Note: Descriptions are shown in the official language in which they were submitted.
CA 02824731 2015-06-19
- 1 -
Smoking Articles
Technical Field
The present invention relates to smoking articles and, in particular, to
smoking
articles which combine two or more technological applications that
individually
reduce the machine measured yields of specific constituents or groups of
constituents in mainstream smoke.
Background
Tobacco smoke is a complex, dynamic mixture of more than 5000 identified
constituents of which approximately 150 have been documented as being
JO undesirable. The constituents are present in the mainstream smoke (MS)
which is
inhaled by a smoker and are also released between puffs as constituents of
sidestream smoke (SS).
In 2001 the Institute of Medicine (TOM) reported that, since smoking related
diseases were dose-related, and because epidemiologic studies show reduction
in the
5 risk of smoking related diseases following cessation, it might be
possible to reduce
smoking related risks by developing potential reduced-exposure products
(PREPs).
These they defined as: (1) products that result in the substantial reduction
in
exposure to one or more tobacco toxicants; and (2) if a risk reduction claim
is made,
products that can reasonably be expected to reduce the risk of one or more
specific
20 diseases or other adverse health effects (Stratton, K et al. (2001).
Clearing the Smoke:
Assessing the Science Base for Tobacco Harm Reduction. National Academy Press,
Washington D.C.) To date, no combustible cigarette product has been shown to
meet
the general requirements outlined by the IOM.
The IOM and other groups (Life Sciences Research Office (LSRO) 2007; World
Health
25 Organisation (WHO) 2007) describe a number of stages of activity which
are likely to be
required for a combustible tobacco product to be recognised as a PREP;
however, the
detailed approach and stages required to provide relevant data have yet to be
agreed
amongst the scientific community. For example, some groups have proposed MS
yield
limits for specific smoke constituents and others have suggested that
biomonitoring should
30 play a role in this assessment
CA 02824731 2013-07-12
WO 2012/110819
PCT/GB2012/050349
- 2 -
Much research has been done into the reduction of specific MS constituents
over
recent years. Appioaches have taigeted different parts of the smoking article.
There
have been efforts to reduce the levels of or to remove certain compounds from
the
starting material, for example by genetic engineering or by blending of
specific
tobaccos. Tobacco treatments have sought to reduce or remove compounds from
tobacco material prior to incorporation into the smoking article. Various ways
of
"diluting" the tobacco in the tobacco rod of a smoking article have been
attempted,
using various types of diluents or fillers. Other approaches have involved
ventilation of the smoking article, where ambient air is drawn into the
smoking
article to dilute the MS. Filtration is obviously another area where much work
has
been done to enhance the removal of MS constituents as they pass through the
filter
section of the smoking article. All of these individual measures have
benefits, but
they generally only address a small part of the picture.
A further issue to be addressed is the importance of pioducing a product which
is
acceptable to the consumer. Much of the sensory impact of a conventional
smoking
article is based upon the constituents of the MS. Removing some of these has
the
potential to provide the smoker with an unsatisfactory smoking experience.
There is, therefore, a challenge to provide a smoking article which shows
significant
reduction in emissions of all MS constituents considered to be undesirable.
However, individual measures to reduce certain constituents will frequently
give rise
to no reduction in other constituents and, in some cases, even an increase in
the
levels of others.
Oven11 reductions in smoking machine measured toxicant yields can be achieved
by
diluting the smoke using filter ventilation or using cigarette papers with
high
permeability, and, in the case of toxicants that are associated with the
particulate
phase of smoke, by increasing the filtration efficiency of the filter. For
many years,
governments and public health autholities in various parts of the world
considered
lower ISO tar yielding cigarettes as a way to reduce the health risks of
smoking for
those smokers who do not quit smoking. However, this product modification
approach has more recently been highly criticised. The Study Group on Tobacco
Product Regulation (TobReg) of the World Health Organization has recently
CA 02824731 2013-07-12
WO 2012/110819
PCT/GB2012/050349
- 3 -
proposed a regulatory approach that would limit the yields of a selected group
of
specific smoke constituents. This group also recommended that the yields of
constituents should be limited on the basis of their yields measured with an
intense
smoking machine regime and determined per mg of nicotine.
Appioaches to selectively reducing specific smoke constituents relative to
machine
measured tar and nicotine yields are very dependent upon the physiochemical
nature
of the individual constituents. Conventional cigarette design parameters offer
limited scope for relative reductions in the smoke constituents. For example,
by
increasing the filter efficiency of a conventional cellulose acetate (CA)
filter, the
particulate phase constituents are reduced with the tar and nicotine and
little ot no
selective reduction occurs. And, since cellulose acetate filters have little
or no effect
on volatile constituents, increasing filtration efficiency increases the
ratios of their
yields relative to tar and nicotine.
Increasing filter ventilation has varied effects on the smoke constituents.
The
absolute yields of all the smoke constituents are reduced, but, relative to
tar or
nicotine, yields of most of the particulate phase constituents are unchanged
or may
even be increased. The yields of some of the volatile constituents, such as
ammonia
and carbon monoxide, are reduced relative to both tar and nicotine, while the
relative yields of some of the semivolatile constituents such as phenols are
increased.
Many of the volatile vapoui phase components, such as the volatile aldehydes
and
hydrogen cyanide may be selectively reduced using adsorbent materials in the
filter
such as activated charcoal or certain resins. However, permanent gases, such
as
carbon monoxide and nitric oxide, are not amenable to adsorption at room
temperature, and toxicants in the particulate phase cannot be selectively
reduced by
filtration since they are largely bound into the aerosol particles.
Since the 1950s, attempts have been made to selectively remove or reduce
constituents from cigarette smoke. Adsorption by porous adsorbents is a
possible
means of removing some of the volatile constituents from smoke. Active Carbon
(AC) is a nonselective adsorbent which is widely used in cigarette filters and
can
reduce a broad range of volatile smoke constituents to a significant extent
via
CA 02824731 2013-07-12
WO 2012/110819
PCT/GB2012/050349
- 4 -
physisorption. However, the difficulty of this challenge should not be
underestimated. With cigarette smoke adsorbents there is a need to operate
under
high flow rate conditions (approximately 1 L per min for typical machine-
smoking
conditions), and therefore very short contact times between smoke constituent
and
filtei adsorbent (of the order of milliseconds). Adsorbents also need to
function at
the gas-sohd interface (i.e. not in solution) and in the presence of thousands
of
other chemicals in both vapour and particulate phases. Adsorbent surfaces are
also
susceptible to blocking by condensing smoke aerosol particles. For permanent
gases, and smoke constituents with high vapour pressures at ambient
temperatures
such as formaldehyde, acetaldehyde or HCN, physical adsorption has been found
to
be less effective and alternative routes are required.
Cigaiette smoke contains a number of volatile aldehydes, both saturated
compounds
such as formaldehyde, acetaldehyde, propionaldehyde and butyraldehyde, and
unsaturated compounds such as acrolein and crotonaldehyde. Carbonyls in
cigarette
smoke are mainly generated by combustion of a number of tobacco constituents,
mostly carbohydrates. In particular it is thought that sugars are major
sources of
formaldehyde in cigarette smoke. Cellulose has been suggested to be the major
precursor of mainstream smoke acetaldehyde. There are some data suggesting
that
glyceiol, a material sometimes added to tobacco as a humectant, is an
additional
precursor for aciolem. Although the boihng point of formaldehyde is sub-
ambient,
30% of formaldehyde in the mainstream smoke exiting a filtered cigarette
resides in
the particulate phase and thus is not available for selective filtration at
room
temperature. Due to the presence of water vapour, formaldehyde in the
particulate
phase of smoke exists as the hydrated foim, CH2(OH)2. Acetaldehyde, one of the
highest yield constituents of cigarette smoke, exists at or around its boiling
point at
ambient temperatures, and therefore has a very high vapour pressure. The
combination of these two factors makes substantial removal of acetaldehyde
from a
smoke stream by filter additives a major challenge.
A promising approach to achieving substantial specific reductions in
particulate
constituents from a conventionally structured cigarette is to modify the
tobacco.
Substitution of different tobacco varieties into the blend can have an impact
on
yields of several smoke constituents. For example there are higher yields of
the
CA 02824731 2013-07-12
WO 2012/110819
PCT/GB2012/050349
- 5 -
nitrogen containing smoke constituents from burley tobacco than from flue
cured
or oriental, and higher yields of formaldehyde and catechol from flue-cured
tobaccos. However, decreases in one constituents or set of constituents are
often
offset by increases in other constituents. To avoid this it would be useful to
be able
to identify and remove precursors to smoke constituents from the tobacco leaf.
With the exception of the metallic constituents (chromium, nickel, arsenic,
selenium, cadmium, mercury and lead) and some of the tobacco specific
nitrosamines (TSNAs), such as NAT and NAB, which are transferred directly from
the leaf, the majority of the smoke constituents are formed by pytosynthesis
from
the leaf components. Thus, the major precursors for the volatile carbonyls,
benzo(a)pyrene, carbon monoxide, benzene and toluene are the structural
carbohydrates such as pectin and cellulose as well as the sugars. The
nitrogenous
smoke constituents are formed from mttogenous precursors in the leaf, and
there is
considerable evidence that ptotem and amino acid combustion contributes to the
generation of several nitrogen containing smoke constituents on the Health
Canada
hst. Proteins and amino acids have been repotted to be precursors for hydrogen
cyanide, pyridine and quinoline, 2-ammonaphthalene and 4-aminobiphenyl.
Tobacco
protein is also strongly correlated with the formation of mutagenic
heterocyclic
amines and the resulting mutagenicity of smoke condensate in the TA98 Ames
assay.
The polyphenols in tobacco are major precursors for phenolic smoke compounds.
Chlorogenic acid, the most abundant polyphenol in flue-cured tobacco, is a
major
precursor for phenol, catechol and the substituted catechols, while
hydroquinone
has also been repotted as a chlorogenic acid pyrolysis product. Rutin and
caffeic
acid also generate catechol and substituted catechols on pyrolysis but because
of
their low concentrations in tobacco and because of their lower pyrolytic
yields their
contributions to catechol in flue-cured tobacco smoke are much less than
chlorogemc acid. Resotcinol is known to be a major product from pyrolysis of
rutin.
Detailed Description
The present invention provides combinations of bespoke tobacco blends with
bespoke adsorbent filter additives, which result in a smoking article having a
CA 02824731 2014-10-01
- 6 -
significant reduction in mainstream smoke constituents considered to be
undesirable.
More specifically, the present invention provides a smoking article
comprising: (a) a
tobacco blend comprising one or more tobaccos or tobacco grades with low TSNA
and/or metal content; and (b) a high activity carbon comprising a synthetic
polymer-
derived carbon material; further comprising one or both of: (c) a tobacco
blend that has
been treated to remove polyphenols and/or peptides; and (d) a tobacco
substitute sheet
comprising a non-combustible inorganic filler, a binder and an aerosol
generating means;
optionally further comprising: (e) an amine-functionalised chelating resin.
In a preferred embodiment, the smoking articles according to the invention
have a reduction in at
least 75%, preferably at least 90% and more preferably in all of the key
constituents of
mainstream smoke, as defined herein.
The so-called "key constituents" of NIS referred to in connection with the
present invention are
those smoke constituents which have been identified in the literature as being
undesirable (see,
for example, The Scientific Basis of Tobacco Product Regulation: Report of a
WHO Study
Group (2007) WHO Technical Report Series 945, Geneva) and/or those whose
yields have been
analysed in the data provided herein (see, for example, Tables 6, 7 and 8).
The reduction is preferably determined using one of the smoking machine
conditions set out in
Table 3. Preferably, the reduced yields are measured under Health Canada
Intense smoking
machine conditions.
The reduction in yield of the key constituents is preferably at least 5% or at
least 10% or more.
Preferably, where the smoking articles of the present invention include a
tobacco blend
comprising one or more tobaccos or tobacco grades with low TSNA and/or
CA 02824731 2013-07-12
WO 2012/110819
PCT/GB2012/050349
- 7 -
metal content, they further comprise two or more other technologies listed as
(b) to
(e).
Brief Description of Figures
In order that the invention may be more fully understood, aspects and
embodiments thereof will be described, by way of non-limiting example only,
with
reference to the accompanying drawings, in which:
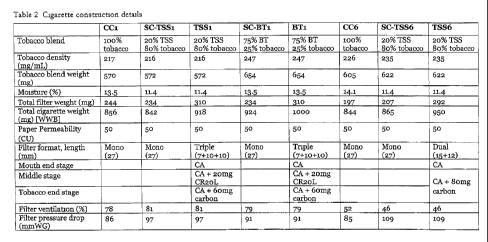
Figure 1 shows Table 2, setting out the cigarette construction details.
