Note: Descriptions are shown in the official language in which they were submitted.
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
1
POUCH, METHOD OF MANUFACTURING A POUCH
AND A METHOD OF DISPENSING A PRODUCT FROM
A POUCH
[0001] This application claims priority to U.S. Provisional Application No.
61/427,526, filed December 28, 2010, the disclosure of which is herein
incorporated by
reference in its entirety.
FIELD OF THE DISCLOSURE
[0002] The field of this disclosure relates generally to packaging for liquid
products and more particularly to a pouch for containing and dispensing
aseptically
processed low acid concentrated liquid (e.g., human milk fortifier), methods
for
manufacturing a hermetically sealed pouch, methods for aseptically packaging
the human
milk fortifier in the pouch, method for testing of seal integrity of the pouch
and methods of
using the pouch to dispense human milk fortifier.
BACKGROUND OF THE DISCLOSURE
[0003] Human milk is generally recognized as an ideal food source for most
infants due to its overall nutritional composition. It is well known and
generally accepted
that human milk provides infants with unique immunologic and developmental
benefits as
compared generally to commercially available infant formulas.
[0004] For some infants, however, especially preterm infants, human milk does
not always meet their complete nutritional needs. Although these infants still
generally
benefit from human milk, it is often desirable to supplement their human milk
feedings
with additional nutrients. Initially, preterm infants may grow more rapidly
than many of
their term counterparts, and accelerated growth often requires additional
nutrition, which
can be made possible by the use of a human milk fortifier in combination with
human milk.
[0005] Human milk fortifiers described in literature and commercially
available
have been formulated as reconstitutable powders rather than liquids in order
to minimize
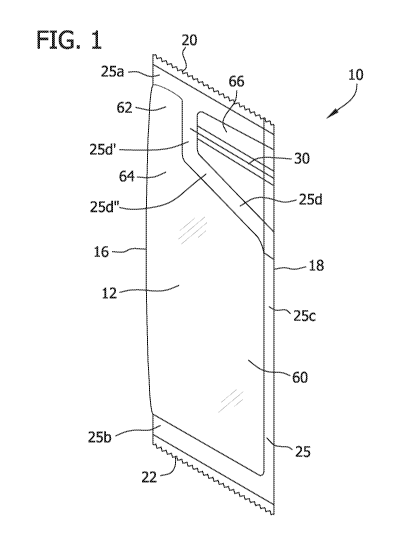
the volume displacement of human milk by the fortifier. Powdered human milk
fortifiers,
however, are not considered commercially sterile therefore microbes can be
present in
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
2
powdered human milk fortifiers and may grow once dispensed from the package
into the
human milk.
[0006] More recently, liquid human milk fortifiers, and specifically highly
concentrated human milk fortifier liquids, have received more attention as an
alternative to
powders. Although these highly concentrated human milk fortifiers do generally
displace
slightly more volume than powders, the liquids are processed to be
commercially sterile,
which is not an option for powders.
[0007] Hydrolyzed proteins are often desirable to utilize in human milk
fortifiers
as they are generally more easily digested and absorbed into the gut of a
preterm infant as
compared to substantially intact proteins. Additionally, the hydrolyzed
proteins may be
hypoallergenic such that they may not predispose the infant to cow's milk
allergies later in
life. However, as compared to intact proteins, extensively hydrolyzed proteins
(i.e.,
proteins having a degree of hydrolysis of about 20% or more) tend to have poor
ability to
form long term stable emulsions. Additionally, the presence of high levels of
insoluble
minerals such as calcium salts may also cause a number of stability issues
when used in
combination with extensively hydrolyzed proteins. As such, manufacturing long
term
stable liquid concentrated human milk fortifiers including extensively
hydrolyzed proteins
have proven difficult.
[0008] To combat this problem, many liquid human milk fortifiers have been
manufactured with stabilizers, such as carrageenan. The stabilizers act to
hold the nutrients
and insolubles in solution over time and thus improve long term stability of
the product.
Although stabilizers, such as carrageenan, have generally proven to retard
precipitation of
many ingredients in the liquid human milk fortifier, these types of
stabilizers are not
permitted in infant formulas and human milk fortifiers in many countries
around the world.
When stabilizers cannot be used in highly concentrated human milk fortifiers,
it can be
very difficult to produce a long term stable highly concentrated human milk
fortifier.
[0009] As such, there is a need for liquid human milk fortifiers that are
commercially sterile, do not require refrigeration, and have relatively low
acidity. In
addition, there is a need for packaging for liquid human milk fortifiers that
is sufficiently
flexible to allow insitu mixing of the fortifier, and transparent so that a
user can visually
observe the human milk fortifier to ensure proper mixing has occurred before
opening the
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
3
packaging to dispense the human milk fortifier. Moreover, the packaging should
be easy to
use and should minimize the amount of residual human milk fortifier remaining
in the
packaging after dosing.
BRIEF DESCRIPTION OF THE DISCLOSURE
[0010] In one aspect, a single-use pouch for liquid product generally
comprises a
front panel and a back panel. The front and back panels at least in part
cooperatively
define an interior space of the pouch. The interior space has a total liquid
capacity. A
volume of liquid product is contained within the interior space. The volume of
liquid
product is less than about 50% of the total liquid capacity of the pouch.
[0011] In another aspect, a single-use pouch for liquid product generally
comprises a front panel and a back panel. The front and back panels at least
in part
cooperatively define an interior space of the pouch. The interior space has a
total liquid
capacity. A volume of liquid product and gas is contained within the interior
space. The
volume of liquid product and gas is less than about 40% of the total liquid
capacity of the
pouch.
[0012] In yet another aspect, a pouch generally comprises a front panel and a
back panel. The front and back panels at least in part cooperatively define an
interior space
of the pouch. At least one of the front panel and the back panel is made at
least in part
from a flexible, transparent material. An aseptically processed liquid product
is contained
within the interior space of the pouch and visually observable through the at
least one of
the front panel and the back panel.
[0013] In still another aspect, a method of packaging an aseptic liquid
product
into a pouch generally comprises sterilizing both sides of a flexible and
transparent web of
sheet material with a sterilant. The web is drawn across forming shoulders,
around filling
tubes, to create longitudinal pouch tubes. Two pouches are formed, one from
each lane.
Each pouch is filled with an aseptically processed liquid product.
[0014] In still yet another aspect, a method of dispensing a liquid product
from a
pouch generally comprises obtaining a pouch having an aseptically processed
liquid
product contained therein. At least a portion of the pouch is transparent for
allowing visual
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
4
observation of the liquid product contained therein. The pouch is manually
kneaded to mix
the liquid product within the pouch. The liquid product is visually observed
through the
transparent portion of the pouch to determine if the liquid product has been
sufficiently
mixed. The pouch is opened and the liquid product is poured from the pouch.
[0015] In still a further aspect, a single-use pouch for product generally
comprises
a body having a front panel and a back panel. The front and back panels at
least in part
cooperatively define an interior space of the pouch for containing the
product. A spout is
in fluid communication with the interior space. Product is dispensed from the
pouch
through the spout. The spout has a width and the body has a width wherein the
ratio of the
width of the body and the width of the spout is between about 3:1 and about
5:1.
[0016] In yet a further aspect, a secondary container for holding a plurality
of
pouches generally comprises a base section and a lid hingely attached to the
base section
for movement between a closed position and an opened position. A pair of hold
downs are
disposed adjacent opposite ends of the hinge.
[0017] In still another aspect, a secondary container for holding a plurality
of
pouches generally comprises a base section and a lid hingely attached to the
base section
for movement between a closed position and an opened position. The base
section includes
a bottom wall, at least one side wall extending up from the bottom wall, a top
wall, and an
interior floor. The interior floor is tented along its center line.
BRIEF DESCRIPTION OF THE DRAWINGS
[0018] Fig. 1 is a perspective of one suitable embodiment of a pouch for
containing and dispensing a liquid product, the pouch being illustrated in a
closed
configuration;
[0019] Fig. 2 is a front elevation thereof;
[0020] Fig. 3 is a back elevation thereof;
[0021] Fig. 4 is a cross-section taken along line 4-4 of Fig. 2;
[0022] Fig. 5 is a cross-section taken along line 5-5 of Fig. 2;
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
[0023] Fig. 6 is a front elevation similar to Fig. 2 but illustrating the
pouch in an
opened configuration;
[0024] Fig. 7A is an enlarged fragmentary cross-section taken along line 7-7
of
Fig. 3 illustrating one suitable laminate for forming the pouch;
[0025] Fig. 7B is an enlarged fragmentary cross-section similar to Fig. 7A but
illustrating another suitable laminate for forming the pouch
[0026] Fig. 8 is a front elevation of another suitable embodiment of a pouch
for
containing and dispensing a liquid product, the pouch being illustrated in a
closed
configuration;
[0027] Fig. 9 is a front elevation of another suitable embodiment of a pouch
for
containing and dispensing a liquid product, the pouch being illustrated in a
closed
configuration
[0028] Fig. 10 is a flow diagram illustrating one suitable embodiment of a
process
for manufacturing the pouch and filling the pouch with a liquid product;
[0029] Figs. 11A ¨ 11C are schematics illustrating sequential aspects of the
process for manufacturing and filling the pouch;
[0030] Fig. 12 is a perspective of one suitable embodiment of a secondary
packaging for containing a plurality of the pouches;
[0031] Fig. 13 is a perspective illustrating the pouch in its opened
configuration
and the liquid product contained therein being dispensed into a nursing bottle
containing
human milk;
[0032] Figs. 14A and 14B are side elevations of pouches similar to the ones
illustrated in Figs. 2 and 9 except that the pouches seen herein are opaque;
[0033] Figs. 15A and 15B illustrate the pouch of Fig. 14A in the process of
being
opened and being tilted as if the product contained therein is being
dispensed;
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
6
[0034] Figs. 16A and 16B illustrate another suitable embodiment of a secondary
packaging for containing a plurality of the pouches illustrating a lid of the
packaging in a
closed position and in an opened position;
[0035] Fig. 17 is a front perspective view of the secondary packaging of Fig.
12
with the lid closed;
[0036] Fig. 18 is another front perspective of the secondary packaging of Fig.
12
with the lid closed;
[0037] Fig. 19 is a rear perspective of the secondary packaging of Fig. 12
with the
lid closed;
[0038] Fig. 20 is a front perspective of the secondary packaging of Fig. 12
with
the lid opened;
[0039] Fig. 21 is a side elevation of the secondary packaging of Fig. 12 with
the
lid opened;
[0040] Fig. 22 is a schematic illustration of a plastic container including a
concentrated liquid human milk fortifier that does not contain any USA-
modified corn
starch or low acyl gellan gum;
[0041] Fig. 23 is a schematic illustration of a plastic container including a
concentrated liquid human milk fortifier that contains OSA-modified corn
starch but does
not contain low acyl gellan gum;
[0042] Fig. 24 is a schematic illustration of a plastic container including a
concentrated liquid human milk fortifier that contains low acyl gellan gum but
does not
contain USA-modified corn starch; and
[0043] Fig. 25 is a schematic illustration of a plastic container including a
concentrated liquid human milk fortifier that contains both OSA-modified corn
starch and
low acyl gellan gum.
[0044] Corresponding reference characters indicate corresponding parts
throughout the drawings.
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
7
DETAILED DESCRIPTION OF THE DRAWINGS
Pouch
[0045] Figs. 1-5 of the drawings illustrate one embodiment of a pouch,
indicated
generally at 10, suitable for packaging and dispensing a liquid product, e.g.,
a liquid
product intended for human consumption. As used herein "liquid product" means
a
product that is a flowable non-solid product including, for example but not
limited to,
aqueous solutions, solutions having a determinable viscosity, emulsions,
colloids, pastes,
gels, dispersions and other flowable non-solid products so as to exclude solid
products such
as bars and particulate products, such as powders.
