Note: Descriptions are shown in the official language in which they were submitted.
CA 02824456 2013-06-25
METHOD OF SEPARATING OFF MAGNETIZABLE CATALYST PARTICLES BY
MEANS OF MAGNETIC FILTERS
Description
The present invention relates to a process comprising at least the steps (A)
chemical reaction of at least one organic compound in the presence of at least
one
heterogeneous catalyst in a reaction mixture and (B) removal of the at least
one
heterogeneous catalyst by means of a magnetic filter, and also the use of a
magnetic
filter for separating off catalyst particles in a process for the
hydrogenation at least one
organic compound.
Methods of separating catalyst particles from reaction mixtures are already
known
from the prior art.
DE 1 081 20 discloses a process for the hydrogenation of adipodinitrile and
epsilon-
aminocapronitrile to form hexamethylenediamine by means of a suspended Raney
catalyst under superatmospheric pressure. After reaction of the organic
compounds
mentioned, the catalyst-rich suspension can be freed of catalyst particles
present in
suspension by means of a cyclone or a magnetic separator.
CA 2,328,391 Al discloses a method of separating off liquid constituents of a
suspension. For this purpose, the suspension, which originates, for example,
from
chemical reactions such as dehydrogenations, hydrogenations,
transhydrogenations,
aromatizations, hydrodenitrations, is firstly treated by means of filtration,
decantation,
separation by means of a hydrocyclone, etc., and a magnetic separation is
subsequently
carried out.
WO 2008/000808 Al discloses a process, for example a hydroformylation,
carbonylation, olefin oligomerization and polymerization reactions, in which
catalysts
comprising cobalt, nickel or iron are used. After carrying out the chemical
reaction, the
solid catalyst components are separated off by means of magnetic filters.
The prior art has not previously described any processes which make it
possible for
the reaction mixture to be freed virtually entirely of solid catalyst
particles in order to
obtain particularly pure reaction mixtures. The processes of the prior art are
therefore still
in need of improvement in respect of the purity of the reaction mixtures
obtained. For this
purpose, it is necessary, in particular, to improve the step of removal of the
suspended
heterogeneous catalyst particles in order to obtain reaction mixtures which
have a
CA 02824456 2013-06-25
2
particularly high purity, so that in further work-up steps for the reaction
mixture, for
example in a work-up by distillation, disadvantages such as coating of the
distillation
columns with catalyst or blockage of the feed lines and/or discharge lines can
be avoided.
It is therefore an object of the present invention to provide a process for
the reaction
of organic compounds in the presence of a heterogeneous catalyst, which makes
it
possible to obtain the reaction mixture, i.e. the reaction output, in a
particularly high
purity, in particular free of residual catalyst particles. A further object is
to provide a
corresponding process which makes it possible to carry out steps following the
process,
in particular the work-up of the reaction mixture by distillation, in an
advantageous
manner. In particular, it is an object of the present invention to provide a
corresponding
process in which the distillation columns used in the work-up of the reaction
mixture by
distillation are not adversely affected by catalyst residues present in the
reaction mixture,
for example as a result of catalyst residues depositing in the distillation
columns. A further
object is to suppress the formation of undesirable by-products during the work-
up of the
product, for example during a distillation.
These objects are achieved according to the invention by a process comprising
at
least the steps:
(A) chemical reaction of at least one organic compound in the presence of
at least one
heterogeneous catalyst in a reaction mixture and
(B) removal of the at least one heterogeneous catalyst by means of a
magnetic filter.
The process of the invention is explained in detail below.
Step (A):
Step (A) of the process of the invention comprises the chemical reaction of at
least
one organic compound in the presence of at least one heterogeneous catalyst in
a
reaction mixture.
In general, the process of the invention can be applied to any chemical
reaction
known to those skilled in the art in which a heterogeneous catalyst, in
particular a
ferromagnetic catalyst, is used.
Examples of chemical reactions which are particularly suitable for the
purposes of
the invention are selected from the group consisting of hydrogenations,
dehydrogenations, isomerizations, aromatizations and combinations thereof.
CA 02824456 2013-06-25
3
In a particularly preferred embodiment, the chemical reaction carried out in
step (A)
of the process of the invention is a hydrogenation of organic compounds.
The present invention therefore provides, in particular, the process of the
invention
in which the chemical reaction is a hydrogenation.
The term "hydrogenation" is known per se to those skilled in the art and
generally
describes the reaction of C-C and/or C-heteroatom double or triple bonds
comprised in
organic compounds with a reducing agent, for example hydrogen in elemental or
molecular form, in order to obtain the respective organic compounds in
correspondingly
reduced form.
According to the invention, all compounds known to those skilled in the art
which
appear to be suitable for a reaction in the presence of at least one
heterogeneous
catalyst can in principle be used as substrates, i.e. as starting compounds or
starting
materials, in step (A) of the process of the invention.
In Schritt (A) of the process of the invention, it is possible to use
monomeric, low
molecular weight organic compounds, for example compounds having 1 to 20
carbon
atoms, preferably from 1 to 12 carbon atoms, particularly preferably from 1 to
8 carbon
atoms, or polymeric organic compounds. If polymeric compounds are used in step
(A) of
the process of the invention, these have, for example, a molecular weight of
from 500 to
100 000 g/mol.
When the chemical reaction in step (A) of the process of the invention is a
hydrogenation of at least one organic compound, the at least one organic
compound
comprises, in particular, at least one functional group selected from the
group consisting
of C-C double bond, C-C triple bond, oxime group, imino group, nitrile group,
carboxylic
acid group, carboxylic ester group, carboxylic anhydride group, aldehyde
group, keto
group and mixtures thereof.
The present invention therefore provides, in particular, the process of the
invention
in which the chemical reaction in step (A) of the process of the invention is
a
hydrogenation of at least one organic compound, where the at least one organic
compound used comprises at least one functional group selected from the group
consisting of C-C double bond, C-C triple bond, oxime group, imino group,
nitrile group,
carboxylic acid group, carboxylic ester group, aldehyde group, keto group and
mixtures
thereof.
CA 02824456 2013-06-25
4
In step (A) of the process of the invention, it is possible to hydrogenate C-C
doubles
to C-C single bonds, C-C triple bonds to C-C double bonds and/or C-C single
bonds.
Furthermore, an oxime, imino or nitrile group can be hydrogenated to the
corresponding
amino group. A carboxylic acid, carboxylic ester, carboxylic anhydride,
aldehyde or keto
group can in each case be hydrogenated to the corresponding alcohol function
in step (A)
of the process of the invention.
