Note: Descriptions are shown in the official language in which they were submitted.
CA 02821241 2013-06-11
WO 2012/082377
PCT/US2011/062608
- 1 -
PHOSPHORUS RECOVERY FROM HYDROTHERMAL TREATMENT OF
BIOMASS
FIELD OF THE INVENTION
[0001] This invention relates to hydrothermal treatment of various types of
biomass, such as algae, to produce hydrocarbon products, such as distillate
fuel.
BACKGROUND OF THE INVENTION
[0002] Conventional production of fuels and lubricants is still dominated
by
conversion of mineral petroleum feeds into desired products. In order to
supplement
and/or replace the conventional sources with renewable forms of energy, a
variety of
problems must be overcome.
[0003] One alternative to conventional fuels and lubricants is to produce
comparable fuels and lubricants based on biomass. One advantage of biomass
based
fuels is that the resulting fuel product may be compatible with existing
infrastructure
and technologies. Ideally, biomass based fuels and lubricants could be used in
a "drop-
in" fashion in place of conventional products, allowing the use of a renewable
product
without having to modify existing equipment.
[0004] One option for processing of a biomass type feed is hydrothermal
processing. Hydrothermal processing involves exposing a feed to water under
elevated
temperature and pressure conditions. U.S. Patent No. 6,180,845 provides an
example
of this type of process. This patent describes a process for transforming
biomass to
hydrocarbon mixtures using near-critical or supercritical water. The process
can be
used on a variety of initial biomass materials. The biomass is processed at
pressures
from 200 bars (20 MPa) to 500 bars (50 MPa) and at temperatures from 320 C to
500 C. The atmosphere in the reactor is described as non-oxidizing, and
hydrogen is
included in an example. About 4 hours is noted as a preferred processing time.
The
hydrothermal processing is described as producing a "petroleum like liquid",
which
appears to include a substantial portion of aromatic and polymeric species, as
well as
some soot and/or carbonized residues. The description mentions that some
metals
present in the biomass feed, such as Ni or Fe, can alter the types of products
generated.
The description also mentions that metals can be used to simplify the
components of
CA 02821241 2013-06-11
WO 2012/082377
PCT/US2011/062608
- 2 -
the product mixture, or to remove unwanted compounds. The only metal
specifically
mentioned as an additive is Cu metal for removal of sulfur compounds such as
thiophenes. Nitrogen compounds are identified as another product that can be
removed
by precipitation with metals, although no examples of a suitable metal are
provided. It
appears from the description that the additive metals used are "reduced
metals", as
opposed to metals in an oxidized state.
[0005] PCT Publication No. WO 96/30464 provides another example of
processing
of biomass at supercritical conditions. The application describes processing
of wet
biomass, such as algae or water hyacinth, to produce gaseous hydrocarbons and
hydrogen. The conversion conditions include contacting the biomass with water
under
supercritical conditions, which is defined as having a temperature of greater
than 374 C
and a pressure greater than 22.1 MPa. The conversion takes place in the
presence of a
carbon based catalyst, such as charcoal or an activated carbon with a high
surface area.
The process is described as providing rapid and virtually complete
gasification of
organic matter in a feedstock.
SUMMARY OF THE INVENTION
[0006] In one aspect of the invention, a method for hydrothermally
processing
biomass is provided. The method includes introducing a biomass feed having a
phosphorus content and a water to biomass ratio of at least 1:1 into a
reaction zone.
The biomass feed can be hydrothermally treated under effective hydrothermal
treatment
conditions to produce a multi-phase product. The multi-phase product can
include a
solids portion containing at least about 80% of the phosphorus content of the
biomass
feed. The multi-phase product can be separated to produce at least a gas phase
portion,
a liquid hydrocarbon product, and the solids portion.
[0007] In another aspect of the invention, another method for
hydrothermally
processing biomass is provided. The method includes adding a multivalent metal
to a
biomass feed having a phosphorus content. The biomass feed can be contacted
with
water in the presence of the multivalent metal under effective hydrothermal
treatment
conditions to produce a multi-phase product. The multi-phase product can
include a
solids portion containing at least about 80% of the phosphorus content of the
biomass
CA 02821241 2013-06-11
WO 2012/082377
PCT/US2011/062608
- 3 -
feed. The multi-phase product can be separated to produce at least a gas phase
portion,
a liquid hydrocarbon product, and the solids portion.
[0008] In still another aspect of the invention, yet another method for
hydrothermally processing biomass is provided. The method includes contacting
an
algae-containing biomass feed having a phosphorus content with water under
effective
hydrothermal treatment conditions to produce a multi-phase product. The multi-
phase
product can include a solids portion containing at least about 80% of the
phosphorus
content of the algae-containing biomass feed. The multi-phase product can be
separated to produce at least a gas phase portion, a liquid hydrocarbon
product, and the
solids portion. Phosphorus from the solids portion can advantageously be
recycled to
an algae growth environment.
BRIEF DESCRIPTION OF THE DRAWINGS
[0009] FIG. I depicts a reaction system suitable for performing a process
according
to an embodiment of the invention.
[0010] FIG. 2 schematically shows a reaction scheme according to an
embodiment
of the invention.
DETAILED DESCRIPTION OF THE EMBODIMENTS
Overview
[0011] One of the difficulties with production of hydrocarbon products from
various types of biomass can be handling of products other than carbonaceous.
In
many instances, the non-carbonaceous products can be viewed as contaminants.
Such
contaminants can include sulfur-containing compounds and nitrogen-containing
compounds formed from sulfur and/or nitrogen present in the biomass.
[0012] For some biomass feeds, such as algae feeds or other feeds where
cellular
material is included in the feed, phosphorus can also represent a noticeable
portion of
the feed. Unlike sulfur, however, it can be beneficial to view phosphorus as
another
product to be harvested from processing of a feed. Phosphorus can be
incorporated into
various cellular structures, such as lipids used for forming cell walls.
Because of the
importance of phosphorus in developing cellular structures, phosphorus can be
a
valuable input for growth of biological organisms. The phosphorus required for
growth
CA 02821241 2013-06-11
WO 2012/082377
PCT/US2011/062608
- 4 -
of biological organisms can represent a significant cost in a growth process.
Even
though phosphorus is not one of the primary saleable products formed from
processing
of a biomass feed to produce hydrocarbon products, the ability to effectively
capture
and re-use phosphorus can greatly improve the economics of a hydrocarbon
production
process.
[0013] In various embodiments, methods are provided for hydrothermal
treatment
of algae feeds (or other biomass based feeds) to produce distillate boiling
range
products while allowing for improved capture and/or recycling of phosphorus.
Hydrothermal treatment of an algae feed can allow for conversion of biomass
into
molecules having a desired boiling range while also removing at least a
portion of
impurities that are not desirable in a distillate product, such as nitrogen
impurities,
oxygen impurities, unsaturated and/or aromatic impurities, metal impurities,
and the
like. In various embodiments, hydrothermal processing conditions can be
adjusted
and/or improved to facilitate recovery of phosphorus. This can include
increasing the
total amount of phosphorus recovered relative to the amount of phosphorus in
the feed.
This can additionally or alternately include increasing the ratio of
phosphorus to carbon
in the phosphorus product formed during processing. The methods for improving
phosphorus recovery can include introducing multivalent metal, such as
multivalent
metal cations, into the reaction environment to form metal phosphates. Another
option
can involve selecting a temperature and/or length of time for hydrothermal
processing
that improves the amount of phosphorus recovered relative to the feed content
and/or
the ratio of phosphorus to carbon in the solids formed during reaction.
[0014] Algae can contain significant amounts of products such as
triglycerides,
fatty acids/alcohols, and isoprenoids, which can be converted to valuable
products such
as transportation fuels and lubricants. However, a number of challenges exist
in
converting an algae feed into a usable product. One challenge is recovering
the desired
hydrocarbon molecules from the algae. An option for recovering hydrocarbon
products
from algae can be to use a solvent extraction based method. Unfortunately,
some
solvent based methods require use of an algae source that contains little or
no water.
Dehydration of an algae source to a sufficient degree to allow for this type
of solvent
extraction can require a high cost of operation. Alternative solvent
extraction methods
can allow for extraction from an algae sample that contains water. However, a
high
CA 02821241 2013-06-11
WO 2012/082377
PCT/US2011/062608
- 5 -
cost step usually remains, as the solvent has to be separated from the water,
for
example by distillation.
[0015] As an alternative to solvent extraction, hydrothermal processing can
be used
to extract hydrocarbon products from an algae source. Hydrothermal processing
has
the advantage that it can be performed without vaporizing water, which can
reduce the
cost of the process. However, another difficulty with using biomass to produce
hydrocarbon products can be the presence of impurities in the biomass. An
algae feed
can have a relatively high concentration of molecules that can contain, inter
alia, sulfur,
nitrogen, oxygen, phosphorus, Group I metals, Group II metals, transition
metals,
olefinic groups, and aromatic groups. Due to the high impurity levels,
additional
processing can be required before the hydrocarbon products from non-catalytic
hydrothermal processing can be used in conventional processes.
Feedstocks
100161 In various embodiments of the invention, an algae feed or another
biomass
based feed can be processed using catalytic hydrothermal processing. In such
embodiments, the feed can typically contain algae and water, and optionally
can
contain additional feed from another biocomponent source, where a biocomponent
source is any source including and/or derived from biological material, such
as from
plants, animals, microbes, algae, or a combination thereof. Additionally or
alternately,
the feed can be a feed derived from a starting mixture containing algae and
water, and
can optionally contain feed from another biocomponent source. Further
additionally or
alternately, the feed can generally be a feed based on biomass.