[0046] As seen therein, the illustrated pouch 10 has a front panel 12 and a
back
panel 14 generally opposed to and sealingly engaged with the front panel to at
least in part
define an interior space 15 sized and shaped for containing the product. The
illustrated
pouch 10 comprises two side edges 16, 18, two end edges 20, 22, a longitudinal
axis LA,
and a transverse axis TA. In the illustrated embodiment, the pouch 10 is
formed from a
single-piece of sheet material that has been folded about a longitudinal fold
line. As seen
in Figs. 2-5, the fold line forms one of the side edges 16 of the pouch 10.
The front panel
12 of the pouch 10 is joined to the back panel 14 along the other side edge 18
and at the
end edges 20, 22 along a plurality of seal lines 25, such as by heat sealing,
to seal the
interior space 15 of the pouch. It is understood, however, that the front
panel 12 and back
panel 14 of the pouch 10 may be joined in other ways without departing from
the scope of
the present invention (e.g., adhesive). It is also understood that the pouch
10 could be
formed from two separate panels that are sealed together along both side edges
16, 18 and
the end edges 20, 22. It is also contemplated that the pouch 10 may include
sidewalls (not
shown) intermediate the front panel 12 and the back panel 14 without departing
from the
scope of this invention.
[0047] With reference to Figs. 2 and 3, the seal lines 25 include end segments
25a, 25b disposed along the margins adjacent the end edges 20, 22 of the pouch
10,
respectively, to fluidly seal the ends of the pouch. A side edge segment 25c
is disposed
adjacent one of the side edges 18 (i.e., opposite the side edge 16 defined by
the fold line)
and extends the longitudinal length of the pouch 10. The side edge segment 25c
intersects
(or otherwise contacts) the end segments 25a, 25b to define the interior space
15 of the
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
8
pouch 10 and to seal the pouch in a fluid-tight manner. As seen in Figs. 2 and
3, the seal
lines 25 further comprise an inboard seal segment 25d that connects one of the
end
segments 25a (the upper seal segment as viewed in Figs. 2 and 3) to the side
edge segment
25c. In the illustrated embodiment, the inboard seal segment 25d includes a
longitudinal
component 25d' extending downward from the end segment 25a and a diagonal
component
25d" extending diagonally from the longitudinal component to the side edge
segment 25c.
It is understood, however, that the inboard seal segment 25d can have
different
configurations (e.g., generally L-shaped) without departing from the scope of
this
invention.
[0048] With reference still to Figs. 2 and 3, the pouch 10 includes a body 60,
a
spout 62, and a transition (or funnel) portion 64 connecting the body to the
spout. The
body 60 is the portion of the pouch 10 below the lower extent of the diagonal
component
25d" of the inboard seal segment 25d of the seal lines 25. The body 60 has a
height H1
and a width W1 . In the illustrated embodiment, the height H1 of the body 60
is
approximately 75 mm and the width W1 of the body is approximately 36 mm. It is
understood that the body 60 can have heights and widths less than or greater
than the
exemplary heights and widths provided herein. The spout 62 is defined by the
end segment
25a and the longitudinal component 25d' of the inboard seal segment 25d of the
seal lines
25. As seen in Figs. 2-4, the spout 62 includes the fold line defining one of
the side edges
16 of the pouch 10. In use, the fold line acts as a channel and guides the
product along the
fold line and towards an opening in the spout. The spout 62 has a height H2
and a width
W2. In the illustrated embodiment, the height H2 of the spout 62 is
approximately 17 mm
and the width W1 of the spout is approximately 10 mm. Thus, the illustrated
body 60 of
the pouch 10 has a width ratio of about 3.6:1 with respect to the width of the
spout 62.
That is, the body has a width that is about 3.6 times larger than the width of
the spout 62.
It is understood that the spout 62 can have heights and widths less than or
greater than the
exemplary heights and widths provided herein. For example, the ratio of the
width of the
body and the width of the spout may be between about 3:1 and about 5:1, such
as, about
4:1.
[0049] The transition portion 64 is a portion of the pouch 10 disposed between
the
spout 62 and body 60, and includes the diagonal component 25d" of the inboard
seal
segment 25d of the seal lines 25. In use, the diagonal component 25d" of the
inboard seal
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
9
segment 25d acts as a funnel-like surface to funnel the product towards the
spout. The
transition portion 64 has a height H3. In the illustrated embodiment, the
height H3 of the
transition portion 64 is approximately 17 mm. The width of the transition
portion 64
reduces along its height as it extends from the body 60 to the spout 62. It is
understood that
the transition portion 64 can have heights and widths less than or greater
than the
exemplary heights and widths provided herein.
[0050] In a sealed (broadly, closed) configuration of the pouch 10, as
illustrated in
Figs. 1-3, the product is sealingly enclosed in the interior space 15 of the
pouch. In one
suitable embodiment, the product is aseptically processed and sealed within
the pouch 10
as described in more detail below. The pouch 10 can be selectively configured
from the
sealed configuration to an opened configuration as illustrated in Fig. 6 to
permit dispensing
of the product from the pouch. In one suitable embodiment, the product is a
liquid and can
be poured from the pouch 10 through the spout 62. It is understood, however,
that the
product can be any suitable, liquid substance including a gel or a paste.
[0051] As illustrated in Figs. 1-3, the pouch 10 has a first line of weakness
30
formed on the front panel 12 of the pouch and a second line of weakness 32
formed on the
back panel 14 of the pouch. The lines of weakness 30, 32 provide a path along
which the
pouch 10 is more readily torn to open the pouch (i.e., configured to the
opened
configuration). It is understood that the pouch 10 may have a line of weakness
30, 32
disposed on only one of the front and back panels 12, 14, with the other panel
being free of
a line of weakness and remain within the scope of this invention. While the
lines of
weakness 30, 32 in the illustrated embodiment are substantially equal in
length, the lengths
of the lines of weakness 30, 32 can be different without departing from the
scope of this
invention. Thus, the line of weakness 30 on the front panel 12 of the pouch 10
may be
longer or shorter than the line of weakness 32 on the back panel 14 of the
pouch.
[0052] In the illustrated embodiment, the lines of weakness 30, 32 comprise
score
lines. The term "line of weakness" is used herein to mean any defined (e.g.,
intended)
structural feature that weakens the pouch 10 along a predetermined path so
that the pouch
is more readily ruptured, or torn, upon application of a tearing force along
the line of
weakness and is not limited to score lines. For example, in other embodiments,
the lines of
weakness 30, 32 may comprise a plurality of separation points, a score line, a
breakaway
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
line or areas, a chain stitch, a thinning of the pouch material, a plurality
of aligned
perforations (e.g., holes, slits, apertures, voids, or the like) or other
suitable line of
weakness. The lines of weakness 30, 32 may be formed by partial pressure
cutting, partial
ultrasonic cutting, partial thermal deformation, mechanical thinning, or other
suitable
techniques.
[0053] As mentioned, the lines of weakness 30, 32 provide a path of low
resistance along which the pouch 10 may be torn. However, the level of
resistance to
tearing provided by the lines of weakness 30, 32 can be altered. Lowering the
tear
resistance would make the pouch 10 easier to open. As a result, less force is
needed to tear
the pouch 10 along the lines of weakness 30, 32. However, lowering the tear
resistance
may increase the risk that the pouch 10 will unintentionally tear apart or
otherwise leak.
On the other hand, increasing the resistance of the lines of weakness 30, 32,
would require
a greater force to tear the pouch 10 along the lines of weakness. In addition,
the lines of
weakness 30, 32 can have varying tear resistance along their length or a
portion of their
length. In addition, the tear resistance of the line of weakness 30 in the
front panel 12 of
the pouch 10 may be equal to or different than the tear resistance of the line
of weakness 32
in the back panel 14 of the pouch.
[0054] In the illustrated embodiment, the lines of weakness 30, 32 begin at
the
side edge 18 (e.g., the side edge not defined by the fold line), extend
through the side edge
segment 25c of the seal lines 25 and generally parallel to but spaced from one
of the end
edges 20, and terminate within the longitudinal component 25d' of the inboard
seal
segment 25d of the seal lines and generally adjacent the spout 62.
Accordingly, the
product can be accessed by tearing the pouch 10 along the lines of weakness
30, 32 as
illustrated in Fig. 6. The spout 62 is torn approximately in half
longitudinally during
tearing of the lines of weakness 30, 32. It is understood, however, that more
or less of the
spout 62 can be torn away.
[0055] In the illustrated embodiment, the portion of the pouch 10 above the
lines
of weakness 30, 32 defines a gripping portion 66 suitable for manually
grasping to
facilitate opening of the pouch 10 by tearing along the lines of weakness 30,
32. In one
suitable embodiment, the gripping portion 66 is removed from the remainder of
the pouch
10 when the pouch is opened (i.e., when the pouch is torn along the lines of
weakness 30,
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
11
32). It is contemplated, however, that the gripping portion 66 can remain
connected to the
pouch 10 so long as the spout 62 is sufficiently open to allow the product to
flow out of the
interior space 15 of the pouch.
[0056] The pouch 10 may be formed from any suitable material including woven
material, non-woven material, films, laminates, or a combination thereof. For
example, in
one suitable embodiment, the pouch 10 comprises a two layered laminate having
an inner
layer 50 and an outer layer 52 (Fig. 7A). In one particularly suitable
embodiment, the inner
layer 50 is formed from a co-extrusion of linear low density polyethylene
(LLDPE) and
ethylene vinyl alcohol (EVOH), and the outer layer 52 from barrier coated
polyethylene
terephthalate (PET). In another suitable embodiment, which is illustrated in
Fig. 7B, the
pouch 10 comprises a three layered laminate having an inner layer 50', an
outer layer 52',
and an intermediate layer 54' disposed between the inner and outer layers
(Fig. 7B). In
one particularly suitable embodiment, the inner layer 50' is formed from a co-
extrusion of
linear low density polyethylene (LLDPE) and ethylene vinyl alcohol (EVOH), and
the
outer layer 52' from barrier coated polyethylene terephthalate (PET). The
intermediate
layer 54' is formed from one of aluminum oxide coated PET, a silicon oxide
coated PET,
or ethylene vinyl alcohol. As explained in more detail below, the pouch 10, in
one suitable
embodiment, is formed from a non-metalic material. That is, the pouch 10 is
substantially
free from metal. It is understood, however, that the layers 50, 50', 52, 52',
54' can be
formed from any suitable materials without departing from some aspects of this
invention.
[0057] As seen in Fig. 7A, the layers 50, 52 of the two layer laminate are
bonded
together using adhesive 51. It is understood, however, that the layers 50, 52
can be bonded
together using other suitable techniques. As also seen in Fig. 7A, indicia 53
is printed on
an inner surface of the inner layer 50 (i.e., the surface that faces and is
bonded to the outer
layer 52) using suitable ink. It is understood, however, that the indicia 53
can be printed on
either surface of the outer layer 52.
[0058] In one suitable embodiment, at least a portion of the pouch 10 is
generally
transparent to permit visual observation of the product contained therein. In
the illustrated
embodiment, for example, the entire pouch 10 is generally transparent. In one
suitable
embodiment, the inner surface of the inner layer 50 of either the front panel
12 or the back
panel 14 can be covered with a white ink to render the front/back panel
generally
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
12
transparent. It is understood, however, that less then the entire pouch can be
transparent.
For example, the front panel 12 could be made from a generally transparent
material and
the back panel 14 formed from a translucent or opaque material, or vise versa.
In another
example, the pouch 10 could include a longitudinally extending strip of
transparent
material (e.g., to form a window) on either one of or both the front and back
panels 12, 14
of the pouch while the remainder of the pouch is formed from a generally
translucent or
opaque material. It is understood, that the pouch 10 can be formed from
generally opaque
material as seen in Figs. 14A and 14B without departing from some aspects of
this
invention.
