
It is particularly preferable to use inert gases, such as nitrogen and carbon
dioxide.
Features of both gases, alongside their suitable physical properties, are low
cost, good
availability, easy handling, and unreactive or inert behavior. By way of
example, in almost
all cases no degradation of the polymer occurs in the presence of the two
gases. Since
CA 02817678 2013-05-10
7
the gases are themselves obtained from the atmosphere, they also have no
effect on the
environment.
The amount of the co-blowing agent F) used here should: (i) be sufficiently
small to
dissolve at the given melt temperatures and given melt pressures during melt
impregnation as far as pelletization; (ii) be sufficiently high to provide
demixing from the
polymer and nucleation at the water pressure and temperature used for
pelletization. In
one preferred embodiment, at least one of the blowing agents used is gaseous
at room
temperature and atmospheric pressure.
It is moreover preferable to use a co-blowing agent F) which, after
prenucleation, escapes
completely from the expandable pellets within a short time and therefore does
not
influence the further foaming process. It is particularly preferable to use
nitrogen, carbon
dioxide, argon, helium, or a mixture thereof as co-blowing agent F).
It is particularly preferable to use talc as nucleating agent D) in
combination with nitrogen
as co-blowing agent F).
Metal drums and octabins, inter alia, can be used for the transport and
storage of the
expandable pellets. If drums are used, a fact that has to be considered is
that the
liberation of the co-blowing agent F) can sometimes increase pressure within
the drum.
Packaging to be used is therefore preferably open packs, such as octabins or
drums,
where these permit dissipation of pressure via permeation of the gas out of
the drum.
Particular preference is given here to drums which permit escape of the co-
blowing agent
F) by diffusion and minimize or inhibit escape of the actual blowing agent E)
by diffusion.
This can be possible by way of example via selection of the sealing material
in a manner
appropriate to the blowing agent and, respectively, co-blowing agent F). It is
preferable
that the permeability of the sealing material to the co-blowing agent F) is
higher by a
factor of at least 20 than the permeability of the sealing material to the co-
blowing agent
E).
The prenucleation, for example via addition of small amounts of nitrogen and
carbon
dioxide, can establish a cellular morphology within the expandable pellets
comprising
blowing agent. The average cell size in the center of the beads can be greater
here than
in the peripheral regions, and the density can be higher in the peripheral
regions of the
beads. Losses of blowing agent are thus minimized as far as possible.
The prenucleation can achieve markedly better cell size distribution and a
reduction of
cell size after prefoaming. The amount of blowing agent needed to achieve a
minimal bulk
CA 02817678 2013-05-10
8
density is moreover smaller, and the storage stability of the material is
moreover better.
Small amounts of nitrogen or carbon dioxide added to the melt can lead to a
marked
reduction of the prefoaming times at constant blowing agent content or to a
marked
reduction of amounts of blowing agent at constant foaming times and at minimal
foam
densities. The prenucleation moreover improves product homogeneity and process
stability.
Reimpregnation of the polymer pellets of the invention with blowing agents is
moreover
possible markedly more rapidly than with pellets of identical constitution and
more
compact, i.e. non-cellular, structure. Firstly, the diffusion times are
smaller, and secondly,
by analogy with direct-impregnated systems, smaller amounts of blowing agent
are
needed for foaming.
Finally, the prenucleation can reduce blowing agent content required to
achieve a certain
density, and can thus reduce the demolding times in the production of moldings
or of
slabs. This can reduce further-processing costs and improve product quality.
The prenucleation process can generally be used on all expandable beads. It is
preferably used on materials with stringent requirements placed upon
mechanical
properties, and on systems where nucleating agents usually used have only
slight effect.
By way of example, in the case of elastified foams it is possible to achieve a
marked
improvement in fine-cell structure via addition of nitrogen or carbon dioxide.
The prenucleation principle can be utilized not only for suspension technology
but also for
melt impregnation technology to produce expandable beads. Preference is given
to the
use in the melt extrusion process, in which the addition of the co-blowing
agents F) is
pelletized via pressure-assisted underwater pelletization after discharge of
the melt which
has absorbed blowing agent. The microstructure of the pellets can be
controlled as
described above via selection of the pelletization parameters and of the co-
blowing agent
F).
Mixing to incorporate the blowing agent E) and co-blowing agent F) into the
polymer melt
can be achieved by way of dynamic mixers, such as extruders, or static mixers.
