Note: Descriptions are shown in the official language in which they were submitted.
CA 02823228 2013-06-27
WO 2012/089776 PCT/EP2011/074179
- 1 -
PROCESS FOR REMOVING SULPHUR-CONTAINING CONTAMINANTS FROM
A GAS STREAM
Field of the invention
The present invention relates to a process for
removing sulphur-containing contaminants, including
hydrogen sulphide, from a gas stream.
Background of the invention
The removal of sulphur-containing compounds from gas
streams comprising such compounds has always been of
considerable importance in the past and is even more so
today in view of continuously tightening environmental
regulations. This holds for hydrogen sulphide-containing
gases that have become available in for example oil
refineries and combustion gases obtained from a coke-
oven. Sulphur contaminants in such gases include apart
from hydrogen sulphide, carbonyl sulphide, carbon
disulphide and mercaptans. Considerable effort has been
spent to find effective and cost-efficient means to
remove these undesired compounds. In addition, such gas
streams may also contain varying amounts of carbon
dioxide which depending on the use of the gas stream
often have to be removed at least partly.
One well-known method that is used to treat certain
process streams that contain hydrogen sulphide to recover
elemental sulphur is the Claus process. The Claus process
is a two-step process that includes a burner (oxidation)
step followed by a catalytic step wherein use is made of
one or more catalytic stages. In the oxidation step, the
hydrogen sulphide of a feed stream is partially oxidized
by combustion with oxygen to form a gas stream containing
sulphur dioxide. The un-reacted hydrogen sulphide and the
formed sulphur dioxide contained in the combustion gas
CA 02823228 2013-06-27
WO 2012/089776 PCT/EP2011/074179
- 2 -
can undergo the Claus reaction whereby they are reacted
to form elemental sulphur. Further in the Claus process,
un-reacted hydrogen sulphide and sulphur dioxide in the
combustion gas are catalytically reacted in accordance
with the Claus reaction by passing the combustion gas
over a Claus catalyst which provides for a lower Claus
reaction temperature. While the Claus process is very
effective at providing for the recovery of a major
portion of the sulphur in its feed stream, conversion is
limited by equilibrium in the Claus process, and so the
Claus tail gas still contains appreciable amounts of
sulphur compounds such as hydrogen sulphide, carbonyl
sulphide and sulphur dioxide. This does not only apply to
sulphur recovery with a two-bed catalytic Claus plant,
but also to Claus plants with three or more catalytic
beds. The tail gas from a Claus process therefore is
usually subjected to a hydrogenation treatment to further
reduce the amounts of sulphur dioxide, COS, CS2, and
mercaptants by converting them into hydrogen sulphide,
followed by a treatment wherein use is made of an amine
that selectively absorbs hydrogen sulphide. Upon
regeneration of the hydrogen sulphide-rich amine stream a
gas stream enriched in hydrogen sulphide can be recovered
which can subsequently be recycled to the Claus unit. The
combination of the hydrogenation and amine treatments can
be a so-called Tail gas Treating (TGT) process. The tail
gas treated in such a TGT process still contains
noticeable amounts of hydrogen sulphide (in the order of
100 ppm sulphur) and other sulphur species such as
carbonyl sulfide. For that reason the tail gas will need
to be oxidized in an incinerator before it can be
discarded into the air as vent gas or used for other
purposes.
CA 02823228 2013-06-27
WO 2012/089776 PCT/EP2011/074179
- 3 -
Hence, there is an ongoing need for improved sulphur
recovery processes that provide for high sulphur recovery
and better operating efficiencies, preferably with lower
capital costs. With increasingly more stringent sulphur
emission standards, there is also a need for sulphur
recovery processes that provide for even greater sulphur
recoveries from process streams containing sulphur
compounds than are provided by conventional sulphur
recovery systems. Simultaneously refineries are under
increasing regulatory pressure to find outlet to high
sulphur residual streams such as heavy residue and FCC
slurries. Some of these are currently used to produce
bunker fuel as maritime fuel. However, future regulatory
pressure, notably MARPOL IV, will limit the use of these
high sulphur fuels for maritime fuel use. Current use
for these high sulphur residual stream is as feed for
gasification units. However, these are complex and
expensive units.