[0059] The pouch 10 illustrated in Figs. 1-6 is suitably configured for
containing
and dispensing a predetermined target dispensing dosage, such as,
approximately 5 ml. It
is understood, however, that the pouch 10 can be configured to hold any
suitable target
dosage. For example, Fig. 8 illustrates a second embodiment of a pouch 110
substantially
similar to the pouch 10 of Figs. 1-6 except that the pouch of this second
embodiment is
smaller and designed to hold a target dosage of approximately 2 ml. More
specifically, the
pouch 110 has a shorter body 160 than the body 60 of the pouch 10 illustrated
in Figs. 1-6.
Otherwise, the pouches 10, 110 are substantially the same including, in one
embodiment,
being of the same width for ease of manufacturing different sized pouches. In
another
example, Fig. 9 illustrates a third embodiment of a pouch 210 substantially
similar to the
previous described pouch 10 of Figs. 1-6 except that the pouch of this
embodiment is larger
and designed to hold a target dosage of approximately 80 ml. More
specifically, the pouch
210 has a longer body 260 than the body 60 of the pouch 10 illustrated in
Figs. 1-6.
Otherwise, the pouches 10, 210 are substantially the same. Depending on the
product and
the desired target dosage, it is understood that the pouch may be sized and
configured for
generally any target dosage.
[0060] In one suitable embodiment, each of the pouches 10, 110, 210 is filled
with a greater quantity of product as compared to its intended target
dispensing dosage to
account for residual product that remains within the pouch after use, such as,
due to
viscosity and stickiness of the product. Testing of the pouch 10 illustrated
in Figs. 1-6
determined that approximately 88 percent of the pouch contents are typically
dispensed
during use. As a result, each of the pouches 10, 110, 210 has an actual fill
volume that is
approximately 12 percent greater than the target dispensing dosage. Thus, the
pouch 110
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
13
intended to have a 2 ml dosage (Fig. 8) has a fill volume of approximately
2.27 ml. The
pouch 10 intended to have a 5 ml dosage (Figs. 1-7) has a fill volume of
approximately
5.69 ml. And the pouch 210 intended to have an 80 ml dosage size (Fig. 9) has
a fill
volume of approximately 90.91 ml. It is understood that the pouches 10, 110,
210 can have
other anticipated residual rates (i.e., besides 88 percent) as a result of
viscosity, stickiness
or other factors and thus other fill volumes without departing from the scope
of this
invention.
[0061] Moreover, it is anticipated that each of the pouches 10, 110, 210 will
have
a distribution ratio within + 4 percent. That is, the actual amount of product
distributed
from each of the pouches 10, 110, 210 will be within 4 percent of the target
dosage for that
pouch. Thus, the pouch 110 intended to have a 2 ml dosage (Fig. 8) will
actually dispense
a quantity of product between about 1.92 ml and about 2.08 ml. The pouch 10
intended to
have a 5 ml dosage (Figs. 1-7) will actually dispense a quantity of product
between about
4.8 ml and about 5.2 ml. And the pouch 210 intended to have an 80 ml dosage
(Fig. 9) will
actually dispense a quantity of product between about 76.8 ml and about 83.2
ml. It is
understood that the pouches 10, 110, 210 can have a different distribution
ratio (i.e.,
besides +4 percent) without departing from the scope of this invention.
[0062] Each of the pouches 10, 110, 210 is capable (e.g., sufficiently
flexible) of
being manually kneaded or otherwise manipulated by a user to ready the product
within the
pouch before opening the pouch. Thus, in one embodiment, the product can be
thoroughly
mixed within the pouch 10, 110, 210 before the pouch is opened and the product
dispensed
therefrom. In other embodiments where the product is more gel-like, kneading
also, or
alternatively, thins the product to render it easier to pour. In one suitable
embodiment, the
front and back panels 12, 14 of the pouch 10 contact each other during the
kneading
process under relatively light, manual pressure and the product is able to
move freely
throughout the interior space 15.
[0063] A qualitative kneadability study was performed on pouches designed for
a
target dispensing dosage of about 5 ml. The pouches had a total (e.g.,
maximum) liquid
capacity of about 20 ml. In Example 1, ten pouches were filled with a various
amount of
air (broadly, a gas) and manually kneaded. The kneadability of the pouch was
rated as
being easy, moderate, difficult or extremely difficult. The amount of air and
the results of
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
14
the testing are provided in the following Table. In Example 2, ten pouches
were filled with
a various amount of liquid and manually kneaded. The kneadability of the pouch
was rated
as being easy, moderate, difficult or extremely difficult. The amount of
liquid and the
results of the testing are provided in the following Table. In Example 3, ten
pouches were
filled with various combinations of liquid and air and manually kneaded. The
kneadability
of the pouch was rated as being easy, moderate, difficult or extremely
difficult. The
amount of liquid and air and the results of the testing are provided in the
following Table.
Example 1
Air Only Sample Air Volume (ml) Total Volume Ease of Kneading
1 6 6 Easy
2 7 7 Easy
3 8 8 Easy
4 9 9 Easy
10 10 Easy
6 11.5 11.5 Moderate
7 16.5 16.5 Moderate
8 19 19 Moderate
9 22 22 Difficult
26.5 26.5 Extremely Difficult
Example 2
Liquid Only Sample Liquid Volume (ml) Total Volume Ease of Kneading
1 4 4 Easy
2 5 5 Easy
3 6 6 Easy
4 7 7 Easy
5 8 8 Easy
6 9 9 Easy
7 10 10 Easy
8 12 12 Moderate
9 16 16 Difficult
10 20 20 Extremely Difficult
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
Example 3
Liquid + Air Air Volume (ml) /
Liquid Vol = 5.56 ml % Air Volume Total Volume Ease of Kneading
1 0.6 / 10% 6.2 Easy
2 1.4 / 20% 7.0 Easy
3 2.4 / 30% 7.9 Easy
4 3.7 / 40% 9.3 Easy
5 5.6 / 50% 11.1 Easy
6 8.3 / 60% 13.9 Moderate
7 10.3 / 65% 15.9 Moderate
8 13 / 70% 18.5 Difficult
9 16.7 / 75% 22.2 Extremely Difficult
10 22.2 / 80% 27.8 Extremely Difficult
[0064] The intent of the kneadability study was to determine suitable packaged
volumes at which kneading of the product/pouch becomes impractical (i.e.,
difficult or
extremely difficult).
[0065] As seen above for Example 2, where no air is present the amount of
liquid
within the pouch should be less than or equal to about 50% of the total liquid
capacity of
the pouch. When the amount of liquid in the pouch exceeded 50%, the
kneadibility of the
pouch was reduced. In one suitable embodiment, the volume of liquid in the
pouch is
between about 20% and about 50%, more suitably between about 30% and about
40%, and
even more suitably about 35% of the total liquid capacity of the pouch.
[0066] As seen above for Example 3, the total volume taken up by liquid and
gas
(e.g., air) within the pouch should be less than or equal to about 50% of the
total liquid
capacity of the pouch. When the combined volume of liquid and gas exceeds
about 50%,
the kneadibility of the pouch is reduced. In one suitable embodiment, the
combined
volume of liquid and gas in the pouch is between about 10% and about 50%, more
suitably
between about 20% and about 40% of the total liquid capacity of the pouch.
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
16
Human Milk Fortifiers
Concentrated Liquid Human Milk Fortifier
[0067] In one suitable use, the pouch 10, 110, 210 can contain liquid human
milk
fortifier capable of being poured directly from the pouch into a container
having human
milk therein. It is understood, however, that the pouch 10, 110, 210 can
contain any
suitable product including other products intended for human consumption. One
suitable
liquid human milk fortifier is a concentrated liquid human milk fortifier
comprising
protein, fat, carbohydrate OSA-modified starch and low acyl gellan gum. The
concentrated
liquid human milk fortifier has a solids content of at least about 20%, or
even at least about
25%, including from about 25% to about 32%, and further including from about
29% to
about 32%. The concentrated liquid human milk fortifier has a caloric density
of at least
about 1.25 kcal/m1 (37 kcal/fl oz), including from about 1.4 kcal/m1 (42
kcal/fl oz) to about
kcal/ml (149 kcal/fl oz), and also including from about 1.5 kcal/ml (44
kcal/fl oz) to
about 2.5 kcal/m1 (74 kcal/fl oz), and also including from about 1.9 kcal/m1
(56 kcal/fl oz)
to about 2.0 kcal/ml (59 kcal/fl oz). The concentrated liquid human milk
fortifiers is
formulated to provide fortified human milk having an osmolality of less than
about 400
mOsm/kg water, preferably from about 300 mOsm/kg water to about 400 mOsm/kg
water.
Extensively Hydrolyzed Casein Protein
[0068] The concentrated liquid human milk fortifier includes hypoallergenic
extensively hydrolyzed casein as a protein source. The term "hypoallergenic"
as used
herein means that the concentrated liquid human milk fortifier has a decreased
tendency to
provoke an allergic reaction in a preterm or term infant as compared to non-
hypoallergenic
fortifiers. Generally, the concentrated liquid human milk fortifier includes
at least about
35%, including at least about 50%, including at least about 60%, including at
least about
75%, including at least about 90% and further including about 100% extensively
hydrolyzed casein, by total weight of protein in the concentrated human milk
fortifier. In
one embodiment, the concentrated liquid human milk fortifier includes 100%
extensively
hydrolyzed casein, by total weight of the protein in the concentrated human
milk fortifier.
In this embodiment, the concentrated liquid human milk fortifier is
hypoallergenic. In
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
17
some other embodiments, the concentrated liquid human milk fortifier will
include from
about 35% to 100%, including from about 50% to 100%, further including from
about 75%
to 100% extensively hydrolyzed casein, by total weight of protein in the
concentrated
human milk fortifier. The concentrated liquid human milk fortifier may
optionally include
other hypoallergenic or non-hypoallergenic proteins in addition to the
extensively
hydrolyzed casein protein.
[0069] Extensively hydrolyzed casein proteins suitable for use in concentrated
liquid human milk fortifiers include those having a degree of hydrolysis of
from about 20%
to about 70%, including from about 30% to about 60%, and further including
from about
40% to about 60%. Generally, the extensively hydrolyzed casein has a ratio of
total amino
nitrogen (AN) to total nitrogen (TN) of from about 0.2 AN to 1.0 TN to about
0.4 AN to
about 0.8 TN. Suitable commercially available extensively hydrolyzed caseins
will
generally have a protein level in the ingredient of from about 50% to about
95%, including
from about 70% to about 90%. One suitable commercially available extensively
hydrolyzed casein is Dellac CE90, which is a spray dried powder casein
hydrolysate
(Friesland Campina Domo, Amersfoort, The Netherlands).
Stabilizer System
[0070] The concentrated liquid human milk fortifier includes a synergistic two
component stabilizer system. The first component is an octenyl succinic
anhydride (OSA)
modified corn starch. The second component is a low acyl gellan gum. These two
components act in a synergistic manner to stabilize the concentrated liquid
human milk
fortifier emulsion and retard the precipitation of nutrients therefrom.
[0071] The OSA-modified corn starch is generally prepared by esterifying a
dextrinized, ungelatinized waxy corn starch with 1-octenyl succinic anhydride.
Methods of
this type are well known in the art. One suitable commercially available USA-
modified
corn starch is N-CREAMERTm 46 (National Starch Food Innovation, Bridgewater,
New
Jersey). Without being bound to a particular theory, it is believed that the
OSA-modified
corn starch adsorbs in the oil and water interface thus preventing the oil
droplets from
coalescence/aggregation by steric hinderance and charge repulsion. The OSA-
modified
corn starch is present in the concentrated liquid human milk fortifier in an
amount of from
about 0.1% to about 3.5%, including from about 0.6% to about 2.0%, including
from about
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
18
0.8% to about 1.5%, and further including about 1.2% by weight of the
concentrated liquid
human milk fortifier.
