Note: Descriptions are shown in the official language in which they were submitted.
CA 02833482 2014-05-20
UTILITY PATENT APPLICATION
Attorney Docket No. 201302670 (101.0675.U01)
PACKAGED MEDICATION ASSEMBLY AND ASSOCIATED METHOD
BACKGROUND OF THE INVENTION
100021 Virtually everyone consumes prescription pharmaceuticals at one time or
another. In each instance, the consumer is faced with a series of procedural
steps and
information. The procedural steps include submitting the prescription, waiting
for it,
picking up the prescription, and signing applicable notices. A large volume of
information about the patient, pharmacy, physician, and drug is provided on
the
prescription sticker on the bottle and on pharmacy transactional papers (e.g.,
on one
or more printed, folded sheets) included with the prescription. In many
instances,
where prescriptions are filled for subsequent patient pick up, the filled
prescriptions
are placed in pharmacy bags with descriptive and other information being
attached to
an external surface of the bag via an adhesive label or one or more staples.
Such
systems aim to position patient identifying information to aid identification
by a
pharmacy employee when a patient arrives to pick up the previously filled
prescription.
100031 While these conventional methods provide for relatively quick
identification,
securement of papers or labels to the external surface or the bag increases
steps and
the required to complete filling and packaging of a prescription and may
present a
1
CA 02833482 2013-11-19
UTILITY PATENT APPLICATION
Attorney Docket No. 201302670 (101.0675.U01)
haphazard overall presentation. In addition, the external identifying papers
may
become separated from the bag, thereby, presenting additional identification
issues.
As such, other methods of packaging and identifying prescriptions for
subsequent
patent pick-up are desired.
SUMMARY
[0004] One embodiment of the invention relates to a packaged medication
assembly
including a packaged medication, a bag, and an information insert. The
packaged
medication includes a container and medication enclosed within the container.
The
bag defines a bottom bag fold line, a first panel adjacent the bottom bag fold
line, a
storage chamber, and an opening to the storage chamber formed at least
partially by a
first edge of the first panel opposite the bottom bag fold line. The
information insert
has a width smaller than and a height greater than a width and a height of the
first
panel of the bag. The height of the information insert is defined between the
first
edge and a second edge of the information insert. The information insert
includes
patient information, which provides identification of a patient that the
medication was
packaged for, and medication information providing at least one of an identity
of and
a description of the medication. The information insert includes a patient
identifying
section including the patient information adjacent the first edge. The
information
insert is placed in the storage chamber such that the second edge is
positioned
adjacent the bottom bag fold line and substantially only the patient
identifying section
extends and remains exposed above the first edge of the first panel such that
a
remainder of the information insert is covered by the first panel of the bag.
The
packaged medication is positioned within the storage chamber. Other packaged
2
CA 02833482 2013-11-19
UTILITY PATENT APPLICATION
Attorney Docket No. 201302670 (101.0675.U01)
medication assemblies, associated combinations, and associated methods are
also
disclosed.
BRIEF DESCRIPTION OF THE DRAWINGS
[0005] Embodiments of the invention will be described with respect to the
figures,
in which like reference numerals denote like elements, and in which:
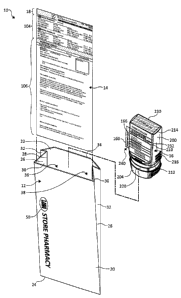
[0006] Figure 1 is an exploded, perspective view illustration of a packaged
medication assembly, according to one embodiment of the invention.
[0007] Figure 2 is a partial front view illustration of an assembled packaged
medication assembly of Figure 1, according to one embodiment of the invention.
[0008] Figure 3 is a rear view illustration of the packaged medication
assembly of
Figure 2, according to one embodiment of the invention.
[0009] Figure 4 is a top view illustration of the packaged medication assembly
of
Figure 2, according to one embodiment of the invention.
[0010] Figure 5 is a front perspective view illustration of a pharmacy system
including a bin maintaining a plurality of packaged medication assemblies,
according
to one embodiment of the invention.
[0011] Figure 6 is a front view illustration of a sheet blank, according to
one
embodiment of the invention.
[0012] Figure 7 is a rear view illustration of a sheet blank, according to one
embodiment of the invention.