Figure 2 shows Table 4, setting out the major constituent yields of test
cigarettes
using different smoking machine condition.
Figure 3 shows Table 5, setting out the blend metal and tobacco-specific
nitrosamine contents.
Figure 4 shows Table 6, setting out the MS yields of metals and TSNAs measured
under Health Canada Intense smoking machine conditions.
13 Figures 5A, 5B and 5C show Table 7, setting out the MS yields of other
smoke
constituents measured under Health Canada Intense smoking machine conditions.
Figure 6 shows Table 8, setting out the MS yields of carbonyl and
miscellaneous
volatile and vapour phase smoke constituents in control and triple stage
filter EC
measured under Health Canada Intense smoking machine conditions.
Figure 7 shows Table 9, setting out the MS yields of carbonyl and
miscellaneous
volatile and vapour phase smoke constituents in control and dual stage filter
EC
measured under Health Canada Intense smoking machine conditions.
Figure 8 shows Table 10, setting out the sidestream smoke yields under ISO
smoking machine conditions
23 Figuie 9 shows a comparison of HCI machine toxicant yields from ECs (lmg
ISO)
with those from published data sources.
CA 02824731 2013-07-12
WO 2012/110819
PCT/GB2012/050349
- 8 -
Figure 10 shows a comparison of HCI machine toxicant yields from ECs (6mg ISO)
with those from published data sources.
Figure 11 shows a comparison of Total Toxicant Yields (TTY) between EC yields
and published HCI yield data.
Figure 12 shows a comparison of total yields from a subset of toxicants (TSY)
between EC yields and published HCI yield data.
Figuie 13 shows a comparison of total normalised toxicant yields (NTT) between
ECs and published HCI yield data.
Figure 14 shows a summary of the process by which high activity polymer-
derived
carbon is prepared.
Figure 15 shows Table 15, setting out the smoke and biomarker changes for test
products as compared with a control cigarette.
Figure 16 shows the in vivo study design.
Figures 17 and 18 show the results of the in vivo study.
Figure 19 shows a smoking article design according to an embodiment of the
invention.
Two low toxicant tobacco blends, featuring a tobacco substitute sheet (TSS) or
a
tobacco blend treatment (BT), were combined with filters containing an amine
functionalised resin material (CR2OL) and/or a high activity carbon adsorbent
(HAC) to generate three experimental cigarettes (ECs). Mainstieam smoke (MS)
yields of smoke constituents were determined under four different smoking
machine
conditions. Health Canada Intense (HCI) machine smoking conditions gave the
highest MS yields for nicotine-free dry particulate matter and for most smoke
constituents measured. Constituent yields from the ECs were compared with
those
from two commercial comparator (CC) cigarettes, three scientific control (SC)
cigarettes and published data on 120 commercial cigarettes. The ECs were found
to
generate some of the lowest machine yields of constituents from cigarettes for
which HCI smoke chemistry is available; these comparisons therefore confirm
that
CA 02824731 2013-07-12
WO 2012/110819
PCT/GB2012/050349
- 9 -
the ECs generate reduced MS machine constituent yields in comparison to
commercial cigarettes.
The first stage in the design of a cigarette-based PREP involved the
development of
technologies which reduce the yields of smoke constituents. Experimental
cigarettes
(ECs) were assembled using these technologies and then assessed for their
constituent yields using smoking machines; comparison to relevant control and
reference products indicated the effectiveness of the cigarette design in
generating
reduced yields of constituents. Those ECs which are found to reduce smoking
machine measured yields of smoke constituents, in comparison to reference
products, are termed "reduced machine-yield prototypes" (RMYPs).
The inventors have described different individual technological approaches to
the
reduction of constituents in cigarette smoke, one of which involves the
selection of
tobacco blend components to provide a blend with reduced levels of the known
precursors of undesirable smoke constituents, two of which modify the tobacco
and
two of which modify the cigarette filter. The tobacco blend (TB), the tobacco-
substitute sheet material (TSS) and the tobacco blend treatment (BT) reduce
the
generation of constituents at source within the burning cigarette. The two
filter
technologies, an amine functionalised resin material (CR2OL) and a high
activity,
polymer-derived, carbon adsorbent (HAC), remove volatile species from the
smoke
stream after formation. These technologies ate discussed in greater detail
below (in
Section 2.1).
Tobacco Blend
This involves the selection of tobacco blend components that exhibit low
levels of
the precursors of undesirable smoke constituents, such as TSNAs and metals.
For
example, the levels of TSNAs may be reduced by using specific (such as
lighter)
tobacco blends and by selecting parts of the tobacco plant that are low in
nitrate, a
precursor of TSNAs. The person skilled in the art would be well aware of the
ways
in which the blending process may be adapted to provide a tobacco blend having
these desired properties.
The tobacco blend may also comprise expanded tobacco, which is cut tobacco
that
has been expanded to reduce the mass of tobacco burnt in a cigarette. The
CA 02824731 2013-07-12
WO 2012/110819
PCT/GB2012/050349
- 10 -
expansion processes are similar to those used to make puffed rice snack food.
One
process used is called dry-ice expanded tobacco (DIET) and involves permeating
the tobacco leaf structure with liquid carbon dioxide before warming. The
resulting
carbon dioxide gas forces the tobacco to expand. Some of the commercially
3 available tobacco brands with low ISO tar yields use some proportion of
expanded
tobacco in the overall blend.
Tobacco Blend Treatment
Treated tobacco blends are described herein which have been treated by
processes
that allow the removal of protein and polyphenols from tobacco, with a
beneficial
effect on the smoke toxicant yields. The tobacco treatment was carried out on
cut,
flue-cured tobacco, and involved extraction of the tobacco with water followed
by
treatment with an aqueous protease enzyme solution. After treatment of the
tobacco
extract with adsorbents and concentration, the solubles were re-applied to the
extracted tobacco. The treated tobacco retained the structure of the original
tobacco
13 and was made into cigarettes using conventional cigarette making
equipment,
without the need for reconstitution into a sheet material.
Tobacco Substitute Sheet
Another approach to reducing smoke toxicant yields is to dilute the smoke with
glycerol and it is proposed to include up to 60% of a glycerol-containing
"tobacco
substitute" sheet in cigarettes. Analysis of mainstream smoke from such
experimental cigarettes showed reductions in yields of most measured
constituents,
other than some volatile species.
Amine-functionalised resin material
It has been found that chemisorption is capable of removing high volatility
aldehydes and HCN from mainstream cigarette smoke. A weakly basic macroporous
polystyrene resin cross-linked with &vinyl benzene, with surface amine
functionality, was identified and assessed as a cigarette filter additive. The
material,
manufactured by Mitsubishi Chemical Corporation is normally supplied in bead
form in an aqueous environment and sold under the trade name DiaionOCR20
(hereafter referred to as CR20). This material offers the potential for the
'
CA 02824731 2015-06-19
nucleophilic capture of aldehydes from mainstream smoke, and due to its weakly
basic nature it may also be used for the removal of HCN from MS.
The amine-functionalised chelating resin material may be incorporated into the
filter
of a smoking article in a cavity, or dispersed (dalmation style) throughout
the filter
material (such as cellulose acetate) in the whole or a section of the filter.
High Activity Carbon
A high activity material comprising spherical particles of polymer-derived
carbon
was prepared by a propriety process (Von Blucher and De Ruiter 2004: US Patent
Application Publication No. 2004/0038802; Von Blucher et el. 2006: EP 1918022
(A1);
Bohringer and Fichtner 2008: International Patent Publication No. WO 2008
110233)
and was available from Blucher GmbH (Germany). The polymer-derived material is
approximately twice as effective, in general, at removing volatile cigarette
smoke
toxicants than the coconut shell-derived carbon commonly used in contemporary
carbon filtered cigarette products. The polymer-derived carbon performed well
at
both ISO and HCI smoking regimes and with regular and smaller circumference
cigarettes. Limitations were also observed under higher flow-rate smoking
conditions in the removal of acetaldehyde.
The high activity carbon may be incorporated into the filter of a smoking
article in a
cavity, or dispersed (dalmation style) throughout the filter material (such as
cellulose
acetate) in the whole or a section of the filter.
The present invention provides ECs made using combinations of the blend and
filter technologies described. The goal of the study of these ECs was to
assess
whether these technologies could be combined into prototypes which reduce
machine yields of toxicants in comparison to commercial products, and have the
potential to reduce exposure of smokers to toxicants in human smoking.
Testing the ECs under a variety of smoking machine conditions and analysing
the
yields of smoke constituents on a per cigarette basis and as a ratio per
milligram of
nicotine yield, permits comparisons with relevant commercial comparator
cigarettes,
and also to a wide range of products reported in the literature. The results
presented
in this work demonstrate that the development of combustible RMYPs is
feasible.
CA 02824731 2013-07-12
WO 2012/110819
PCT/GB2012/050349
- 12 -
2. Materials and Methods
2.1 Design of Experimental, Control and Compaiator Cigarettes
The ECs were constructed from combinations of blend and filter technologies
that
were developed to reduce specific chemical classes of smoke toxicants or their
3 precursors in tobacco (Table 1). For each EC individual tobacco grades
with low
TSNA and metal contents were selected and blended to provide a low toxicant
starting point for the design of experimental cigarettes.
Table 1. Technologies used in the construction of experimental cigarettes
(ECs).
Technological Cigarette Description Potential
Application Component Reduction
Tobacco Blend Blend Selection of tobacco blend Some TSNAs
(TB) components that exhibit and metals
low levels of the
precursors of undesirable
smoke constituents
Tobacco Blend Tobacco-substitute sheet Whole smoke
Substitute reducing tobacco
Sheet (TSS) combustibles and giving
glycerol dilution of smoke
Tobacco Blend Blend Protease treated tobacco, Nitrogen-based
Treatment reducing protein nitrogen constituents:
(BT) and polyphenols in the aromatic
blend amines, NAB,
NAT, NNK,
NNN; phenols
Amine- Filter Amine group HCN, HCHO,
functionalised functionahsed resin acetaldehyde
Resin Beads included in filter stage and other
(CR2OL) caibonyls
High Activity Filter Polymer-derived, spherical Vapoui phase
Carbon carbon beads included in constituents
(HAC) filter stage
Tobacco Blend Treatment
Briefly, the tobacco blend is subjected to an aqueous extraction step and the
extract
is subsequently passed through two stages of filtration to remove polyphenols
and
soluble peptides. The residual tobacco solids are treated with protease to
remove
insoluble proteins. After washing and enzyme deactivation, the tobacco sohds
and
filtered aqueous extract are re-combined. The treatment process iesults in
reduced
smoke yields of phenolics, aromatic amines, HCN, and a number of other
CA 02824731 2013-07-12
WO 2012/110819
PCT/GB2012/050349
- 13 -
nitrogenous smoke constituents; however, there are also increases in the
yields of
formaldehyde and isoprene.
The tobacco material to be extracted may be strip, cut, shredded or ground
tobacco.
In a preferred embodiment, the tobacco is shredded tobacco. Other foims of
tobacco may, however, be extracted using the methods described herein.
The tobacco material may be mixed with a solvent for extraction to form a
slurry.
The solvent may be added to the tobacco material in a ratio of between 10:1
and
50:1, preferably between 20:1 and 40:1 and most preferably between 25:1 and
30:1
by weight. In a particularly preferred embodiment, the solvent is added to the
tobacco material in a ratio of 27:1 by weight.
The solvent may be an organic solution, but preferably is an aqueous solution
or is
water. At the very start of the extraction process, the solvent is usually
water, but it
can also contain alcohols such as ethanol or methanol, or it can contain a
surfactant.
Other solvents could be used, depending on the particular constituents to be
extracted from the tobacco.
The extraction may be performed at 15-85 C, and preferably is performed at 65
C.
It is preferable for the slurry to be continually stirred during extraction,
such that
the tobacco remains in suspension. Extraction should be performed for between
15
minutes and two hours. In a preferred embodiment, extraction is performed for
approximately 20 minutes.