[0072] The low acyl gellan gum (also known as and commonly referred to as
deacylated gellan gum) may be a water-soluble polysaccharide produced by
fermentation
of a pure culture of Sphingomonas elodea. As used herein, "low acyl" means
that the
gellan gum has been treated such that it forms firm, non-elastic, brittle
gels, that are heat
stable, as compared to "high acyl" which forms soft, very elastic, non-brittle
gels. Without
being bound to a particular theory, it is believed that the low acyl gellan
gum creates a
three dimensional gelled network of very small microgels that interact with
each other to
provide a stable suspension. One suitable commercially available low acyl
gellan gum is
Kelcogel F (CP Kelco U.S. Inc., Atlanta Georgia).
[0073] The low acyl gellan gum is present in the concentrated liquid human
milk
fortifier in an amount from greater than 125 ppm to about 800 ppm, including
from about
150 ppm to about 400 ppm, including from about 200 ppm to about 300 ppm and
further
including about 200 ppm.
Macronutrients
[0074] The concentrated liquid human milk fortifier comprises carbohydrate,
fat,
and protein macronutrients of sufficient types and amounts, that when used in
combination
with human milk (or other infant feeding formula), they help meet the
nutritional needs of
infants and especially premature infants. The concentration of these
macronutrients
includes the ranges described hereinafter. The term "infant" as used herein,
refers
generally to individuals less than about 1 year of age, actual or corrected.
The term
"premature infants" are used herein refers to those infants born at less than
37 weeks
gestation, have a birth weight of less than 2500 gm, or both.
Protein
[0075] The concentrated liquid human milk fortifier comprises a protein
suitable
for use with infants, especially preterm infants, at concentrations ranging
from about 5% to
about 50%, including from about 20% to about 40%, including from about 5% to
about
30%, including from about 10% to about 25%, and also including from about 15%
to about
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
19
25%, on a dry weight basis. In some embodiments, the protein may be at a
concentration
of less than 10%, on a dry weight basis. The protein concentration may be from
about 7 to
about 15 grams, including from about 9 to about 12 grams of protein per 100
grams of final
liquid product.
[0076] As noted above, the protein component of the concentrated liquid human
milk fortifier is at least partially comprised of extensively hydrolyzed
casein. In one
particularly suitable embodiment, the protein component of the concentrated
human milk
fortifier is entirely comprised of extensively hydrolyzed casein. In
embodiments wherein
additional proteins sources (i.e., one or more protein sources in addition to
the extensively
hydrolyzed protein source) are to be used in the concentrated liquid human
milk fortifier in
addition to the extensively hydrolyzed casein (i.e., the concentrated human
milk fortifier
protein component is not 100% extensively hydrolyzed casein), the fortifier
may still be
made hypoallergenic by including additional hypoallergenic proteins such as
soy protein
hydrolysate, whey protein hydrolysate, rice protein hydrolysate, potato
protein hydrolysate,
fish protein hydrolysate, egg albumen hydrolysate, gelatin protein
hydrolysate,
combinations of animal and vegetable protein hydrolysates, and combinations
thereof.
[0077] In this context, the terms "protein hydrolysates" or "hydrolyzed
protein"
are used interchangeably herein and include extensively hydrolyzed proteins,
wherein the
degree of hydrolysis is most often at least about 20%, including from about
20% to about
80%, and also including from about 30% to about 80%, even more preferably from
about
40% to about 60%. The degree of hydrolysis is the extent to which peptide
bonds are
broken by a hydrolysis method. The degree of protein hydrolysis for purposes
of
characterizing the extensively hydrolyzed protein component of these
embodiments is
easily determined by one of ordinary skill in the formulation arts by
quantifying the amino
nitrogen to total nitrogen ratio (AN/TN) of the protein component of the
selected
formulation. The amino nitrogen component is quantified by USP titration
methods for
determining amino nitrogen content, while the total nitrogen component is
determined by
the Tecator Kjeldahl method, all of which are well known methods to one of
ordinary skill
in the analytical chemistry art.
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
[0078] In other embodiments, the concentrated liquid human milk fortifier, in
addition to the extensively hydrolyzed protein, may include an additional non-
hypoallergenic protein source including for example, partially hydrolyzed or
non-
hydrolyzed (intact) protein, and can be derived from any known or otherwise
suitable
source such as milk (e.g., casein, whey, lactose-free milk protein isolates),
animal (e.g.,
meat, fish), cereal (e.g., rice, corn), vegetable (e.g., soy), or combinations
thereof. The
protein can include, or be entirely or partially replaced by, free amino acids
known or
otherwise suitable for use in nutritional products, non-limiting examples of
which include
free amino acids including L-alanine, L-arginine, L-asparagine, L-aspartic
acid, L-
carnitine, L-cystine, L-glutamic acid, L-glutamine, glycine, L-histidine, L-
isoleucine, L-
leucine, L-lysine, L-methionine, L-phenylalanine, L-proline, L-serine, L-
taurine, L-
threonine, L-tryptophan, L-tyrosine, L-valine, and combinations thereof.
Carbohydrate
[0079] The concentrated liquid human milk fortifier comprises a carbohydrate
suitable for use with infants, especially preterm infants, at concentrations
most typically
ranging up to about 75% by weight on a dry weight basis, including from about
10% to
about 50%, and also including from about 20% to about 40%, by weight on a dry
weight
basis. Carbohydrates suitable for use in the concentrated liquid human milk
fortifier
include hydrolyzed or intact, naturally and/or chemically modified, starches
sourced from
corn, tapioca, rice or potato, in waxy or non-waxy forms. Other non-limiting
examples of
suitable carbohydrate sources include hydrolyzed cornstarch, maltodextrin
(i.e. non-sweet,
nutritive polysaccharide having a DE value less than 20), corn maltodextrin,
glucose
polymers, sucrose, corn syrup, corn syrup solids (i.e., polysaccharide having
a DE value
greater than 20), glucose, rice syrup, fructose, high fructose corn syrup,
indigestible
oligosaccharides such as fructooligosaccharides (FOS), galactose, glycerol and
combinations thereof. The carbohydrates may comprise lactose or can be
substantially free
of lactose.
[0080] The concentrated liquid human milk fortifier may include a non-reducing
carbohydrate component, which may represent from about 10% to 100%, including
from
about 80% to 100%, and also including 100%, by weight of the total
carbohydrate in the
concentrated liquid human milk fortifier. The selection of a non-reducing
carbohydrate
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
21
may enhance the product stability and is generally better tolerated by
infants, especially
premature infants. Non-limiting examples of non-reducing carbohydrates include
sucrose
or other carbohydrates that do not readily oxidize or react with Tollen's,
Benedict's, or
Fehling's reagents. The concentrated liquid human milk fortifier may have a
carbohydrate
component, wherein the carbohydrate component comprises a mono- and/or
disaccharide
such that at least about 50%, including from about 80% to 100%, and also
including 100%,
of the mono- and/or disaccharide is a non-reducing carbohydrate.
Fat
[0081] The concentrated liquid human milk fortifier comprises a fat component
suitable for use with infants, especially preterm infants, at concentrations
most typically
ranging up to about 40% by weight on a dry weight basis, including from about
10% to
about 40%, and also including from about 15% to about 37%, and also including
from
about 18% to about 30%, by weight on a dry weight basis. Fats suitable for use
in the
concentrated liquid human milk fortifier include coconut oil, soy oil, corn
oil, olive oil,
safflower oil, high oleic safflower oil, MCT oil (medium chain triglycerides),
sunflower
oil, high oleic sunflower oil, structured triglycerides, palm and palm kernel
oils, palm olein,
canola oil, marine oils, cottonseed oils, and combinations thereof.
[0082] Suitable fats for use in the concentrated liquid human milk fortifier
include
emulsifiers to help the various fortifier components readily disperse when
combined with
human milk. Non-limiting examples of suitable emulsifiers include soya bean
lecithin, or
fractions there of, polyoxythylene stearate, mono and di-glycerides, and
combinations there
of, polyoxyethylene sorbitan monopalmitate, polyoxyethylene sorbitan
monostearate,
ammonium phosphatides, polyoxyethylene sorbitan monolaurate, citric acid
esters of mono
and diglycerides of fatty acids, tartaric acid esters of mono and diglycerides
of fatty acids,
and combinations thereof. Natural soy lecithin is especially useful in this
respect. The fat
component of the concentrated liquid human milk fortifier may therefore
optionally include
any emulsifier suitable for use in infant nutritional products. Emulsifier
concentrations in
these products may range up to about 10%, including from about 1% to about
10%, even
more typically from about 1.5% to about 5%, by weight of the total fat
component.
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
22
[0083] The concentrated liquid human milk fortifier also include embodiments
that comprise as part of the fat component one or more of arachidonic acid,
docosahexaenoic acid, or combinations thereof, alone or in further combination
with
linoleic acid, linolenic acid, or both.
[0084] The weight ratio of fat to protein in the concentrated liquid human
milk
fortifier is at least about 0.9, including from about 1 to about 5, and also
including from
about 2 to about 4. These ratios may be helpful in further stabilizing the
concentrated
liquid human milk fortifier.
Vitamins and Minerals
[0085] The concentrated liquid human milk fortifier may further comprise any
of
a variety of vitamins, non-limiting examples of which include vitamin A,
vitamin D,
vitamin E, vitamin K, thiamine, riboflavin, pyridoxine, vitamin B12, niacin,
folic acid,
pantothenic acid, biotin, vitamin C, choline, inositol, salts and derivatives
thereof, and
combinations thereof. The concentrated liquid human milk fortifier includes
embodiments
comprising per 100 kcal of fortifier solids one or more of the following:
vitamin A (from
about 250 to about 6500 IU), vitamin D (from about 40 to about 1200 IU),
vitamin K,
vitamin E (at least about 0.3 IU), vitamin C (at least about 8 mg), thiamine,
vitamin B12,
niacin, folic acid, pantothenic acid, biotin, choline (at least about 7 mg),
and inositol (at
least about 2 mg).
[0086] The concentrated liquid human milk fortifiers may also further comprise
any of a variety of minerals known or otherwise suitable for use in infant or
other
nutritional formulas, non-limiting examples of which include phosphorus,
magnesium,
calcium as described hereinbefore, zinc, manganese, copper, iodine, sodium,
potassium,
chloride, selenium, chromium, and combinations thereof. The concentrated
liquid human
milk fortifier also includes embodiments comprising per 100 kcal of the
fortifier solids one
or more of the following: calcium (at least about 50 mg), phosphorus (at least
about 25
mg), magnesium (at least about 6 mg), iodine, zinc (at least about 0.5 mg),
copper,
manganese, sodium (from about 20 to about 60 mg), potassium (from about 80 to
about
200 mg), chloride (from about 55 to about 150 mg) and selenium (at least about
0.5 mcg).
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
23
Other Optional Ingredients
[0087] The concentrated liquid human milk fortifier may further optionally
comprise other ingredients that may modify the physical, chemical, aesthetic
or processing
characteristics of the formulas or serve as pharmaceutical or additional
nutritional
components when used in the targeted population. Many such optional
ingredients are
known for use in food and nutritional products, including infant formulas, and
may also be
used in the concentrated liquid human milk fortifiers, provided that such
optional materials
are compatible with the essential materials described herein, are safe and
effective for their
intended use, and do not otherwise unduly impair the performance of the
concentrated
liquid human milk fortifier. Non-limiting examples of such optional
ingredients include
preservatives, anti-oxidants, various pharmaceuticals, buffers, carotenoids,
colorants,
flavors, nucleotides and nucleosides, thickening agents, prebiotics,
probiotics, sialic acid-
containing materials, and other excipients or processing aids.