3
CA 02833482 2013-11-19
UTILITY PATENT APPLICATION
Attorney Docket No. 201302670 (101.0675.U01)
[0013] Figure 8 is a front view illustration of a printed guest receipt
section of the
sheet blank of Figures 6 and 7, according to one embodiment of the invention.
[0014] Figure 9 is a front view illustration of a printed prescription
information
section of the sheet blank of Figures 6 and 7, according to one embodiment of
the
invention.
[0015] Figure 10 is a front view illustration of a printed pharmacy use
section of the
sheet blank of Figures 6 and 7, according to one embodiment of the invention.
[0016] Figure 11 is a front view illustration of a printed patient info card
section of
the sheet blank of Figures 6 and 7, according to one embodiment of the
invention.
[0017] Figure 12 is a rear view illustration of a printed guest receipt
section of the
sheet blank of Figures 6 and 7, according to one embodiment of the invention.
[0018] Figure 13 is a rear view illustration of a printed prescription
information
section of the sheet blank of Figures 6 and 7, according to one embodiment of
the
invention.
[0019] Figure 14 is a rear view illustration of a printed pharmacy use section
of the
sheet blank of Figures 6 and 7, according to one embodiment of the invention.
[0020] Figure 15 is a flow chart illustrating a method of assembling and using
the
prescription pharmacy system, according to one embodiment of the invention.
4
CA 02833482 2013-11-19
UTILITY PATENT APPLICATION
Attorney Docket No. 201302670 (101.0675.U01)
DETAILED DESCRIPTION
100211 In the following detailed description, reference is made to the
accompanying
drawings, which form a part hereof, and in which is shown by way of
illustration
specific embodiments in which the invention may be practiced. The following
detailed description, therefore, is not to be taken in a limiting sense, and
the scope of
the present invention is defined by the appended claims.
100221 Embodiments of the invention are directed to a prescription or
medication
packaging system that significantly enhances the efficiency in which a
prescription or
drug order can be filled and prepared for future pick-up by a patient while
also
enhancing identification of pre-filed prescriptions and drug orders at the
time of their
pick up. These improvements also lead to an improved consumer experience in
having a prescription filled at a pharmacy. In one example, the system
includes a bag
or other external container, an information sheet, and a filled prescription
or other
drug. The bag includes a lower edge and an open top. The information sheet is
sized
to a width slightly smaller than an inside width of the bag and a height
greater than a
height of the bag, at least greater than a height of a front panel of the bag.
[00231 Per the above, the information sheet is sized to fit within the bag
such that a
top portion of the information sheet extends above a top of the bag or at
least a top of
the front panel of the bag. The information sheet includes details about the
patient,
the prescription or other medication, etc. More particularly, the portion of
the
information sheet that extends above the bag includes information identifying
the
patient, but, in one embodiment, not identifying details about the
prescription or other
medication specifics. By maintaining such information substantially hidden
within
the bag, the privacy of the patient is respected and details of their health,
etc. are not
CA 02833482 2013-11-19
,
,
UTILITY PATENT APPLICATION
,
,
' Attorney Docket No. 201302670 (101.0675.U01)
exposed for ready viewing by other customers, etc. These embodiments and other
embodiments of the invention are described in greater detail below in
association with
Figures 1-16.
[0024] As shown in Figures 1-4, in one embodiment, a packaged medication
assembly 10 comprises an external container such as a sleeve or a bag 12, an
information insert 14, and a packaged medication 16 (otherwise referred to
herein as a
pharmaceutical item), such as a pre-filed prescription. When a prescription is
filled or
other packaged medication 16 prepared, information insert 14 is printed and
placed in
bag 12 along with packaged medication 16. Information insert 14 generally sits
in
bag 12 in front of packaged medication 16 and a patient identifying section 18
at the
top of information insert 14 extends beyond a top, front edge of bag 12 to
present
information relating to the patient allowing for quick identification of the
particular
bag 12 from a plurality of similarly prepared bags 12 (see Figure 5) for all
pharmacy
customers (i.e., other patients) when that patient or their designee arrives
at the
pharmacy to retrieve the packaged medication 16.