During extraction, soluble tobacco components are removed from the tobacco
material and enter solution. These include nicotine, sugars, some proteins,
amino
acids, pectins, polyphenols and flavours. Up to about 55% of the initial
tobacco
weight may become solubilised. It is important that the pectins in the tobacco
fibre
remain cross-linked throughout the extraction and treatment process in order
to
maintain the fibrous structure of the tobacco. Accordingly, calcium may be
added
to the solvent used to extract the tobacco and to any solutions used in the
downstream processing procedures.
CA 02824731 2013-07-12
WO 2012/110819
PCT/GB2012/050349
- 14 -
Following extraction, the slurry may be drained to allow the liquid filtrate
(the
"mother filtrate") to be collected. Meanwhile, the insoluble tobacco residue
may be
further extracted by counter-current washing as it is conveyed, so that as
many
soluble constituents as possible are removed from the tobacco.
Fresh solvent may be applied to the tobacco and the filtiate (the "wash
filtrate") is
collected. The wash filtrate may be recycled by being applied to the incoming
tobacco residue travelling on the belt at an upstream point. The collection
and
upstream reapplication of wash filtrate to incoming tobacco residue may be
repeated
a number of times, preferably three, four or even five times. Thus, the final
wash
filtrate that is collected at the head of the belt may be concentrated in
those soluble
tobacco constituents that have been removed from the tobacco residue as it
travels
the length of the filter. The final wash filtrate may be further recycled by
being
added to fresh tobacco to form a tobacco slurry, ready for extraction. For
example,
the final wash filtrate may be added into the tobacco mix tank where a tobacco
slurry is formed prior to extraction. The extraction process may thus be a
continual
process in which fresh tobacco is extracted using recycled wash filtrate. Only
at
start-up of this extraction process is tobacco extracted with fresh solvent.
Once the
extraction process has begun, no fresh solvent is used in the extraction, but
the
solvent is solely made up of recycled wash filtrate.
As the extraction process continues, the extract thus becomes more
concentrated in
soluble tobacco constituents. These constituents include those that entered
solution during primary extraction in the extraction tank (forming the mother
filtrate), as well as those that entered solution during secondary extraction
on the
horizontal belt filter (forming the wash filtrate).
The final filtrate thus comprises both the mother and wash filtrates. In so
doing,
the tobacco residue that results after filtration is devoid of those
constituents that
are soluble in the solvent used for extraction. The extracted tobacco may be
squeezed at the end of filtration, so as to remove any excess liquid from it.
The
extracted tobacco emanating from the horizontal belt filter is thus typically
in the
form of a de-watered mat.
CA 02824731 2013-07-12
WO 2012/110819
PCT/GB2012/050349
- 15 -
The final filtrate, hereinafter referred to as the tobacco extract, may be
subsequently
processed to remove those constituents not desired in the final tobacco
product.
Undesirable constituents include proteins, polypeptides, amino acids,
polyphenols,
nitrates, amines, nitrosammes and pigment compounds. The levels of
constituents
3 which may be considered desirable, such as sugar and nicotine, may,
however,
remain unaffected so that the flavour and smoking properties of the extracted
tobacco are comparable to those of the original material.
In a preferred embodiment, the tobacco extract is treated to remove proteins,
polypeptides and/or amino acids. Up to 60% of the proteins contained in the
original tobacco material may be removed using an insoluble adsorbent such as
hydroxyapatite or a Fuller's Earth mineral such as attapulgite or bentonite.
The
tobacco extract is preferably treated with bentonite, to remove polypeptides
therefrom. Bentonite may be added to the extract in an amount of 2-4% of the
weight of tobacco initially extracted. Alternatively, the tobacco extract may
be fed
13 into a tank containing a slurry of bentonite in water. A suitable slurry
contains
approximately 7 kg of bentonite in approximately 64 kg water (quantities per
hour),
for example, 7.13 kg bentonite in 64.18 kg water (quantities per hour). In any
case,
the bentonite concentration should be high enough to substantially reduce the
protein content of the tobacco extract, but not so high as to additionally
adsorb
nicotine from it. Bentonite treatment may also be effective in the removal of
pigment compounds found in tobacco extract which, if not removed, tend to
darken
the extract after concentration. When sufficient bentonite is used to treat
the
extract, the reduced amount of pigment compounds may result in a product that
is
not overly darkened in appearance.
23 Following bentonite treatment, the tobacco extract may be purified from
the slurry
by centrifugation and/or filtration. The tobacco extract may also, or
alternatively,
be treated to remove polyphenols therefrom.
Polyvinylpolypyrrolidone (PVPP) is an insoluble adsorbent for polyphenols,
traditionally used in the brewing industry to remove polyphenols from beer.
PVPP
in an amount of 5-10% of the weight of tobacco initially extracted may be
added to
the extract. This amount of PVPP is capable of removing between 50 and 90% of
CA 02824731 2013-07-12
WO 2012/110819
PCT/GB2012/050349
- 16 -
the polyphenols in solution. The optimum pH for removal of polyphenols from
the
tobacco extract by PVPP is believed to be about 3. The efficiency of
adsorption by
PVPP may therefore be increased by reducing the pH of the extract via the
addition
of a suitable acid, such as hydrochloric acid.
3 As an alternative to using PVPP to adsorb the polyphenols, one or more
enzymes
may be added to the tobacco extract to degrade the polyphenols therein. A
suitable
enzyme is laccase (urishiol oxidase). The invention is not, however, limited
to
methods for removing only proteins and/or polyphenols from tobacco.
Alternative
or additional enzymes, agents or adsorbents may be used to remove other
undesirable tobacco constituents from the tobacco extract. Examples of further
undesirable tobacco constituents that could be removed from the extract
include
nitrates, amines and nitrosamines.
If a plurality of constituents is to be removed from the tobacco extract, a
number of
tanks may be set up in series, each one comprising a different enzyme, agent
or
13 adsorbent, in order for a chosen complement of undesirable constituents
to be
removed. Alternatively, a single tank may contain a plurality of enzymes,
agents or
adsorbents so that the undesirable constituents may be removed in a single
step.
For example, a bentonite or PVPP holding tank could comprise one or more
additional enzymes, agents or adsorbents so as to remove not only protein ot
phenols from the tobacco, but one or more further undesirable constituents
also.
Following treatment of the tobacco extract to remove the selected undesirable
constituents, the extract is preferably concentrated to a solids concentration
of
between 20 and 50% by weight. Concentrations of up to 10% solids are most
efficiently achieved using reverse osmosis. A further concentration to
23 approximately 40% solids may be achieved by means of a falhng film
evaporator.
Othei methods of concentration can be used and will be known to a person
skilled
in the art. The concentrated tobacco extract may be subsequently recombined
with
the extracted tobacco.
The tobacco, having been extracted in an aqueous solution as discussed above,
however, is preferably further extracted to remove one or more further
undesirable
constituents before being recombined with the concentrated tobacco extract.
CA 02824731 2013-07-12
WO 2012/110819
PCT/GB2012/050349
- 17 -
Further extraction of the tobacco may be performed using an enzyme
specifically
selected for removal of the constituent of choice. In a preferred embodiment,
the
enzyme is a proteolytic enzyme for removal of protein from the tobacco. The
enzyme is preferably a bacterial or fungal enzyme and, more preferably, is an
enzyme used commercially in the food and detergent industries. The enzyme may
be selected from the gioup consisting of SavinaseTM, NeutraseTM, EnzobakeTM
and
Alcalasem, which are all available from Novozymes A/S. The proteolytic enzyme
is
preferably added to the tobacco in an amount of between 0.1 and 5% by weight
of
the tobacco material. For example, Savinaseni may be added to the tobacco in
an
amount of approximately 1% by weight. The tobacco may be teslurried in a
solution
of the chosen enzyme. The ratio of water to tobacco in the slurry should be
between 10:1 and 50:1, preferably between 20:1 and 40:1 and most preferably
between 25:1 and 30:1 by weight. In a particularly preferred embodiment, the
ratio
of water to tobacco is 27:1 by weight.
The pH of the tobacco/enzyme mixture should be that which promotes optimal
enzyme activity. Accordingly, it may prove convenient to feed the dewatered
mat of
tobacco into a tank in which the pH is adjusted, for example, by the addition
of a
base such as sodium hydroxide. The pH-adjusted tobacco may then be fed into an
enzyme dosing tank for mixing with the enzyme of choice. The tobacco/enzyme
mixture may subsequently be fed into a plug flow reactor, where the enzymic
extraction is performed. The enzymic extraction should be carried out at the
temperature ptomoting optimal enzyme activity. Preferably, a narrow
temperature
range, such as 30-40 C, should be used to avoid denaturing the enzyme. The
optimum working conditions when SavinaseTM is the chosen enzyme are 57 C and
pH 9-11. The enzymic extraction should be carried out for at least 45 minutes;
any
shorter duration is believed to be insufficient for a proteolytic enzyme to
degrade
tobacco proteins.
Of course, multiple enzymic extractions could be carried out if there are
multiple
constituents to be removed from the tobacco. These could be performed in
series
or multiple enzymes could be added to the tobacco in a single treatment step.
CA 02824731 2013-07-12
WO 2012/110819
PCT/GB2012/050349
- 18 -
It also remains possible for the enzyme to be included in the very first
extraction
step in the treatment process, rather than forming a subsequent separate
extraction
step.
Following enzymic extraction, the insoluble tobacco residue may be washed with
a
salt solution, preferably a sodium chloride solution, to rinse it free of
enzyme. Salt
rinsing may be performed in a sequential, counter-current fashion.
Salt and water rinsing, however, may not be sufficient to remove all of the
enzyme
from the tobacco. The washed tobacco may also be treated to deactivate any
residual enzyme remaining in the tobacco following the salt and water rinses.
This
may be done by steam treating the tobacco sufficiently to deactivate the
enzyme,
but not so much that the tobacco loses its fibrous form. In an embodiment,
steam
treating is carried out at 98 C for four minutes, but the residence time may
be
increased to 10 minutes or so if desired. Alternatively, the tobacco may be
heat
treated to deactivate the enzyme, for example by microwaving or baking the
tobacco. In another embodiment, the enzyme may be deactivated by chemical
denaturation; steps should however be taken to remove the chemical from the
tobacco.
The processed tobacco may then be recombined with the concentrated tobacco
extract. Adding the treated extract back to the extracted tobacco ensures
retention
of water soluble flavour components of tobacco and nicotine in the final
product.
Recombination therefore results in a tobacco product that has similar physical
form
and appearance, taste and smoking pioperties to the original material, but
with
substantially reduced levels of protein, polyphenols or other constituent(s)
of
choice. Recombination may be achieved by spraying the tobacco extract onto the
tobacco. The amount of the original extract being recombined with the
processed
tobacco depends upon the amount that was lost during treatment of the extract
to
remove selected constituents, and will vary from one type of tobacco to the
next.
A standard diying process may be used to dry the treated tobacco, either
before,
during or after recombination with the treated tobacco extiact. The starting
moisture content of the treated tobacco is typically approximately 70-80%. In
a
preferred embodiment, the moisture content after drying should be
approximately
CA 02824731 2013-07-12
WO 2012/110819
PCT/GB2012/050349
- 19 -
14%. A heated dryer, such as an apron dryer, may be used to reduce the
starting
moisture content in the tobacco to approximately 30%. A second heated dryer,
such as an air dryer, may then be used to further reduce the moisture content
to
approximately 14%.
The final dried product may subsequently be processed into a finished form,
such as
a sheet, which, when shredded, can form all or part of a cigarette filler.
Owing to as
much as 30% of the original constituents of tobacco being removed therefrom
during the extraction and treatment process, however, the concentration of
remaining constituents per unit weight of tobacco is increased in the finished
product compared to the original material. These constituents include
cellulose,
which, together with sugars and starches, may produce harmful volatile
materials
such as acetaldehyde and formaldehyde in smoke when combusted.
Tobacco Substitute Sheet
Incorporation of the tobacco substitute sheet (TSS) into a tobacco blend
reduces
the quantity of tobacco in a cigarette, thereby diminishing the overall
potential for
the cigarette to generate toxicants. The TSS also contains glycerol and, when
heated,
the TSS releases glyceiol into the smoke stream contributing to the total
amount of
particulate smoke, measured as nicotine-free dry particulate matter (NFDPM,
also
known as "tar"). As most cigarettes are designed to meet a specific NFDPM
yield
value, incoiporation of glycerol into the smoke stream effectively results in
a
reduced contribution of the tobacco combustion products to the overall NFDPM
value: this process is termed "dilution." The incorporation of TSS into
cigarettes
results in reductions in a wide range of smoke constituents, including both
particulate and vapour phase toxicants. In vitro toxicological tests showed
reductions in the activity of smoke particulates in proportion to their
glycerol
content. Human exposure to nicotine was reduced by a mean of 18% as deteimined
by filter studies and by 14% using 24 hour urinary biomarker analysis. Smoke
particulate exposures were reduced by a mean of 29% in filter studies and by
similar
amounts based on urinary 4-(m ethylnitrosammo)-1-(3- pyridy1)-1-butanol
concentrations. These results show that reducing exposure to some smoke
toxicants
is possible using a tobacco substitute sheet.
