Note: Descriptions are shown in the official language in which they were submitted.
BENZOTHIAZOLE COMPOUNDS AND THEIR PHARMACEUTICAL USE
Background of the Invention
Human immunodeficiency virus (HIV) infection and related diseases are a major
public
health problem worldwide. Human immunodeficiency virus type 1 (HIV-1) encodes
three
enzymes which are required for viral replication: reverse transcriptase,
protease, and integrase.
Although drugs targeting reverse transcriptase and protease are in wide use
and have shown
effectiveness, particularly when employed in combination, toxicity and
development of resistant
strains have limited their usefulness (Palella, et al N Engl. I. Med. (1998)
338:853-860;
Richman, D. D. Nature (2001) 410:995-1001). Accordingly, there is a need for
new agents that
inhibit the replication of HIV. There is also a need for agents that are
directed against alternate
sites in the viral life cycle including agents that target the interaction of
Lens Epithelial Derived
Growth Factor (LEDGF/p75) and HIV-1 integrase.
Summary of the Invention
The present invention provides compounds and methods for the treatment of an
HIV
infection. Accordingly, in one embodiment, the invention provides a compound
of the invention
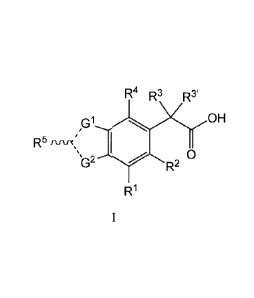
which is a compound of formula I:
R4 R3 R3'
G1 OH
R5 rvvx`I II
0
b2 R2
R1
wherein:
G1 is S, G2 is N, the dashed bond connected to GI is a single bond, the dashed
bond
connected to G2 is a double bond, and the wavy bond connected to R5 is a
single bond; or
GI is N, G2 is S, the dashed bond connected to GI is a double bond, the dashed
bond
connected to G2 is a single bond, and the wavy bond connected to R5 is a
single bond; or
1
CA 2833377 2018-04-17
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
G1 is S, G2 is NR6, the dashed bond connected to Gi is a single bond, the
dashed bond
connected to G2 is a single bond, the wavy bond connected to R5 is a double
bond and R5 is
oxygen (e.g."(wavy bond)-R5" is "=0");
R1 is Ria or Rib;
R2 is R2a or R2b;
R3 is R3a Or R31';
R3' is R3a' or R31f;
R4 is R4a or R4b;
R5 is R5a or R51';
R6 is R6a or R6b;
Ria is selected from:
a) halo;
b) Ri 1, -C(=0)-R11, -C(=0)-0-R11, -0-R11, -S-R11, -S(0)-R11, -S02-R11,
-(C -C6)alkyl-R11, -(Ci-C6)a1kyl-C(=0)-R11, -(C1-C6)alkyl-C(=0)-0-R11, -(CI-
C6)alkyl-O-R11,
-(Ci-C6)alkyl-S-R11, -(Ci-C6)alkyl-S(0)-R" and -(Ci-C6)alkyl-S02-R11, wherein
each Rii is
independently selected from H, (Ci-C6)alkyl, (C2-C6)alkenyl, (C2-C6)alkynyl,
(Ci-C6)haloalkyl,
(C3-C7)cycloalkyl, aryl, heterocycle and heteroaryl, wherein aryl, heterocycle
or heteroaryl are
each optionally substituted with one or more (e.g. 1, 2 or 3) Zil groups; and
c) -N(R9)R1 , -C(=0)-N(R9)R1 , -0-C(=0)-N(R9)R1 , -S02-N(R9)R1 ,
-(C1-C6)alkyl-N(R9)R1 , -(Ci-C6)alkyl-C(=0)-N(R9)R1 , -(Ci-C6)alkyl-O-C(-0)-
N(R9)Ri and
-(Ci-C6)alkyl-S02-N(R9)R1 , wherein each R9 is independently selected from H,
(CI-C6)alkyl
and (C3-C7)cycloalkyl, and each Ri is independently selected from R11, -(Ci-
C6)alkyl-R", -SO2-
Ri1, -C(=0)-R11, -C(=0)0R11 and -C(=0)N(R9)R11, wherein each R" is
independently selected
from H, (C1-C6)alkyl, (C2-C6)alkenyl, (C2-C6)alkynyl, (Ci-C6)haloalkyl, (C3-
C7)cycloalkyl, aryl,
heterocycle and heteroaryl;
R1b is selected from:
a) -(Ci-C6)alky1-0-(Ci-C6)alkyl-(C3-C7)carbocycle, -(C1-C6)alkyl-S-
(CI-
C6)alkyl-(C3-C7) carbocycle, -(Ci-C6)alkyl-S(0)-(CI-C6)alkyl-(C3-C6)
carbocycle, -(C1-C6)alkyl-
S02-(CI-C6)alkyl-(C3-C7)carbocycle, -(CI-C6)alkyl-S02-(Ci-C6)alkyl-Z13, -C(0)-
(C i-C6)alkyl-
3 0 Z13, -0-(C1-C6)alkyl-Z13, -S-(C -C6)alkyl-Z13, -S(0)-(C -C6)alkyl-Z13, -
S 02-(C -C6)alkyl-Z13,
-(C1-C6)alkyl-Z14, -(Ci-C6)alkyl-C(0)-(Ci-C6)alkyl-Z13, -(C1-C6)alkyl-C(0)-
0(Ci-C6)alkyl-Z13,
-(CI-C6)alky1-0-(Ci-C6)alkyl-Z13, -(Ci-C6)alkyl-S-(Ci-C6)alkyl-Z 13, -(C2-
C 6)alkenyl-(C -C6)haloalkyl, -(C2-C 6)alkynyl-(C -C6)haloalkyl, - (C3-
C7)halocarbocycle,
-NRaSO2NR,Rd, -NRaS020(C3-C7)carbocycle, -NRaS020ary1, -(C2-C6)alkenyl-(C3-
C7)carbocycle, -(C2-C6)alkenyl-aryl, -(C2-C6)alkenyl-heteroaryl, -(C2-
C6)alkenyl-heterocycle,
2
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
-(C2-C6)alkynyl-(C3-C7)carbocycle, -(C2-C6)alkynyl-aryl, -(C2-C6)alkynyl-
heteroaryl
-(C2-C6)alkynyl-heterocycle, -(C3-C7)carbocycle-Z1 or -(CI-C6)haloalkyl-Z3,
wherein any
(Ci-C6)alkyl, (Ci-C6)haloalkyl, (C3-C7)carbocycle, (C2-C6)alkenyl, (C2-
C6)alkynyl, aryl or
heteroaryl, either alone or as part of a group, is optionally substituted with
one or more (e.g. 1, 2,
3, 4 or 5) Zi groups;
b) spiro-bicyclic carbocycle, fused-bicyclic carbocycle and bridged-
bicyclic
carbocycle, wherein any spiro-bicyclic carbocycle, fused-bicyclic carbocycle
or bridged-bicyclic
carbocycle is optionally substituted with one or more (e.g. 1, 2, 3, 4 or 5)
Z1 groups, or wherein
two Z1 groups together with the atom or atoms to which they are attached
optionally form a
carbocycle or heterocycle wherein the carbocycle or heterocycle is optionally
substituted with
one or more (e.g. 1, 2, 3, 4 or 5) Z1 groups;
c) (Ci-C6)alkyl, wherein (Ci-C6)alkyl is substituted with one or more (e.g.
1, 2, 3, 4
or 5) Z2 groups and optionally substituted with one or more (e.g. 1, 2, 3, 4
or 5) Zlgroups;
d) -X(Ci-C6)alkyl, -X(Ci-C6)haloalkyl, -X(C2-C6)alkenyl, -X(C2-C6)alkynyl
and
-X(C3-C7)carbocycle, wherein -X(Ci-C6)alkyl and -X(Ci-C6)haloalkyl are each
independently
substituted with one or more Z3 groups and optionally substituted with one or
more Z1 groups,
and wherein -X(C2-C6)alkenyl, -X(C2-C6)alkynyl and -X(C3-C7)carbocycle, are
each
independently substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z4 groups
and optionally
substituted with one or more Zlgroups;
e) aryl, heteroaryl, heterocycle, -Xaryl, -Xheteroaryl and-Xheterocycle;
wherein
aryl heteroaryl and heterocycle, either alone or as part of a group, are each
independently
substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z5 groups and optionally
substituted with one
or more Zlgroups;
(Ci-C6)haloalkyl, (C3-C7)carbocycle, (C2-C6)alkenyl, and (C2-C6)alkynyl,
wherein (Ci-C6)haloalkyl, (C3-C7)carbocycle, (C2-C6)alkenyl and (C2-C6)alkynyl
are each
independently substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z6 groups
and optionally
substituted with one or more Zigroups;
g) NReRf, -C(0)NR,Rf, -0C(0)NReRf, -SO2NReRf, -(Cl-C6)alkyl-NReRfi
-(Cl-C6)allcy1C(0)-NR,Rf, -(CI-C6)alkyl-O-C(0)-NReRf and -(Cl-C6)alkyl-
SO2NReRf; wherein
each (Ci-C6)alkyl, as part of a group, is independently substituted with one
or more (e.g. 1, 2, 3,
4 or 5) Z6 groups and optionally substituted with one or more Zlgroups; and
h) nitro and cyano;
R2a is selected from:
a) halo;
3
CA 02833377 2013-10-16
WO 2012/145728 PCMJS2012/034593
Rii, C(=O)-R'1, _
C(-0)-0-R11, -O-R", -S-R11, -S(0)-R11, -S02-R",
-(Ci-C6)alkyl-R11, -(CI-C6)alkyl-C(=0)-R11, -(C1-C6)alky1-0-R1
1,
-(Ci-C6)alkyl-S-R11, -(CI-C6)alkyl-S(0)-Rn and -(Ci-C6)alkyl-S02-R11, wherein
each R11 is
independently selected from H, (Ci-C6)alkyl, (C2-C6)alkenyl, (C2-C6)alkynyl,
(Ci-C6)haloalkyl,
.. (C3-C7)cycloalkyl, aryl and heterocycle and heteroaryl, wherein aryl,
heterocycle or heteroaryl
are each optionally substituted with one or more (e.g. 1, 2 or 3) Z11 groups;
and
c) _N(R9)Rio, _c(_,0)_N(z9)Rio, -O-C(=O)-N(R9)R' , -S02-N(R9)R' ,
-(C1-C6)alkyl-
N(R9)R1 , -(C1-C6)alkyl-C(=0)-N(R9)R1 , -(C1-C6)alky1-0-C(=0)-N(R9)R1 , and -
(Ci-C6)alkyl-
S02-N(R9)R1 , wherein each R9 is independently selected from H, (Ci-C6)alkyl
and (C3-
wherein each R1 is independently selected from R11, -(Ci-C6)alkyl-R11, -S02-
R11,
-C(=0)-R11, -C(=0)0R11 and -C(---0)N(R9)R11, wherein each R11 is independently
selected from
H, (Ci-C6)alkyl, (C2-C6)alkenyl, (C2-C6)alkynyl, (C1-C6)haloalkyl, (C3-
C7)cycloalkyl, aryl,
heterocycle and heteroaryl;
R21) is selected from:
a) -(C -C6)alky1-0-(C -C6)alkyl-(C3-C7)carbocyc le, -(CI-C6)alkyl-S-(C -
C6)alkyl-(C3-C7)carbocycle, -(Ci-C6)alkyl-S(0)-(Ci-C6)alkyl-(C3-C7)carbocycle,
-(C1-
C6)alkyl-S02-(Ci-C6)alkyl-(C3-C7)carbocycle, -(C2-C6)alkenyl-(Ci-C6)haloalkyl,
-(C2-
C6)alkynyl-(C -C6)haloalkyl, -(C -C6)alkyl-S 02-(C -C6)alkyl-Z13, -C(0)-(C -
C6)alkyl-Z", -0-
(C i-C6)alkyl-Z13, -S-(Ci-C6)alkyl-Z13, -S(0)-(Ci-C6)alkyl-Z13, -S02-(C1-
C6)alkyl-Z13,
-(C1-C6)alkyl-Z14, -(Ci-C6)alkyl-C(0)-(C1-C6)alkyl-Z13, -(Ci-C6)alkyl-C(0)-
0(C1-C6)alkyl-Z13,
-(C1-C6)alky1-0-(C i-C6)alkyl-Z13, -(Ci-C6)alkyl-S-(C1-C6)alkyl-Z13, -(C3-
C7)halocarbocycle,
-NRaSO2NR,R,i, -NRaS020(C3-C7)carbocycle, -NRaS020aryl,
-(C2-C6)alkenyl-(C3-C7)carbocycle, -(C2-C6)alkenyl-aryl, -(C2-C6)alkenyl-
heteroaryl,
-(C2-C6)alkenyl-heterocycle, -(C2-C6)alkynyl-(C3-C7)carbocycle, -(C2-
C6)alkynyl-aryl,
-(C2-C6)alkynyl-heteroaryl, -(C2-C6)alkynyl-heterocycle, -(C3-C7)carbocycle-Z1
or -(C1-
C6)haloalkyl-Z3, wherein any (Ci-C6)alkyl, -(Ci-C6)haloalkyl, (C3-
C7)carbocycle, (C2-
C6)alkenyl, (C2-C6)alkynyl, aryl or heteroaryl, either alone or as part of a
group, is optionally
substituted with one or more (e.g. 1, 2, 3, 4 or 5) Zlgroups;
b) spiro-bicyclic carbocycle, fused-bicyclic carbocycle and
bridged-bicyclic
carbocycle, wherein any spiro-bicyclic carbocycle, fused-bicyclic carbocycle
or bridged-bicyclic
carbocycle is optionally substituted with one or more (e.g. 1, 2, 3, 4 or 5)
Z1 groups, wherein
two Z1 groups together with the atom or atoms to which they are attached
optionally form a
(C3-C7)carbocycle or heterocycle wherein the (C3-C6)carbocycle or heterocycle
is optionally
substituted with one or more (e.g. 1, 2, 3,4 or 5) Z1 groups;
4
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
c) (Ci-C6)alkyl, wherein (Ci-C6)alkyl is substituted with one or more Z2
groups and
optionally substituted with one or more (e.g. 1,2, 3,4 or 5) Zigroups;
d) -X(C1-C6)alkyl, -X(Ci-C6)haloalkyl, -X(C2-C6)alkenyl, -X(C2-C6)alkynyl
and
-X(C3-C7)carbocycle, wherein -X(Ci-C6)alkyl and X(Ci-C6)haloalkyl are each
independently
substituted with one or more Z3 groups and optionally substituted with one or
more (e.g. 1, 2, 3, 4
or 5) Zigroups, and wherein -X(C2-C6)alkenyl, -X(C2-C6)alkynyl and -X(C3-
C7)carbocycle are
each independently substituted with one or more (e.g. 1, 2, 3,4 or 5) Z4
groups and optionally
substituted with one or more Z1groups;
e) aryl, heteroaryl, heterocycle, -Xaryl, -Xheteroaryl and -Xheterocycle,
wherein
aryl heteroaryl and heterocycle, either alone or as part of a group, are each
independently
substituted with one or more (e.g. 1,2, 3,4 or 5) Z5 groups and optionally
substituted with one or
more (e.g. 1, 2, 3, 4 or 5) Zigroups;
0 (Ci-C6)haloalkyl, (C3-C7)carbocycle, (C2-C6)alkenyl, and (C2-
C6)alkynyl,
wherein (Ci-C6)haloalkyl, (C3-C7)carbocycle, (C2-C6)alkenyl and (C2-C6)alkynyl
are each
independently substituted with one or more (e.g. 1,2, 3,4 or 5) Z6 groups and
optionally
substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z1groups;
g) -NReRf, -C(0)NR,Rf, -0C(0)NReRf, -SO2N&Rf, -(Cl-C6)alkyl-NRA6
-(C[-C6)alkylC(0)-NReRf, -(CI-C6)a1kyl-O-C(0)-NReRf and -(Ci-C6)alkyl-
SO2NReRf, wherein
each (C1-C6)alkyl, as part of a group, is independently substituted with one
or more (e.g. 1, 2, 3,
4 or 5) Z6 groups and optionally substituted with one or more (e.g. 1,2, 3,4
or 5) Zlgroups; and
h) nitro and cyano;
or R1 and R2 together with the atoms to which they are attached form a 5 or 6-
membered
carbocycle or a 4, 5, 6 or 7-membered heterocycle, wherein the 5 or 6-membered
carbocycle or a
4, 5, 6 or 7-membered heterocycle are optionally substituted with one or more
Z1 groups;
or R1 and R2 together with the atoms to which they are attached form a 5 or 6-
membered
carbocycle or a 4, 5, 6 or 7-membered heterocycle, wherein the 5 or 6-membered
carbocycle or a
4, 5, 6 or 7-membered heterocycle are each independently substituted with one
or more (e.g. 1, 2
or 3) Z7 or Z8 groups, or wherein when two Z7 groups are on same atom the two
Z7 groups
together with the atom to which they are attached optionally form a (C3-
C7)carbocycle or 4, 5 or
6-membered heterocycle;
R3a is (Ci-C6)alkyl, (CI-C6)haloalkyl, (C2-C6)alkenyl, (C2-C6)alkynyl,
-(C1-C6)alkyl-(C3-C7)cycloalkyl, -(CI-C6)alkyl-aryl, -(CI-C6)alkyl-
heterocycle, -(Ci-C6)alkyl-
heteroaryl, -0(Ci-C6)alkyl, -0(Ci-C6)haloalkyl, -0(C2-C6)alkenyl, -0(C2-
C6)alkynyl,
-0(C3-C7)cycloalkyl, -Oaryl, -0(Ci-C6)alkyl-(C3-C7)cycloalkyl, -0(Ci-C6)alkyl-
aryl,
-0(C i-C6)alkyl-heterocycle and -0(Ci-C6)alkyl-heteroaryl, wherein any (C1-
C6)alkyl,
5
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
(C1-C6)haloalkyl, (C2-C6)alkenyl, (C2-C6)alkynyl, -(C1-C6)alkyl-(C3-
C7)cycloalkyl,
-(C1-C6)alkyl-aryl, -(Ci-C6)alkyl-heterocycle, -(CI-C6)alkyl-heteroaryl, -0(C1-
C6)alkyl,
-0(C i-C6)haloalkyl, -0(C2-C6)alkenyl, -0(C2-C6)alkynyl, -0(C3-C7)cycloalkyl, -
Oaryl,
-0(C -C6)alkyl-(C3-C 7)cycloalkyl, -0 (C -C6)alkyl-aryl, -0(C 1-C6)alkyl-
heterocycle or
-0(CI-C6)alkyl-heteroaryl of R3 is optionally substituted with one or more
(e.g. 1, 2 or 3)
groups selected from (Ci-C6)alkyl, -0(C i-C6)alkyl, halo, oxo and -CN; and
R3'' is H;
R3b is -(C3-C7)carbocycle, aryl, heteroaryl, heterocycle, -(C1-C6)alkylOH, -
(Ci-C6)alky1-
0-(C1-C6)alkyl-Z12, -(C1-C6)alky1-0-(C2-C6)alkenyl-Z12, -(C2-C6)alky1-0-(C2-
C6)alkynyl-Z12,
-(CI-C6)alkyl-S-(C1-C6)alkyl-Z12, -(C1-C6)alkyl-S-(C2-C6)alkenyl-Z12, -(C2-
C6)alkyl-S-(C2-
C6)alkynyl-Z12, -(C1-C6)alkyl-S(0)-(CI-C6)alkyl-Z12, -(Ci-C6)alkyl-S(0)-(C2-
C6)alkenyl-Z12,
-(C2-C 6)alkyl -S(0)-(C2-C6)alkynyl -Z12, -(C -C6)al kyl-S 02-(C i-C6)alkyl -
Z12, -(CI-C6)alkyl-S 02-
(C2-C6)alkenyl-Z12, -(C2-C6)alkyl-S02-(C2-C6)alkynyl-Z12, -(C2-C6)alkyl-NRaRb,
-(C2-C6)alkylOC(0)-NR,Rd, -(C2-C6)alkyl-NRa-C(0)-0R13, -(C2-C6)alkyl-NRa-C(0)-
NRaRb,
-(CI-C6)alkyl-S02(Ci-C6)alkyl, -(C1-C6)alkyl-SO2NR,Rd, -(CI-C6)alkyl-
NRaSO2NReRd,
-(Ci-C6)alkyl-NRaS020(C3-C7)carbocycle, -(CI -C6)alkyl-NRaS020aryl,
-(C1-C6)alkyl-NRa-S02-(C1-C6)alkyl, -(C i-C 6)alkyl-NRa-S 02-(C 1-
C6)haloalkyl,
-(C1-C6)alkyl-NRa-S02-(C2-C6)alkenyl, -(C1-C6)alkyl-NRa-S02-(C2-C6)alkynyl,
-(Ci-C6)alkyl-NRa-S02-(C3-C7)carbocycle, -(Ci-C6)alkyl-NRa-S02-(C3-
C7)halocarbocycle,
-(C1-C6)alkyl-NRa-S02-aryl, -(Ci-C6)alkyl-NRa-S02-heteroaryl,
-(Ci-C6)alkyl-NRa-S02-heterocycle, -0(Ci-C6)alkyl-NRaRb, -0(Ci-C6)a1kylOC(0)-
NRcRth -
0(C1-C6)alkyl-NRa-C(0)-ORb, -0(Cl-C6)alkyl-NRa-C(0)-NRaRb, -0(CI-C6)alkyl-NRa-
S02-(Ci-
C6)alkyl, -0(Ci -C6)alkyl-NRa-S 02-(C i-C6)haloalkyl, -0(Ci-C6)alkyl-NRa-S02-
(C2-C6)alkenyl,
-0(C i-C 6)alkyl-NRa-S02-(C2-C6)alkynyl, -0 (C i-C 6)alkyl-NRa-S02-(C3-
C7)carbocycle,
-0(C i-C6)alkyl-NRa-S02-(C3-C7)halocarbocycle, -0(C i-C 6)alkyl-NRa-S02-aryl,
-0 (C i-C6)al kyl -NRa-S02-heteroaryl, -0(C 1-C6)alkyl-NRa-S 02-heterocycle,
-0 (C -C6)alkyl-NRa-S02-NRaRb, -0(C -C6)alkyl-NRa-S02-(C3-C7)carbocycle,
-0(C -C6)alkyl-NRa-S02-(C3-C7)halocarbocycle, -0 (C i-C6)alkyl-NRa-S02-aryl, -
0(C i-C 6)alkyl-
NRaSO2NRcR,I, -0(Ci-C6)alkyl-NRaS020(C3-C7)carbocycle, -0(C1-C6)alkyl-
NRaS020ary1,
-Oheteroaryl, -Oheterocycle, -Sheteroaryl, -Sheterocycle, -S(0)heteroaryl, -
S(0)heterocycle,
-S02heteroaryl or -S02heterocyc1e, wherein any (Ci-C6)alkyl, (C2-C6)alkenyl,
(C2-C6)alkynyl,
aryl, (C3-C7)carbocyele, heteroaryl or heterocycle of R3b, either alone or as
part of a group, is
optionally substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z1 groups; and
R3b' is H, (Ci-C6)alkyl or -0(CI-C6)alkyl; or
6
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
R31' and R3b' together with the carbon to which they are attached form a
heterocycle or
(C3-C7)carbocycle,which heterocycle or (C3-C7)carbocycle of R3b and R3b'
together with the
carbon to which they are attached is optionally substituted with one or more
(e.g. 1, 2, 3, 4 or 5)
Z1 groups;
R4a is selected from aryl, heterocycle and heteroaryl, wherein any aryl,
heterocycle and
heteroaryl of R4a is optionally substituted with one or more (e.g. 1, 2, 3, 4
or 5) groups each
independently selected from halo, (CI-C6)alkyl, (C2-C6)alkenyl, (Ci-
C6)haloalkyl, (C3-C7)cycloalkyl,
-(Ci-C6)alkyl-(C3-C7)cycloalkyl, -OH, -0(Ci-C6)alkyl, -SH, -S(Ci-C6)alkyl, -
NH2, -NH(C1-C6)alkyl
and -N((Ci-C6)alky1)2, wherein (C1-C6)alkyl is optionally substituted with
hydroxy, -0(C1-
1 0 C6)alkyl, cyano or oxo;
R4b is selected from;
a) (C1-C6)alkyl, (C2-C6)alkenyl and (C2-C6)alkynyl, wherein (Ci-
C6)alkyl,
(C2-C6)alkenyl or (C2-C6)alkynyl are each optionally substituted with one or
more (e.g. 1, 2, 3, 4
or 5) Z1 groups;
b) (C3-Ci4)carbocycle, wherein (C3-C14)carbocycle is optionally substituted
with
one or more (e.g. 1,2, 3,4 or 5) Z1 groups, or wherein two Z1 groups together
with the atom or
atoms to which they are attached optionally form a (C3-C7)carbocycle or
heterocycle;
c) Spiro-heterocycle or bridged-heterocycle, wherein spiro-heterocycle or
bridged-heterocycle is optionally substituted with one or more (e.g. 1, 2, 3,
4 or 5) Z1 groups, or
wherein two Z1 groups together with the atom or atoms to which they are
attached optionally
form a (C3-C7)carbocycle or heterocycle; and
d) aryl, heteroaryl, spiro- heterocycle, fused- heterocycle, or bridged-
heterocycle,
wherein aryl, heteroaryl, Spiro- heterocycle, fused- heterocycle and bridged-
heterocycle are each
independently substituted with one or more Z7 groups and optionally
substituted with one or
more (e.g. 1, 2, 3, 4 or 5) Z1 groups; or
R4 and R3 together with the atoms to which they are attached form a
macroheterocycle or
a macrocarbocycle, wherein any macroheterocycle or macrocarbocycle of R4 and
R3 together
with the atoms to which they are attached may be optionally substituted with
one or more (e.g.
1, 2, 3, 4 or 5) Z1 groups; and R3b' is H or (CI-C6)alkyl, -0(CI-C6)alkyl;
R5a is selected from:
a) halo;
b) R11, -C(=0)-R11, -C(=0)-0-R11,
S(0)-R", -S02-R", -(C1-
C6)alkyl-R11, -(Ci-C6)alkyl-C(=0)-R11, -(Ci-C6)alkyl-C(=0)-0-R", -(Ci-C6)alkyl-
O-R", -(Ci-
C6)alkyl-S-R", -(CI-C6)alkyl-S(0)-R11 and -(Ci-C6)alkyl-S02-R11, wherein each
R" is
independently selected from H, (C1-C6)alkyl, (C2-C6)alkenyl, (C2-C6)alkynyl,
(CI-C6)haloalkyl,
7
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
(C3-C7)carbocycle, aryl, heterocycle and heteroaryl, wherein aryl, heterocycle
and heteroaryl are
each optionally substituted with one or more (e.g. 1,2 or 3) Z" groups; and
c) -N(R9)R10, -C(=0)-N(R9)Rio, -O-C(=O)-N(R9)R' , _s02-N(R9)Rio,
C6)alkyl-
N(R9)R1o,
(u C6)alkyl-C(=0)-N(R9)R1 , -(C1-C6)alkyl-O-C(=0)-N(R9)R1 , and -(C1-C6)alkyl-
S02-N(R9)R1 , wherein each R9 is independently selected from H, (C1-C6)alkyl
and (C3-
C7)cycloalkyl, and each RI is independently selected from R", -(CI-C6)alkyl-
R11, -S02-R", -
C(=0)-R11, -C(=0)0R11 and -C(=0)N(R9)R11, wherein each R" is independently
selected from
H, (C1-C6)alkyl, (C2-C6)alkenyl, (C2-C6)alkynyl, (C1-C6)haloalkyl, (C3-
C7)cycloalkyl, aryl,
heterocycle and heteroaryl;
R5b is selected from:
a) -(Ci-C6)alkyl-0-(CI-C6)alkyl-(C3-C7)carbocycle,
-(C1-C6)alkyl-S-(Ci-C6)alkyl-(C3-C7)carbocycle,
-(CI-C6)alkylS(0)-(Ci-C6)alkyl-(C3-C6)carbocycle,
-(C1-C6)alkylS02(Ci-C6)alkyl-(C3-C7)carbocycle, -(C2-C6)alkenyl-(Ci-
C6)haloalkyl, -(C2-
C6)alkynyl-(C1-C6)haloalkyl, - (C3-C7)halocarbocycle, -NRaSO2NRcIld, -
NRaS020(C3-
C7)carbocycle, -NRaS020aryl, -(C2-C6)alkenyl-(C3-C7)carbocycle, -(C2-
C6)alkenyl-aryl,
-(C2-C6)alkenyl-heteroaryl, -(C2-C6)alkenyl-heterocycle, -(C2-C6)alkynyl-(C3-
C7)carbocycle,
-(C2-C6)alkynyl-aryl, -(C2-C6)alkynyl-heteroaryl, -(C2-C6)alkynyl-heterocycle,
-(C3-
C7)carbocycle-Z1 or -(C1-C6)haloalkyl-Z3, wherein any (Ci-C6)alkyl, (Ci-
C6)haloalkyl,
(C3-C7)carbocycle, (C2-C6)alkenyl, (C2-C6)alkynyl, aryl or heteroaryl, either
alone or as part of a
group is optionally substituted with one or more(e.g. 1, 2, 3, 4 or 5) Z1
groups;
b) spiro-bicyclic carbocycle, fused-bicyclic carbocycle and bridged-
bicyclic
carbocycle, wherein any spiro-bicyclic carbocycle, fused-bicyclic carbocycle
or bridged-bicyclic
carbocycle is optionally substituted with one or more (e.g. 1, 2, 3, 4 or 5)
Z1 groups, or wherein
two Z1 groups together with the atom or atoms to which they are attached
optionally form a (C3-
C7)carbocycle or heterocycle wherein the (C3-C7)carbocycle or heterocycle is
optionally
substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z1 groups;
c) (CI-C6)alkyl, wherein (Ci-C6)alkyl is substituted with one or more Z2
groups and
optionally substituted with one or more (e.g. 1, 2, 3,4 or 5) Z1groups;
d) -X(Ci-C6)alkyl,-X(CI-C6)haloalkyl, -X(C2-C6)alkenyl, -X(C2-C6)allcynyl
and
-X(C3-C7)carbocycle, wherein -X(CI-C6)alkyl and -X(CI-C6)haloalkyl are each
independently
substituted with one or more Z3 groups and optionally substituted with one or
more Z1groups,
and wherein -X(C2-C6)alkenyl, -X(C2-C6)alkynyl and -X(C3-C7)carbocycle are
each
independently substituted with one or more (e.g. 1, 2, 3,4 or 5) Z4 groups and
optionally
substituted with one or more (e.g. 1, 2, 3,4 or 5) Z1groups;
8
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
e) aryl, heteroaryl, heterocycle, -Xaryl, -Xheteroaryl and -
Xheterocycle, wherein
aryl, heteroaryl and heterocycle, either alone or as part of a group, are each
independently
substituted with one or more (e.g. 1,2, 3, 4 or 5) Z5 groups and optionally
substituted with one or
more (e.g. 1, 2, 3, 4 or 5) Zlgroups;
(Ci-C6)haloalkyl, (C3-C7)carbocycle, (C2-C6)alkenyl, and (C2-C6)alkynyl,
wherein (Ci-C6)haloalkyl, (C3-C7)carbocycle, (C2-C6)alkenyl and (C2-C6)alkynyl
are each
independently substituted with one or more (e.g. 1, 2, 3,4 or 5) Z6 groups and
optionally
substituted with one or more (e.g. 1, 2, 3, 4 or 5) Zlgroups;
g) -NReRf, -C(0)NReRf, -0C(0)NReR1, -SO2NReRf, -(CI-C6)alkYl-NReltr,
-(Ci-C6)a1kylC(0)-NR,Rf, -(Ci-C6)alkyl-O-C(0)-NReRf and -(Cl-C6)alkyl-
SO2NReRf, wherein
each (Ci-C6)alkyl is independently substituted with one or more (e.g. 1, 2, 3,
4 or 5) Z6 groups
and optionally substituted with one or more (e.g. 1,2, 3,4 or 5)Z1groups;
h) nitro and cyano;
i) aryl, heteroaryl and heterocycle, wherein aryl, heteroaryl and
heterocycle are
each independently substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z15
groups and optionally
substituted with one or more (e.g. 1, 2, 3, 4 or 5) Zigroups;
and
j) oxo;
R6a is selected from:
a) R", tt, _
C(=0)-0-R11, -0-R11, -S-R", -S(0)-R11, -S02-R11, -(C1-
C6)alkyl-R11, -(Ci-C6)alkyl-C(=0)-R", -(Ci-C6)alkyl-C(=0)-0-R11, -(C1-C6)alkyl-
O-R11, -(C1-
C6)alkyl-S-R", -(CI-C6)alkyl-S(0)-R" and -(CI-C6)alkyl-S02-R", wherein each
R11 is
independently selected from H, (Ci-C6)alkyl, (C2-C6)alkenyl, (C2-C6)alkynyl,
(Ci-C6)haloalkyl,
(C3-C7)cycloalkyl, aryl, heterocycle and heteroaryl, wherein aryl, heterocycle
and heteroaryl are
each optionally substituted with one or more (e.g. 1, 2 or 3) Zll groups; and
b) -C(=0)-N(R9)R10, -S02-N(R9)R10, -(C1-C6)alkyl-N(R9)R10, -(Ci-
C6)alkyl-C(=0)-
N(R9)R1o, _(Ci-C6)alkyl-O-C(=0)-N(R9)Rlo an _
and (Ci-C6)alkyl-S02-N(R9)R1 , wherein each R9
is independently selected from H, (Ci-C6)alkyl and (C3-C7)cycloalkyl, and each
R1 is
independently selected from R", -(Ci-C6)alkyl-R11, -S02-R11, -C(=0)-R", -
C(=0)0R11 and -
.. C(=0)N(R9)R11, wherein each R11 is independently selected from H, (Ci-
C6)alkyl, (C2-
C6)alkenyl, (C2-C6)alkynyl, (Ci-C6)haloalkyl, (C3-C7)cycloalkyl, -(CI-
C6)alkylaryl, aryl,
heterocycle and heteroaryl, wherein aryl, heterocycle and heteroaryl are each
optionally
substituted with one or more (e.g. 1, 2 or 3) Z11 groups;
R6b is selected from:
9
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
a) -(C i-C6)alkyl-S02-(C i-C6)alkyl-Z13, -C(0)-(Ci-C6)alkyl-Z13, -
0-(Ci-C6)alkyl-Z13,
-S-(Ci-C6)alkyl-Z13, -S(0)-(C i-C6)allcyl-Z13, -S 02-(C -C6)alicyl-Z13, -(Ci-
C6)alkyl-Z'4, -(C1_
C6)alkyl-C(0)-(Ci-C6)alkyl-Z13, -(Ci-C6)alkyl-C(0)-0(C i-C6)allcyl-Z13, -(C 1 -
C6)alky1-0-(C 1 -
C6)alicyl-Z13, -(Ci-C6)alkyl-S-(C1-C6)alkyl-Z13, -(C1-C6)alky1-0-(C1-C6)alkyl-
(C3-C7)carbocycle,
-(Ci-C6)alkyl-S-(C1-C6)alkyl-(C3-C7)carbocycle, -(C1-C6)alkyl-S(0)-(C
C6)alkyl-(C3-C7)carbocycle, -(CI-C6)alkyl-S02-(Ci-C6)alkyl-(C3-C7)carbocycle, -
(C2-
C6)alkenyl-(Ci-C6)haloalkyl, -(C2-C6)alkynyl-(Ci-C6)haloalkyl, -(C3-
C7)halocarbocycle,
-(C2-C6)alkenyl-(C3-C7)carbocycle, -(C2-C6)alkenyl-aryl, -(C2-C6)alkenyl-
heteroaryl,
-(C2-C6)alkenyl-heterocycle, -(C2-C6)alkynyl-(C3-C7)carbocycle, -(C2-
C6)allcynyl-aryl,
-(C2-C6)alkynyl-heteroaryl, -(C2-C6)alkynyl-heterocycle, -(C3-C7)carbocycle-
Z1, --(Cr
C6)haloalkyl-Z3, -NRaSO2NRcRd, -NRaS020(C3-C7)carbocycle and -NRaS020ary1,
wherein any
(C1-C6)allcyl, (Ci-C6)haloalkyl, (C3-C7)carbocycle, (C2-C6)alkenyl, (C2-
C6)alkynyl, aryl,
heterocycle and heteroaryl, either alone or as part of a group, is optionally
substituted with one
or more (e.g. 1, 2, 3, 4 or 5) Z1 groups;
b) spiro-bicyclic carbocycle, fused-bicyclic carbocycle and bridged-
bicyclic
carbocycle, wherein any spiro-bicyclic carbocycle, fused-bicyclic carbocycle
and bridged-
bicyclic carbocycle is optionally substituted with one or more (e.g. 1, 2, 3,
4 or 5) Z1 groups, or
wherein two Z1 groups together with the atom or atoms to which they are
attached optionally
form a (C3-C7)carbocycle or heterocycle wherein the (C3-C7)carbocycle or
heterocycle is
.. optionally substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z1 groups;
c) (Ci-C6)alkyl, wherein (Ci-C6)alkyl is substituted with one or more (e.g.
1, 2, 3, 4
or 5) Z2 groups and optionally substituted with one or more (e.g. 1, 2, 3, 4
or 5) Z1 groups;
d) -X(Ci-C6)alkyl, -X(CI-C6)haloalkyl, -X(C2-C6)alkenyl, -X(C2-C6)allcynyl
and
-X(C3-C7)carbocycle, wherein -X(Ci-C6)alky and -X(Ci-C6)haloalkyl, are each
independently
substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z3 groups and optionally
substituted with one
or more (e.g. 1, 2, 3, 4 or 5) Z1 groups, and wherein -X(C2-C6)alkenyl, -X(C2-
C6)alkynyl and
-X(C3-C7)carbocycle, are each independently substituted with one or more (e.g.
1, 2, 3, 4 or 5)
Z4 groups and optionally substituted with one or more (e.g. 1, 2, 3, 4 or 5)
Z1 groups;
e) aryl, heteroaryl, heterocycle, -Xaryl, -Xheteroaryl and -Xheterocycle,
wherein
aryl, heteroaryl and heterocycle, either alone or as part of a group, are each
independently
substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z5 groups and optionally
substituted with one
or more (e.g. 1, 2, 3, 4 or 5) Z1 groups;
(Ci-C6)haloalkyl, (C3-C7)carbocycle, (C2-C6)alkenyl and (C2-C6)alkynyl,
wherein
(Ci-C6)haloalkyl, (C3-C7)carbocycle, (C2-C6)alkenyl and (C2-C6)allcynyl are
each independently
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z6 groups and optionally
substituted with one
or more (e.g. 1,2, 3,4 or 5) Z1 groups; and
g) -C(0)NReRf, -SO2NR,Rf, -(CI-C6)alkyl-NRaRf, -(Cl-C6)alkylC(0)-
NReRf,
-(Cl-C6)alkyl-O-C(0)-NReRf and -(CI-C6)alkyl-SO2NReRf, wherein any (Ci-
C6)alkyl, as part of
a group, is optionally substituted with one or more (e.g. 1,2, 3,4 or 5) Z1
groups;
each X is independently selected from 0, -C(0)-, -C(0)0-, -S-, -S(0)-, -S02_, -
(Ct-
C6)alky10-, -(C1-C6)alkylC(0)-, -(C1-C6)alkylC(0)0-, -(C1-C6)alky1S-, -(Ci-
C6)alkylS(0)- and
-(C1-C6)alkylS02-;
each Z1 is independently selected from halo, -NO2, -OH, =NORa, -SH, -CN, (Ci-
C6)alkyl, (C2-C6)alkenyl, (C2-C6)alkynyl, (CI-C6)haloalkyl, (C3-C7)carbocycle,
(C3-
C7)halocarbocycle, aryl, heteroaryl, heterocycle, -0(Ci-C6)alkyl, -0(C2-
C6)alkenyl, -0(C2-
C6)alkynyl, -0(Ci-C6)haloalkyl, -0(C3-C7)carbocycle, -0(C3-C7)halocarbocycle, -
Oaryl, -
Oheteroaryl, -Oheterocycle, -S(Ci-C6)alkyl, -S(C2-C6)alkenyl, -S(C2-
C6)alkynyl, -S(C1-
C6)haloalkyl, -S(C3-C7)carbocycle, -S(C3-C7)halocarbocycle, -Saryl, -
Sheteroaryl, -
Sheterocycle, -S(0)(Ct-C6)alkyl, -S(0)(C2-C6)alkenyl, -S(0)(C2-C6)alkynyl, -
S(0)(C1-
C6)haloalkyl, -S(0) (C3-C7)carbocycle, -S(0)(C3-C7)halocarbocycle, -S02(Ci-
C6)alkyl, -
S(0)aryl, -S(0)carbocycle, -S(0)heteroaryl, -S(0)heterocycle, -S02(C2-
C6)alkenyl, -S02(C2-
C6)alkynyl, -S02(Ci-C6)haloalkyl, -S02(C3-C7)carbocycle, -S02(C3-
C7)halocarbocycle, -
SO2ary1, -S02heteroaryl, -S02heterocycle, -SO2NRcRd, NRcRd, -NRaC(0)Ra, -
NRaC(0)0Rb,
-NRaC(0)NRcRd -NRaSO2Rb, -NRaSO2NR,Rd, -NRaS020(C3-C7)carbocycle, -
NRaS020aryl,
-0S(0)2Ra, -C(0)Ra, -C(0)0Rb, -C(0)NR,R3, and -0C(0)NRcR4, wherein any (CI-
C6)alkyl,
(C2-C6)alkenyl, (C2-C6)alkynyl, -(C3-C7)halocarbocycle, (C3-C7)carbocycle, (C3-
C7)halocarbocycle, aryl, heteroaryl or heterocycle of Z1, either alone or as
part of a group, is
optionally substituted with one or more (e.g. 1, 2, 3, 4 or 5) halogen, -OH, -
ORb, -CN,
-NRaC(0)2Rb, -heteroaryl, -heterocycle, -Oheteroaryl, -Oheterocycle, -
NHheteroaryl, -
NHheterocycle or -S(0)2NRcitd;
each Z2 is independently selected from -NO2, -CN, spiro-heterocycle, bridge-
heterocycle,
spiro-bicyclic carbocycle, bridged-bicyclic carbocycle, NRaS02(C3-
C7)carbocycle, -NRaS02aryl,
-NRaS02heteroary1, -NRaSO2NRcRd, -NRaS020(C3-C7)carbocycle and -NRaS020aryl;
each Z3 is independently selected from -NO2, -CN, -OH, oxo, =NORa, thioxo,
aryl,-
heterocycle, heteroaryl, (C3-C7)halocarbocycle, -0(Ci-C6)allcyl, -0(C3-
C7)carbocycle, -0(C3-
C7)halocarbocycle, -Oaryl, -Oheterocycle, -Oheteroaryl, -S(Ci-C6)alkyl, -S(C3-
C7)carbocycle, -
S(C3-C7)halocarbocycle, -Saryl, -Sheterocycle, -Sheteroaryl, -S(0)(Ci-
C6)alkyl,
-S(0)(C3-C7)carbocycle, -S(0) (C3-C7)halocarbocycle, -S(0)aryl, -
S(0)heterocycle, -
S(0)heteroaryl, -S02(C1-C6)alkyl, -S02(C3-C7)carbocycle, -S02(C3-
C7)halocarbocycle, SO2aryl,
11
CA 02833377 2013-10-16
WO 2012/145728
PCT/US2012/034593
-S02heterocyc1e, -S02heteroaryl, -NRaRb, -NRaC(0)Rb, -C(0)NRcRd, -SO2NR,Rd,
-NRaSO2NR,R,i, -NRaS020(C3-C7)carbocycle and -NRaS020ary1;
each Z4 is independently selected from halogen, -(C1-C6)alkyl, (C3-
C7)carbocycle, -(C1-
C6)haloalkyl, -NO2, -CN, -0H, oxo, =NORa, thioxo, -aryl, -heterocycle, -
heteroaryl, -(C3-
C7)halocarbocycle, -0(C1-C6)alkyl, -0(C3-C7)carbocycle, -0(C3-
C7)halocarbocycle, -Oaryl, -
heterocycle, -Oheteroaryl, -S(C1-C6)alkyl, -S(C3-C7)carbocycle, -S(C3-
C7)halocarbocycle, -
Saryl, -Sheterocycle, -Sheteroaryl, -S(0)(CI-C6)alkyl, -S(0)(C3-C7)carbocycle,
-S(0)(C3-
C7)halocarbocycle, -S(0)aryl, -S(0)heterocycle, -S(0)heteroaryl, -S02(CI-
C6)alkyl, -S02(C3-
C7)carbocycle, -S02(C3-C7)halocarbocycle, SO2aryl, -S02heterocycle, -
S02heteroaryl, -NRaRb,
-NRaC(0)Ra, -C(0)NRcRd, -SO2NR,Rd, -NRaSO2NRcRd, -NRaS020(C3-C7)carbocycle and
-NRaS020ary1;
each Z5 is independently selected from -NO2, -CN, -NRaSO2NRcR,i, -NRaS020(C3-
C7)carbocycle, -NRaS020aryl, -NRaS02(CI-C6)alkyl, -NRaS02(C2-C6)alkenyl, -
NRaS02(C2-
C6)alkynyl, -NRaS02(C3-C7)carbocycle, -NRaS02(C3-C7)halocarbocycle, -
NRaS02ary1,
-NRaS02heterary1, -NRaS02heteroaryl, -NRaS02heterocycle, -NRaC(0)alkyl, -
NRaC(0)alkenyl,
-NRaC(0)alkynyl, -NRaC(0) (C3-C7)carbocycle, -NRaC(0)(C3-C7)halocarbocycle,
-NRaC(0)aryl, -NRaC(0)heteroaryl, -NRaC(0)heterocycle, -NRaC(0)NRcRd and -
NRaC(0)0Rb;
each Z6 is independently selected from -NO2, -CN, -NRaRa, NRaC(0)Rb,-
C(0)NRcRi,
(C3-C7)halocarbocycle, aryl, heteroaryl, heterocycle, -Oaryl, -Oheteroaryl, -
Oheterocycle,
-0(C3-C7)halocarbocycle, -0(Ci-C6)alkyl, -0(C3-C7)carbocycle, -0(CI-
C6)haloalkyl, -Saryl,
-Sheteroaryl, -Sheterocycle, -S(C3-C7)halocarbocycle, -S(CI-C6)alkyl, -S(C3-
C7)carbocycle,
-S(CI-C6)haloalkyl, -S(0)aryl, -S(0)heteroaryl, -S(0)heterocycle, -S(0)(C3-
C7)halocarbocycle,
-S(0)(Ci-C6)alkyl, -S(0)(C3-C7)carbocycle, -S(0)(C1-C6)haloalkyl, -S02aryl, -
S02heteroaryl,
-S02heterocycle, -S02(Ci-C6)alkyl, -S02(Ci-C6)haloalkyl, -S02(C3-
C7)carbocycle, -S02(C3-
C7)halocarbocycle, -SO2NRcRa, -NRaS02(C3-C7)halocarbocycle, -NRaS02ary1,
-NRaS02heteraryl, -NRaS02heteroary1, -NRaSO2NR,Rd, -NRaS020(C3-C7)carbocycle
and
-NRaS020ary1, wherein any aryl, of Z6, either alone or as part of a group, is
otionally
substituted with one or more (e.g. 1, 2, 3, 4 or 5) halogen, -OH, -0(C i-
C6)alkyl, -CN or -(Ci-
C6)alkyl;
each Z7 is independently selected from -NO2, =NORa, -CN, -(CI-C6)alkyl-Z12, -
(C2-
C6)alkenyl-Z12, -(C2-C6)alkenylOH, -(C2-C6)alkynyl-Z12, -(C2-C6)alkynyl-OH, -
(Ci-
C6)haloalkyl-Z12, -(C -C6)haloalkylOH, -(C3-C7)carbocycle-Z12, -(C3-
C7)carbocycle0H, -(C3-
C7)halocarbocycle, -(Ci-C6)alky1NReRd, -(CI-C6)alkylNRaC(0)Ra, -(Ci-
C6)alky1NRaSO2Ra,
aryl, heteroaryl, heterocycle, -0(CI-C6)alkyl-Z12, -0(C2-C6)alkenyl, -0(C2-
C6)alkynyl, -0(C1-
C6)haloalkyl, -0(C3-C7)carbocycle, -0(C3-C7)halocarbocycle, -Oaryl, -0(C i-
C6)a1ky1NReRd, -
12
CA 02833377 2013-10-16
WO 2012/145728
PCT/US2012/034593
0(Ci-C6)alky1NRaC(0)Ra, -0(C1-C6)a1ky1NRaSO2Ra, -Oheteroaryl, -Oheterocycle, -
S(C1-
C6)alkyl-Z12, -S(C2-C6)alkenyl, -S(C2-C6)alkynyl, -S(Ci-C6)haloalkyl, -S(C3-
C7)carbocycle,
-S(C3-C7)halocarbocycle, -S(Ci-C6)alkylNReRd, -S(CI-C6)a1kylNRaC(0)Ra, -S(C1-
C6)alky1NRaS02Ra, -Saryl, -Sheteroaryl, -Sheterocycle, -S(0)(Ci-C6)alkyl, -
S(0)(C2-
C6)alkenyl, -S(0)(C2-C6)alkynyl, -S(0)(CI-C6)haloalkyl, -S(0)(C3-C7)carbocyle,
-S(0)(C3-
C7)halocarbocycle, -S02(Ci-C6)alkyl, -S(0)(Ci-C6)a1kylNR,Rd, -S(0)(Ci-
C6)alky1NRaC(0)Ra,
-S(0)(CI-C6)alkylNRaSO2Ra, -S(0)aryl, -S(0)heteroaryl, -S(0)heterocycle, -
S02(Ci-C6)alkyl,
-S02(C2-C6)alkenyl, -S02(C2-C6)alkynyl, -S02(Ci-C6)haloalkyl, -S02(C3-
C7)carbocycle,
-S02(C3-C7)halocarbocycle, -S02ary1, -S02heteroaryl, -S02heterocycle, -S02(Ci-
C6)alky1NRcRd, -S02(Ci-C6)alky1NRaC(0)Ra, -S02(Ci-C6)alky1NRaSO2Ra, -SO2NReR4,
-NRaC(0)0Rb, -NRaC(0)NR,R4, -NRaSO2Rb, -NRaSO2NRcRd, 4'4RaS020(C3-
C7)carbocycle, -
NRaS020aryl, -0S(0)2Ra, -C(0)NR,Ra, and -0C(0)NRcRd, wherein any (Ci-C6)alkyl,
(C2-
C6)alkenyl, (C2-C6)alkynyl, (C3-C7)carbocycle, (C3-C7)halocarbocycle, aryl,
heteroaryl or
heterocycle of Z7, either alone or as part of a group, is optionally
substituted with one or more
(e.g. 1, 2, 3, 4 or 5) halogen, -OH, -ORb, -CN, -NRaC(0)2Rb, heteroaryl,
heterocycle,
-Oheteroaryl, -Oheterocycle, -NHheteroaryl, -NHheterocycle or -S(0)2NRcRd.
