Note: Descriptions are shown in the official language in which they were submitted.
PF71248 CA 02817792 2013-05-13
1
Process for mineral oil production using hydrophobically associating
copolymers
The present invention relates to a process for mineral oil production, in
which an aqueous
formulation comprising at least one water-soluble, hydrophobically associating
copolymer is
injected through at least one injection borehole into a mineral oil deposit
having a deposit
temperature of 35 C to 120 C, preferably 40 C to 90 C, and crude oil is
withdrawn from the
deposit through at least one production borehole, wherein the water-soluble,
hydrophobically
associating copolymer comprises at least acrylamide or derivatives thereof, a
monomer
having anionic groups and a monomer which can bring about the hydrophobic
association of
the copolymer.
In natural mineral oil deposits, mineral oil is present in the cavities of
porous reservoir rocks
which are sealed toward the surface of the earth by impermeable top layers.
The cavities
may be very fine cavities, capillaries, pores or the like. Fine pore necks
may, for example,
have a diameter of only approx. 1 p.m. As well as mineral oil, including
fractions of natural
gas, a deposit also comprises water with a greater or lesser salt content.
In mineral oil production, a distinction is drawn between primary, secondary
and tertiary
production.
In primary production, after commencement of drilling of the deposit, the
mineral oil flows of
its own accord through the borehole to the surface owing to the autogenous
pressure of the
deposit. The autogenous pressure can be caused, for example, by gases present
in the
deposit, such as methane, ethane or propane. The autogenous pressure of the
deposit,
however, generally declines relatively rapidly on extraction of mineral oil,
such that usually
only approx. 5 to 10% of the amount of mineral oil present in the deposit,
according to the
deposit type, can be produced by means of primary production. Thereafter, the
autogenous
pressure is no longer sufficient to produce mineral oil.
After primary production, secondary production is therefore typically used. In
secondary
production, in addition to the boreholes which serve for the production of the
mineral oil,
known as the production boreholes, further boreholes are drilled into the
mineral oil-bearing
formation. These are known as injection boreholes, through which water is
injected into the
deposit (known as "water flooding"), in order to maintain the pressure or to
increase it again.
As a result of the injection of the water, the mineral oil is gradually forced
through the cavities
in the formation, proceeding from the injection borehole, in the direction of
the production
borehole. However, this works only for as long as the cavities are completely
filled with oil
and the more viscous oil is pushed onward by the water. As soon as the mobile
water breaks
through cavities, it flows on the path of least resistance from this time
onward, i.e. through
the channel formed, and no longer pushes the oil onward. By means of primary
and
PF71248 CA 02817792 2013-05-13
2
secondary production, therefore, generally only approx. 30 to 35% of the
amount of mineral
oil present in the deposit can be produced.
After the measures of secondary mineral oil production, measures of tertiary
mineral oil
production (also known as "Enhanced Oil Recovery (E0R)") are therefore also
used to
further enhance the oil yield. This includes processes in which suitable
chemicals, such as
surfactants and/or polymers, are used as assistants for oil production. An
overview of tertiary
oil production using chemicals can be found, for example, in the article by D.
G. Kessel,
Journal of Petroleum Science and Engineering, 2(1989) 81 - 101.
The techniques of tertiary mineral oil production include what is known as
"polymer flooding".
Polymer flooding involves injecting an aqueous solution of a thickening
polymer through the
injection boreholes into the mineral oil deposit, the viscosity of the aqueous
polymer solution
being matched to the viscosity of the mineral oil. As a result of the
injection of the polymer
solution, the mineral oil, as in the case of water flooding, is forced through
the cavities
mentioned in the formation, proceeding from the injection borehole, in the
direction of the
production borehole, and the mineral oil is produced through the production
borehole. By
virtue of the fact that the polymer formulation, however, has about the same
viscosity as the
mineral oil, the risk is reduced that the polymer formulation breaks through
to the production
borehole with no effect, and hence the mineral oil is mobilized much more
homogeneously
than in the case of use of mobile water. It is thus possible to mobilize
additional mineral oil in
the formation. Details of polymer flooding and of polymers suitable for this
purpose are
disclosed, for example, in "Petroleum, Enhanced Oil Recovery, Kirk-Othmer,
Encyclopedia of
Chemical Technology, online edition, John Wiley & Sons, 2010".
For polymer flooding, a multitude of different thickening polymers have been
proposed,
especially high molecular weight polyacrylamide, copolymers of acrylamide and
further
comonomers, for example vinylsulfonic acid or acrylic acid. Polyacrylamide may
especially
be partly hydrolyzed polyacrylamide, in which some of the acrylamide units
have been
.. hydrolyzed to acrylic acid. In addition, it is also possible to use
naturally occurring polymers,
for example xanthan or polyglycosylglucan, as described, for example, by US
6,392,596 B1
or CA 832 277.
Also known is the use of hydrophobically associating copolymers for polymer
flooding. These
are understood by the person skilled in the art to mean water-soluble polymers
which have
lateral or terminal hydrophobic groups, for example relatively long alkyl
chains. In aqueous
medium, such hydrophobic groups can associate with themselves or with other
substances
having hydrophobic groups. This forms an associative network by which the
medium is
thickened. Details of the use of hydrophobically associating copolymers for
tertiary mineral oil
production are described, for example, in the review article by Taylor, K. C.
and Nasr-El-Din,
H.A. in J. Petr. Sci. Eng. 1998, 19, 265-280.
PF71248 CA 02817792 2013-05-13
3
EP 705 854 Al, DE 100 37 629 Al and DE 10 2004 032 304 Al disclose water-
soluble,
hydrophobically associating copolymers and the use thereof, for example in the
construction
chemistry sector. The copolymers described comprise acidic monomers, for
example acrylic
acid, vinylsulfonic acid, acrylamidomethylpropanesulfonic acid, basic monomers
such as
acrylamide, dimethylacrylamide, or monomers comprising cationic groups, for
example
monomers having ammonium groups, and also monomers which can bring about the
hydrophobic association of the individual polymer chains.
Our prior application WO 2010/133527 A2 discloses hydrophobically associating
copolymers
which comprise at least hydrophilic, monoethylenically unsaturated monomers,
for example
acrylamide, and monoethylenically unsaturated, hydrophobically associating
monomers. The
hydrophobically associating monomers have a block structure and have ¨ in this
sequence ¨
an ethylenically unsaturated group, optionally a linking group, a first
polyoxyalkylene block
which comprises at least 50 mol /0 of ethyleneoxy groups, and a second
polyoxyalkylene
group which consists of alkyleneoxy groups having at least 4 carbon atoms. The
application
discloses the use of such copolymers as thickeners, for example for polymer
flooding, for
construction chemical applications or for detergent formulations.
Our prior application WO 2011/015520 Al discloses a process for preparing
hydrophobically
associating copolymers by polymerizing water-soluble, monoethylenically
unsaturated
surface-active monomers and monoethylenically unsaturated hydrophilic monomers
in the
presence of surfactants, and the use of such copolymers for polymer flooding.
For polymer flooding, an aqueous polymer solution is injected through a
borehole (called the
injection borehole) into a mineral oil deposit, and the viscosity of the
polymer solution under
formation conditions should correspond approximately to the viscosity of the
mineral oil.
Suitable polymers for polymer flooding must therefore also have the thickening
action under
the conditions of the mineral oil deposit, i.e. at temperatures above room
temperature and in
the presence of formation water with a high salt content. Formation waters may
in the ex-
treme case comprise up to 35% by weight of salts. The salts are, for example,
alkali metal
salts, but also alkaline earth metal salts. Formation temperatures may be up
to 150 C.
Studies on partly hydrolyzed polyacrylamide and copolymers of acrylamide and
acrylamide-
methylpropanesulfonic acid show that the salt tolerance of the polymers can be
enhanced by
the incorporation of sulfo groups (see, for example, Masoud Rashidi, Anne
Marit Blokhus,
Arne Skauge, Journal of Applied Polymer Science, Vol. 117 (2010), pages 1551-
1557). In
the case of such polymers, however, the viscosity decreases with increasing
temperature.
Thus, to achieve a viscosity sufficient for polymer flooding, higher amounts
of polymer have
to be used, which impairs the economic viability of polymer flooding.
4
It was an object of the invention to provide a process for polymer flooding
with which
satisfactory results are achieved even at relatively high formation
temperatures.