CA 02824731 2013-07-12
WO 2012/110819
PCT/GB2012/050349
- 20 -
According to the present invention, a smoking article may be prepared
including a
tobacco substitute sheet material comprising a non-combustible inorganic
filler
material, an alginic binder and aerosol generating means.
Advantageously the tobacco substitute sheet material comprises as the main
components thereof, non-combustible inorganic filler, binder and aerosol
generating
means, with these three components together preferably comprising at least 85%
by
weight of the tobacco substitute sheet material, preferably greater than 90%,
and
even more preferably total about 94% or more by weight of the tobacco
substitute
sheet material. The three components may even be 100% of the tobacco
substitute
sheet material. The remaining components are preferably one or more of
colourant,
fibre, such as wood pulp, or flavourant, for example. Other minor component
materials will be known to the skilled man. The tobacco substitute sheet
material is
therefore a very simple sheet in terms of its constituents.
As used herein, the term' tobacco substitute sheet material' means a material
which
can be used in a smoking article. It does not necessarily mean that the
material itself
will necessarily sustain combustion. The tobacco substitute sheet material is
usually
produced as a sheet, then cut. The tobacco substitute sheet material may then
be
blended with other materials to produce a smokeable filler material.
The present invention further provides a smoking article comprising a wrapped
rod
of a smokeable filler material, the smokeable filler material consisting of a
blend
which incorporates tobacco substitute sheet mateiial comprising a non-
combustible
inorganic filler, an algimc binder and aerosol generating means, the smoking
article
having an aerosol transfer efficiency ratio of greater than 4Ø As used
herein, the
aerosol tiansfer efficiency is measured as the percentage aerosol in the smoke
divided by the percentage aerosol in the smokeable filler material. Preferably
the
aerosol tiansfer efficiency is greater than 5, and more preferably greater
than 6.
The smokeable filler material used in the smoking articles of the present
invention
may comprise a blend consisting of not more than 75% by weight of the tobacco
substitute sheet material.
CA 02824731 2013-07-12
WO 2012/110819
PCT/GB2012/050349
- 21 -
Preferably the inorganic filler material is present in the range of 60-90%,
and is
more preferably greater than 70% of the final sheet material. Advantageously
the
inorganic filler material is present at about 74% by weight of the final sheet
material, but may be present at higher levels, for example, 80%, 85% or 90% by
weight of the final sheet material.
The non-combustible filler advantageously comprises a proportion of material
having a mean particle size in the range of 500[1m to 75p.m. Preferably the
mean
particle size of the inorganic filler is in the range of 4001.tm to 100 m, and
is more
than 125p.m, and preferably more than 150p.m. Advantageously the mean particle
size is at or about 170p.m, and may be in the range of 170 m to 200pim. This
particle size is in contrast to that conventionally used for food grade
inorganic filler
materials in alternative tobacco products, namely a particle size of about 2-
311m.
The iange of particle size seen for each inorganic filler individually may be
from
1 p.m-lmm (100011m). The inorganic filler material may be ground, milled or
precipitated to the desired particle size.
Advantageously the inorganic filler material is one or more of perlite,
alumina,
diatomaceous earth, calcium carbonate (chalk), vermicuhte, magnesium oxide,
magnesium sulphate, zinc oxide, calcium sulphate (gypsum), ferric oxide,
pumice,
titanium dioxide, calcium alummate or other insoluble alummates, or other
inorganic filler materials. The density range of the materials is suitably in
the range
of 0.1 to 5.7 g/cm3. Advantageously, the inorganic filler material has a
density that
is less than 3 g/cm3, and preferably less than 2.5 g/cm3, more preferably less
than
2.0 g/cm3 and even more preferably less than 1.5 g/cm3. An inorganic filler
having a
density of less than 1 g/cm3 is desirable. A lower density inorganic filler
reduces the
density of the product, thus improving the ash characteristics.
If a combination of inorganic filler materials is used, one or more of the
fillers may
suitably be of a small particle size and another may be of a larger particle
size, the
proportions of each filler being suitable to achieve the desired mean particle
size.
The static burn rate required in the finished smoking article may be achieved
using
an appropriate blend of tobacco and tobacco substitute sheet material in the
smokeable filler material.
CA 02824731 2013-07-12
WO 2012/110819
PCT/GB2012/050349
- 22 -
Preferably the inorganic filler material is not in agglomerated form. The
inorganic
filler material should require httle pre-treatment, other than perhaps size
gradation,
before use. Preferably the binder is plesent in the range of about 5-13%, mote
preferably less than 10% and even more preferably less than 8%, by weight of
the
final filler material. Advantageously the binder is about 7.5% by weight or
less of
the final sheet material. Advantageously, if the binder is a mixture of
alginate and
non-alginate binders, then preferably the binder is comprised of at least 50%
alginate, preferably at least 60% alginate and even more preferably at least
70%
alginate. The amount of combined binder requited may suitably decrease when a
non-alginate binder is utilised. The amount of alginate in a binder
combination
advantageously increases as the amount of combined binder decreases. Suitable
algmic binders include soluble alginates, such as ammonium alginate, sodium
alginate, sodium calcium alginate, calcium ammonium alginate, potassium
alginate,
magnesium alginate, triethanol-amine alginate and propylene glycol alginate.
Other
organic binders such as cellulosic binders, gums or gels can also be used in
combination with algmic binders. Suitable cellulosic binders include cellulose
and
cellulose derivatives, such as sodium carboxymethylcellulose, methyl
cellulose,
hydroxypropyl cellulose, hydroxyethyl cellulose or cellulose ethers. Suitable
gums
include gum arabic, gum ghatti, gum tragacanth, Karaya, locust bean, acacia,
guar,
quince seed or xanthan gums. Suitable gels include agar, agarose, canageenans,
furoidan and furcellaran. Starches can also be used as organic binders. Other
suitable gums can be selected by reference to handbooks, such as Industrial
Gums,
E. Whistler (Academic Press). Much preferred as the major proportion' of the
binder
are alginic binders. Alginates are preferred in the invention for their
neutral taste
character upon combustion.
Preferably the aerosol generating means is present in the range of 5-20%, more
preferably is less than 15%, is even more preferably greater than 7% and even
more
preferably is greater than 10%. Preferably the aerosol generating means is
less than
13%. Most preferably the aerosol generating means is between 11% and 13%, and
may advantageously be about 11.25% or 12.5%, by weight of the final sheet
material. Suitably the amount of aerosol genetating means is selected in
combination with the amount of tobacco material to be present in the blend
CA 02824731 2013-07-12
WO 2012/110819
PCT/GB2012/050349
- 23 -
comprising the smokeable filler material of a smoking article. For example, in
a
blend comprising a high proportion of sheet material with a low proportion of
tobacco material, the sheet material may require a lower loading level of
aerosol
generating means therein. Alternatively in a blend comprising a low proportion
of
sheet material with a high proportion of tobacco material, the sheet material
may
require a higher loading level of aerosol generating means therein.
Suitable aerosol generating means include aerosol forming means selected from
polyhydric alcohols, such as glycerol, propylene glycol and triethylene
glycol; esters,
such as triethyl citrate ot triacetin, high boiling point hydrocarbons, or non-
polyols,
such as glycols, sorbitol or lactic acid, foi example. A combination of
aerosol
generating means may be used.
An additional function of the aerosol generating means is the plasticising of
the
sheet material. Suitable additional plasticisers include water. The sheet
material may
suitably be aerated. The cast slurry thereby forms a sheet material with a
cellular
structure.
Advantageously the or a proportion of the aerosol generating means may be
encapsulated, preferably micro-encapsulated, or stabilised in some other way.
In
such cases the amount of aerosol generating means may be higher than the range
given.
Advantageously the smoking material comprises a colourant to darken the
material
and/or a flavourant to impart a particular flavour. Suitable flavouring or
colourant
materials, subject to local regulations, can include cocoa, liquorice,
caramel,
chocolate or toffee, for example. Finely ground, granulated or homogenised
tobacco
may also be used. Industry approved food colorants may also be used, such as
E150a (caramel), E151 (brilliant black BN), E153 (vegetable carbon) or E155
(brown HT). Suitable flavourants include menthol and vanillin, for example.
Other
casing materials may also be suitable. In the alternative, the presence of
vermiculite
or other inorganic filler materials may give a darker colour to the tobacco
substitute
sheet material. Preferably the colourant is present from 0-10% and may be as
much
as 5-7% by weight of the final tobacco substitute sheet material.
Advantageously the
colourant is less than 7%, preferably less than 6% and more preferably less
than 5%
CA 02824731 2013-07-12
WO 2012/110819
PCT/GB2012/050349
- 24 -
of the final tobacco substitute sheet material. Much preferred is use of
colourant at
less than 4%, less than 3% and less than 2%. Cocoa may suitably be present in
a
range of 0-5% and hquorice may be present in a range of 0-4%, by weight of the
final tobacco substitute sheet material. When the colourant is cocoa ot
liquorice, for
example, the minimum amount of cocoa to obtain the desired sheet colour is
about
3% and for liquorice is about 2%, by weight of the final tobacco substitute
sheet
material. Similarly, caramel may suitably be present in a range of 0-5%,
preferably
less than about 2% by weight of the final tobacco substitute sheet material,
and
more preferably about 1.5%. Other suitable colorants include molasses, malt
extract,
coffee extract, tea remolds, St. John's Bread, prune extract oi tobacco
extract.
Mixtures of colorants may also be used.
If permitted under local regulations, flavourants may also be added to alter
the taste
and flavour characteristics of the tobacco substitute sheet material.
Advantageously,
if a food dye is utilised in the alternative it is present at 0.5% by weight
or less of
the final tobacco substitute sheet material. The colourant may alternatively
be
dusted into the sheet after sheet manufacture.
Fibres, such as cellulose fibres, for example wood pulp, flax, hemp or bast
could be
added to provide the sheet material with one or more of a higher strength,
lower
density or higher fill value. Fibres, if added, may be present in the range of
0.5-10%,
preferably less than 5% and even more preferably less than about 3% by weight
of
the final sheet material. Advantageously there is no fibrous material present
in the
sheet material, cellulosic or otherwise.
Advantageously the tobacco substitute sheet material is a non-tobacco
containing
sheet. It shall be understood that at high levels of sheet material inclusion
in the
blend, e.g. at greater than 75% by weight of the blend, the combustibility of
the
blend is poor. This may be overcome by, for example, incorporating low levels
of
up to 5-10% granular carbon in the tobacco substitute sheet material. The
carbon is
prefeiably not an agglomerated carbonaceous material, i.e. the carbon is not
pre-
treated by mixing with another material to produce an agglomerate.
Preferably the tobacco substitute sheet material is blended with tobacco
material to
provide smokeable filler material. Preferably the tobacco material components
in
CA 02824731 2015-06-19
- 25 -
the blend are high quality lamina grades. Advantageously the majority of the
tobacco material is cut tobacco. The tobacco material may comprise between 20-
100 /0 expanded tobacco of a high order expansion process, such as DIET for
example. The filling power of such material is typically in the range of 6-
9cc/g (see
GB 1484536 or US 4,340, 073 for example).
Preferably the blend comprises < 30% of other blend components apart from
lamina, the other blend components being stem cut rolled stem (CRS), water
treated
stern (WTS) or steam treated stem (STS) or reconstituted tobacco. Preferably
the
other components comprise < 200/0, more preferably < 10% and even more
preferably < 5% of the final weight of the tobacco material.
Suitably a smoking article according to the invention comprises tobacco
material
being treated with aerosol generating means. The tobacco material may be
treated
with aerosol generating means, but this is not essential for all blends of
tobacco
material and sheet material.
The amount of aerosol generating means added to the tobacco is m the range of
2-
6 /o by weight of the tobacco. The total amount of aerosol generating means in
the
blend of tobacco material and sheet material after processing is
advantageously in
the range of 4-12 /0 by weight of the smokeable material, preferably less than
10`)/0
and preferably more than 5%.
