Note: Descriptions are shown in the official language in which they were submitted.
CA 02824697 2013-08-23
APPLICATOR HEAD AND METHOD FOR
TREATMENT OF PAIN BY
TRANSCUTANEOUS ELECTRICAL NERVE STIMULATION
FIELD OF THE INVENTION
This disclosure relates to the field of treatment of pain, and more
specifically, to a
TENS unit with a specially configured applicator head and to the more
effective
treatment of pain using that applicator head.
BACKGROUND OF THE INVENTION
This section provides general background information related to the present
disclosure and is not necessarily prior art.
Countless people suffer from chronic, intermittent, or injury related pain.
Often the
body responds to pain by tightening the muscles which can decrease the
circulation in
the affected area and cause the patient continuing painful symptoms.
It is noted that pain is a warning system and is the human body's primary
method of
telling a person that something is wrong. Pain is important because without
it,
abnormal conditions may go undetected, and that can cause significant, and
sometimes permanent, damage or injury to vital body parts.
It is estimated that about 25% of the population suffers from chronic pain.
Several
major medical institutions have also estimated that about 8 - 11% of the
population
suffer from Restless Leg Syndrome (RLS). Additionally, it is also known that
about 2
- 3% of the population has Sciatica and another 2-3% suffer from Fibromyalgia.
There is a long history of using electrical stimulation for relief of pain
beginning with
the ancient Egyptians and Greeks who knew of the electro-analgesic effects of
CA 02824697 2013-08-23
standing in a pool with electric fish. In more recent times, low voltage
pulses of
electric current for pain relief became popular with the U.S. military during
World War
Since 1960 and the advent of the microelectronic age, transcutaneous
electrical
nerve stimulation medical devices have become smaller and more portable. Over
the past 60 years, such devices have become the preferred choice for non-
addictive
drug free control of pain for millions of people.
Traditionally, transcutaneous electrical nerve stimulation makes use of
flexible
electrodes that are attached to the skin or the use of electrical probes
having a variety
of shapes and sizes. These devices all rely on a stimulating pulse current
that travels
between an anode through the skin into the underlying tissues and back though
the
skin to a cathode.
The problem with this arrangement is that the current is diffused or diluted
as it
expands into the various tissues of the body having different resistivities.
As predicted
by Ohm's law (I=V/R), electricity takes the path of least resistance. If the
electrode is
pressed directly over the nerve as current TENS units describe, the results
are
unpredictable and ambiguous because of the variable resistance of the skin,
tissue
and nerve. The effectiveness of the treatment of any given nerve center is
dependent
on the amount of current that actually reaches all the symptomatic portions of
a
particular nerve and this explains the erratic nature of the results when
using prior art
devices.
Therefore, there is a need to provide a more predictable method of treating
pain
that includes a specially designed treatment device that can be easily
positioned
2
CA 02824697 2013-08-23
and repositioned to effectively treat a nerve that is suspected of being the
source
of a patient's pain.
SUMMARY OF THE INVENTION
This section provides a general summary of the disclosure, and is not a
comprehensive disclosure of its full scope or all of its features. More
specifically,
embodiments of the invention as disclosed and discussed herein relate to an
improved TENS unit which can be used to more effectively treat pain such as
results from minor and severe muscle strains, Restless Leg Syndrome, Sciatica
and Fibromyalgia.
Aside from its value in diagnosis, long-lasting persistent pain serves no
useful
purpose. Pain starts as a signal from a distressed biological component which
is
passed along the network of nerves to the brain where it the signal is
decoded,
analyzed, and then reacted to. More specifically, the pain signal travels from
the
injured area along the small nerves leading to the spinal cord where the
signal is
switched or routed to other nerves that connect the spinal cord to the brain.
The brain
then interprets the pain signal as an indication of "pain." That feeling of
"pain" is the
source of the discomfort and hurt felt by a patient. Various embodiments of
the
present invention interrupt these electrical nerve signals at the source and
relieve the
patient's "pain."
