Note: Descriptions are shown in the official language in which they were submitted.
CA 02820717 2013-06-06
WO 2012/087867
PCT/US2011/065723
- 1 -
INCREASING FUEL SMOKE POINT
FIELD OF THE INVENTION
[0001] This invention involves a process for increasing smoke point of a
fuel.
In particular, this invention has an aspect directed to a process for
increasing
smoke point of a fuel using an upgrading catalyst that contains at least one
noble
metal supported on an inorganic, porous crystalline phase material.
BACKGROUND OF THE INVENTION
[0002] Fuels such as kerosene and aviation turbine fuels are required to
meet
smoke point requirements using standard test methods, such as by ASTM D
1322-08. Such a test method provides an indication of the relative smoke
producing properties of the fuel in a diffusion flame.
[0003] The smoke point is quantitatively related to the potential radiant
heat
transfer from the combustion products of the fuel. Because radiant heat
transfer
exerts a strong influence on the metal temperature of combustor liners and
other
hot section parts of gas turbines, the smoke point provides a basis for
correlation
of fuel characteristics with the life of these components.
[0004] Smoke point is related to the hydrocarbon type composition of the
fuel. Generally the more aromatic the fuel the smokier the flame. A high smoke
point indicates a fuel of low smoke producing tendency, which is highly
desirable.
[0005] The aromatics concentration of a fuel type hydrocarbon can be
reduced by catalysts that have hydrogenation, dearomatization, and/or aromatic
saturation functions. A number of such catalysts are known.
CA 02820717 2013-06-06
WO 2012/087867
PCT/US2011/065723
- 2 -
[0006] U.S. Patent Application Publication No. 2002/0112989, for instance,
discloses a hydrocarbon hydrogenation process and a catalytic composition
having such functionality. The catalytic composition includes at least two
noble
metals supported on an inorganic, porous crystalline phase support material.
The crystalline support material includes the M41 S group of mesoporous
crystalline materials, which is described in U.S. Patent No. 5,102,643, and
further includes MCM-41, which is described in U.S. Patent No. 5,098,684, and
MCM-48, which is described in U.S. Patent Nos. 5,102,643 and 5,198,203.
Similar support materials are indicated as being disclosed in U.S. Patent No.
5,573,657. The noble metals are selected from the group consisting of Pd, Pt,
Rh, and Ir, and the crystalline material is a metallosilicate or an
aluminosilicate.
The hydrocarbon hydrogenation process includes contacting a hydrocarbon
feedstock containing aromatics, olefins, or aromatics and olefins with the
catalytic composition under superatmospheric conditions, wherein the
concentration of the aromatics, olefins, or aromatics and olefins in the
product is
reduced.
[0007] What is needed is a process for upgrading fuels that are already
standard quality fuels to premium quality fuels. In particular, what is needed
is a
process for upgrading fuels to increase smoke point to premium quality
standards, without negatively affecting already acceptable fuel properties.
SUMMARY OF THE INVENTION
[0008] This invention provides processes for upgrading fuels to premium
quality fuels. The process is particularly effective in increasing smoke
point,
without negatively affecting already acceptable fuel properties.
CA 02820717 2013-06-06
WO 2012/087867
PCT/US2011/065723
- 3 -
[0009] One aspect of the invention relates to a process for increasing
smoke
point of a fuel, comprising: providing a feedstock fuel having a smoke point
from 18 mm to below 25 mm and a total aromatics content of A, > 68 - 2.6S.,
wherein A, is vol% total aromatics of the feedstock and S. is the smoke point
of
the feedstock, provided that A, is at least about 4 vol%; and contacting the
feedstock fuel with a smoke point upgrading catalyst comprised of at least one
noble metal hydrogenation component disposed on a support having an
inorganic, porous crystalline phase material to provide a fuel product having
a
smoke point of at least 25 mm.
[0010] Another aspect of the invention relates to a process for increasing
smoke point of a fuel, comprising: hydrocracking a mineral oil feedstock;
providing from the hydrocracked mineral oil feedstock a first fuel having a
smoke point from 18 mm to below 25 mm and a total aromatics content of Aõ >
68 - 2.6S., wherein A, is vol% total aromatics of the feedstock and S. is the
smoke point of the feedstock, provided that A, is at least 4 vol%; and
contacting
the first fuel with a smoke point upgrading catalyst comprised of at least one
noble metal hydrogenation component disposed on a support having an
inorganic, porous crystalline phase material to provide a fuel product having
a
smoke point of at least 25 mm.
BRIEF DESCRIPTION OF THE DRAWING
[0017] An example of a preferred embodiment of this invention is shown in
the attached Fig. 1, wherein:
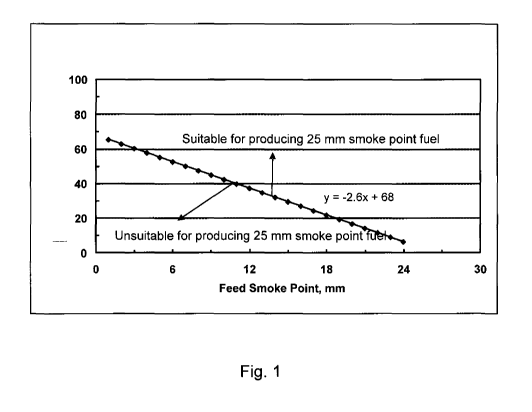
Fig. 1 is a graph showing characteristics of feedstock fuels suitable for
producing at least 25 mm smoke point fuel product according to the invention.
CA 02820717 2013-06-06
WO 2012/087867
PCT/US2011/065723
- 4 -
DETAILED DESCRIPTION OF THE INVENTION
Introduction
[0019] This invention provides a process for increasing the smoke point of a
fuel. The process can be particularly effective in increasing the smoke point
of
fuels of standard quality to relatively high quality fuel.
[0020] The process can include contacting a feedstream that may already be of
at least standard quality (or alternately almost of standard quality) with a
hydrogenation (aromatic saturation, dearomatization) catalyst that contains at
least one noble metal supported on an inorganic, porous crystalline phase
material. The catalytic process can be particularly effective in increasing
smoke
point, while minimizing reduction in naphthalene content, and at relatively
mild
hydrogenation conditions. This can be a particular advantage in upgrading
fuels
such as jet fuels that meet or come close to meeting standard fuel
specifications,
but need to have a higher smoke point in order to meet higher grade fuel
specifications.
Feedstock Fuel Composition for Upgrading to Increase Smoke Point
[0021] The fuel provided as feedstock, or that can be treated according to
this
invention, to be upgraded to increase smoke point can be any one or more of
kerosene, jet, and diesel grades of fuel, including mixtures within or
overlapping
the particular boiling ranges of each indicated fuel. The invention is
particularly
suited to producing jet fuel grades of fuel. Boiling point ranges are
preferably
determined according to ASTM D86-09e1 Standard Test Method for Distillation
of Petroleum Products at Atmospheric Pressure.
