Note: Descriptions are shown in the official language in which they were submitted.
CA 02822163 2013-07-29
CONTROLLER FOR AN EXTREMITY HYPERBARIC DEVICE
BACKGROUND
[0002] Hyperbaric chambers are devices which create sealed
environments for the application of therapeutic gases to
hasten healing of lesions or wounds on a patient's body. See
U.S. Patent No. 5,060,644. The introduction of pressurized
oxygen into such an encapsulated environment promotes healing
of various types of lesions and wounds. In particular, the
treatment of lesions and wounds with hyperbaric chambers, in
conjunction with various stimuli, promotes granulation, raises
the capillary blood oxygen pressure and increases expression
of angiogenesis related growth function VEGF, HE EGI and KGF,
thereby stimulating leukocyte function necessary to suppress
bacterial proliferation. The introduction of humidity into
hyperbaric chambers can also produce positive results.
[0003] When hyperbaric chambers were first introduced for
healing lesions and wounds, they encompassed the entire body.
As time progressed, hyperbaric chambers became more
sophisticated and included multiple functions, and topical
hyperbaric chambers also were developed, such as described in
C.s. Paten'- No. 5,060,644.
[0004] There still exists a need, however, for a hyperbaric
wound treatment apparatus and method for treating a variety of
wounds or lesions on a patient's body with high efficacy and a
short treatment time.
1
CA 02822163 2013-07-29
WO 2008/150466 PCT/US2008/006883
SUMMARY OF INVENTION
0005] In accordance with one aspect of the invention, a
hyperbaric wound treatment device includes a chamber having an
interior and an open end in communication therewith, and an
inflatable limb sleeve coupled to the chamber and which can be
positioned at least partially within the interior of the
chamber adjacent the open end of the chamber.
[0006] In one embodiment of the invention, a method of
operating a hyperbaric wound treatment device includes
inserting a limb through an inflatable limb sleeve and into an
open end of a chamber of the device, where the sleeve is
coupled to the chamber and can be positioned at least
partially within the chamber at the open end.. The method
further includes inflating the sleeve to an inflated condition
when the limb is positioned within the sleeve, thereby sealing
the sleeve about the limb.
[0007] In another embodiment, a hyperbaric wound treatment
device includes a chamber having an open end, and a means
coupled to the chamber for receiving a limb of a patient
therethrough. The means is inflatable from a first condition
whereby the means is capable of receiving the limb to a second
condition whereby the means forms at least a partial seal
about the limb. When in the second condition, the means can
be positioned at least partially within the chamber adjacent
the open end of the chamber.
[0008] In a further embodiment, a hyperbaric wound
treatment device for treatment of a limb of a patient includes
a flexible chamber defining an interior adapted to receive a
portion of a patient's limb to be treated therein, where the
chamber has an open end in communication with the interior of
the chamber. The device further includes an inflatable sleeve
coupled to the chamber adjacent the open end, and the sleeve
includes an outer wall spaced from an inner wall defining an
2
CA 02822163 2013-07-29
WO 2008/150466 PCT/US2008/006883
interior region therebetween. At least
a portion of the
sleeve is extendable into the interior of the chamber adjacent
the open end thereof. The
sleeve is inflatable between a
first condition whereby the patient's limb can be inserted
through the sleeve into a portion of the interior of the
chamber, and a second condition whereby the sleeve forms at
least a partial seal about the patient's limb while received
within the chamber.
[0009] In
another aspect of the invention, a controller
for controlling a hyperbaric wound treatment device includes a
gas conveyance assembly operable for creating a negative
pressure, and a control device which is coupled to the gas
conveyance assembly. The
control device is operable to
control the gas conveyance assembly for providing that a
portion of the gas conveyance assembly is in fluid
communication with the treatment device; and for creating a
negative pressure within the treatment device by evacuating
gas, such as ambient air, at least partially from within the
treatment device.
[0010] In one
embodiment, a hyperbaric wound treatment
control apparatus includes a means for creating a negative
pressure within at least a portion of a hyperbaric wound
treatment device. The apparatus further includes a controller
coupled to and operable for controlling the means for
providing that the means is in fluid communication with the
portion of the device; and for creating a negative pressure
within the portion of the device by evacuating gas at least
partially from within the portion of the device.
[0011] In
another embodiment, a method of conveying gas to
and from a hyperbaric wound treatment device includes creating
a negative pressure in a treatment chamber of the device after
inserting a limb through an open end of the treatment chamber
and sealing the chamber at the open end. The method further
3
=
CA 02822163 2013-07-29
WO 2008/150466 PCT/US2008/006883
includes evacuating gas at least partially from within the
treatment chamber, and supplying a treatment gas to the
treatment chamber following the evacuation of the gas from the
treatment chamber.
