Note: Descriptions are shown in the official language in which they were submitted.
- 1 -
Systems and Methods for the Treatment of Pain Through
Neural Fiber Stimulation
Related Applications
Intentionally left blank.
10
20
CA 2819602 2018-01-19
- 2 -
Background of the Invention
Embodiments according to the present invention
relate generally. to the relief of bodily pain in an
animal, such as a human, and more specifically to the
treatment of pain by action potential activation in
neural fibers.
The peripheral nervous system of an animal,
such as a human, is comprised generally of efferent
(motor) and afferent (sensory) neural fibers. Efferent
fibers generally carry motor action potentials from the
central nervous system, while afferent fibers carry
sensory action potentials to the central nervous system.
Since the 1950's and 1960's and the codification of the
gate theory, it has been generally accepted that bodily
pain results from activity in nociceptive and non-
nociceptive, or somatosensory, afferent nerve fibers, and
the interaction of neural signals and pathways, which are
influenced by several psychological and physiologic
parameters. For instance, in a healthy
person, action
.potentials transmitted along non-nociceptive fibers do
not normally generate or cause a perception of pain.
However, in persons experiencing chronic pain (e.g., when
a person becomes overly sensitized to pain), non-noxious
stimuli, and hence the activity of non-nociceptive
fibers, can cause pain. This means that in a chronic pain
state, sensations that would not be perceived as pain in
a healthy person (e.g. light pressure or touch) may
actually be perceived as painful. That is, in an
individual that experiences chronic pain, the non-noxious
stimuli that are sensed (transducec) by non-nociceptive
receptors can lead to a perception of pain. Generally,
however, while nociceptive afferent activity "opens" a
gate to the transmission of sensory action potentials
related to noxious input, non-nociceptive afferent
CA 2819602 2018-01-19
CA 02819602 2013-05-31
WO 2012/075299
PCT/US2011/062906
- 3 -
act ivi t y "closes" the gate, thereby preventing or
inhibiting the transmission of such sensory signals to
the brain, interrupting or reducing the perception of
pain.
Prior methods of stimulation of nerves for the
reduction of pain, described below, have focused on the
stimulation of afferent neural fibers, and such focus is
perhaps understandable due to the conventional wisdom in
the art for the past five decades related to gate control
theory. However, prior nerve stimulation modalities used
to treat pain, especially with regards to peripheral
nerves, recognized a narrow treatment window between
stimulation settings that may achieve desired analgesia
through sensory stimulation of non-nociceptive afferents
and stimulation settings that reach the threshold for
discomfort or motor stimulation of efferent fibers, the
latter thought to be undesirable for a number of reasons.
Supplementary to such conventional wisdom, and as
described in further detail below, recruitment of
efferent fibers is thought to be actually beneficial in
reducing pain.
The electrical stimulation of nerves, often
afferent nerves, to indirectly affect the stability or
performance of a physiological system can provide
functional and/or therapeutic outcomes, and has been used
for activating target nerves to provide therapeutic
relief of pain. While prior
systems and methods can
provide remarkable benefits to individuals requiring
therapeutic pain relief, many issues and the need for
improvements still remain.
Electrical stimulation systems have been used
for the relief of pain. Despite the recognition and use
of electrical stimulation for the treatment of pain,
widespread use of available systems is limited. Such
limited use is thought to stem from a variety of factors,
CA 02819602 2013-05-31
WO 2012/075299
PCT/US2011/062906
- 4 -
such as invasiveness of required surgical procedures
(e.g. lead placement in epidural space of spinal cord or
surgical dissection), risk of surgical complications
associated with such procedures (e.g. infection,
hemorrhage, neurologic injury, and/or spinal fluid
leaks), the technical skill and training required to
place the electrode(s), the duration of time required to
place the electrode(s) correctly, the supporting
equipment (e.g. imaging equipment such as fluoroscopy)
required for electrode placement, risk of device
complications (e.g. migration of stimulating lead or
catastrophic failure, or breakage, of such lead), and/or
loss of pain relief over time.
Electrical stimulation systems may be provided
as either external or implantable devices, or a
combination thereof, for providing electrical stimulation
to activate nerves to provide therapeutic relief of pain.
These "neurostimulators" are able to provide treatment
and/or therapy to individual portions of the body. The
operation of these devices typically includes the use of
(i) an electrode placed either on the external surface of
the skin, and/or (ii) a surgically implanted electrode.
In most cases, one or more surface electrodes, cuff-style
electrodes, paddle-style electrodes, spinal column
electrodes, percutaneous leads, and/or leadless
microstimulators incorporating integral electrodes, each
having one or more electrodes, may be used to deliver
electrical stimulation to one or more select portions of
a patient's body.
One example of an electrical stimulation
system used to treat pain is a transcutaneous electrical
nerve stimulation (TENS) system, which has been cleared
by the U.S. Food and Drug Administration (FDA) for
treatment of pain. TENS systems are external
neurostimulation devices that employ electrodes placed on
CA 02819602 2013-05-31
WO 2012/075299
PCT/US2011/062906
- 5 -
an external skin surface to activate target afferent
nerve fibers below the skin surface. Advantageously, TENS
has a low rate of serious complications, but
disadvantageously, it also has a relatively low (i.e.,
approximately 25% or less) long-term rate of success, and
some of its success is attributed to a placebo effect.
Additionally, TENS has low long-term patient compliance
because it may cause additional discomfort by generating
cutaneous pain signals due to the electrical stimulation
being applied through the skin, the electrodes may be
difficult to apply, and the overall system is bulky,
cumbersome, and not suited for long-term use.
In addition, several clinical and technical
issues associated with surface electrical stimulation
have prevented it from becoming a widely accepted
treatment method. First, stimulation of cutaneous pain
receptors often cannot be avoided resulting in
stimulation-induced pain that limits patient tolerance
and compliance. Second, electrical stimulation may be
delivered at a relatively high frequency to prevent
stimulation-induced pain, which leads to early onset of
muscle fatigue in turn preventing patients from properly
using their muscle(s). Third, it is difficult to
stimulate deep nerves and/or muscles with surface
electrodes without stimulating overlying, more
superficial nerves and/or muscles resulting in unwanted
stimulation. Finally, clinical skill and intensive
patient training is required to place surface electrodes
reliably on a daily basis and adjust stimulation
parameters to provide optimal treatment. The required
daily maintenance and adjustment of a surface electrical
stimulation system is a major burden on both patient and
caregiver.
Other electrical stimulation systems that have
been employed to treat pain include spinal cord
CA 02819602 2013-05-31
WO 2012/075299
PCT/US2011/062906
- 6 -
s t imul at ion (SCS) systems, which are also FDA approved as
implantable neurostimulation devices marketed in the
United States for treatment of pain. Similar to TENS,
when SCS evokes paresthesias that cover a region of pain,
it confirms that the location of the electrode and the
stimulus intensity should be sufficient to provide pain
relief and pain relief can be excellent initially, but
maintaining sufficient paresthesia coverage is often a
problem due to lead migration along the spinal canal.
Spinal cord stimulation is limited by the
invasive procedure and the decrease in efficacy as the
lead migrates. When it can produce paresthesias in the
region of pain, spinal cord stimulation is typically
successful initially in reducing pain, but over time the
paresthesia coverage and pain reduction is often lost as
the lead migrates away from its target.
