Note: Descriptions are shown in the official language in which they were submitted.
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
INHIBITORS OF INFLUENZA VIRUSES REPLICATION
CROSS REFERENCE TO RELATED APPLICATIONS
[00100] The present application claims priority under 35 U.S.C. 119 to
United States
Provisional Application No. 61/527;276, filed August 25, 2011, entitled
"INHIBITORS OF
INFLUENZA VIRUSES REPLICATION" and United States Provisional Application No.
61/423,925, filed December 16, 2010, entitled "INHIBITORS OF INFLUENZA VIRUSES
REPLICATION", the entire contents of which are incorporated herein by
reference.
BACKGROUND OF THE INVENTION
[00101] Influenza spreads around the world in seasonal epidemics, resulting
in the
deaths of hundreds of thousands annually - millions in pandemic years. For
example, three
influenza pandemics occurred in the 20th century and killed tens of millions
of people, with
each of these pandemics being caused by the appearance of a new strain of the
virus in
humans. Often, these new strains result from the spread of an existing
influenza virus to
humans from other animal species.
[00102] Influenza is primarily transmitted from person to person via large
virus-laden
droplets that are generated when infected persons cough or sneeze; these large
droplets can
then settle on the mucosal surfaces of the upper respiratory tracts of
susceptible individuals
who are near (e.g. within about 6 feet) infected persons. Transmission might
also occur
through direct contact or indirect contact with respiratory secretions, such
as touching
surfaces contaminated with influenza virus and then touching the eyes, nose or
mouth. Adults
might be able to spread influenza to others from 1 day before getting symptoms
to
approximately 5 days after symptoms start. Young children and persons with
weakened
immune systems might be infectious for 10 or more days after onset of
symptoms.
[00103] Influenza viruses are RNA viruses of the family Orthomyxoviridae,
wliich
comprises five genera: Influenza virus A, Influenza virus B, Influenza virus
C, Isavirus and
Thogoto virus.
[00104] The Influenza virus A genus has one species, influenza A virus.
Wild aquatic
birds are the natural hosts for a large variety of influenza A. Occasionally,
viruses are
transmitted to other species and may then cause devastating outbreaks in
domestic poultry or
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
give rise to human influenza pandemics. The type A viruses are the most
virulent human
pathogens among the three influenza types and cause the most severe disease.
The influenza
A virus can be subdivided into different serotypes based on the antibody
response to these
viruses. The serotypes that have been confirmed in humans, ordered by the
number of known
human pandemic deaths, are: H 1N1 (which caused Spanish influenza in 1918),
H2N2 (which
caused Asian Influenza in 1957), H3N2 (which caused Hong Kong Flu in 1968),
H5N1 (a
pandemic threat in the 2007-08 influenza season), H7N7 (which has unusual
zoonotic
potential), H 1N2 (endemic in humans and pigs), H9N2, H7N2 , H7N3 and H I ON7.
[00105] The Influenza virus B genus has one species, influenza B virus.
Influenza B
almost exclusively infects humans and is less common than influenza A. The
only other
animal known to be susceptible to influenza B infection is the seal. This type
of influenza
mutates at a rate 2-3 times slower than type A and consequently is less
genetically diverse,
with only one influenza B serotype. As a result of this lack of antigenic
diversity, a degree of
immunity to influenza B is usually acquired at an early age. However,
influenza B mutates
enough that lasting immunity is not possible. This reduced rate of antigenic
change,
combined with its limited host range (inhibiting cross species antigenic
shift), ensures that
pandemics of influenza B do not occur.
[00106] The Influenza virus C genus has one species, influenza C virus,
which infects
humans and pigs and can cause severe illness and local epidemics. However,
influenza C is
less common than the other types and usually seems to cause mild disease in
children.
[00107] Influenza A, B and C viruses are very similar in structure. The
virus particle
is 80-120 nanometers in diameter and usually roughly spherical, although
filamentous forms
can occur. Unusually for a virus, its genome is not a single piece of nucleic
acid; instead, it
contains seven or eight pieces of segmented negative-sense RNA. The Influenza
A genome
encodes 11 proteins: hemagglutinin (HA), neuraminidase (NA), nucleoprotein
(NP), Ml, M2,
NS1, NS2(NEP), PA, PB1, PB1-F2 and PB2.
[00108] HA and NA are large glycoproteins on the outside of the viral
particles. HA is
a lectin that mediates binding of the virus to target cells and entry of the
viral genome into the
target cell, while NA is involved in the release of progeny virus from
infected cells, by
cleaving sugars that bind the mature viral particles. Thus, these proteins
have been targets for
antiviral drugs. Furthermore, they are antigens to which antibodies can be
raised. Influenza
A viruses are classified into subtypes based on antibody responses to HA and
NA, forming
the basis of the H and N distinctions (vide supra) in, for example, H5N1.
[00109] Influenza produces direct costs due to lost productivity and
associated medical
-2-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
treatment, as well as indirect costs of preventative measures. In the United
States, influenza is
responsible for a total cost of over $10 billion per year, while it has been
estimated that a
future pandemic could cause hundreds of billions of dollars in direct and
indirect costs.
Preventative costs are also high. Governments worldwide have spent billions of
U.S. dollars
preparing and planning for a potential H5N1 avian influenza pandemic, with
costs associated
with purchasing drugs and vaccines as well as developing disaster drills and
strategies for
improved border controls.
[00110] Current treatment options for influenza include vaccination, and
chemotherapy
or chemoprophylaxis with anti-viral medications. Vaccination against influenza
with an
influenza vaccine is often recommended for high-risk groups, such as children
and the
elderly, or in people that have asthma, diabetes, or heart disease. However,
it is possible to
get vaccinated and still get influenza. The vaccine is reformulated each
season for a few
specific influenza strains but cannot possibly include all the strains
actively infecting people
in the world for that season. It takes about six months for the manufacturers
to formulate and
produce the millions of doses required to deal with the seasonal epidemics;
occasionally, a
new or overlooked strain becomes prominent during that time and infects people
although
they have been vaccinated (as by the H3N2 Fujian flu in the 2003-2004
influenza season). It
is also possible to get infected just before vaccination and get sick with the
very strain that
the vaccine is supposed to prevent, as the vaccine takes about two weeks to
become effective.
[00111] Further, the effectiveness of these influenza vaccines is variable.
Due to the
high mutation rate of the virus, a particular influenza vaccine usually
confers protection for
no more than a few years. A vaccine formulated for one year may be ineffective
in the
following year, since the influenza virus changes rapidly over time, and
different strains
become dominant.
[00112] Also, because of the absence of RNA proofreading enzymes, the RNA-
dependent RNA polymerase of influenza vRNA makes a single nucleotide insertion
error
roughly every 10 thousand nucleotides, which is the approximate length of the
influenza
vRNA. Hence, nearly every newly-manufactured influenza virus is a
mutant¨antigenic
drift. The separation of the genome into eight separate segments of vRNA
allows mixing or
reassortment of vRNAs if more than one viral line has infected a single cell.
The resulting
rapid change in viral genetics produces antigenic shifts and allows the virus
to infect new
host species and quickly overcome protective immunity.
[00113] Antiviral drugs can also be used to treat influenza, with
neuraminidase
inhibitors being particularly effective, but viruses can develop resistance to
the standard
-3-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
antiviral drugs.
[00114] Thus, there is still a need for drugs for treating influenza
infections, such as for
drugs with expanded treatment window, and/or reduced sensitivity to viral
titer.
SUMMARY OF THE INVENTION
[00115] The present invention generally relates to methods of treating
influenza, to
methods of inhibiting the replication of influenza viruses, to methods of
reducing the amount
of influenza viruses, to compounds and compositions that can be employed for
such methods.
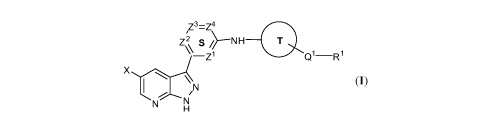
[00116] In one embodiment, the present invention is directed to a compound
represented by Structural Formula (I):
,Z3=Z4
z2 0
Qi
X
N N
or a pharmaceutically acceptable salt thereof, wherein:
X is -Cl, -Br, -F, -CN, -0(C1_4 alkyl), or Cl-C6 aliphatic optionally
substituted with
one or more instances of .11;
Z1, Z2, Z3, and Z4 are each and independently CR2 or N, provided that up to
three N
are selected for Z1, Z2, Z3, and Z4, and provided that when Z3 and Z4 are both
CR2, then Z1
and Z2 are not N at the same time;
Ring S is a 6-membered aromatic ring;
Ring T is a C3-C10 carbocycle optionally further substituted with one or more
instances ofJT;
Q1 is -C(0)-, -0O2-, -0C(0)-, -0(CR1Rs)k-C(0)0-, -C(0)NR'-, -C(0)N(R')-0-,
-C(0)NRC(0)0-, -NRC(0)-, -NRC(0)NR'-, -NRCO2-, -0C(0)NR'-, -0S02NR1-,
-S(0)-, -S02-, -SO2NR'-, -NRS02-, -NRSO2NR'-, -P(0)(0R)0-, -0P(0)(0Ra)0-,
-P(0)20-, -CO2S02-, -B(02)-, or -(CR`Rs)p-Y1--;
Y1 is -C(0)-, -0O2-, -0C(0)-, -0(CR`Rs)k-C(0)0-, -C(0)NR'-, -C(0)N(R')-0-,
-C(0)NRC(0)0-, -NRC(0)-, -NRC(0)NR'-, -NRCO2-, -0C(0)NR'-, -0S02NR1-,
-S(0)-, -S02-, -SO2NR'-,-NRS02-, -NRSO2NR'-, -P(0)(0R)0-, -0P(0)(0Ra)0-,
-P(0)20-, -B(02)-, or -0O2S02-;
R1 is: i) -H; ii) a C1-C6 aliphatic group optionally substituted with one or
more
instances ofJA; iii) a C3-C10 carbocyclic group or 4-10 membered heterocyclic
group, each
-4-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
optionally and independently substituted with one or more instances of j'3; or
iv) a 6-10
membered aryl group or 5-10 membered heteroaryl group, each optionally and
independently
substituted with one or more instances of Jc;
optionally RI, together with R' and the nitrogen to which they are attached,
form a 4-8
membered heterocyclic group optionally substituted with one or more instances
ofJ2; or
optionally -Q'-R' forms, together with Ring T, a 4-10 membered, non-aromatic,
spiro
ring optionally substituted with one or more instances of J4; and
R2 is -H, halogen, -CN, -NO2, -C(0)NH2, -C(0)NH(CH3),-C(0)N(CH3)2, or CI-C6
aliphatic optionally substituted with one or more instances ofJ1;
JA, JB, and JT are each and independently oxo or Jc;
Jc are each and independently selected from the group consisting of halogen,
cyano,
M, Ra, or Ra-M;
M is independently selected from the group consisting of ¨ORb, ¨SRb, -S(0)Ra,
¨SO2Ra, ¨NRbRc, ¨C(0)Ra, -C(=NR)Rc, -C(=NR)NRbR`, -NRC(=NR)NRbRc, ¨C(0)0Rb,
¨0C(0)R', ¨NRC(0)Rb, ¨C(0)NRbRc, ¨NRC(0)NRbRe, ¨NRC(0)0Rb, ¨000NRbRc,
-C(0)NRCO2Rb, -NRC(0)NRC(0)0Rb, -C(0)NR(ORb), -0S02NRbRc,
¨SO2NR`Rb, -NRSO2Rb, -NRSO2NR`Rb, -P(0)(0Rb)2, -0P(0)(0Rb)2, -P(0)20Rb and
-CO2S02Rb; or
optionally, two JT, two JA, two JB, and two Jc, respectively, together with
the atom(s)
to which they are attached, independently form a 4-10-membered ring that is
optionally
substituted with one or more instances of J4; and
Ra is independently:
i) a Cl-C6 aliphatic group optionally substituted with one or more
substituents selected from
the group consisting of halogen, cyano, hydroxy, oxo, -NH2, -NH(Ci-C4 alkyl), -
N(Ci-C4
alky1)2, -000(CI-C4 alkyl), -CO(CI-C4 alkyl), -CO2H, -0O2(CI-C4 alkyl), -0(CI-
C4 alkyl),
C3-C8 carbocyclic group optionally substituted with one or more instances
ofJ2, 4-8
membered heterocyclic group optionally substituted with one or more instances
ofJ2, 5-10
membered heteroaryl group optionally substituted with one or more instances of
J3, and 6-10
membered aryl group optionally substituted with one or more instances of J3;
ii) a C3-C8 carbocyclic group, or 4-8 membered heterocyclic group, each of
which is
optionally and independently substituted with one or more instances ofJ2; or
iii) a 5-10 membered heteroaryl group, or 6-10 membered aryl group, each of
which is
optionally and independently substituted with one or more instances ofJ3; and
-5-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
Rb and Rc are each independently Ra or ¨H; or optionally, Rb and Rc, together
with the
nitrogen atom(s) to which they are attached, each independently form a 4-8
membered
heterocyclic group optionally substituted with one or more instances of J2;
R' and Rs are each independently ¨H, halogen, or CI-C6 alkyl optionally
substituted
with one or more instances of JI, or optionally, R` and Rs, together with the
carbon atom to
which they are attached, form a cyclopropane ring optionally substituted with
one or more
instances of methyl;
R and R' are each independently ¨H or CI-C6 alkyl optionally and independently
substituted with one or more instances of J1, or optionally R and R', together
with the
nitrogen to which they are attached, form a 4-8 membered heterocyclic group
optionally
substituted with one or more instances of J2;
each J1 is independently selected from the group consisting of halogen, cyano,
hydroxy, oxo, -NH2, -NH(CI-Ca alkyl), -N(CI-Ca alky1)2, -000(CI-C4 alkyl), -
CO(Ci-C4
alkyl), -CO2H, -0O2(Ci-C4 alkyl), -0(Ci-C4 alkyl), and phenyl;
each J2 is independently selected from the group consisting of halogen, cyano,
hydroxy, oxo, -NH2, -NH(Ci-Ca alkyl), -N(CI-Ca alky1)2, -000(CI-C4 alkyl), -
CO(CI-C4
alkyl), -CO2H, -0O2(Ci-C4 alkyl), C1-C4 alkyl, CI-Ca haloalkyl, and -0(Ci-C4
alkyl);
each of .13 and J4 is independently selected from the group consisting of
halogen,
cyano, hydroxy, -NH2, -NH(Ci-C4 alkyl), -N(Ci-Ca -000(Ci-C4
alkyl), -CO(Ci-C4
alkyl), -CO2H, -0O2(Ci-C4 alkyl), CI-Ca alkyl, CI-Ca haloalkyl, and -0(CI-C4
alkyl);
p is independently 1, 2, 3 or 4; and
k is independently 1, 2, 3 or 4; and
provided that Q'-R' is not at the same carbon atom to which -NH group that is
attached to
Ring S is attached.
In some embodiments, p is independently 1 or 2; and k is independently 1 or 2.
[00117] In another
embodiment, the present invention is directed to a pharmaceutical
composition comprising a compound disclosed herein (e.g., a compound
represented by
Structural Formula (I) or a pharmaceutically acceptable salt thereof) and a
pharmaceutically
acceptable carrier, adjuvant or vehicle.
[00118] In yet
another embodiment, the present invention is directed to a method of
inhibiting the replication of influenza viruses in a biological sample or
patient, comprising the
step of administering to said biological sample or patient an effective amount
of a compound
disclosed herein (e.g., a compound represented by Structural Formula (I) or a
pharmaceutically acceptable salt thereof).
-6-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
[00119] In yet another embodiment, the present invention is directed to a
method of
reducing the amount of influenza viruses in a biological sample or in a
patient, comprising
administering to said biological sample or patient an effective amount of a
compound
disclosed herein (e.g., a compound represented by Structural Formula (I) or a
pharmaceutically acceptable salt thereof).
[00120] In yet another embodiment, the present invention is directed to a
method of
method of treating influenza in a patient, comprising administering to said
patient an effective
amount of a compound disclosed herein (e.g., a compound represented by
Structural Formula
(I) or a pharmaceutically acceptable salt thereof).
[00121] The present invention also provides use of the compounds described
herein for
inhibiting the replication of influenza viruses in a biological sample or
patient, for reducing
the amount of influenza viruses in a biological sample or patient, or for
treating influenza in a
patient.
[00122] Also provided herein is use of the compounds described herein for
the
manufacture of a medicament for treating influenza in a patient, for reducing
the amount of
influenza viruses in a biological sample or in a patient, or for inhibiting
the replication of
influenza viruses in a biological sample or patient.
[00123] Also provided here in are the compounds represented by Structural
Formula
(XX):
,Z3=z4
Z2 s
Q1 R1
X
or a pharmaceutically acceptable salt thereof, Without being bound to a
particular theory, the
compounds of Structural Formula (XX) can be used for synthesizing the compound
of
Formula (I). The variables of Structural Formula (XX) are each and
independently as
described herein; and G is trityl (Tr) (i.e., C(Ph)3 where Ph is phenyl).
[00124] The invention also provides methods of preparing a compound
represented by
Structural Formula (I) or a pharmaceutically acceptable salt thereof. In one
embodiment, the
= Z3"Z4
QiRi
y"--Z1
L2
method comprises the steps of: i) reacting compound A: (A) with
-7-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
o
X
I \ N
compound (B) : ç (B)to form a compound represented by Structural Formula
(XX); and
ii) deprotecting the G group of the compound of Structural Formula (XX) under
suitable
conditions to form the compound of Structural Formula (I),wherein: the
variables of
Structural Formulae (I) and (XX), and compounds (A) and (B) are each
independently as
described herein; L2 is a halogen (such as CI, Br, or I); and G is trityl. In
another
embodiment, the method comprises the steps of: i) reacting compound (K) or
(L):
z3=Z4 z3=Z4
2 =0
Z \
rx_r Z1
NarZ 1
X X
I \ I \
N N N N H2
(K), or (I-) with compound (D): 01¨R1
under suitable conditions to form a compound represented by Structural Formula
(XX); and
ii) deprotecting the G group of the compound of Structural Formula (XX) under
suitable
conditions to form the compound of Structural Formula (I),wherein: the
variables of
Structural Formulae (I) and (XX), and compounds (K), (L), and (D) are each and
independently as described herein; and G is trityl. In another embodiment, the
method
comprises the steps of: i) reacting Compound (G) with Compound (D):
73=
Zµ,2 s5 L1
X
N N H2 411
Q1____R 1
(G) (D)
under suitable conditions to form a compound represented by Structural Formula
(XX); and
ii) deprotecting the G group of the compound of Structural Formula (XX) under
suitable
conditions to form the compound of Structurafformula (I), wherein: the
variables of
Structural Formulae (I) and (XX), and Compounds (G) and (D) are each and
independently
as described herein; LI is a halogen (such as Cl, Br, or I); and G is trityl.
-8-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
DETAILED DECRIPTION OF THE INVENTION
[00124] The compounds of the invention are as described in the claims. In some
embodiments, the compounds of the invention are represented by any one of
Structural
Formula (I) or pharmaceutically acceptable salts thereof, wherein the
variables are each and
independently as described herein. In some embodiments, the compounds of the
invention
are represented by any chemical formulae depicted in Table 1, or
pharmaceutically
acceptable salts thereof. In some embodiments, the compounds of the invention
are
represented by any chemical formulae depicted in Table 2, or pharmaceutically
acceptable
salts thereof. In some embodiments, the compounds of the invention are
presented by
Structural Formula (I) or a pharmaceutically acceptable salt thereof, wherein
the variables are
each and independently as depicted in the chemical formulae in Table 1. In
some
embodiments, the compounds of the invention are presented by Structural
Formula (I) or a
pharmaceutically acceptable salt thereof, wherein the variables are each and
independently as
depicted in the chemical formulae in Table 2.
[00125] In one embodiment, the compounds of the invention are represented
by Structural
Formula (I) or pharmaceutically acceptable salts thereof, wherein the first
set of values of the
variables of Structural Formula (I) is as follows:
X is ¨C1, -Br, ¨F, ¨CN, -0(C,4 alkyl), or CI-C6 aliphatic optionally
substituted with
one or more instances ail. Typically, X is -F, -Cl, -CN, -0(C1.4 alkyl), C14
alkyl, -or C14
haloalkyl. Typically, X is -F, -Cl, -CN, Ci4 alkyl, -or Ci4 haloalkyl.
Typically, X is -F, -Cl,
-CN, Ci4 alkyl, or C14 haloalkyl. More typically, X is -F, -Cl, -CF3, or -CH3.
More
typically, X is -F, -Cl, or -CF3. Even more typically, X is -F or -Cl.
ZI, Z2, Z3, and Z4 are each and independently CR2 or N, provided that up to
three N
are selected for Z I, Z2, Z3, and Z4, and provided that when Z3 and Z4 are
both CR2, then ZI
and Z2 are both not N at the same time. In one aspect, at least one of Z'-Z4
is N.
Ring S is a 6-membered aromatic ring. Typical examples of Ring S include:
R2
R2r R2 R2
I
127_ N N
R2
N -N
R2
N
µL
NandN
More typical examples of Ring S include:
-9-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
R2 R2
R2,............... R2....,,,...õ,::_..., R2
rrR2 /L
I IN1 I 11 N
'''z.N- .rs-0, ''az.N,,J=ri, \. N.,4-P', µCNI rrij, and
F F.,..r.F FC F3
Specific examples of Ring S include: '2'?-N*-_,J-r', .112. N
ssfj, 'ZZ2.2 IN 1)r.Prj ,
....õ..?õ1:: F
FCN NC F - FN NC
N.r.po , 'z.Ns.sx', 'z.N,,, µlaz. Nr p-P,
X-N
)i NI N
µ N--p, andVN rrsj. ,
Ring T is a C3-C10 carbocycle optionally further substituted with one or more
instances of JT. In one aspect, Ring T is an optionally substituted, bridged,
C5-C10
carbocyclic group. In another aspect, Ring T is an optionally substituted,
monocyclic, C5-C8
R1&(
)e C-R15
1Q1
\
carbocyclic group. A specific example of Ring T is: D '`i2 R13 R1 ,
wherein x is 0, 1 or
2. Typical examples of Ring T include:
,
R23D 22
R23 9 's R23 .ai R25
l R21 --.91IFIlr
R14
Itio .
R21 R14 A3 j R22
R14 R22
0
r R15 µ111. R 15 R24 \ R21
\ Q1 Q1 Q 1 R15
R24 \ \ \
R1, R1R1
, ,
R23
R22 R23 , R22
... R21
R14
III 1 R21
R14 11Sio,'
r R24
Q1 R15
Q1......, \
R1 , and R1 , wherein q is 0, 1 or 2; and
r is 1 or 2.
-10-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
R14
µ2Z, R14 1110
R"
Additional typical examples of Ring T include: Q1 R1 R15, 01R1
R14
R15 R14 = R15
oiRl
01R1 ,and µ111.-
Additional typical examples of Ring T include:
R23 R22
R22 R23 R23 .Al& R25
R21 21
Ria
Ria R14
A3 j R22
52?
R15 CO2R1 R15R24 \ R21
R15
R24 CO2R1 CO2R1
R23
R22 R23 =R22
R21
'
R14 R21 R14 ItWf
R24
R15
CO2R1 , and CO2R1 , wherein q is 0, 1 or 2; and
r is 1 or 2.
Ring A is a 5-10 membered carbocyclic group optionally further substituted
with one
or more instances ofJT; or optionally Ring A and R15, Ring A and R14, or Ring
A and R13
independently and optionally form a 5-10 membered, bridged carbocyclic ring
optionally
further substituted with one or more instances ofJT. In one aspect, Ring A is
optionally and
independently further substituted with one or more substituents selected from
the group
consisting of halogen, cyano, hydroxy, oxo, -NH2, -NH(Ci-Ca alkyl), -N(Ci-Ca
-000(Ci-C4 alkyl), -CO(Ci-Ca alkyl), -CO2H, -COACI-Ca alkyl), CI-Ca alkyl, C,-
C4
haloalkyl, and -0(C,-C4 alkyl); or Ring A and R15, Ring A and R14, or Ring A
and R13
independently and optionally form a bridged carbocyclic group optionally and
independently
substituted with one or more substituents selected from the group consisting
of halogen,
cyano, hydroxy, oxo, -NH2, -NH(CI-C4 alkyl), -N(CI-C4 alky1)2, -000(Ci-C4
alkyl), -CO(Ci-
C4 alkyl), -CO2H, -0O2(Ci-C4 alkyl), CI-Ca alkyl, CI-Ca haloalkyl, and -0(C,-
C4 alkyl). In
another aspect, Ring A and R15, Ring A and R14, or Ring A and R13
independently form an
optionally substituted, bridged carbocyclic group.
-11-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
Each of Rings A1-A5 is independently a 5-10 membered, bridged carbocycle
optionally further substituted with one or more substituents selected from the
group
consisting of halogen, cyano, hydroxy, oxo, -NH2, -NH(C1-C4 alkyl), -N(Ci-C4
alky02,
-000(C1-C4 alkyl), -CO(Ci-Ca alkyl), -CO2H, -0O2(C1-C4 alkyl), C1-C4 alkyl, C1-
C4
haloalkyl, and -0(Ci-C4 alkyl). Typically, each of Rings A1-A5 is
independently and
optionally further substituted with one or more substituents selected from the
group
consisting of halogen, cyano, hydroxy, C1-C4 alkyl, C1-C4 haloalkyl, and -0(Ci-
C4 alkyl).
Each of Rings A8-A11 is independently and optionally substituted with one or
more
substituents selected from the group consisting of halogen, cyano, hydroxy,
oxo, -NH2,
-NH(Ci-Ca alkyl), -N(Ci-Ca alky1)2, -000(Ci-C4 alkyl), -CO(Ci-Ca alkyl), -
CO2H, -0O2(Ci-
C4 alkyl), CI-Ca alkyl, C1-C4 haloalkyl, and -0(Ci-C4 alkyl).
Q1 is -C(0)-, -0O2-, -0C(0)-, -0(CR`Rs)k-C(0)0-, -C(0)NR'-, -C(0)N(R')-0-,
-C(0)NRC(0)0-, -NRC(0)-, -NRC(0)NR'-, -NRCO2-, -0C(0)NR'-, -0S02NR'-,
-S(0)-, -S02-, -SO2NR'-, -NRS02-, -NRSO2NR'-, -P(0)(0R)0-, -0P(0)(0Ra)0-,
-P(0)20-, -0O2S02-, or -(C1111e)p-Y1-. Typically, Q1 is -C(0)-, -0O2-, -0C(0)-
,
-0(CR`Rs)k-C(0)0-, -C(0)NR'-, -C(0)N(R')-0-, -C(0)NRC(0)0-, -NRC(0)-,
-NRC(0)NR'-, -NRCO2-, -0C(0)NR'-, -0S02NR'-, -S(0)-, -S02-, -SO2NR'-,
-NRS02-, -NRSO2NR'-, -B(0)2-, or -(CR1Rs)p-Y1-. More typically, Q1 is -0O2-,
-0(CR1Rs)k-C(0)0-, -P(0)(0R)0-, -0P(0)(01e)0-, -P(0)20-, -0O2S02-, -B(0)2-, or
-(CRIV)p-Y1-. More typically, Q1 is -0O2-, -0(CR111s)k-C(0)0-, -P(0)(0R)0-,
-0P(0)(0Ra)0-, -P(0)20-, -0O2S02-, or -(CRIRs)p-Y1-. More typically, Q1 is -
C(0)0-,
-NRC(0)-, -C(0)NR-, -NRC(0)NR'-, or -(CRIRs)1,2-Y1-. Q1 is -C(0)-, -C(0)0-,
-NRC(0)-, -C(0)NR-, -NRC(0)NR'-, or -(CH2)1,2-Y-. Even more typically, Q1 is
independently -C(0)0-, -NRC(0)-, -C(0)NR-, -NRC(0)NR'-, or -(CH2)1,2-Y-. Even
more
typically, Q1 is -C(0)0-, -NRC(0)-, -C(0)NR-, or -NRC(0)NR'-. Specific
examples of Q1
include -C(0)0-, -NHC(0)-, or -C(0)NH-.
Y1 is -C(0)-, -0O2-, -0C(0)-, -0(CRAs)k-C(0)0-, -C(0)NR'-, -C(0)N(R')-0-,
-C(0)NRC(0)0-, -NRC(0)-, -NRC(0)NR'-, -NRCO2-, -0C(0)NR'-, -0S02NR'-,
-S(0)-, -S02-, -SO2NR'-,-NRS02-, -NRSO2NR'-, -P(0)(0R)0-, -0P(0)(0Ra)0-,
-P(0)20-, -B(0)2-, or -0O2S02-. Typically, Y1 is -C(0)-, -0O2-, -0C(0)-,
-0(CR1R5)k-C(0)0-, -C(0)NR'-, -C(0)N(R')-0-, -C(0)NRC(0)0-, -NRC(0)-,
-NRC(0)NR'-, -NRCO2-, -0C(0)NR'-, -0S02NR'-, -S(0)-, -S02-, -SO2NR'-,
-NRS02-, -6(0)2-, or -NRSO2NR'-. More typically, Y1 is -C(0)-, -0O2-, -0C(0)-,
-12-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
-0(CRIR5)k¨C(0)0-, ¨C(0)NR'¨, ¨C(0)N(R')-0¨, -C(0)NRC(0)0¨, ¨NRC(0)¨,
¨NRC(0)NR'¨, ¨NRCO2¨, -0C(0)NR'¨, -0S02NR'-, -S(0)-, ¨S02¨, -SO2NR'¨,
¨NRS02¨, or -NRSO2NR'-. More typically, YI is ¨0O2¨, -0(CRIR5)k¨C(0)0-,
-P(0)(0R)0-, -0P(0)(01e)0-, -P(0)20-, or -0O2S02-. More typically, YI is -C(0)-
,
-C(0)0-, -NRC(0)-, -C(0)NR-, or ¨NRC(0)NR'¨. More typically, YI is -C(0)0-,
-NRC(0)-, -C(0)NR-, or ¨NRC(0)NR'¨. Specific examples of Y' include -C(0)0-,
-NHC(0)-, -C(0)NH-, or ¨NHC(0)NH¨.
RI is: i) ¨H; ii) a C1-C6 aliphatic group optionally substituted with one or
more
instances of JA; iii) a C3-C10 carbocyclic group or 4-10 membered heterocyclic
group, each
optionally and independently substituted with one or more instances of JB; or
iv) a 6-10
membered aryl group or 5-10 membered heteroaryl group, each optionally and
independently
substituted with one or more instances of f; or
optionally RI, together with R' and the nitrogen to which they are attached,
form a 4-8
membered heterocyclic group optionally substituted with one or more instances
of J2; or
optionally -Q'-R' forms, together with Ring T, a 4-10 membered, non-aromatic,
spiro
ring optionally substituted with one or more instances of J4; and
provided that Q'-R' is not at the same carbon atom to which -NH group that is
attached to Ring S is attached.
In one aspect, RI is independently i)¨H; ii) a C,-C6-aliphatic group
optionally
substituted with one or more instances of JA; iii) a C3-C8 carbocyclic group
or 4-8 membered
heterocyclic group, each of which is optionally and independently substituted
with one or
more instances of JB; iv) a phenyl group or 5-6 membered heteroaryl group,
each of which is
optionally and independently substituted with one or more instances of f;
optionally RI,
together with R' and the nitrogen to which they are attached, form an
optionally substituted,
4-8 membered heterocyclic group; or optionally -Q'-R' forms, together with
Ring T, an
optionally substituted, 4-10 membered, non-aromatic, spiro ring.
In another aspect, RI is independently i) ¨H; ii) a C,-C6-aliphatic group
optionally
substituted with one or more instances of JA; iii) a C3¨C8 carbocyclic group
or 4-8 membered
heterocyclic group, each of which is optionally and independently substituted
with one or
more instances of J'; iv) a phenyl group or 5-6 membered heteroaryl group,
each of which is
optionally and independently substituted with one or more instances of f; or
optionally RI,
together with R' and the nitrogen to which they are attached, form an
optionally substituted,
4-8 membered heterocyclic group.
-13-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
In yet another aspect, R1 is independently: i)¨H; ii) a C1-C6 aliphatic group
optionally
substituted with one or more substituents independently seiected from the
group consisting of
halogen, cyano, hydroxy, oxo, -0(C1¨C4 alkyl), ¨NH2, ¨NH(CI¨C4 alkyl),
¨N(Ci¨C4 alky1)2,
-C(0)(C1¨C4 alkyl), ¨0C(0)(C1¨C4 alkyl), -C(0)0(C1¨C4 alkyl), -CO2H, C3-C8
carbocyclic
group, 4-8 membered heterocyclic group, phenyl, and 5-6 membered heteroaryl;
iii) a C3¨C7
carbocyclic group; iv) a 4-7 membered heterocyclic group; v) a phenyl group;
or vi) a 5-6
membered heteroaryl group; or optionally R1, together with R' and the nitrogen
to which they
are attached, form an optionally substituted, 4-8 membered heterocyclic group;
and
each of said carbocyclic, phenyl, heterocyclic, and heteroaryl groups
represented by
R1 and for the substituents of the Ci-C6-aliphatic group represented by R1,
and said
heterocyclic group formed with R1 and R' is independently and optionally
substituted with
one or more substituents independently selected from the group consisting of
halogen, cyano,
hydroxy, oxo, -NH2, -NH(CI-C4 alkyl), -N(CI-C4 alky1)2, -000(Ci-C4 alkyl), -
CO(C1-C4
alkyl), -CO2H, -0O2(Ci-C4 alkyl), C1-C4 alkyl, C1-C4 haloalkyl, and -0(Ci-C4
alkyl).
In yet another aspect, R1 is independently ¨H or an optionally substituted C1-
C6
aliphatic group, such as -H or optionally substituted Ci_6 alkyl.
In yet another aspect, R1 is independently a 4-7 membered heterocyclic group,
a
phenyl group, or a 5-6 membered heteroaryl group, wherein each of said
heterocyclic, phenyl
and heteroaryl groups is independently and optionally substituted with one or
more
substituents independently selected from the group consisting of halogen,
cyano, hydroxy,
oxo, -NH2, -NH(C1-C4 alkyl), -N(Ci-C4 alky1)2, -000(Ci-C4 alkyl), -CO(Ci-C4
alkyl),
-CO2H, -0O2(C1-C4 alkyl), CI-CI alkyl, C1-C4 haloalkyl, and -0(Ci-C4 alkyl);
or optionally
R1 and R', together with the nitrogen atom to which they are attached, form an
optionally
substituted, 4-8 membered heterocyclic group.
R2 is -H, halogen, -CN, -NO2, -C(0)NH2, -C(0)NH(CH3),-C(0)N(CH3)2, or C1-C6
aliphatic optionally substituted with one or more instances of J1. Typically,
R2 is -H,
halogen, -CN, -NO2, -C(0)NH2, -C(0)NH(CH3),-C(0)N(CH3)2, CI-C6 aliphatic
(e.g., Cl-C6
alkyl), or Ci-C6 haloalkyl. More typically, R2 is -H, halogen, -CN, -NO2, -
C(0)NH2,
-C(0)NH(CH3),-C(0)N(CH3)2, -CH3, or -CF3. More typically, R2 is halogen, -CN, -
NO2,
-C(0)NH2, -C(0)NH(CH3),-C(0)N(CH3)2, -CH3, or -CF3. More typically, R2 is
halogen,
-CN, or -CF3. More typically, R2 is -F, -Cl, -CN, -CH3, or -CF3. More
typically, R2 is -F,
-Cl, -CN, or -CF3. More typically, R2 is -F, -CN, or -CF3.
Each of R12, R13, and R14 is independently ¨H, halogen, cyano, hydroxy, CI-C6
alkyl,
-0(Ci-C6 alkyl), -NH2, -NH(CI-C6 alkyl), -N(Ci-C6 alky1)2, -000(Ci-C6 alkyl), -
CO(CI-C6
-14-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
alkyl), -CO2H, or -0O2(C1-C6 alkyl), wherein each said C1-C6 alkyl is
optionally and
independently substituted with one or more substituents selected from the
group consisting of
halogen, cyano, hydroxy, oxo, -NH2, -NH(Ci-C4 alkyl), -N(Ci-C4 alky1)2, -
000(Ci-C4 alkyl),
-CO(Ci-C4 alkyl), -CO2H, -0O2(CI-C4 alkyl), and -0(CI-C4 alkyl). Typically,
R12, R13, and
R14 are each and independently -H, halogen, cyano, hydroxy, -0(C,-C6 alkyl),
or optionally
substituted C1-C6 alkyl. More typically, R12, R13, and R14 are each and
independently -H,
halogen, hydroxy, Cl-C6 alkyl, CI-C6 haloalkyl, or -0(C1-C6 alkyl).
Each R15 is independently -H, halogen, cyano, hydroxy, or Ci-C6 alkyl
optionally and
independently substituted with one or more substituents selected from the
group consisting of
halogen, cyano, hydroxy, oxo, -NH2, -NH(Ci-C4 alkyl), -N(Ci-C4 alky1)2, -
000(CI-C4 alkyl),
-CO(C1-C4 alkyl), -CO2H, -0O2(C1-C4 alkyl), and -0(Ci-C4 alkyl). Typically,
R15 is -H or
optionally substituted CI-C6 alkyl. More typically, R15 are each independently
-H, C1-C6
alkyl, or CI-C6 haloalkyl.
In one aspect, R12, R13, and R14 are each and independently -H, halogen,
cyano,
hydroxy, -0(Ci-C6 alkyl), or optionally substituted CI-C6 alkyl; and R15 is -H
or optionally
substituted CI-C6 alkyl.
In another aspect, R12 and R13 are each independently -H, halogen, hydroxy, CI-
C6
alkyl, CI-C6 haloalkyl, or -0(CI-C6 alkyl); and R14 and R15 are each
independently -H, Ci-C6
alkyl, or CI-C6 haloalkyl.