High Activity Carbon
The polymer-derived, high activity carbon granules used in the dual and triple
stage
filters possesses a pore structure different from the carbon commonly used in
commercial cigarettes, which is typically derived from coconut shells. As a
result it
has superior adsorption characteristics for a range of volatile smoke
toxicants.
The spherical particle shape polymer-derived carbon was prepared by a
propriety
process (Von Blucher and De Ruiter 2004: US Patent Application Publication No.
2004/0038802, Von Blucher et el 2006: EP 1918022 (A1), Bohrmger and Fichtner
2008: International Patent Publication No. WO 2008 110233), as depicted in
Figure
14. The polymer-derived active carbon is produced using a batch process with
indirect heated rotary kilns, under reduced pressure in an melt atmosphere.
After
preparation of the spherical polymer feed- stock the material is thermally
stabilised
using an excess of oleum. Subsequently, the
CA 02824731 2013-07-12
WO 2012/110819
PCT/GB2012/050349
- 26 -
material is slowly heated to 500 C, resulting in the release of predominantly
SO2 and
H20 and the carbonisation of the polymer. The resulting carbon has an initial
pore
system which is not accessible for typical adsorptives. To create a porous
system
capable for adsorption, the material is further heated to 900 to 1000 C for
activation with oxidising agents (steam). This establishes a pole system
consisting
mainly of micropores with pore sizes between 0.7 and 3 nm. Subsequent
activation
with CO2 leads to the formation of predominantly larger mesopores in the range
of
3 to 80 nm. Combining the steam and CO2 activation steps offers a flexible
strategy
for producing desired pore characteristics.
The polymer-derived carbon, being a synthetic material, possesses a much more
closely defined spherical shape, together with a more uniform particle size.
The
polymer derived material possesses a lower density, and has a lower ash
content
reflecting the synthetic nature of the polymer feedstock in comparison to a
natural
coconut shell as starting materials for the carbonization processes.
Most smoke constituents are adsorbed more effectively by the polymer-derived
carbon under the ISO regime than by activated coconut carbon, with reductions
of
the order of 80-95% observed with smoke constituents other than formaldehyde,
acetaldehyde, hydrogen cyanide (HCN) and toluene (50-60% reductions). Under
HCI conditions, cigarettes with conventional coconut carbon provide reductions
of
the order of 25-45% for most smoke constituents, other than acetaldehyde
(16%).
The cigarettes including polymer-derived carbon reduce most smoke constituent
yields by 60-90%, other than acetaldehyde and HCN (15-30%).
Amine-functionalised Resin Beads
DIAIONO CR20 is a commercially available type of amine-functionalised resin
bead
which may be used in the present invention (manufactured by Mitsubishi
Chemical
Corporation). It has polyamine groups as chelating ligands which are bonded
onto
a highly porous crosslinked polystyrene matrix. CR20 shows large affinity for
transition metal ions. The exact type of amine gioups produced by
functionalization
cannot be precisely controlled and several different types could be present on
the
resins.
CA 02824731 2013-07-12
WO 2012/110819
PCT/GB2012/050349
- 27 -
Commercial grade CR20 (hereafter referred to as CR20C) was found to have a
characteristic odour incompatible with conventional consumer acceptable
cigarette
smoke character when incorporated into cigarettes. However, modification to
the
synthesis conditions by Mitsubishi significantly reduced the intensity of this
odour,
resulting in a "low-odour" grade of CR20 (hereafter referred to as CR2OL). In
this
work, unless otherwise stated, all results obtained refer to CR2OL. This
material
possessed a bead size of 600mm, density of 0.64 g/cm3, a 15% by weight water
content, and total exchange capacity of 0.92 meq/cm3.
Various other types of CR20 are made by Mitsubishi Chemical Corporation,
including CR2OD and CR2OHD. All of the different types or grades of the ion-
exchange resin are encompassed by the term CR20 as used herein.
Some CR20 beads are provided in water and, to make them suitable for use in a
cigarette filter application, it may be necessary to remove at least some of
the water.
In one embodiment, the water is removed and the material is dried to
approximately
15% or less moisture. In an alternative embodiment, a higher moisture content
may
be acceptable in the filter of smoking articles.
CR20, including specifically CR2OL, may be incorporated into cigarette
filters. In
comparison to filters containing conventional carbon, CR2OL offers superior
reductions for HCN, formaldehyde and acetaldehyde. However, carbon is more
efficient than CR2OL in removing other volatile constituents from a smoke
stream.
Experimental Cigarettes
Cigarettes were constructed using these technologies targeting ISO NFDPM (tar)
yields of 1 and 6mg.
Three scientific control cigarettes were also manufactured to allow an
evaluation to
be made of the contribution of the filter technologies to smoke constituent
reductions from ECs. Two commercial comparator cigarettes, a lmg ISO design
and a 6mg ISO design, were also used in these studies. Comparisons with
commercial brands were conducted because realistic control cigarettes are
required
to assess the success with which the different smoke constituent reduction
technologies can be brought together into a coherent and consumer acceptable
CA 02824731 2013-07-12
WO 2012/110819
PCT/GB2012/050349
- 28 -
cigarette design. Also, the use of commercial cigarettes allows examination of
the
extent with which constituent reductions can be realised against real-world
cigarettes, rather than scientific controls. Finally, use of commercial
reference
products allows relevant comparisons to be made of sensory acceptability and
3 human exposure under real-world use.
The commercial comparator products were of similar machine smoked constituent
yields to the market leading brands at 1 mg and 6 mg (ISO) from Germany in
2007-
8. BAT group comparator cigarettes were chosen, rather than the actual market
leading brands, in order that full information was available on blend and
cigarette
design characteristics, and to allow product masking to be conducted for human
sensory and exposure evaluations. Samples of both commercial cigarettes were
therefore manufactured specially for these studies, without brand marking or
other
identification, in order to support human smoking studies.
2.2 Specifications for Experimental, Comparator and Control Cigarettes
Common features were used in the design of the ECs: all were constructed to
the
same basic dimensions, of 84 mm cigarette length (a 57 mm tobacco rod plus a
27
mm filter), 24.6 mm circumference and the filters were all based on cellulose
acetate
(CA) fibres plasticized with triethyl citrate. Tobacco grades with low TSNA
and
metal contents were identified and combined for the tobacco blends used in
these
prototypes. Three different experimental cigarettes were prepared, and the
design
features of the three ECs are summarised and compared with control cigarettes
and
commercial comparators in Table 2 (shown in Figure 1) and described below.
The experimental cigarette BT1, combined a Virginia style tobacco blend
containing
BT treated tobacco (75.4% treated Virginia tobacco, with 4.3% Oriental tobacco
and 20.3% untreated Virginia tobacco) with a filter containing a CR20 stage
(to
reduce formaldehyde, acetaldehyde and HCN yields) and a polymer-derived, high
activity carbon filter containing stage (to reduce yields of isoprene and
other volatile
toxicants). The target NFDPM yield from this cigarette was 1 mg under ISO
machine smoking conditions. The experimental cigarette TSS1 was also designed
to
yield 1 mg of NFDPM under ISO smoking machine conditions and was based on an
US style blend containing TSS (a blend of Viiginia, Burley and Oriental
tobaccos,
CA 02824731 2013-07-12
WO 2012/110819
PCT/GB2012/050349
- 29 -
with the inclusion of approximately 20% TSS and the same filter used in
experimental cigarette BT1. The experimental cigarette TSS6 also used 20%TSS
in a
different US style blend, and was designed to give an NFDPM yield of 6 mg
under
ISO machine smoking conditions. A different filter construction was used with
this
cigarette: a dual segment filter containing 80 mg of the high activity carbon
interspersed amongst CA fibres adjacent to the tobacco rod with a CA stage at
the
mouth end.
The commercial comparator cigarette CC1 contained a US-blended style of
tobacco,
including some Maryland tobacco. The commercial comparator cigarette, CC6, was
also a typical US-blended cigarette but with a different blend to CC1. The
design
features of the thiee ECs are summarised and compared with control cigarettes
and
commercial comparators in Table 2 [shown in Figure 1]. Both commercial
comparator cigarettes used single stage cellulose acetate filters. The three
"scientific
control" (SC) cigarettes had identical construction to the relevant
experimental
cigarettes BT1, TSS1 and TSS6, with the exception that the filter used in each
control cigarette was a single stage 27 mm CA filter without additional filter
adsorbent media.
Table 2 shows that the cigaiette constructions of BT1 and CC1 were very
similar,
with well matched filter ventilation and paper permeability. There were shght
differences in tobacco density and filter pressure drop (the draw resistance
or
impedence to flow of the filter), with BT1 higher than CC1 for both
parameters.
The cigarette constructions of TSS1 and CC1 were also very similar. The filter
pressure drop was higher from TSS1 than the commercial control, but both
tobacco
density and filter pressure drop were higher for CC1. For TSS6 and CC6 less
filter
ventilation was used than with the 1mg (ISO) products. Comparing the two 6 mg
(ISO) products showed shghtly higher tobacco densities, pressure drop values
and
slightly lower filter ventilation for TSS6.
2.3 Smoke Chemistry Analysis
Prior to smoke chemistry analysis, cigarettes were conditioned according to
the
specifications of ISO 3402, 1999. Routine chemical analyses were performed
according to the smoking conditions specified in ISO 4387, 2000 (i.e., a 35 ml
puff
CA 02824731 2015-06-19
- 30 -
of 2 seconds duration taken every 60 seconds, abbreviated as 35/2/60) and ISO
3308, 2000 which was developed for NIFDPM and nicotine analysis.
Approximately 150 smoke constituents have been described as toxicants and a
few
regulatory authorities have requested yield data on a subset (approximately
40) of
them. Yield restrictions for some of these toxicants have been proposed
(Burns, D.,
et al. (2008) Mandated lowering of toxicants in cigarette smoke: a description
of the
World Health Organization TobReg proposal. Tob. Control 17, 132-141) along
with
an approach to their biomonitoring (Hecht, S.S. et al (2010) Applying tobacco
carcinogen and toxicant biomarkers in product regulation and cancer
prevention.
Chem. Res. Toxicol. 23, 1001-1008). For these reasons and in order to
characterise
the ECs more precisely, the MS yields of an extended range (47 analytes) of
smoke
constituents were measured. The other, approximately 100, toxicants not
examined
in this work were not measured due to the lack of available validated
analytical
methods. Values for benzo(a)pyrene yields were obtained twice, through a
direct
measure and also as part of a suite of polycyclic aromatic hydrocarbons
(PAHs).
Slight modification to the ISO smoking parameters was required for the
measurement of other analytes, as described by Gregg, E., et al. (2004) The UK
smoke
constituents testing study. Summary of results and comparison with other
studies. Beitriige
zur Tabakforschung International/Contributions to Tobacco Research, Volume 21,
No. 2,
117-118. Measuring the yield of smoke constituents from a smoking machine does
not mimic human smoking yields and so all RTPs were tested under a range of
different smoking machine settings in order to allow machine yield performance
to
be assessed over a wide range of possible smoking conditions. These modified
smoking conditions are described in Table 3.
Table 3. Smoking machine parameters.
Smoking Abbreviation Puff Puff Puff Filter
Description Volume Duration Interval Vent
(ml) (s) (s) Blocking
c/o
ISO 3308/4387 ISO 35 2 60 0
Health Canada HCI 55 2 3() 100
Intense
Health Canada HCI-VO 55 2 30 0
CA 02824731 2015-06-19
- 31 -
Intense-Filter
Vents Open
ISO WG 9 WG-9B 60 2 30 50
Intense Option B
Sidestream smoke (SS) yields were also measured as described by Health Canada,
(1999). Determination of "Tar" and Nicotine in Sidestream Tobacco Smoke,
Method T-
212 (www.hc-sc.gc.ca) but only under ISO smoke generation parameters and for a
wider range of smoke constituents. The SS testing was conducted by Labstat
International ULC.
2.4 Statistical Analysis
Statistical comparisons of smoke yields between different cigarette types were
conducted using a two-tailed, unpaired, Student's t-test, performed with
Minitab
v16. Levels of significance of P<0.01 and P<0.05 are shown and any P value
>0.05
is shown as nonsignificant (NS).
For comparisons of individual smoke constituent yields across studies, mean
values
from published data sets (Health Canada (2004), Constituents and emissions
reported
for cigarettes sold in Canada (www.hc-sc.gc.ca); Counts, M.E. et al. (2005).