Lead migration is the most common complication
for SCS systems, occurring in up to 40% or more of the
cases. When the lead migrates, the active contact moves
farther from the target fibers and loses the ability to
generate paresthesias in the target area. SCS systems
attempt to address this problem by using leads with
multiple contacts so that as the lead moves, the next
contact in line can be selected to be the active contact.
Additionally, multiple contacts can be used to guide or
steer the current toward the targeted nerve fibers and
away from the non-targeted nerve fibers. Although this
approach may be successful, it often requires time-
intensive and complex programming, adding to the overall
cost of the therapy and the burden on the patient and
caregiver(s).
Peripheral nerve stimulation has been
attempted and may be effective in reducing pain, but it
previously required specialized surgeons to place cuff-
or paddle-style leads on or around the nerves in a time-
CA 02819602 2013-05-31
WO 2012/075299
PCT/US2011/062906
- 7 -
consuming and invasive surgical procedure. Such prior
procedures may include the use of ultrasound-guided lead
placement in an attempt to avoid placement in muscle
tissue in an attempt to coapt intimately an electrode
surface with a target nerve, or approximately 3
millimeters or less from the nerve.
Accordingly, the art of pain reduction by
neural activation would benefit from systems and methods
that improve pain reduction.
Summary of the Invention
Embodiments of the present invention include
improved systems and methods of pain reduction by
inducing action potentials in target neural structures.
Action potentials may be generated or activated in
efferent fibers as an alternative to or in addition to
activation of afferent fibers. If an action potential is
directly induced in select afferent fibers, such action
potentials may be patterned so as to be biomimetic or
stochastic, as explained below. Stimulation may be
applied to targeted neural fibers located (1)
neurologically upstream from a perceived point of pain
(i.e. neurologically between the perceived point of pain
and the central nervous system) such as to target neural
fibers of nerves of passage, (2) at or near a
neurological motor point, and/or (3) neurologically
downstream from such motor point, where such downstream
stimulation may be applied to or near a target region
from which a patient is perceiving pain.
An embodiment of a method according to the
present invention includes a step of stimulating afferent
nerve fibers in a predetermined manner to generate an
action potential in the nerve fibers in an animal, such
as a human, to reduce a perception of pain by the animal.
According to one aspect of a method according
to the present invention, the stimulating step includes
CA 02819602 2013-05-31
WO 2012/075299
PCT/US2011/062906
- 8 -
electrically stimulating the afferent nerve fibers.
According to another aspect of a method
according to the present invention, the afferent nerve
fibers may be located outside a neural receptor and
outside a central nervous system of the human.
According to still another aspect of a method
according to the present invention, the afferent nerve
fibers may be located between a neural receptor and the
central nervous system of the human.
According to yet another aspect of a method
according to the present invention, the afferent nerve
fibers may innervate neural receptors, such as
proprioceptors.
According to a further aspect of a method
according to the present invention, the afferent nerve
fibers may be in neural communication with neural
receptors, such as proprioceptors, and stimulated at a
location that may be between the neural receptors and a
central nervous system of the animal.
According to a still further aspect of a
method according to the present invention, the afferent
nerve fibers may be one or more of a plurality of types
of axons, including Aa axons, such as Ia and lb axons, A13
axons, A5 axons, and/or C axons.
A method according to the present invention
may further include, in addition to afferent nerve fiber
activation, the step of activating efferent nerve fibers.
A method according to the present invention
may further include the step of stimulating efferent
nerve fibers to cause a contraction of a muscle to cause
a series of responsive action potentials to be generated
by afferent nerve fibers. Such responsive
action
potentials may be sensed, and the predetermined manner of
stimulation of the afferent nerve fibers is derived at
least partially by approximating a characteristic of the
- 9 -
responsive action potentials. Such characteristic of the
responsive action potentials may be selected from the group
consisting of: an average frequency of two or more of the
responsive action potentials, an instantaneous frequency
of two of the responsive action potentials, and a pattern
of two or more of the responsive action potentials.
Another method according to the present invention is
a method of reducing a perception of pain by an animal of
a hypersensitized portion of the animal nervous system,
including the step of applying electrical stimulation to
at least a portion of the. nervous system to cause -a
reduction of perception of pain by the animal, the portion
including afferent nerve fibers and the stimulation may
cause action potentials to be generated by or along the
afferent nerve fibers. The afferent nerve fibers may be
located neurologically between and outside a neural
receptor and a'central nervous system of the human.
According to another aspect of a method according to
the present invention, the applying step is performed for
a predetermined treatment time, and the reduction of
perception of pain occurs at least partially during the
treatment time and at least a portion of the reduction of
perception of pain is maintained after the end of the
predetermined treatment time.
In a further aspect, this document discloses a use
of an electrode capable of being inserted in-vivo, wherein
the electrode is for electrically stimulating at least one
of: Type Ia and lb target afferent nerve fibers, in a
predetermined manner to generate action potentials in the
at least one of Type Ia and lb target afferent nerve
fibers, wherein the action potentials reduce a perception
of pain by am animal, wherein use of the electrode avoids
CA 2819602 2019-12-27
- 9a -
generation of action potentials in non-target Type III and
IV nerve fibers in the animal and wherein the at least one
of: Type Ia and. lb target afferent nerve fibers are located
outside a central nervous system of the animal.
In a further aspect, this document discloses a use
an electrode capable of being inserted in-vivo, wherein
said electrode is for a reduction in a perception of pain
by an animal of a hypersensitized portion of the animal
nervous system, where the electrode is for electronically
stimulating at least a portion of the nervous system to
generate action potentials in target Type I afferent nerve
fibers causing said reduction of perception of pain by the
animal, wherein use of the electrode avoids generation of.
action potentials in non-target Type III and IV afferent
nerve fibers in the animal.
Brief Description of the Drawings
Figure 1 depicts various physiological structures for
reference in connection with the following disclosure.
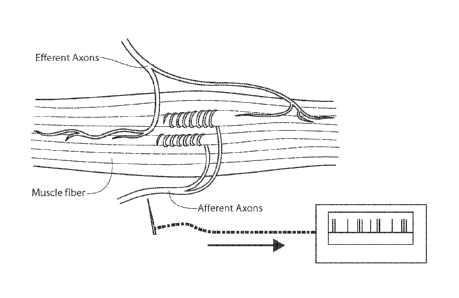
Figure 2A depicts an example of a muscle spindle
(shown contained in the capsule), including the intrafusal
muscle fibers (innervated by type v (gamma) (Class Ay)
motor neurons (efferent axons) and by sensory neurons
(afferent axons)). The efferent (gamma) axons
CA 2819602 2019-12-27
CA 02819602 2013-05-31
WO 2012/075299
PCT/US2011/062906
- 10 -
terminate (shown by Gamma Motor Endings) on and innervate
the spindle's intrafusal muscle fibers. The sensory
endings of the primary (group Ia) afferent axons and a
secondary (group II) afferent axons innervate the
intrafusal fibers.
Figure 2B depicts intrafusal motor fibers
(nuclear chain fibers and nuclear bag fibers) and their
sensory innervation. The group II afferent axons are
shown innervating the nuclear chain fibers and the static
nuclear bag fiber. The group Ia afferent axons are shown
wrapping around and innervating the nuclear chain fibers,
the static nuclear bag fiber, and the dynamic nuclear bag
fiber. The figure also indicates which portions can be
considered contractile and non-contractile.