[0025] For example, similarly prepared bags 12 are placed in a substantially
horizontal stack (e.g., a horizontal array) in a bin 82 or other container as
part of a
pharmacy fulfillment system 80 as shown in Figure 5. In this arrangement,
patient
identifying section 18 extends upwardly above tops of bags 12 allowing ready
identification of a desired one of the packaged medication assembly 10 without
requiring the pharmacy employee to flip through individual ones of the
packaged
medication assemblies 10 in the array to find the desired one.
[0026] More particularly, in one embodiment, bag 12 is a plastic, paper, or
otherwise suitably formed bag including a front panel 20, a rear panel 22
intersecting
6
CA 02833482 2013-11-19
UTILITY PATENT APPLICATION
,
,
' Attorney Docket No. 201302670 (101.0675.U01)
along a bottom bag fold line 24. Front panel 20 and rear panel 22 are,
therefore,
positioned opposite one another. In one embodiment, front panel 20 has a
height (i.e.,
extends a distance from bottom bag fold line 24) less than a height of rear
panel 22
(i.e., distance rear panel 22 extends from bottom bag fold line 24).
100271 A side panel 26 is formed on either side of bag 12 to extend between
front
panel 20 and rear panel 22. In one example, each side panel 26 includes a
center
longitudinal fold line 28 extending from bottom bag fold line 24 to a top the
respective side panel 26 to define a front side panel section 30 adjacent
front panel 20
and a rear side panel section 32 adjacent rear panel 22. Bag 12 folds
substantially flat
when longitudinal fold line 28 is moved into bag 12 (i.e., toward opposite
side panel
26) such that front side panel section 30 is folded over, fully contacts, and
extends
substantially coextensively with rear side panel section 32. A storage chamber
36 is
formed between front panel 20, rear panel 22, and side panels 26. Storage
chamber
36 includes a front storage section 38, which extends forwardly from each
longitudinal fold line 28 to front panel 20, and a rear storage section 40,
which
extends rearwardly from each longitudinal fold line 28 to rear panel 22.
[0028] Information insert 14 is printed while filling a consumer's
prescription,
according to one embodiment of the invention. Referring also to the front
surface
view of Figure 6 and the rear surface view of Figure 7, in one embodiment, a
plurality
of prescription information sheets 100 are initially provided to the pharmacy
in a
blank form, e.g., as illustrated in Figure 6, with very little, if any, text
(e.g., shown in
Figure 1) included, but with various portions, including information insert
14, defined
by at least perforations or other lines of indication. As such, a pharmacist
or other
pharmacy employee is able to feed the plurality of prescription information
sheets 100
7
CA 02833482 2013-11-19
,
UTILITY PATENT APPLICATION
' Attorney Docket No. 201302670 (101.0675.U01)
into a printer programmed or coupled with a processor configured to instruct
the
printer to print the proper text, etc. to predefined portions of individual
ones of the
plurality of prescription information sheets 100 to produce the resultant
information
insert 14 for individual prescriptions and other items as shown, for example,
in
Figures 6-14. In one embodiment, each information sheet 100 defines various
colored
or shaded areas generally indicated with diagonal shading, perforations, or
otherwise
defined areas consistent with various features of the innovation described
herein.
[0029] In one embodiment, prescription information sheet 100 is configured to
be
divided into many pieces configured for use in filling a prescription to meet
statutory
and other regulations, to inform the consumer, to facilitate filling and
prescription
tracking, etc. Such pieces may be positioned in any suitable arrangement to
fit special
and content needs, etc. For example, as illustrated, one side of prescription
information sheet 100 defines one or more of guest receipt section 104, drug
information section 106, a pharmacy processing section 150, compliance section
152,
blank section 158, and an information card 160 each separated by perforation
lines
102, 108, 154, 156, 162, and 164 to be easily separated from one another and
used for
their desired purpose. Perforation lines 102, 108, 154, 156, 162, and 164 are
preformed in the blank versions of prescription information sheets 100 as
shown in
Figures 6 and 7.