[0017] It is noted that the water present in an algae (or other biomass)
feed can
include extracellular water and/or intracellular water. Intracellular water
refers to water
contained within the cell membrane of a cell, such as an algae cell. For an
algae feed, a
feed that appears relatively dry based on extracellular water content, can
still contain a
substantial portion of intracellular water. For algae whose cell walls have
been
ruptured (e.g., substantially dried/dewatered algae), the algae feed can only
contain
extracellular water (as ruptured cells do not have an inside, but only an
outside). For an
algae feed that contains intracellular water, computing the ratio of water to
(dry) algae
requires determining what portion of the algae weight is due to intracellular
water, as
the intracellular water should count toward the weight of water and not the
weight of
CA 02821241 2013-06-11
WO 2012/082377
PCT/US2011/062608
- 6 -
dry algae. As a clarifying example, an algae sample could include no
extracellular
water and still have a water to algae ratio of about 1:1 or greater, for
example about 2:1
or greater, due to the amount of intracellular water in the algae. Thus,
references herein
to the weight of algae refer to the weight of dry algae, excluding
intracellular water.
[0018] For a
feed containing at least algae and water, the algae content of the feed
can be at least about 5 wt%, for example at least about 10 wt%, at least about
20 wt%,
at least about 25 wt%, or at least about 30 wt%. Additionally or alternately,
the algae
content of the feed can be about 50 wt% or less, for example about 30 wt% or
less,
about 25 wt% or less, or about 20 wt% or less. In terms of ratios, the ratio
of water to
algae in the feed can be at least about 1:1, for example at least about 2:1,
at least about
3:1, or at least about 4:1. Additionally or alternately, the ratio of water to
algae can be
about 25:1 or less, for example about 20:1 or less or about 10:1 or less. In
some
embodiments, the algae content of the feed relative to the amount of water can
be based
on practical considerations regarding extraction of water from the source of
the algae.
Thus, in some embodiments, algae can be introduced into a reactor as a mixture
or
paste of algae and water. Additionally or alternately, a dried form of algae
can be
introduced into a reactor along with sufficient water, e.g., to reach a
desired ratio of
algae to water.
[0019] Algae
oils or lipids can typically be contained in algae in the form of
membrane components, storage products, and/or metabolites. Certain algal
strains,
particularly microalgae such as diatoms and cyanobacteria, can contain
proportionally
high levels of lipids. Algal sources for the algae oils can contain varying
amounts, e.g.,
from 2 wt% to 80 wt% of lipids, based on total weight of the biomass itself.
[0020] Algal
sources for algae oils can include, but are not limited to, unicellular
and multicellular algae.
Examples of such algae can include a rhodophyte,
chlorophyte, heterokontophyte, tribophyte, glaucophyte, chlorarachniophyte,
euglenoid,
haptophyte, cryptomonad, dinoflagellum, phytoplankton, and the like, and
combinations thereof. In one embodiment, algae can be of the classes
Chlorophyceae
and/or Haptophyta. Specific species can include, but are not limited to,
Neochloris
oleoabundans, Scenedesmus dimorphus, Euglena gracilis, Phaeodactylum
tricornutum,
Pleurochrysis carterae, Prymnesium parvum, Nannochloropsis gaditiana,
Tetraselmis
chui, Tetraselmis tertiolecta, Dunaliella sauna, various species of Chlorella,
and
CA 02821241 2013-06-11
WO 2012/082377
PCT/US2011/062608
- 7 -
Chlamydomonas reinhardtii, Nonlimiting examples of additional or alternate
algal
sources include one or more microalgae of the Achnanthes, Amphiprora, Amphora,
Ankistrodesmus, Asteromonas, Boekelovia, Borodinella, Botryococcus,
Bracteococcus,
Chaetoceros, Carteria, Chlamydomonas, Chlorococcum, Chlorogonium, Chlorella,
Chroomonas, Chrysosphaera, Cricosphaera, Crypt hecodinium, Cryptomonas,
Cyclotella, Dunaliella, Ellipsoidon, Emiliania, Eremosphaera, Ernodesmius,
Euglena,
Franceia, Fragilaria, Gloeothamn ion, Haematococcus, Halocafeteria,
Hymenomonas,
lsochrysis, Lepocinclis, Micractinium,
Monoraphidium, Nannochloris,
Nannochloropsis, Navicula, Neochloris, Nephrochloris, Nephroselmis, Nitzschia,
Ochromonas, Oedogonium, Oocystis, Ostreococcus, Pavlova, Parachlorella,
Pascheria, Phaeodactylum, Phagus, Platymonas, Pleurochrysis, Pleurococcus,
Prototheca, Pseudochlorella, Pyramimonas, Pyrobotrys, Scenedesmus,
Skeletonema,
Spyrogyra, Stichococcus, Tetraselmis, Thalassiosira, Viridiella, and Vo/vox
species,
and/or one or more cyanobacteria of the Agmenellum, Anabaena, Anabaenopsis,
Anacystis, Aphanizomenon, Arthrospira, Asterocapsa, Borzia, Calothrix,
Chamaesiphon, Chlorogloeopsis, Chroococcidiopsis, Chroococcus, Crinalium,
Cyano bacterium, Cyanobium, Cyanocystis, Cyanospira,
Cyanothece,
Cylindrospermopsis, Cylindrospermum, Dactylococcopsis,
Dermocarpella,
Fischerella, Fremyella, Geitleria, Geitlerinema, Gloeobacter, Gloeocapsa,
Gloeothece,
Halospirulina, Iyengariella, Leptolyngbya, Limnothrix, Lyngbya, Microcoleus,
Microcystis, Myxosarcina, Nodularia, Nostoc, Nostochopsis, Oscillatoria,
Phormidium,
Planktothrix, Pleurocapsa, Prochlorococcus, Prochloron, Prochlorothrix,
Pseudanabaena, Rivularia, Schizothrix, Scytonema, Spirulina, Stan ieria,
Starria,
Stigonema, Symploca, Synechococcus, Synechocystis, Tolypothrix, Trichodesmium,
Tychonema, and Xenococcus species.
[0021] After
catalytic hydrothermal processing, a portion of the products from
catalytic hydrothermal processing can be combined with biocomponent and/or
mineral
based feeds. The combined feedstock can include varying amounts of feedstreams
based on biocomponent sources. When desired, the feed can include at least
about 0.1
wt% of feed based on a biocomponent source, for example at least about 0.5
wt%, at
least about 1 wt%, at least about 3 wt%, at least about 10 wt%, at least about
15 wt%, at
least about 25 wt%, at least about 50 wt%, or at least about 75 wt%. In such
CA 02821241 2013-06-11
WO 2012/082377
PCT/US2011/062608
- 8 -
embodiments, the feed can additionally or alternately include about 100 wt% or
less of
biocomponent, for example about 90 wt% or less, about 75 wt% or less, or about
50
wt% or less. In other embodiments, the amount of biocomponent feed (e.g., for
co-
processing with the mineral oil portion of the feed) can be relatively small,
for instance
with a feed that includes at least about 0.5 wt% of feedstock based on a
biocomponent
source, e.g., at least about 1 wt%, at least about 2.5wt%, or at least about 5
wt%, at
least about 10 wt%, or at least about 20 wt%. In such embodiments, the feed
can
additionally or alternately include about 50 wt% or less of biocomponent based
feedstock, for example about 25 wt% or less, about 20 wt% or less, about 10
wt% or
less, or about 5 wt% or less.
[0022] In various embodiments of the invention, the combined feedstock can
include feeds from various biomass or biocomponent sources, such as vegetable
(higher
plant), animal, fish, and/or algae. Generally, these biocomponent sources can
include
vegetable fats/oils, animal fats/oils, fish oils, pyrolysis oils, and algae
lipids/oils, as
well as components of such materials, and in some embodiments can specifically
include one or more type of lipid compounds. Lipid compounds are typically
biological compounds that are insoluble in water, but soluble in nonpolar (or
fat)
solvents. Non-limiting examples of such solvents include alcohols, ethers,
chloroform,
alkyl acetates, benzene, and combinations thereof.
[0023] Major classes of lipids include, but are not necessarily limited to,
fatty acids,
glycerol-derived lipids (including fats, oils and phospholipids), sphingosine-
derived
lipids (including ceramides, cerebrosides, gangliosides, and sphingomyelins),
steroids
and their derivatives, terpenes and their derivatives, fat-soluble vitamins,
certain
aromatic compounds, and long-chain alcohols and waxes.
100241 In living organisms, lipids generally serve as the basis for cell
membranes
and as a form of fuel storage. Lipids can also be found conjugated with
proteins or
carbohydrates, such as in the form of lipoproteins and lipopolysaccharides.
[0025] Examples of vegetable oils that can be used in accordance with this
invention include, but are not limited to rapeseed (canola) oil, soybean oil,
coconut oil,
sunflower oil, palm oil, palm kernel oil, peanut oil, linseed oil, tall oil,
corn oil, castor
oil, jatropha oil, jojoba oil, olive oil, flaxseed oil, camelina oil,
safflower oil, babassu
oil, tallow oil and rice bran oil.
CA 02821241 2013-06-11
WO 2012/082377
PCT/US2011/062608
- 9 -
[0026] Vegetable oils as referred to herein can also include processed
vegetable oil
material. Non-limiting examples of processed vegetable oil material include
fatty acids
and fatty acid alkyl esters. Alkyl esters typically include C1-05 alkyl
esters. One or
more of methyl, ethyl, and propyl esters are preferred.