Therefore, in accordance with the various embodiments of the present invention
as
disclosed and discussed herein, various embodiments of the present invention
include
a battery powered non-invasive, non-addictive, drug free TENS unit with an
improved
applicator head that works in a similar manner as a spinal stimulator to
control pain,
3
CA 02824697 2013-08-23
except that the present stimulation device does not penetrate the skin or
require
implantation. The applicator head is unique in that when it is pressed against
the body,
the rectangular electrodes and the insulating land in between the electrodes
compress
the body tissue to create an electrical pathway in which the painful nerve
center is
captured and compressed. The shape of the electrodes and the insulating land
and
the electrical properties of body tissue work together to focus the gentle
pulses of
current directly through the compressed nerve to more effectively suppress the
sensation of pain in underlying nerves. This numbing of the nerve may last for
8-36
hours depending on treatment time, severity of symptoms and variations between
patient conditions. Often, as a result of the relaxation of nearby muscle
tissue caused
by gentle massaging with the applicator head, the pain cycle is interrupted on
a
permanent basis and the pain will not return.
More specifically, various embodiments of the present invention only require
the
simple pressing of the TENS unit applicator head with electrodes flanking an
insulating
land on the outer surface of a patient's body by the patient at the area where
the
patient experiences pain without the assistance of specialized medical
personnel. The
block insulator focuses the current through the nerve in a manner that
maximizes the
effective current to the nerve and "patient usability" is enhanced by use of a
very low
current that greatly reduces the possibility of burns to the patient's skin.
Nerves which
lie deep within the body such as those associated with RLS, chronic pain, and
strained
muscles, can be quickly desensitized with treatment times that last only a few
seconds.
4
CA 02824697 2013-08-23
Additionally, certain embodiments of the present invention may be used by
emergency
personnel treating patients with severely painful strained muscles with
dramatic results
in less than a minute. Embodiments of the present invention can be used on
older
patients without significant risk of interference to heart rhythms or
pacemakers. RLS
patients can also use certain embodiments of the present invention before
retiring or
sitting to eliminate the leg jerking that can cause discomfort and serious
sleep
disorders. Patients that suffer from Fibromyalgia can either eliminate or
greatly reduce
their symptoms with self treatment on a daily basis. lmmobolized or bedridden
patients with Sciatica may be able to walk normally again and lead normal
lives.
It is noted that various embodiments of the present invention incorporate a
TENS
unit applicator head having a fiat block insulator or insulating land located
between two essentially parallel electrodes. When the insulator portion is
pressed down over the nerve, the nerve is compressed thus allowing the
electrical current to be focused precisely below the insulating land and
through
the compressed nerve directly below the land and skin. This cannot be done
with prior art flexible electrodes or probes because without the insulating
land,
the nerve will not be compressed and without nerve compression, the current
from the electrodes will pass through only a small portion of the nerve.
With prior art TENS units the individual electrodes are usually widely-placed
over
the body of a patient. With this kind of electrode placement, the current
density
is uncontrollable and the path is unpredictable because the nerves are not in
a
compressed state and the current path does not reach the entire nerve. Current
is either diluted before it reaches the symptomatic nerve giving rise to the
pain
5
CA 02824697 2013-08-23
signals or the current will follow a vein or artery from one electrode to the
other
and miss the nerve entirely. On the other hand, if the two electrodes are
placed
a short distance from each other, current flows just below the uncompressed
skin
and does not reach the distressed nerves lying deeper within the patient's
body.
Thus, if one electrode of prior art TENS units is simply placed over the
nerve, the
current path may be to one side, straight down, or to a large blood vessel
depending on resistivity of the skin, tissue and nerve-- any of which can
prevent
focus of current to the nerve. As a result such electrotherapy treatment
methods
require lengthy treatment times of many hours or all day because of the
dilution
or diffusion of the electrical current or are completely ineffective.