In the case of relatively high amounts of co-blowing agent F), for example in
the range
from 1 to 10% by weight, based on the polymer melt comprising blowing agent,
lowering
of melt temperature is a possibility, or lowering of melt viscosity, with a
resultant marked
increase in throughput. It is therefore also possible to achieve incorporation
of thermally
labile additives under non-aggressive conditions into the polymer melt,
examples being
CA 02817678 2013-05-10
'
9
flame retardants. There is no resultant alteration of the constitution of the
expandable
thermoplastic beads, since the co-blowing agent in essence escapes during the
melt
extrusion process. In order to utilize said effect, it is preferable to use
CO2. The effects on
viscosity are smaller with N2. Nitrogen is therefore mainly used to adjust to
the desired
cell structure.
The chamber comprising liquid for pelletization of the expandable
thermoplastic polymer
beads is preferably operated at a temperature in the range from 20 to 80 C,
particularly
preferably in the range from 30 to 60 C.
Examples of thermoplastic polymer that can be used are styrene polymers,
polyamide
(PA), polyolefins, such as polypropylene (PP) or polyethylene (PE),
polyacrylates, such
as polymethyl methacrylate (PMMA), polycarbonate (PC), polyesters, such as
polyethylene terephthalate (PET) or polybutylene terephthalate (PBT),
polyether sulfone
(PES), polyether ketones (PEK), or polyether sulfides (PES), or a mixture
thereof.
Preference is given to styrene copolymers, such as styrene-butadiene block
copolymers,
styrene-a-methylstyrene copolymer, acrylonitrile-butadiene-styrene (ABS),
styrene-
acrylonitrile (SAN), acrylonitrile-styrene-acrylate (ASA), methyl methacrylate-
butadiene-
styrene (MBS), methyl methacrylate-acrylonitrile-butadiene-styrene (MABS)
polymers,
impact-modified polystyrene (HIPS) or glassclear polystyrene (GPPS) that has
been
polymerized by a free-radical route, or anionically polymerized polystyrene
(APS) or
anionically polymerized impact-resistant polystyrene (AIPS).
The constitution of the polymer pellets can be selected appropriately for the
desired
properties of the molded foam. Styrene-butadiene block copolymers as styrene
copolymer component have particular suitability for improving the elasticity
and the
resilience of the molded foam. Oil resistance, and also solvent resistance, in
particular
with respect to aromatic solvents, and heat resistance, can be improved by
using
acrylonitrile-containing styrene copolymers, such as SAN and ABS.
It is particularly preferable to use, in the process of the invention, a
polymer melt
comprising blowing agent and comprising
A) from 45 to 97.79 percent by weight of a styrene polymer,
B1) from 1 to 45 percent by weight of a polyolefin with a melting point in the
range from
105 to 140 C,
B2) from 0 to 25 percent by weight of a polyolefin with a melting point
below 105 C,
Cl) from 0.1 to 25 percent by weight of a styrene-butadiene block
copolymer or
styrene-isoprene block copolymer,
CA 02817678 2013-05-10
C2) from 0 to 10 percent by weight of a styrene-ethylene-butylene block
copolymer,
D) from 0.1 to 5% by weight of a nucelating agent,
E) from 1 to 10% by weight of a blowing agent which in essence remains
within the
polymer beads, and
5 F) from 0.01 to 5% by weight of a co-blowing agent forming the
cavities,
based in each case on the polymer melt comprising blowing agent.
Component A
10 The polymer beads comprise from 45 to 97.8% by weight, particularly
preferably from 55
to 78.1% by weight, of a styrene polymer A), such as standard polystyrene
(GPPS) or
impact-resistant polystyrene (HIPS), or styrene-acrylonitrile copolymers
(SAN), or
acrylonitrile-butadiene-styrene copolymers (ABS), or a mixture thereof. The
expandable,
thermoplastic polymer beads used to produce the foam beads P1 preferably
comprise, as
styrene polymer A), standard polystyrene (GPPS). Particular preference is
given to
standard polystyrene grades with weight-average molar masses in the range from
120 000 to 300 000 g/mol, in particular from 190 000 to 280 000 g/mol,
determined by gel
permeation chromatography; and with a melt volume rate MVR (200 C/5 kg) to ISO
113
in the range from Ito 10 cm3/10 min, an example being PS 158 K, 168 N, or 148
G from
BASF SE. Free-flowing grades, such as Empera 156L (Innovene) can be added in
order
to improve fusion of the foam beads during processing to give the molding.
Components B
The thermoplastic polymer beads comprise, as components B), polyolefins B1)
with a
melting point in the range from 105 to 140 C, and polyolefins B2) with a
melting point
below 105 C. The melting point is the melting peak determined by means of DSC
(dynamic scanning calorimetry) at a heating rate of 10 C/minute.