Examples
Examples 1-4
[0088] The ingredients for the concentrated liquid human milk fortifiers of
Examples 1-4 are shown in the following table.
Ingredient (Per 1000 Kg) Example 1 Example 2 Example 3 Example 4
Water Q.S. Q.S. Q.S. Q.S.
Casein Hydrolysate 108 Kg 108 Kg 125 Kg 150 Kg
Maltodextrin 104 Kg 104 Kg 104 Kg 104 Kg
MCT Oil 17.3 Kg 17.3 Kg 17.3 Kg 17.3 Kg
Tricalcium Phosphate 16.0 Kg 16.0 Kg 16.0 Kg 16.0 Kg
Soy Oil 10.4 Kg 10.4 Kg 10.4 Kg 10.4 Kg
OSA-Modified Corn Starch 12.0 Kg 10.0 Kg 35.0 Kg 6.0 Kg
Coconut Oil 6.3 Kg 6.3 Kg 6.3 Kg 6.3 Kg
Potassium Citrate 6.9 Kg 6.9 Kg 6.9 Kg 6.9 Kg
Ascorbic Acid 2.9 Kg 2.9 Kg 2.9 Kg 2.9 Kg
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
24
Magnesium Chloride 4.0 Kg 4.0 Kg 4.0 Kg 4.0 Kg
M. Alpina Oil (ARA) 2.6 Kg 2.6 Kg 2.6 Kg 2.6 Kg
Leucine 1.8 Kg 1.8 Kg 1.8 Kg 1.8 Kg
C. Cohnii Oil (DHA) 2.1 Kg 2.1 Kg 2.1 Kg 2.1 Kg
Potassium Chloride 1.1 Kg 1.1 Kg 1.1 Kg 1.1 Kg
Tyrosine 1.4 Kg 1.4 Kg 1.4 Kg 1.4 Kg
Distilled Monoglycerides 800 g 800 g 800 g 800 g
Mixed Carotenoid Premix 551 g 551 g 551 g 551 g
M-Inositol 529 g 529 g 529 g 529 g
Sodium Chloride 861g 861g 861g 861g
L-Carnitine 221 g 221 g 221 g 221 g
Tryptophan 331 g 331 g 331 g 331 g
Zinc Sulfate 309 g 309 g 309 g 309 g
Niacinamide 320g 320g 320g 320g
dl-Alpha-Tocopheryl Acetate 364 g 364 g 364 g 364 g
Gellan Gum 200g 300g 400g 600g
Ferrous Sulfate 106g 106g 106g 106g
Choline Chloride 353 g 353 g 353 g 353 g
Calcium Pantothenate 132g 132g 132g 132g
Vitamin A Palmitate 77 g 77 g 77 g 77 g
Riboflavin 33 g 33 g 33 g 33 g
Vitamin D3 13 g 13 g 13 g 13 g
Copper Sulfate 18 g 18 g 18 g 18 g
Pyridoxine Hydrochloride 20 g 20 g 20 g 20 g
Thiamin Hydrochloride 24 g 24 g 24 g 24 g
Folic Acid 3.3 g 3.3 g 3.3 g 3.3 g
Biotin 2.5g 2.5g 2.5g 2.5g
Manganese Sulfate 1.8g 1.8g 1.8 g 1.8g
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
Phylloquinone 880 mg 880 mg 880 mg 880 mg
Sodium Selenate 90 mg 90 mg 90 mg 90 mg
Cyanocobalamin 88 mg 88 mg 88 mg 88 mg
Potassium Hydroxide Q.S. Q.S. Q.S. Q.S.
[0089] The concentrated liquid human milk fortifier is prepared by
solubilizing
and combining/mixing ingredients into a homogeneous aqueous mixture which is
subjected
to a sufficient thermal treatment and aseptic filling to achieve long term
physical and
microbial shelf stability. The term "shelf stability" as used herein means
that the
concentrated liquid human milk fortifier is resistant to separation and
precipitation for time
period after manufacture of at least three months, and preferably at least six
months.
[0090] To begin the manufacturing process, macronutrients (carbohydrate,
protein, fat, and minerals) are combined in several slurries together and with
water. This
blend is subjected to an initial heat treatment and then tested to verify
proper nutrient
levels. An intermediate aqueous carbohydrate-mineral (CHO-MIN) slurry is
prepared by
heating appropriate amount of water to 140-160 F. With agitation, the
following soluble
ingredients are added: maltodextrin, potassium citrate, magnesium chloride,
potassium
chloride, sodium chloride, and choline chloride. The carbohydrate-mineral
slurry is held at
130-150 F under agitation until added to the blend.
[0091] An intermediate oil slurry is prepared by heating MCT oil and coconut
oil
to 150 to 170 C and then adding distilled monoglycerides with agitation for a
minimum of
10 minutes in order to the ingredient to dissolve. Soy oil, vitamin A
palmitate, vitamin D3,
di-alpha-tocopheryl-acetate, phylloquinone, ARA-containing oil, DHA-containing
oil, and
carotenoid premix are then added with agitation to the oil blend. Insoluble
mineral calcium
source, and ultra micronized tricalcium phosphate is added to the oil. Gellan
gum and
OSA-modified starch are then added to the oil blend with proper agitation. The
oil blend
slurry is maintained at 130-150 F under agitation until added to the blend.
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
26
[0092] The blend is prepared by combining the ingredient water, casein
hydrolysate, all of the CHO-MIN slurry and whole oil blend slurry. The blend
is
maintained at 120 F for a period of time not to exceed two hours before
further
processing.
[0093] The blend is then homogenized using one or more in-line homogenizers at
pressures from 1000-4000 psig with or without a second stage homogenization
from 100-
500 psig followed by heat treatment using a UHTST (ultra-high temperature
short time,
292-297 F for 5-15 seconds) process. After the appropriate heat treatment,
the batch is
cooled in a plate cooler to 33-45 F and then transferred to a refrigerated
holding tank,
where it is subjected to analytical testing.
[0094] The next step in the manufacturing process involves adding vitamins,
trace
minerals, other ingredients, and water in order to reach the final target
total solids and
vitamin/mineral contents. The final batch is filled into a suitable container
under aseptic
conditions or treated with a terminal sterilization process so the product
will be stable at
room temperature for an extended shelf-life. Additional detail on this process
is provided in
the following paragraphs.
[0095] A trace mineral/vitamin/nutrient solution (STD1) is prepared by heating
water to 80-100 F and adding the following ingredients with agitation:
potassium citrate,
ferrous sulfate, zinc sulfate, copper sulfate, manganese sulfate, sodium
selenate, pyridoxine
hydrochloride, riboflavin, thiamine hydrochloride, cyanocobalamin, folic acid,
calcium
pantothenate, niacinamide, biotin, m-inositol, nucleotide/choline premix, L-
carnitine, L-
Leucine, and L-tyrosine.
[0096] A vitamin C solution (STD2) is prepared by adding ascorbic acid to a
water solution with agitation.
[0097] All STD1 and STD2 solutions are then added to the refrigerated batch,
with agitation. The appropriate amount of ingredient dilution water is then
added to the
batch to achieve a target total solids level of 29.0- 32.0%. The final batch
is then subjected
to appropriate thermal treatment and filled into a suitable container (e.g.,
pouches 10, 110,
120) under aseptic packaging conditions and processes. The term "aseptic
packaging" as
used herein, unless otherwise specified, refers to the manufacture of a
packaged product
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
27
without reliance upon "retort packaging", wherein the nutritional liquid and
package are
sterilized separately prior to filling, and then are combined under sterilized
or aseptic
processing conditions to form a sterilized, aseptically packaged, nutritional
liquid product.
The term "retort packaging" as used herein, and unless otherwise specified,
refers to the
common practice of filling a container, most typically a metal can or other
similar package,
with a nutritional liquid and then subjecting the liquid-filled package to the
necessary heat
sterilization step, to form a sterilized, retort packaged, nutritional liquid
product.
Example 5
[0098] In Example 5, four separate concentrated liquid human milk fortifiers
were
prepared and the overall stability in terms of amount of phase separation
(emulsion
stability), sediment at the bottom of the container, and creaming at the top
of the liquid, of
each was evaluated at 24 hours after manufacture. Each of the four tested
concentrated
liquid human milk fortifiers was based on the concentrated liquid human milk
fortifier of
Example 2 above.
[0099] The first concentrated liquid human milk fortifier was identical to
that of
Example 2 except that it did not contain any USA-modified corn starch and did
not contain
any low acyl gellan gum. The second fortifier was identical to that of Example
2 except
that it did not contain any low acyl gellan gum. The third fortifier was
identical to that of
Example 2 except that it did not contain any USA-modified corn starch. The
fourth
fortifier was identical to that of Example 2. Each of the four fortifiers was
prepared in
accordance with the manufacturing process of Examples 1-4.
[00100] Upon evaluation, the first fortifier (no USA-modified corn starch and
no
low acyl gellan gum) showed nearly complete phase separation of the oil and
water phases,
and showed both heavy creaming at the top of the liquid and heavy sediment at
the bottom
of the container. See Figure 22.
[00101] Upon evaluation, the second fortifier (no low acyl gellan gum) showed
both heavy creaming at the top of the liquid and heavy sediment at the bottom
of the
container. See Figure 23.
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
28
[00102] Upon evaluation, the third fortifier (no OSA-modified corn starch)
showed nearly complete phase separation of the oil phase and the water phase.
See Figure
24.
[00103] Upon evaluation, the fourth fortifier (containing both OSA-modified
corn
starch and low acyl gellan gum) showed no phase separation, no creaming, and
no
sediment. See Figure 25. The stabilizing system of a combination of OSA-
modified corn
starch and low acyl gellan gum showed a synergistic interaction and allowed
for the
manufacture of physically stable concentrated liquid human milk fortifier
containing
extensively hydrolyzed casein and a high level of insoluble calcium salts
without causing
defects in emulsion stability and sediment fall out.
Gelled Human Milk Fortifier
[00104] Another suitable human milk fortifier suitable for packaging in the
pouches 10, 110, 210 is a gelled human milk fortifier. The gelled human milk
fortifier
generally comprises protein, fat, and carbohydrate in a stable, concentrated
gel that is shear
thinning and stabilizer-free. The term "gelled human milk fortifier" as used
herein means a
human milk fortifier that is in the form of a colloid in which the dispersed
phase has
combined with the dispersion medium to produce a semisolid material, such as a
jelly,
pudding or yogurt. A "gelled human milk fortifier" has a viscosity at room
temperature of
greater than 800, 900 or even 1000 cps as measured using a Brookfield
Viscometer
(spindle 61, 60 rpm, after 10 seconds of rotation). The term "shear thinning"
as used
herein means an effect where viscosity decreases with increasing rate of shear
stress.
[00105] Various embodiments of the gelled human milk fortifiers can be
substantially free of any optional or selected essential ingredient or feature
described
herein, provided that the remaining gelled human milk fortifier still contains
all of the
required ingredients or features as described herein. In this context, and
unless otherwise
specified, the term "substantially free" means that the selected gelled human
milk fortifier
contains less than a functional amount of the optional ingredient, typically
less than 0.1%
by weight, and also including zero percent by weight of such optional or
selected essential
ingredient. The gelled human milk fortifiers can comprise, consist of, or
consist essentially
of the essential elements, as well as any additional or optional ingredients,
components, or
limitations described herein or otherwise useful in the gelled human milk
fortifier.