In a particularly preferred embodiment, at least one organic compound having
at
least one nitrile group is hydrogenated in step (A) of the process of the
invention. Organic
compounds which have at least one nitrile group and are particularly suitable
for the
purposes of the invention are, for example, selected from the group consisting
of
mononitriles, dinitriles and polynitriles. These particularly preferred
compounds are
preferably hydrogenated to the corresponding amines in step (A). Corresponding
compounds which have more than one nitrile group, for example two nitrile
groups, can
also be hydrogenated only partially according to the invention. For example,
dinitriles can
be hydrogenated to aminonitriles.
Particularly preferred organic compounds which are hydrogenated in step (A) of
the
process of the invention are selected from the group consisting of
acetonitrile,
propionitrile, 3-dimethylaminopropionitrile, i-phoronenitrile, adipodinitrile
and mixtures
thereof. These compounds are preferably hydrogenated to the corresponding
amines; for
example, it is possible to hydrogenate acetonitrile to ethylamine,
propionitrile to
n-propylamine, 3-dimethylaminopropionitrile to 3-dimethylaminopropylamine and
i-phoronenitrile to i-phoronediamine.
The present invention therefore particularly preferably provides the process
of the
invention in which the at least one organic compound which is hydrogenated in
step (A) of
the process of the invention is selected from the group consisting of
acetonitrile,
propionitrile, 3-dimethylaminopropionitrile, i-phoronenitrile, adipodinitrile
and mixtures
thereof.
Particular preference is given to hydrogenating adiponitrile (ADN) to
hexamethylenediamine (HMD) or 6-aminocapronitrile (6-ACN) and hexamethylene-
diamine (HMD) in step (A) of the process of the invention.
The present invention therefore preferably provides the process of the
invention in
which adiponitrile (ADN) is hydrogenated to hexamethylenediamine (HMD) or
6-aminocapronitrile (6-ACN) and hexamethylenediamine (HMD) in step (A).
CA 02824456 2013-06-25
Processes for hydrogenating adipodinitrile are known per se to those skilled
in the
art and are described, for example, in H. J. Arpe, IndustrieIle organische
Chemie, 6th
edition, p. 275, Wiley-VCH-Verlag.
Hexamethylenediamine (HMD) is an important monomer, for example for the
preparation of polyamides. Polyamide-6.6 ("Nylon") can be prepared from adipic
acid and
hexamethylenediamine by polycondensation. Reaction of hexamethylenediamine
with
phosgene gives the diisocyanate which can serve as component for the
production of
polyurethane resins and polyurethane foams.
In a preferred embodiment of the invention, hexamethylenediamine can therefore
be prepared by catalytic liquid-phase hydrogenation of adiponitrile in the
presence of iron,
cobalt or nickel catalysts in step (A) of the process of the invention.
In a preferred embodiment of step (A) of the process of the invention,
adiponitrile is
converted into hexamethylenediamine in a high-pressure process, for example at
a
temperature of from 100 to 200 C, a pressure of from 200 to 400 bar, in the
presence of
ammonia as solvent and an iron-comprising fixed-bed catalyst.
In a further preferred embodiment of step (A) of the process of the invention,
adiponitrile is converted into hexamethylenediamine in a low-pressure
suspension
process. This process is carried out, for example, at from 60 to 100 C and at,
for
example, 20 to 50 bar (pressure), preferably in the presence of alkali metal-
modified
Raney nickel and hexamethylenediamine as solvent.
In the hydrogenation of organic compounds which is preferably carried out
according to the invention, it is generally possible to use any suitable
reducing agent.
Hydrogen is preferably used as reducing agent in step (A) of the process of
the invention.
Both preferred embodiments of step (A) of the process of the invention result
in
formation of hexamethylenediamine, for example with selectivity of 99% at a
conversion
of, for example, 99%. The hydrogenation of adiponitrile to
hexamethylenediamine is
strongly exothermic, see also Winnacker/Kuchler, Chemische Technik, volume 5,
Organische Zwischenverbindungen, Polymere, 5th edition, Wiley-VCH-Verlag 2005,
pages 242 and 244, and also figure 9.2 on page 243. See also figure 2.
In step (A) of the process of the invention, a chemical reaction is carried
out in the
presence of at least one heterogeneous catalyst.
CA 02824456 2013-06-25
6
In general, all heterogeneous catalysts known to those skilled in the art can
be used
in the process of the invention. The at least one heterogeneous catalyst
according to the
invention is, according to the invention, preferably ferromagnetic, in order
to make a
magnetic separation possible in step (B). The at least one heterogeneous
catalyst is
particularly preferably present in emulsified or suspended form.
For example, the heterogeneous catalysts can comprise catalytically active
metals
selected from the group consisting of nickel, cobalt, iron and mixtures
thereof.
The present invention therefore provides, in particular, the process of the
invention
in which the at least one heterogeneous catalyst comprises a metal selected
from the
group consisting of Ni, Fe, Co and mixtures thereof.
The catalytically active metals, in particular the metals stated to be
preferred, can
be present in elemental form. If the heterogeneous catalyst is present in
elemental form, it
is preferably present in the form of particles, for example particles having a
particle size
distribution of from 0.1 to 5000 pm, preferably from 1 to 1000 pm,
particularly preferably
from 1 to 100 pm. According to the present invention these size ranges also
apply to ag-
glomerates which are optionally formed from the particles in an additional
step, i. e. alt-
hough the particle size increases by agglomeration, the size of the obtained
agglomer-
ates is still in the mentioned range. In a further preferred embodiment, these
particles
which are preferably present are suspended in the reaction mixture of step (A)
of the pro-
cess of the invention.
For example, the heterogeneous catalysts can be present in the form of the
Raney
metal. This embodiment of a heterogeneous catalyst is known per se to those
skilled in
the art. In particular, nickel is present in the form of Raney nickel, cobalt
is present in the
form of Raney cobalt and iron is present in the form of Raney iron, see, for
example,
Rompp Chemie Lexikon, 9th edition, volume 5, page 3785, column 2, Georg Thieme
Verlag Stuttgart, New York.
In a preferred embodiment of step (A) of the process of the invention, nickel
is
present in the form of Raney nickel and/or cobalt is present in the form of
Raney cobalt as
heterogeneous catalyst.
In a further preferred embodiment of the process of the invention, the
heterogeneous catalyst is present as a fixed bed in the reaction mixture. A
catalyst
support is preferably not present here. The catalyst precursor comprises, for
example,
iron oxides, cobalt oxides and/or nickel oxides which are reduced to the
corresponding
CA 02824456 2013-06-25
7
metals before the chemical reaction in step (A). This is preferably affected
by reduction by
means of hydrogen.
In this embodiment, the heterogeneous catalyst is preferably present in a
fixed
arrangement in the reactor in which the chemical reaction of step (A) of the
process of the
invention is carried out. In addition, part of the heterogeneous catalyst can
also be
suspended in the reaction mixture in this embodiment.