Summary of the invention
It has now been found that an improved removal of
sulphur-containing contaminants from a gas stream can be
established, as well as a decrease in hardware and an
increase in energy efficiency, when a Claus process is
part of a particularly integrated multi-step process for
removing sulphur-containing contaminants from a gas
stream.
Accordingly, the present invention relates to a
process for removing sulphur-containing contaminants,
including hydrogen sulphide, from a gas stream comprising
the steps of:
(a) subjecting the gas stream to an oxidation treatment;
(b) extracting heat from the gas stream as obtained in
step (a);
CA 02823228 2013-06-27
WO 2012/089776 PCT/EP2011/074179
- 4 -
(c) reacting hydrogen sulphide present in the cooled gas
stream as obtained in step (b) with sulphur dioxide at
elevated temperature and in the presence of a catalyst to
obtain a gas stream which comprises sulphur and water;
(d) separating sulphur from the gas stream obtained in
step (c), thereby obtaining a hydrogen sulphide-lean gas
stream;
(e) subjecting at least part of the hydrogen sulphide-
lean gas stream as obtained in step (d) to an oxidation
treatment to obtain a sulphur dioxide-enriched gas
stream;
(f) extracting heat from the sulphur dioxide-enriched gas
stream as obtained instep (e);
(g) subjecting a sulphur-containing residual hydrocarbon
product to an oxidation treatment to obtain a gas stream
which comprises sulphur dioxide;
(h) extracting heat from the gas stream which comprises
sulphur dioxide as obtained in step (g);
(i) subjecting at least part of the cooled sulphur
dioxide-enriched gas stream as obtained in step (f) and
at least part of the cooled gas stream which comprises
sulphur dioxide as obtained in step (h) to a quench
process;
(j) contacting at least part of the quenched gas stream
as obtained in step (i) with an absorption solvent that
absorbs sulphur dioxide to obtain a sulphur dioxide-
enriched absorption solvent and a sulphur dioxide-
depleted gas stream;
(k) removing sulphur dioxide from at least part of the
sulphur dioxide-enriched absorption solvent as obtained
in step (j) to obtain a sulphur dioxide-depleted
absorption solvent and a sulphur dioxide-enriched gas
stream; and
CA 02823228 2013-06-27
WO 2012/089776 PCT/EP2011/074179
- 5 -
(1) recycling at least part of the sulphur dioxide-
enriched gas stream as obtained in step (k) to step (c).
In accordance with the present invention gas streams
can be obtained that contain such small amounts of
sulphur-containing contaminants that they can
advantageously directly be vented into the air or used
for different purposes.
Detailed description of the invention
The present invention relates to a process for
removing sulphur-containing contaminants, including
hydrogen sulphide, from an acid gas stream. The acid gas
stream to be treated in accordance with the present
invention can be any gas stream comprising sulphur-
containing contaminants. The process according to the
invention is especially suitable for gas streams
comprising sulphur-containing contaminants and optionally
also various amounts of carbon dioxide. Suitably the
total gas stream to be treated comprises in the range of
from 30 to 100 vol% hydrogen sulphide and from 0 to 60
vol% carbon dioxide, based on the total gas stream.
Preferably, the gas stream to be treated comprises from
55 to 100 vol% hydrogen sulphide and from 0 to 45 vol%
carbon dioxide, based on the total gas stream.
In step (a) the oxidation treatment is carried at a
temperature in the range of from 980-2000 C, preferably
in the range of from 1090-1540 C, and a pressure in the
range of from 1.29-2.05 bara, preferably in the range of
from 1.56-2.05 bara. Preferably, the molar ratio of
hydrogen sulphide to oxide in step (a) is in the range of
from 2:1-3:1.
Step (a) can suitably be carried out in a burner.
Prior to such an oxidation treatment in step (a) the gas
stream can suitably be subjected to a hydrogen sulphide
CA 02823228 2013-06-27
WO 2012/089776 PCT/EP2011/074179
- 6 -
enrichment treatment. In such an enrichment treatment
carbon dioxide and hydrocarbons can be removed from the
gas stream, whereas hydrogen sulphide can be retained in
the gas stream which is to be subjected to (a) by using a
particular absorbent that selectively absorbs hydrogen
sulphide. Preferably, the gas stream to be subjected to
the oxidation treatment in step (a) contains hydrogen
sulphide in an amount in the range of 40-100 mole %,
based on total gas stream.