[0012] In still another embodiment, a method of
controlling flow of gas to and from a collapsible hyperbaric
wound device includes inflating an inflatable rib of the
device, which is for retaining the device in a rigid state,
with a gas at least partially, before inserting a limb into a
treatment chamber of the device having an open end. The
method further includes inflating an inflatable sleeve of the
device, which is for receiving the limb of a patient, to an
inflated condition for at least partially sealing against the
limb at the open end of the chamber. In addition, the method
includes evacuating gas at least partially from at least one
of the chamber and rib, and supplying a treatment gas to the
chamber after the evacuating.
[0013] In still
a further embodiment, a controller for
controlling gas flow to and from a collapsible hyperbaric
wound treatment device includes first, second and third
control valves adapted for coupling to an inflatable sleeve, a
treatment chamber and an inflatable rib, respectively, of the
device. Within
the device, the inflatable rib is for
retaining the device in a rigid state, the treatment chamber
includes an open end for receiving a limb of a patient and the
inflatable sleeve is for sealing against the limb at the open
end of the chamber when the sleeve is an inflated condition.
The controller further includes a processor for selectively
controlling the control valves and for selectively providing
that at least one of the first, second and third control
valves is in fluid communication with a gas source or a pump.
The processor is operable for controlling the third valve for
inflating the rib with a gas at least partially, before the
4
CA 02822163 2013-07-29
WO 2008/150466 PCT/US2008/006883
limb is received in the chamber; for controlling the first
valve for inflating the sleeve for at least partially sealing
the sleeve against the limb at the open end of the chamber;
for controlling the second valve for evacuating gas at least
partially from the chamber based on operation of the pump and
after the inflating of the sleeve for sealing the sleeve
against the limb; and for controlling the second valve for
supplying a treatment gas to the chamber from the gas source.
[0014] In
another aspect of the invention, a hyperbaric
wound treatment apparatus includes a chamber having an
interior and an open end in communication therewith, and an
inflatable limb sleeve configured for receiving a limb and
coupled to the chamber. The sleeve can be positioned at least
partially within the interior of the chamber adjacent the open
end. The
apparatus further includes a gas conveyance
assembly, which is coupled to the sleeve and the interior of
the chamber and is for creating a negative pressure, and a
control device coupled to the gas conveyance assembly. The
control device is operable for controlling the gas assembly
for inflating the sleeve to an inflated condition for at least
partially sealing against the limb adjacent the open end of
the chamber, with the sleeve at least partially within the
interior of the chamber at the open end; and for creating a
negative pressure with the interior of the chamber by
evacuating gas from the interior of the chamber, after the
inflating of the sleeve.
[0015] In a
further embodiment, a method of providing a
treatment gas to a hyperbaric wound treatment device includes,
after inserting a limb through an inflatable sleeve and into
an interior of a chamber of the device, where the chamber has
an open end in communication with the interior and the
inflatable sleeve is coupled to the chamber and can be
positioned at least partially within the interior of the
CA 02822163 2013-07-29
WO 2008/150466 PCT/1JS2008/006883
chamber adjacent the open end, inflating the limb sleeve to an
inflated condition for creating at least a partial seal
between the limb and the sleeve adjacent the open end, with
the sleeve at least partially within the interior of the
chamber. The method also includes, after creating the seal,
evacuating at least partially gas from within the interior of
the chamber, and
supplying a treatment gas to the interior
of the chamber, following the evacuation of the gas from the
interior of the chamber.
[0016]
Additional features and advantages of the invention
will become apparent to those skilled in the art upon
consideration of the following detailed description of the
disclosed embodiments.
BRIEF DESCRIPTION OF THE DRAWINGS
[0017] The
various objects, advantages and features of
this invention will be more fully apparent from a reading of
the following detailed description in conjunction with the
accompanying drawings in which like reference numerals refer
to like parts, and in which:
[0018] FIG. I
is a perspective view of an exemplary
controller in accordance with one aspect of the present
invention.
[0019] FIG. 2
is a view of the interior of the controller
of FIG. 1.
[0020] FIG. 3
is a partial schematic, block diagram of
exemplary control circuits under control of a control module
of a controller in accordance with an embodiment of the
present invention.
[0021] FIG. 4
is a flow chart of a process in accordance
with an aspect of the present invention.
[0022] FIG. 5
is a partial schematic, block diagram of a
controller coupled to a hyperbaric wound treatment device, in
accordance with an aspect of the present invention.