The thermoplastic polymer beads comprise from 1 to 45 percent by weight,
preferably
from 4 to 35% by weight, particularly preferably from 7 to 15% by weight, of a
polyolefin
B1). The polyolefin B1) used preferably comprises a homo- or copolymer of
ethylene
and/or propylene with a density in the range from 0.91 to 0.98 g/I (determined
to ASTM
D792), in particular polyethylene. Particular polypropylenes that can be used
are
injection-molding grades. Polyethylenes that can be used are commercially
available
homopolymers made of ethylene, e.g. LDPE (injection-molding grades), LLDPE,
HDPE,
or copolymers made of ethylene and propylene (e.g. Moplen RP220 and Moplen
RP320 from Basell, or Versify grades from Dow), ethylene and vinyl acetate
(EVA),
ethylene acrylates (EA) or ethylene-butylene acrylates (EBA). The melt volume
index MVI
CA 02817678 2013-05-10
11
(190 C/2.16 kg) of the polyethylenes is usually in the range from 0.5 to 40
g/10 min, the
density being in the range from 0.91 to 0.95 g/cm3. It is also possible to use
blends with
polyisobutene (PIB) (e.g. Oppanol B150 from BASF Aktengesellschaft). It is
particularly
preferable to use LLDPE with a melting point in the range from 110 to 125 C
and with a
density in the range from 0.92 to 0.94 g/I.
Other suitable components B1) are olefin block copolymers, where these are
composed
of a polyolefin block PB1 (hard block) and of a polyolefin block PB2 (soft
block), for
example those described in WO 2006/099631. The polyolefin block PB1 is
preferably
composed of from 95 to 100% by weight of ethylene. The PB2 block is preferably
composed of ethylene and a-olefin, where the following can be used as a-
olefins:
styrene, propylene, 1-butene, 1-hexene, 1-octene, 4-methyl-1-pentene,
norbornenes, 1-
decene, 1,5-hexadiene, or a mixture thereof. It is preferable to use, as PB2
block, an
ethylene-a-olefin copolymer block having from 5 to 60% by weight of a-olefin,
in particular
an ethylene-octene copolymer block. Preference is given to multiblock
copolymers of the
formula (PB1-PB2)n, where n represents an integer from 1 to 100. The blocks
PB1 and
PB2 in essence form a linear chain and preferably have alternating or random
distribution. The proportion of the PB2 blocks is preferably from 40 to 60% by
weight,
based on the olefin block copolymer. Preference is particularly given to
olefin block
copolymers having alternating hard PB1 blocks and soft, elastomeric PB2
blocks, these
being obtainable commercially with trademark INFUSE .
Blowing-agent-retention capability increases markedly with a relatively small
proportion of
polyolefin B1). This markedly improves the storage stability and the
processability of the
expandable, thermoplastic polymer beads. In a range from 4 to 20% by weight of
polyolefin, expandable thermoplastic polymer beads are obtained with good
storage
capability, without any impairment of the elastic properties of the molded
foam produced
therefrom. This is apparent by way of example in a relatively low compression
set Eset in a
range from 25 to 35%.
The expandable, thermoplastic polymer beads comprise, as polyolefin B2), from
0 to 25
percent by weight, preferably from 1 to 15% by weight, particularly preferably
from 5 to 10
percent by weight, of a polyolefin B2) with a melting point below 105 C. The
polyolefin
B2) preferably has a density in the range from 0.86 to 0.90 g/I (determined to
ASTM
D792). Thermoplastic elastomers based on olefins (TP0s) are particularly
suitable for this
purpose. Particular preference is given to ethylene-octene copolymers, which
by way of
example are obtainable commercially with trademark Engage 8411 from Dow.
After
processing to give foam moldings, expandable, thermoplastic polymer beads
which
comprise component B2) exhibit a marked improvement in bending energy and
ultimate
CA 02817678 2013-05-10
12
tensile strength.
Components C
In the field of multiphase polymer systems, it is known that most polymers
have no, or
only slight, mutual miscibility (Flory), and demixing to give respective
phases therefore
occurs as a function of temperature, pressure, and chemical constitution. If
incompatible
polymers are linked covalently to one another, the demixing does not occur at
a
macroscopic level, but only at a microscopic level, e.g. on the scale of the
length of a
single polymer chain. The term microphase separation is therefore used in this
case.
There is a wide variety of resultant mesoscopic structures, e.g. lamellar,
hexagonal,
cubic, and bicontinuous morphologies, where these have a strong relationship
to lyotropic
phases.