In step (b) heat is extracted from the gas stream as
obtained in step (a). This can suitably be done by
cooling the gas stream in a two-step heat recovery unit
to a temperature range of from 163-177 C, preferable to
a temperature range of from 168-174 C. In the first
step, a Waste Heat Boiler can suitably be used to
generate high to medium pressure steam by cooling the
gases to a temperature in the range of from 260-370 C,
preferable in the range of from to 315-343 C. In the
second step low pressure steam can suitably be generated
by cooling the gas stream to a temperature range of from
163-177 C, preferable to a temperature in the range of
from 168-174 C. It is believed that at these
temperatures the sulphur dew point will be reached and
the heat exchanger should to be designed for removal of
condensed liquid sulphur.
In step (c) use can be made of one or more catalytic
stages, whereby after each respective catalytic stage
sulphur can be separated from the gas stream as described
in step (d). In step (d) the separation of sulphur from
the gas stream can suitably be carried out by means of a
sulphur condensation unit.
In step (c) hydrogen sulphide present in the gas
stream can be reacted with sulphur dioxide at elevated
CA 02823228 2013-06-27
WO 2012/089776 PCT/EP2011/074179
- 7 -
temperature in a first catalytic stage to obtain a gas
stream which comprises sulphur and water. Suitably step
(c) comprises a catalytic step of a Claus process as
described hereinabove. Suitably, the first catalytic
stage is carried out in a catalytic zone where hydrogen
sulphide reacts with sulphur dioxide to produce more
sulphur. Suitably, the reaction in the first catalytic
stage is carried out with a Claus conversion catalyst at
a temperature in the range of from 204-371 C, preferably
in the range of from 260-343 C, and a pressure in the
range of from 1.29-2.05 bara, preferably in the range of
from 1.56-2.05 bara. Suitably, a second and a third
catalytic stage can be used in step (c) in which stages
use is made of a Claus conversion catalyst. Suitably, in
such a second and third catalytic stage the reaction is
carried out at a temperature which is 5 to 20 C above
the sulphur dew point, preferable at a temperature which
is 6 to 15 C above the sulphur dew point, and a pressure
in the range of from 1.1-2.0 bara, preferably in the
range of from 1.4-1.7 bara. Preferably, the molar ratio
of hydrogen sulphide to sulphur dioxide in step (c) is in
the range of from 2:1-3:1.
Sulphur condensation units can suitably be applied
after each catalytic stage in step (c), which
condensation units can suitably be operated at
temperature in the range of from 160-171 C, preferable
in the range of from 163-168 C.
The remaining gases as obtained after condensation of
sulphur from the gases leaving the final catalytic zone
are usually referred to as "Claus tail gases". These
gases contain nitrogen, water vapour, some hydrogen
sulphide, sulphur dioxide and usually also carbon
dioxide, carbon monoxide, carbonyl sulphide and carbon
CA 02823228 2013-06-27
WO 2012/089776 PCT/EP2011/074179
- 8 -
disulphide, hydrogen, and small amounts of elemental
sulphur.
A suitable Claus catalyst has for instance been
described in European patent application No. 0038741,
which catalyst substantially consists of titanium oxide.
Other suitable catalysts include activated alumina and
bauxite catalysts.
In step (d) sulphur is separated from the gas stream
obtained in step (c), thereby obtaining a hydrogen
sulphide-lean gas stream. To that end the gas stream as
obtained in step (c) can be cooled below the sulphur dew
point to condense and subsequently most of the sulphur
obtained can be separated from the gas stream. Hence,
step (d) can suitably be carried out by cooling the
effluent obtained in step (c) to condense and separate
sulphur, thereby obtaining the hydrogen sulphide-depleted
gas stream.
In step (e) at least part of the hydrogen sulphide-
lean gas stream as obtained in step (d) is subjected to
an oxidation treatment to obtain a sulphur dioxide-
enriched gas stream. At least part, but preferably the
entire hydrogen sulphide-lean gas stream as obtained in
step (d) is subjected to an oxidation treatment to obtain
a gas stream which comprises sulphur dioxide. In step (e)
the oxidation treatment is preferably carried at a
temperature in the range of from 538-1038 C, preferably
in the range of from 648-982 C, and a pressure in the
range of from 1.1-2 bara, preferably in the range of from
1.4-1.7. bara.