6
CA 02822163 2013-07-29
WO 20081150466 PCT/US2008/006883
[0023] FIG. 6
is a schematic illustration of an embodiment
of a hyperbaric wound treatment device.
(0024] FIG. 7
is an illustration of an embodiment of a
portion of the hyperbaric wound treatment device of FIG. 6.
[0025] FIG. 8
is a schematic illustration of another
embodiment of a hyperbaric wound treatment device.
[0026] FIG. 9
is a schematic illustration of an embodiment
of a seal for a hyperbaric wound treatment device.
[0027] FIG. 10
is a schematic illustration of a topical
hyperbaric wound treatment device for use with a controller in
accordance with the present invention.
DETAILED DESCRIPTION
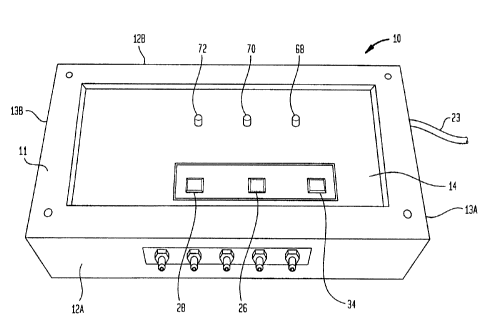
[0028] FIG. 1
is a perspective view of a hyperbaric
controller 10 adapted to control the operations of a flexible
hyperbaric wound treatment device, in accordance with an
aspect of the present invention. The
controller 10 is
desirably portable and can be easily picked up and moved to
different operating locations by an operator. A housing 11
for the controller 10 includes side panels 12A, 12B, end
panels 13A, 133, a bottom panel (not shown) and a top panel
14. Although the embodiment of the invention discussed below
and illustrated in the drawings is a flexible type wound
treatment chamber, it is to be understood that the controller,
in accordance with the present invention, also be may be
utilized with a rigid type of wound treatment chamber, such as
described in U.S. Patent No. 5,060,644.
[0029]
Referring to FIG. 1, the top panel 14 has
indicators or pilot lights 68, 70, 72, which indicate
operating modes or cycles such as "fill ribs," "fill cuff,"
and "hyperbaric therapy," respectively, and switches 26, 28,
34 which control the operation of the controller 10 and are
labeled "cuff fill," "stop" and "start," respectively. Thus,
the controller 10 includes switches that are easy to
7
CA 02822163 2013-07-29
WO 2008/150466 PCT/US2008/006883
understand and easy to use, and indicators that alert the user
as to the particular operation being performed. In an
alternative embodiment, in addition to the pilot lights, the
indicators of the controller 10 may be in any other form, such
as audio, visual or both.
[0030]
Referring also to FIG. 2, air solenoid control
valves 36, 38, 40 and pressure relief valves 42, 43, 44 are
mounted to the rear side panel 12B of the housing 11. A
vacuum pump 41 is mounted to the end panel 13A and a power
supply 58, including a power switch 74 and a 120 VAC power
supply cord 23, is mounted to the end panel 133. The air
solenoid control valves can be Clippard MME-3PDS (Clippard
Instrument Laboratory, Cincinnati, Ohio), the pressure relief
valves can be an Airtrol RV-5300-10-W/K (Airtrol Components
Inc. New Berlin, WI) and the vacuum pump can be a Medo
VP0125-V1005-D2-0511 (Medo USA, Hanover Park, IL). In
addition to the air solenoid control valves, the controller 10
may incorporate any type of valve or the like to perform its
operations, as known in the art. Further, in addition to the
pressure relief valves, other types of valves may be utilized.
In addition to the solenoids mentioned herein, proportional
solenoids may also be utilized.
[0031] Still
referring to FIG. 2 and also referring to
FIGs. 3 and 5, treatment gas, such as oxygen, from a supply
source (not shown) is admitted into the controller 10 via a
port 45b, which is mounted to the side panel 12A, and routed
to the supply ports of the respective control valves 36, 38,
40. Input
and output ports 45c, 45d, and input and output
port assembly 45e, are mounted to the side panel 12A and
connected to the control valves 38, 36 and 40, respectively.