Compatibilizers (components C) are used for controlled adjustment to the
desired
morphology. The invention achieves improved compatibility via use, as
component Cl),
of a mixture of styrene-butadiene block copolymers or styrene-isoprene block
copolymers, and of styrene-ethylene-butylene block copolymers (SEBS) as
component
C2).
The connpatibilizers lead to improved adhesion between polyolefin-rich and
styrene-
polymer-rich phases, and even small amounts improve the elasticity of the foam
markedly
in comparison with conventional EPS foams. Studies of the domain size of the
polyolefin-
rich phase have shown that the compatibilizer stabilizes small droplets by
reducing
surface tension.
The expandable, thermoplastic polymer beads are particularly preferably
composed of a
multiphase polymer mixture which comprises blowing agent and which has at
least one
continuous phase, and which has at least two disperse phases P1 and P2
dispersed
within the continuous phase, where
a) the continuous phase consists essentially of component A,
b) the first disperse phase P1 consists essentially of components BI and
B2, and
c) the second disperse phase P2 consists essentially of component Cl.
Components C2) preferably form a phase boundary between the disperse phase P1
and
the continuous phase.
By virtue of said additional disperse phase it is possible to keep the domain
size of the
CA 02817678 2013-05-10
=,,.. =
13
disperse phase < 2 pm, with relatively high soft-phase content. This leads to
relatively
high bending energy in the molded foam, for identical expandability.
The entirety of components Cl) and C2) in the expandable, thermoplastic
polymer beads
is preferably in the range from 3.5 to 30 percent by weight, particularly
preferably in the
range from 6.8 to 18 percent by weight.
The ratio by weight of the entirety of components B1) and B2) to component C2)
in the
expandable, thermoplastic polymer beads is preferably in the range from 5 to
70.
The ratio by weight of components Cl) to 02) in the expandable, thermoplastic
polymer
beads is preferably in the range from 2 to 5.
The expandable, thermoplastic polymer beads comprise, as component Cl), from
0.1 to
25 percent by weight, preferably from 1 to 15 percent by weight, in particular
from 6 to 9.9
percent by weight, of a styrene-butadiene block copolymer or styrene-isoprene
block
copolymer.
Suitable materials for this purpose by way of example are styrene-butadiene
block
copolymers or styrene-isoprene block copolymers. Total diene content is
preferably in the
range from 20 to 60% by weight, particularly preferably in the range from 30
to 50% by
weight, and total styrene content is accordingly preferably in the range from
40 to 80% by
weight, particularly preferably in the range from 50 to 70% by weight.
It is preferable to use, as compatibilizer, styrene-butadiene-styrene (SBS)
three-block
copolymers having butadiene content of from 20 to 60% by weight, preferably
from 30 to
50% by weight, where these can have been to some extent hydrogenated or not
hydrogenated. They are obtainable commercially by way of example with
trademark
Styroflexe 2G66, Styrolux0 3G55, Styroclear0 GH62, Kraton D 1101, Kraton D
1155,
Tuftec H1043, or Europren SQL T6414. These involve SBS block copolymers with
sharp transitions between B blocks and S blocks.
The expandable, thermoplastic polymer beads comprise, as component 02), from
0.1 to
10 percent by weight, preferably from 1 to 9.9% by weight, in particular from
0.8 to 5
percent by weight, of a styrene-ethylene-butylene block copolymer (SEBS).
Suitable
styrene-ethylene-butylene block copolymers (SEBS) are by way of example those
obtainable via hydrogenation of the olefinic double bonds of the block
copolymers Cl).
Suitable styrene-ethylene-butylene block copolymers are by way of example the
Kraton
G grades obtainable commercially, in particular Kraton G 1650.
CA 02817678 2013-05-10
14
The process of the invention can give expandable thermoplastic polymer bead
material
with cavities with an average diameter in the range from 0.1 to 50 pm,
preferably from 1
to 30 pm.
It is preferable for the expandable thermoplastic polymer bead material to
have an
average diameter in the range from 0.2 to 2.5 mm and to have from 50 to 300
cavities/mm2 of cross-sectional area, preferably from 70 to 150 cavities/mm2.
The number
of cavities can by way of example be determined via counting from a thin layer
through
the polymer bead material under an optical microscope.
The bulk density of the material is preferably in the range from 500 to 590
kg/m3,
preferably from 520 to 580 kg/m3.
The prenucleated structure of the expandable pellets can give better
foamability and
controlled adjustment of cell size, and therefore a significant improvement in
processing
properties and in foam properties.