R21, R22, R23, R24, and R25
are each independently -H, halogen, -OH, CI-C6 alkoxy, or
CI-C6 alkyl optionally substituted with one or more substituents independently
selected from
the group consisting of halogen, cyano, hydroxy, oxo, -NH2, -NH(Ci-C4 alkyl), -
N(Ci-C4
alky1)2, -000(Ci-C4 alkyl), -CO(Ci-C4 alkyl), -CO2H, -0O2(Ci-C4 alkyl), CI-C4
alkyl, CI-C4
haloalkyl, and -0(Ci-C4 alkyl). Typically, R21, R22, R23, R24, and Kr.25
are each independently
-H, halogen, hydroxy, CI-C6 alkoxy, Ci-C6 alkyl, or CI-C6 haloalkyl.
JA, JB, and JT are each and independently oxo or Jc; and Jc are each and
independently
selected from the group consisting of halogen, cyano, M, Ra, or Ra-M.
Optionally, two JT,
two JA, two JB, and two Jc, respectively, together with the atom(s) to which
they are attached,
independently form a 4-10-membered ring (e.g., 5-7-membered or 5-6-memebered)
that is
optionally substituted with one or more instances ofJ4.
M is independently selected from the group consisting of -ORb, -SRb, -S(0)Ra,
-SO2Ra, -NRbR`, -C(0)Ra, -C(=NR)11`, -C(=NR)NRbRc, -NRC(=NR)NRbR`, -C(0)0Rb,
-0C(0)Rb, -NRC(0)Rb, -C(0)NRbR`, -NRC(0)NRbR`, -NRC(0)0Rb, -000NRbre,
-C(0)NRCO2Rb, -NRC(0)NRC(0)0Rb, -C(0)NR(ORb), -0S02NRbRe,
-15-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
-SO2NR`Rb, -NRSO2Rb, -NRSO2NR`Rb, -P(0)(0Rb)2, -0P(0)(0Rb)2, -P(0)20R" and
-CO2S02Rb.
Typically, Jc is selected from the group consisting of halogen, cyano, Ra, -
ORb, -SRb,
-S(0)Ra, -S021=e, -N1-112`, -C(0)Rb, -C(0)0Rb, -0C(0)Rb, -NHC(0)Rb, -
C(0)NH11`,
-NHC(0)NHR`, -NHC(0)0Rb, -000NHR`, -NHC(0)NHC(0)0Rb, -N(CH3)Rc,
-N(CH3)C(0)Rb , -C(0)N(CH3)W, -N(CH3)C(0)NHRc, -N(CH3)C(0)0Rb,
-000N(CH3)Re, -C(0)NHCO2Rb, -C(0)N(CH3)CO2Rb,
-N(CH3)C(0)NHC(0)0Rb, -NHSO2Rb, -SO2NHRb, -SO2N(CH3)Rb, and -N(CH3)S02Rb; or
two Jc, respectively, together with the atom(s) to which they are attached,
independently form
an optionally substituted, 4-10-membered, non-aromatic ring.
, ,
In one aspect, _IA, JB jc
and JT are each independently selected from the group
consisting of halogen, cyano, Ra, -ORb, -1\11-1Rc, -C(0)Rb, -C(0)0Rb, -
0C(0)Rb,
-NHC(0)Rb, -C(0)NHR`, -NHC(0)NH11`, -NHC(0)0Rb, -000NHR`, -N(CH3)R`,
-N(CH3)C(0)Rb , -C(0)N(CH3)Re, -N(CH3)C(0)NHRc, -N(CH3)C(0)0Rb, -NHSO2Rb,
-SO2NHRb, -SO2N(CH3)Rb, and -N(CH3)S02Rb; or =
optionally, two JT, two JA, two JB, and two Jc, respectively, together with
the atom(s)
to which they are attached, independently form a 4-10-membered ring that is
optionally
substituted with one or more substituents selected from the group consisting
of halogen,
cyano, hydroxy, oxo, -NH2, -NH(Ci-Ca alkyl), -N(C1-C4 alky1)2, -000(CI-C4
alkyl), -CO(Ci-
C4 alkyl), -CO2H, -0O2(Ci-C4 alkyl), and -0(Ci-C4 alkyl).
Typically, JA is halogen, cyano, hydroxy, oxo, -0(C,-C4 alkyl), -NH2, -NH(Cr-
Ca
alkyl), -N(Ci-Ca alky1)2, -C(0)(Ci-C4 alkyl), -0C(0)(Ci-C4 alkyl), -C(0)0(CI-
C4 alkyl),
-CO2H, C3-C8 carbocyclic group, 4-8 membered heterocyclic group, phenyl, or 5-
6
membered heteroaryl, wherein each of said carbocyclic, phenyl, heterocyclic,
and heteroaryl
groups is independently and optionally substituted with one or more
substituents
independently selected from the group consisting of halogen, cyano, hydroxy,
oxo, -NH2,
-NH(CI-C4 alkyl), -N(CI-C4 alky1)2, -000(CI-C4 alkyl), -CO(CI-C4 alkyl), -
CO2H, -0O2(Ci-
C4 alkyl), C,-C4 alkyl, CI-C4 haloalkyl, and -0(C,-C4 alkyl). Optionally, two
JA, together
with the atom(s) to which they are attached, form an optionally substituted, 4-
10-membered
(or 5-7 membered, or 5-6 membered) ring.
Typically, JB and Jc are each and independently halogen, cyano, hydroxy, oxo, -
NH2,
-NH(Ci-C4 alkyl), -N(CI-C4 alky1)2, -000(Ci-C4 alkyl), -CO(CI-C4 alkyl), -
CO2H, -0O2(Ci-
C4 alkyl), CI-Ca alkyl, CI-C4 haloalkyl, or -0(CI-C4 alkyl). Optionally, two
JB and two Jc,
-16-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
together with the atom(s) to which they are attached, independently form an
optionally
substituted, 4-10-membered (or 5-7 membered, or 5-6 membered) ring.
Typically, JT is halogen, cyano, hydroxy, oxo, -NH2, -NH(CI-C4 alkyl), -N(C1-
C4
alky1)2, -000(C1-C4 alkyl), -CO(Ci-C4 alkyl), -CO2H, -0O2(CI-C4 alkyl), C1-C4
alkyl, CI-Ca
haloalkyl, or -0(CI-C4 alkyl). More typically, JT is halogen, cyano, hydroxy,
C1-C4 alkyl, C1-
C4 haloalkyl, and -0(C1-C4 alkyl). Optionally, two JT, together with the
atom(s) to which
they are attached, form an optionally substituted, 4-10-membered (or 5-7
membered, or 5-6
membered) ring.
Typically, the ring formed with two JT, two JA, two JB, and two Jc
independently is an
optionally substituted non-aromatic ring, such as carbocycle or heterocycle.
More typically,
the ring is an optionally substituted carbocycle.
Ra is independently:
i) a C1-C6 aliphatic group optionally substituted with one or more
substituents selected from
the group consisting of halogen, cyano, hydroxy, oxo, -NH2, -NH(CI-C4 alkyl), -
N(CI-Ca
alky1)2, -000(CI-C4 alkyl), -CO(CI-C4 alkyl), -CO2H, -0O2(Ci-C4 alkyl), -0(Ci-
C4 alkyl),
C3-C8 carbocyclic group optionally substituted with one or more instances of
J2, 4-8
membered heterocyclic group optionally substituted with one or more instances
of J2, 5-10
membered heteroaryl group optionally substituted with one or more instances of
J3, and 6-10
membered aryl group optionally substituted with one or more instances of J3;
ii) a C3-C8 carbocyclic group, or 4-8 membered heterocyclic group, each of
which is
optionally and independently substituted with one or more instances of J2; or
iii) a 5-10 membered heteroaryl group, or 6-10 membered aryl group, each of
which is
optionally and independently substituted with one or more instances of J3; and
Rb and R` are each independently Ra or ¨H; or optionally, Rb and Rc, together
with the
nitrogen atom(s) to which they are attached, each independently form a 4-8
membered
heterocyclic group optionally substituted with one or more instances of J2.
In one aspect, Ra is independently: i) a C1-C6 alkyl group optionally
substituted with
one or more substituents selected from the group consisting of halogen, cyano,
hydroxy, oxo,
-NH2, -NH(C1-C4 alkyl), -N(CI-C4 alky1)2, -000(CI-C4 alkyl), -CO(CI-C4 alkyl),
-CO2H,
-0O2(CI-C4 alkyl), -0(Ci-C4 alkyl), optionally substituted C3-C8 carbocyclic
group,
optionally substituted 4-8 membered heterocyclic group, optionally substituted
5-6 membered
heteroaryl, and optionally substituted phenyl group; ii) an optionally
substituted C3-C8
carbocyclic group; iii) optionally substituted 4-8 membered heterocyclic
group; iv) an
-17-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
optionally substituted 5-6 membered heteroaryl group; v) or optionally
substituted phenyl
group;
Rb and R` are each independently Ra or ¨H; or optionally, Rb and Rc, together
with the
nitrogen atom(s) to which they are attached, each independently form an
optionally
substituted, 4-8 membered heterocyclic group.
In another aspect, Ra is independently: i) a C1-C6 alkyl group optionally
substituted
with one or more substituents selected from the group consisting of halogen,
cyano, hydroxy,
oxo, -NH2, -NH(CI-C4 alkyl), -N(Ci-Ca alky1)2, -000(C1-C4 alkyl), -CO(CI-Ca
alkyl),
-CO2H, -0O2(Ci-C4 alkyl), -0(CI-C4 alkyl), C3-C8 carbocycle, 4-8 membered
heterocycle, 5-
6 membered heteroaryl, and phenyl; ii) a C3-C8 carbocyclic group or 4-8
membered
heterocyclic group, each of which is independently and optionally substituted
with one or
more substituents selected from the group consisting of halogen, cyano,
hydroxy, oxo, -NH2,
-NH(CI-C4 alkyl), -N(Ci-C4 alky1)2, -000(C1-C4 alkyl), -CO(Ci-Ca alkyl), -
CO2H, -0O2(Ci-
C4 alkyl), CI-Ca alkyl, C1-C4 haloalkyl, and -0(C1-C4 alkyl); or iii) a 5-6
membered
heteroaryl group or phenyl group, each of which is independently and
optionally substituted
with one or more substituents selected from the group consisting of halogen,
cyano, hydroxy,
-NH2, -NH(CI-Ca alkyl), -N(Ci-C4 alky1)2, -000(CI-C4 alkyl), -CO(CI-C4 alkyl),
-CO2H,
-0O2(Ci-C4 alkyl), C1-C4 alkyl, C1-C4 haloalkyl, and -0(C1-C4 alkyl); and
Rb and R` are each independently Ra or ¨H; or optionally, Rb and R`, together
with the
nitrogen atom(s) to which they are attached, each independently form a 4-8
membered
heterocyclic group optionally substituted with one or more substituents
selected from the
group consisting of halogen, cyano, hydroxy, oxo, -NH2, -NH(Ci-Ca alkyl), -
N(Ci-Ca alky1)2,
-000(Ci-C4 alkyl), -CO(CI-Ca alkyl), -CO2H, -0O2(CI-C4 alkyl), C1-C4 alkyl, C1-
C4
haloalkyl, and -0(Ci-C4 alkyl).
R` and Rs are each independently ¨H, halogen, or C1-C6 alkyl optionally
substituted
with one or more instances of J1, or optionally, Ill and Rs, together with the
carbon atom to
which they are attached, form a cyclopropane ring optionally substituted with
one or more
instances of methyl. Typically, R' and Rs are each independently ¨H, halogen,
C1-C6 alkyl,
or C1-C6 haloalkyl. More typically, R' and Rs are each independently ¨H or Ci-
C6 alkyl.
R and R' are each independently ¨H or CI-C6 alkyl optionally and independently
substituted with one or more instances of JI, or optionally R and R', together
with the
nitrogen to which they are attached, form a 4-8 membered heterocyclic group
optionally
substituted with one or more instances 0fJ2. Typically, R and R' are each and
independently
-H or C1-4 alkyl; or optionally RI, together with R' and the nitrogen to which
they are
-18-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
attached, form an optionally substituted, 4-8 membered heterocyclic group.
More typically,
R and R' are each and independently -H or -CH3; or optionally RI, together
with R' and the
nitrogen to which they are attached, form an optionally substituted, 4-8
membered
heterocyclic group.
Each Ji is independently selected from the group consisting of halogen, cyano,
hydroxy, oxo, -NH2, -NH(C1-C4 alkyl), -N(Ci-C4 alky1)2, -000(CI-C4 alkyl), -
CO(Ci-Ca
alkyl), -CO2H, -0O2(CI-C4 alkyl), -0(CI-C4 alkyl), and pheny.1;.
Each J2 is independently selected from the group consisting of halogen, cyano,
hydroxy, oxo, -NH2, -NH(Ci-C4 alkyl), -N(CI-C4 alky1)2, -000(C1-C4 alkyl), -
CO(Ci-C4
alkyl), -CO2H, -0O2(CI-C4 alkyl), CI-Ca alkyl, CI-Ca haloalkyl, and -0(CI-C4
alkyl);
Each of J3 and J4 is independently selected from the group consisting of
halogen,
cyano, hydroxy, -NH2, -NH(Ci-Ca alkyl), -N(CI-Ca alky1)2, -000(Ci-C4 alkyl), -
CO(Ci-Ca
alkyl), -CO2H, -0O2(CI-C4 alkyl), CI-Ca alkyl, CI-Ca haloalkyl, and -0(CI-C4
alkyl).
Each p is independently 1, 2, 3 or 4, and each k is independently 1, 2, 3 or
4.
Typically, each of p and k independently is 1 or 2.
1001261 The second set of values of the variables of Structural Formula (I)
is as follows:
At least one of Z'-Z4 is N; and if Z' and Z4 are both N and Z2 and Z3 are each
independently CR2, or if Z' is N and Z2, Z3 and Z4 are each and independently
CR2, then at
least one of R2 is other than -H. Typically, non-H values of R2 include -F, -
Cl, -CN, -CH3, or
-CF3. More typical non-H values of R2 include -F, -Cl, -CN, or -CF3. More
typical non-H
values of R2 include -F, -CN, or -CF3.
The remaining variables of Structural Formula (I) are each and independently
as
described above in the first set of values of the variables of Structural
Formula (I).
1001271 The third set of values of the variables of Structural Formula (I)
is as follows:
Ring S is selected from:
R2
R
N R2
N
R2
R2 N
N N
11
N
The remaining variables of Structural Formula (I) are each and independently
as
described above in the first set of values of the variables of Structural
Formula (I).
1001281 The fourth set of values of the variables of Structural Formula (I)
is as follows:
-19-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
Values of Ring S are as described above in the third set of values of the
variables of
Structural Formula (I), wherein R2 is -F, -CI, -CN, C1-C4 aliphatic, or CI-Ca
alkyl. More
typically, R2 is -F, -C1, -CN, -CH3, or -CF3.
The remaining variables of Structural Formula (I) are each and independently
as
described above in the first set of values of the variables of Structural
Formula (I).
[00129] The fifth set of values of the variables of Structural Formula (I)
is as follows:
Values of Z1-Z4 and R2 are each and independently as described above in the
second
set of values of the variables of Structural Formula (I).
X is ¨Cl, -Br, ¨F, ¨CN, -CH3, or CF3.
The remaining variables of Structural Formula (I) are each and independently
as
described above in the first set of values of the variables of Structural
Formula (I).
[00130] The sixth set of values of the variables of Structural Formula (I)
is as follows:
Values of Z I-Z4 and R2 are each and independently as described above in the
first or
second set of values of the variables of Structural Formula (I).
Values of Ring S are as described above in the third set of values of the
variables of
Structural Formula (I).
R2 is -F, -C1, -CN, or -CF3.
X is ¨C1, -Br, ¨F, ¨CN, -CH3, or CF3.
The remaining variables of Structural Formula (I) are each and independently
as
described above in the first set of values of the variables of Structural
Formula (I).
[00131] In the seventh set of values of the variables of Structural Formula
(I), QIR1 is
other than ¨C(0)NH2; and values of Z'-Z4, R2, and Ring S are each and
independently as
described above in any one of the first through sixth sets of values of the
variables of
Structural Formula (I).
The remaining variables of Structural Formula (I) are each and independently
as
described above in the first set of values of the variables of Structural
Formula (I).
[00132] The eighth set of values of the variables of Structural Formula (I) is
as follows:
= Values of Z'-Z4, R2, Ring S, and X are each and independently as
described above in
any one of the first through seventh sets of values of the variables of
Structural Formula (I).
Ring T is an optionally substituted, bridged, C5-Ci0 carbocyclic group.
The remaining variables of Structural Formula (I) are each and independently
as
described above in the first set of values of the variables of Structural
Formula (I).
[00133] The ninth set of values of the variables of Structural Formula (I) is
as follows:
Values of Z1LZ4, R2, Ring S, and X are each and independently as described
above in
-20-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
any one of the first through eighth sets of values of the variables of
Structural Formula (I).
Ring T is an optionally substituted, monocyclic, C5-C8 carbocyclic group.
The remaining variables of Structural Formula (I) are each and independently
as
described above in the first set of values of the variables of Structural
Formula (I).
[00134] The tenth set of values of the variables of Structural Formula (I) is
as follows:
Values of Z1-Z4, R2, Ring S, Ring T, and X are each and independently as
described
above in any one of the first through ninth sets of values of the variables of
Structural
Formula (I).
RI is independently i) ¨H; ii) a CI-C6-aliphatic group optionally substituted
with one
or more instances of JA; iii) a C3¨C8 carbocyclic group or 4-8 membered
heterocyclic group,
each of which is optionally and independently substituted with one or more
instances of JB;
iv) a phenyl group or 5-6 membered heteroaryl group, each of which is
optionally and
independently substituted with one or more instances of f; or optionally 12.1,
together with R'
and the nitrogen to which they are attached, form an optionally substituted, 4-
8 membered
heterocyclic group; or optionally -Q1-R1 forms, together with Ring T, an
optionally
substituted, 4-10 membered, non-aromatic, spiro ring.
JA, JB, and JT are each independently oxo or f.
f is selected from the group consisting of halogen, cyano, Ra, ¨ORb, ¨SRb, -
S(0)Ra,
¨SO2Ra, ¨NHR`, ¨C(0)Rb, ¨C(0)0Rb, ¨0C(0)Rb, ¨NHC(0)Rb,*¨C(0)NHR`,
¨NHC(0)NHR`, ¨NHC(0)0Rb, ¨000NHRc, -NHC(0)NHC(0)0Rb, ¨N(CH3)Rc,
¨N(CH3)C(0)Rb , ¨C(0)N(CH3)Rc, ¨N(CH3)C(0)NHie, ¨N(CH3)C(0)0Rb,
¨000N(CH3)Rc, -C(0)NHCO2Rb, -C(0)N(CH3)CO2Rb,
-N(CH3)C(0)NHC(0)0Rb, -NHSO2Rb, -SO2NHRb, -SO2N(CH3)Rb, and -N(CH3)S02Rb.
Optionally, two JT, two JA, two JB, and two f, respectively, together with the
atom(s)
to which they are attached, independently form an optionally substituted, 4-10-
membered,
non-aromatic ring.
The remaining variables of Structural Formula (I) are each and independently
as
described above in the first set of values of the variables of Structural
Formula (I).
[00135] The eleventh set of values of the variables of Structural Formula
(I) is as follows:
Values of Z'-Z4, R2, Ring N¨,
Ring T, X, RI, JA, JB, Jc, and JT are each and
independently as described above in any one of the first through tenth sets of
values of the
variables of Structural Formula (I).
R is independently: i) a CI-C6 alkyl group optionally substituted with one or
more
substituents selected from the group consisting of halogen, cyano, hydroxy,
oxo, -NH2,
-21-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
-NH(CI-C4 alkyl), -N(Cr-C4 alky1)2, -000(C1-C4 alkyl), -CO(Ci-C4 alkyl), -
CO2H, -0O2(C1-
C4 alkyl), -0(CI-C4 alkyl), optionally substituted C3-C8 carbocyclic group,
optionally
substituted 4-8 membered heterocyclic group, optionally substituted 5-6
membered
heteroaryl, and optionally substituted phenyl group; ii) an optionally
substituted C3-C8
carbocyclic group; iii) optionally substituted 4-8 membered heterocyclic
group; iv) an
optionally substituted 5-6 membered heteroaryl group; v) or optionally
substituted phenyl
group.
Rb and Re are each independently Ra or -H; or optionally, Rb and Re, together
with the
nitrogen atom(s) to which they are attached, each independently form an
optionally
substituted, 4-8 membered heterocyclic group.
R and R' are each and independently -H or C1_4 alkyl, or optionally R and R',
together
with the nitrogen to which they are attached, form an optionally substituted 4-
8 membered
heterocyclic group, or optionally R', together with R1 and the nitrogen to
which they are
attached, form an optionally substituted 4-8 membered heterocyclic group.
The remaining variables of Structural Formula (I) are each and independently
as
described above in the' first set of values of the variables of Structural
Formula (I).
1001361 The twelfth set of values of the variables of Structural Formula
(I) is as follows:
Values of Z'-Z4, R2, Ring S, Ring T, X, RI, JA, JE3, jc, JT, Ra, Rb,
K R, and R' are
each and independently as described above in any one of the first through
eleventh sets of
values of the variables of Structural Formula (I).
Q1 is -C(0)-, -0O2-, -0C(0)-, -0(CR`Rs)k-C(0)0-, -C(0)NR'-, -C(0)N(R')-0-,
-C(0)NRC(0)0-, -NRC(0)-, -NRC(0)NR'-, -NRCO2-,
-0C(0)NR'-, -0S02NR'-, -S(0)-, -S02-, -SO2NR'-, -NRS02-, -NRSO2NR'-,
or
Y1 is -C(0)-, -0O2-, -0C(0)-, -0(CR`R5)k-C(0)0-, -C(0)NR'-, -C(0)N(R')-0-,
-C(0)NRC(0)0-, -NRC(0)-, -NRC(0)NR'-, -NRCO2-,
-0C(0)NR'-, -0S02NR'-, -S(0)-, -S02-, -SO2NR'-, -NRS02-, or -NRSO2NR'-.
The remaining variables of Structural Formula (I) are each and independently
as
described above in the first set of values of the variables of Structural
Formula (I).
[00137] The thirteenth set of values of the variables of Structural Formula
(I) is as follows:
Values of Z'-Z4, R2, Ring S, Ring T, X, RI, JA, JB, jc, JT, Ra, Rb, rsc,
K R, and R' are
each and independently as described above in any one of the first through
eleventh sets of
values of the variables of Structural Formula (I).
Q1 is -0O2-, -0(CR'Rs)k-C(0)0-, -P(0)(OR)0-, -0P(0)(0Ra)0-, -P(0)20-,
-22-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
-0O2S02-, or -(CR'Rs)p¨YI¨; and
YI is ¨0O2¨, -0(CR'R5)k¨C(0)0-, -P(0)(0R)0-, -0P(0)(0/e)0-, -P(0)20-, or
-CO2S02-.
The remaining variables of Structural Formula (I) are each and independently
as
described above in the first set of values of the variables of Structural
Formula (I).
[00138] The fourteenth set of values of the variables of Structural Formula
(I) is as
follows:
Values of Z'-Z4, R2, Ring T, X, RI, jA, jB, jc, j-r, Ra, Rb, Rc,
K R', QI, and YI are each
and independently as described above in any one of the first through
thirteenth sets of values
of the variables of Structural Formula (I).
Ring S is
R2 R2
R2 N R2y,...õ,y R2 2
N
N N Prjj, or
The remaining variables of Structural Formula (I) are each and independently
as
described above in the first set of values of the variables of Structural
Formula (I).
[00139] The
fifteenth set of values of the variables of Structural Formula (I) is as
follows:
R2 Ring T, X RI jA jB jc J, Ra, R,
Values of Zi , , , , , , T bR, R', QI, and YI are each
and independently as described above in any one of the first through
thirteenth sets of values
of the variables of Structural Formula (I).
Ring S is selected from:
Fy'F FyCF3 FCN NCF
Nr.Priõ
, FrN NC N
N
N N N or
N N
N
The remaining variables of Structural Formula (I) are each and independently
as
described above in the first set of values of the variables of Structural
Formula (I).
[00140] The
sixteenth set of values of the variables of Structural Formula (I) is as
follows:
-23-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
Values of Zi_za, -2,
K Ring S, X, RI, JA5 jB, jc550-5 Ra, Le, rsc,
R, R', Q1, and Y1 are each
and independently as described above in any one of the first through fifteenth
sets of values
of the variables of Structural Formula (I).
Ring T is:
R1&_
R15
R.R,3 41,
and wherein:
Ring A is a 5-10 membered carbocyclic group optionally further substituted
with one
or more instances of JT; or optionally Ring A and R15, Ring A and R14, or Ring
A and R13
independently and optionally form a 5-10 membered, bridged carbocyclic ring
optionally
further substituted with one or more instances of JT;
each of R12, R13, and R14 is independently -H, halogen, cyano, hydroxy, Ci-C6
alkyl,
-0(Ci-C6 alkyl), -NH2, -NH(Ci-C6 alkyl), -N(Ci-C6 -000(Ci-C6 alkyl), -CO(Ci-
C6
alkyl), -CO2H, or -0O2(Ci-C6 alkyl), wherein each said C,-C6 alkyl is
optionally and
independently substituted with one or more substituents selected from the
group consisting of
halogen, cyano, hydroxy, oxo, -NH2, -NH(Ci-C4 alkyl), -N(Ci-C4 alky1)2, -
000(Ci-C4 alkyl),
-CO(Ci-C4 alkyl), -CO2H, -0O2(Ci-C4 alkyl), and -0(CI-C4 alkyl);
each R15 is independently -H, halogen, cyano, hydroxy, or Ci-C6 alkyl
optionally and
independently substituted with one or more substituents selected from the
group consisting of
halogen, cyano, hydroxy, oxo, -NH2, -NH(Ci-C4 alkyl), -N(Ci-C4 alky1)2, -
000(Ci-C4 alkyl),
-CO(CI-C4 alkyl), -CO2H, -0O2(Ci-C4 alkyl), and -0(Ci-C4 alkyl); and
x is 0, 1 or 2.
The remaining variables of Structural Formula (I) are each and independently
as
described above in the first set of values of the variables of Structural
Formula (I).
[00141] The seventeenth set of values of the variables of Structural
Formula (I) is as
follows:
Values of Z'-Z4, R2, Ring S, Ring T, X, RI, R12, RI3, RI4, R15, Ra, Rb, Rc, R5
R,5 Qi,
y1, and x are each and independently as described above in any one of the
first through
sixteenth sets of values of the variables of Structural Formula (I).
JA, JB, Jc, and JT are each independently selected from the group consisting
of
halogen, cyano, Ra, -ORb, -NHRc, -C(0)Rb, -C(0)0Rb, -0C(0)R", -NHC(0)Rb,
-C(0)NHR`, -NHC(0)NHR`, -NHC(0)0Rb, -000NHR`, -N(CH3)Rc, -N(CH3)C(0)Rb ,
-24-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
¨C(0)N(CH3)R`, ¨N(CH3)C(0)NHIetc, ¨N(CH3)C(0)0Rb, -NHSO2Rb, -SO2NHRb,
-SO2N(CH3)Rb, and -N(CH3)S02Rb; or
optionally, two JT, two JA, two JB, and two Jc, respectively, together with
the atom(s)
to which they are attached, independently form a 4-10-membered ring that is
optionally
substituted with one or more substituents selected from the group consisting
of halogen,
cyano, hydroxy, oxo, -NH2, -NH(CI-C4 alkyl), -N(Ci-Ca alky1)2, -000(CI-C4
alkyl), -CO(C1-
C4 alkyl), -CO2H, -0O2(C1-C4 alkyl), and -0(C1-C4 alkyl).
The remaining variables of Structural Formula (I) are each and independently
as
described above in the first set of values of the variables of Structural
Formula (I).
1001421 The eighteenth set of values of the variables of Structural Formula
(I) is as
follows:
Values of ZI-Z4, R2, Ring S, Ring T, X, RI, Ri2, Ri3, Ria, Ris, JA, jB, jc,
JT, R, R,, Qi,
YI, and x are each and independently as described above in any one of the
first through
seventeenth sets of values of the variables of Structural Formula (I).
Ra is independently: i) a CI-C6 alkyl group optionally substituted with one or
more
substituents selected from the group consisting of halogen, cyano, hydroxy,
oxo, -NH2,
-NH(Ci-Ca alkyl), -N(Ci-C4 alky1)2, -000(CI-C4 alkyl), -CO(Ci-C4 alkyl), -
CO2H, -0O2(Ci-
C4 alkyl), -0(C,-C4 alkyl), C3-C8 carbocycle, 4-8 membered heterocycle, 5-6
membered
heteroaryl, and phenyl; ii) a C3-C8 carbocyclic group or 4-8 membered
heterocyclic group,
each of which is independently and optionally substituted with one or more
substituents
selected from the group consisting of halogen, cyano, hydroxy, oxo, -NH2, -
NH(Ci-Ca alkyl),
-N(Ci-C4 alky1)2, -000(Ci-C4 alkyl), -CO(CI-Ca alkyl), -CO2H, -0O2(Ci-C4
alkyl), CI-Ca
alkyl, CI-C4 haloalkyl, and -0(Ci-C4 alkyl); or iii) a 5-6 membered heteroaryl
group or
phenyl group, each of which is independently and optionally substituted with
one or more
substituents selected from the group consisting of halogen, cyano, hydroxy, -
NH2, -NH(Ci-Ca
alkyl), -N(Ci-C4 alky1)2, -000(Ci-C4 alkyl), '-CO(Ci-Ca alkyl), -CO2H, -0O2(Ci-
C4 alkyl),
CI-Ca alkyl, CI-Ca haloalkyl, and -0(C,-C4 alkyl).
Rb and Rc are each independently Ra or ¨H; or optionally, Rb and Rc, together
with the
nitrogen atom(s) to which they are attached, each independently form a 4-8
membered
heterocyclic group optionally substituted with one or more substituents
selected from the
group consisting of halogen, cyano, hydroxy, oxo, -NH2, -NH(Ci-C4 alkyl), -
N(Ci-C4 alky1)2,
-000(CI-C4 alkyl), -CO(Ci-C4 alkyl), -CO2H, -0O2(CI-C4 alkyl), CI-Ca alkyl, C,-
C4
haloalkyl, and -0(C,-C4 alkyl).
The remaining variables of Structural Formula (I) are each and independently
as
-25-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
described above in the first set of values of the variables of Structural
Formula (I).
[00143] The nineteenth set of values of the variables of Structural Formula
(I) is as
follows:
Values of Z'-Z4, R2, Ring S, Ring T, X, RI, R12, R13, R14, Ris, JA, Jes, Jc,
jT, Ra, Rb, Rc,
R, and R' are each and independently as described above in any one of the
first through
eighteenth sets of values of the variables of Structural Formula (I).
Q1 is -C(0)0-, -NRC(0)-, -C(0)NR-, ¨NRC(0)NR'¨, or -(CR`Rs)i,,¨Y I¨.
Y1 is -C(0)0-, -NRC(0)-, -C(0)NR-, or ¨NRC(0)NR'¨.
The remaining variables of Structural Formula (I) are each and independently
as
described above in the first set of values of the variables of Structural
Formula (I).
[00144] The twentieth set of values of the variables of Structural Formula (I)
is as follows:
Values of Z'-Z4, R2, Ring T, X, RI, R12, R13, R14, R15, JA, jB, Jc, jT, Ra,
Rb, Rc, R, Qi,
and Y1 are each and independently as described above in any one of the first
through
nineteenth sets of values of the variables of Structural Formula (I).
Ring S is selected from:
CF3
F F./rCN NC
por
N =
The remaining variables of Structural Formula (I) are each and independently
as
described above in the first set of values of the variables of Structural
Formula (I).
[00145] The twenty first set of values of the variables of Structural
Formula (I) is as
follows:
Values of Z'-Z4, R2, Ring S, Ring T, X, RI, JA, Jet, JC, =T,
J R, R', QI, and YI are each
and independently as described above in any one of the first through twentieth
sets of values
of the variables of Structural Formula (I).
K12,
RI3, and R14 are each and independently ¨H, halogen, cyano, hydroxy, -0(C,-C6
alkyl), or optionally substituted CI-C6 alkyl.
R15 is ¨H or optionally substituted CI-C6 alkyl.
IV and Rs are each independently ¨H, halogen, C)-C6 alkyl, or CI-C6 haloalkyl.
The remaining variables of Structural Formula (I) are each and independently
as
described above in the first set of values of the variables of Structural
Formula (I).
[00146] The twenty second set of values of the variables of Structural Formula
(I) is as
-26-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
follows:
Values of Z1-Z4, R2, Ring S, Ring T, X, R1, JA, JB, Jc, JT, R, R', Q1, and Y1
are each
and independently as described above in any one of the second through twenty
first sets of
values of the variables of Structural Formula (I).
R12 and R13 are each independently ¨H, halogen, hydroxy, CI-C6alkyl, CI-C6
haloalkyl, or -0(CI-C6 alkyl).
R14 and R15 are each independently ¨H, C,-C6 alkyl, or C1-C6 haloalkyl.
fe and Rs are each independently ¨H or C1-C6 alkyl.
The remaining variables of Structural Formula (I) are each and independently
as
described above in the first set of values of the variables of Structural
Formula (I).
1001471 The twenty third set of values of the variables of Structural
Formula (I) is as
follows:
Values of Z'-Z4, .-. 2,
K Ring S, Ring T, X, JA, JB, Jc, JT, R, R', Q1, yl, R12, R13, R14, R15,
Rs and Ware each and independently as described above in any one of the first
through
twenty second sets of values of the variables of Structural Formula (I).
R1 is independently: i) ¨H; ii) a CI-C6 aliphatic group optionally substituted
with one
or more substituents independently selected from the group consisting of
halogen, cyano,
hydroxy, oxo, -0(C,¨C4 alkyl), ¨NH,. ¨NH(CI¨C4 alkyl), ¨N(Ci¨C4 alky1)2, -
C(0)(CI¨C4
alkyl), ¨0C(0)(Ci¨C4 alkyl), -C(0)0(C,¨C4 alkyl), -CO2H, C3-C8 carbocyclic
group, 4-8
membered heterocyclic group, phenyl, and 5-6 membered heteroaryl; iii) a C3¨C7
carbocyclic
group; iv) a 4-7 membered heterocyclic group; v) a phenyl group; or vi) a 5-6
membered
heteroaryl group;
optionally R1, together with R' and the nitrogen to which they are attached,
form an
optionally substituted, 4-8 membered heterocyclic group; and
each of said carbocyclic, phenyl, heterocyclic, and heteroaryl groups
represented by
R' andfor the substituents of the CI-C6-aliphatic group represented by R1, and
said
heterocyclic group formed with R1 and R is independently and optionally
substituted with
one or more substituents independently selected from the group consisting of
halogen, cyano,
hydroxy, oxo, -NH2, -NH(Ci-Ca alkyl), -N(Ci-Ca alky1)2, -000(CI-C4 alkyl), -
CO(Ci-C4
alkyl), -CO2H, -0O2(CI-C4 alkyl), CI-Ca alkyl, CI-Ca haloalkyl, and -0(Ci-C4
alkyl).
The remaining variables of Structural Formula (I) are each and independently
as
described above in the first set of values of the variables of Structural
Formula (I).
1001481 The twenty fourth set of values of the variables of Structural Formula
(I) is as
follows:
-27-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
Values of Z1-Z4, R1, R2, Ring S, X, JA, JB, Jc, JT, R, R', Q1, Y1, Ri3,
Ria, Ris, Rs
and Fe are each and independently as described above in any one of the first
through twenty
third sets of values of the variables of Structural Formula (I).
Ring T is:
Ri4(;)
C -R15
Ri2R13 \R1
and wherein:
Ring A is a 5-10 membered carbocyclic group optionally further substituted
with one
or more substituents selected from the group consisting of halogen, cyano,
hydroxy, oxo,
-NH2, -NH(Ci-Ca alkyl), -N(Ci-C4 alky1)2, -000(CI-C4 alkyl), -CO(Ci-Ca alkyl),
-CO2H,
-0O2(C1-C4 alkyl), CI-Ca alkyl, CI-Ca haloalkyl, and -0(CI-C4 alkyl); or Ring
A and R15,
Ring A and R14, or Ring A and R13 independently and optionally form a bridged
carbocyclic
group optionally and independently substituted with one or more substituents
selected from
the group consisting of halogen, cyano, hydroxy, oxo, -NH2, -NH(Ci-C4 alkyl), -
N(Ci-Ca
alky1)2, -000(CI-C4 alkyl), -CO(CI-Ca alkyl), -CO2H, -0O2(Ci-C4 alkyl), CI-Ca
alkyl, CI-Ca
haloalkyl, and -0(Ci-C4 alkyl);
each of R12, R13, and R14 is independently -H, halogen, cyano, hydroxy, CI-C6
alkyl,
-0(Ci-C6 alkyl), -NH2, -NH(Ci-C6 alkyl), -N(Ci-C6 alky1)2, -000(Ci-C6 alkyl), -
CO(Ci-C6
alkyl), -CO2H, or -0O2(Ci-C6 alkyl), wherein each said CI-C6 alkyl is
optionally and
independently substituted with one or more substituents selected from the
group consisting of
halogen, cyano, hydroxy, oxo, -NH2, -NH(Ci-C4 alkyl), -N(CI-C4 alky1)2, -
000(Ci-C4 alkyl),
-CO(Ci-C4 alkyl), -0O21-1, -0O2(Ci-C4 alkyl), and -0(Ci-C4 alkyl);
each R15 is independently -H, halogen, cyano, hydroxy, or C1-C6 alkyl
optionally and
independently substituted with one or more substituents selected from the
group consisting of
halogen, cyano, hydroxy, oxo, -NH2, -NH(CI-C4 alkyl), -N(CI-Ca alky1)2, -
000(Ci-C4 alkyl),
-CO(CI-C4 alkyl), -CO2H, -COACI-Ca alkyl), and -0(CI-C4 alkyl); and
x is 0, 1 or 2.