[0027] Examples of animal fats that can be used in accordance with the
invention
include, but are not limited to, beef fat (tallow), hog fat (lard), turkey
fat, fish fat/oil,
and chicken fat. The animal fats can be obtained from any suitable source
including
restaurants and meat production facilities.
[0028] Animal fats as referred to herein also include processed animal fat
material.
Non-limiting examples of processed animal fat material include fatty acids and
fatty
acid alkyl esters. Alkyl esters typically include C1-05 alkyl esters. One or
more of
methyl, ethyl, and propyl esters are preferred.
[0029] Other biocomponent feeds usable in the present invention can include
any of
those which comprise primarily triglycerides and free fatty acids (FFAs). The
triglycerides and FFAs typically contain aliphatic hydrocarbon chains in their
structure
having from 8 to 36 carbons, preferably from 10 to 26 carbons, for example
from 14 to
22 carbons. Types of triglycerides can be determined according to their fatty
acid
constituents. The fatty acid constituents can be readily determined using Gas
Chromatography (GC) analysis. This analysis involves extracting the fat or
oil,
saponifying (hydrolyzing) the fat or oil, preparing an alkyl (e.g., methyl)
ester of the
saponified fat or oil, and determining the type of (methyl) ester using GC
analysis. In
one embodiment, a majority (i.e., greater than 50%) of the triglyceride
present in the
lipid material can be comprised of C10 to C26 fatty acid constituents, based
on total
triglyceride present in the lipid material. Further, a triglyceride is a
molecule having a
structure identical to the reaction product of glycerol and three fatty acids.
Thus,
although a triglyceride is described herein as being comprised of fatty acids,
it should
be understood that the fatty acid component does not necessarily contain a
carboxylic
acid hydrogen. In one embodiment, a majority of triglycerides present in the
biocomponent feed can preferably be comprised of C12 to C18 fatty acid
constituents,
based on total triglyceride content. Other types of feed that are derived from
biological
raw material components can include fatty acid esters, such as fatty acid
alkyl esters
(e.g., FAME and/or FAEE).
CA 02821241 2013-06-11
WO 2012/082377
PCT/US2011/062608
- 10 -
[0030] Biocomponent based diesel boiling range feedstreams can have a wide
range
of nitrogen and/or sulfur contents. For example, a biocomponent based
feedstream
based on a vegetable oil source can contain up to about 300 wppm nitrogen. In
contrast, a biomass based feedstream containing whole or ruptured algae can
sometimes
include a higher nitrogen content. Depending on the type of algae, the
nitrogen content
of an algae based feedstream can be at least about 2 wt%, for example at least
about 3
wt%, at least about 5 wt%, or at least about 10 wt%, and algae with still
higher nitrogen
contents are known. The sulfur content of a biocomponent feed can also vary.
In some
embodiments, the sulfur content can be about 500 wppm or less, for example
about 100
wppm or less, about 50 wppm or less, or about 10 wppm or less.
[0031] Aside from nitrogen and sulfur, oxygen can be another heteroatom
component in biocomponent based feeds. A biocomponent diesel boiling range
feedstream based on a vegetable oil, prior to hydrotreatment, can include up
to about 10
wt% oxygen, for example up to about 12 wt% or up to about 14 wt%. Additionally
or
alternately, such a biocomponent diesel boiling range feedstream can include
at least
about 1 wt% oxygen, for example at least about 2 wt%, at least about 3 wt%, at
least
about 4 wt%, at least about 5 wt%, at least about 6 wt%, or at least about 8
wt%.
Further additionally or alternately, a biocomponent feedstream, prior to
hydrotreatment,
can include an olefin content of at least about 3 wt%, for example at least
about 5 wt%
or at least about 10 wt%.
[0032] A mineral hydrocarbon feedstock refers to a conventional (e.g., non-
biocomponent) hydrocarbon feedstock, typically derived from crude oil and that
has
optionally been subjected to one or more separation and/or other refining
processes. In
one preferred embodiment, the mineral hydrocarbon feedstock can be a petroleum
feedstock boiling in the diesel range or above. Examples of suitable
feedstocks can
include, but are not limited to, virgin distillates, hydrotreated virgin
distillates,
kerosene, diesel boiling range feeds (such as hydrotreated diesel boiling
range feeds),
light cycle oils, atmospheric gasoils, and the like, and combinations thereof
[0033] Mineral feedstreams for blending with a biocomponent feedstream can
have
a nitrogen content from about 50 wppm to about 2000 wppm nitrogen, for example
from about 50 wppm to about 1500 wppm or from about 75 to about 1000 wppm. In
some embodiments, the mineral feedstream can have a sulfur content from about
100
CA 02821241 2013-06-11
WO 2012/082377
PCT/US2011/062608
- 11 -
wppm to about 10,000 wppm sulfur, for example from about 200 wppm to about
5,000
wppm or from about 350 wppm to about 2,500 wppm. Additionally or alternately,
the
combined (biocomponent plus mineral) feedstock can have a sulfur content of at
least
about 5 wppm, for example at least about 10 wppm, at least about 25 wppm, at
least
about 100 wppm, at least about 500 wppm, or at least about 1000 wppm. Further
additionally or alternately, the combined feedstock can have a sulfur content
of about
2000 wppm or less, for example about 1000 wppm or less, about 500 wppm or
less,
about 100 wppm or less, or about 50 wppm or less. Still further additionally
or
alternately, the nitrogen content of the combined feedstock can be about 1000
wppm or
less, for example about 500 wppm or less, about 100 wppm or less, about 50
wppm or
less, about 30 wppm or less, about 20 wppm or less, or about 10 wppm or less.
[0034] The content of sulfur, nitrogen, oxygen, and olefins in a feedstock
created
by blending two or more feedstocks can typically be determined using a
weighted
average based on the blended feeds. For example, a mineral feed and a
biocomponent
feed can be blended in a ratio of 80 wt% mineral feed and 20 wt% biocomponent
feed.
If the mineral feed has a sulfur content of about 1000 wppm, and the
biocomponent
feed has a sulfur content of about 10 wppm, the resulting blended feed could
be
expected to have a sulfur content of about 802 wppm.
[0035] Diesel boiling range feedstreams suitable for use in the present
invention
tend to boil within the range of about 215 F (about 102 C) to about 800 F
(about
427 C). Preferably, the diesel boiling range feedstream has an initial boiling
point of at
least about 2I5 F (about 102 C), for example at least about 250 F (about 121
C), at
least about 275 F (about 135 C), at least about 300 F (about 149 C), at least
about
325 F (about 163 C), at least about 350 F (about 177 C), at least about 400 F
(about
204 C), or at least about 451 F (about 233 C). Preferably, the diesel boiling
range
feedstream has a final boiling point of about 800 F (about 427 C) or less, or
about
775 F (about 413 C) or less, or about 750 F (about 399 C) or less. In some
embodiments, the diesel boiling range feedstream can have a boiling range from
about
451 F (about 233 C) to about 800 C (about 427 C). Additionally or alternately,
the
feedstock can be characterized by the boiling point required to boil a
specified
percentage of the feed. For example, the temperature required to boil at least
5 wt% of
a feed is referred to as a "T5" boiling point. In one embodiment, the mineral
oil
CA 02821241 2013-06-11
WO 2012/082377
PCT/US2011/062608
- 12 -
feedstock can have a T5 boiling point of at least about 230 F (about 110 C),
for
example at least about 250 F (about 121 C) or at least about 275 F (about 135
C).
Further additionally or alternately, the mineral hydrocarbon feed can have a
T95
boiling point of about 775 F (about 418 C) or less, for example about 750 F
(about
399 C) or less or about 725 F (about 385 C) or less. In another embodiment,
the diesel
boiling range feedstream can also include kerosene range compounds to provide
a
feedstream with a boiling range from about 250 F (about 121 C) to about 800 F
(about
427 C).
Hydrothermal Processing Conditions
[0036] In various embodiments, catalytic hydrothermal processing can be
performed in a batch, semi-batch, and/or continuous type processing
environment(s).
Regardless of whether the reaction takes place in a batch, semi-batch, or
continuous
reaction system, any system region where the biomass is treated under
hydrothermal
treatment conditions can be referred to as the reaction zone. The reaction
zone can
correspond to a reactor for a batch or semi-batch environment and/or to a
reactor,
conduit, or other location for hydrothermal treatment in a continuous reaction
system.
[0037] In embodiments involving a batch reactor, the reactor can be any
type of
batch reactor suitable for handling the processing conditions. Due to the
potential
presence of water at supercritical conditions, stainless steel can be a
suitable non-
reactive material for the reactor walls. Other materials and/or coatings for
the reactor
surfaces can be used that are compatible with the reaction conditions
described herein.
Examples of suitable reactors can include, but are not limited to, autoclaves,
stirred
tanks, plough mixers, and the like, and combinations thereof Alternately, a
bubble
column could be used. One possible advantage for batch or semi-batch type
processing
can occur for algae feeds that have relatively poor flow characteristics. For
example, at
an algae concentration relative to water of about 20 wt% (i.e., about 4 parts
water to 1
part algae by weight), the resulting mixture can have the consistency of a
paste. Such a
paste could be difficult to move, e.g., using pumps in a continuous flow type
reactor.