[0030] More specifically, in one embodiment, prescription information sheet
100
includes a longitudinal, hard perforation line 102 extending from a top edge
to a
bottom edge of prescription information sheet 100. As used herein, "hard
perforation" refers to perforations configured to facilitate ready separation
of adjacent
sections of information sheet 100 from one another without the use of tools,
while
8
CA 02833482 2013-11-19
UTILITY PATENT APPLICATION
Attorney Docket No. 201302670 (101.0675.U01)
"soft perforation" refers to perforations configured to facilitate folding of
adjacent
sections of information sheet 100 that does not readily result in separation
of those
same adjacent sections unless additional separating force is applied. Up to an
entirety
of a portion of prescription information sheet 100 on one side of
longitudinal, hard
perforation line 102 defines information insert 14. In one example,
information sheet
14 includes guest receipt section 104 and drug information section 106. Guest
receipt
section 104 may provide a single guest receipt or be divided into to more than
one
guest receipt, e.g., guest receipts 104a and 104b in Figures 6 and 7, via
lateral
perforation line 105. Each of guest receipts 104a and 104b provides
information
regarding an insurance company or lack thereof associated with the patient,
prescription number, drug identification, patient name, patient address,
patient phone,
date the prescription was filled, refill information, quantity, amount due to
be paid by
the patient, etc. and provides the patient with records for use as they see
fit, for
example, as proof of purchase for insurance companies, as a record of prior
prescriptions, etc.
[0031] Additionally referring to Figure 8, the top of guest receipt section
104, for
example, the top of guest receipt 104a, and therefore the top of information
insert 14
includes patient identifying section 18. Patient identifying section 18
includes patient
information but is generally characterized by an absence of information
identifying
what prescription or other medication is contained in the corresponding bag 12
to
maintain patient privacy during delivery of packaged medication assembly 10 to
the
patient or the designee of the patient. More particularly, in one embodiment,
patient
identifying section 18 includes a colored or shaded portion 120, which is
preprinted to
information sheets 100 prior to individual printing of the information sheets
100 at the
9
CA 02833482 2013-11-19
UTILITY PATENT APPLICATION
Attorney Docket No. 201302670 (101.0675.U01)
pharmacy, to aid in easy identification of information subsequently printed
thereon.
As illustrated, information individually printed on each information sheet 100
as part
of patient identifying section 18 includes the patient's name, address, phone
number,
and date of birth as generally indicated at 110 in the Figures 2 and 8.
100321 Patient identifying section 18 includes an abbreviated partial patient
identifier 114, for example, provided in a larger, bolder, or otherwise
readily
differentiated and, therefore, readily identifiable font as compared to other
printing on
guest receipt section 104. In one instance, abbreviated partial patient
identifier 114
includes the first two letters in the last name of the patient. Other patient
but non-
prescription information may additionally be included at the top of
information insert
14, such as a color code 116 associated with the patient to differentiate the
patient
from other family or household members as well as other patients having
prescriptions filled at the same location as will be further described below.
100331 In one embodiment, other pharmacy usage data is also included as part
of
patient identifying section 18 such as a package or fill date 118 for the
prescription or
other medication. An amount due 122 by the patient at time of pick-up may also
be
printed to patient identifying section 18. In addition, a bar code 124 or
other
computer readable identifier configured to be read by a point-of-sale terminal
to
process associated prescription(s) or medication(s) for sale without requiring
any
other entry of drug, prescription, or similar information at the patient's
transaction for
purchase of the prescription or medication. Referring to Figure 12, rear
surface of
information insert 14 also includes shaded portion 120 medicine and/or patient
information. In one example, drug information is included on shaded portion
120 for
CA 02833482 2013-11-19
UTILITY PATENT APPLICATION
,
,
. Attorney Docket No. 201302670 (101.0675.U01)
easy identification by pharmacy workers, but in a manner substantially hidden
by rear
panel 22 of bag 12 upon assembly of information insert 14 with bag 12.
[0034] Referring to the front views of Figures 6 and 9 and the rear views of
Figures
7 and 13, drug information section 106 is separated from guest receipt section
104 by
perforation line 108. In one embodiment, perforation line 108 is a soft
perforation
line. While guest receipt section 104 may be separated from drug information
section
106 along perforation line 108 without tools, in one example, perforation line
108 is
formed as a soft perforation line to decrease the likelihood that guest
receipt section
104 would inadvertently be separated from drug information section 106 before
a
desired time by the end patient or their designee thereby maintaining the
integrity of
information insert 14 prior to deliver to the patient. Notably, perforation
line 105 may
be hard or soft depending upon desired use for guest receipts 104a and 104b.
In one
example, perforation line 105 is soft.