each Z8 is independently selected from -NO2 or -CN;
each Z1 is independently selected from
i) halo, oxo, thioxo, (C2-C6)alkenyl, (Ci-C6)haloalkyl, (C3-
C7)cycloalkyl, (C3-C7)cycloalkyl-(C i-C6)alkyl-, -OH, -0(C1-
C6)alkyl, -0(C1-C6)haloalkyl, -SH, -S(C -C6)alkyl, -S0(C i-
C6)alkyl, -S02(Ci-C6)alkyl, -NH2, -NH(Ci-C6)alkyl and
-N((Ci-C6)alky1)2;
ii) (Ci-C6)alkyl optionally substituted with -OH, -0-(CI-C6)haloalkyl, or -
0-
.. (Ci-C6)alkyl; and
iii) aryl, heterocycle and heteroaryl, which aryl, heterocycle and
heteroaryl is
optionally substituted with halo, (C1-C6)alkyl or COOH;
each Z" is independently selected from Z10, -C(0)-
NH(CI-C4)alkyl,
-C(=0)-N((Ci-C4)alkyl)2, -C(=0)-heterocycle and -C(-0)-heteroaryl;
each Z12 is independently selected from -NO2, =NORa, thioxo, aryl,
heterocycle,
heteroaryl, (C3-C7)halocarbocycle, (C3-C7)carbocycle, -0(C3-C7)carbocycle, -
0(C3-
C7)halocarbocyle, -Oaryl, -Oheterocycle, -Oheteroaryl, -S(Ci-C6)alkyl, -S(C3-
C7)carbocyle,
-S(C3-C7)halocarbocyle, -Saryl, -Sheterocycle, -Sheteroaryl, -S(0)(C1-
C6)alkyl,
-S(0)(C3-C7)carbocyle, -S(0)(C3-C7)halocarbocycle, -S(0)aryl, -
S(0)heterocycle,
-S(0)heteroaryl, -S02(Ci-C6)alkyl, -S02(C3-C7)carbocycle, -S02(C3-
C7)halocarbocycle,
13
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
-S02heterocycle, -S02heteroaryl, -NRaRa, -NRaC(0)Rb, -C(0)NRcRd, -SO2NRcRd, -
NRaSO2NReltd, -NRaS020(C3-C7)carbocyle and -NRaS020ary1;
each Z13 is independently selected from -NO2, -OH, =NORa, -SH, -CN, (C3-
C7)halocarbocycle, -0(Ci-C6)alkyl, -0(C2-C6)alkenyl, -0(C2-C6)alkynyl, -0(CI-
C6)haloalkyl, -
0(C3-C7)carbocycle, -0(C3-C7)halocarbocycle, -Oaryl, -Oheteroaryl, -
Oheterocycle, -S(CI-
C6)alkyl, -S(C2-C6)alkenyl, -S(C2-C6)alkynyl, -S(C1-C6)haloalkyl, -S(C3-
C7)carbocycle, -S(C3-
C7)halocarbocycle, -Saryl, -Sheteroaryl, -Sheterocycle, -S(0)(Ci-C6)alkyl, -
S(0)(C2-C6)alkenyl,
-S(0)(C2-C6)alkynyl, -S(0)(Ci-C6)haloalkyl, -S(0)(C3-C7)carbocycle, -S(0)(C3-
C7)halocarbocycle, -S(0)aryl, -S(0)heteroaryl, -S(0)heterocycle, -S02(CI-
C6)alkyl, -S02(C2-
1 0 C6)alkenyl, -S02(C2-C6)alkynyl, -S02(Ci-C6)haloalkyl, -S02(C3-
C7)carbocycle, -S02(C3-
C7)halocarbocycle, -S02aryl, -S02heteroaryl, -S02heterocyc1e, -SO2NR,R4j, -
NRcRd,
-NRaC(0)Ra, -NRaC(0)0Rb, -NRaC(0)NR,Rd -NRaSO2Rb, -NRaSO2NR,,Rd, -NRaS020(C3-
C7)carbocycle, -NRaS020aryl, -0S(0)2Ra, -C(0)Ra, -C(0)0Rb, -C(0)NR,Rd, and -
0C(0)NR,Rd, wherein any (Ci-C6)alkyl, (C2-C6)alkenyl, (C2-C6)allcynyl, (C3-
C7)halocarbocycle, (C3-C7)carbocycle, (C3-C7)halocarbocycle, aryl, heteroaryl
or heterocycle of
Z13, either alone or as part of a group, is optionally substituted with one or
more (e.g. 1, 2, 3, 4
or 5) halogen, -OH, -ORb, -CN, -NRaC(0)2Rb, -heteroaryl, -heterocycle, -
Oheteroaryl, -
Oheterocycle, -NHheteroaryl, -NH-heterocycle, or -S(0)2NRcRd;
each Z14 is independently selected from -NO2, =NORa -CN, -(C3-
C7)halocarbocycle, -
0(C3-C7)halocarbocycle, -S(C3-C7)halocarbocycle, -S(0)(C3-C7)halocarbocycle, -
S02(C3-
C7)halocarbocycle, -NRaSO2NRcRd, -NRaS020(C3-C7)carbocycle, -NRaS0208ryl, -
0S(0)2Ra,
wherein any -(C3-C7)halocarbocycle of Z14, either alone or as part of a group,
is optionally
substituted with one or more (e.g. 1, 2, 3, 4 or 5) halogen, -OH, -ORb, -CN, -
NRaC(0)2R13, -
heteroaryl, -heterocycle, -Oheteroaryl, -Oheterocycle, -NHheteroaryl, -
NHheterocycle, or
-S(0)2NRcRa;
each Z15 is independently selected from aryl, heteroaryl, heterocycle, -Oaryl,
-Oheteroaryl, -Oheterocycle, -0(C i-C6)alkyl-aryl, -0(CI-C6)alkyl-heteroaryl, -
0(C1-
C6)alkyl-heterocycle, wherein aryl, heteroaryl and heterocycle are each
independently
substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z16 groups and optionally
subsituted with one
or more (e.g. 1, 2, 3, 4 or 5) Z1 groups, and wherein any -Oaryl, -
Oheteroaryl, -Oheterocycle,
-0(C i-C6)alkyl-aryl, -0(CI-C6)alkyl-heteroaryl or -0(Ci-C6)alkyl-heterocycle
is optionally
subsituted with one or more (e.g. 1, 2, 3, 4 or 5) Z1 groups;
each Z16 is independently selected from -NO2, -OH, =NORa, -SH, -CN, (C2-
C6)alkenyl,
(C2-C6)alkynyl, (CI-C6)haloalkyl, (C3-C7)carbocycle, (C3-C7)halocarbocycle,
aryl, heteroaryl,
heterocycle, -0(C i-C6)alkyl, -0(C2-C6)alkenyl, -0(C2-C6)alkynyl, -0(CI-
C6)haloalkyl, -0(C3-
14
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
C7)carbocycle, -0(C3-C7)halocarbocycle, -Oaryl, -Oheteroaryl, -Oheterocycle, -
S(Ci-C6)alkyl,
-S(C2-C6)alkenyl, -S(C2-C6)alkynyl, -S(Ci-C6)haloalkyl, -S(C3-C7)carbocycle, -
S(C3-
C7)halocarbocycle, -Saryl, -Sheteroaryl, -Sheterocycle, -S(0)(C1-C6)alkyl, -
S(0)(C2-C6)alkenyl,
-S(0)(C2-C6)alkynyl, -S(0)(C -C6)haloalkyl, -S(0) (C3-C7)carbocycle, -S(0)(C3-
C7)halocarbocycle, -S02(CI-C6)alkyl, -S(0)aryl, -S(0)carbocycle, -
S(0)heteroaryl,
-S(0)heterocycle, -S02(C2-C6)alkenyl, -S02(C2-C6)alkynyl, -S02(Ci-
C6)haloalkyl, -S02(C3-
C7)carbocycle, -S02(C3-C7)halocarbocycle, -S02aryl, -S02heteroaryl, -
S02heterocycle,
-SO2N&Rd, -N&Rd, -NRaC(0)Ra, -NRaC(0)0Rb, -NRaC(0)N&& -NRaSO2Rb,
-NRaSO2N&R4j, -NRaS020(C3-C7)carbocycle, -NRaS020ary1, -0S(0)2Ra, -C(0)Ra, -
C(0)0Rb,
-C(0)N&Rd, and -0C(0)N&R4, wherein any (C1-C6)alkyl, (C2-C6)alkenyl, (C2-
C6)alkynyl,
(C3-C7)halocarbocycle, (C3-C7)carboeycle, (C3-C7)halocarbocycle, aryl,
heteroaryl or
heterocycle of Z16, either alone or as part of a group, is optionally
substituted with one or more
(e.g. 1, 2, 3, 4 or 5) halogen, (Ci-C6)alkyl, -OH, -CN, -NRaC(0)2Rb,
heteroaryl,
heterocycle, -Oheteroaryl, -Oheterocycle, -NHheteroaryl, -NHheterocycle or -
S(0)2NRcRd;
each Ra is independently H, (C1-C6)alkyl, (C2-C6)alkenyl, (C2-C6)alkynyl,
(C3-C7)carbocycle, heterocycle, aryl, aryl(Ci-C6)alkyl-, heteroaryl or
heteroaryl(C1-C6)alkyl-,
wherein any (C1-C6)alkyl, (C2-C6)alkenyl, (C2-C6)alkynyl, (C3-C7)carbocycle,
heterocycle, aryl,
or heteroaryl of Ra, either alone or as part of a group, is optionally
substituted with one or more
(e.g. 1, 2, 3, 4 or 5) halogen, OH or cyano;
each Rb is independently (Ci-C6)alkyl, (C2-C6)alkenyl, (C2-C6)alkynyl,
(C3-C7)carbocycle, heterocycle, aryl, aryl(Ci-C6)alkyl-, heteroaryl or
heteroaryl(Ci-C6)alkyl-,
wherein any (Ci-C6)alkyl, (C2-C6)alkenyl, (C2-C6)alkynyl, (C3-C7)carbocycle,
heterocycle, aryl,
or heteroaryl of Rb, either alone or as part of a group, is optionally
substituted with one or more
(e.g. 1, 2, 3, 4 or 5) halogen, OH and cyano;
Re and Rd are each independently selected from H, (Ci-C6)alkyl, (C2-
C6)alkenyl,
(C2-C6)alkynyl, (C3-C7)carbocycle, aryl, aryl(C1-C6)alkyl-, heterocycle,
heteroaryl or
heteroaryl(Ci-C6)alkyl-, wherein any (C1-C6)alkyl, (C2-C6)alkenyl, (C2-
C6)alkynyl,
(C3-C7)carbocycle, heterocycle, aryl, or heteroaryl of & or Rd, either alone
or as part of a group,
is optionally substituted with one or more (e.g. 1, 2, 3, 4 or 5) halogen, OH
or cyano; or & and
Rd together with the nitrogen to which they are attached form a heterocycle,
wherein any
heterocycle of & and & together with the nitrogen to which they are attached
is optionally
substituted with one or more (e.g. 1, 2, 3, 4 or 5) halogen, OH or cyano;
each R, is independently selected from -0Ra, (C1-C6)alkyl or (C3-
C7)carbocycle, wherein
(Ci-C6)alkyl and (C3-C7)carbocycle are each independently substituted with one
or more (e.g. 1,
2, 3, 4 or 5) Z6 groups and optionally substituted with one or more (e.g. 1,
2, 3, 4 or 5) Z1
groups; (C2-C6)haloalkyl, (C2-C6)alkenyl and (C2-C6)alkynyl, wherein any (C2-
C6)haloalkyl,
(C2-C6)alkenyl or (C2-C6)alkynyl is optionally substituted with one or more
(e.g. 1, 2, 3, 4 or 5)
Zi groups; and aryl, heterocycle and heteroaryl wherein aryl, heterocycle and
heteroaryl are each
independently substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z5 groups;
each Rf is independently selected from -Rg, -0Ra, -(Ci-C6)alkyl-Z6, -S02Rg, -
C(0)Rg,
C(0)0Rg and -C(0)NReRg; and
each Rg is independently selected from (C1-C6)alkyl, (C3-C7)carbocycle (CI-
C6)haloalkyl,
(C2-C6)alkenyl, (C2-C6)alkynyl, aryl, heterocycle and heteroaryl, wherein any
(Ci-C6)alkyl,
(C3-C7)carbocycle -(CI-C6)haloalkyl, (C2-C6)alkenyl, (C2-C6)alkynyl, aryl,
heterocycle or
heteroaryl of Rg is optionally substituted with one or more (e.g. 1, 2, 3, 4
or 5) Zi groups;
or a salt thereof
The invention also provides a compound, or a pharmaceutically acceptable salt
thereof, of
formula Ia:
R4 R3 R3'
OH
R5 _____________________________ (0
R2
Ri
Ia
wherein:
RI is RI or Rib;
R5 is R5a or R5b;
R1a is:
a) halo; or
b) H;
Rib is cyano;
R2 is (Ci-C6)alkyl;
R3 is -0(Ci-C6)alkyl;
R3' is H;
R4 is:
16
CA 2833377 2018-04-17
ci 0
140
HN
CI
, .11,"/ , or
R5a is:
a) H, (Ci-C6)alkyl, (Ci-C6)haloalkyl, (C3-C7)carbocycle, single ring
heterocycle, -C(=0)-R11, -C(=0)-0-R11, -(Ci-C6)alkyl-R11, or -0-R11, wherein
each R11 is
independently H, (Ci-C6)alkyl, (CI-C6)haloalkyl, (C3-C7)carbocycle, or single
ring heterocycle,
and wherein single ring heterocycle is optionally substituted with 1 to 3 Z11
groups; or
b) -N(R9)Rio or -C(=0)-N(R9)R1 , wherein each R9 is independently H or (Ci-
C6)alkyl, and wherein each R1 is independently H, (Ci-C6)alkyl, (C3-
C7)cycloalkyl,
(C6-C2o)aryl, single ring heterocycle, -(Ci-C6)alkyl-R11, or C(=0)-R11, and
wherein each R11 is
independently H, (CI-C6)alkyl, (C3-C7)cycloalkyl, (C6-C2o)aryl, or single ring
heterocycle;
R" is:
a) -(C2-C6)alkynyl-(C3-C7)carbocycle; or
b) -NReRf;
each Z11 is Z1 ;
each Z1 is independently halo, -0(Ci-C6)alkyl, -S02(Ci-C6)alkyl, or
(Ci-C6)alkyl;
each Re is independently (Ci-C6)alkyl;
each Rf is independently -(CI-C6)alkyl-Z6;
each Z6 is independently -NRaRa or -C(0)NReRd;
each Ra is independently H or (CI-C6)alkyl; and
Re and Rd are each independently H or (Ci-C6)alkyl;
wherein each single ring heterocycle has 1 to 6 carbon atoms and 1 to 3
heteroatoms selected
from the group consisting of oxygen, nitrogen and sulfur.
The invention also provides a compound, or a pharmaceutically acceptable salt
thereof,
selected from the group consisting of:
16a
CA 2833377 2018-04-17
,
=
0 0
0 /
/
0
N O'i<
OH
\
*.< \N 0".<
N 0 S 7 OH
Oiii
0 0 0
/ S
)< 0 S 7
OH 0 N 0
N 0 N
¨ _
S OH N
0
0 FE . *
N
* F F
/ , F F , 3 9
0 0
/' .,..
µ,
e<
N
S OH
O=< I 0 S OH
0 0
N
F F NH N
Ili
F , and ¨0 .
The invention also provides a compound, or a pharmaceutically acceptable salt
thereof, of
formula Ia:
R4 R3 R3'
S OH
R5 ________________________________ (0
N R2
R1
Ia
wherein:
RI is Ria or Rib;
R5 is R5a or R51';
Rla is:
a) halo; or
b) H;
Rib is cyano;
16b
CA 2833377 2018-04-17
R2 is (CI-C6)alkyl;
R3 is -0(Ci-C6)alkyl;
R3' is H;
R4 is:
CI 0 0 CI
HN
CI CI
JNAN 3 JUNIN/ USN./ or awv ;
R5a is:
a) H, (CI -C6)alkyl, (CI-C6)haloalkyl, (C3-C7)carbocycle, (C6-C2o)aryl,
heterocycle,
heteroaryl, -C(=O)-R", -C(=0)-0-RH, -O-R" or -(CI-C6)alkyl-R11, wherein each
RH is
independently H, (C1-C6)alkyl, (CI-C6)haloalkyl, (C3-C7)carbocycle, (C6-
C2o)aryl, heterocycle
and wherein (C6-C2o)aryl, heterocycle, heteroaryl are each optionally
substituted with 1 to 3 ZH
groups; or
b) -N(R9)Rio or -C(=0)-N(R9)R1 , wherein each R9 is independently H or (CI-
C6)alkyl, and wherein each R1 is independently H, (CI-C6)alkyl, (C3-
C7)cycloalkyl,
(C6-C20)aryl, heterocycle, -(Ci-C6)alkyl-R11, or C(0)-R", and wherein each RH
is
independently H, (Ci-C6)alkyl, (C3-C7)cycloalkyl, (C6-C2o)aryl, or
heterocycle;
R5b is:
a) -(C2-C6)alkynyl-(C3-C7)carbocycle; or
b) -NReRf;
each ZH is independently halo, (CI-C6)haloalkyl, -0(Ci-C6)alkyl, -SO(CI-
C6)alkyl, (C1-
C6)alkyl, (C6-C2o)aryl, heterocycle or heteroaryl, wherein (C6-C2o)aryl,
heterocycle and
heteroaryl are each optionally substituted with halo, (C1-C6)alkyl or COOH;
each Re is independently (CI-C6)alkyl;
each Rf is independently -(Ci-C6)alkyl-Z6;
each Z6 is independently -NRaRa or -C(0)NRcRd;
each Ra is independently (CI-C6)alkyl; and
Re and Rd are each independently (CI-C6)alkyl;
16c
CA 2833377 2018-04-17
wherein each heteroaryl has 1 to 6 carbon atoms and 1 to 4 heteroatoms
selected from the
group consisting of oxygen, nitrogen and sulfur, and each heterocycle has 1 to
6 carbon atoms
and 1 to 3 heteroatoms selected from the group consisting of oxygen, nitrogen
and sulfur.
The invention also provides a compound, or a pharmaceutically acceptable salt
thereof, of
formula le:
R4 R3
OH
<0
R2
R6
Ie
wherein:
R2 is -CH3;
R3 is -0C(CH3)3;
R4 is:
0
R6 is selected from the group consisting of -CH3, -CH2C6H5, and -CH2C6H4CF3.
The invention also provides a pharmaceutical composition comprising a compound
of
formula I or a pharmaceutically acceptable salt thereof, and a
pharmaceutically acceptable
carrier.
The invention also provides a pharmaceutical composition comprising a compound
as
defined herein, or a pharmaceutically acceptable salt thereof, and a
pharmaceutically acceptable
carrier.
The invention also relates to the use of the compound as defined herein, or a
pharmaceutically acceptable salt thereof, for the prophylactic or therapeutic
treatment of an HIV
infection.
16d
CA 2833377 2018-04-17
=
The invention also relates to the use of the compound as defined herein, or a
pharmaceutically acceptable salt thereof, for the prophylactic or therapeutic
treatment of an HIV
infection in a human.
The invention also relates to the use of the compound as defined herein, or a
pharmaceutically acceptable salt thereof, for the therapeutic treatment of an
HIV infection.
The invention also relates to the use of the compound as defined herein, or a
pharmaceutically acceptable salt thereof, for the therapeutic treatment of an
HIV infection in a
human.
The invention also relates to the use of the pharmaceutical composition as
defined herein,
for the prophylactic or therapeutic treatment of an HIV infection.
The invention also relates to the use of the pharmaceutical composition as
defined herein,
for the prophylactic or therapeutic treatment of an HIV infection in a human.
The invention also relates to the use of the pharmaceutical composition as
defined herein,
for the therapeutic treatment of an HIV infection.
The invention also relates to the use of the pharmaceutical composition as
defined herein,
for the therapeutic treatment of an HIV infection in a human.
The invention also provides a method for treating (e.g. preventing, mediating
or
inhibiting) the proliferation of the HIV virus, treating AIDS or delaying the
onset of AIDS or
ARC symptoms in a mammal (e.g. a human), comprising administering a compound
of formula
I, or a pharmaceutically acceptable salt thereof, to the mammal.
The invention also provides a method of treating an HIV infection in a mammal
(e.g. a
human) comprising administering a compound of formula I, or a pharmaceutically
acceptable
salt thereof, to the mammal.
The invention also provides a method for treating an HIV infection in a mammal
(e.g. a
human) comprising administering to the mammal in need thereof a
therapeutically effective
amount of a compound of formula I, or a pharmaceutically acceptable salt
thereof, in
combination with a therapeutically effective amount of one or more additional
therapeutic agents
selected from the group consisting HIV protease inhibiting compounds, HIV non-
nucleoside
inhibitors of reverse transcriptase, HIV nucleoside inhibitors of reverse
transcriptase, HIV
nucleotide inhibitors of reverse transcriptase, HIV integrase inhibitors, gp41
inhibitors, CXCR4
16e
CA 2833377 2018-07-05
inhibitors, gp120 inhibitors, CCR5 inhibitors, capsid polymerization
inhibitors, and other drug
for treating HIV, and combinations thereof.
The invention also provides a compound of formula I, or a pharmaceutically
acceptable
salt thereof for use in medical therapy (e.g. for use in treating (e.g.
preventing, mediating or
inhibiting) the proliferation of the HIV virus or AIDS or delaying the onset
of AIDS or ARC
symptoms in a mammal (e.g. a human) _____________________________________
16f
CA 2833377 2018-04-17
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
The invention also provides a compound of formula I, or a pharmaceutically
acceptable
salt thereof for use in medical therapy (e.g. for use in treating (e.g.
preventing, mediating or
inhibiting) an HIV infection in a mammal (e.g. a human)).
The invention also provides a compound of formula I, or a pharmaceutically
acceptable
salt thereof for use in the manufacture of a medicament for treating (e.g.
preventing, mediating
or inhibiting) the proliferation of the HIV virus or AIDS or delaying the
onset of AIDS or ARC
symptoms in a mammal (e.g. a human).
The invention also provides a compound of formula I, or a pharmaceutically
acceptable
salt thereof, for use in the prophylactic or therapeutic treatment (e.g.
prevention, mediation or
inhibiting) of the proliferation of the HIV virus or AIDS or for use in the
therapeutic treatment
of delaying the onset of AIDS or ARC symptoms.
The invention also provides a compound of formula I, or a pharmaceutically
acceptable
salt thereof, for the manufacture of a medicament for treating an HIV
infection in a mammal
(e.g. a human).
The invention also provides a compound of formula I or a pharmaceutically
acceptable
salt thereof, for use in the prophylactic or therapeutic treatment of an HIV
infection in a
mammal (e.g. a human).
The invention also provides processes and intermediates disclosed herein that
are useful
for preparing compounds of formula I or salts thereof
Detailed Description of the Invention
Definitions
Unless stated otherwise, the following terms and phrases as used herein are
intended to
have the following meanings.
When trade names are used herein, applicants intend to independently include
the
tradename product and the active pharmaceutical ingredient(s) of the tradename
product.
"Alkyl" is hydrocarbon containing normal, secondary or tertiary atoms. For
example, an
alkyl group can have 1 to 20 carbon atoms (i.e, (Ci-C20)alkyl), 1 to 10 carbon
atoms (L e., (C1-
C10)alkyl), 1 to 8 carbon atoms (i.e., (Ci-C8)alkypor 1 to 6 carbon atoms
(i.e., (C1-C6 alkyl).
.. Examples of suitable alkyl groups include, but are not limited to, methyl
(Me, -CH3), ethyl (Et,
-CH2CH3), 1-propyl (n-Pr, n-propyl, -CH2CH2CH3), 2-propyl (i-Pr, i-propyl, -
CH(CH3)2), 1-
butyl (n-Bu, n-butyl, -CH2CH2CH2CH3), 2-methyl-1-propyl (i-Bu, i-butyl, -
CH2CH(CH3)2),
2-butyl (s-Bu, s-butyl, -CH(CH3)CII2CH3), 2-methyl-2-propyl (t-Bu, t-butyl, -
C(CH3)3), 1-
pentyl (n-pentyl, -CH2CH2CH2CH2CH3), 2-pentyl (-CT(CH3)CH2CH2CH3), 3-pentyl
(-CH(CH2CH3)2), 2-methyl-2-butyl (-C(CH3)2CH2C113), 3-methyl-2-butyl
17
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
(-CH(CH3)CH(CH3)2), 3-methyl-1 -butyl (-CH2CH2CH(CH3)2), 2-methyl-I -butyl
(-CH2CH(CH3)CH2CH3), 1-hexyl (-CH2CH2CH2CH2CH2CH3), 2-hexyl
(-CH(CH3)CH2C1-12CH2CH3), 3-hexyl (-CH(CH2CH3)(CH2CH2CH3)), 2-methyl-2-pentyl
(-C(CH3)2CH2CH2CH3), 3-methy1-2-pentyl (-CH(CH3)CH(CH3)CH2CH3), 4-methyl-2-
pentyl
.. (-CH(CH3)CH2CH(CH3)2), 3-methyl-3-pentyl (-C(CH3)(CH2CH3)2), 2-methyl-3-
pentyl (-
CH(CH2CH3)01(043)2), 2,3-dimethy1-2-butyl (-C(CH3)2CH(CH3)2), 3,3-dimethy1-2-
butyl (-
CH(CH3)C(CH3)3, and octyl (-(CH2)7CH3). "Alkyl" also refers to a saturated,
branched or straight
chain hydrocarbon radical having two monovalent radical centers derived by the
removal of two
hydrogen atoms from the same or two different carbon atoms of a parent alkane.
For example, an
.. alkyl group can have 1 to 10 carbon atoms (i.e., (Ci-Cio)alkyl), or Ito 6
carbon atoms (L e., (Cr
C6)alkyl) or 1-3 carbon atoms (i.e., (Ci-C3)alkyl). Typical alkyl radicals
include, but are not
limited to, methylene (-CH2-), 1,1-ethyl (-CH(CH3)-), 1,2-ethyl (-CH2CH2-),
1,1-propyl
(-CH(CH2CH3)-), 1,2-propyl (-CH2CH(CH3)-), 1,3-propyl (-CH2CH2CH2-), 1,4-butyl
(-CH2CH2CH2CH2-), and the like.
The term "halo" or "halogen" as used herein refers to fluoro, chloro, bromo
and iodo.
The term "haloalkyl" as used herein refers to an alkyl as defined herein,
wherein one or
more hydrogen atoms are each replaced by a halo substituent. For example, a
(Ci-C6)haloalkyl
is a (Ci-C6)alkyl wherein one or more of the hydrogen atoms have been replaced
by a halo
substituent. Such a range includes one halo substituent on the alkyl group t
to complete
halogenation of the alkyl group.
The term "aryl" as used herein refers to a single aromatic ring or a bicyclic
or
multicyclic ring. For example, an aryl group can have 6 to 20 carbon atoms, 6
to 14 carbon atoms,
or 6 to 12 carbon atoms. Aryl includes a phenyl radical or an ortho-fused
bicyclic or multicyclic
radical having about 9 to 14 atoms in which at least one ring is aromatic
(e.g. an aryl fused to
.. one or more aryl or carbocycle). Such bicyclic or multicyclic rings may be
optionally substituted
with one or more (e.g. 1, 2 or 3) oxo groups on any carbocycle portion of the
bicyclic or
multicyclic ring. It is to be understood that the point of attachment of a
bicyclic or multicyclic
radical, as defined above, can be at any position of the ring including an
aryl or a carbocycle
portion of the ring. Typical aryl groups include, but are not limited to,
phenyl, indenyl, naphthyl, 1,
2, 3, 4-tetrahydronaphthyl, anthracenyl, and the like.
"Arylalkyl" refers to an alkyl radical as defined herein in which one of the
hydrogen
atoms bonded to a carbon atom is replaced with an aryl radical as described
herein (i.e., an
aryl-alkyl- moiety). The alkyl group of the "arylalkyl" is typically 1 to 6
carbon atoms (i.e.
18
CA 02833377 2013-10-16
WO 2012/145728 PCMJS2012/034593
aryl(Ci-C6)alkyl). Arylalkyl groups include, but are not limited to, benzyl, 2-
phenylethan- 1 -yl,
1-phenylpropan-1-yl, naphthylmethyl, 2-naphthylethan-1-y1 and the like.
The term "heteroaryl" as used herein refers to a single aromatic ring or a
multiple
condensed ring. The term includes single aromatic rings of from about 1 to 6
carbon atoms and
about 1-4 heteroatoms selected from the group consisting of oxygen, nitrogen
and sulfur in the
rings. The sulfur and nitrogen atoms may also be present in an oxidized form
provided the ring
is aromatic. Such rings include but are not limited to pyridyl, pyrimidinyl,
oxazolyl or fury!.
The term also includes multiple condensed ring systems (e.g. ring systems
comprising 2 or 3
rings) wherein a heteroaryl group, as defined above, can be fused with one or
more heteroaryls
(e.g. naphthyridinyl), carbocycles (e.g. 5,6,7,8-tetrahydroquinoly1) or aryls
(e.g. indazoly1) to
form a multiple condensed ring. Such multiple condensed rings may be
optionally substituted
with one or more (e.g. 1, 2 or 3) oxo groups on the carbocycle portions of the
condensed ring. It
is to be understood that the point of attachment of a heteroaryl multiple
condensed ring, as
defined above, can be at any position of the ring including a heteroaryl, aryl
or a carbocycle
portion of the ring. Exemplary heteroaryls include but are not limited to
pyridyl, pyrrolyl,
pyrazinyl, pyrimidinyl, pyridazinyl, pyrazolyl, thienyl, indolyl, oxazolyl,
thiazolyl,
furyl, oxadiazolyl, thiadiazolyl, quinolyl, isoquinolyl, benzothiazolyl,
benzoxazolyl, indazolyl,
quinoxalyl, quinazolyl, 5,6,7,8-tetrahydroisoquinolinyl benzofuranyl,
benzimidazolyl and
thianaphthenyl.
The term "heterocycly1" or "heterocycle" as used herein refers to a single
saturated or
partially unsaturated ring or a multiple condensed ring. The term includes
single saturated or
partially unsaturated ring (e.g. 3, 4, 5, 6 or 7-membered ring) from about 1
to 6 carbon atoms
and from about 1 to 3 heteroatoms selected from the group consisting of
oxygen, nitrogen and
sulfur in the ring. The ring may be substituted with one or more (e.g. 1, 2 or
3) oxo groups and
the sulfur and nitrogen atoms may also be present in their oxidized forms.
Such rings include
but are not limited to azetidinyl, tetrahydrofuranyl or piperidinyl. The term
also includes
multiple condensed ring systems (e.g. ring systems comprising 2 or 3 rings)
wherein a
heterocycle group (as defined above) can be connected to two adjacent atoms
(fused
heterocycle) with one or more heterocycles (e.g. decahydronapthyridinyl ),
heteroaryls (e.g.
1,2,3,4-tetrahydronaphthyridinyl), carbocycles (e.g. decahydroquinoly1) or
aryls. It is to be
understood that the point of attachment of a heterocycle multiple condensed
ring, as defined
above, can be at any position of the ring including a heterocyle, heteroaryl,
aryl or a carbocycle
portion of the ring. Exemplary heterocycles include, but are not limited to
aziridinyl, azetidinyl,
pyrrolidinyl, piperidinyl, homopiperidinyl, morpholinyl, thiomorpholinyl,
piperazinyl,
tetrahydrofuranyl, dihydrooxazolyl, tetrahydropyranyl, tetrahydrothiopyranyl,
1,2,3,4-
19
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
tetrahydroquinolyl, benzoxazinyl, dihydrooxazolyl, chromanyl, 1,2-
dihydropyridinyl, 2,3-
dihydrobenzofuranyl, 1,3-benzodioxoly1 and 1,4-benzodioxanyl.
The term "bridged-heterocycle" as used herein refers to a 4, 5, 6, 7 or 8-
membered
heterocycle as defined herein connected at two non-adjacent atoms of the 4, 5,
6, 7 or 8-
membered heterocycle with one or more (e.g. 1 or 2) 3, 4, 5 or 6-membered
heterocycles or (C3-
C7)carbocycles as defined herein. Such bridged-heterocycles include bicyclic
and tricyclic ring
systems (e.g. 2-azabicyclo[2.2.1]heptane and 4-a zatricyclo[4.3.1.13'8]
undecane).
The term "spiro-heterocycle" as used herein refers to a 3, 4, 5, 6, 7 or 8-
membered
heterocycle as defined herein connected to one or more (e.g. 1 or 2) single
atoms of the 3, 4, 5,
6, 7 or 8-membered heterocycle with one or more (e.g. 1 or 2) 3, 4, 5, 6-
membered heterocycles
or a (C3-C7)carbocycles as defined herein. Such spiro-heterocycles include
bicyclic and tricyclic
ring systems (e.g. 1,4-dioxaspiro[4.5]dec-7-eny1).
The term "macroheterocycle" as used herein refers to a saturated or partially
unsaturated
8, 9, 10, 11 or 12-membered ring comprising about 5 to 11 carbon atoms and
about 1 to 3
heteroatoms selected from the group consisting of oxygen, nitrogen and sulfur
in the ring which
may be optionally fused at two adjacent atoms of the macroheterocycle to one
or more (e.g. 1, 2
or 3) aryls, carbocycles, heteroaryls or heterocycles. The macroheterocycle
may be substituted
with one or more (e.g. 1, 2 or 3) oxo groups and the sulfur and nitrogen atoms
may also be
present in their oxidized forms.
"Heteroarylalkyl" refers to an alkyl radical as defined herein in which one of
the
hydrogen atoms bonded to a carbon atom is replaced with a heteroaryl radical
as described
herein (i. e. , a heteroaryl-alkyl- moiety). The alkyl group of the
"heteroarylalkyl" is typically 1
to 6 carbon atoms (i.e. heteroaryl(CI-C6)alkyl). Heteroarylalkyl groups
include, but are not
limited to heteroaryl-CH2-, heteroaryl-CH(CH3)-, heteroaryl-CH2CH2-, 2-
(heteroaryl)ethan-l-yl,
and the like, wherein the "heteroaryl" portion includes any of the heteroaryl
groups described
above. One skilled in the art will also understand that the heteroaryl group
can be attached to
the alkyl portion of the heteroarylalkyl by means of a carbon-carbon bond or a
carbon-
heteroatom bond, with the proviso that the resulting group is chemically
stable. Examples of
heteroarylalkyls include by way of example and not limitation 5-membered
sulfur, oxygen,
and/or nitrogen containing heteroaryls such as thiazolylmethyl, 2-
thiazolylethan-1-yl,
imidazolylmethyl, oxazolylmethyl, thiadiazolylmethyl, etc., 6-membered sulfur,
oxygen, and/or
nitrogen containing heteroaryls such pyridinylmethyl, pyridizylmethyl,
pyrimidylmethyl,
pyrazinylmethyl, etc.
"Heterocyclylalkyl" refers to an alkyl radical as defined herein in which one
of the
hydrogen atoms bonded to a carbon atom is replaced with a heterocyclyl radical
as described
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
herein a heterocyclyl-alkyl- moiety). The alkyl group of the
"heterocyclylalkyl" is
typically 1 to 6 carbon atoms (i.e. heterocyclyl(C1-C6)alkyl). Typical
heterocyclylalkyl groups
include, but are not limited to heterocyclyl-CH2-, heterocyclyl-CH(CH3)-,
heterocyclyl-
CH2CH2-, 2-(heterocyclyl)ethan-l-yl, and the like, wherein the "heterocyclyl"
portion includes
any of the heterocyclyl groups described above. One skilled in the art will
also understand that
the heterocyclyl group can be attached to the alkyl portion of the
heterocyclyl alkyl by means of
a carbon-carbon bond or a carbon-heteroatom bond, with the proviso that the
resulting group is
chemically stable. Examples of heterocyclylalkyls include by way of example
and not limitation
5-membered sulfur, oxygen, and/or nitrogen containing heterocycles such
tetrahydrofuranylmethyl and pyrroldinylmethyl, etc., and 6-membered sulfur,
oxygen, and/or
nitrogen containing heterocycles such as piperidinylmethyl, piperazinylmethyl,
morpholinylmethyl, etc.
The term "carbocycle" or "carbocycly1" refers to a saturated (i.e.,
cycloalkyl) or partially
unsaturated (e.g., cycloalkenyl, cycloalkadienyl, etc.) ring having 3 to 7
carbon atoms as a
monocycle or a mutlicyclic ring system. In one embodiment the carbocycle is a
monocycle
comprising 3-6 ring carbons (i.e. (C3-C6)carbocycle). Carbocycle includes
multicyclic
carbocyles having 7 to 12 carbon atoms as a bicycle, and up to about 20 carbon
atoms as a
polycycle provided that the largest single ring of a multicyclic carbocycle is
7 carbon atoms.
The term "spiro-bicyclic carbocycle" refers to a carbocycle bicyclic ring
system wherein the
rings of the bicyclic ring system are connected to a single carbon atom (e.g.
spiropentane,
spiro[4,5]decane, spiro[4.5]decane, etc). The term "fused-bicyclic carbocycle"
refers to a
carbocycle bicyclic ring system wherein the rings of the bicyclic ring system
are connected to
two adjacent carbon atoms such as as a bicyclo [4,5], [5,5], [5,6] or [6,6]
system, or 9 or 10 ring
atoms arranged as a bicyclo [5,6] or [6,6] system (e.g. decahydronaphthalene,
norsabinane,
norcarane). The term "bridged-bicyclic carbocycle" refers to a carbocycle
bicyclic ring system
wherein the rings of the bicyclic ring system are connected to two non-
adjacent carbon (e.g.
norbomane, bicyclo[2.2.2]octane, etc). The "carbocycle" or "carbocycly1" may
be optionally
substituted with one or more (e.g. 1, 2 or 3) oxo groups. Non-limiting
examples of monocyclic
carbocycles include cyclopropyl, cyclobutyl, cyclopentyl, 1-cyclopent-1 -enyl,
1-cyclopent-2-
enyl, 1-cyclopent-3-enyl, cyclohexyl, 1-cyclohex-1-enyl, 1-cyclohex-2-enyl and
1-cyclohex-3-
enyl.
The term "halocarbocycle" as used herein refers to a carbocycle as defined
herein,
wherein one or more hydrogen atoms are each replaced by a halo substituent.
For example, (C3-
C7)halocarbocycle is a (C3-C7)carbocycle wherein one or more of the hydrogen
atoms have been
21
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
replaced by a halo substituent. Such a range includes one halo substituent on
the carbocycle
group to complete halogentation of the carbocycle group.
The term "macrocarbocycle" as used herein refers to a saturated or partially
unsaturated
8, 9, 10, 11 or 12-membered ring comprising 8 to 12 carbon atoms which may be
optionally
fused at two adjacent atoms of the macrocarbocycle to one or more (e.g. 1, 2
or 3) aryls,
carbocycles, heteroaryls or heterocycles. The macrocarbocycle may be
substituted with one or
more (e.g. 1, 2 or 3) oxo groups.
"Carbocyclylalkyl" refers to an alkyl radical as defined herein in which one
of the
hydrogen atoms bonded to a carbon atom is replaced with a carbocyclyl radical
as described
herein (i.e., a carbocyclyl-alkyl- moiety). The alkyl group of the
"carbocyclylalkyl" is typically
1 to 6 carbon atoms (i.e. carbocyclyl(Ci-C6)alkyl). Typical carbocyclyl alkyl
groups include,
but are not limited to carbocyclyl-CH2-, carbocyclyl-CH(CH3)-, carbocyclyl-
CH2CH2-, 2-
(carbocyclyl)ethan-l-yl, and the like, wherein the "carbocyclyl" portion
includes any of the
carbocyclyl groups described above.
It is to be understood that when a variable is substituted, for example, as
described by the
phrase "(CI-C6)alkyl, either alone or as part of a group, is optionally
substituted ", the phrase
means that the variable (C1-C6)alkyl can be substituted when it is alone and
that it can also be
substituted when the variable "(C1-C6)alkyl" is part of a larger group such as
for example an
aryl(Ci-C6)alkyl or a -( CI-C6)alkyl-S02-(C1-C6)alkyl-(C3-C7)carbocycle group.
Similarly,
when stated, other variables (e.g. (Ci-C6)alkenyl, (Ci-C6)alkynyl, aryl,
heteroaryl, heterocycle,
etc.) can also be substituted "either alone or as part of a group."
It is to be understood that certain variables of formula I that connect two
chemical
groups may be oriented in either direction. Thus, for the X group of formula I
(e.g. 0, -C(0)-,
-C(0)0-, -5-, -5 (0)-, -S02, -(Ci-C6)alky10-, -(Ci-C6)alkylC(0)-, -(C1-
C6)alkylC(0)0-, -(C1-
C6)alky1S-, -(Ci-C6)alkylS(0)- and -(Ci-C6)alky1502-) certain values of X that
are not
symmetric can be oriented in either direction. For example, the -C(0)0-, can
be oriented as
either -C(0)0- or -0C(0)-, relative to the groups it connects.
It is to be understood that the nitrogen that is included in the core of the
compound of
formula I can be present in an oxidized form. For example, the thiazole
nitrogen of either G' or
G2 of formula I can be an N-oxide. Accordingly, the invention includes a
compound of formula
I (as defined in the summary of the invention) or a salt or N-oxide thereof.
One skilled in the art will recognize that substituents and other moieties of
the compounds
of formula I should be selected in order to provide a compound which is
sufficiently stable to
provide a pharmaceutically useful compound which can be formulated into an
acceptably stable
22
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
pharmaceutical composition. Compounds of formula I which have such stability
are contemplated
as falling within the scope of the present invention.
The modifier "about" used in connection with a quantity is inclusive of the
stated value
and has the meaning dictated by the context (e.g., includes the degree of
error associated with
measurement of the particular quantity).
The term "chiral" refers to molecules which have the property of non-
superimposability
of the mirror image partner, while the term "achiral" refers to molecules
which are
superimposable on their mirror image partner.
The term "stereoisomers" refers to compounds which have identical chemical
constitution, but differ with regard to the arrangement of the atoms or groups
in space.
"Diastereomer" refers to a stereoisomer with two or more centers or axes of
chirality and
whose molecules are not mirror images of one another. Diastereomers typically
have different
physical properties, e.g., melting points, boiling points, spectral
properties, and reactivities.
Mixtures of diastereomers may separate under high resolution analytical
procedures such as
electrophoresis and chromatography.
"Enantiomers" refer to two stereoisomers of a compound which are non-
superimposable
mirror images of one another.
Certain compounds of the invention can exist as atropisomers. For example, it
has been
discovered that atropisomers exist for certain substituents at the R4 position
of formula I as
marked by an asterisk in the formula below.
R4 R3 R3'
G1 OH
R5 nrvx:
b2 R2 0
R1
The chirality that results from the atropisomers at the asterisk position is a
feature of
certain compounds of the invention. Accordingly, the invention includes all
atropisomers of
compounds of the invention including mixtures of atropisomers and well as
mixtures that are
enriched in an atropisomer as well as single atropisomers, which mixtures or
compounds possess
the useful properties described herein.
In one embodiment, the compounds of the invention of formula I are greater
than 50% a
single atropisomer for the R4 substituent at the asterisk position. In one
embodiment, the
compounds of the invention of formula I are at least 60% a single atropisomer
for the R4
substituent at the asterisk position. In another embodiment, the compounds of
the invention of
23
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
formula I are at least 70% a single atropisomer for the R4 substituent at the
asterisk position. In
another embodiment, the compounds of the invention of formula I are at least
80% a single
atropisomer for the R4 substituent at the asterisk position. In another
embodiment, the
compounds of the invention of formula I are at least 90% a single atropisomer
for the R4
.. substituent at the asterisk position. In another embodiment, the compounds
of the invention of
formula I are at least 95% a single atropisomer for the R4 substituent at the
asterisk position. In
one embodiment the stereochemistry for the R4 substituent at the carbon marked
with an asterisk
as shown above for Formula I is the (R) stereochemistry. In another embodiment
the
stereochemistry for the R4 substituent at the carbon marked with an asterisk
as shown above for
Formula I is the (S) stereochemistry.
For certain compounds of the invention the stereochemistry at the carbon
bearing the R3
substituent of formula I as marked by an asterisk in the formula below is
another aspect of the
invention.
R4 R3
OH
p1
R5 /WY:
0
b2 R2
R1
In one embodiment the stereochemistry at the carbon marked with an asterisk as
shown
above for Formula I is the (S) stereochemistry. In another embodiment the
stereochemistry at
the carbon marked with an asterisk as shown above for Formula I is the (R)
stereochemistry.
In one embodiment, the compounds of the invention of formula I are greater
than 50% a
stereoisomer for the carbon at the asterisk position. In another embodiment,
the compounds of
the invention of formula I are at least 60% a single stereoisomer for the
carbon at the asterisk
position. In another embodiment, the compounds of the invention of formula I
are at least 70%
a single stereoisomer for the carbon at the asterisk position. In another
embodiment, the
compounds of the invention of formula I are at least 80% a single stereoisomer
for the carbon at
the asterisk position. In another embodiment, the compounds of the invention
of formula I are at
least 90% a single steroisomer for the carbon at the asterisk position. In
another embodiment, the
compounds of the invention of formula I are at least 95% a single stereoisomer
for the carbon at
the asterisk position
The term "treatment" or "treating," to the extent it relates to a disease or
condition
includes preventing the disease or condition from occurring, inhibiting the
disease or condition,
24
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
eliminating the disease or condition, and/or relieving one or more symptoms of
the disease or
condition.
Stereochemical definitions and conventions used herein generally follow S. P.
Parker,
Ed., McGraw-Hill Dictionary of Chemical Terms (1984) McGraw-Hill Book Company,
New
York; and Eliel, E. and Wilen, S., Stereochemistry of Organic Compounds (1994)
John Wiley &
Sons, Inc., New York. Many organic compounds exist in optically active forms,
i.e., they have
the ability to rotate the plane of plane-polarized light. In describing an
optically active
compound, the prefixes (D and L) or (R and S) are used to denote the absolute
configuration of
the molecule about its chiral center(s). The prefixes d and 1 or (+) and (-)
are employed to
designate the sign of rotation of plane-polarized light by the compound, with
(-) or 1 meaning
that the compound is levorotatory. A compound prefixed with (+) or d is
dextrorotatory. For a
given chemical structure, these stereoisomers are identical except that they
are mirror images of
one another. A specific stereoisomer may also be referred to as an enantiomer,
and a mixture of
such isomers is often called an enantiomeric mixture. A 50:50 mixture of
enantiomers is
referred to as a racemic mixture or a racemate, which may occur where there
has been no
stereoselection or stereospecificity in a chemical reaction or process. The
terms "racemic
mixture" and "racemate" refer to an equimolar mixture of two enantiomeric
species, devoid of
optical activity.
Protecting Groups
In the context of the present invention, protecting groups include prodrug
moieties and
chemical protecting groups.
"Protecting group" refers to a moiety of a compound that masks or alters the
properties
of a functional group or the properties of the compound as a whole. Chemical
protecting groups
and strategies for protection/deprotection are well known in the art. See
e.g., Protective Groups
in Organic Chemistry, Theodora W. Greene, John Wiley & Sons, Inc., New York,
1991.
Protecting groups are often utilized to mask the reactivity of certain
functional groups, to assist
in the efficiency of desired chemical reactions, e.g., making and breaking
chemical bonds in an
ordered and planned fashion. Protection of functional groups of a compound
alters other
physical properties besides the reactivity of the protected functional group,
such as the polarity,
lipophilicity (hydrophobicity), and other properties which can be measured by
common
analytical tools. Chemically protected intermediates may themselves be
biologically active or
inactive.
Protected compounds may also exhibit altered, and in some cases, optimized
properties
in vitro and in vivo, such as passage through cellular membranes and
resistance to enzymatic
=
degradation or sequestration. In this role, protected compounds with intended
therapeutic effects
may be referred to as prodrugs. Another function of a protecting group is to
convert the parental
drug into a prodrug, whereby the parental drug is released upon conversion of
the prodrug in
vivo. Because active prodrugs may be absorbed more effectively than the
parental drug,
prodrugs may possess greater potency in vivo than the parental drug.
Protecting groups are
removed either in vitro, in the instance of chemical intermediates, or in
vivo, in the case of
prodrugs. With chemical intermediates, it is not particularly important that
the resulting products
after deprotection, e.g., alcohols, be physiologically acceptable, although in
general it is more
desirable if the products are pharmacologically innocuous.
Protecting groups are available, commonly known and used, and are optionally
used to
prevent side reactions with the protected group during synthetic procedures,
i.e. routes or
methods to prepare the compounds of the invention. For the most part the
decision as to which
groups to protect, when to do so, and the nature of the chemical protecting
group "PG" will be
dependent upon the chemistry of the reaction to be protected against (e.g.,
acidic, basic,
oxidative, reductive or other conditions) and the intended direction of the
synthesis. PGs do not
need to be, and generally are not, the same if the compound is substituted
with multiple PG. In
general, PG will be used to protect functional groups such as carboxyl,
hydroxyl, thio, or amino
groups and to thus prevent side reactions or to otherwise facilitate the
synthetic efficiency. The
order of deprotection to yield free deprotected groups is dependent upon the
intended direction of
the synthesis and the reaction conditions to be encountered, and may occur in
any order as
determined by the artisan.
Various functional groups of the compounds of the invention may be protected.
For
example, protecting groups for ¨OH groups (whether hydroxyl, carboxylic acid,
phosphonic
acid, or other functions) include "ether- or ester-forming groups". Ether- or
ester-forming
groups are capable of functioning as chemical protecting groups in the
synthetic schemes set
forth herein. However, some hydroxyl and thio protecting groups are neither
ether- nor ester-
forming groups, as will be understood by those skilled in the art, and are
included with amides,
discussed below.
A very large number of hydroxyl protecting groups and amide-forming groups and
corresponding chemical cleavage reactions are described in Protective Groups
in Organic
Synthesis, Theodora W. Greene (John Wiley & Sons, Inc., New York, 1991, ISBN 0-
471-62301-6)
26
CA 2833377 2018-04-17
("Greene"). See also Kocienski, Philip J.; Protecting Groups (Georg Thieme
Verlag Stuttgart,
New York, 1994). In particular Chapter 1, Protecting Groups: An Overview,
pages 1-20,
Chapter 2, Hydroxyl Protecting Groups, pages 21-94, Chapter 3, Diol Protecting
Groups, pages
95-117, Chapter 4, ___________________________________________________
26a
CA 2833377 2018-04-17
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
Carboxyl Protecting Groups, pages 118-154, Chapter 5, Carbonyl Protecting
Groups, pages 155-
184. For protecting groups for carboxylic acid, phosphonic acid, phosphonate,
sulfonic acid and
other protecting groups for acids see Greene as set forth below.
Stereoisomers
The compounds of the invention may have chiral centers, e.g., chiral carbon or
phosphorus atoms. The compounds of the invention thus include racemic mixtures
of all
stereoisomers, including enantiomers, diastereomers, and atropisomers. In
addition, the
compounds of the invention include enriched or resolved optical isomers at any
or all
asymmetric, chiral atoms. In other words, the chiral centers apparent from the
depictions are
provided as the chiral isomers or racemic mixtures. Both racemic and
diastereomeric mixtures,
as well as the individual optical isomers isolated or synthesized,
substantially free of their
enantiomeric or diastereomeric partners, are all within the scope of the
invention. The racemic
mixtures can be separated into their individual, substantially optically pure
isomers through
well-known techniques such as, for example, the separation of diastereomeric
salts formed with
optically active adjuncts, e.g., acids or bases followed by conversion back to
the optically active
substances. In most instances, the desired optical isomer is synthesized by
means of
stereospecific reactions, beginning with the appropriate stereoisomer of the
desired starting
material.