In step (f) heat is extracted from the gas stream
which comprises sulphur dioxide as obtained in step (e).
The heat extraction in step (f) can be carried out in a
CA 02823228 2013-06-27
WO 2012/089776 PCT/EP2011/074179
- 9 -
similar way as the heat extraction in step (b) as
previously described.
In step (g) a sulphur-containing residual hydrocarbon
product is subjected to an oxidation treatment to obtain
a gas stream which comprises sulphur dioxide. In step (g)
the oxidation treatment is preferably carried at a
temperature in the range of from 538-1038 C, preferably
in the range of from 648-982 C, and a pressure in the
range of from 1.1-2 bara, preferably in the range of from
1.4-1.7 bara.
In step (g) any sulphur-containing residual
hydrocarbon product can be used. The sulphur-containing
residual hydrocarbon product to be used in step (g) is
preferably a product from a thermal gasoil unit having a
V50 (viscosity at 50 C) in the range of from 30-50 cSt,
a product from a catalytic cracking unit, suitably in
slurry form, having a V50 in the range of from 20-50 cSt,
or a heavy residue containing up to 10 wt% organic
sulphur, based on total heavy residue. More preferably,
the residual hydrocarbon product comprises a product from
a thermal gasoil unit having a V50 in the range of from
35-45 cSt. The integration of step (e) advantageously
ensures that the size of the Claus unit to be used can be
reduced or that the capacity of the Claus unit can be
increased.
As the conditions of the steps (e) and (g) are
similar, if desired, steps (e) and (g) can be carried out
in the same oxidation unit. Preferably, steps (e) and (g)
are carried out in the same oxidation unit.
In step (h) heat is extracted from the gas stream
which comprises sulphur dioxide as obtained in step (g).
The heat extraction in step (h) can be carried out in a
similar way as the heat extraction in step (b) as
CA 02823228 2013-06-27
WO 2012/089776 PCT/EP2011/074179
- 10 -
described hereinbefore. Preferably, if the steps (e) and
(g0 are carried out in the same oxidation unit, also the
steps (f) and (h) are carried out in the same unit.
In step (i) at least part of the cooled sulphur
dioxide-enriched gas stream as obtained in step (f) and
at least part of the cooled gas stream comprising sulphur
dioxide as obtained in step (h) are subjected to a
quenching process before they are contacted with the
absorption solvent in step (j). In such a quenching
process the respective gas streams are suitably cooled by
means of water quenching. Preferably, the entire cooled
sulphur dioxide-enriched gas stream as obtained in step
(f) and the entire cooled gas stream comprising sulphur
dioxide as obtained in step (h) are subjected to the
quenching process before they are contacted with the
absorption solvent in step (j). Suitably, these gas
streams are cooled to a temperature in the range of from
40-60 C in the quenching process.
In step (j) at least part of the sulphur dioxide-enriched
gas stream as obtained in step (i) is contacted with an
absorption solvent that absorbs sulphur dioxide to obtain
a sulphur dioxide-enriched absorption solvent and a
sulphur dioxide-depleted gas stream.
Preferably, in step (j) use is made of an aqueous
solution of the absorption solvent.
Suitably, 10-90 vol.% of the gas stream as obtained
in step (i) can be contacted with the absorption solvent
in step (j). Preferably, the entire gas stream as
obtained in step (i) is contacted with the absorption
solvent in step (j).
Preferably, the absorption solvent in step (j)
comprises water and a water-soluble amine absorbent
having at least one amine group with a pKa value greater
CA 02823228 2013-06-27
WO 2012/089776 PCT/EP2011/074179
- 11 -
than about 7 and at least one other amine group with a
pKa value less than 6.5, wherein the at least one amine
group with a pKa value greater than about 7 irreversibly
absorbs sulphur dioxide in salt form to render the amine
absorbent non-volatile, and wherein the at least one
other amine group with a pKa value less than 6.5
reversibly absorbs sulphur dioxide to saturate the
absorption solvent with sulphur dioxide against a partial
pressure of sulphur dioxide of no more than 1 atmosphere
at 25 C.
Preferably, the water-soluble amine absorbent is a
diamine having the general formula:
R2R5NR1NR3R4
wherein R1 is an alkylene group having 1 to 3 carbon
atoms, R2f R3f R4 and Rs are the same or different and
each represent a hydrogen atom, a lower alkyl group
having 1 to 8 carbon atoms or a lower hydroxy-alkyl group
having 2 to 8 carbon atoms, or any of R2f R3f R4 and Rs
form together with the nitrogen atoms to which they are
attached a 6-membered ring.