The assembly 45e includes a conventional pressure sensor 145e,
which is electrically connected to a microprocessor 60 of the
controller 10. Pressure
relief valves 42, 43, 44 are
8
CA 02822163 2013-07-29
W02008/150466 PCT/US2008/006883
connected to the flow paths, respectively, between the control
valves 36, 38 and 40 and the ports 45d, 45c and the port
assembly 45e. The vacuum pump 41 is connected to the exhaust
ports of the air control valves 38, 40. The exhaust ports of
the relief valves 42, 43, 44, the vacuum pump 41 and the cuff
control valve 36 are routed to a vent port 45a mounted to the
side panel 12A. Tubing to interconnect the ports and port
assemblies, the control valves, the relief valves and the pump
41 can be conventional diameter
tubing. Tubing for the
exhaust lines connected to the vent port 45a can be
conventional 1/8" diameter tubing. Any
device or
configuration may be utilized to control the flow of gas,
including air and treatment gas, being introduced into or
evacuated from a chamber, a cuff or a rib of a wound treatment
device.
[0032]
Referring to FIGs. 2 and 3, the power cord 23 is
fed to a switch 74 and to the power supply module 58 to
generate +12V and +5V for powering electronic control circuits
90 in the controller 10. Control module 22 is attached to the
top panel 14 and includes a programmable microprocessor 60
which is coupled to the control circuits 90 of the controller
10. As discussed in detail below, the microprocessor 60 of
the control module 22 is operable to control the control
circuits 90, which are coupled to the valves 36, 38, 40, to
provide that gas may be conveyed to and from a hyperbaric
wound device, through the valves 36, 38, 40. In one
embodiment, the control module 22 can include a portion or all
of the electronic circuitry of the control circuits 90 that
connects to the control valves of the controller 10, and the
operation of the microprocessor, the valves and the control
circuits in combination can provide for conveyance of gas to
and from a hyperbaric wound device. Any type
of power
9
CA 02822163 2013-07-29
WO 2008/150466 PCPUS2008/006883
configuration or power source may be utilized. For instance,
the power source may be a battery.
[0033] Still
referring to FIG. 3 and further referring to
FIG. 5, the control circuits 90 are used to control the
operation of each of the control valves 36, 38, 40 which
provide for flow of gas, such as oxygen, to a flexible
hyperbaric device 100. The 120 VAC power is applied by the
main power switch 74 to power the supply module 58, which
provides +12 V and +5 V for operating the control circuits 90.
The control module 22 operates the functions of the controller
10, such as time, sequence, pressure sequence and intermittent
compression.
Intermittent compression is determined and
regulated by the control module 22, optionally based on the
pressure detected in a chamber 114 of the device 100 by the
pressure sensor 145e, in accordance to techniques well known
in the art.
[0034] The
programmable microprocessor 60 provides for
software program control of the controller 10. Although
described in greater detail below, the microprocessor 60
generally includes instructions for operating the hyperbaric
device 100, including a cuff 112, a rib 110 and the chamber
114, when in use. In one
embodiment, the microprocessor 60
includes instructions on cycling, and cycles the gas in the
chamber 114 of the device 100, desirably based on a signal
received at B7 representative of the pressure in the chamber
114 as detected by the pressure sensor 145e. The
microprocessor 60 receives a start signal from the switch 34,
which is activated when the operator starts to prepare a
patient for hyperbaric therapy. The
microprocessor 60
provides output signals at ports A2, Bl, B2, B3 to control the
base of each transistor 621, 622, 622, 624, respectively. In an
alternative embodiment, cycling may be done according to
information input by the operator. The
operator sets and
CA 02822163 2013-07-29
adjusts the time for the therapy as desired. For example, for
deep vein thrombosis ("DVT"), no cycling is performed.
[0035] The
output current from the output signal ports A2,
El, B2, B3 is current limited with resistors 611, 61,, 613, 614,
respectively. The
value of each of the resistors 614, 612,
613, 614 is desirably 1K ohms. Each control relay 52, 54, 56,
57 has a flyback diode 66 to suppress voltage transients which
could otherwise damage the microprocessor 60. In addition to
controlling the relays 52, 54, 56, 57, the microprocessor 60
also controls the three pilot lights "fill ribs" 68, "fill
cuff" 70 and "hyperbaric therapy" 72. Each
of the pilot
lights 68, 70, 72 indicates to an operator of the controller
an operational cycle in which the controller 10 is actually
operating. The pilot lights 68, 70, 72 are switched by
transistors 623, 62,q, 62-, respectively, in response to an "ON"
and "OFF" signal
from the microprocessor 60. The
microprocessor 60 may be Model PIT 16F84A, A bit
microcontroller, with 1K bytes of internal ROM memory storage,
manufactured by Microchip Technology, Inc. of Chandler,
Arizona. The switching transistors 621 -62, are commonly
available 21\13904. The
control relays may be Model
G2R-1S-ASI-DC12 manufactured by Omron Electronics 110 of
Schaumburg, Illinois. The
microprocessor 60 may be any type
of computer, processor or an electronic component capable of
performing instructions stored within it.
