Note: Descriptions are shown in the official language in which they were submitted.
CA 02829043 2013-09-04
WO 2012/120487 PCT/IB2012/052044
MEDICANT DELIVERY SYSTEM
TECHNICAL FIELD
[0001] This invention relates to devices and methods for vaporizing a
liquid for
inhalation. More specifically, the invention relates to providing a device and
method for
controlling, metering and measuring precise volumes of fluid vaporized and the
vapor
produced by a hand-held vaporizing device each time the device is engaged by
its user that
is reliable and safer to use than current devices relying on lithium ion
chemistry.
BACKGROUND
[0002] Various hand-held, personal vaporizing devices are currently
available. Some
of these have been specifically designed to produce a nicotine-infused vapor
for the purpose
of serving, as an alternative to smoking, a traditional tobacco cigarette
wherein the tobacco
is ignited and the user inhales the smoke and its constituents- including the
nicotine-a
naturally occurring constituent of tobacco. Devices used for the purpose of
cigarette
alternatives produce a vapor devoid of most of the 4000+ chemicals and
byproducts of
tobacco smoke and, therefore, deliver nicotine to the user, through ingestion
of the vapor,
without most of the harm normally associated with tobacco smoke.
[0003] Unfortunately, disadvantages still remain in the design and
performance of these
vaporizing devices. For example, some devices are bulky or cumbersome to use
as a
transportable, hand-held device.
[0004] Other vaporizing devices are incapable of delivering precise,
consistent, and
reliable metered doses of the medicant. Current electronic atomization
cigarettes do not
provide for a method to control the consistency of the volume of liquid
vaporized nor the
CA 02829043 2013-09-04
WO 2012/120487 PCT/IB2012/052044
volume of vapor produced and, as a result, cannot produce a measurable amount
of
nicotine on a per vaporization basis. There are certain circumstances and
situations,
including those where regulations might dictate, where it may well be required
that these
devices be capable of delivering vapor and its nicotine constituent in a
manner that enables
the amount of nicotine present in the vapor be measurable and consistently
repeated with
each and every engagement by the user. In addition to or in lieu of nicotine,
a vaporizer
might be used to deliver other substances to the user, including medicants.
Similarly, a
precise measured "dose" may be desired, or even required for these substances.
[0005] In
addition, because some of the devices on the market use a liquid storage unit
that is "open" to the atmosphere, some devices leak or fail to perform
reliably unless the
vaporizing device is maintained in an upright position during use, or during
the packaging,
shipping, and storage of the device. Furthermore, with such devices, the
liquid may be
subject to contamination, adulteration and/or evaporation under certain
conditions.
[0006] Finally,
most, if not all, current commercially available products use lithium
chemistry batteries as their power source. This is primarily due to three
factors: 1) the
useful life of the battery; 2) the power needed to vaporize the fluid; and, 3)
the requirement
for a small compact device roughly the size of traditional tobacco products-
i.e. cigarettes
and cigars, or in non-tobacco or nicotine formulations, the need for
compactness in order
to be discretely employed by the user in circumstances where discretion is
appropriate.
Lithium chemistry batteries, however, are volatile, hazardous (both in that
they can release
noxious vapors as well as potential for explosion under certain conditions)
and
environmentally challenging with respect to storage, reliability, and
disposability.
[0007] It is
anticipated that the lithium chemistry power source of hand-held
portable devices will become an issue for U.S. regulators, distributors,
retailers, and
2
CA 02829043 2013-09-04
WO 2012/120487 PCT/IB2012/052044
consumers as the current product gets more widely distributed and used and as
more uses
for the devices are identified, manufactured, distributed, sold and consumed.
[0008] Therefore, there is still a need for a device and method for
providing an
improved hand-held vapor delivery system that reliably and consistently
produces a
repeatable metered dose of a medicant in a safe, efficient, and effective
manner.
SUMMARY
[0009] In one aspect, a method and device for improving hand-held vapor
delivery
devices to generate reliable, consistent, repeatable metered doses of a
medicament or
medicant comprises a power control system utilizing an integrated circuit
capable of
determining and delivering the precise amount of power for the precise
duration of time
that is just enough to completely vaporize a predetermined volume of a liquid.
[0010] In another aspect, the method and device for an improved hand-held
vapor
delivery device may comprise a fluid delivery system, a vaporizing or
atomizing system,
and a power control system contained in a housing, wherein the fluid delivery
system
consistently, repeatably, and reliably delivers a precise metered dose to the
atomizing
system, and the power delivery system supplies just enough electrical power to
the
atomizing system to completely atomize or vaporize the exact volume of liquid
delivered to
the atomizing system.
[0011] In another aspect, the hand-held vapor delivery device has an
ability to operate
independent of orientation, and/or an ability to deliver a repeatable dose of
medicant,
and/or an ability to be stored in any orientation, and/or an ability to
maximize energy
efficiency.
[0012] In another aspect, the invention provides a device and method that
enables vapor
3
CA 02829043 2013-09-04
WO 2012/120487 PCT/IB2012/052044
delivery devices to use more stable, more reliable, less environmentally
hazardous, and
safer sources of battery chemistry without significantly affecting the
portability and
discreteness of the devices.
BRIEF DESCRIPTION OF DRAWINGS
[0013] Figure 1 is a perspective view of an embodiment of a medicant
delivery device
of the present invention.
[0014] Figure 2 is a top view of the device shown in Figure 1.
[0015] Figure 3 is a section view taken along line 3-3 of Figure 2.
[0016] Figure 4 is an enlarged detail section view of the upper section of
the device
shown in Figure 3.
[0017] Figure 5 is an exploded perspective view of the device shown in
Figures 1-4.
[0018] Figure 6 is an enlarge perspective view of elements of the device
shown in
Figures 3-5.
[0019] Figure 7 is a perspective view of another embodiment of the present
invention
with the housing removed for purpose of illustration.
[0020] Figure 8 is an exploded perspective view of the embodiment shown in
Figure 7.
[0021] Figure 9 is an enlarged side view showing details of elements shown
in Figures
7 and 8.
[0022] Figures 10-13 are side views of the device shown in Figures 7-9
illustrating
sequential steps of operation.
[0023] Figure 14 is an enlarged perspective view of the vaporizing system
shown in
Figures 7-9.
4
CA 02829043 2013-09-04
WO 2012/120487 PCT/IB2012/052044
[0024] Figure 15 is a schematic diagram of a "one-shot" circuit that may be
used in an
embodiment of the power control system of the present invention.
[0025] Figure 16 and Figure 17 are schematic diagrams of similar modified
circuits that
may be used in an embodiment of the power control system.
[0026] Figure 18 is an enlarged side view of an embodiment of a vaporizing
element.
[0027] Figure 19 is a perspective view of another embodiment of the
vaporizing device.
[0028] Figure 20 is a section view of the vaporizing device shown in Figure
19.
[0029] Figure 21 is an exploded perspective view of the vaporizing device
shown in
Figures 19 and 20.
[0030] Figure 22 is an enlarged perspective view of elements shown in
Figure 20.
[0031] Figure 23 is an isometric view of another embodiment of the medicant
delivery
device.
[0032] Figure 24 is an exploded view of the device shown in Figure 23.
[0033] Figure 25 is close-up isometric view of an embodiment of a fluid
delivery
system shown in Figure 24.
[0034] Figure 26 is a section view through line 26-26 of the fluid delivery
system
shown in Figure 25.
[0035] Figure 27 is a close-up isometric view of an embodiment of a plunger
of the
fluid delivery system shown in Figure 28.
[0036] Figure 28 is a close-up isometric view of an embodiment of a drive
nut of the
fluid delivery system shown in Figure 24.
[0037] Figure 29 is a close-up isometric view of an embodiment of the
outlet cap and
vaporizing system of the medicant delivery device shown in Figure 24.