More preferably, the diamine is selected from the
group consisting of N,NY,NY-(trimethyl)-N-(2-
hydroxyethyl)-ethylenediamine;
N,N,N',N'-tetramethyl-ethylenediamine;
N,N,N',N'-tetramethyl-diaminomethane;
N,N,NY,NY-tetrakis-(2-hydroxyethyl)-ethylenediamine;
N,N'-dimethylpiperazine;
N,N,NY,N'-tetrakis-(2-hydroxyethyl)-1,3-diaminopropane;
N',N'-dimethyl-N,N-bis-(2-hydroxyethyl)-ethylenediamine;
N-methyl N'-(2-hydroxyethyl)-piperazine;
N-(2-hydroxyethyl)-piperazine;
N,NY-bis(2-hydroxyethyl)-piperazine;
N-methyl-piperazine; and piperazine.
CA 02823228 2013-06-27
WO 2012/089776 PCT/EP2011/074179
- 12 -
It will be understood that the sulphur dioxide-
enriched absorption solvent as obtained in step (j) will
need to be regenerated in order to ensure that the
absorption solvent can be used again in step (j). For
that purpose the sulphur dioxide-enriched absorption
solvent as obtained in step (j) will normally be passed
to a regeneration unit step (k) where the absorption
solvent will be freed from sulphur dioxide and the
sulphur dioxide-depleted absorption solvent so obtained
can suitably be recycled to step (j).
Step (j) can suitably be carried at a temperature in
the range of from 20-80 C, preferably in the range of
from 30-60 C, and a pressure in the range of from 1.1-
2.0 bara, preferably in the range of from 1.4-1.7 bara.
Suitably, use is made in step (j) of an aqueous solution
that comprises the absorption solvent in a concentration
in the range of from 30-55 wt%.
In a particular attractive embodiment of the present
invention at least part of the sulphur dioxide-depleted
gas stream as obtained in step (j) is contacted with an
absorption solvent to absorb carbon dioxide to obtain a
carbon dioxide-enriched absorption solvent and a sulphur
dioxide-depleted gas stream which is lean in carbon
dioxide, and carbon dioxide is removed from at least part
of the carbon dioxide-enriched adsorption solvent to
obtain a carbon dioxide-depleted absorption solvent and a
carbon dioxide-enriched gas stream. Preferably, the
entire sulphur dioxide-depleted gas stream as obtained in
step (j) is contacted with an absorption solvent to
absorb carbon dioxide to obtain a carbon dioxide-enriched
absorption solvent and a sulphur dioxide-depleted gas
stream which is lean in carbon dioxide, and carbon
dioxide is removed from at least part of the carbon
CA 02823228 2013-06-27
WO 2012/089776 PCT/EP2011/074179
- 13 -
dioxide-enriched adsorption solvent to obtain a carbon
dioxide-depleted absorption solvent and a carbon dioxide-
enriched gas stream.
The sulphur dioxide-depleted gas stream as obtained
in step (j) is highly attractive since it is lean in
respect of sulphur-containing contaminants. Suitably, the
sulphur dioxide-depleted gas stream as obtained in step
(j) contains no hydrogen sulphide and less than 50 ppmv
sulphur dioxide. Preferably, said gas stream contains
less than 30 ppmv sulphur dioxide.
In step (k) sulphur dioxide is removed from at least part
of the sulphur dioxide-enriched absorption solvent as
obtained in step (j) to obtain a sulphur dioxide-depleted
absorption solvent and a sulphur dioxide-enriched gas
stream. Step (k) can suitably be carried at a temperature
in the range of from 110-150 C, preferably in the range
of from 120-140 C, and a pressure in the range of from
1.1-1.9 bara, preferably in the range of from 1.2-1.7
bara.
Suitably, in a step (m) at least part of the sulphur
dioxide-depleted absorption solvent as obtained in step
(k) is recycled to step (j). Preferably, the entire
sulphur dioxide-depleted absorption solvent as obtained
in step (k) is recycled to step (j).