[0038] In one embodiment, a batch reactor can be used for catalytic
hydrothermal
processing of an algae feed. A portion of algae feed mixed with water can be
introduced into the reactor, which can then be purged (if necessary), e.g., to
remove any
oxygen containing gases. Additionally or alternately, a catalyst can also be
introduced
CA 02821241 2013-06-11
WO 2012/082377
PCT/US2011/062608
- 13 -
into the reactor. The catalyst can be included as part of the mixture of algae
and water,
or the catalyst can be introduced into the reactor as part of a separate
input.
Additionally or alternately, a partial pressure of an inert gas and/or a
reducing gas can
then be introduced into the reactor. Examples of suitable reducing gases can
include
hydrogen, while suitable inert gases can include nitrogen. Additional or
alternate
examples of suitable reducing gases can include any gas that does not add
molecular
oxygen to the reaction atmosphere, whether prior to the start of the reaction
or from
dissociation forming oxygen during the hydrothermal processing. The partial
pressure
of additional gas introduced into the reactor, when present, can be at least
about 1 bar
(about 0.1 MPa), for example at least about 25 bar (about 2.5 MPa), at least
about 40
bar (about 4.0 MPa), or at least about 50 bar (about 5.0 MPa). Additionally or
alternately, the partial pressure of gas introduced into the reactor, when
present, can be
about 100 bar (about 10 MPa) or less, for example about 75 bar (about 7.5 MPa)
or less
or about 50 bar (about 5.0 MPa) or less. Note that introducing a reducing gas
can
correspond to at least partially dissolving a reducing gas in the water (e.g.,
saturating
the water) for the hydrothermal treatment.
10039] After introducing the algae, water, catalyst, and any
additional reducing
and/or inert gases, the batch reactor can be sealed. The temperature of the
reactor can
then be raised to at least about 50 C, for example at least about 80 C, at
least about
,
100 C, at least about 150 C, at least about 200 C, at least about 250 C, at
least about
275 C, or at least about 300 C. Additionally or alternately, the temperature
of the
reactor can be raised to about 500 C or less, for example about 400 C or less,
about
380 C or less, about 350 C or less, about 300 C or less, or about 275 C or
less.
Further additionally or alternately, the pressure in the reactor can be at
least about 1
barg (about 0.1 MPag), for example at least about 4.5 barg (about 450 kPag),
at least
about 25 barg (about 2.5 MPag), at least about 40 barg (about 4.0 MPag), at
least about
50 barg (about 5.0 MPag), or at least about 100 barg (about 10 MPag).
Additionally or
alternately, the partial pressure of gas introduced into the reactor, when
present, can be
about 300 barg (about 30 MPag) or less, for example about 250 barg (about 25
MPag)
or less, about 225 barg (about 22.5 MPag) or less, or about 200 barg (about 20
MPag)
or less.
CA 02821241 2013-06-11
WO 2012/082377
PCT/US2011/062608
- 14 -
[0040] In some embodiments, the combination of pressure and temperature
within
the reactor can be selected so that the water in the reactor substantially
does not
undergo a phase change (e.g., completely does not undergo a phase change). In
a phase
diagram for water, the critical point is located at a temperature of about 374
C and a
pressure of about 22 MPa. At temperature and pressure combinations beyond this
point
in the phase diagram, water does not experience a phase transition between a
liquid
phase and a gaseous phase. Instead, beyond the critical point, water behaves
as a single
fluid phase. Thus, in some embodiments, the combination of pressure and
temperature
can be selected so that the liquid water in the reactor remains the stable
phase until
conditions beyond the critical point are achieved. One way of satisfying this
condition
can be to select reaction temperatures and pressures that are less than the
critical point
and thus that do not lead to a phase transition. Note that in some
embodiments, a
partial pressure of additional gas can be introduced into the reactor (in
which case,
some minimal amount of water may become vapor, but this situation is
contemplated in
the invention not to be a "substantial" phase change). If the partial pressure
of
additional gas is greater than about 22 MPa, then the pressure is already
beyond the
critical point for water and substantially no phase transition is possible.
Note also that,
in a closed reactor, e.g., which can have a partial pressure of another gas,
substantial
phase transitions of water are not likely to occur, so long as the volume of
liquid water
is sufficient relative to the volume of the reactor.
[0041] Additionally or alternately, the pressure within a reactor can be
set by
selecting a temperature for the water. In some embodiments, the reactor can be
sealed
or closed after introduction of water and any additional gases, if present. A
partial
pressure of water vapor should develop in the reactor to correspond to the
temperature
of the water in the reactor. As the temperature of the reactor increases, a
corresponding
higher partial pressure of water should develop in the reactor. The
hydrothermal
processing can be performed at a pressure that represents the combination of
the partial
pressure of water at the reaction temperature and the partial pressure of any
additional
inert and/or reducing gases, as well as the partial pressure of any gases
generated or
evolved during processing. Examples of water partial pressures at various
temperatures
can include about 0.01 MPa at about 50 C; about 0.05 MPa at about 80 C; about
0.1
MPa at about 100 C; about 0.5 MPa at about 150 C; about 1.6 MPa at about 200
C;
CA 02821241 2013-06-11
WO 2012/082377
PCT/US2011/062608
- 15 -
about 4.0 MPa at about 250 C; about 5.9 MPa at about 275 C; about 8.6 MPa at
about
300 C; about 16.5 MPa at about 350 C; and about 22.1 MPa at about 374 C.
Because
about 22.1 MPa and about 374 C corresponds to the critical point in the phase
diagram
for water, it is not meaningful to refer to the partial pressure of "water
vapor" in a
reactor at temperatures beyond that point.
[0042] In some embodiments, the hydrothermal processing can be performed in
a
continuous flow type reactor. An example of a continuous flow type reactor can
be a
pipe or other conduit that can be heated to raise the temperature of the feed
in the
conduit to the desired hydrothermal processing temperature. For example, a
conduit
passing through a furnace could be used, and/or a conduit surrounded by steam.
The
conduit can have any convenient shape for passing through the heating zone.
For
example, a conduit having the shape of a spiral can be used to increase the
size of the
portion of the conduit within the heating zone.
[0043] It has been noted that the amount of water needed in order to
perform
hydrothermal processing may not be sufficient to provide the type of flow
characteristics desired for a continuous flow environment. In a continuous
flow
processing environment, one option for improving the fluid flow
characteristics of the
algae can be to increase the water content of the algae feed. However,
increasing the
water content can also result in a corresponding decrease in the yield per
volume of the
reaction system, due to the reduction in the amount of algae in the feed.
[0044] FIG. 1 schematically shows an example of a reactor suitable for use
in an
embodiment of the invention. In FIG. 1, hydrothermal processing reactor 100
can
represent any type of reactor suitable for performing a catalytic hydrothermal
process
for treatment of an algae (or other biomass) feed. Input flows into reactor
100 can
include a gas input 102, such as an inert gas input, a hydrogen gas input,
another type
of reducing gas input, or a combination thereof Another input flow can be an
algae or
biomass input 104. If algae input 104 has poor flow properties, such as due to
a
sufficiently low water content, algae input 104 may alternately represent a
non-flow
input, such as extrusion, pouring, or dumping of the algae input 104 into
reactor 100.
Optionally, a supplemental input flow 105 can be provided for various reasons.
One
option for a supplemental input flow 105 can be to include additional water,
so that
hydrothermal processing conditions can be maintained. An additional or
alternate
CA 02821241 2013-06-11
WO 2012/082377
PCT/US2011/062608
- 16 -
component for supplemental input flow 105 can be an "inert" hydrocarbon stream
(that
can undergo minimal reaction under hydrothermal processing conditions) and/or
a
product recycle stream. Such a hydrocarbon stream and/or recycle stream could
be
used as a carrier for a catalyst or a catalyst precursor. As an alternative,
algae input 104
and supplemental input 105 can be combined into a single stream prior to
entering the
reactor 100. The hydrothermal treatment can generate an output flow 107, e.g.,
which
can be a mixture of various phases. Phases that can comprise output flow 107
can
include a gas phase, a hydrocarbon based phase, an aqueous based phase, and
one or
more solid phases. These phases may optionally be mixed with each other, such
as
mixing of the solids with the aqueous phase.
Catalyst for Catalytic Hydrothermal Processing
100451 Another option during processing can be the use of a hydrothermal
processing catalyst. A hydrothermal processing catalyst can be in a form that
is soluble
in the hydrothermal reaction environment (or in at least one feed introduced
thereinto),
or the catalyst can be in the form of catalyst particles in the hydrothermal
reaction
environment. Catalyst particles in the reaction environment can have any
suitable
particle size and/or particle size distribution. The catalyst particles can
optionally be a
supported catalyst, with a catalytic material supported on a substrate.
100461 In an embodiment involving a catalyst that is soluble in the
hydrothermal
reaction environment, the catalyst can be introduced into the reaction either
as a
catalyst or a catalyst precursor. The soluble catalyst can be soluble in water
or in
another solvent introduced into the hydrothermal reaction environment.
Examples of
solvents can include but are not limited to alcohols, acids, hydrocarbons, or
other oils.
Additionally or alternately, the solvent can correspond to a product that is
generated by
the hydrothermal treatment process. Examples of suitable catalysts or catalyst
precursors can include, but are not limited to, transition metal salts such as
metal
acetates, metal carbonates, metal acetyl acetonates, or combinations thereof.
Examples
of suitable metals for such metal salts can include, but are not limited to,
Cr, V, Mo, Ni,
Cu, Fe, Co, Mn, and a combination thereof. Additionally or alternately, a
suitable
metal can include a Group VIB metal or a Group VIII metal, or a combination of
one or
more Group VIB metals and one or more Group VIII non-noble metals. Further
additionally or alternately, a catalyst precursor can be activated to form a
metal sulfide
CA 02821241 2013-06-11
WO 2012/082377
PCT/US2011/062608
- 17 -
by introducing a sulfur-containing stream into the reaction environment, such
as a
stream of H2S.