CA 02820717 2013-06-06
WO 2012/087867
PCT/US2011/065723
-5-
100221 In an embodiment, the fuel or feedstock that is treated according to
this
invention can have an initial and final boiling point within the range from
about
90 C to about 360 C, for example from about 100 C to about 340 C, from about
110 C to about 320 C, or from about 120 C to about 300 C. Additionally or
alternately, the process can be carried out by contacting a catalyst with a
feedstock fuel having an ASTM D86 10% distillation point (i.e., a TIO, which
represents the temperature at which ¨10% of the feedstock would have boiled)
within the range from about 110 C to about 190 C, for example from about
115 C to about 180 C or from about 120 C to about 160 C.
[0023] Further additionally or alternately, the process can be carried out by
contacting a catalyst with a feedstock fuel having an ASTM D86 90%
distillation point (i.e., a T90, which represents the temperature at which
¨90% of
the feedstock would have boiled) within the range from about 200 C to about
290 C, for example from about 210 C to about 280 C or from about 220 C to
about 270 C.
[0024] The feedstock fuel to be upgraded to increase smoke point according to
the present invention can typically already have a relatively high smoke
point,
but which may not be high enough to meet the minimum smoke point
qualifications for Jet A or Jet A-1 aviation turbine fuels (according to ASTM
D1655-09a Standard Specification for Aviation Turbine Fuels). Thus, in an
embodiment, the feedstock fuel to be upgraded can have a smoke point of at
least 18 mm, for example at least 19 mm, at least 20 mm, at least 21 mm, at
least
22 mm, at least 23 mm, or at least 24 mm. Due to the fact that a feedstock
fuel
having a 25 mm smoke point or greater is considered of the highest grade, the
feedstock fuel should typically also have a smoke point below 25mm.
[0025] Due to the type of catalyst used to upgrade the feedstock according to
this invention, there can be acceptable/minimal loss of naphthenics content
CA 02820717 2013-06-06
WO 2012/087867
PCT/US2011/065723
- 6 -
during the upgrade process. However, since there should nevertheless be some
loss of naphthenics, as well as total aromatics, due to
saturation/hydrogenation
of a portion of the aromatic components, the feedstock fuel to be upgraded to
increase smoke point can preferably have a total aromatics content defined by
the following inequality: Ar > 68 - 2.6S,n, wherein A, is vol% total aromatics
of
the feedstock and Si, is the smoke point of the feedstock.
[0026] In any event, however, the feedstock should contain a sufficient level
of aromatics compounds to allow for at least partial saturation/hydrogenation
of
aromatics and corresponding smoke point increase upon treatment in the
presence of the catalyst according to the invention. As a result, the
feedstock A,
can typically be at least about 4 vol%, based on the total feedstock volume.
In
some cases, the feedstock A, can be at least about 6 vol%, for example at
least
about 10 vol%, at least about 15 vol%, or at least about 20 vol%, based on the
total feedstock volume. Although there may not necessarily be an upper limit
for Aõ limits on A, can be desirable in some embodiments. For example, in
various embodiments, the feedstock A, can be no greater than about 50 vol%,
based on the total weight of the feedstock, for example no greater than about
45
vol%, no greater than about 40 vol%, no greater than about 35 vol%, no greater
than about 30 vol%, or no greater than about 25 vol%.
[00271 The feedstock to be upgraded according to the inventive process can
generally have a relatively low sulfur content. However, the catalyst used
according to this invention to upgrade the feedstock fuel can be relatively
more
tolerant of sulfur (and/or nitrogen), as compared to conventional catalysts
capable of increasing smoke point. Thus, in an embodiment, the feedstock fuel
to be upgraded according to this invention can have a sulfur content not
greater
than about 5000 wppm, for example not greater than about 3000 wppm, not
greater than about 2000 wppm, or not greater than about 1000 wppm, as
CA 02820717 2013-06-06
WO 2012/087867
PCT/US2011/065723
- 7 -
measured according to at least one of the following standard test methods:
ATSMs D1266, D2622, D4294, and D5453.
Hydrocracked Mineral oil as Feedstock
[0028] The feedstock provided according to this invention for upgrading to
increase smoke point can be obtained from a mineral oil that has been
hydrocracked. The term "mineral oil," as used herein, should be understood to
represent a fossil/mineral fuel source, such as crude oil, and not the
commercial
organic product, such as sold under CAS number 8020-83-5, e.g., by Aldrich.
Non-limiting examples of mineral oils can include straight run gas oils,
vacuum
gas oils, atmospheric gas oils, demetallized oils, coker distillates, cat
cracker
distillates (including light cycle oils and heavy cycle oils), atmospheric
resids,
vacuum resids, coal liquids, and combinations thereof that would be suitable
for
hydrocracking.
100291 If desired, the present invention can include a step of hydrocracking
(preferably through contact with hydrogen and an appropriate hydrocracking
catalyst) a mineral oil feedstock, particularly in (but not limited to) the
situation
where the mineral oil feedstock had not been previously hydrocracked and/or
where the mineral oil feedstock could benefit from being hydrocracked (again).
The product of any hydrocracking of a mineral oil feedstock, whether within a
step according to the invention or separately, can be referred to as a first
fuel
when it meets the feedstock fuel requirements as described herein. This first
fuel
can then be contacted with the catalyst according to the present invention to
increase smoke point.
[0030] The mineral oil feedstock to be hydrocracked can generally contain
some amount of nitrogen compounds (nitrogen content), which can in some
embodiments be at least about 5 wppm, based on total weight of the feedstock,
CA 02820717 2013-06-06
WO 2012/087867
PCT/US2011/065723
- 8 -
for example at least about 10 wppm, at least about 20 wppm, at least about 30
wppm, or at least about 50 wppm. Additionally or alternately, the mineral oil
feedstock to be hydrocracked can have a nitrogen content of about 2.0 wt% or
less, based on total weight of the feedstock, for example about 1.5 wt% or
less,
about 1.0 wt% or less, about 0.8 wt% or less, about 0.6 wt% or less, or about
0.5
wt% or less. In general, at least a majority of the nitrogen content can be in
the
form of organic nitrogen compounds.
[00311 Furthermore, the mineral oil feedstock to be hydrocracked can
generally contain some amount of sulfur-containing compounds (sulfur content),
which can in some embodiments be at least about 300 wppm, based on total
weight of the feedstock, for example at least about 500 wppm, at least about
700
wppm, at least about 1000 wppm, at least about 1500 wppm, at least about 2000
wppm, or at least about 2500 wppm. Additionally or alternately, the mineral
oil
feedstock to be hydrocracked can have a sulfur content of about 6.0 wt% or
less,
based on total weight of the feedstock, for example about 4.0 wt% or less,
about
2.5 wt% or less, about 1.5 wt% or less, about 1.0 wt% or less, or about 0.9
wt%
or less.