Smoke
composition and predicting relationships for international commercial
cigarettes smoked
with three machine-smoking conditions. Regulat. Toxicol. Pharmacol. 41(3):185-
227;
Department of Health and Ageing ¨ Australia (2002
http://www.health.gov.au/internet/main/publishing.nsf/Content/tobacco-emis)
were
examined for normal distribution using the Anderson Darling statistic.
Percentile
distributions within the toxicant data were calculated using an empirical
cumulative
distribution analysis within Minitab v16.
3. Results and Discussion
Testing of the ECs was conducted in order to examine the actual performance of
the ECs from a blend and smoke chemistry perspective, by quantifying the MSThe
SS emissions from the ECs were also measured using the ISO smoking profile.
The
tests were conducted on a comparative basis with two commercial cigarettes and
with three scientific control cigarettes. As a final step, the overall
performance of
the ECs was assessed both in comparison to previously published MS yield data
on
CA 02824731 2015-06-19
- 31a -
cigarettes from several countries and as ratios of specific toxicant yields to
nicotine
yields.
3.1 Mainstream Smoke Constituent Yields
The yields of the major smoke constituents (NFDPM, nicotine and CO) and
CA 02824731 2013-07-12
WO 2012/110819
PCT/GB2012/050349
- 32 -
glycerol under four smoking machine conditions are shown in Table 4 (shown in
Figure 2). Glycerol measurements are included in this table because it has
been
incorporated into the tobacco-substitute sheet used in the ECs TSS1 and TSS6,
to
dilute other smoke constituents in the smoke particulate phase.
Table 4 shows that BT1 and CC1 were well matched across the four smoking
regimes for MS NFDPM and nicotine yields, but that BT1 had lower CO yields
than
CC1. TSS1 and CC1 were well matched across the four smoking regimes for
NFDPM and nicotine yields but TSS1 had lower CO yields than CC1. The higher
glycerol yield from TSS1 is consistent with the intended dilution effect due
to the
glycerol content of TSS. The MS NFDPM and nicotine yields from TSS6 and CC6
were well matched across the four smoking regimes, other than higher CO yields
from CC6 and the expected higher glyceiol yields from TSS6.
For these major smoke analytes the yields measured followed the same rank
order
based on smoking machine conditions: ISO <HCI-VO <WG9B <HCI. The yield
differences between the different regimes were substantially greater with the
1mg
products than with the 6mg products, as the level of ventilation was higher
and the
impact of ventilation blocking for the WG9B and HCI regimes is therefore more
profound for the lmg products. For the 6mg products the differences in the
major
smoke measures (NFDPM, nicotine and CO) between some of the regimes were
small (in the order of 5-10%).
The 47 toxicants quantified in this work were also measured under all of the
smoking machine conditions shown in Table 3, except that data for the ECs TSS1
and BT1 under ISO machine smoking conditions were not collected because
preliminary runs showed the yields of many constituents to be below the LOQ
for
the methods. The machine smoked yields of these toxicants generally followed
the
lank order noted for NFDPM, nicotine and CO shown in Table 4 and so, for the
remainder of this paper, only the yields obtained under HCI conditions are
described. Some consistent exceptions to the general yield trend were
observed.
With all products the volatile phenols, quinoline, and fluotene did not
increase
systematically with increasing intensity of the smoking regime and the yields
of the
majoi smoke measures; arsenic, phenanthrene and the measure for benzo(a)pyrene
CA 02824731 2013-07-12
WO 2012/110819
PCT/GB2012/050349
- 33 -
from the PAH suite also displayed this behaviour for the majority of the
products.
In particular the yields of these species were greater under the WG9B tegime
than
with the HCI regime despite the greater overall amounts of smoke generated by
the
HCI regime. Volatile phenols are known to be selectively removed from smoke by
cellulose acetate filters; the consistent behaviour obsetved here may
represent some
change in filtration efficiency for these species between the WG9B and HCI
regimes. Alternatively it may represent some analytical weakness with the
measurement method at high intensity smoking regimes. Similar changes were
observed on a more occasional basis for some analytes (e.g. 1,3-butadiene
yields
with CC1 were lower than expected from the trends across smoking regimes found
for the other five products); this was found in particular with the 6mg
products
when similar amounts of NFDPM were generated between the different smoking
regimes, and these observations are likely due to analytical errors, or
reflect hmits in
the discriminatory power of the analytical techniques.
The use of the HCI smoking regime in this work represents the strictest test
of the
ECs and the commercial comparatot cigarettes. Although these smoking
conditions
inactivate a design featute used in the ECs and commercial cigarettes (filter
ventilation), they address criticism of the machine yield values obtained from
ventilated cigarettes.
3.1.1 Metal and TSNA Yields
Two groups of toxicants included on regulatory lists are the metals and the
tobacco
specific nitrosamines (TSNAs). Both these groups of toxicants are primarily
affected
by the tobacco blend used in cigarette manufacture and so careful blend
selection is
a major contributor to their reduction in smoke. The chemical analysis of
blend
metals and TSNAs are described in Table 5 (shown in Figure 3) and their MS
yields
under HCI smoking machine conditions are shown in Table 6 (shown in Figure 4).
The yields are discussed for each EC in Sections 3.1.2.1 to 3.1.2.3 below.
3.1.2 Other Toxicant Yields
Smoke constituent yield comparisons between ECs and commercial controls, under
HCI smoking machine conditions, are shown in Table 7 (shown in Figure 5). The
yields are discussed for each EC in Sections 3.1.2.1 to 3.1.2.3 below.
CA 02824731 2013-07-12
WO 2012/110819
PCT/GB2012/050349
-34-
3.1.2.1 BT1
Measurement of blend chemistries (Table 5) showed the blend arsenic and
chromium contents of BT1 were statistically significantly higher than the
commercial cigarette CC1; whereas lead and nickel contents of the BT1 blend
were
lower. The MS yields for metals from BT1 were comparable to or lower than the
yields from CC1, except that the arsenic and mercury yield were higher. The
higher
arsenic yield may be explained by the higher blend content of this metal but
the
mercury yield is not explained by blend content and may represent an artefact
because the BT1 blend content of mercury was comparable to or lower than CC1,
being below the LOQ for this metal (Table 5).
Blend nitrosamme content of BT1 was lower than US-blended commercial
comparator CC1, as has been seen previously in comparison of Virginia and US-
blended cigarettes. The MS yields of nitrogenous constituents were expected to
be
lower from BT1 than from CC1 for two reasons: first the tobacco treatment
reduces
precursors of nitrogenous smoke compounds; and, second, Virginia style
tobaccos
typically generate lower yields of nitrogenous smoke constituents than US-
blended
cigarettes. Measurement of the yields of nitrogenous compounds showed the
anticipated differences: yields of the TSNAs were statistically significantly
(83-96%)
lower from BT1 than from CC1 (Table 6); aromatic amine yields from BT1 were 26-
57% lower than from CC1 (Table 7); and the yields of other nitrogenous
compounds from BT1 were also substantially lower (HCN by 82%, NO by 79%,
ammonia by 75%, pyridine by 97%, quinolene by 67% and acrylonitrile by 69%)
than the respective yields from CC1 (Table 7). These data confirm that the
blend
selection, use of the BT process (and incorporation of CR20 in the filter in
the case
of HCN yields) produced the expected lower yields of toxicants from the EC.
The BT process also reduces blend polyphenol levels and so reductions in MS
phenols yields would be expected; however, higher yields of phenolics are
generally
expected from Virginia style products than from US-blended products and this
tobacco type difference could mitigate any reductions from the BT process.
Comparison between phenolic compound yields from CC1 and from BT1 showed a
mixed picture: phenol, p-cresol and iesorcinol yields were lower from BT1,
whereas
m-cresol, catechol and hydroquinone yields were higher from BT1 (Table 7).
CA 02824731 2013-07-12
WO 2012/110819
PCT/GB2012/050349
- 35 -
The BT process does not influence benzo(a)pyrene yields and analysis of PAHs
in
the current study showed comparable yields from BT1 and CC1 for fluorene,
phenanthrene, pyrene and benzo(a)pyrene. Lower carbonyl yields (26 to 74%
lower)
were obtained from cigarette BT1, apart from formaldehyde, which showed a
higher
3 (41%) yield from BT1. The volatile hydrocarbon yields from BT1 were
lower, with a
range from 21 to 78% for isoprene, benzene, toluene and naphthalene, when
compared to the respective constituent yields from CC1; however, the 1,3-
butadiene
yield was 35% higher from BT1 compared to CC1. The 1,3-butadiene yields from
CC1 are lower than expected under the HCI regime, and this observation may
therefore be unreliable. Most of the observed differences in volatile
constituent
yields are consistent with the use of an effective vapour phase adsorbent in
the filter
of BT1. Formaldehyde yields are driven in part by sugar levels, which are
normally
higher in Virginia blends than in US blends. Formaldehyde yields are also
increased
by the blend treatment process. Hence the higher formaldehyde yields from BT1
are
understandable on the basis of knowledge of formaldehyde generation in
cigarettes.
The apparent higher yield of 1,3-butadiene from BT1 is possibly due to an
error in
the yield measurement of CC1 as there is no obvious mechanistic factor to
support
this difference (the tobacco treatment process does not give statistically
significant
changes in 1,3-butadiene yields and the use of the vapour phase adsorbent in
BT1
filters should result in lower 1,3 butadiene yields from BT1). The
contribution of
the blend and the selective filter used in BT1 to the overall reductions in
smoke
toxicants are addressed in Section 3.2 and the results are consistent with the
higher
yield values for formaldehyde observed in Table 7 being due to blend chemistry
factors.
3.1.2.2 TSS1
The overall blend metal content was higher in TSS1 than in CC1 for some metals
(arsenic, chromium and nickel), lower for cadmium content and not different
for
other metals (Table 5). The TSS contains a high proportion of chalk, which
would
contribute some portion of the blend metals. Analysis of the TSS showed a
higher
level of chromium and comparable or lower levels of the other measured metals
than the TSS1 blend. Hence, the higher chromium content of TSS1 than CC1 most
likely reflects the inclusion of TSS material in the blend; whereas, the
higher arsenic
CA 02824731 2013-07-12
WO 2012/110819
PCT/GB2012/050349
- 36 -
and nickel levels were most likely due to the different tobacco types used in
the
blend. It should be noted that the transfer of metals from the TSS would not
necessauly occur with the same efficiency as from tobacco, due to possible
differences in the chemical form (and therefore volatility) of trace metals in
chalk
and in tobacco. Thus, the metal yields in MS under HCI smoking machine
conditions were either lower or not statistically significantly different when
TSS1
was compared to CC1 (Table 6). The blend nitrosamme content of TSS1 was lower
(23-72%) than that of CC1 (Table 5) and the MS yields of the TSNAs under HCI
machine smoking conditions were correspondingly lower (17 to 69%) for TSS1
than
CC1 (Table 6).
Statistically significantly lower yields were found from TSS1 than from CC1
for
most of the phenohcs (29-57%), carbonyls (44-86%), PAHs (8 to 71%) and
miscellaneous volatile constituents (27 to 94%); although for catechol,
hychoquinone and benzo(a)pyrene, these differences did not achieve statistical
significance (Table 7). These data demonstrate lower toxicant yields from TSS1
across all of the analyte classes examined, and therefore support the
expectation
that the TSS, and the three stage filter, should function to give overall MS
toxicant
yield reductions in an EC.
3.1.2.3 T556
The blend metal contents of TSS6 and CC6 were similar, other than
statistically
significantly higher chromium and cadmium blend levels in TSS6. As noted
above,
the higher chromium level was most hkely due to the high inorganic content of
the
TSS; whereas, the higher cadmium content most likely reflects a difference in
the
tobacco types used between the two blends. The MS yields of cadmium and
chromium, determined under HCI smoking machine conditions, were not elevated
in TSS6 compared to CC6 (Table 6), which again supports the contention that
the
chemical form of these metals was different between the EC and the commercial
comparator, and less likely to transfer into MS.
The blend nitrosamme contents were lower (39 to 54%) from TSS6 than those
measured for the CC6 blend (Table 5). Again, this lower blend nitrosamme
content
CA 02824731 2013-07-12
WO 2012/110819
PCT/GB2012/050349
- 37 -
translated to 37 to 50% lower MS yields for these TSNAs under HCI smoking
machine conditions (Table 6).