In order to improve processability, the finished expandable thermoplastic
polymer beads
can be coated with glycerol esters, antistatic agents, or anticaking agents.
The resultant round or oval beads are preferably foamed to a diameter in the
range from
0.2 to 10 mm. Their bulk density is preferably in the range from 10 to 100
g/I.
The fusion of the prefoamed foam beads to give the molding, and the resultant
mechanical properties, are in particular improved via coating of the
expandable
thermoplastic polymer beads with a glycerol stearate. It is particularly
preferable to use a
coating made of from 50 to 100% by weight of glycerol tristearate (GTS), from
0 to 50%
by weight of glycerol monostearate (GMS), and from 0 to 20% by weight of
silica.
The expandable, thermoplastic polymer beads can be prefoamed by means of hot
air or
steam to give foam beads with a density in the range from 8 to 200 kg/m3,
preferably in
the range from 10 to 80 kg/m3, in particular in the range from 10 to 50 kg/m3,
and then
fused in a closed mold to give foam moldings. A gauge pressure in the range
from 0.5 to
1.5 bar, in particular from 0.7 to 1.0 bar, is usually used.
Use of this concept can markedly reduce blowing agent contents in comparison
with
standard EPS for achieving comparable densities, and can thus use less of the
blowing
agents that cause a greenhouse effect. Lower minimum bulk densities can thus
be
CA 02817678 2013-05-10
achieved with identical blowing agent content. Furthermore, it is
substantially easier to
reinnpregnate prenucleated pellets with blowing agents, for example in the
event of
blowing agent loss during storage or transport. Since the prenucleation
process can use
nitrogen or other inert gases which have previously been obtained from the
atmosphere,
5 this concept for improving the expansion capability of thermoplastic
moldable foams and
for better adjustment of cell structure protects the environment and conserves
resources.
The co-blowing agents F) used, forming cavities, generally have marked
plastifying effect
if their amounts are relatively large. The viscosity-lowering effect of the co-
blowing agent
10 F) therefore permits an increase in throughput at identical temperature
profile or reduced
melt temperature for identical throughput, for a formulation which is
otherwise identical.
The pressure drop in pressurized apparatuses, such as pelletizing dies or
mixers,
remains identical here, since the material has identical melt viscosity. In
the first instance,
therefore, the thermal stress placed on the material can be reduced, and it is
also
15 possible to incorporate heat-sensitive materials, such as flame
retardants. In the second
instance, the increase in throughput obtained with identical plant
equipment/pressurization of the apparatuses permits more cost-effective
production of
the expandable beads.
Another aspect is that the proportion of the actual blowing agent can be
reduced without
changing melt viscosity, and without any need to adjust the throughput of the
plant or the
conduct of the process. Preference is given to carbon dioxide as plastifying
co-blowing
agent F), because of relatively high solubility in polymers.
The expandable thermoplastic polymer beads obtained by the process of the
invention
can be processed to give foams with relatively high cell number, i.e. fine
cell structure.
The homogeneous foam structure improves the mechanical properties and thermal
insulation properties of the foams.
A further effect is reduction of energy costs for foam processing. The faster
prefoaming
process can achieve higher throughputs. The lower blowing agent contents in
conjunction
with the prenucleation process can markedly reduce demolding times, and can
shorten
cycle times for the complete foaming process.
Examples
Starting materials:
Component A:
Polystyrene with a melt viscosity index MVI (200 C/5kg) of 2.9 crn3/10 min (PS
158K from
CA 02817678 2013-05-10
16
BASF SE, M,, = 280 000 g/mol, intrinsic viscosity number IV 98 ml/g)
Component B:
BI: LLDPE polyethylene (LL1201 XV, Exxon Mobile, density 0.925 g/I, MVI
= 0.7 g/10
min, melting point 123 C)
B2: Ethylene-octene copolymer polyethylene (Engage 8402 from Dow,
density 0.880
g/I, MVI = 18 g/10 min, melting point 72 C)
Component C:
C1.1: Styr lux 3G55, styrene-butadiene block copolymer from BASF SE,
C1.2: Styroflex 2G66, thermoplastic elastic styrene-butadiene block copolymer
(STPE)
from BASF SE,
C2: Kraton G 1651, styrene-ethylene-butylene block copolymer from Kraton
Polymers
LLC
Component D:
Nucleating agent: talc
Component E:
Component F:
Nitrogen co-blowing agent (Examples El ¨ E17), carbon dioxide co-blowing agent
(Examples E19 ¨ E36)
Production of expandable pellets El ¨ El 1
The expandable pellets were produced by a melt impregnation process using
static
mixing apparatuses. For this, the polymers were first plastified in an
extruder and
conveyed by way of a melt pump into a series of static mixers and heat
exchangers. At
the inlet of the first static mixer, technical-grade isopentane (95%
isopentane / 5 % n-
pentane) was added together with co-blowing agent F), and the melt was
impregnated.