In the various embodiments of the present invention, the use of the block
insulator or insulating land compresses the nerve and focuses the current
through the compressed nerve below the insulating land. More particularly with
the present invention, there is a laminar flow of current passing between the
electrodes under the block insulator just below the skin and through the
compressed nerve. This greatly decreases the treatment time and greatly
increases the effectiveness of treatment using transcutaneous nerve
stimulation
and because of the present embodiment's greater efficiency, also allows the
use
of lower skin currents for safer operation and treatment.
As is more particularly described below, the cross sectional area of the
current
pathway under the block insulator is also many time less than the cross
sectional
area of the individually placed electrodes. This allows for a focused laminar
flow
of electrical current under the skin and through the entire nerve which is
6
CA 02824697 2013-08-23
compressed under the land. The laminar current flow evenly distributes the
current throughout that part of the nerve that is compressed under the
insulating
block. Due to the laminar current flow through the nerve, the patient can feel
the
silhouette and location of the nerve center during treatment. This allows the
patient to immediately make intuitive judgments as to the best placement of
the
electrodes in the applicator head. A patient can initially determine the exact
location of the painful nerve caused by chronic pain, muscle strains, RLS,
Fibromyalgia or Sciatica by pressing a finger on and around the nerve that is
the
suspected source of the pain. The same results can be obtained by pressing the
center of the insulating block to the nerve area while applying power to
determine
the location of the nerve sensation that is felt during treatment.
The amplifying effect of current on the treatment of the nerve area compressed
under the insulative land may be analogized to the power from an FM radio
transmitter which may have an output of 6,000 watts to the antenna, but the
antenna has an output of 30,000 watts of effective power. This amplification
occurs because radio frequency energy from the radio's transmitter is
redirected
by the antenna into the electromagnetic medium in a focused manner. Similarly,
a flashlight may be rated at 1,000,000 candlepower while the bulb in the
flashlight is only a fraction of that amount. The parabolic mirror of the
flashlight
focuses the bulb to become 1,000,000 candlepower. Similarly, nerves are three
dimensional and spread out over a given area, but when they are compressed
into an essentially flat plane, the treatment current is focused through the
same
flat plane and the entire selected area of the nerve can receive a uniform
7
CA 02824697 2016-09-26
current flow with much faster and far greater desensitization of the nerve. It
is
this "amplifying effect" that assists the embodiments of the present invention
to
have increased effectiveness in pain treatment by transcutaneous electrical
nerve stimulation.
For larger nerves, after the initial treatment, the patient can re-position
the block
to either side of the larger nerve for treatment of the entire nerve. In
preferred
embodiments, the insulating block and flanking electrodes are assembled into a
treatment applicator as a single unit and the battery operated power supply
can
be located in the handle of the applicator.
In accordance with the various embodiments of the present invention, this
invention relates to an apparatus and method for treatment of pain by
transcutaneous electrical nerve stimulation. Further areas of applicability
will
become apparent from the description provided herein. The description and
specific examples in this summary are intended for purposes of illustration
only
and are not intended to limit the scope or the claims of the present
disclosure.
According to one aspect of the present invention, there is provided a
transcutaneous electrical nerve stimulation (TENS) unit for treatment of pain
comprising:
a hand held applicator having an applicator head;
a set of rectangular electrodes disposed on the applicator head operatively
connected to an electric pulse provider, said electrodes having a length
between
0.25 in. and 2.5 in. wherein lengths below 0.25 in. do not provide an
effective
8
CA 02824697 2016-09-26
. .
current density to a treatment area and lengths above 2.5 in. make it
difficult to
apply enough pressure on the treatment area; and,
a rectangular insulative land disposed between the electrodes on the
applicator
head wherein a surface of the insulative land is coplanar with a surface of
the set
of electrodes, said land having a length between 0.5 in. and 3.5 in. wherein
lengths below 0.5 in. result in current flow across the skin in the treatment
area
and lengths above 3.5 in. make it difficult to position the applicator head on
the
nerves in the treatment area.