Accordingly, a process for mineral oil production has been found, in which an
aqueous
formulation comprising at least one water-soluble, copolymer is injected
through at least one
injection borehole into a mineral oil deposit, and crude oil is withdrawn from
the deposit
through at least one production borehole, and wherein
= the water-soluble, copolymer comprises
(a) 0.1 to 15% by weight of at least one monoethylenically unsaturated,
monomer (a) selected from the group of
H2C=C(R1)-R2-0-(-CH2-CH(R3)-0-)k+CH2-CH(R4)-0-)I-R5 (I),
H2C=C(R)-0-(-CH2-CH(R3)-0-)k-R6 (II), or
H2C=C(R1)-(C=0)-0-(-CH2-CH(R3)-0-)k-R6 (Ill),
where the -(-CH2-CH(R3)-0-)k and -(-CH2-CH(R4)-04 units are arranged in
block structure in the sequence shown in formula (I) and the radicals and
indices are each defined as follows:
k: a number from 10 to 150, in particular from 15 to 30,
I: a number from 5 to 25,
R1: H or methyl,
R2: a single bond or a divalent linking group selected from the group
consisting of -(CnH2n)- , -0-(Cn=H2)- and ¨C(0)-0-(Cn-H2n+ , where n,
n' and n" are each natural numbers from 1 to 6,
R3: each independently H, methyl or ethyl, wherein at least 50 mol% of the
R3 radicals are H,
R4: each independently a hydrocarbyl radical having at least 2 carbon atoms
or an ether group of the general formula ¨CH2-0-R4', where R4' is a
hydrocarbyl radical having at least 2 carbon atoms,
R5: H or a hydrocarbyl radical having 1 to 30 carbon atoms,
R6: an aliphatic and/or aromatic, straight-chain or branched hydrocarbyl
radical having 8 to 40 carbon atoms, such as substituted phenyl groups
having 8 to 40 carbon atoms,
and also
CA 2817792 2019-07-30
5
(b) 85 to 99.9% by weight of at least two monoethylenically unsaturated,
hydrophilic monomers (b) different than (a), where the monomers (b)
comprise
(b1) 30 to 95% by weight of at least one uncharged, monoethylenically
unsaturated, hydrophilic monomer (b1), selected from the group
consisting of (meth)acrylamide, N-methyl(meth)acrylamide, N,N'-
dimethyl(meth)acrylamide and N-methylol(meth)acrylamide, and
(b2) at least one anionic, monoethylenically unsaturated, hydrophilic
monomer (b2) which comprises at least one acidic group selected
from the group consisting of ¨COOH, ¨S03H and ¨P03H2 and salts
thereof, using at least one monomer comprising ¨S03H groups,
where the proportions are each based on the total amount of all monomers in
the
copolymer,
= the copolymer has a weight-average molecular weight Mw of 1*106 g/mol to
30*106 g/mol,
= the amount of the copolymer in the formulation is 0.02 to 2% by weight,
and
= the temperature of the mineral oil deposit is 35 C to 120 C.
In a preferred embodiment of the invention, the temperature of the mineral oil
deposit is 40 C
to 90 C.
In a further preferred embodiment, the aqueous formulation further comprises
salts in an
amount of 20 000 ppm to 350 000 ppm.
It has been found that, surprisingly, the viscosity of the aqueous polymer
formulations used
for the process at first does not decrease with rising temperature, but
actually increases. The
viscosity passes through a maximum and begins to decrease again only at higher
temperatures. This achieves a particularly good ratio of viscosity achieved to
amount of
substance used.
With regard to the invention, the following should be stated specifically:
Hydrophobically associating copolymers used
For the process according to the invention for mineral oil production, an
aqueous formulation
of at least one water-soluble, hydrophobically associating copolymer is used
and is injected
through an injection borehole into a mineral oil deposit.
CA 2817792 2019-07-30
PF71248
CA 02817792 2013-05-13
6
The term "hydrophobically associating copolymers" is known in principle to
those skilled in
the art.
This comprises water-soluble copolymers which, as well as hydrophilic
molecular
components, have hydrophobic groups. In aqueous solution, the hydrophobic
groups can
associate with themselves or with other substances having hydrophobic groups
due to
intermolecular forces. This gives rise to a polymeric network joined by
intermolecular forces,
which thickens the aqueous medium.
In the ideal case, the copolymers used in accordance with the invention should
be miscible
with water in any ratio. According to the invention, however, it is sufficient
when the
copolymers are water-soluble at least at the desired use concentration and at
the desired pH.
In general, the solubility of the copolymer in water at room temperature under
the use
conditions should be at least 25 g/I.
According to the invention, the water-soluble, hydrophobically associating
copolymer
comprises 0.1 to 15% by weight of at least one monoethylenically unsaturated,
hydrophobically associating monomer (a) and 85 to 99.9% by weight of at least
two
monoethylenically unsaturated, hydrophilic monomers (b) different than (a). In
addition, it is
optionally possible for further, ethylenically unsaturated, preferably
monoethylenically
unsaturated, monomers (c) different than the monomers (a) and (b) to be
present in an
amount of up to 14.9% by weight. The amounts mentioned are based in each case
on the
sum of all monomers in the copolymer. Preference is given to using exclusively
monoethylenically unsaturated monomers.
Monomers (a)
The water-soluble, hydrophobically associating copolymer used comprises at
least one
monoethylenically unsaturated monomer (a) which imparts hydrophobically
associating
properties to the copolymer and shall therefore be referred to hereinafter as
"hydrophobically
associating monomer". According to the invention, the monomers (a) are
selected from the
group of
1
H2C=C(R1)-R2-0-(-CH2-CH(R3)-0-)k-(-CH2-CH(R4)-0-)1-R5 (I),
H2C=C(R1)-0-(-CH2-CH(R3)-0-)k-R6 (II),
H2C=C(R1)-(C=0)-0-(-CH2-CH(R3)-0-)k-R6 (III).
PF71248 CA 02817792 2013-05-13
7
Monomers (a) of the formula (I)
In the monomers (a) of the formula (I), an ethylenic group H2C=C(R1)- is
bonded via a
divalent linking group ¨R2-0- to a polyoxyalkylene radical with block
structure
-(-CH2-CH(R3)-0-)k-(-CH2-CH(R4)-0-)I-R5, where the two blocks -(-CH2-CH(R3)-0-
)k and
-(-CH2-CH(R4)-0-)1 are arranged in the sequence shown in formula (I). The
polyoxyalkylene
radical has either a terminal OH group (for R5=H) or a terminal ether group
¨0R5 (if R5 is a
hydrocarbyl radical).
In the abovementioned formula, R1 is H or a methyl group.
R2 is a single bond or a divalent linking group selected from the group of
¨(Cnh12)- [R2a
group], -0-(C0-120- [R2b group]- and ¨C(0)-0-(Cn-H2n)- [R2c group]. In the
formulae
mentioned, n, n' and n" are each a natural number from 1 to 6. In other words,
the linking
group comprises straight-chain or branched aliphatic hydrocarbyl groups having
1 to 6
hydrocarbon atoms, which are joined to the ethylenic group H2C=C(R1)-
directly, via an ether
group ¨0- or via an ester group ¨C(0)-0-. The -(CnH2n)-, -(Cn.1-12)- and -(C,-
,..H2n..)- groups are
preferably linear aliphatic hydrocarbyl groups.
The R2a group is preferably a group selected from ¨CH2-, -CH2-CH2- and ¨CH2-
CH2-CH2-,
more preferably a methylene group ¨CH2-.
The R2b group is preferably a group selected from -0-CH2-CH2-, -0-CH2-CH2-CH2-
and
-0-CH2-CH2-CH2-CH2-, more preferably ¨0-CH2-CH2-CH2-CH2-=
The R2c group is preferably a group selected from ¨C(0)-0-CH2-CH2-, -C(0)0-
CH(CH3)-
0H2_, -C(0)0-CH2-CH(CH3)-, -C(0)0-CH2-CH2-0H2-CH2- and -C(0)0-CH2-CH2-0H2-CH2-
CH2-CH2-, more preferably ¨C(0)-0-CH2-CH2- and -C(0)0-0H2-CH2-CH2-CH2-, and
most
preferably ¨C(0)-0-CH2-CH2-.
The R2 group is more preferably an R2a or R28 group, more preferably an R2b
group.
In addition, R2 is more preferably a group selected from ¨CF-I2- and -0-CH2-
CH2-CH2-CH2-,
most preferably -0-CH2-CH2-CH2-CH2-.
The monomers (I) also have a polyoxyalkylene radical which consists of the
units
-(-CH2-CH(R3)-0-)k and -(-CH2-CH(R4)-0-)i where the units are arranged in
block structure in
the sequence shown in formula (I). The transition between the two blocks may
be abrupt or
else continuous.