Figure 2C depicts an example of how stretch
alone or in combination with stimulation of a static
gamma fiber or a dynamic gamma fiber can change the
neural activity of the respective afferents axons
innervating the fibers of the muscle spindle. Activation
of gamma motor neurons (efferent axons), which activate
the intrafusal muscle fibers, can change the frequency
(Imp/s) of neural activity and stretch-sensitivity of the
afferent neurons. The figure also depicts an example of
the possible relative steady-state and dynamic responses
that may be achieved in terms of the neural activity of
an afferent neuron innervating a muscle spindle fiber.
Figure 3 depicts a Golgi tendon organ
including collagen fibers that physically interact with
afferent axons to generate an action potential thereon
during stretch.
Figure 4A provides a diagrammatic view of
electrode placement near a targeted sensory neural
structure according to a sensing step of a second
embodiment of a method according to the present
invention.
CA 02819602 2013-05-31
WO 2012/075299
PCT/US2011/062906
- 11 -
Figure 4B provides a diagrammatic view of
electrode placement near a targeted sensory neural
structure according to a stimulating step to occur after
or without the sensing step of Figure 4A.
Figure 5 provides a diagrammatic view of
electrode placement near a targeted sensory neural
structure according to a first embodiment of a method
according to the present invention.
Figure 6A provides a diagrammatic view of
electrode placement near a targeted neural structure
according to a third embodiment of a method according to
the present invention.
Figure 6B provides a diagrammatic view of a
muscle contraction caused by electrical stimulation by
the electrode of Figure 6A.
Figure 7A provides a diagrammatic view of
afferent neural structure activation or firing in
response to a muscle stretch.
Figure 7B provides a diagrammatic view of
stretch receptor afferent neural structure activation, as
perhaps by a weighted stretch, and stretch receptor
afferent neural fiber deactivation during muscle
contraction caused by electrical stimulation of efferent
neural structures.
Figure 7C provides a diagrammatic view of a
method of continuing afferent activation during the
contraction of Figure 7B through stimulation of
additional or alternative efferent neural structures to
those efferent structures stimulated in Figure 7B.
Description of the Preferred Embodiment
Although the disclosure hereof is detailed and
exact to enable those skilled in the art to practice the
invention, the physical embodiments herein disclosed
merely exemplify the invention, which may be embodied in
other specific structures. While the preferred
CA 02819602 2013-05-31
WO 2012/075299
PCT/US2011/062906
- 12 -
embodiment has been described, the details may be changed
without departing from the invention, which is defined by
the claims.
As described in the Background section, above,
the nervous system of an animal generally comprises
efferent and afferent neural fibers, and prior pain
reduction modalities have focused on action potential
generation or activation in non-nociceptive afferent
neural fibers to inhibit, or "close the gate" to, the
transmission of nociceptive pain signals to the brain.
This has come to be known as the gate control theory of
pain management. Most afferent fibers, however, are not
bundled only with other afferent fibers; rather, the
majority of nerves found amenable to peripheral nerve
stimulation are nerve bundles comprising both afferent
and efferent fibers.
With reference also to Figures 1-3, electrical
stimulation provided according to systems and methods of
the present invention may mediate pain relief by
activating somatosensory pathways that may be associated
with mechanoreceptors, thermoreceptors, proprioceptors,
and/or chemoreceptors. Generally, types of neural cells,
axons, nerve fibers, or physiological structures that may
be affected, such as by intra- or extra-muscle (e.g., in
subcutaneous, connective, adipose, or other tissue)
electrical stimulation, include functional afferent types
A and C axons and efferent type A axons.
The afferent axons may be classified as An
(type Ia or Ib), TO (type II), A5 (type III), or C (type
IV). An (type Ia) fibers
are generally recognized as
being associated with the primary sensory receptors of
the muscle spindle, such as for transducing muscle length
and speed. These fibers are myelinated, usually having a
diameter from about 9 to about 22 micrometers (pm),
although other diameters have been observed and may be
CA 02819602 2013-05-31
WO 2012/075299
PCT/US2011/062906
- 13 -
included, and a conduction velocity of about 50 to about
120 meters per second (m/s), which is known to be
proportional to the diameter of the fiber for both this
type and other types of myelinated fibers. Au (type Tb)
fibers are generally recognized as being associated with
Golgi tendon organs, such as for transducing muscle
contraction. These fibers are
myelinated, having a
diameter from about 9 to about 22 micrometers (pm) and a
conduction velocity of about 50 to about 120 meters per
second (m/s). Ap (type II) fibers are generally
recognized as being associated with the secondary sensory
receptors of the muscle spindle, such as for transducing
muscle stretch. These fibers are
also associated with
joint capsule mechanoreceptors (as transduces joint
angle) and all cutaneous mechanoreceptors. The cutaneous
mechanoreceptors may include Meissner's corpuscles,
Merkel's discs, Pacinian corpuscles, Ruffini corpuscles,
hair-tylotrich (for sensing stroking/fluttering on the
skin or hair), and the field receptor (for sensing skin
stretch).
Meissner's corpuscles are nerve endings that
can be found in the skin, which transmit afferent
information regarding touch (such as soft, or light,
touch) and/or vibration, especially at vibration
frequencies of less than 50 Hertz. These fibers are
rapidly adaptive receptors that are often located below
the epidermis within the dermal papillae. The corpuscles
may be found as encapsulated unmyelinated nerve endings,
comprising flattened supportive cells arranged as
horizontal lamellae surrounded by a connective tissue
capsule. Examples of this corpuscle have been described
as having a length of about 30 to about 140 pm and a
diameter of about 40 to about 60 pm.
Merkel's discs are a type of mechanoreceptor
found in the skin, hair follicles, and in the oral and
CA 02819602 2013-05-31
WO 2012/075299
PCT/US2011/062906
- 14 -
anal mucosa. The discs
transmit afferent information
regarding pressure and texture. Sometimes referred to as
a Merkel disc receptor or Merkel cell-neurite complex,
the nerve ending comprises a Merkel cell next to a nerve
terminal. A single afferent
nerve fiber may innervate
multiple nerve endings, such as 50-100 endings. This
mechanoreceptor is an unencapsulated, slowly adapting
type I mechanoreceptor that will provide a non- or
minimally-decaying response to pressure. The Merkel disc
receptor may have two phases of firing, dynamic and
static. In the static
phase, an irregular activity may
be observed, which may be typical of slowly adapting type
I mechanoreceptors but contrasts with the regular pattern
of slowly adapting type II mechanoreceptors.
Pacinian corpuscles are nerve endings that may
be found in the skin. They may also be
found in the
mesentery, between layers of muscle, and on interosseous
membranes between bones. Pacinian
corpuscles transmit
afferent information regarding pain and pressure. For
instance, these corpuscles may detect gross pressure
changes and vibrations and may fire in response to quick
changes in joint position. They are phasic
tactile
mechanoreceptors that can detect deep pressure because
they are found below the skin surface, usually in the
dermis, and comprise some free nerve endings.
Ruffini corpuscles are slowly adapting
mechanoreceptors that may be present in the glabrous
dermis (hairless skin) and subcutaneous tissue of humans.
These corpuscles transmit afferent information regarding
skin stretch, movement, position (such as position of the
fingers), and sense of control (such as slipping of
objects along the skin surface). This type of
receptor
may have a spindle shape, and they may be found in the
deep layers of the skin, allowing them to indicate
continuous pressure states and mechanical joint
CA 02819602 2013-05-31
WO 2012/075299 PCT/US2011/062906
- 15 -
deformation, such as joint angle change.