According to another aspect of the present invention, there is provided a
transcutaneous electrical nerve stimulation (TENS) unit for treatment of pain
comprising:
a hand held applicator having an applicator head;
a set of rectangular electrodes disposed on the applicator head operatively
connected to an electric pulse provider, said electric pulse provider
providing:
an electric pulse at a peak current range of between 0 and 80 milliamps;
an average current of between 0 and 0.8 milliamps at 500 ohm resistance and a
peak voltage range of between 0 and 40 volts at 500 ohms resistance;
at a voltage of between 40 volts and 5 volts;
at a width of between 150 microseconds and 250 microseconds and having a
waveform that is an asymmetrical bi-phase square pulse; and,
a rectangular insulative land area disposed between the electrodes wherein a
surface of the insulative land area is coplanar with the set of electrodes,
said
electrodes having a length between 0.25 in. and 2.5 in. wherein lengths below
8a
CA 02824697 2016-09-26
. '
0.25 in. do not provide an effective current density to a treatment area and
lengths above 2.5 in. make it difficult to apply enough pressure on the
treatment
area; and, said land having a length between 0.5 in. and 3.5 in. wherein
lengths
below 0.5 in. result in current flow across the skin in the treatment area and
lengths above 3.5 in. make it difficult to position the applicator head on the
nerves in the treatment area.
DESCRIPTION OF THE DRAWINGS
In the accompanying drawings which form part of this specification:
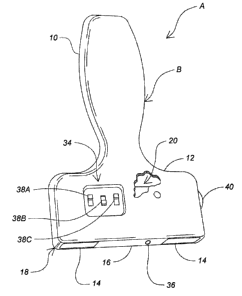
Figure 1 shows a perspective view of one embodiment of a TENS unit with an
applicator head in accordance with the present invention;
Figure 2 shows an end view of the applicator head for one embodiment of the
present invention; and
8b
CA 02824697 2013-08-23
Figure 3 shows a pictorial representation of a cross-section of the applicator
head showing how the electric current is applied to the patient's body during
use
of one embodiment of the present invention.
Corresponding reference numerals indicate corresponding steps or parts
throughout the several figures of the drawings.
DETAILED DESCRIPTION OF AT LEAST ONE EMBODIMENT OF THE INVENTION
In the following description, numerous specific details are set forth such as
examples of some embodiments, specific components, devices, methods, in
order to provide a thorough understanding of embodiments of the present
disclosure. It will be apparent to a person of ordinary skill in the art that
these
specific details need not be employed, and should not be construed to limit
the
scope of the disclosure. In the development of any actual implementation,
numerous implementation-specific decisions must be made to achieve the
developer's specific goals, such as compliance with system-related and
business-related constraints. Such a development effort might be complex and
time consuming, but is nevertheless a routine undertaking of design,
fabrication,
and manufacture for those of ordinary skill.
An embodiment of the present invention is illustrated in the drawings and
figures
contained within this specification.
Referring now to Figure 1, an embodiment of the improved TENS unit A
comprises an applicator B that includes a handle 10 with a paddle portion 12.
A
set of two electrodes 14 separated by an insulative land or block insulator 16
is
9
CA 02824697 2013-08-23
provided in a head 18 of applicator B. In alternative embodiments of the
present
invention, handle 10 and paddle portion 12 can be manufactured from plastic,
wood, or any other material as long as the material selected is an
electrically
insulating material. For example, a polystyrene plastic material could be used
and still remain within the scope of the present invention. It will be
understood
that applicator B may be of any shape as long as the handle allows a user to
be
able to comfortably grip the device for a period of about 15 minutes, and as
long
as the applicator head 18 allows at least one surface of electrodes 14 and
insulative land 16 to be coplanar such that they readily contact the skin
surface of
a patient during treatment with the improved TENS unit A.
The electrodes 14 of a preferred embodiment are made from an electrically
conducting material. In the present embodiment, the two electrodes 14 are made
from medical grade stainless steel. In alternative embodiments, the electrodes
14 are made from electrically conductive metal such as zinc, steel, or other
conductive material which is harmless to the body. It is understood by those
of
skill in the art that the set of two electrodes 14 is conductively attached to
an
electrical connection point, with one electrode being in electrical contact to
an
positive terminal and the other electrode is electrically connected to a
negative
terminal such that a DC current can flow between electrodes 14 if the two
electrodes were conductively connected.