In the -(-CH2-CH(R3)-0-)k block, the R3 radicals are each independently H,
methyl or ethyl,
preferably H or methyl, with the proviso that at least 50 mol% of the R3
radicals are H.
PF71248 CA 02817792 2013-05-13
8
Preferably at least 75 mol% of the R3 radicals are H, more preferably at least
90 mol%, and
they are most preferably exclusively H. The block mentioned is thus a
polyoxyethylene block
which may optionally also have certain proportions of propylene oxide and/or
butylene oxide
units, preferably a pure polyoxyethylene block.
The number of alkylene oxide units k is a number from 10 to 150, preferably 12
to 100, more
preferably 15 to 80, even more preferably 20 to 30 and, for example, approx.
22 to 25. It is
clear to the person skilled in the art in the field of the polyalkylene oxides
that the numbers
mentioned are averages of distributions.
In the second +CH2-CH(R4)-0-)1- block, the R4 radicals are each independently
hydrocarbyl
radicals of at least 2 carbon atoms, preferably at least 3, more preferably 3
to 10 and most
preferably 3 to 8 carbon atoms and for example 3 to 4 carbon atoms. This may
be an
aliphatic and/or aromatic, linear or branched carbon radical. It is preferably
an aliphatic
radical.
Examples of suitable R4 radicals comprise ethyl, n-propyl, n-butyl, n-pentyl,
n-hexyl, n-heptyl,
n-octyl, n-nonyl or n-decyl, and phenyl. Examples of preferred radicals
comprise n-propyl,
n-butyl, n-pentyl, particular preference being given to an n-propyl radical.
The R4 radicals may also be ether groups of the general formula ¨CH2-0-R4
where R4' is an
aliphatic and/or aromatic, linear or branched hydrocarbyl radical having at
least 2 carbon
atoms, preferably at least 3 and more preferably 3 to 10 carbon atoms.
Examples of R3'
radicals comprise n-propyl, n-butyl, n-pentyl, n-hexyl, 2-ethylhexyl, n-
heptyl, n-octyl, n-nonyl
n-decyl or phenyl.
The -(-CH2-CH(R4)-0-)1- block is thus a block which consists of alkylene oxide
units having at
least 4 carbon atoms, preferably at least 5 carbon atoms, especially 5 to 10
carbon atoms,
and/or glycidyl ethers having an ether group of at least 2, preferably at
least 3, carbon atoms.
Preferred R3 radicals are the hydrocarbyl radicals mentioned; the units of the
second terminal
block are more preferably alkylene oxide units comprising at least 5 carbon
atoms, such as
pentene oxide units or units of higher alkylene oxides.
The number of alkylene oxide units I is a number from 5 to 25, preferably 6 to
20, more
preferably 8 to 18, even more preferably 10 to 15 and, for example, approx.
12,
The R5 radical is H or a preferably aliphatic hydrocarbyl radical having 1 to
30 carbon atoms,
preferably 1 to 10 and more preferably 1 to 5 carbon atoms. R5 is preferably
H, methyl or
ethyl, more preferably H or methyl and most preferably H.
In the monomers of the formula (I), a terminal monoethylenic group is joined
to a
polyoxyalkylene group with block structure, specifically firstly to a
hydrophilic block having
PF71248
CA 02817792 2013-05-13
9
polyethylene oxide units, which is in turn joined to a second terminal
hydrophobic block
formed at least from butene oxide units, preferably at least pentene oxide
units, or units of
higher alkylene oxides, for example dodecene oxide. The second block has a
terminal ¨0R5-
group, especially an OH-group. The terminal -(-CH2-CH(R4)-0-)i block with the
R4 radicals is
responsible for the hydrophobic association of the copolymers prepared using
the monomers
(a). Etherification of the OH end group is an option which may be selected by
the person
skilled in the art according to the desired properties of the copolymer. A
terminal hydrocarbyl
group is, however, not required for the hydrophobic association, and the
hydrophobic
association also works with a terminal OH group.
It is clear to the person skilled in the art in the field of polyalkylene
oxide block copolymers
that the transition between the two blocks, according to the method of
preparation, may be
abrupt or else continuous. In the case of a continuous transition, there is a
transition zone
between the two blocks, which comprises monomers of both blocks. When the
block
boundary is fixed at the middle of the transition zone, the first block -(-CH2-
CH(R3)-0-)k may
accordingly also have small amounts of -CH2-CH(R4)-0- units and the second
block
-(-CH3-CH(R4)-0-)1- small amounts of -CH2-CH(R3)-0- units, though these units
are not
distributed randomly over the block but arranged in the transition zone
mentioned.
Preparation of the monomers (a) of the formula (I)
The hydrophobically associating monomers (a) of the formula (I) can be
prepared by
methods known in principle to those skilled in the art.
.. To prepare the monomers (a), a preferred preparation process proceeds from
suitable
monoethylenically unsaturated alcohols (IV) which are subsequently alkoxylated
in a two-
stage process such that the block structure mentioned is obtained. This gives
monomers (a)
of the formula (I) where R5 = H. These can optionally be etherified in a
further process step.
The type of ethylenically unsaturated alcohols (IV) to be used is guided here
especially by
the R2 group.
When R2 is a single bond, the starting materials are alcohols (IV) of the
general formula
H2C=C(R1)-0-(-CH2-CH(R7)-0-)d-H (IVa) where R1 is as defined above, R7 is H
and/or CH3,
.. preferably H, and d is from 1 to 5, preferably 1 or 2. Examples of such
alcohols comprise
diethylene glycol vinyl ether H2C=CH-O-CH2-CH2-0-CH2-CH2-0H or dipropylene
glycol vinyl
ether H2C=CH-O-CH2-CH(CH3)-0-CH2-CH(CH3)-0H, preferably diethylene glycol
vinyl ether.
To prepare monomers (a) in which R2 is not a single bond, it is possible to
use alcohols of
.. the general formula H2C=C(R1)-R2-0H (IVb) or alcohols which already have
alkoxy groups
and are of the formula H2C=C(R1)-R2-0-(-CH2-CH(R7)-0-)d-H (IVc), where R7 and
d are each
as defined above, and R2 in each case is selected from the group of R2a, R2b
and R2b.
PF71248 CA 02817792 2013-05-13
The preparation of the monomers with a linking R2a group preferably proceeds
from alcohols
of the formula H2C=C(R1)¨(CnH2n)-0H, especially H2C=CH¨(CnH2n)-0H, or alcohols
of the
formula H2C=C(R1)-0-(-CH2-CH(R7)-0-)d-H. Examples of preferred alcohols
comprise ally!
5 .. alcohol H2C=CH-CH2-0H or isoprenol l-I2C=C(CH3)-CH2-CH2-0H.
The preparation of the monomers with a linking R2b group proceeds from vinyl
ethers of the
formula H2C=C(R1)-0-(Cn.H2n.)-0H, preferably H2C=CH-0-(C,y1-12)-0H. It is more
preferably
possible to use co-hydroxybutyl vinyl ether H2C=CH¨O-CH2-CH2-CH2-CH2-0H.
The preparation of the monomers with a linking R2c group proceeds from
hydroxyalkyl
(meth)acrylates of the general formula H2C=C(R1)-C(0)-0-(C0-12n..)-0H,
preferably
H2C=C(R1)-C(0)-0-(Cn-H2,,..)-0H. Examples of preferred hydroxyalkyl
(meth)acrylates
comprise hydroxyethyl (meth)acrylate H2C=C(R1)-C(0)-0-CH2-CH2-0H and
hydroxybutyl
(meth)acrylate H2C=C(R1)-C(0)-0-CH2-CH2-CH2-CH2-0H.
The starting compounds mentioned are alkoxylated, specifically in a two-stage
process, first
with ethylene oxide, optionally in a mixture with propylene oxide and/or
butylene oxide, and
in a second step with alkylene oxides of the general formula (Xa) or (Xb)
0 0
¨R4
(Xa)
/C) (Xb)
R4
where R4 in (Xa) and R4' in (Xb) are each as defined at the outset.
The performance of an alkoxylation including the preparation of the block
copolymers from
different alkylene oxides is known in principle to those skilled in the art.
It is likewise known to
those skilled in the art that the reaction conditions, especially the
selection of the catalyst,
can influence the molecular weight distribution of the alkoxylates and the
orientation of the
alkylene oxide units in a polyether chain.