[0047]
Relative to the amount of algae, the amount of metal in a soluble catalyst or
catalyst precursor in the reactor (reaction zone) can be at least about 0.01
wt% (100
wppm), for example at least about 0.05 wt%, at least about 0.1 wt%, at least
about 0.25
wt%, or at least about 0.5 wt%. Additionally or alternately, the amount of
catalyst in
the reactor (reaction zone) can be about 5.0 wt% or less relative to the
amount of algae,
for example about 3.0 wt% or less, about 2.0 wt% or less, about 1.0 wt% or
less, about
0.5 wt% or less, or about 0.25 wt% or less.
[00481 Aside
from the soluble catalyst option, a supported catalyst can be used
including a noble metal (e.g., Pt, Pd, Rh, Ru, Ir, or a combination thereof).
Additionally or alternately, the support for the catalyst can be a
hydrothermally stable
support. Examples of suitable supports can include, but are not limited to,
refractory
oxides such as titania and/or zirconia; silica; activated carbon; carbon on
which is
deposited one or more metals selected from titanium, zirconium, vanadium,
molybdenum, manganese, and cerium; magnesium oxides; hydrotalcites; other
various
types of clays; and combinations thereof, such as a mixture of two or more of
titania,
zirconia, and silica.
Additionally or alternately, the support material can be
substantially free of alumina. As used herein, "substantially free" of alumina
should be
understood to mean less than 1 wt% alumina, preferably less than 0.1 wt%
alumina, for
example less than 0.01 wt% of alumina, completely no added alumina, or
completely
no alumina.
[0049] Still
another catalyst option can be to use a basic metal or mixed metal oxide
with or without a noble metal. Examples of such catalysts without a noble
metal can
include, but are not limited to, magnesium oxide, hydrotalcites, potassium
supported on
titania and/or zirconia, and combinations thereof.
[0050] Yet
another catalyst option can be to use hydroprocessing type metals
supported on a suitable support. Examples of hydroprocessing type metals can
include,
but are not limited to, a combination of a Group VIII metal (such as Co and/or
Ni) with
a Group VIB metal (such as Mo and/or W). Combinations of three or more Group
VIII
and/or Group VI metals can additionally or alternately be used (e.g., NiMoW,
CoNiMo,
CoMoW, and the like). Suitable support materials include those identified
hereinabove.
CA 02821241 2013-06-11
WO 2012/082377
PCT/US2011/062608
- 18 -
[00511 Yet another catalyst option can be to select a catalyst that
includes
biocompatible materials. For example, a biocompatible material can be a
material that
can serve as a nutrient for growth of biomass, such as algae, and/or a
material that does
not harm a biomass growth environment at the concentrations of the material
used for
the hydrothermal treatment. A biocompatible catalyst can optionally include a
biocompatible support. Examples of suitable metals in biocompatible catalysts
can
include K, Na, Mg, Ca, Fe, Zn, Mn, Mo, Cu, and combinations thereof. The
biocompatible catalysts can be in the form of a hydroxide, oxide, carbonate,
or an
organometallic derivative such as an acetate or acetylacetonate (acac).
Additionally or
alternately, the catalyst can be impregnated on a support such as activated
carbon. The
biomass being processed, such as algae, can alternatively serve as a support
for the
catalyst. In some embodiments, these biocompatible catalyst materials can be
recycled
either as a nutrient feed for biomass growth or as an input into the
hydrothermal
treatment reaction.
[0052] Relative to the amount of algae, the amount of catalyst in the
reactor
(reaction zone) can be at least about 0.05 wt%, for example at least about 0.1
wt%, at
least about 1 wt%, at least about 2.5 wt%, or at least about 5 wt%.
Additionally or
alternately, the amount of catalyst in the reactor (reaction zone) can be
about 20 wt% or
less relative to the amount of algae, for example about 15 wt% or less or
about 10 wt%
or less.
[0053] The amount of metal supported on the catalyst can be varied.
Relative to
the weight of the catalyst, the amount of noble metal supported on the
catalyst, when
present, can be at least about 0.1 wt%, for example at least about 0.5 wt%, at
least
about 0.6 wt%, at least about 0.75 wt%, or at least about 1.0 wt%, based on
the total
catalyst weight. Additionally or alternately, the amount of noble metal
supported on
the catalyst, when present, can be about 1.5 wt% or less, for example about
1.0 wt% or
less, about 0.75 wt% or less, or about 0.6 wt% or less, based on the total
catalyst
weight. More generally, the amount of metal(s), individually or in mixtures,
on the
catalyst support can be at least about 0.1 wt%, for example at least about
0.25 wt%, at
least about 0.5 wt%, at least about 0.6 wt%, at least about 0.75 wt%, at least
about 1
wt%, at least about 2.5 wt%, or at least about 5 wt%, based on the total
catalyst weight.
Additionally or alternately, the amount of metal(s), individually or in
mixtures, on the
CA 02821241 2013-06-11
WO 2012/082377
PCT/US2011/062608
- 19 -
catalyst support can be about 35 wt% or less, for example about 20 wt% or
less, about
15 wt% or less, about 10 wt% or less, or about 5 wt% or less, based on the
total catalyst
weight.
[0054] Use of a catalyst can present additional issues for hydrothermal
processing.
For a catalyst or catalyst precursor that is initially soluble in the reaction
environment,
one issue can be separation of the catalyst from the reaction products. One
separation
method can be filtration. If the catalyst is not soluble in the reaction
products, the
resulting catalyst particles can be filtered out of the product with which the
catalyst
particles are mixed. One reason the catalyst may be insoluble in the reaction
products
is if the catalyst has been converted to another form, such as conversion of a
catalyst
precursor to a metal sulfide.
[0055] Supported (or particulate) catalysts can also present additional
considerations. Additionally or alternately, the particle size for the
catalyst particles
can be varied, e.g., selected to facilitate separation of the catalyst
particles from other
solids. In such an embodiment, the catalyst particles can have an average
particle size
of at least about 1000 pm, for example at least about 1500 pm or at least
about 2000
pm. To achieve a desired catalyst particle size, catalysts can optionally be
formulated
to include a hydrothermally stable binder material, in addition to the support
material
and any active metals, if present. Suitable hydrothermally stable binder
materials can
be similar to materials used as a support material and/or can include, but are
not
necessarily limited to, an oxide of one or more metals selected from silicon,
titanium,
zirconium, vanadium, molybdenum, manganese, and cerium. For a supported
catalyst
that is formulated with a binder, the support material can function as a
binder, or a
different material can be used as a binder.
[0056] Supported catalysts can be contacted with a feed under hydrothermal
processing conditions using a variety of reactor types. Batch or semi-batch
reactors as
described above can be used with a particulate catalyst. For example, the
catalyst can
be added to such reactors when the algae, water, and other optional gases are
added to
the reactor. A continuous flow conduit can additionally or alternately be
used. In this
type of embodiment, the flow through the conduit may resemble a slurry of
catalyst
particles suspended in the flow of algae and water.
CA 02821241 2013-06-11
WO 2012/082377
PCT/US2011/062608
- 20 -
[0057] In addition to the reactors suitable for non-catalytic treatment,
other types of
continuous flow reactors can potentially be used for hydrothermal treatment of
an algae
feed, such as a fixed bed reactor, a moving bed, an ebullating bed reactor, or
the like. If
a fixed bed reactor is used, one concern could be fouling of the catalyst bed,
e.g., due to
solids present in the biomass or algae feed. Fouling of a catalyst bed can
result in a
higher than expected pressure drop across a catalyst bed, due to restrictions
in flow of
feed through the bed. Fixed bed reactors can often handle feeds with particle
sizes up
to about 150 p.m without significant fouling issues. Nevertheless, any fouling
of a
catalyst bed can be somewhat mitigated, e.g., by having bypass tubes to
control the
pressure drop across the catalyst bed. Unfortunately, although individual
algae cells
have small diameters, relative to 150 p.m, hydrothermally treated algae can
have an
increased tendency to agglomerate. As a result, 5% or more of the algae based
solids
resulting from hydrothermal treatment of an algae feed can be in the form of
agglomerated particles with a particle size greater than 150 gm. Nevertheless,
in some
embodiments, a fixed bed reactor may be used, particularly when agglomerative
behavior of the product algae solids can be mitigated, e.g., by using a
sufficient space
velocity and/or through other means.
[0058] As an alternative to a fixed bed reactor, an ebullating bed reactor
can be
used for hydrothermal processing. In a conventional ebullating bed reactor,
both the
feedstock (water and algae) and a treat gas (hydrogen-containing reducing gas)
can be
introduced into the reactor from the bottom of the reactor. In such reactors,
a recycled
feed containing a portion of the reactor effluent can also be introduced into
the bottom
of the reactor. These feed flows can travel up into the reactor and pass
through a
catalyst support grid designed to prevent catalyst from entering the areas at
the bottom
of the reactor where the feed pumps are located. The catalyst in such
ebullating bed
reactors is typically located above the catalyst support grid.