CA 02829043 2013-09-04
WO 2012/120487 PCT/IB2012/052044
[0038] Figure 30 is a close-up isolmetric view of an embodiment of a fluid
discharge
atctuator of the medicant delivery device shown in Figure 24.
[0039] Figure 31A is a close-up isometric view of a proximal end of the
fluid delivery
system of the medicant delivery device shown in Figure 24.
[0040] Figures 31B is the medicant delivery device shown in Figure 31A with
the
bottom depressed.
[0041] Figure 32 is an isometric view of an embodiment of an anti-rotation
feature of
the fluid delivery system shown in Figure 24.
[0042] Figure 33 is an isometric view of another embodiment of the delivery
device.
[0043] Figure 34 is an isometric view of the delivery device shown in
Figure 33 with
the housing removed.
[0044] Figure 35 is a close up view of the top of the delivery device shown
in Figure
33 showing the vaporization system.
[0045] Figure 36 is a block diagram of an embodiment of the power control
system.
DETAILED DESCRIPTION OF THE INVENTION
[0046] The detailed description set forth below in connection with the
appended
drawings is intended as a description of presently-preferred embodiments of
the invention
and is not intended to represent the only forms in which the present invention
may be
constructed or utilized. The description sets forth the functions and the
sequence of steps
for constructing and operating the invention in connection with the
illustrated embodiments.
It is to be understood, however, that the same or equivalent functions and
sequences may
be accomplished by different embodiments that are also intended to be
encompassed within
the spirit and scope of the invention.
6
CA 02829043 2013-09-04
WO 2012/120487 PCT/IB2012/052044
[0047] To improve the ability to meter a precise dose of a medicant in
vapor form for
inhalation from a vapor delivery device, the vapor delivery device requires
either a power
control system that can control the amount and duration of heat applied to a
liquid form of
the medicant, or a fluid delivery system that can accurately, consistently,
and repeatably
discharge a precise volume of a medicant. These two methods: (a) controlling
the amount
of heat applied to the liquid, and (b) controlling the volume of liquid to be
vaporized, can
be used alone or in combination to improve the accuracy of the medicant "dose"
provided
by the vaporizer. As used in the claims, the term "medicant" means a
medicament,
medication, medicine, pharmaceutical, drug, and the like used for healing,
treating,
altering, improving, restoring, relieving, and/or curing a particular
condition, disease, or
mental or physical state, which includes the active ingredient or combination
of active
ingredients and inactive ingredients infused into an expedient or dissolved in
some other
carrier.
[0048] The amount and duration of the heat applied correlates with the
amount of
power supplied to the vapor delivery device. Therefore, in order to improve
the
functionality of current vapor delivery devices, the current devices must be
implemented
with a power control system that comprises a means for providing a precise
amount of
power from a power source to heat a heating element to a minimum required
temperature
that completely vaporizes a predetermined volume of a liquid. Based on the
properties of a
medicant, in particular, the expedient or carrier, the minimum required
temperature to
completely vaporize a predetermined volume can be calculated. By knowing the
minimum
temperature required to vaporize a predetermined volume of a medicant, energy
resources
can be conserved by not using more energy than is necessary, which is one of
the problems
with current devices.
7
CA 02829043 2013-09-04
WO 2012/120487 PCT/IB2012/052044
[0049] The means for providing a precise amount of power from a power
source to
heat a heating element to a minimum required temperature to completely
vaporize a
predetermined volume of a liquid comprises a control circuit or integrated
circuit 82 having
a processor 500 that controls the power sent to a heating element 152 to
ensure that only
the necessary amount of power is provided to vaporize the specific volume
discharged.
Since the amount of power supplied to a heating element 152 correlates with
the resistance
through the heating element, the processor 500 may be programmed to monitor
the
resistance of the heating element 152 as a proxy for the amount of power being
supplied to
the heating element 152. Knowing the resistance, the processor 500 can govern
the amount
of power to supply to the heating element 152. Measuring the resistance at the
heating
element has several advantages. First, power may be accurately measured and
maintained.
Second, it measures the resultant voltage from the circuit, rather than
measuring it from the
battery, which conserves battery life. Third, it insures that vaporization
remains constant,
allowing for measured dosages irrespective of the life cycle of the battery
and degradation
of the heating element.
[0050] In some embodiments, the means for providing a precise amount of
power may
also comprise a boost converter that is a switched DC/DC converter, in
conjunction with
supercapacitors 368a, 368b. The boost converter uses a charge converter that
functions
with an H bridge and inductor/capacitor system. By using the boost converter
the charge
current is limited to preserve the batteries and a much higher discharge
current from the
supercap is allowed, but for a shorter duration. By way of example only, it
make take 3-5
seconds to charge but only 0.5 second to discharge. Thus, the battery may only
see 100-
200mA load, but the capacitor might see 1A or more. By utilizing this system,
alkaline
batteries 364 can be used, thereby improving the safety of this device.
8
CA 02829043 2013-09-04
WO 2012/120487 PCT/IB2012/052044
[0051] A supercapacitor ("supercap") 368a, 368b is an electrochemical
capacitor with
relatively high energy density. Its energy density is typically hundreds of
times greater
than conventional electrolytic capacitors. A supercap 368a, 368b can store up
to two
orders of magnitude the capacitance a standard electrolytic capacitor can
maintain.
[0052] The described invention circuitry charges a supercap 368a, 368b from
a set of
alkaline batteries 364 using a DC/DC boost converter. When charging a supercap
368a,
368b, numerous parameters must be taken into consideration. For illustrative
purposes, a
300 farad capacitor bank that is to be charged to 6V DC, using a 6V power
source (4 -
1.5V AA batteries) capable of sourcing 1.2A MAX current could be used. Note
that a
resistor can be used in this circuit to limit the current to a maximum
amperage - e.g. 1A,
etc., as an additional control of the heating circuit.
[0053] To define how the invention charging circuitry works, the Ohm's law
equation
is used - Charge resistor value = 6V / IA = 6 Ohms. This is determined using
Ohm's
law: R =E/I, where R is the resistance in ohms, E is the energy in volts, and
I is the
current in amps.
[0054] To determine what it takes to charge the capacitor bank, 'Power' is
utilized,
which electrically is described as 'Wattage'. This Power equation is described
as:
[0055] Resistor Power = 6V x 1A = 6W (Power = Voltage x Current)
[0056] Thus, in order to charge a 6V capacitor bank at 1A with a 6V power
supply (4
AA/AAA batteries), a 6 Ohm resistor with a wattage rating of 6W or higher is
needed. In
certain designs, fewer batteries, such as one, two, or three, can be used to
supply sufficient
power.
[0057] Using this approach, this invention solves the standard problem of
battery life
issues that current electronic cigarettes (e-cigarettes) have. Additionally,
this approach
9
CA 02829043 2013-09-04
WO 2012/120487 PCT/IB2012/052044
provides the ability to maintain sufficient power to vaporize the liquid using
standard
alkaline chemistry batteries, which current e-cigarette devices are incapable
of utilizing.
[0058] Figure 37 shows a block diagram of the process. There is an energy
or power
source 600 that supplies the input power. This source can be one of several
types but in
general fits in two types. Type 1 may be a low power source not capable of the
higher
current to function directly. This type of power source requires additional
conditioning to
support full function, thus requires a power conversion stage 602 and a power
storage stage
608. Type 2 may be a high current source that allows a direct drive of the
vaporization
element.
[0059] State or control logic 604, which may be dedicated logic or a
processor,
supplies the control, measurement and drive functions. One embodiment may use
a Texas
Instrument MS P430 processor, but this could be any processor or ASIC-like
device. GPIO
and A/D functions may also be used to allow the measurement of either the
current flow
(direct drive) or voltage in the power storage (supercap). When all conditions
are met, the
control logic 604 activates the discharge switch 610 to heat the vaporization
element 612.