The Ap fibers are myelinated, usually having a
diameter from about 6 to about 12 micrometers (pm),
although other diameters have been observed and may be
included, and a conduction velocity of about 33 to about
75 meters per second (m/s).
A6 (type III) fibers are generally recognized
as being associated with free nerve endings of touch and
pressure (for sensing excess stretch or force), hair-down
receptors (for sensing soft, or light, stroking),
nociceptors of the neospinothalamic tract, and cold
thermoreceptors. These fibers are
thinly myelinated,
having a diameter from about 1 to about 5 micrometers
(pm) and a conduction velocity of about 3 to about 30
meters per second (m/s).
C (type IV) fibers are generally recognized as
being associated with nociceptors of the
paleospinothalamic tract, and warmth thermoreceptors.
These fibers are unmyelinated, having a diameter from
about 0.2 to about 1.5 micrometers (pm) and a conduction
velocity of about 0.5 to about 2.0 meters per second
(m/s).
As mentioned above, most nerve bundles include
both afferent and efferent fibers. The efferent
axons
may be classified as An or Ay. An efferent fibers are
generally recognized as being associated with extrafusal
muscle fibers. These fibers are
myelinated, having a
diameter from about 13 to about 20 micrometers (pm) and a
conduction velocity of about 50 to about 120 meters per
second (m/s). Ay efferent fibers
are generally
recognized as being associated with intrafusal muscle
fibers. These fibers are
myelinated, having a diameter
from about 5 to about 8 micrometers (pm) and a conduction
velocity of about 20 to about 40 meters per second (m/s).
A first method according to the present
CA 02819602 2013-05-31
WO 2012/075299
PCT/US2011/062906
- 16 -
invention includes activating afferent fibers (e.g. type
Ia, Ib, and/or II, which may also be called An and/or Ap
afferent fibers), which are physically located in an area
from or in which an animal is perceiving pain. When a
fiber is referred to herein as "activated," it is to be
understood that at least one action potential is
generated or initiated by or along, or propagated along,
such fiber in response to some form of stimulation. Such
afferent fiber activation may mediate pain relief by
activation of afferent pathways associated with primary
receptors of muscle spindles, Golgi tendon organs,
secondary receptors of muscle spindles, joint receptors,
touch receptors (e.g. Meissner's corpuscles, Merkel disk
receptors, Pacinian corpuscles, Ruffini endings, etc.)
other types of mechanoreceptors (e.g. joint capsule
mechanoreceptors), and/or proprioceptors. As a non-
limiting example, stimulation may activate one or more AP
fibers that carry afferent information from a
mechanoreceptor (i.e. a sensory receptor) that responds
to mechanical pressure or distortion. The stimulation may
be applied in muscle or in non-muscle tissue (e.g.
subcutaneous, connective, adipose or other tissue). Non-
limiting examples of mechanoreptor pathways that may be
activated by stimulation include (1) one or more Pacinian
corpuscles; (2) one or more Meissner's corpuscles; (3)
one or more Merkel disc receptors; and/or (4) one or more
Ruffin' corpuscles. The applied stimulation may mediate
pain relief through the activation of nerve fibers
associated with, and/or innervating, receptors that are
rapidly adapting, intermediate adapting, and/or slowly
adapting. While
stimulation may be applied directly to
target nerves, an electrode, as more fully described
below, is preferably spaced a predetermined distance, or
within a predetermined range of distances, from the
target nerve fibers.
CA 02819602 2013-05-31
WO 2012/075299
PCT/US2011/062906
- 17 -
A second method according to the present
invention comprises the step of activating one or more
afferent nerve fibers that may be located outside an area
from or in which an animal is perceiving pain, and may or
may not be associated with the mentioned receptors. Such
stimulation may be beneficial to patients experiencing
pain in regions no longer innervated or that were not
previously innervated by the target fibers, such as those
patients that may have had removal of, or damage to,
their afferent receptors. Examples of such situation may
be amputee phantom limb pain or tissue damage due to
trauma, such as burns, or surgery. Other indications in
which such stimulation may provide beneficial perceived
reduction in pain are pathological or disease states
(e.g. induced by chemotherapy, vascular insufficiency,
cancer, or diabetes) or other considerations that may
prevent activation of receptors by physiological
transduction. Other
considerations my include areas of
the body that are sensory-only areas, such as the sural
nerve, or areas in which the receptors may be intact, but
it may be preferable not to activate them. For instance,
if a nerve trunk (e.g. femoral or sciatic nerve) is being
stimulated, large contractions may be undesirable due to
the physical effect of same. Additionally or
alternatively, tissue damage or disease progression
dictate or influence the placement of needles and/or
electrodes; for instance, if a patient suffers from
complex regional pain syndrome, it may be desirable to
prevent insertion of a needle in the affected area, as it
may make symptoms of the syndrome worse, but a needle may
be inserted outside of the affected area with less risk.
In any method according to the present
invention involving direct stimulation of afferent nerve
fibers and/or afferent receptors, with or without
efferent fiber recruitment, the stimulation is preferably
CA 02819602 2013-05-31
WO 2012/075299
PCT/US2011/062906
- 18 -
provided in one or both of two ways: (1) direct mimicked
(or biomimetic) afferent stimulation and/or (2) modulated
high frequency-induced stochastic response. With respect
to direct mimicked afferent stimulation, stimulation is
applied in a predetermined, random, or pseudo-random
manner to mimic afferent neural activity that otherwise
may naturally occur in response to activity normally
sensed by the target afferents. For example,
afferents,
including type Ia fibers associated with the muscle
spindle (as shown in Figures 2A-B) and type lb fibers
associated with Golgi tendon organs (as shown in Figure
3), and possibly others, normally respond, and fire
multiple, temporally patterned action potentials in
response to a muscle contraction. To predetermine a
stimulation pattern, afferent neural activity¨in response
to applied efferent stimulation or cued, or prompted,
voluntarily generated muscle activity, such as
contraction or stretch¨may be recorded and/or analyzed,
as diagrammatically depicted in Figure 4A. The recorded
or analyzed pattern may be obtained directly from the
animal to be relieved of pain, may be obtained directly
from an animal that is not the animal to he relieved of
pain (live model), may be calculated or modeled from one
or more patterns obtained from one or more animals
(including or excluding the animal to be relieved of
pain), and/or may be mathematically or otherwise
artificially generated (i.e., without sampling). The
predetermined pattern of afferent stimulation to be
applied according to the present invention may then be
established to approximate or identically mimic at least
a portion of the recorded or analyzed pattern, as
diagrammatically shown in Figure 4B. Additionally or
alternatively, a random or pseudo-random stimulation
pattern may be applied to the afferent fibers to mimic
natural afferent activity. The stimulation
patterns
CA 02819602 2013-05-31
WO 2012/075299
PCT/US2011/062906
- 19 -
applied may include variations in duty cycle and/or in
stimulation waveform shape and/or pulse parameters, such
as frequency, pulse width, and/or amplitude, which may be
varied between applied pulses, or during a pulse, which
may have the effect of modifying waveform shapes.
Altered stimulation patterns may additionally or
alternatively utilize a pre-pulse, which may be the same
or opposite polarity as a treatment pulse, and may have
the same or opposite polarity thereof. With respect to
the second way, specified as modulated, high-frequency-
induced stochastic response and as diagrammatically
depicted in Figure 5, naturally occurring afferent action
potentials (occurring in response to stimuli) may be
generated or approximated as a result of an applied
electrical stimulation at a modulated high frequency.