100591 When the feedstock (and optionally additional gas) flow(s) reach the
catalyst bed, the bed generally becomes fluidized, leading to expansion of the
bed as
well as mixing within the bed. The feed (and hydrogen) can react within the
bed to
form products, including liquid products, solid products, and gaseous
products. The
flow in a conventional ebullating bed reactor can continue upward until an
effluent is
drawn off at the top. This effluent can be a combination of desired products,
unreacted
CA 02821241 2013-06-11
WO 2012/082377
PCT/US2011/062608
- 21 -
hydrogen (when present), and byproduct gases, including contaminant gases such
as
H2S or NH3 that may have formed during the reaction. In preferred embodiments,
a
portion of the liquid effluent can be recycled, e.g., to the bottom of the
reactor. If
desired, the gases can be separated from the liquid portion of the effluent.
Phosphorus Content in Solids Fraction
100601 Additionally or alternately to recovery of a hydrocarbon product,
recovery
of other algae solids (or other biomass solids) can be beneficial. For
example,
phosphorus can be recovered from the residual algae solids after hydrothermal
treatment. One potential use for recovered phosphorus can be as a nutrient for
growth
of additional algae or other biomass.
100611 Improving the recovery of phosphorus from hydrothermal processing of
biomass can involve balancing several factors. One benefit of various
embodiments
can be that phosphorus forms a solid product, e.g., that can be filtered out
from the
liquid product streams. Any phosphorus that remains as part of the liquid
hydrocarbon
product and/or any phosphorus that becomes solublized in a solvent could be
recovered
in one or more separate, additional processes. In the discussion below, the
recovery of
phosphorus from products of hydrothermal treatment can be evaluated based on
the
amount of phosphorus recovered as solids.
[0062] Because the recovery of phosphorus can be evaluated based on the
amount
of phosphorus in the solids product, an initial goal can be to develop
processing
conditions that result in a large percentage of phosphorus in the solids
product. One
conventional way of processing a biomass feed, such as an algae feed, can be
to extract
a desired hydrocarbon product from the feed using an extraction solvent (e.g.,
such as a
mixture of CHC13 and CH3OH). An extraction solvent can advantageously produce
yields of phosphorus in the solids product of greater than 90 wt% relative to
the amount
of phosphorus in the feed. For an efficient phosphorus recovery process, it
can be
desirable to have a phosphorus yield in the solids product, relative to the
feed
phosphorus content, of at least 80 wt%, for example at least 85 wt% or at
least 90 wt%.
[0063] One option for improving the yield of phosphorus in the solids
product can
be to increase the amount multivalent cations in the hydrothermal reaction.
Many
biomass feeds can contain at least some multivalent cations, such as Ca, Mg,
and/or Fe.
These multivalent cations can form phosphates or other phosphorus solids as
part of the
CA 02821241 2013-06-11
WO 2012/082377
PCT/US2011/062608
- 22 -
solids product. For some feeds, increasing the amount of available multivalent
cations
may increase the amount of phosphorus in the solids product, such as by adding
extra
cations selected from Ca, Mg, Fe, Al, or a combination thereof In some such
embodiments, sufficient multivalent cations can be added to provide at least
about a 1:1
molar ratio of multivalent cations to phosphorus atoms. This can correspond to
adding
at least about 0.1 wt%, for example at least about 0.2 wt% or at least about
0.3 wt% of
a multivalent metal. Additionally or alternately, the amount of added
multivalent metal
can be about 1.0 wt% or less, for example about 0.8 wt% or less, about 0.6 wt%
or less,
or about 0.5 wt% or less. Note that the amount of multivalent metal can be
reduced in a
feed that already contains some multivalent metal.
[0064] Another consideration in selecting conditions for hydrothermal
processing
can be the relative amount of phosphorus in the solids product. As noted
above,
solvent extraction can produce a solids product that has greater than 90 wt%
of the
initial phosphorus in the feed. Unfortunately, such conventional solvent
processing can
also result in a relatively large amount of carbonaceous solids, e.g., in
which product
phosphorus can be present in amounts as low as 5 wt% or below. This can
present a
number of problems. First, additional processing can be required to extract
the
phosphorus from the much larger proportion of carbon solids and/or other
solids.
Another problem can be that relatively high carbon content in the solids
product can
increase the difficulty of using/selling the solids for an economically
valuable purpose.
To say it another way, a large proportion of carbon in the solids product can
mean that
a noticeable amount of carbon may be lost, rather than being converted into a
desired
product.
[0065] The amount of phosphorus recovered in the solids product relative to
carbon
can depend in part on the reaction conditions. Without being bound by any
particular
theory, it is believed that relatively low severity reaction conditions can
lead to
incomplete reaction of the biomass feed. This can result in algae (or other
biomass)
solids that are unreacted and/or only partially reacted. The algae is
initially solid, so
unreacted and/or partially reacted algae can still be a solid after an
incomplete reaction.
The unreacted and/or partially reacted algae can thus add to the carbon
content of the
solids product, which can therefore reduce the ratio of phosphorus to carbon.
It is
CA 02821241 2013-06-11
WO 2012/082377
PCT/US2011/062608
- 23 -
noted that incomplete reaction may additionally or alternately lead to a
reduction in the
amount of phosphorus in the solids relative to the initial amount of
phosphorus.
100661 Also without being bound by theory, it is believed that reaction
conditions
that are too severe may lead to increased carbon in the solids product.
Hydrothermal
processing of biomass feeds can lead to increased production of some heavier
molecules, including aromatics. A portion of these heavier molecules can
correspond
to insoluble compounds that tend to form solids. These additional solids can
thus
contribute to lowering the ratio of phosphorus to carbon in the solids
products.
[0067] In some embodiments, the hydrothermal processing temperature can be
selected to improve the ratio of phosphorus to carbon in the solids product.
For
example, the reaction temperature can, in on embodiment, range from about 275
C to
about 325 C. Additionally or alternately in catalytic hydrothermal processing
embodiments, the presence of catalyst can reduce the processing temperature
that leads
to an increase in the ratio of phosphorus to carbon in the solids product. In
such
embodiments, the reaction temperature can range from about 250 C to about 300
C.
[0068] Additionally or alternately, improving the ratio of phosphorus to
carbon in
the solids product for hydrothermal processing, either in the presence or
absence of a
catalyst, can be based on a combination of processing temperature and reaction
time.
For example, for a processing time of about 60 minutes to about 105 minutes,
the
reaction temperature can be about 250 C to about 300 C. For a processing time
of
about 45 minutes to about 90 minutes, the reaction temperature can be about
275 C to
about 325 C. For a processing time of about 30 minutes to about 60 minutes,
the
reaction temperature can be about 285 C to about 335 C. For a processing time
of
about 24 minutes to about 48 minutes, the reaction temperature can be about
300 C to
about 350 C. For a processing time of about 15 minutes to about 30 minutes,
the
reaction temperature can be about 325 C to about 375 C. For a processing time
of
about 6 minutes to about 24 minutes, the reaction temperature can be about 350
C to
about 400 C.
[0069] Further additionally or alternately, improving the ratio of
phosphorus to
carbon in the solids product for catalytic hydrothermal processing can be
based on a
combination of processing temperature and reaction time. For example, for a
processing time from about 60 minutes to about 105 minutes, the reaction
temperature
CA 02821241 2013-06-11
WO 2012/082377
PCT/US2011/062608
- 24 -
can be from about 225 C to about 275 C; for a processing time from about 45
minutes
to about 90 minutes, the reaction temperature can be from about 250 C to about
300 C;
for a processing time from about 30 minutes to about 60 minutes, the reaction
temperature can be from about 275 C to about 325 C; for a processing time from
about
24 minutes to about 48 minutes, the reaction temperature can be from about 285
C to
about 335 C; for a processing time from about 15 minutes to about 30 minutes,
the
reaction temperature can be from about 300 C to about 350 C; and for a
processing
time from about 6 minutes to about 24 minutes, the reaction temperature can be
from
about 325 C to about 375 C. It is noted that, in a continuous reaction
environment, a
reaction time can more accurately be described in terms of a residence time or
a space
velocity.
Separation of Products from Catalytic Hydrothermal Processing
[0070] Hydrothermal processing can result in a multi-phase product. The
multi-
phase product can include a gas phase, a hydrocarbon or oil phase, and an
aqueous
phase that can include solids. The gas phase, oil phase, aqueous phase, and
solids
phase can be separated from each other by any convenient method, such as by
use of a
three phase separator. Characterization of the oil phase is described further
below. In
some embodiments, the solids phase can initially be together with aqueous
phase. For
example, the solids phase can be suspended in the aqueous phase or can be a
precipitate
slurried in and/or settling out of the aqueous phase. The solids phase can
also be
valuable, containing one or more of: phosphorus and other potential nutrients
for algae
and/or other microorganisms; unreacted and/or only partially reacted biomass;
and
optionally catalyst particles if the process is a catalytic hydrothermal
process; inter alia.
In some embodiments, the catalyst particles can be separated from the other
solids to
allow for their recycle, as well as for recycle of the nutrients, if present.