The corresponding formulations can be found in Table I. The melt temperature
was then
reduced by way of a heat exchanger, and the melt temperature was homogenized
by way
of a further static mixer. Pressure was applied via a further melt pump, in
order to
pelletize the material by way of a pelletizing die (49 0.60 mm holes) using
pressurized
underwater pelletization (water pressure 12 bar, water temperature 50 C).
Average bead
CA 02817678 2013-05-10
101 =
17
size was about 1.25 mm. Total throughput was 70 kg/h. Melt temperature on
discharge
from the die was about 203 C.
Table 1: Constitution (parts by weight) of expandable pellets El - Ell
El E2 E3 E4 E5 E6
E7 E8 E9 El 0 Ell
Component 70.2 70.25 70.2 70.15 70.1 70.05 70 70.1 70.1 71.3 71.2
A
Component 11.7 11.7 11.7 11.7 11.7 11.7 11.7 11.7 11.7 11.7 11.7
B1
Component 3.9 3.9 3.9 3.9 3.9 3.9 3.9 3.9 3.9 3.9 3.9
B2
Component
7.8 7.8 7.8 7.8
C1.1
Component 7.8 7.8 7.8 7.8 7.8 7.8 7.8
C1.2
Component
1.0 1.0 1.0
C2
Component 0.5 0.5 0.5 0.5 0.5 0.5 0.5 0.5 0.5 0.5 0.5
D (talc)
Component 5.9 5.8 5.8 5.8 5.8 5.8 5.8 5.8 5.8 4.8 4.8
E (blowing
agent)
Component
0.05 0.10 0.15 0.20 0.25 0.30 0.20 0.20 0.05 0.10
F (nitrogen)
Analysis of expandable pellets
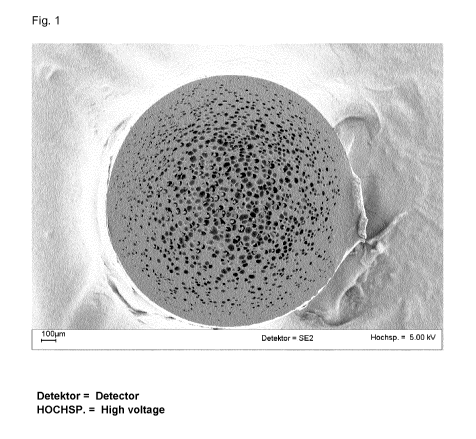
The transmission electron micrographs (TEMs) show the cellular structures of
the pellets
comprising blowing agent in the form of spheroidal cells (dark regions, Fig.
1), and these
subsequently contribute to better expansion capability and finer cell
structure in the foam.
The order of size of the cells of these pellets that have absorbed blowing
agent is below
50 pm, and cell sizes extending to 1 pm are clearly discernible in the
recorded images.
Processing and characterization of expandable pellets
Coating components used were 70% by weight of glycerol tristearate (GTS) and
30% by
weight of glycerol monostearate (GMS).
CA 02817678 2013-05-10
18
The pellets comprising blowing agent were prefoamed in an EPS prefoamer to
give foam
beads of low density (from 15 to 25 g/1), and were processed in an automatic
EPS
molding machine at a gauge pressure of from 0.7 - 1.1 bar to give moldings.
The moldings were subjected to various mechanical measurements. Marked
elastification
is observed in the examples of the invention in comparison with straight EPS,
and is
discernible in very high resilience. Compressive strength was determined at
10%
compression to DIN EN 826 and flexural strength was determined to DIN EN
12089.
Bending energy was determined from the values measured for flexural strength.
Table 2 shows the processing parameters, such as prefoaming time and demolding
time.
It can be clearly seen that the addition of nitrogen reduces prefoaming time
and
demolding time. It was also possible to achieve a marked reduction in cell
size. Blowing
agent content could moreover be markedly reduced in comparison with the
reference,
without impairment of properties.