For example, a relatively high frequency from about 1 kHz
to about 20 kHz, preferably about 4 kHz, modulated at a
reduced frequency, such as about 0.1 Hz to about 1 kHz,
more preferably less than 50 Hz, such as 1-30 Hz, and
more preferably at about 12-16 Hz, may be used. Such
stimulation may generate pseudo-random patterns of
activity in the affected afferent nerve fibers.
Example of Direct Afferent Action Potential Stimulation
The treatment of pain through direct afferent
fiber stimulation may demonstrate dis-sensitization of
the afferent neural tissues that naturally respond to
such stimulation. That is, it is generally recognized
that the perception of pain, especially non-acute pain
such as sub-acute or chronic pain, in mammals can be
caused, worsened, and/or sustained in duration by a
sensitization of afferent sensory receptors and/or the
central nervous system fibers that receive direct and/or
indirect signals from the afferent sensory receptors,
CA 02819602 2013-05-31
WO 2012/075299
PCT/US2011/062906
- 20 -
including free nerve endings, to noxious or conventional
or previously non-noxious stimuli. Sensitization is
the
process whereby previously non-noxious stimuli are
perceived as painful, and this is an integral part of the
development and maintenance of chronic pain (as opposed
to the acute, healthy pain response). Such sensitization
may result from non-nociceptive primary afferents (e.g.
Am sprouting to make inappropriate and/or additional
connections in the spinal cord, from the loss of
inhibition in the central nervous system (e.g. spinal
cord, and/or brain), and from plasticity resulting from
changes in functional connectivity. However, what has
been demonstrated by certain afferent fiber stimulation
for the treatment of pain is that such stimulation may
actually permanently, or at least long-term, reverse the
sensitization process that formed the basis for the
chronic pain being treated. Accordingly, the effects of
the afferent stimulation for the treatment of pain
chronologically outlast the treatment duration, and such
effects may exponentially outlast the treatment duration.
For example, it is common for patients that have a
reduced level of pain measured, observed or reported at
the end of one month after a treatment cycle, such as a
three-week treatment cycle, to demonstrate the same level
of pain reduction up to one year or longer after the
treatment cycle has concluded. Thus, dis-sensitization
may be demonstrated and the pain reduction experienced at
approximately the stimulation treatment duration after
the end of the treatment cycle is maintained for more
than 17 times the treatment duration. For example, if a
patient reported a pain level of 6 prior to treatment,
and a pain level of 2 at a time that is about one month
after a treatment cycle (such as a three-week treatment
cycle), there has been demonstrated a high probability
that the patient will report a pain level of 2 at a time
- 21 -
that is about one year after the completion of the
treatment cycle. In any event, at one year after
treatment, if the pain level reported by the patient is
less than the pain level reported prior to treatment,
then at least some dis-sensitization is thought to have
occurred. Systems and methods according to the present
inventicn may be used to treat pain felt in a given
region of the body by stimulating neural fibers
associated with, disposed on, or innervating muscle,
subcutaneous, connective, adipose, or other tissue that
may be close to or some distance away from a "nerve of
passage" in a region Lhat is superior (i.e., cranial or
upstream toward the spinal column) to the region where
pain is felt. Neural impulses comprising pain felt 1n a
given muscle, organ, or cutaneous region of the body pass
through spinal nerves that arise from one or more nerve
plexuses. The spinal nerves in a nerve plexus, which
comprise trunks that divide by divisions and/or cords
into branches, comprise "nerves of passage." It has been
discovered that applying stimulation in a muscle near a
targeted nerve of passage relieves pain that manifests
itself in a region that is interior (i.e., caudal or
downstream from the spinal column) from where stimulation
is actually applied. An example of nerves of passage
stimulation mdy be found in U.S. Patent Application
Number 12/653,023, filed on December 7, 2009, and
entitled "Systems and Methods to ?lace One or More Leads
in Tissue to Electrically Stimulate Nerves of Passage to
Treat Pain," published as US2010/0152809.
Alternatively or additionally, to relieve pain
in a target muscle, the percutaneous or implanted lead
and/or electrode may be placed in the muscle (e.g.
deltoid) that is experiencing the pain near, or within a
therapeutically effective distance from, the point where
CA 2819602 2018-01-19
- 22 -
a motor nerve enters the muscle (i.e., the motor point).
Phantom pain (a type pain that may be
experienced, e.g., post-amputation) is one example of the
effectiveness of "nerves of passage" stimulation, because
the bodily area in which phantom pain is perceived to
originate does not physically exist. A lead and/or
electrode cannot be physically placed in the muscles that
hurt, because those muscles were amputated. Still, by
applying stimulation in a muscle, subcutaneous,
connective, adipose, or other tissue that has not been
amputated at a therapeutically effective distance from a
targeted nerve of passage that, before amputation,
preferably natively innervated the amputated muscles,
phantom pain can be treated. An example of the treatment
of post-amputation pain may be found in U.S. Patent
Application Number 12/653,029, filed December 7, 2009,
and entitled "Systems and Methods To Place One or More
Leads in Tissue for Providing Functional and/or
Therapeutic Stimulation," published as uS2010/0152809.
Chronic, sub-acute, or acute pain in existing,
non-amputated muscle, subcutaneous, connective, adipose,
or other tissue can also be treated by "nerves of
passage" stimulation. By applying stimulation to or near
a targeted nerve of passage that innervates the region
where chronic, sub-acute, or acute pain is manifested,
the pain can be treated.
In "nerves of passage" stimulation, a lead
and/or electrode can be placed in muscle, subcutaneous,
connective, adipose, or other tissue that is conveniently
located near a nerve trunk that passes by the electrode
and/or lead on the way to the painful area. In "nerves of
passage" stimulation, the lead and/or electrode may be
placed in a muscle, subcutaneous, connective, adipose, or
CA 2819602 2018-01-19
CA 02819602 2013-05-31
WO 2012/075299
PCT/US2011/062906
- 23 -
other tissue that is not necessarily the target (painful)
tissue, but rather in a muscle or other tissue that is
upstream from the painful region, because the proximal
muscle or other tissue presents a convenient and useful
location to place the lead and/or electrode.
Additionally or alternatively, the lead and/or electrode
may be placed in a muscle, subcutaneous, connective,
adipose, or other tissue having more than one region, to
stimulate a nerve to treat the perception of pain from a
different region of the same muscle or tissue. For
instance, with respect to a Sartorius muscle, an
electrode may be placed generally near the top of the leg
(near femoral nerve (1-2 cm below femoral crease)) in a
first region of the Sartorius muscle, to relieve pain
felt in the inner thigh near the knee (downstream), in a
second region of the Sartorius muscle.
The systems and methods make possible the
treatment of chronic or acute pain in which muscle
contraction cannot or should not be evoked (e.g. in the
case of amputation pain in which the target area has been
amputated is no longer physically present) or is
otherwise undesirable, or other cases of nerve damage
either due to a degenerative diseases or condition such
as diabetes of impaired vascular function (in which the
nerves are degenerating, and may be progressing from the
periphery), or due to trauma. The systems and methods
make possible the placement of one or more stimulation
leads and/or electrodes in regions distant from the motor
point or region of pain, e.g., where easier access or
more reliable access or a clinician-preferred access be
accomplished; or in situations where the motor nerve
point is not available, damaged, traumatized, or
otherwise not desirable; or in situations where it is
desirable to stimulate more than one motor point with a
single lead and/or electrode; or for cosmetic reasons; or
CA 02819602 2013-05-31
WO 2012/075299
PCT/US2011/062906
- 24 -
to shorten the distance between the lead and its
connection with a pulse generator; or to avoid tunneling
over a large area or over or across a joint, where the
latter may contribute to device failure.