The MS yields from TSS6, across all of the other chemical classes measured
(aromatic amines (13-20%), phenolics (8-32%), carbonyls (35-85%), PAHs (18-
81%)
and miscellaneous volatile toxicants (41-96%)) were statistically
significantly lower
than the yields from CC6, except for 1- and 2-ammonaphthalene and m- and p-
cresol where the values were not significantly different and for ammonia where
the
higher yield (13%) was not statistically significantly different to that of
CC6 (Table
7). These data again demonstrate reductions in all classes of measured
toxicants, and
therefore it is apparent that the TSS is functioning as expected in the EC, to
give
overall MS toxicant yield reductions.
3.2 Filter Comparisons
From the MS yield data shown in Table 7 all the ECs gave lower yields of
carbonyls
and vapour phase constituents than the respective commercial comparator
cigarettes, with the exception of formaldehyde and 1,3-butadiene yields for
BT1. To
understand better the contribution of the blend and the selective filters used
in the
ECs to the overall reductions in these smoke constituents, direct comparisons
were
made between the ECs and control cigarettes (SC-BT1, SC-TSS1 and SC-TSS6),
which were identical in all aspects to the appropriate EC, except for the use
of a
mono-stage CA filter without adsorbents. The comparisons of the yields from EC
and control cigarettes for the carbonyls and other vapour phase constituents
are
shown in Tables 8 and 9 (shown in Figures 6 and 7, respectively).
From these data it is clear that the yields of the carbonyls and the other
vapour
phase constituents were all reduced by the presence of the triple stage filter
containing CR2OL and high activity carbon used in ECs BT1 and TSS1 (Table 8).
The mean change in MS yield across all volatile constituents measured from BT1
was a reduction of 50% compared to the control cigarette SC-BT1, with a range
of
23% reduction for acetaldehyde to 79% reduction for crotonaldehyde. Very
similar
reductions were obtained with TSS1, which gave a mean reduction of 50%, with a
range from 10% reduction in formaldehyde yield to 79% reduction for
crotonaldehyde yield in comparison to SC-TSS1.
CA 02824731 2015-06-19
- 38 -
From Table 9 it is apparent that the dual filter containing additional polymer
derived carbon but without the CR2OL resin (as used in TSS6), also reduced the
yields of the vapour phase smoke constituents by a mean of 48%, with a range
from
11% reduction in acetaldehyde yield to 79% reduction for crotonaldehyde yield.
3 Together, these data confirm that the selective filters used in the ECs
removed
substantial quantities of volatile smoke constituents from cigarette MS,
confirming
previous studies with the filter adsorbents. For all of the ECs, the MS yields
of both
formaldehyde and 1,3-butadiene were lower than measured with the scientific
control cigarettes. The superior performance of the CR2OL resin compared to
the
high activity carbon at formaldehyde removal from MS can be seen by the
greater
reduction in the yield of formaldehyde from a higher starting value (53n/cig
or
53%) in the BT1 / SC-BT1 pair compared to the 1.9i.tg/cig reduction (10%)
found
with the TSSI / SC-TSS1 pair. Thus, it is clear that the greater formaldehyde
yield
seen when comparing BT1 with the commercial cigarette CC1 (Table 7) must be
due
13 to differences in blend between these cigarettes. A similar comparison
also confirms
that the higher 1,3-butadiene yield from BT1 compared to CC1 is most likely
due to
an analytical error in the measurement of 1,3 butadiene with CC1.
3.3 Comparison of EC toxicant yields with those from published cigarette brand
data
This paper has focused on a comparison of EC toxicant yields with the yields
from
two commercial comparator cigarettes. However, to fully establish whether the
ECs
offer reduced machine yields in comparison to conventional commercial
cigarettes it
is necessary to compare their yields with those from a wider range of
cigarettes. The
absolute yield values of the ECs described here can be compared with other
published data obtained under HCI smoking conditions, namely: (I) (Health
Canada
(2004) Constituents and emissions reported for cigarettes sold in Canada
(http://www.hc-sc.gc.ca; (2) Counts, M.E. et al. (2005) Smoke composition and
predicting relationships for international commercial cigarettes smoked with
three
machine-smoking conditions. Regul. Toxicol. Pharmacol. 41, 185-227; and (3);
Department of Health and Ageing Australia:
(http://www.health.gov.au/intemet/main/publishing.nsf/Content/tobacco-emis) .
It
should, however, be noted that such comparisons must be treated with caution
due
CA 02824731 2015-06-19
- 39 -
to the known difficulties based on limited standardisation between
laboratories for
the analysis of smoke constituents other than NFDPNI, nicotine and CO.
The three data sources above were compiled into one dataset to provide a
reference
set of global cigarette yield data with which to compare the toxicant yields
from the
ECs described in this study. The full dataset was truncated as follows: first,
arsenic,
methyl ethyl ketone, nickel and selenium yields were removed from the dataset
because yields were not provided by all three sources; second, a number of
brands
were removed from the dataset due to incomplete, duplicated or erroneous data
(two brands in the HC dataset appear to have erroneous (exchanged) toluene and
styrene yields; tar, nicotine and CO yields were not provided in the HC
dataset for
Gitanes KS, and multiple instances of the same yield data were observed in the
HC
dataset). Finally, reference products were removed from the dataset to ensure
that
only commercial brands were included. This resulted in a dataset of 120
cigarette
brands covering 16 countries or regions. While extensive it is unlikely that
this
dataset is fully representative of the range of cigarette products on-sale
globally,
either with respect to the range of design features, or as a representative
sample of
global brands. However, while it is limited in these respects, it does
constitute a
valid comparator set for the toxicant yields for these ECs.
The data was examined to see if it was normally distributed; while a number of
toxicants in
the dataset were normally distributed the majority (and in particular
nitrogenous toxicants
such as TSNAs and aromatic amines) were not. Consequently the reference
dataset was
subject to an empirical cumulative distribution analysis, producing a
percentile distribution
within the toxicant yields. Yields from the ECs were then compared to the
empirical
cumulative distribution to identify the position of these yields in comparison
to the
commercial brands (Figures 8 and 9). In these comparisons, the yields of the
ECs described
here fall at the low end of the range for numerous toxicants and often give
lower values for
specific toxicants than any of the products in the commercial brand dataset.
Exceptions to
this are catechol yields from BT1 and NO and TSNA yields from TSS1 and TSS6,
where
the yields are approximately equivalent to the median values for the
commercial product
CA 02824731 2013-07-12
WO 2012/110819
PCT/GB2012/050349
- 40 -
dataset. In contrast, the yields of the commercial comparator cigarettes CC1
and
CC6 are generally distributed over the range of yields observed with the
commercial
dataset.
A further comparison was conducted, examining the total toxicant levels from
the
ECs and each of the commercial products in the dataset. This was conducted in
three ways. The first method was to sum the yields of the 39 toxicants for
each
cigarette to give a total toxicant yield (TTY) for each brand. This approach
is of
limited utility because the TTY value for each brand is dominated by tar, CO
and
nicotine, and many other toxicants do not contribute significantly to the
total value.
A second approach was to sum the yields of all toxicants (but excluding tar,
nicotine
and CO yields) for each cigarette to give a total for the toxicant subset of
yields
(TSY). A third, normalisation method gave greater insight into the
contribution of
all toxicants, wherein a median value was calculated for each toxicant in the
commercial dataset. The median value was normalised to 100 for each toxicant,
and
the yields of toxicants scaled against this value of 100. Totalling the scaled
values
for all toxicants gave a normahsed toxicant total (NTT) for each brand. The
TTY,
TSY and NTT values for the ECs are compared to and tanked against the values
for
all of the brands in the commercial dataset in Figures 10 to 12. The
comparisons
show, with each of the approaches, that the ECs were at the low end of the
ranking
order. The lmg ECs were found to have the lowest total toxicant yields under
each
of the three approaches, and the 6mg EC was also lower than any of the
commercial
brands for the TSY and NTT. In the TTY analysis two of the 120 commercial
products have lower TTY values than TSS6 due to their lower tar and nicotine
values. The commercial comparator cigarettes CC1 and CC6 were also reasonably
low in total toxicant values in comparison with the dataset of commercial
brands,
falling around the lower quartile of values.
Together these analyses show that the ECs offer some of the lowest machine
toxicant yields of cigarettes for which pubhshed HCI smoke chemistry is
available;
these comparisons therefore confirm that the ECs generate reduced machine
toxicant yields in comparison to known levels of commercial cigarettes.
CA 02824731 2015-06-19
- 41 -
3.4 Comparisons of EC yields as a ratio to nicotine yields
The analysis described above is restricted to assessment of machine yields of
toxicants. However, it has been proposed that the ratio of smoke toxicants to
the
MS nicotine yield of cigarettes gives a better predictor of smokers' exposure
to the
3 toxicant than the MS yield value alone. Therefore, the ratio of MS
constituents
yields measured in this study to the MS nicotine yields, all measured under
HCI
smoking machine conditions, has been calculated and is given as a supplemental
table (Table 10, Figures 8A and 8B). Under Health Canada Intense machine
smoking conditions, the NFDPM yields from 13T1, TSS1 and CC1 were comparable,
but the nicotine yield from BT1 was slightly higher and the nicotine yield
from
TSS1 slightly lower than from CC1 (Tables 4 and 7). When the yield values for
the
EC were calculated as a ratio to the nicotine yield, and compared to those
from CC1
and CC6, they followed the same trends as found when comparing the yields per
cigarette, but the lower values from BT1 when compared to CC1 are more
pronounced and the lower values from TSS1 when compared to CC1 are slightly
less pronounced.
3.5 Sidestream Smoke Yields
To complete the chemical analysis of smoke emissions from the EC, SS yields
for
the expanded list of smoke constituents were measured, under ISO smoking
parameters. The ISO smoking parameters were chosen because they generate
higher
SS yields than any of the other smoking regimes. In general, under any smoking
regime, the quantity of sidestream smoke can be expected to be dependent on
the
amount of tobacco consumed in the static burn or smoulder phase of cigarette
smoking. The SS yield results are presented as a comparison between the ECs
BT1
and TSS1 and the commercial cigarette CC1, in Table 10.
Statistically significantly higher yields of sidestream NFDPM (219/o), and
several
constituents such as benzo(a)pyrene (28%), phenolics (28-77%), carbonyls (22-
63%)
and volatile hydrocarbon (20-24%) constituents were found with BTI than from
CC1. In contrast lower yields of nitrogenous SS smoke constituents such as
TSNAs
(31-82%), HCN (47%), aromatic amines (21-40%) nitrogen oxides, pyridine and
quinolene (19-35%) were found with BT1 than with CC1. Most of these changes
were described previously (Liu et al, (2011), The use of a novel tobacco
treatment
process to reduce toxicant yields in cigarette smoke. Food and Chemical
Toxicology 49;
CA 02824731 2015-06-19
- 42 -
1904-1917), however, the higher SS phenolic yields and lower than anticipated
TSNA
yields from BT1 suggest that chemical differences between Virginia and US-
blended
tobaccos also influence the SS yields of individual constituents. Finally, the
13%
higher tobacco weight from BT1 than from CC1 will also contribute across the
3 board to the observed increases.
Many SS smoke constituent yields were lower from the EC cigarette TSS1 than
from
CC1. The greatest numerical differences in SS yields were observed for the
TSNAs
which were 28 to 52% lower from TSS1 than CC1; these observations are
consistent
with the observed trends in MS yields of these species. The wide range of
reductions most likely reflects the reduction in tobacco mass in the
cigarettes
resulting from incorporation of the TSS, and consequent decrease in the total
amount of smoke generated. The one constituent with a statistically
significantly
higher sidestream yield from TSS1 than from CC1 was formaldehyde (19% higher).
Higher SS formaldehyde yields were also observed with higher levels of TSS
13 inclusion in the blend (McAdam K. G. et al., 2011). The use of a novel
tobacco-
substitute sheet and smoke dilution to reduce toxicant yields in cigarette
smoke. Food
Chem. Toxicol. 49,1684-1696), suggesting that formaldehyde might be a
combustion
by-product of the organic materials used in TSS manufacture.
4. Conclusions
Three ECs were made using a combination of technological approaches, and
chemical testing under four different machine smoking parameters has confirmed
overall reductions of MS toxicants yields from the ECs. When compared with
published values of MS toxicant yields from conventional cigarettes, despite
elevated formaldehyde yields with BT1, the performance of these ECs appears to
be
superior, even if they are ranked on a nicotine ratio basis. The data
presented in this
study support a designation of these ECs as reduced machine-yield prototypes,
and
previous data with EC made using the TSS approach suggest that lower
biomarkers
of exposure to MS toxicants could be achieved with these RMYPs when used by
smokers.