The heterogeneous catalysts present in the process of the invention are, in a
further
preferred embodiment, applied to an appropriate support material, for example
metal
oxides and/or semimetal oxides, zeolites, activated carbons, ceramics, etc.,
and
suspended or arranged as a fixed bed in the reaction mixture, in particular in
a liquid
reaction medium. The catalytically active metals are preferably present in
metallic form on
the support material.
The at least one heterogeneous catalyst present according to the present
invention
generally has a particle size distribution of from 0.1 to 5000 pm, preferably
from 1 to
1000 pm, particularly preferably from 1 to 100 pm. According to the present
invention the
size ranges apply to particles as well as to agglomerates that are optionally
formed.
The present invention therefore preferably relates to the process according to
the
present invention, wherein the at least one heterogeneous catalyst has a
particle size
distribution of 0.1 to 5,000 pm, preferably 1 to 1,000 pm, particularly
preferably 1 to 100
pm.
Step (A) of the process of the invention comprises a chemical reaction which
takes
place in a reaction mixture. The reaction mixture is formed here at least by
the organic
compounds to be reacted in the chemical reaction, optionally further reagents,
for
example in liquid, solid, dissolved, suspended or gaseous form, the at least
one
heterogeneous catalyst and products formed during the chemical reaction. In
one
possible embodiment of step (A) of the process of the invention, the reaction
mixture
additionally comprises at least one solvent. The solvent can in principle be
selected from
among all solvents known to those skilled in the art, for example inorganic
solvents, for
example water or liquid ammonia, or organic solvents, for example alcohols,
open-chain
and cyclic ethers, alkanes and mixtures thereof.
In the hydrogenation of adiponitrile to hexamethylenediamine or 6-
aminocapronitrile
and hexamethylenediamine which is preferably, according to the invention,
carried out in
step (A) of the process of the invention, preference is given to using no
solvent. If a
CA 02824456 2013-06-25
8
solvent is nevertheless added, it can be selected from among the
abovementioned
solvents.
Step (A) of the process of the invention is, for example, carried out
continuously or
batchwise, preferably continuously.
In the reaction mixture of step (A) of the process of the invention, the at
least one
heterogeneous catalyst is generally present in an amount which is known to be
suitable to
those skilled in the art, for example from 1 to 50% by weight, preferably from
2 to 40% by
weight, particularly preferably from 5 to 30% by weight, in each case based on
the total
reaction mixture.
In a preferred embodiment of the process of the invention, part of the at
least one
heterogeneous catalyst is separated off after step (A) and before step (6)
according to
the invention.
The present invention therefore preferably provides the process of the
invention in
which part of the at least one heterogeneous catalyst is separated off before
step (B).
When fixed-bed catalysts are used, this removal is preferably omitted. Any
abraded
catalyst material formed is, according to the invention, preferably removed by
means of a
magnetic filter, preferably with a back-flushable magnetic filter, for example
a high
gradient-magnetic filter or an automag magnetic filter, or a wet drum
separator.
For the purposes of the present invention, "part" means, for example, from 80
to
99.99% by weight of the total amount of at least one heterogeneous catalyst
present,
preferably from 90 to 99.99% by weight, particularly preferably from 95 to
99.99% by
weight.
This first separation step which is preferably carried out according to the
invention
in the present process is, for example, carried out in order to separate off a
major part of
the at least one heterogeneous catalyst after step (A) and before step (B).
If a fixed-bed catalyst is used in step (A) of the process of the invention,
this
additional removal after step (A) and before step (B) is, according to the
invention,
preferably not carried out. If a heterogeneous catalyst in suspension is used
in step (A) of
the process of the invention, this additional removal after step (A) and
before step (B) is,
according to the invention, preferably carried out.
CA 02824456 2013-06-25
9
The removal of part of the at least one heterogeneous catalyst can in
principle be
carried out by all methods known to those skilled in the art.
In a preferred embodiment of the process of the invention, the removal before
step
(B) is effected by filtration, for example by means of candle filters, filter
presses, backflush
filters, belt filters, drum filters, rotary pressure filters, etc., by
crossflow filtration, for
example by means of Dyno Filters, and MSD separator, wound membrane module,
hollow fiber module, etc., by separation in the earth's gravitational field,
for example by
means of clarification vessels/settling vessels, lamellar clarifiers, etc., by
separation in a
centrifugal field, for example by means of a plate separator, hydrocyclone,
decanter
centrifuge, solid wall screw centrifuge, sieve decanter, peeler centrifuge,
invertible filter
centrifuge, gyratory centrifuge, pusher centrifuge, etc., by sieving, for
example by means
of a vibratory sieve, vibratory chute, arc sieve, etc., or combinations
thereof.
The present invention therefore preferably provides the process of the
invention in
which the removal before step (B) is carried out by filtration, by crossflow
filtration, by
separation in the earth's gravitational field, by separation in a centrifugal
field, by sieving
or a combination thereof.
Furthermore, the present invention preferably provides the process of the
invention
in which the removal before step (B) is carried out by means of a
hydrocyclone, at least
one settling vessel, at least one clarification vessel and/or at least one
plate separator.
Furthermore, the present invention preferably provides the process of the
invention
in which the removal before step (B) is carried out using a clarification
vessel/settling
vessel.
Furthermore, the present invention preferably provides the process of the
invention
in which the removal before step (B) is carried out using a clarification
vessel/settling
vessel and a hydrocyclone.
A combination of clarification vessel/settling vessel and hydrocyclone is
preferably
used according to the invention. Furthermore, the reaction mixture can be
treated by
means of steps which are known per se to those skilled in the art; for
example, gaseous
components of the reaction mixture can be separated off by lowering the
pressure.
In a preferred embodiment, the reaction mixture which is obtained after the
chemical reaction taking place in step (A) of the process of the invention and
comprising,
for example, the organic compound to be reacted in the chemical reaction,
optionally
further reagents, for example in liquid, solid, dissolved, suspended or
gaseous form, the
CA 02824456 2013-06-25
at least one heterogeneous catalyst and products formed during the chemical
reaction,
optionally in an appropriate solvent, is freed of part of the catalyst by
means of at least
one clarification vessel, i.e. settling vessel, preferably one clarification
vessel, and a first
hyd rocyclone.
For the purposes of the present invention, the formulation "a first
hydrocyclone"
refers either to a single first hydrocyclone or preferably to a formation of
hydrocyclones
operated in parallel, for example at least two hydrocyclones, preferably, for
example, five
hydrocyclones. A plurality of hydrocyclones operated in parallel are used, for
example,
depending on the reaction mixture to be treated. For example, a hydrocyclone
stage
made up of five hydrocyclones operated in parallel is used for a capacity of
120 kt/a. In all
separation apparatuses mentioned in the context of the present invention, a
plurality of
identical apparatuses can, according to the invention, be connected in
parallel in order to
be able to treat the appropriate amount of reaction mixture.