[0035] Drug information section 106 includes at least information about the
medication that will be included in medicine package assembly 10 (see, e.g.,
Figure 1)
and/or information about the patient themselves and is intended to be an
educational
and/or reference document for the patient. In one example the information
printed to
drug information section 106 includes drug name identification 130 on pre-
printed
color or shaded portion 132. More specific, drug information 136, e.g.,
details
regarding the medication, use, possible side effects, dosage, etc., is
provided on other
portions of drug information section 106. In one embodiment, drug information
section 106 of information insert 14 will be maintained substantially within
chamber
16 of bag 12 behind front panel 20 of bag 12 such that information on drug
information section 106 will be substantially concealed when information
insert 14 is
11
CA 02833482 2013-11-19
UTILITY PATENT APPLICATION
,
,
' Attorney Docket No. 201302670 (101.0675.U01)
part of medicine package assembly 10. Accordingly, drug name identification
130
and/or drug information 136 can be included without the concern for patient
privacy
as considered for inclusion of information on patient identifying section 18
of guest
receipt section 104, which is visible over a top of bag 12. In one example,
drug name
identification 130 and other text on drug information section 106 is printed
in a font
that is compliant with all federal or other associated regulations.
[0036] "Substantially hidden" as used herein refers to portions of information
insert
14 that are entirely hidden and/or otherwise hidden that ones ability to read
indicia on
such portions is greatly hindered or obstructed making it very unlikely that a
nearby
patron other than the patient will be able to read indicia from such portion,
e.g., drug
name identification 130. For example, while when bag 12 is not overfilled,
drug
information section 106 is entirely hidden behind front panel 20 of bag 12,
when bag
12 is overfilled, a portion of drug information section 106 may be partially
visible, but
will largely remain unreadable by nearby patrons other than the patient.
[0037] Referring again to Figures 6 and 7, the portion of information sheet
100 on
an opposite side of longitudinal, hard perforation line 102 as compared to
information
insert 14 includes various sections such as pharmacy use section 150,
compliance or
notes section 152, blank section 158, and/or information card 160. Referring
to 10,
pharmacy use section 150 includes retail information relating to processing of
the
prescription and future prescriptions such as refill prescriptions. In one
embodiment,
pharmacy use section 150 is defined adjacent to longitudinal, hard perforation
line
102 near a top edge of information sheet 100 and/or includes indicia
indicating details
regarding a refill, if applicable, including whether the consumer is enrolled
in an
associated automated refill program, an invitation to enroll in an associated
automated
12
CA 02833482 2013-11-19
UTILITY PATENT APPLICATION
Attorney Docket No. 201302670 (101.0675.U01)
refill program, any refills remain, and/or other notes that the pharmacy
wishes to
communicate to the consumer and/or the pharmacy employees.
[0038] Compliance or notes section 152 is, in the illustrated embodiment,
positioned
adjacent pharmacy use section 150 opposite longitudinal, hard perforation line
102
and, in one example, is separated from pharmacy use section 150 by a
longitudinal,
hard perforation line 154 extending downwardly from a top edge of information
sheet
100 about one-third of the way toward a bottom or opposite edge thereof to
intersect a
horizontal hard perforation line 156 extending substantially parallel to the
top edge of
information sheet 100 from a side edge to longitudinal, hard perforation line
102.
Compliance or notes section 152 may include state compliance information for
the
applicable state in which the prescription is being filled and/or may be left
blank for
notes, etc. Blank section 158 is defined adjacent horizontal hard perforation
line 156
opposite pharmacy use section 150 and compliance or notes section.
[0039] Referring to Figures 6, 7, 11, and 14, information card 160 is defined
adjacent longitudinal, hard perforation line 102 and a lateral hard
perforation line 162,
which is positioned adjacent blank section 158 and extends from longitudinal,
hard
perforation line 102 to a side edge of information sheet opposite information
insert
14, according to one embodiment. In one example, upon assembly of medication
package assembly 10 (Figures 1-5), information card 160 is maintained as part
of
packaged medication 16 as will be described in additional detail below.
