Note: Descriptions are shown in the official language in which they were submitted.
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
TITLE OF THE INVENTION
NOVEL CYCLIC AZABENZIMIDAZOLE DERIVATIVES USEFUL AS ANTI-DIABETIC
AGENTS
BACKGROUND OF THE INVENTION
Diabetes is characterized by elevated levels of plasma glucose (hyperglycemia)
in the
fasting state or after administration of glucose during an oral glucose
tolerance test. In type 1
diabetes, or insulin-dependent diabetes mellitus (IDDM), patients produce
little or no insulin, the
hormone which regulates glucose utilization. In Type 2 diabetes, or noninsulin-
dependent
diabetes mellitus (NIDDM), insulin is still produced by islet cells in the
pancreas. Patients
having Type 2 diabetes have a resistance to the effects of insulin in
stimulating glucose and lipid
metabolism in the main insulin-sensitive tissues, including muscle, liver and
adipose tissues.
These patients often have normal levels of insulin, and may have
hyperinsulinemia (elevated
plasma insulin levels), as they compensate for the reduced effectiveness of
insulin by secreting
increased amounts of insulin (Polonsky, Int. J. Obes. Relat. Metab. Disord. 24
Suppl 2:S29-31,
2000). Insulin resistance is not primarily caused by a diminished number of
insulin receptors but
rather by a post-insulin receptor binding defect that is not yet completely
understood. This lack
of responsiveness to insulin results in insufficient insulin-mediated
activation of uptake,
oxidation and storage of glucose in muscle, and inadequate insulin-mediated
repression of
lipolysis in adipose tissue and of glucose production and secretion in the
liver. Eventually, a
patient may be become diabetic due to the inability to properly compensate for
insulin resistance.
In humans, the beta cells within the pancreatic islets initially compensate
for insulin resistance by
increasing insulin output. The onset of Type 2 diabetes due to insufficient
increases (or actual
declines) in beta cell mass is apparently due to increased beta cell apoptosis
relative to non-
diabetic insulin resistant individuals (Butler et al., Diabetes 52:102-110,
2003).
Persistent or uncontrolled hyperglycemia is associated with increased and
premature
morbidity and mortality. Often abnormal glucose homeostasis is associated both
directly and
indirectly with obesity, hypertension, and alterations of the lipid,
lipoprotein and apolipoprotein
metabolism, as well as other metabolic and hemodynamic disease. Patients with
Type 2 diabetes
mellitus have a significantly increased risk of macrovascular and
microvascular complications,
including atherosclerosis, coronary heart disease, stroke, peripheral vascular
disease,
hypertension, nephropathy, neuropathy, and retinopathy. Therefore, effective
therapeutic control
of glucose homeostasis, lipid metabolism, obesity, and hypertension are
critically important in
the clinical management and treatment of diabetes mellitus.
Patients who have insulin resistance often exhibit several symptoms that
together are
referred to as Syndrome X or Metabolic Syndrome. Patients with Metabolic
Syndrome have an
increased risk of developing atherosclerosis and coronary heart disease.
- 1 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
There are several available treatments for Type 2 diabetes, each of which has
its own
limitations and potential risks. Physical exercise and a reduction in dietary
intake of calories
often dramatically improve the diabetic condition and are the usual
recommended first-line
treatment of Type 2 diabetes and of pre-diabetic conditions associated with
insulin resistance.
Compliance with this treatment is generally very poor because of well-
entrenched sedentary
lifestyles and excess food consumption, especially of foods containing high
amounts of fat and
carbohydrates. Pharmacologie treatments for diabetes have largely focused on
three areas of
pathophysiology: (1) hepatic glucose production (biguanides, such as
phenformin and
metformin), (2) insulin resistance (PPAR agonists, such as rosiglitazone,
troglitazone,
engliazone, balaglitazone, MCC-555, netoglitazone, T-131, LY-300512, LY-818
and
pioglitazone), (3) insulin secretion (sulfonylureas, such as tolbutamide,
glipizide and
glirnipiride); (4) incretin hormone mimetics (GLP-1 derivatives and analogs,
such as exenatide
and liraglitide); and (5) inhibitors of incretin hormone degradation (DPP-4
inhibitors, such as
sitagliptin).
Many of the current treatments for diabetes have unwanted side effects.
Phenformin and
metformin can induce lactic acidosis, nausea/vomiting, and diarrhea. Metformin
has a lower risk
of side effects than phenformin and is widely prescribed for the treatment of
Type 2 diabetes.
The currently marketed PPAR gamma agonists are modestly effective in reducing
plasma
glucose and hemoglobinAlC, and do not greatly improve lipid metabolism or the
lipid profile.
Sulfonylureas and related insulin secretagogues can cause insulin secretion
even if the glucose
level is low, resulting in hypoglycemia, which can be fatal in severe cases.
The administration of
insulin secretagogues must therefore be carefully controlled. There remains a
need for treatments
for diabetes that work by novel mechanisms of action and that exhibit fewer
side effects.
AMP-activated protein kinase (AMPK) has been identified as a regulator of
carbohydrate
and fatty acid metabolism that helps maintain energy balance in response to
environmental and
nutritional stress. There is evidence that activation of AMPK results in a
number of beneficial
effects on lipid and glucose metabolism by reducing glucogenesis and de novo
lipogenesis (fatty
acid and cholesterol synthesis), and by increasing fatty acid oxidation and
skeletal muscle
glucose uptake. Inhibition of ACC, by phosphorylation by AMPK, leads to a
decrease in fatty
acid synthesis and to an increase in fatty acid oxidation, while inhibition of
HMG-CoA
reductase, by phosphmylation by AMPK, leads to a decrease in cholesterol
synthesis (Carling, D.
et.al., FEBS Letters 223:217 (1987)).
In the liver, AMPK activation results in a decrease in fatty acid and
cholesterol synthesis,
inhibiting hepatic glucose production and increasing fatty acid oxidation. lt
has been shown that
AMP-activated protein kinase regulates triacylglycerol synthesis and fatty
acid oxidation in liver
and muscle via glycerol-3-phosphate acyltransferase (Muoio, D. M.
Biochem. J. 338:783
(1999)). Another substrace of AMPK, hepatocyte nuclear factor-4a, has been
shown to be
- 2 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
involved in type-1 maturity onset diabetes (Leclerc, I. et.al., Diabetes
50:1515 (2001)).
Additional processes believed to be regulated through AMPK activation include
the stimulation
of glucose transport in skeletal muscle and the regulation of key genes in
fatty acid and glucose
metabolism in the liver (Hardie, D. G. and Hawley, S. A., Bioessays 23: 1112
(2001), Kemp, B.
E. et.al., Biochem. Soc. Transactions 31:162 (2003), Musi, N. and Goodyear, L.
J.. Current Drug
Targets-Immune, Endocrine and Metabolic Disorders 2:119 (2002); Lochhead, P.
A. et.al.,
Diabetes 49:896 (2000); and Zhou, G. et.al., J. of Clin. Invest, 108: 1167
(2001).
In vivo studies have demonstrated the following beneficial effects of both
acute and
chronic administration of AICAR, an AMPK activator, in rodent models of
obesity and type 2
diabetes: 1) an improvement in glucose homeostasis in insulin-resistant
diabetic (ob/ob) mice; 2)
a decrease in blood glucose concentrations in ob/ob and db/db mice and a blood
glucose
reduction of 35% following 8 weeks of administration; and 3) a reduction in
metabolic
disturbances and a reduction of blood pressure in rats displaying
characteristics of insulin
resistance syndrome (Bergeron, R. et.al., Diabetes 50:1076 (2001); Song, S. M.
et.al.,
Diabetologia 45:56 (2002); Halseth, A. E. et.al., Biochem. and Biophys. Res.
Comm. 294:798
(2002); and Buhl, E. S. et.al., Diabetes 51: 2199 (2002)). A farther study of
7 week AICAR
administration in obese Zucker (fa/fa) rats lead to a reduction in plasma
triglycerides and free
fatty acids; an increase in HDL cholesterol; and a normalization of glucose
metabolism as
assessed by an oral glucose tolerance test (Minokoshi, Y. et.al., Nature 415:
339 (2002)).
Expression of dominant negative AMPK in skeletal muscle of transgenic mice has
demonstrated
that the AICAR effect on stimulation of glucose transport is dependent on AMPK
activation
(Mu, J. et.al., Molecular Cell 7: 1085 (2001)).
Recent data also suggest that AMPK activation is involved in the glucose and
lipid-
lowering effects of the anti-diabetic drug metformin. It has been shown that
the diabetes drug
tnetformin can activate AMPK in vivo at high concentrations (Zhou, G. et.al.,
J. of Clin. Invest.
108: 1167 (2001); Musi, N. et.al. Diabetes 51: 2074 (2002)).
Based on these studies, it is expected that the in vivo activation of AMPK in
the liver
may result in the reduction of hepatic glucose output, an improvement in
overall glucose
homeostasis, a decrease in fatty acid and cholesterol synthesis, and an
increase in fatty acid
oxidation. Stimulation of AMPK in skeletal muscle is expected to result in an
increase in
glucose uptake and fatty acid oxidation with resulting improvement of glucose
homeostasis, and
an improvement in insulin action. Finally, the resulting increase in energy
expenditure should
lead to a decrease in body weight. The lowering of blood pressure has also
been reported to be a
consequence of AMPK activation.
Increased fatty acid synthesis is a characteristic of many tumor cells,
therefore decreasing
the synthesis of fatty acids via AMPK activation may also be useful as a
cancer therapy.
- 3 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
Activation of AMPK may also be useful to treat ischemic events in the brain
(Blazquez, C. etal.,
J. Neurochem. 73: 1674 (1999)); to prevent damage from reactive oxygen species
(Zhou, M.
etal., Am. J. Physiol. Endocrinol. Metab. 279: E622 (2000)); and to improve
local circulatory
systems (Chen, Z.-P., etal. AMP-activated protein kinase phosphorylation of
endothelial NO
synthase. FEBS Letters 443: 285 (1999)).
Compounds that activate AMPK are expected to be useful to treat type 2
diabetes
mellitus, obesity, hypertension, dyslipidemia, cancer, and metabolic syndrome,
as well as
cardiovascular diseases, such as myocardial infarction and stroke, by
improving glucose and lipid
metabolism and by reducing body weight. There is a need for potent AMPK
activators that have
pharmacokinetic and pharmacodynamic properties suitable for use as human
pharmaceuticals.
Benzimidazole compounds are disclosed in WO 2010/051206; WO 2010/051176; WO
2010/047982; WO 2010/036613; WO 93/07124; WO 95/29897; WO 98/39342; WO
98/39343;
WO 00/03997; WO 00/14095; WO 01/53272; WO 01/53291; WO 02/092575; WO 02/40019;
WO 03/018061; WO 05/002520; WO 05/018672; WO 06/094209; US 6,312,662; US
6,489,476;
US 2005/0148643; DE 3 316 095; JP 6 298 731; EP 0 126 030; EP 0 128 862; EP 0
129 506; and
EP 0 120 403. AMPK activators are disclosed in WO 08/006432; WO 05/051298; WO
05/020892; US 2007/015665; US 2007/032529; US 2006/287356; and US 2005/038068.
SUMMARY OF THE INVENTION
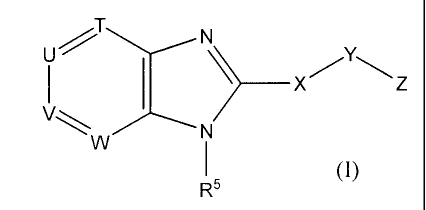
The present invention is concerned with novel benzimidazole derivatives of
structural Formula I:
____________________________________________ x
(I)
R5
and pharmaceutically acceptable salts thereof. The compounds of structural
formula I, and
embodiments thereof, are activators of AMP-activated protein kinase (AMPK) and
are useful in
the treatment, prevention and suppression of diseases, disorders and
conditions mediated by
activation of AMP-activated protein kinase, such as Type 2 diabetes mellitus,
insulin resistance,
hyperglycemia, dyslipidemia, lipid disorders, obesity, hypertension, Metabolic
Syndrome and
atherosclerosis.
The present invention also relates to pharmaceutical compositions comprising
the
compounds of the present invention and a pharmaceutically acceptable carrier.
The present
invention also relates to methods for the treatment, control or prevention of
disorders, diseases,
- 4 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
and conditions responsive to activation of AMP-activated protein kinase in a
subject in need
thereof by administering the compounds and pharmaceutical compositions of the
present
invention. The present invention also relates to the use of compounds of the
present invention
for manufacture of a medicament useful in treating diseases, disorders and
conditions responsive
to the activation of AMP-activated protein kinase. The present invention is
also concerned with
treatment of these diseases, disorders and conditions by administering the
compounds of the
present invention in combination with a therapeutically effective arnount of
another agent known
to be useful to treat the disease, disorder and condition. The invention is
further concerned with
processes for preparing the compounds of this invention.
DETAILED DESCRIPTION OF THE INVENTION
The present invention is concerned with novel compounds of structural Formula
I:
N
V
(I)
R5
or a pharmaceutically acceptable salt thereof, wherein:
T is selected from the group consisting of: CR3, N and N-oxide;
U is selected from the group consisting of: CR1, N and N-oxide;
V is selected from the group consisting of: CR2, N and N-oxide;
W is selected from the group consisting of: CR4, N and N-oxide,
provided that at least one of T, U, V and W is N or N-oxide;
X is absent or selected from:
(1) -CI12-,
(2) -CHF-,
(3) -CF2-,
(4) -S-,
(5) -0-,
(6) ¨0-CH2-,
(7) -NH-,
(8) -C(0)-,
(9) -NHC(0)-,
(10) -C(0)NH-,
(11) -NHS02-,
(12) -SO2NH-, and
- 5 -
CA 02826649 2013 08 05
WO 2012/116145
PCT/US2012/026261
(13) -CO2-,
wherein each CH2 is unsubstituted or substituted with 1 or 2 substituents
selected from:
hydroxy, halogen, N112, Ci -6a1ky1, CO2H, CO2C1-6a1ky1, COC1-6alkyl, phenyl
and -
CH2phenyl3 and wherein each NH is unsubstituted or substituted with 1
substituent selected
from: Ci-6alkyl, CO2H, CO2C1-6alkyl, COCi_6alkyl, phenyl and -CH2phenyl;
Y is selected from:
(1) C3.1 ocycloalkyl,
(2) C3 -iocycloalkenyl,
(3) C2_10cycloheteroalkyl,
(4) C2-1ocyc1oheteroa1keny1,
(5) aryl, and
(6) heteroaryl,
wherein cycloalkyl, cycloalkenyl, cycloheteroalkyl, cycloheteroalkenyl, aryl
and heteroaryl are
unsubstituted or substituted with 1, 2, 3 or 4 substituents selected from Rb;
Z is selected from:
(1) oxo,
(2) -CN,
(3) -CF3,
(4) -Ci_6alkyl,
(5) -(CH2)t-halogen,
(6) --(CH2)nC0C1-6alky1,
(7) -(CH2)nCO2H,
(8) -(CH2)nOCOH,
(9) --(C112)nCO2R1,
(10) -(CH2)nOCOR1,
(11) -(CH2)n0H,
(12) --(CH2)nC(0)N(Rg)2,
(13) --(CH2)nC(0)(CH2)nN(Rg)2,
(14) -(CH2)n0C(0)(CH2)nN(Rg)2,
(15) -(CH2)nNHC(0)C1 -6alkyl,
(16) -(CH2)nNHSO2Ri,
(17) -(CH2)nS02C1-6a1ky1,
(18) -(CH2)nS02NHRg,
(19) -(CH2)nSO2NHC(0)R1,
(20) -(CH2)002NHCO2R1,
(21) -(CH2)nS02NHCON(R02,
(22) -(CH2)11C(0)NHSO2Ri,
- 6 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
(23) ¨(CH2)nNHC(0)N(Rg)2,
(24) ¨(CH2)nC3-1ocycloa1ky1-0O2Re,
(25) heteroaryl,
(26) ¨C2-10cycloheteroalkenyl, and
(27) ¨C2-locycloheteroalkyl,
wherein each CH2 is unsubstituted or substituted with 1 or 2 substituents
selected from C1.
6alky1, -OH and -NH2, wherein each NH is unsubstituted or substituted with 1
substituent
selected from Rc, and wherein each alkyl, cycloalkyl, cycloheteroalkyl,
cycloheteroalkenyl, aryl
and heteroaryl is unsubstituted or substituted with 1, 2, 3 or 4 substituents
selected from Re;
each R1 and R2 is independently selected from:
(1) hydrogen,
(2) halogen,
(3) CN,
(4) CF3,
(5) -C1_6alkyl,
(6) -C2-6alkenyl,
(7) -C2_6alkyny1,
(8) -(CH2)pC3-10cyc1oa1ky1,
(9) -(CH2)pC3-7cycloallcyl-aryl.
(10) -(CH2)pC3-7cyc1oalky1-heteroary16
(11) -(CH2)pC4- 1 ocycloalkenyl,
(12) -(CH2)pC4-7cycloalkenyl-aryl,
(13) -(CH2)pC4-7eycloa1keny1-heteroa1y1,
(14) -(CH2)pC2-iocycloheteroalkyl,
(15) -(CH2)pC2-10cycloheteroalkenyl,
(16) -(CH2)paryl,
(17) -(CH2)paryl-Ci_8a11cy18
(18) -(CH2)pary1-C2-8alkeny18
(19) -(CH2)paryl-C2-8alkynyl-Ci-8alkyl,
(20) -(CH2)paryl-C2-8alkynyl-C3_7cycloalkyl,
(21) -(CH2)pary1-C2_8a1kynyl-C3_7cycloalkeny1,
(22) -(C1{2)pary1-C2-8a1kyny1-C2-10cycloheteroalkyl,
(23) -(C112)paryl-C2-galkYnYl-C2-10cycloheteroalkenyl,
(24) -(CH2)paryl-C2-8alkyny1-ary1,
(25) -(CH2)paryl-C2-8alkynyl-heteroaryl,
(26) -(CH2)pary1-C3_7cyc1oa1ky1,
(27) -(CH2)paryl-C2-10cycloheteroalkyl,
- 7 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
(28) -(CH2)paryl-C2- l ocycloheteroalkenyl,
(29) -(CH2)paryl-ary16
(30) -(CH2)paryl-heteroaryl,
(31) -(CH2)pheteroatyl,
(32) -C2-6alkenyl-alkyl,
(33) -C2-6a1keny1-aty1,
(34) -C2-6alkeny1-heteroary1,
(35) -C2-6a1keny1-C3-7cyc1oa1ky1,
(36) -C2-6a1keny1-C3-7cycloalkenyl,
(37) -C2-6a1kenyl-C2-7cyc1oheteroa1ky1,
(38) -C2-6a1keny1-C2-7cyc1oheteroa1keny1,
(39) -C2-6 alkynyl-(CH2)1-3-0-aryl,
(40) -C2-6a1kyny1-allcy1,
(41) -C2-6a1kyny1-ary1,
(42) -C2-6a11cyny1-heteroary1,
(43) -C2-6alkynyl-C3.7cycloalkyl,
(44) -C2-6a1kynyl-C3-7cyc1oa1kenyl,
(45) -C2-6a1kynyl-C2-7cyc1oheteroa1ky1,
(46) -C2-6a1kyny1-C2-7cyc1oheteroalkeny1, and
(47) ¨C(0)NH-(CH2)0-3Pheny1,
wherein each CH2 is unsubstituted or substituted with 1 or 2 substituents
selected from: halogen,
CF36 -OH, -NH2, ¨C1-6alkyl, -0C1-6alky1, ¨NHCi -6alkyl, and ¨N(C1-6a11cy1)2,
wherein each
alkyl, alkenyl, alkynyl, cycloalkyl, cycloalkenyl, cycloheteroalkyl,
cycloheteroalkenyl, phenyl,
aryl and heteroaryl is unsubstituted or substituted with 1, 2, 3 or 4
substituents independently
selected from Ra,
provided that at least one of and only one of R1 and R2 is selected from the
group consisting of:
hydrogen, halogen, -CN, -CF3> -C1-6alkyl, --C2-6alkenyl and¨C2-6alkynyl;
R3 and R4 are each independently selected from:
(1) hydrogen,
(2) halogen,
(3) ¨Ci _6a1ky1,
(4) ¨C2-6alkenyl3
(5) ¨C2-6alicYnY1,
(6) ¨C3-jocycloalkyl,
(7) ¨C3-iocyc1oalkeny1,
(8) aryl,
(9) heteroaryl,
- 8 -
CA 02826649 2013 08 05
WO 2012/116145
PCT/US2012/026261
(10) ¨CN,
(11) ¨CF3,
(12) ¨OH,
(13) -0C1-6alkyl,
(14) H2,
(15) ¨NHC1_6alkyl,
(16) ¨N(C1-6a110)2,
(17) -SC i_6alkyl,
(18) -SOC _6alkyl,
(19) ¨S02C1_6a1ky1,
(20) ¨NHSO2Ci _6alky1,
(21) ¨NHC(0)Ci_6alkyl,
(22) ¨SO2NHC1_6a1kyl, and
(23) ¨C(0)NHC1-6alkyl;
R5 is selected from:
(1) hydrogen,
(2) -Ci_6a1ky1,
(3) -CH2CO2H, and
(4) -CH2CO2C1_6alkyl;
each Ra is independently selected from the group consisting of:
(1) -(CH2)m-ha1ogen,
(2) oxo,
(3) -(C112)Tri0H,
(4) -(CF12)mN(Ri)2,
(5) -(CH2)mNO2,
(6) -(CH2)mCN,
(7) -Ci -6alkyl,
(8) -(CH2)mCF3,
(9) -(CH2)m0CF3,
(10) -0-(CH2)m-OC1 -6 alkyl,
(11) -(CH2)mC(0)N(Ri)2,
(12) -(CH2)mC(=N-OH)N(Ri)2,
(13) -(CH2)m0C1_6a1kyl,
(14) -(CH2)m0-(CH2)m-C3-7cycloalkyl,
(15) -(CH2)m0(C112)m-C2-7cyc1oheteroalky1,
(16) -(CH2)m0-(CF12)rn-ary1,
(17) -(CH2)1110-(CH2)m-heteroaryl,
- 9 -
CA 02826649 2013 08 05
WO 2012/116145
PCT/US2012/026261
(18) -(CH2)0C1-6alkyl,
(19) -(CH2)mS (0)Ci
(20) -(CH2)mS02C1-6a1icyl,
(21) -(CH2)mSO2C3-7eyc1oa1ky1,
(22) -(CH2)mS02C2-7cycloheteroalkyl,
(23) -(CH2)mS02-aryl,
(24) -(CH2)mS02-heteroaryl,
(25) -(CH2)mS02NHC1-6a1lcy1,
(26) -(CH2)mS02NHC3-7cyc1oa1ky1,
(27) -(CH2)mS02NHC2-7cyc10heteroa1ky1,
(28) -(CH2)mS02NH-aryl,
(29) -(CH2)002NH-heteroaryl,
(30) -(CH2)mNHS02-C1-6a1ky1,
(31) -(CH2)mNHS02-C3-7cycloalkyl,
(32) -(CH2)mNHS02-C2-7cyc1oheteroa1ky1,
(33) -(CH2)mNHS02-aryl,
(34) -(CH2)mNHSO2NH-heteroaryl,
(35) -(CH2)mN(Ri)-C1-6a1ky1,
(36) -(CH2)mN(Ri)-C3-7cyc1oa1lcy1,
(37) -(CH2)mN(Rj)-C2.7cycloheteroalkyl,
(38) -(CH2)mN(Ri)-C2-7cycloheteroalkenyl,
(39) -(C1-12)InN(Ri)-aryl,
(40) -(CH2)mN(Ri)-heteroaryl,
(41) -(CH2)mC(0)Rf,
(42) -(CH2)mC(0)N(Rj)2,
(43) -(CH2)mN(Ri)C(0)N(Ri)2,
(44) -(CH2)mCO2H,
(45) -(CH2)m000H,
(46) -(CH2)mCO2Rf,
(47) -(CF12)m000R1,
(48) -(CH2)mC3-7cyeloa1ky1,
(49) -(CH2)mC3.7cyc1oa1keny1,
(50) -(CH2)mC2-6cyc1oheteroa1ky1,
(51) -(CH2)mC2..6cyc1oheteroa1keny1,
(52) -(CH2)maryi, and
(53) -(CH2)mheteroatyl,
wherein each CH2 is unsubstituted or substituted with 1 or 2 substituents
selected from: ow,
10-
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
(C112)0-30H, -CN, -NH2, -NH(C1-6alkY1), -N(C1-6alky1)2, -Ci-6a1ky1, -0C1-
6a1ky1, halogen, -
CH2F, -CHF2, -CF3, -CO2H, -CO2C1-6a1ky1, -C3_7cycloalkyl, phenyl, CH2phenyl,
heteroaryl
and CH2heteroary1, and wherein alkyl, cycloalkyl, cycloalkenyl,
cycloheteroalkyl,
cycloheteroalkenyl, aryl and heteroaryl are unsubstituted or substituted with
1, 2, 3 or 4
substituents selected from: oxo, -(CH2)0-50H, -CN, -NH2, -NH(C1-6a1kY1), -N(C1-
6a1kY1)2, -
C1_6a1ky1, -0C1-6a1ky1, halogen, -CH2F, -CHF28 -CF3, -0O211, -CO2C1-6a1ky1, -
S02C1-
6alkYl, -C3-7cyc1oa1ky1, phenyl, CH2phenyl8 heteroaryl and CH2heteroaryl;
each Rb is independently selected from:
(1) hydrogen,
(2) -Ci_6a1ky1,
(3) -C3-6cycloa1ky1,
(4) -C3-6cycloa1keny1,
(5) -C2-6cyc1oheteroa1ky1,
(6) -C2_6cyc1oheteroa1kenyl,
(7) aryl,
(8) heteroaryl,
(9) -(CH2)t-halogen,
(10) -(CH2)s-OH,
(11) -NO2,
(12) -NH2,
(13) -NH(C1-6alkY1),
(14) -N(C i-6a1icY1)2,
(15) -0C1_6alkyl,
(16) -(CH2)qCO2H,
(17) -(CH2)qCO2Ci -6alkyl,
(18) -CF3,
(19) -CN,
(20) -S02C1-6alkyl, and
(21) -(CH2)sCON(R92,
wherein each CH2 is unsubstituted or substituted with 1 or 2 halogens, and
wherein each alkyl,
cycloalkyl, cycloalkenyl, cycloheteroalkyl, cycloheteroalkenyl, aryl and
heteroaryl is
unsubstituted or substituted with 1, 2 or 3 halogens;
each Rc is independently selected from:
(1) halogen,
(2) oxo,
(3) -(CH2)r0H,
(4) -(CH2)rN(Re)2,
- 11 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
(5) -(CH2)rCN,
(6) -Ci-6alkyl,
(7) -CF3,
(8) -Ci-6a1ky1-OH,
(9) -OCH2OC1_6a1lcy1,
(10) -(CH2)r0C1_6a1lcyl,
(11) -OCH2a1Y1,
(12) -(CH2)rSC1-6alky1,
(13) -(CH2)rC(0)Rf,
(14) -(CH2)rC(0)N(Re)2,
(15) -(CH2)rCO2H,
(16) -(CH2)rCO2Rf,
(17) -(CH2)rC3-7cycloa1ky1,
(18) -(CH2)rC2-6cycloheteroalkyl,
(19) -(CH2)raryl, and
(20) -(CH2)rheteroaryl,
wherein each CH2 is unsubstituted or substituted with 1 or 2 substituents
selected from: oxo, -
OH, -CN, -N(Rh)2, -C _6alkyl, -0C1-6alky1, halogen, -CH2F, -CHF2, -CF3, -CO2H,
-CO2C1-
6alkyl, -C3.7cycloalkyl and heteroaryl, and wherein alkyl, cycloallcyl,
cycloheteroalkyl, aryl and
heteroaryl are unsubstituted or substituted with 1, 2, 3 or 4 substituents
selected from: oxo, -OH,
-CN, -N(Rh)2, -C1-6alkyl, -OC _6alkyl, halogen, -CH2F, -CHF2, -CF3, -0O211, -
CO2C1-
6alkyl, -C3-7cycloalkyl and heteroaryl;
each Re, Rg and Rh is independently selected from:
(1) hydrogen,
(2) -Ci_6alky1, and
(3) -0-Ci-6alkyl,
wherein alkyl is unsubstituted or substituted with 1, 2, 3 or 4 substituents
selected from: -OH,
oxo, halogen, C1..6alkyl, -0Ci -6alkyl, --NH2, -NH(C1-6alkyl), and -
N(Ci_6alky1)2;
each Ri is independently selected from:
(1) hydrogen,
(2) C1-6alkyl,
(3) C3.6cycloalkyl,
(4) -C(0)Ri, and
(5) -SO2Ri,
wherein alkyl and cycloalkyl are unsubstituted or substituted with 1, 2, 3 or
4 substituents
selected from: -OH, oxo, halogen, C1-6alkyl, -0C1-6alky1, -NH2, -NH(C1-
6a1lcy1), and -N(C1-
6alkY1)2;
- 12-
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
each Rf and Ri is independently selected from:
(1) Ci_6alkyl,
(2) C4-7cycloalkyl,
(3) C4_7eycloalkenyl,
(4) C3_7cycloheteroalkyl,
(5) C3_7cyc1oheteroa1kenyl,
(6) aryl, and
(7) heteroaryl,
wherein alkyl, cycloalkyl, cycloalkenyl, cycloheteroalkyl, cycloheteroalkenyl,
aryl and heteroaryl
are unsubstituted or substituted with 1, 2, 3 or 4 substituents selected from:
oxo, -OH, -CN, -
NH2, -Ci-6a1ky1, -0C1-6a11cy1, halogen, -CH2F, -CHF2, -CF3, -0O211, -CO2C1-
6a1ky1, -C3_
7cycloalkyl, and heteroaryl;
n is 0, 1,2, 3 or 4;
m is 0, 1, 2, 3 or 4;
p is 0, 1, 2, or 3;
Os 0, 1, 2,3 or 4;
r is 0, 1 or 2;
s is 0, 1, 2, 3 or 4; and
tis 0,1, 233 or 4.
In one embodiment of the present invention, the present invention is concerned
with novel
compounds of structural Formula I:
> ___________________________________________ x
N
(I)
R5
or a pharmaceutically acceptable salt thereof, wherein:
T is selected from the group consisting of: CR3, N and N.-oxide;
U is selected from the group consisting of: CR1, N and N-oxide;
V is selected from the group consisting of: CR2, N and N-oxide;
W is selected from the group consisting of: CR4, N and N-oxide,
provided that at least one of T, U, V and W is N or N-oxide;
X is absent or selected from: -CH2-, -CHF-, -CF2-, -S-, -0-, -O-CH2-, -NH-, -
C(0)-, -NHC(0)-,
-C(0)NH-, -NHS02-, -SO2NH-, and -0O2-, wherein each CH2 is unsubstituted or
substituted
with 1 or 2 substituents selected from: hydroxy, halogen, NH2, C1-6a1ky1,
CO2H, CO2C1-
6alkyl, COCI-6a1ky1, phenyl and -CH2phenyl, and wherein each NH is
unsubstituted or
- 13
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
substituted with 1 substituent selected from: CI-6a1ky1, CO2H8 CO2C1-6a1kyl,
COC1-6a1kyl,
phenyl and -CH2phenyl;
Y is selected from: C3_10eye1oa1ky1, C3-10eye1oa1keny1, C2-1
ocycloheteroalkyl, C2-
locycloheteroalkenyl, aryl, and heteroaryl, wherein cycloalkyl, cycloalkenyl,
cycloheteroalkyl,
cycloheteroalkenyl, aryl and heteroaryl are unsubstituted or substituted with
1, 2, 3 or 4
substituents selected from Rb;
Z is selected from: oxo, -CN, -CF3, -C1-6a11(371, -(CH2)t-halogen3 -
(CH2)nCOCi_6a1ky1, -
(CH2)nCO2H, -(CH2)nOCOH, -(CH2)nCO2Ri, -(CH2)n000Ri, -(CH2)n0H, -
(CH2)nC(0)N(Rg)2, -(CH2)nC(0)(CH2)nN(Rg)2, -(CH2)n0C(0)(CH2)nN(R02,
(CH2)nNHC(0)C1-6a1ky1, -(CH2)nNHSO2Ri, -(CH2)002C1-6a1ky1, --(CH2)002NHRg, -
(C112)nS02NHC(0)Ri, -(CH2)nS02NHCO2Ri, -(CH2)nS02NHCON(Rg)2, -
(CH2)nC(0)NHSO2Ri, --(CH2)nNHC(0)N(Rg)2, -(CH2)nC3-10cyc1oalkyl-CO2Re,
heteroaryl,
-C2-locycloheteroalkenyl, and -C2_10cycloheteroalkyl, wherein each CH2 is
unsubstituted or
substituted with 1 or 2 substituents selected from C1-6a1ky1, -OH and -NH28
wherein each NH is
unsubstituted or substituted with 1 substituent selected from Re, and wherein
each alkyl,
cycloalkyl, cycloheteroalkyl, cycloheteroalkenyl, aryl and heteroaryl is
unsubstituted or
substituted with 1, 2, 3 or 4 substituents selected from Re;
each RI and R2 is independently selected from: hydrogen, halogen, CN, CF38 -C1-
6alkyl, -C2-
6alkenYl, -C2-6a1kyny1, -(CH2)pC3- i ocycloalkyl, -(CH2)pC3-7eyc1oa1ky1-aryl8 -
(CH2)pC3_
7cycloalkyl-heteroaryl, -(CH2)pC4-10cycloalkenyl, -(CH2)pC4-7cyc1oa1keny1-
ary1, -(CH2)pC4-
7cycloalkenyl-heteroatyl, -(CH2)pC2-10eycloheteroalkyl, -(CH2)pC2-1
ocycloheteroalkenyl, -
(CH2)paryl, -(C112)paryl-C3-7cycloalkyl, -(CH2)pa1Y1-C2-7cycloheteroa1ky1, -
(CH2)paryl-aryl,
-(CH2)paryl-heteroatyl8 -(C112)pheteroatyl8 -C2-6a1keny1-a1ky1, -C2-6a1keny1-
aryl, -C2-6a1keny1-
heteroaryl, -C2-6a1keny1-C3-7cyc1oa1ky1, -C2-6alkenyl-C3-7cycloalkenyl, -C2-
6alkenyl-C2-
7cycloheteroalkyl, -C2-6alkenyl-C2_7cycloheteroalkenyl, -C2-6 alkynyl-(CH2)1-3-
0-aryl, -C2-
6akYnyl-alkyl, -C2-6a1kyny1-aryl, -C2-6alkyny1-heteroary1, -C2-6alkYnYl-C3-
7eycloalkyl, -C2-
6alkynyl-C3-7cycloalkenyl, -C2-6a1kyny1-C2-7cyc1oheteroa1kyl, -C2-6a1lgn371-C2-
7cycloheteroalkenyl, and -C(0)NH-(CH2)0-3pheny1, wherein each CH2 is
unsubstituted or
substituted with 1 or 2 substituents selected from: halogen, CF3, -OH, -NH2, -
C1-6alkyl, -0C1-
6alkyl, -NHCi -6alkyl, and -N(C1-6alky1)2, wherein each alkyl, alkenyl and
alkynyl is
unsubstituted or substituted with 1, 2 or 3 substituents selected from:
halogen, CF3, -OH, -NH2,
-C1-6alkyl, -0C1.6alkyl, -NHC1-6alkyl, and -N(C1-6alky1)2, and wherein each
cycloalkyl,
cycloalkenyl, cycloheteroalkyl, cyeloheteroalkenyl, phenyl, aryl and
heteroaryl is unsubstituted or
substituted with 1, 2, 3 or 4 substituents independently selected from Ra,
provided that at least one of and only one of R1 and R2 is selected from the
group consisting of:
hydrogen, halogen, -CN, -CF3, -C1-6allcyl, -C2.6alkenyl and-C2-6alkynyl;
R3 and R4 are each independently selected from: hydrogen, halogen, -Ca1ky1, -
C2-6alkenyl,
- 14 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
-C2_6alkyny1, -C3_10cycloalkyl, -C3-i0cycloalkenyl, aryl, heteroaryl, -CN, -
CF3, -OH, -0C1-
6alkYl, -NH29 -NHCi-6alkyl, -N(Ci _6alky1)2, -SC1-6a1ky1, -SOC1-6a1ky1, -S02C1-
6a1ky1, -
NHSO2C1-6alkyl, -NHC(0)Ci -6a1ky1, -SO2NHC l _6alkyl, and -C(0)NHCi _6alky1;
R5 is selected from: hydrogen, -C1 -6alkyl, -CH2CO2H, and -CH2CO2C1_6a1ky1;
each Ra is independently selected from the group consisting of: halogen, oxo, -
(CH2)m0H, -
(CH2)mN(Ri)2, -(CH2)naNO2, -(CH2)mCN, -C _6a1ky1, -(CH2)mCF3, -(CH2)m0CF3,
OCE120C1.6 alkyl, -(CH2)mC(0)N(Ri)2, -(CH2)mC(=N-OH)N(Ri)2, -(CH2)mOCI-6a1ky1,
-
(C1-12)m0-(CH2)m-C3-7cyc1oa1ky1, -(CH2)/n0-(CH2)m-C2-7cyc1oheteroa1ky1, -
(CH2)m0-
(CH2)m-arY1, -(CH2)m0-(CH2)m-heteroaryl, -(CH2)mSC1-6alkyl, -(CH2)mS(0)C1-
6a1ky1,
(CH2)m$02C1-6a1icY1, -(CH2)mS02C3-7cyc1oalky1, -(CH2)mS02C2-7cyc1oheteroa1ky1,
-
(CH2)mS02-aryl, -(CH2)mS02-heteroaryl, -(CH2)mS02NHC1_6a1kyl, -(CH2)mS02NHC3_
7cycloalkyl, -(CH2)mS02NHC2-7cyc1oheteroa1ky1, -(CH2)mS02NH-aryl, -(CH2)mS02NH-
heteroatyl, -(CF12)mNHS02-C1_6a1ky1, -(CH2)mNHS02-C3-7cycloalkyl, -
(CH2)niNHS02-C2-
7cycloheteroalkyl, -(CH2)mNHS02-aryl, -(CH2)mNHSO2NH-heteroary1, -
(CH2)mC(0)Rf, -
(CH2)mgONRi)2, -(CH2)mN(Ri)C(0)N(Ri)2, -(CH2)mCO2H, -(CH2)m000H, -
(CH2)mCO2Rf, -(CH2)m000Rf, -(CH2)naC3-7cycloalkyl, -(CH2)mC3_7cyc1oalkeny1, -
(CH2)mC2-6cycloheteroa1ky1, -(CH2)/nC2-6cycloheteroalkenyl, -(CH2)maryl, and -
(CH2)mheteroaryl, wherein each CH2 is unsubstituted or substituted with 1 or 2
substituents
selected from: oxo, -(CH2)0-30H, -CN, -NH2, -NH(C1-6alkyl), -N(C1-6alky1)2, -
Ci-6a1ky1,
OC1_6a1ky1, halogen, -CH2F, -CHF2, -CF3, -CO2H, -CO2Ci _6alkyl, -
C3_7cyc1oa1ky1, phenyl,
CH2phenyl, heteroaryl and CH2heteroaryl, and wherein alkyl, cycloalkyl,
cycloalkenyl,
cycloheteroalkyl, cyeloheteroalkenyl, aryl and heteroaryl are unsubstituted or
substituted with 1,
2, 3 or 4 substituents selected from: oxo, -(CH2)0-30H, -CN, -NH2, -NII(C1-
6alkyl), -N(C1-
6alkY1)2, -C1-6alkyl, -0Ci_6alkyl, halogen, -CH2F, -CHF2, -CF3, -CO2H, -CO2C
_6alkyl, -
SO2C1.6a1ky1, -C3-7cycloalkyl, phenyl, CH2phenyl, heteroaryl and
CH2heteroaryl;
each Rb is independently selected from: hydrogen, -C1-6alkyl, -C3-6cyc1oa1ky1,
-C3-
6cycloalkenyl, -C2-6cyc1oheteroa1kyl, -C2-6cycloheteroalkenyl, aryl,
heteroaryl, -(CH2)t-
halogen, -(CH2)s-OH, -NO2, -NH2, -NH(Ci -6alkyl), -N(Ci_6alky1)2, -0Ci
õ6alkyl, -
(C112)qCO2H, -(CH2)qCO2C1.6alky1, -CF3, -CN, -S02C1-6alkyl, and -
(CH2)sCON(R92,
wherein each CH2 is unsubstituted or substituted with 1 or 2 halogens, and
wherein each alkyl,
cycloalkyl, cycloalkenyl, cycloheteroalkyl, cycloheteroalkenyl, aryl and
heteroaryl is
unsubstituted or substituted with 1, 2 or 3 halogens;
each Re is independently selected from: halogen, oxo, -(CH2)r0H, -
(CH2)rN(Re)2, -(C112)rCN,
-Ci_6alkyl, -CF3, -Ci -6alkyl-OH, -OCH2OCi -6alkyl, -(CH2)r0C1-6a1ky1, -
OCH2aryl, -
(CH2)rSC1-6a1kY1, --(CH2)rC(0)Rf, -(CH2)/C(0)N(Re)2, -(CH2)rCO2H3 -
(CH2)rCO2Rf, -
(CH2)rC3-7cycloalkyl, -(CH2)rC2-6cyc1oheteroa1ky1, -(CH2)raryl, and -
(CH2)rheteroaryl,
wherein each CH2 is unsubstituted or substituted with 1 or 2 substituents
selected from: oxo, -
- 15 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
OH, -CN, -N(Rh)2, -C .6alkyl, -0C1.6alkyl, halogen, -CH2F, -CHF2, -CF3, -
CO2C1-
6a1kYl, -C3-7cycloalkyl and heteroaryl, and wherein alkyl, cycloalkyl,
cycloheteroalkyl, aryl and
heteroaryl are unsubstituted or substituted with 1, 2, 3 or 4 substituents
selected from: oxo, -01-1,
-CN, .N(Rh)2, -C1 -6alkyl, -OC ..6alkyl, halogen, -CH2F, -CHF2, -CF3, -0O21-1,
-CO2C1-
6a1ky1, -C3-7cycloalkyl and heteroaryl;
each Re, kg and Rh is independently selected from: hydrogen, -Ci..6a1ky1, and -
0-C1-6alkyl,
wherein alkyl is unsubstituted or substituted with 1, 2, 3 or 4 substituents
selected from: -OH,
oxo, halogen, Ca11cy1, -0C1-6a1ky18 -NH2, -NH(C1-6alky1)3 and -N(C1-6alky1)2;
each Ri is independently selected from: hydrogen, Ci_6alkyl, C3-6cycloalkyl, -
C(0)R1, and -
SO2R1, wherein alkyl and cycloalkyl are unsubstituted or substituted with 1,
2, 3 or 4
substituents selected from: -OH, oxo, halogen, C1-6alkyl, -0C1-6a1ky1, --NH2, -
NH(C1-6alkY1),
and -N(C1-6alky1)2;
each Rf and R1 is independently selected from: C1_6alkyl, C4_7cyc1oa1ky1,
C4_7cycloalkenyl,
C3_7cycloheteroalkyl, C3-7cycloheteroalkenyl, aryl, and heteroaryl, wherein
alkyl, cycloalkyl,
cycloalkenyl, cycloheteroalkyl, cyeloheteroalkenyl, aryl and heteroaryl are
unsubstituted or
substituted with 1, 2, 3 or 4 substituents selected from: oxo, -0H, -CN, -
1\1112, -C1-6a1ky1, -OC1-
6alkyl, halogen, -CH2F, -CHF2, -CF3, -CO2H, -CO2C1-6a1kyl, -C3_7eycloalkyl,
and heteroaryl;
n is 0, 1, 2, 3 or 4; m is 0, 1, 2, 3 or 4; p is 0, 1, 2, or 3; q is 0, 1, 2,
3 or 4; r is 0, 1 or 2; s is 0, 1,
2, 3 or 4; and t is 0, 1, 2, 3 or 4.
In another embodiment of the present invention, T is selected from the group
consisting
of: -CR3-, N, and N-oxide. In a class of this embodiment, T is -CR3-. In
another class of this
embodiment, T is selected from the group consisting of: N, and N-oxide. In
another class of this
embodiment, T is N. In another class of this embodiment, T is N-oxide.
In another embodiment of the present invention, U is selected from the group
consisting
of: -CR1-, N, and N-oxide. In a class of this embodiment, U is -CR1-. In
another class of this
embodiment, U is selected from the group consisting of: N, and N-oxide. In
another class of this
embodiment, U is N. In another class of this embodiment, U is selected from
the group
consisting of: N-oxide.
In another embodiment of the present invention, V is selected from the group
consisting
of: -CR2-3 N, and N-oxide. In a class of this embodiment, V is -CR2-. In
another class of this
embodiment, V is selected from the group consisting of: N, and N-oxide. In
another class of this
embodiment, V is N. In another class of this embodiment, V is N-oxide.
In another embodiment of the present invention, W is selected from the group
consisting
of:-CR4-, N, and N-oxide. In a class of this embodiment, W is selected from
the group
consisting of: -CR4-. In another class of this embodiment, W is selected from
the group
consisting of: N, and N-oxide. In another class of this embodiment, W is N. In
another class of
this embodiment, W is N-oxide.
- 16 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
In another embodiment of the present invention, one of T and W is N or N-
oxide, U is
CR1 and V is CR2, provided that if W is N or N-oxide then R1 is selected from
hydrogen,
halogen, -CN, -CF3, -C1-6a1ky1, -C2-6alkenyl and-C2_6alkynyl, and if T is N or
N-oxide then
R2 is selected from hydrogen, halogen, -CN, -CF3, -Ci -6alkyl, -C2-6alkenyl
and-C2-6alkynyl.
In another embodiment of the present invention, one of T and W is N or N-
oxide, U is
CR1 and V is CR2, provided that if W is N or N-oxide then R1 is halogen, and
if T is N or N-
oxide then R2 is halogen.
In another embodiment of the present invention, T is N or N-oxide; U is -CR1-;
V is -
CR2-; and W is -CR4-. In a class of this embodiment, T is N or N-oxide; U is -
CR1-; V is -
CR2-, wherein R2 is halogen; and W is -CR4-. hi another class of this
embodiment, T is N; U is
-CR1-; V is -CR2-, wherein R2 is halogen; and W is -CR4-.
In another embodiment of the present invention, one of T and W is N or N-
oxide, U is
CR1 and V is CR2, provided that if W is N or N-oxide then R1 is halogen, and
if T is N or N-
oxide then R2 is chloride.
In another embodiment of the present invention, T is N or N-oxide, U is CR1, V
is CR2,
and W is CR4. In a subclass of this class, T is N, U is CR1, V is CR2, and W
is CR4. In another
subclass of this class, T is N, U is CR1, V is CR2, W is CR4, and R2 is
halogen. In another
subclass of this class, T is N, U is CR1, V is CR2, W is CR4, and R2 is
chloride. In another
subclass of this class, T is N, U is CR1, V is CR2, W is CR4, R2 is chloride,
and R4 is hydrogen.
In another embodiment of the present invention, X is absent.
In another embodiment of the present invention, X is selected from: -CH2-, -
CHF-, -CF2-
, -S-, -0-, -0-CH2-, -NH-, -C(0)-, -NHC(0)-, -C(0)NH-, -NHS02-, -SO2NH-, and -
CO2-,
wherein each CH2 is unsubstituted or substituted with I or 2 substituents
selected from:
hydroxy, halogen, NH2, Ci-6al1cy1, CO2H, CO2Ci COC1_6alkyl, phenyl and -
CH2phenyl, and wherein each NH is unsubstitute,d or substituted with 1
substituent selected
from: Ci.6alky1, CO2H, CO2C1-6alky1, COCI-6alkyl, phenyl and -CH2phenyl. In a
class of
this embodiment, X is absent or selected from: -CH2-, -CHF-, -CF2-, -S-, -0-, -
0-CH2-, and -NH-
In another class of this embodiment, X is absent or selected from: -CH2-, -0-,
and -0-CH2-. In
another class of this embodiment, X is absent or selected from: -0-, and -0-
CH2-. In another
class of this embodiment, X is selected from: -0-, and -0-CH2-. In another
class of this
embodiment, X is -0-. In another class of this embodiment, X is -0-CH2-. In
another class of
this embodiment, X is absent or selected from: -C(0)-, -NHC(0)-, -C(0)NH-, -
NHS02-, -
SO2NH-, and -0O2-.
In another embodiment of the present invention, X is -0,
In another embodiment of the present invention, Y is selected from: C34
ocycloalkyl, C3_
lOcycloalkenyl, C2-iocycloheteroalkyl, C2_1()cycloheteroalkenyl, aryl, and
heteroaryl, wherein
cycloalkyl, eycloalkenyl, cycloheteroalkyl, cycloheteroalkenyl, aryl and
heteroaryl are
-17-
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
unsubstituted or substituted with 1, 2, 3 or 4 substituents selected from Rb.
In a class of this
embodiment, Y is selected from: C3-10cycloalkyl8 C2-10cycloheteroalkyl, and
aryl, wherein
cycloalkyl, cycloheteroalkyl and aryl are unsubstituted or substituted with 1,
2, 3 or 4 substituents
selected from Rb. In another class of this embodiment, Y is selected from:
Cmocycloalkyl, C2-
iocycloheteroalkyl, and phenyl, wherein cycloalkyl, cycloheteroalkyl and
phenyl are
unsubstituted or substituted with 1, 2, 3 or 4 substituents selected from Rb.
In another class of
this embodiment, Y is selected from: cyclohexyl, cyclobutyl, cyclopropyl,
cyclopentyl,
pyrrolidine, piperidine, tetrahydrofuran, tetrahydropyran and phenyl, wherein
cycloalkyl,
cycloheteroalkyl and phenyl are unsubstituted or substituted with I, 2, 3 or 4
substituents
selected from Rb.
In another class of this embodiment, Y is selected from: C3.7cyc1oalky1, and
aryl,
wherein each cycloalkyl and aryl is unsubstituted or substituted with 1, 2, 3
or 4 substituents
selected from Rb. In a subclass of this class, Y is selected from: cyclohexyl,
and phenyl, wherein
each cycloalkyl, and phenyl is unsubstituted or substituted with 1, 2, 3 Or 4
substituents selected
from Rb.
In another class of this embodiment, Y is aryl, wherein each aryl is
unsubstituted or
substituted with 1, 2, 3 or 4 substituents selected from Rb. In a subclass of
this class, Y is
phenyl, wherein each phenyl is unsubstituted or substituted with 1, 2, 3 or 4
substituents selected
from Rb.
In another class of this embodiment, Y is selected from: Cmocycloalkyl,
wherein each
cycloalkyl is unsubstituted or substituted with 1, 2, 3 or 4 substituents
selected from Rb. In a
subclass of this class, Y is cyclohexyl, wherein each cyclohexyl is
unsubstituted or substituted
with 1, 2, 3 or 4 substituents selected from Rb.
In another embodiment of the present invention, Y is selected from: C3-
7cyc1oalkyl, C2-
I. ocycloheteroalkyl, and phenyl, wherein each cycloalkyl, cycloheteroalkyl,
and phenyl is
unsubstituted or substituted with 1, 2, 3 or 4 substituents selected from Rb.
In a class of this
embodiment, Y is selected from: cyclobutyl, cyclohexyl, 1, 4:3, 6-dianhydro-D-
mannitol,
tetrahydropyran, and phenyl, wherein each cyclobutyl, cyclohexyl,
tetrahydropyran, and phenyl is
unsubstituted or substituted with 1, 2, 3 or 4 substituents selected from Rb.
In another class of
this embodiment, Y is selected from: cyclobutyl, cyclohexyl, 1, 4:3, 6-
dianhydro-D-mannitol5
2,3,3a,5,6,6a-hexahydrofuro[3,2-b]furan, tetrahydropyran, and phenyl, wherein
each cyclobutyl,
cyclohexyl, tetrahydropyran, and phenyl is unsubstituted or substituted with
1, 2, 3 or 4
substituents selected from Rb. In another class of this embodiment, Y is
selected from:
cyclobutyl, cyclohexyl, 2,3,3a,5,6,6a-hexahydrofuro[3,2-b]furan,
tetrahydropyran, and phenyl,
wherein each cyclobutyl, cyclohexyl, tetrahydropyran, and phenyl is
unsubstituted or substituted
with 1, 2, 3 or 4 substituents selected from Rb.
In another embodiment of the present invention, Y is selected from: C2_
- 18-
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
10eycloheteroalkyl, wherein each cycloheteroalkyl is unsubstituted or
substituted with 1, 2, 3 or
4 substituents selected from Rb. In a class of this embodiment, Y is
hexahydrofuro[3,2-b]furan.
In another class of this embodiment, Y is 2,3,3a,5,6,6a-hexahydrofuro[3,2-
b]furan.
In another embodiment of the present invention, Z is selected from: oxo, -CN, -
CF3, -
C1-6alkYl, -(C1-12).halogen, -(CH2)nCOCi- 6a1kY1, -(C112)nCO214, -(CH2)nOCOH, -
(C112)11CO2Ri, -(CH2)11OCORi, -(CH2)n0H, -(CH2)nC(0)N(Rg)2, -
(CH2)11C(0)(CH2)11N(Rg)25 -(CH2)n0C(0)(CH2)nN(Rg)2, -(CH2)nNHC(0)C1-6alkyl, -
(CH2)11NHSO2Ri, -(CH2)002C1-6alkyl, 4CH2)11S02N1-1Rg, -(CH2)002NHC(0)Ri, -
(CH2)nS02NHCO2Ri, -(CH2)002NHCON(Rg)2, -(CH2)nC(0)NHS02Ri, -
(CH2)11NHC(0)N(Rg)2, -(C112)nC3-10cycloallcyl-0O2Re, heteroaryl, -C2-
iocycloheteroalkenyl, and -C2-1ocycloheteroalkyl, wherein each CH2 is
unsubstituted or
substituted with 1 or 2 substituents selected from Ci -6a1ky1, -OH and -NH2,
wherein each NH is
unsubstituted or substituted with 1 substituent selected from Re, and wherein
each alkyl,
cycloalkyl, cycloheteroalkyl, cycloheteroalkenyl, aryl and heteroaryl is
unsubstituted or
substituted with 1, 2, 3 or 4 substituents selected from Re.
In a class of this embodiment, Z is selected from: oxo, -CN, -CF3, -
(C1{2)t-
halogen, -(CH2)11COCi -6alkyl, -(CH2)nCO2H, -(CH2)nOCOH, --(CH2)nCO2Ri, -
(CH2)nOCORi, -(CH2)n0H, -(CH2)11C(0)N(Rg)2, --(C112)11C(0)(CF12)nN(Rg)2, -
(CH2)n0C(0)(CH2)nN(Rg)2, -(C112)11NI1C(0)C1-6alkYl, -(CH2)11NHSO2Ri, -
(CH2)nS02C1-
6alkyl8 -(CH2)002NHRg, -(CH2)11S02NHC(0)Ri, -(CH2)11S02NHCO2Ri, -
(CH2)002NHCON(Rg)2, ---(CH2)nC(0)NHSO2Ri, -(CH2)11NHC(0)N(Rg)2, -(CH2)11C3_
1ocycloalkyl-CO2Re, heteroaryl, -C240cyeloheteroalkenyl, and -
C2_10cycloheteroalkyl,
wherein each CH2 is unsubstituted or substituted with 1 or 2 substituents
selected from CI-
6alkyl, -OH and -NH2, wherein each NH is unsubstituted or substituted with 1
substituent
selected from Re, and wherein each alkyl, cycloalkyl, cycloheteroalkyl,
cycloheteroalkenyl, and
heteroaryl is unsubstituted or substituted with 1, 2, 3 or 4 substituents
selected from Re.
In another class of this embodiment of the present invention, Z is selected
from: oxo, -
CF3,-Ci -6alky1, -(CH2)t-halogen, -(CH2)nC0C1-6alkY1, -(CH2)11CO2H, -(CH2)n0H,
-
(CH2)11C(0)N(Rg)2, -(CH2)11C(0)(CH2)nN(Rg)2, -(CH2)002C1-6a1ky1, and
heteroaryl,
wherein each CH2 is unsubstituted or substituted with 1 or 2 substituents
selected from C1-
6alkyl, -OH and -NH2, wherein each NH is unsubstituted or substituted with 1
substituent
selected from Re, and wherein each alkyl and heteroaryl is unsubstituted or
substituted with 1, 2,
3 or 4 substituents selected from Re.
In another class of this embodiment of the present invention, Z is selected
from: oxo,
CF3, -(CH2)t-halogen, -(CH2)nC0C1 -6a1ky1, -(CH2)nOCOH, -(CH2)11CO2H, -
(CH2)n0F1, -(CH2)nC(0)N(Rg)2, -(CH2)11C(0)(0-12)nN(Rg)2, -
(CH2)110C(0)(C1{2)11N(Rg)2,
and -(CH2)002C1_6alky1, wherein each CH2 is unsubstituted or substituted with
1 or 2
- 19-
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
substituents selected from Ci_6alkyl, -OH and -NH2, wherein each NH is
unsubstituted or
substituted with 1 substituent selected from Re, and wherein each alkyl is
unsubstituted or
substituted with 1, 2, 3 or 4 substituents selected from Re.
In another class of this embodiment of the present invention, Z is selected
from: oxo, -
CF3, -CH3, -CH2F, -COCH3,-CO2H, -OH, -CH2OH, -CH(CH3)0H, -C(CH3)20H, -
C(0)N(OCI-13)(CH3), -C(0)(CH2)NH2, ), -0C(0)CH(CH3)NH2,and -S02CH3, wherein
each
CH2 is unsubstituted or substituted with 1 or 2 substituents selected from C1-
6alkyl, -OH and -
NH2, wherein each NH is unsubstituted or substituted with 1 substituent
selected from Re, and
wherein each alkyl is unsubstituted or substituted with I, 2, 3 or 4
substituents selected from RC.
In another class of this embodiment of the present invention, Z is selected
from: oxo,
CF3 -CI-6a1ky1, -(C112)thalogen, -(CH2)11CO211, --(C112)n0H, and -
(CH2)nS02C1.6a1ky1,
wherein each CH2 is unsubstituted or substituted with 1 or 2 substituents
selected from Ci_
6alkyl, -011 and -N112, wherein each NH is unsubstituted or substituted with 1
substituent
selected from Re, and wherein each alkyl is unsubstituted or substituted with
1, 2, 3 or 4
substituents selected from Re. In another class of this embodiment of the
present invention, Z is
selected from: oxo, -CF3, -CH3,-CH2F, -0O211, -OH, -CH2OH, -CH(C113)011, -
C(CH3)20H, and -S02CH3, wherein each CH2 is unsubstituted or substituted with
1 or 2
substituents selected from C i_6alkyl, -OH and -N112, and wherein each alkyl
is unsubstituted or
substituted with 1, 2, 3 or 4 substituents selected from R.C.
In another class of this embodiment of the present invention, Z is selected
from: oxo,
CF3, -CH3,-CH2F, -CO2H, -011, -CH2011, -CH(CH3)0H, -C(CH3)20H, and -S02C113.
In another class of this embodiment, Z is selected from: oxo, CN, -(CH2)nCO2H,
-
(CH2)nCO2Ri, and -(CH2)n0H. In a subclass of this class, Z is selected from:
oxo, CN, -
CO211, -CO2Ri, and -OH.
In another class of this embodiment, Z is selected from: -(C112)nCO2H, and -
(CH2)nCO2Ri. In a subclass of this class, Z is selected from: -0O211, and -
CO2Ri.
In another embodiment of this invention, Z is selected from: -(CH2)nCO2H, and -
(C1tt2)110H, wherein each C112 is unsubstituted or substituted with 1 or 2
substituents selected
from C1-6alkyl, -011 and -NI12, and wherein each NH is unsubstituted or
substituted with 1
substituent selected from Re. In a class of this embodiment, Z is selected
from: -(C112)nCO2H,
and -(CH2)11011, wherein each CH2 is unsubstituted or substituted with 1 or 2
substituents
selected from Ci..6alkyl, and -01-1. In another class of this embodiment, Z is
selected from: -
(CH2)nCO2H, and -(CH2)n0H. In another class of this embodiment, Z is selected
from: -
CO2H, -OH, -CH2OH, and -C(C113)20H. In another class of this embodiment, Z is
selected
from: -0O2H, -CH2OH, and -C(CH3)2011.
In another embodiment of the present invention, Z is -CO2H.
In another embodiment of the present invention, Z is selected from: -(CH2)n0H,
wherein
- 20 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
each CH2 is unsubstituted or substituted with 1 or 2 substituents selected
from Ci.6allcyl, -OH
and -NH2, and wherein each NH is unsubstituted or substituted with 1
substituent selected from
Re. In a class of this embodiment, Z is selected from: -(CH2)110H, wherein
each CH2 is
unsubstituted or substituted with 1 or 2 substituents selected from Ci_6alkyl,
and -OH. In
another class of this embodiment Z is selected from: -(CH2)n0H. In another
class of this
embodiment, Z is selected from: -OH, -CH2OH, and -C(CH3)20H.