The compounds of the invention can also exist as tautomeric isomers in certain
cases.
Although only one delocalized resonance structure may be depicted, all such
forms are
contemplated within the scope of the invention. For example, ene-amine
tautomers can exist for
purine, pyrimidine, imidazole, guanidine, amidine, and tetrazole systems and
all their possible
tautomeric forms are within the scope of the invention.
Salts and Hydrates
Examples of pharmaceutically acceptable salts of the compounds of the
invention
include salts derived from an appropriate base, such as an alkali metal (for
example, sodium), an
alkaline earth metal (for example, magnesium), ammonium and NX4+ (wherein X is
C i¨C4
alkyl). Pharmaceutically acceptable salts of a hydrogen atom or an amino group
include for
example salts of organic carboxylic acids such as acetic, benzoic, lactic,
fumaric, tartaric,
maleic, malonic, malic, isethionic, lactobionic and succinic acids; organic
sulfonic acids, such as
methanesulfonic, ethanesulfonic, benzenesulfonic and p-toluenesulfonic acids;
and inorganic
acids, such as hydrochloric, hydrobromic, sulfuric, phosphoric and sulfamic
acids.
Pharmaceutically acceptable salts of a compound of a hydroxy group include the
anion of said
27
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
compound in combination with a suitable cation such as Na + and NX4+ (wherein
X is
independently selected from H or a C1¨C4 alkyl group).
For therapeutic use, salts of active ingredients of the compounds of the
invention will
typically be pharmaceutically acceptable, i.e. they will be salts derived from
a physiologically
acceptable acid or base. However, salts of acids or bases which are not
pharmaceutically
acceptable may also find use, for example, in the preparation or purification
of a compound of
formula I or another compound of the invention. All salts, whether or not
derived from a
physiologically acceptable acid or base, are within the scope of the present
invention.
Metal salts typically are prepared by reacting the metal hydroxide with a
compound of
this invention. Examples of metal salts which are prepared in this way are
salts containing Li+,
Na+, and K+. A less soluble metal salt can be precipitated from the solution
of a more soluble
salt by addition of the suitable metal compound.
In addition, salts may be formed from acid addition of certain organic and
inorganic
acids, e.g., HCl, HBr, 112SO4, 113PO4 or organic sulfonic acids, to basic
centers, typically
amines, or to acidic groups. Finally, it is to be understood that the
compositions herein comprise
compounds of the invention in their un-ionized, as well as zwitterionic form,
and combinations
with stoichiometric amounts of water as in hydrates.
Also included within the scope of this invention are the salts of the parental
compounds
with one or more amino acids. Any of the natural or unnatural amino acids are
suitable,
especially the naturally-occurring amino acids found as protein components,
although the amino
acid typically is one bearing a side chain with a basic or acidic group, e.g.,
lysine, arginine or
glutamic acid, or a neutral group such as glycine, serine, threonine, alanine,
isoleucine, or
leucine.
Specific values listed below for radicals, substituents, and ranges in the
embodiments of
the invention are for illustration only; they do not exclude other defined
values or other values
within defined ranges for the radicals and substituents.
Isotopes
It is understood by one skilled in the art that this invention also includes
any compound
claimed that may be enriched at any or all atoms above naturally occurring
isotopic ratios with
one or more isotopes such as, but not limited to, deuterium (2H or D). As a
non-limiting
example, a -CH3 group may be substituted with -CD3.
Compounds of formula I.
A specific group of compounds of formula 1 are compounds of formula Ia:
28
CA 02833377 2013-10-16
WO 2012/145728
PCT/US2012/034593
R4 R3 R3'
OH
R5 ___________________________ (
R2 0
R1
la
or a salt thereof.
Another specific group of compounds of formula I are compounds of formula Ib:
R4 R3 R3'
OH
R5 ___________________________ <0
R2
W
Ib
or a salt thereof.
Another specific group of compounds of formula I are compounds of formula Ic:
R4 R3
SLL011
R5 ___________________________ (0
R2
Ic
wherein R3 is-0(C i-C6)alkyl or a salt thereof.
Another specific group of compounds of formula I are compounds of formula Ic':
R4 R3
OH
R5 ___________________________ (
R2 0
Ic'
wherein R3 is-0(C i-C6)allcyl or a salt thereof.
29
CA 02833377 2013-10-16
WO 2012/145728
PCT/US2012/034593
Another specific group of compounds of formula I are compounds of formula Id:
R4 R3
OH
R5 ___________________________ <
R20
Id
wherein R3 is-0(C i-C6)allcyl, or a salt thereof
Another specific group of compounds of formula I are compounds of formula Id':
R4 R3
OH
R5 ___________________________ <0
R2
Id'
wherein R3 is-0(C i-C6)alkyl, or a salt thereof.
Another specific group of compounds of formula I are compounds of formula le:
R4 R3
OH
0
0
R2
R6
Ie
or a salt thereof.
Another specific group of compounds of formula I are compounds of formula le':
R4 R3
7
0 ____________________________ <
00 H
R2
R6
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
le'
or a salt thereof.
Another specific group of compounds of formula I are compounds of formula If:
R4 R3
OH
0
0
R2
R6
If
wherein R3 is-0(C i-C6)alkyl, or a salt thereof.
Another specific group of compounds of formula I are compounds of formula If:
R4 R3
OH
0 ____________________________
0
R2
R6
If
wherein R3 is-O(CI-C6)alkyl, or a salt thereof.
Another specific group of compounds of formula I are compounds of formula Ig:
R4 R3 R3'
OH
R5 Arvi
0
R2
R1
Ig
wherein:
G2 is N, the dashed bond connected to G2 is a double bond, and the wavy bond
connected
to R5 is a single bond; or
G2 is NR6, the dashed bond connected to G2 is a single bond, the wavy bond
connected to
R5 is a double bond and R5 is oxygen (e.g."(wavy bond)-R5" is "=0").
Another specific group of compounds of formula I are compounds of formula Ig':
31
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
R4 R3
OH
R5 ftrv/s
1
2 11101 0
G R2
R1
Ig'
wherein:
G2 is N, the dashed bond connected to G2 is a double bond, and the wavy bond
connected
to R5 is a single bond; or
G2 is NR6, the dashed bond connected to G2 is a single bond, the wavy bond
connected to
R5 is a double bond and R5 is oxygen (e.g."(wavy bond)-R5" is "=0").
Another specific group of compounds of formula I are compounds of formula Ih:
R4 R3
OH
R5 rtrd
\G2 R2
Ih
wherein:
G2 is N, the dashed bond connected to G2 is a double bond, and the wavy bond
connected
to R5 is a single bond; or
G2 is NR6, the dashed bond connected to G2 is a single bond, the wavy bond
connected to
R5 is a double bond and R5 is oxygen (e.g."(wavy bond)-R5" is "=0").
Another specific group of compounds of formula I are compounds of formula Ih':
R4 R3
OH
R5 AAP1/4
b2
R2
Ih'
wherein:
32
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
G2 is N, the dashed bond connected to G2 is a double bond, and the wavy bond
connected
to R5 is a single bond; or
G2 is NR6, the dashed bond connected to G2 is a single bond, the wavy bond
connected to
R5 is a double bond and R5 is oxygen (e.g."(wavy bond)-R5" is "=0").
Another specific group of compounds of formula I are compounds of formula Ii:
R4 R3 Rs
G1 OH
%G2 R2 0
R1
Ii
wherein:
GI is S; 02 is N; the dashed bond connected to GI is a single bond and the
dashed bond
connected to G2 is a double bond; or
GI is N; G2 is S; the dished bond connected to GI is a double bond and the
dashed bond
connected to G2 is a single bond;
or a salt thereof.
Another specific group of compounds of formula I are compounds of formula Ij:
R4 R3
G1 zOH
R5 Ann:
0
`G2
R2
R1
Ii
or a salt thereof.
Specific embodiments of the invention (e.g. embodiments) and specific values
listed
below are embodiments and values for compounds of formula I including all of
the compounds
of sub-fomulas of formula I (e.g. the compounds of formulas Ia, Ib, Ic, Ic',
Id, Id', le, le,' If, If',
Ig, Ig', Ih, Ih' and Ia100-1a145)
A specific group of compounds of formula I are compounds wherein at least one
of RI,
, R3b, R3b.,
R2, R3, R3', R4 or R5 is selected from R",, R2b R41' or R5b.
33
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
Another specific group of compounds of formula I are compounds wherein at
least two
3
2b, Rb, R3b, RI, R2, R3, R3', R4 or R5 is selected from WI', R R41' or R5b.
Another specific group of compounds of formula I are compounds wherein at
least three
of RI, R2, R3, R3', R4 or R5 is selected from R1', R2b R3b, R4b or R5b.
Another specific group of compounds of formula I are compounds wherein at
least four
of RI, R2, R3, R3', R4 or K-5
is selected from RI", R2b, R31', R3b, R4b or R5b.
Another specific group of compounds of formula I are compounds wherein at
least five
of RI, R2, R3, R3', R4 or R5 is selected from R11', R2b, R3b, R31,', R4bor
R5b.
Another specific group of compounds of formula I are compounds wherein at RI,
R2, R3,
.. R3', R4 and R5 are Rib, R21', R3b, R31', R4b and R5b.
A specific value for RI is H.
Another specific value for RI is H or halo.
Another specific value for RI is H or F.
A specific value for R3' is H.
A specific value for R3 is R3b.
A specific value for R3b is -0C(CH3)2CH2OH, -0C(C113)2CH20H,
-0(Ci-C6)alkyl-O-C(0)-NH2, -0(C 1-C6)alkyl-O-C(0)-N(CH3)2or
-0(Ci-C6)alkyl-O-C(0)-NH(pheny1).
Another specific value for R31' is -(C1-C6)a1kylOH or -0(CI-C6)alkyl-O-C(0)-
NReRd.
3 i 3a
Another specific value for R s R .
A specific value for R3a is (Ci-C6)alkyl, (C2-C6)alkenyl or -0(CI-C6)alkyl
wherein any
(Ci-C6)alkyl or (C2-C6)alkenyl of R3a is optionally substituted with one or
more groups selected
from -0(CI-C6)alkyl, halo, oxo and -CN.
Another specific value for R3a is -0C(CH3).
A specific value for R3' is R3b.
A specific value for R3I" is (Ci-C6)alkyl or -0(Ci-C6)alkyl.
A specific value for R3' is R3a'.
A specific value for R3ais H.
A specific value for R3 is (Ci-C6)alkyl, (C2-C6)alkenyl or -0(C1-C6)alkyl,
wherein any
(Ci-C6)alkyl or (C2-C6)alkenyl of R3a is optionally substituted with one or
more groups selected
from -0(Ci-C6)alkyl, halo, oxo and -CN.
A specific value for R3 is -0C(CH3)3.
A specific group of compounds of fomula I are compounds wherein the compounds
of
formula I are compounds of formula Ih:
34
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
R4 R3
OH
R6 fvl-ri
R20
Ih
wherein:
G2 is N, the dashed bond connected to G2 is a double bond, and the wavy bond
connected
to R5 is a single bond; or
G2 is NR6, the dashed bond connected to G2 is a single bond, the wavy bond
connected to
R5 is a double bond and R5 is oxygen.
A specific group of compounds of fomula I are compounds wherein R3b and R313
together
with the carbon to which they are attached form a (C3-C7)carbocycle or
heterocycle; wherein the
(C3-C7)carbocycle or heterocycle is optionally substituted with one or more Z1
groups.
Another specific group of compounds of fomula I are compounds wherein R3b and
R3b'
together with the carbon to which they are attached form a (C3-C7)carbocycle
or a 4, 5 or 6-
membered heterocycle; wherein the (C3-C6)carbocycle or the 4, 5 or 6-membered
heterocycle is
optionally substituted with one or more Z1 groups.
Another specific group of compounds of fomula I are compounds wherein R3b and
R3b'
together with the carbon to which they are attached form a (C4-C6)carbocycle
or a 5 or 6-
membered heterocycle; wherein the (C4-C6)carbocycle or the 5 or 6-membered
heterocycle is
optionally substituted with one or more Z1 groups.
Another specific group of compounds of fomula I are compounds wherein R3b and
R31"
together with the carbon to which they are attached form a 5 or 6-membered
heterocycle;
wherein the 5 or 6-membered heterocycle is optionally substituted with one or
more Z1 groups.
Another specific group of compounds of fomula I are compounds wherein R3b and
R31'
together with the carbon to which they are attached form a tetrahydropyran or
tetrahydrofuran
optionally substituted with one or more Z1 groups.
Another specific group of compounds of fomula I are compounds wherein R3b and
R3b'
together with the carbon to which they are attached form:
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
or
x0
\ -PrPi 611-t.
each of which is optionally substituted with one or more Z1 groups; and
wherein "*"
denotes the point of attachment to the carbon of the compound of formula I.
A specific value for R4 is R4b.
A specific value for R41' is (Ci-C6)alkyl, (C2-C6)alkenyl or (C2-C6)alkynyl;
wherein
(Ci-C6)alkyl, (C2-C6)alkenyl or (C2-C6)alkynyl are each optionally substituted
with one or more
Z1 groups.
Another specific value for R4b is:
7
0 optionally substituted with one or more Z1 groups.
Another specific value for R4b is (C3-C7)carbocycle; wherein (C3-C7)carbocycle
is
optionally substituted with one or more Z1 groups; or wherein two Z1 groups
together with the
atom or atoms to which they are attached optionally form a (C3-C6)carbocycle
or 5-6-membered
heterocycle.
Another specific value for R4b is:
1110 F-1
0 0 CO
0 01 0 111
Or
,
'NW
..rt/V=I
=IVW
each of which is optionally substituted with one or more Z1 groups.
Another specific value for R41' is aryl, heterocycle or heteroaryl; wherein
aryl,
heterocycle and heteroaryl are each independently substituted with one or more
Z7 groups and
optionally substituted with one or more Z1 groups.
Another specific value for R4b is:
36
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
OCF3 r..... 0
0 OCF3
0 OCF3 0 N. F
NH2
, 0 ,
NOV
JVVV
00F3
1
0 0 1 0F3
8 s 0õ p
;-s--
N H2N
''' , 1161 H2N 0
, S
0'1,1,
H 1/4., ¨ '
JVVV 0
'
0
H
0 Ny 0 NH2 0
NH2
1 aVvv
JVVV
0 0 õ 0
0, ,c,
0 \S ;NH2 F 0 0
OCF3 0 N---k.
m
1101 H la H
F
F
, ,
'
...VW JVVV
F 0
CF3 or F Y yN * F
NC
0 s 0 H2N 0 NH2
0 H
0' .
' Irsx
L., ¨ ' 0 ¨ 0
Another specific value for R4 is lea.
A specific value for R4a is:
CI
0 0 CI
s, s , 40
J-VVV JVVV
CI
0 0
(LrrL õ, le
< F
N,r Ny.- , N
H '
,
JVVV
JVVV
...VVV
CF3 F
F
..-,'
-. -,
cc, -,,
JVVV
J-VIIV
,ONAI JW,
JVLIll
37
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
N
0
'
0
1,101 14111
Another specific value for R4a is:
C,
[1101 (110 0 ci
JVVV JVVV ' CI
JVVV
JVVV JVVV
Or
~A/
Another specific value for R4a is:
CI
40 0
Nuns,
4VVV
A specific value for R4 is selected from:
a) aryl, heterocycle and heteroaryl, wherein any aryl, heterocycle and
heteroaryl of R4 is
optionally substituted with one or more (e.g. 1, 2, 3, 4 or 5) groups each
independently selected from
halo, (Ci-C6)alkyl, (C2-C6)alkenyl, (Ci-C6)haloalkyl, (C3-C7)cycloalkyl,
-(C -C6)alkyl-(C3-C7)cycloalkyl, -OH, -0(C -C6)alkyl, -SIT, -S(C -C6)alkyl, -
NH2, -NH(C -C6)alkyl
and -N((CI-C6)a1ky1)2, wherein (Ci-C6)allcyl is optionally substituted with
hydroxy, -0(C1-
C6)alkyl, cyano or oxo; and
b) aryl, heteroaryl, spiro-, fused-, or bridged-heterocycle; wherein aryl,
heteroaryl, or
spiro-, fused-, or bridged-heterocycle are each independently substituted with
one or more Z7
groups and optionally substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z1
groups.
Another specific value for R4 is selected from:
38
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
a) aryl, heterocycle and heteroaryl, wherein any aryl, heterocycle and
heteroaryl of R4 is
optionally substituted with one or more (e.g. 1, 2, 3, 4 or 5) groups each
independently selected from
halo, (Ci-C6)alkyl, (C2-C6)alkenyl, (Ci-C6)haloalkyl, (C3-C7)cycloalkyl,
-(Ci-C6)alkyl-(C3-C7)cycloalkyl, -OH, -0(CI-C6)allcyl, -SH, -S(CI-C6)alkyl, -
NH2, -NH(Ci-C6)allcyl
and -N((CI-C6)alky1)2, wherein (Ci-C6)alkyl is optionally substituted with
hydroxy, -0(C1-
C6)alkyl, cyano or oxo; and
b) aryl and heteroaryl, wherein aryl and heteroaryl are each independently
substituted
with one or more Z7 groups and optionally substituted with one or more (e.g.
1, 2, 3, 4 or 5) Zi
groups.
Another specific value for R4 is selected from aryl, heterocycle and
heteroaryl, wherein
any aryl, heterocycle and heteroaryl of R4 is optionally substituted with one
or more (e.g. 1,2, 3,4 or
5) groups each independently selected from halo, (Ci-C6)alkyl, (C2-C6)alkenyl,
(Ci-C6)hgoallcyl,
(C3-C7)cycloallcyl, -(CI-C6)alkyl-(C3-00cycloalkyl, -OH, -0(Ci-C6)alkyl, -SH, -
S(CI-C6)alicyl, -
NH2, -NH(Ci-C6)alkyl and -N((Ci-C6)alky1)2, wherein (Ci-C6)alkyl is optionally
substituted with
hydroxy, -0(Ci-C6)alkyl, cyano or oxo.
Another specific value for R4 is:
CI
1:101
Or HN
CI
JVW ~UN VW V JNIVV
Another specific value for R4 is:
CI 0
H N 401
CI
0
0
or
JF
A specific group of compounds of formula I are compounds wherein R4 and R3
together
with the atoms to which they are attached form a macroheterocycle or a
macrocarbocycle
.. wherein any macroheterocycle or macrocarbo cycle of R4 and R3 together with
the atoms to
39
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
which they are attached may be optionally substituted with one or more Z1
groups; and R3' is H,
(Ci-C6)allcyl or -0(Ci-C6)alkyl.
Another specific group of compounds of formula I are compounds wherein R4 and
R3
together with the atoms to which they are attached form a macroheterocycle or
a
macrocarbocycle wherein any macroheterocycle or macrocarbocycle of R4 and R3
together with
the atoms to which they are attached may be optionally substituted with one or
more Z1 groups;
and R3' is H.
Another specific group of compounds of formula I are compounds wherein R4 and
R3
together with the atoms to which they are attached form the macroheterocycle
or a
macrocarbocycle which is further fused to a Z group;
wi--,(CH2)0
VV2
c5s
wherein:
Z is aryl, heteroaryl or (C3-C6)carbocycle;
n3 is 2,3 or 4;
W1 and W2 are each independently 0, NH or CH2; and
wherein "*" denotes the R4 point of attachment of the macroheterocycle or
macrocarbocycle to the compound of formula I and "**" denotes the R3 point of
attachment of
the macroheterocycle or macrocarbocycle to the compound of formula I; and
wherein the
macroheterocycle or a macrocarbocycle is optionally substituted with one or
more Z1 groups.
Another specific group of compounds of formula I are compounds wherein, R4 and
R3
together with the atoms to which they are attached form the macroheterocycle:
1101 (CH2)n1 * ,(cH2)n2
o w (C H 2 )n3
0
* or
0- **
(72. c55 cs5
,rvv
wherein:
n1 is 3 or 4; n2 is 2, 3 or 4; n3 is 2, 3 or 4; W is 0, NH or N(CI-C4)alkyl;
and wherein
"*" denotes the R4 point of attachment of the macroheterocycle to the compound
of formula I
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
and "**" denotes the R3 point of attachment of the macroheterocycle to the
compound of formula
I; and wherein the macroheterocycle or a macrocarbocycle is optionally
substituted with one or
more Z1 groups
A specific value for R2 is R2b.
Another specific value R2 is R2a.
A specific value for R2a is H, halo or -CH3.
Another specific value for R2a is Cl.
A specific value for R2 is halo, H or (Ci-C6)alkyl.
Another specific value for R2 is halo, H or -CH3.
Another specific value for R2 is H or -CH3.
Another specific value for R2 is H or (Ci-C6)alkyl.
Another specific value for R2 is (Ci-C6)alkyl.
Another specific value for R2 is -CH3.
Another specific value for R5 is R5a.
Another specific value for R5a is H, (Ci-C6)alkyl, (C3-C7)carbocycle, -(Ci-
C6)alkyl-R11,
_N(R9)Rio, _C(=0)-N(R9)R1 , heterocycle or heteroaryl, wherein heteroaryl is
optionally substituted with one or more Zi 1 groups.
Another specific value for R5a is H, (Ci-C6)alkyl, (C3-C7)carbocycle, -C(=0)-
R", -
N(R9)R' ,
C(=0)-N(R9)R1 or heterocycle.
Another specific value for R5a is H, (Ci-C6)alkyl, (C3-C7)cycloalkyl or -(CI-
C6)alkyl-R11.
A specific value for R11 is aryl.
Another specific value for R11 is carbocycle or aryl.
Another specific value for R" is carbocycle.
Another specific value for R5' is -N(R9)R' .
A specific value for R9 is H or (C1-C6)alkyl.
A specific value for R1 is H or (Ci-C6)alkyl.
Another specific value for R9 is H, (Ci-C6)alkyl or -C(=0)-R11.
Another specific value for R1 is H, (Ci-C6)alkyl or -C(=0)-R".
A value for Z9 is "each Z9 is independently selected from -(Ci-C6)alkyl, -0(CI-
C6)alkyl".
A specific value for R5a is:
41
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
1 \
04-1
) 1 H-1
' '
0
,
-i, -N
410 \
4 '
N\ ,
0
/
,
0
Or H
H2N
Another specific value for R5a is:
( 1 \
1>-1 N-1
,
\ ,
H \--i , H-
0
0
1 CN-1
or
H2N,
=
A specific value for R5 is R5b.
Another specific value for R51' is-(C2-C6)alkynyl-(C3-C7)carbocycle.
Another specific value for R51' is:
> ____ 1
A specific value for R5 is H, (Ci-C6)alkyl, (C3-C7)carbocycle, -C(=0)-R11, -
N(R9)R' , _
C(=0)-N(R9)R1 , heterocycle or -(C2-C6)alkynyl-(C3-C7)carbocycle.
A specific value for R5 is:
42
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
11-1 H --C. > __ 1 \
1N-1
,
0
H2N, ______________________________________________ 1 Of > __ = i
,
,
Another specific value for R5 is:
o
\ \
, ____________ 1 NA N1 N¨
HO I
, ,
, C , (1 ,
\ , _.....\
)
F/\____ 0-01¨ 1 F¨N---/ /N¨
, N
----IN¨i
, F- / ,
0µ ,\ s \ s \
0 __ 14-1
CNA \S¨c19¨
/
¨N
'
\
0 \N-1
¨isl--/ . /0-1
, 0=1
\
F>¨I H
N-
0 ,
0 0\\
,---1 r--i
¨NH HN NH
o i ' or
. .
¨so
Another specific value for R5 is:
H1 ¨1, CI > __ 1 \
N-1
, / ,
,
0
,
O
H2N __________________________________________________ ,
43
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
0 \ \ \
HC?\ N1 N-1 N1
, ( ' cNi ,
' ---- ,
\
) /N- , CN-1 , FF>CN- 10-CN-1 F-CNA
'
0, \ s \
--1 N-i
0 N \
N-
-rsjj , . ,
,
\
_ 0-1 ,
e i
,
-N
\
OH, 0-1 , >-1 F
F>-1 H
N-
,
0 0
HNH H
-NH NH
oH or
' 41 =
A specific value for R5 is selected from:
a) R11, -C(=0)-Rn, -C(=0)-0-R11, -O-R", -S-R11, -S(0)-R11, -S02-R11,
-(C1-C6)alkyl-R11, -(Ci-C6)alkyl-C(=0)-R11, -(Ci-C6)alkyl-C(=0)-0-R11, -(Ci-
C6)alkyl-O-R11, -
(C1-C6)alkyl-S-R11, -(C1-C6)alkyl-S(0)-R" and -(Ci-C6)alkyl-S02-R11, wherein
each R11 is
independently selected from H, (C1-C6)alkyl, (C2-C6)alkenyl, (C2-C6)alkynyl,
(Ci-C6)haloalkyl,
(C3-C7)carbocycle, aryl, heterocycle and heteroaryl, wherein aryl, heterocycle
and heteroaryl are
each optionally substituted with one or more (e.g. 1, 2 or 3) Zn groups;
b) -N(R9)R10, -C(=0)-N(R9)R10, -0-C(=0)-N(R9)R1 , -S02-N(R9)Rio,
-(Ci-C6)alkyl-
N(R9)Rio, -(Ci-C6)alkyl-C(=0)-N(R9)Rio, _(k.,-1_
C6)alkyl-O-C(=0)-N(R9)R1 , and -(Ci-C6)alkyl-
S02-N(R9)R1 ; wherein each R9 is independently selected from H, (Ci-C6)alkyl
and (C3-
C7)cycloalkyl; and each R1 is independently selected from Rn, -(Ci-C6)alkyl-
R11, -S02-R11, -
C(=0)--K11, _ C(=0)0R11 and -C(=0)N(R9)R11, wherein each R11 is independently
selected from
H, (Ci-C6)alkyl, (C2-C6)alkenyl, (C2-C6)alkynyl, (C1-C6)haloalkyl, (C3-
C7)cycloalkyl, aryl,
heterocycle and heteroaryl;
44
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
-(Ci-C6)alky1-0-(CI-C6)allcyl-(C3-C7)carbocycle,
-(Ci-C6)alkyl-S-(Ci-C6)alkyl-(C3-C7)carbocycle,
-(C1-C6)alkylS(0)-(Ci-C6)alkyl-(C3-C6)carbocycle,
-(C1-C6)alky1S02(Ci-C6)alkyl-(C3-C7)carbocycle, -(C2-C6)alkenyl-(Ci-
C6)haloalkyl, -(C2-
C6)alkynyl-(CF-C6)haloalkyl, - (C3-C7)halocarbocycle, -NRaSO2N&Rd, -NRaS020(C3-
C7)carbocycle, -NRaS020aryl, -(C2-C6)alkenyl-(C3-C7)carbocycle, -(C2-
C6)alkenyl-aryl,
-(C2-C6)alkenyl-heteroaryl, -(C2-C6)alkenyl-heterocycle, -(C2-C6)alkynyl-(C3-
C7)carbocycle,
-(C2-C6)alkynyl-aryl, -(C2-C6)alkynyl-heteroaryl, -(C2-C6)alkynyl-heterocycle,
-(C3-
C7)carbocycle-Z1 or -(Ci-C6)haloalkyl-Z3, wherein any (C1-C6)alkyl, (Ci-
C6)haloalkyl,
(C3-C7)carbocycle, (C2-C6)alkenyl, (C2-C6)alkynyl, aryl or heteroaryl, either
alone or as part of a
group, is optionally substituted with one or more(e.g. 1, 2, 3, 4 or 5) Z1
groups;
d) -NReRf, -C(0)NR,Rf, -0C(0)NReRf, -S02NReRf, -(Cl-C6)alkyl-NRAf,
-(CI -C6)alkylC(0)-NReRf, -(Cl-C6)alkyl-O-C(0)-NReRf and -(CI-C6)alkyl-
SO2NReRf, wherein
each (Ci-C6)alkyl is independently substituted with one or more (e.g. 1,2, 3,4
or 5) Z6 groups
and optionally substituted with one or more (e.g. 1,2, 3,4 or 5) Zlgroups; and
e) oxo.
Another specific value for R5 is selected from:
-
K C(=0)-R", -C(=0)-0-R" and -0-R11; wherein each It" is
independently
selected from H, (C1-C6)alkyl, (C2-C6)alkenyl, (C2-C6)alkynyl, (Ci-
C6)haloalkyl, (C3-
C7)carbocycle, aryl, heterocycle and heteroaryl, wherein any aryl, heterocycle
or heteroaryl is
each optionally substituted with one or more (e.g. 1, 2 or 3) Z" groups;
b) -N(R9)Rio and -C(=0)-N(R9)R19; wherein each R9 is independently selected
from
H, (Ci-C6)alkyl and (C3-C7)cycloalkyl; and each R1 is independently selected
from R11, -(C1-
C6)alkyl-R", -S02-R", -C(=0)-R11, -C(=0)0R11 and -C(=0)N(R9)R11, wherein each
R" is
independently selected from H, (CI-C6)alkyl, (C2-C6)alkenyl, (C2-C6)alkynyl,
(CI-C6)haloalkyl,
(C3-C7)cycloalkyl, aryl, heterocycle and heteroaryl;
c) -(C2-C6)alkynyl-(C3-C7)carbocycle, wherein -(C2-C6)alkynyl-(C3-
C7)carbocycle
is optionally substituted with one or more(e.g. 1, 2, 3, 4 or 5) Z1 groups;
d) -NReRf and-C(0)NR,Rf; and
e) oxo.
Another specific value for R5 is selected from:
a) Ril, -C(=0)-R11, -C(=0)-0-R" and -0-R11; wherein each R" is
independently
selected from H, (Ci-C6)alkyl, (C2-C6)alkenyl, (C2-C6)alkynyl, (Ci-
C6)haloalkyl, (C3-
C7)carbocycle, aryl, heterocycle and heteroaryl, wherein aryl, heterocycle and
heteroaryl are
each optionally substituted with one or more (e.g. 1,2 or 3) ZI I groups;
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
b) -N(R9)Rio and -C(=0)-N(R9)R1 ; wherein each R9 is independently selected
from
H, (Ci-C6)alkyl and (C3-C7)cycloalkyl; and each R1 is independently selected
from R11, -(C1-
C6)alky1-12.11, -S02-R11, -C(=0)-R", -C(---0)0R11 and -C(=0)N(R9)R11, wherein
each R" is
independently selected from H, (C1-C6)alkyl, (C2-C6)alkenyl, (C2-C6)alkynyl,
(C1-C6)haloalkyl,
(C3-C7)cycloalkyl, aryl, heterocycle and heteroaryl;
c) -(C2-C6)allcynyl-(C3-C7)carbocycle, wherein -(C2-C6)alkynyl-(C3-
C7)carbocycle
is optionally substituted with one or more(e.g. 1,2, 3,4 or 5) Z1 groups; and
d) -NRAf and-C(0)NReRf.
Another specific value for R5 is selected from:
a) H, (Ci-C6)alkyl, (Ci-C6)haloalkyl, (C3-C7)carbocycle, heterocycle, -
C(=0)-R", -
C(=0)-0-1e1 and -0-R11, wherein heterocycle is optionally substituted with one
or more (e.g. 1,
2 or 3) Z" groups and wherein each R" is independently selected from H, (Ci-
C6)alkyl, (C2-
C6)alkenyl, (C2-C6)alkynyl, (CI-C6)haloalkyl, (C3-C7)carbocycle, aryl,
heterocycle and
heteroaryl, wherein aryl, heterocycle and heteroaryl are each optionally
substituted with one or
more (e.g. 1, 2 or 3) Z11 groups;
b) -N(R9)R1 and -C(=0)-N(R9)R1 ; wherein each R9 is independently selected
from
H, (C1-C6)alkyl and (C3-C7)cycloalkyl; and each R1 is independently selected
from R", -(Ci-
C6)alkyl-R", -S02-R11, -C(=0)-R", -C(=0)0R11 and -C(=0)N(R9)R11, wherein each
R" is
independently selected from H, (C1-C6)alkyl, (C2-C6)alkenyl, (C2-C6)alkynyl,
(C1-C6)haloalkyl,
(C3-C7)cycloalkyl, aryl, heterocycle and heteroaryl;
c) -(C2-C6)alkynyl-(C3-C7)carbocycle, wherein -(C2-C6)alkynyl-(C3-
C7)carbocycle
is optionally substituted with one or more(e.g. 1, 2, 3, 4 or 5) Z1 groups;
and
d) -NR,Rf and-C(0)NReRf.
Another specific value for R5 is selected from:
, > \ 5
/
0
> __________________________________________________________________ -
= H2N
46
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
0 \ s \ \
F110 N1 ( N-1 N-1
, C' cN-1
/0-CNA F-CN-i
-----/ -N 1
C
,..-0 N- /¨NA
7 b , ) / / 40,
, -ID
\
, * 1
,
-N
\
0-1, 0-1 >-1 F
N-
,
0 ,
0 0,
HNH H
-NH NH
0--1 or
-0 .
A specifc group of compounds of formula I are compounds wherein R5 is oxo and
R6 is
selected from R" and -(C1-C6)alkyl-R", wherein each R" is independently
selected from H,
(Ci-C6)alkyl, (C2-C6)alkenyl, (C2-C6)alkynyl, (Ci-C6)haloalkyl, (C3-
C7)cycloalkyl, aryl,
heterocycle and heteroaryl, wherein aryl, heterocycle and heteroaryl are each
optionally
substituted with one or more (e.g. 1, 2 or 3) Z" groups.
Another specifc group of compounds of formula I are compounds wherein R5 is
oxo and
R6 is selected from:
I
F F
. F
F
F F
F
FF
A specific group of compounds of formula I are compounds wherein R41' is
selected
from;
47
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
a) (Ci-C6)alkyl, (C2-C6)alkenyl and (C2-C6)alkynyl, wherein any (Ci-
C6)alkyl,
(C2-C6)alkenyl or (C2-C6)alkynyl is optionally substituted with one or more
(e.g. 1, 2, 3, 4 or 5)
Z1 groups;
b) (C3-Ci4)carbocycle, wherein (C3-C14)carbocycle is optionally substituted
with
one or more (e.g. 1, 2, 3, 4 or 5) Z1 groups;
c) Spiro-heterocycle or bridged-heterocycle, wherein spiro-heterocycle or
bridged-heteroocycle is optionally substituted with one or more (e.g. 1,2, 3,4
or 5) Z1 groups;
and
d) aryl, heterocycle and heteroaryl, wherein aryl, heterocycle and
heteroaryl are
each independently substituted with one or more Z7 groups and optionally
substituted with one
or more (e.g. 1, 2, 3, 4 or 5) Z1 groups.
Another specific group of compounds of formula I are compounds wherein km is
selected from;
a) (Ci-C6)alkyl, (C2-C6)alkenyl and (C2-C6)alkynyl, wherein any (C1-
C6)alkyl,
(C2-C6)alkenyl or (C2-C6)allcynyl is optionally substituted with one or more
(e.g. 1, 2, 3, 4 or 5)
Z1 groups;
b) (C3-Cm)carbocycle, wherein (C3-C14)carbocycle is optionally substituted
with
one or more (e.g. 1,2, 3, 4 or 5) Z1 groups; wherein two Z1 groups together
with the atom or
atoms to which they are attached optionally form a (C3-C7)carbocycle or
heterocycle; and
c) aryl, heterocycle and heteroaryl, wherein aryl, heterocycle and
heteroaryl are
each independently substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z7
groups and optionally
substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z1 groups.
Another specific group of compounds of formula I are compounds wherein km is
selected from;
a) (Ci-C6)alkyl, (C2-C6)alkenyl and (C2-C6)alkynyl, wherein any (Ci-
C6)alkyl,
(C2-C6)alkenyl or (C2-C6)alkynyl is optionally substituted with one or more
(e.g. 1,2, 3,4 or 5)
Z1 groups;
b) (C3-C14)carbocycle, wherein (C3-C14)carbocycle is optionally
substituted with
one or more (e.g. 1,2, 3, 4 or 5) Z1 groups; and
c) aryl, heterocycle and heteroaryl, wherein aryl, heterocycle and
heteroaryl are
each independently substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z7
groups and optionally
substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z1 groups.
In another embodiment, the invention provides a compound of the invention
which is a
compound of formula I:
48
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
R4 R3 R3'
,P1 OH
R5-<\
0
`G2
R2
R1
wherein:
Gi is S; G2 is N; the dashed bond connected to G1 is a single bond and the
dashed bond
connected to G2 is a double bond; or
Gi is N; G2 is S; the dashed bond connected to G1 is a double bond and the
dashed bond
connected to G2 is a single bond;
Ri is Ria or Rib;
R2 is R2a or R21';
R3 is R3a or R3b;
3' is '
R s R3 or R3t) ;
R4 is R4a or R4b;
R5 is R5a or R5b;
Rla is selected from:
a) halo;
b) R", -C(=0)-R11, -C(=0)-0-R", -0-R", -S-R11, -S(0)-R11, -S02-R11,
-(C -C6)alkyl-R11, -(C -C6)alkyl-C(=0)-R11, -(C -C6)alkyl-C(=0)-0-R11, -(C 1 -
C6)alkyl-O-R11, -
(C1-C6)alkyl-S-R11, -(CI-C6)alkyl-S(0)-R" and -(C1-C6)alkyl-S02-R"; wherein
each Ril is
independently selected from H, (Ci-C6)alkyl, (C2-C6)alkenyl, (C2-C6)alkynyl,
(Ci-C6)haloalkyl,
(C3-C7)cycloalkyl, aryl, heterocycle and heteroaryl, wherein aryl, heterocycle
or heteroaryl are
each optionally substituted with one or more (e.g. 1, 2 or 3) Z11 groups; and
c) -N(R9)R1 , -C(=0)-N(R9)R10, -0-C(=0)-N(R9)R1 , -S02-N(R9)R10
,
-(CI-C6)alkyl-N(R9)R10, -(Ci-C6)alkyl-C(=0)-N(R9)R1 , -(Ci-C6)alkyl-O-C(=0)-
N(R9)R1 and -
(Ci-C6)alkyl-S02-N(R9)Rm; wherein each R9 is independently selected from H,
(C1-C6)allcyl
and (C3-C7)cycloalkyl; and each R1 is independently selected from R11, -
S02-R11, -C(=0)-R", -C(=0)0R11 and -C(=0)N(R9)R", wherein each RH is
independently
selected from H, (Ci-C6)alkyl, (C2-C6)alkenyl, (C2-C6)alkynyl, (Ci-
C6)haloalkyl, (C3-
C7)cycloalkyl, aryl, heterocycle and heteroaryl;
Rth is selected from:
a) -(Ci-C6)allcy1-0-(Ci-C6)alkyl-(C3-C7)carbocycle, -(C1-C6)alkyl-
S-(Ci-
C6)alkyl-(C3-C7) carbocycle, -(Ci-C6)alkyl-S(0)-(Ci-C6)alkyl-(C3-C6)
carbocycle, -(Ci-C6)alkyl-
49
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
S02-(Cl-C6)allcyl-(C3-C7)carbocycle, -(CI-C6)alkyl-S02-(CI-C6)alkyl-Z13, -C(0)-
(C i-C6)alkyl-
Z13, -0-(Ci-C6)alkyl-Z13, -S-(CI-C6)alkyl-Z13, -S(0)-(Ci-C6)alkyl-Z13, -S02-
(Ci-C6)alkyl-Z13,
-(C1-C6)alkyl-Z14, -(C1-C6)alkyl-C(0)-(Ci-C6)alkyl-Z13, -(Ci-C6)alkyl-C(0)-
0(Ci-C6)alkyl-Z13,
-(C1-C6)alky1-0-(Ci-C6)alkyl-Z13, -(Ci-C6)alkyl-S-(Ci-C6)alkyl-Z13, -(C2-
.. C6)alkenyl-(Ci-C6)haloalkyl, -(C2-C6)alkynyl-(Ci-C6)haloalkyl, - (C3-
C7)halocarbocycle,-
NRaSO2NR,124, -NRaS020(C3-C7)carbocycle, -NRaS020aryl, -(C2-C6)alkenyl-(C3-
C7)carbocycle, -(C2-C6)alkenyl-aryl, -(C2-C6)alkenyl-heteroaryl, -(C2-
C6)alkenyl-heterocycle,
-(C2-C6)alkynyl-(C3-C7)carbocycle, -(C2-C6)alkYnYl-aryl, -(C2-C6)allcynyl-
heteroaryl
-(C2-C6)alkynyl-heterocycle, -(C3-C7)carbocycle-Z1 or -halo(Ci-C6)alkyl-Z3;
wherein
(Ci-C6)alkyl, (Ci-C6)haloalkyl, (C3-C7)carbocycle, (C2-C6)alkenyl, (C2-
C6)alkynyl, aryl or
heteroaryl are each optionally substituted with one or more (e.g. 1, 2, 3, 4
or 5) Z1 groups;
b) spiro-bicyclic carbocycle, fused-bicyclic carbocycle and bridged-
bicyclic
carbocycle; wherein spiro-bicyclic carbocycle, fused-bicyclic carbocycle or
bridged-bicyclic
carbocycle are optionally substituted with one or more (e.g. 1, 2, 3, 4 or 5)
Z1 groups; wherein
two Z1 groups together with the atom or atoms to which they are attached
optionally form a
carbocycle or heterocycle wherein the carbocycle or heterocycle is optionally
substituted with
one or more (e.g. 1, 2, 3, 4 or 5) Z1 groups;
c) (Ci-C6)alkyl; wherein (Ci-C6)alkyl is substituted with one or more Z2
groups and
optionally substituted with one or more (e.g. 1, 2, 3, 4 or 5) Zlgroups;
d) -X(Ci-C6)alkyl, -X(Ci-C6)haloalkyl, -X(C2-C6)alkenyl, -X(C2-C6)allcynyl
and
-X(C3-C7)carbocycle; wherein (Ci-C6)alkyl and (Ci-C6)haloalkyl are each
substituted with one
or more Z3 groups and optionally substituted with one or more Zigroups; and
wherein
(C2-C6)alkenyl, (C2-C6)alkynyl and (C3-C7)carbocycle are each substituted with
one or more
(e.g. 1, 2, 3, 4 or 5) Z4groups and optionally substituted with one or more
Zlgroups;
e) aryl, heteroaryl, heterocycle, -Xaryl, -Xheteroaryl and-Xheterocycle;
wherein
aryl heteroaryl and heterocycle are each substituted with one or more (e.g. 1,
2, 3, 4 or 5) Z5
groups and optionally substituted with one or more Zigroups;
f) (Ci-C6)haloalkyl, (C3-C7)carbocycle, (C2-C6)alkenyl, and (C2-C6)alkynyl;
wherein (Ci-C6)haloalkyl, (C3-C7)carbocycle, (C2-C6)alkenyl and (C2-
C6)allcynyl are each
substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z6 groups and optionally
substituted with one
or more Zigroups;
g) -NiZeRf, -C(0)NReltf, -0C(0)NR,Rf, -SO2NReRf, -(Ci-C6)a1kyl-NReRf,
-(C1-C6)alkylC(0)-NR,Rf, -(CI-C6)a1kyl-O-C(0)-NReRf and -(C1-C6)alkyl-
SO2NReRf; wherein
each (C1-C6)alkyl is substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z6
groups and optionally
substituted with one or more Zlgroups; and
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
h) nitro and cyano
R2a is selected from:
a) halo;
b) R", C(=0)-R11, -C(=0)-0-R", -0-R11, -S-R", -S(0)-R", -S02-R11,
-(C -C6)alkyl-R" , -(C i-C6)alkyl-C(=0)-R1 1, -(C -C6)alkyl-g=0)-0-R11, -(Ci-
C6)alkyl-O-R11, -
(Ci-C6)alkyl-S-R11, -(Ci-C6)alkyl-S(0)-R" and -(CI-C6)alkyl-S02-R11; wherein
each R11 is
independently selected from H, (CI-C6)alkyl, (C2-C6)alkenyl, (C2-C6)alkynyl,
(Ci-C6)haloalkyl,
(C3-C7)cycloalkyl, aryl and heterocycle and heteroaryl, wherein aryl,
heterocycle or heteroaryl
are each optionally substituted with one or more (e.g. 1, 2 or 3) Z11 groups;
and
c) -N(R9)R1 , -C(=0)-N(R9)R1 , -0-C(=0)-N(R9)R1 , -S02-N(R9)R1 , -(C1-
C6)alkyl-
N(R9)R1 , -(Ci-C6)alkyl-C(=0)-N(R9)R1 , -(Ci-C6)alky1-0-C(=0)-N(R9)R1 , and -
(CI-C6)alkyl-
S02-N(R9)R10, wherein each R9 is independently selected from H, (Ci-C6)alkyl
and (C3-
C7)cycloalkyl;
each Rm is independently selected from R", -(Ci-C6)alkyl-R", -S02-R11, -C(=0)-
R11, -
C(=0)0R11 and -C(=0)N(R9)R11; wherein each R" is independently selected from
H, (C1-
C6)alkyl, (C2-C6)alkenyl, (C2-C6)alkynyl, (C1-C6)haloallcyl, (C3-
C7)cycloalkyl, aryl, heterocycle
and heteroaryl;
R21' is selected from:
a) -(C 1-C6)allcyl-0-(C -C6)alkyl-(C3-C7)carbocycle, -(C1 -C6)alkYl-S-(C 1-
C6)alkyl-(C3-C7)carbocycle, -(C1-C6)alkyl-S(0)-(C1-C6)alkyl-(C3-C7)carbocycle,
-(C1-
C6)alkyl-S02-(Ci-C6)alkyl-(C3-C7)carbocycle, -(C2-C6)alkenyl-(C1-C6)haloalkyl,
-(C2-
C6)alkynyl-(C1-C6)haloalkyl, -(Ci-C6)alkyl-S02-(Ci-C6)alkyl-Z13, -C(0)-(CF-
C6)alkyl-Z13, -0-
(CI-C6)alky1-Z13, -S-(Ci-C6)alkyl-Z13, -S(0)-(Ci-C6)alkyl-Z13, -S02-(CI-
C6)allcyl-Z13,
-(C1-C6)alkyl-Z14, -(C1-C6)alkyl-C(0)-(Ci-C6)alkyl-Z13, -(Ci-C6)alkyl-C(0)-
0(Ci-C6)alkyl-Z13,
-(C1-C6)alky1-0-(Ci-C6)alkyl-Z13, -(Ci-C6)alicyl-S-(C1-C6)alkyl-Z13, -(C3-
C7)halocarbocycle,-
NRaSO2NR,R4i, -NRaS020(C3-C7)carbocycle, -NRaS020ary1,
-(C2-C6)alkenyl-(C3-C7)carbocycle, -(C2-C6)alkenyl-aryl, -(C2-C6)alkenyl-
heteroaryl,
-(C2-C6)alkenyl-heterocycle, -(C2-C6)alkynyl-(C3-C7)carbocycle, -(C2-
C6)alkynyl-aryl,
-(C2-C6)alkynyl-heteroaryl, -(C2-C6)alkynyl-heterocycle, -(C3-C7)carbocycle-Z1
or -halo(Ci-
C6)alkyl-Z3; wherein (Ci-C6)alkyl, -(C1-C6)haloalkyl, (C3-C7)carbocycle, (C2-
C6)alkenyl, (C2-
C6)alkynyl, aryl or heteroaryl are each optionally substituted with one or
more (e.g. 1, 2, 3, 4 or
5) Z' groups;
b) spiro-bicyclic carbocycle, fused-bicyclic carbocycle and bridged-
bicyclic
carbocycle; wherein spiro-bicyclic carbocycle, fused-bicyclic carbocycle or
bridged-bicyclic
carbocycle are optionally substituted with one or more (e.g. 1, 2, 3, 4 or 5)
Z1 groups; wherein
51
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
two Z1 groups together with the atom or atoms to which they are attached
optionally form a
(C3-C7)carbocycle or heterocycle wherein the (C3-C6)carbocycle or heterocycle
is optionally
substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z1 groups;
c) (Ci-C6)alkyl; wherein (CI-C6)alkyl is substituted with one or more Z2
groups and
optionally substituted with one or more (e.g. 1, 2, 3, 4 or 5) Zigroups;
d) -X(Ci-C6)alkyl, X(C1-C6)haloalkyl, X(C2-C6)alkenyl, -X(C2-C6)alkynyl and
-X(C3-C7)carbocycle; wherein (C1-C6)alkyl and (Ci-C6)haloalkyl are each
substituted with one
or more Z3 groups and optionally substituted with one or more (e.g. 1,2, 3, 4
or 5) Zlgroups; and
wherein (C2-C6)alkenyl, (C2-C6)alkynyl and (C3-C7)carbocycle are each
substituted with one or
more (e.g. 1, 2, 3,4 or 5) egroups and optionally substituted with one or more
Zigroups;
e) aryl, heteroaryl, heterocycle, -Xaryl, Aheteroaryl and -Xheterocycle;
wherein
aryl heteroaryl and heterocycle are each substituted with one or more (e.g. 1,
2, 3, 4 or 5) Z5
groups and optionally substituted with one or more (e.g. 1, 2, 3,4 or 5)
Zlgroups;
(Ci-C6)haloalkyl, (C3-C7)carbocycle, (C2-C6)alkenyl, and (C2-C6)alkynyl;
wherein (Ci-C6)haloalkyl, (C3-C7)carbocycle, (C2-C6)alkenyl and (C2-C6)alkynyl
are each
substituted with one or more (e.g. 1, 2, 3,4 or 5) Z6 groups and optionally
substituted with one or
more (e.g. 1, 2, 3, 4 or 5) Zigroups;
g) -NReRf, -C(0)NRAf, -0C(0)NReRf, -SO2NReltf, -(CI-C6)a1kyl-NReRf,
-(CI-C6)alkylC(0)-NReRf, -(Cl-C6)alkyl-O-C(0)-NR,Rf and -(Cl-C6)alkyl-
S02NReRf; wherein
each (Ci-C6)alkyl is substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z6
groups and optionally
substituted with one or more (e.g. 1,2, 3, 4 or 5) Zlgroups; and
h) nitro and cyano;
R3a is (C1-C6)alkyl, (Ci-C6)haloalkyl, (C2-C6)alkenyl, (C2-C6)alkynyl,
-(C1-C6)alkyl-(C3-C7)cycloalkyl, -(Ci-C6)alkyl-aryl, -(Ci-C6)alkyl-
heterocycle, -(C1-C6)alkyl-
heteroaryl, -0(Ci-C6)alkyl, -0(CI-C6)haloalkyl, -0(C2-C6)alkenyl, -0(C2-
C6)alkynyl,
-0(C3-C7)cycloalkyl, -Oaryl, -0(CI-C6)alkyl-(C3-C7)cycloalkyl, -0(Ci-C6)alkyl-
aryl,
-0(C1-C6)alkyl-heterocycle and -0(Ci-C6)alkyl-heteroaryl; wherein any (CF-
C6)alkyl,
(C1-C6)haloalkyl, (C2-C6)alkenyl, (C2-C6)alkynyl, -(Ci-C6)alkyl-(C3-
C7)cycloalkyl,
-(Ci-C6)alkyl-aryl, -(CI-C6)alkyl-heterocycle, -(Ci-C6)alkyl-heteroaryl, -0(Ci-
C6)alkyl,
-0(CI-C6)haloalkyl, -0(C2-C6)alkenyl, -0(C2-C6)alkynyl, -0(C3-C7)cycloalkyl, -
Oaryl,
-0(CI-C6)alkyl-(C3-C7)cycloalkyl, -0(Ci-C6)alkyl-aryl, -0(CI-C6)alkyl-
heterocycle or
-0(C1-C6)alkyl-heteroaryl of R3a is optionally substituted with one or more
(e.g. 1, 2 or 3)
groups selected from (C1-C6)alkyl, -0(C1-C6)alkyl, halo, oxo and -CN; and
R3a'
52
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
R3b is -(C3-C7)carbocycle, aryl, heteroaryl, heterocycle, -(C1-C6)alkylOH, -
(CI-C6)alky1-
0-(Ci-C6)alkyl-Z12, -(Ci-C6)alky1-0-(C2-C6)alkenyl-Z12, -(C2-C6)alky1-0-(C2-
C6)alkynyl-Z12, -
(C1-C6)alkyl-S-(Ci-C6)alkyl-Z12, -(CI-C6)alkyl-S-(C2-C6)alkenyl-Z12, -(C2-
C6)alkyl-S-(C2-
C6)allcynyl-Z12, -(Ci-C6)alkyl-S(0)-(CI-C6)alkyl-Z12, -(Ci-C6)alkyl-S(0)-(C2-
C6)alkenyl-Z12, -
(C2-C6)alkyl-S(0)-(C2-C6)alkynyl-Z12, -(C1-C6)alkyl-S02-(C1-C6)alkyl-Z12, -(Ci-
C6)alkyl-S02-
(C2-C6)alkenyl-Z12, -(C2-C6)alkyl-S02-(C2-C6)alicynyl-Z12, -(C2-C6)alky1-
NRaRb,
-(C2-C6)a1kylOC(0)-NR,Rd, -(C2-C6)alkyl-NRa-C(0)-ORb, -(C2-C6)alkyl-NRa-C(0)-
NRaRb, -
(CI-C6)alkyl-S02(C1-C6)alkyl, -(CF-C6)alkyl-SO2NRcRd, -(Cl-C6)alkyl-
NRaSO2NRAth
-(C1-C6)alkyl-NRaS020(C3-C7)carbocycle, -(CI-C6)alkyl-NRaS020aryl,
-(Ci-C6)alkyl-NRa-S02-(Ci-C6)alkyl, -(CI-C6)alkyl-NRa-S02-halo(Ci-C6)alkyl,
-(Ci-C6)alkyl-NRa-S02-(C2-C6)alkenyl, -(Ci-C6)alkyl-NRa-S02-(C2-C6)alkynyl,
-(C1-C6)alkyl-NRa-S02-(C3-C7)carbocycle, -(Ci-C6)alkyl-NRa-S02-halo(C3-
C7)carbocycle,
-(CI-C6)alkyl-NRa-S02-aryl, -(CI-C6)alkyl-NRa-S02-heteroaryl,
-(C1-C6)alkyl-NRa-S02-heterocycle, -0(Ci-C6)alkyl-NRaRb, -0(Ci-C6)alkylOC(0)-
NRcitd, -
0(Ci-C6)alkyl-NRa-C(0)-ORb, -0(Ci-C6)alkyl-NRa-C(0)-NRaRb, -0(C1-C6)alkyl-NRa-
S02-(Ci-
C6)alkyl, -0(C i-C6)alkyl-NRa-S02-halo(Ci-C6)alkyl, -0(CI-C6)alkyl-NRa-S02-(C2-
C6)alkenyl,
-0(Ci-C6)alkyl-NRa-S02-(C2-C6)alkynyl, -0(C i-C6)alkyl-NRa-S02-(C3-
C7)carbocycle,
-0(Ci-C6)alkyl-NRa-S02-halo(C3-C7)carbocycle, -0(Ci-C6)alkyl-NRa-S02-aryl,
-0(Ci-C6)alkyl-NRa-S02-heteroaryl, -0(Ci-C6)alkyl-NRa-S02-heterocycle,
-0(Ci-C6)alkyl-NRa-S02-NRaRb, -0(Ci-C6)alkyl-NRa-S02-(C3-C7)carbocycle,
-0(CI-C6)alkyl-NRa-S02-halo(C3-C7)carbocycle, -0(C i-C6)alkyl-NRa-S02-aryl, -
0(C1-C6)alkyl-
NRaSO2NR,R4, -0(Ci-C6)alkyl-NRaS020(C3-C7)carbocycle, -0(Ci-C6)alkyl-
NRaS020aryl,
-Oheteroaryl, -Oheterocycle, -Sheteroaryl, -Sheterocycle, -S(0)heteroaryl, -
S(0)heterocycle, -
SO2heteroaryl or -S02heterocyc1e; wherein any (Ci-C6)alkyl, aryl, (C3-
C7)carbocycle, heteroaryl
or heterocycle of R3b is optionally substituted with one or more (e.g. 1, 2,
3, 4 or 5) Z1 groups;
and
R31'' is H, (C1-C6)alkyl or -0(Ci-C6)alkyl; or
R3b and R3b' together with the carbon to which they are attached form a
heterocycle or
(C3-C7)carbocycle which heterocycle or (C3-C7)carbocycle of R3b and R3b'
together with the
carbon to which they are attached is optionally substituted with one or more
(e.g. 1, 2, 3, 4 or 5)
Z1 groups;
R4a is selected from aryl, heterocycle and heteroaryl, wherein any aryl,
heterocycle and
heteroaryl of R4a is optionally substituted with one or more (e.g. 1, 2, 3, 4
or 5) groups each
independently selected from halo, (Ci-C6)alkyl, (C2-C6)alkenyl, (Ci-
C6)haloalkyl, (C3-C7)cycloalkyl,
-(Ci-C6)alkyl-(C3-C7)cycloalkyl, -OH, -0(C1-C6)alkyl, -SH, -S(CI-C6)alkyl, -
NH2, -NH(C1-C6)alkyl
53
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
and -NRCI-C6)allcy1)2; wherein (C1-C6)alkyl is optionally substituted with
hydroxy, -0(C1-
C6)alkyl, cyano or oxo;
R4b is selected from;
a) (Ci-C6)alkyl, (C2-C6)alkenyl and (C2-C6)alkynyl; wherein (Ci-C6)alkyl,
(C2-C6)alkenyl or (C2-C6)alkynyl are each optionally substituted with one or
more (e.g. 1, 2, 3, 4
or 5) Z1 groups;
b) (C3-C14)carbocycle; wherein (C3-Ci4)carbocycle is optionally substituted
with
one or more (e.g. 1,2, 3,4 or 5) Z1 groups; wherein two Z1 groups together
with the atom or
atoms to which they are attached optionally form a (C3-C7)carbocycle or
heterocycle;
c) Spiro-heterocycle or bridged-heterocycle; wherein spiro-heterocycle or
bridged-heterocycle is optionally substituted with one or more (e.g. 1, 2, 3,
4 or 5) Z1 groups; or
wherein two Z1 groups together with the atom or atoms to which they are
attached optionally
form a (C3-C7)carbocycle or heterocycle; and
d) aryl, heteroaryl, Spiro-, fused-, or bridged-heterocycle; wherein aryl,
heteroaryl,
or spiro-, fused-, or bridged-heterocycle are each independently substituted
with one or more Z7
groups and optionally substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z1
groups; or
R4 and R3 together with the atoms to which they are attached form a
macroheterocycle or
a macrocarbocycle wherein any macroheterocycle or macrocarbocycle of R4 and R3
together
with the atoms to which they are attached may be optionally substituted with
one or more (e.g.