The subject embodiment of the improved TENS unit A includes an electric pulse
provider 20 that is generally disposed within the handle 10. In alternative
embodiments, the electric pulse provider 20 may be located separately from the
CA 02824697 2013-08-23
handle 10 and then electrically connected to the improved TENS unit A by
electrical conductors.
As shown in Figs. 2 and 3, electrodes 14 flank insulative land 16. In a
preferred
embodiment, the skin contacting portion of each electrode measures 1.65 cm
(0.65 in) by 2.54 cm (1.0 in) and has an area of 4.19 cm2. The insulative land
16
measures 3.30 cm (1.3 in) by 1.65 cm (0.65 in). It is noted that human skin
has
a resistance of roughly 6,000 ohms/cm2. The resistivity of skin on electrodes
14
is pictorially represented in Figure 3 by indicating one 6,000 ohm resistor
for
each square centimeter of the electrode surface.
In contrast, flesh 26 beneath skin 22 has very low resistance compared with
the
skin. This resistance varies but is about 5 ¨ 40 ohms/cm2. Resistors 24 are
connected to flesh 26. Electrons passing from the cathode to the anode will
take
the path of least resistance, that pathway under land 16 being a laminar flow
of
electrons through flesh 26 below skin 22. With the electric pulse providers 20
as
described below, the current pathway beneath land 16 is about 0.2 cm (0.08 in)
to about 0.7 cm (0.28 in) deep and the area of the pathway 30 is 0.3 cm2.
Electrons from the cathode 14 having an area of 4.19 cm2 are funneled through
the current pathway under the land 16 having an area of 0.3 cm2 resulting in a
current density theoretically 14 times greater than the current density from
the
electrode. This improvement in current density makes it possible to operate
improved TENS unit A at lower skin currents for safer operation and treatment.
While the theoretical increase in current density could be as high as 14, in
practice it will be less because not all of the current will be perfectly
channeled
11
CA 02824697 2013-08-23
through the compressed area under land 16 but conservatively the increase in
current density will in the order of 2 to 5 times the current density coming
from
the cathode.
The current density in the pathway 30 can be increased by lengthening the
electrodes 14. For example, if the length of the electrode is doubled to 5.08
cm
and the width remains 1.65 cm, the area of the electrode would double to 8.38
cm2 but the area of current pathway 30 would remain the same. The ratio
between the current pathway 30 area and the electrode 14 area is 8.38/0.3 and
the theoretically the gain in current density under the land would be 28. The
width of the electrode is not a factor in this formula because any change in
width
will affect the pathway area by the same factor and the current gain ratio
will
remain the same.
There is a practical limit on the length of the electrodes 14 as the total
area of the
electrodes 14 and the length of land 16 determines the amount of force
required
to compress and flatten the nerve 28 under the land 16. It is preferred that
the
spacing between electrodes 14 and length of land 16 be between about 8.89 cm
(3.5 in) and 1.27 cm (0.5 in). If the land is longer than about 3.5 inches,
there is
a detrimental effect on the laminar flow of the electrons thus reducing the
current
density and the effectiveness of the treatment because it is difficult to
flatten the
treatment area over that length. On the other hand, if the land is shorter
than
about 1.25 cm (0.5 in), the current flow will tend to be on the surface of the
skin
and not under the skin. This is due to the resistance of the surface of the
skin
being in parallel with the resistance from the electrode down through and
under
12
CA 02824697 2013-08-23
the insulating land and skin. If the resistance across the surface of the skin
is R1
and resistance through the skin and under the land and skin is R2, the
parallel
resistance formula R1R2/(R1+R2) will be the determining factor in the path of
least resistance for land lengths of less than 0.5-1 inch. With increasingly
shorter
land length, at some point, the path of least resistance is across the surface
of
the skin with little or no current flowing under the skin, thereby losing
therapeutic
effect.