The remaining variables of Structural Formula (I) are each and independently
as
described above in the first set of values of the variables of Structural
Formula (I).
100149] The twenty fifth set of values of the variables of Structural
Formula (I) is as
follows:
Values of Z'-Z4, R1, R2, Ring S, X, JA, JB, Jc, JT, R, R', Q1, yi, Ri3,
Ria, Ris,.Rs
and fe are each and independently as described above in any one of the first
through twenty
-28-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
fourth sets of values of the variables of Structural Formula (I).
Ring T is:
( A
C -R15
Nol
D12
R13 R1 ,
and wherein Ring A and R15, Ring A and R14, or Ring A and R13 independently
form an
optionally substituted, bridged carbocyclic group.
The remaining variables of Structural Formula (I) are each and independently
as
described above in the first set of values of the variables of Structural
Formula (I).
1001501 The twenty sixth set of values of the variables of Structural
Formula (I) is as
follows:
Values of Z'-Z4, RI, R2, Ring S, X, jA, is, jc, jT, R, R,, Qi, y1, R14, R15,
Rs and R'
are
each and independently as described above in any one of the first through
twenty fourth sets
of values of the variables of Structural Formula (I).
Ring T is:
R23 R23 q R22 R23 4111W
R25
r
R22 R21
Ria
Ria
R21 A3 Rn
Ria 0
R15 \ R15 R24 R21
µZ?? Q1 Q1 Q1 R15
R24
R1, R1 R1
R23
R14
R22
IN li
R22 R14 R23
===. R21 t 1 R21 W
R24
R15
Q1 Q1\
R1 ,or R1
wherein:
each of Rings A1-A5 is independently a 5-10 membered, bridged Carbocycle
optionally further substituted with one or more substituents selected from the
group
consisting of halogen, cyano, hydroxy, oxo, -NH2, -NH(CI-C4 alkyl), -N(CI-C4
-000(Ci-C4 alkyl), -CO(CI-C4 alkyl), -CO2H, -0O2(Ci-C4 alkyl), CI-CI alkyl, CI-
CI
haloalkyl, and -0(C,-C4 alkyl);
-29-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
R14 is -H, halogen, cyanb, hydroxy, CI-C6 alkyl, -0(Ci-C6 alkyl), -NH2, -NH(Ci-
C6
alkyl), -N(Ci-C6 alky1)2, -000(Ci-C6 alkyl), -CO(Ci-C6 alkyl), -CO2H, or -
0O2(CI-C6 alkyl),
wherein each said CI-C6 alkyl is optionally and independently substituted with
one or more
substituents selected from the group consisting of halogen, cyano, hydroxy,
oxo, -NH2,
-NH(Ci-C4 alkyl), -N(CI-C4 alky1)2, -000(Ci-C4 alkyl), -CO(C1-C4 alkyl), -
CO2H, -0O2(Ci-
C4 alkyl), and -0(C,-C4 alkyl);
each RI5 is independently -H, halogen, cyano, hydroxy, or Ci-C6 alkyl
optionally and
independently substituted with one or more substituents selected from the
group consisting of
halogen, cyano, hydroxy, oxo, -NH2, -NH(Ci-C4 alkyl), -N(CI-C4 alky1)2, -
000(CI-C4 alkyl),
-CO(C1-C4 alkyl), -CO2H, -0O2(CI-C4 alkyl), and -0(CI-C4 alkyl); and
R21, R22, R23, R24, and
R25 are each independently -H, halogen, -OH, CI-C6 alkoxy, or
CI-C6 alkyl optionally substituted with one or more substituents independently
selected from
the group consisting of halogen, cyano, hydroxy, oxo, -NH2, -NH(Ci-C4 alkyl), -
N(Ci-C4
alky1)2, -000(Ci-C4 alkyl), -CO(Ci-C4 alkyl), -CO2H, -0O2(Ci-C4 alkyl), CI-C4
alkyl, C,-C4
haloalkyl, and -0(C1-C4 alkyl);
q is 0, 1 or 2; and
r is 1 or 2.
The remaining variables of Structural Formula (I) are each and independently
as
described above in the first set of values of the variables of Structural
Formula (I).
[00151] The twenty seventh set of values of the variables of Structural
Formula (I) is as
follows:
Values of Z1-Z4, RI, R2, Ring S, Ring T, X, JA, jB,jc, jT, R, R,, Qi, yi, Ri2,
Ri3, Rs
and RI are each and independently as described above in the twenty sixth set
of values of the
variables of Structural Formula (I).
R14 and each R15 are each independently -H, CI-C6 alkyl, or CI-C6 haloalkyl.
R21, R22, R23, R24, and R25
are each independently -H, halogen, hydroxy, CI-C6
alkoxy, CI-C6 alkyl, or Ci-C6 haloalkyl.
The remaining variables of Structural Formula (I) are each and independently
as
described above in the first set of values of the variables of Structural
Formula (I).
[00152] The twenty eighth set of values of the variables of Structural Formula
(I) is as
follows:
Values of Z'-Z4, RI, R2, Ring S, Ring T, X, JA, JB, jc, jT, R, R,, Ri2, R13,
R14, Ris, Rs,
Rt, R21, R22, R23, R24, and K.-.25
are each and independently as described above in the twenty
sixth or twenty seventh set of values of the variables of Structural Formula
(I).
-30-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
Q1 is independently -C(0)0-, -NRC(0)-, -C(0)NR-, -NRC(0)NR'-,
or -(CH2)1,2-Y-.
YI is independently -C(0)0-, -NRC(0)-, -C(0)NR-, or -NRC(0)NR'-.R14 and each
RI5 are each independently -H, CI-C6 alkyl, or C1-C6 haloalkyl.
The remaining variables of Structural Formula (I) are each and independently
as
described above in the first set of values of the variables of Structural
Formula (I).
[00153] The twenty ninth set of values of the variables of Structural Formula
(I) is as
follows:
Values of Z'-Z4, RI, R2, Ring S, Ring T, X, jA, jB, jc, jT, R, R,, R12, RI3,
R14, R15, Rs,
Rt, R21, R22, R23, R24, and K-25
are each and independently as described above in the twenty
sixth or twenty seventh set of values of the variables of Structural Formula
(I).
Q1 is independently -C(0)0-, -NRC(0)-, or -C(0)NR-.
The remaining variables of Structural Formula (I) are each and independently
as
described above in the first set of values of the variables of Structural
Formula (I).
[00154] The thirtieth set of values of the variables of Structural Formula
(I) is as follows:
Values of Z'-Z4, RI, R2, Ring S, Ring T, X, JA, jB, jc, jT, R, R,, Rt2, R13,
R14, R15, Rs,
Rt, R21, R22, R23, K-24,
and R25 are each and independently as described above in the twenty
sixth or twenty seventh set of values of the variables of Structural Formula
(I).
Q1 is independently -C(0)0-, -NHC(0)-, or -C(0)NH-.
The remaining variables of Structural Formula (I) are each and independently
as
described above in the first set of values of the variables of Structural
Formula (I).
[00155] The thirty first set of values of the variables of Structural Formula
(I) is as
follows:
Values of Z1-Z4, R2, Ring S, Ring T, X, jA, je, jc, j-r, Q1, yl, R12, R.13,
R14, R15, RS, Fe,
R21, R22, R23,
K and R25 are each and independently as described above in any one
of the
twenty sixth through thirtieth sets of values of the variables of Structural
Formula (I).
RI is independently -H or an optionally substituted C1-C6 aliphatic group; and
R and R' are each and independently -H or -CH3; or
optionally RI, together with R' and the nitrogen to which they are attached,
form an
optionally substituted, 4-8 membered heterocyclic group.
The remaining variables of Structural Formula (I) are each and independently
as
described above in the first set of values of the variables of Structural
Formula (I).
[00156] The thirty second set of values of the variables of Structural Formula
(I) is as
follows:
-31-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
Values of Z1-Z4, Ri, R2, Ring s, jA, jn, jc, jT, Qi, yl, R, R,, R12, R13,
R14, R15, Rs,
Rt, R21, R22, R23, R24, and K-25
are each and independently as described above in any one of
the twenty sixth through thirty first sets of values of the variables of
Structural Formula (I).
Ring T is:
=
R23 R22
R22 R23 R23 Ali.. R25
I
R14
R21 21 tto R r
Ria
R14
A3 R22
R15
(227 CO 2R 1 " R15R24 R21
R15
R24 CO2R1 C07R1
3
R23
, R22
R22 R23
=-=. R21
1 R21 R14 ve
R14
\Iõ R15R24
'111-
CO2R1 ,or CO2R1
wherein each of Rings A1-A5 is independently and optionally further
substituted with one or
more substituents selected from the group consisting of halogen, cyano,
hydroxy, C1-C4 alkyl,
CI-Ca haloalkyl, and -0(CI-C4 alkyl).
The remaining variables of Structural Formula (I) are each and independently
as
described above in the first set of values of the variables of Structural
Formula (I).
[00157] The thirty third set of values of the variables of Structural Formula
(I) is as
follows:
Values of Z1-Z4, RI, R2, Ring S, Ring T, X, JA, JB, Jc, JT, Q1, Y1, R, R',
R12, R13, Rs,
and RI, are each and independently as described above in any one of the twenty
sixth through
thirty second sets of values of the variables of Structural Formula (I).
R14 and each R15 are each independently ¨H or C1.6 alkyl.
R21, R22, R23, 24,
and R25 areeach independently ¨H or C1_6 alkyl.
The remaining variables of Structural Formula (I) are each and independently
as
described above in the first set of values of the variables of Structural
Formula (I).
[00158] The thirty fourth set of values of the variables of Structural
Formula (I) is as
follows:
Values of Z1-Z4, R2, Ring S, Ring T, X, JA, JB, Jc, JT, QI, YI, R, R', Rt2,
R13, Rs, Rt,
are each and independently as described above in any one of the twenty sixth
through thirty
second sets of values of the variables of Structural Formula (I).
RI is H or optionally substituted C1-6 alkyl.
-32-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
R14, RI5, R2I, R22, R23, R24, and K.-.25
are each independently ¨H.
The remaining variables of Structural Formula (I) are each and independently
as
described above in the first set of values of the variables of Structural
Formula (I).
1001591 The thirty fifth set of values of the variables of Structural
Formula (I) is as
follows:
Values of Z'-Z4, RI, R2, Ring S, Ring T, X, jA, js, jc, jT, Qi, yi, R, R.,
R12, R13, Rs,
RI, R21, R22, R23, R24, and R25
are each and independently as described above in any one of the
twenty sixth through thirty fourth sets of values of the variables of
Structural Formula (I).
q is 1.
The remaining variables of Structural Formula (I) are each and independently
as
described above in the first set of values of the variables of Structural
Formula (I).
[00160] The thirty sixth set of values of the variables of Structural
Formula (I) is as
follows:
Values of Z'-Z4, RI, R2, Ring S, X, JA, j8, JC, JT, Q1, yl, R, Rt, ¨s,
and re are each and
independently as described above in any one of the second through twenty fifth
sets of values
of the variables of Structural Formula (I).
Ring T is selected from:
Ria
R14 co A10
'721 R14
R15 µ711,- R15
01R1 R 1 5 IS , 3 t2Z, Q1R1 01R1 ,or
R14 R15
.11/4 Q Ri
wherein:
R14 and each R15 are each independently ¨H, CI-C6 alkyl, or CI-C6 haloalkyl;
and
each of Rings A8-A11 is independently and optionally substituted with one or
more
substituents selected from the group consisting of halogen, cyano, hydroxy,
oxo, -NH2,
-NH(CI-C4 alkyl), -N(Ci-C4 -000(Ci-C4 alkyl), -CO(CI-C4 alkyl), -CO2H, -
CNC!'
C4 alkyl), CI-Ca alkyl, CI-Ca haloalkyl, and -0(C,-C4 alkyl).
The remaining variables of Structural Formula (I) are each and independently
as
described above in the first set of values of the variables of Structural
Formula
[00161] The thirty seventh set of values of the variables of Structural
Formula (I) is as
follows:
Values of Z1-Z4, RI,
R2, Ring S, Ring T, X, JA, JB, Jc, JT, R, R', R5, Ft', R'4,
and R15
-33-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
are each and independently as described above in the thirty sixth set of
values of the variables
of Structural Formula (I).
Q1 is independently -C(0)-, -C(0)0-, -NRC(0)-, -C(0)NR-, ¨NRC(0)NR'¨,
or -(CH2)1,2¨Y¨; and
Yi is independently -C(0)-, -C(0)0-, -NRC(0)-, -C(0)NR-, or ¨NRC(0)NR'¨.
The remaining variables of Structural Formula (I) are each and independently
as
described above in the first set of values of the variables of Structural
Formula
1001621 The thirty eighth set of values of the variables of Structural
Formula (I) is as
follows:
Values of Z1-Z4, RI, R2, Ring S, Ring T, X, jA, jB, jc, jT, Qi, y 1 R, R,,
K¨$3
and Ware
each and independently as described above in the thirty sixth or thirty
seventh set of values of
the variables of Structural Formula (I).
R14 and each RI5 are each independently ¨H or C1_6 alkyl.
Each of Rings A8-A11 is independently and optionally substituted with one or
more
substituents selected from the group consisting of halogen, cyano, hydroxy, CI-
Ca alkyl, CI-
C4 haloalkyl, and -0(C1-C4 alkyl).
The remaining variables of Structural Formula (I) are each and independently
as
described above in the first set of values of the variables of Structural
Formula
[00163] The thirty ninth set of values of the variables of Structural
Formula (I) is as
follows:
Values of Z1-Z4, RI, R2, Ring S, Ring T, X, JA, JB, Jc, JT, R, R', Rs, le,
R14, and R15
are each and independently as described above in the thirty sixth set of
values of the variables
of Structural Formula (I).
Q1 is independently -NRC(0)-, -C(0)NR-, or ¨NRC(0)NR'¨.
The remaining variables of Structural Formula (I) are each and independently
as
described above in the first set of values of the variables of Structural
Formula
[00164] The fortieth set of values of the variables of Structural Formula
(I) is as follows:
Values of Z'-Z4, RI, R2, Ring S, Ring T, X, jA, jB, jc, jT, Qi,
Y R, R', Rs, Rt, RI4,
and R15 are each and independently as described above in any one of the thirty
sixth through
thirty ninth sets of values of the variables of Structural Formula (I).
R and R' are each and independently -H or -CH3.
The remaining variables of Structural Formula (I) are each and independently
as
described above in the first set of values of the variables of Structural
Formula
[00165] The forty first set of values of the variables of Structural
Formula (I) is as follows:
-34-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
Values of Z1-Z4, RI, R2, Ring S, Ring T, X, JA, JB, Jc, jT, Q1, Y-1,
R, R', Rs, Rt, R14,
and Ri5 are each and independently as described above in the thirty sixth
through thirty ninth
sets of values of the variables of Structural Formula (I).
R and R' are each and independently -H or -CH3.
RI is independently a 4-7 membered heterocyclic group, a phenyl group, or a 5-
6
membered heteroaryl group, wherein each of said heterocyclic, phenyl and
heteroaryl groups
is independently and optionally substituted with one or more substituents
independently
selected from the group consisting of halogen, cyano, hydroxy, oxo, -NH2, -
NH(CI-C4 alkyl),
-N(C1-C4 alky1)2, -000(C1-C4 alkyl), -CO(Ct-C4 alkyl), -0O2H, -0O2(CI-C4
alkyl), CI-CI
alkyl, CI-CI haloalkyl, and -0(Ct-C4 alkyl); or
optionally Ri and R', together with the nitrogen atom to which they are
attached, form
an optionally substituted, 4-8 membered heterocyclic group.
The remaining variables of Structural Formula (I) are each and independently
as
described above in the first set of values of the variables of Structural
Formula
[00166] In the forty second set of values of the variables of Structural
Formula (I), p is 1
or 2, k is 1 or 2, and the remaining variables are each and independently as
described above
in any one of the sets of values of the variables of Structural Formula (I).
[00167] In the forty third set of values of the variables of Structural
Formula (I), X is -F,
-Cl, -CH3, or -CF3, and the remaining variables are each and independently as
described
above in any one of the sets of values of the variables of Structural Formula
(I).
[00168] In the forty fourth set of values of the variables of Structural
Formula (I), X is -F,
or -Cl, and the remaining variables are each and independently as described
above in any one
of the sets of values of the variables of Structural Formula (I).
[00169] Specific examples of the compounds represented by Structural Formula
(I)
include:
-35-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
F F
F F
....L..
H H
N,
F F tH
...-
at H
'
F
0
i
\ NH,4-OH o¨OH
/ \ NH
F --N F --N ,--)
F F
1 \
,N I N
'
N N N N
' H
F F
/ \ NH,o-
i \ NH
F --- N Na' F --N Na+
,
F F
, \ 1 '====-. \
I ,N j..., ,N =
,= N.1 N HN
, ,
F F
0
\
I NH OH o`X,--OH
C CI
, "--.. \
I N I N
*...... = .- =
N N N N
H
' H ,
CI CI
F F. F F
, 1
\ . \ , ,
, ,
N i Ns
H N H T
HN-N(-)%(=)- Na+, HN-N 0 0- Na,
' 0
/ \ NH OH
¨ N ¨ N
F F
, \
I N I N .
-... =
N HN N [1
-36-
CA 02822059 2013-06-17
WO 2012/083121 PCT/US2011/065388
a
NI/ "N...NH,
F
N OH
I F
\
H -
HN-NN N
0 ON a, H ,
0
7 Ý\ NH-OH
--N \)' NC --N
F F
I \ N I \ N
k;
N N N "
H
0
i \ NH ,- ft ii--)t
O
N H
F
NC --N
F
\N
, \ I
I
, N
.--...õ, H
N "
H ,and ,
and pharmaceutically acceptable salts thereof.
[00170] Additional specific examples of the compounds represented by
Structural Formula
(I) include:
=
CI CI
F F F F
I I
N N
HN-N HN-N
0 OH, 0 OH,
F
F F
F
F. I
--F \ i
I N / i NNCL NH
H
\ /
N i N N NH HN-N
H t
HN-N O sOCs
CI,
,
F
F NC io F
NC 401 F j.)
\
\ /
/ N I he'sp
N : H
N I H - HN-N
HN-N 0 OH
0 OH
,and ,and
-37-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
pharmaceutically acceptable salts thereof.
[00171] In some embodiments, the compounds of the invention are selected from
any one
of the compounds depicted in Tables 1 and 2, or pharmaceutically acceptable
salts thereof.
[00172] As used herein, a reference to compound(s) of the invention (for
example, the
compound(s) of Structural Formula (I), or compound(s) of claim 1) will include
pharmaceutically acceptable salts thereof.
[00173] The compounds of the invention described herein can be prepared by any
suitable
method known in the art. For example, they can be prepared in accordance with
procedures
described in WO 2005/095400, WO 2007/084557, WO 2010/011768, WO 2010/011756,
WO
2010/011772, WO 2009/073300, and PCT/US2010/038988 filed on June 17, 2010. For
example, the compounds shown in Tables 1 and 2, and the specific compounds
depicted
above can be prepared by any suitable method known in the art, for example, WO
2005/095400, WO 2007/084557, WO 2010/011768, WO 2010/011756, WP 2010/011772,
WO 2009/073300, and PCT/US2010/038988, and by the exemplary syntheses
described
below under Exemplification.
[00174] The present invention provides methods of preparing a compound
represented by
Structural Formula (I). In one embodiment, the compounds of the invention can
be prepared
as depicted in General Schemes 1-5. Any suitable condition(s) known in the art
can be
employed in the invention for each step depicted in the schemes.
[00175] In a specific embodiment, as shown in General Scheme 1, the methods
comprise
the step of reacting Compound (A) with Compound (B) under suitable conditions
to form a
compound of Structural Formula (XX), wherein L2 is a halogen (F, Cl, Br, or
I), G is trityl
(Tr), and the remaining variables of Compounds (A), (B) and Structural Formula
(XX) are
each and independently as described herein. Typically, L2 is F, Cl or Br. More
typically, L2
is Cl or Br. The methods further comprise the step of deprotecting the G group
under suitable
conditions to form the compounds of Structural Formula (I). Any suitable
condition(s)
known in the art can be employed in the invention for each step depicted in
the schemes. For
example, any suitable condition described in WO 2005/095400 and WO 2007/084557
for the
coupling of a dioxaboraolan with a chloro-pyrimidine can be employed for the
reaction
between Compounds (A) and (B). Specifically, the reaction between compounds
(A) and (B)
can be performed in the presence of Pd(PPh3)4 or Pd2(dba)3 (dba is
dibenzylidene acetone).
For example, the de-tritylation step can be performed under an acidic
condition (e.g.,
trifluoroacetic acid (TFA)) in the presence of, for example, Et3SiH (Et is
ethyl). Specific
exemplary conditions are described in the Exemplification below
-38-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
[00176] Optionally, the method further comprises the step of preparing
Compound (A) by
reacting Compound (E) with Compound (D). Any suitable conditions know in the
art can be
employed in this step, and Compounds (E) and (D) can be prepared by any
suitable method
known in the art. Specific exemplary conditions are described in the
Exemplification below.
General Scheme 1
,Z3= Z4 iz3=z4
e s + NH,= ___________ _ z2, S
Q1-R1
1
L2 L2
(E) (D) (A)
0)1-*
0--
X
I ,N
N
(B)
/3=Z4
=
Z,2 S
Q1-R1
(XX)
1Z3=Z4
Z,2 S
x Z1 Q1- R1
I ,
N
(I)
[00177] In another specific embodiment, as shown in General Scheme 2, the
methods
comprise the step of reacting Compound (G) with Compound (D) under suitable
conditions
to form a compound of Structural Formula (XX), wherein LI is a halogen (F, Cl,
Br, or I), G
is trity I (Tr), and the remaining variables of Compounds (G), (D) and
Structural Formula
(XX) are each and independently as described herein. Typically, LI is F, CI or
Br. More
typically, LI is Cl or Br. The methods further comprise the step of
deprotecting the G group
under suitable conditions to form the compounds of Structural Formula (I). Any
suitable
condition(s) known in the art can be employed in the invention for each step
depicted in the
schemes. For example, any suitable amination condition known in the art can be
employed in
-39-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
the invention for the reaction of Compounds (G) and (D), and any suitable
condition for
deprotecting a Ts group can be employed in the invention for the deprotection
step. For
example, the amination step can be performed in the presence of a base, such
as NEt3 or
1\1(3r)2Et. For example, the de-tritylation step can be performed under an
acidic condition
(e.g., trifluoroacetic acid (TFA)) in the presence of, for example, Et3SiH (Et
is ethyl).
Additional specific exemplary conditions are described in the Exemplification
below
1001781 Optionally, the method further comprises the step of preparing
Compound (G) by
reacting Compound (F) with Compound (B). Any suitable conditions know in the
art can be
employed in this step. For example, any suitable condition described in WO
2005/095400
and WO 2007/084557 for the coupling of a dioxaboralan with a chloro-pyrimidine
can be
employed for the reaction between Compounds (F) and (B). Specifically, the
reaction
between compounds (F) and (B) can be performed in the presence of Pd(PPh3)4 or
Pd2(dba)3
(dba is dibenzylidene acetone). Specific exemplary conditions are described in
the
Exemplification below.
General Scheme 2
-40-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
73=
0>L(¨ Z2 s 5--L1
1z3=4 B--0
Z2 S
(F) L1 4. X
I
z ¨D. X /N
L2
N N
(B)
(G)
NH2 0Q1--R1
(D)
3_ 4
Z2 /
-Z)
NH 0
x
N (XX)
73=
Z2 S 5¨NH
x Q1¨R1
(I)
1001791 In yet another specific embodiment, as shown in General Scheme 3, the
methods
comprise the step of reacting Compound (K) with Compound (D) under suitable
conditions
to form a compound of Structural Formula (XX), wherein G is tosyl or trityl,
and the
remaining variables of Compounds (K), (D) and Structural Formula (XX) are each
and
independently as described herein. Typically G is tosyl. The methods further
comprise the
step of deprotecting the G group under suitable conditions to form the
compounds of
Structural Formula (I). Any suitable condition(s) known in the art can be
employed in the
invention for each step depicted in the schemes. For example, any suitable
reaction condition
known in the art, for example, in WO 2005/095400 and WO 2007/084557 for the
coupling of
an amine with a sulfinyl group can be employed for the reaction of Compounds
(K) with
Compound (D). For example, Compounds (D) and (K) can be reacted in the
presence of a
base, such as NEt3 or N(1l3r)2(Et). For example, the de-tritylation step can
be performed
-41-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
under an acidic condition (e.g., trifluoroacetic acid (TFA)) in the presence
of, for example,
Et3SiH (Et is ethyl). Additional specific exemplary conditions are described
in the
Exemplification below
[00180] Optionally, the method further comprises the step of preparing
Compound (K) by
oxidizing Compound (J), for example, by treatment with meta-chloroperbenzoic
acid.
[00181] Optionally, the method further comprises the step of preparing
Compound (J) by
reacting Compound (H) with Compound (B). Any suitable conditions know in the
art can be
employed in this step. For example, any suitable condition described in WO
2005/095400
and WO 2007/084557 for the coupling of a dioxaboraolan with a chloro-
pyrimidine can be
employed for the reaction between Compounds (H) and (B). Specifically, the
reaction
between compounds (H) and (B) can be performed in the presence of Pd(PPh3)4 or
Pd2(dba)3
(dba is dibenzylidene acetone) Specific exemplary conditions are described in
the
Exemplification below.
General Scheme 3
,z3=z
,Z3=Z4 13--()
X X
Z2 S +
L"--Z1 /N
' (J)
(H)
(B)
73=<4 ,Z3=7.1
Zs,2 S
Z2 S
Qi 0
X X
(xx) NH2
=
Ql¨R1
G (K)
(D)
/Z3=Z
S /1--NH 411
,
(1)
-42-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
[00182] In yet another specific embodiment, as shown in General Scheme 4, the
methods
comprise the step of reacting Compound (L) with Compound (D) under suitable
conditions to
form a compound of Structural Formula (XX), wherein G is trityl (Ts), and the
remaining
variables of Compounds (L), (D) and Structural Formula (XX) are each and
independently as
described herein. The methods further comprise the step of deprotecting the G
group under
suitable conditions to form the compounds of Structural Formula (I). Any
suitable
condition(s) known in the art can be employed in the invention for each step
depicted in the
schemes. For example, any suitable reaction condition known in the art, for
example, in WO
2005/095400 and WO 2007/084557 for the coupling of an amine with a sulfonyl
group can
be employed for the reaction of Compounds (L) with Compound (D). For example,
Compounds (D) and (L) can be reacted in the presence of a base, such as NEt3
or N(lpr)2(Et).
For example, the de-tritylation step can be performed under an acidic
condition (e.g.,
trifluoroacetic acid (TFA)) in the presence of, for example, Et3SiH (Et is
ethyl). Additional
specific exemplary conditions are described in the Exemplification below
[00183] Optionally, the method further comprises the step of preparing
Compound (L) by
oxidizing Compound (J), for example, by treatment with meta-chloroperbenzoic
acid.
[00184] Optionally, the method further comprises the step of preparing
Compound (J) by
reacting Compound (H) with Compound (B). Reaction conditions are as described
above for
General Scheme 3.
General Scheme 4
=
=
-43-
CA 02822059 2013-06-17
WO 2012/083121 PCT/US2011/065388
4¨ z3=Z4
--
ZS 0 1
73=z4 ...... x..4-Z
XI X
2 i>"-S +
I N
L2--Z1 I N
/
N N N N GI)
(H) G G
(B)
/
/Z3= Z4 Z3=Z4
Z,2 S --NH 0 .
\\ i z2 s __,õ
\\ i _ 0
.,.e..4_z Q,
_,,...4_z. 1/4., _
x x
I N I N
0
N N
(XX) NH2
Q1¨R1
G G (L) .
(D)
i
Z3=Z4
Zõ2 \S --NH 0
\
- z , = Q 1 -R1
X
I N
N ----NI
H (I)
1001851 In yet another specific embodiment, as shown in General Scheme 5, the
methods
comprise the step of reacting Compound (G) with Compound (D) under suitable
conditions
to form a compound of Structural Formula (XX), wherein G is trityl (Tr), and
the remaining
variables of Compounds (G), (D) and Structural Formula (XX) are each and
independently as
described herein. Typical examples for LI is -F, CI or Br. More typical
examples for LI are
-F or Cl. The methods further comprise the step of deprotecting the G group
under suitable
conditions to form the compounds of Structural Formula (I). Any suitable
condition(s)
known in the art can be employed in the invention for each step depicted in
the schemes. For
example, any suitable amination condition known in the art can be employed in
the invention
for the reaction of Compounds (G) and (D), and any suitable condition for
deprotecting a Tr
group can be employed in the invention for the deprotection step. For example,
the
amination step can be performed in the presence of a base, such as NEt3 or
N('Pr)2Et. For
example, the de-tritylation step can be performed under an acidic condition
(e.g.,
trifluoroacetic acid (TFA)) in the presence of, for example, Et3SiH (Et is
ethyl). Additional
specific exemplary conditions are described in the Exemplification below
-44-
CA 02822059 2013-06-17
WO 2012/083121 PCT/US2011/065388
[00186] Optionally, the method further comprises the step of preparing
Compound (G) by
conversion of Compound (0) under suitable conditions. Any suitable conditions
known in
the art can be employed in this step. For example, cyclocondensation with
hydrazine
hydrate. Optionally, the method further comprises the step of preparing
Compound (0) by
reacting Compound (M) and Compound (N) under suitable conditions. For example,
lithiation of Compound (M) with lithium diisopropylamide (LDA) and addition of
the
resulting lithio species into Compound (N). Specific exemplary conditions are
described in
the Exemplification below.
General Scheme 5
z3=z4
.0S
N
73=Z4 ¨Z1
Z2µ + x
>µ___ 1
N F N F (0)
(M) (N)
/3= zttZ3=Z4
/)--NH 410 Z\ S
Qi
X X
iN
411
(XX) NH2
Q1¨R1 N (G)
(D)
,Z3=Z4
S
Q1¨R1
X
(1)
1001251 Compounds (A) -(0) can be prepared by any suitable method known in
the
art. Specific exemplary synthetic methods of these compounds are described
below in the
Exemplification. In one embodiment, Compounds (A), (G), (J), (K), (L) and (0)
can be
prepared as described in General Schemes 1-5.
[00126] In some embodiments, the present invention is directed to a
compound
-45-
CA 02822059 2013-06-17
WO 2012/083121 PCT/US2011/065388
represented by Structural Formula (XX), wherein the variables of Structural
Formula (XX)
are each and independently as described herein, and G is trityl. The compounds
represented
by Structural formula (XX) can be prepared as described above. In one
embodiment, the
compounds of the invention can be prepared as depicted in General Schemes 1-5.
Specific
examples include:
= F CI F
F F NC F),,i
\ I I
*\ J
/ C /
N i N N N N : N i N N :
H - H - H -
J\I-N CO2Me -N O2Me N-N CO2
Ph Ph Ph)4, Me
Ph )
2Ph Ph Ph
, Ph , Ph ,
F F
H H
/\N., 0 ,\ N, ll0
F
F
¨N ¨"N 0=,"4c
F OEt F OH
, \
I N I
n; *---....;N
N 9 N 9
Tr , Tr ,
\
0
0
F F
0
H cy-OH/ H FNI".
/ \ N, --- N,
= H
- N \ N "N)r. N, ,
F F 0. N \ /11
, ',.. \ F \ 0
I,N I ,N
Ni I F
-......,
N
N " ..N )ç-Ph A-Ph N N
Ph-`PhPh
, Ph , (Ph)1C ,
\
0
n---/
w---,
0
H
N'====(D p1".").__\ ,.c"-(3\
/z.---___( t=ii N -
N ¨
L...N...
F N F N
i F I N I N
N 1 .- =
N N N..---N'
N N õ\---Ph A--Ph
, Ph- \ Ph- \
(Ph)1C Ph , and Ph , and
pharmaceutically acceptable salts thereof. Additional specific examples
include:
-46-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
0\ 0
0
F F
N H 0
CN F CN
/
N.1N
\ N \N
N 6,1 N Ph¨f-ph
Tr Tr Ph ,and
F N CI
N H 0
/ 1
-N
Ph¨t-ph
Ph , and pharmaceutically acceptable salts thereof.
Definitions and General TerminoloRT
[00127] For purposes of
this invention, the chemical elements are identified in
accordance with the Periodic Table of the Elements, CAS version, Handbook of
Chemistry
and Physics, 75th Ed. Additionally, general principles of organic chemistry
are described in
"Organic Chemistry", Thomas Sorrell, University Science Books, Sausolito:
1999, and
"March's Advanced Organic Chemistry", 5th Ed., Ed.: Smith, M.B. and March, J.,
John
Wiley & Sons, New York: 2001, the entire contents of which are hereby
incorporated by
reference.
[00128] As described herein, compounds of the invention may optionally be
substituted with one or more substituents, such as illustrated generally
below, or as
exemplified by particular classes, subclasses, and species of the invention.
It will be
appreciated that the phrase "optionally substituted" is used interchangeably
with the phrase
"substituted or unsubstituted." In general, the term "substituted", whether
preceded by the
term "optionally" or not, refers to the replacement of one or more hydrogen
radicals in a
given structure with the radical of a specified substituent. Unless otherwise
indicated, an
optionally substituted group may have a substituent at each substitutable
position of the
group. When more than one position in a given structure can be substituted
with more than
one substituent selected from a specified group, the substituent may be either
the same or
different at each position. When the term "optionally substituted" precedes a
list, said term
-47-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
refers to all of the subsequent substitutable groups in that list. lf a
substituent radical or
structure is not identified or defined as "optionally substituted", the
substituent radical or
structure is unsubstituted. For example, if X is optionally substituted
Ci.C3alkyl or phenyl; X
may be either optionally substituted C1-C3 alkyl or optionally substituted
phenyl. Likewise,
if the term "optionally substituted" follows a list, said term also refers to
all of the
substitutable groups in the prior list unless otherwise indicated. For
example: if X is Ci.
C3alkyl or phenyl wherein X is optionally and independently substituted by Jx,
then both C1_
C3alkyl and phenyl may be optionally substituted by Jx.
1001291 The phrase "up to", as used herein, refers to zero or any integer
number that is
equal or less than the number following the phrase. For example, "up to 3"
means any one of
0, 1, 2, and 3. As described herein, a specified number range of atoms
includes any integer
therein. For example, a group having from 1-4 atoms could have I, 2, 3, or 4
atoms.
1001301 Selection of substituents and combinations of substituents
envisioned by this
invention are those that result in the formation of stable or chemically
feasible compounds.
The term "stable", as used herein, refers to compounds that are not
substantially altered when
subjected to conditions to allow for their production, detection, and,
specifically, their
recovery, purification, and use for one or more of the purposes disclosed
herein. In some
embodiments, a stable compound or chemically feasible compound is one that is
not
substantially altered when kept at a temperature of 40 C or less, in the
absence of moisture or
other chemically reactive conditions, for at least a week. Only those choices
and
combinations of substituents that result in a stable structure are
contemplated. Such choices
and combinations will be apparent to those of ordinary skill in the art and
may be determined
without undue experimentation.
1001311 The term "aliphatic" or "aliphatic group", as used herein, means a
straight-
chain (i.e., unbranched), or branched, hydrocarbon chain that is completely
saturated or that
contains one or more units of unsaturation but is non-aromatic. Unless
otherwise specified,
aliphatic groups contain 1-20 aliphatic carbon atoms. In some embodiments,
aliphatic groups
contain 1-10 aliphatic carbon atoms. In other embodiments, aliphatic groups
contain 1-8
aliphatic carbon atoms. In still other embodiments, aliphatic groups contain 1-
6 aliphatic
carbon atoms, and in yet other embodiments, aliphatic groups contain 1-4
aliphatic carbon
atoms. Aliphatic groups may be linear or branched, substituted or
unsubstituted alkyl,
alkenyl, or alkynyl groups. Specific examples include, but are not limited to,
methyl, ethyl,
isopropyl, n-propyl, sec-butyl, vinyl, n-butenyl, ethynyl, and tert-butyl and
acetylene.
1001321 The term "alkyl" as used herein means a saturated straight or
branched chain
-48-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
hydrocarbon. The term "alkenyl" as used herein means a straight or branched
chain
hydrocarbon comprising one or more double bonds. The term "alkynyl" as used
herein
means a straight or branched chain hydrocarbon comprising one or more triple
bonds. Each
of the "alkyl", "alkenyl" or "alkynyl" as used herein can be optionally
substituted as set forth
below. In some embodiments, the "alkyl" is C1-C6 alkyl or CI-Ca alkyl. In some
embodiments, the "alkenyl" is C2-C6 alkenyl or C2-C4 alkenyl. In some
embodiments, the
"alkynyl" is C2-C6 alkynyl or C2-C4 alkynyl.
1001331 The term "cycloaliphatic" (or "carbocycle" or "carbocycly1" or
"carbocyclic")
refers to a non-aromatic carbon only containing ring system which can be
saturated or
contains one or more units of unsaturation, having three to fourteen ring
carbon atoms. In
some embodiments, the number of carbon atoms is 3 to 10. In other embodiments,
the
number of carbon atoms is 4 to 7. In yet other embodiments, the number of
carbon atoms is 5
or 6. The term includes monocyclic, bicyclic or polycyclic, fused, spiro or
bridged
carbocyclic ring systems. The term also includes polycyclic ring systems in
which the
carbocyclic ring can be "fused" to one or more non-aromatic carbocyclic or
heterocyclic
rings or one or more aromatic rings or combination thereof, wherein the
radical or point of
attachment is on the carbocyclic ring. "Fused" bicyclic ring systems comprise
two rings
which share two adjoining ring atoms. Bridged bicyclic group comprise two
rings which
share three or four adjacent ring atoms. Spiro bicyclic ring systems share one
ring atom.