A third method according to the present
invention, as diagrammatically depicted in Figures 6A-B,
comprises the step of activating one or more motor
(efferent) axons (type An or Ay) which can, in turn,
mediate pain relief by activating extrafusal muscle
fibers and/or intrafusal muscle fibers. Activation of
extrafusal muscle fibers (e.g. via activation of motor
(Au) axons) can generate and/or modulate responsive
afferent activity by contracting muscle fibers, producing
tension, and/or causing skeletal movement. The action
(e.g. contraction, tension, movement, etc) produced by
efferent activity may be transduced by sensory endings or
fibers and transmitted via afferent fibers to the central
nervous system, which can mediate pain relief.
Activation of intrafusal muscle fibers (e.g. via
activation of motor (Ay) axons) can modulate and/or
generate afferent activity by changing afferent firing
rate or pattern (e.g. the relative base or steady-state
firing frequency, average thereof, and/or the transient
firing frequency such that the running average may or may
not vary over time according to a pattern or non-
patterned sequence) and/or the afferent's sensitivity to
mechanical or other stimuli such as stretch, vibration,
muscle contraction, etc. One method of providing pain
relief is to activate neurons (or neural structures)
innervating (or considered part of) proprioceptors,
modifying proprioception. In either case,
of activation
of intrafusal (via Ay efferent axons) and/or extrafusal
(via An efferent axons) muscle fibers, the neural
receptors (associated with or innervated by afferent
axons) are allowed to naturally perceive and transduce
CA 02819602 2013-05-31
WO 2012/075299
PCT/US2011/062906
- 25 -
the effects of such stimulation. Accordingly, methods
according to the third embodiment of a method according
to the present invention may be said to enhance a
reduction in pain perception through muscle contraction,
which may or may not be perceptible to the naked eye. It
may be possible to detect the muscle contraction with
electromyography (EMG) equipment. The muscle
contraction, in turn, may cause natural afferent neural
activity in response, thereby mediating pain relief.
Electrical stimulation of efferent neural structures may
or may not recruit afferent fiber activation. That is, a
method according to the present invention may include a
step of recruiting or activating one or more sensory,
afferent axons while generating or causing a generation
of an action potential in one or more motor, efferent
axons. Alternatively,
only efferent axons may be
recruited by stimulation. For instance,
when disease
(e.g. diabetes or vascular insufficiency), trauma, or
another disorder has impaired (or eliminated) the
response of large diameter afferents (which are typically
thought to respond "first" - at low levels of
stimulation), electrical stimulation activation of only
efferent fibers may achieve "physiological" activation of
other afferents in response to the evoked extrafusal
(muscle contraction) or intrafusal activity.
Indeed, the treatment of pain through efferent
fiber stimulation may demonstrate at least partial dis-
sensitization (e.g., partial, or complete, temporary or
permanent reduction of neurological hypersensitization)
of at least a portion of the nervous system through
activation of afferent neural tissues that naturally
respond to such stimulation. That is, it is
generally
recognized that the perception of pain in mammals is
caused by a sensitization of afferent sensory receptors,
including free nerve endings, to noxious or conventional
CA 02819602 2013-05-31
WO 2012/075299
PCT/US2011/062906
- 26 -
or previously non-noxious stimuli. Sensitization is
the
process whereby previously non-noxious stimuli are
perceived as painful, and this is an integral part of the
development and maintenance of chronic pain (as opposed
to the acute, healthy pain response). Such sensitization
may result from non-nociceptive primary afferents (e.g.
Ap) afferents sprouting to make additional connections in
the spinal cord, from the loss of inhibition in the
spinal cord, and/or from central (brain) plasticity
resulting from changes in functional connectivity.
However, what has been demonstrated by efferent fiber
stimulation for the treatment of pain is that such
stimulation may actually permanently, or at least long-
term, reverse the sensitization process that formed the
basis for the chronic pain being treated. Dis-
sensitization resulting from efferent fiber stimulation
may reverse these changes through alterations in the
peripheral and/or central nervous systems, including but
not limited to changes in the sensitivity of peripheral
sensory receptors, changes in synaptic connectivity,
changes in synaptic strength, and changes in the rate and
pattern of neural activity. In response to therapy
according to the present invention, the firing pattern
and rate of peripheral nervous system (PNS) (e.g.
afferent) fibers may change, the firing pattern and rate
of central nervous system (CNS) fibers may change, and/or
there may be changes in both the PNS & CNS. Additionally
or alternatively, there may be changes in the threshold
required to active the fibers (in the PNS, CNS, &/or both
PNS & CNS). Accordingly, the
effects of the efferent
stimulation for the treatment of pain chronologically
outlast the treatment duration, and such effects may
exponentially outlast the treatment duration. For
example, it is common for patients that have a reduced
level of pain measured, observed or reported at the end
- 27 -
of one month after a treatment cycle, such as a three-
week treatment cycle, to demonstrate the same level of
pain reduction up to one year or longer after the
treatment cycle has concluded. Thus, this lasting effect
is thought to demonstrate dis-sensitization, and the pain
reduction experienced at approximately the stimulation
treatment duration after the end of the treatment cycle
may be maintained for more than 17 times the Lfeatment
duration. For example, if a
patient reported a pain
level of 6 prior to treatment, and a pain level of 2 at a
time that is about one month after a treatment cycle
(such as a three-week treatment cycle), there has been
demonstrated that the patient may report a pain level of
2 at a time that is about one year after the completion
13 of the treatment cycle. In any event, at one year after
treatment, if the pain level reported by the patient is
less than the pain level reported prior to treatment,
then at least some dis-sensitization is thought to have
occurred.
Systems and Methods
Various systems may be utilized to implement
the stimulation methods provided herein. The methods may
be carried out in a staged progression, which may include
a percutaneous and/or transcutancous phase. The
percutaneous and/or transcutaneous stimulation phase may
be followed by an implanted, percutaneous, and/or
transcutaneous stimulation phase. Preferred percutaneous
systems may be found in U.S. Patent Application Serial
No. 12/462,384, published as U.S. Patent Application
Publication 2010/0036445A1,
and/or U.S. Patent
Application Serial No. 11/595,596, published as U.S.
Patent Application Publication 2007/0123952A1,
CA 2819602 2018-01-19
- 28 -
and
U.S. Patent Application Serial No. 13/095,616,
A
preferred implanted system may be found in U.S. Patent
7,239,918.
Another preferred percutaneous stimulation
system may be found in U.S. Patent Application Serial No.
12/324,044, published as U.S. Patent Application
Publication 2009/0157151A1.
Control of a stimulator and/or stimulation
parameters according to the present invention may be
provided by one or more external controllers. In the case
of an external stimulator, the controller may be
integrated with the external stimulator. In the case of
an implanted stimulator, an implanted pulse generator
external controller (i.e., clinical programmer) may be a
remote unit that uses RE (Radio Frequency) wireless
telemetry communications (rather than an inductively
coupled telemetry) to control the implanted pulse
generator. The external or implantable pulse generator
may use passive charge recovery to generate the
stimulation waveform, regulated voltage (e.g., 10 mV to
20 V), and/or regulated current (e.g., about 10 A to
about 50 mA). Passive charge recovery is one method of
generating a biphasic, charge-balanced pulse as desired
for tissue stimulation without severe side effects due to
a CC component of the current.