In another embodiment of the present invention, Z is selected from: -(CH2)t-
halogen, and
-(C112)n0H, wherein each CH2 is unsubstituted or substituted with 1 or 2
substituents selected
from Ci.6alky1, -OH and -NH2. In a class of this embodiment, Z is selected
from: -(CH2)t-
halogen, and --(CHAOH. In a class of this embodiment, Z is selected from: -
halogen and -OH.
In another class of this embodiment, Z is selected from: fluorine and-OH. In
another class of
this embodiment, Z is halogen. In another class of this embodiment, Z is
fluorine. In another
class of this embodiment, Z is -OH.
In another embodiment of this invention, Z is selected from: -(CH2)n.0O2H, -
(CH2)t-
halogen, and -(CH2)110H, wherein each CH2 is unsubstituted or substituted with
1 or 2
substituents selected from Calky1, -OH and -NH2, and wherein each NH is
unsubstituted or
substituted with 1 substituent selected from Re. In a class of this
embodiment, Z is selected
from: -(CH2)nCO2H, -(CH2)t-halogen, and -(CH2)flOH, wherein each CH2 is
unsubstituted or
substituted with 1 or 2 substituents selected from C1-6a1ky1, and -OH. In
another class of this
embodiment, Z is selected from: -(CH2)nCO21-1, halogen and -(CH2)n0H. In
another class of
this embodiment, Z is selected from: -CO2H, F, -OH, -CH2OH, and -C(CH3)20H. In
another
class of this embodiment, Z is selected from: -CO2H, F, -OH, -CH2OH, and -
C(CH3)20H.
In another embodiment of the present invention, each R1 and R2 is
independently
selected from: hydrogen, halogen, CN, CF3, -C1-6alkyl, -C2-6alkenyl, -C2-
6a1kyny1,
(CH2)pC3-locycloalkyl, -(CH2)pC3-7cyc1oa1ky1-aty1, -(CH2)pC3-7cyc1oa1ky1-
heteroaryl, -
(CH2)pC4-10cycloalkenyl, -(C112)pC4-7cycloalkenyl-aryl, -(CH2)pC4-
7cycloalkenyl-heteroaryl,
-(CH2)pC2-10cycloheteroalkyl, -(CH2)pC2-locycloheteroalkenyl, -(CH2)paryl, -
(CH2)paryl-
C3_7cycloalkyl, -(CH2)parYl-C2-7cycloheteroalkyl, -(CH2)paryl-aryl, -
(CH2)paryl-heteroaryl, -
(CH2)pheteroarY1, -C2-6a1keny1-alky1, -C2-6a1keny1-ary1, -C2-6a1keny1-
heteroary1, -C2_6alkenyl-
C3_7cycloalkyl, -C2-6alkenyl-C3-7cycloalkenyl, -C2-6alkenyl-C2-
7cycloheteroalkYL -C2-
6alkenyl-C2-7cycloheteroalkenyl, -C2-6 alkYnY1-(CH2)1-3-0-aryl, -C2-6alkYnyl-
alkyl, -C2-
6alkynyl-aryl, -C2-6a1kynyl-heteroaryl, -C2-6a1icYnYI-C3-7cyc1oalkyl, -C2-
6alkynyl-C3..
7cycloalkenyl, -C2-6a1kynyl-C2_7cyc1oheteroalkyl, -C2-6a1kyny1-C2-
7cyc1oheteroa1keny1, and -
C(0)NH-(CH2)0-3phenyl, wherein each CH2 is unsubstituted or substituted with 1
or 2
substituents selected from: halogen, CF3, -OH, -NH2, -C-1-6a1ky1, -0C1 -
6alkyl, -NHCi-6alkyl,
and -N(C1-6alky1)2, wherein each alkyl, alkenyl and alkynyl is unsubstituted
or substituted with
I, 2 or 3 substituents selected from: halogen, CF3, -OH, -N112, -C1-6alkyl, -
0Ct _6alkyl, -
-21 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
NHCi..6alkyl, and -N(C1-6alky1)2, and wherein each cycloalkyl, cycloalkenyl,
cycloheteroalkyl,
cycloheteroalkenyl, phenyl, aryl and heteroaryl is =substituted or substituted
with 1, 2, 3 or 4
substituents independently selected from Ra, provided that at least one of and
only one of R1 and
R2 is selected from the group consisting of: hydrogen, halogen, -CN, -CF3, -Ci-
6alkyl, -C2-
6alkenyl and-C2-6a1kynyl. In a class of this embodiment of the present
invention, each RI and
R2 is independently selected from: halogen, -(CH2)pC4-1 oeycloalkenyl, -
(CH2)paryl, -
(CH2)paryl-C3-7cycloalkyl3 -(CH2)paryl-C2-7cycloheteroalkyl, -(CH2)paryl-aryl,
-(CH2)paryl-
heteroaryl, -(CH2)pheteroaryl, -C2-6a1kyny1-ary1, wherein each C112 is
=substituted or
substituted with 1 or 2 substituents selected from: halogen, CF3, -OH, -NH2, -
C1-6alicyl, -0C1-
6alkyl, -NHCi-6a1ky1, and -N(C1-6a1ky1)2, wherein each alkynyl is =substituted
or substituted
with 1, 2 or 3 substituents selected from: halogen, CF3, -OH, -NH2, -C1-
6alkyl, -0C1_6alkyl, -
NHC1.6alkyl, and -N(C1-6alky1)2, and wherein each cycloalkyl, cycloalkenyl,
cycloheteroalkyl,
aryl and heteroaryl is =substituted or substituted with 1, 2, 3 or 4
substituents independently
selected from Ra, provided that at least one of and only one of RI and R2 is
selected from the
group consisting of halogen.
In another class of this embodiment of the present invention, each RI and R2
is
independently selected from: halogen, -C4-1ocyc1oa1keny1, -aryl, -aryl-
C3_7cycloalkyl, -aryl-C2-
7cycloheteroalkyl, -aryl-aryl, -aryl-heteroaryl, -heteroaryl, -C2.6alkynyl-
aryl, wherein each
alkynyl is unsubstituted or substituted with 1, 2 or 3 substituents selected
from: halogen, CF33
OH, -NH2, -Ci_6alkyl, -0Ci -6a1kyl, -NHCi-6alkyl, and -N(C1-6alky1)2, and
wherein each
cycloalkyl, cycloalkenyl, cycloheteroalkyl, aryl and heteroaryl is
=substituted or substituted with
1, 2, 3 or 4 substituents independently selected from Ra, provided that at
least one of and only
one of RI and R2 is selected from the group consisting of halogen. In another
class of this
embodiment of the present invention, each RI and R2 is independently selected
from: halogen, -
C4-10cycloalkenyl, -phenyl, -phenyl-C3_7cycloalkyl, -phenyl-
C2_7eycloheteroalkyl, -phenyl-
heteroaryl, -heteroaryl, -C2_6alkynyl-phenyl, wherein each alkynyl is
unsubstituted or substituted
with 1, 2 or 3 substituents selected from: halogen, CF3, -OH, -N112, -C1-
6alkyl, -0C1-6alkyl, -
NHC1_6alkyl, and -N(Ci..6alky1)2, and wherein each cycloalkyl, cycloalkenyl,
cycloheteroalkyl,
phenyl and heteroaryl is =substituted or substituted with 1, 2, 3 or 4
substituents independently
selected from Ra, provided that at least one of and only one of RI and R2 is
selected from the
group consisting of halogen. In another class of this embodiment of the
present invention, each
RI and R2 is independently selected from: Cl, F, cyclohexenyl, -phenyl, phenyl-
cyclopropyl,
phenyl-piperazine, phenyl-pyrrolidine, -phenyl-phenyl, phenyl-triazole, phenyl-
thiazole, phenyl-
pyrazole, phenyl-oxadiazole, phenyl-furan, -pyridine, benzodioxole, indole,
azaindole,
benzofuran, benzopyrazole, benzodioxane, tetrahydroisoquinoline,
azabenzimidazole, -C2-
alkynyl-phenyl, wherein each alkynyl is =substituted or substituted with I, 2
or 3 substituents
selected from: halogen, CF3, -OH, -NH2, -C1-6a1ky1, -0C1_6alicyl, -NHCi
_6alkyl, and -N(C1-
- 22 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
6alky1)2, and wherein each cycloalkyl, cycloalkenyl, cycloheteroalkyl, phenyl
and heteroaryl is
unsubstituted or substituted with 1, 2, 3 or 4 substituents independently
selected from Ra,
provided that at least one of and only one of R1 and R2 is selected from the
group consisting of
CI and F. In another class of this embodiment of the present invention, each
R1 and R2 is
independently selected from: CI, cyclohexenyl, -phenyl, phenyl-cyclopropyl,
phenyl-piperazine,
phenyl-pyrrolidine, -phenyl-phenyl, phenyl-triazole, phenyl-thiazole, phenyl-
pyrazole, phenyl-
oxadiazole, phenyl-furan, -pyridine, benzodioxole, indole, azaindole,
benzofuran, benzopyrazole,
benzodioxane, tetrahydroisoquinoline, -C2-alkynyl-phenyl, wherein each alkynyl
is unsubstituted
or substituted with I, 2 or 3 substituents selected from: halogen, CF36 -OH, -
NH2, -C1.6alkyl,
OCi_6alkyl, -NHC1-6alkyl, and -N(C1-6alky1)2, and wherein each cycloalkyl,
cycloalkenyl,
cycloheteroalkyl, phenyl and heteroaryl is unsubstituted or substituted with
1, 2, 3 or 4
substituents independently selected from Ra, provided that at least one of and
only one of R1 and
R2 is Cl.
In another class of this embodiment of the present invention, R1 is
independently selected
from: -(CH2)pC4-10cycloalkenyl, -(CH2)parY1, -(CH2)parYl-C3-7cyc1oa1lcy1, -
(CH2)parY1-C2-
7cycloheteroalkyl, -(CH2)pary1-ary1, -(CH2)paryl-heteroaryl, -
(CH2)pheteroatyl, -C2-6alkyny1-
aryl, wherein each CH2 is unsubstituted or substituted with I or 2
substituents selected from:
halogen, CF3, -OH, -N1-12, -C1 -6allcY1, -OC1-6a1ky1, -NHCi-6a1kyl, and -N(Ci
..6alky1)2,
wherein each alkynyl is unsubstituted or substituted with 1, 2 or 3
substituents selected from:
halogen, CF3, -OH, -NH2, -C1-6alkyl, -0C1-6a1ky1, -NHC1-6a1kyl, and -
N(Ci_6alky1)2, and
wherein each cycloalkyl, cycloalkenyl, cycloheteroalkyl, aryl and heteroaryl
is unsubstituted or
substituted with 1, 2, 3 or 4 substituents independently selected from Ra, and
R2 is selected from
the group consisting of halogen. In another class of this embodiment of the
present invention, R1
is independently selected from: -C4-10cycloalkenyl, -aryl, -aryl-C3-
7cycloalkyl, -aryl-C2-
7cycloheteroalkyl, -aryl-aryl, -aryl-heteroaryl, -heteroaryl, -C2-6a1kyny1-
ary1, wherein each
alkynyl is unsubstituted or substituted with I, 2 or 3 substituents selected
from: halogen, CF3, -
OH, -NH2, -C1-6a11cyl, -0C1 -6alkyl, -NHC1-6alkyl, and -N(C1-6a1ky1)2, and
wherein each
cycloalkyl, cycloalkenyl, cycloheteroalkyl, aryl and heteroaryl is
unsubstituted or substituted with
1, 2, 3 or 4 substituents independently selected from Ra, and R2 is selected
from the group
consisting of halogen. In another class of this embodiment of the present
invention, R1 is
independently selected from: -C4-iocycloalkenyl, -phenyl, -phenyl-
C3.7cycloalkyl, -phenyl-C2-
7eycloheteroalkyl, -phenyl-heteroaryl, -heteroaryl, -C2_6a11cyny1-pheny1,
wherein each alkynyl is
unsubstituted or substituted with 1, 2 or 3 substituents selected from:
halogen, CF3, -OH, -NH2,
-C _6alkyl, -0Ci_6alkyl, -NHCI -6alkyl, and -N(Ci -6alky1)2, and wherein each
cycloalkyl,
cycloalkenyl, cycloheteroalkyl, phenyl and heteroaryl is unsubstituted or
substituted with I, 2, 3
or 4 substituents independently selected from Ra, and R2 is selected from the
group consisting of
halogen. In another class of this embodiment of the present invention, R1 is
independently
- 23 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
selected from: cyclohexenyl, -phenyl, phenyl-cyclopropyl, phenyl-piperazine,
phenyl-pyrrolidine,
-phenyl-phenyl, phenyl-triazole, phenyl-thiazole, phenyl-pyrazole, phenyl-
oxadiazole, phenyl-
furan, -pyridine, benzodioxole, indole, azaindole, benzofuran, benzopyrazole,
benzodioxane,
tetrahydroisoquinoline, -C2-alkynyl-phenyl, wherein each alkynyl is
unsubstituted or substituted
with 1, 2 or 3 substituents selected from: halogen, CF3, -OH, -N112, -C1-
6a1kyl, -0C1-6alkyl, -
NHCi .6alkyl, and -N(C1-6alky1)2, and wherein each cycloalkyl, cycloalkenyl,
cycloheteroalkyl,
phenyl and heteroaryl is unsubstituted or substituted with 1, 2, 3 or 4
substituents independently
selected from Ra, and R2 is selected from the group consisting of Cl and F. In
another class of
this embodiment of the present invention, Ri is independently selected from:
cyclohexenyl,
phenyl, phenyl-cyclopropyl, phenyl-piperazine, phenyl-pyrrolidine, -phenyl-
phenyl, phenyl-
triazole, phenyl-thiazole, phenyl-pyrazole, phenyl-oxadiazole, phenyl-furan, -
pyridine,
benzodioxole, indole, azaindole, benzofuran, benzopyrazole, benzodioxane,
tetrahydroisoquinoline, -C2-alkynyl-phenyl, wherein each alkynyl is
unsubstituted or substituted
with 1, 2 or 3 substituents selected from: halogen, CF3, -OH, -N112, -
C1..6alkyl, -0C1_6alky1,
NHC i_6a1ky1, and -N(C1-6alky1)2, and wherein each cycloalkyl, cycloalkenyl,
cycloheteroalkyl,
phenyl and heteroaryl is unsubstituted or substituted with 1, 2, 3 or 4
substituents independently
selected from Ra, and R2 is Cl.
In another embodiment of the present invention, each R1 and R2 is
independently
selected from: halogen, -C4.40cycloa1keny1, -phenyl, -phenyl-C3_7cycloalkyl, -
phenyl-C2-
7cycloheteroalkyl, -phenyl-aryl, -phenyl-heteroaryl, -heteroaryl, and -C2-
6a1kynyl-pheny1,
wherein each alkynyl is unsubstituted or substituted with 1, 2 or 3
substituents selected from:
halogen, CF3, -OH, -NH2, -Ci-6alkyl, -0C1-6alkyl, -NHC1-6alkyl, and -
N(Ci..6alkyl)2, and
wherein each cycloalkyl, cycloalkenyl, cycloheteroalkyl, phenyl, aryl and
heteroaryl is
unsubstituted or substituted with 1, 2, 3 or 4 substituents independently
selected from Ra,
provided that at least one of and only one of R1 and R2 is selected from the
group consisting of
halogen. In another class of the embodiment, each R1 and R2 is independently
selected from:
halogen, -pheny1-C2_7cyc1oheteroa1ky1, and -phenyl-aryl, wherein each
cycloheteroalkyl, aryl
and phenyl is unsubstituted or substituted with 1, 2, 3 or 4 substituents
independently selected
from Ra, provided that at least one of and only one of R1 and R2 is selected
from the group
consisting of halogen; or a pharmaceutically acceptable salt thereof. In
another class of the
embodiment, each R1 and R2 is independently selected from: halogen, -phenyl-
pyrrolidine, and -
phenyl-phenyl, wherein each pyrrolidine and phenyl is unsubstituted or
substituted with 1, 2, 3 or
4 substituents independently selected from Ra, provided that at least one of
and only one of R1
and R2 is selected from the group consisting of halogen; or a pharmaceutically
acceptable salt
thereof. In another class of the embodiment, each R1 is independently selected
from: -phenyl-
C2_7cyc1oheteroa1ky1, and -phenyl-aryl, wherein each cycloheteroalkyl, aryl
and phenyl is
unsubstituted or substituted with 1, 2, 3 or 4 substituents independently
selected from Ra, and R2
- 24 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
is selected from the group consisting of halogen; or a pharmaceutically
acceptable salt thereof.
In another class of the embodiment, each R1 is independently selected from: -
phenyl-C2..
7cycloheteroalkyl, and -phenyl-phenyl, wherein each cycloheteroalkyl and
phenyl is
unsubstituted or substituted with 1, 2, 3 or 4 substituents independently
selected from Ra, and R2
is selected from the group consisting of halogen; or a pharmaceutically
acceptable salt thereof.
In another class of the embodiment, each R1 is independently selected from: -
phenyl-
pyrrolidine, and -phenyl-phenyl, wherein each pyrrolidine and phenyl is
unsubstituted or
substituted with 1, 2, 3 or 4 substituents independently selected from Ra, and
R2 is selected from
the group consisting of halogen; or a pharmaceutically acceptable salt
thereof.
In another class of this embodiment of the present invention, R2 is
independently selected
from: -(C112)pC4-10cycloalkenyl, -(CH2)parY1, 4C112)parY1-C3-7cycloa1ky1, -
(CH2)parYl-C2-
7cycloheteroallcyl, -(CH2)paryl-aryl, -(CH2)paty1-heteroary1, -
(CH2)pheteroaryl, -C2-6alkynyl-
aryl, wherein each CH2 is unsubstituted or substituted with 1 or 2
substituents selected from:
halogen, CF3, -OH, -NH2, ---C1-6al1cY1, -0C1-6alkyl, -NHCi-6a1ky1, and -N(C1-
6alkyl)2,
wherein each alkynyl is unsubstituted or substituted with 1, 2 or 3
substituents selected from:
halogen, CF3, -OH, -NH2, -C1-6a1kyl, -OCI -6alkyl, -NHCI -6alkyl, and -N(C1-
6a1lcy1)2, and
wherein each cycloalkyl, cycloalkenyl, cycloheteroalkyl, aryl and heteroaryl
is unsubstituted or
substituted with I, 2, 3 or 4 substituents independently selected from Ra, and
R1 is selected from
the group consisting of halogen. In another class of this embodiment of the
present invention, R2
is independently selected from: -C4_10cycloalkenyl, -aryl, -aryl-
C3_7cycloalkyl, -aryl-C2-
7cycloheteroalkyl, -aryl-aryl, -aryl-heteroaryl, -heteroaryl, -C2-6alkynyl-
aryl, wherein each
alkynyl is unsubstituted or substituted with 1, 2 or 3 substituents selected
from: halogen, CF3, -
OH, -NH2, -C -6alkyl, -0Ci _6alkyl, -NHC -6alkyl, and -N(C1-6alky1)2, and
wherein each
cycloalkyl, cycloalkenyl, cycloheteroalkyl, aryl and heteroaryl is
unsubstituted or substituted with
1, 2, 3 or 4 substituents independently selected from Ra, and R1 is selected
from the group
consisting of halogen. In another class of this embodiment of the present
invention, R2 is
independently selected from: -C4-1 ocycloalkenyl, -phenyl, -phenyl-
C3_7cycloalkyl, -phenyl-C2_
7cycloheteroalkyl, -phenyl-phenyl, -phenyl-heteroaryl, -heteroaryl, -C2-
6alkynyl-phenyl, wherein
each alkynyl is unsubstituted or substituted with 1, 2 or 3 substituents
selected from: halogen,
CF3, -OH, -N112, -Ci-6a1kyl, -OCI-6alkyl, -NHCi-6a11cy1, and -N(Ci -6alky1)2,
and wherein
each cycloalkyl, cycloalkenyl, cycloheteroalkyl, phenyl and heteroaryl is
unsubstituted or
substituted with I, 2, 3 or 4 substituents independently selected from Ra, and
R1 is selected from
the group consisting of halogen. In another class of this embodiment of the
present invention, R2
is independently selected from: cyclohexenyl, -phenyl, phenyl-cyclopropyl,
phenyl-piperazine,
phenyl-pyrrolidine, -phenyl-phenyl, phenyl-triazole, phenyl-thiazole, phenyl-
pyrazole, phenyl-
oxadiazole, phenyl-furan, -pyridine, benzodioxole, indole, azaindole,
benzofuran, benzoprazole,
benzodioxane, tetrahydroisoquinoline, -C2-alkynyl-phenyl, wherein each alkynyl
is unsubstituted
- 25 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
or substituted with 1, 2 or 3 substituents selected from: halogen, CF3, -OH, -
NH2, -Ci..6alkyl, -
OCi 6alkyl, --NHC I -6a1ky1, and --N(C1_6a1ky1)2, and wherein each cycloalkyl,
cyeloalkenyl,
cycloheteroalkyl, phenyl and heteroaryl is unsubstituted or substituted with
1, 2, 3 or 4
substituents independently selected from Ra, and R1 is selected from the group
consisting of Cl
and F. In another class of this embodiment of the present invention, R2 is
independently selected
from: cyelohexenyl, -phenyl, phenyl-cyclopropyl, phenyl-piperazine, phenyl-
pyrrolidine, -phenyl-
phenyl, phenyl-triazole, phenyl-thiazole, phenyl-pyrazole, phenyl-oxadiazole,
phenyl-furan, -
pyridine, benzodioxole, indole, azaindole, benzofuran, benzopyrazole,
benzodioxane,
tetrahydroisoquinoline, -C2-alkynyl-phenyl, wherein each alkynyl is
unsubstituted or substituted
with 1, 2 or 3 substituents selected from: halogen, CF3, -OH, -NH2, -C -
6alkyl, -0C1-6a1ky1, -
NHCi-6alkyl, and -N(C1-6alky1)2, and wherein each cycloalkyl, cycloalkenyl,
cycloheteroalkyl,
phenyl and heteroaryl is unsubstituted or substituted with 1, 2, 3 or 4
substituents independently
selected from Ra, and R1 is Cl.
In another embodiment of the present invention, each R1 is independently
selected from:
-(CH2)paryl-C .8 alkyl, -(C112)paryl-C2-8alkenyl, -(CH2)paryl-C2.8alkynyl-Ci-
8alkyl, -
(CH2)pary1-C2-8allcynyl-C3-7cyc1oalky1, -(CH2)paryl-C2.galkynyl-
C3_7cycloalkenyl, -
(CH2)paryl-C2-8alkynyl-C2-10cycloheteroalkyl, -(CH2)paryl-C2-8alkynyl-C2..
10cycloheteroalkeny1, -(CH2)pary1-C2-8a1kynyl-ary1, -(CH2)paryl-C2-8alkynyl-
heteroaryl, -
(CH2)paryl-C2-1 ocycloheteroalkyl, -(CH2)parYl-C2-10cycloheteroalkenyl, -
(CH2)paryl-aryl, and
-(CH2)paryl-heteroaryl, wherein each CH2 is unsubstituted or substituted with
1 or 2 substituents
selected from: halogen, CF3, -OH, -NH2, -C -6alkyl, -0C1-6alkyl, -NHC1-6alkyl,
and --N(C1-6alkY1)2, wherein each alkyl, alkenyl, alkynyl, cycloalkyl,
cycloalkenyl, cycloheteroalkyl,
eycloheteroalkenyl, paryl and heteroaryl is unsubstituted or substituted with
1, 2, 3 or 4
substituents independently selected from Ra.
In another embodiment of the present invention, each R1 is independently
selected from:
-(CH2)paryl-C2-galkynyl-C1_8alkyl, -(CH2)paryl-C2-8alkynYl-C3-7eycloalkyl, -
(CH2)parYI-C2-
8a1kYnY1-C2-10cyc1oheteroa1ky1, -(CH2)parY1-C2-10cycloheteroalkenyl, -
(CH2)paryl-aryl, and -
(CH2)pary1-heteroatyl, wherein each CH2 is unsubstituted or substituted with 1
or 2 substituents
selected from: halogen, CF3, -OH, -NH2, -C1-6a1ky1, -0C1-6alkyl, -NITC1-
6alky1, and -N(C1-
6a1ky1)2, wherein each alkyl, alkynyl, cycloalkyl, cycloheteroalkyl,
cycloheteroalkenyl, aryl and
heteroaryl is unsubstituted or substituted with 1, 2, 3 or 4 substituents
independently selected
from Ra.
In another embodiment of the present invention, each R1 is independently
selected from:
-phenyl-C2-8alkYnYl-C1-8a1kyl, -pheny1-C2-3alkyny1- C3-7cycloalkyl5 -phenyl-C2-
3a1kynYI-C2-
iocycloheteroalkyl, -pheny1-C2-locycloheteroalkenyl, biphenyl, and -phenyl-
heteroaryl, wherein
each alkyl, alkynyl, cycloalkyl, cycloheteroalkyl, cycloheteroalkenyl, phenyl,
biphenyl and
heteroaxyl is unsubstituted or substituted with 1, 2, 3 or 4 substituents
independently selected
- 26
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
from Ra,
In another embodiment of the present invention, each R1 is independently
selected from:
-phenyl-C2alkyny1C1-5alkyl, -pheny1-C2-3a1kynyl- C3-7cyc1oa1ky1, -pheny1-C2-
3a1kYnYi-C2-
10cycloheteroalkyl, -phenyl-C2-10cyc1oheteroalkenyl, biphenyl, and -phenyl-
heteroaryl, wherein
each alkyl, alkynyl, cycloalkyl, cycloheteroalkyl, phenyl, biphenyl,
cycloheteroalkenyt and
heteroaryl is =substituted or substituted with I, 2, 3 or 4 substituents
independently selected
from Ra. In another embodiment of the present invention, each Ri is
independently selected
from: phenyl-C2alkynylC1-5alkyl, phenyl-C2-3alkynyl- C3-7cycloalkyl, phenyl-C2-
3alkynyl-
C2-locyeloheteroalkyl, phenyl-dihydropyrrolo[3,4-e]pyrazole, biphenyl, phenyl-
pyridine,
wherein each alkyl, alkynyl, cycloalkyl, cycloheteroalkyl, phenyl, biphenyl
dihydropyrrolop,4-
c3pyrazole and pyridine is unsubstituted or substituted with 1, 2, 3 or 4
substituents
independently selected from Ra. In another embodiment of the present
invention, each R1 is
independently selected from: phenyl-C2alkynyl-CH(OH)CH3, phenyl-C2alkynyl-
CH2CH2OH,
phenyl-C2alkynyl-C(CH3)20H, phenyl-C2alkynyl-CH2OH, phenyl-C2alkynyl-
CH2CH2CH2OH, phenyl-C2alkynyl-(CH2)4CH3, pheny1-C2alkyny1-CH2CH2-NH-
pyrimidine,
and phenyl-C2alkynyl-CH2OCH2CH2OCH3, phenyl-C2alkynyl-cyclopentyl, pheny1-
C2a11cyny1-
cyclopentyl-OH, phenyl-C3alkynyl-cyclopentyl, phenyl-C2alkynyl-cyclohexyl,
phenyl-
C3alkynyl-morpholine, phenyl-C2alkynyl-piperidine, phenyl-C3alkynyl-
pyrrolidine-OH, phenyl-
C3alkynyl-piperazine-CH3, pheny1-4,6-dihydropyrrolo[3,4-c]pyrazole, phenyl-4,6-
dihydropyrrolo[3,4-c}pyrazole-CH2C(CH3)20H, pheny1-4,6-dihydropyrrolop,4-
c3pyrazole-
CH2C(CH3)2F, phenyl-4,6-dihydropyrrolo[3,4-c]pyrazole-CH2cyclopropyl, pheny1-
4,6-
dihydropyrrolo[3,4-c]pyrazole-CH2CF3, and pheny1-4,6-dihydropyrrolo[3,4-
c3mazo1e-S02NH_
cyclopropyl, biphenyl, biphenyl-pyrazole, biphenyl-pyrazole-CH3, biphenyl-
pyrazole-
cyclopropyl, biphenyl-pyrazole-CH2C(CH3)20H, biphenyl-imidazole, biphenyl-
imidazole-CH3,
biphenyl-oxazole, biphenyl-oxadiazole, biphenyl-oxadiazole-CH3, biphenyl-
oxadiazole-
cyclopropyl, biphenyl-oxadiazole-CF3, biphenyl-oxadiazole-OH, biphenyl-
thiazole, biphenyl-
triazole, biphenyl-tetrazole, biphenyl-dihydroimidazole, biphenyl-
tetrahydropyrimidine, phenyl-
pyridine, phenyl-pyridine-triazole, phenyl-pyridine-tetrazole, phenyl-pyridine-
pyrazole, and
phenyl-pyridine-pyrazole-CH2C(CH3)20H.
In another embodiment of the present invention, each g1 is independently
selected from:
-phenyl-C2-10cycloheteroalkenyl, biphenyl, -phenyl-heteroaryl, wherein each
cycloheteroalkenyl, phenyl, biphenyl and heteroaryl is =substituted or
substituted with 1, 2, 3 or
4 substituents independently selected from Ra. In a class of this embodiment,
each R1 is
independently selected from: -phenyl-dihydropyrrolo[3,4-C]pyrazole, biphenyl, -
phenyl-pyridine,
wherein each phenyl, dihydropyrrolo[3,4-c]pyrazole, biphenyl, and pyridine, is
=substituted or
substituted with 1, 2, 3 or 4 substituents independently selected from Ra. In
a subclass of this
class, each Ri is independently selected from: phenyl-4,6-dihydropyrrolo[3,4-
c]pyrazole,
- 27 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
pheny1-4,6-dihydropyrrolo[3,4-c]pyrazole-CH2C(C113)20H, pheny1-4,6-
dihydropyrrolo[3,4-
c]pyrazo1e-CH2C(CH3)2F, phenyl-4,6-dihydropyrrolo[3,4-c]pyrazole-
CH2cyclopropyl, phenyl-
4,6-dihydropyrrolo[3,4-c]pyrazole-CH2CF3, and pheny1-4,6-dihydropyrrolo[3,4-
cjpyrazole-
SO2NH_cyc1opropy1, biphenyl, biphenyl-pyrazole, biphenyl-pyrazole-CH3,
biphenyl-pyrazole-
cyclopropyl, biphenyl-pyrazole-CH2C(CH3)20H, biphenyl-imidazole, biphenyl-
imidazole-CH3,
biphenyl-oxazole, biphenyl-oxadiazole, biphenyl-oxadiazole-CH3, biphenyl-
oxadiazole-
cyclopropyl, biphenyl-oxadiazole-CF3, biphenyl-oxadiazole-OH, biphenyl-
thiazole, biphenyl-
triazole, biphenyl-tetrazole, biphenyl-dihydroimidazole, biphenyl-
tetrahydropyrimidine, phenyl-
pyridine, phenyl-pyridine-triazole, phenyl-pyridine-tetrazole, phenyl-pyridine-
pyrazole, and
phenyl-pyridine-pyrazole-CH2C(C113)20H.
In another embodiment of the present invention, each R1 and R2 is
independently
selected from: halogen, -C4-iocycloalkenyl, -phenyl, phenyl-C2-galkYnY1-C1-
8a1ky1, pheny1-C2-
3alkynyl- C3_7cyc1oa11cy1, pheny1-C2_3a11cyny1-C2_10cycloheteroalkyl, -phenyl-
C3_7cycloalkyl, -
pheny1-C2-7cyc1oheteroa11cy1, phenyl-C2-1ocycloheteroalkenyl, -phenyl-aryl, -
phenyl-heteroaryl,
-heteroaryl, and -C2_6a11cynyl-pheny1, and wherein each alkyl, alkynyl,
cycloalkyl, cycloalkenyl,
cycloheteroalkyl, cycloheteroalkenyl, phenyl, aryl and heteroaryl is
unsubstituted or substituted
with 1, 2, 3 or 4 substituents independently selected from Ra, provided that
at least one of and
only one of R1 and R2 is selected from halogen; or a pharmaceutically
acceptable salt thereof.
In another embodiment of the present invention, R1 is independently selected
from: -C4-
10cycloalkenyl, -phenyl, phenyl-C2alkynylC1 -5a1ky1, pheny1-C2-3alkyny1- C3-
7cyc1oa1ky1,
phenyl-C2-3alkylV1-C2-10cycloheteroalkyl, -phenyl-C3-7cycloalkyl, -phenyl-C2-
7cycloheteroalkyl, phenyl-C2-10cycloheteroalkenyl, -phenyl-phenyl, -phenyl-
heteroaryl, -
heteroaryl, and -C2_6a1kynyl-pheny1, wherein each alkyl, alkynyl, cycloalkyl,
cycloalkenyl,
cycloheteroalkyl, cycloheteroalkenyl, phenyl and heteroaryl is unsubstituted
or substituted with
1, 2, 3 or 4 substituents independently selected from R. In another embodiment
of the present
invention, R1 is independently selected from: -phenyl-C2_7cyc1oheteroa1ky1, -
phenyl-C2-
10cycloheteroalkenyl, -phenyl-phenyl, and -phenyl-heteroaryl, wherein each
cycloheteroalkyl,
cycloheteroalkenyl, heteroaryl and phenyl is unsubstituted or substituted with
1, 2, 3 or 4"
substituents independently selected from Ra,
hi another embodiment of the present invention, R3 and R4 are each
independently
selected from: hydrogen, halogen, -Ci_6alkyl, -C2-6a1keny1, -C2-6alkynyl, -
C3_10cycloalkyl, -
C3-10cyc1oa1keny1, aryl, heteroaryl, -CN, -CF3, -OH, -0C1-6a11cyl, -NH2, -NHC1-
6aikyl, -
N(C 1.6alky02, -SC 1.6alkyl, -SOCi..6alkyl, -S 02C l -6alky1, -NH SO2C1-
6alkyl, -NHC(0)C1-
6alkyl, -S 02NHCi-6alkyl, and -C(0)NHC1 _6alkyl.
In another embodiment of the present invention, each R3 is absent or
independently
selected from: hydrogen, halogen, -C1.6 alkyl, -C2-6 alkenyl, -C2-6 alkYnYI, -
C3-1ocyc1oa11cy1, -
C3-iocycloalkenyl, aryl, heteroaryl, -CN, -CF3, -OH, -0C1-6alkyl, -NH2, -
NHC1_6a1ky1, -
- 28 -
CA 02826649 2013 08 05
WO 2012/116145
PCT/US2012/026261
N(Ci_6alicy1)2, -SC1-6alkyl,
_6a11yl, - S 02Ci -6a1ky1, -NHSO2C1-6alkyl, -NHC(0)C1-6alkyl, -SO2NHe1-6alkyl,
and -C(0)NHC1-6alkyl. In a class of this embodiment, each R3 is
independently selected from: hydrogen, halogen, -C1-6 alkyl, -C2-6 alkenyl, -
C2-6 alkynyl, -C3_
10eyc1oa1ky1, -C3-10eye1oa1keny1, aryl, heteroaryl, -CN, -CF3, -OH, -0ei
_6alkyl, -NH2, -
NHCi-6alkyl, -N(C1-6alky1)2, -SC1_6a1ky1, -SOCi_6a1ky1, -S02C1_6a1ky1, -
NHSO2Ci _6allcyl,
-NHC(0)Ci_6a1ky1, -SO2NHC1_6a1kyl, and -C(0)NHC1-6alkyl. In another class of
this
embodiment, each R3 is independently selected from: hydrogen, halogen, -C1-6
alkyl, -C2-6
alkenyl, -C2-6 alkynyl, -CN, -CF3, -OH, -0C1-6a1ky1, -NH2, -NHC1-6alkY1, -N(C1-
6alkY1)2, -
SC1-6a1kyl, SOCi-6alkyl, -S02C1-6alkyl, -NHSO2C1-6alkyl, -NHC(0)C1-6alkyl,
SO2NHCi..6alkyl3 and -C(0)NHC1-6alkyl. In another class of this embodiment,
each R3 is
independently selected from: hydrogen, halogen, -C1-6 alkyl, -CN, -CF3, -OH, -
0C1.6alky1, -
NH2, -NHC1-6a1ky1, and -N(C1-6alky1)2. In another class of this embodiment,
each R3 is
independently selected from: hydrogen, halogen, -C1_6 alkyl, -CN, -CF3, -OH,
and -0C1_6a1ky1.
In another class of-this embodiment, each R3 is independently selected from:
hydrogen, and-el_
6 alkyl. In another class of this embodiment, each R3 is hydrogen. In another
class of this
embodiment, each R3 is -C1-6 alkyl.
In another embodiment of the present invention, R3 is hydrogen or absent. In a
class of
this embodiment, R3 is hydrogen. In another class of this embodiment of the
present invention,
R3 is absent.
In another embodiment of the present invention, each R4 is absent or
independently
selected from: hydrogen, halogen, -C1-6 alkyl, -C2-6 alkenyl, -C2-6 alkynyl, -
C3-ioeye1oa1ky1, -
C3_10eyeloalkenyl, aryl, heteroaryl, -CN, -CF3, -OH, -0C1_6a1ky1, -NH2, -
NHei_6alkyl, -
N(C1_6alky1)2, -SCi-6alkyl, -S0e1-6alkyl, -S02Ci -6alky1, -NHSO2C1-6alkyl, -
NHC(0)C1-
6alkyl, -SO2NHC1-6alkyl, and -C(0)NHC1_6alkyl. In a class of this embodiment,
each R4 is
independently selected from: hydrogen, halogen, -C1-6 alkyl, -C2-6 alkenyl, -
C2_6 alkynyl, -C3..
10cYcloalkYl, -C3- ocycloalkenyl, aryl, heteroaryl, -CN, -CF3, -OH, -0e
..6alkyl, -NH2, -
NHC1_6alkyl, -N(C1-6alky1)2, -Sei-6alky1, -SOC1-6alkyl, -S02C1 -6alkyl, -
NHSO2C1-6a1ky1,
-NHC(0)C1-6a1ky1, -SO2NHC1-6a1ky18 and -C(0)NHCI-6a1ky1. In another class of
this
embodiment, each R4 is independently selected from: hydrogen, halogen, -C1-6
alkyl, -C2-6
alkenyl, -C2-6 alkynyl, -CN, -CF3, -OH, -0Ci_6alky1, -NH2, -NHe -6alkyl, -
N(C1.6alky1)2, -
SC _6allcyl, -SOC1_6a1ky1, -S02C1_6a1ky1, -NHSO2C1-6alky1, -NHC(0)C1..6alkyl, -
SO2NHe1-6alkyl, and -C(0)NHC1_6a1ky1. In another class of this embodiment,
each R4 is
independently selected from: hydrogen, halogen, -C1-6 alkyl, -CN, -CF3, -OH, -
0Ci_6alkyl, -
NI-12, -NHC1-6alky1, and -N(C1_6a1ky1)2. In another class of this embodiment,
each R4 is
independently selected from: hydrogen, halogen, -Ci-6a1ky1, -CN, -CF3, -OH,
and -0C-1-6a1kyl.
In another class of this embodiment, each R4 is independently selected from:
hydrogen, and-C1-
6alkyl. In another class of this embodiment, each R4 is hydrogen. In another
class of this
- 29 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
embodiment, each R4 is -C1.6alkyl.
In another embodiment of the present invention, R4 is hydrogen or absent. In a
class of
this embodiment, =R4 is hydrogen. In another class of this embodiment of the
present invention,
R4 is absent.
In another embodiment of the present invention, R5 is selected from the group
consisting
of: hydrogen, -C1-6 alkyl, -CH2CO211, and -CH2CO2C 1_6alkyl. In a class of
this embodiment,
R5 is selected from the group consisting of: hydrogen, and -Ci_ alkyl. In
another class of this
embodiment, R5 is hydrogen.
In another embodiment of the present invention, Ra is independently selected
from the
group consisting of: -(CH2)m-ha1ogen, oxo, -(CH2)m0H, -(CF12)mN(02, -(C1-
12)mNO2, -
(CH2)mCN, -Ci -6alkyl, -(CH2)mCF3, -(CF12)m0CF3, -0-(CH2)m-OC1 -6 alkyl, -
(CH2)mC(0)N(Ri)2, --(CH2)mC(=N-0H)N(Ri)2, -(CH2)m0C1-6a1kyl, -(CH2)m0-(CH2)m-
C3-
7cycloalkyl, -(CI-12)m0-(CH2)m-C2-7cyc1oheteroa1kyl, -(CH2)m0-(CH2)m-aryl, -
(CH2)m0-
(CH2)m-heteroaryl, -(CH2)mSC1-6alkyl, -(CH2)mS(0)C1-6a1ky1, -
(CH2)mS02C1_6a1ky1,
(CH2)mS02C3-7cycloalkyl, -(CH2)mS02C2-7cycloheteroalkyl, -(CH2)mS02-ary1, -
(CH2)mS02-heteroaryl, -(CH2)mS02NHC1_6alky1õ -(CH2)mS02NHC3-7cyc1oalky1, -
(CH2)mS02NHC2-7cycloheteroalkyl, -(CH2)mS02NH-aryl, -(CH2)mS02NH-heteroaryl, -
(CH2)/uNHS02-C1-6alkYl, -(CH2)mNHSO2-C3.7cyc1oalky1, -(CH2)mNHS02-C2-
7cycloheteroalkyl, -(CH2)flaNHS02-aryl, -(CH2)mNHSO2NH-heteroary1, -
(CH2)mN(Ri)-C1-
6alkyl, -(CH2)mN(Ri)--C3-7eyc1oa1kyl, -(CH2)mN(Ri)-C2-7cycloheteroa1ky1, -
(CH2)mN(Ri)-
C2.7cyc1oheteroalkeny1, -(CH2)mN(Rj)-arY1, -(CH2)mN(Rj)-heteroaryl, -
(CH2)mC(0)Rf, -
(CH2)mC(0)N(Ri)2, -(CH2)mN(Rj)C(0)N(Ri)2, -(CH2)mCO2H, -(C112)m000H, -
(CH2)mCO2Rf, -(CH2)m000Rf, -(CH2)mC3-7cycloaIkyl, -(CH2)mC3-7cycloalkenyl, -
(CH2)mC2-6cycloheteroalkyl, -(CH2)mC2-6cyc1oheteroa1keny1, -(CH2)maryl, and -
CH2)mheteroaryl, wherein each CH2 is unsubstituted or substituted with 1 or 2
substituents
selected from: oxo, -(CH2)0-30H, -CN, -NH2, -NH(C1-6alkyl), -N(C1-6a1kY023 -C1-
6a1ky1, -
0C1-6alkyl, halogen, -CH2F, -CHF2, -CF3, -0O2H, -0O2C1-6alkyl, -C3-
7cyc1oalky1, phenyl,
CH2phenyl, heteroaryl and CH2heteroaryl, and wherein alkyl, cycloalkyl,
cycloalkenyl,
cycloheteroalkyl, cycloheteroalkenyl, aryl and heteroaryl are unsubstituted or
substituted with 1,
2, 3 or 4 substituents selected from: oxo, -(CI-12)0-50H, -CN, -NH2, -NH(C1-
6alkyl), -N(C1-
6alky1)2, -Ci-6a1kyl, -0C1-6alkyl, halogen, -CH2F, -CHF2, -CF3, -CO2H, -
CO2C1_6a11cy1, -
S02C1-6a1ky1, -C3-7cycloalkyl, phenyl, CH2phenyl, heteroaryl and
CH2heteroaryl.
In another embodiment of the present invention, each Ra is independently
selected from
the group consisting of: halogen, oxo, -(CH2)mOIL -(CH2)mN(Ri)2, -(CH2)/nNO2, -
(CH2)mCN, -Ci _6alkyl, -(CH2)mCF3, -(CH2)m0CF3, -OCH2OC1_6 alkyl, -
(CH2)mC(0)N(Ri )2, -(CH2)mC(=N-OH)N(Ri)2, -(CI12)m0C1-6alkyl, -(CH2)m0-(CH2)m-
C3..
7cycloalkyl, -(CH2)m0-(0-12)m-C2-7cycloheteroalkyl8 -(CH2)m0-(CH2)m-aryl, -
(CH2)m0-
- 30 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
(CH2)m-heteroaryl3 -(CH2)mSCi..6alky1, -(C112)mS(0)Ci_6alky1, -
(CH2)mS02C1_6alkyl, -
(CH2)m802C3-7cycloalkyl, -(CH2)mS02C2-7cycloheteroalkyl, -(CH2)m802-aryl, -
(CH2)002-heteroaryl, -(CH2)002NHC1-6alkyl, -(CH2)002NFIC3-7cycloalkyl, -
(CH2)m802NHC2-7cyc1oheteroa1ky1, -(CH2)mS02NH-aryl3 -(CH2)mS02NH-heteroaryl,
(CH2)mNHS02-C1_6alkyl, -(CH2)mNH802-C3-7cycloalkyl, -(CH2)mNHS02-C2-
7cycloheteroalkyl, -(CH2)mNH802-aryl, -(CH2)N1-1802NH-heteroary1, -
(CH2)mC(0)Rf, -
(CH2)mC(0)N(R.)2, -(CH2)mN(Rj)C(0)N(Rj)2, -(CH2)mCO2H, -(CH2)m000H, -
(CH2)mCO2Rf, -(CH2)m000Rf, -(CH2)mC3-7cyc1oa1ky1, -(CH2)mC3-7cycloalkenyl, -
(CH2)mC2-6cyc1oheteroalky1, -(CH2)mC2-6cyc1oheteroa1keny1, -(CH2)matyl, and -
(CH2)ffiheteroaryl, wherein each CH2 is unsubstituted or substituted with 1 or
2 substituents
selected from: oxo, -(CH2)0-30H, -CN, -NH2, -NH(C1-6alkY1), -N(C -6alky1)2, -
C1-6alkyl,
OCI ..6alkyl, halogen, -CH2F, -CHF2, -CF3, -CO2H, -CO2C1_6a1ky1, -
C3_7cyeloalkyl3 phenyl,
CH2phenyl, heteroaryl and CH2heteroaryl, and wherein alkyl, cycloalkyl,
cycloalkenyl,
cycloheteroalkyl, cycloheteroalkenyl, aryl and heteroaryl are unsubstituted or
substituted with 1,
2, 3 or 4 substituents selected from: oxo, -(CH2)0-30H, -CN, -NH2, -NH(C1-
6alkyl), -N(C1-
6alkY1)2, -C1-6alkyl, -0Ci -6alkyl, halogen, -CH2F, -CHF2, -CF3, -CO2H, -
CO2C1_6alkyl, -
SO2C1_6a1ky1, -C3-7cycloalky1, phenyl, CH2phenyl3 heteroaryl and
CH2heteroaryl.
In a class of this embodiment, each Ra is independently selected from the
group
consisting of: halogen, -(CH2)rn0H, -(CH2)mN(Ri)2, -(CH2)mCN, -C1-6a1kY1, -
(C142)mCF3,
(CH2)m0CF3, -(CH2)InC( )N(Rj)2, -(CH2)m0C1-6a1ky1, -(CH2)m0-(CH2)m-C3-
7cyc1oa1ky1,
-(CH2)m0-(CH2)m-C2_7cyc1oheteroa1ky1, -(CH2)mS02C1-6alky1, -(CH2)002C2-
7cycloheteroalkyl, -(CH2)m802NHC1-6alkyl, -(CH2)mS02NHC3_7eye1oalky1, -
(CH2)mNH802-C1-6alkyl, -(CH2)mC(0)Rf, -(CH2)mCO2H, -(CH2)mCO2Rf,-(CH2)mC3_
7cycloalkyl, wherein each CH2 is unsubstituted or substituted with 1 or 2
substituents selected
from: oxo, -(CH2)0-30H, -CN, -NH2, -NH(Ci -6alky1), -N(C1-6a1ky1)2, -Ci -
6alkyl, halogen, -CH2F, -CHF2, -CF3, -CO2H, -CO2C1-6alkyl, -C3-7cycloa1ky1,
phenyl,
CH2phenyl3 heteroaryl and CH2heteroaryl, and wherein alkyl, cycloalkytand
cycloheteroalkyl
are unsubstituted or substituted with 1, 2, 3 or 4 substituents selected from:
oxo, -(CH2)0-30H, -
CN, -NH2, -NH(C1-6alkY1), -N(C1-6a1ky1)2, -Ci_6alkyl, -0C1-6alkyl, halogen, -
CH2F, -CHF2,
-CF3, -CO2H, -CO2C1-6alkY1, -SO2C1-6alk)71, -C3-7cyeloalkyl, phenyl,
CH2phenyl, heteroaryl
and CH2heteroaryl.
In another class of this embodiment, each Ra is independently selected from
the group
consisting of: halogen, -(CH2)m0H, -N(Ri)2, -CN, -C1-6a1ky1, -(CH2)mCF3, -
0CF3, -
(CH2)mC(0)N(1102, -(CH2)m0C1-6a1kYl, 4CH2)m0-(CH2)ru-C3-7cycloa1ky1, -(CH2)m0-
(CH2)m-C2-7cycloheteroalkyl, -802C1-6alky1, -802C2-7cycloheteroalky1, -
SO2NHC1.6a1ky1, -
802NHC3-7eycloalkyl, -N11802-C1-6a1ky1, -C(0)Rf, -CO2H, -CO2Rf, -C3-
7cyeloalkyl,
wherein each CH2 is unsubstituted or substituted with 1 or 2 substituents
selected from: oxo, -
- 31 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
(CH2)0_30H, -CN, -NH2, -NH(Ci..6alkyl), -N(C1.6a1ky1)2, -C1.6a1kyl, -OCI-
6a1ky1, halogen, -
CH2F, -CHF2, -CF3, -CO2H, -CO2C -
C3_7cycloalkyl, phenyl, CH2phenyl, heteroary.1
and CH2heteroaryl, and wherein alkyl, cycloalkyl and cycloheteroalkyl are
unsubstituted or
substituted with 1, 2, 3 or 4 substituents selected from: oxo, -(CH2)0-30H, -
CN, -NH2, -NH(C1-
6alkY1), -N(C1-6a1ky1)2, -C1-6al1YL -OCI-6a1ky1, halogen, -CH2F3 -CHF2, -CF3, -
CO2H, -
CO2C1-6a1ky1, -S02C1-6alkyl, -C3_7cycloalkyl, phenyl, CH2phenyl, heteroaryl
and
CH2heteroaryl.
In another class of this embodiment, each Ra is independently selected from
the group
consisting of: halogen, -(CH2)m0H3 -N(Ri)2, -CN, -C1-6alkyl, -(CH2)mCF3, -
0CF3,
1 0 (CH2)mC(0)N(Ri)2, -(012)m0C 1 -6alkyl, -(CH2)m0-(CH2)m-C3 -7cycloalkyl,
-(CH2)m0-
(CH2)m-C2-7cycloheteroalkyl, -S02C1-6a1ky1, -S02C2-7cyc1oheteroalkyl, -SO2NHCI-
6alkyl, -
SO2NHC3_7cycloalkyl, -NHS02-C1-6alky1, -C(0)Rf, -0O2H, -CO2Rf, -
C3_7cycloalkyl,
wherein each CH2 is unsubstituted or substituted with 1 or 2 substituents
selected from: -OH, -
C1_6a1ky1, -0C1-6alky1 and halogen, and wherein alkyl, cycloalkyl and
cycloheteroalkyl are
unsubstituted or substituted with 1, 2, 3 or 4 substituents selected from: -
C1_6alkyl, halogen, -
SO2C -6alkyl,and -C 3 _7cycloalkyl.
In another class of this embodiment, each Ra is independently selected from
the group
consisting of: F, CI, Br, -C(CH3)20H, -OH, -CH2OH, -CH(OH)CHF2, CH(OH)CF3, -
(CH2)2C(CH3)2-0H, -N(CH3)23 -CN, -CH3, -CH2CH3, -C(CH3)3 -CF3, -CH2CF3 -0CF3 -
C(0)NH-cyclopropyl -OCH2CH3, -0CH3, -0(CH2)3-S02CH3, OCH2CH2F3 -CH2OCH3 -0-
cyclobutyl, -0-cyclopentyl, -0-azetidine, -0-CH2-dioxolane, -S02CH(CH3)2, -
S02CH3 -S02-
PYrrolidine, -S02-azetidine -SO2NHCH3, -SO2NHC(CH3)3, -SO2NH-cyclopropyl, -
NHS02-
CH3, -C(0)CH(CH3)2, Q0)-pyrrolidine, -C(0)-morpholine, -0O2H, -0O2-CH(CH3)2, -
0O2-
C(CH3)3, and cyclopropyl, wherein each CH2 is unsubstituted or substituted
with 1 or 2
substituents selected from: -OH, -Ci_6alkyl, -0C1-6alkyl and halogen, and
wherein alkyl,
cycloalkyl and cycloheteroalkyl are unsubstituted or substituted with 1, 2, 3
or 4 substituents
selected from: -C1-6alkyl, halogen, -S02C1-6alkyl,and -C3-7cycloalkyl.
In another class of this embodiment, each Ra is independently selected from
the group
consisting of: F, CI, Br, -C(CH3)20H, -OH, -CH2OH, -CH(OH)CHF2, CH(OH)CF3, -
(CH2)2C(CH3)2-0H, -N(CH3)23 -CN, -CH38 -CH2CH3, -C(CH3)3 -CF3, -CH2CF3 -0CF3 -
C(0)NH-cyclopropyl -OCH2CH3, -OCH3, -0(CH2)3-S02CH3, OCH2CH2F, -CH2OCH3 -0-
cyclobutyl, -0-eyclopentyl, -0-azetidine, -0-CH2-dioxolane, -S02CH(CH3)2, -
502CH3 -S02-
PYrrolidine, -S02-azetidine -SO2NHCH33 -SO2NHC(CH3)3, -SO2NH-cyclopropyl, -
NHS02-
CH3, -C(0)CH(CH3)2, C(0)-pyrrolidine, -C(0)-morpholine, -CO2H, -0O2-CH(C113)23
-0O2-
C(CH3)3, and cyclopropyl.
In another embodiment of the present invention, Ra is independently selected
from the
group consisting of: halogen, oxo, -(CH2)m0H, -(CH2)rnN(Rj)2, -(CH2)mNO2, -
(CH2)mCN, -
-32-
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
C _6alkyl, -(CH2)mCF3, -(CH2)m0CF3, -OCH20C1-6 alkyl, -OCH2-aryl, -(CH2)mC(=N-
OH)N(Ri )2, -(CH2)m0C1.6alky1, -(CH2)m0-aryl, -(CH2)mSC1-6alkYl, -(CH2)0(0)C1-
6alicYl, -(CH2)002C1-6a1kY1, -(CH2)002C3-7cycloalkyl8 -(CH2)002C2-
7cycloheteroalkyl, -(CH2)002-aryl, -(CH2)002-heteroaryl, -
(CH2)002N11C1.6a1ky1,
(CH2)mS02NHC3_7cycloalkyl, -(CH2)002NHC2-7cycloheteroalkyl, -(CH2)mS02NH-aryl,
-
(C112)mS02NH-heteroaryl, -(C112)mNFIS02-C1.6alkyl, -(CH2)mNHS02-C3-
7cycloalkyl, -
(CH2)mNHS02-C2_7cyc1oheteroalky1, -(CH2)mNHS02-aryl, -(CH2)mNHSO2NH-
heteroaryl, -
(CH2)mC(0)Rf, -(CH2)mC(0)N(Rj)2, -(CH2)mN(Ri)C(0)N(Ri)2, -(CH2)mCO2H, -
(C1-12)m000H, -(CH2)mCO2Rf, -(CH2)mOCORf, -(CH2)mC3-7cyc1oa1ky1, -(CH2)mC3_
7cycloalkenyl, -(CH2)mC2-6cyc1oheteroa1ky1, -(CH2)mC2-6cyc1oheteroa1keny1, -
(CH2)maryl,
and -(CH2)mheteroaryl, wherein each CH2 is unsubstituted or substituted with 1
or 2
substituents selected from: oxo, -(CH2)0-30H, -CN, -NH2, -NH(C1-6alkyl), -N(C1-
6a141)2, -
C1-6a1ky1, -0Ci -6alkyl, halogen, -CH2F, -CHF2, -CF3, -CO2H, -CO2C1-6alkyl, -
C3-
7cycloalkyl, phenyl, CH2phenyl, heteroaryl and CH2heteroaryl, and wherein
alkyl, cycloalkyl,
cycloalkenyl, cycloheteroalkyl, cycloheteroalkenyl, aryl and heteroaryl are
unsubstituted or
substituted with 1, 2, 3 or 4 substituents selected from: oxo, -(CH2)0-301-1, -
CN, -NH2, -
NH(C1-6a1ky1), -N(C1-6a1lcy1)2, -C1-6a1lcy1, -0C -6alkyl, halogen, -CH2F, -
CHF2, -CF3, -
CO2H, -CO2C1-6a1ky1, -C3-7cycloalkyl, phenyl, CH2phenyl, heteroaryl and
CH2heteroaryl.
In a class of this embodiment, each Ra is independently selected from the
group
consisting of: halogen, -(CH2)m0H, -(CH2)mN(Ri)2, -(CH2)mCN, -C1-6a11(34, -
(CH2)mCF3, -
(CH2)m0C1-6a1ky1, -(CH2)002C1_6alkyl, -(CH2)mSO2C2-7cyc1oheteroa1ky1, -
(CH2)002NHC1-6a1icY1, -(CH2)002NHC3-7cycloalkyl, -(CH2)mNHS02-C1-6a1ky1, -
(CH2)mC(0)Rf, and -(CH2)mCO2H, wherein each CH2 is unsubstituted or
substituted with 1 or
2 substituents selected from: oxo, -(CH2)0-3014, -CN, -NH2, -NIAC1-6alkyl), -
N(C1-6alicY1)2,
C1-6alkyl, -0C1-6alky1, halogen, -CH2F, -CHF2, -CF3, -CO2H, -CO2C1.6a1ky1, -
C3_
7cycloalkyl, phenyl, CH2phenyl, heteroaryl and CH2heteroaryl, and wherein
alkyl, cycloalkyl,
and cycloheteroalkyl are unsubstituted or substituted with 1, 2, 3 or 4
substituents selected from:
oxo, -(CH2)0-30H8 -CN, -NH2, -NH(C1_6alkyl), -N(C1-6alky1)2, -C1_6alkyl, -
0Ci_6alkyl,
halogen, -CH2F, -CHF2, -CF3, -CO2H, -CO2C _6alkyl.
In a subclass of this class, each Ra is independently selected from the group
consisting of:
halogen, -(CH2)m0H, -N(Rj)2, -CN, -C1-6alky1, -CF3, -0C1 ..6alkyl, -S02C1-
6a1kY1, -S02C2-
7cycloheteroalkyl, -SO2NHC1.6alky1, -SO2NHC3-7eyc1oa1ky1, -NHS02-C1-6a1ky1, -
C(0)Rf,
and -CO2H, wherein each alkyl, cycloalkyl, and cycloheteroalkyl is
unsubstituted or substituted
with 1, 2, 3 or 4 substituents selected from: oxo, -(CH2)0-30H, -CN, -NH2, -
NH(C1-6a1kY1), -
N(Ci_6alky1)2, -C1-6alicYl, -0C -6alkyl, halogen, -CH2F, -CHF2, -CF3, -CO2H, -
CO2C1-
6alky1. In another subclass of this class, each Ra is independently selected
from the group
consisting of: F, Cl, -CH2OH, -OH, -N(CH3)2, -CN, -CH3, -CF3, -OCH3, -OCH2CH3,
33 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
C(OH)(CH3)2, -CH(OH)CHF2, -CH(OCH3)CH3, -SO2CH3, -S02CH(CH3)2, -S02-azetidine,
-
S02-pyrrolidine, -SO2NH-tert-butyl, -SO2NH-cyclopropyl, -NHSO2CH3, -
C(0)CH(CH3)2, and
-CO2H, wherein each CH2 is unsubstituted or substituted with 1 or 2
substituents selected from:
oxo, -(CH2)0- 30H, -CN, -NH2, -NI-1(C 1.-6alkY1), -N(C1-6a1ky1)2, -C -6alkyl, -
0C1_6a1ky1,
halogen, -CH2F, -CHF2, -CF3, -CO2H, -CO2C1-6a1kyl, -C3-7cyc1oa1ky1, phenyl,
CH2phenyl,
heteroaxyl and CH2heteroary1, and wherein alkyl, cycloallcyl, and
cycloheteroalkyl are
unsubstituted or substituted with 1, 2, 3 or 4 substituents selected from:
oxo, -(CH2)0-30H, -
CN, -N1-12, -N1-1(C1-6a1kyl), -N(C 1..6alky1)2, -C -6alkyl, -0C1.6a1ky1,
halogen, -CH2F, -CHF2,
-CF3, -CO2H, -CO2C1-6a1ky1. In a subclass of this class, each Ra is
independently selected
from the group consisting of: F, Cl, -CH2OH, -OH, -N(CH3)2, -CN, -CH3, -CF3, -
OCH3, -
OCH2CH3, -C(OH)(CH3)2, -CH(OH)CHF2, -CH(OCH3)CH3, -SO2CH3, -SO2CH(CH3)2, -
S02-azetidine, -S02-pyrrolidine, -SO2NH-tert-butyl, -SO2NH-cyclopropyl, -
NHSO2CH3, -
C(0)CH(CH3)2, and -CO2H. In another class of this embodiment, each Ra is
independently
selected from the group consisting of: -S02C1-6a1ky1, and -NHS02-C1-6alkyl,
wherein alkyl is
unsubstituted or substituted with 1, 2, or 3 substituents selected from: oxo, -
(CH2)0-30H, -CN, -
NH2, -NH(C -6alkyl), -
Ci_6a1kyl, -OC _6alkyl, halogen, -CH2F, -CHF2, -CF3,
-CO2H, and -CO2C1_6alkyl. In a subclass of this class, each Ra is
independently selected from
the group consisting of: -SO2CH3, -S02CH(CH3)2, and -NHSO2CH3, wherein each
alkyl is
unsubstituted or substituted with 1, 2, or 3 substituents selected from: oxo,
4CH2)0-301-1, -CN,
NH2, -NH(C -N(C 1 -6alkY1)2, -C -6alkyl, -OC -6alkyl, halogen, -CH2F, -
CHF2, -CF3,
-CO2H, and -CO2C1_6a1ky1. In another subclass of this class, each Ra is
independently selected
from the group consisting of: -SO2CH3, -S02CH(CH3)2, and -NHSO2CH3. In another
subclass
of this class, each Ra is independently selected from the group consisting of:
-SO2CH3 and -
NHSO2CH3.