The alkoxylates can be prepared, for example, by base-catalyzed alkoxylation.
For this
purpose, the alcohol used as the starting material can be admixed in a
pressure reactor with
alkali metal hydroxides, preferably potassium hydroxide, or with alkali metal
alkoxides, for
example sodium methoxide. By means of reduced pressure (e.g. <100 mbar) and/or
.. increasing the temperature (30 to 150 C), water still present in the
mixture can be removed.
Thereafter, the alcohol is present as the corresponding alkoxide. This is
followed by
inertization with inert gas (e.g. nitrogen) and, in a first step, stepwise
addition of ethylene
oxide, optionally in a mixture with propylene oxide and/or butylene oxide, at
temperatures of
60 to 180 C, preferably 130 to 150 C. The addition is typically effected
within 2 to 5 h, though
the invention should not be restricted thereto. After the addition has ended,
the reaction
PF71248 CA 02817792 2013-05-13
11
mixture is appropriately allowed to continue to react, for example for 1/2 h
to 1 h. In a second
step, alkylene oxides of the general formula (Xb) are subsequently metered in
stepwise. The
reaction temperature in the second stage can be maintained or else altered. A
reaction
temperature lower by approx. 10 to 25 C than in the first stage has been found
to be useful.
The alkoxylation can also be undertaken by means of techniques which lead to
narrower
molecular weight distributions than the base-catalyzed synthesis. For this
purpose, the
catalysts used may, for example, be double hydroxide clays as described in
DE 43 25 237 Al. The alkoxylation can more preferably be effected using double
metal
cyanide catalysts (DMC catalysts). Suitable DMC catalysts are disclosed, for
example, in
DE 102 43 361 Al, especially paragraphs [0029] to [0041] and the literature
cited therein.
For example, it is possible to use catalysts of the Zn-Co type. To perform the
reaction, the
alcohol used as the starting material can be admixed with the catalyst, and
the mixture can
be dewatered as described above and reacted with the alkylene oxides as
described.
Typically, not more than 250 ppm of catalyst based on the mixture are used,
and the catalyst
can remain in the product due to this small amount.
The alkoxylation can additionally also be undertaken under acid catalysis. The
acids may be
Bronsted or Lewis acids. To perform the reaction, the alcohol used as the
starting material
can be admixed with the catalyst, and the mixture can be dewatered as
described above and
reacted with the alkylene oxides as described. At the end of the reaction, the
acidic catalyst
can be neutralized by addition of a base, for example KOH or NaOH, and
filtered off if
required.
It is clear to the person skilled in the art in the field of the polyalkylene
oxides that the
orientation of the hydrocarbyl radicals R4 and optionally R3 may depend on the
conditions of
the alkoxylation, for example on the catalyst selected for the alkoxylation.
The alkylene oxide
groups can thus be incorporated into the monomer either in the -(-CH2-CH(R4)-0-
) orientation
or else in the inverse -(-CH(R4)¨CH2-0-)- orientation. The description in
formula (I) should
therefore not be considered to be restricted to a particular orientation of
the R3 or R4 groups.
When the terminal OH group of the monomers (a) of the formula (I) (i.e. R5 =
H) is to be
etherified, this can be accomplished with customary alkylating agents known in
principle to
those skilled in the art, for example alkyl sulfates. For etherification, it
is especially possible
to use dimethyl sulfate or diethyl sulfates.
The preferred preparation process described for the monomers (I) has the
advantage that
the formation of potentially crosslinking by-products with two ethylenically
unsaturated
groups is substantially avoided. Accordingly, it is possible to obtain
copolymers with a
particularly low gel content.
PF71248 CA 02817792 2013-05-13
12
Monomers (a) of the formulae (II) and (III)
In the monomers of the formulae (II) and (III), R1, R3 and k are each defined
as already
outlined.
R6 is an aliphatic and/or aromatic, straight-chain or branched hydrocarbyl
radical having 8 to
40 carbon atoms, preferably 12 to 32 carbon atoms. For example, it may
comprise n-alkyl
groups such as n-octyl, n-decyl or n-dodecyl groups, phenyl groups, and
especially
substituted phenyl groups. Substituents on the phenyl groups may be alkyl
groups, for
example Cl-C6-alkyl groups, preferably styryl groups. Particular preference is
given to a
tristyrylphenyl group.
The hydrophobically associating monomers of the formulae (II) and (III) and
the preparation
thereof are known in principle to those skilled in the art, for example from
EP 705 854 Al.
Amounts of monomers (a)
The amount of the monoethylenically unsaturated, hydrophobically associating
monomers (a)
is 0.1 to 15% by weight, based on the total amount of all monomers in the
copolymer,
especially 0.1 to 10% by weight, preferably 0.2 to 5% by weight and more
preferably 0.5 to
2% by weight.
Particular preference is given to using monomers (a) of the general formula
(I) to prepare the
inventive copolymers, most preferably monomers (a) of the general formula (I)
in which R2 is
an R2b radical.
Monomers (b)
Over and above the monomers (a), the hydrophobically associating copolymer
used in
accordance with the invention comprises at least two monoethylenically
unsaturated,
hydrophilic monomers (b) different than (a).
More preferably, the hydrophilic monomers (b) used are miscible with water in
any ratio, but
it is sufficient for execution of the invention that the inventive,
hydrophobically associating
copolymer possesses the water solubility mentioned at the outset. In general,
the solubility of
the monomers (b) in water at room temperature should be at least 50 g/I,
preferably at least
150 g/I and more preferably at least 250 g/I.
According to the invention, the copolymer comprises at least one uncharged,
monoethylenically unsaturated, hydrophilic monomer (b1) selected from the
group of
(meth)acrylamide, N-methyl(meth)acrylamide, N,N'-dimethyl(meth)acrylamide or N-
methylol-
(meth)acrylamide. Preference is given to (meth)acrylamide, especially
acrylamide. When
PF71248 CA 02817792 2013-05-13
13
mixtures of different monomers (b1) are used, at least 50 mol% of the monomers
(b1) should
be (meth)acrylamide, preferably acrylamide.
According to the invention, the copolymer used further comprises at least one
hydrophilic,
rnonoethylenically unsaturated anionic monomer (b2) which comprises at least
one acidic
group selected from the group of ¨COOH, ¨S03H and ¨P03H2 and salts thereof.
Preference
is given to monomers comprising COOH groups and/or ¨S03H groups, particular
preference
to monomers comprising ¨S03H groups. The monomers may of course also be the
salts of
the acidic monomers. Suitable counterions comprise especially alkali metal
ions such as Lit,
Nat or Kt, and ammonium ions such as NH4 + or ammonium ions with organic
radicals.
Examples of monomers comprising COOH groups comprise acrylic acid, methacrylic
acid,
crotonic acid, itaconic acid, maleic acid or fumaric acid. Preference is given
to acrylic acid.
Examples of monomers comprising sulfo groups comprise vinylsulfonic acid,
allylsulfonic
acid, 2-acrylamido-2-methylpropanesulfonic acid, 2-methacrylamido-2-
methylpropanesulfonic
acid, 2-acrylamidobutanesulfonic acid, 3-acrylamido-3-methylbutanesulfonic
acid or
2-acrylamido-2,4,4-trimethylpentanesulfonic acid. Preference is given to
vinylsulfonic acid,
allylsulfonic acid or 2-acrylamido-2-methylpropanesulfonic acid, and
particular preference to
2-acrylamido-2-methylpropanesulfonic acid.
Examples of monomers comprising phospho groups comprise vinylphosphonic acid,
allylphosphonic acid, N-(meth)acrylamidoalkylphosphonic acids or
(meth)acryloyloxyalkyl-
phosphonic acids, preference being given to vinylphosphonic acid.
For the sake of completeness, it should be mentioned that the monomers (b1)
can be
hydrolyzed at least partly to (meth)acrylic acid under some circumstances in
the course of
preparation and use. The copolymers used in accordance with the invention may
accordingly
comprise (meth)acrylic acid units, even if no (meth)acrylic acid units at all
have been used for
the synthesis. The tendency to hydrolysis of the monomers (b1) decreases with
increasing
content of sulfo groups. Accordingly, the presence of sulfo groups in the
copolymer used in
accordance with the invention is advisable.
The copolymers used in accordance with the invention may additionally
optionally comprise
at least one monoethylenically unsaturated, cationic monomer (b3) having
ammonium ions.
Suitable cationic monomers (b3) comprise especially monomers having ammonium
groups,
especially ammonium derivatives of N-(w-aminoalkyl)(meth)acrylamides or w-
aminoalkyl-
(meth)acrylic esters.