1, 2, 3, 4 or 5) Z1 groups; and R3b' is H or (C1-C6)allcyl, -0(Ci-C6)alkyl.
R5a is selected from:
a) halo;
_c(=0)-Ri _c(=0)-0-Ri _o_Ri _S-- 11, _
b) R", S(0)-R", -S02-R11, -(C1-
C6)alkyl-R11,
C6)alkyl-C(=0)-R11, -(Ci-C6)alkyl-C(=0)-0-R11, _(ci-
-(Ci-C6)alkyl
C6)alkyl-S-R", -(CI-C6)alkyl-S(0)-R11 and -(Ci-C6)alkyl-S02-R11; wherein each
R11 is
independently selected from H, (Ci-C6)alkyl, (C2-C6)alkenyl, (C2-C6)allcynyl,
(Ci-C6)haloalkyl,
(C3-C7)carbocycle, aryl, heterocycle and heteroaryl, wherein aryl, heterocycle
and heteroaryl are
each optionally substituted with one or more (e.g. 1, 2 or 3) Z" groups; and
c) -N(R9)R10, -C(=0)-N(R9)R10, -0-C(=0)-N(R9)R1 , -S02-N(R9)R1 , -(Ci-
C6)alkyl-
N(R9)R1 , -(C1-C6)alkyl-C(=0)-N(R9)R1 , -(C1-C6)alkyl-0-C(=0)-N(R9)R10, and -
(C1-C6)alkyl-
S02-N(R9)R1 ; wherein each R9 is independently selected from H, (Ci-C6)alkyl
and (C3-
C7)cycloalkyl; and each R1 is independently selected from RH, -(CI-C6)alkyl-
R11, -S02-R11, -
C(=0)-R11, -q=0)0R11 and -C(=0)N(R9)R11; wherein each R" is independently
selected from
H, (C1-C6)alkyl, (C2-C6)alkenyl, (C2-C6)alkynyl, (Ci-C6)haloalkyl, (C3-
C7)cycloalkyl, aryl,
heterocycle and heteroaryl;
54
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
R5b is selected from:
a) -(CI-C6)alkyl-0-(C1-C6)allcyl-(C3-C7)carbocycle,
-(C1-C6)alkyl-S-(CI-C6)alkyl-(C3-C7)carbocycle,
-(C1-C6)alkylS(0)-(Ct-C6)alkyl-(C3-C6)carbocycle,
-(CI-C6)alkylS02(Ct-C6)alkyl-(C3-C7)carbocycle, -(C2-C6)alkenyl-(Ct-
C6)haloalkyl, -(C2-
C6)alkynyl-(C t-C6)haloalkyl, - (C3-C7)halocarbocycle, -NRaSO2NRcRch -
NRaS020(C3-
C7)carbocycle, -NRaS020aryl, -(C2-C6)alkenyl-(C3-C7)carbocycle, -(C2-
C6)alkenyl-aryl,
-(C2-C6)alkenyl-heteroaryl, -(C2-C6)alkenyl-heterocycle, -(C2-C6)alkynyl-(C3-
C7)carbocycle,
-(C2-C6)alkynyl-aryl, -(C2-C6)alkynyl-heteroaryl, -(C2-C6)alkynyl-heterocycle,
-(C3-
C7)carbocycle-Z1 or -halo(CI-C6)alkyl-Z3; wherein (Ct-C6)alkyl, (C1-
C6)haloalkyl,
(C3-C7)carbocycle, (C2-C6)alkenyl, (C2-C6)alkynyl, aryl or heteroaryl are each
optionally
substituted with one or more(e.g. 1, 2, 3, 4 or 5) Z1 groups;
b) spiro-bicyclic carbocycle, fused-bicyclic carbocycle and bridged-
bicyclic
carbocycle; wherein spiro-bicyclic carbocycle, fused-bicyclic carbocycle or
bridged-bicyclic
carbocycle are optionally substituted with one or more (e.g. 1, 2, 3, 4 or 5)
Z1 groups; wherein
two Z1 groups together with the atom or atoms to which they are attached
optionally form a (C3-
C7)carbocycle or heterocycle wherein the (C3-C7)carbocycle or heterocycle is
optionally
substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z1 groups;
c) (C t-C6)alkyl; wherein (Ct-C6)alkyl is substituted with one or more Z2
groups and
optionally substituted with one or more (e.g. 1,2, 3,4 or 5) Zigroups;
d) -X(C1-C6)alkyl,-X(C1-C6)haloalkyl, -X(C2-C6)alkenyl, -X(C2-C6)alkynyl
and
-X(C3-C7)carbocycle; wherein (CI-C6)alkyl or (CI-C6)haloalkyl are each
substituted with one or
more Z3 groups and optionally substituted with one or more Zlgroups; and
wherein
(C2-C6)alkenyl, (C2-C6)alkynyl and (C3-C7)carbocycle are each independently
substituted with
one or more (e.g. 1,2, 3,4 or 5) Z4 groups and optionally substituted with one
or more (e.g. 1, 2,
3, 4 or 5) Zigroups;
e) aryl, heteroaryl, heterocycle, -Xaryl, -Xheteroaryl and -Xheterocycle;
wherein
aryl heteroaryl are heterocycle are each independently substituted with one or
more (e.g. 1, 2, 3,
4 or 5) Z5 groups and optionally substituted with one or more (e.g. 1, 2, 3,4
or 5) Zlgroups;
f) (C t-C6)haloalkyl, (C3-C7)carbocycle, (C2-C6)alkenyl, and (C2-
C6)alkynyl; where
(CI-C6)haloalkyl, (C3-C7)carbocycle, (C2-C6)alkenyl and (C2-C6)alkynyl are
each independently
substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z6 groups and optionally
substituted with one or
more (e.g. 1, 2, 3, 4 or 5) Zigroups;
g) -NIZeRf, -C(0)NReRf, -0C(0)NReRf, -SO2NReRf,
-(Cl-C6)alkylC(0)-N&Rf, -(Cl-C6)alkyl-O-C(0)-NReRf and -(Cl-C6)alkyl-SO2NR,Rf;
wherein
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
each (Ci-C6)alkyl is independently substituted with one or more (e.g. 1, 2, 3,
4 or 5) Z6 groups
and optionally substituted with one or more (e.g. 1, 2, 3, 4 or 5)
Z1groups; and
h) nitro and cyano;
or R1 and R2 together with the atoms to which they are attached form a 5 or 6-
membered
carbocycle or a 4, 5, 6 or 7-membered heterocycle; wherein the 5 or 6-membered
carbocycle or
a 4, 5, 6 or 7-membered heterocycle are each independently substituted with
one or more (e.g. 1,
2 or 3) Z7 or Z8 groups; wherein when two Z7 groups are on same atom the two
Z7 groups
together with the atom to which they are attached optionally form a (C3-
C7)carbocycle or 4, 5 or
6-membered heterocycle;
X is independently selected from 0, -C(0)-, -C(0)0-, -S-, -S(0)-, -S02_, -(C1-
C6)alky10-, -(CI-C6)alkylC(0)-, -(Ci-C6)alkylC(0)0-, -(Ci-C6)alky1S-, -(CI-
C6)alkylS(0)-,
-(Ci-C6)alkylS02-;
each Z1 is independently selected from halo, -NO2, -0H, =NORa, -SH, -CN, -(Ci-
C6)alkyl, -(C2-C6)alkenyl, -(C2-C6)alkYnYl, -(C1-C6)haloalkyl, (C3-
C7)carbocycle, - (C3-
C7)halocarbocycle, -aryl, -heteroaryl, -heterocycle, -0(Ci-C6)alkyl, -0(C2-
C6)alkenyl, -0(C2-
C6)alkynyl, -0(Ci-C6)haloalkyl, -0(C3-C7)carbocycle, -0(C3-C7)halocarbocycle, -
Oaryl, -
Oheteroaryl, -Oheterocycle, -S(CI-C6)alkyl, -S(C2-C6)alkenyl, -S(C2-
C6)allcynyl, -S(CI-
C6)haloalkyl, -S(C3-C7)carbocycle, -S(C3-C7)halocarbocycle, -Saryl, -
Sheteroaryl, -
Sheterocycle, -S(0)(Ci-C6)alkyl, -S(0)(C2-C6)alkeny1, -S(0)(C2-C6)alkynyl, -
S(0)(C1-
C6)haloalkyl, -S(0) (C3-C7)carbocycle, -S(0)(C3-C7)halocarbocycle, -S02(Ci-
C6)alkyl,
-S(0)aryl, -S(0)carbocycle, -S(0)heteroaryl, -S(0)heterocycle, -S02(C2-
C6)alkenyl, -S02(C2-
C6)alkynyl, -S02(C1-C6)haloalkyl, -S02(C3-C7)carbocycle, -S02(C3-
C7)halocarbocycle,
-S02aryl, -S02heteroary1, -S02heterocycle, -SO2NReRd, -NRcRa, -NRaC(0)Ra, -
NRaC(0)0R1õ
-NRaC(0)NRAI -NRaSO2Rb, -NRaSO2NRcRd, -NRaS020(C3-C7)carbocycle, -NRaS020aryl,
-0S(0)2Ra, -C(0)Ra, -C(0)0Rb, -C(0)NRcR4, and -0C(0)NR,Rd, wherein any (C1-
C6)alkyl,
(C2-C6)alkenyl, (C2-C6)alkynyl, -(C3-C7)halocarbocycle, (C3-C7)carbocycle, (C3-
C7)halocarbocycle, aryl, heteroaryl or heterocycle of Z1 is optionally
substituted with one or
more (e.g. 1, 2, 3, 4 or 5) halogen, -OH, -ORb, -CN, -NRaC(0)2Rb, -heteroaryl,
-heterocycle,
-Oheteroaryl, -Oheterocycle, -NHheteroaryl, -NHheterocycle, or -S(0)2NReRd;
each Z2 is independently selected from -NO2, -CN, Spiro- heterocycle, bridge-
heterocycle, spiro-bicyclic carbocycle, bridged-bicyclic carbocycle, NRaS02(C3-
C7)carbocyc1e,
-NRaS02ary1, -NRaS02heteroaryl, -NRaSO2NR,,Rd, -NRaS020(C3-C7)carbocycle and -
NRaS020aryl;
56
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
each Z3 is independently selected from -NO2, -CN, -OH, oxo, =NORa, thioxo,
aryl,
heterocycle, -heteroaryl, -(C3-C7)halocarbocycle, -0(Ci-C6)alkyl, -0(C3-
C7)carbocycle,
-Ohalo(C3-C7)carbocycle, -Oaryl, -Oheterocycle, -Oheteroaryl, -S(Ci-C6)alkyl, -
S(C3-
C7)carbocyc1e, -S(C3-C7)halocarbocycle, -Saryl, -Sheterocycle, -Sheteroaryl, -
S(0)(Ci-C6)alkyl,
-S(0)(C3-C7)carbocycle, -S(0) (C3-C7)halocarbocycle, -S(0)aryl, -
S(0)heterocycle,
-S(0)heteroaryl, -S02(Ci-C6)alkyl, -S02(C3-C7)carbocycle, -S02(C3-
C7)halocarbocycle,
SO2aryl, -S02heterocyc1e, -S02heteroary1, -NRaRb, -NRaC(0)Rb, -C(0)NRcR3, -
SO2NRcR3,
-NRaSO2NRAd, -NRaS020(C3-C7)carbocycle and -NRaS020aryl;
each Z4 is independently selected from halogen, -(C1-C6)alkyl, (C3-
C7)carbocycle,
-halo(Ci-C6)alkyl, -NO2, -CN, -OH, oxo, =NORa, thioxo, -aryl, -heterocycle, -
heteroaryl, -(C3-
C7)halocarbocycle, -0(Ci-C6)alkyl, -0(C3-C7)carbocycle, -0(C3-
C7)halocarbocycle, -Oaryl,
-Oheterocycle, -Oheteroaryl, -S(Ci-C6)alkyl, -S(C3-C7)carbocycle, -S(C3-
C7)halocarbocycle,
-Saryl, -Sheterocycle, -Sheteroaryl, -S(0)(Ci-C6)alkyl, -S(0)(C3-
C7)carbocycle, -S(0)(C3-
C7)halocarbocycle, -S(0)aryl, -S(0)heterocycle, -S(0)heteroaryl, -S02(Ci-
C6)alkyl, -S02(C3-
C7)carbocycle, -S02(C3-C7)halocarbocycle, SO2aryl, -S02heterocyc1e, -
S02heteroaryl, -NRaRb,
-NRaC(0)Ra, -C(0)NRAI, -SO2NRcRd, -NRaSO2NRcRd, -NRaS020(C3-C7)carbocycle and -
NRaS020aryl;
each Z5 is independently selected from -NO2, -CN, -NRaSO2NRcR4, -NRaS020(C3-
C7)carbocycle, -NRaS020ary1, -NRaS02(Ci-C6)alkyl, -NRaS02(C2-C6)alkenyl, -
NRaSO2(C2-
-NRaS02(C3-C7)carbocycle, -NRaS02(C3-C7)halocarbocycle, -NRaS02aryl,
-NRaS02heteraryl, -NRaS02heteroaryl, -NRaS02heterocyc1e, -NRaC(0)alkyl, -
NRaC(0)alkenyl,
-NRaC(0)alkynyl, -NRaC(0) (C3-C7)carbocycle, -NRaC(0)(C3-C7)halocarbocycle,
-NRaC(0)aryl, -NRaC(0)heteroaryl, -NRaC(0)heterocycle, -NRaC(0)NR,Rd and -
NRaC(0)0Rb;
each Z6 is independently selected from -NO2, -CN, -NRaRa, NRaC(0)Rb,-
C(0)NRcRd,
.. -(C3-C7)halocarbocycle, -aryl, -heteroaryl, -heterocycle, -Oaryl, -
Oheteroaryl, -Oheterocycle,
-0(C3-C7)halocarbocycle, -0(Ci-C6)alkyl, -0(C3-C7)carbocycle, -Ohalo(Ci-
C6)alkyl, -Saryl,
-Sheteroaryl, -Sheterocycle, -S(C3-C7)halocarbocycle, -S(Ci-C6)alkyl, -S(C3-
C7)carbocycle,
-S(Ci-C6)haloalkyl, -S(0)aryl, -S(0)heteroaryl, -S(0)heterocycle, -S(0)(C3-
C7)halocarbocycle,
-S(0)(Ci-C6)alkyl, -S(0)(C3-C7)carbocycle, -S(0)halo(CI-C6)alkyl, -SO2aryl, -
S02heteroaryl,
-S 02heterocycle, -S 02(C -C6)alkyl, -S 02halo(C -C6)alkyl, -S 02(C3-
C7)carbocycle, -S02(C3-
C7)halocarbocycle, -S 02NRcltd, -NRaS02(C3-C7)halocarbocycle, -NRaS02aryl,
-NRaS02heteraryl, -NRaS02heteroaryl, -NRaSO2NReftd, -NRaS020(C3-C7)carbocycle
and
-NRaS020aryl.
each Z7 is independently selected from -NO2, =NORa, -CN, -(Ci-C6)alkyl-Z12, -
(C2-
C6)alkenyl-Z12, -(C2-C6)alkenylOH, -(C2-C6)alkynY1-Z12, -(C2-C6)alkynyl-OH, -
(C1-
57
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
C6)haloalkyl-Z12, -(CI-C6)haloalkylOH, -(C3-C7)carbocycle-Z12, -(C3-
C7)carbocycle0H, -(C3-
C7)halocarbocycle, -(CI-C6)alky1NR,Rd, -(CI-C6)alky1NRaC(0)Ra, -(C1-
C6)a1ky1NRaSO2Ra,
aryl, -heteroaryl, -heterocycle, -0(C1-C6)alkyl-Z12, -0(C2-C6)alkenyl, -0(C2-
C6)alkynyl, -0(C1-
C6)haloalkyl, -0(C3-C7)carbocycle, -0(C3-C7)halocarbocycle, -Oaryl, -0(C -
C6)alkylN&Rd,
-0(C i-C6)alky1NRaC(0)Ra, -0(C i-C6)alkylNRaSO2Ra, -Oheteroaryl, -
Oheterocycle, -S(Ci-
C6)alkyl-Z12, -S(C2-C6)alkenyl, -S(C2-C6)alkynyl, -S(CI-C6)haloalkyl, -S(C3-
C7)carbocycle,
-S(C3-C7)halocarbocycle, -S(Ci-C6)alkyINRcki, -S(C -C6)alky1NRaC(0)Ra, -S(Ci-
C6)alky1NRaSO2Ra, -Saryl, -Sheteroaryl, -Sheterocycle, -S(0)(Ci-C6)alkyl, -
S(0)(C2-
C6)alkenyl, -S(0)(C2-C6)alkynyl, -S(0)(Ci-C6)haloalkyl, -S(0)(C3-C7)carbocyle,
-S(0)(C3-
C7)halocarbocycle, -S02(Ci-C6)alkyl, -S(0)(Ci-C6)alkylNKR,I, -S(0)(Ci-
C6)alky1NRaC(0)Ra, -
S(0)(Ci-C6)alky1NRaSO2Ra, -S(0)aryl, -S(0)heteroaryl, -S(0)heterocycle, -
S02(Ci-C6)alkyl,
-S02(C2-C6)alkenyl, -S02(C2-C6)alkynyl, -S02(C1-C6)haloalkyl, -S02(C3-
C7)carbocycle, -
S02(C3-C7)halocarbocycle, -S02aryl, -S02heteroaryl, -S02heterocycle, -S02(CI-
C6)alky1NRcRth
-S02(Ci-C6)alky1NRaC(0)Ra, -S02(CI-C6)alky1NRaS 02Ra, -SO2NRcRi, -NRaC(0)0Rb,
-NRaC(0)NRcRi, -NRaSO2Rb, -NRaSO2NR,RA, -NRaS020(C3-C7)carbocycle, -
NRaS020aryl,
-05(0)2Ra, -C(0)NRcRd, and -0C(0)NRcR4j, wherein any (Ci-C6)alkyl, (C2-
C6)alkenyl, (C2-
C6)alkynyl, (C3-C7)carbocycle, (C3-C7)halocarbocycle, aryl, heteroaryl or
heterocycle of Z7 is
optionally substituted with one or more (e.g. 1, 2, 3, 4 or 5) halogen, -OH, -
ORb, -CN,
-NRaC(0)2Rb, -heteroaryl, -heterocycle, -Oheteroaryl, -Oheterocycle, -
NHheteroaryl,
-NHheterocycle, or -S(0)2NRcR3
each Z8 is independently selected from -NO2 or -CN;
each Z9 is independently selected from -(Ci-C6)alkyl, -0(C1-C6)alkyl;
each Z19 is independently selected from
i) halo, oxo, thioxo, (C2-C6)alkenyl, (Ci-C6)haloalkyl, (C3-
C7)cycloalkyl, (C3-C7)cycloalkyl-(Ci-C6)alkyl-, -OH, -0(C -
C6)alkyl, -0(C i-C6)haloalkyl, -SH, -S(Ci-C6)alkyl, -SO(C1-
C6)alkyl, -S02(C1-C6)alkyl, -NH2, -NH(C1-C6)alkyl and
-N((CI-C6)alky1)2;
ii) (CI-C6)alkyl optionally substituted with -OH, -0-(C i-C6)haloalkyl, or -
0-
(Ci-C6)alkyl; and
iii) aryl, heterocycle and heteroaryl, which aryl, heterocycle and
heteroaryl is
optionally substituted with halo, (Ci-C6)alkyl or COOH;
each Z11 is independently selected from Z19, -C(=0)-NH2, -C(0)-NH(CI-C4)alkyl,
-C(=0)-N((C i-C4)alky1)2, -C(=0)-aryl, -C(=0)-heterocycle and -C(0)-
heteroaryl;
58
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
each Z12 is independently selected from -NO2, =NOR,a, thioxo, -aryl, -
heterocycle,
-heteroaryl, -(C3-C7)halocarbocycle, -(C3-C7)carbocycle, -0(C3-C7)carbocycle, -
Ohalo(C3-
C7)carbocyle, -Oaryl, -Oheterocycle, -Oheteroaryl, -S(CI-C6)alkyl, -S(C3-
C7)carbocyle,
-Shalo(C3-C7)carbocyle, -Saryl, -Sheterocycle, -Sheteroaryl, -S(0)(Ci-
C6)alkyl,
-S(0)(C3-C7)carbocyle, -S(0)halo(C3-C7)carbocycle, -S(0)aryl, -
S(0)heterocycle,
-S(0)heteroaryl, -S02(Ci-C6)alkyl, -S02(C3-C7)carbocycle, -S02(C3-
C7)halocarbocycle,
SO2aryl, -S02heterocyc1e, -S02heteroaryl, -NRaRa, -NRaC(0)Rb, -C(0)NRcR1, -
SO2NRcitd,
-NRaSO2NR,Rd, -NRaS020(C3-C7)carbocyle and -NRaS020aryl;
each Z13 is independently selected from -NO2, -OH, =NORa, -SH, -CN, -(C3-
C7)halocarbocycle, -0(CI-C6)alkyl, -0(C2-C6)alkenyl, -0(C2-C6)alkynyl, -0(C i-
C6)haloalkyl,
-0(C3-C7)carbocycle, -0(C3-C7)halocarbocycle, -Oaryl, -Oheteroaryl, -
Oheterocycle, -S(C1-
C6)alkyl, -S(C2-C6)alkenyl, -S(C2-C6)alkynyl, -S(C1-C6)haloalkyl, -S(C3-
C7)carbocycle, -S(C3-
C7)halocarbocycle, -Saryl, -Sheteroaryl, -Sheterocycle, -S(0)(Ci-C6)alkyl, -
S(0)(C2-C6)alkenyl,
-S(0)(C2-C6)alkynyl, -S(0)(Ci-C6)haloalkyl, -S(0)(C3-C7)carbocycle, -S(0)(C3-
C7)halocarbocycle, -S(0)aryl, -S(0)heteroaryl, -S(0)heterocycle, -S02(CI-
C6)alkyl, -S02(C2-
C6)alkenyl, -S02(C2-C6)allcynyl, -S02(Ci-C6)haloalkyl, -S02(C3-C7)carbocycle, -
S02(C3-
C7)halocarbocycle, -S02aryl, -S02heteroaryl, -S02heterocycle, -SO2NRelta, -
NRcRd,
-NRaC(0)Ra, -NRaC(0)0Rb, -NR,C(0)NR,Rd -NRaSO2Rb, -NRaSO2NRAd, -NRaS020(C3-
C7)carbocycle, -NRaS020aryl, -0S(0)2Ra, -C(0)Ra, -C(0)ORb, -C(0)NR,Rd, and
-0C(0)NReRd; wherein any (C1-C6)alkyl, (C2-C6)alkenyl, (C2-C6)alkynyl, -(C3-
C7)halocarbocycle, (C3-C7)carbocycle, (C3-C7)halocarbocycle, aryl, heteroaryl
or heterocycle of
Z13 is optionally substituted with one or more (e.g. 1, 2, 3, 4 or 5) halogen,
-OH, -ORb, -CN,
-NR,C(0)2Rb, -heteroaryl, -heterocycle, -Oheteroaryl, -Oheterocycle, -
NHheteroaryl, -
NHheterocycle, or -S(0)2N&Rd;
each Z14 is independently selected from -NO2, =NORa, -CN, -(C3-
C7)halocarbocycle,
-0(C3-C7)halocarbocycle, -S(C3-C7)halocarbocycle, -S(0)(C3-C7)halocarbocycle, -
S02(C3-
C7)halocarbocycle, -NRaSO2NReRd, -NRaS020(C3-C7)carbocycle, -NRaS020aryl, -
0S(0)2Ra;
wherein any -(C3-C7)halocarbocycle of Z14 is optionally substituted with one
or more (e.g. 1, 2,
3, 4 or 5) halogen, -OH, -ORb, -CN, -NRaC(0)2R1,, -heteroaryl, -heterocycle, -
Oheteroaryl,
-Oheterocycle, -NITheteroaryl, -NHheterocycle, or -S(0)2NRcRd;
each Ra is independently H, (Ci-C6)alkyl, -(C2-C6)alkenyl, -(C2-C6)alkynyl,
(C3-C7)carbocycle, heterocycle, aryl, aryl(Ci-C6)alkyl-, heteroaryl or
heteroaryl(Ci-C6)alkyl-;
wherein any (Ci-C6)alkyl, (C2-C6)alkenyl, (C2-C6)alkynyl, (C3-C7)carbocycle,
heterocycle, aryl,
or heteroaryl of Ra is optionally substituted by halogen, OH and cyano;
59
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
each Rb is independently -(Ci-C6)alkyl, -(C2-C6)alkenyl, -(C2-C6)alkynyl,
(C3-C7)carbocycle, heterocycle, aryl, aryl(Ci-C6)alkyl-, heteroaryl or
heteroaryl(Ci-C6)alkyl-;
wherein any (Ci-C6)alkyl, -(C2-C6)alkenyl, -(C2-C6)alkynyl, (C3-C7)carbocycle,
heterocycle,
aryl, or heteroaryl of RI, is optionally substituted by halogen, OH and cyano;
R, and Rd are each independently selected from H, (Ci-C6)alkyl, (C2-
C6)alkenyl,
(C2-C6)allcynyl, (C3-C7)carbocycle, aryl, aryl(Ci-C6)alkyl-, heterocycle,
heteroaryl or
heteroaryl(Ci-C6)alkyl- wherein any (Ci-C6)alkyl, -(C2-C6)alkenyl, -(C2-
C6)alkynyl,
(C3-C7)carbocycle, heterocycle, aryl, or heteroaryl of R., or Rd is optionally
substituted by
halogen, OH and cyano; or &and Rd together with the nitrogen to which they are
attached form
a heterocycle; wherein any hetereocycle of Re and Rd together with the
nitrogen to which they
are attached is optionally substituted by halogen, OH or cyano;
each R, is independently selected from -0Ra, (Ci-C6)alkyl or (C3-C7)carbocycle
wherein
(Ci-C6)alkyl or (C3-C7)carbocycle is substituted by one or more Z6 and
optionally substituted
with one or more Z1; -(C2-C6)haloalkyl, -(C2-C6)alkenyl, or -(C2-C6)allcynyl
wherein any
haloalkyl, alkenyl or alkynyl is optionally substituted with one or more Z1;
aryl, heterocycle or
heteroaryl wherein aryl, heterocycle or heteroaryl is substituted by one or
more Z5;
each Rf is independently selected from -Rg, -0Ra, -(Ci-C6)alkyl-Z6, -S02R8, -
C(0)Rg,
C(0)04 or -C(0)NR,Rg; and
each Rg is independently selected from (C1-C6)alkyl, (C3-C7)carbocycle
(C1-C6)haloallcyl, (C2-C6)alkenyl, (C2-C6)alkynyl, aryl, heterocycle or
heteroaryl wherein any
(CI-C6)alkyl, (C3-C7)carboeycle -(C1-C6)haloalkyl, (C2-C6)alkenyl, (C2-
C6)alkynyl, aryl,
heterocycle or heteroaryl of Rg is optionally substituted with one or more Zi
groups;
or a salt thereof.
In one embodiment, the compounds of formula I are selected from:
60
CA 02833377 2013-10-16
WO 2012/145728 PCMJS2012/034593
CI CI
_
S OH S - OH S OH
lII
____________________________________________ ( __ /%1
CI a CI
e< )<
0 0J<
,
S = OH \ __ S - OH S OH
(rsi 0 , >------<\
N 0 ,
C
CI i
CI
e< e<
0j<
- S OH ________ S ' OH 0 S OH
" -
N--µ I> ______ ¨ N \
N 0
/ N 0 0
'
0 0 0
r r r
N .< N. -< N
N 0 NY 0 N 0--
_
S : OH S - OH
N 0 '
N 0
N 0 '
cii
0 0 0
r r r
N 0
N 0j< N 0
S : OH 0 __ S ,
OH and 0
CN4 I ' ) I
2
HN __________________________________________________ N I
0 0 0
N H2N N
and salts thereof.
In one embodiment, the compounds of formula I are selected from:
61
CA 02833377 2013-10-16
WO 2012/145728
PCT/US2012/034593
0 0 0
----
,
--<
NY 0'K
N
0 S OH \ 0 S : OH
S ' OH
HC?0\' N /14---
0 N 0 \
N-<\jJ
0
N
0 0 0
N 0 N 0 N.
- N
\ S 0.<
- OH
OH
( N 0 ( N 0
, N---.
N 0
,
,
0 0 0
.7 .7
N. N.
N.
N 0"-< N 0 N 0 _
\ S = OH \ S OH
\
N---.. , ) p-1%,
=-= N 0 0 , ) /N-4,N 0
,
0
0 0
7'
.7 7-
N.
N 0
e< N. -k
N N 0
_
,..---\ S ' OH N.
N- ----\ SJ5Y " OH F>CN-e
, OH
----.../ N 0 N-, I N 0 F 0
----/ , N
,
'
62
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
0 0 0
..--- ...--- ----
....
N 0.< N 0--.< N 0.<
-.. -...
: OH S ' OH 7 OH
N 0 /
0-CN-
N 0 /
0-CN-s
N 0
0 0
0
..---
..--'
=-=..
N Cd< -..
S : OH
SL-OH N O<
F-N4 0, S OH
,
'
0 0 0
,.
---' .--
--... ----
-... --...
N 0.<
:
\ S 7 OH \
0
: > ______ / N 0 C > ____ / N 0
, /---/ N 0
0 0 -N
, \ ,
0
0 0
/
.----*
-.. ---"*
N 0.< -..
\ S ' OH N 0j< ,...
N Cr<
N-<\ I I II\ S 7 OH 7 OH
\ S
0
/-/ N N4
-N
. N 0 , 0 __ N--<\
/ N 0
jJ
\ ,
-N
\
63
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
0 0 0
,. ---i -k
N 0< N 0 N 0-j<
0-4s ' OH
os - OH S = OH
/ N 0 0 N , 0--. I
, 0 ,
/ N
0 0 0
,- ,--
--"< ,.
N 0 N LJ CY< N e<
os ' OH
os ' OH
N 0 N o
0 N 0
F F
* , ,
,
F
F
F
F
0
0
,--
N (Y
S - OH N 0-<
0 S ' OH
0
S - OH
0
*
N , 0
, N
III
F '
F
F
F
0 0 0
.=
.--
-.
N Cl.< ,...
Y<
* \S OH N C N 0
OH
S -<
: OH
III 0 ,
N
64
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
0 0 0
.= .--
-. J -' --
N 0 N 0 N 0
S OH S 7 OH
0--<\
( N 0
,
HN HN
0-< a 0-.< i j<
s = OH S - OH S - OH
[>--. >---(µ
N 0 0 , 0 ,
, N N
0 0 0
.-
so ,--
.. 0 N N 0 _
' OH
> S OH
N
0 N¨JJJ
N 0 ,
,
0 0 0
.=
-. J
,,
N 0 N 0 N 0
FL/S ' OH S ' OH ¨NH s v OH
N----\\
0 N 0 .
N 0 , ,--4,
0 N 0 ,
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
0 0
V< NY
0
0 S OH 0
HN, 0 0
and NH N
410
¨0
and salts thereof.
In one embodiment, the compounds of formula I are selected from:
66
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
CI CI
0-<
CY< CY<
_
S r OH S r OH S = OH
N N 0 , ( N
CI CI CI
0j< 0-r< CY<
S " OH OH S OH
( 0 , \ ,S
C.
N
N 0 .
,
CI CI
CI
CY< 0j<
-
0
- S OH
S - OH 0 S OH
\ -
N<\ > __ -
- \
N 0
/ N 0 N
0 0 0
N 0-k N CYl< -- N 0
S r OH
OH
>-- >--<,
N 0 '
N 0
N 0 '
0 0 0
..==
,.. -. -. -
N 0-K N e< N 0 '
_
S - OH 0,\ IS r OH 0 S - OH
---, I ) __ I 0
0 N 0 H2N N H2N N ,
67
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
0 0 0
.-
-'
N 0 N e<
N e<
/
0 ______ S OH S : OH
\ S : OH
HO N 0 , \N4
N 0 -N4
/ N 0
0 0 0
..= .'" .-
-,. ..
N 03(< N Cf< _
OH N
\ S tYl<
: OH
( N 0 ( N 0
, N-
0
, N
,
0 0 0
.,' .r
., ,..
N O<N 0 N e<
\ \ S : OH
\ S :
OH
N4 , ) 71-i, j
N 0
0
,
0
0 0
.,-
0
N N oZY
CN---- 0 ,..---\ S - OH F,,/\ S '
OH
N N----
N FN
68 0
---/ 0 ,
68
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
0 0 0
,-
=,.
N 0" N CY.< N 0-"i<
_
S = OH S = OH 0-C iS 0-CN-
,
0 0 0
.. .-
,..
N ak tsi
0j< ,
S : OH OH N 0j<
F-ON4 cN_e V
Os S : OH
N 0
N 0 µS-CN4
0
,
,
0 0 0
..' ,-
JLJ
N 0" N 0"k N 0-k
\ S : OH \ S : OH
, ,..
--O ______________________ N- --<, ________ N4
L > / N 0 C0 N > / N 0 r---/ N 0
'
0 0 -N ,
, \
0
0 0
-, ..
N 0'< , ,
N 0 N 0'.<
-
N- \ S = OH OH
\ S
0
-N
\ ,
-N
\
69
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
0 0 0
,. --i< -k ,.
N 0 N 0 N el<
s
S r OH
/ N 0 N 0 , 0--.. 1 0 ,
,
/ -- N
0 0 0
,- ,--
N 0".< OH N e< N e<
os os OH S OH
N 0 N 0 0
N 0
F F
=
,
F F
F
F
0
0
0
-. ..-
--'
S OH
NY OH
-. -<
os - OH N 0
N e<
-
N 0 CN4 S - OH
N 0
19 \N
, 0
,
F '
F
F
F
0 0
0
-. .71 --
. -,
N 0 N 0
= III
OH N 0
S OH
N
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
0 0 0
,.. -. , - <
N 0 N 0 N 0
S - OH S OH S OH
,
0 ro ro
HN HN
0" CI 0-k I '.
CI * 0,..<
OH S ' OH
0 {:>--. ---4, 0 r), 0 ,
, N N
0 0 0
r r
,. J
>
-..
NY 0 ,.
N 0 N 0
S OH
F S - OH
0 N > N 0 ,
N F
,
0 0 0
r r r
.. -< -k -K
..
N N 0 N 0
H ,S ' OH
S : OH ¨NH s -
= OH
0
N---
0- N 0 , µ
N 0 , ¶
71
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
0 0
N
(30' 0-<
0 S ' OH 0 S ' OH
----
0 0
HN N and NH N
II .
¨0
and salts thereof.
In another embodiment, the compounds of formula I are selected from:
o 0
o
,- ,--
Q--.N - ,-
-. .
0
9-=< N
N :
\ S OH
\ I 04 1 - OH
o_iN--N I
0 0
N 0
0
CI CI
0
0j< S ' OH S - OH
[-)_e 1 : OH > I -4 I N 0
0 , N
N 0 , F CN
CI CI CI
I ,
0 S : OH S OH N S OH
<0
-N N 0 I 0 and C __ <'J L)
N N
, \
F
b F
;
and salts thereof
General Synthetic Procedures
Schemes 1, 2, 3, 4, 5, 6 and 7 are provided as further embodiments of the
invention and
illustrate general methods which were used to prepare compounds of formula I
and which can be
used to prepare additional compounds of formula I.
72
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
Scheme 1
Br2, S
0 KSCN, H2N-4\ 0
101 .. ________________ 0 1. H3PO4, NeNO2 /
, 0
HOAG, it N R2
H2N R2 2. H2P02 N R2
R1
Br2, R1 R1
1B
1A 1C
X-R4
Br
, \s la OH ..
BBr3 < S OH
DCM N WI R2 _____,,.
HOAc N ill R2 PD1APEP/Hh3)8, K2CO3,
R1 R1 HCI
1D 1E (X = boronic acid, halogen)
R4 R4 R4
Bu3Sri
s Ail OH Tf20, S OTf _____ , S
_____________________ . PdC12(PPh3)2 ,,
N igr R2 Pyr, DCM N 1110 R2 DMF, 120 C N R2
R1 R1 R1
1G
IF 1H
R4 OH R4 OH- 1BuOAc
N
AD-mix a S " OH PivCI S - Oy< __________ .-
Pyr/DCM N R2 0 HC104
R2
R1
R1
II 1J
R4 Cr< R4 CI"µ<
R4 e< NaOH Cr03, H5I06 ,
. OH ___________________________________________________________
% wet ACN µ
0 N R2 N R2
N R2
R1 R1
R1
1L 1M
1K
73
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
Scheme 2
0
S 0 R5-)(
t-butylnitrite, Br
H2N¨(
R2 CuBr2, ACN R2 K3PO4, PdC12dppf
R1 R1 dioxane
(X = boronic acid, halogen)
1B 2A
R4 0"
OH
R5-4j1 R5¨JJ
R2
R2
R1
R1
2B 2C
The benzothiazole intermediate 2B can be converted to the final compound 2C by
the
methods used to convert 1C to 1M as outlined in Scheme 1.
74
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
Scheme 3
Br
,S . (). NBS, S sCo, R4-X
H2N¨ ______________ , H2N-- _____________________ .
N R2 H2SO4 N R2
Pd(PPh3)4, K2CO3,
R1 R1
1B 3A DME/H20
(X = boronic acid, halogen)
R4 R4
Br¨.
H2N¨
/S Cis-- t-butylnitrite, S 401 0 R5-X
___________________________________ . _______________________________ _
N R2 CuBr2, MeCN N
R2 K3PO4, PdC12(dPPf),
R1 R1 DME/H20
3B 3C (X = boronic acid,
halogen)
4 R4 0
R -
R20
R
R5 5¨
______________________________________ y N
N R2
R1
Ri
3E 2C
The benzothiazole intermediate 3E can be converted to the final compound 2C by
the
methods used to convert 1C to 1D and 1F to 1M as outlined in Scheme 1.
Scheme 4
R4 R4 R4 CY<
S 0.
HNRR
, RRN4 0 - _... RRN--e - OH
Br¨<\
N R2 N R2 N R2
R1 R1 R1
3C 4A 4B
The benzothiazole intermediate 4A can be converted to the final compound 4B by
the
methods used to convert 1C to 1D and 1F to 1M as outlined in Scheme 1 wherein
HNRR
represents an HNR9R10, HNReRf or a heterocycle (when R and R taken together
with the
nitrogen to which they are attached form a ring).
Scheme 5
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
Br Br
s Ai R3 1. Bn0H, NaH S di R3 KOt-Bu,
CI ____________________________ ' HO-
2. H2, Pd/C N 14r R2 RraX
N 14r1 R2
R1 R1
4C
4D
Br R4
os la R3 R4B(OH)2 S R3
PdPR K2CO3
"- C)
(I13)4., N R2
N ilgri R2
0 R1
0 R1
4E
4F
76
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
Scheme 6
Br Br Br
401 02N OH Tf20 OTf .-1'"SnBu3 ,.,. AD-mix a Br OH
R2 Et3N
-
= OH
Pd , LiCI, 02N
02N R2 R2
R1 R1 DMF R1 02N R2
4G 4H 41 4J
Br OH Br CY.< Br e<
PivCI ' ()my tBuOAc r OPiv H2 ' OPiv KSCN
Py HC104 Pt/C
HOAc, Br2
02N R2 02N R2 H2N R2
R1 R1 RI
4K 4L 4M
Br C;$"< Br 0'<
_ Br 0"-<
,S = OPiv NaNO2 S - OPiv
1. Bn0H, NaH S ,
OPiv
¨1" CI-- I
CuCl2 N R2 _________ ' HO¨ I
N R2 2. H2, Pd/C N R2
II
R1 R1
R1
4P
4N 40
Br e<
R4 1:)<
KOt-Bu, S 7 OPiv R4B(OH)2 ,
_______________________________________________ ,
R6X 11 R2 Pd(PPh3)4, K2CO3 S OPiv
0 1
N R2
R6 R1
A6 R1
4R
R4 e< 4S
OH
_____________________________ . 0 SI
N R2 0
FL R1
4T
The benzothiazoline intermediate 4S can be converted to the final compound 4T
by the
methods used to convert 1C to 1M as outlined in Scheme 1.
77
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
Scheme 7
Br 0j<
Br 0--k
OPiv
Pd , K2CO3 OPiv
R2
R2
R1 R5B(OF02
R1
4P 4U
R4 R4 Co-<
Pd , K2CO3 OPiv OH
\,
R4B(OH)2 R5-4I R5_jj
<
R2 R2
R1 R1
4V 4W
The benzothiazoline intermediate 4V can be converted to the final compound 4W
by the
methods used to convert 1C to 1M as outlined in Scheme 1.
A specific value for R5 is selected from:
a) aryl, heterocycle and heteroaryl, wherein aryl, heterocycle and
heteroaryl are
each optionally substituted with one or more (e.g. 1, 2 or 3) Z" groups;
b) aryl, heteroaryl and heterocycle, wherein aryl, heteroaryl are
heterocycle, are
each independently substituted with one or more (e.g. 1, 2, 3,4 or 5) Z5
groups and optionally
substituted with one or more (e.g. 1, 2, 3,4 or 5) Z1 groups; and
c) aryl, heteroaryl and heterocycle, wherein aryl, heteroaryl and
heterocycle, are
each independently substituted with one or more (e.g. 1, 2, 3,4 or 5) Z15
groups and optionally
substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z1 groups.
A specific group of compounds of formula I are compounds wherein:
R5 is selected from:
a) aryl, heterocycle and heteroaryl, wherein aryl, heterocycle and
heteroaryl are
each optionally substituted with one or more (e.g. 1, 2 or 3) Z" groups;
b) aryl, heteroaryl and heterocycle, wherein aryl, heteroaryl are
heterocycle, are
each independently substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z5
groups and optionally
substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z1 groups; and
c) aryl, heteroaryl and heterocycle, wherein aryl, heteroaryl and
heterocycle, are
each independently substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z15
groups and optionally
substituted with one or more (e.g. 1, 2, 3,4 or 5) Z1 groups;
and
78
CA 02833377 2013-10-16
WO 2012/145728
PCT/US2012/034593
R3' is H.
Another specific group of compounds of formula I are compounds wherein:
R5 is selected from:
a) aryl, heterocycle and heteroaryl, wherein aryl, heterocycle and
heteroaryl are
each optionally substituted with one or more (e.g. 1,2 or 3) Zn groups;
b) aryl, heteroaryl and heterocycle, wherein aryl, heteroaryl are
heterocycle, are
each independently substituted with one or more (e.g. 1,2, 3,4 or 5) Z5 groups
and optionally
substituted with one or more (e.g. 1,2, 3, 4 or 5) Zlgroups; and
c) aryl, heteroaryl and heterocycle, wherein aryl, heteroaryl and
heterocycle, are
each independently substituted with one or more (e.g. 1, 2, 3,4 or 5) Z15
groups and optionally
substituted with one or more (e.g. 1,2, 3, 4 or 5) Zigroups;
R3' is H; R1 is H; and
R2 is H or (CI-C6)alkyl.
Another specific group of compounds of formula I are compounds wherein:
R5 is selected from:
a) aryl, heterocycle and heteroaryl, wherein aryl, heterocycle and
heteroaryl are
each optionally substituted with one or more (e.g. 1,2 or 3) Z11 groups;
b) aryl, heteroaryl and heterocycle, wherein aryl, heteroaryl are
heterocycle, are
each independently substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z5
groups and optionally
substituted with one or more (e.g. 1, 2, 3,4 or 5) Z' groups; and
c) aryl, heteroaryl and heterocycle, wherein aryl, heteroaryl and
heterocycle, are
each independently substituted with one or more (e.g. 1, 2, 3,4 or 5) Z15
groups and optionally
substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z1 groups;
R3' is H; R1 is H;
R2 is H or (Ci-C6)alkyl; and
R3 is -0(Ci-C6)alkyl.