In another embodiment of the present invention, each Ra is independently
selected from
the group consisting of: -(CH2)mCN, -(CH2)roNHS02-C1-6a1kyl, and -(CH2)mS02C1-
6alkyl.
In a class of this embodiment, each Ra is independently selected from the
group consisting of: -
CN, -NHS02-Ca1ky1, and -S02C1-6a1ky1. In another class of this embodiment,
each Ra is
independently selected from the group consisting of: -CN, -NHSO2CH3, and -
S02C1_6a1ky1.
In another embodiment of the present invention, each Ra is independently
selected from
the group consisting of: -(CH2)mCN, -(CH2)niNHS02-C1-6a1ky1, and -(CH2)naS02C1-
6a11<y1.
In a class of this embodiment, each Ra is independently selected from the
group consisting of: -
CN, -NHS02-C1-6a1kyl, and -S02C1,5a1kyl. In another class of this embodiment,
each Ra is
independently selected from the group consisting of: -CN, -NHSO2CH3, -SO2CH3,
and -
SO2CH(CH3)2. In another class of this embodiment, each Ra is independently
selected from the
group consisting of: -CN, -NHSO2CH3 and -SO2CH3.
In another embodiment of the present invention, each Ra is independently
selected from
- 34 -
CA 02826649 2013 08 05
WO 2012/116145
PCT/US2012/026261
the group consisting of: -(CH2)mCN, and -(CH2)mS02C1-6alkyl. In a class of
this
embodiment, each Ra is independently selected from the group consisting of: -
CN, and -S02C1-
6alkyl. In another class of this embodiment, each Ra is independently selected
from the group
consisting of: -CN and -S02CH3.
In another embodiment of the present invention, Ra is independently selected
from the
group consisting of:-(CH2)m-halogen, -(CH2)m0H, -(C142)mCF3, -0-(CH2)m-
OC1_6a1ky1, -
(C112)mS02NHC3-7cycloalkyl, -(CH2)mN(Ri)-heteroaryl, -(CH2)mC3-7cyc1oa1ky1, -
(CH2)mC2-6cyc1oheteroa1keny1, and -(CH2)mheteroaryl, wherein each CH2 is
unsubstituted or
substituted with I or 2 substituents selected from: oxo, -(CH2)0-30H, -CN, -
NH2, -NH(C I-
6alkyl), -N(C -6alky1)2, -C -6alkyl, -0C1_6alkyl, halogen, -CH2F, -CHF2, -CF3,
-0O21-1, -
CO2Ci -6alkyl, -C3-7cycloalkyl, phenyl, CH2phenyl, heteroaryl and
CH2heteroaryl, and wherein
alkyl, cycloalkyl, cycloheteroalkenyl and heteroaryl are unsubstituted or
substituted with 1, 2, 3
or 4 substituents selected from: oxo, -(CH2)0-50H, -CN, -NH2, -NH(C1-6alkyl), -
N(C1-
6alicY1)2, -CI -6alkyl, -0C1-6a1ky1, halogen, -CH2F, -CHF2, -CF3, -CO2H, -
CO2C1-6a1ky1, -
SO2C1-6a1ky1, -C3-7cycloalkyl, phenyl, CH2phenyl, heteroaryl and
CH2heteroaryl. In a class of
this embodiment, each CH2 is unsubstituted or substituted with 1 or 2
substituents selected from:
-C1-6alkyl, and each alkyl, cycloalkyl, cyeloheteroalkenyl and heteroaryl is
unsubstituted or
substituted with I, 2, 3 or 4 substituents selected from: oxo, -(CH2)0-50H, -
CN, -NH2, -
NH(Ci -6alkyl), -N(Ci..6a1ky1)2, -C1.6a1ky1, -0C1-6alkyl, halogen, -CH2F, -
CHF2, -CF3,
CO2H, -CO2C1-6a1ky1, -S02C1-6alky1, -C3-7cycloalkyl, phenyl, CH2phenyl,
heteroaryl and
CH2heteroaryl. In another class of this embodiment, each CH2 is unsubstituted
or substituted
with 1 or 2 substituents selected from: -Ci_6alkyl, and each alkyl,
cycloalkyl, cycloheteroalkenyl
and heteroaryl is unsubstituted or substituted with I, 2, 3 or 4 substituents
selected from: oxo, -
(CH2)0-50H, -C1-6alkyl, -CF3, and -C3_7cycloalkyl. In another class of this
embodiment, each
CH2 is unsubstituted or substituted with 1 or 2 substituents selected from: -
CH3, and each alkyl,
cycloalkyl, cycloheteroalkenyl and heteroaryl is unsubstituted or substituted
with I, 2, 3 or 4
substituents selected from: -OH, -CH2C(CH3)20H, -CH3, CH2CH3, CH(CH3)2, -CF3,
cyclopropyl, cyclohexyl, and cyclopentyl. In another class of this embodiment,
each heteroaryl is
unsubstituted or substituted with 1, 2, 3 or 4 substituents selected from: -
CH2C(CH3)20H.
In another embodiment of the present invention, Ra is independently selected
from the
group consisting of: -CH2CH2F, -OH, -CH2CH2OH, -CH2C(CH3)20H, -CH2CF3, -
OCH2CH2OCH3, -SO2NHcyclopropyl, -NH-pyrimidine, -CH2cyclopropyl, 2,5 dihydro
IH
imidazole, 1,4,5,6-tetrahydropyrimidine, imidazole, oxazole, thiazole,
triazole, tetrazole, and
oxadiazole, wherein each CH2 is unsubstituted or substituted withl or 2
substituents selected
from: -Ci_6allcyl, and wherein alkyl, cycloalkyl, cycloheteroalkenyl and
heteroaryl are
unsubstituted or substituted with I, 2, 3 or 4 substituents selected from:
oxo, -(CH2)0-50H, -
CN, -NH2, -NH(C1-6a1ky1), -N(C1-6a1ky1)2, -C1-6a1ky1, -0C -6alkyl, halogen, -
CH2F, -CHF2,
- 35 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
-CF3, -0O2H, -CO2C1_6a1ky1, -S02C1-6alkyl, -C3_7cycloalkyl, phenyl, CH2phenyl,
heteroaryl
and CH2heteroary1. In a class of this embodiment, each CH2 is =substituted or
substituted with
1 or 2 substituents selected from: -Ci-6alkyl, and each alkyl, cycloalkyl,
cycloheteroalkenyl and
heteroaryl is =substituted or substituted with I, 2, 3 or 4 substituents
selected from: oxo,
(CH2)0.50H, -CN, -NH2, -NH(C1_6alkyl), -N(C _6alky1)2, -C1_6alkyl, -
0Ci_6a1ky1, halogen, -
CH2F, -CHF2, -CF31 -CO2H, -CO2C1-6a1ky1, -S02C1-6a1lcy1, -C3-7cycloalkyl,
phenyl,
CH2phenyl, heteroaryl and CH2heteroaty1. In another class of this embodiment,
each CH2 is
=substituted or substituted with 1 or 2 substituents selected from: -Ci-
6a1ky1, and each alkyl,
eyeloalkyl, cycloheteroalkenyl and heteroaryl is =substituted or substituted
with 1, 2, 3 or 4
substituents selected from: oxo, -(CH2)0-50H, -C1-6alkyl, -CF3, and -C3-
7cycloalkyl. In
another class of this embodiment, each CH2 is unsubstituted or substituted
with 1 or 2
substituents selected from: -CH3, and each alkyl, cycloalkyl,
cycloheteroalkenyl and heteroaryl is
=substituted or substituted with 1, 2, 3 or 4 substituents selected from: -OH,
-CH2C(CH3)20H,
-CH3, CH2C1I3, CH(CH3)2, -CF3, cyclopropyl, cyclohexyl, and cyclopentyl. In
another class of
this embodiment, each heteroaryl is =substituted or substituted with 1, 2, 3
or 4 substituents
selected from: -CH2C(CH3)20H.
In another embodiment of the present invention, Ra is independently selected
from the
group consisting of: -(CH2)m0H, and -(CH2)mheteroaryl, wherein each CH2 is
unsubstituted or
substituted with 1 or 2 substituents selected from: oxo, -(CH2)0-30H, -CN, -
NH2, -NIACI-
N 6alkyl), -N(C1-6alky1)2, -C1-6a1ky1, -0C1-6alkyl, halogen, -CH2F, -CHF2, -
CF3, -CO2H, -
CO2C1-6alkyl, -C3-7cycloa1ky1, phenyl, CH2phenyl, heteroaryl and
CH2heteroaryl, and wherein
heteroaryl is =substituted or substituted with 1, 2, 3 or 4 substituents
selected from: oxo, -
(CH2)0-50H, -CN, -NH2, -NH(C1-6alkyl), -N(C1-6a1kY02, -C -6alkyl, -0C1-6a1ky1,
halogen, -
CH2F, -CHF2, -CF3, -CO2H, -0O2C1 .6alkyl, -S02C1-6alkyl, -C3-7cyeloalkyl,
phenyl,
CH2phenyl, heteroaryl and CH2heteroaryl. In a class of this embodiment, each
CH2 is
=substituted or substituted with 1 or 2 substituents selected from: -
Ci_6alkyl, and each
heteroaryl is =substituted or substituted with 1, 2, 3 or 4 substituents
selected from: oxo, -
(CH2)0-50H, -CN, -NH2, -NH(C1-6a1ky1), -N(C1-6a1k311)2, -C1-6alkyl, -0C1-
6alkyl, halogen, -
CH2F, -CHF2, -CF3, -CO2H, -CO2Ci -6alkyl, -S02C1-6a1ky1, -C3-7cycloalkyl,
phenyl,
CH2phenyl, heteroaryl and CH2heteroaryl. In another class of this embodiment,
each CH2 is
=substituted or substituted with 1 or 2 substituents selected from: -C1-
6alkyl, and each
heteroaryl is =substituted or substituted with 1, 2, 3 or 4 substituents
selected from: oxo, -
(CH2)0-50H, -C1-6alkyl, -CF3, and -C3-7cyc10alky1. In another class of this
embodiment, each
CH2 is unsubstituted or substituted with 1 or 2 substituents selected from: -
CH3, and each
heteroaryl is unsubstituted or substituted with 1, 2, 3 or 4 substituents
selected from: -OH, -
CH2C(CH3)20H, -CH3, CH2CH3, CH(CH3)2, -CF3, cyclopropyl, cyclohexyl, and
cyclopentyl.
In another class of this embodiment, each heteroaryl is unsubstituted or
substituted with 1, 2, 3 or
-36-
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
4 substituents selected from: -CH2C(CH3)20H.
In another embodiment of the present invention, Ra is independently selected
from the
group consisting of: -OH, -CH2CH2OH, -CH2C(C143)2011, pyrazole, imidazole,
oxazole,
thiazole, triazole, tetrazole, and oxadiazole, wherein each CH2 is
unsubstituted or substituted
with 1 or 2 substituents selected from: oxo, -(CH2)0-30H, -CN, -NH2, -NH(C1-
6a110), -N(C1-
6alky1)2, -C1 -6alkYl, -0C1-6alkyl, halogen, -CH2F, -CHF2, -CF3, -CO2H, -CO2Ci
-6alkyl, -C3_
7cycloalkyl, phenyl, CH2phenyl, heteroaryl and CH2heteroaryl, and wherein
heteroaryl are
=substituted or substituted with I, 2, 3 or 4 substituents selected from: oxo,
-(CH2)0-50H, -
CN, -NI42, -NH(C1-6alkyl), -N(C1-6alky1)2, -C1-6alky1, -OC _6alkyl, halogen, -
CH2F, -CHF2,
-CF3, -CO2H, -CO2C1 -6alkyl, -S02C1 _6alkyl, -C3-7cyc1oa1ky1, phenyl,
CH2phenyl, heteroaryl
and CH2heteroary1. In a class of this embodiment, each CH2 is =substituted or
substituted with
1 or 2 substituents selected from: -Ci -6a1ky1, and each heteroaryl is
unsubstituted or substituted
with 1, 2, 3 or 4 substituents selected from: oxo, -(CH2)0-50H, -CN, -NH2, -
NH(Ci_olkyl), -
N(C1-6alky1)2, -Ci-6alkyl, -OC -6alkyl, halogen, -CH2F, -CHF2, -CF36 -0O21-1, -
CO2C1-
6alky1, -S02C1-6a1ky1, -C3-7eycloalkyl, phenyl, CH2phenyl, heteroaryl and
CH2heteroary1. In
another class of this embodiment, each CH2 is unsubstituted or substituted
with 1 or 2
substituents selected from: -C1-6alkyl, and each heteroaryl is =substituted or
substituted with 1,
2, 3 or 4 substituents selected from: oxo, -(CH2)()-50H, -C1-6alky1, -CF3, and
-C3.7cycloalkyl.
In another class of this embodiment, each CH2 is unsubstituted or substituted
with 1 or 2
substituents selected from: -C113, and each heteroaryl is =substituted or
substituted with 1, 2, 3
or 4 substituents selected from: -OH, -CH2C(C113)20H, -CH3, CH2CH3, CH(CH3)2, -
CF3,
cyclopropyl, cyclohexyl, and cyclopentyl. In another class of this embodiment,
each heteroaryl is
unsubstituted or substituted with 1, 2, 3 or 4 substituents selected from: -
CH2C(CH3)2011.
In another embodiment of the present invention, Ra is independently selected
from the
group consisting of: -(CH2)m-halogen, -(CH2)m0H, -(CH2)m -N(Ri)2, -(CH2)m -CN,
--
(CH2)m C1.6alkyl, --(CH2)m OCF3, -(0-12)mC(0)N(Ri)2, -(042)m0-(CH2)m-C3-
7cycloalkyl, -(CH2)m0-(CH2)m-C2-7cycloheteroalkyl, --(CH2)m S02C1-6alkyl, --
(CH2)m
S02C2-7cycloheteroalkyl, --(CH2)m SO2NHC1-6alkyl, --(CH2)m NHS02-C1-6alkyl, --
(CH2)m C(0)Rf, --(CH2)m CO2H, --(CH2)m CO2Rf, -(C112)mCF3, -0-(C112)m-0C1-
6alkyl, -
(CH2)mS02NHC3-7cycloalkyl, -(CH2)mN(Ri)-heteroaryl, -(CH2)mC3-7eyc1oa1kyl, -
(CH2)mC2_6cycloheteroalkenyl, and -(CH2)mheteroatyl, wherein each CH2 is
=substituted or
substituted with 1 or 2 substituents selected from: oxo, -(CH2)0-30H, -CN, -
NH2, -NH(C I-
6alkyl), -N(C1-6alky1)2, -C -6alkyl, -0C1-6a1ky1, halogen, -CH2F, -CHF2, -CF3,
-0O211, -
CO2C1_6a1ky1, -C3-7cycloalkyl, phenyl, CH2phenyl, heteroaryl and
CH2heteroaryl, and wherein
each alkyl, cycloalkyl, cycloheteroalkyl, cycloheteroalkenyl and heteroaryl
are =substituted or
substituted with 1, 2, 3 or 4 substituents selected from: oxo, -(C112)0-50H, -
CN, -NH2, -
NH(C1-6a1kyl), -6alky1)2, -C -6alkyl, -0C1-6a1ky1, halogen, -CH2F, -
CHF2, -CF3,
-37-
CA 02826649 2013 08 05
WO 2012/116145
PCT/US2012/026261
CO2H, -CO2C1_6a1ky1, -S02Ci -6alkyl, -C3-7cycloalkyl, phenyl, CH2phenyl,
heteroaryl and
CH2heteroaryl. In a class of this embodiment, each Ra is independently
selected from the group
consisting of: F, CI, Br, -C(CH3)20H, -OH, -CH2OH, -CH2CH2OH, -CH2C(CH3)20H, -
CH(OH)CHF2, CH(OH)CF3, -(CH2)2C(CH3)2-0H, -N(CH3)2, -CN, -CH3, -CH2CH3,
C(CH3)3 -CF3, -CH2CF3 -0CF3 -C(0)NH-eyclopropyl -OCH2CH3, -OCH3, -0(CH2)3-
S02CH3, OCH2CH2F, -CH2OCH3 -0-cyclobutyl, -0-cyclopentyl, -0-azetidine, -0-CH2-
dioxolane, -SO2CH(CH3)2, -S02CH3 -S02-pyrrolidine, -S02-azetidine -SO2NHCH3, -
SO2NHC(CH3)3, -SO2NH-cyclopropyl, -NHS02-CH3, -C(0)CH(CH3)2, C(0)-pymlidine, -
C(0)-morpholine, -CO2H, -0O2-CH(CH3)2, -0O2-C(CH3)3, cyclopropyl, pyrazole,
imidazole,
oxazole, thiazole, triazole, tetrazole, and oxadiazole, wherein each
heteroaryl is unsubstituted or
substituted with 1, 2, 3 or 4 substituents selected from: oxo, -(CH2)0-50H, -
CN, -NH2, -
NH(C 1..6a lkyl), -N(C1-6a1ky1)2, -C1-6alkYl3 -0C 1-6alkyl, halogen, -CH2F, -
CHF2, -CF3, -
CO2H, -CO2C1-6a1kyl, -S02C1-6alky1, -C3-7cycloalkyl, phenyl, CH2phenyl,
heteroaryl and
CH2heteroaryl.
In another embodiment of the present invention, Ra is independently selected
from the
group consisting of: -(CH2)m-halogen, N(R)2, -CN, -C1-6a1kyl, -(CH2)m0H, -
(CH2)mCF3, -
0-(CH2)m-OC 1 -6a1ky1, -C(0)R, -CO2H, -(CH2)mS02NHC3-7cyc1oa1ky1, -NHS 02-C1 -
6allcyl,
-(CH2)mN(Ri)-heteroary1, -(CH2)mC3-7cycloal1cy1, -(CH2)mC2-
6cycloheteroalkenyl, and -
(CH2)mheteroary1, wherein each CH2 is unsubstituted or substituted with 1 or 2
substituents
selected from: oxo, -(CH2)0-3 OH, -CN, -NH2, -NH(C -6alky1), -N(C _6alky1)2, -
C -6alkyl, -
0C1-6alkyl, halogen, -CH2F, -CHF2, -CF3, -CO2H, -CO2C1-6alkyl, -
C3_7cycloalkyl, phenyl,
CH2phenyl, heteroaryl and CH2heteroary1, and wherein alkyl, cycloallcyl,
cycloheteroalkyl
cycloheteroalkenyl and heteroaryl are unsubstituted or substituted with 1, 2,
3 or 4 substituents
selected from: oxo, -(CH2)0-50H, -CN, -NH2, -NH(Ci.6a1ky1), -N(C1_6allcy1)2, -
C1_6alkyl,
0C1-6alkyl, halogen, -CH2F, -CHF2, -CF3, -CO2H, -CO2C1-6alkyl, -S02C1-6a1ky1, -
C3_
7cycloalkyl, phenyl, CH2phenyl, heteroaryl and CH2heteroaryl. In a class of
this embodiment,
each Ra is independently sekcted from the group consisting of: F, CI, -OH, -
CH2OH, -
CH2CH2OH, -CH2C(CH3)20H, -N(CH3)2, -CN, -CH3, -CF3, -OCH3, -OCH2CH3, -
C(OH)(CH3)2, -CH(OH)CHF2, -CH(OCH3)CH3, -S02CH3, -S02CH(CH3)2, -S02-azetidine,
S02-pyrrolidine, -SO2NH-tert-buty1, -SO2NH-cyclopropyl, -NHSO2CH3, -
C(0)CH(CH3)2, -
CO2H, pyrazole, imidazole, oxazole, thiazole, triazole, tetrazole, and
oxadiazole, wherein each
CH2 is unsubstituted or substituted with 1 or 2 substituents selected from: -
Ci..6alkyl, and each
heteroaryl is unsubstituted or substituted with 1, 2, 3 or 4 substituents
selected from: oxo, -
(CH2)0-50H, -Ci-6alkyl, -CF3, and -C3_7eycloalkyl.
In another embodiment of the present invention, Ra is independently selected
from the
group consisting of: -(CH2)m0H, -(CH2)mCN, -(CH2)mNHS02-C1_oallcyl, -
(CH2)mS02C 1-
6alkyl and -(CH2)mheteroary1, wherein each CH2 is unsubstituted or substituted
with 1 or 2
-38-
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
substituents selected from: -Ci-6alkyl, and each heteroaryl is unsubstituted
or substituted with 1,
2, 3 or 4 substituents selected from: oxo, -(CH2)0-50H, -C1-6alkyl, -CF3, and -
C3-7cycloalkyl.
In another embodiment of the present invention, Ra is independently selected
from the
group consisting of: -OH, -CH2CH2OH, -CH2C(C113)20H, -CN, -NHSO2CH3 and -
S02CH3,
PYrazole, imidazole, oxazole, thiazole, triazole, tetrazole, and oxadiazole,
wherein each CH2 is
unsubstituted or substituted with 1 or 2 substituents selected from: -Ci-
aalkyl, and each
heteroaryl is unsubstituted or substituted with 1, 2, 3 or 4 substituents
selected from: oxo, -
(CH2)0-50H, -C1-6a1kyl, -CF3, and -C3-7cycloalkyl.
In another embodiment of the present invention, each Rb is independently
selected from:
hydrogen, -C1-6alkYl, -C3-6cyeloalkyl, -C3-6cycloalkenyl, -C2-
6cyc1oheteroa1ky1, -C2-
6cycloheteroalkenyl, aryl, heteroaryl, -(CH2)t-halogen, -(CH2)s-0H3 -NO2, -
NH2, -NH(C1-
6alkyl), -N(C -6alky1)2, -0C 1-6alkyl, -(CH2)qCO2H, -(CH2)cICO2C -6alkyl, -
CF3, -CN, -
S 02C .6alkyl, and -(CH2)sCON(Re)2, wherein each CH2 is unsubstituted or
substituted with 1
or 2 halogens, and wherein each alkyl, eyeloalkyl, cycloalkenyl,
cycloheteroalkyl,
cycloheteroalkenyl, aryl and heteroaryl is unsubstituted or substituted with
1, 2 or 3 halogens.
In a class of this embodiment, each Rb is independently selected from:
hydrogen,-Ci_
6alkyl, -(CH2)t-halogen, -(CH2)s-OH, -NO2, -NH2, -NH(C1-6alkyl), -N(C1-
6alkyl)2, -OC i _
-(CH2)cle02H, -(CH2)qCO2C1-6a1ky1, -CF3, -CN, -S02C1-6alkyl, and -
(CH2)sCON(R92, wherein each CH2 is unsubstituted or substituted with 1 or 2
halogens, and
wherein each alkyl is unsubstituted or substituted with 1, 2 or 3 halogens, In
a class of this
embodiment, each Rb is independently selected from: hydrogen, -C1_6alkyl, -
(CH2)t-halogen, -
(CH2)s-OH, -NO2, -NH2, -NH(C1_6alkyl), -N(C _6alky1)2, -OC .6alkyl, -
(CH2)qCO2H, -
(CH2)qCO2C1-6a1ky1, -CF3, -SO2C1-6alkyl, and -(CH2)sCON(Re)2, wherein
each CH2
is unsubstituted or substituted with 1 or 2 halogens, and wherein each alkyl
is unsubstituted or
substituted with 1, 2 or 3 halogens. In another class of this embodiment, each
Rb is
independently selected from: hydrogen, -C1-6alky1, -(CH2)t-halogen, -(CH2)s-
OH, -CO2H, and -
CO2C1_6a1ky1, wherein each CH2 is unsubstituted or substituted with 1 or 2
halogens, and
wherein each alkyl is unsubstituted or substituted with 1, 2 or 3 halogens. In
another class of this
embodiment, each Rb is independently selected from: hydrogen, -C1_6a1ky1, -
(CH2)t-halogen,
(CH2)s-OH, -CO2H, and -CO2C1-6alky1, wherein each CH2 is unsubstituted or
substituted with
1 or 2 halogens, and wherein each alkyl is unsubstituted or substituted with
1, 2 or 3 halogens. In
another class of this embodiment, each Rb is independently selected from:
hydrogen, -C113,-
CH2F, -CH2OH, -C(CH3)2-0H, -CO2H and -CO2C(CH3)3, wherein each CH2 is
unsubstituted
or substituted with 1 or 2 halogens, and wherein each alkyl is unsubstituted
or substituted with 1,
2 or 3 halogens.
In another embodiment of the present invention, each Rb is independently
selected from:
hydrogen, -C1-6a1ky1, and -(CH2)s-OH. in a class of this embodiment, each Rb
is independently
-39-
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
selected front hydrogen, -CH3, -OH, and -CH2OH. In a class of this embodiment,
each Rb is
independently selected from: hydrogen, -CH3, and -CH2OH.
In another embodiment of the present invention, each Re is independently
selected from:
halogen, oxo, -(CH2)r0H, -(CH2)N(Re)2, -(CH2)rCN, -Ci_6a11cy1, -CF3, -
C1_6a1ky1-OH,
OCH2OCi -6alkyl, -(CHAOCi .6alkyl, -OCH2aryl, -(CH2)rSC1 -6alkyl, -
(CH2)rC(0)Rf, -
(CH2)rC(0)N(Re)2, -(CH2)rCO21-18 -(C112)rCO2Rf, -(CH2)rC3-7cycloa11cy1, -
(CH2)rC2-
6cycloheteroalkyl, -(CH2)raryl, and -(CH2)rheteroaryl, wherein each CH2 is
unsubstituted or
substituted with 1 or 2 substituents selected from: oxo, -OH, -CN, -N(Rh)2, -
Ci-6alkyl, -OC1-
6alkyl, halogen, -CH2F, -CHF2, -CF3, -CO2H, -CO2C1-6alkyl, -C3_7cycloalkyl and
heteroaryl,
and wherein alkyl, cycloalkyl, cycloheteroalkyl, aryl and heteroaryl are
unsubstituted or
substituted with 1, 2, 3 or 4 substituents selected from: oxo, -OH, -CN, -
N(Rh)2, -Cl_6alkyl, -
0C1-6alkyl, halogen, -CH2F, -CHF2, -CF3, -CO2H, -CO2C1-6alkyl, -C3-7cyc1oa1ky1
and
heteroaryl. In a class of this embodiment, each Re is independently selected
from: halogen, oxo,
-(CH2)OH, -(CH2)rN(R92, -(CH2)reN, -Ci-6alkyl, -CF3, -C1-6alkyl-OH, -OCH20C1-
6a1ky1,
-(CH2)rOCi -6a1ky1, -(CH2)rSC1-6al1cy1, -(CH2)rC(0)Rf, -(CH2)rC(0)N(Re)2, -
(CH2)rCO2H,
and -(CH2)rCO2Rf, wherein each CH2 is unsubstituted or substituted with 1 or 2
substituents
selected from: oxo, -OH, -CN, -N(Rh)2, -C1.6alkyl, -0C1-6a1ky1, halogen, -
CH2F, -CHF2,
CF3, -CO2H, -0O2C1_6alky1, -C3_7cycloalkyl and heteroaryl, and wherein alkyl
is unsubstituted
or substituted with 1, 2, 3 or 4 substituents selected from: oxo, -OH, -CN, -
N(Rh)2, -C1_6allcyl, -
OC1-6a1ky1, halogen, -CH2F, -CHF2, -CF3, -CO2H, and -CO2C1-6a1ky1. In a class
of this
embodiment, each RC is independently selected from: halogen, oxo, -OH, -
N(Re)2, -CN, -C1-
6ancyl, -CF3, -C -6alkyl-OH, -0C1-6a1kyl, -SC I -6alkyl, -C(0)Rf, -C(0)N(Re)2,
-CO2H, and -
CO2Rf, wherein alkyl is unsubstituted or substituted with 1, 2, 3 or 4
substituents selected from:
oxo, -OH, -CN, -N(Rh)2, -C1-6alkyl, -0Ci -6alkyl, halogen, -CH2F, -CHF2, -CF3,
-CO2H, and
-CO2C l_6alkyl. In another class of this embodiment, each RC is independently
selected from:
halogen, oxo, -OH, -N(Re)2, -CN, -Ci..6a1kyl, -CF3, -C1_6a1ky1-OH, and -
0C1_6alkyl, wherein
alkyl is unsubstituted or substituted with I, 2, 3 or 4 substituents selected
from: oxo, -OH, -CN, -
N(Rh)2, -Calky1, -0C1-6a1kyl, halogen, -CH2F, -CHF2, -CF3, -CO2H, and -CO2C1-
6alky1.
In another embodiment of the present invention, each Re, Rg and Rh is
independently
selected from: hydrogen, -C1-6alkyl, and -0-Ci -6alky1, wherein alkyl is
unsubstituted or
substituted with 1, 2, 3 or 4 substituents selected from: -OH, oxo, halogen,
C1-6alkyl, -0C1-
6alkYl, -NH2, -NH(Ci -6alkyl), and -N(C1-6alky1)2. In a class of this
embodiment of the present
invention, each Re, Rg and Rh is independently selected from: hydrogen, -CH3
,and -OCH3,
wherein alkyl is unsubstituted or substituted with 1, 2, 3 or 4 substituents
selected from: -OH,
oxo, halogen, Ci _6alkyl, -0Ci -6alkyl, -NH2, -NH(C1.6a1ky1), and -N(C1-
6a1kY02.
In another embodiment of the present invention, each Re is independently
selected from:
hydrogen, -CI -6a1ky1, and -0-Cl -6alkyl, wherein alkyl is unsubstituted or
substituted with 1, 2,
- 40 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
3 or 4 substituents selected from: -OH, oxo, halogen, Ci_6alkyl, -0Ci_6alkyl, -
NH2, -NH(C1-
6alkyl), and -N(C1-6alky1)2. In a class of this embodiment of the present
invention, each Re is
independently selected from: hydrogen, -CH3 ,and -OCH3, wherein alkyl is
unsubstituted or
substituted with 1, 2, 3 or 4 substituents selected from: -OH, oxo, halogen,
Ci -6a1k34, -0C I-
6a1kyl, -NH2, -NH(C1-6alkyl), and -N(C1-6a141)2.
In another embodiment of the present invention, each Rg is independently
selected from:
hydrogen, -Ci_6alkyl, and -0-Ci_6alkyl, wherein alkyl is unsubstituted or
substituted with 1, 2,
3 or 4 substituents selected from: -OH, oxo, halogen, C1-6a1kyl, -0C1.6alkyl, -
NH2, -NH(C1-
6alkY1), and -N(C1-6a1ky1)2. In a class of this embodiment of the present
invention, each Rg is
independently selected from: hydrogen, -CH3 ,and -OCH3, wherein alkyl is
unsubstituted or
substituted with 1, 2, 3 or 4 substituents selected from: -OH, oxo, halogen,
C1-6alkyl, -0C1-
6alkyl, -NH2, -NH(C1-6alkyl), and -N(C1-6a1kY1)2.
In another embodiment of the present invention, each Rh is independently
selected from:
hydrogen, -C1-6alkyl, and -0-Ci-6alkyl, wherein alkyl is unsubstituted or
substituted with 1, 2,
3 or 4 substituents selected from: -OH, oxo, halogen, Ci-6alkyl, -0C1-6alkyl, -
NH2, -N11(C1-
6alky1), and -N(C1-6alky1)2. In a class of this embodiment of the present
invention, each Rh is
independently selected from: hydrogen, -CH3, and -OCH3, wherein alkyl is
unsubstituted or
substituted with 1, 2, 3 or 4 substituents selected from: -OH, oxo, halogen,
Ci-6alkyl, -0C
6alkyl, -NH2, -NH(C1_6alkyl), and -N(C1-6alkYD2.
In another embodiment of the present invention, each Re, Rg and Rh is
independently
selected from: hydrogen, and Ci.6alkyl, wherein alkyl is unsubstituted or
substituted with 1, 2, 3
or 4 substituents selected from: -OH, oxo, halogen, CI -6alkyl, -0Ci _6a11cyl,
-NH2, -NI-(C1-
6alkY1), and -N(C1-6ancy1)2. In a class of this embodiment, each Re is
independently selected
from: hydrogen, and C1-6alkyl, wherein alkyl is unsubstituted or substituted
with 1, 2 or 3
substituents selected from: -OH, oxo, halogen, Ci_6a1ky1, -OCI-6a1ky1, -NH2, -
NH(C1-6alkyl),
and -N(C1-6a1ky1)2. In a subclass of this class, Re is hydrogen. In another
subclass of this class,
Re is Ci-6alkyl. In another class of this embodiment, each Rg is independently
selected from:
hydrogen, and Ci -6a11cyl, wherein alkyl is unsubstituted or substituted with
1, 2 or 3 substituents
selected from: -OH, oxo, halogen, C1-6alkyl, -0C1-6alkyl, -NH2, -MAC1-6alkyl),
and -N(C1-
6a1ky1)2. In a subclass of this class, Rg is hydrogen. In another subclass of
this class, Rg is Ci..
Galkyl. In another class of this embodiment, each Rh is independently selected
from: hydrogen,
and Ci-6alkyl, wherein alkyl is unsubstituted or substituted with 1, 2 or 3
substituents selected
from: -OH, oxo, halogen, C1-6a1ky1, -0C1-6a1kyl, -NH2, -NH(C1.6a1ky1), and -
N(C1-6a1kY1)2.
In a subclass of this class, Rh is hydrogen. In another subclass of this
class, Rh is Ci.6a1ky1.
In another embodiment of the present invention, each Ri is independently
selected from:
hydrogen, C1-6alkyl, C3-6cycloalkyl, -C(0)Ri, and -SO2Ri,wherein alkyl and
cycloalkyl are
unsubstituted or substituted with 1, 2, 3 or 4 substituents selected from: -
OH, oxo, halogen, C1_
- 41 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
6alkyl, -0C1-6alkyl, -NH2, -NH(C1-6a1kyl), and -N(C1-6alky1)2. In a class of
this embodiment,
each Ri is independently selected from: hydrogen, C1-6alkyl, and C3-
6cyc1oa1ky1, wherein alkyl
and cycloalkyl are unsubstituted or substituted with 1, 2, 3 or 4 substituents
selected from: -OH,
oxo, halogen, Ca1kyl, -0C i_6alkyl, -NH2, -NH(C1-6a1ky1), and -N(C1-6alky1)2.
In another
class of this embodiment, each Ri is independently selected from: hydrogen, -
CH3 and
cyclopropyl, wherein methyl and cyclopropyl are unsubstituted or substituted
with 1, 2, 3 or 4
substituents selected from: -OH, oxo, halogen, Cl-6alkyl, -0C1 -6alkyl, -NH2, -
NH(C1-6alkyl),
and -N(Ci -6alkY1)2.
In another embodiment of the present invention, each Ili is independently
selected from:
hydrogen, C1_6alkyl, C3-6cycloalkyl, -C(0)Ri, and -SO2Ri, wherein alkyl and
cycloalkyl are
unsubstituted or substituted with 1, 2, 3 or 4 substituents selected from: -
OH, oxo, halogen, C1-
6alkYl., -0Ca1ky1, -NH2, -NH(C1_6alkyl), and -N(C1-6a1ky1)2. In a class of
this embodiment,
each Ri is independently selected from: hydrogen, C1-6alkyl, -C(0)Ri, and -
SO2Ri, wherein
alkyl is unsubstituted or substituted with 1, 2, 3 or 4 substituents selected
from: -OH, oxo,
halogen, Cl 6alkyl, -0Ci _6alkyl, -NH2, -NH(C1-6alkyl), and -N(Ci -6alkY02.
In another class of this embodiment, each Ri is independently selected from:
hydrogen, and Ci_
6alkyl, wherein alkyl is unsubstituted or substituted with 1, 2, 3 or 4
substituents selected from:
OH, oxo, halogen, Ci-6a1ky1, -0C1-6alkyl, -NH2, -NFI(Ci_6a1ky1), and -
N(Ci_6alky1)2. In
another class of this embodiment, each Ri is independently selected from:
hydrogen, and Ci-
6alkyl. In another class of this embodiment, Ri is hydrogen. In another class
of this
embodiment, Ri is C1 -6alkyl, wherein alkyl is unsubstituted or substituted
with 1, 2 or 3
substituents selected from: -OH, oxo, halogen, C1-6alkyl, -0C1-6alkyl, -NH2, -
NH(C1_6alkyl),
and -N(C1-6alky1)2. In another class of this embodiment, Ri is C -6alkyl.
In another embodiment of the present invention, each Rf and Ri is
independently selected
from: Ci-6alkyl, C4-7cycloalkyl, C4.7cycloalkenyl, C3_7cycloheteroalkyl, C3-
7eycloheteroalkenyl, aryl, and heteroaryl, wherein alkyl, cycloalkyl,
cycloalkenyl,
cycloheteroalkyl, cycloheteroalkenyl, aryl and heteroaryl are unsubstituted or
substituted with 1,
2, 3 or 4 substituents selected from: oxo, -OH, -CN, -NH2, -Ci -6alkyl, -0C
_6a1ky1, halogen, -
CH2F, -CHF2, -CF3, -CO2H, -CO2C1-6a1ky1, -C3-7cyeloalkyl, and heteroaryl. In a
class of this
embodiment, each Rf and Ri is independently selected from: C1-6alkyl, and C3..
7cycloheteroalkyl, wherein alkyl and cycloheteroalkyl are unsubstituted or
substituted with 1, 2,
3 or 4 substituents selected from: oxo, -OH, -CN, -NH2, -C1-6alky1, -0C1-
6alkyl, halogen, -
CH2F, -CHF2, -CF3, -CO2H, -0O2C1-6alkyl, -C3-7cycloalkyl, and heteroaryl. In
another class
of this embodiment, each Rand Ri is independently selected from: C1_6alkyl,
and C3_
7cycloheteroalkyl, wherein alkyl and cycloheteroalkyl are unsubstituted or
substituted with 1, 2
or 3 substituents selected from: oxo, -OH, -CN, -NH2, -Ci-6a1ky1, -0C1-6alky1,
halogen, -
CH2F, -CHF2, -CF3, -CO2H and -CO2C1-6alkyl. In another class of this
embodiment, each Rf
- 42 -
CA 02826649 2013 08 05
WO 2012/116145
PCT/US2012/026261
and Ri is independently selected from: -CH(CH3)2, -C(CH3 morpholine,
pyrrolidine, and
piperazine, wherein each alky, morpholine, pyrrolidine, and piperazine is
unsubstituted or
substituted with 1, 2 or 3 substituents selected from: oxo, -OH, -CN, -NH2, -
Ci-6alkyl,
halogen, -012F, -CHF2, -CF3, -CO2H and -CO2C1_6alkyl.
In another embodiment of the present invention, each Rf is independently
selected from:
Ci..6alkyl, C4_7cycloalkyl, C4-7cycloalkenyl, C3.7cycloheteroalkyl, C3-
7cycloheteroalkenyl,
aryl, and heteroaryl, wherein alkyl, cycloalkyl, cycloalkenyl,
cycloheteroalkyl,
cycloheteroalkenyl, aryl and heteroaryl are unsubstituted or substituted with
1, 2, 3 or 4
substituents selected from: oxo, -OH, -CN, -NH2, -Ci-6alkyl, -0Ci_6alkyl,
halogen, -CH2F, -
CHF2, -CF3, -CO2H, -CO2C1-6alkyl, -C3_7cye1oa1ky1, and heteroaryl. In a class
of this
embodiment, each Rf is independently selected from: C1-6alkyl, and C3-
7cyc1oheteroa1ky1,
wherein alkyl and cycloheteroalkyl are unsubstituted or substituted with 1, 2,
3 or 4 substituents
selected from: oxo, -OH, -CN, -N112, -Ci-6a1ky1, -0C1-6allcyl, halogen, -CH2F,
-CHF2, -CF3, -
CO2H, -CO2Ci _6alkyl, -C3_7cycloalkyl, and heteroaryl. In another class of
this embodiment,
each Rf is independently selected from: Ci-6alkyl, and C3-7cycloheteroalkyl,
wherein alkyl and
cycloheteroalkyl are unsubstituted or substituted with 1, 2 or 3 substituents
selected from: oxo,
OH, -CN, -NH2, -C1-6alkY1, -0C1-6a1lcy1, halogen, -CH2F, -CHF2, -CF3, -CO2H
and -CO2C1-
6alkYl. In another class of this embodiment, each Rf is independently selected
from: -
CH(CH3)2, -C(CH3 morpholine, pyrrolidine, and piperazine, wherein each alkyl,
morpholine,
pyrrolidine, and piperazine is unsubstituted or substituted with 1, 2 or 3
substituents selected
from: oxo, -OH, -CN, -
C1-6a1ky1, -0C1-6a11cy1, halogen, -CH2F, -CHF2, -CF3, -CO2H
and -CO2C1_6alkyl.
In another embodiment of the present invention, each Ri is independently
selected from:
Ci..6alkyl, C4.7cyc1oa1ky1, C4.7cycloa1kenyl, C3_7cycloheteroalkyl,
C3_7cycloheteroalkenyl,
aryl, and heteroaryl, wherein alkyl, cycloalkyl, cycloalkenyl,
cycloheteroalkyl,
cycloheteroalkenyl, aryl and heteroaryl are unsubstituted or substituted with
I, 2, 3 or 4
substituents selected from: oxo, -OH, -CN, -NH2, -C1-6alky1, -0C1-6alkyl,
halogen, -CH2F, -
CHF2, -CF3, -CO2H, -CO2C1-6a1kyl, -C3-7eyc1oalky1, and heteroaryl. In a class
of this
embodiment, each Ri is independently selected from: C1_6alkyl, and
C3_7cycloheteroalkyl5
wherein alkyl and cycloheteroalkyl are unsubstituted or substituted with 1, 2,
3 or 4 substituents
selected from: oxo, -OH, -CN, -NH2, -C1-6allcyl, -0C1_6alkyl, halogen., -CH2F,
-CHF2, -CF3, -
CO2H, -CO2C1-6alkyl, -C3-7cycloalkyl, and heteroaryl. In another class of this
embodiment,
each Ri is independently selected from: Ca1ky1, and C3_7cycloheteroalkyl,
wherein alkyl and
cycloheteroalkyl are unsubstituted or substituted with 1, 2 or 3 substituents
selected from: oxo, -
OH, -CN, -NH2, -C1-6alkyl, -0C1-6a1ky1, halogen, -CH2F, -CHF2, -CF3, -CO2H and
-CO2C1-
6a1kyl. In another class of this embodiment, each Ri is independently selected
from: -
CH(CH3)2, -C(CH3 , morpholine, pyrrolidine, and piperazine, wherein each alky,
morpholine,
- 43 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
pyrrolidine, and piperazine is unsubstituted or substituted with 1, 2 or 3
substituents selected
from: oxo, -OH, -CN, -NH2, -C1-6a1ky1, -OCI-6a1kyl, halogen, -CH2F, -CHF2, -
CF3, -CO2H
and -CO2C1_6alkyl.
In another class of this embodiment, each Rf arid Ri is independently selected
from: CI_
6alkyl, wherein alkyl is unsubstituted or substituted with 1, 2, 3 or 4
substituents selected from:
oxo, -OH, -CN, -NH2, -Ci-6alkyl, -0C1-6alkyl, halogen, -CH2F, -CHF2, -CF3, -
CO2H,
CO2C1_6alkyl, -C3_7cycloalkyl, and heteroaryl. In another class of this
embodiment, each Rf
and Ri is independently selected from: C4_7cycloalkyl, C4_7cycloalkenyl,
C3.7cycloheteroalkyt,
C3_7cyc1oheteroa1keny1, aryl, and heteroaryl, wherein cycloalkyl,
cycloalkenyl, cycloheteroalkyl,
cycloheteroalkenyl, aryl and heteroaryl are unsubstituted or substituted with
I, 2, 3 or 4
substituents selected from: oxo, -OH, -CN, -NH2, -C1_6alkyl, -0C1-6alkyl,
halogen, -CH2F, -
CHF2, -CF3, -CO2H, -CO2C1-60kY1, -C3-7cycloalkyl, and heteroaryl.
In another embodiment of the present invention, n is 0, 1, 2, 3 or 4. In a
class of this
embodiment, n is 1, 2 or 3. In another class of this embodiment, n is 0, 1 or
2. In another class
of this embodiment, n is O. In another class of this embodiment, n is 1. In
another class of this
embodiment, n is 2.
In another embodiment of the present invention, m is 0, 1, 2, 3, or 4. In a
class of this
embodiment, m is 0, I, 2 or 3. In another class of this embodiment, m is 1, 2
or 3. In another
class of this embodiment, m is 0, 1 or 2. In another class of this embodiment,
m is 0 or 1. In
another class of this embodiment, m is O. In another class of this embodiment,
m is 11.
In another embodiment of the present invention, p is 0, 1, 2 or 3. In a class
of this
embodiment, p is 1, 2 or 3. In another class of this embodiment, p is 0, 1 or
2. In another class
of this embodiment, p is 0 or 2. In another class of this embodiment, p is O.
In another class of
this embodiment, p is 1. In another class of this embodiment, p is 2.
In another embodiment of the present invention, q is 0, 1, 2, 3 or 4. In a
class of this
embodiment, q is 1, 2 or 3. In another class of this embodiment, q is 0, 1 or
2. In another class
of this embodiment, q is 1 or 2. In another class of this embodiment, q is O.
In another class of
this embodiment, q is 1. In another class of this embodiment, q is 2.
In another embodiment of the present invention, r is 0, 1 or 2. In a class of
this
embodiment, r is 0 or 1. In another class of this embodiment, r is 0. In
another class of this
embodiment, r is 1. In another class of this embodiment, r is 2.
In another embodiment of the present invention, s is 0, 1, 2, 3 or 4. In a
class of this
embodiment, s is 0, 1, 2 or 3. In a class of this embodiment, s is 0, 1 or 2.
In another class of
this embodiment, s is 0 or 1. In another class of this embodiment, s is 1 or
2. In another class of
this embodiment, s is 0 or 2. In another class of this embodiment, s is O. In
another class of this
embodiment, s is 1. In another class of this embodiment, s is 2. In another
class of this
embodiment, s is 3.
- 44 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
In another embodiment of the present invention, t is 0, 1, 2, 3 or 4. In a
class of this
embodiment, t is 0, 1, 2 or 3. In a class of this embodirnent, t is 0, 1 or 2.
In another class of this
embodiment, t is 0 or 1. In another class of this embodiment, t is 1 or 2. In
another class of this
embodiment, t is 0 or 2. In another class of this embodiment, t is O. In
another class of this
embodiment, t is 1. In another class of this embodiment, t is 2. In another
class of this
embodiment, t is 3.
Another embodiment of the present invention relates to compounds of structural
formula
I wherein:
T is N;
U is ¨CR1-;
V is -CR2-;
W is -CR4-;
X is selected from:
(1) -0-, and
(2) -0-CH2-;
Y is selected from:
(1) C3_10cyc1oa1ky1,
(2) C2-locyc1oheteroalky1, and
(3) phenyl,
wherein cycloalkyl, cycloheteroalkyl and phenyl are unsubstituted or
substituted with 1, 2, 3 or 4
substituents selected from Rb;
Z is selected from:
(1) oxo,
(2) ¨CF3,
(3) ¨C ..6alkyl,
(4) ¨(CH2)t-halogen,
(5) ¨(CH2)ELCO2H,
(6) ¨(CH2)n0H, and
(7) ¨(C112)nSO2C1-6a1ky1,
wherein each CH2 is unsubstituted or substituted with 1 or 2 substituents
selected from C1-
6alkyl, -OH and -NH2, and wherein each alkyl is unsubstituted or substituted
with 1, 2, 3 or 4
substituents selected from Itc;
R1 is independently selected from:
(1) -C4_10cycloalkenyl,
(2) -phenyl,
(3) phenyl-C2alkyny1C1-5a1kyl,
(4) phenyl-C2_3alkynyl- C3-7cycloalkyl,
- 45 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
(5) phenyl-C2-3alkynyl-C2-iocycloheteroalkyl,
(6) -phenyl-C3_7cyc1oa1ky1,
(7) -phenyl-C2-7cycloheteroalkyl,
(8) pheny1-C2-10cycloheteroalkenyl,
(9) ¨phenyl-phenyl,
(10) -phenyl-heteroaryl,
(11) -heteroaryl, and
(12) -C2.6a1kyny1-pheny1,
wherein each alkyl, alkynyl, cycloalkyl, cycloalkenyl, eyeloheteroalkyl,
cycloheteroalkenyl,
phenyl and heteroaryl is unsubstituted or substituted with 1, 2, 3 or 4
substituents independently
selected from Ra;
R2 is selected from halogen;
R4 is hydrogen; and
R5 is hydrogen;
or a phamtaceutically acceptable salt thereof.
Another embodiment of the present invention relates to compounds of structural
formula
1 wherein:
T is N;
U is ¨CR1-;
V is -CR2-;
W is -CR4-;
X is -0-;
Y is selected from C2-10cyc1oheteroa1ky1, wherein each cycloheteroalkyl is
unsubstituted or
substituted with 1, 2, 3 or 4 substituents selected from Rb;
Z is selected from: ¨(CH2)t-halogen, and ¨(CH2)110H;
R1 is independently selected from:
(1) pheny1-C2-8a1kyny1-Ci- galkyl,
(2) pheny1-C2-3a1kyny1- C3-7cycloalkyl,
(3) pheny1-C2-3a1kyny1-C2-iocyeloheteroalkyl,
(4) phenyl-C2-1ocycloheteroalkenyl,
(5) biphenyl, and
(6) phenyl-heteroaryl,
wherein each alkyl, allcynyl, cycloalkyl, cycloheteroalkyl,
cycloheteroalkenyl, phenyl, biphenyl
and heteroatyl is unsubstituted or substituted with 1, 2, 3 or 4 substituents
independently selected
from Ra;
R2 is selected from halogen;
R4 is hydrogen; and
- 46 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
R5 is hydrogen;
or a pharmaceutically acceptable salt thereof.
Another embodiment of the present invention relates to compotmds of structural
formula
I wherein:
T is N;
U is ¨CR1-;
V is -CR2-;
W is -CR4-;
X is selected from:
(1) -0-, and
(2) -0-C112-;
Y is selected from:
(1) C3_1 ocycloalkyl,
(2) C2-iocycloheteroalkyl3 and
(3) phenyl,
wherein cycloalkyl, cycloheteroalkyl and phenyl are unsubstituted or
substituted with 1, 2, 3 or 4
substituents selected from Rb;
Z is selected from:
(1) oxo,
(2) ¨CF3,
(3) ¨Ci_6a1lcyl,
(4) ¨(CH2)t-halogen,
(5) ¨(CH2)nCO2H,
(6) --(CH2)n0H, and
(7) --(CH2)nSO2C1-6a1kyl,
wherein each CH2 is unsubstituted or substituted with 1 or 2 substituents
selected from C1-
6alkyl, -011 and -NH2, wherein each NH is unsubstituted or substituted with 1
substituent
selected from Re, and wherein each alkyl is unsubstituted or substituted with
1, 2, 3 or 4
substituents selected from Re;
R1 is independently selected from:
(1) -C4-1 oeyeloalkenyl,
(2) -phenyl,
(3) -phenyl-C3_7cycloalkyl,
(4) -phenyl-C2-7cycloheteroalkyl,
(5) -phenyl-phenyl,
(6) -phenyl-heteroaryl,
(7) -heteroaryl, and
- 47 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
(8) -C2_6alkyny1-pheny1,
wherein each alkynyl is unsubstituted or substituted with 1, 2 or 3
substituents selected from:
halogen, CF3, -OH, -NH2, -C1-6a1ky1, -0C1.6a1ky1, -NHCI .6alkyl, and -
N(C1_6alky1)2, and
wherein each cycloalkyl, cycloalkenyl, cycloheteroalkyl, phenyl and heteroaryl
is unsubstituted
or substituted with 1, 2, 3 or 4 substituents independently selected from Ra;
R2 is selected from: halogen;
R4 is hydrogen; and
R5 is hydrogen;
or a pharmaceutically acceptable salt thereof.
Another embodiment of the present invention relates to compounds of structural
formula
I wherein:
T is N;
U is -CR1-;
V is -CR2-;
W is -CR4-;
X is -0-;
Y is selected from:
(1) C3.7cyc1oa1kyl,
(2) C2-1ocycloheteroalkyl, and
(3) phenyl,
wherein each cycloalkyl, cycloheteroalkyl and phenyl is unsubstituted or
substituted with 1, 2, 3
or 4 substituents selected from Rb;
Z is selected from:
(1) -(CH2)nCO2H,
(2) -(CH2)t-ha1ogen, and
(3) --(CH2)n0H,
wherein each CH2 is unsubstituted or substituted with 1 or 2 substituents
selected from CI-
6alkyl, and -OH; or a pharmaceutically acceptable salt thereof.
R1 is independently selected from:
(1) -pheny1-C2-7cycloheteroa1ky1,
(2) phenyl-C2-11ocycloheteroalkenyl,
(3) -phenyl-phenyl, and
(4) phenyl-heteroaryl,
wherein each cycloheteroalkyl, cycloheteroalkenyl, heteroaryl and phenyl is
unsubstituted or
substituted with 1, 2, 3 or 4 substituents independently selected from Ra;
R2 is selected from halogen;
R4 is hydrogen; and
- 48-
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
R5 is hydrogen;
or a pharmaceutically acceptable salt thereof.
Another embodiment of the present invention relates to compounds of structural
formula
wherein:
T is N;
U is ¨CR1-;
V is -CR2-;
W is -CR4-;
X is -0-;
Y is selected from C2-10cycloheteroalkyl, wherein each cycloheteroalkyl is
unsubstituted or
substituted with 1, 2, 3 or 4 substituents selected from Rb;
Z is selected from: --(CH2)n0H;
R1 is independently selected from:
(1) -phenyl-C2-10cyc1oheteroa1keny1,
(2) biphenyl, and
(3) phenyl-heteroaryl,
wherein each cycloheteroalkenyl, phenyl, biphenyl and heteroaryl is
unsubstituted or substituted
with 1, 2, 3 or 4 substituents independently selected from Ra;
R2 is selected from halogen;
R4 is hydrogen; and
R5 is hydrogen;
or a pharmaceutically acceptable salt thereof.
Another embodiment of the present invention relates to compounds of structural
formula
I wherein:
T is N;
U is -CR1-;
V is -CR2-;
W is -CR4-;
X is -0-;
Y is selected from:
(1) C3..7cycloalkyl,
(2) C2-10cycloheteroalkyl, and
(3) phenyl,
wherein each cycloalkyl, cycloheteroalkyl and phenyl is unsubstituted or
substituted with 1, 2, 3
or 4 substituents selected from Rb;
Z is selected from:
(1) --(CH2)nCO2H, and
- 49 -
CA 02826649 2013 08 05
WO 2012/116145
PCT/US2012/026261
(2) ¨(CH2)n0H3
wherein each CH2 is unsubstituted or substituted with 1 or 2 substituents
selected from C1..
6alkyl, and -OH;
R1 is selected from:
(1) -pheny1-C2-7cyc1oheteroa1ky1, and
(2) ¨phenyl-phenyl,
wherein each cycloheteroalkyl and phenyl is unsubstituted or substituted with
1, 2, 3 or 4
substituents independently selected from Ra;
R2 is selected from: halogen;
R4 is hydrogen; and
R5 is hydrogen;
or a pharmaceutically acceptable salt thereof.
In another embodirnent of the present invention, the invention relates to
compounds of
structural formula Ia.:
R1
R2 N
(la)
R4 R5
=
or a pharmaceutically acceptable salt thereof.
In another embodiment of the present invention, the invention relates to
compounds of
structural formula lb:
R3
R2 N
(lb)
R4 R5 =
or a pharmaceutically acceptable salt thereof.
In another embodiment of the present invention, the invention relates to
compounds of
structural formula Ic:
- 50 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
R3
Ri
(k)
R4 R5 =
or a pharmaceutically acceptable salt thereof.
In another embodiment of the present invention, the invention relates to
compounds of
structural formula Id:
R3
R1
______________________________________________ X
(Id)
R5 =
or a pharmaceutically acceptable salt thereof.
The compound of structural formula I, includes the compounds of structural
formulas Ia,
lb, Ic and Id, and pharmaceutically acceptable salts, hydrates and solvates
thereof.
Illustrative, but non-limiting, examples of the compounds of the present
invention that are
useful as activators of AMP-protein kinase are the following compounds:
N
N
ci H
0
10 N N
0 OH
HO CI NH 0
- 51 -
CA 02826649 2013-08-05
WO 2012/116145 PCT/US2012/026261
NC 0
0 is
N...,.... N 01011
---...,
) N N 1 _,.., ) __ 0
.."--' N / N
CI
Cl H it 14 ii
=
0 0
HO HO
5.
0 /
/ 411
4111
0,, /
ip
N N Oil 01111
) __ 0
0 ?"---0F1
t Vi
CI ..."--. FIN ,,,/
11 N - k
,......
I )
OH ..---- N
Cl
0 H
1.1 HO
11411 HO
HO
14111 N
N õ...54-----OH
II N
N 0
1 0
-- N Cl -.."-' N>
CI H H
5 5
5
--- N
C. N
le N N NI OH
)-- 0 ....... 0
--- N * N N
0
Cl H
. 1
OH ../.
CI N
= ; H 5
jrzr. N
N \
OH
N 0
11' 411
so N N
N --
HZ" 0
NI 0
i s..
I 0
Ci
H CE H
5 5
- 52 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
HO
N
OH
0
0
N
and C1 =
and pharmaceutically acceptable salts thereof.
"Alkyl", as well as other groups having the prefix "alk", such as alkoxy,
alkanoyl, means
carbon chains of up to 10 carbons which may be linear or branched or
combinations thereof.
Examples of alkyl groups include methyl, ethyl, n-propyl, isopropyl, butyl,
isobutyl, sec- and tett-
butyl, pentyl, hexyl, heptyl, octyl, nonyl, and the like.
"Alkenyl" means carbon chains up to 10 carbons which contain at least one
carbon-
carbon double bond, and which may be linear or branched or combinations
thereof. Examples of
alkenyl include vinyl, ally', isopropenyl, pentenyl, hexenyl, heptenyl, 1-
propenyl, 2-butenyl, 2-
methyl-2-butenyl, and the like. In one embodiment of the present invention,
alkenyl is vinyl.
"Alkynyl" means carbon chains up to 10 carbons which contain at least one
carbon-
carbon triple bond, and which may be linear or branched or combinations
thereof. In one
embodiment, C2.5alkynyl means a carbon chain with 2 to 8 carbons that contains
one carbon-
carbon triple bond. Examples of alkynyl include ethynyl, propazgyl, 3-methyl-1-
pentynyl, 2-
heptynyl and the like. In one embodiment of the present invention, alkynyl is
ethynyl. In another
embodiment, alkynyl is propargyl.
"Cycloalkyl" means mono- or bicyclic or bridged saturated carbocyclic rings,
each having
from 3 to 14 carbon atoms. Examples of cycloalkyl include cyclopropyl,
cyclobutyl, cyclopentyl,
cyclohexyl, cycloheptyl, cyclooctyl, and decahydronaphthyl, and the like. In
one embodiment of
the present invention, cycloalkyl is selected from cyclopentyl and cyclohexyl.
In another
embodiment of the present invention, cycloalkyl is selected from cyclopropyl,
cyclopentyl, and
cyclohexyl.
"Cycloalkenyl" means nonarornatic, mono- or bicyclic or bridged carbocyclic
rings, each
having from 3 to 14 carbon atoms and containing at least one double bond.
Examples of
cycloalkyl include cyclopropenyl, cyclobutenyl, cyclopentenyl, cyclohexenyl,
cycloheptenyl,
cyclooxtenyl, decahydronaphthyl, bicyclo[2.2.1]hept-5-en-2-yl, and the like.