Table 2: Processing and properties of the foam beads obtained from Examples El
- El 5
El E2 E3 E4 E5 E6 E7 E8 E9 El0 Eli
Bulk density of 615 605 600 560 550 530 520 530 530 560 540
beads
[g/1]
Bead size d' 1.26 1.27 1.26 1.28 1.26 1.27 1.28 1.29 1.27 1.26 1.27
[mm]
Prefoaming time at 218 178 154 119 108 89 90 92 85
193 157
0.1 bar [s]
Bulk density of foam 21.3 20.8 20.6 21.3 20.5 20.8 20.5 20.4 20.8 20.5 20.9
bead [g/I]
Time for minimum 660 650 630 540 360 300 240 290 250 360 420
bulk density [s]
Minimum bulk 20.0 19.2 17.9 17.5 17.2 17.2 16.7 17.2 17.2 19.2 17.9
density [WI]
Density of molding 22.2 20.9 20.5 22.0 20.4 21.3 21.0 22.1 21.2 21.4 21.1
[g/I]
Demolding time [s] 454 426 402 370 344 297 236 289 273 219 195
Cell number 1.5 1.7 2.1 4.8 5.6 6.0 7.2 6.1
6.0 4.5 5.0
[1/mm]
Flexural strength 295 291 270 268 265 252 261 283 292 279 268
[kPa]
Bending energy 5.3 5.1 4.8 4.5 4.8 4 4.2 4.5
4.9 5.3 5.1
CA 02817678 2013-05-10
19
[Nm]
Compressive 103 99 9 96 92 93 90 100 98 92 91
strength s = 10%
[kPa]
Compression set 23 23 23 31 31 30 39 28 25
32 34
rYol
Production of expandable pellets E 12 - E 17
The expandable pellets were produced by a melt impregnation process. For this,
polystyrene
158 K (component A) was first plastified in an extruder. Within the extruder,
the melt was
impregnated with technical-grade isopentane (95% isopentane / 5 n-pentane)
together
with the co-blowing agent F), and was homogenized. The corresponding
formulations can
be found in Table 3. Pressure was applied via a melt pump at the extruder
head, in order
to pelletize the material by way of a pelletizing die (2 0.65 mm holes) using
pressurized
underwater pelletization (water pressure 12 bar, water temperature 47 C).
Average bead
size was about 1.25 mm. Total throughput was 4.5 kg/h. Melt temperature on
discharge
from the die was about 210 C.
Table 3: Constitution of expandable pellets, processing and properties of
foam beads
obtained from Examples E12 - E17
E12 E13 I E14 I E15 E16 E17
Component A (GPPS) 93.4 93.3 93.1 93.9 93.8 93.6
Component D (talc) 0.5 0.5 0.5 - - -
Component E (blowing agent) 6.1 6.1 6.1 6.1 -
Component F (nitrogen) - 0.10 0.30 0.15 0.10 0.30
Bulk density of beads g/I 550 470 360 620 610 420
Bead size d" mm 1.57 1.58 1.59 1.59 1.60 1.58
Prefoaming time at 0.1 bar s 63 40 31 256 240 38
Bulk density of foam beads g/I 20.5 21.3 21.2 20.6 20.2 21.3
Time for minimum bulk density s 420 240 120 540 360 150
Minimum bulk density g/I 13.5 12.2 13.5 20.0 17.9 12.8
Density of molding g/I 21.4 22.7 21.6 20.3 15.2 22.6
Demolding time s 56 114 52 36 87 469
Cell number 1/mm 6.1 9.3 16.8 0.6 1.2 7.4
Flexural strength kPa 314 342 263 118 99 317
Bending energy Nm 3.3 4.2 3.3 1.8 1.4 3.5
CA 02817678 2013-05-10
Compressive strength C3=10% kPa 139 138 116 41 33 140
Compression set 34 31 52 57 48 36
Fig. 1 and Fig. 2 show transmission electron micrographs at various
magnifications of a
thin section through an expandable pellet from Example 13 with homogeneously
5 distributed cavities in the interior of the pellet bead.
CA 02817678 2013-05-10
21
Production of expandable pellets
The expanable pellets were produced by a melt impregnation process using
static mixing
apparatuses. For this, the polymers were first plastified in an extruder and
metered by
way of a melt pump into a series of static mixers and heat exchangers. At the
inlet of the
first static mixer, technical-grade isopentane (95% isopentane / 5% n-pentane)
was
added together with the co-blowing agent F), and the melt was impregnated. The
corresponding formulations can be found in the table. The melt temperature was
then
reduced by way of a heat exchanger, and the melt temperature was homogenized
by way
of a further static mixer. Pressure was applied via a further melt pump in
order to pelletize
the material by way of a pelletizing die (2 0.65 mm holes) using pressurized
underwater
pelletization (for water pressure see table, water temperature 47 C). Average
bead size
was about 1.25 mm. Total throughput was 4.5 kg/h. Melt temperature on
discharge from
the die was about 207 C.