A specific value for R5 is selected from:
a) aryl,
heterocycle and heteroaryl, wherein aryl, heterocycle and heteroaryl are
each optionally substituted with one or more (e.g. 1, 2 or 3) Z11 groups; and
b) aryl,
heteroaryl and heterocycle, wherein aryl, heteroaryl and heterocycle, are
each independently substituted with one or more (e.g. 1, 2, 3,4 or 5) Z15
groups and optionally
substituted with one or more (e.g. 1,2, 3,4 or 5) Z' groups.
A specific group of compounds of formula I are compounds wherein:
R5 is selected from:
79
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
a) aryl, heterocycle and heteroaryl, wherein aryl, heterocycle and
heteroaryl are
each optionally substituted with one or more (e.g. 1,2 or 3) Z11 groups; and
b) aryl, heteroaryl and heterocycle, wherein aryl, heteroaryl and
heterocycle, are
each independently substituted with one or more (e.g. 1, 2, 3,4 or 5) Z15
groups and optionally
substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z1 groups;
and
R3' is H.
Another specific group of compounds of formula I are compounds wherein:
R5 is selected from:
a) aryl, heterocycle and heteroaryl, wherein aryl, heterocycle and
heteroaryl are
each optionally substituted with one or more (e.g. 1, 2 or 3) Z" groups; and
b) aryl, heteroaryl and heterocycle, wherein aryl, heteroaryl and
heterocycle, are
each independently substituted with one or more (e.g. 1, 2, 3,4 or 5) Z15
groups and optionally
substituted with one or more (e.g. 1, 2, 3, 4 or 5) Zigroups;
R3' is H; R1 is H; and
R2 is H or (C1-C6)alkyl.
Another specific group of compounds of formula I are compounds wherein:
R5 is selected from:
a) aryl, heterocycle and heteroaryl, wherein aryl, heterocycle and
heteroaryl are
each optionally substituted with one or more (e.g. 1,2 or 3) Z11 groups; and
b) aryl, heteroaryl and heterocycle, wherein aryl, heteroaryl and
heterocycle, are
each independently substituted with one or more (e.g. 1, 2, 3,4 or 5) Z15
groups and optionally
substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z1 groups;
R3' is H; R1 is H;
R2 is H or (Ci-C6)alkyl; and
R3 is -0(C i-C6)alkyl.
Another specific value for R5 is aryl, heteroaryl, heterocycle, wherein aryl,
heteroaryl
and heterocycle, are each independently substituted with one or more (e.g. 1,
2, 3, 4 or 5) Z15
groups and optionally substituted with one or more (e.g. 1,2, 3,4 or 5) Z'
groups;
Another specific group of compounds of formula I are compounds wherein:
R5 is selected from aryl, heteroaryl and heterocycle, wherein aryl, heteroaryl
and
heterocycle, are each independently substituted with one or more (e.g. 1, 2,
3,4 or 5) Z15 groups
and optionally substituted with one or more (e.g. 1, 2, 3,4 or 5) Zlgroups;
and
R3' is H.
Another specific group of compounds of formula I are compounds wherein:
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
R5 is selected from aryl, heteroaryl and heterocycle, wherein aryl, heteroaryl
and
heterocycle, are each independently substituted with one or more (e.g. 1, 2,
3, 4 or 5) Z15 groups
and optionally substituted with one or more (e.g. 1,2, 3, 4 or 5) Z' groups;
R3' is H; Ri is H; and
R2 is H or (Ci-C6)allcyl.
Another specific group of compounds of formula I are compounds wherein
R5 is selected from aryl, heteroaryl and heterocycle, wherein aryl, heteroaryl
and
heterocycle, are each independently substituted with one or more (e.g. 1, 2,
3, 4 or 5) Z15 groups
and optionally substituted with one or more (e.g. 1,2, 3,4 or 5) Z1groups;
R3' is H; le is H;
R2 is H or (Ci-C6)alkyl; and
R3 is -0(Ci-C6)alkyl.
Another specific value for R5 is selected from:
a) aryl, wherein aryl is optionally substituted with one or more (e.g. 1, 2
or 3) Z11
groups;
b) aryl, heteroaryl and heterocycle, wherein aryl, heteroaryl are
heterocycle, are
each independently substituted with one or more (e.g. 1, 2, 3,4 or 5) Z5
groups and optionally
substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z1 groups; and
c) aryl, heteroaryl and heterocycle, wherein aryl, heteroaryl and
heterocycle, are
each independently substituted with one or more (e.g. 1,2, 3,4 or 5) Z15
groups and optionally
substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z1 groups.
Another specific group of compounds of formula I are compounds wherein:
R5 is selected from:
a) aryl, wherein aryl is optionally substituted with one or more (e.g. 1, 2
or 3) Z"
groups;
b) aryl, heteroaryl and heterocycle, wherein aryl, heteroaryl are
heterocycle, are
each independently substituted with one or more (e.g. 1,2, 3,4 or 5) Z5 groups
and optionally
substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z1 groups; and
c) aryl, heteroaryl and heterocycle, wherein aryl, heteroaryl and
heterocycle, are
each independently substituted with one or more (e.g. 1,2, 3,4 or 5) Z15
groups and optionally
substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z1 groups;
and
R3' is H.
Another specific group of compounds of formula I are compounds wherein:
R5 is selected from:
81
CA 02833377 2013-10-16
WO 2012/145728
PCT/US2012/034593
a) aryl, wherein aryl is optionally substituted with one or more (e.g. 1, 2
or 3) Z"
groups;
b) aryl, heteroaryl and heterocycle, wherein aryl, heteroaryl are
heterocycle, are
each independently substituted with one or more (e.g. 1, 2, 3,4 or 5) Z5
groups and optionally
substituted with one or more (e.g. 1,2, 3, 4 or 5) Zlgroups; and
c) aryl, heteroaryl and heterocycle, wherein aryl, heteroaryl and
heterocycle, are
each independently substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z15
groups and optionally
substituted with one or more (e.g. 1,2, 3,4 or 5) Zlgroups;
R3' is H; RI is H; and
2 i R s H or (Ci-C6)alkyl.
Another specific group of compounds of formula I are compounds wherein:
R5 is selected from:
a) aryl, wherein aryl is optionally substituted with one or more (e.g. 1, 2
or 3) Z"
groups;
b) aryl,
heteroaryl and heterocycle, wherein aryl, heteroaryl are heterocycle, are
each independently substituted with one or more (e.g. 1, 2, 3,4 or 5) Z5
groups and optionally
substituted with one or more (e.g. 1, 2, 3,4 or 5) Zigroups; and
c) aryl, heteroaryl and heterocycle, wherein aryl, heteroaryl and
heterocycle, are
each independently substituted with one or more (e.g. 1,2, 3,4 or 5) Z'5
groups and optionally
substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z1groups;
R3' is H; R1 is H;
R2 is H or (CI-C6)allcyl; and
R3 is -0(Ci-C6)alkyl.
Another specific value for R5 is selected from:
a) aryl,
heterocycle and heteroaryl, wherein aryl, heterocycle and heteroaryl are
each optionally substituted with one or more (e.g. 1, 2 or 3) Z11 groups;
b) aryl, heteroaryl and heterocycle, wherein aryl, heteroaryl are
heterocycle, are
each independently substituted with one or more (e.g. 1,2, 3,4 or 5) Z5 groups
and optionally
substituted with one or more (e.g. 1, 2, 3, 4 or 5) Zlgroups; and
c) aryl,
heteroaryl and heterocycle, wherein aryl, heteroaryl and heterocycle, are
each independently substituted with one or more (e.g. 1,2, 3,4 or 5) Z15
groups and optionally
substituted with one or more (e.g. 1, 2, 3, 4 or 5) Zigroups;
each Z11 is independently selected from Zw, -C(=0)-NH2, -C(=0)-NH(Ci-C4)alkyl,
-C(=0)-N((C1-C4)alky1)2, -C(=0)-aryl, -C(=0)-heterocycle and -C(=0)-
heteroaryl;
wherein each Z1 is independently selected from:
82
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
i) halo, oxo, thioxo, (C2-C6)alkenyl, (CI-C6)haloalkyl, (C3-
C7)cycloalkyl, (C3-C7)cycloalkyl-(Ci-C6)alkyl-, -OH, -0(C1-
C6)alkyl, -0(Ci-C6)haloalkyl, -SH, -S(Ci-C6)alkyl, -SO(C1-
C6)alkyl, -S02(CI-C6)allcyl, -NH2, -NH(Ci-C6)alkyl and
-N((Ci-C6)alicY1)2;
ii) (Ci-C6)alkyl substituted with -OH, -0-(Ci-C6)haloalkyl, or -0-(C1-
C6)allcyl; and
iii) aryl, heterocycle and heteroaryl, which aryl, heterocycle and
heteroaryl is
optionally substituted with halo, (C1-C6)alkyl or COOH; and
each Zu is independently selected from Z10, -C(=0)-NH2, -C(=0)-NH(Ci-C4)alkyl,
-C(=0)-N((CI-C4)alky1)2, -C(=0)-aryl, -C(=0)-heterocycle and -C(=0)-
heteroaryl.
Another specific group of compounds of formula I are compounds wherein:
R5 is selected from:
a) aryl, heterocycle and heteroaryl, wherein aryl, heterocycle and
heteroaryl are
each optionally substituted with one or more (e.g. 1, 2 or 3) Zu groups;
b) aryl, heteroaryl and heterocycle, wherein aryl, heteroaryl are
heterocycle, are
each independently substituted with one or more (e.g. 1, 2, 3,4 or 5) Z5
groups and optionally
substituted with one or more (e.g. 1, 2, 3, 4 or 5) Zlgroups; and
c) aryl, heteroaryl and heterocycle, wherein aryl, heteroaryl and
heterocycle, are
each independently substituted with one or more (e.g. 1, 2, 3,4 or 5) Z15
groups and optionally
substituted with one or more (e.g. 1,2, 3, 4 or 5) Z' groups;
R3' is H;
each Z1 is independently selected from:
i) halo, oxo, thioxo, (C2-C6)alkenyl, (CI-C6)haloalkyl, (C3-
C7)cycloalkyl, (C3-C7)cycloalkyl-(Ci-C6)alkyl-, -OH, -0(CI-
C6)alkyl, -0(C -C6)hal alkyl , -SH, -S(C -C6)alkyl, -SO(C1-
C6)alkyl, -S02(Ci-C6)alkyl, -NH2, -NH(Ci-C6)alkyl and
-N((CI-C6)alky1)2;
ii) (Ci-C6)alkyl substituted with -OH, -0-(C i-C6)haloalkyl, or -0-(C1-
C6)alkyl; and
iii) aryl, heterocycle and heteroaryl, which aryl, heterocycle and
heteroaryl is
optionally substituted with halo, (C1-C6)alkyl or COOH; and
each Zu is independently selected from Z1 , -C(=0)-NH2, -C(=0)-NH(Ci-C4)alkyl,
-C(=0)-N((C1-C4)alky1)2, -C(=0)-aryl, -C(=0)-heterocycle and -C(=0)-
heteroaryl.
Another specific group of compounds of formula I are compounds wherein:
83
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
R5 is selected from:
a) aryl, heterocycle and heteroaryl, wherein aryl, heterocycle and
heteroaryl are
each optionally substituted with one or more (e.g. 1,2 or 3) Z11 groups;
b) aryl, heteroaryl and heterocycle, wherein aryl, heteroaryl are
heterocycle, are
each independently substituted with one or more (e.g. 1, 2, 3,4 or 5) Z5
groups and optionally
substituted with one or more (e.g. 1, 2, 3, 4 or 5) Zlgroups; and
c) aryl, heteroaryl and heterocycle, wherein aryl, heteroaryl and
heterocycle, are
each independently substituted with one or more (e.g. 1, 2, 3,4 or 5) Z15
groups and optionally
substituted with one or more (e.g. 1,2, 3, 4 or 5) Zlgroups;
R3' is H; Ri is H;
R2 is H or (Ci-C6)alkyl;
each Z10 is independently selected from:
i) halo, oxo, thioxo, (C2-C6)alkenyl, (Ci-C6)haloalkyl, (C3-
C7)cycloalkyl, (C3-C7)cycloalkyl-(Ci-C6)alkyl-, -OH, -0(C1-
C6)alkyl, -0(Ci-C6)haloalkyl, -SH, -S(CI-C6)alkyl, -SO(C 1-
C6)alkyl, -S02(C1-C6)alkyl, -NH2, -NH(Ci-C6)alkyl and
-N((CI-C6)alky1)2;
ii) (C1-C6)alkyl substituted with -OH, -0-(C i-C6)haloalkyl, or -0-(C1-
C6)alkyl; and
iii) aryl, heterocycle and heteroaryl, which aryl, heterocycle and
heteroaryl is
optionally substituted with halo, (Ci-C6)alkyl or COOH; and
each Z11 is independently selected from Z1 , -C(=0)-NH2, -C(=0)-NH(Ci-
C4)alkyl,
-C(=0)-N((Ci-C4)alky1)2, -C(=0)-aryl, -C(=0)-heterocycle and -C(=0)-
heteroaryl.
Another specific group of compounds of formula I are compounds wherein:
R5 is selected from:
a) aryl, heterocycle and heteroaryl, wherein aryl, heterocycle and
heteroaryl are
each optionally substituted with one or more (e.g. 1, 2 or 3) Z11 groups;
b) aryl, heteroaryl and heterocycle, wherein aryl, heteroaryl are
heterocycle, are
each independently substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z5
groups and optionally
substituted with one or more (e.g. 1, 2, 3, 4 or 5) Zlgroups; and
c) aryl, heteroaryl and heterocycle, wherein aryl, heteroaryl and
heterocycle, are
each independently substituted with one or more (e.g. 1, 2, 3, 4 or 5) Z15
groups and optionally
substituted with one or more (e.g. 1, 2, 3, 4 or 5) Zlgroups;
R3' is H; R1 is H;
R2 is H or (C1-C6)alkyl;
84
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
R3 is -0(C1-C6)alkyl;
each Zi is independently selected from:
i) halo, oxo, thioxo, (C2-C6)alkenyl, (Ci-C6)haloalkyl, (C3-
C7)cycloalkyl, (C3-C7)cycloalkyl-(Ci-C6)alkyl-, -OH, -0(C1-
C6)alkyl, -0(Ci-C6)haloalkyl, -SH, -S(Ci-C6)alkyl, -SO(C1-
C6)alkyl, -S02(CI-C6)alkyl, -NH2, -NH(Ci-C6)alkyl and
-NOCI-C6)alicY02;
ii) (Ci-C6)alkyl substituted with -OH, -0-(Ci-C6)haloalkyl, or -0-(C1-
C6)alkyl; and
iii) aryl, heterocycle and heteroaryl, which aryl, heterocycle and
heteroaryl is
optionally substituted with halo, (Ci-C6)alkyl or COOH; and
each Z11 is independently selected from Z1 , -C(=0)-NH2, -C(=0)-NH(Ci-
C4)alkyl,
-C(=0)-N((Ci-C4)alky1)2, -C(=0)-aryl, -C(=0)-heterocycle and -C(=0)-
heteroaryl.
Another specific value for R5 is:
N \
'
0
N¨
0 \
N \
, '
CA 02833377 2013-10-16
WO 2012/145728
PCMJS2012/034593
F F F
N¨
\ /
F
1 , 1
,
,
. . F *
9
, ,=0 ,
,
i = 0*
F
¨0
=0 * CI
*
' 0
. 1 = . 1
CI
* * * *
0 0 0
lipN¨
,
,
Isrl\ s
¨
, ,
86
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
,N
_N F ----N N-
\
,
F -0
-N
CI \ /
41 1 or
'
In one embodiment of the invention the compound of formula I is selected from
a
compound of formulas Ia100-Ia145 (e.g. compounds Ia100, Ia101, Ial 02, Ia103,
Ia104, Ia105,
Ia106, Ia107, Ia108, Ia109, Ia110, Ial 11, Ia112, Ia113, Ia114, Ia115, Ia116,
Ia117, Ia118, Ia119,
Ia120, Ia121, Ia122, Ia123, Ia124, Ia125, Ia126, Ia127, Ia128, Ia129, Ia130,
Ia131, Ia132, Ia133,
Ia134, Ia135, Ia136, Ia137, Ia138, Ia139, Ia140, Ia141, Ia142, Ia143, Ia144,
Ia145):
F
0:c3
_
R4 R3 R3 N \ / \ R4 R3 R3' _
S OH S OH N \ /
R4 R3 R3'
\
N R20 N R20 S OH
R1 R1 \
N R2.0
Ia100
I R1
a102
Ia101
_N \ N_ F
\ / R4 R3 R3'
R4 R3 R3' FF R4 R3
R3'
-0 S OH S OH S OH
N R20 R20
\ \
\ R2 N N
R1 R1 R1
1a103 Ia104 Ia105
-o I
_ N.N
N \ / µ /
R4 R3 R3' R4 R3
R3'
R4 R3 R3'
F S OH S OH S OH
\ \
N R2 N R- R2
W W R1
Ia106
Ia107 Ia108
87
CA 02833377 2013-10-16
WO 2012/145728
PCT/US2012/034593
F
N¨
\ /f<f R4 R3 R3'
R4 R3 R3'
R4 R3 R3'
S OH S OH S OH
\ \
, 0 \
R20
N
N R2 , N R- , ,
R1 R1 R1
Ia109 Iall0 lain
F F
......
\
R4 R3 R3' N \ /
R4 R3 R3' /0 R4 R3 R3'
S OH S S 0
H
\ \
N R2 N R20 OH
111 ' R1 , R1
1a112 1a113 1a114
F
R4 R3 R3' el N-Nµ R4 R3 R3
S OH ¨ R4 R3 R3'
S 0
\ S O H
N R, 0
H
-
R1
R1
R1
Ia115
Ia116
Ia117
/¨ N-
-N N \ / R4 R3 R3
R4 R3 R3
S OH S OH
\
\ N R2
R1 '
R1
1a118 Ia119
88
CA 02833377 2013-10-16
WO 2012/145728 PCMJS2012/034593
R4 R3 R3' R4 R3 R3'
F
OH OH R4 R3 R3'
. \S \S
J111S OH
N R2 0. N R2 \
R20
R1 R1 N
R1
1a120 Ia121
F Ia122
I/ li
R4 R3 R3'
0 R4 R3 R3' R4 R3 R3'
OH
OH P. S OH . \S
0 0 \
R20 41 \S
N
N R2 N R20
R1 ,
R1 , R1 ,
1a123 Ia124 ¨0 Ia125
41 0 R4 R3 R3'
OH41 CI
0 R4 R3 R3' .
S OH
0 R4 R3 R3'
OH
N R2 = \ . \S
R1 , NR-,0
, N R20
R1 R1 ,
Ia126
1a127
Ia128
CI
11 . .
0 R4 R3 R3' 0 R2 R3 R3' 0 R4 R3 R3'
OH
) OH OH ii ,s
N/ __ ) e
44I \Ns R20 N R2 \¨ N R2
R1 ' R1 ,
Rl
Ia129 1a130 Ia131
(F___.)s R4 R3 R3'
R4 R3 R3
OH
e ,
N¨ N R2 \
S OH
R20 N
R1 ,
R1 ,
Ia132 Ia133
N' s
R4 R3 R3' ¨ R4 R3 R3'
¨0 S OH S OH
\ \
R1 ,
R1 '
Ia134 1a135
89
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
¨ \
N
\ / R4 R3 R3' R4 R3 R3' F
R4 R3 R3'
¨0 S OH F S OH \ S OH
I ' \
\
NR2
0
R20 S N
' N ,
R1 R1 R1 ,
Ia136 Ia137
Ia138
N¨
\ / R4 R3 R3'
R4 R3 R3' S OH
S OH
\
\ N R20
,
' Ri
R1
Ia139 Ia140
CI R4 R3 R3'
R4 R3 R3'
OH
. \S
N R20 \
S 0 OH
R1
R1
1a141 Ia142
F
¨N ¨0
\ / R4 R3 R3' R4 R3 R3'
S OH R4 R3 R3'
S OH
\ S OH
R-,0 \
N
R2 \
R
, N 0 and N 20
Rl R1 R1
Ia143 Ia144 Ia145
and salts thereof.
In one embodiment, the compounds of formula I are selected from the compounds
of
formulas Ia100-1a145 wherein:
RI is H; R2 is methyl, R3' is H; R3 is ¨0tBu; and
R4 is:
CI
S
and salts thereof.
In another embodiment, the compounds of formula I are selected from the
compounds of
formulas Ia100-1a145 wherein:
RI is H; R2 is methyl, R3' is H; R3 is ¨0tBu; and
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
R4 is:
0
JVVV
and salts thereof.
In another embodiment, the compounds of formula I are selected from the
compounds of
formulas Ia100-1a145 wherein:
RI is H; R2 is methyl, R3' is H; R3 is ¨0tBu; and
R4 is:
0
JVVV
and salts thereof.
In another embodiment of the invention, the compounds of formula I are
selected from
the compounds of formulas Ia100-1a145 wherein:
Rl is H; R2 is methyl, R3' is H; R3 is ¨0tBu; and
R4 is:
and salts thereof.
In one embodiment of the invention the compounds of formula I are selected
from the
compounds of formulas Ia100-1a145 wherein R3' is H; R3 is -0(Ci-C6)alkyl and
the
stereochemistry of the carbon bearing the R3 (-0(Ci-C6)alkyl) group is (S).
In another embodiment of the invention the compounds of formula I are selected
from
the compounds of formulas Ia100-1a145 wherein R3' is H; R3 is -0(Ci-C6)allcyl
and the
stereochemistry of the carbon bearing the R3 (-0(C1-C6)alkyl) group is (R).
In one embodiment of the invention, the compounds of formula I are selected
from:
91
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
CI
0
,N
¨N
OH F - OH
- OH
¨0
0 , 0
0 ,
0
-N 0
CY<
OH 0 0j<
OH
and
0
N
and salts thereof.
Prodrugs
In one embodiment, the invention provides for a prodrug of a compound of the
invention. The term "prodrug" as used herein refers to any compound that when
administered to
a biological system generates a compound of the invention that inhibits the
replication of HIV
("the active inhibitory compound"). The compound may be formed from the
prodrug as a result
of: (i) spontaneous chemical reaction(s), (ii) enzyme catalyzed chemical
reaction(s), (iii)
photolysis, and/or (iv) metabolic chemical reaction(s).
"Prodrug moiety" refers to a labile functional group which separates from the
active
inhibitory compound during metabolism, systemically, inside a cell, by
hydrolysis, enzymatic
cleavage, or by some other process (Bundgaard, Hans, "Design and Application
of Prodrugs" in A
Textbook of Drug Design and Development (1991), P. Krogsgaard-Larsen and H.
Bundgaard,
Eds. Harwood Academic Publishers, pp. 113-191). Enzymes which are capable of
an enzymatic
activation mechanism with the prodrug compounds of the invention include, but
are not limited
to, amidases, esterases, microbial enzymes, phospholipases, cholinesterases,
and phosphases.
Prodrug moieties can serve to enhance solubility, absorption and lipophilicity
to optimize drug
delivery, bioavailability and efficacy. A prodrug moiety may include an active
metabolite or
drug itself.
Exemplary prodrug moieties include the hydrolytically sensitive or labile
acyloxymethyl
esters ¨CH20C(=0)R99 and acyloxymethyl carbonates ¨CH20C(=0)0R99 where R99 is
C1¨C6
alkyl, Ci¨C6 substituted alkyl, C6¨C20 aryl or C6¨C20 substituted aryl. The
acyloxyalkyl ester
was first used as a prodrug strategy for carboxylic acids and then applied to
phosphates and
phosphonates by Farquhar et al. (1983)J Pharm. Sci. 72: 24; also US Patent
Nos. 4816570,
92
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
4968788, 5663159 and 5792756. Subsequently, the acyloxyalkyl ester was used to
deliver
phosphonic acids across cell membranes and to enhance oral bioavailability. A
close variant of
the acyloxyalkyl ester, the alkoxycarbonyloxyalkyl ester (carbonate), may also
enhance oral
bioavailability as a prodrug moiety in the compounds of the combinations of
the invention. An
exemplary acyloxyrnethyl ester is pivaloyloxymethoxy, (POM) ¨CH20C(=0)C(CH3)3.
An
exemplary acyloxymethyl carbonate prodrug moiety is pivaloyloxymethylcarbonate
(POC)
¨CH20C(=0)0C(CH3)3.
Aryl esters of phosphorus groups, especially phenyl esters, are reported to
enhance oral
bioavailability (De Lombaert et al. (1994)J Med Chem. 37: 498). Phenyl esters
containing a
carboxylic ester ortho to a phosphate have also been described (Khamnei and
Torrence, (1996)
J Med Chem. 39:4109-4115). Benzyl esters are reported to generate parent
phosphonic acids.
In some cases, substituents at the ortho- or para- position may accelerate the
hydrolysis. Benzyl
analogs with an acylated phenol or an alkylated phenol may generate the
phenolic compound
through the action of enzymes, e.g., esterases, oxidases, etc., which in turn
undergoes cleavage
at the benzylic C-0 bond to generate phosphoric acid and a quinone methide
intermediate.
Examples of this class of prodrugs are described by Mitchell et al. (1992) J
Chem. Soc. Perkin
Trans. 112345; Glazier WO 91/19721. Still other benzylic prodrugs have been
described
containing a carboxylic ester-containing group attached to the benzylic
methylene (Glazier WO
91/19721). Thio-containing prodrugs are reported to be useful for the
intracellular delivery of
phosphonate drugs. These proesters contain an ethylthio group in which the
thiol group is either
esterified with an acyl group or combined with another thiol group to form a
disulfide.
Deesterification or reduction of the disulfide generates the free thio
intermediate which
subsequently breaks down to the phosphoric acid and episulfide (Puech et al.
(1993) Antiviral
Res., 22: 155-174; Benzaria et al. (1996)J Med. Chem. 39: 4958).
Combination Therapy
In one embodiment, the invention provides for a method for treating an HIV
infection,
comprising administering to a patient in need thereof a therapeutically
effective amount of a
compound of the invention, or a pharmaceutically acceptable salt, thereof, in
combination with a
.. therapeutically effective amount of one or more additional therapeutic
agents which are suitable
for treating an HIV infection.
In one embodiment, the invention provides pharmaceutical compositions
comprising a
compound of the present invention, or a pharmaceutically acceptable salt
thereof, in
combination with at least one additional therapeutic agent, and a
pharmaceutically acceptable
93
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
carrier. For example, the therapeutic agent used in combination with the
compound of the
present invention can be any anti-HIV agent.
In one embodiment, the invention provides pharmaceutical compositions
comprising a
compound of the present invention, or a pharmaceutically acceptable salt
thereof, in
combination with at least one additional therapeutic agent selected from the
group consisting of
HIV protease inhibiting compounds, HIV non-nucleoside inhibitors of reverse
transcriptase,
HIV nucleoside inhibitors of reverse transcriptase, HIV nucleotide inhibitors
of reverse
transcriptase, HIV integrase inhibitors, gp41 inhibitors, CXCR4 inhibitors,
gp120 inhibitors,
CCR5 inhibitors, capsid polymerization inhibitors, and other drug for treating
HIV, and
.. combinations thereof, and a pharmaceutically acceptable carrier.
In another embodiment, the invention provides pharmaceutical compositions
comprising
a compound of the present invention, or a pharmaceutically acceptable salt
thereof, in
combination with at least one additional therapeutic agent selected from the
group consisting of:
(1) HIV protease inhibiting compounds selected from the group consisting of
.. amprenavir, atazanavir, fosamprenavir, indinavir, lopinavir, ritonavir,
nelfinavir, saquinavir,
tipranavir, brecanavir, darunavir, TMC-126, TMC-114, mozenavir (DMP-450), JE-
2147
(AG1776), L-756423, R00334649, KNI-272, DPC-681, DPC-684, GW640385X, DG17, PPL-
100, DG35, and AG 1859;
(2) HIV non-nucleoside inhibitors of reverse transcriptase selected from the
group
consisting of capravirine, emivirine, delaviridine, efavirenz, nevirapine, (+)
calanolide A,
etravirine, GW5634, DPC-083, DPC-961, DPC-963, MIV-150, and TMC-120,
rilpivirene, BILR
355 BS, VRX 840773, UK-453061, and RDEA806;
(3) HIV nucleoside inhibitors of reverse transcriptase selected from the group
consisting
of zidovudine, emtricitabine, didano sine, stavudine, zalcitabine, lamivudine,
abacavir,
amdoxovir, elvucitabine, alovudine, MIV-210, -FTC, D-d4FC, emtricitabine,
phosphazide,
fozivudine tidoxil, apricitibine (AVX754), amdoxovir, KP-1461, and fosalvudine
tidoxil
(formerly HDP 99.0003),;
(4) HIV nucleotide inhibitors of reverse transcriptase selected from the group
consisting
of tenofovir, tenofovir disoproxil fumarate, GS-7340 (Gilead Sciences),
adefovir, adefovir
dipivoxil, CMX-001 (Chimerix) or CMX-157 (Chimerix)
(5) HIV integrase inhibitors selected from the group consisting of curcumin,
derivatives
of curcumin, chicoric acid, derivatives of chicoric acid, 3,5-dicaffeoylquinic
acid, derivatives of
3,5-dicaffeoylquinic acid, aurintricarboxylic acid, derivatives of
aurintricarboxylic acid, caffeic
acid phenethyl ester, derivatives of caffeic acid phenethyl ester, tyrphostin,
derivatives of
94
CA 02833377 2013-10-16
WO 2012/145728 PCMJS2012/034593
tyrphostin, quercetin, derivatives of quercetin, S-1360, AR-177, L-870812, and
L-870810,
raltegravir, BMS-538158, GSK364735C, BMS-707035, MK-2048, BA 011 and
dolutegravir;
(6) gp41 inhibitors selected from the group consisting of enfuvirtide,
sifuvirtide,
FB006M, and TRI-1144;
(7) the CXCR4 inhibitor AMD-070;
(8) the entry inhibitor SPO1A;
(9) the gp120 inhibitor BMS-488043;
(10) the G6PD and NADH-oxidase inhibitor itnmunitin;
(11) CCR5 inhibitors selected from the group consisting of aplaviroc,
vicriviroc,
maraviroc, PRO-140, INCB15050, PF-232798 (Pfizer), and CCR5mAb004;
(12) other drugs for treating HIV selected from the group consisting of BAS-
100, SPI-
452, REP 9, SP-01A, TNX-355, DES6, ODN-93, ODN-112, VGV-1, PA-457 (bevirimat),
HRG214, VGX-410, KD-247, AMZ 0026, CYT 99007A-221 HIV, DEBIO-025, BAY 50-4798,
MDX010 (ipilimumab), PBS 119, ALG 889, and PA-1050040 (PA-040).
In another embodiment, the invention provides pharmaceutical compositions
comprising
a compound of the present invention, or a pharmaceutically acceptable salt
thereof, in
combination with two, three, four or more additional therapeutic agents. For
example, a
compound of the present invention, or a pharmaceutically acceptable salt,
thereof, is combined
with two, three, four or more additional therapeutic agents selected from the
classes of HIV
protease inhibiting compounds, HIV non-nucleoside inhibitors of reverse
transcriptase, HIV
nucleoside inhibitors of reverse transcriptase, HIV nucleotide inhibitors of
reverse transcriptase,
HIV integrase inhibitors, gp41 inhibitors, CXCR4 inhibitors, gp120 inhibitors,
CCR5 inhibitors,
capsid polymerization inhibitors and other drug for treating HIV. The two,
three four or more
additional therapeutic agents can be different therapeutic agents selected
from the same class of
therapeutic agents, or they can be selected from different classes of
therapeutic agents.
In one embodiment, the invention provides for a combination pharmaceutical
agent
comprising:
a) a compound of the invention (e.g. a compound of Formula I), or
a
pharmaceutically acceptable salt, thereof; and
b) at least one additional active agent which is suitable for treating an
HIV infection.
In another embodiment, the invention provides a combination pharmaceutical
agent
comprising:
a) a compound of the invention (e.g. a compound of Formula I), or
a
phaimaceutically acceptable salt thereof; and
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
b) at least one additional therapeutic agent selected from the
group consisting of
HIV protease inhibiting compounds, HIV non-nucleoside inhibitors of reverse
transcriptase,
HIV nucleoside inhibitors of reverse transcriptase, HIV nucleotide inhibitors
of reverse
transcriptase, HIV integrase inhibitors, gp41 inhibitors, CXCR4 inhibitors,
gp120 inhibitors,
CCR5 inhibitors, capsid polymerization inhibitors and other drug for treating
HIV.
It is also possible to combine any compound of the invention with one or more
other
active therapeutic agents in a unitary dosage form for simultaneous or
sequential administration
to a patient. The combination therapy may be administered as a simultaneous or
sequential
regimen. When administered sequentially, the combination may be administered
in two or more
administrations.
It is also possible to co-administer a compound of the invention with one or
more other
active therapeutic agents. Co-administration of a compound of the invention
with one or more
other active therapeutic agents generally refers to simultaneous or sequential
administration of a
compound of the invention and one or more other active therapeutic agents,
such that
therapeutically effective amounts of the compound of the invention and one or
more other active
therapeutic agents are both present in the body of the patient.
Co-administration includes administration of unit dosages of the compounds of
the
invention before or after administration of unit dosages of one or more other
active therapeutic
agents, for example, administration of the compounds of the invention within
seconds, minutes,
or hours of the administration of one or more other active therapeutic agents.
For example, a
unit dose of a compound of the invention can be administered first, followed
within seconds or
minutes by administration of a unit dose of one or more other active
therapeutic agents.
Alternatively, a unit dose of one or more other therapeutic agents can be
administered first,
followed by administration of a unit dose of a compound of the invention
within seconds or
.. minutes. In some cases, it may be desirable to administer a unit dose of a
compound of the
invention first, followed, after a period of hours (e.g., 1-12 hours), by
administration of a unit
dose of one or more other active therapeutic agents. In other cases, it may be
desirable to
administer a unit dose of one or more other active therapeutic agents first,
followed, after a
period of hours (e.g., 1-12 hours), by administration of a unit dose of a
compound of the
invention.
The combination therapy may provide "synergy" and "synergistic effect", i.e.
the effect
achieved when the active ingredients used together is greater than the sum of
the effects that
results from using the compounds separately. A synergistic effect may be
attained when the
active ingredients are: (1) co-formulated and administered or delivered
simultaneously in a
combined formulation; (2) delivered by alternation or in parallel as separate
formulations; or (3)
96
CA 02833377 2013-10-16
WO 2012/145728 PCMJS2012/034593
by some other regimen. When delivered in alternation therapy, a synergistic
effect may be
attained when the compounds are administered or delivered sequentially, e.g.,
in separate tablets,
pills or capsules, or by different injections in separate syringes. In
general, during alternation
therapy, an effective dosage of each active ingredient is administered
sequentially, i.e. serially,
whereas in combination therapy, effective dosages of two or more active
ingredients are
administered together.
In yet another embodiment, the present application provides a method for
treating an
HIV infection comprising administering to a patient in need thereof a
therapeutically effective
amount of a compound of the present invention, or a pharmaceutically
acceptable salt thereof, in
combination with a therapeutically effective amount of one or more additional
therapeutic
agents selected from the group consisting of HIV protease inhibiting
compounds, HIV non-
nucleoside inhibitors of reverse transcriptase, HIV nucleoside inhibitors of
reverse transcriptase,
HIV nucleotide inhibitors of reverse transcriptase, HIV integrase inhibitors,
gp41 inhibitors,
CXCR4 inhibitors, gp120 inhibitors, CCR5 inhibitors, capsid polymerization
inhibitors, and
other drug for treating HIV.
In yet another embodiment, the present application provides a method for
treating an
HIV infection comprising administering to a patient in need thereof a
therapeutically effective
amount of a compound of the present invention, or a pharmaceutically
acceptable salt, thereof,
in combination with a therapeutically effective amount of one or more
additional therapeutic
agents selected from the group consisting of:
(1) HIV protease inhibiting compounds selected from the group consisting of
amprenavir, atazanavir, fosamprenavir, indinavir, lopinavir, ritonavir,
nelfinavir, saquinavir,
tipranavir, brecanavir, darunavir, TMC-126, TMC-114, mozenavir (DMP-450), JE-
2147
(AG1776), L-756423, R00334649, KNI-272, DPC-681, DPC-684, GW640385X, DG17, PPL-
100, DG35, and AG 1859;
(2) HIV non-nucleoside inhibitors of reverse transcriptase selected from the
group
consisting of capravirine, emivirine, delaviridine, efavirenz, nevirapine, (+)
calanolide A,
etravirine, GW5634, DPC-083, DPC-961, DPC-963, MN-150, and TMC-120,
rilpivirene, BILR
355 BS, VRX 840773, UK-453061, and RDEA806;
(3) HIV nucleoside inhibitors of reverse transcriptase selected from the group
consisting
of zidovudine, emtricitabine, didano sine, stavudine, zalcitabine, lamivudine,
abacavir,
amdoxovir, elvucitabine, alovudine, MIV-210, -FTC, D-d4FC, emtricitabine,
phosphazide,
fozivudine tidoxil, apricitibine (AVX754), amdoxovir, KP-1461, and fosalvudine
tidoxil
(formerly HDP 99.0003),;
97
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
(4) HIV nucleotide inhibitors of reverse transcriptase selected from the group
consisting
of tenofovir, tenofovir disoproxil fumarate, GS-7340 (Gilead Sciences),
adefovir, adefovir
dipivoxil, CMX-001 (Chimerix) or CMX-157 (Chimerix)
(5) HIV integrase inhibitors selected from the group consisting of curcumin,
derivatives
of curcumin, chicoric acid, derivatives of chicoric acid, 3,5-dicaffeoylquinic
acid, derivatives of
3,5-dicaffeoylquinic acid, aurintricarboxylic acid, derivatives of
aurintricarboxylic acid, caffeic
acid phenethyl ester, derivatives of caffeic acid phenethyl ester, tyrphostin,
derivatives of
tyrphostin, quercetin, derivatives of quercetin, S-1360, AR-177, L-870812, and
L-870810,
raltegravir, BMS-538158, GSK364735C, BMS-707035, MK-2048, BA 011 and
dolutegravir;
(6) gp41 inhibitors selected from the group consisting of enfuvirtide,
sifuvirtide,
FB006M, and TRI-1144;
(7) the CXCR4 inhibitor AMD-070;
(8) the entry inhibitor SPO1A;
(9) the gp120 inhibitor BMS-488043;
(10) the G6PD and NADH-oxidase inhibitor immunitin;
(11) CCR5 inhibitors selected from the group consisting of aplaviroc,
vicriviroc,
maraviroc, PRO-140, INCB15050, PF-232798 (Pfizer), and CCR5mAb004;
(12) other drugs for treating HIV selected from the group consisting of BAS-
100, SPI-
452, REP 9, SP-01A, TNX-355, DES6, ODN-93, ODN-112, VGV-1, PA-457 (bevirimat),
HRG214, VGX-410, KD-247, AMZ 0026, CYT 99007A-221 HIV, DEB10-025, BAY 50-4798,
MDX010 (ipilimumab), PBS 119, ALG 889, and PA-1050040 (PA-040).
Pharmaceutical Formulations
The compounds of this invention are formulated with conventional carriers and
excipients, which will be selected in accord with ordinary practice. Tablets
will contain
excipients, glidants, fillers, binders and the like. Aqueous formulations are
prepared in sterile
form, and when intended for delivery by other than oral administration
generally will be
isotonic. All formulations will optionally contain excipients such as those
set forth in the
Handbook of Pharmaceutical Excipients (1986). Excipients include ascorbic acid
and other
antioxidants, chelating agents such as EDTA, carbohydrates such as dextrin,
hydroxyalkylcellulose, hydroxyalkylmethylcellulose, stearic acid and the like.
The pH of the
formulations ranges from about 3 to about 11, but is ordinarily about 7 to 10.
While it is possible for the active ingredients to be administered alone it
may be
preferable to present them as pharmaceutical formulations. The formulations,
both for
veterinary and for human use, of the invention comprise at least one active
ingredient, as above
98
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
defined, together with one or more acceptable carriers and optionally other
therapeutic
ingredients. The carrier(s) must be "acceptable" in the sense of being
compatible with the other
ingredients of the formulation and physiologically innocuous to the recipient
thereof.
The formulations include those suitable for the foregoing administration
routes. The
formulations may conveniently be presented in unit dosage form and may be
prepared by any of
the methods well known in the art of pharmacy. Techniques and formulations
generally are
found in Remington's Pharmaceutical Sciences (Mack Publishing Co., Easton,
PA). Such
methods include the step of bringing into association the active ingredient
with the carrier which
constitutes one or more accessory ingredients. In general the formulations are
prepared by
uniformly and intimately bringing into association the active ingredient with
liquid carriers or
finely divided solid carriers or both, and then, if necessary, shaping the
product.
Formulations of the present invention suitable for oral administration may be
presented
as discrete units such as capsules, cachets or tablets each containing a
predetermined amount of
the active ingredient; as a powder or granules; as a solution or a suspension
in an aqueous or
non-aqueous liquid; or as an oil-in-water liquid emulsion or a water-in-oil
liquid emulsion. The
active ingredient may also be administered as a bolus, electuary or paste.
A tablet is made by compression or molding, optionally with one or more
accessory
ingredients. Compressed tablets may be prepared by compressing in a suitable
machine the
active ingredient in a free-flowing form such as a powder or granules,
optionally mixed with a
binder, lubricant, inert diluent, preservative, surface active or dispersing
agent. Molded tablets
may be made by molding in a suitable machine a mixture of the powdered active
ingredient
moistened with an inert liquid diluent. The tablets may optionally be coated
or scored and
optionally are formulated so as to provide slow or controlled release of the
active ingredient
therefrom.
For administration to the eye or other external tissues e.g., mouth and skin,
the
formulations are preferably applied as a topical ointment or cream containing
the active
ingredient(s) in an amount of, for example, 0.075 to 20% w/w (including active
ingredient(s) in
a range between 0.1% and 20% in increments of 0.1% w/w such as 0.6% w/w, 0.7%
w/w, etc.),
preferably 0.2 to 15% w/w and most preferably 0.5 to 10% w/w. When formulated
in an
ointment, the active ingredients may be employed with either a paraffinic or a
water-miscible
ointment base. Alternatively, the active ingredients may be formulated in a
cream with an oil-
in-water cream base.
If desired, the aqueous phase of the cream base may include, for example, at
least 30%
w/w of a polyhydric alcohol, i.e. an alcohol having two or more hydroxyl
groups such as
propylene glycol, butane 1,3-diol, mannitol, sorbitol, glycerol and
polyethylene glycol
99
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
(including PEG 400) and mixtures thereof. The topical formulations may
desirably include a
compound which enhances absorption or penetration of the active ingredient
through the skin or
other affected areas. Examples of such dermal penetration enhancers include
dimethyl
sulphoxide and related analogs.
The oily phase of the emulsions of this invention may be constituted from
known
ingredients in a known manner. While the phase may comprise merely an
emulsifier (otherwise
known as an emulgent), it desirably comprises a mixture of at least one
emulsifier with a fat or
an oil or with both a fat and an oil. Preferably, a hydrophilic emulsifier is
included together with
a lipophilic emulsifier which acts as a stabilizer. It is also preferred to
include both an oil and a
fat. Together, the emulsifier(s) with or without stabilizer(s) make up the so-
called emulsifying
wax, and the wax together with the oil and fat make up the so-called
emulsifying ointment base
which forms the oily dispersed phase of the cream formulations.
Emulgents and emulsion stabilizers suitable for use in the formulation of the
invention
include Tween 60, Span 80, cetostearyl alcohol, benzyl alcohol, myristyl
alcohol, glyceryl
mono-stearate and sodium lauryl sulfate.
The choice of suitable oils or fats for the formulation is based on achieving
the desired
cosmetic properties. The cream should preferably be a non-greasy, non-staining
and washable
product with suitable consistency to avoid leakage from tubes or other
containers. Straight or
branched chain, mono- or dibasic alkyl esters such as di-isoadipate, isocetyl
stearate, propylene
glycol diester of coconut fatty acids, isopropyl myristate, decyl oleate,
isopropyl palmitate, butyl
stearate, 2-ethylhexyl palmitate or a blend of branched chain esters known as
Crodamol CAP
may be used, the last three being preferred esters. These may be used alone or
in combination
depending on the properties required. Alternatively, high melting point lipids
such as white soft
paraffin and/or liquid paraffin or other mineral oils are used.
Pharmaceutical formulations according to the present invention comprise one or
more
compounds of the invention together with one or more pharmaceutically
acceptable carriers or
excipients and optionally other therapeutic agents. Pharmaceutical
formulations containing the
active ingredient may be in any form suitable for the intended method of
administration. When
used for oral use for example, tablets, troches, lozenges, aqueous or oil
suspensions, dispersible
powders or granules, emulsions, hard or soft capsules, syrups or elixirs may
be prepared.
Compositions intended for oral use may be prepared according to any method
known to the art
for the manufacture of pharmaceutical compositions and such compositions may
contain one or
more agents including sweetening agents, flavoring agents, coloring agents and
preserving
agents, in order to provide a palatable preparation. Tablets containing the
active ingredient in
100
CA 02833377 2013-10-16
WO 2012/145728
PCT/US2012/034593
admixture with non-toxic pharmaceutically acceptable excipient which are
suitable for
manufacture of tablets are acceptable. These excipients may be, for example,
inert diluents,
such as calcium or sodium carbonate, lactose, lactose monohydrate,
croscarmellose sodium,
povidone, calcium or sodium phosphate; granulating and disintegrating agents,
such as maize
starch, or alginic acid; binding agents, such as cellulose, microcrystalline
cellulose, starch,
gelatin or acacia; and lubricating agents, such as magnesium stearate, stearic
acid or talc.
Tablets may be uncoated or may be coated by known techniques including
microencapsulation
to delay disintegration and adsorption in the gastrointestinal tract and
thereby provide a
sustained action over a longer period. For example, a time delay material such
as glyceryl
monostearate or glyceryl distearate alone or with a wax may be employed.
Formulations for oral use may be also presented as hard gelatin capsules where
the active
ingredient is mixed with an inert solid diluent, for example calcium phosphate
or kaolin, or as
soft gelatin capsules wherein the active ingredient is mixed with water or an
oil medium, such as
peanut oil, liquid paraffin or olive oil.
Aqueous suspensions of the invention contain the active materials in admixture
with
excipients suitable for the manufacture of aqueous suspensions. Such
excipients include a
suspending agent, such as sodium carboxymethylcellulose, methylcellulose,
hydroxypropyl
methylcelluose, sodium alginate, polyvinylpyrrolidone, gum tragacanth and gum
acacia, and
dispersing or wetting agents such as a naturally occurring phosphatide (e.g.,
lecithin), a
condensation product of an alkylene oxide with a fatty acid (e.g.,
polyoxyethylene stearate), a
condensation product of ethylene oxide with a long chain aliphatic alcohol
(e.g.,
heptadecaethyleneoxycetanol), a condensation product of ethylene oxide with a
partial ester
derived from a fatty acid and a hexitol anhydride (e.g., polyoxyethylene
sorbitan monooleate).
The aqueous suspension may also contain one or more preservatives such as
ethyl or n-propyl p-
hydroxy-benzoate, one or more coloring agents, one or more flavoring agents
and one or more
sweetening agents, such as sucrose or saccharin.
Oil suspensions may be formulated by suspending the active ingredient in a
vegetable
oil, such as arachis oil, olive oil, sesame oil or coconut oil, or in a
mineral oil such as liquid
paraffin. The oral suspensions may contain a thickening agent, such as
beeswax, hard paraffin
or cetyl alcohol. Sweetening agents, such as those set forth above, and
flavoring agents may be
added to provide a palatable oral preparation. These compositions may be
preserved by the
addition of an antioxidant such as ascorbic acid.
Dispersible powders and granules of the invention suitable for preparation of
an aqueous
suspension by the addition of water provide the active ingredient in admixture
with a dispersing
or wetting agent, a suspending agent, and one or more preservatives. Suitable
dispersing or
101
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
wetting agents and suspending agents are exemplified by those disclosed above.
Additional
excipients, for example sweetening, flavoring and coloring agents, may also be
present.
The pharmaceutical compositions of the invention may also be in the form of
oil-in-
water emulsions. The oily phase may be a vegetable oil, such as olive oil or
arachis oil, a
mineral oil, such as liquid paraffin, or a mixture of these. Suitable
emulsifying agents include
naturally-occurring gums, such as gum acacia and gum tragacanth, naturally
occurring
phosphatides, such as soybean lecithin, esters or partial esters derived from
fatty acids and
hexitol anhydrides, such as sorbitan monooleate, and condensation products of
these partial
esters with ethylene oxide, such as polyoxyethylene sorbitan monooleate. The
emulsion may
also contain sweetening and flavoring agents. Syrups and elixirs may be
formulated with
sweetening agents, such as glycerol, sorbitol or sucrose. Such formulations
may also contain a
demulcent, a preservative, a flavoring or a coloring agent.
The pharmaceutical compositions of the invention may be in the form of a
sterile
injectable preparation, such as a sterile injectable aqueous or oleaginous
suspension. This
suspension may be formulated according to the known art using those suitable
dispersing or
wetting agents and suspending agents which have been mentioned above. The
sterile injectable
preparation may also be a sterile injectable solution or suspension in a non-
toxic parenterally
acceptable diluent or solvent, such as a solution in 1,3-butane-diol or
prepared as a lyophilized
powder. Among the acceptable vehicles and solvents that may be employed are
water, Ringer's
solution and isotonic sodium chloride solution. In addition, sterile fixed
oils may conventionally
be employed as a solvent or suspending medium. For this purpose any bland
fixed oil may be
employed including synthetic mono- or diglycerides. In addition, fatty acids
such as oleic acid
may likewise be used in the preparation of injectables.
The amount of active ingredient that may be combined with the carrier material
to
produce a single dosage form will vary depending upon the host treated and the
particular mode
of administration. For example, a time-release formulation intended for oral
administration to
humans may contain approximately 1 to 1000 mg of active material compounded
with an
appropriate and convenient amount of carrier material which may vary from
about 5 to about
95% of the total compositions (weight:weight). The pharmaceutical composition
can be
.. prepared to provide easily measurable amounts for administration. For
example, an aqueous
solution intended for intravenous infusion may contain from about 3 to 500 fig
of the active
ingredient per milliliter of solution in order that infusion of a suitable
volume at a rate of about
30 mL/hr can occur.
Formulations suitable for administration to the eye include eye drops wherein
the active
.. ingredient is dissolved or suspended in a suitable carrier, especially an
aqueous solvent for the
102
CA 02833377 2013-10-16
WO 2012/145728 PCMJS2012/034593
active ingredient. The active ingredient is preferably present in such
formulations in a
concentration of 0.5 to 20%, advantageously 0.5 to 10% particularly about 1.5%
w/w.
Formulations suitable for topical administration in the mouth include lozenges
comprising the active ingredient in a flavored basis, usually sucrose and
acacia or tragacanth;
pastilles comprising the active ingredient in an inert basis such as gelatin
and glycerin, or
sucrose and acacia; and mouthwashes comprising the active ingredient in a
suitable liquid
carrier.
Formulations for rectal administration may be presented as a suppository with
a suitable
base comprising for example cocoa butter or a salicylate.