[0060] The ability to accurately measure and meter the power that energizes
the
vaporization element 612 allows the accurate metering of the vapor phase
transition and
dosage amounts. In the direct drive system the current and time of drive are
used to
compute and meter the energy that is used to heat the vaporization element
612. In the
stored energy system the formula 1/2CV2 is used to compute the energy in the
system and
the desired end voltage to meter the energy used to heat the vaporization
element 612,
where C is the capacitance and V is the voltage.
[0061] An alternative to controlling the amount power would be to control
the amount
of time the heating element is energized as the power source begins to
dissipate. The
CA 02829043 2013-09-04
WO 2012/120487 PCT/IB2012/052044
processor 500 can be configured to monitor the resistance and adjust the time
the heating
element remains on so as to completely vaporize a given volume of medicant.
[0062] In some embodiments, a flow switch 614 may be used to signal the
requested
start of a vaporization phase. When implemented into a vaporization device,
the device
may have a fluid delivery system (discussed below). The fluid delivery system
deposits a
required amount of fluid on the vaporization element 612 prior to activation
of the flow
switch 614.
[0063] In some embodiments, a fluid discharge activator 616 may be used to
"wake
up" the processor 604 and charge 606 the supercap 608 (in the stored energy
system). In
the direct drive system, the fluid discharge activator would be used to "wake"
the
processor 604 from an ultra-low power sleep mode. The fluid discharge actuator
616 may
be a mechanical device to activate the system, such as a turn switch, a
button, knob, lever,
and the like.
[0064] In some embodiments, diodes 618, 620 may be used to indicate to the
operator
the status of the system operation. For example, one diode may be an LED 618
to signal
when the vaporization element 612 is being driven. Another LED 620 may be used
to blink
specified patterns to indicate system status, e.g. power up, low battery,
exhausted fluid
state, or other system specific status states (i.e. max dosage per unit time,
etc...). In other
embodiments, a display, such as an LCD screen, may be used to show the system
status or
other information, such as the type of substance or medicant contained in the
delivery
device, the amount and/or doses remaining, the battery level, a user ID in
case the device
is lost, etc. A button or similar device could be used to actuate and scroll
through the
display.
11
CA 02829043 2013-09-04
WO 2012/120487 PCT/IB2012/052044
[0065] In a Type 1 configuration (low current, alkaline batteries etc.) the
charge
current may be limited to preserve the battery lifecycle. In many batteries,
if large
amounts of current are drawn, it will significantly reduce the battery life or
state of charge.
Therefore, using the lower current draw from the batteries and the power
storage stage 608
allows for a high current event without unduly draining the batteries.
[0066] In a Type 2 configuration, the power storage 608 can be used to
extend the life
of the battery (lithium polymer, lithium-ion) if desired. The power storage
608 also makes
it easier to very accurately meter the precise amount of energy into the
vaporization
element 612 with a simple voltage measurement. Accurately metering precise
amounts of
energy into the vaporization element can be done with a voltage and current
measurement
but it is harder to accurately measure current than to measure voltage. Thus,
it may be
advantageous to use a simpler-voltage only process.
[0067] An additional power saving feature that is shared with the control
logic 604 and
the power conversion 602, namely, the power state (on switch) of the system.
This power
saving feature with the power state can be accomplished via either an ultra-
low power
mode of the conversion/cpu or a power disconnect/latch function. This is used
to extend
the operation life of the device after the first use.
[0068] The energy required to completely vaporize a predetermined volume of
liquid is
a function of the amount of power and the duration of time the power is
present.
Therefore, the power control system 306 may also comprise a means for
controlling a
precise duration of time to supply the precise amount of power to completely
vaporize the
predetermined volume of liquid at the required temperature. The means for
controlling a
precise duration of time to supply the power may comprise a "one-shot" control
circuitry
170, 172, or 174 that can be integrated with the circuit for controlling the
amount of power
12
CA 02829043 2013-09-04
WO 2012/120487 PCT/IB2012/052044
described above. Examples of "one-shot" circuits 170, 172, or 174 are shown in
Figures
15-17 and described below in more detail. A "one-shot" circuit may be used to
limit the
electric current delivery time interval regardless of how long the user holds
the lever down.
The power control system 306 is completely "off" in between uses; therefore,
there is no
drain on the battery during idle time. As a result, battery life is prolonged.
[0069] In some embodiments, the integrated circuit may be configured to
actuate the
power source a predetermined number of times. This number should be low enough
such
that each actuation results in the same amount of power each time. In some
embodiments,
the integrated circuit may be configured to monitor the battery life and not
actuate the
power when a predetermined amount of battery life has been detected.
[0070] This power control system 306 can be implemented in existing vapor
delivery
devices. For example, the control system 306 can be installed into handles of
current
vapor delivery devices to be implemented with existing heating systems to
improve the
energy efficiency and accuracy of dosing of current devices.
[0071] Besides, or in addition to, controlling the amount and duration of
the power to
significantly improve the efficiency and effectiveness of metering precise
doses from
vaporization devices, a means for consistently metering a precise volume of a
liquid to be
vaporized can be used as an alternative or additional layer of precision.
Therefore, an
efficient medicant delivery device may comprise a power control system 34
utilizing
various embodiments of the circuitry described above to control the efficient
and effective
use of power, and/or a fluid delivery system 30, 302, or 402 as a means for
consistently
metering a precise volume of a liquid from the fluid reservoir to precisely
control the
volume of the liquid discharged for vaporization. Various combinations of
these systems
may be used to achieve the desired level of accuracy. An atomization or
vaporization
13
CA 02829043 2013-09-04
WO 2012/120487 PCT/IB2012/052044
system 32 may also be required to vaporize the medicant. In this application,
atomization
and vaporization are referred to interchangeably to indicate that the state of
the medicant is
a form that can be inhaled and absorbed by the lungs.
[0072] The precise volume of liquid that can be completely vaporized at a
given
temperature and duration of exposure can be calculated. Therefore, the precise
volume
required to be discharged from a fluid delivery system may be predetermined
because the
temperature of the wire and the duration the wire is energized can be fixed.
Alternatively,
in some embodiments, the precise volume may vary depending on the temperature
of the
wire and how long the wire remains energized at that temperature.
[0073] The embodiments of the power control system described above offer an
advantageous way for more precisely metering a specific dose of a medicant.
Controlling
the volume of the medicant discharged also improves the metering accuracy.
Examples of
devices for controlling the volume of medicants to a heating element for
vaporization are
described below. These devices can be used alone or in combination with the
power
control system to further improve the accuracy of metered doses of medicants.
[0074] In one embodiment, as shown in Figures 1 and 2, a medicant delivery
device 20
has an elongated housing 22 with a mouthpiece 24 and a lever 28 adjacent to a
back or top
end of the housing. A mouthpiece opening 26 extends into the mouthpiece 24.
Referring
further to Figures 3-5, an embodiment of the device 20 includes a fluid or
liquid delivery
system 30 as the means for consistently metering a precise volume of a liquid
to precisely
control the volume of the liquid discharged for vaporization, and a vaporizing
system 32,
as well as an electrical power control system 34. The electrical power control
system 34
may include batteries 44 within a battery compartment 42 of the housing 22,
and with the
batteries electrically connected to a flexible circuit board 82 via a spring
46 and contacts
14
CA 02829043 2013-09-04
WO 2012/120487 PCT/IB2012/052044
48. As shown in Figure 5, the housing may be provided with left and right
sides, in a
clamshell design. The lever 28 may be attached to the housing 22 at a pivot
58.