Examples of cycloaliphatic groups include, but are not limited to, cycloalkyl
and
cycloalkenyl groups. Specific examples include, but are not limited to,
cyclohexyl,
cyclopropenyl, and cyclobutyl.
1001341 The term "heterocycle" (or "heterocyclyl", or "heterocyclic" or
"non-aromatic
heterocycle") as used herein refers to a non-aromatic ring system which can be
saturated or
contain one or more units of unsaturation, having three to fourteen ring atoms
in which one or
more ring carbons is replaced by a heteroatom such as, N, S, or 0 and each
ring in the system
contains 3 to 7 members. In some embodiments, non-aromatic heterocyclic rings
comprise
up to three heteroatoms selected from N, S and 0 within the ring. In other
embodiments,
non-aromatic heterocyclic rings comprise up to two heteroatoms selected from
N, S and 0
within the ring system. In yet other embodiments, non-aromatic heterocyclic
rings comprise
up to two heteroatoms selected from N and 0 within the ring system. The term
includes
monocyclic, bicyclic or polycyclic fused, spiro or bridged heterocyclic ring
systems. The
term also includes polycyclic ring systems in which the heterocyclic ring can
be fused to one
or more non-aromatic carbocyclic or heterocyclic rings or one or more aromatic
rings or
-49-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
combination thereof, wherein the radical or point of attachment is on the
heterocyclic ring.
Examples of heterocycles include, but are not limited to, piperidinyl,
piperizinyl, pyrrolidinyl,
pyrazolidinyl, imidazolidinyl, azepanyl, diazepanyl, triazepanyl, azocanyl,
diazocanyl,
triazocanyl, oxazolidinyl, isoxazolidinyl, thiazolidinyl, isothiazolidinyl,
oxazocanyl,
oxazepanyl, thiazepanyl, thiazocanyl, benzimidazolonyl, tetrahydrofuranyl,
tetrahydrofuranyl, tetrahydrothiophenyl, tetrahydrothiophenyl, morpholino,
including, for
example, 3-morpholino, 4-morpholino, 2-thiomorpholino, 3-thiomorpholino, 4-
thiomorpholino, 1-pyrrolidinyl, 2-pyrrolidinyl, 3-pyrrolidinyl, 1-
tetrahydropiperazinyl, 2-
tetrahydropiperazinyl, 3-tetrahydropiperazinyl, 1-piperidinyl, 2-piperidinyl,
3-piperidinyl, 1-
pyrazolinyl, 3-pyrazolinyl, 4-pyrazolinyl, 5-pyrazolinyl, 1-piperidinyl, 2-
piperidinyl, 3-
piperidinyl, 4-piperidinyl, 2-thiazolidinyl, 3-thiazolidinyl, 4-thiazolidinyl,
1-imidazolidinyl,
2-imidazolidinyl, 4-imidazolidinyl, 5-imidazolidinyl, indolinyl,
tetrahydroquinolinyl,
tetrahydroisoquinolinyl, benzothiolanyl, benzodithianyl, 3-(1-alkyl)-
benzimidazol-2-onyl,
and 1,3-dihydro-imidazol-2-onyl.
[00135] The term "aryl" (or "aryl ring" or "aryl group") used alone or as
part of a
larger moiety as in "aralkyl", "aralkoxy", "aryloxyalkyl", or "heteroaryl"
refers to
carbocyclic aromatic ring systems. The term "aryl" may be used interchangeably
with the
terms "aryl ring" or "aryl group".
[00136] "Carbocyclic aromatic ring" groups have only carbon ring atoms
(typically six
to fourteen) and include monocyclic aromatic rings such as phenyl and fused
polycyclic
aromatic ring systems in which two or more carbocyclic aromatic rings are
fused to one
another. Examples include 1-naphthyl, 2-naphthyl, 1-anthracyl and 2-anthracyl.
Also
included within the scope of the term "carbocyclic aromatic ring" or
"carbocyclic aromatic",
as it is used herein, is a group in which an aromatic ring is "fused" to one
or more non-
aromatic rings (carbocyclic or heterocyclic), such as in an indanyl,
phthalimidyl,
naphthimidyl, phenanthridinyl, or tetrahydronaphthyl, where the radical or
point of
attachment is on the aromatic ring.
[00137] The terms "heteroaryl", "heteroaromatic", "heteroaryl ring",
"heteroaryl
group", "aromatic heterocycle" or "heteroaromatic group", used alone or as
part of a larger
moiety as in "heteroaralkyl" or "heteroarylalkoxy", refer to heteroaromatic
ring groups
having five to fourteen members, including monocyclic heteroaromatic rings and
polycyclic
aromatic rings in which a monocyclic aromatic ring is fused to one or more
other aromatic
ring. Heteroaryl groups have one or more ring heteroatoms. Also included
within the scope
of the term "heteroaryl", as it is used herein, is a group in which an
aromatic ring is "fused"
-50-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
to one or more non-aromatic rings (carbocyclic or heterocyclic), where the
radical or point of
attachment is on the aromatic ring. Bicyclic 6,5 heteroaromatic ring, as used
herein, for
example, is a six membered heteroaromatic ring fused to a second five membered
ring,
wherein the radical or point of attachment is on the six membered ring.
Examples of
heteroaryl groups include pyridyl, pyrazinyl, pyrimidinyl, pyridazinyl,
imidazolyl, pyrrolyl,
pyrazolyl, triazolyl, tetrazolyl, oxazolyl, isoxazolyl, oxadiazolyl,
thiazolyl, isothiazolyl or
thiadiazolyl including, for example, 2-furanyl, 3-furanyl, N-imidazolyl, 2-
imidazolyl, 4-
imidazolyl, 5-imidazolyl, 3-isoxazolyl, 4-isoxazolyl, 5-isoxazolyl, 2-
oxadiazolyl, 5-
oxadiazolyl, 2-oxazolyl, 4-oxazolyl, 5-oxazolyl, 3-pyrazolyl, 4-pyrazolyl, 1-
pyrrolyl, 2-
pyrrolyl, 3-pyrrolyl, 2-pyridyl, 3-pyridyl, 4-pyridyl, 2-pyrimidinyl, 4-
pyrimidinyl, 5-
pyrimidinyl, 3-pyridazinyl, 2-thiazolyl, 4-thiazolyl, 5-thiazolyl, 2-
triazolyl, 5-triazolyl,
tetrazolyl, 2-thienyl, 3-thienyl, carbazolyl, benzimidazolyl, benzothienyl,
benzofuranyl,
indolyl, benzotriazolyl, benzothiazolyl, benzoxazolyl, benzimidazolyl,
isoquinolinyl, indolyl,
isoindolyl, acridinyl, benzisoxazolyl, isothiazolyl, 1,2,3-oxadiazolyl, 1,2,5-
oxadiazolyl,
1,2,4-oxadiazolyl, 1,2,3-triazolyl, 1,2,3-thiadiazolyl, 1,3,4-thiadiazolyl,
1,2,5-thiadiazolyl,
purinyl, pyrazinyl, 1,3,5-triazinyl, quinolinyl (e.g., 2-quinolinyl, 3-
quinolinyl, 4-quinolinyl),
and isoquinolinyl (e.g., 1-isoquinolinyl, 3-isoquinolinyl, or 4-
isoquinoliny1).
1001381 As used herein, "cyclo", "cyclic", "cyclic group" or "cyclic
moiety", include
mono-, bi-, and tri-cyclic ring systems including cycloaliphatic,
heterocycloaliphatic,
carbocyclic aryl, or heteroaryl, each of which has been previously defined.
1001391 As used herein, a "bicyclic ring system" includes 8-12 (e.g., 9,
10, or 11)
membered structures that form two rings, wherein the two rings have at least
one atom in
common (e.g., 2 atoms in common). Bicyclic ring systems include
bicycloaliphatics (e.g.,
bicycloalkyl or bicycloalkenyl), bicycloheteroaliphatics, bicyclic carbocyclic
aryls, and
bicyclic heteroaryls.
1001401 As used herein, a "bridged bicyclic ring system" refers to a
bicyclic
heterocycloalipahtic ring system or bicyclic cycloaliphatic ring system in
which the rings are
bridged. Examples of bridged bicyclic ring systems include, but are not
limited to,
adamantanyl, norbomanyl, bicyclo[3.2.1]octyl, bicyclo[2.2.2]octyl,
bicyclo[3.3.1]nonyl,
bicyclo[3.2.3]nonyl, 2-oxa-bicyclo[2.2.2]octyl, 1-aza-bicyclo[2.2.2]octyl, 3-
aza-
bicyclo[3.2.1]octyl, and 2,6-dioxa-tricyclo[3.3.1.03,7]nonyl. A bridged
bicyclic ring system
can be optionally substituted with one or more substituents such as alkyl
(including
carboxyalkyl, hydroxyalkyl, and haloalkyl such as trifluoromethyl), alkenyl,
alkynyl,
cycloalkyl, (cycloalkyl)alkyl, heterocycloalkyl, (heterocycloalkyl)alkyl,
carbocyclic aryl,
-51-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
heteroaryl, alkoxy, cycloalkyloxy, heterocycloalkyloxy, (carbocyclic aryl)oxy,
heteroaryloxy,
aralkyloxy, heteroaralkyloxy, aroyl, heteroaroyl, nitro, carboxy,
alkoxycarbonyl,
alkylcarbonyloxy, aminocarbonyl, alkylcarbonylamino, cycloalkylcarbonylamino,
(cycloalkylalkyl)carbonylamino, (carbocyclic aryl)carbonylamino,
aralkylcarbonylamino,
(heterocycloalkyl)carbonylamino, (heterocycloalkylalkyl)carbonylamino,
heteroarylcarbonylamino, heteroaralkylcarbonylamino, cyano, halo, hydroxy,
acyl, mercapto,
alkylsulfanyl, sulfoxy, urea, thiourea, sulfamoyl, sulfamide, oxo, or
carbamoyl.
[00141] As used herein, "bridge" refers to a bond or an atom or an
unbranched chain of
atoms connecting two different parts of a molecule. The two atoms that are
connected
through the bridge (usually but not always, two tertiary carbon atoms) are
denotated as
"bridgeheads".
[00142] = As used herein, the term "spiro" refers to ring systems having
one atom
(usually a quaternary carbon) as the only common atom between two rings.
[00143] The term "ring atom" is an atom such as C, N, 0 or S that is in the
ring of an
aromatic group, cycloalkyl group or non-aromatic heterocyclic ring.
[00144] A "substitutable ring atom" in an aromatic group is a ring carbon
or nitrogen
atom bonded to a hydrogen atom. The hydrogen can be optionally replaced with a
suitable
substituent group. Thus, the term "substitutable ring atom" does not include
ring nitrogen or
carbon atoms which are shared when two rings are fused. In addition,
"substitutable ring
atom" does not include ring carbon or nitrogen atoms when the structure
depicts that they are
already attached to a moiety other than hydrogen.
[00145] The term "heteroatom" means one or more of oxygen, sulfur,
nitrogen,
phosphorus, or silicon (including, any oxidized form of nitrogen, sulfur,
phosphorus, or
silicon; the quaternized form of any basic nitrogen or; a substitutable
nitrogen of a
heterocyclic ring, for example N (as in 3,4-dihydro-2H-pyrroly1), NH (as in
pyrrolidinyl) or
NR+ (as in N-substituted pyrrolidinyl)).
[00146] As used herein an optionally substituted aralkyl can be substituted
on both the
alkyl and the aryl portion. Unless otherwise indicated as used herein
optionally substituted
aralkyl is optionally substituted on the aryl portion.
[00147] In some embodiments, an aliphatic or heteroaliphatic group, or a
non-aromatic
heterocyclic ring may contain one or more substituents. Suitable substituents
on the saturated
carbon of an aliphatic or heteroaliphatic group, or of a heterocyclic ring are
selected from
those listed above. Other suitable substitutents include those listed as
suitable for the
unsaturated carbon of a carbocyclic aryl or heteroaryl group and additionally
include the
-52-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
following: =0, =S, =NNHR*, =NN(R*)2, =NNHC(0)R*, =NNHCO2(alkyl),
=NNHS02(alkyl), or =NR*, wherein each R* is independently selected from
hydrogen or an
optionally substituted C1.6 aliphatic. Optional substituents on the aliphatic
group of R* are
selected from NH2, NH(C1_4 aliphatic), N(C1.4 aliphatic)2, halogen, C1-4
aliphatic, OH, 0(C1-4
aliphatic), NO2, CN, CO2H, CO2(C1.4 aliphatic), 0(halo C1-4 aliphatic), or
halo(C1_4 aliphatic),
wherein each of the foregoing C1.4aliphatic groups of R* is unsubstituted.
1001481 In some embodiments, optional substituents on the nitrogen of a
heterocyclic
ring include those used above. Other suitable substituents include -R+, -
N(R)2, -C(0)R+,
-CO2R+, -C(0)C(0)R+, -C(0)CH2C(0)R+, -SO2R+, -SO2N(R+)2, -C(=S)N(R4)2, -C(---
NH)-
N(R+)2, or -NR+SO2R+; wherein R+ is hydrogen, an optionally substituted Ci.6
aliphatic,
optionally substituted phenyl, optionally substituted -0(Ph), optionally
substituted -CH2(Ph),
optionally substituted -(CH2)1_2(Ph); optionally substituted -CH=CH(Ph); or an
unsubstituted
5-6 membered heteroaryl or heterocyclic ring having one to four heteroatoms
independently
selected from oxygen, nitrogen, or sulfur, or, two independent occurrences of
R+, on the same
substituent or different substituents, taken together with the atom(s) to
which each R+ group
is bound, form a 5-8-membered heterocyclyl, carbocyclic aryl, or heteroaryl
ring or a 3-8-
membered cycloalkyl ring, wherein said heteroaryl or heterocyclyl ring has 1-3
heteroatoms
independently selected from nitrogen, oxygen, or sulfur. Optional substituents
on the
aliphatic group or the phenyl ring of R+ are selected from NH2, NH(C1.4
aliphatic), N(C1-4
aliphatic)2, halogen, C1-4 aliphatic, OH, 0(C1.4 aliphatic), NO2, CN, CO2H,
CO2(C1-4
aliphatic), 0(halo C1-4 aliphatic), or halo(C1_4 aliphatic), wherein each of
the foregoing
Ci_rialiphatic groups of R4- is unsubstituted.
1001491 In some embodiments, an aryl (including aralkyl, aralkoxy,
aryloxyalkyl and
the like) or heteroaryl (including heteroaralkyl and heteroarylalkoxy and the
like) group may
contain one or more substituents. Suitable substituents on the unsaturated
carbon atom of a
carbocyclic aryl or heteroaryl group are selected from those listed above.
Other suitable
substituents include: halogen; -R ; -OR ; -SRO; 1,2-methylenedioxy; 1,2-
ethylenedioxy;
phenyl (Ph) optionally substituted with le; -0(Ph) optionally substituted with
le;
-(CH2)1_2(Ph), optionally substituted with R ; -CH=CH(Ph), optionally
substituted with le;
-NO2; -CN; -N(R )2; -NR C(0)R ; -NR C(S)R ; -NR C(0)N(R )2; -NR C(S)N(R )2;
-NR CO2R ; -NR NR C(0)R ; -NR NR C(0)N(R )2; -NR NR CO2R ; -C(0)C(0)R ;
-C(0)CH2C(0)R ; -CO2R ; -C(0)R ; -C(S)R ; -C(0)N(R )2; -C(S)N(R )2; -0C(0)N(R
)2;
-0C(0)R ; -C(0)N(OR ) R ; -C(NOR ) R ; -S(0)2R ; -S(0)3R ; -SO2N(R )2; -S(0)R
; -
NR S02N(R )2; -NR S02R ; -N(OR )R ; -C(=NH)-N(R )2; or -(CH2)0_2NHC(0)R ;
wherein
-53-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
each independent occurrence of R is selected from hydrogen, optionally
substituted C1-6
aliphatic, an unsubstituted 5-6 membered heteroaryl or heterocyclic ring,
phenyl, -0(Ph), or
-CH,(Ph), or, two independent occurrences of R , on the same substituent or
different
substituents, taken together with the atom(s) to which each R group is bound,
form a 5-8-
membered heterocyclyl, carbocyclic aryl, or heteroaryl ring or a 3-8-membered
cycloalkyl
ring, wherein said heteroaryl or heterocyclyl ring has 1-3 heteroatoms
independently selected
from nitrogen, oxygen, or sulfur. Optional substituents on the aliphatic group
of R are
selected from NH2, NH(Ci_4aliphatic), N(C1_4aliphatic)2, halogen,
Ci_4aliphatic, OH, 0(Ci_
4aliphatic), NO2, CN, CO2H, CO2(C1.4aliphatic), 0(haloCi_4 aliphatic), or
haloCi_4aliphatic,
CHO, WOK 1-4 aliphatic), C(0)N(Ci_4 aliphatic), wherein each of the foregoing
CI-
4aliphatic groups of R is unsubstituted.
[00150] Non-aromatic nitrogen containing heterocyclic rings that are
substituted on a
ring nitrogen and attached to the remainder of the molecule at a ring carbon
atom are said to
be N substituted. For example, an N alkyl piperidinyl group is attached to the
remainder of
the molecule at the two, three or four position of the piperidinyl ring and
substituted at the
ring nitrogen with an alkyl group. Non-aromatic nitrogen containing
heterocyclic rings such
as pyrazinyl that are substituted on a ring nitrogen and attached to the
remainder of the
molecule at a second ring nitrogen atom are said to be N' substituted-N-
heterocycles. For
example, an N' acyl N-pyrazinyl group is attached to the remainder of the
molecule at one
ring nitrogen atom and substituted at the second ring nitrogen atom with an
acyl group.
[00151] The term "unsaturated", as used herein, means that a moiety has one
or more
units of unsaturation.
[00152] As detailed above, in some embodiments, two independent occurrences
of R
(or R+, or any other variable similarly defined herein), may be taken together
with the atom(s)
to which each variable is bound to form a 5-8-membered heterocyclyl,
carbocyclic aryl, or
heteroaryl ring or a 3-8-membered cycloalkyl ring. Exemplary rings that are
formed when
two independent occurrences of R (or R+, or any other variable similarly
defined herein) are
taken together with the atom(s) to which each variable is bound include, but
are not limited to
the following: a) two independent occurrences of R (or R+, or any other
variable similarly
defined herein) that are bound to the same atom and are taken together with
that atom to form
a ring, for example, N(R )2, where both occurrences of R are taken together
with the
nitrogen atom to form a piperidin-l-yl, piperazin-l-yl, or morpholin-4-y1
group; and b) two
independent occurrences of R (or R+, or any other variable similarly defined
herein) that are
bound to different atoms and are taken together with both of those atoms to
form a ring, for
-54-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
OR
OR
example where a phenyl group is substituted with two occurrences of OR
these two occurrences of R are taken together with the oxygen atoms to which
they are
Oj
bound to form a fused 6-membered oxygen containing ring: 0 . It will be
appreciated that a variety of other rings can be formed when two independent
occurrences of
R (or R+, or any other variable similarly defined herein) are taken together
with the atom(s)
to which each variable is bound and that the examples detailed above are not
intended to be
limiting.
[00153] The term "hydroxyl"or "hydroxy" or "alcohol moiety" refers to ¨OH.
[00154] As used herein, an "alkoxycarbonyl," which is encompassed by the
term
carboxy, used alone or in connection with another group refers to a group such
as
(alkyl-0)-C(0)-.
[00155] As used herein, a "carbonyl" refers to -C(0)-.
[00156] As used herein, an "oxo" refers to =0.
[00157] As used herein, the term "alkoxy", or "alkylthio", as used herein,
refers to an
alkyl group, as previously defined, attached to the molecule through an oxygen
("alkoxy"
e.g., ¨0¨alkyl) or sulfur ("alkylthio" e.g., ¨S-alkyl) atom.
[00158] As used herein, the terms "halogen", "halo", and "hal" mean F, CI,
Br, or I.
[00159] As used herein, the term "cyano" or "nitrile" refer to ¨CN or ¨CEN.
[00160] The terms "alkoxyalkyl", "alkoxyalkenyl", "alkoxyaliphatic", and
"alkoxyalkoxy" mean alkyl, alkenyl, aliphatic or alkoxy, as the case may be,
substituted with
one or more alkoxy groups.
[00161] The terms "haloalkyl", "haloalkenyl", "haloaliphatic", and
"haloalkoxy" mean
alkyl, alkenyl, aliphatic or alkoxy, as the case may be, substituted with one
or more halogen
atoms. This term includes perfluorinated alkyl groups, such as ¨CF3 and -
CF2CF3.
[00162] The terms "cyanoalkyl", "cyanoalkenyl", "cyanoaliphatic", and
"cyanoalkoxy" mean alkyl, alkenyl, aliphatic or alkoxy, as the case may be,
substituted with
one or more cyano groups. In some embodiments, the cyanoalkyl is (NC)-alkyl-.
[00163] The terms "aminoalkyl", "aminoalkenyl", "aminoaliphatic", and
"aminoalkoxy" mean alkyl, alkenyl, aliphatic or alkoxy, as the case may be,
substituted with
one or more amino groups, wherein the amino group is as defined above. In some
embodiments, the aminoaliphatic is a C1-C6 aliphatic group substituted with
one or more
-55-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
-NH2 groups. In some embodiments, the aminoalkyl refers to the structure
(RxRY)N-alkyl-,
wherein each of Rx and RI' independently is as defined above. In some specific
embodiments, the aminoalkyl is CI-C6 alkyl substituted with one or more ¨NH,
groups. In
some specific embodiments, the aminoalkenyl is CI-C6 alkenyl substituted with
one or more
-NH2 groups. In some embodiments, the aminoalkoxy is -0(C I-C6 alkyl) wherein
the alkyl
group is substituted with one or more -NH, groups.
[00164] The terms "hydroxyalkyl", "hydroxyaliphatic", and "hydroxyalkoxy"
mean
alkyl, aliphatic or alkoxy, as the case may be, substituted with one or more
¨OH groups.
[00165] The terms "alkoxyalkyl", "alkoxyaliphatic", and "alkoxyalkoxy" mean
alkyl,
aliphatic or alkoxy, as the case may be, substituted with one or more alkoxy
groups. For
example, an "alkoxyalkyl" refers to an alkyl group such as (alkyl-0)-alkyl-,
wherein alkyl is
as defined above.
[00166] The term "carboxyalkyl" means alkyl substituted with one or more
carboxy
groups, wherein alkyl and carboxy are as defined above.
1001671 The term "protecting group" and "protective group" as used herein,
are
interchangeable and refer to an agent used to temporarily block one or more
desired
functional groups in a compound with multiple reactive sites. In certain
embodiments, a
protecting group has one or more, or specifically all, of the following
characteristics: a) is
added selectively to a functional group in good yield to give a protected
substrate that is b)
stable to reactions occurring at one or more of the other reactive sites; and
c) is selectively
removable in good yield by reagents that do not attack the regenerated,
deprotected functional
group. As would be understood by one skilled in the art, in some cases, the
reagents do not
attack other reactive groups in the compound. In other cases, the reagents may
also react
with other reactive groups in the compound. Examples of protecting groups are
detailed in
Greene, T. W., Wuts, P. G in "Protective Groups in Organic Synthesis", Third
Edition, John
Wiley & Sons, New York: 1999 (and other editions of the book), the entire
contents of
which are hereby incorporated by reference. The term "nitrogen protecting
group", as used
herein, refers to an agent used to temporarily block one or more desired
nitrogen reactive
sites in a multifunctional compound. Preferred nitrogen protecting groups also
possess the
characteristics exemplified for a protecting group above, and certain
exemplary nitrogen
protecting groups are also detailed in Chapter 7 in Greene, T.W., Wuts, P. G
in "Protective
Groups in Organic Synthesis", Third Edition, John Wiley & Sons, New York:
1999, the
=
entire contents of which are hereby incorporated by reference.
[00168] As used herein, the term "displaceable moiety" or "leaving group"
refers to a
-56-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
group that is associated with an aliphatic or aromatic group as defined herein
and is subject to
being displaced by nucleophilic attack by a nucleophile.
[00169] Unless otherwise indicated, structures depicted herein are also
meant to
include all isomeric (e.g., enantiomeric, diastereomeric, cis-trans,
conformational, and
rotational) forms of the structure. For example, the R and S configurations
for each
asymmetric center, (Z) and (E) double bond isomers, and (Z) and (E)
conformational isomers
are included in this invention, unless only one of the isomers is drawn
specifically. As would
be understood to one skilled in the art, a substituent can freely rotate
around any rotatable
N
bonds. For example, a substituent drawn as also represents .
[00170] Therefore, single stereochemical isomers as well as enantiomeric,
diastereomeric, cis/trans, conformational, and rotational mixtures of the
present compounds
are within the scope of the invention.
[00171] Unless otherwise indicated, all tautomeric forms of the compounds
of the
invention are within the scope of the invention.
[00172] Additionally, unless otherwise indicated, structures depicted
herein are also
meant to include compounds that differ only in the presence of one or more
isotopically
enriched atoms. For example, compounds having the present structures except
for the
replacement of hydrogen by deuterium or tritium, or the replacement of a
carbon by a 13C- or
14C-enriched carbon are within the scope of this invention. Such compounds are
useful, for
example, as analytical tools or probes in biological assays. Such compounds,
especially
deuterium analogs, can also be therapeutically useful.
[00173] The terms "a bond" and "absent" are used interchangeably to
indicate that a
group is absent.
1001741 The compounds of the invention are defined herein by their chemical
structures and/or chemical names. Where a compound is referred to by both a
chemical
structure and a chemical name, and the chemical structure and chemical name
conflict, the
chemical structure is determinative of the compound's identity.
Pharmaceutically Acceptable Salts, Solvates, Chlatrates, Prodru2s and Other
Derivatives
[00175] The compounds described herein can exist in free form, or, where
appropriate,
as salts. Those salts that are pharmaceutically acceptable are of particular
interest since they
are useful in administering the compounds described below for medical
purposes. Salts that
are not pharmaceutically acceptable are useful in manufacturing processes, for
isolation and
-57-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
purification purposes, and in some instances, for use in separating
stereoisomeric forms of the
compounds of the invention or intermediates thereof.
[00176] As used herein, the term "pharmaceutically acceptable salt" refers
to salts of a
compound which are, within the scope of sound medical judgment, suitable for
use in contact
with the tissues of humans and lower animals without undue side effects, such
as, toxicity,
irritation, allergic response and the like, and are commensurate with a
reasonable benefit/risk
ratio.
[00177] Pharmaceutically acceptable salts are well known in the art. For
example, S.
M. Berge et al., describe pharmaceutically acceptable salts in detail in J.
Pharmaceutical
Sciences, 1977, 66, 1-19, incorporated herein by reference. Pharmaceutically
acceptable salts
of the compounds described herein include those derived from suitable
inorganic and organic
acids and bases. These salts can be prepared in situ during the final
isolation and purification
of the compounds.
[00178] Where the compound described herein contains a basic group, or a
sufficiently
basic bioisostere, acid addition salts can be prepared by 1) reacting the
purified compound in
its free-base form with a suitable organic or inorganic acid and 2) isolating
the salt thus
formed. In practice, acid addition salts might be a more convenient form for
use and use of
the salt amounts to use of the free basic form.
[00179] Examples of pharmaceutically acceptable, non-toxic acid addition
salts are
salts of an amino group formed with inorganic acids such as hydrochloric acid,
hydrobromic
acid, phosphoric acid, sulfuric acid and perchloric acid or with organic acids
such as acetic
acid, oxalic acid, maleic acid, tartaric acid, citric acid, succinic acid or
malonic acid or by
using other methods used in the art such as ion exchange. Other
pharmaceutically acceptable
salts include adipate, alginate, ascorbate, aspartate, benzenesulfonate,
benzoate, bisulfate,
borate, butyrate, camphorate, camphorsulfonate, citrate,
cyclopentanepropionate, digluconate,
dodecylsulfate, ethanesulfonate, formate, fumarate, glucoheptonate,
glycerophosphate,
glycolate, gluconate, glycolate, hemisulfate, heptanoate, hexanoate,
hydrochloride,
hydrobromide, hydroiodide, 2-hydroxy-ethanesulfonate, lactobionate, lactate,
laurate, lauryl
sulfate, malate, maleate, malonate, methanesulfonate, 2-naphthalenesulfonate,
nicotinate,
nitrate, oleate, oxalate, palmitate, palmoate, pectinate, persulfate, 3-
phenylpropionate,
phosphate, picrate, pivalate, propionate, sal icy late, stearate, succinate,
sulfate, tartrate,
thiocyanate, p-toluenesulfonate, undecanoate, valerate salts, and the like.
[00180] Where the compound described herein contains a carboxy group or a
sufficiently acidic bioisostere, base addition salts can be prepared by 1)
reacting the purified
-58-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
compound in its acid form with a suitable organic or inorganic base and 2)
isolating the salt
thus formed. In practice, use of the base addition salt might be more
convenient and use of
the salt form inherently amounts to use of the free acid form. Salts derived
from appropriate
bases include alkali metal (e.g., sodium, lithium, and potassium), alkaline
earth metal (e.g.,
magnesium and calcium), ammonium and N+(Cma1ky1)4 salts. This invention also
envisions
the quatemization of any basic nitrogen-containing groups of the compounds
disclosed
herein. Water or oil-soluble or dispersible products may be obtained by such
quatemization.
[00181] Basic addition salts include pharmaceutically acceptable metal and
amine
salts. Suitable metal salts include the sodium, potassium, calcium, barium,
zinc, magnesium,
and aluminum. The sodium and potassium salts are usually preferred. Further
pharmaceutically acceptable salts include, when appropriate, nontoxic
ammonium,
quaternary ammonium, and amine cations formed using counterions such as
halide,
hydroxide, carboxylate, sulfate, phosphate, nitrate, lower alkyl sulfonate and
aryl sulfonate.
Suitable inorganic base addition salts are prepared from metal bases which
include sodium
hydride, sodium hydroxide, potassium hydroxide, calcium hydroxide, aluminium
hydroxide,
lithium hydroxide, magnesium hydroxide, zinc hydroxide and the like. Suitable
amine base
addition salts are prepared from amines which are frequently used in medicinal
chemistry
because of their low toxicity and acceptability for medical use. Ammonia,
ethylenediamine,
N-methyl-glucamine, lysine, arginine, omithine, choline, N, N'-
dibenzylethylenediamine,
chloroprocaine, dietanolamine, procaine, N-benzylphenethylamine, diethylamine,
piperazine,
tris(hydroxymethyp-aminomethane, tetramethylammonium hydroxide, triethylamine,
dibenzylamine, ephenamine, dehydroabietylamine, N-ethylpiperidine,
benzylamine,
tetramethylammonium, tetraethylammonium, methylamine, dimethylamine,
trimethylamine,
ethylamine, basic amino acids, dicyclohexylamine and the like.
[00182] Other acids and bases, while not in themselves pharmaceutically
acceptable,
may be employed in the preparation of salts useful as intermediates in
obtaining the
compounds described herein and their pharmaceutically acceptable acid or base
addition
salts.
[00183] It should be understood that this invention includes
mixtures/combinations of
different pharmaceutically acceptable salts and also mixtures/combinations of
compounds in
free form and pharmaceutically acceptable salts.
[00184] In addition to the compounds described herein, pharmaceutically
acceptable
solvates (e.g., hydrates) and clathrates of these compounds may also be
employed in
compositions to treat or prevent the herein identified disorders.
-59-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
[00185] As used herein, the term "pharmaceutically acceptable solvate," is
a solvate
formed from the association of one or more pharmaceutically acceptable solvent
molecules to
one of the compounds described herein. The term solvate includes hydrates
(e.g.,
hemihydrate, monohydrate, dihydrate, trihydrate, tetrahydrate, and the like).
[00186] As used herein, the term "hydrate" means a compound described
herein or a
salt thereof that further includes a stoichiometric or non-stoichiometric
amount of water
bound by non-covalent intermolecular forces.
[00187] As used herein, the term "clathrate" means a compound described
herein or a
salt thereof in the form of a crystal lattice that contains spaces (e.g.,
channels) that have a
guest molecule (e.g., a solvent or water) trapped within.
[00188] In addition to the compounds described herein, pharmaceutically
acceptable
derivatives or prodrugs of these compounds may also be employed in
compositions to treat or
prevent the herein identified disorders.
[00189] A "pharmaceutically acceptable derivative or prodrug" includes any
pharmaceutically acceptable ester, salt of an ester or other derivative or
salt thereof of a
compound described herein which, upon administration to a recipient, is
capable of
providing, either directly or indirectly, a compound described herein or an
inhibitory active
metabolite or residue thereof. Particularly favoured derivatives or prodrugs
are those that
increase the bioavailability of the compounds when such compounds are
administered to a
patient (e.g., by allowing an orally administered compound to be more readily
absorbed into
the blood) or which enhance delivery of the parent compound to a biological
compartment
(e.g., the brain or lymphatic system) relative to the parent species.
[00190] As used herein and unless otherwise indicated, the term "prodrug"
means a
derivative of a compound that can hydrolyze, oxidize, or otherwise react under
biological
conditions (in vitro or in vivo) to provide a compound described herein.
Prodrugs may
become active upon such reaction under biological conditions, or they may have
activity in
their unreacted forms. Examples of prodrugs contemplated in this invention
include, but are
not limited to, analogs or derivatives of compounds of the invention that
comprise
biohydrolyzable moieties such as biohydrolyzable amides, biohydrolyzable
esters,
biohydrolyzable carbamates, biohydrolyzable carbonates, biohydrolyzable
ureides, and
biohydrolyzable phosphate analogues. Other examples of prodrugs include
derivatives of
compounds described herein that comprise -NO, -NO2, -ONO, or -0NO2 moieties.
Prodrugs
can typically be prepared using well-known methods, such as those described by
BURGER'S
MEDICINAL CHEMISTRY AND DRUG DISCOVERY (1995) 172-178, 949-982 (Manfred
-60-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
E. Wolff ed., 5th ed).
[00191] A "pharmaceutically acceptable derivative" is an adduct or
derivative which,
upon administration to a patient in need, is capable of providing, directly or
indirectly, a
compound as otherwise described herein, or a metabolite or residue thereof.
Examples of
pharmaceutically acceptable derivatives include, but are not limited to,
esters and salts of
such esters. Pharmaceutically acceptable prodrugs of the compounds described
herein
include, without limitation, esters, amino acid esters, phosphate esters,
metal salts and
sulfonate esters.
Uses of Disclosed Compounds
[00192] One aspect of the present invention is generally related to the use
of the
compounds described herein or pharmaceutically acceptable salts, or
pharmaceutically
acceptable compositions comprising such a compound or a pharmaceutically
acceptable salt
thereof,' for inhibiting the replication of influenza viruses in a biological
sample or in a
patient, for reducing the amount of influenza viruses (reducing viral titer)
in a biological
sample or in a patient, and for treating influenza in a patient.
[00193] In one embodiment, the present invention is generally related to
the use of
compounds represented by Structural Formula I or pharmaceutically acceptable
salts thereof
for any of the uses specified above:
[00194] In yet another embodiment, the present invention is directed to the
use of any
compound selected from the compounds depicted in Tables 1 and 2, or a
pharmaceutically
acceptable salt thereof, for any of the uses described above.
[00195] In some embodiments, the compounds are represented by any one of
Structural Formula I and the variables are each independently as depicted in
the compounds
of Tables I and 2.
[00196] In yet another embodiment, the compounds described herein or
pharmaceutically acceptable salts thereof can be used to reduce viral titre in
a biological
sample (e.g. an infected cell culture) or in humans (e.g. lung viral titre in
a patient).
[00197] The terms "influenza virus mediated condition", "influenza
infection", or
"Influenza", as used herein, are used interchangeable to mean the disease
caused by an
infection with an influenza virus.
[00198] Influenza is an infectious disease that affects birds and mammals
caused by
influenza viruses. Influenza viruses are RNA viruses of the family
Orthomyxoviridae, which
comprises five genera: lnfluenzavirus A, lnfluenzavirus B, lnfluenzavirus C,
Isavirus and
-61-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
Thogotovirus. Influenzavirus A genus has one species, influenza A virus which
can be
subdivided into different serotypes based on the antibody response to these
viruses: H1N1,
H2N2, H3N2, H5N1, H7N7, HIN2, H9N2, H7N2 , H7N3 and H 1 ON7. Influenzavirus B
genus has one species, influenza B virus. Influenza B almost exclusively
infects humans and
is less common than influenza A. Influenzavirus C genus has one species,
lnfluenzavirus C
virus, which infects humans and pigs and can cause severe illness and local
epidemics.
However, Influenzavirus C is less common than the other types and usually
seems to cause
mild disease in children.
[00199] In some embodiments of the invention, influenza or influenza
viruses are
associated with Influenzavirus A or B. In some embodiments of the invention,
influenza or
influenza viruses are associated with Influenzavirus A. In some specific
embodiments of the
invention, Influenzavirus A is H I NI, H2N2, H3N2 or H5N1.
[00200] In humans, common symptoms of influenza are chills, fever,
pharyngitis,
muscle pains, severe headache, coughing, weakness, and general discomfort. In
more serious
cases, influenza causes pneumonia, which can be fatal, particularly in young
children and the
elderly. Although it is often confused with the common cold, influenza is a
much more severe
disease and is caused by a different type of virus. Influenza can produce
nausea and
vomiting, especially in children, but these symptoms are more characteristic
of the unrelated
gastroenteritis, which is sometimes called "stomach flu" or "24-hour flu".