Formulations suitable for intrapulmonary or nasal administration have a
particle size for
example in the range of 0.1 to 500 microns (including particle sizes in a
range between 0.1 and
500 microns in increments microns such as 0.5, 1, 30 microns, 35 microns,
etc.), which is
administered by rapid inhalation through the nasal passage or by inhalation
through the mouth
so as to reach the alveolar sacs. Suitable formulations include aqueous or
oily solutions of the
active ingredient. Formulations suitable for aerosol or dry powder
administration may be
prepared according to conventional methods and may be delivered with other
therapeutic agents.
Formulations suitable for vaginal administration may be presented as
pessaries, tampons,
creams, gels, pastes, foams or spray formulations containing in addition to
the active ingredient
such carriers as are known in the art to be appropriate.
Formulations suitable for parenteral administration include aqueous and non-
aqueous
sterile injection solutions which may contain anti-oxidants, buffers,
bacteriostats and solutes
which render the formulation isotonic with the blood of the intended
recipient; and aqueous and
non-aqueous sterile suspensions which may include suspending agents and
thickening agents.
The formulations are presented in unit-dose or multi-dose containers, for
example sealed
ampoules and vials, and may be stored in a freeze-dried (lyophilized)
condition requiring only
the addition of the sterile liquid carrier, for example water for injection,
immediately prior to
use. Extemporaneous injection solutions and suspensions are prepared from
sterile powders,
granules and tablets of the kind previously described. Preferred unit dosage
formulations are
those containing a daily dose or unit daily sub-dose, as herein above recited,
or an appropriate
fraction thereof, of the active ingredient.
It should be understood that in addition to the ingredients particularly
mentioned above
the formulations of this invention may include other agents conventional in
the art having regard
to the type of formulation in question, for example those suitable for oral
administration may
include flavoring agents.
The invention further provides veterinary compositions comprising at least one
active
103
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
ingredient as above defined together with a veterinary carrier.
Veterinary carriers are materials useful for the purpose of administering the
composition
and may be solid, liquid or gaseous materials which are otherwise inert or
acceptable in the
veterinary art and are compatible with the active ingredient. These veterinary
compositions may
be administered orally, parenterally or by any other desired route.
Compounds of the invention can also be formulated to provide controlled
release of the
active ingredient to allow less frequent dosing or to improve the
pharmacokinetic or toxicity
profile of the active ingredient. Accordingly, the invention also provides
compositions
comprising one or more compounds of the invention formulated for sustained or
controlled
release.
Effective dose of active ingredient depends at least on the nature of the
condition being
treated, toxicity, whether the compound is being used prophylactically (lower
doses), the method
of delivery, and the pharmaceutical formulation, and will be determined by the
clinician using
conventional dose escalation studies.
Routes of Administration
One or more compounds of the invention (herein referred to as the active
ingredients) are
administered by any route appropriate to the condition to be treated. Suitable
routes include
oral, rectal, nasal, topical (including buccal and sublingual), vaginal and
parenteral (including
subcutaneous, intramuscular, intravenous, intradermal, intrathecal and
epidural), and the like. It
will be appreciated that the preferred route may vary with for example the
condition of the
recipient. An advantage of the compounds of this invention is that they are
orally bioavailable
and can be dosed orally.
The antiviral properties of a compound of the invention may be determined
using Test A
described below.
Test A: Antiviral Assays in MT4 Cells
For the antiviral assay utilizing MT-4 cells, 0.4 uL of 189X test
concentration of 3-fold
serially diluted compound in DMSO was added to 40 p.L of cell growth medium
(RPMI 1640,
10%FBS, 1% penicilline/Streptomycine, 1% L-Glutamine, 1% HEPES) in each well
of 384-well
assay plates (10 concentrations) in quadruplicate.
1 mL aliquots of 2x10e6 MT-4 cells are pre-infected for 1 and 3 hrs
respectively, @
37 C with 25 uL (MT4) or of either cell growth medium (mock-infected) or a
fresh 1:250
dilution of an HIV-IIIb concentrated ABI stock (0.004m.o.i. for MT4 cells).
Infected and
104
CA 02833377 2013-10-16
WO 2012/145728 PCMJS2012/034593
uninfected cells are diluted in cell growth medium and 35 uL of 2000 (for MT4)
cells is added to
each well of the assay plates.
Assay plates were then incubated in a 37 C incubator. After 5 days of
incubation, 25 Ill
of 2X concentrated CellTiter-GloTm Reagent (catalog # G7573, Promega
Biosciences, Inc.,
Madison, WI) was added to each well of the assay plate. Cell lysis was carried
out by
incubating at room temperature for 2-3 min and then chemiluminescence was read
using the
Envision reader (PerkinElmer).
Compounds of the present invention demonstrate antiviral activity in this
assay (Test A)
as depicted in the table below. Accordingly, the compounds of the invention
may be useful for
treating the proliferation of the HIV virus, treating AIDS or delaying the
onset of AIDS or ARC
symptoms
105
CA 02833377 2013-10-16
WO 2012/145728
PCT/US2012/034593
Compound Number EC50 (nM)
5L 2950
7 257
8K 641
9 118
121
12 113
14 718
14b 480
21 14.9
22 170
35 12.7
36 6211
40 722
41 923
42 10.3
43 5090
44 18.7
45 67.0
46 16.8
47 26500
48 67.0
49 13300
50 52.8
51 5250
52 53.4
53 37500
54 274
55 53000
56 62.4
57 147
58 3520
106
CA 02833377 2013-10-16
WO 2012/145728
PCT/US2012/034593
59 149
60 34510
61 987
62 4880
63 351
64 53000
66 22.5
68 292
70 80.8
71 984
72 29.8
73 52.2
74 650
76 26
78 726
79 45900
80 136
81 27400
82 40.2
83 93.2
84 14900
85 66.4
86 61.7
87 1570
88 13.3
89 36.6
92 353
93 1420
95 655
97 1240
98 2510
99 560
107
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
In certain embodiments, the compounds demonstrate an EC50 of < 50 M. In
certain
embodiments, the compounds demonstrate an EC50 of < 30 M. In certain
embodiments, the
compounds demonstrate an EC50 of < 10 M. In certain embodiments, the
compounds
demonstrate an EC50 of < 1 M.
The specific pharmacological responses observed may vary according to and
depending
on the particular active compound selected or whether there are present
pharmaceutical carriers,
as well as the type of formulation and mode of administration employed, and
such expected
variations or differences in the results are contemplated in accordance with
practice of the
present invention.
The invention has been described with reference to various specific and
preferred
embodiments and techniques. However, it should be understood that many
variations and
modifications may be made while remaining within the spirit and scope of the
invention.
The invention will now be illustrated by the following non-limiting Examples.
Example 1: Preparation of tert-butoxy-[7-chloro-5-(4-chloro-pheny1)-2-methyl-
quinolin-6-y1]-
acetic acid (5L):
108
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
0 H2N 0
H2N4
S 0
N
5A
OH
<
----' N
N
5C
5B
CI
Br
S OH
N
µ
S OH
N
5D
5E
CI CI CI
0 OH
S Ai OTf , S --a.- S - OH
<\
N lir N N
5F 5G 5H
CI CI
OH yi< 0-<
,.. /
Y<
0 0
N N
51 5J
CI CI
0- 0*"
S : OH s 7 OH
--,...-
0
N N
5K 5L
(S)-2-tert-butoxy-2-(7-(4-chloropheny1)-5-methylbenzo[d]thiazol-6-ypacetic
acid (5L):
A stock solution of periodic acid/chromium trioxide was prepared according to
WO 99/52850 by
dissolving periodic acid (11.4g, 50.0 mmol) and chromium trioxide (23 mg, 1.2
mol %) in wet
109
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
acetonitrile (0.75% H20) to a volume of 114 mL. This stock solution (0.090 mL)
was added to a
solution of (S)-2-tert-butoxy-2-(7-(4-chloropheny1)-5-methylbenzo[d]thiazol-6-
ypethanol (5K)
(5 mg, 0.013 mmol) in wet acetonitrile (1.0 mL, 0.75% H20) at 0 C. Reaction
mixture was
stirred for 0.5 hour at 0 C. Then more stock solution (0.2 ml) was added and
the reaction
mixture was stirred for 1 h at 0 C . The reaction mixture was filtered and
purified by reverse
phase HPLC (Gemini, 10 to 95% ACN/H20 + 0.1% TFA). Product lyophilized to give
a white
powder. 1H-NMR: 300 MHz, (CD30D) 5: 9.06 (s, 1H), 7.78 (s, 1H), 7.57-7.42 (m,
4H), 5.16
(s, 1H), 2.52 (s, 3H), 0.86 (s, 9H). LCMS-ESI+: calc'd for C201-120C1NO3S:
390.0 (M+H+);
Found: 390.1 (M+H+).
Preparation of (S)-2-tert-butoxy-2-(7-(4-chloropheny1)-5-methylbenzo[d]thiazol-
6-
ypethanol (5K):
Step 1.
Preparation of 6-methoxy-5-methylbenzo[d]thiazol-2-amine (5A): To a solution
of 4-
methoxy-3-methylbenzenamine (1.05g, 7.66 mmol) in acetic acid (30 ml) at 0 C
as added
KSCN with heavy stirring. The reaction mixture was then stirred at room
temperature for 45
min. The reaction was cooled to 0 C and bromine was added dropwise. The
reaction was stirred
at room temperature overnight. The precipitate was collected, washed by acetic
acid,
dichloromethane, minimal water and dried under high vacuum to give the product
as brown
yellow solid. LCMS-ESI+: calc'd for C9H10N20S: 195.0 (M+H+); Found: 195.1
(M+H+).
Step 2.
Preparation of 6-methoxy-5-methylbenzo[d]thiazole (5B): To a solution of 6-
methoxy-5-
methylbenzo[d]thiazol-2-amine (5A) (1.24g, 6.42 mmol) in H3PO4 (5 mL) at 0 C,
was added
NaNO2 (2.2 g, 32 mmol) in minimal amount of water. The reaction mixture was
stirred at 0 C
for 20 mm. The reaction mixture was then transferred to ice-cold
hypophosphorous acid (50 %,
5 ml) and slowly warmed to room temperature and stirred at room temperature
until gas
evolution ceases. Solid Na2CO3 was added to neutralize the reaction and the
mixture was
extracted by ethyl acetate. The organic phase was dried over MgSO4, filtered,
concentrated and
purified by silica gel column (0-100% ethyl acetate/hexanes). LCMS-ESI+:
calc'd for
C9H9NOS: 180.0 (M+H ); Found: 180.1 (M+H+).
Step 3.
Preparation of 5-methylbenzo[d]thiazol-6-ol (5C): To a suspension of 6-methoxy-
5-
methylbenzo[d]thiazole (5B) (160 mg, 0.89 mmol) in dichloromethane (5 mL) was
added boron
tribromide (1 M in dichlormethane, 1.8 ml) at 0 C. The reaction mixture was
stirred at 0 C for
2 h. The reaction was quenched by adding a saturated NaHCO3 solution,
extracted with
110
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
dichlormethane and trace Me0H. The organic layer was dried over MgSO4,
filtered,
concentrated and purified by silica gel column (0-100% Ethyl acetate/hexanes).
LCMS-ESI :
calc'd for C8H7N0S: 166.0 (M+H+); Found: 166.2 (M+H+).
Step 4.
Preparation of 7-bromo-5-methylbenzo[d]thiazol-6-ol (5D): To a suspension of 5-
methylbenzo[d]thiazol-6-ol (5C) (140 mg, 0.84 mmol) in acetic acid (5 ml), was
added bromine
(40 ilL) slowly. The reaction mixture was stirred at room temperature for 1 h.
The precipitate
was collected, washed with acetic acid, water and dried under high vacuum.
LCMS-ESI : calc'd
for C81-16BrNOS: 244.0 (M+H+); Found: 244.1 (M+H+).
Step 5.
Preparation of 7-(4-chloropheny1)-5-methylbenzo[d]thiazol-6-ol (5E): The
reaction
mixture of 7-bromo-5-methylbenzo[d]thiazol-6-ol (5D) (90 mg, 0.37 mmol), 4-
chlorophenyl
boronic acid (86 mg, 0.55 mmol), Pd(PPh3)4 (40 mg, 0.037nuno1), K2CO3 (153 mg,
1.11 mmol)
in 1,2-dimethoxyethane (1 ml)/1120(0.5 ml) was heated at 110 C in the
microwave for 10 min.
Then the reaction mixture was diluted with water, extracted with ethyl
acetate. The organic layer
was dried over MgSO4, filtered, concentrated and purified by silica gel column
(0-100% ethyl
acetate/hexanes). LCMS-ESI+: calc'd for C14ll10C1NOS: 276.0 (M+H+); Found:
276.2 (M+H+).
Step 6.
Preparation of 7-(4-chloropheny1)-5-methyl-6-vinylbenzo[d]thiazole (5G): To a
solution
of 7-(4-chloropheny1)-5-methylbenzo[d]thiazol-6-ol (5E) (107 mg, 0.39 mmol) in
dichloromethane (3 mL)/pyridine (1 mL) at 0 C, was added
trifluoromethanesulfonyl acid
anhydride (130 pt, 0.80 mmol). The reaction mixture was stirred at 0 C for 1
h. Then the
reaction was quenched by adding saturated NaHCO3 solution, extracted by ethyl
ecetate. The
organic layer was dried over MgSO4, filtered, concentrated to give 7-(4-
chloropheny1)-5-
methylbenzo[d]thiazol-6-y1 trifluoromethanesulfonate (5F) which was used in
next step without
purification.
7-(4-chloropheny1)-5-methylbenzo[d]thiazol-6-y1 trifluoromethariesulfonate
(5F) from
above reaction was dissolved in DMF (3 m1). Tributylvinyltin (130 }IL),
PdC12(PPh3)2 (27 mg,
0.039 mmol) and LiC1 (49 mg, 1.17 mmol) were added. The reaction mixture was
stirred at 120
C in microwave for 30 min. The reaction mixture was diluted by ethyl acetate,
washed with
saturated NaHCO3 solution and extracted with ethyl acetate. The organic layer
was dried over
MgSO4, filtered, concentrated and purified by silica gel column (0-50% ethyl
acetate/hexanes).
LCMS-ESI : calc'd for C16H20C1NS: 286.0 (M+H+); Found: 286.1 (M+H+).
Step7.
111
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
Preparation of (S)-1-(7-(4-chloropheny1)-5-methylbenzo[d]thiazol-6-ypethane-
1,2-diol
(5H): A biphasic mixture of AD mix-a (1.5 g) in tert-butanol (5 mL)/H20 (5 mL)
was cooled to
0 C and 7-(4-chloropheny1)-5-methyl-6-vinylbenzo[d]thiazole (5G) (0.050 g,
0.175 mmol) was
added. Reaction mixture was stirred overnight at 0 C. Sodium sulfite (1.5 g)
was added at 0 C,
then warmed to room temperature and stirred for 30 min to give a white
mixture. Mixture was
diluted with ethyl ecetate and H20. Extracted with ethyl ecetate (3x) and
combined organic
layer was dried (MgSO4), concentrated and purified by flash column
chromatography (silica gel,
0 to 100% ethyl acetate/hexanes) to give the product.
LCMS-ESI+: calc'd for CI61114C1NO2S: 320.0 (M+H ); Found: 320.1 (M+H ).
Step 8.
Preparation of (S)-2-(7-(4-chloropheny1)-5-methylbenzo[d]thiazol-6-y1)-2-
hydroxyethyl
pivalate (51): To a solution of (S)-1-(7-(4-chloropheny1)-5-
methylbenzo[d]thiazol-6-ypethane-
1,2-diol (5H) (0.018 g, 0.056 mmol) in pyridine (0.5 mL)/dichoromethane (1 mL)
was added
trimethylacetyl chloride (0.010 mL, 0.081 mmol). Reaction mixture was stirred
for 1 h at room
temperature and additional trimethylacetyl chloride (0.020m1 0.081 mmol) was
added and left it
overnight at room temperature. More trimethylacetyl chloride (0.030 ml, 0.242
mmol) was
added to the mixture and stirred at room temperature for 30 min. Reaction
mixture was diluted
with ethyl acetate. Organic layer was washed with saturated sodium bicarbonate
solution, dried
(MgSO4), concentrated and purified by flash column chromatography (silica gel,
0 to 50% ethyl
acetate/hexanes)._LCMS-ESI+: calc'd for C21H22C1N035: 404.1 (M+H+); Found:
404.1 (M+H ).
Step 9.
Preparation of (S)-2-tert-butoxy-2-(7-(4-chloropheny1)-5-methylbenzo[d]thiazol-
6-
ypethyl pivalate (5J): A solution of (S)-2-(7-(4-chloropheny1)-5-
methylbenzo[d]thiazol-6-y1)-2-
hydroxyethyl pivalate (5I) (0.016 g, 0.040 mmol) and perchloric acid, 70% (6
jil, 0.1 mmol) in
tert-butyl acetate (1 mL) was stirred at room temperature for 2 h. Reaction
mixture was
quenched with solid sodium bicarbonate (0.05 g) for lh. Saturated sodium
bicarbonate solution
was added and extracted with ethyl acetate (3x). The combined organic layer
was dried
(MgSO4), concentrated and purified by flash column chromatography (silica gel,
0 to 50% ethyl
acetate/hexanes)._LCMS-ESI+: calc'd for C25H30C1NO3S: 460.2 (M+H+); Found:
460.2 (M+H+).
Step 10.
Preparation of (S)-2-tert-butoxy-2-(7-(4-chloropheny1)-5-methylbenzo[d]thiazol-
6-
ypethanol (5K): To a solution of (S)-2-tert-butoxy-2-(7-(4-chloropheny1)-5-
methylbenzo[d]thiazol-6-ypethyl pivalate (5J) (8 mg, 0.0174 mmol) in Me0H (0.5
mL) and
THF (1 mL) was added sodium hydroxide (2 M, 0.1 mL, 0.2 mmol) and the reaction
mixture
was stirred at room temperature overnight. Reaction mixture diluted with ethyl
acetate and
112
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
washed with saturated sodium bicarbonate solution. Aqueous layer back-
extracted with ethyl
acetate and combined organic layer was dried (MgSO4), concentrated and
purified by flash
column chromatography (silica gel, 0 to 50% ethyl acetate/hexanes). LCMS-ESI+:
calc'd for
C201122C1NO2S: 376.1 (M+H+); Found: 376.1 (M+I-14).
Example 2: Preparation of 2-cyclopropy1-6-methoxy-5-methylbenzo[d]thiazole
(6B).
H2N¨ Br
5A 6A
0
N
6B
Step 1.
To a solution of 2-bromo-6-methoxy-5-methylbenzo[d]thiazole (6A) (720 mg, 2.8
mmol)
in dioxane (10 ml), was added cyclopropyl boronic acid (722 mg, 8.4 mmol),
potassium
phosphate (2.3 g, 10.9 mmol), PdC12dppf (294 mg, 0.40 mmol). The mixture was
reacted at 100
C overnight. The reaction mixture was cooled to room temperature, washed with
water,
extracted with Et0Ac. The organic phase was combined, dried over MgSO4,
filtered,
concentrated and purified by silica gel column, eluting by 0-50% Et0Ac in
hexanes. LCMS-
ESI+: calc'd for C12H13N0S: 220.1 (M+H+); Found: 220.2 (M+I-1+).
Step 2.
Preparation of (2-bromo-6-methoxy-5-methylbenzo[d]thiazole (6A):
To a solution of t-butylnitrite (5.17 ml, 43.5 mmol) in acetonitrile (50 ml)
was added coppeer
(II) bromide (7.2 g, 32.2 mmol) slowly. The reaction mixture was stirred at
room temperature
for half hour. Then the reaction mixture was put to a 60 C oil bath and 6-
methoxy-5-
methylbenzo[d]thiazol-2-amine (5A) (4.2g, 21.76 mmol) was added slowly. The
reaction
mixture was stirred at 60 C for 1 h. The reaction was cooled to room
temperature; washed with
water and extracted with Et0Ac. The organic phase was combined, dried over
MgSO4, filtered,
concentrated and purified by silica gel column, eluting by 0-50% Et0Ac in
hexanes. LCMS-
ESI : calc'd for C9H8BrNOS: 257.9 (M+H+); Found: 258.0 (M+11 ).
113
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
Example 3: Preparation of (S)-2-tert-butoxy-2-(7-(4-chloropheny1)-2,5-
dimethylbenzo[d]thiazol-
6-ypacetic acid (7).
CI
CY.<
7 0 H
0
7
Compound 7 was synthesized from compound 6A according to the procedure used to
prepare compound 68 (except that trimethylboxine was used instead of
cyclopropyl boronic acid
) followed by the procedures to convert compound 5B to compound 5L as outlined
in Example
1. 1H-NMR: 400 MHz, (CD30D) 8: 7.69 (s, 1H), 7.65-7.51 (m, 4H), 5.22 (s, 1H),
2.76 (s, 3H),
2.57 (s, 3H), 0.94 (s, 9H). LCMS-ESr: calc'd for C21H18C1NO3: 404.1 (MAT);
Found: 404.1
(M+H+).
Example 4: Preparation of (S)-2-tert-butoxy-2-(7-(4-chloropheny1)-2-isobuty1-5-
methylbenzo[d]thiazol-6-ypacetic acid (83) and (S)-2-tert-butoxy-2-(2-isobuty1-
5-methy1-7-
phenylbenzo[d]thiazol-6-yl)acetic acid (8K).
114
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
Br
H2N 0
5A
8A
CI CI
11101
S O S
Br-(
8B 8C
CI CI
1110
S 0 e OH __
N
8D 8E
CY<
OH
__________________________________________________ S 0)(<
( 0
8F R = CI 8H R = CI
8G R = H 81 R = H
CY<
7 OH
--( 0
8J R = CI
8K R = H
The mixture of 7-(4-chloropheny1)-5-methy1-2-(2-methylprop-1-
enyl)benzo[d]thiazol-6-
ol (8E) (56 mg, 0.13 mmol), Pd/C (200 mg) in Et0H (5m1) and Et0Ac( 5 ml) was
stirred at
room temperature under an atmosphere of H2 for 30 mm, which gave a mixture of
7-(4-
chloropheny1)-2-isobuty1-5-methylbenzo[d]thiazol-6-ol (8F) and 2-isobuty1-5-
methy1-7-
115
phenylbenzo[d]thiazol-6-ol (8G). The reaction mixture was filtered over
celiteTM, concentrated
and taken on to next step without purification.
The mixture was converted to a mixture of compound 8J and compound 8K by the
same
steps used to convert compound 5E to compound 5L as outlined in Example 1. The
mixture of
compounds 8J and 8K were separated by reverse phase HPLC to provide the pure
compounds.
Compound 8J: 11-1-NMR: 400 MHz, (CD30D) 8: 7.72 (s, 1H), 7.64-7.50 (m, 4H),
5.22 (s,
1H), 2.92 (d, J = 3.6 Hz, 21-1), 2.58 (s, 3H), 2.17-2.13 (m, 1H), 1.01-0.99
(m, 611), 0.95 (s, 9H).
LCMS-ESr: calc'd for C211118C1NO3: 446.1 (M+H+); Found: 446.2 (M+H+).
Compound 8K: 1H-NMR: 400 MHz, (CD30D) 8: 7.70 (s, 111), 7.64-7.50 (m, 5H),
5.29
(s, 1H), 2.92 (d, J = 3.6 Hz, 2H), 2.57 (d, J = 0.4Hz, 3H), 2.17-2.13 (m, 1H),
1.01-0.99 (m, 6H),
0.92 (s, 9H). LCMS-ESI+: calc'd for C21H18C1NO3: 412.1 (M+H+); Found: 412.2
(M+H+).
Preparation of 7-(4-chloropheny1)-5-methy1-2-(2-methylprop-1-
enyl)benzo[d]thiazol-6-ol (8E):
Step 1.
Preparation of 7-bromo-6-methoxy-5-methylbenzo[d]thiazol-2-amine (8A). To a
solution of 6-methoxy-5-methylbenzo[d]thiazol-2-amine (5A) (1.0 g, 5.15 mmol)
in H2SO4 at 0
C, was added NBS (550 mg, 3.07 mmol). The reaction mixture was stirred at 0 C
for 2h. Then
the reaction mixture was poured to ice-water, neutralized by 50% KOH solution
to pH about 3.
The precipitation was collected, washed by water and dried over high vacuum.
LCMS-ESr:
calc'd for C91-19BrN2OS: 273.0 (M+1-); Found: 273.0 (M+11+).
Step 2.
Preparation of 7-(4-chloropheny1)-6-methoxy-5-methylbenzo[d]thiazol-2-amine
(8B).
The mixture of 7-bromo-6-methoxy-5-methylbenzo[d]thiazol-2-amine (8A) (1.72 g,
6.32 mmol),
4-chlorophenyl boronic acid (1.2 g, 7.67 mmol), K2CO3 (2.63 g, 18.9 mmol),
Pd(PPh3)4 (364 mg,
0.315 mmol) in DME (8 ml) and H20 (4m1) was reacted in microwave at 110 C for
lh. Then 4-
chlorophenyl boronic acid (100 mg, 0.64 mmol), Pd(PPh3).4 (100 mg, 0.086 mmol)
were added
and reacted in microwave at 110 C for 0.5 h and 120 C for 20 min. The
reaction mixture was
washed by water, extracted by Et0Ac. The organic phase was combined, dried
over MgSO4,
filtered, concentrated down and purified by silica gel column, eluting by 0-
100% Et0Ac in
.. hexanes. LCMS-ESI+: calc'd for C15Hi3C1N2OS: 305.0 (M+Fr); Found: 305.1
(M+fr).
Step 3.
116
CA 2833377 2018-04-17
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
Preparation of 2-bromo-7-(4-chloropheny1)-6-methoxy-5-methylbenzo[d]thiazole
(8C).
Compound 8C was synthesized from 88 according to the procedure used to prepare
compound
6A of Example 2. LCMS-ESI+: calc'd for C15H11BrC1NOS: 367.9 (M+H+); Found:
368.0
(M+H+).
Step 4.
Preparation of 7-(4-chloropheny1)-6-methoxy-5-methy1-2-(2-methylprop-1-
enyl)benzo[d]thiazole (8D). The mixture of 2-bromo-7-(4-chloropheny1)-6-
methoxy-5-
methylbenzo[d]thiazole (8C) (0.153 g, 0.417 mmol), 4,4,5,5-tetramethy1-2-(2-
methylprop-1-
eny1)-1,3,2-dioxaborolane (0.256 ml, 1.24 mmol), K3PO4 ( 0.35 g, 1.66 mmol),
PdC12(dppf) (45
mg, 0.062 mmol) in DME (1 ml) and H20 ( 0.5m1) was reacted in microwave at 120
C for 0.5h.
The reaction mixture was washed by water, extracted by Et0Ac. The organic
phase was
combined, dried over MgSO4, filtered, concentrated down and purified by silica
gel column,
eluting by 0-100% Et0Ac in hexanes. LCMS-ESI+: calc'd for C19H18C1NOS: 344.1
(M+H );
Found: 344.1 (M+H+).
Step 5.
Preparation of 7-(4-chloropheny1)-5-methy1-2-(2-methylprop-1-
enyl)benzo[d]thiazol-6-
ol (8E). Compound 8E was synthesized from compound 8D according to the
procedure used to
prepare compound 5C of Example 1.
LCMS-ESI+: calc'd for C19H16C1N0S: 330.0 (M+H+); Found: 330.2 (M+H+).
Example 5: Preparation of compound (9).
CI
CY.<
- OH
0
9
Compound 9 was synthesized from 8C by the method to used convert compound 8C
to
compound 8J as outlined in Example 4, except that tributylvinyltin was used in
first the cross
coupling reaction according to the procedure used to prepare compound 5G of
Example 1. 1H-
NMR: 400 MHz, (CD30D) 8: 7.71 (s, 1H), 7.65-7.51 (m, 4H), 5.22 (s, 1H), 3.11-
3.07 (m, 2H),
2.58 (s, 3H), 1.40 (t, J = 7.6 Hz, 3H), 0.94 (s, 9H). LCMS-ESI+: calc'd for
C211118C1NO3: 418.1
(M+H+); Found: 418.1 (M+H+).
117
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
Example 6: Preparation of compound (10).
CI
e<
7 0 H
0
Compound 10 was synthesized from compound 8C by the method used to convert
compound 8C to compound 8J as outlined in Example 4, except that
cyclopropylboronic acid,
5 was used in first the cross coupling reaction. 'H-NMR: 400 MHz, (CD30D)
.5: 7.63 (s, 1H),
7.61-7.49 (m, 411), 5.20 (s, 111), 2.55 (s, 3H), 2.41-2.36 (m, 1H), 1.26-1.22
(m, 2H), 1.14-1.10
(m, 2H), 0.94 (s, 9H).
LCMS-ESI+: calc'd for C21H18C1NO3: 430.1 (M+H+); Found: 430.1 (MAI).
10 Example 7: Preparation of compound 12.
CI CI
S
N--<\
N
8C CI 11
(J(.<
S OH
N 0
12
Compound 12 was synthesized from compound 11 according to the procedure used
to
prepare compound 8J from compound 8D as outlined in Example 4. 1H-NMR: 400
MHz,
(CD30D) .5: 7.62-7.49 (m, 411), 7.34 (s, 1H), 5.15 (s, 111), 3.27 (s, 6H),
2.53 (s, 311), 0.94 (s,
9H). LCMS-ESI+: calc'd for C21fl18C1NO3: 433.1 (M+H); Found: 433.1 (M+1-1 ).
118
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
Preparation of 6-methoxy-N,N,5-trimethylbenzo[d]thiazol-2-amine (11). To a
solution
of 2-bromo-7-(4-chlorophenyI)-6-methoxy-5-methylbenzo[d]thiazole (8C) (135 mg,
0.37 mmol)
in DMF (2 ml), was added dimethylamine in THF (2M, 0.46 ml, 0.92 mmol). The
reaction
mixture was stirred at 80 C. After the reaction finished, the reaction was
cooled and
concentrated. The residue was purified by silica gel column, eluting by 0-100%
Et0Ac in
hexanes. LCMS-ES[: calc'd for C17H17C1N2OS: 333.1 (M+H ); Found: 333.1 (M+H+).
Example 8: Preparation of compound 14.
CI CI
110
S s
Br---µ 5
> ___________________________________________
N
8C 13
CI
OH
> ___________________________________________ 0
14
Compound 14 was synthesized from compound 13 according to the procedure used
to
prepare compound 8J from compound 8D as outlined in Example 4. 11-1-NMR: 400
MHz,
(CD30D) 6: 7.74 (s, 1H), 7.62-7.50 (m, 4H), 5.22 (s, 1H), 2.58 (s, 3H), 1.61-
1.59 (m, 1H), 1.03-
1.01 (m, 2H), 0.94 (s, 1H), 0.91-0.88 (m, 2H). LCMS-ESI+: calc'd for
C211118C1NO3: 454.1
(M+H+); Found: 454.1 (MAO
CI
o Cr<
s = OH
0
14b
Compound 14b was obtained as a side-product of Compound 14.
119
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
1H-NMR: 400 MHz, (CD30D) 8: 7.99 (s, 111), 7.64-7.55 (m, 5H), 5.26 (s, 1H),
2.91 (t, J = 3
Hz, 211), 2.70 (m, 2H), 2.62 (s, 3H), 0.95 (s, 9H).
LCMS-ES14: calc'd for C25H24C1N04S: 470.1 (M+H+); Found: 470.1 (M+H+).
Preparation of 2-(2-cyclopropylethyny1)-6-methoxy-5-methylbenzo[d]thiazole
(13). To
a solution of 2-bromo-7-(4-chloropheny1)-6-methoxy-5-methylbenzo[d]thiazole
(8C) (188 mg,
0.512 mmol) in THF (3m1), was added ethynylcyclopropane (0.09 ml, 1.2 mmol),
CuI (10 mg,
0.052 mmol), Et3N (0.36 ml, 2.58 mmol) and PdC12(dppf) (19 mg, 0.026 mmol).
The reaction
mixture was stirred at 60 C for 2hs. The reaction mixture was washed by
water, extracted by
Et0Ac. The organic phase was combined, dried over MgSO4, filtered,
concentrated and purified
by silica gel column, eluting by 0-50% Et0Ac in hexanes.
LCMS-ESI+: calc'd for C201116C1N0S: 354.0 (M+11 ); Found: 354.1 (M+H+).
Example 9: Preparation of (S)-2-tert-butoxy-24(S)-2-cyclopropy1-7-(2,3-
dihydropyrano[4,3,2-
de]quinolin-7-y1)-5-methylbenzo[d]thiazol-6-ypacetic acid (21) and (S)-2-tert-
butoxy-2-((R)-2-
cyclopropy1-7-(2,3-dihydropyrano[4,3,2-de]quinolin-7-y1)-5-
methylbenzo[d]thiazol-6-ypacetic
acid (22).
120
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
Br Br Br
OH OTf
17
15 16 0
Br e<
01(<
HO-B4OH
0
18
0 0
j< and
0 /S = 0
0
0
19
0 0
(Y-
= OH = OH
and
0
0
22
21
Compounds 21 and 22 were prepared from compounds 19 and 20 by the method to
used
convert compound 5J to compound 5L as outlined in Example 1.
Compound 21: 1H-NMR: 400 MHz, (CD30D) 5: 8.77 (d, J = 3Hz, 1H), 7.87-7.80 (m,
5 311), 7.40 (d, J = 4.2 Hz, 1H), 5.21 (s, 1H), 4.72-4.68 (m, 2H), 3.64 (t,
J = 6Hz, 2H), 2.73 (s,
3H), 2.35-2.33 (m, 1H), 1.23-1.20 (m, 2H), 1.10-1.07 (m, 2H), 0.90 (s, 9H).
LCMS-ESI : calc'd
for C2iHisC1NO3: 489.1 (M+H ); Found: 489.1 (M+H+).
Compound 22: 1H-NMR: 400 MHz, (CD30D) 5: 8.66 (d, J ¨ 2.6 Hz, 1H), 8.13 (d, J
= 4
Hz, 1H), 7.82 (s, 1H), 7.66 (d, J = 2.8 Hz, 1H), 7.39 (d, J = 4Hz, 1H), 2.52
(s, 1H), 4.66 (t, J = 6
10 .. Hz, 2H), 3.57 (t, J = 5.8 Hz, 2H), 2.68 (s, 3H), 2.37-2.31 (m, 1H), 1.22-
1.19 (m, 211), 1.08-1.06
(m, 2H), 0.89 (s, 911).
LCMS-ESI+: calc'd for C211-118C1NO3: 489.1 (M+H+); Found: 489.1 (M+H+).
121
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
Preparation of compound 19 and compound 20.
Step 1.
Preparation of 7-bromo-2-cyclopropy1-5-methylbenzo[d]thiazol-6-ol (15).
Compound 15
was prepared from compound 6B by the method used to prepare compound 5D from
compound
5B as outlined in Example 1. LCMS-ESI+: calc'd for CicHioBrNOS: 284.0 (M+H+);
Found:
284.2 (M-41 ).
Step 2.
Preparation of 7-bromo-2-cyclopropy1-5-methylbenzo[d]thiazol-6-y1
trifluoromethanesulfonate (16). To a solution of 7-bromo-2-cyclopropy1-5-
methylbenzo[d]thiazol-6-ol (15) (500 mg, 1.766 mmol) in DCM (8 ml) and 2,6-
lutine (2 ml) at -
78 C was slowly added trifluoromethanesulfonic anhydride (0.59 ml, 3.51
mmol). The
temperature was allowed to slowly warm to 0 C over 2h. The reaction mixture
was washed by
saturated NaHCO3 solution, extracted with DCM. The organic phase was combined,
dry over
MgSO4, filtered, concentrated and purified by silica gel column, eluting by 0-
50% Et0Ac in
hexanes. LCMS-ESI+: calc'd for C12H9BrF3NO3S2: 415.9 (M+H+); Found: 415.9
(M+H+).
Step 3.
Preparation of 7-bromo-2-cyclopropy1-5-methyl-6-vinylbenzo[d]thiazole (17). To
a
solution of 7-bromo-2-cyclopropy1-5-methylbenzo[d]thiazol-6-y1
trifluoromethanesulfonate (16)
( 410 mg, 0.988 mmol) in DMF (4m1), was added tributylvinyltin (0.43 ml, 1.47
mmol), LiC1
(125 mg, 2.94 mmol) and PdC12(PPh3)2 ( 70 mg, 0.096 mmol). The reaction
mixture was reacted
at 80 C overnight. The reaction was cooled down, washed by saturated NaHCO3
solution,
extracted by Et0Ac. The organic phase was combined, dry over MgSO4, filtered,
concentrated
and purified by silica gel column, eluting by 0-50% Et0Ac in hexanes.
LCMS-ESI : calc'd for C13H12BrNS: 294.0 (M+1-I'); Found: 294.1 (M+H+).
Step 4.
Preparation of (S)-2-(7-bromo-2-cyclopropy1-5-methylbenzo[d]thiazol-6-y1)-2-
tert-
butoxyethyl pivalate (18). Compound 18 was prepared from compound 17 by the
method used
to convert compound 5G to compound 5J as outlined in Example 1. LCMS-ESI+:
calc'd for
C22H30BrN203S: 468.1 (M+H+); Found: 468.2 (M+H+).
Step 5.
Preparation of the (S)-2-tert-butoxy-2-(2-cyclopropy1-7-(2,3-
dihydropyrano[4,3,2-
de]quinolin-7-y1)-5-methylbenzo[d]thiazol-6-ypethyl pivalate isomers (19 and
20) . To a
solution of (S)-2-(7-bromo-2-cyclopropy1-5-methylbenzo[d]thiazol-6-y1)-2-tert-
butoxyethyl
pivalate (18) (23 mg, 0.047 mmol) in DMA (2m1), was added 2,3-
dihydropyrano[4,3,2-
de]quinolin-7-ylboronic acid ( 25 mg, 0.099 mmol), 2N K2CO3 solution (0.11 ml,
0.22 mmol)
122
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
and Pd(PPh3)4 ( 6 mg, 0.005 mmol). The reaction mixture was reacted at 85 C
for 2hs. The
reaction was cooled down, washed by saturated NaHCO3 solution, extracted by
Et0Ac. The
organic phase was combined, dry over MgSO4, filtered, concentrated and
purified by silica gel
column, eluting by 0-50% Et0Ac in hexanes. Two isomers were separated and went
through the
chemistry sequence as above. LCMS-ESI+: calc'd for C33H381\1204S: 559.2
(M+H+); Found:
559.1 (M+H+).
Example 10. Preparation of (S)-2-((S)-2-(azetidin-l-y1)-7-(2,3-
dihydropyrano[4,3,2-de]quinolin-
7-y1)-5-methylbenzo[d]thiazol-6-y1)-2-tert-butoxyacetic acid (35) and (S)-2-
((R)-2-(azetidin-1-
y1)-7-(2,3-dihydropyrano[4,3,2-delquinolin-7-y1)-5-methylbenzo[d]thiazol-6-y1)-
2-tert-
butoxyacetic acid (36).
123
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
0 0 0
Br
s
H2 N ---<\ al,....,..,
N
0
23 24 25
0 OH OH OH
S
Br-- 33,..--'' Br¨<. 1401
Br¨i11(
N N 0
N
26 27 28
OTf OH OTf 0
OEt S OEt
Br-- I
0 0
N N
29 30
OTf OH OTf CYk
S 7 Br-41 OEt
N S _
= --4.-
Br--- I
0
N 00Et
31 32
0
.,--
OTf 0-< -.
_
CN--- I S = OEt
0 CN- I
N 0
N
33
34
0 0
--=-
-. ''<
N 0
N 0j<
OH
S OH c ,S
CN
0 N
N
36
To a solution of 34 (23 mg, 0.043 mmol) in THF (1 mL) and Me0H (1 mL) was
added a
solution of NaOH (2 M, ¨400 lit). The reaction mixture was heated at 70 C for
4 h. The
reaction was brought to --pH 5 with TFA and was then purified by reverse phase
HPLC
5 (MeCN/H20 containing 0.1% TFA) to give 6 mg of compound 35 and 10 mg of
compound 36.
124
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
Compound 35: 11-1-NMR: 400 MHz, (CD30D) 8: 8.75 (d, J = 2.6 Hz, 1H), 7.80 (d,
J =
4.0 Hz, 1H), 7.72 (d, J = 2.6 Hz, 1H), 7.47 (s, 1H), 7.34 (d, J = 4.0 Hz, 1H),
5.13 (s, 1H), 4.67-
4.65 (m, 2H), 4.17 (t, J = 7.6 Hz, 411), 3.59-3.58 (m, 211), 2.66 (s, 311),
2.52-2.50 (m, 2H), 0.88
(s, 9H). LCMS-ESI4: calc'd for C281129N304S: 504.2 (M+1-1 ); Found: 504.0
(M+H+).
Compound 36: 111-NMR: 400 MHz, (CD30D) 8: 8.67 (d, J = 2.2 Hz, 1H), 8.01 (d, J
=
4.0 Hz, 1H), 7.49 (d, J = 2.6 Hz, 1H), 7.40 (s, 1H), 7.27 (d, J -= 4.2 Hz,
111), 5.18 (s, 1H), 4.60-
4.57 (m, 2H), 4.27 (t, J = 7.8 Hz, 4H), 3.48-3.45 (m, 2H), 2.61 (s, 3H), 2.58-
2.54 (m, 2H), 0.80
(s, 9H). LCMS-ESI+: calc'd for C28H29N304S: 504.2 (M+H+); Found: 504.1 (M+H+).
Preparation of (2S)-ethyl 2-(2-(azetidin-1-y1)-7-(2,3-dihydropyrano[4,3,2-
de]quinolin-7-
y1)-5-methylbenzo[d]thiazol-6-y1)-2-tert-butoxyacetate (34).
Step 1.
Preparation of 2-bromo-5-methylcyclohexane-1,3-dione (24). To a solution of 5-
methyl-
1,3-cyclohexanedione (23) (45.4 g, 360 mmol) in acetic acid (540 mL) was added
bromine (19.4
mL, 378 mmol) over 5 min. After 30 min of stirring (with mechanical stirrer),
the reaction
mixture was filtered. The solid was left under high vacuum overnight and used
in the subsequent
step without further purification.
Step 2.
Preparation of 2-amino-5-methyl-5,6-dihydrobenzo[d]thiazol-7(4H)-one (25). To
a
solution of 24 in acetic acid (540 mL) was added sodium acetate (44.3 g, 540
mmol) and
thiourea (28.8 g, 378 mmol). The reaction mixture was stirred with a
mechanical stirrer at 100
C for 3 h. The reaction mixture was partially concentrated in vacuo. Et0Ac was
added (500
mL). The mixture was made basic with 1 M NaOH, and the layers were separated.
The aqueous
layer was extracted with Et0Ac (2 x 300 mL). The combined organic layers were
dried, filtered,
and concentrated in vacuo to give 49.3 g of 25, which was taken on without
further purification.
LCMS-ESI+: calc'd for C8111 IN2OS: 183.1 (M+114); Found: 183.1 (MAT).
Step 3.
Preparation of 2-bromo-5-methy1-5,6-dihydrobenzo[d]thiazol-7(4H)-one (26). To
a
solution of 25 (53.9 g, 296 mmol) in MeCN (600 mL) at 0 C, while mechanically
stirred), was
added copper (II) bromide (79.2 g, 355 mmol) then t-butyl nitrite (46.8 mL,
355 mmol). The
reaction mixture was stirred from 0 C to room temperature over 2 h and was
then partially
concentrated. Et0Ac (400 mL) and a 0.5 M HCl solution were added. The layers
were
separated, and the organic layer was washed with a brine solution. The
combined organic layers
were dried, filtered, and concentrated in vacuo. The crude product was
adsorbed on ¨150 g of
silica then run through a plug of silica with 40% Et0Ac/hexanes to give 58.3 g
of 26. 1H-NMR:
125
CA 02833377 2013-10-16
WO 2012/145728
PCT/US2012/034593
400 MHz, (CDC13) 8: 3.16 (dd, 1H. J= 18, 4 Hz), 2.66 (m, 2H), 2.47 (m, 1H),
2.34 (dd, 1H, J=
16, 12 Hz), 1.19 (d, 311,J= 7 Hz). LCMS-ESI+: calc'd for C8H9BrNOS: 245.9
(M+H+); Found:
246.1 (M+11 ).
Step 4.
Preparation of 2-bromo-5-methylbenzo[d]thiazol-7-ol (27). To a solution of 26
(7.38 g,
30.0 mmol) in CC14 (90 mL) was added NBS (5.61 g, 31.5 mmol) and dibenzoyl
peroxide (727
mg, 3.0 mmol). The reaction was heated at 90 C in a sealed reaction vessel
for about 4 h. Then
DBU (6.73 mL, 45.0 mmol) in CH2C12 (15 mL) was added. The mixture was heated a
reflux for
30 min, then a 1 M HC1 solution was added. The layers were separated, and the
aqueous layer
was extracted with CH2C12. The combined organic layers were washed with a
brine solution.
The organic layer was then dried, filtered, and concentrated in vacuo. The
crude product was
adsorbed on -30 g of silica then run through a plug of silica with 40%
Et0Ac/hexanes to give
5.2 g of 27. 11-1-NMR: 400 MHz, (CD3OH) 8: 7.25 (s, 1H), 6.69 (s, 1H), 2.40
(s, 3H). LCMS-
ESI : calc'd for C8H7BrNOS: 243.9 (M+H+); Found: 244.1 (M+H+).
Step 5.
Preparation of ethyl 2-(2-bromo-7-hydroxy-5-methylbenzo[d]thiazol-6-y1)-2-
hydroxyacetate (28). To a solution of 27 (3.90 g, 16.0 mmol) in CH2C12 (80 mL)
at 0 C was
added triethylamine (2.45 mL, 16.8 mmol) then a solution of titanium
tetrachloride in CH2C12
(1.0 M, 16.8 mL, 16.8 mmol). After 15 min, ethyl glyoxalate (50% in toluene,
3.49 mL, 17.6
mmol) was added. The reaction mixture was stirred for 2 h while warming to
room temperature.
Water (50 mL) and a saturated solution of potassium sodium tartrate (50 mL)
were added. The
mixture was stirred vigorously for 2 h. The layers were separated, and the
aqueous layer was
extracted with CH2C12. The combined organic layers were dried, filtered, and
concentrated in
vacuo. The crude material was purified by column chromatography to give 2.48 g
of 28 and
recovered -500 mg of 27. 111-NMR: 400 MHz, (CD3OH) 8: 7.33 (s, 1H), 5.69 (s,
111), 4.17 (m,
2H), 2.50 (s, 3H), 1.18 (t, 311, J= 7 Hz). LCMS-ESI+: calc'd for C12H13BrN04S:
346.0
(M+H+); Found: 346.1 (M+H+).
Step 6.
Preparation of ethyl 2-(2-bromo-5-methy1-7-
(trifluoromethylsulfonyloxy)benzo[d]thiazol-6-y1)-2-hydroxyacetate (29). To a
solution of 28
(2.42 g, 7.00 mmol) in CH2C12 (30 mL) at -78 C was added triethylamine (1.02
mL, 7.70
mmol) followed by trifluoromethanesulfonic anhydride (1.24 mL, 7.35 mmol).
After 15 min,
saturated NH4C1 was added. The layers were separated. The organic layer was
dried, filtered,
126
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
and concentrated in vacuo. The crude material was purified by column
chromatography to give
2.17 g of 29. 1H-NMR: 400 MHz, (CDC13) 8: 7.84 (s, 1H), 5.67 (s, 1H), 4.27 (m,
2H), 2.50 (s,
311), 1.23 (t, 3H, J= 7 Hz). LCMS-ESI+: calc'd for C13H12BrF3NO6S2: 477.9
(M+11 ); Found:
478.2 (M+H+).
Step 7.
Preparation of ethyl 2-(2-bromo-5-methy1-7-
(trifluoromethylsulfonyloxy)benzo[d]thiazol-6-y1)-2-oxoacetate (30). To a
solution of 29 (9.85
g, 20.6 mmol) in C112C12 (100 mL) was added Dess-Martin periodinane (9.61 g,
22.6 mmol).
After 30 min, water (75 mL) and saturated Na2S204 solution (75 mL) was added.
The mixture
was stirred vigorously for 30 min. The layers were separated, and the aqueous
layer was
extracted with C112C12. The combined organic layers were dried, filtered, and
concentrated in
vacuo. The crude material was purified by column chromatography to give 8.32 g
of 30. 1H-
NMR: 400 MHz, (CDC13) 8: 7.91 (s, 1H), 4.40 (q, 211, J= 7 Hz), 2.49 (s, 3H),
1.39 (t, 3H, J= 7
Hz).
LCMS-ESI+: calc'd for Ci3Hi0BrF3NO6S2: 475.9 (M+H ); Found: 476.1 (M+11 ).
Step 8.
Preparation of (S)-ethyl 2-(2-bromo-5-methy1-7-
(trifluoromethylsulfonyloxy)benzo[d]thiazol-6-y1)-2-hydroxyacetate (31). To a
solution of 30
(8.30 g, 17.4 mmol) in toluene (70 mL) was added ((R)-2-methyl-CBS-
oxazaborolidine (725
mg, 2.61 mmol). The reaction mixture was then cooled to -35 C and a solution
of
catecholborane (freshly distilled) (1 M in toluene, 20.9 mL, 20.9 mmol) was
added via addition
funnel over 30 min. The reaction was stirred for 20 min while warrning to -20
C. A 2 M
solution of Na2CO3 was added (50 mL). The layers were separated, and the
organic layer was
washed with additional Na2CO3 solution (3 x 25 mL). The organic layer was
dried, filtered, and
concentrated in vacuo to give 31, which had analytical data to match 29. The
compound was
taken on to the next step without further purification.
Step 9.
Preparation of (S)-ethyl 2-(2-bromo-5-methy1-7-
(trifluoromethylsulfonyloxy)benzo[d]thiazol-6-y1)-2-tert-butoxyacetate (32).
To a solution of 31 (-17 mmol) in t-butylacetate (70 mL) was added perchloric
acid (1.23 mL,
20.4 mmol). After 3 h, water was added (50 mL). The layers were separated. The
organic layer
was washed with a saturated solution of NaHCO3. The organic layer was dried,
filtered, and
concentrated in vacuo. The crude material was purified by column
chromatography
(Et0Ac/hexanes) to give 7.22 g of 32 and 1.58 g of 31. 1H-NMR: 400 MHz,
(CD3OH) 8: 7.82
127
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
(s, 1H), 5.59 (s, 1H), 4.08-4.25 (m, 2H), 2.55 (s, 31-1), 1.20 (s, 91-1), 1.16
(t, 3H, J= 7 Hz).
LCMS-ESI+: calc'd for C17H20BrF3N06S2: 534.0 (M+H+); Found: 534.1 (M+11 ).
Step 10.
Preparation of (S)-ethyl 2-(2-(azetidin-1-y1)-5-methy1-7-
(trifluoromethylsulfonyloxy)benzo[d]thiazol-6-y1)-2-tert-butoxyacetate (33).
To a solution of 32
(50 mg, 0.094 mmol) in THF (1 mL) was added azetidine (204). The reaction
mixture was
heated at 70 C for 30 min. A saturated solution of NH4C1 (3 mL) was added,
and the layers
were separated. The aqueous layer was extracted with Et0Ac. The combined
organic layer were
dried, filtered, and concentrated in vacuo. The crude material was purified by
column
chromatography (Et0Ac/hexanes) to give 38 mg of 33. LCMS-ESI+: calc'd for
C201125F3N206S2: 511.1 (M+H+); Found: 511.0 (M+1-1 ).