In step (1) at least part of the sulphur dioxide-
enriched gas stream as obtained in step (k) is recycled
to step (c). Preferably, the entire sulphur dioxide-
enriched gas stream as obtained in step (k) is recycled
to step (c). However, if part of the sulphur dioxide-
enriched gas stream as obtained in step (k) is not
recycled to step (c), a part of that stream might
suitably be recycled to step (a).
CA 02823228 2013-06-27
WO 2012/089776 PCT/EP2011/074179
- 14 -
Suitably, at least part of the sulphur dioxide-
depleted absorption solvent as obtained in step (k) is
recycled to step (j).
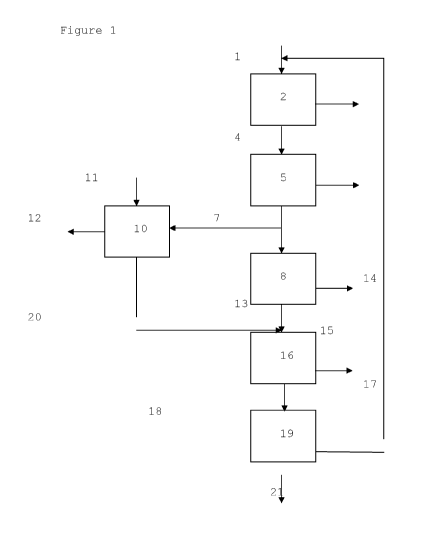
One embodiment of the present invention is
illustrated in Figure 1.
In Figure 1, a gas stream comprising sulphur-
containing contaminants, including hydrogen sulphide is
led via line 1 to an oxidation and heat recovery unit 2
(e.g. a combined burner and heat recovery unit) wherein
the gas stream is oxidized and cooled while producing
steam which is removed via line 3. Liquid sulphur which
is condensed at the low temperature range is also removed
via line 3. From unit 2 the gas stream obtained is
passed via line 4 to a catalyst and sulphur separation
unit 5, the hydrogen sulphide in the gas stream is
reacted with sulphur dioxide in the presence of a
catalyst to obtain a gas stream which comprises sulphur
and water, whereby sulphur is withdrawn via line 6. The
hydrogen sulphide-lean gas stream (tail gas) so obtained
is passed via line 7 into oxidation unit 8. However a
fraction of this gas is passed via line 9 to oxidation
and recovery unit 10 (e.g. combine oxidation and heat
recovery unit). In oxidation unit 8 the hydrogen
sulphide-lean gas stream is subjected to an oxidation
treatment to obtain a sulphur dioxide-enriched gas
stream. Heat can be removed via line 14. A residual
hydrocarbon product is introduced via line 11 into
oxidation and heat recovery unit 10. In oxidation and
heat recovery unit 10 the residual hydrocarbon product is
subjected to an oxidation treatment to obtain a gas
stream which comprises sulphur dioxide. Heat recovered in
the oxidation and heat recovery unit 10 is then removed
via line 12. The gas stream obtained in oxidation and
CA 02823228 2013-06-27
WO 2012/089776 PCT/EP2011/074179
- 15 -
heat recovery unit 10 is passed via line 13 into a
quenching unit 16, whereas the gas stream obtained in the
unit 8 is introduced via line 15 into the quenching unit
16. The quenching medium, e.g. water, can be withdrawn
from the quenching unit 16 via line 17. The gas stream
obtained in the quenching unit 16 is subsequently passed
via line 18 to absorption/regeneration unit 19 where it
is contacted with an absorption solvent that absorbs
sulphur dioxide to obtain a sulphur dioxide-enriched
absorption solvent and a sulphur dioxide-depleted gas
stream. The sulphur dioxide-enriched absorption solvent
is then regenerated and sulphur dioxide-enriched gas
stream thereby obtained is separated from the absorption
solvent and recycled via line 20 to the oxidation unit 2.
The treated gas stream is recovered via line 21.
The invention is illustrated using the following non-
limiting Examples.