Hydrocyclones are known per se to those skilled in the art and are described,
for
example, in ROmpp Chemie Lexikon, 9th expanded and revised edition, 1990, page
1912,
G. Thieme Verlag Stuttgart New York.
According to the invention, the reaction mixture obtained from step (A) is
preferably
firstly conveyed through at least one clarification vessel in order to
separate off part of the
at least one heterogeneous catalyst suspended in the reaction mixture. In a
preferred
embodiment, at least one clarification vessel is used first, then at least one
first
hydrocyclone. An overflow stream from the first hydrocyclone which, according
to the
invention, preferably has a low content of at least one heterogeneous catalyst
of from 5 to
10 000 ppm by weight, particularly preferably from 10 to 1000 ppm by weight,
very
particularly preferably from about 20 to 500 ppm by weight, in each case based
on the
reaction mixture obtained at the overflow of the first hydrocyclone, is
obtained. This
stream is preferably fed to step (B), i.e. the remaining at least one
heterogeneous catalyst
is separated off by means of a magnetic filter. Furthermore, a bottom outlet
stream which
comprises part of the at least one heterogeneous catalyst which is separated
off in this
separation step which is preferred according to the invention is obtained. To
discharge or
recover the at least one heterogeneous catalyst and preferably recirculate it
to step (A) of
the process of the invention, this bottom outlet stream is, according to the
invention,
preferably fed to at least one second hydrocyclone. Here, a second overflow
stream
which can, for example, comprise solvents, starting material and/or product is
obtained.
Furthermore, a second bottom outlet stream which comprises a major part of at
least one
heterogeneous catalyst is obtained. This can, optionally after work-up, be
reused in step
(A) of the process of the invention. Furthermore, the overflow stream of the
second
CA 02824456 2013-06-25
11
hydrocyclone is preferably fed to the inlet of the first hydrocyclone in order
to be able to
feed more product to the subsequent distillation stage.
In a preferred embodiment, the residual amount of the at least one
heterogeneous
catalyst, in particular the iron, cobalt and nickel catalyst, remaining after
the preferred
removal according to the invention of part of the at least one heterogeneous
catalyst is
present in particulate form dispersed in the reaction mixture.
In a preferred embodiment, this reaction mixture optionally comprises solvent,
which can also be separated off in a further step, and predominantly the
desired product
of the chemical reaction carried out in step (A), particularly preferably the
desired
hydrogenation product. The reaction mixture can, after the preferred removal
of part of
the at least one heterogeneous catalyst, also comprise as yet unreacted
starting
materials or intermediates and/or from 0.1 to 50% by weight of water,
preferably from 0.5
to 40% by weight, particularly preferably from 1 to 30% by weight, of water,
in each case
based on the total reaction mixture.
In a preferred embodiment of the process of the invention, this reaction
mixture
from which part of the at least one heterogeneous catalyst and optionally
solvent has
preferably been separated off is transferred directly to step (B) of the
process of the
invention. In the use which is preferred according to the invention of a
clarification vessel
or a clarification vessel and a hydrocyclone, this stream which is transferred
to step (B) of
the process of the invention preferably corresponds to the overflow stream
from the
clarification vessel or the overflow stream from the first hydrocyclone.
After the preferred removal of part of the at least one heterogeneous catalyst
from
the reaction mixture before step (B) of the process of the invention, the
content of at least
one heterogeneous catalyst in the reaction mixture which is used in step (B)
of the
process of the invention is preferably from 5 to 10 000 ppm by weight,
particularly
preferably from 10 to 1000 ppm by weight, very particularly preferably from
about 20 to
500 ppm by weight, in each case based on the reaction mixture to be treated.
The at least one heterogeneous catalyst that is present in the reaction
mixture ac-
cording to step (A) is in a further preferred embodiment agglomerated.
In a further preferred embodiment, the at least one heterogeneous catalyst
that is
present in the reaction mixture according to step (A) is agglomerated after
separation of
the part of the at least one heterogeneous catalyst from the reaction mixture.
CA 02824456 2013-06-25
12
Therefore, the present invention preferably relates to the process according
to the
present invention, wherein the at least one heterogeneous catalyst that is
present in the
reaction mixture according to, preferably after, step (A), preferably after
separation of a
part of the least one heterogeneous catalyst from the reaction mixture, is
agglomerated.
According to the present invention, agglomeration means that small parts, i.
e. the
particles of the heterogeneous catalysts, accumulate to bigger particles, i.
e. agglomer-
ates, based on their attractive interactions.
In a particularly preferred embodiment, the agglomeration of the at least one
heter-
ogeneous catalyst that is present in the reaction mixture according to step
(A) according
to the present invention, takes place after step (A), i. e. after completion
of the chemical
reaction that is conducted in step (A). In a further preferred embodiment of
the process
according to the present invention, the agglomeration of the at least one
heterogeneous
catalyst that is present in the reaction mixture according to step (A)
according to the pre-
sent invention takes place before step (B).
Due to the preferably conducted agglomeration of catalyst particles to bigger
ag-
glomerates according to the present invention, the separability of the at
least one hetero-
geneous catalyst from the reaction mixture can further be improved, wherein
the rate of
separation in step (B) of the process according to the present invention can
further be
increased. A further advantage of the optional agglomeration of the catalyst
particles ac-
cording to the present invention is that for separation of the formed
agglomerates mag-
netic filters, for example back-flushable magnetic filters, in particular
automag magnetic
filters, that are shaped in an easier way and that are therefore more cost
effective, can be
used in step (B) of the process according to the present invention.
The agglomeration of catalyst particles, which are present in the reaction
mixture
according to step (A) of the process according to the present invention, can
in general be
conducted by any processes that are known to the skilled artisan.
For example, according to the present invention, the agglomeration of catalyst
par-
ticles can be obtained by slow stirring of the reaction mixture comprising the
catalyst par-
ticles from step (A), in order to let the single particles get into contact
with each other and
agglomerate.
According to the present invention, the wording "slow stirring" means that
during
stirring the input of power is for example 0.05 to 0.5 kW/m3 and/or the speed
at the tip of
the outmost stirrer perimeter (tip-speed) is 0.1 to 1 m/s.
CA 02824456 2013-06-25
13
The agglomeration of catalyst particles that is optionally conducted according
to the
present invention can for example be done using a panel stirrer
(Scheibenruhrer) that is
known to the skilled artisan, which is preferably provided in an adequate
distance to the
bottom of the container. According to the present invention an "adequate
distance" means
for example that already agglomerated particles can settle at the bottom,
without being
dispersed again, for example the distance between panel stirrer
(Scheibenruhrer) and
bottom is 1 m.
In a further preferred embodiment flow breakers that are known to the skilled
artisan
are provided in an adequate distance to the bottom of the container and/or the
bottom of
the container comprises a cone-shaped form.