Information
card 160 includes a colored or shaded portion 166 along one edge thereof and
defines
a longitudinal and a laterals soft perforation line 164 collectively dividing
information
card 160 in to four quadrants such that information card 160 is readily folded
into a
smaller overall dimension or footprint. According to one embodiment,
information
13
CA 02833482 2013-11-19
,
UTILITY PATENT APPLICATION
,
, Attorney Docket No. 201302670 (101.0675.U01)
card 160 is printed with one or more of an additional drug identifier,
directions for
medication use, prescription number, medication side effects, medication
common
uses, missed dosage instructions, pharmacy information, etc. While described
with a
particular sections, perforation lines, information, etc., other variations to
information
sheet 100 will be apparent to those of skill in the art upon reading this
application.
100401 Returning to Figure 1, packaged medication 16 can take any of a variety
of
forms as commonly presented in pharmacies including medication contained in
boxes,
droppers, bottles, blister packages, vials, plastic zipper close bags, stock
bottles, etc.
One example of packaged medication 16 is illustrated in Figure 1 and includes
a
container such as a bottle 200, a label 230, and a cap 204 covering an opening
to a
storage compartment defined within bottle 200. Bottle 200 comprises a front
portion
210, side portions 212, a spine portion 214, and a rear portion 216, an
opening (not
shown) opposite spine portion 214. Front portion 210 is positioned opposite
rear
portion 216, and one of side portions 212 extends between front portion 210
and rear
portion 216 on either side of bottle 200 to define a storage chamber (not
shown)
therebetween maintain a prescription or other medication (not shown).
[0041] In one embodiment, each packaged medication 16 comprises a ring 220.
Ring 220 encircles a portion of a neck (not shown) of bottle and, in one
example,
includes a color component for uniquely distinguishing between different
bottles 200.
In one embodiment, one color ring 220 represents a first type of medication
while a
second color ring 220 represents a second type of medication. In another
embodiment, one color ring 220 represents a first member of a patient's family
while
a second, different colored ring 220 represents a second member of the same
patient's
family. In one example, the color or pattern of colored ring 220 corresponds
with the
14
CA 02833482 2013-11-19
UTILITY PATENT APPLICATION
' Attorney Docket No. 201302670 (101.0675.U01)
color code 116 (Figure 2 and Figure 8) to facilitate correspondence between
information insert 14 and packaged medication 16 and to provide an additional
check
to the pharmacy worker to quickly be sure the proper packaged medication 16 is
placed in bag 12 with information inset 14.
[0042] Additional uniquely colored rings 220 can represent additional types of
medication or additional family members, respectively. In another embodiment,
different colored rings 220 represent other parameters useful for uniquely
identifying
each single bottle among a plurality of bottles 200. In another embodiment,
ring 220
is not mounted to bottle 200 for using color differentiation via ring 220. In
another
embodiment, ring 220 is removably mounted to bottle 200 but comprises a
neutral
color that does not differentiate between different bottles, such as the color
of bottle
200, differentiate rings 220 of different family members, etc. Accordingly,
ring 200
further contributes to easy-to-follow presentation of information to the
patient and
others. In one example, other color identifiers are used in addition to or as
an
alternative to differently colored rings 200 to differentiate bottles housing
medications
for different family members.
[00431 In one embodiment, a label 230 is applied to substantially planar
surfaces of
front portion 210 and rear portion 216 of bottle 200. Label 230 includes
sections set
off with lines and/or color shading 232. In one embodiment, the presentation
of
information and the use of shading 232 to correspond with medication names,
etc. on
label 230 presents the same general aesthetic look as information insert 14.
In one
embodiment, both information insert 14 and label 230 include, for example, the
drug
name printed over the shading 232 to effectively highlight the drug name. In
this
manner one comparing the information inset 14 to the label 230 of bottle 200
can
CA 02833482 2013-11-19
UTILITY PATENT APPLICATION
,
' Attorney Docket No. 201302670 (101.0675.U01)
quickly determine that the information insert 14 corresponds with the
medication in
bottle 200.
[0044] Label 230 extends over front portion 210 and/or rear portion 216. In
one
embodiment, a portion of label 230 extending over front portion 210 is
adhesive free
such that a slot 240, which is generally indicated in Figure 1, is defined
between front
portion 210 and label 230. Slot 240 is sized and shaped to selectively receive
information card 160 from information sheet 100 when information card 160 is
folded
about soft perforation lines 164. In this manner, information card 160 can
laterally
slide into and out of slot 240. In one instance, shaded portion 166 of
information card
160 always remains outside of slot 240 to allow for easier identification of
information card 160 and easier gripping of information card 160 to pull it
out of slot
240.