[00201] Symptoms of influenza can start quite suddenly one to two days
after
infection. Usually the first symptoms are chills or a chilly sensation, but
fever is also
common early in the infection, with body temperatures ranging from 38-39 C
(approximately 100-103 F). Many people are so ill that they are confined to
bed for several
days, with aches and pains throughout their bodies, which are worse in their
backs and legs.
Symptoms of influenza may include: body aches, especially joints and throat,
extreme
coldness and fever, fatigue, Headache, irritated watering eyes, reddened eyes,
skin (especially
face), mouth, throat and nose, abdominal pain (in children with influenza B).
Symptoms of
influenza are non-specific, overlapping with many pathogens ("influenza-like
illness).
Usually, laboratory data is needed in order to confirm the diagnosis.
[00202] The terms, "disease", "disorder", and "condition" may be used
interchangeably here to refer to an influenza virus mediated medical or
pathological
condition.
[00203] As used herein, the terms "subject" and "patient" are used
interchangeably.
The terms "subject" and "patient" refer to an animal (e.g., a bird such as a
chicken, quail or
-62-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
turkey, or a mammal), specifically a "mammal" including a non-primate (e.g., a
cow, pig,
horse, sheep, rabbit, guinea pig, rat, cat, dog, and mouse) and a primate
(e.g., a monkey,
chimpanzee and a human), and more specifically a human. In one embodiment, the
subject is
a non-human animal such as a farm animal (e.g., a horse, cow, pig or sheep),
or a pet (e.g., a
dog, cat, guinea pig or rabbit). In a preferred embodiment, the subject is a
"human".
[00204] The term "biological sample", as used herein, includes, without
limitation, cell
cultures or extracts thereof; biopsied material obtained from a mammal or
extracts thereof;
blood, saliva, urine, feces, semen, tears, or other body fluids or extracts
thereof.
[00205] As used herein, "multiplicity of infection" or "MO1" is the ratio
of infectious
agents (e.g. phage or virus) to infection targets (e.g. cell). For example,
when referring to a
group of cells inoculated with infectious virus particles, the multiplicity of
infection or MO1
is the ratio defined by the number of infectious virus particles deposited in
a well divided by
the number of target cells present in that well.
[00206] As used herein the term "inhibition of the replication of influenza
viruses"
includes both the reduction in the amount of virus replication (e.g. the
reduction by at least 10
%) and the complete arrest of virus replication (i.e., 100% reduction in the
amount of virus
replication). In some embodiments, the replication of influenza viruses are
inhibited by at
least 50%, at least 65%, at least 75%, at least 85%, at least 90%, or at least
95%.
[00207] Influenza virus replication can be measured by any suitable method
known in
the art. For example, influenza viral titre in a biological sample (e.g. an
infected cell culture)
or in humans (e.g. lung viral titre in a patient) can be measured. More
specifically, for cell
based assays, in each case cells are cultured in vitro, virus is added to the
culture in the
presence or absence of a test agent, and after a suitable length of time a
virus-dependent
endpoint is evaluated. For typical assays, the Madin-Darby canine kidney cells
(MDCK) and
the standard tissue culture adapted influenza strain, A/Puerto Rico/8/34 can
be used. A first
type of cell assay that can be used in the invention depends on death of the
infected target
cells, a process called cytopathic effect (CPE), where virus infection causes
exhaustion of the
cell resources and eventual lysis of the cell. In the first type of cell
assay, a low fraction of
cells in the wells of a microtiter plate are infected (typically 1/10 to
1/1000), the virus is
allowed to go through several rounds of replication over 48-72 hours, then the
amount of cell
death is measured using a decrease in cellular ATP content compared to
uninfected controls.
A second type of cell assay that can be employed in the invention depends on
the
multiplication of virus-specific RNA molecules in the infected cells, with RNA
levels being
directly measured using the branched-chain DNA hybridization method (bDNA). In
the
-63-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
second type of cell assay, a low number of cells are initially infected in
wells of a microtiter
plate, the virus is allowed to replicate in the infected cells and spread to
additional rounds of
cells, then the cells are lysed and viral RNA content is measured. This assay
is stopped early,
usually after 18-36 hours, while all the target cells are still viable. Viral
RNA is quantitated
by hybridization to specific oligonucleotide probes fixed to wells of an assay
plate, then
amplification of the signal by hybridization with additional probes linked to
a reporter
enzyme.
[00208] As used herein a "viral titer (or titre)" is a measure of virus
concentration.
Titer testing can employ serial dilution to obtain approximate quantitative
information from
an analytical procedure that inherently only evaluates as positive or
negative. The titer
corresponds to the highest dilution factor that still yields a positive
reading; for example,
positive readings in the first 8 serial twofold dilutions translate into a
titer of 1:256. A
specific example is viral titer. To determine the titer, several dilutions
will be prepared, such
as 10-1, 10-2, 10-3,...,10-8. The lowest concentration of virus that still
infects cells is the viral
titer.
[00209] As used herein, the terms "treat", "treatment" and "treating" refer
to both
therapeutic and prophylactic treatments. For example, therapeutic treatments
includes the
reduction or amelioration of the progression, severity and/or duration of
influenza viruses
mediated conditions, or the amelioration of one or more symptoms
(specifically, one or more
discernible symptoms) of influenza viruses mediated conditions, resulting from
the
administration of one or more therapies (e.g., one or more therapeutic agents
such as a
compound or composition of the invention). In specific embodiments, the
therapeutic
treatment includes the amelioration of at least one measurable physical
parameter of an
influenza virus mediated condition. In other embodiments the therapeutic
treatment includes
the inhibition of the progression of an influenza virus mediated condition,
either physically
by, e.g., stabilization of a discernible symptom, physiologically by, e.g.,
stabilization of a
physical parameter, or both. In other embodiments the therapeutic treatment
includes the
reduction or stabilization of influenza viruses mediated infections. Antiviral
drugs can be
used in the community setting to treat people who already have influenza to
reduce the
severity of symptoms and reduce the number of days that they are sick.
[00210] The term "chemotherapy" refers to the use of medications, e.g.
small molecule
drugs (rather than "vaccines") for treating a disorder or disease.
[00211] The terms "prophylaxis" or "prophylactic use" and "prophylactic
treatment"
as used herein, refer to any medical or public health procedure whose purpose
is to prevent,
-64-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
rather than treat or cure a disease. As used herein, the terms "prevent",
"prevention" and
"preventing" refer to the reduction in the risk of acquiring or developing a
given condition, or
the reduction or inhibition of the recurrence or said condition in a subject
who is not ill, but
who has been or may be near a person with the disease. The term
"chemoprophylaxis" refers
to the use of medications, e.g. small molecule drugs (rather than "vaccines")
for the
prevention of a disorder or disease.
[00212] As used herein, prophylactic use includes the use in situations in
which an
outbreak has been detected, to prevent contagion or spread of the infection in
places where a
lot of people that are at high risk of serious influenza complications live in
close contact with
each other (e.g. in a hospital ward, daycare center, prison, nursing home,
etc). It also includes
the use among populations who require protection from the influenza but who
either do not
get protection after vaccination (e.g. due to weak immune system), or when the
vaccine is
unavailable to them, or when they cannot get the vaccine because of side
effects. It also
includes use during the two weeks following vaccination, since during that
time the vaccine
is still ineffective. Prophylactic use may also include treating a person who
is not ill with the
influenza or not considered at high risk for complications, in order to reduce
the chances of
getting infected with the influenza and passing it on to a high-risk person in
close contact
with him (for instance, healthcare workers, nursing home workers, etc).
[00213] According to the US CDC, an influenza "outbreak" is defined as a
sudden
increase of acute febrile respiratory illness (AFRI) occurring within a 48 to
72 hour period, in
a group of people who are in close proximity to each other (e.g. in the same
area of an
assisted living facility, in the same household, etc) over the normal
background rate or when
any subject in the population being analyzed tests positive for influenza. One
case of
confirmed influenza by any testing method is considered an outbreak.
[00214] A "cluster" is defined as a group of three or more cases of AFRI
occurring
within a 48 to 72 hour period, in a group of people who are in close proximity
to each other
(e.g. in the same area of an assisted living facility, in the same household,
etc).
[00215] As used herein, the "index case", "primary case" or "patient zero"
is the initial
patient in the population sample of an epidemiological investigation. When
used in general to
refer to such patients in epidemiological investigations, the term is not
capitalized. When the
term is used to refer to a specific person in place of that person's name
within a report on a
specific investigation, the term is capitalized as Patient Zero. Often
scientists search for the
index case to determine how the disease spread and what reservoir holds the
disease in
between outbreaks. Note that the index case is the first patient that
indicates the existence of
-65-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
an outbreak. Earlier cases may be found and are labeled primary, secondary,
tertiary, etc.
[00216] In one embodiment, the methods of the invention are a preventative
or "pre-
emptive" measure to a patient, specifically a human, having a predisposition
to complications
resulting from infection by an influenza virus. The term "pre-emptive" as used
herein as for
example in pre-emptive use, "pre-emptively", etc, is the prophylactic use in
situations in
which an "index case" or an "outbreak" has been confirmed, in order to prevent
the spread of
infection in the rest of the community or population group.
[00217] In another embodiment, the methods of the invention are applied as
a "pre-
emptive" measure to members of a community or population group, specifically
humans, in
order to prevent the spread of infection.
[00218] As used herein, an "effective amount" refers to an amount
sufficient to elicit
the desired biological response. In the present invention the desired
biological response is to
inhibit the replication of influenza virus, to reduce the amount of influenza
viruses or to
reduce or ameliorate the severity, duration, progression, or onset of a
influenza virus
infection, prevent the advancement of an influenza viruses infection, prevent
the recurrence,
development, onset or progression of a symptom associated with an influenza
virus infection,
or enhance or improve the prophylactic or therapeutic effect(s) of another
therapy used
against influenza infections. The precise amount of compound administered to a
subject will
depend on the mode of administration, the type and severity of the infection
and on the
characteristics of the subject, such as general health, age, sex, body weight
and tolerance to
drugs. The skilled artisan will be able to determine appropriate dosages
depending on these
and other factors. When co-administered with other anti viral agents, e.g.,
when co-
administered with an anti-influenza medication, an "effective amount" of the
second agent
will depend on the type of drug used. Suitable dosages are known for approved
agents and
can be adjusted by the skilled artisan according to the condition of the
subject, the type of
condition(s) being treated and the amount of a compound described herein being
used. In
cases where no amount is expressly noted, an effective amount should be
assumed. For
example, compounds described herein can be administered to a subject in a
dosage range
from between approximately 0.01 to 100 mg/kg body weight/day for therapeutic
or
prophylactic treatment.
[00219] Generally, dosage regimens can be selected in accordance with a
variety of
factors including the disorder being treated and the severity of the disorder;
the activity of the
specific compound employed; the specific composition employed; the age, body
weight,
general health, sex and diet of the patient; the time of administration, route
of administration,
-66-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
and rate of excretion of the specific compound employed; the renal and hepatic
function of
the subject; and the particular compound or salt thereof employed, the
duration of the
treatment; drugs used in combination or coincidental with the specific
compound employed,
and like factors well known in the medical arts. The skilled artisan can
readily determine and
prescribe the effective amount of the compounds described herein required to
treat, to
prevent, inhibit (fully or partially) or arrest the progress of the disease.
[00220] Dosages of the compounds described herein can range from between
about
0.01 to about 100 mg/kg body weight/day, about 0.01 to about 50 mg/kg body
weight/day,
about 0.1 to about 50 mg/kg body weight/day, or about 1 to about 25 mg/kg body
weight/day.
It is understood that the total amount per day can be administered in a single
dose or can be
administered in multiple dosing, such as twice a day (e.g., every 12 hours),
tree times a day
(e.g., every 8 hours), or four times a day (e.g., every 6 hours).
[00221] For therapeutic treatment, the compounds described herein can be
administered to a patient within, for example, 48 hours (or within 40 hours,
or less than 2
days, or less than 1.5 days, or within 24 hours) of onset of symptoms (e.g.,
nasal congestion,
sore throat, cough, aches, fatigue, headaches, and chills/sweats). The
therapeutic treatment
can last for any suitable duration, for example, for 5 days, 7 days, 10 days,
14 days, etc. For
prophylactic treatment during a community outbreak, the compounds described
herein can be
administered to a patient within, for example, 2 days of onset of symptoms in
the index case,
and can be continued for any suitable duration, for example, for 7 days, 10
days, 14 days, 20
days, 28 days, 35 days, 42 days, etc.
[00222] Various types of administration methods can be employed in the
invention,
and are described in detail below under the section entitled "Administration
Methods."
Combination Theraov
[00223] An effective amount can be achieved in the method or pharmaceutical
composition of the invention employing a compound of the invention (including
a
pharmaceutically acceptable salt or solvate (e.g., hydrate)) alone or in
combination with an
additional suitable therapeutic agent, for example, an antiviral agent or a
vaccine. When
"combination therapy" is employed, an effective amount can be achieved using a
first amount
of a compound of the invention and a second amount of an additional suitable
therapeutic
agent (e.g. an antiviral agent or vaccine).
[00224] In another embodiment of this invention, a compound of the
invention and the
additional therapeutic agent, are each administered in an effective amount
(i.e., each in an
-67-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
amount which would be therapeutically effective if administered alone). In
another
embodiment, a compound of the invention and the additional therapeutic agent,
are each
administered in an amount which alone does not provide a therapeutic effect (a
sub-
therapeutic dose). In yet another embodiment, a compound of the invention can
be
administered in an effective amount, while the additional therapeutic agent is
administered in
a sub-therapeutic dose. In still another embodiment, a compound of the
invention can be
administered in a sub-therapeutic dose, while the additional therapeutic
agent, for example, a
suitable cancer-therapeutic agent is administered in an effective amount.
[00225] As used herein, the terms "in combination" or "co-administration"
can be used
interchangeably to refer to the use of more than one therapy (e.g., one or
more prophylactic
and/or therapeutic agents). The use of the terms does not restrict the order
in which therapies
(e.g., prophylactic and/or therapeutic agents) are administered to a subject.
[00226] Coadministration encompasses administration of the first and second
amounts
of the compounds of the coadministration in an essentially simultaneous
manner, such as in a
single pharmaceutical composition, for example, capsule or tablet having a
fixed ratio of first
and second amounts, or in multiple, separate capsules or tablets for each. In
addition, such
coadministration also encompasses use of each compound in a sequential manner
in either
order.
[00227] In one embodiment, the present invention is directed to methods of
combination therapy for inhibiting Flu viruses replication in biological
samples or patients, or
for treating or preventing Influenza virus infections in patients using the
compounds or
pharmaceutical compositions of the invention. Accordingly, pharmaceutical
compositions of
the invention also include those comprising an inhibitor of Flu virus
replication of this
invention in combination with an anti-viral compound exhibiting anti-Influenza
virus activity.
[00228] Methods of use of the compounds and compositions of the invention
also
include combination of chemotherapy with a compound or composition of the
invention, or
with a combination of a compound or composition of this invention with another
anti-viral
agent and vaccination with a Flu vaccine.
[00229] When co-administration involves the separate administration of the
first
amount of a compound of the invention and a second amount of an additional
therapeutic
agent, the compounds are administered sufficiently close in time to have the
desired
therapeutic effect. For example, the period of time between each
administration which can
result in the desired therapeutic effect, can range from minutes to hours and
can be
determined taking into account the properties of each compound such as
potency, solubility,
-68-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
bioavailability, plasma half-life and kinetic profile. For example, a compound
of the
invention and the second therapeutic agent can be administered in any order
within about 24
hours of each other, within about 16 hours of each other, within about 8 hours
of each other,
within about 4 hours of each other, within about 1 hour of each other or
within about 30
minutes of each other.
[00230] More, specifically, a first therapy (e.g., a prophylactic or
therapeutic agent
such as a compound of the invention) can be administered prior to (e.g., 5
minutes, 15
minutes, 30 minutes, 45 minutes, 1 hour, 2 hours, 4 hours, 6 hours, 12 hours,
24 hours, 48
hours, 72 hours, 96 hours, 1 week, 2 weeks, 3 weeks, 4 weeks, 5 weeks, 6
weeks, 8 weeks, or
12 weeks before), concomitantly with, or subsequent to (e.g., 5 minutes, 15
minutes, 30
minutes, 45 minutes, 1 hour, 2 hours, 4 hours, 6 hours, 12 hours, 24 hours, 48
hours, 72
hours, 96 hours, 1 week, 2 weeks, 3 weeks, 4 weeks, 5 weeks, 6 weeks, 8 weeks,
or 12 weeks
after) the administration of a second therapy (e.g., a prophylactic or
therapeutic agent such as
an anti-cancer agent) to a subject.
[00231] It is understood that the method of co-administration of a first
amount of a
compound of the invention and a second amount of an additional therapeutic
agent can result
in an enhanced or synergistic therapeutic effect, wherein the combined effect
is greater than
the additive effect that would result from separate administration of the
first amount of a
compound of the invention and the second amount of an additional therapeutic
agent.
[00232] As used herein, the term "synergistic" refers to a combination of a
compound
of the invention and another therapy (e.g., a prophylactic or therapeutic
agent), which is more
effective than the additive effects of the therapies. A synergistic effect of
a combination of
=
therapies (e.g., a combination of prophylactic or therapeutic agents) can
permit the use of
lower dosages of one or more of the therapies and/or less frequent
administration of said
therapies to a subject. The ability to utilize lower dosages of a therapy
(e.g., a prophylactic or
therapeutic agent) and/or to administer said therapy less frequently can
reduce the toxicity
associated with the administration of said therapy to a subject without
reducing the efficacy
of said therapy in the prevention, management or treatment of a disorder. In
addition, a
synergistic effect can result in improved efficacy of agents in the
prevention, management or
treatment of a disorder. Finally, a synergistic effect of a combination of
therapies (e.g., a
combination of prophylactic or therapeutic agents) may avoid or reduce adverse
or unwanted
side effects associated with the use of either therapy alone.
[00233] When the combination therapy using the compounds of the present
invention
is in combination with a Flu vaccine, both therapeutic agents can be
administered so that the
-69-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
period of time between each administration can be longer (e.g. days, weeks or
months).
[00234] The presence of a synergistic effect can be determined using
suitable methods
for assessing drug interaction. Suitable methods include, for example, the
Sigmoid-Emax
equation (Holford, N.H.G. and Scheiner, L.B., Clin. Pharmacokinet. 6: 429-453
(1981)), the
equation of Loewe additivity (Loewe, S. and Muischnek, H., Arch. Exp. Pathol
Pharmacol.
114: 313-326 (1926)) and the median-effect equation (Chou, T.C. and Talalay,
P., Adv.
Enzyme Regul. 22: 27-55 (1984)). Each equation referred to above can be
applied with
experimental data to generate a corresponding graph to aid in assessing the
effects of the drug
combination. The corresponding graphs associated with the equations referred
to above are
the concentration-effect curve, isobologram curve and combination index curve,
respectively.
1002351 Specific examples that can be co-administered with a compound
described
herein include neuraminidase inhibitors, such as oseltamivir (Tamiflue) and
Zanamivir
(Relenza0), viral ion channel (M2 protein) blockers, such as amantadine
(Symmetrele) and
rimantadine (Flumadinee), and antiviral drugs described in WO 2003/015798,
including T-
705 under development by Toyama Chemical of Japan. (See alsoRuruta et al.,
Antiviral
Research, 82: 95-102 (2009), "T-705 (flavipiravir) and related compounds:
Novel broad-
spectrum inhibitors of RNA viral infections.") In some embodiments, the
compounds
described herein can be co-administered with a traditional influenza vaccine.
Pharmaceutical Compositions
[00236] The compounds described herein can be formulated into
pharmaceutical
compositions that further comprise a pharmaceutically acceptable carrier,
diluent, adjuvant or
vehicle. In one embodiment, the present invention relates to a pharmaceutical
composition
comprising a compound of the invention described above, and a pharmaceutically
acceptable
carrier, diluent, adjuvant or vehicle. In one embodiment, the present
invention is a
pharmaceutical composition comprising an effective amount of a compound of the
present
invention or a pharmaceutically acceptable salt thereof and a pharmaceutically
acceptable
carrier, diluent, adjuvant or vehicle. Pharmaceutically acceptable carriers
include, for
example, pharmaceutical diluents, excipients or carriers suitably selected
with respect to the
intended form of administration, and consistent with conventional
pharmaceutical practices.
1002371 An "effective amount" includes a "therapeutically effective amount"
and a
"prophylactically effective amount". The term "therapeutically effective
amount" refers to an
amount effective in treating and/or ameliorating an influenza virus infection
in a patient
infected with influenza. The term "prophylactically effective amount" refers
to an amount
effective in preventing and/or substantially lessening the chances or the size
of influenza
-70-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
virus infection outbreak. Specific examples of effective amounts are described
above in the
section entitled Uses of Disclosed Compounds.
[00238] A pharmaceutically acceptable carrier may contain inert ingredients
which do
not unduly inhibit the biological activity of the compounds. The
pharmaceutically acceptable
carriers should be biocompatible, e.g., non-toxic, non-inflammatory, non-
immunogenic or
devoid of other undesired reactions or side-effects upon the administration to
a subject.
Standard pharmaceutical formulation techniques can be employed.
[00239] The pharmaceutically acceptable carrier, adjuvant, or vehicle, as
used herein,
includes any and all solvents, diluents, or other liquid vehicle, dispersion
or suspension aids,
surface active agents, isotonic agents, thickening or emulsifying agents,
preservatives, solid
binders, lubricants and the like, as suited to the particular dosage form
desired. Remington's
Pharmaceutical Sciences, Sixteenth Edition, E. W. Martin (Mack Publishing Co.,
Easton, Pa.,
1980) discloses various carriers used in formulating pharmaceutically
acceptable
compositions and known techniques for the preparation thereof. Except insofar
as any
conventional carrier medium is incompatible with the compounds described
herein, such as
by producing any undesirable biological effect or otherwise interacting in a
deleterious
manner with any other component(s) of the pharmaceutically acceptable
composition, its use
is contemplated to be within the scope of this invention. As used herein, the
phrase "side
effects" encompasses unwanted and adverse effects of a therapy (e.g., a
prophylactic or
therapeutic agent). Side effects are always unwanted, but unwanted effects are
not
necessarily adverse. An adverse effect from a therapy (e.g., prophylactic or
therapeutic
agent) might be harmful or uncomfortable or risky. Side effects include, but
are not limited
to fever, chills, lethargy, gastrointestinal toxicities (including gastric and
intestinal ulcerations
and erosions), nausea, vomiting, neurotoxicities, nephrotoxicities, renal
toxicities (including
such conditions as papillary necrosis and chronic interstitial nephritis),
hepatic toxicities
(including elevated serum liver enzyme levels), myelotoxicities (including
leukopenia,
myelosuppression, thrombocytopenia and anemia), dry mouth, metallic taste,
prolongation of
gestation, weakness, somnolence, pain (including muscle pain, bone pain and
headache), hair
loss, asthenia, dizziness, extra-pyramidal symptoms, akathisia, cardiovascular
disturbances
and sexual dysfunction.
[00240] Some examples of materials which can serve as pharmaceutically
acceptable
carriers include, but are not limited to, ion exchangers, alumina, aluminum
stearate, lecithin,
serum proteins (such as human serum albumin), buffer substances (such as twin
80,
phosphates, glycine, sorbic acid, or potassium sorbate), partial glyceride
mixtures of saturated
-71-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
vegetable fatty acids, water, salts or electrolytes (such as protamine
sulfate, disodium
hydrogen phosphate, potassium hydrogen phosphate, sodium chloride, or zinc
salts), colloidal
silica, magnesium trisilicate, polyvinyl pyrrolidone, polyacrylates, waxes,
polyethylene-
polyoxypropylene-block polymers, methylcellulose, hydroxypropyl
methylcellulose, wool
fat, sugars such as lactose, glucose and sucrose; starches such as corn starch
and potato
starch; cellulose and its derivatives such as sodium carboxymethyl cellulose,
ethyl cellulose
and cellulose acetate; powdered tragacanth; malt; gelatin; talc; excipients
such as cocoa butter
and suppository waxes; oils such as peanut oil, cottonseed oil; safflower oil;
sesame oil; olive
oil; corn oil and soybean oil; glycols; such a propylene glycol or
polyethylene glycol; esters
such as ethyl oleate and ethyl laurate; agar; buffering agents such as
magnesium hydroxide
and aluminum hydroxide; alginic acid; pyrogen-free water; isotonic saline;
Ringer's solution;
ethyl alcohol, and phosphate buffer solutions, as well as other non-toxic
compatible
lubricants such as sodium lauryl sulfate and magnesium stearate, as well as
coloring agents,
releasing agents, coating agents, sweetening, flavoring and perfuming agents,
preservatives
and antioxidants can also be present in the composition, according to the
judgment of the
formulator.
Administration Methods
1002411 The compounds and pharmaceutically acceptable compositions
described
above can be administered to humans and other animals orally, rectally,
parenterally,
intracisternally, intravaginally, intraperitoneally, topically (as by powders,
ointments, or
drops), bucally, as an oral or nasal spray, or the like, depending on the
severity of the
infection being treated.
1002421 Liquid dosage forms for oral administration include, but are not
limited to,
pharmaceutically acceptable emulsions, microemulsions, solutions, suspensions,
syrups and
elixirs. In addition to the active compounds, the liquid dosage forms may
contain inert
diluents commonly used in the art such as, for example, water or other
solvents, solubilizing
agents and emulsifiers such as ethyl alcohol, isopropyl alcohol, ethyl
carbonate, ethyl acetate,
benzyl alcohol, benzyl benzoate, propylene glycol, 1,3-butylene glycol,
dimethylformamide,
oils (in particular, cottonseed, groundnut, corn, germ, olive, castor, and
sesame oils),
glycerol, tetrahydrofurfuryl alcohol, polyethylene glycols and fatty acid
esters of sorbitan,
and mixtures thereof. Besides inert diluents, the oral compositions can also
include adjuvants
such as wetting agents, emulsifying and suspending agents, sweetening,
flavoring, and
perfuming agents.
[00243] Injectable preparations, for example, sterile injectable aqueous or
oleaginous
-72-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
suspensions may be formulated according to the known art using suitable
dispersing or
wetting agents and suspending agents. The sterile injectable preparation may
also be a sterile
injectable solution, suspension or emulsion in a nontoxic parenterally
acceptable diluent or
solvent, for example, as a solution in 1,3-butanediol. Among the acceptable
vehicles and
solvents that may be employed are water, Ringer's solution, U.S.P. and
isotonic sodium
chloride solution. In addition, sterile, fixed oils are conventionally
employed as a solvent or
suspending medium. For this purpose any bland fixed oil can be employed
including
synthetic mono- or diglycerides. In addition, fatty acids such as oleic acid
are used in the
preparation of injectables.
[00244] The injectable formulations can be sterilized, for example, by
filtration
through a bacterial-retaining filter, or by incorporating sterilizing agents
in the form of sterile
solid compositions which can be dissolved or dispersed in sterile water or
other sterile
injectable medium prior to use.
[00245] In order to prolong the effect of a compound described herein, it
is often
desirable to slow the absorption of the compound from subcutaneous or
intramuscular
injection. This may be accomplished by the use of a liquid suspension of
crystalline or
amorphous material with poor water solubility. The rate of absorption of the
compound then
depends upon its rate of dissolution that, in turn, may depend upon crystal
size and crystalline
form. Alternatively, delayed absorption of a parenterally administered
compound form is
accomplished by dissolving or suspending the compound in an oil vehicle.
Injectable depot
forms are made by forming microencapsule matrices of the compound in
biodegradable
polymers such as polylactide-polyglycolide. Depending upon the ratio of
compound to
polymer and the nature of the particular polymer employed, the rate of
compound release can
be controlled. Examples of other biodegradable polymers include
poly(orthoesters) and
poly(anhydrides). Depot injectable formulations are also prepared by
entrapping the
compound in liposomes or microemulsions that are compatible with body tissues.
[00246] Compositions for rectal or vaginal administration are specifically
suppositories
which can be prepared by mixing the compounds described herein with suitable
non-irritating
excipients or carriers such as cocoa butter, polyethylene glycol or a
suppository wax which
are solid at ambient temperature but liquid at body temperature and therefore
melt in the
rectum or vaginal cavity and release the active compound.
[00247] Solid dosage forms for oral administration include capsules,
tablets, pills,
powders, and granules. In such solid dosage forms, the active compound is
mixed with at
least one inert, pharmaceutically acceptable excipient or carrier such as
sodium citrate or
-73-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
dicalcium phosphate and/or a) fillers or extenders such as starches, lactose,
sucrose, glucose,
mannitol, and silicic acid, b) binders such as, for example,
carboxymethylcellulose, alginates,
gelatin, polyvinylpyrrolidinone, sucrose, and acacia, c) humectants such as
glycerol, d)
disintegrating agents such as agar--agar, calcium carbonate, potato or tapioca
starch, alginic
acid, certain silicates, and sodium carbonate, e) solution retarding agents
such as paraffin, 0
absorption accelerators such as quaternary ammonium compounds, g) wetting
agents such as,
for example, cetyl alcohol and glycerol monostearate, h) absorbents such as
kaolin and
bentonite clay, and i) lubricants such as talc, calcium stearate, magnesium
stearate, solid
polyethylene glycols, sodium lauryl sulfate, and mixtures thereof. In the case
of capsules,
tablets and pills, the dosage form may also comprise buffering agents.
1002481 Solid compositions of a similar type may also be employed as
fillers in soft
and hard-filled gelatin capsules using such excipients as lactose or milk
sugar as well as high
molecular weight polyethylene glycols and the like. The solid dosage forms of
tablets,
dragees, capsules, pills, and granules can be prepared with coatings and
shells such as enteric
coatings and other coatings well known in the pharmaceutical formulating art.
They may
optionally contain opacifying agents and can also be of a composition that
they release the
active ingredient(s) only, or preferentially, in a certain part of the
intestinal tract, optionally,
in a delayed manner. Examples of embedding compositions that can be used
include
polymeric substances and waxes. Solid compositions of a similar type may also
be employed
as fillers in soft and hard-filled gelatin capsules using such excipients as
lactose or milk sugar
as well as high molecular weight polethylene glycols and the like.
1002491 The active compounds can also be in microencapsulated form with one
or
more excipients as noted above. The solid dosage forms of tablets, dragees,
capsules, pills,
and granules can be prepared with coatings and shells such as enteric
coatings, release
controlling coatings and other coatings well known in the pharmaceutical
formulating art. In
such solid dosage forms the active compound may be admixed with at least one
inert diluent
such as sucrose, lactose or starch. Such dosage forms may also comprise, as is
normal
practice, additional substances other than inert diluents, e.g., tableting
lubricants and other
tableting aids such a magnesium stearate and microcrystalline cellulose. In
the case of
capsules, tablets and pills, the dosage forms may also comprise buffering
agents. They may
optionally contain opacifying agents and can also be of a composition that
they release the
active ingredient(s) only, or preferentially, in a certain part of the
intestinal tract, optionally,
in a delayed manner. Examples of embedding compositions that can be used
include
polymeric substances and waxes.
-74-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
[00250] Dosage forms for topical or transdermal administration of a
compound
described herein include ointments, pastes, creams, lotions, gels, powders,
solutions, sprays,
inhalants or patches. The active component is admixed under sterile conditions
with a
pharmaceutically acceptable carrier and any needed preservatives or buffers as
may be
required. Ophthalmic formulation, eardrops, and eye drops are also
contemplated as being
within the scope of this invention. Additionally, the present invention
contemplates the use
of transdermal patches, which have the added advantage of providing controlled
delivery of a
compound to the body. Such dosage forms can be made by dissolving or
dispensing the
compound in the proper medium. Absorption enhancers can also be used to
increase the flux
of the compound across the skin. The rate can be controlled by either
providing a rate
controlling membrane or by dispersing the compound in a polymer matrix or gel.
[00251] The compositions described herein may be administered orally,
parenterally,
by inhalation spray, topically, rectally, nasally, buccally, vaginally or via
an implanted
reservoir. The term "parenteral" as used herein includes, but is not limited
to, subcutaneous,
intravenous, intramuscular, intra-articular, intra-synovial, intrasternal,
intrathecal,
intrahepatic, intralesional and intracranial injection or infusion techniques.
Specifically, the
compositions are administered orally, intraperitoneally or intravenously.
1002521 Sterile injectable forms of the compositions described herein may
be aqueous
or oleaginous suspension. These suspensions may be formulated according to
techniques
known in the art using suitable dispersing or wetting agents and suspending
agents. The
sterile injectable preparation may also be a sterile injectable solution or
suspension in a non-
toxic parenterally-acceptable diluent or solvent, for example as a solution in
1,3-butanediol.
Among the acceptable vehicles and solvents that may be employed are water,
Ringer's
solution and isotonic sodium chloride solution. In addition, sterile, fixed
oils are
conventionally employed as a solvent or suspending medium. For this purpose,
any bland
fixed oil may be employed including synthetic mono- or di-glycerides. Fatty
acids, such as
oleic acid and its glyceride derivatives are useful in the preparation of
injectables, as are
natural pharmaceutically-acceptable oils, such as olive oil or castor oil,
especially in their
polyoxyethylated versions. These oil solutions or suspensions may also contain
a long-chain
alcohol diluent or dispersant, such as carboxymethyl cellulose or similar
dispersing agents
which are commonly used in the formulation of pharmaceutically acceptable
dosage forms
including emulsions and suspensions. Other commonly used surfactants, such as
Tweens,
Spans and other emulsifying agents or bioavailability enhancers which are
commonly used in
the manufacture of pharmaceutically acceptable solid, liquid, or other dosage
forms may also
-75-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
be used for the purposes of formulation.
[00253] The pharmaceutical compositions described herein may be orally
administered
in any orally acceptable dosage form including, but not limited to, capsules,
tablets, aqueous
suspensions or solutions. In the case of tablets for oral use, carriers
commonly used include,
but are not limited to, lactose and corn starch. Lubricating agents, such as
magnesium
stearate, are also typically added. For oral administration in a capsule form,
useful diluents
include lactose and dried cornstarch. When aqueous suspensions are required
for oral use,
the active ingredient is combined with emulsifying and suspending agents. If
desired, certain
sweetening, flavoring or coloring agents may also be added.
[00254] Alternatively, the pharmaceutical compositions described herein may
be
administered in the form of suppositories for rectal administration. These can
be prepared by
mixing the agent with a suitable non-irritating excipient which is solid at
room temperature
but liquid at rectal temperature and therefore will melt in the rectum to
release the drug. Such
materials include, but are not limited to, cocoa butter, beeswax and
polyethylene glycols.
[00255] The pharmaceutical compositions described herein may also be
administered
topically, especially when the target of treatment includes areas or organs
readily accessible
by topical application, including diseases of the eye, the skin, or the lower
intestinal tract.
Suitable topical formulations are readily prepared for each of these areas or
organs.
[00256] Topical application for the lower intestinal tract can be effected
in a rectal
suppository formulation (see above) or in a suitable enema formulation.
Topically-
transdermal patches may also be used.
[00257] For topical applications, the pharmaceutical compositions may be
formulated
in a suitable ointment containing the active component suspended or dissolved
in one or more
carriers. Carriers for topical administration of the compounds of this
invention include, but
are not limited to, mineral oil, liquid petrolatum, white petrolatum,
propylene glycol,
polyoxyethylene, polyoxypropylene compound, emulsifying wax and water.
Alternatively,
the pharmaceutical compositions can be formulated in a suitable lotion or
cream containing
the active components suspended or dissolved in one or more pharmaceutically
acceptable
carriers. Suitable carriers include, but are not limited to, mineral oil,
sorbitan monostearate,
polysorbate 60, cetyl esters wax, cetearyl alcohol, 2 octyldodecanol, benzyl
alcohol and
water.
[00258] For ophthalmic use, the pharmaceutical compositions may be
formulated as
micronized suspensions in isotonic, pH adjusted sterile saline, or,
specifically, as solutions in
isotonic, pH adjusted sterile saline, either with or without a preservative
such as
-76-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
benzylalkonium chloride. Alternatively, for ophthalmic uses, the
pharmaceutical
compositions may be formulated in an ointment such as petrolatum.
1002591 The pharmaceutical compositions may also be administered by nasal
aerosol
or inhalation. Such compositions are prepared according to techniques well-
known in the art
of pharmaceutical formulation and may be prepared as solutions in saline,
employing benzyl
alcohol or other suitable preservatives, absorption promoters to enhance
bioavailability,
fluorocarbons, and/or other conventional solubilizing or dispersing agents.
1002601 The compounds for use in the methods of the invention can be
formulated in
unit dosage form. The term "unit dosage form" refers to physically discrete
units suitable as
unitary dosage for subjects undergoing treatment, with each unit containing a
predetermined
quantity of active material calculated to produce the desired therapeutic
effect, optionally in
association with a suitable pharmaceutical carrier. The unit dosage form can
be for a single
daily dose or one of multiple daily doses (e.g., about 1 to 4 or more times
per day). When
multiple daily doses are used, the unit dosage form can be the same or
different for each dose.
EXEMPLIFICATION
Preparation of Compounds
The compounds disclosed herein can be prepared by any suitable method known in
the art, for example, WO 2005/095400, WO 2007/084557, WO 2010/011768, WO
2010/011756, WP 2010/011772, WO 2009/073300, and PCT/US2010/038988 filed on
June
17, 2010. For example, the compounds shown in Tables 1 and 2 can be prepared
by any
suitable method known in the art, for example, WO 2005/095400, WO 2007/084557,
WO
2010/011768, WO 2010/011756, WP 2010/011772, WO 2009/073300, and
PCT/US2010/038988, and by the exemplary syntheses described below. Generally,
the
compounds of the invention can be prepared as shown in those syntheses
optionally with any
desired appropriate modification.