[0036] FIG. 6
shows a topical hyperbaric wound treatment
device 100, as disclosed in U.S. Patent Pub. No. 2006/0185670,
having a main chamber 114 including an interior 214 and an
exterior 216. The chamber 114 is closed at one end 218 and
open at the other end 220, and sized and shaped to define the
interior 214 for receiving a patient's limb, for example, a
leg 222.
11
CA 02822163 2013-07-29
W02008/150466 PCT/US2008/006883
Adjacent the other end 220 is an inflatable cuff seal 112 for
sealing against the limb 222.
[0037] Referring also to FIG. 7, the chamber 114 is
defined by a collapsible bag including an outer sheet 224 and
an inner sheet 226 defining a space 225 between the two sheets
224 and 226. The device 100 includes fluid ports 120, 121 and
122 which are in communication with the interior of the cuff
112, the space 225 and the interior 214 of the chamber 114,
respectively, and through which gas can be conveyed based on
operation of the controller 10. The ports 45c, 45d and port
assembly 45e of the controller 10 are connected in fluid
communication with the ports 121, 120, 122, respectively, of
the device 100.
[0038] In one embodiment, gas can be delivered by the
controller 10 to the space 225 between the sheets 224, 226 to
inflate the device 100 to a rigid state and maintain the
device 100 in the rigid state when gas pressure in the
interior of the chamber 114 is -cycled between about ambient
pressure and above ambient pressure. In another embodiment,
treatment gas inside the chamber 114 may be cycled by the
controller 10 between at least about atmospheric or ambient
pressure to a pressure of about up to 50 mm of mercury above
atmospheric or ambient pressure.
[0039] Referring to FIG. 6, the device 100 may further
include a plurality of interconnected pockets 230 or miniature
chambers formed between the sheets 224, 226. The pockets can
be formed by securing portions of the sheets of material
together at selected, discrete locations. The sheets can be
secured together at selected portions by any suitable means,
such as by adhesively sealing the sheets together, heat
sealing or ultrasonically welding the sheets together at
selected, discrete points in an array resembling a waffle
pattern. The present invention is not limited to a particular
12
CA 02822163 2013-07-29
WO 2008/150466 PCT/US2008/006883
pattern for forming the interconnected pockets 230, and other
patterns can be utilized.
[0040] FIG. 8 shows an additional embodiment of the
present invention. Rather than utilize interconnected pockets
230, a hyperbaric treatment device 210 can have inflatable
ribs 110 that extend at least partially along the sides of the
device 210. In this embodiment, the two sheets 224 and 226
may be affixed together in a linear fashion creating long
passages or inflatable ribs 110 between the two sheets 224 and
226. The ribs 110 can encircle the chamber 114 entirely or
partially, and there may be any number of such ribs 110. The
ribs 110 may be formed in any of the manners listed
previously.
[0041] FIG. 4 is a flow chart 75 including operations that
the microprocessor 60 may perform to control the operation of
the controller 10. For purposes of illustrating the features
of the invention, the operation of the controller 10 is
described in connection with the hyperbaric device 100.
Referring to FIG. 4, and also to FIGs. 1, 5 and 6, at block 76
an operator initiates a hyperbaric bag preparation cycle by
turning on the main power switch 74, which initializes the
microprocessor 60 and associated electronic control circuits
90. The operator presses the "start" switch 34, which sends a
signal to the microprocessor 60. A signal is provided at the
port 53 to turn-on the rib-fill valve 38, and also at the port
A3 to turn-on the "fill ribs" pilot light 68. Oxygen, another
gas, such as nitrogen, or ambient air, is supplied at the port
45c and inflates the ribs 110, with any excess oxygen flowing
through the rib pressure relief valve 43 to the vent port 45a.
In one embodiment, the valve 38 may control gas flow to and
from the interconnected pockets 230 of the device 100
[0042] Next at block 78, the microprocessor 60 starts a
timer for five minutes and checks to determine if the "stop"
13
CA 02822163 2013-07-29
W02008/150466 PCT/1JS2008/006883
switch 28 is activated, which indicates that an operator
wishes to arrest the preparation procedure. In the case where
the "stop" switch 28 has been pressed, the microprocessor 60
commands the air solenoid control valves 36, 38 and 40 to the
rest state, turns off the vacuum pump 41, if it is running,
and extinguishes the pilot lights 68, 70 and 72, if they are
illuminated. After
the five minute timer has expired, in
block 80 the microprocessor 60 commands the ports B3 and A3 to
turn off the rib-fill air solenoid control valve 38 and
extinguish the "fill ribs" pilot light 68, respectively.