More particularly, monomers (b3) having ammonium groups may be compounds of
the
general formulae H2C=C(R8)-CO-NR9-R10-NR113+X- (Va) and/or H2C=C(RB)-COO-R10-
PF71248 CA 02817792 2013-05-13
14
NR113*X- (Vb). In these formulae, R8 is H or methyl, R9 is H or a Cl-C4-alkyl
group, preferably
H or methyl, and R1 is a preferably linear Cl-C4-alkylene group, for example
a 1,2-ethylene
group ¨CH2-CH2- or a 1,3-propylene group ¨CH2-CH2-CI-12-
The R11 radicals are each independently Ci-04-alkyl radicals, preferably
methyl, or a group of
the general formula ¨R12-S03H where R12 is a preferably linear C1-04-alkylene
group or a
phenyl group, with the proviso that generally not more than one of the R"
substituents is a
substituent having sulfa groups. More preferably, the three R11 substituents
are methyl
groups, i.e. the monomer has a ¨N(CH3)3* group. X- in the above formula is a
monovalent
anion, for example Cl-. X- may of course also be a corresponding fraction of a
polyvalent
anion, though this is not preferred. Examples of preferred monomers (b3) of
the general
formula (Va) or (Vb) comprise salts of 3-
trimethylammoniopropyl(meth)acrylamides or
2-trimethylammonioethyl (meth)acrylates, for example the corresponding
chlorides such as
3-trimethylammoniopropylacrylamide chloride (DIMAPAQUAT) and 2-trimethyl-
.. ammoniomethyl methacrylate chloride (MADAME-QUAT).
The copolymers used in accordance with the invention may additionally also
comprise further
monoethylenically unsaturated hydrophilic monomers (b4) different than the
hydrophilic
monomers (b1), (b2) and (b3). Examples of such monomers comprise monomers
comprising
hydroxyl groups and/or ether groups, for example hydroxyethyl (meth)acrylate,
hydroxypropyl
(meth)acrylate, ally' alcohol, hydroxyvinyl ethyl ether, hydroxyvinyl propyl
ether, hydroxyvinyl
butyl ether, or compounds of the formula H2C=C(R1)-000-(-CH2-CH(R13)-0-)b-R14
(Vla) or
H2C=C(R1)-0-(-CH2-CH(R13)-0-)D-R14 (Vlb), where R1 is as defined above and b
is a number
from 2 to 200, preferably 2 to 100. The R13 radicals are each independently H,
methyl or
ethyl, preferably H or methyl, with the proviso that at least 50 mol% of the
R13 radicals are H.
Preferably at least 75 mol% of the R13 radicals are H, more preferably at
least 90 mol%, and
they are most preferably exclusively H. The R14 radical is H, methyl or ethyl,
preferably H or
methyl. Further examples of monomers (b4) comprise N-vinyl derivatives, for
example N-
vinylformamide, N-vinylacetamide, N-vinylpyrrolidone or N-vinylcaprolactam,
and vinyl
esters, for example vinyl formate or vinyl acetate. N-Vinyl derivatives can be
hydrolyzed after
polymerization to give vinylamine units, and vinyl esters to give vinyl
alcohol units.
The amount of all hydrophilic monomers (b) in the inventive copolymer is, in
accordance with
the invention, 85 to 99.9% by weight, based on the total amount of all
monomers in the
copolymer, preferably 90 to 99.8% by weight.
The amount of the uncharged, hydrophilic monomers (b1) here is generally 30 to
95% by
weight, preferably 30 to 85% by weight and more preferably 30 to 70% by
weight, based on
the total amount of all monomers used.
When the copolymer comprises only uncharged monomers (b1) and anionic monomers
(b2),
it has been found to be useful to use the uncharged monomers (b1) in an amount
of 30 to
PF71248 CA 02817792 2013-05-13
95% by weight and the anionic monomers (b2) in an amount of 4.9 to 69.9% by
weight, each
amount being based on the total amount of all monomers used. In this
embodiment, the
monomers (bl) are preferably used in an amount of 30 to 80% by weight and the
anionic
monomers (b2) in an amount of 19.9 to 69.9% by weight, and the monomers (b1)
are more
5 preferably used in an amount of 40 to 70% by weight and the anionic
monomers (b2) in an
amount of 29.9 to 59.9% by weight
When the copolymer comprises uncharged monomers (b1), anionic monomers (b2)
and
cationic monomers (b3), it has been found to be useful to use the uncharged
monomers (b1)
10 in an amount of 30 to 95% by weight, and the anionic (b2) and cationic
(b3) monomers
together in an amount of 4.9 to 69.9% by weight, with the proviso that the
molar (b2)/(b3)
ratio is 0.7 to 1.3. The molar (b2)/(b3) ratio is preferably 0.8 to 1.2 and,
for example, 0.9 to
1.1. This measure makes it possible to obtain copolymers which are
particularly insensitive to
salt burden. In this embodiment, the monomers (b1) are used in an amount of 30
to 80% by
15 weight, and the anionic and cationic monomers (b2) + (b3) together in an
amount of 19.9 to
69.9% by weight, and the monomers (b1) are more preferably used in an amount
of 40 to
70% by weight and the anionic and cationic monomers (b2) + (b3) together in an
amount of
29.9 to 59.9% by weight, where the molar ratio already mentioned should be
observed in
each case.
Monomers (c)
In addition to the hydrophilic monomers (a) and (b), the inventive copolymers
may optionally
comprise ethylenically unsaturated monomers different than the monomers (a)
and (b),
preferably monoethylenically unsaturated monomers (c). Of course, it is also
possible to use
mixtures of a plurality of different monomers (c).
Such monomers can be used for fine control of the properties of the copolymer
used in
accordance with the invention. If present at all, the amount of such
optionally present
monomers (c) may be up to 14.9% by weight, preferably up to 9.9% by weight,
more
preferably up to 4.9% by weight, based in each case on the total amount of all
monomers.
Most preferably, no monomers (c) are present.
The monomers (c) may, for example, be monoethylenically unsaturated monomers
which
have more hydrophobic character than the hydrophilic monomers (b) and which
are
accordingly water-soluble only to a minor degree. In general, the solubility
of the monomers
(o) in water at room temperature is less than 50 WI, especially less than 30
g/I. Examples of
such monomers comprise N-alkyl- and N,Ni'-dialkyl(meth)acrylamides, where the
number of
carbon atoms in the alkyl radicals together is at least 3, preferably at least
4. Examples of
such monomers comprise N-butyl(meth)acrylamide, N-cyclohexyl(meth)acrylamide
or
N-benzyl(meth)acrylamide.
PF71248 CA 02817792 2013-05-13
16
Preparation of the hydrophobically associating copolymers
The copolymers used in accordance with the invention can be prepared by
methods known
in principle to those skilled in the art, by free-radical polymerization of
the monomers (a), (b)
and optionally (c), for example by solution or gel polymerization in the
aqueous phase.
For polymerization, the monomers (a), (b), optionally (c), initiators and
optionally further
assistants for polymerization are used in an aqueous medium.
In a preferred embodiment, the preparation is undertaken by means of gel
polymerization in
the aqueous phse. For gel polymerization, a mixture of the monomers (a), (b)
and optionally
(c), initiators and optionally further assistants with water or an aqueous
solvent mixture is first
provided. Suitable aqueous solvent mixtures comprise water and water-miscible
organic
solvents, where the proportion of water is generally at least 50% by weight,
preferably at
least 80% by weight and more preferably at least 90% by weight. Organic
solvents in this
context include especially water-miscible alcohols such as methanol, ethanol
or propanol.
Acidic monomers can be fully or partly neutralized before the polymerization.
The
concentration of all components except the solvents in the course of the
polymerization is
typically approx. 20 to 60% by weight, preferably approx. 30 to 50% by weight.
The
polymerization should especially be performed at a pH in the range from 5.0 to
7.5 and
preferably at a pH of 6Ø
Polymerization in the presence of a nonpolymerizable, interface-active
compound
In a preferred embodiment of the invention, the copolymers used are prepared
in the
presence of at least one nonpolymerizable, surface-active compound (T).
The nonpolymerizable, surface-active compound (T) is preferably at least one
nonionic
surfactant, but anionic and cationic surfactants are also suitable to the
extent that they do not
take part in the polymerization reaction. They may especially be surfactants,
preferably
nonionic surfactants, of the general formula R13-Y' where R13 is a hydrocarbyl
radical having
8 to 32, preferably 10 to 20 and more preferably 12 to 18 carbon atoms, and Y'
is a
hydrophilic group, preferably a nonionic hydrophilic group, especially a
polyalkoxy group.