Methodology for Synthesis and Characterization of Compounds
1002611 Syntheses of certain exemplary compounds of the invention are
described
below. NMR and Mass Spectroscopy data of certain specific compounds are
summarized in
Tables 1 and 2. As used herein the term RT (min) refers to the LCMS retention
time, in
minutes, associated with the compound.
Synthetic Scheme 1
-77-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
0 0
NH2
a FC--1)NH b FN
FrxCN
FOH
I I 2CI-4N
rµr CI N CI N CI N N
1 2 3
Br Br
FB-C)
I e I \ N f Nr1-41 N
N
N N N N
6
N ph N
A¨Ph A¨Ph
4 5 Ph ph
Ph
(a) (C0)2C12, DMF/CH2C12, NI-140H; (b) Et3N, TFAA, CH2Cl2 (c) N2H4 H20,
nBuOH,
reflux; (d) tBuNO2, Br3CH, 60-90 C; (e) Ph3CCI, K2CO3, DMF (0 KOAc, 4,4,5,5-
tetramethy1-2-(4,4,5,5-tetramethy 1-1,3,2-dioxaborolan-2-yI)-1,3,2-
dioxaborolane,
Pd(dpPO2C12, DMF, 100 C.
Formation of 2-chloro-5-fluoropyridine-3-carboxamide (1)
To the suspension of 2-chloro-5-fluoropyridine-3-carboxylic acid (37.0 g,
210.8
mmol) in dichloromethane (555 mL) was added oxalyl chloride (56.2 g, 442.7
mmol) under
nitrogen. DMF (1.54 g, 21.08 mmol) was added slowly to the reaction mixture.
The mixture
was stirred at room temperature for 2 h and dichloromethane was removed under
reduced
pressure. The residue was dissolved in THF (300 mL) and cooled down to 0 C by
ice bath.
Ammonium hydroxide (28-30%, 113.0 mL, 1.8 mmol) was added in one portion. The
mixture was stirred for another 15 min. The mixture was diluted into ethyl
acetate (300 mL)
and water (300 mL) and the phases were separated. The organic layer was washed
with
brine and dried over Na2SO4, filtered, and concentrated in vacuo to afford
29.8 g desired
product as white solid: IH NMR (300 MHz, DMSO-d6) ö 8.53 (d, J = 3.0 Hz, 1H),
8.11 (s,
1H), 8.00 (dd, J= 8.0, 3.0 Hz, 1H), 7.89(s, IH); LCMS Gradient 10-90%, 0.1%
formic acid,
5min, C18/ACN, RT = 1.11 min, (M+H) 175.02.
Formation of 2-chloro-5-fluoropyridine-3-carbonitrile (2)
To a suspension of 2-chloro-5-fluoropyridine-3-carboxamide, 1, (29.8 g, 170.4
mmol) in
dichloromethane (327 mL) was added triethylamine (52.3 mL, 374.9 mmol). This
mixture
was cooled down to 0 C. Trifluoroacetic anhydride (26.1 mL, 187.4 mmol) was
added
slowly over period of 15 min. The mixture was stirred at 0 C for 90 min. The
mixture was
diluted into dichloromethane (300 mL) and the resulting organic phase was
washed with
aqueous saturated NaHCO3 solution (300 mL) and brine (300 mL). The organic
layer was
dried over Na2504, filtered, concentrated in vacuo. The product was purified
by silica gel
chromatography (40% to 60% ethyl acetate/hexanes gradient) giving 24.7 g of
product as a
white solid: 11-1 NMR (300 MHz, CDC13) 8 8.50 (d, J = 3.0 Hz, 1H), 7.77 (dd, J
= 6.8, 3.0
Hz, 1H); LCMS Gradient 10-90%, 0.1% formic acid, 5min, C18/ACN, Retention Time
=
2.50 min, (M+H) 157.06.
Formation of 5-fluoro-1H-pyrazolo[3,4-b]pyridin-3-amine (3)
To the mixture of 2-chloro-5-fluoropyridine-3-carbonitrile, 2, (29.6 g, 157.1
mmol) in
n-butanol (492 mL) was added hydrazine hydrate (76.4 mL, 1.6 mol). This
mixture was
heated to reflux for 4.5 h and cooled down. n-Butanol was removed under
reduced pressure
and water (300 mL) was added resulting in a yellow precipitate. The suspension
was filtered
-78-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
and washed with water twice, followed by a MTBE wash. The yellow solid was
dried in a
vacuum oven to give 18 g of the desired product: NMR (300 MHz, DMSO-d6) 8
12.08 (s,
1H), 8.38 (dd, J = 2.7, 1.9 Hz, 1H), 7.97 (dd, J = 8.8, 2.7 Hz, 1H), 5.56 (s,
2H). LCMS
Gradient 10-90%, 0.1% formic acid, 5min, C18/ACN, Retention Time = 1.25 min
(M+H)
152.95.
Formation of 3-bromo-5-fluoro-1H-pyrazolo[3,4-b]pyridine (4)
To a mixture of 5-fluoro-1H-pyrazolo[3,4-b]pyridin-3-amine, 3, (0.88 g, 5.79
mmol)
in bromoform (8.8 mL) was added tert-butyl nitrite (1.38 mL, 11.57 mmol). This
mixture
was heated to 61 C for 1 h and then heated to 90 C for an additional hour.
The mixture was
cooled to room temperature and bromoform was removed under reduced pressure.
The
resulting crude = residue was purified by silica gel chromatography (5-50%
ethyl
acetate/hexanes) to afford 970 mg of the desired product as a white solid: 1H
NMR (300
MHz, DMSO-d6) 8 14.22 (s, 1H), 8.67 (dd, J= 2.7, 1.9 Hz, 1H), 8.07 (dd, J =
8.2, 2.7 Hz,
1H); LCMS Gradient 10-90%, 0.1% formic acid, 5min, C18/ACN, Retention Time =
2.42
min (M+H) 216.11.
Formation of 3-bromo-5-fluoro-1-trity1-1H-pyrazoloI3,4-blpyridine (5)
A mixture of 3-bromo-5-fluoro-1H-pyrazolo[3,4-b]pyridine, 4, (0.97 g, 4.49
mmol)
and K2CO3 (1.86 g, 13.47 mmol) in DMF (9.7 mL) was cooled to 0 C.
Chlorodiphenylmethylbenzene (1.38 g, 4.94 mmol) was added. The mixture was
stirred at
room.temperature overnight. The mixture was diluted into ethyl acetate (40 mL)
and water
(30 mL) and the layers were separated. The organic layer was washed with
brine, dried over
Na2SO4, filtered and concentrated in vacuo. The product was purified by silica
gel
chromatography (40% ethyl acetate/hexanes) to afford 1.68 g of the desired
product as a
white solid: 114 NMR (300 MHz, DMSO-d6) ö 8.45 ¨ 8.38 (m, 1H), 8.04 (dd, J =
8.0, 2.7
Hz, 1H), 7.35 ¨7.16 (m, 15H); LCMS Gradient 10-90%, 0.1% formic acid, 5min,
C18/ACN,
Retention Time = 3.03 min (M+H) 459.46.
Formation of 5-fluoro-3-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-y1)-1-
trityl4H-
pyrazolo[3,4-b]pyridine (6)
A solution of 3-bromo-5-fluoro-1-trityl-pyrazolo[3,4-b]pyridine, 5, (3.43 g,
7.48
mmol), KOAc (2.20 g, 22.45 mmol) and 4,4,5,5-tetramethy1-2-(4,4,5,5-
tetramethy1-1,3,2-
dioxaborolan-2-yI)-1,3,2-dioxaborolane (2.85 g, 11.23 mmol) in DMF (50 ml) was
degassed
under a stream of nitrogen for 40 min. To the mixture was added Pd(dppf)2C12
(0.610 g,
0.748 mmol) The reaction mixture was heated at 100 C for 90 minutes. The
reaction
mixture was filtered through a pad of Celite. To the resulting filtrate was
added ether and
brine. The organic phase was dried over Mg504, filtered and concentrated in
vacuo to afford
4.0 g crude product that was used in the next step without further
purification (note, the
product decomposes if purification is attempted via silica gel
chromatography).
Synthetic Scheme 2
-79-
CA 02822059 2013-06-17
WO 2012/083121 PCT/US2011/065388
0 0
+ a fr0
-0Me
0 OH
0 0
7 8 0
(+/-)
0 0
-0Me d -0Me
NHCbz NH2
(+0 9 (+/-) 10
(a) CHCI3; (b) Na0Me, Me0H; (c) DPPA, Et3N, Bn0H; (d) H2, Pd/C;
Formation of endo-tetrahydro-4,7-ethanoisobenzofuran-1,3-dione (7)
To a cold (0 C) solution of maleic anhydride (210.0 g, 2.1 mol) in CHCI3 (2.3
L) was
added cyclohexa-1,3-diene (224.5 mL, 2.4 mol) slowly over 50 minutes. The
reaction was
warmed to room temperature and stirred overnight in the dark. After removing
the solvent
under reduced pressure, 2.1 L of Me0H was added to the mixture and the mixture
was heated
to 50 C for 10 min and then cooled down to 0 C. The resulting precipitate
was filtered and
dried in a vacuum oven at 45 C overnight to afford 283 g of a white solid.
The resulting
endo (meso) Diels-Alder cycloaddition product was used without further
purification.
Formation of (+/-)-trans-3-(methoxycarbonyl)bicyclo[2.2.2]oct-5-ene-2-
carboxylic acid
(8)
A solution of endo-(+/-)-tetrahydro-4, 7-ethanoisobenzofuran-1,3-dione, 7,
(74.5 g,
418.1 mmol) in Na0Me (764.9 mL of 25 %w/w solution in Me0H, 3.3 mol) was
stirred at
room temperature for 4 days yielding a white suspension. The reaction mixture
was
concentrated in vacuo to remove approximately 300 mL of Me0H. In another
flask, HCI
(315.9 mL of 36.5 %w/w, 3763.0 mmol) in 300 mL of water was cooled to 0 C.
The
reaction mixture was added slowly into this HC1 solution resulting in a white
precipitate. The
remaining methanol was removed under reduced pressure. The mixture was cooled
to 0 C
and stirred for 30 minutes. The precipitate was filtered, washed with water 3
times, giving
off-white solid. The remaining water was removed under reduced pressure to
afford 82 g of a
white solid.
Formation of (+/-)-trans-methyl 3-
(((benzyloxy)carbonyl)amino)bicyclo12.2.21oct-5-ene-
2-carboxylate (9)
To a solution of (+/-)-trans-3-(methoxycarbonyl)bicyclo[2.2.2]oct-5-ene-2-
carboxylic
acid, 8, (100.0 g, 475.7 mmol) in toluene (1.0 L) was added diphenylphosphoryl
azide (112.8
mL, 523.3 mmol) and triethylamine (72.9 mL, 523.3 mmol). The reaction mixture
was
heated to 90 C for 2 hours. Benzyl alcohol (49.2 mL, 475.7 mmol) was added
and the ixture
was heated to 90 C for 3 days. The mixture was cooled to room temperature and
diluted
with Et0Ac (500 mL) and aqueous saturated NaHCO3 solution. The organic phase
was
washed with brine, dried (MgSO4), filtered and concentrated in vacuo. The
resulting crude
material was purified by silica gel chromatography (100% CH2C12) to afford 115
g oil.
NMR show it contains BnOH (about 0.05 equiv). Product was used without further
purification: 11-1 NMR (300 MHz, CDCI3) 5 7.40 - 7.24 (m, 5H), 6.41 (t, J =
7.4 Hz, 1H),
6.21 - 6.04 (m, 1H), 5.15 - 4.94 (m, 2H), 4.63 - 4.45 (m, 1H), 4.30 - 4.18 (m,
1H), 3.70 (s,
= -80-
CA 02822059 2013-06-17
WO 2012/083121 PCT/US2011/065388
2H), 3.49 (s, 1H), 2.81 (br s, 1H), 2.68 (br s, I H), 2.08 (s, 1H), 1.76 -
1.56 (m, 1H), 1.52 -
1.35(m, 1H), 1.33- 1.14(m, 1H), 1.12 - 0.87 (m, IF1).
=
Formation of (+/-)-trans-methyl 3-aminobicyclo[2.2.21octane-2-carboxylate (10)
To a solution of racemic trans-methyl 3-(abenzyloxy)carbonypamino)-
bicyclo[2.2.2]oct-5-ene-2-carboxylate, 9, (115.0 g, 364.7 mmol) in THF (253
mL) and
Me0H (253 mL) was added Pd/C and the suspension was placed stirred under 40
psi
hydrogen atmosphere overnight. Some exotherm was observed. 1H NMR shows the
reaction
is complete and there is BnOH present. Filtered reaction mixture through
Celite, and washed
with Me0H. Concentrated filtrate in vacuo to afford 69 g oil: IFINMR (400 MHz,
CDC13) 8
3.63 (d, J = 5.6 Hz, 3H), 3.30 (d, J = 6.7 Hz, 1H), 2.11 (d, J = 6.6 Hz, I H),
1.91 (t, J= 7.3
Hz, 1H), 1.80¨ 1.64 (m, 1H), 1.63 ¨ 1.38 (m, 6H), 1.36¨ 1.23 (m, 2H).
Synthetic Scheme 3: Preparation of I-8
FF a, b F.
H2N ji
Br N F
CO2Me Br N N
H
CO2Me
(+0 10 11 B-0
6
F NPh
N
Ph- \
Ph
H = N N
NN CO2Me
Ph HN-N CO2Me
Ph 12 Ph 13
61
N NN)>1
H
00- Na+
1-8
a) Et3N, THF; (b) chiral SFC separation; (c) 5-fluoro-3-(4,4,5,5-tetramethy1-
1,3,2-
dioxaborolan-2-y1)-1-trityl-pyrazolo[3,4-b]pyridine, X-Phos, Pd2(dba)3, K3PO4,
2-MeTHF,
135 C; (d) Et3SiH, TFA, CH2C12; (e) Li0H, H20, THF, H20, then NaOH, Me0H.
Formation of (2S, 3S)-methyl 3-(6-bromo-3,5-difluoropyridin-2-ylamino)bicycle-
[2.2.2]
octane-2-carboxylate (11)
A solution of 2-bromo-3,5,6-trifluoro-pyridine (3.18 g, 15.00 mmol), racemic
trans-
methy1-3-aminobicyclo[2.2.2]octane-2-carboxylate, 10, (3.02 g, 16.50 mmol) and
triethylamine (6.2 mL, 33.0 mmol) was heated in a pressure tube at 140 C for
1 day. The
reaction mixture was diluted with ethyl acetate and brine. The organic phase
was dried over
MgSO4, filtered and the solvent was removed under reduced pressure. The
product was
purified by silica gel chromatography (15% Et0Ac/hexanes) to afford 4.1g of
desired
product: IFI NMR (400 MHz, CDC13) 8 7.08 (dd, J= 9.6, 6.8 Hz, 1H), 4.66 (s, I
H), 4.34 (s,
-81-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
1H), 3.79 (s, 31-1), 2.39 (d, J = 5.4 Hz, 1H), 1.97 (d, J = 2.4 Hz, 1H), 1.86
(d, J = 2.4 Hz, 1H),
1.78 (s, 1H), 1.75 ¨ 1.61 (m, 5H), 1.54 (s, 1H), 1.43 (t, J = 11.5 Hz, 1H);
LCMS Gradient 10-
90%, 0.1% formic acid, 5min, C18/ACN, RT = 3.85 min, (M+H) 375.06.
Separation of the racemic mixture using chiral SFC chromatographic resolution
provided the
individual enantiomers. 1.93 grams of the desired (2S, 3S)-enantiomer, 11, was
obtained
along with 2.01 g of the (2R, 3R) enantiomer.
Formation of (2S, 3S)-methyl 3-(3,5-difluoro-6-(5-fluoro-1-trity1-1H-
pyrazolo[3,4-
I)] pyridin-3-yl)pyridin-2-ylamino)bicyclo[2.2.21octane-2-ca rboxylate (12)
A solution of methyl (2S, 3S)-3-[(6-bromo-3,5-difluoro-2-
pyridyl)amino]bicyclo[2.2.2]octane-2-carboxylate, 11, (1.93 g, 5.14 mmol), 5-
fluoro-3-
(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-y1)-1-trityl-pyrazolo[3,4-
b]pyridine, 6, (3.12 g,
6.17 mmol) and K3PO4 (3.28 g, 15.43 mmol) in 2-MeTHF (38.6 mL) and H20 (3.9
mL) was
degassed under a stream of nitrogen for lh. To the mixture was added X-Phos
(0.29 g, 0.62
mmol) and Pd2(dba)3 (0.12 g, 0.13 mmol). The reaction mixture was heated at
135 C in a
pressure tube for 2 hours. The reaction mixture was cooled to room temperature
and the
aqueous phase was discarded. The organic phase was filtered through a pad of
celite and the
solvent was removed under reduced pressure. The crude residue was purified by
silica gel
chromatography (20%Et0Ac/hexanes) to afford 3.0g pure product: 11-1 NMR (400
MHz,
CDC13) 8 8.50 (dd, J = 8.5, 2.7 Hz, IH), 8.15 (s, 1H), 7.27 (s, 15H), 7.12 (t,
J = 9.6 Hz, 1H),
4.75 (s, I H), 4.58 (d, J= 6.6 Hz, I H), 3.56 (s, 3H), 2.37 (d, J= 6.1 Hz,
1H), 1.90 (s, 1H),
1.70 (dd, J = 22.1, 11.7 Hz, 5H), 1.49 (m, 1H); LCMS Gradient 10-90%, 0.1%
formic acid,
5min, C18/ACN, RT = 4.10 min, (M+H) 674.29.
Formation of (2S, 3S)-methyl 3-(3,5-difluoro-6-(5-fluoro-1H-pyrazolo13,4-
blpyridin-3-
yl)pyridin-2-ylamino)bicyclo[2.2.2]octane-2-carboxylate (13)
To a solution of (2S, 3S)-methyl 3-(3,5-difluoro-6-(5-fluoro- 1 -trity1-1H-
pyrazolo[3,4-
b]pyridin-3-y ppyridin-2-y lamino)bicyclo[2.2.2]octane-2-carboxy late, 12,
(3.00 g, 4.45
mmol) in dichloromethane (30 mL) was added triethylsilane (3.56 mL, 22.26
mmol) followed
by trifluoroacetic acid (3.43 mL, 44.53 mmol). The reaction mixture was
stirred at room
temperature for 1 hour. The solvent was removed under reduced pressure and the
resulting
crude residue was purified by silica gel chromatography (Et0Ac/hexanes). The
solvent was
removed and the product was washed with ether and filtered to afford 1.9g of
desired
product: 11-1 NMR (400 MHz, CDC13) 8 8.77 ¨ 8.67 (m, 1H), 8.56 (s, 1H), 7.24
(d, J = 9.8
Hz, I H), 4.80 (d, J= 6.1 Hz, I H), 3.64 (s, 3H), 2.42 (d, J= 6.4 Hz, 1H),
2.11 (s, 1H), 2.03 (s,
1H), 1.89 (d, J= 14.3 Hz, IH), 1.81 ¨ 1.62 (m, 5H), 1.54 (dt, J= 24.1, 12.2
Hz, 2H); LCMS
Gradient 10-90%, 0.1% formic acid, 5min, C18/ACN, RT = 3.52 min, (M+H) 432.45.
Formation of sodium (2S, 3S)-3-(3,5-difluoro-6-(5-fluoro-1H-pyrazolo[3,4-
b]pyridin-3-
y1)pyridin-2-ylamino)bicyclo[2.2.2]octane-2-carboxylate (1-8)
To a solution of (2S, 35)-methyl 3-(3,5-difluoro-6-(5-fluoro-1H-pyrazolo[3,4-
b]pyridin-3-y 1)py ridin-2-y lamino)bicyclo[2.2.2]octane-2-carboxy late, 13,
(1.90 g, 4.40
mmol) in THF (20 mL) was added a solution of lithium hydroxide hydrate (0.74
g, 17.62
mmol) in H20 (5 mL). The reaction mixture was stirred at 75 C for 5h. The
reaction
mixture was cooled to room temperature and to the mixture was added HC1 (1.10
mL of 12 M
solution, 13.21 mmol) dropwise. The product precipitated and was filtered. The
resulting
solid was washed with CH3CN and dried on high vacuum to afford 1.46 g of
desired product:
11-1 NMR (400 MHz, DMSO-d6) 8 8.59 (d, J= 11.3 Hz, 2H), 7.68 (t, J = 10.3 Hz,
1H), 6.42
(s, 1H), 4.67 (s, 11-1), 2.39 (s, 1H), 1.99 (s, 1H), 1.91 (s, 1H), 1.84 ¨ 1.48
(m, 5H), 1.44 (s,
1H), 1.29 (d, J= 13.4 Hz, 2H).
-82-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
1.46 g of product was converted to the sodium salt by dilution into methanol
followed
by addition of 3.51 mL of IN NaOH solution. The suspension clarified and was
stirred at
room temperature for 1 hour. The solvent was removed under reduced pressure to
afford
1.34 g of the sodium salt: IFI NMR (400 MHz, DMSO-d6) 6 8.37 (d, J= 7.0 Hz,
1H), 8.23
(s, 1H), 7.50 (t, J= 10.5 Hz, 1H), 5.87 (d, J= 5.7 Hz, 1H), 4.69 (s, 1H), 2.18
(d, J= 5.8 Hz,
1H), 1.96 (d, J = 16.0 Hz, 2H), 1.86 ¨ 1.57 (m, 4H), 1.56 ¨ 1.33 (m, 2H), 1.25
(d, J = 11.4
Hz, 2H); LCMS Gradient 10-90%, 0.1% formic acid, 5min, C18/ACN, RT = 3.05 min,
(M+H) 417.89.
Preparation of Compounds 1-6
The following compounds can be prepared in the same fashion using the
procedures above:
CI
F.õ(F
NN
N
HN-N
0 0- Na. (+V-) (I-6)
(2S, 3S)-34(6-(5-ehloro-1H-pyrazolo[3,4-blpyridin-3-y1)-3,5-difluoropyridin-2-
y1)-
amino)bicyclo[2.2.21octane-2-carboxylate (I-6)
IH NMR (400 MHz, DMSO-d6) 6 8.81 (s, 1H), 8.45 (s, 1H), 7.62 (t, J = 10.4 Hz,
1H),
6.22 (d, J = 6.0 Hz, IH), 4.70 (s, 1H), 2.28 (d, J = 6.0 Hz, 1H), 2.00 (s,
1H), 1.91 (s, 2H),
1.69 (d, J= 11.9 Hz, 3H), 1.53 (d, J= 5.2 Hz, 1H), 1.43 (s, 1H), 1.27 (d, J =
12.1 Hz, 2H);
LCMS Gradient 10-90%, 0.1% formic acid, 5min, C18/ACN, RT = 3.26 min, (M+H)
434.44.
Synthetic Scheme 3: Preparation ofI-15
eNH2 0
NCF I NCr.F c NC F f<1 a, b
I ¨ I
)\.1
CIIµr CI CI N 6 N N
H
(+I-) 10 CO2Me N-N CO2Me
15 Ph
Phi -Ph 16
F
NC F
I
\ \
HN-N CO2Me HN-N
0 OH
17 1-15
(a) (a) Et3N, CH3CN; (b) SFC chiral separation; (c) 5-fluoro-3-(4,4,5,5-
tetramethy1-1,3,2-
dioxaborolan-2-y1)-1-trityl-pyrazolo[3,4-b]pyridine, 6, X-Phos, Pd2(dba)3,
K3PO4, 2-MeTHF,
135 C; (d) Et3SiH, TFA, CH2Cl2: (e) Li0H, H20, THF.
Formation of (2S, 3S)-methyl 3-(6-chloro-5-cyano-3-fluoropyridin-2-ylamino)-
bicyclo[2.2.2]-octane-2-carboxylate (15)
A solution of racemic trans-methyl-3-aminobicyclo[2.2.2]octane-2-carboxylate,
10,
(2.00 g, 10.91 mmol), 2,6-dichloro-5 fluoro-pyridine-3-carbonitrile (2.29 g,
12.00 mmol) and
triethylamine (3.35 mL, 24.00 mmol) in acetonitrile (25 mL) was refluxed for 4
h. The
reaction mixture was diluted into Et0Ac and brine. The organic phase was dried
over
MgSO4, filtered and the solvent was removed under reduced pressure. The
resulting crude
residue was purified by silica gel chromatography (20%Et0Ac/hexanes) to afford
3.15g of
desired product as a racemic mixture: NMR (400
MHz, CDC13) 6 7.32 ¨ 7.28 (m, 1H),
5.32 (s, 1H), 4.48 (s, 1H), 3.77 (s, 3H), 2.39 (d, J = 5.6 Hz, 1H), 2.03 ¨
1.97 (m, 1H), 1.88 (d,
-83-
CA 02822059 2013-06-17
WO 2012/083121 PCT/US2011/065388
J= 2.2 Hz, 1H), 1.81 (d, J = 13.5 Hz, 1H), 1.74 ¨ 1.62 (m, 5H), 1.47 (d, J=
13.2 Hz, 1H);
LCMS Gradient 10-90%, 0.1% formic acid, 5min, C18/ACN, Retention Time = 3.60
minutes, (M+H) 338.35.
The racemic mixture of trans isomers, 3-(6-chloro-3-fluoropyridin-2-
ylamino)bicyclo[2.2.2]octane-2-carboxylic acid, was separated by SFC chiral
purification to
afford (2R, 3R)-methyl 34(6-chloro-5-cyano-3-fluoropyridin-2-y0amino)-
bicyclo[2.2.2]octane-2-carboxylate and (2S, 3S)-methyl 34(6-chloro-5-cyano-3-
fluoropyridin-2-yDamino)bicyclo[2.2.2]octane-2-carboxylate, 15.
Formation of (2S, 3S)-methyl 3-06-(5-cyano-1-trity1-1H-pyrazolo[3,4-b]pyridin-
3-y1)-
3,5-difluoropyridin-2-yl)amino)bicyclo[2.2.2Joctane-2-carboxylate (16)
A solution of (2S, 3S)-methyl 34(6-chloro-5-cyano-3-fluoropyridin-2-
yDamino)bicyclo[2.2.2]octane-2-carboxylate, 15, (0.86 g, 2.55 mmol), 5-fluoro-
3-(4,4,5,5-
tetramethy1-1,3,2-dioxaborolan-2-y1)-1-trityl-pyrazolo[3,4-b]py ridine, 6,
(1.54 g, 3.06 mmol)
and K3PO4 (1.62 g, 7.64 mmol) in 2-methyl THF (17.2 mL) and H20 (1.7 mL) was
degassed
under a stream of nitrogen for 1h. To the reaction mixture was added X-Phos
(0.15 g, 0.31
mmol) and Pd2(dba)3 (0.06 g, 0.06 mmol). The reaction mixture was heated at
125 C in a
pressure tube for 2 hours. The reaction mixture was cooled to room temperature
and the
aqueous phase was removed. The organic phase was filtered through a pad of
Celite and the
solvent was removed under reduced pressure. The resulting crude residue was
purified by
silica gel chromatography (20%Et0Ac/hexanes) to afford 1.05 g of desired
product:
NMR (400 MHz, CDC13) 5 8.33 (d, J= 5.6 Hz, 1H), 8.18 (s, 1H), 7.40 (d, J= 10.5
Hz, 1H),
7.35 ¨ 7.20 (m, 15H), 5.18 (d, J= 6.3 Hz, 1H), 4.85 (t, J= 6.9 Hz, 1H), 3.51
(s, 3H), 2.40 (d,
J= 5.5 Hz, 1H), 2.09 (s, 1H), 2.03 (s, 1H), 1.87 (s, 1H), 1.79 ¨ 1.58 (m, 6H),
1.51 (d, J= 11.3
Hz, 1H).
Formation of (2S, 3S)-methyl 34(5-cyano-3-fluoro-6-(5-fluoro-1H-pyrazolo[3,4-
b]pyridin-3-y1)pyridin-2-y1)amino)bicyclo[2.2.21octhne-2-carboxylate (17)
To a solution of (2S, 3S)-methyl 34(6-(5-cyano-1-trity1-1H-pyrazolo[3,4-
b]pyridin-3-
y1)-3,5-difluoropyridin-2-yDamino)bicyclo[2.2.2]octane-2-carboxylate, 16,
(1.00 g, 1.47
mmol) in dichloromethane (40 mL) was added triethylsilane (1.17 mL, 7.35 mmol)
and
trifluoroacetic acid (1.13 mL, 14.69 mmol). The reaction mixture was stirred
at room
temperature for 15min. The solvent was removed under reduced pressure. The
crude residue
was purified by silica gel chromatography (3% Me0H/CH2C12) to afford the
desired product:
LCMS Gradient 10-90%, 0.1% formic acid, 5min, C18/ACN, Retention Time = 3.46
min,
(M+H) 439.43.
Formation of (2S, 3S)-34(5-cyano-3-fluoro-6-(5-fluoro-1H-pyrazolo13,4-
b]pyridin-3-
Apyridin-2-y1)amino)bicyclo12.2.21octane-2-carboxylic acid (I-15)
To a solution of (2S, 3S)-methyl 34(5-cyano-3-fluoro-6-(5-fluoro-1H-
pyrazolo[3,4-
b]pyridin-3-yppyridin-2-yDamino)bicyclo[2.2.2]octane-2-carboxylate, 17, (0.60
g, 1.37
mmol) in THF (20 mL) was added a solution of lithium hydroxide hydrate (0.23
g, 5.48
mmol) in H20 (2 mL). The reaction mixture was stirred at 60 C for 4h. Organic
solvent of
the reaction mixture was removed under reduced pressure. The aqueous phase pH
was
adjusted to 6 by adding HCI (0.34 mL of 12 M, 4.10 mmol). The resulting
precipitate was
filtered and dried under vacuum overnight to afford 500 mg of desired product.
1H NMR
(300 MHz, DMSO-d6) 5 8.75 ¨ 8.63 (m, 1H), 8.49 (dd, J= 8.8, 2.8 Hz, 1H), 7.94
(d, J= 11.3
Hz, 1H), 7.87 (d, J= 8.0 Hz, I H), 4.79 (d, J= 7.0 Hz, 1H), 2.91 (d, J= 7.2
Hz, 1H), 2.03 (s,
1H), 1.87 (s, 1H), 1.77 (s, 2H), 1.62 (d, J= 8.5 Hz, 2H), 1.43 (d, J= 30.8 Hz,
4H); LCMS
Gradient 10-90%, 0.1% formic acid, 5min, C18/ACN, Retention Time = 3.06 min,
(M+H)
-84-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
425.06.
Synthetic Scheme 4
0
a
EtO'VLOH EtO)Car NH'Cbz=Et0jtrNH2
18 19
i.
DPPA, Et3N, toluene, 110 C; ii. Bn0H, 85 C (b) Pd/C (wet, Degussa),
hydrogen,
Et0H
Formation of (1S, 3R)-3-(ethoxycarbonyl)cyclohexanecarboxylic acid
(1S, 3R)-3-(ethoxycarbonyl)cyclohexanecarboxylic acid starting material can be
prepared following the literature procedures described in : Barnett, C. J.,
Gu, R. L.,
Kobierski, M. E., WO-2002024705, Stereoselective process for preparing
cyclohexyl amine
derivatives.
Formation of ethyl (1R, 3S)-3-benzyloxycarbonylaminocyclohexanecarboxylate
(18)
(IS, 3R)-3-(Ethoxycarbonyl)cyclohexanecarboxylic acid (10.0 g, 49.9 mmol) was
dissolved in toluene (100 mL) and treated with triethylamine (7.6 mL, 54.9
mmol) and DPPA
(12.2 mL, 54.9 mmol). The resulting solution was heated to 110 C and stirred
for 1 hour.
After cooling to 70 C, benzy 1 alcohol (7.7 mL, 74.9 mmol) was added, and the
mixture was
heated to 85 C overnight. The resulting solution was cooled to room
temperature, poured
into Et0Ac (150 mL) and water (150 mL) and the layers were separated. The
aqueous layer
was extracted with Et0Ac (2x75 mL) and the combined organic extracts were
washed with
water (100mL) and brine (100 mL), dried over Na2SO4 and concentrated in
vactio. The crude
material was purified by silica gel chromatography (0%-50% Et0Ac/hexanes) to
provide 26
(15.3 g, containing ¨25% benzyl alcohol), which was used for the next step
without further
purification.
Formation (1R, 3S)-ethyl 3-aminocyclohexanecarboxylate (19)
To a solution of (1R, 3S)-ethyl 3-(benzy loxycarbonylamino)cyclohexane-carboxy
late,
18, (14.0 g, 45.9 mmol) in ethanol (3 mL) was added Pd/C (wet, Degussa (2.4 g,
2.3 mmol).
The mixture was evacuated and then stirred under atmosphere of hydrogen at
room
temperature overnight. The reaction mixture was filtered through a pad of
celite and the
resulting filtrate concentrated in vacuo to provide an oil that was used
without further
purification.
Synthetic scheme 5: Preparation of compound 1-2
-85-
CA 02822059 2013-06-17
WO 2012/083121 PCT/US2011/065388
Br
0
N)-F a
F N 0 __________
\ Ns
¨N
¨ 14 0 OEt
F)Y 14
19
OEt
Br = 0
13-
"
20 N 21
F.õIN 6
N -
Tr
F 14% 0 \ 1.1µ 0
¨N ¨N
0 . (
OH F 0 .....
OH
,
I \ N 1
=
I41 N N
Tr 22 1-2
(a) (IR, 3S)-ethyl 3-aminocyclohexanecarboxylate, 19, Et3N, THF, Me0H; (b) 5-
fluoro-3-
(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-y1)-1-trity 1-pyrazolo[3,4-
b]pyridine, 6, X-Phos,
Pd2(dba)3, K3PO4, 2-MeTHF, 130 C; (c) Li0H.H20, THF, H20; (d) Et3SiH, TFA,
CH2C12
Formation of (1R, 3S)-ethyl 3-(6-bromo-3,5-difluoropyridin-2-ylamino)-
cyclohexanecarboxylate (20)
A solution of ethyl (IR, 3S)-3-aminocyclohexanecarboxylate, 19, (1.88 g, 11.00
mmol),
2-bromo-3,5,6-trifluoro-pyridine (2.12 g, 10.00 mmol) and triethylamine (3.07
mL, 22.00
mmol) in THF/Me0H mixture was heated at 100 C in a pressure tube overnight.
The
reaction mixture was diluted into Et0Ac and brine. The organic phase was dried
over
MgSO4, filtered and the solvent was removed under reduced pressure. The
product was
purified by silica gel chromatography (10% Et0Ac/hexanes) to afford 1.08g of
desired
product: 1H NMR (300 MHz, CDC13) .5 7.06 (ddd, J = 43.8, 23.6, 20.2 Hz, 1H),
4.23 ¨ 4.07
(m, 2H), 4.02 ¨ 3.86 (m, 1H), 2.51 (tt, J = 11 .8, 3.6 Hz, 1H), 2.41 ¨ 2.28
(m, 1H), 2.16 ¨ 2.07
(m, 1H), 2.04 ¨ 1.96 (m, 1H), 1.95 ¨ 1.84 (m, 1H), 1.58 ¨ 1.44 (m, 1H), 1.43 ¨
1.30 (m, 2H),
1.30 ¨ 1.23 (m, 4H), 1.23 ¨ 1.08 (m, 1H); LCMS Gradient 10-90%, 0.1% formic
acid, 5min,
C18/ACN RT = 3.89 min (M+H) 363.30.
Formation of (IR, 3S)-ethyl 3-(3,5-difluoro-6-(5-fluoro-1-trity1-1H-
pyrazolo13,4-
b]pyridin-3-yOpyridin-2-ylamino)cyclohexanecarboxylate (21)
A solution of 5-fluoro-3-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-y1)-1-
trityl-
pyrazolo[3,4-b]pyridine, 6, (0.76 g, 1.50 mmol) and (1R, 3S)-ethyl 3-(6-bromo-
3,5-
difluoropyridin-2-ylamino)-cyclohexanecarboxylate, 20, (0.65 g, 1.80 mmol) in
2-methyl
THF and H20 was degassed under a stream of nitrogen for 30 minutes. To the
mixture was
added X-Phos (0.09 g, 0.180 mmol), Pd2(dba)3 (0.03 g, 0.04 mmol) and K3PO4
(1.27 g, 6.00
mmol) and degassed the reaction mixture for another 20 minutes. The reaction
mixture was
heated in a pressure tube at 130 C for 45 minutes. The reaction mixture was
filtered through
celite and the solvent was removed under reduced pressure. The crude residue
was purified
by silica gel chromatography (30%Et0Ac/hexanes) to afford 220 mg of desired
product: IF1
NMR (300 MHz, CDC13) 5 8.43 (dd, J = 8.5, 2.9 Hz, 1H), 8.18 (dd, J = 2.8, 1.1
Hz, 1H), 7.33
¨ 7.22 (m, 15H), 7.12 (dd, J = 23.5, 13.5 Hz, 1H), 4.54 (d, J = 6.3 Hz, 1H),
4.23 ¨ 4.08 (m,
2H), 4.11 ¨3.99 (m, 1H), 2.61 (ddd, J = 12.4, 6.8, 3.1 Hz, 2H), 2.28 (d, J =
12.4 Hz, 1H),
2.04 (ddd, J = 17.0, 10.1, 9.5 Hz, 2H), 1.68 ¨ 1.38 (m, 3H), 1.37 ¨ 1.28 (m,
1H), 1.24 (m,
3H).