(0043] With the
ribs 110 now fully inflated, the patient's
limb is placed in the chamber 114 at block 83 and the "cuff
fill" switch 26 is activated. When the signal from the cuff
fill switch is received by the microprocessor 60, the
microprocessor 60 provides a signal at the port A2 to turn on
the cuff-fill valve 36 and also provides a signal at the port
Al to turn on the "Fill Cuff" pilot light 70. It is to
be
understood that the cuff 112 may be inflated using air from
the surrounding atmosphere, or other gas, such as nitrogen and
the like.
(0044] Next, at
block 85, the microprocessor 60 starts a
timer for two minutes and checks to determine if the "stop"
switch 28 is activated, which indicates that an operator
wishes to arrest the preparation procedure. After the two
minute timer has expired, the microprocessor 60 leaves both
ports Al and A2 switched on, which maintains oxygen flowing
through the cuff-fill air solenoid control valve 36 and the
valve 45d, and keeps the "Fill cuff" pilot light 70
illuminated. Excess oxygen flowing to the cuff 112 is vented
by the pressure relief valve 42 and exits the controller 10
through the vent port 45a. With the
cuff 112 now fully
inflated, the flexible hyperbaric bag 100 is now sealed to the
patient's limb.
14
CA 02822163 2013-07-29
WO 2008/150466 PCT/US2008/006883
[0045] Next, at block 86, the vacuum pump 41 is utilized
to remove existing ambient air from the chamber 114. In some
instances, the inflated ribs 110 can withstand this ambient
air evacuation and stay rigid.
[0046] However, in other instances, depending on the size
of the vacuum pump chosen, it may be advantageous to
simultaneously evacuate the gas in both the chamber 114 as
well as the ribs 110. This simultaneous evacuation can occur,
because the evacuation of the chamber 114 places pressure on
the chamber 114 walls and pulls them inwardly. Although the
ribs 110 can remain inflated while the chamber 114 is
evacuated, it has been found that this can place undue stress
on the ribs 110. This stress results from the ribs 110 trying
to stay rigid while the evacuation of the chamber 114 pulls
the ribs 110 inwardly toward the wound. Therefore, to remove
this undue stress on the ribs 110, evacuating the ribs 110 for
a short period allows the ribs 110 to be pulled inwardly
without the gas in the ribs 110 trying to counteract the
pressure on the walls as the chamber 114 evacuation occurs.
The ribs 110 and the chamber 114 can be evacuated in a manner
such that the walls of the chamber 114 will not contact the
wound. Thus, at block 86, the microprocessor 60 port BI is
commanded to +5 V, which in turn saturates the junction of
transistor 624 which engages the relay 57 causing the vacuum
pump 41 to start removing gas from the ribs 110 and also gas
from the therapy chamber 114. The gas may be evacuated up to
about 95% of the gas initially within the chamber 114 prior to
the commencement of the evacuation, such that about 5% of the
gas, such as ambient air, initially within the chamber 114
remains within the chamber 114 following evacuation.
[0047] Alternatively, additional gas may be supplied to
the ribs 110 to overcome the pressures within the chamber 114
during evacuation.
CA 02822163 2013-07-29
WO 2008/150466 PCT/US2008/006883
[0048] Next, at
block 87, the microprocessor 60 starts a
timer for five minutes and checks to determine if the "stop"
switch 28 is activated, which indicates that an operator
wishes to arrest the preparation procedure. At block
88,
after the five-minute timer has expired, the microprocessor 60
commands the port Bl to turn off the vacuum pump 41. Now that
the ribs 110 have been deflated and the chamber 114 has been
evacuated, at block 89, the microprocessor 60 commands the
port 32 to activate therapy chamber air solenoid control valve
40, and the port B3 to activate the valve 38. Treatment gas,
such as oxygen, flows from the port 45b, through the valve 40
and the port assembly 45e and into the therapy chamber 114.
In one embodiment, the ribs 110 are simultaneously inflated
with air or gas when the treatment gas is supplied to the
chamber 114. However, in the event that the ribs 110 had not
been deflated, in block 89, only the chamber 114 is filled
with oxygen and the treatment begins.
[0049] Next, at
block 90, the microprocessor GO starts a
timer for five minutes and checks to determine if the "stop"
switch 28 is activated. If during this block or at any time
during the hyperbaric therapy session, the pressure in therapy
chamber 114 exceeds 50 mm Hg above one atmosphere of pressure
("ATA") or 810 mm Hg, oxygen is vented by the pressure relief
valve 44 and exits the control box 10 through the vent port
45a.