[0075] As shown in Figure 4, a means for consistently metering a precise
volume of a
liquid from a fluid reservoir to precisely control the volume of the liquid
discharged for
vaporization is achieved by the liquid delivery system 30, in the example
shown, which
includes a resilient or flex wall liquid chamber or reservoir 64 connected via
a tube 66 to a
lever valve 70. The reservoir 64 may be a thin walled flexible pouch made of
polyethylene
film. The reservoir 64 is positioned between two rigid surfaces, with a plate
62 on one
side and an inner wall of the housing 22 on the other side. Springs 60 within
the housing
22 press on a plate 62, which in turn presses on the reservoir 64. This
pressurizes the
liquid in the reservoir.
[0076] A tube 66 extends from the reservoir 64 to a lever valve 70 which
may include a
valve post 74, a valve spring 72 and valve washer 76. A valve section 80 of
the tube 66 in
this design extends through an opening the valve post 74, as shown in Figure
6. The valve
spring 72 urges the valve washer 76 against the valve section 80 of the tube
pinching it
closed.
[0077] Referring to Figures 4-6, an embodiment of the vaporizing system 32
includes a
heater 150 which is electrically connected to the electrical power control
system 34. The
vaporizing system 32 is also connected to, and receives liquid from, the
liquid delivery
system 30. The heater 150 may be an electrical resistance heater formed by an
open coil of
wire 152, such as ni-chrome wire. In this design, the electric current is
supplied to the coil
wire 152 via connectors 156 on, or linked to, the flexible circuit board 82,
which in turn in
connected to the batteries 44. Figure 14 shows the connectors 156 for
providing electrical
power to the heating element.
[0078] An outlet segment 154 of the tube 66 extending out of the lever
valve 70
towards the mouthpiece or back end of the device is inserted into the front
end of a wire
coil 152. Referring momentarily to Figure 14, solid wire inserts 159 may be
inserted into
the ends of the wire coil 152 and the outlet segment 154 to provide internal
support, so that
they do not distort or collapse when pressed down into connectors 156. The
outlet segment
154 at the front end of wire coil heater 152 provides liquid into the bore of
coil with each
actuation of the device 20.
[0079] The tube 66 is connected to the reservoir 64 with a liquid-tight
connection so
that liquid can only flow from the reservoir only through tube 66. The tube 66
may be a
resilient, flexible material such that its inner lumen can in general be
completely flattened
when compressed, and then generally fully recover to its original shape when
released. A
lever segment 67 of the tube 66 is positioned beneath the lever 28 and a fixed
rigid surface
inside of the housing, which optionally may be part of the circuit board 82 on
which power
management circuitry is located. Locating features 112 may be provided in, on,
or through
the circuit board 82 to ensure desired positioning is maintained. The lever 28
is retained
by lever pivot 116 and can pivot through a controlled range of motion.
[0080] In use, the mouthpiece 24 is placed into the mouth and the user
presses or
squeezes the lever 28. The tube 66 is pre-filled or primed with liquid during
manufacture.
Referring to Figure 4, as the lever 28 pivots down about the pivot 58, a
pincher 86 located
on a first section 90 pivotally attached to the housing pinches the pump
segment 67 of the
tube 66 against an inside surface of the housing 22, adjacent to the pivot 58
and the
reservoir 64. This temporarily closes off the tube 66 at the pincher 86. As
the lever 28
continues to pivot down (or inward towards the centerline of the device) a
ramp surface
88 on a second section 92 of the lever 28, flexibly attached to the first
section,
progressively squeezes the pump segment 67 of the tube 66 between the pincher
86 and
the lever valve 70. This creates a squeegee type of
16
CA 2829043 2018-08-20
CA 02829043 2013-09-04
WO 2012/120487 PCT/IB2012/052044
movement which pumps liquid towards the lever valve 70 using a peristaltic
action. As the
lever 28 continues to pivot inwardly, posts on the lever press the valve
washer 76 down
against the force of the valve spring 72. This temporarily opens the lever
valve 70 by
allowing the valve section 80 of the tube 66 to open. With the valve section
80 of the tube
open, and with liquid in the tube being pumped via the ramp surface 88, a
bolus of liquid
flows through the valve section 80 and the outlet segment 154 and into the
wire coil 152.
[0081] The constant positive pressure exerted on the reservoir 64 by the
springs 60
pressurizes the liquid in the tube 66. However, since the tube 66 is pinched
closed by the
pincher 86, no liquid flows out of the reservoir when the lever is depressed
and the lever
valve is opening. Rather, the liquid already present in the tube 66 between
the pincher 86
and the lever valve 70 provides the measured bolus which is unifoimly
delivered to the
wire coil.
[0082] The downward movement of the lever 28 also closes a switch 158
linked to or
located on the circuit board 82. Electric current then flows from the
batteries 44, or other
power source, to the wire coil 152. The wire coil heats up causing the liquid
to vaporize.
The current supplied to the wire coil, and the temperature of the wire coil
when operating,
may be regulated by the circuit board, depending on the liquid used, the
desired dose, and
other factors. The switch 158 may be positioned to close only when the lever
28 is fully
depressed. This avoids inadvertently heating the wire coil. It also delays
heating the wire
coil until the bolus of liquid is moved into the wire coil via the pivoting
movement of the
lever, to help prolong battery life. A "one-shot" control circuit 170, for
example, as
shown in Figure 15 described below, may be used to limit the electric current
delivery time
interval regardless of how long the user holds the lever down. The power is
completely
"off" in between uses. There is no drain on the battery during idle time. As a
result,
17
CA 02829043 2013-09-04
WO 2012/120487 PCT/IB2012/052044
battery life is prolonged.
[0083] As is apparent from this description, the liquid delivery system 30,
using a
linear peristaltic pumping action, delivers a consistent, fixed, repeatable
bolus of liquid to
vaporizing system 32 with each actuation of the device 20. The liquid delivery
system 30
further seals the reservoir 64 between actuations via the pincher 86,
maintains the contents
of the reservoir in a pressurized state, and controls electric power delivery
to the
vaporizing system 32. The liquid delivery system is designed so that as liquid
is used, air
is not introduced into the system.
[0084] The diameter and length of the wire coil 152 forms a cylindrical
volume within
the inside diameter of the coil that is sufficient to capture a single
expressed dose of liquid
from the liquid delivery system. The adjacent loops of wire of the wire coil
152 may also
be positioned so that liquid surface tension holds the liquid within the bore
of the coil.
This allows the device 20 to be used in any orientation, since gravity is not
needed to keep
the released dose of liquid in place.
[0085] The use of an open coil offers the further advantage that the vapor
may be
generated and escape anywhere along the length of the coil, without
inadvertently affecting
vaporization of the balance of the bolus of liquid in the coil. The wire coil
also provides a
large surface area for heat transfer and minimizes energy loss resulting from
heating
ancillary components.
[0086] Upon application of electric power, liquid in the coil vaporizes and
passes
through gaps between coils. The coil can be sized and shaped and positioned in
the
housing so that the vapor generated can be entrained into an air stream drawn
through the
device 20 when the user inhales on the mouthpiece. "Inhale" here means drawing
the
vapor at least into the mouth.
18
CA 02829043 2013-09-04
WO 2012/120487 PCT/IB2012/052044
[0087] Figures 7-13 show a second device embodiment 100 which may be
similar to the
device 20, but with the following differences. In the device 100, the means
for consistently
metering a precise volume of a liquid from the fluid reservoir to precisely
control the
volume of the liquid discharged for vaporization comprises a foam pad 106 that
is
compressed and inserted between a reservoir 64 and one of the rigid walls of
the housing.
Force exerted on the reservoir 64 by the foam trying to recover to its relaxed
state exerts
compressive force on the reservoir which maintains the liquid in the reservoir
under
pressure. The foam pad 106 may be used in place of the springs 60 shown in
Figure 4.