"Cyclobeteroalkyl" means nonaromatic, mono- or bicyclic or bridged saturated
carbocyclic rings, each having from 2 to 14 carbon atoms and containing 1, 2,
3, 4 or 5
heteroatoms selected from N, NH, 0 and S. In one embodiment,
C2.40cyc1oheteroalkyl means
non-aromatic, mono- or bicyclic or bridged saturated carbocyclic rings, having
from 2 to 10
carbon atoms and containing, 1, 2, 3, 4 or 5 heteroatorns selected from N, NH,
0 and S.
Examples of cycloheteroalkyl include tetrahydrofuranyl, azetidinyl,
perhydroazepinyl,
- 53 -
CA 02826649 2013 08 05
WO 2012/116145
PCT/US2012/026261
dihydrofaranyl, dioxanyl, oxanyl, morpholinyl, 1,4-dithianyl, piperazinyl,
piperidinyl, 1,3-
dioxolanyl, imidazolidinyl, imidazolinyl, pyrrolinyl, pyrrolidinyl, pyranyl,
tetrahydropyranyl,
dihydropyranyl, oxathiolanyl, dithiolanyl, 1,3-dithianyl, oxathianyl,
thiomorpholinyl,
dioxidoisothiazolidinyl, azacycloheptyl, diazobicyclo[3.2.1i-octane, and
hexahydroindazolyl.
The cycloheteroalkyl ring may be substituted on the ring carbons and/or the
ring nitrogens. In
one embodiment of the present invention, cycloheteroalkyl is selected from
piperidine,
pyrrolidine, oxazolidine, 1,3-oxazolidine-254-dione, thiazolidine, 1,3-
thiazolidine-2,4-dione,
imidazolidine, and hydantoin, and the like. In another embodiment of the
present invention
cycloheteroalkyl is selected from: morpholine, pyrrolidine, piperazine, and
piperldine. In another
embodiment of the present invention, cycloheteroalkyl is pyrrolidine.
In another embodiment, C2.10cyc1oheteroalky1 is a non-aromatic, bicyclic
saturated
carbocyclic ring having from 2 to 10 carbon atoms, and containing 1 or 2
heteroatonas selected
from O. In another embodiment of the present invention, cycloheteroalkyl is
dianhydro-
mannitol. In another embodiment of the present invention, cycloheteroalkyl is
1, 4:3, 6-
dianhydro-mamtitol. In another embodiment of the present invention,
cycloheteroalkyl is 1, 4:3,
6-clianhydro-D-marmitol. In another embodiment of the present invention,
cycloheteroalkyl is
hexahydrofuro[3,2-b]faran. In a class of this embodiment, cycloheteroalkyl is
2,3,3a,5,6,6a-
hexahydrofuro[3,2-b]furan.
"Cycloheteroalkenyl" means nonaromatic mono- or bicyclic or bridged rings each
having
from 2 to 14 carbon atoms containing at least one double bond and containing
1, 2, 3, 4 or 5
heteroatoms selected from N, NH, O and S. Examples of cycloheteroalkenyl
include 1,2,4-
oxadiazol-5-one, 1,2,4-thiadiazol-5-one, 1,2,4-triazol-3-one, and 1,2,3,6-
tetrahydropridine,
dihydro-1,3,4-oxadiazole, and [1,61-dihydropyridine and the like. In one
embodiment of the
present invention, cycloheteroalkenyl is dihydro-1,3,4-oxadiazole. In another
embodiment of the
present invention, cycloheteroalkenyl is [1,61-dihydropyridine.
In another embodiment, C2_10cycloheteroalkenyl is a non-aromatic, bicyclic
carbocyclic
ring having from 2 to 10 carbon atoms, and containing 1, 2 or 3 heteroatoms
selected from N,
and NH. In a class of this embodiment, cycloheteroalkenyl is
dihydropyrrolo[3,4-c]pyrazole. In
another class of this embodiment, cycloheteroalkenyl is 4,6-dihydropyrrolo[3,4-
clpyrazole.
In another embodiment, C2_6cycloheteroalkenyl is a non-aromatic, bicyclic
carbocyclic
ring having from 2 to 6 carbon atoms, and containing I or 2 heteroatorns
selected from N, and
NH. In a class of this embodiment, cycloheteroalkenyl is dihydroimidazole or
tetrahydropyrimidine. In another class of this embodiment, cycloheteroalkenyl
is 2,5 dihydro-
1H-imidazole or 1,4,5,6-tetrahydropyrimidine. In another class of this
embodiment,
cycloheteroalkenyl is dihydroimidazole. In another class of this embodiment,
cycloheteroalkenyl
is 2,5 dihydro-1H-imidazole. In another class of this embodiment,
cycloheteroalkenyl is
tetrahydropyrimidine. In another class of this embodiment, cycloheteroalkenyl
is 1,4,5,6-
- 54 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
tetrahydropyrimidine.
"Aryl" means a monocyclic, bicyclic or tricyclic ring system containing 5-14
carbon
atoms, wherein at least one of the rings is aromatic. Aryl thus includes ring
systems in which an
aromatic ring is fused to a non-aromatic ring, such as a cycloalkyl or
cycloalkenyl ring.
Examples of aryl include phenyl, naphthalene, biphenyl, indane and 5,6,7,8-
tetrahydronaphthalene, and the like. In one embodiment of the present
invention, aryl is phenyl,
naphthalene, biphenyl, indane, and 5,6,7,8-tetrahydronaphthalene. In another
embodiment of the
present invention, aryl is phenyl, naphthalene, indane and 5,6,7,8-
tetrahydronaphthalene. In one
class of this embodiment, aryl is phenyl and naphthalene. In another class of
this embodiment,
aryl is phenyl. In another class of this embodiment, aryl is naphthalene.
"Heteroaryl" means a monocyclic, bicyclic or tricyclic ring system containing
5-14
carbon atoms and containing 1, 2, 3, 4 or 5 heteroatoms selected from N, NH, O
and S wherein at
least one of the heteroatom containing rings is aromatic. Heteroaryl thus
includes ring systems in
which an aromatic heteroatom containing ring is fused to a non-aromatic ring,
such as a
cycloalkyl, cycloalkenyl, cyclohetemalkyl or cycloheteroalkenyl ring, and also
includes ring
systems in which an aryl ring is fused to a non-aromatic heteroatom containing
ring, such as
acycloheteroalkyl or cycloheteroalkenyl ring. Examples of heteroaryls include:
pyrazole,
pyridine, pyrazine, pyrimidine, thiazole, thiophene, benzoimidazole,
quinoline, isoquinoline,
indole, indazole, carbazole, benzotriazole, benzofuran, benzothiazole,
benzothiophene,
benzoisooxazole, oxazole, furan, benzoxazole, isoxazole, indoline,
isoindoline, tetrazole,
imidazole, oxadiazole, thiadiazole, triazole, benzothiazole, bemzopyrazole,
imidazopyridine,
benzodioxole, dihydropyridine, dihydropyrrolopyridine, dihydrobenzooxazine,
benzodioxole,
benzodioxine, pyrrolopyridine, triazolopyridine, dihydropyridooxazine,
dihydrobenzoxazine,
dihydroindole, dihydroisoindole, dihydrobenzoimidazole, dihydroquinoline,
tetrahydroisoquinoline, tetrahydrocyclopentaindole, tetrahydroquinoxaline, and
tetrahydropyridine. In one embodiment of the present invention, heteroaryl is
selected from:
imidazole, pyrazole, pyridine, pyrazine, pyrimidine, thiazole, thiophene,
benzonnidazole,
quinoline, isoquinoline, indole, indazole, carbazole, benzotriazole,
benzofuran, benzothiazole,
benzo[b]thiophene, benzo[d]isooxazole, 3,4-dihydro-2H-benzo[1,4]oxazine,
benzo[1,31dioxole,
benzo[l ,4]dioxine, 1H-pyrrolo[2,3-b]pyridine, 1,6-dihydro-pyridine,
[1,2,4]triazolo[4,3-
a]pyridine, 3,4 dihydropyrido [3,2-b][1,41oxazine, 3,4-dihydro-2H-1,4-
benzoxazine, 2,3-dihydro-
1H-indole, 2,3-dihydro-1H-isoindole, 2,3-dihydrobenzoimidazole, 1,2-
dihydroquinoline, 1,2,3,4-
tetrahydroisoquinoline, 1,2,3,4-tetrahydrocyclopenta[b]indole, 1,2,3,4-
tetrahydroquinoxaline, and
1,2,3,6-tetrahydropyridine. In another embodiment of the present invention,
heteroaryl is
tetrazole. In another embodiment, heteroaryl is selected from: pyrazole,
pyridine, pyrimidine,
isoxazole, imidazole, oxazole, triazole, tetrazole, oxadiazole, thiazole,
thiadiazole, and
benzoxazole. In another embodiment of this invention, heteroaryl is tetrazole.
-55-
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
"Halogen" includes fluorine, chlorine, bromine and iodine. In one embodiment
of the
present invention, halogen is selected from fluorine, chlorine, and bromine.
In another
embodiment of the present invention, halogen is selected from fluorine, and
chlorine. In another
embodiment of the present invention, halogen is fluorine. In another
embodiment of the present
invention, halogen is chlorine.
When any variable (e.g., R1, Ra, etc.) occurs more than one time in any
constituent or in
formula I, its definition on each occurrence is independent of its definition
at every other
occurrence. Also, combinations of substituents and/or variables are
permissible only if such
combinations result in stable compounds. A squiggly line across a bond in a
substituent variable
represents the point of attachment.
Under standard nomenclature used throughout this disclosure, the terminal
portion of the
designated side chain is described first, followed by the adjacent
functionality toward the point of
attachment. For example, a Ci _5 alkylcarbonylamino C1-6 alkyl substituent is
equivalent to:
o
Ci_5a1kyl -
In choosing compounds of the present invention, one of ordinary skill in the
art will
recognize that the various substituents, i.e. R1, R2, etc., are to be chosen
in conformity with well-
known principles of chemical structure connectivity and stability.
The term "substituted" shall be deemed to include multiple degrees of
substitution by a
named substitutent. Where multiple substituent moieties are disclosed or
claimed, the
substituted compound can be independently substituted by one or more of the
disclosed or
claimed substituent moieties, singly or plurally. By independently
substituted, it is meant that the
(two or more) substituents can be the same or different.
Compounds of Formula I may contain one or more asymmetric centers and can thus
occur
as racemates and racemic mixtures, single enantiomers, diastereomerie mixtures
and individual
diastereomers. The present invention is meant to comprehend all such isomeric
forms of the
compounds of Formula 1.
Some of the compounds described herein contain olefinic double bonds, and
unless
specified otherwise, are meant to include both E and Z geometric isomers.
Tautomers are defined as compounds that undergo rapid proton shifts from one
atom of
the compound to another atom of the compound. Some of the compounds described
herein may
exist as tautomers with different points of attachment of hydrogen. Such an
example may be a
ketone and its enol form known as keto-enol tautomers. The individual
tautomers as well as
mixture thereof are encompassed with compounds of Formula I.
In the compounds of general formula I, the atoms may exhibit their natural
isotopic
abundances, or one or more of the atoms may be artificially enriched in a
particular isotope
having the same atomic number, but an atomic mass or mass number different
from the atomic
- 56-
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
mass or mass number predominately found in nature. The present invention is
meant to include
all suitable isotopic variations of the compounds of structural formula I. For
example, different
isotopic forms of hydrogen (H) include protium (III) and deuterium (2H).
Protium is the
predominant hydrogen isotope found in nature. Enriching for deuterium may
afford certain
therapeutic advantages, such as increasing in vivo half-life or reducing
dosage requirements, or
may provide a compound useful as a standard for characterization of biological
samples.
Isotopically-enriched compounds within structural formula I, can be prepared
without undue
experimentation by conventional techniques well known to those skilled in the
art or by
processes analogous to those described in the Schemes and Examples herein
using appropriate
isotopically-enriched reagents and/or intermediates.
Compounds of the Formula I may be separated into diastereoisomeric pairs of
enantiomers by, for example, fractional crystallization from a suitable
solvent, for example
MeOH or ethyl acetate or a mixture thereof. The pair of enantiomers thus
obtained may be
separated into individual stereoisomers by conventional means, for example by
the use of an
optically active amine as a resolving agent or on a chiral HPLC column.
Alternatively, any enantiomer of a compound of the general Formula I may be
obtained
by stereospeeitic synthesis using optically pure starting materials or
reagents of known
configuration.
Furthermore, some of the crystalline forms for compounds of the present
invention
may exist as polyrnorphs and as such are intended to be included in the
present invention. In
addition, some of the compounds of the instant invention may form solvates
with water or
common organic solvents. Such solvates are encompassed within the scope of
this invention.
It is generally preferable to administer compounds of the present invention as
enantiomerically pure formulations. Racemic mixtures can be separated into
their individual
enantiomers by any of a number of conventional methods. These include chiral
chromatography,
derivatization with a chiral auxiliary followed by separation by
chromatography or
crystallization, and fractional crystallization of diastereomeric salts.
The term "pharmaceutically acceptable salts" refers to salts prepared from
pharmaceutically acceptable non-toxic bases or acids including inorganic or
organic bases and
inorganic or organic acids. Salts derived from inorganic bases include
aluminum, ammonium,
calcium, copper, ferric, ferrous, lithium, magnesium, manganic salts,
manganous, potassium,
sodium, zinc, and the like. Particularly preferred are the ammonium, calcium,
magnesium,
potassium, and siadiurn salts. Salts derived from pharmaceutically acceptable
organic non-toxic
bases include salts of primary, secondary, and tertiary amines, substituted
amines including
naturally occurring substituted amines, cyclic amines, and basic ion exchange
resins, such as
arginine, betaine, caffeine, choline, N,N'-dibenzylethylenediarnine,
diethylatnine, 2-
diethylaminoethanol, 2-dimethylaminoethanol, ethanolamine, ethylenediamine, N-
ethyl-
- 57 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
morpholine, N-ethylpiperidine, glucamine, glueosamine, histidine, hydrabamine,
isopropylamine,
lysine, methylglucarnine, morpholine, piperazine, piperidine, polyarnine
resins, procaine, purines,
theobromine, triethylamine, trimethylamine, tripropylamine, tromethamine, and
the like. The
term "pharmaceutically acceptable salt" further includes all acceptable salts
such as acetate,
trifluoroacetate, lactobionate, benzenesulfonate, laurate, benzoate, malate,
bicarbonate, maleate,
bisulfate, mandelate, bitartrate, mesylate, borate, rnethylbromide, bromide,
methylnitrate,
calcium edetate, methylsulfate, camsylate, mucate, carbonate, napsylate,
chloride, nitrate,
clavulanate, N-methylglucamine, citrate, ammonium salt, dihydrochloride,
oleate, edetate,
oxalate, edisylate, pamoate (embonate), estolate, palmitate, esylate,
pantothenate, fumarate,
phosphate/diphospinate, gluceptate, polygalacturonate, gluconate, salicylate,
glutamate, stearate,
glycollylarsanilate, sulfate, hexylresorcinate, subacetate, hydrabamine,
succinate, hydrobromide,
tannate, hydrochloride, tartrate, hydroxynaphthoate, teoclate, iodide,
tosylate, isothionate,
triethiodide, lactate, panoate, valerate, and the like which can be used as a
dosage form for
modifying the solubility or hydrolysis characteristics or can be used in
sustained release or pro-
drug formulations.
It will be understood that, as used herein, references to the compounds of
Formula I are
meant to also include the pharmaceutically acceptable salts.
Compounds of the present invention are activators of the AMP-activated protein
kinase.
The methods of treatment of this invention comprises a method of activating
AMPK-activated
protein kinase and treating AMPK-activated protein kinase mediated diseases by
administering to
a patient in need of such treatment a non-toxic therapeutically effective
amount of a compound of
this invention that activate AMPK-activated protein kinase.
AMP-activated protein kinase (AMPK) is a heterotrimeric enzyme composed of a
catalytic a subunit and regulatory p and 7 subunits. There are two genes
encoding isoforms of
both the a and 13 subunits (al, a2, 131 and 132) and three genes encoding
isoforms of the y subunit
(y1 , y2 and y3) leading to 12 possible heterotrimeric combinations. The a2
isoform is
predominately found in skeletal and cardiac muscle AMPK; both the al and ot2
isoforms are
found in hepatic AMPK; while in pancreatic islet t3-cells the al isoform AMPK
predominates.
In particular, the compounds of structural formula I are activators of at
least one heterotrimeric
isoform of AMP-activated protein kinase.
An "activator" is a compound that either increases the activity
(phosphorylation of
downstream substrates) of fully phosphorylated AMPK or that increases the
phosphorylation of
AMPK.
The compounds of the present invention are efficacious in the treatment and
prevention
of diseases, disorders and conditions responsive to the activation of AMP-
activated protein
kinase, including but not limited to: type 2 diabetes, insulin resistance,
hyperglycemia, obesity,
hyperinsulinemia, glucose intolerance, atherosclerosis, Metabolic Syndrome,
hypertension, high
- 58 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
hepatic glucose output, high blood glucose concentrations, nonalcoholic
steatohepatitis,
protection against ischemia and reperfitsion damage, and lipid disorders, such
as dyslipidernia,
elevated levels of plasma triglycerides, elevated levels of free fatty acids,
elevated levels of
cholesterol, high levels of low density lipoprotein (LDL) and low levels of
high density
lipoprotein (HDL). The compounds are also useful for the treatment of cancer,
hypoxia and
glucocorticoid-induced apoptosis.
One or more of the following diseases may be treated by the administration of
a
therapeutically effective amount of a compound of Formula 1, or a
pharmaceutically acceptable
salt thereof, to a patient in need of treatment: (1) non-insulin dependent
diabetes mellitus (Type
2 diabetes); (2) hyperglycemia; (3) Metabolic Syndrome; (4) obesity; (5)
hypercholesterolemia; (6) hypertriglyceridemia (elevated levels of
triglyceride-rich-
lipoproteins); (7) mixed or diabetic dyslipidemia; (8) low HDL cholesterol;
(9) high LDL
cholesterol; (10) atherosclerosis; and (11) hypertension.
Also, the compounds of Formula 1 may be used for the manufacture of a
medicament for
treating one or more of the above diseases.
One embodiment of the uses of the compounds is directed to the treatment of
one or more
of the following diseases by administering a therapeutically effective amount
to a patient in need
of treatment: (1) Type 2 diabetes; (2) hyperglycemia; (3) Metabolic Syndrome;
(4) obesity; (5)
hypercholesterolemia; and (6) hypertension.
The compounds may also be used for manufacturing a medicament for use in the
treatment of one or more of the above diseases.
The compounds are expected to be effective in lowering glucose and lipids in
diabetic
patients and in non-diabetic patients who have impaired glucose tolerance
and/or are in a pre-
diabetic condition. The compounds may ameliorate hyperinsulinernia, which
often occurs in
diabetic or pre-diabetic patients, by modulating the swings in the level of
serum glucose that
often occurs in these patients. The compounds may also be effective in
treating or reducing
insulin resistance. The compounds may be effective in treating or preventing
gestational
diabetes.
The compounds, compositions, methods and medicaments as described herein may
also
be effective in reducing the risks of adverse sequelae associated with
metabolic syndrome, and in
reducing the risk of developing atherosclerosis, delaying the onset of
atherosclerosis, and/or
reducing the risk of sequelae of atherosclerosis. Sequelae of atherosclerosis
include angina,
claudication, heart attack, stroke, and others. By keeping hyperglycemia under
control, the
compounds may also be effective in delaying or preventing vascular restenosis
and diabetic
retinopathy.
The compounds of this invention may also have utility in improving or
restoring 13-ce11
function, so that they may be useful in treating type I diabetes or in
delaying or preventing a
- 59 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
patient with Type 2 diabetes from needing insulin therapy.
Other possible outcomes of treatment with the compounds of the present
invention
include, but are not limited to: 1) a decrease in fatty acid synthesis; 2) an
increase in fatty acid
oxidation and ketogenesis; 3) a decrease in cholesterol synthesis,
lipogenesis, and triglyceride
synthesis; 4) a decrease in blood glucose levels and concentration; 5) an
improvement in
glucose homeostasis; 6) a normalization of glucose metabolism; 7) a decrease
in blood pressure;
8) an increase in HDL; 9) a decrease in plasma triglycerides; 10) a decrease
in free fatty acids;
11) a decrease in hepatic glucose output; 12) an improvement in insulin
action; 13) a decrease
in blood pressure; 14) an improvement in insulin sensitivity; 15) a
suppression of hepatic glucose
output; 15) an inhibition of de novo lipogenesis; 16) stimulation of muscle
glucose uptake; 17)
modulation of insulin secretion by pancreatic 13 cells; and 16) a decrease in
body weight.
The compounds generally may be efficacious in treating one or more of the
following
diseases: (1) Type 2 diabetes (also known as non-insulin dependent diabetes
mellitus, or
NIDDM), (2) hyperglycemia, (3) impaired glucose tolerance, (4) insulin
resistance, (5) obesity,
(6) lipid disorders, (7) dyslipidemia, (8) hyperlipidemia, (9)
hypertriglyceridemia, (10)
hypercholesterolemia, (11) low HDL levels, (12) high LDL levels, (13)
atherosclerosis and its
sequelae, (14) vascular restenosis, (15) abdominal obesity, (16) retinopathy,
(17) metabolic
syndrome, (18) high blood pressure (hypertension), and (19) insulin
resistance.
One aspect of the invention provides a method for the treatment and control of
mixed or
diabetic dyslipidemia, hypercholesterolemia, atherosclerosis, low HDL levels,
high LDL levels,
hyperlipidemia, and/or hypertriglyceridemia, which comprises administering to
a patient in need
of such treatment a therapeutically effective amount of a compound having
fommla L The
compound may be used alone or advantageously may be administered with a
cholesterol
biosynthesis inhibitor, particularly an HMG-CoA reductase inhibitor such as
lovastatin,
simvastatin, rosuvastatin, pravastatin, fluvastatin, atorvastatin, rivastatin,
itavastatin, or ZD-4522.
The compound may also be used advantageously in combination with other lipid
lowering drugs
such as cholesterol absorption inhibitors (for example stanol esters, sterol
glycosides such as
tiqueside, and azetidinones such as ezetimibe), ACAT inhibitors (such as
avasimibe), CETP
inhibitors (for example anamtrapib, torcetrapib, and those described in
published applications
W02005/100298, W02006/014413, and W02006/014357), niacin and niacin receptor
agonists,
bile acid sequestrants, microsomal triglyceride transport inhibitors, and bile
acid reuptake
inhibitors. These combination treatments may be effective for the treatment or
control of one or
more related conditions selected from the group consisting of
hypercholesterolemia,
atherosclerosis, hyperlipidemia, hypertriglyceridemia, dyslipidemia, high LDL,
and low HDL.
The present invention also relates to methods and medicaments for the
treatment, control,
or prevention of Type 2 diabetes by administering the compounds and
pharmaceutical
compositions of the present invention. The present invention also relates to
methods and
- 60 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
medicaments for the treatment, control, or prevention of Type 2 diabetes by
administering the
compounds of the present invention in combination with a therapeutically
effective amount of
another agent known to be useful to treat the condition. The present invention
also relates to
methods and medicaments for the treatment, control, or prevention of diabetes
related disorders
by administering the compounds and pharmaceutical compositions of the present
invention
alone, or in combination. The present invention also relates to methods and
medicaments for the
treatment and prevention of diabetes in pre-diabetic subject by administering
the compounds and
pharmaceutical compositions of the present invention alone, or in combination.
The present invention also relates to methods and medicaments for the
treatment, control,
or prevention of obesity by administering the compounds and pharmaceutical
compositions of
the present invention. The present invention also relates to methods and
medicaments for the
treatment, control, or prevention of obesity by administering the compounds of
the present
invention in combination with a therapeutically effective amount of another
agent known to be
useful to treat the condition. The present invention also relates to methods
and medicaments for
the treatment, control, or prevention of obesity related disorders by
administering the compounds
and pharmaceutical compositions of the present invention alone, or in
combination. The present
invention also relates to methods and medicaments for the treatment and
prevention of obesity in
overweight subject by administering the compounds and pharmaceutical
compositions of the
present invention alone, or in combination. The compounds are also useful for
the treatment of
obesity related disorders, or eating disorders associated with excessive food
intake, and
complications associated therewith, including left ventricular hypertrophy, as
well as treating or
preventing obesity in other mammalian species, including canines and felines.
The present invention also relates to methods and medicaments for the
treatment, control,
or prevention of hyperglycemia by administering the compounds and
pharmaceutical
compositions of the present invention. The present invention also relates to
methods and
medicaments for the treatment, control, or prevention of hyperglycemia by
administering the
compounds of the present invention in combination with a therapeutically
effective amount of
another agent known to be useful to treat the condition.
The present invention also relates to methods and medicaments for the
treatment, control,
3 0 or prevention of insulin resistance by administering the compounds and
pharmaceutical
compositions of the present invention. The present invention also relates to
methods and
medicaments for the treatment, control, or prevention of insulin resistance by
administering the
compounds of the present invention in combination with a therapeutically
effective amount of
another agent known to be useful to treat the condition.
The present invention also relates to methods and medicaments for the
treatment, control,
or prevention of lipid disorders by administering the compounds and
pharmaceutical
compositions of the present invention. The present invention also relates to
methods and
- 61 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
medicaments for the treatment, control, or prevention of lipid disorders by
administering the
compounds of the present invention in combination with a therapeutically
effective amount of
another agent known to be useful to treat the condition. The present invention
also relates to
methods and medicaments for the treatment, control, or prevention of
dyslipidemia related
disorders and lipid disorder-related disorders by administering the compounds
and
pharmaceutical compositions of the present invention alone, or in combination.
The present invention also relates to methods and medicaments for the
treatment, control,
or prevention of atherosclerosis by administering the compounds and
pharmaceutical
compositions of the present invention. The present invention also relates to
methods and
medicaments for the treatment, control, or prevention of atherosclerosis by
administering the
compounds of the present invention in combination with a therapeutically
effective amount of
another agent known to be useful to treat the condition. The present invention
also relates to
methods and medicaments for the treatment, control, or prevention of
atherosclerosis related
disorders by administering the compounds and pharmaceutical compositions of
the present
invention alone, or in combination.
The present invention also relates to methods and medicaments for the
treatment, control,
or prevention of hypertension by administering the compounds and
pharmaceutical compositions
of the present invention. The present invention also relates to methods and
medicaments for the
treatment, control, or prevention of hypertension by administering the
compounds of the present
invention in combination with a therapeutically effective amount of another
agent known to be
useful to treat the condition. The present invention also relates to methods
and medicaments for
the treatment, control, or prevention of hypertension related disorders by
administering the
compounds and pharmaceutical compositions of the present invention alone, or
in combination.
The present invention also relates to methods and medicaments for the
treatment and prevention
of hypertension in pre-hypertensive subject by administering the compounds and
pharmaceutical
compositions of the present invention alone, or in combination.
The present invention also relates to methods and medicaments for the
treatment, control,
or prevention of Metabolic Syndrome by administering the compounds and
pharmaceutical
compositions of the present irwention. The present invention also relates to
methods and
medicaments for treating Metabolic Syndrome by administering the compounds of
the present
invention in combination with a therapeutically effective amount of another
agent known to be
useful to treat the condition.
The compounds of the present invention wherein at least one of T, U, V and W
is N or N-
oxide have the unexpected benefit of increased potency in enzyme activation
assays using
recombinant human AMPK complex (see Biological Example 1) compared to
compounds
wherein T is CR3, U is CR1-, V is CR2 and W is CR4.
Additionally, the compounds of the present invention wherein at least one of
T, U, V and
- 62 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
W is N or N-oxide have the unexpected benefit of reduced binding to human
plasma proteins
compared to compounds wherein T is CR3, U is CR1-, V is CR2 and W is CR4.
Pharmacological
activity in vivo is associated with the concentration of drug unbound to
plasma proteins. Plasma
proteins, by virtue of their high concentration, control the concentration of
drug unbound to
plasma proteins in plasma and in compartments in equilibrium with plasma,
thereby, effectively
attenuating drug potency in vivo (See Trainor, G.L. (2007), Expert Opin. Drug
Discov. 2(1), 51-
64). A higher concentration of drug unbound to plasma proteins results in an
increase in
pharmacological activity in vivo. Due to their increased potency and their
higher unbound
fraction in plasma, the compounds of the present invention are expected to
exhibit glucose
lowering efficacy at reduced plasma exposures.
The term "diabetes," as used herein, includes both insulin-dependent diabetes
mellitus
(i.e., IDDM, also known as type 1 diabetes) and non-insulin-dependent diabetes
mellitus (i.e.,
NIDDM, also known as Type 2 diabetes). Type 1 diabetes, or insulin-dependent
diabetes, is the
result of an absolute deficiency of insulin, the hormone which regulates
glucose utilization. Type
2 diabetes, or insulin-independent diabetes (i.e., non-insulin-dependent
diabetes mellitus), often
occurs in the face of normal, or even elevated levels of insulin and appears
to be the result of the
inability of tissues to respond appropriately to insulin. Most of the Type 2
diabetics are also
obese. The compositions of the present invention are useful for treating both
Type 1 and Type 2
diabetes. The term "diabetes associated with obesity" refers to diabetes
caused by obesity or
resulting from obesity. The compositions are especially effective for treating
Type 2 diabetes.
The compositions of the present invention are also useful for treating and/or
preventing
gestational diabetes mellitus.
Diabetes is characterized by a fasting plasma glucose level of greater than or
equal to 126
mg/d1. A diabetic subject has a fasting plasma glucose level of greater than
or equal to 126
mg/d1. A pre diabetic subject is someone suffering from prediabetes.
Prediabetes is
characterized by an impaired fasting plasma glucose (PPG) level of greater
than or equal to 110
mg/di and less than 126 mg/di; or impaired glucose tolerance; or insulin
resistance. A prediabetic
subject is a subject with impaired fasting glucose (a fasting plasma glucose
(FPG) level of greater
than or equal to 110 mg/d1 and less than 126 mg/di); or impaired glucose
tolerance (a 2 hour
plasma glucose level of >140 mg/d1 and <200 mg/di); or insulin resistance,
resulting in an
increased risk of developing diabetes.
Treatment of diabetes mellitus refers to the administration of a compound or
combination
of the present invention to treat a diabetic subject. One outcome of treatment
may be decreasing
the glucose level in a subject with elevated glucose levels. Another outcome
of treatment may be
decreasing insulin levels in a subject with elevated insulin levels. Another
outcome of treatment
may be decreasing plasma triglycerides in a subject with elevated plasma
triglycerides. Another
outcome of treatment is decreasing LDL cholesterol in a subject with high LDL
cholesterol
- 63 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
levels. Another outcome of treatment may be increasing HDL cholesterol in a
subject with low
HDL cholesterol levels. Another outcome of treatment is increasing insulin
sensivity. Another
outcome of treatment may be enhancing glucose tolerance in a subject with
glucose intolerance.
Yet another outcome of treatment may be decreasing insulin resistance in a
subject with
increased insulin resistance or elevated levels of insulin. Prevention of
diabetes mellitus, in
particular diabetes associated with obesity, refers to the administration of a
compound or
combination of the present invention to prevent the onset of diabetes in a
subject in need thereof.
A subject in need of preventing diabetes is a prediabetic subject that is
overweight or obese.
The term "diabetes related disorders" should be understood to mean disorders
that are
associated with, caused by, or result from diabetes. Examples of diabetes
related disorders
include retinal damage, kidney disease, and nerve damage.
The term "atherosclerosis" as used herein encompasses vascular diseases and
conditions
that are recognized and understood by physicians practicing in the relevant
fields of medicine.
Atherosclerotic cardiovascular disease, coronary heart disease (also known as
coronary artery
disease or ischernic heart disease), cerebrovascular disease and peripheral
vessel disease are all
clinical manifestations of atherosclerosis and are therefore encompassed by
the terms
"atherosclerosis" and "atherosclerotic disease" The combination comprised of a
therapeutically
effective amount of an anti-obesity agent in combination with a
therapeutically effective amount
of an anti-hypertensive agent may be administered to prevent or reduce the
risk of occurrence, or
recurrence where the potential exists, of a coronary heart disease event, a
cerebrovascular event,
or intermittent claudication. Coronary heart disease events are intended to
include CHI) death,
myocardial infarction (i.e., a heart attack), and coronary revascularization
procedures.
Cerebrovascular events are intended to include ischernic or hemorrhagic stroke
(also known as
cerebrovascular accidents) and transient ischemic attacks, Intermittent
claudication is a clinical
manifestation of peripheral vessel disease. The term "atherosclerotic disease
event" as used
herein is intended to encompass coronary heart disease events, cerebrovascular
events, and
intermittent claudication. It is intended that persons who have previously
experienced one or
more non-fatal atherosclerotic disease events are those for whom the potential
for recurrence of
such an event exists, The term "atherosclerosis related disorders" should be
understood to mean
disorders associated with, caused by, or resulting from atherosclerosis.
The term "hypertension" as used herein includes essential, or primary,
hypertension
wherein the cause is not known or where hypertension is due to greater than
one cause, such as
changes in both the heart and blood vessels; and secondary hypertension
wherein the cause is
known. Causes of secondary hypertension include, but are not limited to
obesity; kidney disease;
hormonal disorders; use of certain drugs, such as oral contraceptives,
corticosteroids,
cyclosporin, and the like. The term "hypertension" encompasses high blood
pressure, in which
both the systolic and diastolic pressure levels are elevated (?_140 minflg/>90
mmHg), and
- 64 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
isolated systolic hypertension, in which only the systolic pressure is
elevated to greater than or
equal to 140 mm Hg, while the diastolic pressure is less than 90 mm Hg. Normal
blood pressure
may be defined as less than 120 mmHg systolic and less than 80 mmHg diastolic.
A hypertensive
subject is a subject with hypertension. A pre-hypertensive subject is a
subject with a blood
pressure that is between 120 mmHg over 80 mmHg and 139 mmHg over 89 mmHg. One
outcome of treatment is decreasing blood pressure in a subject with high blood
pressure.
Treatment of hypertension refers to the administration of the compounds and
combinations of the
present invention to treat hypertension in a hypertensive subject. Treatment
of hypertension-
related disorder refers to the administration of a compound or combination of
the present
invention to treat the hypertension-related disorder. Prevention of
hypertension, or a
hypertension related disorder, refers to the administration of the
combinations of the present
invention to a pre-hypertensive subject to prevent the onset of hypertension
or a hypertension
related disorder. The hypertension-related disorders herein are associated
with, caused by, or
result from hypertension. Examples of hypertension-related disorders include,
but are not limited
to: heart disease, heart failure, heart attack, kidney failure, and stroke.
Dyslipidemias and lipid disorders are disorders of lipid metabolism including
various
conditions characterized by abnormal concentrations of one or more lipids
(i.e. cholesterol and
triglycerides), and/or apolipoproteins (i.e., apolipoproteins A, B, C and E),
and/or lipoproteins
(i.e., the macromolecular complexes formed by the lipid and the apolipoprotein
that allow lipids
to circulate in blood, such as LDL, VLDL and IDL). Hyperlipidemia is
associated with
abnormally high levels of lipids, LDL and VLDL cholesterol, and/or
triglycerides. Treatment of
dyslipidemia refers to the administration of the combinations of the present
invention to a
dyslipidemic subject. Prevention of dyslipidemia refers to the administration
of the
combinations of the present invention to a pre-dyslipidemic subject. A pre-
dyslipidemic subject
is a subject with higher than normal lipid levels, that is not yet
dyslipidemic.
The terms "dyslipidemia related disorders" and "lipid disorder related
disorders" should
be understood to mean disorders associated with, caused by, or resulting from
dyslipidemia or
lipid disorders. Examples of dylipidemia related disorder and lipid disorder
related disorders
include, but are not limited to: hyperlipidemia, hypertriglyceridemia,
hypercholesterolemia, low
high density lipoprotein (HDL) levels, high plasma low density lipoprotein
(LDL) levels,
atherosclerosis and its sequelae, coronary artery or carotid artery disease,
heart attack, and stroke.
The term "obesity" as used herein is a condition in which there is an excess
of body fat.
The operational definition of obesity is based on the Body Mass Index (BMI),
which is calculated
as body weight per height in meters squared (kg/m2). "Obesity" refers to a
condition whereby an
otherwise healthy subject has a Body Mass Index (BMI) greater than or equal to
30 kg/m2, or a
condition whereby a subject with at least one co-morbidity has a BMI greater
than or equal to 27
kg/m2. An "obese subject" is an otherwise healthy subject with a Body Mass
Index (BMI)
- 65 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
greater than or equal to 30 kg/m2 or a subject with at least one co-morbidity
with a BMI greater
than or equal to 27 kg/m2. An overweight subject is a subject at risk of
obesity. A "subject at
risk of obesity" is an otherwise healthy subject with a BMI of 25 kg/m2 to
less than 30 kg/m2 or
a subject with at least one co-morbidity with a BMI of 25 kg/m2 to less than
27 kg/m2.
The increased risks associated with obesity occur at a lower Body Mass Index
(BMI) in
Asians. In Asian countries, including Japan, "obesity" refers to a condition
whereby a subject
with at least one obesity-induced or obesity-related co-morbidity, that
requires weight reduction
or that would be improved by weight reduction, has a BMI greater than or equal
to 25 kg/m2. In
Asian countries, including Japan, an "obese subject" refers to a subject with
at least one obesity-
induced or obesity-related co-morbidity that requires weight reduction or that
would be improved
by weight reduction, with a BMI greater than or equal to 25 kg/m2. In Asia-
Pacific, a "subject at
risk of obesity" is a subject with a BMI of greater than 23 kg/m2 to less than
25 kg/m2.
As used herein, the term "obesity" is meant to encompass all of the above
definitions of
obesity.
Obesity-induced or obesity-related co-morbidities include, but are not limited
to, diabetes
mellitus, non-insulin dependent diabetes mellitus - type 2, diabetes
associated with obesity,
impaired glucose tolerance, impaired fasting glucose, insulin resistance
syndrome, dyslipidemia,
hypertension, hypertension associated with obesity, hyperuricacidemia, gout,
coronary artery
disease, myocardial infarction, angina pectoris, sleep apnea syndrome,
Pickwickian syndrome,
fatty liver; cerebral infarction, cerebral thrombosis, transient ischemic
attack, orthopedic
disorders, arthritis deformans, 1-umbodynia, emmeniopathy, and infertility. In
particular, co-
morbidities include: hypertension, hyperlipidemia, dyslipidemia, glucose
intolerance,
cardiovascular disease, sleep apnea, and other obesity-related conditions.
Treatment of obesity and obesity-related disorders refers to the
administration of the
compounds of the present invention to reduce or maintain the body weight of an
obese subject.
One outcome of treatment may be reducing the body weight of an obese subject
relative to that
subject's body weight immediately before the administration of the compounds
of the present
invention. Another outcome of treatment may be preventing body weight regain
of body weight
previously lost as a result of diet, exercise, or pharmacotherapy. Another
outcome of treatment
may be decreasing the occurrence of and/or the severity of obesity-related
diseases. The
treatment may suitably result in a reduction in food or calorie intake by the
subject, including a
reduction in total food intake, or a reduction of intake of specific
components of the diet such as
carbohydrates or fats; and/or the inhibition of nutrient absorption; and/or
the inhibition of the
reduction of metabolic rate; and in weight reduction in patients in need
thereof. The treatment
may also result in an alteration of metabolic rate, such as an increase in
metabolic rate, rather
than or in addition to an inhibition of the reduction of metabolic rate;
and/or in minimization of
the metabolic resistance that normally results from weight loss.
- 66 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
Prevention of obesity and obesity-related disorders refers to the
administration of the
compounds of the present invention to reduce or maintain the body weight of a
subject at risk of
obesity. One outcome of prevention may be reducing the body weight of a
subject at risk of
obesity relative to that subject's body weight immediately before the
administration of the
compounds of the present invention. Another outcome of prevention may be
preventing body
weight regain of body weight previously lost as a result of diet, exercise, or
pharmacotherapy.
Another outcome of prevention may be preventing obesity from occurring if the
treatment is
administered prior to the onset of obesity in a subject at risk of obesity.
Another outcome of
prevention may be decreasing the occurrence and/or severity of obesity-related
disorders if the
treatment is administered prior to the onset of obesity in a subject at risk
of obesity. Moreover, if
treatment is commenced in already obese subjects, such treatment may prevent
the occurrence,
progression or severity of obesity-related disorders, such as, but not limited
to, arteriosclerosis,
Type 11 diabetes, polycystie ovarian disease, cardiovascular diseases,
osteoarthritis,
dermatological disorders, hypertension, insulin resistance,
hypercholesterolemia,
hypertriglyceridemia, and cholelithiasis.
The obesity-related disorders herein are associated with, caused by, or result
from obesity.
Examples of obesity-related disorders include overeating and bulimia,
hypertension, diabetes,
elevated plasma insulin concentrations and insulin resistance, dyslipidemias,
hyperlipidemia,
endometrial, breast, prostate and colon cancer, osteoarthritis, obstructive
sleep apnea,
cholelithiasis, gallstones, heart disease, abnormal heart rhythms and
anythmias, myocardial
infarction, congestive heart failure, coronary heart disease, sudden death,
stroke, polycystic
ovarian disease, craniopharyngioma, the Prader-Willi Syndrome, Frohlich's
syndrome, GH-
deficient subjects, normal variant short stature, Turner's syndrome, and other
pathological
conditions showing reduced metabolic activity or a decrease in resting energy
expenditure as a
percentage of total fat-free mass, e.g, children with acute lymphoblastic
leukemia. Further
examples of obesity-related disorders are metabolic syndrome, also known as
syndrome X,
insulin resistance syndrome, sexual and reproductive dysfunction, such as
infertility,
hypogonadism in males and hirsutism in females, gastrointestinal motility
disorders, such as
obesity-related gastro-esophageal reflux, respiratory disorders, such as
obesity-hypoventilation
syndrome (Pickwickian syndrome), cardiovascular disorders, inflammation, such
as systemic
inflammation of the vasculature, arteriosclerosis, hypercholesterolemia,
hyperuricaemia, lower
back pain, gallbladder disease, gout, and kidney cancer. The compounds of the
present invention
are also useful for reducing the risk of secondary outcomes of obesity, such
as reducing the risk
of left ventricular hypertrophy.
The compounds of formula 1 are also useful for treating or preventing obesity
and
obesity-related disorders in eats and dogs. As such, the term "mammal"
includes companion
animals such as cats and dogs,
- 67 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
The term "metabolic syndrome", also known as syndrome X, is defined in the
Third
Report of the National Cholesterol Education Program Expert Panel on
Detection, Evaluation
and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III,
or ATP III),
National Institutes of Health, 2001, NIH Publication No. 01-3670. E.S. Ford et
al., JAMA, vol.
287 (3), Jan. 16, 2002, pp 356-359. Briefly, a person is defined as having
metabolic syndrome if
the person has three or more of the following disorders: abdominal obesity,
hypertriglyceridemia,
low HDL cholesterol, high blood pressure, and high fasting plasma glucose. The
criteria for
these are defined in ATP-III. Treatment of metabolic syndrome refers to the
administration of the
combinations of the present invention to a subject with metabolic syndrome.
Prevention of
metabolic syndrome refers to the administration of the combinations of the
present invention to a
subject with two of the disorders that define metabolic syndrome. A subject
with two of the
disorders that define metabolic syndrome is a subject that has developed two
of the disorders that
define metabolic syndrome, but has not yet developed three or more of the
disorders that define
metabolic syndrome.
Left ventricular hypertrohpy (LVH) is identified based on left ventricular
mass index
(LVMI) and relative wall thickness (RWT). Left ventricular mass index is
defined as left
ventricular mass in grams divided by body surface area in meters2. Relative
wall thickness is
defined as 2 x posterior wall thickness/left ventricular end diastolic
diameter. Normal LVMI
values are typically 85 and normal RWT approximately 0.36. A male subject with
LVH has a
LVMI greater than 131 g/m2; a female subject with LVH has a LVMI greater than
100 g/m2. A
subject with an elevated LVMI value is a male subject with a LVMI between 85
g/m2 and 131
g/m2, or a female subject with a LVMI between 85 g/m2 and 100 g/m2.
Treatment of cardiac hypertrophy, or left ventricular hypertrophy, refers to
the
administration of the combinations of the present invention to a subject with
cardiac hypertrophy
or left ventricular hypertrophy. Prevention of cardiac hypertrophy, or left
ventricular
hypertrophy, refers to the administration of the combinations of the present
invention to decrease
or maintain the LVMI in a subject with an elevated LVMI value or to prevent
the increase of
LVMI in a subject with a normal LVMI value.
One outcome of treatment of cardiac hypertrophy or left ventricular
hypertrophy may be a
decrease in ventricular mass. Another outcome of treatment of cardiac
hypertrophy or left
ventricular hypertrophy may be a decrease in the rate of increase of
ventricular mass. Another
outcome of treatment of cardiac hypertrophy or left ventricular hypertrophy
may be a decrease in
ventricular wall thickness. Another outcome of treatment of cardiac
hypertrophy of left
ventricular hypertrophy may be the decrease in the rate of increase in
ventricular wall thickness.
The terms "administration of and or "administering a" compound should be
understood
to mean providing a compound of the invention or a prodrug of a compound of
the invention to
the individual or mammal in need of treatment.
- 68 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
The administration of the compound of structural formula I in order to
practice the
present methods of therapy is carried out by administering an effective amount
of the compound
of structural formula I to the mammal in need of such treatment or
prophylaxis. The need for a
prophylactic administration according to the methods of the present invention
is determined via
the use of well known risk factors. The effective amount of an individual
compound is
determined, in the final analysis, by the physician or veterinarian in charge
of the case, but
depends on factors such as the exact disease to be treated, the severity of
the disease and other
diseases or conditions from which the patient suffers, the chosen route of
administration other
drugs and treatments which the patient may concomitantly require, and other
factors in the
physician's judgment.
The usefulness of the present compounds in these diseases or disorders may be
demonstrated in animal disease models that have been reported in the
literature.
The magnitude of prophylactic or therapeutic dose of a compound of Formula I
will, of
course, vary with the nature of the severity of the condition to be treated
and with the particular
compound of Formula I and its route of administration. It will also vary
according to the age,
weight and response of the individual patient. In general, the daily dose
range lie within the
range of from about 0.001 mg to about 100 mg per kg body weight of a mammal,
preferably 0.01
mg to about 50 mg per kg, and most preferably 0.1 to 10 mg per kg, in single
or divided doses.
On the other hand, it may be necessary to use dosages outside these limits in
some cases.
For use where a composition for intravenous administration is employed, a
suitable
dosage range is from about 0.001 mg to about 100 mg in one embodiment from
about 0.01 mg to
about 50 mg, and in another embodiment from 0.1 mg to 10 mg of a compound of
Formula I per
kg of body weight per day.
In the case where an oral composition is employed, a suitable dosage range is,
e.g. from
about 0.01 mg to about 1000 mg of a compound of Formula I per day. In one
embodiment, the
range is from about 0.1 mg to about 10 mg per day. For oral administration,
the compositions are
preferably provided in the form of tablets containing from 0.01 to 1,000 rng,
preferably 0.01,
0.05, 0.1, 0.5, 1, 2, 2.5, 3, 4, 5, 6, 7, 8, 9, 10, 12, 12.5, 15, 20, 25, 30,
40, 50, 100, 250, 500, 750
or 1000 milligrams of the active ingredient for the symptomatic adjustment of
the dosage to the
patient to be treated.
Another aspect of the present invention provides pharmaceutical compositions
which
comprises a compound of Formula I and a pharmaceutically acceptable carrier.
The term
"composition", as in pharmaceutical composition, is intended to encompass a
product comprising
the active ingredient(s), and the inert ingredient(s) (pharmaceutically
acceptable excipients) that
make up the carrier, as well as any product which results, directly or
indirectly, from
combination, cornplexation or aggregation of any two or more of the
ingredients, or from
dissociation of one or more of the ingredients, or from other types of
reactions or interactions of
- 69 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
one or more of the ingredients. Accordingly, the pharmaceutical compositions
of the present
invention encompass any composition made by admixing a compound of Formula 1,
additional
active ingredient(s), and pharmaceutically acceptable excipients.
Any suitable route of administration may be employed for provioling a mammal,
particularly a human or a companion animal such as a dog or cat, with an
effective dosage of a
compound of the present invention. For example, oral, rectal, topical,
parenteral, ocular,
pulmonary, and nasal routes of administration, and the like may be employed.
Dosage forms
include tablets, troches, dispersions, suspensions, solutions, capsules,
creams, ointments,
aerosols, and the like.
The pharmaceutical compositions of the present invention comprise a compound
of
Formula I as an active ingredient or a pharmaceutically acceptable salt
thereof, and may also
contain a pharmaceutically acceptable carrier and optionally other therapeutic
ingredients. By
"pharmaceutically acceptable" it is meant the carrier, diluent or excipient
must be compatible
with the other ingredients of the formulation and not deleterious to the
recipient thereof. The
compositions include compositions suitable for oral, rectal, topical,
parenteral (including
subcutaneous, intramuscular, and intravenous), ocular (ophthalmic), pulmonary
(aerosol
inhalation), or nasal administration, although the most suitable route in any
given case will
depend on the nature and severity of the conditions being treated and on the
nature of the active
ingredient. They may be conveniently presented in unit dosage form and
prepared by any of the
methods well-known in the art of pharmacy.
For administration by inhalation, the compounds of the present invention are
conveniently delivered in the form of an aerosol spray presentation from
pressurized packs or
nebulizers, or as powders which may be formulated and the powder composition
may be inhaled
with the aid of an insufflation powder inhaler device. The preferred delivery
systems for
inhalation are metered close inhalation (MDI) aerosol, which may be formulated
as a suspension
or solution of a compound of Formula I in suitable propellants, such as
fluorocarbons or
hydrocarbons and dry powder inhalation (DPI) aerosol, which may be formulated
as a dry
powder of a compound of Formula I with or without additional excipients.
Suitable topical formulations of a compound of formula I include transdermal
devices,
aerosols, creams, solutions, ointments, gels, lotions, dusting powders, and
the like. The topical
pharmaceutical compositions containing the compounds of the present invention
ordinarily
include about 0,005% to 5% by weight of the active compound in admixture with
a
pharmaceutically acceptable vehicle. Transdermal skin patches useful for
administering the
compounds of the present invention include those known to those of ordinary
skill in that art.
In practical use, the compounds of Formula I can be combined as the active
ingredient in
intimate admixture with a pharmaceutical carrier according to conventional
pharmaceutical
compounding techniques. The carrier may take a wide variety of forms depending
on the form of
- 70 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
preparation desired for administration, e.g., oral or parenteral (including
intravenous). In
preparing the compositions for oral dosage form, any of the usual
pharmaceutical media may be
employed, such as, for example, water, glycols, oils, alcohols, flavoring
agents, preservatives,
coloring agents and the like in the case of oral Liquid preparations, such as,
for example,
suspensions, elixirs and solutions; or carriers such as starches, sugars,
microcrystalline cellulose,
diluents, granulating agents, lubricants, binders, disintegrating agents and
the like in the case of
oral solid preparations such as, for example, powders, capsules and tablets,
with the solicioral
preparations being preferred over the liquid preparations. Because of their
ease of
administration, tablets and capsules represent the most advantageous oral
dosage unit form in
which case solid pharmaceutical carriers are obviously employed. If desired,
tablets may be
coated by standard aqueous or nonaqueous techniques.
In addition to the common dosage forms set out above, the compounds of Formula
I may
also be administered by controlled release means and/or delivery devices such
as those described
in U.S. Patent Nos. 3,845,770; 3,916,899; 3,536,809; 3,598,123; 3,630,200 and
4,008,719.
Pharmaceutical compositions of the present invention suitable for oral
administration
may be presented as discrete units such as capsules (including timed release
and sustained release
formulations), pills, cachets, powders, granules or tablets each containing a
predetermined
amount of the active ingredient, as a powder or granules or as a solution or a
suspension in an
aqueous liquid, a non-aqueous liquid, an oil-in-water emulsion or a water-in-
oil liquid emulsion,
including elixirs, tinctures, solutions, suspensions, syrups and emulsions.
Such compositions
may be prepared by any of the methods of pharmacy but all methods include the
step of bringing
into association the active ingredient with the carrier which constitutes one
or more necessary
ingredients. In general, the compositions are prepared by uniformly and
intimately admixing the
active ingredient with liquid carriers or finely divided solid carriers or
both, and then, if
necessary, shaping the product into the desired presentation. For example, a
tablet may be
prepared by compression or molding, optionally with one or more accessory
ingredients.
Compressed tablets may be prepared by compressing in a suitable machine, the
active ingredient
in a free-flowing form such as powder or granules, optionally mixed with a
binder, lubricant,
inert diluent, surface active or dispersing agent. Molded tablets may be made
by molding in a
suitable machine, a mixture of the powdered compound moistened with an inert
liquid diluent.
Desirably, each tablet cachet or capsule contains from about 0.01 to 1,000 mg,
particularly 0.01,
0.05, 0.1, 0.5, 1.0, 2, 2.5, 3, 4, 5, 6, 7, 8, 9, 10, 12, 15, 25, 30, 40, 50,
75, 100, 125, 150, 175,
180, 200, 225, 250, 500, 750 and 1,000 milligrams of the active ingredient for
the symptomatic
adjustment of the dosage to the patient to be treated.
Additional suitable means of administration of the compounds of the present
invention
include injection, intravenous bolus or infusion, intraperitoneal,
subcutaneous, intramuscular,
intranasal, and topical, with or without occlusion.
-71-
CA 02826649 2015-01-29
Exemplifying the invention is a pharmaceutical composition comprising any of
the
compounds described above and a pharmaceutically acceptable carrier. Also
exemplifying the
invention is a pharmaceutical composition made by combining any of the
compounds described
above and a pharmaceutically acceptable carrier. An illustration of the
invention is a process for
making a pharmaceutical composition comprising combining any of the compounds
described
above and a pharmaceutically acceptable carrier.
The dose may be administered in a single daily dose or the total daily dosage
may be
administered in divided doses of two, three or four times daily. Furthermore,
based on the
properties of the individual compound selected for administration, the dose
may be administered
less frequently, e.g., weekly, twice weekly, monthly, etc. The unit dosage
will, of course, be
correspondingly larger for the less frequent administration.
When administered via intranasal routes, transdermal routes, by rectal or
vaginal
suppositories, or through a continual intravenous solution, the dosage
administration will, of
course, be continuous rather than intermittent throughout the dosage regimen.
The following are examples of representative pharmaceutical dosage forms for
the
compounds of Formula I:
Injectable Suspension (I.M.) mg/mL Tablet
mg/tablet
Compound of Formula I 10 Compound of Formula I 25
Methylcellulose 5.0 Microcrystalline Cellulose 415
Tween 8OTM 0.5 Povidone 14.0
Benzyl alcohol 9.0 Pregelatinized Starch 43.5
Benzalkonium chloride 1.0 Magnesium Stearate 2.5
Water for injection to a total volume of 1 mL 500
Capsule mg/capsule Aerosol Per canister
Compound of Formula I 25 Compound of Formula I 24 mg
Lactose Powder 573.5 Lecithin, NF Liq. Conc. 1.2 mg
Magnesium Stearate 1.5 Trichlorofluoromethane, NF 4.025 g
600 Dichlorodifluoromethane, NF 12.15 g
Compounds of Formula I may be used in combination with other drugs that are
used in the
treatment/prevention/suppression or amelioration of the diseases, disorders or
conditions for
which compounds of Formula I are useful. Such other drugs may be administered,
by a route and
in an amount commonly used therefor, contemporaneously or sequentially with a
compound of
Formula I. When a compound of Formula I is used contemporaneously with one or
more other
drugs, a pharmaceutical composition containing such other drugs in addition to
the compound of
Formula I is preferred. Accordingly, the pharmaceutical compositions of the
present invention
include those that also contain one or more other active ingredients, in
addition to a compound of
- 72 -
CA 02826649 2013-08-05
WO 2012/116145 PCT/US2012/026261
Formula I. Examples of other active ingredients that may be combined with a
compound of
Formula I include, but are not limited to: other anti-diabetic agents, anti-
dylipidemic agents, and
anti-hypertensive agents, anti-obesity agents, and anorectic agents, which may
be administered
separately or in the same pharmaceutical compositions,
The present invention also provides a method for the treatment or prevention
of an
AMPK-activated protein kinase (AMPK) mediated disease, which method comprises
administration to a patient in need of such treatment or at risk of developing
an AMPK mediated
disease of an amount of an AMPK activator and an amount of one or more active
ingredients,
such that together they give effective relief.
In a further aspect of the present invention, there is provided a
pharmaceutical
composition comprising an AMPK activator and one or more active ingredients,
together with at
least one pharmaceutically acceptable carrier or excipient.
Thus, according to a further aspect of the present invention there is provided
the use of an
AMPK activator and one or more active ingredients for the manufacture of a
medicament for the
treatment or prevention of an AMPK mediated disease. In a further or
alternative aspect of the
present invention, there is therefore provided a product comprising an AMPK
activator and one
or more active ingredients as a combined preparation for simultaneous,
separate or sequential use
in the treatment or prevention of an AMPK mediated disease. Such a combined
preparation may
be, for example, in the form of a twin pack.
It will be appreciated that for the treatment or prevention of diabetes,
obesity,
hypertension, Metabolic Syndrome, dyslipidemia, cancer, atherosclerosis, and
related disorders
thereof, a compound of the present invention may be used in conjunction with
another
pharmaceutical agent effective to treat that disorder.
The present invention also provides a method for the treatment or prevention
of diabetes,
obesity, hypertension, Metabolic Syndrome, dyslipidemia, cancer,
atherosclerosis, and related
disorders thereof, which method comprises administration to a patient in need
of such treatment
an amount of a compound of the present invention and an amount of another
pharmaceutical
agent effective to threat that disorder, such that together they give
effective relief.
The present invention also provides a method for the treatment or prevention
of diabetes,
obesity, hypertension, Metabolic Syndrome, dyslipidemia, cancer,
atherosclerosis, and related
disorders thereof, which method comprises administration to a patient in need
of such treatment
an amount of a compound of the present invention and an amount of another
pharmaceutical
agent useful in treating that particular condition, such that together they
give effective relief.
Suitable pharmaceutical agents of use in combination with a compound of the
present
invention, include, but are not limited to:
(a) anti-diabetic agents such as (1) PPARy agonists such as glitazones (e.g.
ciglitazone;
darg1ita7one; englita7one; isaglita7one (MCC-555); pioglitazone (ACTOS);
rosiglitazone
-73-
CA 02826649 2013-08-05
WO 2012/116145 PCT/US2012/026261
(AVAND1A); troglitazone; rivoglitazone, BRL49653; CLX-0921; 5-BTZD, 13W-0207,
LG-
100641, R483, and LY-300512, and the like and compounds disclosed in
W097/10813,
97/27857, 97/28115, 97/28137, 97/27847, 03/000685, and 03/027112 and SPPARMS
(selective
PPAR gamma modulators) such as T131 (Amgen), FK614 (Fujisawa), netoglitazone,
and
metaglidasen; (2) biguanides such as buformin; metformin; and phenfonnin, and
the like; (3)
protein tyrosine phosphatase-1B (PTP-1B) inhibitors such as ISIS 113715, A-
401674, A-
364504, IDD-3, IDD 2846, KP-40046, KR61639, MC52445, MC52453, C7, 0C-0600628
OC-
86839, 0C29796, TTP-277BC1, and those agents disclosed in WO 04/041799,
04/050646,
02/26707, 02/26743, 04/092146, 03/048140, 04/089918, 03/002569, 04/065387,
04/127570, and
US 2004/167183; (4) sulfonylureas such as acetohexamide; chlorpropamide;
diabinese;
glibenclanaide; glipizide; glyburide; glimepiride; gliclazide; glipentide;
gliquidone; glisolamide;
tolazamide; and tolbutamide, and the like; (5) meglitinides such as
repaglinide, metiglinide
(GLUFAST) and nateglinide, and the like; (6) alpha glucoside hydrolase
inhibitors such as
acarbose; adiposine; camiglibose; emiglitate; rniglitol; voglibose; pradimicin-
Q; salbostatin;
CKD-711; MDL-25,637; MDL-73,945; and MOR 14, and the like; (7) alpha-amylase
inhibitors
such as tendamistat, trestatin, and A1-3688, and the like; (8) insulin
secreatagogues such as
linogliride nateglinide, mitiglinide (GLUFAST), ID1101 A-4166, and the like;
(9) fatty acid
oxidation inhibitors, such as clomoxir, and etornoxir, and the like; (10) A2
antagonists, such as
inidaglizole; isaglidole; deriglidole; idazoxan; earoxan; and fluparoxan, and
the like; (11) insulin
or insulin mimetics, such as biota, LP-100, novarapid, insulin detemir,
insulin lispro, insulin
glargine, insulin zinc suspension (lente and ultralente); Lys-Pro insulin, GLP-
1 (17-36), GLP-1
(73-7) (insulintropin); GLP-1 (7-36)-NH2) exenatide/Exendin-4, Exenatide LAR,
Linaglutide,
AVE0010, CJC 1131, BIM51077, CS 872, TH0318, BAY-694326, GP010, ALBUGON (GLP-1
fused to albumin), HGX-007 (Epac agonist), S-2352I, and compounds disclosed in
WO
04/022004, WO 04/37859, and the like; (12) non-thiazolidinediones such as JT-
501, and
farglitazar (GW-2570/GI-262579), and the like; (13) PPARaly dual agonists such
as AVE 0847,
CLX-0940, GW-1536, GW1929, GW-2433, KRP-297, L-796449, LBM 642, LR-90,
LY510919,
MK-0767, ONO 5129, SB 219994, TAK-559, TAK-654, 677954 (GlaxoSmithkline), E-
3030
(Eisai), LY510929 (Lilly), AK109 (Asahi), DRF2655 (Dr. Reddy), DRF8351 (Dr.