Formation of (IR, 3S)-3-(3,5-difluoro-6-(5-fluoro-1-trity1-1H-pyrazolo13,4-
b]pyridin-3-
yflpyridin-2-ylamino)cyclohexanecarboxylic acid (22)
-86-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
A solution of (1R, 35)-ethyl 3-(3,5-difluoro-6-(5-fluoro-1-trity1-1H-
pyrazolo[3,4-
b]pyridin-3-yppyridin-2-ylamino)cyclohexanecarboxylate, 21, (0.220 g, 0.333
mmol) lithium
hydroxide hydrate (0.070 g, 1.662 mmol) in THF (15 mL) and H20 (2 mL) was
stirred at
room temperature overnight. The reaction mixture was diluted into Et0Ac and
brine. The
aqueous phase was adjusted to pH 6. The organic phase was separated, dried
over MgSO4,
filtered and the solvent was removed under reduced pressure to afford 200 mg
of desired
product: 111 NMR (300 MHz, CDCI3) 8 8.37 (ddd, J= 8.5, 3.9, 2.9 Hz, 1H), 8.14
(d, J = 2.8
Hz, 1H), 7.31 ¨ 7.19 (m, 15H), 7.15 ¨ 7.01 (m, 1H), 4.49 (s, 1H), 4.01 (d, J =
11.1 Hz, 1H),
2.68 ¨ 2.45 (m, 2H), 2.24 (d, J = 10.8 Hz, 1H), 2.01 ¨ 1.92 (m, I H), 1.61 ¨
1.35 (m, 3H);
LCMS Gradient 10-90%, 0.1% formic acid, 5min, C18/ACN, RT = 4.13 min (M-H)
632.51.
Formation of (1R, 3S)-ethyl 3-(3, 5-difluoro-6-(5-fluoro-1H-pyrazolo[3,4-
blpyridin-3-
yl)pyridin-2-ylamino)cyclohexanecarboxylate (4) and (1R,3S)-3-(3,5-difluoro-6-
(5-
fluoro-1H-pyrazolo[3,4-b]pyridin-3-yl)pyridin-2-ylamino)cyclohexanecarboxylic
acid (I-
2)
To a solution of (IR, 3S)-3-(3,5-difluoro-6-(5-fluoro-l-trity1-1H-pyrazolo[3,4-
b]pyridin-3-yppyridin-2-ylamino)cyclohexanecarboxylic acid, 22, (0.110 g,
0.174 mmol) in
dichloromethane was added triethylsilane (0.554 mL, 3.472 mmol) followed by
trifluoroacetic acid (0.535 mL, 6.944 mmol). The reaction mixture was stirred
at room
temperature for 1 hr. The reaction mixture was diluted into Et0Ac and aqueous
saturated
Na2CO3 and the organic phase was washed with brine, dried over MgSO4, filtered
and the
solvent was removed under reduced pressure. The crude residue was purified by
silica gel
chromatography (Me0H/CH2C12) to afford 58 mg of desired product: 11-1 NMR (300
MHz,
CDC13) 8 8.54 ¨ 8.38 (m, 2H), 7.22 (d, J = 9.7 Hz, 1H), 4.05 (dd, J = 13.5,
9.8 Hz, 1H), 2.66
¨2.48 (m, 3H), 2.26 (d, J = 13.0 Hz, 1H), 2.11 (d, J = 11.9 Hz, 1H), 2.00 (d,
J = 15.2 Hz,
1H), 1.50 (dt, J= 24.2, 12.8 Hz, 3H), 1.25 (dd, J= 15.8, 8.9 Hz, 2H); LCMS
Gradient 10-
90%, 0.1% formic acid, 5min, C18/ACN, RT = 2.70 min (M+H) 392.42.
Synthetic scheme 6: Preparation of compound 1-11
N NH2 0 N 0
=,\
A 0". a
1
H2N) F
CI fsr CI ¨4" CI N NH 0 CI N 0
(+0
(41-) 23 .(31
24 (+/-)
0
0
k OH
HOF OH )XI
d, e ¨N
CI
CI N NH 0 6 F N NH 0
OH I N
isr
25 (+/-) 26 ph Ph
)\--Ph 28
0
kOH
¨N
¨=- F
I N
Nj
I-1 1-11
(a) Et3N, CH3CN, reflux; (b) conc. H2SO4; (c) 9M H2SO4 (d) Ag2CO3, HOAc, DMSO,
100
C; (e) SFC chiral separation (f) 5-fluoro-3-(4,4,5,5-tetramethy1-1,3,2-
dioxaborolan-2-y1)-1-
tosy1-1H-pyrrolo[2,3-b]pyridine, 6, Pd2(dba)3, K3PO4, X-Phos, 2-Me-THF/H20,
120 C; (g)
triethylsilane, TFA, CH2C12
-87-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
Formation of racemic-trans-methyl 34(6-chloro-5-cyano-3-fluoropyridin-2-
yl)amino)-
bicyclo[2.2.2joctane-2-carboxylate (23)
A solution of racemic-trans-methyl-3-aminobicyclo[2.2.2]octane-2-carboxylate,
10,
(2.00 g, 10.91 mmol), 2,6-dichloro-5 fluoro-pyridine-3-carbonitrile (2.29 g,
12.00 mmol) and
Et3N (3.35 mL, 24.00 mmol) in acetonitrile (25 mL) was refluxed for 4 h. The
reaction
mixture was diluted into Et0Ac and brine. The organic phase was dried over
MgSO4,
filtered and the solvent was removed under reduced pressure. The resulting
crude residue
was purified by silica gel chromatography (20% Et0Ac/hexanes) to afford 3.15 g
of desired
product: IF1 NMR (400 MHz, CDC13) 8 7.32 ¨ 7.28 (m, 1H), 5.32 (s, 1H), 4.48
(s, 1H), 3.77
(s, 3H), 2.39 (d, J= 5.6 Hz, 1H), 2.03 ¨ 1.97 (m, 1H), 1.88 (d, J= 2.2 Hz,
1H), 1.81 (d, J =
13.5 Hz, 1H), 1.74¨ 1.62(m, 5H), 1.47 (d, J= 13.2 Hz, 1H); LCMS Gradient 10-
90%, 0.1%
formic acid, 5min, C18/ACN, Retention Time = 3.60 minutes (M+H) 338.35.
Forma tion of racemic-trans-3-(5-carbamoy1-6-chloro-3-fluoropyridin-2-yla m
ino)-
bicyclo[2.2.21octa ne-2-ca rboxylic acid (24)
To H2SO4 (35 mL of 18 M solution, 630 mmol) was added racemic-trans-methyl 3-
((6-chloro-5-cyano-3-fluoropyridin-2-yl)amino)bicyclo[2.2.2]octane-2-
carboxylate, 35, (3.15
g,.9.33 mmol). The reaction mixture was heated at 80 C for lh. The reaction
mixture was
taken on directly into next step without purification: LC/MS Gradient 10-90%,
formic 5min,
C18/can, Retention Time = 2.39 minutes (M+H) 342.28.
Formation of racemic-trans-64-3-carboxybicyclo[2.2.2loctan-2-ylamino)-2-chloro-
5-
fluoropyridine-3-carboxylic acid (25)
A solution of racemic-trans-3-(5-carbamoy1-6-chloro-3-
fluoropyridin-2-
ylamino)bicyclo[2.2.2]octane-2-carboxylic acid, 24, in concentrated H2SO4 (35
mL of 18 M
solution) at room temperature was transferred to a flask with 35 mL H20
slowly. The
reaction mixture was then heated and stirred at 100 C for 5 hours. The
reaction mixture was
cooled to room temperature and to it was added ice to total 250 mL volume. The
resulting
precipitate was filtered. The filtration cake was dissolved in CH2C12 and
purified by silica gel
chromatography (40%Et0Ac/hexanes) to afford 2.0g product: 1H NMR (400 MHz,
DMSO-
d6) 8 7.76 (d, J = 11.2 Hz, 1H), 7.69 (d, J= 6.9 Hz, 1H), 4.42 (t, J= 6.8 Hz,
1H), 2.78 (d, J=
6.8 Hz, 1H), 1.95 (s, 1H), 1.74 (s, 1H), 1.69 (d, J = 8.5 Hz, 2H), 1.62 ¨ 1.36
(m, 5H), 1.32 (t,
J = 10.4 Hz, 1H); LCMS Gradient 10-90%, 0.1% formic acid, 5min, C18/ACN,
Retention
Time = 2.84 minutes (M+H) 343.07.
Formation of (2S, 38)-34(6-chloro-3-fluoropyridin-2-
yl)amino)bicyclo12.2.21octane-2-
carboxylic acid (26)
A solution of racemic-trans-64-3-carboxybicyclo[2.2.2]octan-2-ylamino)-2-
chloro-5-
fluoropyridine-3-carboxylic acid, 25, (2.00 g, 5.84 mmol), Ag2CO3 (0.16 g,
0.58 mmol) and
acetic acid (0.02 mL, 0.29 mmol) in DMSO (20 mL) was heated and stirred at 120
C for 5h.
The reaction mixture was diluted with Et0Ac and aqueous saturated NH4C1
solution. The
organic phase was dried over MgSO4, filtered and the solvent was removed under
reduced
pressure. The product was purified by silica gel chromatography (20%
Et0Ac/hexanes) to
afford 1.34 g of racemic-trans-3-(6-chloro-3-fluoropyridin-2-
ylamino)bicyclo[2.2.2]octane-
2-carboxylic acid: IF1 NMR (400 MHz, CDC13) 8 7.19 (dd, J = 10.0, 8.2 Hz, 1H),
6.59 (dd, J
= 8.1, 2.9 Hz, 1H), 5.22 (s, 1H), 4.03 (d, J = 4.3 Hz, 1H), 2.50 (s, 1H), 2.17
(s, 1H), 2.04 (dd,
J= 17.6, 7.1 Hz, 1H), 1.87 (s, 1H), 1.82 ¨ 1.64 (m, 4H), 1.63 ¨ 1.50 (m, 6H),
1.44 (dd, J =
19.8, 11.4 Hz, 1H); LCMS RT = 3.22 (M+H) 299.07.
-88-
CA 02822059 2013-06-17
WO 2012/083121 PCT/US2011/065388
The racemic mixture (880 mg) was separated by SFC chiral purification to 400mg
of the (S,
S) enantiomer, 26, and 438 mg of the (R, R) enantiomer, 27. The (S, S)-
enantiomer, 26, was
taken onto the next step.
Formation of (2S, 3S)-3-(3-fluoro-6-(5-fluoro-1-trity1-1H-pyrazolo[3,4-
b]pyridin-3-
yl)pyridin-2-ylamino)bicyclo[2.2.2]octane-2-carboxylic acid (28)
A solution of 5-fluoro-3-(4,4,5,5-tetramethy I-1,3,2-d ioxaborolan-2-
y1)-1-trity I-
pyrazolo[3,4-b]pyridine, 6, (0.812 g, 1.607 mmol), (2S, 3S)-3-(6-chloro-3-
fluoropyridin-2-
ylamino)bicyclo[2.2.2]octane-2-carboxylic acid, 26, (0.400 g, 1.339 mmol) and
K3PO4 (1.137
g, 5.356 mmol) in 2-MeTHF (10.0 mL) and H20 (1.43 mL) was degassed under a
stream of
nitrogen for 1 hour. To the mixture was added X-Phos (0.076 g, 0.161 mmol) and
Pd2(dba)3
(0.030 g, 0.033 mmol). The reaction mixture was heated in a pressure tube at
135 C for 2
hours. The reaction mixture was cooled to room temperature and the organic
phase was
filtered through a pad of celite and concentrated under reduced pressure. The
crude residue
was purified via silica gel chromatography (15% Et0Ac/hexanes) to afford 313
mg of desired
product: 1H NMR (400 MHz, CDC13) 6 8.53 ¨ 8.43 (m, 1H), 8.12 (s, 1H), 7.27 (s,
15H), 7.21
¨ 7.15 (m, 2H), 4.79 (s, 1H), 4.69 (s, 1H), 2.43 (d, J = 5.4 Hz, 1H), 2.18 (s,
1H), 2.09 (d, J =
11.3 Hz, 1H), 1.92¨ 1.60(m, 7H), 1.59¨ 1.42(m, 2H).
Formation of (2S, 3S)-3-(3-fluoro-6-(5-fluoro-1H-pyrazolo13,4-b]pyridin-3-
yl)pyridin-2-
ylamino)bicyclo[2.2.2]octane-2-carboxylic acid (I-11)
To a solution of (2S, 3S)-3[[3-fluoro-6-(5-fluoro- 1 -trityl-pyrazolo[3,4-
b]pyridin-3-yI)-
2-pyridyl]amino]bicyclo[2.2.2]octane-2-carboxylic acid, 28, (0.313 g, 0.488
mmol) in
dichloromethane (25 mL) was added triethylsilane (0.390 mL, 2.439 mmol)
followed by
trifluoroacetic acid (0.376 mL, 4.878 mmol). The reaction mixture was stirred
at room
temperature for 1.5h. The solvent was removed under reduced pressure and the
product was
purified by silica gel chromatography (5% Me0H/CH2C12) to afford 110 mg of
desired
product: 114 NMR (400 MHz, Me0D) 6 8.72 (dd, J = 8.6, 2.7 Hz, 1H), 8.46 (d, J
= 1.7 Hz,
1H), 7.32 (ddd, J= 19.0, 9.5, 5.8 Hz, 2H), 2.72 (d, J = 6.8 Hz, 1H), 2.10 (s,
1H), 2.02 (d, J =
5.5 Hz, 1H), 1.97 ¨ 1.79 (m, 3H), 1.77 ¨ 1.58 (m, 3H), 1.57 ¨ 1.40 (m, 2H);
LCMS RT = 3.10
min (M+H) 400.45.
Note, using the above procedures, the (R, R) intermediate carboxylic acid, 27,
can be used to
synthesize the corresponding (R, R)-enantiomer, I-10.
Synthetic Scheme 7
Yr1, a
HO 0
0
Et0 o[s1Cbz N 'Cbz H2NjtrN'Cbz
18 29 30
c H2N d, e Boc,1=110r,NH2
Cbz
31 32
(a) Li0H, THF: H20; (b) Boc20, pyridine, NH4HCO3, dioxane; (c) BTIB,
CH3CN:H20; (d)
Boc20, K2CO3, THF; (e) Pd/C, H2, Me0H
Formation of (IR, 3S)-3-benzyloxycarbonylaminocyclohexanecarboxylic acid (29)
Ethyl (IR, 3S)-3-benzyloxycarbonylaminocyclohexanecarboxylate, 18, (36.0 g,
117.9
mmol) was dissolved in THF (144.0 mL) and treated with a solution of LiOH (5.7
g, 235.8
-89-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
mmol) in water (216.0 mL). After stirring overnight, the reaction mixture was
diluted with
water (100 mL), washed with MTBE (150 mL) and brought to pH 3 by addition of
3N HCI.
The acidic solution was extracted with Et0Ac (3x100 mL), and the combined
organic layers
were washed with water and brine, dried on Na2SO4 and concentrated in vacuo.
The crude product was triturated with MTBE (30 mL) and filtered to provide a
first
crop of crystals. The filtrate was treated with heptane (20 mL), concentrated
to 30 mL and
allowed to stand at room temperature for 3 hours to provide a second crop of
crystals that
were collected by filtration for a total of 14.4 g (44%yield) of desired
product: 1H NMR (300
MHz, CDC13) 8 7.38 - 7.33 (m, 5H), 5.11 (s, 2H), 4.68 (s, IH), 3.55 (s, IH),
2.44 (d, J =11.0
Hz, 1H), 2.32 (d, J =11.7 Hz, 1H), 2.03 - 1.86 (m, 3H) and 1.48 - 0.88 (m, 4H)
ppm.
Formation of benzyl N-R1S, 3R)-3-carbamoylcyclohexylIcarbamate (30)
To a solution of (IR, 3S)-3-Benzyloxycarbonylaminocyclohexanecarboxylic acid,
29,
(10.0 g, 36.1 mmol) in 1,4-dioxane (300 mL) was added pyridine (2.9 mL, 36.1
mmol),
followed by di-tert-butyl dicarbonate (10.7 mL, 46.9 mmol) and ammonium
bicarbonate
(10.1 g, 126.2 mmol). After 3 hours, another portion of di-tert-butyl
dicarbonate (1.5 g, 6.8
mmol) and ammonium bicarbonate (1.5 g, 6.8 mmol) was added and stirring was
continued
overnight. The reaction was quenched by addition of 2N HC1 (400 mL) and
stirred for 1
hour. The resulting suspension was filtered under reduced pressure, washed
with 2N HC1 (50
mL), water (8x50 mL) and hexanes (3x50 mL) and vacuum dried to provide benzyl
N-[(1S,
3R)-3-carbamoylcyclohexyl]carbamate, 30, (9.1 g, 91%) as a white solid: IF1
NMR (300
MHz, CDCI3) 8 7.40 - 7.24 (m, 5H), 5.08 (s, 2H), 3.58 - 3.44 (m, 1H), 2.38 -
2.21 (m, 1H),
2.17 (d, J =12.7, 1H), 2.05- 1.78(m, 8H), 1.54 - 0.97 (m, 5H).
Formation of benzyl N-1(1S, 3R)-3-aminocyclohexyl]carbamate (31)
Benzyl N-[(IS, 3R)-3-carbamoylcyclohexyl]carbamate, 30, (9.1 g, 32.9 mmol) was
suspended in a mixture of acetonitrile (100 mL) and water (100 mL) and treated
with .
bis(trifluoroacetoxy)iodobenzene (15.5 g, 36.1 mmol). The suspension was
allowed to stir at
room temperature overnight and was then quenched with IN HC1 (100mL). After
evaporation of the acetonitrile, the acidic aqueous solution was washed with
Et0Ac
(2x150mL). The pH was adjusted to basic by addition of solid KOH and the
resulting
emulsion was extracted with Et0Ac (3x200 mL). The combined organic layers were
dried
over Na2SO4 and concentrated in vacuo to provide 6.2 g of the desired product:
NMR
(300 MHz, CDC13) 8 7.31 - 7.45 (m, 5H), 5.11 (s, 2H), 4.90 (br. s., 1H), 3.58
(br. s., 1H),
2.72 - 2.97 (m, 1H), 2.14 (d, J =11.90 Hz, 1H), 1.87 -2.02 (m, I H), 1.73 -
1.87 (m, 2H), 1.21
- 1.46 (m, 1H), 0.89 - 1.18 (m, 3H).
Formation of benzyl tert-butyl (1R, 3S)-cyclohexane-1,3-diyidicarbamate
To a solution of of benzyl N-[(1S, 3R)-3-aminocyclohexyl]carbamate, 31, (2.04
g,
8.22 mmol) in T1-IF (20 mL) was added potassium carbonate (3.41 g, 24.64 mmol)
followed
by di-tert-butyldicarbonate (1.97 g, 9.04 mmol). The reaction mixture was
stirred overnight
at room temperature. The solids were filtered and the filtrate was
concentrated in vacuo. The
crude residue was purified by silica gel chromatography (10%-25%
Et0Ac/hexanes) to give
the desired Boc-protected intermediate.
Formation of tert-butyl (OR, 3S)-3-aminocyclohexyl)carbamate (32)
To a solution benzyl tert-butyl (IR, 35)-cyclohexane-1,3-diyldicarbamate
(168.0 g, 0.5 mol) in Me0H (2 L) was added Pd/C 10% (24 g). After flushing
with nitrogen.
The mixture was stirred under 1 bar hydrogen pressure. Conversion had reached
80%
overnight according to NMR. After an additional 48 h the conversion was
complete. The
mixture was filtered through Celite and the filter cake was washed with Me0H.
-90-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
Concentration of the filtrate gave the final product (103 g) that was used
without further
purification.
Synthetic scheme 8: Preparation of Compound 1-3
Fn F
Br F
H2No...
NHBoc a
F \ N 0...NHBoc
F \ =ANH2
Isr
Br Br
32 33 34
N
N4. H Hi
F \ r;j 0141/11 e F 0"")rr
/ [41
F 0
6 I N
Br N N 1-3
Ph' 'Ph
(a) Et3N, THF, Me0H; (b) TFA, CH2C12; (c) 1-methylimidazole-4-carboxylic acid,
HATU,
'Pr2NEt, THF; (d) 5-fluoro-3-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-y1)-1-
tosy1-1H-
PYrrolo[2,3-b]pyridine, 6, X-Phos, Pd2(dba)3, K3PO4, 2-methyl THF, H20, 120
C; (e)
Et3SiH, TFA, CH2C12
Formation of tert-butyl (1R, 3S)-3-aminocyclohexylcarbamate (33)
A solution of tert-butyl N-[( 1 R,3S)-3-aminocyclohexyl]carbamate, 32, (1.0 g,
4.7
mmol), 2-bromo-3,5,6-trifluoro-pyridine (1.2 g, 5.6mmol) and triethylamine
(1.3 mL, 9.3
mmol) in THF (20 mL) and Me0H (5 mL) was heated in a pressure tube at 80 C
for 17
hours. The reaction mixture was diluted into Et0Ac and brine. The organic
phase was dried
over MgSO4, filtered and the solvent was removed under reduced pressure which
resulted in
precipitation of the product. The solid was filtered to yield 1.6g of 33: Ili
NMR (300 MHz,
CDC13) ö 7.08 (dd, J = 9.6, 6.7 Hz, 1H), 4.37 (d, J= 7.2 Hz, 1H), 4.08 ¨ 3.88
(m, 1H), 3.56
(s, 1H), 2.41 (d, J = 12.2 Hz, 1H), 2.05 (dd, J = 30.3, 18.2 Hz, 2H), 1.83
(dd, J = 13.8, 3.3
Hz, 1H), 1.46 (d, J= 3.3 Hz, 12H), 1.15 ¨ 0.88 (m, 2H); LCMS Gradient 10-90%,
0.1%
formic acid, 5min, C18/ACN, Retention Time = 3.78 min, (M+H) 406.57.
Formation of (1S, 3R)-N1-(6-bromo-3,5-difluoropyridin-2-yl)cyclohexane-1,3-
diamine
(34)
To a solution of tert-butyl (IR, 3S)-3-aminocyclohexylcarbamate, 33, (0.93 g,
2.29
mmol) in dichloromethane was added trifluoroacetic acid (3.53 mL, 45.78 mmol).
The
reaction mixture was stirred at room temperature for lhr. The reaction mixture
was diluted
into Et0Ac and brine, and the aqueous phase was adjusted to pH 8. The organic
phase was
separated, dried over MgSO4, filtered and concentrated in vacuo to afford 530
mg of product
that was used without further purification: LCMS Gradient 10-90%, 0.1% formic
acid, 5min,
CI 8/ACN, Retention Time = 1.68 minutes (M+H) 306.28.
Formation of N-((1R, 3S)-3-(6-bromo-3,5-difluoropyridin-2-ylamino)cyclohexyl)-
1-
methyl-1H-imidazole-4-carboxamide (35)
To a suspension of 1-methylimidazole-4-carboxylic acid (0.35 g, 2.77 mmol) and
[dimethylamino(triazolo[4,5-b]pyridin-3-yloxy)methylenel-dimethyl-ammonium
hexafluorophosphate (1.05 g, 2.77 mmol) in THF (10 mL) was added a THF
solution of (IS,
3R)-N1-(6-bromo-3,5-difluoro-2-pyridypcyclohexane-1,3-diamine, 34, (0.53 g,
1.73 mmol)
followed by N,N-diisopropylethylamine (0.97 mL, 5.54 mmol). The reaction
mixture was
stirred at room temperature overnight. The reaction mixture was diluted into
Et0Ac and
brine. The organic phase separated, dried over MgSO4, filtered and
concentrated in vacuo.
-91-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
The crude residue was purified by silica gel chromatography (10% Me0H/CH2C12)
to afford
589 mg of desired product: Ili NMR (300 MHz, CDCI3) 8 7.52 (d, J = 1.3 Hz,
1H), 7.38 (d,
J= 1.1 Hz, 1H), 7.11 ¨ 7.02 (m, 1H), 6.97 (d, J= 8.4 Hz, 1H), 4.42 (d, J= 6.8
Hz, 1H), 4.12
¨ 3.95 (m, 2H), 3.73 (s, 3H), 2.51 ¨ 2.37 (m, 1H), 2.18 ¨ 2.05 (m, 2H), 1.92 ¨
1.78 (m, 1H),
1.65 ¨ 1.37 (m, 2H), 1.24 ¨ 1.00 (m, 3H); LCMS Gradient 10-90%, 0.1% formic
acid, 5min,
C 1 8/ACN, RT = 2.38 minutes (M+H) 414.31.
Formation of N-((1R, 3S)-3-(3,5-difluoro-6-(5-fluoro-1-trity1-1H-pyrazolo[3,4-
b]pyridin-
3-yl)pyridin-2-ylamino)cyclohexyl)-1-methyl-1H-imidazole-4-carboxamide (36)
A solution of 5-fluoro-3-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-y1)-1-
trityl-
pyrazolo[3,4-b]pyridine, 6, (0.505 g, 1.000 mmol) and N-((1R, 3S)-3-(6-bromo-
3,5-
difluoropyridin-2-ylamino)cyclohexyl)-1-methy1-1H-imidazole-4-carboxamide, 35,
(0.290 g,
0.700 mmol) in 2-MeTHF and H20 was degassed under a stream of nitrogen for 40
min. To
the mixture was added K3PO4 (0.637 g, 3.000 mmol), X-Phos (0.057 g, 0.120
mmol) and
Pd2(dba)3 (0.023 g, 0.025 mmol). The reaction mixture was heated in a pressure
tube at 120
C for lh. The aqueous phase was removed and the organic phase was filtered
through celite.
The filtrate was concentrated in vacuo. The resulting crude residue was
purified by silica gel
chromatography (5%Me0H/CH2C12) to afford 402nng of product: NMR (300 MHz,
CDC13) ö 8.43 (dd, J= 8.4, 2.6 Hz, 1H), 8.16 (d, J= 2.0 Hz, 1H), 7.54 (s, I
H), 7.41 (s, 1H),
7.28 (d, J = 2.9 Hz, 15H), 7.11 (dd, J = 12.6, 6.6 Hz, 2H), 4.45 (t, J = 13.0
Hz, 1H), 4.29 ¨
4.07 (m, 2H), 3.77 (d, J= 21.7 Hz, 3H), 2.57 (d, J= 10.9 Hz, 1H), 2.38 (d, J =
12.7 Hz, 1H),
2.18 (d, J= 9.7 Hz, 2H), 1.95 (d, J = 14.0 Hz, 1H), 1.66 (dd, J = 26.6, 13.1
Hz, 1H), 1.34 (dt,
J = 15.1, 7.7 Hz, 2H); LCMS Gradient 10-90%, 0.1% formic acid, 5min, C18/ACN,
Retention Time = 3.87 minutes (M+H) 713.00.
Formation of N-((1R, 3S)-3-(3,5-difluoro-6-(5-fluoro-1H-pyrazolo[3,4-b]pyridin-
3-
y1)pyridin-2-ylamino)cyclohexyl)-1-methyl-1H-imidazole-4-carboxamide (1-3)
To a solution of N-[(1R, 3S)-34[3,5-difluoro-6-(5-fluoro-l-trityl-pyrazolo[3,4-
b]pyridin-3-y1)-2-pyridyl]amino]cyclohexyl]-1-methy 1-i midazole-4-carboxamide
(0.400 g,
0.561 mmol) in dichloromethane was added triethylsilane (0.448 mL, 2.806 mmol)
followed
by trifluoroacetic acid (0.432 mL, 5.612 mmol). The reaction was stirred at
room
temperature for 30 min. The reaction solvent was removed and the resulting
crude residue
was dissolved in 5m1 Me0H and the mixture.was purified by reverse phase
chromatography
(43g Isco C18 column, H20 (0.05%TFA), CH3CN (0.05%TFA) to afford 17 mg of
product:
LCMS Gradient 10-90%, 0.1% formic acid, 5min, C18/ACN, Retention Time = 2.11
minutes
(M+H) 471.29.
=
Synthetic Scheme 9: Preparation of Compound 1-14 and 1-13
-92-
CA 02822059 2013-06-17
WO 2012/083121 PCT/US2011/065388
\
0
0 0
CI 0 0.S H
NI¨
(L N + Or""'--'= HN* b
I -/-----=.<
r
\ /
INL...s.:1õ.õ......___
N 6
CI H2W N
I 1 F
NCI /
N I
µN1 NI"
37 (+0 (Ph)3C
38 (+/-)
\
0 OH OH
0 0 0---=e
:
I\1/<--/-----<
N --/----.K
c \ / N d, e
-- F -- NF / N F
N/ I
--
\Ir
/ '=
N I /
N I
N N '1µ1-"N"-- '1\1-"N
H H H
39 (+/-) 1-14 1-13
(a) 'Pr2NEt, THF, 60 C; (b) 5-fluoro-3-(4,4,5,5-tetramethy1-1,3,2-
dioxaborolan-2-y1)-1-tosy1-
1H-pyrrolo[2,3-b]pyridine, 6, Pd2(dba)3, X-Phos, K3PO4, 2-MeTHF-H20, 125 C;
(c) Et3SiH,
TFA, CH2C12, 0 C; (d) NaOH, THF-Me0H- H20; (e) chiral SFC separation.
Formation of (+/-)-trans-(2,3)-methyl 3-((6-chloropyrazin-2-yl)amino)-
bicyclo[2.2.2Joctane-2-carboxylate (37)
A solution of 2,6-dichloropyrazine (0.339 g, 2.274 mmol) and (+1-)-trans-(2,3)-
methyl 3-aminobicyclo[2.2.2]octane-2-carboxylate, 10, (0.500 g, 2.729 mmol)
and IV,N-
diisopropylethylamine (0.792 mL, 4.548 mmol) in anhydrous acetonitrile was
heated to 70 C
for 16 hr. The reaction was still incomplete as judged by LCMS. Then, the
temperature was
raised to 110 C for an additional 24 hr. The mixture was diluted with Et0Ac,
washed with
half saturated brine (2 x), dried over Na2SO4, filtered and concentrated in
vacuo. Flash
chromatography (Si02, 0-100% Et0Ac-hexanes, gradient elution) provided the
desired
product (217 mg, 32% yield): Ili NMR (400 MHz, CDC13) 8 8.02 (s, 1H), 7.74 (s,
1H), 5.71
(s, 1H), 4.32 (s, 1H), 3.80-3.68 (m, 3H), 2.40 (d, J= 5.6 Hz, 1H), 2.03 (d, J
= 2.5 Hz, 1H),
1.87 (d, J= 2.7 Hz, 1H), 1.76 (d, J = 10.1 Hz, 2H), 1.71 ¨ 1.40(m, 6H).
(+/-)-trans-(2,3)-methyl 3-06-(5-fluoro-1-trity1-1H-pyrazolo[3,4-b]pyridin-3-
y1)pyrazin-
2-y1)amino)bicyclo[2.2.2]oetane-2-earboxylate (38)
To a solution of (+/-)-trans-(2,3)-methyl 34(6-chloropyrazin-2-yDamino)-
bicyclo[2.2.2]octane-2-carboxylate, 37, (0.11 g, 0.36 mmol) and K3PO4 (0.23 g,
1.09 mmol)
in water (0.54 mL) was added 5-fluoro-3-(4,4,5,5-tetramethy1-1,3,2-
dioxaborolan-2-y1)-1-
trity1-1H-pyrazolo[3,4-b]pyridine, 6, (0.22 g, 0.43 mmol) in THF (2.14 mL).
The mixture .
was degassed with a stream of nitrogen for 5 min. Then, X-Phos (0.02 g, 0.04
mmol) and
Pd2(dba)3 (0.01 g, 0.01 mmol) was added to the mixture. The vessel was sealed
and heated to
90 C for 16 hr. Flash chromatography (Si02, 0-35% Et0Ac-hexanes, gradient
elution)
provided 190 mg of the desired product which was sufficiently pure for use in
the next
-93-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
reaction: LCMS Gradient 10-90%, 0.1% formic acid, 5min, C18/ACN, RT = 3.39 min
(M+H) 640.21.
Formation of (+/-)-trans-(2,3)-34(6-(5-fluoro-1H-pyrazo1o13,4-Npyridin-3-
y1)pyrazin-2-
yl)amino)bicyclo[2.2.21octane-2-carboxylic acid (1-14 and 1-13)
To a solution of (+/-)-trans-(2,3)-methyl 34(6-(5-fluoro-1-trity1-1H-
pyrazolo[3,4-
b]pyridin-3-yppyrazin-2-yDamino)bicyclo[2.2.2]octane-2-carboxylate (0.190 g,
0.298 mmol)
in dichloromethane (4.75 mL) was added Et3SiH (0.238 mL, 1.490 mmol), followed
by TFA
(0.229 mL, 2.975 mmol). After the reaction was deemed complete as judged by
TLC, the
mixture was concentrated in vacuo. The crude was taken up in dichloromethane,
washed
with aqueous saturated NaHCO3, dried over Na2SO4, filtered and concentrated in
vacuo to
provide crude racemic-trans-methyl 34(6-(5-fluoro-1H-pyrazolo[3,4-b]pyridin-3-
yppyrazin-
2-yDamino)bicyclo[2.2.2]octane-2-carboxylate, 39. The crude material was taken
directly
into the next reaction without further characterization or purification.
The crude material, 39, was dissolved in THF (3 mL) and Me0H (1 mL) and
treated
with 2N NaOH (0.74 mL, 1.49 mmol) and stirred at room temperature for 24 hr.
The
reaction mixture was diluted with water (3 mL) and concentrated in vacuo to
remove the
volatile organic solvents. The aqueous layer was washed with MTBE, neutralized
to a pH 4-
with 2N HC1 and the resulting solid precipitate was filtered and rinsed with
additional water
and acetonitrile. The wet solid was dried in vacuo to provide the desired
product (65 mg,
55% yield over 2 steps) as an amorphous solid: 1H NMR (300 MHz, Me0D) 8 8.70
(dd, J =
8.5, 2.8 Hz, 1H), 8.51 (dd, J= 2.7, 1.7 Hz, 1H), 8.44 (s, 1H), 7.83 (s, 1H),
4.74 - 4.64 (m,
1H), 2.60 - 2.52 (m, 1H), 2.15 - 2.06 (m, 1H), 2.06 - 1.98 (m, 1H), 1.98 -
1.61 (m, 6H), 1.61 -
1.44 (m, 2H); LCMS Gradient 10-90%, 0.1% formic acid, 5min, C18/ACN, RT = 2.54
minutes (M+H) 383.11.
Separation of the racemic mixture using chiral SFC: 25% Me0H, 75% CO2 (10
mL/min) on
Chiralpak IB (10 x 250) provided the individual enantiomers.
(2R, 3R)-34(6-(5-fluoro-1H-pyrazolo[3,4-blpyridin-3-yl)pyrazin-2-
yDamino)bicyclo-
12.2.2Joctane-2-carboxylic acid (1-13)
Fast eluting enantiomer: 25% Me0H, 75% CO2(10 mL/min,) on Chiralpak 1B (10 x
250), RT
= 7.24 min.
114 NMR (400 MHz, Me0D) 8 8.70 (dd, J= 8.5, 2.6 Hz, 1H), 8.50 (s, 1H), 8.43
(s, 1H), 7.83
(s, 1H), 4.71 (d, J= 6.1 Hz, 1H), 2.55 (d, J= 6.4 Hz, 1H), 2.10 (s, 1H), 2.02
(s, 1H), 1.97 -
1.62 (m, 6H), 1.62 - 1.42 (m, 2H); LCMS Gradient 10-90%, 0.1% formic acid,
5min,
C18/ACN, RT = 2.54 minutes (M+H) 383.14.
(2S, 3S)-34(6-(5-fluoro-1H-pyrazolo[3,4-131pyridin-3-yl)pyrazin-2-yl)a
mino)bicyclo-
12.2.21octa ne-2-carboxylic acid
Slow eluting enantiomer: 25% Me0H, 75% CO2(10 mL/min,) on Chiralpak IB (10 x
250),
RT = 8.39 min.
NMR (400 MHz, Me0D) 8 8.75 - 8.66 (m, 1H), 8.51 (s, 1H), 8.44 (s, 1H), 7.83
(s, 1H),
4.71 (d, J = 6.4 Hz, 1H), 2.55 (d, J = 6.7 Hz, 1H), 2.10 (s, 1H), 2.02 (s,
1H), 1.96 - 1.61 (m,
6H), 1.61 - 1.43 (m, 2H); LCMS Gradient 10-90%, 0.1% formic acid, 5min,
C18/ACN, RT =
2.55 minutes (M+H) 383.14.
Synthetic Scheme 9: Preparation of Compound 1-17
-94-
CA 02822059 2013-06-17
WO 2012/083121 PCT/US2011/065388
CI0
"
HN A a N
NH N( ¨N
CI)N -14 NH 0
) 6
01:1 +/-
I N
ao 41 N N 42
(+/-) )_Ph (+/-)
Ph
Ph
0 0
OH .
¨N rxr-N
I \ N I N
N 43 I-17
(+/-) 1.r N
(+/-)
(a) P0C13, /V,N-dimethylaniline, 90 C; (b) racemic trans-methyl 3-
aminobicyclo[2.2.2]
octane-2-carboxylate, 10, 113r2NEt, THF, 60 C; (c) 5-fluoro-3-(4,4,5,5-
tetramethy1-1,3,2-
dioxaborolan-2-y1)-1-tosy1-1H-pyrrolo[2,3-b]pyridine, 6, Pd2(dba)3, X-Phos,
K3PO4,
MeTHF-H20, 80 C; (d) Et3SiH, TFA, CH2C12; (d) Li0H, THF- H20, 80 C
Formation of 3,5-dichloro[1,2,4]triazine (40)
To 6-azauracil (1.0 g, 8.9 mmol) was added phosphorus oxychloride (10.0 mL,
108.0
mmol) and /V,N-dimethylaniline (2.0 mL, 16.0 mmol). The reaction mixture was
heated in a
microwave reactor at 90 C for 20 minutes. The mixture was extracted with
hexane (200
mL) twice and filtered through Celite and sodium sulfate. The organic solvent
was
evaporated in vacuo to give 530 mg of the title compound which was used
without further
purification.