[0032] In an embodiment, the mineral oil feedstock to be hydrocracked can
have an initial boiling point of at least about 100 C, for example at least
about
150 C, at least about 180 C, or at least about 200 C. Additionally or
alternately,
the mineral oil feedstock to be hydrocracked can have T5 boiling point (i.e.,
the
temperature below which about 5 wt% of the composition boils) of at least
about
100 C, for example at least about 130 C, at least about 150 C, at least about
180 C, at least about 200 C, or at least about 210 C. Further additionally or
alternately, the mineral oil feedstock to be hydrocracked can have a final
boiling
point of about 600 C or less, for example about 550 C or less, about 500 C or
less, about 450 C or less, about 425 C or less, or about 400 C or less.
Additionally or alternately, the mineral oil feedstock to be hydrocracked can
CA 02820717 2013-06-06
WO 2012/087867
PCT/US2011/065723
- 9 -
have T95 boiling point (i.e., the temperature below which about 95 wt% of the
composition boils) of about 590 C or less, for example about 550 C or less,
about 500 C or less, about 450 C or less, about 425 C or less, about 400 C or
less, or about 375 C or less. The basic test method of determining the boiling
points/ranges of such feedstocks, as well as of fuel compositions produced
according to the present invention, involves performing batch distillation
according to ASTM D86-09e1, Standard Test Method for Distillation of
Petroleum Products at Atmospheric Pressure.
[0033] It is noted that, although the feedstock (e.g., to be hydrocracked) is
described herein as being a "mineral oil" feedstock, it may optionally contain
an
additional biocomponent portion. As used herein, an additional biocomponent
portion refers to a hydrocarbonaceous component derived from a biological raw
material component, from biocomponent sources such as higher plant
(vegetable), animal, fish, and/or algae. Generally, these biocomponent sources
can include higher plant (vegetable) fats/oils, animal fats/oils, fish oils,
pyrolysis
oils, and algae lipids/oils, as well as components of such materials, and in
some
embodiments can specifically include one or more type of lipid compounds.
Lipid compounds are typically biological compounds that are relatively
insoluble in water, but can be soluble in relatively nonpolar (or fat)
solvents.
Non-limiting examples of such solvents include alcohols, ethers, chloroform,
alkyl acetates, benzene, and combinations thereof.
[0034] Major classes of lipids include, but are not necessarily limited to,
fatty
acids, glycerol-derived lipids (including fats, oils and phospholipids),
sphingosine-derived lipids (including ceramides, cerebrosides, gangliosides,
and
sphingomyelins), steroids and their derivatives, terpenes and their
derivatives,
fat-soluble vitamins, certain aromatic compounds, and long-chain alcohols and
waxes.
CA 02820717 2013-06-06
WO 2012/087867
PCT/US2011/065723
- 10 -
[0035] In living organisms, lipids generally serve as the basis for cell
membranes and as a form of fuel storage. Lipids can also be found conjugated
with proteins or carbohydrates, such as in the form of lipoproteins and
lipopolysaccharides.
[0036] Examples of higher plant (vegetable) oils that can be used in
accordance with this invention include, but are not limited to rapeseed
(canola)
oil, soybean oil, coconut oil, sunflower oil, palm oil, palm kernel oil,
peanut oil,
linseed oil, tall oil, corn oil, castor oil, jatropha oil, jojoba oil, olive
oil, flaxseed
oil, camelina oil, safflower oil, babassu oil, tallow oil and rice bran oil.
[0037] Higher plant (vegetable) oils as referred to herein can also include
processed plant (vegetable) oil material. Non-limiting examples of processed
vegetable oil material include fatty acids and fatty acid alkyl esters. Alkyl
esters
typically include C1-05 alkyl esters. One or more of methyl, ethyl, and propyl
esters are preferred.
[0038] Examples of animal fats that can be used in accordance with the
invention include, but are not limited to, beef fat (tallow), hog fat (lard),
turkey
fat, fish fat/oil, and chicken fat. The animal fats can be obtained from any
suitable source including restaurants and meat production facilities.
[0039] Animal fats as referred to herein also include processed animal fat
material. Non-limiting examples of processed animal fat material include fatty
acids and fatty acid alkyl esters. Alkyl esters typically include C1-05 alkyl
esters. One or more of methyl, ethyl, and propyl esters are preferred.
[0040] Algae oils or lipids are typically contained in algae in the form of
membrane components, storage products, and metabolites. Certain algal strains,
particularly microalgae such as diatoms and cyanobacteria, contain
CA 02820717 2013-06-06
WO 2012/087867
PCT/US2011/065723
- 11 -
proportionally high levels of lipids. Algal sources for the algae oils can
contain
varying amounts, e.g., from 2 wt% to 40 wt% of lipids, based on total weight
of
the biomass itself.
[00411 Algal sources for algae oils include, but are not limited to,
unicellular
and multicellular algae. Examples of such algae include a rhodophyte,
chlorophyte, heterokontophyte, tribophyte, glaucophyte, chlorarachniophyte,
euglenoid, haptophyte, cryptomonad, dinoflagellum, phytoplankton, and the
like,
and combinations thereof. In one embodiment, algae can be of the classes
Chlorophyceae and/or Haptophyta. Specific species can include, but are not
limited to, Neochloris oleoabundans, Scenedesmus dimorphus, Euglena gracilis,
Phaeodactylum tricornutum, Pleurochrysis carterae, Prymnesium parvum,
Tetraselmis chui, and Chlamydomonas reinhardtii. Additional or alternate algal
sources can include one or more microalgae of the Achnanthes, Arnphiprora,
Amphora, Ankistrodesmus, Asteromonas, Boekelovia, Borodinella,
Botryococcus, Bracteococcus, Chaetoceros, Carteria, Chlamydomonas,
Chlorococcum, Chlorogonium, Chlorella, Chroomonas, Chrysosphaera,
Cricosphaera, Crypthecodinium, Cryptomonas, Cyclotella, Dunaliella,
Elltpsoidon, Emiliania, Eremosphaera, Ernodesmius, Euglena, Franceia,
Fragilaria, Gloeothamnion, Haematococcus, Halocafeteria, Hymenomonas,
Isochrysis, Lepocinclis, Micractinium, Monoraphidium, Nannochloris,
Nannochloropsis, Navicula, Neochloris, Nephrochloris, Nephroselmis,
Nitzschia, Ochromonas, Oedogonium, Oocystis, Ostreococcus, Pavlova,
Parachlorella, Pascheria, Phaeodactylum, Phagus, Platymonas, Pleurochrysis,
Pleurococcus, Prototheca, Pseudochlorella, Pyramimonas, Pyrobotrys,
Scenedesmus, Skeletonema, Spyrogyra, Stichococcus, Tetraselmis,
Thalassiosira, Viridiella, and Vo/vox species, and/or one or more
cyanobacteria
of the Agmenellum, Anabaena, Anabaenopsis, Anacystis, Aphanizomenon,
Arthrospira, Asterocapsa, Borzia, Calothrix, Chamaesiphon, Chlorogloeopsis,
Chroococcidiopsis, Chroococcus, Crinalium, Cyanobacterium, Cyanobium,
CA 02820717 2013-06-06
WO 2012/087867
PCT/US2011/065723
- 12 -
Cyanocystis, Cyanospira, Cyanothece, Cylindrospermopsis, Cylindrospermum,
Dactylococcopsis, Dermocarpella, Fischerella, Fremyella, Geitleria,
Geitlerinema, Gloeobacter, Gloeocapsa, Gloeothece, Halospirulina,
Iyengariella, Leptolyngbya, Limnothrix, Lyngbya, Microcoleus, Microcystis,
Myxosarcina, Nodular/a, Nostoc, Nostochopsis, Oscillator/a, Phormidium,
Planktothrix, Pleurocapsa, Prochlorococcus, Prochloron, Prochlorothrix,
Pseudanabaena, Rivularia, Schfrothrix, Scytonema, Spirulina, Stanieria,
Starr/a,
Stigonema, Syrnploca, Synechococcus, Synechocystis, Tolypothrix,
Trichodesmium, Tychonema, and Xenococcus species.