[0045] Figure 15 illustrates a method 300 of using medication packaging system
10
to fill a prescription or other order for medication (or a method of
administering
pharmaceuticals to patients), according to one embodiment of the present
invention.
At 302, a pharmacist or technician enters or recalls patient and prescription
information into a computer control module (via a graphical user interface)
and then
directs printing of that information onto information sheets 100, which is
loaded into
or has already been loaded into the associated printer. A hardware processor
then
directs printing of the appropriate portions of front and back surfaces of one
of the
information sheets, with information relating to the particular prescription
being filed
in the particular sections of information sheet 100 described above (e.g., one
or more
of guest receipt section 104, drug information section 106, pharmacy
processing
16
CA 02833482 2013-11-19
UTILITY PATENT APPLICATION
,
, Attorney Docket No. 201302670 (101.0675.U01)
section 150, compliance section 152, blank section 158, and information card
160)
and/or other suitable sections.
[0046] Following printing, at 304, information sheet 304 is torn along
longitudinal,
hard perforation lines 102 to separate information insert 14 from a remainder
of
information sheet 100. Other sections, such as pharmacy processing section
150,
compliance section 152, blank section 158, and information card 160 may also
be
separated at 304 and/or separated at a future time as the various sections are
needed.
In one embodiment, one or more of pharmacy processing section 150, compliance
section 152, and blank section 158 may be left attached to information insert
14 and
simply be folded back about longitudinal, hard perforation 102 for placement
in bag
12 and easy access to those sections during processing the prescription or
other
medication therein for sale.
100471 Then, at 306, information insert 14 is placed in storage chamber 36 of
bag
12, more particularly, within front storage section 38 of storage chamber 36
between
front panel 20 and front side panel sections 30 of side panels 26 of bag.
Information
insert 14 is sized to fit within bag 12 without folding or other manipulation
of the
overall dimensions of information insert 14, in one example. The height of
information insert 14 is configured such that a bottom edge of information
insert 14 is
placed in bag immediately adjacent and in very near or direct contact with an
internal
side of bottom bag fold line 24. Substantially only patient identifying
section 18 of
information insert 14 extends above a top edge of front panel 20 of bag 12 as
illustrated, for example, in Figure 2. Since at least a front surface of
patient
identification section 18 is characterized by an absence of human readable
prescription or medication identifying information as only bar code 124 of
patient
17
CA 02833482 2013-11-19
UTILITY PATENT APPLICATION
Attorney Docket No. 201302670 (101.0675.U01)
identifying section 18 includes prescription identifying information, this
positioning
of information insert 18 in bag 12 preserves the privacy of the patient from
prying or
wandering eyes of other patients, customers, etc. In other words,
substantially all of
drug information section 106 and/or the drug information 136 or other medicine
identifying indicia on drug information section 106 are concealed from view by
front
panel 20 and rear panel 22 of bag 12.
100481 Concurrently, before, or after steps 302, 304, and 306, at 308, label
230 of
bottle 200 is printed. As for step 302, pharmacist or technician enters or
recalls
patient and prescription information into a computer control module (via a
graphical
user interface) and then directs printing of that information onto a blank
label sheet
(not shown), which is loaded into or has already been loaded into the
associated
printer. In one embodiment, the label sheet is a multiple layer sheet and
configured
such that individual label sections can be peeled out of the label sheet and
adhered to
bottle 200 via adhesive already present as part of the label sheet. A hardware
processor then directs printing of the appropriate portions label 230, with
information
relating to the particular prescription being filled. In one embodiment, a
standard
black and white laser printer is used for printing at 302 while a thermal-
printing is
used at 308. In one example, when the pharmacist or technician enters or
recalls
patient and prescription information into the computer control module (via a
graphical
user interface) and then directs printing, information sheet 100 is
automatically or can
be designated to be printed substantially simultaneously with printing label
230 at 308
without requiring the pharmacist or technical to separate recall prescription
information at each printing step 302 and 308.