The reservoir may alternatively be pressurized using a syringe with a spring-
biased
plunger. With any of these designs, the reservoir may optionally be provided
as a
replaceable cartridge.
[0088] As shown in Figure 8, in the device 100, a lever valve 118 is
provided (in place
of the pincher 86 in the device 20) to compress the front end of the tube 66,
preventing
liquid from flowing out from the pressurized reservoir in between uses. The
lever valve
118 may be a stamped sheet metal form soldered to a rigid circuit board 114
containing the
same or similar circuitry as described above for the power control system 34.
[0089] Figures 10-13 show additional features that can be used for a means
for
consistently metering a precise volume of a liquid from the fluid reservoir to
precisely
control the volume of the liquid discharged for vaporization, specifically,
the pumping
action of the liquid delivery system in the device 100. When a dose of vapor
is desired,
the user places the mouthpiece in the mouth and inhales while pressing a
button 109 on the
lever 110, causing the lever to rotate downward (counter-clockwise). As the
lever 110
initially rotates as shown in Figure 10, a lever pinch projection 132 clamps
or pinches the
tube 66 closed at a pinch point 140, closing off the pressurized liquid
reservoir. Continued
19
rotation of lever 110 causes the lever 110 to flex at a flex point 124 having
reduced
thickness, as shown in Figure 11. This allows over-travel rotation of the
lever while the
tube 66 remains closed off at the pinch point 140, without crushing the tube.
[0090] Further rotation of lever 110 then compresses the lumen of the pump
segment
68 of the tube 66. This pumps liquid from the pump segment 68 towards the
lever valve
118. This movement also moves projections on the lever which push valve
flanges 120
down, deflecting and opening the lever valve 118, and allowing a pressurized
bolus of
liquid to move through the tube and into the vaporizing system 32. The dotted
lines in
Figure 12 show the lever valve 118 deflected down and away from the bottom
surface of
the circuit board 114, to open the valve. Lastly, at end of the lever stroke,
a lever switch
protrusion contacts a switch 158, switching the power delivery system on.
[0091] When lever 110 is released, it pivots back up to its original
position. As the
lever returns, the lever valve 118 reseats first, sealing the back end of pump
segment 68 of
the tube 66 and preventing air from being drawn back into the pump segment. As
the lever
110 continues to rotate clockwise, the pump segment 68 decompresses, creating
a negative
pressure within the tube lumen. Lastly, at pinch point 140 the tube 66
reopens, allowing
pressurized liquid from the reservoir to enter, refilling pump segment 68 with
liquid to
provide the next dose.
[0092] The volume of liquid expressed with each stroke can be controlled
by selection
of desired pump segment 67 tube diameter and length. Maintenance of a positive
pressure
on the liquid reservoir ensures that the system always stays primed with
liquid, and that
"short shots" resulting from air bubbles in the tube do not occur.
Furthermore, sealing of
the vaporizer system with a valve such as the valve 70 or 118 that is only
actuated at the
time of delivery, and positive pressure dispensing prevents inadvertent
leakage of liquid
CA 2829043 2018-08-20
CA 02829043 2013-09-04
WO 2012/120487 PCT/IB2012/052044
irrespective of orientation of the device during storage or use, thereby
providing a means
for consistently metering a precise volume of a liquid from the fluid
reservoir to precisely
control the volume of the liquid discharged for vaporization.
[0093] Figure 15 is a schematic diagram for a "one-shot" circuit 170 for
the power
control system that delivers a fixed time interval of electric current to the
heater 150
regardless of how long the lever is depressed by the user. In Figure 15,
CD4047 is a
CMOS low power monostable/astable multivibrator available for example from
Texas
Instruments. Ul is a common CD4047 which operates from a 12V battery voltage
with
very low quiescent current drain. When pushbutton SW1 is depressed, Ul is
triggered, Q
(pin 10) goes high and Cl is rapidly charged to near the supply voltage
through a FET
within Ul. At the same time, resistor R1 is switched to a logical "0" state
and
immediately begins discharging capacitor Cl with the time constant of 1/RC.
[0094] A wide range of pulse durations may be selected. Using a typical ni-
chrome
wire coil, pulse durations ranging from approximately 0.2 to 2 seconds are
sufficient to
fully vaporize the bolus of liquid. When the voltage on pin 3 reaches the
threshold for
logic "0" ( ¨1/3 supply voltage), the logic levels switch and Q (pin 10)
returns to a logic
low level. Q2 is an emitter follower that provides current amplification to
enable Q1 to be
fully saturated during the desired current pulse. D1 and R4 provide a visual
indication of
the heater current. R2 is a "pull down" resistor for SW1, and C2 prevents
induced noise
from falsely triggering the circuit. Other choices of IC may be employed, such
as the
Toshiba TC7WH123, depending upon battery voltage, package size, and cost.
[0095] The battery voltage gradually decreases over the lifespan of the
device. For
many applications, the circuit described in Figure 15 provides the necessary
control.
However, more precise metering of the medicant may be accomplished by
increasing the
21
CA 02829043 2013-09-04
WO 2012/120487 PCT/IB2012/052044
current pulse duration as the current decreases over the discharge life of the
battery. In the
circuit 172 shown in Figure 16, an additional OP amp IC serves as a voltage
controlled
current source for the power control system. The input voltage is sampled from
Pin 10 of
U1. A constant current is generated in Q3 and used to discharge the timing
capacitor, Cl,
at a constant rate. Once the voltage across Cl reaches the logic threshold, CD
4047 trips
and the output pulse width is complete. As the battery voltage decreases the
constant
current generated in Q3 decreases, causing the time to discharge Cl to
increase. This
lengthens the output pulse to maintain a relatively constant heater power per
inhalation
cycle as the battery voltage declines over the lifetime of the device. The
various current
setting and sense resistor values may be adjusted to provide optimal
performance. Other
circuits may be employed to provide the same function such as voltage to
frequency
converters.
[0096] Figure 17 shows another circuit 174 for the power control system
where a
voltage regulator U2 is inserted between the output transistor Q1 and the
heater filament.
This keeps the filament voltage constant throughout the battery life. The
regulated voltage
may be chosen to optimize the heater operation near end of life. A low dropout
regulator
is desired to maximize the lifespan before regulation is no longer maintained.
A simple
linear regulator is shown, but a high efficiency, switching regulator may also
be employed
to improve efficiency. The pulse duration is maintained as described above or
an
equivalent "one shot" circuit and the heater current is kept constant by the
voltage
regulator.
[0097] In another alternative design, the electrical power control system
34 may be
configured to provide consistent power by timing the power to provide the
minimum
energy needed to vaporize the liquid. The power control system 34 may also be
22
CA 02829043 2013-09-04
WO 2012/120487 PCT/IB2012/052044
programmed to do this. For example, the electrical power control system 34 may
be
programmed to power the source down to the voltage required to vaporize the
liquid, so as
to extend its useful life. Here, the power source may include a capacitor that
builds,
retains and provides a charge necessary to vaporize the liquid to be
vaporized, again, so as
to extend the useful life of the power source. In some embodiments,
supercapacitors may
be employed as discussed above to further enhance the functionality of the
power source.
[0098] In an additional alternative design shown in Figure 18, the liquid
to be atomized
is delivered into a small diameter tube 180 via capillary action, as distinct
from providing
the liquid via pressure into the heating coil, where it is stabilized for
vaporization due to
surface tension. The tube 180 can be glass, polyaniline or metal, e.g.,
stainless steel. A
heating element such as ni-chrome wire can be coiled around the tube, coiled
into the tube
or inserted into a tube in a V-shape so as to heat the entire volume of liquid
at the same
time.