100421 When present in mineral oil feedstocks, the biocomponent portion can
comprise less than 50 wt%, based on the total weight of the feedstock
material,
for example about 40 wt% or less, about 30 wt% or less, about 25 wt% or less,
about 20 wt% or less, about 15 wt% or less, about 10 wt% or less, or about 5
wt% or less. Additionally, when present in mineral oil feedstocks, the
biocomponent portion can optionally comprise at least about 0.1 wt%, for
example at least about 0.5 wt%, at least about 1.0 wt%, at least about 1.5
wt%, at
least about 2.0 wt%, at least about 2.5 wt%, or at least about 5 wt%.
[0043] Hydrocracking refers to a process by which certain hydrocarbon
molecules in a provided feedstock are broken into simpler molecules to produce
a fuel product. Typically, the feedstock to be upgraded can include one or
more
fuels such as gasoline, kerosene, jet fuel, and diesel, and these individual
fuels
can be separated into their component parts, if desired, e.g., by
fractionation.
100441 A hydrocracking step can be carried out by contacting the mineral oil
feedstock with a hydrocracking catalyst in the presence of hydrogen to form
the
product. The addition of hydrogen can provide further benefit to the cracking
aspect of the step in that the fuel product produced can be more saturated
and/or
reduced in aromatic content, and/or can typically have a reduced content of
CA 02820717 2013-06-06
WO 2012/087867
PCT/US2011/065723
- 13 -
undesirable components such as heteroatoms (e.g., nitrogen, oxygen, and/or
sulfur).
[0045] In an embodiment, the hydrocracking catalyst can be comprised of an
amorphous and/or zeolitic support/base and a hydrogenation component
comprising one or more metals from Groups 6 and 8-10 of the Periodic Table of
Elements (e.g., Fe, Co, Ni, Mo, and/or W). Zeolitic cracking supports/bases
can
also be referred to as molecular sieves, which may be composed of silica,
alumina, and typically (but not necessarily) one or more exchangeable cations,
such as sodium, magnesium, calcium, a rare earth metal, or a combination
thereof. Examples of zeolitic molecular sieves that can be used in the
hydrocracking catalyst can include, but are not limited to, Zeolite Beta,
Zeolite
X, Zeolite Y, faujasite, Ultrastable Y (USY), Dealuminized Y (Deal Y),
Mordenite, ZSM-3, ZSM-4, ZSM-5, ZSM-18, ZSM-20, and combinations
thereof.
[0046] In one embodiment, the hydrocracking catalyst can comprise a large
pore crystalline molecular sieve, e.g., having a Constraint Index of less than
2, or
less than 1. The method by which the Constraint Index can be determined is
fully described in U.S. Patent No. 4,016,218, which is incorporated herein by
reference.
100471 Additionally or alternately, the hydrocracking catalyst can comprise a
molecular sieve having a pore size of at least about 7A, for example at least
about 7.4A or at least about 8A. Further additionally or alternately, the
hydrocracking catalyst can comprise a molecular sieve having a pore size of
about 18A or less, for example about 15A or less.
[00481 It can be preferred in some embodiments for the hydrocracking catalyst
to have at least some acidity. The alpha value is a measure of zeolite acidic
CA 02820717 2013-06-06
WO 2012/087867
PCT/US2011/065723
- 14 -
functionality and is described in greater detail in U.S. Patent No. 4,016,218
and
in J. Catalysis, Vol. VI, pages 278-287 (1966). Thus, in various embodiments,
the hydrocracking catalyst can have an alpha value greater than 1, for example
greater than 5 or greater than 10. It is not necessary that the hydrocracking
catalyst be highly acidic, although a highly acidic catalyst can be used.
Therefore, additionally or alternately, the hydrocracking catalyst can have an
alpha value of not greater than 200, for example not greater than 100.
100491 Hydrocracking can be carried out under conditions effective for
producing the desired fuel product, which can include one or more of an
average reaction temperature from about 300 F (about 149 C) to about 900 F
(about 482 C), for example from about 550 F (about 289 C) to about 800 F
(about 427 C); an average reaction pressure from about 400 psia (about 2.8
MPaa) to about 3000 psia (about 20.7 MPaa), for example from about 500 psia
(about 3.5 MPaa) to about 2000 psia (about 13.8 MPaa); a hydrogen-containing
treat gas rate from about 300 scf/bbl (about 51 Sm3/m3) to about 5000 scf/bbl
(about 850 Sm3/m3), for example from about 1000 scf/bbl (about 170 Sm3/m3) to
about 4000 scfibbl (about 680 Sm3/m3); and a liquid hourly space velocity
(LHSV), in volumes/volume/hour (v/v/hr or hr-1), from about 0.1 hr-1 to about
20
hr-1, for example from about 1 hr-1 to about 10 hr-1
100501 Treat gas, as referred to in this invention, can be either pure
hydrogen
or a hydrogen-containing treat gas, so long as hydrogen is present in an
amount
at least sufficient for the intended reaction(s). If the hydrogen is not pure,
other
gas(es) can be present (e.g., nitrogen, light hydrocarbons such as methane,
and
the like, and combinations thereof), advantageously which gas(es)
substantially
do not adversely interfere with or affect either the reactions or the
products.
Impurities, such as H2S and NH3, can be generally undesirable and can thus be
removed from the treat gas before it is conducted to the reactor. The treat
gas
CA 02820717 2013-06-06
WO 2012/087867
PCT/US2011/065723
- 15 -
stream can contain at least about 50 vol% hydrogen, preferably at least about
70
vol%, at least about 80 vol%, or at least about 90%.
[0051] Any type of reactor suitable for hydrocracking can be used to carry out
the step. Examples of such reactors can include, but are not limited to,
trickle
bed, ebullating bed, moving bed, and slurry reactors.