The neurostimulation pulse may by monophasic,
biphasic, and/or multi-phasic. In the case of the
biphasic or multi-phasic pulse, the pulse may be
symmetrical or asymmetrical. Its shape may be rectangular
or exponential or a combination of rectangular and
exponential waveforms. The pulse width of each phase may
range between e.g., about 0.1 psec. to about 1.0 sec., as
CA 2819602 2018-01-19
CA 02819602 2013-05-31
WO 2012/075299 PCT/US2011/062906
- 29 -
non-limiting examples. The preferred neurostimulation
waveform is cathodic stimulation (though anodic may
work), biphasic, and asymmetrical.
Pulses may be applied in continuous or
intermittent trains (i.e., the stimulus frequency changes
as a function of time). In the case of intermittent
pulses, the on/off duty cycle of pulses may be
symmetrical or asymmetrical, and the duty cycle may be
regular and repeatable from one intermittent burst to the
next or the duty cycle of each set of bursts may vary in
a random (or pseudo random) fashion. Varying the stimulus
frequency and/or duty cycle may assist in warding off
habituation because of the stimulus modulation.
The stimulating frequency may range from e.g.,
about 1 Hz to about 300 Hz, or even as high as about 20
kHz to obtain a stochastic response, and the frequency of
stimulation may be constant or varying. In the case of
applying stimulation with varying frequencies, the
frequencies may vary in a consistent and repeatable
pattern or in a random (or pseudo random) fashion or a
combination of repeatable and random patterns.
In a representative embodiment, the stimulator
is set to an intensity (e.g. 1-2mA (or 0.1-40mA, or 0.01-
2001nA), 100-300us (or 40-1000us, or 1-10,000us))
sufficient to activate the targeted efferent or afferent
neural structures, using an electrode that is preferably
spaced at some distance (e.g. 1 mm) away from the
targeted structure. Additionally or alternatively, an
electrode may be placed in direct contact with a target
neural structure. If the stimulus
intensity is too
great, it may generate large muscle twitches or
contractions sufficient to disrupt correct placement of
the lead. If stimulus intensity is too low, the lead may
be advanced too close to the targeted nerve of passage
(beyond the optimal position), possibly leading to
CA 02819602 2013-05-31
WO 2012/075299
PCT/US2011/062906
- 30 -
incorrect guidance, nerve damage, mechanically evoked
sensation (e.g. pain and/or paresthesia) and/or muscle
contraction, inability to activate the target nerve
fiber(s) without activating non-target nerve fiber(s),
improper placement, and/or improper anchoring of the lead
(e.g. the lead may be too close to the neural structure
and no longer able to anchor appropriately in the
targeted anchoring tissue, such as muscle or adipose
tissue).
In a representative embodiment, the stimulator
may be set to a frequency (e.g. 0.5-12Hz (or 0.1-20Hz, or
0.05-40Hz)) low enough to evoke visible muscle twitches
(i.e. non-fused muscle contraction) and/or muscle
contraction(s) of the targeted muscle(s) innervated by
the target nerve of passage, but high enough that that
the targeted nerve will be activated before the lead is
advanced beyond an optimal position, preferably spaced
from the nerve. An Example of
preferred stimulation
parameters are as follows:
Parameter Default Minimum Maximum Adjusts in
increments
of
Amplitude 20 mA 1 mA 20 mA 1 mA
Frequency 12 Hz 5 Hz 25 Hz 1 Hz
Pulse 20 psec 20 psec 200 psec 10 psec
Duration
Minimum
Pulse Pulse Pulse 200 psec 10 psec
Duration Duration Duration
Maximum Minimum Minimum
Pulse Pulse Pulse Pulse 10 psec
Duration Duration Duration Duration
Normal Minimum Minimum Maximum
Therapy 6 hours 15 min 12 hours 15 min
Time
Duty Cycle 50 N/A
To position an electrode in vivo, preferably
while stimulation is being applied, the lead (non-
CA 02819602 2013-05-31
WO 2012/075299
PCT/US2011/062906
- 31 -
limiting examples of the lead could include a single or
multi-contact electrode that is designed for temporary
(percutaneous) or long-term (implant) use or a needle
electrode (used for in-office testing only)) may be
advanced (e.g. slowly advanced) towards the targeted
nerve until a desired indicator response (e.g. muscle
twitch, muscle contraction, patient sensation, and/or
some combination) is obtained, thereby defining an
optimal placement position. The intensity may then be
decreased (e.g. gradually decreased) as the lead is
advanced (e.g. advanced slowly) closer to the targeted
nerve until the desired indicator response(s) may be
obtained at smaller intensity(ies) within the target
range (e.g. 0.1-1.0mA (or 0.09-39mA, or 0.009-199mA),
100-300us (or 40-1000us, or 1- 10,000us)) at some
distance (e.g. X2 mm, where X2 < Xl, and (as a non-
limiting example) X1 may be multiple times larger than
X2, such as X1 2*X2, or X1 5*X2, or X1 20*X2 ) from
the target nerve. If specific response(s) (e.g. desired
response(s) and/or undesired response(s)) can be obtained
at a range of intensities that are too low, then the lead
may be located in a non-optimal location (e.g. too close
to the target nerve(s)). Non-limiting examples of ranges
of intensities that may be considered too low Include
those that are a fraction (e.g. < 2/3, or < 1/5, or <
1/10) of the Intensities that obtained the desired
response(s) at Xl.
The preferred stimulus intensities are a
function of many variables, are meant to serve as non-
limiting examples only, and may need to be scaled
accordingly. As an example, if electrode shape, geometry,
or surface area were to change, then the stimulus
intensities may need to change appropriately. For
example, if the intensities were calculated for a lead
with an electrode surface area of approximately 20 mm
CA 02819602 2013-05-31
WO 2012/075299
PCT/US2011/062906
- 32 -
then they may need to be scaled down accordingly to be
used with a lead with an electrode surface area of 0.2 mm2
because a decrease in stimulating surface area may
increase the current density, increasing the potential to
activate excitable tissue (e.g. target and non-target
nerve (s) and/or fiber(s)). Alternatively, if the
intensities were calculated for a lead with an electrode
surface area of approximately 0.2 =2, then the
intensities may need to be scaled up accordingly to be
used with a lead with an electrode surface area of 20 mm.
Alternatively, stimulus intensities may need to be scaled
to account for variations in electrode shape or geometry
(between or among electrodes) to compensate for any
resulting variations in current density. In a non-
limiting example, the electrode contact surface area may
be 0.1-20=2, 0.01-40=2, or 0.001-200=2. In a non-
limiting example, the electrode contact configuration may
include one or more of the following characteristics:
cylindrical, conical, spherical, hemispherical, circular,
triangular, trapezoidal, raised (or elevated), depressed
(or recessed), flat, and/or borders and/or contours that
are continuous, intermittent (or interrupted), and/or
undulating.
Stimulus intensities may need to be scaled to
account for biological factors, including but not limited
to patient body size, weight, mass, habitue, age, and/or
neurological condition(s). As a non-limiting example,
patients that are older, have a higher body-mass index
(BMI), and/or neuropathy (e.g. due to diabetes) may need
to have stimulus intensities scaled higher (or lower)
accordingly.