Reddy),
MC3002 (Maxocore), TY51501 (ToaEiyo), farglitazar, naveglitazar, muraglitazar,
peliglitazar,
tesaglitamr (GALIDA), reg1ita7ar (JT-501), chiglitazar, and those disclosed in
WO 99/16758,
WO 99/19313, WO 99/20614, WO 99/38850, WO 00/23415, WO 00/23417, WO 00/23445,
WO
00/50414, WO 01/00579, WO 01/79150, WO 02/062799, WO 03/033481, WO 03/033450,
WO
03/033453; and (14), insulin, insulin mimetics and other insulin sensitizing
drugs; (15) VPAC2
receptor agonists; (16) GLK modulators, such as PSN105, RO 281675, RO 274375
and those
disclosed in WO 03/015774, WO 03/000262, WO 03/055482, WO 04/046139, WO
04/045614,
WO 04/063179, WO 04/063194, WO 04/050645, and the like; (17) retinoid
modulators such as
- 74 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
those disclosed in WO 03/000249; (18) GSK 3beta/GSK 3 inhibitors such as 44242-
bromopheny1)-4-(4-fluoropheny1-1H-irnidazol-5-yllpyridine, CT21022, CT20026,
CT-98023,
SB-216763, SB410111, SB-675236, CP-70949, XD4241 and those compounds disclosed
in WO
03/037869, 03/03877, 03/037891, 03/024447, 05/000192, 05/019218 and the like;
(19) glycogen
phosphorylase (HGLPa) inhibitors, such as AVE 5688, PSN 357, GPi-879, those
disclosed in
WO 03/037864, WO 03/091213, WO 04/092158, WO 05/013975, WO 05/013981, US
2004/0220229, and JP 2004-196702, and the like; (20) ATP consumption promotors
such as
those disclosed in WO 03/007990; (21) fixed combinations of PPAR 7 agonists
and metformin
such as AVANDAMET; (22) PPAR pan agonists such as GSK 677954; (23) GPR40 (G-
protein
coupled receptor 40) also called SNORF 55 such as BG 700, and those disclosed
in WO
04/041266, 04/022551, 03/099793; (24) GPR119 (G-protein coupled receptor 119,
also called
RUP3; SNORF 25) such as RUP3, HGPRBMY26, PFI 007, SNORF 25; (25) adenosine
receptor
28 antagonists such as ATL-618, AT1-802, E3080, and the like; (26) camitine
palmitoyl
transferase inhibitors such as ST 1327, and ST 1326, and the like; (27)
Fructose 1,6-
bisphospohatase inhibitors such as CS-917, MB7803, and the like; (28) glucagon
antagonists
such as AT77077, BAY 694326, GW 4123X, NN2501, and those disclosed in WO
03/064404,
WO 05/00781, US 2004/0209928, US 2004/029943, and the like; (30) glucose-6-
phosphase
inhibitors; (31) phosphoenolpyruvate carboxykinase (PEPCK) inhibitors; (32)
pymvate
dehydrogenase kinase (PDK) activators; (33) RXR agonists such as MC1036,
CS000185 JN.1
10166806, and those disclosed in WO 04/089916, US 6759546, and the like; (34)
SGLT
inhibitors such as AVE 2268, KGT 1251, T1095/RWT 394718; (35) BLX-1002; (36)
alpha
glucosidase inhibitors; (37) glucagon receptor agonists; (38) glucokinase
activators; 39) G1P-1;
40) insulin secretagogues; 41) GPR-40 agonists, such as TAK-875, 544-[[(1R)-
446-(3-hydroxy-3-
methylbutoxy)-2-methylpyridine-3-y1]-2,3-dihydro-1H-indene-1-
ylioxylphenyllisothiazo1e-3-o1 1-oxide,
5-(443-(2,6-dimethy1-4-(3-
(methylsulfonyl)propoxy)phenyl)phenyl)methoxy)phenypiso, 5-(4-((3-(2-
methy1-6-(3-hydroxypropoxy)pyridine-3-y1)-2-
methy1pheny1)mettioxy)pheny1)isothiazo1e-3-ol 1-oxide,
and 5-[4-[[344-(3-antinopropoxy)-2,6-
diniethylplienyl]plienylimethoxy]phenyllisothiazole-3-ol 1-oxide),
and those disclosed in WO 11/078371.
(b) anti-dyslipidemic agents such as (1) bile acid sequestrants such as,
cholestyramine,
colesevelem, colestipol, dialkylaminoalkyl derivatives of a cross-linked
dextran; Colestidg;
LoCholeste; and Questran , and the like; (2) HMG-CoA reductase inhibitors such
as
atorvastatin, itavastatin, pitavastatin, fluvastatin, lovastatin, pravastatin,
rivastatin, simvastatin,
rosuvastatin (Z1)-4522), and other statins, particularly simvastatin; (3) HMG-
CoA synthase
inhibitors; (4) cholesterol absorption inhibitors such as FMVP4 (Forbes Medi-
Tech), KT6-971
(Kotobuki Pharmaceutical), FM-VA12 (Forbes Medi-Tech), FM-VP-24 (Forbes Medi-
Tech),
stanol esters, beta-sitosterol, sterol glycosides such as tiqueside; and
azetidinones such as
ezetimibe, and those disclosed in WO 04/005247 and the like; (5) acyl coenzyme
A -cholesterol
-75-
CA 02826649 2013-08-05
WO 2012/116145 PCT/US2012/026261
acyl transferase (ACAT) inhibitors such as avasimibe, eflucimibe, pactimibe
(KY505), SMP 797
(Sumitomo), SM32504 (Sumitomo), and those disclosed in WO 03/091216, and the
like; (6)
CETP inhibitors such as anacetrapib, JTT 705 (Japan Tobacco), torcetrapib, CP
532,632,
BAY63-2149 (Bayer), SC 591, SC 795, and the like; (7) squalene synthetase
inhibitors; (8) anti-
oxidants such as probucol, and the like; (9) PPARa agonists such as
beclofibrate, bezafibrate,
ciprofibrate, clofibrate, etofibrate, fenofibrate, gemcabene, and gemfibrozil,
GW 7647, BM
170744 (Kowa), LY518674 (Lilly), GW590735 (GlaxoSmithkline), KRP-101 (Kyorin),
DRF10945 (Dr. Reddy), NS-220/R1593 (Nippon Shinyaku/Roche, ST1929 (Sigma Tau)
MC3001/MC3004 (MaxoCore Pharmaceuticals, gemcabene calcium, other fibric acid
derivatives, such as Atromid , Lopid and Tricor , and those disclosed in US
6,548,538, and
the like; (10) FXR receptor modulators such as GW 4064 (GlaxoSmithkline), SR
103912,
QRX401, LN-6691 (Lion Bioscience), and those disclosed in WO 02/064125, WO
04/045511,
and the like; (11) LXR receptor modulators such as GW 3965 (GlaxoSmithkline),
T9013137,
and XTC0179628 (X-Ceptor Therapeutics/Sanyo), and those disclosed in WO
03/031408, WO
03/063796, WO 04/072041, and the like; (12) lipoprotein synthesis inhibitors
such as niacin;
(13) renin angiotensin system inhibitors; (14) PPAR 8 partial agonists, such
as those disclosed in
WO 03/024395; (15) bile acid reabsorption inhibitors, such as BARI 1453,
SC435, PHA384640,
S8921, AZD7706, and the like; and bile acid sequesterants such as colesevelam
(WELCHOL/
CHOLESTAGEL), colestipol, cholestyramine, and dialkylarninoalkyl derivatives
of a cross-
linked dextran, (16) PPAR 8 agonists such as GW 501516 (Ligand, GSK), GW
590735, GW-
0742 (GlaxoSmithkline), T659 (Amgen/Tularik), LY934 (Lilly), NNC610050 (Novo
Nordisk)
and those disclosed in W097/28149, WO 01/79197, WO 02/14291, WO 02/46154, WO
02/46176, WO 02/076957, WO 03/016291, WO 03/033493, WO 03/035603, WO
03/072100,
WO 03/097607, WO 04/005253, WO 04/007439, and JP10237049, and the like; (17)
triglyceride
synthesis inhibitors; (18) rnicrosomal triglyceride transport (MTTP)
inhibitors, such as
implitapide, LAB687, JTT130 (Japan Tobacco), CP346086, and those disclosed in
WO
03/072532, and the like; (19) transcription modulators; (20) squalene
epoxidase inhibitors; (21)
low density lipoprotein (LDL) receptor inducers; (22) platelet aggregation
inhibitors; (23) 5-LO
or FLAP inhibitors; and (24) niacin receptor agonists including HM74A receptor
agonists; (25)
PPAR modulators such as those disclosed in WO 01/25181, WO 01/79150, WO
02/79162, WO
02/081428, WO 03/016265, WO 03/033453; (26) niacin-bound chromium., as
disclosed in WO
03/039535; (27) substituted acid derivatives disclosed in WO 03/040114; (28)
infused HDL such
as LUV/ETC-588 (Pfizer), APO-A1 Milano/ETC216 (Pfizer), ETC-642 (Pfizer),
ISIS301012,
D4F (Bruin Pharma), synthetic ttimeric ApoAl, Bioral Apo Al targeted to foam
cells, and the
like; (29) IBAT inhibitors such as BARI143/HMR145A/ HMR1453 (Sanofi-Aventis,
PHA384640E (Pfizer), S8921 (Shionogi) AZD7806 (AstrZeneca), AK105 (Asah
Kasei), and the
like; (30) Lp-PLA2 inhibitors such as SB480848 (GlaxoSmithkline), 659032
(GlaxoSmithkline),
- 76 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
677116 (GlaxoSmithkline), and the like; (31) other agents which affect lipic
composition
including ETC1001/ESP31015 (Pfizer), ESP-550I6 (Pfizer), AG11067
(AtheroGenics), AC3056
(Anaylin), AZD4619 (AstrZeneca); and
(c) anti-hypertensive agents such as (1) diuretics, such as
thiazides, including
chlorthalidone, chlorthiazide, dichlorophenamide, hydroflumethiazide,
indapamide, and
hydrochlorothiazide; loop diuretics, such as burnetanide, ethaciynic acid,
furosemide, and
torsemide; potassium sparing agents, such as amiloride, and triamterene; and
aldosterone
antagonists, such as spironolactone, epirenone, and the like; (2) beta-
adrenergic Mockers such as
acebutolol, atenolol, betaxolol, bevantolol, bisoprolol, bopindolol,
carteolol, carvedilol,
celiprolol, esmolol, indenolol, metaprolol, nadolol, nebivolol, penbutolol,
pindolol, propanolol,
sotalol, tertatolol, tilisolol, and timolol, and the like; (3) calcium channel
blockers such as
amlodipine, aranidipine, azelnidipine, barnidipine, benidipine, bepridil,
cinaldipine, clevidipine,
diltiazem, efonidipine, felodipine, galloparnil, isradipine, lacidipine,
lemildipine, lercanidipine,
nicardipine, nifedipine, nilvadipine, nimodepine, nisoldipine, nitrendipine,
manidipine,
pranidipine, and veraparnil, and the like; (4) angiotensin converting enzyme
(ACE) inhibitors
such as benazepril; captopril; cilazapril; delapril; enalapril; fosinopril;
imidapril; losinopril;
moexipril; quinapril; quinaprilat; ramipril; perindopril; perindropril;
quanipril; spirapril;
tenocapril; trandolapril, and zofenopril, and the like; (5) neutral
endopeptidase inhibitors such as
omapatrilat, cadoxatril and ecadotril, fosidotril, sampatrilat, AVE7688,
ER4030, and the like; (6)
endothelin antagonists such as tezosentan, A308165, and YM62899, and the like;
(7)
vasodilators such as hydralazine, clonidine, minoxidil, and nicotinyl alcohol,
nicotinic acid or
salt thereof, and the like; (8) angiotensin 11 receptor antagonists such as
candesartan, eprosartan,
irbesartan, losartan, pratosartan, tasosartan, telmisartan, valsartan, and EXP-
3137, FI6828K, and
RNH6270, and the like; (9) el adrenergie blockers as nipradilol, arotinolol
and amosulalol, and
the like; (10) alpha 1 blockers, such as terazosin, urapidil, prazosin,
Immazosin, trimazosin,
doxazosin, naftopidil, indorarnin, WHIP 164, and XEN010, and the like; ((11)
alpha 2 agonists
such as lofexidine, tiamenidine, moxonidine, rilmenidine and guanobenz, and
the like; (12)
aldosterone inhibitors, and the like; (13) angiopoietin-2-binding agents such
as those disclosed in
WO 03/030833; and
(d) anti-obesity agents, such as (1) 5HT (serotonin) transporter
inhibitors, such as
paroxetine, fluoxetine, fenfluramine, fluvoxamine, sertraline, and imipramine,
and those
disclosed in WO 03/00663, as well as serotonin/noradrenaline re uptake
inhibitors such as
sibutrarnine (MERID(1A/REDUCTIL) and dopamine uptake inhibitor/Norepenephrine
uptake
inhibitors such as radafaxine hydrochloride, 353162 (GlaxoSmithicline), and
the like; (2) NE
(norepinephrine) transporter inhibitors, such as GW 320659, despiramine,
talsupram, and
nomifensine; (3) CB1 (cannabinoid-1 receptor) antagonist/inverse agonists,
such as taranabant,
rimonabant (ACCOMPLIA Sanofi Synthelabo), SR-147778 (Sanofi Synthelabo),
AVE1625
- 77 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
(Sanofi-Aventis), BAY 65-2520 (Bayer), SLV 319 (Solvay), SLV326 (Solvay),
CP945598
(Pfizer), E-6776 (Esteve), 01691 (Organix), ORG14481 (Organon), VER24343
(Vernalis),
NE5S0327 (Univ of SassarifUniv of Cagliari), and those disclosed in US Patent
Nos. 4,9735587,
5,013,837, 5,081,122, 5,112,820, 5,292,736, 5,532,237, 5,624,941, 6,028,084,
and 6,509367; and
WO 96/33159, W097/29079, W098/31227, WO 98/33765, W098/37061, W098/41519,
W098/43635, W098/43636, W099/02499, W000/10967, W000/10968, WO 01/09120, WO
01/58869, WO 01/64632, WO 01/64633, WO 01/64634, WO 01/70700, WO 01/96330, WO
02/076949, WO 03/006007, WO 03/007887, WO 03/020217, WO 03/026647, WO
03/026648,
WO 03/027069, WO 03/027076, WO 03/027114, WO 03/037332, WO 03/040107, WO
04/096763, WO 04/111039, WO 04/111033, WO 04/111034, WO 04/111038, WO
04/013120,
WO 05/000301, WO 05/016286, WO 05/066126 and EP-658546 and the like; (4)
ghrelin
agonists/antagonists, such as BVT81-97 (BioVitrum), RC1291 (Rejuvenon), SRD-
04677
(Sumitomo), unacylated ghrelin (TheraTechnologies), and those disclosed in WO
01/87335, WO
02/08250, WO 05/012331, and the like; (5) H3 (histamine H3) antagonist/inverse
agonists, such
as thioperamide, 3-(1H-imidazol-4-yl)propyl N-(4-pentenyl)carbarnate),
clobenpropit,
iodophenpropit, imoproxifan, GT2394 (Gliatech), and A331440, and those
disclosed in WO
02/15905; and 043-(1H-imidazo1-4-y1)propano1learbamates (Kiec-Kononowicz, K.
et al.,
Pharmazie, 55:349-55 (2000)), piperidine-containing histamine H3-receptor
antagonists
(Lazewska, D. et al., Pharmazie, 56:927-32 (2001), benzophenone derivatives
and related
compounds (Sasse, A. et al., Arch. Pharm.(Weinheim) 334;45-52 (2001)),
substituted N-
phenylcarbamates (Reidemeister, S. et al., Pharmazie, 55:83-6 (2000)), and
proxifan derivatives
(Sasse, A. et al., J. Med, Chem.. 43:3335-43 (2000)) and histamine H3 receptor
modulators such
as those disclosed in WO 03/024928 and WO 03/024929; (6) melanin-concentrating
hormone 1
receptor (MCH1R) antagonists, such as T-226296 (Takeda), T71 (Takeda/Arngen),
AMGN-
608450, AMGN-503796 (Amgen), 856464 (GlaxoSmithkline), A224940 (Abbott), A798
(Abbott), ATC0175/AR224349 (Arena Pharmaceuticals), GW803430 (GlaxoSmithkine),
NBI-
1A (Neurocrine Biosciences), NGX-1 (Neurogen), SNP-7941 (Synaptic), SNAP9847
(Synaptic),
T-226293 (Schering Plough), TPI-1361-17 (Saitama Medical School/University of
California
Irvine), and those disclosed WO 01/21169, WO 01/82925, WO 01/87834, WO
02/051809, WO
02/06245, WO 02/076929, WO 02/076947, WO 02/04433, WO 02/51809, WO 02/083134,
WO
02/094799, WO 03/004027, WO 03/13574, WO 03/15769, WO 03/028641, WO 03/035624,
WO
03/033476, WO 03/033480, WO 04/004611, WO 04/004726, WO 04/011438, WO
04/028459,
WO 04/034702, WO 04/039764, WO 04/052848, WO 04/087680; and Japanese Patent
Application Nos. JP 13226269, JP 1437059, JP2004315511, and the like; (7)
MCH2R (melanin
concentrating hormone 2R) agonistlantagonists; (8) NPY1 (neuropeptide Y Y1)
antagonists, such
as BMS205749, BIBP3226, J-115814, BIBO 3304, LY-357897, CP-671906, and GI-
264879A;
and those disclosed in U.S. Patent No. 6,001,836; and WO 96/14307, WO
01/23387, WO
-78-
CA 02826649 2013-08-05
WO 2012/116145
PCT/US2012/026261
99/51600, WO 01/85690, WO 01/85098, WO 01/85173, and WO 01/89528; (9) NPY5
(neuropeptide Y Y5) antagonists, such as 152,804, S2367 (Shionogi), E-6999
(Esteve), GW-
569180A, GW-594884A (GlaxoSmithkline), GW-587081X, GW-548118X; FR 235,208;
FR226928, FR 240662, FR252384; 1229U91, G1-264879A, CGP71683A, C-75 (Fasgen)
LY-
377897, LY366377, PD-160170, SR-120562A, SR-120819A,S2367 (Shionogi), JCF-104,
and
H409/22; and those compounds disclosed in U.S. Patent Nos. 66140,354,
61,191,160, 6,258,837,
6,3131,298, 6,326,375, 6,329,395, 6,335,345, 6,337,332, 6,329,395, and
6,340,683 ; and EP-
01010691, EP-01044970, and FR252384; and PCT Publication Nos. WO 97/19682, WO
97/20820, WO 97/20821, WO 97/20822, WO 97/20823, WO 98/27063, WO 00/107409, WO
00/185714, WO 00/185730, WO 00/64880, WO 00/68197, WO 00/69849, WO 01/09120,
WO
01/14376, WO 01/85714, WO 01/85730, WO 01/07409, WO 01/02379, WO 01/02379, WO
01/23388, WO 01/23389, WO 01/44201, WO 01/62737, WO 01/62738, WO 01/09120, WO
02/20488, WO 02/22592, WO 02/48152, WO 02/49648, WO 02/051806, WO 02/094789,
WO
03/009845, WO 03/014083, WO 03/022849, WO 03/028726, WO 05/014592, WO
05/01493;
and Norman et al., J. Med. Chem. 43:4288-4312 (2000); (10) leptin, such as
recombinant
human leptin (PEG-0B, Hoffman La Roche) and recombinant methionyl human leptin
(Amgen);
(11) leptin derivatives, such as those disclosed in Patent Nos. 5,552,524;
5,552,523; 5,552,522;
5,521,283; and WO 96/23513; WO 96/23514; WO 96/23515; WO 96/23516; WO
96/23517;
WO 96/23518; WO 96/23519; and WO 96/23520; (12) opioid antagonists, such as
nalmefene
(Revex ), 3-methoxynaltrexone, naloxone, and naltrexone; and those disclosed
in WO
00/21509; (13) orexin antagonists, such as SB-334867-A (GlaxoSmithkline); and
those disclosed
in WO 01/96302, 01/68609, 02/44172, 02/51232, 02/51838, 02/089800, 02/090355,
03/023561,
03/032991, 03/037847, 04/004733, 04/026866, 04/041791, 04/085403, and the
like; (14) BRS3
(bombesin receptor subtype 3) agonists; (15) CCK-A (cholecystokinin-A)
agonists, such as AR-
R 15849, 01 181771, IMV-180, A-71378, A-71623, PD170292, PD 149164, SR146131,
SR125180, butabindide, and those disclosed in US 5,739,106; (16) CNTF (ciliary
neurotrophic
factors), such as GI-181771 (Glaxo-SmithKline); SR146131 (Sandi Synthelabo);
butabindide;
and 1'D170,292, PD 149164 (Pfizer); (17) CNTF derivatives, such as axokine
(Regeneron); and
those disclosed in WO 94/09134, WO 98/22128, and WO 99/43813; (18) GHS (growth
hormone secretagogue receptor) agonists, such as NN703, hexarelin, MK-0677, SM-
1306861, CP-
424,391, L-692,429 and L-163,255, and those disclosed in U.S. Patent No.
6358951, U.S. Patent
Application Nos. 2002/049196 and 2002/022637; and WO 01/56592, and WO
02/32888; (19)
5HT2c (serotonin receptor 2c) agonists, such as APD3546/AR10A (Arena
Pharmaceuticals),
ATH88651 (Athersys), ATH88740 (Athersys), 8VT933 (Biovitrwn/GSK), DPCA37215
(BMS),
1K264; LY448100 PNU 22394; WAY 470 (Wyeth), WAY629 (Wyeth), WAY161503
(Biovitrum), R-1065, VR1065 (Vemalis/Roche) YM 348; and those disclosed in
U.S. Patent No.
3,914,250; and PCT Publications 01/66548, 02/36596, 02/48124, 02/10169,
02/44152;
- 79 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
02/51844, 02/40456, 02/40457, 03/057698, 05/000849, and the like; (20) Mc3r
(melanoeoitin 3
receptor) agonists; (21) Mc4r (melanocortin 4 receptor) agonists, such as
CHIR86036 (Chiron),
CH1R915 (Chiron); ME-10142 (Melacure), ME-10145 (Melacure), HS-131 (Melacure),
NBI72432 (Neurocrine Biosciences), NNC 70-619 (Novo Nordisk), TTP2435
(Transtech)and
those disclosed in PCT Publications WO 99/64002, 00/74679, 01/991752,
01/0125192,
01/52880, 01/74844, 01/70708, 01/70337, 01/91752, 01/010842, 02/059095,
02/059107,
02/059108, 02/059117, 02/062766, 02/069095, 02/12166, 02/11715, 02/12178,
02/15909,
02/38544, 02/068387, 02/068388, 02/067869, 02/081430, 03/06604, 03/007949,
03/009847,
03/009859, 03/013509, 03/031410, 03/094918, 04/028453, 04/048345, 04/050610,
04/075823,
04/083208, 04/089951, 05/000339, and EP 1460069, and US 2005049269, and
JP2005042839,
and the like; (22) monoarnine reuptake inhibitors, such as sibutratmine
(Meridia VReductile)
and salts thereof, and those compounds disclosed in U.S. Patent Nos.
4,746,680, 4,806,570, and
5,436,272, and U.S. Patent Publication No. 2002/0006964, and WO 01/27068, and
WO
01/62341; (23) serotonin reuptake inhibitors, such as dexfenfluramine,
fluoxetine, and those in
U.S. Patent No. 6,365,633, and WO 01/27060, and WO 01/162341; (24) GLP-1
(glucagon-like
peptide 1) agonists; (25) Topiramate (Topimax0); (26) phytopharm compound 57
(CP 644,673);
(27) ACC2 (acetyl-CoA carboxylase-2) inhibitors; (28)133 (beta adrenergic
receptor 3) agonists,
such as rafebergron/AD9677/TAK677 (Dainippon/ Takeda), CL-316,243, SB 418790,
BRL-
37344, L-796568, BMS-196085, BRL-35135A, CGP12177A, BTA-243, GRC1087 (Glenmark
Pharmaceuticals) GW 427353 (solabegron hydrochloride), Trecadrine, Zeneca
D7114, N-5984
(Nisshin Kyorin), LY-377604 (Lilly), KT07924 (Kissei), SR 59119A, and those
disclosed in US
Patent Nos. 5,705,515, US 5,451,677; and W094/18161, W095/29159, W097/46556,
W098/04526 W098/327536 WO 01/74782, WO 02(32897, WO 03/014113, WO 03/016276,
WO 03/016307, WO 03/024948, WO 03/024953, WO 03/037881, WO 04/108674, and the
like;
(29) DGAT1 (diacylglycerol acyltransferase 1) inhibitors; (30) DGAT2
(diacylglycerol
acyltransferase 2)inhibitors; (31) FAS (fatty acid synthase) inhibitors, such
as Cerulenin and
C75; (32) PDE (phosphodiesterase) inhibitors, such as theophylline,
pentoxifylline, zaprinast,
sildenafil, amrinone, milrinone, cilostamide, rolipram, and citomilast, as
well as those described
in WO 03/037432, WO 03/037899; (33) thyroid hormone 13 agonists, such as KB-
2611
(KaroBioBMS), and those disclosed in WO 02/15845; and Japanese Patent
Application No. JP
2000256190; (34) UCP-1 (uncoupling protein 1), 2, or 3 activators, such as
phytanic acid, 4-[(E)-
2-(5,6,7,8-tetrahydro-5,5,8,8-tetramethy1-2-na,pthaleny1)-1-propenyljbenzoie
acid (TTNPB), and
retinoic acid; and those disclosed in WO 99/00123; (35) acyl-estrogens, such
as oleoyl-estrone,
disclosed in del Mar-Grasa, M. et al., Obesity Research, 9:202-9 (2001); (36)
glucocorticoid
receptor antagonists, such as CP472555 (Pfizer), KB 3305, and those disclosed
in WO
04/000869, WO 04/075864, and the like; (37) llfi HSD-1 (11-beta hydroxy
steroid
dehydrogenase type 1) inhibitors, such as BVT 3498 (AMG 331), BVT 2733, 3-(1-
adamantyD-4-
- 80 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
ethyl-5-(ethylthio)-4H-1,2,4-triazole, 3-(1-adamanty1)-5-(3,4,5-
trimethoxypheny1)-4-methy1-4H-
1,2,4-triazole, 3-adamantany1-4,5,6,7,8,9,10,11,12,3a-decahydro-1,2,4-
triazolo[4,3-
a][111annulene, and those compounds disclosed in WO 01/90091, 01/90090,
01/90092,
02/072084, 04/011410, 04/033427, 04/041264, 04/027047, 04/056744, 04/065351,
04/089415,
04/037251, and the like; (38) SCD-1 (stearoyl-CoA desaturase-1) inhibitors;
(39) dipeptidyl
peptidase IV (DPP-4) inhibitors, such as isoleucine thiazolidide, valine
pynolidide, sitagliptin
(Januvia), saxagliptin, alogliptin, NVP-DPP728, LAF237 (vildagliptin), P93/01,
TSL 225, TMC-
2A/2B/2C, FE 999011, P9310/K364, VIP 0177, SDZ 274-444, GSK 823093, E 3024,
SYR 322,
TS021, SSR 162369, GRC 8200, K579, NN7201, CR 14023, PHX 1004, PHX 1149, PT-
630,
SK-0403; and the compounds disclosed in WO 02/083128, WO 02/062764, WO
02/14271, WO
03/000180, WO 03/000181, WO 03/000250, WO 03/002530, WO 03/002531, WO
03/002553,
WO 03/002593, WO 03/004498, WO 03/004496, WO 03/005766, WO 03/017936, WO
03/024942, WO 03/024965, WO 03/033524, WO 03/055881, WO 03/057144, WO
03/037327,
WO 04/041795, WO 04/071454, WO 04/0214870, WO 04/041273, WO 04/041820, WO
04/050658, WO 04/046106, WO 04/067509, WO 04/048532, WO 04/099185, WO
04/108730,
WO 05/009956, WO 04/09806, WO 05/023762, US 2005/043292, and EP 1 258 476;
(40)
lipase inhibitors, such as tetrahydrolipstatin (orlistatrXENICAL), ATL962
(Alizyme/Takeda),
GT389255 (Genzyme/Peptirnmune)Triton WR1339, RHC80267, lipstatin, teasaponin,
and
diethylumbelliferyl phosphate, FL-386, WAY-121898, Bay-N-3176, valilactone,
esteracin,
ebelactone A, ebelactone B, and RHC 80267, and those disclosed in WO 01/77094,
WO
04/111004, and U.S. Patent Nos. 4,598,089, 4,452,813, 5,512,565, 5,391,571,
5,602,151,
4,405,644, 4,189,438, and 4,242,453, and the like; (41) fatty acid transporter
inhibitors; (42)
dicarboxylate transporter inhibitors; (43) glucose transporter inhibitors; and
(44) phosphate
transporter inhibitors; (45) anorectic bicyclic compounds such as 1426
(Aventis) and 1954
(Aventis), and the compounds disclosed in WO 00/18749, WO 01/32638, WO
01/62746, WO
01/62747, and WO 03/015769; (46) peptide YY and PYY agonists such as PYY336
(Nastech/Merck), AC162352 (IC Innovations/Curis/Amylin), TM30335/TM30338 (7TM
Phanna), PYY336 (Ernisphere Tehcnologies), pegylated peptide YY3-36, those
disclosed in WO
03/026591, 04/089279, and the like; (47) lipid metabolism modulators such as
maslinic acid,
erythrodiol, ursolic acid uvaol, betulinic acid, betulin, and the like and
compounds disclosed in
WO 03/011267; (48) transcription factor modulators such as those disclosed in
WO 03/026576;
(49) Mc5r (melanocortin 5 receptor) modulators, such as those disclosed in WO
97/19952, WO
00/15826, WO 00/15790, US 20030092041, and the like; (50) Brain derived
neutotropic factor
(BDNF), (51) Melr (melanocortin 1 receptor modulators such as LK-184 (Proctor
& Gamble),
and the like; (52) 5HT6 antagonists such as BVT74316 (BioVitrum), BVT5182c
(BioVitrum), E-
6795 (Esteve), E-6814 (Esteve), SB399885 (GlaxoSmithkline), SB271046
(GlaxoSmithldine),
RO-046790 (Roche), and the like; (53) fatty acid transport protein 4 (FATP4);
(54) acetyl-CoA
- 81 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
carboxylase (ACC) inhibitors such as CP640186, CP610431, CP640188 (Pfizer);
(55) C-terminal
growth hormone fragments such as A0D9604 (Monash Univ/Metabolic
Pharmaceuticals), and
the like; (56) oxyntomodulin; (57) neuropeptide FF receptor antagonists such
as those disclosed
in WO 04/083218, and the like; (58) amylin agonists such as
Symlin/pramlintide/AC137
(Amylin); (59) Hoodia and trichocaulon extracts; (60) 8VT74713 and other gut
lipid appetite
suppressants; (61) dopamine agonists such as bupropion
(WELLBUTRIN/GlaxoSmithkline);
(62) zonisamide (ZONEGRAN/Dainippon/Elan), and the like; and
(e) anorectic agents suitable for use in combination with a compound of the
present
invention include, but are not limited to, aminorex, amphechloral,
amphetamine, benzphetamine,
chlorphentermine, clobenzorex, cloforex, clominorex, clortermine,
cyclexedrine,
dexfenfluramine, dextroamphetamine, diethylpropion, diphemethoxidine, N-
ethylamphetamine,
fenbutrazate, fenfiuramine, fenisorex, fenproporex, fludorex, flumin.orex,
furfurylmethylamphetamine, levamfetamine, levophacetoperane, mazindol,
mefenorex,
metarnfepramone, methamphetamine, norpseudoephedrine, pentorex,
phendimetrazine,
phenmetrazine, phentermine, phenylpropanolamine, picilorex and sibutramine;
and
pharmaceutically acceptable salts thereof. A particularly suitable class of
anorectic agent are the
halogenated amphetamine derivatives, including chlorphentermine, cloforex,
clortermine,
dexfenfluramine, fenfluramine, picilorex and sibutramine; and pharmaceutically
acceptable salts
thereof. Particular halogenated amphetamine derivatives of use in combination
with a compound
of the present invention include: fenfluramine and dexfenfluramine, and
pharmaceutically
acceptable salts thereof.
Specific compounds of use in combination with a compound of the present
invention
include: simvastatin, mevastatin, ezetimibe, atorvastatin, sitagliptin,
metformin, sibutramine,
orlistat, Qnexa, topiramate, naltrexone, bupriopion, phentermine, and
losartan, losartan with
hydrochlorothiazide. Specific CB1 antagonists/inverse agonists of use in
combination with a
compound of the present invention include: those described in W003/077847,
including: N43-
(4-chloropheny1)-2(S)-phenyl-1(S)-methylpropyl]-2-(4-trifluoromethyl-2-
pyrimidyloxy)-2-
methylpropariamide, N43-(4-chloropheny1)-2-(3-cyanopheny1)-1-methylpropyl]-2-
(5-
trifluoromethy1-2-pyridyloxy)-2-methylpropanamide, N-[3-(4-chlorophenyI)-2-(5-
chloro-3-
pyridy1)-1-methylpropy11-2-(5-trifluoromethy1-2-pyridyloxy)-2-
methylpropanamide, and
pharmaceutically acceptable salts thereof; as well as those in W005/000809,
which includes the
following: 3-{1-[bis(4-chlorophenyOmethyl]azetidin-3-ylidene)-3-(3,5-
difluoropheny1)-2,2-
dimethylpropanenitrile, 1-{1.11-(4-chlorophenyl)pentyliazetidin-3-y11-1-(3,5-
difluoropheny1)-2-
methylpropan-2-ol. 34(S)-(4-chloropheny1){3-[(1S)-1-(3,5-difluoropheny1)-2-
hydroxy-2-
methylpropyl]azetidin-l-y1) methyl)benzonitrile, 3-((S)-(4-chloropheny1){3-
[(1S)-1-(3,5-
difluoropheny1)-2-fluoro-2-methylpropyljazetidin-1-y1)methypbenzonitrile, 34(4-
chloropheny1){3-[1-(3,5-difluoropheny1)-2,2-dimethylpropyl]azetidin-1-
yllmethyObenzonitrile,
- 82 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
3-((1S)-1-{1-[(S)-(3-cyartophenyl)(4-cyanophenyl)methyl]azetidin-3-y1}-2-
fluoro-2-
methylpropy1)-5-fluorobe3rizonitrile, 3-[(S)-(4-chlorophenyl)(3-{(1S)-2-fluoro-
143-fluoro-5-
(4H-1,2,4-triazo1-4-y1)phenyli-2-methy1propyl}azetidin-1-
3/1)methylibenzonitrile, and 54(4-
chloropheny1){34(1S)-1-(3,5-difluoropheny1)-2-fluoro-2-methylpropyljazetidin-1-
yllmethyl)thiophene-3-carbonitrile, and pharamecueitcally acceptable salts
thereof; as well as:
3-[(S)-(4-ehlorophenyl)(3-{(1S)-2-fluoro-1-[3-fluoro-5-(5-oxo-4,5-dihydro-
1,354-oxadiazol-2-
yl)phenyli-2-methylpropyllazetidin-1-y1)methylibenzonitrile, 3- [(S)-(4-
chlorophenyl)(3- {(1S)-2-
fluoro-1-[3-fluoro-5-(1,3,4-oxadiazo1-2-yl)phenylj-2-methylpropyl}azetidin-l-
yl)methyllbenzonitrile, 3-[(S)-(3- {(1S)-1-[3-(5-amino-1,3,4-oxadiazol-2-y1)-5-
fluorophenyl]-2-
fluoro-2-methylpropyll azetidin-l-y1)(4-chlorophenyl)methylibenzonitrile, 3-
[(S)-(4-
cyanophenyl)(3- {(1S)-2-fluoro-1-[3-fluoro-5-(5-oxo-4,5-dihydro-1,3,4-
oxadiazol-2-yl)phenyl]-2-
methylpropyl}azetidin-1-y1)methyl]benzonitrile, 3-[(S)-(3-{(18)-143-(5-amino-
1,3,4-oxadiazol-
2-y1)-5-fluoropheny1]-2-fluoro-2-methylpropyl) azetidin-1-y1)(4-
eyanophenyl)methyl]benzonitrile, 3-[(S)-(4-cyanophenyl)(3-{(1S)-2-fluoro-1-[3-
fluoro-5-(153,4-
oxadiazol-2-yl)pheny1]-2-methylpropyllazetidin-1-yOmethylibenzonitrile, 3-[(S)-
(4-
ehlorophenyl)(3-{(1S)-2-fluoro-1-[3-fluoro-5-(1,2,4-oxadiazol-3-yl)phenyl]-2-
methylpropyl}azetidin-1-yl)methyl]benzonitrile, 3-[(1S)-1-(1-{(S)-(4-
cyanopheny1)[3-(1,2,4-
oxadiazol-3-yl)phenyl]-methyl}azetidin-3-y1)-2-fluoro-2-methylpropyli-5-
fluorobenzonitrile, 5-
(3- {1- [1-(diphenylmethyDazetidin-3-y11-2-fluoro-2-methylpropyl}-5-
fluoropheny1)-1H-tetrazole,
543- {1- [1-(diphenylmethyl)azetidin-3-y11-2-fluoro-2-methylpropyl) -5-
fluoropheny1)-1-methyl-
1H-tetrazole3 5-(3-{141-(diphenylmethyl)azetidin-3-y1:1-2-fluoro-2-
methylpropy1)-5-
fluoropheny1)-2-methyl-2H-tetrazole, 3-[(4-chlorophenyl)(3- {2-fluoro-143-
fluoro-5-(2-methyl-
2H-tetrazol-5-yl)phenyl]-2-methylpropyl} azetidin-l-yl)methyljbenzonitrile, 3-
[(4-
ehlorophenyl)(3- {2-fluoro-l-[3-fluoro-5-(1-methyl-1H-tetrazol-5-yl)phenylj-2-
methylpropyl}azetidin-1-ypmethyllbenzonitrile, 3-[(4-cyanophenyl)(3- {2-fluoro-
143-fluoro-5-
(1-methy1-1H-tetrazol-5-y1)pheny1}-2-methylpropyllazetidin-1-
ypmethyl]benzonitrile, 34(4-
cyanophenyl)(3-{2-fluoro-143-fluoro-5-(2-methyl-2H-tetrazol-5-y1)phenyl3-2-
methylpropyl}azetidin-1-yOmethyllbenzonitrile, 5-{3-[(S)-{3-[(1S)-1-(3-bromo-5-
fluoropheny1)-
2-fluoro-2-methylpropyliazetidin-1-y1}(4-chlorophenyl)methyljpheny1}-1,3,4-
oxadiazol-2(3H)-
one, 3-[(1S)-1-(1-{(S)-(4-chloropheny1)[3-(5-oxo-4,5-clihydro-1,3,4-oxacliazol-
2-
yl)phenyl]methyl) azetidin-3-y1)-2-fluoro-2-methylpropy1]-5-
fluorobenzonitrile, 3-[(1S)-1-(1-
{(S)-(4-cyanopheny1)[3-(5-oxo-4,5-dihydro-1,3,4-oxadiazol-2-yl)phenylimethyl)
azetidin-3-y1)-
2-fluoro-2-methylpropy1]-5-fluorobenzonitrile, 3-[(1S)-1-(1-{(S)-(4-
cyanopheny1)[3-(1,3,4-
oxadiazol-2-yl)phenyl]methyl}azetidin-3-y1)-2-fluoro-2-methylpropyl]-5-
fluorobenzonitrile, 3-
[(1S)-1-(1-{(S)-(4-chloropheny1)[3-(1,3,4-oxadiazol-2-
yl)phenylimethyllazetidin-3-y1)-2-fluoro-
2-methylpropyli-5-fluorobenzonitrile, 3 -((lS)-1-{1-[(S)-[3-(5-amino-1,3,4-
oxadiazol-2-
yl)phenyl](4-chlorophenyl)methyllazeti din-3-y1}-2-fluoro-2-methylpropy1)-5-
fluorobenzonitrile,
- 83 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
3 -((1 S)- 1-{ 1 - [(S )43-(5-amino- 1 ,3,4-oxadiazol-2-yl)phenyli(4-
cyanophenyl)methyll azetidin-3-
yl} -2-fluoro-2-methylpropyI)-5-fluorobenzonitrile, 3 -[(1S)-1-(1- [(S)-(4-
eyanopheny1)[3-(1,2,4-
oxadiazol-3-yDphenyl]methyl}azetidin-3-y1)-2-fluoro-2-methylpropyl]-5-
fluorobenzonitrile, 3-
[(1S)-1-(1- {(S)-(4-chloropheny1)[3-(1,2,4-oxadiazol-3-
y1)phenyl}methyllazetidin-3-y1)-2-fluoro-
2-methylpropy1]-5-fluorobenzonitrile, 5- [34(S)-(4-chloropheny1){3-[(1S)-1-
(3,5-
difluoropheny1)-2-fluoro-2-methylpropyl] azetidin-l-y1} methyl)pheny1]-1,3,4-
oxadiazol-2(3H)-
one, 543((S)-(4-chlorophenyl) f 3- [(1 S)- 1-(3,5-difluorophenyI)-2-fluoro-2-
methylpropyl] azetidin- 1-y1} methyl)pheny1}-1,3 ,4-oxadiazol-2 (311)-one, 4-
{(S)- { 3- [(1 S)- 1 -(3 ,5 -
difluoropheny1)-2-fluoro-2-methylpropyl]azetidin-1-y1}[3-(5-oxo-4,5-dihydro-
1,3,4-oxadiazol-2-
yl)phenylimethyl }-benzonitrile, and pharmaceutically acceptable salts
thereof.
Specific NPY5 antagonists of use in combination with a compound of the present
invention include: 3-oxo-N-(5-pheny1-2-pyraziny1)-spiro[isobenzofuran-1(3H),4'-
piperidine]-1'-
carboxamide, 3 -oxo-N-(7-tritfluoromethylpyrido [3,2-b]pyridin-2-yl)spiro-
Psobenzofuran-
1(3H),4%piperidine}-1 '--carboxamide, N45-(3-fluorophenyD-2-pyrimidiny1]-3-
oxospiro-
[isobenzofuran-1(311),4'-piperidinel-1'-carboxamide, trans-3' -oxo-N-(5-pheny1-
2-
primidinyl)spiro[cyclohexane-1,1'(3'H)-isobenzofuran]-4-carboxamide, trans-3 '
-oxo-N41-(3-
quinoly1)-4-imidazolyll spiro[cyclohexane-1,1'(3'H)-isobenzothrani-4-
carboxamide, trans-3-oxo-
N-(5-pheny1-2-pyrazinyl)spiro[4-azaiso-benzofuran- 1(3H),1'-cyclohexane]-4'-
carboxamide,
trans-N45-(3-fluoropheny1)-2-pyrimidinyli-3 -oxospiro [5-azaisobenzofuran-
1(3H),1 ' -
cyclohexane]-4'-carboxamide, trans-N45-(2-fluoropheny1)-2-pyrimidiny1}-3-
oxospiro[5-
azaisobenzoftu=an-1(311),1'-cyclohexanci-4'-carboxamide, trans-N-[1-(3,5-
difluoropheny1)-4-
imidazoly1]-3-oxospiro[7-azaisobenzofiiran-1(311),1'-cyclohexanej-
4%carboxamide, trans-3-oxo-
N-(1-pheny1-4-pyrazolybspiro[4-azaisobenzofuran-1(3H),1'-cyclohexane]-4'-
carboxamide,
trans-N- [1 -(2-fluoropheny1)-3 -pyrazoly1]-3 -oxospiro [6-az aisobenzo furan-
1 (3 H),1 '-cyclohexane]-
4' -carboxamide, trans-3-oxo-N-(1-phenyl-3-pyrazolyl)spiro [6-azaisobenzofuran-
1 (3 H),1 -
cyclohexane] -carboxamide, trans-3 -oxo-N-(2-pheny1-1,2,3-triazol-4-yl)spiro[6-
azaisobenzofuran-1(3H), I ' -cyclohexane] -carboxamide, and pharmaceutically
acceptable salts
and esters thereof.
Specific ACC-1/2 inhibitors of use in combination with a compound of the
present
invention include: 1'-[(4,8-dirnethoxyquinolin-2-yl)carbonyli-6-(1 H-tetrazol-
5-yl)spiro[chroman-
2,4'-piperidin] -4-one; (5- { 11- [(4,8-dimetho xyquinol in-2-yl)carbonyll-4-
oxospiro [chromari-2,4'-
piperidin] -6-y1} -2H-tetrazol-2-yOmethyl pivalate; 5- { 14(8-cyclopropy1-4-
methoxyquinolin-2-
yl)carbony11-4-oxospiro[chroman-2,4*-piperidin]-6-y1}nicotinic acid; 1'-(8-
rnethoxy-4-
morpholin-4-y1-2-naphthoy1)-6-(1H-tetrazol-5-yl)spiro [chroman-2,48-piperidin]
-4-one; and 1'-
[(4-ethoxy-8-ethylquinolin-2-ypearbony1]-6-(1H-tetrazol-5-yDspiro[chroman-2,4'-
piperidinj-4-
one; and pharmaceutically acceptable salts and esters thereof.
Specific MCH1R antagonist
compounds of use in combination with a compound of the persent invention
include: 1-144(1-
- 84 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
ethylazetidin-3-ypoxylpheny1}-4-[(4-fluorobenzypoxy]pyridin-2(1H)-one, 4-[(4-
fluorobenzyl)oxy]-1- f 4-[(1-isopropylazetidin-3 -yl)oxy]phenyl [ pyridin-
2(1}1)-one, 144-
(azetidin-3-y1oxy)pheny11-4-[(5-ch1oropyridin-2-y1)methoxy]pyridin-2(1H)-one,
44(5-
chloropyridin-2-ypinethoxy] -1-{4-[(1-ethylazetidin-3-yl)oxy]phenyllpyridin-
2(1H)-one, 4-[(5-
chloropyridin-2-yl)methoxy1-144-[(1-propylazetidin-3-y1)oxy]phenyl}pyridin-
2(1H)-one, and 4-
[(5-chloropyridin-2-yl)methoxy]-1 -(4- f [(2S)-1-ethylazetidin-2-
yl]methoxy)phenyl)pyridin-
2(1H)-one, or a pharmaceutically acceptable salt thereof.
Specific DP-Iv inhibitors of use in combination with a compound of the present
invention
are selected from Januvia, 7-[(3R)-3-amino-4-(2,4,5-trifluorophenyl)butanoy1]-
3-
(trifluoromethyl)-5,6,7,8-tetrahydro-1,2,4-triazolo[4,3-a]pyrazine. In
particular, the compound of
formula I is favorably combined with 7-[(3R)-3-amino-4-(2,4,5-
trifluorophenyl)butanoy111-3-
(trifluoromethyl)-5,6,7,8-tetrahydro-1,2,4-triazolo[4,3-a]pyrazine, and
pharmaceutically
acceptable salts thereof.
Specific H3 (histamine 113) antagonists/inverse agonists of use in combination
with a
compound of the present invention include: those described in W005/077905,
including:344-
[(1-cyclobuty1-4-piperidinyl)oxy]pheny1}-2-ethylpyrido[2,3-d]-pyrimidin-4(3H)-
one, 3- f 4-[(1-
cyclobuty1-4-piperidinyl)oxy}pheriy1}-2-methylpyrido[4,3-d]pyrimidin-4(3H)-
one, 2-ethy1-3-(4-
{3-[(3S)-3-methylpiperidin-1-yl]propoxy}phenyppyrido[2,3-d]pyrimidin-4(3H)-one
2-methyl-3 -
(4- f 3-[(3S)-3-methylpiperidin-1-Apropoxy}phenyl)pyrido[4,3-d]pyrimidin-4(3H)-
one, 3-{4-
[(1-cyclobuty1-4-piperidinyl)oxy]pheny1}-2,5-dimethy1-4(3H)-quinazolinone, 3-
f 44(1-
cyclobuty1-4-piperidinypoxy]pheny1}-2-methy1-5-trifluoromethyl-4(3H)-
quinazolinone, 3- f 4-
[(1-cyclobuty1-4-piperidinyDoxy]pheriy1}-5-methoxy-2-methy1-4(3H)-
quinazolinone, 3- {44(1-
cyclobutylpiperidin-4-ypoxylpheny1}-5-fluoro-2-methy1-4(3H)-quinazolinone, 3-
(4-[(1-
cyclobutylpiperidin-4-ypoxy]pheny1}-7-fluoro-2-methyl-4(3H)-quinazolinone, 3-
{4-[(1-
cyclobutylpiperidin-4-yl)oxy]pheny1}-6-methoxy-2-methy1-4(3H)-quinazolinone, 3-
f 4-[( I-
cyc1obutylpiperidin-4-ypoxylpheny1}-6-fluoro-2-methyl-4(3H)-quinazo1inone, 3-
f4-[(1-
cyc1obuty1piperidin-4-y1)oxyipherry1}-8-fluoro-2-methy1-4(3H)-quinazolinone,
3-{4-[(1-cyclopenty1-4-piperidinyl)ox]phenyl) -2-methylpyrido[4,3-d]pyrimidin-
4(3H)-one, 3-
{4-[(1-cyclobutylpiperidin-4-y1)oxy]pheny1}-6-fluoro-2-methylpyrido[3,4-
dlpyrimidin-4(311)-
one, 3- (4-[(1-cyclobuty1-4-piperidinyl)oxy]phenyl}-2-ethylpyridof4,3-
djpyrimidin-4(3H)-one, 6-
methoxy-2-methy1-3- f 4-[3-(1-piperidinyl)propoxy]phenyl}pyrido[3,4-
d]pyrimidin-4(3H)-one, 6-
methoxy-2-methy1-3- f 4-[3 -(1-pyrrolidinyl)propoxy] phenyl pyrido [3 ,4-
dipyrimidin-4(3H)-one,
2,5-dimethy1-3-{443-(1-pyrrolidinyl)propoxylpheny1}-4(3H)-quinazolinone, 2-
methy1-3-{443-
(1-pyrrolidinyl)propoxy]pheny1}-5-trifluoromethy1-4(3H)-quinazolinone, 5-
fluoro-2-inethyl-3-
{443-(1-piperidinyl)propoxylpheny1}-4(3H)-quinazolinone, 6-methoxy-2-methy1-3-
{4-[3-(1-
piperidinyl)propoxy]pheny1}-4(3H)-quinazolinone, 5-methoxy-2-methy1-3-(4-f3-
[(3S)-3-
rnethylpiperidin-1-yl]propoxy}pheny1)-4(3H)-quinazolinone, 7-methoxy-2-methyl-
3-(4- f 3-[(3S)-
- 85 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
3-methylpiperidin-1-yllpropoxylpheny1)-4(3H)-quinazolinone, 2-methy1-3-(4-{3-
[(3S)-3-
methylpiperidin-1-yllpropoxy}phenyppyrido[2,3-d]pyrimidin-4(3H)-one, 5-fluoro-
2-methy1-3-
(4-{3-[(2R)-2-methylpyrrolidin-1-yl]propoxylpheny1)-4(3H)-quinazolinone, 2-
methyl-3 -(4- (3-
[(2R)-2-methylpyrrolidin-1-y1lpropoxy}phenyppyrido[4,3-d]pyrimidin-4(3H)-one,
6-methoxy-2-
methyl-3-(4-(3-[(2R)-2-methylpyrrolidin-1-yllpropoxylpheny1)-4(3H)-
quinazolinone, 6-
methoxy-2-methy1-3 -(4- {3- [(2 S)-2-methylpyrrolidin-1 -yl]propoxy) pheny1)-
4(3H)-quinazolinone,
and pharmaceutically acceptable salts thereof.
Specific CCKIR agonists of use in combination with a compound of the present
invention include: 3-(4-([1-(3-ethoxypheny1)-2-(4-tnethylpheny1)-1H -imidazol-
4-Acarbonyl) -
1-piperaziny1)-1-naphthoic acid; 3-(4-([1-(3-ethoxypheny1)-2-(2-fluoro-4-
methylpheny1)-1H -
imidazol-4-yllicarbony1}-1-piperaziny1)-1-naphthoic acid; 3-(4-{[1-(3-
ethoxypheny1)-2-(4-
fluoropheny1)-1H -imidazol-4-yl]carbony11-1-piperaziny1)-1-naphthoic acid; 3-
(4-{[1-(3-
ethoxypheny1)-2-(2,4-difluorophenyl)-1H -imidazol-4-yl]carbony1}-1-
piperaziny1)-1-naphthoic
acid; and 3-(4-([1-(2,3-dihydro-1,4-benzodioxin-6-y1)-2-(4-fluoropheny1)-1H-
imidazol-4-
Acarbony1}-1-piperaziny1)-1-naphthoic acid; and pharmaceutically acceptable
salts thereof.
Specific 1C4R agonists of use in combination with a compound of the present
invention
include: 1) (5S)-1'- ( [(3R,4R)-1-tert-buty1-3-(2,3,4-
trifluorophenyl)piperidin-4-yl3carbony1}-3-
chloro-2-methy1-541-methyl- 1-(1-methy1-1H-1,2,4-triazol-5-ypethyl]-5H-spiro
[Rao [3,4-
b]pyridine-7,4'-piperidine]; 2) (5R)-1'- [(3R,4R)-1-tert-buty1-3-(2,3,4-
trifluoropheny1)-
piperidin-4-yl]carbony1}-3-chloro-2-methyl-541-methy1-1-(1-methyl-1H-1,2,4-
niazol-5-
y1)ethyll-5H-spiro[furo[3,4-blpyridine-7,4'-piperidine]; 3) 2-(1'-{ [(3S,4R)-1-
tert-buty1-4-(2,4-
difluorophenyppyrrolidin-3-yl]carbony1}-3-chloro-2-methyl-5H-spiro[fitro[3,4-
b]pyridine-7,4'-
piperidin]-5-y1)-2-methylpropanenitrile; 4) 1'-{[(3S,4R)-1-tert-buty1-4-(2,4-
difluorophenyl)pyrrolidin-3-yl]carhony11-3-chloro-2-methy1-541-methyl-1-(1-
methyl-1H-1,2,4-
triazol-5-yl)ethyl]-5H-spiro[fitro[3,4-b]pyridine-7,4'-piperidine]; 5) N-
[(3R,4R)-3-((3-chloro-2-
methy1-5-[1-methyl-1-(1-methyl-1H-1,2,4-triazol-5-yl)ethyl]-1111,5H-spiro[furo-
[3,4-b3pyridine-
7,4'-piperidin]-11-y1)carhony1)-4-(2,4-difluoropheny1)-cyclopentyll-N-
methyltetrahydro-2H-
pyran-4-amine; 6) 2-[3-chloro-1'-({(1R,2R)-2-(2,4-difluoropheny1)-4-
[methyl(tetrahydro-2H-
pyran-4-yl)amino]-cyclopenty1}-carbony1)-2-methyl-5H-spiro[furo[3,4-b]pyridine-
7,4'-
piperidin]-5-y1]-2-methyl-propane-nitrile; and pharmaceutically acceptable
salts thereof.
Suitable neurokinin-1 (NK-1) receptor antagonists may be favorably employed
with the
AMP-kinase activators of the present invention. NK-1 receptor antagonists of
use in the present
invention are fully described in the art. Specific neurokinin-1 receptor
antagonists of use in the
present invention include: ( )-(2R3R,2S3S)-N- ([2-cyclopropoxy-5-
(trifluoromethoxy)-
phenyl]methy1}-2-phenylpiperidin-3-amine; 2-(R)-(1-(R)-(3,5-
bis(trifluoromethyl)-
phenyl)ethoxy)-3-(S)-(4-fluoropheny1)-4-(3-(5-oxo-1H,4H-1,2,4-
triazolo)methyl)morpholine;
aperpitant; 017493; GW597599; GW679769; R673; R067319; R1124; R1204;
SSR146977;
-86-
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
SSR240600; T-2328; and T2763.; or a pharmaceutically acceptable salts thereof.
The term "therapeutically effective amount" means the amount the compound of
structural formula I that will elicit the biological or medical response of a
tissue, system, animal
or human that is being sought by the researcher, veterinarian, medical doctor
or other clinician,
which includes alleviation of the symptoms of the disorder being treated. The
novel methods of
treatment of this invention are for disorders known to those skilled in the
art. The term
"mammal" includes humans, and companion animals such as dogs and cats.
The weight ratio of the compound of the Formula I to the second active
ingredient may be
varied and will depend upon the effective dose of each ingredient. Generally,
an effective dose
of each will be used. Thus, for example, when a compound of the Formula I is
combined with a
DP1V inhibitor the weight ratio of the compound of the Formula I to the DPIV
inhibitor will
generally range from about 1000:1 to about 1:1000, preferably about 200:1 to
about 1:200.
Combinations of a compound of the Formula I and other active ingredients will
generally also be
within the aforementioned range, but in each case, an effective dose of each
active ingredient
should be used.
The compounds of structural formula I of the present invention can be prepared
according
to the procedures of the following Schemes, Intermediates and Examples, using
appropriate
materials and are further exemplified by the follo-wing specific examples.
Moreover, by utilizing
the procedures described in the disclosure contained herein, one of ordinary
skill in the art can
readily prepare additional compounds of the present invention claimed herein.
The compounds
illustrated in the examples are not, however, to be construed as forming the
only genus that is
considered as the invention. The Examples further illustrate details for the
preparation of the
compounds of the present invention. Those skilled in the art will readily
understand that known
variations of the conditions and processes of the following preparative
procedures can be used to
prepare these compounds. The instant compounds are generally isolated in the
form of their
pharmaceutically acceptable salts, such as those previously described herein.
The use of
protecting groups for the amine and carboxylic acid fiinctionalities to
facilitate the desired
reaction and minimize undesired reactions is well documented. Conditions
required to remove
protecting groups are found in standard textbooks such as Greene, T, and Wuts,
P. G. M.,
Protective Groups in Organic Synthesis, John Wiley ct Sons, Inc., New York,
NY, 1991. CBZ
and 130C are commonly used protecting groups in organic synthesis, and their
removal
conditions are known to those skilled in the art. All temperatures are degrees
Celsius unless
otherwise noted. Mass spectra (MS) were measured by electron-spray ion-mass
spectroscopy.
Abbreviations used in the description of the preparation of the compounds of
the present
invention: ACN is acetonitrile; AcOH is acetic acid; C is carbon; CV is column
volume(s);
DAST is (diethylamino)sulfur trifluoride; D13U is 1,8-diazabicyclo[5.4.0]undec-
7-ene; DIBAL-FI
is di-isobutyl aluminum hydride; DCM is dichloromethane; DIPEA is
diisopropylethyl amine;
- 87 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
DMA is dimethyl acetal; DME is 1,2-dimethoxyethane; DMF is dimethyl formamide;
DMSO is
dimethyl sulfoxide; dppf DCM complex is 1,11-bis(diphenyl-phosphino)ferrocene
dichloromethane complex; Et20 is diethyl ether; Et0Ac is ethyl acetate; dppf
is 1,1c-
Bis(diphenyl-phosphino)ferrocene; Et0H is ethanol; Et3N is triethyl amine; h
is hour(s); HPLC
is high pressure liquid chromatography; ISCO Rf is the Rf determined via
medium pressure
liquid chromatography using aTeledyne ISCO RediSep column; isomannide is
1,4:3,6-Di-
anhydro-marmitol; KOAc is potassium acetate; L is liter; LC/MS and LC-MS is
liquid
chromatography/mass spectroscopy; KOTMS is potassium trimethylsilanolate; LAH
is lithium
aluminum hydride; M is molar; ml and mL is milliliter; Me is methyl, MeCN is
acetonitrile; Mei
is methyl iodide; MeMgBr is methyl magnesium bromide; Me0H is methanol; MgBr
is
magnesium bromide; min is minutes; rnmol is millimole(s); m-CPBA is meta
chloro per benzoic
acid; MTBE is tert-butyl methyl ether; N is normal; Na0Ac is sodium acetate;
NBS is N-bromo
succinarnide; NIS is N-iodo succinamide; PPh3 is triphenyl phosphine; PhSiH is
phenyl silane;
wt % is weight percent; psi is pounds per square inch; RT and rt is room
temperature; Rt is
retention time; Rochelles Salt is potassium sodium tartrate; SEM is 2-
(trimethylsilyl)ethoxyrnethyl; SEMC1 is 2-(trimethylsily1)-ethoxymethyl
chloride; TBAF is
tetrabutyl ammonium fluoride; TMS is trimethylsily1;TFA is tailor() acetic
acid; and THE is
tetrahydrofuran.