100521 One or more fractions can be removed/recovered from the
hydrocracked product as the first fuel composition to be upgraded to increase
smoke point according to the present invention. Like the mineral oil
feedstock,
the first fuel for upgrading to increase smoke point can be fractionated from
hydrocracked product into at least one fractionated first fuel component,
e.g.,
selected from the group consisting of gasoline, kerosene, jet fuel, diesel,
and
combinations thereof.
100531 In one embodiment, the smoke point upgrading process can be carried
out to produce and/or recover a kerosene type or a gasoline type jet fuel. For
instance, a kerosene type jet fuel can be produced/recovered according to the
inventive process so as to exhibit an ASTM D86 90% distillation point within
the range from about 250 C to about 290 C, for example from about 260 C to
about 280 C. Additionally or alternately in such an embodiment, a kerosene
type jet fuel can be produced/recovered according to the inventive process so
as
to exhibit an ASTM D86 10% distillation point within the range from about
150 C to about 200 C, for example from about 160 C to about 180 C. In an
alternate embodiment, a gasoline type jet fuel can be produced/recovered
according to the inventive process so as to exhibit an ASTM D86 90%
distillation point within the range from about 200 C to about 240 C, for
example
from about 210 C to about 230 C. Additionally or alternately in such an
embodiment, a gasoline type jet fuel can be produced/recovered according to
the
inventive process so as to exhibit an ASTM D86 10% distillation point within
CA 02820717 2013-06-06
WO 2012/087867
PCT/US2011/065723
- 16 -
the range from about 110 C to about 140 C, for example from about 120 C to
about 130 C.
[0054] In another embodiment, the smoke point upgrading process can be
carried out to produce and/or recover a diesel fuel. For instance, a diesel
fuel
can be produced/recovered according to the inventive process so as to exhibit
an
ASTM D86 90% distillation point within the range from about 260 C to about
350 C, for example from about 280 C to about 340 C. Additionally or
alternately, a diesel fuel can be produced/recovered according to the
inventive
process so as to exhibit an ASTM D86 10% distillation point within the range
from about 200 C to about 240 C, for example from about 210 C to about
230 C.
Smoke Point Upgrading Catalyst
[0055] Highly active upgrading catalysts, which can be more sulfur and/or
nitrogen tolerant than other, can be used to increase the smoke point of the
feedstock fuel according to the process of the invention. Such upgrading
catalysts can generally contain a hydrogenation component comprising at least
one Group VIII noble metal (e.g., Pt, Pd, Ru, Rh, Ir, or a combination
thereof),
for example at least two Group VIII noble metals, disposed on a support having
an inorganic, porous crystalline phase material.
[0056] Crystalline support materials suitable for use in the upgrading
catalyst
can include the M41S group of mesoporous crystalline materials, which are
described in U.S. Patent No. 5,102,643. Specific examples of such support
materials can include, but are not limited to, MCM-41 and MCM-48. MCM-41,
which is described in U.S. Patent No. 5,098,684, is characterized by a
microstructure with a relatively uniform, hexagonal arrangement of pores with
CA 02820717 2013-06-06
WO 2012/087867
PCT/US2011/065723
- 17 -
diameters of at least about 13A. MCM-48 has a cubic structure and is described
in U.S. Patent Nos. 5,102,643 and 5,198,203.
[0057] In one preferred embodiment, the smoke point upgrading catalyst can
comprise an MCM-41 support material on which both platinum and palladium
hydrogenation metals are disposed. The Pt/Pd/MCM-41 catalyst can produce
high-quality jet and diesel fuels by reducing smoke point highly efficiently.
100581 Additionally or alternately, the upgrading catalytic material according
to the invention can include an ultra-large pore size crystalline phase as a
support for the hydrogenation component, which crystalline phase material can
be characterized (in its calcined form) by an X-ray diffraction (XRD) pattern
having at least one peak corresponding to a d-spacing greater than about 18A
with a relative intensity normalized to about 100 (e.g., which can represent
the
largest resolvable peak in the XRD spectrum) and/or can be characterized by an
equilibrium benzene sorption capacity of at least about 15 grams of benzene
per
100 grams of material at about 50 ton (about 6.7 kPaa) and at about 25 C.
Further additionally or alternately, the upgrading catalytic material
according to
the invention can include an inorganic, porous support material having a
hexagonal pore with a maximum perpendicular cross-section pore dimension of
not less than about 13A, for example from about 13A to about 200A (also
termed "mesoporous") or from about 15A to about 110A.
[0059] Equilibrium benzene adsorption capacity can be determined by
contacting a material at about 25 C and at about 50 ton (about 6.7 kPaa) with
benzene until equilibrium is reached, after which the weight of sorbed benzene
can then be determined. The material, e.g., usable as support material
according
to the invention, should generally be tested only after thermal treatment at a
temperature of about 540 C for at least one hour, e.g., in an attempt to
remove
any pore blocking contaminants.
CA 02820717 2013-06-06
WO 2012/087867 PCT/US2011/065723
- 18 -
[0060] Still further additionally or alternately, the upgrading catalytic
material
according to the invention can include an inorganic mesoporous crystalline
material as a support having the following composition:
Mn/q(WaXbYeZdOn)
(1)
wherein: W is an element having a divalent ion, such as a divalent first row
transition metal (e.g., manganese, cobalt, iron, and/or magnesium, preferably
containing cobalt); X is an element having a trivalent ion, such as aluminum,
boron, iron, and/or gallium (preferably containing aluminum); Y is an element
having a tetravalent ion, such as silicon and/or germanium (preferably
containing silicon); Z is an element having a pentavalent ion, such as
phosphorus; M is one or more ions whose valence is an integer multiple of n/q,
and which can include, but is not limited to, ammonium, a mono-, di-, tri-, or
tetra- alkylammonium, one or more ions of Groups IA, IIA, and VIIB of the
Periodic Table of Elements (e.g., hydrogen, sodium, and/or fluoride); the
subscript n represents the charge of the composition, excluding M, expressed
as
oxides; the subscript q represents the weighted molar average valence of M;
the
subscripts a, b, c, and d represent the mole fractions of W, X, Y and Z,
respectively; the subscript his a number of from 1 to 2.5; and (a+b+c+d) = 1.
[0061] In one such embodiment, the inorganic mesoporous crystalline material
defined by equation (1) can be further defined by (a+b+c) being greater than
d,
and by h being 2. Additionally or alternately, both a and d can be 0, and h
can
be 2. Examples of materials that satisfy such definitions can include
aluminosilicates, although other metallosilicates can be suitable for use
according to the invention.
[0062] In the as-synthesized form, the support material having the
composition defined by equation (1) can additionally have a composition, on an
anhydrous basis, expressed empirically as follows:
rRMõig(WaX bY,Zd0h)
(2)
CA 02820717 2013-06-06
WO 2012/087867
PCT/US2011/065723
- 19 -
wherein M, W, X, Y, Z, n, q, a, b, c, d, and hare defined as above; R is the
total
organic material not included in M as an ion; and r is the coefficient for R
(i.e.,
the number of moles, or mole fraction, of R). The M and R components can be
associated with the material as a result of the presence during
crystallization of
components that may not be present in the dried and/or calcined product, and
which can thus be easily removed or, in the case of M, replaced by post-
crystallization.