Despite the low overall machine yields of toxicants obtained from the current
RMYP and
their performance against commercial comparators and other published toxicant
yield data,
substantial amounts of scientific data would need to be acquired, including
biomarkers of
exposure and biomarkers of biological effect, to determine
CA 02824731 2013-07-12
WO 2012/110819
PCT/GB2012/050349
- 43 -
whether such products might be associated with lower health risks, and
therefore
there is no certainty that these RMYP will meet the IOM definitions of a PREP.
Nonetheless, we believe that the results from this study are sufficient to
encourage
further work, including human biomarket studies of these RMYP and further
application and refinement of the technologies used in their manufacture.
5. Prototype Smoking Articles
Three prototype RTP smoking articles were produced according to the present
invention. The cigarettes are of king size format with a filter length of 27mm
and a
tobacco rod of 56mm. The prototypes have a tobacco rod comprising a mix of
lamina, Expanded Tobacco and non tobacco sheet or modified tobacco.
Conventional cigarette paper is used to form the tobacco rod and ensure the
achievement of burn rate and subsequent puff number.
The filter for two of the prototypes is a triple filter composed of a CA mouth
end
segment (7 mm in length), a CA central segment containing CR20 HD ion exchange
resin (10 mm in length) and a dalmation style tobacco end segment containing
carbon beads with an engineered microstructure (10 mm in length). The filter
for
the third prototype is a dual filter composed of a CA mouth end segment (15mm
in
length) and a dalmation style tobacco end segment containing high activity,
polymer-derived carbon beads (12 mm in length).
The prototype cigarettes were manufactured to give ISO NFDPM yields of 1 (T562
and H671) and 6 mg (F752). The specification of the prototype cigarettes is
described in more detail in Tables 11 to 13.
Table 11. Tobacco blend specifications
Prototype T562 (1mg) H671 (1mg) F752 (6mg)
Lamina (Vowwb) 40 12.5 55.0
Expanded Tobacco' 40 12.5 -
(Vowwb)
Expanded Tobaccob - - 25.0
(Vowwb)
Modified Tobaccoc (Vowwb) - 75 -
CA 02824731 2013-07-12
WO 2012/110819
PCT/GB2012/050349
- 44 -
Non tobacco sheetd (/owwb) 20 20
Added Top Flavour 0.8 0.8 0.8
(AWOLSA) (%wwb)
'Aurora - 100% flue cured tobacco
bSCB - 50% flue cured, 50% Burley
'Tobacco processed using the tobacco blend treatment
dThe non tobacco sheet is TSS with the following specification: Chalk (78.5%),
Kelvis Alginate (7.5%), Glycerol (12.5%) and Caramel colourant (1.5%)
(manufacturer; Deli-HTL).
Table 12. Cigarette specifications
Prototype T562 (1mg) H671 (1mg) F752
(6mg)
Circumference (mm) 24.6 24.6 24.6
Total length (mm) 83 83 83
Tobacco rod length (mm) 56 56 56
Cigarette paper CP 50-23 VGM CP 50-23 VGM CP 50-23 VGM
2.0 KCM 2.0 KCM 2.0 KCM
Ventilation type OML OML OML
Ventilation total 80 80 46
(ST+OML) (%)
Density (mg/cc) 216 247 235
Cigarette pressure drop 97 91 109
(mmWG)
Cigarette firmness (%) TBC TBC TBC
Tar (NFDPM) (mg) 1.0 1.2 5.3
Nicotine (mg) 0.08 0.10 0.43
Carbon monoxide (mg) 1.0 1.0 4.9
Table 13. Filter specification
Code T562 (1mg) H671 (1mg) F752
(6mg)
Filter Code (Filtrona, USA) SAM 013108- SAM 013108- SAM
020608-
031 031 040
CA 02824731 2013-07-12
WO 2012/110819
PCT/GB2012/050349
- 45 -
Total length (mm) 27 27 27
Mouth end segment length 7 7 15
(mm)
Central segment length 10 10
(mm)
Tobacco segment length 10 10 12
(mm)
Mouth end segment tow Mono CA Mono CA Mono
CA
Central segment tow a CA + 20mg CA + 20mg
CR20 HD CR20 HD
Tobacco end segment tow b CA + 60mg CA + 60mg CA +
80mg
Blucher carbon Blucher carbon Blucher
carbon
Total filter PD (mmWG) 150 142 114
Plugwrap` PW600043 PW600043
PW600043
n CR20 HD = amine functionalised resin (manufacturer: Mitsubishi)
b Bliichet carbon = spherical carbon beads (manufacturer: Adsor Tech.)
Tlugwrap for completed Dual or Triple filter
6. Smoke Toxicant Exposure Study
This study looked at the evaluation of biomarkers of exposure (BoE) to
toxicants in
smokers who switched from conventional cigarettes to reduced toxicant
prototype
(RTP) cigarettes according to the present invention.
The technologies discussed in detail above were combined to produce one 6mg
and
two 1mg ISO tar yield RTPs as detailed in Table 14 below.
Table 14: Tested prototype products
Product identifier & Tobacco Tobacco ISO* tar yield HCI# tar
description blend filter target yield
(actual) (actual)
CC6 100% US Single 6mg (5.0mg) 24.4mg
Control based on 6mg style tobacco segment:
ISO conventional blend CA
cigarette
TSS6 80% US style Dual 6mg (5.3mg) 20.7mg
6mg ISO tar tobacco segment:
prototype blend CA +
20% tobacco Carbon
substitute (80mg)
sheet
CA 02824731 2013-07-12
WO 2012/110819
PCT/GB2012/050349
- 46 -
CC1 100% US Single 1mg (1.2mg) 18.9mg
Control based on 1mg style tobacco segment:
ISO tar commercial blend CA
cigaiette
TSS1 80% US style Triple 1mg (1.0mg) 17.3mg
1mg ISO tar tobacco segment:
prototype blend CA +
20% tobacco Carbon
substitute (60mg) +
sheet Resin (20mg)
BT1 25% Virginia Tiiple 1mg (1.2mg) 17.8mg
1mg ISO tar style tobacco segment:
prototype with blend CA +
tobacco blend (untreated) Carbon
modification 75% Virginia (60mg) +
style tobacco Resin (20mg)
blend
(treated)
* ISO regime = 35mL puff volume, 2 second duration, 60 second interval, filter
ventilation 100% open
# HCI (Health Canada Intense) regime = 55mL puff volume, 2 second duration, 30
second interval, filter ventilation 100% blocked
Smoke chemistry indicated good reductions in toxicants compared to control
cigaiettes of conventional design, see Table 15 (Figure 15).
A six week single-centie, single-blinded, iandomised controlled switching
study with
occasional clinical confinement, as illustrated in Figure 16, was conducte. A
total of
301 healthy adult subjects were recruited into the study; 100 smokers of 6-7
mg ISO
tar yield cigarettes (assigned to the 6 mg groups), 151 smokers of 1-2 mg ISO
tar
yield cigarettes (assigned to the 1 mg groups) and 50 non-smokers. Recruited
smokers were randomly assigned to a control or test group within their tar
band,
with approximately 50 per group. All smokers smoked a supplied control product
for 2 weeks after which Day 14 baseline measurements were made. Control group
smokers continued to smoke the control product for a further 4 weeks, while
test
group smokers were switched to an RTP for 4 weeks. In each case, measurements
were made at Days 28 (two weeks) and 41 (four weeks). The non-smoker group
provided an indication of background levels of biomarkers.
CA 02824731 2013-07-12
WO 2012/110819
PCT/GB2012/050349
- 47 -
Collection of 24 hour urine samples occurred during three (for smokers) and
two
(for non-smokers) short periods of clinical confinement (see Figure 16), and
exposure to a number of smoke constituents was estimated by analysis of levels
of
urinary biomarkers of exposure. Analysis of biomarkers of exposure was
achieved
using validated LC-MS/MS methods.
When the RTP smoke chemistry was compared to that of the control cigarette,
most
measured toxicants were substantially lower (10-96%) with actual levels
dependant
on design and toxicant (see Table 15). The only higher yields were for one
product
(BT1) which delivered 16% more nicotine and 35% more 1,3-butadiene, although
this was also the product that showed the greatest overall reductions for all
other
toxicants. Direction and relative magnitude of changes in corresponding
biomarkers
were largely in-keeping with the changes in smoke chemistry (Table 15 and
Figures
17 and 18) although in a few cases a reduction in the smoke was accompanied by
an
increase in the biomarker (nicotine and NNK for TSS1) or an increase in the
smoke
but a reduction in biomarker (1,3-butadiene for BT1). Reasons for these
discrepancies are unknown, but may involve analytical variability or smoker
behavioui.
Figure 17 shows the biomarker results for Group 2 who switched ftom control
cigarette CC6 (Day 14) to test cigarette TSS6 (Day 41). * denotes a
statistically
significant difference (p 5. _ 0.01) between day 14 and 41 results. Non-smoker
biomarker levels are shown for reference. All non-smoker levels were
significantly
lower than day 14 values
Figure 18 shows the biomarker results for Group 4, who switched from control
cigarette CC1 (Day 14) to test cigarette TSS1 (Day 41) and Group 5, who
switched
from control cigarette CC1 (Day 14) to test cigarette BT1 (Day 41).
The study found that, on average, gtoups of cigarette smokers who switched to
reduced toxicant prototype cigarettes had reduced levels in the corresponding
biomarker of exposure (BoE). These included BoEs for particulate and vapour
phase toxicants. Different prototypes resulted in different levels of
reductions to
the BoE, in some cases with reductions substantially greater than 50%,
depending
upon which combination of technologies was used. Generally most of the
reduction
CA 02824731 2013-07-12
WO 2012/110819 PCT/GB2012/050349
- 48 -
in biomarket level was apparent two weeks after switching. In all cases the
average
biomarker level was lower in the non-smoker group
This study demonstrates for the first time significant reductions in a range
of BoE
of tobacco smoke toxicants in smokers following a switch from conventional
cigarettes to reduced toxicant prototype cigarettes according to the present
invention.
Figure 19 shows a smoking article design according to an embodiment of the
present invention. The smoking article 1 comprises a tobacco rod 2 and a
filter 3.
The tobacco rod comprises a rod of smokeable material, the composition of
which
is 75% blend treated tobacco, 12.5% leaf and 12.5% expanded tobacco.
The blend treated tobacco is a tobacco with reduced protein and polyphenol
content which results from the following process: (i) aqueous exttaction of a
tobacco; (n) passing the aqueous extract through a clay and a resin; (iii)
treatment of
the fibre with an enzyme and deactivation; and (iv) recombining the extract
and
fibre and drying. The leaf is tobacco as is used in conventional commercial
cigatettes. The expanded tobacco is a tobacco that has been expanded using a
supeicritical CO2 process which is used in conventional commercial cigarettes.
The filter 3 is attached to the tobacco rod 2 by a tipping paper which is a
non-
porous paper.
The filter 3 is made up of three sections, as indicated by the inset. The
section 4
adjacent the end of the tobacco rod is 10 mm in length and contains 60 mg of
synthetic carbon. This is a form of carbon which has an engineered porous
structuie. The middle section 5 is 10 mm in length and contains 20 mg (that
is,
2 mg/mm) of CR2OHD, an amine functionahzed resin having a water content of 12-
17%. The mouth-end section 6 of the filter is 7 mm in length. This may
comprise,
for example, cellulose acetate tow as used in conventional commercial
cigarettes.
CA 02824731 2014-10-01
- 49 -
In possible variations of the smoking article design shown in Figure 19, the
smokeable material
may further include tobacco substitute sheet. Tobacco substitute sheet is a
chalk-based sheet
containing glycerol that reduces the quantity of tobacco in a cigarette when
incorporated into the
tobacco blend. The tobacco substitute sheet may replace some or all of any or
all of the different
materials making up the smokeable material of the smoking article design
discussed above.
A further variation may be to use CR2OD in the filter. CR2OD is an amine
functionalized resin
having a water content of 0-5%. For example, CR2OD may partially or completely
replace the
CR2OHD used in the design discussed above.
The foregoing description and examples have been set forth merely to
illustrate the invention and
are not intended to be limiting. Since modifications of the described
embodiments may occur to
persons skilled in the art, the invention should be construed broadly to
include all variations
within the scope of the appended claims.