Microwave (MW) reactions were performed with a single mode operating Biotage
Enarys
Optimizer in sealed reaction vials at the indicated fixed temperature held
constant for the
designated reaction time. The medium pressure liquid chromatography (MPLC)
purifications
were performed with Teledyne ISCO RediSept normal-phase columns pre-packed
with 35-60
micron silica gel. The LC-MS system contained an Applied Biosystems API150EX
MS
operating in a positive ion mode receiving 0.1 mL/min flowrate with a Shimadzu
UV detector
receiving 0.1 mL/min flowrate. Unless specified, the LC conditions were
solvent A = 0.03%
TEA in acetonitrile; solvent B = 0.05% TEA in water; flowrate ¨ 10 mL/min;
column:
Chromolith Performance RP-18e, 100x4.6 mm; gradient program: min (%B) 0 (95),
1.6 (5), 2.6
(5), 2.7 (95)3 3.0 (95). Unless specified, the Ili NMRs were obtained in DMSO-
d6 at 300 or 500
MHz and spectra were recorded in units 8 with CD2HS(0)CD3 (8 2.504) as the
reference line
internal standard. C, H, N microanalyses were performed by Robertson Microlit
Laboratories,
Inc., Madison, NJ.
The following reaction schemes illustrate methods which may be employed for
the
synthesis of the compounds of structural formula I described in this
invention. All substituents
are as defined above unless indicated otherwise. Several strategies based upon
synthetic
transformations known in the literature of organic synthesis may be employed
for the preparation
- 88 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
of the title compounds of general formula I.
GENERAL SCHEME
1) R1-H or R1-M
2) protection PG
PG = SEM or ailyi
Intermediate
R NRNN
-Z 1) deprotection X'
DBU, DMF PG 2) hydrolysis
25 C
INTERMEDIATE 1
6-chloro-5-iodo-2-(rnethylsulfony1)-11-1-imidazo [4,5-b] pyridine
Step A 5, 6-dich1oro-3-nitropyridin-2-amine. To a solution of 5-chloro-3-
nitropyridin-2-amine
(16 g, 92 mrnol) in AcOH (70 mL) was added N-chlorosuccinimide (14.8 g 111
mmol). The
mixture was stirred overnight at 80 C for 3 h, cooled to rt, diluted with
Me0H (30 mL) and
filtered. The solid residue was washed with AcOH, water, and then dried to
afford the desired
product as a white solid, which was used in the next step without further
purification. LC-MS:
calculated for C5H3C12N302 208.0, observed m/e: 208.07 (M+H) (Rt 1.48/5 min).
Step B 5-chloro-6-iodo-3-nitropyridin-2-amine. To a solution of 5,6-dichloro-3-
nitropyridin-2-
amine (15 g, 72.1 mmol) in AcOH (70 mL) was added sodium iodide (43.2 g 149.9
mmol). The
mixture was stirred at 90 C for 2 h, cooled to rt, diluted with water (70 mL)
and filtered. The
solid residue was washed with water, and then dried under vacuum to afford the
desired product
as a pale yellow solid, which was used in the next step without further
purification. LC-MS:
calculated for C5H3C11N302 299.45, observed m/e: 299.94 (M+H)+ (Rt 2.18/5
min).
Step C 5-chloro-6-iodopyridine-2,3-diamine. To a suspension of 5-chloro-6-iodo-
3-nitropyridin-
2-amine (18.9 g, 63.1 mmol) irì Et0H (100 mL) was added tin (11) chloride
dihydrate (57 g, 252
mmol). The mixture was heated at 70 C for 0.5 h. The rxn was warmed to rt and
treated with a
slurry of 150 mL water and 60 g KF and stirred for 0.5 h. The mixture was then
partitioned
between ethyl acetate (300 mL) and water (300 mL). The ethyl acetate layer was
washed with
brine, dried over magnesium sulfate and filtered through a 100 g pad of silica
gel. The filtrate
- 89 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
was concentrated and dried under vacuum to give an off-white solid, which was
used in next step
without further purification. LC-MS: calculated for C5H5C1IN3 269.47, observed
rn/e: 269.99
(M+H)+ (Rt 1.35/5 min).
Step D 6-chloro-5-iodo-1,3-dihydro-2H-imidazo [4,5-b] pyridine-2-thione. [)MAP
(15.4 g, 126
mmol) was added to a THF (200 mL) solution of 5-chloro-6-iodopyridine-2,3-
diamine (17 g,
63.1 mmol). Thiophosgene (4.9 mL, 63.1 mmol) was then added drop-wise via
addition funnel
under nitrogen and allowed to stir at rt for 1 h. The mixture was then
partitioned between ethyl
acetate (500 mL) and 2N HC1 (100 mL). The ethyl acetate layer was washed with
brine, dried
over magnesium sulfate and concentrated to give the desired product as a white
powder, which
was used in the next step without further purification. LC-MS: calculated for
C6H3C1IN3S 311.5,
observed mie: 311.91 (M+H)4- (Rt 1.69/5 min).
Step E 6-chloro-5-iodo-2-(methysulfany1)-1H-imidazo[4,5-blpyridine. A
suspension of 6-
chloro-5-iodo-1,3-dihydro-2H-imidazo [4,5-11 pyridine-2-thione (11.0 g, 35.3
mmol) and KOH
(2.38 g, 42.4 mmol) in ethanol (200 mL) was stirred at rt for 0.5 h.
lodomethane (2.2 mL, 35.3
rnmol) was then added and the reaction was allowed to stir for 1 h at rt. The
ethanol was
removed in vacuo and the resulting residue was partitioned between ethyl
acetate (250 mL) and
2N HC1 (50 mL). The ethyl acetate layer was washed with brine, dried over
magnesium sulfate,
filtered through a 100 g pad of silica gel and concentrated to give the
desired product as a white
solid. LC-MS: calculated for C7H5C11N3S 325.56, observed rn/e: 325.88 (M+11)4-
(Rt 2.05/5
min).
Step F 6-ch1oro-5-iodo-2-(methysu1fony1)-1H-imidazo[4,5-11 pyridine. Oxone
(20.8 g, 33.8
mmol) was added to an acetonitrile (100 mL)/water (100 mL) suspension of 6-
chloro-5-iodo-2-
(methysulfany1)-1H-imidazo[4,5-b] pyridine (5.0 g, 15.4 mmol) and the reaction
was allowed to
stir for 18 h at rt. The suspension was filtered through a sintered glass
funnel and the filtrate was
partitioned between ethyl acetate and saturated sodium bisulfate. The ethyl
acetate layer was
washed with brine, dried over magnesium sulfate and concentrated to afford the
title compound
as a white solid that was used in subsequent steps without further
purification. Solubility
precludes purification and this was used as is. LC-MS: calculated for
C7115CIN302S 357.56,
observed rrile: 357.07 (M+H) (Rt 1.36/4 min) 11-1 NMR 8 (ppm)(DMSO-d6 ): 8.44
(1 H, s),
3.53 (3 H, s).
INTERMEDIATE 2
N
I
EM
6-ohloro-5-iodo-2-(inethylsulfony1)- 1-([2-(trimethylsilyl)ethoxy]methy1}-
imidazo f4,5-131 pyridine
- 90 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
SEM-C1 (2.48 m, 14 mmol) was added to a THF (100 mL) solution of Intermediate
1 (5.0 g, 14 mmol)
and triethyIamine ( 2.92 mL, 21 mmol) at 0 C under nitrogen atmosphere. The
reaction was warmed to rt
over 30 min. The reaction was partitioned between ethyl acetate and saturated
aqueous ammonium
chloride. The organic layer was washed with water, brine, dried over magnesium
sulfate and
concentrated, Flash chromatography of the resulting residue utilizing a
Biotagerm 100G SNAP cartridge
and employing a linear gradient: 0-20% Et0Ac/hexane and then 20-100%
Et0Ac/hexane; afforded the
title compound as a clear oil. LC-MS: calculated for C33H19C1N303SSi 487.8,
observed in/e: 428.9
(WHY (Rt 2,54/4 min).
INTERMEDIATE 3
I
)-0
COOCH3
SEM
Methyl 5-[(6-chloro-5-iodo-1-{12-(trimethy1si1y1)ethoxylmethy1}-1 H imidazo
[4,5-b] pyridin-2-
yboxy1-2-metk lbenzoate. Cesium carbonate (2.67 g, 8.2 mmol) was added to a
DMA (10 mL)
solution of Intermediate 2 (1.6 g, 3.28 mmol) and methyl 5-hydroxy-2-
methylbenzoate (0,82 g,
4.92 mmol) at rt under a nitrogen atmosphere. The reaction was stirred for 15
min, then
partitioned between ethyl acetate and 10% aqueous citric acid. The organic
layer was washed
with water, brine, dried over magnesium sulfate and concentrated. Flash
chromatography of the
resulting residue utilizing a Biotagerm 100G SNAP cartridge and employing a
linear gradient:
0-100% Et0Ac/hexane afforded the title compound as a white solid. LC-MS:
calculated for
C211-125CIN304Si 573.89, observed rn/e: 573.93 (M+H)+ (Rt 2.92/4 min).
INTERMEDIATE 4
CO2Et
Ethyl trans-44(6-chloro-5-iodo-1qprop-2-en-1-y11-1 H imidazo f4,5-b1
oxy}cyclohexanecarboxylate. Sodium hydride (475 mg, 19.8 mmol) was added to a
DMF
solution of Intermediate 1 (5.9 g, 16.5 mmol) and allyl bromide (1.7 mL, 19.8
mmol) at rt. The
reaction was allowed to stir at rt for 16 h. The reaction was then treated
sequentially with ethyl-
4-hydroxycyclohexanecarboxylate (10.64 mL, 66 mmol) and DBU (9.95 mL, 66
mmol). The
reaction was partitioned between Et0Ac and 10% aqueous citric acid. The Et0Ac
layer was
washed with water, brine, dried over magnesium sulfate and concentrated. Flash
chromatography
-91-
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
of the resulting residue utilizing a Biotagerm 100g SNAP cartridge and
employing a gradient: 0-
20% Et0Ac/hexane afforded the title compound as a colorless oil, which
solidified after
overnight vacuum drying. LC-MS: calculated for C1sH2/C1N303 489.7, observed:
m/e: 489.9
(M+H) (Rt 2.75/4 min).
INTERMEDIATE 5
Br =N N
,
Cl N
0
Ethyl trans-4-{ [(5-(4-bromopheny1)-6-ch1oro-1-{prop-2-en-1-y1}-1 H imidazo
0.5-b] pyridin-2-
y1] oxy}eyclohexanecarboxylate. 1,1r-bis(dipheny1phosphinoferrocene-Pa11aditmt
(II) dichloride
dichloromethane complex (183 mg, 0.22 mmol) was added to a DMSO (5 mL)
solution of
Intermediate 4 (550 mg, 1.12 mmol), 4-bromophenylboronie acid (271 mg, 1.35
namol) and
cesium carbonate (1.1 g, 3.37 mmol) at rt under a nitrogen atmosphere. The
reaction was heated
to 90 C for 3 h, then partitioned between Et0Ac and 10% aqueous citric acid.
The organic layer
was washed with water and brine, dried over magnesium sulfate and
concentrated. Flash
chromatography of the resulting residue utilizing a BiotageTm 50G SNAP
cartridge and
employing a linear gradient: 0-100% Et0Ac/hexane afforded the title compound
as a white solid.
LC-MS: calculated for C24H25BrC1N303 518.8, observed m/e: 520.3 (M+H) (Rt
1.4/2 min).
INTERMEDIATE 6
N
I
CI N 0
ll
5-(bipheny1-4-y1)-6-chloro-2-(methylsulfony1)-1H-imidazo[4,5-blpyridine
Intermediate 1 (50 g, 140 mmol), 4 - Biphenylboronic acid (33.2 g, 168 mmol)
and tripotassium
phosphate (89 g, 212.3 mmol) were dissolved in THF (500 mL) and water (50 mL),
then sparged
with N2 for 20 min. A solution of palladium acetate (3.14g, 14.0 mmol) and n-
butyldiadamantylphosphine (Catacxium A, 10g, 28 mmol) in THF (30 mL) was
sparged with N2
for 20 minutes, and then added to the mixture of Intermediate 1,
biphenylboronic acid and base.
The reaction was heated to 45 C for 18 h. An additional aliquot of palladium
acetate (3.14g,
14.0 mmol) and n-butyldiadamantylphosphine (Catacxium A, 10g, 28 mmol) in THF
(30 mL)
- 92.
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
was sparged with N2 for 20 minutes and added to the reaction mixture. After 24
h at 45 C, the
reaction was cooled to rt and diluted with Et0Ac and brine. The organic layer
was concentrated
and triturated with THF/MTBE to provide the title compound as a tan solid. LC-
MS: calculated
for C19E114C1N302S 383.05; observed m/e: 383.9 (M+H)+ (Rt 2.01/4 min).
SCHEME 1
1)1.05 Sem-CI 1.3 Et3N
9
THF @ 0 C - rt
¨N 8R2 Y-
2) 3.0 DBU, 3.1 X-Y-Z
rt SEM
1.2 R1B(OH)2, 3.0 Cs2CO3
Y¨Z 0.2 PdCl2 dppf DCM complex Ri
SEM DMSO (0.1M) @ 90 C 1.5h R2 ---- 1?1
Y¨Z
SEM
N 1)4.0 KOTMS, THF
(0,2M) @ rt RiNN
R2 11 "(--z
R2 N
SEM 2) 10.0 TBAF, THF
@ 80 C 4-8 h
EXAMPLE 1
411I
N
,¨O
CICO H
N 2
5- ([5-(biphenyl-4-y1)- 6-chloro-1-11 imidozo 1-4,5-b] pyridin-2-ylioxy}-2-
methylbenzoic acid.
Step A. 1,11-Bis(diphenylphosphinoferrocene-Palladium (11) dichloride
dichloromethane complex
(142 mg, 0,17 mmol) was added to a dioxane (1.8 mL)/ water (0.2 mL) solution
of Intermediate 3
(200 mg, 0.35 mmol), 4-biphenylboronic acid (104 mg, 0.52 mmol) and lithium
hydroxide ( 20
mg, 0.87 mmol) at rt under a nitrogen atmosphere. The reaction was heated to
80 C for 20 min,
then partitioned between ethyl acetate and 10% aqueous citric acid. The
organic layer was
washed with water, brine, dried over magnesium sulfate and concentrated. Flash
chromatography
of the resulting residue utilizing a BiotageTm 10G SNAP cartridge and
employing a linear
gradient: 0-100% Et0Ac/hexane afforded methyl 5-{[5-(bipheny1-4-y1)- 6-chloro-
1-{ [2-
- 93 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
(trimethylsilypethoxy]methyl} -1 H imidazo [4,54)] pyridin-2-yl]oxyl-2-
methylbenzoate as a
white solid. LC-MS: calculated for C33H34C1N304Si 600.1 8, observed m/e:
600.93 (M+H)+ (Rt
3.2/4 min),
Step B. A formic acid (2.7 mL, 70.4 mmol) solution of methyl 5-{[5-(bipheny1-4-
yI)- 6-chloro-1-
{ [2-(trimethylsilyl)ethoxy]methy1}-1 H imidazo [4,5-1)] pyridin-2-y1loxy}-2-
methylbenzoate
(140 mg, 0.23 mmol) and saturated potassium bisulfate (0.27 mL) was stirred at
80 C for 1 h.
The reaction was partitioned between Et0Ac and brine. The organic layer was
separated, and
concentrated to give methyl 5-{[5-(bipheny1-4-y1)- 6-chloro-1-1-1 imidazo [4,5-
b] pyridin-2-
yl]oxy}-2-methylbenzoate as a white solid, which was used in the next step
without further
purification. LC-MS: calculated for C271120C1N303 469.89, observed rn/e: 470.2
(M+H)+ (Rt
2.3/4 min).
Step C. 2N KOH (1.1 mL, 2.2 mmol) was added to a methanol (10 mL) solution of
methyl 5-
{[5-(bipheny1-4-y1)- 6-chloro-1-H imidazo [4,5-b] pyridin-2-ylloxy}-2-
methylbenzoate (104 mg,
0.22 mmol) and the reaction was stirred at 80 C for 2 h. The reaction was
partitioned between
Et0Ac and 10% aqueous citric acid. The organic layer was separated, washed
with brine, dried
over magnesium sulfate and concentrated. Flash chromatography of the resulting
residue utilizing
a BiotageTm 10G SNAP cartridge and employing a gradient: 0-100%Et0Ac/hexane
afforded the
title compound as a white solid. LC-MS: calculated for C261-118CIN303 455.893
observed m/e:
456.2 (1V1+H) (Rt 2.0/4 min). 111NMR 8 (ppm)(DMSO-d6 ): 8.00 (1H, s), 7.82
(1H,m) 7.79-
7.69 (7H, m), 7.48 (2 H, m), 7.38 (2 H,m), 2.56 (3 H, s).
Table 1. Compounds prepared according to the methods described in Example 1.
HPLC-mass
Example spectrum
Number Structure
m/e
N
2 419.93
CI N 0
411 OH
CN
111W N
3 448.13
ci N 0
II OH
- 94 -
CA 02826649 2013-08-05
WO 2012/116145
PCT/US2012/026261
N,.... N
4 1 --0 464.1
CIN
H . 0
OH
---- N N
I '....j. ,----0 404.05
ci ' N . OH
H
0
NI N( . 0
_
OH 534.09
CI
HO
Si N,.., N
71 -C) 437.92
Cl N
H . OH
0
-....õ,.0 0 F
N. N
1 -.--0
441.83
CI ii OH
H
0
OH
it OH
F
,,,, H
9 F 411I pi N 0 460.0
I --0
Cl ---- N
,
OH
F
Fo
H
F 1410 OH
N N 0 478.0
1 --0
ci ---- N
- 95 -
CA 02826649 2013-08-05
WO 2012/116145
PCT/US2012/026261
Nzz=N
.,- . OH
11 0 N 1-N1 0 447.1
CI ----. N
0
01 N
N OH
12
EN1 4110 o 570.2
I >----0
CI N
_
le
13 40 N N , O'r",OH 420.25
CI --- II
H
4.,.o
14 io N N 0"r". \ 448.31
I , cf OH
Ci NI
I-1
Me026.õ---=.õ..0 40 F
N
0
N 0
I , OH 534.11
Cl N
H
A 0
--- ,
1
16 N. N N = OH
, --. 421.05
I-.--Ci
CI r-' N
H
r-----N
N-1µ\1 411 AL o
17 N N 1111-r OH
447.12
, .
I ¨C)
N
' CI H
NC 0O
18 II N N 411 OH
481.19
I ,-0
CI --- N
H
- 96 -
CA 02826649 2013-08-05
WO 2012/116145
PCT/US2012/026261
(11
0
19 40 N N __OH
463.12
I ¨Ci
ci
H
410 0
20 10 N N = OH
470.02
I --0
Cl N
H
A=-=
0 OH
0
21 14111 N N . OH
485.95
I ,-0
Cl ''' N
H
0
N'j114-Th
22 40 N N . OH
548.01
1 --c,1
ci ---- N
H
0
,g1.
Of N I
23
. N N . OH 584.06
Cl '''' N
H
0
HO S'
* 0
24
N N OH 514.04
=
1 -----0
CI '' N
H
- 97 -
CA 02826649 2013-08-05
WO 2012/116145
PCT/US2012/026261
NC 00
25 0 N N 4411
0H 494.88
CI
H
\ N 0
N"." 41
ii
26 N N OH 461.05
, .
I ,-0
CI
_
ON
4110
27 =N N OH 463.09
I --0
CI
H
1N 0
28 \ lel N
.,. N . .
OH 447.03
, .
I ---0
CI --- N
H
SCHEME 2
1)1.0 NaH, 1,2 allyl-Br 1....,,N,...õN
I N N 0 D1V1F (0.5 M) @ rt ( 16 h)
Y-Z
R2------N 0 2) 2,0 DBU, 2.1 X-Y-Z
1..--.
H I
@ 11
I
N_ 1) 1.2 R1-B(OH)2, 3,0 Cs2CO3 ---X, 0.2 Pd012
clppf DCM complex
R.
R2 NI Y-Z DMSO (0.1 M) @ 90 C 1.5h
'--,.. ________________________________________ ).- j
R2" --=-) -N Y-Z
1 2) 3.0 PhS1I-1, 1-2 h @ 90 C H
R1 N N R1 N,_,N
---....- ...,........ ¨ 4.0 MIMS
I ---X,
R2--'-----5.---N Y-Z THF (0.2M) @ rt R2'---;;--N
Y-Z
H H
DaMPLE 29
- 98 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
114111
N N
0õ
CI N Ci.sr
OH
0
Trans-4-{ [(5-(biphenyl-4-y1)-6-chloro-1 H imidazo [4õ5-131pyridin-2-yll
oxylcyclohexanecarboxylic acid
Step A. Ethyl trans-4-1 [(5-(biphenyl-4-v1)-6-ehloro-1 H imidazo f4,5-
blnyridin-2-y1l
oxy}cyclohexanecarboxylate. 1,15-bis(diphenylphosphinoferrocene-Palladium (II)
dichloride
dichloromethane complex (1.0 g, L22 mmol) was added to a DMSO (50 mL) solution
of
Intermediate 4 (3.0 g, 6.13 mmol), 4-biphenylboronic acid (1.46 g, 7.35 mmol)
and cesium
carbonate (1.99 g, 18.4 mmol) at rt under nitrogen atmosphere. The reaction
was heated to 90 C
for 2 h. The reaction was kept under nitrogen, then phenylsilane (2.27 mL,
18.4 mmol) was
added, and reaction was allowed to stir at 90 C for 1 h. After cooling to rt,
the reaction was
diluted with Et0Ac (200 mL) and poured into 10% aqueous citric acid (200 mL).
The organic
layer was washed with water and brine, dried over magnesium sulfate and
concentrated. Flash
chromatography of the resulting residue utilizing a BiotageTm 100G SNAP
cartridge and
employing a linear gradient: 0-100% ethyl acetate/hexane afforded ethyl trans-
4-{ [(5-(biphenyl-
4-y1)-6-chloro-1 H imidazo [4,5-b] pyridin-2-yl] oxylcyclohexanecarboxylate as
a crystalline
white solid. LC-MS: calculated for C271126C1N303 475.9, observed nee: 476.4
(M+H) (Rt L5/2
min).
Step B. Potassium trimethylsilanolate (1.48 g, 11.5 mmol) was added to a THF
(10 mL) solution
of ethyl trans-44 [(5-(bipheny1-4-y1)-6-chloro-1 H imidazo [455-b] pyridin-2-
yl]
oxylcyclohexanecarboxylate (2.5 g, 5.25 mmol), and the reaction was stirred at
rt for 16 h. The
reaction mixture was then partitioned between Et0Ac and 10% aqueous citric
acid. The organic
layer was washed with brine, dried over magnesium sulfate and concentrated
rinsing with THF
(40 mL). The combined organic layers were concentrated, and then triturated
with acetonitrile
(50 mL) to afford the title compound as an off-white solid. LC-MS: calculated
for C25H22CIN303
447.9, observed m/e: 448.4 (M+H)+ (Rt 1.15/2 min). 1H NMR (ppm)(DMSO-d6 ):
7.91 (1 H,
s), 7.78-7.71 (6 H, m), 7.49 (2 H, t, J = 7.60 Hz), 7.39 (1 H, t, .1= 7.38
Hz), 5.02-4.95 (1 H, m),
2.35-2.27 (1 H, m), 2.24 (2 H, m), 1.99 (2 H, m), 1.57-1.46 (4 H, m).
Table 2. Compounds prepared according to the methods described in Example 29.
HPLC-mass
Example
spectrum
Number Structure
m/e
- 99 -
CA 02826649 2013-08-05
WO 2012/116145
PCT/US2012/026261
ON
* N N
CI H
0
HO
* .
* N N
)--0
31 448.3
--"-- N
CI H
0
HO
\N 010
\ Nc N
32)-----0,
---- N --- 425.9
CI H Q
CO2H
A
140/ N N
33 1>----Q 412.1
CI H Cia,
CO2H
F
N N
1
1 > Q
34 CI ----' N
H
0
HO
- 100 -
CA 02826649 2013-08-05
WO 2012/116145
PCT/US2012/026261
_
%N,N 40
N N
I
439.3
CI
OH
0
,
Cil
N N
1 ) 0
36
CI
H
OH
0
\
INI Olt
N N2
\\
I Q
37 ------ N '5" 438.3
CI
H qii___
OH
0
ON
N 40
N N
I 2 Q
438.4
CI
H Qtti_
OH
0 _
- 101 -
CA 02826649 2013-08-05
WO 2012/116145 PCT/US2012/026261
/CL.c
r
0 ii
N N :
',.
39I\? Q-' 450.2
Cl H "OH
0
NiP
0 0
N N
I 0
40 N) 505.3
CI H it
OH
0
rs
N---- 40
N N
1 .-.
i
41 I\I)
CI 11
OH
O
R
\¨OH
416.4
CI N p (402.1
42
I ) 0 strong
N hl fragment)
0
\--0
- 102 -
CA 02826649 2013-08-05
WO 2012/116145
PCT/US2012/026261
o
43 CI N) p 456,4
0
FM)
0\
CI N p 420.4
) 0 (M-CH2
44 410 N
fragment
strong)
o
Cl N p
45 ) 0 430.4
411 N
o
H
C N p46 I
) 0 434.4
011
\--0
- O3-
CA 02826649 2013-08-05
WO 2012/116145
PCT/US2012/026261
0,
CI ) N p
47 0 426.3
48 CI N p 425.4
) 0
N N
\
CI \ N p
49 1o
454.3
Nr
NN
0,
50 Cl N) p 416.3
0
,0
- 104-
CA 02826649 2013-08-05
WO 2012/116145
PCT/US2012/026261
o
51 Cl N p 430.3
N-` N
o
0
CI N p
>-0
52 110 f\r' N 430.4
HO
o
N p
53
455.3
a N
V 0
Cl N p
54
) 0 411.3
\ NH
- 105 -
CA 02826649 2013 08 05
WO 2012/116145
PCT/US2012/026261
0,
Cl N p 425.4
) 0
N NH
OH
0,
ci N p
56 452.3
1.11
01-1
Cl N p
57 ) 0 432.4
N
0
=
Cl N p
58
458.4
N
HO
-J06-
CA 02826649 2013-08-05
WO 2012/116145
PCT/US2012/026261
1. 40
FLN .õõ
59
535.19
NxQ\ro
o
0CiN
474.1
o
1.1 N N
0
OH
CI N
61 485.1
0-Th 4110
0
o
Ct N p
62 1 478.1
Clo
OH
N p
63 455.1
N
\ 0
- 107 -
CA 02826649 2013-08-05
WO 2012/116145
PCT/US2012/026261
o
Ci N p
64 486.1
oN 40 N
O
o
CI N p
65 412.1
001 N
HN-N
o
CI N
66 528.1
N
oyN
o
CI N p
67 465.1
N N
N.
0
0
CI N p
68 402.1
N
HO
- 108 -
CA 02826649 2013-08-05
WO 2012/116145
PCT/US2012/026261
0
N
69 01 402.1
OH
0
$¨OH
CI N p
70 =
441,1
çN
71 p 412.1
0
Me028..õ----y.0 40 F
72 õd H 526.01
N
,
e¨O
CI N
4111 HO 0
0
73 N
N OH 464.24
ci N
SCHEME 3
- 109-
CA 02826649 2013-08-05
WO 2012/116145 PCT/US2012/026261
1NjF
x tith
1)1.2 R-B(OH)2, 3.0 2 M KR04
NN
0.2 PdCl2 dppf DCM complex
clioxane (0.1M) @ 80 C 2 h
R2 N Y¨Z
F a46,
N mu N 2) 3,0 PhSH, 1-2 h @ 90 C
R'C) raki
1) R-OH 5.0, Cs2CO3, DMS0 (0.2 M), 4h @ 90 C KL.,
Nõ, N
R2 N
2) 4.0 LICH, THF (0.2 M) rt 16h R2
N Y¨Z
EXAMPLE 74
//
k0
v=CI
N\
OH
0
Trans-4-[(6-chloro-5- 3-cyano-4- [(3-methylazetidin-3-yl)oxylpheny1}-1H-
imidazo [4,5-
b1pyridin-2-y1)oxy]cyc1ohexanecarboxy1ic acid
Step A. Intermediate 4 was dissolved in DMSO ( 3.7 mL) and cesium carbonate
(1996 mg, 6.13
mmol), then 3-cyano-4-fluorophenylboronic acid (674 mg, 4.08 mmol) and 1,11-
bis(diphenylphosphinoferrocene-Palladium (II) dichloride dichloromethane
complex (149 rng,
0.204 mmol) were added. The reaction was heated to 80 C and stirred for 4 h.
The reaction was
quenched with citric acid (3 mL, 10% w/v). The organic layer was collected,
concentrated in
vacua and then purified via flash chromatography (40g column, 0-50%
Et0Ac/hexane) to afford
ethyl trans-4-{[6-chloro-5-(3-cyano-4-fluorophenyI)-1-(prop-2-en-1-y1)-1H -
imidazo[4,5-b
]pyridin-2-ylloxy}cyclohexanecarboxylate as a tan solid.
Step B. To a solution of ethyl trans-4- f [6-chloro-5-(3-cyano-4-fluoropheny1)-
1-(prop-2-en-l-y1)-
I H -imidazo[4,5-b ]pyridin-2-y1loxy}cyclohexanecarboxylate (980 mg, 2.029
mmol) in DMSO
(4 mL) was added 1.,1 -bis(dipheny1phosphinoferrocene-Pa11adium (II)
dichloride
dichloromethane complex (148 mg, 0.203 mmol) followed by phenylsilane (220 mg,
2.029
mmol). The reaction was heated at 90 C for 1 h. The reaction was then cooled,
quenched with
citirc acid (10% w/v, 5 mL) and then extracted with Et0Ac (2 x 10 mL). The
organic layers were
collected, dried and then concentrated in vacuo. The residue was purified via
flash
chromatography (40g column, 40 mL/min flow, 0-100% Et0Ac / hexane to afford
ethyl trans-4-
f [6-chloro-5-(3-cyano-4-fluoropheny1)-1H-irnidazo [4,5-b]pyridin-2-ylloxy} -
- 110-
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
cyclohexanecarboxylate as a yellow foam.
Step C. To a solution of ethyl trans-4-{[6-chloro-5-(3-cyano-4-fluoropheny1)-
1H-irnidazo[4,5-
b]pyridin-2-yl3oxy}cyclohexanecarboxylate (30 mg, 0.075 mmol) in DMSO (400 uL)
was added
cesium carbonate (48.9 mg, 0.150 mmol) and 3-methylazetidin-3-ol (2.55 mg,
0.150 mmol). The
reaction was heated to 90 C over 4 h. The reaction was cooled, then quenched
with citric acid,
and extracted with Et0Ac. LiOH (100 pL, 0.200 mmol) was added and the reaction
was stirred
overnight. The reaction was then quenched with citric acid (2 mL, 10% w/v) and
extracted with
Et0Ac (2x5 mL). The organic layers were combined and concentrated in vacuo to
give a crude
residue, which was purified via reverse phase HPLC to afford the title
compound as white
powder. LC-MS: calculated for C241124C1N504 481.2, observed m/e: 482.1 (M-FH)+
(Rt 0.8/2
min).
Table 3. Compounds prepared according to the methods described in Example 74.
HPLC-mass
Example spectrum
Number Structure
m/e
>c 0
\N") = 01
75 N 482.1
NH
N
Oft-0 OH
0
CI
76 N\ 467.1
NH
0
- 111 -
CA 02826649 2013-08-05
WO 2012/116145 PCT/US2012/026261
_
N
//
--4\---O
. CI
77 N /
= 481.1
Nz,.....(NFI
,
0i4.0
.4 i õel-I
0
N
/I
0-0
. CI
78 N\ i 481.1
N<. -,:-. NH
0
SCHEME 4
1...,...R...z......_N Br 0
1.2 R-B(OH)2, 3.0 2 M K3PO4
R2---.5:-----' N \f--.Z 0.2 PdC12 dppf
DCM complex N N
dioxane (0.1 M) @ 80 C 2 h )1h
I .
-.---X,
I R2 N Y¨Z
L.
Br 0 1
1) 1.2 R-B(OH)2, 3.0 Cs2CO3 R 0
N N 0.2 PdC12 dppf DCM complex
, .
I ---)C DM%) (0.1 M) @ 90 C 1.5 h N N
, .
R2 -- ,- N Y¨Z r.
I ---x,
L. 2) 3.0 PhSiH, 1-2 h @ 90oC R2 '' N
Y¨Z
1 3) 4.0 KOTMS, THE (0.2 M) @ rt 18h H
EXAMPLE 79
- 132 -
CA 02826649 2013 08 05
WO 2012/116145
PCT/US2012/026261
Ot
N
CI N
OH
0
Trans-4-( t6-chloro-514'-(methy1su1fony1)bipheny1-4-y1l -1H-imidazo[4,5-
b]pyridin-2-
ylloxy)cyclohexanecarboxylic acid
Step A. 1,1'-bis(dipheny11phosphinoferrocene-Pal1adium (11) dichloride
dichloromethane complex
(260 mg, 0.32 mmol) was added to a dioxane (6 mL) solution of Intermediate 5
(550 mg, 1.06
mmol), 4-(methylsulfonyl)phenylboronic acid (233 mg, 1.17 mmol) and 2 M
potassium
phosphate tribasic (1.59 mL, 3.18 mmol) at rt under nitrogen atmosphere. The
reaction was
heated to 80 C for 4 h. Then phenylsilane (688 mg, 6.36 mmol) was added and
reaction was
stirred at 80 C for 1 h. After cooling to rt, the reaction was diluted with
Et0Ac (200 mL) and
poured into 10% aqueous citric acid (200 mL). The organic layer was separated,
washed with
water and brine, dried over magnesium sulfate and concentrated. Flash
chromatography of the
resulting residue utilizing a SiotageTm 50G SNAP cartridge and eluting with 0-
100%
Et0Ac/hexane afforded ethyl trans-4-({ 6-chloro-5-[4'-(methylsulfony1)-
bipheny1-4-y11-1 11
imidazo [4,5-b] pyridin-2-y1) oxy) cyclohexanecarboxylate as a pale yellow
oil. LC-MS:
calculated for C281128C1N305S 554.05, observed m/e: 555.04 (M+H)+ (Rt 2.2/4
min).
Step B. Potassium trimethylsilanolate (278 mg, 2.16 mmol) was added to a
tetrahydrofuran (3
mL) solution of ethyl trans-4-(1 6-chloro-5[4'-(methylsulfonyl)bipheny1-4-y1]-
1 H imidazo [4,5-
pyridin-2-yll oxy) cyclohexanecarboxylate (240 mg, 0.43 mmol), and the mixture
was stirred
at rt for 16 h. The reaction mixture was partitioned between Et0Ac and 10%
aqueous citric acid.
The organic layer was separated, washed with brine, dried over magnesium
sulfate and
concentrated. Trituration of the resulting crude product with acetonitrile (10
mL) provided the
title compound as an off-white solid. LC-MS: calculated for C26H24CIN305S
526.0, observed
rn/e: 526.3 (M+H)+ (Rt 0.8/2 min). 'H NMR 5 (ppm)(DMSO-d 6 ): 8.03-7.99 (5 H,
m), 7.86 (2
H, d, J ¨ 8.16 Hz), 7.79 (2 H, d, J = 8.12 Hz), 5.01-4.97 (1 H, m), 2.30 (1 1-
1, m), 2.24 (3 H, d, J =
10.17 Hz), 2.00 (3 H, m), 1.58-1.49 (4 H, m).
Table 4. Compounds prepared according to the methods described in Example 79.
HPLC-mass
Example spectrum
Number Structure
m/e
- 113 -
CA 02826649 2013-08-05
WO 2012/116145
PCT/US2012/026261
,N
0'4'% = 0
le N.,
80 541.1
ct =N
OH
0
0
11,0
u011
ell N
81 l)¨(3%. 567.3
et N
H
OH
L
N'
011 N
82 583.4
ct
H
OH
0
N 0
1
N KI
"
83 I 479.3
CI N
H
OH
0
- 114 -
CA 02826649 2013-08-05
WO 2012/116145
PCT/US2012/026261
/\ 04
rir 0
84 1 )---0, 567.8
ci --- N '''
H qOH
-
I
:
41111 N N
85 1 ) 4. 478.3
ci 7 N '
H ,
(11--OH
0
FN,,,,,..
1
..... 40
KL, N
86 I . )---4. 467.3
CI --- N "
H
OH
')
0
* I N...., N
87 492.3
ci ,7 N -..'
H QOH
0
- 115 -
CA 02826649 2013-08-05
WO 2012/116145
PCT/US2012/026261
F
N N
I 466.3
88
CI N
H
OH
0
C)
N
1
i N N
479.3
89
CI N
H
OH
0
OH
F
N
)
90 527.1
N
H 0OH
0
HO I.
N
N
506.3
91
N
H
OH
0
- 116 -
CA 02826649 2013-08-05
WO 2012/116145
PCT/US2012/026261
poio N N
92
506.4
N
H
OH
0
N
N N
93 I 492.3
CI N
H C)\))._
OH
0
0 N
N
0:
94 I 479.3
ci N
H
OH
401 N N
95 473.3
N
H
o
OH
i
- l7-
CA 02826649 2013-08-05
WO 2012/116145
PCT/US2012/026261
o.µ
96
I -02 480.3
N
VON
CI
97
0
491.4
4\ ¨OH
P
98 ill II' 493.3
0 FrO
'''.
-
CI
oP
99 485.3
H
F N F
- 118 -
CA 02826649 2013-08-05
WO 2012/116145
PCT/US2012/026261
o
CI \N>
, 00 492.3
HO ell
0
0\ -
--OFf
Pc. p
101 ) 462.3
4111
OH
CI > op
1027-7 483.4
14
411
CI
\--OH
CI
P
,03
496.1
- 119-
CA 02826649 2013-08-05
WO 2012/116145
PCT/US2012/026261
o
OH
104
473.3
Oil
CI
N
105 463.3
N
VOH
CI
P
106
527.3
111
- 120-
CA 02826649 2013-08-05
WO 2012/116145
PCT/US2012/026261
\
o
ci p
107 tr- N 516.3
Lai
F F
o
4.
01 N p
108 s 517.1 i fkr
ci
ci
0
CI N p
109
N 1E1 517.1
CI 111
CI
0
0
110 N 498.1
110 N
)-0
CI N
SCHEME 5
- 121 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
R1 N 1) LAH, THF N
R2 'Y¨ Y-4(
R2 N
H ¨\OH
EXAMPLE 111
HC;" joH
010 N N
CI N
(3-{[5-(bipheny1-4-y11-6-chloro-1H-imidazo[4,5-b]pyridin-2-ylloxyjcyclobutane-
1,1-diy1)dimethanol.
To a stirred solution of diethyl 2,2'-(34[5-(bipheny1-4-y1)-6-chloro-1H-
imidazo[4,5-b]pyridin-2-
ylloxy}cyclobutane-1,1-diyOdiacetate (ester precursor to example 73, 590 mg,
1,14 mmol) in anhydrous
TRF (13.0 mL) under nitrogen at ambient temperature was added lithium aluminum
hydride (215 mg,
5.67 mmol) in one portion. The mixture exhibited an exotherm and was allowed
to stir at ambient
temperature for 45 minutes. The mixture was diluted with anhydrous THF (20 mL)
and cooled to 5 C.
Saturated aqueous ammonium chloride was added dropwise until gas evolution
ceased. The mixture was
then diluted with a saturated aqueous solution of Rochelle's salt (50 mL) and
stirred for 2 hours. The
mixture was extracted with ethyl acetate (3 x 150 mL). The organic layers were
combined and washed
with a saturated aqueous solution of Rochelle's salt (3 x 70 mL) and brine
(100 mL). The organic layer
was filtered through a thin pad of CeliteTm and concentrated under reduced
pressure. The resultant white
solid was dried under high vacuum at 50 C for 1 hour to provide the title
compound. LC-MS : calculated
for C24H22C1N303 435.13, observed mile: 436.19 (M+H)+ (Rt 2.0/4.0 min). 1H NMR
6 (ppm) (DMSO-d6):
7.90 (111, s), 7.77-7.72 (6H, m), 7.49 (2H, t, .1=7.6 Hz), 7.39 (1H, 6, J=7.2
Hz), 5.27 (1H, t, 7.1 Hz), 4.74
(1H, t, 5.4 Hz), 4.67 (1H, t, 5.5 Hz), 3.41 (2H, d, 5.3 Hz), 3.34 (2H, d, 5.3
Hz), 2.30 (2H, m), 2.07 (2H,
m).
Table 5. Compounds prepared according to the methods in Example 111.
HPLC-mass
Example spectrum
Number Structure
rn/e
434.16
112 N N
CI
- 122-
CA 02826649 2013-08-05
WO 2012/116145
PCT/US2012/026261
._
A
0 N N
113 I 406.19
o .
ot N
H
OH
114 0 N N \ 434.30
OH
CI / N
...--,OH
:
----......--0 0 F
115 2 420.18
, ,0
a - N
,--OH
a,
.=
116 410 N Fri p 427.23
l -----0
ci --' N
c,--AN 0 I" OH
H
N N 435.22
117
i ---0
ci N
OH
N H
118 iN N 520.10
Cl
,---OH
MeO2S......õ----,...õ..0 0
119 m H p
... N 512.14
, ..
I ,--o
Cl N
D
40 Hr
D
120 410 N N OH 440.25
1 ,--o
N
Cl H
..,
,
- 123 -
CA 02826649 2013-08-05
WO 2012/116145
PCT/US2012/026261
HO
121 01 40
N N
, ---,, r.:I---\OH
429.27
I --0
CI ---- N
H
HO
Br I.
I
5:10H
122 N 440.11
-..
N ,--0
CI .'-' N
H
Me02S 0 HO
123 olo 14,...... N OH 514.27
1 --0
CI --- N
H
OH
JOH
124 410 Nõ.. N 464.07
1 --0
--"- N
CI
H
0 H;5\,,,
125 4101 N.,... N OH 450.30
I
CI
H
,
OH
F
126 F fili N I N it
OH 446.08
, -...
,---0
CI -'.. N
H
OH
F
F 410 N 11.
127 F , N OH 464.06
I ¨C) -
CI ----- N
H
- 124-
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
C1NN _______________________________________________________________
128 N 4110
N N = OH 433.26
CI N
1-1
OH
129 N N 448.35
01 N
SCHEME 6
RI N N Ri N N
1) RMgBr
0 ________________________________________________
R pp,
R2 N Y-4
R2 N \t4"-
H
O OH
EXAMPLE 130
0 0
''sjf
0110 N N
Cl N
24cis-3-({6-chloro-5-[48-(methylsulfonyl)bipheny1-4-v11-1H-imidazor4,5-
blnyridin-2-
yl}oxy)cyclobutylipropan-2-ol. To a solution of ethyl cis -3-({6-chloro-544'-
(methylsulfony1)-
biphenyl-4-y11-1H -imidazo[4,5-b] pyridin-2-ylIoxy)cyclobutanecarboxylate
(precursor to
example 110, 19 mg, 0.036 mmol) in 0.5 InL anhydrous THF at 0 C was added
1004L of
MeMgBr solution (3.0 M in THF, 0.3 mrnol). The resulting clear solution was
stirred at 0 C for
46 min, and then quenched by the addition of saturated aqueous ammonium
chloride solution.
The mixture was then partitioned between Et0Ac and water. The aqueous portion
was back-
extracted once with Et0Ac. The combined organic extracts were further washed
once with
saturated aqueous ammonium chloride solution, once with brine, dried over
Na2SO4, filtered, and
concentrated. The resulting crude material was chromatographed on a BiotageTm
10 g SNAP
cartridge, with a linear gradient of 0-20% of Et0Ac in hexanes. The desired
fractions were
combined to provide the desired product as a white foam. LC-MS: calculated for
C26H26C1N304S = 511,13, observed mie: 512.2 (M-FH)+ (Rt 1.97/4 min). 'H NMR
(500 MHz,
- 125 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
CDC15): & 11.21 (s, 1 H), 8.04 (d, J = 8.0 Hz, 2 H); 7.92 (s, 1 H); 7.87-7.80
(m, 4 H); 7.71 (d, .1
¨ 7.9 Hz, 2 H); 5.20 (s, 1 H); 3.12 (s, 3 H); 2.46 (s, 2 H); 2.11 (s, 2 11);
1.94 (m, 1 II); 1.11 (s,
6H).
Table 6. Compounds prepared according to the methods M Example 130.
t 'HPLC-mass
Example spectrum
Number Structure
mie .
_
110
131 10 N N 462.21
i )
! N 04:H
CI H
101
132 0 N N 0 462.32
=
CI ''' N
133 I. 110 N N 04 H 434.31
GI - N
H
IP
134 01 04'-'s-oH
" N , 434.33
:
CI NI
H
1.1 H044,_
135 011111 N N OH 492.31
1 )-0
Ci . îi
H ,
40 Tt
136 410 N N OH 464.42
1 ,>_....0
Ci -" N
H
SCHEME 7
- 126 -
CA 02826649 2013 08 05
WO 2012/116145
PCT/US2012/026261
RI N Nf21 N I'd
1) DIBAL-H -...,- -
...z.....-..
=-=.--- ,..,--,...-
R2'-'!----N sY---<( ________ ).
R2--7----"N \(--(
H H
2 2) RMgBr 0
R H
EXAMPLE 137
N N
0,_,
C I '"- 1\.. ë,
OH
5 Step A : trans-4-{r5-(bipheny1-4-y1)-6-chloro-1H imidazo [4,5-bt pyridin-
2-yll-oxyl
cyclohexanecarbaldehyde. To a solution of the ester precursor to Example 29
(200 mg, 0.420
mmol) in THF (10mL) under N2 at -78 C was added DIBAL-H in toluene (0.364 ml,
0.546
mmol). The mixture was stirred at -78 C for 2 hours, then additional DIBAL-H
(0.840 ml,
1.261 mmol) was added. The reaction was stirred overnight (from -78 C to -
200C), and then
10 quenched by the addition of a few drops of saturated NH4C1 at -78 C.
Precipitate was formed and
removed by filtration through CeliteTm. The filtrate was concentrated under
reduced pressure,
the resulting residue was purified by column chromatography on silica gel
Biotage 25S, eluting
with Et0Ac/isohexane (gradient from 20% to 65%) to give the title compound as
a white solid.
LC-MS : calculated for C25H22C1N302 431.914, observed trie: 432.14 (WM+ (Rt
3.54/5.5
15 min).
Step B: 1-(trans-4-{15-(bipheny1-4-y1)-6-ch1oro-1H imidazo r4,5-bi pyridin-2-
yll-
oxy)cyc1ohexy1ethano1. To a solution of the product from step A (70 mg, 0.162
mmol) in THF
(10 mL) under N2 at 0 C was added methylmagnesium bromide in ether (0.108 ml,
0.324 mmol).
The mixture was stirred at 0 C for 15 min and then for 2 hours at room
temperature. The reaction
20 was quenched by the addition of a few drops of saturated NH4CI at O'C.
Precipitate was formed
and removed by filtration through CeliteTm. The filtrate was concentrated
under reduced
pressure, and the resulting residue was purified by preparative TLC, eluting
with
Et0Ac/isohexane (6:4) to give the title compound as 1:1 mixture of two
isomers. LC-MS:
calculated for C26H26CIN302 447.957, observed tn/e: 448.21 (M+H)+ (Rt 3.59/5.5
min). 11-1
25 NMR 8 (ppm) (CD30D): 7.78 (1H, s), 7.68-7.75 (6H, m), 7.46 (211, t,
J=7.5 Hz), 7.36 (1H, t,
J=7.5 Hz), 4.96 (1H, m), 3.54 (111, qn, J= 6.0 Hz), 2.05 (1 H, m), 1.86 (1 H,
m), 1.55 (2 H, m),
121-1.40 (3 H, m), 1.17(3 H, d, .1= 6 Hz).
Table 7. Compound prepared according to the method in Example 137.
- 127-
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
HPLC-mass
Example spectrum
Number Structure
m/e
1101
138 I. 1'1 N 448.21
N
CI
SCHEME 8
R1 N N
1) PAST
I )¨X
H or F
Y-7\
OH
EXAMPLE 139
010
N N
CI N '"g
Step A: 5-(bipheny1-4-y1)-6-chloro-2-{ftrans-4-(fluoromethyl)cyclohexylloxy)-
1H imidazo 14,5-
1)] pyridine. To a stirred solution of the alcohol (55.6 mg, 0.128 =not) in
CH2Cl2 (10 ml) at -78
C was added DAST (0.085 ml, 0.641 mmol), and the mixture was allowed to stir
at room
temperature for 2 hours. The crude product precipitated from solution and was
isolated by
filtration. The crude product was purified by column chromatography eluting
with
EtOAC/Hexanes (1/1) to give the title compound as a white solid. LC-MS :
calculated for
C25H23CIFN30 435.921, observed m/e: 436.15 (M+11)+ (Rt 3.86/5.5 min). 1H NMR 5
(ppm)
(CD30D): 7.78 (1H, s), 7.67-7.77 (6H, m), 7.46 (2H, t, J-7.5 Hz), 7.36 (1H, t,
J=7.5 Hz), 4.99
(1H, m), 4.33 (111, d, 6.0 Hz), 4.23 (1H, d, 6.0 Hz), 2.35 (2 1i, m), 1.93 (2
H, m), 1.58 (2 H, m),
1.21-1.42 (3 H, m).
SCHEME 9
- 128 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
1) R1B(OH)2
PdC12 Opt DCM complex RI N N
R2- -IN 0 2) Sem-CI, Et3N R2 N
SEM
1) Cs2CO3, x-Y-Z R1 N
N
2) SEM removal "(--Z
SEM
EXAMPLE 140
HO
HEL?
I
Cl N
(2R,3S,5R)-54[6-chloro-5-(4-phenylpheny1)-1H-imidazo[4,5-blpyridin-2-yl]oxy]-2-
(hydroxymethyl)tetrahydropyran-3-ol
Step A: 2-R6-chloro-2-methy1su1fony1-5-(4-pherty1pheny1)imidazo14,5-b1,pyridin-
1-
ylimethoxylethyl-trimethyl-silane To a 0 'V solution of intermediate 6 (4g,
10.42 mmol) in THF
(21 mL) was added triethylamine (1.89 mL, 13.5 mmol) and SEM-C1 (2.03 mL, 11.4
'mop.
The reaction was warmed to rt and stirred 15 min, then diluted with Et0Ac and
water. The
aqueous layer was extracted with Et0Ac (x 2) and the combined extracts were
washed with
brine, dried over Na2SO4, filtered, and concentrated. The resulting crude
material was purified
by column chromatography using a BiotageTm 100g column eluted with a 5% to 50%
Et0Ac/Hexane gradient. The fractions containing product were collected and
concentrated in
vacuo to provide the desired product as a white solid. LC-MS : calculated for
C25H28C1N303SSi
513.13, observed mie: 514.16 (M+11)+; (Rt 1.4/2.0 min).
Step B: 24[241-(4aR3R,8aS)-2-pheny1-4,4a,6,7,8,8a-hexahydropyrano[3,2-
d1[1.3]dioxin-7-
ylioxyl-6-chloro-5-(4-phenylphenypimidazo[4,5-blpyridin-1-ylimethoxyjethyl-
trimethyl-silane
1,5-Anhydro-4,6-0-benzylidene-3-deoxy-]i)-glucitol (266 mg, 1.125 mmol,
Carbosynth, CAS
Number: 152613-20-2), 24[6-chloro-2-methylsulfony1-5-(4-
phenylphenyl)imidazo[4,5-
b]pyridin-1 -yl]methoxy]ethyl-trimethyl-silane from step A (386 mg, 0.75
mmol), and cesium
carbonate (489 mg, 1.5 mmol) were dissolved in DMF (1.5 mL). The reaction
mixture was
stirred for 3 h at rt, then diluted with water and Et0Ac. The aqueous layer
was separated,
extracted with Et0Ac (x 1) and the combined organic layers were washed with
saturated
NaHCO3 and brine, dried over Na2SO4, filtered and concentrated. The resulting
crude material
- 129-
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
was purified by column chromatography using a BiotageTM 25g column eluted with
a 20% to
80% Et0Ac:Hexane ramp. The fractions containing product were collected and
concentrated in
vacuo to provide the desired product as a white foam. LC-MS : calculated for
C37H40C1N305Si
669.24, observed rn/e: 670.34 (M+H)+; (Rt 1.4/2.0 min).
Step C: (2R,3S,5R)-5-[1-6-ch1oro-5-(4-pheny1pheny1)-1H-imidazo[4,5-b]pyridin-2-
y11oxy1-2-
chydroxymethy1)tetrahydropyran-3-ol
24[2-[[(4aR,7R,8aS)-2-pheny1-4,4a,6,7,8,8a-hexahydropyrano[3,2-d][1,3]dioxin-7-
ylioxy]-6-
chloro-5-(4-phenylphenyl)imidazo[4,5-blpyridin-l-ylimethoxy]ethyl-trimethyl-
silane from step
B (190mg, 0.283 mmol) was dissolved in 4M HO in dioxane (4 mL, 16 mmol). The
reaction
was stirred 3 h at rt and additional 4M HC1 in dioxane (2 rriL, 8 mmol) was
added. The reaction
was stirred an additional hour, then diluted with Et0Ac and neutralized with 3
M NaOH and
saturated NaHCO3. The aqueous layer was separated, extracted with Et0Ac (x 2),
and the
combined extracts were washed with brine, dried over Na2SO4, filtered, and
concentrated. The
resulting crude material was purified by column chromatography using a
BiotageTM 25g column
eluted with 10% to 100% Et0Ac/Hexane, then 0% to 5% Me0H/CH2C12. The fractions
containing the desired product were collected and concentrated in vacua. The
resulting foam was
freeze dried from MeCN/water to provide the title compound ai a white solid.
LC-MS:
calculated for C24H22C1N304 451.13, observed rn/e: 452.13 (M+H); (Rt 2.9/5.5
min); 'HNMR 8
(ppm) (CD30D): 7.82 (S, 1H), 7.74-7.68 (m, 6H), 7.46 (t, J--- 7.5 Hz, 2H),
7.36 (t, J = 7.5 Hz,
1H), 5,12 (septet, J = 5.5 Hz, 1H), 4.34 (ddd, J = 10.5, 5.0, 1.5 Hz, 1H),
3.88 (dd, J = 12.0, 2.0
Hz, 1H), 3.67-3.59 (m, 2H), 3.37 (t, J = 10 Hz, 111), 3.18-3.15 (m, 1H), 2.76-
2.74 (m, 1H), 1.69
(q, J = 11.0 Hz, 1H).
Table 8. Compound prepared according to the methods in Example 140.
HPLC-mass
Example
spectrum
Structure
Number m/e
11101
141 101 N N 418.20
Me
142 401 N N 472.17
N
- 130-
CA 02826649 2013-08-05
WO 2012/116145
PCT/US2012/026261
0 0
143 40 N NO 418.1
1 ,--0
CI .'- N
H
0 OH
144 411 N NO 420.2
1 -----0
Cl '' N
H
=
0\
N
145 4111 410 N 7---
N ) 483.1
CI '.- N
H .
40 HO
H0,õN
146 1110 N N. - 438.1
"
N
H
0
147 0 N , N CI 406.18
I .,
7- N
H 0
H
HO
,,,)HO v 1-110
148 =, N L\O 446.14
i ,----d`
ci N
H
Me02S 0 HO
1-1(?)
101 N N c___ 530.13
149
1 .
ci ---- N
H
- 131 -
CA 02826649 2013-08-05
WO 2012/116145
PCT/US2012/026261
_
HO
HQ_ )
F
40
150 N N CO 438.07
, N
CI
N
H
H(? HO
._)Me02S.,õ-,0 00 F
151 N N U0 530.20
CI -- N
H
101
152 op N N 418.20
1 __.,,c,õ,õõ0....õõMe
ci N
H
410
153 410 N N 472.17
oim.... c3
=-- N
CI H
00
154 40 N N,-0\_____t 406.18
1
CI N
H 0
H
40 0
155 0 NNO 418.1
1 )-0
CI --"' N
H
40 czcH
156 SI N. N 420.2
1)-0
CI --- N
H
- 132 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
0
157 111111 N N 483.1
CI N
HO
HO0
158 N N 438.1
N
SCHEME 10
RN N DBU, X-Y-Z
\ ¨ SO2Me __________________________________
N
R2 2) Formic Acid R2NY¨Z
SEM KHSO4
EXAMPLE 159
N N Hi ,() OH
"H
Ci N
1, 4:3, 6-dianhydro-2-045-(bipheny1-4-y1)-6-chloro-1H-irnidazo[4,5-b-lpyridin-
2-y1]-D-mannitol
Step A: Mixture of 1, 4:3, 6-dianhydro-2-0-1-5-(bipheny1-4-y1)-6-chloro-1-{[2-
ftrimethy1si1y1)ethoxylmethy1}-1H-imidazo[4,5-b] pyridin-2-y11-5-0-ftert-
butyl(dimethypsilyli-
D-mannitol and 1, 4:3, 6-dianhydro-2-045-(bipheny1-4-y1)-6-chloro-1- { [2-
itrimethylsilybethox_ylmethy1}-1H-imidazof4,5-b] pyridin-2-y11-D-mannitol
A mixture of 2-11[6-chloro-2-methylsulfony1-5-(4-phenylphenyl)imidazo[4,5-
b]pyridin-l-
yl]methoxylethyl-trimethyl-silane (from step A in Example 147, 2023 mg, 3.93
mmol), 1, 4:3, 6-
dianhydro-2-0-[tert-butyl(dimethyl)sily1]-D-mannitol (2049 mg, 7.87 mmol, from
WuXi
PharmaTech Co., Ltd. ) and DBU (1.186 mL, 7.87 mmol) in DMA (30 mL) was
stirred at r.t.
overnight. Then the cincle mixture was extracted with Et0Ac and washed with
water. The
organic layer was dried over anhydrous sodium sulfate and concentrated under
reduced pressure.
The resulting residue was purified by column chromatography on silica gel
BiotageTM 40M,
eluting with a 5% to 100% Et0Ac:Hexane ramp. The fractions containing product
were collected
and concentrated in vacuo to provide the desired products as white solid. LC-
MS : calculated for
- 133 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
C36H48C1N3OsSi2 693.28, observed mile: 694.05 (M+H)+; (Rt 3.17/4.0 min) and
calculated for
C301134C1N3O5Si 579.20, observed m/e: 579.97 (M+H)+; (Rt 2.59/4.0 min).
Step B:1, 4:3, 6-dianhydro-2-0-[5-(bipheny1-4-y1)-6-chloro-1H-imidazo[4,5-
blpyridin-2-y1]-D-
mannitol The product from step A (2160mg, 1.695 mmol) was dissolved in formic
acid (27 mL),
and saturated aqueous potassium hydrogen sulfate (3 mL, 1.695 mmol) was added.
The reaction
was stirred at room temperature overnight, then cooled to 0 C, and basified
to pH-12 with
NaOH (50/50% by weight). The mixture was diluted with THF (10 mL) and stirred
at room
temperature for 45 min. Then resulting mixture was acidified to pH=7 with 2N
HC1, and
extracted with Et0Ac. The combined organic layers were washed with water
twice, once with
brine, then dried over anhydrous sodium sulfate, filtered and concentrated to
give the crude
product. The crude product was purified by column chromatography on silica gel
Biotage TM
40M, eluting with CH2C12/Et0H/NH4-95/4/1 (gradient from 5% to 9%) to give
crude product,
which was recrystallized from CH2C12/Me0H to give the title compound as a
white solid. LC-
MS : calculated for C24H20C1N304 449.11, observed mile: 449.96 (M+H); (Rt
3.2/5.5 min);
NMR 8 (ppm) (DMSO-d6): 7.91 (S, 1H), 7.70-7.80 (m, 6H), 7.48 (t, J = 7.5 Hz,
2H), 7.38 (t, J =
7.5 Hz, 1H), 5.47 (qt, J = 6.0 Hz, 1H), 5.05-4.90 (m, br,1H), 4.82 (t, J =
5.0Hz, 1H), 4.35 (t, J =
5.0 Hz, 1H), 4.20-4.00 (m, 2H), 3.90 (m, 1H), 3.77 (t, J = 7.5Hz, 1H), 3.42
(t, J = 8.511z, 1H),
Table 9. Compound prepared according to the methods in scheme 10 and the
procedure of
Example 159.
Example HPLC-mass
Number Structure spectrum
m/e
o
160 410 N N ,,"'OH
449.89(M+1)
'
CI N 0
0
161 401 , N 11/,
OH 449.89(M+1)
'
CI N 0
162 N N '',t0H
449.89(M+1)
N ''H
CI
H H
0
- 134-
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
INTERIVIEDIATE 7
OH
Br 0
µ+'
N N 0
CI
o
(3R,3aR,6R,6aR)-645-(4-bromopheny1)-6-chloro-1-(2-
trimethy1sily1ethoxymethypimidazo[4,5-
bipyridin-2-y1]oxy-2,3,3aõ5,6,6a-hexahydrofuro[3,2-b]furan-3-ol
Step A 5-(4-bromopheny1)-6-chloro-2-(methylsulfony1)-1H-irnidazo[4,5-
blpyridine.