[0050] At block
91, after the five minute timer has
expired, the microprocessor 60 commands the ports B2 and Al to
turn off the air solenoid control valve 40 and extinguish the
"Fill Cuff" pilot light 70, respectively. The microprocessor
60 also commands port 36 on to illuminate "Hyperbaric Therapy"
pilot light 72. Then the
microprocessor 60 continues to
determine if the "stop" button 28 has been pressed in block
92. Finally, at block 93, if the "stop" button 28 has been
16
CA 02822163 2013-07-29
depressed because of either an emergency situation or the
hyperbaric therapy treatment is completed, the microprocessor
60 commands ports A2 and B6 to shut off the oxygen flow to the
cuff 112 and extinguish "hyperbaric therapy" pilot light 72.
The oxygen in the cuff 112 now vents to the atmosphere via the
exhaust port of air solenoid control valve 36, leaving
controller 10 through the vent port 45a. Although timers can
be used throughout, in an embodiment of the present invention,
a timer may not be required or utilized for some or all of the
blocks described herein. In the event a timer is not
incorporated, depressing the stop button may simply halt the
process currently underway. In another embodiment, instead of
timers, event driven sensors, such as pressure sensors, or the
like may be used.
[0051] The
objects and illustrative embodiments of the
hyperbaric therapy are fully disclosed in U.S. Pat. Pub.
No. 20061018567A1 entitled "Hyperbe.ric oxygen devices and
delivery methods".
[0052] In one
embodiment, the controller 10 may also have
a built-in safety feature should the pressure in the chamber
114 during treatment exceed its preset pressure, for example,
a maximum pressure of 100 mm Hg above ATA, or 860 mm Hg. In
such embodiment, referring to FIGs. 3 and 3, the controller 10
includes a port B8 connected through a IK resistor 613 to a
base of a transistor 52, and a control relay 59 with a flyback
diode 66 couples the collector of the transistor 62. to a dump
(exhaust) air solenoid control valve 39. The controller 10
commands the port B8 to open the valve 39, which is in fluid
communication with the port assembly 45e, when the "Stop"
switch 78 is activated, to cause the chamber 114 to
automatically decompress to 3 mm Hcf and avoid the risk of a
tourniquet effect. The tourniquet effect may be caused by the
17
!
CA 02822163 2013-07-29
WO 2008/150-166 PCT/US2008/006883
therapy pressure being set above capillary closure in the
human body (16-33 mm Hg), or if a malfunction occurs, such
that the pressure sensor does not operate correctly or the
chamber 114 stays at a constant pressure above about 22 mm Hg.
This safety feature of the controller 10 offers benefits to
patients who suffer from chronic wounds, have very fragile
vascular systems in their lower extremities and are at high
risk of capillary closure. In an alternative embodiment, the
controller 10 commands the port B8 to open the valve 39, based
on a signal provided by the pressure sensor 145e, which is
representative of the pressure within the chamber 114.
[0053] In another aspect of the present invention as shown
in FIG. 9, the cuff 112 of the hyperbaric treatment device 100
can be positioned partially or wholly inside the chamber 114
when in an inflated condition. Referring to FIG. 9, the cuff
112 includes a tubular inflatable sleeve 240 that can provide
a hermetic seal against the limb when the limb is inserted
through the sleeve 240 and into the chamber 114. The sleeve
has a length L as well as an inside diameter Id and an outside
diameter Od. The sleeve 240 further includes an inside wall
242, an outside wall 244 and a side wall 246 that connects the
inside and outside walls, 242, 244. The inside diameter Id is
formed from the inside wall 242, and the outside diameter Od
is formed from the outside wall 244.
[0054] The sleeve 240 is inflated using an air valve 248
disposed on the sleeve 240. In one embodiment, the valve 248
is coupled in fluid communication with the valve 45d of the
controller 10. Air or any suitable gas is introduced between
the inside and outside walls, 242, 244 to inflate the sleeve
240. Prior to inflation, the inside diameter Id is X. Upon
inflation, the inside diameter is less than X. This ensures
that the sleeve 240 diameter, prior to inflation, is large
enough to accommodate a limb sliding through the sleeve 240,
18
CA 02822163 2013-07-29
WO 2008/150466 PCT/US2008/006883
but the diameter can be decreased enough to snugly encircle
another portion of the limb that is not for treatment. In one
embodiment, the sleeve 240 is configured to have a
sufficiently large diameter in a non-inflated or partially
inflated condition, such that a portion of a limb to be
inserted into the chamber 114 for treatment can be slid
through the sleeve 240, when the sleeve 240 is in such
condition, without the portion of the limb contacting the
sleeve inside wall 242, while providing that the diameter also
can be decreased enough to snugly encircle another portion of
the limb that is not for treatment.