Step 11.
Preparation of (2S)-ethyl 2-(2-(azetidin-1-y1)-7-(2,3-dihydropyrano[4,3,2-
de]quinolin-7-
y1)-5-methylbenzo[d]thiazol-6-y1)-2-tert-butoxyacetate (34). To a solution of
33 (38 mg, 0.075
mmol) in freshly distilled DME (1 mL) was added 2,3-dihydropyrano[4,3,2-
de]quinolin-7-
ylboronic acid hydrochloride (24 mg, 0.097 mmol), ch1oro(2-
dicyclohexylphosphino-2',6'-
dimethoxy-1,1'-bipheny1)[2-(2-aminoethylphenyl)]palladium(H) methyl-t-
butylether adduct,
[SPhos Palladacycle] (5 mg, 0.0075 mmol), and cesium fluoride (46 mg, 0.3
mnunol). The
reaction mixture was heated in the microwave at 110 C for 45 min. A saturated
solution of
NaHCO3 (3 mL) was added, and the layers were separated. The aqueous layer was
extracted
with Et0Ac. The combined organic layer were dried, filtered, and concentrated
in vacuo. The
crude material was purified by column chromatography (Et0Ac/hexanes) to give
21 mg of 34.
LCMS-ESI+: calc'd for C301133N304S: 532.2 (M+H+); Found: 532.0 (M+H+).
Example 11. Preparation (S)-2-tert-butoxy-24(S)-2-carbamoy1-7-(2,3-
dihydropyrano[4,3,2-
de]quinolin-7-y1)-5-methylbenzo[d]thiazol-6-yDacetic acid (40).
128
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
OTf 0=.< OTf 0-<
= OEt 0 S OEt
I I
0 Et0 N 0
37
32
0 0
Oj<
0 S OEt
I 0 S OEt
0
H2N I Et0 N 0
38 39A
0 0
0-<
OEt = OH
N- I 0
0
H2N N
39B 40
Compound 40 was prepared from compound 39. To a solution of compound 39A (200
mg) in CH2C12 (5 mL) was added triethylamine (2 mL) and trifluoroacetic acid
anhydride (100
L). After 3 h, a saturated solution of NH4C1 was added. The layers were
separated, and the
aqueous layer was extracted with CH2C12. The combined organic layers were
dried, filtered, and
concentrated in vacuo. A solution of THF and Me0H was added (1:1, 5 mL)
followed by NaOH
solution (2 M, 200 W. The reaction mixture was stirred at 45 C for 6 h. The
mixture was made
acidic with 1 M HC1. The crude mixture was purified by reverse phase HPLC to
give 10.8 mg of
compound 40.
Compound 40. 1H-NMR: 400 MHz, (CD30D) 8: 8.79 (d, J=5.2 Hz, 1H); 8.21 (s, 1H);
7.92 (d, J=8.0 Hz, 1H); 7.87(d, J=6.0 Hz, 1H); 7.46(d, J=8.0 Hz, 1H); 5.27 (s,
111); 4.74-4.72
(m, 2H); 3.68 (t, J=6.0 Hz, 2H); 2.80 (s, 3H); 0.93 (s, 9H).
LCMS-ESI+: calc'd for C26H25N305S: 492.1 (M+11 ); Found: 492.1 (M+H+).
0
0
0 S = OH
HO' N0
41
129
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
Compound 41 was a by-product in the preparation of 40.
LCMS-ESI+: calc'd for C26H24N206S: 493.1 (M+11 ); Found: 493.1 (M+H+).
Preparation of (S)-ethyl 2-tert-butoxy-2-((S)-2-carbamoy1-7-(2,3-
dihydropyrano[4,3,2-
de]quinolin-7-y1)-5-methylbenzo[d]thiazol-6-ypacetate (39).
Step 1.
Preparation of (S)-ethyl 6-(1-tert-butoxy-2-ethoxy-2-oxoethyl)-5-methy1-7-
(trifluoromethylsulfonyloxy)benzo[d]thiazole-2-carboxylate (37). To a a
solution of (S)-ethyl 2-
(2-bromo-5-methy1-7-(trifluoromethylsulfonyloxy)benzo[d]thiazol-6-y1)-2-tert-
butoxyacetate
(32) (1.07 g, 2.00 mmol) in DMF (10 mL) was added tributy1(1-
ethoxyvinyl)stannane (867 mg,
2.40 mmol), copper iodide (38 mg, 0.20 mmol), and Pd(PPh3)4 (116 mg, 0.10
mmol). The
reaction mixture was stirred at 45 C for 2.5 h. A saturated solution of NH4C1
was added and
Et0Ac. The layers were separated, and the aqueous layer was extracted with
Et0Ac. The
combined organic layers were dried, filtered, and concentrated in vacuo.
Methanol and CH2C12
(1:1, 20 mL) added. The mixture was cooled to -78 C and ozone (03) was
bubbled through the
solution for about 15 min until the reaction mixture was blue-green.
Dimethysulfide (1 mL) was
added and the reaction was stirred at rt for 20 min. The mixture was
concentrated in vacuo and
purified by column chromatography (Et0Ac/hexanes) to give 811 mg of 37.
11-1-NMR: 400 MHz, (CDC13): 6 8.06 (s, 1H), 5.65 (s, 1H), 4.56 (q, J = 7 Hz,
2H), 4.14 (m, 2H),
2.59 (s, 3H), 1.49 (t, J = 7 Hz, 3H), 1.21 (s, 9H), 1.16 (t, J = 7 Hz, 3H).
Step 2.
Preparation of (S)-ethyl 64(S)-1-tert-butoxy-2-ethoxy-2-oxoethyl)-7-(2,3-
dihydropyrano[4,3,2-de]quinolin-7-y1)-5-methylbenzo[d]thiazole-2-carboxylate
(38). To a
solution of 37 (807 mg, 1.53 mmol) and CsF (1.02 g, 6.73 mmol) in distilled
dimethoxyethane
(15 mL) was added 2,3-dihydropyrano[4,3,2-de]quinolin-7-ylboronic acid (HC1
salt, 770 mg,
3.06 mmol) and chloro(2-dicyclohexylphosphino-2',6'-dimethoxy-1,1'-bipheny1)[2-
(2-
aminoethylphenyl)jpalladium(II) methyl-t-butylether adduct, [SPhos
Palladacycle] (206 mg,
0.31 mmol). The reaction mixture was heated at 110 C in a sealed tube for 2
h. The reaction
was cooled to it and saturated solution of NaHCO3 was added. The layers were
separated, and
the aqueous layer was extracted with Et0Ac. The combined organic layers were
dried, filtered,
and concentrated in vacuo. The crude material was purified by column
chromagraphy
(increasing Et0Ac w/ 5% Me0H to hexanes) to give 224 mg of 38 and 348 mg of
undesired
atropisomer.
130
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
1H-NMR: 400 MHz, (CDC13): 6 8.54 (d, J = 4 Hz, 114), 8.04 (s, 114), 7.55 (d, J
= 8 Hz, 1H), 7.29
(d, J = 4 Hz, 1H), 7.10 (d, J = 8 Hz, 111), 5.18 (s, 1H), 4.55 (m, 2H), 4.41
(q, J = 7 Hz, 2H), 4.01
(m, 114), 3.88 (m, 1H), 3.37 (m, 2H), 2.77 (s, 3H), 1.36 (t, J = 7 Hz, 3H),
1.00 (t, J = 7 Hz, 3H),
0.90 (s, 9H).
Step 3.
Preparation of (S)-ethyl 2-tert-butoxy-24(S)-2-carbamoy1-7-(2,3-
dihydropyrano[4,3,2-
de]quinolin-7-y1)-5-methylbenzo[d]thiazol-6-yl)acetate (39). To a solution of
38 (224 mg) in
Me0H (5 mL) was added NH4OH (500 [LW. The reaction mixture was stirred at rt
for 2 h and
then concentrated in vacuo to give 220 mg of 39.
LCMS-ESI+ (m/z): [M+H} calcd for C28H29N305S: 520.2 (MAT); Found: 520.1,
493.07(M+H+).
Example 12. Preparation of (S)-2-tert-butoxy-2-((S)-7-(2,3-dihydropyrano[4,3,2-
de]quinolin-7-
y1)-2-(dimethylamino)-5-methylbenzo[d]thiazol-6-yDacetic acid (42) and (S)-2-
tert-butoxy-2-
((R)-7-(2,3-dihydropyrano[4,3,2-de]quinolin-7-y1)-2-(dimethylamino)-5-
methylbenzo[d]thiazol-
6-yDacetic acid (43).
Compounds 42 and 43 were prepared from compound 32 according to the procedure
used to prepare compound 35 (except that dimethylamine was used instead of
azetidine) in
Example 10.
131
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
OTf OTf 0-"
Br
OEt \s - OEt
0 N 0
32 42A
0
0-r<
OEt
S
N-<\
N 0
42B
0 0
1:Y*<
- OH OH
\N--
0 / 0
42 43
0
00H
\
0
42
Compound 42: 11-1-NMR: 400 MHz, (CD30D)15: 8.76 (d, J=4.8 Hz, 1H); 7.82 (d,
J=8.0
Hz, 1H); 7.73 (d, J=5.2 Hz, 1H); 7.50 (s, 1H); 7.35 (d, J=7.6 Hz, 1H); 5.14
(s, 1H); 4.67 (m,
2H); 3.61 (t, J=5.8 Hz, 2H); 3.13 (s, 6H); 2.66 (s, 3H); 0.89 (s, 9H). LCMS-
ESI+ (m/z): [M+H]
calcd for C27H30N304S: 492.20 (M+H+); Found: 492.00, 493.07(M+H+).
0
0j<
- OH
SL
43
132
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
Compound 43:1H-NMR: 400 MHz, (CD30D) 8 8.67 (d, J= 4.9 Hz, 1H), 8.04 (d, J=
8.1
Hz, 1H), 7.51 (d, J= 4.9 Hz, 11-1), 7.45 (s, 1H), 7.30 (d, J= 8.1 Hz, 1H),
5.19 (s, 1H), 4.67 ¨
4.55 (m, 211), 3.21 (s, 6H), 2.62 (s, 3H), 0.81 (s, 9H).
LCMS-ESI+ (m/z): [M+H] calcd for C27H29N304S: 492.20 (M+H+); Found: 491.98,
492.96(M+H+).
OTf CY<
S OEt
N 0
42A
Compound 42A: LCMS-ESI+ (m/z): [M+H] calcd for C19H25F3N206S2: 499.1 (M+H+);
Found: 499.0(M+H+).
0
CY.<
S 7 OEt
N 0
428
Compound 42B: 1H-NMR: 400 MHz, (CDC13) 8: 8.75 (d, J = 1.8 Hz, 1H); 7.54 (d, J
=
4.0 Hz, 111), 7.48 (s, 111), 7.11 (d, J = 2.2 Hz, 1H), 7.06 (d, J = 3.8 Hz,
1H), 4.94 (s, 1H), 4.54 (t,
J= 5.6 Hz, 211), 4.00-4.03 (m, 2H), 3.31-3.30 (m, 2H), 3.08 (s, 6H), 2.64 (s,
3H), 1.25-1.27 (m,
311), 0.88 (s, 9H).
LCMS-ESI+ (m/z): [M+Hr calcd for C29H33N304S: 520.2 (M+H+); Found: 520.0 (M+H
).
Example 13. Preparation of (S)-2-tert-butoxy-24(S)-7-(2,3-dihydropyrano[4,3,2-
de]quinolin-7-
y1)-2-(ethyl(methyDamino)-5-methylbenzo[d]thiazol-6-ypacetic acid (44).
Compound 44 was prepared from compound 32 according to the procedure used to
prepare compound 35 (except that methylethylamine was used instead of
azetidine) in Example
10.
133
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
0
ç D0
OH
\N-µ
N 0
44
Compound 44:11-1NMR (400 MHz, CD30D) 8 8.77 (d, J= 5.4 Hz, 1H), 7.82 (d, J=
8.1
Hz, 1H), 7.72 (d, J= 5.3 Hz, 1H), 7.49 (s, 1H), 7.35 (d, J= 8.1 Hz, 1H), 5.14
(s, 1H), 4.67 (t, J=
5.9 Hz, 2H), 3.59 (t, J= 5.9 Hz, 2H), 3.52 (dd, J= 14.3, 7.1 Hz, 2H), 3.12 (s,
3H), 2.66 (s, 3H),
1.20 (t, J= 7.1 Hz, 3H), 0.90 (s, 9H). LCMS-ESI+ (m/z): [M+Hr calcd for
C28H3IN304S:
506.21 (M+H+); Found: 506.05, 507.00 (M+H+).
Example 14. Preparation of (S)-2-tert-butoxy-24(S)-2-(diethylamino)-7-(2,3-
dihydropyrano[4,3,2-de]quinolin-7-y1)-5-methylbenzo[d]thiazol-6-ypacetic acid
(45).
Compound 45 was prepared from compound 32 according to the procedure used to
prepare compound 35 (except that diethylamine was used instead of azetidine)
in Example 10.
0
S - OH
( N 0
Compound 45: IHNMR (400 MHz, CD30D) 8 8.90 (s, 1H), 7.90 (d, J= 7.9 Hz, 2H),
7.61 (s, 1H), 7.44 (d, J= 7.9 Hz, 1H), 5.17 (s, 1H), 4.71 (m, 2H), 3.66 (s,
6H), 2.73 (s, 3H), 1.95
15 (s, 4H), 1.29 (d, J= 5.9 Hz, 6H), 0.90 (s, 8H).
LCMS-ESI+ (m/z): [M+H]+ calcd for C29H34N304S: 520.23(M+H+); Found: 520.05,
521.13
(M--1-11+).
Example 15. Preparation of (S)-2-tert-buto4-24(S)-7-(2,3-dihydropyrano[4,3,2-
de]quinolin-7-
20 y1)-2-(isopropyl(methyl)amino)-5-methylbenzo[d]thiazol-6-y0acetic acid
(46) and (S)-2-tert-
butoxy-2-((R)-7-(2,3-dihydropyrano[4,3,2-de]quinolin-7-y1)-2-
(isopropyl(methypamino)-5-
methylbenzo[d]thiazol-6-ypacetic acid (47).
134
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
Compounds 46 and 47 were prepared from compound 32 according to the procedure
used to
prepare compound 35 (except that N-methyl-N-isopropylamine was used instead of
azetidine) in
Example 10.
0
\ S OH
N 0
46
Compound 46: 1H-NMR: 400 MHz, (CD30D) 8 8.76 (d, J= 5.4 Hz, 1H), 7.82 (d, J=
8.2
Hz, 111), 7.73 (d, J= 5.2 Hz, 1H), 7.49 (s, 1H), 7.35 (d, J= 8.1 Hz, 1H), 5.14
(s, 1H), 4.67 (t, J=
5.7 Hz, 2H), 4.23 -4.06 (m, 1H), 3.59 (t, J= 5.8 Hz, 214), 3.00 (s, 311), 2.67
(s, 3H), 1.23 (t, J=
6.5 Hz, 6H), 0.89 (s, 911). LCMS-ESI4 (m/z): [M-FH]1 calcd for C29H34N304S:
520.23 (M+H );
Found: 519.95, 521.00 (M+H+).
0
0j<
\ S 7 OH
N 0
47
Compound 47: 1H-NMR: 400 MHz, (CD30D) 8 8.67 (d, J= 4.9 Hz, 1H), 8.02 (d, J=
8.1
Hz, 111), 7.48 (d, J= 4.6 Hz, 1H), 7.44 (s, 111), 7.28 (d, J= 8.1 Hz, 114),
5.19 (s, 1H), 4.67 -
4.52 (m, 2H), 4.11 -4.00 (m, 1H), 3.50 - 3.43 (m, 111), 3.08 (s, 514), 2.62
(s, 411), 1.26 (d, J=
6.1 Hz, 6H), 0.80 (s, 911).
LCMS-ESI (m/z): [M+H] calcd for C271134N304S: 520.23 (M+H ); Found: 520.05,
521.08(M+H+).
Example 16a. Preparation of (S)-2-tert-butoxy-2-((S)-7-(2,3-
dihydropyrano[4,3,2-delquinolin-7-
y1)-2-(isobutyl(methypamino)-5-methylbenzo[d]thiazol-6-ypacetic acid (48) and
(S)-2-tert-
butoxy-24(R)-7-(2,3-dihydropyrano[4,3,2-de]quinolin-7-y1)-2-
(isobutyl(methypamino)-5-
methylbenzo[d]thiazol-6-ypacetic acid (49).
Compounds 48 and 49 were prepared from compound 32 according to the procedure
used to
prepare compound 35 (except that N-methyl-N-isobuytlarnine was used instead of
azetidine) in
Example 10.
135
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
0
çD0"--<
S OH
0
48
Compound 48: 11-1-NMR: 400 MHz, (CD30D) 8 8.77 (d, J= 5.4 Hz, 1H), 7.83 (d, J=
8.3
Hz, 1H), 7.75 (d, J= 5.1 Hz, 1H), 7.49 (s, 1H), 7.36 (d, J= 8.0 Hz, 1H), 5.13
(s, 1H), 4.68 (dd, J
= 9.9, 6.0 Hz, 2H), 3.60 (t, J= 6.0 Hz, 211), 3.27 (m, 2H), 3.13 (s, 411),
2.66 (s, 311), 2.08
(m,1H), 0.89-0.87 (m, 1511). LCMS-ESI+ (m/z): [M+H] calcd for C301136N304S:
534.24
(M+H+); Found: 533.9 (M+H+).
0
CD4-<
S - OH
0
49
Compound 49: 11I-NMR: 400 MHz, (CD30D) 8 8.69 (d, J= 5.0 Hz, 1H), 8.06 (d, J=
8.1
Hz, 1H), 7.54 (d, J= 4.9 Hz, 1H), 7.46 (s, 1H), 7.32 (d, J= 8.1 Hz, 1H), 5.19
(s, 1H), 4.62 (m,
Hz, 211), 3.50 (t, J= 5.8 Hz, 211), 3.22 (s, 311), 2.63 (s, 311), 2.21 ¨ 2.04
(m, 1H), 0.91 (d, J = 6.6
Hz, 6H), 0.83 (s, 9H). LCMS-ESI+ (m/z): [M+H] calcd for C30H36N304S: 534.24
(M+H+);
Found: 534.04, 535.05 (M+H+).
Example 16b. Preparation of (S)-2-tert-butoxy-2-((5)-7-(2,3-
dihydropyrano[4,3,2-de]quinolin-7-
y1)-5-methy1-2-(pyrrolidin-1-yObenzo[d]thiazol-6-ypacetic acid (50) and (S)-2-
tert-butoxy-2-
((R)-7-(2,3-dihydropyrano[4,3,2-de]quinolin-7-y1)-5-methy1-2-(pyrrolidin-l-
yObenzo[d]thiazol-
6-ypacetic acid (51) .
Compounds 50 and 51 were prepared from compound 32 according to the procedure
used to
prepare compound 35 (except that pyrrolidine was used instead of azetidine) in
Example 10.
136
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
0
S 7 OH
N 0
Compound 50: 111-NMR: 400 MHz, (CD30D) 8 8.76 (d, J= 5.3 Hz, 111), 7.80 (d, J=
8.1
Hz, 1H), 7.69 (d, J= 5.1 Hz, 1H), 7.50 (s, 1H), 7.33 (d, J= 8.0 Hz, 1H), 5.15
(s, 1H), 9.03 ¨
0.64 (m, 79H), 4.70 ¨ 4.60 (m, 2H), 3.56 (dd,J= 13.8, 7.7 Hz, 6H), 2.68 (s,
3H), 2.10 (t, J= 6.7
5 Hz, 4H), 0.89 (s, 10H). LCMS-ES1+ (m/z): [M+Hr calcd for C291132N304S:
518.21 (M+H+);
Found: 517.99, 518.97(M+H+).
0
el<
CN
OH
0
51
Compound 51: 1H-NMR: 400 MHz, (CD30D) 8 8.67 (d, J= 4.7 Hz, 111), 8.01 (d, J=
8.1
Hz, 1H), 7.46 (d, J= 4.8 Hz, 1H), 7.44 (s, 1H), 7.27 (d, J= 8.1 Hz, 111), 5.20
(s, 1H), 4.68 ¨
10 4.50 (m, 2H), 3.57 (s, 311), 3.45 (t, J= 5.8 Hz, 211), 2.63 (s, 4H),
2.14 (t, J= 6.3 Hz, 4H), 0.79
(s, 911). LCMS-ESI+ (m/z): [M+H] calcd for C29H32N304S: 518.21 (M+H+); Found:
518.07,
519.07(M+H+).
Example 17. Preparation of (S)-2-tert-butoxy-24(S)-2-(3,3-difluoroazetidin-l-
y1)-7-(2,3-
15 dihydropyrano[4,3,2-de]quinolin-7-y1)-5-methylbenzo[d]thiazol-6-ypacetic
acid (52) and (S)-2-
tert-butoxy-24(R)-2-(3,3-difluoroazetidin-l-y1)-7-(2,3-dihydropyrano[4,3,2-
de]quinolin-7-y1)-5-
methylbenzo[d]thiazol-6-ypacetic acid (53).
Compounds 52 and 53 were prepared from compound 32 according to the procedure
used to prepare compound 35 (except that 2,2-difluoroazetidine was used
instead of azetidine) in
20 Example 10.
137
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
0
Cr<
OH
F 0
52
Compound 52: 1H-NMR: 400 MHz, (CD30D) 5 8.80 (d, J= 5.6 Hz, 1H), 7.87 (d, J=
8.2
Hz, 1H), 7.83 (d, J= 5.6 Hz, 1H), 7.61 (s, 1H), 7.41 (d, J= 8.2 Hz, 1H), 5.17
(s, 1H), 4.76 -
4.64 (m, 2H), 4.56 -4.43 (m, 411), 3.65 (t, J= 5.9 Hz, 2H), 2.69 (s, 3H), 0.91
(s, 911). 19F NMR
(377 MHz, CD30D) b -77.88 (s). LCMS-ESI+ (m/z): [M+H] calcd for
C281128F2N304S: 540.18
(M+H+); Found: 539.96, 540.96 (M+H+).
0
- OH
0
53
Compound 53: 1H-NMR: 400 MHz, (CD30D) 5 8.71 (d, J= 5.4 Hz, 111), 8.14 (d, J=
8.2
Hz, 1H), 7.69 (d, J= 5.4 Hz, 1H), 7.57 (s, 111), 7.40 (d, J= 8.2 Hz, 111),
5.21 (s, 1H), 4.72 -
4.60 (m, 2H), 4.56 - 4.42 (m, 411), 3.58 (t, J= 6.0 Hz, 211), 2.65 (s, 3H),
0.91 (s, 911). LCMS-
ESI+ (m/z): [M+H] calcd for C28H28F2N304S: 540.18 (M+H+); Found: 539.98,
541.02 (MAO
Example 18. Preparation of (S)-2-tert-butoxy-24(S)-7-(2,3-dihydropyrano[4,3,2-
de]quinolin-7-
y1)-2-(3-methoxyazetidin-1-y1)-5-methylbenzo[d]thiazol-6-ypacetic acid (54)
and (S)-2-tert-
butoxy-24(R)-7-(2,3 -dihydropyrano [4,3,2-de] quinolin-7-y1)-2-(3 -
methoxyazetidin-l-y1)-5-
methylbenzo[d]thiazol-6-yOacetic acid (55).
Compounds 54 and 55 were prepared from compound 32 according to the procedure
used to
prepare compound 35 (except that 2-methoxyazetidine was used instead of
azetidine) in
Example 10.
138
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
0
CY<
OH
0
54
Compound 54: 'H-NAM: 400 MHz, (CD30D) 8: 8.78 (d, J= 5.5 Hz, 1H), 7.84 (d, J=
8.5 Hz, 1H), 7.77 (d, J= 6.1 Hz, 1H), 7.52 (s, 1H), 7.37 (d, J= 7.8 Hz, 1H),
5.16 (s, 1H), 4.73 -
4.64 (m, 2H), 4.41 (ddd, J= 9.9, 6.2, 3.4 Hz, 1H), 4.31 (td, J=7.7, 1.0 Hz,
211), 4.02 - 3.90 (m,
2H), 3.62 (t, J= 5.7 Hz, 2H), 2.68 (s, 4H), 0.91 (s, 1111). LCMS-ESI+ (m/z):
[M+H]1 calcd for
C29H32N305S: 534.21 (M+H+); Found: 533.95, 534.97(M+H+).
0
OH
Compound 55: 1H-NMR: 400 MHz, (CD30D) 8 8.67 (d, J= 5.1 Hz, 1H), 8.04 (d, J=
8.2
Hz, 1H), 7.52 (d, J= 4.7 Hz, 1H), 7.43 (s, 1H), 7.30 (d, J= 8.1 Hz, 1H), 5.19
(s, 1H), 4.66 -
10 4.56 (m, 2H), 4.42 (m, 1H), 4.38 -4.32 (m, 2H), 4.08 -4.01 (m, 2H), 3.49
(t, J= 6.0 Hz, 3H),
2.61 (s, 3H), 0.82 (s, 1014). LCMS-ESI (m/z): [M-FfIr calcd for C29H32N305S:
534.21 (M+H+);
Found: 534.03, 535.08 (M+H+).
Example 19. Preparation of (S)-2-tert-butoxy-2-((S)-7-(2,3-dihydropyrano[4,3,2-
de]quinolin-7-
15 y1)-2-(3-fluoroazetidin-1-y1)-5-methylbenzo[d]thiazol-6-ypacetic acid
(56).
Compound 56 was prepared from compound 32 according to the procedure used to
prepare compound 35 (except that 2-fluoroazetidine was used instead of
anticline) in Example
10.
0
: OH
FN
0
56
139
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
Compound 56: 111-NMR: 400 MHz, (CD30D) 8 8.79 (d, J= 5.5 Hz, 1H), 7.85 (d, J=
8.1
Hz, 1H), 7.79 (d, J= 5.1 Hz, 1H), 7.55 (s, 1H), 7.39 (d, J= 7.9 Hz, 1H), 5.58 -
5.38 (m, 1H),
5.16 (s, 1H), 4.70 (td, J= 5.9, 3.1 Hz, 2H), 4.49 - 4.35 (m, 211), 4.28 - 4.12
(m, 2H), 3.63 (t, J=
6.0 Hz, 2H), 2.68 (s, 3H), 0.91 (s, 9H).
LCMS-ESI+ (m/z): [M+H] calcd for C28H29FN1304S: 522.19 (M+H ); Found: 521.97,
523.02
(M+H+).
Example 20a. Preparation of (S)-2-tert-butoxy-24(S)-7-(2,3-dihydropyrano[4,3,2-
delquinolin-7-
y1)-5-methyl-2-(3-methylazetidin-1-yObenzo[d]thiazol-6-ypacetic acid (57).
Compound 57 was prepared from compound 32 according to the procedure used to
prepare compound 35 (except that 2-methylazetidine was used instead of
azetidine) in Example
10.
0
0j<
- OH
CN 0
57
Compound 57: 111-NMR: 400 MHz, (CD30D) 8: 8 8.92 (s, 111), 7.90 (d, J= 7.6 Hz,
211),
7.56 (s, 1H), 7.44 (d, J= 7.3 Hz, 111), 5.18 (s, 1H), 4.73 (s, 2H), 4.48 (s,
211), 3.99 (s, 2H), 3.68
(s, 2H), 3.12 (m, 1H), 2.73 (s, 3H), 1.35 (d, J= 5.6 Hz, 3H), 0.91 (s, 9H).
LCMS-ESI+ (m/z):
[M+Hr calcd for C291132N304S: 518.21(M+H+); Found: 518.09, 519.12(M+H+).
Example 20b. Preparation of (S)-2-tert-butoxy-2-((S)-7-(2,3-
dihydropyrano[4,3,2-de]quinolin-7-
y1)-5-methyl-2-(3-(methylsulfonypazetidin-1-yObenzo[d]thiazol-6-yDacetic acid
(58).
Compound 58 was prepared from compound 32 according to the procedure used to
prepare compound 35 (except that 2-methylsulfonylazetidine was used instead of
azetidine) in
Example 10.
0
CY<
()µµS N
N 0
58 OH
140
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
Compound 58: 1H-NMR: 400 MHz, (CD30D) 6:1H NMR (400 MHz, cd3od) 6 8.85 (d, J
= 5.3 Hz, 1H), 7.89 (t, J= 6.7 Hz, 2H), 7.61 (s, 1H), 7.44 (d, J= 8.1 Hz, 1H),
5.18 (s, 1H), 4.72
(dd, J= 9.0, 6.2 Hz, 2H), 4.59 ¨4.35 (m, 5H), 3.01 (s, 3H), 2.72 (s, 3H), 0.92
(s, 9H). LCMS-
ESI+ (m/z): [M+Hr calcd for C29H32N306S: 582.17 (M+H+); Found: 581.95,
583.02(M+H+).
Example 21. Preparation of (S)-24(S)-2-0(1,3-dioxolan-2-
y1)methyl)(methypamino)-7-(2,3-
dihydropyrano[4,3,2-de]quinolin-7-y1)-5-methylbenzo[d]thiazol-6-y1)-2-tert-
butoxyacetic acid
(59) and (S)-2-((R)-2-(((1,3-dioxolan-2-yl)methyl)(methyl)amino)-7-(2,3-
dihydropyrano[4,3,2-
de]quinolin-7-y1)-5-methylbenzo[d]thiazol-6-y1)-2-tert-butoxyacetic acid (60).
Compounds 59 and 60 were prepared from compound 32 according to the procedure
used to
prepare compound 35 (except that 1-(1,3-dioxolan-2-y1)-N-methylmethanamine was
used
instead of azetidine) in Example 10.
Cr.<
S OH
Co) N 0
59
Compound 59: 1H-NMR: 400 MHz, (CD30D) 6: 8.81 (d, J = 6 Hz, 1H), 7.85 (d, J =
8
Hz, 111), 7.81 (d, J = 6 Hz, 1H), 7.54 (s, 1H), 7.40 (d, J = 8 Hz, 1H), 5.15
(s, 111), 5.07 (m, 1H),
4.69 (m, 2H), 3.91 (m, 2H), 3.82 (m, 2H), 3.63 (m, 2H), 3.30 (s, 3H), 2.68 (s,
3H), 0.89 (s, 9H).
LCMS-ESI+ (m/z): [M+H] calcd for C30H33N306S: 564.2 (M+H+); Found: 564.1
(M+H+).
0
0'<
S : OH
,0
_____________________________________ N 0
0 60
Compound 60: 1H-NMR: 400 MHz, (CD30D) 6: 8.70 (d, J = 6 Hz, 1H), 8.09 (d, J =
8
Hz, 1H), 7.60 (d, J = 6 Hz, 111), 7.48 (s, 1H), 7.35 (d, J = 8 Hz, 1H), 5.19
(s, 111), 5.07 (m, 1H),
4.65 (m, 2H), 3.92 (m, 2H), 3.83 (m, 211), 3.53 (m, 2H), 3.23 (s, 3H), 2.63
(s, 3H), 0.85 (s, 9H).
LCMS-ES[+ (m/z): [M+Hr calcd for C301133N306S: 564.2 (M+H+); Found: 564.1
(M+H+).
Example 22. Preparation of (S)-2-tert-butoxy-24(S)-7-(2,3-dihydropyrano[4,3,2-
de]quinolin-7-
y1)-242-(dimethylamino)ethyl)(methypamino)-5-methylbenzo[d]thiazol-6-ypacetic
acid (61)
141
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
and (S)-2-tert-butoxy-24(R)-7-(2,3-dihydropyrano[4,3,2-de]quinolin-7-y1)-2-02-
(dimethylamino)ethyl)(methypamino)-5-methylbenzo[d]thiazol-6-yeacetic acid
(62).
Compounds 61 and 62 were prepared from compound 32 according to the procedure
used to prepare compound 35 (except that N1,N1,N2-trimethylethane-1,2-diamine
was used
instead of azetidine) in Example 10.
0
0-j<
S : OH
0
N
-N
61
Compound 61: 1H-NMR: 400 MHz, (CD30D) 8: 8.78 (d, J = 6 Hz, 111), 7.80 (m,
2H),
7.57 (s, 1H), 7.38 (d, J = 8 Hz, 1H), 5.15 (s, 111), 4.67 (m, 2H), 4.11 (m,
1H), 3.94 (s, 1H), 3.63
(t, J = 6 Hz, 2H), 3.47 (m, 2H), 3.02 (s, 6H), 2.66 (s, 3H), 0.89 (s, 9H).
LCMS-ESI+ (m/z):
[M+Hr calcd for C301136N404S: 549.3 (M+H+); Found: 549.0 (M+H+).
0
0'<
S OH
N-4 0
¨N N
62
Compound 62: 1H-NMR: 400 MHz, (CD30D) 8: 8.71 (d, J = 6 Hz, 1H), 8.13 (d, J =
8
Hz), 7.70 (d, J = 6 Hz, 214), 7.54 (s, 11-1), 7.41 (d, J = 8 Hz, 111), 5.18
(s, 1H), 4.66 (m, 2H), 4.06
(m, 1H), 3.98 (s, 114), 3.59 (t, J = 6 Hz, 2H), 3.47 (m, 2H), 3.02 (s, 3H),
3.00 (s, 311), 2.63 (s,
311), 0.89 (s, 9H).
LCMS-ESI (m/z): [M+H]+ calcd for C301-136N404S: 549.3 (M+H+); Found: 549.0
(M+11+).
Example 23. Preparation of (2S)-2-(2-(benzyl(methyl)amino)-7-(2,3-
dihydropyrano[4,3,2-
de]quinolin-7-y1)-5-methylbenzo[d]thiazol-6-y1)-2-tert-butoxyacetic acid (63).
Compound 63 was prepared as a mixture of atropisomers from compound 32
according to the
procedure used to prepare compound 35 (except that N-methyl-N-benzylamine was
used instead
of azetidine) in Example 10.
142
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
0
0"/<
S : OH
0
63
Compound 63: LCMS-ESI+ (m/z): [M+H1+ calcd for C33H33N304S: 568.2 (M+H+);
Found: 568.1 (M+H+).
Examle 24. Preparation of (S)-2-tert-butoxy-2-((R)-7-(2,3-dihydropyrano[4,3,2-
de]quinolin-7-
y1)-2-02-(dimethylamino)-2-oxoethyl)(methypamino)-5-methylbenzo[d]thiazol-6-
ypacetic acid
(64).
Compound 64 was prepared from compound 32 according to the procedure used to
prepare compound 35 (except that N,N-dimethy1-2-(methylamino)acetamide was
used instead of
azetidine) in Example 10.
0
CY<
\ S 7 OH
0
_____________________________________ N 0
-N 64
Compound 64: 11-I-NMR: 400 MHz, (CD30D) 5: 8.72 (d, J = 6 Hz, 1H), 8.14 (d, J
= 8
Hz), 7.70 (d, J = 6 Hz, 2H), 7.48 (s, 1H), 7.41 (d, J = 8 Hz, 1H), 5.19 (s,
1H), 4.66 (m, 2H), 3.58
(t, J = 6 Hz, 2H), 3.42 (s, 2H), 3.10 (s, 3H), 3.00 (s, 3H), 2.94 (s, 311),
2.63 (s, 3H), 0.91 (s, 911).
LCMS-ESI+ (m/z): [M+H]+ calcd for C301-134N405S: 563.2 (M+H+); Found: 563.1
(M+H+).
Example 25. Preparation of (S)-2-tert-butoxy-24(S)-7-(2,3-dihydropyrano[4,3,2-
de]quinolin-7-
y1)-2-methoxy-5-methylbenzo[d]thiazol-6-ypacetic acid (66).
0
c D0
7 OH
N 0
66
143
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
Compound 66: 1H-NMR: 400 MHz, (CD30D) 8: 8.63 (d, J=4.4 Hz, 1H); 7.68 (d,
J=8.0
Hz, 1H); 7.54 (s, 1H); 7.38 (d, J=4.8 Hz, 1H); 7.14 (d, J=7.6 Hz, 1H); 5.08
(s, 1H); 4.58-4.53
(m, 2H); 4.11 (s, 3H); 3.39 (t, J=6.0 Hz, 2H); 2.61 (s, 3H); 0.87 (s, 9H).
LCMS-ESI+ (m/z):
[M+Hi+ calcd for C26H27N205S: 479.16 (M+H+); Found: 479.00, 480.02(M+H+).
Br Br Br
401 OH OTf
02N 02N 02N
65A 65B 65C
Br OH Br OH Br i:Y<
OH OPiv OPiv
02N 02N 02N
65D 65E 65F
Br (:)"'< Br CY-<
OPiv OPiv
H2N¨ I
H2N
65G 65H
Br 0-< Br CY*-<
OPiv OPiv
MeO-4. I
651 65J
0 0
0-k
LNY
7 OPiv OH
Me04 I Me0--
0
65K 66
Step 1.
Preparation of 2-bromo-6-methyl-4-nitrophenyl trifluoromethanesulfonate (65B).
To a
solution of 2-bromo-6-methyl-4-nitrophenol (65A) (58.0 g, 250 mmol) in CH2C12
(500 mL) at -
70 C was added triethylamine (45.3 mL, 325 mmol) then
trifluoromethanesulfonic acid
anhydride (46.3 mL, 275 mmol). After 20 min, a solution of HCI was added (0.5
M, 500 mL).
The layers were separated. The organic layer was dried, filtered, and
concentrated in vacuo. The
144
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
crude oil was run through a plug of SiO2 and celite with 10% Et0Ac in hexanes
to give 90 g of
65B.
11-1-NMR: 400 MHz, (CDC13) 8: 8.40 (d, J = 2.4 Hz, 1H), 8.15 (d, J = 2.4 Hz,
1H), 2.58 (s, 3H).
Step 2.
Preparation of 1-bromo-3-methyl-5-nitro-2-vinylbenzene (65C): The reaction
mixture of
2-bromo-6-methyl-4-nitrophenyl trifluoromethanesulfonate (65B) (10.1g, 27.7
mmol),
tributylvinyltin (8.18 ml, 27.7 mmol), LiC1 (1.4g, 33.2 mmol), PdC12dppf (607
mg, 0.83 mmol)
in DMF (50 ml) was reacted at 70 C for 3h. Then 2N NaOH was added and stirred
at 70 C for
5 min. The reaction mixture was cooled down, washed by sat. NaHCO3, extracted
by Et0Ac,
dry over MgSO4, filtered, concentrated down and purified by silica gel column,
eluting by 0-
100% Et0Ac in Hexanes to give 65C (1.9 g, 30%). 111-NMR: 400 MHz, (CDC13) 8:
8.30 (d, J =
0.8 Hz, 114 8.03 (d, J = 1 Hz, 1H), 6.71-6.64 (dd, J = 18, 12 Hz, 1H), 5.77-
5.74 (d, J = 12
Hz,1H), 5.51-5.46 (d, J = 18 Hz,1H), 2.48 (s, 3H).
Step 3.
Preparation of (S)-1-(2-bromo-6-methy1-4-nitrophenyl)ethane-1,2-diol (65D):
The
reaction mixture of 1-bromo-3-methyl-5-nitro-2-vinylbenzene (65C) (12.3g,
50.83 mmol), AD-
mix a (71 g) , MeS02NH2 (4.8 g, 50.8 mmol) in t-butyl alcohol/H20 (1:1) (200
ml) was stirred
at 0 C for 3 days. Na2S03 (- 6 g) was added to quench the reaction, stirred
at rt for 40 min. The
reaction mixture was washed by water, extracted by Et0Ac, dry over MgSO4,
filtered,
concentrated down and purified by silica gel column, eluting by 0-100% Et0Ac
in Hexanes to
give 6.96 g of 65D and recovered 2.3 g of 65C.
1H-NMR: 400 MHz, (CDC13) 8: 8.25 (d, J = 2 Hz, 1H), 7.98 (d, J = 2 Hz, 1H),
5.55 (m, 1H),
3.93 (dd, J = 11, 9 Hz, 1H), 3.77 (dd, J = 11, 4 Hz), 2.66 (s, 3H).
Step 4.
Preparation of (S)-2-(2-bromo-6-methyl-4-nitropheny1)-2-hydroxyethyl pivalate
(65E):
To a suspension of (S)-1-(2-bromo-6-methy1-4-nitrophenypethane-1,2-diol (65D)
(6.96 g, 25.22
mmol) in DCM (100 ml), was added pyridine (5 mL) at 0 C. To the solution was
added
pivaloyl chloride (PivC1) slowly at 0 C. The reaction mixture was stirred at
0 C for 5 min, then
raised to rt, stirred at rt for 5h. The reaction mixture was washed by sat
NaHCO3, extracted by
DCM, dry over MgSO4, filtered, purified by silica gel column, eluting by 0-40%
Et0Ac in
Hexanes to give 9.13 g of 65E. The product taken on without full
characterization.
Step 5.
Preparation of (S)-2-(2-bromo-6-methyl-4-nitropheny1)-2-tert-butoxyethyl
pivalate
(65F): To a solution of (S)-2-(2-bromo-6-methyl-4-nitropheny1)-2-hydroxyethyl
pivalate (65E)
in t-butyl acetate at 0 C, was added HC104 (perchloric acid) (5.45 ml)
slowly, stirred at 0 C for
145
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
min, then the reaction mixture was warmed up to it and stirred for 3hs. The
mixture was
diluted by Et0Ac, washed by sat. NaHCO3, extracted by Et0Ac, dry over MgSO4,
filtered,
concentrated down and purified by silica gel column, eluting by 0-100% Et0Ac
in Hexanes to
give 65F (9g, 85 %).
5 1H-NMR: 400 MHz, (CDC13) 5: 8.23 (d, J = 1 Hz, 1H), 7.96 (d, J =1.2 Hz,
111), 5.58-5.54(m,
1H), 4.30-4.25 (m, 1 H), 4.16-4.12 (m, 1H), 2.71 (s, 3H), 1.154 (s, 9H), 1.151
(s, 9H).
Step 6.
Preparation of (S)-2-(2-amino-7-bromo-5-methylbenzo[d]thiazol-6-y1)-2-tert-
butoxyethyl pivalate (6511): To a solution of S)-2-(2-bromo-6-methy1-4-
nitropheny1)-2-tert-
butoxyethyl pivalate (9 g, 21.63 mmol) in Et0H (50 ml) and Et0Ac (50 ml) was
added Pt/C (
1.5g), attached with a balloon of112. More Pt/ C (500 mg) was added after 3h.
Then the reaction
mixture was stirred at rt for another 2h. The reaction mixture was filtered
over celite,
concentrated down to give product (S)-2-(4-amino-2-bromo-6-methylpheny1)-2-
tert-butoxyethyl
pivalate (65G) and went to next step without purification. To a solution of
(S)-2-(4-amino-2-
bromo-6-methylpheny1)-2-tert-butoxyethyl pivalate (65G) (21.63 mmol) in HOAc/
THF (80 ml,
1:1) was added KSCN at 0 C. The reaction mixture was stirred at 0 C for 0.5
h. Then Br2 was
added slowly, reacted at 0 C. The reaction was quenched by adding sat.
NaHS03, extracted by
Et0Ac, dried over MgSO4, purified by silica gel column, eluting by 0-40% Et0Ac
in Hexanes
to give 65H (2.3 g, 24% over 2 steps).
1H-NMR: 400 MHz, (CD30D) 5: 7.15 (s, 1H), 5.51 (t, J = 7 Hz, 1H), 4.28 (m,
111), 4.14 (m,
1H), 2.62 (s, 3H), 1.15 (s, 911), 1.10 (s, 911).
LCMS-ESI+: calc'd for C19H27BrN204S: 443.1 (M+H+); Found: 443.1 (M+H+).
Step 7.
Preparation of (S)-2-(7-bromo-2-chloro-5-methylbenzo[d]thiazol-6-y1)-2-tert-
butoxyethyl pivalate (651): The reaction mixture of (S)-2-(2-amino-7-bromo-5-
methylbenzo[d]thiazol-6-y1)-2-tert-butoxyethyl pivalate (6511) (100 mg, 0.226
mmol), t-butyl
nitrite (32 ul, 0.271 mmol), CuC12 (36 mg, 0.271 mmol) in acetonitrile (1.5m1)
was reacted at rt.
The reaction mixture was diluted by Et0Ac, washed by water, extracted by Et0Ac
, dried over
MgSO4, filtered, concentrated down and purified by silica gel column, eluting
by 0-40% Et0Ac
in Hexanes to give 651 (90 mg, 86 %).
LCMS-ESI+: calc'd for C19H25C1BrNO3S: 462.0 (M+H ); Found: 462.1 (M+H+).
Step 8.
Preparation of (S)-2-(7-bromo-2-methoxy-5-methylbenzo[d]thiazol-6-y1)-2-tert-
butoxyethyl pivalate (65J): The reaction mixture of (S)-2-(7-bromo-2-ehloro-5-
methylbenzo[d]thiazol-6-y1)-2-tert-butoxyethyl pivalate (651) (90 mg, 0.195
mmol), Na0Me in
146
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
Me0H (25 % wt, 66 ul) in Me0H (3 ml) was heated at 50 C for 20 min in sealed
microwave
vial. The reaction mixture was washed by sat. NaHCO3, extracted by Et0Ac,
dried over MgSO4,
filtered, concentrated down and purified by silica gel column, eluting by 0-
100% Et0Ac in
Hexanes to give 65J (70 mg, 79 %).
LCMS-ESI+: calc'd for C201-128BrNO4S: 458.1 (M+H+); Found: 458.1 (M+H+).
Step 9.
Preparation of (S)-2-tert-butoxy-24(S)-7-(2,3-dihydropyrano[4,3,2-de]quinolin-
7-y1)-2-
methoxy-5-methylbenzo[d]thiazol-6-yDethyl pivalate (65K): The reaction mixture
of (S)-2-(7-
bromo-2-methoxy-5-methylbenzo[cl]thiazol-6-y1)-2-tert-butoxyethyl pivalate
(65K) (70 mg,
0.153 mmol), 2,3-dihydropyrano[4,3,2-de]quinolin-7-ylboronic acid
hydrochloride (58 mg, 0.23
mmol), 2N K2CO3 ( 380u1), Pd(PPh3)4 (17 mg, 0.015 mmol) in DME (3 ml) was
heated at 90 C
overnight. The reaction mixture was washed by sat. NaHCO3, extracted by Et0Ac,
dried over
MgSO4, filtered, concentrated down and purified by silica gel column, eluting
by 0-100%
Et0Ac in Hexanes to give 65K.
LCMS-ESI+: calc'd for C31H36N205S: 549.2 (M+H+); Found: 549.0 (M+H+).
Step 10.
Preparation of (S)-2-tert-butoxy-24(S)-7-(2,3-dihydropyrano[4,3,2-de]quinolin-
7-y1)-2-
methoxy-5-methylbenzo[d]thiazol-6-ypethanol : The mixture of (S)-2-tert-butoxy-
24(S)-7-(2,3-
dihydropyrano[4,3,2-de]quinolin-7-y1)-2-methoxy-5-methylbenzo[d]thiazol-6-
ypethyl pivalate
(65K) (20 mg, 0.036 mmol), 2N NaOH (360 ul) in THF/Me0H (1:1, 2 ml) was
stirred at 40 C
overnight. The reaction mixture then was washed by sat. NaHCO3, extracted by
Et0Ac, dried
over MgSO4, filtered, concentrated down and purified by silica gel column,
eluting by 0-100%
Et0Ac in Hexanes to give the product (11 mg).
LCMS-ESI+: calc'd for C26H28N204S: 465.2 (M+H+); Found: 465.7 (M+H+).
Step 11.
Preparation of (S)-2-tert-butoxy-24(S)-7-(2,3-dihydropyrano[4,3,2-de]quinolin-
7-y1)-2-
methoxy-5-methylbenzo[d]thiazol-6-ypacetic acid (66): To a solution of (S)-2-
tert-butoxy-2-
((S)-7-(2,3-dihydropyrano[4,3,2-de]quinolin-7-y1)-2-methoxy-5-
methylbenzo[d]thiazol-6-
ypethanol (11 mg, 0.024 mmol) in wet acetonitrile (0.75 % v H20), was added
stock solution of
H6105/Cr03 (0.439 mmol, 500 ul) at 0 C. After the reaction was finished, the
reaction was
quenched by adding 1.5 M K2HPO4, extracted by Et0Ac, the organic phase was
washed by
NaHS03/brine(1:1), dried over MgSO4, filtered, concentrated down and purified
by silica gel
column, eluting by 20-80% Et0Ac in hexanes to give 66 (3.1 mg).
147
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
114-NMR: 400 MHz, (CD30D) 5: 8.63 (d, J=4.4 Hz, 114); 7.68 (d, J=8.0 Hz, 1H);
7.54 (s, 1H);
7.38 (d, J=4.8 Hz, 1H); 7.14 (d, J=7.6 Hz, 1H); 5.08 (s, 114); 4.58-4.53 (m,
2H); 4.11 (s, 3H);
3.39 (t, J=6.0 Hz, 2H); 2.61 (s, 3H); 0.87 (s, 9H)ppm.
LCMS-ESI+ (m/z): [M+11} calcd for C26H271\1205S: 479.16 (M+H+); Found:
479.00,
480.02(M+H+).
Example 26. Preparation of (S)-2-tert-butoxy-24(S)-7-(2,3-dihydropyrano[4,3,2-
de]quinolin-7-
y1)-3,5-dimethy1-2-oxo-2,3-dihydrobenzo[d]thiazo1-6-y1)acetic acid (68).
0
os O
0
68 H
Compound 68:1H-NMR: 400 MHz, (CDC13) 5: 8.66 (d, J=4.0 Hz, 1H); 7.69 (d, J=8.4
Hz, 1H); 7.29 (d, J=4.0 Hz, 1H); 7.16 (d, J=8.4 Hz, 1H); 6.97 (s, 111); 4.94
(s, 111); 4.59 (dd, J1=
5.2 Hz, J2=9.6 Hz, 2H); 3.44 (s, 3H); 3.39 (t, J=5.6 Hz, 214); 2.64 (2, 3H);
0.90 (s, 9H). LCMS-
ESI+ (m/z): [M+H] calcd for C26H271\1205S: 479-.16 (M+H+); Found: 479.04,
480.06(M+H+).
Br 0<
7 OPiv
OPiv
I Bn0-µ I
67A
651
Br 0
HO OPiv
OPiv
I
-- I
1
6
67B 7C
0
c D0
OPiv
I
1
67D
Step 1.
148
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
Preparation of (S)-2-(2-(benzyloxy)-7-bromo-5-methylbenzo[d]thiazol-6-y1)-2-
tert-
butoxyethyl pivalate (67A): NaH (415 mg, 10.38 mmol) was added to Bn0H,
stirred at rt for 0.5
h. The Na0Bn solution was transferred to a flask charged with (S)-2-(7-bromo-2-
chloro-5-
methylbenzo[d]thiazol-6-y1)-2-tert-butoxyethyl pivalate (651) (1.6g, 3.46
mmol). The reaction
.. mixture was heated at 60 C for 45 min. The reaction mixture was washed by
sat NaHCO3,
extracted by Et0Ac, the organic phase was washed by brine, dry over MgSO4,
filtered,
concentrated down, distilled most NaOH off. The residue was purified by silica
gel column,
eluting by 0-50% Et0Ac in hexanes.