The nonionic surfactant is preferably an ethoxylated long-chain aliphatic
alcohol which may
optionally comprise aromatic components.
Examples include: C12C14-fatty alcohol ethoxylates, C16C18-fatty alcohol
ethoxylates, 013-oxo
alcohol ethoxylates, Clo-oxo alcohol ethoxylates, Cl3C15-oxo alcohol
ethoxylates,
C10-Guerbet alcohol ethoxylates and alkylphenol ethoxylates. Useful compounds
have
especially been found to be those having 5 to 20 ethyleneoxy units, preferably
8 to 18
PF71248
CA 02817792 2013-05-13
17
ethyleneoxy units. It is optionally also possible for small amounts of higher
alkyleneoxy units
to be present, especially propyleneoxy and/or butyleneoxy units, though the
amount in the
form of ethyleneoxy units should generally be at least 80 mol% based on all
alkyleneoxy
units.
Especially suitable are surfactants selected from the group of the ethoxylated
alkylphenols,
the ethoxylated, saturated iso-C13-alcohols and/or the ethoxylated C10-Guerbet
alcohols,
where in each case 5 to 20 ethyleneoxy units, preferably 8 to 18 ethyleneoxy
units, are
present in alkoxy radicals.
Surprisingly, the addition of nonpolymerizable, interface-active compounds (T)
during the
polymerization leads to a distinct improvement in performance properties of
the copolymer in
polymer flooding. More particularly, the thickening action is increased and
the gel content of
the copolymer is also reduced. This effect can probably be explained as
follows, without any
intention that the invention thus be tied to this explanation. In the case of
polymerization
without presence of a surfactant, the hydrophobically associating comonomers
(a) form
micelles in the aqueous reaction medium. In the polymerization, this leads to
blockwise
incorporation of the hydrophobically associating regions into the polymer. If,
in accordance
with the invention, an additional surface-active compound is present in the
preparation of the
copolymers, mixed micelles form. These mixed micelles comprise polymerizable
and
nonpolymerizable components. As a result, the hydrophobically associating
monomers are
then incorporated in relatively short blocks. At the same time, the number of
these relatively
short blocks is greater per polymer chain. Thus, the structure of the
copolymers prepared in
the presence of a surfactant differs from those without the presence of a
surfactant.
The nonpolymerizable, interface-active compounds (T) can generally be used in
an amount
of 0.1 to 5% by weight, based on the amount of all monomers used.
The weight ratio of the nonpolymerizable, interface-active compounds (T) used
to the
monomers (a) is generally 4:1 to 1:4, preferably 2:1 to 1:2, more preferably
1,5:1 to 1:1.5
and, for example, approx. 1:1.
Performance of the polymerization
For the polymerization, the components required are first mixed with one
another. The
sequence with which the components are mixed for polymerization is
unimportant; what is
important is merely that, in the preferred polymerization method, the
nonpolymerizable,
interface-active compound (T) is added to the aqueous polymerization medium
before the
initiation of the polymerization.
The mixture is subsequently polymerized thermally and/or photochemically,
preferably at
-5 C to 80 C. If polymerization is effected thermally, preference is given to
using
PF71248
CA 02817792 2013-05-13
18
polymerization initiators which can initiate the polymerization even at
comparatively low
temperature, for example redox initiators. The thermal polymerization can be
undertaken
even at room temperature or by heating the mixture, preferably to temperatures
of not more
than 50 C. The photochemical polymerization is typically undertaken at
temperatures of -5 to
10 C. It is also possible to combine photochemical and thermal polymerization
with one
another, by adding both initiators for the thermal and photochemical
polymerization to the
mixture. In this case, the polymerization is first initiated photochemically
at low temperatures,
preferably -5 to +10 C. The heat of reaction released heats the mixture, which
additionally
initiates the thermal polymerization. By means of this combination, it is
possible to achieve a
conversion of more than 99%.
In a further preferred embodiment of the polymerization, it is also possible
to perform the
reaction with a mixture of a redox initiator system and a thermal initiator
which does not
decompose until relatively high temperatures. This may, for example, be a
water-soluble azo
initiator which decomposes within the temperature range from 40 C to 70 C. The
polymerization here is at first initiated at low temperatures of, for example,
0 to 10 C by the
redox initiator system. The heat of reaction released heats the mixture, and
this additionally
initiates the polymerization by virtue of the initiator which does not
decompose until relatively
high temperatures.
The gel polymerization is generally effected without stirring. It can be
effected batchwise by
irradiating and/or heating the mixture in a suitable vessel at a layer
thickness of 2 to 20 cm.
The polymerization gives rise to a solid gel. The polymerization can also be
effected
continuously. For this purpose, a polymerization apparatus is used, for
example, which
possesses a conveyor belt to accommodate the mixture to be polymerized. The
conveyor
belt is equipped with devices for heating and/or for irradiating with UV
radiation. In this
method, the mixture is poured onto one end of the belt by means of a suitable
apparatus, the
mixture is polymerized in the course of transport in belt direction, and the
solid gel can be
removed at the other end of the belt.
The gel obtained is preferably comminuted and dried after the polymerization.
The drying
should preferably be effected at temperatures below 100 C. To prevent
conglutination, it is
possible to use a suitable separating agent for this step. This gives the
hydrophobically
associating copolymer as granules or powder.
Further details of the performance of a gel polymerization are disclosed, for
example in
DE 10 2004 032 304 Al, paragraphs [0037] to [0041].
Since the polymer powder or granules obtained are generally used in the form
of an aqueous
solution in the course of application at the site of use, the polymer has to
be dissolved in
water on site. This may result in undesired lumps with the high molecular
weight polymers
described. In order to avoid this, it is possible to add an assistant which
accelerates or
PF71248 CA 02817792 2013-05-13
19
improves the dissolution of the dried polymer in water to the inventive
polymers as early as in
the course of synthesis. This assistant may, for example, be urea.
The resulting copolymers generally have a weight-average molecular weight M,
of
1*106 g/mol to 30*106 g/mol, preferably 5*106 g/mol to 20*106 g/mol.
Processes for mineral oil production
To execute the process according to the invention, at least one production
borehole and at
least one injection borehole are sunk into the mineral oil deposit. In
general, a deposit is
provided with several injection boreholes and with several production
boreholes. An aqueous
formulation of the copolymer described is injected into the mineral oil
deposit through the at
least one injection borehole, and mineral oil is withdrawn from the deposit
through at least
one production borehole. The term "mineral oil" in this context of course does
not only mean
single-phase oil, but the term also comprises the customary crude oil-water
emulsions, As a
result of the pressure generated by the formulation injected, known as the
"polymer flood",
the mineral oil flows in the direction of the production borehole and is
produced via the
production borehole.
The deposit temperature of the mineral oil deposit to which the process
according to the in-
vention is applied is, in accordance with the invention, 35 to 120 C,
preferably 40 C to 90 C,
more preferably 45 C to 75 C and, for example, 50 C to 70 C.
It is clear to the person skilled in the art that a mineral oil deposit may
also have a certain
temperature distribution. The deposit temperature mentioned relates to the
region of the de-
posit between the injection and production boreholes which is covered by the
polymer flood-
ing. Methods for determining the temperature distribution of a mineral oil
deposit are known
in principle to those skilled in the art. The temperature distribution is
generally undertaken
from temperature measurements at certain sites in the formation in combination
with simula-
tion calculations, which take account, inter alia, of the amounts of heat
introduced into the
formation and the amounts of heat removed from the formation.
The process according to the invention can be employed especially in the case
of mineral oil
deposits having an average permeability of 10 mD to 4 D, preferably 100 mD to
2 D and
more preferably 200 mD to 1 D. The permeability of a mineral oil formation is
reported by the
person skilled in the art in the unit "darcy" (abbreviated to "D" or "mD" for
"millidarcies") and
can be determined from the flow rate of a liquid phase in the mineral oil
formation as a
function of the pressure differential applied. The flow rate can be determined
in core flooding
tests with drill cores taken from the formation. Details on this subject can
be found, for
example, in K. Weggen, G. Pusch, H. Rischm011er in "Oil and Gas", pages 37 if,
Ulmann's
Encyclopedia of Industrial Chemistry, online edition, Wiley-VCH, Weinheim
2010. It is clear
to the person skilled in the art that the permeability in a mineral oil
deposit need not be
PF71248 CA 02817792 2013-05-13
homogeneous, but generally has a certain distribution, and the reported
permeability of a
mineral oil deposit is accordingly an average permeability.