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
29
Product Form
[00106] The gelled human milk fortifier is shear thinning such that they can
easily
be converted from the gelled form to a liquid form by shaking and/or kneading
prior to
being poured from the pouch 10, 110, 210. Generally, the gelled human milk
fortifier has a
viscosity of greater than 1000 cps at room temperature as measured using a
Brookfield
Viscometer Model DVII (spindle 61, 60 rpm, after 10 seconds rotation). The
gelled human
milk fortifier has a shaken viscosity, as defined herein, of from about 20 cps
to about 200
cps, or even from about 20 cps to about 150 cps, or even from about 20 cps to
about 100
cps, or even from about 20 cps to about 80 cps, or even from about 50 cps to
about 95 cps.
Generally, as the gelled human milk fortifier ages, the shaken viscosity will
increase
slightly.
[00107] The gelled human milk fortifier has a gel strength, as defined herein,
of
from about 25 grams to about 200 grams, or even from about 50 grams to about
200 grams,
or even from about 75 grams to about 150 grams. The gelled human milk
fortifier has a
shaken gel strength of less than 10, or even less than 5 or even zero. In one
suitable
embodiment, the shaken gel strength is zero.
[00108] The gelled human milk fortifiers can be stabilizer free. That is, they
may
be formulated to not include any stabilization agent for keeping precipitation
and/or settling
from occurring in the fortifier. By formulating the gelled human milk
fortifier to be
stabilizer free, it becomes more acceptable worldwide. Specifically, the
gelled human milk
fortifier can be formulated to be carrageenan-free.
[00109] The gelled human milk fortifier is generally formulated to have a
caloric
density of at least about 1.25 kcal/m1 (37 kcal/fl oz), including from about
1.4 kcal/ml (42
kcal/fl oz) to about 5 kcal/ml (149 kcal/fl oz), and also including from about
1.5 kcal/m1
(44 kcal/fl oz) to about 2.5 kcal/m1 (74 kcal/fl oz), and also including from
about 1.9
kcal/ml (56 kcal/fl oz) to about 2.0 kcal/ml (59 kcal/fl oz). The gelled human
milk fortifier
is preferably formulated to provide fortified human milk having an osmolality
of less than
about 400 mOsm/kg water, preferably from about 300 mOsm/kg water to about 400
mOsm/kg water.
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
Macronutrients
[00110] The gelled human milk fortifiers of the present disclosure comprise
carbohydrate, fat, and protein macronutrients of sufficient types and amounts,
that when
used in combination with human milk or other infant feeding formula, they help
meet the
nutritional needs of the infant, especially the premature infant. The
concentration of these
macronutrients in the various embodiments of the present disclosure includes
the ranges
described hereinafter.
Protein
[00111] The gelled human milk fortifier comprises a protein suitable for use
with
infants, especially preterm infants, at concentrations ranging from about 10%
to about
30%, including from about 10% to about 25%, and also including from about 15%
to about
25%, on a dry weight basis. In some embodiments, the protein may be at a
concentration
of less than 10%.
[00112] In one suitable embodiment, the gelled human milk fortifier is
prepared
by aseptic processing, which comprise the requisite protein concentrations
with a specific
blend of casein and whey protein. The blend includes from about 40% to about
80% by
weight of whey protein, including from about 50% to about 70% by weight whey
protein,
including from about 55% to about 70% by weight whey protein, and including
from about
60% to about 70% by weight whey protein, in combination with from about 20% to
about
60% by weight of casein protein, including from about 30% to about 50% by
weight of
casein protein, including from about 20% to about 50% by weight casein
protein, including
from about 20% to about 45% by weight casein protein, including from about 20%
to about
40% by weight casein protein, including from about 20% to about 30% casein
protein. It
has been found that these particular blends of whey protein and casein protein
provide for a
suitable gelled human milk fortifier that can be prepared by aseptic
processing.
[00113] In some embodiments, in addition to the whey protein and casein
protein
outlined above, the gelled human milk fortifier may contain additional
protein. Suitable
additional protein may include soy protein hydrolysate, casein protein
hydrolysate, whey
protein hydrolysate, rice protein hydrolysate, potato protein hydrolysate,
fish protein
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
31
hydrolysate, egg albumen hydrolysate, gelatin protein hydrolysate,
combinations of animal
and vegetable protein hydrolysates, and combinations thereof.
[00114] Proteins suitable for use in the gelled human milk fortifier may
include
intact or hydrolyzed proteins, free amino acids, or combinations thereof. Non-
limiting
examples of suitable proteins include hydrolyzed, partially hydrolyzed or non-
hydrolyzed
protein, and can be derived from any known or otherwise suitable source such
as milk (e.g.,
casein, whey, lactose-free milk protein isolates), animal (e.g., meat, fish),
cereal (e.g., rice,
corn), vegetable (e.g., soy), or combinations thereof. The protein can
include, or be
entirely or partially replaced by, free amino acids known or otherwise
suitable for use in
nutritional products, non-limiting examples include L-alanine, L-arginine, L-
asparagine, L-
aspartic acid, L-carnitine, L-cystine, L-glutamic acid, L-glutamine, glycine,
L-histidine, L-
isoleucine, L-leucine, L-lysine, L-methionine, L-phenylalanine, L-proline, L-
serine, L-
taurine, L-threonine, L-tryptophan, L-tyrosine, L-valine, and combinations
thereof.
Carbohydrate
[00115] The gelled human milk fortifiers comprises a carbohydrate suitable for
use with infants, especially preterm infants, at concentrations most typically
ranging up to
about 75% by weight on a dry weight basis, including from about 10% to about
50%, and
also including from about 20% to about 40%, by weight on a dry weight basis.
Carbohydrates suitable for use in the gelled human milk fortifiers include
hydrolyzed or
intact, naturally and/or chemically modified, starches sourced from corn,
tapioca, rice or
potato, in waxy or non-waxy forms. Other non-limiting examples of suitable
carbohydrate
sources include hydrolyzed cornstarch, maltodextrin (i.e. non-sweet, nutritive
polysaccharide having a DE value less than 20), corn maltodextrin, glucose
polymers,
sucrose, corn syrup, corn syrup solids (i.e., polysaccharide having a DE value
greater than
20), glucose, rice syrup, fructose, high fructose corn syrup, indigestible
oligosaccharides
such as fructooligosaccharides (FOS), and combinations thereof The
carbohydrates may
comprise lactose or can be substantially free of lactose.
[00116] One embodiment of the gelled human milk fortifier includes a non-
reducing carbohydrate component, which may represent from about 10% to 100%,
including from about 80% to 100%, and also including 100%, by weight of the
total
carbohydrate. The selection of a non-reducing carbohydrate may enhance the
product
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
32
stability and is generally better tolerated by infants, especially premature
infants. Non-
limiting examples of non-reducing carbohydrates include sucrose or other
carbohydrates
that do not readily oxidize or react with Tollen's, Benedict's, or Fehling's
reagents. The
gelled human milk fortifier therefore includes embodiments comprising a
carbohydrate
component, wherein the carbohydrate component comprises a mono- and/or
disaccharide
such that at least about 50%, including from about 80% to 100%, and also
including 100%,
of the mono- and/or disaccharide is a non-reducing carbohydrate.
Fat
[00117] The gelled human milk fortifiers also comprises a fat component
suitable
for use with infants, especially preterm infants, at concentrations most
typically ranging up
to about 40% by weight on a dry weight basis, including from about 10% to
about 40%,
and also including from about 15% to about 37%, and also including from about
18% to
about 30%, by weight on a dry weight basis. Fats suitable for use in the
gelled human milk
fortifier may include coconut oil, soy oil, corn oil, olive oil, safflower
oil, high oleic
safflower oil, MCT oil (medium chain triglycerides), sunflower oil, high oleic
sunflower
oil, structured triglycerides, palm and palm kernel oils, palm olein, canola
oil, marine oils,
cottonseed oils, and combinations thereof.
[00118] Suitable fats for use in the gelled human milk fortifier include
emulsifiers
to help the various fortifier components readily disperse when combined with
human milk.
Non-limiting examples of suitable emulsifiers include soya bean lecithin,
polyoxythylene
stearate, polyoxyethylene sorbitan mono-oleate, polyoxyethylene sorbitan
monopalmitate,
polyoxyethylene sorbitan monostearate, ammonium phosphatides, polyoxyethylene
sorbitan monolaurate, citric acid esters of mono and diglycerides of fatty
acids, tartaric acid
esters of mono and diglycerides of fatty acids, and combinations thereof.
Natural soy
lecithin is especially useful in this respect. The fat component of the gelled
human milk
fortifier may therefore optionally include any emulsifier suitable for use in
infant
nutritional products. Emulsifier concentrations in these products may range up
to about
10%, including from about 1% to about 10%, even more typically from about 1.5%
to
about 5%, by weight of the total fat component. The weight ratio of fat to
protein
(fat:protein, by weight) in the human milk fortifier is at least about 0.9,
including from
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
33
about 1 to about 5, and also including from about 2 to about 4. These ratios
may be helpful
in further stabilizing the gelled human milk fortifier.
[00119] The gelled human milk fortifier also include embodiments that
comprise,
as part of the fat component, one or more of arachidonic acid, docosahexaenoic
acid, or
combinations thereof, alone or in further combination with linoleic acid,
linolenic acid, or
both.
Vitamins and Minerals
[00120] The gelled human milk fortifier may further comprise any of a variety
of
vitamins, non-limiting examples of which include vitamin A, vitamin D, vitamin
E, vitamin
K, thiamine, riboflavin, pyridoxine, vitamin B12, niacin, folic acid,
pantothenic acid,
biotin, vitamin C, choline, inositol, salts and derivatives thereof, and
combinations thereof.
The gelled human milk fortifier includes embodiments comprising per 100 kcal
of fortifier
solids one or more of the following: vitamin A (from about 250 to about 750
IU), vitamin
D (from about 40 to about 100 IU), vitamin K, vitamin E (at least about 0.3
IU), vitamin C
(at least about 8 mg), thiamine, vitamin B12, niacin, folic acid, pantothenic
acid, biotin,
choline (at least about 7 mg), and inositol (at least about 2 mg).
[00121] The gelled human milk fortifier may also further comprise any of a
variety of minerals known or otherwise suitable for use in infant or other
nutritional
formulas, non-limiting examples of which include phosphorus, magnesium,
calcium as
described hereinbefore, zinc, manganese, copper, iodine, sodium, potassium,
chloride,
selenium, and combinations thereof. The gelled human milk fortifier also
include
embodiments comprising per 100 kcal of the fortifier solids one or more of the
following:
calcium (at least about 50 mg), phosphorus (at least about 25 mg), magnesium
(at least
about 6 mg), iodine, zinc (at least about 0.5 mg), copper, manganese, sodium
(from about
20 to about 60 mg), potassium (from about 80 to about 200 mg), chloride (from
about 55 to
about 150 mg) and selenium (at least about 0.5 mcg).
Other Optional Ingredients
[00122] The gelled human milk fortifier may further optionally comprise other
ingredients that may modify the physical, chemical, aesthetic or processing
characteristics
CA 02823150 2013-06-26
WO 2012/091886 PCT/US2011/064247
34
of the formulas or serve as pharmaceutical or additional nutritional
components when used
in the targeted population. Many such optional ingredients are known for use
in food and
nutritional products, including infant formulas, and may also be used in the
gelled human
milk fortifiers of the present disclosure, provided that such optional
materials are
compatible with the essential materials described herein, are safe and
effective for their
intended use, and do not otherwise unduly impair product performance. Non-
limiting
examples of such optional ingredients include preservatives, anti-oxidants,
various
pharmaceuticals, buffers, carotenoids, colorants, flavors, nucleotides and
nucleosides,
thickening agents, prebiotics, probiotics, sialic acid-containing materials,
and other
excipients or processing aids.
Examples
[00123] The following examples illustrate specific embodiments and/or features
of the gelled human milk fortifier. The examples are given solely for the
purpose of
illustration as many variations thereof are possible. All exemplified amounts
are weight
percentages based upon the total weight of the formulation, unless otherwise
specified.