As mentioned above, if the lead is too far
away from the targeted nerve, then stimulation may be
unable to evoke the desired response (e.g. muscle
contraction(s), comfortable sensation(s), and/or pain
CA 02819602 2013-05-31
WO 2012/075299
PCT/US2011/062906
- 33 -
relief) in the desired region(s) at the desired stimulus
intensity(ies). If the lead is too close to the targeted
nerve, then stimulation may be unable to evoke the
desired response(s) (e.g. muscle contraction(s),
comfortable sensation(s), and/or pain relief) in the
desired region(s) at the desired stimulus intensity(ies)
without evoking undesirable response(s) (e.g. unwanted
and/or painful muscle contraction(s), sensation(s)),
increase in pain, and/or generation of additional pain in
related or unrelated area(s)). In some cases, it may
difficult to locate the optimal lead placement (or
distance from the targeted nerve) and/or it may be
desirable to increase the range stimulus intensities that
evoke the desired response(s) without evoking the
undesired response(s) so alternative stimulus waveforms
and/or combinations of leads and/or electrode contacts
may be used. A non-limiting example of alternative
stimulus waveforms may include the use of a pre-pulse to
Increase the excitability of the target fiber(s) and/or
decrease the excitability of the non-target fiber(s).
Those skilled in the art will recognize that,
for simplicity and clarity, the full structure and
operation of all devices and processes suitable for use
with the present invention is not being depicted or
described herein. Instead, only so much of an implantable
pulse generator and supporting hardware as is unique to
the present invention or necessary for an understanding
of the present invention is depicted and described. The
remainder of the construction and operation of the IPGs
described herein may conform to any of the various
current implementations and practices known in the art.
Pain relief provided by systems and methods
according to the present invention may be correlated to
an analysis of quality of life of the animal receiving
such relief. It may be important to measure the health-
CA 02819602 2013-05-31
WO 2012/075299
PCT/US2011/062906
- 34 -
related quality of life (HRQOL), as pain is known to
impact even otherwise simple, daily activities. There is
a plurality of generally accepted methodologies for
measuring improvements in a patient's quality of life.
One methodology includes analysis of patient responses to
one or more questions from an 55-36 Health Survey,
available from Quality Metric, Inc., of Lincoln, Rhode
Island. The SF-36 is a generic health survey of 36 items
designed to assess basic physical functioning and
emotional well-being regardless of the disease or
treatment. The 36 items are grouped into eight domains:
physical functioning, role limitations due to physical
problems, social functioning, bodily pain, general mental
health, role limitations due to emotional problems,
vitality, and general health perceptions. The items
include questions related to the following:
present and comparative general health;
frequency and severity of physical health or
emotional limitations on typical daily activities, such
as stair-climbing, personal positioning such as squatting
or kneeling, walking, and maintenance of personal
hygiene;
amount of bodily pain and interference of such
pain on daily and social activities;
comparison of general health to others; and
feelings such as nervousness, peacefulness,
amount of energy, depression or happiness, and
exhaustion.
Generally, the ratings provided by the patient
are on scales of, e.g., 1 to 3, or 1 to 5.
Another methodology of correlating treatment
to quality of life involves an analysis of data from the
Pain Disability Index (PDI), which is a validated survey
that measures the degree to which pain disrupts
activities such as work and athletics. Many patients
CA 02819602 2013-05-31
WO 2012/075299
PCT/US2011/062906
- 35 -
with chronic pain believe increased ability to function
physically is an important objective for pain treatment,
and assessment of the impact of pain on physical
functioning is recommended as one of the core outcome
measures in chronic pain studies. The PDI is considered a
simple and rapid tool for evaluating the impact that pain
has on physical functioning. The PDI provides
patient
feedback related to the impact of pain on seven
categories of life activity. Generally, each category is
rated on a zero to ten scale, where zero indicates no
disability at all in such life activity and ten Indicates
that a patient has been prevented from engaging in all
activities of the category. The seven categories of the
PDI are:
Chores and errands: This category refers to
activities of related generally to home life and/or
family. It includes chores or duties performed around the
house (e.g., yard work, dusting, laundry) and errands or
favors for other family members (e.g., driving the
children to school, grocery shopping);
Leisure time: This category includes
athletics, hobbies, and other similar recreation;
Social activity: This category refers to
interaction with friends and acquaintances other than
family members, such as attendance at parties, a theater,
concerts, restaurants, and other social functions;
Job-related activities: This category refers
to activities that are a part of or directly related to
one's job, whether or not it is a paying, non-paying or
volunteer career;
Sexual behavior: This category refers to the
frequency and quality of one's sex life;
Personal maintenance: This category includes
activities related to independent daily living (e.g.
taking a shower, driving, getting dressed, shaving,
CA 02819602 2013-05-31
WO 2012/075299
PCT/US2011/062906
- 36 -
etc.); and
Life-sustaining behavior: This category refers
to basic behaviors such as eating, sleeping, and
breathing.
Alternatively or additionally, other validated
measurements or surveys, such as the Brief Pain
Inventory-Short Form (BPI-SF) or Brief Pain Inventory-
Long Form (BPI-LF), may be used. For instance,
BPI-SF
(or -IF) question number 3 requests that patients rate
the worst pain they have experienced in the past week on
a scale from zero to ten, where zero indicates "no pain"
and ten indicates "pain as bad as you can imagine."
Alternatively or additionally, BPI-SF question 9 requests
that patients rate, on a scale of zero to ten, the
interference of pain on various activities. A rating of
zero indicates that pain has no interference on the
activity and a rating of ten indicates that pain
completely interferes with such activity. Such survey
may include ratings of interference on activities such as
general activity, walking ability, normal work (inside
and outside the home), sleep, and interpersonal
relations. Further such
survey may include ratings of
personal feelings, such as the pain interference with
mood or enjoyment of life. Where all seven eleven-point-
scale ratings are included, a mean score between zero and
ten may be calculated by summing the seven ratings and
dividing by seven.
The quality of life surveys are preferably
administered both before and after a treatment period,
and the results thereof are compared. For instance, if
the BPI-SF (or -LF) question 9 is used, a comparison of
the post-treatment mean and the pre-treatment mean may
indicate a level of success of the treatment. As used
herein, post-treatment may refer to any time after the
start of treatment, including but not limited to after
CA 02819602 2013-05-31
WO 2012/075299
PCT/US2011/062906
- 37 -
completion of a treatment period, duration, regime, or
protocol. On the eleven-
point (0-10) mean scale of the
BPI-SF question 9, it is preferable to have an
improvement (reduction) of more than one point out of the
total ten possible points, more preferably to have an
improvement of more than two points, and most preferred
to have an improvement of more than three points. After
any mid- or post-treatment quality of life analysis,
stimulation parameters or methodologies may be altered,
and the quality of life may again be examined, and
compared to baseline (prior to receipt of any treatment)
and/or to other post-treatment results to determine
whether the altered parameters were any more or less
effective than the first in improving quality of life.
The foregoing is considered as illustrative
only of the principles of the invention. Furthermore,
since numerous modifications and changes will readily
occur to those skilled in the art, it is not desired to
limit the invention to the exact construction and
operation shown and described. While the preferred
embodiment has been described, the details may be changed
without departing from the invention, which is defined by
the claims.