[0063] To the extent desired, the original M (e.g., sodium and/or chloride)
ions of the as-synthesized material can be replaced in accordance with
conventional ion-exchange techniques. Examples of ions for replacing the
original M can include, but are not limited to, metal ions, hydrogen ions,
hydrogen precursor (e.g., ammonium) ions, and mixtures thereof. When
utilized, the replacing ions can, in some embodiments, provide at least a
significant portion of the desired metal (hydrogenation component)
functionality
in the final catalyst. Such ions can therefore include hydrogen, rare earth
metals,
Group 7 metals (e.g., Mn), Group 8-10 metals (e.g., Ni), Group 11 metals
(e.g.,
Cu), Group 14 metals (e.g., Sn) (each from the Periodic Table of Elements),
and
mixtures thereof
[0064] Such inorganic mesoporous crystalline materials can be distinguished
from other porous inorganic solids by the regularity of its large open pores,
whose pore size more nearly resembles that of amorphous or paracrystalline
materials, but whose regular arrangement and uniformity of size (e.g., having
a
pore size distribution within a single phase of, for instance, 25% or less,
usually
15% or less, of the average pore size of that phase) can tend to resemble more
those of crystalline framework materials such as zeolites. The term
"hexagonal," with reference to pores, should be understood herein to encompass
not only materials that exhibit mathematically perfect hexagonal symmetry
within the limits of experimental measurement, but also those with significant
CA 02820717 2013-06-06
WO 2012/087867
PCT/US2011/065723
- 20 -
observable deviations from that ideal state, so long as most channels in the
material are surrounded by six nearest neighbor channels at roughly the same
distance. Defects and imperfections can cause significant numbers of channels
to violate this criterion to varying degrees, depending on the quality of the
material's preparation. However, samples which exhibit as much as 25%
random deviation from the average repeat distance between adjacent channels
can still show recognizable XRD and/or neutron scattering order. Comparable
variations can similarly be observed in the d 100 values from electron/x-ray
diffraction patterns.
[0065] The most regular preparations of support material suitable for
upgrading catalysts according to the invention can show XRD patterns with a
few distinct maxima in the extreme low (2-theta) angle region. The positions
of
such maxima (peaks) approximately fit the positions of certain hk0 reflections
from a hexagonal crystalline lattice. XRD spectra, however, may not always be
a sufficient indicator of the presence of such order, as the degree of
regularity in
the microstructure and/or the extent of repetition of the structure within
individual particles can affect the number and/or position of peaks observed.
For instance, preparations with only one distinct peak in the low angle region
of
an XRD spectrum have been found to contain substantial amounts of
mesoporous material. Thus, other techniques, such as transmission electron
microscopy (TEM) and electron diffraction, can be used to elucidate details of
the microstructure. In some embodiments, properly oriented specimens of
mesoporous material can show a hexagonal arrangement of large channels, and
the corresponding electron diffraction pattern can yield an approximately
hexagonal arrangement of diffraction maxima. The d100 spacing of the electron
diffraction patterns can be described as the distance between adjacent spots
on
the hk0 projection of the hexagonal lattice and can be related to the repeat
distance, a0, between channels observed in electron micrographs through the
formula d100 a0(3/2)1/2. This (1100 spacing observed in certain electron
CA 02820717 2013-06-06
WO 2012/087867
PCT/US2011/065723
- 21 -
diffraction patterns can correspond to the d-spacing of an XRD low angle peak
of mesoporous material.
[0066] In some embodiments, in its calcined form, the crystalline support
material may be further characterized by an XRD spectrum with at least one
peak at a position corresponding to at least about an 18A d-spacing (<4.91
20,
for Cu-Ka radiation), which can correspond to the d100 value of an electron
diffraction pattern, and by an equilibrium benzene adsorption capacity of at
least
than about 15 grams of benzene per 100 grams of material at about 50 ton
(about 6.7 kPaa) and at about 25 C (which equilibrium benzene adsorption
capacity is measured on the basis of no pore blockage by incidental
contaminants, such as water and/or inorganic amorphous materials such as
silica,
which can be removed by dehydration techniques, e.g., thermal treatment,
and/or
chemical techniques, e.g., acid/base treatments, such that any detrital
material
can be removed, preferably without significant detrimental effect).
[0067] In an embodiment, a calcined, crystalline, non-layered material that
can be used as a support material according to this invention can be
characterized by an XRD spectrum with at least two peaks at positions
corresponding to at least about a 10A d-spacing (<8.84 20, for Cu-Ka
radiation), at least one of which can be at a position corresponding to at
least
about an 18A d-spacing (<4.91 20, for Cu-Ka radiation), and none of which
peaks can correspond to at least about a 10A d-spacing having a relative
intensity greater than about 20% of the magnitude of the strongest XRD peak,
for example having a relative intensity greater than about 10%. In this
embodiment, at least one peak in the XRD spectrum can advantageously have a
d-spacing that corresponds to the (1100 value of the electron diffraction
pattern of
the material.
CA 02820717 2013-06-06
WO 2012/087867
PCT/US2011/065723
-22 -
[0068] If an ammonium-containing form of catalytic support material is made,
it can be converted to the hydrogen form, e.g., by thermal treatment
(calcination), which can be performed at a temperature from about 400 C to
about 750 C for a time from about 1 minute to about 20 hours, for example from
about 1 hour to about 10 hours. While sub-atmospheric pressure can be
employed for such thermal treatments, atmospheric pressure can be preferable
in
some embodiments, for reasons of convenience, in an environment comprising
air, nitrogen, and/or ammonia, optionally with one or more other gases, as
desired.
[0069] Where sulfur and other contaminants such as phosphorus are present
only in relatively low concentrations in the feedstock (e.g., a sulfur content
of
about 50 wppm or less, such as about 30 wppm or less, about 20 wppm or less,
or about 10 wppm or less, and a phosphorous content about 20 wppm or less,
such as about 10 wppm or less or about 5 wppm or less), a combination of at
least two noble metals can be preferred. Where at least two noble metals are
used as hydrogenation components, a preferred example includes the
combination of Pd and Pt. In addition to the synergistic benefit which a
combination of platinum and palladium provides with respect to hydrogenation
activity, the palladium metal can optionally also provide the catalyst
composition with an added resistance to nitrogen poisoning.
100701 The content of the hydrogenation component can vary according to
catalytic activity. Thus, highly active (noble) metals may be used in
relatively
smaller amounts. However, increasing loading may nevertheless be desirable,
e.g., to the extent that the catalyst has low cracking activity, to maintain a
relatively high activity for smoke point upgrading, e.g., through
hydrogenation,
dearomatization, and/or aromatic saturation, which can improve product quality
by increasing API density, increasing cetane index/number, or the like, or a
combination thereof.