1H-NMR: 400 MHz, (CDC13) 8: 7.52-7.24 (m, 6H),5.58 (s, 2H), 5.57-5.45 (m, 1H),
4.35-4.27
(m, 1H), 4.18-4.12 (m, 1H), 2.68 (s, 3H), 1.07 (s, 1811).
Step 2.
Preparation of (S)-2-(7-bromo-2-hydroxy-5-methylbenzo[d]thiazol-6-y1)-2-tert-
butoxyethyl pivalate (67B): The mixture of (S)-2-(2-(benzyloxy)-7-bromo-5-
methylbenzo[d]thiazol-6-y1)-2-tert-butoxyethyl pivalate (67A), Pd/C (800 mg)
in Et0Ac/Et0H
(10 ml, 1:1) was charged into a flask with a H2 balloon, and stirred at rt for
lh. The reaction
mixture was filtered over celite, concentrated down, purified by silica gel
column, eluting by 0-
50% Et0Ac in hexanes to give 67B (850 mg).
111-NMR: 400 MHz, (CDC13) 8: 8.99 (s, 1H), 6.88 (s, 1H), 5.45 (t, J = 7 Hz,
4.26-4.22 (m, 1H),
4.14-4.09 (m, 1H), 2.62 (s, 3H), 1.13 (s, 18H).
Step 3.
Preparation of (S)-2-(7-bromo-3,5-dimethy1-2-oxo-2,3-dihydrobenzo[d]thiazol-6-
y1)-2-
tert-butoxyethyl pivalate (67C): To a solution of (S)-2-(7-bromo-2-hydroxy-5-
methylbenzo[d]thiazol-6-y1)-2-tert-butoxyethyl pivalate (67B) (40 mg, 0.090
mmol) in THF
(1m1) was added KOtBu (0.14 ml, 0.135 mmol, 1M in THF) slowly at -78 C. After
15 min, Mel
(8.5 ul, 0.135 mmol) was added at -78 C and stirred at -78 C for 15 min.
Then the reaction was
reacted at rt for 3hs. The reaction mixture was washed by sat. NaHCO3,
extracted by Et0Ac,
dried over MgSO4, filtered, concentrated down and purified by silica gel
column, eluting by 0-
40% Et0Ac in hexanes to give 67C (30 mg, 73%).
'H-NMR: 400 MHz, (CDC13) 8: 6.79 (s, 1H), 5.49-5.45 (m, 1H), 4.27-4.23 (m,
1H), 4.14-4.10
(m, 1H), 3.40 (s, 3H), 2.66 (s, 3H), 1.46 (s, 18H).
Step 4.
Preparation of (S)-2-tert-butoxy-24(S)-7-(2,3-dihydropyrano[4,3,2-de]quinolin-
7-y1)-
3,5-dimethyl-2-oxo-2,3-dihydrobenzo[d]thiazol-6-ypethyl pivalate (67D): The
reaction mixture
of (S)-2-(7-bromo-3,5-dimethy1-2-oxo-2,3-dihydrobenzo[d]thiazol-6-y1)-2-tert-
butoxyethyl
pivalate (67C) (20 mg, 0.044 mmol), 2,3-dihydropyrano[4,3,2-de]quinolin-7-
ylboronic acid
149
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
hydrochloride (16.5 mg, 0.066 mmol), 2N K2CO3 (0.12 ml, 0.22 mmol), Pd(PPh3)4
(5.0 mg,
0.0044 mmol) in DME(1 ml) was heated at 120 C in sealed microwave vial for
3hs. The
reaction mixture was washed by sat. NaHCO3, extracted by Et0Ac, the organic
phase was dried
over MgSO4, filtered, concentrated down and purified by silica gel column,
eluting by 0-60%
Et0Ac in hexanes to give the product (15 mg, 62%).
LCMS-ESI : calc'd for C31H36N205S: 549.2 (M+H+); Found: 549.0 (M+H+).
The remainder of the synthesis of compound 68 is analogous to the preparation
of compound 66
from compound 65K in example 25.
Example 27. Preparation of compound (S)-2-tert-butoxy-24(S)-7-(2,3-
dihydropyrano[4,3,2-
de]quinolin-7-y1)-2-isopropoxy-5-methylbenzo[d]thiazol-6-ypacetic acid (70).
0
7 H
I
N 0
Compound 70:11I-NMR: 400 MHz, (CDC13) 5: 8.60 (d, J=4.8 Hz, 1H); 7.76 (d,
J=7.6
15 Hz, 1H); 7.57 (s, 1H); 7.28-7.26 (m, 1H); 7.15 (d, J=8.0 Hz, 1H); 5.37-
5.30 (m, 1H); 4.97 (s,
1H); 4.61-4.57 (m, 2H); 3.39 (t, J=6.2 Hz, 211); 2.64 (s, 3H); 1.39 (dd,
J1=6.4 Hz, J2= 14 Hz,
6H); 0.91 (s, 911).
LCMS-ESI+ (m/z): [M+Hr calcd for C28113IN205S: 507.19 (M+H+); Found: 507.01,
508.07(M+H+).
150
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
Br CY.< Br O'v<
= OPiv = OPiv
I
SEM
67B
69A
0 0
= OPiv
= OPiv
I
69B 69C
0
Oer<
= OPiv
I
N
69D
Step 1.
Preparation of (S)-2-(7-bromo-5-methy1-2-oxo-34(2-
(trimethylsilypethoxy)methyl)-2,3-
dihydrobenzo[d]thiazol-6-y1)-2-tert-butoxyethyl pivalate (69A): Prepared by
the similar method
to make (S)-2-(7-bromo-3,5-dimethy1-2-oxo-2,3-dihydrobenzo[d]thiazol-6-y1)-2-
tert-
butoxyethyl pivalate (67C) in example 26 from 67B using 2-
(trimethylsilyl)ethoxymethyl
chloride (SEMC1) instead of methyl iodide.
1H-NMR: 400 MHz, (CDC13) (5: 7.05 (s, 1H); 5.53-5.49 (s, 1H), 5.34 (s, 2H),
4.32-4.27 (m, 1H),
4.18-4.14 (m, 1H), 3.66 (t, J = 8 Hz, 2H), 2.68 (s, 3H), 1.19 (s, 1811), 0.97-
0.89 (m, 211), 0.00 (s,
9H).
Step 2.
Preparation of (S)-2-tert-butoxy-2-((S)-7-(2,3-dihydropyrano[4,3,2-de]quinolin-
7-y1)-3,5-
dimethy1-2-oxo-2,3-dihydrobenzo[d]thiazol-6-ypethyl pivalate (69B): prepared
by the similar
method to make 67D from 67C in Example 26.
LCMS-ESI+: calc'd for C36[148N206SSi: 665.3 (M+H+); Found: 664.9 (M+H+).
Step 3.
Preparation of (S)-2-tert-butoxy-24(S)-7-(2,3-dihydropyrano[4,3,2-delquinolin-
7-y1)-5-
methy1-2-oxo-2,3-dihydrobenzo[d]thiazol-6-yDethyl pivalate (69C): The reaction
mixture of
151
CA 02833377 2013-10-16
WO 2012/145728
PCT/US2012/034593
(S)-2-tert-butoxy-24(S)-7-(2,3-dihydropyrano[4,3,2-de]quinolin-7-y1)-3,5-
dimethyl-2-oxo-2,3-
dihydrobenzo[d]thiazol-6-yDethyl pivalate (69B) (250 mg, 0.376 mmol), TBAF (1M
in THF,
1.1 ml, 1.1 mmol) in DME was heated at 120 C in sealed microwave vial for 3
h. The reaction
mixture was cooled down, washed by sat. NaHCO3, extracted by Et0Ac, the
organic phase was
dried over MgSO4, filtered, concentrated down and purified by silica gel
column, eluting by 0-
100% Et0Ac in hexanes to give 69C (30 mg, 15 %).
LCMS-ESI+: calc'd for C30H34N205S: 535.2 (M+H+); Found: 535.0 (M+H+).
Step 4.
Preparation of (S)-2-tert-butoxy-24(S)-7-(2,3-dihydropyrano[4,3,2-de]quinolin-
7-y1)-2-
isopropoxy-5-methylbenzo[d]thiazol-6-ypethyl pivalate (69D): The reaction
mixture of (S)-2-
tert-butoxy-24(S)-7-(2,3-dihydropyrano[4,3,2-de]quinolin-7-y1)-5-methyl-2-oxo-
2,3-
dihydrobenzoklithiazol-6-yDethyl pivalate (69C) (30 mg, 0.056 mmol), Ag2CO3
(50% wt on
celite, 310 mg, 0.56 mmol), isopropy bromide (160 ul, 1.68 mmol) in
benzene/DME (1:1, 2 ml)
was heated at 70 C overnight. The reaction mixture was washed by water,
extracted by Et0Ac,
the organic phase was dried over MgSO4, filtered, concentrated down and
purified by silica gel
column, eluting by 0-60% Et0Ac in hexanes to give the product (15 mg, 46 %).
LCMS-ESI+: calc'd for C33H40N205S: 577.3 (M+H+); Found: 577.0 (M+H+).
The remainder of the synthesis of compound 70 is analogous to the preparation
of compound 66
from compound 65K in example 25.
Example 28. Preparation of (S)-2-tert-butoxy-24(S)-7-(2,3-dihydropyrano[4,3,2-
de]quinolin-7-
y1)-5-methyl-2-oxo-3-(2-(trifluoromethypbenzy1)-2,3-dihydrobenzo[d]thiazol-6-
yflacetic acid
(71).
Compound 71 was prepared from compound 69C according to the procedure used to
prepare compound 67C (except that 1-(bromomethyl)-2-(trifluoromethyl)benzene
was used
instead of methyl iodide) in Example 26, and the remainder of the synthesis of
compound 71 is
analogous to the preparation of compound 66 from compound 65K in example 25.
152
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
0
ç D0
os OH
0
F F 71
Compound 71:1H-NMR: 400 MHz, (CDC13) 8: 8.72 (s, 111); 7.76-7.73 (m, 2H); 7.50
(t,
J=7.6 Hz, 1H); 7.42 (t, J=7.4 Hz, 111); 7.33 (t, J=4.0 Hz, 1H); 7.2 (d, J=8.0
Hz, 1H); 7.13 (d,
J=6.8 Hz, 1H); 6.72 (s, 1H); 5.34 (s, 111); 4.95 (s, 111); 4.65-4.60 (m, 211);
3.42 (t, J=5.4 Hz,
2H); 2.52 (2, 3H); 0.88 (s, 911).
19F NMR (377 MHz, CDC13) 8 -60.73. LCMS-ESI+ (m/z): [M+Hr calcd for
C33H30F3N204S:
623.18 (M+H+); Found: 623.09, 624.09(M+H+).
Example 29. Preparation of (S)-24(S)-3-benzy1-7-(2,3-dihydropyrano[4,3,2-
de]quinolin-7-y1)-5-
methyl-2-oxo-2,3-dihydrobenzo[d]thiazol-6-y1)-2-tert-butoxyacetic acid (72).
Compound 72 was prepared from compound 69C according to the procedure used to
prepare compound 67C (except that benzyl bromide was used instead of methyl
iodide) in
Example 26, and the remainder of the synthesis of compound 71 is analogous to
the preparation
of compound 66 from compound 65K in example 25.
0
ç D0
os OH
0
72
Compound 72:11-1-NMR: 400 MHz, (CDC13) 8: 8.67 (d, J=4.0 Hz, 1H); 7.70 (d,
J=8.4
Hz, 111); 7.37-7.36 (m, 411); 7.34-7.29 (m, 211); 7.16 (d, J=8.4 Hz, 1H); 6.92
(s, 1H); 5.21-5.01
(dd, J1=15.6 Hz, J2= 79.6 Hz, 2H); 4.92 (s, 111); 4.63-41.56 (m, 211); 3.39
(t, J=5.8 Hz, 2H); 2.55
(s, 3H); 0.88 (s, 911). LCMS-ESI+ (m/z): [M+H] calcd for C32H311\1205S: 555.19
(MAI);
.. Found: 555.08, 556.12 (M+11+).
153
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
Example 30. Preparation of (S)-2-tert-butoxy-24(S)-7-(2,3-dihydropyrano[4,3,2-
delquinolin-7-
y1)-5-methyl-2-oxo-3-(3-(trifluoromethyl)benzy1)-2,3-dihydrobenzo[d]thiazol-6-
yDacetic acid
(73).
Compound 73 was prepared from compound 69C according to the procedure used to
prepare compound 67C (except that 1-(bromomethyl)-3-(trifluoromethyl)benzene
was used
instead of methyl iodide) in Example 26, and the remainder of the synthesis of
compound 71 is
analogous to the preparation of compound 66 from compound 65K in example 25.
0
0-<
os : OH
0
73
FF
Compound 73:111-NMR: 400 MHz, (CDC13) 6: 8.68 (s, 1H); 7.71 (d, J=7.6 Hz, 1H);
7.64 (s, 1H); 7.59 (d, J=6.4 Hz, 1H); 7.51-7.48 (m, 2H); 7.32 (d, J=3.2 Hz,
1H); 7.18 (d, J=7.6
Hz, 1H); 6.87 (s, 1H); 5.25-5.06 (dd, J1= 16Hz, .12= 63.2 Hz, 2H); 4.94 (s,
1H); 4.65-4.59 (m,
2H); 3.43 (t, J=5.2 Hz, 1H); 2.56 (s, 3H); 0.88 (s, 9H). LCMS-ES1+ (m/z):
[M+11]+calcd for
C27H29N304S: 623.18 (M+H+); Found: 623.04, 624.09(M+H+).
Example 31. Preparation of (S)-2-tert-butoxy-24(S)-7-(2,3-dihydropyrano[4,3,2-
delquinolin-7-
y1)-5-methyl-2-oxo-3-(4-(trifluoromethyDbenzy1)-2,3-dihydrobenzo[d]thiazol-6-
yDacetic acid
(74).
Compound 74 was prepared from compound 69C according to the procedure used to
prepare compound 67C (except that 1-(bromomethyl)-4-(trifluoromethypbenzene
was used
instead of methyl iodide) in Example 26, and the remainder of the synthesis of
compound 71 is
analogous to the preparation of compound 66 from compound 65K in example 25.
154
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
0
CY<
: OH
0
74
Compound 74:1H-NMR: 400 MHz, (CDC13) 6: 8.67 (d, J=4.4 Hz, 1H); 7.70 (d, J=8.0
Hz, 111); 7.63 (d, J=8.0 Hz, 1H); 7.46 (d, J=7.6 Hz, 1H); 7.31 (d, J=4.0 Hz,
1H); 6.86 (s, 1H);
5.25-5.07 (dd, J1=16, J2=56.8 Hz, 2H); 4.93 (s, 1H); 4.63-4.58 (m, 2H); 3.40
(t, J=5.8 Hz, 2H);
2.56 (s, 3H); 0.88 (s, 9H). LCMS-ESI+ (m/z): [M+H] calcd for C33H30F3N205S:
623.18
(M+H+); Found: 623.06, 624.14(M+H+).
Example 32. Preparation of (S)-2-((S)-2-(azetidin-1-y1)-7-(2,3-
dihydropyrano[4,3,2-de]quinolin-
7-y1)-4-fluoro-5-methylbenzo[d]thiazol-6-y1)-2-tert-butoxyacetie acid (76).
0
0".<
OH
0
76
Compound 76:1H-NMR: 400 MHz, (CD30D) 6: 8.65 (d, J=4.4 Hz, 1H); 7.70 (d, J=7.6
Hz, 1H); 7.39 (d, J=4.4 Hz, 11-1); 7.16 (d, J=7.6 Hz, 1H); 5.04 (s, 1H); 4.57
(t, J=6.0 Hz, 2H);
4.15-4.10 (m, 4H); 3.41 (t, J=6.0 Hz, 211); 2.50-2.46 (m, 6H); 0.90 (s, 9H).
LCMS-ESI+ (m/z):
[M+111+ calcd for C28H29FN304S: 522.19 (M+H ); Found: 521.99, 523.00(M+H+).
155
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
OTf 0-< OH
OEt OEt
Br -4N
0
0
32 75A
OH CY.< OH O''<
OEt OEt
Br¨. I I
0
0
75B 75C
OTf 0-<
OEt
CN-4,
0
75D
Step 1.
Preparation of (S)-ethyl 2-(2-bromo-7-hydroxy-5-methylbenzo[d]thiazol-6-y1)-2-
tert-
butoxyacetate (75A): To a solution of (S)-ethyl 2-(2-bromo-5-methy1-7-
(trifluoromethylsulfonyloxy)benzo[d]thiazol-6-y1)-2-tert-butoxyacetate (32):
(500 mg, 0.938
mmol) in THF (5m1) was added TBAF (1.0 M in THF, 4 ml) slowly. The reaction
mixture was
stirred at rt for lb. The reaction mixture was washed by a mixture of 1120 (20
ml) and
H0Ac(200 ul), extracted by Et0Ac, the organic phase was washed by sat. NaHCO3,
dried over
MgSO4, filtered, concentrated down and purified by silica gel column, eluting
by 0-40% Et0Ac
in hexanes to give 75A (380 mg). LCMS-ESI+: calc'd for C16H2oBrN04S: 402.0
(M+H+);
Found: 401.9 (M+H+).
Step 2.
Preparation of (S)-ethyl 2-(2-bromo-4-fluoro-7-hydroxy-5-methylbenzo[d]thiazol-
6-y1)-
2-tert-butoxyacetate (75B): The reaction mixture of (S)-ethyl 2-(2-bromo-7-
hydroxy-5-
methylbenzo[d]thiazol-6-y1)-2-tert-butoxyacetate (75A) (380 mg, 0.948 mmol),
Selectfluor (1.9
g, 4.74 mmol) in acetonitrile (7 ml) was reacted at 0 C for 5 days. The
reaction mixture was
washed by 1.5 M KH2PO4, extracted by Et0Ac, the organic phase was dried over
MgSO4,
filtered, concentrated down and purified by silica gel column, eluting by 0-
40% Et0Ac in
hexanes to give 75B (137 mg, 35%). LCMS-ESI : calc'd for CI6110FN04S: 420.0
(M+H+);
Found: 420.1 (M+H+).
Step 3.
156
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
Preparation of (S)-ethyl 2-(2-(azetidin-1-y1)-4-fluoro-7-hydroxy-
5methylbenzo[d]thiazol-6-y1)-2-tert-butoxyacetate (75C): Prepared by the
similar method to
make (S)-ethyl 2-(2-(azetidin-1-y1)-5-methy1-7-
(trifluoromethylsulfonyloxy)benzo[d]thiazol-6-
y1)-2-tert-butoxyacetate (33) in Example 10. LCMS-ESI+: calc'd for
C19H25FN204S: 397.2
(M+H+); Found: 397.0 (M+H ).
Step 4.
Preparation of (S)-ethyl 2-(2-(azetidin-l-y1)-4-fluoro-5-methy1-7-
(trifluoromethylsulfonyloxy)benzo[d]thiazol-6-y1)-2-tert-butoxyacetate (75D):
The reaction
mixture of S)-ethyl 2-(2-(azetidin-1 -y1)-4-fluoro-7-hydroxy-
5methylbenzo[d]thiazol-6-y1)-2-
tert-butoxyacetate (75C) (50 mg, 0.126 mmol), N-phenyl triflimite (90 mg,
0.252 mmol),
Cs2CO3 ( 82 mg, 0.126 mmol) in THF (2 ml) was stirred at rt. After the
reaction finished, the
reaction was washed by sat NaHCO3, extracted by Et0Ac, the organic phase was
dried over
MgSO4, filtered, concentrated down and purified by silica gel column, eluting
by 0-40% Et0Ac
in hexanes to give 75D (50 mg, 75%). LCMS-ESI+: calc'd for C201-124F4N206S2:
529.1 (M+H+);
Found: 529.0 (M+H+).
The remainder of the synthesis of compound 76 is analogous to the preparation
of compound 35
from compound 33 in example 10.
Example 33. Preparation of (S)-2-tert-butoxy-24(S)-2-cyclopenteny1-7-(2,3-
dihydropyrano[4,3,2-de]quinolin-7-y1)-5-methylbenzo[d]thiazol-6-yl)acetic acid
(78) and (S)-2-
tert-butoxy-24(R)-2-cyclopenteny1-7-(2,3-dihydropyrano[4,3,2-de]quinolin-7-y1)-
5-
methylbenzo[d]thiazol-6-ypacetic acid (79).
0
0j<
- OH
0
78
Compound 78:11-1-NMR: 400 MHz, (CD30D) 8:8.78 (d, J=5.6 Hz, 1H); 7.99 (s, 1H);
7.90 (d, J=8.0 Hz, 1H); 7.85 (d, J=5.6 Hz, 111); 7.43 (d, J=7.6 Hz, 1H); 6.58
(s, 1H); 5.23 (s,
111); 4.72-4.69 (m, 211); 3.66 (t, J=5.8 Hz, 2H); 2.85-2.83 (m, 2H): 2.76 (s,
3H); 2.56 (m, 2H),
2.07-2.02 (m, 2H); 0.941 (s, 9H).
157
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
0
=
\S = OH
0
79
Compound 79:11-1-NMR: 400 MHz, (CD30D) 3:8.71 (d, J=5.2 Hz, 1H); 8.22 (d,
J=8.0
Hz, 111); 7.97 (s, 1H); 7.77 (d, J=6.0 Hz, 1H); 7.47 (d, J=8.0 Hz, 1H); 6.56
(s, 1H); 5.27 (s, 1H);
4.70 (t, J=6.0 Hz, 2H); 3.63 (t, J=6.2 Hz, 2H); 2.84-2.83 (m, 2H); 2.72 (s,
3H); 2.55-2.54 (m,
.. 2H); 2.07-2.03 (m, 2H); 0.94 (s, 9H).
OTf OTf
== OEt OEt
Br I \ I
0 0
77
32
Preparation of (S)-ethyl 2-tert-butoxy-2-(2-cyclopenteny1-5-methy1-7-
(trifluoromethylsulfonyloxy)benzo[d]thiazol-6-y1)acetate (77). To a solution
of 32 (100 mg, 0.19
mmol) in toluene (1 mL), ethanol (0.5 mL), water (0.5 mL) was added potassium
carbonate (77
mg, 0.56 mmol), cyclopentenylboronic acid (25 mg, 0.22 mmol), and Pd(PPh3)4
(11 mg, 0.0094
mmol). The reaction mixture was stirred at 90 C for 2 h. The reaction was
cooled to rt and
diluted with water and Et0Ac. The layers were separated, dried, filtered, and
concentrated in
vacuo. The crude material was purified by column chromatography
(Et0Ac/hexanes) to give 96
mg of 77. 11-1-NMR: 400 MHz, (CDC13) .3: 7.82 (s, 1H), 6.72 (m, 1H), 5.60 (s,
I H), 4.17 (m,
1H), 4.11 (m, 1H), 2.92 (m, 211), 2.63 (m, 2H), 2.53 (s, 31), 2.10 (m, 2H),
1.17 (s, 9H), 1.13 (t, J
= 7 Hz, 314).
The remainder of the synthesis of 78 and 79 follows the same route as Example
10 from
compound 33.
Example 34. Preparation of (S)-2-tert-butoxy-2-((S)-2-cyclopenty1-7-(2,3-
dihydropyrano[4,3,2-
de]quinolin-7-y1)-5-methylbenzo[d]thiazol-6-yDacetic acid (80) and (S)-2-tert-
butoxy-24(R)-2-
cyclopenty1-7-(2,3-dihydropyrano[4,3,2-de]quinolin-7-y1)-5-
methylbenzo[d]thiazol-6-yDacetic
acid (81).
158
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
Compound 80 was prepared from compound 78 according to the procedure used to
prepare
compound 8F from 8E in Example 4.
- OH
0
Compound 80: 'H-NMR: 400 MHz, (CD30D) 8: 8.77 (d, J=6.0 Hz, 111); 7.96 (s,
1H);
5 7.88 (d, J=8.0 Hz, 1H); 7.83 (d, J=6.0 Hz, 1H); 7.42 (d, J=8.0 Hz, 1H);
5.23 (s, 1H); 4.73-4.69
(m, 2H); 3.67-3.64 (m, 2H); 3.53-3.44 (m, 1H); 2.75 (s, 1H); 2.17-2.14 (m,
211); 1.81-1.71 (m,
6H); 0.90 (s, 9H).
Compound 81 was prepared from compound 79 according to the procedure used to
prepare compound 77 from 8E in Example 4.
0
CY.<
OH
10 81
Compound 81: 1H-NMR: 400 MHz, (CD30D) 8:8.67 (d, J=5.2 Hz, 111); 8.15 (d,
J=8.4
Hz, 111); 7.90 (s, 111); 7.68 (d, J=5.6 Hz, 111); 7.41 (d, J=8.4 Hz, 1H); 5.27
(s, 111); 4.69-4.65
(m, 2H); 4.67 (t, J=6.2 Hz, 2H); 3.59 (t, J=6.0 Hz, 2H); 3.50-3.42 (m, 1H);
2.71 (s, 3H); 2.16-
2.13 (m, 2H); 1.78-1.70 (m, 6H); 0.90 (s, 9H).
Example 35. Preparation of (S)-2-tert-butoxy-2-((S)-2-cyclobuty1-7-(2,3-
dihydropyrano[4,3,2-
de]quinolin-7-y1)-5-methylbenzo[d]thiazol-6-ypacetic acid (82).
Compound 82 was prepared from compound 32 according to the procedure used to
prepare compound 77 in Example 33, except cyclobutyl zinc bromide was used
instead of
cyclopentenylboronic acid.
159
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
0
ç D0
OH
0
82
Compound 82:1H-NMR: 400 MHz, (CD30D) 8 8.79 (d, J= 5.6 Hz, 1H), 7.98 (s, 1H),
7.87 (dd, J= 13.1, 6.9 Hz, 2H), 7.44 (d, J= 8.4 Hz, 1H), 5.23 (s, 1H), 4.71
(dt, J= 11.5, 5.8 Hz,
2H), 3.91 (p, J= 8.3 Hz, 1H), 3.67 (t, J= 5.8 Hz, 3H), 2.76 (s, 3H), 2.50¨
2.40 (m, 2H), 2.39 ¨
2.27 (m, 2H), 2.19 ¨ 2.05 (m, 1H), 0.91 (s, 9H). LCMS-ESI+ (m/z): [M+Hr calcd
for
C291131N204S: 503.20 (M+H+); Found: 503.07, 504.10(M+H+).
Example 36. Preparation of (S)-2-tert-butoxy-24(S)-7-(2,3-dihydropyrano[4,3,2-
de]quinolin-7-
y1)-2-isobuty1-5-methylbenzo[d]thiazol-6-ypacetic acid (83) and (S)-2-tert-
butoxy-2-((R)-7-
(2,3-dihydropyrano[4,3,2-de]quinolin-7-y1)-2-isobuty1-5-methylbenzo[d]thiazol-
6-ypacetic acid
(84).
Compounds 83 and 84 were prepared from compound 32 according to the procedure
used to prepare compound 77 in Example 33, except tributy1(2-methylprop-1-
enypstannane was
used instead of cyclopentenylboronic acid. Also, hydrogenation was performed
according to the
procedure used to prepare 8F from 8E in Example 4.
0
OH
0
83
Compound 83:1H-NMR: 400 MHz, (CD30D) 8: 8.74 (d, J= 5.4 Hz, 111), 7.95 (s,
1H),
7.85 (d, J= 8.0 Hz, 1H), 7.74 (d, J= 5.7 Hz, 1H), 7.37 (d, J= 8.3 Hz, 1H),
5.22 (s, 1H), 4.69
(m, 2H), 3.61 (t, J= 5.9 Hz, 2H), 2.90 (d, J= 7.2 Hz, 2H), 2.75 (s, 3H), 0.97
(d, J= 6.5 Hz, 6H),
0.91 (s, 9H). LCMS-ESI+ (m/z): [M+Hr calcd for C29H33N204S: 505.22 (M+H+);
Found:
505.06, 506.06(M+H+).
160
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
0
- OH
( N 0
84
Compound 84: 'H-NMR: 400 MHz, (CD30D) 8 8.65 (d, J= 5.2 Hz, 1H), 8.12 (d, J=
8.1
Hz, 1H), 7.90 (s, 1H), 7.61 (d, J= 5.1 Hz, 111), 7.37 (d, J= 8.2 Hz, 1H), 5.28
(s, 1H), 4.65 (t, J=
6.1 Hz, 2H), 3.55 (t, J= 5.9 Hz, 2H), 2.89 (d, J= 7.2 Hz, 2H), 2.70 (s, 4H),
0.97 (dd, J= 6.6, 3.2
Hz, 7H), 0.88 (s, 10H).
LCMS-ESI4 (m/z): [M+H] calcd for C29H33N204S: 505.22(M+11+); Found: 505.01,
506.07(M+H+).
Example 37. Preparation of (S)-2-tert-butoxy-24(S)-2-cyclopropy1-7-(2,3-
dihydrobenzo[de]chromen-7-y1)-5-methylbenzo[d]thiazol-6-ypacetic acid (85).
Compound 85 was prepared from compound 18 according to the procedure used to
prepare compounds 19 and 20 (except that 2,3-dihydrobenzo[de]chromen-7-
ylboronic acid was
used instead of 2,3-dihydropyrano[4,3,2-de]quinolin-7-ylboronic acid) in
Example 9.
0
r OH
0
15 Compound 85:111-NMR: 400 MHz, (CD30D) 8: 7.70 (s, 1H); 7.29-7.19 (m,
4H), 6.95
(d, J = 4 Hz, 1H), 5.07 (s, 1H), 4.48-4.45 (m, 2H), 3.29-3.27 (m, 2H), 2.64
(s, 3H), 2.32-2.28 (m,
1H), 1.20-1.18 (m, 2H), 1.056-1.03 (m, 2H), 0.96 (s, 9H).
LCMS-ESI+ (m/z): [M+H] calcd for C291129N04S: 488.2 (M+H+); Found:
488.1(M+H+).
20 Example 38. Preparation of (S)-2-tert-butoxy-2-((S)-7-(5-chloro-3,4-
dihydro-2H-
benzo[b][1,4]oxazin-6-y1)-2-cyclopropy1-5-methylbenzo[d]thiazol-6-yDacetic
acid (86) and (S)-
2-tert-butoxy-24(R)-7-(5-chloro-3,4-dihydro-211-benzo[b][1,4]oxazin-6-y1)-2-
cyclopropy1-5-
methylbenzo[d]thiazol-6-ypacetic acid (87).
161
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
Compounds 86 and 87 was prepared from compound 18 according to the procedure
used
to prepare compounds 19 and 20 (except that 5-chloro-3,4-dihydro-2H-
benzo[b][1,4]oxazin-6-
ylboronic acid was used instead of 2,3-dihydropyrano[4,3,2-de]quinolin-7-
ylboronic acid) in
Example 9.
HN
CI
OH
0
86
Compound 86:1H-NMR: 400 MHz, (CD30D) 8: 7.63 (d, J = 0.4 Hz, 1H); 6.74 (d, J =
4.2
Hz, 1H), 6.44 (d, J = 4.2 Hz, 1H), 5.21 (s, 1H), 4.26 (t, J = 4.6 Hz, 2H),
3.51-3.49 (m, 2H), 2.65
(d, J = 0.4 Hz, 3H), 2.40-2.36 (m, 1H), 1.26-1.23 (m, 2H), 1.13-1.11 (m, 2H),
1.09 (s, 9H).
LCMS-ESI+ (m/z): [M+Hr calcd for C251127C1N204S: 487.1 (M+H+); Found:
487.1(M+H+).
HN
Iõ
CI = 0
OH
0
87
Compound 87:1H-NMR: 400 MHz, (CD30D) 8: 7.60 (s, 111); 6.83-6.78 (m, 2H), 5.27
(s,
1H), 4.27-4.24 (m, 211), 3.51-3.48 (m, 2H), 2.55 (s, 3H), 2.40-2.36 (m, 1H),
1.28-1.23 (m, 2H),
1.13-1.12 (m, 211), 1.01 (s, 9H)..
LCMS-ESI+ (m/z): [M+Hr calcd for C25H27C1N204S: 487.1 (M+H4); Found: 487.1(M+H
).
Example 39. Preparation of (S)-2-tert-butoxy-24(S)-7-(2,3-dihydropyrano[4,3,2-
delquinolin-7-
y1)-2-isopropy1-5-methylbenzo[d]thiazol-6-ypacetic acid (88).
Compound 88 was prepared from compound 32 according to the procedure used to
prepare
compound 77 in Example 33, except propen-2-y1-(tri-n-butyptin was used instead
of
cyclopentenylboronic acid. Also, hydrogenation was performed according to the
procedure used
to prepare 8F from 8E in Example 4.
162
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
0
0-<
\ - OH
0
88
Compound 88:1H NMR (400 MHz, CD30D) 6 8.70 (d, J= 5.4 Hz, 1H), 7.90 (s, 1H),
7.80 (d, J= 7.9 Hz, 1H), 7.66-7.60 (m, 1H), 7.31 (d, Jr 8.0 Hz, 1H), 5.20 (s,
1H), 4.70-4.61 (m,
2H), 3.59-3.51 (m, 211), 3.15-3.09 (m, 111), 2.72 (s, 3H), 1.36 (m, 6H), 0.90
(s, 9H). LCMS-
ESI+: calc'd for C28H30N204S: 491.2 (M+H+); found: 491.4 (M+H+).
Example 40. Preparation of (S)-2-((S)-2-(azetidin-1-y1)-7-(2,3-
dihydropyrano[4,3,2-de]quinolin-
7-y1)-5-methylbenzo[d]thiazol-6-y1)-2-(tert-pentyloxy)acetic acid (89).
0
ç D0
OH
CN-4
0
89
Compound 89:1H NMR (400 MHz, CD30D) 6 8.75 (d, J= 5.1 Hz, 111), 7.82 (d, J=
8.3
Hz, 1H), 7.71 (d, J= 5.6 Hz, 1H), 7.47 (s, 1H), 7.35 (d, J= 8.1 Hz, 1H), 5.09
(d, J= 0.6 Hz,
111), 4.69 ¨ 4.62 (m, 2H), 4.17 (t, J= 7.7 Hz, 4H), 3.61 ¨3.55 (m, 211), 2.66
(s, 3H), 2.58 ¨2.42
(m, 2H), 0.87 (d, J= 2.9 Hz, 6H), 0.59 (t, J= 7.0 Hz, 3H). 19F NMR (377 MHz,
CD30D) 6 -
77.77. LCMS: calc'd= 518.64, observed: 518.08
OTf
OTf OH
OEt OEt
Br-4.
Br-4. 0
0
IIIJIJT
31 90
Preparation of 90: A slurry of 31 (740 mg, 1.55 mmol) in tert-amyl acetate
(7.0 mL) was
treated with 70% aq. HC104 (5 ut) was added at 23 C. Reaction became cloudy,
but LCMS
analysis indicated minimal conversion. More 70% aq. HC104 (50 1.10 was
introduced. After 2 h,
the reaction was added dropwise over 5 min to sat. aq. NaHCO3 (20 mL). 1120
(10 mL) was
added, and the system was extracted with DCM (3 x 20 mL). Combined organic
layers were
dried (Na2SO4), filtered, concentrated, and treated with hexane (10 mL). The
system was
163
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
concentrated again to remove some residual t-amyl alcohol. The residue was
treated with PhH
and loaded onto a 12 gram "gold" ISCO silica gel column. Chromatography
(eluent: Hexanes /
Ethyl Acetate) gave 90 (134 mg, 16% yield) along with some recovered 31.
H-NMR: 400 MHz, (CDC13) 5: 7.80 (s, 1H), 5.49 (s, 1H), 4.24-4.06 (m, 211),
2.57 (s, 311),
1.60-1.40 (m, 2H), 1.17 (s, 3H), 1.16 (t, J = 7.0 Hz, 3H), 1.05 (s, 3H), 0.80
(t, J = 7.0 Hz, 3H).
19F-NMR: 376 MHz, (CDC13) 8: ¨73.8
The remainder of the synthesis of 89 follows the same route as Example 10 from
compound 32.
Example 41. Preparation of (S)-2-tert-butoxy-2-((S)-2-(difluoromethyl)-7-(2,3-
dihydropyrano[4,3,2-de]quinolin-7-y1)-5-methylbenzo[d]thiazol-6-ypacetic acid
(92).
0
0-j<
F S OH
F N 0
92
Compound 92:1H NMR (400 MHz, CD30D) 5 8.67 (d, J= 5.6 Hz, 1H), 8.08 (d, J= 5.9
Hz, 1H), 7.78¨ 7.75 (m, HA 7.29 ¨ 7.22 (m, 111), 7.04 (s, 1H), 6.89 (s, 1H),
5.23 (s, 114), 4.66
¨4.61 (m, 2H), 3.69 (s, 111), 3.64 (s, 211), 3.17 ¨ 3.16 (m, 1H), 3.13 (dd, J=
4.1, 2.4 Hz, 2H),
2.75 (s, 3H), 0.90 (s, 911).
OTf 0`
OTf
OEt
Br 0 s5yOEt
0
32 91A
OTf 0 OTf
OEt F S OEt
0
0 N F N
91B 91C
Step 1.
Preparation of (S)-ethyl 2-tert-butoxy-2-(5-methy1-7-
(trifluoromethylsulfonyloxy)-2-
vinylbenzo[d]thiazol-6-ypacetate (91A): A microwave vial was charged with CuI
(9.4 mg, 49
mop, Pd(PPh3)4 (29 mg, 25 p.mol), and 32 (250 mg, 0.494 mmol). The vial was
sealed and
placed under a vacuum. The vessel was backfilled with argon and charged with
DMF (1.0 mL)
164
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
followed by vinyl-(tri-n-butyl)tin (173 L, 0593 mmol). Reaction was stirred
at 65 C for 1 h,
then cooled to 23 C. Sat. aq. N114C1 (40 mL) was added and the reaction was
extracted with
Et0Ac (2 x 20 mL). Combined organic layers were dried (Na2SO4), filtered, and
concentrated.
Hexane was added, and the slurry was concentrated again. The residue was
treated with Phil and
purified by silica gel column chromatography (eluent: Hexanes / Ethyl Acetate)
giving 91A (173
mg, 77% yield). 111-NMR: 400 MHz, (CDC13) 8: 7.80 (s, 1H), 7.00 (dd, J = 18.6,
10.9 Hz, 1H),
6.24 (d, J = 18.6 Hz, 1H), 5.82 (d, J = 10.9 Hz, 1H), 5.60 (s, 1H), 4.24-4.06
(m, 2H), 2.54 (s,
3H), 1.22 (s, 9H), 1.19 (t, J = 6.8 Hz, 3H). 19F-NMR: 376 MHz, (CDC13) 8:
¨73.8.
Step 2.
Preparation of (S)-ethyl 2-tert-butoxy-2-(2-formy1-5-methy1-7-
(trifluoromethylsulfonyloxy)benzo[d]thiazol-6-ypacetate (91B): A solution of
91A (170 mg,
0.353 mmol), DCM (5.0 mL), and Me0H (5.0 mL) was cooled to ¨78 C and perfused
with
oxygen gas for 3 mm. Then using an ozonator, a stream of 03 in oxygen gas was
bubbled
through the solution for 5 min. After this, the reaction was stirred for 10
min, then sparged with
oxygen gas for 2 min to drive out unreacted ozone in solution. While the
reaction was still at ¨78
C, dimethylsulfide (200 L) was added and the reaction allowed to warm to 0
C. After 30 min,
10% w/v aq Na2S203 (5 mL) was added and the reaction was warmed to 23 C and
stirred for 10
min. The reaction was diluted with H20 (20 mL) and extracted with DCM (3x 15
mL).
Combined organic layers were dried (Na2SO4), filtered, and concentrated. More
DCM was
added and the reaction was concentrated once more to remove residual methanol,
giving 91B
(165 mg, 97% yield). 1H-NMR: 400 MHz, (CDC13) 8: 10.04 (s, 1H), 8.00 (s, 1H),
5.60 (s, 1H),
4.22-4.00 (m, 211), 2.51 (s, 311), 1.16 (s, 9H), 1.14 (t, J = 6.8 Hz, 3H). 19F-
NMR: 376 MHz,
(CDC13) 8: ¨73.6.
Step 3.
Preparation of (S)-ethyl 2-tert-butoxy-2-(2-(difluoromethyl)-5-methy1-7-
(trifluoromethylsulfonyloxy)benzo[d]thiazol-6-ypacetate (91C): A solution of
Fluolead (318
mg, 1.27 mmol) in DCM (1.0 mL) was cooled to 0 C and treated with a solution
of 91B (123
mg, 0.254 mmol) in DCM (1.5 mL). The reaction was allowed to warm to 23 C.
Absolute
Et0H (5 L) was added to initiate the reaction. After 1 h, additional Fluolead
(318 mg, 1.27
mmol) was added. Once 4 h had passed, 0.5 M aq. NaOH (5 mL) was added, and the
reaction
reached a pH of ¨2. DCM (10 mL) was introduced. 1.0 M aq NaOH (-5 mL) was
added
dropwise until the pH reached 12. The system was extracted with DCM (3x 10
mL). The
combined organic layers were dried (Na2SO4), filtered, and carefully
concentrated to a volume
of ¨3 mL. The system became a suspension, which was then filtered. The
filtrate was directly
165
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
loaded onto a 12 gram "gold" ISCO silica gel column. Purification by
chromatography (eluent:
Hexanes / Ethyl Acetate) gave 91C (67 mg, 52% yield).
1H-NMR: 400 MHz, (CDC13) 8: 7.97 (s, 1H), 6.92 (t, JHF = 44.5 Hz, 1H), 5.62
(s, 1H), 4.24-
4.08 (m, 211), 2.59 (s, 3H), 1.27 (s, 9H), 1.19 (t, J = 6.8 Hz, 3H). 19F-NMR:
376 MHz, (CDC13)
8: -73.7 (3F), -110.6 (app. dd, JFF = 4.0 Hz, JHF = 44.5 Hz, 2F).
The remainder of the synthesis of 92 follows the same route as Example 10 from
compound 33.
Example 42. Preparation of (S)-2-((S)-2-acetatnido-7-(2,3-dihydropyrano[4,3,2-
de]quinolin-7-
y1)-5-methylbenzo[d]thiazol-6-y1)-2-tert-butoxyacetic acid (93).
0
c D0
OH
0 N4SN
0
93
Compound 93: '11-NMR: 400 MHz, (CD30D) 8:11-INMR (400 MHz, cd3od) 6 8.73 (d, J
= 5.4 Hz, 111), 7.85 (d, J= 8.2 Hz, 111), 7.76- 7.73 (m, 1H), 7.72 (d, J= 5.6
Hz, 1H), 7.39 (t, J
= 10.0 Hz, 1H), 5.20 (s, 1H), 4.68 (m, 4H), 3.64 - 3.57 (m, 211), 2.71 (s,
311), 2.17 (s, 3H), 0.91
(s, 911). LCMS-ESI+ (m/z): [M+Hi+ calcd for C271128N305S: 506.17 (M+H );
Found: 506.02,
507.03(M+H+).
Br 0-.< Br 0-r<
OPiv s OPiv
H2N-- I HN4
65H 94
Preparation of compound (S)-2-(2-acetamido-7-bromo-5-methylbenzo[d]thiazol-6-
y1)-2-
tert-butoxyethyl pivalate (94). To a solution of 65H in CH2C12 was added
pyridine, acetic
anyhydride, and trace DMAF'. Upon consumption of starting material by LC-MS,
the mixture
was concentrated in vacuo and purified by column chromatography to give 94.
LCMS-ESI+ (m/z): [M+Hr calcci for C211-129BrN204S: 487.1 (M+H+); Found: 486.9
(M+H+).
The remainder of the synthesis of compound 93 is analogous to the preparation
of compound 66
from compound 65J in example 25
166
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
Example 43. Preparation of (S)-2-tert-butoxy-24(S)-7-(2,3-dihydropyrano[4,3,2-
de]quinolin-7-
y1)-5-methylbenzo[d]thiazol-6-ypacetic acid (95).
Compound 95 was a by-product in the preparation of 40.
0
c D9
7 OH
0
95
Compound 95:111-NMR: 400 MHz, (CD30D) 8:9.40 (s, 1H); 8.82 (d, J=6.0 Hz, 114);
8.17 (s, 1H); 7.93 (d, J=8.0 Hz, 111); 7.89 (d, J=6.0 Hz, 111); 7.46 (d, J=8.0
Hz, 1H); 5.27 (s,
1H); 4.76-4.71 (m, 2H); 3.69 (t, J=6.0 Hz, 2H); 2.81 (s, 3H); 0.92 (s, 9H).
LCMS-ESI+ (m/z):
[M+H] calcd for C251124N204S: 449.2 (M+111); Found: 449.1 (M+H+).
Example 44. Preparation of (S)-2-tert-butoxy-24(S)-7-(2,3-dihydropyrano[4,3,2-
de]quinolin-7-
y1)-5-methy1-2-(methylcarbamoyDbenzo[d]thiazol-6-yDacetic acid (97).
0
-NH S 7 OH
0 0
97
Compound 97:111-NMR: 400 MHz, (CD30D) 8: 8.77 (d, J=6.0 Hz, 1H); 8.18-8.15 (m,
111); 7.91 (m, 111); 7.84 (d, J=5.2 Hz, 1H); 7.45 (d, J=8.4 Hz, 1H); 5.26 (s,
111); 4.75-4.71 (m,
2H); 3.67 (t, J=6.0 Hz, 2H); 2.93 (s, 3H); 2.79 (s, 311); 0.92 (s, 9H). LCMS-
ESI (m/z): [M+H]
calcd for C271127N305S: 506.2 (MAI); Found: 506.0 (M+H+).
Step 1.
Preparation of (S)-6-((S)-1-tert-butoxy-2-ethoxy-2-oxoethyl)-7-(2,3-
dihydropyrano[4,3,2-de]quinolin-7-y1)-5-methylbenzo[d]thiazole-2-carboxylic
acid (96A): To a
solution of compound 38 (40 mg) in THF/Me0H (1:1, 2 mL) was added a NaOH
solution (2 M,
100 4). The reaction mixture was stirred at rt for 1 h. A saturated solution
of NH4C1 was
added, and the aqueous was extracted with Et0Ac. The organic layer was dried,
filtered, and
concentrated in vacuo to give 95A. 111-NMR: 400 MHz, (CD30D) 8: 8.63 (d, J = 6
Hz, 1H),
.. 8.05 (br s, 1H), 7.63 (d, J = 8 Hz, 1H), 7.46 (d, J = 6 Hz), 7.21 (d, J = 8
Hz, 1H), 5.20 (s, 1H),
167
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
4.58 (m, 2H), 4.04 (m, 1H), 3.91 (m, 1H), 3.46 (m, 2H), 2.76 (s, 3H), 1.03 (t,
J = 7 Hz, 3H), 0.89
(s, 9H).
Step 2.
To a solution of 96A (15 mg) in CH2C12 (1 mL) was added carbonyldiimidazole
(10 mg)
and then methylamine (solution in Me0H, 100 4). Once conversion is complete by
LC-MS,
the solution was concentrated to give crude 96B. Then THF/Me0H added (1:1, 1
mL) followed
by NaOH solution (2 M, 100 4). The reaction mixture was stirred at 55-60 C
for 4 h. Purified
by reverse phase HPLC to give 3.8 mg of 97.
0 0
LNLJ
0-< 0".<
0 S OEt 0 S = OEt
EtO) I 0 HO) N I 0
38 96A
0 0
0j<
0µµ N OEt 0 S
-NI OH
I 0 0
96B 97
Example 45. Preparation of (S)-2-tert-butoxy-24(S)-7-(2,3-dihydropyrano[4,3,2-
de]quinolin-7-
y1)-5-methy1-2-(phenethy1carbamoyDbenzord1thiazo1-6-y1)acetic acid (98).
Compound 98 was prepared from compound 96A according to the procedure used to
prepare compound 97 (except that 2-phenylethanamine was used instead of
methylamine) in
Example 44.
0
0j<
0 S OH
0
HN N
98
Compound 98:1H-NMR: 400 MHz, (CD30D) 8: 8.76 (d, J=6.0 Hz, 1H); 8.17 (s, 1H);
7.90 (d, J = 8 Hz, 1H); 7.82 (d, J = 6 Hz, 1H); 7.43 (d, J = 8 Hz, 1H); 7.20
(m, 5H), 5.26 (s, 1H);
168
CA 02833377 2013-10-16
WO 2012/145728 PCT/US2012/034593
4.71 (m, 2H); 3.64 (t, J=6 Hz, 211); 2.91 (t, J = 7 Hz, 2H), 2.77 (m, 2H),
2.72 (s, 311), 0.92 (s,
9H). LCMS-ESI+ (m/z): [M+Hr calcd for C341133N305S: 596.2 (M+H+); Found: 596.1
(M+H+).
Example 46. Preparation of (S)-2-tert-butoxy-2-((S)-7-(2,3-dihydropyrano[4,3,2-
de]quinolin-7-
y1)-2-(4-methoxybenzylcarbamoy1)-5-methylbenzo[d]thiazol-6-yDacetic acid (99).
Compound 99 was prepared from compound 96A according to the procedure used to
prepare compound 97 (except that 4-methoxybenzylamine was used instead of
methylamine) in
Example 44.
0
N 0j<
0 S - OH
0
NH N
= 99
-0
Compound 99: LCMS-ESI+ (m/z): [M+H] calcd for C34H33N306S: 612.2 (M+H+);
Found: 612.1 (M+H+).
Example 47.
The following illustrate representative pharmaceutical dosage forms,
containing a compound of
formula I ('Compound X'), for therapeutic or prophylactic use in humans.
(i) Tablet 1 mg/tablet
Compound X= 100.0
Lactose 77.5
Povidone 15.0
Croscarmellose sodium 12.0
Microcrystalline cellulose 92.5
Magnesium stearate 3.0
300.0
(ii) Tablet 2 mg/tablet
Compound X= 20.0
Microcrystalline cellulose 410.0
Starch 50.0
Sodium starch glycolate 15.0
Magnesium stearate 5.0
500.0
169
(iii) Capsule mg/capsule
Compound X= 10.0
Colloidal silicon dioxide 1.5
Lactose 465.5
Pregelatinized starch 120.0
Magnesium stearate 3.0
600.0
(iv) Injection 1 (1 mg/ml) mg/m1
Compound X= (free acid form) 1.0
Dibasic sodium phosphate 12.0
Monobasic sodium phosphate 0.7
Sodium chloride 4.5
1.0 N Sodium hydroxide solution
(pH adjustment to 7.0-7.5) q.s.
Water for injection q.s. ad 1 mL
(v) Injection 2 (10 mg/ml) mg/ml
Compound X= (free acid form) 10.0
Monobasic sodium phosphate 0.3
Dibasic sodium phosphate 1.1
Polyethylene glycol 400 200.0
1.0 N Sodium hydroxide solution
(pH adjustment to 7.0-7.5) q.s.
Water for injection q.s. ad 1 mL
(vi) Aerosol mg/can
Compound X= 20.0
Oleic acid 10.0
Trichloromonofluoromethane 5,000.0
Dichlorodifluoromethane 10,000.0
Dichlorotetrafluoroethane 5,000.0
The above formulations may be obtained by conventional procedures well known
in the
pharmaceutical art.
The invention has been described with reference to various specific and
preferred
embodiments and techniques. However, it should be understood that many
variations and
modifications may be made while remaining within the scope of the invention.
170
CA 2833377 2018-04-17