In a further preferred embodiment of the present invention, stirring for
agglomera-
tion of the particles is conducted in a container having a cone-shaped double
bottom. In
this embodiment according to the present invention, a second cone-shaped
bottom is
provided in a distance to the bottom in the container, wherein stirring is
only conducted in
the space above the second bottom. Agglomerates that are preferably obtained
in the
upper space, settle down and get into the space between first and second
bottom through
a correspondingly provided hole. The size of the hole can be adjusted by the
skilled arti-
san and depends preferably on the amount of the reaction mixture that is
stirred, on the
amount of catalyst particles that are to be agglomerated and on the size of
the obtained
agglomerates. For example, the diameter of the hole that is present in the
second bottom
is 0.02 to 0.3 m.
In a further preferred embodiment of the present invention agglomeration of
catalyst
particles can be obtained by addition of at least one flocculant to the
reaction mixture of
step (A). Examples of flocculants that are known to the skilled artisan are
for example
Sedipul&products. Depending on the composition of the flocculant, it can be
either anion-
ic, cationic or non-ionic. Examples of anionic flocculants are anionic,
substituted poly-
acrylamides with low and high molecular masses. Examples for non-ionic
flocculants are
non-ionic polyacrylamides. Examples of cationic flocculants are cationic,
substituted poly-
acrylamides, polyethleneimines, polyamines or polyDADMAC
(polydiallyldimethylammo-
nium chloride). The at least one flocculant is added in an amount that is
known to the
skilled artisan, for example 10 to 5,000 weight ppm, preferably 50 to 1,000
weight ppm,
particularly preferably 50 to 200 weight ppm, in each case in relation to the
product of the
chemical reaction of at least one organic compound in step (A) of the process
according
to the present invention, for example hexamethylenediamine (HMD). For example,
the
optionally used at least one flocculant is used as 1 % solution, preferably in
water. At
least one flocculant can be used for agglomeration in this case, wherein the
product that
CA 02824456 2013-06-25
14
is obtained in step (A) or the process according to the present invention
itself is not dis-
turbed.
The present invention therefore further relates to the process according to
the pre-
sent invention, wherein agglomeration of the catalyst particles is obtained by
slow stirring
of the reaction mixture from step (A) comprising catalyst particles and/or
addition of at
least one flocculant.
Step (B):
Step (B) of the process of the invention comprises removal of the at least one
het-
erogeneous catalyst by means of a magnetic filter, preferably a back-flushable
magnetic
filter, for example a high gradient magnetic filter or an automag magnetic
filter, or a wet
drum separator is used.
Step (B) of the process of the invention is carried out in order to obtain a
reaction
mixture which has a particularly low residual content of at least one
heterogeneous
catalyst. The preferred use according to the invention of a back flushable
magnet filter, for
example a high-gradient magnetic filter or an automag magnetic filter or a wet
drum
separator makes it possible to obtain a reaction mixture having a particularly
low residual
content of at least one heterogeneous catalyst after step (B).
In a preferred embodiment, the content of at least one heterogeneous catalyst
in
the reaction mixture which is used in step (B) of the process of the invention
is preferably
from 5 to 10 000 ppm by weight, particularly preferably from 10 to 1000 ppm by
weight,
very particularly preferably from about 20 to 500 ppm by weight, in each case
based on
the reaction mixture to be treated.
The present invention therefore preferably provides the process of the
invention in
which the content of at least one heterogeneous catalyst in the reaction
mixture which is
used in step (B) of the process of the invention is preferably from 5 to 10
000 ppm by
weight, particularly preferably from 10 to 1000 ppm by weight, very
particularly preferably
from about 20 to 500 ppm by weight, in each case based on the reaction mixture
to be
treated.
The at least one heterogeneous catalyst to be separated off according to the
invention usually has a particle size distribution of from 0.1 to 5000 pm,
preferably from
about 0.1 to 1000 pm, particularly preferably from about 0.1 to 100 pm.
CA 02824456 2013-06-25
The separation in step (B) of the process of the invention is preferably
carried out at
a temperature of from 0 to 200 C, preferably from 20 to 100 C. The separation
in step (B)
of the process of the invention is preferably carried out at a pressure of
from 0.2 to
200 bar (absolute), particularly preferably from 0.4 to 50 bar (absolute),
very particularly
preferably from 1 to 10 bar (absolute).
The separation in step (B) of the process of the invention is carried out
continuously
or batchwise, preferably continuously. In the case of continuous operation,
the volume
flow of the reaction output to be filtered is generally from 0.2 to 10 000
m3/h, preferably
from 3 to 1000 m3/h and particularly preferably from 5 to 100 m3/h.
The viscosity of the reaction mixture treated in step (B) is generally from
0.05 to 50
mPa * s, preferably from 0.6 to 5 mPa * s, in particular from 0.8 to 2 mPa *
s.
The removal of the at least one heterogeneous catalyst is carried out in step
(B) of
the process of the invention using a magnetic filter. Preferably a back-
flushable magnetic
filter, for example a high gradient magnetic filter or an automag magnetic
filter, or a wet
drum separator is used.
The present invention therefore preferably relates to the process according to
the
present invention, wherein the magnetic filter is a back-flushable magnetic
filter, for ex-
ample a high-gradient-magnetic filter or an automag magnetic filter, or a wet
drum sepa-
rator.
Back flushable magnetic filters, for example high-gradient magnetic filters or
automag magnetic filters, are known per se to those skilled in the art. These
are
"switchable" permanent magnet filters whose action is based on the deposition
of
magnetizable particles in highly homogeneous magnetic fields.
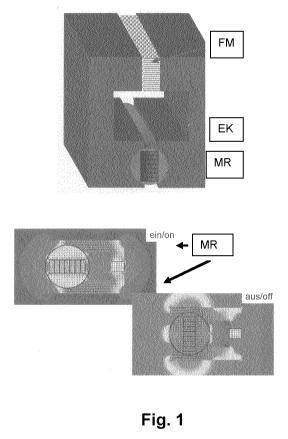
In the switched-on state, the permanent magnets are preferably aligned so that
they generate the magnetic flux to the filter chamber by means of an iron
yoke. The
magnetic flux density of the filter at a pole spacing of, for example, 25 mm
is, for example,
about 0.3 T. In this position, separation of the iron-comprising particles
from the liquid
flowing through occurs. The particles are transported in the magnetic field to
ferromagnetic collectors, for example fine wires of the material X6Cr17, which
can also be
referred to as filter matrix, and accumulated there, see also figure 1.