[0055] In one
embodiment, the outside wall 244 and the
side wall 246 may have a thickness greater than the thickness
of the inside wall 242. This difference in thickness ensures
that when the sleeve 240 is inflated, the thicker walls
generally resist flexure and maintain their size and
dimension, allowing the inside wall 242 to absorb the
inflation. Thus,
due to its smaller thickness, the inside
wall 242 will stretch and accommodate the inflation, allowing
the inside diameter Id to decrease to a size sufficient to
seal against a limb. This also allows the sleeve 240 to seal
well against any variations in the limb size or shape, such as
a knee or ankle.
[0056] In one
embodiment, the sleeve wall thicknesses are
larger than the thickness of the chamber walls, formed by the
sheets 224 and 226. This is
because the sleeve 240 must
withstand the pressures within the sleeve due to inflation of
the sleeve 240 and pressure on the outside of the sleeve from
the gas in the chamber 114. Thus, the sleeve 240 is acted on
by pressures from inside the sleeve 240 and inside the chamber
114. The pressure within the sleeve 240 is much smaller than
the maximum pressure in the chamber 114.
Therefore, the
19
CA 02822163 2013-07-29
pressures internal and external to the sleeve 240 do not
cancel out.
[0057] An
advantage of placing the sleeve 240 within the
chamber 114 is to ensure that the incidence of the sleeve 240
sliding off the limb is reduced. The gas inside the chamber
114 places positive pressure on the outside wall 244 and
retains the sleeve 240 in place on the limb. During
inflation, the sleeve 240 can be inflated up to 1 psi of
pressure. Thus, less pressure is required to maintain the
sleeve 240 on the limb than with other types of wound
treatment seals or sleeves placed on the outside of the
chamber 114.
(0058] This
type of sleeve 240, placed inside the chamber
114, can be incorporated with reusable chambers, chambers
having rigid structures such as disclosed in U.S. Pat. No.
5,060,644, as well as single use chambers where these sleeves
24U can replace the latex seals that are now used. This
is
especially advantageous in that some patients have an adverse
reaction to latex.
[0059] Still referrin to FIG. 9, in an alternative
embodiment of the device 100, the outside wall 244 of the
sleeve 240 is attached to an interior wall surface 249 of the
chamber 114. In addition, the sleeve 240 is made of
sufficiently flexible material, such that the sleeve 240 can
be folded or rolled into itself, when the sleeve 240 is not
inflated or minimally inflated. In one
desired embodiment,
the sleeve 240 can be folded so that the sleeve 240 is not
within the interior 114 of the device 100 when the interior
114 is not inflated or minimally inflated.
[0060]
References to the hyperbaric chamber device 100 are
exemplary only and it should be noted that the cone-roller 10
described herein can be used with any type of hyperbaric
CA 02822163 2013-07-29
chamber. For example, the controller 10 can be used with
reusable chambers, a topical hyperbaric chamber such as a
torso or abdominal chamber or a single use hyperbaric chamber
having several internal rings that form a plurality of
chambers within the chamber 114. The controller can also be
configured for use with chambers that treat wounds by means of
evacuation or chambers that perform compression therapy or a
combination of a variety of treatments.
[0061] in an
embodiment of the present invention, the
controller 10 described herein can be utilized with a topical
hyperbaric chamber device 300, as illustrated in FIG. 10. See
U.S. Patent No. 5,134,697.
Referring to FIG. 10, the topical
device 300 includes a top sheet 302 and a bottom sheet 304
defining an interior region 314. In addition, the topical
chamber 302 includes an opening 306 having a seal 308 for
affixing to a patient and in communication with the region
314.
Further, the topical chamber 300 includes couplers 310
that connect to the vacuum pump 41 and a valve of the
controller 10. Once affixed to a patient, the topical chamber
300 can be operated by the controller 10 in a manner similar
to that of the device 100.
[0062]
Although the invention herein has been described
with reference to particular embodiments, it is to be
understood that these embodiments are merely illustrative of
the principles and application of the present invention, it
is therefore to be understood that numerous modifications may
be made to the illustrative embodiments and that other
arrangements may be devised without departing from the scope
of the present invention as defined by the appended claims,
which should be given the broadest interpretation consistent
with the description as a whole.
21