Preparation of (+1-)-trans-methyl 34(3-chloro-1,2,4-triazin-5-yl)amino)bicyclo-
[2.2.21octane-2-carboxylate (41)
To a solution of 3,5-dichloro[1,2,4]triazine, 40, (0.75 g, 5.00 mmol) in
anhydrous
dioxane (50 mL) was added /V,N-diisopropylethylamine (1.74 mL, 10.00 mmol) and
racemic
trans-methyl 3-aminobicyclo[2.2.2]octane-2-carboxylate, 10, (0.92 g, 5.00
mmol). The
reaction stirred at room temperature for 4 hours. Ethyl acetate (200 mL) was
added. The
organic phase was washed with aqueous saturated ammonium chloride solution,
water and
brine. The organic phase was dried over sodium sulfate, filtered and
concentrated in vacuo.
The crude product was purified by silica gel chromatography (25-75% Ethyl
Acetate in
Hexane) to give 500 mg of the title compound.
Preparation of (+0-trans-methyl 3-43-(5-fluoro-1-trity11-1H-pyrazolo[3,4-
b]pyridin-3-
y1)-1,2,4-triazin-5-yl)amino)bicyclo[2.2.2]octane-2-carboxylate (42)
To a solution of 5-fluoro-3-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-y1)-1-
tosyl- 1 H-
pyrrolo[2,3-b]py ridine, 6, (0.255 g, 0.506 mmol) and racemic trans-methyl 3-
((3-chloro-
1,2,4-triazin-5-yl)amino)bicyclo[2.2.2]octane-2-carboxylate, 41, (0.150 g,
0.506 mmol) in 2-
MeTHF (5 mL) and water (1 mL) was added Pd2(dba)3 (0.032g 0.035 mmol) and X-
Phos
(0.036 g 0.075 mmol). The mixture was degassed under flow of nitrogen for 5
minutes.
K3PO4. (0.375 g, 1.770 mmol) was then added and solution was sealed in vial
and heated to
80 C for 2 hours. The mixture was diluted with Ethyl acetate (20 mL) and
washed with
brine and water. The organic phase was dried over sodium sulfate and
concentrated in vacuo.
The resulting crude residue was purified by silica gel chromatography (0-7%
Me0H [2N]
NH3 in Et0Ac) to give 50 mg of the title compound.
-95-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
Preparation of (+1-)-trans-methyl 34(3-(5-fluoro-1H-pyrazolo[3,4-b]pyridin-3-
y1)-1,2,4-
triazin-5-y1)a mino)bicyclo[2.2.2]octa ne-2-carboxylate (43)
To a solution
of racemic-trans-methyl 34(3-(5-fluoro- 1 -trity1-1H-pyrazolo[3,4-
b]pyridin-3-y1)-1,2,4-triazin-5-yDamino)bicyclo[2.2.2]octane-2-carboxylate,
42, (0.050 g
0.078 mmol) in dichloromethane (10 mL) was added triethylsilane (0.375 mL 2.35
mmol)
and trifluoroacetic acid (0.090 mL 1.170 mmol). The reaction mixture was
stirred at room
temperature for 1 hour. The mixture was concentrated in vacuo and crude was
purified by
reverse phase-HPLC to give 10 mg of the title compound as a TFA salt.
Preparation of (+/-)-trans-34(3-(5-fluoro-1H-pyrazolo[3,4-b]pyridin-3-y1)-
1,2,4-triazin-
5-yl)amino)bicyclo[2.2.21octane-2-carboxylic acid (I-17)
To a solution of racemic trans-methyl 34(3-(5-fluoro-1H-pyrazolo[3,4-b]pyridin-
3-
y1)-1,2,4-triazin-5-yDamino)bicyclo[2.2.2]octane-2-carboxylate, 43, (0.010 g,
0.025 mmol) in
THF (5 mL) was added LiOH (0.50 mL of 2N solution, 1.00 mmol). The reaction
mixture
was heated to 80 C for 2 hours. Ethyl acetate (25 mL) was added and solution
was washed
with brine and water. The organic phase was dried over sodium sulfate,
filtered and
concentrated in vacuo. The resulting crude residue was purified by reverse
phase-HPLC to
give 5 mg of the title compound as a TFA salt: NMR (300
MHz, DMSO-d6) 8 14.87 (s,
1H), 9.62 (s, 1H), 8.76 (s, 1H), 8.53 - 8.34 (m, 2H), 4.79 (s, 2H), 2.62 (d,
J= 6.0 Hz, 1H),
2.07 (s, 1H);1.96 (s, 1H), 1.81 - 1.31 (m, 9H).
Synthetic Scheme 10
o o o o o 0 Bd'NH 0 NI-12 0
a)0Et a ULOBn b OBn c OBn d
47 48 (+4-) 49 (+/-) 50
(a) Benzyl
alcohol, toluene, 4 angstrom molecular sieves, reflux (b) NaH, Mel, DMF (c)
benzylamine, TiC14, CH2C12, then NaCNBH3, Me0H, 0 C (d) H2, Pd-C, Me0H
A general method for the synthesis of trans-2-amino- 1 -alkyl-
cyclohexanecarboxylic acids is
shown in the scheme above.
Compound 47 was prepared following literature procedures described in: Matsuo,
J. etal.
Tetrahedon: Asymmetry 2007, 18, 1906-1910.
Formation of Benzyl 1-methyl-2-oxocyclohexanecarboxylate (48)
This compound was prepared following the literature procedures described in:
(a)
Hayashi, Y.; Shoji, M.; Kishida, S. Tetrahedron Lett. 2005, 46, 681-685.
(Winfield, C. J.;
Al-Mahrizy, Z.; Gravestock, M.; Bugg, T. D. H. J. Chem. Soc., Perkin Trans. 1,
2000, 3277.
Formation of (+/-) Trans-Benzyl 2-(benzylamino)-1-methylcyclohexanecarboxylate
(49)
To a solution of benzyl 1-methyl-2-oxo-cyclohexanecarboxylate, 48, (0.50 g,
2.03
mmol) and benzylamine (0.63 mL, 5.75 mmol) in dichloromethane (10.0 mL) was
added
TiC14 (1.93 mL of 1 M solution, 1.93 mmol) dropwise at room temperature. The
mixture was
stirred for 2 hours. The mixture was cooled to 0 C and a solution of NaBH3CN
(0.21 g, 3.34
mmol) in Me0H was added dropwise over a period of 3 minutes. After 15 min, the
solution
was warmed to room temperature and stirred for an additional 45 min. Then, the
mixture was
diluted with Et0Ac, quenched with 10 mL 1M NaOH. The mixture was partitioned
with
Et20 and the aqueous layer was extracted several times with Et20 (2 x) and
Et0Ac (1 x).
-96-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
The combined organic phases were dried over MgSO4, filtered and concentrated
in vacuo.
Flash chromatography (Si02, 0-50% Et0Ac-Hexanes gradient elution) and
isolation of the
major component provided the desired product (320 mg) as a single racemic
trans isomer:
11-1 NMR (300 MHz, Me0D) 8 7.34 - 7.16 (m, 10H), 5.07 (dd, J= 12.4, 31.2 Hz,
2H), 3.78
(d, J= 13.0 Hz, 1H), 3.57 (d, J= 13.0 Hz, 1H), 2.96 (m, 1H), 1.86 (m, 1H),
1.74 - 1.57 (m,
3H), 1.52 - 1.25 (m, 4H) and 1.20 (s, 3H) ppm.
Formation of (+/-)-Trans-2-Amino-1-methylcyclohexanecarboxylic acid (50)
To a solution of racemic trans-benzyl (1S, 25)-2-(benzylamino)-1-ethyl-
cyclohexanecarboxylate, 49, (0.32 g, 0.91 mmol) in Me0H (12.8 mL), was added
Pd (5% Pd
on carbon, 0.07 g). The solution was degassed and placed under 50 PSI H2
atmosphere (Parr
shaker) overnight. The mixture was filtered through celite and the filtrate
was rinsed with
Me0H. Concentration of the mother liquor followed by acetonitrile azeotrope
(2x) to remove
residual Me0H provided the desired product (162 mg): 1H NMR (300 MHz, Me0D) 8
3.22
(m, 1H), 1.93 (m, 1H), 1.77 (m, 2H), 1.57 - 1.23 (m, 5H) and 1.17 (s, 3H) ppm.
Preparation of Compounds 1-18 and 1-19
F
\ ,
N N N NH
HN-N -0
Formation of N-((1R,3S)-3-((3,5-difluoro-6-(5-fluoro-1H-pyrazolo[3,4-b]pyridin-
3-
yl)pyridin-2-yl)amino)cyclohexyl)thiophene-3-carboxamide (I-19)
The compound was prepared in a similar manner as described above for Compound
1-3.
LCMS Gradient 10-90%, 0.1% formic acid, 5min, C18/ACN, RT = 2.90 min, (M+H)
472.99.
NH
N N
HN-N
CI
Formation of 5-chloro-N-41R,3S)-34(3,5-difluoro-6-(5-fluoro-1H-pyrazolo[3,4-
b] pyridin-3-yl)pyridin-2-yl)amino)cyclohexyl)thiophene-3-ca rboxa mide (I-18)
The compound was prepared in a similar manner as described above for Compound
1-3.
LCMS Gradient 10-90%, 0.1% formic acid, 5min, C18/ACN, RT = 3.25 min, (M+H)
507.19.
-97-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
Preparation of Compound 1-20
\ NC F4D
,
N
H
HN-N
0 OH
Formation of (2S,3S)-34(4-cyano-2-fluoro-5-(5-fluoro-1H-pyrazolo13,4-blpyridin-
3-
yl)phenyl)amino)bicyclo[2.2.2Joctane-2-carboxylic acid (I-20)
The compound is the free acid form of Compound 1-8.
11-1 NMR (400 MHz, DMSO) 8 14.24 (s, 1H), 12.36 (s, 1H), 8.67 (s, 1H), 8.06
(d, J =
8.7 Hz, 1H), 7.72 (d, J = 11.9 Hz, 1H), 6.97 (d, J = 8.2 Hz, 1H), 6.73 (d, J=
5.9 Hz, 1H), 4.05
(d, J = 6.7 Hz, 1H), 2.80 (d, J = 6.9 Hz, 1H), 1.98 (s, 1H), 1.75 (d, J = 18.5
Hz, 3H), 1.50 (dd,
J = 54.4, 32.5 Hz, 6H); LCMS Gradient 10-90%, 0.1% formic acid, 5min, C18/ACN,
RT =
2.99, (M+H) 424.14.
Synthetic scheme 11: Preparation of compound 1-21
0
oo
= H
F CN a :r.N
CI CI CN 6 CN
\04,o NH2 Cl \,N
(+/-) 51 N 52
(+/-) 10 Tr (+0
0
HO
z
(1).--NH F ===='NH
CN F CN
I \ N \,N
N N
H 53 (+/-) N N i+9 1_21
H k
(a) methyl 3-aminobicyclo[2.2.2]octane-2-carboxylate (10), xantphos, Pd(OAc)2,
Cs2CO3 and
dioxane, 120 C; (b) 5-fluoro-3-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-y1)-
1-trity1-1H-
PYrazolo[3,4-Npyridine (6) x-phos, Pd2(dba)3, K3PO4, 2-methyl THF and H20, 130
C; (c)
Et3SiH, TFA, CH2C12; (d) NaOH and THF, 120 C.
-98-
,
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
Formation of (+/-)-trans-methyl 34(5-chloro-4-cyano-2-fluorophenyl)amino)-
bicyclo[2.2.21octane-2-carboxylate (51)
To a solution of methyl racemic-trans-3-aminobicyclo[2.2.2]octane-2-
carboxylate
(0.550 g, 3.000 mmol) and 2,4-dichloro-5-fluoro-benzonitrile (0.570 g, 3.000
mmol) in 1,4-
dioxane (12 mL) was added (5-diphenylphosphany1-9,9-dimethyl-xanthen-4-y1)-
diphenyl-
phosphane (0.087 g, 0.150 mmol), diacetoxypalladium (0.040 g, 0.180 mmol) and
Cs2CO3
(1.955 g, 6.000 mmol). The reaction mixture was heated a t 120 C in a
pressure tube for 1.5
hours. The reaction mixture was filtered through a pad of celite and the
filtrate concentrated
in vacuo. The
resulting residue was purified by silica gel chromatography
(30%Et0Ac/Hexanes) to afford 860mg of the desired product: 114 NMR (400 MHz,
CDC13)
8 7.19 (d, J= 11.0 Hz, 1H), 6.77 (d, J= 7.5 Hz, 1H), 4.58 (d, J= 4.0 Hz, IH),
4.09 (t, J = 6.6
Hz, 1H), 3.75 (d, J = 1.9 Hz, 3H), 2.34 (d, J = 5.8 Hz, 1H), 2.11 (d, J= 2.4
Hz, 1H), 1.85 (d,
J= 2.2 Hz, 1H), 1.78 ¨ 1.62 (m, 5H), 1.60 ¨ 1.41 (m, 4H); LCMS Gradient 10-
90%, 0.1%
formic acid, 5min, C18/ACN, RT = 3.76, (M+H) 337.02.
Formation of (+1-)-trans-methyl 34(4-cyano-2-fluoro-5-(5-fluoro-1-trity1-1H-
pyrazolo[3,4-b]pyridin-3-y1)phenyl)amino)bicyclo[2.2.21octane-2-carboxylate
(52)
A solution of racemic trans-
methyl 3-(5-chloro-4-cyano-2-fluoro-
anilino)bicyclo[2.2.2]octane-2-carboxylate, 51, (0.400 g, 1.188 mmol), 5-
fluoro-3-(4,4,5,5-
tetramethy1-1,3,2-dioxaborolan-2-y1)-1-trity1-1H-pyrazolo[3,4-13]pyridine, 6,
(0.660 g, 1.307
mmol) and K3PO4 (0.757 g, 3.564 mmol) in 2-Methyl THF (25.44 mL) and H20
(3.393 mL)
was degassed under a stream of nitrogen for 40 minutes. To the reaction
mixture was added
x-phos (0.068 g, 0.143 mmol) and Pd2(dba)3 (0.027 g, 0.030 mmol). The reaction
mixture
was heated at 130 C in a pressure tube for 45 minutes. The aqueous phase was
removed and
the organic phase was filtered through a pad of celite and concentrated in
vacuo. The
resulting residue was purified by silica gel chromatography (30%Et0Ac/Hexanes)
to afford
540 mg of the desired product: IH NMR (400 MHz, CDC13) 8 8.22 ¨ 8.14 (m, 1H),
7.79 (dd,
J= 8.1, 2.7 Hz, 1H), 7.38 ¨ 7.25 (m, 16H), 7.00 (d, J= 8.2 Hz, IH), 4.60 (d, J
= 4.6 Hz, IH),
4.15 (t, J = 5.9 Hz, IH), 3.63 (s, 3H), 2.39 (d, J= 5.5 Hz, 1H), 2.09 (d, J=
16.5 Hz, 1H), 1.87
(s, 1H), 1.79 ¨ 1.62 (m, 5H), 1.56 ¨ 1.41 (m, 3H); LCMS Gradient 60-98%, 0.1%
formic
acid, 5min, C18/ACN, RT = 3.58 minutes (M+H) 680.52.
Formation of (+/-)-trans-methyl 3-((4-cyano-2-fluoro-5-(5-fluoro-1H-
pyrazolo[3,4-
13] pyrid in-3-yl)phenyl)a m ino)bicyclo[2.2.21octane-2-ca rboxylate (53)
To a solution
of racemic trans-methyl 344-cyano-2-fluoro-5-(5-fluoro- 1 -trityl-
pyrazolo[3,4-b]pyridin-3-yl)anilino]bicyclo[2.2.2]octane-2-carboxylate, 52,
(0.84 g, 1.24
mmol) in dichloromethane (20 mL) was added triethylsilane (0.99 mL, 6.18 mmol)
followed
by trifluoroacetic acid (0.95 mL, 12.36 mmol). The reaction mixture was
stirred at room
temperature for 10 minutes. The reaction mixture was concentrated in vacuo and
the
resulting residue was purified by silica gel chromatography (40%Et0Ac/Hexanes)
to afford
490 mg of the desired product: 1H NMR (400 MHz, CDCI3) 8 8.56 (s, 1H), 7.95
(dd, J = 8.0,
2.6 Hz, 1H), 7.41 (d, J = 11.2 Hz, 1H), 7.07 (t, J= 18.3 Hz, 1H), 4.23 (s,
1H), 3.72 (d, J= 7.8
Hz, 3H), 2.43 (d, J= 5.3 Hz, 1H), 2.13 (s, 1H), 1.91 (s, 1H), 1.85 ¨ 1.60 (m,
5H), 1.55 (dd, J
= 21.5, 10.9 Hz, 3H); LCMS Gradient 10-90%, 0.1% formic acid, 5min, C18/ACN,
RT =
3.42 minutes (M+H) 438.05.
Formation of (+/-)-trans-3-04-cyano-2-fluoro-5-(5-fluoro-1H-pyrazolo13,4-
b]pyridin-3-
yl)phenyl)amino)bicyclo[2.2.2]octane-2-carboxylic acid (1-21)
To a solution
of racemic trans-methyl 3-[4-cyano-2-fluoro-5-(5-fluoro-I H-
pyrazolo[3,4-b]pyridin-3-ypanilino]bicyclo[2.2.2]octane-2-carboxy late, 53,
(0.24 g, 0.55
-99-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
mmol) in THF (8 mL) was added NaOH (5.49 mL of 1 M solution, 5.49 mmol). The
reaction
mixture was stirred at 120 C in a pressure tube for 2 hours. To the reaction
mixture was
added HCI to pH 6. The product was extracted into Et0Ac and the organic phase
was dried
over MgSO4, filtered and concentrated in vacuo. The resulting residue was
purified by silica
Influenza Antiviral Assay
[00262] Antiviral assays were performed using two cell-based methods:
A 384-well microtiter plate modification of the standard cytopathic effect
(CPE) assay
method was developed, similar to that of Noah, et al. (Antiviral Res. 73:50-
60, 2006).
Briefly, MDCK cells were incubated with test compounds and influenza A virus
(A/PR/8/34),
at a low multiplicity of infection (approximate M01=0.005), for 72 hours at 37
C, and cell
viability was measured using ATP detection (CellTiter Glo, Promega Inc.).
Control wells
containing cells and virus show cell death while wells containing cells,
virus, and active
antiviral compounds show cell survival (cell protection). Different
concentrations of test
compounds were evaluated, in quadruplicate, for example, over a range from
approximately
201.1M to 1 nM. Dose-response curves were prepared using standard 4-parameter
curve
fitting methods, and the concentration of test compound resulting in 50% cell
protection, or
cell survival equivalent to 50% of the uninfected wells, was reported as the
IC50.
A second cell-based antiviral assay was developed that depends on the
multiplication
of virus-specific RNA molecules in the infected cells, with RNA levels being
directly
measured using the branched-chain DNA (bDNA), hybridization method (Wagaman et
al, J.
Virol Meth, 105:105-114, 2002). In this assay, cells are initially infected in
wells of a 96-
well microtiter plate, the virus is allowed to replicate in the infected cells
and spread to
additional rounds of cells, then the cells are lysed and viral RNA content is
measured. This
assay is stopped earlier that the CPE assay, usually after 18-36 hours, while
all the target cells
are still viable. Viral RNA is quantitated by hybridization of well lysates to
specific
oligonucleotide probes fixed to wells of an assay plate, then amplification of
the signal by
hybridization with additional probes linked to a reporter enzyme, according to
the kit
manufacturer's instructions (Quantigene 1.0, Panomics, Inc.). Minus-strand
viral RNA is
measured using probes designed for the consensus type A hemagglutination gene.
Control
wells containing cells and virus were used to define the 100% viral
replication level, and
dose-response curves for antiviral test compounds were analyzed using 4-
parameter curve
-100-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
fitting methods. The concentration of test compound resulting in viral RNA
levels equal to
that of 50% of the control wells were reported as EC50.
Virus and Cell culture methods: Madin-Darby Canine Kidney cells (CCL-34
American Type Culture Collection) were maintained in Dulbecco's Modfied Eagle
Medium
(DMEM) supplemented with 2mM L-glutamine, 1,000U/m1 penicillin, 1,000 ug/ml
streptomycin, 10 rnM HEPES, and 10% fetal bovine medium. For the CPE assay,
the day
before the assay, cells were suspended by trypsinization and 10,000cells per
well were
distributed to wells of a 384 well plate in 50 JAI. On the day of the assay,
adherent cells were
washed with three changes of DMEM containing lug/ml TPCK-treated trypsin,
without fetal
bovine serum. Assays were initiated with the addition of 30 TCID50 of virus
and test
compound, in medium containing 1 ,g/m1TPCK-treated trypsin, in a final volume
of 501.11.
Plates were incubated for 72 hours at 37 C in a humidified, 5% CO2 atmosphere.
Alternatively, cells were grown in DMEM + fetal bovine serum as above, but on
the day of
the assay they were trypsinized, washed 2 times and suspended in serum-free EX-
Cell
MDCK cell medium (SAFC Biosciences, Lenexa, KS) and plated into wells at
20,000 cells
per well. These wells were then used for assay after 5 hours of incubation,
without the need
for washing.
Influenza virus, strain A/PR/8/34 (tissue culture adapted) was obtained from
ATCC
(VR-1469). Low-passage virus stocks were prepared in MDCK cells using standard
methods
(WHO Manual on Animal Influenza Diagnosis and Surveillance, 2002), and TCID50
measurements were performed by testing serial dilutions on MDCK cells in the
384-well
CPE assay format, above, and calculating results using the Karber method.
Mean IC50 values (mean all) for certain specific compounds are summarized in
Tables
1 and 2:
A: IC50 < 3.3 uM;
IC50 > 3.3 M.
Mean EC50 values (mean all) for certain compounds are also summarized in
Tables 1
and 2:
A: EC50 < 3.3 IrM;
EC50 > 3.3 pM.
For example, IC50and EC50 values of Compound 1-8 are 3.57 11.M and 0.007 M,
respectively. IC50and EC50 values of Compound 1-9 are 0.017 M and 0.034 M,
respectively.
1002631 Table 1: 1050, EC50, NMR and LCMS Data of Compounds of Invention.
-101-
CA 02822059 2013-06-17
WO 2012/083121 PCT/US2011/065388
Flu,
bDNA
Comp MDCK
Molecule EC50 RT M+1 NMR
1050
(uM)
(uM)
1H NMR (300 MHz,
CDCI3) 8 8.58 - 8.51 (m,
2H), 7.26 (t, J = 9.8 Hz,
F H
//--11 2H), 7.28 (s, H),
4.20 -
1-1 F
A A 3.45 420.1
4.01 (m, 3H), 2.66 - 2.55
õII 4 "/(/
(m, 2H), 2.28 (d, J = 12.2
Hz, 1H),-2.13 - 1.98 (m,
co3
2H) and 1.66 - 1.19 (m,
7H) ppm
1H NMR (300 MHz,
CDCI3) 8 8.49 - 8.45 (m,
2H), 7.29 (s, H), 7.24 (t, J
F = 9.8 Hz, 1H), 4.14
- 3.98
F H
), (n, 1H), 2.63 - 2.52 (m,
2H 2.26 d J = 13.0 Hz
1-2 A A 2.7 392.4
F N
1H), 2.13 - 2.02 (m, 2H),
2.13 - 1.95 (m, 2H), 1.58
"
(d, J = 12.8 Hz, 2H), 1.34
- 1.30 (m, 2H), 1.22 (dd,
J = 6.9, 10.2 Hz, 2H) and
-0.00 (s, H) ppm
F
// 'NH
1-3 F 0 A A 2.11 471.3
j;
õN
cr.) 1-17,N -CH3
1H NMR (400 MHz,
DMSO-d6) 8 8.60 (d, J =
9.9 Hz, 2H), 7.66 (t, J =
10.3 Hz, 1H), 6.31 (d, J =
1-5o
V-iot A A 3.05 417.9
F \eµri-7( "-cr F
2.28 (d, J = 6.0 Hz, 1H),
,N 03
1.98 (s, 1H), 1.90 (s, 1H),
H se
1.82 - 1.57 (m, 4H), 1.52
(s, 1H), 1.43 (s, 1H), 1.27
(d, J = 11.1 Hz, 2H).
-102-
CA 02822059 2013-06-17
WO 2012/083121 PCT/US2011/065388
1H NMR (400 MHz,
DMSO-d6) 8 8.81 (s, 1H),
8.45 (s, 1H), 7.62 (t, J =
i
10.4 Hz, 1H), 6.22 (d, J =
, ,..,(------.:
6.0 Hz, 1H), 4.70 (s, 1H),
1-6 ' N i '.--..
/).--101 .i.; a -",!';'M .i/L. ..,,,s a A A
3.26 434.4 2.28 (d, J = 6.0 Hz, 1H),
q-0 ,.,-1-.-11
cji 2.00 (s, 1H), 1.91 (s, 2H),
.,......õ . H
õ
1.69 (d, J = 11.9 Hz, 3H),
1.53 (d, J = 5.2 Hz, 1H),
1.43 (s, 1H), 1.27 (d, J =
12.1 Hz, 2H).
1H NMR (400 MHz,
F
DMSO-d6) 8 8.64 (dd, J =
//z-----k
2.6, 8.9 Hz, 1H), 8.56 (s,
V. .,. o
kl -.,, "NH
\\ 1H), 7.37 (dd, J = 8.1,
r,/ - N --, 40.--.0-
.
11.4 Hz, 1H), 7.26 - 7.17
N N .1'1
0
(n, 1H), 6.25 (d, J = 5.4
H
Hz, 1H), 4.76 - 4.73 (m,
F
1H), 2.50 (s, H), 2.28 (d,
1-7A A 3.11
400 J = 5.1 Hz, 1H), 1.98 (d, J
(.-:
I, ).-NH V
= 10.3 Hz, 2H), 1.80 (d, J
F õy2õs"..., //----N
= 11.8 Hz, 1H), 1.72 (d, J
1 Irii </-''
= 11.7 Hz, 1H), 1.67 (s,
N' 'N'
H .----i
2H), 1.57 (d, J = 11.1 Hz,
1H), 1.43 (m, H), 1.31 (d,
J = 12.4 Hz, 1H), 1.24 (d,
J = 9.3 Hz, 1H) and -0.00
(s, H) ppm
1H NMR (400 MHz,
DMSO) 8 8.39 - 8.36 (m,
1H), 8.23 (s, 1H), 7.50 (t,
F
J = 10.5 Hz, 1H), 5.87 (d,
F---_(--1.
J = 5.7 Hz, 1H), 4.70 (d, J
0
1-8 V. / ---Na \\ B A
3.05 418.4 = 6.4 Hz, 1H), 2.50 (s, H),
F .,.....,....õ7-"N ,:.
?,.-Ø 2.18 (d, J = 5.8 Hz, 1H),
1 0
'-=:: 1/ 1 .
1.96 (d, J= 16.0 Hz 2H),
N ----,N -...1
1.86 - 1.57 (m, 4H), 1.56
H
- 1.33 (m, 2H), 1.27 (t, J
= 11.2 Hz, 2H) and -0.00
(s, H) ppm
1H NMR (300 MHz,
Me0D) d 8.70 (dd, J =
0H GI
0,1) 0,--,/
8.5, 2.8 Hz, 1H), 8.51
,
L' õ(..,\ - i----
., c. \
8(dd, J = 2.7, 1.7 Hz, 1H),
.44 (s, 1H), 7.83 (s, 1H),
N -"-- \-/ N ----\\ \------Vµ
1-9 A A 2.54 383.1
4.74 - 4.64 (m, J = 6.8
Hz, 1H), 2.60 - 2.52 (m, J
,F
2
/
= 6.7 Hz, 1H), 2.15 - 2.06 /...,I
N I I
(in, 1H), 2.06 - 1.98 (m,
H H II
1H), 1.98 - 1.61 (m, 6H),
1.61 - 1.44 (m, 2H).
-103-
CA 02822059 2013-06-17
WO 2012/083121 PCT/US2011/065388
1H NMR (400 MHz,
'
Me0D) 5 8.75 (dd, J =
2.7, 8.6 Hz, 1H), 8.46 (S,
F
1H), 7.40 - 7.35 (m, 1H),
/-------'(
\\.,,,L-. . 0
.,. N N \ 7.29 (dd, J = 8.2, 11.0
Hz, 1H), 3.31 (s, H), 2.72
1-10 3 ____F -.-.-, ..., N _
.,:,--Ø,
.L.,'-: 1.. ,iN ,./.' \ A 3.1 400.4
(d, J = 6.8 Hz, 1H), 2.10
N Li µ ----/>
(S, 1H), 1.99 (dd, J = 9.4,
28.4 Hz, 1H), 1.90 - 1.81
(m, 3H), 1.78 - 1.61 (m,
3H), 1.53 - 1.42 (m, 2H)
,
and 0.00 (s, H) ppm
1H NMR (400 MHz,
Me0D) 8 8.74 - 8.66 (m,
1H), 8.46 (d, J = 1.7 Hz,
F
.."------'"-c.
1H), 7.39 - 7.20 (m, 2H),
\\...,õ._ 0
3.32 - 3.31 (m, H), 2.72
0õ
1-11 *.1 1 " ---i----N,
k___.=_. A A 3.1 400.5
(d, J = 6.8 Hz, 1H), 2.10
" (s, 1H), 2.02
(d, J = 5.4
Hz, 1H), 1.86- 1.81 (m,
H), 1.73 - 1.61 (m, 3H),
1.57 - 1.45 (m, 2H) and
0.00 (s, H) ppm
N /"
1H NMR (300 MHz,
DMSO-d6) 8 14.37 (s,
c!,
/-* N -:.
1H), 12.38 (s, 1H), 8.68
_ ,,..j\ --.
iõ ,t IN 651
(s, 1H), 8.49 (dd, J = 8.6,
3.0 Hz, 1H), 7.93 (dd, J =
1-12 H A A 3.05 425.3
18.0, 9.5 Hz, 2H), 4.79
F
(s, 1H),2.91 (d, J = 6.4
Hz, 1H), 2.02 (s, 1H),
(?
;.\
1.88 (s, 1H), 1.70 (d, J =
..---,oti
h II 41.2 Hz,
3H), 1.43 (d, J =
--z.--N.--kL.N.N
0
29.4 Hz, 3H).
H
1H NMR (400 MHz,.
01-1
0 ---,
Me0D) 8 8.70 (dd, J =
H 8.5, 2.6 Hz,
1H), 8.50 (s,
N
1H), 8.43 (s, 1H), 7.83 (s,
N
1-13 4%-"-\<. ..._,"
N A A 2.54 383.1 1H), 4.71
(d, J = 6.1 Hz,
\--K. 1H), 2.55
(d, J = 6.4 Hz,
/1----.. -------------._..-F
1H), 2.10 (s, 1H), 2.02 (s,
N ji
1H), 1.97- 1.62 (m, 6H),
si, -- -
-N...-7--1
.T
" 1.62 - 1.42
(m, 2H).
1H NMR (400 MHz,
0.4
Me0D) 8 8.75 - 8.66 (m,
or ......---
.
- 1
1H), 8.51 (s, 1H), 8.44 (S,
H
1H), 7.83 (s, 1H), 4.71 (d,
1-14 N\1_ N A A 2.55
383.1 J = 6.4 Hz, 1H), 2.55 (d, J
..-
-"------Ci F= 6.7 Hz, 1H), 2.10 (s,
1H), 2.02 (s, 1H), 1.96 -
N µ
'11 ----- N ------)
1.61 (m, 6H), 1.61 - 1.43
11
(m, 2H).
-104-
CA 02822059 2013-06-17
WO 2012/083121 PCT/US2011/065388
1H NMR (300 MHz,
=DMSO-d6) 8 8.75 - 8.63
(m, 1H), 8.49 (dd, J = 8.8,
2.8 Hz, 1H), 7.94 (d, J =
N
NH ,
11.3 Hz, 1H), 7.87 (d, J =
8.0 Hz, 1H), 4.79 (d, J =
1-15
F 1(
A A 3.02 425.1
7.0 Hz, 1H), 3.17 (s, 1H),
2.91 (d, J = 7.2 Hz, 1H),
2.03 (s, 1H), 1.87 (s, 1H),
1.77(s, 2H), 1.62 (d, J =
8.5 Hz, 2H), 1.43 (d, J =
30.8 Hz, 4H).
1H NMR (300 MHz,
DMS0) 6 8.68 (dd, J =
2.6, 1.5 Hz, 1H), 8.49
(dd, J = 8.7, 2.8 Hz, 1H),
N
7.94 (d, J = 11.3 Hz, 1H),
7.86 (d, J = 7.1 Hz, 1H),
O
1-16 A A 3.02 425.1
4.80 (t, J = 7.0 Hz, 1H),
F . -
'NH
N
3.31 (s, 2H), 2.90 (d, J =
6.9 Hz, 1H), 2.03 (s, 1H),
N N
1.87 (s, 1H), 1.75 (d, J =
14.7 Hz, 2H), 1.61 (d, J =
5.5 Hz, 1H), 1.43 (d, J =
30.9 Hz, 4H).
1H NMR (300 MHz,
DMSO-d6) 8 14.87 (s,
N,
1H), 9.62 (s, 1H), 8.76 (s,
1-17 F A A 1.83 384.3
1H), 8.53 - 8.34 (m, 2H),
\ N
4.79 (s, 2H), 2.62 (d, J =
6.0 Hz, 1H), 2.07 (s, 1H),
1.96 (s, 1H), 1.81 - 1.31
(m, 9H).
[00264] Table 2: 1050, EC50, NMR and LCMS Data of Compounds of
Invention.
Flu,
bDNA
MDCK LCMS
Molecule , EC50 M+1 NMR
IC50 RT
(
(uM) uM)
=
-105-
CA 02822059 2013-06-17
WO 2012/083121 PCT/US2011/065388
0 -SI
----\,,
1,--\411 Cl
I i Fl
1-18 1:--N A A 3.25 507.19
H
F "--t=I'
I ,1 ill
N11"1
Fl
=
F,
H
= 8 --\ Ii, rs,
F \\
1-19 FI_N 1 VII\ it 1
- ! 1 , Nr---\,/,,... A A 2.9 472.99 --- ii ,,..4,2\......k
0
I \N
\',--/-'--- /
N = N ,
1.1
=
F HO =
1H NMIR' (300 MHz,
H \.,.-0
DMSO) ? 14.09 (s, 1H),
,,,,.. r
12.27 (s, 1H), 8.65 (dd, J
= 2.7, 1.5 Hz, 1H), 8.54
1-20 F -
--\ N / 1-"` A A 3.08 418.44
(dd, J = 8.8, 2.8 Hz, 1H),
F / '--____/
=7.76 (t, J = 10.4 Hz, 1H),
6.85 (d, J = 7.0 Hz, 1H),
I I \H
4.65 (t, J = 6.7 Hi, 1H),
NN-7-'11/
2.82 (d, J = 6.8 Hz, 1H),
H
2.06 - 1.23 (m, 10H).
-
=
-106-
CA 02822059 2013-06-17
WO 2012/083121 PCT/US2011/065388
1H NMR (400 MHz,
DMSO) ? 14.24 (s, 1H),
12.36 (s, 1H), 8.67 (s,
1H), 8.06 (d, J = 8.7 Hz,
N
1H), 7.72 (d, J = 11.9 Hz,
0
1H), 6.97 (d, J = 8.2 Hz,
1-21 F NH \\
/13/--011 A A 2.99 424.14
1H), 6.73 (d, J = 5.9 Hz,
1H), 4.05 (d, J = 6.7 Hz,
I IN
1H), 2.80 (d, J = 6.9 Hz,
N N
1H), 1.98(s, 1H), 1.75(d,
J = 18.5 Hz, 3H), 1.50
(dd, J = 54.4, 32.5 Hz,
6H).
In Vivo AssaV
[00265] For efficacy studies, Balb/c mice (4-5 weeks of age) were
challenged with
5x103TCID50 in a total volume of 50 1 by intranasal by intranasal
instillation (25 1/nostril)
under general anesthesia (Ketamine/Xylazine). Uninfected controls were
challenged with
= tissue culture media (DMEM, 50 IA total volume). 48 hours post infection
mice began
treatment with Compound 1-8 at 30 mg/kg bid for 10 days. Body weights and
survival is
scored daily for 21 days. In addition, Whole Body Plethysmography is conducted
approximately every third day following challenge (Penh). Total Survival,
Percent Body
Weight Loss on post challenge day 8 and Penh on study day 6/7 are reported.
Table 3. Influneza Therapeutic Mouse Model (Dosing @ 48 hours post infection
with 30
mg/kg BID X 10 days)
Compounds Percent Survival Percent Weight Loss WBP (Penh; Day 6)2
(Day 8)1
1-8 100 20.8 2.03
'Average weight loss for untreated controls on day 8 is 30-32%.
2 Average Penh scores for untreated controls on study day 6 or 7 is 2.2-2.5,
and for uninfected
mice is ¨0.35-0.45.
[00266] All references provided herein are incorporated herein in its
entirety by
reference. As used herein, all abbreviations, symbols and conventions are
consistent with
those used in the contemporary scientific literature. See, e.g., Janet S.
Dodd, ed., The ACS
Style Guide: A Manual for Authors and Editors, 2nd Ed., Washington, D.C.:
American
Chemical Society, 1997.
1002671 It is to be understood that while the invention has been
described in
-107-
CA 02822059 2013-06-17
WO 2012/083121
PCT/US2011/065388
conjunction with the detailed description thereof, the foregoing description
is intended to
illustrate and not limit the scope of the invention, which is defined by the
scope of the
appended claims. Other aspects, advantages, and modifications are within the
scope of the
following claims.
-108-