Production of expandable pellets, Examples 19 to 36
The expandable pellets were produced by a melt impregnation process using
static
mixing apparatuses. Table 4 gives an overview of the constitution of the
materials - the
quantitative proportions of the polymers and, respectively, of the talc
(components A-D)
were identical with those of Examples 12 and 1, and the proportion of the
blowing agent
E) and of the co-blowing agent F) was varied. For this, the polymers were
first plastified in
an extruder, and metered by way of a melt pump into a series of static mixers
and heat
exchangers. At the inlet of the first static mixer, technical-grade isopentane
(95%
isopentane/5% n-pentane) was added together with the co-blowing agent F), and
the melt
was impregnated. The procedure was analogous to that of Example 12 and Example
9,
but instead of nitrogen CO2 was used as component F) to reduce thermal stress.
The
corresponding formulations can be found in the table. The melt temperature was
then
reduced by way of a heat exchanger, and the melt temperature was homogenized
by way
of a further static mixer. Pressure was applied via a further melt pump in
order to pelletize
the material by way of a pelletizing die (2 0.65 mm holes) using pressurized
underwater
pelletization (for water pressure see table, water temperature 47 C). Average
bead size
was about 1.25 mm. Total throughput was 4.5 kg/h.
In order to demonstrate the plastifying action and the throughput increases
and,
respectively, reduced melt temperatures that can be achieved, pressure loss
across a
static mixer was in each case used as a measure of melt viscosity. The
diameter of the
static mixer used was 25 mm and its L/D ratio was 15. The relationship between
the
pressure loss here and the viscosity in the laminar region is as follows:
CA 02817678 2013-05-10
. .
22
Ap = Re Ne Fi\-47¨L
D2
_
where Re, Ne, 77, w, L, and D are the Reynolds number, the Ne number, the
average
shear viscosity, the average flow rate, the length of the static mixer, and
the diameter of
the static mixer. For CSE-X/8 static mixers, the product of Ne and Re is
constant and is
1200. The average flow rate is:
¨ V (ril /P)
w = = ,
A P2 I 4)
where V, hi , P and A are the volumetric throughput, the mass-based
throughput, the
melt density, and the cross-sectional area of the mixer. The average shear
viscosity of
_ 7
the polymer melt, ii at the average shear rate 7 is calculated as follows:
¨/\ ¨(64 (rii /p)
77V1)= 11
D 77- (D2/4)/
On the basis of these principles, the shear viscosity of the melt was
determined (Table 4)
at various temperatures and throughputs. Examples 19 to 36 in each case give
the effect
of CO2 on viscosity and on pressure loss (at the static mixer/additive mixer).
Pressure
loss here is a variable involving technical restrictions, since there is a
maximum
permissible pressure loss at the mixer and a permissible total system
pressure. By using
CO2, it is possible to reduce the thermal stress (24/25, 33/34) or to increase
the
throughput (26/27, 35/36) for identical pressure loss in comparison with the
system
comprising only pentane. The use of CO2 here has no adverse effect on foaming
performance.
CA 02817678 2013-05-10
, . =
23
Example Components Total Pentane CO2 : Pressure
Average Temperature
without throughput : loss viscosity
blowing
agent
(kg/h) (% by wt.) (% by wt.)
(bar) (Pa.$) ( C)
E19 B12 4.5 6.5% 0.0% 26 1.276
183
E20 B12 4.5 3.8% 0.0% 65 3.212
183
E21 B12 4.5 3.8% 0.5% 56 2.756
183
E22 B12 4.5 3.8% 1.0% 47 2.304
182
E23 B12 4.5 3.7% 2.8% . 30 1.467
183
E24 B12 4.5 3.8% 1.0% 64 3.140
175
E25 B12 4.5 3.7% 2.8% 66 3.238
158
E26 B12 6.2 3.8% 1.0% 62 2.208
183
E27 B12 11.0 3.7% 2.8% . 64 1.285
183
E28 B1 4.5 6.1% 0.0% 48 2.314
187 '
E29 B1 4.5 6.1% 0.5% 44 2.127
186
E30 B1 4.5 6.0% 0.9% 40 1.906
186
E31 B1 4.5 6.0% 1.4% 36 1.703
186
E32 B1 4.5 6.0% 1.8% 32 1.546
186
E33 B1 4.5 6.0% 0.9% . 46 2.207
178
E34 B1 4.5 6.0% 1.8% 47 2.255
169
E35 B1 6.1 6.0% 0.9% 48 1.699
186
E36 B1 8.0 6.0% 1.8% . 46 1.241
186