PalladiumTetrakis (1.63 g, 1.411 mmol) was added to a stirred hazy solution of
intermediate 1
(10.08 g, 28.2 mmol), 4-bromophenylboronic acid (6.23 g, 31.0 mmol), and
potassium phosphate
(18.44 g, 87 mmol) in dioxane (150 mL) and water (30 mL). The reaction mixture
was degassed
(3x) and placed under nitrogen before being heated to 100 C. After 17 hours,
the reaction
mixture was cooled to room temperature before being evaporated under reduced
pressure. The
resulting residue was stripped with toluene (2 x 60 mL) to afford the product
as a brown / white
solid, which was used in the next step without further purification. LC-MS:
calculated for
Ci3H9BrCIN302S 384.93, 386.93 observed m/e: 385.81, 387.84 (M+H)+ (Rt 1.15 / 2
min).
Step B 2-1I5-(4-bromopheny1)-6-chloro-2-methylsulfonyl-imidazo[4,5-blpyridin-1-
yl]methoxylethyl-trimethyl-silane. N,N-diisopropylethylamine (15 mL, 86 mmol)
was added to
a stirred suspension of the unpurified 5-(4-bromopheny1)-6-chloro-2-
(methylsulfony1)-1H-
imidazo[4,5-blpyridine from the previous step in THF (150 mL). The reaction
mixture was
cooled to 0 C in an ice bath prior to the slow addition of SEM-CI (10 mL,
56.4 mmol) over 9
minutes. Ten minutes after the addition was complete, the reaction mixture was
removed from
the ice bath and allowed to warm to room temperature. After 16 hours, the
reaction mixture was
partitioned following the addition of water (200 mL). The aqueous layer was
extracted with
Et0Ac (3 x 200 mL). The organic layers were combined, washed with brine (1 x
100 mL), dried
over Na2SO4, filtered, and evaporated under reduced pressure to give a yellow
/ brown solid.
Flash chromatography of the solid utilizing two 165 g silica RediSep R(l)
columns and
employing a 0-30% Et0Ac / hexane gradient with a 30% Et0Ac / hexane hold
afforded the
desired product as a yellow solid. LC-MS: calculated for C19H23BrC1N303SSi
515.01, 517.01
observed tn/e: 515.85, 517.86 (114 1-1)+ (Rt 1.33 / 2 min).
Step C (3R,3aR,6R,6aR)-6-1-5-(4-bromopheny1)-6-chloro-1-(2-
- 135-
CA 02826649 2013-08-05
WO 2012/116145 PCT/US2012/026261
trimethylsilylethoxymethyDimidazo14,5-bIpyridin-2-yl]oxy-2,3,3a,5,6,6a-
hexahydrofurof3,2-
hifuran-3-01. DBU (4.2 mL, 27.9 mmol) was added to a stirred solution of
isomannide (4.11 g,
28.1 mmol) iri DMF (60 mL). The reaction mixture was a yellow solution that
was stirred at
room temperature. A suspension of 2-[{5-(4-bromopheny1)-6-chloro-2-
methylsulfonyl-
imidazo[4,5-b]pyridin-l-yllmethoxylethyl-trimethyl-silane (4.78 g, 9.25 mmol)
in DMF (34 mL)
was added dropwise to the reaction mixture over 54 minutes. After 1.5 hours,
the reaction
mixture was partitioned between Et0Ac (500 mL) and water (200 mL). The organic
layer was
washed with water (4 x 100 nit) and brine (1 x 100 mL), dried over Na2SO4,
filtered, and
evaporated under reduced pressure to give a yellow foam. Flash chromatography
of the foam
utilizing a 120 g silica RediSep Rf column and employing a 0-70% Et0Ac /
hexane gradient
with a 70% Et0Ac / hexane hold afforded the title compound as a white foam. LC-
MS:
calculated for C241129BrCIN305Si 581,07, 583.07 observed m/e: 582.20, 584.23
(M+H)+ (Rt 1.32
/ 2 min).
INTERMEDIATE 8
H0F1
O
4111) 0
N N 0 z
CI
o
(3R,3aR,6R,6aR)-646-chloro-544-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-
yDphenyll-1-(2-
trirnethylsilyiethoxymethyDimidazof4õ5-blpyridin-2-ylloxy-2,3,3a,5,6,6a-
hexahydrofuro[3,2-
b]furan-3-ol
1,1'-bis(diphenylphosphino)ferrocene-palladium(IDdichloride dichloromethane
complex (0.5137
g, 0.629 narnol) was added to a stirred suspension of intermediate 7 (3.1823
g, 5.46 mmol),
bis(pinacolato)diboron (4.09 g, 16A1 mmol), and potassium acetate (2.79 g,
28.4 mmol) in
dioxane (50 mL). The reaction mixture was degassed (3x) and placed under
nitrogen before
being heated to 80 'C. After 2 hours, the reaction mixture was cooled to room
temperature
= before being partitioned between Et0Ac (200 mL), water (200 mL), and enough
brine to break
an emulsion. The aqueous layer was extracted with Et0Ac (3 x 100 mL). The
organic layers
were combined, washed with brine (1 x 100 mL), dried over Na2SO4, filtered,
and evaporated
under reduced pressure to give a dark brown residue. Flash chromatography of
the residue
- 136-
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
utilizing a 120 g silica RediSep Rf column and employing a 0-70% Et0Ac /
hexane gradient
with a 70% Et0Ac / hexane hold afforded the title compound as a white foam. LC-
MS:
calculated for C30H4113C1N307Si 629.25 observed mie: 630.46 (M+H)+ (Rt 1.34 /
2 min).
INTERMEDIATE 9
Br
N
OH
0
,µµH
N N HN"
el
o
Si
f3R3aR,6R,6aR)-6-15-14-(5-bromo-2-pyridyl)nheny1}-6-chloro-1-(2-
trimethy1si1y1ethoxymethv1)imicin70E4,5-b1pyridin-2-y11oxy-2,3.,3a,5,6,6a-
hexahydrofuror3,2-
b]furan-3-ol
PalladiumTetrakis (189.5 mg, 0.164 mmol) was added to a hazy stirred solution
of intermediate 8
(0.98 g, 1.556 mmol), 2,5-dibromopyridine (375.5 mg, 1.585 mmol), and sodium
carbonate
(669.9 mg, 6.32 mmol) in 1,4-dioxane (80 mL) and water (20 mL). The reaction
mixture was
degassed (3x) and placed under nitrogen before being heated to 80 C. After 2
hours, the
reaction mixture was cooled to room temperature before being partitioned
between Et0Ac (150
nth) and water (150 mL). The aqueous layer was extracted with Et0Ac (3 x 75
mL). The
organic layers were combined, washed with thine (1 x 100 mL), dried over
Na2SO4, filtered, and
evaporated under reduced pressure to give an amber foam. Flash chromatography
of the foam
utili7ing an 80 g silica RediSep Rfe column and employing a 0-80% Et0Ac /
hexane gradient
with a 80% Et0Ac / hexane hold afforded the title compound as a white solid.
LC-MS:
calculated for C29H32BrCIN405Si 658.1, 660.1 observed m/e: 659.36, 661.33
(M+11)1- (Rt 1.33 /
2 min).
INTERMEDIATE 10
- 137-
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
Br N
\ I I.
:HH
Hkk
N N
\
N
CI
0
Si
(3R,3aR,6R.6aR)-64544-(6-brorno-3-pyridyflphenyli-6-chloro-1-(2-
trimethylsilylethoxymethypimidazo[4,5-blpyridin-2-ylloxy-2,3,3a,5,6,6a-
hexah.ydrofuro[3,2-
bifuran-3-ol PalladiumTetralcis (134.9 mg, 0.117 mmol) was added to a stirred
hazy solution of
intermediate 8 (625.8 mg, 0.993 rnmol), 2-brorno-5-iodopyridine (313.1 mg,
1.103 mmol), and
potassium phosphate (631.3 mg, 2.97 mmol) in 1,4-dioxarie (8 mL) and water (2
mL). The
reaction mixture was degassed (3x) and placed under nitrogen before being
heated to 80 C.
After 7.5 hours, the reaction mixture was cooled to room temperature before
being partitioned
between Et0Ac (150 mL) and water (150 mL). The aqueous layer was extracted
with Et0Ac (3
x 75 mL). The organic layers were combined, washed with brine (1 x 100 mL),
dried over
Na2SO4, filtered, and evaporated under reduced pressure to give an amber foam.
Flash
chromatography of the foam utilizing a 40 g silica RediSep Rf column and
employing a 0-80%
Et0Ac / hexane gradient with a 80% Et0Ac / hexane hold afforded the title
compound as an off-
white solid following lyophilization from ethanol and benzene. LC-MS:
calculated for
C29H3zEirC1N405Si 658.1, 660.1 observed rnie: 659.30, 661.33 (M-FH)+ (Rt 1.31
/ 2 min).
INTERMEDIATE 1 1
N
0 OH
" "kH
N N 0
I
01 N
4441241(3R,3alt6R,6aR)-3-hydroxy-2,3,3a,5,6,6a-hexahydrofuro[3,2-b1firan-6-
yl1oxy1-6-
chloro-1H-imidazo[4,5-bluridin-5-yliphenyllbenzonitrile
Step A 44442-1[(3R,3aR,6R,6aR)-3-hydroxv-2,3,3a,5,6,6a-hexahydrofuro13,2-
b]furan-6-
ylloxyl-6-chloro-1-(2-trimethylsilylethoxymethyDimidazo[4.5-blpyridin-5-
yllphenylibenzonitrile. LiOH (0.22 mL, 0.660 mmol) and 1,1'-
bis(dipheny1phosphino)-
ferrocene-palladium(II)dichlofide dichloromethane complex (24.1 mg, 0.030
mmol) were added
- 138 -
CA 02826649 2013 08 05
WO 2012/116145
PCT/US2012/026261
to a stirred solution of intermediate 7 (153.1 mg, 0.263 mmol) and 4-(4,4,5,5-
tetramethy1-1,3,2-
dioxaborolan-2-yl)benzonitrile (126.4 mg, 0.552 mmol) in 1,4-dioxane (2.1 mL)
and water (0.31
mL). The reaction mixture was degassed (3x) and placed under nitrogen before
being heated to
80 C. After 16 hours, the reaction mixture was cooled to room temperature
before being
partitioned between Et0Ac (40 mL) and water (40 mL). The aqueous layer was
extracted with
Et0Ac (2 x 20 mL). The organic layers were combined, washed with brine (1 x 20
mL), dried
over Na2SO4, filtered, and evaporated under reduced pressure to give a brown
residue. This
material was purified by flash chromatography utilizing a 4 g silica RediSep
Rfe column and the
following conditions: a 0-60% Et0Ac / hexane gradient, a 60% Et0Ac / hexane
hold, a 60%-
70% Et0Ac / hexane gradient, and a 70% Et0Ac / hexane hold. The desired
fractions were
combined, evaporated under reduced pressure, and lyophilized from ethanol and
benzene to give
the desired compound as a yellow solid. LC-MS: calculated for: C3
11133C1N405Si 604.19
observed in/e: 605.21 (M-I-H)+ (Rt 1.31 / 2 min).
Step B 4-14424[(3R.3aR,6R,6aR)-3-hydroxy-2,3,3a,5,6,6a-hexahydroftirof3,2-
b]furan-6-
yl]oxyl-6-chloro-1H-imidazo[4,5-b]oyridin-5-yllphenyljbenzonitrile. A mixture
of 4-[4-[2-
[[(3R,3aR,6R,6aR)-3-hydroxy-2,3,3a,5,6,6a-hexahydrofuro[3,2-b]ftiran-6-yl]oxy1-
6-chloro-1-(2-
. trimethylsilylethoxymethyt)imidazo[4,5-b]pyridin-5-
yliphenyllbenzonitrile (49.4 mg, 0.082
mrnol), formic acid (1.0mL, 26.1 rnmol), and saturated aqueous KHSO4 (0.06 mL)
was heated to
40 C with stirring. After 6.5 hours, the reaction mixture was cooled to room
temperature and
placed in the refrigerator overnight, and then cooled to 0 C in an ice bath.
The pH was adjusted
to pH 14 through the addition of 5 N NaOH (5.2 mL, 26 mmol). THF (2 nip was
added to the
reaction mixture, which was removed from the ice bath and allowed to warm to
room
temperature. After stirring for 45 minutes at room temperature, the pH was
adjusted to 7 through
the addition of 2 N HCI. The reaction mixture was partitioned between Et0Ac
(30 mL) and
water (30 mL). The aqueous layer was extracted with Et0Ac (2 x 20 mL). The
organic layers
were combined, washed with brine (1 x 20 mL), dried over Na2SO4, filtered, and
evaporated
under reduced pressure to give a pale yellow solid. Purification by HPLC
reverse phase (C-18),
using a 30 x 150 mm SunfireTM column and eluting with a 20%-100% acetonitrile
/ water +
0.05% TFA gradient followed by a 100% acetonitrile -4- 0.05% TFA flush
afforded the title
compound as a white solid following lyophilization from ethanol and benzene.
LC-MS:
calculated for: C251119C1N404 474.11 observed in/e: 475.12 (M+H)+ (Rt 1.15 / 2
min).
INTERMEDIATE 12
HO
Br
N
1-(1-(4-bromopheny1)-1H-pyrazol-4-y1)-2-methylpropan-2-ol
- 139-
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
Step A methyl 2-(1H-pyrazol-4-yflacetate. 2-(1H-pyrazol-4-yl)acetic acid (970
mg, 7.69 mmol)
was placed under nitrogen in anhydrous methanol (80 mL). The mixture was
sonicated until solid
completely dissolved. To this solution was added dropwise over 30 minutes a
2.0M solution of
trimethylsilyldia2omethane in hexanes (3.85 mL, 7.69 mmol). A small excess of
trimethylsilyidia2:omethane was added until the yellow color persisted. The
mixture was allowed
to stir for 20 minutes at room temperature, and then concentrated under
reduced pressure. The
resulting orange oil was chrornatographed using a Biotage 25g silica gel
cartridge eluted with 0-
5% methanol in dichloromethane over 10 column volumes with a 10 column volume
hold at 5%
methanol. The product fractions were combined and concentrated under reduced
pressure to
afford the desired compound as a clear, colorless oil. 1H NMR 8 (ppm)
(CJ)C13): 7.54 (2H, s),
3.70 (3H, s), 3.55 (2H, s).
Step B 2-methyl-1-(1H-pyrazol-4-yl)propan-2-ol. Methyl 2-(1H-pyrazol-4-
yl)acetate (700 mg,
4.99 mmol) was placed under nitrogen in anhydrous THF (100 mL). The mixture
was cooled to
0 C and a 3.0M solution of MeMgBr (15.38 mL, 46.10 mmol) was added dropwise
over 5
minutes. The mixture was allowed to warm to room temperature. After 16 hours
the mixture was
poured into saturated aqueous sodium bicarbonate (100 mL). The mixture was
extracted with
Et0Ac ( 4 x 100 mL). The combined organic layers were washed with brine (100
mL), dried over
sodium sulfate, and concentrated under reduced pressure. The resulting orange
oil was
chromatographed using a Biotage 25g silica gel cartridge eluted with 0-10%
methanol in
dichloromethane over 15 column volumes with a 5 column volume hold at 10%
methanol. The
product fractions were combined and concentrated under reduced pressure to
provide the desired
product as a white solid. 111 NMR 8 (ppm) (CDC13): 7.40 (2H, s), 2.63 (2H, s),
L21 (6H, s).
Step C 141-(4-bromopheny1)-1H-pyrazol-4-y1)-2-methylpropan-2-ol. 2-methy1-1-
(1H-pyrazol-4-
yl)propan-2-ol (21 mg, 0.15 mmol), copper (II) acetate (27 mg, 0.15 mm.o1),
and 4-
bromophenylboronic acid (30 mg, 0.15 mmol) were placed under nitrogen in 1,2-
dichloroethane
(1 mL). Added 4A molecular sieves (-20 mg) and pyridine (36 uL, 36 mg, 0.45
mmol). The
mixture was stirred at 50 C for 9 hours while open to the atmosphere. The
mixture was allowed
to cool to room temperature, filtered through CeliteTm , rinsed with methanol,
and concentrated
under reduced pressure. The resulting orange oil was chromatographed using a
Biotage 50g (2 x
25g in series) silica gel cartridge eluted with 0-5% methanol in
dichloromethane over 30 column
volumes. The desired product fractions were combined and concentrated under
reduced pressure
to provide a colorless oil (25 mg, 54%). LC-MS: calculated for C131-115BrN20;
295.17 observed
m/e: 296.87 (M+H)+ (Rt 1.75 / 4 min). NMR 5 (ppm) (CL)C13):' 7.77 (1H, s),
7.58 (1H, s),
7.55 (4H, s), 2.67 (2H, s), 1.26 (6H, s).
EXAMPLE 163
- 140-
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
N
0 OH
=O INI 0
CI
(3R,3aR,6R,6aR)-641-6-chloro-5-[4-(4-pyrazo1-1-y1pheny1)pheny11-1H-imidazo[4,5-
b1pyridin-2-
ylloxy]-2,33a,5,6,6a-hexahydrofuro[3,2-b]furan-3-ol
Step A f3R,3aR,6R,6aR)-646-chloro-5-[4-(4-pyrazol-1-ylphenyl)phenyll-1-(2-
trimethylsilylethoxymethypimidazo[4,5-blpyridin-2-yl]oxy-21313a,5,6,6a-
hexahydrofurof3,2-
b1fura.n-3-ol. (4-pyrazol-1-ylphenyl)boronic acid (127 mg, 0.675 mmol), 1,1t-
bis(diphenylphosphino)ferrocene-palladiumaDdichloride diehloromethane complex
(45.9 mg,
0.056 mmol), and LiOH (0.469 mL, 1.407 mmol) were added to a stirred mixture
of intermediate
7 (328 mg, 0.563 mmol) in 1,4-dioxane (3 mL) and water (0.8 la). The reaction
mixture was
placed under nitrogen before being heated 90 'C. After 2 hours, the reaction
mixture was cooled
to room temperature before being partitioned between Et0Ac (50 mL) and
saturated aqueous
ammonium chloride (50 mL). The aqueous layers were extracted with Et0Ac (2 x
50 mL). The
organic layers were combined, washed with brine (1 x 50 mL), dried over
Na2SO4, filtered, and
evaporated under reduced pressure. Flash chromatography of the resulting
residue utilizing a
silica gel BiotageTM 255 column and employing a 0-80% Et0Ac / hexane gradient
afforded the
desired compound as a light yellow solid. LC-MS: calculated for C331-
136C1N505Si 645.22
observed m/e: 646.48 (M+H)+ (Rt 1.32 / 2 min).
Step B (3R,3aR,6R,6aR)-641-6-chloro-544-(4-pyrazo1-1-ylpheny1)phenyli-1H-
imidazo[4,5-
blpyridin-2-yl]oxy]-2,313a55,6,6a-hexahydrofuro[3,2-b]furan-3-ol. Combined
formic acid (3 mL,
78 mmol), saturated aqueous KHSO4 (0.33 mL, 0.289 mmol), and (3R,3aR,6R,6aR)-
646-ehloro-
544-(4-pyrazol-1-ylphenyl)phenyll -1-(2-trimethylsilylethoxyrnethypimidazo-
[4,5-13]pyridin-2-
ylioxy-2,3,3a,5,6,6a-hexahydrofuroP,2-bifuran-3-ol (187 mg, 0.289 mmol). The
reaction
mixture was stirred at 60 C overnight. The reaction mixture was cooled to 0
C in an ice bath.
The pH of the reaction mixture was adjusted to >11 by the addition of NaOH
(3120 mg, 78
mmol) in water (5 mL). THF (5 mL) was added to the reaction mixture, then the
reaction was
removed from the ice bath and allowed to warm to room temperature. After 30
minutes, the pH
of the reaction mixture was adjusted to 6 through the addition of concentrated
HC1. The reaction
mixture was partitioned between Et0Ac (100 mL) and water (50 mL). The aqueous
layer was
extracted with Et0Ac (2 x 100 mL). The organic layers were combined, washed
with brine (1 x
50 mL), dried over Na2SO4, filtered and evaporated under reduced pressure. The
resulting
residue was purified by preparative HPLC reverse phase (C-18), using a 19 x
100 mm SunfireTM
column and eluting with a 10%-90% acetonitrile / water + 0.05% TFA gradient
followed by a
- 141 -
CA 02826649 2013-08-05
WO 2012/116145 PCT/US2012/026261
90% acetonitrile / water + 0.05% TFA flush. The desired fractions were
combined, evaporated
under reduced pressure, and lyophilized from acetonitrile and water to give
the title compound as
a white solid. LC-MS: calculated for C271122C1N504 515.14 observed mile:
515.92 (M+H)+ (Rt
1.16 / 2 min); 111 NMR 5 (ppm) (CD3OD): 8.30 (d, .1 = 2.5 Hz, 1H), 7.87 (m,
4H), 7.85 (s, 1H),
7.79 (m, 4H), 7.77 (d, J - 1.5 Hz, 1H), 6.58 (t, J = 2 Hz, 111), 5.56 (qt, J 5
Hz, 1H), 4.99 (t, J =
5.3 Hz, 111), 4.49 (t, J --- 5.3 Hz, 1H), 4.28-4.31 (m, 111), 4.19 (dd, J -
5.5 Hz, 10.5 Hz, 111), 4.12
(dd, J = 4.5 Hz, 10 Hz, 1H), 3.92 (t, J = 7.5 Hz, 1H), 3.62 (t, J - 8.8 Hz,
1H).
OR
Br
H "tH A.) Pd(II)dppf-CH2C12
(4-pyrazol-1 Ilphenyl)boronie tkl
N
\>-o
01
0 water 1.1 :
0o
90 C 114111 N N H".
0
ler
13.) Formic Acid
KEIS04
CI
600C
The isomannide alcohol starting material was either unprotected (R=H) or TBS
protected
(R-OTBS) during the 1,1'-bis(diphenylphosphino)ferrocene-palladium(I1)-
dichloride
dichloromethane complex coupling reaction used to prepare Example 163, as
shown above. The
TBS group was observed to substantially deblock during the reaction giving the
unprotected
alcohol.
EXAMPLE 164
rth,
N 410
OH
0
H
N N 0
CI
(3R,3aR,6R,6aR)-64[6-chloro-5-14-[4-(1-methylimidazol-2-y1)phenyl]nhenyl]-111-
imidazof4,5-
b]pyridin-2-yljoxyl-2,3,3a,5,6,6a-hexahydrofurof3,2-bjfuran-3-ol
Step A (3R,3aR,6R,6aR)-6-115-(4-bromophenv1)-6-chloro-1H-imidazo[4,5-blpyridin-
2-yl]oxy]-
2,3,3a,5,6,6a-hexahydrofuro[3,2-b]furan-3-ol. A mixture of formic acid (4.5
mL, 117 mmol),
saturated aqueous 1(1-B0.4(0.5 mL, 0.940 rarnol), and intermediate 7 (548 mg,
0.940 mrnol) was
heated at 60 C overnight. The reaction mixture was cooled to 0 C in an ice
bath. The pH of
the reaction mixture was adjusted to > pH 11 through the addition of NaOH
(4680 mg, 117
mmol) in water (10 mL). THF (10 mL) was added to the reaction mixture, which
was removed
from the ice bath and allowed to warm to room temperature. After 30 minutes,
the pH of the
reaction mixture was adjusted to pH 6 through the addition of concentrated
HC1. The reaction
- 142-
CA 02826649 2013 08 05
WO 2012/116145
PCT/US2012/026261
mixture was extracted with Et0Ac (3 x 80 mL). The organic layers were
combined, washed with
brine (1 x 100 mL), dried over Na2SO4, filtered, and evaporated under reduced
pressure. Flash
chromatography of the resulting residue utilizing a silica gel BiotageTM 25M
column and
employing a 0-10% Me0H / DCM gradient afforded the desired product as a white
solid. LC-
MS: calculated for CI8F115BrCIN304 450.99, 452.99 observed tn/e: 452.00,
454.02 (M+H) (Rt
1.14 / 2 min).
Step B (3R,3aR66R,6aR)-64[6-chloro-5-[444-(1-methylimidazol-2-
yl)phenyl]phenyl]-111-
imidazo[4,5-blpyridin-2-ylioxyl-2,3,3a,5,6,6a-hexahydrofuror3,2-blfuran-3-ol.
1-methy1-244-
(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-y1)phenyliimida7ole (26.4 mg, 0.093
mmol), 1,1'-
bis(diphenylphosphino)ferrocene-palladium(Il)dichloride dichloromethane
complex (6.33 mg,
7.75 !mop, and Li0H (0.065 mL, 0.194 mmol) were added to a stirred mixture of
(3R,3aR36R56aR)-64[5-(4-bromopheny1)-6-chloro-1H-imidazo[4,5-b]pyridin-2-
y1]oxy]-
2,3,3a,5,6,6a-hexahydrofuro[3,2-b]furan-3-ol (35.1 mg, 0.078 mmol) in 164-
dioxane (3 mL) and
water (0.8 mL). The reaction mixture was placed under nitrogen and heated to
90 C. After 2
hours, the reaction mixture was cooled to room temperature before being
partitioned between
Et0Ac (30 mL) and saturated NH4C1 (30 mL). The aqueous layer was extracted
with Et0Ac (1 x
30 mL). The organic layers were combined, washed with brine (1 x 40 mL), dried
over Na2SO4,
filtered, and evaporated under reduced pressure. The resulting residue was
purified by
preparative thin layer chromatography using a 500 micron 20 cm x 20 cm silica
gel plate, which
was developed using 10% Me0H / DCM. The material isolated from the plate was
further
purified by preparative HPLC reverse phase (C-18), using a 19 x 100 min
SunfireTM column and
eluting with a 10%-90% acetonitrile / water + 0.05% TFA gradient, followed by
a 90%
acetonitrile / water + 0.05% TFA flush. The desired fractions were combined,
concentrated
under reduced pressure and lyophilized from acetonitrile and water to afford
the title compound
as a white solid. LC-MS: calculated for C28H24C1N504 529.15 observed m/e:
530.28 (M+H) (Rt
1.00 / 2 min); IHNMR 6 (pprn) (CD30D): 8.04 (d, J = 8 Hz, 2H), 7.81-7.88 (m,
7H), 7.69 (d, J =
1.5 Hz, 1H), 7.66 (d, J-- 2.0 Hz, 1H), 5.55 (qt, J = 5.5 Hz, 1H), 4.97 (t, J =
5.3 Hz, 1H), 4.47 (t, J
= 5.0 Hz, 1H),4.28-4.29 (m, 1H), 4.17 (dd, J = 5.8 Hz, 10.3 Hz, 1H),4.11 (dd,
J = 4.5 Hz, 10.0
Hz, 1H), 3.99 (s, 3H), 3.90 (t, J = 7.5 Hz, 1H), 3.62 (t, J - 8.5 Hz, 1H).
Table 10. The compounds in Table 10 were prepared according to the methods in
Examples 163
and 164, starting with the appropriate starting materials.
Example Number Structure
HPLC-mass
spectum tn/e
- 143 -
CA 02826649 2013-08-05
WO 2012/116145 PCT/US2012/026261
'
(IN ____________________________________________________________________
0 .0 OH
1110 N N Hµ" *I1H
165 1 --o 517.16
,,,,'
CI N R=H*
H
e\I
$ isi0 OH
01 N N 111" :
166 I --c) 533.16
CI N R=H*
H _
(NH
N
N ,
i OH
=,, 0
167
1.1 N N HI" '01µ H
I --0 517.28
el N
H R= ii*
N -NH
i
.--
Si 0 OH
01 N N 1
168
l o 516.21
ci N
..--- R= TBS*
H
_
N...-.:N
0 0 OH
0
H " .1111
N N µ
169
l ,¨o 517.20
ci N R- TBS*
H
- 144 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
Crµµ1
OH
1
HO tµH 4111 N N 0
170 >--0 517.20
c N TBS*
*The isomannide alcohol starting material was either unprotected (as -OH) or
TBS
protected (as -OTBS) during the 1,1'-bis(diphenylphosphino)ferrocene-
palladium(II)dichloride
dichloromethane complex coupling reaction used to prepare Examples 164-170 in
Table 10. The
TBS group was observed to substantially deblock during the reaction giving the
unprotected
alcohol (-OH). The use of TBS protection is noted in the mass spectrum entry
of Examples 164-
170 in Table 10.
The substituted dihydropyrrolo[3,4-c]pyrazole starting materials used in the
coupling
reactions of Examples 171-178 were prepared using procedures described in WO
2011/028455,
and modifications of the procedures known to those skilled in the art. Example
173 uses a
mixture of regioisomers 1-(cyclopropylrnethyl)-5,6-dihydro-4H-pyrrolo[3,4-
c]pyrazole and 2-
(cyclopropylmethyl)-5,6-dihydro-4H-pyrrolo[3,4-e]pyrazole as the starting
material. Example
177 is derived from starting material 2,4,5,6-tetrahydropyrrolo[3,4-
e]pyrazole. Example 176
uses N-ethy1-5,6-dihydro-4H-pyrrolo[3,4-c]pyrazo1e-2-su1fonamide as the
starting material; the
sulfonamide of N-ethy1-5,6-dihydro-4H-pyrrolo[3,4-c]pyrazole-2-sulfonamide
hydrolyzed under
the reaction conditions to give Example 176.
EXAMPLE 171
N
NctOH
N HO IµH
lk'
N kl 0
"
et
(3R,3aR,6R,6aR)-6-[[6-chloro-51442-(2-hydroxy-2-methyl-propy1)-4,6-
dihydropyrrolo[3,4-
clpyrazol-5-yl]pheny11-1H-imidazo[4,5-blpyrklin-2-ylloxy]-2,3,3a,5,6,6a-
hexahydrofuro[3,2-
b]furan-3-ol
Step A (3R,3aR,6R,6aR)-6-[6-ch1oro-54442-(2-hydroxy-2-methyl-Tropy1)-4,6-
dihydropyrrolo[3,4-c]pyrazol-5-yl]pheny1]-1-(2-
trimethylsilylethoxymethyl)imidazo[4,5-
b]pyridin-2-ylloxy-2,3,3a,5,6,6a-hexahydrofuro[3,2-b]furan-3-ol. A mixture of
- 145 -
CA 02826649 2013 08 05
WO 2012/116145
PCT/US2012/026261
tris(dibenzylideneacetone)dipalladium(0) (3.8 mg, 0.0041 mmol) and 1-pheny1-2-
(di-tert-
butylphosphino)-1H-pyrrole (3.0 mg, 0.010 mmol) in dioxane (0.2 mL) was
stirred at room
temperature under a nitrogen atmosphere for 30 minutes. A solution of 1-(5,6-
dihydro-4H-
pyrrolo[3,4-cipyrazol-2-y1)-2-methyl-propan-2-ol; 2,2,2-trifluoroacetic acid
(68.7 mg, 0.233
mmol), 2 M aqueous K3PO4 (0.2 mL, 0.4 mmol), and intermediate 7 (72.0 mg,
0.124 mmol) in
dioxane (1.2 mL) was added to the above catalysts and the mixture was
blanketed with nitrogen
and placed in a 110 C oil bath for 20 hours. The resulting mixture was added
to ethyl acetate
(30 mL) and water (20 niL), the organic layer was separated, washed with
saturated aqueous
sodium chloride (1 x 10 mL), dried with anhydrous magnesium sulphate, filtered
and evaporated
to an oil. The residue was dissolved in ethyl acetate and placed onto a
preparative silica plate (1
x 1000u) which was developed and the UV active band eluted with ethyl acetate
to give an oil
upon evaporation. LC-MS: calculated for C33H43C1N606Si 682.27observed m/e:
683.57 (M+H)+
(Rt 1.25/ 2.0 min).
Step B (3R,3aR,6R,6aR)-64[6-chloro-51442-(2-hydroxy-2-methyl-propy1)-4,6-
dihydropyrrolo[3,4-clpyrazol-5-yllpheny1]-1H-imidazo[4,5-blpyridin-2-y11oxyl-
23,3a,5,6,6a-
hexahydrofuro[3,2-blfuran-3-ol. A mixture of (3R,3aR,6R,6aR)-646-chloro-54412-
(2-hydroxy-
2-methyl-propy1)-4,6-dihydropyrrolo[3,4-c]pyrazol-5-yliphenyll-1-(2-
trimethylsilylethoxymethypimidazo[4,5-b]pyridin-2-ylioxy-2,3,3a,5,6,6a-
hexahydrofuro[3,2-
b]furan-3-ol (48.8 mg, 0.07 mmol), formic acid (1.5 mL), and saturated aqueous
KHSO4 (0.2
mL) was stirred at ambient temperature for 40 minutes and then placed in a
refrigerator. After 18
hours, the reaction mixture was partitioned between Et0Ac (50 mL) and
saturated aqueous
sodium bicarbonate (25 mL). The aqueous layer was extracted with Et0Ac (2 x 20
mL). The
organic layers were combined, washed with brine (1 x 10 mL), dried over MgSO4,
filtered, and
evaporated under reduced pressure. The residue was dissolved in Me0H (1 mL)
and 7 drops of 3
N aqueous NaOH were added over 15 minutes. The mixture was purified by HPLC
reverse
phase (C-18), using a 30 x 150 mm SunfireTm column and eluting with a 20%-80%
acetonitrile /
water gradient to give the title compound as a light yellow solid following
lyophilization. LC-
MS: calculated for C271129C1N605 552.19 observed ink: 553.44 (M+H) (Rt 1.09 /
2.0 min); 11-1
NMR 5 (ppm) (CD301): 7.96 (s, 1H), 7.62 (d, 1H), 7.52 (s, 1H), 6.81 (d, 1H),
5.59 (m,1H), 4.98
(dd,1H), 4.48 (dd,1H), 4.30 (m, 1H), 4.16 (m, 2H), 4.14 (s, 2H), 3.91 (dd.
1H), 3.60 (dd, 1H) and
1.20 (s, 6H).
Table 11. The compounds in Table 11 were prepared according to the methods in
Example 171,
starting from the appropriate starting materials.
Example Structure
HPLC-mass
Number
spectum m/e
- 146-
CA 02826649 2013-08-05
WO 2012/116145
PCT/US2012/026261
FN^.....NctiN ..
OH
N 0
172 Si " MI.1
N N 00
1 ..,.. 555.08
I --0
...---
ci N
H
OH
N sil
N N 0
I ----0
CI N
H
4) 535,07
N'N
173
ki.... OH mixture
of
N
N
00 ,
0 \ H
N N H\µµ 0
regioisomers
,--"
CF N
H 1
F>e,....---..., õ...N
F NL...t1
F OH
N
N N
174 0110 HO ,titi
IP
0
1 µ... 562.99
I --o
...---
ci N
H _
rkF FF
I t -i OH
N 0
175
Si 563.08 ,AH
N N 0
I ¨0
..,"
Cl N
H
- 147 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
õNI _________________________________________________________________________
HNcti
oz0H
---
H'
176 N 481.07
N 0
CI
HN
OH
,N
N
HµCIP "IH 480.92
177 N N 0
CI
7 0
,N
0 // NL.t1
178
OH
585.13
Hµµ` 'Iµ H
N N 0
CI
EXAMPLE 179
N N
OH
0
,µµH
N N
1
CI
(3R,3aR,6R,6aR)-64[6-chloro-544-(6-pyrazol-1-y1-3-pyridyl)pheny11-1H-
imidazo[4,5-bipyridin-
2-y1loxyl-2,3,3a,5,6,6a-hexahydrofuro[3,2-b]furan-3-o1
Step A (3R,3aR,6R,6aR)-616-chloro-544-(6-pyrazol-1-y1-3-pyridyl)pheny11-1-(2-
trimethy1si1y1ethoxymethy1)imidazo[4,5-b1pyridin-2-y1joxy-2,3,3a,5,6,6a-
hexahydrofuro[3.2-
blfuran-3-ol. 5-bromo-2-pyrazol-1-yl-pyridine (0.879 g, 3.92 mmol), 1,1'-
bis(diphenylphosphino)ferrocene-palladiumaDdichloride dichloromethane complex
(0.267 g,
0.327 mmol), and LiOH (2.72 mL, 8.17 mmol) were added to a stirred mixture of
intermediate 8
(2.06 g, 3.27 mmol) in 1,4-dioxane (9 mL) and water (2A mL). The reaction
mixture was
placed under nitrogen, and then heated to 90 C. After 4 hours, the reaction
mixture was cooled
- 148 -
CA 02826649 2013-08-05
WO 2012/116145 PCT/US2012/026261
to room temperature before being partitioned between saturated aqueous
ammonium chloride
(200 mL) and Et0Ac (150 mL). The aqueous layer was extracted with Et0Ac (2 x
150 mL).
The organic layers were combined, washed with brine (1 x 100 mL), dried over
Na2SO4, filtered,
and evaporated under reduced pressure. Flash chromatography of the resulting
residue utilizing a
silica gel BiotageTM 40M column and employing a 0-80% Et0Ac / hexane gradient
afforded the
desired product as a light yellow solid. LC-MS: calculated for C32H35C1N605Si
646.21 observed
tn/e: 647.53 (M+H)4- (Rt 1.32 / 2 min).
Step B (3R,3aR,6R,6aR)-6416-ehloro-5-f4-(6-pyrazol-1-y1-3-pyridyl)pheny11-1H-
imidazof4,5-
blpyridin-2-yljoxv]-2,3,3a,5,6,6a-hexa.hydrofuro[3,2-b]furan-3-ol. A mixture
of formic acid (6
mL, 156 mmol), saturated aqueous KHSO4 (0.66 mL, 2.58 mmol), and
(3R,3aR,6R,6aR)-646-
chloro-544-(6-pyrazol-1-y1-3-pyridyl)phenyli-1-(2-
trimethylsilylethoxymethypimidazo[4,5-
b]pyridin-2-ylloxy-2,3,3a35,6,6a-hexahydro-furo[3,2-13]furan-3-ol (1.67 g,
2.58 mmol) was
stirred at 60 C overnight, and then cooled to 00 C in an ice bath. The pH of
the reaction mixture
was adjusted to pH >11 through the addition of NaOH (6.24 g, 156 mmol) in
water (5 mL). THF
(10 mL) was added to the reaction mixture, and the reaction was removed from
the ice bath and
allowed to warm to room temperature. After 30 minutes, the pH of the reaction
mixture was
adjusted to pH 6 through the addition of concentrated HC1. The biphasic
mixture was separated.
The organic layer and the resulting white precipitate were concentrated under
reduced pressure,
redissolved in DMSO, and filtered before being purified by preparative HPLC
Reverse phase (C-
18), using a 19 x 100 mm SunfireTM column and eluting with a 10%-90%
acetonitrile / water +
0.05% TFA gradient followed by a 90% acetonitrile / water + 0.05% TFA flush.
The desired
fractions were combined, and evaporated under reduced pressure. The resulting
residue was
washed with Me0H and lyophilized from acetonitrile and water to afford the
title compound as a
white solid. LC-MS: calculated for C26H21C1N604 516.13 observed m/e: 517.22
(M+H)+ (Rt
1.16 / 2 min); 1H NMR 8 (ppm) (CD30D): 8.80 (d, J = 2.5 Hz, 1H), 8.67 (d, J =
2.0 Hz, 1H),
8.30 (dd, J - 2.8 Hz, 8.5 Hz, 111), 8.06 (d, J = 9.0 Hz, 1H), 7.81-7.83 (m,
6H), 6.84 (t, J = 2.3 Hz,
1H), 5.56 (qt, J = 5.5 Hz, 111), 4.99 (t, J - 5.3 Hz, 1H), 4.49 (t, J 5.0 Hz,
1H), 4.28-4.32 (m,
1H), 4.19 (dd, J = 6.0 Hz, 10 Hz, 1H), 4.13 (dd, J = 4.8 Hz, 10.3 Hz, 1H),
3.92 (dd, J = 7.0 Hz, J
= 8.0 Hz, 11-1), 3.62 (t, J = 8.5 Hz, 1H).
OR A ) Pr1(11)dppf-CH2C12
N N 00, oµH 5-,bromo-2-pyrazol-111-pyridine it
140
0 8
L1014
1,4-dioxane -f,=,.õ-1,1 14
oz0H
water d41-i
N 90
______________________________________________ 1-1W C )12 N
0 B.) Forme Acid 40\>--0
KUM,
60 C 0 N
The isomannide alcohol was either unprotected (R=H) or TBS protected (R=TBS)
during
- 149-
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
the coupling reaction used to prepare Example 179, as shown above. The TBS
group was
observed to substantially deblock during the reaction giving the unprotected
alcohol (R=H).
EXAMPLE 180
N
OH
Hµ t"
10111 N N 0
CI
(3R,3aR,6R,6aR)-64[6-chloro-5-14-(4-(1,2,4-triazol-1-v1)phenyllpheny1}-1H-
imidazo [4,5-
b1pyridin-2-y1]oxy]-2,3,3a,5,6,6a-hexahydrofuroi3,2-b}furan-3-01
Step A (3R,3aR,6R,6aR)-6- f6-chloro-5-[4-[4-(1,2,4-triazol-1-yl)phenyllpheny11-
1-(2-
trimethylsilylethoxymethyl)imidazo[4,5-blpyridin-2-yl]oxy-2,33a,5,6,6a-
hexahydrofuro(3,2-
bifuran-3-ol. 1-(4-bromopheny1)-1,2,4-tiazole (0.557 g, 2.486 mmol),
PalladiumTetrakis (0.383
g, 0.331 mmol), and potassium phosphate (1.407 g, 6.63 mmol) were added to a
stirred mixture
of intermediate 8 (1.044 g, 1.657 mmol) in dioxane (9 mL) and water (2.4 mL).
The reaction
mixture was placed under nitrogen before being heated to 90 C. After 4 hours,
the reaction
mixture was cooled to room temperature, and then partitioned between Et0Ac
(100 mL) and
saturated aqueous ammonium chloride (100 mL). The aqueous layer was extracted
with ethyl
acetate (2 x 100 mL). The organic layers were combined, washed with brine (1 x
50 mL), dried
over Na2SO4, filtered, and evaporated under reduced pressure. Flash
chromatography of the
resulting residue utilizing a silica gel BiotageTM 401v1 column and employing
a 0-100% Et0Ac /
hexane gradient afforded the desired product as a light yellow solid. LC-MS:
calculated for
C32H35C1N605S1 646.21 observed m/e: 647.01 (M-1-11)+ (Rt 1.26 / 2 min).
Step Bi3R.3aR,6R,6aR)-6-r[6-chloro-5-1-4-1-4-(1,2,4-triazol-1-
yi)phenyllphenyl]-1H-
imidazo[4,5-b]pyridin-2-yljoxv1:2,3,3a,5,6,6a-hexahydrofitro[32-b}furan-3-ol.
A mixture of
formic acid (3 mL, 68.8 mmol), saturated aqueous KHSO4 (0.33 mL, 1.777 mmol),
and
(3R,3aR,6R,6aR)-6-[6-chloro-54444-(1,2,4-triazol-1-y1)phenylipheny11-1-(2-
trimethylsilylethoxymethyl)imidazo[4,5-b]pyridin-2-ylloxy-2,3,3a,5,6,6a-
hexahydrofuro[3,2-
b]furan-3-ol (1.15 g, 1.777 mmol). The reaction mixture was stirred at 60 C
overnight, and then
cooled to 0 C in an ice bath. The pH of the reaction mixture was adjusted to
pH >11 through the
addition of NaOH (2.75 g, 68.8 mmol) in water (5 mL). THF (5 mL) was added to
the reaction
mixture, and the reaction was removed from the ice bath and allowed to warm to
room
temperature. After 30 minutes, the pH of the reaction mixture was adjusted to
pH 6 through the
addition of concentrated HC1. The biphasic mixture was separated. The organic
layer and the
resulting white precipitate that had formed were concentrated under reduced
pressure,
- 150-
CA 02826649 2013-08-05
WO 2012/116145
PCT/US2012/026261
redissolved in DMSO, and filtered before being purified by preparative HPLC
Reverse phase (C-
18), using a 19 x 100 mm Sunfireirm column and eluting with a 10%-90%
acetonitrile / water
gradient + 0.05% TFA followed by a 90% acetonitrile / water + 0.05% TFA flush.
The desired
fractions were combined, and evaporated under reduced pressure. The resulting
residue was
washed with 1e0H and lyophilized from acetonitrile and water to afford the
title compound as a
white solid. LC-MS: calculated for C26H21C1N604 516.13 observed m/e: 516.85
(M+H)+ (Rt
1.11 / 2 min); Ill NMR 8 (ppm) (CD30D): 9.17 (s, 111), 8.21 (s, 1H), 7.82-7.97
(m, 4H), 7.78-
7.80 (m, 5H), 5.56 (qt, J = 5,5 Hz, 1H), 4.98 (t, J = 5.3 Hz, 1H), 4.49 (t, J
= 5 Hz, 1H), 4.28-4.32
(m, 1H), 4.19 (dd, 6.0 Hz, 10.0 Hz, 1H), 4.12 (dd, J = 5.0 Hz, 10.0 Hz,
1H), 3.92 (dd, J = 7.0
Hz, 8,0 Hz, 1H), 3.62 (t, J = 8.8 Hz, 1H).
tL) 144-bromop1reny1)-1,2,4-triazole
0".
PalladiurnTetrakis
B ,6114
N K3PO4
0
1,4-thoxane N
Water
N
90 C N N 1-1".
o B }Formic Acid rn(D
KES04
60 C CI
--Sr"
The isomannide alcohol starting material was either unprotected (12.41) or TBS
protected
15 (R=TBS) during the coupling reaction used to prepare Example 180, as
shown above. The TBS
group was observed to substantially deblock during the reaction giving the
unprotected alcohol
(R H).
Table 12. The compounds in Table 12 were prepared according to the methods in
Examples 179
20 and 180, starting from the appropriate starting materials.
Example Structure
HPLC-mass
Number spectum
/Die
si
OH
0
s"
181 N
532.28
N 1-1µ 0
R¨TBS*
CI
- 151 -
CA 02826649 2013-08-05
WO 2012/116145
PCT/US2012/026261
, _______________________________________________________________________
o.. N.--. 0
OH
182 o
401 N , 0. 'Ikfl 558.36
I ---o R=H*
..---
a N
H
)7"-N
N 1
OH
0
183 401 N N H". µ0"H 532.29
I --o R¨H*
,---
a N
H
F
F.......F__N
N I
=cf 0
OH
0
184 N
H µ` '" 586.32
411 N µ
i-.--o R=H*
..--
Cl N
H
NN
HO--- I
0 4110 OH
0
Si N N IP' µ011
185
l --o 534.34
...-- R=H*
a N
H
N 1 I
= '
1' SI OH
0
0 NN Hk" :
186 1 --,o 529.97
...-=
N R=H*
cr
H _
- 152 -
CA 02826649 2013-08-05
WO 2012/116145
PCT/US2012/026261
,
,
¨N
= ---'
N 0
OH
0
187 4111 N N 1-1µv ."
l .---o 529.96
a N
H R=H*
,
l>"---N=N,--' 0
0
HZ
N N 0
188 1 ---c)
556.43
''-' N R=H*
ci ii
\
N
Ki
\
lin0z0H
,µ \ H
189 4111 N N HI" 0 530.29
1 ,¨o R=H*
ci N
H
HO 7
1
\ N
Olt 0 OH
0111 N N Hµ1' 01111 587.97
190
I ,--o R=H*
' N
CI H
HN.- N
µ lip
OH
0
. N N 'Pill
l ,¨o 517.27
191
..." R=11*
Cl N
H
- 153 -
CA 02826649 2013-08-05
WO 2012/116145
PCT/US2012/026261
N
Nµ,"......... N 00
0 N N
192 OH
HOZ
,µµH
i --o 517.34
..--- R=TBS*
ci N
H
N
.----- N
I OH
,kµ H
193 Hg
. N N =Y" 0
518.26
I --o
.---"' N R-TBS*
a H
N
N,N,',....N N
.." 1
i
OB114 H
,µµH
. N N
194
l --o 518.41
ci N
H R-1-1*
71_,..-N
N µ
'N.......N 00
oli
likl N N "OW
o 517.99
195 1
..--' R=H*
Cl N
H
Nz-..N
/ ,
N 1
1 OH
-...... I 0
09g
H
Hµ"
N N
196 o
1 ,---o 518.98
..---- R *
C N
H
- 154-
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
NN
N
N
\
OH2
,µµH
197
OOP N N 0
519.35
1
ci R=H*
*The isomannide alcohol starting material was either unprotected (as -OH) or
TBS
protected (as -OTBS) during the coupling reaction used to prepare Examples 181-
197 in Table
12. The TBS group was observed to substantially deblock during the reaction
giving the
unprotected alcohol (-011). The use of TBS protection is noted in the mass
spectrum entry of
Examples 181-197 in Table 12.
EXAMPLE 198
N
tizOH
0
01H
N N 0
CI
(3R,3aR,6R,6aR)-6-4 1-6-chloro-514-(5-pyrazol-1-y1-2-pyridyl)pheny1]-111-
imidazo[4,5-b]pyridin-
2-vijoxyl-2,3,3a15,6,6a-hexahydrofuro13,2-11ffiran-3-ol
Step A (3R,3aR,6R,6aR)-646-chloro-5-14-(5-pyrazol-1-y1-2-pyridyl)pheny11-1-(2-
trimethylsilylethoxymethyl)imidazo[4,5-blpyridin-2-ylloxy-2,3,3a,5,6,6a-
hexahydroftiro[3,2-
blfuran-3-ol. A mixture of intermediate 9 (81.0 mg, 0.123 mmol), pyrazole
(11.2 mg, 0.165
mmol), potassium phosphate (76.1 mg, 0.359 mmol), and copper(I) iodide (5.0
mg, 0.026 mmol)
was evacuated and backfilled with nitrogen (3x). Trans-(1R,2R)-N,N-bismethy1-
1,2-
cyclohexanediamine (100, 0.063 mmol) and DMF (0.25 mL) were added, and the
suspension
was heated to 110 C with stirring. After 24 hours, the reaction mixture was
cooled to room
temperature, and then filtered through a pad of CeliteTM, and rinsed with
Et0Ac (75 mL). The
filtrate was washed with water (3 x 30 mL) and brine (1 x 20 mL), dried over
Na2SO4, filtered,
and evaporated under reduced pressure to give an amber residue. Preparative
thin layer
chromatography of the residue using two 500 micron 20 cm x 20 cm silica gel
plates, which were
developed using 80% Et0Ac / Hexanes afforded the desired product as a
colorless residue. LC-
MS: calculated for C32H35C11\1"605Si 646.21 observed m/e: 647.45 (M-I-HY' (Rt
1.27 / 2 min).
Step B (3R,3aR,6R,6aR)-6-0-6-chloro-544-(5-pyrazol-1-y1-2-pyridyl)phenyll-1H-
imidazo[4,5-
blpyridin-2-yl]oxyl-2,3,3a,5,6,6a-hexahydrofaro[3,2-11furan-3-ol. A mixture of
- 155-
CA 02826649 2013 08 05
WO 2012/116145
PCT/US2012/026261
(3R,3aR,6R,6aR)-646-chloro-544-(5-pyrazol-1-y1-2-pyridy1)pheny11-1-(2-
trimethylsilylethoxymethypimidazo[4,5-b]pyridin-2-Aoxy-2,3,3a,5,6,6a-
hexahydrofuro[3,2-
b]furan-3-ol (32.7 mg, 0.051 mmol), formic acid (1.0 mL, 26.1 mmol), and
saturated aqueous
ICHSO4 (0.05 mL) was heated to 40 C with stirring. After 16.5 hours, the
reaction mixture was
cooled to room temperature, and then cooled to 0 C in an ice bath. The pH of
the reaction
mixture was adjusted to pH 14 through the addition of 5 N NaOH (5.8 mL, 29
mmol). THF (2
mL) was added to the reaction mixture, which was removed from the ice bath and
allowed to
warm to room temperature. After 1.5 hours, the pH of the reaction mixture was
adjusted to pH 6
through the addition of 2 N HC1. The reaction mixture was partitioned between
Et0Ae (30 mL)
and water (30 mL). The aqueous layer was extracted with Et0Ac (2 x 20 mL). The
organic
layers were combined, washed with brine (1 x 20 mL), dried over Na2SO4,
filtered, and
evaporated under reduced pressure to give a white residue. Purification of the
residue by HPLC
reverse phase (C-18), using a 30 x 150 mm SunfireTM column and eluting with a
20%-70%
acetonitrile / water 0.05% TFA gradient, followed by a 70% acetonitrile /
water + 0.05% TFA
flush solid followed by lyophilization from ethanol and benzene afforded the
title compound as a
white. LC-MS: calculated for C26H21C1N604 516.13 observed i-n/e: 517.35 (M+H)+
(Rt 1.11 / 2
min); 11-1 NMR 8 (ppm) (CD30D): 9.21 (d, J = 2.6 Hz, 1H), 8.52 (dd, J = 2.6
Hz, 8.6 Hz, 1H),
8.47 (d, J = 2.6 Hz, 1H), 8.24 (d, J = 8.7 Hz, 1H), 8.19 (d, J = 8.4 Hz, 2H),
8.04 (s, 1H), 7.89 (d,
J = 8.3 Hz, 2H), 7.87 (d, J = 1.8 Hz, 1H), 6.66 (t, J - 2.2 Hz, 111), 5.61
(qt, J = 5.2 Hz, 1H), 4.99
(t, J = 5.2 Hz, 111), 4.48 (t, J = 5.1 Hz, 111), 4.28-4.32 (m, 1H), 4.18 (d, J
= 5.1 Hz, 2H), 3.91 (dd,
J - 6.8 Hz, 8.2 Hz, 1H), 3.61 (t, J = 8.6, 1H).
Table 13. The compounds in Table 13 were prepared according to the methods in
Example 198,
starting from the appropriate starting materials.
Example Number Structure
HPLC-mass
spectum in/e
====-= N
OH
0 ,I1H
N N 0
199 518.36
cl N
- 156 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
1 _
1,--z-.NµI
c\' ,N
N .-." N
I OH
\ 0 0
,%\ H
N N Hlv 0
200
1 ----0 518.36
/
ci N
H
/7"----.-N
N \
=,`,\õ.....N .......,, N
I OH
\
ON N
201
1
,-0 518.32
./
ci N
H
EXAMPLE 202
HO -- N
N N
N
I OH
\ 0 0
1-I
N N Hµ" 0
I ----0
-/-
c' N
H
(3R,3aR,6R,6aR)-64[6-chloro-544-[544-(2-hydroxy-2-methyl-propyl)pyrazol-1-y11-
2-
pyridyliphenyl]-1H-imidazo[4,5-blpyridin-2-ylioxy]-2,3,3a,5,6,6a-
hexahydrofitro[3,2-blfuran-3-
ol
Step A 2-1-146-1412-013R,3aR,6R,6aR)-3-hydroxy-2,3,3a,5,6,6a-hexahydrofuro[3,2-
b]furan-6-
yl]oxy1-6-ehloro-1-(2-trimethylsilylethoxymethyl)imidazo[4,5-b]pyridin-5-
yl]phenyl]-3-
pvridyllovrazol-4-vilacetic acid. To a mixture of intermediate 9 (196.6 mg,
0.298 mmol), methyl
2-(1H-pyrazol-4-ybacetate (87.2 mg, 0.622 mmol), potassium phosphate (191.9
mg, 0.904
mmol), and copper(1) iodide (11.9 mg, 0.062 mmol) in an 8 mL vial was added
trans-(1R,2R)-
N,N1-bismethy1-1,2-cyclohexanediamine (20.0 t.t1, 0.127 mmol) and DMF (0.6
mL). The
resulting suspension was heated to 110 'C. After 24 hours, the reaction
mixture was cooled to
room temperature before being partitioned between Et0Ac (50 rnL) and 2 N HC1
(50 mL). The
biphasic mixture was filtered and the solids were collected. The biphasic
filtrate was partitioned,
while the solids were washed with Et0Ae (2 x 30 mL). Each of these Et0Ac
washes was used to
extract the aqueous layer. The organic layers were combined, washed with brine
(1 x 30 mL),
- 157 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
dried over Na2SO4, filtered, and evaporated under reduced pressure to give a
yellow residue. The
residue was dissolved in Et0Ac (50 mL) and water (20 mL). The biphasic mixture
was
partitioned and the aqueous layer was extracted with Et0Ac (1 x 50 mL). The
organic layers
were combined, washed with brine (1 x 20 mL), dried over MgSO4, filtered, and
evaporated
under reduced pressure to give a yellow residue. The filtered solids were
washed with Me0H
(30 mL), which was combined with the residue from the workup and evaporated
under reduced
pressure to give a yellow residue. Purification of the residue by HPLC reverse
phase (C-18),
using a 30 x 150 mm SunfireTM column and eluting with a 20%400% acetonitrile /
water +
0.05% TFA gradient followed by a 100% acetonitrile + 0.05% TFA flush afforded
the desired
compound as a yellow residue. LC-MS: calculated for C34H37C11\1607Si 704.22
observed m/e:
705.32 (M+H) (Rt 1.20 / 2 min).
Step B methyl 2-[1464442-f[(3R,3aR,6R,6aR)-3-hydroxy-263õ3a,5 6,6a-
hexahydrofuro[3,2-
b]furan-6-yl]oxy]-6-chloro-1-(2-trimethy1si1y1ethoxymethy1)imidazo[4,5-
b]pyrklin-5-y1lpheny1]-
3-pyridylipyrazol-4-yllacetate. TMS-Diazornethane (2 M in hexanes, 0.15 mL,
0.300 mmol) was
added to a stirred solution of 2-[1-[6-[4-[2-[[(3R,3aR,6R,6aR)-3-hydroxy-
2,3,3a,5,6,6a-
hexahydrofuro[3,2-b]furan-6-yl]oxy]-6-chloro-1-(2-trimethylsilylethoxymethyl)-
imidazo[4,5-
b]pyridin-5-yl]pheny11-3-pyridyl]pyrazol-4-yllacetic acid (27.3 mg, 0.039
mmol) in Me0H (0.5
mL) and DCM (0.5 mL). The reaction mixture was stirred at room temperature.
After 3.5 hours,
the reaction mixture was evaporated under reduced pressure to give the desired
compound as a
yellow residue. This material was used in the next step without further
purification. LC-MS:
calculated for C35H39C1N607Si 718.23 observed m/e: 719.31 (M+H)+ (Rt 1.26 / 2
min).
Step C (3R,3aR,6R,6aR)-646-chloro-54445-14-(2-hydroxy-2-methyl-propyl)pyrazol-
1-y1]-2-
pyridyllpheny11-1-(2-trimethylsilylethoxymethyDimidazol4,5-blnyridin-2-ylloxy-
2,3,3a,5,6,6a-
hexahydrofuro[3,2-b]furan-3-o1. Methylmagnesium bromide (0.2 mL, 0.600 minol)
was added
to a stirred solution of methyl 2-[1464442-[[(3R,3aR,6R,6aR)-3-hydroxy-
2,3,3a,5,6,6a-
hexahydrofuro[3,2-b]furan-6-ylioxy]-6-chloro-1-(2-
trimethylsilylethoxymethyl)imidazo[4,5-
b]pyridin-5-yl]phenyl]-3-pridyl]pyrazol-4-yllacetate from Step B in THF (1
mL). The reaction
mixture was stirred at room temperature. After 2.5 hours, the reaction mixture
was partitioned
between Et0Ac (40 mL) and saturated aqueous NH4C1 (40 mL). The aqueous layer
was
extracted with Et0Ac (2 x 20 mL). The organic layers were combined, washed
with brine (1 x
20 mL), dried over Na2SO4, filtered, and evaporated under reduced pressure to
give a yellow
residue. Purification of the residue by HPLC reverse phase (C-18), using a 30
x 150 rnm
SunfireTM column and eluting with a 20%400% acetonitrile / water + 0.05% TFA
gradient
followed by a 100% acetonitrile 0.05% TFA flush afforded the desired
compound as a yellow
residue. LC-MS: calculated for C361143C1N606Si 718.27 observed m/e: 719.52
(M+II)+ (Rt 1.23 /
2 min).