Example 1
[00124] In this Example, a gelled human milk fortifier is prepared with the
ingredients shown in the following table.
Ingredients Qty. per 32000 lb
Water Q.S.
Condensed Skim Milk 5250 Kg
Non-Fat Milk Solids 1365 Kg
Corn Maltodextrin 1450 Kg
Corn Syrup Solids 1388 Kg
Medium Chain triglycerides 694 Kg
Whey Protein Concentrate 634 Kg
Calcium Phosphate 271 Kg
Ascorbic Acid 152 Kg
Magnesium Chloride 38.0 Kg
Potassium Citrate 12.2 Kg
Sodium Chloride 12.1 Kg
Soy Lecithin 8.84 Kg
M-Inositol 7.98 Kg
Magnesium Phosphate 5.55 Kg
M. Alpina Oil 5.35 Kg
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
Niacinamide 4.35 Kg
.Alpha-Tocopheryl Acetate 4.21 Kg
Zinc Sulfate 3.51 Kg
C. Cohnii Oil 3.45 Kg
Choline Chloride 2.90 Kg
Calcium Pantothenate 1.89 Kg
Potassium Phosphate 1.62 Kg
Ferrous Sulfate 1.64 Kg
Vitamin A PaImitate 900
Cupric Sulfate 678
Riboflavin 572
Thiamine Hydrochloride 373
Pyridoxine Hydrochloride 232
Vitamin D3 152
Folic Acid 47.9
Biotin 34.1
Manganese Sulfate 23.2
Phylloquinone 11.6
Cyanocobalamin 1.60
Sodium Selenate 0.798
Calcium Carbonate as needed
Sodium Citrate as needed
Potassium Hydroxide as needed
[00125] The gelled human milk fortifier is prepared by solubilizing and
combining ingredients into a homogeneous aqueous mixture which is subjected to
an
adequate heat treatment to achieve long term shelf stability. To begin the
manufacturing
process, the ingredients that supply the macronutrients (carbohydrate,
protein, fat and
minerals) are combined in multiple slurries together and with water. This
blend is
subjected to an initial heat treatment and then tested to verify proper
nutrient levels.
Additional detail on this process is provided in the following paragraphs.
[00126] An intermediate aqueous carbohydrate-mineral slurry is prepared by
heating water to 60-66 C. With agitation, the following soluble minerals are
added:
magnesium chloride, potassium citrate, sodium chloride, monopotassium
phosphate and
magnesium phosphate. Once fully dissolved, corn maltodextrin and corn syrup
solids are
added to the mineral solution. The carbohydrate-mineral slurry is held at 54 C
under low
agitation until added to the blend.
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
36
[00127] An intermediate oil and protein slurry is prepared by heating MCT oil
to
32-43 C and then adding DHA oil and AA oil, with agitation. A soy lecithin
emulsifier
(8.84 kg) is added with agitation to the heated oils and allowed to dissolve.
Vitamin A,
vitamin D, and vitamin K, and natural vitamin E are then added with agitation
to the oil
blend. Whey protein concentrate and tricalcium phosphate are added to the oil.
The oil
and protein slurry is maintained at 38 C under low agitation until added to
the blend.
[00128] An intermediate aqueous protein slurry is prepared by heating
ingredient
water to 49-54 C, and then adding whey protein concentrate with moderate
agitation. The
aqueous protein slurry is held at 52 C under low agitation until added to the
blend.
[00129] The blend is prepared by combining the carbohydrate-mineral slurry
with
condensed skim milk and non-fat milk solids and then adding the oil and
protein slurry and
the aqueous protein slurry. After no less than five minutes, the blend pH is
adjusted to 6.8-
7.0 using a 1N KOH solution, and thereafter maintained at 52-60 C, for a
period of time
not to exceed two hours before further processing.
[00130] The pH adjusted blend is then homogenized using one or more in-line
homogenizers at pressures from 1000-4000 psig with or without a second stage
homogenization from 100-500 psig followed by heat treatment using a HTST (high
temperature short time, 74 C for 16 seconds). After the appropriate heat
treatment, the
batch is cooled in a plate cooler to 1.0-5.0 C and then transferred to a
refrigerated holding
tank, where it is subjected to analytical testing.
[00131] The next step in the manufacturing process involves adding vitamins,
trace minerals and water to the target total solids. The final batch is
sterilized and filled
into a suitable container under aseptic conditions or treated with a terminal
sterilization
process so the product will be stable at room temperature for an extended
shelf life.
Additional detail on this process is provided in the following paragraphs.
[00132] A trace mineral solution is prepared by heating water to 27-38 C and
adding the following minerals with agitation: potassium citrate, ferrous
sulfate, zinc sulfate,
cupric sulfate, manganese sulfate, sodium selenate.
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
37
[00133] A water-soluble vitamin solution is prepared by heating water to 27-38
C. The following vitamins are added to the water with agitation: choline
chloride,
niacinamide, riboflavin, calcium pantothenate, pyridoxine hydrochloride,
thiamine
hydrochloride, m-inositol, biotin, folic acid, and cyanocobalamin.
[00134] A vitamin C solution is prepared by adding ascorbic acid tolN KOH
solution with agitation.
[00135] All three vitamin or mineral solutions are then added to the
refrigerated
batch, with agitation. The appropriate amount of ingredient dilution water is
then added to
the batch to achieve a target total solids level of 32%, and the pH is
adjusted to 7.0 with a
1N KOH solution.
Example 2
[00136] In this Example, the unshaken viscosity, shaken viscosity, unshaken
get
strength and shaken gel strength of the human milk fortifier prepared in
Example 1 is tested
at a sample aged three months and a sample aged six months.
[00137] The viscosities were measured using a Brookfield Viscometer Model
DV11+ (spindle 61, 60 rpm, after 10 second of rotation). The gel strengths
were measure
using a Stable Micro Systems TA.XT plus Texture Analyzer (1 inch ball probe,
20 mm
depth). For the shaken samples, each sample was shaken vigorously by hand for
five
seconds prior to testing.
[00138] The results of the viscosity measurements and gel strengths are shown
in
the following Table.
Sample A Sample B
(aged 3 months) (aged 6 months)
Unshaken Shaken Unshaken Shaken
Viscosity > 1000 cps 56 cps > 1000 cps 95 cps
Gel Strength 78 g 0 g 133 g 0 g
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
38
[00139] As can be seen from the data in the Table, the unshaken viscosities
for
both samples are greater than 1000 cps, while the viscosities of both shaken
samples are
substantially less (56 cps for 3 months and 95 cps for 6 months). This
indicates that in
unshaken form, a gel is present whereas after shear is applied (by shaking)
the gel easily
breaks for forms a liquid of relatively low viscosity that could easily be
poured from one of
the pouches 10, 110, 210.
[00140] Additionally as can be seen from the data in the Table, the unshaken
gel
strength for both samples is relatively high (78 grams at 3 months and 133
grams at 6
months), while the gel strengths after shaking for both samples is zero grams.
This
indicates that after shaking, the gel has transformed into a liquid that could
easily be poured
from one of the pouches 10, 110, 210.
Dose Pouches
[00141] The concentrated liquid human milk fortifier and the gel human milk
fortifier can be packaged in suitable unit dose pouches (e.g., pouches 10,
110, 210). The
term "unit dose" as used herein refers to individual, single-use, pouches of
concentrated
human milk fortifier containing a predetermined amount of human milk fortifier
that can be
used in a preparation of a predetermined amount of human milk. The unit dose
pouches
10, 110, 210 are single use containers that alone, or in combination with
other unit dose
pouches, provide sufficient human milk fortifier to supplement human milk for
immediate
use, e.g., preferably within 8-24 hours, more preferably within 0-3 hours, of
mixing with
human milk.
[00142] The amount or volume of concentrated liquid human milk fortifier or
gel
human milk fortifier in each unit dose pouch 10, 110, 210 includes those
embodiments in
which the package contains an amount suitable to prepare an infant's feeding.
In one
suitable embodiment, the unit dose pouches 10, 110, 210 typically contain
sufficient
fortifier to provide from about 0.5 g to about 10 g of fortifier solids, more
typically from
about 0.8 g to about 5.0 g of fortifier solids, and even more typically from
about 0.85 g to
about 2.0 g, of fortifier solids. The terms "fortifier solids" or "total
solids", unless
otherwise specified, are used interchangeably herein and refer to all material
components
of the compositions of the present disclosure, less water.
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
39
[00143] The amount of fortified human milk prepared for a premature infant,
for
example, typically ranges from 25 ml to 150 ml a day. Consequently, in one
suitable
embodiment, a single unit dose is the appropriate amount of fortifier solids
to fortify a 25
ml preparation. Multiple pouches 10, 110, 210 can be used to prepare larger
feeding
volumes, especially for term infants.
Aseptic Packaging
[00144] The concentrated liquid human milk fortifier and the gel human milk
fortifier can be sterilized and aseptically packaged into the pouches 10, 110,
210. The
aseptic packaging can be accomplished using any of a variety of techniques
well known to
those of ordinary skill in the formulation art, so long as the technique is
sufficient to
achieve long term shelf stability of the fortifier. Fig. 10 is a flow diagram
of one suitable
process for manufacturing a plurality of aseptically sterilized pouches 10,
110, 210 suitable
for containing the concentrated liquid human milk fortifier, the gel human
milk fortifier, or
any other suitable aseptic product. While the following description of the
aseptic
packaging process is provided with respect to the pouch 10 illustrated in
Figs. 1-6, it is
understood that the pouches 110, 210 of Figs. 8 and 9 can be processed in
substantially the
same manner.
[00145] In this embodiment, a web of plastic sheeting (e.g., the two layered
laminate illustrated in Fig. 7A) is fed from a suitable web feeding device 80
(e.g., unwound
from a roll) to a web alignment device 82 as indicated in the flow chart in
Fig. 10. In one
suitable embodiment, the web has a width sufficient to make four pairs of
pouches 10 in
side-by-side relationship (Fig 11A-C). It is understood, however, that the
width of the web
can be sufficient to make more or fewer pairs of pouches 10 in side-by-side
relationship.
From the web alignment device 82 and as indicated in Fig. 10, the web is
directed to a
coding station 84 wherein the web is laser coded (or otherwise printed) with
indicia, e.g.,
batch number, expiration date, current time and date. It is contemplated that
other indicia
can be printed on the pouch 10 including, for example, the manufacturer's
name, the trade
name of the product, the generic name of the product, direction of use,
nutritional
information of the product, and/or quantity of the product. The web is then
fed to a laser
scoring station 86 wherein the web is scored along three longitudinal lines
(Figs. 10 and
11A) to delineate the four separate pairs of pouches.
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
[00146] The web next enters a sterilization station 88 wherein the web passes
through a peroxide bath, thereby sterilizing the entire web, as both sides of
the web are
brought into direct contact with a peroxide solution. It is contemplated that
other sterilants
(e.g., oxonia) or forms of sterilization (e.g., UV light, electron beam) can
be used. Once
the web has passed through the peroxide bath, the web is dried by blowing
sterile air
thereon at a drying station 90. While still in a sterile environment, the web
is directed to a
web separation station 92 and a web folding station 94. More specifically, the
web is
separated into four lanes at the web separation station 92 as it is pulled
across respective
forming collars. Each of the four lanes is defined by segments of the web.
Each of the
web segments are folded by the respective forming collar. Thus, in the
described
embodiment, the four forming collars both separate the web into segments and
fold the
segments. In other words, the four forming collars collectively define both
the web
separation station 92 and the web folding station 94. It is understood,
however, that the
web separation station 92 and the web folding station 94 can be separate,
discrete stations.
It is also understood that the forming collars can be any suitable device(s)
capable of
dividing the web into a plurality of web segments and folding each of the web
segments.