To execute the process, an aqueous formulation which comprises, in addition to
water, at
5 least the hydrophobically associating copolymer described is used. It is
of course also
possible to use mixtures of different hydrophobically associating copolymers.
The formulation can be made up in fresh water, or else in water comprising
salts. Of course,
it can also comprise mixtures of different salts. For example, it is possible
to use sea water to
10 make up the aqueous formulation, or it is possible to use produced
formation water, which is
reused in this manner. In the case of offshore production platforms, the
formulation is
generaly made up in sea water. In the case of onshore production units, the
polymer can
advantageously first be dissolved in fresh water, and the resulting solution
can be diluted to
the desired use concentration with formation water. The formulation can
preferably be
15 prepared by initially charging the water, sprinkling in the copolymer as
a powder and mixing it
with the water.
The salts may especially be alkali metal salts and alkaline earth metal salts.
Examples of
typical cations comprise Na, K*, Mg2+ or Ca2+, and examples of typical anions
comprise
20 chloride, bromide, hydrogencarbonate, sulfate or borate.
When the formulation comprises salts, generally at least one or more than one
alkali metal
ion, especially at least NAP, is present. In addition, it is also possible for
alkaline earth metal
ions to be present, where the weight ratio of alkali metal ions/alkaline earth
metal ions is
generally 2, preferably 3. The anions present are generally at least one or
more than one
halide ion, especially at least Cl-. In general, the amount of Cl- is at least
50% by weight,
preferably at least 80% by weight, based on the sum of all anions.
The total amount of all salts in the aqueous formulation is generally 20 000
ppm to
350 000 ppm (parts by weight), based on the sum of all components of the
formulation.
When sea water is used to make up the formulation, the salt content is
generally 20 000 ppm
to 50 000 ppm, and, when formation water is used, generally 100 000 ppm to 250
000 ppm.
The amount of alkaline earth metal ions may preferably be 1000 to 53 000 ppm.
The aqueous formulation may of course comprise further components. Examples of
further
components comprise biocides, stabilizers or inhibitors.
The concentration of the copolymer is fixed such that the aqueous formulation
has the
desired viscosity for the end use. The viscosity of the formulation should
generally be at least
5 mPas (measured at 25 C and a shear rate of 7 s-1), preferably at least 10
mPas.
PF71248 CA 02817792 2013-05-13
21
According to the invention, the concentration of the polymer in the
formulation is 0.01 to 2%
by weight based on the sum of all components of the aqueous formulation. The
amount is
preferably 0.05 to 0.5% by weight, more preferably 0.04 to 0.2% by weight and,
for example,
approx. 0.1% by weight.
The injection of the aqueous copolymer formulation can be undertaken by means
of
customary apparatus. The formulation can be injected into one or more
injection boreholes
by means of customary pumps. The injection boreholes are typically lined with
cemented
steel tubes, and the steel tubes are perforated at the desired site. The
formulation exits
through the perforation from the injection borehole into the mineral oil
formation. The
pressure applied by means of the pumps, in a manner known in principle, fixes
the flow rate
of the formulation and hence also the shear stress with which the aqueous
formulation enters
the formation. The shear stress on entry into the formation can be calculated
by the person
skilled in the art in a manner known in principle on the basis of the Hagen-
Poiseuille law
using the area flowed through on entry into the formation, the mean pore
radius and the
volume flow. The average porosity of the formation can be determined in a
manner known in
principle by measurements on drill cores. By its nature, the greater the
volume flow of
aqueous copolymer formulation injected into the formation, the greater the
shear stress.
The rate of injection can be fixed by the person skilled in the art according
to the conditions
in the formation. Preferably, the shear rate on entry of the aqueous polymer
formulation into
the formation is at least 30 000 s-1, preferably at least 60 000 and more
preferably at least
90 000 s-1.
Copolymers particularly preferred for execution of the process comprise
monomers (a) of the
general formula H2C=CH-0-(CH2),,-0-(-CH2-CH2-0-)k+CH2-CH(R4)-0-)I-H (la) where
n' is 2
to 6, preferably 2 to 4 and more preferably 4. R4 in the preferred variant is
a hydrocarbyl
radical having 3 to 10 carbon atoms, especially an n-propyl radical. In
addition, in formula
(la), k is a number from 20 to 30 and I is a number from 6 to 20, preferably 8
to 18. The
amount of the monomers (a) of the formula (la) is 0.2 to 5% by weight,
preferably 0.5 to 2%
by weight. As monomer (b1), the preferred copolymer comprises 40 to 60% by
weight of
acrylamide and, as monomer (b2), 35 to 55% by weight of a monomer (b2) having
sulfo
groups, preferably 2-acrylamido-2-methylpropanesulfonic acid or salts thereof.
Further copolymers preferred for execution of the process likewise comprise
0.2 to 5% by
weight, preferably 0.5 to 2% by weight, of monomers (a) of the general formula
(la) and 30 to
40% by weight of acrylamide. They additionally comprise 25 to 35% by weight of
at least one
monomer (b2) having sulfo groups, preferably 2-acrylamido-2-
methylpropanesulfonic acid or
salts thereof, and 25 to 35% by weight of at least one cationic monomer having
ammonium
ions, preferably salts of 3-trimethylammoniopropyl(meth)acrylamides and 2-
trimethyl-
ammonioethyl (meth)acrylates.
PF71248 CA 02817792 2013-05-13
22
The examples which follow are intended to illustrate the invention in detail:
Monomers (a) used
.. Monomer M1
Hydroxybutyl vinyl ether alkoxylate with 22 EO units and 12 Pe0 units
H2C=CH-0-(CH2)4-0-(-CH2-CH2-0-)22-(-CH2-CH(C3H7)-0-)12-H
A 1 I stirred stainless steel autoclave is initially charged with 44.1 g of
hydroxybutyl vinyl
ether. Subsequently, 3.12 g of KOMe (32% in Me0H) are metered in and the
methanol is
drawn off at 80 C and approx. 30 mbar. This is followed by heating to 140 C,
purging of the
reactor with nitrogen and establishment of a nitrogen pressure of 1.0 bar.
Then 368 g of EO
are metered in within approx. 3 h. After continued reaction at 140 C for a
half hour, the
reactor is cooled to 125 C, and a total of 392 g of pentene oxide are metered
in over the
course of 3.5 h. The reaction continues overnight.
The product has an OH number of 31.9 mg KOH/g (theory: 26.5 mg KOH/g). The OH
number is determined by means of the ESA method.
Preparation of the copolymers
Polymer 1:
Preparation of a copolymer from 2% by weight of monomer Ml, 50% by weight of
acrylamide
and 48% by weight of 2-acrylamido-2-methylpropanesulfonic acid
A plastic bucket with magnetic stirrer, pH meter and thermometer is initially
charged with
121.2 g of a 50% aqueous solution of NaATBS (2-acrylamido-2-
methylpropanesulfonic acid,
sodium salt), and then 155 g of distilled water, 0.6 g of a defoamer (Surfynol
DF-58), 0.2 g
of a silicone defoamer (Baysilon EN), 2.3 g of monomer Ml, 114.4 g of a 50%
aqueous
solution of acrylamide, 1.2 g of pentasodium diethylenetriaminepentaacetate
(complexing
agent, as a 5% aqueous solution) and 2.4 g of a nonionic surfactant
(nonylphenol,
alkoxylated with 10 units of ethylene oxide) are added successively.
After adjusting the pH with a 20% or 2% sulfuric acid solution to a value of 6
and adding the
rest of the water, the monomer solution is adjusted to the start temperature
of 5 C. The total
amount of water is such that ¨ after the polymerization ¨ a solids
concentration of approx. 30
to 36% by weight is attained. The solution is transferred to a thermos flask,
a temperature
sensor for the temperature recording is provided and the solution is purged
with nitrogen for
.. 30 minutes. The polymerization is then initiated by adding 1.6 ml of a 10%
aqueous solution
of a water-soluble cationic azo initiator 2,2'-azobis(2-amidinopropane)
dihydrochloride (Wako
V-50), 0.12 ml of a 1% aqueous solution of tett-butyl hydroperoxide and 0.24
ml of a 1%
CA 02817792 2013-05-13
PF71248
23
sodium sulfite solution. After the initiators have been added, the temperature
rises to approx.