[0099] Figures 19-22 show an alternative vaporizing device 200 having a
housing
formed from a base 202 including a mouthpiece 206, and a cover 204 attached to
the base
202. Pivot arms 209 on a button 208 are pivotally attached to pivot posts 226
on a bridge
224, as shown in Figure 21 to provide another means for consistently metering
a precise
volume of a liquid from the fluid reservoir 234 to precisely control the
volume of the liquid
discharged for vaporization. The radius 244 of the pincher 238 can flex when
the tube 236
is compressed. The bridge 224 has pins for securely attaching it to the base
202. The
positive electrode of each battery 44 is held into contact with center contact
212 by a spring
46. A positive conductor strip 214 connects the center contact to a printed
circuit board
216.
[00100] Referring to Figure 22, a wick 220 extends from the printed circuit
board 216
23
(containing the same or similar circuitry as described above for the power
control system
34) up to a vaporizing coil 222 and optionally over a raised wall 240. The
wick may be a
strip or sheet of ceramic tape 220 that serves as a wick and a heat sink. The
wick 220 is
positioned between the heating element, such as the vaporizing coil 222, and
the outlet of
the tube 236. The wick 200 may rest on top of the heating element, or be
positioned
adjacent to it, and the tube outlet may also be on top of the heating element
and the wick
220 (when the device 200 is in the upright position, with the button 208 on
top).
[00101] Brass posts 218 or similar contacts are attached to the printed
circuit board 216
and to opposite ends of the coil 222. The button 208 has a pincher arm 209
positioned to
pinch and close off flow in a tube 236 connecting a liquid reservoir to an
outlet location on,
adjacent to or overlying the wick 220. The tube 236 may be held in place by
molded in
tube clips 242 on the bridge 224. Arms 233 on a normally closed pinch valve
232 extend
up through openings in the bridge 224. A valve spring 230 around a post 228
holds the
valve 232 into the normally closed position. A bottom surface of the valve 232
may act as
a switch with the printed circuit board 216, or actuate a separate switch on
the printed
circuit board 216, to switch on electrical current to the coil 222 when the
button 208 is
pressed.
[00102] In use, the vaporizing device 200 operates on the same principals as
described
above, with the following additions. A slot 210 may be provided in the housing
to
accommodate an insulating tab. The insulating tab is installed during
manufacture and
prevents electrical contact between the center contact 212 and the batteries.
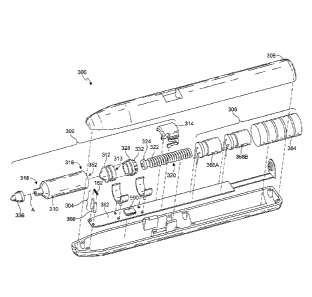
As a result,
the device cannot be inadvertently turned on during shipping and storage.
Battery life is
therefore better preserved. Before operating the vaporizing device 200 for the
first time,
the user pulls the tab out of the slot 210. As shown in Figures 19 and 20, the
mouthpiece
24
CA 2829043 2018-08-20
CA 02829043 2013-09-04
WO 2012/120487 PCT/IB2012/052044
is round. The dimension LL in Figure 20 between the coil 222 and the
mouthpiece tip may
be minimized to 15, 10 or 5 mm. The liquid reservoir may have a volume
exceeding 0.8
or 1.0 ml to allow foam compression to pressurize the pump. In the device 200,
the liquid
supplied from the reservoir via the tube 236 is not delivered into the coil
222. Rather the
liquid is delivered onto the wick 220. The heating coil 222 abuts the wick 220
and heats
the wick, which then vaporizes substantially all of the liquid on or in the
wick.
[00103] In each of the vaporizing devices described above, the open coil
heater 152 or
222 of e.g., ni-chrome wire may be encased in a porous ceramic material, so
that the vapor
produced when the fluid is atomized must pass through the ceramic material in
order to be
ingested or inhaled. The ceramic material can be manufactured with techniques
that
control the size of the pores through which the vapor will pass. This can help
to regulate
the size of the vapor molecules or droplets produced for inhalation. By
controlling the
amount of electrical power and the duration of power to the coil heater, the
heater
continues to vaporize the fluid at the heater until the vapor droplets or
particles are small
enough to pass through the ceramic material, effectively utilizing all the
fluid delivered to
the coil and controlling the dose in addition to regulating the molecule size.
By regulating
the size of the vapor molecule produced, the vaporizing devices can be used
with more
precision and with fluids and medicants that require carefully controlled
dosages particle
sizes. In some cases, smaller molecules may be advantageous as they can be
inhaled more
deeply into the lungs, providing better a more effective delivery mechanism.
[00104] The wire coil heater may alternatively be encased in a heat resistant
fabric-like
material, such as Kevlar , so that the vapor must pass through the fabric to
be ingested.
The fabric can be manufactured with a desired mesh opening size, to regulate
the size of
the vapor particles and/or molecules delivered by the vaporizer. By
controlling the amount
CA 02829043 2013-09-04
WO 2012/120487 PCT/IB2012/052044
of electrical power and the duration of power to the heater, the heater
continues to vaporize
the fluid delivered to the heater until the vapor particles are small enough
to pass through
the mesh of the fabric. Containing the fluid inside the fabric with the heater
until the
particles are sufficiently small enough to pass through the fabric can help to
effectively
atomize and deliver all the fluid delivered to the heater, with little or no
waste, in turn
controlling the dose.
[00105] Although the switch 158 is described above as a mechanical contact
switch,
other forms of switches may optionally be used, including switches that
optically or
electrically sense the movement of position of an element, or a switch that
senses the
presence of liquid in the heater 150. In addition, though the lever and pinch
valves are
shown as clamping type of valves, other forms of mechanically or electrically
operated
valves may be used. Similarly, the peristaltic pumping action created by the
pivoting
movement of the lever may be optionally replaced with alternative forms of
pumping or
fluid movement. Various types of equivalent heating elements may also be used
in place of
the wire coils described. For example, solid state heating elements may be
used. The
heating element may also be replaced by alternative vaporizing elements, such
as electro-
hydrodynamic or piezo devices that can convert liquid into a vapor without
heating.
[00106] In another embodiment, a delivery device 300 utilizes a plunger-style
liquid
delivery system 302 as another means for consistently metering a precise
volume of a liquid
from a fluid reservoir to precisely control the volume of the liquid
discharged for
vaporization. As shown in Figure 23, the delivery device 300 comprises a new
liquid
delivery system 302, but utilizes the same or similar an atomization or
vaporization system
32 and power control system 34 described above, all contained in a housing
308,
preferably cylindrical in shape to mimic a cigarette or cigar.
26
CA 02829043 2013-09-04
WO 2012/120487 PCT/IB2012/052044
[00107] The fluid delivery system 302 has a fluid reservoir 310 to contain the
medicant
and a pressure generator, such as a piston 312 that indexes forward inside the
fluid
reservoir 310 in a consistent, fixed, repeatable amount every time fluid
discharge actuator,
such as a button 314, is pressed or actuated. Preferably, the fluid reservoir
310 is
cylindrical in shape, and more preferably, shaped like a syringe. The delivery
device 300
is completely sealed between applications such that the medicant cannot
evaporate during
storage or between actuation cycles.
[00108] The fluid reservoir 310 has a proximal end 316 and a distal end 318.
The
proximal end 316 is configured to accept the piston 312 which forms a
hydraulic seal
against the walls of the reservoir 310, such that the medicant cannot leak
past the piston
312. The piston 312 may have a hollow core 313. A plunger 320 is provided to
couple
with the piston 312 to drive the piston 312 forward in a controlled and step-
like manner.
The plunger 320 comprises a shaft 322 having a head 324 at one end. In a
preferred
embodiment, the head 324 is flanged. The head 324 is configured to engage with
mating
geometry on the inside of the piston 312, securing the piston 312 to the
plunger 320. The
shaft 322 of the plunger is configured with a male screw thread 326,
preferably, for its
entire length.