While the specifics of a preferred embodiment are given above, the electrodes
14
may have a length between about 0.635 cm (0.25 in.) and 6.35 cm (2.5 in.)
Dimensions below about 0.25 in. may not provide enough current density and
dimensions above about 2.5 in. make it difficult to apply enough pressure on
the
treatment area as discussed below. The width of electrodes 14 may be between
about 0.328 cm (0.125 in.) and 6.35 cm (2.5 in.). While the width of
electrodes 14
does not affect the current density, if the electrodes 14 are too wide it may
be
difficult to apply effective pressure to the treatment area. The width of the
insulative
land 16 preferably matches the width of electrodes 14 but may be wider than
the
electrodes but preferably not narrower. While not shown in the drawings, it is
possible that a plurality of applicators B may be grouped together for
treatment of a
larger area.
With continuing reference to Fig. 3, a second key to the high efficiency of
improved TENS unit A is that the nerves 28 under the land 16 and electrodes 14
are compressed and flattened. By flattening the branches of the nerves 28, the
nerve is placed in the dense current pathway. Regular TENS units with
13
CA 02824697 2013-08-23
electrodes in a flexible pad or in a probe do not compress the nerves between
the electrodes and therefore much of the current does not reach the nerve
fibers.
As a result, poor results are often obtained or it may take hours to
desensitize the
nerves.
Those of skill in the art will also appreciate the fact that the edges of each
electrode 14, El and E2 of Figure 2, may tend to work better in some instances
if
those nearest edges are parallel. Thus, in certain preferred embodiments, the
edges of the electrodes 14 that are closest together on applicator head 18 are
positioned to be parallel. The current pathway through the tissue under the
land
16 is thus made constant across the width of electrodes 14.
The electric pulse provider 20 provides an electrical pulse that is
transmitted to
the set of electrodes 14. In the present embodiment, the electric pulse
provider
has the following specifications:
Peak Current 0 - 80 milliamps (500 Ohms)
Average Current 0 - 0.80 milliamps (500 Ohms)
Pulse Rate 40 Hz
Pulse Width 150 or 250 microsecond (selectable)
Waveform Asymmetrical bi-phase square pulse
Peak Voltage 0 -40 Volts (500 Ohms load)
Maximum Charge Per Pulse 20 microcoulombs
¨Power Supply 9 volt battery (alkaline)
14
CA 02824697 2013-08-23
In other embodiments, the above specifications for the electric pulse provider
20
may be adjusted as needed to reap the best benefit as necessary depending
upon the type of pain being treated as long as the electric pulse provider
selected
is capable of performing as described herein.
For use in improved TENS unit A, the electric pulse provider 20 of the present
embodiment includes an intensity control 32 for adjusting the pulse amplitude,
and an array of selector switches 34. The circuitry for electric pulse
provider 20
may be either analog or digital.
In another alternative embodiment, the improved TENS unit A includes an
internal safety interlock switch 36 that is activated by pressing the paddle
portion
12 with applicator head 18 against the patient's leg or other body part. When
the
safety interlock switch 36 is activated, it allows the voltage from the
electric pulse
provider 20 to be transmitted to the electrodes 14. It is understood by those
of
skilled in the art that the design, activation style, and location of the
interlock
safety switch 36 on the improved TENS unit A may be of any appropriate design
as required to meet the unique specifications and applications of alternative
embodiments of the present invention, and as long as no power is transmitted
to
the electrodes 14 until the interlock safety switch 36 detects applicator head
18 is
being pressed against a patient's leg or other body part. In present
embodiment,
the array of selector switches 34 includes an ON/OFF switch 38A a pulse width
switch 386, and a voltage selection switch 38C. The present embodiment also
includes an intensity control 40 on the side of the paddle 12 that can rotated
to
CA 02824697 2013-08-23
either increase or decrease the voltage intensity applied to the patient
during
treatment.