CA 02820717 2013-06-06
WO 2012/087867
PCT/US2011/065723
- 23 -
[0071] At least one noble metal can be disposed on the support material in a
total noble metal concentration from about 0.01 wt% to about 10 wt%, based on
the total weight of the catalyst, with individual noble metal concentrations
being
from about 0.01 wt% to about 5 wt%, from about 0.05 wt% to about 3 wt%, or
from about 0.1 wt% to about 2 wt%. In embodiments where more than one
noble metal is present, each individual noble metal can have a concentration
from about 0.01 wt% to about 5 wt%, based on the total weight of the catalyst.
100721 The hydrogenation component metal can be exchanged onto the
support material, impregnated into it, physically admixed with it, or a
combination thereof. If the metal is impregnated into or exchanged onto the
support material, it can accomplished in one embodiment by treating the
support
with a metal-containing ion, e.g., containing Pt and/or Pd. Suitable platinum
ion
forming compounds can include, but are not limited to, chloroplatinic acid,
platinous chloride, various compounds containing a platinum amine complex,
and combinations thereof. The metal-containing ion can additionally or
alternately manifest in a cation or anion form. Non-limiting examples of such
palladium and/or platinum compounds can include ammonia chlorides
complexes (e.g., Pd(N113)4C12and Pt(NH3)4C12), anionic nitrate complexes,
anionic vanadate complexes, and anionic metatungstate ions. Ionic forms of
hydrogenation metals can be rather useful, as they may be exchanged onto the
crystalline material or impregnated into it.
[0073] In various embodiments, in addition to a support material on which at
least one noble metal can be disposed, the smoke point upgrading catalyst can
contain a binder material. The binder material can, in many embodiments,
comprise or be a refractory inorganic oxide, for example one or more of
alumina, silica, silica-alumina, titania, zirconia, and magnesia, inter alia.
CA 02820717 2013-06-06
WO 2012/087867
PCT/US2011/065723
-24 -
Smoke Point Upgrading Conditions
[0074] Smoke point upgrading of the feedstock/fuel into a premium feedstock
can preferably be carried out at conditions including a relatively mild
average
reactor temperature, for example from about 100 C to about 300 C, from about
125 C to about 275 C, or from about 175 C to about 225 C. Additionally or
alternately, since the feedstock to be upgraded to increase smoke point can
typically be relatively low in sulfur content, low to moderate pressures can
be
used, for example from about 50 psig (about 450 kPag) to about 2000 psig
(about 17.8 MPag) or from about 300 psig (about 2.1 MPag) to about 1000 psig
(about 6.9 MPag). Further additionally or alternately, smoke point upgrading
can be effectively carried out at LHSVs from about 0.3 hfl to about 10 V', for
example from about 1 hr-1 to about 5 hel. Still further additionally or
alternately, smoke point upgrading can be effectively carried out by applying
a
hydrogen-containing treat gas, for example at a hydrogen treat gas rate from
about 34 Sm3/m3 (about 200 scf/bbl) to about 1700 Sm3/m3 (about 10000
scf/bbl) or from about 85 Sm3/m3 (about 500 scf/bbl) to about 850 Sm3/m3
(about 5000 scf/bbl).
Upgraded Product
[0075] The upgraded fuel product produced according to the inventive process
can have a relatively high smoke point, for example at least 25 mm, which is
believed to be indicative of premium quality jet fuels, such as Jet A and/or
Jet A-
1 aviation turbine fuels.
[0076] Additionally or alternately, the smoke point upgrading process can be
carried out to produce and/or recover a kerosene type or a gasoline type jet
fuel.
For instance, a kerosene type jet fuel can be produced/recovered according to
the
CA 02820717 2013-06-06
WO 2012/087867
PCT/US2011/065723
- 25 -
inventive process so as to exhibit an ASTM D86 90% distillation point within
the range from about 250 C to about 290 C, for example from about 260 C to
about 280 C. Additionally or alternately in such an embodiment, a kerosene
type jet fuel can be produced/recovered according to the inventive process so
as
to exhibit an ASTM D86 10% distillation point within the range from about
150 C to about 200 C, for example from about 160 C to about 180 C. In an
alternate embodiment, a gasoline type jet fuel can be produced/recovered
according to the inventive process so as to exhibit an ASTM D86 90%
distillation point within the range from about 200 C to about 240 C, for
example
from about 210 C to about 230 C. Additionally or alternately in such an
embodiment, a gasoline type jet fuel can be produced/recovered according to
the
inventive process so as to exhibit an ASTM D86 10% distillation point within
the range from about 110 C to about 140 C, for example from about 120 C to
about 130 C.
Additional Embodiments
[0077] The present invention can additionally or alternately include one or
more of the following embodiments.
[0078] Embodiment 1. A process for increasing smoke point of a fuel,
comprising: providing a feedstock fuel having a smoke point from 18 mm to
below 25 mm and a total aromatics content of Ar > 68 - 2.6Sm, wherein A, is
vol% total aromatics of the feedstock and Sir, is the smoke point of the
feedstock,
provided that A, is at least about 4 vol%; and contacting the feedstock fuel
with
a smoke point upgrading catalyst comprised of at least one noble metal
hydrogenation component disposed on a support having an inorganic, porous
crystalline phase material to provide a fuel product having a smoke point of
at
least 25 mm.
CA 02820717 2013-06-06
WO 2012/087867
PCT/US2011/065723
-26 -100791 Embodiment 2. The process of embodiment 1, further comprising
hydrocracking a mineral oil feedstock under conditions sufficient to provide
the
feedstock fuel.
100801 Embodiment 3. The process of embodiment 1 or embodiment 2,
wherein the feedstock fuel exhibits one or more of: an initial and final
boiling
point within the range from about 90 C to about 360 C; an ASTM D86 10%
distillation point within the range from about 110 C to about 190 C; a total
aromatics content of at least about 6 vol%; a total aromatics content of not
greater than about 25 vol%; a smoke point of at least 19 mm; and a sulfur
content of not greater than about 3000 wppm.
100811 Embodiment 4. The process of any one of the previous embodiments,
wherein the smoke point upgrading catalyst comprises a support material
comprising an inorganic, porous crystalline phase material having pores with
diameters of at least about 13A and exhibiting, after thermal treatment, an
XRD
spectrum having at least one peak corresponding to a d-spacing greater than
about 18A with a relative intensity of about 100, and having an equilibrium
benzene sorption capacity of at least about 15 grams of benzene per 100 grams
of material at about 50 toff (about 6.7 kPaa) and at about 25 C.
10082] Embodiment 5. The process of any one of the previous embodiment,
wherein the at least one noble metal is selected from the group consisting of
Pd,
Pt, Rh, Ru, Ir, and combinations thereof.
100831 Embodiment 6. The process of any one of the previous embodiments,
wherein crystalline phase material is a metallosilicate or an aluminosilicate.