[00109] A drive nut 328 is disposed at the proximal end 316 of the reservoir
310.
Various features of the housing 308 and reservoir 310 constrain the position
of the drive
nut 328 such that it is free to move rotationally concurrent to the axis A of
the plunger 320,
but prevent translation in any other direction. The drive nut 328 has a mating
female screw
thread 330 to the plunger 320 and is threaded onto the plunger 320. The drive
nut 328 is
further configured with ratchet teeth 332, which interact with a pawl 334 on a
button 314
27
CA 02829043 2013-09-04
WO 2012/120487 PCT/IB2012/052044
described later such that during operation, the drive nut 328 will rotate in a
single
direction.
[00110] A cap 336 is disposed at the distal end 318 of reservoir 310. The cap
336 may
be an elastomeric component with an outlet 338 comprising a self-collapsing
slit/hole.
Preferably, the cap 336 is made of silicone. The outlet 338 is responsive to
pressure from
the medicant within the reservoir 310 such that when the medicant is at a
higher pressure
than the ambient pressure outside of the reservoir 310, the outlet 338 will
open 338A,
allowing medicant to escape the reservoir 310. Once enough medicant has
escaped the
reservoir 310 to equilibrate with ambient pressure, the outlet 338 will
automatically
collapse, sealing the remaining contents of the reservoir 310 from ambient,
thereby,
preventing loss of medicant to evaporation. So, the measured dose is
determined by proper
calibration of the pressure needed to properly form and maintain a droplet of
the medicant
at the outlet 338 until vaporization is initiated. The nature of seal is such
that pressure
changes external to the device will not cause the reservoir to come "unsealed"
the external
pressure changes would not be focused enough nor forceful enough to "unseal."
And, the
natural elasticity of the reservoir would cause the seal to "re-seal"
irrespective of external
pressure changes.
[00111] Based on the surface tension of the liquid medicant, the volume of the
medicant
discharged from the outlet 338 should be small enough that it forms a droplet
at the outlet
338 that adheres to the outlet 338 without dropping or dripping off from the
cap 336. The
distance from the outlet 338 to the coiled wire 152 should also be small
enough that a
droplet of the liquid formed at the outlet bridges the gap between the outlet
338 and the
coiled wire 152 thereby allowing the droplet to transfer to the coiled wire
152 or the wick
28
CA 02829043 2013-09-04
WO 2012/120487 PCT/IB2012/052044
360 within the coiled wire 152. This configuration allows the vapor delivery
device 300 to
be used in any orientation; thereby, improving the versatility over current
devices.
[00112] As shown in Figure 30, the button 314 functions to provide controlled
rotational
indexing of the drive nut 328. The button 314 includes a control surface 340
protruding
through the upper housing for the user to actuate the button 314. In its
neutral (home)
position, the button 314 is normally protruding slightly from the housing 308.
The button
314 is constrained such that it can translate in a direction normal to the
control surface 340
when it is pressed. The button 314 is configured with two spring elements
341a, 341b
which bias the button back to its neutral position in the absence of pressure
on the control
surface 340. The spring elements 341a, 341b are designed to deform under
pressure on
the load surface and return to their original shape upon release of that
pressure. The range
of button travel motion is limited by a stop 342 having an upper surface 343a
and a lower
surface 343b. The stop surfaces 343a, 343b engage opposing surfaces on lower
and upper
housings at limits of button travel, creating a fixed range of displacement
for the button 314
when it is pressed/released. The button 314 is further configured with a pawl
334 to
engage ratchet teeth 332 on the drive nut 328. When the button 314 is
depressed, the pawl
334 engages ratchet teeth 332, causing the drive nut 328 to rotate. Upon
release, a sloped
surface 344 of a pawl 334 and an opposing surface 346 of the ratchet 332
oppose one
another, deflecting the pawl 334 at a web allowing the pawl 334 to ride over
the adjacent
ratchet tooth and the button 314 to return to its neutral position. In this
manner, the ratchet
332 allows the drive nut 328 to rotate in a single direction.
[00113] In some embodiments, the button 314 may function to initiate the
delivery of
power to the heating system 304 synchronous to the delivery of a bolus of
medicant to the
vaporization system 32. As shown in Figures 31A and 31B a contact pin 348 is
provided
29
CA 02829043 2013-09-04
WO 2012/120487 PCT/IB2012/052044
spanning the button spring elements 341a, 341b. Deflection of the spring
elements 341a,
341b during actuation of the button 314 lowers the contact pin 348 relative to
contacts
350a, 350b, closing the circuit across the contacts 350a, 350b, as shown in
Figure 31B.
This closure serves to initiate a power cycle to the vaporization system 32 as
described
later. In some embodiments, the contacts 350a, 350b may be directly under the
spring
elements 341a, 341b. The underside of the spring elements 341a, 341b may have
independent contact pins 348 to connect with the contacts 350a, 350b to close
the circuit.
In some embodiments, a single contact pin 348 and a single contact 350a may be
used.
[00114] The pitch of thread 326 is selected in consideration of the bore 352
of the
reservoir 310, and controlled angular indexing of the drive nut 328 to
displace the desired
bolus of medicant from the reservoir 310 with each indexing of the drive nut
328. All of
the rotational motions of the drive nut 328 are converted to linear motion on
the plunger
320 to provide a consistent, fixed, and repeatable dose of the medicant. To
ensure that the
plunger 320 does not spin with the rotating drive nut 328, the plunger 320 is
further
provided with a groove 354 running down its length, said groove 354 accepting
anti-
rotation tang 356 protruding from the lower portion of the housing 308, as
shown in Figure
32.
[00115] The atomization or vaporization system 32, similar to what was
described
above, comprises a tightly coiled wire heater element 152 positioned adjacent
to the fluid
delivery system outlet 338. In the preferred embodiment, the coiled wire 152
is a
nichrome wire. In some embodiments, the nichrome wire coil 152 may be wound
around a
high temperature fiber wicking element 360 to distribute the received medicant
dose across
the coil 152.
CA 02829043 2013-09-04
WO 2012/120487 PCT/IB2012/052044
[00116] The power control system 34 comprises a circuit board 362 (containing
the same
or similar circuitry as described above) and associated battery 364 that
delivers a fixed and
precise amount of power to the nichrome wire 152 with each actuation, the
amount of
power delivered being that necessary to atomize or vaporize the precise volume
of
delivered bolus of the medicant. For optimal system efficiency, it is
desirable to maximize
energy density of the heater. Thus the coils of the heater are ideally spaced
as close
together as possible. Furthermore, it is desirable to distribute the dose of
medicant that is
to be vaporized as uniformly as possible across the heater elements. To that
end, the
heater coils 152 are wrapped around a wick 360 comprising material tolerant of
high
temperature, said material compelling the medicant to distribute evenly
throughout the wick
360. The coil 152 is connected to the power control system 34 via crimp
connectors 366.
In the preferred embodiment, the circuit board 362 comprises a one-shot
circuit (similar to
or same as the circuitry described above) that delivers a fixed and precise
amount of power
to the nichrome heater 152 with each actuation, the amount of power delivered
being that
necessary to atomize the delivered bolus of medicant.
[00117] In some embodiments, to further provide a means for delivering a
precise
amount of power to the vaporization system 32, the power control system may
comprise
one or more supercaps 368a, 368b connected to the power source and the
circuit. Using
supercaps 368a, 368b prevents the vaporization system 32 from receiving
varying amounts
of power as the batteries 364 approach there end. In particular, supercaps
368a, 368b
prevent the power to the vaporization system 32 from decreasing as the
batteries die.
Without the circuitry to precisely control the power, decreased battery power
would lead to
a lower temperature wire 152 for a given activation. In such case, if the
volume of the
medicant remains the same, then there may be incomplete vaporization of the
medicant.