When a limiting loading of the filter matrix is reached, i.e. in the case of a
no longer
operable pressure drop over the filter matrix or unsatisfactory separation of
magnetizable
particles from the suspension to be filtered, the magnetic field is briefly
switched off by
rotating the permanent magnet and the filter matrix is cleaned by means of a
flushing
CA 02824456 2013-06-25
16
pulse. This backflushing pulse should preferably be in the direction opposite
to the flow
direction of the filtrate, using organic components or water, but can also be
in the flow
direction of the filtration using water, organic components or filtrate. The
backflushing
pulse can, for example, be generated by means of a pump or from a vessel under
superatmospheric pressure, for example blanketed with a gas cushion.
The magnetic action can alternatively be produced by means of electromagnets
which are switched to the no-current state during the above-described
backflushing. High-
gradient magnetic filters which are suitable for the purposes of the invention
can be
obtained, for example, from Steinert.
Wet drum separators are also known per se to those skilled in the art. Here,
the
liquid preferably flows through a semicircular separation space under a
stainless steel
drum to which the magnetizable particles become attached. The strong, high-
gradient
magnetic field is, for example, generated by means of a cylinder comprising
permanently
magnetic disks within the drum. The special permanent magnets generate
particularly
high field gradients. When the drum is rotated, the filtercake is preferably
moved upward
from the suspension and out from the magnetic field. Despite the strong
magnetic field in
the separation space, the filtercake can easily be discharged since the
cylinder with the
permanent magnets is preferably arranged concentrically in the rotating drum.
As a result,
the magnetic field in the separation space is strong and that in the cake
offtake zone is
very weak. Wet drum separators which are suitable for the purposes of the
invention can
be obtained, for example, from Steinert.
Further preferred examples of back-flushable magnetic filters are automag
magnet-
ic filters, in particular automag skid AM6/SKID1 or AM12/SKID1, of Eclipse
Magnetics
Ltd.
In a preferred embodiment according to the present invention, beside the men-
tioned magnetic filters, can, in the case that the at least one heterogeneous
catalyst that
is present in the reaction mixture according to step (A), is agglomerated,
preferably be-
fore step (B) according to the present invention is conducted, even simpler
designed and
therefore more cost effective magnetic filters be used, that are known to the
skilled arti-
san, for example back flushable magnetic filters like Automag skid AM6/SKID1
or
AM12/SKID1 of Eclipse Magnetics LTD.
After the step (B) according to the invention of the process of the invention,
a
reaction mixture which comprises the at least one heterogeneous catalyst in a
particularly
low concentration, for example from 0.1 to 100 ppm by weight, preferably from
0.1 to
20 ppm by weight, particularly preferably from 0.1 to 5 ppm by weight, is
obtained.
CA 02824456 2013-06-25
17
This particularly low content according to the invention of at least one
heterogeneous catalyst makes it possible to carry out a work-up step, for
example a
distillation of the reaction mixture obtained after step (B), particularly
advantageously.
Firstly, deposition of the at least one heterogeneous catalyst or degradation
products
thereof in the distillation columns is avoided. As a result, cleaning of the
distillation
column and an associated downtime of the plant are avoided. Furthermore, the
formation
of undesirable by-products formed from the desired product by reactions in the
presence
of the at least one heterogeneous catalyst or degradation products thereof
during the
distillation is suppressed, so that the desired product can be obtained in
higher yield and
purity.
Furthermore, the combination which is preferred according to the invention of
a first
removal of part of the at least one heterogeneous catalyst, for example by
means of a
clarification vessel and/or a hydrocyclone, in combination with step (B), viz,
removal of
the at least one heterogeneous catalyst using a magnetic filter, provides a
process which
consumes particularly little energy compared to known processes of the prior
art. In
addition, with the process according to the present invention, lower
investment costs
apply.
In a preferred embodiment of the process of the invention, step (B) is
followed by
the step (C) below:
(C) work-up of the reaction mixture from step (B), preferably by distillation,
in
order to obtain the desired product of the chemical reaction in step (A).
Processes for working up reaction mixtures are known per se to those skilled
in the
art. Furthermore, processes for distilling reaction mixtures are known per se
to those
skilled in the art and are described, for example, in Winnacker Kuchler,
Chemische
Technologie, 4th edition, 1984, pages 180-199, Carl-Hanser Verlag, Munich,
Vienna.
A particularly preferred embodiment of the process of the invention is
described
below, see also figure 3.
A catalyst suspension comprising from 5 to 30% by weight of Raney nickel is
obtained from the hydrogenation of adipodinitrile (ADN) by means of hydrogen
to
hexamethylenediamine (HMD) or 6-aminocapronitrile (6-ACN)
and
hexamethylenediamine (HMD). This suspension is firstly fed to a gas separation
vessel
from which a suspension stream runs into a clarification vessel. The lower
outlet of the
clarification vessel goes back to the reactor. The clarified upper offtake
stream
CA 02824456 2013-06-25
18
corresponding to from about 5 to 20% of the feed still comprises from 10 to
1000 ppm by
weight of catalyst. The overflow stream is depressurized from reactor pressure
level to a
slightly superatmospheric pressure via a throttle valve. The gas liberated
here is
separated off in a separator. The liquid runs into a buffer vessel and from
there is fed to a
two-stage hydrocyclone battery.
First hydrocyclone
The overflow stream from the first hydrocyclone stage has a residual content
of
from 10 to 1000 ppm by weight of Raney nickel and goes into the "crude HMD
tank" and
subsequently goes, after the removal according to the invention of Raney
nickel by
means of a high-gradient magnetic filter, into the first distillation column
of the HMD work-
up. According to the invention, the overflow stream from the first
hydrocyclone stage is
conveyed to the first work-up column. The nickel content of this stream can,
according to
the invention, be reduced to less than 5 ppm by weight.
Second hydrocyclone
The bottom outlet stream from the first hydrocyclone stage is fed to the
second
hydrocyclone stage whose overflow stream goes back into the buffer vessel. The
bottom
outlet stream from the second stage, which is enriched in catalyst, goes into
a settling
vessel and from there to catalyst discharge.
The catalyst sediment accumulating in the catalyst discharge vessel
("hydrocyclone
tails settler") is periodically drained into a second stirred vessel in which
the catalyst is
freed of HMD in a multistage batch process and is resuspended in water. The
used
catalyst is discharged via a settling tank from which the catalyst is
dispensed while moist
with water by means of a discharge screw into drums and passed to recycling.
The
supernatant aqueous liquid is preferably, according to the invention, likewise
conveyed
via a magnetic filter. Here too, the nickel content of the supernatant liquid
can be reduced
to less than 5 ppm.
The present invention also provides for the use of a magnetic filter,
preferably of a
back-flushable magnetic filter, for example a high-gradient magnetic filter or
an automag
magnetic filter, or a wet drum separator, for separation of catalyst particles
in a process
for the hydrogenation of at least one organic compound.
The present invention preferably provides the use according to the invention
in
which adiponitrile (ADN) is hydrogenated to hexamethylenediamine (HMD) or 6-
aminocapronitrile (6-ACN) and hexamethylenediamine (HMD) in the process.