18
CA 02833482 2013-11-19
UTILITY PATENT APPLICATION
Attorney Docket No. 201302670 (101.0675.U01)
100491 At 310, the selected label 230 is removed from a remainder of its label
sheet,
and label 230 is placed on bottle 200. For example, for some bottles 200,
label 230 is
applied over and pressed to adhere it to each of front portion 210, spine
portion 214,
and rear portion 216. In one embodiment, a portion of label 230 corresponding
with a
portion of front portion 210 is characterized by an absence of adhesive such
that slot
240 is defined between label 230 and front portion 210 with an opening thereto
formed on side of label 230, e.g., near a side portion 212.
100501 Information card 160, which was separated from a remainder of
information
sheet 100 at 304, is folded along soft perforation lines 164 and slid through
the
opening into slot 240 to be substantially maintained between label 230 and
front
portion 210 at 312. Once bottle 200 is properly labeled at 310 and 312, then
at 314,
the pharmacist or technician fills bottle 200 with the appropriate kind,
dosage, and
amount of medication as indicated on the patient's prescription or other drug
order
previously received. In other examples, bottle 200 may be filled with
medication
prior to labeling bottle 200 at 310 and 312. At 316, bottle 200 and all of
packaged
medication 16 is placed into bag 12 behind information insert 14, that is,
between
information insert 14 and rear panel 22 of bag 12. In this manner, insertion
of
packaged medication 16 does not significantly impact the amount of information
insert 14 covered by front panel 20 of bag 12, which continues the integrity
of the
patient privacy measures achieved by packaged medication assembly 10.
100511 At 318, packaged medication assembly 10 is placed in bin 82 or other
container, file, stack, array, etc. to await pick up by the patient or her
designee as
illustrated, for example, in Figure 5. In one embodiment, packaged medication
assembly 10 is placed in bin 82 with other packaged medication assemblies 10
19
CA 02833482 2013-11-19
UTILITY PATENT APPLICATION
Attorney Docket No. 201302670 (101.0675.U01)
arranged in alphabetical order. In this manner, the resultant array of
packaged
medication assemblies 10 are presented with the respective patient identifying
sections 18 extending above bags 12 for easy viewing. Notably, while one bin
82 is
illustrated, it should be understood that a pharmacy will likely have many
bins 82, for
example, one or more for each first letter of patient last names, with the
resultant
array of packaged medication assemblies 10 therein all being arranged
alphabetically
or in some other designated order.
[0052] After time has passed since 318, at 320, the pharmacist, technician, or
other
pharmacy employee locates and selects one of the previously assembled packaged
medication assemblies 10 that corresponds to a patient who has arrived at the
pharmacy (or whose designee has arrived at the pharmacy) to pick up the
packaged
medication 16. In locating the desired one of the previously assembled
packaged
medication assemblies 10, the appropriate bin 82 is determined, and patient
identifying sections 18 of the array of packaged medication assemblies 10 are
viewed
to determine the ones that have an partial patient identifier 114
corresponding to the
patient. If more than one of the array of packaged medication assemblies 10
has the
desired partial patient identifier 114, then the patient identifying sections
18 of the
array of packaged medication assemblies 10 are viewed closer, for example,
patient
information 110 is viewed to select the one or more packaged medication
assemblies
corresponding to the current pick up request. This method of identification is
simplified as compared to prior art as the one or more packaged medication
assemblies 10 being picked up can be visually identified without flipping
through the
array of the packaged medication assemblies 10 to verify their inclusion in
the one or
more packaged medication assemblies 10 being picked up. Once identified, the
one
CA 02833482 2013-11-19
UTILITY PATENT APPLICATION
Attorney Docket No. 201302670 (101.0675.U01)
or more packaged medication assemblies 10 are taken to the patient or designee
and
are processed for sale at the point-of-sale terminal (not shown) at 322. In
one
example, processing the one or more packaged medication assemblies 10 includes
scanning bar code 124 at the point-of-sale terminal.
100531 Although specific embodiments have been illustrated and described
herein, it
will be appreciated by those of ordinary skill in the art that a variety of
alternate
and/or equivalent implementations may be substituted for the specific
embodiments
shown and described without departing from the scope of the present invention.
This
application is intended to cover any adaptations or variations of the specific
embodiments discussed herein. Therefore, it is intended that this invention be
limited
only by the claims and the equivalents thereof.
21