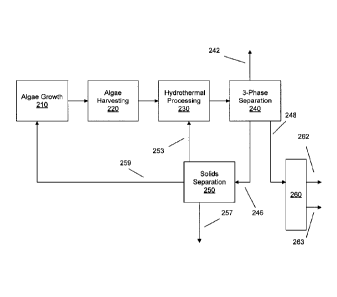
[0071] FIG. 2 shows a schematic example of a processing flow for an
embodiment
of the invention involving algae as the form of biomass for processing. In
FIG. 2, an
integrated scheme is shown where products from the (optionally catalytic)
hydrothermal processing are recycled for further use. In FIG. 2, the biomass
input for
the hydrothermal processing can be from an algae source. This algae can be
produced
by an algae growth process 210, which can include any convenient and/or known
process. The algae can be harvested 220 for conversion into hydrocarbon
products. As
CA 02821241 2013-06-11
WO 2012/082377
PCT/US2011/062608
- 25 -
part of algae harvesting 220, some amount of water can optionally be removed
from the
algae. For example, water can be completely removed from the algae as part of
production of freeze-dried algae. Alternately, water can be removed using only
physical processes, such as by centrifuge, which can advantageously result in
an algae
feed with a water to algae weight ratio of about 10:1 or less, for example
about 7.5:1 or
less, or about 5:1 or less. Additionally or alternately, the water to algae
weight ratio
can be at least about 2:1, for example at least about 2.5:1, or at least about
3:1. One
advantage of performing only a partial separation of algae and water can be
that less
energy is needed to perform only a partial separation, as compared to complete
separation.
[0072] After harvesting, the harvested algae can be used as a feed for
hydrothermal
processing 230. The algae feed can be optionally combined with a catalyst, a
partial
pressure of gas such as hydrogen, and optionally water, e.g., if sufficient
water is not
included with the algae feed. The hydrothermal processing 230 can generate a
variety
of products. An initial separation of these products can be performed in three-
phase
separator 240. Three-phase separator 240 can be used to generate a gas phase
product
242, a hydrocarbon or oil product 248, and a product including water and
various solids
246. The gas phase product 242 can include hydrogen, inert gases that may have
been
present during hydrothermal processing 230, product gases from the
hydrothermal
processing 230 (such as CO2, CO, H2S, NH3, and the like, and combinations
thereof),
and low boiling hydrocarbons produced during catalytic hydrothermal processing
230.
The low boiling hydrocarbons can include hydrocarbons that are gases at room
temperature (such as methane, ethane, or the like, or combinations thereof)
and/or
hydrocarbons that are gases at the temperature of the three-phase separation.
If the
three-phase separation is performed at an elevated temperature, this could
include
higher boiling aliphatic hydrocarbons and/or other species (such as methanol).
Note
that some of the above products may be at least partially solublized in the
water phase,
such as the product gases from the hydrothermal processing.
[0073] In the products from hydrothermal processing 230, the desired
hydrocarbon
or oil product can form a phase separate from an aqueous phase containing
various
solids. These distinct phases can be separated in three-phase separation 240.
The
resulting hydrocarbon product 248 can represent the desired oil product from
the
CA 02821241 2013-06-11
WO 2012/082377
PCT/US2011/062608
- 26 -
catalytic hydrothermal treatment. The hydrocarbon product 248 may, if desired,
undergo a variety of additional processing, which can include an optional
distillation
260 to isolate desired boiling ranges 262 and 263 of the product and/or
hydroprocessing
to upgrade the hydrocarbon product 248 or a distillation cut 262 or 263 for
use.
Additionally or alternately, at least a portion of hydrocarbon product 248
and/or of
distillation cut(s) 262 and/or 263 may optionally be recycled to hydrothermal
processing 230, e.g., for combination with the algae/water input feed, which
may
improve the input feed flow characteristics.
[0074] In some embodiments, the water and solids 246 from the three-phase
separation 240 can include several types of solids, which can include but are
not limited
to solids derived from the algae, solids comprising phosphorus and/or various
metals,
unreacted and/or partially reacted biomass, and optionally catalyst particles,
including
spent catalyst particles. The water and solids 246 can be further processed in
solids
separation 250 to separate the solids for further use. Solids separation 250
can generate
an aqueous stream 257, optional catalyst particles 253, and algae-derived
solids 259.
Note that separation of the optional catalyst particles from the algae-derived
solids may
occur prior to separation of the aqueous phase from the solids. In a preferred
embodiment, the optional catalyst particles 253 can be returned to the
catalytic
hydrothermal processing for further use. Additionally or alternately, the
algae-derived
solids 259 can be returned to the algae growth process 210, e.g., as raw
material for
developing a new batch of algae feed. Further additionally or alternately, at
least a
portion of aqueous stream 257 and/or of the water from water and solids 246
can be
recycled to the algae growth process 210, e.g., to provide additional
nutrients such as
nitrogenated species (like NH3).
[0075] Although the scheme in FIG. 2 implies a series of processes located
together, the algae growth 210 and harvesting 220 could take place at a
location remote
from the catalytic hydrothermal processing 230. In such an embodiment, several
of the
arrows in FIG. 2 could represent transport steps, such as transport of the
harvested
algae to the location for catalytic hydrothermal processing and transport of
the algae-
derived solids to the algae growth site.
CA 02821241 2013-06-11
WO 2012/082377
PCT/US2011/062608
- 27 -
Processina of Product Solids for Recycle of Nutrients
[0076] As noted above, some of the product solids can be recycled for use
as
nutrients for growth of further algae or other biomass. An example of this
type of
recycle can be recycling of phosphorus compounds. In order to recycle the
phosphorus,
the phosphorus can be converted from the solid form into a precursor form that
can be
readily processed into a suitable nutrient. An example of this type of
conversion can be
conversion of phosphorus in the product solids into a more easily
distributable form,
such as phosphoric acid. The phosphoric acid can then be used either as a
nutrient, or
as a precursor or reagent to make a suitable nutrient.
[0077] Phosphorus can be contained in the product solids in a variety of
forms,
such as phosphates and/or phosphites, and may be coordinated by Ca, Mg, or
other
multivalent cations. The solids can also contain carbon compounds. In order to
separate the phosphorus from the carbon, the phosphorus in the solids can, in
one
embodiment, be converted to phosphoric acid. Conversion of phosphorus to
phosphoric acid is a known reaction, and can be performed by treating the
phosphorus
containing solids with sulfuric acid. The sulfuric acid can react with the
phosphorus to
form phosphoric acid. The sulfate ions from the sulfuric acid can combine with
Ca or
Mg cations and precipitate out. In such situations, the carbon may remain as
additional
solid product. The sulfate solids and carbon can be separated from the
phosphoric acid
by physical and/or known/conventional means, e.g., using filtration or a
settling pond.
Evaluation of Products from Hydrothermal Processing
[0078] Hydrothermal processing can be used to extract various hydrocarbon
fractions from an algae (or other biomass) feed. One example of a hydrocarbon
fraction that can be extracted from an algae feed can include and/or be a
distillate
fraction. In the discussion below, a distillate fraction refers to a fraction
that has a
boiling range between about 193 C and about 360 C, or alternately to a
fraction having
at least 90 wt% of its boiling range between about 193 C and about 360 C
(e.g., the T5
could be about 193 C and the T95 about 360 C, or the T2 could be about 193 C
and
the T98 about 360 C, or the like).
[0079] One way to evaluate the products of a hydrothermal treatment
process,
whether catalytic or non-catalytic, can be to consider the hydrocarbon yield
from the
process. A total yield can be defined for a hydrothermal treatment process
based on the
CA 02821241 2013-06-11
WO 2012/082377
PCT/US2011/062608
- 28 -
weight of hydrocarbon product captured relative to the initial weight of the
algae or
other biomass. A distillate yield can also be defined for a hydrothermal
treatment
process. One yield characterization can be the total distillate boiling range
yield for a
process relative to the starting weight of algae or biomass. Another
characterization
can be the percentage of distillate produced relative to the total hydrocarbon
yield.
[0080] An additional or alternate way to evaluate the products of a
hydrothermal
treatment process can be based on the levels of various impurities in the
products. In a
non-catalytic hydrothermal treatment process (or in a catalytic hydrothermal
process,
analyzed on a catalyst-free basis), the hydrocarbon products can tend to
incorporate
impurities such as nitrogen, oxygen, carbon-carbon double bonds, and aromatic
groups.
Thus, the percentage of heteroatoms (nitrogen and/or oxygen) in the total
hydrocarbon
product and/or the distillate product can be of interest. The percentage of
carbon-
carbon double bonds and aromatic groups can be measured using techniques such
as
13C NMR, and/or other metrics can be used such as the ratio of hydrogen to
carbon in
the products.
Additional Embodiments
[0081] Additionally or alternately, the present invention can include one
or more of
the following embodiments.
[0082] Embodiment 1. A method for hydrothermally processing biomass,
comprising: introducing a biomass feed having a water to biomass ratio of at
least 1:1
into a reaction zone, the biomass feed having a phosphorus content;
hydrothermally
treating the biomass feed under effective hydrothermal treatment conditions to
produce
a multi-phase product, the multi-phase product including a solids portion
containing at
least about 80% of the phosphorus content of the biomass feed; and separating
the
multi-phase product to produce at least a gas phase portion, a liquid
hydrocarbon
product, and the solids portion.
100831 Embodiment 2. A method for hydrothermally processing biomass,
comprising: adding a multivalent metal to a biomass feed having a phosphorus
content;
contacting the biomass feed with water in the presence of the multivalent
metal under
effective hydrothermal treatment conditions to produce a multi-phase product,
the
multi-phase product including a solids portion containing at least about 80%
of the
phosphorus content of the biomass feed; and separating the multi-phase product
to
CA 02821241 2013-06-11
WO 2012/082377
PCT/US2011/062608
- 29 -
produce at least a gas phase portion, a liquid hydrocarbon product, and the
solids
portion.
[0084] Embodiment 3. The method of embodiment 2, wherein the multivalent
metal comprises Ca, Mg, Fe, or a combination thereof, for example comprises Ca
and/or Mg.
[0085] Embodiment 4. The method of embodiment 2 or embodiment 3, wherein
the multivalent metal is added to the biomass feed in a reaction zone for the
contacting
of the biomass feed with water under effective hydrothermal conditions.