31
CA 02829043 2013-09-04
WO 2012/120487 PCT/IB2012/052044
[00118] Figures 33-35 show another embodiment of a delivery device 400. Figure
34
shows the delivery device 400 with the housing 408 removed. Delivery device
400
comprises the same or similar vaporization system 32 and power control system
34 as
described above with another embodiment of a fluid delivery system 402 as a
means for
consistently metering a precise volume of a liquid from a fluid reservoir. The
housing 408
of delivery device 400 also differs from that of delivery device 300. The
housing 408
generally has an elongated box-like configuration. The housing 408 can take on
other
shapes as well, such as a cylinder or any shape or size desired for a
particular application.
The housing 408 has a top end 410 and a bottom and 412 opposite the top end
410. The
top end 410 comprises a cover 414.
[00119] Protruding from the top end 410 is an inhaler tube 416. The inhaler
tube 416 is
operatively connected to the fluid delivery system 402. Medicants from the
fluid delivery
system 402 are vaporized by the vaporization system 32 and the vapors flow
through the
inhaler tube 416 and into the user's mouth. The cover 414 is used to protect
the inhaler
tube 416 when not in use. Figure 33 shows a sliding cover; however, the cover
414 can be
flip top, detachable, slidable, and the like. As the cover 414 is pushed back,
away, off, or
otherwise removed from the top and, the inhaler tube 416 is released and
rotates upward.
The user can then begin the process of inhaling through the inhaler, which
starts the
heating process by activating a flow sensor.
[00120] At the bottom end 412 of the housing 408 is a knob 418 to deliver a
precise
volume of medicant from the fluid delivery system 402 to the vaporization
system 32. Like
the button 314 of device 300, the knob 418 at the bottom 412 of device 400 is
used to
advance a plunger (not shown) through a syringe (not shown) repeatedly in a
step-like
manner to deliver a precise, fixed, and consistent volume of a mendicant from
the syringe
32
CA 02829043 2013-09-04
WO 2012/120487 PCT/IB2012/052044
and deposit it onto the coiled wire 152 of the vaporization system 32. Each
rotation of the
knob 418 advances an exact, metered amount of drug with a consistently
repeatable
volume.
[00121] Like the previous versions, the device 400 utilizes a circuit board
420
(containing the same or similar circuitry as described above for the power
control system
34) with associated processor (not shown), super caps 368a, 368b, and other
electronic
components utilized to deliver a consistent, precise and sufficient amount of
power to the
heating system to vaporize or atomize a predetermined volume of a liquid. The
circuit
board 420 is located at the top end 410 adjacent to the fluid delivery system
402 and the
vaporization system 32. A through-hole 430 is provided to allow the inhaler
tube 416 to be
passed through the circuit board 420 allowing the fluid reservoir 422 to be
attached to this
inhaler tube 416 and present the inhaler tube 416 to the user.
[00122] Below the circuit board 420, the fluid delivery system 402 is mounted.
This
assembly provides a secure, tamper resistant chamber for retaining the fluid.
The fluid
delivery system 402 is then connected to a gear reduction assembly 424 that
allows the
linear syringe actuator to be advanced through the reservoir 422 in a
consistent amount for
each rotation of the knob 418.
[00123] The vaporization system 32 is placed into the path of the fluid that
is delivered
via the fluid delivery system 402 each time the knob 418 is rotated. The
vaporization 32
comprises a heating coil 152. In some embodiments, the heating coil 152 may be
wrapped
around a wick 360, which helps retain the liquid after it has been discharged
from the fluid
delivery system 402. After the fluid is advanced, the fluid wets the wick 428
that is placed
inside the heating coil assembly 152. Once this wick 360 is wetted, the coil
152 can be
heated once the user begins to inhale (suck) on the inhaler tube 416. To
trigger the heating
33
CA 02829043 2013-09-04
WO 2012/120487 PCT/IB2012/052044
mechanism, a flow sensor (not shown) is placed in the inhalation path, which
is the path
between the inlet of the inhaler tube 416 and the outlet 417 of the inhaler
tube 416.
[00124] As flow is sensed when the user begins to inhale/suck on the inhaler
tube 416,
the coil heating is begun by applying voltage to the coil 152. The power
applied to the coil
wire 152 is supplied via the supercap assembly 368a, 368b, which is charged
via the
device batteries 364.
[00125] To further improve the delivery and efficacy of the medicant delivered
by the
present invention, plume chemistry of the medicant delivered to the lungs must
be
analyzed. Depending on the size of the vapor product released by the vapor
delivery
device, the medicant may have effects at various places; thereby dictating the
effectiveness
and speed with which the medicant can work on a user. For example, the larger
vapor
products are more likely to get caught inside the mouth, which would result in
the medicant
travelling through the digestive track. Small vapor products can be inhaled
into the lungs,
but may get caught in the upper lungs. Even finer vapor products can reach the
lower
lungs where absorption of the medicant is more effective and faster.
[00126] Again, to control the size of the vapor product, a permeable membrane
of
ceramic, fabric, or the like may be placed between the heating system and the
mouthpiece.
The heating element allows the medicant to vaporize; however, prior to exiting
through the
mouthpiece, the vapor product is filtered through the permeable membrane to
govern the
size of the vapor product delivered to the user. The membrane should be made
of a
material that is resilient to heat, such as ceramic or Kevlar material.
[00127] Due to the consistent, reliable, and precise control of dosage offered
by the
present invention, its application goes far beyond just as a substitute for
tobacco products.
The device can be used to deliver dietary supplements, sleep aids, weight loss
products,
34
CA 02829043 2013-09-04
WO 2012/120487 PCT/IB2012/052044
pain killers, and many other prescription or over-the-counter pharmaceutical
products
where precise dosing is required. The present invention can even be
implemented in a non-
pharmaceutical context, such as for dispensing liquid candies for consumption,
breath
fresheners, room fresheners, and any other application where vaporization of a
liquid in
consistent, reliable, and precise doses are needed.
[00128] While the system and device have been described in terms of what are
presently
considered to be the most practical and effective embodiments, it is to be
understood that
the disclosure need not be limited to the disclosed embodiments. It is
intended that all
permutations, enhancements, equivalents, combinations, and improvements
thereto that are
apparent to those skilled in the art upon a reading of the specification and a
study of the
drawings are included within the true spirit and scope of the present
invention. The scope
of the disclosure should thus be accorded the broadest interpretation so as to
encompass all
such modifications and similar structures. It is therefore intended that the
application
includes all such modifications, permutations and equivalents that fall within
the true spirit
and scope of the present invention. Thus, multiple embodiments and methods
have been
shown and described. Various modifications and substitutions may of course be
made
without departing from the spirit and scope of the invention. The invention,
therefore,
should not be limited except by the following claims and their equivalents.
INDUSTRIAL APPLICABILITY
[00129] This invention may be industrially applied to the development,
manufacture, and
use of a medicant delivery system that can consistently, reliably, and
repeatably deliver a
precise dose of a medicant to a user in vapor form in an energy efficient
manner. The
delivery system comprises a power control system, a vaporization system, and a
fluid
delivery system. The power control system utilizes a circuitry that allows the
system to
CA 02829043 2013-09-04
WO 2012/120487 PCT/IB2012/052044
deliver just enough power to vaporize or atomize a known volume of a medicant.
To avoid
changes in the current due to power drainage, the control system utilizes
supercapacitors
connected to the circuitry. The power source and/or the resistance at the
heating element
can be monitored so that the system knows how much power needs to be supplied
to
efficiently vaporize the known volume of medicant. The fluid delivery system
utilizes a
reservoir and dispensing mechanism that dispenses the same volume of medicant
with each
actuation. The heating system utilizes a nichrome wire.
36