Example 1 (according to the invention)
In a process as described in Figure 1, an acid gas
comprising 60 % (v/v) hydrogen sulphide, 40 % (v/v)
carbon dioxide, 0 % (v/v) sulphur dioxide, 50 ppmv
carbonyl sulphide (COS), 200 ppmv mercaptans and 20 ppmv
carbon disulphide is routed via line 1 to a burner unit 2
with a flow rate of 6.74 Nm3/s. In the oxidation and
heat recovery unit 2 the acid gas is oxidized at a
temperature of 980 C and a pressure of 1.5 bara. The
molar ratio of hydrogen sulphide to oxide in the
oxidation and heat recovery unit 2 is 2:1. The gas stream
so obtained is reacted in the catalyst unit 5 with
sulphur oxide at a temperature of 300 C in a first stage
and 220 C in a second and a third stage, and a pressure
of 1.4 bara using a Claus process catalyst which
comprises activated alumina. Sulphur and steam are
CA 02823228 2013-06-27
WO 2012/089776 PCT/EP2011/074179
- 16 -
withdrawn from the catalyst unit via line 6. The molar
ratio of hydrogen sulphide to sulphur dioxide in the
catalyst unit 5 is 2:1. The hydrogen sulphide-lean gas
stream (tail gas) so obtained is passed via line 7 into
oxidation unit 8. In oxidation unit 8 the hydrogen
sulphide-lean gas stream is subjected to an oxidation
treatment to obtain a sulphur dioxide-enriched gas
stream.
A residual hydrocarbon product is introduced via line
11 into oxidation and heat recovery unit 10. In oxidation
and heat recovery unit 10 the residual hydrocarbon
product is subjected to an oxidation treatment which is
carried out at a temperature of 900 C and a pressure of
1.3 bara to obtain a sulphur dioxide-enriched gas stream
which contains no hydrogen sulphide nor organic sulphur
compounds. The gas stream obtained in oxidation and heat
recovery unit 10 is passed via line 13 into quenching
unit 16 and the gas stream obtained in the oxidation unit
8 is introduced via line 15 into quenching unit 16. In
the quenching unit 16 the gas stream is cooled with water
to a temperature of 50 C, and the water is withdrawn from
the quenching unit via line 17. The gas stream obtained
in the quenching unit 16 is subsequently contacted at a
temperature of 50 C and a pressure of 1.3 bara in
absorption/regeneration unit 19 with a Cansolv SO2
solvent that selectively absorbs sulphur dioxide to
obtain a sulphur dioxide-enriched absorption solvent and
a gas stream depleted in sulphur dioxide. The sulphur
dioxide-enriched absorption solvent is then regenerated
and sulphur dioxide-enriched gas stream thereby obtained
is separated from the absorption solvent and recycled via
line 20 to the oxidation unit 2. The gas stream which is
lean in sulphur-containing contaminants is eventually
CA 02823228 2013-06-27
WO 2012/089776 PCT/EP2011/074179
- 17 -
obtained from the absorption/regeneration unit 19 via
line 21 comprises 0 ppmv hydrogen sulphide, 50 % (v/v)
carbon dioxide, 45 % (v/v) nitrogen, 0 ppmv carbonyl
sulphide (COS), 0 ppmv mercaptans, 0 ppmv carbon
disulphide, and 40 ppmv sulphur dioxide.
Example 2 (comparative Example)
A similar process as described in Example 1 was
carried out, except that (a) oxidation unit 8 is replaced
by a hydrogenation unit wherein the gas stream is
contacted at a temperature of 320 C and a pressure of
1.2 bara with a reducing agent hydrogen and a SCOT
catalyst, and that (b) no use is made of an integrated
oxidation unit 10 wherein a residual hydrocarbon product
is subjected to a oxidation treatment and heat recovery.
The oxidation and heat recovery unit 2 is operated at a
temperature of 1040 C, a pressure of 1.5 bara, and a
molar ratio of hydrogen sulphide to oxide of 2:1. The gas
stream so obtained gas is reacted in the catalyst unit 5
with sulphur oxide at a temperature of 280 C and a
pressure of 1.4 bara. The molar ratio of hydrogen
sulphide to sulphur dioxide in catalyst unit 5 is 2:1 The
gas stream eventually obtained from the
absorption/regeneration unit 19 comprises 200 ppmv
hydrogen sulphide, 50 % (v/v) carbon dioxide, 45 % (v/v)
nitrogen, 20 ppmv carbonyl sulphide (COS), 0 ppmv
mercaptans, 0 ppmv carbon disulphide, and 0 ppmv sulphur
dioxide.
From the above, it will be clear that the process in
accordance with the present invention (Example 1)
constitutes an improvement in terms of efficient removal
of sulphur-containing contaminants when compared to
comparative Example 2.