In alternative embodiments of the improved TENS unit A, a standby-off switch
(not shown) may be included. The standby-off switch would turn on the internal
circuits of the electric pulse provider 20, but would not permit the treatment
voltage provided by the electric pulse provider to be fully transmitted to the
electrodes 14 on the applicator head 18. Instead, the stand-by off switch
would
enable the interlock safety switch 36 to function as noted above. In other
words,
the interlock safety switch 36 would not allow treatment voltage from the
electric
pulse provider 20 to be transmitted to the electrodes 14, regardless of the
disposition of the interlock safety switch 36, unless the standby-off switch
was
disposed in the "STANDBY" position. It is understood that in certain
embodiments of the present invention, the standby-off switch would be placed
in
the "STANDBY" position just prior to use of the improved TENS unit A to treat
a
patient.
In an additional alternative embodiment, the improved TENS unit A may be
programmed to ramp up the electrical energy at the electrodes 14 from a very
low voltage to the final treatment voltage for the patient. This is to say,
when
applicator head 18 is placed into position against a patient's leg or other
body
part, the initial voltage potential between the two electrodes 14 may be at or
near
zero volts. Then, over a specified period of time, the voltage potential
between
the two electrodes 14 is increased at a specified rate until the final voltage
potential is reached. It is understood that the final voltage potential in
most
16
CA 02824697 2013-08-23
embodiments would be the actual treatment pulse as necessary for the treatment
of pain for each specific patient. This ramping of the voltage potential
between
the electrodes 14 would provide a sensitive patient with more comfort during
the
pain treatment.
METHOD OF TREATMENT
The improved TENS unit A is intended to be used for the treatment of a wide
variety of pain conditions. As noted above, electric pulse provider 20 may
have
an adjustable output voltage of 0-40 volts/0-80 milliamps and a selectable
pulse
width of 150/250 microseconds at a frequency of 40 Hz. The output from the
electric pulse provider 20 is electrically connected to the set electrodes 14
flanking insulative land 16. As applicator head 18 is pressed against the
treatment area, electrodes 14 and land 16 compress the flesh and flatten the
nerves 28 under land 16. The electron flow in the pathway 30 under land is
channeled in a laminar flow just below the skin and through compressed nerves
28. The skin under the electrodes 14 may be moistened to facilitate the flow
of
electrons through the skin and pathway 30 under land 16.
With this arrangement it is possible to use low voltage with corresponding low
current levels through the high resistance skin to prevent skin bums. For a
given
current, the power developed across a resistance is proportional to the amount
of
the resistance. This is why the skin, with its high resistance, can more
easily be
damaged by electrical current than the low resistance tissue within the body.
After the current passes through the larger cross section of the electrodes 14
and
17
CA 02824697 2013-08-23
through the skin, it recombines just below the compressed skin area in the
smaller cross section of pathway 30. This appears to result in a current
concentration of at least 3 - 5 times as it travels through the flesh under
the
insulating land 16. When the applicator head 18 is pressed against the skin,
the
three dimensional nerve 28 is entirely compressed within an essentially two
dimensional laminar current pathway under the insulating land 16. As a result,
the entire three dimensional nerve can be desensitized with an extremely short
treatment time.
Referring now to Figures 2 and 3, the understood path of the electrical
current
during treatment with the improved TENS unit A and the effect on the general
resistivity of the treated area are illustrated. It is noted that human skin
22 has a
resistance of very roughly 6,000 ohms/cm2. That resistivity is pictorially
represented in Figure 2 and 3 by indicating one 6,000 ohm resistor 24 for each
cm2 attached to the electrode surface. In contrast, flesh 26 beneath the skin
22
has a very low resistance compared with the skin. This resistance is varied,
but
is about 5 -40 ohms/cm2. The other ends of the 6,000 ohm resistors 24 are
connected to the flesh 26.