[00147] As illustrated in Fig. 11B, the respective forming collar folds each
of the
side edges of the respective web segment inward (i.e., in the direction of the
arrows of Fig.
11B) toward the longitudinal center line of the web segment at the folding
station 94. As
seen in Fig. 11B, each of the web segments are folded about a fill pipe. After
the web
segment is folded longitudinally, each of the web segments are longitudinally
heat sealed at
a longitudinal seal station 96 wherein the overlying portion of the web
segment is bonded
to the underlying portion of the web segment along each of the side edges to
form the side
edge segments 25c of the seal lines 25.
[00148] Next, each of the web segments is perforated along a longitudinal
perforation line located between the tubes of each of the web segments at a
longitudinal
perforation station 97 (Figures 10 and 11B). Once each of the web segments
move past a
fill nozzle disposed on the respective fill pipe, the web segments are
directed to a
horizontal sealing station 99 wherein each of the web segments are heat sealed
to sealingly
bond the overlying portion to the underlying portion of the blank to form one
of the end
segments 25a and the inboard seal segment 25d of the seal lines 25. As seen in
Fig. 11C,
two pouches 10, which are separated by the perforated center line, are formed
from each of
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
41
the web segments and the respective fill nozzle is disposed within the
interior space of the
pouch. The pouches 10 are then filled at a filling station 98 wherein both of
the pouches of
each of the four web segments are filled with a predetermined amount of
sterilized product.
Next, each of the pouches 10 is moved past the respective fill nozzle and is
heat sealed
shut, which forms the other end segment 25b of the seal lines 25, at the
horizontal sealing
station 99. The lines of weakness 30, 32 for each of the pouches 10 are formed
at a tear
notch and cutting station 302.
[00149] After the pouches 10 are filled with product and sealed, they are
transferred to weight and leak inspection stations 304 wherein each of the
pouches 10 are
weighed and checked for leakage. Pouches 10 that pass inspection are incubated
at an
incubation station 305 and tested for spoilage at a spoilage inspection
station 306. Then,
pouches are packaged in pluralities into suitable secondary packaging, e.g.,
opaque
cardboard box 500, 500' as illustrated in Figs. 12, 16A and 16B at a secondary
packaging
station 307. Figs. 12,16A and 16B illustrate different embodiments of suitable
secondary
packaging 500, 500' for the pouches 10. Pouches 10 that fail inspection are
discarded.
[00150] When the product is a liquid human milk fortifier (e.g., the
concentrated
liquid human milk fortifier or the gelled human milk fortifier described
above), the product
can be sterilized by heat treatment via a high temperature short time (HTST)
process or an
ultra high temperature (UHT) process to sufficiently reduce the bioburden
before the
pouches 10 are filled. The above described packaging process of a sterile
product, allows
some products (e.g., some embodiments of the concentrated liquid human milk
fortifier and
the gelled human milk fortifier described above) to maintain commercially
sterility over an
extended shelf-life without the need for refrigeration even if the product is
low acid (i.e.,
has a pH greater than 4.6) and has water activity greater than 0.85.
[00151] In one embodiment, the liquid human milk fortifier is photosensitive.
That is, the vitamins in liquid human milk fortifier will degrade more slowly
when not
exposed to light, and conversely, will degrade more rapidly when exposed to
light. When
the liquid human milk fortifier is photosensitive, the opaque cardboard box
500 inhibits the
pouches 10 container therein from being exposed to light and thereby extends
the shelf life
of the liquid human milk fortifier.
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
42
Leak Detection Inspection System
[00152] In one suitable inspection station 304, each of the pouches 10 are
transferred through an in-line checkweigher were it is weighed. Any pouch 10
having a
weight outside an acceptable weight range is rejected. The pouches 10 that
pass the inline
checkweigher are aligned and conveyed into a high voltage leak detection
(HVLD)
inspection system. In this system, the seal integrity of each of the pouches
10 is non-
destructively inspected by applying high voltage to the sealed liquid-filled
pouch. The
system is designed to conduct electric current through the pouch 10 and
measure the
amount of current that passes through the pouch. A pouch 10 with a leak (i.e.,
a faulty
seal) will transfer more current to a ground electric than a pouch having a
seal with good
integrity. The seals of the pouch 10 act as an insulator to the liquid inside.
Any pouch 10
that does not pass inspection (i.e., has a current above an acceptable range)
is automatically
rejected.
[00153] More specifically, once the pouches 10 enter the high voltage leak
detection inspection system, they pass to a press that applies a compression
force to each of
the pouches. The compression force pushes liquid into any weak areas of the
pouch body
and/or pouch seal. While compressed, each of the pouches 10 is conveyed past a
series of
rollers and metal electrode brushes in the inspection station wherein high
voltage power is
applied the pouches. In one suitable configuration, the voltage is transferred
from an upper
electrode positioned above the pouch through the pouch 10 to ground electrode
positioned
beneath the pouch. In other words, the pouch 10 completes the circuit between
the upper
electrode and the ground electrode, which provides a measurable volume of
electric current
through the pouch.
[00154] A pouch 10 with good seal integrity will provide a lower voltage
output
as compared to a pouch with poor seal integrity, which provides a higher
voltage output.
Thus, the high voltage leak detection inspection machine determined if each of
the pouches
is "good" or "bad" based on the measured voltage relative to a voltage
threshold, which
is a pre-determined set point. If the measured voltage is below the threshold,
the pouch
will be transferred to an outfeed conveyor for subsequent secondary packaging.
If the
measured voltage is above the threshold, the pouch 10 will be transferred to a
reject bin.
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
43
Secondary Container
[00155] In one suitable embodiment, the cardboard box 500 (broadly, the
secondary container) includes a generally rectangular base section 502 and a
lid 501
hingely attached to the base (Figs. 12 and 17-21). The base section 502 and
lid 501 are
indicated generally by their respective reference numbers. The base section
502 includes a
bottom wall 504, four side walls 506 extending up from the bottom wall, and a
top wall
508. As seen in Fig. 21, the top wall 508 of the base section 502 extends
along only a
portion of a length of the box 500. For example, in the illustrated
embodiment, the box 500
has a length L of about 12 cm and the top wall 508 has a length L' of 2.5 cm.
It is
understood that the box 500 and top wall 508 can have different lengths. It is
also
understood that the ratio between the length of the box 500 and the length of
the top wall
508 can be different. It is further understood that the box 500 can be shaped
other than
rectangular and be constructed from other suitable materials (e.g., plastic).
[00156] The lid 501, which is formed integrally with the base section 502, has
an
upper wall 510 and a pair of tapered sidewalls 503 extending downward from the
upper
wall. An end wall 505 extends downward from the upper wall 510 and between the
sidewalls 503.. The lid 501 is pivotally about a living hinge 507 between a
closed position
(Figs. 17-19) and an opened position (Figs. 12, 20 and 21). The living hinge
507 is located
between the top wall 508 of the base section 502 and the upper wall 510 of the
lid 501. In
one suitable embodiment, the weight of the lid 501 is sufficient to bias the
lid about the
living hinge toward to the closed position. The end wall 505 of the lid 501
includes a tab
511 adapted for insertion into a slot 513 in one of the side walls (i.e., a
front wall) of the
box 500 for holding the lid 501 in the closed position. The tab 511 can be
seen inserted
into the slot 513 in Fig. 17. It is understood that the lid 501 can be hingely
attached to the
base section 502 in other suitable manners besides the illustrated living
hinge 507. It is
further understood that the lid 501 can be formed separate from the base
section 502 and
attached thereto.
[00157] A pair of hold-downs 509 are located adjacent the ends of the living
hinge 507 to provide rigidity and support to the box 500 about the living
hinge. In the
illustrated embodiment, each of the hold-downs 509 are flaps that extend
outward from the
top wall of the base section 502. Each of the flaps are folded about a pair of
fold-lines and
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
44
inserted into an associated slot in one of the sidewalls of the base section
(Fig. 21). One of
the fold-lines is adjacent the top wall 508 of the base section 502 and the
other is adjacent
the slot in the respective sidewall. Each of the flaps includes a head portion
(not shown) to
inhibit the flap from being pulled (or otherwise withdrawn) from the
associated slot.
[00158] As seen in Figs. 12 and 20, an interior floor 521 of the box 500 is
tented
or peaked along its center line 533. That is, the interior floor 521 is
highest at its center
and slopes downward toward each of its sides. In one suitable embodiment, the
interior
floor 521 of the box 500 is defined by an insert that is formed separate from
the other
components of the box and rest on top of the bottom wall of the base section.
It is,
understood, however, that the interior floor 521 can be formed integrally with
another
component of the box 500, such as, the bottom wall of the base section.
Method of Use
[00159] In use, a user removes a pair of the joined pouches 10 from the
cardboard
box 500 of Fig. 12 (or the cardboard box 500' of Figs. 16A and 16B) and
separates them by
tearing along the perforated center line that divides the two, joined pouches.
Once the
pouches 10 are separated, the user inspects the contents of one of the pouches
through the
transparent front and back panels to determine if the product has separated or
spoiled. If
the product has separated (or mixing is otherwise desired), the user can
manually knead (or
otherwise manipulate) the product within the pouch 10 as described above to
thoroughly
mix the product insitu. Once the user observes that the product is thoroughly
mixed, the
user manually grips the pouch 10 by its grip portion 66 and tears the grip
portion along the
lines of weakness 30, 32 to completely remove the grip portion from the pouch
10. In
doing so, the user opens the pouch 10 by tearing through the spout 62 to form
the spout
opening 63 (Figs. 6 and 15).
[00160] The product can be poured or squeezed from the pouch 10. In one
embodiment, the product is a consumable product that can be consumed directly
from the
pouch 10. In another embodiment, the product is a consumable product intended
to be
mixed with another product. For example, if the product is a human milk
fortifier (e.g., the
concentrated liquid human milk fortifier or the gelled human milk fortifier
described
above), the human milk fortifier can be dispensed directly into a container
(e.g., infant
bottle B) containing human milk M (or other suitable infant formula) as
illustrated in Fig.
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
13. In such an embodiment, the resulting fortified human milk or fortified
infant formula is
suitable for oral feeding to an infant, including a premature infant.
General
[00161] All percentages, parts and ratios as used herein, are by weight of the
total
composition, unless otherwise specified. All such weights as they pertain to
listed
ingredients are based on the active level and, therefore, do not include
solvents or by-
products that may be included in commercially available materials, unless
otherwise
specified.
[00162] Numerical ranges as used herein are intended to include every number
and subset of numbers within that range, whether specifically disclosed or
not. Further,
these numerical ranges should be construed as providing support for a claim
directed to any
number or subset of numbers in that range. For example, a disclosure of from 1
to 10
should be construed as supporting a range of from 2 to 8, from 3 to 7, from 5
to 6, from 1
to 9, from 3.6 to 4.6, from 3.5 to 9.9, and so forth.
[00163] All references to singular characteristics or limitations of the
present
disclosure shall include the corresponding plural characteristic or
limitation, and vice versa,
unless otherwise specified or clearly implied to the contrary by the context
in which the
reference is made.
[00164] All combinations of method or process steps as used herein can be
performed in any order, unless otherwise specified or clearly implied to the
contrary by the
context in which the referenced combination is made.
[00165] When introducing elements of the present invention or the preferred
embodiment(s) thereof, the articles "a", "an", "the" and "said" are intended
to mean that
there are one or more of the elements. The terms "comprising", "including" and
"having"
are intended to be inclusive and mean that there may be additional elements
other than the
listed elements.
CA 02823150 2013-06-26
WO 2012/091886
PCT/US2011/064247
46
[00166] As various changes could be made in the above constructions without
departing from the scope of the invention, it is intended that all matter
contained in the
above description or shown in the accompanying drawings shall be interpreted
as
illustrative and not in a limiting sense.