Step D (3R,3aR,6R,6aR)-64[6-chloro-544-1.544-(2-hydroxy-2-methyl-
propyl)pyrazol-1-y1]-2-
- 158 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
pyridyfjphenyli-1H-imidazo[4,5-blpyridin-2-yl]oxy]-2,3,3a,5,6,6a-
hexahydrofuro[3,2-blfuran-3-
ol. A mixture of (3R,3aRi6R,6aR)-646-chloro-5444544-(2-hydroxy-2-methyl-
propyl)pyrazol-
1-yli-2-pyridyl3phenyl]-1-(2-trimethylsilylethoxy-methypimidazo[4,5-b3pyridin-
2-ylioxy-
2,3,3a,5,6,6a-hexahydrofuro[3,2-b]furan-3-ol (10.1 mg, 0.014 mmol), formic
acid (1.0 mL, 26.1
.. rnmol), and saturated aqueous KHSO4 (0.05 mL) was heated to 40 C with
stirring. After 16
hours, the reaction mixture was cooled to room temperature before being cooled
to 0 C in an ice
bath. The pH of the reaction mixture was adjusted to pH 14 through the
addition of 5 N NaOH
(5.8 mL, 29.0 mmol). THF (2 mL) was added to the reaction mixture, and the
reaction was
removed from the ice bath and allowed to warm to room temperature. After 30
minutes, the pH
.. of the reaction mixture was adjusted to pH 7 through the addition of 2 N
HCI. The reaction
mixture was partitioned between Et0Ac (30 mL) and water (20 mL). The aqueous
layer was
extracted with Et0Ac (2 x 20 mL). The organic layers were combined, washed
with brine (1 x
mL), dried over MgSO4, filtered, and evaporated under reduced pressure to give
a white
residue. This material was dissolved in DMSO / Me0H, and purified by HPLC
reverse phase
15 .. (C-18), using a 30 x 150 mm SunflreTM column and eluting with a 20%400%
acetonitrile / water
+ 0.05% TFA gradient followed by a 100% aeetonitrile + 0,05% TFA flush. The
desired
fractions were combined, evaporated under reduced pressure, and lyophilized
from ethanol and
benzene to afford the title compound as a pale yellow solid. LC-MS: calculated
for
C30H29C1N605 588.19 observed m/e: 589.28 (M+H) (Rt 1.10 / 2 min); 1H NMR 5
(PPIn)
20 .. (CD30D): 9.15 (d, J = 2.5 Hz, 1H), 8.46 (dd, J = 2.7 Hz, 8.9 Hz, 1H),
8.26 (s, 111), 8.19 (d, J=
8.7 Hz, 1H), 8.14 (d, J = 8.4 Hz, 2H), 7.97 (s, 111), 7.86 (d, J --- 8.4 Hz,
1H), 7.73 (s, 1H), 5.58
(qt, J = 5.2 Hz, 1H), 4.98 (t, J = 5.2 Hz, 1H), 4.47 (t, J = 5.0 Hz, 1H), 4.27-
4.31 (m, 1H), 4.17
(dd, J = 5.4 Hz, 10.2 Hz, 111), 4.14 (dd, J = 4.7 Hz, 10.2 Hz, 1H), 3.91 (m,
111), 3.60 (t, J = 8.6
Hz, 1H), 2.73 (s, 2H), 1.25 (s, 6H).
EXAMPLE 203
N
iztN
,N N
OH
\
0
Hkt' "µEl
N 0
CI
(3R,3aR,6R,6aR)-64[6-ehloro-544-16-(1,2,4-triazol-1-y1)-3-pridyl]pheny11-1H-
imidazo[4,5-
b1pyridin-2-y1]oxy1-2,3,3a,5,6,6a-hexahydrofuro[3,2-b]furan-3-o1
.. Step A (3R,3aR,6R,6aR)-646-chloro-544-1-6-(1,2,4-triazol-1-y1)-3-
pyrid_yllphenyl]-1-(2-
trimethy1si1y1ethoxymethy1)imidazo[4,5-blpyridin-2-y1]oxy-2,3,3a,5,6,6a-
hexahydrofuxo[3,2-
b]furan-3-ol. To a mixture of intemiediate 10 (89.7 mg, 0.136 mmol), 1,2,4-
triazole (11.5 mg,
- 159-
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
0.167 nunol), copper(I) iodide (5.4 mg, 0.028 mmol), and potassium phosphate
(71.8 mg, 0.338
mmol) under nitrogen was added trans-(1R,2R)-N,Nt-bismethy1-1,2-
cyclohexanediamine (10 pi,
0.063 mmol) and DMF (0.27 mL). The resulting suspension was heated to 110 C
with stirring.
After 25 hours, the reaction mixture was cooled to room temperature and then
filtered through a
pad of CeliteTM, and rinsed with Et0Ac (75 mL). The filtrate was washed with
water (3 x 20
mL) and brine (1 x 20 mL), dried over Na2SO4, filtered, and evaporated under
reduced pressure
to give a pale yellow residue. The residue was purified by preparative thin
layer
chromatography using a 500 micron 20 cm x 20 cm silica gel plate, which was
developed using
85% Et0Ac / Hexanes to give a colorless residue. The residue was further
purified by HPLC
reverse phase (C-18), using a 30 x 150 mm SunfireTM column and eluting with a
20%400%
acetonitrile / water + 0.05% TFA gradient, followed by a 100% acetonitrile +
0.05% TFA flush
to give the desired compound as a colorless residue. LC-MS: calculated for C3
11434C1N705 Si
647.21 observed ni/e: 648.45 (M+H)+ (Rt 1.27 / 2 min).
Step B (3R,3aR,6R,6aR)-6-116-chloro-54446-(152,4-tria2ol-1-y1)-3-
pyridA1nhenvil-IH-
imidazo[4,5-b]pyridin-2-yl]oxyl-2,3,3a,5,6,6a-hexahydrofuro[3,2-b]furan-3-ol.
A mixture of (3R,3aR,6R,6aR)-646-chloro-544-[6-(1,2,4-triazol-1-y1)-3-
pyridyl]pheny11-1-(2-
trirnethylsilylethoxymethyl)imidazo[4,5-b]pyridin-2-ylioxy-2,3,3a,5,6,6a-
hexahydrofuro[3,2-
b]ftnan-3-ol (29.2 mg, 0.045 mmol), formic acid (1.0 mL, 26.1 mmol), and
saturated aqueous
KHSO4 (0.05 mL) was heated to 40 'C. After 15 hours, the reaction mixture was
cooled to room
temperature before being cooled to 0 C in an ice bath. The pH of the reaction
mixture was
adjusted to pH 14 through the addition of 5 N NaOH (5.8 mL, 29.0 mmol). THF (2
mL) was
added to the reaction mixture, and the reaction was removed from the ice bath
and allowed to
warm to room temperature. After 30 minutes, the pH of the reaction mixture was
adjusted to pH
6 through the addition of 2 N HCI. The reaction mixture was partitioned
between Et0Ac (40
mL) and water (30 mL). The aqueous layer was extracted with Et0Ac (2 x 20 mL).
The organic
layers were combined, washed with brine (1 x 20 mL), dried over Na2SO4,
filtered, and
evaporated under reduced pressure to give a white residue. Purification of the
residue by HPLC
reverse phase (C-18), using a 30 x 150 mm SunfireTM column and eluting with a
20%-80%
acetonitrile / water + 0.05% TFA gradient, followed by a 80% acetonitrile /
water + 0.05% TFA
flush and followed by lyophilization from ethanol and benzene afforded the
title compound as an
off-white solid. LC-MS: calculated for C251120C1N704 517.13 observed rn/e:
518,30 (M+H) (Rt
1.10 / 2 min); 1H NMR 6 (ppm) (CD30D): 9.42 (s, 1H), 8.89 (d, J = 2.2 Hz, IH),
8.39 (dd, J =
2.4 Hz, 8.6 Hz, 1H), 8.25 (s, 1H), 8.07 (d, J = 8.5 Hz, 1H), 7.97 (s, 1H),
7.86 (m, 4F1), 5.59 (qt,
5.2 Hz, IH), 4.99 (t, J = 5.2 Hz, 1H), 4.48 (t, J = 5.0 Hz, 1H), 4.28-4.32 (m,
1H), 4.14-4.20 (m,
2H), 3.92 (dd, .1= 6.8 Hz, 8.2 Hz, 1H), 3.61 (t, .1 = 8.7 Hz, 1H).
Table 14, The compounds in Table 14 were prepared according to the methods in
Example 203,
- 160-
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
starting from the ap ropriate starting materials.
Example Number Structure HPLC-mass
spectum m/e
Nz.N
N
OH
10111
N N 0
204 51836
ci
,N N
N
OH
HIC:* l"H
N 0
205 N
518.36
ot
EXAMPLE 206
H03(
__________________________ N N
C Nµj
OH
0
ci N
(3R,3aR,6R56aR)-6-[l6-ehloro-544-[644-(2-hydroxy-2-methyl-propyl)pyrazol-1-y1]-
3-
pyridyllpheny11-1H-imidazo[455-1Apyridin-2-ylioxy]-2,3,3a,5,6,6a-
hexahydrof1ro[3,2-b1fican-3-
ol
Step A 2-[145-[4-[2-[[(3R53aR,6R,6aR)-3-hydroxy-2,3,3a,5,6,6a-
hexahydrofuro[3,2-b]furan-6-
ylloxy]-6-chloro-1-(2-trimethylsilylethoxymethyDimidazo[4,5-b]pyridin-5-
yl]pheny1:1-2-
pyridylipyrazol-4-yl]acetic acid. To a mixture of methyl 2-(11-1-pyrazol-4-
yDacetate (36.9 mg,
0.263 =not), intermediate 10 (147.3 mg, 0.223 mmol), potassium phosphate
(152.9 mg, 0.720
mmol), and copper(11) iodide (8.8 mg, 0.046 mmol) under nitrogen was added
trans-(1R,2R)-
N,N-bismethy1-1,2-cyclohexanediamine (15 0.095 mmol) and DMF (0.45 mL). The
resulting
suspension was heated to 110 <V with stiffing. After 24 hours, the reaction
mixture was cooled
to room temperature before being partitioned between Et0Ac (100 mL) and 2 N 1-
1C1 (30 mL).
- 161 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
The biphasic mixture was filtered through a pad of Celiterm. The solid from
the top of the
CeliteTM pad was dissolved in DMA and combined with the biphasic filtrate. The
resulting solid
was collected by filtration of the aqueous layer. The organic layer was washed
with water (3 x
30 mL) and brine (1 x 30 mL). The organic layer was dried over Na2SO4,
filtered, and
evaporated under reduced pressure to give a pale yellow residue. The solid
collected from the
filtration of the aqueous layer and the residue from the organic layer were
dissolved in DM80
and Me0H and purified by preparative HPLC Reverse phase (C-18), using a 30 x
150 111111
SunfireTm column and eluting with a 20%400% acetonitrile / water + 0.05% TFA
gradient
followed by a 100% acetonitrile + 0.05% TFA flush. The desired fractions were
combined and
evaporated under reduced pressure to give the title compound as a yellow
residue. LC-MS:
calculated for C34H37C1N607Si 704.22 observed m/e: 705.13 (M-F-H)+ (Rt 1.24 /
2 min).
Step B methyl 2-[14544-1241(3R,3aR,6R,6aR)-3-hydroxy-2,3,3a,5,6,6a-
hexahydrofuro[3,2-
b]furan-6-ylloxyl-6-chloro-1-(2-trimethy1si1y1ethoxyrnethy1)imidazor4,5-
b1npidin-5-y1jpheny1}-
2-pyridyllpyrazol-4-yl]acetate. TMS-Diazomethane (2 M in hexanes, 0.06 mL,
0.120 mmol) was
added to a stirred suspension of the 2-[1-[54442-[[(3R,3aR,6R,6aR)-3-hydroxy-
2,3,3a,5,6,6a-
hexahydrofuro[3,2-11furan-6-ylioxy]-6-chloro-1-(2-trimethylsilylethoxymethyl)-
imidazo[4,5-
b]pyridin-5-y1]pheny11-2-pyridy1ipyrazo1-4-y1iacetic acid (60.4 mg, 0.086
mmol) in Me0H (1
mL). DCM (0.6 mL) was added to the reaction mixture to give a yellow solution.
Additional
TMS-Diazomethane (2 M in hexanes, 0.04 mL, 0.080 mmol) was added to the
reaction mixture.
After 1 hour, the reaction mixture was evaporated under reduced pressure to
give a yellow
residue. The residue was purified by preparative thin layer chromatography
using two 500
micron 20 cm x 20 cm silica gel plates, which were developed using 75% Et0Ac /
hexanes to
afford the title compound as a colorless residue. LC-MS: calculated for
C35H39C1N607Si 718.23
observed wife: 719.03 (M+H)+ (Rt 1.29 / 2 min).
Step C 2-[145-[4-(241(3R,3aR,6R,6aR)-3-hydroxy-2,3,3a,5,6,6a-
hexahydrofiiro[3,2-blfuran-6-
Ylloxy]-6-chloro-1H-imi1a7o14,5-blpyridin-5-y11pheny1i-2-pyridy1]pyrazo1-4-
y1jacetic acid. A
mixture of methyl 2-[1-[5-[4-[2-[[(3R,3aR,6R,6aR)-3-hydroxy-2,3,3a,5,636a-
hexahydrofuro[3,2-
b]furan-6-ylloxy]-6-chloro-1-(2-trimethylsilyl-ethoxymethyl)imidazo[4,5-
b]pyridin-5-Apheny1]-
2-pyridyl]pyrazol-4-yljacetate (28.5 mg, 0.040 mmol), formic acid (1.0 mL,
26.1 mmol), and
saturated aqueous KHSO4 (0.05 mL) was heated to 40 C with stirring. After 16
hours, the
reaction mixture was cooled to room temperature before being partitioned
between Et0Ac (40
mL) and saturated aqueous NaHCO3 (40 mL). A white precipitate fomed during the
partition.
The aqueous layer was extracted with Et0Ac (2 x 20 mL). The organic layers
were combined
and washed with brine (1 x 20 mL). 2N HC1 (20 mL) was added to the brine
layer, dissolving
the white precipitate. The brine / 2N HC1 layer was repartitioned with the
combined organic
layers. The aqueous layers were combined and the pH was adjusted to ¨ pH 3
through the
addition of 2 N HC1. The combined aqueous layers were extracted with Et0Ac (3
x 20 mL).
- 162-
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
The combined Et0Ac extracts were washed with brine (1 x 20 mL) before being
combined with
the original organic layers. The combined organic layers were dried over
Na2SO4, filtered, and
evaporated under reduced pressure to give a mixture of: 24145-[442-
11(3R,3aR,6R,6aR)-3-
hydroxy-2,3,3a,5,6,6a-hexahydrofuro[3,2-b]furan-6-ylloxyl-6-chloro-1H-imidazo
[4,5-b]pyri din-
5-yllipheny11-2-pyridyflpyrazol-4-yl]acetic acid and methyl 2-[1454442-
[[(3R,3aR,6R,6aR)-3-
hydroxy-2,3,3a,5,6,6a-hexahydrofuro[3,2-b]furan-6-yl]oxy}-6-chloro-1H-
imidazo[4,5-b]pyridin-
5-ylipheny1]-2-pyridyl]pyrazol-4-yl]acetate as a pale yellow residue. This
residue was used in
the next step without Anther purification. LC-MS: calculated for C281123C1N606
574.14 observed
m/e: 574.90 (M+H) (Rt 1.11 / 2 min).
Step D methyl 2-[1-[544-(2-[[(3R,3aRõ6R,6aR)-3-hydroxy-2,3,3a,5,6,6a-
hexahydrofuro[3,2-
bifuran-6-yfloxyl-6-chloro-114-imidazo[4õ5-blpyridin-5-yllphenyl]-2-
pyridyllpyrazol-4-
vilacetate. TMS-Diazomethane (2 M in hexanes, 25 121, 0.050 mmol) was added to
a stirred hazy
solution of the mixture of 2-[1-[5-[4-[2-[[(3R,3aR,6R,6aR)-3-hydroxy-
2,3,3a,5,6,6a-
hexahydrofuro[3,2-b]furan-6-ylioxy1-6-chloro-1H-irnidazo[4,5-b]pyridin-5-
yllphenyl]-2-
pyridyllpyrazol-4-yljacetic acid and methyl 2-[1-[54442-[[(3R,3aR,6R,6aR)-3-
hydroxy-
2,3,3a,5,6,6a-hexahydrofuro[3,2-133finun-6-yl]oxy]-6-chloro-1H-imidazo[4,5-
b]pyridin-5-
ylipheny11-2-pyridyljpyrazol-4-yllacetate (23.1 mg) from Step C in Me0H (0.7
mL) and DCM
(0.7 mL). Additional Me0H (0.5 mL), DCM (0.7 mL) and TMS-Diazomethane (20 Al,
0.040
mmol) were added to the reaction mixture, and the reaction mixture was stirred
at room
temperature. After 50 minutes, additional TMS-Diazomethane (20 111, 0.040
mmol) was added to
the reaction mixture. After 35 minutes, the reaction mixture was evaporated
under reduced
pressure to give the title compound as a white solid, which was used in the
next step without
further purification. LC-MS: calculated for C29H25C1N606 588.15 observed m/e:
588.95 (M+H)+
(Rt 1.15 / 2 min).
Step E k3R.,3aR,6R,6aR)-611-6-chloro-5444644-(2-hydroxy-2-methyl-
propyl)pyrazol-1-y1]-3-
pyridylipheny1)-1H-imidazo[4,5-blpyridin-2-ylloxyl-2,3,3a,5,6,6a-
hexahydrofuror3,2-blfuran-3-
ol. Methylmagnesium bromide (0.13 mL, 0.390 rnrnol) was added dropwise to a
stirred solution
of methyl 2-{1454442-[[(3R,3aR,6R,6aR)-3-hydroxy-2,3,3a,5,6,6a-
hexahydrofuro[3,2-b]furan-
6-ylioxyl-6-chloro-1H-imidazo[4,5-bippidin-5-yl]pheny11-2-pyridyljpyrazol-4-
yllacetate from
Step D in THF (1 mL). The reaction mixture was stirred at room temperature.
After 1 hour,
additional methyl-magnesium bromide (0.05 mL, 0.15 mmol) was added to the
reaction mixture.
One hour later, the reaction mixture was partitioned between Et0Ac (40 JILL)
and saturated
aqueous NH4C1 (40 mL). The aqueous layer was extracted with Et0Ac (2 x 20 mL).
The
organic layers were combined, washed with brine (1 x 20 mL), dried over
Na2SO4, filtered, and
evaporated under reduced pressure to give a pale yellow residue. Purification
by HPLC reverse
phase (C-18), using a 30 x 150 mm SunfireTM column and eluting with a 20%400%
acetonitrile /
water + 0.05% TFA gradient followed by a 100% acetonitrile 4- 0.05% TFA flush
and followed
- 163 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
by lyophilization from ethanol and benzene afforded the title compound as a
white solid. LC-
MS: calculated for C30H29C1N605 588.19 observed mile: 588.95 (M+H) (Rt L13 /
2 min); IH
NMR 8 (ppm) (CD30D): 8.78 (broad s, 1H), 8.50 (broad s, 8.28 (dd, J = 2.0
Hz, 8.6 Hz,
1H), 8.02 (broad d, J= 8.6 Hz, 1H), 7.93 (s, 1H), 7.81-7.85 (in, 4H), 7.69
(broad s, 1H), 5.58 (qt,
J = 5.3 Hz, 1H), 4.99 (t, J = 5.2 Hz, 1H), 4.48 (t, J = 5.0 Hz, 1H), 4.28-4.32
(m, 1H), 4.18 (dd, J
= 5.6 Hz, 10.3 Hz, 1H), 4.14 (dd, J = 4.7 Hz, 10.2 Hz, 1H), 3.92 (dd, J = 6.9
Hz, 8.3 Hz, 1H),
3.61 (t, J = 8.6 Hz, 1H), 2.73 (s, 2H), 1.25 (s, 6H).
EXAMPLE 207
N-N
N
4/0 OH
0
N N 11µ" 'µ 0111
CI N
(3R,3aR,6R,6aR)-6-f[6-ch1oro-51444-(1H-tetrazol-5-yl)phenyllphenyli-1H-
imidazo[4,5-
blpyridin-2-yl]oxy]-2,3,3a,5,6,6a-hexahydrofurop,2-151furan-3-01
A mixture of intermediate 11 (27.9 mg, 0.059 mmol), azidotrimethyltin (153.5
mg, 0.746 mrnol),
and toluene (1 ml..) was heated to 110 C with stirring. After 16 hours, the
reaction mixture was
cooled to room temperature. The reaction mixture was purified by preparative
thin layer
chromatography using two 1000 micron 20 cm x 20 cm silica gel plates, which
were developed
using 90: 9: 1 DCM / Me0H / acetic acid to give a white solid. The product was
purified again
by preparative thin layer chromatography using a 500 micron 20 cm x 20 cm
silica gel plate,
which was developed twice using 90 : 9: 1 DCM / Me0H / acetic acid to give a
white solid. This
material was further purified by HPLC reverse phase (C-18), using a 30 x 150
min Sunfirevm
column and eluting with a 20%400% acetonitrile / water + 0.05% TFA gradient
followed by a
100% acetonitrile + 0.05% TFA flush. The desired fractions were combined,
evaporated under
reduced pressure, and lyophilized from ethanol and benzene to give the title
compound as a white
solid. LC-MS: calculated for: C25H20CIN704 517.13 observed m/e: 518.20 (M+H)+
(Rt 1.08 / 2
min); IH NMR 8 (ppm) (CD30D): 8.14 (d, J = 8.3 Hz, 2H), 7.95 (d, J = 8.3 Hz,
2H), 7.87 (s,
1H), 7.79-7.84 (m, 4H), 5.56 (qt, J = 5.3 Hz, 1H), 4.97 (t, J = 5.2 Hz, 1H),
4.47 (t, J = 5.0 Hz,
1H), 4.26-4.30 (m, 1H), 4.17 (dd, J = 5.7 Hz, 10.1 Hz, 1H), 4.12 (dd, J 4.8
Hz, 10.3 Hz, 1H),
3.90 (dd, J = 7.0 Hz, 8.3 Hz, 1H), 3.60 (t, J - 8.6 Hz, 1H).
EXAMPLE 208
- 164 -
CA 02826649 2013 08 05
WO 2012/116145
PCT/US2012/026261
H2 40 OH
O 401 N N Hµ''
0-1,1<F
0
CI
(3R,3aR,6R,6aR)-6-R6-chloro-5-1-444-(2,5-dihydro-1H-imidazo1-2-
y1)phenyl]phenyij-1H-
imidazo[4,5-blpyridin-2-yl]oxyl-2,3,3a,5,6,6a-hexahydrofuro[3,2-b]furan-3-o1
A mixture of intermediate 11 (28.4 mg, 0.060 ramol), ethylenecliamine (0.5 mL,
7.46 mmol), and
carbon disulfide (6 0.100 nunol) was heated to 50 C with stirring. After
18 hours, the
reaction mixture was cooled to room temperature before being evaporated under
reduced
pressure to give a yellow solid. Purification of the solid by HPLC reverse
phase (C-18), using a
30 x 150 mm SunfireTm column and eluting with a 20%400% acetonitrile / water +
0.05% TFA
gradient followed by a 100% acetonitrile + 0.05% TFA flush and followed by
lyophilization
from ethanol and benzene afforded the title compound as a white solid. LC-MS:
calculated for:
C271124CIN504 517.15 observed mote: 518.14 (M+H)+ (Rt 0.98 / 2 min); 1H NMR 8
(ppm)
(CD30D): 7.96-8.01 (m, 4H), 7.81-7.85 (m, 5H), 5.55 (qt, J = 5.4 Hz, 1H), 4.97
(t, J¨ 5.2 Hz,
1H)5 4.47 (t, J = 5.0 Hz, 1H), 4.26-4.30 (in, 1H), 4.09-4.19 (m, 6H), 3.90 (t,
J = 7.0 Hz, 1H), 3.60
(t, J = 8.6 Hz, 1H),
Table 15. The compounds in Table 15 were prepared according to the methods in
Example 208,
starting from the appropriate starting materials.
Example Number Structure
HPLC-mass
spectum rnie
r-N
i$3?
NH
isso0H
0
N N
209 9,..11.õ CI 0 1<F 532.22
0
6:1N
NH
0 .0,,OH
0 N 0
210546.26
e,A,1<F
0
CI
- 165 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
(IN
NH =
O
N N
211)-0 560.31
CI
111)2 4111 OH
\ 0, ,IµH
0 N
212 532.20
eo N HNµ 0
>---0
CI
EXAMPLE 213
41)
.õ.
N
CI
241.(3R,3aR,6S,6aS)-6-fluoro-2,33a,5,6,6a-hexahydrofuro[3,2-blfuran-3-y1]oxy]-
6-ch1oro-5-(4-
phen_ylpheny1)-1H-imiclazo[415-blpyridine
Step A 2-112-rf(3R,3aR,6S.6aS)-6-fluoro-2,3,3a,5,6,6a-hexahydroftiro[3,2-
b]furan-3-yl3oxyl-6-
ch1oro-5-(4-pheny1phenv1)imidazo[4,5-blpyridin-1-yl]methoxy]ethyl-trimeth,1-
silane. A stirred
solution of the intermediate from Example 159 Step A (85.8 mg, 0.148 mmol) in
DCM (1.5 mL)
(in a 20 mL plastic vial) was cooled to 0 C in an ice bath. DAST (0.12 mL,
0.908 mmol) was
added to the reaction mixture dropwise. After 10 minutes, the reaction mixture
was removed
from the ice bath and allowed to warm to room temperature. After 21.5 hours,
the reaction
mixture was cooled to 0 C in an ice bath prior to the slow addition of
saturated aqueous
NaHCO3 (30 mL). The resulting biphasic suspension was extracted with Et0Ac (3
x 30 mL).
The organic layers were combined, dried over NazSO4, filtered, and evaporated
under reduced
pressure to give an amber residue. Flash chromatography of the residue
utilizing a 4 g silica
RediSep Rfik column and employing a 0-50% Et0Ac / hexane gradient with a 50%
Et0Ac /
hexane hold afforded the desired compound as a colorless residue. LC-MS:
calculated for:
C30H33C1FN304Si 581.19 observed m/e: 582.21 (M-FH)+ (Rt 1.41 / 2 min).
Step 13 2-11(3R,3aR,6S,6aS)-6-fluoro-2,3,3a,5,6,6a-hexahydrofuro[3,2-b]furan-3-
y1]oxy]-6-
- 166 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
chloro-5-(4-phenylpheny1)-1H-imidazo[4,5-blpyridine. A mixture of 24[2-
[[(3R,3aR,6S,6aS)-6-
fluoro-2,3,3a,5,6,6a-hexahydrofuro[3,2-b]furan-3-y1]oxy]-6-chloro-5-(4-
phenylphenypimidazo[4,5-b]pyridin-1-yllimethoxylethyl-trimethyl-silane (23.2
mg, 0.040
mmol), formic acid (1.0 mL, 26.1 mmol), and saturated aqueous KHSO4 (0.05 mL)
was heated to
40 'V with stirring. After 4 hours, the reaction mixture was cooled to room
temperature before
being concentrated under reduced pressure. The concentrated reaction mixture
was partitioned
between Et0Ac (20 mL) and saturated aqueous NaHCO3 (20 mL). The aqueous layer
was
extracted with Et0Ac (2 x 20 mL), The organic layers were combined, dried over
Na2SO4,
filtered, and evaporated under reduced pressure to give a colorless residue.
Purification of the
residue by HPLC reverse phase (C-18), using a 30 x 150 mm SunfireTM column and
eluting with
a 20%400% acetonitrile / water + 0.05% TFA gradient followed by a 100%
acetonitrile + 0.05%
TFA flush and followed by lyophilization from ethanol and benzene afforded the
title compound
as a white solid. LC-MS: calculated for: C241119C1FN303 451.11 observed m/e:
452.14 (M+H)+
(Rt 1.23 / 2 min); 1H NMR 6 (ppm) (CD30D): 7.86 (s, 1H), 7.71-7.75 (m, 411),
7.69 (d, J - 7.3
Hz, 211), 7.46 (t, J - 7.7 Hz, 2H), 7.36 (t, J -- 7.4 Hz, 111), 5.59 (qt, J =
4.8 Hz, 1H), 5.13 (dd, J =
2.4 Hz, 50.4 Hz, 111), 5,13 (t, J = 5.3 Hz, 11I), 4.67 (dd, J = 5.0 Hz, 11.4
Hz, 1H), 4.11 (t, J =
11.5 Hz, 1H), 4.03-4.08 (m, 2H), 3.97 (ddd, J = 2.5 Hz, 11.3 Hz, 41.1, 11-1).
INTERMEDIATE 13
CF 3 N
y\ Br
N
5-(4-bromopheny1)-3-(trifluoromethyl)-1,2,4-oxadiazole Carbonyldiimidazole
(202 mg, 1.244
mmol) was added to a solution of 4-bromobenzoic acid (208.4 mg, 1.037 mmol) in
anhydrous
methylene chloride (2 mL). Then 2,2,2-trifluoro-N'-hydroxy-acetamidine (173
mg, 1.348 mmol)
was added and the mixture was stirred for 1 hour at room temperature. The
mixture was
evaporated and the resulting residue was dissolved in toluene (2 mL) and
heated in a 100 degree
oil bath for 18 hours. The solution was evaporated and the reside was purified
on a silica gel
Biotage 25S, eluting with Et0Ac/isohexane (0-5% Et0Ac in hexane) to give the
title compound
as a light yellow oil. 1H NMR & (ppm) (CDC13): 8.08 (d, 211) and 7.76 (d,
211).
INTERMEDIATE 14
, Br
- 167 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
3-(4-bromopheny1)-5-cyclopropy1-1,2,4-oxadiazole Triethylamine (0.204 mL,
1.463 mmol) and
cyclopropanecarbonyl chloride (0.089 mL, 0.976 mmol) were added to a stirred
mixture of 4-
bromo-Nt-hydroxy-benzamidine
(104.9 mg, 0.488 mmol) in methylene chloride (2 mL) and the mixture was
stirred at room
temperature for 30 min. The mixture was evaporated, dissolved in toluene and
re-evaporated.
The resulting residue was dissolved in toluene (3 mL) and heated in a 110 C
oil bath for 18
hours. Evaporation and purification of the resulting mixture by preparative
TLC gave the title
compound. LC-MS: calculated for: CiiH9BrN20 263.99 observed m/e: 265.18/
267.18 (M-FH)+
(Rt 1.25 / 2 min).
INTERMEDIATE 15
OTBS
,
Br 0--
Br
N N O H j N N
SO2Nle Cesium carbonate
N N
CI DMF CI
SEM 25 C 'SEM
5-(4-bromopheny1)-24(3R,3aR,6R,6aS)-6-((tert-butyldimethylsilyppxy)hexa-
hydrofilro[3,2-
15 b]furan-3-yl)oxy)-6-chloro-142-(trimethylsilyflethoxy)methyl)-1H-
imidazot4,5-b]p_ ridine. 5-
(4-bromopheny1)-6-chloro-2-(methylsulfony1)-1-02-
(trimethylsilyl)ethoxy)methyl)-1H-
imidazo [435-bjpyridine (2.00 g, 3.87 mmol) and (3R,3aR,6R,6aS)-6-((tert-
butyldimethylsilyl)oxy)hexahydrofuro[3,2-blfuran-3-ol (2.02 g, 7.74 mmol) were
placed under
nitrogen in anhydrous DMF (15 mL). Then cesium carbonate (3.78 g, 11.61 mmol)
was added
20 and the mixture was allowed to stir at room temperature for 2 hours.
Then the mixture was
poured into water (200 mL) and extracted with ethyl acetate (2 x 150 mL). The
combined organic
layers were washed with brine (100 mL), dried over sodium sulfate, and
concentrated under
reduced pressure to afford a dark oil. The oil was chromatographed using a
Biotage 100 g silica
gel cartridge eluted with 0-75% ethyl acetate in a 1:1 mixture of
dichloromethane/hexane. The
25 desired product fractions were combined and concentrated under reduced
pressure to afford the
title compound as an off-white solid. LC-MS: calculated for C301-
143BrC1N305Si2 697.21
observed m/e: 698.17 (M+H)+ (Rt 3.19 / 4 min).
INTERMEDIATE 16
- 168 -
CA 02826649 2013-08-05
WO 2012/116145 PCT/US2012/026261
OTEIS ho
Br is
OZH OTBS
N N D N B N 0
D )Pci(II)dppf-C1-12C12 o-
Bis(pinacolato)dibeion
Cl N I
o) Potassium Acetate
N
1,4=Dioxatte
a)
R() OC
,--
Bis(pinaeolato)diboron (1.573 g, 6.20 mmol), 1,1'-
bis(diphenylphosphino)ferrocene-
palladium(IDdichloride dichloromethane complex (0.337 g, 0.413 mmol) and
potassium acetate
(1.014 g, 10.33 mmol) were added Co a stirred solution of [(3R,3aR,6R,6aS)-3-
[5-(4-
bromopheny1)-6-chloro-1-(2-trimethylsilylethoxyrnethypirnidazo[4,5-bipyridin-2-
y1loxy-
2,3,3a,5,6,6a-hexahydroffiro[3,2-b]furan-6-y1]oxy-tert-buty1-dirnethyl-si1ane
(intermediate 15,
1.44 g, 2.065 mmol) in DMF (15 mL). The mixture was heated in a 80 C oil bath
for 18 hours,
and after cooling to room temperature, was diluted with Et0Ac (100 mL),
filtered through
CeliteTm, and the filter pad was washed with ethyl acetate (100 mL). The
combined organic layer
was washed with brine (100 mL), dried with Na2SO4, filtered and evaporated
under reduced
pressure. The resulting residue was purified by column chromatography on
silica gel Biotage
40M, eluting with Et0Ac/isohexane (0-30% Et0Ac in hexane) to give the title
compound as a
light yellow solid. LC-MS: calculated for: C361-155BC1N307Si2743.34 observed
mile: 744.52
(M+H) (Rt 1.53 / 2 min)
INTERMEDIATE 17
"
N
Br
2-bromo-5-(tetrazol-1-yflpyridine. Sodium azide (241.6 mg, 3.72 mmol) and
triethyl
orthoformate (1.0 mL, 6.01 mmol) were added to a stirred solution of 5-amino-2-
brornopyridine
(511.8 mg, 2.96 mmol) in acetic acid (3 mL, 52.4 minol), and the reaction
mixture was heated to
80 C. After 7 hours, the reaction mixture was cooled to room temperature
before being
evaporated under reduced pressure. The resulting red / brown solid was
partitioned between
Et0Ac (50 mL) and water (50 mL). The aqueous layer was extracted with Et0Ac (2
x 50 mL).
The organic layers were combined, washed with 1 N HC1 (2 x 50 mL), saturated
aqueous
NaHCO3 (1 x 50 mL), and brine (1 x 50 mL), dried over Na2SO4, filtered, and
evaporated under
reduced pressure to give an amber solid. Flash chromatography of the solid
utilizing an 40 g
silica RediSep Rf column and employing a 0-2% Me0H / DCM gradient with a 2%
Me0H /
DCM hold afforded the title compound as a light yellow solid. LC-MS:
calculated for C6H4BrN5
- 169 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
224.97, 226.96 observed m/e: 226.22, 228.21 (M+H)+ (Rt 0.42 / 2 min).
INTERMEDIATE 18
B ______________________________________________
\O-
1-methy1-244-(4,4,5.5-tetramethy1-1,3,2-dioxaborolan-2-yl)phenyllimidazole. A
solution of 2-
(4-bromopheny1)-1-methy1-1H-imidazole (71.1 g, 300 mmol, 1 equiv) in DMSO (660
mL),
4,415,5-tetramethy1-2-(4,4,5,5-tetramethyl-1,3,2-dioxaboro1an-2-y1)-1,3,2-
dioxaborolane (83.8 g,
329.92 mmol, 1.1 equiv), dppthiC12(6.6 g, 9.03 innaol, 0.03 equiv) and KOAc
(88.2 g, 900
mmol, 3 equiv) was stirred overnight at 85 C in an oil bath. The reaction
mixture was cooled and
then quenched by the addition of 1200 mL of water. The resulting solution was
extracted with 2
x 500 mL of dichloromethane. The organic layers were combined, dried over
anhydrous
magnesium sulfate and concentrated under vacuum. The resulting residue was
applied onto a
silica gel column and eluted with ethyl acetate/petroleum ether (1:5-1:2) to
afford the title
compound as a gray powder. LC-MS: calculated for C16H2113N202 284.17 observed
m/e: 285
(M+11)+; IHNMR 8 (ppm) (CDC13): 7.89(2H, d, J=8.0 Hz), 7.65(2H1 d, J=8.0 Hz),
7.14(1H, d,
J=1.2 Hz), 6.97(1H, d, J=1.2Hz,), 3.76(3H, s), 1.36(12H, s).
INTERMEDIATE 19
let
, 13\
'N 0
Intermediate 19 may be prepared from 2-(4-bromophenyl)thiazole according to
the procedure
described in Intermediate 18.
INTERMEDIATE 20
SEm
/OH
OH
Intermediate 20 may be prepared by reacting [6-(1H-imidazol-2-y1)-3-
pyridyilboronie acid with
SEMC1.
INTERMEDIATE 21
- 170-
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
N
ci
Inteimediate 21may be prepared by reacting NAP-bis(dimethylarninomethylene)-
hydrazine with
6-chloropyridin-3-amine.
INTERMEDIATE 22
Br
N-N
3-(4-bromopheny1)-1-cyclopropyl-pyrazole. A solution of 3-(4-bromopheny1)-1H-
pyrazole (302
g, 1.35 mol), cyclopropylboronic acid (233 g, 2.71 mol), copper(II) acetate
(246 g, 1.35 mot), 4-
(dirnethylamino)pyridine (662 g, 5.42 mol), cesium carbonate (1103 g, 3.39
mol), and 1,4-
dioxane (8 L) was stirred at 90 C for 36 h. The reaction mixture was cooled
to room
temperature before being filtered through Celiterm, which was washed with
Et0Ac (4 L). The
filtrate was acidified to pH 5 with 2 N HC1. The aqueous layer was extracted
with Et0Ac (12 L).
The organic layers were combined, washed with brine, dried over MgSO4,
filtered, and
evaporated under reduced pressure. The resulting residue was purified on an
ISCO 1500g
column eluting with a 0-20% Et0Ac/Heptane gradient to afford the title
compound. LC-MS:
calculated for C12H11BrN2 262.01, 264.01, observed m/e: 263.04, 265.06 (M-H)
(Rt 1.19 / 2
min).
EXAMPLE 214
HN OH
f?/-1
0 , 0
N N
C N
I
(3R,3aR,6R,6aR)-646-chloro-5-(4-(niperidin-4-ylethynyl)pheny1)-1H-imidazo[4,5-
blpyridin-2-
yflox_y)hexahydrofirol3,2-blfiiran-3-ol
Step A : tert-butyl 44(4-(2-(((3R,3aR,6R,6aS)-6-((tert-
butyldimethylsilyl)oxy)hexa-
hydrofuro[3,2-1)] furan-3-yl)oxy)-6-chloro-14(2-
(trimethylsilyflethoxy)rnethyl)-1H-imidazot4,5-
blpyridin-5-yOphenyflethynyl)piperidine-1-carboxylate.
5-(4-bromopheny1)-24(3R,3aR,6R,6aS)-6-((tert-butyldimethylsilypoxy)hexa-hydro-
furo[3,2-
- 171 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
bilfuran-3-ypoxy)-6-chloro-14(2-(trimethylsilyl)ethoxy)methyl)-1H-imidazo-[4,5-
b]pyridine
(intermediate 15, 63 mg, 0.090 mmol), tert-butyl 4-ethynyl-piperidine-1-
carboxylate (23 mg,
0.108 mmol), bis(triphenylphospine)palladium(II)dichloride (10 mg, 0.014
mmal), and copper(I)
iodide (1.4 mg, 0.007 mmol) were placed under nitrogen in anhydrous
triethylamine (0.5 mL).
While cooling the mixture in a dry-ice/acetone bath, a hard vacuum was applied
and nitrogen
was subsequently introduced (3x). The mixture was removed from the ice-bath,
sealed with a
teflon cap, and heated to 50 C for 24 hours. After cooling to room
temperature, the mixture was
filtered through CeliteTm, and rinsed with ethyl acetate until the fifixate
was colorless. The filtrate
was then concentrated under reduced pressure to provide an orange oil, which
was used directly
in the next step without further purification. LC-MS: calculated for C421-
161CIN407Si2 824.38
observed ni/e: 825.45 (M1-11)4 (Rt 3.34 / 4 min).
Step B : (3R,3aR,6R,6aR)-6-06-ch1oro-5-(4-(piperidin-4-y1ethynynpheny1)-1H-
imidazo[4,5-
blpyridin-2-yl)oxy)hexahydrofuro[352-bifuran-3-ol.
Unpurified tert-butyl 44(4-(2-(((3R,3aR,6R,6aS)-6-((tert-
butyldimethylsilyl)oxy)-
hexahydrofuro[3,2-b]furan-3-yl)oxy)-6-chloro-14(2-
(trimethylsilyl)ethoxy)methyl)-1H-
imidazo[4,5-b]pyridin-5-y1)phenyl)ethynyl)piperidine-1-carboxylate (0.090
theoretical mol) from
Step A was placed under nitrogen in formic acid (1.0 mL). A saturated aqueous
solution of
potassium hydrogen sulfate (0.2 mL) was added and the mixture was allowed to
stir at 50 C for 4
hours. The mixture was allowed to cool to room temperature. Then the mixture
was diluted with
methanol (5 mL), cooled to 5 C in an ice bath, and basified to pH 14 using an
aqueous solution
of 6N NaOH. The mixture was stirred at pH 14 for 10 minutes, then concentrated
aqueous HC1
was added dropwise until the pH was pH 7. The mixture was poured into
saturated aqueous
sodium bicarbonate (40 mL) and extracted with ethyl acetate (2 x 70 mL). The
combined organic
layers were concentrated under reduced pressure. The resultant yellow solid
was dissolved in
DMSO and ehromatographed using a Gilson reverse phase preparatory HPLC eluted
with 0-60%
aeetonitrile in water (0.1% TFA) over 10 minutes. The desired product
fractions were combined,
frozen at -78 C, and lyophilized to dryness to provide a yellow solid. LC-MS:
calculated for
C25H25C1N404 480.16 observed m/e: 481.14 (M+H)+ (Rt 1.43 / 4 min). 1H NMR 8
(pm)
(CD30D): 7.80 (111, s), 7.62 (2H, d, J ¨ 8.5 Hz), 7.49 (2H, d, J = 8.5 Hz),
5.53 (1H, m), 4.95
(1H, t, J = 5.5 Hz), 4.46 (1H, t, J = 5.0 Hz), 4.27 (1H, m), 4.15 (1H, dd, J =
9.5, 6.0 Hz), 4.09
(1H, dd, J = 10.0, 4.9 Hz), 3.88 (111, dd, J 8.0, 7.0 Hz), 3.58 (1H, t, J =
8.5 Hz), 3.42 (2H, m),
3.18 (2H, m), 3.09 (1H, m), 2.17 (2H, m), 1,96 (2H, m)
Alternatively, (3R,3aR,6R,6aR)-64(6-chloro-5-(4-(piperidin-4-ylethynyl)pheny1)-
1H-
imidazo[4,5-b]pyridin-2-yl)oxy)hexahydrofuro[3,2-b]furan-3-ol may be prepared
according to
the following procedure:
Step A: (3R3aR,6R,6aR)-6-((5-(4-bromophenyl)-6-chloro-1H-imidazo[4,5-blpyridin-
2-
- 172-
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
ypoxy)hexahydrofuro[3,2-blfuran-3-ol.
(3R,3aR,6R,6aR)-64(5-(4-bromopheny1)-6-chloro-1-02-
(trimethylsilypethoxy)methyl)-1H-
irnidazo[4,5-b]pyridin-2-y1)oxy)hexahydrofirro[3,2-h3firran-3-ol (intermediate
7, 527 mg, 0.904
mmol) was placed under nitrogen in formic acid (2.5 mL). A saturated aqueous
solution of
potassium hydrogen sulfate (0.4 mL) was added and the resulting mixture was
allowed to stir at
50 C for 9 hours. The mixture was cooled and added slowly to saturated aqueous
sodium
bicarbonate (200 mL). The mixture was extracted with ethyl acetate (2 x 150
mL) and
concentrated under reduced pressure. The resulting white solid was dissolved
in methanol (5 mL)
and basified with 3N aqueous sodium hydroxide (2 mL). The mixture was stirred
at room
temperature for 10 minutes, then neutralized with IN aqueous hydrochloric acid
(6 mL). The
mixture was poured into saturated aqueous sodium bicarbonate (25 mL) and
extracted with ethyl
acetate (2 x 50 mL). The combined organic layers were dried over sodium
sulfate and
concentrated under reduced pressure. The resulting white solid was
chromatographed using a
Biotage 25 g silica gel cartridge eluted with 0-5% methanol in dichloromethane
over 20 minutes.
The product fractions were combined, concentrated under reduced pressure, and
dried under high
vacuum to obtain a white solid. LC-MS: calculated for C 181115BrC1N304 452.69
observed m/e:
454.02 (M+H)+ (Rt 1.84 / 4 min).
Step B : (3R,3aR,6R,6aR)-6-(16-chloro-5-(4-(piperidin-4-ylethynyl)uheny1)-1H-
imidazo14,5-
blpyridin-2-yl)oxy)hexahydrofitro[3,2-b]furan-3-ol. 3R,3aR,6R,6aR)-64(5-(4-
bromopheny1)-6-
chloro-1H-imidazo[4,5-bipyridin-2-ypoxy)hexahydrofuro[3,2-b]fitran-3-ol (25
mg, 0.055
mmol), 4-ethynylpiperidine (10.5 mg, 0.072 mmol), copper (I) iodide (2.1 mg,
0.011 mmol),
chloro(tri-tert-buty1)-2[-(21amino-1,1'-biphenyl)]palladiurn (II) (5.7 mg,
0.011 mmol, prepared by
a procedure analogous to procedures described in J. AM, CHEM. SOC. 2010, 132,
14073-
14075), and cesium carbonate (54.0 mg, 0.166 mmol) were placed under nitrogen
in dry,
degassed toluene (138 gl.) and dimethylacetamide (1380. The mixture was
stirred at 50 C for
2 hours. The resulting residue was diluted with dimethylfonnamide (0.7 mL),
then filtered
through a filter disc (0.45 micron). The material was purified by reverse
phase HPLC purification
using 0-100% acetonitrile in water over 10 minutes. The desired product
fraction was
concentrated under reduced pressure to afford the title compound as an off-
white solid. LC-MS:
calculated for C251125C1N404 480.16 observed m/e: 481.14 (M H)+ (Rt 1.43 Ý4
min). 'HNNIR 8
(ppm) (CD30D): 7.80 (1H, s), 7.62 (2H, d, J = 8.5 Hz), 7.49 (2H, d, J ¨ 8.5
Hz), 5.53 (1H, m),
4.95 (111, t, J = 5.5 Hz), 4.46 (1H, t, J = 5.0 Hz), 4.27 (IH, m), 4.15 (1H,
dd, J = 9.5, 6.0 Hz),
4.09 (1H, dd, J ¨ 10.0, 4.9 Hz), 3.88 (1H, dd, J = 8.0, 7.0 Hz), 3.58 (1H, t,
J = 8.5 Hz), 3.42 (2H,
m), 3.18 (2H, m), 3.09 (1H, m), 2.17 (2H, m), 1.96 (2H, m)
Table 16. The compounds in Table 16 were prepared according to the methods
described in
Example 214, starting from the appropriate starting materials.
- 173 -
CA 02826649 2013-08-05
WO 2012/116145
PCT/US2012/026261
HPLC-mass
Example
spectrum
Number Structure
/We
_
= OH
...,
Offil'sso
466.12
215
140 N N Fr.
I.......0
N
H
I. OH -
0
216 =
480.12
Su N N F .i
rs'
1 --0
CI ---- N
H
. OH
HO1-1
0 , 0
217 N N Fr 497.18
1 .---0
"-- N
CI H
A ,
OH
j::: jiH ,
= '*.. ,., 0
218 Si N N 1.4s. 480.19
1 --o
N
H
,
OH OH
\
\
0 0 442.15
219 0 N
I --.0
CI ...--- N
H
46. OH OH
IV \
\ Of?
220 o
II NN Fr .
., 482.18
I--.0
ci .--- N
H -
OH
\
\Of?
221 01-1 =
0
=N N Frs' 442.15
I ----AD
ci --"" N
H
- 174 -
CA 02826649 2013-08-05
WO 2012/116145
PCT/US2012/026261
- -
OH
HO "..,
0?
o
222 0 N N Fr. 428.13
ci N
OH
-,
0
0?0
223 HO Ki , N 11 . 456.15
---o
1 ci i ---- N
L H
OH
=-.,,, 01?
. o
224 * N,.., N 1-1' 468.20
1 --0
CI '--. N
H
OH
(1),,,) orlile,
225 0 N N Elµµ. 497.18
i --o
01 --- N
H
OH
=,,,
226 , N rNH
0 N N H)_1
C.519.17
1 --0
CI ="" N
H
OH
,-----, ,,
1\1,..) Orf1,0
227 140 N N H _1 510.20
a 7 N
H
OH
228 r3 * N N 1?- 1-1 486.15
A, .
I --o
a 7 N
H
OH
HOfifil
0 0
229 0 N N Ws' 455.13
I --0
. CI '' N
H
- 175 -
CA 02826649 2013-10-02
BIOLOGICAL EXAMPLE 1
AMPKSAMSF (in vitro AIVIPK activation assay) =
The recombinant htunan AMPK complex 1 (containing al p 1y1). (SEQ ID NO: 1 to
3)
was obtained front baculovirus expression system. Recombinant viruses were
generated by
cotransfection of AMPK/pBacPak9 clones with Baculogold baculovints DNA
(Pharmingen) in
spodoptera frugiperda 21 cells according to the manufacturer's instructions.
Each round of virus
amplification was performed for 5 days in Grace's medium containing 10% serum.
Virus
that had been subjected to three rounds of amplification was used for all
protein
production procedures. To express the AMPK complex, sf21 cells were adapted to
serum
free medium (SF900 II, Invitrogen) by sequential dilution from serum
containing stocks
into SF900II medium and maintained in shaker flasks at 90 rpm at 27`)C. The
recombinant AMPK enzyme complex was produced by triple infection, one
recombinant
virus for each of the subunits, in sf21 cells under serum free conditions.
Cells were
infected in log phase, 1 x 106 cells/ml, at a multiplicity of infection of ¨5.
Cells were
harvested by centrifugation at 10,000 x g for 15 minutes after 72 hours of
infection with
viruses. The insect cell pellet from 2 liters of culture was resuspended in 50
ml lysis
buffer (20 mM Tris-HC1, 50 mM NaC1, 50 mM NaF, 30 mM Na PPi, 0.25 M sucrose,
10
mM ZnCl2, 2 mM D'FT, 0.4 mg/mIdigitonin) and subjected to two cycles of freeze-
thaw
lysis in a dry-ice ethanol bath. Insoluble material was removed by
centrifugation at
10,000 x g and the supernatant was fractionated with use of polyethylene
glycol (PEG).
The protein fraction precipitating between 2.5 and 6% PEG was used for further
purification using a Blue-Sepharose step (Zhou et al, J. Clin. Invest. 108,
1167-1174,
2001).
The in vitro AMPK activation assay is performed in a volume of 30 I in a 384-
well plate. Enzyme reactions were assembled in the microtiter plate by adding
15 1 of
2X enzyme in assay buffer (20 mM HEPES, pH 7.3, 5 mM MgC12, 3mM DTT, 0.01%
Brij 35 and CamK. Kinase, to activate AMPK) to wells which contained either
DMSO or
compound. The reaction was initiated with the addition of 15 1 2X substrate
mixture
containing 200 M ATP, and 3.0 M fluorescently labeled SAMS (5-FAM-
HMRSAMSGUELVICRR-COOH, SEQ ID NO : 4) in assay buffer. After 43--minute
incubation
at 25 C, the reaction was stopped by the addition of 70 p.1 stop buffer (100mM
HEPES, pH 7.3,
40mM bDTA, 0.015% Brij 35). Phosphorylated 5-FAM SAMS product is assessed
using
a Caliper EZ Reader LabChip microfluidics reader. Product conversion is
determined by
calculating the peak heights of the substrate and product and reporting the
produot/(product + substiate) peak ratio. The 10-point titration data were
expressed as %
maximum AMP activation. The resuhs were plotted using 4 parameter fit and the
- 176 -
CA 02826649 2013 08 05
WO 2012/116145
PCT/US2012/026261
inflection point reflecting 50% of the maximum activation was reported as the
EC50. The
% maximum AMP activation for selected compounds is provided in the table
below.
The compounds of present invention, including the compounds of Examples 1-
229, were tested in the in vitro AMPK activation assay using recombinant human
AMPK
complex 1 (containing al ply1) and found to have greater than 50% maximum AMP
activation of human AMPK complex 1 (containing cc1131y1), and EC50 values of
less than
micromolar. Preferred compounds of the present invention were found to have
EC50
values of less than 0.1 micromolar in the in vitro AMPK activation assay using
10 recombinant human AMPK complex 1.
Maximum AMP Activation for Selected Compounds
Example No. % Maximum AMP EC50 (nM)
Activation of
human AMPK
Complex 1
3 681% 1
s,
29 798% 7
30 852% 24
79 661% 5
111 813% 28
130 440% 47
140 668% 38
159 373% 4
=
163 362% 0.3
171 252% 1
172 249% 2
177 368% 18
178 223% 1
179 292% 0.9
180 350% 0.3
190 374% 1
202 372% 1
207 289% 0.5
214 386% 6
BIOLOGICAL EXAMPLE 2
- 177-
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
Phosphoroylation of Acetyl CoA Carboxylase by AMPK activators in db/+ Mice:
To assess the potential for AMPK activators to increase the phosphorylation of
Acetyl COA
Carboxylase (ACC) in liver and skeletal muscle, db/+ mice were dosed with AMPK
activators at
either 2 or 7 h prior to evaluation where phosphoiylated ACC (p-ACC)/ total
ACC levels were
compared in the tissues of vehicle and compound treated mice. Briefly, mice
were anesthetized
using gas anesthesia with 1-4% isoflurane administered to effect via nose
cone. Once
anesthetized, samples of liver and skeletal muscle (gastrocnemius) are
removed, snap frozen in
liquid nitrogen, and homogenized. Homogenates are analyzed for protein
concentration and equal
amounts of protein are assayed for total and phosphorylated ACC (p-ACC) levels
using Meso
Scale Discovery's Multi-array assay kit. MSD assay plates contain an electrode
surface that is
coated with streptavidin. Protein sample binds to streptavidin. The primary
ACC or p-ACC
specific antibody binds to protein and a secondary antibody labeled with MSD
SULFO-TAG
then binds to the primary antibody. The electrode surface of the MSD plate
responds to an
electrical stimulus and causes the SULFO-TAG labels bound to ACC and p-ACC to
emit a light
signal in proportion to the amount of p-ACC or total ACC present. The ratio of
p-ACC/ total
ACC levels are determined for each sample and the ratio of p-ACC/ total ACC
levels for mice
treated with AMPK activators is significantly elevated compared to the ratio
of those treated with
the vehicle control (significant elevations are described as differences where
p< 0.05).
BIOLOGICAL EXAMPLE 3
Inhibition of Fatty Acid Synthesis (FAS) by AMPK activators in db/+ Mice:
To determine the effect of AMPK activators on Fatty Acid Synthesis (FAS) in
the liver,
the effect of oral pre-dosing of compounds on the amount of 3H incorporated
into hepatic
triglyceride is determined as described by Sakurai T, Miyazawa S, Shindo Y,
and T. Hashimoto
(Biochim Biophys Acta. 1974 Sep 19; 360 (3):275-88). Briefly, mice (db/+,
Jackson Laboratory,
Maine) axe orally dosed with AMPK activators at time = -8 h. Then at time = -1
h, mice are
injected with 0.5 ml of 0.15 M NaCI containing 0.2 mCi of3H water per 100 g of
body weight.
At time 0, mice are sacrificed via cervical dislocation and livers are
harvested for FAS analysis.
To analyze livers for FAS, samples of liver are heated at 90 C for 5 hours in
a 4 M KOH / 50%
ethanol solution. Then the alkaline hydrolysate of liver is extracted with
hexane and acidified to a
pH <2 with 10 M H2SO4. The fatty acids of liver are then extracted from
acidified hydrolysate
with additional hexane, dried down with a stream of warm air, then re-
suspended in scintillation
fluid, and counted on a beta counter. The amount of fatty acids synthesized
per gram of liver is
calculated based on the amount of 3H inemporated into hepatic triglyceride.
The amount of 311
radiolabelled fatty acids synthesized in mice with treated with an AMPK
activator is significantly
less than the amount of3F1 radiolabelled fatty acids synthesized in the
control mice.
- 178 -
CA 02826649 2013 08 05
WO 2012/116145 PCT/US2012/026261
BIOLOGICAL EXAMPLE 4
In vivo study for therapy with an AMPK activator in Mice (Glucose Tolerance
Test):
DIO mice are treated simultaneously with an effective dose of an AMPK-
activated
protein kinase activator.
Materials and Methods: Male C57BL/6NT mice (Taconic, 16-18 weeks old at the
beginning of
the drug administration) are used. Mice are given water and high fat diet
D12492 (Research Diet
Inc.) ad libitum. They are kept in an animal room which is maintained at 23
2 C temperature,
55 15 % relative humidity and on a 12-hr light-dark cycle (7:00-19:00)
during a quarantine and
acclimatization period of 1 week. Animals are then administered vehicle
(5m1/kg of 0.5%
methylcellulose in distilled water) by oral gavage twice-daily at 9 AM and 5
PM. After 9 days,
stable body weight is observed. The following day (day -1), the mice are
fasted for 4 hours and
tail bled to determine the glucose and insulin levels. Animals are sorted into
groups based on
plasma glucose, insulin levels and body weight (n=8). The body weight and food
in the hopper
are recorded on day 0 before compound dosing is initiated. One of the groups
is orally
administered vehicle while the second group is administered an AMPK-activated
protein kinase
activator of the present invention at a dose of 30 mg/kg (5 ml/kg) twice-daily
for 12 days by
gavage. Body weight and food intake are measured every other day. On day 5,
the animals are
fasted 4 hours for measuring plasma glucose and insulin levels after morning
dosing. At day 12,
body weight and food intake are measured and animals receive their last
morning dose. Mice
again are fasted 4 hours, blood is collected at a set time point (t ¨ 0 min),
and then challenged
with dextrose orally (2 g/kg) Plasma glucose and insulin levels are determined
from tail bleeds
taken at 20 and 90 minutes after dextrose challenge. The plasma glucose and
insulin excursion
profile from t = 0 to t ¨ 90 min is used to integrate an area under the curve
(AUC) for each
treatment. Percent inhibition values for each treatment are generated from the
AUC data
normalized to the C57BL/6NT mice feed with D7012. Preferred compounds of the
present
invention significantly reduce day 12 glucose and/or insulin AUC during the
Oral Glucose
Tolerance Test after an oral dose in the range of 0.1 to 100 mg/kg.
BIOLOGICAL EXAMPLE 5
Acute food intake studies in Diet Induced Obese (DIO) mice: General Procedure
Adult DIO mice are used in these studies. After at least 2 days of acclimation
to the
vivarium conditions (controlled humidity and temperature, lights on for 12
hours out of 24 hours)
food (D12492 (Research Diet Inc.) is rernoved from rodent cages. An AMPK
activator of the
present invention or the vehicle is administered orally, intraperitoneally,
subcutaneously or
intravenously before the return of a known amount of food to cage. The optimal
interval
between dosing and food presentation is based on the half-life of the compound
based on when
brain concentrations of the compound is the highest. Food remaining is
measured at several
- 179-
CA 02826649 2015-01-29
intervals. Food intake is calculated as grams of food eaten per gram of body
weight within each
time interval and the appetite-suppressant effect of the AMPK activator is
compared to the effect
of the vehicle. The food intake of mice treated with an AMPK activator is
significantly less than
the food intake of control mice.
BIOLOGICAL EXAMPLE 6
Chronic weight reduction studies in Diet Induced Obese (DIO) mice: General
Procedure
Adult DIO mice are used in these studies. Upon or soon after weaning, rats or
mice are
made obese due to exclusive access to diets containing fat and sucrose in
higher proportions than
in the control diet. The diet used to induce obesity is Research Diets D12451
chow (45% fat).
The rodents ingest chow until they are significantly heavier and have a higher
proportion of body
fat than control diet rats, often 9 weeks. The rodents receive injections (1
to 4 per day) or
continuous infusions of an AMPK activator of the present invention or the
vehicle either orally,
intraperitoneally, subcutaneously or intravenously. Food intake and body
weights are measured
daily or more frequently. Food intake is calculated as grams of food eaten per
gram of body
weight within each time interval and the appetite-suppressant and weight loss
effect of the
AMPK activator of the present invention is compared to the effect of the
vehicle. The weight
loss of mice treated with an AMPK activator is significantly greater than the
weight loss of
control mice.
While the invention has been described and illustrated with reference to
certain particular
embodiments thereof, those skilled in the art will appreciate that various
changes, modifications
and substitutions can be made therein. For example, effective dosages other
than the particular
dosages as set forth herein above may be applicable as a consequence of
variations in the
responsiveness of the mammal being treated for any of the indications for the
compounds of the
invention indicated above. Likewise, the specific pharmacological responses
observed may vary
according to and depending upon the particular active compound selected or
whether there are
present pharmaceutical carriers, as well as the type of formulation and mode
of administration
employed, and such expected variations or differences in the results are
contemplated in
accordance with the objects and practices of the present invention. It is
intended, therefore, that
the invention be defined by the scope of the claims which follow and that such
claims be
interpreted as broadly as is reasonable.
- 180 -