In a representative treatment for pain, a set of 0-40 volt pulses are applied
by the
applicator head 18 at some low frequency and at some convenient pulse width
across the electrodes. During that application, the electrical current travels
from
the anode 14, through the parallel resistors 24 in skin 22, through the flesh
26 in
pathway 30 under land 16, through the nerve 28 which is compressed by the
insulator land 16, back through the resistors 24 in skin 22 and into the
cathode
18
CA 02824697 2013-08-23
14. The total current possibly flowing through the compressed nerve 28 is the
sum of the currents through the resistors 24.
If the electrodes 14 are made longer as discussed above, the sum of the
current
is increased through the compressed nerve 28 in pathway 30. Likewise, if the
electrodes 14 are shorter in length, the current is reduced through the nerve
28.
It is noted that the above results are generally attributable to the unique
design of
the applicator head 18 with an insulative land 16 flanked with electrodes 14,
all
lying in a plane. In other prior art TENS units the individual electrodes that
are
placed at generally random points on the body and there is no compression of
the flesh 26 between the electrodes. Thus there is no concentration of the
electrical current more directly to the nerve 28. In other words, one aspect
that
makes the present invention unique over the prior art is the fact that
compressing
the nerve 28 with applicator head 18 increases the "effective" current
directed
toward the nerve 28. In addition, the more direct treatment of the nerve 28
can
result in generally reduced treatment time of that nerve than when treated
with
prior art devices.
Referring now back to Figure 1, the general treatment of pain using
embodiments
of improved TENS unit A includes first turning the device on using the
selector
switch 38A. Then, pulse width selector switch 38B is set to 150 microseconds
and the voltage selector switch 38C is set to 4 volts. The applicator head 18
is
then is pressed against the moistened skin of the patient over the center of
the
pain spot. After the flesh 26 under land 16 has been compressed for a moment,
the intensity of the treatment can be gradually increased by rotating the
intensity
19
CA 02824697 2013-08-23
control 32. This is done by slowly increase the intensity control 32 for the
amplitude until a stinging is felt at the targeted pain center. Normally,
holding the
applicator head 18 in this position for about 15 - 45 seconds is usually
sufficient,
but it may take a longer time and require a higher pulse amplitude for
aggressively active pain centers. Longer periods cause no harm but normally
are only necessary when treating aggressive pain centers. During treatment the
patient will continue to feel a stinging sensation in the symptom center until
the
pain and pain symptoms from that pain center are eliminated. This stinging is
the
effect of the ribbon-like flow of pulsating electrical current acting on the
flattened
nerve 28 in the pain or symptom center. The stinging may feel somewhat like
scratching an itch or in aggressively active clusters the stinging may be more
intense. This stinging actually feels satisfying because it "scratches the
itch"
caused by the pain center. Sometimes the portion of the patient's body being
compressed by the applicator head 18 will jerk, but pressure should be
continued
and the applicator head 18 should not be removed during the jerking. When
sensitive patients are being treated, embodiments of the present invention as
noted above that include a gradual ramping upward of the voltage provided by
the electrodes 14 can be used to reduce any discomfort felt by the patient
during
pain treatments.
The object of improved TENS unit A is to stimulate and confuse the nerve at
the
pain center which should provide immediate temporary relief from pain
symptoms. The process may be repeated for any other pain symptom centers
within the problem area. For the pain and nerve problems associated with RLS,
CA 02824697 2013-08-23
Fibromyalgia, and Sciatica, treatment will normally last for 8-36 hours for
each
symptom center depending on the patient. With strained or pulled muscles,
TENS unit A simultaneously relaxes the muscle and desensitizes the painful
nerve centers in the muscle strain. This immediately relaxes the muscle which
allows normal blood circulation to return to the muscle and enables much
faster
healing of the strain in the muscle.
In the preceding description, numerous specific details are set forth such as
examples of specific components, devices, methods, in order to provide a
thorough understanding of embodiments of the present disclosure. It will be
apparent to a person of ordinary skill in the art that these specific details
need not
be employed, and should not be construed to limit the scope of the disclosure.
The scope of the invention should be determined by any appended claims and
their legal equivalents, rather than by the examples given.
21