CA 02824456 2013-06-25
. .
19
As regards the magnetic filter, the wet drum separator, the catalysts used,
the
process for the hydrogenation of at least one organic compound and also
further details
and preferred embodiments, what has been said in respect of the process of the
invention, applies.
Figures
Figure 1 shows the functional principle of a high-gradient magnetic filter
which can
be used according to the invention. In figure 1, the abbreviations have the
following
meanings:
FM Filter matrix
IC Iron circle
MR Magnetic rotor
Figure 2 shows a process scheme for the preparation of HMD from adiponitrile,
without the separation process according to the invention. In figure 2, the
abbreviations
have the following meanings:
H2 Hydrogen
ADN Adipodinitrile
H2 (R) Hydrogen for recirculation
LB Low boilers
HB High boilers
UC Used catalyst
Figure 3 shows a process scheme for the preparation of HMD from ADN with
subsequent catalyst removal.
Figure 4 schematically shows the experimental set-up used in the examples. In
figure 4, the abbreviations have the following meanings:
R1 Temperature-controllable reservoir
Fl Magnetic filter
M Stirrer
Figure 5 shows the magnetic filter degree of deposition curves for example 1.
The
experimental conditions are: HMD, fine matrix, about 90 ppm by weight of
catalyst.
CA 02824456 2013-06-25
Figure 6 shows the magnetic filter degree of deposition curves for example 2.
The
experimental conditions are: water, X50 = 3.4 pm, about 130 ppm by weight of
catalyst.
In figures 5 and 6, the degree of deposition is in each case plotted on the y
axis in
`)/0 by weight and the suspension volume in I is in each case plotted on the x
axis.
Examples
The present examples illustrate the present invention without constituting a
restriction.
Example 1
Raney nickel-comprising crude HMD from an industrial plant for the low-
pressure
hydrogenation of ADN, specifically from the overflow of the first
hydrocyclone, is used as
starting material. The starting material comprises 90 ppm by weight of Raney
nickel,
determined by AAS (atomic absorption spectrometry) analysis. The average
particle size
of the Raney nickel is from 5 or 13 pm, determined by laser light scattering
measurement
(Master Sizer 2000, from Malvern).
The starting material is purified by means of a high-gradient magnetic filter
(model
HGF10) from Steiner.
Figure 4 schematically shows the experimental set-up. The crude HMD is placed
in
a stirred, temperature-controllable 400 I reservoir (R1). The magnetic filter
(F1) is located
in a pumped circuit operated by means of a pump. Backflushing of the loaded
filter is
carried out by means of deionized water into a collection vessel. Appropriate
instrumentation makes it possible to set the amounts in the pumped circuit, to
measure
the pressure drop in the filter and the amount of backflushing water and also
the
temperature of the plant. The following filter matrix is used in this filter.
Matrix (L>< B x T): 20 x 80 X 100 mm
Matrix area: 1600 mm2
Matrix mesh opening: 0.5 mm, 1.0 mm, 1.5 mm
All experiments are carried out at 25 C and a filter pressure of about 1 bar
(absolute). However, the experimental results achieved are independent of the
filtrate
pressure. The filtrate pressure is limited only by the maximum permissible
operating
pressure of the filter housing (minus the differential pressure during
filtration). The
deposition performance is limited by the magnetic material properties of the
filter matrix
CA 02824456 2013-06-25
21
and of the iron circle of the high-gradient magnetic filter. For a good degree
of separation,
the operating temperature should therefore not be above 80 C in the case of
the
materials used at present. When other materials whose magnetic materials
properties are
impaired only slightly, if at all, above 80 C are used according to the
invention, higher
operating temperatures can be employed.
The magnetic flux density of the test filter is 0.3 T (25 mm pole spacing).
The filter
matrix is packed with wire mesh layers made of the material X6Cr17
(ferromagnetic
stainless steel).
Experimental results
A volume flow of from 0.290 to 0.855 m3/h of HMD comprising about 90 ppm by
weight of catalyst particles is conveyed through the magnetic filter at a
temperature of
25 C and 1 bar (abs.). The residence time in the filter matrix is from 0.68 to
2 seconds.
The degree of deposition for a stream of 0.29 m3/h comprising catalyst
particles
having an average particle size of 5 pm is from 90 to 95%, corresponding to a
residual
amount of 4.5 ppm by weight (see figure 5).
The degrees of deposition are as expected better for the fine-structured
filter matrix
having a mesh opening of 0.5 mm (triangular symbols) than for the larger
filter matrix
having a mesh opening of 1.0 mm (square symbols). With increasing loading, the
degree
of deposition decreases due to the increasing weakening of the magnetic field
gradient
and impairment of the flow conditions in the filter matrix. Likewise, the
degrees of
deposition decrease with higher liquid throughput through the matrix (inflow
velocity
achieved of from 3.2 to 9.4 cm/s).
The degrees of deposition of the magnetic filter are determined by determining
the
particulate nickel concentration downstream of the filter (no dissolved nickel
fraction in the
HMD) with and without an applied magnetic field. This procedure ensures that
exclusively
the magnetic degree of deposition is taken into account. In addition, a long-
term
experiment carried out without a magnetic field shows that mechanical
filtration in any
case does not take place to a significant extent (stable exit concentration
since there is no
increase in the pressure drop).
The storage capacity of the filter matrix is, for the region of still moderate
deteriorations in the degree of deposition, about 5 g of catalyst (coarser
matrix) to about
7.5 g of catalyst (fine matrix) per 100 cm3 of matrix volume.
CA 02824456 2013-06-25
22
In all cases, the loaded matrix can be largely cleaned by means of a
backflushing
pulse with about 1 I of water per second (duration: 3 to 4 seconds).
Repetitions of the
backflushing operation give only a slight additional catalyst discharge. After
the end of the
experiments, no permanent increase in the basic pressure drop of the matrix
can be
observed.
Example 2
Milled Raney nickel (x50 = about 3.4 pm) suspended in water serves as starting
material. Milling is carried out in a stirred mill.
The experimental apparatus corresponds to example 1.
Experimental results
A volume flow of from 0.385 to 0.77 m3/h of aqueous suspension comprising
about
130 ppm by weight of catalyst particles having an average particle size of 3.4
pm is
conveyed through the magnetic filter at a temperature of 25 C and 1 bar. The
residence
time in the filter matrix is from 0.75 to 1.5 seconds.
The degree of deposition for a stream of 0.385 m3/h is from 90 to 95%,
corresponding to a residual amount of 6.5 ppm by weight. The degrees of
deposition are
as expected better for the finely structured filter matrix having a mesh
opening of 0.5 mm
(triangular symbols) than for the coarser filter matrix having a mesh opening
of 1.0
(square symbols). The degree of deposition decreases with increasing loading,
owing to
the increasing weakening of the magnetic field gradients and the impairment of
the flow
conditions in the filter matrix. The degrees of deposition likewise decrease
with higher
liquid throughput through the matrix (inflow velocity achieved from 4.3 to 8.6
cm/s).