CA 02820717 2013-06-06
WO 2012/087867
PCT/US2011/065723
- 27 -
[0084] Embodiment 7. The process of any one of the previous embodiments,
wherein the at least one noble metal is present on the catalyst in an amount
from
about 0.01 wt% to about 5 wt%, based on total catalyst weight.
[0085] Embodiment 8. The process of any one of the previous embodiments,
wherein the at least one noble metal is bound by a refractory inorganic oxide
selected from the group consisting of alumina, silica, silica-alumina,
titania,
zirconia, magnesia, and combinations thereof.
[0086] Embodiment 9. The process of any one of the previous embodiments,
wherein the smoke point upgrading catalyst is a Pt/Pd-containing M4 1S
catalyst
or a Pt/Pd-containing MCM-41 catalyst.
EXAMPLES
Example 1
[0087] An ¨11,500 barrel per day kerosene upgrading process was designed to
upgrade a vacuum gas oil (VG0) hydrocracker kerosene feed to 25 mm smoke
point jet fuel according to computer simulation. In this Example, two
catalysts
were compared: a Pt/Pd upgrading catalyst on a support having an inorganic,
porous crystalline phase material according to the invention (e.g., a Pt/Pd-
modified MCM-41) and a commercial Pt/Pd catalyst on alumina support. Table
1 shows the feed properties.
CA 02820717 2013-06-06
WO 2012/087867
PCT/US2011/065723
- 28 -
Table 1
Feed Rate, BPSD 11550
Feed Rate, ms/hr 76.5
API Gravity 43.8
Specific Gravity -15 C 0.807
Total Sulfur, wppm <1
Total Nitrogen, wppm <1
Smoke Point, mm 18
Freeze Point, C <-47
Flash Point D93, C 51
Kinematic Visc @-40 C,cSt 1.5
Total Aromatics, vol% 25
Naphthenics, vol% 1.5
ASTM D86 distillation, C
IBP 170
5% 171
10% 171
30% 182
50% 192
70% 201
90% 210
95% 216
FBP 221
[0088] In order to control the temperature rise across the catalyst bed, e.g.,
due
to the high heat release associated with the aromatic saturation reaction,
some of
the product was recycled back to the reactor. The recycle rate was set to be
about 50 vol% of the feed rate. For this case, the total feed to the reactor
was
about 17250 barrels per day, as shown below in Table 2:
Table 2
B/D I mithr
Raw Kerosene 11500 76.5
Product Recycle 5750 38.3
Total Feed 17250 115
[0089] A large number of catalyst beds could be used to control the heat
release for each catalyst bed. However, this approach could be impractical,
since bed length can be too short to give acceptable flow dynamics, and since
it
would require the use of a very large recycle compressor to provide the amount
of quench gas needed to control the overall reactor temperature across the
entire
reactor.
CA 02820717 2013-06-06
WO 2012/087867
PCT/US2011/065723
- 29 -
[0090] The total catalyst volume used was 30 m3, divided into two catalyst
beds for the both cases, where the first bed volume was about 10 m3 and the
second bed volume was about 20 m3.
[0091] The operating conditions were set to be as follows:
Product Recycle Rate, vol% ¨50
Inlet Total Pressure, barg ¨50
Make-Up H2 Gas Rate, Sm3/m3 ¨71.2
Make-Up 112 Purity, % ¨98.2
Recycle Gas Rate, Sm3/m3 ¨350
Purge Gas Rate, Sm3/m3 ¨3
It should be noted that gas flow rates in these operating conditions are based
on
the total feed rate.
[00921 The comparison between the catalysts in this Example was made at the
same reaction temperature. For commercial operation, the reaction temperature
can be defined as WABT (weighted average bed temperature), which is defined
as follows:
WABT = Catalyst bed inlet temperature + 2/3 AT,
wherein AT represents the temperature rise across the catalyst bed. As shown
in
Table 3, the operating conditions were simulated at the same WABT for each
catalyst bed for the both catalysts.
CA 02820717 2013-06-06
WO 2012/087867
PCT/US2011/065723
- 30 -
Table 3
Commercial Catalyst Pt./Pd MCM-41
Catalyst
Catalyst Recycle Rate on Feed vol A) ¨50 ¨50
Bed-1
Temperature - Inlet C ¨214 ¨186
Temperature Outlet C ¨238 ¨210
DT C ¨24 ¨24
WABT C ¨230 ¨202
Bed-2
Temperature - Inlet C ¨224 ¨196
Temperature - Outlet C ¨257 ¨234
DT C ¨33 ¨38
WABT C ¨246 ¨221
Cumulative WABT C ¨240 ¨215
100931 As can be seen from Table 4 below, which shows fuel yields and
product properties, the smoke point attained with the catalyst used according
to
this invention was substantially higher than the comparative commercial
catalyst. Although total aromatics content of the catalyst used according to
this
invention was substantially lower than that of the comparative commercial
catalyst, the naphthalenes content was a bit higher.
Table 4
Pt/Pd MCM-41
Commercial Catalyst
Catalyst
Recycle Rate on Feed vol% ¨50 ¨50
API Gravity ¨45.9 ¨46.6
Specific Gravity -60 F ¨0.798 ¨0.794
Freeze Point C <-47 <-47
Smoke Point mm ¨24 ¨26
Total Aromatics content vol% ¨8.4 ¨3.8
Naphthalenes content wt% ¨0.003 ¨0.012
Example 2
[00941 Unexpectedly, it has also been found that not all fuels in the jet fuel
boiling point range can be upgraded to produce a smoke point greater than 25,
as
shown in Table 5 below.
CA 02820717 2013-06-06
WO 2012/087867
PCT/US2011/065723
- 31 -
Table 5
Can Pr >25 m
Sample # Smoke Point Aromatic Content oduce
Smoke Point Fuel?m
mm vol% Yes or No
1 7 70.5 Yes
2 7 78.6 Yes
3 9 58.3 Yes
4 12.5 31.5 No
12.5 55.7 Yes
6 13 54.2 Yes
7 13 46 Yes
8 13 54.7 Yes
9 15 31.5 Yes
16 9.2 No
11 21 22.6 Yes
12 22 2.9 No
13 22 14.7 Yes
14 23 16.5 Yes
23 22.7 Yes
16 24 22.6 Yes
17 24 4 No
[00951 To produce at least a 25 mm smoke point fuel, the minimum aromatic
content required can be a function of the original smoke point of the feed,
e.g.,
as shown in Fig. 1. As shown in the Fig. 1, the feeds suitable for producing
at
least a 25 mm smoke point fuel should have an aromatic content as follows: A,
> 68 - 2.6Sõõ wherein Ar is voI% aromatics of the feedstock and Sm is the
smoke
point of the feedstock.
[0096] The principles and modes of operation of this invention have been
described above with reference to various exemplary and preferred
embodiments. As understood by those of skill in the art, the overall
invention,
as defined by the claims, may encompass other preferred embodiments not
specifically enumerated herein.