80 C within 15 to 30 min. After 30 min, the reaction vessel is placed into a
drying cabinet at
approx. 80 C for approx. 2 h to complete the polymerization. The total
duration of the
polymerization is approx. 2 h to 2.5 h.
A gel block is obtained, which, after the polymerization has ended, is
comminuted with the
aid of a meat grinder. The gel granules obtained are dried in a fluidized bed
dryer at 55 C for
two hours. This gives white, hard granules which are converted to a
pulverulent state by
means of a centrifugal mill. This gives a copolymer with a weight-average
molecular weight
of approx. 1*106 g/mol to 30*106 g/mol.
Polymer 2:
Preparation of a copolymer from 5% by weight of monomer Ml, 50% by weight of
acrylamide
and 45% by weight of 2-acrylamido-2-methylpropanesulfonic acid
The procedure is as in Example 1, except that the amount of monomer M1 is
increased from
2% by weight to 5% by weight based on the sum of all monomers, and the amount
of
2-acrylamido-2-methylpropanesulfonic acid is reduced from 48% by weight to 45%
by weight.
The amount of the surfactant (proportions by mass) corresponds to that of
monomer Ml.
Comparative polymer 1:
This is a commercially available copolymer for polymer flooding, formed from
approx. 50% by
weight of acrylamide and approx. 50% by weight of 2-acrylamido-2-
methylpropanesulfonic
acid with a weight-average molecular weight Mõ, of approx. 8 to 13*106 g/mol.
Comparative polymer 2:
This is a commercially available copolymer for polymer flooding, formed from
approx. 72% by
weight of acrylamide and approx. 28% by weight of sodium acrylate units,
having a weight-
average molecular weight M, of approx. 20 000 000 g/mol.
Comparative polymer 3:
A commercially available xanthan polymer was used for the tertiary mineral oil
production.
Performance tests
Determination of viscosity
The viscosity measurements were carried out with a Brookfield LVDV-UL
viscometer at a
shear rate of 7 s-1.
CA 02817792 2013-05-13
24
For the viscosity measurements, aqueous solutions of the polymers were used.
To dissolve the
polymers, the following aqueous media were used:
Tap water:
Total salinity 123 mg/I
Sea water (synthetic):
Total salinity: approx. 35 000 mg/I
Na+ 10 692 mg/I, K+ 420 mg/I, Mg2+ 1295 mg/I, Ca2+ 422 mg/I, Cl- 19218 mg/I,
HCO3- 145 mg/I,
S042- 2697 mg/I
Ratio of alkali metal ions/alkaline earth metal ions: 6.2
Deposit water (synthetic):
Total salinity: 185 548 mg/I
Na+ 52 079 mg/I, Mg2+ 2681 mg/I, Ca2+ 15 383 mg/I, CI- 115 105 mg/I, borate
117 mg/I, S042-
1 83 mg/I.
Ratio of alkali metal ions/alkaline earth metal ions: 2.9; deposit water with
high Ca2+ content
The following tests were carried out:
Test series 1:
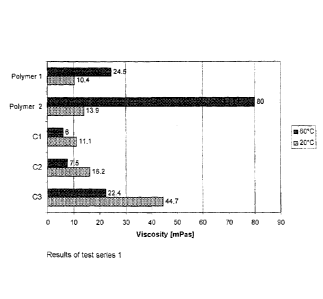
Solutions of polymers 1, 2 and Cl, C2 and C3 were made up in a concentration
of in each case
1500 ppm in sea water. The viscosity of the solutions was measured at 20 C and
at 60 C.
Figure 1 shows the results obtained.
The aqueous solutions of comparative polymers 2 and 3 have a higher viscosity
at 20 C than
the polymers 1 and 2 used in accordance with the invention. For all
comparative polymers, the
viscosity at 60 C is, however, much lower than at 20 C. For polymers 1 and 2,
in contrast, the
viscosity at 60 C is significantly higher than at 20 C.
Test series 2:
Solutions of polymers 1 and Cl were made up in a concentration of in each case
1200 ppm in
tap water, and the viscosity of each of the solutions was measured at 30 C, 60
C, 90 C and
120 C. Figure 2 shows the results obtained.
The solution of polymer 1 has, at 30 C, a viscosity approx. 4 x higher than
the solution of
polymer Cl. The viscosity of the latter solution decreases with increasing
temperature. The
CA 02817792 2013-05-13
viscosity of the aqueous solution of polymer 1 increases very significantly
between 30 C and
60 C, and decreases only when the temperature is increased further. Even at
120 C, the
viscosity of the solution of polymer 1 is still greater than that of
comparative polymer Cl.
Test series 3 to 5:
5 Solutions of polymer 1 were made up at different concentrations in tap
water (test series 3), sea
water (test series 4) and deposit water (test series 5), and the viscosity of
each of the solutions
was measured at 30 C, 60 C, 90 C and 120 C. The results are shown in figures 3
to 5. The
figures also comprise the information with regard to the concentrations used
in each case.
In all tests, the viscosity of the solutions increases significantly from 30 C
to 60 C and then
10 .. decreases again. The viscosity maximum is in the range from approx. 50 C
to 70 C.
Test series 6:
Solutions of comparative polymer Cl were made up at different concentrations
in tap water, and
the viscosity of each of the solutions was measured at 30 C, 60 C, 90 C and
120 C. The results
are shown in figure 6. The figure also comprises the information with regard
to the
15 concentrations used in each case.
For the comparative polymer, the viscosity level is firstly lower than for
inventive polymer 1. In
addition, the viscosity does not pass through a maximum, but decreases
continuously with
increasing temperature.
Core flooding tests
20 The copolymer of example 1 and comparative polymer Cl were also used to
carry out core
flooding tests.
In each case, sandstone cores (composition 99% by weight of quartz) with an
average porosity
of approx. 2 darcies were used. The properties of the sandstone cores used are
compiled in
table 3 below.
CA 02817792 2013-05-13
26
Core 1 Core 2
Copolymer used polymer 1 Cl
Length 8.53 cm 8.56 cm
Cross-sectional area 7.02 cm2 7.02 cm2
Gas permeability 1993 mD 2350 mD
Water permeability 1734 mD 2077 mD
Porosity 23.6% 24.6%
Pore volume 14.2 cm3 14.8 cm3
Table 3: Properties of the cores used
For the core flooding tests, solutions of the polymers in deposit water of the
composition detailed
above with a total salt content of 186 g/l were prepared. The concentration of
polymer 1 was
1200 ppm and that of comparative polymer Cl 3000 ppm.
For the tests, a customary apparatus for core flooding was used, in which the
core is introduced
into a pressure-resistant steel shell sealed at both ends, one end having an
orifice for injection of
gases and aqueous solutions and the other end an outlet orifice. Gases or the
aqueous
formulations to be tested are injected with a particular pressure through the
inlet orifice and flow
through the core under the influence of the pressure. The entire apparatus is
stored in a bath for
temperature control. The tests were carried out at 55 C.
By varying the injection rate (i.e. variation of the pressure applied), it is
possible to calculate the
apparent viscosity of the aqueous formulations according to equations 1 to 3:
RF = X(water)/ X(polymer solution) where X = k/ (equation 1)
RF = resistance factor, X = mobility, k = permeability, = viscosity
RRF = ?.(water)/ X(water after the polymer solution has flowed through)
RRF = residual resistance factor
j.Lapp = (RF/RRF)* PAvaler
Figure 7 shows the apparent viscosity of the two polymer solutions as a
function of the flow rate
in the core in m/day.
CA 02817792 2013-05-13
27
The apparent viscosities of the solutions determined by means of the core
flooding test show
that the viscosity efficiency of the polymer used in accordance with the
invention at low flow
rates is much better than that of comparative polymer 1, which does not have
any
hydrophobically associating monomers but apart from that is of similar
structure to polymer 1.
Even at a concentration of 1200 ppm, a much higher viscosity is achieved than
with comparative
polymer 1 at a concentration of 3000 ppm.
The core flooding tests also show that the solution of polymer 1 used in
accordance with the
invention has highly shear-diluting (thixotropic) behavior, i.e. the viscosity
of the polymer solution
decreases very significantly with increasing flow rate. This is particularly
advantageous for
polymer flooding since ¨ as already stated above ¨ the flow rate is at its
highest on entry into the
formation and decreases again with increasing distance from the injection
site. Advantageously,
the viscosity of the solution decreases specifically at this point, and thus
enables easy injection
into the formation. The solution of comparative polymer 1, in contrast,
exhibits shear-thickening
(dilatant) behavior, i.e. the viscosity increases with increasing flow rate.