The degrees of deposition of the magnetic filter are again determined by
determining the particulate nickel concentration downstream of the filter (no
dissolved
nickel fraction in the water) with and without an applied magnetic field.
In all cases, the loaded matrix could be largely cleaned by means of a
backflushing
pulse of about 1 I of water per second (duration: about 3 to 4 seconds).
Repetitions of the
backflushing operation give only a slight additional catalyst discharge.
The experiment shows, for the example of Raney nickel, that ferromagnetic
catalyst
particles can be separated off from aqueous suspensions or emulsions by means
of
magnetic filters.
CA 02824456 2013-06-25
23
Example 3:
Example 3 shows that a significantly smaller amount of by-products is formed
on
thermal stressing of hexamethylenediamine (HMD) in the presence of a small
amount
according to the invention of catalyst (e.g. <5 ppm by weight) coming from the
hydrogenation previously carried out, as occurs, for example, in a
distillation column used
for purifying the product, so that, firstly, the purity of the product and
also the yield of
desired product increases. HMD was stirred under nitrogen as inert gas in the
presence
of suspended Raney nickel. The concentration of tetrahydroazepine (THA)
CN
__________________________________ THA
as by-product formed is determined by way of example after particular times.
The by-
product THA can generally only be separated off from hexamethylenediamine with
a high
outlay for distillation, see WO 01/66514, page, lines 31 to 39.
The amount of THA in pure HMD should not exceed 100 ppm (Ullmann's
Encyclopedia of Industrial Chemistry, 6th completely revised edition, volume
16, page
435, column 1).
Example 3.1 (comparative example)
Pure HMD is stirred at 180 C with 1% by weight, corresponding to 10 000 ppm by
weight, of Raney Ni, at atmospheric pressure under nitrogen as protective gas.
t (min) c(THA) in % by weight
0 0.05
34 2.08
82 3.35
292 5.72
This implies a rate of formation of THA of about 0.7% by weight/h
Example 3.2 (comparative example)
CA 02824456 2013-06-25
24
Pure HMD is stirred at 150 C with 1% by weight, corresponding to 10 000 ppm by
weight, of Raney Ni, at atmospheric pressure under nitrogen as protective gas.
t(min) c(THA) in % by weight
0 0.14
8 0.42
75 1.69
221 2.26
363 4.19
Example 3.3 (according to the invention)
Pure HMD is stirred at 200 C without Raney Ni (i.e. <5 ppm by weight of Raney
nickel), at atmospheric pressure under nitrogen as protective gas.
t (min) c(THA) in % by weight
0 0.06
1440 0.10
Example 3.4 (comparative example)
Pure HMD is stirred at 180 C with 65 weight-ppm Raney-Ni without pressure
under
nitrogen as inert gas.
t(min) c(THA) in % by weight
0 0.06
0.15
60 0.40
180 0.60
420 1.14
This experiment shows that significant amounts of tetrahydroazepine (THA) are
obtained even with a low concentration of Raney-Ni. The THA-concentration of
HMD is
obtained in all experiments of example 3 by gas chromatography.
Example 4 (according to the invention)
Two back-flushable magnetic filters of type automag SKID AM12/SKID1 of Eclipse
Magnetics ltd. which are arranged in parallel, were tested in an industrial
facility for low
pressure-hydrogenation of ADN. Therefore, the overflow stream of the first
hydrocyclone
CA 02824456 2013-06-25
(Raney-nickel comprising crude HMD) is fed into a container, wherein a part of
the cata-
lyst particles agglomerated. Out of this container, Raney-nickel comprising
crude HMD
was fed to each of the two magnetic filters 6 t/h (about 7.3 m3/h) with 70 to
100 C contin-
uously. The retention time in the container having a volume of 55 m3 was 3.5
h.
The feed of the container did comprise 30 to 60 ppm by weight Raney-nickel
during
the experiments, analyzed by AAS-(atom-adsorption spectrometry)-analysis. The
Raney-
nickel particle size in the feed to the container was about 5 or 13 pm.
The drain of the container did comprise about 30 to 60 ppm by weight Raney-
nickel.
The average particle size of the Raney-nickel in the drain of the container
was 30 to 80
pm. The drain of the container was purified from catalyst by a magnetic filter
(type au-
tomag SKID AM12/SKID12, Eclipse Magnetics ltd.).
For cleaning, the magnetic filters were back-flushed with feed suspension each
12
to 24 hours. For this, the permanent magnets were lifted at the inside of the
magnetizable
metal tubes in the filters, and the valve for draining the solid was opened.
The results obtained are independent from the pressure of the filtrate. The
pressure
of the filtrate according to this example is only limited by the at most
admissible operating
pressure of the filter housing. The filtration efficiency according to this
example is limited
by the magnetic material properties of the filter built-in components and of
the permanent
magnets of the magnetic filter. According to this example, the separation
efficiency is not
decreased, although the temperature of the feed is lowered. Because the
magnetic flux
density, which acts onto the particles, increases at temperatures < 70 C, the
viscosity
that increases at lower temperatures, which degrades the particle preparation
a little bit,
is compensated.
Other materials according to the present invention, of which magnetic material
properties do not or only insignificantly worsen above 100 C, according to the
present
invention can also be operated at higher operating temperatures.
Experimental results
A volume flow rate of about 7.3 m3/h HMD with 30 to 60 ppm by weight catalyst
par-
ticles is fed at a temperature of 70 to 100 C and 1 to 5 bar (abs.) to a
magnetic filter (au-
tomag SKID AF12/SKI D1).
CA 02824456 2013-06-25
26
The separation efficiency for this stream of 7.3 m3/h, containing catalyst
particles
having an average particle size of 30 to 80 pm, is about 75%, according to the
remaining
amount of 8 to 15 ppm by weight.
With increasing loading of the filter, the separation efficiency decreases,
caused by
increasing weakening of the magnetic field gradient and degradation of the
streaming
conditions in the filter. Further, the separation efficiency decreases with a
higher feed
stream to the filter. Vice versa, the separation efficiency increases at a
reduced feed. Fur-
thermore, with a lower feed, smaller catalyst particles can be separated.
The storage capacity of the magnetic filter (automag SKID AN12/SKID1) is as a
function of particle size about 4 to 6 kg, 12 kg at most.
In all cases the loaded magnetic filters can be cleaned with a flushing
stream. The
efficiency of this back-flushing operation only gives a marginal additional
catalyst
discharge. After ending of the experiments, no enduring increased of the space
pressure
loss can be detected.