[0086] Embodiment 5. The method of any one of the previous embodiments,
wherein the biomass feed comprises algae.
[0087] Embodiment 6. A method for hydrothermally processing biomass,
comprising: contacting an algae-containing biomass feed having a phosphorus
content
with water under effective hydrothermal treatment conditions to produce a
multi-phase
product, the multi-phase product including a solids portion containing at
least about
80% of the phosphorus content of the algae-containing biomass feed; separating
the
multi-phase product to produce at least a gas phase portion, a liquid
hydrocarbon
product, and the solids portion; and recycling phosphorus from the solids
portion to an
algae growth environment.
[0088] Embodiment 7. The method of embodiment 6, wherein recycling
phosphorus from the solids portion comprises: extracting phosphorus from the
solids
portion to form a phosphorus based nutrient or nutrient precursor; and
introducing the
phosphorus based nutrient or nutrient precursor into the algae growth
environment.
[0089] Embodiment 8. The method of embodiment 7, wherein the phosphorus
based nutrient or nutrient precursor is phosphoric acid.
[0090] Embodiment 9. The method of any one of the previous embodiments,
wherein the weight ratio of water to algae is from about 2:1 to about 10:1,
for example
from about 3:1 to about 5:1.
[0091] Embodiment 10. The method of any one of the previous embodiments,
wherein the effective hydrothermal treatment conditions include a temperature
from
about 150 C to about 500 C, for example from about 250 C to about 375 C, and a
pressure from about 25 barg (about 2.5 MPag) to about 300 barg (about 30
MPag).
CA 02821241 2013-06-11
WO 2012/082377
PCT/US2011/062608
- 30 -
[0092]
Embodiment 11. The method of any one of the previous embodiments,
wherein the effective hydrothermal treatment conditions include hydrothermal
treatment in the presence of a catalyst, and wherein one of the following is
satisfied:
the processing time is from about 45 minutes to about 90 minutes when the
temperature
is from about 250 C to about 300 C; the processing time is from about 30
minutes to
about 60 minutes when the temperature is from about 275 C to about 325 C; or
the
processing time is from about 15 minutes to about 30 minutes when the
temperature is
from about 300 C to about 350 C.
[0093]
Embodiment 12. The method of any one of the previous embodiments,
wherein contacting the algae based feed with water under effective
hydrothermal
processing conditions substantially does not result in a phase change for the
water.
[00941
Embodiment 13. The method of any one of the previous embodiments,
further comprising separating the hydrocarbon liquid product to produce a
fraction
having at least 90% of its boiling range between about 193 C to about 360 C.
[0095]
Embodiment 14. The method of any one of the previous embodiments,
wherein the phosphorus to carbon molar ratio of the solids portion is at least
about 0.2,
for example at least about 0.25, and wherein the solids portion optionally
includes at
least about 90% of the phosphorus content of the biomass feed.
Examples of Phosphorus Recovery
[0096] A
series of experiments were performed to test phosphorus recovery from
conventional solvent processing of an algae feed and from hydrothermal
treatment of
an algae feed. A commercially available freeze-dried Nannochloropsis algae
sample
was used for the experiments.
[0097] For the
solvent processing, the solvent was a 50:50 mixture on a volume
basis of CHC13 and CH3OH. One part of the freeze-dried Nannochloropsis algae
was
combined with five parts of the CHC13/CH3OH solvent and vigorously stirred for
about
24 hours at room temperature (i.e., about 20-25 C). Two distinct phases were
apparent,
a first phase containing the solvent and a solublized product, and a second
phase
containing solid remnants suspended in and/or settled to the bottom of the
solvent. The
solids remnants were isolated and analyzed; the results of these
characterizations are
shown in Table 3 below.
CA 02821241 2013-06-11
WO 2012/082377
PCT/US2011/062608
-31 -
[0098] For the hydrothermal treatment experiments, samples of the freeze-
dried
algae were mixed with water in a ratio of about four parts water to one part
algae. The
algae and water mixture was placed in 316SS stainless steel ¨1-inch outer
diameter
reactors (Swagelok cap and plug). A nitrogen partial pressure of about 50 bar
(about
5.0 MPa) was added to the reactor. A separate catalyst was not added to the
reactor.
The reactor was placed into a pre-heated ebullated sandbath. The reactors
remained in
the sandbath for about 60 minutes. Thereafter, the reactors were removed from
the
sandbath and quenched to approximately room temperature. The hydrocarbon
products
were recovered using methylene chloride extraction and phase separation. In
the
experiments described below, the temperature of the sandbath (and therefore
the
reactor) was about 200 C, about 300 C, or about 350 C.
[0099] Table 3 shows examples of processing of algae samples using solvent
extraction and at the three hydrothermal processing temperatures. In the
table, the term
"phosphorus yield" refers to the weight percent of phosphorus from the initial
sample
that was contained in the solids product. Phosphorus concentration refers to
the weight
percent of phosphorus in the solids product. The P / C molar ratio refers to
the molar
ratio of phosphorus to carbon in the solids product. The phosphorus recovery
efficiency is a measure of the relative amounts of phosphorus and carbon in
the solids
product. The phosphorus recovery efficiency is defined as PfCCOV eff = Pyield
X [Pmoles
(Pmoles + Cmoles)]=
[00100] In Table 3, Column A shows the results from analysis of the product
solids
from the solvent extraction. Columns B, C, and D show the results from
analysis of the
solids fraction from the hydrothermal treatments at about 200 C, about 300 C,
and
about 350 C, respectively.
Table 1
A
(Solvent only) (200 C) (300 C) (350 C)
P Yield (%) 97 34 91 95
P Conc. (wt %) 1.55 2.16 30.8 21.8
P / C molar ratio 0.014 0.015 0.56 0.26
P recovery effic. (%) 1.3 0.5 32.5 19.8
CA 02821241 2013-06-11
WO 2012/082377
PCT/US2011/062608
- 32 -
[001011 As shown in Table 1, solvent extraction resulted in a relatively
high
phosphorus yield in the solids product of 97%. However, the solids product
also
included a large amount of other material, as shown by the overall weight
percentage of
phosphorus (1.55%). A large portion of this additional material was carbon, as
shown
by the phosphorus to carbon molar ratio (0.014). As a result, the phosphorus
recovery
efficiency, as defined above, was only 1.3%.
[00102] For the hydrothermal processing at about 200 C, the phosphorus yield
was
lower at about 34%. Because of the low initial recovery, and a relatively low
concentration of phosphorus in the solids, the phosphorus recovery efficiency
at about
200 C was less than 1%.
[00103] At the higher processing temperatures, the phosphorus recovery
efficiency
was notably higher. At both ¨300 C and ¨350 C, the phosphorus yield was
greater
than about 90%, indicating a good capture of the initial phosphorus in the
solids
product. Both the ¨300 C and ¨350 C experiments showed dramatically improved
phosphorus recovery efficiencies, relative to the solvent extraction. This was
due in
part to the lower carbon content of the solids product, as the phosphorus to
carbon
molar ratio at both ¨300 C and ¨350 C was greater than about 0.25.
[00104] Additionally, the experiment at about 300 C showed an unexpectedly
improved result even relative to the experiment at about 350 C. Although the
experiment at ¨300 C had a slightly lower phosphorus yield, the amount of
carbon and
other materials in the solids product was dramatically lower, as shown by the
¨30.8
wt% phosphorus concentration and the phosphorus to carbon molar ratio of
¨0.56.
Without being bound by any particular theory, it is believed that the
additional carbon
present in the solids product at ¨350 C may be due to excess reaction with the
feed. In
an embodiment, the additional improved phosphorus recovery efficiency shown
here at
a ¨300 C processing temperature can be maintained for other feeds and at other
reaction conditions by selecting reaction conditions that maintain a
phosphorus yield of
around 90%, such as a phosphorus yield from about 87% to about 93%.
[00105] The solids product generated by the experiment at ¨300 C was also
analyzed using X-ray diffraction (XRD). Compounds that could be identified
from the
XRD spectrum included phosphates and phosphites. Some compounds identified in
the
scan were Ca18Mg2H2(PO4)14; Ca288Fe32(PO4)2108,6; Mg(P03)2; Ca2P207; and
CaCO3.
CA 02821241 2013-06-11
WO 2012/082377
PCT/US2011/062608
- 33 -
Prophetic Example of Hydrothermal Processing
[00106] An algae feed is processed under hydrothermal treatment conditions in
a
continuous flow reaction system. The reaction zone for the hydrothermal
treatment
includes a coiled conduit surrounded by an oven. The coiling of the conduit
increases
the path length of the conduit within the oven. The flow rate within the
conduit is
selected so that feed has a residence time within the reaction zone of about
15 minutes.
The temperature in the reaction zone is about 350 C. The feed passing through
the
reaction zone includes a mixture of algae and water with a water to algae
weight ratio
from about 10:1 to about 2.5:1. The pressure in the conduit is determined in
part by the
vapor pressure of water at the reaction temperature. If an optional catalyst
is used (e.g.,
included with the feed), the pressure is also increased by the addition of
about 2.5 MPa
of hydrogen gas. After passing through the coiled conduit, the flow is passed
into a
separator. A gas phase product, a hydrocarbon product, an aqueous product, and
a
solids product are separated out. The solids product can have a phosphorus
content that
is at least 85% of the initial phosphorus content of the feed. The solids
product can also
have a phosphorus content that is at least about 20% of the total solids
product.